
Chemical and Functional
Properties of Food
Components
Over three editions, this book described the contents of food raw materials and prod-
ucts, the chemistry/biochemistry of food components, as well as the changes occur-
ring during post-harvest storage and processing affecting the quality of foods. The
fourth edition of Chemical and Functional Properties of Food Components discusses
the role of chemical compounds in the structure of raw materials and the formation
of different attributes of food quality, including nutritional value, safety, and sensory
properties. This new edition contains four new chapters: “Non-Protein Nitrogenous
Compounds”; “Prooxidants and Antioxidants in Food”; “Non-Nutritive Bioactive
Compounds in Food of Plant Origin”; and “Analytical Methods Used for Assessing
the Quality of Food Products.”
These chapters have been included because new research results have brought
increasing knowledge on the effect of non-protein nitrogenous compounds, espe-
cially bioactive peptides, nucleic acids, and biogenic amines on the biological prop-
erties of foods; the role of natural and added prooxidants and antioxidants in the
processing and biological impact of foods; numerous benecial and harmful effects
of bioactive components of plant foods; and new systems for control of food composi-
tion and the safety of foods.
Features:
• Stresses the effect of the chemical/biochemical reactions on the selection
of optimum parameters of food processing without presenting details of the
technological processes
• Describes naturally occurring elements and compounds as well as those
generated during food handling in view of health hazards they may bring
to consumers
• Discusses the risks and benets of reactions occurring during food handling
The knowledge of the chemistry and biochemistry of the components and their inter-
actions presented in this book aids food scientists in making the right decisions for
controlling the rate of benecial and undesirable reactions, selecting optimal storage
and processing parameters, as well as the best use of food raw materials.
Chemical and Functional Properties of Food Components Series
SERIES EDITOR
Zdzisław E. Sikorski
Chemical and Functional Properties of Food Components, Fourth Edition
Edited by Hanna Staroszczyk and Zdzisław E. Sikorski
Meat Quality: Genetic and Environmental Factors
Edited by Wiesław Przybylski and David Hopkins
Food Oxidants and Antioxidants: Chemical, Biological, and Functional Properties
Edited by Grzegorz Bartosz
Fermentation: Effects on Food Properties
Edited by Bhavbhuti M. Mehta, Afaf Kamal-Eldin and Robert Z. Iwanski
Methods of Analysis of Food Components and Additives, Second Edition
Edited by Semih Otles
Food Flavors: Chemical, Sensory and Technological Properties
Edited By Henryk Jelen
Environmental Effects on Seafood Availability, Safety, and Quality
Edited by E. Grazyna Daczkowska-Kozon and Bonnie Sun Pan
Chemical and Biological Properties of Food Allergens
Edited By Lucjan Jedrychowski and Harry J. Wichers
Chemical, Biological, and Functional Aspects of Food Lipids, Second Edition
Edited by Zdzisław E. Sikorski and Anna Kołakowska
Food Colorants: Chemical and Functional Properties
Edited by Carmen Socaciu
Mineral Components in Foods
Edited by Piotr Szefer and Jerome O. Nriagu
Chemical and Functional Properties of Food Components, Third Edition
Edited by Zdzisław E. Sikorski
Carcinogenic and Anticarcinogenic Food Components
Edited by Wanda Baer-Dubowska, Agnieszka Bartoszek and Danuta Malejka-Giganti
Toxins in Food
Edited by Waldemar M. Dąbrowski and Zdzisław E. Sikorski
Chemical and Functional Properties of Food Saccharides
Edited by Piotr Tomasik
Chemical and Functional Properties of Food Proteins
Edited by Zdzisław E. Sikorski

Chemical and Functional
Properties of Food
Components
Fourth Edition
Edited by
Hanna Staroszczyk and Zdzisław E. Sikorski
Fourth edition published 2023
by CRC Press
6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487-2742
and by CRC Press
4 Park Square, Milton Park, Abingdon, Oxon, OX14 4RN
CRC Press is an imprint of Taylor & Francis Group, LLC
© 2023 selection and editorial matter, Hanna Staroszczyk and Zdzislaw E. Sikorski; individual chapters, the
contributors
First edition published by CRC Press 1997
Second edition published by CRC Press 2002
Third edition published by CRC Press 2007
Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot
assume responsibility for the validity of all materials or the consequences of their use. The authors and publish-
ers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to
copyright holders if permission to publish in this form has not been obtained. If any copyright material has not
been acknowledged please write and let us know so we may rectify in any future reprint.
Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmit-
ted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented,
including photocopying, microlming, and recording, or in any information storage or retrieval system, without
written permission from the publishers.
For permission to photocopy or use material electronically from this work, access www.copyright.com or con-
tact the Copyright Clearance Center, Inc. (CCC), 222 Rosewood Drive, Danvers, MA 01923, 978-750-8400. For
works that are not available on CCC please contact mpkbookspermissions@tandf.co.uk
Trademark notice: Product or corporate names may be trademarks or registered trademarks and are used only
for identication and explanation without intent to infringe.
Library of Congress Cataloging-in-Publication Data
Names: Staroszczyk, Hanna, editor. | Sikorski, Zdzisław E., editor.
Title: Chemical and functional properties of food components / edited by
Hanna Staroszczyk, Zdzislaw Sikorski.
Description: Fourth edition. | Boca Raton, FL : CRC Press, 2023. | Includes
bibliographical references and index. | Contents: Food components and
quality / Zdzisław E. Sikorski and Barbara Piotrowska, Merck -- Chemical
composition and structure of foods / Jolanta Tomaszewska-Gras -- Water
and food quality / Peter Edward Doe and Emilia Barbara Cybulska.
Identiers: LCCN 2022049021 (print) | LCCN 2022049022 (ebook) | ISBN
9781032199221 (hbk) | ISBN 9781032209227 (pbk) | ISBN 9781003265955
(ebk)
Subjects: LCSH: Food--Analysis. | Food--Composition.
Classication: LCC TX545 .C44 2023 (print) | LCC TX545 (ebook) | DDC
664/.07--dc23/eng/20221214
LC record available at https://lccn.loc.gov/2022049021
LC ebook record available at https://lccn.loc.gov/2022049022
ISBN: 978-1-032-19922-1 (hbk)
ISBN: 978-1-032-20922-7 (pbk)
ISBN: 978-1-003-26595-5 (ebk)
DOI: 10.1201/9781003265955
Typeset in Times
by Deanta Global Publishing Services, Chennai, India

v
Contents
Preface......................................................................................................................vii
About the Editors......................................................................................................ix
Contributors ..............................................................................................................xi
Chapter 1 Food Components and Quality ............................................................1
Zdzisław E. Sikorski and Barbara Piotrowska
Chapter 2 Chemical Composition and Structure of Foods ................................. 13
Jolanta Tomaszewska-Gras
Chapter 3 Water and Food Quality.....................................................................39
Peter Edward Doe and Barbara Emilia Cybulska
Chapter 4 The Role of Mineral Components......................................................73
Małgorzata Grembecka
Chapter 5 Saccharides....................................................................................... 105
Hanna Staroszczyk
Chapter 6 The Role of Proteins in Food ........................................................... 155
Zdzisław E. Sikorski and Izabela Sinkiewicz
Chapter 7 Non-Protein Nitrogenous Compounds .............................................203
Edyta Malinowska-Pańczyk
Chapter 8 Lipids and Food Quality ..................................................................225
Izabela Sinkiewicz
Chapter 9 Factors Affecting the Rheological Properties of Foods...................265
Robert Tylingo
Chapter 10 Food Colorants .................................................................................285
Anna Podsędek
vi Contents
Chapter 11 Prooxidants and Antioxidants in Food.............................................303
Ronald B. Pegg and Ryszard Amarowicz
Chapter 12 Food Allergens ................................................................................. 339
Barbara Wróblewska
Chapter 13 Food Flavors..................................................................................... 363
Shwu-Pyng Joanna Chen and Bonnie Sun Pan
Chapter 14 The Role of Food Additives ............................................................. 401
Joanna Le Thanh-Blicharz and Jacek Lewandowicz
Chapter 15 Food Safety ...................................................................................... 419
Agata Witczak and Kamila Pokorska-Niewiada
Chapter 16 Probiotics and Prebiotics in Food..................................................... 433
Edyta Malinowska-Pańczyk
Chapter 17 Mood Food ....................................................................................... 457
Maria H. Borawska and Sylwia K. Naliwajko
Chapter 18 Mutagenic and Carcinogenic Compounds in Food..........................469
Agnieszka Bartoszek and Serhii Holota
Chapter 19 Non-Nutritive Bioactive Compounds in Food of Plant Origin.........497
Barbara Kusznierewicz
Chapter 20 Analytical Methods Used for Assessing the Quality of Food
Products............................................................................................ 535
Widiastuti Setyaningsih

vii
Preface
Water, saccharides, proteins, lipids, mineral compounds, colorants, other constituents,
and additives contribute to the nutritional value and sensory properties of food.
During post-harvest storage and processing, these components change. The extent
and nature of the alterations depend on the chemical properties of the compounds
themselves. Knowledge of the chemistry and biochemistry of food components and
their behavior in the face of various stressors aids in making the right decisions for
controlling the rate of benecial and undesirable reactions, selecting optimal storage
and processing parameters, and dening the best use of food raw materials.
The book draws from the personal research and teaching experience of scientists
from universities and research institutions around the world. Beginning with
an examination of food components both natural and added, this volume, like
its predecessors, details the role of chemical compounds in the structure of raw
materials and the formation of different attributes of food quality. All chapter authors
are renowned specialists in their eld and the editors have extensive experience in
editing food science books.
This new edition contains the following new chapters: “Non-Protein Nitrogenous
Compounds,” “Prooxidants and Antioxidants in Food,” “Non-Nutritive Bioactive
Compounds in Food of Plant Origin,” and “Analytical Methods Used for Assessing
the Quality of Food Products.” These chapters have been included because new
research results have brought increasing knowledge on the effect of non-protein
nitrogenous compounds, especially bioactive peptides, nucleic acids, and biogenic
amines on the quality as well as biological properties of foods, the role of natural and
added prooxidants and antioxidants in the processing and biological impact of foods,
numerous benecial and harmful effects of bioactive components of plant foods, and
new systems for control of food composition and the safety of foods.
It was possible to prepare the book only thanks to the dedication of all persons
involved. As the editors we thank all of them for their contributions. These thanks
are primarily addressed to those who followed strictly all our editorial suggestions
and delivered their chapters well ahead of the deadline. We dedicate this volume
to all scientists, who cooperated in writing the numerous books of the CRC series
Chemical and Functional Properties of Food Components.
Hanna Staroszczyk and Zdzisław E. Sikorski
Gdańsk University of Technology

ix
About the Editors
Hanna Staroszczyk earned her BS and MS at the Cracow University of Technology,
her PhD at the Hugon Kołłątaj University of Agriculture in Cracow, and her DSc at
the Gdańsk University of Technology (GUT), Poland. Currently, she is head of the
Department of Chemistry, Technology, and Biotechnology of Food at GUT. She also
worked as a researcher/postdoc/visiting professor at the Institute of Food Research,
Norwich, UK, Academia Sinica, Taipei, Taiwan, and the University of Arkansas,
Fayetteville, AR. Her research is focused mainly on biopolymer chemistry. She
is an author/co-author of original papers on the modication of proteins and
polysaccharides, as well as an editor of books on food chemistry. She is a member of
the Polish Society of Food Technologists.
Zdzisław E. Sikorski received his BS, MS, PhD, and DSc from the Gdańsk
University of Technology (GUT), a doctorate honoris causa from the Agricultural
University in Szczecin, and is a fellow of the International Academy of Food Science
and Technology. He gained industrial experience in breweries, in sh, meat, and
vegetable processing plants, and on a deep-sea shing trawler. He was the organizer,
professor, and head of the Department of Food Chemistry and Technology at GUT,
served as dean of the Faculty of Chemistry, was chairman of the Committee of
Food Technology and Chemistry of the Polish Academy of Sciences, chaired the
scientic board of the Sea Fisheries Institute in Gdynia, and was an elected member
of the Main Council of Science and Tertiary Education in Poland. He worked also
as a researcher/professor at Ohio State University, OH; CSIRO in Hobart, Australia;
DSIR in Auckland, New Zealand; and National Taiwan Ocean University in Keelung,
Taiwan. Currently, he is an honorary professor emeritus in the Faculty of Chemistry
at GUT. His research concentrated on the technology of sh processing and changes
in food proteins due to storage and handling. He published numerous papers contain-
ing the results of his investigations in the eld of food chemistry and technology and
is the author/editor of a number of books in English, Polish, Russian, and Spanish.

xi
Contributors
Ryszard Amarowicz
Institute of Animal Reproduction and
Food Research
Polish Academy of Sciences
Olsztyn, Poland
Agnieszka Bartoszek
Gdańsk University of Technology
Department of Chemistry, Technology
and Biotechnology of Food
Gdańsk, Poland
Maria H. Borawska
Medical University of Białystok
Department of Bromatology
Białystok, Poland
Shwu-Pyng Joanna Chen
Chinese University of Hong Kong,
Honorary Research Fellow
State Key Laboratory of
Agrobiotechnology
Hong Kong, China
Emilia Barbara Cybulska
Gdańsk University of Technology
formerly Department of
Pharmaceutical Technology and
Biochemistry
Gdańsk, Poland
Peter Edward Doe
University of Tasmania
School of Engineering
Tasmania, Australia
Małgorzata Grembecka
Medical University of Gdańsk
Department of Bromatology
Gdańsk, Poland
Serhii Holota
Danylo Halytsky Lviv National Medical
University
Department of Organic, Bioorganic and
Pharmaceutical Chemistry
Lviv, Ukraine
Barbara Kusznierewicz
Gdańsk University of Technology
Department of Chemistry, Technology
and Biotechnology of Food
Gdańsk, Poland
Jacek Lewandowicz
Poznań University of Technology
Institute of Logistics
Poznań, Poland
Edyta Malinowska-Pańczyk
Gdańsk University of Technology
Department of Chemistry, Technology
and Biotechnology of Food
Gdańsk, Poland
Sylwia K. Naliwajko
Medical University of Białystok
Department of Bromatology
Białystok, Poland
Bonnie Sun Pan
National Taiwan Ocean University
Department of Food Science
Keelung City, Taiwan
Ronald B. Pegg
University of Georgia
Department of Food Science and
Technology
Athens, Georgia, USA
xii Contributors
Barbara Piotrowska
Merck Life Science
Poznań, Poland
Anna Podsędek
Lodz University of Technology
Institute of Molecular and Industrial
Biotechnology
Łódź, Poland
Kamila Pokorska-Niewiada
West Pomeranian University of
Technology
Department of Toxicology, Dairy
Technology and Food Storage
Szczecin, Poland
Widiastuti Setyaningsih
Gadjah Mada University
Department of Food and Agricultural
Product Technology
Yogyakarta, Indonesia
Zdzisław E. Sikorski
Gdańsk University of Technology
Department of Chemistry,
Technologyand Biotechnology
of Food
Gdańsk, Poland
Izabela Sinkiewicz
Gdańsk University of Technology
Department of Chemistry,
Technologyand Biotechnology
of Food
Gdańsk, Poland
Hanna Staroszczyk
Gdańsk University of Technology
Department of Chemistry, Technology
and Biotechnology of Food
Gdańsk Poland
Joanna Le Thanh-Blicharz
Prof. Waclaw Dabrowski Institute of
Agriculture and Food Biotechnology
– State Research Institute
Warszawa, Poland
Jolanta Tomaszewska-Gras
Poznań University of Life Sciences
Department of Food Quality and Safety
Management
Poznań, Poland
Robert Tylingo
Gdańsk University of Technology
Department of Chemistry, Technology
and Biotechnology of Food
Gdańsk, Poland
Agata Witczak
West Pomeranian University of
Technology
Department of Toxicology, Dairy
Technology and Food Storage
Szczecin, Poland
Barbara Wróblewska
Institute of Animal Reproduction and
Food Research
Polish Academy of Sciences
Olsztyn, Poland

