INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-1
CHAPTER 8 - INVESTIGATIONS
8.1 - INVESTIGATIONS AND INSPECTIONS ........................................................................................8-4
8.1.1 – BACKGROUND – WHAT IS AN INVESTIGATION? ................................................................................8-4
8.1.2
- INVESTIGATIONS, INSPECTIONS, AND FORM 482? – WHEN DO YOU ISSUE AN FDA 482? ...........................8-4
8.1.3 - EXTERNAL REQUESTS FOR INVESTIGATIVE INFORMATION – WHAT IF SOMEONE ASKS YOU ABOUT AN
INVESTIGATION? .................................................................................................................................8-4
8.1.4
- OFFICE OF CRIMINAL INVESTIGATIONS – WHO IS OCI? .......................................................................8-4
8.1.4.1
- OCI RESPONSIBILITIES ....................................................................................................................... 8-4
8.1.4.2
- REPORTS OF CRIMINAL ACTIVITY ......................................................................................................... 8-4
8.1.4.3
- LIAISON WITH LAW ENFORCEMENT / INTELLIGENCE COMMUNITY ............................................................. 8-5
8.1.5
- TYPES OF INVESTIGATIONS – WHAT SITUATIONS LEAD TO INVESTIGATIONS? ............................................8-5
8.1.5.1
- DEFECTIVE PRODUCTS ....................................................................................................................... 8-5
8.1.5.2
- INJURY, ILLNESS, DEATH ..................................................................................................................... 8-6
8.1.5.3
- CRIMINAL INVESTIGATIONS ................................................................................................................. 8-6
8.1.5.4
- SURVEILLANCE .................................................................................................................................. 8-6
8.1.5.5
- WASHOUTS ..................................................................................................................................... 8-7
8.1.5.6
- FOR CAUSE/FACT-FINDING/INFORMATION GATHERING .......................................................................... 8-7
8.1.5.7
- COMPLAINTS .................................................................................................................................... 8-7
8.1.5.8
- DISASTER/EMERGENCY RESPONSE – HOW DO WE PROTECT THE CONSUMER DURING A DISASTER OR EMERGENCY?
................................................................................................................................................................. 8-10
8.1.5.9
- COUNTERFEITING AND TAMPERING .................................................................................................... 8-18
8.1.6
- GENERAL INVESTIGATIVE TECHNIQUES – WHAT DO I DO DURING AN INVESTIGATION? ............................. 8-22
8.1.6.1
- INTERVIEWS ................................................................................................................................... 8-22
8.1.6.2
- MEDICAL RECORDS ......................................................................................................................... 8-24
8.1.6.3
- SAMPLE COLLECTION ....................................................................................................................... 8-26
8.1.6.4
- INTERNET INVESTIGATIONS ............................................................................................................... 8-26
8.1.7
- LOCATIONS FOR INVESTIGATIONS – WHERE COULD YOU CONDUCT AN INVESTIGATION? ............................ 8-27
8.1.8
- INTERNAL AND EXTERNAL ORGANIZATIONS INVOLVED - WHO ELSE WILL I ENCOUNTER DURING AN
INVESTIGATION
? WHY ARE THEY INVOLVED? ............................................................................................ 8-27
8.1.8.1
- INTERAGENCY REFERRAL .................................................................................................................. 8-27
8.1.8.2
- INTRA-AGENCY/CROSS-PROGRAM ..................................................................................................... 8-27
8.1.9
- GENERAL INVESTIGATION REPORTING - HOW DO I REPORT MY INVESTIGATION? HOW DO I GET CREDIT FOR THE
TIME
I SPENT ON IT?........................................................................................................................... 8-28
8.1.9.1
- ENTERING INVESTIGATION OPERATIONS IN ENSPECT ............................................................................. 8-28
8.1.9.2
- INVESTIGATION MEMO: FORMAT, CONTENT, ENDORSEMENT, AND ROUTING ............................................. 8-29
8.1.9.3
- REPORTING COMPLAINTS/FOLLOW-UPS .............................................................................................. 8-29
8.1.9.4
- REPORTING INFORMATION OBTAINED FROM A CONFIDENTIAL INFORMANT ............................................... 8-30
8.1.9.5
- REPORTING INVESTIGATIONS CONDUCTED DURING DISASTER RESPONSE ................................................... 8-30
8.2 - HUMAN AND ANIMAL FOOD INVESTIGATIONS ....................................................................... 8-32
8.2.1 - COORDINATION ...................................................................................................................... 8-32
8.2.2
- FOODBORNE ILLNESS OUTBREAK INVESTIGATIONS ........................................................................... 8-32
8.2.2.1
- COOPERATION WITH OTHER AGENCIES ............................................................................................... 8-32
8.2.2.2
- OUTBREAKS ON FOREIGN FLAG VESSELS ............................................................................................. 8-33

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-2
8.2.2.3 - OUTBREAKS INVOLVING INTERSTATE CONVEYANCES ............................................................................. 8-33
8.2.2.4
- OUTBREAK MANAGEMENT ............................................................................................................... 8-34
8.2.2.5
- CONDUCTING FOODBORNE ILLNESS FOLLOW-UP .................................................................................. 8-34
8.2.2.6
- REPORTING .................................................................................................................................... 8-41
8.2.3
- INJURY, ILLNESS, DEATH ........................................................................................................... 8-43
8.2.3.1
- PROCEDURES ................................................................................................................................. 8-43
8.2.3.2
- SPECIFIC PRODUCT REPORTING (FOOD, DIETARY SUPPLEMENT, AND COSMETIC – INJURY OR REACTION) ...... 8-44
8.2.3.3
- VETERINARY PRODUCTS-COMPLAINTS/ADVERSE REACTIONS ................................................................. 8-47
8.2.3.4
- SAMPLE COLLECTION ....................................................................................................................... 8-47
8.2.4
- SPECIAL EVENTS ...................................................................................................................... 8-47
8.2.5
- FARM INVESTIGATIONS ............................................................................................................. 8-48
8.2.6
- INFANT FORMULA AND BABY FOOD ............................................................................................. 8-49
8.2.7
- TAMPERING INVOLVING ALCOHOLIC BEVERAGES ............................................................................. 8-50
8.3- DRUG INVESTIGATIONS .......................................................................................................... 8-51
8.3.1 - INVESTIGATIONS COORDINATION ................................................................................................ 8-51
8.3.2
- ILLNESS/INJURY ...................................................................................................................... 8-51
8.3.2.1
- REPORTING .................................................................................................................................... 8-52
8.3.3
- COMPLAINTS .......................................................................................................................... 8-52
8.4 - DEVICE INVESTIGATIONS ....................................................................................................... 8-54
8.4.1 - INJURY/ILLNESS ...................................................................................................................... 8-54
8.4.1.1
- TYPES OF DEVICE INJURIES OR ILLNESSES INCLUDE: ................................................................................ 8-54
8.4.2
- CONFIDENTIAL INFORMANTS ...................................................................................................... 8-56
8.4.3
- COMPLAINTS .......................................................................................................................... 8-56
8.4.4
- REPORTING ........................................................................................................................... 8-56
8.4.5 – MEDICAL DEVICE SAMPLING .............................................................................................. 8-57
8.5 - BIOLOGICS INVESTIGATIONS .................................................................................................. 8-58
8.5.1 - ILLNESS/INJURY ...................................................................................................................... 8-58
8.5.1.1
- REPORTING .................................................................................................................................... 8-58
8.5.2
- SURVEILLANCE ........................................................................................................................ 8-60
8.5.3
- CONFIDENTIAL INFORMANTS ...................................................................................................... 8-60
8.5.4
- COMPLAINTS .......................................................................................................................... 8-60
8.5.4.1
- BIOLOGICAL PRODUCTS ............................................................................................................. 8-60
8.5.4.2
-BIOLOGICAL SAMPLES ...................................................................................................................... 8-61
8.5.4.3
- BIOLOGICS INJURY/ADVERSE REACTION REPORTS .................................................................... 8-61
8.6 - BIORESEARCH MONITORING INVESTIGATIONS ....................................................................... 8-62
8.6.1 - ILLNESS/INJURY ...................................................................................................................... 8-62
8.6.1.1
- REPORTING .................................................................................................................................... 8-62
8.6.2
- SURVEILLANCE ........................................................................................................................ 8-62
8.6.3
- COMPLAINTS .......................................................................................................................... 8-62
8.7 - TOBACCO INVESTIGATIONS ................................................................................................... 8-63
8.7.1 - INVESTIGATIONS COORDINATION ................................................................................................ 8-63
8.7.2
- COMPLAINTS .......................................................................................................................... 8-63
EXHIBITS ....................................................................................................................................... 8-64
8-1 FEDERAL ANTI-TAMPERING ACT FULL LANGUAGE.................................................................. 8-64
8-2 LETTER TO HEALTHCARE PROVIDER FOR MEDICAL RECORDS.................................................. 8-65
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-3
8-3 INVESTIGATION MEMO ........................................................................................................ 8-66
8-4 TABLE OF INCIDENT COORDINATION ..................................................................................... 8-67
8-5 CIFOR OUE AGENT LIST ......................................................................................................... 8-69
8-6 – VAERS FORM ..................................................................................................................... 8-76
8-7 MEDWATCH FORM ............................................................................................................... 8-80
8-8 POTENTIAL TOBACCO PRODUCT VIOLATIONS FORM.............................................................. 8-83
8-9 – DISASTER RESPONSE FLOW DIAGRAM ................................................................................ 8-86
INDEX ........................................................................................................................................... 8-91
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-4
8.1 - Investigations and Inspections
8.1.1 – Background – What is an investigation?
An investigation is an information-gathering activity conducted for several reasons and this definition
applies across ORA programs. The purpose of an investigation is to determine and document facts
concerning an issue to inform the agency in making sound decisions. Used informally, investigation can
apply to a very general activity. It may refer to a response to a more formal request for specific
information. Information obtained during an investigation may lead to other operations such as sample
collections or inspections.
This chapter contains specific information on many types of investigations, and each section provides
guidance on how to conduct those investigations, special reporting requirements, and where additional
assistance can be obtained. Recall work, a special type of investigation, is covered in Chapter 7.
Reporting an investigation is covered in Section 8.1.9 of this chapter.
8.1.2 - Investigations, Inspections, and Form 482? – When do you issue an
FDA 482?
Investigations generally do not require an FDA 482, but there will be times when you need to issue an
FDA 482, such as when you are at a manufacturing site or doing work like an inspection (e.g., collecting
records at a manufacturer or shipper to document interstate commerce). Consult with your supervisor
to determine the proper course of action for these situations. Investigations may be performed at a
location not subject to FDA inspection.
8.1.3 - External Requests for Investigative Information – What if someone
asks you about an investigation?
Investigations will naturally lead to interest from outside groups. Consumers, industry, press, and other
external stakeholders may want information about your investigations. Do not reveal any information
about an investigation to anyone outside of the agency without express permission. Direct any requests
for information to the FDA’s How to Make a FOIA Request webpage (
https://www.fda.gov/regulatory-
information/freedom-information/how-make-foia-request). Refer all media inquiries to the ORA Press
Office at ORAPress@fda.hhs.gov (see IOM 1.7).
8.1.4 - Office of Criminal Investigations – Who is OCI?
8.1.4.1 - OCI Responsibilities
ORA’s Office of Criminal Investigations (OCI) has the primary responsibility for all criminal
investigations conducted by the FDA, including suspected tampering incidents and suspected
counterfeit products. Similarly, OCI has primary responsibility — and is the primary point of contact
for — all law enforcement and intelligence matters.
8.1.4.2 - Reports of Criminal Activity
All reports of suspected or confirmed criminal activity, including suspected tampering or
counterfeiting incidents, must be reported to the appropriate OCI field office or resident office

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-5
without delay. Additionally, all threats or perceived threats against FDA-regulated products are to be
referred immediately to the local OCI field office or to OCI headquarters. In those instances where
OCI does not, or cannot initiate a criminal investigation in a timely manner, the division offices will
consult with OCI to determine the proper follow-up.
8.1.4.3 - Liaison with Law Enforcement / Intelligence Community
OCI is the FDA's liaison with the law enforcement community for criminal investigations and
related matters. In addition, OCI serves as the primary point of contact between the FDA and
the intelligence community on all matters of mutual interest. OCI participates in numerous law
enforcement and intelligence task forces both nationally and internationally including as a full-
time representative at Interpol.
All contacts regarding requests or questions received from federal, state, or local law
enforcement agencies or intelligence agencies are to be referred without delay to the local OCI
field office. Similarly, law enforcement contacts to FDA headquarters or centers should be
referred to OCI headquarters.
When FDA personnel receive information or requests from law enforcement or other agencies,
they should obtain the caller's name, organization, and the details of the request. The caller
should then be referred to the appropriate OCI component. After referring the caller to OCI,
contact the affected OCI unit to provide the caller's information. This will prepare OCI of the
expected contact. FDA personnel should not respond to inquiries concerning criminal
investigations, including questions seeking confirmation of whether FDA is or is not conducting a
criminal investigation.
8.1.5 - Types of Investigations – What situations lead to investigations?
You may conduct a variety of investigations in your career. Some types of investigations include, but are
not limited to complaint investigations, disaster investigations, health fraud investigations, and product
tampering investigations. When conducting any investigation, keep an open mind. Each investigation
will be unique.
8.1.5.1 - Defective Products
A defective product is one that fails to do what it is expected to do. For example, a diabetes
medication that fails to adequately control blood sugar levels that is prescribed for that reason. A
defective product will typically result in a recall where the product may be destroyed or
reconditioned.
Investigations into defective products could be initiated as a result of consumer or industry
complaints that may indicate the need for follow up with the consumer or industry representative,
which would be conducted as an investigation. Investigations are initiated in order to determine
facts surrounding a claim related to the status or disposition of a subject FDA-regulated product.
Subsequent findings would determine necessary follow-up and/or FDA action (e.g., inspection,
sampling, product recall).

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-6
8.1.5.2 - Injury, Illness, Death
Immediate follow up action should be made when there is indication of a serious injury or adverse
reaction, including illness and death. Follow up may vary depending on the situation. You may be
asked to conduct investigations at complainants’ residences or at firms to investigate any potential
causes for the adverse reaction. Inspections at firms may also be warranted. These investigations
could be assigned by Office of Emergency Operations (OEO), the Coordinated Outbreak Response
and Evaluation (CORE) Network, or other agency components.
You may need to collect medical records or in some cases autopsy reports during these
investigations. (See Section 8.1.6.2 of this Chapter for guidance on obtaining medical records.)
When discussing complaints with a firm representative, do not provide any identifying information
of the complainant, for example, name, phone number, or city or state of residence. Reports of
adverse reactions may be received from consumers or health care professionals through voluntary
reporting such as MedWatch. Reports may be received from state or federal partners. These reports
should be treated as confidential.
NOTE: Follow any program specific guidance related to investigation preparation, collection of these
records, etc.
8.1.5.3 - Criminal Investigations
During your work, you may encounter situations that involve criminal or fraudulent activity as
defined under Title 18 USC and Title 21 USC. Criminal activity noted by FDA consumer safety officers
(CSO) is typically cases of individuals and/or firms making false statements or providing false
documents during the course of an inspection or other official activity. There are other violations of
Title 18 and criminal violations of Title 21 USC that you may encounter.
Fraud is a separate criminal act from false statements and involves a false representation of a
matter of fact whether by words or by conduct, including concealment of information, intended to
deceive another for advantage.
In all cases of criminal activity including fraud, OCI is the primary investigative office for FDA. Gather
as much initial information as possible and notify your supervisor. You may be asked to assist OCI in
its investigation. If so, follow their directions and do not discuss the investigation with anyone
outside of the investigation.
8.1.5.4 - Surveillance
During your inspectional, investigational, and other activities, be alert to anything which may be
new or unusual or interesting from FDA's viewpoint such as:
• New firms.
• New products.
• New production and distribution practices.
• New equipment and industrial processes.
• Seasonal practices.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-7
• Industry trends.
• Recent or on-going construction and plans for future expansion.
• Proposed products.
• New ideas the firm is contemplating.
• New products in the development stage.
• Activities about a firm's competitor.
• Plans for consolidation, mergers, diversification, etc.
If this information relates to a firm you are not currently inspecting, report the information using a
Memo of Investigation and route through your supervisor appropriately. If the information relates
to a firm being inspected report in the Establishment Inspection Report (EIR). (See Section 8.1.9 for
details on reporting your investigation.)
8.1.5.5 - Washouts
A “washout” is defined as an operation where you are unable to complete an assigned inspection.
When you encounter a washout, you should determine the reason you are unable to conduct the
inspection. For example, a firm that operates seasonally may be available for inspection later in the
year. If a firm has moved, attempt to find the forwarding address of the firm. If the firm remains in
the local area, do not treat it as a washout but conduct the inspection at the new location. Each
washout should be investigated so that you are able to explain why you could not conduct the
inspection. (See Section 8.1.9 for details on reporting your investigation.)
8.1.5.6 - For Cause/Fact-Finding/Information Gathering
A for cause, fact-finding, or information gathering investigation is generally received by the division
from an outside source like a center, ORA headquarters, or another division. It will generally be a
request to obtain specific information from a firm or other source. One example could be obtaining
interstate documentation from a shipper of a product to support a regulatory action, such as a
seizure in another division.
8.1.5.7 - Complaints
A complaint is a notification that a product may be adulterated or misbranded. A complaint may be
related to the following areas:
• Economic problems/misbranding (i.e., labeling).
o Short weight.
o Deceptive or misleading packaging and labeling.
o Fraudulent products.
• Filth, decomposition, foreign objects, microbial or chemical contamination
• Animal/plant/insect material.
• Off appearance, off odor, or off taste.
• Glass, metal, plastic or other foreign objects.
• Bacteria, yeasts, molds, or fungi.
• Pesticides, industrial, or other chemicals.
• Defective products

