
State Operations Manual
Appendix M - Guidance to Surveyors: Hospice -
Table of Contents
(Rev. 222; Issued: 06-07-24)
Transmittals for Appendix M
Part I – Investigative Procedures
I - Introduction
II. Regulatory and Policy References
III. Tasks in the Survey Protocol
Introduction
Task 1 Pre-Survey Preparation
Task 2 Entrance Conference
Task 3 Sample Selection
Task 4 Information Gathering—Phase 1 & Phase 2
Task 5 Preliminary Decision Making and Analysis of Findings
Task 6 Exit Conference
Task 7 Post-Survey Activities
C - Post Survey Revisit
Part II – Interpretive Guidelines
Subpart C - Conditions of Participation: Patient Care
§418.3 Definitions
§418.52 Condition of Participation: Patient's Rights
§418.52(a) Standard: Notice of Rights and Responsibilities
§418.52(b) Standard: Exercise of Rights and Respect for Property and Person
§418.52(c) Standard: Rights of the Patient
§418.54 Condition of Participation: Initial and Comprehensive Assessment of the Patient
§418.54(a) Standard: Initial Assessment
§418.54(b) Standard: Time Frame for Completion of the Comprehensive Assessment
§418.54(c) Standard: Content of the Comprehensive Assessment
§418.54(d) Standard: Update of the Comprehensive Assessment
§418.54(e) Standard: Patient Outcome Measures
§418.56 Condition of Participation: Interdisciplinary Group, Care Planning, and Coordination of
Services
§418.56(a) Standard: Approach to Service Delivery
§418.56(b) Standard: Plan of Care
§418.56(c) Standard: Content of the Plan of Care
§418.56(d) Standard: Review of the Plan of Care
§418.56(e) Standard: Coordination of Services
§418.58 Condition of Participation: Quality Assessment and Performance Improvement
§418.58(a) Standard: Program Scope
§418.58(b) Standard: Program Data
§418.58(c) Standard: Program Activities
§418.58(d) Standard: Performance Improvement Projects
§418.58(e) Standard: Executive Responsibilities
§418.60 Condition of Participation: Infection Control
§418.60(a) Standard: Prevention
§418.60(b)Standard: Control
§418.60(c) Standard: Education
§418.62 Condition of Participation: Licensed Professional Services
§418.64 Condition of Participation: Core Services
§418.64(a) Standard: Physician Services
§418.64(b) Standard: Nursing Services
§418.64(c) Standard: Medical Social Services
§418.64(d) Standard: Counseling Services
§418.66 Condition of Participation: Nursing Services Waiver Of Requirement That
Substantially All Nursing Services Be Routinely Provided Directly by a Hospice
§418.70 Condition of Participation: Furnishing of Non-core Services
§418.72 Condition of Participation: Physical Therapy (PT), Occupational Therapy (OT), and
Speech-Language Pathology (SLP)
§418.74 Waiver of Requirement-Physical Therapy, Occupational Therapy, Speech-language
Pathology and Dietary Counseling
§418.76 Condition of Participation: Hospice Aide and Homemaker Services
§418.76(a) Standard: Hospice Aide Qualifications
§418.76(b) Standard: Content and Duration of Hospice Aide Classroom and Supervised
Practical Training
§418.76(c) Standard: Competency Evaluation
§418.76(d) Standard: In-service Training
§418.76(e) Standard: Qualifications for Instructors Conducting Classroom And Supervised
Practical Training
§418.76(f) Standard: Eligible Competency Evaluation Organizations
§418.76(g) Standard: Hospice Aide Assignments and Duties
§418.76(h) Standard: Supervision of Hospice Aides
§418.76(i) Standard: Individuals Furnishing Medicaid Personal Care Aide-Only Services under
a Medicaid Personal Care Benefit
§418.76(j) Standard: Homemaker Qualifications
§418.76(k) Standard: Homemaker Supervision and Duties
§418.78 Condition of participation: Volunteers
§418.78(a) Standard: Training
§418.78(b) Standard: Role
§418.78(c) Standard: Recruiting and Retaining
§418.78(d) Standard: Cost Saving
§418.78(e) Standard: Level of Activity
Subpart D --Conditions of Participation: Organizational Environment
§418.100 Condition of Participation: Organization and Administration of Services
§418.100(a) Standard: Serving the Hospice Patient and Family
§418.100(b) Standard: Governing Body and Administrator
§418.100(c) Standard: Services
§418.100(d) Standard: Continuation of Care
§418.100(e) Standard: Professional Management Responsibility
§418.100(f) Standard: Hospice Multiple Locations
§418.100(g) Standard: Training
§418.102 Condition of Participation: Medical Director.
§418.102(a) Standard: Medical Director Contract
§418.102(b)Standard: Initial Certification of Terminal Illness
§418.102(c) Standard: Recertification of the Terminal Illness
§418.102(d) Standard: Medical Director Responsibility
§418.104 Condition of participation: Clinical Records
§418.104(a) Standard: Content
§418.104(b) Standard: Authentication
§418.104(c) Standard: Protection of Information
§418.104(d) Standard: Retention of Records
§418.104(e) Standard: Discharge or Transfer of Care
§418.104(f) Standard: Retrieval of Clinical Records
§418.106 Condition of Participation: Drugs and Biologicals, Medical Supplies, and Durable
Medical Equipment
§418.106(a) Standard: Managing Drugs and Biologicals
§418.106(b) Standard: Ordering of Drugs
§418.106(c) Standard: Dispensing of Drugs and Biologicals
§418.106(d) Standard: Administration of Drugs and Biologicals
§418.106(e) Standard: Labeling, Disposing, and Storing of Drugs and Biologicals
§418.106(f) Standard: Use and Maintenance of Equipment and Supplies
§418.108 Condition of Participation: Short-term Inpatient Care
§418.108(a) Standard: Inpatient Care for Symptom Management and Pain Control
§418.108(b) Standard: Inpatient Care for Respite Purposes
§418.108(c)Standard: Inpatient Care Provided under Arrangements
§418.108(d) Standard: Inpatient Care Limitation
§418.108(e) Standard: Exemption from Limitation.
§418.110 Condition of Participation: Hospices that Provide Inpatient Care Directly
§418.110(a) Standard: Staffing
§418.110(b) Standard: Twenty-four Hour Nursing Services
§418.110(c) Standard: Physical Environment
§418.110(d) Standard: Fire Protection
§418.110(e) Standard: Patient Areas
§418.110(f) Standard: Patient Rooms
§418.110(g) Standard: Toilet/Bathing Facilities
§418.110(h) Standard: Plumbing Facilities

§418.110(i) Standard: Infection Control
§418.110(j) Standard: Sanitary Environment
§418.110(k) Standard: Linen
§418.110(l) Standard: Meal Service and Menu Planning
§418.110(m) Standard: Restraint or Seclusion
§418.110(n) Standard: Restraint or Seclusion Staff Training Requirements
§418.110(o) Standard: Death Reporting Requirements
§418.112 Condition of Participation: Hospices that Provide Hospice Care to Residents of a
SNF/NF or ICF/IID
§418.112(a) Standard: Resident Eligibility, Election, and Duration of Benefits
§418.112(b) Standard: Professional Management
§418.112(c) Standard: Written Agreement
§418.112(d) Standard: Hospice Plan of Care
§418.112(e) Standard: Coordination of Services
§418.112(f) Standard: Orientation and Training of Staff
§418.113 Condition of participation: Emergency preparedness.
§418.114 Condition of Participation: Personnel Qualifications
§418.114(a) Standard: General Qualification Requirements
§418.114(b) Standard: Personnel Qualifications for Certain Disciplines
§418.114(c) Standard: Personnel Qualifications When No State Licensing, Certification or
Registration Requirements Exist
§418.114(d) Standard: Criminal Background Checks
§418.116 Condition of Participation: Compliance with Federal, State, and Local Laws and
Regulations Related to the Health and Safety of Patients
§418.116(a) Standard: Multiple Locations
§418.116(b) Standard: Laboratory Services
_____________________________________________________________________
Part I – Survey Protocol
I – Introduction
Hospice care is a comprehensive, holistic approach to treatment that recognizes the needs of a
terminally ill individual, and warrants focus on palliative care for relief of pain and symptom
management. Medicare regulations define “palliative care” as patient and family-centered care
that optimizes quality of life by anticipating, preventing, and treating suffering. Palliative care
throughout the continuum of illness involves addressing physical, intellectual, emotional, social,
and spiritual needs and facilitating patient autonomy, access to information, and choice (42 CFR
§ 418.3). The goal of hospice care is to help terminally ill individuals continue life with minimal
disruption to normal activities while remaining primarily in the home environment. A hospice
program uses an interdisciplinary approach to deliver medical, nursing, social, psychological,
emotional, and spiritual services through a collaboration of professionals and other caregivers,
intending to make the beneficiary as physically and emotionally comfortable as possible. The
interdisciplinary group (IDG) works with the patient, family, and caregivers, and the patient’s
attending physician (if any) to develop a coordinated, comprehensive care plan; reduce the use of
diagnostics and therapies that are not conducive to achieving the patient’s end-of-life goals of
care; and maintain ongoing communication with the patient, family, and caregivers about
changes in the patient’s condition. The plan of care will shift over time to meet the changing
needs as the patient approaches the end of life.
The Hospice Survey
Survey protocols and Interpretive Guidelines (IGs) are established to provide guidance to
personnel conducting surveys of hospices and serve to clarify and/or explain the intent of the
regulations. All surveyors are required to use them in assessing compliance with Federal
requirements. The purpose of the protocols and guidelines is to direct the surveyor’s attention to
avenues of investigation in preparation for the survey, conducting the survey, and evaluating the
survey findings.
These protocols represent the view of the Centers for Medicare & Medicaid Services (CMS) on
relevant areas and items that must be inspected/reviewed under each regulation. The use of these
protocols promotes efficiency and consistency in the survey process by providing surveyors with
direction on how to gather sufficient information to make compliance decisions.
All mandatory requirements for hospices are set forth in relevant provisions of the Social
Security Act and in the Code of Federal Regulations (CFR). Although surveyors use the
information contained in the IGs to help to make a determination about compliance with the
requirements, the IGs are not binding and do not replace or supersede the law or regulations.
The IGs contain authoritative interpretations and clarification of statutory and regulatory
requirements and are used to assist surveyors in making determinations about a hospice’s
compliance, however IGs may not be used alone as the sole basis for a citation.
II. Regulatory and Policy References
• Subpart A of 42 CFR part 418 sets forth the statutory basis and scope and defines terms used in
42 CFR part 418. Subpart B (42 CFR §§ 418.20 through 418.30) specifies the eligibility and
election requirements and the benefit periods. Subparts C and D (42 CFR §§ 418.52 through
418.116) specify the conditions of participation (CoPs) for hospices.
• Should an individual or entity that is seeking certification to participate in Medicare refuse to
allow immediate access to a State Survey Agency (SA) or CMS surveyor, a surveyor from a
national accreditation organization (AO) with a CMS-approved hospice program, or the Office
of Inspector General (OIG), then that entity may be excluded from participation in all Federal
healthcare programs, in accordance with 42 CFR § 1001.1301. If a surveyor intends to request
immediate access with the threat of possible exclusion for non-compliance, the SA must first
contact the applicable CMS Location, which must then contact the OIG Administrative and Civil
Remedies Branch at 202-619-1306. In addition, failure to grant immediate access to a surveyor
is a basis for CMS to terminate the provider under 42 CFR § 489.53(a)(18), and failure to permit
copying of any records or information during the survey under 42 CFR § 489.53(a)(13) is also
grounds for CMS to terminate the provider.
• The CMS State Operations Manual (SOM), Publication 100-07, in which this guidance
(Appendix M) is located, provides CMS policy regarding survey and certification activities.
III - Tasks in the Survey Protocol
The hospice survey process evaluates the hospice’s compliance with all applicable CoPs and,
ultimately, its impact on safety and quality of care. All hospice initial certification and
recertification surveys are full surveys, i.e., surveys that evaluate compliance with all CoPs.
The hospice survey process consists of seven standard tasks, listed below:
• Task 1 Pre-Survey Preparation;
• Task 2 Entrance Conference;
• Task 3 Sample Selection;
• Task 4 Information Gathering—Phase 1 & Phase 2
• Task 5 Preliminary Decision Making and Analysis of Findings;
• Task 6 Exit Conference; and
• Task 7 Post-Survey Activities.
Task 1 –Pre-Survey Preparation
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives
The objectives of the pre-survey preparation are to review historical information about the
hospice that may assist in identifying areas of potential concern and planning the logistics of the
survey. The primary pre-survey activities include:
A. Select the survey team;
B. Review background information about the hospice;
C. Review CMS hospice quality measures and other performance data; and
D. Review recent CMS-2567 and other relevant CMS forms.
Types of Hospice Surveys
There are six types of hospice surveys. All hospice surveys are unannounced and must verify
compliance with regulatory requirements contained in §418.52 through §418.116. (See 42 CFR
§ 488.1105; §2700A.), unless there is complaint allegation being assessed, in which case a focal
set of CoPs may be considered.
1. Initial Certification Survey: Before the SA or the CMS-approved national accrediting
organization (AO) with deeming authority conducts the initial Medicare certification survey, the
surveying entity must have received written documentation submitted by the prospective hospice
requesting an initial certification survey. At the time of the survey, the prospective hospice
must:
• Have completed the Medicare Enrollment Application Form CMS-855A and had this
form verified by the assigned Medicare Administrative Contractor (MAC);
• Be operational;
• Have provided care to a minimum of 5 hospice patients (not required to be Medicare
patients);
• Have at least 3 hospice patients receiving care at the time of the initial Medicare
certification survey. If the hospice is located in a medically underserved area, as
determined by the CMS Location, the CMS Location may reduce the minimum number
of patients serviced from 5 to 2. At least 1 of the 2 required patients should be receiving
care from the hospice at the time of the initial Medicare survey;
• Be providing all services needed by the patient(s) actually being served; and
• Be capable of demonstrating the operational capability of all facets of its operations.
In the event that the hospice patient(s) being served at the time of the survey do not require the
full scope of hospice services, verify that the hospice is fully prepared to provide all services
necessary to meet the hospice Medicare CoPs.
The effective date of Medicare participation can be no earlier than the date the hospice is
prepared to provide all of the required services and meets all hospice CoPs. In no case can the
effective date of certification be earlier than the date the hospice meets all the Federal
requirements (42 CFR § 489.13).
2. Standard Survey: Hospices are subject to standard surveys by an SA or a CMS approved
accrediting organization (AO), every three years. If an existing certified hospice has a new
inpatient unit or an inpatient unit that it wishes to relocate, compliance with the regulations at
§418.110 (Hospices that Provide Inpatient Care Directly) must also be verified on-site, during
the survey.
Surveyors should routinely conduct the standard survey, also known as the recertification survey,
at a multiple location of the hospice, if applicable, when that location serves more patients than
the initial location issued the CMS certification number. Whenever possible, visit as many
multiple locations as practical. Deficiencies found at any multiple location(s) are applicable to
the entire hospice organization, which includes the primary hospice that is assigned the CCN and
any identified multiple locations.
3. Complaint Survey (Investigation): Investigation and resolution of complaints is a critical
certification activity. A complaint investigation looks into substantial allegations of
noncompliance. Refer to the SOM, Chapter 5, for additional guidance regarding complaint
surveys. If surveyors find the hospice has one or more condition-level deficiencies during the
complaint investigation, they may elect to review some or all CoPs as needed and relevant to the
investigation.
4. Post-Survey Revisit (Follow-up Survey): When the SA cites deficiencies during a survey,
the SA may conduct a post-survey revisit to determine if the hospice has made corrections to
meet the requirements for participation for those cited deficiencies. However, the existence of
condition-level deficiencies requires an on-site post-survey revisit to determine if the hospice has
corrected these deficiencies. See also SOM Chapter 2, Section 2732A - Post-Survey Revisit.
5. Change in Ownership (CHOW): When the Medicare Administrative Contractor (MAC)
receives a notification of a CHOW, the CMS Location and/or SA determine whether a desk audit
and/or on-site survey is required to approve the CHOW.
6. Validation Survey for Deemed Hospices: Section 1865(a)(1) of the Social Security Act
(Act) provides that, under the direction of the designated CMS Location, SAs conduct validation
surveys as a component of CMS’ oversight of an AO’s deeming program. CMS Headquarters
selects the hospices for validation survey and notifies the applicable CMS Location. The CMS
Location then requests that the SA conduct a validation survey.
Note: Survey types one and two (initial certification and standard) are considered full surveys.
Survey types three through six (i.e., Complaint, Post-Survey Revisit, CHOW, and Validation
Survey) are referred to as abbreviated surveys. The abbreviated standard survey is a highly
focused survey that evaluates the hospice’s compliance with specific CoPs or standards, as
determined by the reason or purpose of the survey. An abbreviated survey can become a full
standard survey based on additional information and the surveyor’s on-site concerns.
Survey Team Size and Composition
Surveyors must successfully complete the CMS Basic Hospice Surveyor Training Course and
any additional training specified by CMS (e.g., associated prerequisites) before they serve on a
hospice survey team (except as a trainee). Surveyor trainees may accompany the survey team
under the supervision of an experienced surveyor. Each hospice survey team should include at
least one registered nurse (RN) with hospice survey experience. When there is more than one
surveyor, the team should be multidisciplinary, incorporating other areas of professional practice
as are typically represented on the inter-disciplinary team. When needed, surveyors who have
special expertise to determine whether the hospice is in compliance may be included. The SA, or
the CMS Location for Federal teams, decides the size of the team.
Hospice surveys will vary in
duration, dependent on the size of the survey team.
The survey team size will vary depending on the size and characteristics of the hospice. The
following factors may have an influence:
1. The hospice patient census, number of unduplicated admissions, and number of multiple
locations at the time of the last survey;
2. The settings the hospice serves (each of which requires visits whenever possible), including:
a. home,
b. inpatient hospice,
c. nursing home,
d. respite settings,
e. intermediate care facilities and assisted living facilities;
3. The pattern of past deficiencies or complaints;
4. Whether new surveyors are to accompany the surveyor as part of their training.
Prohibition of Conflicts of Interest
Prior to finalizing the survey team, SAs, federal teams, and AOs must ensure that no conflicts of
interest are present between the team and the hospice being surveyed. Section 488.1115(b) sets
out the circumstances that would disqualify a surveyor from surveying a particular hospice. It
also notes that surveyor(s) must disclose actual or perceived conflicts of interest prior to
participating in a hospice program survey and be provided the opportunity to recuse themselves
as necessary.
Additionally, any of the following circumstances disqualifies a surveyor from surveying a
particular hospice program.
• The surveyor currently serves, or, within the previous 2 years has served, with the
hospice program to be surveyed as a direct employee; an employment agency staff at the
hospice program; or an officer, consultant, or agent for the hospice program to be
surveyed.
• The surveyor has a financial interest or an ownership interest in the hospice program to
be surveyed.
• The surveyor has an immediate family member, as defined at 42 CFR 411.35, who has a
financial interest or an ownership interest with the hospice program to be surveyed.

• The surveyor has an immediate family member, as defined at 42 CFR 411.351, who is a
patient of the hospice program to be surveyed.
Assembling Background Information
In preparation for the survey/resurvey, review documents of record including licensure records,
fire inspection reports, previous survey reports including Life Safety Code (LSC) and complaint
investigations, media reports about the facility, and other publicly available information about
the facility (e.g., the hospice’s website; CMS Care Compare – Hospice; information from the
Quality, Safety and Oversight Reports (QCOR)). The background material that is reviewed in the
SA’s files assists in determining the composition of the survey team and the time that may be
required for the survey, as well as identifying potential concerns for a focused review.
Review the following files:
• The most recent Form CMS-417, Hospice Request for Certification in The Medicare
Program;
• The most recent Form CMS-643 Hospice Survey and Deficiencies Report;
• The most recent Form CMS-2567, Statement of Deficiencies and Plan of Correction; and
• All complaint investigations since the last recertification survey to evaluate for patterns
of deficient practice;
• Complaints triaged at non-IJ/medium that should be investigated during this survey;
• Change of ownership or additional multiple locations documents or information; and
• Data including information from QCOR and the CMS Care Compare – Hospice website.
CMS Hospice Quality Measures and Publicly Available Information
Surveyors should review the information available on the CMS Care Compare – Hospice website
as part of the off-site survey preparation to help identify potential concerns to examine during the
hospice survey. This website includes the following information:
• General Information: This section presents the level of care provided by the hospice.
Routine home care and other levels of care including general inpatient care, continuous
home care, and respite care.
• Conditions: This section presents the average daily census as well as the medical
conditions the hospice most commonly treated based on their patients’ primary diagnoses
from a calendar year. This information may assist the surveyor to stratify the survey
sample.
• Locations of Care: This section presents the locations of patients served by the hospice
and includes, home, assisted living facility, nursing facility, skilled nursing facility,
inpatient hospital facility, and all other locations. This information will help to select
patients for home visits, and to assess for coordination of care in the various locations.
• Family Experience of Care: Implemented in 2015, the Consumer Assessment of
Healthcare Providers and Systems (CAHPS) Hospice Survey focuses on the experiences
of patients who have died in hospice care, and their primary informal caregivers.

Surveyors can use CAHPS information as indications of things to observe during home visits or
ask about during patient/caregiver interviews. Its purpose is to:
• Provide a source of information on patient/caregiver experiences that can be publicly
reported to beneficiaries and their family members to help them select a hospice program,
• Support hospices with their internal quality improvement efforts through external
benchmarking, and;
• Provide CMS with information for monitoring the care provided by hospices.
The CAHPS Hospice instrument is composed of a series of questions used to develop quality
measures regarding:
• Communication with Family;
• Getting Timely Help
• Treating Patient with Respect
• Emotional and Spiritual Support
• Help for Pain and Symptoms
• Training Family to Care for Patient
• Rating of Hospice
• Willingness to Recommend this Hospice
NQFs
• NQF #1617 Patients Treated with an Opioid who are Given a Bowel Regimen
• NQF #1637 Pain Assessment
• NQF #1634 Pain Screening
• NQF #1639 Dyspnea Screening
• NQF #1638 Dyspnea Treatment
• NQF #1641 Treatment Preferences
• NQF #1647 Beliefs/Values Addressed (if desired by the patient)
Survey Forms to Review During the Pre-Survey
Hospice survey-related forms are available on the internet and designed to capture information
for the current survey as well as information needed for other Medicare administrative purposes.
These forms from the most recently completed survey include:
•
Quality of Care: This section is intended to assess the extent to which the hospice visits
patients in the last three days of life and how the hospice scores on the seven Hospice Item
Set measures from the CMS Hospice Quality Reporting Program.
• CMS-417 – Hospice Request for Certification in the Medicare Program
• CMS-643 – Hospice Survey and Deficiencies Report
• CMS-2567 – Statement of Deficiency (and CMS-807-Surveyor Notes Worksheet if
available)
Form CMS-417 provides basic information about the hospice, which is necessary to schedule a
survey, including type of hospice, control, location, staffing, and services provided. Review of
this form pre-survey allows the surveyor to plan how they will conduct the survey. Once onsite,
the hospice completes a new Form CMS-417 and gives it to the surveyors at the start of the
survey. The FTE (full time equivalent) requirement for each staff type, is the total number of
hours for the category (including employees and volunteers), divided by 2,080 hours.
Form CMS-643 collects information about the survey, and additional hospice characteristics
that will assist in planning for the survey. Review the form to ascertain:
• If an inpatient hospice was reviewed for requirements under §418.110, (hospices that
provide inpatient care directly);
• The number of home visits and the number of records reviewed in the most recent
recertification survey;
• If the hospice has a waiver for core services;
• The type of setting(s) in which the hospice provides routine home care; and
• The number of multiple locations that the surveyors will attempt to visit, including
determining the potential number of home visits.
Form CMS-2567, Statement of Deficiencies and Plan of Correction is the primary
documentation of the survey results, delineating findings of non-compliance. Surveyors should
review CMS-2567s from the most recent standard recertification survey, as well as complaint
investigations, for repeated deficiencies, condition-level findings, and immediate jeopardy
situations.
Task 2 – Entrance Conference
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives
The objectives of this task are, generally, to inform the hospice administrator or designee of the
survey activities that will take place and request specific information needed to conduct the
survey. Surveyors must be professional—organized, prepared, and courteous. The entrance
conference should be informative, concise, and brief. SOM, Chapter 2 at section 2833C.2a,
addresses more detail on the entrance protocol.
Surveyors should investigate discrepancies in information obtained during the entrance
conference that are uncovered through observation, interviews with key staff, and a review of
source documents.
General Procedures
Arrival
For surveys requiring more than one surveyor, the entire survey team should enter the hospice
together. Upon arrival, the surveyor(s) must present identification. If the hospice denies
entrance to the facility or otherwise tries to limit required survey activities, explain that this may
be grounds for OIG to exclude the hospice from participation in Medicare. (See 42 CFR
§ 1001.1301.) Denying entrance to the facility (42 CFR § 489.53(a)(18)) and refusing to permit
copying of any records or information during the survey (42 CFR § (a)(13)), may also be
grounds for CMS to terminate the hospice’s provider agreement.
If the surveyor(s) encounter any problems on-site, they should feel free to contact the SA
manager or the CMS Location for guidance. For instance, if hospice staff will not let a surveyor
into the facility even after they’re informed of the possible consequences that may result for
restricting access to their facility, a call to the SA or CMS Location would be appropriate.
The surveyor, or team coordinator, when the team has more than one surveyor, announces to the
hospice’s administrator (or person in charge) that they are there to conduct a survey. If the
administrator (or person in charge) is not on-site or readily available, the surveyor or team
coordinator asks that the administrator be notified that a Federal survey is underway. Do not
delay the survey because the administrator is not available.
Entrance Conference
During the entrance conference:
• Introduce the survey team and their roles if there are multiple members of the team, and
explain the survey process, including the estimated duration of the survey, and visits to
multiple locations (if there are any).
• Request assistance with the following from the administrator:
• A private space for the survey team to work;
• Location of a copier and operation instructions;
• Identify and assign hospice staff who:
• Will be a resource to respond to the surveyor’s questions and who can obtain additional
information for the surveyor;
• Are most knowledgeable about clinical supervision, in-service training, and hospice aide
supervision;
• Can respond to any questions or assist the surveyor as needed in accessing the electronic
health record (EHR) in a timely fashion
• Orientation to the electronic and/or paper clinical records that includes the comprehensive
assessment, the plan of care, physician’s orders, progress notes and home visits,
supervisory visits, IDG meeting minutes, medication lists, medication administration
records;
• Computer terminals where the surveyors may access all patients’ EHRs.
• Request the following patient information:
• The number of unduplicated admissions for the entire hospice (all payer sources, parent,
and all multiple locations) during the last 12 months;
• A complete list of current patients (all payer sources and multiple locations), including, at
a minimum, the following information for each patient:
• Patient names;
• Date of hospice benefit election;
• Terminal diagnosis;
• Location of care—home, including assisted living facility (ALF), SNF/NF, or ICF/IID),
or inpatient facility on a short-term basis; and
• Current level of care (routine or continuous home care, general inpatient care, or respite);
• Request the schedule of home visits scheduled during the survey for all locations,
including parent and the multiple locations;
• Lists of patients who, in the last 12 months;
• Revoked the hospice benefit (live discharges);
• Died while receiving hospice care (and provide access to bereavement records for those
patients);
• Request the following agency information:
• A Form CMS-417, Hospice Request for Certification in the Medicare Program and CMS-
643, Hospice Survey and Deficiencies Report, to be completed by the hospice within an
hour of the entrance conference;
• A list of all multiple locations (including addresses) that the hospice operates under the
CCN;
• If the hospice has an inpatient facility;
• Interdisciplinary Group (IDG) meeting schedule and location;
• Location of IDG minutes;
• Documentation of grievances/complaints, including complaint logs and investigations
with their outcomes during the past 12 months;
• A copy of the hospice’s charter and organizational chart;
• Information regarding how the hospice provides 24-hour services;
• Personnel documents:
• Comprehensive current personnel list to include the medical director(s), volunteers, and
all staff under contract or arrangement including names and titles;
• The identity of, and governing body authorization for, the person who is authorized in
writing to act on behalf of the administrator;
• Staffing schedules for the week of survey in order for surveyors to plan their staff
interviews;

• A list of RN coordinators who are responsible for the coordination of care and
implementation of the interdisciplinary plan of care;
• Names of key staff and persons most knowledgeable about the hospice aides,
homemakers, volunteer coordination, pastoral services, infection control, quality
assessment and performance improvement (QAPI), in-service training, clinical
supervision, bereavement;
• Documentation of hospice aide training and/or competency evaluations and in-service
training;
• If a core nursing services waiver has been granted, and the date of the waiver;
• If a waiver of requirements for physical therapy, occupational therapy, speech-language
pathology and dietary counseling services has been granted, and date of the waiver;
• List of contracts/agreements as applicable (e.g., SNF/NF, DME, pharmacy, inpatient
facilities);
• Written agreements with all long-term care facilities (nursing homes, ICF/IIDs) where
the hospice is currently treating patients;
• The Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver for
the agency and CLIA licenses for clinical laboratories where the agency sends
specimens;
• The emergency preparedness plan (to include documented exercises or records);
• Information given to the patient on admission to hospice;
• Policies and training documentation on the prevention of abuse, neglect, and patient
harm; and
• The Quality Assessment and Program Improvement (QAPI) program activities and
performance improvement projects including infection control.
• Policies and procedures related to:
• Advanced directives
• Plan of Care
• IDG Coordination of services
• Infection control
• Training
• Clinical records
• Management and disposal of controlled drugs
• Use and maintenance of equipment and supplies
• Pain and symptom management
• Emergency preparedness
Determine if the hospice is providing laboratory testing as set forth at 42 CFR Part 493. If the
hospice is performing testing, request to see the Clinical Laboratory Improvement Amendments
(CLIA) certificate for the level of testing being performed, i.e., a certificate of waiver, certificate
for provider-performed microscopy procedures, certificate of accreditation, certificate of
registration, or certificate of compliance (issued upon the determination of compliance after an
on-site survey).
• Short-term inpatient care documentation
• Surveyors should review information documenting how and where the hospice provides general
inpatient short-term care—under arrangement or, directly, including addresses of all locations
and the written agreements.
• If the hospice provides inpatient care directly, request the following information:
• The current active inpatient census and the level of care they are receiving, i.e., GIP, respite
care including:
• Date of admission
• Diagnosis
• Reason for admission
• The last 30 days of inpatient admissions and reason for admission (GIP, respite) including:
• Date of admission
• Diagnosis
• Reason for admission
• Date of Discharge
• The working schedules for licensed and registered nursing staff for the last 30 days.
• The visitor policy.
• Information about the facility’s emergency water source (verbal confirmation is acceptable).
• A copy of an updated facility floor plan.
• Schedule of mealtimes, locations of dining room(s).
• Location of medication storage rooms and medication carts (if any), and medication
administration times.
• List of IDG personnel location and phone numbers.
• List of patients who were placed in restraints or seclusion in the past 12 months.
• Restraint/seclusion policy and procedures.
• Access to all resident electronic health records – do not exclude any information that should
be a part of the resident’s medical record. Provide specific information on how surveyors
can access the EHRs outside of the conference room.
• If the inpatient entrance interview was not conducted in the inpatient facility, confirm
that the above information was obtained.
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives
The sampling of records and selection of patients for hospice home visits is multilayered to
capture the variety of settings where patients receive care. Sample selection identifies a
representative sample, including levels of services from all operating locations (parent and

multiple locations) and in all patient care settings. Selection should be random and from
complete lists, where all cases of the described list are included.
Sample Representativeness
Patient Care Setting
In order to evaluate the care and services provided by a hospice, the survey sample must include
patients receiving care in each setting (patient care setting, or PCS) to the extent possible. The
sample must include patients who received care in the following settings, when applicable:
• Private home;
• Long term care facilities, including SNFs, NFs, and ICF/IIDs;
• Inpatient hospice facilities;
• Hospitals and long-term acute care hospitals; and
• Assisted living facilities.
Hospice Parent and Multiple Locations
Hospices also provide care from multiple locations associated with the “parent” agency under a
single provider number. The sample must include at least one record from each multiple
location. Surveyors must make an effort to conduct a home visit to patients from each hospice
multiple location. If this is not feasible, review at least one record (active or closed) from the
parent and each multiple location. It may be necessary to increase the sample size to include at
least one record review of each multiple location.
Level of Care
Three of the four levels of care are included in the sampling strategy—routine home care,
continuous home care and general inpatient care.
• Routine home care (RHC)—RHC is the usual care provided based on the plan of care,
consisting of periodic nursing and aide visits as well as other services.
• Continuous home care (CHC)—CHC is a benefit that enables a hospice to provide a higher
level of care during periods of brief crisis, consisting predominantly of nursing care to
achieve palliation or management of acute medical symptoms, to maintain the beneficiary at
home to avoid inpatient care.
• CHC must total a minimum of eight hours or more of care within a 24-hour period.
• General inpatient care (GIP—Care for symptom management and pain control);
• If, in the judgment of the hospice interdisciplinary team, which includes the hospice
physician, management of the patient’s symptoms are not effective at home, then the
patient is eligible for general inpatient care (GIP), a more medically intense level of care.
• GIP must be provided in a Medicare certified hospice inpatient facility, SNF, or hospital
that meets the 24-hour nursing requirement.
• GIP ensures intensive management of new or worsening symptoms, so that the beneficiary
can return to his or her home.

• Limited, short-term, intermittent, inpatient respite care (IRC)—Care provided because of the
absence or need for relief of the family or other caregivers. Respite care must be provided in
one of the following settings:
• Medicare-certified hospice that provides inpatient care directly;
• Medicare-certified hospital; or
• SNF that meets §418.110(b) and (e) regarding 24-hour nursing and patient areas.
Diagnoses and Services
Include patients who have a wide range of terminal diagnoses, as well as those patients who
receive clinically complex services or treatments.
Include a variety of terminal diagnoses in the sample to assess the care and services provided to
patients with a variety of diagnoses, including but not limited to:
• Dementia
• Circulatory/Heart
• Cancer
• Respiratory
• Stroke
• Chronic Kidney Disease
Use the following criteria for the active patient sample selection for both record review only as
well as home visits to include patients who receive clinically complex services or treatments:
• Infusion therapies including infusion pumps delivering patient controlled analgesia;
• Wound and ulcer care, including negative pressure wound therapy;
• Dementia care;
• Complex pain and symptom management unique to hospice patients, such as intractable
nausea, pain, anxiety/agitation;
Documents and Information Used for Sample Selection
The following documents identify opportunities to select a representative sample:
1. Confirmed list of all multiple locations (refer to the Form CMS-417);
2. Number of unduplicated admissions for the entire hospice (parent and all multiple locations)
during the recent 12-month period, all payer sources;
3. The following patient lists:
1. Active patients, from all payer sources, including the parent hospice and all multiple
locations containing, at a minimum, the following information for each patient:
• Patient name;
• Date of hospice benefit election;
• Terminal diagnosis;
• Location of the patient, i.e., receiving hospice care at home (e.g., assisted living facility), in
an inpatient facility, SNF/NF, ICF/IID or other facility;

• Core services (physician services, nursing services, medical social services, and counseling
services (bereavement, dietary, and spiritual));
• Non-core services (physical and occupational therapy, or speech language pathology)
b. Patients currently on a short-term inpatient stay for pain or symptom management;
c. Patients who received respite care in the past year;
d. Patients who received continuous home care in the past year;
e. Patients who revoked the hospice benefit (live discharges from hospice);
f. Deceased patients (last 12 months); and
A list of family members currently receiving bereavement counseling.
Patient Sample Size and Criteria
Survey Sample (Table 1) determines the minimum sample size based on five categories: 1) size
of the hospice; 2) closed record reviews of patients who revoked the hospice benefit; 3) closed
records for bereavement; 4) current patient home visit with record review, and 5) current patient
record review only.
Surveyors can expand the sample, during the survey, to investigate findings as needed. (The
sample for an inpatient hospice survey is in this document, under the description of §418.110, at
Table 2. Inpatient Hospice Sample.)
Table 1. Survey Sample Table
Number of
Admissions
(Past 12
Months)
Closed
Records
(Live
Discharges)
Closed
Records
(Bereaveme
nt Records)
Record
Review—No
Home Visit
(RR-NHV)
Record
Review
with Home
Visit
(RR-HV)
Total
Minimu
m Sample
Inclusion of
Records from
Multiple-
Location(s)
< 150
2
2
7
3
14
The number of
records from each
multiple location
should be
proportionate.
Include at least one
RR-NHV or RR-HV
from each location
1
150-750
2
3
10
4
19
751-1250
2
3
12
6
23
1251 or
more
3
4
14
6
27
Selection Criteria for Record Review
The sample consists of both closed records (former patients) and active (current) patients, for all
payer sources. The active patient sample is comprised of two groups: record review only and
record review with home visit. The closed record review includes patients who revoked the
hospice benefit (withdrew from hospice care, also referred to as live discharges), as well as post-
death bereavement counseling records.
1
Example. For hospices with < 150 admissions. if there are three locations and 50% of patients are from location A, 25% from
location B, and 25% from location B, then, from the total minimum number of 14 records, 7 records should come from location
A, 3-4 records from location B and 3-4 records from location C. If there is a large number of multiple locations, the surveyor
should distribute the total minimum sample across the locations as most feasible.

