
How smart was T. rex? Testing claims of exceptional cognition in dinosaurs and the 1
application of neuron count estimates in palaeontological research 2
3
Caspar, Kai R.
1,2
* , Gutiérrez-Ibáñez, Cristián
3
* , Bertrand, Ornella C.
4
, Carr, Thomas
5
, 4
Colbourne, Jennifer A. D.
6
; Erb, Arthur
7,8
, George, Hady
9
, Holtz, Thomas R., Jr.
10,11
, Naish, 5
Darren
12
; Wylie, Douglas R.
3
; Hurlburt, Grant R.
13
* 6
7
*These authors contributed equally; corresponding authors 8
9
1
Institute of Cell Biology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany;
2
Department of 10
Game Management and Wildlife Biology, Faculty of Forestry and Wood Sciences, Czech University of 11
Life Sciences, Prague, Czech Republic;
3
Department of Biological Sciences, University of Alberta, 12
Edmonton, Canada;
4
Institut Català de Paleontologia Miquel Crusafont, Universitat Autònoma de 13
Barcelona, Barcelona, Spain
5
Department of Biology, Carthage College, Kenosha, Wisconsin, USA 14
6
Comparative Cognition Unit, Messerli Research Institute, University of Veterinary Medicine Vienna, 15
Vienna, Austria;
7
School of GeoSciences, Grant Institute, University of Edinburgh, Edinburgh, 16
Scotland, UK;
8
Center for Science, Teaching, and Learning, Rockville Centre, New York, USA;
9
School 17
of Earth Sciences, University of Bristol, Bristol, UK;
10
Department of Geology, University of Maryland, 18
College Park, Maryland, USA;
11
Department of Paleobiology, National Museum of Natural History, 19
Washington, DC, USA;
12
School of Biological Sciences, Faculty of Environment and Life Sciences, 20
University of Southampton, Southampton, United Kingdom;
13
Department of Natural History, Royal 21
Ontario Museum, Toronto, Ontario, Canada 22
23
Abstract 24
Recent years have seen increasing scientific interest in whether neuron counts can act as 25
correlates of diverse biological phenomena. Lately, Herculano-Houzel (2023) argued that 26
fossil endocasts and comparative neurological data from extant sauropsids allow to 27
reconstruct telencephalic neuron counts in Mesozoic dinosaurs and pterosaurs, which might 28
act as proxies for behaviors and life history traits in these animals. According to this analysis, 29
large theropods such as Tyrannosaurus rex were long-lived, exceptionally intelligent animals 30
equipped with “macaque- or baboon-like cognition” whereas sauropods as well as most 31
ornithischian dinosaurs would have displayed significantly smaller brains and an ectothermic 32
physiology. Besides challenging established views on Mesozoic dinosaur biology, these 33
claims raise questions on whether neuron count estimates could benefit research on fossil 34
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
animals in general. Here, we address these findings by revisiting Herculano-Houzel’s (2023) 35
work, identifying several crucial shortcomings regarding analysis and interpretation. We 36
present revised estimates of encephalization and telencephalic neuron counts in dinosaurs, 37
which we derive from phylogenetically informed modeling and an amended dataset of 38
endocranial measurements. For large-bodied theropods in particular, we recover significantly 39
lower neuron counts than previously proposed. Furthermore, we review the suitability of 40
neurological variables such as neuron numbers and relative brain size to predict cognitive 41
complexity, metabolic rate and life history traits in dinosaurs, coming to the conclusion that 42
they are flawed proxies of these biological phenomena. Instead of relying on such 43
neurological estimates when reconstructing Mesozoic dinosaur biology, we argue that 44
integrative studies are needed to approach this complex subject. 45
Key words: endocast, palaeoneurology, brain evolution, comparative cognition, graphic 46
double integration 47
48
Introduction 49
The Late Cretaceous North American theropod dinosaur Tyrannosaurus rex is a superlative 50
predator, being among the largest, heaviest, and most powerful (in terms of bite force) 51
terrestrial carnivores of all time (Gignac and Erickson 2017; Sakamoto 2022; Henderson, 52
2023). Recently, Herculano-Houzel (2023) proposed that anthropoid primate-level 53
intelligence should be added to T. rex’s already impressive predatory resume based on high 54
estimated numbers of telencephalic neuron counts in large-bodied theropod taxa. This 55
conclusion emerged from a paradigm whereby neurological variables estimated from 56
endocasts can, so it is claimed, be used to infer metabolic parameters, social behaviors, and 57
longevity in fossil species. Here, we test whether this approach and its remarkable prospects 58
withstand scrutiny. 59
The hypothesis of exceptional intelligence in dinosaurs such as T. rex challenges the 60
consensus of crocodile-like cognition in these animals, a position informed by comparative 61
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
anatomical data (Rogers, 1998; Witmer & Ridgely, 2009; Hurlburt et al., 2013). Moreover, 62
this claim bears ramifications that extend beyond specialized biological disciplines due to its 63
potential to create long-lasting impacts on the public’s perspective on dinosaurs, evolution, 64
and the scientific process. Given the extreme contrast between Herculano-Houzel’s (2023) 65
proposal and more traditional perspectives on dinosaur biology, we revisit the claim of 66
exceptional intelligence in these animals through an assessment of her methodology and a 67
reanalysis of the underlying data. By integrating perspectives from both paleontology and 68
neontology, we evaluate the potential benefits and limitations of neuron count estimation in 69
research on the behavior and physiology of fossil species. We begin with a brief review of 70
dinosaur paleoneurology and a discussion of how Herculano-Houzel’s (2023) approach aims 71
to expand the field's methodological tool kit. 72
73
Dinosaur paleoneurology and the prospects of neuron count estimates for the field 74
Paleoneurology is a subfield of paleontology dedicated to research on the nervous systems 75
of extinct animals. Because soft tissues are not readily preserved in the fossil record, 76
paleobiologists rely on endocasts when studying the brains of extinct species (Paulina-77
Carabajal et al. 2023). An endocast can be a natural (infilling), artificial (mold) or virtual 78
(digitally reconstructed) cast of the endocranial cavity that is formed by the bones of the 79
braincase. 80
The study of extinct species’ endocasts, including those of dinosaurs, can be traced back to 81
the 1800s (e.g., Cuvier, 1812; Marsh, 1879). However, the field was truly defined by Edinger 82
(1929) who effectively introduced the concept of geological time to neurobiological studies. 83
Before her, anatomists made comparisons between endocasts and fresh brains, but without 84
considering the stratigraphic context (Buchholtz & Seyfarth, 2001). Jerison (1973) built on 85
Edinger’s work by studying brain evolution in a quantitative manner and developed the 86
encephalization quotient (EQ) as an estimate of relative brain size, applicable to both extant 87
and extinct species. Later, the advent of X-ray computed tomography at the end of the 88
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
1990’s transformed the field and provided novel ways in which the neurosensory systems of 89
extinct species could be studied (e.g., Knoll et al., 1999; Witmer et al., 2008). Despite these 90
crucial innovations, however, paleoneurology has so far remained largely restricted to the 91
measurement and comparison of gross morphology, limiting our understanding of how the 92
brains of Mesozoic dinosaurs and other extinct animals worked. 93
94
95
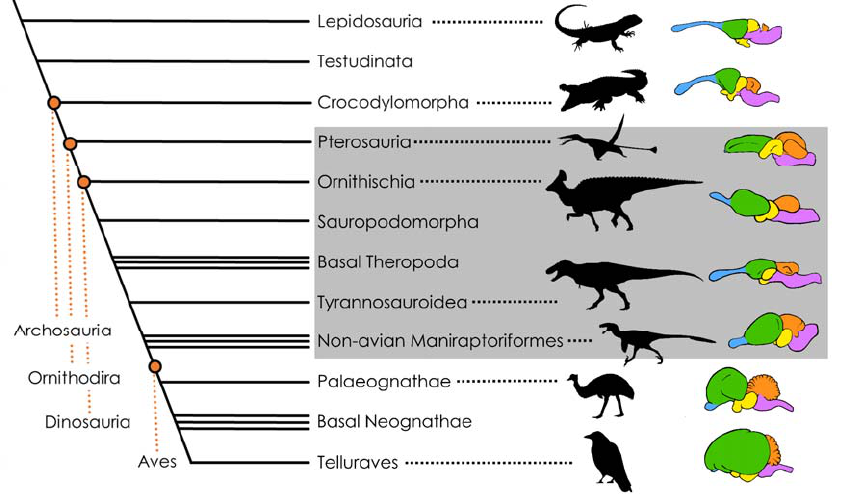
Figure 1: Simplified phylogeny of the Sauropsida (= total group Reptilia) with a focus on the taxon
96
Ornithodira (the least inclusive clade containing pterosaurs and dinosaurs, see revised definition of
97
Nesbitt, 2011) and representative color-coded brain morphologies, excluding the pituitary (not to
98
scale). Blue: olfactory bulb and tracts, Green: pallium (homologous to the mammalian cerebral
99
cortex), Orange: cerebellum, Yellow: diencephalon and optic tectum; Violet: brain stem. Olfactory
100
structures, pallium and subpallium comprise the telencephalon. The gray overlay indicates extinct
101
taxa, the brain morphologies of which are approximated. Note that the brain morphology in T. rex and
102
its relatives (Tyrannosauroidea) is conspicuously plesiomorphic when compared to the other
103
ornithodirans pictured here (see e.g., Giffin, 1989). Definitions of notable dinosaur clades: Ornithischia
104
- a large group of primarily herbivorous dinosaurs, excluding the long-necked sauropodomorph
105
dinosaurs, defined as the most inclusive clade including Triceratops but not Diplodocus nor
106
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Tyrannosaurus. Most popular representatives of this group include horned or otherwise heavily 107
armored forms such as Triceratops, Stegosaurus and Ankylosaurus as well as the hadrosaurs, 108
colloquially known as duck-billed dinosaurs. Sauropodomorpha - the long-necked and often 109
particularly large-bodied herbivorous dinosaurs, defined as the most inclusive clade including 110
Diplodocus but not Triceratops nor Tyrannosaurus; Theropoda - the bipedal, mostly carnivorous 111
dinosaurs, the most inclusive clade including Tyrannosaurus but not Diplodocus or Triceratops. The 112
birds are part of this clade (see Baron et al., 2017 for definitions of Ornithischia, Sauropodomorpha 113
and Theropoda); Tyrannosauroidea, the most inclusive clade of theropods containing Tyrannosaurus 114
but not more bird-like taxa such as Velociraptor and Ornithomimus (Sereno et al., 2009); 115
Maniraptoriformes, the least inclusive clade containing Velociraptor and Ornithomimus but not earlier-116
diverging theropods like Tyrannosaurus (Holtz, 1996). Silhouettes were taken from PhyloPic (listed 117
from top to bottom): Morunasaurus (in public domain), Crocodylus (in public domain), 118
Rhamphorhynchus (by Scott Hartman), Olorotitan (by , vectorized by T. Michael Keesey), 119
Tyrannosaurus (by Matt Dempsey), Dromaeosaurus (by Pranav Iyer), Dromaius (by Darren Naish), 120
Corvus (in public domain). 121
122
Pterosaurs and dinosaurs (the latter including birds) form the clade Ornithodira (Fig. 1), the 123
closest extant relatives of which are crocodilians (Fig. 1). Together, both lineages, which 124
separated about 250 million years ago, comprise the clade Archosauria (e.g., Legendre et 125
al., 2016). Next to birds, crocodilians therefore represent a critical reference point in 126
reconstructing the nervous systems of extinct ornithodirans. 127
Interestingly, highly disparate patterns of endocranial tissue organization are realized in 128
these two extant clades. One fundamental difference relates to the portion of the endocranial 129
cavity which is occupied by the brain rather than by the associated meningeal tissues 130
(including the dura mater and arachnoid mater) and cerebrospinal fluid (Fig. 2). In 131
crocodilians, nervous tissue only fills a fraction of the braincase (Hopson, 1979; Jirak & 132
Janacek, 2017; Watanabe et al. 2019). Longitudinal venous sinuses course along the dorsal 133
and ventral aspect of the brain, obscuring its true shape in casts of the braincase. 134
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Furthermore, the size of the brain relative to both the endocranial volume and total body 135
size, decreases during crocodilian ontogeny, even over the course of adulthood (Hurlburt et 136
al., 2013; but note that absolute brain volume increases with body size, even in adults - 137
Ngwenya et al., 2013). 138
Endocast morphology indicates that the endocranial cavity in most non-avian dinosaurs was 139
organized in crocodilian-like fashion and comparative studies suggest that this configuration 140
was indeed ancestral for the clade Archosauria (Witmer et al., 2008; Hurlburt et al., 2013; 141
Fabbri & Bhullar, 2022). For tyrannosauroids specifically, which are among the best-studied 142
dinosaurs when it comes to palaeoneurology, endocasts representing different ontogenetic 143
stages suggest that brain size (relative to endocranial volume) decreased with age (Brusatte 144
et al., 2009; Witmer & Ridgely, 2009; Bever et al., 2013), as is the case in modern 145
crocodilians. As in, crocodilians, most dinosaurian endocasts do not faithfully capture the 146
volume and anatomy of the brain, particularly its posterior regions such as the cerebellum 147
(Watanabe et al., 2019). This contrasts with the situation in birds and most mammals for 148
which endocasts represent excellent brain size proxies (e.g., Iwaniuk and Nelson, 2002; 149
Bertrand et al., 2022). 150
The avian pattern probably evolved at the root of the theropod dinosaur clade 151
Maniraptoriformes, which includes ornithomimosaurs (‘ostrich-mimic’ dinosaurs) and 152
maniraptorans (the bird-like oviraptorosaurs, dromaeosaurids and kin, and birds themselves) 153
(Osmólska, 2004; Balanoff et al., 2013; Fig. 1). Maniraptoriform brains have enlarged 154
cerebral and cerebellar regions that almost fully occupy the endocranial cavity, as evidenced 155
by brain contours faithfully captured by the endocranium and extensive vascular imprints. 156
There is no evidence that the brains of other dinosaurs similarly contacted the endocranial 157
surface (pachycephalosaurs pose an exception to this pattern but are not covered in this 158
article, their endocranial anatomy is described in Hopson, 1979, Giffin, 1989, and Evans, 159
2005; we discuss other suggested cases of secondarily increased endocranial fills in 160
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
dinosaurs in Supplementary File 1). Pterosaurs are similar to maniraptoriforms in also 161
possessing brains that fit tightly into the endocranial cavity (Witmer et al., 2003). 162
Aside from general endocranial tissue organization, the neuroarchitecture and circuitry of 163
the forebrain in birds and crocodilians differs notably from one another (Ulinski & Margoliash, 164
1990; Briscoe et al., 2018; Briscoe & Ragsdale, 2018). Comparisons with other sauropsids 165
demonstrate that again the crocodilian condition is more plesiomorphic (Briscoe & Ragsdale, 166
2018). To which extent non-avian dinosaurs and pterosaurs resembled the two extant 167
archosaur groups in these regards cannot be reliably reconstructed, since they lack 168
osteological correlates. 169
The inferred brain anatomy of various dinosaur groups has been discussed elsewhere 170
(Paulina-Carabajal et al. 2023) and reviewing it here is beyond the scope of this article. We 171
aim instead to focus on what endocast-based methods potentially reveal about the behavior 172
and cognition of extinct species. While considering the aforementioned limitations, 173
endocasts from fossil ornithodirans allow us to reasonably estimate basic neuroanatomical 174
measures such as EQ, as well as to deduce specific sensory specializations (e.g., Witmer et 175
al., 2003; Witmer & Ridgely, 2009; Zelenitsky et al., 2011). Nonetheless, it is generally 176
assumed that the predictive power of these data in elucidating the cognitive capacities of a 177
fossil species is low (Paulina-Carabajal et al., 2023). Researchers have long sought to 178
identify robust morphological correlates of cognition but have found traditional proxies such 179
as EQ and absolute brain size to be limited and problematic regarding their conceptual 180
justifications (Van Schaik et al., 2021). Current debates focus on whether refined 181
neuroanatomical measures such as “cognitive brain size” (Van Schaik et al., 2021) and brain 182
region-specific neuron counts (Herculano-Houzel, 2011; Kabadayi et al., 2016; Logan et al., 183
2018; Sol et al., 2022) might be able to overcome these issues. The quantification of these, 184
however, seemed out of reach for vertebrate palaeontology. 185
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
With this in mind, the approach proposed by Herculano-Houzel (2023) is of great potential 186
significance: It entails that endocasts of extinct taxa can be used to model neuron counts if 187
neurological data from related extant species can be taken into account. If valid, this 188
technique would potentially allow researchers to elucidate aspects of brain physiology that 189
cannot be inferred from endocast morphology alone. Herculano-Houzel & Kaas (2011) and 190
Herculano-Houzel et al. (2011) pioneered this approach for fossil hominins and extinct giant 191
rodents, but Herculano-Houzel (2023) was first in applying this methodology to fossil 192
sauropsid groups separated from their extant relatives by hundreds of millions of years of 193
evolution, namely pterosaurs and Mesozoic dinosaurs. 194
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
195
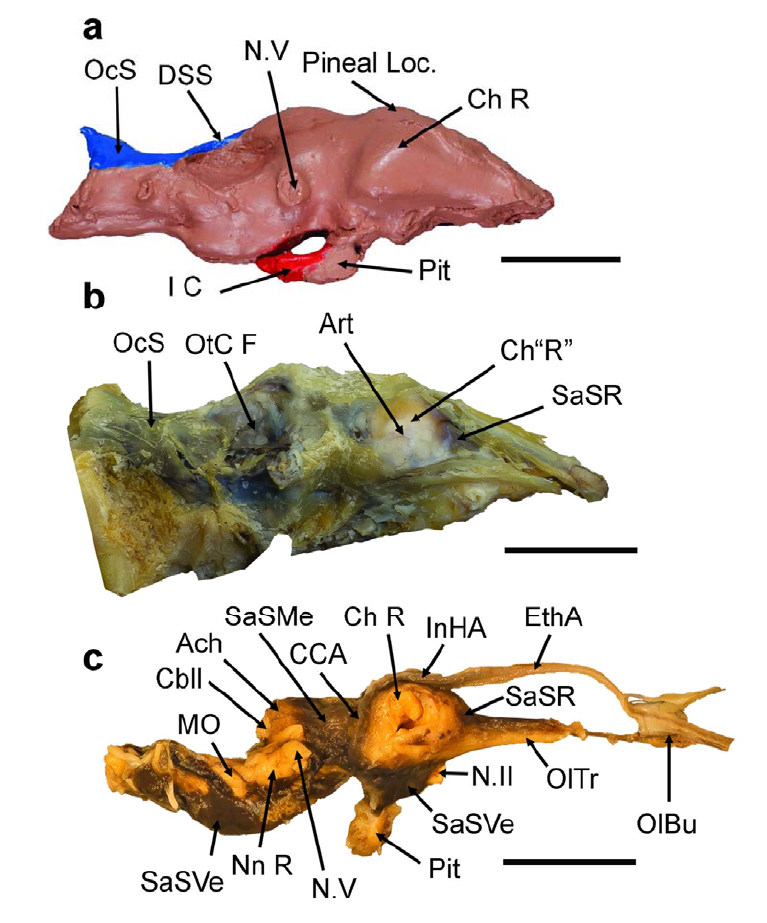
Figure 2: The endocast and endocranial tissue organization in the American alligator
196
(Alligator mississippiensis), illustrating the plesiomorphic condition within the clade Archosauria. Scale
197
bar = 2 cm in all cases. a: Endocast of a wild A. mississippiensis (Fla.F&G.HarvestTag 937095),
198
Dorsal cranial length (DCL), 342.90 mm, right lateral view. Reduced in size to match proportions of
199
brain in b & c. b: Dura mater around the brain of A. mississippiensis, specimen CITES FLM 12-
200
29409, DCL, 380 mm, left lateral view (reversed). c: Brain within arachnoid of FLM 12-29409. Brown-
201
red material is dried blood filling the subarachnoid space (SaS), right lateral view. Abbreviations:
202
Ach, arachnoid mater (covering the cerebellum); Art, artery on external wall of dura mater over the
203
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
