
Final
1
project report - Template
[Full Project Title]
[Acronym]
[Grant Agreement No]
[Name of the scientific representative of the Coordinator]
2
[Coordinator Institution]
[Coordinator Contact Details]
Last Period [month/year] - [month/year]
Reporting Period [number]
Duration of the project [start project month/year] - [end project month/year]
Description of work - [date/version]
Submission deadline
1
The Final project report also includes the periodic report of the last period (see Articles II.4 and II.4.2 of the IMI Model
Grant Agreement).
2
Usually the person mentioned in the coordinator A2.4 form (in SOFIA IT tool).
(IMI Ref. IMI/INT/2014-02186)

Declaration of the coordinator
I, the coordinator of the <GA number>-<Acronym> project, declare that,
The periodic report submitted is in line with the obligations as stated in Article 20 of the Grant
Agreement:
The attached periodic report represents an accurate description of the work carried out in this
project for this reporting period;
The project (tick as appropriate):
□ has fully achieved its objectives and technical goals for the period
□ has achieved most of its objectives and technical goals for the period with relatively minor
deviations
□ has failed to achieve critical objectives and/or is not at all on schedule
The public project website <address> is up to date, if applicable.
To my best knowledge, the financial statements which are being submitted as part of this report are
in line with the actual work carried out and are consistent with the report on the resources used for
the project and if applicable with the certificate on financial statement.
All participants have declared to have verified their legal status. Any changes or deviations have
been reported to the Beneficiary Register, and to the IMI2 JU via the coordinator in accordance
with Article 17.2 of the Grant Agreement.
Name of the Coordinator: Firstname Secondname
Date: dd Mmm YYYY
Signature of the Coordinator: …………………………….
<PROJECT ACRONYM> 2
Table of content
Declaration of the coordinator ........................................................................4
1. Executive summary .............................................................................5
1.1. Project rationale and overall objectives of the project ........................................................... 5
1.2. Overall deliverables of the project .......................................................................................... 5
1.3. Summary of progress versus plan since last period................................................................. 5
1.4. Significant achievements since last report .............................................................................. 5
1.5. Scientific and technical results/foregrounds of the project .................................................... 5
1.6. Potential impact and main dissemination activities and exploitation of results ..................... 5
1.7. Lessons learned and further opportunities for research ......................................................... 5
2. Summary of progress against objectives ..............................................6
2.1. Summary table ......................................................................................................................... 6
2.2. Description of progress for delayed milestones/deliverables not completed partially
completed during the last reporting period ............................................................................ 6
2.3. Follow-up of recommendations and comments from previous review(s) (if applicable) ....... 6
2.4. Deviations from Description of Work during the last reporting period .................................. 6
2.5. Summary statement on all Work Packages ............................................................................. 7
3. Summary of Major Achievements and key dissemination activities .....8
3.1. Major achievements for the last reporting period .................................................................. 8
3.2. Key dissemination activities for the last reporting period ....................................................... 8
4. Summary of project outcomes ............................................................8
5. Research use and dissemination of Foreground ................................ 15
5.1. Current Status .............................................................................................................................. 15
5.2. Plan for Research use and dissemination of Foreground ............................................................ 15
5.3. Plan for sustainability................................................................................................................... 20
6. Management of Project and Consortium ........................................... 20
7. Finance - Cost .................................................................................... 21
7.1. Cost summary for the last reporting period ................................................................................ 21
7.2. Description of deviation from original budget ............................................................................ 23
8. Form C and Summary Financial Report .............................................. 24
<PROJECT ACRONYM> 3

Declaration of the coordinator
I, the coordinator of this project, declare that,
The final report submitted is in line with the obligations as stated in Article II.2.3 of the Grant
Agreement:
The attached report represents an accurate description of the work carried out in this project for
the last reporting period as well as for the whole duration of the project;
For the last period, the project
(tick as appropriate):
has fully achieved its objectives and technical goals; has achieved most of its objectives and
technical goals for the period with relatively minor deviations
3
;
has failed to achieve critical objectives and/or is not at all on schedule
3
.
For the whole duration of the project, the project
(tick as appropriate):
has fully achieved its objectives and technical goals;
has achieved most of its objectives and technical goals with relatively minor deviations
3
;
has failed to achieve critical objectives and/or is not at all on schedule
3
.
The public project website <address>
4
is up to date.
To my best knowledge, the financial statements which are being submitted as part of this final
report are in line with the actual work carried out and are consistent with the report on the
resources used for the project (section 7) and if applicable with the certificate on financial
statement.
All participants, in particular non-profit public bodies, secondary and higher education
establishments, research organisations and SMEs, have declared to have verified their legal
status. Any changes or deviations have been reported under section 6 (Project Management) in
accordance with Article II.3.f of the Grant Agreement.
Name of the Coordinator: ……………………………………………………
Date: ............/ ............/ ............
Signature of the Coordinator: …………………………………………..….
3
If either of these boxes is ticked, the report should reflect these and any remedial actions taken.
4
Please add the address of the public project website. The home page of the website should contain the generic IMI
logo which is available in electronic format at the IMI website. The area of activity of the project should also be
mentioned.
