
Saudi Arabian Handbook for
Healthcare Guideline Development
April 2014
The Saudi Center for
Evidence Based Health Care
Version 1.0

2
Saudi Arabian Handbook for Healthcare
Guideline Development
Contents
1. Preamble ......................................................................................................................................... 5
2. Introduction .................................................................................................................................... 6
Guideline definition ......................................................................................................................... 6
When is a guideline the right approach? ...................................................................................... 6
Context for guideline development in Saudi Arabia .................................................................. 6
Information reviewed for this handbook ..................................................................................... 7
3. Overview of the guideline enterprise ......................................................................................... 8
Guideline panel ................................................................................................................................ 9
Guideline panel chair .................................................................................................................... 11
Guideline support unit .................................................................................................................. 11
4. Topic proposal and selection ..................................................................................................... 13
By whom and how are topics proposed? ................................................................................... 13
What is the process for making a proposal? .............................................................................. 13
Who selects the topic? ................................................................................................................... 13
How is topic selection done? ........................................................................................................ 13
5. Defining the scope of the guideline .......................................................................................... 15
What is the scope of the GL? ........................................................................................................ 15
Who prepares the scope? .............................................................................................................. 15
Drafting the initial scope ............................................................................................................... 15
Informing other stakeholders about the initial scope ............................................................... 17
Formulating questions for the scope ........................................................................................... 17
Choosing and rating outcomes .................................................................................................... 19
Identifying resource implications ................................................................................................ 20
Finalising the scope ........................................................................................................................ 20
6. Panel meetings ............................................................................................................................. 21
Management of conflict of interests ............................................................................................ 21
7. Evidence retrieval ........................................................................................................................ 22
What is ‘evidence’ for guideline development? ........................................................................ 22
Evidence to decision tables ........................................................................................................... 23
Prioritizing evidence retrieval ...................................................................................................... 23
The process of evidence retrieval ................................................................................................. 24
Retrieving and assessing existing guidelines ............................................................................. 25
Retrieving existing systematic reviews ....................................................................................... 26
Adequacy of systematic reviews.................................................................................................. 26
8. Grading the quality of evidence ................................................................................................ 27
Limitations that can reduce the quality of the evidence: .......................................................... 30
Factors that can increase the quality of the evidence: ............................................................... 37
Presenting the evidence to the Guideline Panel ........................................................................ 38
9. Assessing cost implications ........................................................................................................ 38
10. Developing recommendations ................................................................................................... 40
How a panel decides on recommendations ............................................................................... 40
Grading recommendations ........................................................................................................... 40
Indicators for implementation ..................................................................................................... 43

3
Saudi Arabian Handbook for Healthcare
Guideline Development
11. Producing and disseminating the guideline ............................................................................ 44
12. Guideline authorship .................................................................................................................. 45
13. Updating a guideline................................................................................................................... 45
14. Glossary and references .............................................................................................................. 46
16. Appendices ................................................................................................................................... 63
Appendix 1: Template for topic proposal ................................................................................... 63
Appendix 2: The GRADE process in developing guidelines ................................................... 64
Appendix 3: WHO Conflict of Interest Form ............................................................................. 66
Appendix 4.1. Example of an evidence profile .......................................................................... 70
Appendix 4.2. Example of evidence to decision framework ................................................... 72

4
Saudi Arabian Handbook for Healthcare
Guideline Development
Acronyms and Terms
A full glossary of terms and their definitions may be found at the end of this handbook.
AMSTAR Assessment of Multiple Systematic Reviews
AGREE
Appraisal
of
Guidelines for Research
and
Evaluation
AHRQ Agency for Healthcare Research and Quality
CADTH Canadian Agency for Drugs and Technologies in Health
COI Conflict of interest
DOI Declaration of Interest
GAB Guideline Advisory Board
GRADE Grading of Recommendations Assessment, Development and Evaluation
GDT Guideline Development Tool
GIN Guideline International Network
GL Guideline
GP Guideline Panel
ICER Incremental cost-effectiveness ratio
IOM Institute of Medicine
MeSH Medical Subject Headings
MoH Ministry of Health
NGC National Guideline Center
NICE National Institute for Health and Care Excellence (UK)
PICO Patient/Population-Intervention-Comparison-Outcome
QALY Quality-adjusted life years
WHO World Health Organization

5
Saudi Arabian Handbook for Healthcare
Guideline Development
1. Preamble
Guidelines, if based on the best available evidence for the decision criteria that determine the
direction and strength of a recommendation, are an ideal tool to support health care decision
makers. The handbook for guideline development in Saudi Arabia establishes a framework for
guideline development in the Kingdom of Saudi Arabia that addresses organizational and
practical issues to ensure practice is evidence based and addresses the need of the population.
The aim of Healthcare Guidelines is to reduce unnecessary variation in practice through
involvement of all relevant groups including health care professionals, such as nurses, physicians,
allied health workers and patients in the development of health care recommendations based on
the best available evidence. Such guidelines must provide support rather than dictate care; they
will not be cookbooks. For that reason, much of the suggested methodology focuses on
approaches, such as the Grading of Recommendations Assessment, Development and
Recommendations (GRADE) approach with its rationale approach to decision determinants.
The handbook places emphasis on using existing evidence syntheses, sometimes from existing
guidelines, as a means to develop recommendations for Saudi Arabia. Emphasis is also placed on
using decision determinants (so called evidence to decision frameworks) to ensure that
information that is relevant for the target populations is sought for and integrated rather than
placing undue emphasis on existing guidelines that may be outdated or not considerate of the
context.
The approach and methodology are also based on the work done for the World Health
Organization in 2006, modelled on country specific advice given to the country of Estonia and
advice given to numerous professional societies and other organizations. It is based on research
in the field of evidence to decisions using existing highly credible systematic reviews rather than
de novo reviews and placing emphasis on transparency, including conflict of interest
management. Existing standards by the Guideline International Network and authorities such as
the Institute of Medicine are carefully integrated. Finally, the experience collected during a large
guideline development effort in Saudi Arabia in December 2013 helped fine tune the approach
described here.
This handbook has been developed by a team of researchers at McMaster University, Hamilton
Canada (headed by Dr. Holger Schünemann with collaboration from Drs. Reem Mustafa, Alonso
Carrasco, Romina Brignardello-Petersen, Jan Brozek and Wojtek wiercioch) with involvement of
key informants in Saudi Arabia who commented on an early draft and provided invaluable advice
during in-depth interviews. In particular the authors would like to thank Prof. Lubna Al-Ansary,
Dr. Noha Dashash, Dr. Sohail Bajammal, Prof. Hassan Baaqeel, Dr. Zulfa Al Rayess, Dr. Yaser Adi
and Dr. Rajaa Alraddadi who provided important insights.
The ministry of health welcomes the opportunity to introduce this new era of supporting care
providers in the Kingdom.
Preamble written by Holger Schünemann, Chair of the Department of Clinical Epidemiology and
Biostatistics at McMaster University, Hamilton, Canada.

6
Saudi Arabian Handbook for Healthcare
Guideline Development
2. Introduction
2.1 Guideline definition
A guideline (GL) is a product that contains recommendations about health interventions,
including interventions focusing on health care related tests and test strategies.
1-3
As defined by
the World Health Organization, the recommendation is a statement that assists health care
providers and recipients of health care in making the best possible health care decision. A
recommendation implies a choice between different interventions that may have an impact on
health and that have influence on resource use as well as other consequences. The direction and
strength of a health care recommendation depends not only on the magnitude of anticipated
benefits and harms or burden, but also on the certainty in the intervention effects, the value the
population places on these associated outcomes and interventions and the impact on resources,
including considerations around feasibility, acceptability and equity.
4,5
Such considerations may
be highly context or setting specific and may require local information or evidence.
The main difference between a guideline and a typical textbook is that a guideline provides
answers as actionable statements to foreground questions; advice about “what to do” rather
than background questions which deal with “how or why does it work“. There is broad
agreement that these statements should be based on systematic reviews.
6-8
Systematic reviews
are transparent syntheses of the available best evidence for a given question following
established methodology. Given the best available evidence should be used, the synthesis of
evidence may be derived from different types of studies, such as randomized trials and various
types of observational studies, depending on the type of questions and availability of evidence.
Detailed processes on guideline development are available through various resources, such as
the guideline development checklist on: http://cebgrade.mcmaster.ca/guidecheck.html.
9
2.2 When is a guideline the right approach?
Before beginning the process of guideline development, prioritization should include
considerations if and what type of a guideline is the correct approach to solving the problem.
10,11
The need for rapid responses may lead to providing interim or preliminary advice or guidelines
that will later be supported by a fully developed guideline.
2.3 Context for guideline development in Saudi Arabia
Several groups have supported or carried out the development of guidelines in Saudi Arabia with
limited co-ordination. There has been no uniformly accepted approach to guideline development
and this has resulted in a wide array of different guideline formats and compilation processes.
The Ministry of Health of Saudi Arabia (MoH) has embarked on standardizing and coordinating
guideline development nationally.
This handbook has two main goals: 1) to summarize the internationally accepted methods and
approaches related to the health care guideline enterprise; and 2) to provide an approach on
how to successfully implement and sustain the guideline enterprise in Saudi Arabia. It intends to

7
Saudi Arabian Handbook for Healthcare
Guideline Development
cover all aspects of the guideline enterprise, starting with assessing the need for one
(prioritization) and finishing with the distribution and implementation covered in the following 18
topics found to be relevant for guideline development.
9
1. Organization, Budget, Planning and Training
2. Priority Setting
3. Guideline Group Membership
4. Establishing Guideline Group Processes
5. Identifying Target Audience and Topic Selection
6. Consumer and Stakeholder Involvement
7. Conflict of Interest Considerations
8. (PICO) Question Generation
9. Considering importance of outcomes and interventions, values, preferences and
utilities
10. Deciding what Evidence to Include and Searching for Evidence
11. Summarizing Evidence and Considering Additional Information
12. Judging Quality, Strength or Certainty of a Body of Evidence
13. Developing Recommendations and Determining their Strength
14. Wording of Recommendations and of Considerations of Implementation, Feasibility
and Equity
15. Reporting and Peer Review
16. Dissemination and Implementation
17. Evaluation and Use
18. Updating
Although the need for country-specific guidelines is envisaged in most areas of health care due to
the need to consider costs and values in addition to the health care evidence, the use of
international resources is encouraged.
2.4 Information reviewed for this handbook
Writing this handbook involved multiple steps and a mixed methods approach. First, critically
reviewing different sources to develop a first draft of this handbook. A list of these sources
include: A series of articles on “Improving the use of research evidence in guideline development
(16-article series)”,
12
another series entitled “Integrating and Coordinating Efforts in COPD
Guideline Development (14-article series)”,
13
articles published in Implementation Science on
developing CPGs (3-article series),
14
Estonian Handbook for guideline Development (work done
by Dr. Schünemann and colleagues on that handbook served as partial template for this
handbook),
11
AGREE II: Advancing guideline development, reporting and evaluation in health
care,
15
Conference on Guideline Standardization (COGS): Standardized Reporting of Clinical
Practice Guidelines, Institute of Medicine: Clinical Practice Guidelines We Can Trust,
2
and
Guideline International Network (GIN): Toward International Standards for CPGs.
16
Additionally,
multiple manuals of guideline developers were reviewed. These manuals include: Argentina
National Academy of Medicine, Colombia Ministry of Health and Social Security, Peru Ministry of
Health, Spain Ministry of Health, American College of Cardiology-American Heart Association,
Cancer Care Ontario, Canadian Task Force on Preventative Health Care, Kaiser Permanente,
National Health and Medical Research Council (NHMRC), National Institute for Health and Clinical
Excellence (NICE), New Zealand Guidelines Group, Scottish Intercollegiate Guidelines Network
8
Saudi Arabian Handbook for Healthcare
Guideline Development
(SIGN), United States Preventive Services Task Force (USPSTF), Centers for Disease Control and
Prevention (CDC), and the World Health Organization (WHO). Second, using information from
interviews with key Saudi stakeholders to obtain feedback on local needs and requirements that
are relevant for the adaptation. Third, critical revision of initial draft based on comments, written
response, and development work based on the KSA context following a workshop in KSA.
3. Overview of the guideline enterprise
A formal process to support guideline development under the auspices of the MoH is a
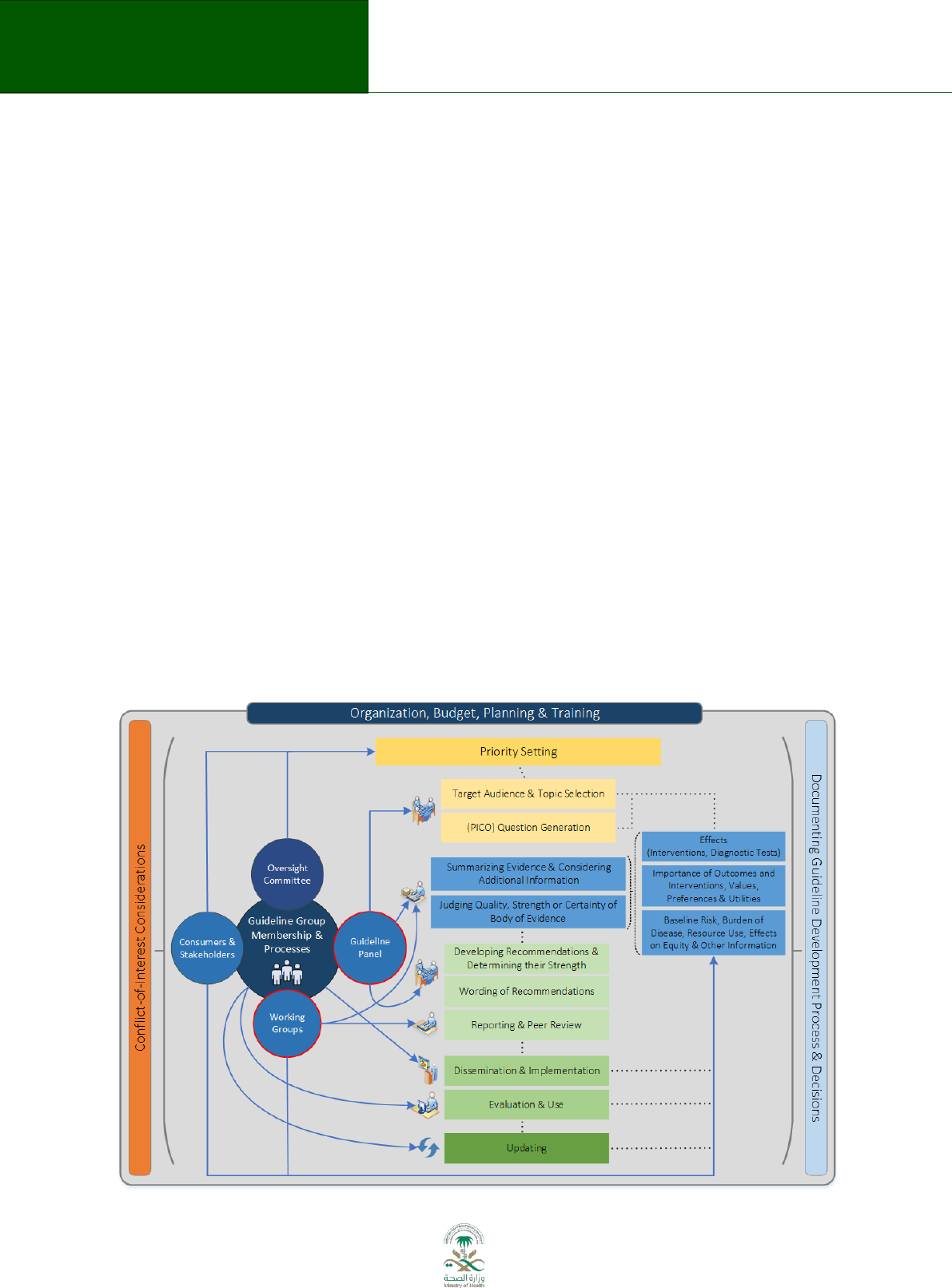
prerequisite for consistent application of the guideline development process. Figure 3.1
describes this process (from reference
9
). A national guideline center (NGC) will be established. A
10 person advisory board to oversee organization, budget, planning and training will include
representation of various stakeholders (e.g. MoH, medical societies, care providers, National
Guard, Military hospitals) and act as advisory board to support organization, planning and
training. Members of the advisory board will be selected based on qualification and background.
The advisory board is chaired by an elected member. The NGC is chaired by a member who is
nominated by the MoH and approved by the advisory board. The NGC will nominate one or
more oversight committees with representation from various stakeholders for guideline projects
depending on the type of guidelines. Further roles of the NGC will be described below and are
represented by the blue circles in Figure 3.1.
9
Figure 3.1:

9
Saudi Arabian Handbook for Healthcare
Guideline Development
Legend: Flow diagram of the guideline development process. The steps and involvement of
various members of the guideline development group are interrelated and not necessarily
sequential. The guideline panel and supporting groups (e.g. methodologist, health economist,
systematic review team, a secretariat for administrative support) work collaboratively, informed
through consumer and stakeholder involvement. They report to the oversight committee. While
deciding how to involve stakeholders early for priority setting and topic selection, the guideline
group must also consider how developing formal relationships with the stakeholders will enable
effective dissemination and implementation to support uptake of the guideline. Furthermore,
considerations for organization, planning and training encompass the entire guideline
development project, and steps such as documenting the methodology used and decisions made,
as well as considering conflict-of-interest occur throughout the entire process.
The need for a guideline can be identified by any body (e.g., professional medical society, patient
group, academic institution etc.), but requires coordination by the NCG. This need will be
described in a topic proposal (see Chapter 4) and a draft scope (see chapter 5), and a proposal for
the guideline panel membership needs to be made, bearing in mind the panel requirements for
multidisciplinary.
A guideline topic proposal is submitted to the NGC advisory board. Guidelines intended to be
financed by the MoH must be submitted to the NGC for approval. Despite the effort of
centralizing guideline development through the NGC, other entities in Saudi Arabia may wish to
develop guidelines independently. Such guideline developers using other financing mechanisms
and not developing guidelines as part of the activity of the NGC are encouraged to submit their
proposals as well to benefit from methodological advice of the NGC.
The roles of the NGC are:
developing an oversight committee for guideline topics
proposing and evaluating guideline topic(s) to be financed;
consulting on and approving the composition of guideline panel;
evaluating conflict of interests of panel members;
overseeing and acting as an advisory resource for the work of the guideline panel;
finalizing the initial scope with the panel;
signing off on the final scope;
being an arbiter in situations of lack of agreement on issues other than recommendations
(e.g. authorship issues);
approving the final guideline (note, this function is not to alter recommendations but to
ensure that the guidelines are methodologically sound).
3.1 Guideline panel
The guideline is drafted by a guideline panel. The panel should be multidisciplinary and should
incorporate representatives of specialities involved in the relevant guideline.
15,17-22
The panel
should include representatives of patient and/or consumer groups.
2,17,19,23,24
Patients may be
familiar with the topic and its treatments based on personal experience and may be able to

10
Saudi Arabian Handbook for Healthcare
Guideline Development
provide information and evidence relative to the guideline. Conflicts of interests of all panel
members must be managed appropriately as described below.
The initiator of the guideline presents the potential composition of the Panel and the name of
the proposed chair to the NGC for approval. The NGC may deliberate on the composition of the
Panel.
The Panel should include:
medical experts;
methodologists;
health economist;
representatives from key stakeholders and organizations involved in implementation,
including:
representatives from consumer or patient associations;
representatives from the medical faculty of a university;
representatives of organizations involved in the health-care process and who are likely to
be end-users of the guideline;
The size of the panel depends on the topic of the guideline, but is generally up to
20 persons. The size of a guidelines panel should be small enough for effective group interaction,
but large enough to ensure adequate representation of relevant views.
11,16,18,19,23,25-27
The roles of the Guideline Panel Members include:
11,16-18,25,28,29
Comment on the initial scope selected by the NGC and finalize it (including the
formulation of clinical questions and choosing outcomes), taking into account the views
of stakeholders. During the development of the questions for the guideline, the guideline
panel has to consider which clinical questions may require information from existing
guidelines or from systematic reviews.
Review draft recommendations based on the presented evidence, with explicit
consideration of the overall balance of risks and benefits. The assumption for the Panel is
that the research evidence to support a particular recommendation is global, whereas
costs, values and preferences, feasibility, acceptability and equity of recommendations
are local considerations, and therefore should be the basis of adaptation of international
recommendations for local situations.
Approve recommendations according to the GRADE approach, taking into account values
and preferences and resource implications.
Decide on consultation needed for the draft guideline.
Plan and agree on the primary methods for implementation and indicators for measuring
the use of the guideline.
After guideline finalization, facilitate the process of implementation (i.e. to act as opinion
leaders for and advocates of the guideline).
Work closely with the guideline support groups (see below).

11
Saudi Arabian Handbook for Healthcare
Guideline Development
Specifically, the backgrounds of various panel members would be:
2,10,11,17,19,23,25,30,31
experts who should represent the perspective(s) of health-care professionals, as well as
social care and other professionals, where relevant.
involved in the care of patients affected by the guideline topic; detailed evidence
research expertise is not necessary, although an understanding of evidence-based
medicine is essential.
methodologists in assessing clinical evidence and developing guidelines, should be
included as appropriate, ideally as a panel co-chair. Inclusion of a methodologist in a
leading role, particularly one with experience in the guideline development process, is
recommended to explain to the panel the evidence retrieval process and to guide the
process of formulating recommendations.
patient representatives, e.g. from patient organizations (or a representative of the
patient with the relevant chronic condition) – they will represent the view of the
patient(s) with the relevant condition.
managers and other health professionals may represent the view of the health-care
services and provide expert opinion on the implementation of guidelines.
health economists and/or bio-statisticians can provide an analysis or explanation of the
costs of health services, cost-effectiveness, data on the provision of health care services
and medicines.
Panel members are asked to make a commitment to attend meetings for the guideline
development process, in order to ensure continuity and effective participation in the
process.
11,18,26,27,32
3.2 Guideline panel chair
The choice of the co-chairs (ideally one content expert and a methodologist) of the panel is
important to ensure that the panel will be able to work effectively. In most situations, groups
work most effectively if the chairs have knowledge of the content, but there must be particular
expertise in facilitating groups, interpreting evidence and developing guidelines. People who are
experts in the content area of the guideline and who have strong views about interventions or
aspects that may be included should not chair a guidelines panel. A panel would be chaired
jointly by a methodologist and a content expert with appropriate division of tasks and
labor.
2,16,17,26,32-36
3.3 Guideline support unit
The panel is supported in its work by a guideline support unit within the NGC, consisting of
experts in methodological aspects of guideline development, evidence retrieval and assessment
and health economics. If the guideline is financed by the MoH, the MoH provides the guideline
support unit for the panel
2,17,18,27,29,32,36
. The guideline support unit reports to the NGC advisory
board and works with the guideline oversight committee forming various working groups.

