INTENDED USE
HepaSphere™ Microspheres are indicated
for use in embolization of blood vessels
with or without delivery of doxorubicin HCl
for therapeutic or preoperative
purposes in the following procedures:
• Embolization of hepatocellular
carcinoma
• Embolization of metastases to the liver.
HepaSphere Microspheres loaded with
irinotecan are indicated for use in:
• Embolization of metastatic colorectal
cancer (mCRC) to the liver.
DESCRIPTION
HepaSphere Microspheres are part of a
family of embolic agents based on pro-
prietary technologies. They are designed
for controlled, targeted embolization. The
HepaSphere Microspheres can be loaded
with doxorubicin HCl or irinotecan, and are
able to release the drug locally at the em-
bolization site. HepaSphere Microspheres
are biocompatible, hydrophilic, non-re-
sorbable, expandable, and conformable
microspheres. HepaSphere Microspheres
swell upon exposure to aqueous solutions.
They are available in a range of sizes.
DEVICE PACKAGING
HepaSphere Microspheres are contained
in a sterile, 10 ml vial, with a crimped cap,
packaged in a sealed pouch.
Contents: 25 mg or 50 mg of dry
HepaSphere Microspheres per vial to be
reconstituted before use.
CONTRAINDICATIONS
• Patients intolerant to vascular occlusion
procedures
• Vascular anatomy or blood ow pre-
cluding correct catheter placement or
embolic injection
• Presence or suspicion of vasospasm
• Presence or likely onset of haemorrhage
• Presence of severe atheromatous
disease
• Presence of collateral vessel pathways
potentially endangering normal territo-
ries during embolization
• High ow arteriovenous shunts or
stulae with luminal diameter greater
than the selected size of HepaSphere
Microspheres
• Vascular resistance peripheral to the
feeding arteries precluding passage
of HepaSphere Microspheres into the
lesion
• Do not use in pulmonary vasculature,
coronary and central nervous system
vasculature
• Known sensitivity to poly vinyl alco-
hol-co-sodium acrylate
WARNINGS
• HepaSphere Microspheres size must be
chosen after consideration of the arte-
riovenous angiographic appearance.
HepaSphere Microspheres size should
be selected both to be appropriate for
the size of the vessel feeding the target
and to prevent passage from artery
to vein.
• Some of the HepaSphere Microspheres
may be slightly outside of the range,
so the physician should be sure to
carefully select the size of HepaSphere
Microspheres according to the size of
the target vessels at the desired level of
occlusion in the vasculature and after
consideration of the arteriovenous
angiographic appearance.
• Because of the signicant compli-
cations of untargeted embolization,
extreme caution should be used for any
procedures involving the extracranial
circulation encompassing the head
and neck, and the physician should
carefully weigh the potential benets
of using embolization against the risks
and potential complications of the
procedure. These complications can
include blindness, hearing loss, loss of
smell, paralysis, and death.
• Serious radiation induced skin injury
may occur to the patient due to long
periods of uoroscopic exposure, large
patient, angled x-ray projections and
multiple image recording runs or radio-
graphs. Refer to your facility’s clinical
protocol to ensure the proper radiation
dose is applied for each specic type of
procedure performed.
• Onset of radiation injury to the patient
may be delayed. Patients should be
counselled on potential radiation
eects, what to look for and whom to
contact if symptoms occur.
• HepaSphere Microspheres MUST NOT
be reconstituted in sterile water for
injection. Reconstitution in sterile
INSTRUCTIONS FOR USE
English
Dry(μm) 30-60 50-100 100-150 150-200
730142001_001_hepasphere_irinotecan.indd 1 1/7/16 8:22 AM

water results in extensive swelling that
renders the injection of HepaSphere
Microspheres very dicult or may
prevent injection.
• Do not reconstitute HepaSphere Micro-
spheres with Lipiodol / Ethiodol.
• Pay careful attention for signs of untar-
geted embolization. During injection
carefully monitor patient vital signs to
include SaO
2
(e.g. hypoxia, CNS chang-
es). Consider terminating the procedure,
investigating for possible shunting, or
increasing Microspheres size if any signs
of untargeted embolization occur or
patient symptoms develop.
• Consider upsizing the Microspheres if
angiographic evidence of embolization
does not quickly appear evident during
injection of the Microspheres.