<PROJECT ACRONYM> 4

1. Executive summary
The executive summary will be made publically available, and therefore should not include
information deemed as confidential by the consortium. It should be concise (preferably no more than
40 pages), comprehensive and should capture the updates for the last reporting period as well as the
overall outputs of the project and its impact. It shall at least cover the following items:
1.1. Project rationale and overall objectives of the project
(max 1 page)
1.2. Overall deliverables of the project
(max 1 page)
1.3. Summary of progress versus plan since last period
(Any major deviations, risks should be highlighted in this section)
1.4. Significant achievements since last report
1.5. Scientific and technical results/foregrounds of the project
1.6. Potential impact and main dissemination activities and exploitation of
results
Please explain how the project scientific/technical outputs contribute to the overall IMI objectives:
- to provide socio-economic benefits for European citizens,
- to contribute to the health of European citizens,
- to increase the competitiveness of Europe and help to establish Europe as the most
attractive place for biopharmaceutical research and development.
Please outline how the project outputs have/will have the potential to be rapidly and broadly spread
and taken up within the scientific/industrial community and healthcare professionals.
1.7. Lessons learned and further opportunities for research
Please indicate how the collaboration in a public private partnership (PPP) has been an added value
to achieve the objectives of the project.
From your experience, please propose any recommendations/ solutions which could be useful for a
PPP.
In view of your project achievements, please provide your views on potential new research to further
advance the field.
<PROJECT ACRONYM> 5
2. Summary of progress against objectives
2.1. Summary table
Please include the complete list of all milestones and deliverables of the project including those due
for the last reporting period, and any outstanding ones from the previous reporting period(s).
Description of the milestones/deliverables should be short and concise reflecting the status.
Please align these milestones and deliverables with the objectives listed above.
Work -
Package
Number
Milestone/
Deliverable
Due Date
(Annex I-
description
of work)
Completed
(Yes/Not
yet/Partially)
Dissemin.
level
5
Related document
attached
(Yes/No/Not
applicable)
2.2. Description of progress for delayed milestones/deliverables not completed
partially completed during the last reporting period
For those milestones and deliverables “not completed-partially completed” for the last reporting
period, please explain the reasons for non-completion and the impact on achieving the overall
objectives of the project. Description should be no more than 1/2 page for each
milestone/deliverable.
[No further description is needed for the completed milestones/deliverables for which related
document(s) listed in the table above has been provided].
2.3. Follow-up of recommendations and comments from previous review(s) (if
applicable)
Include in this section the list of recommendations and comments from previous reviews and give
information on how they have been followed up.
2.4. Deviations from Description of Work during the last reporting period
(max 2 pages)
5
PU = Public, fully open, e.g. web CO = Confidential, restricted under conditions set out in Model Grant
Agreement CI = Classified, information as referred to in Commission Decision 2001/844/EC
<PROJECT ACRONYM> 6

In case of any major deviations during the last reporting period, please provide the reason for such
deviations as well as measures taken to achieve the objectives of the project. Please focus on major
deviations which impact on success of the project, including budget.
2.5. Summary statement on all Work Packages
For this final report, please provide a summary statement for each work package (max 3 pages for
work-package). In case of major deviations, please explain.
<PROJECT ACRONYM> 7
3. Summary of Major Achievements and key dissemination activities
3.1. Major achievements for the last reporting period
(max 1 page)
For the last reporting period, please present the major achievements that really capture the impact
of your project in adding to the knowledge in this research area using tangible results.
Achievements should be described as a standalone success for the project e.g. major results in
publications, successful ‘qualification for use’ approval, successful course launch/completion as well
as feedback from attendees and raising awareness of patients.
Please avoid repeating progress against milestones and deliverables.
These major achievements may be used by the IMI JU to communicate success stories.
3.2. Key dissemination activities for the last reporting period
Please report major activities undertaken during the last reporting period to disseminate the project
results including patent application, publications, abstracts, conferences, project website using the
table below and specify for each activity the target group (e.g. scientific community, patients’
organisations, policy makers, the general public).
Nature of
Communication
Title
Responsible Participant
Date
Target audience
4. Summary of project outcomes
Please fill the below table for your project. Some sections of the form may not be relevant to your
project. The information on your project will provide IMI with statistics and indicators on societal and
socio-economic issues addressed by projects. It will help to feed Key Performance Indicators (KPIs)
for the measurement of performance and results against strategic overarching priorities identified as
critical for overall success of IMI. The replies for individual project will not be made public.
Where appropriate please document the resources produced by the project (with the exclusion of
deliverable reports and publications) and where they are archived for the purpose of
reproducibility/verifiability. If the resource is destroyed (e.g. bio samples) please indicate.
<PROJECT ACRONYM> 8
4.1. Project general information
Research area
Type of impact Methodology, model, tool, process, drug etc.
Stage in drug development
pathway
Lead discovery, lead optimisation, Pre-clinical, clinical, manufacturing,
etc.
4.2. Staff statistics
Please indicate in the table below the number of people who worked on the project (on a headcount basis).
Type of position Number of Women Number of Men
Scientific Coordinator
Work Package leaders
Experienced researchers (i.e. PhD
holders)
PhD Students
Other
Number of personnel hired for the
project
Number of staff changing positions
within and between partners (staff
mobility)
Specify organisations Specify organisations
4.3. Resource Input from the Project Partners
Number of
resources
pooled
Size
Unit
(data, samples
subjects,
compounds, etc.)
Comments
Data sets
6
Briefly describe resource
Biobanks
7
Briefly describe resource
Biologicals Samples
8
Briefly describe resource
Cohorts
9
/ Patient registries
10
Briefly describe resource
Software
11
Briefly describe resource
Models, tools
Briefly describe resource
Compounds
Briefly describe resource
Other (please specify)
Briefly describe resource
6
Any organised collection of data
7
A collection of biological material and the associated data and information stored in an organised system, for a
population or a large subset of a population.