Record Review with a Home Visit (RR-HV)—Surveyors may conduct home visits to any patient
of the hospice who gives their permission for the surveyor to observe care and services. The
home visit sample should represent the variety of services that the hospice provides. Surveyors
conduct home visits to patients served by each multiple location.
1
Example. For hospices with < 150 admissions. if there are three locations and
50% of patients are from location A, 25% from location B, and 25% from
location B, then, from the total minimum number of 14 records, 7 records
should come from location A, 3-4 records from location B and 3-4 records
from location C. If there is a large number of multiple locations, the surveyor
should distribute the total minimum sample across the locations as most
feasible.
1. The surveyor must select the patient records for review and patients who will receive a
home visit. If feasible, a survey team member should contact the patient/family directly
to ensure that the family understands the reason for the visit.
a) Use the number of admissions in the last 12 months (Table 1) to determine the
number RR-HV. Select a few more patients than required to accommodate
possible refusals or other unanticipated conflicts. Provide the hospice with the
home visit sample as soon as possible so that securing consent starts early in the
process.
b) Conducting additional home visits to address concerns identified during the survey
findings are encouraged, as needed.
c) If a sufficient number of home visits are not available (low census) to meet the
sample requirement, the surveyor may first, substitute record reviews of current
patients, then closed records, to meet the minimum sample size.
2. Record Review—No Home Visit (RR-NHV)—Use the same criteria for RR-NHV as
used for the RR-HV sample. Expand the sample as needed to ensure that at least one
patient from each operating location is included.
3. Closed Record Sample Selection—Use the revocation list (live discharges) and list of
deceased patients whose family members currently receive bereavement counseling to select
the closed records for review. Again, include multiple locations if possible.
Task 4 – Information Gathering – Phase 1 & Phase 2
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives

Information gathering is a systematic process to assess the hospice’s compliance with CoPs,
consistently and accurately. During a hospice survey, surveyors gather information using a set of
procedures, common across provider types, including observations, interviews, and record
reviews. Surveyors gather information through home or facility visit observations, interviews
with patients, caregivers and families, hospice caregiving personnel including the IDG, as well as
reviews of clinical records and other hospice documents, such as relevant policies and
procedures. Surveyors validate all findings with additional hospice document review and/or
interviews with the hospice staff and administration. Check specific patient/family complaints
concerning the hospice’s delivery of items and services with the hospice to be sure that there are
no misunderstandings and that the patient’s plan of care implementation is as stated in the record.
Clinical Record and Other Hospice Documentation Review
Using the complaint log, requested during the Entrance Conference, verify that the hospice is
tracking complaints and review the documentation of complaints made by patients or patients’
families for the previous 12 months, to determine how the hospice received, recorded,
investigated, and resolved these complaints.
When surveyors identify concerns that indicate actual or potential findings of noncompliance,
surveyors should review additional documentation, as needed, to assist in making a compliance
determination. A few non-clinical documents are required in conjunction with specific CoP
guidance. However, not all non-clinical documents are routinely reviewed unless the surveyor(s)
identifies(y) concerns during interviews, home visits, and clinical record reviews, in which case
surveyors may review additional non-clinical documents such as service contracts, clinical
practice guidelines, CLIA waiver, and/or other materials.
The clinical record is the enduring evidence of care for patients and families. A review of the
record assures the surveyor that the hospice provides services in compliance with the plan of care
and CoPs.
If time permits, the surveyor should review the clinical record prior to the home visit to be
prepared to observe care and services (e.g., the most current plan of care, medication list, and
aide instructions). After the home visit, review the record in more detail to address concerns
identified during the home visit. Give special attention to the quality of care and coordination of
care, based on a person and family-centered plan of care with individualized goals of care.
Detailed guidance for clinical record reviews is presented in this Appendix, under the description
of §418.104 -Clinical Records.
Closed Record Review
A review will be conducted of closed clinical records for patients who are no longer in hospice
care due to death, revocation of the hospice benefit or transfer (live discharges). After the death
of the patient, the focus of the review is on care provided in the final days of life, post-death
bereavement counseling and services for the family and caregivers. For live discharges, explore
the circumstances leading to the cessation of hospice services.

1. Post-Death Bereavement Counseling and Services- While bereavement and grief assessment
should begin with the initial and comprehensive assessments, and be continually integrated
into the plan of care, closed records are reviewed to verify bereavement services provided to
family/caregivers, following the death of the patient. A plan of care should reflect periodic
and ongoing monitoring of bereavement/grief support for family and caregivers, similar to
that used for pain and symptom management for the patient before their death.
Determine if:
• Family and caregivers were offered and furnished (if desired) bereavement services
counseling, for up to one year following the death of the patient, using an established plan of
care, under the supervision of a qualified professional with experience or education in grief
or loss.
• Bereavement plan of care contains the type and frequency of services offered.
• Bereavement services met the needs of the bereaved (i.e., needs assessment, scope of
services, plan of care, etc.).
• Hospice evaluates the outcomes and effectiveness of the bereavement services provided.
2. Appropriate care- During the last few days before death, patients experience many physical
and emotional symptoms, requiring close care and attention from the interdisciplinary
hospice team. Review the record to confirm that the agency was responsive and available to
provide care and services in the last three days of life. Examples of situations that would
require more investigation would be: 1) no visits made in the last week of life; 2) missed
visits in the last week of life; 3) pain and symptom management not addressed; or 4) hospice
not addressing patient’s or caregivers’ concerns. Consider citing findings with non-
compliance regarding care in the last three days of life, under §418.52 or §418.56.
3. Live Discharge- The hospice benefit is for individuals who are considered terminally ill,
where life expectancy is not six months or less if the illness runs its normal course. There are
situations where discharge occurs while the patient is still living. Common reasons for live
discharge are both beneficiary and provider driven.
An unusually high rate of live discharges could indicate that a hospice provider is not
meeting the needs of patients and families or is admitting patients who do not meet the
eligibility criteria. Review the record to detect whether or not the patient or the hospice
initiated the hospice discharge, looking for any indication of potentially inappropriate
discharge, (e.g., discharge during a weekend or holiday, discharge associated with ER use or
hospitalization).
a. Beneficiary driven live discharge:
• The beneficiary revokes the hospice benefit.
• The patient moves out of the hospice’s service area or transfers to another hospice.
b. Agency driven live discharge:
• The hospice determines that the patient is no longer terminally ill;
• Discharge for cause: The hospice discharges the beneficiary citing that the behavior of the
patient, or other persons in the patient’s home, is disruptive, abusive, or uncooperative to the
extent that delivery of care to the patient or the ability of the hospice to operate effectively is
seriously impaired. The hospice should attempt to resolve these problems satisfactorily
before it considers discharge. Review that the hospice has documented its efforts to resolve
the problem(s) in detail, in the patient’s clinical record.
Closed records reviews determine whether the record includes:
• A signed statement that the individual revokes the election for Medicare coverage of hospice
care for the remainder of that election period.
• The effective date of revocation is not earlier than the date the actual revocation is made.
• Indication(s) whether or not the hospice was responsive to the patients’ needs and
patient/caregiver was unhappy with hospice care.
• If the patient and family decided they were not ready for hospice care.
• Rescinding of the terminal diagnosis by the hospice physician if the patient is no longer
terminally ill.
Transitions of care due to relocation, or transitions to another provider type (hospital, nursing
home, etc.) are reviewed for presence of a discharge summary that includes:
• A summary of the patient’s stay including treatments, symptoms, and pain management;
• Patient’s current plan of care and latest physician’s orders; and
• Any other documentation that will assist in post-discharge continuity of care;
• Evidence that the hospice considered the effect on the current plan of care before discharging
the patient and responded appropriately to ensure a safe transition of care.
Interviews with Patients and Family, and Agency Staff
The objective of hospice interviews with patients, family/caregivers and staff is to further
investigate and confirm findings identified during record reviews, observations, and to clarify
other interviews. Information from interviews may lead to the review of additional records,
observations, and/or need for additional staff interviews to determine compliance with the CoPs.
Surveyors must ensure the inclusion of staff members who provide clinical care directly such as
the RN coordinator who is coordinating the care for the beneficiary as core members of the IDG,
and others identified in the plan of care. Administrative/Organizational staff are interviewed,
only as necessary, to assess facets of the hospice that impact their ability to provide a high
quality of care.

Among staff interviews, the RN whose primary function is the care of the patient and
coordination of care between the patient and the IDG, is the most critical. As established in the
42 CFR Part 418 preamble, “The unique skills of registered nurses, who are educated to assess
and manage the overall aspects of a patient’s physical and psychosocial care, can be used to
oversee the coordination and implementation of the care identified by the IDG”. This individual
may have a variety of titles such as RN coordinator, primary care nurse or case manager.
Surveyors should ensure a comprehensive interview of the RN Coordinator, as well as other
direct-care providers.
Whenever possible, ask open-ended questions to obtain detailed information regarding specific
events, how care is delivered, or if there are apparent lapses in care. For example, if concerns are
identified with the frequency of hospice aide visits or hospice aide training, ask the hospice aide
about her background or the RN coordinator about the most recent plan of care.
Patient and Family Interviews during Home Visits
The purpose of the home visit is to evaluate whether the care provided by the hospice meets the
health and safety standards of the Medicare program and to confirm that the agency protects and
promote patient’s rights, the comprehensive assessment is current and accurate, and the care
provided is consistent with the patient’s plan of care. The home visit is the only opportunity for
the surveyor to observe the direct care provided by the hospice personnel and is thus the most
important means of information gathering during the hospice survey. The surveyor uses
observational and interview skills to assess the hospice’s adherence to the requirements.
Planning the Home Visit with the Agency
After the survey team selects the sample, a member of the survey team should contact the
patient, family, or caregiver to request permission and arrange for the home visit. If the patient
or caregiver/guardian refuses to allow the surveyor to visit, the surveyor should select an
alternate patient.
Clinical records should be reviewed prior to and after the home visit. Prior to the home visit,
obtain the information relevant to the home visit, such as copies of the most current plan of care,
medication list, and aide instructions.
Conducting Home Visits and Patient Interviews
As a guest in a patient’s residence, courtesy, respect, and sensitivity to the patient’s clinical
status (physical and emotional) are necessary. Explain to the patient that the purpose of the visit
is to ensure that the care provided by the hospice meets the health and safety standards of the
Medicare program and is provided in accordance with the plan of care ordered by the patient’s
interdisciplinary group. Prior to asking the patient to sign the home visit consent, confirm with
the beneficiary that the hospice explained that the home visit and interview are voluntary, and
that refusal would not affect their benefits.

• Ask the patient or caregiver to sign a home visit consent (Exhibit 128, Model Consent for
Hospice Home Visit Form at https://www.cms.gov/Regulations-and-
Guidance/Guidance/Manuals/downloads/som107_exhibit_128.pdf).
• Provide a copy of the signed consent form to the patient, a copy to the hospice for the
patient’s clinical record and retain a copy for the survey file.
• Prior to interviewing the patient/family/caretaker, the surveyor reassures them that any
discussion is voluntary and confidential, and refusal to participate will not affect his or
her Medicare/Medicaid or other health benefits, to which they may be entitled.
Observe, but do not interfere with, the delivery of care and the interactions between the hospice
representative and the patient/family and/or caregiver. The plan of care determines the focus and
depth of questions asked of the patient and hospice staff by the surveyor. It may be appropriate
to ask questions during patient care if it does not interfere with care or disturb the rapport of the
hospice staff with the patient. The surveyor should ask the patient’s permission to review the
patient’s information packet and written information that the hospice provided to the patient at
the start of care and subsequent updates. If the patient is not able to locate the information
readily, do not press the issue with the patient and continue the visit.
The surveyor should use discretion to end the interview or home visit if the patient indicates a
desire or need to conclude the interview or home visit. The surveyor should end the interview or
home visit if the patient requests it. Observe if the patient displays reluctance to speak in front of
hospice staff, or appears fatigued or distressed, as these behaviors may indicate an unexpressed
concern. Surveyors should remain after the hospice staff leave to give the patient and family an
opportunity to share information with them confidentially. The surveyor should discontinue the
visit if conditions in the patient’s home raise concerns for the surveyor’s physical safety.
Organizing the Survey Using Hospice Core Requirements and Protocol Phases
Hospice regulations contain 23 CoPs that hospices must comply with to participate in the
Medicare programs. As a means of organizing the survey, Task 4 – Information Gathering –
Phase 1 & Phase 2, focuses on four core requirements and 19 associated CoPs. The hospice final
rule (73 FR 32088) specified a set of four CoPs as core requirements for hospice services:
§418.52 Condition of participation: Patient's rights.
§418.54 Condition of participation: Initial and comprehensive assessment of the patient.
§418.56 Condition of participation: Interdisciplinary group, care planning, and coordination of
services.
§418.58 Condition of participation: Quality assessment and performance improvement.
The survey has two phases that consist of four core requirements and associated CoPs.
• Protocol Phase 1 consists of reviewing three core CoPs and six associated CoPs related to direct
care of the patient and family, and that require home visits, observations, and interviews.

• Protocol Phase 2 consists of one core CoP and 13 associated CoPs, including administrative and
structural matters, such as review of the development and execution of the QAPI plan, review of
waivers, furnishing core and special services, etc.
Overarching these requirements is a quality assessment and performance improvement program
that builds on the philosophy that a provider’s own quality management system is key to
improved patient care performance. The objective is to achieve a balanced regulatory approach
by ensuring that a hospice furnishes health care that meets essential health and quality standards,
while ensuring that it monitors and improves its own performance.
The protocol phases are sequential. Surveyors should initially gather information for Phase I
CoPs that entail the predominant level of effort/priority, before CoPs where administrative
elements are considered in the Phase 2 CoPs. Phase 1 findings regarding direct care services can
inform Phase 2 in terms of pointing to potentially systemic issues/deficiencies.
Information gathering strategies are suggested below for home visit observation and interview,
and review of clinical records and other documents. Surveyor discretion and specific findings
ultimately determine the direction of the investigation.
Information Gathering – Survey Protocol Phase 1
Survey Protocol Phase 1 Core Requirements CoPs
§418.52 Condition of participation: Patient's rights.
§418.54 Condition of participation: Initial and comprehensive assessment of the patient.
§418.56 Condition of participation: Interdisciplinary group, care planning, and coordination of
services.
Survey Protocol Phase 1 Associated Quality of Care CoPs
§418.60 Condition of participation: Infection control.
§418.76 Condition of participation: Hospice aide and homemaker services.
§418.102 Condition of participation: Medical director.
§418.108 Condition of participation: Short-term inpatient care.
§418.110 Condition of participation: Hospices that provide inpatient care directly.
§418.112 Condition of participation: Hospices that provide hospice care to residents of a
SNF/NF or ICF/IID.

Information Gathering Survey Protocol Phase 1: Core Requirements CoPs
§418.52 Condition of participation: Patient’s rights
Ensuring that patients are aware of their rights and how to exercise them is vital to quality of care
and patient satisfaction. Hospices must inform patients of their rights and protect and promote
the exercise of these rights, e.g., by informing the patient how to exercise those rights.
A. Observation
Review documents in the home provided by the hospice to the patient if the patient (or
authorized representative) can provide them.
Determine if the hospice gave information to the patient, and if the patient understood
information about approaches to palliative care/symptom management related to the individual’s
terminal illness, and that the patient waives certain Medicare services by this election. Surveyors
are not to advise the patient about finances, or coverage, or payment issues, but rather confirm if
the hospice has provided this information.
While observing care and interactions between hospice personnel and the patient, note the
following:
1. If the patient is actively participating in his or her treatment;
2. If the patient is being treated with respect;
3. If the staff encourages the patient’s feedback; and
4. If hospice personnel are providing the care and services as specified in the plan of care.
5. Verify that agency staff maintain the confidentiality of protected health information that they
transport and use.
B. Patient/Family Interview
Assess whether the patient or caregiver(s) (if any), were informed that, as a Medicare
beneficiary, they are entitled to certain rights. Interview the patient or caregiver to determine:
1. If they received a verbal description and a copy of their rights. If the patient has difficulty
recalling information about the written notice of rights, ask if the patient kept any written
information that the hospice may have provided to them and review that material with the
patient, if the patient agrees.
2. If the patient/family know how and whom to contact if they have a complaint. Ask the
patient, the patient’s family, guardian, or other legal representative, if they have any
comments or concerns, or have registered any grievances or complaints about the hospice or
its services. If this has already occurred, ask how it was handled and what were the results or
outcomes.
3. Whether the hospice informed the beneficiary of the following patient rights in a language
and manner that the patient understands.
a. Informed the patient concerning its policies on advance directives, and provided the patient
with written information;
b. Informed the beneficiary about the scope of services that the hospice identified on the
election statement.
c. Informed patients of their specific rights to:
• Receive effective pain management and symptom control for conditions related to the
terminal illness.
• Be involved in developing the plan of care;
• Have his or her property treated with respect;
• Have the right to refuse care or treatment; probe further if a trend emerges where a
majority or all patients are refusing a particular service (e.g., social work, spiritual
counseling, volunteers, etc.,) to assure the hospice is fully prepared to provide the service
with qualified personnel;
• Choose an attending physician;
• Have a confidential clinical record;
• Be free from mistreatment, neglect, or verbal, mental, sexual, and physical abuse,
including injuries of unknown origin;
• Be free from misappropriation of property;
• Receive information about services covered by the hospice benefit;
• Receive information about the scope of services the hospice will provide and any
limitations;
• Express dissatisfaction or concerns (voice grievances) regarding treatment or care, and
not be subject to discrimination or reprisal for exercising his or her rights and if
patient/caregiver was encouraged to provide input into the plan of care and the type of
services they receive; and
• File a complaint and how to do so; ascertain that the hospice election form used by the
hospice includes the name and phone number of the appropriate Beneficiary and Family-
Centered Care Quality Organization (BFCC-QIO) and is signed by the beneficiary and/or
legal representative.
4. During the home visit, ask patient/family how quickly the hospice satisfies the patient’s
request for pain medication or symptom control, during the daytime hours, nights, and
weekends.
5. Observe the patient for any signs of discomfort. Ask the patient or family, as appropriate, if
the patient has been experiencing pain or other symptoms, and if so, did they report this to
the hospice? If reported, what was the hospice’s response?
6. Determine if there have been any instances where the hospice failed to respond promptly to
the patient’s request for pain medication or symptom management?
7. During home visits, ask the patient/family if they know how and whom to contact if they
have a complaint. Ask the patient, the patient’s family, guardian, or other legal

representative, if they have any comments or concerns, or have registered any grievances or
complaints about the hospice or its services. If this has already occurred, ask how it was
handled and what the results or outcomes were.
8. Determine if the rights of a patient adjudged incompetent or who has a representative acting
on his/her behalf are exercised by the legally appointed individual. If the hospice is currently
caring for a patient who has been adjudged incompetent, and you have questions concerning
the exercise of the patient’s rights, you may contact the patient’s legal representative about
their involvement in planning care, treatment, and services decisions. If the patient is
selected for a home visit, obtain the legal representative’s approval for the visit.
9. If the patient is informed about the services they are receiving and when they will receive
them, for example, who is scheduled to visit, how often and for how long;
10. If the hospice informed them of any uncovered services by Medicare and if so, and options to
address them. If a notice of Medicare non-coverage was provided to the patient, confirm that
it was received prior to the care being provided.
11. How often the patient/caregiver feels that the hospice team listens carefully when discussing
problems with hospice care?
12. Was the patient advised that they could keep their own physician when hospice was elected?
C. Interview key staff
1. Ask about the hospice’s system of documentation and retrieval of patient specific data
elements.
2. Ask to see a copy of the data elements that comprise the hospice’s comprehensive
assessment.
3. Have the hospice explain how they use these data elements in care planning, coordination of
services and in their quality assessment and performance improvement (QAPI) program.
4. Ask the hospice to describe its policy for assessing, managing, and reassessing pain and other
symptoms, and how it defines effective pain management and symptom control.
5. Determine how the hospice assures that the patient receives the needed medications in a
timely fashion.
D. Clinical Record Review
The clinical record review aids in reconciling concerns identified
during the patient/family interview or follow-up for unverified concerns, during the interview.
Clinical record review should confirm that the hospice provided the required written notice of
patient rights to the patient. Details of the clinical record review are found below at §418.104.
E. Management of Complaints and Alleged Abuse
Obtain the complaint log (requested during the Entrance Interview) to verify that the hospice is
tracking complaints from receipt of complaint through resolution.
Review the documentation of complaints made by patients or patients’ families for the previous
12 months to determine how the hospice received, recorded, investigated, and resolved these

complaints. Is there evidence that the hospice staff is aware of and follows the hospice’s policy
for complaint investigation when a patient/family makes a complaint to a staff member? Pay
close attention to staff remarks and staff behavior that may represent deliberate actions to
promote or to limit a patient’s autonomy or choice. Who in the hospice is ultimately accountable
for receiving, investigating, and resolving any patient concerns or problems that cannot be
resolved at the staff level?
Interview with administrator/staff regarding patient abuse and neglect policies.
The hospice must ensure that all hospice employees and contracted staff are trained on how and
when to report allegations involving mistreatment, neglect, or verbal, mental, sexual, and
physical abuse by anyone furnishing services on behalf of the hospice. This includes reporting
injuries of unknown origin, as well as misappropriation of patient property. Determine how the
agency complies with these requirements:
• Is there evidence that the hospice staff is aware of and follows the hospice’s policy for
complaint investigation when a patient/family makes a complaint to a staff member?
• Pay close attention to staff remarks and staff behavior that may represent deliberate actions to
promote or to limit a patient’s autonomy or choice.
• Who in the hospice is ultimately accountable for receiving, investigating, and resolving any
patient concerns or problems that cannot be resolved at the staff level?
2. Discovery of Current Abuse or Neglect
If, during a survey, you identify the possibility of mistreatment, neglect, abuse, or injuries of
unknown source or misappropriation of patient property, investigate the circumstances. The
surveyor will review and confirm that the hospice (including its contracted suppliers that have
patient contact), and its employees, identified and reported violations to appropriate authorities in
accordance with applicable state and Federal requirements under Medicare or Medicaid.
States commonly have mandatory reporting requirements for providers, suppliers, and
individuals making them legally responsible to report suspicions of abuse and neglect to
appropriate State authorities. These entities and individuals should follow existing mandatory
reporting requirements in their State in addition to any applicable Federal requirements. Action
or inaction on the part of a provider or supplier to follow mandatory reporting requirements does
not preclude an employee from fulfilling their reporting obligations.
If a report was made to a State or local body (including the State survey and certification agency
or law enforcement), the surveyor should request a copy of the report and determine whether the
report was made within five days of the incident.
For suspected violations discovered during the survey, for which the surveyor cannot verify that
a report was made to the appropriate authorities or law enforcement, the surveyor must consult
with his/her supervisor immediately. The SA should report the suspected violations, including
abuse and neglect, to the appropriate authorities or law enforcement immediately.

§418.54 Condition of participation: Initial and comprehensive assessment of the patient
Accurate and timely patient assessments are crucial to the development of an effective plan of
care that addresses the physical, psychosocial, emotional, and spiritual needs associated with the
patient’s terminal illness to promote well-being, comfort, and dignity throughout the dying
process.
Home Visit Observations
Verify if the current comprehensive assessment and plan of care were completed and updated
timely and accurately reflect the patient’s status. Review the prescriptions and over-the-counter
medications, herbal remedies, and other alternative treatments with the patient or caregiver and
compare your findings with the drug profile in the patient’s plan of care.
Patient/Family Interview
• Identify what grief assessments, surveys, questionnaires the provider uses to screen/identify
bereavement needs of the patient and family/caregiver;
• Ask the patient/family if they were involved in identifying goals of care;
• Identify how follow-up is conducted including frequency and method (phone, in-person,
email/mail);
• Review other resources (e.g., organizations, group therapy, programs etc.) that are provided
to patient family/caregiver;
• Ask how the hospice determines the need to refer a patient or family member(s) to
appropriate health professionals for further evaluation.
Clinical Record Review
• Timing of the assessment: Determine if the initial assessment took place within the required
timeframes.
• Confirm that the initial assessment took place within the required period to identify the
immediate care and support needs of the patient. The hospice RN must conduct the initial
assessment within 48 hours, unless the physician, patient, or representative requests that the
initial assessment be completed in less than 48 hours.
• The hospice must complete the first comprehensive assessment no later than five calendar
days after the start of care.
• Confirm that the comprehensive assessment is updated by the hospice IDG in collaboration
with the patient’s attending physician, if any, as frequently as the condition of the patient
requires, but no less frequently than every 15 days.
• Is there evidence in the clinical record and during home visits that the reasons for admission,
complications and risk factors that could affect care planning, functional status, imminence
of death, and symptom severity have been identified and are being addressed?
• Review the comprehensive assessment to assure it is person-centered and individualized to
meet the needs of the unique patient.
• Does the comprehensive assessment include the patient/caregiver goals of care?

• Are the goals of care measurable/quantifiable where appropriate?
• Does the record reflect regular assessments for pain, symptom management to include
spiritual and psychosocial needs?
• Review the medications the patient indicated that they were taking against the medical record
documentation to verify that the hospice identified all medications that the patient is
currently taking, both prescription and non-prescription. (e.g., over-the-counter drugs, herbal
remedies, and other alternative treatments that could affect drug therapy). The
documentation in the clinical record should verify that the hospice nurse assessed the list of
medications for potential side effects and drug interactions. The hospice should monitor for
medication effectiveness, actual or potential medication-related effects, duplicate drug
therapy, and untoward interactions during each update to the comprehensive assessment, and
as needed as new medications are added or changed, or the patient’s condition changes.
Where indicated, note if side effect preventive measures are implemented, e.g., bowel
regimen when opioids are used, to avoid constipation. If medication concerns were
identified, confirm that the physician was notified.
Bereavement Component of the Comprehensive Assessment
An initial bereavement assessment of the needs of the patient's family and caregivers, focusing
on the social, spiritual, and cultural factors that may affect their ability to cope with the patient's
death, is required. Information gathered from the initial bereavement assessment must be
incorporated into the plan of care and considered in the bereavement plan of care.
Through record review and interview, confirm the hospice conducted an initial bereavement
assessment.
In addition to the bereavement tools used for the initial assessment and plan of care, review for
the following:
• Procedures for providing bereavement services until one year post-death
• Identification of what grief assessments, surveys, questionnaires utilized to screen
bereavement needs of the patient and family/caregiver.
• Identify mode and frequency of follow-up is conducted.
• Review other resources (e.g., organizations, group therapy, programs, etc.) provided to the
patient family/caregiver and ask how the need for referrals and further evaluation by
appropriate health professionals is determined by the hospice IDG.
Staff Interview
• Ask clinical staff to describe how they obtain all relevant information necessary to complete
the comprehensive assessment.
Providing post-death bereavement services is further discussed under “Closed Record Review,”
under Task 4.

§418.56 Condition of participation: Interdisciplinary group, care planning, and
coordination of services
Once a terminally ill patient elects to receive hospice care, a hospice interdisciplinary group
(IDG) must direct, coordinate and monitor the services that are based on the ongoing
comprehensive assessment and a patient-specific, individualized plan of care. Due to the number
of settings where hospice care is provided, the coordination of care is especially significant in
providing consistent and responsive care to a patient who is at the end of life.
This interdisciplinary care model requires frequent communication between disciplines of care
and patient settings, as well as between the hospice, the patient, and the family to formulate an
effective plan of care that is continually monitored by the IDG. There should be a continuous
feedback loop between the needs identified in the comprehensive assessment and an updated
individualized plan of care. The RN, who is a member of the IDG, monitors the effectiveness of
the plan of care and serves as the liaison between the patient and the IDG. The 2008 hospice rule
(73 FR 32088 (June 5, 2008) provides that the hospice must designate an RN that is a member of
the IDG to provide coordination of care (418.56(a)(1), but a given hospice may identify this RN
by a different title like case manager. The essential element is that he/she is the RN providing
direct patient care and is a member of the IDG.
Home Visit Observations
Verify that the care provided during observation is consistent with the plan of care. Examples
include:
• Observing treatments to confirm if the care is provided according to the current plan of care;
• Determining the patient’s understanding of the purpose of the hospice services, and if they
had input into setting the goals or objectives that were established for their care;
• Determining if written instructions were provided to the patient or caregiver;
• If education was conducted, did the hospice staff provide education and training to the patient
and any caregivers, when appropriate, and according to the plan of care?
• Whether a pain assessment is included in the plan of care and is completed as indicated;
• If equipment, supplies, and assistive devices were indicated in the plan of care, determining if
the patient received the items timely; and
• Investigating medication discrepancies in the comprehensive assessment, the plan of care, and
the written information to the patient.
Patient/Caregiver Interview
Consider the following interview questions:
• What are the purposes of the hospice services you are receiving?
• What is your experience with contacting the hospice team during evenings, weekends, or
holidays with questions or concerns?

• How often do you get the help you need from the hospice team during evenings, weekends,
or holidays?
• Do you receive the care and support that you need to manage your illness?
• When you call with an urgent need, how long does it take for someone from the hospice team
to respond?
• How does the hospice team keep you informed about when they will arrive to care for you?
• When there is an unexpected delay or re-scheduling of a visit, how does the hospice notify
you? How often do either of these situations occur?
• Are you aware of the IDG?
• (When applicable) Does the hospice team give you the training you need about if and when
to take more pain medicine?
• (For dementia terminal diagnosis) How has the hospice educated you on the death and dying
process of a patient with dementia?
• How much support for your religious and spiritual beliefs do you get from the hospice team
if you have indicated that you wanted that?
IDG Meeting Observation
Schedule at least one IDG meeting and observe for the following:
• Are all relevant core services staff present (remote or in person)?
• Are other hospice staff members involved in the patient’s care participating?
• Are family/caregivers encouraged to attend?
• Are the goals of care being reviewed revised as needed?
• Is the plan of care being reviewed and revised as needed?
• How are documented missed visits being addressed?
Hospice Staff Interview
Ask clinical staff to describe their process/policy of drug regimen/medication review including:
• What process is followed when a patient/family is found not to be following the patient’s
drug/medication regimen?
• What non-pharmacological methods are considered to relieve pain and other symptoms?
• How are patients and families educated about effective pain and symptom management?
• What process does the hospice utilize to assess and measure pain and other uncomfortable
symptoms?
• How does the hospice monitor a patient when they begin a new medication,
increase/decrease a dosage, or discontinue a medication?
(Also see Interviews with Patients and Family, and Agency Staff regarding the RN Coordinator
Interview, under this task.)
Clinical Record Review

• Verify that the plan of care is established timely and updated as necessary, that the patient is
informed of changes, and that the hospice provides all of the care and services that are
identified in the plan.
• Track how the plan is implemented and monitored by the hospice interdisciplinary team.
• Verify that the hospice began services as ordered, within the specified time frame, and at the
frequency ordered.
• There should be evidence in the clinical record and on home visits that the hospice treats
patient’s symptoms such as pain, nausea, vomiting, dehydration, constipation, dyspnea,
emotional distress, insomnia, neuropsychiatric symptoms, and spiritual needs using accepted
professional standards of practice.
• If a surveyor detects a pattern of missed visits by any discipline, determine how the IDG and
RN coordinator are tracking these missed visits. How are missed visits communicated to the
patient/caregiver? Is there evidence that visit frequencies are reduced due to staffing
shortages? If the missed visits occur on weekends and holidays, conduct additional
interviews with the RN coordinator and administrator to determine if the hospice employs
sufficient staff to meet patients’ needs.
• Verify that the clinical record reflects ongoing pain/symptom assessment using
measurable/quantifiable tools to provide relevant data for the IDG to assess if goals of care
are being appropriately addressed.
• Verify that patient-specific measurable goals and instructions for care are tailored to the
individual patient.
• Verify that the patient (representative or caregiver as appropriate) is receiving education and
training as planned, and patient comprehension is documented.
• How does the plan of care reflect the patient and family-specific needs of the patient based
on the terminal diagnosis?
• Does the patient receive the appropriate level of care, for example, does the hospice offer
continuous home care for symptom management when indicated?
Information Gathering Survey Protocol Phase 1: Associated Quality of Care CoPs
§418.60 Condition of participation: Infection control
Home Visit Observations
Infection control practices by the hospice staff are observed during home visits and inpatient care
observations. Observe hand hygiene and wound care to see how clean/ sterile wound supplies
are stored/ protected in the home and during transport by staff, and how soiled/ contaminated
dressings are handled by hospice staff.
Observe for adherence to standard precautions, which apply to all patient care, regardless of the
patient’s suspected or confirmed infectious state. These practices protect healthcare personnel
and prevent healthcare personnel or the environment from transmitting infections to patients.
Hospices typically provide an agency-specific policy and procedure for a “bag technique” to
describe the management of patient care equipment and supplies that are transported into patient
homes.

Infection control patient/caregiver education may be provided by the hospice during the visit
(when indicated) or may have been addressed during prior treatments. When provided in prior
treatments, verify the education is documented in the record. Observe that the hospice staff
follow accepted standards of practice to prevent the transmission of infections and
communicable diseases, including the use of standard precautions during the provision of care
(see also Interpretive Guidance at tag L582).
Core Infection Prevention and Control Practices
For example, the six (6) core practices described below are based on the Center for Disease
Control and Prevention’s (CDC), “Core Infection Prevention and Control Practices for Safe
Healthcare Delivery in All Settings –Recommendations of The Healthcare Infection Control
Practices Advisory Committee (HICPAC)” published in 2016 and periodically updated. These
recommendations are a core set of infection prevention and control practices that are
recommended in all healthcare settings, regardless of the type of healthcare provided. Also, refer
to “Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care”
published by the National Center for Emerging and Zoonotic Infectious Diseases Division of
Healthcare Quality Promotion, Version 2.3.
1. Hand Hygiene adherence: Surveyors are advised to review the most current Center for
Disease Control’s hand hygiene recommendations for correct procedures.
2. Environmental Cleaning and Disinfection: The hospice staff have little control over the
home environment but must protect their equipment and supplies from potential contamination
during the home visit. Examples of how this might be accomplished include, but are not limited
to:
• Cleaning and disinfecting; and
• Placing a clean barrier on the surface in the home where clean equipment will be placed
and/or preparation of injectable medications will be performed.
3. Injection and Medication Safety: During direct care observation, note whether:
• Caregivers/Staff use aseptic techniques to avoid contamination of sterile medications and
injection equipment. This includes preparing injectable medications on a clean surface away
from potential sources of contamination, such as sinks;
• Caregivers/Staff do not reuse single-use equipment (e.g., needles, lancets, syringes, IV
tubing) either for more than one patient or repeated use on an individual patient;
• Single-dose or single-use vials for parenteral medications are used whenever possible;
• Medication from a single-dose or single-use container (e.g., vial, ampule, bag) is only
administered to a single patient;
• Contents from opened single-dose or single-use containers are not stored for future use on the
same patient;
• If multi-dose vials are used, they are dedicated for single-patient use whenever possible. If
multi-dose vials are used for more than one patient, they do not enter the patient treatment

area (e.g., home). If they enter a patient treatment area, they should be dedicated for single-
patient use only.
• Insulin pens are dedicated for a single patient and never shared even if the needle is changed;
• Sharps disposal complies with applicable state and local laws and regulations.
4. Appropriate Use of Personal Protective Equipment (PPE) based on current HHS and CDC
guidelines for prevailing situations:
• Examples of PPE include gloves, gowns, face protection (facemask and goggles, or face
shields).
• The selection of PPE is determined by:
• The type of exposure anticipated, i.e., splash/spray versus touch;
• Category of isolation precautions;
• Durability and appropriateness for the task;
• Fit.
5. Minimizing Potential Exposures:
• Staff should be prudent in not working or entering a patient’s home when the staff have a
respiratory infection or other communicable infection;
• Staff should properly handle, transport, and store any healthcare related items (e.g.,
medications, fluids, specimens, and body fluids) using methods that promote safety and
prevent the spread of pathogens, including but not limited to the following methods:
• Maintain the appropriate environmental temperature of medication and specimens
during transport;
• Transport urine specimens in a protective container to prevent spillage; and
• Transport blood specimens in a protective container to prevent breakage of tubes.
6. Reprocessing, storage, transport, and usage/operation of equipment or devices used for
patient care:
• Staff follows the manufacturer’s instructions for reprocessing (i.e., cleaning and disinfection
or cleaning and sterilization) and uses current standards of practice for transport and storage
of patient care equipment;
• Single-use equipment is discarded after use;
• Reusable medical equipment (e.g., blood glucose meters and other point-of-care meters,
blood pressure cuffs, oximeter probes) are reprocessed prior to use on another patient and
when soiled;
• Staff maintains separation between clean and soiled equipment to prevent cross-
contamination in the patient care environment and during transport.
Patient/Family Interview

If infection control education has been provided to the patient in prior treatments, inquire with
the patient regarding the information to assess their knowledge and recall of the information.
Ask the patient if hospice staff perform hand hygiene, use personal protective equipment, clean
reusable equipment, and handle/dispose of needles and sharps safely.
Clinical Record Review
Review for evidence of patient/caregiver infection control education pertinent to the patient’s
condition and per the plan of care.
Staff Interview
Ask the staff what training they received in infection control and how often they receive the
training. Training should include but not be limited to identification of infection signs and
symptoms, routes of infection transmission, and the components of standard precautions.
§418.76 Condition of participation: Hospice aide and homemaker services
Home Visit Observations
If direct care observations are made during a hospice aide visit, confirm that the hospice aide:
• Safely and effectively provides services as indicated on the plan of care and in the written
patient care instructions for the hospice aide, as devised by the IDG;
• Communicates effectively with the patient, representative (if any), caregivers, and family;
• Demonstrates competency with assigned tasks;
• Practices standard precautions when providing care;
• Observes for and reports changes in the patient’s condition;
• Honors patient rights consistent with §418.52, including respecting the patient and their
property, and ensuring patients have the opportunity to voice grievances regarding treatment
or care ; and
• Reports changes in the patient’s medical, nursing, rehabilitative, and social needs to the RN.
Patient/Family Interview
• Interview the patient to determine how satisfied she/he is with the services provided by the
aide.
• Determine if the patient is aware of the aide’s visit schedule if the visits are made as
scheduled, and if the hospice communicates any changes to that schedule in advance.
• Inquire if the patient feels that the hospice aide is respectful of her/him and their property.
Clinical Record Review
• The clinical record should indicate that hospice aides are provided with specific written
patient care instructions by a RN for services that are ordered by the physician, documented
in the plan of care, permitted under state law, and consistent with hospice aide training. The
record review should confirm these requirements are met and the instructions do not exceed
the scope of duties permitted for aides. Documentation should include evidence of the
hospice aide reporting changes in the patient’s condition to the RN when observed. Look for
hospice aide documentation that describes changes in the patient’s medical, nursing,
rehabilitative, and social needs and to whom he or she reported the information. Clinical
notations should be dated and signed.
Hospice Aide Supervisory Visits: Review supervisory visit documentation to confirm that a RN
makes an on-site supervisory visit at least every 14 days; this documentation must be present in
the clinical record. The aide does not need to be present during a supervisory visit, however, if
the RN identifies areas of concern, the clinical record should indicate that direct on-site
supervision of the aide took place during the aide’s next home visit. If the RN confirms the
concern through direct observation, the aide must complete a competency evaluation according
to the requirements at §418.76(c).
The elements of hospice aide supervision ensure that aides furnish care safely and effectively,
including, but not limited to, the following:
• Following the patient’s plan of care for completion of tasks assigned to a hospice aide;
• Creating a successful interpersonal relationship with the patient and family;
• Demonstrating competency with assigned tasks;
• Complying with infection prevention and control policies and procedures;
• Reporting changes in the patient’s condition; and
• Honoring patients’ rights.
Hospice aides play an integral role in the delivery of hospice services and have frequent and/or
prolonged encounters with patients. Their input is important for information sharing and their
participation in furnishing services should be reflected in the visit notes of the clinical record.
Hospice Aide Training: When aide services are observed during the surveyor home visit or are
included in the patient sample, review documentation of the hospice aide competency testing for
those hospice aides to confirm that it was completed. The competency evaluation consists of
those subject areas specified in §418.76(b)(3).
Ask how the hospice schedules training to assure that every aide receives at least 12 hours of in-
service training within each 12-month period.
• Review a sample of 3-4 hospice aide training files to validate that aides are receiving the
required number of training hours. If concerns arise, interview the aides regarding in-service
trainings received.
• Are aides direct employees of the hospice or provided by arrangement?