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-8
o Sub potency or super potency.
o Particulate matter.
o Failure to operate as intended.
• Adverse reactions
o Allergic reactions.
o Expected reactions.
o Birth defects and problem pregnancies.
o Death.
• Tampering
Complaints are received from various sources, including consumers, other government agencies,
Congress on behalf of their constituents, trade associations, etc.
SOP-000544 – Consumer Complaint
Procedure describes the receipt and processing of consumer complaints in detail.
The FDA Office of Emergency Management/Office of Emergency Operations (OEM/OEO), 1 (866)
300-4374 and Emergency.Operations@fda.hhs.gov
, must be notified immediately of all significant
injury, illness, and suspected tampering complaints. OEM/OEO must also be notified of all
complaints regarding infant formula/baby food. Advise OEM/OEO of the status of all such follow-up
investigations.
As unique situations arise, OEM may provide guidance concerning the type of follow-up to be made.
Note: Link to SOP-000544 is only available to ORA users on the FDA intranet. The link is:
http://qmis.fda.gov/mc/Main/MASTERControl/vault/view_pdf.cfm?ui=062321012030&infocardID=7
QYTPC6FZFEZBO7GPP. Users who need a copy of the SOP outside FDA should use the Freedom of
Information Process described in Section 8.1.3 to get a copy of the SOP.
8.1.5.7.1 - Types of Complaints
8.1.5.7.1.1 - Injury/Illness Complaints
A complaint indicating a serious injury, illness, hospitalization, or death requires immediate
reaction. It will most likely require immediate investigation.
There are additional considerations with injury/illness complaints. The prior medical history
of the complainant may provide indications regarding allergies, drug side effects or drug-
food/drug-drug interactions which may be responsible for the illness or injury. Medical
verification should be sought in these situations.
8.1.5.7.1.2 - Non-Injury/Illness Complaints
Generally, these do not require immediate follow-up at the consumer level. Follow-up may
include examining the parent lot, referral to another FDA division, state, or local agency, or
deferral until the next regularly scheduled inspection. Examples include mold in beverages,
obvious filth or insects in canned goods, etc. It may be possible that adequate investigation
would be contacting the dealer, advising them of the nature of the complaint and
requesting notification of any action taken. Non-injury/illness complaints do not need to be
reported to the OEM/OEO unless product tampering is suspected, or the product is a baby
food or infant formula.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-9
8.1.5.7.2 - Sources of Complaints – Who provides us with complaints?
8.1.5.7.2.1 - Consumer
Consumers contacting field offices with complaints of injury or illness should receive a
prompt, courteous response and assurance that their complaints will receive appropriate
consideration. (See SOP-000544 – Consumer Complaint Procedure.) As the procedure
describes, if the complainant cannot reach the complaint line, be sure to obtain all pertinent
information (see SOP-00054 step 6.1.1 C). You cannot rely on consumers responding to
follow-up calls or providing additional information later.
8.1.5.7.2.2 - Industry
An industry official who contacts the field offices with complaints should receive a prompt
and courteous response and consideration. Industry complaints should be treated in the
same manner as consumer complaints.
8.1.5.7.2.3 - Confidential Informant
A confidential informant is typically an employee at a firm providing information they
believe is a violation of FDA regulations. It is important to avoid the disclosure of a
confidential informant to a firm. The investigator conducting the investigation or inspection
should not disclose the complainant’s information or report the information in the EIR. The
complaint itself should be treated in the same manner as consumer complaints.
To maintain confidentiality, a memorandum regarding confidential information should be
submitted as a separate operation, linked to the original report or submitted as an
attachment to the EIR. There may be times when the report may be discussed in the EIR but,
it will not disclose the source of the information. Discuss with your supervisor before
including information about a confidential source complaint in the EIR.
8.1.5.7.2.4 - Whistleblower
A whistleblower is a person, usually an employee or ex-employee, who discloses
information or activity within a private, public, or government organization that is deemed
illegal, illicit, unsafe, or a waste, fraud, or abuse of taxpayer funds. The complaint itself
should be treated in the same manner as consumer complaints. It is important in these
types of complaints that the identity of the whistleblower is not disclosed. The investigator
should follow the same protocol as dictated in the Confidential Informant section above by
not disclosing the complainant’s information or reporting the information in the EIR or any
format where the information could possibly be released under the Freedom of Information
Act.
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-10
8.1.5.8 - Disaster/Emergency Response – How do we protect the consumer
during a disaster or emergency?
The objective of FDA investigations in the aftermath of disasters is to determine whether or not
foods, drugs including biologics, cosmetics, and devices affected by the catastrophe are safe for
human and animal use; and if not, to effectively have them removed from commerce.
In disaster operations, FDA may assist state, local, and other federal agencies in removing
contaminated or unfit merchandise from the market.
State and local officials usually assume direct responsibility for facilities and products under their
jurisdiction, as their laws and regulations can be immediately invoked; however, FDA assistance is
sometimes requested. Based on the size and scale of the disaster, FDA may receive an official
request for assistance through FEMA, FDA/state Rapid Response Teams, or ad hoc through
traditional state contacts.
If contacted by emergency response personnel for follow-up assignments, please work with your
supervisor to engage district Emergency Response Coordinator (ERC) for further coordination.
8.1.5.8.1 - Preparedness
Disaster preparedness is the first step to ensure personal safety and response efficiency.
Measures taken to prepare for and reduce the effects of disasters both personally and
professionally are crucial before an incident occurs.
It is recommended as a preparedness measure that you familiarize yourself with your
local Continuity of Operations Plan (COOP). COOP is the initiative that ensures that federal
government departments and agencies can continue operation of their essential functions
under a broad range of circumstances including all-hazard emergencies, natural, man-made, and
technological threats, and national security emergencies. Today's threat environment makes
COOP planning even more critical. Your local COOP will alert you to likely disasters for your
geographic area.
Preparedness Resources:
FDA’s Emergency Operations Plan
(http://inside.fda.gov:9003/PolicyProcedures/SOPsbyProgram/EmergencyResponse/ucm381
277.htm)
FEMA Preparedness (www.Ready.gov)
8.1.5.8.2 - Safety
ORA considers the safety of staff to be of the utmost importance.
In a disaster or pending disaster the personal protection of yourself and your family is
your primary concern. Provide for your own safety as you perform your assigned FDA
duties in a disaster area. Inoculations and protective clothing should be considered.
See Chapter S-Safety. Particularly S.8.1 - General Preventive and Protective Measures,
https://fda.sharepoint.com/sites/insideFDA-EmployeeResources/SitePages/Occupational-
Health-Services.aspx, S.17.2 -Immunizations, and S.9- PPE.
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-11
Disasters produce dangerous situations (e.g., high water, escaping gases, fallen electrical lines,
damaged buildings, falling rubble, etc.), so care and extra safety precautions must be observed.
A Personal Safety Plan may be developed when dealing with disaster situations.
Be aware of hazards you may encounter while traveling in an affected zone such as power
outages, damaged or impassable roads, and a lack of available supplies in the area.
Personal Protective Equipment (PPE) should be considered where appropriate. For example,
appropriately fit-tested respirators such as N95 masks should be worn where there is a risk of
inhaling pathogens. Each situation requires a careful evaluation and determination of effective
PPE. Your supporting industrial hygienist should be consulted for guidance.
Safety Resources:
• DFI Field Alert #16
(http://inside.fda.gov:9003/downloads/policyprocedures/guidanceregulations/fiel
dinvestigations/ucm010162.doc)
• ORA Safety Contacts
(http://inside.fda.gov:9003/ORA/Offices/OORS/Safety/default.htm)
• ORA Radiation and Laser Safety Resources
(http://inside.fda.gov:9003/ora/offices/oors/safety/ucm655438.htm)
8.1.5.8.3 - Response
CAUTION: Although procedures in this subchapter do not cover disasters resulting from
a radiological event (presence or release of radioactive materials), it is possible you
may discover products suspected of contamination by radioactive materials in the
disaster area. If you suspect the presence of radioactive materials, take no action on
the materials yourself, but have the area cordoned off at once. Notify the command
official (official in charge) and immediately contact your IMT or supervisor, as applicable, to alert
the radiological health representative and the state radiation control agency
. Follow their
instructions.
8.1.5.8.3.1 - Use of Incident Command System (ICS)
During some disasters, FDA may implement an Incident Command System (ICS) for
response. ICS is a standardized approach to managing incidents at the on-scene level. It is
the combination of procedures, personnel, facilities, equipment, and communications
operating within a common organizational structure. ICS is scalable and flexible and can be
used for small, as well as large and complex, incidents and planned events.
As a CSO, you will typically be assigned under the Operations Section of the Incident
Management Team (IMT). All operations you conduct, and your reporting structure will be
provided by the IMT and shared via an Incident Action Plan (IAP). An IAP contains the
incident objectives, the overall strategy for managing an incident, personal safety guidance,
a comprehensive listing of the tactics, resources, and support needed to accomplish the

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-12
objectives. (Note: Some CSOs with ICS position specific training may serve in a leadership
role on the IMT.)
While serving on an IMT, your reporting will be to your team leader and not to your
supervisor. The IMT will provide specific guidance for reporting. Your activities will be
reported through the IMT and not through normal channels. Reporting may vary depending
on the incident and its objectives. You will not be following reporting guidance later in this
chapter.
8.1.5.8.3.2 - Management of Disasters without ICS
Specific investigation assignments should come from your supervisor and reporting will be
through the normal means, unless directed otherwise.
Response Resources:
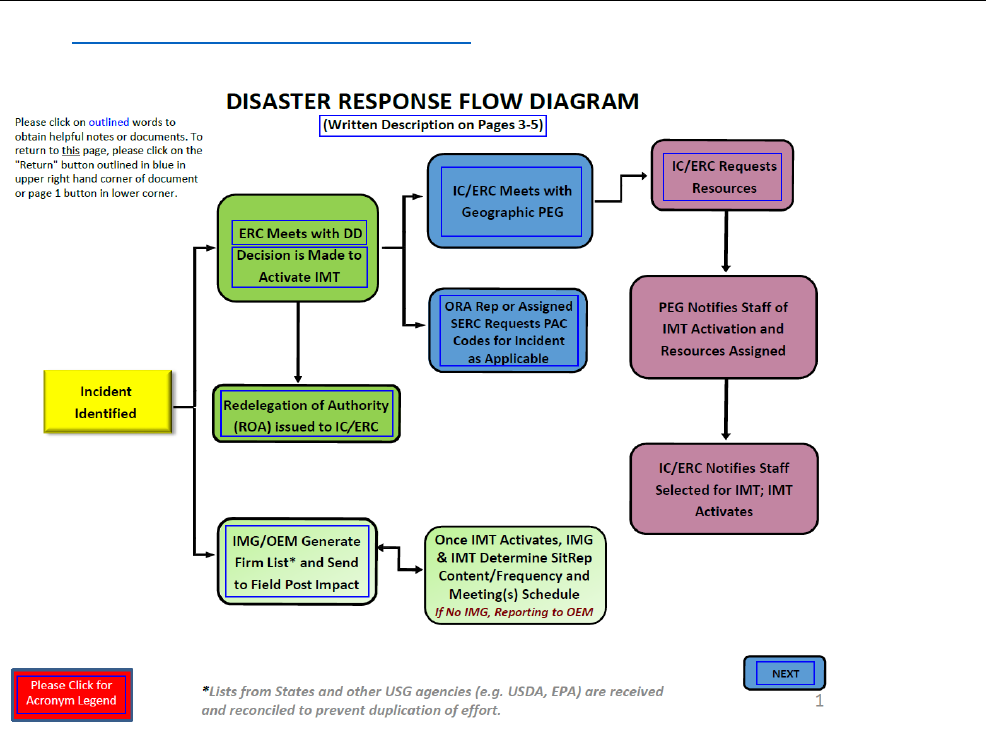
• Disaster Response Flow Diagram (DRFD) package
(Exhibit 8-9)
• Incident Management Handbook (IMH)
(http://inside.fda.gov:9003/downloads/policyprocedures/sopsbyprogram/emerge
ncyresponse/ucm391230.pdf)
• Emergency Operations Plan (EOP)
(http://inside.fda.gov:9003/downloads/PolicyProcedures/SOPsbyProgram/Emerge
ncyResponse/UCM228297.pdf)
• Homeland Security Presidential Directive 5
(https://www.dhs.gov/publication/homeland-security-presidential-directive-5)
8.1.5.8.4 - Disaster Types
The types of natural and man-made disasters that affect FDA operations are:
8.1.5.8.4.1 - Floods
All flood water, regardless of its source, must be considered a polluting medium because of
overflowing sewers, outhouses, decomposing livestock, street run-off water, etc.
Depending on the extent of the flood, first determine the locations of the major stocks of
regulated products. Food and drugs will normally receive first priority. As stocks of goods
are located, rapidly survey the extent of damage, then concentrate on affected materials.
Use your camera extensively. Examine the walls of buildings, storage areas, and the top and
sides of stacked or tiered goods for flood water residue, debris, and a well-defined high-
water mark. Finished products, ingredients, and containers stacked above this line are still
of concern because other problems probably exist (e.g., vermin defilement, failure of
refrigeration, thawing of frozen items, etc.).
Any suspect material should be embargoed by local officials or held pending final
disposition. Management is usually cooperative and willing to do things it may not normally
do to get back to normal operations as quickly as possible. Cooperate with management but
avoid hasty decisions.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-13
Many products are quickly rendered unsuitable for human consumption by flood water.
Items such as bread, cakes, cookies, candies, bulk flour, sugar, bulk liquids, and similar items
not in jars or hermetically sealed containers can often be immediately hauled to disposable
areas and destroyed.
Determine areas which have lost power. In facilities such as frozen food firms, and frozen or
refrigerated warehouses, check the sites for length of down-power and condition of the
products. If power is restored in time to avoid thawing, or prevent spoilage of refrigerated
items, and products were not inundated, or otherwise affected, there is no need for further
examination.
Even though flood waters may not have inundated the firm, the situation may have caused
sewer and waste lines to backflush into basements and immediately drain out again. Debris
or sewage particles along walls and on low floor surfaces or presence of sewage odors are
evidence of backflushing.
Grain, cottonseed, soybeans, dried bean products, peanuts, and similar products may
become flood damaged in terminal elevators, on farms, and in flat storage facilities. In
addition to flood water contamination, molding products may develop mycotoxin
contamination. Examine susceptible products and facilities for damage, inundation, and
mold.
Rodent activity may increase in flooded areas as the vermin seek food and shelter. Be alert
to rodent defilement on products.
As lots of products are checked, embargoed, or released and the immediate situation
returns to normal, firms will want to start operating. Prior to beginning operations, examine
equipment and processing facilities for pollution and its aftermath. Plant operation must not
be permitted unless proper cleanup and sanitizing is performed.
8.1.5.8.4.2 - Earthquakes
Extreme care must be exercised when working in earthquake areas. Do not enter
severely damaged buildings.
Most damage from an earthquake comes from the aftershocks, falling debris, and
resulting fires and flooding. Items under FDA jurisdiction are most likely to suffer physical
damage, spoilage from lack of refrigeration, and/or fire and flood damage.
8.1.5.8.4.3 - Hurricanes and Tornadoes
Investigate following the guidance in Flooding Section above. In addition, examine products
for evidence of physical damage caused by flying objects and crushing by debris. Physical
damage to product containers may be extensive. Broken or leaking containers of materials
such as chemicals, oils, fertilizers, etc., may have contaminated FDA-regulated products. See
the Chemical Spills, Hazardous Waste Sites, Wrecks section below on chemical
contamination from various sources.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-14
8.1.5.8.4.4 - Chemical Spills, Hazardous Waste Sites, Wrecks
Chemical spills occurring on land or water can pose a serious threat to the environment and
contaminate FDA-regulated products both directly and indirectly. See IOM 3.2.11 for
information.
In wrecks, the physical impact usually causes most damage. Toxic items in the same load
may rupture and add to the contamination. In train wrecks, other railcars loaded with
chemicals, oils, or other contaminating materials may rupture and contaminate food and
drug products in otherwise undamaged cars. Removal of the wreckage may cause further
physical damage or chemical contamination. Exposure to weather may also adversely affect
the products.
Do not overlook the possibility that runoff of toxic chemicals from wrecked and ruptured
cars may contaminate adjacent or nearby streams supplying water to downstream firms
under FDA jurisdiction.
Hazardous waste sites also pose a hazard to the immediate environment and other locations
off-site, if runoff contaminates nearby surface waters or, if leachate, contaminates ground
water supplies.
8.1.5.8.4.5 - Fires, Explosions, Riots
FDA operations following these disasters are usually localized and do not normally involve
many personnel or extended resources.
Examine products for exposure to excessive heat, physical damage from flying objects,
falling debris, and lack of refrigeration in down-power areas. Examine for water damage
from firefighting activities and handle these as a flooding situation. Be alert for possible
pollution from using non-potable water in firefighting.
Firefighting often involves use of chemicals. Examine products for residues from possible
toxic fire extinguishing materials and question fire authorities regarding this issue.
In addition, chemical contamination in fire disasters can also be present from other sources,
including:
1. Stored chemicals rupturing from heat or from impact of falling debris.
2. Spraying or leaking chemicals (liquid, powder, dust, granules) as damaged
containers are being removed or salvaged from the fire area.
3. Tracking of chemical material from contaminated areas to other areas by fire
crews or others.
4. Burning or melting plastic containers, insulation, and other building materials.
5. Leaking fuels, storage batteries, anti-freeze, etc., from burning, damaged or
overheated equipment.
6. Chemicals from melting or vaporizing electrical insulation and, in particular,
cooling chemicals from leaking or exploding electrical transformers. Large

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-15
commercial transformers are often directly involved in the fire area and may leak
or explode from the heat, spreading toxic liquid chemicals (some transformer oils
contain con
-c
entrations of PCB) over a large area, even contaminating products
in non-fire areas.
8.1.5.8.5 - Bioterrorism
The field was issued guidance from 2001 which includes the following:
If a bioterrorism act is suspected, FDA staff should not collect or accept samples from any local,
state, or law enforcement agency as such actions will be coordinated by OCI and the FBI, as
appropriate. If an FDA-regulated product is suspected in a tampering, please call OEM/OEO
immediately. In the FBI/OCI determines the product is not suspect, OEM/OEO will issue further
guidance to the division office.
Office of Emergency Operations / Office of Emergency Management (OEM/OEO) emergency
operations 24-hour phone number is 1 (866) 300-4374. The e-mail is
emergency.operatio[email protected]v
.
For additional information see Guidance to the Field on Bioterrorism (10/17/2001)
(http://inside.fda.gov:9003/downloads/policyprocedures/guidanceregulations/fieldinvestigation
s/ucm023333.doc). (Note: This link is only available on the FDA Intranet site and cannot be
accessed by individuals outside the FDA network. Requests can be made through the FOI process
described in Section 8.1.3)
8.1.5.8.6 - Embargoes
See IOM 3.3.1 and IOM 2.7.1.
FDA does not have embargo authority, but does have administrative detention authority as
specified in:
• The Federal Meat Inspection Act (
https://www.fsis.usda.gov/policy/food-safety-
acts/federal-meat-inspection-act)
• The Poultry Products Inspection Act (https://www.fsis.usda.gov/policy/food-safety-
acts/poultry-products-inspection-act)
• The Egg Products Inspection Act (https://www.fsis.usda.gov/policy/food-safety-
acts/egg-products-inspection-act
• Certain parts of the FD&C Act, namely Section 304(g) [21 U.S.C. 334(g)](g) [21 U.S.C.
334(g)] for medical devices, drugs, and tobacco and Section 304(h) [21 U.S.C. 334(h)] for
human and animal food
States and local jurisdictions have embargo authority over FDA-regulated products. Embargoes
are an effective tool for keeping adulterated and misbranded products from the consumer
market. State and local embargoes can be employed immediately requiring the merchandise be
held, destroyed, or reconditioned without time consuming delays. Some state and local
embargo powers are limited to the length of time the product can be embargoed and a minimal
quantity or value. In these cases, the use of federal administrative detention, injunction, and

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-16
seizure action should still be considered. Your division will determine if embargoes are
warranted and work with state or local authorities to obtain them.
8.1.5.8.7 - Field Operations
On-site inspectional and investigational activities will normally be conducted with other FDA
personnel and state or local counterparts.
An assessment must first be made of the disaster area to determine the extent of damage, and
the amounts and kinds of merchandise involved. This may be done by contacting local
Emergency Operation Centers on current conditions, and from firm and mapping details of the
impacted area provided by the OEO Geographic Information System (GIS). If an IMT is activated
the Planning Section and Safety Officer will perform this assessment.
Whether operating within an IMT or not, once personnel are mobilized and assignments are
issued, operational procedures will be similar, regardless of the type of disaster. Normally, you
will search, identify, and investigate foods, drugs, devices, and cosmetics for actual or possible
contamination and taking the necessary steps to preclude their use until they are released,
reconditioned, or destroyed.
CAUTION: Although procedures in this subchapter do not cover disasters resulting from
a radiological event (presence or release of radioactive materials), it is possible you
may discover products suspected of contamination by radioactive materials in the
disaster area. If you suspect the presence of radioactive materials, take no action on
the materials yourself, but have the area cordoned off at once. Notify the command
official (official in charge) and immediately contact your IMT or supervisor, as applicable, to alert
the radiological health representative and the state radiation control agency. Follow their
instructions.
When in doubt as to the condition of any materials affected, request holds or embargoes
pending final outcome of further examinations. See Section 8.1.5.8.6.
8.1.5.8.8 - Field Examination and Samples
Field examinations are an effective tool for determining adulteration or misbranding during
disaster investigations. Judge the extent of field examination and sample collections necessary,
based on the nature and magnitude of the disaster.
In major catastrophes, large numbers of samples may not be necessary because of obvious
visible contamination and the emergency disposition powers invoked by state and local officials.
In minor local disasters, such as fires, riots, train wrecks, truck accidents, or shipwrecks, lots may
be held pending outcome of examinations and extensive sampling may be required.
Field examinations should focus on obvious adulteration, such as physical damage to products
or containers, or damage to labeling.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-17
Examine bulk containers and their contents, including underground storage tanks. Examine
material in rail cars, truck trailers, and storage silos. Be especially alert for rail car and trailer
movement. These may quickly disappear, as clean-up crews arrive.
8.1.5.8.9 - Product Disposition
Lots under embargo, or voluntarily held pending examination or analysis, must be secured until
the examination or analysis is completed, and a release decision is made. If the material can be
released, it is returned to the owner.
Depending on the circumstances and the magnitude of the disaster, segregation, destruction, or
reconditioning of affected goods may be accomplished in the immediate area or the materials
may be moved to distant locations for further manipulation.
FDA normally opposes movement of affected goods since control of the lots is difficult.
However, in cases of widespread disasters, reconditioning centers established in non-disaster
areas may be the most efficient way to handle the problem.
8.1.5.8.9.1 - Segregation
The segregation process often creates a multitude of problems, especially when insurance
claims agents and salvage firms become involved. You are not to segregate materials
yourself. This is the responsibility of the owner or his agent. You should advise them what
constitutes releasable conditions. After segregation, you may be instructed to advise them
about product release based on your examination and/or laboratory results.
8.1.5.8.9.2 - Destruction
It is not your responsibility to say how condemned products are to be destroyed. This is a
concern of the owner and the state or local health agencies that condemned the products.
FDA may be asked to aid in or recommend destruction methods. The most common
destruction method is crushing and dumping in a land fill in approved areas. See IOM 2.6.1.
Destruction methods usually are worked out with state or local officials. The final decision in
major operations may be required of the command officials or higher headquarters,
especially if the environmental impact is significant.
Control products to be destroyed and protect them from pilfering at destruction sites.
8.1.5.8.9.3 - Reconditioning
Affected products may often be reconditioned depending on the condition of the product,
its container, type of product, intended use, and extent and type of contamination.
Any reconditioning must be closely supervised, with proper safeguards for product
accountability. Control must be maintained over the complete operation, with proper
disposition of the rejected portion and the reconditioning of the acceptable portion
performed to the satisfaction of all health officials.
Certain food products which cannot be salvaged for human or animal use might be of use in
non-food or non-feed industries. However, these must be denatured to render them unfit