8
A biological specimen including, for example, blood, tissue, urine, etc. taken from a participant.
9
A cohort is a group of persons who experience a certain event in a specified period of time. For example, the birth
cohort of 1985 would be the people born in that year.
10
An application which stores metadata for querying, and which can be used by any other application in the network
with sufficient access privileges.
11
Programmes, procedures and data associated with the operation of a computer system.
<PROJECT ACRONYM> 9

4.4. Resource Outputs of the project
Models, tools, technologies, molecules, protocols
Number/size and type
Stage of
development
Resource location
and identifier, future
maintenance
Provide unique identifier, DOI
or data citation
Biomarkers
Efficacy, safety, prognostic,
etc.
Identified, validated,
qualified, etc.
Preclinical models (in vitro)
Standardised,
validated, qualified,
etc.
Preclinical models (in vivo)
Standardised,
validated, qualified,
etc.
In silico models
Standardised,
validated, qualified,
etc.
Tools (diagnostic)/assays
Standardised,
validated, qualified,
etc.
Patient reported outcomes
Standardised,
validated, qualified,
etc.
Modelling and Simulation
technologies
Standardised,
validated, qualified,
etc.
New drug targets
Discovered, validated,
qualified, etc.
Novel hit and lead molecules
Novel clinical protocols
New disease related definitions
Other (specify)
Infrastructure (operations)
Patient registries/cohorts
Number of patients
included
Clinical Networks
Number of centres
Biobanks
Number of samples
Other (specify)
<PROJECT ACRONYM> 10
‘Big data’ solutions to leverage knowledge
12
Number/size and type
Comments / Resource location and
identifier, future maintenance
Provide unique identifier, DOI or data citation
Databases
size
Data citation including Data model description,
data quality description, interoperability through
format and content standards
New data collection
# of studies with new data
collection
Data Citation
Harmonization of existing data
from multiple sources (pooling)
# of data fields reviewed
and harmonized
Data Citation
Linking different databases (linked
data)
13
number of data &
information sources linked
Data Citation
Software applications
# deployed /# releases /
#newly developed
Please specify internal / public
Validated, Data Citation
Mathematical/Statistical Model
Repositories for reuse
# of models curated and
loaded
Data Citation
Other (specify)
Implementation of Standards
Number/size and type
Comments / Resource location and
identifier, future maintenance
Provide unique identifier, DOI or data citation
Data Format and Content
Standards and Vocabularies
(including ontologies)
adopted/adapted or
developed; references
Data Citation; In collaboration with a standards
development organization (e.g. CDISC) Yes/NO
Have the standards and vocabularies been cited in
project publications? yes/no
Standard Operating Procedures
# developed; application
area
Data Citation; Are the procedures Findable/
Accessible / Reusable)?
Other (specify)
12
Any record which can be used to support a scholarly research argument. The term "data" is meant to be broadly
inclusive with the exclusion of digital manifestations of text. Data refers to forms of data and databases that are not
self-describing -- that require the assistance of metadata, computational machinery and/or software in order to be
useful, such as various types of laboratory data including spectrographic, genomic sequencing, and electron
microscopy data; observational data; clinical trial data, assay data; as well as other forms of data either generated or
compiled by humans or machines. Source: modified from https://www.force11.org/datacitation Glossary.
13
Linking databases maintained by two organisations in different geographical locations, or simply heterogeneous
systems within one organisation that, historically, have not easily interoperated at the data level.
Source: modified from http://eprints.soton.ac.uk/271285/1/bizer-heath-berners-lee-ijswis-linked-data.pdf
<PROJECT ACRONYM> 11
Education and Training Programme outputs
Number Comments
Courses conducted
Training type, face to face or e-
course, masters, stand alone, etc.
Trainees who completed continuous
professional development training programs
Trainee type; EFPIA, academia,
regulators, patients
Students graduated from different training
programmes
Trainee type; EFPIA, academia,
regulators, patients
Teachers involved in the training
programmes
Trainee type; EFPIA, academia,
regulators, patients
Training centres labelled “excellence”
Countries covered by training centres
List countries
Other (specify)
Business related outputs
Number Comments
Implementation of project results in industry
Brief description
Patents or other IP rights
Filled, awarded, etc.
Spin offs created or planned
Partners involved, etc.
Buy outs, take overs
Partners involved, etc.
Material Transfer Agreement (MTA),
licencing deals with industry
Type of deal and partners
involved
Number of additional EFPIA companies and
funding attracted (after GA signature)
List entities
Number of additional beneficiaries attracted
(after GA signature)
List entities
Additional funding sources and amounts
Other (specify)
Impact on regulatory framework
Regulators part of the consortium
Yes or no List entities
Regulators part of advisory board
Yes or no List entities
Qualification advice completed or in progress
Yes or no Comments
Qualification opinion completed or in
progress
Yes or no Comments
Impact/input into regulatory practices
Yes or no Details
Impact on Health Technology Assessment framework
HTA bodies part of the consortium
Yes or no List entities
HTA bodies part of advisory board
Yes or no List entities
HTA opinion completed or in progress
Yes or no Comments
Impact/input into HTA practices
Yes or no Comments
Sustainability plans
Sustainability/business plan in place (yes/no)
Brief description
<PROJECT ACRONYM> 12
4.5. Stakeholder engagement
SMEs
Number Comments
SMEs as consortium partners
Type of SME; research,
management, etc.
