
Authors:
Triona Fortune, Elaine O’ Connor and Barbara Donaldson
Guidance on Designing
Healthcare External
Evaluation Programmes
including Accreditation
2015

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
I
Table of contents
Acknowledgements iii
Foreword ISQua iv
Foreword World Bank & WHO vi
List of Tables vii
Glossary of Terms viii
Introduction 2
Chapter 1: Why develop an external evaluation programme? 4
1.1 The growing demand for external evaluation in health and social care 4
1.2 Models of external evaluation 5
1.3 Benefits of external evaluation 8
1.4 Challenges for external evaluation programmes 10
Chapter 2: Establishing the fundamentals 12
2.1 Defining the purpose of the external evaluation programme 12
2.2 Defining the scope of the external evaluation programme 15
2.3 Establishing the role of government 18
2.4 Determining incentives 21
2.5 Developing relationships with stakeholders 24
Chapter 3: Setting up the external evaluation organisation 27
3.1 Establishing a preliminary board or advisory committee 27
3.2 Proposing a governance board and framework 28
3.3 Funding of the programme 30
3.4 Setting up strategic, operational and financial management systems 33
3.5 Timeframes 35
Chapter 4: Developing the standards 37
4.1 The role of standards 37
4.2 Principles for standards 38
4.3 Referencing to quality dimensions 39
4.4 Developing the measurement system 40
Chapter 5: Developing assessment methodologies 43
5.1 Selection, training and evaluation of surveyors 43
5.2 Developing the survey management process 46
5.3 Establishing the accreditation / certification process 48
5.4 Quality Assurance 51
Chapter 6: Evaluating systems and achievements 52
6.1 Measuring performance internally 52
6.2 Evaluating independently 53
6.3 Monitoring by regulatory agencies 53
6.4 Accrediting the external evaluation bodies 53

II
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Table of contents
Conclusions 54
References 55
Bibliography 58
Useful web resources 62
Appendix 1: Case Studies 64
Appendix 1a. Danish Case Study 64
Appendix 1b: Jordanian Case Study 67
Appendix 1c. New Zealand Case Study 69
Appendix 1d: Practice Incentive Program (PIP) 72
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
© 2015 Publisher: The International Society for Quality in Health Care, Joyce House,
8-11 Lombard Street East, Dublin 2, D02 Y729, Ireland.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
III
Acknowledgements
This document is based on the Toolkit for Accreditation Programs, 2004 developed by Charles
Shaw. The International Society for Quality in Health Care would like to thank the following for
their contributions to the development of this document:
Reviewers
Mark Brandon, AACQA - Australia
Stephen Clark, AGPAL - Australia
Heleno Costa Junior, CBA - Brazil
Carsten Engel, IKAS - Denmark
Eric de Roodenbeke, International Hospital Federation
Carlos Goes de Souza, CHKS - UK
Helen Healey, DAP BC - Canada
Salma Jaouni, HCAC - Jordan
Thomas Leludec, HAS - France
Hung-Jung Lin, JCT - Taiwan
Lena Low, ACHS - Australia
Kadar Marikar, MSQH - Malaysia
Wendy Nicklin, Accreditation Canada
BK Rana, NABH - India
Charles Shaw, Independent Consultant
Paul van Ostenberg, JCI - USA
Kees van Dun, NIAZ - The Netherlands
Stuart Whittaker, COHSASA - South Africa
Hongwen Zhao, WHO
Nittita Prasopa-Plaizier, WHO
Akiko Maeda, World Bank
Dinesh Nair, World Bank
Rafael Cortez, World Bank
A special acknowledgement to Akiko Maeda and the World Bank for supporting this project.

IV
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Foreword
Accreditation is an important tool for improving the care delivered by healthcare systems, and
one of the key roles of the International Society for Quality in Health Care (ISQua) has been to
accredit the accreditors. However, accreditation has to evolve to be beneficial. An increase in
requests - especially from developing economies - for advice on establishing an accreditation
programme prompted ISQua to review two of its major tools: the Toolkit for Accreditation
Programs, 2004
1
, and Checklist for Development of New Healthcare Accreditation Programs,
2006
2
. The last decade has seen considerable changes, worldwide, to healthcare systems
and external evaluation programmes. To reflect these changes, a revision to the existing
guidance was deemed inadequate and this new Guidance manual was therefore developed. We
believe this document will be suitable for a much wider audience; it is designed for countries,
governments and policy makers within public or private, primary, secondary or tertiary
healthcare systems. It is also intended as an aid for funding and development agencies such as
the World Bank, international aid agencies, the World Health Organization (WHO), Ministries of
Health, other government agencies, groups and organisations who want to improve the quality
and safety of healthcare in their country, region or specialty area.
It has now been almost 100 years since the first external evaluation programme, known as
accreditation, was established. Nearly every country currently has some form of external
evaluation, whether voluntary or mandatory. There are both “aficionados” and critics of
healthcare accreditation. Anyone who has dealt with accreditors coming into their site has
likely felt that they were arbitrary, or focused on things that were less than important. However,
accreditation gets organisations to pay attention to things they might otherwise prefer to ignore
or put off. While it is sometimes voluntary, following a series of adverse events policymakers
then change it to mandatory in response. While traditionally accreditation was a programme
for developed economies, developing countries are now equally as interested. This document
has extended its scope beyond healthcare accreditation programmes to include other external
evaluation programmes such as certification and licensing as they apply to organisations, not
individual practitioners. These programmes have different scopes and organisational coverage
but are based on the same principle of evaluating and improving performance against a defined
set of standards, using external evaluators, to improve the safety and quality of health services
for the public.
Accreditation is not a panacea to address all quality improvement issues but it can provide
a systematic approach that identifies areas where improvements are necessary, and when
mandatory, can “lift all the boats”, including some of the less strong entities within our
healthcare systems. When used with tools such as checklists and supported by technology, it
can become a powerful instrument for healthcare reform.
Developing an external evaluation system is a process that should be designed according to
each countries’ profile. Firstly, the purpose should be clear and secondly, depending on the
desired outcome, a decision should be made as to whether a voluntary or mandatory system
is appropriate. This document is not designed as a rigid guideline, rather as a diverse range
of practices which should be discussed. It includes advice on best practices for governance,
developing standards and assessment methodologies. It also includes real case studies from
both developed and developing countries.
Healthcare continues to evolve; some of the key changes occurring today are that populations
are ageing, while technology is becoming smarter and the relationships between providers
and patients are tilting so that patients are much more empowered, and they are becoming
our partners. We all need to strive to reach country specific and global goals such as the World
Health Organization’s mandate on Universal Health Coverage (UHC) by 2020.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
V
Governments will ultimately be responsible for providing UHC and they will be required to
demonstrate efficient use of limited public funds while providing safe quality healthcare.
External evaluation systems can provide this assurance.
ISQua believes that accreditation can continue to be a powerful force for improvement in the
quality of care that is delivered. However, like all quality improvement initiatives, it must evolve
with the times to reflect the needs of our healthcare systems.
Professor David W. Bates
President International Society for Quality in Health Care
August 2015
VI
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Foreword
World Bank and the World Health Organization
The public has a growing awareness of and expectation for their healthcare to be accountable,
safe, of high quality and responsive to their needs. Globally, healthcare costs are rising, putting
increasing burdens on both governments and healthcare organisations, as they try to meet
the growing challenges with limited resources. Governments are working towards Universal
Health Coverage (UHC) as a way to ensure that their populations have equitable access to safe,
high quality services, without suffering financial hardship. The critical question remains: how
can countries maximise access whilst maintaining safe and quality services within affordable
margins?
External evaluation programmes, which include accreditation, certification and licensing of
healthcare institutions, are among measures that can help improve organisational efficiency and
effectiveness as well as the safety and quality of services. However, implementation of these
programmes is not uniform. This may be due to a lack of resources or expertise or, importantly,
due to a lack of operational ‘know-how’ on the implementation of such programmes.
This report aims to provide a practical guide for setting up an external evaluation programme
at both a national and an organisational level. It will help governments and policy makers to
identify and determine health systems’ priorities and gaps, so they can re-orient healthcare
systems and policies to meet such growing challenges. The report offers a range of approaches
and practical steps on the setting up of external evaluation programmes, including creating an
enabling environment and developing human and system capacities.
Better implementation of external evaluation programmes can contribute to improved safety
by requiring services to meet standards, and by encouraging quality improvement through
organisational and individual professional development. Such programmes, if adopted
and implemented appropriately and consistently, will contribute to a more resilient, more
accountable, and more effective healthcare system in the long run.
It is hoped that this report will encourage governments and healthcare organisations to adopt
and implement external evaluation programmes in order to achieve safe, high quality, resilient
and sustainable health systems and services.
Timothy Grant Evans
Senior Director
Health Nutrition and Population Global Practice
The World Bank Group
Marie-Paule Kieny
Assistant - Director General
Health Systems and Innovation
World Health Organization

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
VII
List of Tables
Table Page No.
Table 1: Definitions of accreditation, certification and licensing 8
Table 2: Comparison of capacity building and regulatory external evaluation 13
Table 3: Potential composition of a preliminary / interim board or advisory
committee
28

VIII
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Glossary of Terms
Accountability
Responsibility and requirement to answer for tasks or activities. This
responsibility may not be delegated and should be transparent to all
stakeholders.
Accreditation
A self-assessment and external peer review process used by health and
social care organisations to accurately assess their level of performance
in relation to established standards and to implement ways to
continuously improve the health or social care system.
Assessment
Process by which the characteristics and needs of patients, groups,
populations, communities, organisations or situations are evaluated or
determined so that they can be addressed. The assessment forms the
basis of a plan for services or action.
Assessor
Person who evaluates characteristics and needs. For external evaluation,
an assessor identifies and evaluates evidence that set criteria are being
met and makes recommendations for action to address any gaps. Also
auditor, surveyor, external evaluator.
Benchmarking
Comparing the results of services’ or organisations’ evaluations to
the results of other interventions, programmes or organisations, and
examining processes against those of others recognised as excellent, as a
means of making improvements. Also benchmark.
Certification
Process by which an authorised body, either a governmental or non-
governmental organisation (NGO), evaluates and recognises either an
individual, organisation, object or process as meeting pre-determined
requirements or criteria. The pre-determined requirements are set out in
standards which are developed specifically for the purpose of assessment.
The standards assess the performance of the organisation, object, process
or person, may focus on specific aspects of performance and may address
more than legal requirements.
Clients
Individuals or organisations being served or treated by the organisation.
Also patients, consumers, service users.
External
evaluation
Process in which an objective independent assessor gathers reliable
and valid information in a systematic way by making comparisons to
standards, guidelines or pathways for the purpose of enabling more
informed decisions and for assessing if pre-determined and published
requirements such as goals, objectives or standards have been met. An
organisation, object, process or individual may be assessed and evaluation
may be undertaken by peers, including organisations and professionals,
private professional auditors or consultants, purchasers / funders /
insurers, consumers / patients or governments.
Health Outcome
Health state or condition attributable to treatment, care or service
provided.
Leader
An individual who sets expectations, develops plans and implements
procedures to assess and improve the quality of the organisation’s
governance, management, clinical and support functions and processes.
Leadership
Ability to provide direction and cope with change. It usually involves
establishing a vision, developing strategies for producing the changes
needed to implement the vision, aligning people, motivating and inspiring
people to overcome obstacles.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
IX
Licensing
Process by which a governmental authority grants permission to an
individual practitioner or health and social care organisation to operate or
engage in an occupation or profession. Licensing regulations are generally
established to ensure that an organisation or individual meets minimum
standards to protect health and safety. The output of licensing is the
awarding of a document or licence allowing an organisation or person to
provide a service within a specified scope.
Medical tourism
Travel of people to another country for the purpose of obtaining medical
treatment in that country.
Organisational
peer assessment
A process whereby the performance of an organisation is evaluated by
members of similar organisations. Also peer review.
Outcome
standards
Standards which address the results, consequences or outcomes of the
performance and measurement of activities, systems and functions.
Patient
centredness
Focus on the experience of the patient / client from their perspective,
minimising vulnerability and maximising control and respect. Also patient
/ client focus.
Patient / Client
journey
The patient / client path through the care or treatment process – entry,
assessment, planning, delivery of care or treatment, evaluation, follow-up
and across services and providers. Also client continuum of care.
Process
standards
Standards which address the interrelated processes of different
organisational and clinical functions and activities.
Quality
improvement
Ongoing response to quality assessment data about a service, in ways
that improve the processes by which services are provided to clients. Also
continuous quality improvement (CQI).
Regulation
Is a form of external evaluation by which a body, who is authorised
by law, assesses an organisation or a person against pre-determined
requirements. The pre-determined requirements are derived from
legislation and therefore, the regulator may take a number of actions in
the event of non-compliance.
Risk mitigation
A systematic reduction in the extent of exposure to a risk and / or the
likelihood and consequences of its occurrence.
Self-assessment
A process by which an organisation evaluates its own performance against
set criteria or standards, identifies strengths and gaps, and plans actions
for improvement.
Standardisation
Process of developing and implementing technical, service or other
standards; that can help to maximize compatibility, interoperability, safety,
repeatability or quality.
Structure
standards
Standards which address the relatively stable characteristics of healthcare
providers, their staff, tools and resources, and physical and organisational
settings.
System
A set of interacting or interdependent processes forming an integrated,
whole function or activity.
Transparency
Operating in such a way that it is easy for others to see what actions
are performed; a principle that allows those affected by administrative
decisions, business transactions or charitable work to know not only the
basic facts and figures but also the mechanisms and processes. Usually
requires documented policies and procedures.
Universal health
coverage
The goal of all people having access to and obtaining health promotion,
preventive, curative, rehabilitative and palliative health services they need,
of sufficient quality to be effective, without suffering financial hardship to
avail of them.

2
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Introduction
The purpose of this document is to guide countries, agencies and other groups in the process of
setting up new health or social care external evaluation organisations or programmes. It is also
intended as an aid for funding and development agencies such as the World Bank, international
aid and technical cooperation agencies, World Health Organization, Ministries of Health, other
government agencies, groups and organisations who want to improve the quality and safety
of healthcare in their country, region or specialty area. It revises the International Society for
Quality in Health Care (ISQua) Toolkit for Accreditation Programs, 2004
1
, and ISQua Checklist for
Development of New Healthcare Accreditation Programs, 2006
2
. This document has extended
its scope beyond healthcare accreditation programmes to include other external evaluation
programmes such as certification and licensing as they apply to organisations, not individual
practitioners. These programmes have different scopes and organisational coverage but are
based on the same principle of evaluating and improving performance against a defined set
of standards or criteria, using external evaluators, to improve the safety and quality of health
services for the public.
Accreditation can be defined as a self-assessment and external peer review process used by
health and social care organisations to accurately assess their level of performance in relation
to established standards and to implement ways to continuously improve the health or social
care system. Certification is a process by which an authorised body, either a governmental or
non-governmental organisation, evaluates and recognises an organisation as meeting pre-
determined requirements or criteria. Licensing is a process by which a governmental authority
grants permission for a healthcare organisation to operate. Licensing regulations are generally
established to ensure that an organisation or individual meets minimum standards to protect
health and safety. For the purpose of this document we will refer to an accreditation body but
this includes any external evaluation programme as the principles remain the same.
The document refers mainly to healthcare organisations but is also applicable to social care
organisations. In it, the term external evaluation is used to cover accreditation, certification,
licensing and other standards based assessment programmes. The term survey is used to refer
to survey, assessment and audit. The term surveyor is used to include surveyors, assessors and
auditors.
Research and experience have identified the benefits of external evaluation programmes such
as improved organisational efficiency and effectiveness, improved safety and quality, better risk
mitigation, improved leadership, reduced liability costs, better communication and teamwork,
increased satisfaction of users and staff, and better patient care. However, there are challenges
in setting up these programmes. The principal threats to new external evaluation programmes
appear to be inconsistency of government policy, unstable politics, unrealistic expectations
and lack of professional / stakeholder support, continuing finance and / or incentives. The
effectiveness and sustainability of an external evaluation organisation or programme depends
ultimately on many variable factors in the particular healthcare environment of the country
or organisation involved. It also depends on the kind of programme concerned, and how it is
implemented.
To be sustainable, external evaluation programmes need ongoing government and / or private
support, a sufficiently large healthcare market size, stable programme funding, diverse
incentives to encourage participation, and continual refinement and improvement in the external
evaluation organisation’s operations and service delivery.
This guide addresses the variables of policy, organisation, methods and resources. It outlines
the reasons why an external evaluation programme might be developed, describes the different
models, and highlights the benefits and challenges associated with external evaluation.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
3
It then provides guidance on the steps that need to be taken in establishing a new external
evaluation organisation including:
Establishing the fundamentals of scope and purpose, and defining the important roles of
government and incentives in the external evaluation organisation / programme.
Setting up of the external evaluation organisational structure including: establishing an
advisory committee; developing relationships with stakeholders; designing a governance
framework; embedding the values of fairness and transparency; and getting outside
assistance and funding.
Establishing governance and management systems including: staffing; financial and
information systems; and risk management and performance improvement systems. It also
highlights the importance of allowing enough time for these stages.
Developing the standards to be used by the organisation and the system for measuring their
achievement.
Developing the surveyor and survey management systems including: the selection and
training of surveyors; the designing of processes and technology for managing surveys
and other events; developing and establishing education services; and determining and
establishing the process for awarding accreditation or certification status.
Integrating into all these systems and processes ways of measuring and evaluating
performance.
This document reflects the best practice guidelines and standards developed by the
International Society for Quality in Health Care (ISQua) as part of its International Accreditation
Programme (IAP): ISQua Guidelines and Standards for External Evaluation Organisations, 4th
Edition Version 1.1, 2014
3
; ISQua Guidelines and Principles for the Development of Health and
Social Care Standards, 4th Edition Version 1.1, 2014
4
; and ISQua Surveyor Training Standards
Programme, 2nd Edition 2009
5
.
The appendices include case studies outlining how three different healthcare external
evaluation organisations were established. Two of the organisations featured are accreditation
organisations. The third featured organisation is an assessment organisation established
primarily to assess against government-mandated standards for compulsory certification.
Appendix 1d describes an Australian Practice Incentive Programme that demonstrates how
accreditation can be used as a lever to encourage quality improvement.

4
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Chapter 1: Why develop
an external evaluation
programme?
This chapter introduces what a healthcare external evaluation programme is; describes some
of the different models of external evaluation; outlines the benefits of such programmes; and
highlights the challenges which may be encountered in establishing such programmes.
1.1 The growing demand for external evaluation in health and
social care
There is growing worldwide demand, concern and focus on quality and safety in
healthcare. UUniversal Health Coverage (UHC) is now a key agenda item for the World
Bank and the World Health Organization and many countries have adopted or are about
to adopt this system of equal healthcare for all. The goal of universal health coverage is
to ensure that all people obtain the health services they need without suffering financial
hardship when paying for them. This requires:
A strong, efficient, well-run health system with good governance
A system for financing health services in an efficient and equitable way
Access to essential medicines and technologies and good health information
systems
A sufficient capacity of well-trained, motivated health workers
6
.
There is increasing support from governments, and from funding agencies, for
mechanisms, such as accreditation, to support UHC. Governments will ultimately be
responsible for providing UHC and they will be required to demonstrate efficient use
of limited public funds while providing safe quality healthcare. External evaluation
provides assurances that healthcare facilities have quality systems in place and
the data to demonstrate the required level of service provision. Depending on the
comprehensiveness of the standards against which health service performance is being
measured, external evaluation programmes such as accreditation and certification can
contribute to quality improvement, risk mitigation, patient safety, improved efficiency and
accountability, and can contribute to the sustainability of the healthcare system. They
can provide information on how well health services are being delivered, identify issues,
and assist the decision-making of funders, regulators, healthcare professionals and the
public. External evaluation supports transparency, benchmarking and accountability, so
that government funding is allocated in a fair and equitable way and supports a culture of
change and quality and an increased focus on risk.
Patients expect to receive safe care and are demanding quality services that meet their
needs. They expect to be treated with respect, to receive services of an appropriate and
consistent standard that are delivered with care and skill, that minimise risk and harm,
comply with legal, professional and ethical standards, and that facilitate continuity of
care. Patients need to receive information about their condition and treatment in a way
they can understand, to be able to make informed choices about their treatment and to
be communicated with openly and honestly. They want the right to complain if services do
not meet their needs and expect action to be taken to address the problem.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
5
They have a right to trust that their health provider or hospital has systems and
processes in place to provide such patient-centred, reliable, efficient, effective and
responsive care. An external evaluation programme based on best practice standards
will make a significant contribution to achieving this.
With preventable error rates estimated to be 83% in developing and transitional countries
and a 30% rate of adverse events associated with deaths, these countries require not only
more resources to improve the safety and quality of care, but a political environment,
policies and mechanisms that support quality initiatives. The contribution of external
evaluation organisations centred on promoting improvements, applying standards and
providing feedback is being increasingly recognised in these countries. Preventable
error rates of over 10% in developed countries are also unacceptable. A flourishing
accreditation programme is one element of the institutional basis for high quality
healthcare
7
.
1.2 Models of external evaluation
External evaluation
Is a process by which an objective independent assessor gathers reliable and valid
information in a systematic manner by making comparisons to standards, guidelines
or pathways for the purpose of enabling more informed decisions and for assessing
if pre-determined and published requirements such as goals, objectives or standards
have been met. An organisation, object, process or individual may be assessed and
evaluation may be undertaken by peers, including organisations and professionals,
private professional auditors or consultants, purchasers / funders / insurers, consumers
/ patients or governments.
The distinguishing features of external evaluation are as follows:
It is a formal process
The object being assessed is an organisation, object, process or individual person
Assessment is undertaken by an objective, independent assessor
Assessment is against pre-determined and published requirements / criteria
It is designed so that decisions are not influenced by those being assessed
The assessment results in a defined output
There are a number of models of external evaluation and it should be acknowledged
that there can be confusion regarding terminology due to the diverse applications of
the external evaluation models. Examples of external evaluation models include the
following:
Accreditation
Accreditation may be defined as a self-assessment and external peer review process
used by health and social care organisations to accurately assess their level of
performance in relation to established standards and to implement ways to continuously
improve the health or social care system. Although primarily applied in relation to
organisations, processes may also be accredited. Accreditation standards assess
the organisation’s or process’s ability to fulfil its core mission and may address more
than legal requirements. They are usually recognised as optimal, evidence-based and
achievable and are designed to encourage continuous improvement
8
. The output of
accreditation is a report summarising the findings of the assessment and a recognition
decision regarding the accreditation status.

