Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences.
General Best Practice Guidelines for Immunization
Chapter 2, Page 9

–
–
–
–
–
–
–
–
–
–
Timing and spacing
Contraindications and precautions
Preventing and managing adverse reactions to immunization
Vaccine administration
Storage and handling
Altered immunocompetence
Special situations
Vaccination records
Vaccination programs
Vaccine information sources
General Best Practice Guidelines for Immunization

General “Recommendations” on Immunization
Pink Book chapter
• Timing and spacing
• Contraindications and precautions

Timing and Spacing Issues
Interval between receipt of antibody- containing blood
products and live vaccines
Interval between doses of different vaccines not
administered simultaneously
Interval between subsequent doses of the same vaccine

Antibody-containing
Blood Products
Used to restore a needed component of blood or provide a
passive immune response following disease exposure.
Sometimes circumstance dictates the use of antibody-
containing blood products along with a vaccine.

Antibody and Live Vaccines
Inactivated vaccines are generally not affected by circulating
antibody to the antigen
Live, attenuated vaccines might be affected by circulating
antibody to the antigen – an effectiveness concern
General Rule

Antibody Products and
Measles- and Varicella-containing Vaccines
Product given first
Vaccine
Antibody
Action
Wait 2 weeks
before giving antibody
Wait at least 3 months before giving
vaccine
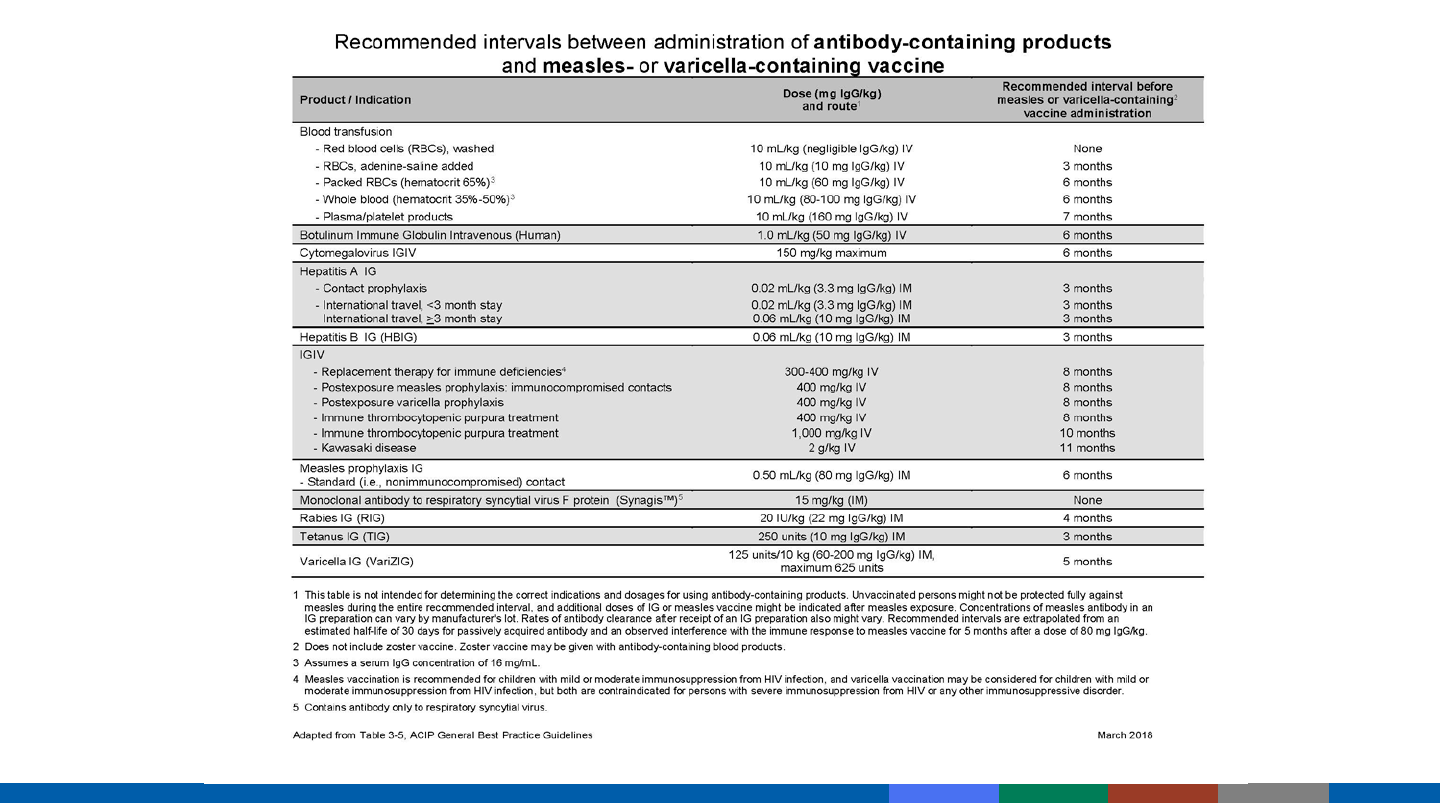
Spacing of Antibody-containing Products and MMR and
Varicella Vaccines
Product
Hepatitis A (IG)
Washed red blood cells
Measles prophylaxis (IG)
(immunocompetent recipient)
Plasma/platelet products
Intravenous immune globulin (IGIV)
0 months
3 months
6 months
7 months
7-11 months
Interval

