
Chapter 6
Chirality: The Handedness
of Molecules

Table of Contents
1. Chirality and Stereochemistry (Chiral vs. achiral)
2. Isomerism: Constitutional Isomers and
Stereoisomers
3. Enantiomers and Chiral Molecules
4. Molecules Having One Chirality Center Are Chiral
5. More about the Biological Importance of Chirality
6. How to Test for Chirality: Planes of Symmetry
7. Naming Enantiomers: The R,S-System
8. Properties of Enantiomers: Optical Activity
9. The Origin of Optical Activity
10. Chiral Drugs
11. Molecules with More than One Chirality Center
12. Fischer Projection Formulas
13. Stereoisomerism of Cyclic Compounds

In this chapter we will consider:
How to identify, codify, and name the
three-dimensional arrangement of
atoms and molecules
How such arrangements can lead to
unique properties and behaviors

1. Chirality & Stereochemistry
An object is
achiral
(not chiral) if the
object and its mirror image are
identical
chiral
!

A
chiral
object is one that cannot be
superposed on its mirror image

Which are chiral objects?

1A. The Biological Significance of
Chirality
Chiral molecules are molecules that
cannot be superimposed onto their
mirror images
● One enantiomer
causes birth defects,
the other cures
morning sickness
Thalomide
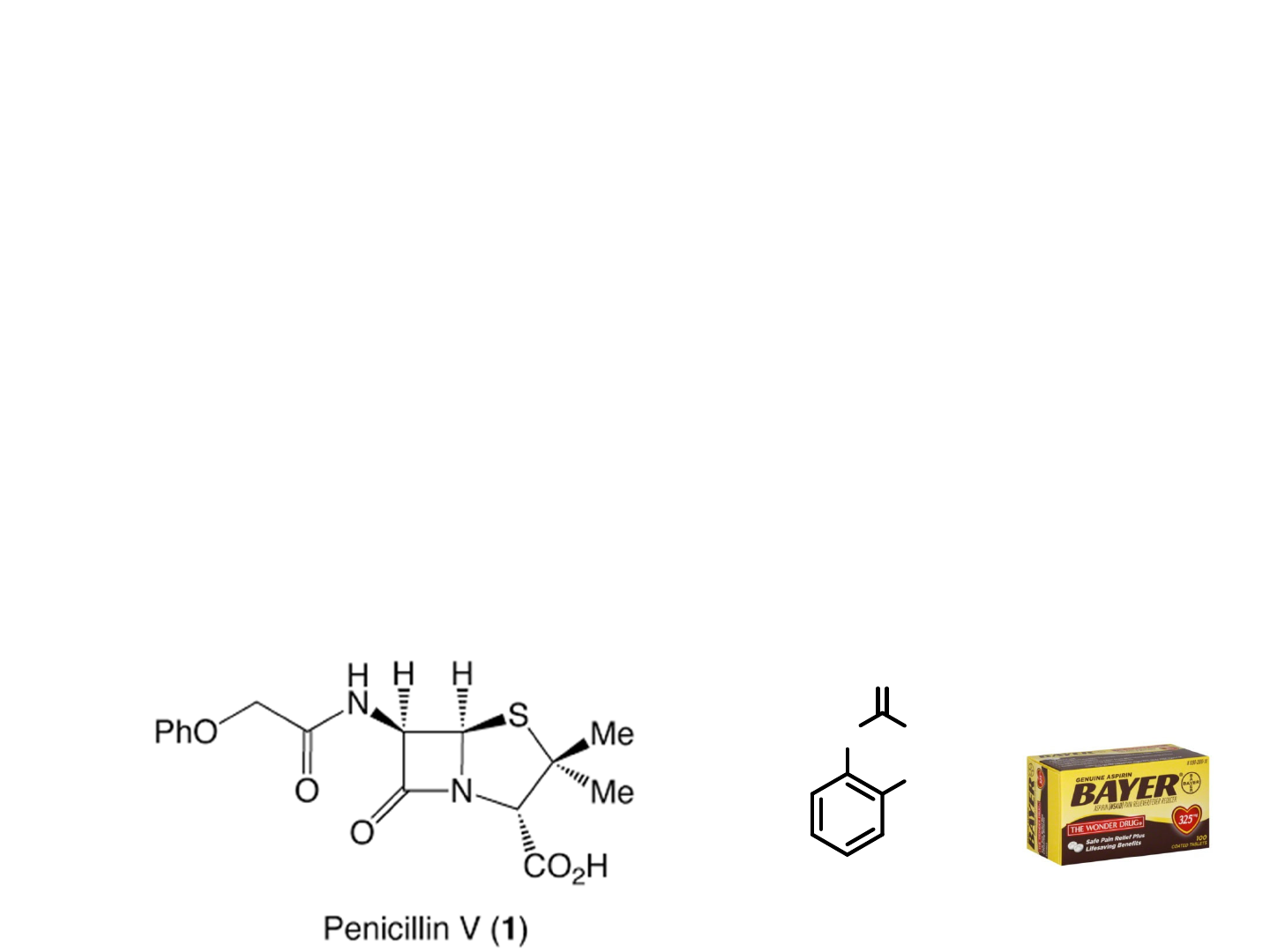
66% of all drugs in development are
chiral, 51% are being studied as a single
enantiomer
Of the $475 billion in world-wide sales of
formulated pharmaceutical products in
2008, $205 billion was attributable to
single enantiomer drugs
O
CO
2
H
O
CH
3
Aspirine