6
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Accreditation is one of the longest established models of external evaluation. It is a
self-assessment and external peer review process that assesses the entire organisation
including both clinical and management processes and activities. Traditionally, health
and social care organisations engaged in accreditation on a voluntary basis and
accreditation schemes were provided by non-governmental agencies. However, there
has been a shift over time towards greater governmental involvement in accreditation
with the development of national government funded accreditation programmes and a
shift from voluntary to mandatory participation in such schemes. For example, in 2011
the Australian Health Ministers endorsed the National Safety and Quality Health Service
(NSQHS) Standards and a national accreditation scheme. As a result, all hospitals and
day procedure services and the majority of public dental services across Australia now
need to be accredited to the NSQHS Standards. Private health service organisations are
required to confirm their requirements for accreditation to any standards in addition to
the NSQHS Standards with the relevant
health department. Prior to 2011, participation in accreditation was voluntary for
Australian hospitals
9
.
Certification
Certification is a process by which an authorised body, either a governmental or non-
governmental organisation, evaluates and recognises either an individual, organisation,
object or process as meeting pre-determined requirements or criteria. The pre-
determined requirements are set out in standards which are developed specifically
for the purpose of assessment. The standards assess the performance of the
organisation, object, process or person, may focus on specific aspects of performance
and may address more than legal requirements. The output of certification is a report
summarising the findings of the assessment and a recognition decision regarding the
certification status.
Certification may be used by governments or other authorised agencies to assess the
compliance of healthcare facilities or specific departments / services within those
facilities with a set of standards. The focus is usually on essential elements being in
place rather than on continuous quality improvement. The standards and certification
may not be organisation-wide, but may apply to a particular service, e.g. physiotherapy.
Governments may authorise independent assessment organisations to assess health and
social care providers’ compliance with government-mandated standards.
An example of a certification scheme is ISO: the International Organization for
Standardization. ISO provides standards, e.g. ISO 9000 Quality Management, against
which organisations or functions may be certified by ISO accredited certification bodies or
organisations
10
. Although originally designed for the manufacturing industry, e.g. medical
devices, these have been primarily applied to radiology and laboratory systems in
healthcare, and more generally to quality systems in hospitals and clinical departments.
Conformance with ISO standards is assessed by professional quality auditors and any
non-conformance is followed up with a subsequent audit.
When applied to individuals, certification usually implies that the individual has received
additional education and training, and demonstrated competence in a specialty area
beyond the minimum requirements set for registration or licensing. For example, a
doctor may be certified by a professional specialty board in the practice of obstetrics
8
.
There can be confusion between the terms accreditation and certification and they are
often used interchangeably. However, accreditation usually applies only to organisations,
while certification may apply to individuals, as well as organisations.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
7
Regulation
Regulation is a form of external evaluation by which a body, authorised by law, assesses
an organisation or a person against pre-determined requirements. The pre-determined
requirements are derived from legislation and therefore, the regulator may take a
number of actions in the event of non-compliance.
Licensing
Licensing is a process by which a governmental authority grants permission to an
individual practitioner or health or social care organisation to operate or engage in an
occupation or profession. Licensing regulations are generally established to ensure that
an organisation or individual meets minimum standards to protect public health and
safety.
The output of licensing is the awarding of a document or licence allowing an organisation
or person to provide a service within a specified scope.
Organisational licensing or registration is granted following an on-site inspection
to determine if minimum health and safety standards have been met. Maintenance
of registration or licensure is an ongoing requirement for the health or social care
organisation to continue to operate and care for patients or clients.
Individual or professional licensing or registration is usually granted after some form of
examination or proof of education and may be renewed periodically through payment of a
fee and / or proof of continuing education or professional competence
8
.
Countries may have more than one model of external evaluation in operation in specific
sectors. For example, hospitals may be required to be licensed and meet specific
government-mandated standards in order to be able to provide health services in a
particular country, but may still engage voluntarily in organisational accreditation
or certification programmes for specific departments in the facility e.g. laboratory
certification programmes. Individual healthcare practitioners may need to be registered
with their professional body in order to be employed in a hospital but they may also
voluntarily undergo additional education in order to be certified in a respective field by a
professional specialty board.
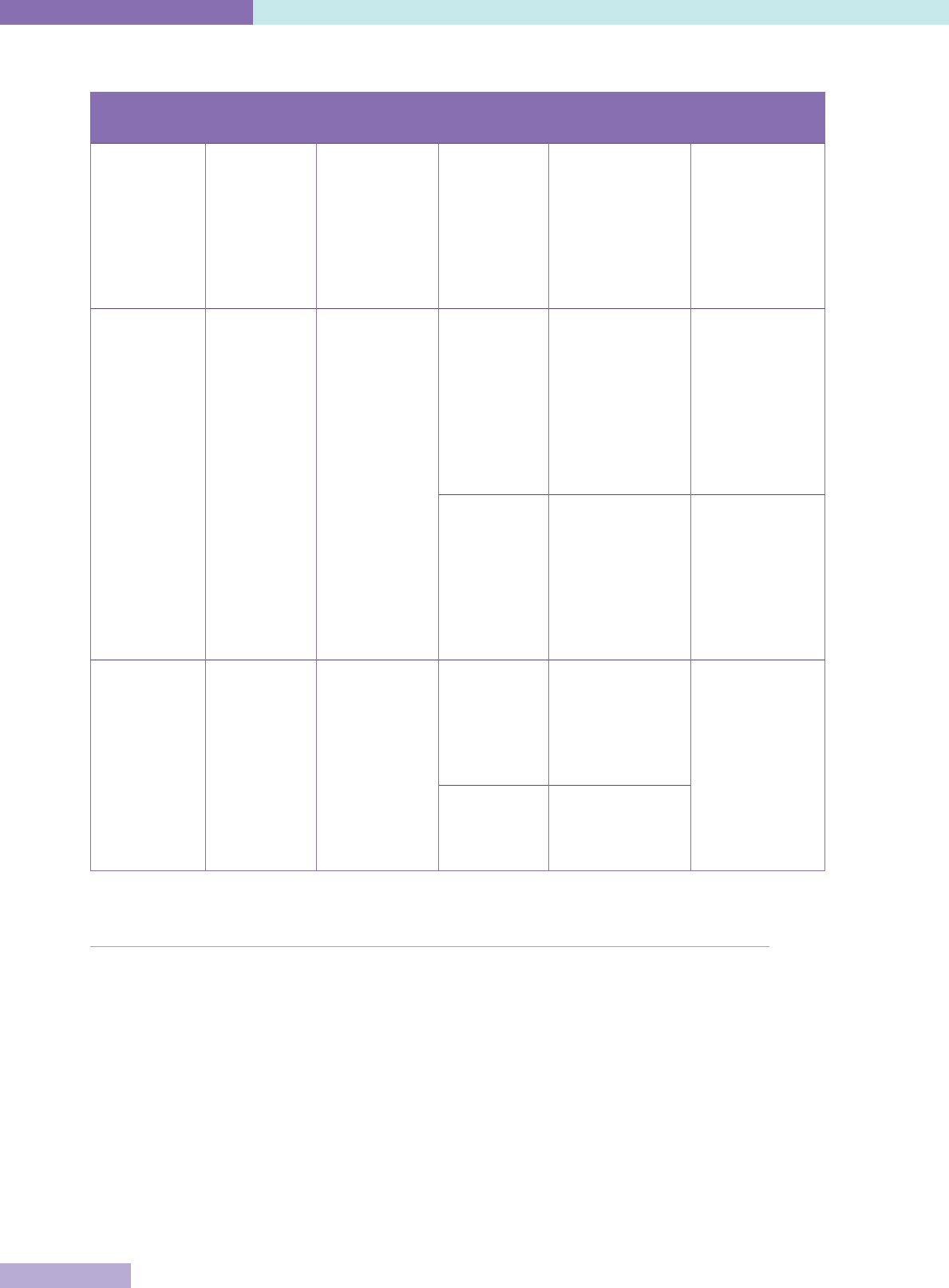
The key characteristics of accreditation, licensing and certification are set out in Table 1.
8
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Table 1: Definitions of accreditation, certification and licensing
Process Participation Issuing
organisation
Object of
evaluation
Components /
Requirements
Standards
Accreditation Voluntary or
mandatory
Non-
governmental
organisation
(NGO) or
government
authority
Organisation Compliance
with published
standards, on-
site evaluation;
compliance may
not be required
by law and / or
requlations
Set at a
maximum level
to stimulate
improvement
over time
Certification Voluntary or
mandatory
Authorised
body, either
government or
NGO
Individual Evaluation of
pre-determined
requirements,
additional
education
/ training,
demonstrated
competence in
speciality area
Set by national
professional or
speciality boards
Organisation
or component
Demonstration
that the
organisation
has additional
services,
technology or
capacity
Industry
standards
(e.g. ISO 9000
standards)
evaluate
conformance
to design
specifications
Licensing Mandatory Governmental
authority
Individual Regulations to
ensure minimum
standards,
exam, or proof
of education /
competence
Set at a
minimum level
to ensure an
environment
with minimum
risk to health
and safety
Organisation Regulations to
ensure minimum
standards, on-site
inspection
1.3 Benefits of external evaluation
External evaluation has contributed to improving the quality and safety of healthcare
for nearly 100 years and the majority of the published literature relates to accreditation.
Research on the benefits of certification, regulation and licensing is sparse. It must
be acknowledged that historically there has been limited evidence of the impact of
accreditation but in recent years more empirical research has been undertaken to
identify and quantify the benefits.
Some of the specific benefits of accreditation identified in the literature include impacts
on structural elements of quality improvement in healthcare organisations such as
leadership, governance and management, and process elements such as organisational
performance
11
.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
9
From a leadership, governance and management perspective, accreditation is
perceived as: providing a framework for helping to create and implement systems and
processes that improve operational effectiveness and advance positive health outcomes;
providing organisations with a well-defined vision for sustainable quality improvement
initiatives; and as a means of demonstrating credibility and a commitment to quality and
accountability.
From an organisational performance perspective, some of the identified benefits include:
Increases healthcare organisations’ compliance with quality and safety standards
Stimulates sustainable quality improvement efforts and continuously raises the bar
with regard to quality improvement initiatives, policies and processes
Decreases variances in practice among healthcare providers and decision-makers
Highlights practices that are working well. Promotes the sharing of policies,
procedures and best practices among healthcare organisations
11
.
Accreditation has also been perceived as having an impact on team working by
strengthening interdisciplinary team effectiveness and promoting capacity building,
professional development and organisational learning
11
.
Similarly, a recent synthesis of 122 empirical studies that examined either the processes
or impacts of accreditation programmes concluded that research evidence generally
presents health service accreditation as a useful tool to stimulate improvement in
health service organisations and to promote high quality organisation processes.
Some of the cited studies found that accreditation promotes standardisation of care
processes; increased compliance with external programmes or guidelines; development
of organisational cultures conducive to quality and safety; implementation of continuous
quality improvement (CQI) activities; and superior leadership. There was limited evidence
showing positive associations between accreditation and patient outcome measures.
However, this was attributed to poor research design
12
.
A comparison of accreditation in low- and middle-income countries versus higher-
income countries showed all programmes promote improvements, apply standards
and provide feedback. Accreditation programmes are contributing to incremental
improvements in quality systems and clinical processes in health systems around the
world and are one element of the institutional basis for high-quality healthcare
7
.
A recent review examining the use of economic evaluation techniques in health services
accreditation research identified that no formal economic evaluation of health services
accreditation has been carried out to date. It also highlighted that the impact or
effectiveness of accreditation has been researched with a variety of foci and to differing
degrees. The research design of some studies, particularly those that are observational
or qualitative in nature, makes it difficult to provide statistically robust evidence for
the efficacy of accreditation or causality. The lack of a clear relationship between
accreditation and the outcomes measured in benefit studies makes it difficult to design
and conduct economic appraisal studies where a more robust understanding of the costs
and benefits involved is required. In turn, the absence of formal economic appraisal
means it is challenging to appraise accreditation in comparison to other methods to
improve patient safety and quality of care
13
.
While the evidence for the direct impact of accreditation on patient / client outcomes
is inconclusive, the available research suggests that accreditation may contribute to
improving health outcomes by strengthening interdisciplinary team effectiveness and
communication and by enhancing the use of indicators for evidence-based decision
making
14
. The challenge for mature external evaluation systems is to become more
outcome driven. This reduces the burden of audit but also helps to highlight its benefits.

10
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
1.4 Challenges for external evaluation programmes
The principal threats to new external evaluation programmes include: inconsistency of
government policy; unstable politics; unrealistic expectations; and lack of professional /
stakeholder support, continuing finance and / or incentives. To be sustainable, external
evaluation programmes need a number of elements to be in place, including some of
the following: ongoing government and / or private support; a sufficiently large health
or social care market size; stable programme funding; diverse incentives to encourage
participation; and continual refinement and improvement in the external evaluation
organisation’s operations and service delivery
15
(refer Chapter 2).
To be sustainable and credible, new programmes need sufficient numbers of trained
and skilled personnel and a realistic timeframe for the development of the programme.
They need to demonstrate objectivity and independence with transparent procedures for
the assessment of healthcare services and for decisions on accreditation or certification
awards. The expectations of governments and stakeholders about what the external
evaluation programme can achieve need to be realistic, in line with the purpose and
scope for which it has been designed and resourced, and in line with the government’s
broader strategy or policy for healthcare quality and safety. Within that strategy or policy
there needs to be a balance between the objectives of external control or regulation
and internal organisational development or improvement. Attempts to prescribe and
control every process of a complex system like a healthcare organisation or service,
which cannot be understood as simply a sum of a number of discrete and predictable
processes, will evoke resistance from staff, and can be counterproductive in terms of
quality and safety. Health and social care staff need to be motivated and committed to
improving quality rather than directed and sanctioned.
Expectations of accredited or certified health or social care services can be
unrealistically high. The external assessment of organisations for the purposes of
accreditation or certification is based on an on-site survey or assessment of compliance
with, or achievement of, standards. This is a snapshot in time and does not guarantee,
nor is it meant to guarantee, ongoing performance at the same level. However, external
evaluation organisations who themselves engage in an external evaluation process, such
as ISQua’s International Accreditation Programme (IAP) are expected, as part of this
process to monitor the continued maintenance of standards and quality improvements by
the organisations they have accredited or certified, e.g. submission of action plans and
reports of their implementation, periodic self-assessment or external reviews, random
reviews, follow-up of significant complaints or sentinel events.
Given the amount of effort and money invested worldwide in external evaluation and
regulation of healthcare delivery, and the common pursuit of valid standards and
reliable measurement, there are economic and technical reasons to share research and
experience more actively in the international community.
A study comparing European hospitals in terms of quality and safety was found to be
challenging because of the different hospital accreditation and licensing systems in each
country; the different indicators collected; different definitions of the same indicators;
different mandatory versus voluntary data collection requirements; different types of
organisations overseeing data collection; different levels of aggregation of data (country,
region, hospital); and different levels of public access to such data.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
11
This means that patients are unable to make informed choices about where they receive
their healthcare in different countries and some governments will remain in the dark
about the quality and safety of care available to their citizens as compared to that
available in neighbouring countries
16
.
Ongoing research is needed into the benefits and limitations of external evaluation
in healthcare. To measure the impact of any new programme, before and after
measurements are needed of the indicators that the programme is intended to address.
This chapter has introduced different external evaluation models and has outlined the
benefits of external evaluation and the challenges associated with establishing a new
programme. The following chapters will present the factors that need to be considered
when deciding which external evaluation model to adopt in a country and the steps to be
undertaken when setting up an external evaluation organisation and programme.

12
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Chapter 2: Establishing
the Fundamentals
This chapter outlines the initial decisions that need to be made when a new external
evaluation programme is being established: the purpose of the programme; its scope; the
role of government; and the incentives that may be needed to ensure health and social
care organisations participate. It also highlights the importance of identifying who the main
stakeholders may be and what external influences for the programme will look like.
2.1 Defining the purpose of the external evaluation programme
One of the first steps in the development of a new external evaluation programme is to
determine its purpose.
Factors to consider in determining the purpose of an external evaluation programme or
organisation include the following:
Developmental or regulatory
According to the World Bank
17
, governments regulate health services in order to guide
private activity and achieve national health objectives. Regulation can be used for control,
with instruments that use the force of law to ensure that services provided adhere to
legal requirements. Instruments that aim to control include: licensing, restrictions on
dangerous clinical practice and registration. Examples include: basic legislation on
health personnel such as registration and licensing requirements, which can also be
used to set minimum requirements for health services or facilities to operate. Regulation
can also use financial or non-financial incentives that change the behaviour of private
healthcare providers. The advantages of using incentive-based regulation is that it
avoids the informational, administrative and political constraints that control-based
interventions entail. Accreditation, certification and contracts are examples of incentive-
based regulation. However, in developing countries, regulation is often ineffective
because of the low level of enforcement and insufficient resources.
Standards-based external evaluation
Standards-based accreditation is a programme that contributes to developing an
organisation, and is designed to improve the quality as well as the safety of health
services.
Accreditation programmes monitor and promote, via self and external assessment,
healthcare organisation performance against pre-determined optimal standards
18
. They
also aim to contribute to the provision of high quality and safe healthcare services and to
improve patient health outcomes.
Certification may be similarly standards-based and use a rating system that encourages
improvement over time but its focus is usually more on continuing compliance with
criteria and the standards may be more limited. Licensing may be used when the priority
is ensuring basic health and safety requirements are met in order for a healthcare
organisation to operate and will usually be facility focused.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
13
Values and objectives underpinning a new programme
A survey of healthcare accreditation organisations revealed that quality improvement
was the reason healthcare organisations participated in accreditation. On the other hand,
the government agenda commonly focused more on the protection of public money and
public health as a priority, meaning reducing variation in practice to increase efficiency
and improve patient safety, consistent with WHO global initiatives
15
.
Values or principles may relate to features such as leadership, a system and process
approach, multidisciplinary teamwork, capacity building and training, patient
centredness, devolved decision-making and accountability, evidence-based decisions for
continuous improvement and performance-based incentives.
Objectives of external evaluation programmes identified in some developing countries
have included: improving leadership of a quality health system; improving resources and
capacity of the system and staff; improving performance by clearly defining the roles
and responsibilities of staff at all levels; developing the structures, systems and capacity
to support quality improvement; strengthening the focus and role of health service
consumers and other stakeholders; and improving health services through systematic
implementation of standards.
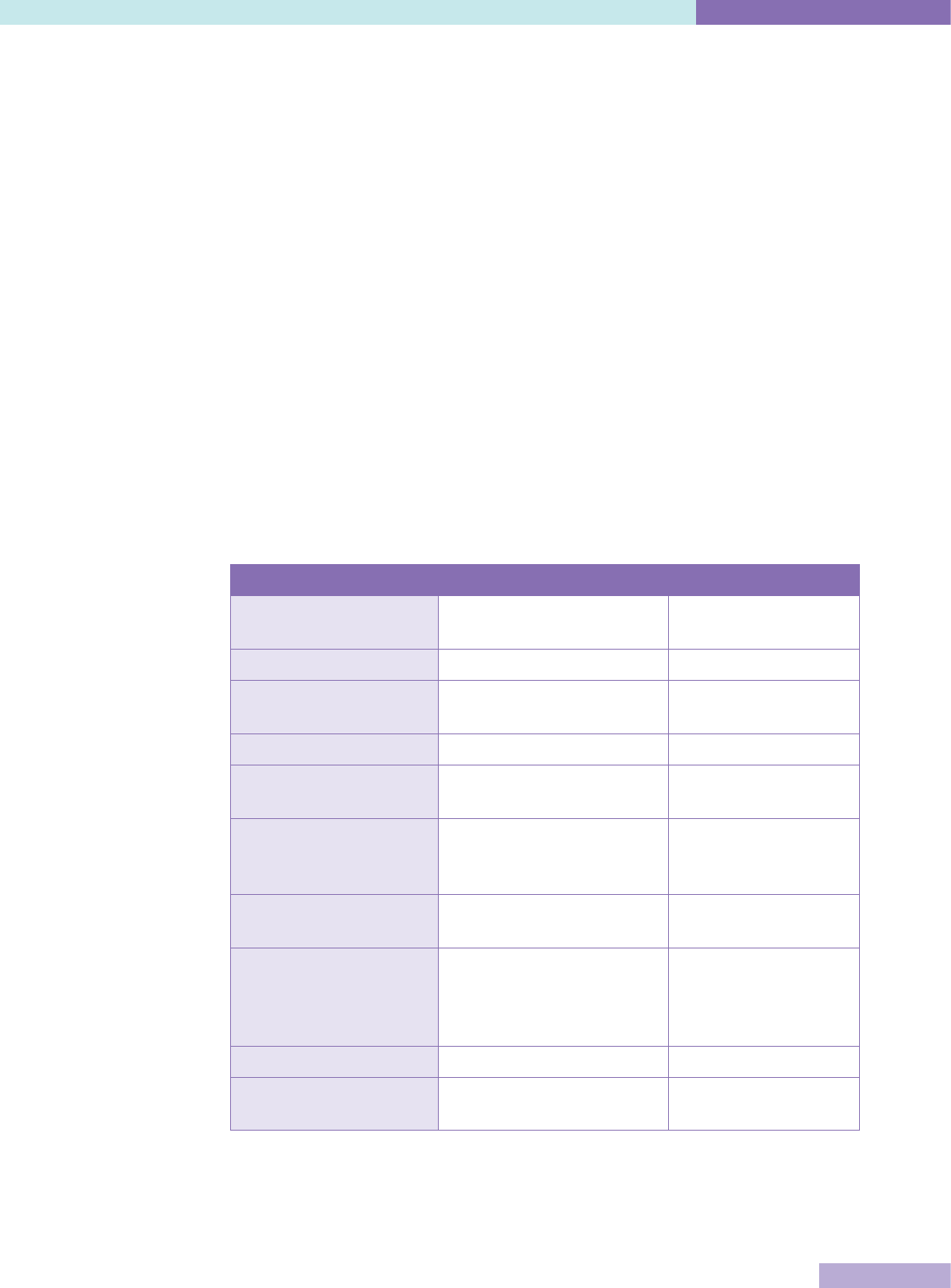
The following table compares capacity building and regulatory external evaluation
approaches
15
.
Table 2: Comparison of capacity building and regulatory external evaluation
Capacity building Regulatory
Purpose Dynamic, organisational
improvement
Static, control
Terminology Accreditation, certification Licensing, regulation
Governance Non-governmental
organisation, stakeholders
National / regional
government agency
Primary Customers Healthcare providers Government
Secondary customers Patients, professions,
healthcare insurers
Population, politicians,
public finance
Incentives for healthcare
organisations to
participate
Ethical, commercial Legal, mandatory
Uptake Voluntary self-selection to
available programs
All institutions in all
sectors
Standards Defined by non-governmental
organisation, optimal,
achievable, encourage quality
improvement
Defined by regulation,
minimal acceptable
Funding Self-financing State
Cross-border mobility Limited by language, culture Limited by political
borders