1
1
Food Components
and Quality
Zdzisław E. Sikorski and Barbara Piotrowska
CONTENTS
1.1 Introduction ......................................................................................................1
1.1.1 Components of Food Raw Materials and Products ..............................1
1.1.2 Factors Affecting Food Composition ...................................................2
1.1.3 The Role of Food Components.............................................................3
1.2 Functional Properties........................................................................................4
1.3 Food Quality.....................................................................................................5
1.3.1 Attributes of Quality.............................................................................5
1.3.2 Safety and Nutritional Value ................................................................6
1.3.3 Sensory Quality .....................................................................7
1.4 Chemical Analysis in Ensuring Food Quality..................................................8
1.4.1 Introduction ..........................................................................................8
1.4.2 Requirements of the Producer ..............................................................8
1.4.3 Requirements of the Consumer .......................................................... 10
1.4.4 Limits of Determination..................................................................... 10
1.5 Conclusion ...................................................................................................... 11
1.1 INTRODUCTION
1.1.1 COMPONENTS OF FOOD RAW MATERIALS AND PRODUCTS
Foods are derived from plants, carcasses of animals, and single-celled organisms.
They are composed of water, saccharides, proteins, lipids, and minerals, as well as
a host of other compounds present in minor quantities, albeit of signicant impact
on the quality of many products. Here belong especially the non-protein nitrogenous
compounds, vitamins, colorants, avorings, functional additives, and numerous
other items generated during the processing or storage of foods.
The content of water in various foods ranges from a few percent in dried com-
modities, e.g. milk powder, through about 15% in grains, 16–18% in butter, 20% in
honey, 35% in bread, 65% in manioc, and 75% in meat, to about 90% in many fresh
fruits and vegetables. Saccharides are present in food raw materials in quantities
ranging from about 1% in meats and sh, through about 4.5% in milk, 18% in pota-
toes, and 15–21% in sugar beets, to about 70% in cereal grains.
DOI: 10.1201/9781003265955-1
2 Sikorski and Piotrowska
The protein content in foods regards mainly crude protein, i.e. N × 6.25. The
nitrogen-to-protein conversion factor 6.25 has been recommended for most plant and
animal foods under the assumption that the N content in their proteins is 16% and
they do not contain non-protein N. The N content in the proteins, however, depends
on the amino acid composition. Furthermore, the total N consists of protein N and N
contained in numerous non-protein compounds, e.g. free peptides and amino acids,
nucleic acids and their degradation products, amines, betaines, urea, vitamins, and
alkaloids. In some foods, the non-protein N may constitute up to 30% of total N. In
many of these compounds the C:N ratio is similar to the average in amino acids.
However, the N content in urea, being 47%, is exceptionally high. The average con-
version factor for estimation of true protein, based on the ratios of total amino acid
residues to amino acid N, is in the range of 5.14–6.61 for different classes of foods.
Crude protein makes up about 1% of the weight of fruits, 2% of potatoes, 3.2% of
bovine milk, 12% of eggs, 12–22% of wheat grain, about 20% of meat, and 25–40%
of different beans. Cereal grain and legume seeds deposit during their development
large quantities of storage proteins in granules known also as protein bodies. In soy-
beans, these proteins constitute 60–70% of the total protein content. The granules
are 80% made of proteins.
The lipid content in foods is given in nutrition information labeling predomi-
nantly as “total fat,” which is often called also “crude fat.” This is a mixture of vari-
ous classes of lipids, mainly different triacylglycerols. The lipids of numerous shes,
such as orange roughy, mullets, codsh, and sharks, as well as some crustaceans and
mollusks, also comprise wax esters. Some shark oils are very rich in hydrocarbons,
particularly in squalene. Furthermore, the lipid fraction of food raw materials har-
bors different sterols, vitamins, and pigments that are crucial for the metabolism.
Thus the composition of the extracted crude fat depends on the kind of food and the
polarity of the solvent used for extraction. Lipids constitute about 1% of the weight
of fruits, vegetables, and lean sh muscle, 3.5% of milk, 6% of beef meat, 32% of
egg yolk, and 85% of butter.
1.1.2 FACTORS AFFECTING FOOD COMPOSITION
The content of different components in food raw materials depends on the species
and variety of the animal and plant crop, the conditions of cultivation and time of
harvesting of the plants, the feeding, conditions of life, and age of the farm ani-
mals, or the season of catching the sh and marine invertebrates. The post-harvest
changes in the crop during storage are also important. The food industry, by estab-
lishing quality requirements for raw materials, can encourage the producers to con-
trol within limits the contents of the main components. This regards, e.g., starch in
potatoes, fat in various meat cuts, pigments in fruits and vegetables and in the esh
of sh, or protein in wheat and barley, as well as the fatty acid composition of lipids
in oilseeds and meats. The contents of desirable minor components, e.g. of natural
antioxidants, can also be effectively controlled to slow the oxidation of pigments and
lipids in beef meat. Contamination of the raw material with organic and inorganic
pollutants can be controlled, e.g. by observing recommended agricultural procedures
3 Food Components and Quality
in using fertilizers, herbicides, and insecticides, and by seasonally closing certain
shing areas to avoid marine toxins. The size of predatory sh like swordsh, tuna,
or sharks that are shed commercially can be limited to reduce the risk of too high a
content of mercury and arsenic in the esh.
The composition of processed foods depends on the applied recipe as well as on
changes taking place due to processing and storage. These are mainly brought about
by added, endogenous, and microbial enzymes, active forms of oxygen, heating,
chemical treatment, and low or high pH. Examples of such changes are:
• leaching of soluble, desirable, and undesirable parts, e.g. vitamins, mineral
components, and toxins during washing, blanching, or cooking,
• dripping after thawing or due to cooking,
• loss of moisture and volatiles by evaporation and sublimation,
• absorption of valuable or harmful compounds, e.g. during salting, pickling,
seasoning, frying, or smoking,
• formation of desirable or unwanted compounds as a result of enzyme
activity, e.g. development of typical avor in cheese or decarboxylation of
amino acids in sh marinades,
• generation of welcome or objectionable products due to interactions of
reactive groups induced by heating or chemical treatment, e.g. avors
or carcinogenic compounds in roasted meats, or trans fatty acids in
hydrogenated fats,
• formation of different products of oxidation of components, mainly of
lipids, pigments, and vitamins,
• loss of nutrients and deterioration, e.g. of dried sh because of attacks by
ies, mites, and beetles.
In recent decades, a novel type of product appeared known as designer food. It is
manufactured using biotechnological/engineering methods and enriched with health-
promoting nutritive additives. Putting this type of product on the market should be
approved by appropriate food legislation authorities.
1.1.3 THE ROLE OF FOOD COMPONENTS
The indigenous water is immobilized in plant and animal tissues by the structural
elements and various solutes; it contributes to buttressing the conformation of the
polymers, serves as a solvent for different constituents, and interacts in metabolic
processes.
Polysaccharides, proteins, and lipids serve as the building materials of different
structures of plant and animal tissues. These structures are responsible for the form
and tensile strength of the tissues and create the necessary conditions for metabolic
processes to occur. The resulting compartmentation plays a crucial biological role
in the organisms. Some of the main components, as well as other constituents, are
either bound to different cell structures or are distributed in soluble form in the tissue
uids.
4 Sikorski and Piotrowska
Many saccharides, proteins, and lipids are stored in living organisms for reserve
purposes. Polysaccharides are present in plants as starch in the form of granules and
in muscles as glycogen. Other saccharides are dissolved in tissue uids or perform
various biological functions, e.g. in free nucleotides or as components of nucleic
acids, or being bound to proteins and lipids. Proteins also play crucial metabolic roles
in plants and animals as enzymes and enzyme inhibitors, participate in the transport
and binding of oxygen and metal ions, and perform immunological functions. Some
plant polysaccharides are only partly utilized for energy. However, as dietary ber,
they affect various processes in the gastrointestinal tract in different ways.
The distribution of lipids in food raw materials depends on their role in living
animals and plants. In an animal body, the lipids occur primarily as an energy-
rich store of neutral fat in the subcutaneous adipose tissue, as well as kidney, leaf,
and crotch fat, the intramuscular fat responsible for marbling, and intermuscular or
seam fat. In fatty animals, most of the lipids are stored as depot fat in the form of
triacylglycerols. In sh of lean species, most of the fat occurs in the liver. The lipids
contained in the food raw materials in low quantities serve mainly as components of
protein-phospholipid membranes and have metabolic functions.
The main food components supply the consumers with the necessary building
material and source of energy, as well as elements and compounds indispensable
for the metabolism. Some polysaccharides are utilized as dietary ber. Many of the
minor components present originally in the raw materials are nutritionally essential,
e.g. vitamins. Some, although not indispensable, can be utilized by the body, e.g.
most free amino acids, or impart valuable sensory properties to the food products.
Numerous groups, including tocopherols, ubiquinone, carotenoids, ascorbic acid,
thiols, amines, and several other non-protein nitrogenous compounds serve as
endogenous muscle antioxidants, playing an essential role in postmortem changes in
meat. Other minor components are useless or even harmful if present in excessive
amounts. Most food raw materials are infected with different microorganisms,
putrefactive and often pathogenic, and some contain parasites and the products
of microbial metabolism. A variety of compounds are added intentionally during
processing to serve as preservatives, antioxidants, colorants, avorings, sweeteners,
and emulsifying agents or to fulll other technological purposes.
1.2 FUNCTIONAL PROPERTIES
The term “functional properties” has evolved to have a broad range of meanings.
The meaning corresponding to the term “technological properties” implies that
the given component present in proper concentration, subjected to processing at
optimum parameters, contributes to the expected desirable sensory characteristics
of the product, usually by interacting with other food constituents. Hydrophobicity,
hydrogen bonds, ionic forces, and covalent bonding are involved in the interactions.
Thus the functional properties of food components are affected by the number of
accessible reactive groups and by the exposure of hydrophobic areas in the material.
Therefore in a system of known water activity, pH, and range of temperature,
the functional properties can be to a large extent predicted from the structure of
5 Food Components and Quality
the respective saccharides, proteins, and lipids. They can also be improved by
appropriate, intentional enzymatic or chemical modications of the molecules,
mainly those that affect the size, charge density, or the hydrophilic/hydrophobic
character of the compounds or change the environment.
The functional properties of food components make it possible to manufacture
products of desirable quality. Thus pectins contribute to the characteristic texture
of ripe apples and make perfect jellies. Different other polysaccharides are efcient
thickening and gelling agents at various ranges of acidity and concentration of
ions. Alginates in the presence of Ca
2+
form protective, unfrozen gels on the
surface of frozen products. Some starches are resistant to retrogradation thereby
retarding staling of bread. Fructose slows moisture loss from biscuits. Mono- and
diacylglycerols, phospholipids, and proteins are used for emulsifying lipids and
stabilizing food emulsions and foams. Antifreeze proteins inhibit ice formation in
various products, and gluten plays a major role in producing the characteristic texture
of wheat bread. Technologically required functional effects can also be achieved by
intentionally employing food additives, i.e. colors, sweeteners, and a host of other
compounds. These additives are not per se regarded as foodstuffs but are used to
modify the rheological properties or acidity, increase the color stability or shelf life,
and act as humectants or avor enhancers. In recent decades, the term “functional”
has been predominantly given to a large group of products and components, also
termed designer foods, pharmafoods, nutraceuticals, or foods for specic health
use, which are regarded as health-enhancing or potentiating the performance of the
human organism. These foods, mainly drinks, meals, confectionery, ice cream, and
salad dressings, contain various ingredients, e.g. oligosaccharides, sugar alcohols, or
choline, which are claimed to have special physiological functions like neutralizing
harmful compounds in the body and promoting recovery and general good health.
Foods containing prebiotics, various oligosaccharides, and probiotics, mainly dairy
products, have been treated in-depth in other chapters of this volume.
1.3 FOOD QUALITY
1.3.1 ATTRIBUTES OF QUALITY
The quality of a food product, i.e. the characteristic properties that determine the
degree of excellence, is the sum of the attributes contributing to the satisfaction and
good health of the consumer. The composition and the chemical nature of the food
components affect all aspects of food quality. The total quality reects at least the
following attributes:
• compatibility with the local or international food law regulations and
standards regarding mainly the proportions of main components, presence
of compounds serving as identity indicators, contents of contaminants and
additives, hygienic standards, packaging, and labeling,
• nutritional aspects, i.e. the contents and availability of nutritionally desir-
able constituents, mainly proteins, essential amino acids, essential fatty
6 Sikorski and Piotrowska
acids, saccharides, vitamins, prebiotics, probiotics, ber, and mineral
components,
• safety affected by the concentration of compounds that may constitute
health hazards for the consumers and affect the digestibility and nutritional
availability of the food, e.g. heavy metals, toxins of different origins, some
enzymes and enzyme inhibitors, factors decreasing the availability of some
metal components, pathogenic microorganisms, and parasites,
• sensory attributes, i.e. the color, size, form, avor, taste, and rheological
properties affected by the chemical composition of the product, as well as
by changes resulting from processing and culinary preparation,
• shelf life at specic storage conditions,
• convenience aspects that are reected by the size and ease of opening/
reclosing the container, suitability of the product for immediate use or for
different types of thermal treatment, ease of portioning or spreading, as
well as transport and storage requirements,
• ecological aspects regarding suitability for recycling of the packaging
material and pollution hazards.
For many foods, one of the most important quality criteria is freshness. This is
especially so in the case of numerous species of vegetables, fruits, and seafood. Fish
of valuable species at a state of prime freshness, suitable to be eaten raw, may have
over ten times higher market price than the same sh after several days of storage in
ice, which is still very t for human consumption.
1.3.2 SAFETY AND NUTRITIONAL VALUE
Food is regarded as safe if it does not contain harmful organisms or compounds
in concentrations above the ofcially accepted limits. The nutritional value of
foods depends primarily on the contents of nutrients and nutritionally objectionable
components in the products. Processing may increase the safety and biological value
of food by inducing chemical changes increasing the digestibility of the components
or by inactivating undesirable compounds, e.g. toxins or enzymes catalyzing the
generation of toxic agents from harmless precursors. Freezing and short-term frozen
storage of sh inactivates the parasites Anisakis that could escape detection during
visual inspection of herring llets used as raw material for cold marinades produced
at mild conditions. Thermal treatment inactivates myrosinase, the enzyme involved
in the hydrolysis of glucosinolates. This arrests the reactions, which lead to the
formation of goitrogenic products in oilseeds of Cruciferae. Heat pasteurization
and sterilization reduce to the acceptable level the number of vegetative forms and
spores, respectively, of pathogenic microorganisms. Several other examples of such
improvements in the safety and biological quality of foods are given in the following
chapters of this book.
There are, however, also nutritionally undesirable side effects of processing, e.g.
destruction of essential food components as a result of heating, chemical treatment,
and oxidation. Generally known side effects are the partial thermal decomposition of
7 Food Components and Quality
vitamins, especially thiamine, loss of available lysine and sulfur-containing amino
acids, or generation of harmful compounds, e.g. carcinogenic heterocyclic aromatic
amines, lysinoalanine, and lanthionine or position isomers of fatty acids, not present
originally in foods. In recent decades new evidence of side effects has been accumu-
lated with respect to the chemical processing of oils and fats. Commercial hydroge-
nation of oils leads not only to the intended saturation of selected double bonds in
the fatty acids and thereby the required change in the rheological properties of the
oil but also to the generation of trans-trans and cis-trans isomers which are absent
in the unprocessed oils.
1.3.3 Sensory Quality
Many of the desirable sensory attributes of foods stem from the properties of the
raw material. The natural color of meat, sh muscles and skin, vegetables, and fruits
depends on the presence of a host of different pigments, which are water or lipid
soluble. Chlorophylls impart a green color to vegetables, but also to olive oil. Some
natural oils are yellow or red due to different carotenoids. Carotenes are present
also in the esh oil of redsh (Sebastes marinus), while different carotenoproteins
are responsible for the vivid colors of sh skin. Many hydroxy carotenoids occur in
plants in form of esters of long-chain fatty acids. The red, violet, or blue color of
fruits and owers is caused by anthocyanins. Betalains impart the color to red beets.
The avor, taste, and texture of fresh fruits and vegetables, as well as the taste of
nuts and milk, depend on the presence of natural compounds. These properties are
in many cases carried through to the nal products.
In numerous other commodities, the characteristic sensory attributes are gener-
ated as a result of processing. The texture of bread develops due to interactions of
proteins, lipids, and saccharides with each other and with various gases, while that
of cooked meats appears as the result of thermal protein denaturation. The bouquet
of wine is due to the presence of volatile components in the grape as well as the
result of fermentation of saccharides and a number of other biochemical and chemi-
cal reactions. The delicious color, avor, texture, and taste of smoked salmon or
sturgeon are generated by enzymatic changes in the tissues and the effect of salt and
smoke. The avor of various processed meats develops due to the thermal degrada-
tion of predominantly nitrogenous compounds, the generation of volatile products
of the Maillard reaction, interactions of lipid oxidation products, and the effect of
added spices. Optimum foam performance of beer depends on the interactions of
peptides, lipids, the surface-active components of hops, and gases. The avor, tex-
ture, and taste of cheese result from fermentation and ripening, while the appealing
color and avor of different fried products are due to reactions of saccharides and
amino acids.
The sensory attributes of foods are related to the contents of many chemically
labile components. These, however, just like most nutritionally essential compounds,
are prone also to deteriorative changes in conditions of severe heat treatment, oxida-
tion, or application of considerably high doses of chemical agents, e.g. acetic acid
or salt, which are often required to ensure safety and sufciently long shelf life of
the products. Thus loss in sensory quality takes place, e.g. in over-sterilized meat
8 Sikorski and Piotrowska
and sh products due to degradation of sulfur-containing amino acids and develop-
ment of off-avor; toughening of the texture of over-pasteurized ham or shellsh due
to excessive shrinkage of the tissues and drip; and deterioration of the texture and
arresting of ripening in herring preserved at too high concentration of salt. Optimum
parameters of storage and processing ensure the retention of the desirable proper-
ties of the raw material and lead to the development of the intended attributes of the
product. In the selection of these parameters, the chemistry of food components and
of the effect of processing must be studied. The eager food technology student can
nd all the necessary information in excellent textbooks on food chemistry and in
numerous books on food lipids, proteins, and saccharides, as well as in the current
international journals.
1.4 CHEMICAL ANALYSIS IN ENSURING FOOD QUALITY
1.4.1 INTRODUCTION
All aspects of food quality described earlier can be assured only by applying
appropriate control in the manufacturing process and storage, based on sensory,
physical, chemical, biochemical, and microbial techniques. According to the purpose
of analysis, appropriate techniques and hardware are used, from the most simple
procedures and gadgets to the very sophisticated analytical instruments known in
analytical chemistry. A rational system of control is necessary for the producer of the
raw material, the food processor, the retailer, and even the consumer organizations.
The results of chemical and microbiological analyses are indispensable for
selecting the most suitable parameters of processing and for their implementation,
for designing and operating the hazard analysis and critical control points system
of quality assurance in processing plants, and for securing the safety of the food
products available on the market.
1.4.2 REQUIREMENTS OF THE PRODUCER
Thanks to the possibility of rapid and reliable determination of food composition
and contaminants by applying appropriate techniques, the raw materials can be
optimally used for manufacturing various products. Furthermore, loss in quality, as
well as health hazards, can be avoided.
In the relations between the primary producer and the food processor, usually
the requirements regarding the contents and characteristics of the most important
components, as well as freshness grades of the raw materials, are agreed upon.
Depending on the commodity it may be, e.g.:
• saccharose in sugar beets,
• fat in milk or in mackerel as raw material for hot smoking,
• color of vegetables and egg yolk depending on the concentration of carot-
enoid pigments,
9 Food Components and Quality
• proportion of lean tissue and marbling in pig or beef carcasses,
• connective tissue in meats used for least-cost formulations of sausages,
• contents and characteristics of gluten in wheat grains,
• starch and protein in barley used for malting,
• extract in tomatoes, oil in oil-bearing raw materials,
• free fatty acids and peroxide value in fat-containing commodities,
• trimethylamine, hypoxanthine, or other freshness indicators in marine sh,
• elasticity of kamaboko, the Japanese-type sh cake.
These components and characteristics are usually determined using standard
chemical or physicochemical analyses or enzymatic sensors. For example, the texture
of kamaboko is commonly determined by folding a 5 mm-thick slice of the product
and observing the formed edge. The highest-quality kamaboko can be folded twice
without any cracking; the lowest-quality product falls apart after the rst folding.
Although this test is very simple, it may decide the price of a large consignment
of surimi or kamaboko. Nowadays many companies supply the hardware, reagents,
and analytical procedures for numerous applications in the food plant and for
water eld analysis. Thanks to enormous progress in analytical methodology and
instrumentation, the food chemist can use automated equipment for assaying, e.g.,
water, proteins, lipids, saccharides, ber, and mineral components. Online analyses
provide for continuous control of processing parameters. Among the rapid tests
for food and beverage analysis are those for the determination of ascorbic acid
in vegetable products, calcium, chromium, nickel, and nitrate in drinking water,
hydroxymethylfurfural in honey and tomato products, as well as saccharose in fruits
and juices.
To assist in routine analyses in dairy production many tests, photometric or
reectometric techniques are offered, like reectometric detection of alkaline
phosphatase for controlling milk pasteurization, photometric control of lactose
fermentation and determination of urea, or photometric assay of ammonia in milk.
The characteristic freshness attributes of different foods are usually evaluated
by sensory methods and by the determination of specic indices, predominantly by
biochemical sensors. A typical example may be the examination of sh freshness by
a taste panel and by chemical tests or biochemical sensors suitable for assaying the
volatile odorous compounds and products of nucleotide catabolism. The results of
these kinds of analyses serve as the basis for technological decisions regarding the
suitability of the raw materials for further storage or the given treatment, as well as
for adjusting the processing parameters. They often decide also on the price of the
commodity.
The producer needs chemical analysis to ascertain that the raw material used in
his plant does not contain any harmful components or contaminants in quantities
higher than those accepted by national or international regulations, e.g. nitrates(V)
and nitrates(III) in vegetables, pesticide residues in various crops, heavy metals in
many plant and animal tissues including Hg in large predatory sh, histamine in sh
meat, or mycotoxins in peanuts.
10 Sikorski and Piotrowska
1.4.3 REQUIREMENTS OF THE CONSUMER
The results of routine analyses performed by the producer and by food inspection
laboratories have to ascertain that most consumer expectations regarding nutritious
and wholesome food of high sensory quality are fullled. The consumer generally
requires that foods offered on the market contain the components typical for the type
of product and that their proportions are those as presented on the label. This regards
e.g. the contents of protein and fat in meat products, milk fat in butter, vitamin C
in fruit juices, the unique fatty acid composition of the product sold as extra virgin
olive oil or as n-3 polyenoic fatty acids rich preparation, absence of pork in produce
declared as made of other meats, or meat or sh species other than that specied in
comminuted commodities. Food adulteration has been known as an age-old vice
and chemical analysis helps to combat it. The nutrition-cautious person looks on
the label for information regarding essential amino acids, polyunsaturated fatty
acids, vitamins, mineral components, ber, and recently also functional additives or
GMO products. Many consumers study carefully the labels on packaged foods, since
their health or even life may depend on the information regarding the presence of
different ingredients rich in allergens in the product, e.g. gluten or peanuts. However,
small amounts of such compounds may originate from residues in processing
machinery or stem from additives used by the processor. The safety of food products
is safeguarded by determining e.g. heavy metals and their speciation, polycyclic
aromatic hydrocarbons in oils, heavy smoked sh and meat products, acrylamide
in French fries, mycotoxins in a variety of commodities, and various additives.
For determination of the very large number of hazardous components, additives,
and impurities, many specialized chromatographic, spectroscopic, and physical
techniques, as well as enzyme, microbial, and immunological sensors are used.
1.4.4 LIMITS OF DETERMINATION
By applying efcient procedures of enrichment and separation of analytes, combined
with the use of highly selective and sensitive detectors, it is now possible to determine
different additives and contaminants, as well as the products of various chemical
and biochemical reactions in foods in extremely low concentrations. This is often
necessary, since the national and international bodies responsible for the safety and
authenticity of foods require that the producers conform to regulations allowing very
low amounts of various characteristic components and natural toxic compounds and
contaminants in their products. These requirements are especially rigorous with
respect to foods destined for young children – e.g. the contents of nitrates in potatoes
and other vegetables should not exceed 250 mg NO
3
/kg. The tolerance for various
pesticide residues ranges in different foods from about 0.01 to 20 mg/kg. The content
of benzo[a]pyrene (BaP), one of the recognized representatives of the carcinogenic
PAHs, should be in smoked meat products no higher than 1 μg/kg; for meats treated
with smoke preparations the upper limit of 0.03 μg/kg has been set by the EU. In
Europe, the countries producing olive residual oil have established a maximum level
of 2 μg/kg for each of the eight highly carcinogenic PAHs, but not above 5 μg/kg
11 Food Components and Quality
for the total amount of all eight compounds. In smoked meat and shery products,
in baby foods, and in food oils, according to the regulation of the EC, the maximum
permissible level of BaP is 5 μg/kg, 1 μg/kg, and 2 μg/kg wet weight, respectively.
The detection limit of acrylamide in foods is actually about 10 μg/kg wet weight.
In selecting the most appropriate analytical procedure suitable for the detection
or determination of a compound in a food sample, the properties of the matrix must
be considered. This is especially important in the step of separation of the analyte
from the food material, be it by digestion, membrane techniques, solvent extraction,
supercritical uid extraction, sorption, headspace technique, or steam distillation.
By using procedures comprising extraction of hydrocarbons from the food matrix,
clean-up, separation by GC or HPLC, followed by detection and quantication by mass
spectrometry or in uorescence detectors, it is possible to determine the individual
carcinogenic PAHs at concentrations of the order of 0.1 or even 0.01 μg/kg wet
weight. The accuracy of the results depends signicantly on the quality of standards
used for calibration. Certied reference materials are now available containing up
to 15 PAHs in food samples. For quantitative analysis, internal GC-MS calibration
with stable isotopes added prior to extraction and an MS detector in selected ion
mode may also be used. In studies and routine monitoring regarding nutritional
requirements and food safety aspects, many toxic elements are determined in trace
concentrations of 0.01–10 mg/kg or even in ultra-trace amounts of below 10 μg/kg
by using mainly spectrometric techniques. The lowest dose inducing symptoms of
allergy in highly sensitive persons is about 0.1 mg of peanut or egg protein. This
means, that the applied chemical examination must guarantee the detection of a few
μg of peanut material in one gram of food.
1.5 CONCLUSION
The contemporary market offers consumers in various parts of the world a very large
variety of foods, obtained, handled, and processed by different methods. The qual-
ity of the products depends upon the characteristics of the raw material and on the
after-harvest treatment at home and in the industry. Traditional and science-based
methods as well as reliable control systems of production make it possible to supply
the population with safe food of high sensory quality. Food chemistry is of crucial
importance for realizing this goal. The interested reader can nd information on the
best available books on food chemistry on the Internet.