Palivizumab (Synagis)
Contains only monoclonal RSV antibody
Does not interfere with live virus vaccination
Red blood cells (RBCs), washed
Negligible antibody content
Products Containing Type-specific
or Negligible Antibody

Interval Between Doses of Different Vaccines
Simultaneous administration
Non-simultaneous administration

Simultaneous Administration
General Rule
All vaccines can be administered at the same visit as all
other vaccines.
Exceptions:
• PCV13 and PPSV23: Give PCV13 first
• MCV4-D
(Menactra only) and PCV13 in asplenic or HIV infected
persons: Give PCV13 first

Combination
2 live injected OR
1 live injected and 1 intranasal
influenza vaccine
A
ll other vaccines
One exception
Menactra and DTaP
Minimum interval
4 weeks
None
6 months
Non-simultaneous Administration:
Live-vaccine Effectiveness

Spacing of Live Vaccines Not
Given Simultaneously
If 2 live parenteral or intranasal vaccines are given less than
28 days apart, the vaccine given second should be repeated.
Antibody response from first vaccine interferes with
replication of second vaccine.

Intervals Between Doses
General Rule
Increasing the interval between doses of a multidose vaccine
does not diminish the effectiveness of the vaccine.

Extended Interval Between Doses
Not all variations among all schedules for all vaccines have
been studied
Available studies of extended intervals have shown no
significant difference in final titer
It is not necessary to restart the series or add doses because
of an extended interval between doses