14
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Possible purposes or objectives of an external evaluation programme might be to:
Improve the performance of health services by setting and measuring the
achievement of standards
Increase public safety and reduce risks associated with injury and infections for
patients / clients and staff
Increase public confidence in the quality of healthcare services
Promote accountability of health services to funders and the public.
How do these values and objectives relate to plans for health reform in
general, and to the national quality strategy in particular?
The next important step is to identify if there are plans for health and / or social care
reform in the country or region and if there are any national or regional quality strategies
or plans in place. Reform plans outline the changes that a government intends to
make to a particular sector and outlines the specific actions that it will take to achieve
those reforms. For example, a government may outline in a reform plan that it intends
to establish an external evaluation organisation and what the role or purpose of this
organisation will be. A quality strategy provides an agreed direction and identifies the
most important activities for improving quality in the health and social care sector in the
country or region. It helps to identify the strengths of the system and also the constraints
that prevent the provision of a quality service. A quality strategy may outline the role
or will help to identify or clarify the role that external evaluation is expected to play in
achieving the country or region’s quality vision.
These factors will guide all further decisions - the role of the government, relationships
with stakeholders, the governance and management framework, the standards or
criteria to be used for assessment, the assessment process, and the outcome of
licensing, certification or accreditation.
The case study examples below provide further insight into the factors that influenced
the establishment of external evaluation agencies in different jurisdictions.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
15
Case Studies – Foundation of the programme
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
The Danish accreditation programme (DDKM) was established as part of the
“National Strategy for Quality Development in the Healthcare System – Joint Goals
and Action Plan 2002-2006”. The strategy was developed by the national, regional and
local political authorities in cooperation with stakeholder organisations, representing
professionals and consumers.
At that time, a number of hospitals already had positive experiences with
accreditation provided by international accreditors – one of the intentions of the
strategy was to spread this to the entire healthcare system, based on a Danish
model.
Health Care Accreditation Council (HCAC)
Country: Jordan
The HCAC is the national healthcare accreditation agency of Jordan. Several reasons
were stated for why the programme was developed including to improve the quality
of hospitals and to enhance medical tourism. In addition, it was a response to public
complaints of poor quality of care and a need to improve the entire healthcare system
in the country.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
The commencement of the Health and Disability Services (Safety) Act on 1 July 2002
represented a significant change in the regulatory environment in the New Zealand
health and disability sector. This Act replaced several previous pieces of legislation
and changed the way in which residential and hospital services were licensed
or registered. In addition, the Act introduced health and disability standards for
hospitals, rest homes and residential disability services aimed at improving safety
levels and quality of care that became mandatory from 1 October 2004. The Act
required that designated audit agencies (DAAs) are approved by the Director General
of Health for the purpose of auditing these services to those standards.
2.2 Defining the scope of the external evaluation programme
Once the purpose is established it is important to define the initial scope of the
programme. The purpose of a new external evaluation programme may depend on
the government’s priorities, the national health reform or quality strategies, available
funding, the commitment of stakeholders and the problems or issues that need to be
addressed.
Factors to consider in defining the scope of the external evaluation programme include
the following:
Primary or hospital care?
Traditionally, accreditation has been developed for hospitals or aged care facilities and
then moved outwards towards home support, hospice and other community services and
then to regional networks or networks of preventive and curative services.

16
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
However, in developing countries the most urgent need may be for improved primary
and community care and the programme will initially be developed to cover primary
care clinics and outreach services, although there may be some resource advantages in
developing primary care and hospital programmes at the same time.
Often it is easier to develop facilities based programmes first, starting with core
standards and external evaluation for single institutions, e.g. acute hospitals, polyclinics
or health centres. Standards can then be developed for more specialised services, e.g.
rest homes or hospice care or mental health, followed by the linkages between them,
preventive health or health networks, and they can then be covered by the programme.
Assessment of single units, services or departments could offer large organisations a
gradual entry to a full programme but it does not carry the benefits of integration and
organisation consistency. It may hide the opportunities for improvement which frequently
lie in communication between services rather than within them. However, there are
many service specific external evaluation programmes which are operated either by a
larger generic programme, or by a provider or association which works only in that area,
e.g. palliative care, laboratory medicine, speech therapy, autism, general practice, aged
care, and community services.
Some programmes have started with tertiary hospitals and services, with the intention
of expanding to secondary care services later. Some programmes in North America (e.g.
Accreditation Canada) accredit entire health networks and regions and are applying
accreditation across the continuum of care. Some governmental programmes in Europe
address public health priorities (such as cardiac health, cancer services) by assessing
local performance of preventive to tertiary services against national service frameworks.
In such programmes, measures may include the application of evidence-based
medicine (process) and the measurement of population health gain (outcome) but many
health determinants, e.g. housing, education and poverty, remain outside the scope of
healthcare external evaluation programmes.
However, current best practice is to provide a programme that focuses on the patient or
client and their journey through the service, hospital, network or care programme and
the continuity of service or care for that individual or family across the entire continuum
of care.
Historically, external evaluation programmes have set their scope in a way which
compartmentalises care and service rather than optimising quality outcomes for the
patient or client.
Public or Private coverage?
Most external evaluation programmes offer services to both public and private sector
services, although some are restricted to either the public or private sector. Evaluating
across sectors has advantages to healthcare organisations in facilitating the focus on the
patient or client journey, providing a level playing field for comparing and benchmarking
potential competitors, to surveyors in learning from another sector and to self-financing
programmes in having a larger potential market. Sometimes either the private or
public sector has the size, resources and incentives such as funding incentives, medical
insurance and competitive advantage to adopt an external evaluation programme earlier.
Medical tourism is another large incentive. To attract patients who are crossing national
borders in search of affordable and timely healthcare, private and public health services
need accreditation or certification to demonstrate their competence and safety.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
17
Many medical tourism companies are now involved in organising cross-border health
services and it has been recommended that the care they arrange should only be at
accredited international health facilities. Other recommendations include the medical
tourism companies themselves having to undergo an accreditation review; standards to
ensure patients make informed choices; and continuity of care as an integral feature of
cross-border care
19
.
The case studies provide some further insights into how the scope of external evaluation
agencies in different jurisdictions was determined.
Case Studies – Scope of the programme
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
Public and private hospitals, pharmacies, municipalities (primary care services,
including long-term care), ambulance providers and General Practitioners (GPs) all
participate in DDKM.
Health Care Accreditation Council (HCAC)
Country: Jordan
The HCAC is the national healthcare accreditation agency of Jordan. The organisation
sets standards for hospitals, primary healthcare centres, family planning and
reproductive health, transport services (ambulances), cardiac care, and diabetes
mellitus. HCAC surveys against the standards and awards accreditation. HCAC also
provides consultation and education to prepare healthcare facilities for accreditation
and offers certification courses.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
The commencement of the Health and Disability Services (Safety) Act on 1 July 2002
represented a significant change in the regulatory environment in the New Zealand
health and disability sector. This Act replaced several previous pieces of legislation
and changed the way in which residential and hospital services were licensed or
registered. HDANZ’s scope was determined by the Safety Act – the assessment of
standards is a legal requirement for public and private hospitals, rest homes and
residential disability services. Standards New Zealand (SNZ) is responsible for the
New Zealand standards and this includes others such as for home support, allied
health, and day surgery procedures.
Critical mass: economy, consistency, equity, objectivity
Larger countries can achieve economies of scale; smaller countries (perhaps with a
population of less than 5 million), or large ones which choose to devolve the process
to regional government, e.g. Italy, or ethnic groups, e.g. Aboriginal, have to share the
considerable costs of infrastructure and development among a smaller number of
healthcare organisations (giving higher unit costs). If the surveyor workforce is voluntary,
this may also mean having a smaller choice of surveyors (giving more potential for
conflict of interest). However, there are options such as contracting or employing a
smaller paid surveyor workforce or contracting surveyors from other countries for
surveys.

18
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Other options for enhancing the opportunities for smaller programmes include:
Sharing a programme with a neighbouring region or state which has similar culture
and language
Designing one national programme, rather than several regional ones
Providing national standards, guidelines or tools for regional agencies or
designated assessment organisations
Using a single organisation to provide multiple accreditation programmes
Using the same organisation or agency as a centre for research and development
of other quality methods, e.g. performance indicators, clinical guidelines, patient
surveys, technological assessment
Obtaining accreditation services from another region or state.
2.3 Establishing the role of government
The development of an external evaluation programme may be part of broader health
reforms, or part of an overall governmental strategy for quality improvement and a
transition from a centralised system to one which is more open and independent. It may
be necessary for the health ministry to re-define its own duties and responsibilities in the
context of a reformed organisational structure of the health system.
The relationships between departments of government which have a major impact on
quality may be unclear. The roles of agencies responsible for such areas as public health,
blood products, pharmaceuticals or medical devices and inspectorates responsible for
such aspects as control of the environment, safety, radiation at national or local level
need to be clarified as part of the overall quality plan. Dissemination of this structure and
plan would also provide an opportunity to develop a strategy for active communication of
the aims and operation of an integrated quality system.
Government controlled or not?
Specific to external evaluation is the question of whether the programme should
be organised and administered directly and solely within the ministry of health, like
licensing, or by an independent body totally unconnected to government, or by something
between these two extremes – which has become more common. The legitimate and
necessary role of government is the licensing of healthcare facilities, using basic safety
standards or criteria. Licensing of individual medical practitioners may be a government
function but is usually carried out by a medical council. However, there are challenges
for governmental external evaluation programmes which include:
Inconsistent policy and management with changes in government
Reviewing and updating standards consistently and in a timely way
Public perception of government that is too low to make them credible assessors of
healthcare
Conflict of interest between government roles as purchaser, regulator and insurer,
and lack of independence and continuity
Delegation of powers to local areas, which may result in multiple government
programmes duplicating development and ongoing costs of running the
programmes.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
19
Some countries, such as France and Saudi Arabia, have made participation in
accreditation by healthcare organisations legally compulsory, but most countries
merely authorise the functions of the external evaluation organisation. Two-thirds of
accreditation organisations surveyed in 2010 were supported by enabling legislation.
However, many independent programmes thrive without it. Five accreditation
organisations were struggling or inactive, despite being supported by a published
government strategy. If enabling legislation is not essential and national strategies often
change with ministers and governments, external evaluation organisations must choose
reliable partners for survival
15
.
Need for government support
To be successful, external evaluation programmes often need government support and
collaboration and to be recognised as an important part of the national health quality
strategy. The support may be through funding, providing incentives for participants
such as limiting other forms of inspection or audit, or recognising the programmes as a
legitimate and essential part of the overall health quality strategy.
Some functions, such as the definition of standards, the assessment of compliance
and the grading of awards may be totally independent or may be shared between
government and independent external evaluation organisations. Some governments,
for example, New Zealand, have developed or approved standards that they require
healthcare organisations to meet. However, the government have devolved the process
of assessment of compliance with the standards and follow-up to ensure the standards
are being maintained to independent designated auditing, accreditation or certification
organisations. These organisations in turn need to be internationally recognised by a
3rd party accreditor such as ISQua. In Australia, a similar system operates through
the Australian Commission on Safety and Quality in Health Care which has developed
national quality and safety standards. The accreditation of healthcare organisations who
meet the national quality and safety standards has been devolved.
The mandatory requirement for external evaluation, as in the above examples, is an
increasing trend as governments seek to improve the quality and safety of health
services.
Key roles which governments might play in supporting external evaluation include:
Enabling the external evaluation process, e.g. through policy decisions such as by
reciprocal recognition of assessments; joint development of standards; avoiding
conflict such as perverse incentives and competing mechanisms for assessment
Providing leverage, e.g. by according preference to accredited or certified facilities,
services or networks such as reimbursement tariffs and payment procedures
Using accreditation or certification as a criterion in its own purchasing decisions,
e.g. in defining preferred providers and contract monitoring
Regulating individuals and institutions, e.g. by ensuring consistency and distinction
between licensing and accreditation
Acknowledging or endorsing accreditation or certification programmes against
defined criteria to maintain standards, avoid duplication and potential exploitation
Providing financial support in establishing programmes and / or contributing to the
funding of programmes’ continuing development.
20
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
The extent of government support and involvement in the external evaluation programme
may also depend on the country’s overall stage of development. In developing countries,
where there may be a more limited health industry or where professional organisations
may not have the resources or financial capacity to initiate an external evaluation
programme, government organisations may be needed to establish such programmes.
For example, in Kenya, the National Health Insurance Fund (the insurer) manages
accreditation; their standards, known as the Kenya Quality Model, were developed by
a broad coalition of professionals outside of the Insurance Fund and are supported by
the Ministry of Health. In Ghana in West Africa, the National Health Insurance Scheme
originally placed responsibility for accreditation within government; that task is now
being transferred to an independent body
20
.
The case studies highlight the nature of the relationships between external evaluation
agencies and governments in different jurisdictions.
Case Studies – Role of the government
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
IKAS and the Danish accreditation programme (DDKM) were established by an
agreement between the regional and local political authorities, who are responsible
for delivering healthcare, and the national government that sets the overarching
political priorities, including the economic frame, and is the healthcare legislator
and regulator. The first step in the development of DDKM was the development of a
cooperation agreement between the government and the regions of a joint model for
quality assessment which included provisions for the funding for DDKM. IKAS is a
formal independent organisation but the government provides part of the funding for
IKAS.
Health Care Accreditation Council (HCAC)
Country: Jordan
The HCAC is a private, not-for-profit shareholding company registered under the
Ministry of Trade and Industry.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
The Safety Act required that designated audit agencies (DAAs) who monitor
compliance with health and disability standards for hospitals, rest homes and
residential disability services are approved by the Director General of Health for
the purpose of auditing these services to those standards. HDANZ is a private,
independently owned company. It is linked to government as a Ministry of Health
(MOH) approved designated auditing agency and for these services HDANZ submits
the audit report to the MoH who issues the certificate. HDANZ was designated as an
approved designated auditing agency in October 2002.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
21
2.4 Determining incentives
If the external evaluation programme is not mandatory, evidence suggests that incentives
are useful to promote and sustain it. Possible incentives for healthcare organisations to
participate in an external evaluation programme include:
Organisational development: self-assessment, team-building, benchmarking,
guided pathways
Increased public funding such as health insurance fund payments moderated
by accreditation or certification status, additional government subsidy, e.g.
per accredited or certified bed, or some other linkage to core funding or
reimbursement
Effective exchange of data between external evaluation programmes and insurance
programmes to inform their purchasing decisions and payments
Preference from private insurers: insurers prefer to deal with facilities or services
whose clinical and management processes have been independently verified; they
also make reimbursement simpler and faster for such organisations
Market advantage: public recognition brings status and advantage in a competitive
market which can attract patients / clients, staff and income
Reduction of liability insurance costs: premiums reflect reduced risk rating
Exemptions from regulatory inspection: e.g. the state issues a licence to an
accredited or certified facility on the basis that accreditation or certification
standards include and exceed licensing standards (“deemed status”); this may be a
condition of receiving public funding
Linkage to training posts: status conditional on accreditation or certification
National quality competitions: for example, making accreditation or certification
status one of the judging criteria.
Healthcare organisations may be discouraged from participating in an external
evaluation programme by:
The cost in terms of time, management, and money
Fears about the outcome - sanctions for shortcomings, loss of staff morale if
denied the award of accreditation or certification, misuse of performance data, and
of gaining the award and then losing it when standards get more demanding
Lack of recognition for the resources invested
Lack of information about the benefits
Resistance from healthcare professionals and other staff and the failure to recruit
clinical and other staff champions
The difficulties of effecting culture change without external support and
Failure to recognise and celebrate the achievements of participating organisations.
Consideration also needs to be given at this time to the issue of consequences when
organisations do not achieve or meet the accreditation or certification standards to
the acceptable level. What are the consequences, if any, for these organisations? For
example, do the consequences include financial sanctions?
The case studies provide examples of some of the incentives put in place for external
evaluation programmes.
22
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Case Studies – Incentives for external evaluation programmes
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
DDKM (Danish accreditation programme) is not required by any legislation, but is
based on agreements as follows:
Public hospitals: all hospitals participate by agreement between National and
Regional governments
Private hospitals: voluntary, but participation is a prerequisite to obtain a contract
to treat patients for the regions (also required by some insurance companies)
Pharmacies: voluntary, financial incentive in place
Municipalities (primary care services, including long-term care): voluntary, no
incentives in place
Ambulance operators: prerequisite to obtain contract with Regions
General practitioners: mandatory (with some minor exceptions) by agreement
between the Regions and the Organisation of General Practitioners in Denmark;
financial compensation as part of the agreement.
Health Care Accreditation Council (HCAC)
Country: Jordan
Accreditation is voluntary. There are no incentives (laws, regulation, insurance
requirements) in the country for accreditation.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
The Safety Act 2002 introduced health and disability standards for hospitals, rest
homes and residential disability services aimed at improving safety levels and quality
of care that became mandatory from 01 October 2004. Under the Safety Act 2002,
service providers such as hospitals, rest homes and residential disability service
providers must be certified. From September 2005, physiotherapy services were
required to be certified if they wished to provide services under the New Zealand
Accident Compensation Scheme (ACC) physiotherapy services contract. From
September 2012, health funders made certification mandatory for home support
providers and from March 2013, a health insurance provider Southern Cross Health
Society made certification mandatory for their affiliated providers.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
23
Practice Incentive Program (PIP)
Country: Australia
The Australian Government introduced the Practice Incentive Program (PIP) in 1998.
The PIP is aimed at supporting general practice activities that encourage continuing
improvements and quality care, enhance capacity and improve access and health
outcomes for patients
21
.
In the 2015-16 Australian Government Budget, in excess of $1.5bn over four years
22
was allocated to the PIP to support the continuation of incentive payments to general
practices.
The PIP is used as a lever by government to influence behavioural change within
the general practice environment. To access payments under the PIP, practices
must meet the eligibility requirements, including that a practice must be accredited
or registered for accreditation against the Royal Australian College of General
Practitioners (RACGP) Standards for general practices and must maintain full
accreditation.
Approximately 80% of all practices that meet the RACGP definition of a general
practice participate in accreditation and, therefore, may access PIP payments.
There are three types of payments available under the PIP
21
:
1. Practice Payments
The majority of payments through the PIP are made to practices and focus on those
aspects of general practice that contribute to quality care. These payments are
intended to support the practice to purchase new equipment, upgrade facilities or
increase remuneration for GPs working at the practice.
2. Service Incentive Payments
Service Incentive Payments (SIPs) are generally made to GPs to recognise and
encourage the provision of specified services to individual patients. The Cervical
Screening, Asthma and Diabetes incentives have service incentive payment
components, and the Aged Care Access Incentive is a service incentive payment
only.
3. Rural Loading Payments
Practices participating in the PIP, with a main practice location situated outside
capital cities and other major metropolitan centres, are automatically paid a rural
loading.
There are ten individual incentives available to general practices and GPs under the
PIP
23
: (See Appendix 1d for further information)
Since the inception of the PIP in 1998, successive Australian Governments have
committed to ongoing funding for the program; and during this time, have retained
the requirement that a practice must be accredited, or registered for accreditation,
and must maintain full accreditation in order to access such payments.
Given the level of participation in accreditation by Australian general practices, it can
be assumed that the highly incentivised PIP has been instrumental in encouraging
practices to engage in the process, and in turn has had a positive impact by
supporting practices to focus on improvements and quality outcomes.