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-18
for food or feed use. Firms must account for the amounts of product denatured, to whom it
was sold, and the final use of the product. Examination of the product at its final destination
and/or a spot check may be required to assure it is utilized in non-food or non-feed
products. Reconditioning plans should be reviewed by the division’s Compliance Branch in
consultation with the appropriate center or by the IMT if ICS is being used for the incident.
It is your responsibility to assure the firm is following the reconditioning plan and that no
product is diverted from the plan.
8.1.5.8.9.4 - Relabeling
Relabeling may be the only reconditioning required if damage is solely to the label and all
the following conditions are met:
• The new label contains all mandatory information, is not misleading in any way,
and conforms with the FD&C Act in all other aspects.
• Label codes are carried over to the new label.
• The product is not contaminated; and
• The container has its original integrity.
8.1.5.9 - Counterfeiting and Tampering
8.1.5.9.1 - Reporting Contacts
All reports of counterfeiting, tampering, or tampering threats must be immediately reported to
the Office of Criminal Investigations (OCI) headquarters office, Special Agent in Charge-
Headquarters Operations (SAIC-HQS OPS) at 240-276-9500 and the Office of Emergency
Management (OEM)/Office of Emergency Operations (OEO) at 1 (866) 300-4374 (24 hours).
If the complaint or report involves a United States Department of Agriculture (USDA) regulated
product, the district office should report it directly to the USDA and notify OCI, SAIC-HQS OPS,
and OEM/OEO immediately. Notification of OCI may be done online at OCI’s
Report Suspect
Criminal Activity website: (https://www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm).
OEM/OEO can be notified by e-mail at emergency.operati[email protected] and by phone 24
hours a day at 1 (866) 300-4374.
Do not conduct any investigation into these reports unless you have been directed to do so by
management following their meeting with OCI.
8.1.5.9.2 - OEM / OEO Responsibility
OEO/OEM is the focal point for communications; especially in those counterfeiting/tampering
cases where regional/national coverage is necessary. Alert OEM immediately to all suspected or
confirmed counterfeiting/tampering incidents, whether or not there is an injury/illness involved,
especially if media attention will be initiated by any source.
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-19
8.1.5.9.3 - Coordination with Other Government Agencies
The Federal Bureau of Investigations (FBI) and the USDA share enforcement of the Federal Anti-
Tampering Act (FATA) with FDA as described below:
1. FBI Responsibility - The FBI has concurrent jurisdiction under the FATA over products
regulated by FDA. The FDA understands the FBI's primary interest in the FATA matters
will be to investigate; particularly, those cases which involve a serious threat to human
life or a death. SAIC-HQS OPS or the local OCI field office will coordinate all referrals to
the FBI in accordance with agency policy.
2. USDA Responsibility - The USDA will investigate and interact with the FBI on
counterfeiting/tampering of products regulated by USDA. If a counterfeiting/tampering
complaint or report is made to an FDA district office and involves a USDA-regulated
product, the district office should report it directly to the USDA and notify OCI, SAIC-
HQS OPS, and OEM/OEO immediately. Notification of OCI may be done online at OCI’s
Report Suspect Criminal Activity website
:
(https://www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm).
Isolated incidents of counterfeiting/tampering not investigated by OCI and not meeting the
criteria for FBI or USDA follow-up, may be referred to the appropriate state or local
investigative agencies, as outlined in section 8.1.5.9.4. The appropriate center should be
consulted in these cases. Assistance should be provided to cooperating officials as necessary or
where requested.
8.1.5.9.4 - Authority & Responsibility
FDA is authorized to investigate reported counterfeiting/tampering of FDA-regulated consumer
products under the FATA, Title 18, USC, Section 1365 and Title 18, USC, Section 2320
. See IOM
Exhibit 8-1. In most cases, the authority for such investigations is also found in the FD&C Act.
OCI has the primary responsibility for all criminal investigations of
counterfeiting/tampering/threat incidents of FDA regulated products. Given that responsibility,
OCI field offices will coordinate responses to counterfeiting/tampering reports with the district
offices they deem appropriate, to ensure initial investigative steps are taken in a timely and
efficient manner.
In those incidents where OCI does not, or cannot, initiate a criminal investigation,
they will inform the division of their decision and the division will determine the
proper follow-up, which could include further investigation by the division or referral
to local or state authorities. The division will keep OCI informed of their follow-up
activities and any relevant changes in its status. Prior to initiation of any tampering
investigation, you and your supervisor should evaluate the situation from a personal safety
perspective. You and your division management may also need to determine if a situational plan
is warranted. Refer to IOM 5.2.1.2 - Personal Safety, and IOM 5.2.1.4 Situational Plan, for more
information.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-20
8.1.5.9.5 - Release of Information
During any investigation related to counterfeiting or tampering, no information should be
released without management approval. If there are inquiries about the investigation, contact
your supervisor.
8.1.5.9.6 - Investigation
The purpose of these investigations is to determine if counterfeiting/tampering has occurred;
the seriousness of the problem; the quantity of affected products on the market; the source of
the counterfeiting/tampering; and quick removal from consumers or commerce of any
contaminated product. OCI will seek to identify and initiate criminal prosecution of those
persons responsible for criminal activity associated with counterfeiting/tampering/threat
incidents.
FDA will investigate reports of counterfeiting/tampering associated with FDA-regulated
products. Priority will be given to reports of death, illness, injury, or a potential health hazard.
Adhere to existing procedures and instructions as outlined in the IOM and RPM when
conducting counterfeiting/tampering investigations, inspections, sample collections, special
investigations, and related activities including interviews, record examination, direct
observation, affidavits, etc.
8.1.5.9.7 - General Procedures
Counterfeiting/Tampering incidents historically have occurred in unpredictable forms and
products. Standard operating procedures (SOPS), in most cases, will suffice for these
investigations. As events take place, specific instructions for some investigations may be
provided by OCI headquarters and/or your division office. Expeditious resolution is important,
especially when a health hazard may be involved.
Attempt to answer the following questions as rapidly as possible:
• Has counterfeiting/tampering occurred, or can the condition of the product be
explained by other means?
• Is death, injury, or illness associated with the report and, if so, does it appear to be
caused by the product counterfeiting/tampering?
• Does the incident appear to be isolated or wide-spread?
• Is it likely other, similarly affected FDA-regulated products remain in distribution, and if
so, what is the extent and magnitude of distribution?
• If the incident involves more than a single container, could counterfeiting/tampering
have occurred at the production facility or in the distribution chain rather than at retail?
• Can specific persons or points in the distribution chain be identified as possibly causing
the problem?
Be sure to coordinate your efforts with OCI SAIC/IOD HQS OPS and OEM/OEO.
In many counterfeiting cases, ORA investigators and OCI agents conduct joint
inspections/investigations at the distributors. It is the purpose of the ORA investigators to
document receipt and distribution of counterfeit products and to discuss voluntary recall of

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-21
those products. OCI agents will at the same time conduct their investigation into the knowledge
and source of the counterfeit products. It is not your purpose to accompany the OCI agent
during his/her investigation.
8.1.5.9.8 - Sampling
8.1.5.9.8.1 - Tampering Cases
Whenever a sample is collected for suspected tampering, you must collect an authentic
sample of the same product. It should be from the same lot and code, if at all possible. The
sample size for the authentic portion is at least six in
-ta
ct units. Follow normal sampling
techniques; however, recognize that there may be forensic evidence available such as
fingerprints and hair that can be lost if the sample is not handled properly.
T
he Forensic Chemistry Center should be contacted prior to sampling. They can give specific
directions regarding sampling in each situation, especially related to the preservation of
forensic evidence like fingerprints.
Samples should be packed to avoid movement of the product container within the
bag. Individual dosage units from previously opened containers can be protected
by removing them from their container utilizing spoons or forceps. Secure them in
separate containers so they do not rub or smear possible evidence. Further
guidance can be found in the FBI "HANDBOOK OF FORENSIC SERVICES"
(https://www.fbi.gov/file-repository/handbook-of-forensic-services-pdf.pdf/view
). As a
precaution, rubber gloves may be worn inside of cotton gloves as protection against toxic or
caustic substances.
Ship samples with extreme care to ensure their integrity. Thoroughly describe your sample
and its characteristics on the collection report (C/R) to facilitate analysis. Include any
descriptive terms used by individuals associated with the complaint. If special instructions to
preserve fingerprints or for further handling are indicated, they should be noted on the C/R.
If speed is imperative, consider hand delivery to the lab.
8.1.5.9.8.2 - Counterfeiting Cases
If sampling is indicated during an investigation of counterfeiting, follow the directions from
OCI or the Forensic Chemistry Center regarding collection, packaging, and shipment of the
sample. Authentic samples should only be collected when requested by OCI in consultation
with FCC.
8.1.5.9.9 - Complainants
Some complaints about “foreign objects” may be tampering complaints. The complainant may
state they found something in a product. You should be aware that any complaint investigation
of foreign objects may become a tampering investigation.
Consumers are likely unaware of the provisions of the Federal Anti-Tampering Act (FATA). A
general discussion of the FATA, its provisions for investigation, filing of false reports, and

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-22
counterfeiting/tampering can be useful and informative to those individuals. Consumers are
often unaware that merely filing a false report is a serious crime and once aware may rescind
previous statements. In general, this would close an investigation, but you should discuss this
with your supervisor.
Prior to concluding your interview of the complainant, obtain a signed affidavit attesting to the
circumstances of the complaint, as directed by IOM 4.4.8. Include a statement in the affidavit
similar to the following, "I have been informed of the provisions of the Federal Anti-Tampering
Act and also that the providing of false information to the federal government is illegal." It is
permissible to pre-type this statement at the bottom of an affidavit, FDA 463a, and photocopy it
before use if you have a large number of counterfeiting/tampering complaints to investigate.
8.1.5.9.10 - Continuance of Investigation
Some investigations may continue after the interview and sample collection from the consumer.
If you are directed to continue the investigation at the retail, distribution, or manufacturing
sites, obtain specific guidance from your management or OCI before proceeding. You may be
conducting an inspection at a firm simultaneously with an OCI investigation. You should not
disclose to the firm officials anything about an OCI investigation.
8.1.5.9.11 - Refusals
All refusals encountered during counterfeiting/tampering investigations should be documented
using existing procedures. Refusals of requests should be documented in detail. Assure the firm
is aware of the non-routine nature of the request. If a search warrant or other court order is
necessary, OCI will lead or direct this part of the investigation. Report all refusals to the local OCI
field office.
8.1.6 - General Investigative Techniques – What do I do during an
investigation?
8.1.6.1 - Interviews
An interview is a one-on-one structured conversation to obtain accurate, reliable information. To
gain the most facts and information, be prepared and conduct the interview methodically with a set
purpose.
8.1.6.1.1 - Preparation
Interviews may be conducted in various agreed upon meeting places. Choose a non-threating
place for the interview, such as a conference room or private office free from distractions or
interruptions. Silence your phone to avoid incoming calls. If possible, conduct the interview away
from the person’s normal area of business. If interviewing a consumer at their home, try to
interview them in an area of their home that has the least distractions. If possible, conduct the
interview sitting directly across from the interviewee.
Begin by researching your topic. Set a specific purpose and objectives for what you want to learn
during the interview.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-23
8.1.6.1.2 - The Interview
• Set the tone. In most cases, you may tell the interviewee what they can expect. Start out
with generic or easy-to-answer questions to establish a baseline and to put the subject at
ease.
• Avoid asking leading questions. Ask open-ended questions that encourage the interviewee
to talk and provide a full answer rather than a “yes” or “no” (e.g., Tell me about…, How did
you…, Why was this…, etc.) Avoid combining more than one idea into the same question.
Frame the question to generate an answer one fact at a time. Avoid questions that are
accusatory or that trigger a defensive response. ‘Yes’ and ‘no’ questions may be used at the
end of the interview to affirm facts.
• Keep an open mind.
• Do not express your opinions, thoughts, and your own conclusions about the situation or
what the interviewee says. You are trying to learn information and facts from the
interviewee so avoid being too familiar with the topic in your responses. Set aside any
potential biases while conducting the interview.
• Take detailed notes or have another CSO present to take notes. This is extremely helpful
since you are focused on the objectivity of the interview. If taking notes makes the
interviewee uncomfortable or hinders the interview, you may take notes immediately after
the interview and identify the time between the interview and your notetaking and explain
the circumstances for not taking contemporaneous notes during the interview. Only use
quotes (“…”) if you are certain they are exact. It is a good practice to read a quote back to
the interviewee to confirm its accuracy.
• Pay attention to the subject’s verbal and non-verbal communication.
• Ask for clarification and more detail if responses are not clear to you during the interview.
Repeat answers back to the subject to ensure you heard the information correctly. Ask if
documents exist and to support any part of the interviewee’s story. Collect any available
relevant documents.
• Follow-up questions may help establish additional facts. If your questions are avoided or
the answers seem evasive, try rephrasing the question and ask it again. You may also
change topics and return to an issue later.
• Allow the interviewee enough time to answer your questions and avoid interrupting them.
Sometimes silence can be a tool to prompt further explanation or reaction. Before
concluding the interview, ask the subject if there is anything else they would like to provide
or discuss. Ensure that the interviewee has your contact information in case they recall any
more material information later.
• Interviews and discussions with complainants where tampering is suspected or alleged,
should include a discussion of the Federal Anti-Tampering Act (Exhibit 8-14). This discussion
needs to be documented in the investigation report/memo. See IOM 4.4.6.3
8.1.6.1.3 - Safety
Developing a Situational Safety Plan may also be required. Refer to IOM 5.3.5.4.2
In preparation for any consumer complaint interviews, you should take your personal
safety into consideration. Refer to IOM 5.2.1.2 for more information.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-24
8.1.6.1.4 - Basic Information to Obtain
Obtain an accurate and complete description of the product, e.g., brand name, product name,
flavor or variety, how packaged, storage conditions required (i.e., refrigerated or shelf stable) etc.
Refer to Consumer Complaint Procedure (SOP-000544) Section 6.1.1 C.
It is important to accurately determine the sequence of events leading up to the complaint.
You cannot rely on consumers responding to follow-up calls or providing additional information
later.
8.1.6.2 - Medical Records
In investigating complaints where the complainant was seen by a health professional, contact the
health professional concerning the nature of the alleged illness/injury, and the relationship to the
product. You may occasionally find the complainant has not mentioned the product to the health
professional as a potential cause of the illness or injury. Use judgment as to the usefulness of
collecting medical records. Examples of medical records to collect include: Admission History and
Physical; Emergency Room/Clinic Record of the event if patient not admitted; Discharge Summary;
Autopsy Report; and Death Certificate. See also IOM 5.3.8.6.
If collection of medical records is necessary, use the letter template found in Exhibit 8-2. It may be
necessary to use multiple letters if medical records are at different locations. If you encounter
resistance from the medical professionals in providing records, you may refer them to 45 CFR
164.512(b) which explains the exemptions allowing FDA access to the medical records.
FDA is exempt from the HIPAA Privacy Rule as a public health authority. If a situation arises in which
information sharing is impeded by the belief that FDA lacks authority to receive this information,
you may share the language below during disease outbreak investigations or consumer complaint
follow-up. References are provided for further information.
“The Health Insurance Portability and Accountability Act (HIPAA), Standards for Privacy of
Individually Identifiable Health Information; Final Rule (Privacy Rule) permits disclosure of privacy
information without a written patient authorization for specific public health purposes. Specifically,
the Privacy Rule permits covered entities to disclose this type of information to ‘a public health
authority that is authorized by law to collect or receive such information for the purpose of
preventing or controlling disease, injury or disability, including…the conduct of…public health
investigations’
1
. Per the Privacy Rule, ‘public health authority means an agency or authority of the
United States…including the employees or agents of such public agency…that is responsible for public
health matters as part of its official mandate’
2
. FDA, as a public health authority responsible for
ensuring the public health and safety with regards to FDA-regulated products, meets this definition.
Our authority to receive information related to FDA-regulated products comes from the Federal
1
45 CFR 164.512, from the Privacy Rule, available at https://www.law.cornell.edu/cfr/text/45/164.512
2
45 CFR 164.501, also from the Privacy Rule, available at https://www.law.cornell.edu/cfr/text/45/164.501

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-25
Food, Drug and Cosmetic Act (FD&C Act), the Public Health Service Act, and regulations issued under
those authorities.
“The Privacy Rule permits covered entities to disclose protected health information (including
personal privacy information) directly to the FDA for certain public health activities and purposes,
provided that the disclosure is limited to the minimum amount necessary. During FDA follow-up to
reports of illnesses potentially associated with FDA-regulated products, access to personal privacy
information including names and contact information is necessary in order to ensure timely follow-
up and, potentially, removal of implicated products from commerce. FDA is also responsible for
safeguarding personal privacy information released to us according to the Freedom of Information
Act and the Privacy Act
3
and our information disclosure regulations
4
, and is obligated to comply with
all applicable protections, procedures and legal requirements against the unauthorized disclosure of
this information.
“Consequently, personal privacy information including case names and contact information should
be shared by state and local health departments with FDA authorities during an investigation of
potentially adulterated FDA-regulated products, including illness outbreaks potentially associated
with FDA-regulated foods. Prompt information sharing speeds the agency’s investigation and can
prevent additional illnesses and/or deaths due to an adulterated FDA-regulated product.”
If the investigation is related to an outbreak/illness and the Office of Emergency Operations or
Coordinated Outbreak Response and Evaluation is coordinating the incident and a medical officer
has been assigned to the investigation it is preferred that the CSO, with supervisory concurrence
communicates with the medical officer about the documents to collect prior to the collection. In the
absence of a medical officer being assigned or available, the CSO in collaboration with the
supervisor, should collect medical records most relevant to the incident. Once collected, the Office
of Emergency Operations or CORE if involved, or the supervisor in consultation with their
management should identify a medical officer to review the records.
The records containing personal identifiable information (PII) and medical information need to be
protected. All medical information sent to the medical officer electronically needs to be encrypted.
Hardcopy records shipped to the medical officer need to include shipment tracking information and
request signature upon receipt. The medical records should be addressed to the attention of the
specific medical officer who will be conducting the review.
Any hard copy medical records in the possession of the CSO after sending to the medical officer or
returned by the medical officer, should be placed in a sealed envelope, identified to contain PII and
medical information and filed with the investigation memo.
3
5 USC 552, 5 USC 552a, from The Privacy Act of 1974 5 USC 552a (as amended), available at
https://www.law.cornell.edu/uscode/text/5/552
and https://www.law.cornell.edu/uscode/text/5/552a
4
21 CFR Parts 20 and 21, from FDA information disclosure regulations 21 CFR Parts 20 and 21, available at
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=20

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-26
When collecting medical records from a Department of Defense (DoD) medical facility, identify
yourself to the commanding officer of the facility or representative and request authorization to
examine and copy records. Please note that DoD Directive 6040.2, Release of Information from
Medical Records authorizing release of medical information to government agencies, has been
rescinded by DoD; if the representative of the facility requests a letter authorizing release, use the
same letter as above.
If the hospital does not accept the FDA letter for Authorization for Medical Records Disclosure,
obtain and complete the one the facility provides.
Collect all medical records pertinent to the investigation. See IOM 5.11.5.
References are available at: https://www.law.cornell.edu/cfr/text/45/164.512
and
https://www.cdc.gov/mmwr/preview/mmwrhtml/m2e411a3.htm.
8.1.6.3 - Sample Collection
Chapter 4 covers general sample collection methods and authority. In general, collection of samples
during an investigation will be directed by the assignment or in discussion with your supervisor.
Opened containers of product are rarely sampled.
Prior to initiating sample collection, you may consider contacting the home division of the
manufacturing plant. They may be aware of an existing issue related to the product and problem.
Samples should be collected immediately, while they are available.
When a consumer portion is collected, intact containers of products of the same lot should be
collected from the retail and wholesale levels if available. When collecting samples at retail or
wholesale, ask if the firm is aware of any other complaints concerning the product. Refer to IOM
4.3.5.1 for additional information concerning collection of consumer portions. Maintain the
confidentiality of the complainant. If the distributor inquires about holding or recalling the product,
refer them to your supervisor.
8.1.6.4 - Internet Investigations
The internet can provide useful information when conducting many types of investigations,
including obtaining basic background information. Often you can use your government issued
computer or cell phone for basic firm information (e.g., hours of operation, key personnel, location,
directions, etc.). In these cases, you are using the internet as a tool to assist as you determine where
and how to collect information and conduct your investigation.
When conducting specific internet investigations and documenting evidence online, refer to
Introduction to Internet Investigations
(http://inside.fda.gov:9003/ORA/Offices/OEIO/Enforcement/ucm560967.htm).
Note: This website is on the FDA Intranet and not accessible outside of the FDA Network.
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-27
8.1.7 - Locations for Investigations – Where could you conduct an
investigation?
Some examples of locations for investigations include:
Retail establishments.
A consumer’s residence.
Other government agencies.
Location agreed upon with a complainant.
Hospitals/physicians’ offices.
Online.
Depending on the circumstances involved, an investigation can be performed at almost any location.
8.1.8 - Internal and External Organizations Involved - Who else will I
encounter during an investigation? Why are they involved?
The agency works cooperatively with many outside organizations, primarily other federal government
agencies and state, local, tribal and territorial (SLTT) authorities. Internally, you may work with
individuals from ORA headquarters, OCI, other programmatic divisions or center employees. Some of
these organizations become involved due to contractual obligation, statutory obligation, request for
expertise, or memorandum of understanding (MOU). Chapter 3 of the IOM provides information about
major organizations that FDA interacts with, both federal and state.
8.1.8.1 - Interagency Referral
One of FDA's functions is to assist SLTT and other federal agencies in conducting investigations,
collecting samples, and conducting plant inspections. If you find information during the course of an
investigation that may be relevant to another federal agency, a referral request can be made by
filling out an online form
https:/www.accessdata.fda.gov/scripts/IRF/.
Primary regulatory authority may belong to FDA or another agency. It is important to be aware of
which organization has primary regulatory authority during an investigation.
For Grade A Milk products, raw molluscan shellfish, and retail food operations, within ORA, the
Office of State Cooperative Programs (OSCP) has lead responsibility. For these cooperative
programs, the state has primary authority for investigations. FDA often accompanies and assists
states during investigations through the Office of State Cooperative Programs. If your investigation
involves Grade A Milk or Milk Products, raw molluscan shellfish, or retail food operations, contact
the Office of State Cooperative Programs before investigating.
8.1.8.2 - Intra-agency/Cross-Program
Outside of ORA, you may be involved other components of FDA. FDA staff work closely with one
another to ensure the safety, efficacy, and security of FDA-regulated products. FDA
functions are organized into the following:

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-28
• The Office of Foods and Veterinary Medicine (OFVM) provides oversight of FDA’s food and
feed programs as well as leads the implementation of the FDA Food Safety Modernization
Act of 2011 (FSMA). OFVM includes the Center for Veterinary Medicine (CVM) and the
Center for Food Safety and Applied Nutrition (CFSAN) which includes the Coordinated
Outbreak Response and Evaluation (CORE) Network.
• The Office of Medical Products and Tobacco (OMPT) provides high-level coordination and
leadership across the centers for drugs, biologics, medical devices, and tobacco products.
This office also oversees special medical programs.
• The Office of Global Operations (OGO) is focused on globalization and import safety of
food and drug production and supply. OGO provides direction and support to ORA and the
Office of International Programs (OIP).
8.1.9 - General Investigation Reporting - How do I report my investigation?
How do I get credit for the time I spent on it?
Reporting an investigation is almost always done using a memorandum (see Exhibit 8-3) and captured as
an operation in eNSpect (explained in more detail below). The format of the investigation memo is not
as defined in sections as an establishment inspection report (EIR). As a general guideline you can first
summarize why or give the reason for the investigation, what was covered, and briefly state the
findings. After this, you can go into detail about how you conducted the investigation and what you
found. Reporting the course of your investigation and your findings chronologically works in most
situations. For long narratives, using headings will make it easier for the reader to follow your reporting.
Some types of investigations have forms that need to be completed in addition to the narrative within
the memo. For example, FDA Form 3623, the Farm Investigation Questionnaire (FIQ), must be
completed for all farm investigations.
For import specific investigations see IOM 6.1.3.8.
8.1.9.1 - Entering investigation operations in eNSpect
eNSpect is used to capture information about the assignment, the establishment, and the
investigation. Investigation operations are reported in eNSpect as either a Domestic Investigation
(OP13) or Foreign Investigation (OP15). General information on how to complete these operations
in eNSpect field client are provided below. (For complete instructions, refer to the
eNSpect
Resources Site for the current eNSpect User Guide and eNSpect training.) The “Investigation” tab
in eNSpect includes the “Details,” “Time & Coverage,” and “Endorsement” pages.
The “Endorsement” page has three sections: Endorsement, Attachments, and Supervisor Feedback.
Attachments, such as the investigation memo, are uploaded to support your findings under the
“Attachments” section of the endorsement page. All three sub-sections must be completed, and
each has a maximum of 4000 characters per text box. The narrative entered in these sub-sections
will depend on several factors (e.g., program/division, type of investigation, assignment). If the
character limit prevents you from describing all relevant facts, an investigation memo should be
prepared and uploaded under the “Attachments” section. If the space is adequate to report your
investigation, you may not need to prepare a memo. For example, reporting OEI improvement
activities and firms determined to be out of business (OOB) are two situations where a memo

