
192 Asia Pac J Clin Nutr 2020;29(1):192-204
Original Article
Reproducibility and construct validity of a food
frequency questionnaire for assessing dietary intake in
rural and urban Asian Indian adults
Vasudevan Sudha MSc
1
, Ranjit Mohan Anjana MD, PhD
2
, Parthasarathy Vijayalakshmi
MSc
1
, Nagarajan Lakshmipriya MSc
1
, Natarajan Kalpana MSc
1
, Rajagopal Gayathri MSc
1
,
Rahavan Durga Priyadarshini MSc
1
, Hutgikar Madhav Malini MSc
1
,
Chandrasekaran Anitha MCA
1
, Mohan Deepa PhD
3
, Sekar Sathish Raj BSc
3
, Kumar
Parthiban MSc
3
, Rajappan Ramakrishnan BSc
3
, Gunasekaran Geetha MSc
1
, Kamala
Krishnaswamy MD
1
, Ranjit Unnikrishnan MD, FRCP
2
, Viswanathan Mohan DSc
2
1
Department of Foods, Nutrition & Dietetics Research, Madras Diabetes Research Foundation, Chennai,
Tamil Nadu, India
2
Department of Diabetology, Madras Diabetes Research Foundation, Chennai, Tamil Nadu, India
3
Department of Epidemiology, Madras Diabetes Research Foundation, Chennai, Tamil Nadu, India
Background and Objectives: To evaluate the reproducibility and construct validity of the Madras Diabetes Re-
search Foundation FFQ (MDRF-FFQ) with biomarkers for its use in epidemiological settings in India. Methods
and Study Design: The MDRF-FFQ was administered to 500 participants representing rural and urban areas of
10 Indian states, twice at an interval of 12 months. Reproducibility was assessed using intra cluster correlation
coefficients (ICC). Construct validity of carbohydrate and fat intake was assessed using baseline serum lipids by
regression analysis. Results: Reproducibility as measured by ICC was 0.50-0.77 for saturated fatty acids (SFA)
and energy in urban and 0.61-0.72 for protein and SFA in rural areas. The ICC for food groups was 0.53-0.77 for
whole grains, fruits and vegetables in urban and 0.50-0.89 for animal foods and whole grains in rural areas. After
adjusting for potential confounders, carbohydrate intake was positively associated with serum triglycerides (TG)
(β [SE]: +2.3 [0.72] mg/dL; p=0.002) and inversely with high density lipoprotein cholesterol (HDL) (β [SE]:-0.48
[0.12], p<0.001), while dietary fat and SFA (% Energy) were positively associated with HDL, low density lipo-
protein (LDL) and total cholesterol and inversely with TG. Conclusions: The MDRF-FFQ can be considered as a
reliable and valid tool to measure the long-term dietary exposure in respect of macronutrient intakes in Indian
populations despite diverse dietary practices.
Key Words: food frequency questionnaire, reproducibility, validity, biomarkers, serum lipid
INTRODUCTION
The increasing burden of non-communicable diseases
(NCD) globally as well as in India
1
can be largely ex-
plained by the adoption of unhealthy dietary practices
2
consequent on the so-called “nutrition transition”. As-
sessment of long-term dietary habits in the population is
an essential first step in devising meaningful nutrition
strategies for prevention and management of NCDs. The
Food Frequency Questionnaire (FFQ) is the most widely
used tool in large nutrition epidemiological studies.
3
The
use of validated FFQs enhances elucidation of the rela-
tionship of the diet to disease risk.
Dietary intake assessments are challenging, especially
in India where there exist diverse cuisines and a wide
variety of regional and cultural food habits and practices.
4
However, Indian diets are comprised, in general, of a ce-
real staple and are meal-based (breakfast, lunch and din-
ner) in most regions of India. Cereal based food choic-
es form the main courses of the daily meals irrespective
of the population’s main dietary habits (vegetarian or
non-vegetarian).
5-7
Most of the National Surveys con-
ducted in India have used dietary records, dietary recalls
or semi-structured interviews to assess the nutritional
status and time trends of food and nutrient intake, in rural
and urban areas.
8,9
Other noteworthy studies like the
Corresponding Author: Dr RM Anjana, Madras Diabetes Re-
search Foundation & Dr. Mohan’s Diabetes Specialities Centre,
WHO Collaborating Centre for Non-Communicable Diseases,
ICMR Centre for Advanced Research on Diabetes, IDF Centre
of Excellence in Diabetes Care, 4, Conran Smith Road, Gopala-
puram, Chennai - 600 086, India.
Tel: (9144)4396 8888; Fax: (9144)2835 0935
Email: dranjana@drmohans.com
Manuscript received 05 September 2019. Initial review com-
pleted 08 October 2019. Revision accepted 16 December 9
doi: 10.6133/apjcn.202003_29(1).0025
Reproducibility and validity of FFQ for Asian Indians 193
Indian Migration study (IMS)
10
Chennai Urban Rural
Epidemiology Study (CURES)
7,11
and Prospective Urban
Rural Epidemiology (PURE) Study
12
have used validated
FFQs for diet-disease risk studies in India.
7,10,12
These
studies, however, have their own limitations; for instance,
the IMS FFQ was restricted to a selected industrial and
rural migrant population while the CURES and PURE
developed FFQs separately for urban and rural popula-
tions in India.
7,10-12
The lack of a single common FFQ
poses challenges in assessing rural and urban diets and
their association with chronic disease prevalence. Consid-
ering the common availability of many regional food
choices in both rural and urban areas
10
today, it was
thought worthwhile to develop a single common FFQ for
both rural and urban areas of India covering north, south,
east, west and northeastern regions.
Reproducibility of an FFQ over longer periods of time
(e.g. 12 months) points to the stability of food and nutri-
ent estimates and is a much-needed feature to assess the
diet and chronic diseases risk in large epidemiological
studies. Biomarkers provide objective assessments, albeit
of an intermediate kind, and may minimize subjective
dietary measurement error (recall from memory) which
often occurs with self -reported dietary intake. The use of
‘gold standard’ recovery biomarkers such as double-
labeled water for energy intake and 24 hr urinary sodium
measurement for sodium intake are impractical in the
epidemiological setting due to the constraints of feasibil-
ity and expense.
3
Studies from the West have attempted
to validate macronutrient (carbohydrate and fats) intake
(as assessed by FFQs) with biochemical markers such as
plasma lipids.
13,14
It is known that obese and overweight
individuals with higher intake of energy from either fat or
carbohydrate or both are likely to have higher blood li-
pids.
13,14
However, such biomarker-based validation stud-
ies have not been carried out in India where carbohydrate
and fat together contribute almost 80-90% to the daily
energy intake.
11,15
In view of this, a comprehensive, structured, quantita-
tive national FFQ covering a wide range of commonly
consumed rural and urban Indian foods was developed
and pre-tested with the help of visual aids by the Madras
Diabetes Research Foundation (MDRF). The present
study aims to evaluate the reproducibility of the Madras
Diabetes Research Foundation FFQ (MDRF-FFQ) over a
12- month period and also to estimate the construct va-
lidity for the measurement of carbohydrate and total fat
intake using serum lipids as a biomarker in Asian Indian
adults from rural and urban areas of all the regions (north,
south, east, west and northeast) of India.