Intervals Between Doses
General Rule
Increasing the interval between doses of a multidose vaccine
does not diminish the effectiveness of the vaccine.
Decreasing the interval between doses of a multidose vaccine
may interfere with antibody response and protection.
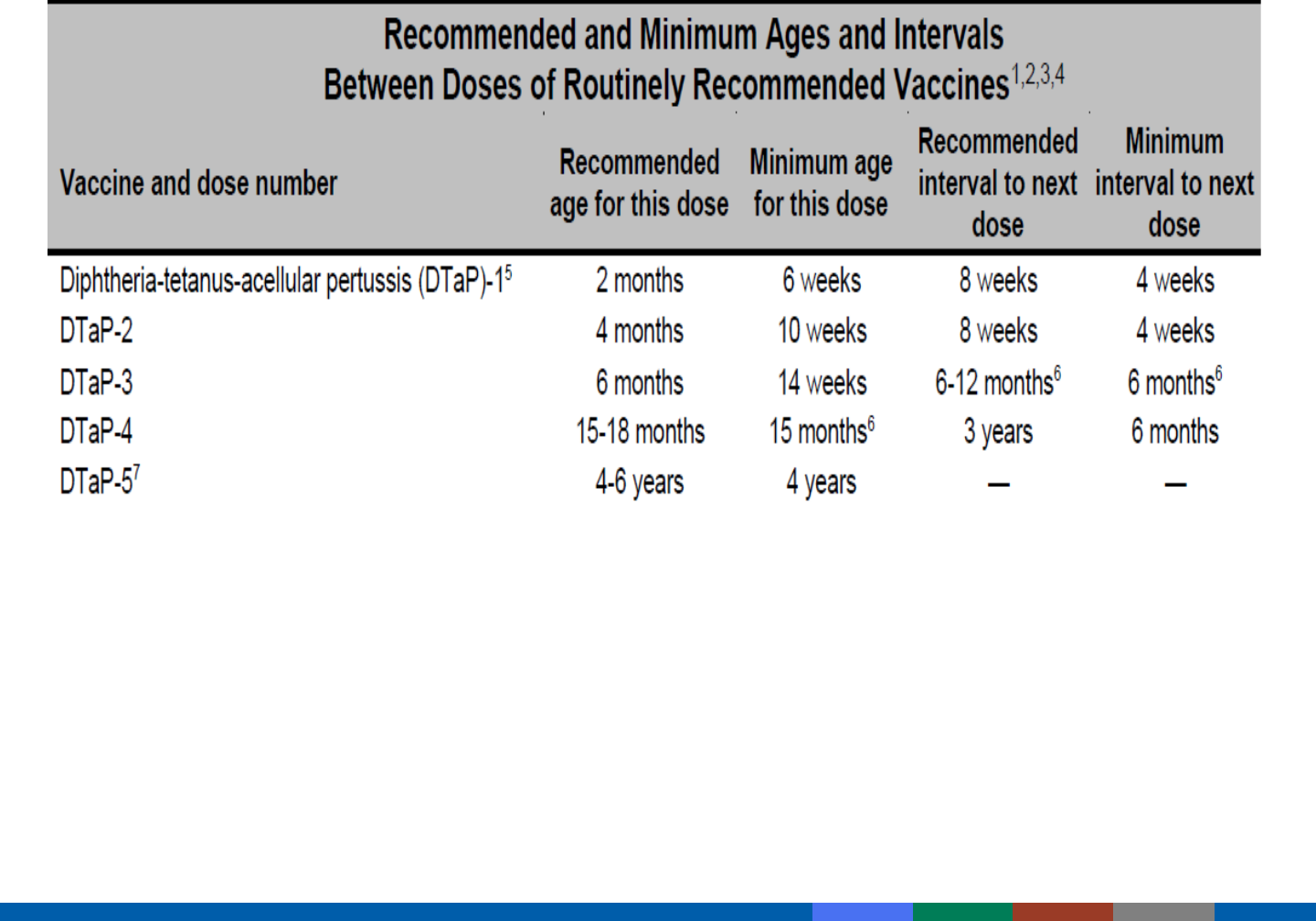
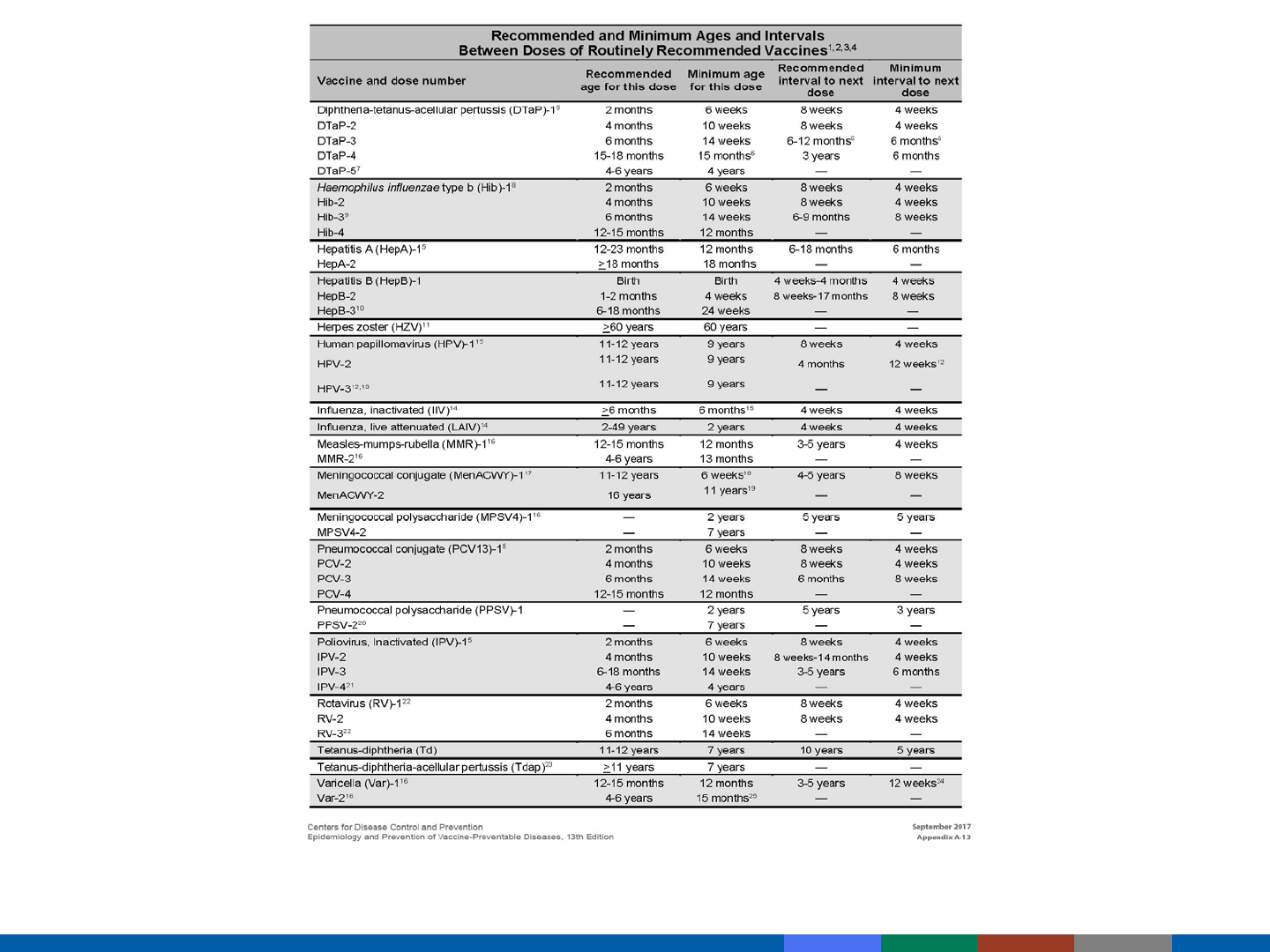
Included in Pink Book Appendix A-13

