
SELECTION GUIDE:
ENVIRONMENTAL CORROSION
PROTECTION
Condenser Coils and Cooling/Heating Coils
for Commercial Products
Carrier Corporation
Syracuse, New York
December 2012
2
TABLE OF CONTENTS
INTRODUCTION .............................................................. 2
CORROSION ................................................................. 2-4
Localized Corrosion .................................................... 2
General Corrosion........................................................ 4
CORROSIVE ENVIRONMENTS ................................. 4-6
Coastal/Marine ............................................................ 4
Industrial ...................................................................... 5
Combination Coastal/Marine and Industrial ................ 5
Urban ........................................................................... 5
Rural ............................................................................ 5
Localized Environment—Corrosivity of
the Surroundings ..................................................... 6
CORROSION PROTECTION ..................................... 7-11
Condenser Coils ........................................................... 7
Cooling/Heating Coils ................................................. 8
Carrier’s E-Coating Process ........................................ 9
E-Coated Material and Chemical Resistance ............... 9
Field-Applied Coatings .............................................. 11
SELECTION SUMMARY ........................................... 11-13
Selection Tables ......................................................... 11
Selection Example ..................................................... 11
JOB SITE COMMISSIONING AND PROPER
EQUIPMENT STORAGE ................................................. 14
COIL MAINTENANCE AND CLEANING
RECOMMENDATIONS ................................................... 14
APPENDIX ........................................................................ 15
E-Coating Chemical Resistance Guide ...................... 15
_________________________________________________________________________________________________________
INTRODUCTION
Corrosion is costly. By definition, corrosion is the
destruction or deterioration of a metal or alloy due to a
reaction with an environment.
In HVAC/R equipment, heat exchangers, including
condensers, evaporators, and hydronic coils, must be
protected from environments that may lead to
localized and/or generalized corrosion. Corrosion of
heat exchangers may lead to performance loss,
unsightly appearance, and possible equipment failure.
Fortunately, the harmful effects of coil corrosion can
be significantly delayed or avoided if the application
environment is correctly identified and the appropriate
corrosion protection option is selected.
This selection guide will provide information on
the causes of corrosion and identify corrosive
environments in order to aid in the selection of the
proper coil.
CORROSION
There are many types of corrosion. The two forms of
corrosion most common to HVAC/R equipment are
known as localized (galvanic, pitting, or formicary
corrosion) and general corrosion. Each of these
corrosion types can lead to equipment failure,
depending on conditions and the material systems
used.
Localized Corrosion
ROUND TUBE PLATE FIN COILS
One form of localized corrosion is galvanic corrosion.
The necessary conditions for galvanic corrosion occur
when dissimilar metals, in contact, are exposed to an
electrolyte, a substance that is electrically conductive
when dissolved in water. The environment creates the
electrolytes necessary for general and localized
corrosion of materials.
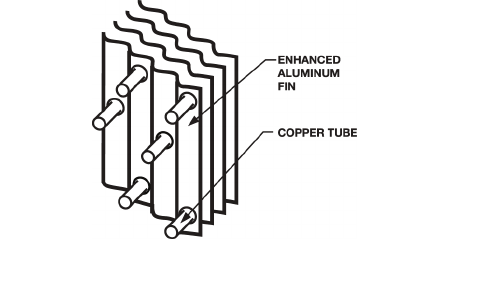
Standard round tube plate fin (RTPF) condenser coils
have copper tubes mechanically bonded to aluminum
fins with wavy enhancements. Figure 1 shows a cross-
section of a copper tube and several aluminum fins.
High thermal efficiency is achieved through direct
metallic contact between the tube and fin. As a result,
maximum thermal performance is achieved with this
high-efficiency coil design, provided there is no
corrosion.
Fig. 1. Standard Coil Construction
3
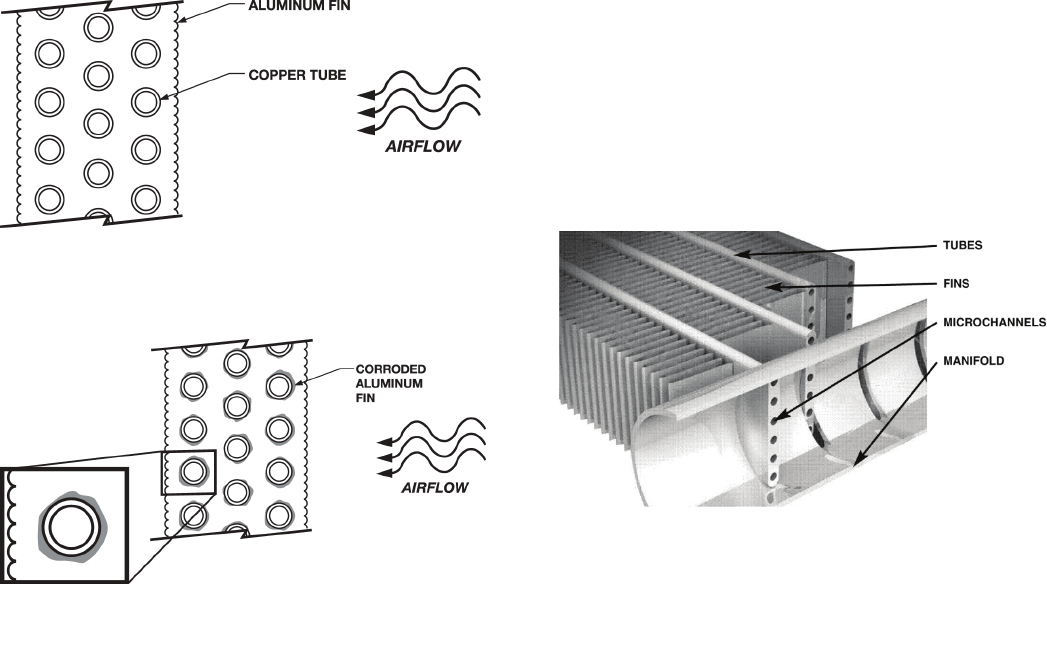
Figure 2 shows a typical RTPF coil prior to galvanic
corrosion.
During galvanic corrosion, the aluminum fin initially
corrodes at the copper/aluminum interface as this is
the point of electrical contact between the dissimilar
metals. As corrosion of the aluminum fin progresses,
the fin conductivity deteriorates which in turn reduces
the coil thermal performance. Aluminum oxide
deposits that are formed in the process (Fig. 3) can
further reduce performance by impeding air flow
through the coil.
One way of preventing galvanic corrosion of RTPF
coils is through the effective elimination of the bi-
metallic couple. An example of this approach is the
all-copper RTPF coil. The use of an all-copper
construction, i.e., copper tube/copper fin, virtually
eliminates the presence of dissimilar metals, one of the
necessary requirements for galvanic corrosion.
Another method commonly used to prevent galvanic
corrosion is to isolate the two dissimilar metals from
the electrolyte through use of a protective coating. The
protective coating in effect creates a barrier between
the dissimilar metallic couple and the electrolyte,
thereby eliminating the electrolyte from this interface.
A third way to prevent galvanic corrosion is to insulate
the electrical connection of the copper and the
aluminum through the use of a pre-coated aluminum
fin. The pre-coating insulation removes the electrical
contact of the dissimilar metals.
