
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences.
General Best Practice Guidelines
Part Two
June 19, 2019
Chapter 2, Page 9

ACIP Table of Contents
– Introduction
– Methods
– Timing a
nd spacing
– Contraindications and precautions
– Preventing and managing adverse reactions to immunization
– Vaccine administration
– Storage and handling
– Altered i
mmunocompetence
– Special situations
– Vaccination records
– Vaccination programs
– Vaccine information sources
General Best Practice Guidelines for Immunization

A chapter in the Pink Book
– Timing and spacing
– Contraindications and precautions
General Best Practice Guidelines for Immunization

A chapter in the Pink Book
– Timing and spacing
– Contraindications and pr
ecautions
• Screening
General Recommendations
Screening
1

Specific questions intended to identify contraindications or precautions
to vaccination
Screening must occur at every immunization encounter (not just before
th
e first dose)
Use of a standardized form will facilitate effective screening
Following questions written from the perspective of the pediatric
p
atient, but can be adjusted for the adult patient
Screening

Is the child sick today?
Does the child have an allergy to any medications, food, or any vaccine?
Has the child had a serious reaction to a vaccine in the past?
Screening Questions

Has the child had a seizure, brain, or nerve problem?
Has the child had a health problem with asthma, lung disease, heart
d
isease, kidney disease, metabolic disease (such as diabetes), or a blood
disorder?
Screening Questions

Does the child have cancer, leukemia, AIDS, or any other immune system
problem?
Has the child taken cortisone, prednisone, other steroids, or anticancer
m
edications, or had x-ray treatments in the past 3 months?
Screening Questions

Has the child received a transfusion of blood or blood products, or been
given a medicine called “immune (gamma) globulin” in the past year?
Is the child/teen pregnant or is there a chance she could become
p
regnant during the next month?
Has the child received vaccinations in the past 4 weeks?
Screening Questions

www.immunize .org

Mild illness
Antimicrobial therapy
Disease exposure or convalescence
Pregnant or immunosuppressed person in the household
Breastfeeding
Preterm birth
Allergy to products not present in vaccine or allergy that is not severe (e.g.,
anaphylactic)
Family history of adverse events
Tuberculin skin testing
Multiple vaccines
Invalid Contraindications

Mild Illness
– Vaccinate with:
• Low -grade fever
• Upper respiratory infection
• Otitis media
• Mild diarrhea
Invalid Contraindications

Susceptible household contacts of pregnant women
– SHOULD receive MMR and varicella vaccines
– SHOULD receive either nonlive influenza vaccine or LAIV
– SHOULD receive zoster and rotavirus vaccines if eligible
Household Contacts and Pregnancy

Preterm birth (less than 37 weeks)
– Generally, infants and children should be vaccinated according to chronologic age
(not gestational age)
– Use full recommended dose
– Birth weight and size not factors but, as with all rules, there are exceptions (HepB)
Invalid Contraindications

Family history of adverse events generally NOT a contraindication
Family history can be a precaution:
– Example: Family history of seizures is a precaution to MMRV
Family history of a condition can also be a contraindication/precaution
– Example: Family history of immunosuppression requires screening to assure the
condition is not inherited prior to receipt of MMR and varicella vaccine
Family History of Adverse Events