Minimum Intervals and Ages
Vaccine doses should not be administered at intervals
less than the minimum intervals or earlier than the
minimum age.

When Can Minimum Intervals
Be Used?
Catch-up for a lapsed vaccination schedule
Impending international travel
NOT to be used routinely

The “Grace Period”
ACIP recommends that vaccine doses given up to four days
before the minimum interval or age be counted as valid
Should not be used for scheduling future vaccination visits
Use for reviewing vaccination records

Use of the “Grace Period”
To schedule a future appointment
When evaluating a vaccination record
C
lient is in the office or clinic early
NO!
Yes
Maybe

Use of the “Grace Period”
Client is in the office or clinic
Client/parent is
known and
dependable
Client/parent is
unknown or
undependable
Reschedule
Vaccinate

Use of the “Grace Period”
Basic principles
• The recommended interval or age is preferred
• The m
inimum interval can be used to catch up
• The g
race period is last resort

Violations of
Minimum Intervals and Minimum Ages
Grace period may conflict with some state school entry
requirements
Immunization programs and/or school entry requirements
may not accept some or all doses given earlier than the
minimum age or interval, particularly varicella and/or MMR
vaccines
Providers should comply with local and/or state
immunization requirements

Violations of
Minimum Intervals and Minimum Ages
Minimum interval/age has been violated
Dose invalid
The repeat dose should be administered at least a minimum
interval from the invalid dose

Contraindications and Precautions

Vaccine Adverse Reaction
Adverse reaction
• Extraneous effect caused by vaccine
• “Side effect"

Vaccine Adverse Reaction
Adverse reaction
Adverse event
• Any medical event following vaccination
• May be true adverse reaction
• May be only coincidental

Vaccine Adverse Reactions
Local
• Pain, swelling, redness at site of injection
• Common with inactivated vaccines
• Usually mild and self-li
mited

Vaccine Adverse Reactions
Local
Systemic
• Fever, malaise, headache
• Nonspecific
• May be unrelated to vaccine