NOVATION
®
HEAT EXCHANGERS WITH
MICROCHANNEL COIL TECHNOLOGY
Novation
®
heat exchangers with microchannel coil
technology utilize several aluminum alloys in
combination with a metallic coating. The alloys are
carefully chosen to extend the life of the coil.
Furthermore, the coil has been designed so that any
galvanic couple within the coil has been carefully
chosen to provide the maximum life possible for the
coil.
The refrigerant carrying tube is essentially flat, with its
interior sectioned into a series of multiple, parallel
flow microchannels that contain the refrigerant (Fig 4).
In between the flat tube microchannels are fins that
have been optimized to increase heat transfer.
The microchannel tubes in the heat exchanger have
excellent heat transfer characteristics on the refrigerant
side. On the air side, heat transfer is improved due to
the enhanced surface area contact and the
metallurgical bond between tube and fin. Fin design is
optimized to enhance the fin heat transfer
performance. The fin-to-tube bond reduces thermal
resistance between tube and fin, resulting in better heat
conduction.
The microchannel heat exchanger (MCHX) coil design
uses zinc-enriched surfaces that perform in a manner
similar to the zinc layer in galvanized steel. The zinc-
enriched surface allows the tube to weather laterally,
preventing corrosion pits from progressing deeply into
the tube. The zinc layer will not be consumed during
the effective service life of the MCHX coil when the
coil is applied properly.
Fig. 2. Standard Copper Tube/Aluminum Fin Coil
Fig. 3. Galvanic Corrosion Begins
Fig. 4. Microchannel/Fin Center

4
In corrosive environments an unprotected coil may
face a rapid direct pitting attack of the tube and/or
tube-to-manifold joint, which may lead to a
catastrophic refrigerant leak and system failure. These
conditions can be found in aggressive marine,
industrial, urban, or highly alkaline environments.
The latter condition can occur, for example, at new
construction sites if the unprotected MCHX coil is
exposed to excessive quantities of concrete dust and
moisture.
A protective coating may be applied to MCHX coils
for use in corrosive application environments.
General Corrosion
General corrosion is the degradation of metal caused
by a reaction with the surrounding environment. Since
general corrosion consumes metal and typically forms
metal oxides, unsightly surface conditions usually
result. Unprotected metal will continue to react with
the contaminant resulting in corrosion. Under severe,
prolonged conditions, the metal continues to corrode
until the integrity of the material and equipment is
jeopardized. Unprotected copper or aluminum tubes in
polluted industrial environments can lead to tube leaks
and failure of the refrigeration system. Sulfur and
nitrogen based electrolytes in combination with
chloride environments are often the cause of
accelerated corrosion of these metals.
The environment in which HVAC/R equipment is
applied varies throughout the globe and in some
instances, even within a local area. Corrosive
environments occur not only in coastal or marine
climates and industrial areas, but also are present in
urban or rural areas, localized microclimates, and
combinations of these conditions. Factors including
but not limited to the presence of flue gas, sewage
vents or open sewage systems and diesel exhaust can
all have a detrimental effect on HVAC/R coils.
These pollutants, in combination with other factors
such as wind direction, humidity, water, fog,
temperature, proximity to pollutant source, and dust or
particle contamination, may result in the premature
failure of equipment.
For both RTPF and MCHX coils it is therefore critical
that the application environment is properly identified,
and if needed, the appropriate corrosion protection is
used.
CORROSIVE ENVIRONMENTS
As previously discussed, potentially corrosive outdoor
environments include areas adjacent to the seacoast,
industrial sites, heavily populated urban areas, some
rural locations, or combinations of any of these
environments. These macro environments are often
characterized as rural, urban, coastal (marine),
industrial, or industrial marine. In addition, some air-
handling applications, indoor environments such as
swimming pool areas, water treatment facilities, and
industrial process areas can also produce corrosive
atmospheres.
Local environments called micro environments must
also be considered. Close proximity to laundry
facilities, diesel-burning devices/exhaust piping, sewer
vents, and traffic can lead to premature failure of
improperly protected equipment, in a similar manner
as the macro environmental conditions.
Contaminants in an environment typically result in the
creation of electrolytes that facilitate the corrosion
process. Electrolytes are substances that are
electrically conductive when dissolved in water.
Common electrolytes may contain chloride
contaminants from sources such as seawater, road
salts, cement dust, pool cleaners, laundry facilities, and
household cleaning agents, which are typically sodium
or calcium chloride-based compounds. Other relevant
contaminants that contribute to the formation of
electrolytes include sulfur and nitrogen bearing
compounds from the combustion of coal and fuel oils.
Chemical contamination from industrial processes,
e.g., ammonia, can also contribute to the formation of
an electrolyte.
In view of this it is necessary to identify each of these
environments so that appropriate corrosion protection
methods may be used.
Coastal/Marine
Many emerging HVAC/R
markets have a majority of
their populations located
in coastal regions, leading
to an increased number of
applications in corrosive
environments. Coastal or
marine environments are
characterized by the
abundance of sodium
chloride (salt) and sulfur
compounds that are
carried by sea spray, mist,
fog, or prevailing winds. Sea spray, mist, and fog
contain tiny droplets of salt water that can be
transported many miles by ocean breezes and result in
equipment contamination. The deposition of salt
water spray onto metallic substances is the most
corrosive aspect of the marine environment.

5
Several factors should be considered when choosing
the best solution for a coastal or marine environment:
land formation (e.g., islands, depending on size, often
are influenced by coastal contaminations); distance
from the coast and the direction of the prevailing
winds (wind direction helps to determine the distance
that contaminants can be carried); corrosion on other
equipment or infrastructure in the area (an excellent
indicator of the corrosiveness of the environment);
common practices that have worked well in the area;
and other pollution sources, such as industrial
influences.
Industrial
Industrial environments
are very diverse, with the
potential to produce a
variety of corrosive
compounds. An industrial
environment can exist on a
macro or micro scale, each
with the same detrimental
effect. Sulfur and nitrogen
containing contaminants
are most often linked but
not limited to industrial
and high-density urban
environments. Combustion of coal and fuel oils release
sulfur oxides (SO
2
, SO
3
) and nitrogen oxides (NO
x
)
into the atmosphere. Other contributors such as
ammonia and its salts and hydrogen sulfide can have a
detrimental effect on materials. Many of these gases
accumulate in the atmosphere and return to the ground
in the form of acid rain or low pH (acidic) dew.
Not only are industrial emissions potentially corrosive,
but many industrial dust particles can be laden with
harmful metal oxides, chlorides, sulfates, sulfuric acid,
carbon and carbon compounds. These particles, in the
presence of oxygen, water, or high humidity can be
highly corrosive and may lead to many forms of
corrosion including general corrosion and localized
corrosion such as pitting and formicary corrosion.
Combination
Coastal/Marine and
Industrial
Salt-laden seawater mist,
combined with the harmful
emissions of an industrial
environment (either on a
macro or micro level), poses
a severe threat to the life of
HVAC/R equipment. The
combined effects of salt
contamination and industrial
emissions will accelerate
corrosion of any improperly protected coil. This harsh
environment requires superior corrosion resistant
properties for HVAC/R components to maintain
acceptable product quality. Complete encapsulation of
the coil surfaces (such as e-coating for MCHX coils) is
strongly recommended. When identifying this type of
environment it is essential that local influences not be
overlooked. Open sewage systems, vents, diesel
exhaust, emissions from dense traffic, landfills, aircraft
and ocean vessel exhaust, industrial manufacturing,
chemical treatment facilities (cooling tower
proximity), and fossil fuel burning power plants are
potential contributors to consider.