13
2
Chemical Composition
and Structure of Foods
Jolanta Tomaszewska-Gras
CONTENTS
2.1 Meat ................................................................................................................ 14
2.1.1 Denition of Meat............................................................................... 14
2.1.2 Structure of Meat................................................................................ 14
2.1.3 The Chemical Composition of Meat................................................... 17
2.2 Eggs ................................................................................................................ 19
2.2.1 Foreword............................................................................................. 19
2.2.2 Egg Structure......................................................................................19
2.2.3 Chemical Composition of a Hen’s Egg............................................... 21
2.3 Milk ................................................................................................................23
2.3.1 Denition............................................................................................23
2.3.2 Chemical Composition of Milk..........................................................23
2.4 Cereals ............................................................................................................25
2.4.1 Foreword.............................................................................................25
2.4.2 The Structure of the Grain .................................................................26
2.4.3 Chemical Composition of Cereals......................................................26
2.5 Legumes..........................................................................................................28
2.6. Fruits...............................................................................................................30
2.6.1 Foreword.............................................................................................30
2.6.2 Structure of Fruits...............................................................................30
2.6.3 The Chemical Composition of Fruits ................................................. 31
2.7 Vegetables.......................................................................................................32
2.7.1 Denitions...........................................................................................32
2.7.2 Chemical Composition of Vegetable .................................................. 33
2.7.3 Potato .................................................................................................. 33
2.8 Oil Seeds and Fruits ....................................................................................... 35
2.9 Honey..............................................................................................................36
References................................................................................................................36
DOI: 10.1201/9781003265955-2
14 Jolanta Tomaszewska-Gras
2.1 MEAT
2.1.1 DEFINITION OF MEAT
Denitions of the term “meat” can differ depending on the intention and purpose for
which it is used. In the context of food legislation, for instance, the term “meat” is
dened in Regulation (EC) No. 853/2004, as edible parts of domestic cattle (beef),
pigs (pork), sheep (mutton), goats (goat), farmed birds (poultry), lagomorphs (rabbit),
and wildlife such as deer, rabbits, and sh. This denition applies not only to the
muscular system but also to other edible parts, e.g. internal organs (offal), as well
as bones. However, from the food science point of view, meat most often refers to
skeletal muscle with adjacent connective tissue and associated fat derived from
slaughtered animals of various mammalian species (pigs, cattle, sheep, goats, etc.),
poultry, and sh, but also of seafood or insects. In the broadest sense, meat is the
edible post-mortem component originating from domesticated live animals, as well
as wildlife. In colloquial language, this term means skeletal muscle tissue containing
more-or-less adhering fat and connective tissue. Skeletal muscle is skeletal striated
tissue, which is one of three types of muscle tissue (cardiac muscle and smooth
tissue).
2.1.2 STRUCTURE OF MEAT
At the macroscopic level, meat consists of muscle tissue, which is the contractile
part, and connective tissue, as an elastic part building the tendons and membranes.
Tendons hold the muscle to the bone and the membranes keep the muscle bers bun-
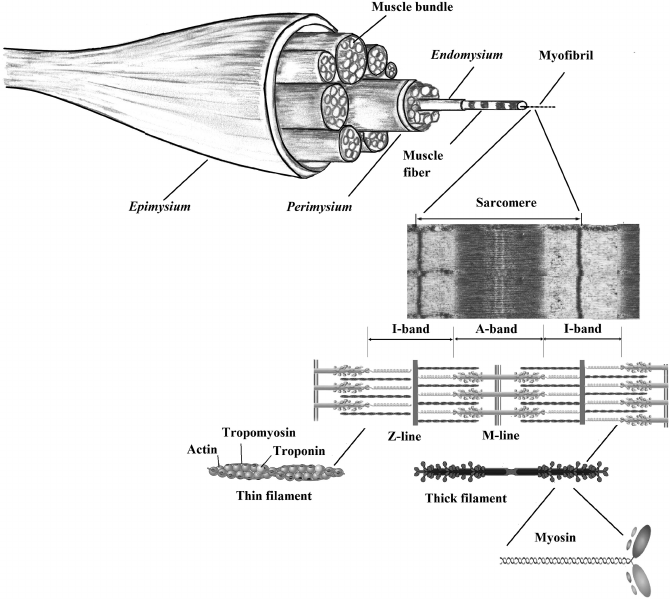
dled together. From the outside, the muscle is covered by the thick and tough mem-
brane called the epimysium, while inside the muscle there are bundles of muscle
bers (multiple fascicles) surrounded by the perimysium, with about 50 muscle bers
in each bundle (Figure 2.1). A single muscle ber is a polynuclear, cylindrical cell,
surrounded on the outside by a connective tissue membrane called the endomysium,
and on the inside by a cell membrane (sarcolemma). The muscle cell, 10–100 µm in
diameter and 1–40 mm long, contains all cellular organelles, such as the nuclei, the
Golgi apparatus, the mitochondria, and the sarcoplasmic reticulum. However, the
most characteristic components of a muscle cell, necessary for motor activities, are
myobrils, about 1 µm in diameter, composed of actin and myosin laments called
myolaments. These are repeated in units called sarcomeres, which are the basic
functional, contractile units of the muscle ber necessary for muscle contraction.
Each myobril consists of:
• thick myolaments composed of myosin,
• thin actin myolaments,
• protein structures of the M and Z lines and cytoskeleton (Figure 2.2).
The segment of myobrils bound on both sides by the Z line constitutes the sarco-
mere, the basic structural unit of the myobrils. The specic arrangement of struc-
tures such as myolaments (along the ber) and the Z line (across the ber) make
15 Chemical Composition and Structure
FIGURE 2.1 Meat structural organization from whole muscle to subcellular myobrils.
the cross-sectional image visible in the longitudinal section under the microscope,
which is caused by differences in refraction. The darker bands of the A (anisotropic)
zone, composed mainly of myosin myolaments with overlapping actin myola-
ments, refract light twice, while the lighter bands of zone I (isotropic), built only of
actin myolaments with the Z line visible in the center, refract light ones. Regulatory
proteins tropomyosin and troponin are bound to the actin chain. The most abun-
dant myobrillar protein is myosin, which forms the thick laments (Figure 2.1) and
makes up about 50% of the total contractile proteins. The myosin with a molecular
weight (MW) of about 500 kDa is composed of two heavy polypeptide chains and
four light polypeptide chains. Contraction of the muscle bers is possible due to the
interaction of myosin with actin by the activity of ATPase, located in the myosin
head. The second major myobrillar protein is actin, a constituent of the thin la-
ment, which can exist in two forms, G-actin, being a small globular molecule (mono-
mer) of about 42 kDa MW, and F-actin, in which the G-actin beads are aggregated
forming a double-stranded helix. In addition to myolaments, the ber also has a
system of structures called the cytoskeleton, which maintains cell integrity, con-
nects the organelles of the cell, and binds them to the sarcolemma. Based on their
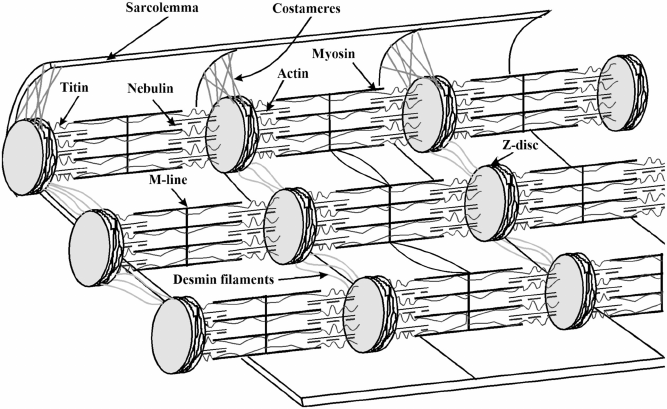
16 Jolanta Tomaszewska-Gras
FIGURE 2.2 Structural arrangement of the muscle cell.
location, the cytoskeleton structures can be divided by whether they are internal or
external to myobrils. Titin and nebulin are among the cytoskeletal proteins forming
the longitudinal skeleton inside the muscle cell, mainly for the contractile proteins of
myosin and actin. As the largest known protein, titin has about 1 µm in length and a
molecular weight of 2,800–3,000 kDa. Intermediate laments, composed of desmin,
synemin, and vimentin, among other proteins, are the transverse skeleton on the
outside of the myobrils that runs crosswise to the muscle ber to integrate and link
adjacent myobrils at the Z line level. Another group of cytoskeletal proteins found
outside myobrils is the submembrane proteins that make up structures called cos-
tameres. Among them are vinculin, dystrophin, ankyrin, talin, and spectrin. They
connect the Z lines of the peripheral sarcomeres with the sarcolemma, i.e. they form
a connection between the cell wall and the entire internal system of interconnected
myobrils, participating in the transmission of nerve impulses.
In general, skeletal muscle can be divided into two types of muscle bers: red,
slow-twitch oxidative (SO) and white, fast-twitch glycolytic (FG). Fibers differ in
composition, structure, metabolism, contractile protein isoforms (e.g. myosin heavy
and light chain), regulatory proteins, muscle contraction rate, and the content of the
sarcoplasmic reticulum, mitochondria, and oxidative phosphorylation enzymes and
glycolytic enzymes and their substrates (Table 2.1).
Muscle bers can be further divided into three subtypes, depending on the activ-
ity of myosin ATPase, the level of metabolic enzymes, and myosin isoforms: I,
IIA, and IIB. Type I (βR), also referred to as SO, are red bers, the thinnest, slow-
contracting muscles, with aerobic metabolism, and therefore containing the most
myoglobin and mitochondria. The second, opposing type is IIB (αW), also referred
to as FG, white bers of the largest diameter, rapidly contracting, with dominant
17 Chemical Composition and Structure
TABLE 2.1
Comparison of the Properties of White and Red Muscle Fibers
Feature of the muscle White fibers IIB, αW (FG) Red fibers I, βR (SO)
Fiber diameter Higher Lower
Glycogen content Higher Lower
Myoglobin content Lower Higher
Content of connective tissue and intramuscular fat Lower Higher
Sarcoplasm content Lower Higher
Number of myobrils per unit Higher
Number of mitochondria Lower Higher
Activity of oxidative enzymes Lower Higher
Activity of glycolytic enzymes Higher Lower
Activity of myosin ATPase enzymes Higher Lower
The rate of contraction Higher Lower
Ca
2+
uptake by the sarcoplasmic reticulum Faster Slower
Time of active contraction state Longer Shorter
Intensity of rigor mortis Lower Higher
Duration of rigor mortis Lower Higher
Metabolism Anaerobic Aerobic
glycolytic metabolism. There is also an intermediate type IIA (αR), referred to as FO
(fast-twitch oxidative), classied as a fast-twitch red ber with oxidative-glycolytic
metabolism. White bers contain more glycogen, have a higher activity of ATPases,
phosphorylases, and glycolytic enzymes, mainly with anaerobic metabolism, and
contract faster and more vigorously. In turn, red bers, mainly those involved in
oxygen metabolism, contract more slowly, and their efciency depends on the rate of
supply of oxygen and substrates for energy transformations. In addition, they show
a high activity of oxidative enzymes and have a greater number of mitochondria.
Most skeletal muscles are heterogeneous and contain all types of bers in varying
proportions, although the pectoral muscle of chickens is an example of a muscle that
contains only white bers of anaerobic metabolism, rapidly contracting.
2.1.3 THE CHEMICAL COMPOSITION OF MEAT
Lean meat contains an average of 70–75% water, 19–21% protein, 1–5% fat, 0.8–
1.8% mineral components, and 0.4–1.2% saccharides. The content of saccharides
decreases after slaughter as they undergo glycolytic changes. The chemical compo-
sition of meat is determined to the greatest extent by the genotype (species, utility
type, breed, breeding line), age, body weight, feeding method, and the conditions
of rearing, as well as the location of the muscles in the animals’ organism. The
variation in meat composition is mainly related to the fat content, which can range
between 0.7% for chicken breast and 29% for beef brisket, as shown in Table 2.2.