24
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
2.5 Developing relationships with stakeholders
Another key exercise at this stage is to identify or map out the other main stakeholders
in the quality and safety arena in the country or region; their role; and their link to the
external evaluation programme. This may be different for each country or region and
this exercise will help to establish what external influences for the programme will look
like and what the nature of the relationship with the other stakeholders should be. For
instance, if the external evaluation organisation does not itself manage related functions
at a national or regional level, then it needs to define communications and relationships
with other departments and agencies to harmonise the setting and assessment of
healthcare standards, to avoid waste and conflict between systems, and to minimise the
“burden of audit” on healthcare organisations. A new organisation should seek where
possible to integrate and build upon existing systems of standards and inspections.
For example, by establishing a process to recognise existing ISO or mandated audits.
In addition, there are a number of organisations internationally who define and assess
standards, and with whom they could usefully collaborate, ISQua being one.
Key stakeholders with whom the external evaluation organisation may consider
developing relationships with include the following:
Consumer groups
Representatives of a recognised consumers’ council or association should be involved in
the creation and support of the proposed external evaluation organisation as a means of
making health services more transparent and accessible to the public. They should help
define what standards and services the public should expect from healthcare providers,
and develop and promote reliable and consistent methods for measuring them. They may
assist with developing a consumer code of rights. Consumer and patient representatives
may also be part of the advisory committee of the external evaluation organisation and
later sit on the governance board.
Regulatory inspectorates and other external agencies
These might include statutory bodies with responsibility for areas such as fire safety,
radiation, medical device safety, hygiene and health data collection agencies. The
relationship between the country’s or region’s ISO accreditation organisation and the
health service accreditation or certification organisation needs to be explored and
defined. Relationships also need to be built with the assessment organisation that
certifies laboratories, x-ray departments or other technical services and organisations to
relevant ISO standards, to understand each other’s needs and requirements and possibly
coordinate activities and assessments.
Key relevant legislative requirements such as for buildings, health and safety in
employment, equal opportunities, consumer rights or waste management can be more
specifically referenced in the external evaluation organisation’s standards in consultation
with the relevant agencies responsible. Specific technical standards or regulatory
requirements relating to safety such as infection control, fire safety, equipment safety
and emergency preparedness can be integrated into the standards as criteria and
assessed as part of the survey or assessment visit.
Most accreditation or certification organisations assume that statutory inspections are
carried out as intended, and expect to examine safety certificates, such as for radiation
protection as part of their own surveys, but in some countries the statutory radiation
protection agency does not have the resources to carry out its own inspections and may
turn to the accreditation or certification organisation to provide its own expertise.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
25
A process needs to be developed to determine which alternative evaluations are robust
enough to be accepted as proof of compliance.
Public and community health bodies
Links between these bodies and the external evaluation organisation would give an
opportunity to share data to describe the impact on population and community health and
on the performance of providers and the healthcare delivery system. Where countries
currently employ inspectors to regulate healthcare facilities, the inspectors’ role could be
modified to include assisting local facilities to prepare for external evaluation surveys by
the organisation when it is established, and to monitor the implementation of the ensuing
recommendations for improvement. This would require initial and continuing education
programmes.
Technical agencies
Relationships with agencies for aspects such as health technology assessment, clinical
guidelines, clinical pathways and patient / consumer safety are useful, especially to
enable consultation and advice on the development of appropriate evidence-based
standards and for keeping information and communications current.
Professional bodies
Independent bodies such as medical academies or councils will offer wisdom and
advice to the organisation and be recognised for that purpose. Other bodies responsible
for such duties as supervising training or licensing or registering clinicians (doctors,
nurses, dentists, pharmacists, allied health professionals) will contribute to the setting of
standards and to their local assessment.
In particular, the role of professional chambers, associations and colleges needs to be
defined with respect to:
Professional regulation
Setting and monitoring of clinical performance standards
Monitoring of clinical practice according to these standards
Development and dissemination of quality improvement methods.
The functions of statutory bodies should be defined in relation to voluntary associations
and to the external evaluation organisation. The organisation should work with local
government ministries, insurance funds and professional associations and chambers to
develop consistent incentives for measurable achievement of agreed national standards
of process and outcome in primary, ambulatory and hospital care.
Health insurance funds
Using contracted service providers offers an alternative to the traditional centralised
model in healthcare management. In several countries, laws on healthcare insurance
specify that only accredited organisations, from either the public or private sector, have
the right to sign contracts to provide services under compulsory insurance. The external
evaluation organisation can work with health insurance funds to help them obtain and
protect best value from available funding by recognising accreditation or certification for
its impact on quality improvement.

26
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
External assistance
A further group of stakeholders with whom an external evaluation organisation may
interact would be individuals, organisations or groups providing external assistance.
External assistance is available from a number of sources including:
International external evaluation businesses or initiatives
International aid organisations and technical corporations
International experts
Neighbouring external evaluation organisations
ISQua.
Assistance may be for any part or all of the components of an external evaluation
programme. Before engaging formal external assistance, it is important that:
The project specifications have been scoped out and are appropriate
Competency criteria for selection of external assistance include relevant
experience with health or social care standards based external evaluation
References and advice are sought from experienced accreditation or similar
organisations and ISQua.
Most accreditation organisations have based their standards on existing research,
clinical practice guidelines, input from experts and other accreditation and technical
standards. New organisations can, in consultation with the owners of these standards,
choose a model that best reflects their purpose, scope and cultural context, and then
adapt those standards or build on them to make them appropriate to the local context.
It is important that the standards adhere to the ISQua Guidelines and Principles for the
Development of Health and Social Care Standards
4
as these are accepted as best practice
by organisations and so that they can become internationally accredited (See Chapter 4
for more information).
ISQua’s Guidelines and Standards for External Evaluation Organisations
3
and for
Surveyor Training Standards Programme
5
provide guidance on what structures, systems,
processes and evaluation methods need to be in place to be a best practice organisation.
When organisations seek ISQua accreditation, they get assistance with their self-
assessment and they can have a mock survey prior to an international accreditation
survey.
Information specific to healthcare external evaluation is widely available - see web links
in the bibliography section.
The next chapter will focus on the initial steps involved in setting up an external
evaluation organisation including how to involve and engage with other stakeholders as
part of this process.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
27
Chapter 3: Setting up
the External Evaluation
Organisation
This chapter focuses on the process of establishing an external evaluation organisation and
the different stages in this process. This process may be different for each country or region
depending on government policy, the stakeholders involved and the size of the health or social
care sector. The case study examples outline the approaches adopted in different countries.
3.1 Establishing a preliminary board or advisory committee
The impetus for setting up an accreditation or certification organisation may come from
a number of possible stakeholders: Ministry of Health, health professional associations,
consumer organisations, private insurers, university departments, voluntary membership
societies, health service charities or aid organisations. The initiative may come from
a company or group of individuals who see a market opportunity, e.g. as assessors
of government standards. If the purpose of the programme is clear, it is not difficult
to identify whom it will serve and whom it will affect. Traditional, profession-driven
programmes have tended to build links with regulators and consumers, thus becoming
more accountable and transparent. More recent programmes have been more influenced
by commercial providers and insurers or actively supported by government.
One way of involving relevant stakeholders who have or will have an interest in the
success of the new organisation is through setting up a preliminary board or an advisory
committee to establish the organisation. This enables them to feel they have a stake in
the organisation and its work and to provide advice and expertise.
The preliminary board or advisory committee will provide guidance and direction on the
practical aspects of establishing the external evaluation programme including:
Clarifying the role of the external evaluation programme in the context of other
departments and agencies working in the quality and safety arena in the country or
jurisdiction e.g. other external evaluation programmes
Funding of the external evaluation programme
Governance framework for the external evaluation organisation
The use of external assistance for development and delivery of the external
evaluation programme.
The composition of the interim board or advisory committee will be unique for each
country depending on government policy and the range of stakeholders working in
the quality and safety arena. Some members from this board or committee may form
the basis for the governance board in the established organisation. Table 3 outlines
suggested members of a preliminary board or advisory committee.

28
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Table 3: Potential composition of a preliminary board or advisory committee
Stakeholder
Group
Examples of representatives
Government Ministry of Health and / or other related departments e.g. Finance.
Local government e.g. municipality, canton, oblast level
Consumer
groups
Recognised national consumer council / association or advocacy
organisation
External
evaluation
organisations
Regulatory and other external evaluation agencies working in the
quality and safety arena in the country or jurisdiction e.g. statutory
bodies with responsibility for areas such as health and safety,
radiation, medical devices, medicines, regulatory inspectorates,
certification agencies
Service
providers
Public and private providers in country or region e.g. national
representative bodies such as national hospital association or
national disability service providers association / forum
Professional
bodies
Independent bodies with responsibility for the licensing or
registration of health and social care professionals or the
supervision of training such as medical academies or councils
Academia Universities or colleges who deliver education and training
programmes for health and social care professionals
Technical
agencies
National agencies with a specific role e.g. health technology
assessments, clinical guidelines and pathways, patient / consumer
safety
Independent Independent experts, neighbouring external evaluation
organisations, international external evaluation initiatives
3.2 Proposing a governance board and framework
One of the first tasks for the interim board will be to develop a draft governance
framework for the external evaluation organisation or programme, with a formal
constitution, governance board and draft policies and procedures. For credibility and
in line with best practice, a commitment should be made that the organisation will be
established in line with the ISQua Guidelines and Standards for External Evaluation
Organisations
3
(currently 4th edition, 2014, but note that these are updated on a regular
basis and the latest ones should always be obtained).
3.2.1 Governance body
If it is to be a non-governmental organisation, it is preferable for the organisation to have
a board comprising and accountable to the various stakeholder organisations rather
than the government. The board should represent professional, public and governmental
interests and bring personal qualities to the governance of the organisation, such as
finance, legal and public relations, but be dominated by none of them. For example, in
Malaysia accreditation programmes are delivered by the Malaysian Society for Quality in
Health (MSQH), which was established by the Malaysian Ministry of Health in association
with the Private Hospital Association and the Malaysian Medical Association. All three
organisations are represented on the board of MSQH
24
.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
29
Typically, independent boards include consumers; representatives of professional
associations such as nurses, managers and doctors; industry associations such as
hospitals or rest homes; funding agencies; and statutory bodies. Some boards are
now appointed according to skillsets, expertise and experience rather than chosen by
representative stakeholder organisations because of the perceived conflicts of interest
the representative members may have, being the provider, consumer and sometimes
also purchaser of the external evaluation. Government representatives in particular may
have a perceived conflict of interest.
Public involvement goes beyond the sharing of information; it also demands the sharing
of authority. Many external evaluation organisations have representatives of patients and
the public in their governance structure to ensure their involvement in the development
of policy and standards and in ensuring that agreed procedures are followed throughout
the external evaluation process.
As per good governance practice, members of the governing body must be oriented to
their roles and have ongoing information and education to assist them in their role. They
should be guided by a set of governance policies.
Case Studies – Composition of governing board
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
IKAS and DDKM were established by an agreement between the regional and local
political authorities, who are responsible for delivering healthcare, and the national
government that sets the overarching political priorities, including the economic
frame, and is the healthcare legislator and regulator. The government is represented
on the board of IKAS; the Chair of the Board is a government representative, a
Director of the Danish Health and Medicines agency.
Health Care Accreditation Council (HCAC)
Country: Jordan
The board of directors is made up of representatives for all healthcare sectors in
Jordan, medical and nursing professions, and education.
3.2.2 Governance framework
The external evaluation organisation needs to be set up as a legal entity, or a part of one,
with clear legal responsibilities for all its external evaluation activities. If it is part of a
Ministry or government agency, this independence is particularly important.
The organisation’s governance arrangements need to be clearly described in a deed,
constitution or similar document that defines powers, accountability and responsibility
including:
The composition of the governing body
The process for appointing its members
Lines of accountability including lines of accountability out of the legal entity
The terms of reference of the governing body and any of its committees
Responsibility and rules for making decisions such as on accreditation or
certification awards.

30
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
The organisation requires a clear vision and mission or purpose and strategic direction
to provide the basis for the organisation’s planning and direction and must be guided by a
defined set of values which are reflected in all services and activities. It is also important
that the organisation has an explicit set of ethical principles to inform all decision-
making and a code of conduct outlining the expected behaviours of those working in and
/ or on behalf of the organisation. Other responsibilities for overseeing, monitoring and
approval also need to be defined
3
.
3.2.3 Committing to fairness and transparency
External evaluation organisations which have succeeded in making improvements
in client healthcare organisations have generally done so by stimulating internal
motivation and commitment to self-assessment and change. This requires a culture
of transparency and acceptance of personal and organisational responsibility among
management, clinicians and other staff. However such a culture is not universal,
especially in hierarchical systems. External evaluation organisations cannot rely on
health professionals’ ethics and self-regulation to ensure an open and fair culture that
promotes quality improvement. The commitment to fairness and transparency must be
built into the governance framework and the ways of leading the organisation.
In setting up the new external evaluation organisation, a commitment must be made that
it will:
Use transparent and objective systems, decision-making and reporting
Be free from undue influence by any party
Avoid conflicts of interest
Establish a fair complaints and appeals system
Design and publish procedures for contracting, facilitation, assessment, reporting
and accreditation or certification decisions to promote confidence and
Put arrangements in place that ensure that external evaluation activities are
strictly separated from consultancy.
This commitment should be defined in policies, including one requiring accreditation or
certification decisions to be made solely based on the relevant standards, the findings
of the surveyors / assessors and other objective evidence related to the standards. A
growing trend is for decisions on accreditation status to be made based on a formulaic,
mathematically oriented approach, which avoids any perception of bias
3
.
3.3 Funding of the programme
Most new external evaluation organisations require at least two years to establish their
organisation and / or programme, longer before they are sustainable, and longer still
before they are self-financing. In short, political and financial support generally needs to
be consistent beyond the term in office of most health ministers and many governments.
External funding from government, health insurers, aid organisations or other partners
will be required for:
Establishment of the external evaluation organisation
Initial development and testing of the standards
Marketing
Possibly subsidising the running of the organisation for the first few years or a year
after break-even.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
31
However, the initial set-up costs may be much less for external evaluation organisations
whose role is to accredit or certify health or social care organisations against
government-mandated standards or similar. In this situation, there is an identified
potential client pool, there will be guaranteed payment of costs of the assessment by
either the clients or the government and there may be a shorter time period in which
clients are required to be assessed (See the example from HDANZ in the Case Studies
section).
For most other organisations the number of potential client health or social care
organisations will be a key determinant of programme costs, as will other factors such
as whether the programme:
Is a single national programme, regional or sector specific
Is limited initially to a priority focus, e.g. nursing homes, or to the entire health
system
Is supplementing or replacing existing external assessments
Is development focused, requiring training and education of clients
Develops its own standards
Employs specialist expertise.
One of the major potential costs for an external evaluation organisation will be the
surveyor workforce and in particular whether they are paid or voluntary. Traditionally,
accreditation organisations have relied upon participating accredited institutions to
provide or loan staff to work as surveyors and to promote the concept of peer review.
Certification agencies usually employ or contract their assessment personnel on a
paid basis, sometimes supplemented by technical experts. However, accreditation
organisations are now also increasingly paying surveyors as employed or contracted
personnel, or using a mix of both paid and voluntary. The organisation would need to
consider factors such as the availability of suitable personnel in the country to act as
surveyors; the feasibility of suitable personnel being released by their organisations to
work as surveyors; and the number of and costs of employing full or part-time surveyors
when deciding on which approach to take.
Thorough system design and testing will be another cost, as will the investment in
communications, information management and marketing.
Although a sustainable external evaluation organisation and its programme are
constantly under development, the start-up costs may last 3-5 years before a tested and
valued product is sufficiently marketable to begin to recover operational costs from client
organisations. Whether they choose to participate, or whether they can afford to, depends
on the incentives and sanctions provided and existing operating budgets.
During the first year, the organisation may manage with a small core staff, several
working groups and low overheads; however costs increase rapidly with the addition
of, surveyor training, document production and the direct costs of field testing. In some
countries external expertise is required and must be factored in to the start-up costs. At
the next stage, when the initial development is completed and the organisation is ready
to offer accreditation or certification, it may face another challenge; the faster the rate
of uptake, the faster it must invest to build capacity. Funding should be profiled to reflect
this growth.
At the same time as obtaining funding, incentives need to be negotiated if possible.
The case studies outline the experiences of external evaluation agencies in different
countries in terms of funding arrangements.
32
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Case Studies – Funding
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
Set-up costs
When IKAS was being established, a decision was made to seek external assistance
to help with the establishment of the organisation and the development of the
accreditation programme. A request for tender was issued to international
accrediting organisations to provide consultancy services for the establishment
of IKAS and the development of DDKM. The United Kingdom based international
accreditation organisation CHKS was awarded the contract to assist with the
establishment of IKAS as an accreditation organisation; the development of
standards; and the training of surveyors.
Funding of the accreditation scheme
IKAS is an independent organisation but receives an index-linked annual grant from
the central government, regions and local government. Public clients such as public
hospitals or pharmacies do not have to pay any fees to participate in DDKM. Other
private clients pay a fee that covers direct expenses plus an overhead.
Health Care Accreditation Council (HCAC)
Country: Jordan
Initial funding
The original funding to develop the HCAC came through the Jordan Healthcare
Accreditation project funded by the United States Agency for International
Development (USAID) and grants. The HCAC is a private, not-for-profit shareholding
company registered under the Ministry of Trade and Industry. Since March 2013,
HCAC has been financially sustainable through charging fees for services offered
including surveys, education and consultation.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
HDANZ is a private, independently owned company. It is linked to government as a
Ministry of Health (MOH) approved designated auditing agency. HDANZ audits these
services on behalf of the MOH and submits audit reports to the MOH who then issues
the certificates to the services.
Service providers pay fees to HDANZ for survey and monitoring visits. Certification
has been mandatory for the MOH Safety Act since October 2002. From September
2005, it became mandatory for physiotherapy services if they wanted to provide
services under the New Zealand Accident Compensation Scheme (ACC)
physiotherapy services contract. From September 2012, health funders made
certification mandatory for home support providers and from March 2013, a health
insurance provider Southern Cross Health Society made certification mandatory for
their affiliated providers.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
33
3.4 Setting up strategic, operational and financial management
systems
Once the governance board has been established and the governance framework has
been developed, the next step is to staff the external evaluation organisation and to
develop the management systems.
3.4.1 Staffing the organisation
The most important task of any board is to appoint the chief executive, with the
appropriate skills and experience for the role. The governing board may delegate
accountability, authority and responsibility for managing the external evaluation
organisation to a chief executive. The responsibilities for managing the organisation, the
level of authority and the chief executive’s relationship and accountability to the board
need to be defined in a job description or similar document. It is also the board’s role to
confirm strategic and operational plans, to receive regular reports on achievement of
goals and targets and to review the chief executive’s performance annually against set
performance targets
3
.
After the chief executive has been employed, personnel need to be selected, trained
and paid, including employed staff, seconded staff, e.g. surveyors, and sub-contractors
e.g. legal, statistical, marketing, communications. Sometimes financial and information
technology staff are contracted.
In larger organisations, staff may be structured into functional units such as:
Survey planning and management
Surveyor recruitment and development
Standards research, development and revision
User education and development
Technical support staff – financial, human resources, information management
Administration.
Smaller organisations can be sustained on very few core staff if they have significant
support from unpaid experts and staff seconded from employment in health and social
care services. Staffing numbers and skill levels need to be planned and transparent
policies developed for recruitment, selection and appointment; orientation; health and
safety; ongoing training; and regular performance assessment. Personnel records with
defined content need to be established for all staff.
It is important that the lines of responsibility within the external evaluation organisation
are clearly defined; made known to all staff; and that there are processes in place to
ensure that staff and surveyors are free from influence by those who have a direct
interest in the services and accreditation / certification decisions. The lines of authority,
responsibility and allocation of functions in the external evaluation organisation may be
outlined in an organisational chart or organogram. The lines of responsibility may be
outlined to staff as part of their orientation and updates provided whenever there is a
change of responsibilities.
A financial system needs to be set up to develop budgets and record and track income
and expenditure and past, current and projected financial positions. It needs to be able to
produce timely reports to assist staff to manage their budgets. Control and audit systems
will be needed to protect assets and ensure the transparency of financial transactions.

34
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
3.4.2 Developing the system for financial sustainability
Initial budgeting is challenging and depends on how much funding is received for
development or how much of the set-up costs need to be included in the budget.
Provision usually needs to be made for external assistance and expertise. Some
organisations consider guided facilitation and / or training on the survey standards
and process as an integral part of the development process. Others provide separate
consultancy (including general education and development) for which they charge a fee
which can be budgeted for.
If client healthcare organisations are required to pay on an event basis, ongoing costs
will depend on the length and depth of surveys (which are influenced by the standards),
length of the survey cycle, mid-term monitoring system, the efficiency of scheduling,
survey logistics, report handling and award adjudication. Budgets have to predict
when events such as training, on-site surveys, and mid-term surveillance visits will
occur and how much they will cost. Any postponement or cancellation can negatively
affect anticipated cash flow. Some organisations include all documentation and direct
survey costs, e.g. surveyor travel and accommodation, into a single-price package per
survey but costs and revenues are still dependent on the event occurring. A number of
accreditation organisations have moved to a membership or subscription based financial
system, whereby clients become members of the accreditation programme and are
charged a regular annual fee based on anticipated costs over the whole accreditation
cycle, including overheads, education, guidance, standards, tools, survey and mid-
term progress visits. While it still requires budget forecasting of the number and type
of clients, it limits the uncertainty of whether and when events will happen and has
contributed to the ongoing sustainability of a number of accreditation organisations.
A marketing programme and budget will be needed by most new external evaluation
organisations to publicise itself, the services it offers and the benefits of its programme
to attract healthcare providers. Getting a sustainable market share of client
organisations will be fundamental to its success. Wider marketing and publicity will be
needed for potential insurers, funders and the general public.
3.4.3 Establishing information systems
Information management covers both technological and paper based information,
including educational and marketing resources. Internal information systems are
essential for planning, operations and finance, but they also need to have the capacity
to collect, aggregate and compare data over time within and between participating
organisations, standards and surveyors, such as:
Data of compliance with achievement of individual criteria or standards
Profiles of participating organisations
Calculation of standard scores, function scores, and overall score for each
organisation
Aggregated results for comparison over time, function and place
Profiles of individual surveyors and their participation
Survey scheduling and management
Overall impact of programme.
Data which show that participating organisations have made improvements associated with
the programme since the first (baseline) contact are essential to demonstrate the value of the
programme to the healthcare system
3
.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
35
3.4.4 Addressing risk management and performance improvement
The external evaluation organisation must model the safety and quality approach
it expects from its client organisations. A robust risk management framework that
identifies and manages risks and promotes safety must be implemented. While most
of these organisations demonstrate a safety culture, it needs to be demonstrated by
establishing a quality improvement policy and framework. Essential to this will be the
documentation of policies and procedures for all functions, the development and use
of key quality indicators which can be monitored and benchmarked over time or with
similar organisations, the use of audits and reviews to ensure compliance with policies
and procedures, documented quality improvement projects and a transparent complaints
system that is available to staff, surveyors, clients and other stakeholders
3
.
3.4.5 Providing education services
Most external evaluation programmes provide a variety of education and training as
an essential component of their services. Education services need to be systematically
designed and implemented to meet quality standards and client needs. These include:
Induction and development of staff
Orientation and ongoing education of members of the governing board
Initial and continuing training of surveyors
General preparation of participating organisations and their staff as a basic
component of their participation
Specific methods of internal quality improvement required to meet external
evaluation standards, such as infection control, risk management, performance
measurement, patient / client surveys – these are usually additional to services
covered by fees and are charged separately
Quality improvement programmes for the health or social care sectors in general.
These training and education programmes and courses and their resources need to be
planned, scheduled and costed. Information provided needs to be kept up-to-date and
based on current research and evidence. Trainers and educators, whether internal or
external, need to have the competence and expertise to deliver the programmes.
3.5 Timeframes
The most commonly underestimated resource is the time needed to plan, design, build
and deliver a sustainable new external evaluation organisation. The pace at which this
can be done is limited largely by factors outside the control of the organisation, notably
by the prevailing culture and attitudes towards leadership, innovation, improvement,
team-working and transparency.
In practice the development stages, which may overlap, are:
Policy decision to develop an external evaluation organisation / programme and
defining its scope
Option appraisal on existing models and their adaptation
Setting up the organisation structure and obtaining of funding
Development and testing of standards
Development and testing of assessment methodologies
Surveyor selection and training

36
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Pilot testing, education and marketing campaigns
Revision of standards and methods based on feedback from piloting
First “live” surveys
First accreditation / certification recognition status decisions.
This process is likely to take at least two years but can take much longer (The case
studies outline the order of development and the timescales involved for the three
different agencies. Please refer to Appendices 1 a, b and c for further information.).
Taking time to establish communication with all stakeholders and the public and
continual updating of information as the organisation develops, is essential for success.
The following chapters focus on and provide more detail in relation to the development
and testing of standards; the development of assessment methodologies and
mechanisms for evaluating systems and performance.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
37
Chapter 4: Developing the
standards
This chapter focuses on the different elements required when developing standards. It includes
the use of quality dimensions and the importance of a reliable and valid measurement scale.
The standards used or developed by external evaluation organisations are the most fundamental
element of their programme. While not always realistic, it is advisable to consider what
evaluation methodology will be used while the standards are still in the development phase. The
standards will help to inform the public what to expect from health and social care providers and
will act as a benchmark against which providers and the government can measure quality. The
standards will form the framework for self-assessment and internal audits.
Standards development can often commence prior to the setting up of governance and
management systems in the external evaluation organisation and can take two or more years
to complete. Funders may want to know the shape and content of the standards before they
commit to funding the organisation. Separate funding is often available for the standards
development process.
4.1 The role of standards
An external evaluation organisation’s standards have to reflect its purpose and cover
the key functions and processes of the healthcare or social care sectors that are being
evaluated. Similarly, if standards are owned or mandated by government, they need to
reflect the purpose for which government intends them. They have to reflect legislative
requirements, safety and good practice. They should be relevant, understandable,
measurable, beneficial and achievable (RUMBA)
25
.
Standards also need to be realistic and reflect the availability of resources, especially in
developing countries where resource limitations can significantly impact a healthcare
organisation’s ability to achieve optimal performance. For example, Malaysia and
Thailand began with relatively achievable accreditation standards but committed to
continue updating and improving these over time. In this context, Malaysia has published
the 4th edition of their hospital standards since the accreditation programme began in
1999. Thailand has also made progressive changes, introducing a stepwise recognition
programme in 2004 and patient safety goals in 2006
20
. Standards can also be prioritised
and incremental improvements made in achieving them can be recognised and rewarded.
In India, the National Accreditation Board for Hospitals & Healthcare Providers (NABH)
has developed Pre-Accreditation Entry Level certification standards, in consultation with
various stakeholders in the country, whose aim is to introduce quality and accreditation
to healthcare organisations as their first step towards awareness and capacity building.
Once organisations have met the Pre-Accreditation Entry Level certification standards,
they can then prepare and move on to the next stage –Progressive Level and can then
work towards Full Accreditation status. This methodology provides a step by step phased
approach for healthcare organisations
26
.
The long-established accreditation organisations generally began with standards and
surveys which reflected management units, e.g. departments. They also tended to focus
on structures, e.g. staffing arrangements, funding, equipment or committees. Most
programmes now focus their standards and assessments on a client focused continuum
of care or patient’s journey rather than management units and on processes and
outcomes rather than structures.