Warnings about use of small micro-
spheres:
• Careful consideration should be given
whenever use is contemplated of em-
bolic agents that are smaller in diameter
than the resolution capability of your
imaging equipment. The presence of
arteriovenous anastomoses, branch ves-
sels leading away from the target area
or emergent vessels not evident prior
to embolization can lead to untargeted
embolization and severe complications.
• Microspheres smaller than 100 microns
are more likely to terminate circulation
to distal tissue. Greater potential of isch-
emic injury results from use of smaller
sized microspheres and consideration
must be given to the consequence of
this injury prior to embolization. The
potential consequences include swell-
ing, necrosis, paralysis, abscess and/or
stronger post-embolization syndrome.
• Post embolization swelling may result
in ischemia to tissue adjacent to target
area. Care must be given to avoid
ischemia of intolerant, non targeted
tissue such as nervous tissue.
PRECAUTIONS
HepaSphere Microspheres must only be
used by physicians trained in vascular
embolization procedures. The size and
quantity of microspheres must be carefully
selected according to the lesion to be
treated and the potential presence of
shunts. Only the physician can decide the
most appropriate time to stop the injection
of HepaSphere Microspheres.
Do not use if the vial, cap, or pouch
appear damaged.
For single patient use only - Contents
supplied sterile - Never reuse, reprocess,
or resterilize the contents of a vial that
has been opened. Reusing, reprocessing or
resterilizing may compromise the structural
integrity of the device and or lead
to device failure, which in turn may result
in patient injury, illness or death. Reusing,
reprocessing or resterilizing may also
create a risk of contamination of the device
and or cause patient infection or cross
infection including, but not limited to,
the transmission of infectious disease(s)
from one patient to another. Contamina-
tion of the device may lead to injury,
illness or death of the patient. All proce-
dures must be performed according to
accepted aseptic technique.
HepaSphere Microspheres MUST NOT be
used in their original dry state. They must
be reconstituted before use. HepaSphere
Microspheres swell in aqueous solution.
The magnitude of swelling depends on the
ionic concentration of the solution. The
microspheres swell to approximately four
times their diameter in 0.9% NaCl aqueous
solution and nonionic contrast media, as
compared to their initial dry diameter. The
magnitude of swelling when loaded with
doxorubicin HCl is dependent upon the
amount of drug with which the product is
loaded. Lyophilized doxorubicin HCl must
be reconstituted in NaCl 0.9 % solution.
HepaSphere Microspheres undergo a
size decrease of about 20% when loaded
with doxorubicin HCl, and 30% when
loaded with irinotecan compared to the
size in pure NaCl 0.9 % aqueous solution.
HepaSphere Microspheres are compress-
ible and can be injected easily through
microcatheters. However, injection of the
HepaSphere Microspheres before they are
fully expanded could result in failure to
reach the intended embolization target
and possible embolization of a larger tissue
area.
Note: Maximum recommended concen-
tration of doxorubicin HCl is 5mg/ml.
Concentrations of doxorubicin HCl above
5mg/ml substantially increase the solution
viscosity and make it dicult to handle
with HepaSphere Microspheres.
Maximum recommended concentration of
irinotecan is 20 mg/ml.
730142001_001_hepasphere_irinotecan.indd 2 1/7/16 8:22 AM
Patients with known allergies to non-ionic
contrast media may require corticosteroids
prior to embolization. Additional evalua-
tions or precautions may be necessary in
managing periprocedural care for patients
with the following conditions:
• Bleeding diathesis or hypercoagulative
state
• Immunocompromise
Note: If loading HepaSphere Microspheres
with doxorubicin HCl or irinotecan, refer to
the appropriate drug IFU for information
concerning contraindications, warnings,
precautions, potential complications, dos-
age, and patient management before use.