• If services are provided under arrangement, how does the hospice ensure that the aides
providing patient care have the appropriate competency skills?
Hospice Homemaker
• Interview key administrative staff regarding which member(s) of the IDG is responsible for
the coordination and supervision of homemaker services.
• Through interview, home visits, and record reviews assure that there are written instructions
for duties to be performed and that any patient and family concerns are being reported to the
homemaker services coordinator.
• The duties of the homemaker and the services performed must be documented in the clinical
record.
Hospice Documentation Review
Annual Supervisory Visit: Perform a random sample of 2 hospice aide personnel records to
confirm the supervisory direct observations are completed annually. Expand this sample if issues
are noted during clinical record reviews.
§418.102 Condition of participation: Medical director
Confirm:
• The identity of the medical director through interview and documentation and the identity of
the individual designated to serve in this capacity in his/her absence.
• That there is a medical director for the hospice, including all multiple locations.
• The medical director may work full time or part time. If the medical director is not a paid
employee or a contracted medical director, he/she is considered a volunteer under the control
of the hospice.
• All other hospice physicians function under the supervision of the medical director.
• In the absence of a medical director (e.g., illness, vacation, etc.), a physician designee (who is
a hospice employee or under contract with the hospice) should be identified. This individual
should be a doctor of medicine or osteopathy designated by the hospice who assumes the
same responsibilities and obligations as the medical director when the medical director is not
available;
• The medical director or physician designee has the responsibility for the medical component
of the hospice’s patient care program, including initial certifications and recertifications of
terminal illness.
• Primary roles of the medical director that can be reviewed through interview of the medical
director and documentation:
• Confirm that the medical director is responsible for supervising and coordinating
entities that comprise the medical component of the hospice’s patient care program.
• The medical director and/or the physician member of the IDG plays an active role in
the coordination of care and care planning for the individuals being served.
• Evidence that the medical director is the only person who admits an individual to the
hospice benefit, in consultation with the individual’s primary and/or attending
physician.
• Confirm that discharge orders are written by the medical director.
• The medical director is involved in the certification of terminal illness, which may
also be done with another hospice physician member of the IDG.
• If the medical director does not participate in every IDG meeting, confirm that each
interdisciplinary team has a physician member to provide direction for medical care.
Table 1: Medical Director Responsibilities Compared to all Hospice Physicians, Nurse Practitioners and Physician Assistants
Medical Director Only
Nurse Practitioner
(NP) Only
Physician Assistant (PA) Only
The hospice must designate a physician to serve as medical director. The
medical director must be a doctor of medicine or osteopathy who is an employee or
is under contract with the hospice. (§418.102). When the medical director is not
available, a physician designated by the hospice assumes the same responsibilities
and obligations as the medical director.
Supervision of all physician employees. All physician employees and those under
contract, must function under the supervision of the hospice medical director.
Admission to hospice care. The hospice admits a patient on the
recommendation of the medical director in consultation with
patient's attending physician (if any). (§418.25(a)).
Discharge from hospice care. Prior to discharging a patient, the hospice must
obtain a written physician's discharge order from the hospice medical director.
(§418.26(b)).
NPs may function as
the “Attending
Physician” and may
write orders within
the scope of their
state practice act. As
a hospice employee,
NPs may do face-to-
face examination
required for the 3
rd
or later hospice
benefit period.
Functioning as the “Attending
Physician,” PAs may write
orders that are unrelated to the
terminal illness, within the scope
of their state practice act. PAs
who are hospice employees or
providing care under
arrangement may not write
orders pertaining to the terminal
illness or the face-to-face
assessment for certifying the
terminal illness.
Medical Director/Physician Designee
NP/PA.
Medical component of patient care program. The medical director or physician
designee, in the absence of the medical director, has responsibility for the medical
component of the hospice's patient care program.
Neither NPs or PAs can function as the physician on the
interdisciplinary team or certify terminal illness.
Certification and Recertification of Terminal Illness. Medical director or
physician designee, in the absence of the medical director, reviews the clinical
information for each hospice patient and provides written certification that the
patient's life expectancy is 6 months or less if the illness runs its normal course
(§418.102(b)).
Physicians
The hospice medical director, physician employees, and contracted physicians, in conjunction with the patient's attending physician, are
responsible for the palliation and management of the terminal illness and conditions related to the terminal illness. (§418.64(a)). If the
attending physician is unavailable, the hospice physician, is responsible for meeting the medical needs of the patient.

§418.108 Condition of participation: Short-term inpatient care
All hospices must provide access to inpatient care in a Medicare or Medicaid facility for
the provision of pain control, symptom management, and respite care. The facility
furnishing inpatient care and staffing must follow the requirements in §418.112 with the
exception that respite care can be provided in a Medicare-certified nursing facility.
Clinical records must reflect all care received in the facility, who was responsible for the
care, and the facility staff’s adherence to palliative care protocols and the patient’s plan
of care established by the hospice.
Hospices may have arrangements with other facilities for the provision of inpatient care.
• Ask the hospice clinical manager what facilities are used and how the care their
patients receive at each facility is monitored. If you have questions concerning the
provision of care or the hospice’s explanation of how they monitor care at the
facility(ies), ask to review a copy of the written agreement.
• Ask how the hospice assures that staff of the external inpatient facility(ies) caring for
hospice patients have been trained in the hospice philosophy of care and can provide
patient care according to the hospice plan of care. If necessary, contact or visit the
facility(ies) to verify compliance.
§418.110 Hospices that provide inpatient care directly
All hospices that provide direct inpatient services must have an on-site survey and a Life
Safety Code (LSC) survey. Inpatient facilities in which hospices provide direct inpatient
services vary widely in size and location. For example, they may be located as a
freestanding hospice facility or leased space within another Medicare certified facility
such as a hospital. The primary focus of this observation is on the quality and safety of
patient care; the survey protocol consists of patient care observations, patient or family
interviews, hospice staff interviews, and facility observations.
During an inpatient hospice survey, all aspects of patient care noted in Phase 1 are
considered. This survey includes an LSC survey (based on the procedures in Appendix I-
Life Safety Code) that must be done both at the time of initial certification of the
inpatient facility and at the time of recertification surveys.
The primary tasks of the inpatient hospice survey include:
1. Entrance Interview with the administrator
2. Facility tour with staff
3. Sample selection
4. Patient care observations and staff interviews
5. Family or other caregiver interviews
6. Meal service observation
7. Medication administration observation
8. Medication room observation
9. Determine that pharmacy services are provided under the direction of a qualified
licensed pharmacist
10. 24-hour nursing staffing evaluation
11. Review use of, reporting of, and staff training on restraint/seclusion
12. Emergency preparedness plan for inpatients
13. Compliance with procedures in Appendix I-Life Safety Code
Entrance Interview with the Administrator—see entrance element
under Survey Protocol.
Facility tour and observation, interviews, and record reviews
During the tour, note the following factors to aid the selection of the patient sample for
record review:
• That the facility is clean, calm, quiet, and homelike, and the patient and family have
privacy;
• There is a comfortable ambient temperature, without strong odors;
• The condition of patient care equipment, and linen storage areas:
• Interview patients/families to determine if linens are promptly changed when
soiled throughout all 24-hour periods, including weekends and holidays;
• Ask management what the hospice’s policy is on the frequency of linen
change and replacement;
• During a tour of the inpatient hospice unit, observe patient bedding to assure
cleanliness;
• Request to see the linen storage area to determine if there is an adequate
supply to meet ongoing patient needs at all times;
• How does the hospice store the clean linen to keep it clean, dry, and dust free?
• Is soiled linen and clothing collected and enclosed in suitable bags or
containers in well-ventilated areas, separate from clean linen and not
permitted to accumulate in the facility?
• There are no potential safety hazards (i.e., obstructed walkways, non-
functioning call system for patient assistance, unattended medication carts
and/or unlocked medication rooms).
• Observe staff providing care. Do they follow acceptable infection control guidelines?
• Complete a more in-depth review if environmental concerns were identified through
observation or patient, patient representative or hospice staff interviews.
3. Inpatient Hospice Sample
Table 2. Inpatient Hospice Sample Selection (added to the total hospice sample)

Number of Patients in the Inpatient Facility
Minimum Number in the Inpatient Sample
1- 4
2
5-16
3
17+
4
Vary the sample considering:
• Diagnoses;
• Reason for inpatient care, i.e., GIP or respite care;
• Length of stay;
• Presence of wounds, ostomies, or other high intensity or complex care.
4. Patient care observations and staff interviews
• Observe patients’ level of pain/discomfort/distress and at least two treatments, in
particular wound care and intravenous therapy or other complex care, if available.
During these observations, note the infection control techniques practiced by staff,
and whether patient care supplies are readily available.
• Observe the patient areas to evaluate if there is a home-like atmosphere: For example,
are furnishings, lighting, personal space, etc.?
• Note how patient care equipment is handled, cleaned, and stored (toothbrush, comb,
urinals, water pitchers, positioning devices, etc.).
• Observe if patients appear comfortable and clean.
• Conduct interviews as necessary to capture:
• How the staff has reviewed and revised the plan of care since the patient was
admitted to hospice to address both physical and psychosocial needs;
• How new interventions/medications are evaluated for effectiveness;
• The patient/family teaching that is being provided;
• The staff’s interaction with the IDG or primary care RN/RN Coordinator;
• How is the social worker (an individual with a Master of Social Work
(MSW)) involved when a patient is admitted for inpatient services?
• Observe for availability of staff.
• Observe for availability of handwashing supplies and hand sanitizers.
• Ask the hospice to describe how they keep the facility clean and sanitary.
• How does the hospice ensure that staff follows current standards of practice for
patient environmental safety, infection control, and security?
5. Patient, family, or other caregiver interviews
Interview patients and/or their family to determine how the hospice is addressing the
reason the patient is receiving inpatient hospice care, such as severe pain management
and abating other symptoms such as shortness of breath, nausea and vomiting,
constipation, pathological fractures, agitation/anxiety.
Ask the patient and/or family member:
• The reason for their inpatient admission and if they received help with these
problems. And, whether the hospice team provides training about how to manage
these symptoms (severe pain, shortness of breath, nausea and vomiting, constipation,
pathological fractures, agitation/anxiety) after discharge?
• Awareness of the respite length of stay and discharge plan.
• Validate that visiting hours are not restricted and accommodations are provided for
family members to stay with the patient during the night.
• If the patient is receiving respite care, ask the patient if they are receiving the care and
services that they would receive at home.
• If patients’ needs are met, whether staff respond timely to calls for assistance, and if
there are enough staff in the evenings and on weekends to meet their care needs.
• Do staff and other patients respect their privacy and provide a private place to meet
with visitors without restriction?
• Do staff members willingly take the time to listen when the patient or family member
want to talk about a problem, and make an effort to resolve the problem? Are the
patient preferences taken into account, i.e., are they given a choice what and where
they can eat, and are satisfied with the food being served?
• Food and meal services.
• If needed, does hospice staff assist with eating?
• Determine how the interdisciplinary group (IDG) is kept informed of a
patient’s response to a therapeutic diet.
• Is food available 24 hours/day 7 days a week? If small frequent meals are
indicated for a patient’s condition to maintain hydration and nutrition, how
does the hospice meet these needs?
6. Meal service observation
• Assess if food is served consistent with the plan of care (including providing the level
of assistance needed), meets the patient’s nutritional needs, is palatable, attractive, at
proper temperature, and is served under sanitary conditions
• If the hospice prepares the meal, conduct a tour of the kitchen and observe for the
following:
• Refrigerator temperatures are monitored and documented (freezer temperature
should be 0° F or below and refrigerator 41° F or below (allow 2-3 degrees’
variance));
• Hot foods temperatures should be maintained at 140° F or above and cold
foods should be maintained at 41° F or below when served;
• Foods should be covered until served;
• Employees wash hands before and after handling food; if used, are gloves changed
after touching unclean surfaces before handling food again?
7. Medication administration observation
Medications may be administered only by a licensed nurse or physician, an employee
who has completed a State-approved training program in medication administration, or
the patient upon approval of IDG.
Medication administration observation will include a minimum of two patients but can be
expanded if there are concerns. Any concerns regarding a medication that is about to be
administered should be brought to the attention of the person administering the
medication. The record of observation should be reconciled with the most current plan of
care.
If the opportunity presents itself, observe medications for a patient in the sample.
Otherwise, observe medications for any patient to whom the nurse is ready to administer
medications. Try to arrange the observation of the administration of opioids/controlled
drugs to observe the facility procedures for the dispensing and recording practices.
8. Medication room observation
This observation confirms that:
• The hospice has a system to ensure that drugs and biologicals are not expired,
mislabeled, unlabeled, or otherwise unusable, and conform to professional standards
of practice.
• The hospice maintains accurate records for all drugs including controlled drugs.
• All drugs and biologicals are stored in secure areas.
• All controlled drugs listed in Schedules II, III, IV, and V are stored in locked
compartments within secured storage areas.
• Sufficiently detailed records of receipt and disposition of controlled medications to
enable an accurate reconciliation.
• All medication records are maintained, and all controlled medications are periodically
reconciled.
• Disposal methods for controlled medications, such as a secure and safe method to
prevent diversion and/or accidental exposure.
9. Review of pharmacy services requirements (qualified licensed pharmacist)

Confirm that the hospice provides pharmacy services under the direction of a qualified
licensed pharmacist who is an employee of or under contract with the hospice. The
provided pharmacist services must include evaluation of a patient’s response to
medication therapy, identification of potential adverse drug reactions, and recommended
appropriate corrective action.
• How does the hospice ensure timely review of inpatients’ drug profiles by the
pharmacy service?
• Evaluate how the IDG works with the pharmacy service, incorporates the
pharmacist’s review of the medications into the plan of care, and adjusts the
medication profile. When pain or agitation/anxiety management is the primary
problem for example, how does the hospice work with the pharmacist to establish an
effective medication-dosing regimen?
10. 24-hour nurse staffing evaluation
This activity confirms staffing of the inpatient unit/facility with at least one RN on every
shift when one or more patients are receiving general inpatient care. Review the past 30
days patient census and corresponding staff schedule to determine that the hospice meets
this requirement.
11. Review of restraint/seclusion reporting and staff training
The regulations ensure, when needed, hospices only impose seclusion or restraints for the
shortest time necessary to ensure the immediate physical safety of the patient, staff
members or others. Select one patient record in which the hospice reported the use of
restraints, if applicable.
Staff must be trained in techniques to identify behaviors, events, and environmental
factors that may trigger the need for seclusion or restraint. Staff training minimizes the
likelihood of a patient death related to the use of seclusion or restraint for a patient and
will thus minimize resulting injury or death. Review the facility’s restraint/seclusion
training materials to confirm compliance.
• Request a copy of the training curriculum for the use of restraints or seclusion. Does
it contain all the required content items as prescribed, at 42 CFR 418.110(o)?
• Request a copy of new employee orientation content to ensure that information on the
use of restraints or seclusion is included.
• Review attendance sheets for initial and periodic training sessions.
• Review 3 new employee (hired within the past 12 months) personnel files to verify
there is evidence of appropriate training in restraint and seclusion use.
• Interview the trainer if additional validation is needed.
• Does the inpatient hospice policy related to the use of restraints or seclusion include
information on reporting to CMS in the event of a death connected to the use of
restraints or seclusion?

• Interview management and staff to assess if any deaths have occurred related to the
use of seclusion or restraint.
• Review any documentation/clinical records if such a death has occurred. Was this
information reported appropriately to CMS within the applicable time frame?
12. Review of Emergency Preparedness Plan for inpatients
This is included as a requirement for the hospice inpatient facility to assure that the
overall hospice emergency preparedness plan includes the inpatient facility procedures.
This review may be performed at the hospice office location if located in a different site
from the inpatient hospice.
For more information on emergency preparedness, see §418.113 and SOM, Appendix Z.
§418.112 Condition of participation: Hospices that provide hospice care to residents
of a SNF/NF, ICF/IID
At least one home visit should take place with a hospice patient in a SNF/NF and ICF/IID
(if any) to determine how the two providers coordinate services, how the hospice
manages the needs of the patient, and to evaluate the impact of hospice services on
patient outcomes.
Hospices are responsible for furnishing and managing a patient’s hospice care related to
the terminal illness and related conditions. They are not responsible for managing every
aspect of a patient’s care in the nursing home.
When assessing hospice care to residents of a SNF/NF or ICF/IID facility, verify the
following:
Home (SNF/NF or ICF/IID Facility) Visit Observation
• Observe care by hospice personnel to confirm that the current plan of care and
coordination of services is delivered as described in the written agreement;
• Determine the patient’s or family’s understanding of the purpose of the hospice
services, and if they had input into setting the goals or objective that were established
for their care;
• Interview the patient, family, or representative if possible, to determine their
involvement in the development of the plan of care, defining the approaches and
goals, and to determine if interventions reflect choices and preferences. Also,
determine how they are involved in developing and revising pain management
strategies (if any) and any necessary revisions if the interventions do not work;
• Determine whether medications or other interventions for symptom control, medical
supplies or DME related to the terminal illness have been arranged and provided by
the hospice and are available for patient use. Determine whether there have been
delays in the provision of medications and/or supplies/equipment, and how this has
been addressed by the hospice and the facility;
• Determine if written instructions were provided to the nursing home staff for hospice-
related interventions;
• Did the hospice staff provide education and training to facility staff regarding hospice
philosophy;
• If pain assessment is included in the plan of care and is completed as indicated;
• If equipment, supplies, and assistive devices are in the plan of care, determine if the
patient has received the items and was instructed in their safe and appropriate use;
• Review the most current medication list maintained by the hospice. Determine if the
medications match those listed in the comprehensive assessment, the plan of care, and
the written information to the patient. Investigate any discrepancies for additions or
deletions to the medications since the information was last updated by the hospice;
• Assess for compliance with infection control requirements.
SNF/NF or ICF/IID Facility Staff Interview
Interview a facility staff person who is knowledgeable about the needs and care of the
patient, and provides direct care to determine:
• Ask how the facility staff is trained in the hospice philosophy of care.
• If the patient/representative and facility staff, are not familiar with hospice
philosophy, policies and procedures regarding methods of comfort, pain control,
symptom management, as well as principles of death and dying, patient rights,
appropriate forms, and record keeping requirements, then interview hospice staff on
how they have provided education to the facility staff in these matters.
• How facility staff communicate with the hospice when there is a change in the
patient’s physical, mental, social, or emotional status.
• If the patient receives pain medication (including PRN and adjuvant medications),
how, when, and by whom the results of medication effectiveness are evaluated
(including the dose, frequency of PRN use, schedule of routine medications, and
effectiveness). Is there evidence that the hospice provides services and medications,
equipment and supplies necessary for pain control and symptom management on a
24-hour basis?
• How staff monitor for the emergence or presence of adverse consequences of
interventions.
• How the hospice and the facility coordinate their approaches, communicate about the
patient’s needs, and monitor the outcomes (both effectiveness and adverse
consequences).
• What system is in place to assure that the facility knows how to notify the hospice
when necessary on a 24/7 basis?
• Is there any evidence that the communication is not occurring as needed during
various times of the day or week or specific shifts?

• How does the hospice ensure that facility staff are able to recognize the individuals
who are receiving hospice services and know that the services provided to this patient
should be in accordance with the coordinated plan of care?
• What evidence is there that the hospice and the facility communicate with each other
during and between patient visits, as appropriate, to share information about the
patient’s needs and response to the plan of care?
• Does the hospice staff have access to and the ability to communicate with facility
staff about the patient’s care as often as needed?
• Is there evidence that facility personnel assist in the administration of prescribed
therapies included in the plan of care that exceed what a hospice family member
might implement?
• How do the hospice and the facility identify the therapies that facility staff will be
allowed to perform?
Hospice Staff Interview
Interview hospice staff who are responsible for coordinating care and overseeing the
direct care to the patient (such as the RN coordinator) to determine:
• How the hospice communicates the hospice plan of care and all updates with the
facility.
• How do the hospice and the facility communicate with each other during and between
patient visits, as appropriate, to share information about the patient’s needs and
response to the plan of care?
• Does the hospice staff have access to and the ability to communicate with facility
staff about the patient’s care as often as needed?
Documentation Review
Hospice plan of care and coordination of services
To provide continuity of care, the hospice, nursing home, and resident/representative
must collaborate in the development of a coordinated plan of care. Does each patient
receive updates to the comprehensive assessment at the required time points according to
§418.54(d) and to the plan of care reviews according to §418.56(d)?
If concerns were identified that changes to the plan of care, without prior hospice
approval, occurred as a result of physician orders received by the facility, determine:
• How the IDG communicates with physicians involved with the patient; and
• If there is evidence that the IDG communicates effectively with all physicians
involved in the patient’s care to ensure that duplicative and/or conflicting physician
orders related to the terminal illness and related conditions are not issued.
Based on the shared communication between providers (as noted at 418.56), both
providers’ portion of the plan of care should include, but not be limited to:
• A common problem list;
• Palliative interventions;
• Palliative outcomes;
• Responsible discipline;
• Responsible provider; and
• Patient goals.
Determine whether medications or other interventions for symptom control, medical
supplies or DME related to the terminal illness have been arranged and provided by the
hospice and are available for patient use. Determine whether there have been delays in
the provision of medications and/or supplies/equipment, and how this has been addressed
by the hospice and the SNF/NF or ICF/IID facility.
The structure of the plan of care is established by the nursing home and the hospice; the
plan may be divided into two portions, one maintained by the nursing home and the other
maintained by the hospice. The nursing home and the hospice must be aware of the
location and content of the coordinated care plan (which includes the nursing home
portion and the hospice portion). The plan must be current and internally consistent to
assure that the resident’s needs, for both hospice care and nursing home care, are met at
all times. Any changes to the plan(s) must be discussed and approved by the nursing
home, hospice staff, and, to the extent possible, the resident and/or representative.
The SNF/NF or ICF/IID and hospice staff must have a procedure that clearly outlines the
chain of communication between the hospice and facility in the event a crisis or
emergency develops, a change of condition occurs, and/or changes to the hospice portion
of the plan of care are indicated.
• Is there a member of the IDG providing overall coordination of the hospice care of
the SNF/NF or ICF/IID - communications surrounding provision of care to the patient
by other physicians)?
• Does each patient receive updates to the comprehensive assessment at the required
time points according to §418.54(d) and to the plan of care reviews according to
§418.56(d)?
• Is there evidence that the patients are receiving the appropriate level of hospice
services to meet their needs?
• Is the SNF/NF or ICF/IID provided with pertinent information such as medication
information, names of hospice personnel involved in hospice care of each patient;
hospice orders specific to each patient?
Review the plan of care to determine if the plan was coordinated between the hospice and
the facility. Determine if symptom management, including pain management
interventions, are included, if needed, and addressed as appropriate:

• Measurable pain/symptom management goals, reflecting patient needs and
preferences;
• Pertinent non-pharmacological and/or pharmacological interventions;
• Time frames and approaches for monitoring the status of the patient’s pain, including
the effectiveness of the interventions;
• Identification of clinically significant medication-related adverse consequences such
as falling, constipation, anorexia, or drowsiness, and a plan to minimize those adverse
consequences; and
• Whether the pain has been reassessed and the plan of care revised as necessary if the
current interventions are not effective or the patient has experienced a change of
condition or status.
• Review and analyze documentation related to patient and staff incidents and accidents
to identify any incidents/accidents or patterns of incidents/accidents concerning a safe
environment. Expand your review if you suspect a problem with a safe environment
in the hospice.
• If the hospice has identified problems, did it evaluate those problems and take steps to
ensure a safe patient environment?
Written Agreement
• Is there a signed current written agreement with each SNF/NF or ICF/IID facility
where care is provided?
• Does the written agreement include the required elements such as assurance that the
needs of the patient are addressed and met 24 hours a day; reporting of alleged
violations to hospice; hospice reporting alleged violations to the facility
administrator; and other requirements as per the standard?
Orientation and Training of Staff
• Is there evidence that the hospice has assessed the need for staff training, and how
frequently the facility staff should be trained?
• Is there evidence that the facility staff furnishing care to hospice patients are trained
in the hospice philosophy of care?
Information Gathering – Survey Protocol Phase 2
Protocol Phase 2 consists of the 4th “Core” CoP (QAPI) to determine if the hospice is
actively engaged in monitoring the effectiveness and safety of services and quality of
care, as well as the remaining 13 CoPs. Protocol Phase 1 CoPs quality of care findings
informs the Protocol Phase 2 CoPs investigation.
Note: It important to note all CoPs continue to have the same weight, be they Phase
1 or Phase 2, in terms of finding noncompliance and citing deficiencies.
Protocol Phase 2 Core Requirement CoP

§418.58 Condition of participation: Quality assessment and performance improvement.
Protocol Phase 2 Associated Quality of Care CoPs
§418.62 Condition of participation: Licensed professional services.
§418.64 Condition of participation: Core services.
§418.66 Condition of participation: Nursing services—Waiver of requirement that
substantially all nursing services be routinely provided directly by a hospice.
§418.70 Condition of participation: Furnishing of non-core services.
§418.72 Condition of participation: Physical therapy, occupational therapy, and speech-
language pathology.
§418.74 Waiver of requirement—Physical therapy, occupational therapy, speech-
language pathology, and dietary counseling.
§418.78 Conditions of participation—Volunteers.
§418.100 Condition of Participation: Organization and administration of services.
§418.104 Condition of participation: Clinical records.
§418.106 Condition of participation: Drugs and biologicals, medical supplies, and
durable medical equipment.
§418.113 Condition of participation: Emergency preparedness.
§418.114 Condition of participation: Personnel qualifications.
§418.116 Condition of participation: Compliance with Federal, State, and local laws and
regulations related to the health and safety of patients.
§418.58 Condition of participation: Quality assessment and performance
improvement (QAPI)
The QAPI review confirms that the hospice has a documented, operational, and effective
QAPI program that focuses on high risk, high volume, or problem prone areas specific to
that hospice. If surveyors discover systemic quality of care deficiencies during Phase 1,
in addition to routine review, closely examine reports and minutes of QAPI meetings to
determine if the hospice is actively identifying and analyzing problem prone areas.
Interview the hospice team to ascertain if the hospice continually analyzes data and
evaluates the effectiveness of its QAPI program.
Hospices are required to collect and analyze patient care and administrative quality data
and to use that data to identify, prioritize, implement, and evaluate performance
improvement projects to improve the quality of services furnished to hospice patients.
To assess compliance with the QAPI requirements and the adequacy and appropriateness
of a hospice’s QAPI program, request the following:
• The hospice’s aggregated data and its analysis of that data;
• The hospice’s QAPI plan;
• The individuals responsible for the QAPI program;
• Evidence that the QAPI system has been implemented and is functioning effectively,
including evidence of:
• Regular meetings;
• Investigation and analysis of sentinel and adverse events;
• Recommendations or options for systemic change to prevent recurrence of
sentinel or adverse events;
• Identified performance measures that are tracked and analyzed; and
• Regular review and use of the QAPI analyses by hospice management and the
governing body to make systemic improvements.
• Any other necessary resources needed to assess a hospice’s compliance.
This information will allow you to match the data provided by the hospice with the actual
experiences of hospice employees and patients to ensure that the QAPI program is
prevalent throughout the hospice’s operations and services, and that it is positively
influencing patient care.
Focus on areas such as how and why the hospice chose its quality measures, how it
ensures consistent data collection, how it uses data in patient care planning, and how it
aggregates and analyzes data. Ask the hospice how it uses the data analysis to select
performance improvement projects, how it implements such projects, and how it uses the
data to evaluate the effectiveness of those projects.
While a copy of QAPI meeting minutes may be an acceptable method of demonstrating
that regular meetings were held, alternate evidence may be acceptable. Surveyors may
not require copies of meeting minutes unless the meeting minutes are judged to be
essential to an assessment of whether the QAPI analyzed an adverse or sentinel event that
is the subject of a complaint investigation or standard survey. Essential in this context
means that there is not alternate evidence that suffices to address the central question of
whether an assessment that meets CMS requirements was conducted. Alternate evidence,
for example, may be a recommendation for systemic change that was sufficiently detailed
that a reasonable person would conclude the recommendation was based on competent
analysis.
• Does the hospice’s QAPI program measure, analyze and track quality indicators
related to processes of care, hospice services and operations?
• Is the hospice’s QAPI program data-driven?
• Is there evidence that the hospice uses the data collected to identify opportunities for
improvement?
• Determine if the hospice has taken appropriate action to correct problems identified
by the QAPI program. Examine reports and minutes of QAPI meetings to determine
if the hospice has documented the remedial action and its outcome. Examples of

appropriate remedial action may include but are not limited to changes in policies and
procedures.
• Is there evidence that the hospice continues to monitor performance to ensure that
improvements are sustained?
• Is the hospice’s QAPI program data-driven?
• Is there evidence that the hospice uses the data collected to identify opportunities for
improvement?
• Do hospice records indicate that the hospice’s governing body is involved in
oversight of the QAPI program?
• Is there an individual appointed by the governing body who is responsible for
operating the QAPI program?
• Interview key staff to determine how the hospice ensures that licensed professionals
participate in their QAPI and in-service training programs.
§418.62 Condition of participation: Licensed professional services
The three standards in this CoP require that qualified licensed professionals, who provide
services to hospice patients, either directly or under arrangement, participate in
coordinating all aspects of the patient’s hospice care. This includes updating
interdisciplinary comprehensive assessments, developing, and evaluating plans of care,
participating in patient and family counseling, participating in the quality assessment and
performance improvement plan, and participating in in-service training.
Home Visit Observations
During direct care observation, confirm that the licensed professional:
• Provides services as indicated in the plan of care;
• Provides patient and caregiver education per plan of care
• Patient, caregiver, and family counseling per the plan of care; and
• Seeks the patient’s, representative’s (if any) and caregiver’s(s’) input and feedback
into their care.
Patient/Family Interview
During patient or caregiver interview, ask questions that address how nurses, social
workers, physicians, and counselors/therapists:
• Provide education (e.g., medication administration) and counseling to the
patient/caregiver as well as counseling to the family;
• Keep the patient informed about and engaged in developing the services that are
specified in the plan of care.
Professional Staff Interview

Interview direct care staff to determine:
• How staff are involved in the coordination of patient care services;
• How often they participate in IDG meetings;
• What kind of training do they receive by the hospice about the hospice philosophy
and approach to care?
Clinical Record Review
• The clinical record should reflect the education and counseling provided by skilled
professionals to the patient, caregiver, and family.
• Verify that skilled professionals communicate changes to the plan of care and
instructions to hospice aides promptly. Determine if the hospice aides identify a
change in the patient’s condition and report this information to the skilled
professional.
§418.64 Condition of participation: Core services
Hospice core services include physician services, nursing services, medical social
services, and counseling services (bereavement, dietary and spiritual). A hospice is
required to routinely provide substantially all core services directly by staff employed by
the hospice (see Definitions at 418.3) The hospice may contract for physician services
who are supervised by the medical director.
Home Visit Observations
If a clinical provider is present during direct care observations, confirm that he or she:
• Adequately and effectively, address the current plan of care as part of their visit.
• Does the clinician assess pain and symptom management issues based on the plan of
care?
• In addition to pain and symptom management does the clinician explore spiritual,
emotional and grief questions/concerns with the patient and caregiver to ensure these
services are made available when they are not in place due to being previously
declined?
Patient/Family Interview
• Has the family been satisfied with communication of hospice staff and availability to
hospice staff after hours and on weekends/holidays?
• Interview the patient and caregiver to determine how satisfied she/he is with the
services provided by the clinicians identified in his/her plan of care.
• Does the patient/caregiver know the visit schedule of the clinician and are visits made
as scheduled. If home visits are missed does the patient/caregiver know why and are
they able to easily contact the hospice if a need arises?
• How does the hospice introduce and offer medical social work services to the
patient/family?

• Is there evidence that each patient receives social work services (unless specifically
refused by the patient) that reflect the needs identified in the psychosocial
assessment?
• Is there evidence that the medical needs of the patients are being met by the hospice
physician for patients who do not have an attending physician or when the attending
physician is unresponsive or unavailable?
Staff Interview
Social Work
• Ask the social worker or clinical manager to describe the factors that are included in
the psychosocial assessment and how this information is used in the care planning
process to benefit the patient/family.
Nursing
• Ask the primary care RN assigned to provide direct care (e.g., RN coordinator) how
he/she maintains an updated plan of care for the patient.
• Review with RN how he/she incorporates the patient/caregiver’s goals of care into the
plan of care developed by the IDG
Nutrition/Dietary Counseling
• Ask the clinical manager how the hospice meets the needs of patients and families
who experience challenges and conflict with end-of-life care dietary issues. This may
include providing education about how the dying process naturally results in lack of
appetite and intake and how this may relate to the patient’s decreasing appetite and
food intolerances during the end of life.
• Ask the clinical manager how the hospice meets the needs of patients who experience
dysphasia, problematic enteral feedings, or unresolved nutritional issues secondary to
nausea, vomiting, or the dying process.
Spiritual Needs
• Determine through clinical record review, interview, and home visits how the hospice
addresses the spiritual needs/concerns of the patients and families.
• How does the hospice introduce the availability of spiritual counseling?
• What mechanisms are in place to meet the patient/family spiritual needs?
Clinical Record Review
The clinical record should indicate what core services are being provided with person-
centered and specific visit schedules defined in the plan of care.

• If the clinical record identifies missed visits is there substantial evidence to describe
why visits were missed and what efforts by the clinician have been made to resolve
missed visits?
• Does the clinical record include measurable and outcome-oriented goals of care that
are person-centered and individualized?
§418.70 Condition of participation: Furnishing of non-core services
If questions arise during home visits or record reviews regarding the availability of
needed clinical services by physical therapists, occupational therapists or speech
language pathologists, or volunteers, and whether these individuals follow professional
standards of practice, ask clinical managers and staff what the hospice's policies are
regarding these issues.
§418.72 Condition of participation: Physical therapy, occupational therapy, and
speech language pathology
If patients require physical therapy (PT), occupational therapy (OT), or speech language
pathology (SLP) and do not receive them, ask whether the hospice received a waiver for
these services (see §417.74)
§418.74 Waiver of requirement - physical therapy, occupational therapy, speech-
language pathology and dietary counseling
If PT, OT, or SLP are needed, but not available on a 24-hour basis, request to see
evidence of the hospice requested a waiver for 24-hour availability of those service, and
evidence that the hospice made efforts to secure the needed services.
Ask how the hospice monitors the professional skills of its non-core services staff to
determine if those skills are appropriate and adequate for its patients.
§418.78 Conditions of participation – Volunteers
• Conduct an interview with the individual designated to supervise the volunteers
regarding the use, training, and supervision of volunteers.
• How does the hospice supervise the volunteers? Is there evidence that all volunteers
receive the supervision necessary to perform their assignments?
• Is there documentation supporting that all the volunteers have received training or
orientation before being assigned to a patient/family?
• How does the hospice program determine where to dispatch volunteers? Does the
IDG consider the patient/family’s need for a volunteer?
• There should be documentation of time spent and the services provided by volunteers.
• Is there documentation or evidence of the hospice’s viable and ongoing efforts to
recruit and retain volunteers?

• Is there documentation of the cost savings achieved through the use of volunteers,
including identification of each position that is occupied by a volunteer, time spent,
and the services provided by volunteers, and estimates of the dollar costs that the
hospice would have incurred if the positions were paid?
• Volunteers should be aware of:
• Their duties and responsibilities;
• The person(s) to whom they report;
• The person(s) to contact if they need assistance and instructions regarding the
performance of their duties and responsibilities;
• Hospice goals, services, and philosophy;
• Confidentiality and protection of the patient’s and family’s rights;
• Family dynamics, coping mechanisms and psychological issues surrounding
terminal illness, death, and bereavement;
• Procedures to be followed in an emergency, or following the death of the patient;
and
• Guidance related specifically to individual responsibilities.
§418.100-Organization and administration of services
Hospice Administrator
Just as the medical director is directly responsible for the medical component of the
hospice program, the administrator assumes full responsibility for the day-to-day
operations of the hospice. The administrator must be a hospice employee who possesses
the education and experience determined to be necessary by the governing body.
Identify through interview and document review the following:
• The hospice demonstrates an understanding of the principles surrounding quality
assessment and implements effective ongoing performance improvement projects
utilizing data collected;
• When the hospice identifies trends that indicate potential or actual problems, it takes
follow-up actions to resolve the issue(s);
• The hospice provides care that optimizes the patient’s comfort and dignity and is
consistent with the patient and family needs and goals;
• The hospice assumes overall professional management responsibility for all services
provided directly and under arrangement;
• How is the governing body informed of the hospice’s ongoing operations, including
patient care delivery issues and quality assessment and performance improvement
activities?
• Ask the administrator or clinical supervisor to describe the relationship between the
governing body, hospice management and staff.
• Ask the hospice how it assures that any multiple locations operating as a part of the
hospice share administration, supervision, and services, and participate in the
hospice’s QAPI activities.
• How does the hospice communicate with any multiple locations to assure that they
are responsible to the same governing body and central administration that governs
the hospice issued the provider agreement, and that the governing body and central
administration are able to adequately manage such locations, resolve any problems
that occur, and assure quality of care for all patients?
• Oversight of Contracted Personnel:
• How does the hospice assure that all contracted personnel (agency, individual
or organization) provide care that is in accordance with the patient’s plan of
care?
• How does the hospice assure that all services provided under arrangement are
authorized by the hospice?
• How does the hospice monitor and exercise control over services provided by
personnel under arrangements or contracts?
• How does the hospice assure professional management of patients that are
receiving inpatient care under arrangement?
• How and when does communication occur between the hospice and
contracted individuals, agencies, or organizations?
• How does the hospice assure that services are furnished by qualified staff?
• Review a sample of personnel records to verify that initial orientation,
assessment of skills and competency, and in-service training was provided to
all employees, contracted staff, and volunteers furnishing care/services to
hospice patients and families.
• Review hospice written agreements and training programs provided for
contracted personnel.
• If concerns are identified, interview the administrator or his/her designee, and
staff regarding the specific issue.
• Nursing services, physician services, drugs and biologicals are routinely available on
a 24-hour basis, 7 days a week. Other covered services are available on a 24-hour
basis when reasonable and necessary to meet the needs of the patient and family;
• The on-call system is operational on a 24-hour basis so that patients can contact the
hospice as necessary;
• Drugs, treatments, and medical supplies are provided as needed for the palliation and
management of the terminal illness and related conditions, and
• The hospice makes arrangements for any necessary inpatient care according to
§418.108 and retains professional management responsibility for services furnished
by inpatient facility staff.