SMEs created
Size of company created and
type
SME growth
Staff hires, opening new sites
Patient organisations
Number
Comments
Participation to the consortium
List entities
Participation to the advisory/ethics board
List entities
Consultations at hoc
List entities
Engagement with healthcare
professionals
Number
Comments
Participation to the consortium
List entities
Participation to the advisory board
List entities
Consultations ad hoc
List entities
4.6. Collaboration
Number Comments
Memoranda of Understanding within IMI
List collaborators
Memoranda of Understanding outside IMI
List collaborators
Staff exchanges and internships
Type; industrial and academic
internship
4.7. Dissemination
Number Comments
Publications
How many were open access
Data citation
External newsletter circulated
Presentations at scientific meetings
Type of meeting, audience type,
size and country
Website for general public (patients)
Press releases
Media (TV, radio, press, multimedia)
Type of media outlet and target
audience
Brochures / posters / flyers
Type of target audience
<PROJECT ACRONYM> 13
4.8. Ethics
Did your project undergo an Ethics Review (and/or Screening)?
If Yes: have you described the progress of compliance with the relevant Ethics
Review/Screening
RESEARCH ON HUMANS Yes or No
Did the project involve children?
Did the project involve patients?
Did the project involve persons not able to give consent?
Did the project involve adult healthy volunteers?
Did the project involve Human genetic material?
Did the project involve Human biological samples?
Did the project involve Human data collection?
RESEARCH ON HUMAN EMBRYO/FOETUS Yes or No
Did the project involve Human Embryos?
Did the project involve Human Foetal Tissue / Cells?
Did the project involve Human Embryonic Stem Cells (hESCs)?
Did the project on human Embryonic Stem Cells involve cells in culture?
Did the project on human Embryonic Stem Cells involve the derivation of cells
from Embryos?
PRIVACY Yes or No
Did the project involve processing of genetic information or personal data (e.g.
health, sexual lifestyle, ethnicity, political opinion, religious or philosophical
conviction)?
Did the project involve tracking the location or observation of people?
RESEARCH ON ANIMALS Yes or No
Did the project involve research on animals?
Were those animals transgenic small laboratory animals?
Were those animals transgenic farm animals?
Were those animals cloned farm animals?
Were those animals non-human primates?
RESEARCH INVOLVING DEVELOPING COUNTRIES Yes or No
Did the project involve the use of local resources (genetic, animal, plant etc.)?
Was the project of benefit to local community (capacity building, access to
healthcare, education
DUAL USE Yes or No
Research having direct military use
Research having the potential for terrorist abuse
<PROJECT ACRONYM> 14

5. Research use and dissemination of Foreground
5.1. Current Status
Please describe what has been done in relation to the research use and dissemination of Foreground
for the consortium as a whole, or for individual or groups of participant(s) (including socio-economic
impact and target groups for the results of the research).
5.2. Plan for Research use and dissemination of Foreground
Please present the plan that the consortium has established at the end of the project. The plan
should consist of two sections:
Section A
This section should describe the planned dissemination measures, including any scientific
publications relating to Foreground (templates A1 and A2 provided hereafter to be filled in). Its
content will be made available in the public domain thus demonstrating the added-value and
positive impact of the project on IMI.
<PROJECT ACRONYM> 15
TEMPLATE A1: LIST OF PLANNED SCIENTIFIC (PEER REVIEWED) PUBLICATIONS, STARTING WITH THE MOST IMPORTANT ONES
NO. Title
Main
author
Title of the
periodical
or the
series
Number, date or
frequency
Publisher
Place of
publication
Year of
publication
Relevant
pages
Permanent
identifiers
14
(if available)
Is/Will open
access
15
provided to
this
publication?
1
Ex: Economic
transformation in Hungary
and Poland’
Ex:
European
Economy
Ex: No x, March
20xx
Ex: Office for
Official
Publications
of the
European
Communities
Ex:
Luxembourg
Ex: 20xx
Ex: pp. 151 -
167
yes/no
2
3
14
A permanent identifier should be a persistent link to the published version full text if open access or abstract if article is pay per view or to the final manuscript accepted for publication
(link to article in repository).
15
Open Access is defined as free of charge access for anyone via Internet. Please answer "yes" if the open access to the publication is already established and also if the embargo period for
open access is not yet over but you intend to establish open access afterwards.