Live, Attenuated Vaccines
Must replicate to produce immunity
Symptoms usually mild
Occur after an incubation period
(usually 3-21 days)

Vaccine Adverse Reactions
Local
Systemic
Allergic
• Due to vaccine or vaccine component
• Rare
• Risk minimized by screening
Contraindication
A condition in a recipient that greatly increases the chance
of a serious adverse event
Precaution
A condition in a recipient that may increase the chance or
severity of an adverse event
May compromise the ability of the vaccine to produce
immunity
Might cause diagnostic confusion

Permanent Contraindications
Severe allergic reaction to a prior dose of vaccine or to a
vaccine component

Permanent Contraindications
Rotavirus vaccines only
• Severe Combined Immunodeficiency disease (SCID)
• History of intussusception
Pertussis vaccines only
• Encephalopathy not due to another identifiable cause occurring
within 7 days of pertussis vaccination
Contraindications and Precautions
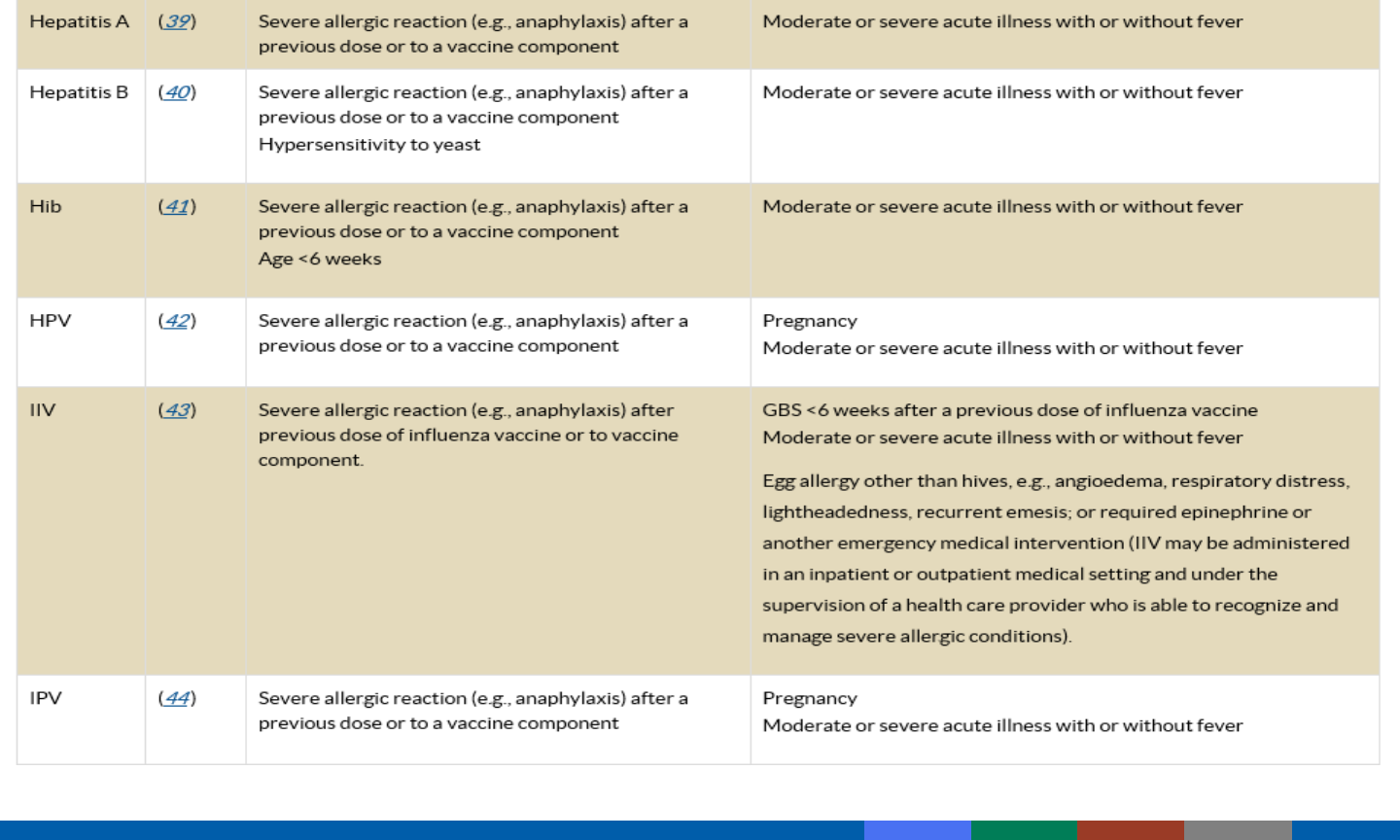
Condition
Allergy to component
Encephalopathy
Pregnancy
Immunosuppression
Moderate/severe illness
Recent blood product
Live
C
---
C
C
P
P**
Inactivated
C
C
V*
V
P
V
C=contraindication
P=precaution
V=vaccinate if indicated
*Except HPV
**MMR and varicella-containing (except zoster vaccine and LAIV)
https://www.cdc.gov/vaccines/hcp/acip-recs/general-
recs/contraindications.html
Included in Pink Book Appendix A-28-30