lateral pole of the cerebrum; Cbll, cerebellum; CCA, caudal cerebral artery; Ch L or R, left or right 204
cerebral hemisphere; DSS, dorsal sagittal sinus; EthA, common ethmoid artery; I C, internal carotid 205
artery; InHA, interhemispheric artery; MO, medulla oblongata; N.II, optic nerve; N.V, (cast of) 206
trigeminal nerve; Nn R, roots of nerves IX-XI; OC, occipital condyle; OcS, occipital sinus; OlBu & Tr, 207
olfactory bulb & tract; OtC F, fossa of otic capsule; Pineal Loc, pineal gland location; Pit, pituitary 208
gland; SaSMe, mesencephalic subarachnoid space; SaSR, rostral SaS; SaSVe, ventral SaS; SN.I, 209
first spinal nerve. The rostral end of the cerebrum is below the arrow for SaSR in B. Both specimens 210
are housed in the private collection of G. R. Hurlburt. 211
212
Indeed, Herculano-Houzel (2011, 2017, 2023) has argued emphatically that neuron counts 213
represent reliable estimates for cognitive abilities in extant vertebrates, markedly 214
outperforming other measures such as relative or absolute brain size. If we accept this 215
premise, accurate modeling of neuron counts in dinosaurs based on endocast volumes and 216
comparative neurological data might appear as a promising new method to elucidate the 217
behavior and cognitive capacities of these animals. 218
219
The methodology and rationale of Herculano-Houzel (2023) 220
Herculano-Houzel (2023) reconstructed relative brain size and neuron counts for 29 221
dinosaur and pterosaur species based on comparative data from extant non-avian and avian 222
sauropsids (“reptiles” and birds respectively; Olkowicz et al., 2016; Kverková et al., 2022). 223
Although we want to avoid lengthy discussions about taxonomy, it is worth noting that some 224
of these are no longer considered valid taxonomic entities (see below; an updated 225
nomenclature for relevant dinosaurs is included in Tab. 1). For instance, Rhamphorhynchus 226
muensteri and R. gemmingi have long been synonymized (Bennett, 1995). Surprisingly, 227
Herculano-Houzel (2023) inferred an ectothermic metabolism for one, and endothermy for 228
the other based on assumptions about relative brain size. 229
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Neuron count estimates for fossil taxa only concerned the telencephalon, a major brain 230
region which is critically involved in cognitive and motor functions as well as the processing 231
of sensory information. It encompasses the pallium (which is homologous to the cerebral 232
cortex in humans and other mammals) and subpallium as well as the olfactory bulbs and 233
tracts (Fig. 1). To understand the rationale behind the approach of reconstructing neuron 234
counts in fossil species, two important matters must be pointed out: firstly, among jawed 235
vertebrates, body size and brain size are highly correlated, exhibiting a constant allometric 236
relationship overall (Tsuboi et al., 2018). It should be noted however, that scaling 237
relationships can vary to some extent between major taxa as well as between early- and 238
late-diverging members of a clade (Ksepka et al., 2020; Bertrand et al., 2022). Secondly, 239
neuronal densities (the number of neurons in a given volume of nervous tissue) can differ 240
profoundly between different vertebrate taxa. Based on current evidence, the highest neuron 241
densities among land vertebrates are found in the bird clade Telluraves, consisting of birds 242
of prey, rollers, parrots, songbirds and kin, while the lowest occur among crocodilians and 243
turtles (Kverková et al., 2022). For instance, the goldcrest (Regulus regulus), short-tailed 244
shrew (Blarina sp.) and painted turtle (Chrysemys picta) have brains of equal mass (ca. 245
0.37 g), but there is remarkable disparity in their whole brain neuron numbers, which range 246
from 14.3 M in the turtle over 58.8 M in the shrew to 164 M in the passerine bird (Olkowicz et 247
al., 2016; Kverková et al., 2022). This example illustrates that brain size alone is not a 248
reliable predictor of neuron counts across distantly related clades (compare Herculano-249
Houzel et al., 2014; Olkowicz et al., 2016), which makes their inference in fossil groups 250
inherently difficult. 251
To decide which neuronal density patterns apply to specific groups of dinosaurs and 252
pterosaurs, Herculano-Houzel (2023) relied on brain x body mass regressions. The brain 253
and body mass datasets used were taken from various literature sources and, as we attempt 254
to show here, both are problematic. In the resulting regression plot, she identified theropods 255
clustering distinctly from most other species. On average, they appeared to exhibit larger 256
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
brains for a given body size than the remaining dinosaur or pterosaur taxa. When comparing 257
the regression lines for extinct groups with those of living birds on the one hand and 258
squamates and turtles on the other, Herculano-Houzel (2023) noted that the theropod 259
regression fits with the avian one, while the remaining ornithodiran groups aligned more with 260
the non-avian sauropsid regression line. 261
Based on these analyses, the author made two critical assumptions: First, since only 262
theropod brain-body data aligned with those of endothermic extant sauropsids, namely birds, 263
the other groups (aside from specific pterosaurs and ornithischians that cluster with 264
theropods) should be considered ectothermic. Second, telencephalic neuron densities in 265
theropod brain tissue should have been comparable to those found in certain extant bird 266
taxa (that is, to those found in a polyphyletic assemblage denoted as “pre-K-Pg birds” that 267
includes Palaeognathae, Galloanserae and Columbiformes and which is considered to form 268
a neurological grade: Kverková et al., 2022), whereas those of the other groups should 269
resemble densities encountered in squamates and turtles. No further justification for these 270
suggestions is provided. 271
Applying the avian scaling regime, Herculano-Houzel (2023) estimated remarkably high 272
telencephalic neuron counts in large-bodied theropods such as Acrocanthosaurus atokensis 273
(2.1 billion) and T. rex (3.3 billion) which would exceed those of any extant bird and be 274
comparable to large-bodied Old World monkeys such as baboons (Papio anubis - Olkowicz 275
et al., 2016). Based on this apparent similarity to anthropoid primates, she further speculated 276
that these giant theropods would have crafted and used tools and exhibited cultural 277
behaviors (Herculano-Houzel, 2023). 278
We regard the methodology of Herculano-Houzel (2023) as problematic and disagree with 279
her physiological and behavioral interpretations. Before we attempt to replicate her findings 280
with a more refined analytical approach, we want to enumerate the most important flaws of 281
the article and how they affect the inferences made. 282
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
283
Issues with Herculano-Houzel’s method and rationale 284
A key problem for paleoneurology lies in the fact that an endocast does not necessarily 285
reflect the morphology of an animal's brain. As discussed in previous sections, the 286
endocasts of most non-avian dinosaurs differ markedly in size and shape from the actual 287
brain, as is the case in crocodilians (Fig. 1). Unfortunately, not all studies from which 288
Herculano-Houzel (2023) derived her raw data considered this issue (see below). In 289
addition, the percentage of endocranial space filled by the brain, as well as its proportions 290
may be further influenced by ontogeny (Bever et al., 2013; Hurlburt et al., 2013; Ngwenya et 291
al., 2013; Jirak & Janacek, 2017; Hu et al., 2021). The latter point is relevant because 292
Herculano-Houzel (2023) included several specimens which corresponded to juveniles 293
rather than adults, and thus might have introduced biases to the dataset (see below). 294
Interestingly, at least in crocodilians, neuronal densities in the brain are also affected by 295
ontogenetic stage (Ngwenya et al., 2016). 296
To arrive at the estimated telencephalic neuron count of > 3 billion for T. rex, Herculano-297
Houzel (2023) assumed a brain mass of 343 g. However, this presupposed that endocast 298
volume equaled brain volume in this species. While it has indeed been claimed that in 299
theropods such as T. rex, the brain filled the entire endocranial cavity (Balanoff et al., 2013), 300
this hypothesis is, as previously discussed, contradicted by multiple lines of evidence. More 301
conservative inferences suggest a brain mass of approximately 200 g (Hurlburt, 1996; 302
Hurlburt, et al, 2013; Morhardt, 2016) or possibly even lower (this study; Tab. 1) for T. rex. 303
Herculano-Houzel (2023) acknowledged these lower estimates but chose to rely on the 304
inflated values for large theropod brain masses in accompanying figures and the article’s 305
Discussion section. 306
Moreover, while the literature-derived brain mass estimates used for their analyses did in 307
some cases include the olfactory tracts and bulbs (Franzosa & Rowe, 2005, Balanoff et al., 308
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
2013), these were omitted in others (Hurlburt 1996; Hurlburt et al., 2013). This incongruence 309
created critical biases, affecting both the inference of telencephalic neuron counts and 310
relative brain size estimates. The latter are additionally skewed by the fact that body masses 311
used by Herculano-Houzel (2023) were not determined via a uniform methodology but 312
compiled from sources applying various approaches. There are several ways to estimate 313
body mass in extinct animals and they can differ greatly regarding their outcomes and 314
precision (Campione & Evans, 2020). When compared to body mass estimates derived from 315
stylopodial circumference, a well-established and robust method (Campione & Evans, 2020), 316
some striking differences become apparent (Tab. 1; Herculano-Houzel, 2023). 317
Another flaw of Herculano-Houzel’s (2023) approach is the neglect of brain morphology to 318
inform its analyses. To estimate telencephalic neuron numbers in fossil species, the mass of 319
the telencephalon needs to be approximated first. For theropods, Herculano-Houzel (2023:6) 320
extrapolated this variable from extant bird data while stating that “within a clade, brain mass 321
has strongly predictive power to arrive at estimates of numbers of telencephalic neurons in a 322
brain of known mass, once the neuronal scaling rules that presumably apply are known.” 323
However, this statement can only hold true if the general proportions of the telencephalon 324
compared to the remaining brain are roughly constant in the group of concern, which is a 325
precondition that Herculano-Houzel (2023) did not test for in the fossil sample. Indeed, avian 326
brains only poorly reflect the brain morphologies found in the majority of Mesozoic dinosaurs 327
(reviewed by Paulina-Carabajal et al. 2023) and their general proportions are only 328
comparable to those found among maniraptoriform theropods (Balanoff et al., 2013; Fig. 1). 329
An important difference concerns the pallium, which crucially contributes to higher cognitive 330
functions, and greatly increased in size within the maniraptoriform radiation (Balanoff et al., 331
2013). The same is true for the cerebellum, a part of the brain which is not encompassed by 332
the telencephalon but is also involved in various aspects of cognition in amniotes (Spence et 333
al., 2009). Thus, the telencephalic mass and proportions of non-maniraptoriform theropods, 334
such as T. rex, cannot be adequately modeled based on extant birds. Similar limitations 335
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
need to be considered when reconstructing traits of, for instance, the pterosaur or 336
sauropodomorph telencephalon based on extant non-avian sauropsids and they also apply 337
to our own empirical approach. 338
Herculano-Houzel (2023) hypothesized that the inferred incongruence in relative brain size 339
between theropods and other dinosaurs reflects differences in thermobiology, which would 340
justify applying avian neuronal scaling schemes to the former and non-avian sauropsid 341
scaling to the latter. Sauropodomorphs as well as selected ornithischians and pterosaurs are 342
instead suggested to be ectothermic due to their relatively smaller brains. Both of these 343
assumptions are problematic: First, multiple lines of evidence suggest that ornithodiran 344
endothermy evolved long before theropods emerged and was likely already present in the 345
Early Triassic common ancestor of dinosaurs and pterosaurs (e.g., Benton, 2012; Grigg et 346
al., 2022). We will revisit this evidence and how it challenges the brain size-derived 347
hypothesis in more detail in the Discussion section of this paper. Herculano-Houzel (2023) 348
only referenced a single article on dinosaur thermobiology, that of Wiemann et al. (2022), to 349
defend her standpoint on the matter. The study in question applies a promising but novel 350
technique to infer endothermy based on lipoxidation end products in fossil bone that still has 351
to prove itself. While it indeed suggests lowered metabolic rates in some ornithischians, it 352
also infers an endothermic metabolism for pterosaurs and sauropodomorphs (Wiemann et 353
al., 2022). Thus, its findings do not align with Herculano-Houzel’s (2023) assumptions. 354
Second comparisons between groups of extant vertebrates, especially birds and mammals, 355
strongly suggest that there is no uniform relationship between neuron density and relative 356
brain size or elevated metabolic rates (Kverkova et al., 2022; see also Estienne et al., 2024). 357
We will elaborate on this aspect in the Discussion section but would like to state at this point 358
already that it is not straight-forward to assume avian neuron densities in Mesozoic 359
theropods simply because they exhibited endothermy or a potential increase in relative brain 360
size. On the other hand, the extensive evidence for endothermy in other dinosaurs and 361
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
pterosaurs does not entail that these groups could not have had neuron densities similar to 362
those found in extant ectothermic sauropsids. 363
Other issues relate to the statistical methods employed by Herculano-Houzel (2023). Despite 364
dealing with a large multi-species dataset, their analyses did not take phylogeny into 365
account, which can produce mathematical artifacts. Phylogenetic relationships among taxa 366
need to be statistically addressed because shared ancestry can result in non-independence 367
of species-specific data points (Revell et al., 2008). Such non-independence is known as the 368
phylogenetic signal, and it has been prominently recovered for relative brain size in extant 369
sauropsids (Font et al., 2019). Hence, phylogenetically-informed modeling is necessary for 370
adequately analyzing such datasets (Font et al., 2019). 371
In light of these substantial shortcomings, we attempt to replicate the findings of Herculano-372
Houzel (2023) with phylogenetically informed models of telencephalic neuron counts in fossil 373
dinosaurs that acknowledge the issues lined out above. Different from her, we do not include 374
pterosaurs into our analysis due to difficulties with estimating their body mass (especially for 375
taxa with incomplete postcrania such as Scaphognathus) and because of the unclear 376
taxonomic and ontogenetic status of some of the few available endocasts. 377
378
Empirical part: modeling neurological variables in dinosaurs 379
Endocast sample composition, with notes on endocranial volumes provided by 380
Hurlburt (1996) 381
We estimated the mass of the brain (MBr, g; excluding the olfactory tracts and bulbs) as well 382
as its size relative to body mass (MBd, g) in 31 Mesozoic dinosaur taxa for which data on 383
endocranial volume (EV, ml) have been published (Tab. 1; Suppl. File 1). Note that this 384
study does not aim to provide a comprehensive dataset of dinosaur brain sizes. Given the 385
questions we want to address, we focus on large-bodied theropods and taxa covered in 386
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
previous comparative analyses. We included one endocast per species, except for T. rex, for 387
which three adult endocasts (AMNH 5029, AMNH 5117, FMNH PR 2081) were considered. 388
We only considered species for which we could calculate body mass based on stylopodial 389
circumference (see below) to reliably infer encephalization. Due to this, our analysis does 390
not cover all dinosaur species for which complete endocasts are available, nor all species 391
that Herculano-Houzel (2023) included in her analyses (namely Conchoraptor gracilis, 392
Tsaagan mangas, Zanabazar junior, and unnamed troodontid IGM 100/1126). Juvenile 393
specimens considered by that study (Alioramus altai IGM 100/1844, Gorgosaurus libratus 394
ROM 1247, and Tyrannosaurus rex CMN 7541
= “Nanotyrannus lancensis”) were omitted in 395
this analysis to eliminate the confounding variable of ontogeny. 396
The only juvenile we include is Bambiraptor feinbergi KUVP 129737, which is one of the few 397
maniraptoriform theropods that we can take into account. For this species, an adult femur 398
(FIP 002) is available, allowing us to estimate body mass in fully grown individuals. Our 399
method of body mass inference suggests that KUVP 129737 had attained about 45% of 400
adult body mass when it died. Data from similar-sized extant rheas (Rhea americana), 401
palaeognath birds which are close neuroanatomical analogs to highly derived theropods 402
such as Bambiraptor (Balanoff et al., 2013), suggest that adult brain mass is already 403