2. Isomerisom: Constitutional
Isomers & Stereoisomers
Isomers: different compounds that
have the same molecular formula
● Constitutional isomers: isomers
that have the same molecular
formula but different connectivity –
their atoms are connected in a
different order
2A. Constitutional Isomers

Examples
and
Butane
2-Methylpropane
Molecular
Formula
Cl
Cl
and
1-Chloropropane
2-Chloropropane
Constitutional
Isomers
C
4
H
10
C
3
H
7
Cl

Examples
Molecular
Formula
and
Butanoic acid
Methyl propanoate
OH
OCH
3
O
O
Constitutional
Isomers
C
2
H
6
O
C
4
H
8
O
2
OH
CH
3
OCH
3
and
Ethanol Methoxymethane

2B. Stereoisomers
Stereoisomers are NOT constitutional
isomers
Stereoisomers have their atoms
connected in the same sequence but
they
differ in the arrangement of their
atoms in space
. The consideration of
such spatial aspects of molecular
structure is called
stereochemistry

2C. Enantiomers & Diastereomers
Stereoisomers can be subdivided into
two general categories:
enantiomers
&
diasteromers
● Enantiomers – stereoisomers
whose molecules are
not
superposable
mirror images of
each other
● Diastereomers – stereoisomers
whose molecules are not mirror
images of each other

Geometrical isomers
(
cis
&
trans
isomers) are:
●
Diastereomers
e.
g
.
(trans)
Ph
Ph
Ph Ph
(cis)
and
Cl
H
Cl
H
H
Cl
Cl
H
(trans)(cis)
and

Subdivision of Isomers
Isomers
(different compounds with same
molecular formula)
Constitutional Isomers
(isomers whose atoms
have a different
connectivity)
Stereoisomers
(isomers that have the same
connectivity but differ in spatial
arrangement of their atoms)
Enantiomers
(stereoisomers that are
nonsuperposable mirror
images of each other)
Diastereomers
(stereoisomers that are
NOT mirror images of
each other)

3. Enantiomers and Chiral
Molecules
Enantiomers occur only with compounds
whose molecules are chiral
A chiral molecule is one that is NOT
superposable on its mirror image
The relationship between a chiral
molecule and its mirror image is one
that is
enantiomeric
. A chiral
molecule and its mirror image are said
to be enantiomers of each other

OH
(2-Butanol)
OH
H
(I)
HO
H
(II)
(I) and (II) are
not superposable
mirror images of
each other

4. Molecules Having One Chirality
Center Are Chiral
A chirality center is a tetrahedral
carbon atom that is bonded to four
different groups
Or stereo center…
A molecule that contains one chirality
center is chiral and can exist as a pair
of enantiomers

The presence of a single chirality
center in a molecule guarantees that
the molecule is chiral and that
enantiomeric forms is a possibility
An important property of enantiomers
with a single chirality center is that
interchanging any two groups at the
chirality center converts one
enantiomer into the other