18 Jolanta Tomaszewska-Gras
TABLE 2.2
Fat and Cholesterol Content in the Meat of Animals of
Different Species
Cholesterol
Type of meat Part of carcass Fat (g/100 g) (mg/100 g)
Poultry chicken Breast without skin 0.7 43.4
Breast with skin 6.2 61.4
Leg without skin 6.45 84.0
Leg with skin 15.1 84.6
Poultry goose Breast without skin 5.9 80.7
Breast with skin 28.2 76.8
Poultry duck Breast without skin 6.7 87.3
Breast with skin 16.1 81.7
Poultry turkey Breast 1.5 53
Thigh 2.4–3.8 37–62
Separable wing meat 0.9 46
Pork Tenderloin 3.3–7.4 45–91
Belly 10.3–35.1 70–120
Loin 1.1–7.1 31–62
Ham 1.6 51.3
Chuck steak 11.9 62.2
Spare ribs 5.1–17.6 46–102
Beef Blade 2.9 56.7
Striploin 11.3 46.7
Chuck 6.8 55
Brisket 14 52
Lamb Longissimus muscle 3.8–6.9 60–70
Source: Honikel and Arneth, 1996; Dinh et al., 2011.
Particularly in the case of sh, there is a large variation in the amount of fat, depend-
ing on the species, which is the basis for their classication as sh:
• lean, containing less than 2% fat (pike, hake, pollock, halibut, cod),
• medium fat, 2–7% (carp, redsh, catsh, tuna, trout),
• fatty, 7–15% (salmon, sprat),
• full-fat, over 15% (herring, mackerel, sardine, eel).
On the other hand, within the same species, differences in the fat content can be
observed between different breeds or genetic lines; for example, in pigs, the differ-
ences between breeds in the average fat content are approx. 2.5%, while in the case of
ducks it is approx. 14%. In addition to the species of animals and the type of culinary
element of the meat, gender has a signicant impact on the chemical composition. In
general, females have more fat than males, since males are more muscular. Likewise,
19 Chemical Composition and Structure
age and body weight have an inuence on fat content. Adipose tissue begins to grow
more intensively after the animals reach their maximum muscle mass. Older animals
tend to have higher fat and lower water content. The presence of fat is related to the
occurrence of another component characteristic of animal tissue, which is cholesterol.
Its content in the muscle tissue of various animal species may range from 43 to 87 mg
in 100 g of tissue, as shown in Table 2.2. Cholesterol is present not only in the meat of
mammals and birds, but also in the meat of sh. For example, cod contains about 57
mg of it, herring 65 mg, and mackerel 72 mg per 100 g of muscle tissue. The cholesterol
content of offal is even higher, for instance, in 100 g of poultry liver, it is 350–700 mg,
and in 100 g of pig or bovine liver 200–400 mg. Meat is also a valuable source of min-
eral compounds and B vitamins. The heme pigments present in the muscle tissue, i.e.
myoglobin and hemoglobin, contain iron ions with high bioavailability. Bovine meat
and breast muscles of ducks and geese contain the most heme pigments, 4–4.5 mg per
1 g, while the breast muscles of chickens or turkeys contain 0.2–0.6 mg per 1 g. Among
the B group vitamins, 100 g of meat contains approx. 0.7–0.85 mg of thiamine, 0.1–0.3
mg of riboavin, 5–7 mg of niacin, and 0.7 mg of pantothenic acid.
2.2 EGGS
2.2.1 FOREWORD
Eggs are edible products whose trade name refers to hen’s eggs, in accordance with
Reg. EC No. 589/2008, although the eggs of other species are also sold. However,
those of other birds (geese, ducks, plovers, seagulls, quail) are of lesser signicance,
thus the term “egg,” without a prex, generally relates to hen’s eggs. The egg
contains all the nutrients necessary for the embryo’s growth, without the need to
access external sources of food. Due to the richness of highly digestible nutrients, it
is a valuable food product for humans. The proteins present in eggs contain all the
essential amino acids.
2.2.2 EGG STRUCTURE
The whole egg is composed of an eggshell (approx. 10%), egg white (albumen) (57%),
and egg yolk (33%). The weight of a hen egg varies considerably within the same
species; it can range from 53 to 73 grams, which is the basis for qualitative classica-
tion (Reg. EC No. 589/2008). There is even greater variation between different bird
species, from 12 grams for a quail egg to 1.5–2 kg for an ostrich egg. In the macro-
scopic structure of an egg (Figure 2.3), the following structures can be distinguished
from the outside:
• the shell with the membrane,
• the egg white (albumen) with chalazae,
• the yolk with embryonic disc (blastoderm).
The eggshell, 0.2–0.4 mm thick, consisting of two layers, spongy (outer) and mam-
millary (inner), makes up 10–12% of the weight of the egg. It is composed mainly
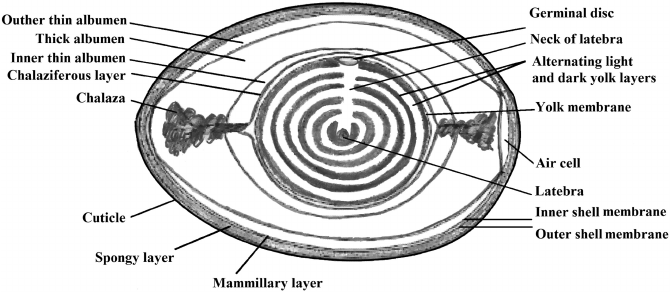
20 Jolanta Tomaszewska-Gras
FIGURE 2.3 Macrostructure of the egg.
of mineral substances (approx. 95%), mostly calcium carbonate. On the outside, it
is covered with a thin lm, called the cuticle or bloom. The shell protects the con-
tents of the egg from damage and contamination and allows gas exchange due to the
pores it contains. Directly under the shell, there is the shell membrane, composed
of two layers (48 and 22 μm, respectively), each of which is an interwoven network
of proteins and polysaccharides bers. As the egg is laid, an air cell is formed at the
large end of the egg, approx. 5 mm in diameter and increases in size during stor-
age, hence it can be used to determine the age of eggs. In the shell, funnel-shaped
minute pores can be seen (7,000–17,000 per egg). They are partially sealed by the
proteins of the cuticle but remain permeable to gases while restricting penetration by
microorganisms. The diameter of the pore canals ranges from 10 to 30 µm. The egg
albumen (egg white), constituting about 57% of the egg mass, protects the embryo
against physical damage, but also against microbial infections. It contains approx.
88% water and consists of four layers:
• external thin albumen,
• thick albumen,
• internal thin albumen,
• chalaziferous layer with chalazae.
These layers represent 23%, 57%, 17%, and 3% of the total protein weight, respec-
tively. The viscosity of thick albumen, much higher than that of thin albumen, is
caused by the four times higher content of ovomucin. In the long axis of the egg,
the chalaziferous layer, mainly composed of mucin bers, is twisted at both sides of
the yolk, forming a thick rope-like structure named chalaza, which holds the yolk in
the center. It is twisted clockwise at the small end of the egg and counterclockwise
at the large end. The yolk, comprising 32% of the weight of the egg, in fact, is one
of the biggest ova. It is surrounded by a vitelline membrane and contains genetic
material in the form of the germinal disc and the nutrient material for the growing
21 Chemical Composition and Structure
embryo. Structurally, the inner content is composed of yellow yolk and white yolk,
whose weight is less than 2% of the total egg yolk. The yellow yolk consists of alter-
nate light layers and deep yellow layers. In the center of the egg yolk, the latebra is
located, which is the basic nutritional material for the embryo in the rst days of life.
It is connected by a tubule (neck of the latebra) to the embryonic disc in the nucleus
of the pander on the surface of the yolk (2–3 mm in diameter).
2.2.3 CHEMICAL COMPOSITION OF A HEN’S EGG
In terms of chemical composition, the egg contains the most water, approx. 73.5%
(Table 2.3), while the content of saccharides and minerals is the lowest – approx.
1%. Proteins are present in both the albumen and yolk. Egg albumen contains oval-
bumin, ovotransferrin, ovomucoid, ovomucin, lysozyme, and avidin, which mainly
have an enzymatic and antibacterial function, while the yolk contains mainly com-
plex proteins. Ovalbumin is the main albumen protein, consisting of 54% of total
egg white proteins. Several albumen proteins have biological activity as enzymes
(e.g. lysozyme), enzyme inhibitors (e.g., ovomucoid, ovoinhibitor) and complex-
forming agents for some coenzymes (e.g., avoprotein, avidin). Egg yolk is an oil-
in-water emulsion with about 50% dry weight. The main proteins of egg yolk are
LDL-lipoproteins (68%), HDL-lipoproteins (16%), livetins (10%), and phosvitins
(4%). Egg yolk can be separated by centrifugation into plasma (90% of yolk lipids
and 50% of yolk proteins) and granules (7% of yolk lipids and 50% of yolk proteins).
Plasma, which is 71–81% of the yolk dry weight, is composed of LDL (85%) and
glycoproteins: α, β, and γ livetins (15%). The granules, constituting 19–23% of the
yolk’s dry weight, are composed of the HDL: α- and β-lipovitellins (70%), phosvitin
(16%), and LDL (12%). Lipids, on the other hand, are found only in the yolk and
account for about 64% of the yolk’s dry weight. They include mainly triacylglycerols
(TAGs), which constitute 65% of all lipids, phospholipids with a share of 31%, and
sterols with 3%. TAGs contain the biggest amount of the following fatty acids:
• oleic, about 42%,
• palmitic, about 30%,
TABLE 2.3
Chemical Composition of Hen Egg (%)
Whole egg Whole egg Egg Egg white
(with shell) (without shell) yolk (albumen) Eggshell
Water 66 73 48 88 1.6
Proteins 12 13 17 11 3.3
Lipids 10 12 33 0.01 –
Saccharides 1.0 1.0 1.0 0.9 –
Mineral compounds 11 1 1.0 0.6 95