approached at that point of somatic development (Picasso et al., 2011; Picasso, 2012;). We 404
therefore combine the juvenile endocranial measurement of Bambiraptor with adult body 405
mass estimates. 406
Just as Herculano-Houzel (2023) did, we derive a significant portion of our EV values from 407
Hurlburt (1996). However, many EV figures communicated in this reference must be 408
considered outdated or otherwise flawed and were carefully bypassed here. We give 409
detailed reasons for discarding or modifying data from Hurlburt (1996) and the references 410
provided therein (Jerison, 1973; Hopson, 1979) in Suppl. File 1 Part A. Given that EVs from 411
this problematic dataset are still widely used (e.g., Müller et al. 2021; Button & Zanno, 2023), 412
we consider their revision an important aspect of this study. In cases where EVs appeared 413
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
doubtful but appropriate illustrations or photographs of specimens were available, we 414
recalculated EV using manual graphic double integration (GDI; see below for methodology). 415
This was done for four species (Allosaurus fragilis, Euoplocephalus tutus, Kentrosaurus 416
aethiopicus & Ornithomimus edmontonicus; see Suppl. File 1 for details on specimens). 417
418
Brain mass estimates 419
We estimated the brain mass (MBr, g) of fossil dinosaurs from endocast volume (EV, mL). 420
Because the specific gravity (density) of living amniote brain tissue approximates one (1.036 421
g/mL - Iwaniuk & Nelson, 2002), we used brain volume and mass interchangeably (compare 422
Hurlburt et al., 2013; Herculano-Houzel, 2023). For maniraptoriform species, because their 423
endocasts preserve brain contours similar to those of avians, we assumed a brain:endocast 424
ratio of 100%. This is consistent with empirical data on the relationship between MBr and EV 425
in modern birds, which suggest contributions of meningeal tissue to endocast volume to be 426
negligible (Iwaniuk & Nelson, 2002). For other dinosaurs, we assumed MBr:EV ratios of 31% 427
and 42%. Many previous studies have assumed a 50% ratio in these groups (reviewed in 428
Morhardt, 2016) while some even assumed 100% (Balanoff et al., 2013) or advocated for 429
intermediate values (e.g., Evans et al., 2008; Knoll & Schwarz-Wings, 2009; Knoll et al., 430
2021). The widely adopted 50% ratio was originally proposed by Jerison (1973) and based 431
on measurements from a likely subadult green iguana (Iguana iguana) and a mere visual 432
estimate of endocranial filling in the tuatara (Sphenodon punctatus; the endocranial fill in this 433
species is now known to be 30% in adults - Roese-Miron et al., 2023). We abandon the 434
problematic 50% estimate and replace it here by the two aforementioned ratios that are 435
based on the morphology of extant crocodilians, the closest extant analogs to most non-436
avian dinosaurs in regards to endocranial tissue organization, body size and braincase 437
ossification. Excluding one anomalous value, the lowest MBr:EV ratio among the three 438
longest American alligators (Alligator mississippiensis) studied by Hurlburt et al (2013) was 439
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
found to be 31% (n
total
= 12, note that this figure excludes the olfactory bulb and tract 440
portions of the endocranial cavity). This
is consistent with observations on the largest Nile 441
crocodile (Crocodylus niloticus; a 16-year-old female) studied by Jirak & Janacek (2017) 442
when excluding the olfactory tracts and bulb portion of the endocast
. The 42% ratio is 443
derived solely from American alligators. An endocranial fill of 42% was found in an adult 444
female with a total length of 2.87 m, which roughly approximates both (a) the maximum 445
length for a female American alligator and (b) the midpoint length within the size spectrum of 446
sexually mature alligators (Woodward et al, 1991; Hurlburt & Waldorf, 2002; Hurlburt et al, 447
2013). 448
The majority of EV data for Mesozoic dinosaurs were taken and modified from the literature 449
(detailed out in Tab. 1). In many cases, original sources communicated measurements that 450
correspond to total EV. This is the volume of the entire endocast, often including the region 451
of the olfactory tract and bulbs as well as portions of the cranial spinal cord among other 452
structures. For our analysis, we exclusively relied on the so-called “brain” endocast volume 453
instead (BrEV; Fig. 3), which was popularized by Jerison (1973) and has been commonly 454
used since then (e.g., Hurlburt, 1996; Larsson et al., 2000; Paulina- Carabajal & Canale, 455
2010; Hurlburt et al., 2013). It excludes the spinal cord portion of the endocast caudal to 456
cranial nerve XII, the volume of nerve trunks from infillings of respective foramina and blood 457
vessel casts, the labyrinth of the inner ear, the infundibulum, the pituitary fossa, and 458
especially the volume of the olfactory bulbs and tracts (Fig. 4). The latter are often only 459
poorly preserved in fossil endocasts, so that relying on specimens with intact casts of 460
olfactory structures would have reduced our sample size. 461
In some dinosaurs, there is an obvious constriction and/or a change in surface morphology 462
at the junction of the cerebrum and olfactory tract (e.g., Euoplocephalus tutus AMNH 5337 - 463
Hopson, 1979; Stegosaurus ungulatus CM 106 - Galton, 2001; Diplodocus longus CM 464
11161- Witmer et al., 2008). If present, this was used as a landmark to delineate these brain 465
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
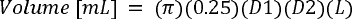
regions from one another. In less obvious cases, the junction between the cerebrum and 466
olfactory tract portion was assumed to be where the ventral curve of the rostral cerebrum 467
flattens out to approach a horizontal orientation. When selecting the boundary, we erred 468
towards a more rostral location, to assign as much of the endocast as part of the BrEV as 469
seemed reasonable. In American alligators, the rostral termination of the cerebrum within the 470
rostral subarachnoid space is clearly visible (Fig. 2C; SaSR) and consistent with the change 471
in curvature referred to above. 472
We used manual GDI to extract BrEV from total EV (relevant details for each specimen are 473
provided in Supplementary File 1 Part B). The method involves drawing an outline around 474
two scaled orthogonal two-dimensional views of an endocast, and adding equally spaced 475
lines perpendicular to the endocast midline (Fig. 3). The mean length (cm) of these lines in 476
each view (i.e., dorsal, lateral) provides diameters D1 and D2. The volume (mL) of the 477
desired region is calculated using these two diameters and the length (L, cm) in the formula 478
for the volume (mL or cm
3
) of a cylinder where (all lengths in cm): 479
480
GDI has been demonstrated to produce reasonable estimates of endocast volumes (Fig. 3). 481
For instance, Jerison (1973) used GDI to calculate a total endocast volume of 536 ml for a T. 482
rex specimen (AMNH 5029), which was 101.13% of the 530 ml volume determined for it by 483
means of water displacement (Osborn, 1912; Fig. 3). For the same specimen, Jerison (1973) 484
calculated a 404 ml volume for the “brain region” of the endocast (extending from cranial 485
nerve XII to the rostral cerebral limit), which was 106.04 % of the 381 ml obtained by CT 486
volumetry (Hurlburt et al., 2013). 487
488
Body mass estimates 489
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
We calculated the body mass (MBd, g) of the selected dinosaur taxa (and its mean absolute 490
percent prediction error - PPE or %PE - Campione & Evans, 2020) in a standardized manner 491
based on the minimum femoral circumference (as well as humeral circumference in case of 492
quadrupedal taxa) by aid of the QE() and cQE() functions from the MASSTIMATE package 493
(Campione, 2020) in R (R Core Team, 2023). Data on relevant long bone dimensions were 494
primarily obtained from Benson et al. (2017). Corresponding specimens as well as additional 495
sources and information on stylopodium circumference measurements are listed in Tab. 1. 496
To the best of our knowledge, all data correspond to adult specimens. 497
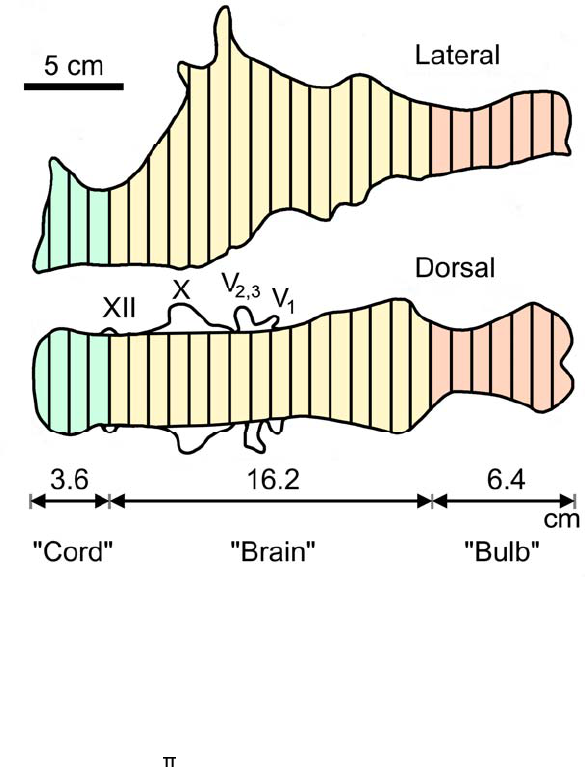
498
Figure 3. Exemplary graphic double integration (GDI) of the endocast of Tyrannosaurus rex AMNH 499
5029. Equally spaced lines are drawn across the right lateral and dorsal views respectively. Mean 500
lengths of the lines drawn across the “brain” portion (BrEV) were 4.8 cm and 6.6 cm for dorsal and 501
lateral views respectively. BrEV = x 0.25 x 4.8 x 6.6 x 16.2 = 404 mL (the volume of the entire 502
endocast was 536 mL). Abbreviations: “Bulb”: Olfactory tract and bulbs; “Cord”: spinal cord; V: 503
Trigeminal nerve with its three branches (V1, V2,& V3); X: vagus nerve; XII: hypoglossal nerve. 504
(Adapted from Fig. 2.7 in Jerison,1973, p. 51). 505
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
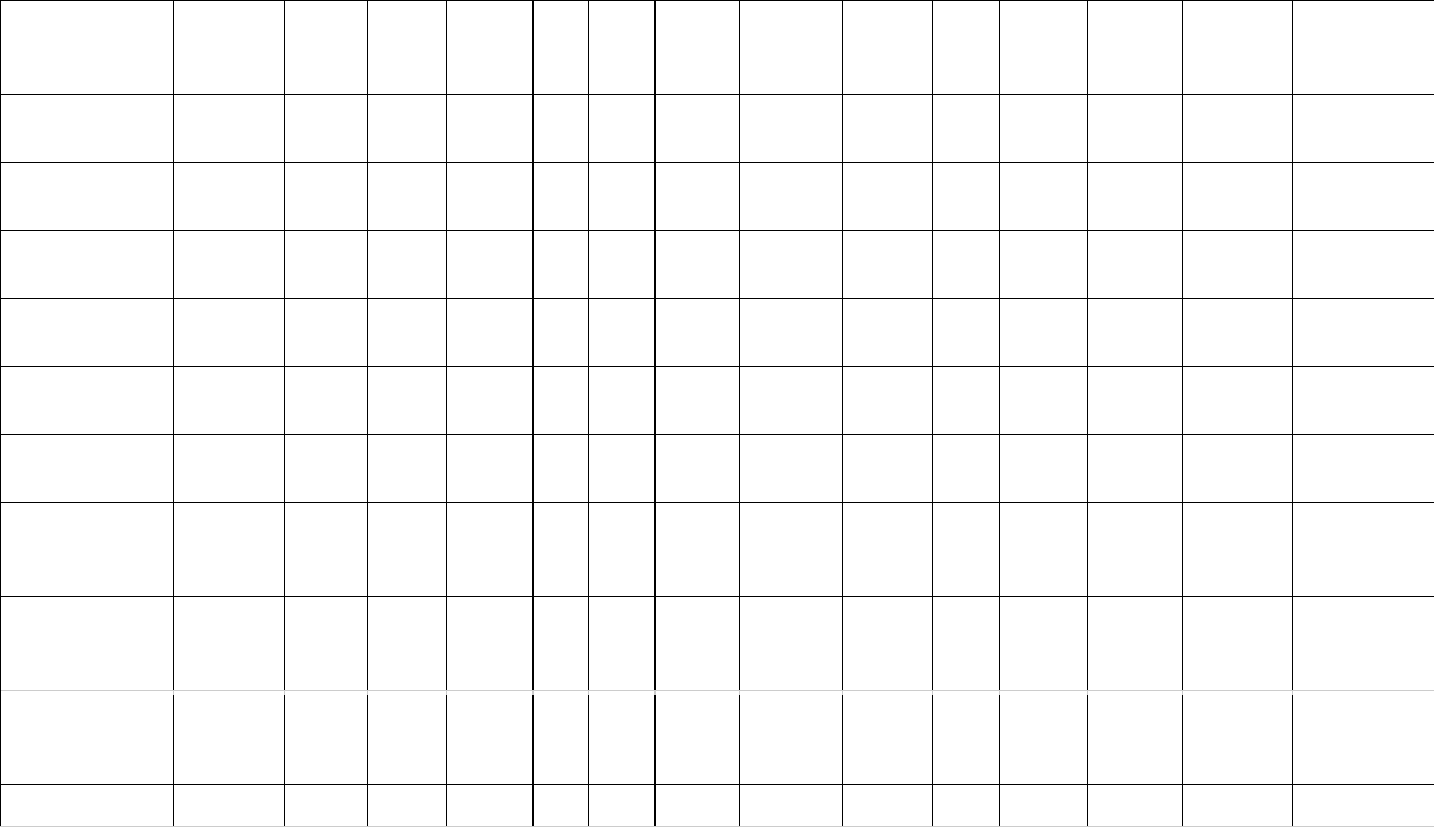
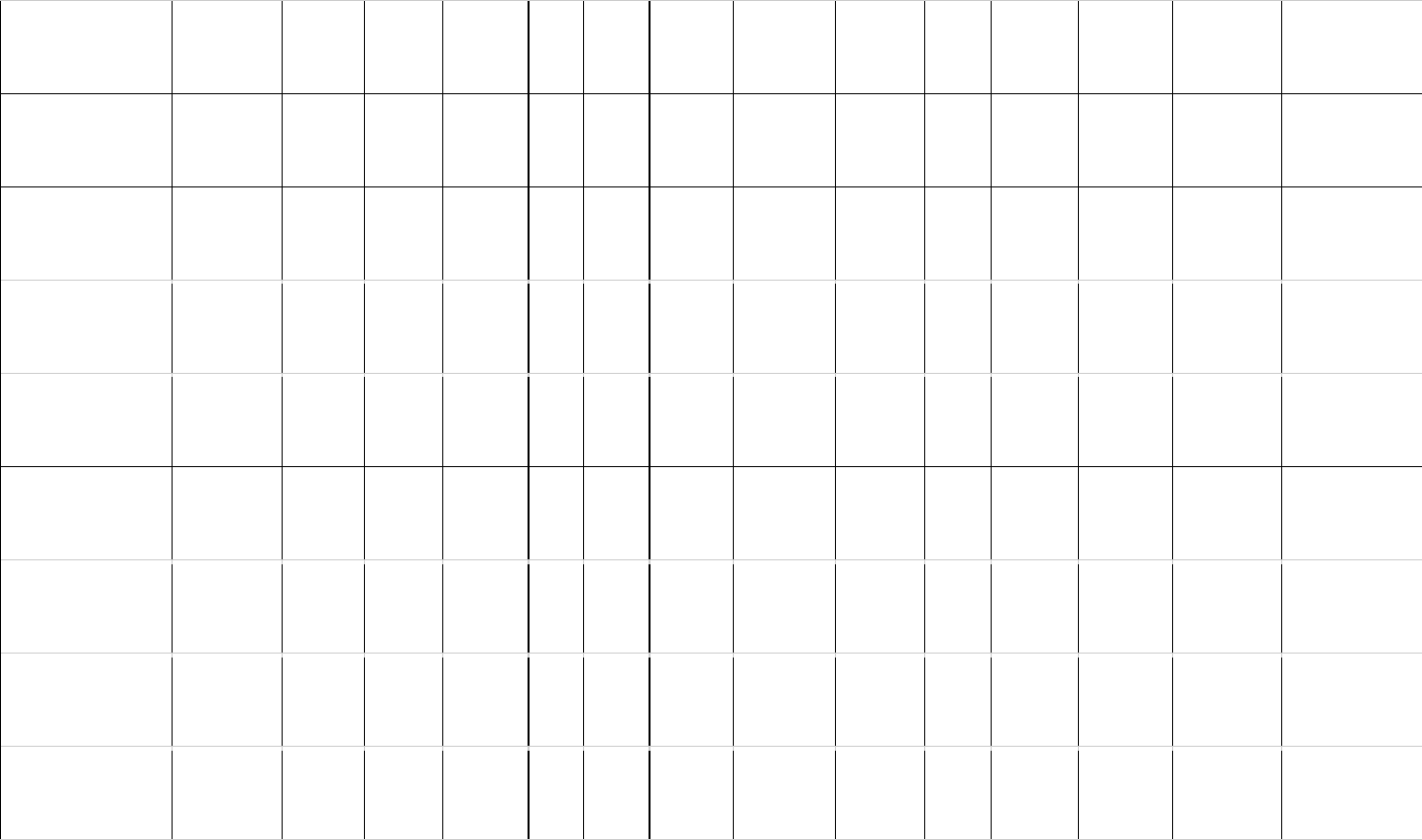
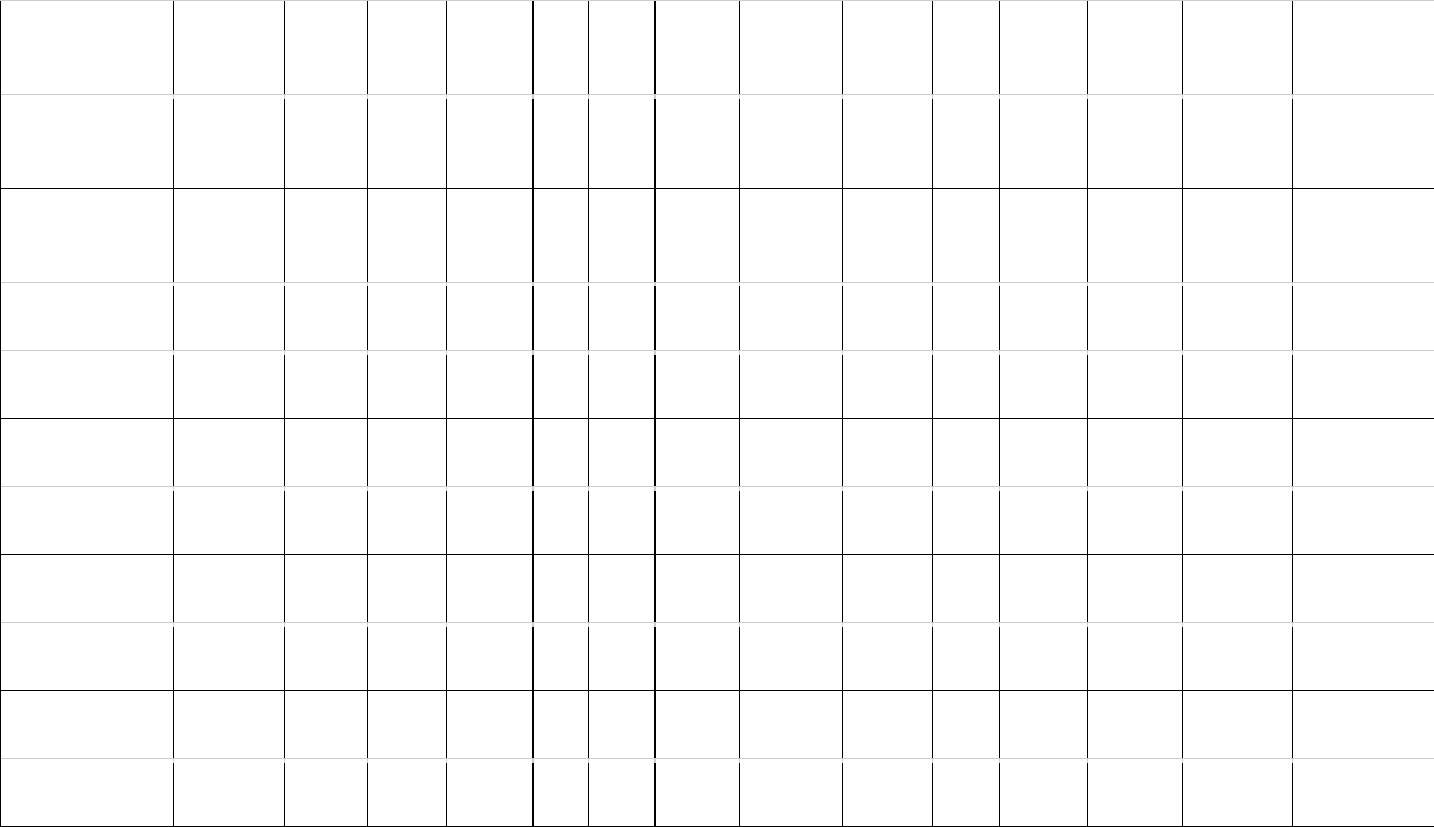
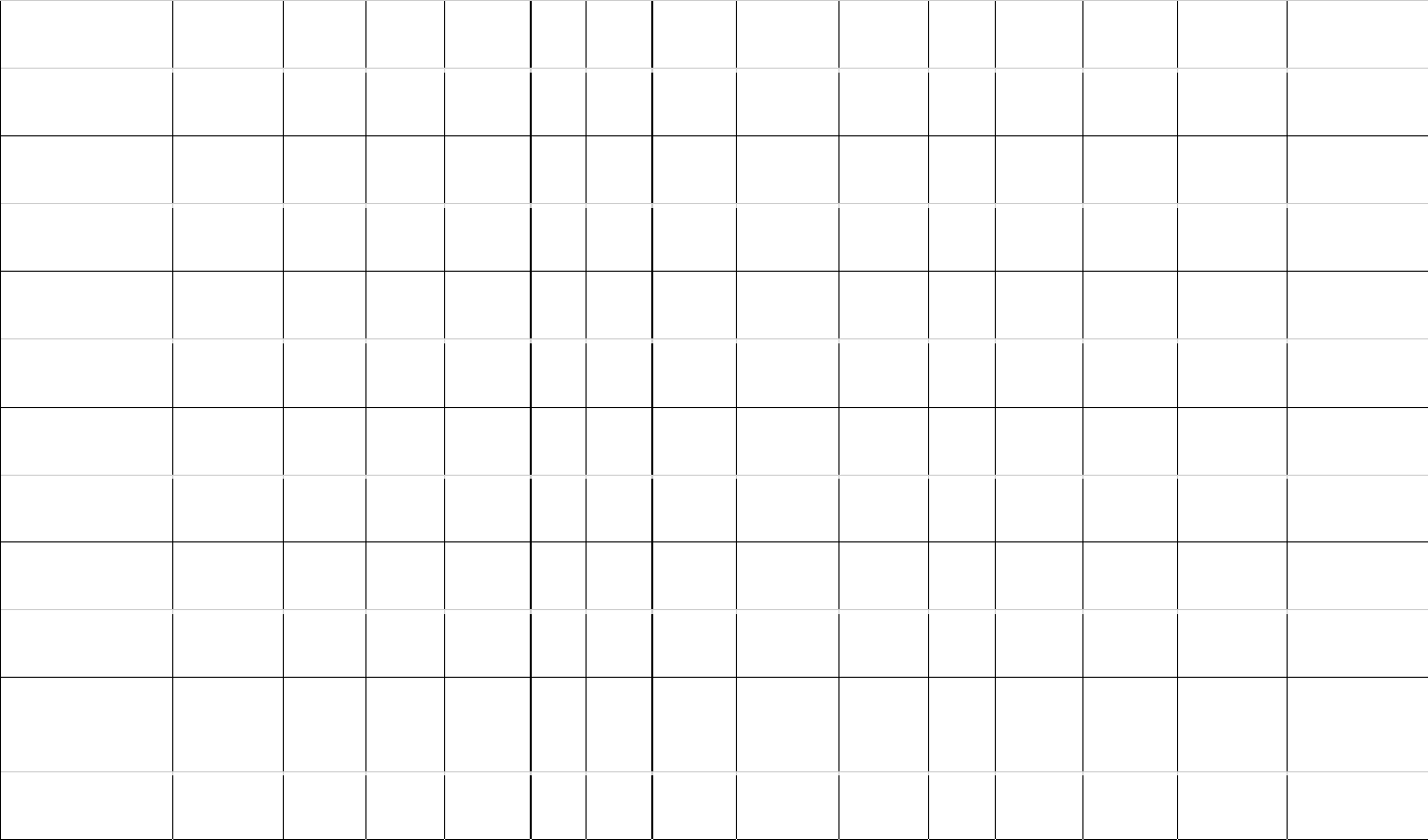
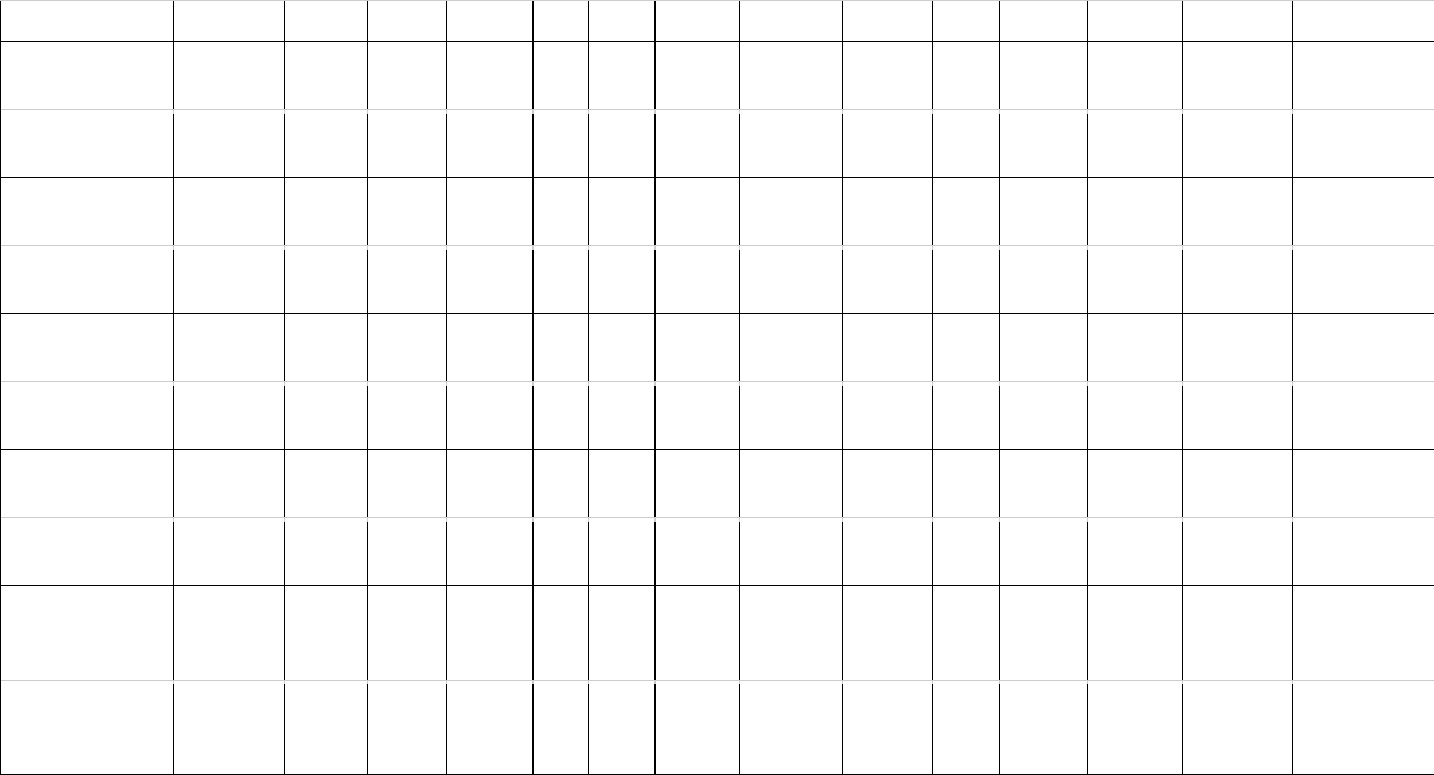
Table 1: Estimates of brain (MBr, g; derived from endocranial volume, EV) and body mass (MBd, kg) in Mesozoic dinosaurs. For maniraptoriform theropods, 506
we assumed that brain endocast volume equals brain volume. For other dinosaurs, we assumed a brain:endocast ratio of 31 - 42 %. Body mass was 507
calculated based on stylopodial circumference (femoral circumference (FC) for bipedal and femoral as well as humeral (HC) circumference for quadrupedal 508
species). Footnotes: [1] Bambiraptor feinbergi. MBd from femur circumference, measured on a cast of the right femur of an adult specimen now in the 509
collection of the Vertebrate Paleontology Division, ROM (FIP 002). Original elements of this specimen are now in the collection of the AMNH. [2] 510
Archaeopteryx lithographica. MBd from FC (14.93 mm) estimated from femur length (60.5mm) of BMNH 37001 (Gatesy, 1991). FC was calculated using the 511
equation from Benson et al. (2017): log10(Femur circumference estimate from femur length) = 1.132 * log10(Femur length) - 0.8429. [3] 512
Carcharodontosaurus saharicus. MBd from estimated femur circumference (FC = 417.52 mm) from Benson et al. (2017) for BSP 1922 X46. The specimen 513
has been destroyed and is no longer accessible. [4] Sinraptor dongi. MBd based on FC (283 mm) from Campione & Evans (2020), measurement taken from 514
TMP 93.115.1 (cast of IVPP 10600). Benson et al. (2017) consider IVPP 10600 a subadult based on reported incomplete fusion of cervical vertebrae; 515
However, Paulina-Carabajal & Currie (2012) noted that the degree of cranial suture fusion indicates that the specimen is an adult or at least a large subadult. 516
We consider it an adult here. [5] Tyrannosaurus rex specimens. MBd based on FC values listed by Persons et al. (2020). For FMNH PR 2081 (“Sue”), but not 517
the other individuals considered here, both FC and EV are available. We associated the EV (313.636 mL) of AMNH 5117 with the MBd (5515 kg) of BHI 3033 518
(“Stan”), as both specimens have been considered proxies for each other (G. M. Erickson, pers comm. to G.R. Hurlburt, 2005). The EV (381.8 mL) of AMNH 519
5029 is here linked to the MBd (6430 kg) of CM 9380 (holotype specimen) because it fell between MBd’s associated with EVs of FMNH PR 3081 and AMNH 520
5117. Besides our brain:endocast ratios, we also apply the 57% ratio proposed by Morhardt (2016) to this species. 521
522
523
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Species Group
Body
mass
(MBd) (kg)
Lower
25% PPE
(kg)
Upper 25%
PPE (kg)
FC
(mm)
HC
(mm)
MBd
Specimen
MBd Source
for FC & HC
Brain Mass
(MBr) (g)
MBr/EV
Br EV
(mL/cm3)
EV
Specimen
EV Meth Original EV Source
Archaeopteryx
lithographica[2]
Non-avian
maniraptoriform
3.446 2.563 4.329 14.93 NA
BMNH
37001
Gatesy, 1991 1.52 1 1.52 BMNH 37001 CT
Dominguez Alonso et
al. (2004)
Bambiraptor feinbergi[1]
Non-avian
maniraptoriform
8.0632 5.9966 10.1298 47 NA FIP 002
Cast of right
femur
14 1 14
KUVP
129737
Water
displacement
Burnham (2004)
Citipati osmolskae
Non-avian
maniraptoriform
123.993 92.214 155.772 127 NA
IGM
100/978
Benson et al.
(2017), #321
22.05 1 22.05 IGM 100/978 CT Balanoff et al. (2013)
Khaan mckennai
Non-avian
maniraptoriform
219.198 163.018 275.378 67.62 NA
IGM
100/1127
Benson et al.
(2017), #452
8.8 1 8.8 IGM 100/973 CT Balanoff et al. (2013)
Ornithomimus edmontonicus
Non-avian
maniraptoriform
835.238 621.167 1.049.309 110 NA ROM 851
Benson et al.
(2017), #522
49.89 1 49.89 NMC 12228 GDI (this study)
Photos of endocast by
GRH
Shuvuuia deserti
Non-avian
maniraptoriform
3.0497 2.2681 3.8313 33 NA
IGM
100/1304
Benson et al.
(2017), #574
1.52 1 1.52 IGM 100/977 CT
Balanoff et al. (2013),
Balanoff et al. (2024)
Stenonychosaurus inequalis
Non-avian
maniraptoriform
473.759 352.335 59.52 89.5 NA
MOR 748
(MTC)
Benson et al.
(2017), #621
38.65 1 38.65
RTMP
86.36.457 &
79.8.1
CT Morhardt (2016)
Acrocanthosaurus atokensis
Non-
maniraptoriform
theropod
3.454.954 2.569.449 4340.46 426 NA
NCSM
14345
Benson et al.
(2017), #242
51.55 0.42 122.74
OMNH
10146
CT
Franzosa & Rowe
(2005)
Acrocanthosaurus atokensis
Non-
maniraptoriform
theropod
3.454.954 2.569.449 4340.46 426 NA
NCSM
14345
Benson et al.
(2017), #242
38.05 0.31 122.74
OMNH
10146
CT
Franzosa & Rowe
(2005)
Allosaurus fragilis Non- 2.541.814 1.890.347 3193.28 381 NA AMNH 680 Benson et al. 41.37 0.42 98.5 UUVP 294 GDI (this study) Photo of cast by GRH
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

maniraptoriform
theropod
(2017), #253
Allosaurus fragilis
Non-
maniraptoriform
theropod
2.541.814 1.890.347 3193.28 381 NA AMNH 680
Benson et al.
(2017), #253
30.54 0.31 98.5 UUVP 294 GDI (this study) Photo of cast by GRH
Carcharodontosaurus
saharicus
Non-
maniraptoriform
theropod
3.269.147 2.431.265 4107.03
417.5
2
NA
BSP 1922
X46
Benson et al.
(2017), #307
69.44 0.31 224 SGM-Din 1 CT Larsson et al. (2000)
Carcharodontosaurus
saharicus[3]
Non-
maniraptoriform
theropod
3.269.147 2.431.265 4107.03
417.5
2
NA
BSP 1922
X46
Benson et al.
(2017), #307
94.08 0.42 224 SGM-Din 1 CT Larsson et al. (2000)
Carnotaurus sastrei
Non-
maniraptoriform
theropod
1.641.829 1.221.028 2062.63 325 NA
MACN CH
894
Benson et al.
(2017), #308
45.49 0.42 108.3
MACN CH-
894
CT
Cerroni & Paulina-
Carabajal (2019)
Carnotaurus sastrei
Non-
maniraptoriform
theropod
1.641.829 1.221.028 2062.63 325 NA
MACN CH
894
Benson et al.
(2017), #308
33.57 0.31 108.3
MACN CH-
894
CT
Cerroni & Paulina-
Carabajal (2019)
Giganotosaurus carolinii
Non-
maniraptoriform
theropod
6.136.771 4.563.916 7.709.625 525 NA
MUCPv-
Ch1
Benson et al.
(2017), #399
94.5 0.42 225
MUCPv-CH
1
CT
Paulina-Carabajal &
Canale (2010)
Giganotosaurus carolinii
Non-
maniraptoriform
theropod
6.136.771 4.563.916 7.709.625 525 NA
MUCPv-
Ch1
Benson et al.
(2017), #399
69.75 0.31 225
MUCPv-CH
1
CT
Paulina-Carabajal &
Canale (2010)
Majungasaurus
crenatissimus
Non-
maniraptoriform
theropod
1.614.201 1.200.481 2027.92 323 NA
FMNH PR
2278
Benson et al.
(2017), #482
37.51 0.42 89.32
FMNH PR
2100
CT
Sampson & Witmer
(2007)
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Majungasaurus
crenatissimus
Non-
maniraptoriform
theropod
1.614.201 1.200.481 2027.92 323 NA
FMNH PR
2278
Benson et al.
(2017), #482
27.69 0.31 89.32
FMNH PR
2100
CT
Sampson & Witmer
(2007)
Sinraptor dongi
Non-
maniraptoriform
theropod
1.122.287 834.645 1.409.929 283 NA
TMP
93.115.1
Campione &
Evans, 2020
29.45 0.31 95 IVPP 10600 CT
Paulina-Carabajal &
Currie (2012)
Sinraptor dongi[4]
Non-
maniraptoriform
theropod
1.122.287 834.645 1.409.929 283 NA
TMP
93.115.1
Campione &
Evans, 2020
39.9 0.42 95 IVPP 10600 CT
Paulina-Carabajal &
Currie (2012)
Tarbosaurus bataar
Non-
maniraptoriform
theropod
2.345.113 1744.06 2.946.165 370 NA
MPC-D
552/1
Benson et al.
(2017), #610
66.86 0.42 159.2
PIN, no. 553-
3/1
Estimated from
latex half-cast
Saveliev & Alifanov
(2007)
Tarbosaurus bataar
Non-
maniraptoriform
theropod
2.345.113 1744.06 2.946.165 370 NA
MPC-D
552/1
Benson et al.
(2017), #610
49.35 0.31 159.2
PIN, no. 553-
3/1
Estimated from
latex half-cast
Saveliev & Alifanov
(2007)
Tyrannosaurus rex
Non-
maniraptoriform
theropod
8070.46 6.002.001 10.138.919 580 NA
FMNH PR
2081
Persons et al.,
2020
128.4 0.31 414.19
FMNH PR
2081
CT Hurlburt et al (2013)
Tyrannosaurus rex
Non-
maniraptoriform
theropod
6.430.357 4.782.256 8.078.457 534 NA CM 9380
Persons et al.,
2020
160.34 0.42 381.76 AMNH 5029 CT Hurlburt et al (2013)
Tyrannosaurus rex
Non-
maniraptoriform
theropod
6.430.357 4.782.256 8.078.457 534 NA CM 9380
Persons et al.,
2020
118.35 0.31 381.76 AMNH 5029 CT Hurlburt et al (2013)
Tyrannosaurus rex
Non-
maniraptoriform
theropod
5.515.247 4101.69 6.928.805 505 NA BHI 3033
Persons et al.,
2020
178.77 0.57 313.64 AMNH 5117 CT Morhardt (2016)
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Tyrannosaurus rex
Non-
maniraptoriform
theropod
5.515.247 4101.69 6.928.805 505 NA BHI 3033
Persons et al.,
2020
131.73 0.42 313.64 AMNH 5117 CT Hurlburt et al (2013)
Tyrannosaurus rex
Non-
maniraptoriform
theropod
5.515.247 4101.69 6.928.805 505 NA BHI 3033
Persons et al.,
2020
97.23 0.31 313.64 AMNH 5117 CT Hurlburt et al (2013)
Tyrannosaurus rex [5]
Non-
maniraptoriform
theropod
8070.46 6.002.001 10.138.919 580 NA
FMNH PR
2081
Persons et al.,
2020
173.96 0.42 414.19
FMNH PR
2081
CT Hurlburt et al (2013)
Amargasaurus cazaui
Non-theropod
dinosaur
10194.61 7581.73 12807.49 505 388
MACN-N
15
Benson et al.
(2017), #20
35.28 0.42 84 MACN-N 15 CT
Paulina Carabajal et
al. (2014)
Amargasaurus cazaui
Non-theropod
dinosaur
10194.61 7581.73 12807.49 505 388
MACN-N
15
Benson et al.
(2017), #20
26.04 0.31 84 MACN-N 15 CT
Paulina Carabajal et
al. (2014)
Apatosaurus sp.
Non-theropod
dinosaur
41.268.719
30.691.54
6
51.845.891 845 640 CM 3018
Benson et al.