A pregnant woman living in the household is a contraindication to measles-mumps-
rubella (MMR) and varicella (VAR) vaccines for a healthy child in the same household.
a. True
b. False
What Do You Think?
Resources
2
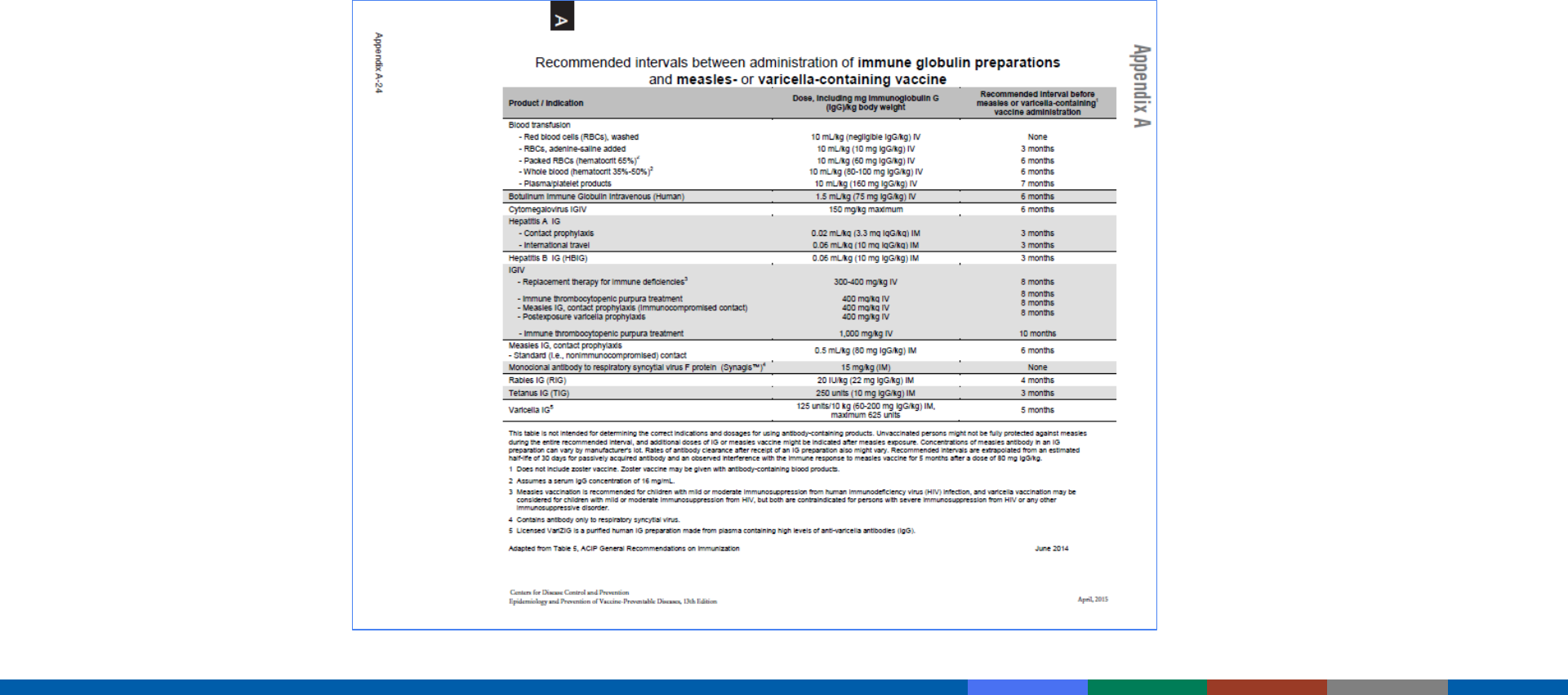
Appendix A24: Interval Between Antibody-Containing Products
and Measles- and Varicella-Containing Vaccines