Urban
Highly populated areas
generally have high levels of
automobile emissions and
high rates of the byproducts
of the combustion of
building heating fuels. Both
conditions elevate sulfur
oxide (SO
x
) and nitrogen
oxide (NO
x
) concentrations.
Corrosion severity in this
environment is a function of
pollution levels, humidity,
average temperature, and equipment usage, which in
turn depend on several factors including population
density for the area, emission control, and local
pollution standards. In areas with rapid growth, such
as many areas in China and India, contamination levels
can change drastically; thus, the future direction of a
region should be considered when looking at the best
corrosion protection system.
Note that any HVAC/R equipment installed near diesel
exhaust, incinerator discharge stacks, fuel-burning
boiler stacks, areas exposed to fossil fuel combustion
emissions, or areas with high automobile emissions
should be considered industrial applications.
Rural
A rural environment typically is unpolluted by exhaust
and sulfur containing gases. Rural environments are
usually sufficiently inland so that contamination and
high humidity from coastal waters are not present. Coil
protection in these environments is typically not
required beyond the standard MCHX coil or aluminum
fin/copper tube offerings.
However, rural environments may contain high levels
of ammonia and nitrogen contamination from animal
excrement, fertilizers, and high concentrations of
diesel exhaust. In this case, these environments should
be considered industrial applications and would
require e-coated coil protection.
6
Localized Environment - Corrosivity of the
Surroundings
All of the above environments are subject to
microclimates that can significantly increase the
corrosivity of the environment. Care should be taken
to ensure that the localized environment surrounding
the HVAC/R equipment does not contain
contaminants that will be detrimental to the
equipment. An example would be equipment placed
near a diesel vehicle loading area or a diesel generator.
Although the general area in which the building is
located may meet the scope of a coastal or marine
environment, the localized elements that surround the
equipment may actually classify the application as
industrial or industrial marine, and protection of the
coil should be planned accordingly.
Localized environments can result from a variety of
contaminants, including but not limited to those
originating with:
Traffic
Airports
Power plants
Power generators
Factories and chemical plants
Breweries and food processing plants
Wastewater treatment plants
Dumps and incineration plants
Cruise ships and shipping traffic
Swampy areas (rotting vegetation)
Farms and nurseries
Fisheries
The contaminants in the preceding list must be
considered in combination with other contributing
factors, including but not limited to:
Distance from contaminant source. The most
detrimental effect in a micro environment occurs
within 50 ft (15 meters); in a macro environment,
within 1 mile (1.6 km)
Prevailing wind direction
Acid rain (note that sources may be hundreds of
miles away)
Condensation
Temperature
Humidity
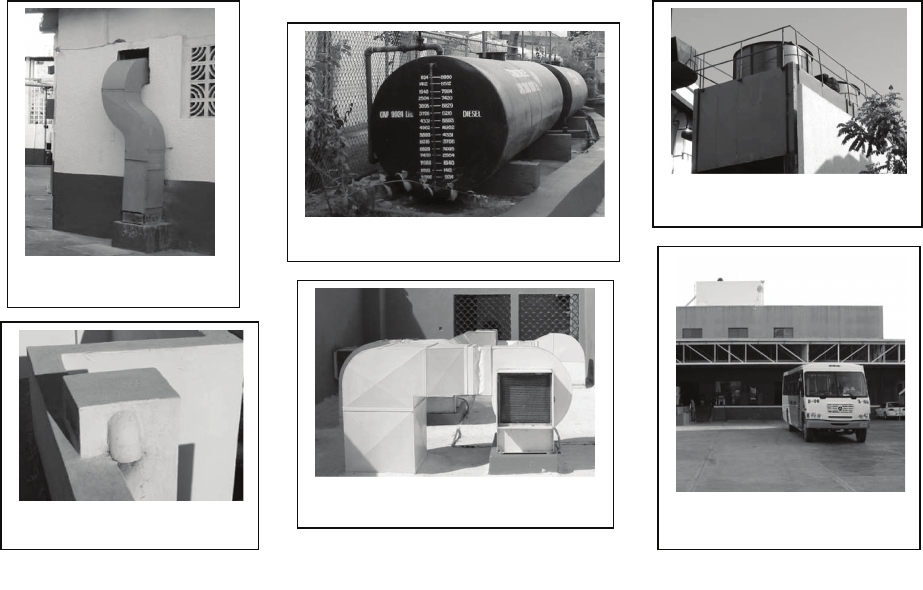
The following are examples of contaminants that can
create a micro environment within 50 ft (15 meters).
(See Fig. 5.):
Heavy/frequent fertilizer or insecticide usage
Chemical/cleaner storage areas
Bus or truck loading areas or heavy traffic
Power generators
Fan-powered exhaust vents
Cooling towers due to drift of chemical treatments
Fig. 5. Sources of Contaminants in Micro Environments
Diesel Bus Loading Area
Diesel Tank/ Loadin
g
Area
Cooling Tower
Plumbin
g
Vent Stack
Laundr
y
Vent
Exhaust Vent
7
CORROSION PROTECTION
The choices available for Carrier’s commercial
products offer protection for most common aggressive
environments. Note that not all options are available
for all products. For information on coil options for
specific products, consult your Carrier sales office.
Condenser Coils
MCHX COILS
MCHX coils are constructed utilizing all-aluminum
alloys with brazed fin construction. Microchannel
heat exchangers provide high thermal performance per
unit volume. Unprotected MCHX coils should never
be applied in corrosive environments.