• The administrator can describe the relationship between the governing body, hospice
management and staff.
Governing Body
The governing body assumes responsibility for ensuring that the hospice is managed by
the administrator and any managers that the administrator appoints. If the hospice is part
of a larger organization (e.g., HHA, hospital) and the governing body is the same, there
must be documented evidence that the governing body is assuming full authority and
responsibility for the management of the hospice specific program.
Confirm through interview and record review:
• Ensure that the governing body is meeting at the specified intervals indicated in the
Governing Body Bylaws (ex. monthly, quarterly, semi-annual, etc.), and reviewing
Governing Body meeting minutes as evidentiary support of the governing body
fulfilling its responsibilities at 418.58 QAPI and 418.100(b).
• How the governing body is informed of the hospice’s ongoing operations, including
patient care delivery issues and quality assessment and performance improvement
activities;
• The governing body assumes responsibility for ensuring that the hospice is managed
by the administrator and any managers that the administrator appoints;
• The governing body ensures the hospice has an ongoing program to promote quality
assessment and performance improvement.
§418.100(f)(2)
Surveyors may conduct the entire survey or part of the survey at the multiple location(s).
When conducting a survey at a multiple location, the surveyor may request that all
necessary documentation for review be transported to that location at the hospice’s
expense. This may include, but not be limited to, a sample of clinical records from all
other locations, QAPI reports, administrative records, personnel files, and policies and
procedures.
There should be evidence that:
• The hospice exerts the supervision and control necessary at each location to assure
that all hospice care and services continue to be responsive to the needs of the
patient/family at all times and in all settings;
• Each location of the hospice provides the same full range of services that is required
of the hospice that was issued the certification number;
• Each patient is assigned to a specific IDG responsible for ongoing assessment,
planning, monitoring, coordination, and provision of care;

• Each location is responsible to the same governing body and central administration
that governs the hospice that was issued the certification number, and the governing
body and central administration must be able to adequately manage the location and
assure quality of care.
§418.104 -Clinical records
A clinical record containing past and current findings is maintained for each hospice
patient. The clinical record must contain correct clinical information that is available to
the patient’s attending physician (if one has been identified by the patient) and hospice
staff. The clinical record should be person-centered and address the goals of care
identified by the IDG and patient.
Home Visit Observations
During the home visit, observe how agency staff maintain the confidentiality of protected
health information (PHI) that they transport and use for patient care encounters as well as
safeguard it against loss or unauthorized use. Suspected PHI compliance issues with PHI
rules should be referred to the appropriate agency as per 45 CFR parts 160 and 164.
Clinical Record Review
All entries must be legible, clear, complete, and appropriately authenticated and dated in
accordance with hospice policy and currently accepted standards of practice. The clinical
record should include:
• During the clinical record review, verify that the clinical information necessary for
hospice certification is present in the record;
• The initial plan of care, updated plans of care, initial assessment, comprehensive
assessment, updated comprehensive assessments, and clinical notes should be
individualized, i.e., adapted to the needs or special circumstances of the patient;
• Signed copies of the notice of patient rights in accordance with §418.52 and election
statement in accordance with §418.24;
• Contact information for the patient and the patient’s primary caregiver;
• Responses to medications, symptom management, treatments, and services;
• Outcome measure data elements;
• Physician certification and recertification of terminal illness as required;
• Any advance directives;
• Physician orders (including verbal orders);
• Plans of care should be unique to each individual (i.e., individualized as noted at
418.56(b));
• Medication monitoring (to support findings related to 418.102(b) Initial certification,
418.106(a)(1) , 418.112(e)(6) coordination of SNF care;

• Treatments and interventions by all disciplines (418.56 related to the
multidisciplinary, individualized plan of care);
• Transfer or discharge summary as applicable (see 418.104(d));
• Is the clinical record up to date and relevant?).
Ask the hospice to explain their system of authentication. Verify that the hospice’s
system meets currently accepted standards of practice, which could include evidence that:
• The hospice has a method to identify the author of each entry. This would include
verification of the author of faxed orders/entries or computer entries.
• If the hospice uses electronic medical records, electronic authentication must have a
user ID and password protections in place.
• Every entry, both written and electronic, must be signed and dated by the person
performing the service.
Confidentiality of Medical Records:
• How does the hospice protect the confidentiality of clinical records?
• What is the hospice’s policy on leaving and protecting clinical record information in
the patient’s home?
• If the hospice uses electronic patient records, what security safeguards are in place to
protect the electronic system against loss, theft, damage, disruption of operations or
unauthorized use?
• Is access to clinical records controlled?
• Are there measures in place to protect the patient from identity theft?
• Observe the hospice’s security practices for patient records. Are patient records (hard
copy or electronic) left unsecured or unattended?
• Verify that adequate precautions are taken to prevent physical or electronic altering.
Review all entries, including physician (written and verbal) orders to determine whether
they are legible, clear, complete, and authenticated and dated in accordance with hospice
policy and currently accepted standards of practice.
§418.106 - Drugs and biologicals, medical supplies, and durable medical equipment
While a Medicare patient is under hospice care, the hospice must provide medical
supplies and appliances, durable medical equipment, and drugs and biologicals related to
the palliation and management of the terminal illness and related conditions, as identified
in the hospice plan of care. During the home visit, evaluate whether drugs and
biologicals, medical supplies, and durable medical equipment are present according to the
written plan of care.
• Verify that the hospice drug orders (written, verbal or via electronic transmission) are
only given to (a licensed nurse, nurse practitioner (where appropriate), pharmacist, or
physician) and signed by (NP, MD/DO, PA) appropriately licensed professionals in
accordance 418.106(b)(1) and 418.106 (b)(2) and State and Federal regulations.
• Has the hospice provided necessary pain/symptom management medications in a
timely manner?
• Verify how the hospice obtains the drugs and biologicals necessary for patient care.
• For a hospice that provides inpatient care, verify there is a written policy in
place that promotes dispensing accuracy, and that the hospice maintains
records for the receipt and disposition of all controlled drugs; verify the policy
is being followed.
• Did the IDG determine, in the plan of care, whether or not the patient or
representative can safely self-administer drugs and biologicals to the patient in his or
her home?
• For an inpatient hospice, verify that drugs and biologicals are administered according
to the CoP.
• Verify the hospice has a system in place to ensure patients are not provided (either
directly or under arrangement) outdated, mislabeled, or otherwise unusable drugs and
biologicals.
• Check the policy and procedures for managing and disposal of controlled drugs in the
patients’ home for evidence of the following:
• A copy of the hospice written policies and procedures on management and
disposal of controlled drugs to patient or patient representative was received.
• Is there evidence a discussion took place in a language and manner understood
by the patient and/or representative that reviewed the hospice policies and
procedures for managing the safe use and disposal of controlled drugs? Is
there documentation to support this finding?
• Did the inpatient hospice facility dispose of controlled drugs in compliance with
hospice policy and in accordance with State and Federal requirements? Are the
records of the receipt and disposition of all controlled drugs current and accurate?
• Has the patient/family had any problems with the equipment received? Does the
DME function as required and intended? Clinical record documentation should
verify/support their responses.
§418.110 Condition of participation: Hospices that provide inpatient care directly.
§418.110(a) Staffing
• How does the hospice ensure that there is adequate staff on duty, especially during
the evening, nighttime, weekends, and holiday shifts, to take care of the individual
needs of all patients?
• Interview patients/family to determine if they were satisfied with the care and
services they received.

• If an on-site visit is conducted, observe if the staff is responsive to patient needs and
if call bells are answered promptly.
• Do patients frequently call for assistance?
• Are patients checked frequently for safety, comfort, and positioning?
• Ask hospice management for the inpatient staffing schedules and patient census for
the past month to determine if staffing was adequate to meet patient needs.
• How does the hospice determine the staff-to-patient ratios on each shift?
• Review at least one clinical record to evaluate if staff provided the treatments,
medications, personal care, and diet in compliance with the patient’s plan of care.
• If questions arise regarding staffing patterns (staff illness, staff not reporting to work,
etc.,) review the facility’s staffing schedule and/or timecards as necessary.
§418.110(h) Toilet and bathing facilities
Assure that each floor has at least one toilet facility and shower stall large enough to
accommodate a wheelchair and patient transfer.
§418.110(j) Infection control
Review disease reporting procedures and evidence of systematic tracking of
communicable and reportable diseases. Is this program part of the hospice’s overall
quality assessment and performance improvement and education program?
Interview management and staff to determine if they are aware of the procedure to be
followed if a patient or staff contracts an infectious or communicable disease.
Determine if there have been a high number of infections unrelated to the patients’
diagnosis. If identified, what were the hospice’s response and actions to prevent these
and future occurrences?
§418.110(o)(3) Trainer requirements
Interview management and review documentation to assure the course trainer has the
appropriate qualifications.
§418.113 Condition of participation: Emergency preparedness
Emergency preparedness applies to institutional settings where patients may receive
short-term inpatient care or other facilities where the patient may be a resident such as
NF or SNFs or ICD/IIDs. See item 12 at §418.108, above. The regulation describes the
requirements for the hospice’s:
• Emergency plan—content and bi-annual review

• Communication plan—contact information for employees, physicians, other hospices,
et.al; status and needs of patients for medical care and evacuation; emergency
management services at all levels
• Policies and procedures—that support the emergency and communications plans
• Annual testing of the plan for both patient homes and the hospice inpatient facility, if
applicable
• Staff training
• Participation in an integrated health system if that is the case
Surveyors should review these plans for appropriate inclusion of all requirements, review
records for evidence of timely completion of required testing and training, as well as
interview staff regarding their knowledge of the emergency plan, and participation in
required test exercises.
§418.114 Condition of participation: Personnel qualifications
Surveyors should review personnel files of a sample of employees from each of the
following
categories to ensure they meet the educational and experiential requirements of this
condition,
including:
• Physicians
• Registered nurses and licensed practical nurses
• Hospice aides
• Physical therapists and assistants
• Occupational therapists and assistants
• Speech language therapists and assistants
• Social workers and assistants
Surveyors should check personnel records to ensure that criminal background checks
(CBCs) were performed in accordance with §418.114(d) and in compliance with State
requirements, or in the absence of state requirements, check that the CBC was completed
within 3 months of employment for all states where the individuals lived or worked for
the three years prior to being employed at the hospice being surveyed.
§418.116 Condition of participation: Compliance with Federal, State, and local laws
and regulations related to the health and safety of patients
Surveyors should ask to see the hospice’s state license, and contract with a certified
reference lab, and if they performed waived laboratory tests, their Clinical Laboratory
Improvement Act (CLIA) certificate. Check to make sure that only appropriately trained
personnel perform only waived tests. These requirements apply to each multiple
location.

If the hospice does not possess the appropriate CLIA certificate, inform the hospice that it
is in violation of CLIA law, that it must apply immediately to the SA for the appropriate
certificate, and that the hospice is out of compliance with 42 CFR § 418.116(b). Also,
refer the hospice’s noncompliance to the department within the SA responsible for CLIA
surveys.
Task 5 – Preliminary Decision Making and Analysis of Findings
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives
The general objectives of this task are to integrate findings, review and analyze all
information collected from observations, interviews, and record reviews, and to
determine whether or not the hospice meets, or is in compliance with, the CoPs. An
assessment of whether a finding is a standard-level, or a condition-level deficiency
should not be made until all pertinent information has been collected.
B. Analysis
Guidance for Citing Standard versus Condition Level Non-compliance
The surveyor’s role is to assess the quality of care and services the hospice provides and
relate those findings to the statutory and regulatory requirements. Surveyors are expected
to use their training and professional judgment, as well as the prevailing standards of
practice, in concert with the federal regulations to assess a hospice’s compliance with the
CoPs. Deficiencies must be based on regulatory requirements.
Analyze your findings relative to each requirement to determine:
• Severity of the effect or potential effect on the patient(s);
• Frequency of occurrence, and
• Impact on the delivery of services.
An isolated incident that has little or no effect on the delivery of patient services may not
warrant a deficiency citation. Conversely, isolated or not, an incident may be considered
deficient if it constitutes a significant or serious problem that adversely affects or has the
potential to adversely affect the patient(s). In each case, the surveyor must determine if
further investigation is warranted. The finding of a deficiency is based on:
• The applicable statutory or regulatory provision and not on a violation of a guideline
• The facts and existing circumstances.
Deciding whether the observed deficiency is cited at a standard or condition level
depends on factors such as frequency of occurrence, poor patient outcomes and impeding
safe and effective delivery of care, and it requires surveyors to use their professional
knowledge of patient care and clinical standards in making a deficiency determination.
When deficiencies are found during a survey, the surveyor should explain to the provider
what the deficiency is, so the provider understands why the requirement is not met. It is
not the surveyor’s job to provide consultation on how to fix the deficiencies. See SOM,
Chapter 4, section 4018 for further information on the regulatory role of the surveyor.
Task 6 – Exit Conference
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives
The general objective of this task is to informally communicate preliminary survey team
findings and provide an opportunity for the exchange of information with the hospice’s
administrator, designee, or other invited staff. The Exit Conference is both a courtesy to
the hospice and a way to expedite the hospice’s planning a response to the Form CMS-
2567, Statement of Deficiencies and Plan of Correction. An Exit Conference is not
always guaranteed, as is noted in SOM, Chapter 2, section 2724.
Prior to the Exit Conference
• The surveyor is responsible for organizing his/her presentation for and facilitating
the exit conference.
• If the surveyor feels he/she may encounter a problem during the exit conference,
he/she should contact the SA manager in advance to discuss the potential
problems and appropriate methods to handle them.
• If the survey is conducted by a survey team, the Team Coordinator would be
responsible for the above tasks.
Discontinuation of an Exit Conference
CMS’ general policy is to conduct an exit conference at the conclusion of all types of
surveys as a courtesy to the provider/supplier and to promote timely remediation of
quality of care for safety problems. However, there are some rare situations that justify
refusal to conduct or continue an exit conference. For example, as noted in SOM Chapter
2, section 2724:
• Surveyors may refuse to conduct or may discontinue the exit conference if the
hospice is represented by an attorney who is present at the conference and the
attorney attempts to turn it into an evidentiary hearing; or
• If hospice staff /administration create an environment that is hostile, intimidating,
or inconsistent with the informal and preliminary nature of an exit conference.
Under such circumstances, it is suggested that the surveyor stop the exit conference and
call the SA for further direction. If a survey team is on-site, the Team Coordinator should
take the above actions.
Recording the Exit Conference
If the facility wishes to audio tape the conference, it must provide two tapes and tape
recorders, recording the meeting simultaneously. The surveyor or Team Coordinator
should select one of the tapes at the conclusion of the exit conference to take back to the
SA. If the recording is electronic, a copy must be submitted to the surveyor immediately
upon ending the conference. Videotaping is also permitted, if: 1) the surveyor/team
agrees to this, and 2) a copy is provided the surveyor/team at the conclusion of the
conference. The surveyor or survey team is under no obligation to consent to videotaping
and is not required to offer a reason if it refuses to permit videotaping.
General Principles
The following general principles apply when conducting an exit conference:
• Hospice management determines which staff will attend the exit conference.
• The identity of individual patients or staff members must not be revealed by
surveyors when discussing the survey results. Identity includes not just the name
of an individual patient or staff member, but also includes any reference or
characterization by which identity may be deduced.
• Because of ongoing dialogue between the surveyor(s) and hospice staff during the
survey, there should be few instances where the hospice is not aware of the
surveyor concerns prior to the exit conference. Accordingly, there should be few
cases where the hospice has not already had the opportunity prior to the exit
conference to present additional information that might be relevant to the survey
findings.
Exit Conference Sequence of Events
Introductory Remarks
• Thank everyone for their cooperation during the survey.
• Reintroduce all surveyors who participated in the survey, even if they are no
longer in the facility.
• Briefly reiterate what was the reason for the survey (i.e., initial, recertification,
representative sample validation, or complaint).
• Explain how the exit conference will be conducted and any ground rules, such as,
• The exit conference is an informal meeting for surveyors to summarize their
preliminary findings;
• Brief comments on the findings may be made by the hospice, but the
surveyor/team will not engage in a debate; or
• Whether comments will be permitted in the middle of a surveyor’s presentation or
only after the presentation has concluded.
Presentation of Findings
• The findings or information conveyed at the Exit Conference are preliminary in
nature and are subject to change pursuant to the State and CMS supervisory
review processes.
• Do not refer to any specific ASPEN tag numbers when describing deficiency
findings as the tags’ numbers often identify the Condition or Standard-level
classification for most non-long-term care (NLTC) deficiencies. Additionally,
such specific details should wait supervisory review. This has been CMS’ long-
standing policy and will continue for NLTC providers and suppliers, including
hospices. In the process of completing the Form CMS-2567 after exiting the
hospice, the SA will establish which tags/regulatory text to cite for each finding.
It would be premature to make such statements during the exit conference.
• Present the findings of noncompliance, explaining why the findings indicate
noncompliance with the regulatory requirement(s). If the hospice asks for the
pertinent regulatory reference, provide the citation for the applicable CoP.
• Do not make any general characterizations about the survey results (e.g., “overall
the facility is very good” or “in general the facility is in compliance with
Medicare requirements.”) Stick to presenting the specific factual findings.
• Do not make any statements about whether the findings represent condition-level
or standard-level deficiencies. Avoid statements such as, “the condition was not
met” or “the standard was not met.” It is better to state “the requirement related
to [applicable regulation number] is not met.”
• If an immediate jeopardy (IJ) situation was identified during the review process
that had not previously been discussed with the hospice’s management, explain
the significance and need for immediate removal of the IJ. Follow instructions in
Appendix Q.
• Do not rank findings. Treat all CoP requirements as equally as possible.
• Ensure each deficiency finding is discussed at the exit conference.
Closure
Explain that the State and/or CMS Location will send the official survey findings
presented in writing to the hospice via the Form CMS-2567, Statement of Deficiencies
and Plan of Correction, which will be prepared and mailed to the hospice within 10
working days from the end of the survey. The Form CMS-2567 is the official report of
survey findings and documents each deficiency found. There will also be a letter
communicating whether or not CMS will be taking enforcement action as a result of the
survey’s findings of noncompliance.
If there are deficiencies cited and the hospice:
• Does not have deemed status, advise the hospice that it will be required to submit
a Plan of Correction (PoC) for any deficiencies cited. Inform the hospice that a
written PoC must be submitted to the survey agency within 10 calendar days
following receipt of the written statement of deficiencies, i.e., the Form CMS-
2567.
• Has deemed status, advise the hospice that it will be required to submit a PoC
(also due within 10 calendar days of receipt of the Form CMS-2567) only if the
statement of deficiencies indicates that there was condition-level noncompliance.
• The deemed status hospice may voluntarily submit a PoC even when there is only
standard-level noncompliance, but the SA will not evaluate the PoC for its
acceptability.
• Explain that pursuant to 42 CFR § 488.7(c), CMS posts inspection reports from
an SA or AO conducted on or after October 1, 2022, for hospice programs,
including copies of a hospice program’s survey deficiencies and enforcement
actions (for example, involuntary terminations) taken as a result of such surveys,
on its public website. When a PoC is required, the hospice’s PoC and timeframes
for implementation of corrective actions are incorporated into the Form CMS-
2567 by the hospice and returned to the SA.
• Explain that, if a PoC is required, the hospice will have the following three
options for each cited deficiency:
• Accept the cited deficiency stated on Form CMS-2567 and submit a PoC;
• Record objections to the cited deficiency on Form CMS-2567 and submit a PoC;
or
• Record objections to cited deficiencies on Form CMS-2567, do not submit a PoC,
and provide convincing arguments and documented evidence that the deficiencies
are invalid. CMS will consider objections and accompanying documentation that
attempt to refute the factual accuracy of the survey findings but will not consider
objections to CMS’s judgment of the level, extent, scope, or severity of a
deficiency.
• CMS reviews additional documentation submitted by an hospice making an
objection and, if the added evidence convincingly demonstrates the deficiency
finding was factually inaccurate, will make a determination about removing the
deficiency citation. In this instance, the SA will be asked to revise the CMS-
2567.
• If CMS disagrees with the hospice’s objections, the hospice must submit an
acceptable PoC. Failure to submit an acceptable PoC or failure to correct a
deficiency may result in termination of the hospice’s provider agreement in
accordance with 42 CFR § 488.1260(a)(2). See section 2728 of the SOM for
more detailed information on PoC requirements and timelines.
• Explain that an acceptable PoC must contain the following:
• Action that will be taken to correct each specific deficiency cited;

• Description of how the actions will correct, and/or improve the processes that
led to, the deficiency cited;
• The procedure for implementing the corrective actions;
• A completion date for correction of each deficiency cited;
• Monitoring and tracking procedures to ensure the PoC is effective in bringing
the hospice into compliance, and that the hospice remains in compliance with
the regulatory requirements;
• The title of the person responsible for implementing the acceptable PoC; and
• The administrator’s signature and the date signed on page 1 of the Form
CMS-2567;
Indicate that any required PoC will be reviewed by the SA, or in some cases, the CMS
Location, to determine whether it is acceptable. If a PoC is determined not to be
acceptable, it will be returned to the hospice for revision. State that in some cases, the
SA will make an unannounced revisit survey to determine whether the hospice has come
into compliance. If the exit conference was audio- or video recorded, obtain a copy of
the tape or recording before exiting the facility.
All survey team members should leave the facility together immediately following the
exit conference. If the facility staff provides further information for review, the surveyor,
or team coordinator if applicable, determines the best way to review the additional
information. It is usually prudent for at least two individuals to remain if all of the team
members do not leave at the same time.
Task 7 – Post-Survey Activities
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
General Objectives
After completing the survey, surveyors must prepare documents that report their findings
and collaborate with their managers for concurrence on survey outcomes. These
outcomes are documented on the Form CMS-2567 and communicated to the hospice in a
timely manner.
General Procedures
Formation of the Statement of Deficiencies
Follow SOM, Chapter 2, section 2728 for preparation of the Statement of Deficiencies
and Plan of Correction. Refer to the document “Principles of Documentation for the
Statement of Deficiencies” for detailed instructions on completing the Form CMS-2567.
If hospice deficiencies are identified as a result of observations, interviews, or record
reviews, cite these deficiencies on the Form CMS-2567. These deficiencies could
include, but are not limited to:
• Failure to promote and protect the patient’s rights;
• Failure to accurately conduct a patient-specific comprehensive assessment that
identifies the patient/family’s need for hospice care and services, and the
patient/family’s need for physical, psychosocial, emotional, and spiritual care;
• Failure to develop and implement a plan of care that meets the needs identified in
the initial or comprehensive assessment;
• Failure of the IDG to meet the physical, medical, psychosocial, emotional, and
spiritual needs of the hospice patient/family;
• Failure to provide all covered services, as necessary, including the continuous
home care level of care, respite care and short-term inpatient care;
• Failure to provide nursing and physician services, drugs, and treatments on a 24-
hour basis;
• Failure to retain professional management responsibility for all hospice services
provided under contract to patients, and
• Failure to develop, implement, and maintain an effective, ongoing, hospice-wide
data-driven QAPI program.

Part II- Interpretive Guidelines
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
NOTE: In the regulations or guidance which follow, in every instance where the
following terms appear:
• “spouse” means an individual who is married to another individual as a result of
marriage lawful where entered into, including a lawful same-sex marriage, regardless
of whether the jurisdiction where the hospice is located, or in which the spouse lives,
permits such marriages to occur or recognizes such marriages.
• “marriage” means a marriage lawful where entered into, including a lawful same-sex
marriage, regardless of whether the jurisdiction where the hospice is located, or in
which the spouse lives, permits such marriages to occur or recognizes such marriages;
• “family” includes, but is not limited to, an individual’s “spouse” (see above); and
• “relative” when used as a noun, includes, but is not limited to, an individual’s
“spouse” (see above).
Except where CMS regulations require an interpretation in accordance with State law,
wherever CMS regulation or guidance uses the above terms, or includes a reference to a
patient’s “representative,” “surrogate,” “support person,” “next-of-kin,” or similar term
as would normally, implicitly or explicitly, include a spouse, the terms are to be
interpreted as in the guidance above.
A hospice is expected to recognize all lawful marriages and spouses for purposes of
compliance with the Conditions of Participation, regardless of any laws to the contrary of
the state or locality or other jurisdiction where the hospice is located or where the spouse
lives.
§418.3 Definitions.
For the purposes of this part—
Attending physician means a —
(1)(i) Doctor of medicine or osteopathy legally authorized to practice medicine
and surgery by the State in which he or she performs that function or action; or
(ii) Nurse practitioner who meets the training, education, and experience
requirements as described in §410.75 (b) of this chapter; or
(iii) Physician assistant who meets the requirements of §410.74(c) of this chapter.

(2) Is identified by the individual, at the time he or she elects to receive hospice
care, as having the most significant role in the determination and delivery of the
individual's medical care.
Bereavement counseling means emotional, psychosocial, and spiritual support and
services provided before and after the death of the patient to assist with issues related to
grief, loss, and adjustment.
BFCC-QIO means Beneficiary and Family Centered Care Quality Improvement
Organization
Cap period means the twelve-month period ending September 30 used in the application
of the cap on overall hospice reimbursement specified in §418.309.
Clinical note means a notation of a contact with the patient and/or the family that is
written and dated by any person providing services and that describes signs and
symptoms, treatments and medications administered, including the patient's reaction
and/or response, and any changes in physical, emotional, psychosocial or spiritual
condition during a given period of time.
Comprehensive assessment means a thorough evaluation of the patient’s physical,
psychosocial, emotional and spiritual status related to the terminal illness and related
conditions. This includes a thorough evaluation of the caregiver’s and family’s
willingness and capability to care for the patient.
Dietary counseling means education and interventions provided to the patient and family
regarding appropriate nutritional intake as the patient’s condition progresses. Dietary
counseling is provided by qualified individuals, which may include a registered nurse,
dietitian or nutritionist, when identified in the patient’s plan of care.
Employee means a person who:
(1) works for the hospice and for whom the hospice is required to issue a W-2 form on
his or her behalf; or
(2) if the hospice is a subdivision of an agency or organization, an employee of the
agency or organization who is assigned to the hospice; or
(3) is a volunteer under the jurisdiction of the hospice.
Hospice means a public agency or private organization or subdivision of either of these
that is primarily engaged in providing hospice care as defined in this section.
Hospice care means a comprehensive set of services described in Section 1861(dd)(1) of
the Act, identified and coordinated by an interdisciplinary group (IDG) to provide for the

physical, psychosocial, spiritual, and emotional needs of a terminally ill patient and/or
family members, as delineated in a specific patient plan of care.
Initial assessment means an evaluation of the patient’s physical, psychosocial and
emotional status related to the terminal illness and related conditions to determine the
patient’s immediate care and support needs.
Licensed professional means a person licensed to provide patient care services by the
State in which services are delivered.
Multiple location means a Medicare-approved location from which the hospice provides
the same full range of hospice care and services that is required of the hospice issued the
certification number. A multiple location must meet all of the conditions of participation
applicable to hospices.
Palliative care means patient and family-centered care that optimizes quality of life by
anticipating, preventing, and treating suffering. Palliative care throughout the continuum
of illness involves addressing physical, intellectual, emotional, social, and spiritual needs
and to facilitate patient autonomy, access to information, and choice.
Physician means an individual who meets the qualifications and conditions as defined in
Section 1861(r) of the Act and implemented at §410.20 of this chapter.
Physician designee means a doctor of medicine or osteopathy designated by the hospice
who assumes the same responsibilities and obligations as the medical director when the
medical director is not available.
Pseudo-patient means a person trained to participate in a role-play situation, or a
computer-based mannequin device. A pseudo-patient must be capable of responding to
and interacting with the hospice aide trainee, and must demonstrate the general
characteristics of the primary patient population served by the hospice in key areas such
as age, frailty, functional status, cognitive status and care goals.
Representative means an individual who has the authority under State law (whether by
statute or pursuant to an appointment by the courts of the State) to authorize or terminate
medical care or to elect or revoke the election of hospice care on behalf of a terminally ill
patient who is mentally or physically incapacitated. This may include a legal guardian.
Restraint means:
(1) Any manual method (physical or mechanical device, material, or equipment) that
immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or
head freely. Manual methods of restraint do not include devices such as orthopedically
prescribed devices, surgical dressings or bandages, protective helmets, or other methods
of physical holding for the purpose of conducting routine physical examinations or tests,

to protect the patient from falling, or to participation in activities without the risk of
physical harm (this does not include a physical escort).
(2) A drug or medication used to restrict behavior or freedom of movement that is not a
standard treatment or dosage for the patient’s condition.
Seclusion means the involuntary confinement of a patient alone in a room or an area
from which the patient is physically prevented from leaving.
Simulation means a training and assessment technique that mimics the reality of the
homecare environment, including environmental distractions and constraints that evoke
or replicate substantial aspects of the real world in a fully interactive fashion, in order to
teach and assess proficiency in performing skills, and to promote decision making and
critical thinking.
Terminally ill means that the individual has a medical prognosis that his or her life
expectancy is 6 months or less if the illness runs its normal course.

L500
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52 Condition of Participation: Patient’s rights
The patient has the right to be informed of his or her rights, and the hospice must
protect and promote the exercise of these rights.
L502
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(a) Standard: Notice of rights and responsibilities.
(1) During the initial assessment visit in advance of furnishing care the hospice must
provide the patient or representative with verbal (meaning spoken) and written
notice of the patient's rights and responsibilities in a language and manner that the
patient understands.
Interpretive Guidelines §418.52(a)(1)
When reference is made to “patient” in the Guidelines, it also refers to any person who
may, under State law, act on the patient’s behalf when the patient is unable to act for him
or herself. That person is referred to as the patient’s surrogate or representative. If a court
has formally declared the patient incompetent, the surrogate or representative is
whomever the court guardian, conservator, or committee appointed. The hospice should
verify that the representative has the necessary authority. For example, a court-appointed
conservator might have the power to make financial decisions, but not health care
decisions.
All hospice patients should be aware of their rights and responsibilities before the hospice
begins to provide care. The hospice must verbally explain the patient rights and
responsibilities to all patients accepted for care (or explain the rights to the patient’s
representative if the patient is physically or mentally incapacitated).
There must be evidence that the hospice conscientiously tried, within the constraints of
the individual situation, to inform the patient/family both verbally (spoken) and in writing
of patient rights and responsibilities. If a patient is able to read and understand written
materials without assistance, an oral summary, along with the complete written
documentation is acceptable.
For the patient who does not speak or understand English, hospices should make all
reasonable efforts to secure a professional, objective translator for hospice-patient
communications, including those involving the notice of patient rights and
responsibilities. The hospice may only use family and friends as translators for the

patient when the hospice cannot secure an objective translator or if the patient
specifically requests this approach. Hospices should make all reasonable efforts to have
written copies of the notice of rights and responsibilities available in the language(s) that
are commonly spoken in the hospice’s service area. For those patients who speak
languages in areas where professional translators for those languages are not readily
available, using family and friends of the patient is an acceptable option if the patient
agrees.
Further information on this topic is available from the Department of Health and Human
Services, Office for Civil Rights Policy Guidance: Title VI Prohibition Against National
Origin Discrimination Affecting Limited English Proficiency Persons
L503
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(a)(2) - The hospice must comply with the requirements of subpart I of part
489 of this chapter regarding advance directives. The hospice must inform and
distribute written information to the patient concerning its policies on advance
directives, including a description of applicable State law.
Interpretive Guidelines §418.52(a)(2)
Advance directives generally refer to written statements or instructions, completed in
advance of a serious illness, about how an individual wants medical decisions made. The
two most common forms of advance directives are a living will and a durable medical
power of attorney for health care. It is the patient’s right to formulate an advance
directive should he/she wish to do so. The patient’s desire not to formulate an advance
directive, nor the contents of an advance directive should not affect admission to hospice.
There may be State specific requirements for advance directives that the hospice must
follow.
The hospices’ obligations under 42 CFR 489.102 include the following requirements:
Hospices must maintain written policies and procedures concerning advance directives
with respect to all adult individuals receiving medical care by or through the provider and
are required to:
(1) Provide written information to such individuals concerning:
(i) An individual's rights under State law (whether statutory or recognized by the
courts of the State) to make decisions concerning such medical care, including
the right to accept or refuse medical or surgical treatment and the right to
formulate, at the individual's option, advance directives. Providers are
permitted to contract with other entities to furnish this information but are still
legally responsible for ensuring that the requirements of this section are met.
Providers are to update and disseminate amended information as soon as
possible, but no later than 90 days from the effective date of the changes to
State law; and
(ii) The written policies of the provider or organization respecting the implementation
of such rights, including a clear and precise statement of limitation if the
provider cannot implement an advance directive based on conscience. At a
minimum, a provider's statement of limitation should:
(A) Clarify any differences between institution-wide conscience objections and
those that may be raised by individual physicians;
(B) Identify the state legal authority permitting such objection, and
(C) Describe the range of medical conditions or procedures affected by the
conscience objection.
(2) Document in a prominent part of the individual's current medical record, or patient
care record in the case of an individual in a religious nonmedical health care
institution, whether or not the individual has executed an advance directive;
(3) Not condition the provision of care or otherwise discriminate against an individual
based on whether or not the individual has executed an advance directive;
(4) Ensure compliance with requirements of State law (whether statutory or recognized
by the courts of the State) regarding advance directives. The provider must inform
individuals that complaints concerning the advance directive requirements may be
filed with the State survey and certification agency;
(5) Provide for education of staff concerning its policies and procedures on advance
directives, and
(6) Provide for community education regarding issues concerning advance directives that
may include material required in paragraph (a)(1) of this section, either directly or in
concert with other providers and organizations. Separate community education
materials may be developed and used, at the discretion of providers. The same
written materials do not have to be provided in all settings, but the material should
define what constitutes an advance directive, emphasizing that an advance directive
is designed to enhance an incapacitated individual's control over medical treatment,
and describe applicable State law concerning advance directives. A provider must
be able to document its community education efforts.
Hospices must furnish this information to the patient at the time of initial receipt of
hospice care by the individual from the hospice. Hospices:

1. Are not required to provide care that conflicts with an advance directive; and
2. Are not required to implement an advance directive if, as a matter of conscience, it
cannot implement an advance directive and State law allows the hospice to
conscientiously object.
If an adult individual is incapacitated at the time of admission or at the start of care and is
unable to receive information (due to the incapacitating conditions or a mental disorder)
or articulate whether or not he or she has executed an advance directive, then the hospice
may give advance directive information to the individual's family or surrogate in the
same manner that it issues other materials about policies and procedures to the family of
the incapacitated individual or to a surrogate or other concerned persons in accordance
with State law. The hospice is not relieved of its obligation to provide this information to
the individual once he or she is no longer incapacitated or unable to receive such
information. Follow-up procedures must be in place to provide the information to the
individual directly at the appropriate time.
Compliance with the advance directives requirements is necessary for continued
participation in the Medicare and Medicaid programs.
L504
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(a)(3) The hospice must obtain the patient’s or representative’s signature
confirming that he or she has received a copy of the notice of rights and
responsibilities.
L505
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b) Standard: Exercise of rights and respect for property and person
(1) The patient has the right:
(i) To exercise his or her rights as a patient of the hospice;
(ii) To have his or her property and person treated with respect;
(iii) To voice grievances regarding treatment or care that is (or fails to be)
furnished and the lack of respect for property by anyone who is furnishing
services on behalf of the hospice; and

(iv) To not be subjected to discrimination or reprisal for exercising his or her
rights.
Interpretive Guidelines §418.52(b)(1)(i)-(iv)
Patients must be free to exercise their rights without fear of reprisal from the hospice.
The hospice must not hamper, compel, treat differentially, or retaliate against a patient or
family for exercising the patient’s rights. The hospice must assure that its staff will
protect patients’ rights and will involve patients in decisions about their care, treatment
and services.
A grievance is a formal or informal written or verbal complaint that is made to any
hospice employee, including volunteers and individuals furnishing hospice services under
arrangement, by a patient or the patient’s representative regarding the patient’s care,
abuse, neglect, or misappropriation of property. Hospices should inform patients and
family/caregivers of accurate information for filing a complaint.
L506
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b)(2) If a patient has been adjudged incompetent under state law by a court
of proper jurisdiction, the rights of the patient are exercised by the person
appointed pursuant to state law to act on the patient's behalf.
L507
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b)(3) - If a state court has not adjudged a patient incompetent, any legal
representative designated by the patient in accordance with state law may exercise
the patient’s rights to the extent allowed by state law.
L508
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b)(4) The hospice must:
(i) Ensure that all alleged violations involving mistreatment, neglect, or verbal,
mental, sexual, and physical abuse, including injuries of unknown source,
and misappropriation of patient property by anyone furnishing services on
behalf of the hospice, are reported immediately by hospice employees and
contracted staff to the hospice administrator;
Interpretive Guidelines §418.52(b)(4)(i)
All patient complaints and alleged or real violations included in this standard must be
reported immediately to the hospice administrator and should be investigated, resolved
and documented. The hospice must ensure that all hospice employees and contracted
staff are trained on how and when to report allegations involving mistreatment, neglect,
or verbal, mental, sexual, and physical abuse by anyone furnishing services on behalf of
the hospice. This includes reporting injuries of unknown origin, as well as
misappropriation of patient property.
• “Abuse” means the willful infliction of injury, unreasonable confinement,
intimidation, or punishment with resulting physical harm, pain or mental anguish.
• “Verbal abuse” includes the use of oral, written or gestured language that
willfully includes disparaging and derogatory terms to patients or their families,
or within their hearing distance, regardless of their age, ability to comprehend, or
disability.
• “Mental abuse” includes, but is not limited to, humiliation, harassment, and
threats of punishment or deprivation.
• “Sexual abuse” includes, but is not limited to, sexual harassment, sexual
coercion, or sexual assault.
• “Physical abuse” includes, but is not limited to, hitting, slapping, pinching and
kicking. It also includes controlling behavior through corporal punishment.
• “Neglect” means failure to provide goods and services necessary to avoid
physical harm or mental anguish.
• “Misappropriation of patient property” means the deliberate misplacement,
exploitation, or wrongful, temporary or permanent use of a patient’s belongings or
money without the patient’s consent.
• “Injuries of unknown source” – An injury should be classified as an “injury of
unknown source” when both of the following conditions are met:
1. The source of the injury was not observed by any person or the source of
the injury could not be explained by the patient; and
2. The injury is suspicious because of the extent of the injury or the location
of the injury (e.g., the injury is located in an area not generally vulnerable
to trauma) or the number of injuries observed at one particular point in
time or the incidence of injuries over time.
• “Immediately” means as soon as possible, but not to exceed 24 hours after
discovery of the incident, in the absence of a shorter State time frame
requirement.
L509
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b)(4)(ii) - Immediately investigate all alleged violations involving anyone
furnishing services on behalf of the hospice and immediately take action to prevent
further potential violations while the alleged violation is being verified.
Investigations and/or documentation of all alleged violations must be conducted in
accordance with established procedures;
L510
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b)(4)(iii) Take appropriate corrective action in accordance with state law if
the alleged violation is verified by the hospice administration or an outside body
having jurisdiction, such as the State survey agency or local law enforcement
agency; and
L511
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(b)(4)(iv) Ensure that verified violations are reported to State and local
bodies having jurisdiction (including to the state survey and certification agency)
within 5 working days of becoming aware of the violation.
Interpretive Guidelines §418.52(b)(4)(iv)
The hospice has 5 working days from becoming aware of the violation to investigate any
alleged violations and, if the alleged violation is verified, it must report the verified
violation to the State and local bodies having jurisdiction within those 5 days. If State
requirements for reporting verified violations are more stringent than Federal
requirements, the more stringent State requirements take precedence. The stringent State
requirements may be those that require violations to be reported regardless of whether
they are verified or not, or requirements that verified violations be reported in less than 5
days. However, if State requirements are less stringent than Federal requirements, the
Federal requirements take precedence.