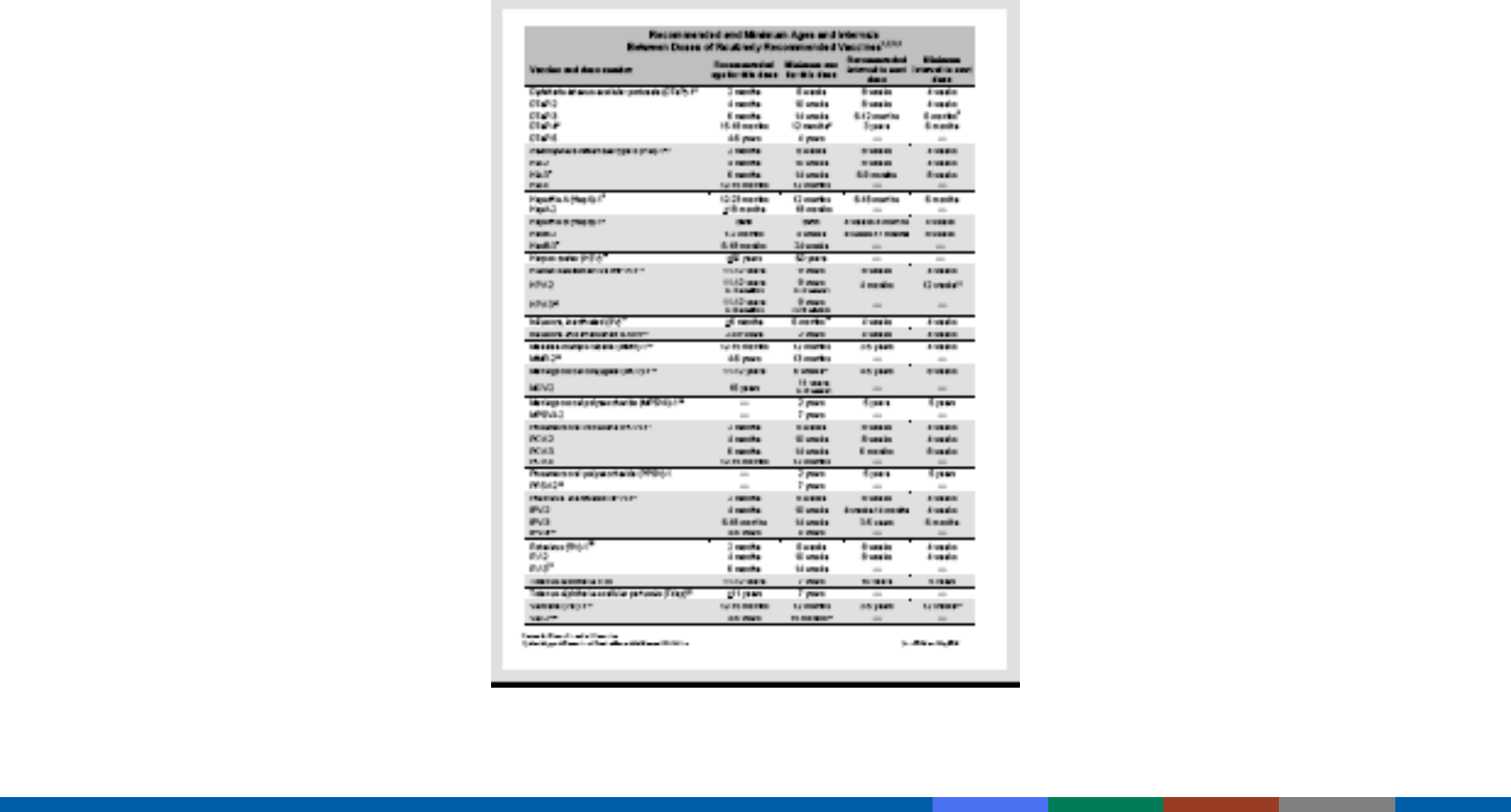
Included in Pink Book Appendix A-13
https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/a/age-interval-table.pdf
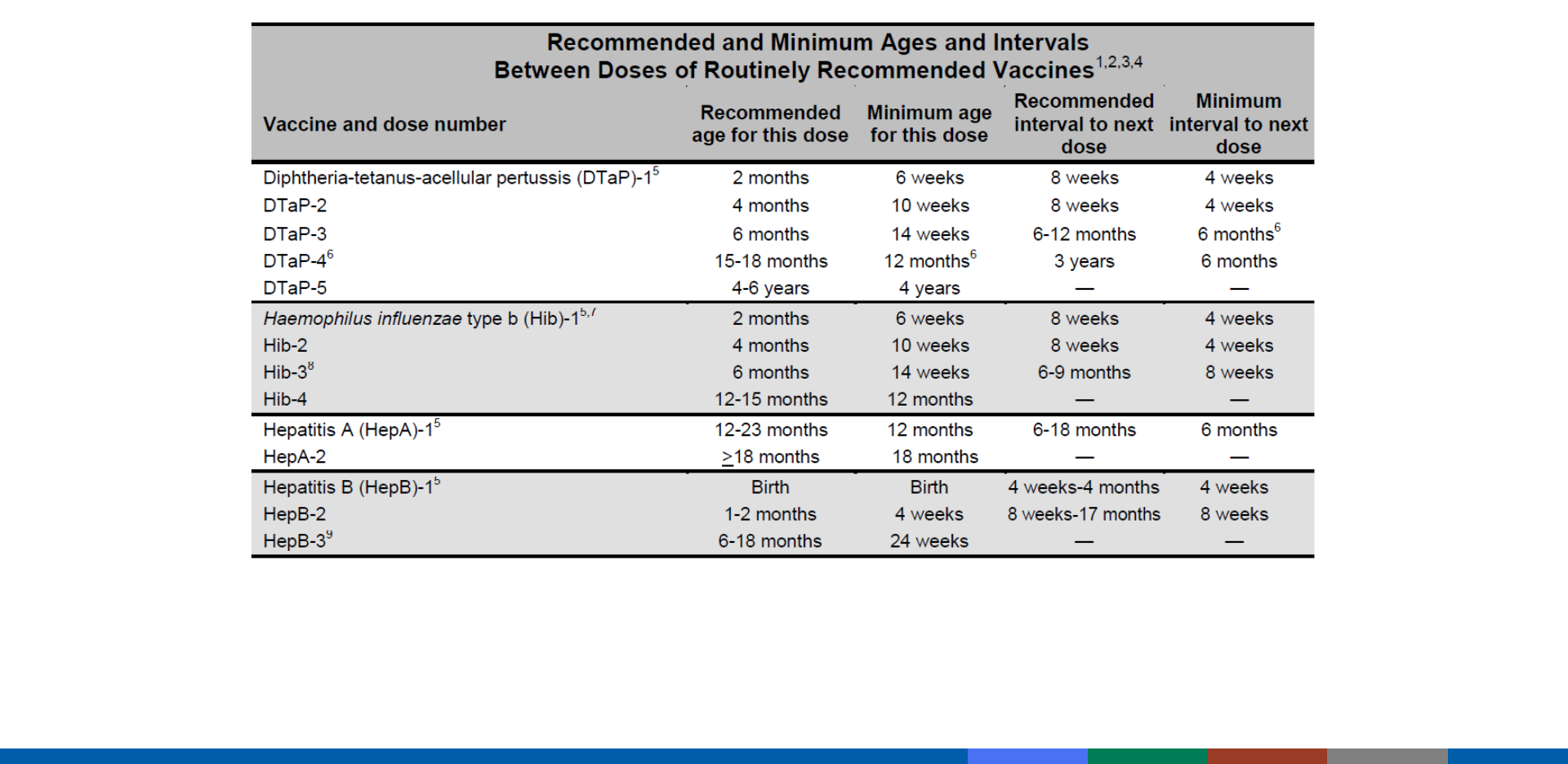
Included in Pink Book Appendix A-13
https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/a/age-interval-table.pdf

Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences.
Vaccine Safety
Chapter 4
June 19, 2019
Background
1
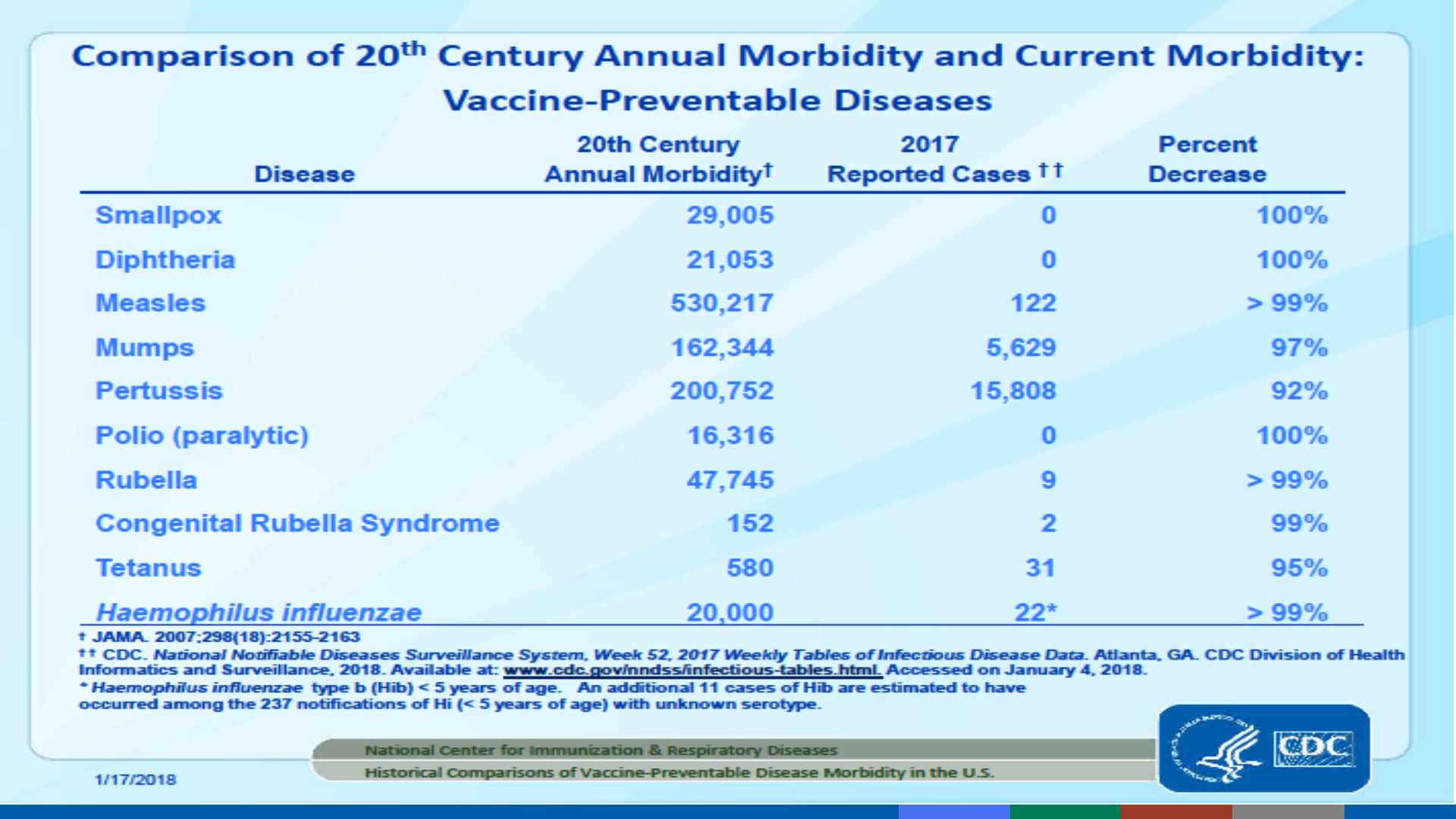
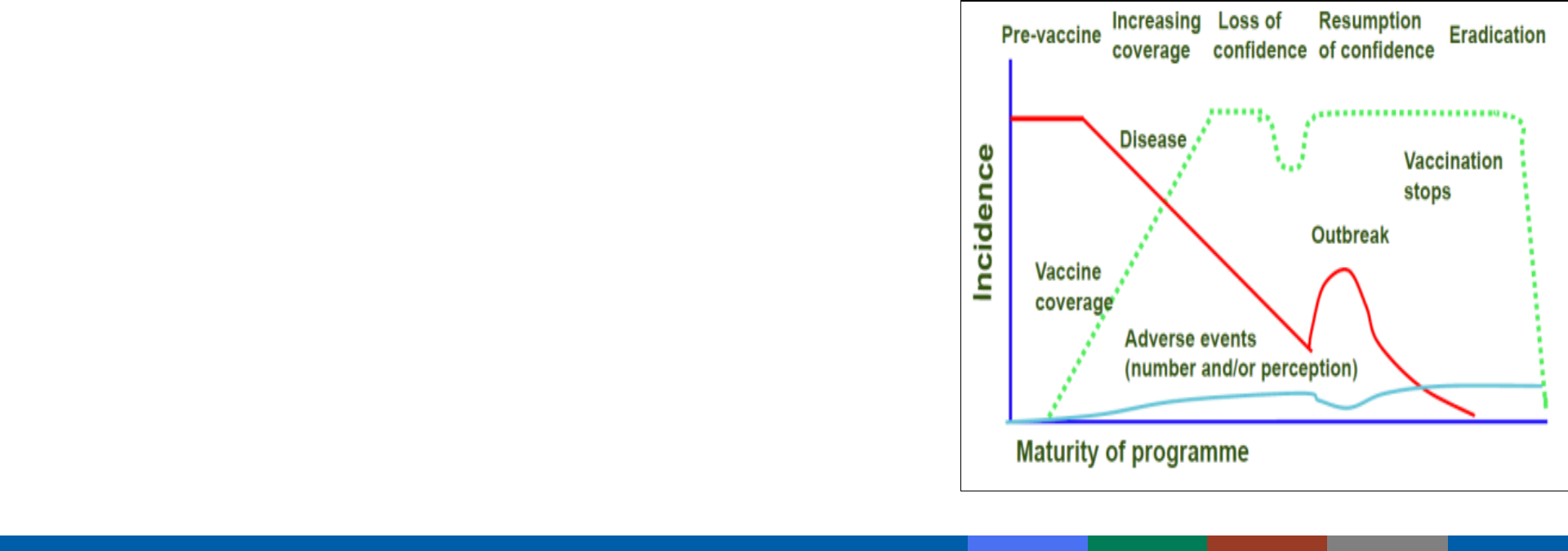
Decreases in disease risks and increased attention on vaccine risks
Public confidence in vaccine safety is critical
– Higher standard of safety is expected of vaccines
– Vaccinees generally healthy (vs. ill for medications)
– Lower risk tolerance = need to search for rare reactions
– Vaccination universally recommended and mandated
Importance of Vaccine Safety
Chen RT, et al. Vaccine 1994;12(6):542–50. Omer SB, et al. N Engl J Med 2013;368(15):1374–6