(2017), #33
43.04 0.42 102.48 BYU 17096 CT Balanoff et al. (2010)
Apatosaurus sp.
Non-theropod
dinosaur
41.268.719
30.691.54
6
51.845.891 845 640 CM 3018
Benson et al.
(2017), #33
31.77 0.31 102.48 BYU 17096 CT Balanoff et al. (2010)
Buriolestes schultzi
Non-theropod
dinosaur
6.424 4.777 08.07 43.27 NA
CAPPA/UF
SM 0035
Müller et al.
(2021)
1.021 0.42 2.43
CAPPA/UFS
M 0035
CT Müller et al. (2021)
Buriolestes schultzi
Non-theropod
dinosaur
6.424 4.777 08.07 43.27 NA
CAPPA/UF
SM 0035
Müller et al.
(2021)
0.753 0.31 2.43
CAPPA/UFS
M 0035
CT Müller et al. (2021)
Diplodocus sp.
Non-theropod
dinosaur
14.813.081
11.016.48
8
18.609.673 563 460
USNM
10865
Benson et al.
(2017), #86
42 0.42 100 CM 11161 CT
L. M. Witmer, pers.
comm. (2023)
Diplodocus sp.
Non-theropod
dinosaur
14.813.081
11.016.48
8
18.609.673 563 460
USNM
10865
Benson et al.
(2017), #86
31 0.31 100 CM 11161 CT
L. M. Witmer, pers.
comm. (2023)
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Edmontosaurus annectens
Non-theropod
dinosaur
6.610.079 4.915.916 8.304.243 512.3 250.5
AMNH
5730
Benson et al.
(2017), #757
126 0.42 300 YPM 618
GDI (Jerison,
1973)
Lull & Wright (1942)
Edmontosaurus annectens
Non-theropod
dinosaur
6.610.079 4.915.916 8.304.243 512.3 250.5
AMNH
5730
Benson et al.
(2017), #757
93 0.31 300 YPM 618
GDI (Jerison,
1973)
Lull & Wright (1942)
Euoplocephalus tutus
Non-theropod
dinosaur
2.329.632 1.732.548 2.926.717 278 244
AMNH
5404
Benson et al.
(2017), #766
34.73 0.42 82.7 AMNH 5337 GDI (this study) Hopson (1979)
Euoplocephalus tutus
Non-theropod
dinosaur
2.329.632 1.732.548 2.926.717 278 244
AMNH
5404
Benson et al.
(2017), #766
25.64 0.31 82.7 AMNH 5337 GDI (this study) Hopson (1979)
Giraffatitan brancai
Non-theropod
dinosaur
34.003.143
25.288.13
7
42.718.148 730 654 HMN SII
Benson et al.
(2017), #107
130.2 0.42 310 MB.R.2223.1 Plasticine cast Janensch (1935-36)
Giraffatitan brancai
Non-theropod
dinosaur
34.003.143
25.288.13
7
42.718.148 730 654 HMN SII
Benson et al.
(2017), #107
96.1 0.31 310 MB.R.2223.1 Plasticine cast Janensch (1935-36)
Hypacrosaurus altispinus
Non-theropod
dinosaur
3.689.151 2.743.622 4.634.681 395 222 CMN 8501
Benson et al.
(2017), #800
85.53 0.31 275.9 ROM 702 CT Evans et al (2009)
Hypacrosaurus altispinus
Non-theropod
dinosaur
3.689.151 2.743.622 4.634.681 395 222 CMN 8501
Benson et al.
(2017), #800
115.88 0.42 275.9 ROM 702 CT Evans et al. (2009)
Iguanodon bernissartensis
Non-theropod
dinosaur
8.268.265 6.149.108 10.387.421 490 337.5
RBINS
R51
Benson et al.
(2017), #805
149.94 0.42 357 RBINS R51 CT Lauters et al. (2012)
Iguanodon bernissartensis
Non-theropod
dinosaur
8.268.265 6.149.108 10.387.421 490 337.5
RBINS
R51
Benson et al.
(2017), #805
110.67 0.31 357 RBINS R51 CT Lauters et al. (2012)
Kentrosaurus aethiopicus
Non-theropod
dinosaur
1.596.86 1.187.585 2.006.136 245 210
HMN
composite
specimen
Benson et al.
(2017), #813
22.092 0.42 52.6 HMN Ki 124 GDI (this study) Galton (1988)
Kentrosaurus aethiopicus
Non-theropod
dinosaur
1.596.86 1.187.585 2.006.136 245 210
HMN
composite
Benson et al.
(2017), #813
16.306 0.31 52.6 HMN Ki 124 GDI (this study) Galton (1988)
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
specimen
Protoceratops andrewsi
Non-theropod
dinosaur
82.695 61.5 103.889 93 62
AMNH
6424
Benson et al.
(2017), #892
12.6 0.42 30 AMNH 6466
GDI (Jerison,
1973)
Brown & Schlaikjer
(1940)
Protoceratops andrewsi
Non-theropod
dinosaur
82.695 61.5 103.889 93 62
AMNH
6424
Benson et al.
(2017), #892
9.3 0.31 30 AMNH 6466
GDI (Jerison,
1973)
Brown & Schlaikjer
(1940)
Psittacosaurus lujiatunensis
Non-theropod
dinosaur
28.61 21.278 35.944 74.5 NA
AMNH
6541
Benson et al.
(2017), #898
6.006 0.42 14.3 PKUP V1060 CT Zhou et al. (2007)
Psittacosaurus lujiatunensis
Non-theropod
dinosaur
28.61 21.278 35.944 74.5 NA
AMNH
6541
Benson et al.
(2017), #898
4.433 0.31 14.3 PKUP V1060 CT Zhou et al. (2007)
Stegosaurus ungulatus
Non-theropod
dinosaur
6.953.916 5.171.627 8.736.205 425 352 YPM 1853
Benson et al.
(2017), #927
26.964 0.42 64.2 CM 106 GDI (this study) Galton (2001)
Stegosaurus ungulatus
Non-theropod
dinosaur
6.953.916 5.171.627 8.736.205 425 352 YPM 1853
Benson et al.
(2017), #927
19.902 0.31 64.2 CM 106 GDI (this study) Galton (2001)
Thescelosaurus neglectus
Non-theropod
dinosaur
338.505 251.746 425.263 183 NA
AMNH
5891
Benson et al.
(2017), #946
11.614 0.42 27.653 NCSM 15728 CT Button & Zanno (2023)
Thescelosaurus neglectus
Non-theropod
dinosaur
338.505 251.746 425.263 183 NA
AMNH
5891
Benson et al.
(2017), #946
8.572 0.31 27.653 NCSM 15728 CT Button & Zanno (2023)
Triceratops sp.
Non-theropod
dinosaur
13.274.61 9.872.328 16.676.893 493 490
AMNH
5033
Benson et al.
(2017), #950
96.075 0.42 228.75 MOR 1194 CT
Morhardt (2016), L. M.
Witmer, pers. comm.
(2024)
Triceratops sp.
Non-theropod
dinosaur
13.274.61 9.872.328 16.676.893 493 490
AMNH
5033
Benson et al.
(2017), #950
70.913 0.31 228.75 MOR 1194 CT
Morhardt (2016), L. M.
Witmer, pers. comm.
(2024)
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
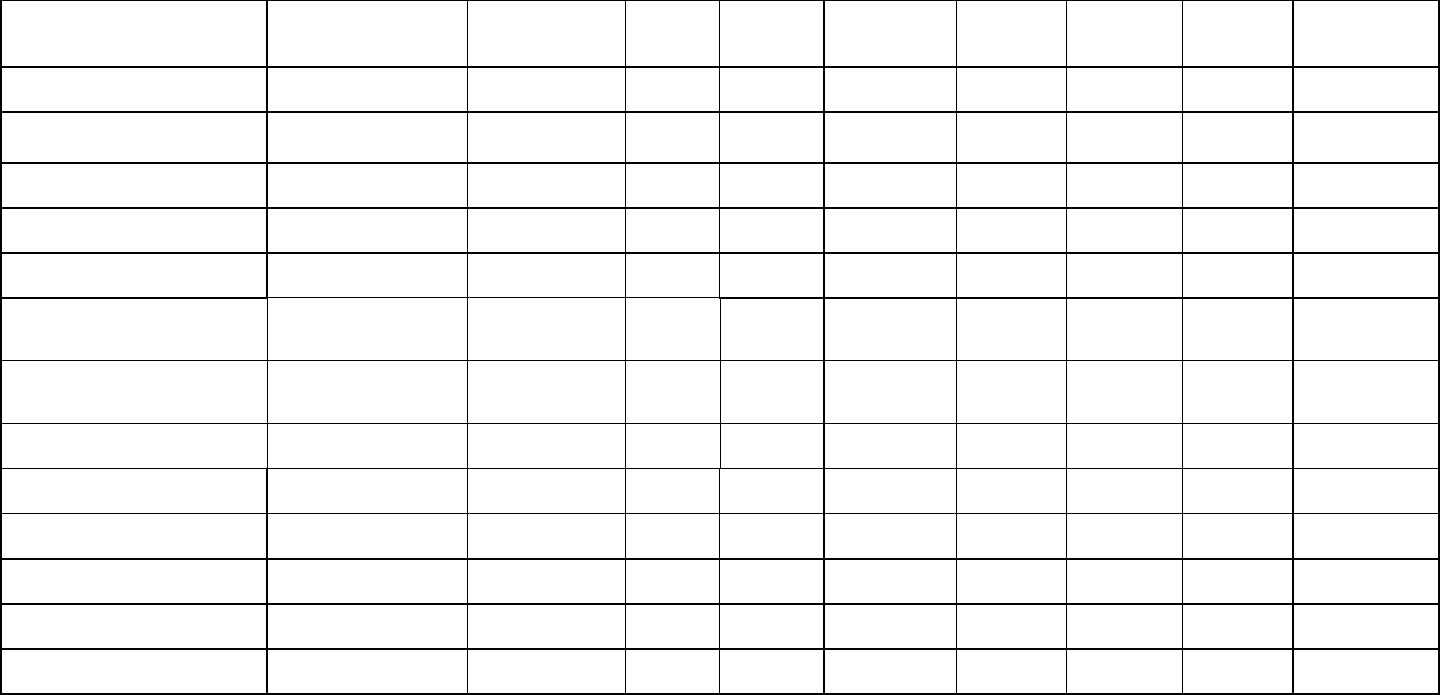
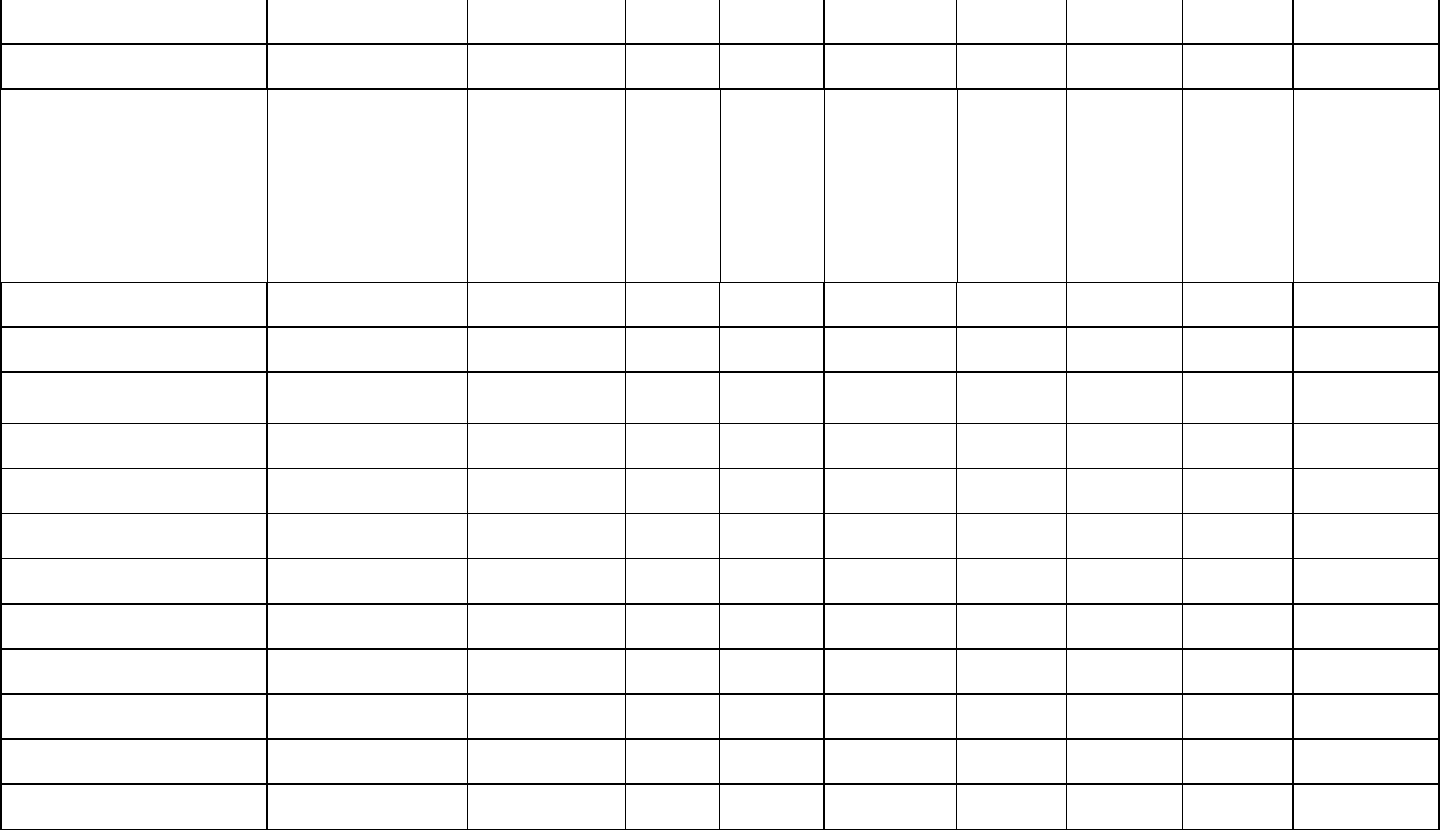
Table 2: Estimates of telencephalic neuron counts (N; excluding the olfactory system) in Mesozoic dinosaurs. Our inferences are compared with those 524
presented by Herculano-Houzel (2023; HH) if respected species were included in both studies (see text for the rationale of our sample composition). 525
Minimum and maximum estimates based on both avian and non-avian sauropsid regressions are provided. 526
Species Group MBr range (g)
N
(non
-
avian_
min)
N
(non
-
avian_m
ax)
N
(avian_min)
N
(avian_m
ax)
MBr (g),
HH
N
(non
-
avian),
HH
N (avian), HH
Archaeopteryx lithographica
Non
-
avian
maniraptoriform
1.52-1.52 16,2M 16,2M 56,5M 56,5M 1.47-1.76
15.8M
-
17.7M
54.2M-62.1M
Bambiraptor feinbergi
Non-avian
maniraptoriform
14.00 69,2M 69,2M 277,7M 277,7M 14 62.9M 295.8M
Citipati osmolskae
Non
-
avian
maniraptoriform
22.05 93,2M 93,2M 384,6M 384,6M 22.62 84.4M 424.5M
Khaan mckennai
Non
-
avian
maniraptoriform
8.80 51,1M 51,1M 199,1M 199,1M 8.83 47.4M 209.1M
Ornithomimus edmontonicus
Non
-
avian
maniraptoriform
49.89-49.89 159,1M 159,1M 690,7M 690,7M 87.85 193.6M 1,179M
Shuvuuia deserti
Non
-
avian
maniraptoriform
1.52
16.1M 16.1M 56.5M 56.5M 0.83 11.2M 35.2M
Stenonychosaurus inequalis
Non-avian
maniraptoriform
38.65
134.1M 134.1M 575.2M 575.2M 41 121.4M 664.4M
Acrocanthosaurus atokensis
Non
-
maniraptoriform
theropod
38.05-51.55 133,2M 162,5M 568,8M 707,2M 191 311.3M 2,116M
Allosaurus fragilis
Non
-
maniraptoriform
theropod
30.54-41.37 115,3M 140,7M 485,8M 603,9M 168 287.8M 1,921M
Carcharodontosaurus
saharicus
Non
-
maniraptoriform
theropod
69.44-94.08 197,5M 241M 875,6M 1,088M
Carnotaurus sastrei
Non
-
maniraptoriform
theropod
33.57-45.49 122,7M 149,7M 519,9M 646,5M
Giganotosaurus carolinii
Non
-
maniraptoriform
theropod
69.75-94.50 198,1M 241,7M 878,4M 1,092M
Majungasaurus
crenatissimus
Non
-
maniraptoriform
theropod
27.69-37.51 108,2M 131,9M 452,9M 563,1M
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Sinraptor dongi
Non
-
maniraptoriform
theropod
29.45-39.90 112,6M 137,4M 473,3M 588,5M
Tarbosaurus bataar
Non
-
maniraptoriform
theropod
49.35-66.86 157,9M 192,7M 685,4M 852,1M
Tyrannosaurus rex
AMNH
5029
Non
-
maniraptoriform
theropod
118.35-160.34 280,1M 341,7M 1,283M 1,595M 343 445.5M 3,289M
Tyrannosaurus rex
AMNH
5117 (Morhardt, 2016)
Non
-
maniraptoriform
theropod
178.37
365M 365M 1722.1M 1722.1M
Tyrannosaurus rex
AMNH
5117
Non
-
maniraptoriform
theropod
97.23-131.73 246,2M 300,4M 1,114M 1,385M
Tyrannosaurus rex
FMNH
PR 2081
Non
-
maniraptoriform
theropod
128.40-173.96 295,4M 360,5M 1,360M 1,691M 202 322.2M 2,207M
Amargasaurus cazaui
Non
-
theropod
dinosaur
26.04-35.28 103,9M 126,8M 433,4M 538,8M
Apatosaurus sp.
Non
-
theropod
dinosaur
31.77-43.04 118,4M 144,4M 499,8M 621,4M
Buriolestes schultzi
Non-theropod
dinosaur
0.75-1.02 10.2M 12.4M 34.1M 42.5M
Diplodocus sp.
Non
-
theropod
dinosaur
31.00-42.00 116,4M 142,1M 491,1M 610,6M 57 148.5M 851.4M
Edmontosaurus annectens
Non
-
theropod
dinosaur
93.00-126.00 239,2M 291,9M 1,079M 1,342M 150 268.5M 1,764M
Euoplocephalus tutus
Non
-
theropod
dinosaur
25.64-34.73 102,9M 125,5M 428,6M 532,8 41 121.4M 664.4M
Giraffatitan brancai
Non
-
theropod
dinosaur
96.10-130.20 244,4M 298,2M 1,105M 1,374M 186 306.3M 2,075M
Hypacrosaurus altispinus
Non
-
theropod
dinosaur
85.53-115.88 226,4M 276,2M 1,016M 1,264M
Iguanodon bernissartensis
Non
-
theropod
dinosaur
110.67-149.94 268,1M 327,1M 1,223M 1,520M 125 240.2 M 1.538 M
Kentrosaurus aethiopicus
Non
-
theropod
dinosaur
16.31-22.09 76,5M 93,3M 309,8M 385,2M 24 87.5M 443.9M
Protoceratops andrewsi
Non
-
theropod
dinosaur
9.30-12.60 52,9M 64,5M 207,1M 257,5M 28 96.1M 498.5M
Psittacosaurus lujiatunensis
Non
-
theropod
dinosaur
4.43-6.01 32.5M 39.6M 121.8M 151.4M
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Stegosaurus ungulatus
Non
-
theropod
dinosaur
19.90-26.96 87,1M 106,3M 357,4M 444,3M 22.5 84.1M 422.8M
Thescelosaurus neglectus
Non-theropod
dinosaur
8.57-11.61 49.9M 60.9M 195.4M 242.9M
Triceratops sp.
Non
-
theropod
dinosaur
70.91-96.08 199.5M 243.4M 888.9M 1105.1M 72.2 171.7M 1,017M
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Phylogenetic modeling of neurological variables 527
We used data from extant sauropsids to place brain size variables for Mesozoic dinosaurs 528
into their phylogenetic context. To examine variations in the relative size of the brain in fossil 529
taxa and to calculate potential neuronal scaling regimes in extinct dinosaurs, we relied on 530
log-transformed published data on brain mass, telencephalon mass and telencephalic 531
neuronal numbers in extant groups (see below). Allometric equations were calculated with 532
least squares linear regressions using phylogenetic generalized least squares (PGLS) to 533
account for phylogenetic relatedness (Garland and Ives, 2000). PGLS allows the covariance 534
matrix to be modified to accommodate the degree to which trait evolution deviates from 535
Brownian motion, through a measure of phylogenetic correlation, Pagel’s
(Pagel, 1999). 536
PGLS and maximum likelihood estimates of
were performed using the ape (Paradis & 537
Schliep, 2019) and nlme (Pinheiro et al., 2017) packages in R.