METHODS
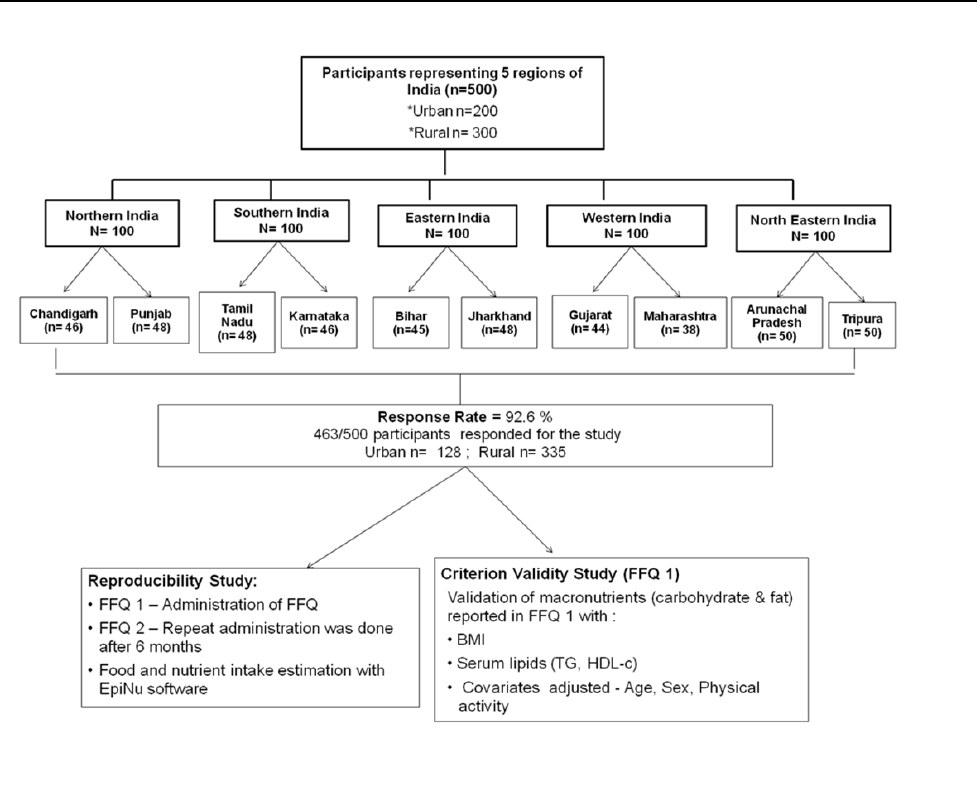
Study participants
The study was conducted during 7th May 2011 to 5th
June 2012. Urban and rural areas of 9 States and 1 Union
Territory representing north, south, east, west and north-
eastern regions of India were randomly chosen for the
study. These were: Karnataka and Tamil Nadu (southern
region), Punjab and Chandigarh (Union Territory) from
the northern region, Bihar and Jharkhand (eastern region),
Gujarat and Maharashtra (western region) and Arunachal
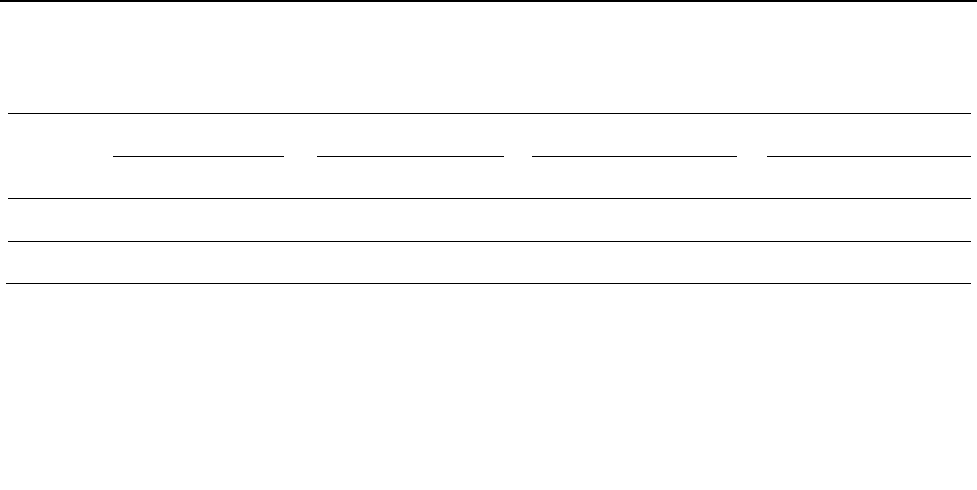
Pradesh and Tripura (northeastern region) (Figure 1). The
Figure 1. Regions across India where the FFQ data was collected.
194 V Sudha, RM Anjana, P Vijayalakshimi, N Lakshmipriva, N Kalpana, R Gayathri et al
participants for this study were chosen using a stratified
multistage sampling design (similar to the one employed
in the India National Family Health Survey-3).
16
From
one district from each State/Union Territory, two census
enumeration blocks (CEB) in urban areas and three vil-
lages in rural areas were randomly selected. From 10 ran-
domly selected households from each selected CEB or
village, 1 adult participant (of either sex) was randomly
selected (2 CEBs x 10 households = 20; 3 villages x 10
households = 30). Therefore, 50 participants (≥20 years)
from each State were selected, making for a total of 500
participants, of whom 463 participated in the study (re-
sponse rate = 92.6%) (Figure 2). The Institutional Ethics
Committee at MDRF approved the study and written in-
formed consent was obtained from each participant before
commencing the study. The study is registered in the
Clinical Trials Registry of India-
(REFCTRI/2008/000174).
Reproducibility and construct validity of MDRF-FFQ
Information on the most frequently consumed foods was
separately collected by a data driven approach using 24 h
dietary recalls among randomly chosen individuals (not
those individuals selected for the present study) from all
the selected states. Single 24-hour recall was collected by
face-to-face interview during the household visit (De-
cember 2010 to February, 2011). The 24-hour recall in-
cluded either a weekday or a weekend day. The partici-
pants were requested to recall all the food and drinks con-
sumed over the last 24 hours in a systematic way (from
morning till night) with the help of visual aids. In addition,
nutritionists and dietitians from each region were contact-
ed to provide missing regional foods, if any, in the 24
hour recall data. Based on these, an extensive list of
foods/food preparation methods was complied. Food
items with similar ingredients and method of preparation
were grouped together to reduce the length of the ques-
tionnaire. Thus, the MDRF-FFQ has a food list contain-
ing 236 food items capturing both rural and urban food
choices of India.
The food items listed in the MDRF-FFQ were catego-
rized into the usually prepared portion sizes (small / me-
dium / large) and portion utensils (ladle / cup / tsp / tbsp
etc.) based on the average weight of the food samples
collected from various commercial and non-commercial
sources. Frequency of intake was measured using 5 cate-
gories (“never,” “daily”, “weekly”, “monthly” and “year-
ly”). Study participants were asked to report the usual
frequency (open ended) as the number of times that best
represented their dietary habits over the past one year [for
instance a food item consumed 3 times weekly was
marked as “3” under the weekly column of the FFQ (us-
ing a visual Food Atlas as an aid)].
MDRF-FFQ is an interviewer administered self- re-
ported paper-based questionnaire.
7
Trained interviewers
(n=6) with good inter-rater agreement (ICC 0.84) admin-
istered the MDRF-FFQ during the period of May 2011 to
June 2012. To test reproducibility, the MDRF-FFQs were
administered 2 times to the same participants (n=463) at
an interval of 12 months (MDRF-FFQ 1 and MDRF-FFQ
2) with the visual aid of photographic Atlas of Indian
Foods containing the pictures of various portion sizes.
17
Figure 2. Reproducibility and Validity of MFFQ – Study design.

Reproducibility and validity of FFQ for Asian Indians 195
In this study, the reported carbohydrate and fat intake
expressed as percentage of energy were compared with
serum lipids-triglycerides; HDL cholesterol, total and low
density lipoprotein (LDL) cholesterol respectively for
estimating construct validity.
Anthropometric assessments
Anthropometric measurements and blood pressure were
assessed using standardized methods.
15
Height (in centi-
meters) was measured using a stadiometer (SECA Model
214, Seca Gmbh Co, and Hamburg, Germany) and weight
(in kilograms) by an electronic weighing scale (SECA
Model 807, SecaGmbh Co). Waist circumference was
measured with a non-stretchable measuring tape. Individ-
uals were asked to keep both feet together and look
straight ahead. The smallest horizontal girth between the
iliac crest and the coastal margins at the end of expiration
was measured as the waist circumference.
18
Body mass
index was calculated using the formula weight in kilo-
grams/height in meters squared. Blood pressure was rec-
orded in the sitting position in the right arm to the nearest
1 mmHg using the electronic OMRON machine (Omron
Corporation, Tokyo, Japan). Two readings were taken 5
minutes apart and their mean was taken as the blood pres-
sure.
Biochemical assessments
Blood samples were obtained after 8-10 hours of fasting
for biochemical assessments only during the FFQ1 die-
tary data collection time point. The fasting venous sample
was centrifuged within 1 hour of collection at the survey
site, and serum was transferred to separate labeled vials
and temporarily stored in cold boxes until they were
transferred to minus 80◦C freezers in the central laborato-
ry of the Madras Diabetes Research Foundation at Chen-
nai. All the analyses for the study were performed at the
National Accreditation Board for Testing and Calibration
Laboratories (NABL) and College of American
Pathologists (CAP)-accredited central laboratory at Dr
Mohan’s Diabetes Specialities Centre in Chennai. Two
percent of the fasting plasma samples were analyzed for
quality control. Accurate coding system was followed to
ensure anonymity of samples and also to facilitate track-
ing of specific samples if the need arose.