L512
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(c) Standard: Rights of the patient
The patient has a right to the following:
§418.52(c)(1) Receive effective pain management and symptom control from the
hospice for conditions related to the terminal illness;
Interpretive Guidelines §418.52(c)(1)
Hospices are responsible for managing the patient’s pain and symptoms related to the
terminal illness and related conditions in a timely fashion. Patients should not have to
experience long waits for pain and symptom management, medications, or interventions
to address the patient’s condition. Hospices should have methods in place to assure that
the patient’s pain, and all other distressing symptoms, are controlled effectively 24 hours
a day/7days per week, in all settings and wherever the patient resides.
L513
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52 (c)(2) Be involved in developing his or her hospice plan of care;
L514
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52 (c)(3) Refuse care or treatment;
L515
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(c)(4) Choose his or her attending physician;
Interpretive Guidelines §418.52(c)(4)
Patients have the right to choose their attending physician (generally a provider for whom
the beneficiary has a relationship with and is not part of the current hospice staff) and to
have this person involved in their medical care in collaboration with the hospice medical

staff. An attending physician (if any) can also manage those aspects of his/her health
care unrelated to the hospice services being provided.
L516
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(c)(5) - Have a confidential clinical record. Access to or release of patient
information and clinical records is permitted in accordance with 45 CFR Parts 160
and 164.
Interpretive Guidelines §418.52(c)(5)
The right to confidential clinical records means safeguarding the content, including paper
records and/or electronically stored information from unauthorized disclosure without the
specific informed consent of the patient or legal representative.
L517
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(c)(6) - Be free from mistreatment, neglect, or verbal, mental, sexual, and
physical abuse, including injuries of unknown source, and misappropriation of patient
property;
Interpretive Guidelines §418.52(c)(6)
States commonly have mandatory reporting requirements for providers, suppliers, and
individuals making them legally responsible to report suspicions of abuse and neglect to
appropriate State authorities. These facilities and individuals should follow existing
mandatory reporting requirements in their State, in addition to any Federal requirements.
Action or inaction on the part of a provider or supplier to follow mandatory reporting
requirements does not preclude an employee from fulfilling their individual reporting
obligations.
Hospices should maintain documentation of any reports filed with law enforcement or
State, local, or Federal authorities related to abuse and neglect.
The hospice should document the following information:
• Who submitted the report, including name and contact information;
• Who did the reporter contact, including the appropriate authority or law
enforcement entity, name, and contact information;
• Date/Time that the report was filed;
• Any copies of the report made to the appropriate authority or law enforcement, if
available;

• What information was conveyed to the appropriate authority or law enforcement;
and
• The police report number provided by the appropriate authority or law
enforcement.
L518
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(c)(7) - Receive information about the services covered under the hospice
benefit;
Interpretive Guidelines §418.52(c)(7)
Medicare covered hospice services are set forth at 42 CFR 418.200-204. The hospice
should fully inform Medicare patients about all Medicare covered hospice services and
fully inform non-Medicare patients about any other hospice services that apply to the
patient (e.g., Medicaid, private insurance).
L519
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.52(c)(8) - Receive information about the scope of services that the hospice will
provide and specific limitations on those services.
L520
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54 Condition of participation: Initial and Comprehensive assessment of the
patient
The hospice must conduct and document in writing a patient-specific comprehensive
assessment that identifies the patient’s need for hospice care and services, and the
patient’s need for physical, psychosocial, emotional, and spiritual care. This
assessment includes all areas of hospice care related to the palliation and
management of the terminal illness and related conditions.
Interpretive Guidelines §418.54
The comprehensive patient assessment must accurately reflect the patient’s current health
status and include information to establish and monitor a plan of care. Hospices are not
required to use specific forms or formats to document their initial or comprehensive
assessments. They may choose to document patient specific comprehensive assessments

in either written or electronic format provided the assessments are complete, readily
identifiable, and available in the patient’s clinical record.
L522
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(a) Standard: Initial assessment
The hospice registered nurse must complete an initial assessment within 48 hours
after the election of hospice care in accordance with §418.24 is complete (unless the
physician, patient, or representative requests that the initial assessment be
completed in less than 48 hours.)
Interpretive Guidelines §418.54(a)
The purpose of the initial assessment is to gather the critical information necessary to
treat the patient/family’s immediate care needs. The assessment needs to take place in
the location where hospice services are being delivered. The initial assessment is not a
“meet and greet” visit whereby the hospice introduces itself to the patient/family and
begins to evaluate the patient’s interest in and appropriateness for hospice care. It must
assess the patient’s immediate physical, psychosocial, emotional and spiritual status
related to the terminal illness and related conditions. The initial assessment is necessary
to gather the essential information necessary to begin the plan of care and provide the
immediate necessary care and services.
The RN must conduct this initial assessment. Hospices may choose to send a social
worker or other discipline along with the RN to complete the initial assessment.
Hospices are free to choose their own method for documenting the initial assessment.
L523
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(b) Standard: Timeframe for completion of the comprehensive assessment
The hospice interdisciplinary group, in consultation with the individual’s attending
physician (if any), must complete the comprehensive assessment no later than 5
calendar days after the election of hospice care in accordance with §418.24.
Interpretive Guidelines §418.54(b)
All members of the IDG must be involved with completing the comprehensive
assessment in order to identify the patient/family’s physical, psychosocial, emotional and

spiritual needs and contribute to the development of the plan of care to address those
needs. The individuals/disciplines that complete the assessment should be consistent
with the hospice's own policies and procedures and the discipline's scope of practice. The
RN, in consultation with the other members of the IDG, considers the information
gathered from the initial assessment as they develop the plan of care and the group
determines who should visit the patient/family during the first 5 days of hospice care in
accordance with patient/family needs and desires and the hospice's own policies and
procedures.
The patient may or may not have an attending physician. If the attending physician is
unavailable or unresponsive, the hospice physician must assume this role. If the patient
does have an attending physician, one or more members of the IDG should consult with
this physician in completing the comprehensive assessment. This consultation can occur
through phone calls or other means of communication (Fax, e-mails, text messages, etc.,)
and will help to acquire a better understanding of the patient and family. Attending
physicians can often provide a history of the patient’s disease process and family
dynamics that can help the hospice make better care planning decisions that address all
areas of need related to the terminal illness and related conditions, resulting in improved
patient outcomes.
The “election of hospice care” is the effective date of the election statement. The patient
may sign the hospice election statement with a later (not earlier) effective date. Hospices
may choose to complete the comprehensive assessment earlier than 5 days after the
effective date of the election (e.g., it may complete the comprehensive assessment at the
same time the initial assessment is completed).
L524
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c) Standard: Content of the comprehensive assessment
The comprehensive assessment must identify the physical, psychosocial, emotional,
and spiritual needs related to the terminal illness that must be addressed in order to
promote the hospice patient’s well-being, comfort, and dignity throughout the dying
process.
Interpretive Guidelines §418.54(c)
The assessment would include, but not be limited to, screening for the following: pain,
dyspnea, nausea, vomiting, constipation, restlessness, anxiety, sleep disorders, skin
integrity, confusion, emotional distress, spiritual needs, support systems, and family need
for counseling and education. The hospice would then gather additional information, as
necessary, to be able to meet the patient/family needs. For example, in addition to
screening the patient for the presence of pain, a comprehensive assessment of the

patient’s pain based on accepted clinical standards of practice may necessitate gathering
the following information, as applicable to the patient:
• History of pain and its treatment (including non-pharmacological and
pharmacological treatment);
• Characteristics of pain, such as:
– Intensity of pain (e.g., as measured on a standardized pain scale);
– Descriptors of pain (e.g., burning, stabbing, tingling, aching);
– Pattern of pain (e.g., constant or intermittent);
– Location and radiation of pain;
– Frequency, timing and duration of pain;
– Impact of pain on quality of life (e.g., sleeping, functioning, appetite, and
mood);
– Factors such as activities, care, or treatment that precipitate or exacerbate
pain;
– Strategies and factors that reduce pain; and
– Additional symptoms associated with pain (e.g., nausea, anxiety).
• Physical examination (may include the pain site, the nervous system, mobility and
function, and physical, psychological and cognitive status);
• Current medical conditions and medications; and
• The patient/family’s goals for pain management and their satisfaction with the
current level of pain control.
L525
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c) The comprehensive assessment must take into consideration the
following factors:
§418.54(c)(1) The nature and condition causing admission (including the presence or
lack of objective data and subjective complaints).

L526
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(2) Complications and risk factors that affect care planning.
L527
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(3) Functional status, including the patient’s ability to understand and
participate in his or her own care.
L528
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(4) Imminence of death.
L529
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(5) Severity of symptoms.
L530
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(6) Drug profile. A review of all of the patient's prescription and over-
the-counter drugs, herbal remedies and other alternative treatments that could
affect drug therapy. This includes, but is not limited to, identification of the
following:
i. Effectiveness of drug therapy
ii. Drug side effects
iii. Actual or potential drug interactions
iv. Duplicate drug therapy
v. Drug therapy currently associated with laboratory monitoring.
Interpretive Guidelines §418.54(c)(6)

In reviewing the patient’s prescribed and over-the-counter medications and any additional
substance that could affect drug therapy, the hospice must consider drug effectiveness,
side effects, interactions of drugs, duplicate drugs and drugs associated with laboratory
testing which could affect the patient. In addition, the hospice should consider both the
use of pharmacological and non-pharmacological interventions to promote the patient’s
comfort level and sense of well-being based on the assessment of patient needs and
desires.
“Medication Interaction” is the impact of another substance (such as another
medication, nutritional supplement (including herbal products), food, or substances used
in diagnostic studies) upon a medication’s action. The interactions may alter absorption,
distribution, metabolism, or elimination. These interactions may decrease the
effectiveness of the medication or increase the potential for adverse consequences.
“Duplicate therapy” refers to multiple medications of the same pharmacological
class/category or any medication therapy that substantially duplicates a particular effect
of another medication that the individual is taking.
“Non-pharmacological interventions” refers to approaches to care that do not involve
medications, generally directed towards stabilizing or improving a person’s mental,
physical or psychosocial well being.
L531
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(7) Bereavement. An initial bereavement assessment of the needs of the
patient's family and other individuals focusing on the social, spiritual, and cultural
factors that may impact their ability to cope with the patient's death. Information
gathered from the initial bereavement assessment must be incorporated into the
plan of care and considered in the bereavement plan of care.
Interpretive Guidelines §418.54(c)(7)
Although a bereavement plan is initiated after the death of the patient, prior to the death,
the hospice must assess any grief/loss issues of the patient’s family through an initial
bereavement risk assessment that is incorporated in the plan of care. Bereavement issues
continue to be part of the ongoing assessments, and the bereavement plan of care after
death is based on all these assessments. Bereavement services may be offered prior to the
death when the initial assessment, comprehensive assessment, or updates to the
assessment identifies the need for the patient/family.
Social, spiritual and cultural factors that may impact a family member or other
individual’s ability to cope with the patient’s death would include, but not be limited to:

• History of previous losses;
• Family problems;
• Financial concerns;
• Communication issues;
• Drug and alcohol abuse;
• Health concerns;
• Legal and financial concern;
• Mental health issues;
• Presence or absence of a support system; and
• Feelings of despair, anger, guilt or abandonment.
These issues may not be readily apparent during the initial bereavement risk assessment,
but should be incorporated into the hospice plan of care if they become evident, and must
be considered in the bereavement plan of care.
L532
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(c)(8) The need for referrals and further evaluation by appropriate health
professionals.
L533
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(d) Standard: Update of the comprehensive assessment
The update of the comprehensive assessment must be accomplished by the hospice
interdisciplinary group (in collaboration with the individual’s attending physician,
if any) and must consider changes that have taken place since the initial assessment.
It must include information on the patient's progress toward desired outcomes, as
well as a reassessment of the patient’s response to care. The assessment update
must be accomplished as frequently as the condition of the patient requires, but no
less frequently than every 15 days.
Interpretive Guidelines §418.54(d)
Hospices are free to choose their own method for documenting updates to the assessment.
The hospice should evaluate and document the patient’s response to the care, treatment
and services provided, and progress toward desired outcomes. The purpose of updating
the assessment is to ensure that the hospice IDG has the most recent accurate information
about the patient/family in order to make accurate care planning decisions. Assessment
updates should be easily identified in the clinical record.

Hospices are required to update the comprehensive assessment as frequently as the
condition of the patient requires, which may be more frequently than every 15 days. The
hospice must ensure that each update is completed no later than 15 days from the
previous one. Hospices are not required to complete, in full, those documents that they
identified as comprising their comprehensive assessment every 15 days, although
hospices are free to do so if they so choose. They are required to identify and document
if there were no changes in the patient/family condition or needs.
L534
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(e) Standard: Patient outcome measures
(1) The comprehensive assessment must include data elements that allow for
measurement of outcomes. The hospice must measure and document data in the
same way for all patients. The data elements must take into consideration aspects of
care related to hospice and palliation.
Interpretive Guidelines §418.54(e)(1)
Examples of data elements that would allow for the measurement of outcomes include,
but are not limited to, patient reported data on outcomes of treatment for pain, dyspnea,
nausea, vomiting, constipation, emotional distress, and spiritual needs. For example, a
hospice may choose to measure patients whose pain is controlled within 48 hours of
admission. Incorporating a data element into the initial assessment and comprehensive
assessment will identify the patients that had pain upon admission and identify the
patients that had their pain controlled within 48 hours.
L535
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.54(e)(2) The data elements must be an integral part of the comprehensive
assessment and must be documented in a systematic and retrievable way for each
patient. The data elements for each patient must be used in individual patient care
planning and in the coordination of services, and must be used in the aggregate for
the hospice’s quality assessment and performance improvement program.
L536
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56 Condition of participation: Interdisciplinary group, care planning, and
coordination of services

The hospice must designate an interdisciplinary group or groups as specified in
paragraph (a) of this section which, in consultation with the patient's attending
physician, must prepare a written plan of care for each patient. The plan of care
must specify the hospice care and services necessary to meet the patient and family-
specific needs identified in the comprehensive assessment as such needs relate to the
terminal illness and related conditions.
Interpretive Guidelines §418.56
The physician member of the IDG may be the hospice medical director or another
hospice physician who is employed by or under contract with the hospice. The nurse,
social worker and counselor members of the IDG must be hospice employees or
employees of the agency or organization of which the hospice is a sub-division (e.g., a
hospital) who are appropriately trained and assigned to the hospice.
There should be a direct link between the needs identified in the patient/family
assessment and the plan of care developed by the hospice. Hospices may identify needs
in the comprehensive assessment that are not related to the terminal illness and related
conditions, and should document that they are aware of these needs and note who is
addressing them. Hospices are not required to provide direct services to meet needs
unrelated to the terminal illness. Hospices are responsible for including services and
treatments in the plan of care that address how they will meet the patient and family-
specific needs related to the terminal illness and related conditions.
The medical director and/or other hospice physician is responsible for meeting the
medical needs of the patient according to §418.64(a)(3) per the patient’s attending
physician’s request or when the hospice is unable to contact the attending physician to
address the patient’s medical needs.
L539
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(a) Standard: Approach to service delivery
§418.56(a)(1) The hospice must designate an interdisciplinary group or groups
composed of individuals who work together to meet the physical, medical,
psychosocial, emotional, and spiritual needs of the hospice patients and families
facing terminal illness and bereavement. Interdisciplinary group members must
provide the care and services offered by the hospice, and the group, in its entirety,
must supervise the care and services.
Interpretive Guidelines §418.56(a)(1)

Members of the IDG must be appropriately trained in the hospice philosophy and
competent to perform in their assigned area(s). The hospice may involve other members
of the care team in the IDG’s activities.
“Supervision” of care by the IDG members may be accomplished by face-to- face or
telephonic conferences, evaluations, discussions and general oversight, as well as by
direct observations.
L540
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(a)(1) The hospice must designate a registered nurse that is a member of the
interdisciplinary group to provide coordination of care and to ensure continuous
assessment of each patient’s and family's needs and implementation of the
interdisciplinary plan of care.
L541
(Rev. 222; Issued: 06-07-24; Effective: 06-07-24; Implementation: 06-07-24)
§418.56(a)(1) …The interdisciplinary group must include, but is not limited to,
individuals who are qualified and competent to practice in the following
professional roles:
(i) A doctor of medicine or osteopathy (who is an employee or under contract
with the hospice).
i. (ii) A registered nurse.
ii. (iii) A social worker, marriage and family therapist, or a mental health
counselor.
iii. (iv) A pastoral or other counselor.
Interpretive Guidelines §418.56(a)(1)(i)-(iv)
The number of individuals on the IDG is not as important as their qualifications and
abilities. For example, if a group member meets the hospice criteria and is licensed as a
RN and also meets the Medicare criteria to be considered a social worker under the
hospice benefit, he/she would be qualified to serve on the IDG as both a nurse and a
social worker.
L542
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.56(a)(2) If the hospice has more than one interdisciplinary group, it must
identify a specifically designated interdisciplinary group to establish policies
governing the day-to-day provision of hospice care and services.
Interpretive Guidelines §418.56(a)(2)
If the hospice has more than one IDG, it may select members from different IDGs to
serve on the IDG that establishes the hospice’s policies, as long as all required disciplines
are represented (e.g., physician, RN, social worker, counselor).
L543
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(b) Standard: Plan of care
All hospice care and services furnished to patients and their families must follow an
individualized written plan of care established by the hospice interdisciplinary
group in collaboration with the attending physician (if any), the patient or
representative, and the primary caregiver in accordance with the patient’s needs if
any of them so desire.
L544
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(b) - The hospice must ensure that each patient and the primary care
giver(s) receive education and training provided by the hospice as appropriate to
their responsibilities for the care and services identified in the plan of care.
L545
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c) Standard: Content of the plan of care
The hospice must develop an individualized written plan of care for each patient.
The plan of care must reflect patient and family goals and interventions based on
the problems identified in the initial, comprehensive, and updated comprehensive
assessments. The plan of care must include all services necessary for the palliation
and management of the terminal illness and related conditions, including the
following:

L546
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c)(1) Interventions to manage pain and symptoms.
Interpretive Guidelines §418.56(c)(1)
The goal of effective pain and symptom management is quality of life. When the pain
and symptoms that cause distress to the patient are effectively managed, the patient and
family are better able to focus on their vision of a “good death.” Effective pain and
symptom management include the ongoing assessment of the patient’s physical,
psychosocial, emotional and spiritual needs and re-evaluating the effectiveness of the
current plan of care in order to address those needs.
The hospice may also include the use of alternative therapies in the plan of care, to
benefit hospice patients/families (e.g., art, yoga, massage, music and light therapy).
L547
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c)(2) A detailed statement of the scope and frequency of services necessary
to meet the specific patient and family needs.
Interpretive Guidelines §418.56(c)(2)
The use of visit ranges in the patient plan of care should follow these parameters:
• The plan of care may include a range of visits and ’provide as needed’ orders for
visit frequencies to ensure the most appropriate level of service is provided to the
patient.
• A range of visits is acceptable as long as it continues to meet the identified needs
of the patient/family.
• Visit ranges with small intervals are acceptable (i.e., 1-3 visits/week; 2-4
visits/week) but ranges that include “0” as a frequency are not allowed.
• The IDG may exceed the number of visits in the range to address patient/family’s
needs. There should be documentation in the record to support the need for the
extra visit(s).
If the patient requires frequent use of PRN visits, the plan of care should be updated to
include the need for additional visits.
Standing orders or routine orders must be individualized to address the specific patient’s
needs and signed by the patient’s physician.

The IDG should be proactive in developing each patient’s plan of care by planning ahead
for anticipated patient changes and needs. Decisions should reflect the patient/family
preferences rather than be solely a response to a crisis.
L548
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c)(3) Measurable outcomes anticipated from implementing and
coordinating the plan of care.
Interpretive Guidelines §418.56(c)(3)
The outcomes should be a measurable result of the implementation of the plan of care.
The hospice should be using data elements as a part of the plan of care to see if they are
meeting the goals of care.
Are the outcomes documented and measurable? Look for movement towards the
expected outcome(s) and revisions to the plan of care that have been made to achieve the
outcomes.
L549
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c)(4) Drugs and treatment necessary to meet the needs of the patient.
Interpretive Guidelines §418.56(c)(4)
See guidance at §418.52(c)(1).
L550
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c)(5) Medical supplies and appliances necessary to meet the needs of the
patient.
L551
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(c)(6) The interdisciplinary group's documentation of the patient’s or
representative’s level of understanding, involvement, and agreement with the plan
of care, in accordance with the hospice’s own policies, in the clinical record.
Interpretive Guidelines §418.56(c)(6)

While the patient/family must be included in developing/updating the plan of care, they
do not need to be present during IDG meetings.
L552
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(d) Standard: Review of the plan of care
The hospice interdisciplinary group (in collaboration with the individual’s attending
physician, if any) must review, revise and document the individualized plan as
frequently as the patient’s condition requires, but no less frequently than every 15
calendar days.
Interpretive Guidelines §418.56(d)
Communication with the attending physician may be through phone calls, electronic
methods, orders received, or other means according to hospice policy and patient needs.
L553
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(d) A revised plan of care must include information from the patient's
updated comprehensive assessment and must note the patient’s progress toward
outcomes and goals specified in the plan of care.
L554
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(e) Standard: Coordination of services
The hospice must develop and maintain a system of communication and integration,
in accordance with the hospice’s own policies and procedures, to-
§418.56(e)(1) Ensure that the interdisciplinary group maintains responsibility for
directing, coordinating, and supervising the care and services provided.
L555
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(e)(2) - Ensure that the care and services are provided in accordance with
the plan of care.

L556
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(e)(3) Ensure that the care and services provided are based on all
assessments of the patient and family needs.
L557
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(e)(4) Provide for and ensure the ongoing sharing of information between all
disciplines providing care and services in all settings, whether the care and services
are provided directly or under arrangement.
L558
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.56(e)(5) Provide for an ongoing sharing of information with other non-hospice
healthcare providers furnishing services unrelated to the terminal illness and
related conditions.
L559
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58 Condition of participation: Quality assessment and performance
improvement.
The hospice must develop, implement, and maintain an effective, ongoing, hospice-
wide data-driven quality assessment and performance improvement program. The
hospice’s governing body must ensure that the program: reflects the complexity of
its organization and services; involves all hospice services (including those services
furnished under contract or arrangement); focuses on indicators related to
improved palliative outcomes; and takes actions to demonstrate improvement in
hospice performance. The hospice must maintain documentary evidence of its
quality assessment and performance improvement program and be able to
demonstrate its operation to CMS.
Interpretive Guidelines §418.58
The condition requires each hospice to develop its own QAPI program to meet its needs.
Hospice outcome measures, data elements, tools, and instructions for using them have
been developed by the hospice industry and quality improvement organizations. Quality
improvement in hospice is a developing field. The methods used by the hospice for self-
assessment are flexible and may include a review of current documentation (e.g., review
of clinical records, incident reports, complaints, patient satisfaction surveys, etc.); patient
care, direct observation of clinical performance, operating systems and interviews with
patients and/or staff. The information gathered by the hospice should be based on criteria
and/or measures generated by the medical and professional/technical staffs and reflect
hospice best practice patterns, staff performance, and patient outcomes.
Ongoing means that there is a continuous and periodic collection and assessment of data.
Assessment of such data enables areas of potential problems to be identified and indicates
additional data that should be collected and assessed in order to identify whether a
problem exists.
The following elements should be considered within the QAPI plan however it is
structured:
• Program objectives;
• All patient care disciplines;
• Description of how the program will be administered and coordinated;
• Methodology for monitoring and evaluating the quality of care;
• Priorities for resolution of problems;
• Monitoring to determine effectiveness of action;
• Oversight responsibility reports to governing body; and
• Documentation of the review of its own QAPI program.
The fundamental purpose of the QAPI CoP is to set a clear expectation that hospices
must take a proactive approach to improve their performance, and focus on improved
patient/family care and activities that impact patient health and safety. CMS stresses the
improvement in systems in order to improve processes and patient outcomes.
Hospices must have all of the components of a QAPI program in place hospice-wide.
CMS expects hospices to demonstrate, with objective data, that improvements have taken
place in actual care outcomes, processes of care, patient/family satisfaction levels,
hospice operations, or other performance indicators.
The QAPI program will be evaluated for its hospice-wide effectiveness on the quality of
care provided and activities that impact upon patient health and safety. The impact of the
program can be assessed by looking at data gathered and compared at different points in
time, and actions taken based on that comparison. The hospice should be analyzing data
and evaluating the effectiveness of their own program continually.

The organized hospice-wide QAPI program must be ongoing and have a written plan of
implementation. Opportunities to improve care should be applied on a hospice-wide
basis, when appropriate. The hospice takes and documents remedial action when
problems are identified and evaluates the outcome of these actions. The results must be
transmitted to the governing body to fulfill its responsibility to ensure an effective QAPI
program.
Quality assessment and performance improvement is a process of continual assessment of
a hospice’s performance with implementation of solutions, assessment of the
effectiveness of the solutions, and evaluations to determine how it can do even better.
The QAPI program fosters the continual striving of improvement of the delivery of care
and services provided by a hospice. Performance improvement fosters a “blame-free”
environment and encourages hospices to evaluate the operating systems and processes in
the agency instead of fixing one problem at a time.
L561
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(a) Standard: Program scope
§418.58(a)(1) The program must at least be capable of showing measurable
improvement in indicators related to improved palliative outcomes and hospice
services.
L562
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(a)(2) The hospice must measure, analyze, and track quality indicators,
including adverse patient events, and other aspects of performance that enable the
hospice to assess processes of care, hospice services, and operations.
Interpretive Guidelines §418.58(a)(2)
Hospices are required to assess quality in all areas of operations that might be adversely
affecting patient care or core hospice services. There is a specific requirement to track
adverse events (as they are defined in hospice policy) and reduce their occurrence where
possible. They must be able to show (using quantitative data or other means) that they
can improve quality, as measured by their own indicators or measures.

L563
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(b) Standard: Program data
§418.58(b)(1) The program must use quality indicator data, including patient care,
and other relevant data, in the design of its program.
Interpretive Guidelines §418.58(b)(1)
Hospices must not limit their QAPI data collection efforts to the data collected during
patient assessments. Data collection must look beyond patient assessment data to
examine all facets of a hospice’s operation. All patient services and all activities that
may impact patient/family care should be evaluated as part of the QAPI program. This
would include but not be limited to:
• physician services,
• nursing services,
• medical social services,
• counseling services,
• clinical records,
• infection control,
• pharmaceutical services,
• durable medical equipment (DME),
• patient rights,
• administrative services,
• contract services,
• volunteers,
• hospice aide and
• adverse events.
Whatever measures the hospice chooses to assess quality should be monitored regularly
so that opportunities for improvement can be identified and prioritized. Data should be
collected in a timely manner so that measures can be reported on the schedule set up by
the hospice.
L564
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(b)(2) The hospice must use the data collected to do the following:
(i) Monitor the effectiveness and safety of services and quality of care.

(ii) Identify opportunities and priorities for improvement.
L565
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(b)(3) The frequency and detail of the data collection must be approved by
the hospice’s governing body.
Interpretive Guidelines §418.58(b)(3)
The governing body may assume hands-on control of the QAPI program to ensure that
the program is in compliance with this rule, or it may choose to appoint one or more
individuals to handle the structure and administration of the QAPI program. The
governing body retains ultimate responsibility for the actions of the designated
individual(s).
L566
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(c) Standard: Program activities
§418.58(c)(1) The hospice’s performance improvement activities must:
§418.58(c)(1)(i) Focus on high risk, high volume, or problem-prone areas.
L567
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(c)(1)(ii) Consider incidence, prevalence, and severity of problems in those
areas.
L568
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(c)(1)(iii) Affect palliative outcomes, patient safety, and quality of care.
Interpretive Guidelines §418.58(c)(1)(iii)
Outcomes are the results of care provided; palliative outcomes are the results of palliative
care provided.

L569
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(c)(2) Performance improvement activities must track adverse patient
events, analyze their causes, and implement preventive actions and mechanisms that
include feedback and learning throughout the hospice.
Interpretive Guidelines §418.58(c)(2)
Hospices may choose to develop their own definition for the term “adverse event” or use
a definition developed by a national accrediting organization or industry organization.
Once a hospice has identified the definition of an adverse event, it is responsible for
adhering to the definition when tracking and analyzing these events and when
implementing preventive actions. In general, an adverse event would be any action or
inaction by a hospice that caused harm to a hospice patient. However, hospices are not
bound to use this generic description.
L570
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(c)(3) The hospice must take actions aimed at performance improvement
and, after implementing those actions, the hospice must measure its success and
track performance to ensure that improvements are sustained.
Interpretive Guidelines §418.58(c)(3)
Hospices must consider how often certain quality issues arise and the severity of potential
harm when prioritizing opportunities for improvement. When adverse event monitoring
reveals a problem area, the hospice must implement changes designed to decrease
occurrence of the adverse event. The hospice must assure that the new process is
implemented hospice-wide and that it is effective in reducing the adverse event. For
performance improvement in all areas of operations, the hospice must monitor the level
of improvement over time to be sure that it is sustained.
L571
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(d) Standard: Performance improvement projects.

Beginning February 2, 2009, hospices must develop, implement and evaluate
performance improvement projects.
L572
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(d)(1) The number and scope of distinct performance improvement projects
conducted annually, based on the needs of the hospice’s population and internal
organizational needs, must reflect the scope, complexity, and past performance of
the hospice's services and operations.
L573
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(d)(2) The hospice must document what performance improvement projects
are being conducted, the reasons for conducting these projects, and the measurable
progress achieved on these projects.
Interpretive Guidelines §418.58(d)(2)
There is no requirement for hospices to conduct a specific number of performance
improvement projects. They must select the number and topics of projects based on the
results of their quality monitoring and other quality information such as the results of
State or accreditation surveys. Performance improvement projects must be documented
in written form and include the elements outlined in the standard.
L574
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(e) Standard: Executive responsibilities
The hospice’s governing body is responsible for ensuring the following:
§418.58(e)(1) That an ongoing program for quality improvement and patient safety
is defined, implemented, and maintained, and is evaluated annually.
L575
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.58(e)(2) That the hospice-wide quality assessment and performance
improvement efforts address priorities for improved quality of care and patient
safety, and that all improvement actions are evaluated for effectiveness.
L576
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.58(e)(3) That one or more individual(s) who are responsible for operating the
quality assessment and performance improvement program are designated.
Interpretive Guidelines §418.58(e)(3)
The governing body is responsible for assuring that the QAPI program is working to
address any problem areas in patient care and hospice operations, and to improve
performance in these areas. The governing body must also appoint individuals who will
operate the QAPI program for the hospice.
L577
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.60 Condition of participation: Infection control
The hospice must maintain and document an effective infection control program
that protects patients, families, visitors, and hospice personnel by preventing and
controlling infections and communicable diseases.
Interpretive Guidelines §418.60
The hospice infection control program must identify risks for the acquisition and
transmission of infectious agents in all settings where patients reside. There needs to be a
system to communicate with all hospice personnel, patients, families and visitors about
infection prevention and control issues including their role in preventing the spread of
infections and communicable diseases through daily activities.
The hospice’s infection control program may include, but not be limited to the following:
• Educating staff on the science of infectious disease transmission;
• Protocols for addressing patient care issues and prevention of infection related to
infusion therapy, urinary tract care, respiratory tract care, and wound care;
• Guidelines on caring for patients with multi-drug resistant organisms;

• Policies on protecting patients, staff and families from blood borne or airborne
pathogens;
• Monitoring staff for compliance with hospice policies and procedures related to
infection control; and
• Protocols for educating staff and families in standard precautions and the
prevention and control of infection.
L579
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.60(a) Standard: Prevention
The hospice must follow accepted standards of practice to prevent the transmission
of infections and communicable diseases, including the use of standard precautions.
Interpretive Guidelines §418.60(a)
Accepted standards of practice for health care providers are typically developed by
government agencies, professional organizations and associations. Examples would
include, but not be limited to, the Centers for Disease Control and Prevention (CDC), the
Agency for Healthcare Research and Quality, State Practice Acts, and commonly
accepted health standards established by national organizations, boards, and councils
(e.g., Association for Professionals in Infection Control and Epidemiology (APIC),
American Nurses’ Association etc.)
Standard Precautions are based on the principle that all blood, body fluids, secretions,
excretions (except sweat), non-intact skin, and mucous membranes may contain
transmissible infectious agents. Standard Precautions include a group of infection
prevention practices that apply to all patients, regardless of suspected or confirmed
infection status, in any setting in which healthcare is delivered. These include: hand
hygiene; use of gloves, gown, mask, eye protection, or face shield, depending on the
anticipated exposure; and safe injection practices. Also, equipment or items in the patient
environment likely to have been contaminated with infectious body fluids must be
handled in a manner to prevent transmission of infectious agents (e.g., wearing gloves for
direct contact, contain heavily soiled equipment, properly clean and disinfect or sterilize
reusable equipment before use on another patient). (Excerpt from CDC “Guideline for
Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare
Settings 2007.”)
Any deficiency cited as a violation of accepted standards of practice must have a copy of
the applicable standard of practice provided to the hospice along with the statement of
deficiencies. A hospice may also be surveyed for compliance with State practice acts for

each relevant discipline. Any deficiency cited as a violation of a State practice act must
reference the applicable section of the State practice act allegedly violated, and a copy of
that section of the act must be provided to the hospice along with the statement of
deficiencies.
L580
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.60(b) Standard: Control
The hospice must maintain a coordinated agency-wide program for the surveillance,
identification, prevention, control, and investigation of infectious and communicable
diseases that—
§418.60(b)(1) Is an integral part of the hospice's quality assessment and
performance improvement program; and
Interpretive Guidelines §418.60(b)(1)
Examples of infection control practices that the hospice may use include monitoring work
related employee illness and infections, analyzing them in relation to patient infections,
and taking appropriate actions when an infection or communicable disease is present to
prevent its spread among staff, patients, family and visitors.
Surveillance data should be routinely reviewed and monitored. Appropriate corrective
actions need to be taken based on the data analysis. The hospice must use this
information as a part of its QAPI program.
L581
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.60(b)(2) Includes the following:
§418.60(b)(2)(i) A method of identifying infectious and communicable disease
problems; and
§418.60(b)(2)(ii) A plan for implementing the appropriate actions that are
expected to result in improvement and disease prevention.
L582
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.60(c) Standard: Education

The hospice must provide infection control education to employees, contracted
providers, patients, and family members and other caregivers.
L583
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.62 Condition of participation: Licensed professional services.
L584
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.62(a) Licensed professional services provided directly or under arrangement
must be authorized, delivered, and supervised only by health care professionals who
meet the appropriate qualifications specified under §418.114 and who practice
under the hospice’s policies and procedures.
Interpretive Guidelines §418.62(a)
Licensed professional services, for purposes of this section, would include, but not be
limited to:
• skilled nursing care,
• physical therapy,
• speech language pathology,
• occupational therapy, and
• medical social services.
L585
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.62(b) Licensed professionals must actively participate in the coordination of all
aspects of the patient’s hospice care, in accordance with current professional
standards and practice, including participating in ongoing interdisciplinary
comprehensive assessments, developing and evaluating the plan of care, and
contributing to patient and family counseling and education; and
L586
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.62(c) Licensed professionals must participate in the hospice’s quality
assessment and performance improvement program and hospice sponsored in-
service training.
L587
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64 Condition of participation: Core services
A hospice must routinely provide substantially all core services directly by hospice
employees. These services must be provided in a manner consistent with acceptable
standards of practice. These services include nursing services, medical social
services, and counseling. The hospice may contract for physician services as
specified in paragraph (a) of this section.
A hospice may use contracted staff, if necessary, to supplement hospice employees in
order to meet the needs of patients under extraordinary or other non-routine
circumstances. A hospice may also enter into a written arrangement with another
Medicare certified hospice program for the provision of core services to supplement
hospice employee/staff to meet the needs of patients. Circumstances under which a
hospice may enter into a written arrangement for the provision of core services
include unanticipated periods of high patient loads, staffing shortages due to illness
or other short-term temporary situations that interrupt patient care; and temporary
travel of a patient outside of the hospice’s service area.
Interpretive Guidelines §418.64
Employee means a person who: (1) works for the hospice and for whom the hospice is
required to issue a W-2 form on his or her behalf; or (2) if the hospice is a subdivision of
an agency or organization, an employee of the agency or organization who is assigned to
the hospice; or (3) is a volunteer under the jurisdiction of the hospice.
If a contracting service or agency pays the individual, and is required to issue a form W-2
on the individual’s behalf, or if the individual is self-employed, the individual is not
considered a hospice employee.
Extraordinary circumstances generally would be a short-term temporary event that was
unanticipated. Examples of such circumstances might include, but are not limited to,
unanticipated periods of high patient loads (such as an unexpectedly large number of
patients requiring continuous care simultaneously), staffing shortages due to illness,
receiving patients evacuated from a disaster such as a hurricane or a wildfire, or
temporary travel of a patient outside the hospice’s service area. If a hospice chooses to
contract with another Medicare-certified hospice or a non-hospice entity, the contracting

hospice must maintain professional management responsibility for the services provided,
in accordance with §418.100(e).
L590
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(a) Standard: Physician services
The hospice medical director, physician employees, and contracted physician(s) of
the hospice, in conjunction with the patient’s attending physician, are responsible
for the palliation and management of the terminal illness and conditions related to
the terminal illness.
(1) All physician employees and those under contract, must function under
the supervision of the hospice medical director.
(2) All physician employees and those under contract shall meet this
requirement by either providing the services directly or through
coordinating patient care with the attending physician.
(3) If the attending physician is unavailable, the medical director,
contracted physician, and/or hospice physician employee is responsible
for meeting the medical needs of the patient.
Interpretive Guidelines §418.64(a)
The medical director may also serve as the physician member of the IDG.
L591
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(b) Standard: Nursing services
§418.64(b)(1) The hospice must provide nursing care and services by or under the
supervision of a registered nurse. Nursing services must ensure that the nursing
needs of the patient are met as identified in the patient’s initial assessment,
comprehensive assessment, and updated assessments.
L592
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.64(b)(2) If State law permits registered nurses to see, treat, and write orders
for patients, then registered nurses may provide services to beneficiaries receiving
hospice care.
Interpretive Guidelines §418.64(b)(2)
If an R.N., including a nurse practitioner, advanced practice nurse, etc., is permitted by
State law and regulation to see, treat, and write orders, then the R.N. may perform this
function while providing nursing services for hospice patients. Hospices are free to use
the services of all types of advanced practice nurses within their respective scopes of
practice to enhance the nursing care furnished to its patients. Services provided by a
nurse practitioner (NP) who is not the patient’s attending physician, are included under
nursing care.
L593
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(b)(3) Highly specialized nursing services that are provided so infrequently
that the provision of such services by direct hospice employees would be
impracticable and prohibitively expensive, may be provided under contract.
Interpretive Guidelines §418.64(b)(3)
Highly specialized services, such as complex wound care and infusion specialties, are
determined by the nature of the service and the nursing skill level required to be
proficient in the service. For example, a hospice may need to contract with a pediatric
nurse because of the very infrequent pediatric patients the hospice cares for and that to
employee a pediatric nurse would be impracticable and expensive. Continuous care is
not a highly specialized service, because while time intensive, it does not require highly
specialized nursing skills.
L594
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(c) Standard: Medical social services
Medical social services must be provided by a qualified social worker, under the
direction of a physician. Social work services must be based on the patient’s
psychosocial assessment and the patient’s and family’s needs and acceptance of
these services.
Interpretive Guidelines §418.64(c)

The social worker’s services are provided in accordance with the plan of care. Because
social work services must be provided under the direction of a physician, physician
approval of the plan of care will satisfy the intent of this requirement.
The psychosocial assessment is an evolving document that is revised as new information
is acquired and as progress toward goals is made. The psychosocial assessment may also
include the bereavement risk assessment. The purpose of the psychosocial assessment is
to help the IDG identify issues that either impede or facilitate the patient’s treatment and
to assist the patient/family in reaching the maximum benefit from hospice care and
services. The assessment should include a wide variety of factors, including but not
limited to, the patient and family’s adjustment to the terminal illness, the social and
emotional factors related to the terminal illness, the presence or absence of adequate
coping mechanisms, the family dynamics and communication patterns, financial
resources or constraints, the caregiver’s ability to function effectively, identifying
obstacles and risk factors which may effect compliance with the plan of care, and
identifying family support systems to help facilitate coping with end of life issues.
L595
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(d) Standard: Counseling services
Counseling services must be available to the patient and family to assist the patient
and family in minimizing the stress and problems that arise from the terminal
illness, related conditions, and the dying process.
L596
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(d) Counseling services must include, but are not limited to, the following:
(1) Bereavement counseling. The hospice must:
(i) Have an organized program for the provision of bereavement services
furnished under the supervision of a qualified professional with
experience or education in grief or loss counseling.
(ii) Make bereavement services available to the family and other individuals
in the bereavement plan of care up to 1 year following the death of the
patient. Bereavement counseling also extends to residents of a SNF/NF or
ICF/MR when appropriate and identified in the bereavement plan of
care.