SAFE = No harm from the vaccine?
No vaccine is 100% safe
SAFE = No harm from the disease?
No
vaccine is 100% effective
Remind parents that to do nothing is to take a risk
What is “Safe”?

Laboratory
Animals
Humans
Prelicensure Vaccine Safety Studies
Phase I, II, III trials
Phase III trials usually include a control group that receives a placebo
Common reactions are identified
Most Phase III trials include 2,000 to 5,000 participants
Largest recent Phase III trial was REST (rotavirus) – around 70,000 infants
Prelicensure Human Studies

Identify rare reactions
Monitor increases in known reactions - identify risk factors for reactions
Identify vaccine lots with increased rates of reactions
Identify “signals”–reports of adverse events more numerous than would be expected
Postlicensure Surveillance
Federal
Vaccine
Safety
Monitoring
2
Jointly administered by CDC and FDA
National reporting system
Receives ~30,000 reports per year
Passive–depends on health care providers and
others to report
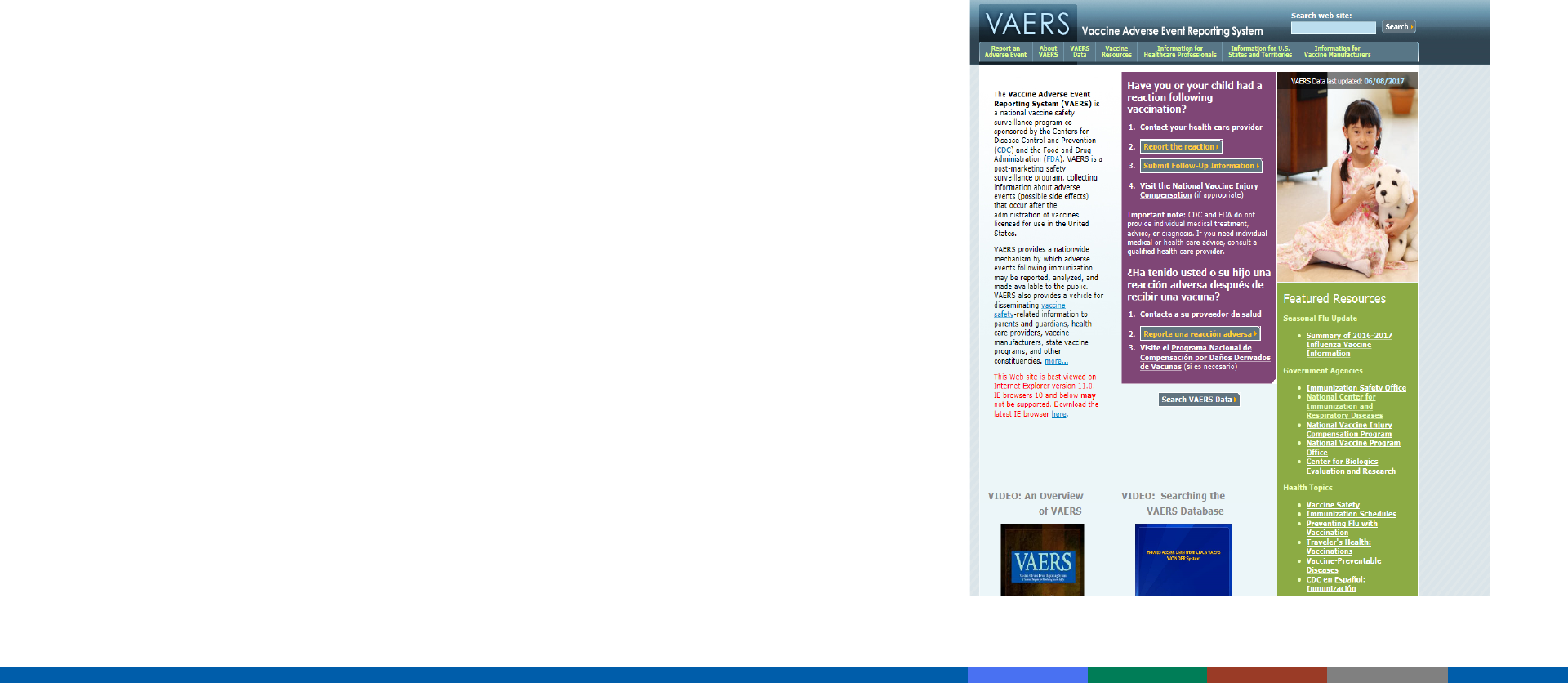
Vaccine Adverse Event Reporting System (VAERS)
https://vaers.hhs.gov/index