POTENTIAL COMPLICATIONS
Vascular embolization is a high-risk
procedure. Complications may occur at
any time during or after the procedure,
and may include, but are not limited to, the
following:
• Paralysis resulting from untargeted
embolization or ischemic injury from
adjacent tissue oedema
• Undesirable reux or passage of
HepaSphere Microspheres into normal
arteries adjacent to the targeted lesion
or through the lesion into other arteries
or arterial beds, such as the internal
carotid artery, pulmonary, or coronary
circulation
• Pulmonary embolism due to arteriove-
nous shunting
• Ischemia at an undesired location,
including ischemic stroke, ischemic
infarction (including myocardial infarc-
tion), and tissue necrosis
• Capillary bed occlusion and tissue
damage
• Vasospasm
• Recanalisation
• Blindness, hearing loss, and loss of smell
• Foreign body reactions necessitating
medical intervention
• Infection necessitating medical inter-
vention
• Complications related to catheterization
(e.g. haematoma at the site of entry, clot
formation at the tip of the catheter and
subsequent dislodgement, and nerve
and/or circulatory injuries which may
result in leg injury)
• Allergic reaction to medications (e.g.
analgesics)
• Allergic reaction to non-ionic contrast
media or embolic material
• Vessel or lesion rupture and hemor-
rhage
• Death
• Additional information is found in the
Warnings section
SWELLING BEHAVIOR
HepaSphere Microspheres swell during
reconstitution with NaCl 0.9% aqueous
solution and non-ionic contrast media.
When hydrated in 100% NaCl 0.9% aque-
ous solution or non-ionic contrast medium,
or 50% non-ionic contrast and 50% NaCl
0.9% aqueous solution, HepaSphere Micro-
spheres swell approximately 4 times their
original dry diameter in approximately 10
minutes. For example, HepaSphere Micro-
spheres with a diameter of approximately
50-100 microns in their dry state will
expand to approximately 200-400 microns
during reconstitution as recommended
below. Because of the inherent variabil-
ity of the swelling process, some of the
HepaSphere Microspheres will be slightly
outside of this range after reconstitution,
so the physician should be sure to carefully
select the size of HepaSphere Microspheres
according to the size of the target vessels
at the desired level of occlusion in the
vasculature and the nature of the aqueous
solution.
Note: To expand properly HepaSphere
Microspheres need to be exposed to a
minimum of 10 ml of solution for doxoru-
bicin HCl or saline and a minimum of 5 ml
for irinotecan. The magnitude of swelling
when loaded with doxorubicin HCl is
dependent upon the amount of drug with
which the product is loaded. HepaSphere
Microspheres undergo a size decrease of
about 20% when loaded with doxorubicin
HCl compared to the size in pure NaCl 0.9%
aqueous solution and about 30 % when
loaded with irinotecan.
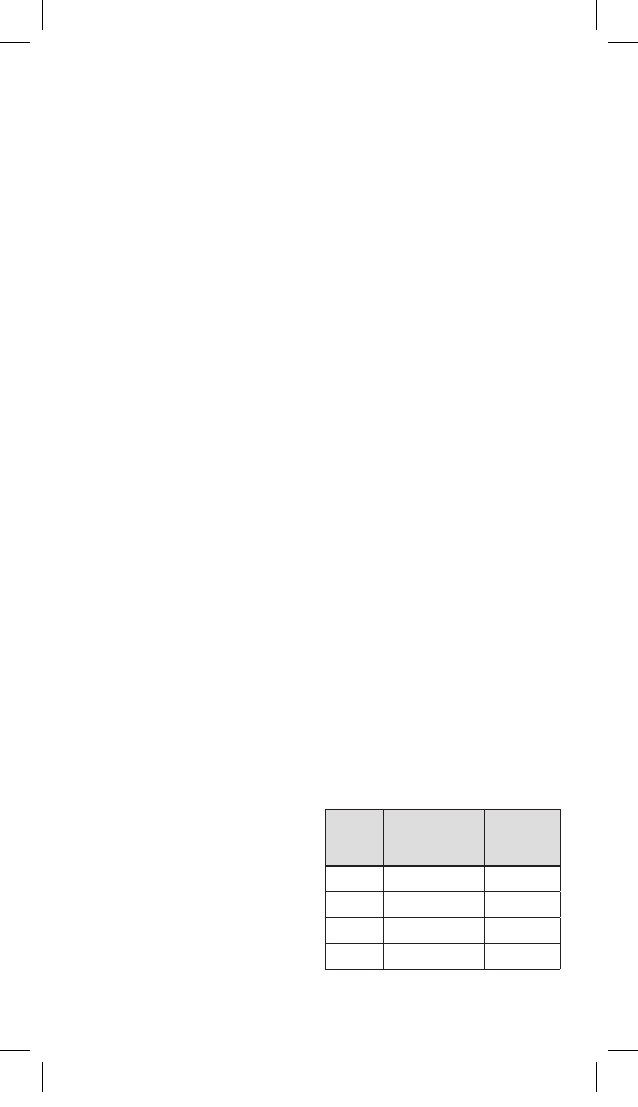
CATHETER COMPATIBILITY
HepaSphere Microspheres can be injected
with microcatheters with the following
specications:
Dry
(μm)
Approximate
Reconstituted
Size range (μm)
Catheter
Size ID(in.)