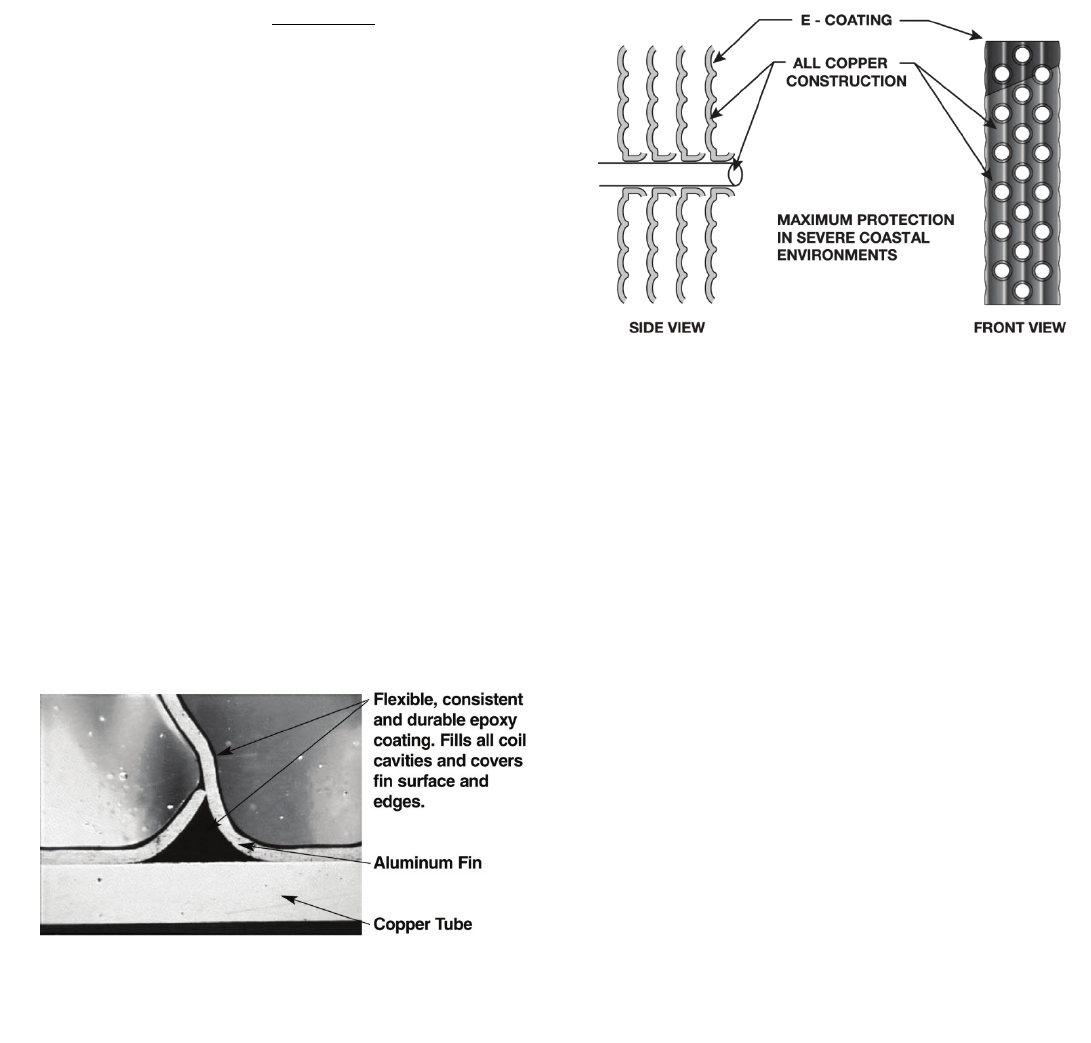
E-Coated MCHX Coils
E-coated coils provide superior protection against
many corrosive atmospheres. E-coated MCHX coils
have an extremely durable and flexible epoxy coating
uniformly applied over all coil surfaces for complete
isolation from the contaminated environment. A
consistent coating is achieved through a precisely
controlled electrocoating process that bonds a thin,
impermeable epoxy coating to the specially prepared
coil surfaces. A detailed description of the proprietary
Carrier e-coating process is provided on page 9.
RTPF COILS
The standard aluminum fin/copper tube coil generally
provides high performance for non-corrosive
environments (e.g., non-polluted rural environments).
Application of this coil in any environment containing
corrosive elements is not recommended because of the
likelihood of deterioration resulting from corrosion.
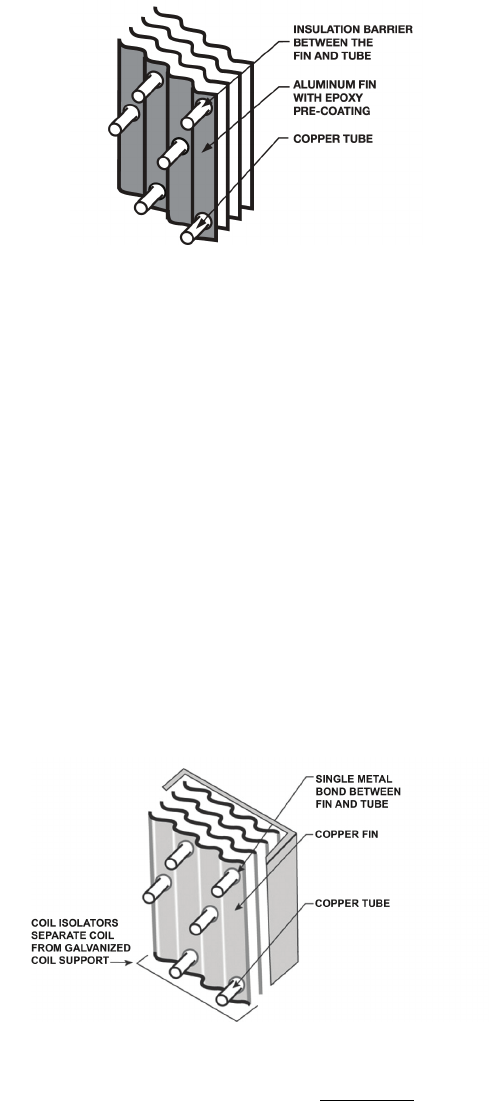
Pre-Coated Aluminum-Fin Coils
Pre-coated aluminum fin/copper tube coils have a
durable coating applied to the fin. This design offers
protection in mildly corrosive coastal environments,
but is not recommended in severe industrial or coastal
environments.
Aluminum fin stock is coated with a baked-on coating
prior to the fin stamping process (Fig. 6). Coating of
the fin material prior to the fin stamping process is
known as “pre-coating.” The pre-coated fin material is
then stamped to form the desired fin pattern for
optimum thermal performance.
A thin layer of a non-metallic pre-coating material
insulates the dissimilar metals of the coil (copper tube
and aluminum fin) from one another. As a result, the
electrical connection between the copper and
aluminum is disrupted, thus minimizing galvanic
corrosion. In mild coastal environments pre-coated
coils are an economical alternative to e-coated coils
and offer improved corrosion protection beyond the
standard uncoated copper tube/aluminum fin coil.
Copper-Fin Coils
Typically, a copper wavy fin pattern is mechanically
bonded to the standard copper tube. Protective
isolators are installed between the coil assembly and
sheet metal coil support pan to further protect the coil
assembly from galvanic corrosion. (See Fig. 7.)
Copper is generally resistant to unpolluted
coastal
environments due to a natural protective film that is
formed on the copper surfaces. Furthermore, galvanic
corrosion is not an issue in these mono-metal coils.
However, copper-fin coils are priced significantly
higher than other coil options since material costs for
copper are greater than those for aluminum. Other
alternatives (see summary guide) provide preferred
solutions for most applications.
Fig. 6. Pre-Coated Coil Assembly
Fig. 7. Copper-Fin Coil Assembly
8
Uncoated copper coils are not suitable
for dense urban,
polluted coastal applications, industrial applications, or
industrial marine applications since many pollutants
attack copper putting both the fin and the tube at risk.
The use of uncoated copper in these applications is not
recommended. E-coated aluminum fin/copper tube or
e-coated MCHX coils should be considered for such
applications.
E-Coated Aluminum-Fin Coils
E-coated coils provide superior protection against
many corrosive atmospheres with the exception of
formic acid and nitric acid environments.
The aluminum fin/copper tube coils are e-coated using
the same proprietary process described above. A very
flexible and durable epoxy coating is uniformly
applied over all coil surfaces for complete isolation
from the contaminated environment (Fig. 8). A
consistent coating is achieved through a precisely
controlled electrocoating process that bonds a thin,
impermeable epoxy coating to the specially prepared
coil surfaces.