<PROJECT ACRONYM> 16
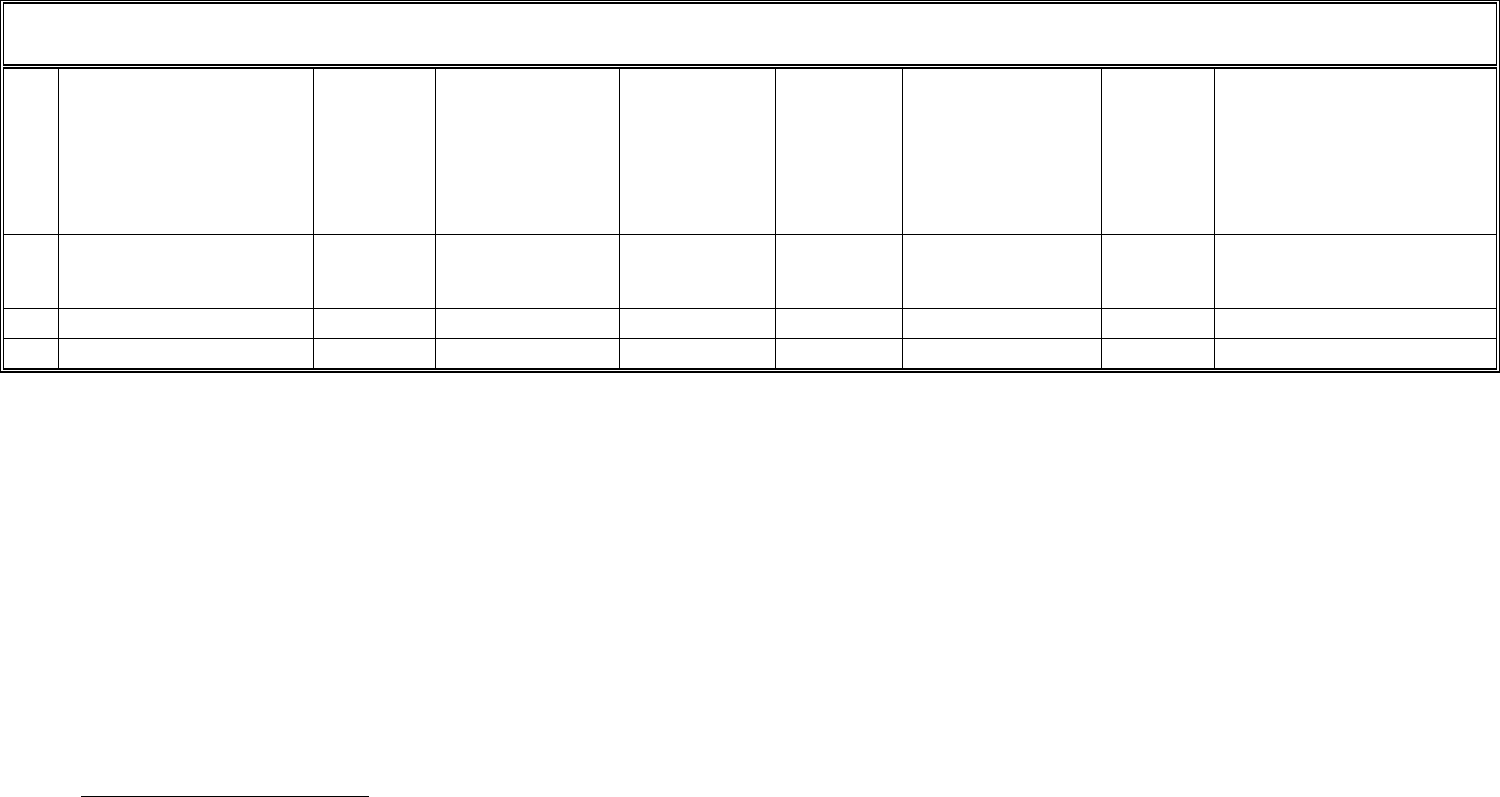
TEMPLATE A2: LIST OF PLANNED DISSEMINATION ACTIVITIES
NO. Type of activities
16
Main leader Title Date/Period Place Type of audience
17
Size of
audience
Countries addressed
1
Ex: Conference
Ex: European
Conference on
Nanotechnologies
Ex: 26 February
20xx
2
3
16
List of dissemination activity: publications, conferences, workshops, web, press releases, flyers, articles published in the popular press, videos, media briefings, presentations, exhibitions,
thesis, interviews, films, TV clips, posters, Other.
17
Type of public: Scientific Community (higher education, Research), Industry, Civil Society, Policy makers, Medias, Other ('multiple choices' is possible).
<PROJECT ACRONYM> 17
Section B
This section should specify the exploitable Foreground and provide the plans for exploitation. All
these data can be public or confidential; the report must clearly mark non-publishable (confidential)
parts that will be treated as such by IMI. Information that is not marked clearly as confidential will be
made available in the public domain thus demonstrating the added-value and positive impact of the
project on IMI.
The applications for patents, trademarks, registered designs, etc. shall be listed according to the
template B1 provided hereafter.
The list should specify at least one unique identifier e.g. European Patent application reference. For
patent applications, only if applicable, contributions to standards should be specified. This table is
cumulative, which means that it should always show all applications from the beginning until after
the end of the project.
Exploitable Foreground shall be listed according to the template B2 provided hereafter. In addition to
the table (template B2), please explain the exploitable Foreground, in particular:
• How the Foreground might be exploited, when and by whom, including IPR measures taken or
intended,
• Further research necessary, if any,
• Potential/expected impact (quantify where possible).