30-60 120-240 ≥0.021
50-100 200-400 ≥0.021
100-150 400-600 ≥0.024
150-200 600-800 ≥0.027
730142001_001_hepasphere_irinotecan.indd 3 1/7/16 8:22 AM

INSTRUCTIONS
HepaSphere Microspheres must be recon-
stituted with 100% NaCl 0.9% aqueous
solution or non-ionic contrast medium,
or 50% non-ionic contrast medium and
50% NaCl 0.9% aqueous solution if using
without delivery of doxorubicin HCl or
irinotecan, or loaded with doxorubicin
HCl solution or irinotecan solution before
positioning the catheter.
• Carefully select the size of HepaSphere
Microspheres according to the size of
the target vessels at the desired level
of occlusion in the vasculature and the
nature of the aqueous solution. See the
description of “SWELLING BEHAVIOR”.
• HepaSphere Microspheres may be
present outside the vial. Therefore, the
vial must be aseptically handled away
from the main sterile eld.
• Ensure the compatibility of the
HepaSphere Microspheres with the
intended size of catheter to be used.
See the table above.
• Inspect the packaging to conrm that
it is intact. Remove the vial from the
pouch. The external surface of the vial
is sterile.
• To prevent coring the rubber stopper,
insert the injection needle as follows:
1. Hold the needle so that the bevel
faces upwards and position the tip
diagonally to the insertion site. Press
the tip against the centre of the
insertion site.
2. Apply a gentle force to the needle in
the opposite direction to the bevel
to ease the needle into the insertion
site until the heel section of the
needle is no longer visible. Be careful
not to scrape o the upper-facing
surface of the rubber cap with the
heel of the needle tip.
3. Continuing to apply a gentle force to
the needle in the opposite direction
to the bevel, slowly insert the needle
vertically through the rubber cap.
4. After preparation, carefully examine
the solution to determine if there are
any rubber impurities present. If the
solution appears contaminated, do
not use it.
HEPASPHERE MICROSPHERES CAN BE
USED WITH OR WITHOUT LOADING OF
DOXORUBICIN HCl OR IRINOTECAN.
OPTION 1: PREPARATION FOR EMBOLI-
ZATION WITHOUT DRUG (BLAND)
The approximate reconstitution time when
used without loading of a drug is 10 min.
• Fill a 10ml syringe with 100% NaCl 0.9%
aqueous solution or non-ionic contrast
medium (or 50% NaCl 0.9% aqueous
solution and 50% contrast). Connect
the syringe to a needle of 20 gauge
diameter or larger.
• To ensure proper reconstitution of
the HepaSphere Microspheres, grasp
the vial horizontally in your ngertips
and roll the vial several times. This will
transfer the dry contents of the vial to
the sidewall.
Note: Pull back only the ip-top cap; do
not remove the crimp ring or the stopper
from the vial.
• Carefully insert the needle from the
syringe through the stopper of the
vial. Continue rolling the vial in your
ngertips and inject the full amount
(10ml) of reconstitution medium into
the vial, then place the vial vertically
and carefully remove the syringe with
the needle attached.
Note: The vial is hermetically closed. If
aspiration from the syringe into the vial
does not automatically occur, then, using
caution, manually aspirate air from the
vial into the syringe prior to injecting the
reconstitution uid. Proper aspiration and/
or venting techniques, as approved by
the healthcare facility, may be used for
easier injection of reconstitution medium
into vial. If aspiration of air from the vial is
performed prior to reconstitution, exercise
caution not to remove the spheres from
the vial.
• To ensure a homogeneous reconstitu-
tion of the HepaSphere Microspheres,
gently invert the vial back and forth
so that the liquid contacts the stopper
5-10 times.
• Note: Vigorous shaking may introduce
micro bubbles, which can cause the
microspheres to aggregate.
• Wait a minimum of 10 minutes to
allow the HepaSphere Microspheres to
reconstitute and expand fully.