The proprietary Carrier e-coating process is described
in further detail on page 9.
E-Coated Copper-Fin Coils (not available with all
products)
E-coated copper fin/copper tube coils have the same
durable and flexible epoxy coating uniformly applied
over all coil surfaces as the e-coated aluminum-fin
coils (Fig. 9). However, these coils combine the
natural resistance of all-copper construction with
complete encapsulation from the e-coat process. As
noted previously, e-coated copper fin/copper tube coils
may be specified for severe marine environments that
are void of industrial contaminants.
Cooling/Heating Coils
Standard Coil Construction
The standard cooling/heating coil (water, steam or
direct expansion) has copper tubes mechanically
bonded to aluminum fins. The fin pack is assembled
with galvanized steel tube sheets and coil case. This
assembly has classic galvanic corrosion components
with multi-metal bonds between the fin-and-tube and
tube-and-tube sheet.
In cooling applications, condensate accumulates on the
coil surfaces when dehumidification occurs. Wet coil
surfaces resulting from condensation in the presence of
a contaminated airstream will lead to galvanic
corrosion if not properly protected.
Potentially corrosive airstreams may not be suitable
for building occupants. If a contaminated airstream
can lead to corrosion, special consideration with
respect to indoor air quality and potentially harmful
side effects to building occupants is recommended.
Copper Fin/Copper Tube Coils
Much like the all-copper condenser coil, all-copper
cooling/heating coils eliminate the bi-metallic bond
found on standard coils. A copper fin with wavy
pattern is mechanically bonded to the standard copper
tube to ensure a single-metal assembly. Most air-
handling equipment is available with copper or
stainless steel tube sheets and coil cases to improve the
corrosion durability of the entire coil assembly. As a
result of the reduction of the bi-metallic couples, the
potential for corrosion within the coil assembly is
reduced.
Fig. 8. Magnification of E-Coated
Aluminum Fin/Copper Tube Coil
Fig. 9. E-Coated Copper Coil
9
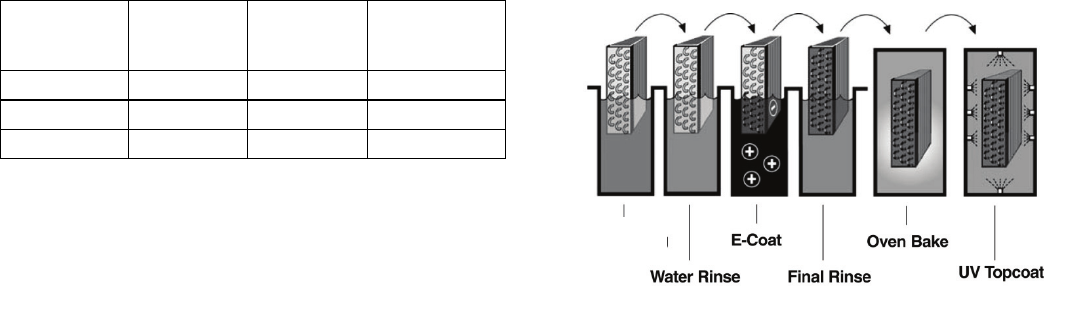
E-Coated Coils
E-coated cooling/heating coils have the same e-coating
as the condenser coils. All e-coated coils have a
durable and flexible epoxy coating uniformly applied
over all coil surfaces, including tubesheets and coil
cases. The coating provides a barrier between the coil
surfaces and the corrosive effects of the atmosphere.
In considering e-coated coils, it is important to also
consider the effects of moisture carryover. Moisture
carryover occurs when accumulated condensation is
blown from the coil surface during cooling coil
applications. The extent of carryover is a function of
airstream velocity across the coil, fin spacing, fin
geometry and material of construction. When e-
coating is applied to a cooling coil, carryover will
occur at lower coil face velocities. Recommendations
shown in Table A should be considered when selecting
chilled water or DX (direct expansion) coils to ensure
moisture carryover will be prevented.
Table A
Maximum Recommended Face Velocity (FPM)*
Fin Spacing
(FPI)
Aluminum-
Fin Coil
Copper-Fin
Coil
E-Coated
Coil
8 650 500 500
11 650 425 425
14 575 375 375
FPI - Fins per Inch
FPM - Feet per Minute
*External fouling on cooling coils will adversely affect the
maximum recommended face velocities. Data based on
clean coils with proper filtration and periodic cleaning of coil
surfaces
.
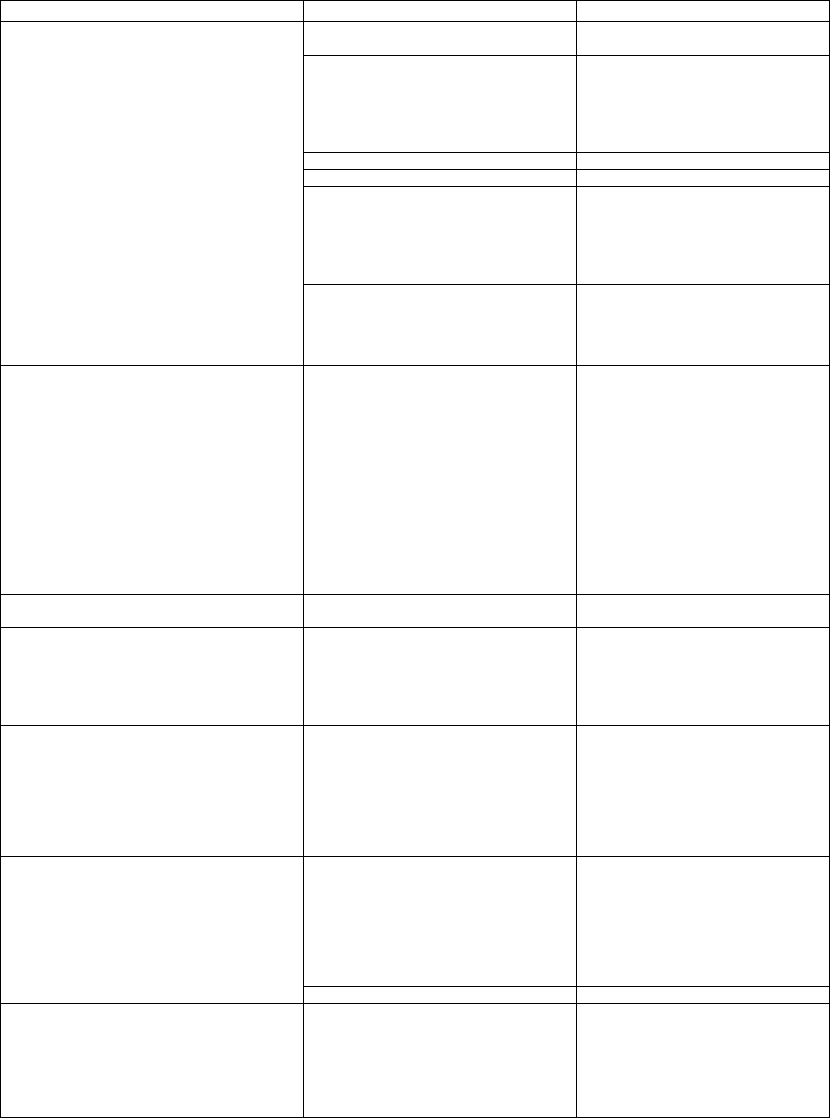
Carrier’s E-Coating Process
Electrocoating is a multi-step process that ensures ultra
clean coils are properly coated, cured, and protected
from environmental attack (Fig. 10). This process
includes complete immersion cleaning to remove
contamination and ensure all surfaces are ultra clean.