538
To compare differences in relative brain size across groups, phylogenetically corrected 539
ANCOVA with Tukey post hoc comparisons were performed using a modified version of the 540
multcomp package (Hothorn et al., 2015; modification allowed outputs of the nlme package 541
(gls objects) to be processed). Because of the uncertainty in estimating both brain mass and 542
body mass in Mesozoic dinosaurs, we opted to test for inter-group differences in two 543
datasets: One with the greatest possible relative brain size i.e., the lowest body mass 544
estimate (lower PPE) for each species and the brain mass estimated from the highest 545
assumed endocranial fill (42%), and one with the lowest relative brain size i.e. the highest 546
body mass estimates (upper PPE) and the lowest assumed endocranial fill (31%). Since we 547
assumed that the brain filled 100% of the endocranial cavity in maniraptoriform theropods, 548
inferred relative brain mass for these species was only affected by differences in the applied 549
body mass estimates. 550
As mentioned above, an important assumption of Herculano-Houzel (2023) is that theropods 551
in general had relative brain sizes similar to birds. However, there is notable discontinuity in 552
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
relative brain size and brain morphology between maniraptoriforms and more basal non-553
maniraptoriform theropods (Fig. 1, Balanoff et al., 2013). Because of this, we divided our 554
sample of Mesozoic theropods into these two groups (Tabs. 1 & 2). For T. rex, mean values 555
for the three available adult brain mass and corresponding body mass estimates were used. 556
We grouped Sauropodomorpha and Ornithischia together as non-theropod dinosaurs and 557
compared relative brain size in this group with that in the two theropod samples. PGLS 558
models of brain mass vs. body mass with clade as a covariate were used to test if relative 559
brain size in these groups differs significantly between them and from extant birds and/or 560
non-avian sauropsids. Relative brain size data for 63 extant non-avian sauropsids (including 561
lepidosaurs, crocodilians, and turtles) and 84 bird species (not including members of the 562
large-brained clade Telluraves) were derived from Hurlburt (1996), Chentanez et al. (1983) 563
and Roese-Miron et al. (2023) and are listed in Supplementary File 2. Importantly, these 564
sources provide brain mass estimates excluding the olfactory tracts and bulbs and thus fit 565
our brain size estimates for Mesozoic dinosaurs. 566
To test for differences in relative brain size we built a phylogenetic tree for all 175 fossil and 567
extant species. To construct the phylogeny of bird species, we extracted 1,000 fully resolved 568
trees from birdtree.org (Jetz et al., 2012) using the Hackett et al. (2008) backbone, and built 569
a maximum clade credibility (MCC) tree using phangorn (Schliep, 2011). For lepidodaurs, we 570
followed Kverková et al. (2022) by using a species level time-calibrated phylogeny (Tonini et 571
al., 2016) and built a MCC tree the same way as we did for birds. For phylogenetic 572
information on turtles and crocodilians, we relied on the Timetree of Life (Kumar et al., 573
2017). We then stitched the trees together manually, using the divergence times from the 574
Timetree of Life. For Mesozoic dinosaurs (31 species) we used an updated version of the 575
composite phylogeny of Benson et al. (2014, 2018). Phylogenies for fossil dinosaurs, extant 576
non-avian sauropsids, and birds were stitched together manually using Mesquite (Maddison 577
& Maddison, 2023). We opted to set all branch lengths to 1. This was done because clade-578
specific trees were obtained from various sources applying different phylogenetic methods, 579
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

which, together with issues related to the precise dating of some of the fossils covered, 580
makes it difficult to have well calibrated branch lengths. Importantly, simulation studies have 581
found that independent contrasts and PGLS are robust to errors in both phylogenetic 582
topology and branch lengths, so that we do not expect uniform branch lengths to 583
compromise our analyses (Díaz-Uriarte & Garland 1998; Symonds 2002; Martins & 584
Housworth 2002; Stone 2011). 585
Tree building procedures were the same for telencephalic neuron count regressions, but 586
trees used here included branch lengths. We calculate regression lines between brain mass 587
and telencephalic number of neurons for extant non-telluravian birds and non-avian 588
sauropsids. Analogous to Herculano-Houzel (2023), these regressions were then used to 589
estimate telencephalic neuron counts in dinosaurs, applying either an avian or a reptilian 590
scaling regime. Since our estimates are based on brain portion endocasts that exclude the 591
olfactory system, our telencephalic neuron counts correspond to the pallium and subpallium. 592
Data on whole brain and telencephalic neuron counts as well as on total telencephalic and 593
brain mass (including olfactory tract and bulbs, since neuron count data excluding these 594
structures are currently unavailable for the taxa considered here) for birds (n = 112) were 595
derived from Kverková et al. (2022) and Sol et al. (2022), for non-avian sauropsids (n = 108) 596
from Kverková et al. (2022) and for mammals (n = 39) from Herculano-Houzel et al. (2015). 597
The dataset is included in Supplementary File 3. 598
To get a more precise estimate of the possible number of telencephalic neurons in T. rex, we 599
also modeled scaling regimes for telencephalon mass vs. telencephalic neuron numbers in 600
extant sauropsids (non-avian sauropsids and non-telluravian birds), using the same 601
references listed above. We then calculated telencephalic neuron numbers in T. rex using 602
the obtained scaling regimes and applying the telencephalic volumes estimated with a 603
comparative 3D landmark approach by Morhardt (2016) (referred to as “cerebral 604
hemispheres” therein, excluding olfactory bulbs and tracts) for specimen AMNH FR 5117. 605
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Based on the estimates of Morhardt (2016), we also comparatively assessed the mass of the 606
telencephalon and cerebellum in T. rex. 607
We want to note that our neuron count estimates might be biased by the fact that we predict 608
neuron numbers in the pallium and subpallium (telencephalon excluding the olfactory 609
system) based on total telencephalic neuron counts (including the olfactory system) here. 610
This is an issue that in parts also applies to Herculano-Houzel (2023) and which we cannot 611
circumvent due to limitations of the available raw data. 612
613
Results 614
Relative brain size. 615
We did not recover notably large relative brain sizes in large-bodied theropods like T. rex. 616
Instead, our analyses suggest that these animals had relative brain dimensions comparable 617
to extant non-avian sauropsids such as lizards and crocodilians, as did Mesozoic dinosaurs 618
outside of the clade Theropoda (Table 3). Relative brain sizes similar to those of extant birds 619
appear to only have emerged among the maniraptoriform theropods: PGLS models showed 620
a significant difference in relative brain size (intercept) between non-maniraptoriform 621
theropods, such as T. rex, and the more bird-like Maniraptoriformes, which tended to have 622
larger brains than other dinosaurs (PGLS, max: F
4,172
= 9.49, p 0.0001, = 0.707); min: 623
F
4,172
= 14.03, p 0.0001, = 0.707; Fig. 4a-b). Post-hoc analysis shows that both the 624
maximum and minimum relative brain size estimates for non-maniraptoriform theropods like 625
T. rex are not significantly different from what would be expected from extant non-avian 626
sauropsids (Tab. 3). However, both minimum and maximum relative brain size estimates for 627
these animals are significantly smaller than what would be expected for extant birds (Tab. 3; 628
Fig. 4). On the other hand, we found that maniraptoriforms show no significant differences in 629
relative brain size compared to birds (Tab. 3) regardless of whether maximum or minimum 630
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
relative brain size was assumed (Tab. 3; Fig 4; note that some maniraptoriforms such as 631
Shuvuuia deserti cluster with non-avian sauropsids rather than with birds, though). In 632
contrast to maniraptoriforms, other theropods did not exhibit significantly larger brains than 633
the non-theropod dinosaurs of the clades Sauropodomorpha and Ornithischia (Tab. 3), data 634
for which we pool here. Relative brain sizes in these dinosaurs were not recovered to differ 635
notably from those of non-avian sauropsids. However, if minimum figures are assumed, their 636
relative brain sizes would have been notably small, approaching a significant difference to 637
extant non-avian sauropsids (Tab. 3). 638
Table 3: Tukey post hoc comparisons for a phylogenetic ANCOVA testing for differences in relative 639
brain size between groups of fossil dinosaurs and extant sauropsids. Significant p-values are shown 640
in bold. 641
Minimum relative brain size
Aves Non-avian
Maniraptoriformes
Non-maniraptoriform
Theropoda
Non-theropod
Dinosauria
Non-avian
Maniraptoriformes
0.57
Non-maniraptoriform
Theropoda
> 0.00001 > 0.00001
Non-theropod
Dinosauria
> 0.00001 > 0.00001
0.103
Non-avian Sauropsida
> 0.00001 0.033
0.96 0.051
Maximum relative brain size
Aves Non-avian
Maniraptoriformes
Non-maniraptoriform
Theropoda
Non-theropod
Dinosauria
Non-Avian 0.61
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
642
Table 4: Regression parameters for different models describing the scaling of neurological traits in 643
extant non-avian sauropsids (“reptiles”) and non-telluravian birds. Pagel’s (ranging between 0 - 1) 644
was used to quantify the phylogenetic signal. See methods for details. SE = standard error. 645
646
Model Slope (SE) Intercept (SE)
Avian GLS log(Tel Neurons N) ~ log(Brain Mass) 0.821 (0.043) 17.5 (0.063) 0
Avian PGLS Log(Tel Neurons N) ~ log(Brain Mass) 0.717 (0.05) 17.55 (0.12) 0.50
Reptilian GLS Log(Tel Neurons N) ~ log(Brain Mass) 0.615 (0.03) 16.347 (0.06) 0
Reptilian PGLS Log(Tel Neurons N) ~ log(Brain Mass) 0.655 (0.03) 16.324 (0.17) 0.82
Avian PGLS log(Brain Mass) ~ log(Body Mass) 0.584 (0.02) -2.584 (0.25) 0.96
Reptilian PGLS log(Brain Mass) ~ log(Body Mass) 0.56 (0.03) -4.077 (0.26) 0.70
647
Numbers of neurons 648
We re-calculated estimates for the number of forebrain neurons in Mesozoic dinosaurs 649
based on PGLS-derived regressions of brain size vs. number of telencephalic neurons in 650
extant non-avian sauropsids and birds. Our neuron count estimates are listed in Table 2 and 651
Maniraptoriformes
Non-Maniraptoriform
Theropoda
0.000106 0.0015
Non-theropod
Dinosauria
> 0.00001 > 0.00001
0.103
Non-avian Sauropsida
> 0.00001 0.0033
0.91 0.79
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
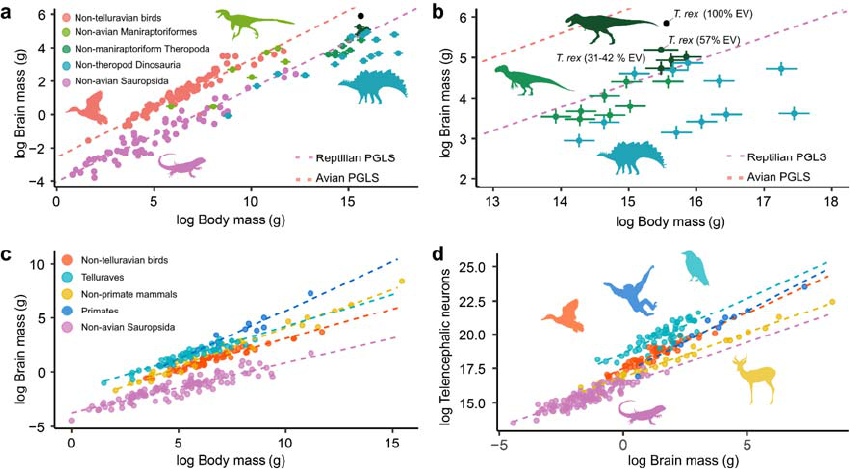
are compared to those of Herculano-Houzel (2023), whereas regression parameters are 652
provided in Table 4. While many of the estimates do not differ notably from one another, the 653
differences for some taxa, especially large theropods, are striking. For T. rex, Herculano-654
Houzel (2023) provided an estimate of 300-450 M forebrain neurons if modeled based on 655
extant non-avian sauropsids, and 2-3B based on an avian regression. In contrast, we 656
estimated a range of 245-360M neurons with a reptilian regression and ~1-2 B with an avian 657
one (Tab. 2, Fig. 5). Using the forebrain volumes estimated for T. rex by Morhardt (2016), we 658
predict 133-166 M telencephalic neurons in this species if applying a reptilian scaling and 659
0.989 to 1.25 B based on an avian scaling (Fig. 5). 660
661
662
Figure 4: Relative brain size and forebrain neuronal numbers in Mesozoic dinosaurs and other
663
amniotes. a.The log-transformed mass of the brain is plotted as a function of the mass of the body for
664
extant and fossil sauropsids. In the case of fossil species, the mean body and/or brain size is shown
665
along with standard deviations. The orange dotted line represents the regression line for avian
666
species (excluding the large-brained clade Telluraves) obtained from PGLS while the pink one
667
represents the same for extant non-avian sauropsids (“reptiles'' in the colloquial sense). b. A detail of
668
the plot shown in a. to illustrate the range of relative brain size in Tyrannosaurus rex and other
669
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
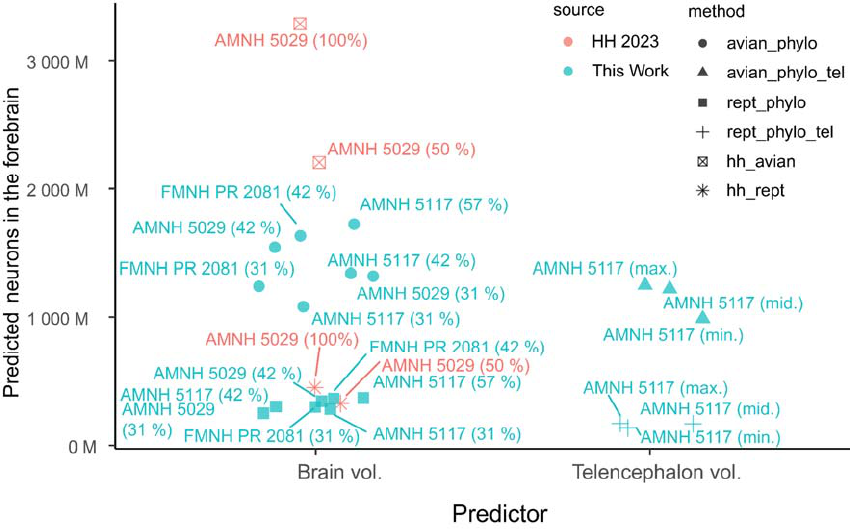
Mesozoic dinosaurs that we consider plausible. Besides our own brain size estimates, the plot
670
contains those from Morhardt (2016) (specimen AMNH FR 5117, endocranial fill = 57%) and Balanoff
671
et al. (2013) (specimen AMNH 5029, endocranial fill = 100%, assumed MBd = 5840 kg) c. Plot
672
showing log-transformed brain mass for different groups of extant amniotes plotted against body
673
mass. d. Plot showing log-transformed numbers of telencephalic neurons as a function of the mass of
674
the forebrain, illustrating neuronal density. Note that non-avian sauropsids and non-primate mammals
675
differ only moderately from one another here, although mammals have markedly larger brains relative
676
to body size, as shown in c. See methods for data sources. Silhouettes were taken from PhyloPic
677
(listed clockwise from top left): Anas (in public domain) Morunasaurus (in public domain),
678
Dromaeosaurus (in public domain), Stegosaurus (by Matt Dempsey), Allosaurus (by Tasman Dixon),
679
Tyrannosaurus (by Matt Dempsey), Corvus (in public domain), Hylobates (by Kai Caspar), Antidorcas
680
(by Sarah Wernig).
681
682
683
Figure 5: Predicted numbers of neurons in telencephalon (excluding olfactory tracts and
684
bulbs) of Tyrannosaurus rex. Points represent the estimated number of neurons in three adult
685
specimens of T. rex using different inference methods. Estimates from this study using a regression
686
that takes phylogenetic relationships into account (PGLS, see methods, filled circle, triangle, square
687
and cross) are plotted in cyan. The estimates from Herculano-Houzel (2023) based on a non-
688
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
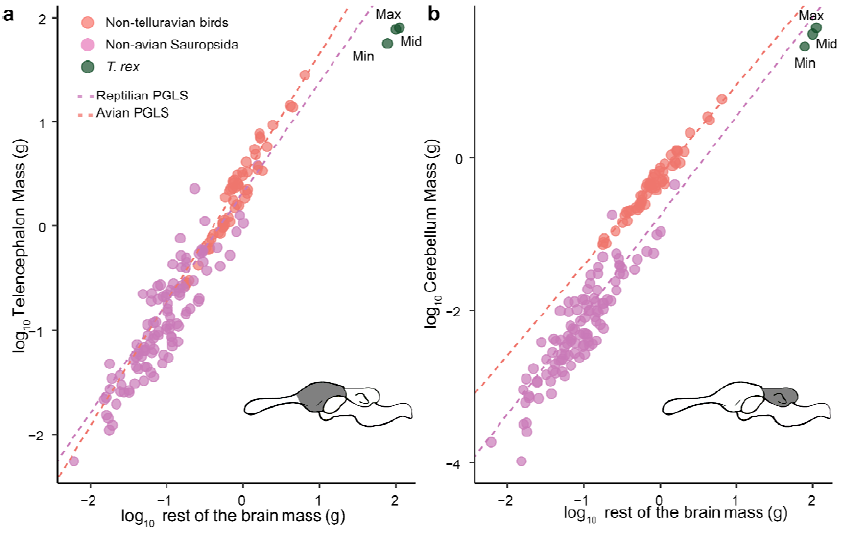
phylogenetic regression are shown in red (crossed square, asterisk). Different underlying ratios of
689
brain volume:endocranial volume are annotated. On the left predicted numbers of forebrain neurons
690
(based on either the avian or extant non-avian sauropsid scaling regime) based on the estimated
691
volume of the brain portion of the endocast are shown. On the right, analogous to that, the predicted
692
numbers of telencephalic neurons based on forebrain volumetric estimates by Morhardt (2016) is
693
plotted.
694
695
696
Figure 6: Relative size of telencephalon (excluding olfactory bulb and tracts) and cerebellum in
697
T. rex. a: The log-transformed mass of the telencephalon in extant non-telluravian birds and non-
698
avian sauropsids is plotted as a function of the mass of the rest of the brain (total brain -
699
telencephalon - cerebellum volume). Green dots show the maximum, mid and minimum estimates for
700
the mass of the T. rex telencephalon as estimated from digital endocasts of AMNH 5117 by
Morhardt
701
(2016).
The orange dotted line represents the regression line for non-telluravian bird species obtained
702
from PGLS while the pink represents the same for non-avian sauropsids. B: Analogous plot to a, but
703
for the cerebellum. Note that telencephalic mass in extant species includes the olfactory bulb and
704
tracts.
705
706
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Discussion of empirical results 707
We want to emphasize two aspects of our empirical findings that contrast with those of 708
Herculano-Houzel (2023). Firstly, we did not find relative brain size to separate non-709
maniraptoriform theropods such as T. rex from extant non-avian sauropsids like crocodilians 710
and lizards or other large-bodied dinosaurs outside the clade Theropoda; rather, our data 711
support a grade shift in this trait between maniraptoriform and non-maniraptoriform 712
theropods, which at least in parts relates to an increase in endocranial fill. As we have 713
argued beforehand, we see no support for the brains of non-maniraptoriform theropods, 714
sauropodomorphs, and most ornithischians to have filled the endocranial cavity in a bird-like 715
fashion. However, we are aware that such a condition, or one that is at least intermediate 716
between modern birds and crocodilians has been proposed for all of these groups at one 717
point (e.g., Knoll & Schwarz-Wings, 2009; Morhardt, 2016; Balanoff et al., 2013; Knoll et al., 718
2021; see Supplementary File 1 Part C for further comments on that topic). Obviously, future 719
research might significantly change our understanding of endocranial tissue organization in 720
Mesozoic dinosaurs and thus challenge the assumptions that we make here. For the time 721
being, however, we consider our crocodilian-based inferences more plausible and 722
parsimonious than the alternative suggestions proposed so far. 723
Our approach suggests that relative brain size in all dinosaurs, except for the majority of 724
maniraptoriform theropods, does not differ significantly from values present in extant non-725
avian reptiles. These results agree with previous conclusions (Hurlburt et al., 2013; 726
Morhardt, 2016). Nonetheless, we want to stress that it remains unclear how meaningful the 727
transfer of brain size scaling rules established for the given extant bird (32g – 120kg) and 728
non-avian sauropsid (1g – 71kg) datasets to large-bodied dinosaurs actually is. Brain-body 729
size ratios in extant cetaceans drop dramatically in taxa that evolved multi-ton body masses 730
(Tartarelli & Bisconti, 2006), suggesting that allometric trajectories need to be accounted for. 731
However, the restricted body mass spectrum of extant birds and reptiles as well as the 732
limited availability of large-bodied crocodilians and turtles for neurological research hinders 733
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
the compilation of such datasets for sauropsids. Furthermore, the scarcity of complete and 734
adult dinosaur endocasts from taxa that also preserve stylopodial elements to derive body 735
mass estimates from, limits our understanding of differences in brain size scaling between 736
taxa. Different clades of mammals and birds have been shown to have distinct allometric 737
relationships for relative brain size (Ksepka et al., 2020; Smaers et al., 2021). The same 738
might have been the case in non-avian dinosaurs, biasing comparisons between the 739
groupings we selected here. In addition to that, there might also be temporal effects on 740
relative brain size. Such a phenomenon appears to be rampant in mammalian evolution 741
during the Cenozoic (Bertrand et al., 2022). To our knowledge, this pattern has not been 742
properly described yet in other vertebrate groups but should be considered in future studies 743
on brain evolution in long-lived clades such as dinosaurs. 744
Secondly, our empirical findings do not support Herculano-Houzel’s (2023) claim of 745
exceptionally high telencephalic neuron counts in dinosaurs, particularly in T. rex and other 746
large theropods. Instead, T. rex likely did not exhibit more than approximately 1.5 B (or at a 747
maximum 2 B) telencephalic neurons, even when an avian neuronal density is assumed. If 748
we assume reptilian neuronal densities, it might even have exhibited neuron numbers an 749
order of magnitude lower than the 3.3 B suggested by Herculano-Houzel (2023). Apart from 750
the difficulty of estimating brain mass from a dinosaurian endocast, there is one additional 751
caveat to our neuron count estimates that needs to be acknowledged and that also applies 752
to Herculano-Houzel’s (2023) study: Telencephalic neuron numbers can only be reliably 753
derived from total brain mass when the proportions of the studied brains are comparable. 754
Since brain morphology in many dinosaurian lineages differs significantly from both extant 755
birds and non-avian sauropsids (Fig. 1, Paulina-Carabajal et al., 2023), biases are thus 756
ingrained into such estimates. Volumetric modeling of brain regions from endocasts, on 757
which we relied here for T. rex exclusively, could potentially ameliorate this problem to some 758
degree (Morhardt, 2016) but it is challenging and not yet widely used. For T. rex, such 759
inferences yield lower telencephalic neuron numbers than would be hypothesized based on 760
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
our total brain volume estimates, if reptilian scaling rules are applied (Fig. 5). They overlap if 761
an avian neuron count scaling is assumed (Fig. 5). 762
We want to emphasize that there is little reason to assume that the brains of non-763
maniraptoriform theropods such as T. rex had a telencephalic neuronal density similar to that 764
of extant birds. In living sauropsids, relative brain size is positively correlated with neural 765
density (Kverková et al., 2022). We show that this measure likely did not differ significantly 766
between those theropods and extant non-avian sauropsids. Consequently, relative brain size 767
cannot be used as an argument to defend elevated neuron densities in these animals. The 768
presence of endothermy in dinosaurs (see below) does also not entail avian neuronal 769
density (contra Herculano-Houzel, 2023): Similar to birds, mammals have evolved 770
endothermy and exhibit large relative brain sizes (Fig. 4C; Tsuboi et al., 2018). Furthermore, 771
they display a unique multilayered cerebral neuroarchitecture (Briscoe & Ragsdale, 2018). 772
Yet their average forebrain neuronal density is only moderately elevated compared to extant 773
non-avian sauropsids (at least if anthropoid primates are not considered) and there is a 774
broad overlap in neuronal density between the groups (Fig. 4D; Kverková et al., 2022), 775
indicating remarkable conservatism in this trait. With respect to birds, however, the typical 776
mammalian telencephalic neuron density is remarkably low (Fig. 4D). Interestingly, brain cell 777
densities (suggestive of high neuron counts but including endothelial and glia cells) on par 778
with or even higher than those of telluravian birds have recently been identified among 779
ectotherm teleost fish, with comparatively small relative brain sizes (Estienne et al., 2024). 780
All of this suggests that metabolic rate and neuronal density are not tightly coupled and that 781
endothermy cannot be used as a proxy for the latter. Finally, the shape of the endocast and 782
volumetric estimates of its forebrain and cerebellar portions (compare Fig. 6) suggest that 783
the brains of T. rex and other large non-maniraptoriform theropods were not dissimilar to 784
those of extant crocodilians (Rogers, 1998; Hurlburt et al., 2013; Morhardt, 2016), which 785
reflect the plesiomorphic archosaurian condition (Fabbri & Bhullar, 2022). Given this 786
morphological conservatism and the rather static neuron densities of non-avian amniotes, it 787
appears appropriate to assume reptilian neuronal densities for these animals. 788
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
It is tempting to speculate that the increased neuronal density that sets extant birds apart 789
from other sauropsids and mammals coevolved with the marked changes in brain 790
morphology and size that occurred in maniraptoriform theropods. If we indeed assume that 791
an avian-like brain organization and high neuronal density emerged early within this clade’s 792
history, it is plausible that the Mesozoic dinosaurs with the highest neuron counts, perhaps 793
above the extant avian range, are represented by the largest-bodied taxa within this group 794
(for which no complete endocasts are currently available). These include bizarre animals 795
such as the immense ornithomimosaur Deinocheirus mirificus (~ 7 t), the scythe-clawed 796
Therizinosaurus cheloniformis (~ 5 t) and the giant oviraptorosaur Gigantoraptor erlianensis 797
(~ 2 t). Alternatively, the emergence of volancy in small maniraptoriforms similar to 798
Archaeopteryx might have driven the evolution of elevated neuron densities, since active 799
flight likely imposes constraints on skull and brain size (Olkowicz et al., 2016; Shatkovska & 800
Ghazali, 2021). However, the lack of reliable morphological markers to infer neuron density 801
renders all these notions speculative. Such a vagueness is inherent to predictions about the 802
biology of extinct taxa without close living relatives and obviously needs to be 803
acknowledged. The main argument for assuming avian neuronal densities in any group of 804
Mesozoic dinosaurs is that the emergence of this trait within the avian stem-lineage cannot 805
be reliably dated and thus might have significantly preceded the origins of crown birds. 806
Hence, both a non-avian sauropsid and an avian neuron density (as well as intermediate 807
conditions) could principally be justified for dinosaurs, although we advocate for the former if 808
taxa outside the Maniraptoriformes are concerned. Importantly, however, even if we had 809
robust evidence for high neuron counts in Mesozoic dinosaurs, this would by no means 810
automatically suggest exceptional cognitive capacities. 811
812
General discussion - implications for neuron count and brain size estimates for 813
vertebrate paleontology 814
1) Are neuron counts good predictors of cognitive performance? 815
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