12
Saudi Arabian Handbook for Healthcare
Guideline Development
The roles of the guideline support unit are:
11,18,25,32,37,38
provide technical and administrative support for developing the guideline;
make panel members aware of items on the guideline checklist;
preparation of materials, evidence retrieval and summary for recommendations;
organizing the panel meetings;
use and manage the guideline development tool (www.guidelinedevelopment.org);
prepare draft records (minutes) of all panel meetings, taking special care to document
areas of controversy and dissent.
Once finalised by the panel, the guideline is endorsed by the NGC, making sure the appropriate
methodology has been followed while developing it.
The principles for the production and dissemination of the guideline are described in the chapter
on implementation and dissemination of this handbook.

13
Saudi Arabian Handbook for Healthcare
Guideline Development
4. Topic proposal and selection
4.1 By whom and how are topics proposed?
General topics for guideline development can be proposed by medical societies, the medical
faculty of a university or MoH (the proposer is subsequently called “the initiator”). Topics
together with initial scope must be presented by the initiator to NGC on October 31 of each year.
4.2 What is the process for making a proposal?
Topics can be triggered by many different inputs: regular audits, feedback from practitioners,
variations in care, guidelines being issued by other entities that need to be adapted, introduction
of new interventions, emerging health problems, etc.
10,18,21,23,36,39
Topic proposal will need an active communication between the initiator and other potential
stakeholders including the MoH and the guideline support unit to provide background
information and statistical data for the proposals.
4.3 Who selects the topic?
The selection of topics for guidelines intended to be financed by the MoH has to be made by the
NGC taking into account the initial scope (see Chapter 3). In process of choosing topic(s) to be
financed and approved applicability of further guideline should be taken into account (including
organisational and potential resource implications of a guideline). This would help to avoid
situation when the NGC chooses to finance a guideline topic which implementation is
organizationally not feasible and evidently not affordable to the health system.
4.4 How is topic selection done?
The NGC will assess the topics together with draft scope documents presented annually based
upon the criteria listed below. The NGC advisory board will evaluate all topics. A nine point Likert-
type scale will be used; were 9 is the score of the most important and useful topic and 1 is the
score of the topics that are not important or useful to address. Following resolution of possible
misunderstandings, the average will be used to determine priority topic. Raters (members of the
NGC) will be encouraged to use the whole range of the scale to allow for differentiation between
topics’ importance.
In general, the NGC will evaluate topics based on an assessment of:
Burden of disease
o the population suffering the disease/condition in Saudi Arabia (incidence,
prevalence, mortality,)
o the resource impact of the disease/condition in Saudi Arabia

14
Saudi Arabian Handbook for Healthcare
Guideline Development
Variations
o practice variation and variations in health outcome by different
regions in Saudi Arabia
providers in Saudi Arabia
level of care (primary care, specialist services)
patient populations (here and after most critical subgroups under the
disease/condition can be identified if necessary)
international practice compared with Saudi Arabia
o variation in treatment costs (regions, providers, level of care, patient populations).
Treatment costs analyses can be conducted using data from databases.
service treatment (all treatment costs in certain period)
pharmaceuticals
hospitalization (rate, length of stay)
Potential
o potential for modernization of current practice
availability of new interventions (including diagnostic tests and strategies)
availability of new evidence that will likely change practice
availability of new service delivery
o potential result of successfully implemented guideline
measurable impact on health (indicators)
more cost-effective use of resources
Problem statement and the purpose of the guideline
o problem statement is completed by the initiator based on the information listed
above eg, “persons having condition X in the Riyadh area are hospitalized more
frequently and their average prescription cost for drug B is different from other
regions in Saudi Arabia.” and the purpose of the guideline: eg, “to guarantee up-to-
date treatment with equitable costs for persons with condition X irrespective of
region”
Initial scope prepared by initiator (See template below)
Relationship of topics and scope to health related government priorities.
The NGC may exclude proposed topics if the topics proposed are not potential subjects for
guidelines. The MoH may propose topics of special importance that receive financing through
purpose directed channels. Topics receiving directed financing should be prioritized.

15
Saudi Arabian Handbook for Healthcare
Guideline Development
5. Defining the scope of the guideline
5.1 What is the scope of the GL?
The scope of a guideline provides a framework within which to conduct the guideline
development work, that is the topics that should generally be addressed.
17-19,25,27,36
Proposing
topics and the scope of the guideline will be influenced by existing guidelines and systematic
reviews. The NGC will make a list of preferred topics available. These topics will result from
surveying existing systematic reviews and guidelines from international organizations to allow for
adaptation of guidelines.
Considering the resources for possible guideline topics, scoping should be conducted in stages:
1. Drafting the scope
2. Consulting with stakeholders about the draft scope
3. Finalizing the scope
5.2 Who prepares the scope?
The initial scope, with questions and preliminary outcomes, is prepared by the initiator of the
clinical guideline. The scope is finalized by the oversight committee for an approved topic, in
cooperation with the NGC, and signed off by NGC, e.g. the advisory board.
5.3 Drafting the initial scope
After the general topic is defined by the group proposing the topic, the aspects of care that the
guideline will cover should also be defined:
17-19,25,27,36
population to be included or excluded (e.g., specific age groups or people with certain
types of disease);
healthcare settings (primary or specialized care);
the different types of interventions and treatments to be included or excluded
(diagnostic tests, surgery, rehabilitation, lifestyle advice). Does the potential guideline
complement other programs or interventions in the particular therapeutic area?
information and support for patients and carers;
the preliminary outcomes that will be considered (benefits and potential harms to
patients, impact on health insurance, society perspective); this list will be completed by
the guideline panel;
links with other relevant guidance. Are there any similar guidelines available in Saudi
Arabia in this particular therapeutic area? If so, will the new guideline replace or
supplement the existing one(s)?
16
Saudi Arabian Handbook for Healthcare
Guideline Development
On the basis of these aspects, formulate two-page document with a scope that:
provides an overview of what the clinical guideline will include and what will not be
covered;
identifies the key questions (clinical, as well as organizational, regulatory, etc). It is useful
to formulate the questions using the PICO format (see below for formulating questions);
chooses and rates the outcomes in the PICO (see below for choosing and rating
outcomes)
sets the boundaries of the development and provides a clear framework to enable the
work to stay within the agreed priorities;
informs the development of the detailed review questions from the key clinical issues
and the search strategy;
provides information about the expected content of guideline;
ensures that a minimum set of essential aspects, questions and recommendations is
covered;
ensures that the guideline will be of reasonable size (no more than 20 key questions are
suggested) and can be developed within a specified time period.
evaluates, if:
- any existing guideline in Saudi Arabia covers this topic?
- up-to-date evidence is likely to be available on the topic (see list of preferred topics)?
finalizes:
- the title of guideline;
- who should be key stakeholders for implementation for further consultation on the
scope, if they have not already been involved in preparing it.
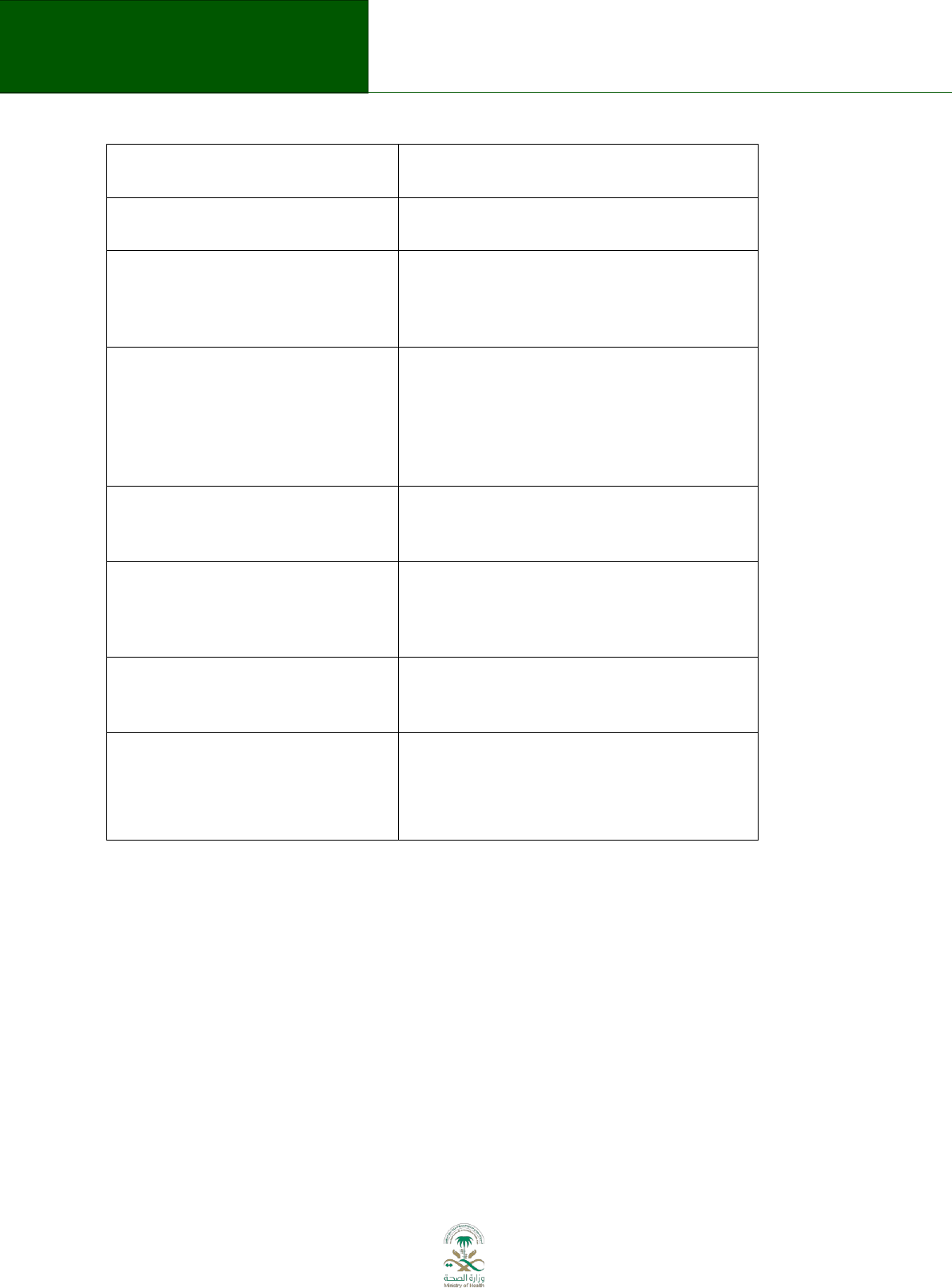
Table 5.1 Submission of topic and scope
Domain
Description
Describe the general topic:
Does the potential guideline
complement other programs or
interventions in the particular
therapeutic area?
Population to be included or excluded
(e.g., specific age groups or people
with certain types of disease):
Healthcare settings (e.g. primary or
specialized care):
The different types of interventions
and treatments to be included or
17
Saudi Arabian Handbook for Healthcare
Guideline Development
excluded (diagnostic tests, surgery,
rehabilitation, lifestyle advice).
Information and support for patients
and carers to be provided:
The preliminary outcomes that will be
considered (benefits and potential
harms to patients, impact on health
insurance, society perspective)
Links with other relevant guidance.
Are there any similar guidelines
available in Saudi Arabia in this
particular therapeutic area? If so, will
the new guideline replace or
supplement the existing one(s)?
Provide an overview of what the
clinical guideline will include and what
will not be covered:
Identify some of the key questions
(clinical, as well as organizational,
regulatory, etc) following PICO
format:
Describe the up-to-date evidence that
is available on the topic (see list of
preferred topics)?
Who are the key stakeholders for
implementation and for further
consultation on the scope, if they
have not already been involved in
preparing it.
5.4 Informing other stakeholders about the initial scope
When the initial scope is prepared the initiator must decide who else should be consulted and
involved
2,18,27,29,33
. This can be an informal process, the main purpose of which is to check that the
initial scope is clearly understood.
5.5 Formulating questions for the scope
The selection of questions (and their components) that are to be addressed in the guideline has
major consequences for the scope of the guideline. The questions will drive the direction
(inclusion and exclusion of data) and determine the type of information that will be searched for
and assessed. The questions are also the starting point for formulating the recommendations. It
is very important that the questions are clear and well defined, and that there is agreement

18
Saudi Arabian Handbook for Healthcare
Guideline Development
about them among panel members. The guideline development tool can be used to complete
this task.
10,19,23,26,35,38-40
Updating a guideline may include a change of scope; not only the questions but also the selection
of critical outcomes may differ from the original guideline.
It is helpful to start by dividing the types of information and questions into three main categories:
Definition/background questions
e.g., What is chronic obstructive pulmonary disease (COPD)?
What are the causes of caries?
Facts/foreground questions
e.g. What is the effect of inhaled steroids in COPD?
What types of public health interventions reduce the incidence of caries?
Recommendation/decision
e.g. Should inhaled steroids in COPD be used?
Should regular dental hygiene visits be included in dental care?
Guidelines should focus on the recommendation and decision and minimize the description of
the definition and background to what is needed to put the recommendations in context.
The questions to be covered by the guideline should be identified on the basis of clinical, public
health or policy needs and input from clinicians and other experts. Input from consumer or
patient groups may also be helpful. Questions should focus on areas where changes in policy or
practice are needed and/or controversy may exist.
41,42
During the development of the questions for the guideline, the guideline panel should consider
which questions may need information from systematic reviews or from existing
guidelines.
7,11,18,19,23,27,33
Questions that may require new systematic reviews will have the
greatest impact on the time taken to complete the guideline. Thus, the preferred approach is
one in which existing, highly credible systematic reviews can be used.
The foreground questions are the most important ones for a guideline and they are used to
inform the recommendation/decision and they may require a systematic review and quality
assessment of the evidence about effects using the GRADE approach. Ideally systematic reviews
inform adaptation issues, values and preferences, clinical needs and baseline risks.
For priority setting and defining the scope, the initial list of types of question may be probably be
a long one. Some examples could be:
What is the frequency of the condition or issue of interest? (background)
What causes the condition or issue of interest? (etiology)
Who has the condition or issue of interest? (diagnosis)
What happens if someone gets the condition or issue of interest? (prognosis)
How can we treat the condition or issue of interest? (interventions)

19
Saudi Arabian Handbook for Healthcare
Guideline Development
What policies should we introduce to alleviate the condition or issue of interest? (policy
intervention)
To formulate these general questions in a way that they can be answered, the PICO framework is
useful:
10,19,23,43,44
Population (What factors are essential?)
In patients with cancer
Intervention (Specific intervention or class?)
what is the impact of blood
thinning with heparin
Comparator (Compared with nothing or with standard treatment)
compared with no heparin
Outcome (Patient-relevant outcomes, including both benefits
and potential side effects and burden and over what
time, e.g. mortality at 2 years)
This format can also be used, with slight modifications, for questions on prevalence and
incidence, etiology (exposure-outcome) and diagnosis. For instance:
In women in Saudi Arabia (P), what is the frequency of breast cancer (O)?
In men over 40 years of age (P), what is the rate of lung cancer (O) in smokers versus non-
smokers (C)?
In babies born (P), does screening with a new rapid diagnostic test
(I, C) accurately detect disease?
5.6 Choosing and rating outcomes
Once the clinical questions for the guideline have been defined, identify the key outcomes that
need to be considered in making the recommendations. Specially define the outcomes for
foreground questions and for the outcomes that will be critical for making decisions and
recommendations. These outcomes will also be used to guide the evidence retrieval and
synthesis. It is important to focus on the outcomes that are important to patients, and to avoid
the temptation to focus on those outcomes that are easy to measure and are often reported
(unless those are also the important outcomes).
11,44,45
Step 1. Create an initial, comprehensive list of possibly relevant outcomes for each question,
including both desirable and undesirable outcomes from the interventions that will be
considered in the recommendations.
45
Step 2. Score the relative importance of each outcome from 1–9. Rating an outcome 7–9
indicates that the outcome is critical for a decision to recommend or not recommend a particular
intervention or diagnostic test, 4–6 indicates that it is important, and 1–3 indicates that it is not
important. The average score for each outcome can be used to determine the relative
importance of each outcome, although it is helpful to provide the range of results as well.
Sometimes people with different perspectives (patients, physicians, researchers, policy-makers)
on…

20
Saudi Arabian Handbook for Healthcare
Guideline Development
have different opinions about which outcomes are important.
44,45
Therefore all these
stakeholders should have an opportunity to contribute to the discussion on the selection of
critical outcomes either by participation in the panel or by consultation.
Please note that meetings are not required to accomplish this task. These ratings can be
conveniently completed using electronic tools.
5.7 Identifying resource implications
Once the key questions are formulated, the initiator should list the resource implications for the
potential interventions that may be recommended. This might include for example, possible
costs of new medicines or diagnostic tests, or possible outcomes, such as admission time to
hospital, that might be associated with costs.
40,46
This step will inform any budget-impact
assessment that will be carried out by one of the working groups of the guideline support unit.
5.8 Finalising the scope
Topics together with initial scope must be presented to NGC according to template (see Table
5.1). The NGC will assess the topics together with initial scope documents and will approve topics
to be proper for guideline development. The NGC will consult and approve the composition of
the guideline panel.
The panel may revise the initial scope based on the clinical importance of some the questions and
outcomes, the potential evidence available or the potential for recommendations that will be
useful in the Saudi Arabian health care context.
11,18,29,43
It is critical to maintain the scope as
narrow as sensible to ensure feasibility of completing the guideline in a timely manner.
Final scope will be approved by NGC advisory board.

21
Saudi Arabian Handbook for Healthcare
Guideline Development
6. Panel meetings
The purpose of the meetings and expected tasks must be clearly laid out at the start,
including:
11,18,32
what is expected from meeting participants;
what needs to be achieved during the meeting;
what can be done afterwards;
what follow-up will take place with meeting participants;
what the ground rules and processes to be followed (there should be no discussion about
the process, i.e. members of the panel agree to the process when they agree to become
a member).
Decisions are made based on consensus and voting as a form of forced consensus is used only in
exceptional situations when consensus cannot be reached through discussion.
47
If voting takes
place, existing models can be used.
48
The panel will usually benefit from 2 to 3 face-to-face meetings with a minimum of one face-to-
face meeting (the meeting to agree on recommendations). The purpose of the first meeting is
generally to finalize the scope of the proposed guideline. At the (essential in person) meeting
when recommendations are formulated, the panel reviews recommendations based on evidence
prepared by guideline support unit. Another meeting might include finalizing plans for
dissemination and for assessment of implementation of the guideline. Additional consultations
(outside group meetings) may be held through electronic communication.
If the purpose of the meeting is to formulate recommendations:
distribute the evidence profiles (see Appendix 4.1 for an example based on the GDT)
prepared by the methodologist before the meeting, ideally two weeks before the
meeting;
distribute the evidence to decision tables (see Appendix 4.2 for an example based on
the GDT) prepared by the methodologist in consultation with the guideline panel
before the meeting, ideally two weeks before the meeting;
at the meeting, present draft recommendations that have been prepared by the
guideline support unit (meeting participants will comment on these and refine them).
6.1 Management of conflict of interests
Nominated Panel members should declare to the NGC their conflict of interests, for
example according to the declaration used by the World Health Organization.
22,41,49,50
The
NGC oversight committee will decide whether any declared interest are such that a
proposed panel member should not be included, for example due to significant financial
or personal ties with a company who has an interest in a product that is the subject of
the guideline; the NGC advisory board will resolve conflicts.