22 Jolanta Tomaszewska-Gras
• linoleic, about 10%,
• stearic and arachidonic, about 3%.
In the total composition of all fatty acids, monounsaturated ones constitute approx.
42%, and polyunsaturated fatty acids approx. 12%. The ratio of n-6 to n-3 acids is
10:1. Among the phospholipids, lecithins (phosphatidylcholine) and cephalins (phos-
phatidylethanolamine) are the most abundant in the yolk (Table 2.4). The yolk also
contains cholesterol, the content of which per 100 g of egg is approximately 370 mg,
while a medium-sized hen’s egg contains approximately 200–215 mg of cholesterol.
Carotenoids, the natural pigments of hen egg yolk, are mainly composed of carotene
and xanthophylls (lutein, cryptoxanthin, zeaxanthin), which are present in the yolk
lipids in an amount of less than 1%.
During storage, a series of chemical and structural changes occurs in eggs,
including a decrease in the acidity of the egg white and yolk. This is caused by
the diffusion of CO
2
through the pores of the shell, which starts soon after the egg
is laid, especially in the egg white. The pH of the albumen varies from 7.6 to 9.7,
while the pH of the yolk varies from 6.0 to 6.8. The chalazae are weakened and the
density of the thick albumen drops, which causes the yolk to deviate from the cen-
tral position. The air cell expands due to the evaporation of water through the shell
pores. There is also diffusion of water from the egg white into the yolk, the volume
of which increases. The yolk also becomes less resilient due to the weakening of
the vitelline membrane, which is seen on the level surface after the egg is cracked
as a attening. This is measured as a yolk index for determining the age of an egg,
expressed as the ratio of yolk height to diameter. Considering chemical changes
in proteins of egg white, during storage the more heat-stable S-ovalbumin (tem-
perature of denaturation 92.5° C) is formed from the native protein (temperature of
denaturation 84.5° C), causing an increase in S-ovalbumin from 5% in fresh eggs to
81% in eggs cold-stored for six months. The whole egg weight loss during storage
ranges from 3.0 to 6.5%.
TABLE 2.4
Phospholipids Composition in Egg Yolk as Percent
of Phospholipid Fraction (%)
Phospholipids %
Phosphatidylcholine 73
Phosphatidyl ethanolamine 15.5
Lysophosphatidylcholine 5.8
Sphingomyelin 2.5
Lysophosphatidylethanolamine 2.1
Plasmalogen 0.9
Phosphatidyl inositol 0.6
Source: Sugino, Nitoda, and Juneja, 1997
23 Chemical Composition and Structure
2.3 MILK
2.3.1 DEFINITION
Long before recorded history, the rst food provided for humankind was from the
woman’s mammary glands. In distant times, when it was not possible for the new-
born child to suckle from the mother, and people found that milk from other animals
was good, they began domesticating milk-producing animals. Milk is the secretion
of the mammary gland of female mammals that appears during the lactation period.
The denition included in Regulation (EC) No. 853/2004 denes raw milk as that
obtained from the mammary glands of farmed animals which has not been heated
to above 40° C or any other treatment having an equivalent effect. For humans, the
most important milk is from cows, goats, sheep, and buffalo, but the milk of the
donkey, reindeer, camel, buffalo, musk ox, and alpaca can also be used for consump-
tion, although these milks are of lesser signicance. Thus, the term “milk,” without
a prex, generally relates to cow’s milk. In terms of composition and structure, milk
is an oil-in-water emulsion consisting of a three-phase system:
• continuous, which is an aqueous solution of lactose, mineral salts, and
water-soluble proteins,
• casein micelles,
• fat globules,
which are stabilized by phospholipids and proteins.
2.3.2 CHEMICAL COMPOSITION OF MILK
In the chemical composition of milk, as shown in Table 2.5, the biggest component is
water (from 82 to 88%), then protein and fat in relatively similar proportions in cow’s
and goat’s milk (from 3.5 to 4.0%), while in sheep’s milk the fat content is approx.
6.5% and the protein content approx. 6%. The composition of milk is determined to
the greatest extent by the species of mammals, and to a lesser extent by the lactation
period, feed composition, and the health condition of the udder.
Cow’s milk proteins can be divided into three groups: caseins, whey proteins,
and blood proteins (Table 2.6). Caseins, belonging to phosphoproteins, are the main
proteins in milk (78–82% of the total amount of milk protein). They play a very
important role in cheese-making due to their ability to form a curd. Proteins, belong-
ing to the group of caseins: calcium-sensitive (α
S1
-casein, α
S2
-casein, and β-casein)
and calcium-insensitive (κ-casein and γ-casein) exist in milk in a unique, highly
hydrated spherical complex with calcium phosphate, named casein micelle. These
micelles have a broad range of diameter from 30 to 600 nm, which are on average ten
times smaller than fat globules. The next group of proteins, known as whey proteins,
consists of β-lactoglobulin and α-lactalbumin, which are, in contrast, soluble in the
nonmicellar, aqueous phase in monomeric and dimeric forms. These two groups of
proteins are separated by agglomeration of casein micelles during the forming of the
curd, for instance, by enzyme treatment in cheese production. In addition, there are
also blood proteins (serum albumin and immunoglobulins [Igs]) in milk, which, in

24 Jolanta Tomaszewska-Gras
TABLE 2.5
Chemical Composition of Mammalian Milk of Various Species (%)
Water Protein Lipid Lactose Ash
Alpaca 83.7 5.8 3.2 5.1 1.6
Buffalo 83.2 4.0 7.4 4.4 0.8
Llama 84.8 4.1 4.2 6.3 0.7
Moose 76.8 10.5 8.6 2.6 1.6
Donkey 82.6 5.2 6.8 4.8 0.8
Man 87.8 1.0 3.8 7.0 0.2
Horse 89.8 2.0 1.6 6.6 0.4
Cow 88,1 3.2 3.3 5.1 0.7
Goat 87.7 2.9 4.5 4.1 0.8
Donkey 90.8 1.6 0.7 6.4 0.4
Sheep 80.7 4.5 7.4 4.8 1.0
Reindeer 67.9 10.4 16.1 2.9 1.5
Dromedary camel 89.0 3.1 3.2 4.3 0.8
Bactrian camel 84.8 3.9 5.0 4.2 0.9
Musk ox 83.6 5.3 5.4 4.1 1.6
Source: Huppertz and Kelly, 2009; Medhammar et al., 2012.
TABLE 2.6
Composition of the Major Proteins of Cow’s Milk
Proteins Concentration (g/100 g) Isoionic point Molecular weight (kDa)
Caseins 24.7–28.9
α
S1
-casein
12.4–15.5 4.92–5.35 23.6
α
S2
-casein
3.1–4.1 25.2
β-casein
9.3–11.3 5.20–5.85 24
κ-casein
3.1–4.1 5.77–6.07 19
γ-casein
1–2.1 5.8–6.0 12–21
Whey proteins 5.2–7.2
β-lactoglobulin
2.1–4.1 5.35–5.41 18.3
α-lactalbumin
1–1.5 4.2−4.5
a
14.2
Proteose-peptones 0.6–1.9 3.3–3.7 4–41
Blood proteins
Serum albumin 0.1–0.4 5.13 66.3
Immunoglobulins 0.6–1.0
IgG1 5.5–6.8 162
IgG2 7.5–8.3 152
IgA 400
b
IgM 950
c
Notes: a: isoelectric point; b: dimer; c: pentamer.
Source: Horne, 2017; Belitz, Grosch, and Schieberle, 2009.