38
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
However, for developing countries, basic structural standards may still be an important
starting point. External evaluation may be primarily a vehicle for taking stock and
developing greater equality of structure and access where the healthcare system
has wide regional and social divisions. In this case, the health system must be able
to mobilise resources in order to respond appropriately to the priorities which are
objectively demonstrated through the external evaluation process. For example,
participants from external evaluation organisations in low and middle-income
countries attending a 2013 workshop in Bangkok, Thailand highlighted that standards
are important in their countries to improve the overall quality of care and not just to
differentiate between hospitals that pass an accreditation visit and those that do not.
In many low and middle-income countries, institutions that fail to meet standards may
still be the only available source of care for parts of the population and therefore, it is
important that there is a focus on improving the care they do provide
20
.
4.2 Principles for standards
Standards are developed and written in many different ways and are designed to meet
the purpose and scope of the particular external evaluation programme, as discussed in
Chapter 2. However, they must be user-friendly, able to meet the purposes for which they
have been designed, and be able to measure achievement in a consistent way. Evidence-
based mechanisms by which standards are developed, promulgated, reinforced, audited
and evaluated are needed. Linking the writing of standards, including the wording,
structure, design, focus and content, to demonstrating improved outcomes requires
further investigation
27
.
ISQua has focused on addressing this gap by developing principles to guide the
development of health and social care standards and enable their assessment and
accreditation. These were originally developed in 2000, and revised on numerous
occasions. The most recent 4th edition was published in 2014
4
. The principles are based
on the Institute for Medicine (IOM) quality dimensions
28
, of effective quality performance,
efficient organisational performance, safety and patient focus. The ISQua Principles
(2014)
4
also give guidance on how to develop and measure standards. ISQua recommends
that the development and content of all standards should meet its internationally
accepted best practice principles.
The purpose of some external evaluation organisations is to assess, and sometimes
certify, health and social care organisations against government standards or the
standards of another external evaluation organisation, perhaps adapted to local
circumstances. For the credibility of its own assessments, these organisations should
encourage the owners of the standards to get them ISQua accredited.
The ISQua Principles cover all the functions of a healthcare or social care organisation,
from governance, to management, to client care, to quality. They are:
1. Standards Development: Standards are planned, formulated and evaluated through
a defined and rigorous process.
2. Standards Measurement: Standards enable consistent and transparent rating and
measurement of achievement.
3. Organisational Role, Planning and Performance: Standards assess the capacity and
efficiency of health and social care organisations.
4. Safety and Risk: Standards include measures to manage risk and to protect the
safety of patients / service users, staff and visitors.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
39
5. Patient / Service User Focus: The standards focus on patients / service users and
reflect the continuum of care.
6. Quality Performance: Standards require service providers to regularly monitor,
evaluate and improve the quality of services
4
.
Steps for developing standards in line with the ISQua Principles for Standards
4
include:
Reviewing other external evaluation organisation standards, current research
and evidence, recognised guidelines, recommendations from WHO and other
professional organisations and experts
Incorporating legislative, technical and safety requirements
Incorporating best practice where evidence is available
Ensuring the standards are client focused, cover the functions or systems of a
whole organisation or service, address the dimensions of quality, and support
quality improvement
Consulting stakeholder groups, including consumer groups
Involving stakeholders in standards development committees and working groups
Developing the rating system for measuring compliance with / against the
standards
Testing the standards and the way they are rated through self-assessment and pilot
surveys
Using feedback from testing to improve the standards and rating system
Developing guidelines to assist users to interpret and apply the standards
Ensuring the standards are approved by the external evaluation organisation
governing body
Applying for ISQua standards accreditation.
This development process may take two years or more if the standards are being fully
developed. With the rapidly changing healthcare environment, 12 months would be an
appropriate timeframe for organisations adapting other organisations’ standards.
4.3 Referencing to quality dimensions
Standards can be grouped around quality dimensions to demonstrate their relationship
to quality. The six quality dimensions as defined within the Institute of Medicine (IOM)
report Crossing the Quality Chasm, are the most commonly referenced
28
.
Safe S
Timely T
Efficient E
Equitable E
Effective E
Patient-centered P
By defining the dimensions of quality, organisations are able to ensure that their
inclusion can be justified but can also measure achievement in relation to those
dimensions, demonstrating that quality is not an optional extra but the essence of a good
and acceptable service. When standards are developed the criteria should address all of
the quality dimensions.

40
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Codes of patient / consumer rights have now been developed or adopted in many
countries. These are designed to protect an individual’s rights when they access
health or social care services and describe what their rights are when accessing such
services. In some jurisdictions, the codes of patient / consumer rights are specified in or
underpinned by legislation and service providers are required to have processes in place
to meet them. In such cases the codes of patient / consumer rights may be referenced
in the standards as this will provide a means of assessing how service providers are
meeting patient / consumer rights. In other countries, codes of patient / consumer
rights have been developed by organisations such as national consumer or advocacy
organisations and service providers may adopt them on a voluntary basis. Referencing
the codes of patient / consumer rights in standards is one way of helping to ensure that
standards are focused on the patient / consumer. This in turn will help service providers
to focus on delivering patient / consumer focused care that meets their needs and
protects their rights.
Mature accreditation organisations have now moved to designing their standards to
reflect the patient / consumer journey or pathway and then surveyors may, as part of the
survey process, trace or follow selected patients’ / consumers’ journeys to check at each
stage if the standards were met for that individual and their family.
Many sets of standards label some criteria as core or compulsory, usually based on
safety and risk. The core criteria are usually then required to be met or a defined ratio
of them met, e.g. 80%. These core criteria may be used for licensing or regulation
purposes.
4.4 Developing the measurement system
The rating scale should reflect the purpose of the standards, be transparent and enable
users to rate and measure standards, criteria or elements consistently. A yes / no scale
is good for determining compliance or non-compliance with a criterion or standard,
especially for measuring structural elements, so its use should reflect the nature of the
standards. It leaves less scope for recommendations for improvement where a criterion
is mainly met, but some elements are missing.
Likert-type rating scales are particularly suited for standards with a strong quality
improvement approach, e.g. 3, 5 or 7 point scales, often with descriptions for each point
or some of the points. These descriptions may relate to principles such as compliance,
consistency, evidence and implementation.
There is a tendency for assessors to favour a middle or neutral point, so an even point
scale such as a four point scale can give a clear cut-off point as to whether the criterion
is met or not but still provide a graduated measure of how well it is met or how badly it is
not met. The clearer the descriptors, the more consistent the assessments are likely to
be.
As well as a measurement system for rating each measureable criterion, element or
standard, a system is needed to determine if the standards are met overall which will
be the basis for awarding accreditation or certification where that is applicable. In a
study comparing the organisational attributes of accreditation programmes in low- and
middle-income countries with those in higher-income countries, it was found that the
low- and middle-income countries’ programmes were more likely to use a formulaic
mathematically oriented approach to make accreditation decisions
7
. Traditionally,
accreditation organisations relied on accreditation panels to make decisions but this
was not always a transparent process, the basis of the decision was not always clear, it
could be more prone to bias or external influence and was also likely to result in appeals
against the decision.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
41
Therefore, best practice is to determine overall achievement of standards based on a
formula which includes the level of achievement of or compliance with the measureable
elements of the standards, risk and other elements of the standards such as core criteria
or high priority criteria.
Some organisations measure only at the criterion level, so their overall decision will
be based on achievement of criteria while others use the overall ratings of the criteria
within each standard to rate achievement of the standard, so their overall decision will
be based on achievement of the standards. For example, the methodology could be that
all core or compulsory criteria must be met, or all criteria or standards must be met at a
defined level such as 3 or 4 on a 4 point scale, or no standards must be rated at below a
certain level.
Like the rest of the standards, the rating scale needs to be developed in consultation with
stakeholders and the satisfaction of users regularly assessed. As with the standards
themselves, the rating scale needs to be tested and piloted before use to ensure it is
reliable and can produce consistent and fair results.
The case study examples highlight the approaches to standards development adopted in
different countries.
Case Studies – Development of standards
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
Range of standards
IKAS has developed all standards used in its programmes. They were first developed
for hospitals and community pharmacies. Standards have since been developed
for primary care services, delivered by municipalities, and for ambulance services.
Currently standards are being developed for general practitioners and specialist
physicians. Over the coming years, all healthcare professions who operate outside of
hospitals in their own office or premises will be covered.
Development process
Standards were developed by theme groups (for related groups of standards) of
standard developers, consisting of senior professionals, appointed by the Regions
and the Association of Danish Pharmacies. IKAS and HQS / CHKS served as advisors
and secretariat for the groups.
Rating scale
Compliance with standards is assessed by scoring a number of elements (for the
hospital standards roughly 450) according to a four point scale (Fully / Largely /
Partially / Not Met), where the two upper levels indicate a satisfactory performance
(except for certain safety critical standards, where only Fully Met is considered
satisfactory). Any element not met to satisfaction will require follow up, and if
not corrected, results in accreditation with comments. An Accreditation Award
Panel decides, guided by certain rules, whether the nature and / or amount of the
comments preclude accreditation – if so, status as “not accredited” is awarded and
published.
42
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Health Care Accreditation Council (HCAC)
Country: Jordan
Range of standards
As the national accreditation agency of Jordan, HCAC sets standards for hospitals,
primary healthcare centres, family planning and reproductive health, transport
services (ambulances), cardiac care, and diabetes mellitus. HCAC surveys against the
standards and awards accreditation.
Development process
All the standards are developed in Jordan. No standards developed by other
organisations are used. Hospital standards were developed first, then standards for
primary care centres, family planning and reproductive health, transport services
(ambulances), cardiac care, and diabetes mellitus.
Rating scale
Standards are classified as critical, core and stretch.100% of critical standards must
be met; and a specified percentage of both core and stretch standards must be met in
order for a service to be accredited.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
HDANZ is a private, independently owned company. It is linked to government as a
Ministry of Health (MOH) approved designated auditing agency. HDANZ audits these
services on behalf of the MOH and submits audit reports to the MOH who then issues
the certificates to the services.
Service providers pay fees to HDANZ for survey and monitoring visits. Certification
has been mandatory for the MOH Safety Act since October 2002. From September
2005, it became mandatory for physiotherapy services if they wanted to provide
services under the New Zealand Accident Compensation Scheme (ACC)
physiotherapy services contract. From September 2012, health funders made
certification mandatory for home support providers and from March 2013, a health
insurance provider Southern Cross Health Society made certification mandatory for
their affiliated providers.
The rating scale for compliance against the health and disability sector standards is:
CI = Continuous improvement
FA = Fully attained
PA = Partially attained
UA = Unattained
The Ministry of Health uses the assessment ratings to determine certification. The
length of certification can vary from one to four years depending on the level of
achievement of the standards.
The next chapter outlines the factors to be considered in developing assessment
methodologies.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
43
Chapter 5: Developing
assessment methodologies
This chapter explores factors to be considered in the development of the assessment
methodology such as the selection, training and evaluation of surveyors; the development
of the survey management process; and the establishment of processes for determining the
accreditation or certification status.
A survey against standards can be achieved by either a desk top review or an on-site survey. Desk
top reviews may be suitable for some specialities such as diagnostic imaging or clinical pathways
such as stroke care. For organisations an on-site survey is recommended, which can be planned
or unannounced.
Surveyors are the main interface of the external evaluation organisation with its clients, and
the survey is the key event on which the clients will judge the organisation. It is essential that
surveyors and the survey and award processes are managed consistently, transparently and well.
5.1 Selection, training and evaluation of surveyors
Accreditation organisations generally use the term “surveyors” while certification
organisations usually use the terms “assessors” or “auditors” to describe the personnel
who visit, assess and draft reports. Regulatory bodies may use the term “inspectors”. They
are central to the credibility, objectivity and sustainability of the organisation. Accreditation
surveyors are generally regarded as peer reviewers – doctors, nurses, managers and
allied health professionals – who understand the work their peers do but their role is
to assess processes and systems rather than their peers’ performance. Auditors are
professional quality auditors, usually certified as such, who can audit or assess across
industries and do not need to be a healthcare professional peer. In this guide the term
“surveyor” is used to cover all assessment personnel and the term “survey” to cover all
external assessments.
Paid or voluntary?
As previously highlighted in Chapter 3 (See Section 3.3 Funding of the programme),
accreditation organisations have traditionally relied upon participating accredited
institutions to provide or loan staff to work as surveyors and to promote the concept
of peer review. This has the advantage of reducing survey costs, maintaining the
acceptability and independence of peer review, and sharing the experience and knowledge
of accreditation widely throughout the health system. However, it assumes that there
are personnel with enough experience who are able and willing to be seconded by their
employers to be trained as surveyors without creating a conflict of interest. To maintain
skill levels and currency with standards and systems, surveyors should be expected to
undertake a minimum number of working days (usually ten) a year. It can be a challenge
for them to get released from their full-time job for this amount of time.
Certification organisations usually employ or contract their assessment personnel on
a paid basis, supplemented by technical experts. However, as highlighted in Chapter 3
(See Section 3.3 Funding of the programme), accreditation organisations are now also
increasingly paying surveyors as employed or contracted personnel, or using a mix of both
paid and voluntary. Surveyors mostly come from a health background and have previously
been involved in accreditation programmes.
44
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
The advantages of having a more stable workforce of paid surveyors is their greater
availability, the reduced number of surveyors needed, reduced demand for recruitment
campaigns and new training programmes and more reliable and consistent performance
of the role because of the increased frequency of undertaking surveys and writing
reports.
Selecting and appointing
The first steps in developing a surveyor workforce are to:
Determine the number, skill mix and mix of paid / employed or voluntary surveyors
needed for the planned programme of work (the numbers will need to be increased
as more organisations join the programme)
Define the required competencies, including personal attributes, professional
qualifications and experience, knowledge and skill sets relevant to the programme.
The number of surveyors to be recruited should be estimated from the volume of
surveys planned, their duration (in terms of surveyor days), the number of days each
surveyor would provide per year, the number of surveyors withdrawing each year and
the paid / voluntary mix of surveyors. Their professional background, culture and skills
should reflect the function and scope of the programme. Recruitment may be done by
advertising in relevant publications, sending notices to all potential client organisations
and professional associations, and directly approaching likely candidates.
Surveyors should be appointed through a clearly stated and fairly applied process in
accordance with the defined competencies and the numbers determined. Competencies
could include:
Personal attributes, including the ability to communicate effectively and to work as
a team member
Professional qualifications and experience, usually at a senior level
Current healthcare or social care sector knowledge
Skills in the areas covered by the programme.
Whether surveyors are seconded (on their usual salary), or employed directly by the
external evaluation organisation, they must be committed to comply with the rules of that
organisation, particularly with respect to confidentiality and independence. If the external
evaluation organisation employs them directly, it may have to accept additional legal
responsibility and have to provide additional liability insurance.
Training to be a surveyor and undertaking the role is a form of professional development
and is recognised as such by many professional colleges and associations. Surveyors
become familiar with the standards and survey processes and are able to learn for their
own practice from what they observe in the organisations in which they survey. They in
turn become educators of the staff they survey, able to identify areas where they can
improve and best practice methods or tools they could use.
Training
After selection, surveyors will then need to be either employed or contracted, and
trained and oriented to the role. Training cannot begin until at least draft standards and
procedures are available. In established organisations, training is provided by existing
surveyors and staff; new organisations generally use expertise from other programmes,
at least for initial training.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
45
The initial training programme can be of one to five days duration and should cover topics
such as:
Standards’ interpretation
Survey process
Interviewing and observation skills
Documentation review
Specific areas, e.g. safety, infection control
Report writing techniques
Trainees then need to be evaluated to determine their suitability for the role. Mock
assessments are often included so that trainees can demonstrate their aptitude. They
then usually go on one or more survey visits as observers or trainees with a mentor to
accustom them to the role and further test their suitability. They need manuals and other
resources to assist them. Programmes are increasingly using technology on-site for the
recording of the assessment and use of this also needs to be part of the training.
The surveyor training programme of accreditation organisations in low- and middle-
income countries tend to be surveyor certification programmes and organisations
in developed countries are also moving in this direction. Such certification provides
a recognised status for the surveyor but may also provide the opportunity for more
rigorous evaluation of performance and ongoing training and development. Certification
programmes generally expect their auditors or assessors to be certified.
Ongoing development and evaluation
Surveyors must be provided with ongoing training and development opportunities,
and be evaluated regularly to ensure their ongoing competence. External evaluation
organisations need to define criteria for selecting, training, retraining and deselecting
surveyors. Some organisations have an independent committee to monitor inter-
rater reliability of the survey and rating performance of surveyors and / or satisfaction
surveys by an independent third party, as well as in-house survey team assessments.
It is common to ask client organisations to evaluate the standards, the survey, and the
performance of the surveyors after the external survey. These evaluations are most
useful if they relate to the individuals rather than just the team. All these reports, and
participation in continuing training, contribute to the systematic appraisal of each
surveyor.
Where there is a surveyor certification programme, surveyors must meet the annual
requirements to maintain their certification.
The ISQua Surveyor Training Programme Standards (2009)
5
provide guidance on setting
up these training programmes which can then be ISQua accredited. The ISQua Guidelines
and Standards for External Evaluation Organisations also contain a standard (Standard 6)
on surveyor management
3
.