(iii) Ensure that bereavement services reflect the needs of the bereaved.
(iv) Develop a bereavement plan of care that notes the kind of bereavement
services to be offered and the frequency of service delivery. A special
coverage provision for bereavement counseling is specified in §418.204(c).
Interpretive Guidelines §418.64(d)(1)
The supervisor of bereavement services may be the IDG social worker or other
professional with documented evidence of experience or education in grief or loss
counseling.
L597
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(d)(2) Dietary counseling.
Dietary counseling, when identified in the plan of care, must be performed by a
qualified individual, which include dietitians as well as nurses and other individuals
who are able to address and assure that the dietary needs of the patient are met.
Interpretive Guidelines §418.64(d)(2)
Hospices are required to assure the dietary needs of the patient are met by a qualified
individual. If an RN is capable of meeting the patient’s needs, then the dietary
counseling can be provided by the RN. If the needs of the patient exceed the expertise of
the nurse, then the hospice must have available an appropriately trained and qualified
individual such as a registered dietitian or nutritionist to meet the patient’s dietary needs.
L598
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.64(d)(3) Spiritual counseling. The hospice must:
(i) Provide an assessment of the patients’ and family’s spiritual needs.
(ii) Provide spiritual counseling to meet these needs in accordance with the
patient’s and family’s acceptance of this service, and in a manner
consistent with patient and family beliefs and desires.
(iii) Make all reasonable efforts to facilitate visits by local clergy, pastoral
counselors, or other individuals who can support the patient’s spiritual
needs to the best of its ability.
(iv) Advise the patient and family of this service.

Interpretive Guidelines §418.64(d)(3)
There should be evidence in the clinical record that the hospice has offered and/or
provided spiritual counseling in accordance with the patient/family’s desires. If a patient
and family desires spiritual counseling, then a hospice should facilitate visits by local
clergy, pastoral counselors, or others to the best of its ability.
L599
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.66 Condition of participation: Nursing services -- Waiver of requirement that
substantially all nursing services be routinely provided directly by a hospice.
L600
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.66(a) CMS may waive the requirement in §418.64(b) that a hospice provide
nursing services directly, if the hospice is located in a non-urbanized area. The
location of a hospice that operates in several areas is considered to be the location of
its central office. The hospice must provide evidence to CMS that it has made a
good faith effort to hire a sufficient number of nurses to provide services. CMS may
waive the requirement that nursing services be furnished by employees based on the
following criteria:
§418.66(a)(1) The location of the hospice’s central office is in a non-urbanized
area as determined by the Bureau of the Census.
§418.66(a)(2) There is evidence that a hospice was operational on or before
January 1, 1983 including the following:
i. Proof that the organization was established to provide hospice services on
or before January 1, 1983.
ii. Evidence that hospice-type services were furnished to patients on or before
January 1, 1983.
iii. Evidence that hospice care was a discrete activity rather than an aspect of
another type of provider's patient care program on or before January 1,
1983.
§418.66(a)(3) By virtue of the following evidence that a hospice made a good
faith effort
to hire nurses:

i. Copies of advertisements in local newspapers that demonstrate recruitment
efforts.
ii. Job descriptions for nurse employees.
iii. Evidence that salary and benefits are competitive for the area.
iv. Evidence of any other recruiting activities (for example, recruiting efforts
at health fairs and contacts with nurses at other providers in the area).
§418.66(b) Any waiver request is deemed to be granted unless it is denied within 60
days after it is received.
§418.66(c) Waivers will remain effective for 1 year at a time from the date of the
request.
§418.66(d) If a hospice wishes to receive a 1-year extension, it must submit a request
to CMS before the expiration of the waiver period, and certify that the conditions
under which it originally requested the initial waiver have not changed since the
initial waiver was granted.
Interpretive Guidelines §418.66
Section 1861(dd)(5)(a)(i) of the Social Security Act specifically references urbanized
areas as defined by the Bureau of the Census. Further information on this topic is
available at http://www.census/gov. Hospices may also contact their assigned Medicare
administrative contractor or check the hospice wage index, which is updated and
published yearly.
If there is any question concerning a waiver, contact the CMS Location.
L601
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.70 Condition of participation: Furnishing of non-core services.
A hospice must ensure that the services described in §418.72 through §418.78 are
provided directly by the hospice or under arrangements made by the hospice as
specified in §418.100. These services must be provided in a manner consistent with
current standards of practice.
Interpretive Guidelines §418.70

The hospice must ensure that all clinical staff members (direct and contractual) are aware
of and follow professional practice standards, laws, hospice policies, and procedures.
L603
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.72 Condition of participation: Physical therapy, occupational therapy, and
speech-language pathology.
Physical therapy services, occupational therapy services, and speech-language
pathology services must be available, and when provided, offered in a manner
consistent with accepted standards of practice.
Interpretive Guidelines §418.72
Rehabilitative services such as training in the use of adaptive equipment, home safety
assessment, and caregiver instruction in use of good body mechanics for turning and
lifting patients, may be appropriate/beneficial for the hospice patient/family.
L605
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.74 Waiver of requirement - Physical therapy, occupational therapy, speech-
language pathology, and dietary counseling.
L606
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.74 (a) A hospice located in a non-urbanized area may submit a written request
for a waiver of the requirement for providing physical therapy, occupational
therapy, speech-language pathology, and dietary counseling services. The hospice
may seek a waiver of the requirement that it make physical therapy, occupational
therapy, speech-language pathology, and dietary counseling services (as needed)
available on a 24-hour basis. The hospice may also seek a waiver of the requirement
that it provide dietary counseling directly. The hospice must provide evidence that
it has made a good faith effort to meet the requirements for these services before it
seeks a waiver. CMS may approve a waiver application on the basis of the following
criteria:
1) The hospice is located in a non-urbanized area as determined by the Bureau
of the Census.

2) The hospice provides evidence that it had made a good faith effort to make
available physical therapy, occupational therapy, speech-language pathology,
and dietary counseling services on a 24-hour basis and/or to hire a dietary
counselor to furnish services directly. This evidence must include the
following:
(i) Copies of advertisements in local newspapers that demonstrate
recruitment efforts.
(ii) Physical therapy, occupational therapy, speech-language
pathology, and dietary counselor job descriptions.
(iii) Evidence that salary and benefits are competitive for the area.
(iv) Evidence of any other recruiting activities (for example, recruiting
efforts at health fairs and contact discussions with physical
therapy, occupational therapy, speech-language pathology, and
dietary counseling service providers in the area).
(a) Any waiver request is deemed to be granted unless it is denied within 60 days
after it is received.
(b) An initial waiver will remain effective for 1 year at a time from the date of the
request.
(c) If a hospice wishes to receive a 1-year extension, it must submit a request to
CMS before the expiration of the waiver period and certify that conditions
under which it originally requested the waiver have not changed since the initial
waiver was granted.
Interpretive Guidelines §418.74
Eligibility for this waiver, as with the nursing waiver, is based on the primary location of
the hospice. If the hospice operates in multiple locations, the primary location is
considered to be location of the central office. This office must be located in a non-
urbanized area as determined by the Bureau of Census.
This waiver does not waive the hospice’s responsibility to provide PT, OT, SLP, and
dietary counseling; but rather waives the requirement to provide them on a 24-hour basis.
There are no limit restrictions to the number of extensions a hospice may request to the
original waiver request.
L607
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.76 Condition of participation: Hospice aide and homemaker services.
All hospice aide services must be provided by individuals who meet the personnel
requirements specified in paragraph (a) of this section. Homemaker services must
be provided by individuals who meet the personnel requirements specified in
paragraph (j) of this section.
L609
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(a) Standard: Hospice aide qualifications
§418.76(a)(1) A qualified hospice aide is a person who has successfully completed
one of the following:
i. A training program and competency evaluation as specified in paragraphs
(b) and (c) of this section respectively.
ii. A competency evaluation program that meets the requirements of
paragraph (c) of this section.
iii. A nurse aide training and competency evaluation program approved by the
State as meeting the requirements of §483.151 through §483.154 of this
chapter, and is currently listed in good standing on the State nurse aide
registry.
iv. A State licensure program.
L610
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(a)(2) A hospice aide is not considered to have completed a program, as
specified in paragraph (a)(1) of this section, if, since the individual's most recent
completion of the program(s), there has been a continuous period of 24 consecutive
months during which none of the services furnished by the individual as described
in §409.40 of this chapter were for compensation. If there has been a 24month lapse
in furnishing services, the individual must complete another program, as specified
in paragraph (a)(1) of this section, before providing services.
L611

(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(b) Standard: Content and duration of hospice aide classroom and
supervised practical training.
§481.76(b)(1) Hospice aide training must include classroom and supervised practical
training in a practicum laboratory or other setting in which the trainee
demonstrates knowledge while performing tasks on an individual under the direct
supervision of a registered nurse, or a licensed practical nurse, who is under the
supervision of a registered nurse. Classroom and supervised practical training
combined must total at least 75 hours.
L612
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(b)(2) A minimum of 16 hours of classroom training must precede a
minimum of l6 hours of supervised practical training as part of the 75 hours.
L613
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(b)(3) A hospice aide training program must address each of the following
subject areas:
(i) Communication skills, including the ability to read, write, and verbally
report clinical information to patients, care givers, and other hospice
staff.
(ii) Observation, reporting, and documentation of patient status and the care
or service furnished.
(iii) Reading and recording temperature, pulse, and respiration.
(iv) Basic infection control procedures.
(v) Basic elements of body functioning and changes in body function that
must be reported to an aide’s supervisor.
(vi) Maintenance of a clean, safe, and healthy environment.
(vii) Recognizing emergencies and the knowledge of emergency procedures
and their application.

(viii) The physical, emotional, and developmental needs of and ways to work
with the populations served by the hospice, including the need for respect
for the patient, his or her privacy, and his or her property.
(ix) Appropriate and safe techniques in performing personal hygiene and
grooming tasks, including items on the following basic checklist:
(A) Bed bath;
(B) Sponge, tub, and shower bath;
(C) Hair shampoo (sink, tub, and bed);
(D) Nail and skin care;
(E) Oral hygiene;
(F) Toileting and elimination;
(x) Safe transfer techniques and ambulation.
(xi) Normal range of motion and positioning.
(xii) Adequate nutrition and fluid intake.
(xiii) Any other task that the hospice may choose to have an aide perform. The
hospice is responsible for training hospice aides, as needed, for skills not
covered in the basic checklist, as described in paragraph (b)(3)(ix) of this
section.
L614
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(b)(4) The hospice must maintain documentation that demonstrates that the
requirements of this standard are met.
Interpretive Guidelines §418.76(b)(4)
A hospice aide may receive training from different organizations if the amount of training
totals 75 hours, the content of training addresses all subjects listed at §418.76(b)(3) and
the organization, training, instructors, and documentation meet the requirements of the
regulation.
Documentation of training should include:
• A description of the training/competency evaluation program, including the
qualifications of the instructors;
• A record that indicates which skills each aide was judged to be competent and that
distinguishes between skills taught at a patient’s bedside with supervision, and

those taught in a laboratory or simulated setting using a pseudo-patient as defined
at §418.3. A pseudo-patient may be a real person trained to participate in a role-
play situation, or a computer-based mannequin device ; and
• How additional skills (beyond the basic skills listed in the regulation) are taught
and tested if the hospice’s admission policies and case-mix of hospice patients
require aides to perform more complex procedures.
L615
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(c) Standard: Competency evaluation.
An individual may furnish hospice aide services on behalf of a hospice only after
that individual has successfully completed a competency evaluation program as
described in this section.
§418.76(c)(1) The competency evaluation must address each of the subjects listed in
paragraph (b)(3) of this section. Subject areas specified under paragraphs (b)(3)(i),
(iii), (ix), (x), and (xi) of this section must be evaluated by observing an aide’s
performance of the task with a patient or pseudo-patient. The remaining subject
areas may be evaluated through written examination, oral examination, or after
observation of a hospice aide with a patient or a pseudo-patient during a simulation.
Interpretive Guideline for §418.76(c)(1)
The hospice may not allow an aide to provide services to patients independently until the
aide has successfully completed competency testing, either at the hospice or at another
training facility, and the hospice has verified successful completion through
documentation provided by the applicant or the training facility.
The relevant subject areas specified under §418.76(b)(3) are listed below.
The listed skills below must be evaluated by observing the aide’s performance while
carrying out the task with a patient or pseudo-patient in a practicum laboratory or other
setting as per §418.76(b)(1). A pseudo-patient is a person who is trained to participate in
a role-play situation or is a computer-based mannequin device. A pseudo-patient must be
capable of responding to and interacting with the hospice aide trainee and must be similar
in characteristics to the primary patient population served by the hospice in key areas
such as age, frailty, functional status, and cognitive status.
When pseudo-patients are used to test hospice aide competency, the simulated
environment must mimic the reality of the homecare environment, including
environmental distractions and constraints that evoke or replicate substantial aspects of

the real world in a fully interactive fashion, in order to assess proficiency in performing
skills.
The following are the relevant subject areas at §418.76(b)(3):
(i) Communication skills, including the ability to read, write, and verbally report
clinical information to patients, representatives, and caregivers, as well as to other
hospice staff;
. . .
(iii) Reading and recording temperature, pulse, and respiration;
. . .
(ix) Appropriate and safe techniques in performing personal hygiene and grooming
tasks that include—
(A) Bed bath;
(B) Sponge, tub, and shower bath;
(C) Hair shampooing in sink, tub, and bed;
(D) Nail and skin care;
(E) Oral hygiene;
(F) Toileting and elimination;
(x) Safe transfer techniques and ambulation;
(xi) Normal range of motion and positioning.
. . .
In accordance with §418.76(c)(3), a registered nurse, in consultation with other skilled
professionals (as appropriate), must observe the hospice aide candidate perform each of
the tasks above to confirm the competence of the candidate.
L616
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(c)(2) A hospice aide competency evaluation program may be offered by any
organization, except as described in paragraph (f) of this section.
Interpretive Guidelines §418.76(c)(1) – (c)(2)
The hospice must ensure that the skills learned or tested elsewhere can be transferred
successfully to care of the hospice patient in all settings. The hospice should give careful
attention to evaluating both employed aides and those aides who provide services under
arrangement or contract. This review of skills could be done when the nurse installs an
aide into a new patient care situation or during a supervisory visit. A mannequin may not
be used for this evaluation.

If the hospice’s admission policies and the case-mix of patients demand that the aide care
for individuals whose needs require additional competency beyond the minimum required
in the regulation, the hospice must document how these additional skills are taught and
tested.
L617
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(c)(3) The competency evaluation must be performed by a registered nurse
in consultation with other skilled professionals, as appropriate.
L618
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(c)(4) A hospice aide is not considered competent in any task for which he or
she is evaluated as unsatisfactory. An aide must not perform that task without
direct supervision by a registered nurse until after he or she has received training in
the task for which he or she was evaluated as “unsatisfactory,” and successfully
completes a subsequent evaluation. A hospice aide is not considered to have
successfully completed a competency evaluation if the aide has an “unsatisfactory”
rating in more than one of the required areas.
Interpretive Guidelines §418.76(c)(4)
A hospice aide who is evaluated as satisfactory in all subject areas except one would be
considered competent. However, this aide would not be allowed to perform the task in
which he or she was evaluated as unsatisfactory except under direct supervision. If a
hospice aide receives an unsatisfactory evaluation in more than one subject area, the aide
would not be considered to have successfully passed a competency evaluation program
and would be precluded from functioning as a hospice aide in any subject area. The
regulations place no restrictions on the number of times or the time frame in which an
aide can be tested in a deficient area.
L619
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(c)(5) The hospice must maintain documentation that demonstrates the
requirements of this standard are being met.
L620
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(d) Standard: In-service training
A hospice aide must receive at least l2 hours of in-service training during each 12-
month period. In-service training may occur while an aide is furnishing care to a
patient.
Interpretive Guidelines §418.76(d)
Hospices may fulfill the annual 12-hour in-service training requirement on a calendar
year basis, an employment anniversary basis, or a rolling 12 month basis as long as each
aide meets this in-service training requirement.
Hospice aide in-service training, that occurs with a patient in a place of residence,
supervised by an RN, can occur as part of the every 14 day supervisory visit, but the
exact new skill or theory taught must be documented. In-service training taught in the
patient’s environment should not be a repetition of a basic skill.
L621
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(d)(1) In-service training may be offered by any organization, and must be
supervised by a registered nurse.
L622
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(d)(2) The hospice must maintain documentation that demonstrates the
requirements of this standard are met.
L623
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(e) Standard: Qualifications for instructors conducting classroom and
supervised practical training
Classroom and supervised practical training must be performed by a registered
nurse who possesses a minimum of 2 years nursing experience, at least 1 year of

which must be in home care, or by other individuals under the general supervision
of a registered nurse.
Interpretive Guidelines §418.76(e)
The required 2 years of nursing experience for the instructor should be “hands on”
clinical experience such as providing care and/or supervising nursing services or teaching
nursing skills in an organized curriculum or in-service program. The required 2 years of
nursing experience may be in home care or in hospice care.
“Other individuals” who may help with hospice aide training would include health care
professionals such as physicians, physical therapists, occupational therapists, medical
social workers, and speech-language pathologists. Dietitians, pharmacists, lawyers and
consumers might also be teaching resources.
L624
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(f) Standard: Eligible competency evaluation organizations.
A hospice aide competency evaluation program as specified in paragraph (c) of this
section may be offered by any organization except by a home health agency that,
within the previous 2 years:
(1) Had been out of compliance with the requirements of §484.80 of this
chapter.
(2) Permitted an individual that does not meet the definition of a “qualified
home health aide” as specified in §484.80(a) of this chapter to furnish home
health aide services (with the exception of licensed health professionals and
volunteers).
(3) Had been subjected to an extended (or partial extended) survey as a result of
having been found to have furnished substandard care (or for other reasons
at the discretion of CMS or the State).
(4) Had been assessed a civil monetary penalty of $5,000 or more as an
intermediate sanction.
(5) Had been found by CMS to have compliance deficiencies that endangered the
health and safety of the home health agency’s patients and had temporary
management appointed to oversee the management of the home health
agency.
(6) Had all or part of its Medicare payments suspended.

(7) Had been found by CMS or the State under any Federal or state law to have:
(i) Had its participation in the Medicare program terminated.
(ii) Been assessed a penalty of $5,000 or more for deficiencies in Federal or
State standards for home health agencies.
(iii) Been subjected to a suspension of Medicare payments to which it
otherwise would have been entitled.
(iv) Operated under temporary management that was appointed by a
governmental authority to oversee the operation of the home health
agency and to ensure the health and safety of the home health agency’s
patients.
(v) Been closed by CMS or the State, or had its patients transferred by the
State.
L625
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(g) Standard: Hospice aide assignments and duties
(1) Hospice aides are assigned to a specific patient by a registered nurse that is a
member of the interdisciplinary group. Written patient care instructions for a
hospice aide must be prepared by a registered nurse who is responsible for the
supervision of a hospice aide as specified under paragraph (h) of this section.
Interpretive Guidelines §418.76(g)(1)
Hospice aide written instructions for patient care prepared by the RN responsible for the
supervision of the aide must be patient specific and not generic.
L626
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(g)(2) A hospice aide provides services that are:
i. Ordered by the interdisciplinary group;
ii. Included in the plan of care;
iii. Permitted to be performed under State law by such hospice aide;
iv. Consistent with the hospice aide training.
L627
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.76(g)(3) The duties of a hospice aide include the following:
(i) The provision of hands on personal care.
(ii) The performance of simple procedures as an extension of therapy or
nursing services.
(iii) Assistance in ambulation or exercises.
(iv) Assistance in administering medications that are ordinarily self-
administered-.
Interpretive Guidelines §418.76(g)(3)(iv)
The IDG determines if there are medications that are appropriate for aides to help
administer based on the needs of the patient and family, training and competency of the
aide, policies of the hospice, and any applicable State and local laws and rules. If State or
local laws and rules prohibit hospice aides from administering medications, they are
precluded from doing this activity. However, if medication administration is within the
bounds of State and local laws and rules, and if hospices choose to have aides perform
this task, the hospice is required to provide aide training in medication administration and
assure that the aide is competent to perform this task before he/she is assigned to the
patient. See also §418.76(b)(3)(xiii).
L628
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(g)(4) Hospice aides must report changes in the patient’s medical, nursing,
rehabilitative, and social needs to a registered nurse, as the changes relate to the
plan of care and quality assessment and improvement activities. Hospice aides must
also complete appropriate records in compliance with the hospice’s policies and
procedures.
L629
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(h) Standard: Supervision of hospice aides.
(1) A registered nurse must make an on-site visit to the patient’s home:
(i) No less frequently than every 14 days to assess the quality of care and
services provided by the hospice aide and to ensure that services ordered by
the hospice interdisciplinary group meet the patient’s needs. The hospice
aide does not have to be present during this visit.
Interpretive Guidelines §418.76(h)(1)(i)

If the RN makes a supervisory visit on a Tuesday, the next supervisory visit is due by the
Tuesday which occurs 14 days later.
In addition to ensuring that hospice aides furnish the care identified in the plan of care,
RN supervisors must assess the adequacy of the aide services in relationship to the needs
of the patient and family. In-person visits by the supervising nurse to the patient’s home
allow the nurse to directly observe the patient and the results of the aide’s care. The
supervisory visits must be documented in the patient’s clinical record.
L630
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(h)(1)(ii) If an area of concern is noted by the supervising nurse, then the
hospice must make an on-site visit to the location where the patient is receiving care
in order to observe and assess the aide while he or she is performing care.
Interpretive Guidelines §418.76(h)(1)(ii)
The supervising RN must conduct an in person supervisory visit with the aide to observe
and assess aide skills if a potential deficiency in care furnished by the aide is noted in the
regular 14 day supervisory visit (during which the aide is not required to be present).
L631
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(h)(1)(iii) If an area of concern is verified by the hospice during the on-site
visit, then the hospice must conduct, and the hospice aide must complete, a
competency evaluation of the deficient skill and all related skill(s) in accordance
with §418.76(c).
L632
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(h)(2) A registered nurse must make an annual on-site visit to the location
where a patient is receiving care in order to observe and assess each aide while he or
she is performing care.
Interpretive Guidelines §418.76(h)(2)
The annual on-site supervision visit is to assess and observe each aide providing care to
one of the patients. There is no requirement for the observation visit to be conducted on
each patient the aide is caring for.

Hospices may determine the appropriate location to document the annual aide on-site
evaluation in accordance with their own policies and procedures.
L633
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(h)(3) The supervising nurse must assess an aide’s ability to demonstrate
initial and continued satisfactory performance in meeting outcome criteria that
include, but is not limited to–
i. Following the patient’s plan of care for completion of tasks assigned to
the hospice aide by the registered nurse;
ii. Creating successful interpersonal relationships with the patient and
family;
iii. Demonstrating competency with assigned tasks;
iv. Complying with infection control policies and procedures;
v. Reporting changes in the patient’s condition.
Interpretive Guidelines §418.76(h)(3)
Supervisory visits may be made in conjunction with a professional visit to provide
services. Documentation of RN supervision should include, but not be limited to, if the
aide is following the plan of care, is competent in performing required tasks and is
satisfactory to the patient/family.
L634
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(i) Standard: Individuals furnishing Medicaid personal care aide-only
services under a Medicaid personal care benefit. An individual may furnish
personal care services, as defined in §440.167 of this chapter, on behalf of a hospice
agency.
§418.76(i)(1) Before the individual may furnish personal care services, the
individual must be found competent by the State (if regulated by the State) to
furnish those services. The individual only needs to demonstrate competency in the
services the individual is required to furnish.
L635
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.76(i)(2) Services under the Medicaid personal care benefit may be used to the
extent that the hospice would routinely use the services of a hospice patient’s family
in implementing a patient’s plan of care.
L636
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(i)(3) The hospice must coordinate its hospice aide and homemaker services
with the Medicaid personal care benefit to ensure the patient receives the hospice
aide and homemaker services he or she needs.
Interpretive Guidelines §418.76(i)(3)
It is up to the State to define the optional Medicaid State Plan personal care services
benefit and to determine if the benefit is more extensive than the homemaker/hospice
aide benefit provided under the Medicare hospice benefit. If the Medicaid personal care
services benefit is more extensive than what is offered under the Medicare hospice
benefit, proper coordination of services must occur. In this instance, the State must pay
for covered Medicaid personal care services that exceed the scope of the Medicare
hospice benefit when a need for those personal care services is indicated in the patient’s
hospice plan of care.
L637
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(j) Standard: Homemaker qualifications.
A qualified homemaker is—
(1) An individual who meets the standards in §418.202(g) and has successfully
completed hospice orientation addressing the needs and concerns of patients and
families coping with a terminal illness; or
(2) A hospice aide as described in §418.76.
Interpretive Guidelines §418.76(j)
Homemaker services may include assistance in maintaining a safe and healthy
environment for the patient/family and services to help the patient/family carry out the
treatment plan. See §418.202(g).
L638

(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(k) Standard: Homemaker supervision and duties.
§418.76(k)(1) Homemaker services must be coordinated and supervised by a
member of the interdisciplinary group.
L639
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(k)(2) Instructions for homemaker duties must be prepared by a member of
the interdisciplinary group.
L640
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.76(k)(3) Homemakers must report all concerns about the patient or family to
the member of the interdisciplinary group who is coordinating homemaker services.
L641
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.78 Conditions of participation: Volunteers.
The hospice must use volunteers to the extent specified in paragraph (e) of this
section. These volunteers must be used in defined roles and under the supervision of
a designated hospice employee.
Interpretive Guidelines §418.78
Volunteers are considered hospice employees to facilitate compliance with the core
services requirement.
L643
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.78(a) Standard: Training

The hospice must maintain, document and provide volunteer orientation and
training that is consistent with hospice industry standards.
Interpretive Guidelines §418.78(a)
All required volunteer training should be consistent with the specific tasks that volunteers
perform.
L644
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.78(b) Standard: Role
Volunteers must be used in day-to-day administrative and/or direct patient care
roles.
Interpretive Guidelines §418.78(b)
Qualified volunteers who provide professional services for the hospice must meet all
requirements associated with their specialty area. If licensure or registration is required
by the State, the volunteer must be licensed or registered.
The hospice may use volunteers to provide assistance in the hospice’s ancillary and office
activities as well as in direct patient care services, and/or help patients and families with
household chores, shopping, transportation, and companionship. Hospices are also
permitted to use volunteers in non-administrative and non-direct patient care activities,
although these services are not considered when calculating the level of activity described
in standard (e).
The duties of volunteers used in direct patient care services or helping patients and
families must be evident in the patient’s plan of care. There should be documentation of
time spent and the services provided by volunteers.
L645
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.78(c) Standard: Recruiting and retaining.
The hospice must document and demonstrate viable and ongoing efforts to recruit
and retain volunteers.
L646

(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.78(d) Standard: Cost saving
The hospice must document the cost savings achieved through the use of volunteers.
Documentation must include the following:
(1) The identification of each position that is occupied by a volunteer.
(2) The work time spent by volunteers occupying those positions.
(3) Estimates of the dollar costs that the hospice would have incurred if paid
employees occupied the positions identified in paragraph (d)(1) of this section
for the amount of time specified in paragraph (d)(2) of this section.
Interpretive Guidelines §418.78(d)
There is no requirement for what the cost savings must be, only on how it is computed.
L647
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.78(e) Standard: Level of activity
Volunteers must provide day-to-day administrative and/or direct patient care
services in an amount that, at a minimum, equals 5 percent of the total patient care
hours of all paid hospice employees and contract staff. The hospice must maintain
records on the use of volunteers for patient care and administrative services,
including the type of services and time worked.
Interpretive Guidelines §418.78(e)
In computing this level of activity, the hospice divides the number of hours that hospice
volunteers spent providing administrative and/or direct patient care services by the total
number of patient care hours of all paid hospice employees and contract staff. For
example, if the hospice provides 10,000 hours of paid direct patient care during a one-
year period the hospice must provide 500 volunteer hours in direct patient care or
administrative activities to meet the required 5 percent total.
A hospice may fluctuate the volume of care provided by volunteers after the hospice
meets the required 5 percent minimum.

L648
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100 Condition of Participation: Organization and administration of services.
The hospice must organize, manage, and administer its resources to provide the
hospice care and services to patients, caregivers and families necessary for the
palliation and management of the terminal illness and related conditions.
L650
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(a) Standard: Serving the hospice patient and family.
The hospice must provide hospice care that-
(1) Optimizes comfort and dignity; and
Is consistent with patient and family needs and goals, with patient needs and goals
as priority.
L651
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(b) Standard: Governing body and administrator.
A governing body (or designated persons so functioning) assumes full legal
authority and responsibility for the management of the hospice, the provision of all
hospice services, its fiscal operations, and continuous quality assessment and
performance improvement. A qualified administrator appointed by and reporting
to the governing body is responsible for the day-to-day operation of the hospice.
The administrator must be a hospice employee and possess education and
experience required by the hospice's governing body.
Interpretive Guidelines §418.100(b)
If the hospice is part of a larger organization (e.g., HHA, hospital) and the governing
body is the same, there must be documented evidence that the governing body is
assuming full authority and responsibility for the management of the hospice and reviews
and addresses the functioning of specific hospice operations, services and QAPI program.

If the administrator is not available to fulfill his or her assigned duties and
responsibilities, the hospice must identify another individual to assume those assigned
duties and responsibilities in accordance with the hospice’s established policies and
procedures. The governing body must assume responsibility for ensuring that the hospice
is managed by the administrator and any managers that the administrator appoints.
L652
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(c) Standard: Services.
(1) A hospice must be primarily engaged in providing the following care and
services and must do so in a manner that is consistent with accepted
standards of practice:
(i) Nursing services.
(ii) Medical social services.
(iii) Physician services.
(iv) Counseling services, including spiritual counseling, dietary counseling,
and bereavement counseling.
(v) Hospice aide, volunteer, and homemaker services.
(vi) Physical therapy, occupational therapy, and speech-language pathology
services.
(vii) Short-term inpatient care.
(viii) Medical supplies (including drugs and biologicals) and medical appliances.
L653
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(c)(2) Nursing services, physician services, and drugs and biologicals (as
specified in §418.106) must be made routinely available on a 24-hour basis 7 days a
week. Other covered services must be available on a 24-hour basis when reasonable
and necessary to meet the needs of the patient and family.
L654
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(d) Standard: Continuation of care
A hospice may not discontinue or reduce care provided to a Medicare or Medicaid
beneficiary because of the beneficiary's inability to pay for that care.

Interpretive Guidelines §418.100(d)
This condition applies to Medicare and Medicaid beneficiaries only.
L655
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(e) Standard: Professional management responsibility
A hospice that has a written agreement with another agency, individual, or
organization to furnish any services under arrangement must retain administrative
and financial management, and oversight of staff and services for all arranged
services, to ensure the provision of quality care. Arranged services must be
supported by written agreements that require that all services be--
(1) Authorized by the hospice;
(2) Furnished in a safe and effective manner by qualified personnel; and
(3) Delivered in accordance with the patient's plan of care.
Interpretive Guidelines §418.100(e)
The hospice must retain administrative and financial management responsibility, and
oversight of staff and services provided under arrangement. For Medicare purposes, the
hospice is reimbursed for all covered services it provides, whether directly or under
arrangement. It is the responsibility of the hospice to pay for those services provided to
Medicare beneficiaries under arrangement. When a hospice provides services under
arrangements to non-Medicare beneficiaries, the hospice is responsible for establishing
how payment for those services will occur, but the standard does not require the hospice
to pay for those services directly or to pay for services for which there is no
reimbursement or for services that another insurer is obligated to pay.
L656
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(f) Standard: Hospice multiple locations
If a hospice operates multiple locations, it must meet the following requirements:
(1) Medicare approval.
(i) All hospice multiple locations must be approved by Medicare before
providing hospice care and services to Medicare patients.
Interpretive Guidelines §418.100(f)(1)(i)

It is inherent in the provider certification process for a hospice to notify CMS of its
proposal to add a location from which it provides services. Absent such notification,
CMS has no way of carrying out the statutorily mandated obligation of determining
whether the hospice is complying with all applicable participation requirements at the
new location. It is a longstanding CMS policy that there is no basis for a provider to bill
Medicare for services provided from a location that has not been determined to meet
applicable requirements of participation.
When an existing hospice intends to add a multiple location, it must notify CMS, the
State Survey Agency (SA), and, if deemed, it should notify its approved national
accreditation organization (AO), in writing of the proposed location if it expects this
location to participate in Medicare or Medicaid. The hospice must also submit a Form
CMS-855A change of information request (including all supporting documentation) to its
Medicare Administrative Contractor (MAC) before CMS approval can be granted. The
provider must also obtain CMS’ approval of the new multiple location before it is
permitted to bill Medicare for services provided from the new location.
NOTE: CMS will not approve a hospice’s inpatient facility or a change of location for a
hospice’s own inpatient facility without a survey to assure that the facility meets
all requirements specified at 42 CFR 418.110.
A hospice may not bill Medicare for services provided from a multiple location until the
new site or location has been approved by CMS. The fact that a national accreditation
organization with deeming authority has approved a new site or location will not affect
CMS’ decision. CMS’ determination will be based on its independent application of its
regulations to the facts in the case. Services provided before the effective date of
approval should not be billed to Medicare.
If the hospice does operate at multiple locations, a deficiency found at any location will
result in a compliance issue for the entire hospice.
L657
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(f)(1)(ii) The multiple location must be part of the hospice and must share
administration, supervision, and services with the hospice issued the certification
number.
L658
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.100(f)(1)(iii) The lines of authority and professional and administrative control
must be clearly delineated in the hospice’s organizational structure and in practice,
and must be traced to the location which was issued the certification number.
L659
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(f)(1)(i)(iv) The determination that a multiple location does or does not
meet the definition of a multiple location, as set forth in this part, is an initial
determination, as set forth in §498.3.
Interpretive Guidelines §418.100(f)(1)(iv)
Initial determinations under 42 CFR 498.3 are subject to administrative review.
L660
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(f)(2) The hospice must continually monitor and manage all services
provided at all of its locations to ensure that services are delivered in a safe and
effective manner and to ensure that each patient and family receives the necessary
care and services outlined in the plan of care, in accordance with the requirements
of this subpart and subparts A and C of this section.
L661
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(g) Standard: Training
(1) A hospice must provide orientation about the hospice philosophy to all
employees and contracted staff who have patient and family contact.
L662
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(g)(2) - A hospice must provide an initial orientation for each employee that
addresses the employee’s specific job duties.

L663
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.100(g)(3) A hospice must assess the skills and competence of all individuals
furnishing care, including volunteers furnishing services, and, as necessary, provide
in-service training and education programs where required. The hospice must have
written policies and procedures describing its method(s) of assessment of
competency and maintain a written description of the in-service training provided
during the previous 12 months.
L664
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.102 Condition of Participation: Medical director
The hospice must designate a physician to serve as medical director. The medical
director must be a doctor of medicine or osteopathy who is an employee, or is under
contract with the hospice. When the medical director is not available, a physician
designated by the hospice assumes the same responsibilities and obligations as the
medical director.
Interpretive Guidelines §418.102
There is only one medical director for the hospice, including all multiple locations, if it
has them. That individual may work full time or part time. If the medical director is not a
paid employee or a contracted medical director, he/she is considered a volunteer under
the control of the hospice. All other hospice physicians function under the supervision of
the medical director.
L666
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.102(a) Standard: Medical director contract.
(1) A hospice may contract with either of the following—
i. A self-employed physician; or
ii. A physician employed by a professional entity or physicians group.

When contracting for medical director services, the contract must specify the
physician who assumes the medical director responsibilities and obligations.
Interpretive Guidelines §418.102(a)
The medical director may also be a volunteer physician under the control of the hospice,
as long as this person meets all Federal and State requirements for a hospice physician.
L667
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.102(b) Standard: Initial certification of terminal illness.
The medical director or physician designee reviews the clinical information for each
hospice patient and provides written certification that it is anticipated that the
patient’s life expectancy is 6 months or less if the illness runs its normal course. The
physician must consider the following when making this determination:
(1) The primary terminal condition;
(2) Related diagnosis(es), if any;
(3) Current subjective and objective medical findings;
(4) Current medication and treatment orders; and
(5) Information about the medical management of any of the patient’s conditions
unrelated to the terminal illness.
Interpretive Guidelines §418.102(b)
• The medical director or physician designee (who is a hospice employee or
under contract with the hospice) has the responsibility for the medical
component of the hospice’s patient care program, including initial
certifications and recertification of terminal illness.
L668
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.102(c) Standard: Recertification of the terminal illness.
Before the recertification period for each patient, as described in §418.21(a), the
medical director or physician designee must review the patient’s clinical
information.