Serum cholesterol (cholesterol esterase oxidase-
peroxidase-amidopyrine method), serum triglycerides
(glycerol phosphate oxidase-peroxidase-amidopyrine
method) and HDL cholesterol (direct method poly-
ethylene-glycol-pretreated enzymes) were measured us-
ing the Beckman Coulter AU 2700/480 Autoanalyser
[Beckman AU (Olympus), Ireland]. LDL cholesterol was
calculated using the Friedewald formula.
19
The coeffi-
cients of variation for the biochemical assays ranged from
3.1 to 7.6%.
20
Other assessments
Demographic data and detailed information on smoking
and alcohol consumption were collected by trained inter-
viewers. The validated MDRF Physical Activity Ques-
tionnaire (MPAQ) was used to assess physical activity
levels [PAL (as covariate)] of the participants.
21
The
physical activity level category of the participants was
determined using the cut-off PAL value.
22
Basal metabol-
ic rate (BMR) of the participants was calculated using
age- and sex-specific equations for Indians
23
to test the
extent of under-reporters of total energy using <1.2 ratio
of EI/BMR as cut off.
24
Statistical analysis
Analyses were carried out using the statistical analysis
software (SAS version 9.0; SAS Institute Inc. Cary, NC).
The individual’s average daily nutrient intake of the listed
food items in the FFQ was computed by multiplying the
reported frequency with serving size and per-portion nu-
trient content using the in-house EpiNu [food and nutrient]
database (Version 1 India: Madras Diabetes Research
Foundation; 2006). The EpiNU database consists of a
collection of a wide range of recipes gathered from dif-
ferent sources. In addition to available data for Indian
foods, other nutrient composition tables like United State
Department of Agriculture (USDA) database and Malay-
sian food composition tables were used to ensure the best
possible assessment of nutrient data. Similarly, glycaemic
index (GI) values of Indian foods were derived from
those available in the International GI table in addition to
published literature on GI of Indian foods (Epinu 2006).
25
All food groups and nutrients were adjusted for total en-
ergy to reduce the measurement error and between-person
variation in the food and nutrient intake, using the residu-
al method with total energy intake as the independent
variable and the absolute nutrient and food group intake
as the dependent variable. Significance of differences
between regions was tested using Kruskal Wallis test as
the data was not normally distributed and Chi square test
was used to test categorical variables. As the data was not
normally distributed it was further log transformed. For
evaluating the reproducibility of the FFQ, intra-cluster
associated correlation coefficients (ICC) were calculated
for both nutrients and food groups using FFQ1 and FFQ2
collected at an interval of 12 months and are presented for
both rural and urban participants. Nutrients and food
group intakes were categorized into quartiles, with the
cut-off points for the FFQ1 (original survey) variables
also applied to the repeat FFQ2 variables. A ‘proc mixed’
model was used to compute the adjusted ICC using the
SAS. Agreement was tested with Bland Altman analy-
sis
26
for the total energy intake reported in FFQ1 and
FFQ2. Multivariate regression analysis was used to assess
the construct validity of FFQ using serum lipid (triglycer-
ides and total, LDL and HDL cholesterol) biomarkers
collected during the FFQ 1 time point as the dependent
variables and carbohydrate (%E); glycemic load, dietary
fat (%E), dietary SFA%E as continuous independent var-
iables. Potential confounders like age, sex, BMI, blood
pressure, fasting blood glucose, blood cholesterol, LDL-C,
physical activity levels, cooking oil, energy intake, re-
fined grains, milk and its products and fruits and vegeta-
bles were adjusted in the model. The ratio of energy in-
take (EI) to basal metabolic rate (BMR)
24
was ascertained
as a measure to identify the proportion of under-reporters
(EI/BMR 1.2) for total energy intake. All tests of signif-
icance were two-tailed and a p value of <0.05 was con-
sidered significant.
196 V Sudha, RM Anjana, P Vijayalakshimi, N Lakshmipriva, N Kalpana, R Gayathri et al
RESULTS
Region-wise demographic, anthropometric and biochemi-
cal assessments of the participants are presented in Table
1. Significant differences between regions were seen with
respect to body weight, BMI, diastolic blood pressure,
serum total cholesterol, LDL-C and physical activity lev-
els.
The region wise intake of nutrients and food groups by
the study participants is given in supplementary table.
The intake of energy was found to be highest in the North
followed by the East, while Northeastern and Southern
regions reported the highest intake of carbohydrate and
protein. The Southern region reported the highest intake
of fat (mainly as saturated fatty acid and poly unsaturated
fatty acids while the intake of mono-unsaturated fatty
acid was highest in the North. With regard to food groups,
the intake of refined cereal and animal foods was reported
to be highest in the Northeast, while intake of whole
grains, milk and milk products was highest in the North.
The intake of fruits, fats and edible oils was found to be
highest in the West.
Reproducibility between the two FFQs (FFQ1 and
FFQ2) collected from the same participant at a 12
months’ interval, assessed using intra-cluster correlation
coefficients (ICC) for energy-adjusted nutrients and food
groups, and stratified as rural /urban is presented in Table
2.
The energy adjusted ICCs ranged from 0.61 for protein
(g/day) to 0.72 for SFA (g/day) for nutrients and from
0.50 for animal foods and fruits to 0.89 for whole grains
among food groups in the rural population. The adjusted
ICCs between the two FFQs in the urban population
ranged from 0.50 for SFA (g/day) to 0.77 for total energy
in nutrients and 0.53 for whole grains to 0.77 for fruits
and leafy vegetables (g/day) (among food groups). The
overall adjusted ICC for nutrients ranged from 0.54 for
dietary fiber (g/day) to 0.87 for energy (kcal/day) respec-
tively, while for food groups, it ranged from 0.50 for an-
imal foods (g/day) to 0.75 for pulses and legumes (g/day).
The average energy-adjusted ICCs with respect to sex
was 0.69 for nutrients and 0.60 for food groups among
males and 0.68 for nutrients and 0.59 for food groups in
females (Table 3).