Detects:
– New or rare events
– Increases in rates of known events
– Patient risk factors
VAERS cannot establish causality
– Additional studies required to confirm VAERS signals and causality
Not all reports of adverse events are causally related to vaccine
Reportable Events Table (Pink Book Appendix D-2)
Vaccine Adverse Event Reporting System (VAERS)
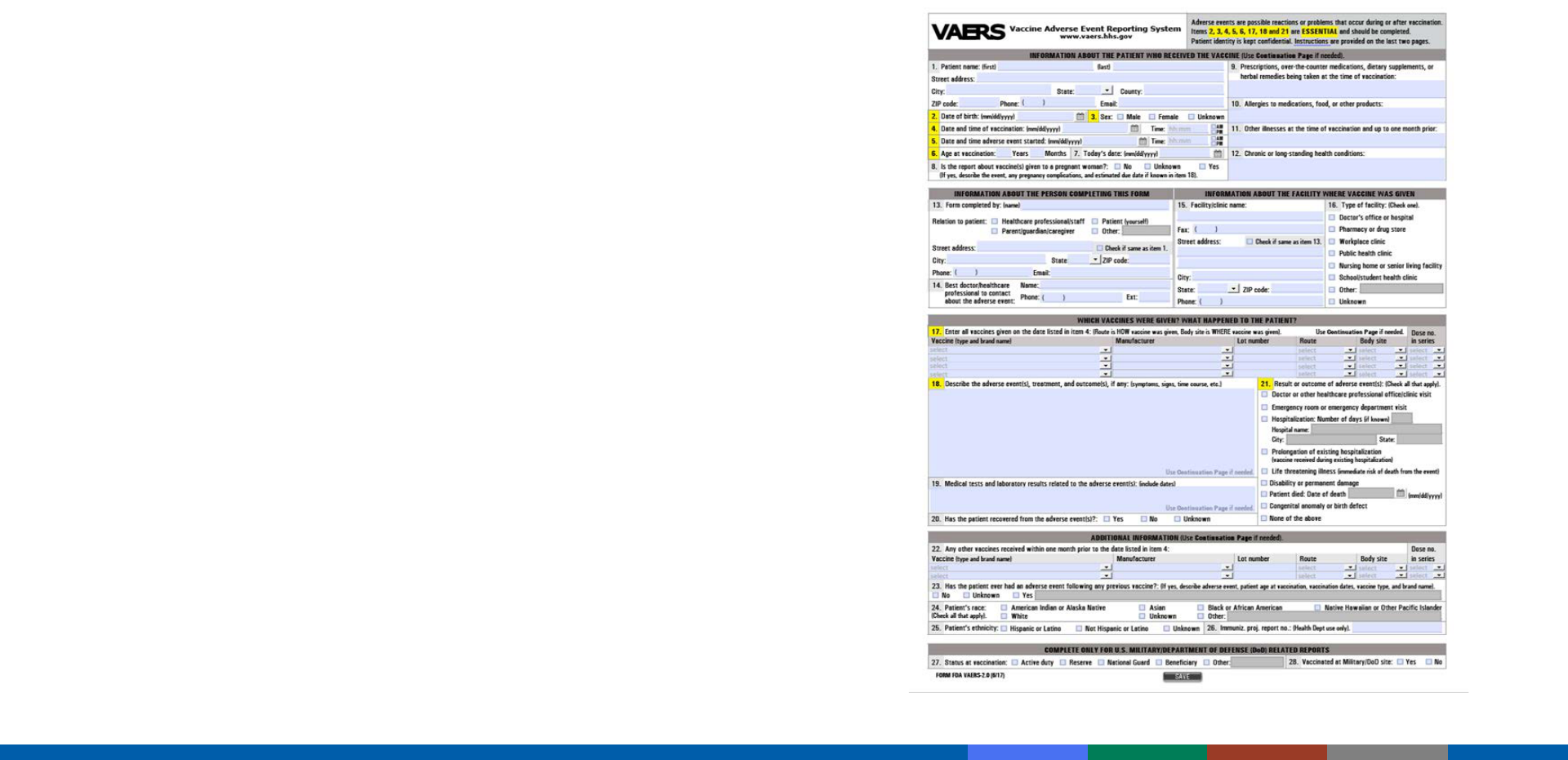
VAERS
– National spontaneous reporting system
for monitoring the safety of U.S.-licensed
vaccines
– Co-managed by CDC and FDA
– Accepts reports from anyone (providers,
patients, etc.)
VAERS Reporting Methods
• Option 1: online reporting tool (preferred)
• Option 2: writable PDF form combined
w
ith electronic document upload
capability
Vaccine Adverse Event Reporting System (VAERS) and VAERS
reporting form

Instructions for reporting to VAERS at
https://vaers.hhs.gov/reportevent.html
Additional assistance
– Email at info@vaers.org
– Phone at 1-800-822-7967
VAERS (additional information)

“After this therefore because of this”
Temporal a
ssociation does not prove causation
Just because one event follows another does not mean that the first
c
aused the second
Causation requires knowledge of
– Correct diagnosis of event
– Clinical and/or laboratory evidence
– Known causal association between event and vaccine
– Any evidence against a causal association?
– Specific laboratory test supporting vaccine role
Post hoc ergo propter hoc

Disease No disease
Vaccine a b
No vaccine c d
Rate in “vaccine” group
=
a /a + b
Rate in “no vaccine” group c/ c + d
If the rate in “vaccine” group is higher than the rate in the “no
vaccine” group, then vaccines may be the cause
Elements Needed To Assess Correlation of Vaccine
Adverse Events