22
Saudi Arabian Handbook for Healthcare
Guideline Development
Once the Guideline Panel is approved by the NGC, the guideline support unit collects
declarations of interest (can be done electronically with the GDT) before the first meeting
and later if there are any changes so that there is enough time to intervene if necessary
(e.g. if any invited participant needs to be excluded owing to major conflicts or to prevent
there being too many participants with potential conflicts of interest).
At each panel meeting each participant reports verbally potential conflicts of interest
(with actions taken if necessary); all panel members and any individuals who have direct
input into the guideline should update their declaration of interests form before each
panel meeting. Any changes to a panel member’s declaration of interests should be
recorded in the minutes of the panel meeting.
Declarations of interests will be published in the final full guideline.
10,11,19,22,41,49-52
Recusal or excusal from certain decisions or recommendations is appropriate. If guideline
panels involve members with (limited) conflict of interest, Chairs and group members on
a guideline group should ensure that committees are reminded of the specific COI before
discussion of individual conclusions or recommendations on which those COI bear. This
will allow recusal from recommendations of those with important COI. Group chairs can
play an active role and excuse group members from discussions or decision-making on
particular recommendations.
Procedures for handling disputes in conflict of interest resolution: Final decision about
inclusion and exclusion from a panel with rest with the NGC. Chairs of guideline panels
will have to review individuals’ conflicts before each panel meeting and evaluate if the
NGC should be involved based on new or changing conflict of interest. Once a member is
approved for participation in a guideline panel, the panel chair will determine if specific
panel members should be excused from individual recommendations or part of the
discussion.
7. Evidence retrieval
7.1 What is ‘evidence’ for guideline development?
A summary of all relevant research evidence is essential when developing a recommendation,
ideally based on systematic reviews.
49,53-56
In contrast to narrative reviews, systematic reviews
address a specific question and apply a rigorous scientific approach to the selection, appraisal
and synthesis of relevant studies. Systematic reviews, if conducted properly, reduce the risk of
selective citation (the 'my favourite study' approach) and improve decisions.
Many guideline organizations rely on groups such as the Cochrane Collaboration for systematic
reviews for use in guideline development. In countries or organizations with limited resources
(including staff and expertise), however, it may be more practical and efficient to use existing
systematic reviews including those used for already existing guidelines as the basis for local
guideline development or to adapt recommendations from existing guidelines, and only
occasionally develop recommendations de novo. The assumption here is that the research
evidence to support a particular recommendation is 'global' whereas costs, values and

23
Saudi Arabian Handbook for Healthcare
Guideline Development
preferences and the feasibility of recommendations are 'local' considerations, and therefore
should be the basis of adaptation of existing, sometimes international, recommendations.
Guidelines and recommendations will therefore be developed from a variety of sources, where
the emphasis in existing guidelines is on the possibility of extracting information from highly
credible systematic reviews from these guidelines:
1. recommendations and systematic reviews developed from published clinical guidelines
that were created by independent organizations or groups that meet specified criteria
(see “Retrieving and assessing existing guidelines”);
2. recommendations developed from existing systematic reviews;
3. recommendations developed from new systematic reviews;
Existing guidelines could be assessed for their credibility using validated tools such as the AGREE
tool.
15
However, the emphasis is on finding systematic reviews that include the information of
interest. If a guideline recommendation is required when there is truly little evidence to support
a decision, then the panel will need to document the reasons for developing the
recommendation based on little evidence and the basis for their judgement.
6,20,42,57,58
Such a
recommendation may also be the basis for a proposal for research.
7.2 Evidence to decision tables
Regardless of the source of a recommendation or a systematic review, evidence to decision
tables should be completed by a guideline panel, ideally for each recommendation or set of
related recommendations based on the obtained information (see appendix 6.2).
57,58
Existing
evidence to decision tables can be used and checked for relevance and possibility of adaptation.
7.3 Prioritizing evidence retrieval
Whatever the source of the evidence, retrieving evidence to support every recommendation in a
guideline may simply not be feasible. This is where it becomes important to identify priority
questions or issues that the guideline should address (see section on defining the scope).
11
To avoid duplication, the process outlined below starts by using existing guideline
recommendations, and checking the evidence that relates to the recommendations (i.e.
availability of systematic reviews supporting them), then describes the full process of developing
recommendations based on systematic reviews, and includes a process for undertaking
systematic reviews. This third option should be carried out only when there is no existing basis
for recommendations and when the question is a major issue for the guideline to cover.
2,10,11,16,18-
21,26,27,29,32,36
The methodology of development of systematic reviews is not covered in this
handbook. Preparation of systematic reviews should follow the Cochrane Handbook for
Systematic Reviews of Interventions (available at:
http://www.cochrane.org/training/cochrane-handbook).
24
Saudi Arabian Handbook for Healthcare
Guideline Development
7.4 The process of evidence retrieval
The process of evidence retrieval, assessment and synthesis is described in further detail below
and is summarized in the figure below (adapted from Estonian Handbook for guideline
development)
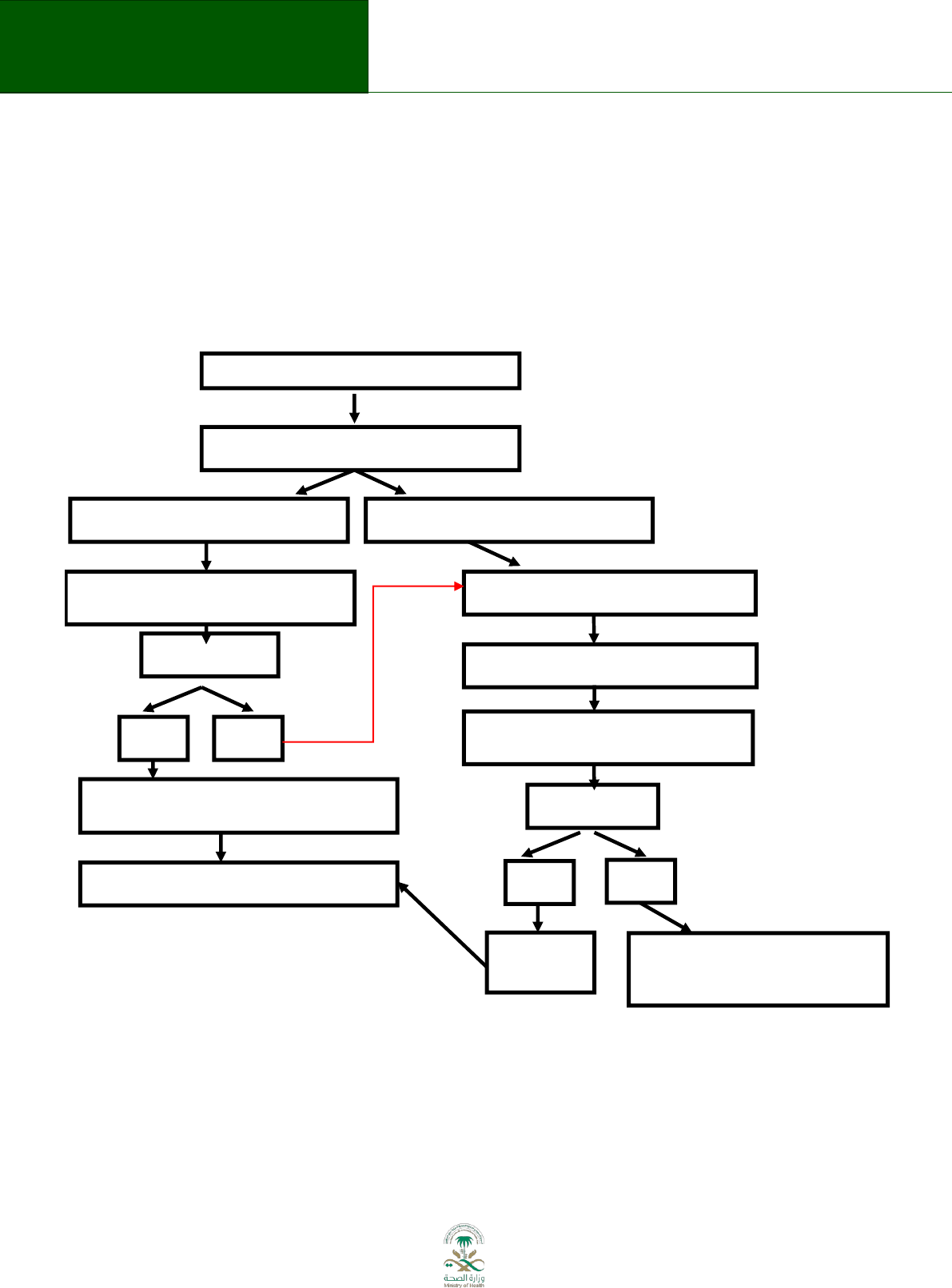
Figure 7.1
Guideline question formulated
Systematic search for existing guidelines
Current, relevant guidelines identified
No relevant guidelines identified
Assess quality, currency, and relevance of
systematic reviews in these guidelines
High credibility?
YES
NO
Create evidence to decision tables of
recommendations
Decide on need for additional evidence
Systematic search for systematic reviews
Relevant systematic reviews identified
High credibility?
YES
NO
Assess the
evidence
Decide whether to do systematic
reviews or wait to make
recommendation
Assess quality, currency, and relevance of
systematic reviews

25
Saudi Arabian Handbook for Healthcare
Guideline Development
7.5 Retrieving and assessing existing guidelines
Start by conducting a systematic search for existing guidelines (generally, those published in the
last 5 years to ensure currency) on the same topic(s).
11
Guidelines can be difficult to find through
electronic databases, so the following sources, in addition to Medline, may be helpful:
the National Guideline Clearinghouse - http://www.guideline.gov/
websites of guideline-producing agencies, such as NICE
the guidelines international network (GIN) database of guidelines
It is strongly recommended to consult with an expert in information retrieval to ensure the use of
a sound search strategy.
The search strategy should include key words for target population, intervention and
comparators if relevant, etc. MeSH terms could be used. See http://www.ncbi.nlm.nih.gov/mesh.
The search strategy should be clearly documented and should specify:
19,23,36
the details of the sources (including web sites) searched, and the search used in each
database including the date when the search was performed;
If relevant guidelines are identified the following aspects should be assessed:
1) are the guidelines based on systematic reviews?
o if not, they should not be used.
o If they are evidence based, are evidence summaries provided and are evidence
to decision tables provided? (including GRADE evidence profiles, summary of
findings tables, or references to systematic reviews)
2) who funded the guideline development?
o what processes were used to manage conflicts of interest? If these are not
described, the guidelines should not be used further, but there may be relevant
systematic reviews or evidence profiles incorporated into them that can be
helpful.
For the assessment tool it is suggested to use AGREE instrument questions 8-11 and 22-23.
15
Once it is decided if the guidelines can be used as the basis for development of local
recommendations, one will need to identify the recommendations in them that are relevant to
the scope of the guideline. One approach that has been used is to make a table that includes all
similar recommendations from different guidelines on the same topic, compare their sources
(who produced them and if there are systematic reviews).
If the recommendations and the sources are the same, the main task is to develop or use
evidence to decision tables (see section on developing recommendations). It is also possible that
new evidence may be available that might need to be considered. Pragmatic decisions will have
to be made about how to supplement the evidence in existing guidelines with new evidence, if

26
Saudi Arabian Handbook for Healthcare
Guideline Development
necessary. Advice on this should also be obtained from the content experts on the guidelines
panel.
If an existing guideline has used GRADE evidence profiles as the basis for evidence presentation
(based on systematic reviews), it may be possible to update the evidence profile and then
reassess the recommendation, adding in considerations such as costs, local values and
preferences, feasibility and other factors in the evidence to decision tables.
7.6 Retrieving existing systematic reviews
Rationale
Systematic reviews, if conducted properly, reduce the risk of selective citation and improve the
reliability and accuracy of decisions. Systematic reviews should be assessed for their quality (see
below “Adequacy of systematic reviews”).
20,27,28,33,35,36
Each systematic review under consideration should have a protocol that describes:
11,55
the search strategy used to identify all relevant published – and unpublished – studies;
the eligibility criteria for the selection of studies;
how studies will be critically appraised for risk of bias;
an explicit method of synthesis of results and, if feasible, a quantitative synthesis of the
results of studies to estimate the overall effect of an intervention (meta-analysis).
The first step is to identify relevant systematic reviews for each of your questions. The most
readily accessible biomedical database is PubMed. The PubMed “Clinical Queries” or “Special
Queries” options permit specific searches to be set up to identify systematic reviews of different
types of studies identified with MeSH terms (see http://www.ncbi.nlm.nih.gov/mesh). This
includes searches of the Cochrane Database of Systematic Reviews. A expert in information
retrieval should be consulted through the NGC.
7.7 Adequacy of systematic reviews
Once the reviews are retrieved, they should be checked for:
11,18,23,59
relevance (to the questions to be addressed in the recommendations);
timeliness (assessed by date of last update);
quality (assessed by a standard critical appraisal instrument).
There are multiple checklists available for critical appraisal of systematic reviews, such as the one
developed by the Oxford Centre for Evidence-Based Medicine
(http://www.cebm.net/index.aspx?o=1157) or, better, the validated AMSTAR toolto assess the
credibility of a systematic review.
60,61
An update of the AMSTAR tool is being prepared (under
the leadership of one of the authors of this handbook) and table 7.1 shows the current draft
version which could be used for the assessment of systematic reviews.

27
Saudi Arabian Handbook for Healthcare
Guideline Development
If there are several relevant systematic reviews, use the most recent one that is of high quality. If
the review is of high quality but more than two years old, consider updating the review to include
more recent evidence and compare if other reviews include more or additional studies.
Table 7.1 Credibility of the Systematic Review Process
8. Grading the quality of evidence
Assessing the retrieved evidence is a crucial step that enables the guideline panel to formulate
recommendations.
62,63
The GRADE system for preparing evidence profiles, assessing quality of
evidence and developing recommendations should be used.
5,63,64
This approach allows for a
structured and transparent assessment of the quality of evidence for each outcome. For each
question, there should be relevant data (from the systematic review) for all the outcomes
(benefits and harms) that were rated as important.
If GRADE tables have already been prepared for the published guidelines they should be used as
the basis for formulating recommendations using the evidence to decision frameworks as
described above.
54,65
If there are no GRADE evidence summaries (evidence profiles or summary
of findings), the guideline panel will have to decide whether to retrieve the systematic reviews on
which the recommendations are based, and to prepare evidence summaries, or simply to use the
existing recommendations, and apply considerations of cost, local values and preferences and
feasibility. For potentially high-cost interventions it is strongly suggested that the systematic
review be retrieved and evidence summaries prepared.
The GRADE handbook contains (electronic help file in the GDT) all the instructions for developing
GRADE evidence profiles, and the software for the entire guideline development process can be
accessed on http://www.guidelinedevelopment.org/.
In the GRADE system, the quality of evidence in the context of clinical practice guidelines reflects
“the extent to which confidence in an estimate of the effect is adequate to support
recommendations”.
63
It is implicit in the definition that guidelines panels have to judge the
quality of the evidence along with the specific context in which the evidence is being used.
Did the Review Explicitly Address a Sensible Clinical Question?
Was the Search for Relevant Studies Exhaustive?
Was the Risk of Bias of the Primary Studies Assessed?
Did the review address possible explanations of between-study
differences in results?
Did the review present results that are ready for clinical application?
Were Selection and Assessments of Studies Reproducible?
Did the Review Address Confidence in Effect Estimates (i.e, quality
of evidence)?

28
Saudi Arabian Handbook for Healthcare
Guideline Development
Table 8.1 explains the quality assessment according to GRADE. Although RCTs start as high quality
evidence, they can be downgraded depending on whether issues of risk of bias, indirectness,
imprecision, inconsistency, and publication bias are detected in the body of evidence. On the
other hand, observational studies start as low quality evidence; however, they can be upgraded
to moderate or high quality evidence if they are methodologically sound and evidence of a large
magnitude of effect, dose-response gradient or plausible confounding, which would reduce a
demonstrated effect, are identified. It is important to highlight that the assessment of the quality
of the evidence should be conducted at an outcome level, across studies.
This handbook will not provide details on grading as this is described in the GDT and in the series
of articles cited below. An overview is provided here.
29
Saudi Arabian Handbook for Healthcare
Guideline Development
Table 8.1 GRADE's approach to rating quality of evidence (aka confidence in effect estimates)
For each outcome based on a systematic review and across outcomes (lowest quality across the outcomes critical for decision making)
1.
Establish initial
level of confidence
2.
Consider lowering or raising
level of confidence
3.
Final level of
confidence rating
Study design
Initial confidence
in an estimate of
effect
Reasons for considering lowering
or raising confidence
Confidence
in an estimate of effect
across those considerations
High
confidence
Risk of Bias
Inconsistency
Indirectness
Imprecision
Publication bias
Large effect
Dose response
All plausible
confounding & bias
would reduce a
demonstrated effect
or
would suggest a
spurious effect if no
effect was observed
High
Moderate
Low
confidence
Low
Very low
*upgrading criteria are usually applicable to observational studies only.

30
Saudi Arabian Handbook for Healthcare
Guideline Development
8.1 Limitations that can reduce the quality of the evidence:
1. Risk of bias or limitations in the detailed study design and execution: RCTs and observational
studies may suffer from limitations in the study design that could increase the risk of misleading
results. Although this assessment is conducted at a study level, the risk of bias can differ across
outcomes.
66
Some of the reasons for downgrading by one or two levels the quality of the evidence
of RCTs are:
• Lack of allocation concealment
• Lack of blinding (particularly if outcomes are subjective and their assessment highly
susceptible to bias)
• Large loss to follow-up
• Failure to adhere to an analysis according to intention-to-treat principle
• Selective reporting of events: investigators neglect to report outcomes that they have
measured (typically those for which they observed no effect).
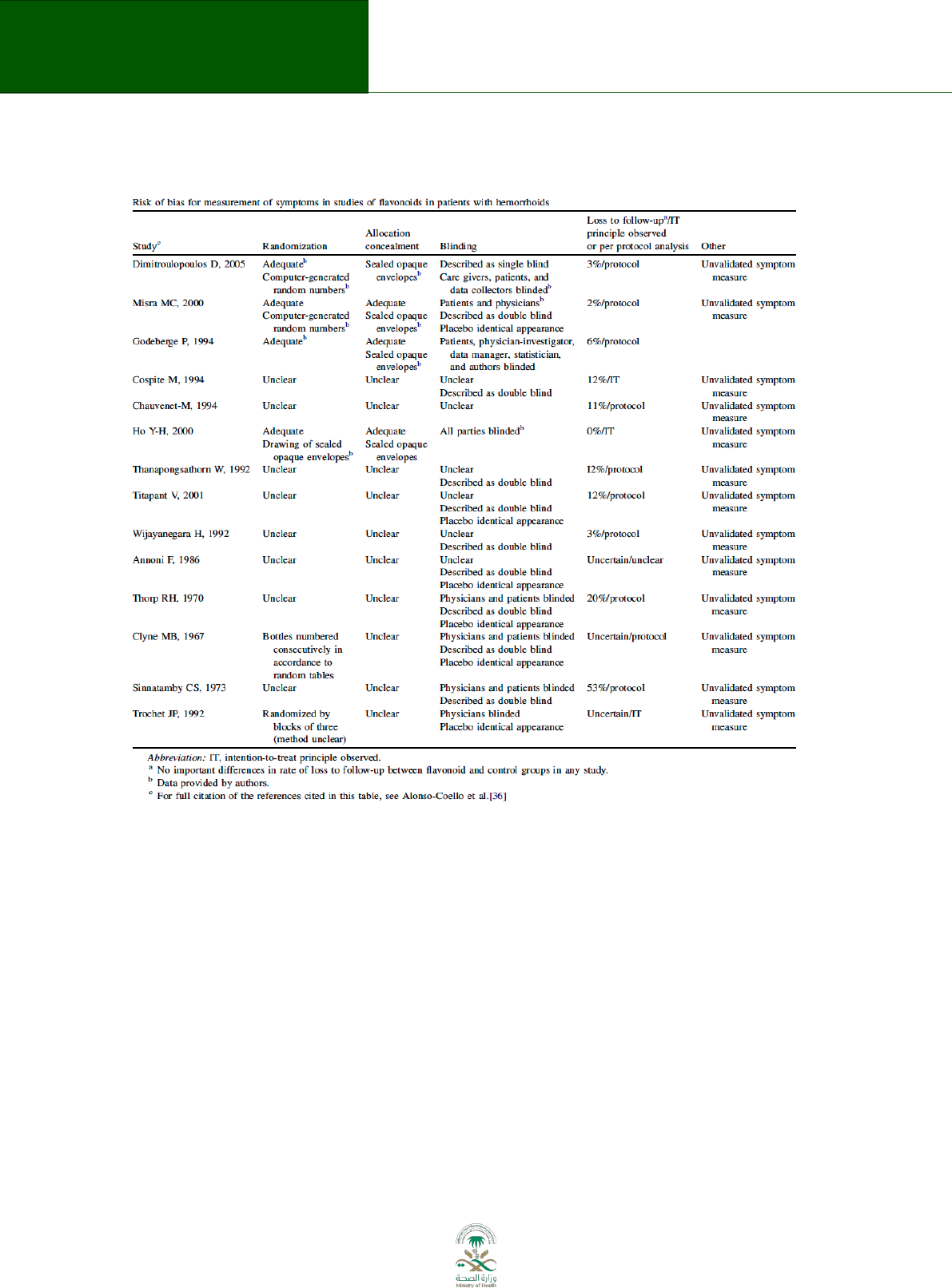
Consider the following example from the GRADE series in the Journal of Clinical Epidemiology.
The table below was extracted from a systematic review summarizing the evidence on the use of
flavonoids for treating haemorrhoids (Figure 8.1)
67
. The table describes the risk of bias
assessment of all the included studies providing evidence for the outcome of persisting
symptoms. Most of the trials did not provide enough information to determine which method
was used to generate the randomization sequence nor the appropriateness of the allocation
concealment. Most of the studies described blinding using the terms double blinding, with no
clear specification of who was blinded. Finally, the majority of the trials failed to conduct an
intention-to-treat analysis and did not report enough data to allow readers to conduct it.
After conducting an assessment of the risk of bias at a study level, it is necessary to obtain an
overall estimate of the risk of bias for the body of evidence informing a particular outcome.
Since we are using RCTs to inform this outcome, the quality of the evidence started as high
quality; however, due to issues of risk of bias it has to be downloaded at least one level going
from high to moderate.
31
Saudi Arabian Handbook for Healthcare
Guideline Development
Figure 8.1 Risk of bias example
2. Inconsistency: Widely differing estimates of the treatment effect (i.e. heterogeneity or variability
in results) across studies suggest true differences in underlying treatment effect. When evidence of
heterogeneity exists, but investigators fail to provide a plausible explanation, the quality of evidence
should be downgraded by one or two levels, depending on the magnitude of the inconsistency in the
results.
66
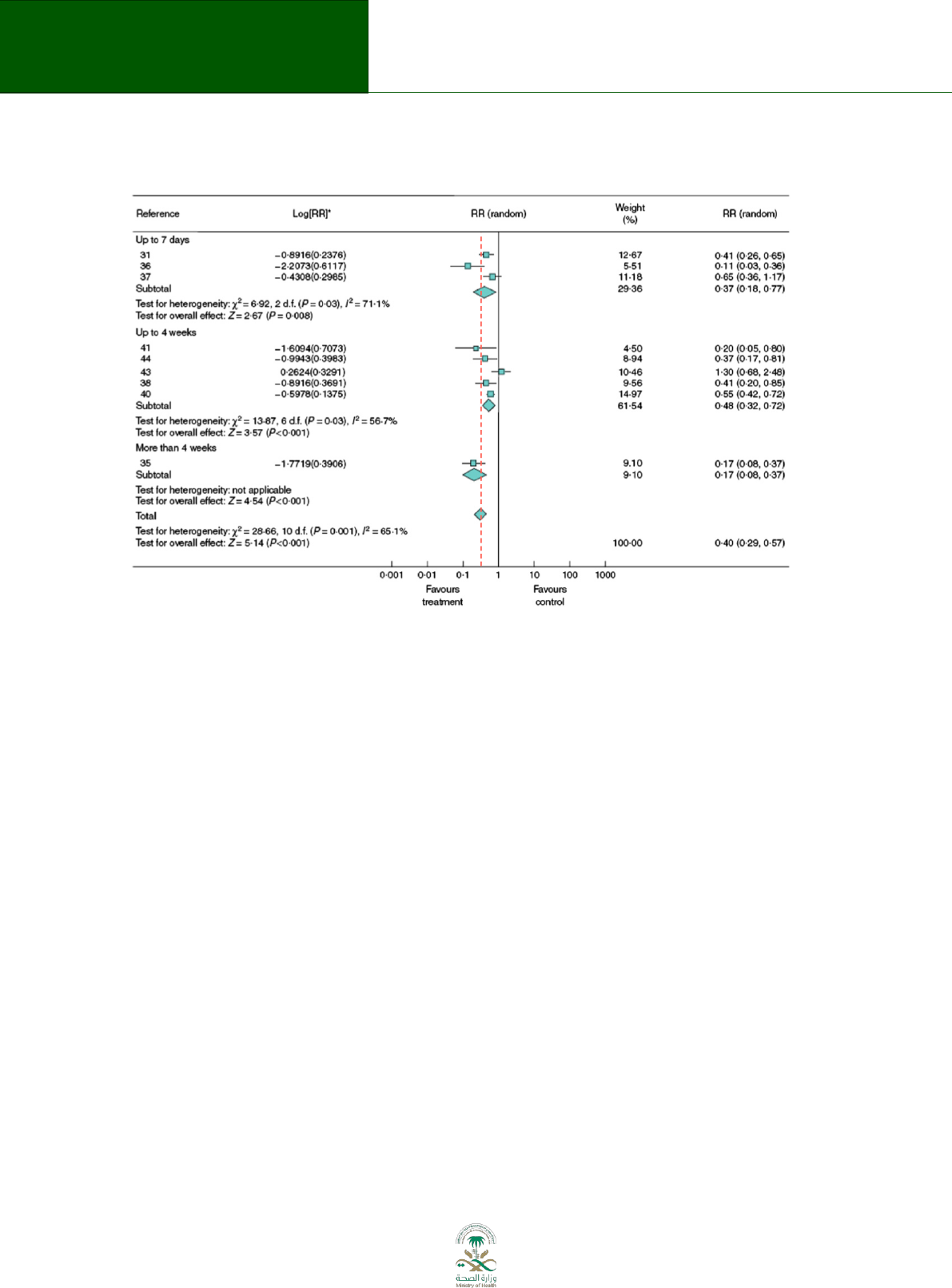
A systematic review provides a summary of the data from the results of a number of individual
studies. If the results of the individual studies are similar meta-analysis is used to combine the
results from the individual studies and an overall summary estimate is calculated. The meta-analysis
gives weighted values to each of the individual studies according to their size. The individual results
of the studies need to be expressed in a standard way, such as relative risk, odds ratio or mean
difference between the groups. Results are traditionally displayed in a figure (see Figure 8.2) called a
forest plot.
32
Saudi Arabian Handbook for Healthcare
Guideline Development
Figure 8.2 Forest plot
The forest plot depicted above represents a meta-analysis of 9 trials that assessed the effects of
flavonoids for the treatment of haemorrhoids
67
. Individual studies are represented by a square and
a horizontal line, which corresponds to the point estimate and 95% confidence interval of the
relative risk. The size of the square reflects the weight of the study in the meta-analysis. The solid
vertical line corresponds to ‘no effect’ of treatment – a relative risk of 1.0. When the confidence
interval includes 1 it indicates that the result is not significant at conventional levels (P > 0.05).
The diamond at the bottom represents the combined or pooled relative risk of all 9 trials with its
95% confidence interval. In this case, it shows that the treatment reduces persisting symptoms by
60% (RR 0.40 95% CI 0.29 to 0.57). Notice that the diamond does not overlap the ‘no effect’ line (the
confidence interval doesn’t include 1) so we can be assured that the pooled RR is statistically
significant. The test for overall effect also indicates statistical significance (p<0.001).
Heterogeneity can be assessed by eyeballing or more formally with statistical tests, such as the
Cochran Q test and the I
2
value. With the “eyeball” test one looks for the similarity of the point
estimates and the overlap of the confidence intervals of the trials with the summary estimate. In the
example above note that the dotted line running vertically through the combined relative risk does
not cross the horizontal lines of all the individual studies (3/9) indicating small to moderate degree
of heterogeneity among the included studies. If Cochran Q is statistically significant there is an
indication for heterogeneity. If Cochran Q is not statistically significant but the ratio of Cochran Q
and the degrees of freedom (Q/df) is > 1 there is possible heterogeneity. If Cochran Q is not
statistically significant and Q/df is < 1 then heterogeneity is very unlikely. In the example above Q/df
is >1 (28.66/10= 2.866) and the p-value is significant (0.001) indicating heterogeneity. Finally, the I
2
,
which quantifies the proportion of the variation in point estimates due to among-study differences is
large (65%). The higher the I
2
the greater the inconsistency (i.e. that differences between studies are
not likely due to chance).