<PROJECT ACRONYM> 18
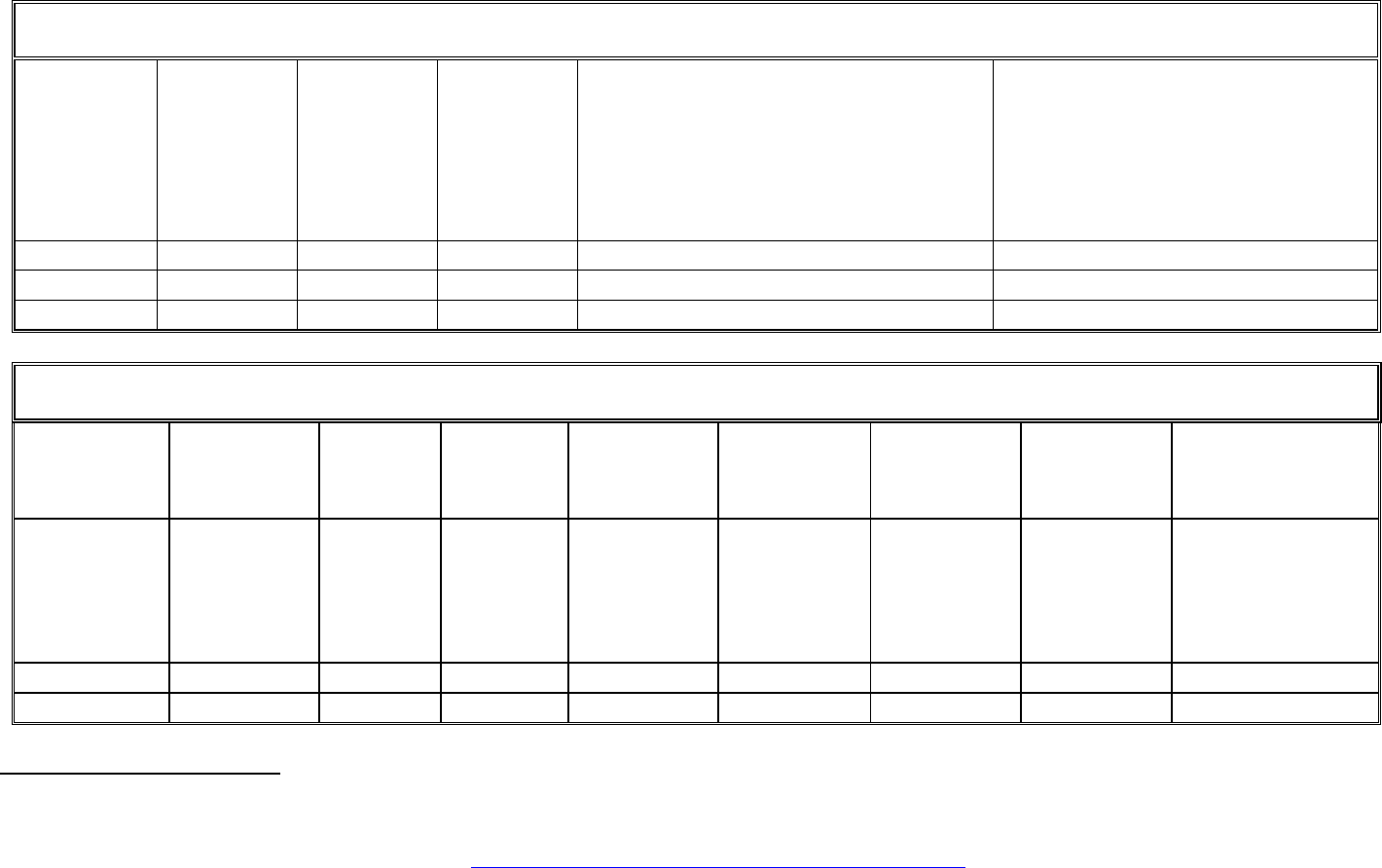
TEMPLATE B1: LIST OF APPLICATIONS FOR PATENTS, TRADEMARKS, REGISTERED DESIGNS, ETC.
Type of IP
Rights
18
Confidential
Click on
YES/NO
Foreseen
embargo date
dd/mm/yyyy
Application
reference(s)
(e.g.
EP123456)
Subject or title of application Applicant (s) (as on the application)
TEMPLATE B2: EXPLOITABLE FOREGROUND
Type of
Exploitable
Foreground
19
Description
of exploitable
Foreground
Confidential
Click on
YES/NO
Foreseen
embargo
date
dd/mm/yyyy
Exploitable
product(s) or
measure(s)
Sector(s) of
application
20
Timetable,
commercial or
any other use
Patents or
other IPR
exploitation
(licences)
Owner & Other
Beneficiary(s) involved
Ex: New
superconductive
Nb-Ti alloy
Ex: MRI
equipment
Ex Medical
Ex 20xx
20xx
Ex A materials
patent is
planned for
2006
Ex Beneficiary X
(owner)
Beneficiary Y,
Beneficiary Z, Poss.
licensing to equipment
manuf. ABC
18
Please choose the type of IP rights: Patents, Trademarks, Registered designs, Utility models, Trade Secrets, Others.
19
Please choose the type of Foreground: General advancement of knowledge, commercial exploitation of R&D results, contribution to the preparation of European or international
standards, impact on EU policies, exploitation of results contributing to innovation.
20
Please indicate the type sector (NACE nomenclature) : http://ec.europa.eu/competition/mergers/cases/index/nace_all.html
<PROJECT ACRONYM> 19
5.3. Plan for sustainability
Please indicate the actions taken to ensure the sustainability beyond the end of the project, when
relevant.
6. Management of Project and Consortium
(max 0.5 page)
Please describe the overall management of the project during the period, highlighting any success
factors and/or challenges that have arisen within the team and indicate how these challenges have
been resolved.
Throughout the lifetime of the project, summarise, if any, the major changes in the composition of
the consortium, and in case these have created difficulties for the progress of the project, please
explain the approach taken to resolve them.
Please indicate if any interactions, synergies with other IMI projects or any other relevant
programmes occurred during the period.
Please describe if any interactions with relevant stakeholders occurred during the period or are
foreseen, including Regulators, Health Technology Assessment Bodies and patients organisations.
In particular, when relevant, please indicate if the consortium has taken any actions to interact with
the Regulators in the context of qualification advice/opinion procedures.