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-29
usually is not necessary. However, this can also depend on your program and/or division
procedures. Programs of divisions may require a memo for all investigations. Consult with your
supervisor if you are unsure whether a memo is required for the investigation.
Your supervisor or other designated individual will review and endorse the investigation report
(OP13 or OP15) in eNSpect. An inspection (OP11 and OP12) can be converted to an investigation
(OP15 and OP13, respectively) in eNSpect when you were unable to complete the inspection (often
referred to as a “washout”). Obtain supervisory concurrence before converting an inspection to an
investigation due to a washout. For example, your supervisor may want you to hold onto an
inspection assignment and inspect a seasonal firm later in the year rather than converting the
inspection to an investigation as a washout.
Reasons to convert an inspection to a washout include the following: Out of Business (OOB); Not
Official Establishment Inventory (NOE); Inactive (INA); Seasonal (SEA); Operational but not an FDA
obligation (OPR); Pre-Production (PRE-PROD), and Firm does not meet assignment criteria (OPR).
The information reported in your investigation, especially the reason for the investigation, may be
helpful to future investigators. If the investigation finds further action is recommended, do not
convert the associated inspection assignment to a “washout” in eNSpect. Report the operation
using an ad-hoc eNSpect investigation (OP13 or OP15). Do not return the associated inspection
operation (OP12 or OP11) to FACTS for conversion to an investigation. An example of a further
action would be a request for Import Alert because of an inspection refusal in a foreign country.
8.1.9.2 - Investigation memo: format, content, endorsement, and routing
Exhibit 8-15 demonstrates the general format of a memorandum of investigation (investigation
memo), which includes the originating division/office; responsible firm; FDA Establishment
Inventory (FEI); to/from; date; and subject. When writing an investigation memo, consider the
following:
• Document all pertinent information (e.g., who, what, when, where, why). At a minimum,
the investigation memo should contain the following information: the reason for the
investigation; background and history, if any; findings; and recommendations.
• Provide details of how you conducted the investigation and describe pertinent data,
references, attachments, etc.
• Headings may be used if it contributes to presenting your report in a clear, logical, and
concise manner.
• Routing for the memorandum should be included. Consult with your supervisor if unsure
of the correct routing information to include.
8.1.9.3 - Reporting complaints/follow-ups
Refer to SOP-000544, Consumer Complaint Procedure, in the Quality Management Information
System (QMiS) for detailed instructions on the screening, evaluation, monitoring and investigation
of consumer complaints of FDA-regulated products received by and/or submitted to ORA personnel.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-30
If you conduct an inspection to follow-up on a complaint, any findings related to the complaint
should be documented in the Establishment Inspection Report or in one of the following ways for an
OP13/OP15, if not involving confidential informants, whistleblowers, etc.,
If you conduct an inspection as above, but it involves a confidential informant or whistleblower,
follow directions for reporting provided in Consumer Complaint section related to Confidential
Informant or Whistleblower.
Information contained in the investigation memo or sub-sections of the “Endorsement” section of
eNSpect, should at a minimum include a general discussion of the complaints that were covered and
the complaint number(s). The complaint numbers should be recorded in the endorsement of the
OP13/OP15. In addition, consumer complaints are linked to these operations in eNSpect by entering
the consumer complaint ID under the “Details” page in eNSpect.
The time spent on the consumer complaint follow-up should be reported in the eNSpect assignment
(OP13 or OP15) under the appropriate complaint Program/Assignment Code (PAC) for any
complaints covered during the investigation. Refer to the PAC Master List for the appropriate PAC.
If a sample is collected during a domestic consumer complaint follow-up investigation, the sample
number is linked to the OP13 in eNSpect by entering the sample number under the “Details” page in
eNSpect. In addition, an OP31 (Sample Collection) with a collection report containing all relevant
information will be completed. If the primary response to the complaint is collection of a sample
and no further investigation, no assignment (OP13 or Op12) is generally created for completion in
eNSpect. In this case all relevant documents would be included with the OP31 collection report. If
unsure of what operation to enter in eNSpect and/or FACTS, consult with your supervisor.
8.1.9.4 - Reporting information obtained from a confidential informant
During an investigation, inspection, or other operation, you may acquire information from a
confidential informant. See IOM 5.2.9 for information on how to interview confidential informants
and document information obtained from them. Information received from a confidential informant
during an investigation should be captured in an investigation memo as an attachment to the
OP13/OP15. See IOM 5.2.9.2 for suggestions on how to protect the identity of the confidential
informant when writing your investigation memo. Information contained within an OP13/OP15 is
outside the scope of FMD-145 (Release of the Establishment Inspection Report (EIR) and should be
reviewed by FOIA personnel for appropriate action before release.
If during an inspection you interview a confidential informant or whistleblower, do not include any
identifiable information in the EIR and prepare a separate memo of investigation to cover this part
of the inspection. Enter as an OP 13 or OP 15. See 5.2.9.2.
8.1.9.5 - Reporting investigations conducted during disaster response
There is no prescribed format for narrative reporting of disaster operations. Consult with your
supervisor as to your division's preference. If operations were conducted as an investigation, you
will likely write an investigation memo to document the activities. The memo should briefly describe

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-31
the onset of the disaster, its magnitude, and your activities. Include cooperation with officials,
planning operations, and the logical sequence of your activities.
Your memo must contain exhibits consisting of photographs, diagrams, records, references to
samples, and any other items necessary for proper presentation of the operation. Refer to RPM
Chapter 8 “Emergency Procedures,” for guidance on reporting natural disasters and civil disorders.
List amounts of materials or products destroyed and the method of destruction. Prepare charts and
lists as necessary to provide documentation of all affected lots destroyed, reconditioned, or
released. Include kinds and amounts of materials segregated, released, reconditioned, and
destroyed and method of reconditioning and/or destruction.
In situations where an ICS structure has been implemented, operations are reported through the
IMT and use of ICS forms, situation reports, after-action reports, or other documents as appropriate
to the operation. The IMT will direct you on reporting your time spent working on the operation.
If a sample of an FDA-regulated product is collected as part of the disaster response under ICS, an
OP31 (Sample Collection) with a collection report containing all relevant information will usually be
completed. In this case, your time spent conducting the sample collection would be reported in
FACTS as part of the OP31 and using the PAC appropriate for the assignment.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-32
8.2 - Human and Animal Food Investigations
8.2.1 - Coordination
The initial step in coordination of a human and animal food investigation is notification of the potential
incident. Notification to FDA may be received from SLTT officials via the district ERC or divisional staff;
consumer complaints or adverse event reporting portals; or from federal entities such as the Centers for
Disease Control and Prevention (CDC) alerting the FDA of illness clusters. Regardless of the source, once
a potential incident is identified, the district ERC (DERC) is the primary point of contact (POC) for
coordination of the response at the field level. (See Communications SOP for ERCs)
If agency-level central coordination is needed, CORE or CVM will most often provide management of the
incident based on whether human or animal foods are suspected. However, there are instances when
food incidents may be coordinated by OEM or a CFSAN office based on the specific commodity and
scope of incident. (See Exhibit 4 for the “Table Depicting Incident Coordination Body by Type of Incident.)
8.2.2 - Foodborne Illness Outbreak Investigations
8.2.2.1 - Cooperation with Other Agencies
One of FDA's functions is to assist SLTT and other federal agencies in conducting investigations,
collecting samples, and conducting firm inspections if warranted.
In addition to state and local health departments, the following federal agencies may also become
involved in investigating foodborne disease outbreaks:
• Centers for Disease Control and Prevention (CDC)
• U.S. Department of Agriculture (USDA)
• Environmental Protection Agency (EPA)
CDC becomes involved in foodborne outbreaks when people in more than one state are sick with
the same germ from contaminated food. Their role involves coordinating the epidemiologic
investigation, including identifying illnesses. CDC works directly with CORE to provide
epidemiological information to help identify a possible food vehicle and focus the scope of FDA’s
investigation. During an outbreak, CORE and CDC coordinate with internal and external partners
(including international governments) to help determine the outbreak source and prevent future
illness.
USDA is responsible for investigating outbreaks involving meat and poultry products under their
jurisdiction. Whenever a complaint is received involving any meat-containing product, including
such items as soups, combination infant foods, frozen dinners, etc., evaluate the need to contact
USDA. Most products containing red meat or poultry are regulated by USDA. The exceptions
include:
• products containing meat from game animals, such as venison, rabbits, etc.
• meat-flavored instant noodles
• "pork and beans" (which contains only a small amount of pork fat and is regulated by FDA)
• Closed face sandwiches

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-33
USDA-regulated products display on their labels a round "shield" with the USDA Establishment
Number. Alternatively, the establishment number may be identified in the lot number. Red meat
products under USDA jurisdiction will often contain the abbreviation "EST" followed by a one to
four-digit number; poultry products under USDA jurisdiction will contain the letter "P" followed by
a number.
IOM 3.2.1 and 3.2.4.3 provide information for reporting suspected outbreaks to USDA and CDC. In
addition, FDA and CDC have an agreement that FDA will be immediately advised whenever CDC
ships botulism antitoxin anywhere in the United States or its possessions.
Whenever a water source is suspected as a likely origin of the agent of an illness outbreak, the EPA
should be notified. For example, when investigating a foodborne outbreak on a vessel passenger
conveyance, you may find the water used in food preparation to be from a land-based source or
from an on-board water treatment plant. Both of these sources would fall under EPA jurisdiction.
See IOM 3.2.11.
When two or more people get the same illness from the same contaminated food or drink, the
event is called a foodborne illness outbreak. For more information related to foodborne illnesses,
please refer to
https://www.fda.gov/food/recalls-outbreaks-emergencies/outbreaks-foodborne-
illness
8.2.2.2 - Outbreaks on Foreign Flag Vessels
If a suspect outbreak involving a foreign flag vessel or a U.S. flag vessel with an international
itinerary comes to your attention, report it to your supervisor and the district ERC immediately. The
district ERC will provide the information to OEM/OEO. The CDC assumes primary jurisdiction for
foreign flag (non-U.S. registry) and U.S. flag vessels with international itineraries entering the U.S.
and traveling in U.S. waters. See IOM 3.2.4.3.
8.2.2.3 - Outbreaks Involving Interstate Conveyances
Reports of illness attributed to travel on an interstate conveyance (plane, bus, train, or vessel) are a
shared responsibility of FDA, CDC, USDA, EPA, and potentially others. When a report of illness is
received, notify the district ERC in your division/district. The ERC will contact the CORE Signals Team
at [email protected]s.gov
. Please include the CFSAN Office of Food Safety Interstate Travel
on any email correspondence (Joseph.morin@fda.hhs.gov ). In addition, you are encouraged to
share the report with state and local public health officials. The following activities are to be
coordinated with local/state public health officials: Interviews with the ill passenger(s), family
members (well and ill), caregivers, and/or health professional (as appropriate) should be sufficiently
probative to hypothesize if the food, water, or an environmental transmission is related to the
illness. Transmission of illnesses, particularly viral diseases, by ill employees and contaminated
environmental surfaces can result in illness carryover between successive trips and should be
considered. Factors such as symptoms, time of onset of symptoms, food history for the 72 hours
prior to onset of the first symptom, any clinical laboratory results, and other potential exposures
should be documented. The carrier should also be contacted to determine if other reports of illness
have been received (passengers and employees). Obtain any illness logs from the carrier. The
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-34
information developed should be evaluated to determine if further follow-up is necessary. On those
carriers where a reservation system is used, obtain the names and phone numbers of passengers,
and a passenger manifest, if available. If a reservation system is used, then a passenger manifest
should also be available. A manifest will provide passenger seating, which will help identify
additional cases based on proximity or in the event of an etiological agent like Norovirus, the
passengers who occupy the seat on the next flight could also be at risk of infection. It may be
necessary for the state/local health authorities, CDC or FDA to contact other passengers to
determine if they became ill.
If additional cases are uncovered during these contacts, immediately notify the appropriate ERC in
your division who will then contact the CORE Signals Team at CORESignalsTeam@fda.hhs.gov
and
the state and local public health authorities in all of the affected states. FDA will work cooperatively
with these authorities and request their assistance in conducting an epidemiological investigation
and collecting patient specimens. Note: If at any time the local/state public health officials are
unable to assist with an investigation, have the district ERC notify CORE Signals Team at
[email protected] who will contact the CDC and request assistance with the
epidemiological investigation.
8.2.2.4 - Outbreak Management
CORE coordinates FDA’s efforts to prevent, detect, investigate, respond to, evaluate and apply
lessons learned from foodborne outbreaks and public health incidents. Along with ORA, CFSAN
subject matter experts (SME), and others in FDA, CORE manages the strategy and implementation of
outbreak response activities and evaluates environmental, epidemiologic, and laboratory data to
inform assignments and direction of outbreak investigations related to foods, cosmetics, and dietary
supplements. ORA’s primary role in the outbreak investigation is to perform activities related to
tracing food from source to destination; food and environmental sample collection and analysis; and
facility investigations.
If you become aware of a foodborne outbreak, contact the appropriate district ERC immediately
who will then contact the CORE Signals Team at CORESig[email protected]
.
8.2.2.5 - Conducting Foodborne Illness Follow-up
A priority for all foodborne illness investigations is to establish the basis for implementing control
measures to stop transmission and prevent additional illnesses.
CDC is the federal agency with primary responsibility for investigating large, multi-state foodborne
illness outbreaks. FDA plays a role in outbreak response generally by collecting samples, obtaining
traceback information, and conducting food establishment inspections. CDC guidance for
investigating foodborne illness is available at Investigating Outbreaks
(https://www.cdc.gov/foodsafety/outbreaks/investigating-outbreaks/index.html). SLTT generally
conducts small, local foodborne illness outbreaks using generally the same process. In FDA, CORE
guides investigations into the cause of foodborne illness outbreaks after notification from CDC that
an outbreak is ongoing.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-35
A resource for conducting epidemiological investigations is the Council to Improve Foodborne
Outbreak Response (CIPHOR). Its website (https://cifor.us/) has many resources available to aid
during an epidemiological investigation.
If you receive a report of a foodborne illness or an outbreak provide details to your district ERC and
determine the extent of investigation you need to conduct. If you are required to respond to a
foodborne illness outbreak use the following as guidance.
8.2.2.5.1 - Preparation
Divisions should maintain enough supplies of equipment used for sampling during a foodborne
illness investigation. Assure all sterile supplies are within expiry. It is important that swabbing
materials be monitored and utilized in a first in, first out manner to prevent the expiry of
supplies.
8.2.2.5.2 - Interviews
Reports of foodborne illness can come from many sources, such as:
• Laboratories
• Hospital-based laboratories
• Clinical laboratories
• National or regional commercial referral laboratories
• Local or state health department laboratories
• CDC laboratories
• Health care institutions
• Hospitals (e.g., hospitalized patients reported by infection control practitioners)
• Emergency departments
• Long-term–care facilities or nursing homes
• Physicians
• Schools and childcare centers
• Food establishments (e.g., restaurants)
• State health departments
Regardless of the source of the report, the diagnosis must be verified by a thorough case history
and, if possible, by examination of appropriate food samples and clinical specimens.
8.2.2.5.2.1 - Conducting Interviews
Normally, conducting foodborne illness interviews is not a role CSOs will perform since food
history/symptoms are typically gathered by CDC and/or SLTT partners. Follow the guidelines
upon contacting the affected person in the General Interview section (Section 8.1.6.1) of
this chapter and the following:
• Identify yourself and explain the purpose of the visit or call.
• Ensure confidentiality.
• Conduct interviews in a private location.
• Be non-judgmental.
• Show empathy and attempt to build rapport with the interviewee.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-36
• Exhibit genuine concern for persons affected and be sincere and respectful
when requesting personal and confidential information.
• Set your level of communication based on the person being interviewed.
• Be tactful. People are sometimes sensitive to questions about age, gender,
special dietary habits, ethnic group, excreta disposal, and housing conditions.
Phrase questions thoughtfully.
• While keeping the interview as conversational and as natural as possible,
communicate a sense of urgency and emphasize the positive contribution
already made by the interviewee toward the control and prevention of
foodborne illness.
• Use open-ended questions.
• Phrase your questions so the interviewee(s) will describe their illness and the
foods and events which they feel are associated.
• Accurately record what interviewees say.
• Gently redirect, as needed.
• Probe if answers are vague, particularly about time of symptom onset.
• Asking references may help their memory, for example, “What did you do prior
to eating lunch?”
• Work with epidemiology staff to provide language interpretation, if needed.
• Thank interviewee at closing and explain how the information will be used.
8.2.2.5.2.2 - Information to Gather
Targeted, effective and pertinent information gathering is critical in a foodborne illness
outbreak investigation. Per the CDC: Health officials use three types of data to generate
hypotheses about the likely source of an outbreak:
epidemiologic, traceback, and food and
environmental testing. Investigators begin by trying to pinpoint how the pathogen spread.
They review details such as:
• The specific pathogen causing illness
• Where sick people live
• How old they are, their sex, and race/ethnicity
• Did they have contact with a sick person
When a contaminated food is suspected, investigators must consider many different foods
that may be causing the illness. Interviews help to establish a list of foods people ate before
getting sick and collect information on other exposures such as restaurants where the ill
person ate and stores where they bought food. This list is used to help investigators
determine what food or ingredients the sick individuals may have in common.
Consult with management, ERCs, CFSAN, SMEs, state liaisons, state partners, FDA, CORE,
and others involved in the outbreak, as necessary, to determine what information is needed
from the interviewee(s). Interview topics can include:
• Interviewee information
• Clinical information
• A standard list of food items

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-37
• Each meal a person ate before becoming ill and all meals and snacks eaten
seventy-two hours before onset of illness. The food, even the meal, which
precipitated the illness, might not be obvious and the type of illness will
sometimes provide clues:
− If the first and predominant symptoms are nausea and vomiting,
concentrate questions on foods eaten recently.
− If the first and predominant symptoms are diarrhea and abdominal
cramps, foods eaten six to twenty hours before onset of illness are
suspect.
− If diarrhea, chills and fever predominate, foods eaten twelve to
seventy-two hours before onset of illness are suspect.
− More unusual illnesses often present different clinical patterns. For
instance, some illnesses such as Typhoid Fever and Hepatitis A, have
incubation periods greater than 72 hours.
• Food allergies, special diets, vitamins, and supplements
• Sources of food at home/outside of the home
• Animal contact and pets
• Specific food categories
• Food shopping habits
• Travel
• Restaurant dining
• Attendance at events where food was served
Although some may not have been ill, use this detailed interview approach with each
individual identified in the initial complaint or alert, until there is sufficient information to
determine the scope and source of the foodborne illness outbreak.
8.2.2.5.3 - Medical Records
Physicians' and hospitals' records can be useful in verifying reported signs, symptoms and other
clinical data and can sometimes rule out the possibility of foodborne illness. See General Section
on Medical Records (Section 8.1.6.2).
8.2.2.5.4 - Sampling Procedures
CAUTION: Never taste any of the food products. Handle all samples with caution to
prevent accidental exposure to and/or ingestion of even minute amounts of the
contaminated or suspect product.
8.2.2.5.4.1 - Sample Collection
During investigations of foodborne illnesses, cooperate with other public health officials in
collecting samples of items that may be associated with the outbreak.
Use interview information and a menu or data from an attack-rate table to determine which
of the foods from the implicated meal are most suspect and collect samples of the suspect
foods. Check storage areas for items that may have been overlooked. Check garbage for
discarded foods or containers. Suspect foods often are discarded by an operator if he thinks
someone may have become ill as a result of eating in his establishment. Because one of the
primary tasks of the investigator is to prevent further illness, take appropriate action to

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-38
prevent distribution or serving of any suspect food. If no foods remain from the suspect
meal or lot, try to collect samples of items prepared in a similar manner, but subsequently
to the suspect lot. Collect ingredients or raw items used in the suspect food. Determine
supplier, distribution, and code information on ingredients and packaged foods to aid any
investigation of the same lot in distribution channels.
Collect samples aseptically. If foods are to be examined for organophosphate pesticides or
heavy metals, do not use plastic containers. Use glass jars with foil-lined lids because
substances from the plastic can leach into the food and interfere with analysis.
The following are examples of articles normally collected:
• Remaining portions of all suspect foods.
• Parent stocks of suspect foods.
• Insecticides, rodenticides, or other poisons which may be involved.
• Suspect food containers such as cans, bottles, etc.
• Utensils or materials used in the preparation and storage of the suspect food.
• Table scrapings and food residues from equipment such as slicing machines,
cutting boards, etc.
NOTE: Clinical specimens such as vomitus, stools, swabs of nasal and throat passages, or
open sores or lesions of food workers are collected by local, state, or CDC health officials or
private physicians. Do not collect these samples.
8.2.2.5.4.2 - Sample Size
In general, follow the IOM SAMPLE SCHEDULE in Charts 1, 2, and 3 (IOM, Chapter 4). Where
only small amounts of items remain, such as partial meals/leftovers, empty containers with
adhering particles, etc., collect all or as much as possible by scraping from utensils,
equipment, or containers. It may also be necessary to collect the empty containers.
8.2.2.5.4.3 - Sample Handling
Record the temperature of the room, refrigerator, or warmer in which the food was stored,
and the temperature of the food that remains after a sample is collected.
Inform the laboratory of the type and number of samples. Discuss methods to preserve and
transport samples, time of arrival, and the person who will receive the shipment.
Follow guidance in Chapter 4 for collecting, handling, and shipping samples. See IOM
4.5.5.8.6.
If the suspect food is a commercial product, examine the original package or container for
coding information to identify the place and time of processing. Your division may notify all
agencies responsible for regulating the products alleged or suspected to have caused the
illness. Collect additional packages bearing the same code number for analyses for
microorganisms, toxins, seam defects, vacuum, leaks, or other conditions. Be as specific as
possible in requesting the type of analysis.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-39
8.2.2.5.5 - Establishment Investigation
After a foodborne illness outbreak is reported and an investigation is initiated, the initial impact
of the incident can create confusion at the facility and could result in conflicting information if
too many entities become involved.
The responsibility for investigating foodborne illness outbreaks rests on a core team of people
who each contribute different knowledge and skills. For FDA-initiated investigations/inspections,
one FDA investigator should be designated as the inspection team leader. The team leader will
set and enforce priorities, coordinate all activities associated with the investigation, serve as the
point of contact about the investigation, communicate with other organizations involved in the
investigation and communicate the recommended course of action determined by team to ORA
management. A supervisor and/or ERC should be the coordinator for overall division activities
and the division contact for headquarters personnel. All communications from FDA field or other
offices to the firm's management should be channeled through the supervisor/ERC. The lead
investigator should be responsible for all phases of the physical inspection of the facilities and
briefing the supervisor about team progress. See IOM 5.1.2.5.2.
Upon arrival at the establishment where the suspect food was processed or prepared, identify
yourself to the person in charge and state the purpose of your visit. Emphasize the purpose of
the investigation is to determine what contributed to the outbreak, so preventive measures can
be taken. Attempt to create a spirit of cooperation. Consider the position, feelings, and concerns
of the manager and facility staff; defensive reactions are common.
Many factors could have contributed to contamination before foods came under the control of
the manager. Assure the manager that these possibilities will be investigated. Inform the
manager of the activities proposed and benefits gained for educating their workers.
When investigating the root cause of the contamination obtain the following documents:
inspection reports (state and/or federal), detailed epidemiological data and traceback
investigation reports to try to pinpoint locations of interest, environmental monitoring records,
verification records of the identity, safety, strength, purity, efficacy, and accuracy of raw
materials and packing materials used, and any analysis of resource availability, (e.g.,
documenting sufficient manpower and prescribed raw materials, packaging materials utilized,
substitutions made, etc.), and historical data on weather events, e.g., flooding, for foods
produced in the open outdoor environment.
Perform the following activities:
1). conduct personnel interviews to determine their qualifications, knowledge, experience, and
training;
2). review related logbooks, records, processes, laboratory data;
3). document observations made with photos and videos whenever possible;
4). visit the facilities or farms, where causes of the event occurred (where possible).