To infer cognitive abilities in extinct animals from neuron count estimates for the whole brain 816
or pallium, we first need to be assured that this measure can give us meaningful insight into 817
behaviors of extant ones. However, while there is some evidence for effects of pallial neuron 818
counts on species-level cognitive performance in primates (Deaner 2007 - but see below) 819
and birds (impulse inhibition - Herculano-Houzel, 2017; but see Kabadayi et al. 2017 for 820
conflicting evidence; foraging-related innovativeness – Sol et al., 2022; but consider 821
limitations on how innovativeness is measured – Logan et al., 2018), the available data do 822
not provide consistent support for the hypothesis that more neurons per se enhance 823
cognition (Barron & Mourmourakis, 2023). As an example, Güntürkün et al. (2017) reviewed 824
the performance of domestic pigeons (Columba livia), corvids and anthropoid primates in a 825
number of cognitive tasks with the aim to determine if a “cognitive hierarchy” between the 826
three groups exists. They note that pallial neuron counts in corvids are about 2–6 times 827
lower than in large-bodied monkeys and apes but 6–17 times higher than in pigeons. Thus, 828
one would predict major increases in cognitive capacities from pigeons to corvids to 829
anthropoids. Yet, corvids typically perform on par with anthropoid primates (see also 830
Kabadayi et al. 2016 and Pika et al. 2020), and pigeons do so as well in some cognitive 831
dimensions, such as numerical competence and short-term memory (Güntürkün et al., 832
2017). In addition, standardized testing of various primate species suggests that small-833
brained lemurs with comparatively low neuronal densities (Kverková et al., 2022), monkeys 834
and great apes rival each other in a number of cognitive dimensions (Schmitt et al., 2012; 835
Fichtel et al., 2020). In fact, findings that report the influence of absolute brain size (and thus 836
neuron number) on cognitive performance in primates (Deaner et al. 2007) have failed 837
replication so far (Fichtel et al. 2020). As a final example, we want to point out large-bodied 838
dolphin species, which have remarkably high neocortical neuron counts (Globicephala melas 839
- 37 B, Orcinus orca - 43 B; Ridgway et al., 2019). Although neuron numbers in these 840
animals vastly exceed those of humans (15-20 B), there is no evidence that cetacean 841
cognition is on par or even superior to that of our species (e.g., Manger, 2013; Güntürkün, 842
2014). Hence, even immense differences in telencephalic neuron numbers do not 843
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

necessarily create cognitive divides and their value in predicting cognitive performance is 844
remarkably limited. 845
The case becomes even more untenable when we take more complex cognitive phenomena 846
into consideration, such as habitual tool use. Remarkably, Herculano-Houzel (2023) 847
suggested that this might be within the realm of possibility for large theropods such as T. rex, 848
as it is for primates and telluravian birds. However, tool use even within these groups is rare, 849
especially if the more rigorous definition of “tooling” (requiring the deliberate management of 850
a mechanical interface, see Fragaszy & Mangalam, 2018) is employed: this occurs in only 9 851
avian and 20 primate genera (Colbourne et al., 2021). While it is true that telencephalon size 852
in birds has an association with tool use (Lefebvre et al., 2002), this correlation does not hold 853
any predictive power in the sense that all birds with a certain-sized telencephalon exhibit tool 854
use. Even within corvids, which telencephalic neuron counts and sophisticated cognitive 855
abilities overlap with those of anthropoid primates (Olkowicz et al., 2016; Ströckens et al., 856
2022), New Caledonian crows (Corvus moneduloides), and Hawaiian ‘alal
crows (C. 857
hawaiiensis) are the only species known to employ and manufacture tools in the wild. 858
Notably, both species inhabit remote islands, and they share unusually straight beaks and 859
greater binocular overlap than other crows, which are thought to be specific morphological 860
adaptations to enable tool use (Troscianko et al., 2012; Rutz et al., 2016). A similar situation 861
can be observed in parrots. These birds display the highest avian telencephalic neuron 862
counts (Olkowicz et al., 2016; Kverková et al., 2022; Ströckens et al., 2022), and a greatly 863
enlarged medial spiriform nucleus, which acts as an interface between the pallium and the 864
cerebellum, enabling enhanced motor cognition (Gutiérrez-Ibáñez et al., 2018). However, 865
the Tanimbar corella (Cacatua goffiniana) is the only parrot known to be a sophisticated tool 866
user in the wild (O’Hara et al., 2021); tellingly, the Tanimbar corella also inhabits an isolated 867
Indonesian archipelago. Cases like these indicate that while there might be a chance that a 868
gross neuron count threshold must be met for such sophisticated vertebrate tool use to 869
emerge (a notion we would reject since ants evolved remarkable tool use skills with brains 870
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
that are small and few in neurons even for the standard of hymenopteran insects - Godfrey 871
et al., 2021), it is highly unlikely to happen without sufficient ecological pressure, and the 872
differences between tool using and non-tool using species are likely too subtle to detect via 873
measurement of neuronal quantities. 874
Considering these findings, it is unsurprising that taxa converging in neuronal counts often 875
differ markedly in cognition and behavior. Herculano-Houzel (2023) ranked her neuronal 876
count estimates for large theropods against those of anthropoid primates, but she might as 877
well have done so for giraffes (1.7 B neurons), which exceed tool-proficient capuchins (1.1 878
B) and corvids (0.4-1.2 B) in telencephalic neuron numbers, rivaling macaques (0.8 - 1.7 B) 879
(Olkowicz et al., 2016). We know little about giraffes’ cognitive abilities (Caicoya et al., 880
2018), but it would be appropriate to be skeptical of any claim that they might exhibit 881
“macaque-like” cognition based simply on that measure. Too many other biological traits 882
divide these taxa, perhaps most strikingly body size. While we agree with many 883
contemporary authors that relative brain size per se is a flawed measure of cognitive 884
complexity (e.g., Van Schaik et al., 2021), it must not be ignored. This is especially true if 885
comparisons between primates and Mesozoic dinosaurs are drawn, since the species 886
concerned may differ in body mass by several orders of magnitude. Contrary to the 887
assumptions of Herculano-Houzel (2023), the size of the telencephalon and number of its 888
neurons must be related to the dimensions of the body, because it processes sensory, 889
visceral, and motoric information, which scale with body size (Chittka & Niven, 2009; Van 890
Schaik et al., 2021). This fact is clearly reflected by the pronounced intra- as well as 891
interspecific body size-dependent scaling of brain size in vertebrates (Tsuboi et al., 2018; 892
Ksepka et al., 2020; Van Schaik et al., 2021; Bertrand et al., 2022), which can hardly be 893
explained otherwise. Relative brain size and body size are thus not negligible variables in 894
comparative cognition and need to be considered in paleoneurology. 895
The confounding factor of body size on neurological measures might be mitigated by 896
calculating clade-specific portions of telencephalic mass dedicated to somatic functions (the 897
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
regulation of visceral, sensory and motor processes unrelated to cognition) based on 898
intraspecific variation (Triki et al., 2021; Van Schaik et al., 2021) or by focusing on neuron 899
counts in brain regions that are evidently not involved in somatic processing (Herculano-900
Houzel, 2017; Logan et al., 2018). In fact, a number of studies, particularly in birds, were 901
able to associate intraspecific differences in certain cognitive dimensions to localized 902
neurological variation, making this approach a promising one (discussed by Logan et al., 903
2018). At the same time however, the great intra- and interspecific heterogeneity in brain 904
tissue architecture and neurochemistry enormously complicates any interspecific 905
extrapolations (Logan et al., 2018; Barron & Mourmourakis, 2023). Thus, researchers cannot 906
translate these findings to extinct species with any tolerable degree of certainty. This issue is 907
of special relevance when comparing sauropsids with mammals. The mammalian forebrain 908
exhibits a layered cortex but the pallium of extant sauropsids (and thus likely Mesozoic 909
dinosaurs) is largely nuclear in organization. As the forebrain increases in size and neuron 910
counts, a cortical organization can reduce axon length (and therefore processing time and 911
energetic demands) by bringing adjacent areas closer together through folding, something 912
that is impossible in a nuclear organization (see Reiner, 2023 for an extensive review). 913
Neuron counts corresponding to major brain regions, whether empirically determined or 914
estimated, dramatically simplify neuronal tissue complexity, as do measures such as 915
absolute brain size or EQ. Based on current evidence, they also represent flawed cognitive 916
proxies that need to be viewed in the broader context of an animal’s ecology, neuroanatomy, 917
connectomics, and neurochemistry (Fields & Stevens-Graham, 2002; Eyal et al., 2016; 918
Logan et al., 2018; Barron & Mourmourakis, 2023, Reiner, 2023). All in all, we want to 919
discourage attempts to predict cognitive performance in extinct species based on endocast-920
derived neuron count estimates. 921
2) Inferring metabolic rate 922
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Apart from inferences about cognition, Herculano-Houzel (2023) claims that relative brain 923
size should be established as a new thermobiological indicator in vertebrate palaeontology: 924
Relatively large brains, as inferred for theropods, should be viewed as indicators of 925
endothermy, while smaller ones, as are found in pterosaurs, sauropodomorphs and many 926
ornithischians, would indicate ectothermy. Whereas overall brain size in vertebrates is 927
indeed correlated with metabolic rate (e.g., Yu et al., 2014 - but also note the extreme 928
variability within ecto- as well as endothermic groups), Herculano-Houzel’s (2023) approach 929
simplifies the matter and ignores a vast body of already available evidence on dinosaur 930
thermobiology. First, as we have extensively discussed here, relative brain size in large 931
theropods was probably markedly smaller than suggested by Herculano-Houzel (2023) and 932
more similar to the condition in extant crocodilians rather than in birds. Second, it is 933
important to point out that there is a spectrum of metabolic rates in vertebrates (Legendre & 934
Davesne, 2020) rather than a dichotomy, as suggested by Herculano-Houzel (2023). 935
Where exactly certain ornithodiran taxa align within this spectrum continues to be debated, 936
but there is consensus that dinosaurs and pterosaurs, despite their in parts iconically small 937
brains, had metabolic rates well above the range of extant ectothermic sauropsids (see 938
references below). Rather than emerging with theropods, contemporary evidence suggests 939
that endothermy evolved in the ornithodiran stem-lineage or even earlier (Legendre et al., 940
2016; Benton, 2021; Grigg et al., 2022) and hence was inherited by pterosaurs and 941
dinosaurs. The extensive data supporting the presence of endothermy across Ornithodira 942
has recently been reviewed by Grigg et al. (2022) and includes the presence of hair-like, 943
sometimes branched, integumentary structures (Benton et al., 2019; Campione et al., 2020), 944
the efficiency of the ornithodiran respiratory system (Wedel, 2006; Butler et al., 2009; 945
Aureliano et al., 2022; Wang et al., 2023), bone histology and high skeletal growth rates (de 946
Ricqlès et al., 2000; Padian et al. 2004; Prondvai et al., 2012; Redelstroff et al. 2013; 947
Legendre et al., 2016), paleoenvironmental data (Druckenmiller et al., 2021), models of 948
locomotor costs (Pontzer et al., 2009) and geochemically-derived thermometric findings 949
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
(Barrick et al., 1996; Dawson et al. 2020; Wiemann et al., 2022). Nevertheless, 950
osteohistological evidence suggests that both theropod and non-theropod ornithodiran taxa 951
varied in their growth and associated metabolic rates (Jenkins et al., 2001; Erickson et al., 952
2009; Redelstroff et al. 2013; D’Emic et al., 2023) and a secondary reduction of metabolic 953
rate in some ornithischian groups appears plausible (Padian et al., 2004; Redelstroff and 954
Sander, 2009; Wiemann et al., 2022), albeit still compatible with an endothermic physiology 955
(Grigg et al., 2022). 956
Overall, we want to emphasize the need for a nuanced perspective on this trait. The 957
assumption that relative brain size alone (even if inferred correctly) can outperform all the 958
aforementioned thermophysiological predictors to infer endothermy appears at best 959
improbable. Its utility to gauge metabolic rate across ornithodiran groups therefore remains 960
highly doubtful and must be viewed in the framework of other, more robust lines of evidence. 961
962
3) Inferring life history traits 963
Finally, Herculano-Houzel (2023) suggests that neuron count estimates can be used to 964
model life history traits in Mesozoic ornithodiran taxa. This notion is based on previous 965
empirical work that showed an association between pallial neuron counts and selected 966
ontogenetic variables in extant mammals and birds (Herculano-Houzel, 2019). Applied to T. 967
rex, the respective equations predict that females reached sexual maturity at an age of 4–5 968
years and that the longevity of the species was 42-49 years (Herculano-Houzel, 2023). 969
These calculations rest on the assumption that T. rex had to have 2.2 – 3.3 billion pallial 970
neurons. As we have shown, this premise appears exceedingly unlikely. Furthermore, the 971
aforementioned life history predictions are contradicted by the fossil evidence: Sexual 972
maturity in extinct nonavian dinosaurs can be estimated histologically by the presence of 973
medullary bone, a tissue that forms as a calcium reservoir for egg shell production and which 974
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
is also seen in female birds (Schweitzer et al., 2005; Woodward et al., 2020). The earliest 975
estimate of sexual maturity in T. rex, as estimated by the presence of medullary bone, is 15 976
years (Woodward et al., 2020; Carr, 2020). If the life history of T. rex was similar to extant 977
American alligators where sexual maturity occurs in animals that attain half of adult size 978
(which would be in line with the available fossil data - Carr, 2020), then the earliest onset of 979
sexual maturity in T. rex happened in its 12th year of life. Based on these lines of evidence, 980
Herculano-Houzel’s (2023) method greatly underestimates the onset of sexual maturity by 8 981
to 11 years. Based on the number of lines of arrested growth in its long bones, which are 982
thought to indicate annual cessations of growth, the chronologically oldest T. rex sampled so 983
far lived up to 33 years (Cullen et al., 2020). Although it is not unreasonable to assume that 984
T. rex lived longer than three decades, there is yet no histological evidence to support that 985
hypothesis. Given that Herculano-Houzel’s (2023) longevity estimate is based on 986
problematic premises, it should not be considered a plausible alternative. 987
In fact, if applied to species other than T. rex, the limitations of the aforementioned approach 988
become even more visible. For instance, if the life history of the sauropod Apatosaurus, a 989
gigantic dinosaur with an adult weight exceeding 30 tonnes, is modeled based on our own 990
neuron count estimates derived from an avian regression and an endocranial fill of 42%, the 991
equations suggest a longevity of only 24.5 years and an onset of sexual maturity at 2.2 years 992
(note that assuming a non-avian sauropsid regression or smaller brain size would result in 993
an even more fast-paced life history prediction). These figures are obviously unfeasible. We 994
are aware that Herculano-Houzel (2023) assumes that sauropods such as Apatosaurus 995
were ectothermic animals so that the given equations could not be applied to them. 996
However, since this notion defies essentially all available evidence on the biology of 997
sauropods (see above), we choose to ignore it here. To conclude, relationships between life 998
history and neurology that were established from a selection of extant mammals and birds 999
cannot be used to reliably infer ontogenetic parameters across non-avian dinosaurs (and 1000
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
potentially other fossil groups). We strongly discourage relying on them in palaeontological 1001
practice. 1002
1003
Beyond endocasts: What are the limits of inference on dinosaur cognition? 1004
If neuron count estimates and other endocast-derived variables do not allow reliable 1005
predictions about the cognitive abilities of non-avian dinosaurs to be made, what other 1006
methods are available? First of all, trace fossils can provide direct evidence on how 1007
dinosaurs exploited their environment and interacted with both hetero- and conspecifics 1008
(e.g., Carpenter et al. 2005; Varricchio et al. 2007; Lockley et al. 2016; Brown et al. 2021). 1009
While such fossils can provide precise and diverse insights into dinosaur behavior, obvious 1010
limitations render perspectives gained from them extremely patchy, nonetheless. 1011
One further way of inferring cognitive traits in dinosaurs is by comparatively studying 1012
relevant behavioral phenomena in living crocodilians and birds, the groups that form their 1013
extant phylogenetic bracket. While such approaches are starting to gain pace (Zeiträg et al., 1014
2023), we are not aware that ethological research could so far identify shared physical or 1015
social cognitive skills in crocodilians and birds that have not also been found in turtles and 1016
squamates (in case such comparative data is indeed available - Zeiträg et al., 2022; Font et 1017
al., 2023). Thus, the behavioral resolution of such approaches appears limited thus far. 1018
Cognitive traits identified exclusively in birds or crocodiles cannot simply be extrapolated to 1019
Mesozoic dinosaurs with any degree of certainty since they might represent crown group 1020
apomorphies. Whereas it might be appealing to hypothesize that cognitive patterns found 1021
among modern palaeognaths are representative for their maniraptoriform forerunners 1022
(Jensen et al., 2023; Zeiträg et al., 2023), this idea is (in most cases) not testable and should 1023
hence not be disseminated uncritically. 1024
Inferences on dinosaur cognition are hindered by the fact that both extant crocodilians and 1025
birds are highly derived in their own ways: Convergently to mammals, birds have not only 1026
evolved an enlarged forebrain and cerebellum, but also extensive connections between 1027
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
these two brain regions (Gutierrez-Ibanez et al., 2018) and descending projections from the 1028
pallium to the brainstem and/or spinal cord (Ulinski and & Margoliash, 1990; Reiner & 1029
Medina, 2000). These circuits are likely essential for enabling various avian behaviors but 1030
are not present in extant non avian sauropsids (Ulinski and & Margoliash 1990; Gutierrez-1031
Ibanez et al., 2023). It remains obscure when they evolved. Crown-group birds also possess 1032
an apomorphic dorsal projection of the telencephalon, the eminentia sagittalis or wulst, 1033
which appears to be absent even from endocasts of derived non-avian maniraptoriforms 1034
such as Archaeopteryx and Stenonychosaurus and is prominently involved in visual 1035
cognition (Walsh & Milner, 2011; Iwaniuk & Wylie, 2020). Crocodilians on the other hand 1036
conserve a plesiomorphic brain morphology and cerebral tissue organization (Briscoe et al., 1037
2018; Briscoe & Ragsdale, 2018). They are unusual in being secondary ectotherms (e.g., 1038
Seymour et al., 2004; Legendre et al., 2016; Botha et al., 2023) and it is unclear how this 1039
might have affected their neurology and cognition. Thus, the extant archosaurian groups 1040
leave us in a rather suboptimal position to infer cognitive traits in non-avian dinosaurs. 1041
Obviously, even the absence of a given cognitive trait in both crocodilians and basal extant 1042
birds like palaeognaths does not refute its existence in Mesozoic dinosaurs, considering the 1043
diversity and long evolutionary history of this group. In fact, a species’ ecology is typically 1044
more indicative of certain behaviors and associated cognitive phenomena than its 1045
phylogenetic affinities. For instance, habitual tool use is an adaptation typically found in 1046
omnivorous extractive foragers (Parker & Gibson, 1977; Parker, 2015) and is only rarely 1047
reported in predators (Shumaker et al., 2011). This is reflected by the fact that the most 1048
common types of tooling actions that have evolved comprise reaching, probing or pounding, 1049
usually in order to access food (Colbourne et al., 2021). It has long been observed that tool 1050
use appears when a species is found in an uncharacteristic niche, for which it lacks the 1051
appropriate morphological adaptations, and thus compensates by using tools to generate a 1052
functionally equivalent behavior (Alcock, 1972; Parker & Gibson, 1977). This is likely why a 1053
number of birds that use tools are found on islands, yet the ability appears absent in their 1054
close mainland relatives (Rutz et al., 2016). Simply put, in order for tool use to evolve, there 1055
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
needs to be a reason for it to evolve, and there are very few ecological contexts where tool 1056
use is a superior adaptation to its morphological equivalent (Hansell & Ruxton, 2008). 1057
Unfortunately, this type of extremely specific contextual information is nearly absent in long 1058
extinct species. From its iconic tooth and jaw morphology, one can confidently predict that a 1059
hypercarnivorous species like T. rex would have no need for tools, but the problem remains 1060
that few assumptions about extinct animal cognition are falsifiable. 1061
In sum, reconstructing cognition in dinosaurs and other fossil taxa without close living 1062
analogs is a challenging endeavor that requires integrative approaches if we are to provide 1063
compelling inferences (de Sousa et al., 2023). Bare neuronal count estimates might be 1064
considered a rather minor contribution to this effort and need to be aligned with data from 1065
comparative anatomy and neurology, ecology, trace fossils, and comparative behavioral 1066
studies on extant animals to offer a plausible picture of cognition in extinct lineages. While 1067
communicating such findings, researchers should acknowledge the limitations of the 1068
presented inferences to allow their audience to delineate between reasoned conclusions and 1069
speculation. In a field such as dinosaur research - avidly followed by popular media and the 1070
public eye - a nuanced view appears especially warranted. 1071
1072
Conclusions 1073
The dinosaurian neuronal count and relative brain size estimates presented by Herculano-1074
Houzel (2023) are inaccurate due to methodological shortcomings, in particular for T. rex. 1075
Accordingly, the biological inferences drawn from them are implausible. As we show here, 1076
there is no compelling evidence that relative brain size in large-bodied theropods differed 1077
significantly from that of extant non-avian sauropsids, and their telencephalic neuron counts 1078
were likely not exceptional, especially for animals of their size. Furthermore, we highlight 1079
issues associated with neuron count estimates in vertebrate paleontology and argue against 1080
their use in reconstructing behavioral and life history variables, especially in animals such as 1081
non-avian dinosaurs, for which disparate neuron densities might be hypothesized based on 1082
different phylogenetic and morphological arguments. 1083
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
For obvious reasons, many inferences we might make about Mesozoic dinosaur behavior 1084
will remain limited. Nevertheless, we can justify certain predictions - to a degree - within 1085
integrative empirical frameworks to which neuron count estimates might well be added in the 1086
future. Before such steps can be taken, however, a substantially improved understanding of 1087
the relationship between neuron counts and other biological variables, especially cognitive 1088
performance, in extant animals is required. 1089
1090
Institutional abbreviations 1091
AMNH = American Museum of Natural History, New York City, New York, United States; 1092
BMNH / NHMUK = Natural History Museum, London, UK; BSP = Bayerische 1093
Staatssammlung für Paläontologie und historische Geologie, Munich, Germany; BYU = 1094
Brigham Young University, Earth Science Museum, Provo, Utah, United States; 1095
CAPPA/UFSM, Centro de Apoio à Pesquisa Paleontológica da Quarta Colônia / 1096
Universidade Federal de Santa Maria, São João do Polêsine, Rio Grande do Sul, Brazil.