Risk of Autism Spectrum Disorder (ASD)
Among Children in Denmark, 1991-1998
Madsen et al. N Eng J Med 2002;347:1477-82
ASD No ASD
Vaccine 345 440,310
No vaccine 77 96,571
Risk in “vaccine” group
=
7.83/10,000
Risk in “no vaccine” group 7.96/10,000
Relative Risk = 0.98

Phase IV trials
– ~10,000 participants
– Better but still limited
Vaccine Safety Data Link
Clinical Immunization Safety Assessment Project (
CISA)
Postlicensure Vaccine Safety Activities

Vaccine Safety Datalink:
– Large linked databases
– Connects
vaccination and health records
– Partnership wi
th large health plans: population under “active surveillance”
• 9 HMOs
• >3% (~12 million) of U.S. population
Plans, executes immunization safety studies
Investigates hypotheses from medical literature, VAERS reports, changes
in
schedules, introduction of new vaccines
Vaccine Safety Datalink

Improve understanding of vaccine safety issues at individual level
Evaluate individual cases with adverse health events
Develop strategies to assess individuals
Conduct studies to identify risk factors
Established by National Childhood Vaccine Injury
Act (1986)
“No fault” program
Covers all routinely recommended childhood
vaccines
Vaccine Injury Table (Appendix D-5, D-6)
Vaccine Injury Compensation Program
Vaccine Injury Compensation Program website: www.hrsa.gov/vaccinecompensation/index.html

Immunization providers can help ensure the safety and efficacy of
vaccines through proper:
– vaccine storage and administration
– timing a
nd spacing of vaccine doses
– screening of contraindications and precautions
– management of adverse reactions
– reporting to VAERS
– benefit and risk communication
The Provider’s Role

Opportunities for questions should be provided before each vaccination
Vaccine Information Statements (VISs)
– Must be provided before each dose of vaccine
– Public and private providers
– Available in multiple languages
Benefit and Risk Communication

For providers:
– If provider recommends it, parents more likely to follow
– Ask, acknowledge, and advise
– Start at prenatal visit, develop trust
– Offer reliable resources
– Know the science
– Do not get defensive
Communicating with Parents
Your Source for VISs
www.immunize.org
omm
Common
Concerns
3

National Academy of Medicine–Mission
– Review scientific findings and stakeholder concerns related to the safety of the
recommended childhood immunization schedule
– Identify potential research approaches, methodologies, and study designs that
could inform this question
– Issue a summary report
Findings
– Committee finds no evidence that the schedule is unsafe
– Following the complete childhood immunization schedule is strongly associated
with reducing vaccine-preventable diseases
– Committee calls for continued study of the immunization schedule using existing
data systems
Childhood Immunization Schedule and Safety - 2013
www.iom.edu/childimmunizationschedule

Committee findings:
– CAUSAL RELATIONSHIP between some vaccines and adverse events
• MMR, VZV, Influenza, etc., and anaphylaxis
– REJECTION OF
5 RELATIONSHIPS
• Including MMR and autism, TIV and as
thma
Overall, the committee concluded that few health problems are caused
by or clearly associated with vaccines
National Academy of Medicine, August 2011
http://nationalacademies.org/HMD/Reports/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality.aspx