• Use a 30ml syringe and 20 gauge or
larger needle to aspirate the contents
of the vial. Rotate the vial to a vertical
position with the bottom of the vial
facing upward. Pull the needle back so
(3)
(1)
(2)
DIRECTION OF FORCE
DIRECTION OF FORCE
DIRECTION OF FORCE
(3)
(1)
(2)
DIRECTION OF FORCE
DIRECTION OF FORCE
DIRECTION OF FORCE
(3)
(1)
(2)
DIRECTION OF FORCE
DIRECTION OF FORCE
DIRECTION OF FORCE
730142001_001_hepasphere_irinotecan.indd 4 1/7/16 8:22 AM

that it is submerged in the liquid but
not occluded by the stopper. Gently
aspirate the entire contents of the vial
into the syringe.
Note: If the air was previously aspirated
from the vial, gentle injection of air using
the syringe prior to aspirating the contents
of the vial will ensure an easier aspiration of
vial contents into the syringe. If all contents
are not withdrawn, introduce an additional
volume of air and repeat the aspiration
process. It is possible to add an additional
amount of non-ionic contrast or NaCl 0.9%
aqueous solution into the syringe in order
to get a higher dispersion of microspheres.
Note: HepaSphere Microspheres reconsti-
tuted as described above can be used in
the presence of chemotherapeutic agents
such as cisplatin, epirubicin, doxorubicin
HCl, uorouracil, irinotecan and mitomycin
after hydration. However for drug delivery,
HepaSphere Microspheres are only
indicated for use with doxorubicin HCl (see
below Option 2) or irinotecan (see below
Option 3).
• If microspheres were reconstituted
using 100% NaCl 0.9%, non-ionic
contrast medium must be added to
the syringe containing the HepaSphere
Microspheres for visualization under
uoroscopy. If non-ionic contrast
medium was used to reconstitute the
microspheres, additional non-ionic
contrast medium may be added.
OPTION 2: PREPARATION FOR
EMBOLIZATION LOADED WITH
DOXORUBICIN HCl
WARNING: Liposomal formulations of
doxorubicin HCl are not suitable for loading
into HepaSphere Microspheres.
As a general guideline the loading of
lyophilized doxorubicin HCl solubilized in
NaCl 0.9% solution into HepaSphere Micro-
spheres will take one hour. The HepaSphere
Microspheres should not be used before
they are fully hydrated and expanded.
Loading kinetics of pre-solubilized doxo-
rubicin HCI may vary, depending on the
concentration and pH of the solution.
• Choose the appropriate dose of doxo-
rubicin HCl to load into the HepaSphere
Microspheres.
Note: A maximum dose of doxorubicin HCl
75mg can be loaded into each vial of 25 mg
HepaSphere Microspheres. Solubilize the
desired dose of lyophilized doxorubicin HCl
in 20ml of NaCl 0.9% solution for injection.
NEVER USE PURE WATER
Note: Maximum recommended concen-
tration of doxorubicin HCl is 5mg/ml.
Concentrations of doxorubicin HCl above
5mg/ml substantially increase the solution
viscosity and make it dicult to handle
with HepaSphere Microspheres.
• Aspirate the 20ml of doxorubicin HCl
solution into two separate 30ml syring-
es. Each 30ml syringe should contain
10ml of doxorubicin HCl solution.
• Connect one of the 30ml syringes
containing 10ml of the doxorubicin
HCl solution to a needle of 20 gauge
diameter or larger.
• To ensure proper reconstitution of the
HepaSphere Microspheres, grasp the
HepaSphere Microspheres vial horizon-
tally in your ngertips and roll the vial
several times. This will transfer the dry
contents of the vial to the sidewall.
• Note: Pull back only the ip-top cap;
do not remove the crimp ring or the
stopper from the vial.
• Carefully insert the needle of one of
the 30ml syringes containing 10ml of
doxorubicin HCl solution through the
stopper of the vial. Continue rolling the
vial in your ngertips and inject the full
10ml of doxorubicin HCl solution into
the vial.
• Place the HepaSphere Microspheres vial
vertically. Carefully remove the syringe
with the needle attached, and allow the
vial to stand for 10 minutes in order to
completely hydrate the spheres.
• During the 10 minutes hydration period,
shake the HepaSphere Microspheres
vial several times back and forth so that
the liquid contacts the grey stopper.