Any atom at which an interchan
g
e of
g
roups
produces a stereoisomer is called a stereo-
genic center (if the atom is a carbon atom
it is usually called a stereogenic carbon,
or
stereocenter
or
chiral carbon
)
If all of the tetrahedral atoms in a molecule
have two or more groups attached that
are
the same
, the molecule does not
have a chirality center. The
molecule is superposable on
its mirror ima
g
e and is achiral

Me Et
Cl
H
(III)
C
H
EtMe
Cl
*
mirror
MeEt
Cl
H
(IV)
(III) and (IV) are non-superposable
mirror images of each other
same
as

4A. Tetrahedral Stereogenic Centers
Chirality centers are
tetrahedral
stereogenic
centers
Me Et
OH
H
*
(A)
mirror
MeEt
HO
H
*
(B)
Tetrahedral
stereogenic
center
⇒ chiral
(A) & (B) are
enantiomers
5. More about the Biological
Importance of Chirality

Chirality in Biomolecules
Figure 6.7 Schematic diagram of the
surface of an enzyme capable of
distinguishing between enantiomers.

6. How to Test for Chirality:
Planes of Symmetry
A molecule will not be chiral if it
possesses a plane of symmetry
A plane of symmetry (mirror plane) is an
imaginary plane that bisects a molecule
such that
the two halves of the molecule
are mirror images
of each other
All molecules with a plane of symmetry
in their most symmetric conformation
are
achiral

Planes of Symmetry
Figure 6.2 Plane of Symmetry: An
imaginary plane passing through an
object and dividing it such that one half
is the mirror image in of the other half.

Me Me
Cl
H
Me Et
Cl
H
Plane of symmetry
No plane of
symmetry
achiral
chiral

Chirality in Cyclic Molecules
2-Methylcyclopentanol
● 2 stereocenters; according to the 2
n
rule, a maximum of 2
2
= 4
stereoisomers are possible.
● How many actually exist? Answer
four; two pairs of enantiomers.

Enantiomers & Diastereomers
2,3,4-Trihydroxybutanal
● Figure 6.4 Two stereocenters; 2
2
= 4
stereoisomers (two pairs of enantiomers)
are possible.
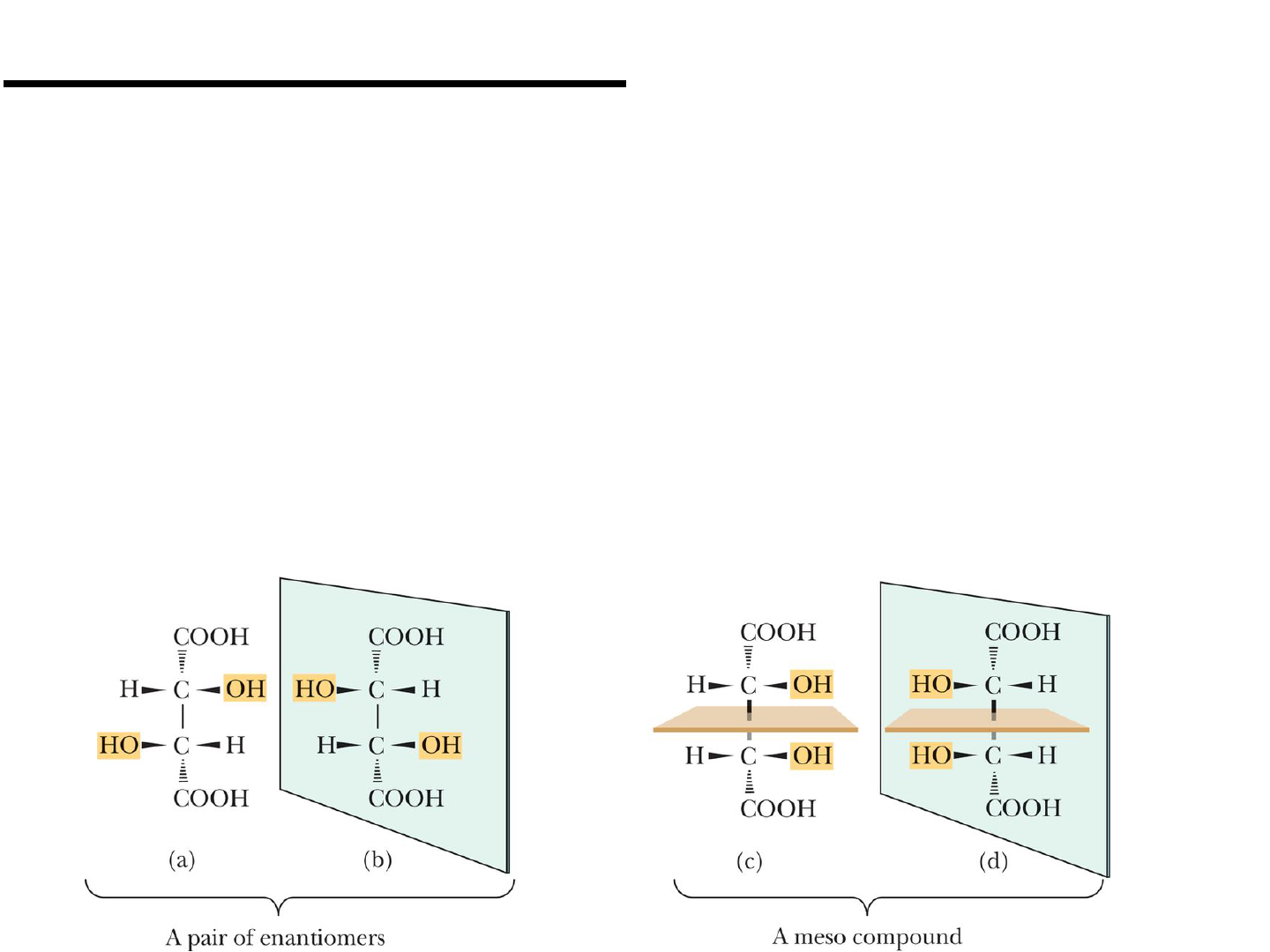
Meso Compounds
Meso compound: an achiral compound
possessing two or more stereocenters.
● Tartaric acid contains two stereocenters.
● Figure 6.5 Two stereocenters; 2
n
= 4,
but only three stereoisomers exist, one
meso compound and one pair of
enantiomers.