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-40
5) Describe the processes, equipment and facilities.
6) Evaluate the following: the suitability of equipment, facilities, and utility systems; the
calibration and preventative maintenance of the equipment and instruments used; the
adequacy and the implementation of the relevant standard operating procedures (SOP) utilized
by the food business operator; and the Good Manufacturing Practices (GMPs), preventive
controls and food safety standards, as applicable, utilized in the area where the product
concerned was produced, processed, packed and/or held.
Include all relevant information in the investigation memo or EIR as appropriate.
Review of distribution records and examination of warehouse stock are two important aspects
of a foodborne illness follow-up inspection. Field examination should include an inventory by
code of all stock on hand. When conducting field examinations, follow instructions in IOM
Sample Schedule Chart 2 (IOM, Chapter 4).
8.2.2.5.5.1- Food Handlers Interviews
If a food is already suspect, interview separately all persons who were directly involved in
processing, preparing, or storing of the food and others who could have observed
preparation and storage. Ask questions in a sequence that discloses the flow of food from
the time it was received until it was served or distributed. Especially inquire about foods
that were prepared several hours or days before being served with the suspect meal and
about foods that have specific temperature requirements. Ask similar questions, suitably
modified, of the managers or workers who were involved in producing, transporting,
processing, preparing, or storing food at other levels of the food chain, as well as individuals
who prepared the food at home.
Food workers who fear criticism or punitive action because of their possible role in the
outbreak do not always accurately describe the food handling as it actually happened. Their
descriptions should be plausible, account for possible sources of contamination, and
indicate possibilities of survival and potential for growth of pathogens. If the description
does not contain all the information desired, rephrase the questions and continue the
inquiry. Seek confirmation of one person's story by talking to others who have knowledge of
the food operation, or by watching the food preparation or processing practices. Be alert for
inconsistencies among the accounts, as told by different individuals.
8.2.2.5.6 - Possible Contamination Source
It is important to understand the pathogen and the factors that contribute to the contamination
that resulted in the foodborne illness. Some pathogens, such as Norovirus, are associated with
human fecal contamination, while other pathogens, may be more commonly associated with a
particular food source (e.g., raw meat and E. coli O157:H7).
CDC has identified the most common causes of foodborne illness:
• Food from unsafe source.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-41
• Poor personal hygiene.
• Improper food holding temperatures.
• Improper cooking temperatures
• Contaminated equipment of cross-contamination of raw with ready-to-eat foods
You may want to familiarize yourself with Factors that Contribute to Outbreaks of Foodborne
Illness (https://www.cdc.gov/nceh/ehs/nears/factors-contribute-to-outbreaks.htm
) before
beginning a foodborne illness investigation.
Exhibit 8-5 (https://cifor.us/uploads/resources/CIFOR-OUE-Agent-List_FINAL.pdf) provides
details about possible food associations with different illness symptoms, latency and factors that
contribute to outbreaks. Although the table lists possible clinical specimens to collect, you
should not collect clinical samples. A SLTT health department may be able to assist and collect
those samples for analysis at a state laboratory.
8.2.2.5.7 – Conducting Traceback Investigations
Traceback investigations are important epidemiological tools that are used to determine the
source of food implicated in foodborne outbreaks. Traceback investigations may prevent further
sale and distribution of contaminated food. Commonly, SLTT agencies conduct the initial
epidemiological investigation of foodborne outbreaks and identify suspect product(s) requiring
tracebacks.
CORE issues traceback assignments to the appropriate division(s) and coordinate inter-division
assignments for traceback investigations. The assignment will generally provide all the guidance
needed to conduct the traceback investigation.
Other resources available include:
• the
FDA Guide to Traceback of Fresh Fruits and Vegetables Implicated in Foodborne
Outbreaks, dated April, 2001
• the Office of Training Education and Development (OTED) training: ER220: Traceback
Investigations.
8.2.2.6 - Reporting
8.2.2.6.1 - Reporting Epidemiological Investigations
Follow the reporting guidance in this chapter to report epidemiological investigations. Promptly
submit a complete narrative of the investigation, including references to exhibits, samples,
medical records, and laboratory reports. There is no prescribed reporting format, but it should
be in a logical order. With the inclusion of investigative memos in eNSpect EIR, eNSpect can be
utilized to prepare these memos. See the eNSpect EIR Quick Reference Guide for detailed
information. See also IOM 8.10.
Submit copies of any written reports and documents for all injury or illness complaints involving
all CFSAN products (see section 8.2 and 8.4.5) using encrypted email, secure fax transmission, or
mailing.
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-42
If using mail, use this address:
Food and Drug Administration
CFSAN/OSAS
CAERS Staff (HFS-700)
5001 Campus Drive
College Park, MD 20740
Attn: CAERS Monitor
Illness/injury complaints involving special nutritional products (refer to IOM 8.2.3.2) must be
accompanied by a completed FACTS Adverse Event Questionnaire when forwarded to CFSAN.
If additional follow-up on any complaint involving a CFSAN product is necessary, the Division of
Field Program Planning and Evaluation (HFS-635) will issue an assignment.
8.2.2.6.2 - Reporting Food Adverse Events
Prompt reporting is essential. You may save the lives of others with prompt reporting. If
consumers contact you to report adverse events including injury, illness, or death related to a
human or animal food, dietary supplement, or cosmetic, they should be directed to report
through the following online reporting systems. If they do not want to report through those
systems, you may report for them.
8.2.2.6.2.1 - Food and Cosmetics
Details on reporting adverse events related to human food can be found at the CFSAN
Adverse Event Reporting System (CAERS) website
(
https://www.fda.gov/food/compliance-enforcement-food/cfsan-adverse-event-
reporting-system-caers).
8.2.2.6.2.2 - Dietary Supplements
Details on reporting adverse events related to dietary supplements can be found at the
How to Report a Problem with Dietary Supplements website
(
https://www.fda.gov/food/dietary-supplements/how-report-problem-dietary-
supplements).
8.2.2.6.2.3 - Cosmetics
Details on reporting adverse events can be found at the How to Report a Cosmetic
Related Complaint website (
https://www.fda.gov/cosmetics/cosmetics-compliance-
enforcement/how-report-cosmetic-related-complaint).
8.2.2.6.2.4 - Veterinary Products
Details on reporting adverse events and complaints for animal food and animal medical
products can be found at the CVM Report a Problem website
(https://www.fda.gov/animal-veterinary/safety-health/report-problem
).

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-43
8.2.2.6.2.5 - Reports of Criminal Activity
If a consumer calls to report criminal activity, they should be directed to the Report
Suspected Criminal Activity website
(https://www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm
).
8.2.3 - Injury, Illness, Death
Injury and adverse reaction complaints should receive a prompt, courteous response, and assurance
their complaints will receive appropriate consideration. An immediate follow-up should be made when
there is an indication of a serious injury or adverse reaction.
When you are investigating injuries or adverse reactions, do not make comments or enter discussions
with firms as to the involvement of particular products, unless specifically instructed to do so. Many
adverse reactions come to FDA from consumers or health care professionals through the voluntary
reporting branch of the MedWatch system. These reports are to be held confidential.
Whenever the press has been informed about a complaint, follow instructions found in Section 1.6.1.
When the responsible firm invites the news media to observe the inspectional process, follow
instructions found in Section 5.1.4.3.
NOTE: CFSAN Adverse Events Reporting System (CAERS) Staff, HFS-845, 240-402-2405, Fax: 301-436-
2452, or email [email protected]hs.gov, can assist with questions pertaining to field follow-up related to
foods, seafood, food additives, dietary supplements, infant formulas, and medical foods. CAERS
personnel can assist in obtaining guidance from CFSAN's experts.
8.2.3.1 - Procedures
When investigating all injuries and adverse reactions the consumer complaint coordinator will
follow SOP-00045 Consumer Complaint Procedure.
Once it is determined by program management that follow-up is deemed necessary, an assignment
will be created and assigned to a CSO, who will then fill out the Follow-up Consumer Compliant
Report in FACTS.
The following should be addressed and confirmed during a follow up investigation with the
complainant.
• Details on the product involved, including brand name, product labeling, and any codes
including lot, expiry, and/or use by codes.
• The source of the product. Where did the consumer obtain it?
• Details of how the product was used, including frequency, in what amounts, any known
previous adverse reactions or pre-existing allergies and whether anyone else used the
product in the household.
• If appropriate, determine if label directions were followed.
• Copies of all labeling/inserts.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-44
• Any research the complainant may have conducted or relied upon and collect copies or
internet web addresses.
• Complete description of the incident (sequence of events) and the nature of the injury
or adverse reaction, including date, time, location, and symptoms or description of
injury.
o Any hospital or physician's records available and identify pre-existing conditions
which may have a bearing on the injury or adverse reaction.
o Photographs of the victim's injuries, if significant. See Section on Medical Records.
• List names of other persons involved, such as beauty salon operators, medical
personnel, lawyers, insurance agents. Obtain their views on the injury or adverse
reaction. The views of an attending physician are important because they may vary
markedly from those of the patient.
• Determine if the consumer reported the adverse reaction to the manufacturer and the
manufacturer's response.
• Any other consumer complaints, injuries or alleged adverse reactions reported to the
manufacturer concerning the product.
• If necessary, obtain distribution information of the implicated lot(s) from the
manufacturer.
8.2.3.2 - Specific Product Reporting (Food, Dietary Supplement, and Cosmetic
– Injury or Reaction)
8.2.3.2.1 - Dietary Supplements
It is extremely important that FDA conducts appropriate investigations and follow-up on adverse
events attributed to dietary supplement products. DSHEA removed dietary supplement and
ingredients from food additive regulations and therefore it is the agency’s burden to prove them
unsafe. An important source of information concerning potentially unsafe dietary supplements
and ingredients is consumer complaints.
Injuries or other adverse reactions may be associated with the use of products which:
• Vary from the declared potency or concentration.
• Contain deleterious substances accidentally included in manufacturing.
• Have changed composition or become contaminated after shipment.
• Are mislabeled as to identity warnings or instructions for use.
• Have not been used according to label instructions or the directions of the manufacturer
or prescriber.
• Are dangerous when used according to directions.
When investigating adverse events attributed to dietary supplements, direct attention to, and
document:
• Details on the product involved, including lot codes and expiration dates.
• Source of the supplement. Where did the consumer obtain it?
• Details on the consumer’s use of the product including frequency, dose used,
concomitant treatments, and whether administered by the user or someone else.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-45
• Details on the directions of use provided with the product or otherwise (on the web or
from a practitioner). Obtain copies of labeling and any additional information
concerning use of the product by the consumer.
• Nature of the injury. Include any hospital or physician's records available and identify
pre-existing conditions which may have a bearing on the injury. Obtain photographs of
the victim's injuries, if significant. See IOM 8.1.6.2 for the procedures used to obtain
medical records.
• Names of other persons involved, such as medical personnel, lawyers, insurance agents,
etc. Obtain their views on the injury. The views of the attending physician are important
because they may vary markedly from those of the patient.
• Complete description of the incident (sequence of events) and the nature of the injury
or adverse reaction, including date, time, location and symptoms or description of
injury.
• Any hospital or physician's records available and identify pre-existing conditions which
may have a bearing on the injury or adverse reaction.
Photographs of the victim's injuries, if significant. See Section on Medical Records
8.2.3.2.2 - Cosmetics
For clarification of the distinction between cosmetics and drugs, refer to the document, “Is it a
cosmetic, a drug or both? (or is it soap?)” located at
https://www.fda.gov/cosmetics/cosmetics-
laws-regulations/it-cosmetic-drug-or-both-or-it-soap
If you are unsure about a products status you may contact the Office of Cosmetics and Colors at
(240) 402-1130.
8.2.3.2.2.1 - Causes
Injuries or adverse reactions may arise from cosmetics which:
• Are inherently dangerous or which may prove harmful or injurious to a consumer.
• Cause primary irritation of skin, eye, or mucous membranes (including the lungs
and urinary tract) or which may be due to an individual sensitization reaction or
allergic response, or due to ingestion.
• Have undergone formulation changes or been chemically or microbiologically
contaminated while in the possession of the manufacturer, dealer, distributor, or
end user.
• Are misbranded because they contain unlisted ingredients, lack instructions for
safe use for certain high-risk products (e.g., depilatories, hair dyes), or lack any
required warning statements.
• Have been misused.
8.2.3.2.3 - Investigation Requirements for Serious Adverse Events of CFSAN Regulated
Products
If the suspect product is a cosmetic, interview the injured person and/or the reporter of the
event and complete the FACTS Consumer Complaint Cosmetic Report. If the suspect product is

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-46
not a cosmetic, interview the injured person and/or the reporter of the event and complete the
FACTS Adverse Event Questionnaire.
If the suspect product is an infant formula or baby food, immediately inform OEM/OEO at 866-
300-4374 or [email protected]
and investigate on a high-priority basis due to
the continued sensitivity to these incidents. This will include follow-up with the doctor or
hospital, sample collection, and analysis of appropriate product. Refer complaints involving baby
food regulated by USDA for appropriate follow-up. See IOM 8.1.8.1 and 3.2.1.2.
Obtain Medical Records as described in Section 8.1.6.2.
If the adverse event is a death, the following medical records should be considered for
collection:
• Admission history and physical or emergency room/clinic record of the event if the
patient was not admitted
• Discharge Summary
• Autopsy Report
• Death Certificate
If you believe a suspect product should be sampled, discuss with your supervisor.
For all events, a memo of investigation will be completed. Send a complete copy, including
copies of all labels and labeling, Medical Records Letter [IOM Exhibit 8-4] and medical records
collected to the CAERS Staff.
8.2.3.2.4- Undeclared Allergen/Allergic Reactions
Suspected undeclared allergen complaints should receive high priority. Undeclared allergens in
food products often result in recalls.
The following should be addressed and confirmed during a follow-up investigation with the
complainant:
• List all the complainant’s food allergies.
• List all foods consumed within approximately an hour prior to reaction.
• Amount of suspect food consumed.
• Time of on-set.
• Specific symptoms experienced and the order they occurred.
• Medical or other treatment received.
• The ingredient statement from product packaging.
• Any label statement related to a “may contain” statement and record the statement.
Inspectional follow up at the manufacturing plant may be warranted to determine if suspect
allergen is added to the product; or if the possibility of cross-contact exists.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-47
8.2.3.3 - Veterinary Products-Complaints/Adverse Reactions
If you become aware of human illnesses associated with CVM-regulated products, contact the
appropriate ERC in your division and/or regional office immediately who will then contact the CORE
Signals Team at CORESignalsTea[email protected]
.
Investigations of complaints of animal food, both medicated and non-medicated, should be
investigated like other complaints. Discuss any investigation with your supervisor. The CVM Office of
Compliance can be consulted concerning appropriate follow-up and sample collection related to
complaints.
8.2.3.4 - Sample Collection
When directed to collect a sample, collect the product which appears to have caused the injury and
an official sample from both the same and other lot codes, if available. Check with your supervisor if
you have any doubt as to the appropriateness of collecting a sample related to an investigation.
See IOM 4.5.5.3 for routing of injury and complaint samples to the laboratory.
8.2.3.4.1 - Cosmetic Samples
Many cosmetic products such as permanent hair dyes, home permanents, deodorants, hair
straighteners, etc. are known to cause adverse reactions. Samples of these products should not
be collected except in cases of alleged severe or unusual injury (e.g., multiple complaints). In
cases of obvious allergic type reactions, samples should not be collected. For example, most
cosmetic products which get into the eye will cause temporary eye irritation and, in such cases,
a sample generally should not be collected.
8.2.3.4.2 - Microbiological Contamination
Collect samples associated with consumer complaints in which microbiological contamination is
suspected.
8.2.3.4.3 - Allergen Samples
Collect a sample if the allergen is visible (i.e., nuts,) is not declared on the label, and if deemed
necessary by division management. In all other cases, collect a sample only after consultation
with OEM/OEO (e.g., national consumer complaint coordinator) and CFSAN. See IOM Sample
Schedule Chart 13 for guidance on sample size. Note: the sample size may be modified
depending on product availability.
8.2.4 - Special Events
Special Events (SEs) are organized, pre-planned mass gatherings of national or international importance
that usually garner significant media coverage and are typically attended by dignitaries or public
personalities. The venues for these events are frequently stadiums, arenas, convention centers, and
hotels which contain retail food establishments that are under the jurisdiction of state and local
agencies. SLTTs, United States Secret Service (USSS), or the event organizer may request FDA assistance.
You may be requested to investigate food suppliers to the SE to verify compliance with regulations. This
investigation, referred to as a Supply Chain Integrity Check (SCIC), may be performed onsite or through

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-48
an online record review. The SCIC entails a review of a firm’s inspectional and compliance history,
including recalls, Reportable Food Registry (RFR) and whether the firm has been involved in a CORE
investigation. (See IOM Chapter 3.2.5.2.9 for full details on SEs)
8.2.5 - Farm Investigations
A farm investigation of a raw agricultural commodity (RAC) may be conducted in response to traceback
information obtained during a foodborne illness outbreak investigation that implicates one or more
farms, ranches, packing houses, or other such operations as being involved in handling the outbreak
suspect RAC. Generally, CORE would request a domestic farm investigation through the district ERC for
the responsible ORA Human and Animal Food (HAF) program division office. HAF program division
offices may also initiate or be assigned by CFSAN or ORA/OHAFO to perform a farm investigation as
needed to protect public health. The goals of a farm investigation are to gather information, to identify
potential environmental sources of the outbreak agent, to identify routes of contamination from
potential outbreak agent sources to the implicated RAC, to observe and document potential
contributing factors to the outbreak such as practices, procedures, or conditions that may facilitate
proliferation, spread, growth, survival, or contamination by the outbreak agent, and to support
regulatory action, if appropriate.
8.2.5.1 - Approach
A team approach is utilized for a farm investigation (see IOM 5.1.2.5 Team Investigations). A lead
CSO should be identified from the Produce Safety Network (PSN) or the responsible HAF division
office that has attended both the FD226 Produce Inspections for Regulators Course, and the FD326
Produce and Sprout Investigations for Regulators Course. A minimum of three team members
should participate and ideally all members should have produce farm training and/or produce farm
inspection experience. The appropriate state regulatory agency having jurisdiction over produce
farms should be notified and invited to participate. Additional SMEs may be added to provide
needed expertise such as wildlife, soils, agricultural water, or epidemiology. CORE, CFSAN Produce
Safety Staff, and/or ORA HQ may assist with identifying appropriate SMEs and providing technical
guidance during the investigation.
The implicated grower should be notified in advance of the investigation as he/she or a
representative of the grower will need to be present to provide information to assist the
investigation. Generally, an FDA 482 will be issued to the grower or packing house, if different. If the
investigation expands to fields not owned by the grower, a new 482 must be issued to those
growers. Please see IOM 5.1.3.5 Team Investigations for additional information.
CORE has implemented formalized outbreak incident operation processes. The CORE operation
guides are available through the inside.FDA.gov website.
8.2.5.2 - Sampling
A variety of environmental samples may be collected during a farm investigation, including
environmental swabs and water from both the field and the packing house, and soil and wildlife scat
samples from the growing environment. Do not collect human fecal matter unless specifically
assigned or pre-approved to do so. In general, FDA laboratories are not prepared to receive human
feces.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-49
Instructions for collecting soil and water samples on farm investigations are found in IOM Ch. 4, in
the Salmonella Sample Schedule Chart 1, and are also covered in FD326 (Produce and Sprout
Investigations for Regulators Training Course). Additional sampling guidance can be found in ORA
Field Bulletin #30; Food Program Area Instructions for Environmental Sampling, and ORA Outbreak
Response Field Guide #1 covering E. coli, Listeria, and Salmonella inspections and investigations at
sprout operations. Specific sample collection instructions or methods may also be included in the
CORE farm investigation assignment.
All environmental samples are investigational. Use the product code builder to identify the proper
code for the type of environmental sample collected, including swabs, soil, water, and animal scat.
Do not use the product code of the implicated produce for environmental samples. Produce samples
collected from the field or prior to packing (i.e., not finished product) are labeled as investigational.
Product that has completed processing on the packing line are labeled official product samples.
8.2.5.3 - Form 3623 Farm Investigation Questionnaire
FDA Form 3623, the Farm Investigation Questionnaire (FIQ), must be completed for all farm
investigations, as covered in FD3263. Some portions may not be applicable, such as the use of
biosolids. These questions may be marked as N/A. However, questions for practices that may be
used but are not currently in use should be completed by use of interview techniques with the
grower to the extent possible. The FIQ should be completed on-site to ensure all information is
collected and submitted to CORE and/or the CFSAN Produce Safety Staff if requested and included in
the Investigation Memo or EIR as an attachment. To avoid duplication, the FIQ may be used to
provide information under the “Manufacturing Processes” section by either reference or cutting and
pasting into that section. A short summary and flow diagram(s) describing the steps from planting
through harvesting and/or packing should be included along with this.
8.2.5.4 - Reporting
Domestic outbreak work assignments will be designated in FACTS as either an operation 12
inspection (OP12) or an operation 13 investigation (OP13). Foreign outbreak work assignments will
be designated in FACTS as either an operation 11 inspection an operation 15 investigation.
For FACTS operation 11 or 12 farm inspections see Chapter 5 for reporting; however, if an outbreak
is ongoing and the information is needed immediately, it may be necessary to prepare a separate
memo to submit to CORE prior to completing the EIR.
For FACTS operation 13 or 15 investigations, follow reporting guidance in this chapter.
8.2.6 - Infant Formula and Baby Food
There is a continued sensitivity to all reported incidents involving infant formula and baby food. All
complaints involving either infant formula or baby food are to be thoroughly investigated on a high-
priority basis. This includes with the consumer, inspection of the manufacturer, and with the doctor or
hospital if appropriate. Samples should be collected as part of the follow-up. Complaints involving baby
food regulated by USDA should be referred to USDA for appropriate follow-up. See IOM 3.2.1.2. There