33
Saudi Arabian Handbook for Healthcare
Guideline Development
3. Indirectness: Two types of indirectness are relevant. First, a review of the evidence comparing the
effectiveness of alternative interventions (say A and B) may find that randomized trials are available,
but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to
indirect comparisons between A and B.
Second, an evidence review may find randomized trials that meet eligibility criteria but which
address a restricted version of the main review question in terms of population, intervention,
comparator or outcomes. For example, suppose that in a review addressing an intervention for
secondary prevention of coronary heart disease, the majority of identified studies happened to be in
people who also had diabetes. Then the evidence may be regarded as indirect in relation to the
broader question of interest because the population is restricted to people with diabetes. The
opposite scenario can equally apply: a review addressing the effect of a preventative strategy for
coronary heart disease in people with diabetes may consider trials in people without diabetes to
provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had
conducted few if any randomized trials in the target population (e.g. people with diabetes). Other
sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical
intervention was implemented by expert, highly trained specialists in specialist centres, then
evidence on the effects of the intervention outside these centres may be indirect), comparators
used (e.g. if the control groups received an intervention that is less effective than standard
treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes
when data on patient-important outcomes are not available, or when investigators sought data on
quality of life but only symptoms were reported). Review authors should make judgements
transparent when they believe downgrading is justified based on differences in anticipated effects in
the group of primary interest. Review authors may be aided and increase transparency of their
judgments about indirectness if they use 8.2 (available in the GDT software).
68
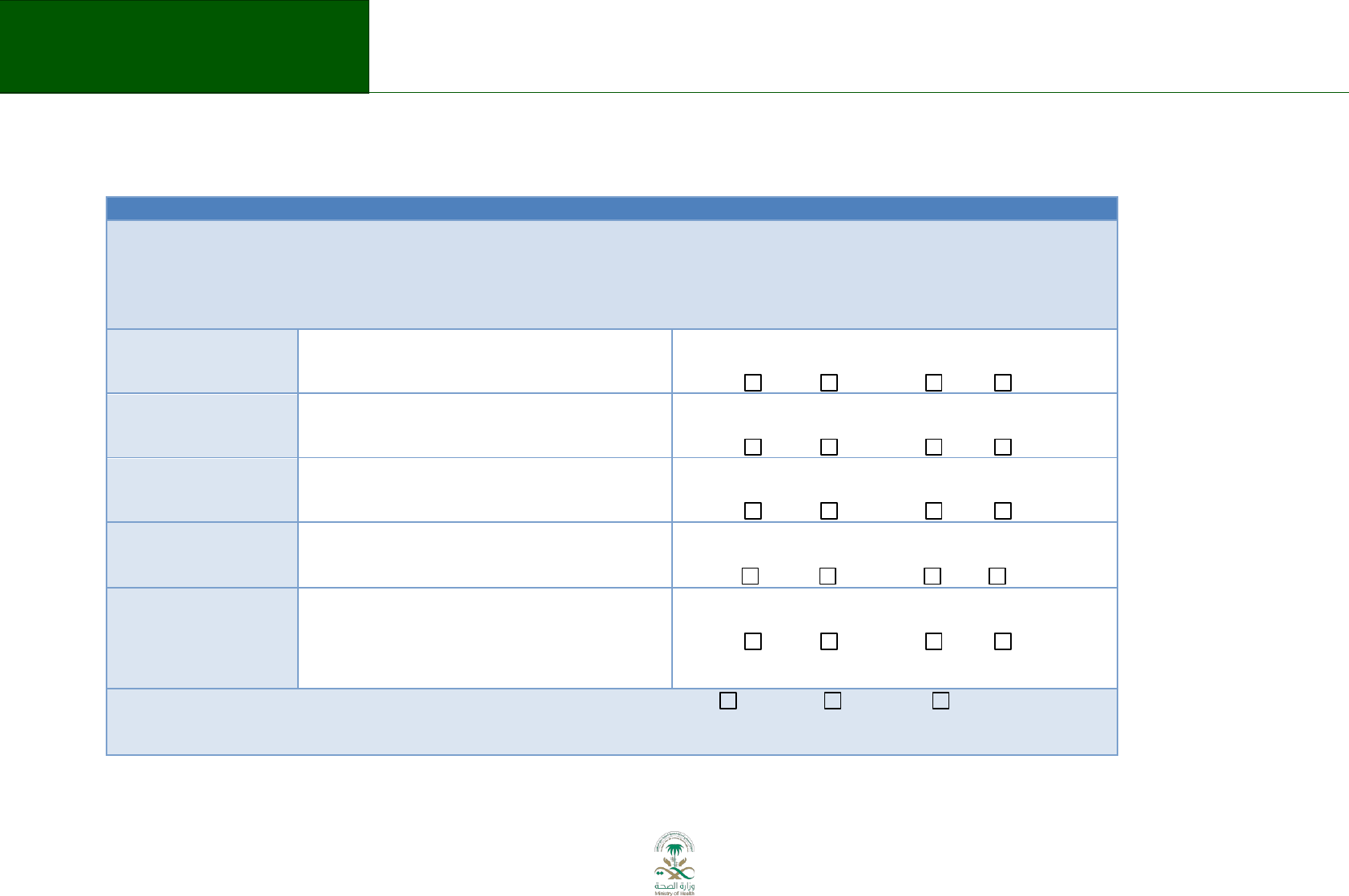
34
Saudi Arabian Handbook for Healthcare
Guideline Development
Table 8.2 Judgements about indirectness by outcome
Outcome
Domain (original
question asked)
Description (evidence found and included,
including evidence from other studies) –
consider the domains of study design and study
execution, inconsistency, imprecision and
publication bias
Judgment - Is the evidence sufficiently direct?
Population:
Yes
Probably
yes
Probably
no
N
o
Intervention:
Yes
Probably
yes
Probably
no
N
o
Comparator:
Yes
Probably
yes
Probably
no
N
o
Direct comparison:
Yes
Probably
yes
Probably
no
N
o
Outcome:
Yes
Probably
yes
Probably
no
N
o
Final judgment about
indirectness across
domains:
No
indirectness
Serious
indirectness
Very serious
indirectness
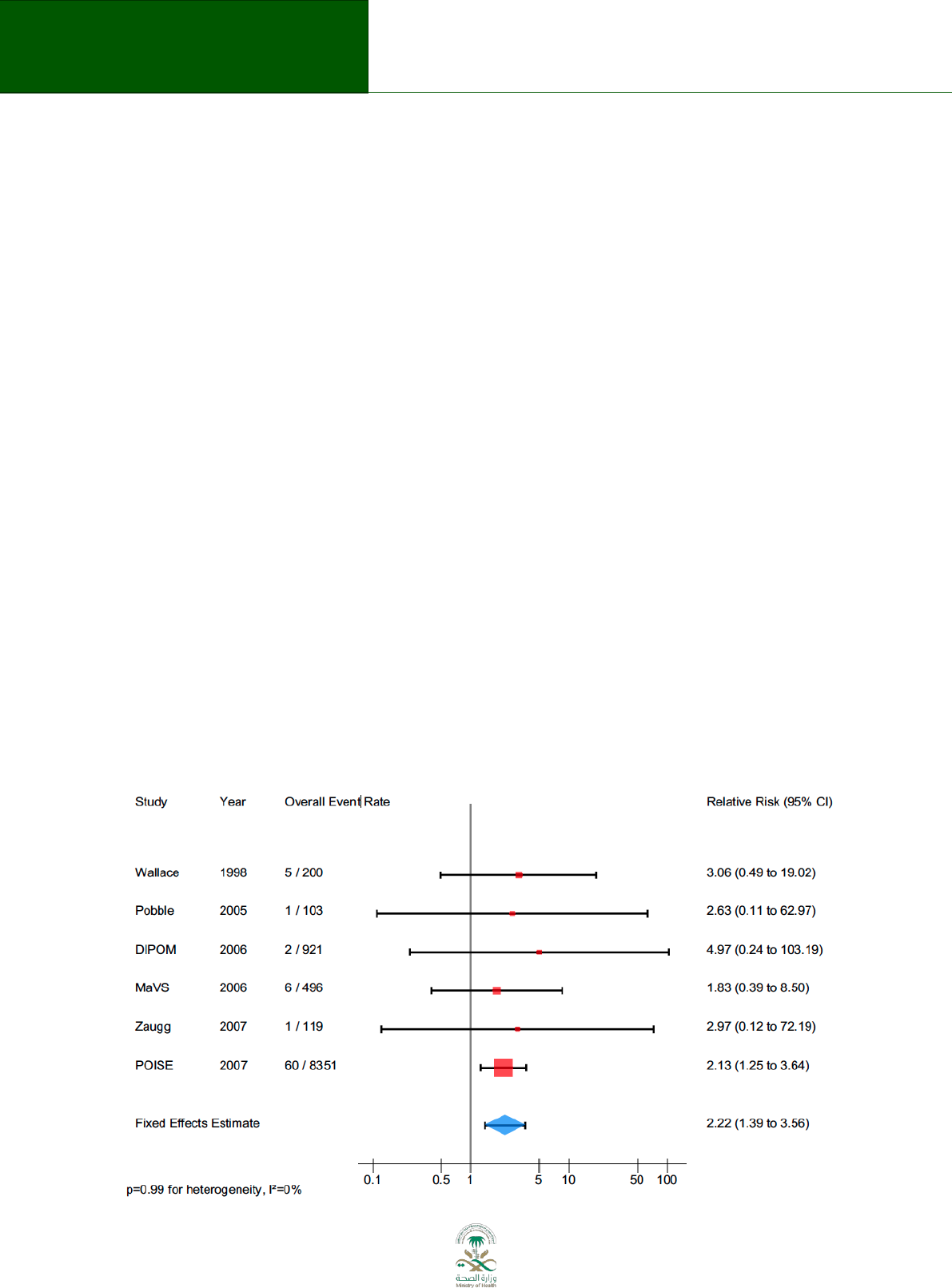
35
Saudi Arabian Handbook for Healthcare
Guideline Development
For example, a panel of a clinical practice guideline was formulating a recommendation about the
use of antiviral therapy for treating avian influenza. The available evidence showed that RCTs of high
quality (low risk of bias) have demonstrated that antiviral treatment is effective for managing
seasonal influenza; however, the panel had concerns about whether the underlying biology of
seasonal influenza is different enough from the avian one to decrease the confidence in the
estimates of effect. In this case, the quality of the evidence can be downgraded by one or two levels
due to indirectness.
4. Imprecision: Results are imprecise when studies include relatively few patients and few events
and thus have wide confidence intervals around the estimate of the effect. In the context of clinical
practice guidelines, if a recommendation or a clinical decision-making would differ if the upper
versus the lower boundary of the CI represented the truth, one should consider to rate down for
imprecision.
69
For example, the forest plot below represents a meta-analysis of 6 studies about the use of β-
blockers for preventing cardiovascular events in patients undergoing noncardiac surgery.
70
The
pooled estimate (blue diamond) suggests a doubling of the risk of stroke using the intervention (RR:
2.22; 95%CI: 1.39-3.56). In this case, two main arguments can support the decision of rating down
the quality of the evidence due to imprecision. First, the lower boundary of the 95% confidence
interval suggests a 39% increase in the risk of stroke, while the upper limit suggests a 256%
reduction on the risk for this cardiovascular event. Since both, appreciable benefit and considerable
harm are being included in the 95% confidence interval. Second, Only 75 events (from 10,889
participants) are informing this outcome. If conventional sample size estimation is conducted,
43,586 participants are required to detect a clinically relevant difference (α 0.05, β 0.20, β-blocker
group’s 1% rate, and an effect size of 0.25). Under these circumstances, a guideline panel may
decide to downgrade due to imprecision.
Figure 8.3 Forrest plot showing imprecision
36
Saudi Arabian Handbook for Healthcare
Guideline Development
5. Publication bias: Corresponds to a systematic underestimate or an overestimate of the underlying
beneficial or harmful effect of an intervention due to the selective publication of studies. That is,
investigators fail to report studies they have undertaken (typically those that can be considered as
“negative” results) or journals are less likely to accept studies that show no effect for publication.
71
Publication bias should be suspected especially when studies are consistently small, and sponsored
by the industry.
Following the example described earlier in this section, a systematic review of the use of flavonoids
for treating haemorrhoids included 9 studies reporting on the outcome persistent symptoms
67
.
Figure 8.4 depicted below is a funnel plot. The x-axis represents the magnitude of the effect size,
while the y-axis is the precision of the estimate of the effect. The small dots populating the figure
represent the point estimate of each included trial. Larger studies tend to be grouped around the
pool estimate (dotted vertical line) and show more precision (located at the top) than smaller
studies (located at the bottom). The ideal funnel plot should be symmetric, which means that small
“positive” and “negative” trials are equally distributed around the point estimate. Asymmetric
funnel plot suggests the presence of publication bias, particularly when studies are missing at the
bottom right quadrant.
The figure suggests the presence of publication bias due to the asymmetry of the funnel plot (studies
at the bottom right quadrant are missing). Likewise, all the included trials recruited on average no
more than 100 participants and all of them were industry sponsored. Thus, collecting these different
pieces of information, the suspicious for publication bias cannot be discarded. Under these
circumstances, the quality of the evidence should be rated down by one level due to publication
bias.
Figure 8.4 Funnel plot to detect publication bias

37
Saudi Arabian Handbook for Healthcare
Guideline Development
8.2 Factors that can increase the quality of the evidence:
1. Large magnitude of effect: When methodologically strong observational studies yield large or very
large and consistent estimates of the magnitude of a treatment or exposure effect, the quality of the
evidence increases. In this particular case, the weaknesses of the observational design is unlikely to
explain all of the apparent benefit or harm, even though observational studies are likely to provide
an overestimate of the true effect. The larger the magnitude of effect, the stronger becomes the
evidence:
72
. A large effect (e.g. RR > 2 or RR < 0.5) in the absence of plausible confounders, or a very
large effect (e.g. RR > 5 or RR < 0.2) in studies with no major threats to validity, might qualify for this.
For example, a systematic review studied the association between infant sleeping position and
sudden infant death syndrome.
73
An impressive 4-fold increase in the risk of sudden death was
found for front compared to back sleep position (OR: 4.1; 95% CI: 3.1-5,5). Subsequent studies
supported these findings increasing even more the confidence in the estimates of effect. When large
magnitude of effect are reported in the body of evidence for a particular outcome, this allows to rate
up the quality of the evidence for observational studies from low to moderate or even high quality of
the evidence.
2. Dose-response gradient: The presence of a dose-response gradient may increase the confidence in
the findings of observational studies. Only studies with no threats to validity or any other of the
criteria for downgrading can be upgraded by one or two levels.
72
For example, observational data shows that patients with supra-therapeutic anticoagulation therapy
levels have an increased risk for bleeding. The strength of this association increases when there is
evidence of a dose-response gradient between the two variables. In this particular case, the higher
the levels of the international normalized ratio, the higher the risk of bleeding.
74
Under this
circumstance, and as long as the quality of the body of evidence from observational studies shows,
otherwise, rigorous methodologies, it can be rated up from low to moderate or high confidence in
the estimates of effect.
3. Plausible confounding, which would reduce a demonstrated effect: All plausible confounding from
observational studies may be working to reduce the demonstrated effect or increase the effect if no
effect was observed.
72
This phenomenon increases the confident in the association demonstrated
and consequently, increases the quality of the evidence.
For example, a systematic review of observational studies summarized the evidence about the
contrast between private for-profit vs. private not-for-profit hospitals and death rates.
75
The review
showed that there was a higher death rate in private for-profit institutions than private non-for
profit ones. One potential source of bias is the severity of the diseases between patients in these
hospitals. Since it is expected that patients in the non-for-profit hospital were sicker than the ones in
for-profit hospitals, this potential bias would work against the review findings, providing more
confidence for the association found. In this case, the quality of the body of the evidence from
observational data informing about death rates and profit hospital characteristic can be upgraded by
one or two levels from low quality to moderate or even high quality evidence.
If further information is required about the process of assessing the quality of the evidence or
grading the recommendations, please visit the GRADE guideline series published in the Journal of
Clinical Epidemiology.
40,44,65,66,68,69,71,72,76-84

38
Saudi Arabian Handbook for Healthcare
Guideline Development
8.3 Presenting the evidence to the Guideline Panel
Draft evidence summaries, along with a draft assessment of values, preferences, and costs, should
be sent to the members of the panel before the meeting. Panel members should be asked to identify
any relevant evidence that is missing from the summaries. The final summaries are then used as the
basis for drafting recommendations.
54,65
9. Assessing cost implications
When developing guideline recommendations the cost implications of alternative strategies have to
be taken into account by the guideline panel.
85,86
Guideline panels should evaluate the potential
consequences of alternative scenarios and consider cost implications in addition to health outcomes.
In the KSA context cost implication analysis are divided into budget impact and economic evaluation
analysis. Economic evaluation of interventions not included in the health care services list financed
by MoH and the reimbursed pharmaceuticals list should be done according to procedures set in
legislation.
It is important to assess the cost implications related to potential changes in current clinical practice
standard related to each developed recommendation. This has to be done in parallel with
developing recommendation enabling to consider cost information as one input into the process
when moving from evidence to recommendations.
10,40,85
Generally, all important resource use
associated with the recommendation (suggested intervention and, if available, the comparator) –
are assessed.
Consideration of the economic consequences of potential guideline recommendations has to be
taken after defining the final scope of the guideline. In this stage it is also suggested to decide which
of the recommendations probably will need economic evaluation in addition to budget impact
analysis. A summary of budget impact analysis should be done for all initial recommendations by
describing also alternatives. A full economic evaluation might be worthwhile if an unbiased
effectiveness measure is available, and a review of country relevant existing economic studies may
be useful to inform the definition of resource use (for a costing exercise only or for a full economic
evaluation).
40,85
The description of resource use and costs should be made from the perspective of the health system
by identifying the main resources required to implement a specific recommendation. It is important
to include resource use associated with the provision of the intervention, subsequent investigations
and care, and adverse effects.
40,85
Implications not only for MoH but also for other stakeholders
(hospitals etc) should be taken into account. These should be grouped as costs incurred by the
patient, the health system and society. Those incurred by the patient and health system should be
described (e.g. drug, admissions, visits, examinations). Other resources, such as patient and care-
giver time, should generally be considered only when they are considered to be very important in
that context as they are difficult to measure and to put a value on reliably. It is also important to
define the time horizon for inclusion of resource use – when are important differences in resource
use likely to occur (in the short term or the long term).
10,40,46,85,86

39
Saudi Arabian Handbook for Healthcare
Guideline Development
Cost-effectiveness analyses must be done selectively. A full economic evaluation of cost-
effectiveness if conducted has to take into account the costs and health outcomes (effects) of an
intervention assessed in relation to its comparator, and present incremental cost-effectiveness ratio
(ICER). Effectiveness measures can be natural units (e.g. disease episodes or deaths prevented), two-
dimensional quality-adjusted life years (QALYs) in a costutility analysis, or can be expressed in
monetary terms in a costbenefit analysis. Cost-effectiveness analyses often use decision-analytic
methods in order to combine evidence from different sources and to extrapolate from the limited
time-horizons of existing studies on health outcomes. Once the cost-effectiveness of an intervention
is established, an evaluation should be made as to whether the intervention is affordable and
represents value for money.
85
Costs must be taken into account additionally to clinical evidence in process of approving specific
recommendation by guideline panel.
46
Wording recommendation as strong is suggested only in
cases if the intervention or pharmaceutical is affordable in Saudi Arabia or accepted for financing by
MOH or some other state agency.
Once resource use is measured, a range of monetary values can be estimated for each item of
resource use. For reporting on this costing exercise, it is important not just to document the
aggregate costs (number of units of resource use x unit costs of resource) associated with an
intervention, but also to report as far as possible disaggregated costing information (i.e. all the
associated resource use and unit costs separately).
Practical guidance:
Refer to interpretation of strong and weak recommendations.
Guideline panels will take a health systems perspective. Panels will label situations in which
resources are a driver of conditional/weak recommendations. When panels feel that they should
make a strong recommendation based on the overwhelming benefits, then it will be up to the
ministry to implement or make a decision about implementation. If a panel feels that there is benefit
but the technology is possibly too costly to implement then they might offer a conditional
recommendation indicating that the resource considerations are the key factor for the conditional
recommendation. This provides appropriate guidance to clinicians and the ministry (as opposed to
misleading guidance if offering strong recommendation for something that is not available) and
allows MoH to prioritize across health conditions and identify recommendations that are
conditional/weak solely bearing on resources/implementability.