L669
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.102(d) Standard: Medical director responsibility.
The medical director or physician designee has responsibility for the medical
component of the hospice’s patient care program.
Interpretive Guidelines §418.102(d)
The single individual who fills the role of the medical director assumes overall
responsibility for the medical component of the hospice’s patient care program. This
responsibility, which extends to all hospice multiple locations, includes overseeing the
implementation of the entire physician, nursing, social work, therapy, and counseling
areas within the hospice to ensure that these areas consistently meet patient and family
needs.
L670
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104 Condition of participation: Clinical records.
A clinical record containing past and current findings is maintained for each
hospice patient. The clinical record must contain correct clinical information that is
available to the patient’s attending physician and hospice staff. The clinical record
may be maintained electronically.
L672
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(a) Standard: Content.
Each patient’s record must include the following:
(1) The initial plan of care, updated plans of care, initial assessment, comprehensive
assessment, updated comprehensive assessments, and clinical notes.
L673
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.104(a)(2) Signed copies of the notice of patient rights in accordance with
§418.52 and election statement in accordance with §418.24.
L674
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(a)(3) Responses to medications, symptom management, treatments, and
services.
L675
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(a)(4) Outcome measure data elements, as described in §418.54(e) of this
subpart.
L676
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(a)(5) Physician certification and recertification of terminal illness as
required in §418.22 and §418.25 and described in §418.102(b) and §418.102(c)
respectively, if appropriate.
L677
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(a)(6) Any advance directives as described in §418.52(a)(2).
L678
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(a)(7) Physician orders.
L679
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.104(b) Standard: Authentication.
All entries must be legible, clear, complete, and appropriately authenticated and
dated in accordance with hospice policy and currently accepted standards of
practice.
Interpretive Guidelines §418.104(b)
A hospice may create its own policy on authentication of clinical records based on
accepted standards of practice. Hospices must follow State laws regarding authentication
of clinical records, and, within this context, alter their policies as often as necessary to
adapt to changing technologies and practices.
Medicare requires a legible identifier for services provided/ordered. This method must
be handwritten (not stamped) or an electronic signature to sign an order or other clinical
record documentation. The noted exception is that facsimiles of original written or
electronic signatures are acceptable for the certifications of terminal illness for hospice.
Stamped signatures are not acceptable.
Providers and physicians using electronic signatures should recognize that there is a
potential for misuse or abuse with alternate signature methods. For example, providers
need a system and software products that are protected against modification, etc., and
should apply administrative procedures that are adequate and correspond to recognized
standards and laws. The individual whose name is on the alternate signature method as
well as the provider bear the responsibility for the authenticity of the information to
which they have attested. Physicians should check with their attorneys and malpractice
insurers in regard to the use of alternative signature methods.
Hospices may not accept stamped physician signatures on orders, treatments, or
other documents that are a part of the patient’s clinical record.
Surveyors must have access to clinical records. If the record is maintained electronically,
the hospice must provide all equipment necessary to read the record in its entirety. The
hospice must also produce a paper copy of the record, if requested by the surveyor.
All State licensure and State practice regulations continue to apply to Medicare-approved
hospices. Where State law is more restrictive than Medicare, the hospice needs to apply
the State law standard.
L680
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(c) Standard: Protection of information

The clinical record, its contents and the information contained therein must be
safeguarded against loss or unauthorized use. The hospice must be in compliance
with the Department’s rules regarding personal health information as set out at 45
CFR parts 160 and 164.
Interpretive Guidelines§418.104(c)
The hospice must ensure that unauthorized individuals cannot gain access to patient
records, and that individuals cannot alter patient records.
L681
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(d) Standard: Retention of records
Patient clinical records must be retained for 6 years after the death or discharge of
the patient, unless State law stipulates a longer period of time. If the hospice
discontinues operation, hospice policies must provide for retention and storage of
clinical records. The hospice must inform its State agency and its CMS Location
where such clinical records will be stored and how they may be accessed.
L682
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(e) Standard: Discharge or transfer of care
(1) If the care of a patient is transferred to another Medicare/Medicaid
certified- facility, the hospice must forward, to the receiving facility, a copy of-
(i) The hospice discharge summary; and
(ii) The patient’s clinical record, if requested.
L683
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(e)(2) If a patient revokes the election of hospice care, or is discharged from
hospice in accordance with §418.26, the hospice must forward to the patient’s
attending physician, a copy of

(i) The hospice discharge summary; and
(ii) The patient’s clinical record, if requested.
L684
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(e)(3) The hospice discharge summary as required by (e)(1) and (e)(2) of
this section must include—
(i) A summary of the patient's stay including treatments, symptoms and pain
management;
(ii) The patient's current plan of care;
(iii) The patient's latest physician orders; and
(iv) Any other documentation that will assist in post-discharge continuity of
care or that is requested by the attending physician or receiving facility.
Interpretive Guidelines §418.104(e)
For further information regarding revocation or discharge of hospice services see Chapter
2, §2081 and §2082, respectively, of this Chapter 2 in this manual.
L685
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.104(f) Standard: Retrieval of clinical records
The clinical record, whether hard copy or in electronic form, must be made readily
available on request by an appropriate authority.
Interpretive Guidelines §418.104(f)
An appropriate authority includes representatives from the SA or other authorized entity,
who visits the hospice for the purpose of determining in accordance with Section 1864(a)
of the Act whether the hospice is meeting all conditions of participation.
If the clinical record is maintained electronically, the hospice must provide all equipment
necessary to read the record in its entirety. The hospice must also produce a paper copy
of the record, if requested by the surveyor. In addition, ascertain how the hospice ensures
that the record is up-to-date including documentation of recent services/visits or
handwritten notes held by staff that were not included in the record when the paper copy
was produced.

L686
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106 Condition of participation: Drugs and biologicals, medical supplies, and
durable medical equipment.
Medical supplies and appliances, as described in §410.36 of this chapter; durable
medical equipment, as described in §410.38 of this chapter; and drugs and
biologicals related to the palliation and management of the terminal illness and
related conditions, as identified in the hospice plan of care, must be provided by the
hospice while the patient is under hospice care.
L688
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(a) Standard: Managing drugs and biologicals.
(1) A hospice that provides inpatient care directly in its own facility must provide
pharmacy services under the direction of a qualified licensed pharmacist who is an
employee of or under contract with the hospice. The provided pharmacist services
must include evaluation of a patient’s response to medication therapy, identification
of potential adverse drug reactions, and recommended appropriate corrective
action.
(2)[Reserved]
L690
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(b) Standard: Ordering of drugs.
(1) Drugs may be ordered by any of the following practitioners:
(i) A physician as defined by Section 1861(r)(1) of the Act,
(ii) A nurse practitioner in accordance with state scope of practice
requirements.
(iii) A physician assistant in accordance with the state scope of practice
requirements and hospice policy who is:
(A) The patient’s attending physician; and
(B) Not an employee of or under arrangement with the hospice.
(2) If the drug order is verbal or given by or through electronic transmission—
(i) It must be given only to a licensed nurse, nurse practitioner (where
appropriate), pharmacist, or physician; and
(ii) The individual receiving the order must record and sign it immediately
and have the prescribing person sign it in accordance with State and
Federal regulations.
L691
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(c) Standard: Dispensing of drugs and biologicals.
The hospice must—
(1) Obtain drugs and biologicals from community or institutional pharmacists or
stock drugs and biologicals itself.
(2) The hospice that provides inpatient care directly in its own facility must:
(i) Have a written policy in place that promotes dispensing accuracy; and
(ii) Maintain current and accurate records of the receipt and disposition of
all controlled drugs.
Interpretive Guidelines §418.106(c)
A biological is any medicinal preparation made from living organisms and their products
including, but not limited to, serums, vaccines, antigens, and antitoxins.
L692
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(d) Standard: Administration of drugs and biologicals.
(1) The interdisciplinary group, as part of the review of the plan of care, must
determine the ability of the patient and/or family to safely self-administer drugs
and biologicals to the patient in his or her home.

(2) Patients receiving care in a hospice that provides inpatient care directly in its
own facility may only be administered medications by the following individuals:
(i) A licensed nurse, physician, or other health care professional in
accordance with their scope of practice and State law;
(ii) An employee who has completed a State-approved training program in
medication administration; and
(iii) The patient, upon approval by the interdisciplinary group.
Interpretive Guidelines §418.106(d)
The patient’s individualized written plan of care should identify if the patient and/or
family are self-administering drugs and biologicals. If the patient and/or family are not
capable of safely administering drugs and biologicals in the home, the hospice must
address this issue in the patient’s plan of care.
L693
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e) Standard: Labeling, disposing, and storing of drugs and biologicals
(1) Labeling. Drugs and biologicals must be labeled in accordance with currently
accepted professional practice and must include appropriate usage and
cautionary instructions, as well as an expiration date (if applicable).
Interpretive Guidelines §418.106(e)(1)
The hospice must have a system to ensure that they do not provide to their patients (either
directly or under arrangement) outdated, mislabeled, or otherwise unusable drugs and
biologicals.
L694
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e)(2) Disposing.
(i) Safe use and disposal of controlled drugs in the patient’s home. The hospice
must have written policies and procedures for the management and disposal of
controlled drugs in the patient’s home. At the time when controlled drugs are
first ordered the hospice must:

L695
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e)(2)(A) - Provide a copy of the hospice written policies and procedures on
the management and disposal of controlled drugs to the patient or patient
representative and family;
L696
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e)(2)(B) - Discuss the hospice policies and procedures for managing the
safe use and disposal of controlled drugs with the patient or representative and the
family in a language and manner that they understand to ensure that these parties
are educated regarding the safe use and disposal of controlled drugs; and
Interpretive Guidelines §418.106(e)(2)(B)
The hospice’s policies and procedures may also address the safe use and disposal of
controlled drugs at other times, such as when a drug is discontinued, a new controlled
drug is ordered, or when the patient dies.
L697
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e)(2)(C) - Document in the patient’s clinical record that the written policies
and procedures for managing controlled drugs was provided and discussed.
L698
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e)(2)(C)(ii) - Disposal of controlled drugs in hospices that provide
inpatient care directly. The hospice that provides inpatient care directly in its
own facility must dispose of controlled drugs in compliance with the hospice
policy and in accordance with State and Federal requirements. The hospice must
maintain current and accurate records of the receipt and disposition of all
controlled drugs.
L699
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.106(e)(3) Storing. The hospice that provides inpatient care directly in its own
facility must comply with the following additional requirements-
(i) All drugs and biologicals must be stored in secure areas. All controlled
drugs listed in Schedules II, III, IV, and V of the Comprehensive Drug Abuse
Prevention and Control Act of 1976 must be stored in locked compartments
within such secure storage areas. Only personnel authorized to administer
controlled drugs as noted in paragraph (d)(2) of this section may have access
to the locked compartments; and
Interpretive Guidelines §418.106(e)(3)(1)
Compartments in the context of these regulations include, but are not limited to, drawers,
cabinets, rooms, refrigerators, and carts. The provisions for “authorized personnel” to
have access to keys must be determined by the hospice management in accordance with
Federal, State, and local laws and facility practice.
L700
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(e)(3)(ii) Discrepancies in the acquisition, storage, dispensing,
administration, disposal, or return of controlled drugs must be investigated
immediately by the pharmacist and hospice administrator and where required
reported to the appropriate State authority. A written account of the
investigation must be made available to State and Federal officials if required by
law or regulation.
L701
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(f) Standard: Use and maintenance of equipment and supplies
(1) The hospice must ensure that manufacturer recommendations for performing
routine and preventive maintenance on durable medical equipment are followed.
The equipment must be safe and work as intended for use in the patient's
environment. Where a manufacturer recommendation for a piece of equipment
does not exist, the hospice must ensure that repair and routine maintenance
policies are developed. The hospice may use persons under contract to ensure
the maintenance and repair of durable medical equipment.

L702
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(f)(2) The hospice must ensure that the patient, where appropriate, as well
as the family and/or other caregiver(s), receive instruction in the safe use of durable
medical equipment and supplies. The hospice may use persons under contract to
ensure patient and family instruction. The patient, family, and/or caregiver must be
able to demonstrate the appropriate use of durable medical equipment to the
satisfaction of the hospice staff.
Interpretive Guidelines §418.106(f)(2)
The instruction given to the patient/family on the use of the DME and supplies must be
documented in the patient’s clinical record, as well as the patient/family’s understanding
of the safe use of the DME and supplies.
L703
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.106(f)(3) Hospices may only contract for durable medical equipment services
with a durable medical equipment supplier that meets the Medicare DMEPOS
Supplier Quality and Accreditation Standards at 42 CFR §424.57.
Interpretive Guidelines §418.106(f)(3)
DMEPOS is the acronym for Durable Medical Equipment Prosthetics, Orthotics and
Supplies. All DMEPOS suppliers are required under separate rulemaking to be
accredited by September 30, 2009, in order to receive Medicare payment. If a hospice
has a contract with a DME supplier (that has a Medicare supplier billing number), the
hospice should have a letter in its file from the DME supplier stating that the DME
supplier is accredited.
If the hospice contracts with a DME supplier that only serves hospices (therefore no
Medicare supplier number), the hospice will still need to have a letter in its file from the
DME supplier stating that the DME is accredited.
L704
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108 Condition of participation: Short-term inpatient care.

Inpatient care must be available for pain control, symptom management, and
respite purposes, and must be provided in a participating Medicare or Medicaid
facility.
L706
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(a) Standard: Inpatient care for symptom management and pain control.
Inpatient care for pain control and symptom management must be provided in one
of the following:
(1) A Medicare-certified hospice that meets the conditions of participation for
providing inpatient care directly as specified in §418.110.
L707
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(a)(2) Medicare-certified hospital or a skilled nursing facility that also
meets the standards specified in §418.110(b) and (f) regarding 24-hour nursing
services and patient areas.
L708
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(b) Standard: Inpatient care for respite purposes
(1) Inpatient care for respite purposes must be provided by one of the following:
(i) A provider specified in paragraph (a) of this section.
L709
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(b)(1)(ii) A Medicare or Medicaid-certified nursing facility that also meets
the standards specified in §418.110 (f).

L710
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(b)(2) - The facility providing respite care must provide 24-hour nursing
services that meet the nursing needs of all patients and are furnished in accordance
with each patient’s plan of care. Each patient must receive all nursing services as
prescribed and must be kept comfortable, clean, well-groomed, and protected from
accident, injury, and infection.
Interpretive Guidelines §418.108(b)(2)
The hospice must assure that the inpatient facility has enough nursing personnel present
on all shifts to guarantee that adequate safety measures are in place for the patients, and
that the routine, special, and emergency needs of all patients are met at all times.
L711
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(c) Standard: Inpatient care provided under arrangements
If the hospice has an arrangement with a facility to provide for short-term inpatient
care, the arrangement is described in a written agreement, coordinated by the
hospice and at a minimum specifies —
(1) That the hospice supplies the inpatient provider a copy of the patient’s plan of
care and specifies the inpatient services to be furnished;
L712
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(c)(2) That the inpatient provider has established patient care policies
consistent with those of the hospice and agrees to abide by the palliative care
protocols and plan of care established by the hospice for its patients;
L713
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(c)(3) That the hospice patient’s inpatient clinical record includes a record
of all inpatient services furnished and events regarding care that occurred at the
facility; that a copy of the discharge summary be provided to the hospice at the time
of discharge; and that a copy of the inpatient clinical record is available to the
hospice at the time of discharge;

L714
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(c)(4) That the inpatient facility has identified an individual within the
facility who is responsible for the implementation of the provisions of the
agreement;
L715
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(c)(5) That the hospice retains responsibility for ensuring that the training
of personnel who will be providing the patient’s care in the inpatient facility has
been provided and that a description of the training and the names of those giving
the training are documented; and
L716
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(c)(6) A method for verifying that the requirements in paragraphs(c)(1)
through (c)(5) of this section are met.
Interpretive Guidelines §418.108(c)(6)
Hospices may have arrangements with more than one facility for the provision of
inpatient care
L717
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(d) Standard: Inpatient care limitation
The total number of inpatient days used by Medicare beneficiaries who elected
hospice coverage in a 12-month period in a particular hospice may not exceed 20
percent of the total number of hospice days consumed in total by this group of
beneficiaries.
Interpretive Guidelines §418.108(d)
This standard applies to Medicare beneficiaries only. Compliance with this regulation is
based on the total number of Medicare beneficiaries enrolled in the hospice program, and
does not include patients from other payor sources.

L718
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.108(e) Standard: Exemption from limitation
Before October 1, 1986, any hospice that began operation before January 1, 1975, is
not subject to the limitation specified in paragraph (d) of this section.
The Condition of Participation at §418.110 has been re-designated from L719-L758 to
L820-L862
L719-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L720-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L721-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L722-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L723-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L724-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L725-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L726-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L727-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

L728-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L729-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L730-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L731-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L732-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L733-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L734-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L735-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L736-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L737-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

L738-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L739-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L740-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L741-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L742-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L743-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L744-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23
L745-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L746-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L747-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L748-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L749-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

L750-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L751-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L752-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L753-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L754-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L755-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L756-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L757-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L758-Reserved for future use
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
L759
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112 Condition of participation: Hospices that provide hospice care to residents
of a SNF/NF or ICF/IID.
In addition to meeting the conditions of participation at §418.10 through §418.116, a
hospice that provides hospice care to residents of a SNF/NF or ICF/IID must abide
by the following additional standards.
Interpretive Guidelines §418.112
For the purposes of this guidance under this condition, "facility" will be used in place of
SNF/NF or ICF/IID.
All references to a "patient" in the guidance under this condition mean a person who is a
resident of a facility and is receiving hospice services from the Medicare certified
hospice.
L761
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(a) Standard: Resident eligibility, election, and duration of benefits.
Medicare patients receiving hospice services and residing in a SNF, NF, or ICF/IID
are subject to the Medicare hospice eligibility criteria set out at §418.20 through
§418
L762
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(b) Standard: Professional management.
The hospice must assume responsibility for professional management of the
resident’s hospice services provided, in accordance with the hospice plan of care and
the hospice conditions of participation, and make any arrangements necessary for
hospice-related inpatient care in a participating Medicare/Medicaid facility
according to §418.100 and §418.108.
Interpretive Guidelines §418.112(b)
The term “professional management” for a hospice patient who resides in a SNF/NF or
ICF/IID has the same meaning that it has if the hospice patient were living in his/her own
home. Professional management involves assessing, planning, monitoring, directing and
evaluating the patient’s/resident’s hospice care across all settings.
Hospices must routinely provide substantially all core services directly by the hospice
employee, and cannot delegate these services to the facility. Hospices should specify that
facility staff should immediately notify the hospice when facility staff must perform
hospice core services in place of hospice staff. The contract between the hospice and the
facility should address potential crisis-situations and temporary emergency measures and
how facility staff should handle them.
Hospice is responsible for providing all hospice services including:
• Ongoing assessment, care planning, monitoring, coordination, and provision of
care by the Hospice IDG.
• Assessment, coordination, and provision of any needed general inpatient or
continuous care.
• Consultation about the patient’s care with facility staff.
• Coordination by the hospice RN for the implementation of the plan of care for the
patient.
• Provision of hospice aide services, if these services are determined necessary by
the IDG to supplement the nurse aide services provided by the facility.
• Provision, in a timely manner, of all supplies, medications, and DME needed for
the palliation and management of the terminal illness and related conditions.
• Financial management responsibility for all medical supplies, appliances,
medications and biologicals related to the terminal illness and related conditions.
• Determination of the appropriate level of care to be given to the patient (routine
homecare, inpatient, or continuous care).
• Arranging any necessary transfers from the facility, in consultation with the
facility staff.
L763
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c) Standard: Written agreement.
The hospice and SNF/NF or ICF/IID must have a written agreement that specifies
the provision of hospice services in the facility. The agreement must be signed by
authorized representatives of the hospice and the SNF/NF or ICF/IID before the
provision of hospice services.
Interpretive Guidelines §418.112(c)
The written agreement is for the provision of hospice services between the two entities.
As the written agreement is not patient specific, it does not need to be rewritten for each
patient. If there are concerns regarding the provision of services, the hospice and the
facility may review and revise this agreement as appropriate for needed changes and/or
improvement in the working relationship between the two entities.
L764
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c) - The written agreement must include at least the following:
(1) The manner in which the SNF/NF or ICF/IID and the hospice are to
communicate with each other and document such communications to ensure that
the needs of patients are addressed and met 24 hours a day.
Interpretive Guidelines §418.112(c)(1)
There should be evidence that the hospice and the facility have reached an agreement on
how to communicate concerns and responses 24 hours a day in order to work together to
meet the needs of the patient identified in the patient’s plan of care. The hospice must
document that this communication has occurred.
L765
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(2) A provision that the SNF/NF or ICF/IID immediately notifies the
hospice if—
(i) A significant change in a patient’s physical, mental, social, or emotional
status occurs;
(ii) Clinical complications appear that suggest a need to alter the plan of
care;
(iii) A need to transfer a patient from the SNF/NF or ICF/IID arises, and
the hospice makes arrangements for, and remains responsible for, any
necessary continuous care or inpatient care necessary related to the
terminal illness and related conditions; or
A patient dies.
L766
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(3) A provision stating that the hospice assumes responsibility for
determining the appropriate course of hospice care, including the determination to
change the level of services provided.
L767
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(4) An agreement that it is the SNF/NF or ICF/IID responsibility to
continue to furnish 24 hour room and board care, meeting the personal care and
nursing needs that would have been provided by the primary caregiver at home at
the same level of care provided before hospice care was elected.
Interpretive Guidelines §418.112(c)(4):
In entering into an agreement with each other, each provider retains responsibility for the
quality and appropriateness of the care it provides in accordance with their respective
laws and regulations. Both providers must comply with their applicable
conditions/requirements for participation in Medicare/Medicaid. The facility’s services
must be consistent with the plan of care developed in coordination with the hospice, (the
hospice patient residing in a facility should not experience any lack of services or
personal care because of his/her status as a hospice patient); and the facility must offer
the same services to its residents who have elected the hospice benefit as it furnishes to
its residents who have not elected the hospice benefit. If a patient is receiving services
from a Medicare/Medicaid certified nursing facility or ICF/IID, and the facility was
advised of concerns by the hospice and failed to address and/or resolve issues related to
coordination of care or implementation of appropriate services, the hospice surveyor will
refer the concerns as a complaint to the State Agency responsible for oversight of the
facility identifying the specific patient(s) involved and the concerns identified.
L768
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(5) An agreement that it is the hospice’s responsibility to provide services
at the same level and to the same extent as those services would be provided if the
SNF/NF or ICF/IID resident were in his or her own home.
Interpretive Guidelines §418.112(c)(5)
Regardless of where a patient resides, a hospice is continually responsible for furnishing
core services, and may not delegate these services to the facility staff.
L769
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(6) A delineation of the hospice’s responsibilities, which include, but are
not limited to the following: providing medical direction and management of the
patient; nursing; counseling (including spiritual, dietary and bereavement); social
work; provision of medical supplies, durable medical equipment and drugs
necessary for the palliation of pain and symptoms associated with the terminal
illness and related conditions; and all other hospice services that are necessary for
the care of the resident’s terminal illness and related conditions.
Interpretive Guidelines §418.112(c)(6)
The agreement should identify how the facility and the hospice determine how all needed
services, professionals, medical supplies, DME and drugs and biologicals necessary for
the palliation and management of pain and symptoms associated with the terminal illness
and related conditions are available to the patient 24 hours a day, 7 days a week,
including who may receive and/or write orders for care, in accordance with State/Federal
requirements.
L770
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(7) A provision that the hospice may use the SNF/NF or ICF/IID nursing
personnel where permitted by State law and as specified by the SNF/NF or ICF/IID
to assist in the administration of prescribed therapies included in the plan of care
only to the extent that the hospice would routinely use the services of a hospice
patient’s family in implementing the plan of care.
L771
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(8) A provision stating that the hospice must report all alleged violations
involving mistreatment, neglect, or verbal, mental, sexual, and physical abuse,
including injuries of unknown source, and misappropriation of patient property by
anyone unrelated to the hospice to the SNF/NF or ICF/IID administrator within 24
hours of the hospice becoming aware of the alleged violation.
L772
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(c)(9) A delineation of the responsibilities of the hospice and the SNF/NF or
ICF/IID to provide bereavement services to SNF/NF or ICF/IID staff.
Interpretive Guidelines §418.112(c)(9)
There are times when facility staff and residents fulfill the role of a patient’s family,
providing caregiver services, being companions, and generally supporting the patient. A
hospice may offer bereavement services to facility staff or residents that fulfill the role of
a hospice patient’s family as identified in the patient’s plan of care.
L773
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(d) Standard: Hospice plan of care.
In accordance with §418.56, a written hospice plan of care must be established and
maintained in consultation with SNF/NF or ICF/IID representatives. All hospice
care provided must be in accordance with this hospice plan of care.
L774
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(d)(1) The hospice plan of care must identify the care and services that are
needed and specifically identify which provider is responsible for performing the
respective functions that have been agreed upon and included in the hospice plan of
care.
L775
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(d)(2) The hospice plan of care reflects the participation of the hospice, the
SNF/NF or ICF/IID, and the patient and family to the extent possible.
Interpretive Guidelines §418.112(d)(2)
The hospice and the facility must develop a coordinated plan of care for each patient that
guides both providers. When a hospice patient is a resident of a facility, that patient’s
hospice plan of care must be established and maintained in consultation with
representatives of the facility and the patient/family (to the extent possible). The hospice
portion of the plan of care governs the actions of the hospice and describes the services
that are needed to care for the patient. In addition, the coordinated plan of care must
identify which provider (hospice or facility) is responsible for performing a specific
service. The coordinated plan of care may be divided into two portions, one of which is
maintained by the facility and the other, which is maintained by the hospice. The facility
is required to update its plan of care in accordance with any Federal, State or local laws
and regulations governing the particular facility, just as hospices need to update their
plans of care according to §418.56(d) of these CoPs. The hospice plan of care must
specifically identify/delineate the provider responsible for each
function/service/intervention included in the plan of care.
NOTE: The providers must have a procedure that clearly outlines the chain of
communication between the hospice and facility in the event a crisis or
emergency develops, a change of condition occurs, and/or changes to the
hospice portion of the plan of care are indicated.
Based on the shared communication between providers, both providers’ portion of the
plan of care should reflect the identification of:
• A common problem list;
• Palliative interventions;
• Palliative outcomes;
• Responsible discipline;
• Responsible provider; and
• Patient goals.
L776
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(d)(3) - Any changes in the hospice plan of care must be discussed with the
patient or representative, and SNF/NF or ICF/IID representatives, and must be
approved by the hospice before implementation.
Interpretive Guidelines §418.112(d)(3)
The hospice and the facility must have a process in which they can exchange information
from the hospice IDG plan of care reviews and assessment updates, and the facility team,
patient and family (to the extent possible) conferences, when updating the plan of care
and evaluating outcomes of care to assure that the patient receives the necessary care and
services. The hospice must authorize all changes to the hospice portion of the plan of
care prior to the change being made.
L777
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(e) Standard: Coordination of services.
The hospice must:
(1) Designate a member of each interdisciplinary group that is responsible for a
patient who is a resident of a SNF/NF or ICF/IID. The designated interdisciplinary
group member is responsible for:
L778
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(e)(1)(i) - Providing overall coordination of the hospice care of the SNF/NF
or ICF/IID resident with SNF/NF or ICF/IID representatives; and
Interpretive Guidelines §418.112(e)(1)(i)
The intent of this regulation is for the hospice IDG to designate a member responsible for
overseeing and coordinating the provision of care between the hospice and the facility.
This person may or may not be the hospice RN responsible for the coordination of
patient’s hospice care in the facility. It may also be the physician, social worker or
counselor member of the IDG. In order to facilitate the coordination and provision of
hospice care to the patient, the hospice and the facility should address how the hospice
staff access and communicate with facility staff. This includes, but is not limited to:
• Development of each provider’s portion of the plan of care to assure that the plans
are complimentary and reflect common goals and the patient’s expressed desire
for hospice care;
• Documentation in both respective entities’ clinical records or other means to
ensure continuity of communication and easy access to ongoing information;
• Role of any hospice vendor in delivering supplies or medications;
• Ordering, renewal, delivery and administration of medications; and
• Role of the attending physician, and process for obtaining and implementing
physician orders.
L779
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(e)(1)(ii) Communicating with SNF/NF or ICF/IID representatives and
other health care providers participating in the provision of care for the
terminal illness and related conditions and other conditions to ensure quality of
care for the patient and family.
L780
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(e)(2) Ensure that the hospice IDG communicates with the SNF/NF or
ICF/IID medical director, the patient’s attending physician, and other physicians
participating in the provision of care to the patient as needed to coordinate the
hospice care of the hospice patient with the medical care provided by other
physicians.
Interpretive Guidelines §418.112(e)(2)
Both providers may document physician orders. Orders are to be dated and signed in
accordance with State laws. Implementation of the plan of care changes resulting from
physician orders received by the facility must have prior hospice approval.
L781
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(e)(3) Provide the SNF/NF or ICF/IID with the following information:
(i) The most recent hospice plan of care specific to each patient;
(ii) Hospice election form and any advance directives specific to each
patient;
(iii) Physician certification and recertification of the terminal illness
specific to each patient;
(iv) Names and contact information for hospice personnel involved in
hospice care of each patient;
(v) Instructions on how to access the hospice’s 24-hour on-call system;
(vi) Hospice medication information specific to each patient; and
(vii) Hospice physician and attending physician (if any) orders specific to
each patient.
Interpretive Guidelines §418.112(e)(3)
The hospice and facility must have a process by which information from the hospice IDG
plan of care reviews, updated assessments, and the facility team and the patient and
family (to the extent possible) will be exchanged when developing and updating the plan
of care and evaluating outcomes of care to assure that the patient receives the necessary
care and services.
L782
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.112(f) Standard: Orientation and training of staff.
Hospice staff, in coordination with SNF/NF or ICF/IID facility staff, must assure
orientation of such staff furnishing care to hospice patients in the hospice
philosophy, including hospice policies and procedures regarding methods of
comfort, pain control, symptom management, as well as principles about death and
dying, individual responses to death, patient rights, appropriate forms, and record
keeping requirements.
Interpretive Guidelines §418.112(f)
It is a shared responsibility of the hospice in conjunction with the SNF/NF or ICF/IID to
assess the need for staff training and coordinate the staff training with representatives of
the facility, and to determine how frequently training needs to be offered in order to
ensure that the facility staff furnishing care to hospice patients are oriented to the
philosophy of hospice care. Facility staff turnover rates should be a consideration in
determining training frequency.
§418.113 Condition of participation: Emergency preparedness.
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
Interpretive Guidelines: § 418.113
Hospice programs must comply with the applicable emergency preparedness
requirements referenced in Appendix Z of the State Operations Manual. For all
applicable requirements, guidance, and survey protocol related to Emergency
Preparedness in hospice programs, please refer to Appendix Z.
L783
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114 Condition of participation: Personnel qualifications
L784
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(a): General qualification requirements
Except as specified in paragraph (c) of this section, all professionals who furnish
services directly, under an individual contract, or under arrangements with a
hospice, must be legally authorized (licensed, certified or registered) in accordance
with applicable Federal, State and local laws, and must act only within the scope of
his or her State license, or State certification, or registration. All personnel
qualifications must be kept current at all times.
L785
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b) Personnel qualifications for certain disciplines. The following
qualifications must be met:
(1) Physician. Physicians must meet the qualifications and conditions as defined in
Section 1861(r) of the Act and implemented at §410.20 of this chapter.
L786
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(2) Hospice aide. Hospice aides must meet the qualifications required by
Section 1891(a)(3) of the Act and implemented at §418.76.
L787
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(3) Social worker. A person who—
(i) (A) Has a Master of Social Work (MSW) degree from a school of
social work accredited by the Council on Social Work Education;
or
(B) Has a baccalaureate degree in social work from an institution
accredited by the Council on Social Work Education; or a
baccalaureate degree in psychology, sociology, or other field
related to social work and is supervised by an MSW as described
in paragraph (b)(3)(i)(A) of this section; and
(ii) Has one year of social work experience in a health care setting; or
(iii) Has a baccalaureate degree from a school of social work accredited by
the Council on Social Work Education, is employed by the hospice
before December 2, 2008, and is not required to be supervised by an
MSW.
Interpretive Guidelines §418.114(b)(3)
A hospice social worker must at least meet one of the following options:
1. Have an MSW degree from a school of social work accredited by the Council on
Social Work Education (CSWE), and one year of experience in a health care
setting.
2. Have a baccalaureate degree in social work (BSW) from a school of social work
accredited by the CSWE, and one year of experience in a health care setting and
be supervised by a MSW from a school of social work accredited by the CSWE
and who has one year of experience in a health care setting. If the BSW is
employed by the hospice before December 2, 2008, he/she is exempted from the
MSW supervision requirement.
3. Have a baccalaureate degree in psychology, sociology, or other field related to
social work, and at least one year of social work experience in a health care
setting and be supervised by a MSW from a school of social work accredited by
the CSWE and who has one year of experience in a health care setting.
The hospice must also defer to State law regarding social work requirements. If State
requirements are more stringent, the hospice must comply with the State requirements.
For example, if the State requires a social worker to have a BSW or an MSW, the hospice
may not employ a person with a baccalaureate degree in psychology, sociology, or other
field related to social work to work as a hospice social worker.
Each hospice must employ or contract with at least one MSW to serve in the supervisor
role as an active advisor, consulting with the BSW on assessing the needs of patients and
families, developing and updating the social work portion of the plan of care, and
delivering care to patients and families. This supervision may occur in person, over the
telephone, through electronic communication, or any combination thereof. The hospice
must allow time for this supervision to happen on a regular basis and provide
documentation as to the nature and scope of supervision. The hospice must also ensure
that non-social work trained bachelor’s prepared employees filling the role of social
worker are supervised by a MSW who graduated from a school of social work accredited
by the CSWE, and who has at least one year of experience in a health care setting.
Social workers with a baccalaureate degree from a school of social work accredited by
the CSWE and who are employed by the hospice before December 2, 2008, are exempted
from the MSW supervision requirement. If a hospice hires a new social worker with a
baccalaureate degree and one year of experience in a health care setting after December
2, 2008, then the baccalaureate social worker must be supervised by an MSW who has
one year of experience in a health care setting.
L788
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(4) - Speech language pathologist. A person who meets either of the
following requirements:
(i) The education and experience requirements for a Certificate of Clinical
Competence in speech-language pathology granted by the American
Speech-Language-Hearing Association.
(ii) The educational requirements for certification and is in the process of
accumulating the supervised experience required for certification.
L789
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(5) - Occupational therapist. A person who—
(i)
(A) Is licensed or otherwise regulated, if applicable, as an occupational
therapist by the State in which practicing, unless licensure does not
apply;
(B) Graduated after successful completion of an occupational therapist
education program accredited by the Accreditation Council for
Occupational Therapy Education (ACOTE) of the American
Occupational Therapy Association, Inc. (AOTA), or successor
organizations of ACOTE; and
(C) Is eligible to take, or has successfully completed the entry-level
certification examination for occupational therapists developed and
administered by the National Board for Certification in Occupational
Therapy, Inc. (NBCOT).
(ii) On or before December 31, 2009—
(A) Is licensed or otherwise regulated, if applicable, as an occupational
therapist by the State in which practicing; or
(B) When licensure or other regulation does not apply—
(1) Graduated after successful completion of an occupational
therapist education program accredited by the accreditation
Council for Occupational therapy Education (ACOTE) of the
American Occupational Therapy Association, Inc. (AOTA) or
successor organizations of ACOTE; and
(2) Is eligible to take, or has successfully completed the entry-level
certification examination for occupational therapists developed
and administered by the National Board for Certification in
Occupational Therapy, Inc., (NBCOT).
(iii) On or before January 1, 2008—
(A) Graduated after successful completion of an occupational therapy
program accredited jointly by the committee on Allied Health
Education and Accreditation of the American Medical Association
and the American Occupational Therapy Association; or
(B) Is eligible for the National Registration Examination of the American
Occupational Therapy Association or the National Board for
Certification in Occupational Therapy.
(iv) On or before December 31, 1977—
(A) Had 2 years of appropriate experience as an occupational therapist;
and
(B) Had achieved a satisfactory grade on an occupational therapist
proficiency examination conducted, approved, or sponsored by the
U.S. Public Health Service.