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-50
are two exceptions for collecting samples as part of the follow-up to infant formula/baby food
complaints. Do not collect samples unless direct for:
• Complaints involving outdated product in the marketplace with no associated injury or illness
only require investigation to ensure all outdated product has been removed from the identified
retail and/or wholesale source.
• Complaints involving an illness associated with normal appearing product when the follow-up
investigation discloses that the event does not appear to be product related or was an allergic
response to a properly labeled product per a physician's diagnosis.
8.2.7 - Tampering Involving Alcoholic Beverages
All tampering complaints involving alcoholic beverages should be entered as a consumer complaint in
FACTS. OEM/OEO and OCI should be notified immediately. OEM/OEO can be notified by e-mail at
emergency.operatio[email protected]v
and by phone 24 hours a day at 1 (866) 300-4374.
For all other complaints involving alcoholic beverages, please see IOM 3.2.8.1 for guidance.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-51
8.3- Drug Investigations
8.3.1 - Investigations Coordination
The following procedures should be followed for investigating suspected adverse drug reactions,
including drug-induced birth defects:
• If you are interviewing the consumer, conduct the normal complaint investigation and
gather all pertinent information regarding the product, patient, adverse event, etc. If the
consumer received medical treatment, obtain a medical records release (Exhibit 8-5).
Reporting of drug adverse experiences is voluntary and you should encourage and assist
complainants and health care providers to complete the MedWatch form (FDA 3500) (see
Exhibit 8-10) and submit to MedWatch. Report your findings in the FACTS Consumer
Complaint follow-up screens and in a memo of investigation.
• If you are investigating an adverse reaction at the manufacturer, conduct your investigation
to determine whether the adverse event was caused by a drug quality defect. Determine if
the manufacturer was aware of the complaint, has investigated, and per IOM 5.5.7 Adverse
Event Reporting has submitted the reportable event to FDA. For additional information
regarding DQRS (MedWatch Reports) and NDA FARS (New Drug Application Field Alert
Report) see the applicable compliance program in the Compliance Program Guidance
Manual (CPGM). Determine if the manufacturer is aware of any similar reported events.
Collect current labeling of the product to determine whether this was an expected or
unexpected adverse event. Your findings will be reported through the FACTS Consumer
Complaint follow-up screens and a memo of investigation or EIR.
• You may also be directed to conduct investigations at other establishments, such as
pharmacies, doctors’ offices, or distributors. Conduct your normal complaint investigation
determining each party’s role and involvement. If individuals interviewed are not required
to report adverse drug reactions, encourage and assist them to complete and submit the
FDA 3500 form to MedWatch.
In all cases of suspect drug-induced adverse reactions, the center will review the information on the FDA
3500 form and will issue assignments to the field if additional information is needed.
8.3.2 - Illness/Injury
Drug injuries or reactions, either human or veterinary, result from the use of products which may:
• Vary markedly from declared potency.
• Contain deleterious substances.
• Be mislabeled as to identity, warnings, or instructions.
• Have been mistaken for other drugs despite proper labeling.
• Have changed composition or become contaminated after shipment.
• Be dangerous when used according to directions.
• Have not been used in accordance with label directions or directions from the prescriber.
• Have been improperly administered or administered without the necessary precautions.
• Have been contaminated with objectionable microorganisms, soaps, or cleaning
solutions.
• Have been misidentified.
• Be labeled as sterile drugs but are found to be non-sterile.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-52
• Have adverse effects that were not identified prior to marketing.
8.3.2.1 - Reporting
8.3.2.1.1 - Reporting Forms – Drugs
Submit drug complaints and injuries to:
MedWatch
The FDA Medical Products Reporting Program (HFD-410)
Food and Drug Administration
5600 Fishers Lane
Rockville, MD 20857
Fax Number: 301-827-7241
8.3.2.1.2 - Reporting Forms – Veterinary Products
Submit veterinary injuries or adverse reaction reports to:
Food and Drug Administration
Center for Veterinary Medicine
Division of Surveillance (HFV-210)
7500 Standish Place
Rockville, MD 20857
In addition, follow specific reporting instructions as indicated per an assignment
8.3.3 - Complaints
The FDA Office of Emergency Management/Office of Emergency Operations (OEM/OEO) HFA-615, 301-
796-8240 must be notified immediately of all significant injury, illness, and suspected tampering
complaints. This may be accomplished via the checkbox indicated in the FACTS Consumer Complaint.
• Injury/illness complaints
o Any illness/injury related to infants should be considered significant. These complaints
are to be thoroughly investigated on a high-priority basis.
• Complaints and adverse reactions associated with veterinary products including animal drugs,
medicated feeds, and medical devices for animals are handled through the FDA CVM Division of
Veterinary Product Safety (HFV - 240). Veterinarians, animal owners, and drug manufacturers
may report problems to their local FDA district offices or directly to CVM. The division should
advise the complainant to complete an FDA 1932a, "Veterinary Drug Adverse Experience, Lack
of Effectiveness or Product Defect Report" for drug adverse events associated with unapproved
animal and approved human drugs and veterinary devices. For approved animal drugs, the
complainant should be instructed to call the manufacturer directly to report the event. Detailed
instructions and options for different case scenarios are available at
https://www.fda.gov/AnimalVeterinary/SafetyHealth/ReportaProblem/default.htm
.
For 3-day Field Alert Reports (FAR), drug sponsors now have the option to electronically submit 3-Day
field alert reports (FARs) directly to CVM. CVM will receive the electronic 3-Day FAR from the sponsors
and will automatically generate and email a .pdf of the FAR with associated attachments to the
appropriate district office. Some sponsors may still send the 3-day FAR through the traditional route to
the district office. The district office should email the form and any other attachments to CVM. The

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-53
drug manufacturer should notify and submit the FAR to their respective district office within three days.
The district offices will ask for additional information if necessary and submit the 3-day FAR to the
Division of Veterinary Product Safety.
Complaints and adverse reactions associated with animal feeds including pet food products are handled
through the Division of Compliance (HFV-230) at CVM. Veterinarians, animal owners, and firms may
report pet food problems to consumer complaint coordinators at their FDA district office or OEM/OEO;
the district will complete a FACTS Consumer Complaint Report. Pet food reports may also be made
directly to CVM using FDA’s Safety Reporting Portal. Instructions for stakeholders to report problems
associated with pet food products are available at
https://www.fda.gov/AnimalVeterinary/SafetyHealth/ReportaProblem/default.htm
. If you become
aware of human illnesses associated with CVM-regulated products, contact the appropriate ERC in your
division and/or regional office immediately who will then contact the CORE Signals Team at

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-54
8.4 - Device Investigations
8.4.1 - Injury/Illness
When investigating incidents implicating medical devices, you must first confirm whether the device was
a contributing factor. An appropriate follow-up, such as an inspection at the manufacturer may be
necessary. The cause of medical device injuries may originate with the manufacturer, operator, user, or
from other factors including, but not limited to, the transportation or installation of the device.
Additionally, an illness may occur as a result of device specifications not being met, such as a device
labeled as sterile not meeting sterility requirements.
Obtain the following information for medical devices:
1. A complete description of the incident (sequence of events) and the injury/illness, including:
a. Type, model, serial number and manufacturer of the device, and copies of any labeling
for the specific device(s) involved including instructions for use or operations manual(s).
b. Details of the alleged incident, including: number of people involved; symptoms, onset
time, duration, and outcome; date and time of occurrence; reports of other
investigating agencies and their conclusions, (e.g., fire marshal or OSHA reports); similar
incidents which may have resulted in injury/illness; and all operational SOPs, written or
unwritten.
2. Copies of medical records and/or laboratory records. Use an FDA 461, Authorization for
Medical Records Disclosure, IOM Exhibit 8-5, signed by the patient or other authorized
person, when obtaining these records.
3. Official cause of death, death certificate, and/or autopsy report, if indicated.
4. Determine if the device malfunctioned, and the cause.
5. The condition of the device at the time of use. Review its maintenance history, including
responsibility for maintenance (past and present), special service calls, repairs, whether
component warning or safety systems were functional, maintenance records, changes or
corrections accomplished just prior to or immediately after the incident, and who
performed the activity. An interview with biomedical engineering department personnel
may be indicated.
6. Who has access to the device? Determine if individuals using the device are familiar with its
operation.
7. The results of any examination or inspection of the device by the hospital or other party to
determine the cause of the incident.
8. Whether there are other devices of the same model number or lot number on the premises.
8.4.1.1 - Types of device injuries or illnesses include:
8.4.1.1.1 - Mechanical, Electrical, or Electromechanical Devices
Injuries caused by mechanical, electrical, or electromechanical devices may result from devices
that:
• Do not conform to specifications due to mistreatment (e.g., damage in transit), or
failure to comply with good manufacturing practices.
• Malfunction due to incorrect installation.
• Have not been used in accordance with labeled instructions.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-55
• Have been used/installed with incompatible accessories or parts which are not
compatible.
• Have been used under conditions which interfere with their ability to function (e.g.,
electromagnetic interference (EMI), fluid seepage into electrical circuits, etc.).
• Have been damaged during use, or random failures.
• Have not been adequately designed for intended use ( unstable, poor structural integrity,
electrical leakage, reusable but unable to thoroughly clean, etc.).
• Do not contain adequate directions or warnings.
• Are intended to be sterile but are non-sterile.
• Failor deteriorate for any reason.
8.4.1.1.2 - Devices for Implant
Causes of injuries which may result from implanted devices include those listed in IOM
8.4.1.1.1.. An injury or illness may also result because the materials used in the implant are not
biocompatible, thereby causing an adverse tissue reaction and/or deterioration of the implant.
It is important to obtain information relating to a medical professional’s interpretation of the
relations
8.4.1.1.3 - In-Vitro Diagnostic Devices
In Vitro Diagnostics (IVD) are instruments that can include, gas chromatographs and automated
blood analyzers, and much of the information under IOM 8.4.1.1.1 is applicable.
Injuries to patients from IVD products may be considered indirect, because they are due to
complications resulting from misdiagnosis or delays in patient treatment due to incorrect test
results. Examples of IVD failures include false positives, false negatives, and erratic results. Poor
performance or failure may be due to poor manufacturing practices or user error.
Manufacturing problems include:
• Process errors and mix-ups (varying fill in kit components, improper ingredient addition,
etc.).
• Labeling does not contain adequate directions or warnings or contains incorrect
information.
• Labeling mix-ups.
• Contamination making the product unusable or causing misdiagnosis.
• User error due to poor directions for use, operator’s manual, or inadequacies in labeling
requirements.
• Use of unclean, not maintained, or improperly calibrated equipment.
• Improper storage or use of reagents.
For In Vitro Diagnostic devices determine:
1. How the results of the test are used; screening, therapeutic drug monitoring,
epidemiological information, monitoring the course of a disease, etc.
2. The role in overall determination of patient clinical care.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-56
8.4.2 - Confidential Informants
FDA may receive external complaints that request their identity to be kept confidential or anonymous.
In this case, the complainant’s information should not be disclosed in the investigation memorandum or
EIR. The complaint should be assessed to determine if it is a non-injury/illness complaint or an
injury/illness complaint as this may impact the urgency of the response. An immediate follow-up may be
warranted if there is illness, injury, or if directed by higher authority. All information should be obtained
in the least intrusive, yet constructive, manner that allows the investigator to collect the evidence
required to evaluate the validity of the complaint to determine if additional action is warranted.
8.4.3 - Complaints
FDA may receive information from various sources, such as a consumer, whistleblower, employee, other
governmental agency, Congress, or competitor alleging a potential violation of the FD&C Act that must
be followed up to confirm the information provided by the complainant. For medical devices, a
complaint means any written, electronic, or oral communication that alleges deficiencies related to the
identity, quality, durability, reliability, safety, effectiveness, or performance of a device after it is
released for distribution.
As with all investigation types, report your findings in a memorandum and include all pertinent
information and any attachments collected as evidence to support the complaint. Using eNSpect, create
an operation 13, domestic investigation, or operation 15, foreign investigation, and complete all
required fields. Upload all labeled attachments and submit for endorsement by your supervisor. Ensure
the endorsement section includes verbiage to share the information with the divisional consumer
complaint coordinator for appropriate follow-up in FACTS and completion of any other required
documents for domestic complaints. If foreign, ensure the center is notified of the investigation and
receives a copy of the investigation memorandum and any attachments.
If the complaint is an adverse reaction to a device, advise the complainant to visit FDA.gov, specifically
the MedWatch Online Voluntary Reporting Form (fda.gov)
to complete an FDA 3500, Medwatch Form;
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm (See IOM Exhibit 8-7) and discuss the need
to have physician complete the form for submission. If the physician will not cooperate by completing
the FDA-3500, request the complainant to do it. Note in the "Remarks" section of the FACTS Consumer
Complaints Report that the FDA 3500 was forwarded to the complainant.
8.4.4 - Reporting
The Medical Device Reporting (MDR) regulation and the changes mandated by the Safe Medical Devices
Act of 1990 (SMDA) is a mandatory information reporting system. It requires manufacturers, importers,
and device user facilities to report to FDA certain adverse experiences caused or contributed to by their
devices.
This program is administered by the Center's MDR Policy Team in the Office of Regulatory Programs.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-57
The regulation requires a report be submitted to FDA whenever a manufacturer or an importer becomes
aware of information that its device: 1. May have caused or contributed to a death or serious injury, or
2. Has malfunctioned and this device or a similar device would be likely to cause or contribute to a death
or serious injury, if the malfunction were to occur.
Under the Safe Medical Devices Act of 1990, user facilities must report device-related deaths to FDA and
to the manufacturer, if known. User facilities must also report device-related serious illnesses and injuries
to the manufacturer, or to FDA if the manufacturer is unknown. In addition, SMDA also requires user
facilities to submit to FDA, on an annual basis, a summary of all reports submitted.
The CDRH Division of Industry and Consumer Education (DI[email protected].gov) and the MDR Team
) in the Office of Regulatory Programs should be contacted for further guidance
about the MDR regulation.
As of August 2018, the agency’s Voluntary Malfunction Summary Reporting program was implemented.
It permits certain manufacturers an alternative method to submit MDRs for eligible product codes in
summary form on a quarterly basis; see 83 FR 40973
.
8.4.5 – Medical Device Sampling
Obtain CDRH and WEAC concurrence prior to collecting any medical device samples.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-58
8.5 - Biologics Investigations
8.5.1 - Illness/Injury
Reactions or symptoms of illness may occur in association with the administration of vaccines and other
biological products. The Center for Biologics Evaluation and Research (CBER) is interested in all
unexpected clinical responses to a biological product, as well as any expected responses of unusual
frequency or severity. In some cases, a reaction or illness could occur because the product may:
• Vary from declared potency.
• Have been contaminated during manufacturing, shipment, or after shipment.
• Be mislabeled.
• Not have been given according to directions.
• Not have been stored under proper conditions.
• Have been provided to the wrong person.
• Contain substances innocuous to most people, but which the recipient is unable to tolerate
(e.g., anti-Kidd, anti-Duffy), or contains substances not usually present in such a product which
stimulate an adverse response in the recipient (e.g., HLA antibodies).
8.5.1.1 - Reporting
8.5.1.1.1 - Investigation/Reporting
When a biologics reaction/injury complaint is received by a CSO or consumer complaint
coordinator, they should forward the complaint to ORABIOBiolgicsInspectionsPO[email protected]
.
All complaints received by the ORA BIO Biologics Inspection POC will be reviewed and upon
determination of initial follow-up status sent to the district consumer complaint coordinator to
be recorded on the FACTS Consumer Complaint Report. When interviewing the complainant
about a biologics complaint /injury, obtain:
• Complete description of the complaint/injury.
• Onset and duration of the reaction/injury.
• Name of product administered, include date and time of administration.
• Manufacturer and lot number of product(s), if available.
At this point, it is generally unnecessary to conduct interviews beyond the complainant, or
obtain records, until a preliminary review has been conducted. It is important to rapidly
communicate the basic information about the incident, implicated product, lot, license
number, manufacturer, and presence of intact units to the ORA BIO Biologics POC email.
Confidential complaints received during an inspection should be captured in a memorandum as
an attachment to the EIR. The confidential informant information should not be referenced in
the EIR. Any findings related to complaints not involving confidential informants should be
documented in the narrative to the EIR. The complaint number for all complaints should be
written in the EIR coversheet in eNSpect. Complaint follow-up assignments will be issued in
eNSpect as determined by OBPO.
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-59
If a complaint related to a vaccine product involves an adverse reaction of any kind, then a
Form VAERS-1 (IOM Exhibit 8-6) should be completed online by complainant or their physician.
If they cannot complete the form online, the VAERS Reporting Form can be mailed to them and
they can send it to the address on the form. When you send a VAERS form to a complainant,
note this fact in the Remarks Section of the FACTS Consumer Complaint Report.
The Vaccine Adverse Event Reporting System (VAERS) is administered under a joint FDA/CDC
contract. For reporting adverse events which occur subsequent to vaccine administration, the
system utilizes a fillable online form (Form FDA VAERS 2.0) or can be directly submitted at:
https://vaers.hhs.gov/reportevent.html See IOM Exhibit 8-6.
8.5.1.1.2 - Professional Reporting System for Vaccine Adverse Reactions
The National Childhood Vaccine Injury Act of 1986, 42 USC 201, was passed to achieve optimal
prevention of childhood infectious diseases through immunization. At the same time, it was
intended to minimize the number and severity of adverse reactions to vaccines routinely
administered to children. This law requires health care providers and vaccine manufacturers to
report certain adverse events which occur following the administration of specific vaccines. The
vaccines and reportable events are listed in the National Childhood Vaccine Injury Act Vaccine
Injury Table. The Department of Health and Human Services (DHHS) has established a Vaccine
Adverse Events Reporting System (VAERS) to accept all reports of suspected adverse events
after the administration of any vaccine, in all age groups, including but not limited to those in
the table.
If the complaint does not involve an adverse reaction, obtain the necessary information to allow
the center to make an informed decision on follow-up at the manufacturer.
If the complaint is an adverse reaction to a biologics device, drug, or HCT/P product, an FDA
3500, MedWatch Form (See IOM Exhibit 8-7) must also be completed and forwarded to the
complainant for completion by their physician. If the physician will not cooperate by completing
the FDA-3500, request the complainant to do it. Assist the complainant in completing the FDA
3500, if necessary. Note in the "Remarks" section of the FACTS Consumer Complaints Report
that the FDA 3500 was forwarded to the complainant. MedWatch forms can be found at
https://www.fda.gov/safety/medical-product-safety-information/medwatch-forms-fda-safety-
reporting.
If the complaint does not involve an adverse reaction, obtain information necessary to permit
OBPO make an informed decision on follow-up at the manufacturer. If a complainant desires
further information, refer them to CBER, Office of Biostatistics and Epidemiology, Division of
Epidemiology, at 301-827-3974.
If a CSO finds that there is a complaint of a fatality where blood or a blood component is
implicated and that was not already reported to CBER, the CSO should notify their supervisor.
The supervisor will then follow-up with OBPO management and CBER. Reporting a fatality is
required of the collecting facility, in the event of a donor reaction, and by the facility which