40
Saudi Arabian Handbook for Healthcare
Guideline Development
10. Developing recommendations
Draft (neutral) recommendations are prepared by guideline support unit and final recommendations
(direction and strength) must be approved by panel.
For each recommendation, the quality of evidence and strength of recommendation should be
presented.
13,57,87,88
Recommendations should specify the perspective that is taken (e.g., individual
patient, health care system or society) and which outcomes were considered (including costs, if
assessed). The language used in recommendations should be clear and direct, indicating an
unambiguous action (e.g., all patients with disease A should be offered treatment B by health
professionals).
11,57,88
Preferably the language should be consistent across recommendations (e.g., all
strong recommendations phrased with “should” – see below for wording of recommendations).
11
10.1 How a panel decides on recommendations
The panel should reach recommendations based on consensus. Consensus does not necessarily
mean unanimity, however, and in some cases, at the discretion of the chair, a vote may need to be
taken.
89
Voting can then be used as a tool to work toward consensus. Panel members collaborate
with the chair to achieve the wording of final recommendations. The group should discuss and agree
on the process at the beginning of the meeting.
It is most effective if the group considers draft recommendations that have been prepared by the
guideline support unit. A suggested process is as follows:
the draft recommendation is presented by the guideline support unit using the evidence to
decision framework or tables, with a justification and reference to the relevant evidence
(evaluated by GRADE) summary;
the evidence is reviewed and discussed by the group, considering the balance of evidence
for benefits and harms;
the panel considers costs or resource utilization, ideally presented by health economists of
the guideline support unit, budget impact, and possibly cost-effectiveness, along with values
and preferences;
if necessary, the first recommendation is modified;
final agreement on the recommendation is reached.
10.2 Grading recommendations
The strength of a recommendation reflects the degree of confidence that the desirable effects of
adherence to the recommendation outweigh the undesirable effects.
4,62,64,87
Desirable effects can include beneficial health outcomes, less burden and greater savings.
Undesirable effects can include harms, greater burden, and increased costs. Burden here refers to
the demands of adhering to a recommendation that patients or care-givers (e.g., family members)
may find onerous – such as having to undergo more frequent tests or opting for a treatment that
may require a longer time for recovery.
57,77

41
Saudi Arabian Handbook for Healthcare
Guideline Development
Although the degree of confidence is a continuum, the GRADE system defines two categories –
strong and conditional (also known as “weak”). A strong recommendation is one for which the
guideline development group is confident that the desirable effects of adherence outweigh the
undesirable effects. This can be either in favour of or against an intervention. A weak
recommendation is one for which the panel concludes that the desirable effects of adherence
probably outweigh the undesirable effects, but the group is not confident about the trade-off.
4,5,62,87
Reasons for not being confident may include:
absence of high-quality evidence;
presence of imprecise estimates of benefit or harm;
uncertainty or variation in how different individuals value the outcomes;
small benefits;
benefits that are not worth the costs (including the costs of implementing the
recommendation).
Despite the lack of a precise threshold for moving from a strong to a conditional (also known as
“weak”) recommendation, the presence of important concerns about one or more of the above
factors make a weak recommendation more likely (see Table 10.1). The Guideline Development
Panel should consider all these factors and make the reasons for their judgments explicit.
Implications of a strong recommendation are:
4,5,62,87
For patients: most people in your situation would want the recommended course of action
and only a small proportion would not.
For clinicians: most patients should receive the recommended course of action. Adherence
to this recommendation is a reasonable measure of good-quality care.
For policy-makers: the recommendation can be adopted as a policy in most situations.
Quality initiatives could use this recommendation to measure variations in quality.
Implications of a conditional recommendation are:
4,5,62,87
For patients: the majority of people in your situation would want the recommended course
of action, but many would not.
For clinicians: be prepared to help patients to make a decision that is consistent with their
own values.
For policy-makers: there is a need for substantial debate and involvement of stakeholders.
42
Saudi Arabian Handbook for Healthcare
Guideline Development
It is strongly suggested to present the implications in a written copy of each guideline to facilitate
interpretation. However, the implications should not be seen as definition of the strength of a
recommendation.
Table 10.1 Factors that may influence the strength of recommendations
Factor
Examples of strong
recommendations
Examples of conditional (weak)
recommendations
Quality of evidence
Many high-quality randomized
trials have demonstrated the
benefit of inhaled steroids in
asthma
Only case series have examined the
utility of pleurodesis in
pneumothorax
Uncertainty about
the balance
between desirable
and undesirable
effects
Aspirin in myocardial infarction
reduces mortality with minimal
toxicity, inconvenience and cost
Warfarin in low-risk patients with
atrial fibrillation results in small
stroke reduction but increased risk
of bleeding and substantial
inconvenience
Uncertainty or
variability in values
and preferences
Young patients with lymphoma will
invariably place a higher value on
the life-prolonging effects of
chemotherapy over treatment
toxicity
Older patients with lymphoma may
not place a higher value on the life-
prolonging effects of
chemotherapy over treatment
toxicity
Uncertainty about
whether the
intervention
represents a wise
use of resources
The low cost of aspirin as
prophylaxis against stroke in
patients with transient ischaemic
attacks
The high cost of clopidogrel and
dipyridamole/aspirin as prophylaxis
against stroke in patients with
transient ischaemic attacks
Many recommendations are labelled as either strong or conditional. However, because the
“conditional” label may sometimes be misinterpreted, other options exist. These include the use of
terms such as “strong/weak” or “strong/qualified”.
The wording of recommendations is important.
57,77
To ensure that end users will understand the
specific linguistic and cultural contexts of the wording, sample text should be validated with them.
The key to the wording must always be attached to the guideline. Some examples are in table 10.2
below.
43
Saudi Arabian Handbook for Healthcare
Guideline Development
Table 10.2 Wording of recommendations
Wording 1
Wording 2
Wording 3
Strong
recommendation for
We recommend…
Clinicians should…
We recommend…
Weak
recommendation for
We suggest
Clinicians might…
We conditionally
recommend…
Weak
recommendation
Against
We suggest...not
Clinicians might
not…
We conditionally
recommend...not
Strong
recommendation
Against
We recommend
…not
Clinicians should
not…
We recommend …not
Example of a conditional recommendation: The KSA MoH panel suggests sublingual immunotherapy
for treatment of adults with seasonal or intermittent allergic rhinitis (conditional recommendation;
Moderate-quality evidence).
Example of a strong recommendation: The KSA MoH panel recommends intranasal corticosteroids
rather than intranasal H1-antihistamines for treatment of adults with seasonal or intermittent
allergic rhinitis (Strong recommendation; High-quality evidence).
Please refer to appendix 6.2 for detailed examples.
10.3 Indicators for implementation
The Guideline Development Panel should suggest indicators for monitoring the implementation of
the guideline and its impact, based on the final recommendations as part of the evidence to decision
tables.
10,11,18,21,23,59
When conditional recommendations are selected (ideally only those based on
high quality evidence) the decision making process (a dyad approach between the patient and the
clinician) can function as quality indicator.
In general, indicators can be process indicators (for example, prescription rates for specific
medicines; length of hospital stay) or outcome indicators, such as readmission to hospital due to a
specific cause, or clinical events (for example, patients experiencing myocardial infarction).
The indicators that are selected by the guideline panel should be events or processes that are
expected to be affected as result of the recommendation and in some instances may be the same as
the critical outcomes used by the Panel in making recommendations.
10,11,18,21,23,59,90-92
They should
also be processes or events that can be measure through routine data collection by the MOH or
through audit that can be done as part of the guideline implementation process. There is no pre-
specified number of indicators required for a guideline, but if there are several strong
recommendations, there may need to be several indicators.
The final selection of indicators should be done in consultation with the MOH and the key societies
likely to be involved in implementing the guideline and approved by the NGC.

44
Saudi Arabian Handbook for Healthcare
Guideline Development
11. Producing and disseminating the guideline
The person or group in charge of producing the guideline must be identified in the early stages of
the guideline development. If a member of the panel group will produce the draft of the guideline,
there must be sufficient time in the timeline to do this. If the services of an external group will be
used, it is important to consider the experience of the group and what are the resources that must
be allocated to this purpose.
The guideline should have three main parts: 1. Executive summary including a summary of the key
recommendations; 2. Main text; and 3. The evidence to recommendation tables and evidence
profiles.
The key recommendations of the guideline should be easily identifiable
15
and should answer to the
guideline questions in a clear and concise manner. The guideline summary must provide an overview
of the guideline. It should briefly describe the scope, aim, target audience, methods and
recommendations. The main text must describe with details the guideline development process. It
must be complemented with appendices that could include the following:
Information about panel members and external reviewers
Conflicts of interest of the panel members
Search strategies and electronic datasets used (with dates and number of hits)
If recommendations from existing guidelines were adapted, quality assessment of the
guidelines from which they were obtained
Risk of bias assessment of the primary studies included, or methodological quality
assessment of the systematic reviews included
Evidence profiles used by the panel members to formulate the recommendations
Evidence to decision frameworks used to formulate the recommendations
Supplementary material for clinicians and patients
The dissemination of the guideline should aim to deliver it to the target audience in order to
facilitate its implementation. Not only a printed version, but also an online version, publication in
peer-reviewed journals, educational interventions such as meeting and conferences, and
introduction to electronic medical record systems should be considered.
The plan for dissemination should consider the scale to which the guideline will be implemented,
and used strategies according to this.