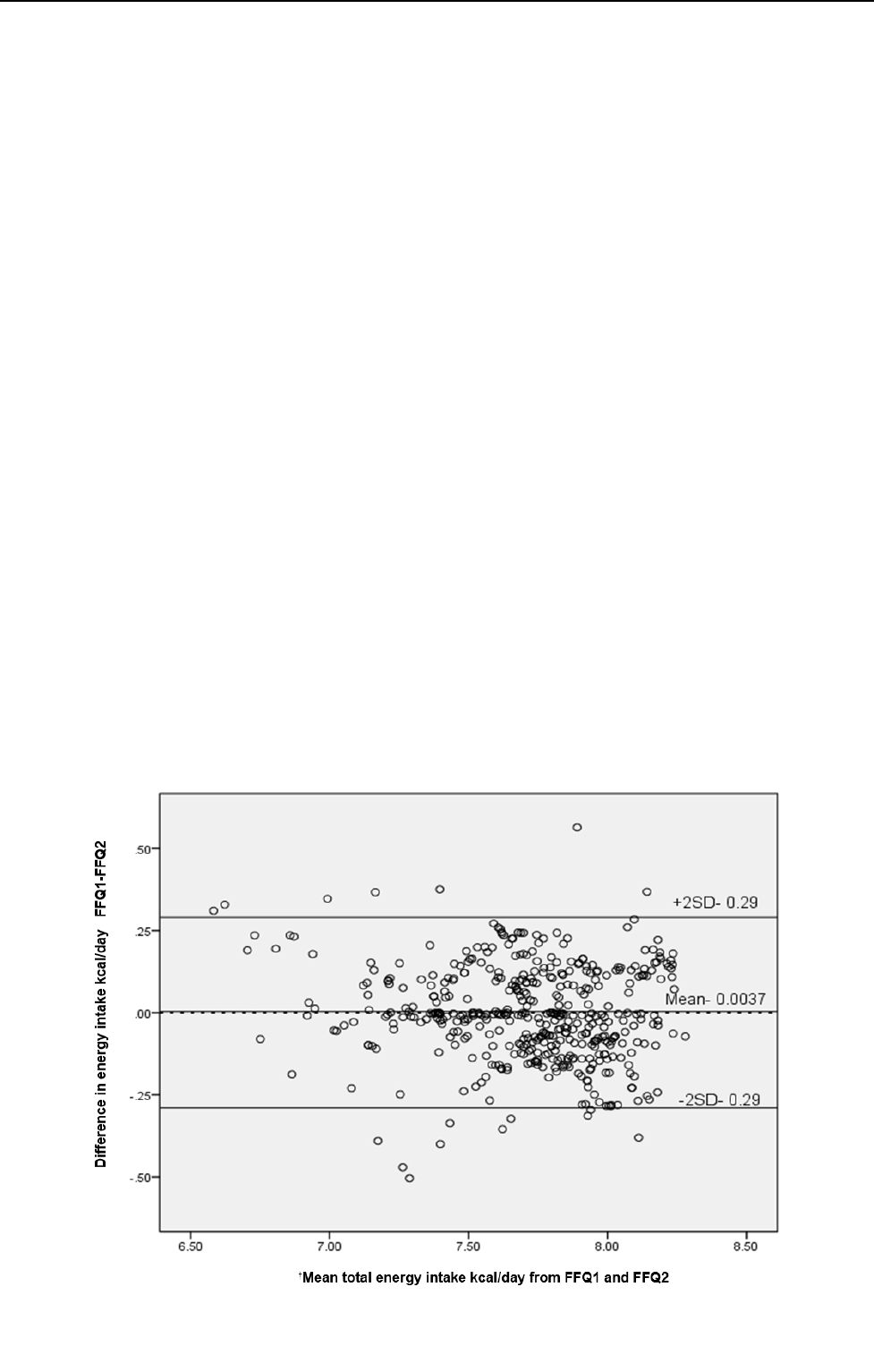
Figure 3 shows the Bland-Altman plot for agreement
between FFQ1 and FFQ2 for reported energy intake. The
agreement plot revealed heteroscedasticity by visual in-
spection using residual plot and Kendall’s Tau test.30
Accordingly, the data was log transformed and the mean
bias and the limits were -0.0037, +0.29 and -0.29.
In the present study, the ratio of self -reported energy
intake (EI) from FFQ1 to the basal metabolic rate (BMR)
was used to measure the extent of under-reporting of en-
ergy intake 3 and the details are presented in Table 4.
About 12% of rural adults, 9% of urban adults, 12% male
and 10% of the female population were found to be un-
der-reporters in the present study.
Table 5 and 6 show the multivariate adjusted associa-
tion of Carbohydrates (%E), glycaemic load (GL), dietary
fat (%E) and SFA (%E) with the lipid profile after adjust-
ing for potential confounders such as age, sex, literacy,
BMI, blood pressure, fasting blood glucose, blood choles-
terol, LDL-C, physical activity levels, cooking oil, energy
intake, refined grains, milk and milk products and fruits
and vegetables. For every unit increase in carbohydrates
(%E), there was a significant rise in triglycerides [β (SE):
+2.29 (0.72), p=0.002] while HDL cholesterol levels de-
Figure 3. Bland-Altman plot showing the difference between total energy intake from the FFQ 1 and FFQ 2 versus the mean of these two
measures.
†
Mean energy intake from FFQ1 and FFQ2 collected at an interval of 12 months from both urban and rural subjects. Data was
log transformed due to heteroscedasticity by visual inspection using residual plot and Kendall’s Tau test
31
Reproducibility and validity of FFQ for Asian Indians 197
Table 1. Region wise demographic, anthropometric and biochemical characteristics of 463 rural and urban participants from 10 Indian states
Description
East
West
North
South
Northeast
Median IQR
Median IQR
Median IQR
Median IQR
Median IQR
Age (yrs) 35.0 25.3 42.0 20.5
36.0 18.0
42.0 24.3 40.0 23.5
Weight (kg)
**
50.0 13.3 54.0 18.5
61.5 20.0
54.0 18.0 54.9 13.9
Height (cm) 156 13.3 156 13.0
160 14.3
157 13.0 158 11.4
Body mass index (kg/m
2
)
**
20.3 5.0 21.9 7.0
23.9 6.0
21.3 6.2 21.4 4.7
Systolic blood pressure (mmHg) 122 21.5 126 19.9
127 19.3
127 20.9 127 24.5
Diastolic blood pressure (mmHg)
**
73.5 15.4 78.5 11.4
77.0 13.3
79.0 14.5 81.0 14.0
Serum cholesterol (mg/dL
†
)
**
140 44.8 161 46.0
165 62.0
155 57.5 149 50.5
Serum triglyceride (mg/dL
†
)
*
115 74.0 101 61.5
102 86.5
118 79.8 132 93.5
Serum high density lipoprotein (mg/dL
†
) 37.0 12.0 40.0 21.0
42.0 14.0
37.0 13.5 37.0 11.0
Serum low density lipoprotein (mg/dL
†
)
**
74.2 34.7 97.8 37.1
93.7 41.8
95.5 41.3 84.8 41.7
Physical activity level (PAL)
‡
§
**
,
n (%)
Sedentary (PAL value 1.40-1.69) 36 (38.3) 39 (60.0)
65 (72.2)
71 (62.3) 42 (42.0)
Moderate (PAL value 1.70-1.99) 35 (37.2) 19 (29.2)
17 (18.9)
30 (26.3) 46 (46.0)
Vigorous (PAL value 2.00-2.40) 23 (24.5) 7 (10.8)
8 (8.9)
13 (11.4) 12 (12.0)
PAL: Physical Activity Level
†
To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.0259. To convert mmol/L cholesterol to mg/dL, multiply mmol/L by 38.7. Cholesterol of 193 mg/dL¼5.00 mmol/L.
‡
p value for categorical variable test using chi-square
§
Based on FAO/WHO cut off for physical activity
26
*
p<0.05,
**
p value <0.001
198 V Sudha, RM Anjana, P Vijayalakshimi, N Lakshmipriva, N Kalpana, R Gayathri et al
Table 2. Reproducibility study: Energy adjusted intra cluster correlation coefficients (ICC) of nutrients and food groups from the self-reported food frequency questionnaires collect-
ed twice at the interval of 12 months in urban and rural Asian Indian adults (n=463)
Urban (n= 128)
Rural (n= 335)
Median (95% CI)
ICC (95% CI)
†
Median (95% CI)
ICC (95% CI)
†
MDRF-FFQ 1
(May 2011)
MDRF-FFQ 2
(June 2012)
MDRF-FFQ 1
(May 2011)
MDRF-FFQ 2
(June 2012)
Energy (kcal) 2426 (2406-2650) 2478 (2374-2594) 0.77 (0.72-0.84) 2302 (2189-2333) 2321(2224-2379) 0.63 (0.61 - 0.66)
Carbohydrate (g/d) 364 (361-374) 366 (362-377) 0.76 (0.71 - 0.83) 369 (367-375) 372 (371.2-381) 0.69 (0.57 - 0.83)
Protein (g/d) 62.8 (62.7-66.7) 65.4 (65.1-70.7) 0.67 (0.60 - 0.77) 61.9 (62.3-64.4) 63.1 (63.2-66.1) 0.61 (0.59 - 0.64)
Total fat (g/d) 66.8 (62.9-68.2) 72.7 (69.4-75.7) 0.69 (0.62 - 0.77) 65.9 (62.8-66) 69 (67.7-71.5) 0.63 (0.61 - 0.66)
Total SFA (g/d) 22.1 (20.5-23.2) 23.9 (23.3-26.5) 0.5 (0.46 - 0.59) 20.6 (20.2-21.9) 20.5 (20.6-22.7) 0.72 (0.7 - 0.75)
Total MUFA(g/d) 21.8 (20.9-23.8) 22.8 (21.1-23.9) 0.63 (0.54 - 0.76) 23.5 (22.4-24.1) 22.3 (21.6-23.4) 0.63 (0.60 - 0.66)
Total PUFA(g/d) 16 (16.9-19.7) 16.8 (16.7-19.8) 0.7 (0.67 - 0.72) 15 (16.3-17.7) 16 (16.7-18.3) 0.69 (0.62 - 0.79)
Dietary fibre (g/d) 33.6 (32.6-36.2) 29.9 (29.8-33.9) 0.76 (0.71 - 0.83) 34.9 (33.2-35.2) 31.4 (31.4-33.7) 0.71 (0.69 - 0.73)