Repeat this process every 2-3 minutes
to ensure a homogenous reconstitution
of the HepaSphere Microspheres.
Note: The vial is hermetically closed. If
aspiration from the syringe into the vial
does not automatically occur, then, using
caution, manually aspirate air from the
vial into the syringe prior to injecting the
reconstitution uid. Proper aspiration and/
or venting techniques, as approved by the
healthcare facility, may be used for easier
injection of reconstitution media into
the vial. If aspiration of air from the vial is
performed prior to reconstitution, exercise
caution not to remove the spheres from
the vial.
• After the 10 minutes hydration period,
attach a 20 gauge or larger needle to
the second 30ml syringe containing
730142001_001_hepasphere_irinotecan.indd 5 1/7/16 8:22 AM

the remaining 10ml of doxorubicin HCl
solution and insert into the HepaSphere
Microspheres vial. Aspirate the contents
of the HepaSphere Microspheres vial
into the 30ml syringe containing the
remaining 10 ml of doxorubicin HCl
solution. Rotate the vial to a vertical
position with the bottom of the vial
facing upward. Pull the needle back so
that it is submerged in the liquid but
not occluded by the stopper. Gently
aspirate the entire contents of the vial
into the syringe.
• Prior to removing the needle from the
HepaSphere Microspheres vial, while
holding the syringe vertically, gently
pull the plunger of the syringe down,
removing any solution that may be in
the hub of the needle.
• Replace the needle with a syringe cap
and invert the syringe back and forth
to disperse the contents within the
syringe.
• Wait a minimum of 60 minutes to
allow the HepaSphere Microspheres to
expand fully and load the doxorubicin
HCl. During the 60 minutes, the syringe
should be inverted every 10 – 15
minutes in order to optimize the drug
distribution into the spheres.
• After 60 minutes, let the syringe stand
for the spheres to settle down and
purge all supernatant and discard it
following facility approved standards.
• Add a minimum of 20ml of non-ionic
contrast medium to the 30ml syringe
containing the doxorubicin HCl loaded
HepaSphere Microspheres, however
larger volume of solution can provide
better control during embolization.
Gently invert the syringe 2 or 3 times
and wait 5 min until solution homoge-
neity is reached.
• Before any injection, check the spheres
are in suspension, if not, invert the
syringe back and forth to disperse
contents within the syringe.
OPTION 3: PREPARATION FOR
EMBOLIZATION LOADED WITH
IRINOTECAN
HepaSphere Microspheres loaded with
irinotecan are only applicable to the 30-60μ
and 50-100μ sizes.
As a general guideline the loading of
irinotecan into HepaSphere Microspheres
will take 30 minutes. The HepaSphere Mi-
crospheres should not be used before they
are fully hydrated and expanded.
• Choose the appropriate dose of
irinotecan solution to load into the
HepaSphere Microspheres. A maximum
dose of 100 mg irinotecan can be load-
ed in each vial of 25 mg HepaSphere
MicroSpheres. Irinotecan solution is
typically available in a concentration of
20 mg/ml.
• Aspirate the irinotecan into a syringe
connected to a needle of 20 gauge
diameter or larger.
• To ensure proper reconstitution of the
HepaSphere Microspheres, grasp the
HepaSphere Microspheres vial horizon-
tally in your ngertips and roll the vial
several times. This will transfer the dry
contents of the vial to the sidewall.
Note: Pull back only the ip-top cap; do
not remove the crimp ring or the stopper
from the vial.
• Carefully insert the needle of the
syringe containing the irinotecan
solution through the stopper of the vial.
Continue rolling the vial in your nger-
tips and inject the irinotecan solution
into the vial.
• Place the HepaSphere Microspheres vial
vertically. Carefully remove the syringe
with the needle attached, and allow the
vial to stand for 30 minutes in order to
completely hydrate the spheres.
• During those 30 minutes, shake the
HepaSphere Microspheres vial several
times back and forth so that the liquid
contacts the grey stopper. Repeat this
process every 2-3 minutes to ensure
a homogenous reconstitution of the
HepaSphere Microspheres.
Note: The vial is hermetically closed. If
aspiration from the syringe into the vial
does not automatically occur, then, using
caution, manually aspirate air from the
vial into the syringe prior to injecting the
reconstitution uid. Proper aspiration
and/or venting techniques, as approved
by the healthcare facility, may be used for
easier injection of reconstitution media
into the vial. If aspiration of air from the
vial is performed prior to reconstitution,
exercise caution not to remove the spheres
from the vial.