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-60
performed the compatibility tests, in the event of a transfusion reaction. An investigation of the
incident shall be conducted by either Healthcare Finance Administration (HCFA) Centers for
Medicare and Medicaid Services (CMS) or FDA, based on the type of facility involved, for
example, transfusion service, blood bank, plasma center or hospital. OBPO CSOs may be
assigned to investigate a fatality through an assignment from CBER.
CSOs should follow OBPO’s procedure as a guide for conducting the investigation. The CSO
should also refer to the eNSpect assignment for additional information regarding the
investigation. If the hospital, medical examiner, or other entity either refuses to provide or
requires a written request in order to provide the CSO with medical history records, a death
certificate, autopsy report, or other needed records, the CSO should complete and provide the
firm with the Records Request Letter, that is referenced in OBPO’s procedure.
8.5.2 - Surveillance
OBPO CSOs should review OSAR Firm 360 to determine if an existing complaint exists in preparation for
conducting an inspection assignment. The CSO will review all firm information in OSAR Firm 360,
including reviewing all complaints and address all complaints that do not have entries under follow-up
disposition and follow-up disposition dates during the inspection assignment. CSO conducts the
establishment inspection and investigates those issues identified in the complaint(s) and includes
observations in the complaints and summary sections in the narrative of the EIR.
8.5.3 - Confidential Informants
In addition to this section, please refer to the general section on Confidential Informants (Section
8.1.7.2.3). Complaints can originate from public sources, including establishment employees at firm’s
we inspect, donors, donor family members, and industry. Confidential complaints can also come
through CBER and through other agencies. If the complaint is from a confidential informant, the
complaint is NOT documented in the EIR. Confidential Informant complaints are documented in an
Investigation Memo and saved as an attachment to the EIR. Findings are considered in the initial
classification of the inspection.
8.5.4 - Complaints
8.5.4.1 - BIOLOGICAL PRODUCTS
OBPO CSOs should follow the OBPO procedure on oversight of consumer complaints. If any ORA
office receives a complaint on a biological product, regardless of licensure status, the receiving office
will notify OBPO at ORABIOBiologicsInspec[email protected]s.gov
. OBPO will provide direction on how to
proceed, and next steps, including instructions on any FACTS entries. For additional information or
inquiries, send an email to the inspection POC address above or contact either of the OBPO division
directors. OBPO staff receiving a complaint from external or internal sources should send the
complaint to
ORABIOBiologicsInspection@fda.hhs.gov. Confidential complaints received during an
inspection should be captured in a memorandum as an attachment to the EIR. The confidential
informant information should not be referenced in the EIR.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-61
Any findings related to complaints not involving confidential informants should be documented in
the narrative to the EIR. The complaint number for all complaints should be written in the EIR
coversheet in eNSpect. Complaint follow-up assignments will be issued in eNSpect as determined by
OBPO.
8.5.4.2 -Biological Samples
Do not collect samples of a suspect product without first consulting with the supervisor. An
evaluation of the preliminary information on the injury/reaction by CBER (for licensed products)
and/or the home district division (for unlicensed products, plasma and blood products) may be
necessary to determine if a sample should be collected.
8.5.4.3 - BIOLOGICS INJURY/ADVERSE REACTION REPORTS
Submit biologics injury and adverse reaction narrative reports using encrypted email or mailing. If
mailing, use this address:
Food and Drug Administration
White Oak Bldg71
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
NOTE: In addition, check the “Notify EO/EOMPS?” box in FACTS for all injury and adverse reaction
complaints. For serious injury/illness reports, please notify the OEM/OEO immediately at 1 (866)
300-4374 and emergency.operatio[email protected].gov
.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-62
8.6 - Bioresearch Monitoring Investigations
8.6.1 - Illness/Injury
8.6.1.1 - Reporting
Submit drug complaints and injuries to:
MedWatch
The FDA Medical Products Reporting Program (HFD-410)
Food and Drug Administration
5600 Fishers Lane
Rockville, MD 20857
Fax Number: 1-800--322-0178
8.6.2 - Surveillance
For Cause assignments issued to OBIMO may require interviewing of subjects to verify their
participation in the clinical trial. These activities would be conducted with supervisor approval. An OP13
(or OP15 for foreign) will be created in eNSpect, for the purpose of subject interviewing, with
information correlating the OP12 (or OP11) For Cause assignment. An investigational memo will be
uploaded as an “Attachment” as per Section 8.1.9 General Investigation Reporting. Additionally, the
investigational memo will be included in the EIR as an “Attachment.”
8.6.3 - Complaints
Complaints are received via assignment memo from the respective center. The memo will have specifics
about the complaint and any special instructions. Reporting of complaints are the same as an inspection
via an EIR unless otherwise instructed (see section regarding For Cause/Fact Finding/Information
Gathering above). See IOM 5.10.2 – BIMO Assignments as complaint information will be included in the
overarching assignment memo.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-63
8.7 - Tobacco Investigations
8.7.1 - Investigations Coordination
Tobacco Products Samples: When collecting tobacco product samples as a result of a product complaint
or adverse report investigation, see IOM 4.5.5.3.8, for sample collection guidance and contact CTP’s
Office of Compliance and Enforcement. (extract from IOM 8.4.7.6)
8.7.2 - Complaints
Consumers who experience a problem with a tobacco product, such as undesired health or quality
problems, may report it online via the FDA Safety Reporting Portal (SRP) at
www.safetyreporting.hhs.gov.
Potential tobacco product violations
include (but are not limited to):
• Sales to minors.
• Flavored cigarette sales.
• Illegal marketing and advertising – The Tobacco Control Act gives the FDA the ability to regulate
certain marketing and advertising activities by the tobacco industry, including
describing tobacco products as “light,” “mild,” or “low” – or claiming a product is safer or less
harmful without an FDA order.
• Distributing t-shirts or other promotional or novelty items with brand names of cigarette or
smokeless tobacco products.
• Sponsoring events using the brand name of a tobacco product.
• Distribution of free samples of tobacco products except in limited circumstances.
• Placement of cigarette or smokeless tobacco product vending machines in prohibited areas (or
providing access to self-service or direct access of tobacco products in prohibited areas).
• Sale of cigarettes in packages of less than 20.
If you see what you believe to be a violation of the Tobacco Control Act or other related regulations, you
can:
• Submit online (https://www.accessdata.fda.gov/scripts/ptvr/index.cfm
)
• Call the Tobacco Call Center using CTP's toll-free number: 1.877.CTP.1373
• Send an email: CTPCompliance@FDA.hhs.gov
• Print and mail:
Paper form
(Form FDA 3779, Potential Tobacco Product Violations Report)
(https://www.accessdata.fda.gov/scripts/ptvr/index.cfm) to:
Potential Tobacco Products Violation Report
Food and Drug Administration
Center for Tobacco Products
Office of Compliance and Enforcement
Document Control Center
Building 71, Room G335
10903 New Hampshire Avenue
Silver Spring, MD 20993

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-64
EXHIBITS
8-1 FEDERAL ANTI-TAMPERING ACT FULL LANGUAGE

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-65
8-2 LETTER TO HEALTHCARE PROVIDER FOR MEDICAL RECORDS
To access the word document, click here. Note: Link to the Letter to Healthcare Provider for Medical
Records is only available to ORA users on the FDA intranet. The link is
http://qmis.fda.gov:80/mc/main/index.cfm?event=showFile&ID=OMIJZHPT3ZE7FJRCSJ&static=
false&mc
uid=ANONYMOUS&mcsid=FPSFER5OBBABVCT55K. Users who need a copy of the template outside FDA
should use the Freedom of Information Process described in Section 8.1.3 to get a copy of the template.

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-66
8-3 INVESTIGATION MEMO
To access the word document, click here. Note: Link to the Investigation Memo is only available to
ORA users on the FDA intranet. The link is
http://qmis.fda.gov:80/mc/main
/index.cfm?event=showFile&ID=C7ZQFEQOANEQ5JXMLX&static=false&
mcuid=ANONYMOUS&mcsid=AQZ6DL5KXFHKBPWISH. Users who need a copy of the SOP outside FDA
should use the Freedom of Information Process described in Section 8.1.3 to get a copy of the SOP.

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-67
8-4 TABLE OF INCIDENT COORDINATION
Incident Type
Coordinating Body
Points of Contact (POCs)
Clusters and outbreaks of 2+
human illnesses
CFSAN / CORE
(Coordinated Outbreak
Response and Evaluation
Network)
CORE Signals Team,
Single human illness (this
includes single case
retrospective incidents but
also individual consumer
complaints)
CFSAN / OC
(Office of Compliance)
CFSANOCSRT@fda.hhs.gov
Clusters and outbreaks of
human illness due to pet
food/feed products
CVM
(Center for Veterinary
Medicine)
David.Rotstein@fda.hhs.gov,
Mark.Glove[email protected]v
Allergen issues (any and all)
CFSAN / OC
(Office of Compliance)
Seafood toxin incidents*
(All toxins; All domestic and
international waters)
CFSAN / OFS / DSS
(Division of Shellfish Safety
and DSST, Division of Seafood
Science and Technology)
Ronald.Benner@fda.hhs.gov
Jonathan.Deeds@fda.hhs.gov
Molluscan shellfish outbreaks
(single and multiple human
illnesses)
CFSAN / OFS / DSS
(Division of Shellfish Safety
and DSST, Division of Seafood
Science and Technology)
[email protected] (goes
by Lizzie; OFS / DSS)
Melissa.Abbott@fda.hhs.gov (OFS /
DSS)
Jessica.Jones@fda.hhs.gov (OFS /
DSST)
Processed shellfish outbreaks
(e.g., non-molluscan shellfish
(crustaceans such as lobster,
crab, crab meat, crawfish,
shrimp, and processed
molluscan shellfish))
CFSAN / CORE
(Coordinated Outbreak
Response and Evaluation
Network)
CORE Signals Team,
Kratom-related / CBD /
psychoactive substance
incidents
OC / OO / OSEM / OEM / OEO
(Office of Emergency
Operations)
FDA Emergency Operations list:
emergency.operatio[email protected]v
Hepatitis A positive samples
(and subsequent coordination
with CDC for PEP); no known
associated HAV illnesses
FDA Liaison to CDC
FDA Liaison to CDC
(Susan.Lance@fda.hhs.gov)

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-68
Infant illnesses* (Salmonella,
Cronobacter, infant botulism
with rule-out investigations for
infant formula or related
infant products such as gripe
water or medicated foods)
CFSAN / OC
(Office of Compliance) -
Powdered Infant Formula (PIF)
CFSAN / ONFL
(Office of Nutrition and Food
Labeling)
NCCC
National Consumer Complaint
Coordinator
OC contact for PIF is
ONFL contact for infant formula are
Andrea.Lotze@fda.hhs.gov
and
NCCC in OEO is
Joan.Trankle@fda.hhs.gov
Disasters (Natural and
Manmade)
OC / OO / OSEM / OEM / OEO
(Office of Emergency
Operations)
FDA Emergency Operations list:
emergency.operatio[email protected]v
Food Defense incidents
(Intentional Contamination)
OC / OO / OSEM / OEM / OEO
(Office of Emergency
Operations)
And
CFSAN / OAO
(Office of Analytics and
Outreach)
CFSAN/OAO/Food Defense and
Emergency Coordination Staff
contact is
Leeanne.jackson@fda.hhs.gov
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-69
8-5 CIFOR OUE Agent List
Agent Name
Median Incubation
Period (Range)
1
Primary Signs and
Symptoms
Primary
Specimen(s)
KEI
§
-Special
group(s)
KEI-Geographic
C
onsiderations
KEI-Notable
Exposures
BACTERIAL
Arcobacter butzleri
32
hrs (6-83 hrs)
2
D (persistent and watery),
abdominal
cramps, N, V
Stool in Cary-Blair,
raw stool
Bacillus anthracis
Usually ≤1
week (Up
to
60
days)
Severe abdominal pain, N, V,
fever, D (may be bloody),
ascites, sepsis,
meningitis
Blood, stool in Cary-
Blair, raw stool
Recent travel to
endemic
areas,
tropical
or sub-
temperate
regions
Undercooked
meat
or hides of
herbivores
Bacillus cereus, diarrheal
toxin
10-16 hours (6-24
hours)
Abdominal cramps, D
(watery), N
Stool in Cary-Blair,
raw stool
Time and/or
temperature
-
abused foods
Bacillus cereus, pre-formed
toxin
30 min- 6 hours
Sudden onset of severe N, V,
D
Stool in Cary Blair
Time and/or
temperature
-
abused foods
Brucella spp.
3
-4 weeks (1 week to
several
months)
Flu-like symptoms including
fever, chills, sweating, HA,
joint pain, weakness; may
cause recurrent fevers and
chronic joint pain/fatigue;
may cause diarrhea and
bloody
stools in acute phase
Blood, serum
Animal
handlers,
especially
farm
workers
and
veterinarians
Ingestion of
raw
milk and
dairy
products
Campylobacter spp.
2-5 days (1-10 days)
D (may be bloody),
abdominal
cramps, Fever,
possible N & V, Guillain-
Barre Syndrome
3
Stool in Cary Blair,
raw stool
Undercooked or raw
meat
or poultry;
raw
milk/ milk-
products
Clostridium botulinum,
foodborne
£
12
-72 hours (6 hours-
10
days)
V, D, blurred vision, diplopia,
dysphagia, "bilateral"
descending
muscle
weakness, cranial nerve
palsies (e.g., blurred vision,
diplopia, dysphagia)
Raw stool,
vomitus
or
serum
(specimens
collected prior to
anti-
toxin
administration)
Improperly
processed
and
canned
foods in
airtight
containers/packagin
g
Clostridium
botulinum,
infantile
£
3
- 30 days
Lethargy, weakness, poor
feeding, constipation,
hypotonia, poor head
control, poor gag reflex and
sucking reflex
Raw stool, serum
Infants
Honey; home
canned
vegetables,
fruits; corn syrup
Clostridium perfringens
8
-16 hours (6-24
hours)
D (watery), abdominal
cramps, N;
fever is rare
Stool in Cary-
Blair, raw stool
Time and / or
temperature
abused
foods
Cronobacter sakazakii
Less
than 28 days
Bacteremia, meningitis,
necrotizing enterocolitis
Blood, stool in
Cary- Blair, raw
stool
Premature
infants
Infant formula
Coxiella burnetii (Acute Q
fever)
2-3 weeks (3-39 days)
Fever, HA, fatigue, malaise,
cough,
anorexia, N, V, D,
abdominal pain, pneumonia
Blood with EDTA/
serum, tissue
Pregnant
women,
immunosuppr
essed,
and
patients
with a
pre
-existing
heart
valve
defects
Consumption of raw
cow
or
goat milk;
contact with
cows
or
goats
Enterohemorrhagic E.
coli
(EHEC)
(including Shiga-toxin
producing
E.
coli (STEC) and
Verotoxin
producing E. coli
(VTEC))
3-4 days (1-10 days)
D (often bloody), abdominal
cramps, V, hemolytic-
uremic syndrome (HUS)
Stool in Cary-
Blair, raw stool
Young children
Consumption of raw
milk;
contact with
cattle/ruminants;
undercooked
ground
beef; leafy
greens
Enterotoxigenic E. coli
(ETEC)
24-72 hours (10
hours
- 6
days)
D (profuse watery),
abdominal
cramps, V
Stool in Cary-
Blair, raw stool
Foreign travel
especially
to
Contaminated water
and
food
sources
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-70
developing
countries
Enteroinvasive E. coli (EIEC)
As short as 10-18 hrs
D (watery), fever, abdominal
cramps, dysentery (in rare
cases)- scant
stools w/
evidence of blood, mucous
or leukocytes in stool
Stool in Cary-
Blair, raw stool
Foreign travel
especially
to
developing
countries
Enteropathogenic
E. coli
(EPEC)
As
short as 9-12 hrs
D (watery with mucous),
fever, V
Stool in Cary-
Blair, raw stool
Children < 2
years
of
age
Enteroaggregative
E. coli
(EAEC)
Estimated
at 20-48
hrs
Chronic or acute D (watery),
V
Stool in Cary-
Blair, raw stool
Diffuse-Adherence E. coli
(DAEC)
D
Stool in Cary-
Blair, raw stool
Young children
Leptospira interrogans
5-14 days (2-30 days)
Anicteric disease (no liver
involvement)- Abrupt onset
of fever, HA, abdominal
pain, N, V, severe
myalgia,
malaise, conjuctival
petechiae and/or
hemorrhage
Icteric disease
(liver involvement)-
Jaundice, upper right
quadrant
pain, N, V,
decreased urine output,
edema, hemorrhage,
vascular
collapse, severe
altered mental
status (AMS)
Blood, CSF, Urine
Farmers,
veterinarians,
slaughterhous
e
and
sewer
workers
Water activities
(swimming,
kayaking)
Listeria monocytogenes
1 day- 3 weeks (3-70
days)
Invasive disease-
Severe HA, N, V, stiff
neck, confusion, and
other neurological
symptoms consistent
with meningitis, sepsis,
bacteremia,
premature
birth or stillbirth
Gastrointestinal disease-
Fever, D,
myalgia
Blood, CSF, Stool
in Cary-Blair
Pregnant
women
€
,
immunosuppr
essed
¥
,
elderly
¥
Raw milk/dairy; soft
cheeses;
deli or RTE
meats,
raw produce
Mycobacterium bovis
Undetermined
Gastrointe
stinal
disease-
Abdominal
pain, D
Lung disease- Fever, weight
loss,
night sweats, cough
Stool in Cary-
Blair, sputum
Foreign born,
immigrants,
immunocompr
omised,
dairy
workers
Raw milk/milk
products;
contact
with cattle, bison,
elk and
deer
Salmonella spp. (non-typhi)
12
-36 hours (6- 72
hours)
D (can be bloody) fever,
abdominal
pain, N, V
Stool in Cary-
Blair, raw stool
Salmonella Typhi/
Paratyphi
Typhi- 7-14 days (3-
60+
days)
Paratyphi- 1-10 days
Fever, HA, malaise, chills,
myalgia, weight loss,
constipation or D,
bacteremia, rash, cough
Stool in Cary-
Blair, raw stool
Recent travel to
endemic areas;
Africa,
Southeast
Asia
Contaminated water
and
food
sources
Shigella
spp.
24
-72 hours (1-7
days)
D (stools can have blood and
mucus), abdominal cramps,
fever, V, tenesmus
Stool in Cary-
Blair, raw stool
Young children
Usually
person to
person,
water or raw milk
Staphylococcus aureus
(preformed
toxin)
1-6 hrs (30 minutes-
8hrs)
Severe N, V, abdominal
cramps, prostration, D, drop
in blood
pressure
Stool in Cary-
Blair, raw stool
Foods handled with
bare
hands
especially
those
without
further
cooking or
inadequate
heating/
refrigeration,
time
and
/ or
temperature
abused
foods
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-71
Streptococcus, Group A
1-5 days
Sore throat (pharyngitis,
tonsillitis), fever, malaise,
rash, cellulitis
Throat swab
Milk/ raw milk,
eggs,
raw
produce
Vibrio parahaemolyticus
12-24 hours (2-96
hours)
D (watery), N, V, abdominal
cramps, HA, fever, chills;
Wound infections are
possible
Stool in Cary-
Blair, blood,
wound culture
Immunocompr
omised,
pre-
existing
liver
conditions
Coastal,
brackish
waters,
estuaries
Raw or
undercooked
seafood
(oysters,
clams,
squid,
mackerel,
tuna,
sardines,
crab,
shrimp)
Vibrio vulnificus
24-72 hours (1-7
days)
V, D, abdominal pain, wound
infections, bacteremia,
shock
Stool in Cary-
Blair, blood,
wound culture
Immuocompro
mised,
pre-
existing
liver
conditions
Coastal,
brackish
waters,
estuaries
Raw or under-
cooked
seafood
(oysters,
clams,
squid,
mackerel,
tuna,
sardines,
crab,
shrimp),
contaminated
water, open wounds.
Vibrio cholerae, toxigenic
24-72 hours (few
hours
to
5
days)
D (profuse watery),
abdominal
cramps, N, V, dehydration,
shock
Stool in Cary-
Blair, rectal swab
Immunocompr
omised,
esp.
pre
-existing
liver
conditions
Coastal,
brackish
waters,
estuaries
esp.
P
acific
Northwest
Seafood, raw or
under
-cooked
oysters,
contaminated
water
Recent
travel to
endemic
areas
Yersinia enterocolitica
3- 7 days (1-14 days)
Fever, abdominal pain, D, V
Stool in Cary-Blair,
raw stool; blood
Children and
elderly
more
susceptible
Undercooked pork
products,
raw milk
Yersinia pseudotuberculosis
3- 7 days (1-14 days)
Fever, abdominal pain, D, V,
(can have scarlatiniform
rash)
Stool in Cary-Blair,
raw stool; blood
Males
FUNGAL
Cryptococcus
2
to 14 months (C.
gattii
)
D, abdominal cramps
CSF, serum
Immunocompr
omised
P
acific
Northwest,
Australia, Africa
Inhalation
PARASITIC
Angiostrongylus
cantonensis
or
A. costaricensis
1
-3 weeks (1 day- 6
weeks
-
cantonensis);
weeks-
1
year
(costaricensis)
Severe HA, N, V, stiff neck,
and other neurological
symptoms consistent
with
meningitis (A. canontensis);
Abdominal pain, fever, N, V
(A. costaricensis)
CSF, blood, serum
Texas,
Pacific
Basin,
SE Asia
(A.
cantonensis);
Latin
America,
Caribbean
(A.
costaricencis)
Raw/undercooked
snails,
slugs;
chopped
vegetables
contaminated
with
infected
snails or
slugs
Cryptosporidium
7
days (1-14 days) D (severe watery; may be
recurrent),
abdominal cramps, N, fever
Stool (2-3
samples
collected over
several
days)
Recreational
water,
drinking
water,
unpasteurized
milk,
contact
with cattle,
children
in daycare
settings
(fecal-oral
transmission)
Cyclospora
cayetanensis
7
days (1-14 days)
D (watery), weight loss,
anorexia, abdominal
cramps, N, V and fatigue;
fever rare
Stool, intestinal
fluid,
tissue biopsy
More
common
in trop
ical and
subtropical
countries,
but
occurs
in
other
areas due
to
contaminated
imported
produce
Fresh
fruit and
vegetables
(e.g.
berries,
basil, snow
peas,
lettuce),
contaminated
water
Entamoeba
histolytica
1
-4 weeks (from a few
days
to
several months or
years)
Fever, chills, lower
abdominal pain, D, bloody
D (amoebic dysentery), liver
(or other organ) abscess
Stool (2-3
samples
over
several days),
blood if
Invasive
amoebiasis
more
common
in
young
adults, liver
Tropical
countries
with
poor
sanitation
(South
and
Central
Human
reservoir,
fecally
contaminated
food
or
water; person-to-
person less common
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-72
disseminated
abscess more
common
in
males,
dysentery
rare
before
age 5
America, Africa,
and
Asia)
Giardia lamblia
1
-3 weeks (3 days- 3
weeks)
D, abdominal cramps,
greasy stools,
gas
Stool (2-3
samples
collected over
several
days)
Drinking
water,
recreational
water,
children
in daycare
settings
(fecal-oral
transmission);
occasional
food
contamination
Toxoplasma
gondii
7
days (4-23 days)
Cervical lymphadenopathy,
flu-like illness; if
immunocompromised,
central nervous system
(CNS) disease, myocarditis,
or pneumonitis
can occur
Serum
Raw
beef
Trichinella
spiralis
GI
symptoms- 1-2
days;
5
days- 8 weeks for
other
symptoms
Muscle soreness
accompanied
by fever
and edema of eyelids
are
characteristic;
eosinophilia, N, V,
chills, D, abdominal cramps,
fatigue
and weakness possible
Serum; biopsy of
tissue
Consumption
of raw
or
undercooked
meat
(particularly
bear,
pork, wild
feline,
fox, dog, wolf,
moose,
horse, seal
or
walrus)
VIRAL
Adenovirus
1
-10 days
D (prolonged), N, V, HA,
fever, malaise, abdominal
pain; Types 40 and 41 can
cause GI outbreaks
Stool in Cary-
Blair, raw stool,
serum,
naso-
pharyngeal
swab,
Children
Astrovirus
1-4 days
D (watery), N, V, fever,
malaise,
abdominal pain,
HA, anorexia
Stool in Cary-
Blair, raw stool,
serum
Children and
immunocompr
omised
Child care facilities,
long
-term
care
facilities
Hepatitis
A
28
days (15-50 days)
Jaundice, dark urine,
fatigue, anorexia, N, D,
fever, HA, abdominal
pain,
weight loss
Stool in Cary-
Blair, raw stool,
Serum
Men
who have
sex
with men,
injection
drug
users,
international
adoptees
Foreign
travel
Water
contaminated
with
infectious
human
waste;
raw,
under-
cooked
mollusks
harvested
from
contaminated
waters
Hepatitis
E
26
-42 days (15- 64
days)
Jaundice, dark urine, D,
fever, abdominal pain,
arthralgia,
rash,
hepatomegaly, altered
consciousness
Stool in Cary-
Blair, raw stool,
Serum
Foreign
travel,
especially
Asia,
Middle
East,
Africa
and
Central
America;
exposure
to pigs
Contaminated
drinking
water;
oysters,
mussels
and
other
shellfish;
pork,
pig liver;
and
raw/rare
deer
and boar
Norovirus
12-48 hours (10- 50
hours)
N, V, D, abdominal cramps,
fever
(low grade), HA,
myalgia, malaise
Stool in Cary-
Blair, raw stool
Institutionalize
d
populations
Parvovirus (Human
Bocavirus,
HBoV 2-4)
Unknown- emerging
pathogen
D, V, fever, abdominal pain,
coryza, cough
Stool in Cary-
Blair, raw stool,
serum, CSF
Children
Rotavirus
1
-3 days
D (watery), V, fever (low
grade),
abdominal pain
Stool in Cary-
Blair, raw stool
Children
Saffold virus (SAFV)
Unknown-emerging
pathogen
D, V, respiratory symptoms
(children); if invasive, then
Stool in Cary-
Blair, raw stool,
Children
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-73
meningitis, encephalitis,
myelitis,
myocarditis,
enanthema,
exanthema,
septicemia
naso-
pharyngeal
swab, CSF
Sapovirus
12
-48 hours
N, V, D, abdominal pain,
fever, HA, myalgia
Stool in Cary-
Blair, raw stool
Infants,
young
children
and
institutionalize
d
populations
(esp.
long-
term
care
facilities)
OTHER
Brainerd D agent
Unknown
D (Profuse, watery,
prolonged 2-36 months)
Stool in Cary-
Blair, raw stool
Toxins
Azaspiracid Poisoning (AZP)
12-24 hours
N, V, D, abdominal cramps
Shellfish, toxin
detection
Europe
Mussels,
oysters
Carchatoxin
< 1-6 hours
N, V, D, and paresthesias
Food
Madagascar
Shark,
particularly
the liver
Ciguatera toxin
GI symptoms- 1-6
hours
(few minutes-
48 hours)
Neurologic
symptoms
- few
minutes
- 48 hours
N, V, D, abdominal cramps,
sweating, HA, muscle aches,
paresthesia of lips, tongue,
face
or extremities and
temperature
sensation
reversal (hot/cold
sensation
flip)
Fish for
purification/
extraction and
mouse
bioassay
Tropical areas
Predatory
fish
like
barracuda,
grouper,
sea
bass,
snapper,
mullet
Scombroid
Few minutes- 3 hours
Rash, D, flushing, sweating,
HA, V, burning/tingling
sensation in mouth,
swelling in mouth,
abdominal pain and
metallic taste
Fish, histamine
testing
Fish such as
tuna
and
mackerel;
(bacterial
action in)
Swiss cheese
Tetrodotoxin
< 30
minutes
Paresthesia of lips,
tongue, face
or
extremities often
following
numbness; floating
sensation,
V, D, abdominal pain,
ascending
paralysis,
respiratory failure
Puffer fish, toxin
testing
Puffer
fish
consumption
Mushroom toxin (short-
acting)
Few minutes- 2 hours
V, D, confusion, vision
problems,
salivation,
diaphoresis,
hallucinations
Mushrooms,
toxin detection
Mushroom
consumption
Mushroom toxin (long-
acting)
4-24 hours
D, abdominal cramps, liver
and
kidney failure
Mushrooms,
toxin detection
Mushroom
consumption
Shellfish toxin (diarrheic)
30 minutes- 2 hours
N, V, D, abdominal pain,
chills, HA, fever
Shellfish, toxin
detection
Mussels,
oysters,
scallops
from
Gulf
of Mexico,
FL
Shellfish toxin (neurotoxic)
Few minutes- 3 hours
Tingling and numbness of
lips, tongue and throat;
muscle aches, dizziness and
reversal of hot/cold
sensation, D, V
Shellfish, toxin
detection
Mussels,
oysters,
scallops
from
Gulf
of Mexico,
FL
Shellfish toxin (amnesic)
< 24 - 48 hours
V, D, abdominal pain and
neurologic
symptoms of
confusion, memory loss,
disorientation, seizure or
coma
Shellfish, toxin
detection
Mussels,
oysters,
scallops
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-74
Shellfish toxin (paralytic
poisoning)
30 minutes- 3 hours
(15
minutes
- 10 hours)
N, V, D, paresthesia of
mouth
and lips, weakness,
dysphasia, dysphoria,
respiratory paralysis
Shellfish or water,
toxin detection
Scallops, mussels,
clams,
cockles
Chemicals
Antimony
<1
hour (5 mins- 8
hours)
V, D, abdominal pain,
metallic taste
Food or beverage
Metallic
container
Arsenic
Few
hours
N, V, D, pins and needles
sensation,
colic
Urine analysis
Cadmium
<1 hour (5 mins
- 8
hours)
N, V, D, myalgia, increased
salivation, abdominal pain;
often a metallic taste
Food
Seafood,
oysters,
clams,
lobsters,
grains and peanuts
Chlorinated
hydrocarbon
insecticides
(aldrin,
chlordane,
DDT, endrin,
lindane,
toxaphene)
30
minutes- 6 hours
N, V, paresthesia, dizziness,
muscular weakness,
anorexia, weight loss,
confusion
Blood, urine, stools,
gastric washings
Storing
insecticides
in
same
areas as
foods;
mistaking
pesticides
for
powdered foods
Copper
<1 hour (5 mins- 8
hours)
N, V (blue or green), D;
often a
metallic taste
Food or beverage
Metallic containers
Mercury
<1 week
N, V, D, numbness, skin
rash, eye irritation,
weakness of legs, spastic
paralysis, impaired vision,
blindness,
coma
Blood, hair
Fish; grains treated
with
mercury
containing
fungicides
Monosodium
glutamate
(MSG)
Few
minutes to 1
hour
Tingling, flushing, dizziness,
HA, N, burning sensation in
back of neck, forearms;
feeling of tightness in chest
N/A
Foods
seasoned
with
MSG
Nicotinic acid/Niacin
Few minutes to 1
hour
Flushing, sensation of
warmth,
itching, abdominal
pain, puffiness of face and
knees
N/A
Meats or other
foods
with sodium
nicotinate
as color
preservative;
high
doses of
dietary
supplements
Nitrite poisoning
1-2 hours
N, V, cyanosis/blue skin, HA,
dizziness, weakness, fatigue,
loss of consciousness,
chocolate-brown colored
blood
Blood, food
Cured meats and
spinach
Organophosphates
or
carbamate
pesticides
(Diazinon,
Malathion,
Parathion,
TEPP; Carbaryl,
Sevin®,
Lannate®,
Aprocarb®)
Few
minutes to few
hours
N, V, abdominal pain, HA,
nervousness, blurred vision,
twitching, convulsions
Blood, food
Spraying
foods just
before
harvesting;
storing
insecticides
in
same areas as
foods;
mistaking
pesticides
for
powdered foods
Sodium
fluoride
Few
minutes to 2
hours
Irritation of skin, eyes, and
respiratory tract, salty or
soapy taste
in mouth,
numbness of mouth, V, D,
dilated pupils, spasms,
pallor, shock, collapse
Vomitus, gastric
washes and food
Dry
goods
(powdered
milk,
flour
, baking
powder,
cake mix),
insecticides
and
rodenticides
Thallium
Few
hours
V, D, hair loss, neurologic
manifestations (paresthesia,
respiratory depression,
bronchospasms, cranial
nerve
palsies)
Urine, hair
Centers
for
Disease
Control
and
Prevention.
Thallium
Poisoning
from
Eating
Contaminated
Cake
-- Iraq, 2008.
MMWR.
September 19,
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-75
2008 / 57(37);1015-
1018.
Tin
Few hours
N, V, D; often a metallic
taste
Food
Metallic container
Triorthocresyl
phosphate
10
days (5-21 days)
N, V, D, leg pain, ungainly
high stepping gait, food and
wrist drop
N/A
Using
compound to
extract
foods or as
cooking or salad oil
Zinc
Few hours
Stomach cramps, N, V, D,
myalgias;
often a metallic taste
Blood, stool, saliva,
urine and food
Metallic container
Notes:
1
Unless otherwise noted, the median incubation period and range were obtained from the following three sources: Heymann, D.L.
(Ed.)(2008). Control of Communicable Diseases Manual (19th ed.). Washington, DC: American Public Health Association; Centers for
Disease Control and Prevention (CDC) (March 26, 2014) A-Z Index for Foodborne Illness. Retrieved from
http://www.cdc.gov/foodsafety/diseases/index.html.
2
Victoria Lappi, John R. Archer, Elizabeth Cebelinski, Fe Leano, John M. Besser, Rachel F. Klos, Carlota Medus, Kirk E. Smith, Collette
Fitzgerald, and Jeffrey P. Davis. Foodborne Pathogens and Disease. March 2013, 10(3): 250-255. doi:10.1089/fpd.2012.1307.
3
B.R. Jackson, J. Alomia Zegarra, H. Lopez-Gatell, J. Sejvar, F. Arzate, S. Waterman, A. Sanchez Nunez, B. Lopez, J. Weiss, R. Quintero Cruz, D.
Y. Lopez Murrieta, R. Luna-Gierke, K. Heiman, A. R. Vieira, C. Fitzgerald, P. Kwan,
M. Zarate-Bermudez, D. Talkington, V. R. Hill and B. Mahon (2014). Binational outbreak of Guillain–Barré syndrome associated with
Campylobacter jejuni infection, Mexico and USA, 2011 . Epidemiology and Infection, 142, pp 1089-1099. doi:10.1017/S0950268813001908.
§- Key epidemiological information
£- Clinical consultation and testing recommendations (including lab collection recommendations) can be obtained through
consultation with CDC.
€- Pregnant women may be more likely to present with mild, flu-like symptoms.
¥- Elderly or immunocompromised may be more likely to present with sepsis or meningitis.
N- nausea, D- diarrhea, V-vomiting, HA- headache