46
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
5.2 Developing the survey management process
Contracting with the client organisation
There should be a defined process to ensure that participating organisations are aware
of their rights and responsibilities in relation to the external evaluation programme, and
that they understand the procedures and responsibilities of the programme. This usually
involves a standard contract or service agreement between the applicant healthcare or
social care organisation and the external evaluation programme.
Training and educational support are often provided by the programme for the staff of the
client organisation as an integral part of the preparatory process. This may include for
example: project manager training, standards interpretation, and internal assessment
and self-assessment training. Where self-assessment is a component of its programme,
the external evaluation organisation’s staff can guide the client as to how to undertake
and complete this. Self-assessment against the published standards develops insight
and commitment, and reduces the burden of external assessment because it helps
organisations to identify, understand and resolve their own problems. Many programmes
consider this internalisation to be a key factor in the rapidly increasing compliance with
standards which can be demonstrated in participating organisations in the months prior
to external survey. It is important to determine what is included within the programme
fees and what training / educational support is provided at an additional fee.
Many programmes provide facilitators, such as programme staff or trained surveyors,
to support client organisations to prepare on first entering the programme, and to feed
back to the programme any problems with systems or processes. This acknowledges that
the early external surveys are as much a test of the standards, surveyors and procedures
as they are of the organisation being visited. The facilitators should not be permitted to
take part in or influence the external survey. They can arrange training, participate as a
trainer, advise clients on interpretation of the standards or what needs to be in place to
meet the standards but they can only provide generic advice that is freely available in the
public domain. They must not give any advice on how things should be done or provide
any technical assistance such as preparing or producing documentation or procedures,
or giving client-specific advice, instructions or solutions. This would be regarded as
consultancy which must be strictly separated from external evaluation activities.
A pre-survey review or mock survey can also be a valuable part of preparation. It
identifies whether the client organisation is interpreting the standards correctly and
has appropriate documentation as evidence of how it meets different criteria as well as
indicating the client’s progress towards survey readiness. It also provides a good practice
run for staff so they know what to expect from the actual survey.
Planning and conducting the survey
Planning the scope of the survey, duration and the size of the survey team should be
transparent, based on the needs of the organisation and the policies of the external
evaluation body. The surveyor team for the external survey should include an appropriate
mix of skills and experience and avoid conflict(s) of interest. A more experienced team
leader is generally chosen to guide the process. Dates for the external survey are usually
set 6-12 months in advance to allow for self-assessment and preparation and possibly a
mock survey.
The standards must be incorporated into a tool in which surveyors can make findings,
ratings and recommendations for improvement. The self-assessment can be included in
the tool if this is part of the process. The tool may be on paper or loaded into a tablet or
similar technological device.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
47
Site visits may extend from half a day for one surveyor for a small rural primary care
clinic to two weeks for large teams for a healthcare network. Small hospitals or rest
homes often use two people for two days (four surveyor days); larger ones commonly use
three people for five days. Time for surveyor preparation, travel, team briefing and report
completion must be added to these “on-site” estimates. At the end of the visit, most
organisations provide time for the team to prepare a report back of findings which they
present at a meeting to the leadership of the client organisation and preferably also to
staff. This enables the client to correct any errors at the time and means there should be
no surprises when they receive the final report.
The efficiency of the survey visit and the transparency and consistency of the process
can be improved through the provision of tools and guidelines to assist the surveyors;
thorough preparation by the organisation being surveyed and the surveyors; the timely
submission of complete and accurate self-assessments and other pre-visit documents;
a realistic survey timetable; explicit sampling procedures; specified documents being
made readily available for review on site; and time management. Increasing the number
of surveyor days may not help, but will certainly increase the complexity and cost of the
visit.
Writing the report
The surveyors write a report of their findings and rating of achievement against the
standards either while still on-site at the end of the visit or afterwards. Doing this
electronically contributes to the speed with which the report can be submitted. New
external evaluation organisations should include the e-generation of the report as part of
their programme if possible. It is important that strict timelines are put on this process,
otherwise the surveyors can get back to their usual workplace and try to catch up on
that work before finishing the report. A delay at this stage leads to a delay in making the
award decision which is frustrating for the client. The report is submitted to the external
evaluation organisation which must have processes for editing and reviewing the reports
to ensure they are complete, accurate, balanced, constructive and consistent with the
intent of the standards.
Performance indicators
The external evaluation organisation should determine what indicators it requires its
client organisations to monitor. These should cover the different management, safety
and clinical functions of the healthcare organisation and may include things such as
complaints, patient / client satisfaction, staff satisfaction, staff turnover, financial ratios,
adverse events, accidents, clinical indicators such as falls and infections, and medication
errors. These demonstrate that the client organisation has the capacity to generate and
analyse performance data as part of an internal quality improvement programme and is
using the results to make improvements.
Sometimes the collection, analysis and publication of the results of indicator data is
part of the scope of the external evaluation organisation. In these cases, there must
be processes to ensure the indicators have standardised definitions and numerators
and denominators, that the data collected is clean, complete, accurate and timely. The
data can then provide comparable measures of achievement over time for a healthcare
organisation or between similar organisations in terms of processes and outcomes in
clinical, safety, financial or other areas
3
.

48
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
5.3 Establishing the accreditation / certification process
Responsibilities for accreditation / certification
The external evaluation organisation is responsible for setting the criteria for
determining accreditation or certification status, and the decision on whether or not to
grant accreditation status is made in accordance with the criteria on the basis of the
findings in the survey report. These criteria should ensure:
Transparency for organisations being accredited or certified, for surveyors and for
the public
Consideration for the clients of the service and their safety
Decisions based on the achievement of the standards
Consideration of how accreditation or certification status will facilitate further
quality improvement
Consistency between award decisions
A non-adversarial process for appeals.
Basis for recognition decisions
Earlier programmes based the recognition decision or accreditation status primarily
on the capacity for good clinical care, demonstrated by compliance with accreditation
standards, but the emphasis has now shifted towards overall performance. Newer
accreditation programmes, especially in developing and under-resourced countries, may
need, at least initially, to focus on and to reward the existence of basic infrastructure
and demonstrated progress towards, rather than absolute compliance with, the
published standards. Different programmes may have different priority concerns, e.g.
critical functional areas such as patient care, infection control, quality improvement or
management of the environment; patient safety goals such as patient identification, high
alert medications, wrong-site surgery or communication among caregivers; or areas of
difficulty such as information flow, patient records or medical equipment surveillance.
In case there is any dispute about the recognition decision, a transparent, independent
and clearly described appeals process is necessary.
Timeframe for recognition decisions
Having worked hard to prepare for the external survey, staff and management of
client organisations are eager to receive a timely decision from the external evaluation
organisation. Many programmes still aim to provide the majority of decisions within
two months of the survey, although those using electronic technology for reports and
formulaic criteria for decision making are able to make the decisions much quicker. As
the delay increases, the report and decision become increasingly irrelevant, staff become
demotivated and improvement is not sustained. The adjudication process must therefore
be transparent and thorough, but also timely.
Duration and maintenance of accreditation
Accreditation status is normally awarded for a period of between one and four years.
Sometimes there are different grades of achievement, e.g. conditional, or with
commendations, or exemplary. ISQua criteria now require monitoring by the external
evaluation organisation of the continued maintenance of standards and quality
improvements by accredited or certified organisations.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
49
Monitoring could include submission of an action plan following the award with
timeframes for making improvements recommended in the report and regular
updates on progress with implementation. In most programmes, the majority of
report recommendations after the external survey are about improving systems in the
organisation rather than about increasing resources and, as with the preparation, the
organisation should be incurring much of that cost anyway so it should not be a barrier
to improvement. Other monitoring may require a review of specified documents that
were deemed incomplete, inadequate or missing; annual or mid-term visits and random
reviews. Longer intervals between external surveys tend to instil a false sense of security
and remove the momentum for internal improvement.
Publication of results
The extent and methods of public disclosure of survey findings and accreditation or
certification awards must be agreed in advance by the external evaluation organisation
and the various stakeholders. The public should have access to information about which
organisations are accredited or certified. Some organisations are now publishing the
survey reports or a summary of them. Regulatory bodies are usually mandated to publish
full reports.
50
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Example: Public disclosure of accreditation reports: Japan
The Japan Council for Quality Health Care (JCQHC) was founded in 1995 and has developed
standards and criteria for accreditation and began carrying out on-site assessments in
1997. Japan has a universal health insurance system and so Japanese people have a right
to receive medical care at any healthcare organisation and hospitals cannot refuse any
patients. Hospital accreditation is voluntary and requires an application fee. Hospitals
receive scores for each item in all areas with comments from JCQHC in the standard
accreditation process. There are two forms of disclosure of hospital accreditation reports in
Japan:
1. Self-disclosure to the public directly by hospitals;
2. Disclosure by the JCQHC with agreement from the hospital concerned.
Hospitals are not permitted to disclose only selected parts of their accreditation report as
the purpose of disclosure of accreditation reports is to give consumers access not only to
favourable aspects of the report but also to information about those aspects of the service
that require improvement. The data disclosed by the JCQHC to the public include summary
comments and accreditation scores for all the items assessed.
A study was performed in Japan to examine the association between accreditation scores
and the disclosure of accreditation reports. This included a questionnaire to hospitals
who disclosed their accreditation reports to gather data about hospital characteristics
along with perceptions about the public disclosure of accreditation reports. A total of 547
of the 817 hospitals accredited by JCQHC participated in the study. Comments about the
disclosure of accreditation reports were categorised into five general subject areas: (1)
impact of disclosure on the public, (2) advantages to the hospital, (3) risks to the hospital,
(4) JCQHC disclosure, and (5) hospital self-disclosure of information—that is, voluntary
disclosure by the hospital by, for example, a pamphlet or a notice on all billboards in
the hospital. Feedback from participating hospitals, highlighted that most hospitals
(60%) perceive disclosure as good for consumers and hospitals; with most hospitals who
disclosed their reports to the JCQHC (80.5%) agreeing that “disclosure provides incentives
for improving the quality of care because consumers in the community read accreditation
reports”.
A total of 508 (93%) of the participating hospitals disclosed their accreditation reports on
the JCQHC website. Public hospitals were significantly more committed to public disclosure
than private hospitals, and larger hospitals were significantly more likely to participate in
public disclosure than smaller hospitals. Accreditation scores were positively related to
the public disclosure of hospital accreditation reports. Scores for patient focused care and
efforts to meet community needs were significantly higher in actively disclosing hospitals
than in non-disclosing hospitals. Among the large hospitals, scores for safety management
were significantly higher in hospitals advocating disclosure than in non-disclosing hospitals.
Most hospitals who agreed to disclosure by the JCQHC (410/508 – 80.7%) reported that
their public disclosure was helpful. A total of 489 of the 547 respondents (89.4%) indicated
that they also disclosed their accreditation reports themselves: 366 disclosed only their
accreditation status and 123 disclosed more than this. The study found that significantly
more of the hospitals who agreed to disclosure of their report by the JCQHC also released
information than those who were not in favour of disclosure by the JCQHC.
The study findings suggest that public disclosure of accreditation reports should be
encouraged to improve public accountability and the quality of care. The authors highlighted
that there is a need for further research to explore the interaction between public
disclosure, processes and outcomes
29
.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
51
5.4 Quality Assurance
External evaluation organisations need to be able to demonstrate their integrity,
objectivity and reliability. Mechanisms include:
The programme’s standards, survey processes and criteria for accreditation or
certification awards are made publicly available
Surveyors are selected, trained and evaluated against explicit published criteria
Survey teams are tailored to each individual client organisation, according to
published criteria, to avoid any conflict of interest
The survey team reports initial findings back to the client organisation before
leaving the site, especially in relation to those likely to generate recommendations,
in order to check the observation and to ensure there are no surprises later
Team reports are prepared and agreed jointly and in compliance with procedures
which are often defined in a surveyors’ handbook
Team reports are independently checked within the external evaluation
organisation for content, consistency and compliance with procedures
Final draft reports are referred to the client organisation for verification before the
accreditation or certification decision
Accreditation or certification awards are made by a panel or staff independent of
the process, based on the team’s report and in line with defined decision-making
criteria or formulae, not by the team itself
3
.
The final chapter will look at evaluation systems that need to be established.

52
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Chapter 6: Evaluating
systems and achievements
External evaluation organisations need to set an example of quality improvement within their
own organisation. This includes defining, monitoring and improving their own performance. This
chapter outlines some of the mechanisms which external evaluation organisations can employ
to do this.
6.1 Measuring performance internally
Internal audits, indicators and quality improvement projects will form part of the overall
quality framework of the organisation.
Indicator data routinely collected by external evaluation organisations and reported to
governing boards include:
Recruitment, drop-out of participating organisations
Denial rate (proportion of organisations refused accreditation or certification)
Report turnaround times (from survey date to final report or to award decision)
Financial performance, such as actual against budget and various financial ratios
Website hits
Surveyor recruitment, training and evaluation
Client satisfaction with surveyors, education services, the survey process, the
survey visit and other products provided by the programme
Staff satisfaction
Surveyor satisfaction
Satisfaction of other stakeholders.
The ISQua organisation standards require the external evaluation organisation to
evaluate the performance of various functions (such as governance, human resources
management, surveyor and survey management and accreditation or certification
processes and outcomes), by collecting data on defined indicators and other measures of
performance, analysing it, making improvements and evaluating achievements
3
.
External evaluation organisations typically undertake many development and
improvement initiatives. These need to be treated as quality projects and the objectives,
actions, timeframes, responsibilities, progress and results documented. These project
documents will form an important part of the evidence needed when the organisation
undergoes its own external evaluation survey through ISQua.
Audits need to be scheduled, results documented and actions taken as a result recorded
and evaluated. Audits can address a number of areas; for example, audits can be
conducted of staff, surveyor and client records; award decisions; health and safety; and
the complaints register.
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
53
6.2 Evaluating independently
Independent evaluations of new accreditation organisations have been commissioned,
often by governments or as conditions of receiving initial development funding. Examples
from Australia, South Africa, Zambia and the UK document benefits perceived by
organisations and their users, but include little data on individual or population health
improvements
1
.
A WHO study of external quality assessment programmes for maternal and child health
concluded in 2002 that these bring benefits to clients, the community, staff and the
service, summarised as
30
:
The linkages, networks and structures which have been developed and / or
improved to influence the political, legislative, economic, socio-cultural and public
health environment within which services operate (enabling mechanisms)
The reorganisation and / or development of the healthcare delivery systems at the
service level
The change in attitude and / or development of skills and knowledge of health
service staff
Improvements to health facilities and equipment
A client-centred and clients’ rights approach to healthcare whereby services
consult with and support clients, are needs based and able to deliver better care to
clients and the community.
6.3 Monitoring by regulatory agencies
Some regulatory bodies, e.g. in USA and Canada, monitor independent accreditation
programmes, primarily by representation on the governing board or by checks on
selected surveys. The federal government follow The Joint Commission into 5% of
surveys in “deemed status” hospitals within a few weeks of the visit to validate reports;
the National Committee for Quality Assurance (NCQA) in USA has a proportion of co-
visits; and the Accreditation Association for Ambulatory Health Care (AAAHC) has a
similar proportion of post-accreditation validation surveys of ambulatory care centres.
In South Africa, the provincial government, which is also the contractor, provides
monitoring by co-visiting. In New Zealand, the Ministry of Health arranges monitoring
audits of 5% of all certification audits undertaken by independent designated audit
agencies
1
.
6.4 Accrediting the external evaluation bodies
The International Society for Quality in Health Care’s (ISQua’s) International Accreditation
Programme has been in existence since 1999 and “accredits the accreditors”. The
scope of the programme has been extended from the evaluation of national healthcare
accreditation organisations, their standards and surveyor training, to include other
standards based certification and audit organisations.
The International Accreditation Programme (IAP) provides three products for health and
social care external evaluation bodies:
Survey and accreditation to international standards for external evaluation
organisations
Standards assessment and accreditation to international principles for healthcare
and social care standards
Assessment and accreditation of surveyor training programmes.

54
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
The international standards for external evaluation organisations are the outcome of
several years of development, testing, peer review and consultation with the international
accreditation community. They were designed to address the quality of all aspects and
functions of an accreditation body, broadly incorporating the International Standards
Organisation (ISO) requirements for certification bodies, the Baldrige criteria for
performance excellence, and criteria for organisational excellence from the accreditation
standards of a number of national accreditation bodies. These standards assess the
key business functions as well as best practice in assessment methodologies, surveyor
management and award recognition.
The standards and principles and their criteria are intended to guide external evaluation
organisations in their development by identifying best practice processes and systems
and providing an assessment process and recognition system for achievement of these.
Many smaller and developing programmes cannot justify the resources required for full
international recognition but they could embark on a defined progression of development
and standardisation starting from self-assessment, to peer review, and aiming eventually
for international accreditation.
ISQua provides technical and advisory services such as self-assessment review and
mock surveys to assist external evaluation organisations develop their programmes and
prepare for international accreditation.
ISQua requires at least one set of the organisation’s standards to be ISQua accredited
before the organisation can enter the organisation accreditation programme.
Conclusions
This document has aimed to highlight some of the questions, issues and challenges which need
to be addressed before deciding on and implementing an external evaluation programme. The
decisions made must be specific to the values, health policies or strategies and organisations of
individual countries, regions and care sectors. Steps have been identified that need to be taken
to ensure that the foundation is set for a sustainable organisation. The order may be different,
but the fundamentals must be established first. Some steps may be done in parallel, for
example obtaining funding, negotiating incentives and developing standards, or establishing the
governance framework and management systems.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
55
References
1 Shaw C. Toolkit for Accreditation Programs. Melbourne: The International Society for
Quality in Healthcare, 2004.
2 International Society for Quality in Health Care. ISQua Checklist for Development of New
Healthcare Accreditation Programs: Guidance for governments, agencies and other
groups. Melbourne: The International Society for Quality in Healthcare, 2006.
3 International Society for Quality in Health Care. Guidelines and Standards for External
Evaluation Organisations 4th Edition Version 1.1 July 2014. Dublin: International Society for
Quality in Health Care, 2014.
4 International Society for Quality in Health Care. Guidelines and Principles for the
Development of Health and Social Care Standards 4th Edition Version 1.1 July 2014. Dublin:
International Society for Quality in Health Care, 2014.
5 International Society for Quality in Health Care. Surveyor Training Standards Programme.
Dublin: International Society for Quality in Health Care, 2009.
6 World Health Organization. Universal Health Coverage [Internet]. Geneva: World Health
Organization. Available from: http://www.who.int/universal_health_coverage/en/
7 Braithwaite J, Shaw C.D, Molodovan M, Greenfield D, Hinchcliff R, Mumford V, Kristensen
MB, Westbrook J, Nicklin W, Fortune T and Whittaker S. Comparison of health service
accreditation programs in low- and middle- income countries with those in higher income
countries: a cross-sectional study. International Journal for Quality in Health Care. 2012; 24
(6): 568-577.
8 Rooney AL and van Ostenberg PR. Licensure, Accreditation and Certification: Approaches
to Health Services Quality. Quality Assurance Methodology Refinement Series. Bethesda:
Center for Human Services, Quality Assurance Project; 1999.
9 Australian Commission on Safety and Quality in Health Care. Accreditation and the NSQHS
Standards [Internet]. Sydney: Australian Commission on Safety and Quality in Health Care.
Available from: http://www.safetyandquality.gov.au/our-work/accreditation-and-the-nsqhs-
standards/
10 International Organization for Standardization (ISO). About ISO [Internet]. Geneva:
International Organization for Standardization. Available from: http://www.iso.org/iso/
home/about.htm
11 Nicklin W. The Value and Impact of Health Care Accreditation: A Literature Review. Ottawa:
Accreditation Canada; 2014. Available from: http://www.accreditation.ca/sites/default/files/
value-and-impact-en.pdf
12 Hinchcliff R, Greenfield D, Moldovan M, Westbrook JI, Pawsey M, Mumford V and
Braithwaite J. Narrative synthesis of health service accreditation literature. BMJ Qual Saf.
2012; 21:979-991.
13 Mumford V, Forde K, Greenfield D, Hinchcliff R and Braithwaite J. Health services
accreditation: what is the evidence that the benefits justify the costs? International Journal
for Quality in Health Care. 2013; 25 (5): 606-620.
14 Beaumont M. Recherche sur l’efficacite du programme d’agrement du Conseil canadien
d’agement des services de sante: Methodologie et resultants. Maitrise en administration
des services de sante: Faculte de medicine, Universite de Montreal; 2002.

56
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
15 Shaw C.D, Braithwaite J, Moldovan M, Nicklin W, Grgic I, Fortune T and Whittaker S.
Profiling health-care accreditation organizations: an international survey. International
Journal for Quality in Health Care. 2013; 25 (3): 222-231.
16 Burnett S, Renz A, Wiig S, Fernandes A, Weggelaar AM, Calltorp J, Anderson J.E., Robert G,
Vincent C and Fulop N. Prospects for comparing European hospitals in terms of quality and
safety: lessons from a comparative study in five countries. International Journal for Quality in
Health Care. 2013; 25 (1): 1-7.
17 The World Bank. Health Systems Regulation [Internet]. Washington D.C.: The World Bank.
Available from: http://www.worldbank.org
18 Al-Awa B, De Wever A, Melot C and Devreux I. An overview of patient safety and
accreditation: a literature review study. RJMS. 2011; 5: 200-223.
19 Turner L. Quality in healthcare and globalisation of health services: accreditation and
regulatory oversight of medical tourism companies. International Journal for Quality in
Health Care. 2011; 23(1): 1-7
20 Smits H, Supachutikul A and Mate KS. Hospital accreditation: lessons from low- and
middle-income countries. Globalization and Health. 2014; 10 (65).
21 Australian Government Department of Human Services. Practice Incentive Program
Guidelines. Adelaide: Australian Government Department of Human Services; 2013.
Available from: http://www.humanservices.gov.au/healthprofessionals/services/practice-
incentives-programme/
22 Australian Government Department of Health. Budget 2015 – 2016 Portfolio Budget
Statements 2015 -16 Budget Related Paper No. 1.10 Health Portfolio. Canberra: Australian
Government Department of Health; 2015. p96. Available from: http://www.health.gov.au/
internet/budget/publishing.nsf/Content/2015-2016_Health_PBS_sup1/$File/2015-16_
Health_PBS_0.0_Complete.pdf
23 Australian Government Department of Human Services. Practice Incentives Program
[Internet]. Adelaide: Australian Government Department of Human Services; (undated).
Available from: http://www.humanservices.gov.au/health-professionals/services/practice-
incentives-programme/
24 Malaysian Society for Quality in Health. MSQH – Committee Members [Internet]. Kuala
Lumpar: Malaysian Society for Quality in Health. Available from: http://www.msqh.com.my/
msqh/ct-menu-item-3/2013-07-29-06-13-01/ct-menu-item-9
25 Shaw CD. Developing hospital accreditation in Europe. Copenhagen: WHO Regional Office
for Europe; 2004. Available from: http://www.unifesp.br/hsp/acred.pdf
26 National Accreditation Board for Hospitals & Healthcare Providers. Introduction – Hospital
Entry Level [Internet]. New Delhi: National Accreditation Board for Hospitals & Healthcare
Providers. Available from: http://nabh.co/Hospital-EntryLevel.aspx
27 Greenfield D, Pawsey M, Hinchcliff R, Moldovan M and Braithwaite J. The standard of
healthcare accreditation standards: a review of empirical research underpinning their
development and impact. BMC Public Health Services Research. 2012; 12: 329.
28 Institute of Medicine (IOM). Crossing the Quality Chasm: A New Health System for the 21st
Century. Washington D.C.: National Academy Press, 2001. Available from: http://www.iom.
edu/Reports/2001/Crossing-the-Quality-Chasm-A-New-Health-System-for-the-21st-
Century.aspx

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
57
29 Ito H and Sugawara H. Relationship between accreditation scores and the public disclosure
of accreditation reports: a cross sectional study. Qual Saf Health Care. 2005; 14 (2): 87-92.
30 Mahaffey A. In Pursuit of Quality Improvement: A review of experiences of external quality
assessments at reproductive health services. Geneva: World Health Organization, 2003.