Glycemic Index 60.7 (60.2-61.3) 61.4 (61.1-62.7) 0.6 (0.57 - 0.66) 61.1 (60.4-61.3) 62 (61.8-62.8) 0.58 (0.56 - 0.61)
Glycemic Load (g/d) 195 (193-204) 194 (186-203) 0.62 (0.58 - 0.67) 199 (200-207) 203 (204-213) 0.63 (0.61 - 0.65)
Refined cereals (g/d) 200 (195-231) 198 (183-223) 0.59 (0.54 - 0.65) 199 (203-227) 203 (199-223) 0.51 (0.48 - 0.54)
Whole grains (g/d) 60.5 (68-95.4) 56.9 (64.7-90.7) 0.53 (0.48 - 0.61) 65.4 (70.1-86) 71.4 (77.7-95.2) 0.61 (0.59 - 0.63)
Pulses and legume (g/d) 44.2 (41.3-50) 44.6 (43.1-52.8) 0.69 (0.66 - 0.72) 40.2 (41.3-46.8) 42.7 (42.2-48.2) 0.52 (0.50 - 0.55)
Milk and milk products (g/d) 244 (246-320) 260 (257-336) 0.61 (0.53 - 0.72) 225 (235-280) 224(237-283) 0.52 (0.48 - 0.57)
Fats and edible oils (g/d) 36.7 (35.8-39.8) 36.2 (35.3-39.4) 0.56 (0.52 - 0.63) 38.9 (36.1-38.7) 36.9 (37.1-39.8) 0.56 (0.54 - 0.59)
Fruits(g/d) 134 (135-173) 133 (137-176) 0.77 (0.72 - 0.84) 129 (132-149) 124 (128-146) 0.89 (0.89 - 0.9)
Leafy vegetables (g/d) 16.1 (21.3-33.3) 18.2 (22.5-35.5) 0.77 (0.72 - 0.84) 13.2 (18.1-24.4) 13.9 (18.5-24.6) 0.69 (0.67 - 0.72)
Other vegetables (g/d) 46.8 (55.8-82.5) 45 (55.5-84.4) 0.74 (0.68 - 0.81) 42.1 (59.3-76.8) 31.3 (49.7-65.4) 0.72 (0.70 - 0.74)
Roots and tuber s(g/d) 224(193-263) 194 (198-256) 0.73 (0.67 - 0.79) 248 (224.0-285) 213 (210-266) 0.71 (0.70 - 0.74)
Animal foods (g/d) 28.1 (33.3-53.4) 27.9 (29.7-60) 0.58 (0.47 - 0.76) 25.7 (35.5-46) 26 (31.5-41.8) 0.5 (0.46 - 0.56)
MDRF-FFQ: Madras Diabetes Research Foundation Food Frequency Questionnaire; ICC: intra cluster correlation coefficients; SFA: Saturated fatty acid; MUFA: Mono-unsaturated fatty acid; PUFA: Poly-
unsaturated fatty acid
†
ICC measures agreement between FFQ1 and FFQ
Reproducibility and validity of FFQ for Asian Indians 199
Table 3. Reproducibility study: Energy adjusted intra class correlation coefficients (ICC) of nutrients and food groups from the self-reported food frequency questionnaires Asian
Indian adults based on gender (n=463)
Nutrient and food group In-
take
Male (n=216)
Female (n= 247)
Median (95% CI)
ICC
†
(95% CI)
‡
Median (95% CI)
ICC
†
(95% CI)
‡
MDRF-FFQ 1
(May 2011)
MDRF-FFQ 2
(June 2012)
MDRF-FFQ 1
(May 2011)
MDRF-FFQ 2
(June 2012)
Energy (kcal)
2410 (2349-2539) 2443 (2357-2543)
0.89 (0.89-0.90)
2267 (2157-2321) 2210 (2179-2354)
0.88 (0.88-0.89)
Carbohydrate (g/d) 371 (366-377) 373 (368-381) 0.72 (0.70-0.74)
365 (365-373) 369 (368-379) 0.74 (0.73-0.76)
Protein (g/d) 62.9 (63.3-66.4) 63.9 (64.7-68.9) 0.75 (0.74-0.77)
61.5 (61.5-63.9) 63.7 (62.8-66) 0.75 (0.73-0.76)
Total fat (g/d) 64.7 (61.2-65.4) 66.8 (66.8-71.7) 0.65 (0.62-0.67)
67.3 (64.1-67.8) 72.3 (69.3-73.6) 0.74 (0.73-0.75)
Total SFA (g/d) 19.8 (19.5-21.6) 20.2 (19.9-22.6) 0.61 (0.58-0.64)
22 (21-22.9) 23 (22.6-24.7) 0.78 (0.77-0.79)
Total MUFA (g/d) 23.1 (21.3-23.5) 22.3 (21.3-23.6) 0. 70 (0.68-0.72)
23.9 (22.5-24.4) 22.6 (21.5-23.5) 0.72 (0.71-0.74)
Total PUFA (g/d) 14.9 (16.1-18) 16.7 (16.8-19) 0.70 (0.69-0.72)
15.7 (16.7-18.5) 16 (16.6-18.4) 0.71 (0.69-0.73)
Dietary fibre (g/d) 33.3 (31.8-34.6) 30.8 (30.4-33.4) 0.64 (0.61-0.67)
35.1 (34-36.3) 31.4 (31.3-34.1) 0.64 (0.62-0.66)
Glycemic Index 61.2 (60.5-61.7) 62.3 (61.9-63.3) 0.66 (0.64-0.69)
60.8 (60.2-61) 61.5 (61.3-62.4) 0.71 (0.70-0.73)
Glycemic Load (g/d) 200(199-209) 201 (199-210) 0.70 (0.68-0.72)
197 (196-204) 197 (199-210) 0.70 (0.69-0.72)
Refined cereals (g/d) 204 (210-240) 215 (203-236) 0.60 (0.57-0.63)
192 (193-219) 197 (187-213) 0.64 (0.62-0.66)
Whole grains (g/d) 55.4 (64.4-85) 59.8 (67.7-90.5) 0.67 (0.65-0.70)
72.8 (73.7-92.1) 72.2 (78.7-97.5) 0.66 (0.64-0.69)
Pulses and legume (g/d) 38 (39.3-46.7) 39.8 (40.5-48.7) 0.71 (0.70-0.73)
42.3 (42.9-48.6) 44.6 (43.9-50.3) 0.68 (0.67-0.71)
Milk and Milk products (g/d) 210.5 (228-289) 216 (231-292) 0.51 (0.47-0.55)
246 (245-295) 256 (252-305) 0.57 (0.54-0.60)
Fats and edible oils (g/d) 37.4 (34.7-37.9) 36.1 (35.5-39.1) 0.60 (0.57-0.63)
39.3 (37.2-40) 37.3 (37.4-40.3) 0.61 (0.58-0.63)
Fruits(g/d) 131 (133-158) 129 (134-161) 0.55 (0.51-0.59)
130 (132-155) 124 (126-148) 0.50 (0.45-0.57)
Leafy vegetables (g/d) 12 (16-22.1) 13.1 (17.1-24.2) 0.60 (0.58-0.63)
16.3 (21.8-30.9) 15.6 (21.9-30.5) 0.50 (0.47-0.55)
Other vegetables (g/d) 46.4 (61.3-83.4) 36.5 (57.9-81.8) 0.60 (0.57-0.63)
41.8 (55.1-74.7) 32.3 (45.6-61.2) 0.54 (0.51-0.57)
Roots and tubers (g/d) 219 (227-266) 202 (213-242) 0.52 (0.47-0.60)
215 (227-262) 195(204-230) 0.52 (0.47-0.58)
Animal foods (g/d) 34.8 (41.8-57.7) 30.9 (36.1-56) 0.51 (0.47-0.55)
19.6 (29-39.5) 22.2 (27-38.4) 0.62 (0.59-0.65)
MDRF-FFQ: Madras Diabetes Research Foundation Food Frequency Questionnaire; ICC: Intra class correlation; SFA: Saturated fatty acid; MUFA: Mono-unsaturated fatty acid; PUFA: Poly-unsaturated fatty acid
†
ICC measures agreement between FFQ1 and FFQ.