• After the 30 minutes hydration and
loading period, attach a 20 gauge
or larger needle to an appropriately
sized syringe and insert it into the
HepaSphere Microspheres vial. Aspirate
the contents of the HepaSphere Mi-
crospheres vial into the syringe. Rotate
the vial to a vertical position with the
730142001_001_hepasphere_irinotecan.indd 6 1/7/16 8:22 AM

bottom of the vial facing upward. Pull
the needle back so that it is submerged
in the liquid but not occluded by the
stopper. Gently aspirate the entire con-
tents of the vial into the syringe.
• Prior to removing the needle from the
HepaSphere Microspheres vial, while
holding the syringe vertically, gently
pull the plunger of the syringe down,
removing any solution that may be in
the hub of the needle.
• Replace the needle with a syringe cap
and invert the syringe back and forth
to disperse the contents within the
syringe.
• Add an equal volume of non-ionic
contrast medium to the syringe contain-
ing the irinotecan loaded HepaSphere
Microspheres immediately before use.
• Larger volume of non-ionic contrast me-
dia can lead to irinotecan release into
the supernatant.
• Gently invert the syringe 2 or 3 times
and wait 5 min until solution homoge-
neity is reached.
• Before any injection, check that the
microspheres are in suspension. If not,
invert the syringe back and forth to
disperse contents within the syringe.
• Do not remove the supernatant.
DELIVERY INSTRUCTIONS
• Carefully evaluate the vascular network
associated with the target lesion utiliz-
ing high resolution imaging.
Note: It is important to determine if any
arteriovenous shunts are present before
beginning embolization.
• Using standard techniques, position
the delivery catheter within the target
vessel and the catheter tip as close as
possible to the embolization target.
• Use an injection syringe no larger than
3ml for the delivery of doxorubicin/
irinotecan/bland loaded HepaSphere
Microspheres. Use of a 1ml injection
syringe is recommended.
• Aspirate the HepaSphere Microspheres
mixture into the injection syringe.
• Two methods for embolic aliquot
sequestering for injection may be used:
Option 1: Connect a 3way-stopcock to
the syringe containing the doxorubicin/
irinotecan/bland loaded HepaSphere
Microspheres to the infusion micro
catheter and use a 1ml syringe for
injection through the open port of the 3
way-stopcock.
Option 2: Serial aliquots of the
doxorubicin/irinotecan/bland loaded
HepaSphere Microspheres can be
drawn from the syringe into a 1ml
injection syringe through a 3 way-stop
cock that is not attached to the infusion
catheter. The 1ml syringe containing
each aliquot can be attached inde-
pendently to the infusion microcatheter
and injected.
• Invert the syringe back and forth to
maintain the homogenous suspen-
sion of the HepaSphere Microspheres
mixture.
• Under continuous uoroscopic guid-
ance, inject the aliquot of HepaSphere
Microspheres in a slow, non forceful,
pulsatile manner over a time period
of approximately 1 minute per ml of
microspheres solution. Always inject
under free-ow conditions and monitor
for reux.
Note: Reux of embolic spheres can induce
immediate ischemia of untargeted tissues
and vessels.
• When stasis in the feeding pedicle oc-
curs while delivering the doxorubicin/
irinotecan/bland loaded HepaSphere
Microspheres, wait a minimum of 5
minutes then perform a selective an-
giogram after the full 5 minutes wait to
verify the cessation of antegrade ow.
• If cessation of antegrade ow has not
occurred, continue infusion under
uoroscopic guidance until the desired
devascularization is obtained.
• After the HepaSphere Microsphere
infusion is completed, remove the
catheter while maintaining gentle aspi-
ration to avoid dislodging any residual
HepaSphere Microspheres that may still
be in the catheter lumen. Discard the
catheter after removal and do not reuse.
• Discard any open vial or unused
HepaSphere Microspheres.
CAUTION
In the event that the catheter becomes
obstructed or signicant infusion resistance
is encountered during injection, do not at-
tempt to ush the catheter with excessive
pressure because reux of embolic material
may occur resulting in untargeted emboli-
zation. Remove the catheter while applying
gentle aspiration and discard.