25 Chemical Composition and Structure
TABLE 2.7
Lipid Composition of Cow’s Milk
Cow’s milk lipid fractions Percent of the total lipid (%)
Triacylglycerols 95–96
Diacylglycerols 1.3–1.6
Monoacylglycerols 0.02–0.04
Keto acid glycerides 0.9–1.3
Hydroxy acid glycerides 0.6–0.8
Free fatty acids 0.1–0.4
Phospholipids 0.8–1.0
Sphingolipids 0.06
Sterols 0.2–0.4
(cholesterol, cholesterol esters)
Source: Belitz, Grosch, and Schieberle, 2009.
contrast to caseins and whey proteins, are not produced by the mammary glands, but
are derived from the blood.
The second signicant component of milk is fat, which is used in the production
of cream, butter, and anhydrous milk fat. Milk fat (milk lipids) consists of 98% TAG,
concentrated in the form of globules of 2–6 µm in diameter. Minor components of fat
globules are diacylglycerols, monoacylglycerols, free fatty acids, polar lipids, sterols,
and fat-soluble vitamins (Table 2.7). The main fatty acids present in TAG are butyric,
capric, lauric, myristic, palmitic, stearic, oleic, and linoleic. However, more than 400
different fatty acids have been detected in cow’s milk lipids. Milk fat is also a valu-
able source of conjugated linoleic – rumenic (CLA) and vaccenic acid (VA), which
have a proven health-promoting effect. Lactose, with sweetness about one-fth of
that of sucrose, is the disaccharide characteristic only for milk. It is present in milk
in α- and β-forms. The α-form can be responsible in dairy products like ice cream
for a sandy mouthfeel. In the dairy manufacturing industry, β-galactosidase is com-
monly used to hydrolyze lactose into glucose and galactose to produce lactose-free
dairy products.
2.4 CEREALS
2.4.1 FOREWORD
Cereals and cereal products are amongst the most important basic foods for humans.
The rst cereal grown systematically was probably barley, which was known as early
as 5000 BC in Egypt and Babylon. Cereals are plants from the Poaceae family that
are of great importance as raw materials for the production of our, groats, beer, and
alcohol, as well as for animal feed, because of their high starch content. The raw
materials for milling and groats processing are common wheat, also known as bread
wheat (Triticum vulgare), durum wheat (Triticum durum), spelt (Triticum spelta L.),
26 Jolanta Tomaszewska-Gras
rye (Secale cereale), triticale, barley (Hordeum vulgare), and oats (Avena sativa L.),
as well as maize (Zea mays L.), rice (Oryza sativa L.), buckwheat (Fagopyrum escu-
lentum Moench), sorghum (Sorghum Moench), and millet (Panicum miliaceum).
Botanically, buckwheat is not a cereal, but because of its widespread use in cereal
products, it will be described in this chapter. Commercially, the most important cere-
als are wheat, maize, and rice. Durum semolina is generally the best type of wheat
for the production of pasta.
2.4.2 THE STRUCTURE OF THE GRAIN
Cereals form a relatively large fruit, termed a caryopsis, in which the pericarp and
seed are adherents. The shape of grains varies from elongated (rye) to spherical (mil-
let), but in structure they are similar. Cereal grain consists of the germ, endosperm,
and seed coat, also known as bran, and consists of three major parts:
• the germ (including the scutellum), which produces the new plant,
• the endosperm, rich in starch, which serves as food for the germinating
seed and is the raw material of our manufacture,
• bran (husk, coat) with various covering layers protecting the grain (Figure 2.4).
The average wheat grain composition is approx. 85% endosperm, 13% bran, and 2%
germ.
The bran is composed of ve layers, which protect the endosperm and embryo
against damage and drying out. The germ is located at the base of the grain, and
it contains the embryo, which is rich in lipids (28%), proteins (34%), and vitamins
(6%). The membranous tissue called the scutellum separates the germ from the
endosperm. The most important part from the technological point of view is the
endosperm, which contains mainly starch and proteins. These serve as food during
germination. The endosperm consists of two elements: the aleurone layer, which
lies directly under the seed coat, and the endosperm cells with starch granules, the
main part of the endosperm. The aleurone layer makes up about 7% of the grain
weight and consists of proteins, fat, vitamins, and enzymes. During milling, this
layer passes along with the coat to the bran. The main component of the endosperm
is starch, which comprises 80% of the total compounds in this structure, and 13% of
proteins. The polysaccharide molecules in starch granules are organized radially and
vary in size and form between different cereals.
2.4.3 CHEMICAL COMPOSITION OF CEREALS
The major saccharide storage form of cereals is starch, which occurs only in the
endosperm cells (Table 2.8). Due to the presence of alternating amorphous (mainly
amylose) and semicrystalline layers (amylopectin), differences in the refraction
index can be observed. Cereal starches consist of about 25% amylose and 75% amy-
lopectin, except waxy corn, which contains only amylopectin. Starch granules swell
when heated in a water suspension and by gelatinizing, they lose their form.
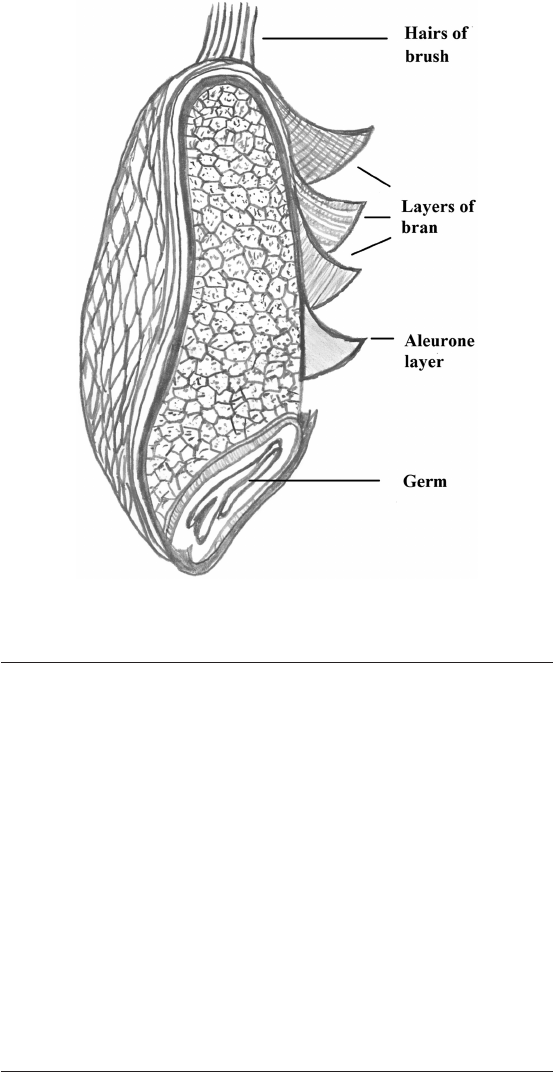
27 Chemical Composition and Structure
FIGURE 2.4 Structure of a wheat grain caryopsis.
TABLE 2.8
Chemical Composition of Cereals (%)
Wheat Rye Corn Barley Oats Rice Millet
Moisture 13.2 13.7 12.5 11.7 13.0 13.1 12.1
Lipids 2.2 1.7 3.8 2.1 7.1 2.4
a
4.05
Saccharides 59.6 60.7 64.2 63.3 55.7 74.1 68.8
Fiber 13.3 13.2 9.7 9.8 9.7 2.2 3.8
Proteins (N × 6.25)
11.7 9.5 9.2 10.6 12.6 7.4 10.6
Protein distribution (%) in Osborne fraction
Albumins 14.7 44.4 4.0 12.1 20.2 10.8 18.2
Globulins 7.0 10.2 2.8 8.4 11.9 9.7 6.1
Prolamins 32.6 20.9 47.9 25.0 14.0 2.2 33.9
Glutelins 45.7 24.5 45.3 54.5 53.9 77.3 41.8
Note: a: polished rice: 0.8%.
Sourc: Belitz, Grosch, and Schieberle, 2009.
28 Jolanta Tomaszewska-Gras
TABLE 2.9
Chemical Composition of Different Types of Wheat Flour
Type of wheat flour 405 550 812 1,050 1,700
Starch 82 82 78 78 69
Proteins (N × 5.8)
12 12 13 13 13
Lipids 1.0 1.2 1.5 2 2.3
Dietary ber 5 5.0 6 6 13
Ash 0.4 0.6 0.8 1 2
The other grain saccharides are glucose, fructose, and sucrose, present mainly in
the embryo, while in the coat, there are also cellulose and hemicellulose, which are
the main components of the dietary ber in the bran (78%). There are also proteins
in the grain, which, despite their low content (7.4–12.6%), play an important role in
the process of making the dough. The main proteins of cereal grains are proteins,
classied by Thomas Burr Osborne according to different solubilities:
• albumins can be removed with water and also remain in solution at the
isoelectric point,
• globulins can be extracted with saline solution,
• prolamines can be extracted with 70% ethanol,
• glutelins are not extractable; they remain in the residue.
Osborne fractions derived from different cereals are often designated by special
names. For instance, glutelins and prolamines of wheat are called glutenin and glia-
din while in corn they are zeanin and zein, in rice they are oryzenin and orizin, and
in barley they are hordenin and hordein. Wheat gluten proteins fractionated by the
Osborne method provide glutenins and ω-, α-, γ-gliadins in a ratio of 1:1. Both frac-
tions, in their hydrated form, have different effects on the rheological characteristics
of dough: prolamins are responsible, preferentially, for viscosity, and glutelins for
dough strength and elasticity. The basic product produced from the wheat grains in
the milling process is our, for which different types are produced, depending on the
composition (Table 2.9).
2.5 LEGUMES
Legumes, also known as pulses, the common feature of which is the fruit in the form
of pods, are annual plants from the family of Fabaceae Lindl. The ripe seeds of the
pods are primarily an important source of proteins. The common pulses used as food
are broad beans (Vicia faba L.), chickpeas (Cicer arietinum L.), beans (Phaseolus
L.), peas (Pisum L.), lupins (Lupinus L.), peanuts (Arachis hypogaea L.), lentils
(Lens culinaris L.), and soybeans (Glycine max). Beans are eaten not only in the
form of seeds but also in the form of pods such as green beans (string beans). There

29 Chemical Composition and Structure
are many varieties of beans, such as the garden bean, black bean, red bean, borlotti
bean, pinto bean, ageolet bean, mung bean, adzuki bean, rice bean, kidney bean,
lima bean, and jack bean.
Legume seeds are relatively high in protein and saccharides, and some also con-
tain fat. Broad beans and soybeans are distinguished by their high protein content,
38% and 44%, respectively (Table 2.10). Due to its amino acid composition most
similar to meat, soybean protein is used in the production of protein concentrates and
isolates as the main component of vegetarian dishes. Moreover, soybeans are char-
acterized by a high fat content, approx. 20%, which also makes them a raw material
for the fat industry. Even more fat is found in peanuts, as much as 50%. Peanuts are
also a good source of niacin, as they contain about ve times more (14.2 mg/100 g)
than the seeds of other legumes. Peanut seeds ripen in the ground to a depth of about
5–8 cm, where the pod is pushed through the peduncle after the ower fades. Peanuts
are the raw material for the production of peanut butter and peanut oil. Generally,
in all legumes, three types of proteins are predominant: albumins, globulins, and
glutelins. With regard to biological value, legume proteins are somewhat decient
in S-containing amino acids. Glycinin (350 kDa MW) and β-conglycinin (156 kDa
MW) are two of the most important allergenic proteins in soybean with medium
thermal stability. In peanuts, two glycoproteins are predominant allergens: Ara h1 (65
kDa MW, 7S globulin, vicilin) with high thermal stability and Ara h2 (17 kDa MW)
with medium thermal stability. The major carbohydrate in legumes is starch, amount-
ing to 75–80%. Oligosaccharides in legumes are present in higher concentrations than
in cereals, with sucrose, stachyose, and verbascose being predominant. After legume
consumption, oligosaccharides might cause atulence, a symptom of gas accumula-
tion in the stomach or intestines as a result of the growth of anaerobic microorgan-
isms in the intestines, which hydrolyze the oligo- into monosaccharides and cause
their further degradation to CO
2
, CH
4
, and H
2
. In terms of nutritional value, legume
seeds contain ingredients with high nutritional value, such as proteins, fat, niacin,
isoavones (phytoestrogens), as well as anti-nutritional components such as trypsin
inhibitors, phenolic compounds, phytates, cyanogenic compounds, lectins, saponins,
as well as allergenic proteins, most of which are inactivate after heat treatment and
can be substantially reduced by milling, cooking, germination, and fermentation.
TABLE 2.10
Chemical Composition of Various Legumes
Type of legume Protein Lipid Saccharide Ash
Broad bean 38.0 1.7 52.0 3.7
White beans 21.0 1.6 62.0 1.9
String bean 22.0 2.0 72.0 1.7
Pea 30.0 1.4 67.0 1.5
Peanut 30.0 50.0 14.0 3.0
Lentils 30.0 3.0 62.0 2.5
Soy 44.0 19.6 33.0 3.4
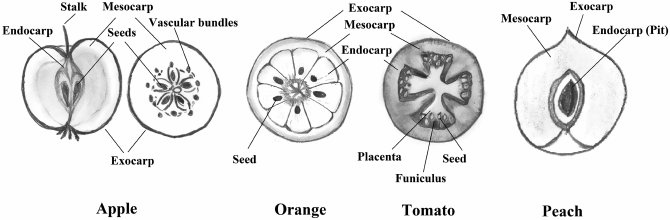
30 Jolanta Tomaszewska-Gras
2.6. FRUITS
2.6.1 FOREWORD
In botanical terms, fruits are organs of angiosperms formed from the ovary of the
pistil, or the ower bottom. However, in the culinary sense, this denition is nar-
rowed down to the edible parts of trees or shrubs, which no longer includes the fruits
of herbaceous plants such as cucumbers or tomatoes. In turn, the exception to this
denition is strawberries, which are the fruit of perennials. Hence, all fruits can be
divided into fruits of fruit trees from the temperate zone (pear, apricot, apple, cherry,
plum, peach, hazelnut, walnut), fruits of fruit trees from the subtropical and tropical
zones (lemon, g, pomegranate, grapefruit, orange, mandarin, mango, kiwi, date,
coconut, avocado), fruit bushes (gooseberry, chokeberry, blueberry, lingonberry, bil-
berry, blackberry, raspberry, blackcurrant, red currant, white currant, grape, cran-
berry), and fruits of perennials (wild strawberry, strawberry). Due to their structure,
they can be divided into fruits as follows:
• pome (apples, pears, quinces), which are formed from the ovary of the pistil
and the bottom of the ower,
• pitted (cherries, peaches, plums), which are formed exclusively from the
ovary of the pistil,
• berry (blueberries, gooseberries, chokeberries), characterized by a pericarp
with numerous seeds.
In terms of nutrition, fruit is one of the most valuable sources of natural antioxidants,
such as ascorbic acid, carotenoids, and phenolic compounds.
2.6.2 STRUCTURE OF FRUITS
The anatomical structure of the fruit distinguishes seeds and the pericarp, which
includes the exocarp, mesocarp, endocarp, and conductive bundles (Figure 2.5). The
outer part of the pericarp is called the exocarp, which is the epidermis of the fruit,
i.e. the skin, which is usually the cuticle, which protects the fruit against water loss,
FIGURE 2.5 Illustration of anatomical structures of different types of fruits.