58
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Bibliography
Alkhenizan A and Shaw C. Impact of accreditation on the Quality of Healthcare Services: a
systematic review of the literature. Ann Saudi Med. 2011; 31 (4): 407-416.
Al-Awa B, De Wever A, Melot C and Devreux I. An Overview of Patient Safety and Accreditation: A
Literature Review Study. RJMS. 2011; 5: 200-223.
Al Tehewy M, Salem B, Habil I, and El Okda S. Evaluation of accreditation program in non-
governmental organizations’ health units in Egypt: Short-term outcomes. International Journal
for Quality in Health Care. 2009; 21 (3): 183-189.
Auras A and Geraedts M. Patient experience data in practice accreditation - an international
comparison. International Journal for Quality in Health Care. 2011; 22 (2); 132-139.
Braithwaite J, Greenfield D, Westbrook J, Pawsey M, Westbrook M, Gibberd R, Naylor J, Nathan
S, Robinson M, Runciman B, Jackson M, Travaglia J, Johnston B, Yen D, McDonald H, Low
L, Redman S, Johnson B, Corbett A, Hennessy D, Clark J and Lancaster J. Health service
accreditation as a predictor of clinical and organisational performance: a blinded, random,
stratified study. Qual Saf Health Care. 2010; 19: 14-21.
Braithwaite J, Westbrook J, Johnston B, Clark S, Brandon M, Banks M, Hughes C, Greenfield
D, Pawsey M, Corbett A, Georgiou A, Callen J, Øvretveit J, Pope C, Suñol R, Shaw C, Debono D,
Westbrook M, Hinchcliff R, and Moldovan M. Strengthening organizational performance through
accreditation research: the ACCREDIT project. BMC Res Notes. 2011; 4: 390.
British Standards Institution. A standard for standards – Principles of standardization. BS 0
2011. London: British Standards Institution; 2011. Available from: http://www.iso.org/sites/PEG/
docs/PEG%20Documents/04_bs02011.pdf
Bukonda N, Tavrow P, Abdallah H, Hoffner K, and Tembo J. Implementing a national hospital
accreditation program: the Zambian experience. International Journal for Quality in Health Care.
2002; 14 (Supplement 1): 1-7.
Chandra A, Glickman SW, Ou F-S, Peacock WF, McCord JK, Cairns CB, Peterson ED, Ohman EM,
Gibler WB, and Roe MT. An Analysis of Society of Chest Pain Centers Accreditation to American
College of Cardiology / American Heart Association Non-ST Segment Elevation Myocardial
Infarction Guidelines Adherence. Annals of Emergency Medicine. 2009; 54 (1): 17-25.
Chung K-P and Yu T-H. Are quality improvement methods a fashion for hospitals in Taiwan?
International Journal for Quality in Health Care. 2012; 24 (4): 371-379.
Cleveland EC, Dahn BT, Lincoln TM, Safer M, Podesta M and Bradley E. Introducing health
facility accreditation in Liberia. Global Public Health. 2014; 10 (65).
Devkaran S and O’Farrell PN. The impact of hospital accreditation on clinical documentation
compliance: a life cycle explanation using interrupted time series analysis. BMJ Open. 2014; 4:
e005240. doi: 10.1136/bmjopen-2014-005240
de Walcque C, Seuntjens B, Vermeyen K, Peeters G, and Vinck I. Comparative study of hospital
accreditation programs in Europe. Health Services Research (HSR). Brussels: Belgian Health
Care Knowledge Centre (KCE); 2008. KCE reports 70C, D/2008/10.273/03. Available from: https://
kce.fgov.be/sites/default/files/page_documents/d20081027303.pdf
El-Jardali F, Jamal D, Dimassi H, Ammar W, and Tchaghchaghian V. The impact of hospital
accreditation on quality of care: Perception of Lebanese nurses. International Journal for Quality
in Health Care. 2008; 20 (5): 363-371.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
59
Fortes MT, de Mattos RA, and de Faria Baptista TW. Accreditation or accreditations? A
comparative study about accreditation in France, United Kingdom and Cataluña. Rev Assoc Med
Bras. 2011; 57 (2): 234-241.
Gratwohl A, Brand R, Niederwieser D, Baldomero H, Chabannon C, Cornelissen J, de Witte
T, Ljungman P, McDonald F, McGrath E, Passweg J, Peters C, Rocha V, Slaper-Cortenbach I,
Sureda A, Tichelli A, and Apperley J. Introduction of a Quality Management System and Outcome
after Hematopoietic Stem-Cell Transplantation. J Clin Oncol. 2011; 29 (15): 1980-1986.
Greenfield D, Travaglia J, Braithwaite J, and Pawsey M. Unannounced Surveys and Tracer
Methodology: Literature Review. Sydney: Centre for Clinical Governance Research, University of
New South Wales, 2007.
Greenfield D, Travaglia J, Braithwaite J, and Pawsey M. Intra-rater and Inter-rater Reliability in
Health Care Accreditation: Literature Review. Sydney: Centre for Clinical Governance Research,
University of New South Wales, 2007.
Greenfield D and Braithwaite J. Health sector accreditation research: a systematic review.
International Journal for Quality in Health Care. 2008; 20 (3): 172-183.
Greenfield D, Pawsey M, and Braithwaite J. What motivates professionals to engage in the
accreditation of healthcare organizations? International Journal for Quality in Health Care. 2011;
23 (1): 8-14.
Greenfield D, Hinchcliff R, Westbrook M, Jones D, Low L, Johnston B, Banks M, Pawsey M,
Moldovan M, Westbrook J, and Braithwaite J. An empirical test of accreditation patient journey
surveys: randomized trial. International Journal for Quality in Health Care. 2012; 24 (5): 495-500.
Greenfield D, Hinchcliff R, Moldovan M, Mumford V, Pawsey M, Westbrook JI, and Braithwaite
J. A multimethod research investigation of consumer involvement in Australian health service
accreditation programmes: the ACCREDIT-SCI study protocol. BMJ Open. 2012; 2: e002024. doi:
10.1136/bmjopen-2012-002024
Greenfield D, Moldovan M, Westbrook M, Jones D, Low L, Johnston B, Clark S, Banks M, Pawsey
M, Hinchcliff R, Westbrook J, and Braithwaite J. An empirical test of short notice surveys in two
accreditation programmes. International Journal for Quality in Health Care. 2012; 24 (1): 65-71.
Greenfield D, Hinchcliff R, Pawsey M, Westbrook J, and Braithwaite J. The public disclosure of
accreditation information in Australia: stakeholder perceptions of opportunities and challenges.
Health Policy. 2013; 113 (1-2): 151-159.
Greenfield D, Pawsey M, Naylor J, and Braithwaite J. Researching the reliability of accreditation
survey teams: Lessons learnt when things went awry. Health Information Management Journal.
2013; 42 (1): 4-10.
Greenfield D, Civil M, Donnison A, Hogden A, Hinchcliff R, Westbrook J, and Braithwaite J. A
mechanism for revising accreditation standards: A study of the process, resources required and
evaluation outcomes. BMC Health Services Research. 2014; 14: 571.
Greenfield D, Kellner A, Townsend K, Wilkinson A, and Lawrence SA. Health service
accreditation reinforces a mindset of high-performance human resource management: Lessons
from an Australian study. International Journal for Quality in Health Care. 2014; 26 (4): 372-377.
Greenfield D, Hinchcliff R, Hogden A, Mumford V, Debono D, Pawsey M, Westbrook J, and
Braithwaite J. A hybrid health service accreditation program model incorporating mandated
standards and continuous improvement: Interview study of multiple stakeholders in Australian
healthcare. Int J Health Plann Mgmt. 2015. doi: 10.1002/hpm.2301

60
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Guérin S, Le Pogam M-A, Robillard B, Le Vaillant M, Lucet B, Gardel C, Grenier C, and Loira
P. Can we simplify the hospital accreditation process? Predicting accreditation decisions
from a reduced dataset of focus priority standards and quality indicators: results of predictive
modelling. BMJ Open. 2013; 3: e003289. doi: 10.1136/bmjopen-2013-003289.
Gyani GJ and Krishnamurthy B. The National Accreditation Board for Hospital and Health Care
Providers Accreditation Programme in India. World Hospitals and Health Services. 2014; 50 (1):
9-12.
Hinchcliff R, Greenfield D, Westbrook JI, Pawsey M, Mumford V and Braithwaite J. Stakeholder
perspectives on implementing accreditation programs: a qualitative study of enabling factors.
BMC Health Services Research. 2013; 13 (437).
Jaafaripooyan E, Agrizzi D, and Akbari-Haghighi F. Healthcare accreditation systems: further
perspectives on performance measures. International Journal for Quality in Health Care. 2011; 23
(6): 645-656.
Jaafaripooyan E. Potential pros and cons of external healthcare performance evaluation
systems: real-life perspectives on Iranian hospital evaluation and accreditation program. Int J
Health Policy Manag. 2014; 3 (4): 191-198.
Leatherman S, Ferris T, Berwick D, Omaswa F, and Crisp N. The role of quality improvement in
strengthening health systems in developing countries. International Journal for Quality in Health
Care. 2010; 22 (4): 237-243.
Lind A, Edward A, Bonhoure P, Mustafa L, Hansen P, Burnham G, and Peters DH. Quality of
outpatient hospital care for children under 5 years in Afghanistan. International Journal for
Quality in Health Care. 2011; 23 (2): 108-116.
Menachemi N, Chukmaitov A, Brown LS, Saunders C, and Brooks RG. Quality of care in
accredited and nonaccredited ambulatory surgical centers. The Joint Commission Journal on
Quality and Patient Safety. 2008; 34 (9): 546-551.
Mumford V, Greenfield D, Hinchcliff R, Moldovan M, Forde K, Westbrook JI, and Braithwaite
J. Economic evaluation of Australian acute care accreditation (ACCREDIT-CBA (Acute)): study
protocol for a mixed method research project. BMJ Open. 2013; 3: e002381. doi:10.1136/
bmjopen-2012-002381
Mumford V, Greenfield D, Hogden A, Debono D, Gospodarevskaya E, Forde K, et al. Disentangling
quality and safety indicator data: A longitudinal, comparative study of hand hygiene compliance
and accreditation outcomes in 96 Australian hospitals. BMJ Open. 2014; 4: e005284. doi: 10.1136/
bmjopen-2014-005284
National Advisory Group on the Safety of Patients in England. A promise to learn – a
commitment to act: Improving the safety of patients in England. 2013. Available from: https://
www.gov.uk/government/uploads/system/uploads/attachment_data/file/226703/Berwick_
Report.pdf
Pomey MP, Lemieux-Charles L, Champagne F, Angus D, Shabah A and Contandriopoulos AP.
Does accreditation stimulate change? A study of the impact of the accreditation process on
Canadian healthcare organizations. Implement Sci. 2010; 5 (31). doi: 10.1186/1748-5908-5-31
Ruelas E, Gómez-Dantés O, Leatherman S, Fortune T, and Gay-Molina JG. Strengthening the
quality agenda in health care in low- and middle-income countries: questions to consider.
International Journal for Quality in Health Care. 2012; 24 (6): 553-557.
Sack C, Scherag A, Lütkes P, Günther W, Jöckel K-H, and Holtmann G. Is there an association
between hospital accreditation and patient satisfaction with hospital care? A survey of 3700
patients treated by 73 hospitals. International Journal for Quality in Health Care. 2011; 23 (3): 1-6.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
61
Sack C, Lütkes P, Günther W, Jöckel K-H, and Holtmann GJ. Challenging the holy grail of
hospital accreditation: A cross sectional study of inpatient satisfaction in the field of cardiology.
BMC Health Services Research. 2010; 10: 120.
Sax S and Marx M. Local perceptions on factors influencing the introduction of international
healthcare accreditation in Pakistan. Health Policy Plan [Internet]. 2013 November [cited 2014
June 11]; doi:10.1093/heapol/czt084
Shaw C, Bruneau C, Kutryba B, De Jongh G, and Suñol R. Towards hospital standardization in
Europe. International Journal for Quality in Health Care. 2010; 22 (4): 244-249.
Shaw C, Groene O, Botje D, Sunol R, Kutryba B, Klazinga N, Bruneau C, Hammer A, Wang A,
Arah OA and Wagner C. The effect of certification and accreditation on quality management in 4
clinical services in 73 European hospitals. International Journal for Quality in Health Care. 2014;
26: Number S1: 100-107.
Shaw C, Groene O, Mora N, and Sunol R. Accreditation and ISO certification: do they explain
differences in quality management in European hospitals? International Journal for Quality in
Health Care. 2010; 22 (6): 445 – 451.
Shaw C, Kutryba B, Braithwaite J, Bedlicki M, and Warunek A. Sustainable healthcare
accreditation: messages from Europe in 2009. International Journal for Quality in Health Care.
2010; 22 (5): 341-350.
Siddiqi S, Elasady R, Korshid I, Fortune T, Leotsakos A, Letaief M, Qsoos S, Aman R, Mandhari A,
Sahel A, El-Tehewy M, and Abdellatif A. Patient Safety Friendly Hospital Initiative: from evidence
to action in seven developing country hospitals. International Journal for Quality in Health Care.
2012; 24 (2): 144-151.
Tabrizi JS, Gharibi F and Wilson AJ. Advantages and Disadvantages of Health Care Accreditation
Models. Health Promotion Perspectives. 2011; 1 (1): 1-31.
The Mid Staffordshire NHS Foundation Trust Public Inquiry. Report of the Mid Staffordshire NHS
Foundation Trust Public Inquiry. London: The Stationery Office; 2013. Available from: http://www.
midstaffspublicinquiry.com/report
Touati N and Pomey MP. Accreditation at a crossroads: Are we on the right track? Health Policy.
2009; 90 (2-3): 156-165.
University Research Co., LLC. Jordan Healthcare Accreditation Project Final Report June 17,
2007 – March 17, 2013. Bethesda: University Research Co., LLC, 2013.
Webster TR, Mantopoulos J, Jackson E, Cole-Lewis H, Kidane L, Kebede S, Abebe Y, Lawson
R, and Bradley EH. A brief questionnaire for assessing patient healthcare experiences in low
income settings. International Journal for Quality in Health Care. 2011; 23 (3): 258-268.
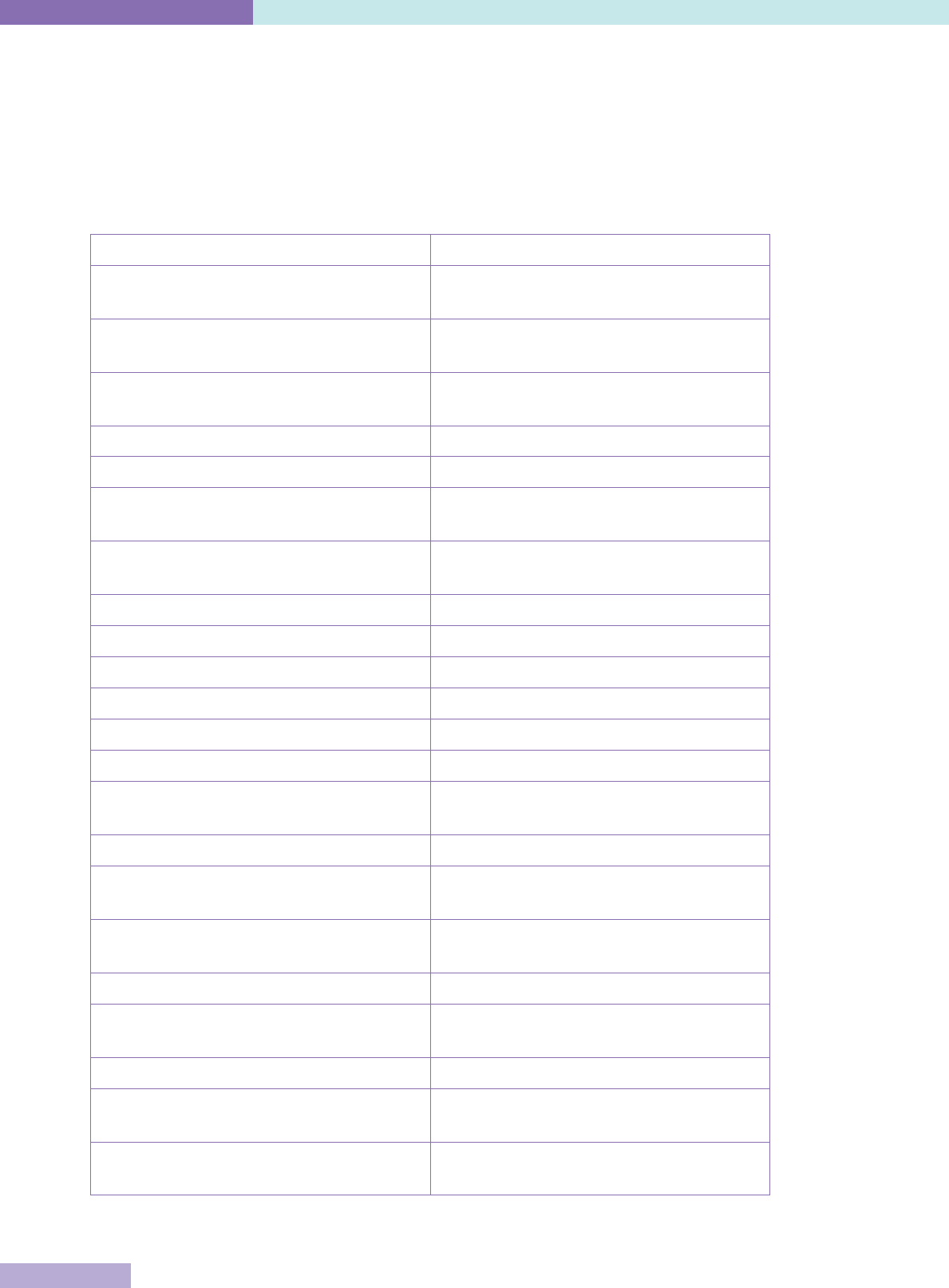
62
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Useful web resources
The International Society for Quality in Health Care (ISQua) is not responsible for external
website content. Please note that many organisations have English language content on their
websites and where possible the direct link to such material is provided. However, in some
instances the website content is only available in the native language.
Accreditation Canada http://accreditation.ca/
Agency for Quality and Accreditation in
Health and Social Welfare, Croatia
http://aaz.hr/
American Accreditation Council http://www.americanaccreditationcouncil.
com/
American Association for Accreditation of
Ambulatory Surgery Facilities International
http://www.aaaasfi.org/
American Association of Blood Banks http://www.aabb.org/
Australian Aged Care Quality Agency http://www.aacqa.gov.au/
Australian Commission on Safety and Quality
in Health Care
http://www.safetyandquality.gov.au/
Australian General Practice Accreditation Ltd
(AGPAL)
http://www.agpal.com.au/
Canadian Accreditation Council http://www.cacohs.com/
CHKS, United Kingdom http://www.chks.co.uk/
Consortium for Brazilian Accreditation (CBA) http://www.cbacred.org.br/
DAA Group Ltd http://www.daagroup.co.nz/
DNV GL Business Assurance http://www.dnvba.com/
Global-Mark Pty Ltd http://www.global-mark.com.au/
Haute Authorité de Santé, France http://www.has-sante.fr/portail/
jcms/r_1455134/fr/about-has
Health Accreditation Service, Columbia http://www.icontec.org/
Health and Disability Auditing New Zealand
Ltd (HDANZ)
http://www.healthaudit.co.nz/
Health and Disability Auditing Australia Pty
Ltd
http://www.hdaau.com.au/
Health Care Accreditation Council, Jordan http://www.hcac.jo/
IKAS, The Danish Institute for Quality and
Accreditation in Healthcare
http://www.ikas.dk/IKAS/English.aspx
Japan Council for Quality Health Care http://jcqhc.or.jp/pdf/top/english.pdf
Joint Commission International http://www.jointcommissioninternational.
org/
Joint Commission of Taiwan http://www.tjcha.org.tw/FrontStage/aboutus_
en.html
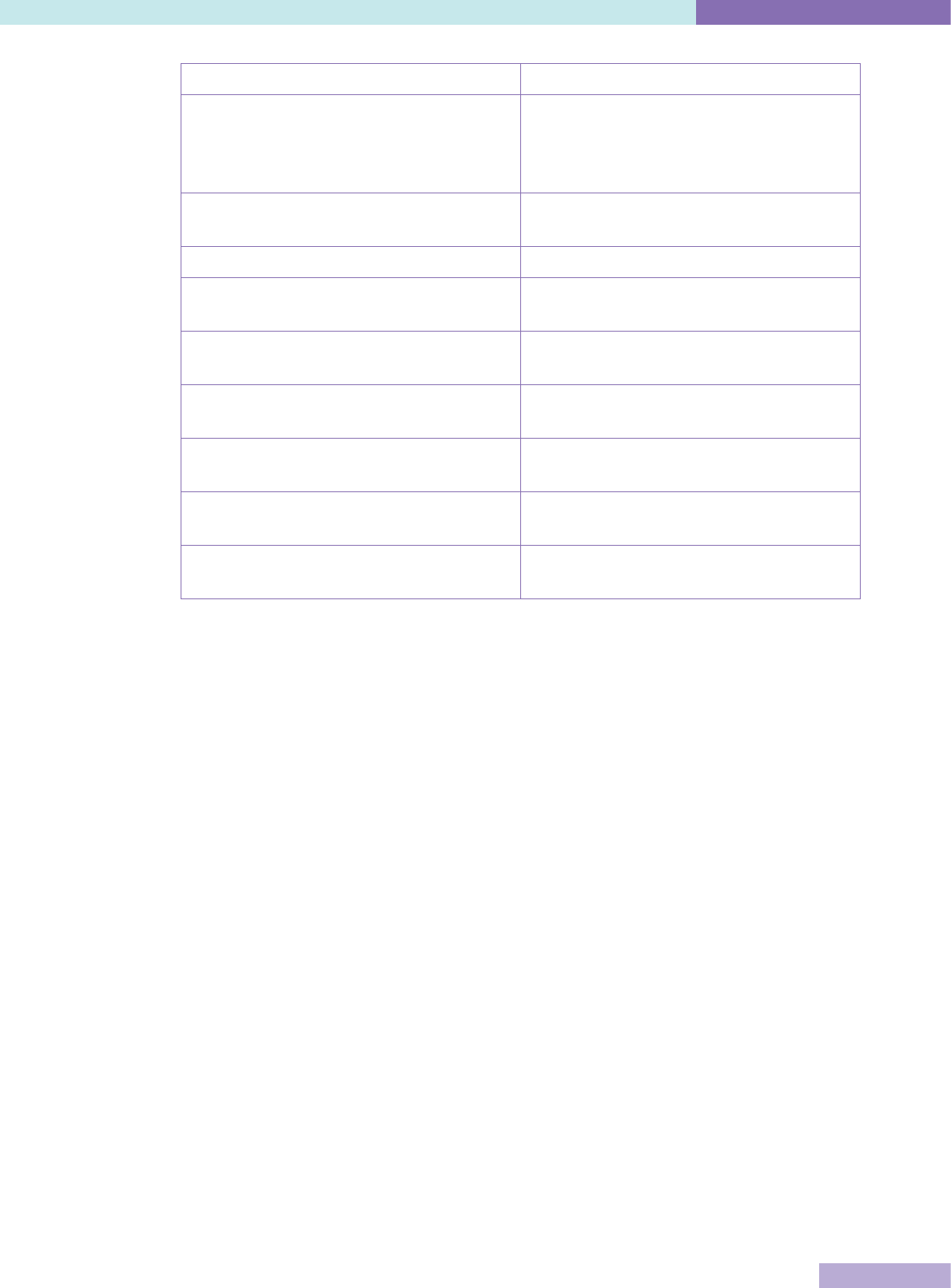
International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
63
Malaysian Society for Quality in Health http://www.msqh.com.my/
Ministry of Health New Zealand – Health and
Disability Services Standards
http://www.health.govt.nz/our-work/
regulation-health-and-disability-system/
certification-health-care-services/health-
and-disability-services-standards
National Accreditation Board for Hospitals
and Healthcare Providers, India
http://www.nabh.co/
Quality Innovation Performance, Australia http://www.qip.com.au/
Joint Commission of Taiwan http://www.tjcha.org.tw/FrontStage/aboutus_
en.html
The Australian Council on Healthcare
Standards
http://www.achs.org.au/
The Healthcare Accreditation Institute (Public
Organisation), Thailand
http://www.ha.or.th/
The Council for Health Service Accreditation
of Southern Africa
http://www.cohsasa.co.za/
The Diagnostic Accreditation Program,
British Columbia, Canada
http://www.dap.org/
The Netherlands Institute for Accreditation in
Healthcare (NIAZ)
http://en.niaz.nl/
64
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Appendix 1 - Case Studies
Appendix 1a.
IKAS – Danish Institute for Quality and Accreditation in Healthcare
Country: Denmark
Contributed by: Carsten Engel
Foundation of the programme
The Danish accreditation programme (DDKM) was established as part of the “National Strategy
for Quality Development in the Healthcare System – Joint Goals and Action Plan 2002-2006”. The
strategy was developed by the national, regional and local political authorities in cooperation
with stakeholder organisations, representing professionals and consumers.
At that time, a number of hospitals already had positive experiences with accreditation provided
by international accreditors – one of the intentions of the strategy was to spread this to the
entire healthcare system, based on a Danish model.
IKAS is formally an independent organisation, but IKAS and DDKM were established by an
agreement between the regional and local political authorities, who are responsible for
delivering healthcare, and the national government that sets the overarching political priorities,
including the economic frame, and is the healthcare legislator and regulator.
The government provides part of the funding for IKAS. The government is represented on the
Board of IKAS; the Chair of the Board is a government representative (a director of the Danish
Health and Medicines Authority).
Development steps
The following steps describe the initial development of DDKM. The programme has since been
extensively developed, based on the experiences obtained.
1. Cooperation agreement between the government and the regions on the establishment of a
joint model for quality assessment, including provisions for the funding for DDKM (2004)
2. Appointment of a Board by the parties to the cooperation agreement and endorsement of
bylaws for IKAS
3. Establishment of IKAS as an organisation (2005)
4. Tender for consultancy by an established international accreditor, resulting in a contract
with HQS / CHKS for support to develop standards, establish IKAS as an accreditation
organisation, and train surveyors
5. Development of first two sets of standards (hospitals and pharmacies) by theme groups
(for related groups of standards) of standard developers, consisting of senior professionals,
appointed by the Regions and the Association of Danish Pharmacies. IKAS and HQS / CHKS
served as advisors and secretariat for the groups.
6. Public hearing, which for the hospital standards resulted in an extended revision by an
editorial group with members from IKAS and the Regions, followed by a second hearing.
7. Pilot testing of standards for usability (for clients) and understandability
8. Submission of standards for ISQua accreditation