‡
ICC- Nutrients and food groups were log transformed and further adjusted for covariates age (in years), sex (male/female), BMI (kg/m
2
), regions (North, South, East, west and North east), income (INR) (>2000,
2000-5000, 5000-10000, >10000) and education (illiterate, primary, higher secondary and college education).

200 V Sudha, RM Anjana, P Vijayalakshimi, N Lakshmipriva, N Kalpana, R Gayathri et al.
creased [β (SE): -0.48 (0.12), p<0.001]. Energy adjusted
glycemic load (a measure of the carbohydrate quantity
and quality) also showed a trend similar to that of carbo-
hydrates (%E). However, for a unit increase in dietary fat,
total cholesterol [β (SE): 1.10 (0.51), p=0.032], LDL [β
(SE): 0.96 (0.43), p=0.025] and HDL cholesterol [β (SE):
0.57 (0.12), p<0001] significantly increased while tri-
glycerides decreased [β (SE): -1.88 (0.76), p=0.014]. A
similar trend was observed for SFA (%E).
DISCUSSION
The present study evaluates, for the first time in India, the
reproducibility and construct validity of an interviewer
administered comprehensive quantitative national FFQ
for adults of both sex residing in rural and urban areas of
all regions of India including the Northeast. The MDRF-
FFQ is a reliable tool to assess the dietary measures of
macronutrients and food groups reported by Asian Indian
urban and rural adults. Moderate to good correlation coef-
ficients were found between FFQ1 and the repeat FFQ2
collected at an interval of 12 months for both nutrients
and food groups even when stratified by sex, suggesting
consistent performance. Construct validity was assessed
only with limited biomarkers of blood lipids with the re-
ported intakes of macronutrients such as carbohydrates
(%E), glycemic load, total dietary fat (%E) and SFA
(%E).
The results of the present study by and large agree with
other studies that have reported a moderate to substantial
agreement (>0.40 to <0.80) for both nutrients and food.
However, SFA, milk and milk products and roots and
tubers in this study showed a lower agreement than that
cited elsewhere
27,28
especially among urban participants.
Several national studies have reported similar ICCs for
majority of nutrients (0.40-78) and food groups (0.86-
0.99).
7,11,29
Similarly, international studies had shown
moderate to high ICC for majority of nutrients (0.42-0.91)
and food groups (0.28-0.91).
30,31
The results of the pre-
sent study are in agreement with the above stated studies
(Table 2), thereby reiterating the reliability of the MDRF-
FFQ.
Validation of food and nutrient intake by FFQ against
biomarkers has enormous value in nutritional epidemio-
logical studies. Biomarkers could reduce subjectivity
compared to validation studies with diet records or multi-
ple 24 hour recalls as reference method as the latter is
prone to subjectivity like the FFQ. While biomarkers are
also prone to errors of estimation and physiological varia-
tions, these are unrelated to errors with self-reported die-
tary assessments
3
and can thereby reflect relationships
with nutrient intake as they are based on the biological
processes in the body (construct validity). Several studies
have shown the effect of intake of carbohydrates and fats
on blood lipids.
13,14,32
Hence, in the present study, the
energy intake from carbohydrates, fat and SFA reported
in FFQ1 were tested against lipid parameters such as tri-
glycerides and HDL, total and LDL cholesterol to evalu-
ate construct validity, which is a first for any FFQ in In-
dia.
Nettleton et al. 2009
14
and Ma et al. 2006
33
reported
that an increase in carbohydrate calories was associated
with a significant increase in triglyceride concentration
and decrease in HDL cholesterol concentration. The pre-
sent study also reported similar findings, after adjusting
for potential non-dietary and dietary factors that may af-
fect these lipids. Furthermore, an increase in triglyceride
levels and decrease in HDL-c was observed with an in-
crease in GL evaluated from the FFQ (Table 5 ), similar
to reports from the West
34,35
Ours is the first study to as-
sess dietary glycemic load (GL) from the MDRF-FFQ
across rural and urban areas of different regions of India.
The GL is relevant in diet-disease relationship as it has
been well associated with risk of diabetes both in Indian
and western populations.
36,37
Willett et al. 2001
13
reported an increase in HDL-C
and a decrease in triglyceride when carbohydrate energy
T
able
4
.
Percent u
nder reporters from the ratio of energy intake and BMR in rural and urban India (n=450)
FFQ 1 n
EI/BMR ratio
(mean±SD)
Percentage below the EI/BMR (<1.2)
†
n (%)
Rural 330 1.75 (0.52) 38 (11.5)
Urban 120 1.83 (0.48) 11 (9.2)
Male 216 1.73 (0.53) 26 (12.0)
Female 234 1.81 (0.49) 23 (9.8)
Overall 450 1.77 (0.51) 49 (10.9)
EI/BMR: energy intake/basal metabolic rate.
†
EI/BMR <1.2 are considered as under-reporters and the BMR was calculated from the age- and gender- specific prediction equation
23
Table 5. Multivariate adjusted regression coefficients for association of carbohydrates (%E), glycemic load with TG
and HDL as a measure of construct validity from MDRF FFQ 1 (May 2011))
Description
TG (mg/dL
†
)
HDL (mg/dL
†
)
β SE
p
value
β SE
*
p
value
Carbohydrates (% Energy) 2.29 0.72 0.002 -0.48- 0.12 <0.001
Energy adjusted glycemic load 0.38 0.15 0.01 -0.11 0.02 <0.001
TG: triglyceride; HDL: High density lipoprotein; MDRF FFQ: Madras Diabetes Research Foundation Food Frequency Questionnaire
†
To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.0259. To convert mmol/L cholesterol to mg/dL, multiply mmol/L by
38.7. Cholesterol of 193 mg/dL¼5.00 mmol/L.
*
p<0.001 considered to be statistically significant
Reproducibility and validity of FFQ for Asian Indians 201
is replaced by fat in a Western population. Recent find-
ings from 18 countries in the Prospective Urban Rural
Epidemiology study (PURE) have shown that a higher
percentage energy from fat and SFA was associated with
a higher total cholesterol, HDL and LDL-C and lower
triglyceride.
38
These agree with the present study findings.
Similar findings were also reported in studies done else-
where.
39-41
This reiterates the MDRF-FFQ’s ability to
provide a valid measure of dietary fat that could further
show the physiologically relevant associations with total
cholesterol, TG, LDL and HDL-C (Table 6).
Generally, misreporting of dietary intakes affects the
construct validity of the assessment tool and is a barrier in
understanding diet-disease relationship.
42
The underesti-
mation of nutrient intakes may be associated with under-
reporting of total energy intake. Livingstone and Black
2003 and Black et al. 1991,
43,44
reported widespread prev-
alence of under-reporting in various nutritional studies.
However, the proportion of under-reporters in this study
(11%) is much lower than that reported by Bedard et al
(43%).
45
In general, however, studies on FFQ validity are chal-
lenging to undertake in a sufficiently large and repre-
sentative sample of the population for which they have
been developed. In addition, there is no gold standard
reference method to validate FFQ. One of the important
strengths of the MDRF-FFQ is its ability to assess dietary
habits of the population in both rural and urban settings of
all the regions of India. One may argue that regional diets
are diverse and hence separate FFQs are needed for each
region. In fact, diets in all regions of India including both
rural and urban areas are high in carbohydrates and bulk
of the carbohydrate calories is derived from cereal staples
(though the choice of grain could differ from region to
region) (NSSO 2011-2012). MDRF-FFQ questionnaire is
unique as it has been validated using serum triglycerides
and HDL-C as construct validity for assessing the dietary
GL of the population. However, the FFQ has a few limi-
tations as well. The test-retest for reproducibility assump-
tion that true intake did not change between the 2 admin-
istrations of FFQ1 and FFQ2 cannot be confirmed with
certainty. The accuracy of responses was dependent on
the memory of each individual and would be subject to
recall bias.
46
Micronutrients were not evaluated for repro-
ducibility and construct validity owing to budgetary con-
straints and incomplete micronutrient composition pro-
vided in food composition tables. Besides, another limita-
tion was that the serum lipids were measured at only one
time, which might not account for intra-individual varia-
bility in these parameters. Further, due to budget con-
straints and feasibility challenges, assessment of recovery
biomarkers like doubly-labelled water for energy intake
and 24 –hour urine for sodium intake could not be carried
out.