The water bath rinses residual dust and contamination
away in preparation for the e-coating process. The
fundamental principle of electrocoating is that the
materials with opposite electrical charges attract each
other. An electrocoating system applies a DC charge to
the coil immersed in a bath of oppositely charged
epoxy molecules. The molecules are drawn to the
metal, forming an even, continuous film over the
entire surface. At a certain point, the coating film
insulates the metal, stopping the attraction, and
preventing further coating deposition (self-limiting
nature of the coating process).
The final rinse bath removes and recovers residual
coating material to ensure a smooth coating and
minimize process waste. A precisely controlled oven
bake cures the coating uniformly to ensure consistent
adhesion on all coil surfaces. This electrocoating
process creates a smooth, consistent, and flexible
coating that penetrates deep into all coil cavities and
covers the entire coil assembly including the fin edges.
The process in conjunction with the coating material
results in a less brittle, more resilient, and more
durable coating without bridging between adjacent
fins. E-coated coils provide superior protection in the
most severe environments.
Finally, a UV protective topcoat is applied to shield
the finish from ultraviolet degradation and to ensure
coating durability and long life.
E-Coated Material and Chemical Resistance
Chemical resistance of the e-coating material is
described in the appendix, “E-Coating Chemical
Resistance Guide.” Application of an e-coated coil
should only be considered when the contaminant is
listed in the Appendix guide. If the e-coating is NOT
resistant to the contaminant listed or if the contaminant
is not listed in the appendix, application in this
environment is not recommended. Contact your
Carrier representative for further guidance.
Some common industrial processes and their related
contaminants that are resisted by the e-coated coils are
shown in Table B.
Fig. 10. E-Coating Process
Cleaning
10
Table B
Industrial Contaminants
Type of Industry/Application Source of Contaminant Contaminant
Pulp, Paper and Lumber Plants Process Emissions Nitrogen Oxides
Sulfur Oxides
Pulp Bleaching Dichloromethane
Chloroform
Methyl Ethyl Ketone
Carbon Disulphide
Chlormethane
Trichloroethane
Sulphite Mill Operations Sulfur Oxides
Kraft Pulping and Recovery Processes Volatile Organic Compounds
Chip Digester and Liquid Evaporator Terpenes
Alcohols
Phenols
Methanol
Acetone
Methyl Ethyl Ketone
Products of Combustion Nitrogen Oxides
Sulfur Oxides
Carbon Monoxide
Particulate Matter
Fly Ash
Incineration Facilities
Fuel Burning Power Generation
Diesel/Gasoline Engine Operation
Products of Combustion Sulfur Oxides
Nitrogen Oxides
Sulfur Trioxide
Sulfuric Acid
Ammonium Sulfate
Ammonium Bisulfate
Carbon Dioxide
Sulphate
Nitrate
Hydrochloric Acid
Hydrogen Fluoride
Particulate Matter
Ozone
Volatile Organic Compounds
Cleaning Agent Processing Process Emissions
Chlorine
Chlorides
Salt Mining/Processing
Swimming Pool Agents
Process By-Products Bromine
Chlorine
Sulfate
Sodium Bisulfate
Phosphate
Chlorides
Fertilizer Manufacturers Process By-Products Hydrogen Fluoride
Sulfites
Sulfuric Acid
Hydrofluoric Acid
Phosphoric Acid
Fluorosiliac Acid
Ammonia
Ammonia Salts
Waste Water Treatment Facilities Waste Digestion Methane
Sulfur Dioxide
Nitrogen Oxides
Volatile Organic Compounds
Chlorine
Chlorine Dioxide
Ammonia
Ammonia Salts
Sludge Processing Hydrogen Sulfide
Agriculture Animal Waste and Fertilizers Sulfu
r
Nitrous Oxide
Nitrogen Oxides
Methane
Hydrogen Sulfide
Ammonia
Ammonia Salts
11
Field-Applied Coatings
Field-applied sprayed-on coatings will not provide
sufficient protection in corrosive environments and
should not be used on Carrier coils. The use of field-
applied coatings on Novation
®
heat exchangers may
negatively affect the Carrier warranty. Possible
reasons for inadequate protection include:
Coil cleanliness is crucial for proper adhesion.
Adequate field cleaning techniques are often
overlooked. In addition, the coil must be void
of any corrosion. Encapsulation of existing
corrosion makes the coating ineffective by
leading to continued deterioration and
eventual coating delamination.
Field application cannot ensure continuous
coating of coil surfaces on multiple row coils.
It is difficult to ensure uniform coating quality
throughout the depth of the fin pack.
Interior coil surfaces remain untreated when
sprayed-on from unit exterior; often, spray
applicators cannot reach deep into the coil
assemblies, leaving inconsistent thickness or
areas of no coverage.
Inconsistent coating thickness can minimize
or negate coating protection. Recommended
coating thickness cannot be ensured with field
application on multiple row coils. Film
thickness measurements are often overlooked.
___________________________________________________________________________________________
SELECTION SUMMARY
Selection Tables
Tables C through F provide guidelines for coil
selection. To use the tables, first clearly identify the
operating environment for the intended installation.
Then determine the severity of each environmental
factor associated with the installation site. Choose the
protection option based on the most severe
environmental factor anticipated for the given site.
NOTE: In these tables, acceptability of a coil option is
based solely on corrosion performance. Other factors,
including cost, should be considered in making the final
coil selections.
For MCHX coils, the Carrier Electronic Catalog
(E-CAT) can be used to obtain confirmation on
whether or not corrosion protection is recommended
for particular applications in coastal/marine
environments.
Selection Example
Following is an example of the selection process as
applied to a coastal environment.
Step 1 – Identify the operating environment
according to the factors described in Table C.
For this example:
a. Site is on the coastline (distance from the coast is
<0.01 miles).
b. Condenser coil is facing the ocean, with the
direction of the prevailing wind from the coast to
the unit.
c. No noticeable corrosion on other equipment.
Step 2 – Determine the severity of each
environmental factor; always choose the option for
the most severe environmental factor present.
There is no noticeable corrosion on the unit, which
means low severity for this factor; however, the
distance from the coast and the prevailing wind are
both in the severe category, so coil selection should be
guided by recommendations in the last column on the
right, at the severe end of the range.
Step 3 – Identify coil options.
The acceptable coil options are as follows: copper
fin/copper tube, e-coated aluminum fin/copper tube, e-
coated microchannel heat exchanger coil, or e-coated
copper fin/copper tube coil.