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
65
9. Development of an IT system to support implementation and external assessment
10. Development of a rating system
11. Development of information for hospitals and pharmacies and holding a series of courses
for key persons in client organisations
12. Development of a survey methodology, described in a handbook
13. Selection and training of surveyors
14. Appointment and training of an Accreditation Awards Panel
15. Development and implementation of processes to process survey reports
16. Preparation for ISQua accreditation as an external evaluation organisation and of the
surveyor training programme (obtained early 2011).
The standards actually led the development process; steps 9 – 15 overlapped each other and the
later phases of standard development, but continued up to the commencement of surveys, 1½
years after finalising the standards.
The first survey was conducted 4½ years after the establishment of IKAS.
Funding & incentives
In terms of funding, IKAS has an index-linked annual grant from the central government,
regions and local government. There are no fees for public clients or pharmacies. Other private
clients pay a fee that covers direct expenses plus an overhead.
The programme is not required by any legislation, but is based on agreements as follows:
Public hospitals: all hospitals participate by agreement between National and Regional
governments
Private hospitals: voluntary, but participation is a prerequisite to obtain a contract to treat
patients for the regions (also required by some insurance companies)
Pharmacies: voluntary, financial incentive in place
Municipalities (primary care services, including long-term care): voluntary, no incentives in
place
Ambulance operators: prerequisite to obtain contract with Regions
General practitioners: mandatory (with some minor exceptions) by agreement between the
Regions and the Organisation of General Practitioners in Denmark; financial compensation
as part of the agreement.
Standards and measurement
IKAS has developed all standards used in its programmes. They were first developed for
hospitals and for community pharmacies. Standards have since been developed for primary care
services, delivered by municipalities, and for ambulance services. Currently standards are being
developed for general practitioners and specialist physicians. Over the coming years, all health
care professions providing office-based services, outside of hospitals, will be covered.
Compliance with standards is assessed by scoring a number of elements (for the hospital
standards roughly 450) according to a four point scale (Fully / Largely / Partially / Not Met),
where the two upper levels indicate a satisfactory performance (except for certain safety
critical standards, where only Fully Met is considered satisfactory). Any element not met to
satisfaction will require follow up, and if not corrected, results in accreditation with comments.
An Accreditation Award Panel decides, guided by certain rules, whether the nature and / or
amount of the comments preclude accreditation – if so, status as “not accredited” is awarded
and published.

66
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Assessment methodology and focus
The assessment methodology used is external survey with extensive use of tracer methodology.
The focus is on exploring the implementation of safe processes and investigating the use of
quality data for improvement activities.
Quality improvement is fundamental. There is an extensive set of national quality registers
in Denmark, and one of the purposes of DDKM is to support and assess that data is not just
collected, but also used for quality improvement. Demonstration of completed and evaluated
improvement activities is required from the second accreditation cycle.
Surveyors are active senior health care professionals who are contracted for 15 survey days per
year. In addition, they are obligated to participate in continuous training activities.
Barriers
Development of an accreditation programme from scratch is much like building a bridge while
you are crossing it. Even with the best support from consultants, there are a lot of lessons to
be learned when the programme is applied in practice. A full pilot test, including complete
implementation and external assessment, would be ideal, but would add a considerable delay.
Lessons learned
One lesson learned is that while it adds to the legitimacy of the programme that standards
are developed involving a large number of healthcare professionals as standard developers, a
strong editorial process is needed if this is to result in a uniform and balanced standard set.
Furthermore, these types of standard developers will almost exclusively focus on the standards
as implementation guides; it may be a challenge to assess performance in a reliable and
uniform way. To support reliable assessment, the standards must include a lot of guidance for
surveyors, both as to methodology and to rating, while avoiding surveys becoming exercises of
“ticking check boxes”.
We have underestimated the need to communicate that the standards are different from
regulatory rules. The latter contain specific directions that must be strictly adhered to, whereas
many (albeit not all) standards express a goal to strive for or require the client to define the
specifics, according to local needs and priorities. You will meet clients asking to be told exactly
what to do, and you will meet examples of “over implementation”, where clients demand their
staff to rigidly apply the same standardised procedures to all patients; an example could be
hospitals believing that the standards require them to screen all patients for malnutrition,
regardless of the likelihood for a certain patient or type of patient to be malnourished. This is, in
our experience, an important source of resistance to accreditation among staff.
Our surveys are announced and are preceded by a lot of preparation by the clients. Many of
their staff perceive this as building a nice picture to show the surveyors, but not necessarily
giving a fair picture of the real performance; the risk is that preparing for accreditation is
seen by staff as a show, designed to obtain a certificate, more than as a value adding activity.
Doing unannounced or partially unannounced surveys would no doubt add to the face validity
of accreditation. We are currently preparing a controlled study to investigate the merits of
unannounced surveys.
One typical way to articulate resistance is to ask for the evidence for accreditation. While you
must argue that accreditation is a complex intervention that cannot be backed by evidence of the
same type as a drug treatment, design of a formal evaluation as part of the programme should
be considered.
More information, including accreditation standards, can be found at http://www.ikas.dk/IKAS/
English.aspx

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
67
Appendix 1b.
Health Care Accreditation Council (HCAC)
Country: Jordan
Contributed by: Ed Chappy
Foundation of the programme
Several reasons were stated for why the programme was developed including to improve the
quality of hospitals and to enhance medical tourism. In addition, it was a response to public
complaints of poor quality of care and a need to improve the entire healthcare system in the
country.
The HCAC is the national healthcare accreditation agency of Jordan. The organisation sets
standards for hospitals, primary healthcare centres, family planning and reproductive health,
transport services (ambulances), cardiac care, and diabetes mellitus. HCAC surveys against the
standards and awards accreditation. HCAC also provides consultation and education to prepare
healthcare facilities for accreditation and offers certification courses.
The HCAC is a private, not-for-profit shareholding company registered under the Ministry of
Trade and Industry. The board of directors is made up of representatives for all healthcare
sectors in Jordan, medical and nursing professions, and education.
Development steps
1. Decision on funding and incentives
2. Standards or criteria development if applicable
3. Survey / Assessment management processes
4. Development of manuals, tools, education programmes for clients or others
5. Selection and training of surveyors / assessors
6. Type of proposed governance board and framework, constitution
7. Setting up of governance board, governance policies and procedures
8. Development of management systems, strategic and operational plans
9. Accreditation / Certification processes
10. Monitoring, review and evaluation systems
11. Development and use of website, portal or other electronic aids
A decision was taken to develop standards, prepare 17 pilot hospitals from the public, private,
university, and military sectors for accreditation and then create the agency based on demand
for accreditation.
The first set of hospital standards were developed in 2005, surveyors trained in 2006 and
the agency (HCAC) established in December 2007. The first hospital accredited using HCAC
standards was in March 2008.
The first services developed were consultation and education services to prepare hospitals
for accreditation and mock and accreditation surveys. Then preparation of primary healthcare
centres to meet standards and mock and accreditation surreys for them were added. Later,
local and regional consultation and education surveys and certification courses for infection
prevention staff, risk managers, and quality improvement coordinators were added.
68
© Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
Funding and incentives
The original funding to develop the HCAC came through the Jordan Healthcare Accreditation
project funded by the United States Agency for International Development (USAID) and grants.
Since March 2013, HCAC has been financially sustainable through charging fees for services
offered including surveys, education and consultation.
Accreditation is voluntary. There are no incentives (laws, regulation, insurance requirements) in
the country for accreditation.
Standards and measurement
All the standards are developed in Jordan. No standards developed by other organisations are
used. Hospital standards were developed first, then standards for primary care centres, family
planning and reproductive health, transport services (ambulances), cardiac care, and diabetes
mellitus.
Standards are classified as critical, core and stretch.100% of critical standards must be met;
and a specified percentage of both core and stretch standards must be met in order for a service
to be accredited.
Assessment methodology & focus
Mock and accreditation surveys are used. The focus is on quality improvement.
Surveyors are certified for two years and are paid per survey. Staff are trained as surveyors but
are only used in emergencies when a surveyor is ill or for other reasons cannot do a survey.
Challenges
The main challenge was deciding where the organisation was going to be placed in the country
– Ministry of Health, other government agency, professional association, or as an independent
company. The second challenge was to determine how it would be funded.
Lessons learned
Every country must develop their system based on their needs and goals.
See what other countries are doing but create your own system.
Many activities can be done in parallel and you do not need to wait until one task is done
before proceeding to the next (do not have to wait for the agency to be developed before
standards are developed).
Recognise that accreditation is a business and look at the agency as any other business with
strategic, business, and operational plans and business processes.
Do not neglect the need to market accreditation to the population as well as healthcare
facilities and professionals.
Partner with clients and maintain a relationship after and between accreditations.
Look at accreditation as a means of improving the entire healthcare system, not just
hospitals.
See accreditation as one means to quality, not the only means.
Always seek ways to do things better, which may be different from what everyone else is
doing.
ISQua Accreditation International Accreditation Programme (IAP)

© Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
69
Appendix 1c.
Health and Disability Auditing New Zealand Ltd (HDANZ)
Country: New Zealand
Contributed by: Jim duRose
Foundation of the programme
The commencement of the Health and Disability Services (Safety) Act on 1 July 2002 represented
a significant change in the regulatory environment in the New Zealand health and disability
sector. This Act replaced several previous pieces of legislation and changed the way in which
residential and hospital services were licensed or registered. In addition, the Act introduced
health and disability standards for hospitals, rest homes and residential disability services
aimed at improving safety levels and quality of care that became mandatory from 1 October
2004. The Act required that designated audit agencies (DAAs) are approved by the Director
General of Health for the purpose of auditing these services to those standards.
HDANZ became designated in October 2002. In 2004 3rd party accreditation was with
International Accreditation New Zealand (IANZ). Due to a change in IANZ’s legislation they
could no longer accredit HDANZ and in December 2008 HDANZ decided to proceed with ISQua
accreditation. The objective was to have a seamless transition from IANZ and this was achieved
by August 2009. Also, as of December 2008, the Ministry of Health did not require 3rd party
accreditation but a few months later this became a requirement to maintain designation.
HDANZ’s scope was determined by the Safety Act – the assessment of standards is a legal
requirement for public and private hospitals, rest homes and residential disability services.
Standards New Zealand (SNZ) is responsible for the New Zealand standards and this includes
others such as for Home Support, Allied Health, and Day surgery procedures.
HDANZ is also 3rd party accredited with ISQua in order to audit and certify services to these
standards.
HDANZ is a private, independently owned company. It is linked to the government as a MoH
approved designated auditing agency and for these services, HDANZ submits the audit report to
the MoH who issues the certificate
Development steps
1. Type of proposed governance board and framework, constitution
2. Decision on funding and incentives
3. Development of management systems, strategic and operational plans
4. Setting up of governance board, governance policies and procedures
5. Survey / Assessment management processes
6. Accreditation / Certification processes
7. Selection and training of surveyors / assessors
8. Monitoring, review and evaluation systems
9. Development of manuals, tools, education programmes for clients or others
10. Development and use of website, portal or other electronic aids – HDANZ had a website
early on but web based assessment tools were introduced in 2008.
The first assessment was undertaken approximately 6-8 months after HDANZ was established.
International Accreditation Programme (IAP) ISQua Accreditation

70
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
There was no trial period but pre-audit “gap analysis” work was commonplace for most services
before they completed their first assessment in 2003 / 04.
At first, HDANZ provided assessment services for all services under the Safety Act: rest homes,
geriatric hospitals, maternity, surgical, hospice, mental health, disability services and addiction
services. These continue but also HDANZ certifies home care, allied health / physiotherapy
services, day surgery / office-based services and community services. HDANZ also completes
funder contract auditing with NGO providers for a wide range of personal health and mental
health and addiction services. General practice reviews are completed on behalf of Primary Care
Organisations (PHO). HDANZ also assists the Royal College of General Practitioners (RNZCGP)
with their Cornerstone general practice accreditation programme by independently reviewing
reports and issuing a recommendation for accreditation.
Funding & incentives
Service providers pay fees to HDANZ for survey and monitoring visits. Certification has been
mandatory for the MoH Safety Act since October 2002. From September 2005, it became
mandatory for physiotherapy services if they wanted a special contract from the Accident
Compensation Corporation (ACC). From September 2012, health funders made it mandatory
for Home Support providers. From March 2013, Southern Cross Health Society insurance made
certification mandatory for their affiliated providers.
Standards and measurement
Standards New Zealand is responsible for the standards. In 2003, the main standards were
Health and Disability Sector Standards and this includes Infection Control and Restraint
Minimisation. These were updated in 2008. In 2003, Home and Community Support Standards
were issued by SNZ and these were updated in 2012. In 2005, Allied Health Standards and Day
stay surgery standards were issued by SNZ.
The rating scale is:
CI = Continuous improvement
FA = Fully Attained
PA = Partially attained
UA = Unattained
The Ministry of Health uses the assessment ratings to determine certification. The length
of certification can vary from one to four years depending on the level of achievement of the
standards.
Assessment methodology & focus
Audit teams are formed for on-site visits and reporting to the relevant standards. This includes
documentation, observation, client records sampling, tracer methodology, and interviewing of
staff, management, clients and family.
Quality improvement is the focus and at the same time the provider has to have achieved the
standards being assessed, noting that areas identified for further work (PA / UA ratings) have to
have progress reported and are reviewed at the surveillance audit.
Assessors are paid per event and in addition to the two operational company Directors who
audit there is one employee auditor. HDANZ maintains two separate auditor networks; one
is for DAA / other services which includes about 20 assessors and is a mix of lead, clinical,
consumers, technical experts, cultural and financial auditors and the other is for Physiotherapy
services with an auditor network of 8 auditors.

International Accreditation Programme (IAP) ISQua Accreditation
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
71
Challenges
In October 2002 there were 10 DAAs and all but HDANZ had a formal status in either ISQua
health accreditation at the time or non-health ISO certification.
Development of HDANZ’s services and the infrastructure to deliver a range of audits.
Setting up the quality management system.
Lessons learned
Early investment in a customer relationship database was very important and then later
improved at identifying sub-groups for marketing and other information.
The two key drivers for this business are a) operational efficiency with competent
administration staff and b) assessor competency.
Added value for governance and robust organisational management from maintaining a 3rd
party accreditation status.
Sound decision as growth occurred to structure into programmes.
Costs need to be closely monitored and managed as they can easily escalate otherwise.
Outsourcing the financials in 2009 was a positive decision.
Maintaining NZQA auditor training course approval for credibility and HDANZ purpose
despite not being a revenue generator.
2008 investment into a marketing course reaped substantial dividends.
To be perceived as the expert.
Board / governance development in hindsight could have been more of a priority earlier on.
Appendix 1d.
Practice Incentive Program (PIP)
Country: Australia
Contributed by: Steve Clark
The Australian Government introduced the Practice Incentive Program (PIP) in 1998. The PIP is
aimed at supporting general practice activities that encourage continuing improvements and
quality care, enhance capacity and improve access and health outcomes for patients
21
.
In the 2015-16 Australian Government Budget, in excess of $1.5bn over four years
22
was
allocated to the PIP to support the continuation of incentive payments to general practices.
The PIP is used as a lever by government to influence behavioural change within the general
practice environment. To access payments under the PIP, practices must meet the eligibility
requirements, including that a practice must be accredited or registered for accreditation
against the Royal Australian College of General Practitioners (RACGP) Standards for general
practices and must maintain full accreditation.
Approximately 80% of all practices that meet the RACGP definition of a general practice
participate in accreditation and, therefore, may access PIP payments.

72
ISQua Accreditation International Accreditation Programme (IAP)
Guidance on Designing Healthcare External Evaluation Programmes including Accreditation
There are three types of payments available under the PIP
21
:
1. Practice Payments
The majority of payments through the PIP are made to practices and focus on those aspects
of general practice that contribute to quality care. These payments are intended to support
the practice to purchase new equipment, upgrade facilities or increase remuneration for GPs
working at the practice.
2. Service Incentive Payments
Service Incentive Payments (SIPs) are generally made to GPs to recognise and encourage
the provision of specified services to individual patients. The Cervical Screening, Asthma and
Diabetes incentives have service incentive payment components, and the Aged Care Access
Incentive is a service incentive payment only.
3. Rural Loading Payments
Practices participating in the PIP, with a main practice location situated outside capital cities
and other major metropolitan centres, are automatically paid a rural loading.
There are ten individual incentives available to general practices and GPs under the PIP
23
:
After-hours Incentive, supporting general practices to have appropriate arrangements in
place that ensure their patients have access to quality after-hours care.
Asthma Incentive, which aims to encourage GPs to better manage the clinical care of people
with moderate to severe asthma.
Cervical Screening Incentive, which aims to encourage GPs to screen under-screened
women for cervical cancer, and to increase overall screening rates.
Diabetes Incentive, which aims to encourage GPs to provide earlier diagnosis and effective
management of people with established diabetes mellitus.
eHealth Incentive, which aims to encourage general practices to keep up-to-date with the
latest developments in eHealth and adopt new eHealth technology as it becomes available.
GP Aged Care Access Incentive, which aims to encourage GPs to provide increased and
continuing services in Residential Aged Care Facilities.
Indigenous Health Incentive, which aims to support general practices and Indigenous health
services to provide better healthcare for Aboriginal and / or Torres Strait Islander patients,
including best practice management of chronic disease.
Procedural GP payment, which aims to encourage GPs in rural and remote areas to maintain
local access to surgical, anaesthetic and obstetric services.
Quality Prescribing Incentive, which aims to encourage practices to keep up-to-date with
information on the quality use of medicines.
Teaching payments, which aim to encourage general practices to provide teaching sessions
to undergraduate and graduate medical students who are preparing for entry into the
Australian medical profession.
Since the inception of the PIP in 1998, successive Australian Governments have committed to
ongoing funding for the programme; and during this time, have retained the requirement that a
practice must be accredited, or registered for accreditation, and must maintain full accreditation
in order to access such payments.
Given the level of participation in accreditation by Australian general practices, it can be
assumed that the highly incentivised PIP has been instrumental in encouraging practices to
engage in the process, and in turn has had a positive impact by supporting practices to focus on
improvements and quality outcomes.

International Society for Quality in Health Care
Joyce House, 8-11 Lombard Street East
Dublin 2, Ireland
Ph: +353 1 670 6750 Fax: +353 1 671 0395
Web: www.isqua.org