45
Saudi Arabian Handbook for Healthcare
Guideline Development
12. Guideline authorship
The decision about guideline authorship rests with the NGC. Generally, the guidelines will be
authored by the “The Saudi Center for EBHC”. Panel members will be acknowledged by name as
members of the “Saudi Expert Panel” if they participate meaningfully in the guideline development
process, e.g. in meetings, question development, developing recommendations, review of the final
guideline document. Other contributors, such as systematic review authors or support staff will be
listed under appropriate headings, such as working groups. The address for correspondence of the
guidelines will be the “The Saudi Center for Evidence Based Health Care”.
13. Updating a guideline
The process for updating a guideline must already be planned during the guideline development,
and it should be reported in the guideline publication. The aim of updating a guideline is to include
any new evidence relevant to the guideline questions, and to reflect any important change in the
setting for which the recommendation was developed.
Some of the main reasons for updating a guideline are:
93
Availability of new evidence regarding the benefits and harms of the interventions
recommended in the guideline
Availability of new evidence regarding critical and important outcomes, for which there was
not high quality evidence when the recommendations were formulated
Development of new intervention that could be a valid alternative to those recommended in
the guideline
Changes in the health system, which modify the setting for which the guideline was
developed
Changes is issues related to the resources needed to implement the recommendations
The updating plan should specify a time period after a complete revision of the evidence included in
the guideline would be done. On average, this revision should be done every 3-5 years.
11,94
The
specific time depends on the speed to which the evidence in a specific medical area evolves.
When updating a guideline, the same process for guideline development described in this handbook
should be used.
46
Saudi Arabian Handbook for Healthcare
Guideline Development
14. Glossary and references
The glossary (from reference
9
) includes definitions of terms and acronyms appearing throughout the
handbook to help with interpretation of the items included. Related terms in the list are grouped
into categories describing the various aspects of guideline development.
Term
Definition
Groups, individuals, and organizations involved in the guideline development process
Guideline development panel
The entire group of healthcare and other professionals,
stakeholders, patients and carers, research and technical staff
who develop a guideline. The guideline development group
may consist of several task-specific subgroups or committees
such as the oversight committee, guideline panel, stakeholder
and consumer consultants, and working group. Certain
individuals may be members of more than one subgroup or
committee (e.g. a clinician scientist as a member of the working
group and guideline panel).
11,95
Oversight committee
A body overseeing the guideline development process, whose
tasks include the priority setting, and selection of potential
guidelines for development out of proposed topics, recruitment
and appointment of members for the guideline panel, and
approval of the final guideline for publication and
dissemination. May also be referred to as an executive
committee or guideline advisory board.
11
Guideline panel
Decides on topics to be covered within the guideline,
formulates questions, develops and agrees on the
recommendations in the guideline using evidence summaries
prepared by the working group, and endorses the final
guideline document for approval by the oversight committee.
Members of the guideline panel may often be referred to as
‘panelists’.
11
Chair (of the guideline panel)
The leading member of the guideline panel. This person is
neutral and has an expertise in coordinating groups of
healthcare professionals and patients and caregivers.
Someone who is qualified and experienced in strategies and
facilitation of optimal group processes, ensuring all members of
the panel have equal opportunity to contribute and freely
express their opinion without feeling intimidated. This
individual is not necessarily an expert of any specific clinical
domain.
2,25
Co-chair (of the guideline panel)
Should be appointed when the guideline panel is especially
large or the task particularly complex. Co-chairs should also
have experience leading groups but should represent a
47
Saudi Arabian Handbook for Healthcare
Guideline Development
different discipline (clinical or methodological) than the
Chair.
2,25
Working group
A group of individuals tasked with the preparation and
technical aspects of guideline development such as assisting
the guideline panel in formulating PICO questions, conducting
systematic reviews, rating quality of evidence, preparing
evidence summaries and background documents for guideline
panel discussions, writing the guideline, and reviewing
comments from stakeholders and public consultation. Works
closely with the guideline panel to ensure the work to achieve
goals and objectives for the guideline is completed.
Secretariat
A group of individuals tasked with supporting the guideline
development group in preparing for the development and
writing of the guideline. The Secretariat provides technical
support as well as administrative support (e.g. scheduling
meetings and teleconferences, distributing documents).
11
Stakeholder
An individual, group or an organization that has an interest in
the organization and delivery of health care and will have an
interest in the content of or the outcome of a guideline. This
may include health care providers, professional societies and
colleges, experts in a disease or condition, research institutions,
and policy makers.
11,95
Consumer
Consumers of healthcare include: (a) individual patients, (b)
carers, including patients’ family and friends, (c) members of
the public (both as potential patients and as funders of
healthcare through taxation, insurance or direct payments), (d)
voluntary and community organizations that represent the
interests of patients, carers and the public, (e) advocates
representing the interests of patients, carers and other client
groups.
They are described collectively as ‘consumers’ (without
implying consumerist assumptions about health services) and
are distinct from other consumers of guidelines such as health
professionals, commissioners and providers of services.
96
Carer
Provide non-reimbursed care and/or support to patients (e.g.
family members, friends) and have knowledge of the issues
that are important to patients and carers. May also be referred
to as caregivers.
Advocate
Someone who speaks on behalf of a patient, or a group of
patients to help them make their wishes known.
97
Sponsoring organization
The organization that funds the development of a guideline and
will endorse it for publication and dissemination.
48
Saudi Arabian Handbook for Healthcare
Guideline Development
Professional societies
Not-for-profit organizations whose membership consists of
healthcare professionals working in a specific field or specialty
and whose work focuses on a specific area or topic in health
care (e.g. American College of Chest Physicians, European
Society of Cardiology). Professional societies are often involved
in the development of guidelines for their members and often
take policy stances on medical issues and health promotion.
May also be referred to as professional organizations or
medical societies or associations.
Third party organizations
Organizations or groups that wish to adopt or adapt a guideline
for which they were not directly involved in its development.
This may often include government departments or ministries
of health that do not have sufficient resources to develop
guidelines de novo, or whose populations and health care
settings are similar to those covered in an existing guideline.
Guidelines and topics
Guideline
A document that focuses on a disease or condition and includes
recommendations for appropriate management of patients
with this disease or condition. The guideline should be based
on the best available evidence and should help healthcare
providers by supplementing their knowledge and skills.
Guidelines can be tailored to clinical, health policy, health
systems or public health settings, among others.
11
Target audience
The specific group or range of health care provider for whom
the clinical practice guidelines are intended, to inform their
work in a health care setting. The target audience will have an
influence on the breadth and depth of the guideline content.
24
The primary audience consists of the intended end users of the
guideline. For example, if the guideline is for primary care, then
the target audience will comprise of primary care physicians
and nurses. Secondary audiences may include any other groups
to whom the guideline content will be applicable, such as
health care managers, hospital administrators, and policy
makers.
98
Guideline topic
The guideline topic specifies the disease, condition or overall
area that will be covered by the guideline (e.g. chronic
obstructive pulmonary disease). Guideline developers must
consider prioritizing the guideline topics with the greatest
potential to improve health care and health outcomes.
39
Topics within guidelines
Topics within the guideline encompass the content that the
guideline will cover. For example, whether the guideline will
cover diagnosis of a condition, treatment of a condition, or
both, or whether it will focus on topics where there is most
uncertainty or variation in practice. Guideline panels must
consider and decide on the many issues that may be addressed
49
Saudi Arabian Handbook for Healthcare
Guideline Development
within a guideline that will be important to the target audience.
May also be referred to as the scope of the guideline, and will
be interrelated with the PICO questions addressed in the
guideline.
39
Steps and processes in guideline development
Priority setting
Priority setting is the identification, balancing and ranking of
priorities by stakeholders. It ensures that resources and
attention are devoted to those general areas (e.g. chronic
obstructive pulmonary disease, diabetes, cardiovascular
disease, cancer, prevention) where health care
recommendations will provide the greatest benefit to the
population, a jurisdiction, or a country. A priority-setting
approach needs to contribute to future plans while responding
to existing potentially difficult circumstances.
99
Peer review
A process of subjecting scholarly works, research, or ideas to
the scrutiny of others. Peer review of a guideline and
recommendations by those with similar interests and expertise
to the people who produced it is intended to ensure the
guideline is accurate and valid. Peer review may be internal,
conducted by colleagues from the same organization not
directly involved in the production of the guideline, or external,
conducted by individuals fully independent and removed from
the development of the guideline.
11,97
Dissemination
The active process of distributing information, such as
guidelines, to the target end users to ensure maximum
exposure, uptake, and implementation. Various methods for
dissemination may be used such as a printed version of the full
guideline, online version of the guideline, a quick reference
guide, mobile application of the guideline, incorporation of
guideline recommendations into clinical decision support
systems, consumer version of the guideline, education
materials detailing the recommendations, conference meetings
with target end users, etc. Products other than the main
guideline document that are developed are commonly referred
to as derivative products.
92
Implementation
The uptake and incorporation of guideline recommendations
into practice by the target end users. An implementation plan
should include the identification of potential barriers, criteria
and indicators for success, baseline data for the indicators,
required resources, training and education needs, identification
of existing mechanisms or networks, methods for monitoring
the implementation process, reporting and feedback
mechanisms, and milestones with timescales.
11,92
Guideline Adaptation
A systematic approach to using and adjusting existing
guidelines produced in one setting for use in a new setting with
50
Saudi Arabian Handbook for Healthcare
Guideline Development
a different cultural or organizational context. The process of
adapting a guideline and its recommendations must ensure
that the adapted guideline addresses specific health questions
relevant to the context of use and that it is suited to the needs,
priorities, legislation, policies, and resources in the new target
setting.
10
Group processes
Group processes encompass how and when members of a
group interact. For example, the interaction of guideline panel
members during a consensus meeting to formulate
recommendations.
25
Consensus methods
Techniques used in decision-making to reach agreement on a
particular issue. Consensus may be informal or formal, with
examples of formal consensus methods including the
Delphi and nominal group techniques.
95
Quorum
The smallest number of group members that must be present
to constitute a valid meeting or voting or consensus process.
95
Milestones
When major steps are achieved during the guideline
development process. Examples include completing the
systematic review, having recommendations developed, and
publishing the guideline report.
25
Considerations in the development of a guideline
Declaration of interest (or
disclosure of interest)
A declaration of interest is the disclosure of any potential or
actual conflicts of interest that include financial, professional,
intellectual or other interests relevant to the subject of the
work or meeting to determine possible conflicts of interest. The
declaration of interest must also include any relevant interests
of others who may, or may be perceived to, unduly influence
the expert’s judgment, such as immediate family members,
employers, close professional associates, or any others with
whom the expert has a substantial common personal, financial,
or professional interest.
11
Conflict of interest
A divergence between or individual’s private interests and his
or her professional obligations such that an independent
observer might reasonably question whether the individual’s
professional actions or decisions are motivated by personal
gain, such as financial, academic advancement, clinical revenue
streams or community standing. This definition includes a
financial or intellectual relationship that may impact an
organization’s or individual’s ability to approach a scientific
question with an open mind.
50
Commercial sponsorship
May apply to individuals or organizations, including funding for
the development of a guideline. Of particular concern is the
possibility that guideline developers will feel, or be perceived
to be, beholden to or pressured by the commercial sponsor to
make recommendations favorable to the sponsor’s interests.
51
Saudi Arabian Handbook for Healthcare
Guideline Development
Commercial sponsorship may be in the form of industry-
sponsored research, clinical services from which a committee
member derives a substantial proportion of his or her income,
consulting, board membership for which compensation of any
type is received.
100
Barriers to change
Should be identified and considered prior to developing a
guideline where recommendations suggest changes in health
care practice(s). Barriers to change can exist at various levels of
the health care system and include structural barriers (e.g. lack
of resources, financial disincentives), organizational barriers
(e.g. inappropriate skill mix, lack of facilities or equipment),
peer group barriers (e.g. local standards of care not in line with
desired practice), professional-patient interaction barriers (e.g.,
communication and information-processing issues), and
competing priorities. There are diverse methods to identify
barriers that vary in their formality. Barriers may vary for given
resources, across settings, and for different guidelines.
92
Equity (in health)
Equity in health, or health equity, is a measure of the degree to
which health policies are able to distribute well-being fairly. It is
the absence of systematic or potentially remediable differences
in health status, access to healthcare and health-enhancing
environments, and treatment in one or more aspects of health
across populations or population groups defined socially,
economically, demographically or geographically. Health
inequity results from a gap in health status and in access to
health services between different social classes, ethnic groups,
and between populations in different geographical areas.
Guideline panels must consider whether and the extent to
which recommendations will have an impact on health equity.
May also be referred to as health inequality.
95,99,101
Values, preferences and utilities
These include patient and carer knowledge, attitudes,
expectations, moral and ethical values and beliefs; patient
goals for life and health; prior experience with the intervention
and the condition; symptom experience (for example
breathlessness, pain, dyspnoea, weight loss); preferences for
and importance of desirable and undesirable outcomes;
perceived impact of the condition or interventions on quality of
life, well-being or satisfaction and interactions between the
work of implementing the intervention, the intervention itself,
and other contexts the patient may be experiencing;
preferences for alternative courses of action; and preferences
relating to communication content and styles, information and
involvement in decision-making and care. This can be related to
what in the economic literature is considered utilities. An
intervention itself can be considered a consequence of a
52
Saudi Arabian Handbook for Healthcare
Guideline Development
recommendation (e.g. the burden of taking a medication or
undergoing surgery) and a level of importance or value is
associated with that. The values and preferences of those who
will be affected by the recommendations should be integrated
into the process of developing the guideline.
96
Transparency
Transparency involves clearly documenting and presenting
details of the entirety of the methods and process that were
used to develop a guideline, including the participants involved,
the evidence and information reviewed, and judgements made
during any decision-making, especially formulating the
recommendations. Transparency would allow others to follow
and arrive at the same guideline product if replicating the
guideline development process.
Credibility of guidelines
The degree to which a guideline’s conclusions and
recommendations can be trusted. Determined by the methods
and approaches used, including timing and editorial
dependence such as described by the AGREE II tool, the
Institute of Medicine’s report on guidelines and the Guideline
International Network. May also be referred to as
trustworthiness or quality of guidelines.
2,16,102
Evidence review and consideration of additional information
Protocol
A document that outlines the plan or set of steps that defines
how a guideline will be produced and the methodology that
will be used. Before carrying out a guideline, for example, the
protocol sets out what questions to be answered, how
information will be collected and analyzed, and the framework
and consensus methods to be used to formulate
recommendations.
PICO question
Population/Patient-Intervention-Comparison-Outcome; a
mnemonic used in developing specific health care questions to
be answered in a guideline. A question generated using the
PICO framework will guide which evidence is reviewed and is
meant to elicit information about the patient and their
condition, interventions of interest that have been undertaken
or should be taken, any comparisons between the current
intervention and possible alternatives, and outcomes to be
desired or achieved.
11
Population
A group of people with a common link, such as the same
medical condition or living in the same area or sharing the
same characteristics. The population identified for a guideline is
all the people the recommendations are intended to apply to
(e.g. adults with diabetes mellitus).
103
Comorbidity
A disease or condition that exists in a patient in addition to the
principal disease of interest being studied or treated (e.g.
chronic obstructive pulmonary disease and diabetes mellitus).
53
Saudi Arabian Handbook for Healthcare
Guideline Development
Comorbidities may influence the clinical manifestations and
natural history of a disease. May also be referred to as
concomitant conditions.
97,104
Clinical pathway (or care
pathway)
The sequence of practices, procedures, tests, interventions and
treatments that should be used to provide care for people with
a particular clinical condition.
97
Outcomes
The impact that a test, treatment, policy, program or
other intervention has on a person, group or population.
Outcomes from interventions to improve the public's health
could include a change in people's health and wellbeing
or health status. In clinical terms, outcomes could include the
number of patients who fully recover from an illness or the
number of hospital admissions, and an improvement or
deterioration in someone's health, functional ability, symptoms
or situation.
97
Patient-important outcomes
An outcome defined by answering “yes” to the following
question: “If one knew that this outcome was the only thing to
change with treatment, would the patient consider receiving
this treatment even if it was associated with adverse effects,
inconvenience, or cost?” Such outcomes include mortality,
morbidity, and outcomes reported by patients.
44,105
Health-related quality of life
A combination of a person's physical, mental and social well-
being; not merely the absence of disease. An example of a
patient-important outcome.
95
Surrogate outcomes
Outcomes that are not themselves important health outcomes
but may be correlated with patient-important health outcomes
(e.g. bone density as surrogate for fractures as the patient-
important outcome). May be referred to as substitute or
indirect outcomes.
44
Importance of outcomes
Ranking the relative importance of desirable (e.g. reduced
mortality, improvement in health-related quality of life) and
undesirable outcomes (e.g. side effects, costs) for the
intervention in question allows a guideline panel to determine
how much influence the particular outcomes and the
results/estimates of effect for those outcomes will have in
formulating a recommendation. The relative importance of
outcomes is likely to vary according to different values and
preferences or when considered from the perspective of
patients, clinicians or policy-makers. In the GRADE framework,
outcomes are rated as critical for decision-making, important
but not critical for decision-making, or low importance for
decision-making.
44
Magnitude of effect
A measure of the difference or relative effect of an
intervention on the outcome in the intervention group
compared with that in a control group. Also referred to as the
54
Saudi Arabian Handbook for Healthcare
Guideline Development
effect size.
97
Systematic review
A comprehensive review of the published literature that
focuses on a healthcare topic and answers a specific question.
An extensive literature search is conducted based on a search
strategy to identify all studies. The studies are reviewed, their
quality is assessed, and the results are summarized according
to the review question.
11
Evidence retrieval
In the context of systematic reviews, the process of
systematically searching for all scientific studies relevant to a
particular question, and obtaining them for review. The process
also includes obtaining evidence from other sources that may
be unpublished.
11
Selection criteria
The criteria used to decide which studies and study types
should be included and excluded from consideration as
potential sources of evidence when retrieving evidence during
the development of a guideline. Also referred to as inclusion
and exclusion criteria.
97
Expert opinion
An interpretation of evidence. Sometimes based on high quality
evidence, such as from randomized controlled trials or well-
done observational studies, and other times based on
unsystematically collected information, ideally summarized in
writing. Expert opinion is often confused with the notion of
evidence that is either not available from systematic research
or not systematically summarized. Also often used as excuse
for not collecting evidence systematically.
Economic evaluation
A set of formal, quantitative methods used to asses one or
more interventions, programs, or strategies with respect to
their resource use and their expected outcomes. Economic
evaluation may involve different study types such as cost-
effectiveness analysis, cost-benefit analysis, and economic
models.
11
Quality of evidence
Describes the level of confidence or certainty in the estimates
of the effect of an intervention on a specific outcome in a given
population. Also called strength of evidence, confidence in
estimates, certainty in evidence, levels of evidence.
79
Evidence table or profile or
summary of findings table
A table summarizing the results/estimate of effect from studies
for each outcome of interest and the associated quality of
evidence. The table provides a concise summary of the key
information that is needed by someone making a decision and,
in the context of a guideline, provides a summary of the key
information underlying a recommendation.
65,97
Recommendations and formulation of recommendations
Analytic framework
A framework outlining the criteria that guideline panels use to
review the evidence and analyze relevant information to arrive
at a recommendation. The analysis may focus on the balance
55
Saudi Arabian Handbook for Healthcare
Guideline Development
between desirable and undesirable consequences, informed by
the quality of evidence, magnitude of the difference between
the benefits and harms, the certainty about or variability in
values and preferences, resource use, equity and other factors
(e.g. GRADE/DECIDE Evidence-to-Recommendation
framework).
58
Recommendation
A course of action recommended by the guideline based on
clinical questions, evidence retrieval, and consideration of
other information in the analytic framework.
Recommendations in guidelines may relate to clinical
interventions, public health activities, or government policies.
11
Conditional recommendation
A recommendation for which a guideline panel rested with
more uncertainty about whether implementation of the
recommended action leads to more desirable than undesirable
consequences. Specific conditions may have to be described.
Also known as weak recommendation in the GRADE
framework.
77
Research recommendation
A recommendation resulting from a guideline process for use in
the context of research only. Guideline panels should consider
making research recommendations when there is important
uncertainty about the desirable and undesirable effects of an
intervention, further research could reduce that uncertainty,
and the potential benefits and savings of reducing the
uncertainty outweigh the potential harms of not making the
research recommendation. The formulation of
recommendations for additional research should be as precise
and specific as possible. Defining the population, intervention,
comparator and outcomes (PICO) explicitly will make research
recommendations more helpful.
58,106
Strength of recommendation
The strength of a recommendation reflects the extent to which
guideline developers are confident that the desirable effects of
adherence to the recommendation outweigh the undesirable
effects.
58,77
Performance measures
Performance measures are criteria that can be measured to
assess the quality-of-care (e.g. a physician following a specific
management option). Management options associated with
strong recommendations are particularly good candidates for
quality criteria.
58

56
Saudi Arabian Handbook for Healthcare
Guideline Development
15. References
1. Academy of Medicine of Malaysia. Clinical Practice Guidelines. 2013;
http://www.acadmed.org.my/index.cfm?&menuid=67. Accessed April 22, 2013.
2. Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice
Guidelines. Clinical Practice Guidelines We Can Trust. 2011;
http://www.nap.edu/openbook.php?record_id=13058. Accessed April 22, 2013.
3. Appraisal of Guidelines Research and Evaluation. Introduction to AGREE II. 2013;
http://www.agreetrust.org/about-the-agree-enterprise/introduction-to-agree-ii/.
4. Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of
recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE
approach and grading quality of evidence about interventions. Allergy. May 2009;64(5):669-
677.
5. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of
evidence and strength of recommendations. Bmj. Apr 26 2008;336(7650):924-926.
6. Ahmed F, Temte JL, Campos-Outcalt D, Schunemann HJ. Methods for developing evidence-
based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the
U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29(49):9171-9176.
7. Oxman A, Schunemann H, Fretheim A. Improving the use of research evidence in guideline
development: 7. Deciding what evidence to include. Health Research Policy and Systems.
2006;4(1):19.
8. Qaseem A, Snow V, Owens DK, Shekelle P. The development of clinical practice guidelines
and guidance statements of the American College of Physicians: summary of methods.
Annals of internal medicine. Aug 3 2010;153(3):194-199.
9. Schunemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of
a comprehensive checklist for a successful guideline enterprise. CMAJ : Canadian Medical
Association journal = journal de l'Association medicale canadienne. Dec 16 2013.
10. The ADAPTE Collaboration. The ADAPTE Process: Resource Toolkit for Guideline Adaptation.
Version 2.0. 2009; http://www.g-i-n.net/document-store/working-groups-
documents/adaptation/adapte-resource-toolkit-guideline-adaptation-2-0.pdf. Accessed July
5, 2013.
11. World Health Organization. Estonian Handbook for Guidelines Development. 2011;
http://whqlibdoc.who.int/publications/2011/9789241502429_eng.pdf. Accessed April 22,
2013.
12. Oxman AD, Fretheim A, Schunemann HJ. Improving the use of research evidence in guideline
development: introduction. Health Res Policy Syst. 2006;4:12.
13. Schunemann HJ, Woodhead M, Anzueto A, et al. A guide to guidelines for professional
societies and other developers of recommendations: introduction to integrating and
coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.
Proceedings of the American Thoracic Society. Dec 2012;9(5):215-218.
14. Eccles MP, Grimshaw JM, Shekelle P, Schunemann HJ, Woolf S. Developing clinical practice
guidelines: target audiences, identifying topics for guidelines, guideline group composition
and functioning and conflicts of interest. Implementation science : IS. 2012;7:60.
15. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: Advancing guideline development,
reporting and evaluation in health care. Canadian Medical Association Journal.
2010;182(18):E839-E842.

57
Saudi Arabian Handbook for Healthcare
Guideline Development
16. Qaseem A, Forland F, Macbeth F, Ollenschlager G, Phillips S, van der Wees P. Guidelines
International Network: Toward international standards for clinical practice guidelines.
Annals of internal medicine. 2012;156(7):525-531.
17. Fretheim A, Schunemann H, Oxman A. Improving the use of research evidence in guideline
development: 3. Group composition and consultation process. Health Research Policy and
Systems. 2006;4(1):15.
18. National Institute for Health and Clinical Excellence. The guidelines manual. 2012;
http://publications.nice.org.uk/the-guidelines-manual-pmg6. Accessed April 22, 2013.
19. World Health Organization. WHO Handbook for Guideline Development. 2012;
http://apps.who.int/iris/bitstream/10665/75146/1/9789241548441_eng.pdf. Accessed April
22, 2013.
20. Centers for Disease Control and Prevention. Guidelines and Recommendations: A CDC
Primer. Atlanta, Georgia: Office of the Associate Director for Science Centers for Disease
Control and Prevention;2012.
21. New Zealand Guidelines Group. Handbook for the preparation of explicit evidence-based
clinical practice guidelines. Wellington: New Zealand Guidelines Group;2001.
22. Eccles M, Grimshaw J, Shekelle P, Schunemann H, Woolf S. Developing clinical practice
guidelines: target audiences, identifying topics for guidelines, guideline group composition
and functioning and conflicts of interest. Implementation Science. 2012;7(1):60.
23. Scottish Intercollegiate Guidelines Network. SIGN 50: A guideline developer's handbook -
Annex D: Completed considered judgement form. 2013;
http://www.sign.ac.uk/guidelines/fulltext/50/compjudgement.html.
24. National Health and Medical Research Council. Procedures and requirements for meeting
the 2011 NHMRC standard for clinical practice guidelines. 2011;
http://www.nhmrc.gov.au/guidelines/publications/cp133-and-cp133a. Accessed April 22,
2013.
25. Kunz R, Fretheim A, Cluzeau F, et al. Guideline Group Composition and Group Processes:
Article 3 in Integrating and Coordinating Efforts in COPD Guideline Development. An Official
ATS/ERS Workshop Report. Proceedings of the American Thoracic Society. 2012;9(5):229-
233.
26. Agency for Healthcare Research and Quality. U.S. Preventive Services Task Force Procedure
Manual. 2008;
http://www.uspreventiveservicestaskforce.org/uspstf08/methods/procmanual.htm.
Accessed April 22, 2013.
27. Canadian Task Force on Preventive Health Care. Canadian Task Force on Preventive Health
Care Procedure Manual. 2011; http://canadiantaskforce.ca/methods/methods-manual/.
Accessed April 22, 2013.
28. Cancer Care Ontario. Program in Evidence-Based Care Handbook. 2012;
http://www.cancercare.on.ca/about/programs/pebc/pebc-products/. Accessed April 22,
2013.
29. Scottish Intercollegiate Guidelines Network. SIGN 50: A guideline developer’s handbook.
2011; http://www.sign.ac.uk/guidelines/fulltext/50/. Accessed April 22, 2013.
30. Cancer Care Ontario. Program in Evidence-Based Care Document Assessment and Review
Protocol. 2012; http://www.cancercare.on.ca/about/programs/pebc/document__review/.
Accessed April 22, 2013.
31. European Society of Cardiology. Recommendations for Guidelines Production. 2010;
http://www.escardio.org/guidelines-surveys/esc-guidelines/about/Pages/rules-writing.aspx.
Accessed April 22, 2013.
32. American College of Cardiology Foundation and American Heart Association. Methodology
Manual and Policies from the ACCF/AHA Task Force on Practice Guidelines. 2010;

58
Saudi Arabian Handbook for Healthcare
Guideline Development
http://my.americanheart.org/professional/StatementsGuidelines/PoliciesDevelopment/Dev
elopment/Methodologies-and-Policies-from-the-ACCAHA-Task-Force-on-Practice-
Guidelines_UCM_320470_Article.jsp. Accessed April 22, 2013.
33. Davino-Ramaya C, Krause LK, Robbins CW, et al. Transparency matters: Kaiser Permanente's
National Guideline Program methodological processes. The Permanente journal. Winter
2012;16(1):55-62.
34. Esandi ME, Luca MD, Chapman E, Schapochnik N, Bernztein R, Otheguy L. Guía para la
adaptación de Guías de Práctica Clínica. 2008;
http://publicaciones.ops.org.ar/publicaciones/otras pub/GuiadeGuias.pdf. Accessed April
22, 2013.
35. Gutiérrez GC, Bossert T, Espinosa JQ, et al. Guía Metodológica para la elaboración de Guías
de Atención Integral en el Sistema General de Seguridad Social en Salud Colombiano. 2010;
http://www.minsalud.gov.co/Documentos y Publicaciones/GUIA METODOLOGICA PARA LA
ELABORACI%C3%93N DE GU%C3%8DAS DE ATENCI%C3%93N INTEGRAL.pdf. Accessed April
22, 2013.
36. Rosenfeld RM, Shiffman RN, Robertson P. Clinical Practice Guideline Development Manual,
Third Edition: A Quality-Driven Approach for Translating Evidence into Action.
Otolaryngology -- Head and Neck Surgery. 2013;148(1 suppl):S1-S55.
37. American College of Rheumatology. Policy and Procedure Manual for Clinical Practice
Guidelines. 2012;
http://www.rheumatology.org/Practice/Clinical/Guidelines/Clinical_Practice_Guidelines.
Accessed July 5, 2013.
38. Ministerio de Sanidad y Consumo. Elaboración de guías de práctica clínica en el sistema
nacional de salud: Manual metodológico. 2007;
http://www.guiasalud.es/emanuales/elaboracion/index-02.html. Accessed April 22, 2013.
39. Atkins D, Perez-Padilla R, MacNee W, Buist AS, Cruz AA. Priority Setting in Guideline
Development: Article 2 in Integrating and Coordinating Efforts in COPD Guideline
Development. An Official ATS/ERS Workshop Report. Proceedings of the American Thoracic
Society. 2012;9(5):225-228.
40. Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and
rating the quality of economic evidence. Journal of clinical epidemiology. Feb
2013;66(2):140-150.
41. National Health and Medical Research Council. Guideline Development and Conflicts of
Interest: Identifying and Managing Conflicts of Interest of Prospective Members and
Members of NHMRC Committees and Working Groups Developing Guidelines. 2012;
http://www.nhmrc.gov.au/guidelines-and-publications/information-guideline-
developers/guideline-development-and-conflicts. Accessed April 22, 2013.
42. Woolf S, Schunemann H, Eccles M, Grimshaw J, Shekelle P. Developing clinical practice
guidelines: types of evidence and outcomes; values and economics, synthesis, grading, and
presentation and deriving recommendations. Implementation Science. 2012;7(1):61.
43. Burford BJ, Rehfuess E, Schunemann HJ, et al. Assessing evidence in public health: the added
value of GRADE. J Public Health (Oxf). Dec 2012;34(4):631-635.
44. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding
on important outcomes. Journal of clinical epidemiology. Apr 2011;64(4):395-400.
45. Schunemann H, Oxman A, Fretheim A. Improving the use of research evidence in guideline
development: 6. Determining which outcomes are important. Health Research Policy and
Systems. 2006;4(1):18.
46. Guyatt GH, Oxman AD, Kunz R, et al. Incorporating considerations of resources use into
grading recommendations. Bmj. May 24 2008;336(7654):1170-1173.