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-76
8-6 – VAERS Form
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-77

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-78

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-79
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-81

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-82

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-83
8-8 Potential Tobacco Product Violations Form

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-84

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-85
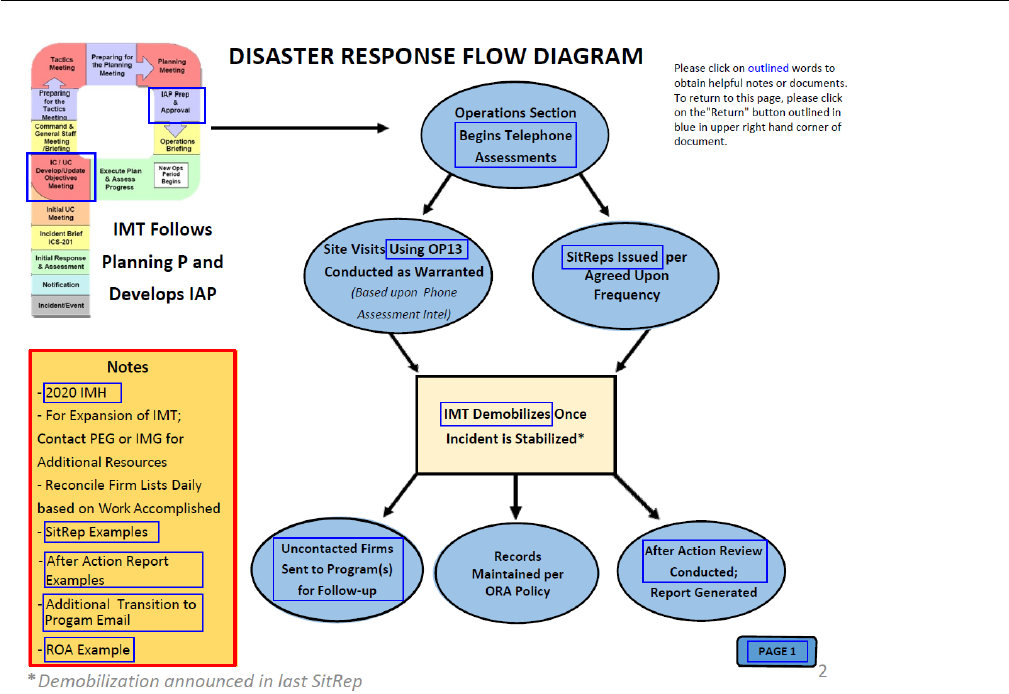
INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-87
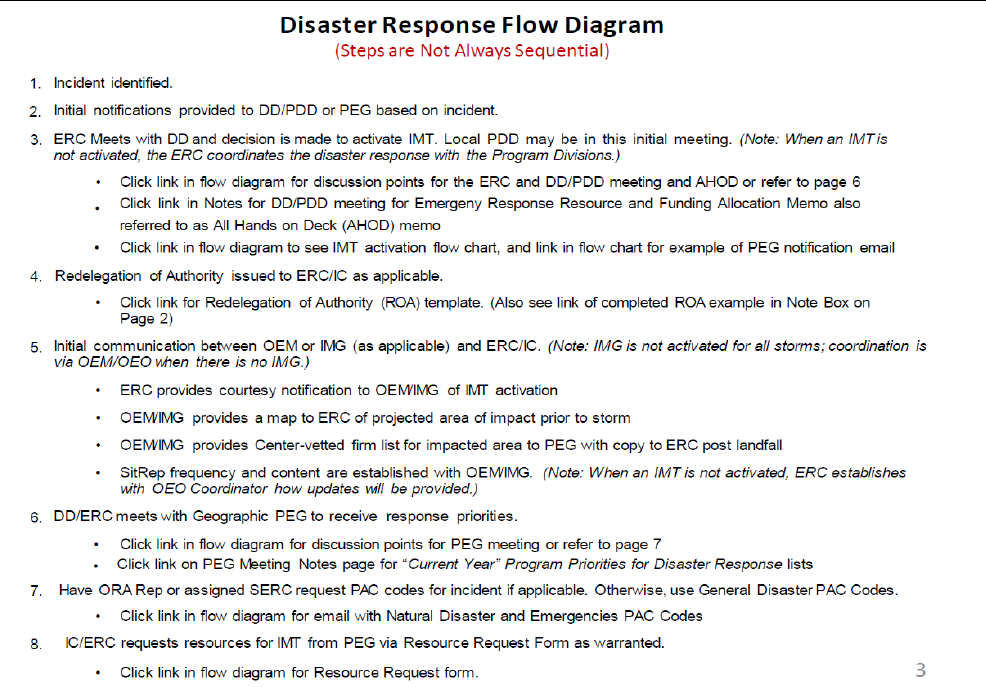
CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-88

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-89

CHAPTER 8 INVESTIGATIONS OPERATIONS MANUAL 2022
8-90

INVESTIGATIONS OPERATIONS MANUAL 2022 CHAPTER 8
8-91
INDEX
A
Adverse……………. . 8-5, 8-31, 8-40, 8-41, 8-42, 8-43, 8-44, 8-
45, 8-49, 8-50, 8-51, 8-56, 8-58
Alcohol .......................................................................... 8-48
B
Baby Food .............................................. 8-7, 8-8, 8-44, 8-48
C
CBER ................................................... 8-55, 8-56, 8-57, 8-58
CFSAN …………..…….8-27, 8-31, 8-32, 8-33, 8-35, 8-40, 8-41,
8-44, 8-45, 8-46, 8-47, 8-64, 8-65
Complainant…………… ... 8-5, 8-8, 8-9, 8-21, 8-23, 8-25, 8-26,
8-41, 8-42, 8-44, 8-51, 8-53, 8-54, 8-55, 8-56
Complaint…..…. ... 8-4, 8-5, 8-7, 8-8, 8-9, 8-18, 8-20, 8-21, 8-
23, 8-24, 8-25, 8-29, 8-31, 8-36, 8-40, 8-41, 8-42, 8-44,
8-45, 8-48, 8-49, 8-50, 8-51, 8-53, 8-54, 8-55, 8-56, 8-
57, 8-58, 8-59, 8-60, 8-64, 8-65
Complaints………. .... 8-7, 8-8, 8-45, 8-48, 8-50, 8-51, 8-54, 8-
56, 8-57, 8-59, 8-60
Cooperation ....................................... 8-30, 8-31, 8-38, 8-50
Counterfeit......................... 8-4, 8-17, 8-18, 8-19, 8-20, 8-21
Criminal Activity ..................................... 8-4, 8-6, 8-19, 8-41
CVM ................................. 8-27, 8-31, 8-41, 8-45, 8-51, 8-64
D
Death .. ………………………….8-5, 8-7, 8-18, 8-19, 8-20, 8-23, 8-
40, 8-41, 8-44, 8-52, 8-57
Detention ............................................................. 8-14, 8-15
Disaster.. ............ 8-4, 8-9, 8-10, 8-11, 8-15, 8-16, 8-30, 8-83
E
Embargo ...................................................... 8-14, 8-15, 8-16
F
Farm .......................................... 8-12, 8-27, 8-46, 8-47, 8-66
FATA ..................................................................... 8-18, 8-21
I
Illness …………………….8-5, 8-7, 8-8, 8-31, 8-33, 8-39, 8-40, 8-
41, 8-49, 8-52, 8-55, 8-59, 8-72
Infant Formula ......................8-7, 8-8, 8-41, 8-44, 8-48, 8-65
Injury……………………………… 8-5, 8-7, 8-8, 8-18, 8-19, 8-20, 8-
23, 8-24, 8-40, 8-41, 8-42, 8-43, 8-45, 8-48, 8-49, 8-50,
8-51, 8-52, 8-53, 8-55, 8-56, 8-58, 8-59
Internet ....................................................... 8-25, 8-26, 8-42
Interview………………………………..8-19, 8-21, 8-22, 8-23, 8-29,
8-32, 8-34, 8-35, 8-36, 8-38, 8-44, 8-47, 8-52, 8-55
M
Medical Records .. ………………..8-5, 8-23, 8-24, 8-25, 8-36, 8-
39, 8-42, 8-43, 8-44, 8-49, 8-52
O
OCI ………………………………..8-4, 8-6, 8-14, 8-17, 8-18, 8-19, 8-
20, 8-21, 8-26, 8-48
Outbreak………………… ... 8-5, 8-24, 8-27, 8-31, 8-32, 8-33, 8-
34, 8-35, 8-36, 8-38, 8-39, 8-46, 8-47, 8-64, 8-69, 8-72
R
Reconditioning ............................................ 8-16, 8-17, 8-30
Relabeling ...................................................................... 8-17
Report…………………………………… .. 8-3, 8-4, 8-5, 8-6, 8-8, 8-9,
8-11, 8-17, 8-18, 8-19, 8-20, 8-21, 8-23, 8-24, 8-27, 8-
28, 8-29, 8-30, 8-31, 8-32, 8-34, 8-39, 8-40, 8-41, 8-42,
8-44, 8-47, 8-48, 8-49, 8-50, 8-51, 8-52, 8-54, 8-55, 8-
56, 8-57, 8-58, 8-59, 8-60
S
Sample………………….… ... 8-3, 8-14, 8-16, 8-19, 8-20, 8-21, 8-
25, 8-26, 8-29, 8-30, 8-31, 8-33, 8-34, 8-36, 8-37, 8-38,
8-39, 8-44, 8-45, 8-47, 8-48, 8-58, 8-60, 8-64, 8-68, 8-69
Surveillance .......................................... 8-6, 8-50, 8-57, 8-59
T
Tamper………………… . 8-4, 8-7, 8-8, 8-14, 8-17, 8-18, 8-19, 8-
20, 8-21, 8-23, 8-48, 8-50
Tobacco .............................................. 8-15, 8-27, 8-60, 8-80
W
Washout ................................................................. 8-6, 8-28