1097
CM = Carnegie Museum of Natural History, Pittsburgh, Pennsylvania. CMN = Canadian 1098
Museum of Nature, Ottawa, Ontario, Canada. DINO = Dinosaur National Monument, 1099
Jensen, Utah, United States. FIP - Florida Institute of Paleontology, Palm Beach, Florida, 1100
United States; FMNH = Field Museum of Natural History, Chicago, Illinois, United States; 1101
FPDM = Fukui Prefectural Dinosaur Museum, Fukui, Japan. HMN / MB.R. = Museum für 1102
Naturkunde, Berlin, Germany; IGM = Mongolian Institute of Geology, Ulaan Bator, 1103
Mongolia; IRSNB / RBINS = Institut Royal des Sciences Naturelles de Belgique, Brussels, 1104
Belgium; IVPP = Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, 1105
China; KUVP = Kansas University Natural History Museum, Lawrence, Kansas, United 1106
States; MACN = Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos 1107
Aires, Argentina; MPC-D = Institute of Paleontology and Geology, Mongolian Academy of 1108
Sciences, Ulaan Bator, Mongolia; MUCPv-CH = Museo de la Universidad Nacional del 1109
Comahue, colección del Museo Ernesto Bachmann, Villa El Chocón, Argentina; MOR = 1110
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Museum of the Rockies, Bozeman, Montana, United States; NMC = Canadian Museum of 1111
Nature, Ottawa, Canada; NCSM = North Carolina Museum of Natural Sciences, Raleigh, 1112
North Carolina, United States; OMNH = Sam Noble Museum at the University of 1113
Oklahoma, Norman, Oklahoma, United States; PIN = Paleontological Institute, Russian 1114
Academy of Sciences, Moscow, Russia; PKUP: Peking University Paleontological 1115
Collections, Beijing, China
. ROM = Royal Ontario Museum, Toronto, Ontario, Canada; 1116
RTMP/TMP = Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada; SGM 1117
= Ministere de l’Energie et des Mines, Rabat, Morocco; USNM = Smithsonian National 1118
Museum of Natural History, Washington, D.C., United States; UUVP = University of Utah, 1119
Salt Lake City, Utah, United States; YPM = Yale Peabody Museum, New Haven 1120
Connecticut, United States. 1121
1122
Acknowledgements 1123
We want to thank David Burnham, Gregory M. Erickson, Ariana Paulina-Carabajal, and 1124
Lawrence M. Witmer for sharing valuable information on fossil specimens and Nicolas E. 1125
Campione for recommendations on body mass calculations. Andrew N. Iwaniuk is 1126
acknowledged for helpful discussions on the methodology and structure of the study and 1127
Jonathan Stone for allowing GRH to perform alligator dissections in his lab. Finally, we thank 1128
Stig Walsh and two anonymous reviewers for their thoughtful and constructive comments on 1129
earlier drafts of this manuscript. 1130
1131
References 1132
Alcock, J. (1972). The evolution of the use of tools by feeding animals. Evolution, 26(3), 464-1133
473. 1134
1135
Alonso, P. D., Milner, A. C., Ketcham, R. A., Cookson, M. J., & Rowe, T. B. (2004). The 1136
avian nature of the brain and inner ear of Archaeopteryx. Nature, 430(7000), 666-669. 1137
1138
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Balanoff, A. M., Bever, G. S., & Ikejiri, T. (2010). The braincase of Apatosaurus (Dinosauria: 1139
Sauropoda) based on computed tomography of a new specimen with comments on variation 1140
and evolution in sauropod neuroanatomy. American Museum Novitates, 2010(3677), 1-32. 1141
1142
Balanoff, A. M., Bever, G. S., Rowe, T. B., & Norell, M. A. (2013). Evolutionary origins of the 1143
avian brain. Nature, 501(7465), 93-96. 1144
1145
Balanoff, A., Ferrer, E., Saleh, L., Gignac, P. M., Gold, M. E. L., Marugán-Lobón, J., ... & 1146
Vaska, P. (2024). Quantitative functional imaging of the pigeon brain: implications for the 1147
evolution of avian powered flight. Proceedings of the Royal Society B, 291(2015), 20232172. 1148
1149
Barrick, R. E., Showers, W. J., & Fischer, A. G. (1996). Comparison of thermoregulation of 1150
four ornithischian dinosaurs and a varanid lizard from the Cretaceous Two Medicine 1151
Formation: evidence from oxygen isotopes. Palaios, 11, 295-305. 1152
1153
Baron, M.G., Norman, D.B., & Barrett, P.M. (2017). A new hypothesis of dinosaur 1154
relationships and early dinosaur evolution. Nature, 543, 501-506. 1155
1156
Barron, A. B., & Mourmourakis, F. (2023). The relationship between cognition and brain size 1157
or neuron number. Brain, Behavior and Evolution. https://doi.org/10.1159/000532013 1158
1159
Bennett, S. C. (1995). A statistical study of Rhamphorhynchus from the Solnhofen 1160
Limestone of Germany: year-classes of a single large species. Journal of Paleontology, 1161
69(3), 569-580. 1162
1163
Benton, M. J. (2021). The origin of endothermy in synapsids and archosaurs and arms races 1164
in the Triassic. Gondwana Research, 100, 261-289. 1165
1166
Benton, M.J., Dhouailly, D., Jiang, B., & McNamara, M. (2019). The early origin of feathers. 1167
Trends in Ecology and Evolution. 34(9), 856-869. 1168
1169
Benson, R. B. J., Hunt, G., Carrano, M. T. & Campione, N. (2017). Data from: Cope’s rule 1170
and the adaptive landscape of dinosaur body size evolution. Dryad Digital Repository. 1171
https://doi.org/10.5061/dryad.1t3r4
1172
1173
Benson, R.B., Campione, N. E., Carrano, M. T., Mannion, P. D., Sullivan, C., Upchurch, P. & 1174
Evans, D. C. (2014). Rates of dinosaur body mass evolution indicate 170 million years of 1175
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
sustained ecological innovation on the avian stem lineage. PLoS Biology, 12(5), 1176
p.e1001853. 1177
1178
Benson, R.B., Hunt, G., Carrano, M. T. & Campione, N. (2018). Cope's rule and the adaptive 1179
landscape of dinosaur body size evolution. Palaeontology, 61(1), 13-48. 1180
1181
Bertrand, O. C., Shelley, S. L., Williamson, T. E., Wible, J. R., Chester, S. G., Flynn, J. J., ... 1182
& Brusatte, S. L. (2022). Brawn before brains in placental mammals after the end-1183
Cretaceous extinction. Science, 376(6588), 80-85. 1184
1185
Bever, G. S., Brusatte, S. L., Carr, T. D., Xu, X., Balanoff, A. M., & Norell, M. A. (2013). The 1186
braincase anatomy of the late Cretaceous dinosaur Alioramus (Theropoda: 1187
Tyrannosauroidea). Bulletin of the American Museum of Natural History, 2013(376), 1-72. 1188
1189
Botha, J., Weiss, B. M., Dollman, K., Barrett, P. M., Benson, R. B., & Choiniere, J. N. (2023). 1190
Origins of slow growth on the crocodilian stem lineage. Current Biology, 33(19), 4261-4268. 1191
1192
Briscoe, S. D., & Ragsdale, C. W. (2018). Homology, neocortex, and the evolution of 1193
developmental mechanisms. Science, 362(6411), 190-193. 1194
1195
Briscoe, S. D., Albertin, C. B., Rowell, J. J., & Ragsdale, C. W. (2018). Neocortical 1196
association cell types in the forebrain of birds and alligators. Current Biology, 28(5), 686-696. 1197
1198
1199
Brown, C.M., Currie, P.J., & Therrien, F. (2021) Intraspecific facial bite marks in 1200
tyrannosaurids provide insight into sexual maturity and evolution of bird-like intersexual 1201
display. Paleobiology 48(1), 12-43. 1202
1203
Brusatte, S. L., Carr, T. D., Erickson, G. M., Bever, G. S., & Norell, M. A. (2009). A long-1204
snouted, multihorned tyrannosaurid from the Late Cretaceous of Mongolia. Proceedings of 1205
the National Academy of Sciences, 106(41), 17261-17266. 1206
1207
Buchholtz, E. A., & Seyfarth, E.-A. (2001.) The study of “fossil brains”: Tilly Edinger (1897–1208
1967) and t
he beginnings of paleoneurology. BioScience, 51(8), 674-682. 1209
1210
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Burnham, D. A. (2004). New information on Bambiraptor feinbergi (Theropoda: 1211
Dromaeosauridae) from the Late Cretaceous of Montana. In P. J. Currie, E. B. Koppelhus, 1212
M. A. Shugar & J. L. Wright (eds.), Feathered dragons: studies on the transition from 1213
dinosaurs to birds (pp. 67-111). Indiana University Press, Bloomington. 1214
1215
Butler, R. J., Barrett, P. M., & Gower, D. J. (2009). Postcranial skeletal pneumaticity and air-1216
sacs in the earliest pterosaurs. Biology Letters, 5(4), 557-560. 1217
1218
Button, D. J., & Zanno, L. E. (2023). Neuroanatomy of the late Cretaceous Thescelosaurus 1219
neglectus (Neornithischia: Thescelosauridae) reveals novel ecological specialisations within 1220
Dinosauria. Scientific Reports, 13(1), 19224. 1221
1222
Caicoya, Á. L., Amici, F., Ensenyat, C., & Colell, M. (2019). Object permanence in Giraffa 1223
camelopardalis: First steps in giraffes’ physical cognition. Journal of Comparative 1224
Psychology, 133(2), 207. 1225
1226
Campione, N. E. (2020) MASSTIMATE: Body Mass Estimation Equations for Vertebrates. 1227
Available at: https://cran.r-project.org/web/packages/MASSTIMATE/MASSTIMATE.pdf 1228
1229
Campione, N. E., & Evans, D. C. (2020). The accuracy and precision of body mass 1230
estimation in non
avian dinosaurs. Biological Reviews, 95(6), 1759-1797. 1231
1232
Campione, N. E., Barrett, P. M., & Evans, D. C. (2020). On the ancestry of feathers in 1233
Mesozoic dinosaurs. In C. Foth & O. W. M. Rauhut (eds.), The Evolution of Feathers: From 1234
Their Origin to the Present (pp. 213-243). Springer International Publishing. 1235
1236
Carpenter, K., Sanders, F., McWhinney, L.A., & Wood, L. (2005). Evidence for predator-prey 1237
relationships: examples for Allosaurus and Stegosaurus. In K. Carpenter (ed.), The 1238
Carnivorous Dinosaurs (pp. 325-350). Indiana University Press, Bloomington. 1239
1240
Carr, T. D. (2020). A high-resolution growth series of Tyrannosaurus rex obtained from 1241
multiple lines of evidence. PeerJ, 8, e9192. 1242
1243
Cerroni, M. A., & Paulina-Carabajal, A. (2019). Novel information on the endocranial 1244
morphology of the abelisaurid theropod Carnotaurus sastrei. Comptes Rendus Palevol, 1245
18(8), 985-995. 1246
1247
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Chentanez, T., Huggins, S. E., & Chentanez, V. (1983). Allometric relationships for the 1248
Siamese crocodile, Crocodylus siamensis. Journal of The Science Society of Thailand, 9, 5–1249
26. 1250
1251
Chittka, L., & Niven, J. (2009). Are bigger brains better?. Current Biology, 19(21), R995-1252
R1008. 1253
1254
Colbourne, J. A. D., Auersperg, A. M. I., Lambert, M. L., Huber, L., & Völter, C. J. (2021). 1255
Extending the reach of tooling theory: a neurocognitive and phylogenetic perspective. Topics 1256
in Cognitive Science, 13(4), 548–572. 1257
1258
Cullen, T. M., Canale, J. I., Apesteguía, S., Smith, N. D., Hu, D., & Makovicky, P. J. (2020). 1259
Osteohistological analyses reveal diverse strategies of theropod dinosaur body-size 1260
evolution. Proceedings of the Royal Society B, 287(1939), 20202258. 1261
1262
Cuvier, G. (1812). Recherches sur les ossemens fossiles de quadrupèdes, Vol. 3. Deterville, 1263
Paris. 1264
1265
Dawson, R. R., Field, D. J., Hull, P. M., Zelenitsky, D. K., Therrien, F., & Affek, H. P. (2020). 1266
Eggshell geochemistry reveals ancestral metabolic thermoregulation in Dinosauria. Science 1267
Advances, 6(7), eaax9361. 1268
1269
Deaner, R. O., Isler, K., Burkart, J., & Van Schaik, C. (2007). Overall brain size, and not 1270
encephalization quotient, best predicts cognitive ability across non-human primates. Brain, 1271
Behavior and Evolution, 70(2), 115-124. 1272
1273
D'Emic, M. D., O’Connor, P. M., Sombathy, R. S., Cerda, I., Pascucci, T. R., Varricchio, D., 1274
... & Curry Rogers, K. A. (2023). Developmental strategies underlying gigantism and 1275
miniaturization in non-avialan theropod dinosaurs. Science, 379(6634), 811-814. 1276
1277
Diaz-Uriarte R, Garland T (1998) Effects of branch length errors on the performance of 1278
phylogenetically independent contrasts. Systematic Biology 47: 654–672. 1279
1280
de Ricqlès, A., Padian, K., Horner, J.R., & Francillon-Vieillot, H. 2000. Palaeohistology of the 1281
bones of pterosaurs (Reptilia: Archosauria): anatomy, ontogeny, and biomechanical 1282
implications. Zoological Journal of the Linnean Society 129: 349-385. 1283
1284
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

de Sousa, A. A., Beaudet, A., Calvey, T., Bardo, A., Benoit, J., Charvet, C. J., ... & Wei, Y. 1285
(2023). From fossils to mind. Communications Biology, 6(1), 636. 1286
1287
Druckenmiller, P. S., Erickson, G. M., Brinkman, D., Brown, C. M., & Eberle, J. J. (2021). 1288
Nesting at extreme polar latitudes by non-avian dinosaurs. Current Biology, 31(16), 3469-1289
3478. 1290
1291
Edinger T. (1929) Die fossilen Gehirne. J. Springer, Berlin. 1292
1293
Erickson, G.M., Rauhut, O.W.M., Zhou, Z., Turner, A.H., Inouye, B.D., Hu, D., and Norell, 1294
M.A. (2009). Was dinosaurian physiology inherited by birds? reconciling slow growth in 1295
Archaeopteryx. PLoS ONE 4(10): e7390. 1296
1297
Estienne, P., Simion, M., Hagio, H., Yamamoto, N., Jenett, A., & Yamamoto, K. (2024). 1298
Different ways of evolving tool-using brains in teleosts and amniotes. Communications 1299
Biology, 7(1), 88. 1300
1301
Evans, D.C. 2005. New evidence on brain−endocranial cavity relationships in ornithischian 1302
dinosaurs. Acta Palaeontologica Polonica 50 (3): 617–622. 1303
1304
Evans, D. C., Ridgely, R., & Witmer, L. M. (2009). Endocranial anatomy of lambeosaurine 1305
hadrosaurids (Dinosauria: Ornithischia): a sensorineural perspective on cranial crest 1306
function. The Anatomical Record, 292(9), 1315-1337. 1307
1308
Eyal, G., Verhoog, M. B., Testa-Silva, G., Deitcher, Y., Lodder, J. C., Benavides-Piccione, 1309
R., ... & Segev, I. (2016). Unique membrane properties and enhanced signal processing in 1310
human neocortical neurons. Elife, 5, e16553. 1311
1312
Fabbri, M., & Bhullar, B. A. S. (2022). The endocast of Euparkeria sheds light on the 1313
ancestral archosaur nervous system. Palaeontology, 65(6), e12630. 1314
1315
Fragaszy, D. M., & Mangalam, M. (2018). Tooling. Advances in the Study of Behavior, 1316
2018(50), 177–241. 1317
1318
Franzosa, J., & Rowe, T. (2005). Cranial endocast of the Cretaceous theropod dinosaur 1319
Acrocanthosaurus atokensis. Journal of Vertebrate Paleontology, 25(4), 859-864. 1320
1321
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Fields, R. D., & Stevens-Graham, B. (2002). New insights into neuron-glia communication. 1322
Science, 298(5593), 556-562. 1323
1324
Font, E., García-Roa, R., Pincheira-Donoso, D., & Carazo, P. (2019). Rethinking the effects 1325
of body size on the study of brain size evolution. Brain Behavior and Evolution, 93(4), 182-1326
195. 1327
1328
Font, E., Burghardt, G. M., & Leal, M. (2023). Brains, behaviour, and cognition: multiple 1329
misconceptions. In C. Warwick, P. C. Arena, & G. M. Burghardt (eds.), Health and Welfare of 1330
Captive Reptiles (pp. 211-238). Springer International Publishing, Cham. 1331
1332
Galton, P. M. (2001). Endocranial casts of the plated dinosaur Stegosaurus (Upper Jurassic, 1333
Western USA): a complete undistorted cast and the original specimens of Othniel Charles 1334
Marsh. In K. Carpenter (ed.), The Armored Dinosaurs (pp. 103–129). Indiana University 1335
Press, Bloomington. 1336
1337
Garland Jr, T., Harvey, P. H., & Ives, A. R. (1992). Procedures for the analysis of 1338
comparative data using phylogenetically independent contrasts. Systematic Biology, 41(1), 1339
18-32. 1340
1341
Garland, Jr, T., & Ives, A. R. (2000). Using the past to predict the present: confidence 1342
intervals for regression equations in phylogenetic comparative methods. The American 1343
Naturalist, 155(3), 346-364. 1344
1345
Gatesy, S. M. (1991). Hind limb scaling in birds and other theropods: implications for 1346
terrestrial locomotion. Journal of Morphology, 209(1), 83-96. 1347
1348
Giffin, E. B. (1989). Pachycephalosaur paleoneurology (Archosauria: Ornithischia). Journal 1349
of Vertebrate Paleontology, 9(1), 67-77. 1350
1351
Gignac, P.M. & Erickson, G.M. (2017). The biomechanics behind extreme osteophagy in 1352
Tyrannosaurus rex. Scientific Reports 7(1), 2012. 1353
1354
Godfrey, R. K., Swartzlander, M., & Gronenberg, W. (2021). Allometric analysis of brain cell 1355
number in Hymenoptera suggests ant brains
diverge from general trends. Proceedings of 1356
the Royal Society B, 288(1947), 20210199. 1357
1358
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Grigg, G., Nowack, J., Bicudo, J. E. P. W., Bal, N. C., Woodward, H. N., & Seymour, R. S. 1359
(2022). Whole
body endothermy: ancient, homologous and widespread among the 1360
ancestors of mammals, birds and crocodylians. Biological Reviews, 97(2), 766-801. 1361
1362
Güntürkün, O. (2014). Is dolphin cognition special?. Brain, Behavior and Evolution, 83(3), 1363
177-180. 1364
1365
Güntürkün, O., Ströckens, F., Scarf, D., & Colombo, M. (2017). Apes, feathered apes, and 1366
pigeons: differences and similarities. Current Opinion in Behavioral Sciences, 16, 35-40. 1367
1368
Gutiérrez-Ibáñez, C., Iwaniuk, A. N., & Wylie, D. R. (2018). Parrots have evolved a primate-1369
like telencephalic-midbrain-cerebellar circuit. Scientific Reports, 8(1), 1-11 1370
1371
Gutiérrez-Ibáñez, C., Kettler, L., Pilon, M.C., Carr, C.E. & Wylie, D.R., (2023). Cerebellar 1372
inputs in the American alligator (Alligator mississippiensis). Brain Behavior and Evolution, 1373
98(1):.44-60. 1374
1375
Hackett, S. J., Kimball, R. T., Reddy, S., Bowie, R. C. K., Braun, E. L., Braun, M. J., … Yuri, 1376
T. (2008). A phylogenomic study of birds reveals their evolutionary history. Science, 1377
320(5884), 1763–1768. 1378
1379
Hansell, M., & Ruxton, G. (2008). Setting tool use within the context of animal construction 1380
behaviour. Trends in Ecology & Evolution, 23(2), 73–78. 1381
1382
Henderson, D. M. (2023). Growth constraints set an upper limit to theropod dinosaur body 1383
size. The Science of Nature, 110(1), 4. 1384
1385
Herculano
Houzel, S. (2011). Brains matter, bodies maybe not: the case for examining 1386
neuron numbers irrespective of body size. Annals of the New York Academy of Sciences, 1387
1225(1), 191-199. 1388
1389
Herculano-Houzel, S. (2017). Numbers of neurons as biological correlates of cognitive 1390
capability. Current Opinion in Behavioral Sciences, 16(2017):1–7. 1391
1392
Herculano
Houzel, S. (2019). Longevity and sexual maturity vary across species with 1393
number of cortical neurons, and humans are no exception. Journal of Comparative 1394
Neurology, 527(10), 1689-1705. 1395
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

1396
Herculano-Houzel, S. (2023). Theropod dinosaurs had primate-like numbers of telencephalic 1397
neurons. Journal of Comparative Neurology, 531(9), 962-974. 1398
1399
Herculano-Houzel, S., & Kaas, J. H. (2011). Gorilla and orangutan brains conform to the 1400
primate cellular scaling rules: implications for human evolution. Brain, Behavior and 1401
Evolution, 77(1), 33-44. 1402
1403
Herculano-Houzel, S., Ribeiro, P., Campos, L., Valotta da Silva, A., Torres, L. B., Catania, K. 1404
C., & Kaas, J. H. (2011). Updated neuronal scaling rules for the brains of glires 1405
(Rodents/Lagomorphs). Brain, Behavior and Evolution, 78(4), 302-314. 1406
Herculano-Houzel, S., Manger, P. R., & Kaas, J. H. (2014). Brain scaling in mammalian 1407
evolution as a consequence of concerted and mosaic changes in numbers of neurons and 1408
average neuronal cell size. Frontiers in Neuroanatomy, 8, 77. 1409
1410
Herculano-Houzel, S., Catania, K., Manger, P. R., & Kaas, J. H. (2015). Mammalian brains 1411
are made of these: a dataset of the numbers and densities of neuronal and nonneuronal 1412
cells in the brain of glires, primates, scandentia, eulipotyphlans, afrotherians and 1413
artiodactyls, and their relationship with body mass. Brain, behavior and evolution, 86(3-4), 1414
145-163. 1415
1416
Holtz, T.R. (1996). Phylogenetic taxonomy of the Coelurosauria (Dinosauria: Theropoda). 1417
Journal of Palaeontology, 70(3), 536-538. 1418
1419
Hopson, J.A. (1979). Paleoneurology. In C. Gans, R. G. Northcutt, & P. Ulinski (eds.), 1420
Biology of the Reptilia, Volume 9 (pp. 39–146). Academic Press, New York. 1421
1422
Hothorn, T., Bretz, F., Westfall, P., Heiberger, R. M., Schuetzenmeister, A., & Scheibe, S. 1423
(2015). Package “multcomp”. Available at: 1424
https://cran.r-project.org/web/packages/multcomp/multcomp.pdf
1425
1426
Hu, K., King, J. L., Romick, C. A., Dufeau, D. L., Witmer, L. M., Stubbs, T. L., ... & Benton, 1427
M. J. (2021). Ontogenetic endocranial shape change in alligators and ostriches and 1428
implications for the development of the non
avian dinosaur endocranium. The Anatomical 1429
Record, 304(8), 1759-1775. 1430
1431
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Hurlburt, G. R. (1996). Relative brain size in recent and fossil amniotes: determination and 1432
interpretation. PhD thesis, University of Toronto, Toronto, Canada (pp. 250). 1433
1434
Hurlburt, G.R., & Waldorf, L. (2002). Endocast volume and brain mass in a size series of 1435
alligators. Journal of Vertebrate Paleontology, 23, (Supplement to 3), 69A. 1436
1437
Hurlburt, G. R., Ridgely, R. C., & Witmer, L. M. (2013) Relative size of brain and cerebrum in 1438
tyrannosaurid dinosaurs: an analysis using brain-endocast quantitative relationships in 1439
extant alligators. In J. M. Parrish, R. E. Molnar, P. J.Currie, & E. B. Koppelhus (eds.), 1440
Tyrannosaurid Paleobiology (pp. 134–154). Indiana University Press, Bloomington. 1441
1442
Iwaniuk, A. N., & Nelson, J. E. (2002). Can endocranial volume be used as an estimate of 1443
brain size in birds? Canadian Journal of Zoology, 80(1), 16-23. 1444
1445
Iwaniuk, A. N., & Wylie, D. R. (2020). Sensory systems in birds: What we have learned from 1446
studying sensory specialists. Journal of Comparative Neurology, 528(17), 2902-2918. 1447
1448
Jenkins Jr, F. A., Shubin, N. H., Gatesy, S. M., & Padian, K. E. V. I. N. (2001). A diminutive 1449
pterosaur (Pterosauria: Eudimorphodontidae) from the Greenlandic Triassic. Bulletin of the 1450
Museum of Comparative Zoology, 156(1), 151-170. 1451
1452
Jensen, T. R., Zeiträg, C., & Osvath, M. (2023). The selfish preen: absence of allopreening 1453
in Palaeognathae and its socio-cognitive implications. Animal Cognition, 26, 1467–1476. 1454
1455
Jerison, H. J. (1973). Evolution of the Brain and Intelligence. Academic Press, New York. 1456
1457
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., & Mooers, A. O. (2012). The global 1458