59
Saudi Arabian Handbook for Healthcare
Guideline Development
47. Carling CL, Kristoffersen DT, Oxman AD, et al. The effect of how outcomes are framed on
decisions about whether to take antihypertensive medication: a randomized trial. PLoS ONE.
2010;5(3):e9469.
48. Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical
practice guidelines when consensus is elusive. Bmj. 2008;337:a744.
49. Boyd E, Bero L. Improving the use of research evidence in guideline development: 4.
Managing conflicts of interests. Health Research Policy and Systems. 2006;4(1):16.
50. Schunemann HJ, Osborne M, Moss J, et al. An official American Thoracic Society Policy
statement: managing conflict of interest in professional societies. American journal of
respiratory and critical care medicine. Sep 15 2009;180(6):564-580.
51. Cancer Care Ontario. Program in Evidence-Based Care Conflict of Interest Policy. 2011;
http://www.cancercare.on.ca/cms/one.aspx?objectId=7582&contextId=1377. Accessed
April 22, 2013.
52. Oxman A, Schunemann H, Fretheim A. Improving the use of research evidence in guideline
development: 14. Reporting guidelines. Health Research Policy and Systems. 2006;4(1):26.
53. Cincinnati Children’s Hospital Medical Center Evidence-Based Care Group. Let Evidence
Guide Every New Decision (LEGEND) Evidence-Based Care Guideline Development and
Update Process. 2006; http://www.cincinnatichildrens.org/service/j/anderson-
center/evidence-based-care/legend. Accessed April 22, 2013.
54. Guyatt G, Akl EA, Oxman A, et al. Synthesis, Grading, and Presentation of Evidence in
Guidelines: Article 7 in Integrating and Coordinating Efforts in COPD Guideline Development.
An Official ATS/ERS Workshop Report. Proceedings of the American Thoracic Society.
2012;9(5):256-261.
55. National Heart Lung and Blood Institute. Systematic Evidence Reviews and Clinical Practice
Guidelines. 2013; http://www.nhlbi.nih.gov/guidelines/index.htm. Accessed April 22, 2013.
56. Oxman A, Schunemann H, Fretheim A. Improving the use of research evidence in guideline
development: 8. Synthesis and presentation of evidence. Health Research Policy and
Systems. 2006;4(1):20.
57. Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. Bmj. May 10
2008;336(7652):1049-1051.
58. Schünemann HJ, Oxman AD, Akl EA, et al. Moving from Evidence to Developing
Recommendations in Guidelines: Article 11 in Integrating and Coordinating Efforts in COPD
Guideline Development. An Official ATS/ERS Workshop Report. Proceedings of the American
Thoracic Society. 2012;9(5):282-292.
59. World Health Organization. Declaration of interests for WHO experts.
http://www.who.int/ipcs/methods/harmonization/areas/mutagenicity_doi.pdf.
60. AMSTAR. Assessing the methodological quality of systematic reviews. 2012;
http://amstar.ca. Accessed July 21, 2013.
61. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess
the methodological quality of systematic reviews. Journal of clinical epidemiology. Oct
2009;62(10):1013-1020.
62. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of
recommendations. Bmj. Jun 19 2004;328(7454):1490.
63. Guyatt GH, Oxman AD, Kunz R, et al. What is "quality of evidence" and why is it important to
clinicians? Bmj. May 3 2008;336(7651):995-998.
64. Brozek JL, Akl EA, Compalati E, et al. Grading quality of evidence and strength of
recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to
developing recommendations. Allergy. May 2011;66(5):588-595.

60
Saudi Arabian Handbook for Healthcare
Guideline Development
65. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence
profiles and summary of findings tables. Journal of clinical epidemiology. Apr
2011;64(4):383-394.
66. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence--
inconsistency. Journal of clinical epidemiology. Dec 2011;64(12):1294-1302.
67. Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF. Meta-
analysis of flavonoids for the treatment of haemorrhoids. Br J Surgery. 2006;93:909e920.
68. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence--
indirectness. Journal of clinical epidemiology. Dec 2011;64(12):1303-1310.
69. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--
imprecision. Journal of clinical epidemiology. Dec 2011;64(12):1283-1293.
70. POISE Study Group, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol
succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled
trial. Lancet. 2008;371(9627):1839-1847.
71. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence-
-publication bias. Journal of clinical epidemiology. Dec 2011;64(12):1277-1282.
72. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of
evidence. Journal of clinical epidemiology. Dec 2011;64(12):1311-1316.
73. Gilbert R, Salanti G, Harden M, See S. Infant sleeping position and the sudden infant death
syndrome: systematic review of observational studies and historical review of
recommendations from 1940 to 2002. International journal of epidemiology. Aug
2005;34(4):874-887.
74. Schulman S, Beyth RJ, Kearon C, Levine M. Hemorrhagic complications of anticoagulant and
thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical
Practice Guidelines. Chest. 2008;133(257):Se98S.
75. Devereaux PJ, Choi PT, Lacchetti C, et al. A systematic review and meta-analysis of studies
comparing mortality rates of private for-profit and private not-for-profit hospitals. CMAJ :
Canadian Medical Association journal = journal de l'Association medicale canadienne.
2002;166:1399e1406.
76. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to
recommendations: the significance and presentation of recommendations. Journal of clinical
epidemiology. Jul 2013;66(7):719-725.
77. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to
recommendations: the significance and presentation of recommendations. Journal of clinical
epidemiology. 2013;66(7):719-725.
78. Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence
to recommendation-determinants of a recommendation's direction and strength. Journal of
clinical epidemiology. Jul 2013;66(7):726-735.
79. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of
evidence. Journal of clinical epidemiology. Apr 2011;64(4):401-406.
80. Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of
confidence in effect estimates for a single outcome and for all outcomes. Journal of clinical
epidemiology. Feb 2013;66(2):151-157.
81. Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of
findings tables-binary outcomes. Journal of clinical epidemiology. Feb 2013;66(2):158-172.
82. Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new
series of articles in the Journal of Clinical Epidemiology. Journal of clinical epidemiology. Apr
2011;64(4):380-382.
83. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--
study limitations (risk of bias). Journal of clinical epidemiology. Apr 2011;64(4):407-415.

61
Saudi Arabian Handbook for Healthcare
Guideline Development
84. Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of
findings tables and evidence profiles-continuous outcomes. Journal of clinical epidemiology.
Feb 2013;66(2):173-183.
85. Edejer T. Improving the use of research evidence in guideline development: 11.
Incorporating considerations of cost-effectiveness, affordability and resource implications.
Health Research Policy and Systems. 2006;4(1):23.
86. Hill SR, Olson LG, Falck-Ytter Y, et al. Incorporating Considerations of Cost-Effectiveness,
Affordability, and Resource Implications in Guideline Development: Article 6 in Integrating
and Coordinating Efforts in COPD Guideline Development. An Official ATS/ERS Workshop
Report. Proceedings of the American Thoracic Society. 2012;9(5):251-255.
87. GRADE working group. Grading the quality of evidence and the strength of
recommendations. 2013; http://www.gradeworkinggroup.org/intro.htm.
88. Hsu J, Brozek JL, Terracciano L, et al. Application of GRADE: Making evidence-based
recommendations about diagnostic tests in clinical practice guidelines. Implementation
science : IS. 2011;6:62.
89. Murad MH, Montori VM, Sidawy AN, et al. Guideline methodology of the Society for
Vascular Surgery including the experience with the GRADE framework. Journal of vascular
surgery. May 2011;53(5):1375-1380.
90. Fretheim A, Schunemann H, Oxman A. Improving the use of research evidence in guideline
development: 15. Disseminating and implementing guidelines. Health Research Policy and
Systems. 2006;4(1):27.
91. Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw JM. How can we
improve guideline use? A conceptual framework of implementability. Implementation
science : IS. 2011;6:26.
92. Grimshaw JM, Schünemann HJ, Burgers J, et al. Disseminating and Implementing Guidelines:
Article 13 in Integrating and Coordinating Efforts in COPD Guideline Development. An
Official ATS/ERS Workshop Report. Proceedings of the American Thoracic Society.
2012;9(5):298-303.
93. Canadian Health Services Research Foundation. Reader Frindly Writing- 1:3:25. 2010.
94. Wollersheim H, Burgers J, Grol R. Clinical Guidelines to Improve Patient Care. Neth J Med.
2005;63(6):188-192.
95. Shekelle PG, Ortiz E, Rhodes S, Morton SC, et al. Validity Of the Agency For Healthcare
Research And Quality Clinical practice guidelines: how quickly do guidelines become
outdated? . JAMA. 2001;286(12):1461-1467.
96. National Institute for Health and Clinical Excellence. The guidelines manual: Appendix L –
Abbreviations and Glossary. 2012; http://publications.nice.org.uk/the-guidelines-manual-
appendix-l-abbreviations-and-glossary-pmg6d/l2-glossary. Accessed July 21, 2013.
97. Kelson M, Akl EA, Bastian H, et al. Integrating Values and Consumer Involvement in
Guidelines with the Patient at the Center: Article 8 in Integrating and Coordinating Efforts in
COPD Guideline Development. An Official ATS/ERS Workshop Report. Proceedings of the
American Thoracic Society. 2012;9(5):262-268.
98. National Institute for Health and Clinical Excellence. Glossary. 2013;
http://www.nice.org.uk/website/glossary/glossary.jsp. Accessed July 21, 2013.
99. Yawn BP, Akl EA, Qaseem A, Black P, Campos-Outcalt D. Identifying Target Audiences: Who
Are the Guidelines For?: Article 1 in Integrating and Coordinating Efforts in COPD Guideline
Development. An Official ATS/ERS Workshop Report. Proceedings of the American Thoracic
Society. 2012;9(5):219-224.
100. World Health Organization. Health Systems Strengthening Glossary. 2013;
http://www.who.int/healthsystems/hss_glossary/en/index.html. Accessed July 26, 2013.

62
Saudi Arabian Handbook for Healthcare
Guideline Development
101. Boyd EA, Akl EA, Baumann M, et al. Guideline Funding and Conflicts of Interest: Article 4 in
Integrating and Coordinating Efforts in COPD Guideline Development. An Official ATS/ERS
Workshop Report. Proceedings of the American Thoracic Society. 2012;9(5):234-242.
102. Oxman A, Schunemann H, Fretheim A. Improving the use of research evidence in guideline
development: 12. Incorporating considerations of equity. Health Research Policy and
Systems. 2006;4(1):24.
103. AGREE Research Trust. The AGREE Enterprise Website. 2013; http://www.agreetrust.org.
Accessed July 21, 2013.
104. Wilt TJ, Guyatt G, Kunz R, et al. Deciding What Type of Evidence and Outcomes to Include in
Guidelines: Article 5 in Integrating and Coordinating Efforts in COPD Guideline Development.
An Official ATS/ERS Workshop Report. Proceedings of the American Thoracic Society.
2012;9(5):243-250.
105. Fabbri LM, Boyd C, Boschetto P, et al. How to Integrate Multiple Comorbidities in Guideline
Development: Article 10 in Integrating and Coordinating Efforts in COPD Guideline
Development. An Official ATS/ERS Workshop Report. Proceedings of the American Thoracic
Society. 2012;9(5):274-281.
106. Guyatt G DP, Montori V, Schünemann HJ, Bhandari M,. Putting the patient first: In our
practice, and in our use of language. ACP Journal Club. Jan-Feb 2004;140(A11).
107. Brown P BK, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al,. How to formulate research
recommendations. Bmj. Oct 14 2006;333(7572):804-806.
63
Saudi Arabian Handbook for Healthcare
Guideline Development
16. Appendices
Appendix 1: Template for topic proposal
Factors
Considerations per each factor
Data provided by
Burden of disease
(problem)
mortality (per 1000)
the initiator
Incidence
the initiator
Prevalence
the initiator
resource impact (MOH spending,
per year)
the initiator in
cooperation with
MOH
Variations
Practice variation
the initiator in
cooperation with
MOH
Health outcome variation
the initiator in
cooperation with
MOH
Variation in treatment costs
the initiator in
cooperation with
MOH
Potential
Potential for modernization
the initiator
Potential result on health
the initiator
Potential impact on resources
the initiator in
cooperation with
MOH
Problem statement
Based on the information listed
above
the initiator
Purpose of the
guideline
Based on problem statement
the initiator
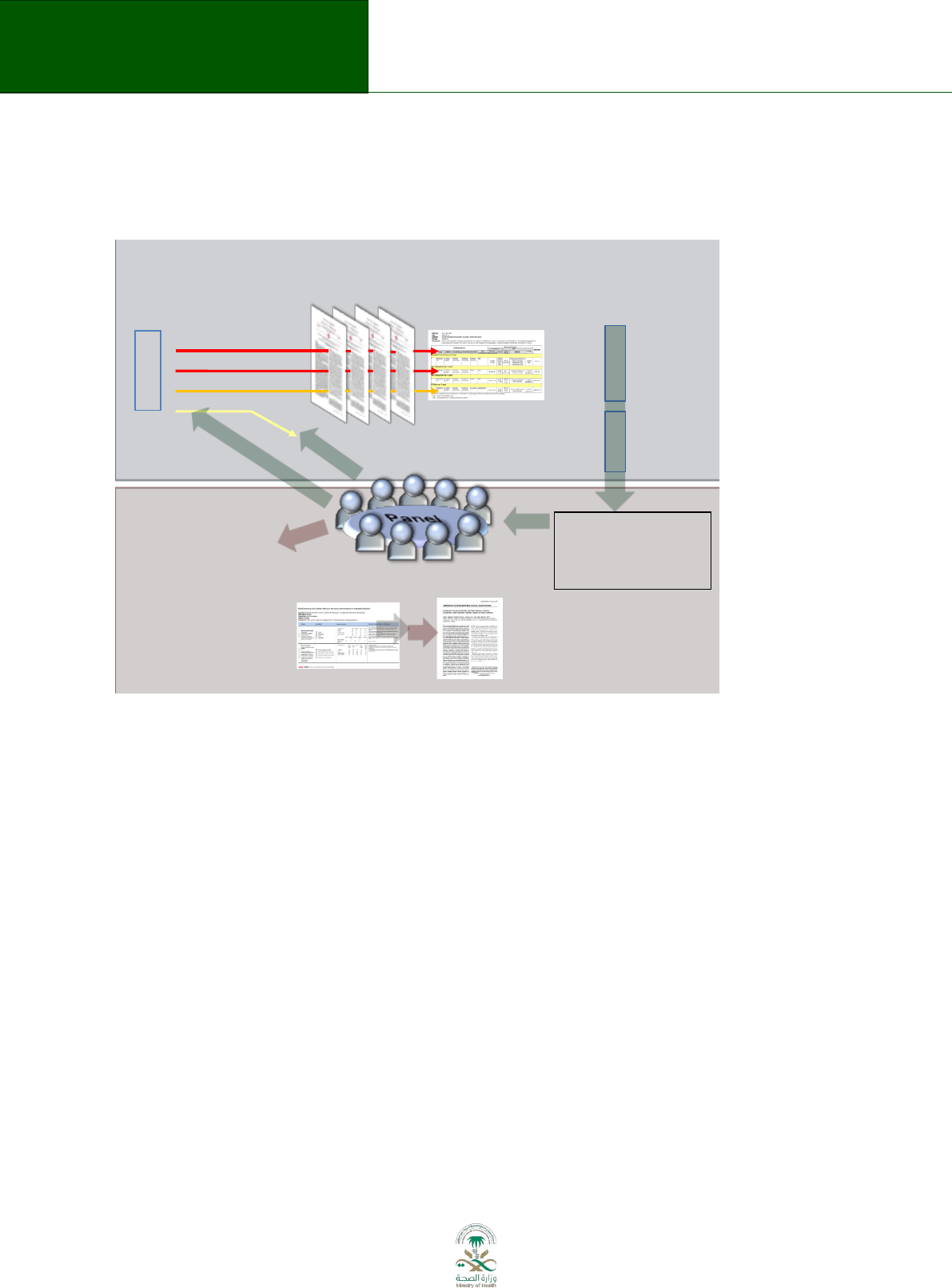
64
Saudi Arabian Handbook for Healthcare
Guideline Development
Appendix 2: The GRADE process in developing guidelines
This figure describes the GRADE process
The figure demonstrates an optimal process for integrating the GRADE approach into guideline
development. It also highlights the relation between the conduct of systematic reviews (evidence
synthesis) and guideline development (decision making by a panel). The requirement for a close
relationship between systematic reviewers and guideline panels is highlighted. Multidisciplinary
guideline panels should be involved in the development of the guideline questions using the PICO
framework. The panels are also involved in the process of selecting critical and important outcomes
upon which the evidence assessment is focused and prioritizing their importance for decision
making.
Outcomes that are considered critical and important for decision-making should be evaluated in a
systematic review. Outcomes rated as less important do not serve as the substrate for
recommendations and generally do not need to be considered further for making a
recommendation. One of the innovations inherent to the GRADE approach is the evaluation of
outcomes across, rather than within, studies. As a consequence, different bodies of evidence may
contribute information towards the different critical and important outcomes that are being
considered. Those who review the evidence will then assign a degree of confidence or certainty in
the estimates of effect of a body of evidence (i.e. the quality of evidence) for each outcome. The
degree of certainty can be one of four categories: high, moderate, low or very low on the basis of 8
factors that either increase or decrease the initial quality assignment.
With regards to the initial assignment, randomization is considered the best method to protect
against bias and confounding and as such the initial quality rating for a body of evidence from
randomized control trials starts as high, but there are 5 factors that lower the quality, and for
observational studies 3 factors that can increase the quality. Therefore, an alternative way to
conceptualize the use of the factors that influence the rating of the evidence begins with
Recommendation/Decision
Evidence synthesis (SR, HTA)
P
I
C
O
Outcome
Outcome
Outcome
Outcome
F
o
r
m
u
l
a
t
e
q
u
e
s
t
i
o
n
R
a
t
e
i
m
p
o
r
t
a
n
c
e
Critical
Important
Critical
Not
i
m
p
o
r
t
a
n
t
Summary of findings &
estimate of effect for
each outcome
Grade overall
quality of evidence
across outcomes based on
lowest quality
of critical outcomes
Randomization raises
initial quality
RCTs: high
Observational: low
1. Risk of bias
2. Inconsistency
3. Indirectness
4. Imprecision
5. Publication bias
Grade down Grade up
1. Large effect
2. Dose response
3. Opposing bias &
Confounders
R
a
t
e
q
u
a
l
i
t
y
o
f
e
v
i
d
e
n
c
e
f
o
r
e
a
c
h
o
u
t
c
o
m
e
S
e
l
e
c
t
o
u
t
c
o
m
e
s
Very low
Low
Moderate
High
Grade recommendations
(Evidence to Decision)
• For or against (direction) ¯-
• Strong or conditional/weak (strength)
By considering balance of consequences
(evidence to recommendations):
q Quality of evidence
q Balance benefits/harms
q Values and preferences
q Feasibility, equity and acceptability
q Resource use (if applicable)
Formulate Recommendations/Decision
“The panel recommends that ….should...”
“The panel suggests that ….should...”
“The panel suggests to not ...”
“The panel recommends to not...”
Transparency, clear, actionable
Research?
O
u
t
c
o
m
e
s
a
c
r
o
s
s
s
t
u
d
i
e
s
Guideline/Decision
E
t
D
f
r
a
m
e
w
o
r
k
w
i
t
h
G
D
T
C
r
e
a
t
e
e
v
i
d
e
n
c
e
p
r
o
f
i
l
e
w
i
t
h
G
D
T

65
Saudi Arabian Handbook for Healthcare
Guideline Development
acknowledging that any confident in estimates starts as low and randomization is one of four factors
that lead to an increase in the confidence of effects.
Both the statistical information and the assessment of the quality of evidence are presented in
standardized evidence tables organized by outcome and ideally created using the GRADE profiler
Guideline Development Tool (GDT) software. Information can either be presented in typical detailed
‘evidence profiles’ or in the more succinct ‘Summary of Findings (SoF)’.
Once all of the outcomes that are critical for decision-making have been evaluated in terms of the
quality of the supporting evidence an overall grade of the quality of evidence is assigned. This overall
grade is equivalent to the rating for the lowest quality evidence base linked to a critical outcome.
This information is then provided to the panel for the purpose of formulating recommendations
based on the evidence profiles.
A guideline panel then moves to developing recommendations, the endproduct of the guideline
development exercise. Development of recommendations requires considering the following
factors: the quality of the evidence, the balance between benefits and harms, a consideration of
patient values and preferences, equity, feasibility, acceptability and resource implications. These
factors should be seen as broad domains with many items being considered within. Guideline panels
will determine and express the fundamental nature of the recommendation either for or against an
intervention or diagnostic test or strategy on the basis of evaluating the desirable and undesirable
consequences that result across these domains. Panels will then formulate recommendations in a
clear and unambiguous way categorising the strength of the recommendation as either a strong or a
conditional (weak) recommendation. They will give implementation and research due consideration
as part of this process.