(v) If educated outside the United States—
(A) Must meet both of the following:
(1) Graduated after successful completion of an occupational therapist
education program accredited as substantially equivalent to
occupational therapist assistant entry level education in the United
States by one of the following:
(i) The Accreditation Council for Occupational Therapy
Education (ACOTE).
(ii) Successor organizations of ACOTE.
(iii) The World Federation of Occupational Therapists.
(iv) A credentialing body approved by the American
Occupational Therapy Association.
(v) Successfully completed the entry level certification
examination for occupational therapists developed and
administered by the National Board for Certification in
Occupational Therapy, Inc. (NBCOT).
(2) On or before December 31, 2009, is licensed or otherwise regulated, if
applicable, as an occupational therapist by the State in which
practicing.
L790
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(6) - Occupational therapy assistant. A person who—
(i) Meets all of the following:
(A) Is licensed or otherwise regulated, if applicable, as an occupational
therapy assistant by the State in which practicing, unless licensure does
apply.
(B) Graduated after successful completion of an occupational therapy
assistant education program accredited by the Accreditation Council for
Occupational Therapy Education, (ACOTE) of the American
Occupational Therapy Association, Inc. (AOTA) or its successor
organizations.
(C) Is eligible to take or successfully completed the entry-level certification
examination for occupational therapy assistants developed and
administered by the National Board for Certification in Occupational
Therapy, Inc. (NBCOT).
(ii) On or before December 31, 2009—
(A) Is licensed or otherwise regulated as an occupational therapy assistant, if
applicable, by the State in which practicing; or any qualifications defined
by the State in which practicing, unless licensure does not apply; or
(B) Must meet both of the following:
(1) Completed certification requirements to practice as an
occupational therapy assistant established by a credentialing
organization approved by the American Occupational Therapy
Association.
(2) After January 1, 2010, meets the requirements in paragraph
(b)(6)(i) of this section.
(iii) After December 31, 1977 and on or before December 31, 2007—
(A) Completed certification requirements to practice as an occupational
therapy assistant established by a credentialing organization approved by
the American Occupational Therapy Association; or
(B) Completed the requirements to practice as an occupational therapy
assistant applicable in the State in which practicing.
(iv) On or before December 31, 1977—
(A) Had 2 years of appropriate experience as an occupational therapy
assistant; and
(B) Had achieved a satisfactory grade on an occupational therapy assistant
proficiency examination conducted, approved, or sponsored by the U.S.
Public Health Service.
(v) If educated outside the United States, on or after January 1, 2008—
(A) Graduated after successful completion of an occupational therapy
assistant education program that is accredited as substantially equivalent
to occupational therapist assistant entry level education in the United
States by—
(1) The Accreditation Council for Occupational Therapy Education
(ACOTE).
(2) Its successor organizations.
(3) The World Federation of Occupational Therapists.
(4) By a credentialing body approved by the American Occupational
Therapy Association; and
(5) Successfully completed the entry level certification examination
for occupational therapy assistants developed and administered by
the National Board for Certification in Occupational Therapy, Inc.
(NBCOT).
L791
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(7) - Physical therapist. A person who is licensed, if applicable, by the
State in which practicing, unless licensure does not apply and meets one of the
following requirements:
(i) Graduated after successful completion of a physical therapist education
program approved by one of the following:
(A) The Commission on Accreditation in Physical Therapy Education
(CAPTE).
(B) Successor organizations of CAPTE.
(C) An education program outside the United States determined to be
substantially equivalent to physical therapist entry level education in the
United States by a credentials evaluation organization approved by the
American Physical Therapy Association or an organization identified in
8 CFR 212.15(e) as it relates to physical therapists.
(D) Passed an examination for physical therapists approved by the State
in which physical therapy services are provided.
(ii) On or before December 31, 2009—
(A) Graduated after successful completion of a physical therapy curriculum
approved by the Commission on Accreditation in Physical Therapy
Education (CAPTE); or
(B) Meets both of the following:
(1) Graduated after successful completion of an education program
determined to be substantially equivalent to physical therapist
entry level education in the United States by a credentials
evaluation organization approved by the American Physical
Therapy Association or identified in 8 CFR 212.15(e) as it relates
to physical therapists.
(2) Passed an examination for physical therapists approved by the
State in which physical therapy services are provided.
(iii) Before January 1, 2008—
(A) Graduated from a physical therapy curriculum approved by one of the
following:
(1) The American Physical Therapy Association.
(2) The Committee on Allied Health Education and Accreditation of
the American Medical Association.
(3) The Council on Medical Education of the American Medical
Association and the American Physical Therapy Association.
(iv) On or before December 31, 1977 was licensed or qualified as a physical
therapist and meets both of the following:
(A) Has 2 years of appropriate experience as a physical therapist.
(B) Has achieved a satisfactory grade on a proficiency examination
conducted, approved, or sponsored by the U.S. Public Health Service.
(v) Before January 1, 1966—
(A) Was admitted to membership by the American Physical Therapy
Association;
(B) Was admitted to registration by the American Registry of Physical
Therapists; and
(C) Graduated from a physical therapy curriculum in a 4-year college or
university approved by a State department of education.
(vi) Before January 1, 1966 was licensed or registered, and before January 1,
1970, had 15 years of fulltime experience in the treatment of illness or injury
through the practice of physical therapy in which services were rendered
under the order and direction of attending and referring doctors of medicine
or osteopathy.
(vii) If trained outside the United States before January 1, 2008, meets the
following requirements:
(A) Was graduated since 1928 from a physical therapy curriculum approved
in the country in which the curriculum was located and in which there is
a member organization of the World Confederation for Physical
Therapy.
(B) Meets the requirements for membership in a member organization of the
World Confederation for Physical Therapy.
L792
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(b)(8) - Physical therapist assistant. A person who is licensed, registered or
certified as a physical therapist assistant, if applicable, by the State in which
practicing, unless licensure does not apply and meets one of the following
requirements:
(i) Graduated from a physical therapist assistant curriculum approved by
the Commission on Accreditation in Physical Therapy Education of the
American Physical Therapy Association; or if educated outside the
United States or trained in the United States military, graduated from an
education program determined to be substantially equivalent to physical
therapist assistant entry level education in the United States by a
credentials evaluation organization approved by the American Physical
Therapy Association or identified at 8 CFR 212.15(e); and
(ii) Passed a national examination for physical therapist assistants.
(A) On or before December 31, 2009, meets one of the following:
(1) Is licensed, or otherwise regulated in the State in which
practicing.
(2) In States where licensure or other regulations do not apply,
graduated before December 31, 2009, from a 2-year college-
level program approved by the American Physical Therapy
Association and after January 1, 2010, meets the requirements
of paragraph (b)(8) of this section.
(3) Before January 1, 2008, where licensure or other regulation
does not apply, graduated from a 2-year college level program
approved by the American Physical Therapy Association.
(4) On or before December 31, 1977, was licensed or qualified as
a physical therapist assistant and has achieved a satisfactory
grade on a proficiency examination conducted, approved, or
sponsored by the U.S. Public Health Service.
L793
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(c) -Personnel qualifications when no State licensing, certification or
registration requirements exist. If no State licensing laws, certification or
registration requirements exist for the profession, the following requirements must
be met:
(1) Registered nurse. A graduate of a school of professional nursing.
L794
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(c)(2) - Licensed practical nurse. A person who has completed a practical
nursing program.
L795
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(d) Standard: Criminal background checks
(1) The hospice must obtain a criminal background check on all hospice employees
who have direct patient contact or access to patient records. Hospice contracts
must require that all contracted entities obtain criminal background checks on
contracted employees who have direct patient contact or access to patient
records.
L796
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.114(d)(2) - Criminal background checks must be obtained in accordance with
State requirements. In the absence of State requirements, criminal background
checks must be obtained within three months of the date of employment for all
states that the individual has lived or worked in the past 3 years.
L797
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.116 Condition of participation: Compliance with Federal, State, and local laws
and regulations related to the health and safety of patients.
The hospice and its staff must operate and furnish services in compliance with all
applicable Federal, State, and local laws and regulations related to the health and
safety of patients. If State or local law provides for licensing of hospices, the hospice
must be licensed.

L799
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.116(a) Standard: Multiple locations.
Every hospice must comply with the requirements of §420.206 of this chapter
regarding disclosure of ownership and control information. All hospice multiple
locations must be approved by Medicare and licensed in accordance with State
licensure laws, if applicable, before providing Medicare reimbursed services.
L800
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.116(b) Standard: Laboratory services.
(1) If the hospice engages in laboratory testing other than assisting a patient in self-
administering a test with an appliance that has been approved for that purpose by
the FDA, the hospice must be in compliance with all applicable requirements of part
493 of this chapter.
Interpretive Guidelines §418.116(b)(1)
Hospices holding a certificate of waiver are limited to performing only those tests
determined to be in the waived category. Some tests that a hospice may perform that fall
into the waived category include:
• Dipstick/tablet reagent urinalysis;
• Blood glucose by glucose monitoring devices cleared by the Food and Drug
Administration (FDA) specifically for home use;
• Some prothrombin time tests; and
• Some glycosylated hemoglobin tests.
For a complete listing of waived tests, refer to CMS’ website at
http://www.cms.hhs.gov/CLIA/10_Categorization_of_Tests.asp#TopOfPage
Hospices holding a certificate for provider-performed microscopy procedures are limited
to performing only those tests determined to be in the provider-performed microscopy
procedure category or in combination with waived tests.
The tests in the provider-performed microscopy procedures category (e.g., wet mounts,
urine sediment examinations, and nasal smears for granulocytes) are not typical of those
performed in a hospice. However, if they are conducted by hospice staff under a
certificate for provider-performed microscopy procedures, they must be performed by a
practitioner as specified at §493.19 (i.e., a physician, nurse midwife, nurse practitioner,

physician assistant, or dentist). If not performed by these personnel, the hospice would
require a registration certificate (which allows the performance of such testing until a
determination of compliance is made), certificate of accreditation, or certificate of
compliance.
For a complete listing of provider-performed microscopy procedures, refer to CMS’
website at http://www.cms.hhs.gov/CLIA/10_Categorization_of_Tests.asp#TopOfPage
A registration certificate, a certificate of accreditation, or a certificate of compliance is
required if the hospice performs any other testing procedures, (i.e., moderate or high
complexity testing). While some prothrombin testing is in the waived category, as
mentioned above, other prothrombin testing is considered moderate complexity testing
depending on the skill level required to operate the instrument.
For a complete listing of moderate and high complexity tests, refer to CMS’ website at
http://www.cms.hhs.gov/CLIA/10_Categorization_of_Tests.asp#TopOfPage
Assisting individuals in administering their own tests, such as fingerstick blood glucose
or prothrombin testing, is not considered testing subject to the CLIA regulations.
However, if the hospice staff is actually responsible for measuring the blood glucose
level or prothrombin times of patients with an FDA-approved blood glucose or
prothrombin time monitor, and no other tests are being performed, request to see the
facility’s certificate of waiver, since glucose testing with a blood glucose meter
(approved by the FDA specifically for home use) and some prothrombin time tests are
waived tests under the provisions at 42 CFR 493.15.
L801
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.116(b)(2) - If the hospice chooses to refer specimens for laboratory testing to a
reference laboratory, the reference laboratory must be certified in the appropriate
specialties and subspecialties of services in accordance with the applicable
requirements of Part 493 of this chapter.
Interpretive Guidelines §418.116(b)(2)
The hospice is required to comply with applicable State law and secure a CLIA certificate
of waiver for any waived testing performed by staff. Lab specimens obtained in the
patient’s home must be taken to laboratories that meet CLIA and state law requirements.
The hospice should have a copy of the reference laboratory’s CLIA certificate in its
administrative records.
L820
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110 Condition of participation: Hospices that provide inpatient care directly
A hospice that provides inpatient care directly in its own facility must demonstrate
compliance with all of the following standards:
L821
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(a) Standard: Staffing
The hospice is responsible for ensuring that staffing for all services reflects its
volume of patients, their acuity, and the level of intensity of services needed to
ensure that plan of care outcomes are achieved and negative outcomes are avoided.
Interpretive Guidelines 418.110(a)
The intent of this regulation is to ensure that the hospice provides staffing that is adequate
to meet patient needs. Adequate staff means that the numbers and types of qualified,
trained, and experienced staff on the inpatient unit meet the care needs of every patient.
L822
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(b) Standard: Twenty-four hour nursing services
§418.100(b)(1) The hospice facility must provide 24-hour nursing services that meet
the nursing needs of all patients and are furnished in accordance with each patient’s
plan of care. Each patient must receive all nursing services as prescribed and must
be kept comfortable, clean, well-groomed, and protected from accident, injury, and
infection.
L823
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(b)(2) - If at least one patient in the hospice facility is receiving general
inpatient care, then each shift must include a registered nurse who provides direct
patient care.
Interpretive Guidelines §418.110(b)(2)
The general inpatient care provided in a facility for pain control or acute or chronic
symptom management, which cannot be managed in other settings, is a different level of
care than respite care. It is not automatically necessary to have an RN assigned to every
shift to provide direct patient care if the only hospice patients in a facility are receiving
the respite or routine levels of care. Staffing for a facility solely providing the respite or
routine home care levels of care to hospice patients should be based on each patient’s
care needs. The requirements for nursing services for respite care are located at
§418.108(b)(2).
L824
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(c) Standard: Physical environment.
The hospice must maintain a safe physical environment free of hazards for patients,
staff, and visitors.
L825
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(c)(1) - Safety management.
The hospice must address real or potential threats to the health and safety of
the patients, others, and property.
L826
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(c)(2) - Physical plant and equipment. The hospice must develop
procedures for controlling the reliability and quality of--
(i) The routine storage and prompt disposal of trash and medical
waste;
(ii) Light, temperature, and ventilation/air exchanges throughout the
hospice;
(iii) Emergency gas and water supply; and
(iv) The scheduled and emergency maintenance and repair of all
equipment.
Interpretative Guidelines §418.110(c)(2)
The storage and disposal of trash and medical waste should be in accordance with
Federal, State and local laws and regulations (i.e., the Environmental Protection Agency,
Occupational Health and Safety Administration (OSHA), CDC, State environmental,
health and safety regulations).
The hospice must have a system to provide emergency gas and water as needed to
provide care to inpatients. This includes making arrangements with local utility
companies and others for the provision of emergency sources of water and gas. The
hospice should consider nationally accepted references or calculations made by qualified
staff when determining the need for at least water and gas. For example, one source for
information on water is the Federal Emergency Management Agency (FEMA).
L827
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110 (d) Standard: Fire protection
(1) Except as otherwise provided in this section--
(i) The hospice must meet the applicable provisions and must proceed in
accordance with the Life Safety Code (NFPA 101 and Tentative Interim
Amendments TIA 12-1, TIA 12-2, TIA 12-3, and TIA 12-4.)
(ii) Notwithstanding paragraph (d)(1)(i)) of this section, corridor doors and
doors to rooms containing flammable or combustible materials must be
provided with positive latching hardware. Roller latches are prohibited
on such doors.
(1) In consideration of a recommendation by the State survey agency or
Accrediting Organization or at the discretion of the Secretary, may waive, for
periods deemed appropriate, specific provisions of the Life Safety Code which
would result in unreasonable hardship upon a hospice facility, but only if the
waiver will not adversely affect the health and safety of the patients.
(1) The provisions of the adopted edition of the Life Safety Code do not apply in a
State if CMS finds that a fire and safety code imposed by State law adequately
protects patients in hospices.
(1) A hospice may place alcohol-based hand rub dispensers in its facility if the
dispensers are installed in a manner that adequately protects against access by
vulnerable populations.
(1) When a sprinkler system is shut down for more than 10 hours, the hospice
must:
(i) Evacuate the building or portion of the building affected by the system
outage until the system is back in service, or
(ii) Establish a fire watch until the system is back in service.
(1) Buildings must have an outside window or outside door in every sleeping room,
and for any building constructed after July 5, 2016 the sill height must not
exceed 36 inches above the floor. Windows in atrium walls are considered
outside windows for the purposes of this requirement.
L828
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(e) Standard: Building Safety.
Except as otherwise provided in this section, the hospice must meet the applicable
provisions and must proceed in accordance with the Health Care Facilities Code
(NFPA 99 and Tentative Interim Amendments TIA 12-2, TIA 12-3, TIA 12-4, TIA
12-5 and TIA 12-6).
(1) Chapters 7, 8, 12, and 13 of the adopted Health Care Facilities Code do not
apply to a hospice.
(2) If application of the Health Care Facilities Code required under paragraph (e)
of this section would result in unreasonable hardship for the hospice, CMS may
waive specific provisions of the Health Care Facilities Code, but only if the
waiver does not adversely affect the health and safety of patients.
L829
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(f) Standard: Patient areas.
The hospice must provide a home-like atmosphere and ensure that patient areas are
designed to preserve the dignity, comfort, and privacy of patients.
(1) The hospice must provide—
(i) Physical space for private patient and family visiting;
(ii) Accommodations for family members to remain with the patient
throughout the night; and
(iii) Physical space for family privacy after a patient's death.
(2) The hospice must provide the opportunity for patients to receive visitors at any
hour, including infants and small children.
Interpretive Guidelines §418.110(f)
A homelike atmosphere de-emphasizes the institutional character of the setting to the
extent possible.
L830
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(g) Standard: Patient rooms.
(1)
The hospice must ensure that patient rooms are designed and equipped for
nursing care, as well as the dignity, comfort, and privacy of patients.
(2)
The hospice must accommodate a patient and family request for a single room
whenever possible.
(3)
Each patient's room must—
(i) Be at or above grade level;
(ii) Contain a suitable bed and other appropriate furniture for each
patient;
(iii) Have closet space that provides security and privacy for clothing and
personal belongings;
(iv) Accommodate no more than two patients and their family members;
(v) Provide at least 80 square feet for each residing patient in a double
room and at least 100 square feet for each patient residing in a single
room; and
(vi) Be equipped with an easily-activated, functioning device accessible to
the patient, that is used for calling for assistance.
(4) For a facility occupied by a Medicare-participating hospice on December 2,
2008, CMS may waive the space and occupancy requirements of paragraphs
(g)(2)(iv) and (g)(2)(v) of this section if it determines that—
(i) Imposition of the requirements would result in unreasonable hardship
on the hospice if strictly enforced; or jeopardize its ability to continue to
participate in the Medicare program; and
(ii) The waiver serves the needs of the patient and does not adversely affect
their health and safety.
Interpretive Guidelines §418.110(g)
In addition to a clean, comfortable bed, each patient should have at least a place to put
personal effects, such as pictures and a clock, furniture suitable for the comfort of the
patient and visitors (i.e., a chair) and adequate lighting suitable to the tasks the patient
chooses to perform, or the inpatient staff needs to perform.
Hospices must submit the waiver requests mentioned in this requirement, in writing, to
the CMS Location.
L831
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(h) Standard: Toilet and bathing facilities.
Each patient room must be equipped with, or conveniently located near, toilet
and bathing facilities.
Interpretive Guidelines §418.110(h)
“Toilet facilities” means a space that contains a lavatory and a toilet. There should be at
least one toilet facility and shower stall large enough to accommodate a wheelchair and
patient transfer, that is conveniently located near patient rooms that require such
accommodations.
L832
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(i) Standard: Plumbing facilities.
The hospice must—
(1)
Have an adequate supply of hot water at all times; and
(2)
Have plumbing fixtures with control valves that automatically regulate the
temperature of the hot water used by patients.
Interpretive Guidelines §418.110(i)
The intent of this regulation is that the temperature of water at fixtures and in showers
and tubs used by patients shall be automatically regulated by control valves and delivered
for use at the appropriate temperature.
There is a risk that patients or staff may be scalded by excessively hot water discharged
by plumbing fixtures. Water that is too hot may scald individuals who are exposed to it.
This danger is particularly significant for patients who may have circulatory or other
neurological deficits that prevent the instantaneous recoil from water that is too hot.
The chart below shows the estimated time for persons to receive second and third degree

burns at various temperatures.
Water Temperature
Time to Receive Second
Degree Burn
Time to Receive Third
Degree Burn
120 degrees
8 minutes
10 minutes
124 degrees
2 minutes
4 minutes
131 degrees
17 seconds
30 seconds
140 degrees
3 seconds
5 seconds
150 degrees
<1 second
1 second
The recommended water temperatures at the plumbing fixtures should be maintained at
or below 110 degrees.
L833
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(j) Standard: Infection control.
The hospice must maintain an infection control program that protects patients, staff
and others by preventing and controlling infections and communicable disease as
stipulated in §418.60.
Interpretive Guidelines §418.110(j)
The hospice inpatient facility must have an active surveillance program that includes
specific measures for prevention, early detection, control, education, and investigation of
infections and communicable diseases in the hospice. There must be a mechanism to
evaluate the effectiveness of the program(s) and take corrective action when necessary.
The program must include implementation of nationally recognized systems of infection
control guidelines to avoid sources and transmission of infections and communicable
diseases (e.g., the CDC’s Healthcare Infection Control Guidelines, the CDC Guidelines
for Preventing the Transmission of Mycobacterium Tuberculosis in Health Care Settings,
OSHA regulations, and APIC guidelines on infection control, etc.).
The active infection control program should have policies that address the following:
• Definition of nosocomial infections and communicable diseases;
• Measures for identifying, investigating, and reporting nosocomial infections and
communicable diseases;
• Measures for assessing and identifying patients and health care workers, including
hospice personnel, contract staff (e.g., agency nurses, housekeeping staff) and
volunteers, at risk for infections and communicable diseases;
• Measures for the prevention of infections;
• Measures for prevention of communicable disease outbreaks, such as airborne
diseases (TB, SARS, etc.), food borne diseases (Hepatitis A, Salmonella, etc.),
blood borne diseases (HIV, Hepatitis B, etc.), and others (VRE, MRSA,
pseudomonas, etc.);
• Provision of a safe environment consistent with nationally recognized infection
control precautions, such as the current CDC recommendations for the identified
infection and/or communicable disease;
• Isolation procedures and requirements for infected or immunosuppressed patients;
• Use and techniques for standard precautions;
• Education of patients, family members and caregivers about infections and
communicable diseases;
• Techniques for hand washing, respiratory protections, asepsis as well as other
means for limiting the spread of contagion;
• Orientation of all new hospice personnel to infections, communicable diseases,
and to the infection control program;
• Measures for the screening and evaluation of health care workers, including all
hospice staff, contract workers (e.g., agency nurses, housekeeping staff, etc.), and
volunteers, for communicable diseases, and for the evaluation of staff and
volunteers exposed to patients with non-treated communicable diseases; and
• Employee health policies regarding infectious diseases and when infected or ill
employees, including contract workers and volunteers, must not render patient
care and/or must not report to work.
L834
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(k) Standard: Sanitary environment.
The hospice must provide a sanitary environment by following current standards of
practice, including nationally recognized infection control precautions, and avoid
sources and transmission of infections and communicable diseases.
Interpretive Guidelines §418.110(k)
“Sanitary” includes, but is not limited to, preventing the spread of disease-causing
organisms by keeping patient care equipment clean and properly stored. Patient care
equipment includes, but is not limited to, toothbrushes, dentures, denture cups, glasses,
water pitchers, emesis basins, hairbrushes, combs, bed pans, urinals, and positioning or
assistive devices.
L835
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(l) Standard: Linen.
The hospice must have available at all times a quantity of clean linen in sufficient
amounts for all patient uses. Linens must be handled, stored, processed, and
transported in such a manner as to prevent the spread of contaminants.
L836
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(m) Standard: Meal service and menu planning.
The hospice must furnish meals to each patient that are—
Interpretive Guidelines §418.110(m)
The intent of this regulation is to assure that the nutritive value of food is not
compromised and destroyed because of prolonged food storage, light, and air exposure.
Food should be palatable, attractive, and served at the proper temperature as determined
by the type of food.
• Food-palatability refers to the taste and/or flavor of the food.
• Food attractiveness refers to the appearance of the food when served.
• Food temperature is food served at preferable temperature (hot foods are served
hot and cold foods are served cold) as discerned by the patient and customary
practice.
L837
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(m)(1) - Consistent with the patient’s plan of care, nutritional needs, and
therapeutic diet;
L838
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(m)(2) - Palatable, attractive, and served at the proper temperature; and
L839
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(m)(3) - Obtained, stored, prepared, distributed, and served under sanitary
conditions.
L840
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n) Standard: Restraint or seclusion
All patients have the right to be free from physical or mental abuse, and corporal
punishment. All patients have the right to be free from restraint or seclusion, of any
form, imposed as a means of coercion, discipline, convenience, or retaliation by
staff. Restraint or seclusion may only be imposed to ensure the immediate physical
safety of the patient, a staff member, or others and must be discontinued at the
earliest possible time.
Interpretive Guidelines §418.110(n)
The hospice is responsible for creating a culture that supports a patient’s right to be free
from restraint or seclusion and ensures patients are free from physical or mental abuse
and corporal punishment. The hospice must also ensure that systems and processes are
developed, implemented, and evaluated that support the patients’ rights addressed in this
standard, and that eliminate the inappropriate use of restraint or seclusion.
If restraint or seclusion is necessary within the parameters of this regulation (§488.110),
it must be discontinued as soon as possible based on an individualized patient assessment
and re-evaluation. A violation of any of these patients’ rights constitutes an inappropriate
use of restraint or seclusion and would be subject to a condition level deficiency.
The use of restraints for the prevention of falls must not be considered a routine part of a
falls prevention program. Although restraints have been traditionally used as a falls
prevention approach, they have major, serious drawbacks and can contribute to serious
injuries. There is no evidence that the use of physical restraint, (including, but not
limited to, raised side rails) will prevent or reduce falls. Additionally, falls that occur
while a person is physically restrained often result in more severe injuries and/or death.
L841
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(1) - Restraint or seclusion may only be used when less restrictive
interventions have been determined to be ineffective to protect the patient, a staff
member, or others from harm.

L842
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(2) The type or technique of restraint or seclusion used must be the least
restrictive intervention that will be effective to protect the patient, a staff member,
or others from harm.
L843
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(3) - The use of restraint or seclusion must be--
(i) In accordance with a written modification to the patient’s plan of care; and
(ii) Implemented in accordance with safe and appropriate restraint and
seclusion techniques as determined by hospice policy in accordance with
State law.
L844
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(4) - The use of restraint or seclusion must be in accordance with the order of
a physician authorized to order restraint or seclusion by hospice policy in accordance
with State law.
L845
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(5 ) – Orders for the use of restraint or seclusion must never be written
as a standing order or on an as needed basis (PRN).
Interpretive Guidelines §418.110(n)(5)
This regulation prohibits the use of standing or PRN (Latin abbreviation for pro re nata -
as needed; as circumstances require) orders for the use of restraint or seclusion. The
ongoing authorization of restraint or seclusion is not permitted. Each episode of restraint
or seclusion must be initiated in accordance with the order of a physician. If a patient
was recently released from restraint or seclusion, and exhibits behavior that can only be
handled through the reapplication of restraint or seclusion, a new order would be
required. Staff cannot discontinue a restraint or seclusion intervention, and then re-start it
under the same order. This would constitute a PRN order.
L846
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(6) - The medical director or physician designee must be consulted as
soon as possible if the attending physician did not order the restraint or seclusion.
L847
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(7) - Unless superseded by State law that is more restrictive —
(i) Each order for restraint or seclusion used for the management of violent
or self-destructive behavior that jeopardizes the immediate physical
safety of the patient, a staff member, or others may only be renewed in
accordance with the following limits for up to a total of 24 hours:
(A) 4 hours for adults 18 years of age or older;
(B) 2 hours for children and adolescents 9 to 17 years of age; or
(C) 1 hour for children under 9 years of age; and
After 24 hours, before writing a new order for the use of restraint or
seclusion for the management of violent or self-destructive behavior, a
physician authorized to order restraint or seclusion by hospice policy in
accordance with State law must see and assess the patient.
(ii) Each order for restraint used to ensure the physical safety of the non-
violent or non-self-destructive patient may be renewed as authorized by
hospice policy.
L848
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(8) - Restraint or seclusion must be discontinued at the earliest possible
time, regardless of the length of time identified in the order.
L849
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(9) - The condition of the patient who is restrained or secluded must be
monitored by a physician or trained staff that have completed the training criteria
specified in paragraph (o) of this section at an interval determined by hospice policy.

L850
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(10) - Physician, including attending physician, training requirements
must be specified in hospice policy. At a minimum, physicians and attending
physicians authorized to order restraint or seclusion by hospice policy in accordance
with State law must have a working knowledge of hospice policy regarding the use of
restraint or seclusion.
L851
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(11) - When restraint or seclusion is used for the management of violent
or self-destructive behavior that jeopardizes the immediate physical safety of the
patient, a staff member, or others, the patient must be seen face-to-face within 1
hour after the initiation of the intervention --
(i) By a—
(A) Physician; or
(B) RN who has been trained in accordance with the requirements
specified in paragraph (n) of this section.
(ii) To evaluate—
(A) The patient’s immediate situation;
(B) The patient’s reaction to the intervention;
(C) The patient’s medical and behavioral condition; and
(D) The need to continue or terminate the restraint or seclusion.
L852
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(12) - States are free to have requirements by statute or regulation that
are more restrictive than those contained in paragraph (m)(11)(i) of this section.
L853
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(13) - If the face-to-face evaluation specified in §418.110(n)(11) is
conducted by a trained registered nurse, the trained registered nurse must consult
the medical director or physician designee as soon as possible after the completion
of the 1-hour face-to-face evaluation.
L854
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.110(n)(14) - All requirements specified under this paragraph are applicable to
the simultaneous use of restraint and seclusion. Simultaneous restraint and
seclusion use is only permitted if the patient is continually monitored--
i. Face-to-face by an assigned, trained staff member; or
ii. By trained staff using both video and audio equipment. This monitoring
must be in close proximity to the patient.
L855
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(n)(15) - When restraint or seclusion is used, there must be documentation
in the patient’s clinical record of the following:
i. The 1-hour face-to-face medical and behavioral evaluation if restraint or
seclusion is used to manage violent or self-destructive behavior;
ii. A description of the patient’s behavior and the intervention used;
iii. Alternatives or other less restrictive interventions attempted (as applicable);
iv. The patient’s condition or symptom(s) that warranted the use of the restraint
or seclusion; and the patient’s response to the intervention(s) used, including
the rationale for continued use of the intervention.
L856
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(o) Standard: Restraint or seclusion staff training requirements.
The patient has the right to safe implementation of restraint or seclusion by trained
staff.
L857
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(o)(1) - Training intervals. All patient care staff working in the hospice
inpatient facility must be trained and able to demonstrate competency in the
application of restraints, implementation of seclusion, monitoring, assessment, and
providing care for a patient in restraint or seclusion—
(i) Before performing any of the actions specified in this paragraph;
(ii) As part of orientation; and
(iii) Subsequently on a periodic basis consistent with hospice policy.
Interpretive Guidelines §418.110(o)(1)

All staff designated by the hospice as having direct patient care responsibilities, including
contract or agency personnel, must demonstrate the competencies specified in standard
(o) prior to participating in the application of restraints, implementation of seclusion,
monitoring, assessment, or care of a patient in restraint or seclusion. These competencies
must be demonstrated initially as part of hospice orientation and subsequently on a
periodic basis consistent with hospice policy. Hospices have the flexibility to identify a
time frame for ongoing training based on the level of staff competency, and the needs of
the patient population(s) served.
All staff working in a hospice that precludes the use of restraints or seclusion would not
have to be trained or demonstrate competencies specified in this standard since no staff in
a restraint free facility would be applying restraints or placing patients in seclusion. In
this situation, the hospice should ensure that all staff are aware of its restraint and
seclusion free philosophy and provide ongoing training in this philosophy. The hospice
should also closely monitor patients to be sure that the use of any restraint or seclusion
technique is not used.
L858
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(o)(2) - Training content. - The hospice must require appropriate staff to
have education, training, and demonstrated knowledge based on the specific needs
of the patient population in at least the following:
(i) Techniques to identify staff and patient behaviors, events, and
environmental factors that may trigger circumstances that require the
use of a restraint or seclusion.
(ii) The use of nonphysical intervention skills.
(iii) Choosing the least restrictive intervention based on an individualized
assessment of the patient’s medical, or behavioral status or condition.
(iv) The safe application and use of all types of restraint or seclusion used
in the hospice, including training in how to recognize and respond to
signs of physical and psychological distress (for example, positional
asphyxia).
(v) Clinical identification of specific behavioral changes that indicate that
restraint or seclusion is no longer necessary.
(vi) Monitoring the physical and psychological well-being of the patient
who is restrained or secluded, including but not limited to, respiratory
and circulatory status, skin integrity, vital signs, and any special
requirements specified by hospice policy associated with the 1-hour face-
to-face evaluation.
(vii) The use of first aid techniques and certification in the use of
cardiopulmonary resuscitation, including required periodic
recertification.
Interpretive Guidelines §418.110(o)(2)

The term “appropriate staff” includes all staff that apply restraint or seclusion, monitor,
assess, or otherwise provide care for patients in restraint or seclusion.
Staff needs to be able to employ a broad range of clinical interventions to maintain the
safety of the patient and others. The hospice is expected to provide education and
training at the appropriate level, to the appropriate staff, based upon the specific needs of
the patient population(s) being served.
L859
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(o)(3) - Trainer requirements. Individuals providing staff training must be
qualified as evidenced by education, training, and experience in techniques used to
address patients’ behaviors.
Interpretive Guidelines §418.110(o)(3)
Hospices may develop and implement their own training programs or use an outside
training program.
L860
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(o)(4) - Training documentation. The hospice must document in the staff
personnel records that the training and demonstration of competency were
successfully completed.
L861
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)
§418.110(p) - Standard: Death reporting requirements.
Hospices must report deaths associated with the use of seclusion or restraint.
(1) The hospice must report the following information to CMS:
(i) Each unexpected death that occurs while a patient is in restraint or
seclusion.
(ii) Each unexpected death that occurs within 24 hours after the patient has
been removed from restraint or seclusion.

(iii) Each death known to the hospice that occurs within 1 week after restraint or
seclusion where it is reasonable to assume that use of restraint or placement
in seclusion contributed directly or indirectly to a patient's death.
"Reasonable to assume" in this context includes, but is not limited to, deaths
related to restrictions of movement for prolonged periods of time, or death
related to chest compression, restriction of breathing or asphyxiation.
(2) Each death referenced in this paragraph must be reported to CMS by telephone
no later than the close of business the next business day following knowledge of
the patient’s death.
(3) Staff must document in the patient's clinical record the date and time the death
was reported to CMS.
Interpretive Guidelines §418.110(p)
If a patient has an unexpected death that occurs while in restraint or seclusion, or an
unexpected death occurs within 24 hours after restraint or seclusion has been
discontinued, the death must be reported to CMS Location. Additionally, if a death
occurs within one week after the use of restraint or seclusion and it is reasonable to
assume the death was associated with restraint and/or seclusion, the death should be
reported to CMS Location.
Restraint means:
(1) Any manual method, physical or mechanical device, material, or equipment that
immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or
head freely, not including devices, such as orthopedically prescribed devices, surgical
dressings or bandages, protective helmets, or other methods that involve the physical
holding of a patient for the purpose of conducting routine physical examinations or tests,
or to protect the patient from falling out of bed, or to permit the patient to participate in
activities without the risk of physical harm (this does not include a physical escort); or
(2) A drug or medication when it is used as a restriction to manage the patient’s behavior
or restrict the patient’s freedom of movement and is not a standard treatment or dosage
for the patient’s condition.
Seclusion means the involuntary confinement of a patient alone in a room or an area
from which the patient is physically prevented from leaving. Patients who request private
rooms would not be considered in seclusion.
L862
(Rev. 210; Issued:02-03-23; Effective:02-03-23; Implementation:02-03-23)

§418.110(q) – The standards incorporated by reference in this section are approved
for incorporation by reference by the Director of the Office of the Federal Register
in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. You may inspect a copy at
the CMS Information Resource Center, 7500 Security Boulevard, Baltimore, MD or
at the National Archives and Records Administration (NARA). For information on
the availability of this material at NARA, call 202–741–6030, or go to:
http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
If any changes in this edition of the Code are incorporated by reference, CMS will
publish a document in the Federal Register to announce the changes.
(1) National Fire Protection Association, 1 Battery march Park, Quincy, MA 02169,
www.nfpa.org, 1.617.770.3000.
(i) NFPA 99, Standards for Health Care Facilities Code of the National
Fire Protection Association 99, 2012 edition, issued August 11, 2011.
(ii) TIA 12–2 to NFPA 99, issued August 11, 2011.
(iii) TIA 12–3 to NFPA 99, issued August 9, 2012.
(iv) TIA 12–4 to NFPA 99, issued March 7, 2013.
(v) TIA 12–5 to NFPA 99, issued August 1, 2013.
(vi) TIA 12–6 to NFPA 99, issued March 3, 2014.
(vii) NFPA 101, Life Safety Code, 2012 edition, issued August 11, 2011.
(viii) TIA 12–1 to NFPA 101, issued August 11, 2011.
(ix) TIA 12–2 to NFPA 101, issued October 30, 2012.
(x) TIA 12–3 to NFPA 101, issued October 22, 2013.
(xi) TIA 12–4 to NFPA 101, issued October 22, 2013.
(2) [Reserved]
L901
(Rev. 222; Issued: 06-07-24; Effective: 06-07-24; Implementation: 06-07-24)
§418.114(b)(9)
Marriage and family counselor as defined at § 410.53.
L902
(Rev. 222; Issued: 06-07-24; Effective: 06-07-24; Implementation: 06-07-24)
§418.114(b)(10)
Mental health counselor as defined at § 410.54.
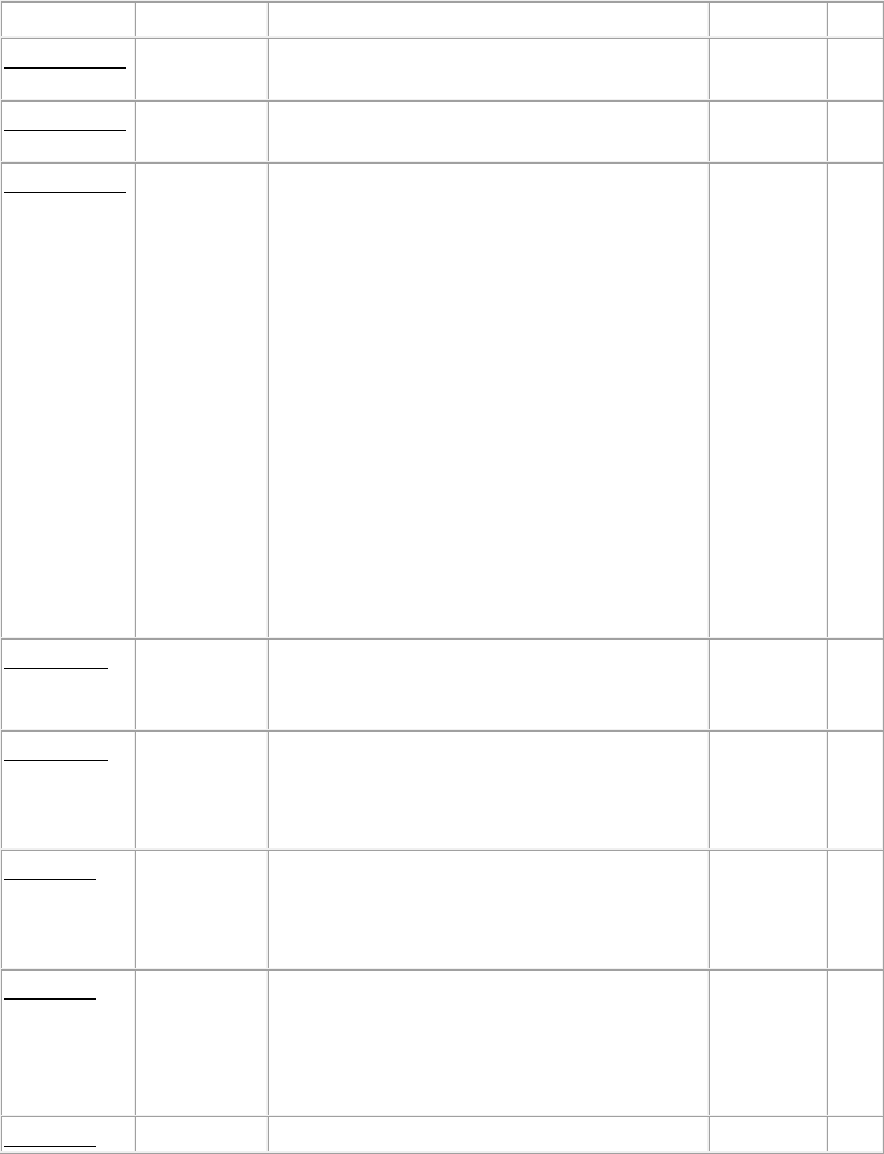
Transmittals Issued for this Appendix
Rev #
Issue Date
Subject
Impl Date
CR#
R222SOMA
06/07/2024
Revisions to the State Operations Manual
(SOM) Appendix M-Hospice
06/07/2024
N/A
R210SOMA
02/03/2023
Revisions to the State Operations Manual
(SOM) Appendix M – Hospice
02/03/2023
N/A
R200SOMA
02/21/2020
Revisions to the State Operations Manual
(SOM) Appendix A -
Hospitals, Appendix AA – Psychiatric
Hospitals, Appendix B – Home Health
Agency, Appendix D - Portable X-Ray,
Appendix G - Rural Health
Clinics/Federally Qualified Health Centers,
Appendix H- End Stage Renal Disease
Facilities (ESRD), Appendix K –
Comprehensive Outpatient Rehabilitation
Facility, Appendix L - Ambulatory Surgical
Centers, Appendix M – Hospice, Appendix
U - Religious Nonmedical Healthcare
Institutions, Appendix W - Critical Access
Hospitals (CAHs), Appendix X-Organ
Transplant Program and Appendix Z -
Emergency Preparedness
02/21/2020
N/A
R149SOM
10/09/2015
State Operations Manual (SOM) for All
Types of Providers and Suppliers Subject to
Certification
10/09/2015
N/A
R104SOM
03/072014
State Operations Manual (SOM) Appendix
M revisions for Intermediate Care Facilities
for Individuals with Intellectual Disabilities
(ICF/IID)
03/07/2014
N/A
R69SOM
12/15/2010
Revisions to Chapter 2, “The Certification
Process”, Sections 2080-2089-“Hospices,”
and Appendix M, “Guidance to Surveyors,
Hospices”
10/01/2010
N/A
R65SOM
10/01/2010
Revisions to Chapter 2, “The Certification
Process”, Sections 2080-2089-“Hospices,”
and Appendix M, “Guidance to Surveyors,
Hospices” - Rescinded and replaced by
Transmittal 69
10/01/2010
N/A
R01SOM
05/21/2004
Initial Release of Pub 100-07
N/A
N/A