diversity of birds in space and time. Nature, 491(7424), 444-448. 1459
1460
Jirak, D., & Janacek, J. (2017). Volume of the crocodilian brain and endocast during 1461
ontogeny. PloS ONE, 12, 6, e0178491. 1462
1463
Kabadayi, C., Taylor, L. A., von Bayern, A. M., & Osvath, M. (2016). Ravens, New 1464
Caledonian crows and jackdaws parallel great apes in motor self-regulation despite smaller 1465
brains. Royal Society Open Science, 3(4), 160104. 1466
1467
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Kabadayi, C., Krasheninnikova, A., O'Neill, L., van de Weijer, J., Osvath, M., & von Bayern, 1468
A. M. (2017). Are parrots poor at motor self-regulation or is the cylinder task poor at 1469
measuring it?. Animal Cognition, 20, 1137-1146. 1470
1471
Knoll, F., Buffetaut, E., & Buelow, M. (1999). A theropod braincase from the Jurassic of the 1472
Vaches Noires cliffs (Normandy, France); osteology and palaeoneurology. Bulletin de la 1473
Société géologique de France, 170(1), 103-109. 1474
1475
Knoll, F., Lautenschlager, S., Kawabe, S., Martínez, G., Espílez, E., Mampel, L., & Alcalá, L. 1476
(2021). Palaeoneurology of the early cretaceous iguanodont Proa valdearinnoensis and its 1477
bearing on the parallel developments of cognitive abilities in theropod and ornithopod 1478
dinosaurs. Journal of Comparative Neurology, 529(18), 3922-3945. 1479
1480
Knoll, F. & Schwarz-Wings, D. (2009) Palaeoneuroanatomy of Brachiosaurus. Annales de 1481
Paléontologie, 95(3), 165–175. 1482
1483
Ksepka, D. T., Balanoff, A. M., Smith, N. A., Bever, G. S., Bhullar, B. A. S., Bourdon, E., ... & 1484
Smaers, J. B. (2020). Tempo and pattern of avian brain size evolution. Current Biology, 1485
30(11), 2026-2036. 1486
1487
Kumar, S., Stecher, G., Suleski, M., & Hedges, S. B. (2017). TimeTree: a resource for 1488
timelines, timetrees, and divergence times. Molecular Biology and Evolution, 34(7), 1812-1489
1819. 1490
1491
Kverková, K., Marhounová, L., Polonyiová, A., Kocourek, M., Zhang, Y., Olkowicz, S., ... & 1492
N
mec, P. (2022). The evolution of brain neuron numbers in amniotes. Proceedings of the 1493
National Academy of Sciences, 119(11), e2121624119. 1494
1495
Larsson, H. C., Sereno, P. C., & Wilson, J. A. (2000). Forebrain enlargement among 1496
nonavian theropod dinosaurs. Journal of Vertebrate Paleontology, 20(3), 615-618. 1497
1498
Legendre, L. J., & Davesne, D. (2020). The evolution of mechanisms involved in vertebrate 1499
endothermy. Philosophical Transactions of the Royal Society B, 375(1793), 20190136. 1500
1501
Legendre, L.J., Guénard, G., Botha-Brink, J., & Cubo, J. (2016). Palaeohistological evidence 1502
for ancestral high metabolic rate in archosaurs. Systematic Biology 65(6): 989-996. 1503
1504
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Lauters, P., Coudyzer, W., Vercauteren, M., & Godefroit, P. (2012). The brain of Iguanodon 1505
and Mantellisaurus: perspectives on ornithopod evolution. In P. Godefroit (ed.), Bernissart 1506
dinosaurs and early Cretaceous terrestrial ecosystems (pp 213–224). Indiana University 1507
Press, Bloomington. 1508
1509
Lefebvre, L., Nicolakakis, N., & Boire, D. (2002). Tools and brains in birds. Behaviour, 1510
139(7), 939–973. 1511
1512
Lockley, M.G., McCrea, R.T., Buckley, L.G., Lim, J.D., Matthews, N.A., Breithaupt, B.H., 1513
Houck, K.J., Gierli
ski, G.D., Surmik, D., Kim, K.S., Xing, L., Kong, D.Y., Cart, K., Martin, J., 1514
& Hadden, G. (2016). Theropod courtship: large scale physical evidence of display arenas 1515
and avian-like scrape ceremony behaviour by Cretaceous dinosaurs. Scientific Reports 1516
6:18952 1517
1518
Logan, C. J., Avin, S., Boogert, N., Buskell, A., Cross, F. R., Currie, A., ... & Montgomery, S. 1519
H. (2018). Beyond brain size: Uncovering the neural correlates of behavioral and cognitive 1520
specialization. Comparative Cognition & Behavior Reviews. 1521
1522
Maddison, W. P. & Maddison, D. R. (2023). Mesquite: a modular system for evolutionary 1523
analysis. Available at: http://www.mesquiteproject.org
1524
1525
Manger, P. R. (2013). Questioning the interpretations of behavioral observations of 1526
cetaceans: is there really support for a special intellectual status for this mammalian order?. 1527
Neuroscience, 250, 664-696. 1528
1529
Marsh, O. C. (1879). History and methods of palaeontological discovery. American Journal 1530
of Science, 3(107), 323-359. 1531
1532
Martins, E. P., & Housworth, E. A. (2002). Phylogeny shape and the phylogenetic 1533
comparative method. Systematic biology, 51(6), 873-880. 1534
1535
Medina, L. & Reiner, A. (2000). Do birds possess homologues of mammalian primary visual, 1536
somatosensory and motor cortices?. Trends in Neurosciences, 23(1): 1-12. 1537
1538
Morhardt, A. C. (2016). Gross anatomical brain region approximation (GABRA): assessing 1539
brain size, structure, and evolution in extinct archosaurs. PhD thesis, Ohio University, Ohio, 1540
United States. 1541
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

1542
Müller, R. T., Ferreira, J. D., Pretto, F. A., Bronzati, M., & Kerber, L. (2021). The endocranial 1543
anatomy of Buriolestes schultzi (Dinosauria: Saurischia) and the early evolution of brain 1544
tissues in sauropodomorph dinosaurs. Journal of Anatomy, 238(4), 809-827. 1545
1546
Nesbitt, S.J. (2011). The early evolution of archosaurs: relationships and the origin of major 1547
clades. Bulletin of the American Museum of Natural History, 2011(352), 1-292. 1548
1549
Ngwenya, A., Patzke, N., Spocter, M. A., Kruger, J. L., Dell, L. A., Chawana, R., ... & 1550
Manger, P. R. (2013). The continuously growing central nervous system of the Nile crocodile 1551
(Crocodylus niloticus). The Anatomical Record, 296(10), 1489-1500. 1552
1553
Ngwenya, A., Patzke, N., Manger, P. R., & Herculano-Houzel, S. (2016). Continued growth 1554
of the central nervous system without mandatory addition of neurons in the Nile crocodile 1555
(Crocodylus niloticus). Brain Behavior and Evolution, 87(1), 19-38. 1556
1557
O’Hara, M., Mioduszewska, B., Mundry, R., Yohanna, Haryoko, T., Rachmatika, R., 1558
Prawiradilaga, D. M., Huber, L., & Auersperg, A. M. I. (2021). Wild Goffin’s cockatoos flexibly 1559
manufacture and use tool sets. Current Biology, 31(20), 4512-4520.e6. 1560
1561
Olkowicz, S., Kocourek, M., Lu
an, R. K., Porteš, M., Fitch, W. T., Herculano-Houzel, S., & 1562
N
mec, P. (2016). Birds have primate-like numbers of neurons in the forebrain. Proceedings 1563
of the National Academy of Sciences, 113(26), 7255-7260. 1564
1565
Osborn, H. F. (1912). Crania of Tyrannosaurus and Allosaurus. Memoirs of the American 1566
Museum of Natural History, Volume. 1, New Series, 1, 1-30. 1567
1568
Osmólska, H. (2004). Evidence on relation of brain to endocranial cavity in oviraptorid 1569
dinosaurs. Acta Palaeontologica Polonica, 49(2), 321-324. 1570
1571
Padian, K., Horner, J.R., & De Ricqlès, A. 2004. Growth in small dinosaurs and pterosaurs: 1572
the evolution of the archosaurian growth strategies. Journal of Vertebrate Paleontology 1573
24(3): 555-571. 1574
1575
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401(6756), 1576
877-884. 1577
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Paradis, E., & Schliep, K. (2019). ape 5.0: an environment for modern phylogenetics and 1578
evolutionary analyses in R. Bioinformatics, 35(3), 526-528. 1579
1580
Parker, S. T. (2015). Re-evaluating the extractive foraging hypothesis. New Ideas in 1581
Psychology, 37, 1–12. 1582
1583
Parker, S. T., & Gibson, K. R. (1977). Object manipulation, tool use and sensorimotor 1584
intelligence as feeding adaptations in cebus monkeys and great apes. Journal of Human 1585
Evolution, 6(7), 623–641. 1586
1587
Paulina-Carabajal, A., Bronzati, M., & Cruzado-Caballero, P. (2023) Paleoneurology of non-1588
avian dinosaurs: an overview. In M. T. Dozo, A. Paulina-Carabajal, T. E. Macrini, & S. Walsh 1589
(eds.), Paleoneurology of Amniotes : New Directions in the Study of Fossil Endocasts (pp. 1590
267-332). Springer International Publishing, Cham. 1591
1592
Paulina-Carabajal, A., & Canale, J. I. (2010). Cranial endocast of the carcharodontosaurid 1593
theropod Giganotosaurus carolinii Coria & Salgado, 1995. Neues Jahrbuch fur Geologie und 1594
Palaontologie, 258, 249-256. 1595
1596
Paulina-Carabajal, A., & Currie, P.J. (2012). New information on the braincase and endocast 1597
of Sinraptor dongi (Theropoda: Allosauroidea): Ethmoidal region, endocranial anatomy and 1598
pneumaticity. Vertebrata PalAsiatica, 50, 85–101 1599
1600
Paulina Carabajal, A., Carballido, J. L., & Currie, P. J. (2014). Braincase, neuroanatomy, and 1601
neck posture of Amargasaurus cazaui (Sauropoda, Dicraeosauridae) and its implications for 1602
understanding head posture in sauropods. Journal of Vertebrate Paleontology, 34(4), 870-1603
882. 1604
1605
Persons IV, W. S., Currie, P. J., & Erickson, G. M. (2020). An older and exceptionally large 1606
adult specimen of Tyrannosaurus rex. The Anatomical Record, 303(4), 656-672. 1607
1608
Picasso, M. B., Tambussi, C. P., & Degrange, F. J. (2011). Virtual reconstructions of the 1609
endocranial cavity of Rhea americana (Aves, Palaeognathae): postnatal anatomical 1610
changes. Brain Behavior and Evolution, 76(3-4), 176-184. 1611
1612
Picasso, M. B. J. (2012). Postnatal ontogeny of the locomotor skeleton of a cursorial bird: 1613
greater rhea. Journal of Zoology, 286(4), 303-311. 1614
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

1615
Pika, S., Sima, M. J., Blum, C. R., Herrmann, E., & Mundry, R. (2020). Ravens parallel great 1616
apes in physical and social cognitive skills. Scientific Reports, 10(1), 1-19. 1617
1618
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., Heisterkamp, S., and Van Willigen, B. 1619
(2017). Package "nlme". Available at: https://cran.r-project.org/web/packages/nlme/nlme.pdf. 1620
1621
Pontzer, H., Allen, V., & Hutchinson, J.R. (2009). Biomechanics of running indicates 1622
endothermy in bidepal dinosaurs. PLoS ONE 4(12): 10.1371. 1623
1624
Prondvai, E., Stein, K.,
si, A., & Sander, P. M. (2012). Life history of Rhamphorhynchus 1625
inferred from bone histology and the diversity of pterosaurian growth strategies. PLoS ONE 1626
7(2): e31392. 1627
1628
R Core Team (2023). R: A language and environment for statistical computing. R 1629
Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-1630
project.org/. 1631
1632
Redelstroff, R. & Sander, P.M. (2009). Long and girdle bone histology of Stegosaurus: 1633
implications for growth and life history. Journal of Vertebrate Paleontology 29(4): 1087-1099. 1634
1635
Redelstroff, R., Hübner, T.R., Chinsamy, A., & Sander, P. M. (2013). Bone histology of the 1636
stegosaur Kentrosaurus aethiopicus (Ornithischia: Thyreophora) from the Upper Jurassic of 1637
Tanzania. Evolutionary Biology 296(6): 933-952. 1638
1639
Reiner, A. (2023). Could theropod dinosaurs have evolved to a human level of intelligence?. 1640
Journal of Comparative Neurology, 531(9), 975–1006. 1641
1642
Revell, L. J., Harmon, L. J., & Collar, D. C. (2008). Phylogenetic signal, evolutionary 1643
process, and rate. Systematic Biology, 57(4), 591-601. 1644
1645
Roese-Miron, L., Jones, M. E. H., Ferreira, J. D., & Hsiou, A. S. (2023). Virtual endocasts of 1646
Clevosaurus brasiliensis and the tuatara: Rhynchocephalian neuroanatomy and the oldest 1647
endocranial record for Lepidosauria. The Anatomical Record, 1–24. https://doi.org/10.1002/ 1648
ar.25212 1649
1650
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Ridgway, S. H., Brownson, R. H., Van Alstyne, K. R., & Hauser, R. A. (2019). Higher neuron 1651
densities in the cerebral cortex and larger cerebellums may limit dive times of delphinids 1652
compared to deep-diving toothed whales. PLoS One, 14(12), e0226206. 1653
1654
Rogers, S. W. (1998). Exploring dinosaur neuropaleobiology: computed tomography 1655
scanning and analysis of an Allosaurus fragilis endocast. Neuron, 21(4), 673-679. 1656
1657
Rutz, C., Klump, B. C., Komarczyk, L., Leighton, R., Kramer, J., Wischnewski, S., 1658
Sugasawa, S., Morrissey, M. B., James, R., St Clair, J. J. H., Switzer, R. A., & Masuda, B. 1659
M. (2016). Discovery of species-wide tool use in the Hawaiian crow. Nature, 537(7620), 1660
403–407. 1661
1662
Saveliev, S. V., & Alifanov, V. R. (2007). A new study of the brain of the predatory dinosaur 1663
Tarbosaurus bataar (Theropoda, Tyrannosauridae). Paleontological Journal, 41(3), 281-289. 1664
1665
Sakamoto, M. (2022). Estimating bite force in extinct dinosaurs using phylogenetically 1666
predicted physiological cross-sectional areas of jaw adductor muscles. PeerJ, 10: e13731. 1667
1668
Sampson, S. D. & Witmer, L. M. (2007) Craniofacial anatomy of Majungasaurus 1669
crenatissimus (Theropoda: Abelisauridae) From the Late Cretaceous Of Madagascar, 1670
Journal of Vertebrate Paleontology, 27:S2, 32-104. 1671
1672
Schliep, K., Paradis, E., de Oliveira Martins, L., Potts, A., White, T. W., Stachniss, C., & 1673
Kendall, M. (2019). Package ‘phangorn’. Available at: https://cran. r-project. 1674
org/web/packages/phangorn/phangorn. pdf 1675
1676
Schmitt, V., Pankau, B., & Fischer, J. (2012). Old world monkeys compare to apes in the 1677
primate cognition test battery. PloS one, 7(4), e32024. 1678
1679
Schweitzer, M. H., Wittmeyer, J. L., & Horner, J. R. (2005). Gender-specific reproductive 1680
tissue in ratites and Tyrannosaurus rex. Science, 308
(5727), 1456-1460. 1681
1682
S
eymour, R. S., Bennett-Stamper, C. L., Johnston, S. D., Carrier, D. R., & Grigg, G. C. 1683
(2004). Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. 1684
Physiological and Biochemical Zoology, 77(6), 1051-1067. 1685
1686
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Sereno, P.C., Tan, L., Brusatte, S.L., Kriegstein H.J., Zhao, X., & Cloward K. (2009). 1687
Tyrannosaurid skeletal design first evolved at small body size. Science, 326(5951), 418-422. 1688
1689
Shatkovska, O. V., & Ghazali, M. (2021). Relative skull size as one of the factors limiting 1690
skull shape variation in passerines. Canadian Journal of Zoology, 99(12), 1054-1066. 1691
1692
Shumaker, R. W., Walkup, K. R., & Beck, B. B. (2011). Animal Tool Behavior: The Use and 1693
Manufacture of Tools by Animals (revised and updated edition edition). Johns Hopkins 1694
University Press, Baltimore. 1695
1696
Smaers, J. B., Rothman, R. S., Hudson, D. R., Balanoff, A. M., Beatty, B., Dechmann, D. K., 1697
... & Safi, K. (2021). The evolution of mammalian brain size. Science Advances, 7(18), 1698
eabe2101. 1699
1700
Sol, D., Olkowicz, S., Sayol, F., Kocourek, M., Zhang, Y., Marhounová, L., ... & N
mec, P. 1701
(2022). Neuron numbers link innovativeness with both absolute and relative brain size in 1702
birds. Nature Ecology & Evolution, 6(9), 1381-1389. 1703
1704
Spence, R. D., Zhen, Y., White, S., Schlinger, B. A., & Day, L. B. (2009). Recovery of motor 1705
and cognitive function after cerebellar lesions in a songbird – role of estrogens. European 1706
Journal of Neuroscience, 29(6), 1225-1234. 1707
1708
Stone, E. A. (2011). Why the phylogenetic regression appears robust to tree 1709
misspecification. Systematic Biology, 60(3), 245-260. 1710
1711
Ströckens, F., Neves, K., Kirchem, S., Schwab, C., Herculano-Houzel, S., & Güntürkün, O. 1712
(2022). High associative neuron numbers could drive cognitive performance in corvid 1713
species. Journal of Comparative Neurology, 530(10), 1588–1605. 1714
1715
Symonds, M. R. (2002). The effects of topological inaccuracy in evolutionary trees on the 1716
phylogenetic comparative method of independent contrasts. Systematic Biology, 51(4), 541-1717
553. 1718
1719
Tartarelli, G., & Bisconti, M. (2006). Trajectories and constraints in brain evolution in 1720
primates and cetaceans. Human Evolution, 21, 275-287. 1721
1722
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Tonini, J. F. R., Beard, K. H., Ferreira, R. B., Jetz, W., & Pyron, R. A. (2016). Fully-sampled 1723
phylogenies of squamates reveal evolutionary patterns in threat status. Biological 1724
Conservation, 204, 23-31. 1725
1726
Triki, Z., Aellen, M., van Schaik, C. P., & Bshary, R. (2021). Relative brain size and cognitive 1727
equivalence in fishes. Brain, Behavior and Evolution, 96(3), 124-136. 1728
1729
Troscianko, J., von Bayern, A. M. P., Chappell, J., Rutz, C., & Martin, G. R. (2012). Extreme 1730
binocular vision and a straight bill facilitate tool use in New Caledonian crows. Nature 1731
Communications, 3(1), 1110. 1732
1733
Tsuboi, M., van der Bijl, W., Kopperud, B. T., Erritzøe, J., Voje, K. L., Kotrschal, A., ... & 1734
Kolm, N. (2018). Breakdown of brain–body allometry and the encephalization of birds and 1735
mammals. Nature Ecology & Evolution, 2(9), 1492-1500. 1736
1737
Ulinski, P. S. & Margoliash, D. (1990). Neurobiology of the reptile-bird transition. In E. G. 1738
Jones & A. Peters (eds.), Comparative Structure and Evolution of Cerebral Cortex, Part I 1739
(pp. 217-265). Springer, Boston. 1740
1741
Van Schaik, C. P., Triki, Z., Bshary, R., & Heldstab, S. A. (2021). A farewell to the 1742
encephalization quotient: a new brain size measure for comparative primate cognition. Brain, 1743
Behavior and Evolution, 96(1), 1-12. 1744
1745
Varricchio, D.J., Martin, A.J., Katsura, Y. (2007). First trace and body fossil evidence of a 1746
burrowing, denning dinosaur. Proceedings of the Royal Society B 274:1361–1368. 1747
1748
Walsh, S., & Milner, A. (2011). Halcyornis toliapicus (Aves: lower Eocene, England) 1749
indicates advanced neuromorphology in Mesozoic Neornithes. Journal of Systematic 1750
Palaeontology, 9(1), 173-181. 1751
1752
Wang, Y., Claessens, L.P.A.M., & Sullivan, C. (2023). Deep reptilian evolutionary roots of a 1753
major avian respiratory adaptation. Communications Biology 6:3. 1754
1755
Watanabe, A., Gignac, P.M., Balanoff, A.M., Green, T.L., Kley, N.J. & Norell, M.A. (2019). 1756
Are endocasts good proxies for brain size and shape in archosaurs throughout ontogeny?. 1757
Journal of Anatomy, 234, 3, 291-305. 1758
1759
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
Wedel, M.J. (2006). Origin of postcranial skeletal pneumaticity in dinosaurs. Integrative 1760
Zoology, 1(2): 80-85. 1761
1762
Wiemann, J., Menéndez, I., Crawford, J. M., Fabbri, M., Gauthier, J. A., Hull, P. M., ... & 1763
Briggs, D. E. (2022). Fossil biomolecules reveal an avian metabolism in the ancestral 1764
dinosaur. Nature, 606(7914), 522-526. 1765
1766
Witmer, L. M., Chatterjee, S., Franzosa, J., & Rowe, T. (2003). Neuroanatomy of flying 1767
reptiles and implications for flight, posture and behaviour. Nature, 425(6961), 950-953. 1768
1769
Witmer, L. M., Ridgely, R. C., Dufeau, D. L., & Semones, M. C. (2008). Using CT to peer into 1770
the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian 1771
dinosaurs. In H. Endo & R.Frey (eds.), Anatomical Imaging: Towards a New Morphology (pp. 1772
67–87). Springer, Tokyo. 1773
1774
Witmer, L. M., & Ridgely, R. C. (2009). New insights into the brain, braincase, and ear region 1775
of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and 1776
behavior. The Anatomical Record, 292(9), 1266-1296. 1777
1778
Woodward, A., C. T. Moore, & M. F. Delaney. (1991). Experimental Alligator Harvest: Final 1779
Report. Study no. 7567. Bureau of Wildlife Research, Florida Game and Fresh Water Fish 1780
Commission, Tallahassee, 118pp. 1781
1782
Woodward, H. N., Tremaine, K., Williams, S. A., Zanno, L. E., Horner, J. R., & Myhrvold, N. 1783
(2020). Growing up Tyrannosaurus rex: Osteohistology refutes the pygmy “Nanotyrannus” 1784
and supports ontogenetic niche partitioning in juvenile Tyrannosaurus. Science Advances, 1785
6(1), eaax6250. 1786
1787
Yu, Y., Karbowski, J., Sachdev, R. N., & Feng, J. (2014). Effect of temperature and glia in 1788
brain size enlargement and origin of allometric body-brain size scaling in vertebrates. BMC 1789
Evolutionary Biology, 14(1), 1-14. 1790
1791
Zeiträg, C., Jensen, T. R., & Osvath, M. (2022). Gaze following: A socio-cognitive skill rooted 1792
in deep time. Frontiers in Psychology, 13:950935. 1793
1794
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

Zeiträg, C., Reber, S. A., & Osvath, M. (2023). Gaze following in Archosauria — Alligators 1795
and palaeognath birds suggest dinosaur origin of visual perspective taking. Science 1796
Advances, 9(20), eadf0405. 1797
1798
Zelenitsky, D. K., Therrien, F., Ridgely, R. C., McGee, A. R., & Witmer, L. M. (2011). 1799
Evolution of olfaction in non-avian theropod dinosaurs and birds. Proceedings of the Royal 1800
Society B: Biological Sciences, 278(1725), 3625-3634. 1801
1802
Zhou, C. F., Gao, K. Q., Fox, R. C., & Du, X. K. (2007). Endocranial morphology of 1803
psittacosaurs (Dinosauria: Ceratopsia) based on CT scans of new fossils from the Lower 1804
Cretaceous, China. Palaeoworld, 16(4), 285-293. 1805
1806
Supplementary Files 1807
Suppl. File 1: Information on the selection and determination of brain endocast 1808
measurements in this study, including comments on endocast data presented in Jerison 1809
(1973), Hopson (1979) and Hurlburt (1996) and discussions on endocranial fill in non-1810
maniraptoriform dinosaurs. 1811
1812
Suppl. File 2: Brain and body mass data of extant sauropsid species, excluding the olfactory 1813
tracts and bulbs. Brain mass data are derived from Hurlburt (1996), except for those of the 1814
Siamese crocodile, which derive from Chentanez et al. (1983). All birds considered here 1815
belong to non-telluravian taxa. 1816
1817
Suppl. File 3: Neuron count data, brain size measures and respective references for extant 1818
sauropsid and mammalian species. 1819
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
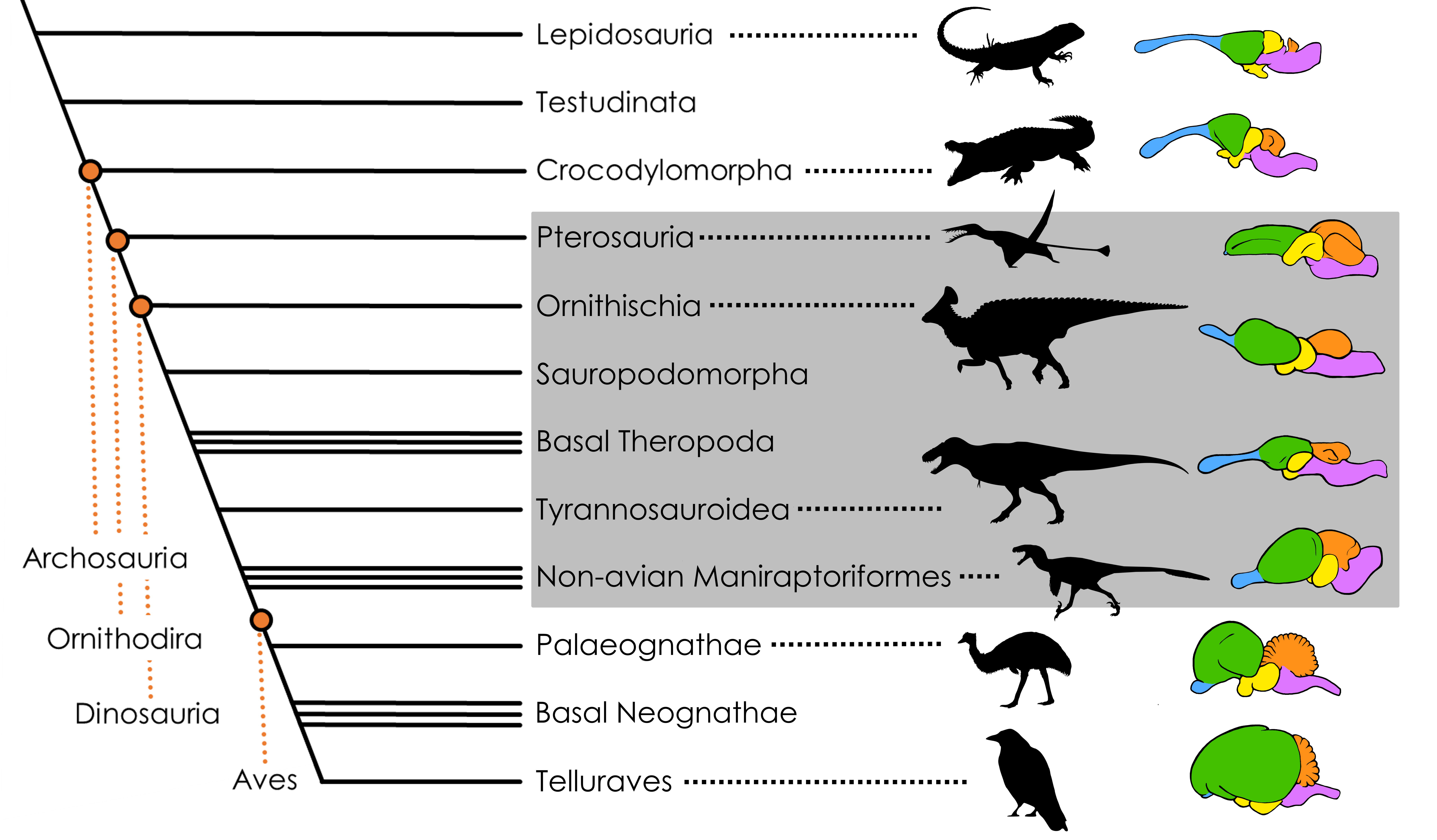
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
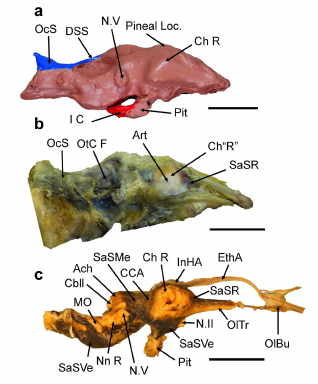
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
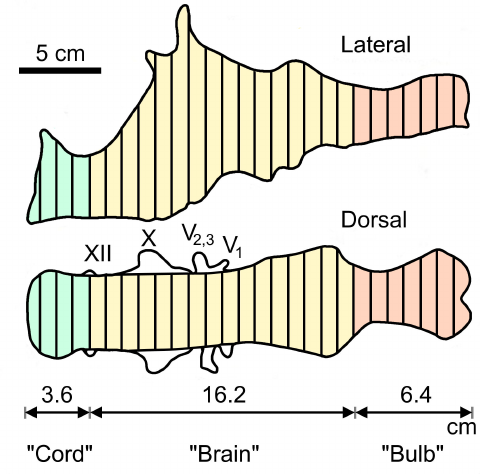
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint

.CC-BY-NC 4.0 International licenseavailable under a
was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprint (whichthis version posted April 3, 2024. ; https://doi.org/10.1101/2024.01.10.575006doi: bioRxiv preprint