31 Chemical Composition and Structure
and sometimes it is additionally a hair (e.g. peaches). The middle part of the pericarp
is the mesocarp, which is the pith tissue of the fruit, and the inner part is the endo-
carp, which in the case of stone fruit hardens and becomes woody, forming a stone
with the seed inside, unlike the berry, where this part is completely eshy.
2.6.3 THE CHEMICAL COMPOSITION OF FRUITS
Water is the most abundant in fruit (up to 96%) and saccharides are also key com-
ponents of the fruit, with content ranging from 7.2% in strawberries to 23.5% in
bananas (Table 2.11). Glucose and fructose are the most abundant saccharides, which
are stored in the parenchyma tissue. The fruit peel contains cellulose, hemicellulose,
and pectins, which are components of dietary ber. Fruits are also a rich source
of other nutrients, such as vitamins (vitamin C, carotenoids), phenolic compounds,
organic acids (citric, malic), pigments (chlorophylls, carotenoids, anthocyanins), and
TABLE 2.11
Chemical Composition of Fruits per 100 g of Edible Tissue
Type of fruit Protein (g) Lipid (g) Saccharide (g) Vitamin C (mg) Fiber (g)
Acerola 0.4 0.3 6.8 1300 0.4
Apple 0.2 0.6 14.1 7 2.0
Apricot 1.0 0.2 12.8 10 0.6
Avocado 2.1 16.4 6.3 14 1.6
Banana 1.2 0.2 29.0 15 0.4
Blackberry 1.2 0.9 12.9 21 4.1
Blackcurrant 1.7 0.1 13.1 200 2.4
Blueberry 0.7 0.5 15.3 14 1.5
Cherry, sweet 1.3 0.3 17.4 10 0.4
Cherry, sour 0.9 0.4 10.9 12 1.0
Gooseberry 0.8 0.2 9.7 30 1.9
Grape 0.6 0.3 17.3 4.0 0.5
Grapefruit 0.5 0.1 10.6 30 0.2
Hazelnuts 14.4 63.0 14.9 3.0 8.9
Kiwi 0.9 0.5 13.9 71 2.1
Lemon 1.1 0.3 8.2 53 0.4
Orange 0.9 0.2 12.2 50 0.5
Pear 0.7 0.4 15.3 4.0 1.4
Pineapple 0.3 0.2 13.7 17.0 0.4
Plum 0.8 0.2 19.7 4.0 0.4
Pomegranate 0.5 0.3 16.4 4.0 0.2
Raspberry 1.5 1.4 15.7 18 5.1
Strawberry 0.7 0.5 8.4 59 1.3
Walnut 16.0 60.3 18.0 5.8 6.5
Source: Ensminger et al., 1995.
32 Jolanta Tomaszewska-Gras
aromatic compounds giving them a characteristic aroma. Carotenoids are wide-
spread in fruits; 26 different carotenoids have been detected and are the main factor
determining color in some fruits, such as citrus fruits, peaches, and sweet melons.
In fruits, several hundred polyphenols (classied into six groups according to the
number of phenol rings: hydroxybenzoic acids, hydroxycinnamic acids, avonoids,
chalcones, stilbenes, and lignans) have also been identied, which are antioxida-
tively active and contribute to the color and taste of many types of fruit.
A special kind of fruit is nuts, consisting of a hard nutshell protecting a kernel,
which is usually edible. A wide variety of dry seeds are called nuts in general usage
or in a culinary sense, but in the botanical meaning, “nut” implies that the shell does
not open to release the seed, like in the case of walnuts, hazelnuts, or chestnuts.
The storage time of fruits varies from four to eight months for apples, two to six
months for pears, two to three months for grapes, and a few days for strawberries,
raspberries, and cherries. Commonly used conditions are a temperature of about
2–4° C at 80–90% relative humidity. Some fruits, like apples, kiwis, and pears, have
high ethylene production (the volatile promoter of fruit ripening), in contrast to cher-
ries, grapes, and pineapples, which can be used to ripen the fruit by enclosing both
types of fruit in a paper bag. Weight losses occur during fruit storage due to moisture
losses of 3–10%.
2.7 VEGETABLES
2.7.1
DEFINITIONS
Vegetables are a group of annual, biennial, and perennial plants, which are a rich
source of dietary ber, vitamins, phenolic compounds, and glucosinolates (crucifer-
ous vegetables).
The whole group of vegetables can be divided into:
• alliums (onion, garlic, leek),
• cucurbits (pumpkin, cucumber),
• brassicas (cauliower, broccoli, kohlrabi, head cabbage, Chinese cabbage,
savoy cabbage, Brussels sprout),
• root (beetroot, carrot, parsley),
• leafy (lettuce, spinach, lamb’s lettuce, arugula),
• nightshades (aubergine, tomato, pepper, potato),
• turnips (radish, turnip).
The structure of root vegetables (carrots, beetroot) is characterized by vascular bun-
dles in the form of wood (xylem) and phloem, through which water is delivered to
other parts of the plant (Figure 2.6). In carrots, the vascular bundles are formed due
to the activity of the creative pulp (cambium), which causes secondary root growth.
The beetroot, on the other hand, is characterized by secondary growths, visible in
the cross-section in the form of centrally located rings of the creative pulp, produc-
ing phloem and wood. The vascular bundles in the stalks of monocotyledons, e.g.
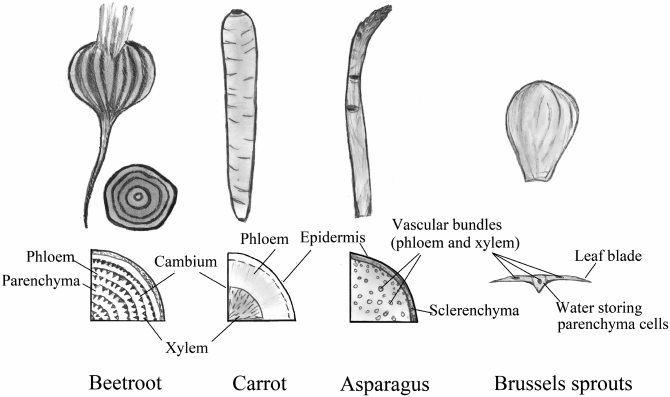
33 Chemical Composition and Structure
FIGURE 2.6 Illustration of anatomical structures of different types of vegetables.
asparagus, for example, are arranged differently, where they are dispersed through-
out the crumb.
2.7.2 CHEMICAL COMPOSITION OF VEGETABLE
Vegetables are more diverse in terms of chemical composition than fruit (Table 2.12).
In vegetables, water is the most abundant component, from 60% in garlic to 98% in
asparagus. Protein is relatively low, with the exception of Brussels sprouts, which
are 4.2% protein. The lipid content of vegetables is also negligible (less than 1%).
On the other hand, saccharides are present in greater amounts, from 3.2% in cucum-
bers to 21.1% in potatoes, which contain the most starch of all saccharides. Like
fruit, vegetables are a valuable source of potassium (broccoli, Brussels sprout, leaf
parsley, potato – over 400 mg/100 g), calcium (leaf parsley, kale – 150–190 mg/100
g), magnesium (leaf parsley, spinach – 50–70 mg/100 g), phosphorus (garlic up to
150 mg/100 g), iron (spinach, parsley – 3–5 mg/100 g), vitamins, e.g. vitamin C
(celery parsley, red pepper, kale over 100 mg/100 g), provitamin A (pumpkin, kale,
carrot, red pepper, leaf parsley, spinach over 300 µg of β-carotene/100 g). Among
the most valuable components of vegetables are also polysaccharides, comprising
dietary ber.
2.7.3 POTATO
Since the potato is the fourth largest crop in the world after maize, rice, and wheat,
it is described separately from other vegetables. Potato (Solanum tuberosum) is
the name of the tuber of a plant belonging to the nightshade family, which is very

34 Jolanta Tomaszewska-Gras
TABLE 2.12
Chemical Composition of Vegetables per 100 g of Edible Tissue
Type of vegetable Protein (g) Lipid (g) Saccharide (g) Vitamin C (mg) Fiber (g)
Asparagus 2.2 0.2 3.6 26 0.7
Beets 1.1 0.1 7.2 6.0 0.8
Broccoli 3.1 0.3 4.5 90 1.5
Brussels sprouts 4.2 0.4 6.4 87 1.6
Cabbage 1.1 0.2 4.3 33 0.8
Carrot 1.2 0.2 9.7 – 1.0
Cauliower 2.7 0.2 5.2 78 1.0
Cucumber 0.6 0.1 3.2 11 0.3
Onion 1.5 0.2 8.2 32 1.2
Red pepper 1.2 0.2 4.8 128 1.4
Potato 2.6 0.1 21.1 20 0.3
Spinach 3.2 0.3 4.3 51 0.6
Tomato 1.0 0.2 4.7 23 0.5
Source: Ensminger et al., 1995.
FIGURE 2.7 Structure of a potato.
widespread in Europe. Potato tubers are used as food and animal feed. The main
components of the potato tuber structure are the periderm, pith (inner medulla),
perimedulla, cortex, and vascular ring (Figure 2.7). New potato tubers are covered
with an epidermis, i.e. a thin skin that is gradually exfoliated, and in its place, sec-
ondary tissue is formed, which is called the periderm. This has a thickness of 80
to 200 μm, which protects against mechanical damage and water loss, with many
spiracles allowing gas exchange. Below this layer is the primary cortex, the outer
part of which is rich in proteins, lipids, and pigments, while the inner part is rich in
starch. Under the cortex there is a vascular ring, supplying nutrients from the shoot
35 Chemical Composition and Structure
to the tuber and to the plants germinating from it. The bundles form a vascular ring
surrounding the perimedulla, the outer part of which is the main starch storage loca-
tion in the tuber, while the interior of the inner medulla (pith) is more watery and
less starchy.
The potato tuber mostly contains water, about 75%, and then starch. Its starch
content depends on the variety and ranges from 10 to 30% and is a store for the
plant. The potato contains a relatively high amount of vitamin C (15–50 mg in 100
g), and there are also potassium 415 mg/100 g, magnesium 24 mg/100 g, and a small
quantity of B vitamins. Green or sprouted potato tubers can cause gastrointestinal
disturbances due to the formation of solanine, which is a toxic glycoalkaloid.
2.8 OIL SEEDS AND FRUITS
Oil plants, the seeds of which are used to obtain oil, are primarily rapeseed (Brassica
napus), sunower (Helianthus annuus), palm oil kernel (Elaeis guineensis), axseed
(Linum usitatissimum), sesame (Sesamum indicum), cottonseed (Gossypium), cocoa
(Theobroma cocao), and coconut (Cocos nucifera). The source of the oil can also be
the pulp of fruit, e.g. olives (Olea europaea sativa) or oil palm (Elaeis guineensis).
Oil is also produced from legumes like soybean (Glycine max) or peanut (Arachis
hypogaea) or from cereals, for instance, from corn (Zea mays). In Europe, olive oil
is classied into eight categories according to Commission Regulation (EEC) No.
2568/91, i.e. extra virgin olive oil, virgin olive oil, Lampante olive oil, rened olive
oil, olive oil composed of rened olive oil and virgin olive oils, crude olive-pomace
oil, rened olive-pomace oil, and olive-pomace oil. Rapeseed, sunower, and soy-
bean oils are mostly produced and sold as rened oils since olive oil or axseed
oil are mainly sold as cold-pressed oils. In the past, rapeseed oil was characterized
by a high content of erucic acid (20:1), which was hazardous in human nutrition.
Nowadays new cultivars have been developed, called canola, “double zero” cultivars,
with low levels of this fatty acid. Rapeseed oil is distinguished from other oils by a
very favorable n-6 to n-3 fatty acid ratio of 2.2:1. However, the best ratio was noted
for axseed oil, as it is 1:3.5. Due to the high content of linolenic acid (18:3, n-3), i.e.
58%, the oil is very susceptible to peroxidation, a process involving polymerization
reactions, making the oil solidify (“fast drying oil”). Therefore, axseed oil is also
used as a base for oil paints, varnishes, and linoleum manufacturing. Recently, in
order to extend the shelf life and to reduce peroxidation, new genotypes of soybean,
rapeseed, sunower, and axseed with low linolenic and high oleic acid have been
developed, named “high oleic.” Palm oil, cocoa fat, and coconut oil belong to fats
that are solid or semi-solid at room temperature. The fruits from the oil palm, the
utilization of which is constantly increasing, provide two different oils – the rst
from the pulp (palm oil) and the second from the seeds (palm kernel oil). Crude
palm oil has a high carotene content, hence the color of the oil is red. Oil palm is the
largest source of natural carotenes – there are 500–700 mg/kg of carotenes in crude
palm oil (CPO). The raw palm kernel oil lacks carotenoids and is not red. Palm oil
and palm kernel oil also differ in saturated fat content: palm mesocarp oil is 49%
saturated, while palm kernel oil is 82% saturated fats. However, crude red palm oil
36 Jolanta Tomaszewska-Gras
is mainly processed, i.e. rened, neutralized, bleached, and deodorized, which is
called RBD (rened, bleached, and deodorized) palm oil and does not contain carot-
enoids. Many industrial food applications of palm oil use fractionated components
of palm oil (stearin, olein). Cocoa butter, named confectionery fat, is the fat from
cocoa beans, which is solid at room temperature. The specic feature of this fat is the
ability to crystallize in six different polymorphic forms, while the best form melts at
body temperature, giving a pleasant, cooling sensation in the mouth.
2.9 HONEY
Honey is produced by honeybees from the sugary secretions of plants (oral nectar)
or from secretions of other insects (such as honeydew). Bees suck up nectar, store it in
their honey sac, and enrich it with some enzymes. Honey is essentially an oversatu-
rated aqueous solution of inverted sugar (glucose and fructose), very hygroscopic and
sticky with a density of about 1.4 g/cm
3
. It also contains a very complex mixture of
other carbohydrates, several enzymes (for instance, peroxidases), amino and organic
acids, aroma substances, pigments, waxes, and pollen grains. Fructose (30–44%) and
glucose (25–40%) are the predominant sugars in honey. Other monosaccharides have
not been found. However, more than 20 di- and oligosaccharides have been identied,
with maltose predominating, followed by kojibiose. The composition of disaccharides
depends largely on the plants, from which the honey was derived. The water content of
honey should be less than 20%, otherwise, it can be readily fermented by osmophilic
yeasts. The crystallization of honey is inuenced mainly by the ratio of two main
sugars, glucose and fructose, which varies depending on the assortment of honey.
Glucose, due to its low solubility in water, accelerates crystallization, while fructose
slows it down, and it is 4.4 times more soluble in water. Honey with a high glucose/
fructose ratio crystallizes more rapidly (rapeseed and sunower honey), while honey
with a lower glucose/fructose ratio does so slowly (acacia, lime).
REFERENCES
Belitz, H.-D., Grosch, W., Schieberle, P. Food Chemistry, Springer-Verlag, Berlin Heidelberg,
2009.
Commission Regulation (EC) No 589/2008 of 23 June 2008, laying down detailed rules for
implementing Council Regulation (EC) No 1234/2007 as regards marketing standards
for eggs.
Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil
and olive-residue oil and on the relevant methods of analysis.
Dinh, T.T.N., Thompson, L.D., Galyean, M.L., Brooks, J.C., Patterson, K.Y., Boylan, L.M.
Cholesterol content and methods for cholesterol determination in meat and poultry.
Comprehensive Reviews in Food Science and Food Safety, 10: 269–289, 2011.
Ensminger, A.H, Ensminger, M.E., Konlande, J.E., Robson, J.R.K. The Concise Encyclopedia
of Food and Nutrition, CRC Press, Boca Raton, London, Tokyo, 1995.
Honikel, K.O., Arneth, W. Cholesteringehalt in Fleich und Eiern. Fleischwirtschaft, 1996.
Horne, D.S Charactersitics of milk. In: Fennema’s Food Chemistry, Ed. S. Damodaran, K.L.
Parkin, CRC Press, Taylor & Francis Group, Boca Raton, London, New York, 907–953,
2017.
37 Chemical Composition and Structure
Huppertz, T., Kelly, A.L. Properties and constituents of cow’s milk. In: Milk Processing and
Quality Management, Ed. A.Y. Tamine, Wiley-Blackwell, Oxford, 23-43, 2009.
Medhammar, E., Wijesinha-Bettoni, R., Stadlmayr, B., Nilsson, E., Charrondiere, U.R.,
Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A
biodiversity perspective. Journal of the Science of Food and Agriculture, 92(3): 445–
474, 2012, nr 3.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January
2002 laying down the general principles and requirements of food law, establishing the
European Food Safety Authority and laying down procedures in matters of food safety.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April
2004, laying down specic hygiene rules for food of animal origin.
Sugino, H., Nitoda, T., Juneja, L.R. General chemical composition of hen eggs. In: Hen Eggs:
Their Basic and Applies Science, Ed. T. Yamamoto, L.R. Juneja, H. Hatta, M. Kim,
CRC Press LLC, Boca Raton, Boston, London, New York, Washington, DC, 13–24,
1997.