Vaccination During Pregnancy
Live vaccines should not be administered to women known
to be pregnant.
In general, inactivated vaccines may be administered to
pregnant women for whom they are indicated.
HPV vaccine should be deferred during pregnancy.

Vaccination During Pregnancy
Inactivated vaccines
• Routine
o
o
Influenza – any trimester
Tdap – 27
to 36 weeks
• Vaccinate if indicated (HepA, HepB, MenACWY)
• Withhold (HPV, HepB-C
pG)
• Va
ccinate if increased risk (all others except PCV13, Hib, MenB, RZV)

Vaccination of
Immunosuppressed Persons
Live vaccines should not be administered to severely
immunosuppressed persons.
Persons with isolated B-cell deficiency (i.e. deficiency in
humoral immunity) may receive varicella and zoster
vaccine.
Inactivated vaccines are safe to use in immunosuppressed
persons, but the response to the vaccine may be decreased.

Immunosuppression
Disease
• Congenital immunodeficiency
• Leukemia or lymphoma
• Generalized m
alignancy
Cancer Therapy
• Alkylating agents
• Antimetabolites
• Radiation

Immunosuppressive Drugs
Alkylating agents
Antimetabolites
Radiation Therapy
Immune mediators
Immune modulators
Iso-antibodies (therapeutic monoclonal antibodies)
• Antitumor necrosis factor agents
• B-lymphocyte depleting agent
Checkpoint inhibition

Corticosteroids and Immunosuppression
The amount or duration of corticosteroid therapy needed
to increase adverse event risk is not well-defined.
Dose generally believed to be a concern:
20 mg or more/day of prednisone for 2 weeks or longer
2 mg/kg per day or more of prednisone for 2 weeks or longer

Corticosteroids and Immunosuppression
Does NOT apply to aerosols, topical, alternate-day, short
courses (less than 2 weeks), physiologic replacement
schedules
Delay live vaccines for at least 1 month after discontinuation
of high-dose therapy

Vaccination of Immunosuppressed Persons
Safety:
Immunocompromised persons are at increased risk of
adverse events following live vaccines.
Live vaccines may be administered at least 3 months
following termination of chemotherapy (at least 1
month after high -dose steroid use of 2 weeks or more).
LAIV, MMR, varicella, and rotavirus vaccines may be
administered to susceptible household and other close
contacts.

Vaccination of Immunosuppressed Persons
Safety and efficacy
Anti-tumor necrosis factor inhibitors
• Wait 3 months after stopping medication before administering live vaccines
• Do not initiate medication until 1 months after the live vaccine
Other iso-antibodies (e.g., anti-B cell antibodies aka
lymphocyte depleting agents, checkpoint inhibition)
• Some experts recommend up to 6 months

Persons with HIV Infection
Persons with HIV/AIDS are at increased risk for
complications of measles, varicella, influenza, and
pneumococcal disease.
Vaccine
Varicella
Zoster
MMR
MMRV
LAIV
Rotavirus
Yellow Fever
Asymptomatic
Yes
No
Yes
No
No
Consider
Consider
Symptomatic*
No
No
No
No
No
Consider
No
Yes=vaccinate No=do not vaccinate
*
See specific ACIP recommendations for details
.
Live, Attenuated Vaccines for
Persons with HIV/AIDS*