7. Naming Enantiomers:
The
R
,
S
-System
Using only the IUPAC naming that we have
learned so far, these two enantiomers will
have the same name:
● 2-Butanol
This is undesirable because each compound
must have its own distinct name
OH
H
(I)
HO
H
(II)
OH
Recall:

Rule 1
● Assign priorities to the four different
groups on the stereocenter from
highest to lowest (priority bases on
atomic number, the higher the
atomic number, the higher the
priority)
7A. How to Assign (R) and (S)
Configurations

Rule 2
● When a priority cannot be assigned
on the basis of the atomic number
of the atoms that are directly
attached to the chirality center, then
the next set of atoms in the
unassigned groups is examined.
This process is continued until a
decision can be made.

Rule 3
● Visualize the molecule so that the
lowest priority group is directed
away from you, then trace a path
from highest to lowest priority. If
the path is a clockwise motion, then
the configuration at the asymmetric
carbon is (
R
) “
Rectus
.” If the path
is a counter-clockwise motion, then
the configuration is (
S
) “
Sinister
.”

C
H
2
C
H
3
C
O
H
Step 2:
CH
3
Example
HO
H
(2-Butanol)
①
③
④
② or ③ ② or ③
CC
O
H
Step 1:
① ④
②
(H, H, H) (C, H, H)

OH
EtMe
①
④
②③
OH
Et
Me
H
OH
EtMe
EtMe
HO
H
=
OH
Et
Me
H
Arrows are clockwise
(
R
)-2-Butanol
OCH
3
H
3
C
CH
2
CH
3
Br
Other examples
①
④
②
③
Cl
H
CH
3
HO
Cl
CH
3
HO
Counter-
clockwise
(
S
)
①
④
②
③
OCH
3
CH
2
CH
3
Br
Clockwise
(
R
)

Cl
H
OH
Br
Cl
Br
H
HO
Other examples
①
④
②
③
Cl
OHBr
Clockwise
(
R
)
● Rotate C–Cl bond such that H is pointed
to the back

OCH
3
H
I
H
3
C
H
I
OCH
3
H
3
C
Other examples
①
④
②
③
OCH
3
IH
3
C
● Rotate C–CH
3
bond such that H is
pointed to the back
Counter-clockwise
(
S
)