12
Table C
Coastal Environment Protection Option
Global Coil and Coating Options*
Severity of Environmental Factors
Low
Severe
Distance from Coast**
Inland
> 5 mi 5 to 2 mi
Coastline
< 2mi to <0.01 mi
Direction of Prevailing Winds
From Unit to Coast From Coast to Unit
Corrosion Present on Other Equipment
None Present Noticeable Corrosion
Standard: Aluminum Fin / Copper Tube ACC ACC NR
Microchannel Heat Exchanger† ACC ACC NR
Pre-Coated Aluminum Fin / Copper Tube ACC ACC NR
Copper Fin / Copper Tube ACC ACC ACC
E-Coated Aluminum Fin / Copper Tube ACC ACC ACC
E-Coated Microchannel Heat Exchanger† ACC ACC ACC
E-Coated Copper Fin / Copper Tube ACC ACC ACC
ACC - Indicates that the option is acceptable for the application
and conditions shown; in some cases, the level of
corrosion protection provided by this option may be higher
than required. Acceptability is based solely on corrosion
performance. Other factors, including cost, should be
considered in making the final selections.
NR - Not recommended
*Other coating options may be available within a given region.
†Information in this table is provided as a guide; contact a Carrier
Sales Engineer for an E-CAT selection.
**Refer to the E-CAT program for exact distance requirements
for MCHX coils.
Note: The distances stated relate to land distances to ocean.
Additional coating may be required for Marine Applications on-
board a ship.
Environments immediately adjacent to diesel exhaust,
incinerator discharge stacks, fuel burning boiler stacks, or areas
exposed to fossil fuel combustion emissions should be
considered a Combined Coastal/Industrial application.
Recommendations presented for Industrial and Combined
Coastal/Industrial Environments should be followed.
Table D
Industrial Environment Protection Option
Global Coil and Coating Options*
Severity of Environmental Factors
Low
Severe
Contaminant Concentration††
0 to 50 ppm 51 to 100 ppm >100 ppm
Corrosion Present on Other Equipment
None Present Noticeable Corrosion
Standard: Aluminum Fin / Copper Tube ACC NR NR
Microchannel Heat Exchanger† ACC NR NR
Copper Fin / Copper Tube NR NR NR
Pre-Coated Aluminum Fin / Copper Tube ACC ACC NR
E-Coated Aluminum Fin / Copper Tube ACC ACC ACC
E-Coated Microchannel Heat Exchanger† ACC ACC ACC
E-Coated Copper Fin / Copper Tube NR NR NR
ACC - Indicates that the option is acceptable for the
application and conditions shown; in some cases, the
level of corrosion protection provided by this option
may be higher than required. Acceptability is based
solely on corrosion performance. Other factors,
including cost, should be considered in making the final
selections.
NR - Not recommended
*Other coating options may be available within a given region.
†Information in this table is provided as a guide; contact a
Carrier Sales Engineer for an E-CAT selection.
††See “E-Coating Chemical Resistance Guide” in Appendix.
Testing for contaminants may be performed by Draeger tube
procedure.
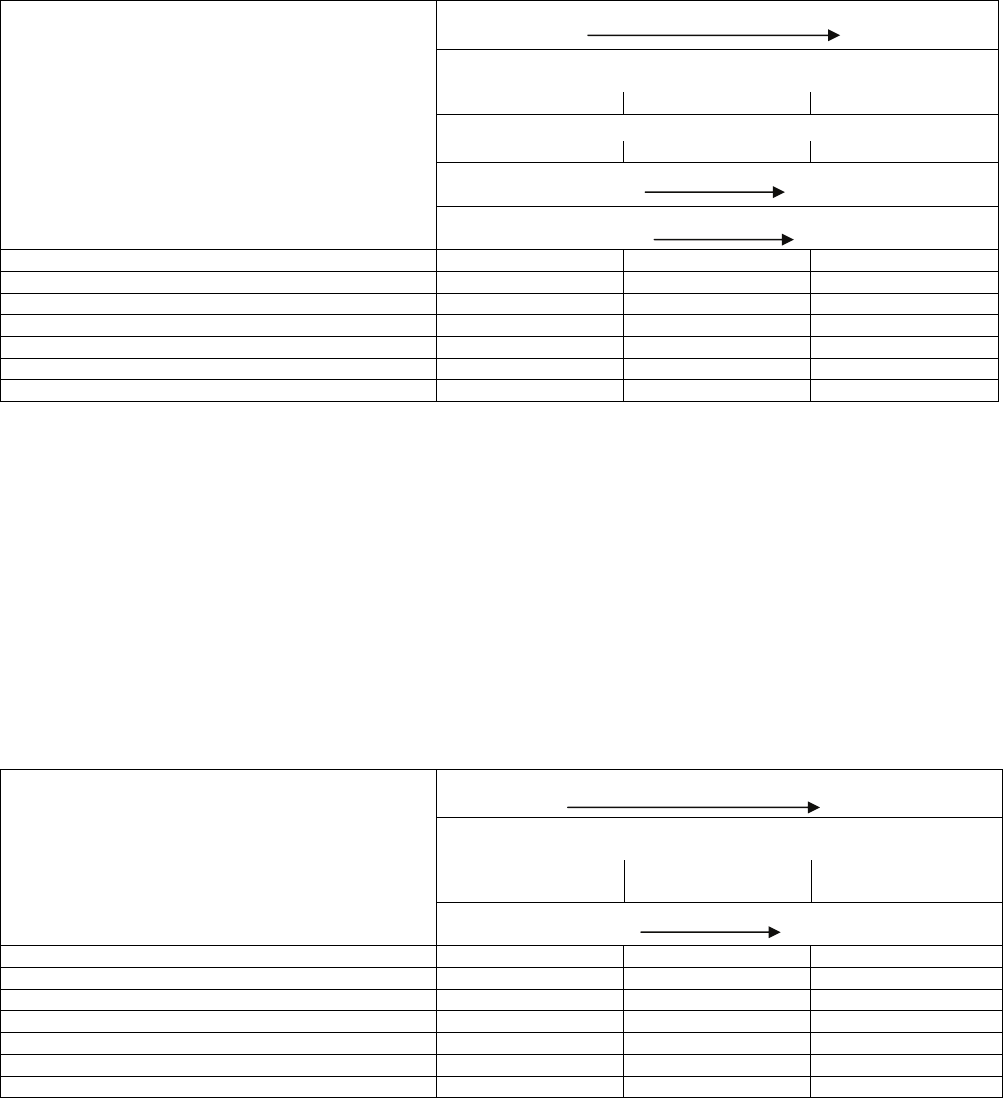
13
Table E
Combined Coastal/Industrial Environment Protection Option
Global Coil and Coating Options*
Severity of Environmental Factors
Low
Severe
Distance from Coast**
Inland Coastline
> 5 mi 5 to 2 mi < 2 mi to <0.01 mi
Contaminant Concentration††
0 to 50 ppm 51 to 100 ppm >100 ppm
Direction of Prevailing Winds
From Unit to Coast From Coast to Unit
Corrosion Present on Other Equipment
None Present Noticeable Corrosion
Standard: Aluminum Fin / Copper Tube ACC NR NR
Microchannel Heat Exchanger† ACC NR NR
Copper Fin / Copper Tube NR NR NR
Pre-Coated Aluminum Fin / Copper Tube ACC ACC NR
E-Coated Aluminum Fin / Copper Tube ACC ACC ACC
E-Coated Microchannel Heat Exchanger† ACC ACC ACC
E-Coated Copper Fin / Copper Tube NR NR NR
ACC - Indicates that the option is acceptable for the application
and conditions shown; in some cases, the level of
corrosion protection provided by this option may be
higher than required. Acceptability is based solely on
corrosion performance. Other factors, including cost,
should be considered in making the final selections.