39
3
Water and Food Quality
Peter Edward Doe and Barbara Emilia Cybulska
CONTENTS
3.1 Introduction ....................................................................................................39
3.2 Structure and Properties of Water ..................................................................40
3.2.1 The Water Molecule ...........................................................................40
3.2.2 Hydrogen Bonds ................................................................................. 41
3.2.3 Properties of Bulk Water .................................................................... 43
3.2.4 Thermal Properties of Water .............................................................. 47
3.2.5 Water as a Solvent............................................................................... 47
3.2.6 Water in Biological Materials............................................................. 51
3.2.6.1 Properties ............................................................................. 51
3.2.6.2 Water Transport ...................................................................54
3.3 Water in Food ................................................................................................. 55
3.3.1 Introduction ........................................................................................ 55
3.3.2 Sorption Isotherms and Water Activity ..............................................56
3.3.2.1 Principle...............................................................................56
3.3.2.2 Measurement of Water Activity........................................... 58
3.3.2.3 Water Activity and Shelf Life of Foods...............................60
3.3.3 Bottled Water...................................................................................... 61
3.3.3.1 Classication........................................................................ 61
3.3.3.2 Natural Mineral Water......................................................... 61
3.3.4 Bottled Water Other Than Natural Mineral Water.............................63
3.3.4.1 Denition .............................................................................63
3.3.4.2 Water Dened by Origin......................................................63
3.3.4.3 Hygiene, Labeling, and Health Benets ..............................64
3.3.5 Water Supply, Quality, and Disposal..................................................65
3.3.5.1 Water Supply........................................................................65
3.3.5.2 Water Quality.......................................................................65
3.3.6 Water Pollution ...................................................................................67
3.3.7 Wastewater Treatment and Disposal...................................................68
References................................................................................................................69
3.1 INTRODUCTION
Water is the most popular and most important chemical compound on our planet.
It is a major chemical constituent of the Earth’s surface and it is the only substance
that is abundant in solid, liquid, and vapor forms. Because it is ubiquitous, it seems
to be a mild and inert substance. In fact, it is a very reactive compound characterized
DOI: 10.1201/9781003265955-3
40 Doe and Cybulska
by unique physical and chemical properties that make it very different from other
popular liquids. The peculiar water properties determine the nature of the physical
and biological world.
Water is the major component of all living organisms. It constitutes 60% or more
of the weight of most living things, and it pervades all portions of every cell. It
existed on our planet long before the appearance of any form of life. The evolution of
life was doubtlessly shaped by the physical and chemical properties of the aqueous
environment. All aspects of living cells’ structure and function seem to be adapted
to water’s unique properties.
Water is the universal solvent and dispersing agent, as well as a very reactive
chemical compound. Biologically active structures of macromolecules are sponta-
neously formed only in aqueous media. Intracellular water is not only a medium in
which structural arrangement and all metabolic processes occur, but an active part-
ner of molecular interactions, participating directly in many biochemical reactions
as a substrate or a product. Its high heat capacity allows water to act as a heat buffer
in all organisms. Regulation of water contents is important in the maintenance of
homeostasis in all living systems.
Only 0.003% of all freshwater reserve participates in its continuous circulation
between the atmosphere and the hydrosphere. The remaining part is conned to the
Antarctic ice. The geography of water availability has determined, to a large degree,
the vegetation, food supply, and habitation in the various areas of the world. For
example, Bangladesh has one of the world’s highest population densities, made pos-
sible through the regular ooding of the Ganges River and the rich silts it deposits
in its wake. In Bangladesh, the staple food – rice – grows abundantly and is readily
distributed. In other societies, the food must be transported long distances or kept
over winter.
Human well-being is closely linked to the availability of water and food. An
expected increase in the world population by the year 2050 (65% or 3.7 billion) will
create enormous pressure on freshwater resources and food production. Agriculture
is by far the largest consumer of water and the key issue is to look for ways to
improve water use efciency. The solution lies in producing more food from existing
water and land resources (Wallace and Gregory, 2002).
Stability, wholesomeness, and shelf life are signicant features of foods that are,
to a large degree, inuenced by the water content. Dried foods were originally devel-
oped to overcome the constraints of time and distance before consumption. Canned
and frozen foods were developed next. The physical properties, quantity, and quality
of water within food have a strong impact on food effectiveness, quality attributes,
shelf life, textural properties, and processing.
3.2 STRUCTURE AND PROPERTIES OF WATER
3.2.1 THE WATER MOLECULE
Water is a familiar material, but it has been described as the most anomalous of chem-
ical compounds. Although its chemical composition, HOH or H
2
O, is universally

41 Water and Food Quality
known, the simplicity of its formula belies the complexity of its behavior. Its physical
and chemical properties are very different from compounds of similar complexity,
such as HF and H
2
S. To understand the reasons for water’s unusual properties, it is
necessary to examine its molecular structure in some detail.
Although a water molecule is electrically neutral as a whole, it has a dipolar
character. The high polarity of water is caused by the direction of the H-O-H bond
angle, which is 104.5°, and by an asymmetrical distribution of electrons within the
molecule. In a single water molecule, each hydrogen atom shares an electron pair
with the oxygen atom in a stable covalent bond. However, the sharing of electrons
between H and O is unequal because the more electronegative oxygen atom tends to
draw electrons away from the hydrogen nuclei. The electrons are more often in the
vicinity of the oxygen atom than in the vicinity of the hydrogen atom. The result of
this unequal electron sharing is the existence of two electric dipoles in the molecule,
one along each of the H-O bonds. The oxygen atom bears a partial negative charge
δ
–
, and each hydrogen bears a partial positive charge δ
+
. Because the molecule is not
linear, H-O-H has a dipole moment (Figure 3.1). Because of this, water molecules
can interact through electrostatic attraction between the oxygen atom of one water
molecule and the hydrogen of another.
3.2.2 HYDROGEN BONDS
Such interactions, which arise because the electrons on one molecule can be partially
shared with the hydrogen on another, are known as hydrogen bonds. The H
2
O
molecule, which contains two hydrogen atoms and one oxygen atom in a nonlinear
arrangement, is ideally suited to engage in hydrogen bonding. It can act both as a
donor and as an acceptor of hydrogen. The nearly tetrahedral arrangement of the
orbital about the oxygen atom allows each water molecule to form hydrogen bonds
with four of its neighbors (Figure 3.2).
An individual, isolated hydrogen bond is very labile. It is longer and weaker
than a covalent O-H bond (Figure 3.3). The hydrogen bond’s energy, that is, the
energy required to break the bond, is about 20 kJ/mol. These bonds are intermediate
between those of weak van der Waals interactions (about 1.2 kJ/mol) and those of
covalent bonds (460 kJ/mol).
FIGURE 3.1 Water molecule as an electric dipole.
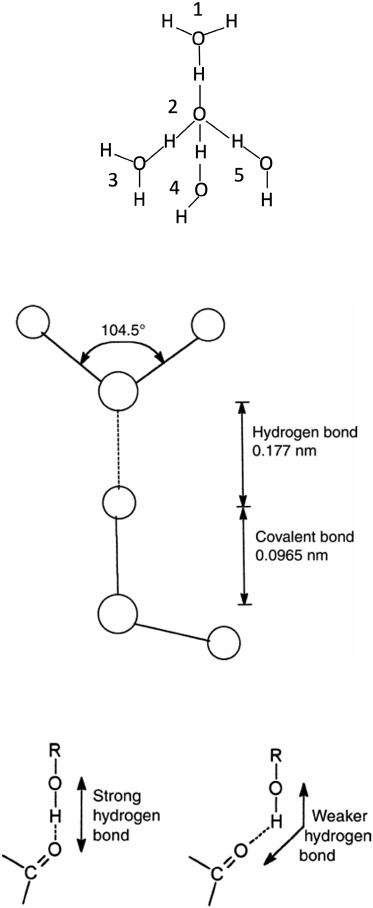
42 Doe and Cybulska
FIGURE 3.2 Tetrahedral hydrogen bonding of ve water molecules.
FIGURE 3.3 Two water molecules connected by hydrogen bonds.
FIGURE 3.4 Directionality of the hydrogen bonds.
Hydrogen bonds are highly directional; they are stronger when the hydrogen and
the two atoms that share it are in a straight line (Figure 3.4).
Hydrogen bonds are not unique to water. They are formed between water and
different chemical structures, as well as between other molecules (intermolecular) or
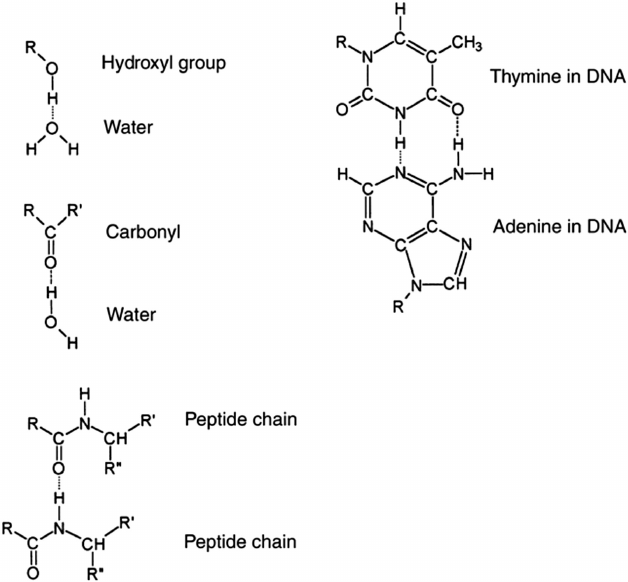
43 Water and Food Quality
FIGURE 3.5 Some hydrogen bonds of biological importance.
even within a molecule (intramolecular). They are formed wherever an electronega-
tive atom (oxygen or nitrogen) comes in close proximity to a hydrogen atom cova-
lently bonded to another electronegative atom. Some representative hydrogen bonds
of biological importance are shown in Figure 3.5.
Intra- and intermolecular hydrogen bonding occurs extensively in biological mac-
romolecules. A large number of hydrogen bonds and their directionality confer very
precise three-dimensional structures upon proteins and nucleic acids.
3.2.3 PROPERTIES OF BULK WATER
The key to understanding water structure in solid and liquid form lies in the con-
cept and nature of the hydrogen bonds. In the crystal of ordinary hexagonal ice
(Figure 3.6), each molecule forms four hydrogen bonds with its nearest neighbors.
Each HOH acts as a hydrogen donor to two of the four water molecules, and as a
hydrogen acceptor from the remaining two. These four hydrogen bonds are spatially
arranged according to tetrahedral symmetry (Bjerrum, 1952).
The crystal lattice of ice occupies more space than the same number of H
2
O
molecules in liquid water. The density of solid water is thus less than that of liquid
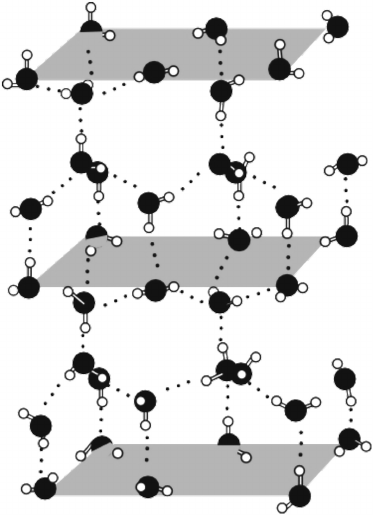
44 Doe and Cybulska
FIGURE 3.6 Structure of ice.
water, whereas simple logic would have the more tightly bound solid structure be
denser than its liquid. One explanation for ice being lighter than water at 0° C pro-
poses a reforming of intermolecular bonds as the ice melts, so that on average, a
water molecule is bound to more than four of its neighbors, thus increasing its den-
sity. But as the temperature of liquid water increases, the intermolecular distances
also increase, giving a lower density. These two opposite effects explain the fact
that liquid water has a maximum density at a temperature of 4° C. At any given
instant in liquid water at room temperature, each water molecule forms hydro-
gen bonds with an average of 3.4 other water molecules (Nelson and Cox, 2021).
The average translational and rotational kinetic energies of a water molecule are
approximately 7 kJ/mol, the same order as that required to break hydrogen bonds;
therefore, hydrogen bonds are in a continuous state of ux, breaking and reform-
ing with high frequency on a picosecond time scale. A similar dynamic process
occurs in aqueous media with substances that are capable of forming hydrogen
bonds.
At 100° C liquid water still contains a signicant number of hydrogen bonds, and
even in water vapor, there is a strong attraction between water molecules. The very
large number of hydrogen bonds between molecules confers great internal cohesion
in liquid water. This feature provides a logical explanation for many of its unusual
properties. For example, its large values for heat capacity, melting point, boiling