Rule 4
● For groups containing double or
triple bonds, assign priorities as if
both atoms were duplicated or
triplicated
e.g.
CO
C
O
O
C
as
CC
C
C
C
C
as
CC C
C
C
C
C
as
C

Example
CH
3
H
CH=CH
2
HO
①
④
②
③
(
S
)
CH
3
CH CH
2
C
H
C
C
C
H
H
CH
3
CH CH
2
Compare
&
:
equivalent to
Thus,
(H, H, H)
(C, C, H)
CH CH
2

Other examples
OH
H
Cl
CH
3
O
①
④
②
③
(
R
)
H
2
C
H
Cl
CH
3
O
OH
①
④
②
③
(
S
)
C (O, O, C)
C (O, H, H)
②
③

8. Properties of Enantiomers:
Optical Activity
Enantiomers
● Mirror images that are not
superposable
mirror
H
3
CH
2
C
*
CH
3
H
Cl
CH
2
CH
3
*
H
3
C
H
Cl
Enantiomers have identical physical
properties (e.g. melting point, boiling
point, refractive index, solubility etc.)
Compound bp (
o
C) mp (
o
C)
(–)-(
R
)-2-Butanol 99.5
(+)-(
S
)-2-Butanol 99.5
(+)-(
R,R
)-Tartaric Acid 168 – 170
(–)-(
S,S
)-Tartaric Acid 168 – 170
(+/–)-Tartaric Acid 210 – 212

Enantiomers
● Have the same chemical properties
(except reaction/interactions with chiral
substances)
● Show different behavior only when they
interact with other chiral substances
● Rotate plane-polarized light in opposite
direction
Optical activity
● The property possessed by chiral
substances of rotating the plane of
polarization of plane-polarized light

The electric field (like the magnetic
field) of light is oscillating in all
possible planes
When this light passes through a
polarizer (Polaroid lens), we get plane-
polarized light (oscillating in only one
plane)
Polaroid
lens
8A. Plane-Polarized Light

A device for measuring the optical
activity of a chiral compound
8B. The Polarimeter
=observed
optical rotation

8C. Specific Rotation
D
[] =
25
c
x
temperature
observed
rotation
wavelength
of light
(e.g. D-line
of Na lamp,
=589.6 nm)
concentration
of sample
solution
in g/mL
length of cell
in dm
(1 dm = 10 cm)
ℓ

The value of depends on the
particular experiment (since there are
different concentrations with each run)
● But specific rotation [
] should be
the same regardless of the
concentration

Two enantiomers should have the
same value of specific rotation, but the
signs are opposite
mirror
HO
*
CH
2
CH
3
CH
3
H
[] = + 13.5
o
25
D
OH
*
H
3
CH
2
C
CH
3
H
[] = 13.5
o
25
D

An equimolar mixture of two enantiomers
is called a racemic mixture (or
racemate
or
racemic form
)
A racemic mixture causes no net rotation
of plane-polarized light
9. The Origin of Optical Activity
9A Racemic Forms
H
C
2
H
5
CH
3
OH
(R)-2-Butanol
H
C
2
H
5
H
3
C
HO
(S)-2-Butanol
(if present)
rotation
equal & opposite
rotation by the
enantiomer

A sample of an optically active
substance that consists of a single
enantiomer is said to be
enantiomerically pure or to have an
enantiomeric excess of 100%
9B. Racemic Forms and Enantiomeric
Excess