CONSERVATION AND STORAGE
HepaSphere Microspheres must be stored
in a dry, dark place in their original vials
and packaging. Use by the date indicated
on the labeling.
730142001_001_hepasphere_irinotecan.indd 7 1/7/16 8:22 AM
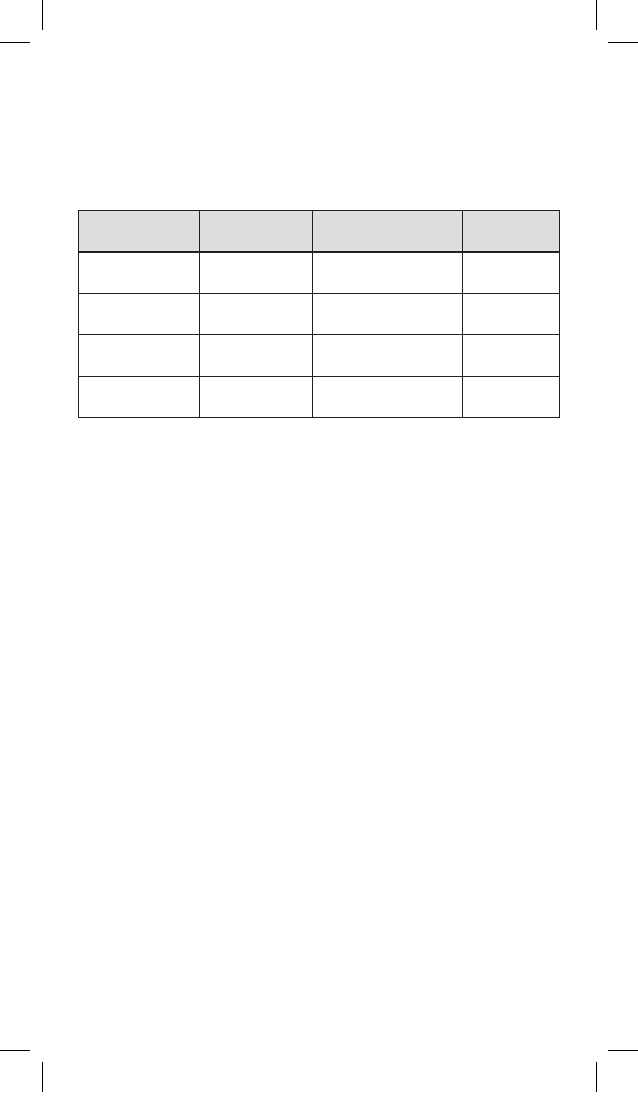
Size of dry
products (μm)
Colour code
(label borders)
Quantity of
microspheres (mg) Reference
30-60 Orange
25
50
V 225 HS
V 250 HS
50-100 Yellow
25
50
V 325 HS
V 350 HS
100-150 Blue
25
50
V 525 HS
V 550 HS
150-200 Red
25
50
V 725 HS
V 750 HS
When the procedure of reconstitution
is completed, store the solution of
HepaSphere Microspheres in 2 to 8°C con-
ditions and use within 24 hours, IF not used
immediately. Do not store HepaSphere
Microspheres after contrast medium has
been added.
730142001_001_hepasphere_irinotecan.indd 8 1/7/16 8:22 AM

Symbol Designation
Manufacturer: Name & Address
Use by date: year-month-day
LOT
Batch code
Catalog number
Do not resterilize
Do not use if package is damaged
Keep away from sunlight
Keep dry
Do not re-use
Caution: Consult accompanying documents
Non-pyrogenic
Sterilized using irradiation
EC mark logo - Notied body identication: 0459
Size of dry microspheres / Size of hydrated microspheres
All serious or life threatening adverse events or deaths associated with use of
HepaSphere Microspheres should be reported to the device manufacturer.
INFORMATION ON PACKAGING
Biosphere Medical, S.A.
Parc des Nations - Paris Nord 2
383 rue de la Belle Etoile
95700 Roissy en France
France
Manufactured for:
Merit Medical Systems, Inc.
1600 West Merit Parkway
South Jordan, Utah 84095, U.S.A.
1-801-253-1600
U.S.A. Customer Service 1-800-356-3748
www.merit.com
730142001_001 2015-11-24
0459_2004
730142001_001_hepasphere_irinotecan.indd 9 1/7/16 8:23 AM