66
Saudi Arabian Handbook for Healthcare
Guideline Development
Appendix 3: WHO Conflict of Interest Form
DECLARATION OF INTERESTS FOR WHO EXPERTS
WHO's work on global health issues requires the assistance of external experts who may have
interests related to their expertise. To ensure the highest integrity and public confidence in its
activities, WHO requires that experts serving in an advisory role disclose any circumstances that
could give rise to a potential conflict of interest related to the subject of the activity in which they
will be involved.
All experts serving in an advisory role must disclose any circumstances that could represent a
potential conflict of interest (i.e., any interest that may affect, or may reasonably be perceived to
affect, the expert's objectivity and independence). You must disclose on this Declaration of Interest
(DOI) form any financial, professional or other interest relevant to the subject of the work or
meeting in which you have been asked to participate in or contribute towards and any interest that
could be affected by the outcome of the meeting or work. You must also declare relevant interests
of your immediate family members (see definition below) and, if you are aware of it, relevant
interests of other parties with whom you have substantial common interests and which may be
perceived as unduly influencing your judgement (e.g. employer, close professional associates,
administrative unit or department).
Please complete this form and submit it to WHO Secretariat if possible at least 4 weeks but no later
than 2 weeks before the meeting or work. You must also promptly inform the Secretariat if there is
any change in this information prior to, or during the course of, the meeting or work. All experts
must complete this form before participation in a WHO activity can be confirmed.
Answering "Yes" to a question on this form does not automatically disqualify you or limit your
participation in a WHO activity. Your answers will be reviewed by the Secretariat to determine
whether you have a conflict of interest relevant to the subject at hand. One of the outcomes listed in
the next paragraph can occur depending on the circumstances (e.g, nature and magnitude of the
interest, timeframe and duration of the interest).
The Secretariat may conclude that no potential conflict exists or that the interest is irrelevant or
insignificant. If, however, a declared interest is determined to be potentially or clearly significant,
one or more of the following three measures for managing the conflict of interest may be applied.
The Secretariat (i) allows full participation, with public disclosure of your interest; (ii) mandates
partial exclusion (i.e., you will be excluded from that portion of the meeting or work related to the
declared interest and from the corresponding decision making process); or (iii) mandates total
exclusion (i.e., you will not be able to participate in any part of the meeting or work).
All potentially significant interests will be disclosed to the other participants at the start of the
activity and you will be asked if there have been any changes. A summary of all declarations and
actions taken to manage any declared interests will be published in resulting reports and work
products. Furthermore, if the objectivity of the work or meeting in which you are involved is
subsequently questioned, the contents of your DOI form may be made available by the Secretariat
to persons outside WHO if the Director-General considers such disclosure to be in the best interest
of the Organization, after consulting with you. Completing this DOI form means that you agree to
these conditions.
67
Saudi Arabian Handbook for Healthcare
Guideline Development
If you are unable or unwilling to disclose the details of an interest that may pose a real or perceived
conflict, you must disclose that a conflict of interest may exist and the Secretariat may decide that
you be totally recused from the meeting or work concerned, after consulting with you.
Name:
Institution:
Email:
Date and title of meeting or work, including description of subject matter to be considered (if a number of
substances or processes are to be evaluated, a list should be attached by the organizer of the activity):
________________________________________________________________________
________________________________________________________________________
Please answer each of the questions below. If the answer to any of the questions is "yes", briefly describe the
circumstances on the last page of the form.
The term "you" refers to yourself and your immediate family members (i.e., spouse (or partner with
whom you have a similar close personal relationship) and your children). "Commercial entity" includes any
commercial business, an industry association, research institution or other enterprise whose funding is
significantly derived from commercial sources with an interest related to the subject of the meeting or work.
"Organization" includes a governmental, international or non-profit organization. "Meeting" includes a series
or cycle of meetings.
EMPLOYMENT AND CONSULTING
Within the past 4 years, have you received remuneration from a commercial entity or other
organization with an interest related to the subject of the meeting or work?
1a
Employment
Yes | No |
1b
Consulting, including service as a technical or other advisor
Yes | No |
RESEARCH SUPPORT
Within the past 4 years, have you or has your research unit received support from a commercial
entity or other organization with an interest related to the subject of the meeting or work?
2a
Research support, including grants, collaborations, sponsorships, and other funding
Yes| No|
2b
Non-monetary support valued at more than US $1000 overall (include equipment, facilities,
research assistants, paid travel to meetings, etc.)
Support (including honoraria) for being on a speakers bureau, giving speeches or training for a
commercial entity or other organization with an interest related to the subject of the meeting or
work?
Yes |No|
INVESTMENT INTERESTS
Do you have current investments (valued at more than US $10 000 overall) in a commercial entity
with an interest related to the subject of the meeting or work? Please also include indirect
investments such as a trust or holding company. You may exclude mutual funds, pension funds
or similar investments that are broadly diversified and on which you exercise no control.
3a
Stocks, bonds, stock options, other securities (e.g., short sales)
Yes| No |
3b
Commercial business interests (e.g., proprietorships, partnerships, joint ventures, board
memberships, controlling interest in a company)
Yes| No |
INTELLECTUAL PROPERTY
Do you have any intellectual property rights that might be enhanced or diminished by the
outcome of the meeting or work?
4a
Patents, trademarks, or copyrights (including pending applications)
Yes | No|
4b
Proprietary know-how in a substance, technology or process
Yes |No |
PUBLIC STATEMENTS AND POSITIONS (during the past 3 years)
5a
As part of a regulatory, legislative or judicial process, have you provided an expert opinion or
Yes |No |
68
Saudi Arabian Handbook for Healthcare
Guideline Development
testimony, related to the subject of the meeting or work,
for a commercial entity or other organization?
5b
Have you held an office or other position, paid or unpaid, where you represented interests or
defended a position related to the subject of the meeting or work?
Yes | No|
ADDITIONAL INFORMATION
6a
If not already disclosed above, have you worked for the competitor of a product that is
the subject of the meeting or work, or will your participation in the meeting or work
enable you to obtain access to a competitor's confidential proprietary information, or
create for you a personal, professional, financial or business competitive advantage?
Yes |No |
6b
To your knowledge, would the outcome of the meeting or work benefit or adversely affect
interests of others with whom you have substantial common personal, professional, financial or
business interests (such as your adult children or siblings, close professional colleagues,
administrative unit or department)?
Yes| No|
6c
Excluding WHO, has any person or entity paid or contributed towards your travel costs in
connection with this WHO meeting or work?
Yes| No|
6d
Have your received any payments (other than for travel costs) or honoraria for speaking publicly
on the subject of this WHO meeting or work?
Yes| No|
6e
Is there any other aspect of your background or present circumstances not addressed above that
might be perceived as affecting your objectivity or independence?
Yes| No|
7.
TOBACCO OR TOBACCO PRODUCTS (answer without regard to relevance to the subject
of the meeting or work)
Within the past 4 years, have you had employment or received research support or
other funding from, or had any other professional relationship with, an entity directly
involved in the production, manufacture, distribution or sale of tobacco or tobacco
products or representing the interests of any such entity?
Yes| No|
EXPLANATION OF "YES" RESPONSES: If the answer to any of the above questions is "yes", check above and
briefly describe the circumstances on this page. If you do not describe the nature of an interest or if you do
not provide the amount or value involved where relevant, the conflict will be assumed to be significant.
Nos. 1 - 4:
Type of interest,
question number and
category (e.g.,
Intellectual Property
4.a copyrights) and
basic descriptive
details.
Name of company,
organization, or
institution
Belongs to you, a
family member,
employer, research
unit or other?
Amount of
income or
value of
interest (if not
disclosed, is
assumed to be
significant)
Current
interest (or
year ceased)

69
Saudi Arabian Handbook for Healthcare
Guideline Development
Nos. 5-6: Describe the subject, specific circumstances, parties involved, time frame and other relevant details
CONSENT TO DISCLOSURE. By completing and signing this form, you consent to the disclosure of any relevant
conflicts to other meeting participants and in the resulting report or work product.
DECLARATION. I hereby declare on my honour that the disclosed information is true and complete to the
best of my knowledge.
Should there be any change to the above information, I will promptly notify the responsible staff of WHO
and complete a new declaration of interest form that describes the changes. This includes any change that
occurs before or during the meeting or work itself and through the period up to the publication of the final
results or completion of the activity concerned.
Date: ________________ Signature________________________________
70
Saudi Arabian Handbook for Healthcare
Guideline Development
Appendix 4.1: Example of an evidence profile
Evidence profile: Sublingual immunotherapy vs usual care in adults with seasonal/intermittent AR
Author(s): Itziar Etxeandia Date: 2013-11-16
Quality assessment
No of patients
Effect
Quality
Importance
No of
studies
Design
Risk of bias
Inconsistency
Indirectness
Imprecision
Other
considerations
SLIT
Control
Relative
(95% CI)
Absolute
Allergic rhinitis symptom scores (SS) (follow-up median 7 months
1
) (Better indicated by lower values)
33
randomised
trials
No serious
2
Serious
3
no serious
indirectness
no serious
imprecision
none
1768
1708
-
SMD 0.38 lower
(0.49 to 0.27 lower)
4
MODERATE
CRITICAL
Ocular symptoms (follow-up median 7 months
5
; Better indicated by lower values)
8
randomised
trials
serious
6
no serious
inconsistency
7
no serious
indirectness
serious
none
597
616
-
SMD 0.26 lower
(0.06 to 0.46 lower)
LOW
IMPORTANT
Medication scores (MS) (follow-up median 7 months
1
) (Better indicated by lower values)
27
randomised
trials
No serious
2
Serious
3
no serious
indirectness
no serious
imprecision
none
1353
1438
-
SMD 0.35 lower
(0.47 to 0.23 lower)
9
MODERATE
IMPORTANT
Combined SS and MS (SMS) (follow-up median 7 months
10
) (Better indicated by lower values)
5
randomised
trials
No serious
Serious
11
no serious
indirectness
no serious
imprecision
none
541
546
-
SMD 0.44 lower
(0.62 to 0.27 lower)
12
MODERATE
IMPORTANT
QoL (disease specific RQLQ) (follow-up median 7 months
10
) (Better indicated by lower values)
6
randomised
trials
No serious
Serious
13
no serious
indirectness
no serious
imprecision
none
818
840
-
SMD 0.36 lower
(0.46 to 0.26 lower)
14
MODERATE
CRITICAL
Serious adverse effects (follow-up median 7 months
1
)
36
randomised
trials
no serious
limitations
no serious
inconsistency
no serious
indirectness
no serious
imprecision
none
0/2253 (0%)
0/1906 (0%)
not pooled
15
not pooled
HIGH
IMPORTANT
Withdrawal due to adverse effect (follow-up median 7 months
1
)
25
randomised
trials
no serious
limitations
no serious
inconsistency
no serious
indirectness
serious
16
none
70/1691 (4.1%)
16/1430 (1.1%)
RR 2.91 (1.72
to 4.92)
21 more per 1000
(from 8 more to 44 more)
MODERATE
CRITICAL
Oral pruritus or burning (follow-up median 7 months
1
)
17
19
randomised
trials
no serious
limitations
no serious
inconsistency
no serious
indirectness
no serious
imprecision
strong association
18
481/1304 (36.9%)
73/1152 (6.3%)
RR 4.92 (3.16
to 7.67)
248 more per 1000x
(from 137 more to 423
more)
HIGH
CRITICAL
Oral oedema (follow-up median 8 months
1,19
)
7
randomised
trials
no serious
limitations
no serious
inconsistency
no serious
indirectness
Serious
20
very strong
association
21
113/763 (14.8%)
4/702 (0.6%)
RR 11.47
(4.66 to
28.24)
60 more per 1000 (from
21 more to 155 more)
HIGH
CRITICAL
Gastrointestinal adverse effects (follow-up median 7 months
1
; nausea, vomiting, stomach upset, diarrhoea)
9
randomised
trials
no serious
limitations
no serious
inconsistency
no serious
indirectness
serious
22
none
40/482 (8.3%)
10/413 (2.4%)
RR 2.85 (1.44
to 5.65)
45 more per 1000 (from
11 more to 113 more)
MODERATE
CRITICAL

71
Saudi Arabian Handbook for Healthcare
Guideline Development
1
The duration of maintenance treatment and the period of follow up varied considerably between studies, largely reflecting pre-seasonal, co-seasonal and perennial administration. Range of
follow-up was 1 to 48 months
2
Most studies were at low or unclear risk of bias, mostly because they did not report the sequence generation and in some cases allocation concealment. Majority of studies did not report
following intention-to-treat principle and was analysed per-protocol.
3
There was some inconsistency in the results with I2= -48%49%.
4
Moderate effect sizes favouring active SLIT in the adults subgroup analysis, and these did not differ significantly in the subgroups analysis of the 42 studies with age (children and adults
together (SMD: -0.33 (95%IC -0.42 to-0.25)) , study duration (42 studies) ( <6 months, 6-12 months,>12monts), major allergen content (31 studies) (5µg, 5-20 µg, >20 µg) or type of allergen
(42 studies) (Grass, Ragweed, Parietaria, tree).
5
Range: 3.5 to 18 months.
6
In all studies but one between 10% and 20% of patients withdrew from the study. Majority of studies did not report following intention-to-treat principle and was analysed per-protocol.
7
There was some inconsistency in results, but removing the studies with extreme results did not substantially change the estimate of effect.
9
Combined SMD of the 35 studies which included Children and adults was –0.27 (95% CI –0.37 to –0.17) but MSs in children were not significantly better than with placebo treatment (see
GRADE profile in the next question).On the other hand small to moderate effect sizes favouring active SLIT were found in all subgroup analyses of the 35 studies, study duration ( <6 months,
6-12 months,>12monts), MAC (5µg, 5-20 µg, >20 µg) and type of allergen (Grass, Ragweed, Parietaria, tree).
10
Range of follow-up was 3 to 10 months
11
Some heterogeneity between Studies I2: 41%.
12
When all 6 studies of Children and adults are taking together the combined SMD was similar (–0.40 (95% CI –0.55 to –0.25)), furthermore moderate effect sizes favouring active SLIT were
found in all subgroup analyses of those 6 studies conducted in children and adults [study duration (6 studies) ( <6 months, 6-12 months,>12monts), MAC (3 studies) (5-20 µg) or type of
allergen (4 studies) (Grass)], and these were similar between studies.
13
Some heterogeneity between Studies I2: 69%. Four of the included studies used the full version of the disease-specific RQLQ to measure QoL, the others an alternative version.
Nevertheless the subgroup analysis of those four studies showed a similar combined SMD – 0.34 (95%IC -0.49 to -0.18).
14
When all 7 studies of Children and adults are taking together the combined SMD was similar -0.37 (95%IC -0.52 to -0.22), moderate effect sizes favouring active SLIT were found in all
subgroup analyses of those 7 studies conducted in children and adults [study duration (6 studies) ( <6 months, >12monts) or MAC (4 studies) (5-20 µg, >20 µg).
15 There were no serious adverse observed in any of the 36 studies and five new trials added in the Meadows et al. meta-analysis reported a total of 20 SAEs in a total of 1565 study
participants, of which only one, abdominal pain in a placebo-treated patient, was considered likely to be treatment related.
16
Only 86 events
17
In the new RCT added in the Meadows et al. meta-analysis the numbers of adverse events were generally not reported. The most commonly reported local reactions were itching, swelling
and burning in the oral cavity. Four trials (n = 890), one in children (n= 307) and three in adults (n=583) reported oral pruritus (39% in active group vs. 5% placebo); two trials (n = 782)
reported throat irritation ( 33% active vs. 4% of control), and mild erythema (11% active vs. 1% control ); and three trials (n = 863) reported oral paraesthesia (10% in SLIT vs. 2% in placebo)
and mouth oedema (9% in SLIT vs. 1% in placebo).
18
Lower confidence limit was 3.16.
19
Range: 4 to 24 months.
20
Only 117 events.
21
Lower confidence limit was 4.66 21
22
Only 50 events.
72
Saudi Arabian Handbook for Healthcare
Guideline Development
Appendix 4.2: Example of evidence to decision framework
Question 3: Should sublingual specific immunotherapy be used for treatment of allergic rhinitis in adults without concomitant asthma?
Problem: Adults with Allergic Rhinitis
Option: sublingual specific immunotherapy
Comparison: No treatment
Setting: Outpatient
Perspective: Health Care system
Background: Background: Allergic rhinitis (AR) is defined clinically by nasal hypersensitivity symptoms induced by
an immunologically mediated (most often IgE-dependent) inflammation after the exposure of the nasal mucous
membranes to an offending allergen. Symptoms of rhinitis include rhinorrhea, nasal obstruction or blockage, nasal
itching, sneezing, and postnasal drip that are reversible spontaneously or under treatment. Allergic conjunctivitis
often accompanies allergic rhinitis.
Allergic rhinitis has been traditionally subdivided into seasonal, perennial, and occupational rhinitis. Perennial
allergic rhinitis is most frequently, although not necessarily, caused by indoor allergens such as house dust mites,
moulds, cockroaches, and animal dander. Seasonal allergic rhinitis is most often caused by outdoor allergens such
as pollens or moulds. As in a 2010 edition of ARIA guideline in this document we retained the terms “seasonal” and
“perennial” to enable the interpretation of published studies, and we also include the terms used to classify AR
according to the duration of symptoms as “intermittent” rhinitis (symptoms are present less than 4 days a week or
for less than 4 weeks) or “persistent” (symptoms are present at least 4 days a week and for at least 4 weeks).
These guidelines do not address the issues related to diagnosis of allergic rhinitis and it is assumed that the correct
diagnosis had been established before commencing treatment.
CRITERIA
JUDGEMENTS
RESEARCH EVIDENCE
ADDITIONAL CONSIDERATIONS
PR O BL E M
Is the
problem a
priority?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
1. Overall risk of AR in adults Saudi Arabia is 90 per 1000 (79% SAR)
Overall in the Middle East:
Runny nose, nasal and throat itching, postnasal drip, and nasal congestion or
stuffed up nose were the most common and bothersome symptoms of AR.
58% of participants with AR reported that the condition had an impact on
their daily private and professional life.
72% reported that limitations on their work/school activities
35% reported that interfered with and caused them to miss work or
Sleep disturbances were shown in this survey to be extremely troubling in
15% of AR patients.
(Abdulrahman H, 2012. Survey conducted in Middle East including KSA)
2. A high percentage of patients with AR surveyed missed work or had their
The guideline panel estimates a
prevalence of 20% to 40% of AR in
KSA. They consider that due to the lack
of an appropiate data base with this
data, the self- reporting studies could
underestimate the prevalence (for not
recognize the symptoms or not having
a medical diagnosis) or overestimate
(for considering any kind of rhinitis not
only the allergic one).
73
Saudi Arabian Handbook for Healthcare
Guideline Development
CRITERIA
JUDGEMENTS
RESEARCH EVIDENCE
ADDITIONAL CONSIDERATIONS
work performance affected by allergies: work productivity decreasing by 23%
in AIA, 24% in AIAP, 33% in AILA and 30% in Middle East when allergy
symptoms were at their worst.
Nasal allergies also interfered with many patients' sleep, and were associated
with feelings of depression, anxiety, irritability and tiredness.
(Blaiss 2012, America, Asia pacific, Latin America, and Middle East)
Seasonal / Intermittent Allergic Rhinitis
CRITERIA
JUDGEMENTS
RESEARCH EVIDENCE
ADDITIONAL
CONSIDERATIONS
B E NEF I TS & H A R M S OF T HE O PTI ONS
What is the
overall
certainty of
this
evidence?
No
included
studies
Very low
Low
Moderate
High
X
Outcome
Relative importance
Certainty of the evidence
(SAR)
Nasal symptoms
Critical
Moderate
Ocular symptoms
Important
Low
Medication score
Important
Moderate
Symptom-medication
score
Important
Moderate
Quality of life
Critical
Moderate
Serious adverse effects
Important
High
Withdrawal due to
adverse effect
Critical
High
Oral pruritus or burning
Critical
High
Oral oedema
Critical
High
Gastrointestinal adverse
effects
Critical
Moderate
Summary of the evidence for patients’ values and preferences:
- There is a concern that some
patients in KSA would not
accept SLIT with some allergens
of animal origin.
- Also considered that most
people initially do not accept
SLIT but when the symptoms do
not decrease with all other
regular options, they accept this
medication with its adverse
effects.
- It is considered that the lack of
adherence with the medication
use is not related with its
adverse effects but with the
long duration of treatment.
Is there
important
uncertainty
about how
much
people
value the
main
outcomes?
Important
uncertainty
or
variability
Possibly
important
uncertainty
or
variability
Probably
no
important
uncertainty
or
variability
No
important
uncertainty
or
variability
No known
undesirable
outcomes
X
Are the
desirable
anticipated
effects
large?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
74
Saudi Arabian Handbook for Healthcare
Guideline Development
CRITERIA
JUDGEMENTS
RESEARCH EVIDENCE
ADDITIONAL
CONSIDERATIONS
Are the
undesirable
anticipated
effects
small?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
This recommendation places a relatively high value on alleviating the
symptoms of rhinitis, and relatively low value on avoiding adverse effects and
resource expenditure.
Local adverse effects are relatively frequent (~35%). An alternative choice may
be equally reasonable, if patients’ values or preferences differ from those
described here.
Summary of findings: see evidence table and reference list
Are the
desirable
effects
large
relative to
undesirable
effects?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
CRITERIA
JUDGEMENTS
RESEARCH EVIDENCE
ADDITIONAL CONSIDERATIONS
RE S OUR CE U SE
Are the
resources
required
small?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
1. SLIT was compared with standard therapy, It was
(just) more effective or, in some cases, both more
effective and cost-effective
- SLIT is likely to be cost-effective at thresholds of
£20,000;
(Meadows A, 2013. SR)
- These studies did not, however, report all of the
utility data in a disaggregated form and all were
funded by a manufacturer of SIT products (Meadows
A, 2013. SR)
- Average annual cost per patient: around 35 K SAR
- Average cost per treatment (3 years) and patient:
around 100K SAR
Average maintenance vial/ allergen/ month =707 SAR.
Average 4 allergens/patient:
Annual cost= 707 X 4 X 12 = 33, 936 SAR
Is the
incremental
cost small
relative to
the net
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
75
Saudi Arabian Handbook for Healthcare
Guideline Development
CRITERIA
JUDGEMENTS
RESEARCH EVIDENCE
ADDITIONAL CONSIDERATIONS
benefits?
EQU I TY
What would
be the
impact
on health
inequities?
Increased
Probably
increased
Uncertain
Probably
reduced
Reduced
Varies
X
Comments from the panel members:
1. If sublingual immunotherapy use were to be
recommended, the health inequity will increase so the
indications and the applications of SLIT should be
determined: The SLIT should be used only when all other
regular options do not work
2. Impact: Few patients will be affected
AC C EP T A B I LI TY
Is the option
acceptable
to key
stakeholders
?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
Uncertain acceptance from patients and likely not for
health care system because of cost consideration reasons
FE A SI B I L I TY
Is the option
feasible to
implement?
No
Probably
No
Uncertain
Probably
Yes
Yes
Varies
X
Implementation would require expertise and resources
(i.e. skin tests, relevant allergen) not readily available in
most areas.

76
Saudi Arabian Handbook for Healthcare
Guideline Development
Balance of
consequences
Undesirable
consequences
clearly
outweigh
desirable
consequences
in most
settings
Undesirable
consequences probably
outweigh
desirable consequences
in most settings
The balance between
desirable and
undesirable
consequences
is closely balanced or
uncertain
Desirable
consequences
probably
outweigh
undesirable
consequences
in most
settings
Desirable
consequences
clearly
outweigh
undesirable
consequences
in most
settings
X
Type of
recommendation
We recommend
against
offering this option
We suggest not
offering
this option
We suggest offering
this option
We recommend
offering
this option
X
Recommendation
(text)
The KSA MoH panel suggests sublingual immunotherapy for treatment of adults
with seasonal or intermittent allergic rhinitis (conditional recommendation;
Moderate-quality evidence).
Justification
The evidence, with an overall moderate certainty, shows that the desirable effects
probably are not large relative to undesirable effects. Furthermore, possibly there is an
important variability about how much people value its effectiveness because there is a
concern that some patients in KSA would not accept SLIT with some allergens of animal
origin, however others would accept it as the last option when the symptoms do not
decrease with all other regular options. On the other hand the incremental cost is not
small relative to the net benefits, and the implementation would require personnel
experts and resources (i.e. skin tests, specific allergen) which are not readily available in
most areas. Reasons to formulate a conditional rather than a strong recommendation.
It is considered that the lack of adherence with the medication use is not related with its
adverse effects but with the long duration of treatment. For this reason in the cases
when the SLIT would be the treatment of choice clinicians should provide an adequate
educational instruction to the patient.
Subgroup
considerations
The SLIT should be used only when all other regular options do not work: It is more
appropriate for those with moderate to severe AR who does not respond to first line
therapy.
The SLIT Should not be started during pregnancy, but could be continued if the woman has
already started the treatment.
Implementation
considerations
SLIT should only be prescribed by allergy specialists who have expertise in diagnosis of AR,
proper identification of the allergens, providing immunotherapy and treatment of
potentially serious adverse effects.
Monitoring and
evaluation
If patients receiving SLIT do not respond within 6-12 m consider discontinuation SLIT