NR - Not recommended
*Other coating options may be available within a given region.
†Information in this table is provided as a guide; contact a
Carrier Sales Engineer for an E-CAT selection.
**Refer to the E-CAT program for exact distance requirements
for MCHX coils.
††See “E-Coating Chemical Resistance Guide” in Appendix.
Testing for contaminants may be performed by Draeger tube
procedure.
Note: The distances stated relate to land distances to ocean.
Additional coating may be required for Marine Applications on-
board a ship.
Environments immediately adjacent to diesel exhaust,
incinerator discharge stacks, fuel burning boiler stacks, or areas
exposed to fossil fuel combustion emissions should be
considered a Combined Coastal/Industrial application.
Recommendations presented for Industrial and Combined
Coastal/Industrial Environments should be followed.
Table F
Urban Environment Protection Option
Global Coil and Coating Options*
Severity of Environmental Factors
Low
Severe
Pollution Levels
(SO
2
levels in Cities with >50K Inhabitants)***
Low
<20 to 50 µg/m
3
51 to 125 µg/m
3
High
>125 µg/m
3
Corrosion Present on Other Equipment
None Present Noticeable Corrosion
Standard: Aluminum Fin / Copper Tube ACC ACC NR
Microchannel Heat Exchanger† ACC ACC NR
Copper Fin / Copper Tube NR NR NR
Pre-Coated Aluminum Fin / Copper Tube ACC ACC NR
E-Coated Aluminum Fin / Copper Tube ACC ACC ACC
E-Coated Microchannel Heat Exchanger† ACC ACC ACC
E-Coated Copper Fin / Copper Tube NR NR NR
ACC - Indicates that the option is acceptable for the application
and conditions shown; in some cases, the level of
corrosion protection provided by this option may be
higher than required. Acceptability is based solely on
corrosion performance. Other factors, including cost,
should be considered in making the final selections.
NR - Not recommended
*Other coating options may be available within a given region.
†Information in this table is provided as a guide; contact a
Carrier Sales Engineer for an E-CAT selection.
***SO
2
levels shall be determined in accordance with ASTM
D2914, ISO/FDIS 10498.
Note: Environments immediately adjacent to diesel exhaust,
incinerator discharge stacks, fuel burning boiler stacks, or
areas exposed to fossil fuel combustion emissions should be
considered an Industrial application. Recommendations
presented for Industrial Environments should be followed.
14
JOB SITE COMMISSIONING AND PROPER
EQUIPMENT STORAGE
An important factor that is often overlooked is the
proper storage of HVAC/R equipment, including
equipment with coated or uncoated coils, prior to start-
up at new installations. It is not unusual for equipment
to arrive on site several months prior to the actual
installation and start-up, resulting in a potential for
premature corrosion to occur if the equipment is not
stored in a proper manner. Equipment should be stored
so that it is not exposed to excessive construction
debris and concrete dust, industrial contaminants,
coastal contaminants, or high levels of humidity and
moisture.
Improper storage can lead to premature corrosion prior
to start-up and can reduce the overall life of the
equipment.
Extra care should be taken to ensure that equipment
located on the ground level remains free from debris
prior to start-up.
COIL MAINTENANCE AND CLEANING
RECOMMENDATIONS
Routine cleaning of coil surfaces is essential to
maintain proper operation of the unit. Elimination of
contamination and removal of harmful residues will
greatly increase the life of the coil, optimize
equipment performance, and extend the life of the unit.
Maintenance requirements and correct cleaning
procedures for MCHX and RTPF coils may be found
in the Service instructions provided with each unit and
should be followed carefully.
15
APPENDIX
E-Coating Chemical Resistance Guide
The coating material used for e-coat is resistant to
fumes from the chemicals listed below. However,
Carrier does not recommend direct coil immersion
service for any of these chemicals. The chemical
resistance guidelines were determined by a 24-hour
spot test exposure of the chemical listed. Resistance
was determined for these chemicals at the
concentrations identified.
E-coat is resistant to the following fumes:
NOTE: All data, statements, and recommendations are based on research
conducted by the e-coat manufacturer and are believed to be accurate. It is
the responsibility of the user to evaluate the accuracy, completeness or
usefulness of any content in this paper. Neither Carrier nor its affiliates
make any representations or warranties regarding the content contained in
this paper. Neither Carrier nor its affiliates will be liable to any user or
anyone else for any inaccuracy, error or omission, regardless of cause, or
for any damages resulting from any use, reliance or reference to the content
in this paper.
Acetates (ALL)
Cresol
Lauryl Alcohol Propylene Glycol
Acetic Acid 99%
Dichloromethane
Magnesium Chloride Salicylic Acid
Acetone
Diesel Fuel
Magnesium Sulfate Salt Water
Alcohols Diethanolamine Maleic Acid Sodium Bisulfate
Amines (ALL) Ethyl Acetate
Menthanol
Sodium Bisulfite
Amino Acids Ethyl Alcohol
Menthol
Sodium Chloride
Ammonia Ethyl Ether
Methane
Sodium Hypochlorite 5%
Ammonia Salts Fatty Acid Methyl Ethyl Ketone Sodium Hydroxide 10%
Ammonium Bisulfate Fluoride Methyl Isobutyl Ketone Sodium Hydroxide 25%
Ammonium Sulfate Fluorine Gas Methylene Chloride Sodium Sulfate
Ammonium Hydroxide
Fluorosiliac Acid
Mustard Gas Sorbitol
Benzene
Formaldehyde 27%
Naphthol Stearic Acid
Borax Fructose
Nitrate Sucrose
Boric Acid Gasoline Nitric Acid 25%
Sulfates (ALL)
Bromine Glucose Nitrogen Oxides Sulfides (ALL)
Butric Acid Glycol Nitrous Oxide Sulfites (ALL)
Butyl Alcohol Glycol Lither Olale Acid Sulfur Dioxide
Butyl Cellosolve Hydrazine
Oxalic Acid
Sulfur Oxides
Calcium Chloride Hydrochloric Acid 37% Ozone Sulfur Trioxide
Calcium Ilypochloric Hydrofluoric Acid 30% Perchloric Acid Sulfuric Acid 25-85%
Carbon Dioxide Hydrogen Fluoride Phenol 85% Starch
Carbon Disulphide Hydrogen Peroxide 5% Phosgene Terpenes
Carbon Monoxide Hydrogen Sulfide Phosphate Toluene
Chlorides Hydroxylamine Phosphoric Acid Trichloroethane
Chlorine Iodine Phenolphthalein Triethanolamine
Chlorine Dioxide Isobutyl Alcohol Phosphoric Acid Urea
Chlorine Gas Isopropyl Alcohol Potassium Chloride
Vinegar
Chloroform Kerosene Potassium Hydroxide
Volatile Organic Compounds
Chromic Acid 25% Lactic Acid Propionic Acid Xylene
Citric Acid
Lactose Propyl Alcohol

5
This paper is provided for informational and marketing purposes only and shall not be deemed to create any implied or express warranties
or covenants with respect to the products of Carrier Corporation or those of any third party.
© Carrier Corporation 2012 www.carrier.com 04-581061-01 Printed in U.S.A. 12-12 Replaces 04-581042-01,
04-581043-01