An enantiomerically pure sample of (
S
)-(+)-
2-butanol shows a specific rotation of
+13.52
D
[] = +13.52
25
A sample of (S)-(+)-2-butanol that contains
less than an equimolar amount of (
R
)-(–)-2-
butanol will show a specific rotation that is
less than 13.52 but greater than zero
Such a sample is said to have an
enantiomeric excess
less than 100%
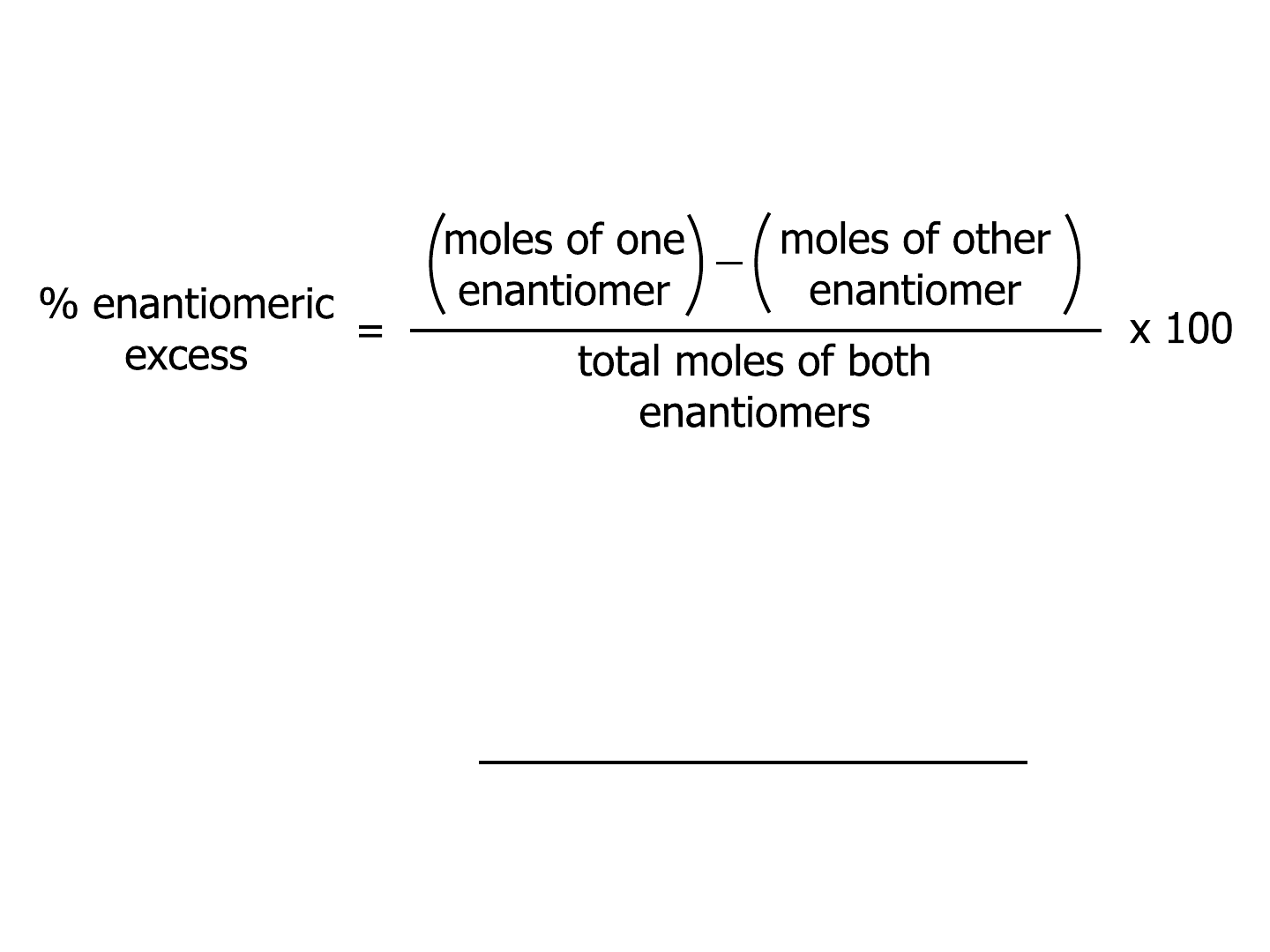
Enantiomeric excess (ee)
● Also known as the optical purity
% enantiomeric
excess *
observed speci
f
ic rotation
specific rotation of the
pure enantiomers
=
x 100
● Can be calculated from optical
rotations

Example
● A mixture of the 2-butanol
enantiomers showed a specific
rotation of +6.76. The
enantiomeric excess of the (S)-(+)-
2-butanol is 50%
% enantiomeric
excess *
+6.76
+13.52
=
x 100 = 50%

Three Or More Stereocenters
Problem:
● How many stereocenters are present in the
molecule on the left?
● How many stereoisomers are possible?
● One of the possible stereoisomers is menthol.
● Assign an R or S configuration to each
stereocenter in menthol.