Please comment on the aspects related to the public private partnership (PPP) during the period
i.e. added value of the collaboration on the project or leverage effect if any.
<PROJECT ACRONYM> 20
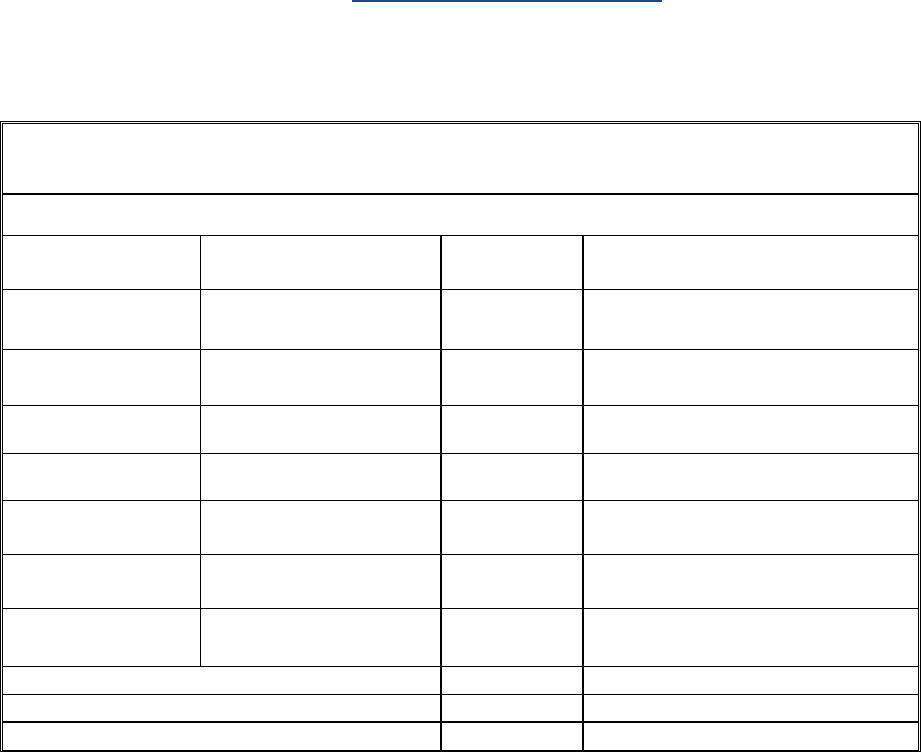
7. Finance - Cost
7.1. Cost summary for the last reporting period
Please provide a cost summary for the last reporting period by filling the following tables (one table
per participant; for adjustment to previous periods a separate table should be added per adjusted
period).
Any deviations from original budget should be highlighted and explanation given in section 7.2.
• Reporting of costs incurred by IMI beneficiaries and third parties
Please note that the table may also be used to report costs declared by participant special clause 11
(participant which are neither a beneficiary nor an EFPIA company).
T
ABLE: PERSONNEL AND OTHER MAJOR COST ITEMS INCLUDING SUBCONTRACTING
[Beneficiary number and name] – [if applicable, adjustment to Period n]
Work relevant to Work-
Package(s)
Item description
Amount in €
Explanations of the use of resources
Personnel direct costs
e.g. salaries of 2 postdoctoral students
6PM each, or 50% each
Subcontracting [if foreseen
in Description of Work]
Other direct costs
Consumables [if applicable]
Equipment depreciation
[if applicable]
depreciation of important equipment
(provide detail)
Other [if applicable]
e.g. maintenance of the web site , animal
costs
Indirect costs e.g. 20% flat rate, actual indirect costs
TOTAL COSTS
Budget for the last period
Deviation
Direct financial contribution
In case of direct financial contribution (‘’in-cash”) received from EFPIA company(ies), please provide
the details of the amounts received as well as the name(s) of the EFPIA company(ies).
<PROJECT ACRONYM> 21
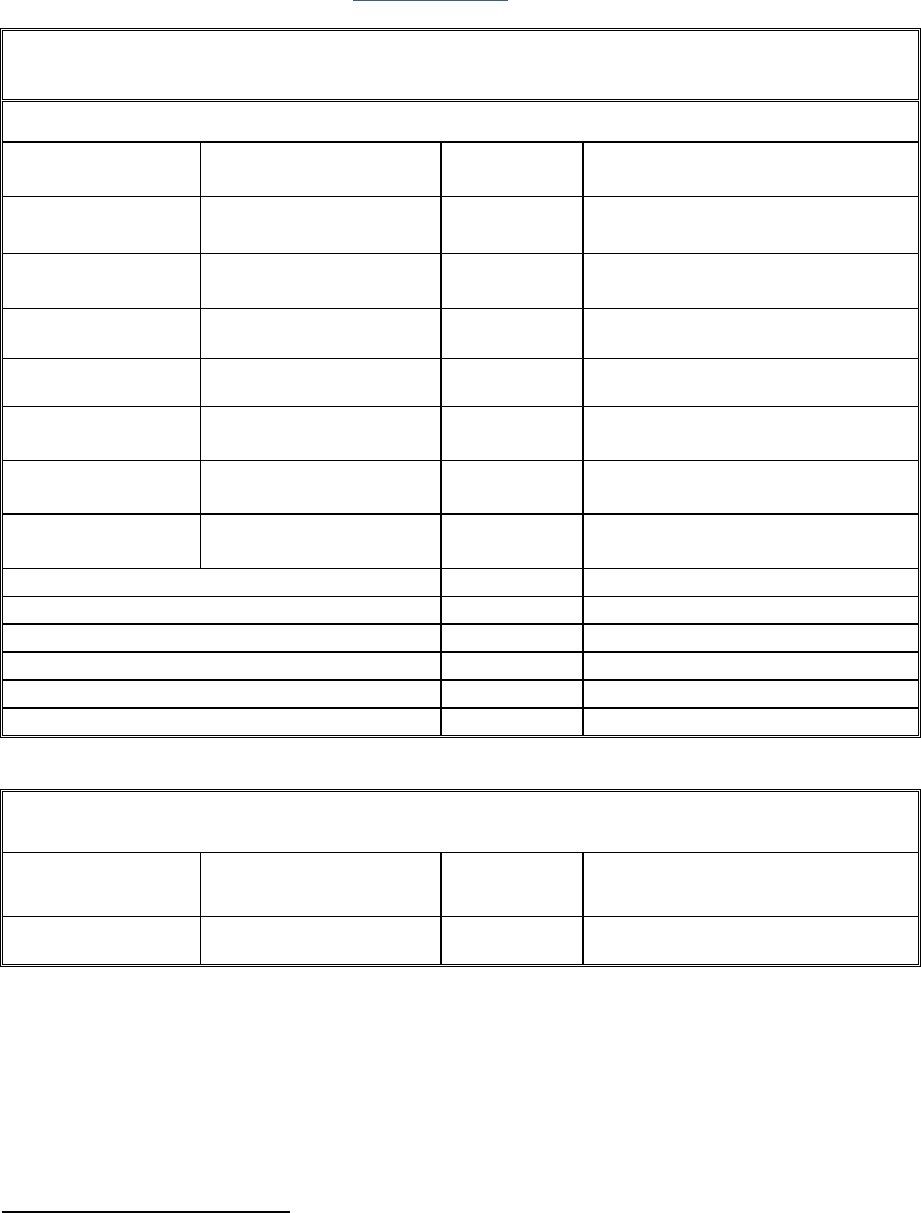
• Reporting of costs incurred by EFPIA companies
TABLE: PERSONNEL AND OTHER MAJOR COST ITEMS INCLUDING SUBCONTRACTING
[EFPIA participant number and name] – [if applicable, adjustment to Period n]
Work relevant to Work-
Package(s)
Item description
Amount in €
Explanations of the use of resources
Personnel direct costs
e.g. salaries of 2 postdoctoral students
Subcontracting [if foreseen
in Description of Work]
Other direct costs
Consumables [if applicable]
Equipment depreciation [if
applicable]
depreciation of important equipment
(provide detail)
Other [if applicable]
e.g. maintenance of the web site , animal
costs
Indirect costs
only if not included in FTE, according to
certified methodology
Sub-total in kind contribution