Conclusion
MDRF-FFQ can be considered a realistic, practical and
economical tool for assessing usual dietary habits of
Asian Indian populations, based on the evaluation of the
major macronutrients, carbohydrates and fat, that pro-
vides 3/4th of daily energy. MDRF-FFQ has validity for
the measurement of physiologically important variations
in macronutrient intake and presumptively, in the assess-
ment of long-term dietary exposure in studies of chronic
disease in India.
ACKNOWLEDGEMENTS
We would like to acknowledge the laboratory team for their
support in biochemical assessments as well as the information
technology team for their assistance in the dietary data extrac-
tion.
AUTHOR DISCLOSURES
All authors declare that there is no conflict of interest.
REFERENCES
1. Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K,
Das HK et al. Prevalence of diabetes and pre diabetes in 15
states of India: results from the ICMR-INDIAB population-
based cross-sectional study. Lancet Diabetes Endocrinol.
2017; 5:585-96. doi: 10.1016/S2213-8587(17)30174-2.
2. Chauhan S, Aeri BT. Prevalence of cardiovascular disease in
India and its economic impact-A review. International
Journal of Scientific and Research Publications. 2013;3:
2250-3153.
3. Willett W. Nutritional epidemiology. 3rd ed. New York,
USA: Oxford University Press; 2013.
T
able
6
.
Multivariate adjusted regression coefficients for association of total dietary Fat, SFA with lipid profile as a
measure of construct validity from MDRF FFQ 1 (May 2011)
Description
Cholesterol
(mg/dL
†
)
TG (mg/dL
†
)
HDL (mg/dL
†
)
LDL (mg/dL
†
)
β SE
p
value
β SE
p
value
β SE
p
value
β SE
*
p
value
Total Fat
(% Energy)
1.10
0.51
0.03
-1.88
0.76
0.01
0.57 0.121
<0.001
0.96 0.43
0.03
Total SFA
(% Energy)
0.68
0.30
0.02
-1.34
0.59
0.02
0.28 0.094
0.003
0.60 0.25
0.026
TG: triglyceride; HDL: high density lipoprotein cholesterol, LDL: Low density lipoprotein cholesterol; MDRF FFQ: Madras Diabetes
Research Foundation Food Frequency Questionnaire
†
To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.0259. To convert mmol/L cholesterol to mg/dL, multiply mmol/L by
38.7. Cholesterol of 193 mg/dL¼5.00 mmol/L.
‡
Adjusted for: age (yrs), sex (M/F), BMI (kg/m
2
), region, rural/urban, physical activity category (sedentary 1.40-1.69; moderate: 1.70-
1.99; vigorous: 2.0-2.40), systolic and diastolic blood pressure (mmHg), fasting blood glucose (mg/dL), literacy (yes/ no), main cooking
oil, total energy (kcal/d) intake
*
p<0.001 considered to be statistically.

202 V Sudha, RM Anjana, P Vijayalakshimi, N Lakshmipriva, N Kalpana, R Gayathri et al.
4. Dubey KG. The Indian Cuisine. New Delhi,: PHI Learning
Pvt. Ltd; 2010.
5. Report of Third Repeat Rural Survey. Hyderabad,India:
National Nutrition Monitoring Bureau, National Institute of
Nutrition; 2012.
6. Ramachandran. P. The double burden of malnutrition in
India. Rome: Food and Agriculture organization; 2006.
7. Sudha V, Radhika G, Sathya RM, Ganesan A, Mohan V.
Reproducibility and validity of an interviewer-administered
semi-quantitative food frequency questionnaire to assess
dietary intake of urban adults in southern India. Int J Food
sci nutr. 2006;57:481-93. doi: 10.1080/09637480600969220.
8. Nutritional Intake in India. NSS 68th Round. India: National
Sample Survey Organization, Ministry of Statistics and
Program Implementation, Government of India. [25th April
2018]; Available from:
http://mospi.nic.in/sites/default/files/publication_reports/nss
_report_560_19dec14.pdf. 2011-2012.
9. International Institute for Population Sciences (IIPS) and
ICF. National Family Health Survey (NFHS-4), 2015-16:
India. Mumbai: IIPS; 2017.
10. Bowen L, Bharathi AV, Kinra S, DeStavola B, Ness A,
Shah E. Development and evaluation of a semi quantitative
food frequency questionnaire for use in urban and rural
India. Asia Pac J Clin Nutr. 2012;21:355-60.
11. Sowmya N, Lakshmipriya N, Arumugam K, Venkatachalam
S, Vijayalakshmi P, Ruchi V et al. Comparison of dietary
profile of a rural south Indian population with the current
dietary recommendations for prevention of non-
communicable diseases. Indian J Med Res. 2016;144:112-9.
doi: 10.4103/0971-5916.193297.
12. Bharathi AV, Kurpad AV, Thomas T, Yusuf S, Saraswathi
G, Vaz M. Development of food frequency questionnaires
and a nutrient database for the Prospective Urban and Rural
Epidemiological (PURE) pilot study in South India:
methodological issues. Asia Pac J Clin Nutr. 2008;17:178-
85.
13. Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M,
Rimm E. Assessment of questionnaire validity for
measuring total fat intake using plasma lipid levels as
criteria. Am J Epidemiol. 2001;154:1107-12. doi: 10.1093/
aje/154.12.1107.
14. Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR.
Associations between dietary macronutrient intake and
plasma lipids demonstrate criterion performance of the
Multi-Ethnic Study of Atherosclerosis (MESA) food-
frequency questionnaire. Br J Nutr. 2009;102:1220-7. doi:
10.1017/S0007114509382161.
15. Radhika G, Sathya RM, Ganesan A, Saroja R,
Vijayalakshmi P, Sudha V, Mohan V. Dietary profile of
urban adult population in South India in the context of
chronic disease epidemiology (CURES–68). Public Health
Nutr. 2011;14:591-8. doi: 10.1017/S136898001000203X.
16. International Institute for Population Sciences (IIPS) and
ICF. National Family Health Survey (NFHS-4), 2005-2006:
India. Mumbai: IIPS; 2007.
17. Sudha V, Mohan V, Anjana RM, Kamala Krishnaswamy. Dr.
Mohan’s Atlas of Indian. Chennai,India: ; 2015.
18. Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V,
Unnikrishnan R et al. The Indian Council of Medical
Research—India Diabetes (ICMR-INDIAB) Study:
Methodological Details. J Diabetes Sci Technol. 2011;5:
906-14. doi: 10.1177/1932296 81100500413.
19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the
concentration of low-density lipoprotein cholesterol in
plasma, without use of the preparative ultracentrifuge. Clin
Chem. 1972;18:499-502.
20. Anjana RM, Rani CS, Deepa M, Pradeepa R, Sudha V, Nair
HD et al. Incidence of diabetes and pre diabetes and
predictors of progression among Asian Indians: 10-year
follow-up of the Chennai Urban Rural Epidemiology Study
(CURES). Diabetes Care. 2015;38:1441-8. doi: 10.2337/dc
14-2814.
21. Anjana RM, Sudha V, Lakshmipriya N, Subhashini S,
Pradeepa R, Geetha L et al. Reliability and validity of a new
physical activity questionnaire for India. Int J Behav Nutr
Phys Acty. 2015; 12:40. doi: 10.1186/s12966-015-0196-2.
22. Human energy requirements. Scientific background papers
from the Joint FAO/WHO/UNU Expert Consultation. Public
Health Nutr. 8: 929–1228. 2005. [15th April 2018];
Available from: http://www.fao.org/3/a-y5686e.pdf
23. Indian Council of Medical Research. Nutrient Requirements
and Recommended Dietary Allowances for Indians: A
Report of the Expert Group of the Indian Council of Medical
Research. NewDelhi, India: Indian Council of Medical
Research; 2010.
24. Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR,
Coward WA, Prentice AM. Critical evaluation of energy
intake data using fundamental principles of energy
physiology: 1. Derivation of cut-off limits to identify under-
recording. Eur J Clin Nutr. 1991;45:569-81.
25. EpiNu [food and nutrient database]. Version 1. India:
Madras Diabetes Research Foundation; 2006
26. BREHM MA, Scholtes VA, Dallmeijer AJ, Twisk JW,
Harlaar J. The importance of addressing heteroscedasticity
in the reliability analysis of ratio-scaled variables: an
example based on walking energy-cost measurements. Dev
Med Child Neurol. 2012;54:267-73.