Early vaccination is important to prevent diseases
Vaccines are given at a young age because infants and children are at highest risk of
getting sick or dying if they get these diseases
Newborn babies have antibodies to some diseases from their mothers. BUT
– Maternal antibodies lasts a few months–passive immunity
– Most babies do not get protective antibodies against diphtheria, pertussis polio, tetanus, hepatitis B, or Hib
from their mothers.
– Therefor should vaccinate a child before she or he is exposed to a disease.
Multiple Vaccines
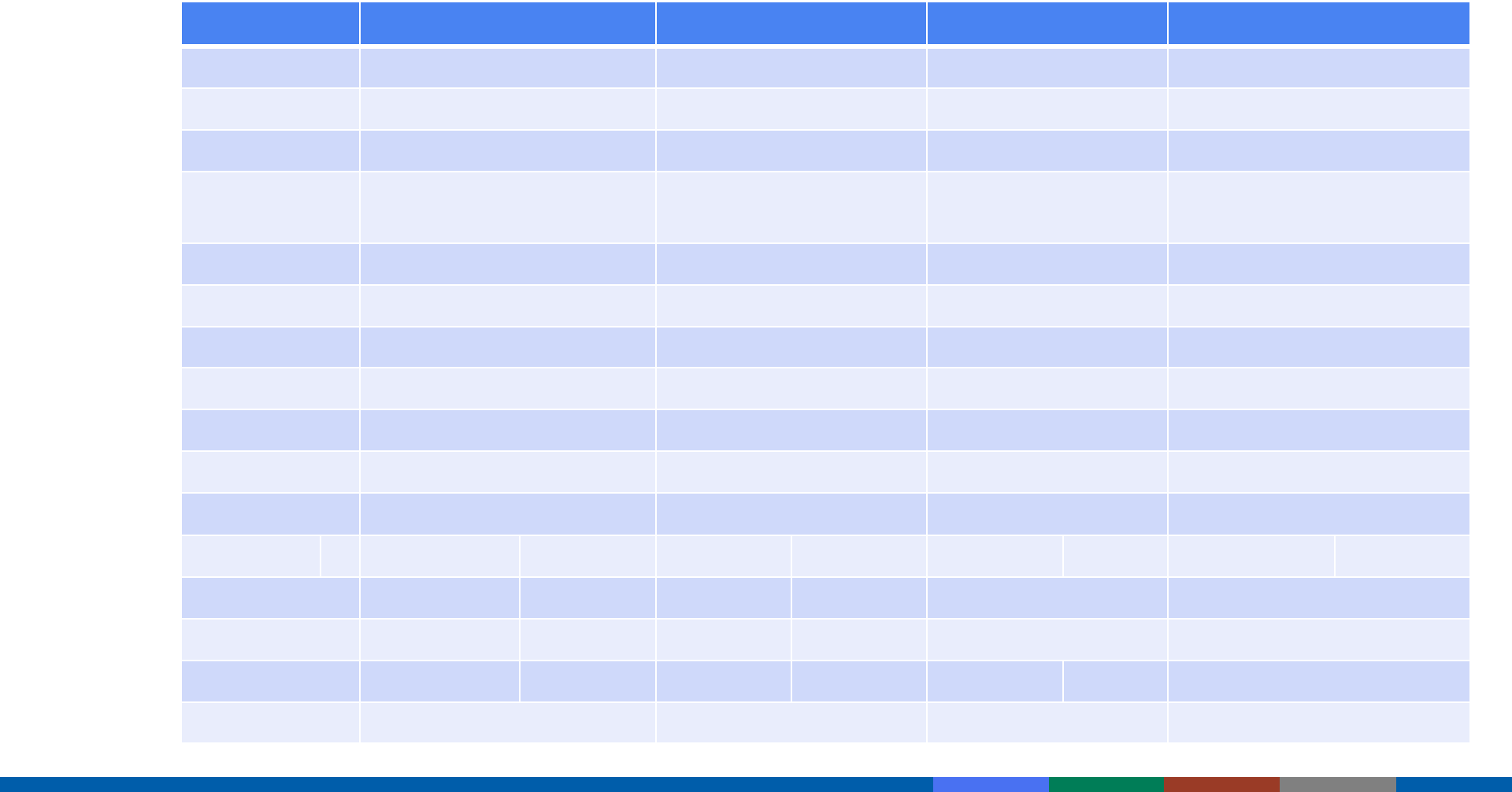
Antigens in Vaccines for Children, 1960-2019
Vaccine 1960 1980 2000 2019
Smallpox ~200 Not recommended
Diphtheria 1 1 1 1
Tetanus 1 1 1 1
W cell
pertussis
~3,000 ~3,000 Acellular pertussis 2-
5
2-5
Polio 15 15 15 15
Measles 10 10 10
Mumps 9 9 9
Rubella 5 5 5
Hib 2 2
Varicella 69 69
Pneumococcal 8 8
Hep B 1 1
Hep A 4
Rotavirus 11-16
Influenza 11 11
Total ~3,217 ~3,041 134-137 149-157
Adapted from
https://www.chop.edu/centers-programs/vaccine-education-center/vaccine-safety/immune-system-and-health

Babies are exposed to thousands of germs and other antigens in the environment
from the time they are born
– When a baby is born, his or her immune system is ready to respond to the many antigens in the environment
and the selected antigens in vaccines
– Vaccines contain weakened or killed versions of the germs that cause a disease
Getting multiple vaccines at the same time has been shown to be safe
– The recommended vaccines have been shown to be as effective in combination as they are individually
ACIP childhood vaccination schedule ensures children get the best protection
Multiple Vaccines

Multiple population-based studies have examined the rate of autism
among vaccinated and unvaccinated children
Available evidence does not indicate that autism is more common
am
ong children who receive MMR or thimerosal-containing vaccines
than among children who do not receive vaccines
Autism and Vaccines
http://www.cdc.gov/vaccinesafety/Concerns/Autism/Index.html

Kaye JA, et al. Measles, mumps, and rubella vaccine and incidence of autism recorded by general practitioners: a
time-trend analysis. Brit Med J 322:460-463, 2001.
Madsen KM, et al. A population-bas
ed study of measles, mumps, and rubella vaccination and autism. N Engl J
Med. 2002;347:1477-1482.
Frambonne E,
et al. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with
immunizations. Pediatrics 118:e139-50, 2006.
Thompson WW, et al. Early thimerosal exposure and neuro-psychological outcomes at 7 to 10 years. N Engl J Med
2007; 357(13):1281-92.
Schechter R, Gr
ether JK. Continuing increases in autism reported to California's developmental services system:
mercury in retrograde. Arch Gen Psychiatry 2008;65(1):19-24.
Taylor LE, Swerdfeger AL
, Eslick GD. Vaccines are not associated with autism: An evidence-based meta-analysis
of case-control and cohort studies. Vaccine. 2014 June;32(29):3623–3629
Studies of Autism and Vaccines*
*Partial listing of representative studies
“... given what the scientific literature tells us today,
there is no evidence that thimerosal or the MMR
vaccine cause autism. Evidence does not support the
theory that vaccines are causing an autism
epidemic.“
- Dr. Geri Dawson, July 30, 2009

The Vaccine Adverse Event Reporting System (VAERS) detects new or rare events,
increases in rates of known events, and patient risk factors associated with vaccination.
VAERS cannot establish causality.
a. True
b. False
What Do You Think?