Direct financial contribution
Total in kind contribution
Of which Non-EU in kind contribution
21
Please specify the type of costs
Budget for the last period
Deviation
NON-EU IN KIND CONTRIBUTION NOT ELIGIBLE
22
Work relevant to Work-
Package(s)
Item description Amount in € Explanations of the use of resources
Major cost item 'Y'
21
when there is a special clause 13 in the Grant Agreement
22
when the non-EU in kind exceeds the maximum limit set in special clause 13 or when there is no special clause 13 in
the Grant Agreement
<PROJECT ACRONYM> 22
7.2. Description of deviation from original budget
(max 0.5 page)
Please fill-in for each IMI beneficiary and each third party the below table.
B
ENEFICIARY
–
D
EVIATION FOR ORIGINAL BUDGET
A1
A2
A3
A1+ A2
B
C
A3-B
D
A3/B
Participant
no and
name
IMI JU
contribution
for the
previous
reporting
periods(*)
IMI JU
requested
contribution
for the last
reporting
period
Total IMI JU
contribution
Budget (IMI
contribution)
over the
project life-
time
Deviation
Current
budget
Status
1
2
3
Total IMI
Contribution
Σ IMI
contribution
Σ IMI
contribution
Σ IMI
contribution
Σ Budget
Σ deviation
A3/B
(*) for previous reporting period: accepted IMI contribution.
Please fill-in for each EFPIA company the below table.
EFPIA
C
OMPANY
–
D
EVIATION FOR ORIGINAL BUDGET
A
A2
A3
A1+ A2
B
C
A3-B
D
A3/B
Participant
no and
name
Cumulative
in kind
for the
previous
reporting
periods
Cumulative
in kind
for the last
reporting
period
Total
Cumulative
in kind
Cumulative
Budgeted
in kind
over the
project life-
time
Deviation
Current
budget
Status
1
2
3
Total EFPIA
in kind
Σ in kind
Σ in kind
Σ in kind
Σ Budget
Σ deviation
A3/B
In addition, if any, please explain only the major deviations from original budget for the last reporting
period (e.g. redistribution of resources from one participant to another) which has an impact on the
overall project.
<PROJECT ACRONYM> 23

8. Form C and Summary Financial Report
For the last reporting period:
The following must be submitted as separate PDF files (originals should be sent by surface mail):
• Summary financial report, extracted from SOFIA (Submission OF Information Application),
• Form Cs for each participant (beneficiary, third party, EFPIA companies), extracted from
SOFIA,
• Certificate on financial statements
23
.
As this is the final report, please note that 30 days after receipt of the final payment the managing
entity shall submit a report on the distribution of the IMI JU financial contribution between
beneficiaries.
23
To be submitted for the final reporting period.
<PROJECT ACRONYM> 24