27. Ibiebele TI, Parekh S, Mallitt KA, Hughes MC, O’Rourke
PK, Webb PM. Reproducibility of food and nutrient intake
estimates using a semi-quantitative FFQ in Australian adults.
Public Health Nutr. 2009;12:2359-65. doi: 10.1017/S136898
0009005023.
28. Hebden L, Kostan E, O’Leary F, Hodge A, Allman-Farinelli
M. Validity and reproducibility of a food frequency
questionnaire as a measure of recent dietary intake in young
adults. PLoS One. 2013;8:e75156. doi: 10.1371/journal.pone.
0075156.
29. Shaikh NI, Frediani JK, Ramakrishnan U, Patil SS, Yount
KM, Martorell R, Narayan KV, Cunningham SA.
Development and evaluation of a Nutrition Transition-FFQ
for adolescents in South India. Public Health Nutr. 2017;
20:1162-72. doi: 10.1017/S1368980016003335.
30. Esfahani FH, Asghari G, Mirmiran P, Azizi F.
Reproducibility and relative validity of food group intake in
a food frequency questionnaire developed for the Tehran
Lipid and Glucose Study. J Epidemiol. 2010;20:150-8. doi:
10.2188/jea.je20090083.
31. Dehghan M, del Cerro S, Zhang X, Cuneo JM, Linetzky B,
Diaz R, Merchant AT. Validation of a semi-quantitative
Food Frequency Questionnaire for Argentinean adults. PLoS
One. 2012;25:7:e37958. doi: 10.1371/journal.pone.0037958.
32. Mohseni-Takalloo S, Mirmiran P, Hosseini-Esfahani F,
Azizi F. Dietary fat intake and its relationship with serum
lipid profiles in tehranian adolescents. J Food Nutr Res.
2014;2:330-4.
33. Ma Y, Li Y, Chiriboga DE, Olendzki BC, Hebert JR, Li W,
Leung K, Hafner AR, Ockene IS. Association between
carbohydrate intake and serum lipids. J Am Coll Nutr.
2006;25:155-63.
34. Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB,
Hankinson SE, Willett WC. Dietary glycemic load assessed
by food-frequency questionnaire in relation to plasma high-
density-lipoprotein cholesterol and fasting plasma

Reproducibility and validity of FFQ for Asian Indians 203
triacylglycerols in postmenopausal women. Am J Clin Nutr.
2001;73:560-6. doi: 10.1093/ajcn/73.3.560.
35. Brand-Miller JC. Glycemic load and chronic disease. Nutr
Rev. 2003;61:S49-55. doi: 10.1301/nr.2003.may.S49-S55.
36. Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A,
Sudha V. Dietary carbohydrates, glycaemic load, food
groups and newly detected type 2 diabetes among urban
Asian Indian population in Chennai, India (Chennai Urban
Rural Epidemiology Study 59). Br J Nutr. 2009;102:1498-
506. doi: 10.1017/S0007114509990468.
37. Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik
VS, Willett WC, Hu FB. White rice, brown rice, and risk of
type 2 diabetes in US men and women. Arch Intern Med.
2010;170:961-9. doi: 10.1001/archinternmed.2010.109.
38. Mente A, Dehghan M, Rangarajan S, McQueen M,
Dagenais G, Wielgosz A et al. Association of dietary
nutrients with blood lipids and blood pressure in 18
countries: a cross-sectional analysis from the PURE study.
Lancet Diabetes Endocrinol. 2017;5:774-87. doi: 10.1016/
S2213-8587(17)30283-8.
39. Sharlin J, Posner BM, Gershoff SN, Zeitlin MF, Berger PD.
Nutrition and behavioral characteristics and determinants of
plasma cholesterol levels in men and women. J Am Diet
Assoc. 1992;92:434-40.
40. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention
for overweight and obese adults: comparison of low-
carbohydrate and low-fat diets. A meta-analysis. PLoS One.
2015;10:e0139817. doi: 10.1371/journal.pone.0139817.
41. Schwingshackl L, Hoffmann G. Comparison of effects of
long-term low-fat vs high-fat diets on blood lipid levels in
overweight or obese patients: a systematic review and meta-
analysis. J Acad Nutr Diet. 2013;113:1640-61. doi: 10.
1016/j.jand.2013.07.010.
42. Mendez MA. Invited commentary: dietary misreporting as a
potential source of bias in diet-disease associations: future
directions in nutritional epidemiology research. Am J
Epidemiol. 2015;181:234-6. doi: 10.1093/aje/kwu306
43. Livingstone MB, Black AE. Markers of the validity of
reported energy intake. J Nutr. 2003;133:895S-920S. doi: 10.
1093/jn/133.3.895S.
44. Black AE, Goldberg GR, Jebb SA, Livingstone MB, Cole
TJ, Prentice AM. Critical evaluation of energy intake data
using fundamental principles of energy physiology:
2.Evaluating the results of published surveys. Eur J Clin
Nutr. 1991;45:583-99.
45. Bedard D, Shatenstein B, Nadon S. Underreporting of
energy intake from a self-administered food-frequency
questionnaire completed by adults in Montreal. Public
Health Nutr. 2004;7:675-81. doi: 10.1079/PHN2003578.
46. Vaz S, Falkmer T, Passmore AE, Parsons R, Andreou P.
The case for using the repeatability coefficient when
calculating test–retest reliability. PLoS One. 2013;8:e73990.
doi: 10.1371/journal.pone.0073990.
204 V Sudha, RM Anjana, P Vijayalakshimi, N Lakshmipriva, N Kalpana, R Gayathri et al
Supplementary table 1. Region-wise intake of nutrients and food groups
Nutrient and food group intake
East West North South Northeast
Median IQR Median IQR Median IQR Median IQR Median IQR
Energy (kcal) 2412 1014 2158 676 2681 592 2263 750 2062 903
Carbohydrate (g/d) 373 29 351 29.6 355 27.5 375 37.8 380 53.1
Protein (g/d) 58.4 8.2 58.0 10.2 61.2 9.6 63.9 11.6 66.6 12.7
Fat (g/d) 65.1 12.2 77.2 13.4 72.7 11.5 63.8 14.7 56.3 19.3
SFA (g/d) 19.1 7.0 28.4 5.5 24.7 7.9 20.8 8.7 15.7 8.4
MUFA (g/d) 28.9 6.6 21.5 8.1 30.1 6.2 21.0 8.0 16.3 8.4
PUFA (g/d) 13.1 2.5 22.5 9.8 13.5 3.5 16.2 10.5 18.2 7.4
Dietary fibre (g/d) 37.9 8.2 40.3 8.4 41.0 10.7 29.7 9.3 25.1 10.4
Glycemic Index 62.0 3.5 57.8 5.1 58.9 3.4 61.4 2.8 63.1 4.2
Glycemic load (g/d) 203.0 48.7 179 37.6 1736 41.5 212 37.3 227 56.0
Refined cereals (g/d) 197.1 81.2 118 86.8 118 48.4 229 79.6 311 162
Whole grains (g/d) 120.4 80.0 89.1 108 157 61.2 31.5 39.8 22.3 54.1
Pulses and legumes (g/d) 43.7 25.8 44.3 32.3 42.6 30.4 46.1 20.6 30.5 21.5
Milk and milk products (g/d) 157 189 302 250 345 229 246 199 138.3 166.6
Fats and edible oils (g/d) 39.8 9.4 46.2 9.1 41.3 10.7 34.5 13.7 32.5 14.4
Fruits (g/d) 139 70.7 152 71.4 151 100 117 65.2 108 59.7
Leafy vegetables (g/d) 6.9 10.8 17.3 16.7 10.4 31.4 23.1 19.5 11.7 15.3
Other vegetables (g/d) 29.9 48.9 23.7 27.6 38.5 53.7 47.1 32.1 104 164
Roots and tuber s(g/d) 98.5 53.2 110 51.5 96.0 48.8 85.6 33.6 110 59.2
Animal foods
†
(g/d) 17.0 31.9 8.1 22.0 5.1 24.0 40.2 54.0 60.5 67.8
IQR: Inter Quartile Range; SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: Polyunsaturated fatty acids
†
Animal foods include meat, poultry, egg, fish and other sea foods.
