
Wiggling Through the World: The mechanics of slithering locomotion depend on the
surroundings
Author(s): Daniel I. Goldman and David L. Hu
Source:
American Scientist
, July-August 2010, Vol. 98, No. 4 (July-August 2010), pp.
314-323
Published by: Sigma Xi, The Scientific Research Honor Society
Stable URL: https://www.jstor.org/stable/27859538
JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide
range of content in a trusted digital archive. We use information technology and tools to increase productivity and
facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected].
Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at
https://about.jstor.org/terms
is collaborating with JSTOR to digitize, preserve and extend access to
American Scientist
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms

Wiggling Through the World
The mechanics of slithering locomotion depend on the surroundings
Daniel I. Goldman and David L. Hu
Movement is critical for the surviv
al of animals. Eels swim, hawks
soar,' moles tunnel and squirrels leap
to perform vital tasks such as foraging,
mating and escaping pr?dation. These
feats of locomotion involve the coordi
nation of complex biophysical process
es that span scales from the tiny (ion
channels in nerve fibers that depolar
ize to send and receive information) to
the intermediate (muscles and tendons
coupling to skeletal elements to gener
ate motion of body parts) to the large
(interaction of body parts such as feet
and hands with their surroundings to
effectively generate traction). A goal of
locomotion science is to uncover gen
eral principles of movement through
the development of models across
sizes. This challenge requires the col
laboration of biologists, physicists, math
ematicians and engineers. Such animal
locomotion studies are also inspiring the
design of vehicles with mobility equal to
or greater than that of animals.
Many animals move effectively with
out the use of limbs. Some legged liz
Daniel I. Goldman is an assistant professor in the
School of Physics at the Georgia Institute of Tech
nology. He received his PhD. in physics from the
University of Texas at Austin in the Center for Non
linear Dynamics, studying fluidization of granular
media. He followed with postdoctoral training at the
University of California at Berkeley in the Depart
ment of Integrative Biology, studying biomechan
ics of climbing and running cockroaches. He has
been awarded a Burroughs Wellcome Fund Career
Award at the Scientific Interface and a Sigma Xi
Young Faculty Award. David L. Hu is an assistant
professor of mechanical engineering and biology at
the Georgia Institute of Technology. He earned his
doctorate in mathematics from the Massachusetts
Institute of Technology, then served as an NSF
postdoctoral fellow and an instructor at the Applied
Math Lab of New York University. Address for
Goldman: School of Physics, 837 State Street NW,
Georgia Institute of Technology, Atlanta, G A 30332
0430. E-mail: [email protected]
ards, for example, are known to forsake
their limbs entirely and wiggle their
bodies to move through dense grasses
or sandy environments. For other crea
tures, such as the thousands of species
of snakes, slugs and worms (and a few
lizards), legs were so superfluous that
they have been completely limbless
for millions of years. Their long, flex
ible bodies enable them* to enter tight
crevices and to traverse long distances
through complex and often tortuous
substrates such as the tops of trees, un
derneath the soil or inside the digestive
tracts of other organisms.
Our recent laboratory work and
models of terrestrial limbless locomo
tion have elucidated the mechanisms
that make undulatory locomotion effec
tive in two distinct environments: above
ground where snakes slither (Hu) and
within flowing substrates such as sand
where sandfish lizards "swim" (Gold
man). We hope to give a glimpse of
how such animals can move at speeds
of several body-lengths per second by
describing the movement of snakes and
sandfish in turn,' and drawing attention
to the specific adaptations these animals
use to enhance their performance in par
ticular habitats. Although the motion of
these snakes and sandfish may appear
similar, these animals propel themselves
using substrate interactions that are dis
tinct to their respective environments, as
we have determined with mathematical
and physical modeling.
Making the Model
The common features of our approaches
to mathematical modeling are derived
from applying what's called the resistive
force technique, developed to model the
locomotion of small organisms in fluids.
Consider a small rod-shaped "slice" of
an undulating reptile. Muscular forces
acting on the segment, combined with
the inertia of the body, generate equal
and opposite reaction forces from the an
imal's environment. If the animal moves
its body in particular ways, the sum of all
the reaction forces on the animal's body
can propel the animal's center of mass
forward. To simplify our model, we will
assume that our animals undulate in a
plane. Thus we can decompose the force
acting on the segment into two pieces,
one tangential and the other perpendicu
lar to the segment's surface. These are
called the axial and normal forces, respec
tively (see Figure 2). For an undulating
animal to move, the summation of the
forward components of the normal forc
es on the animal must exceed the back
ward components of the axial forces. To
determine if that is the case, we first must
calculate the reaction forces from the
animal's environment. Although math
ematically this approach seems straight
forward, it requires input of models of
the environment, and this is where the
new modeling challenge begins.
In fluid environments, such as those
typically encountered by swimming
spermatozoa, nematodes or sea snakes,
we can use what are called the Navier
Stokes equations to account for force pro
duced by flows. These equations, named
after physicists Claude-Louis Navier and
George Gabriel Stokes, apply Newton's
second law to fluid motion, and account
for pressure and viscosity, in order to
describe fluid movement. However,
these partial differential equations are
often impossible to solve analytically,
so there are many approximate models
(such as Stokes' Law for viscously dom
inated disturbances) that can be used to
rapidly calculate forces experienced by
swimmers and fliers.
In contrast, environmental models for
terrestrial locomotion can be more com
plex. Although dry friction can describe
interactions with spme surfaces, we lack
validated equations for many materials
such as mud and sand. For surfaces that
314 American Scientist, Volume 98
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms
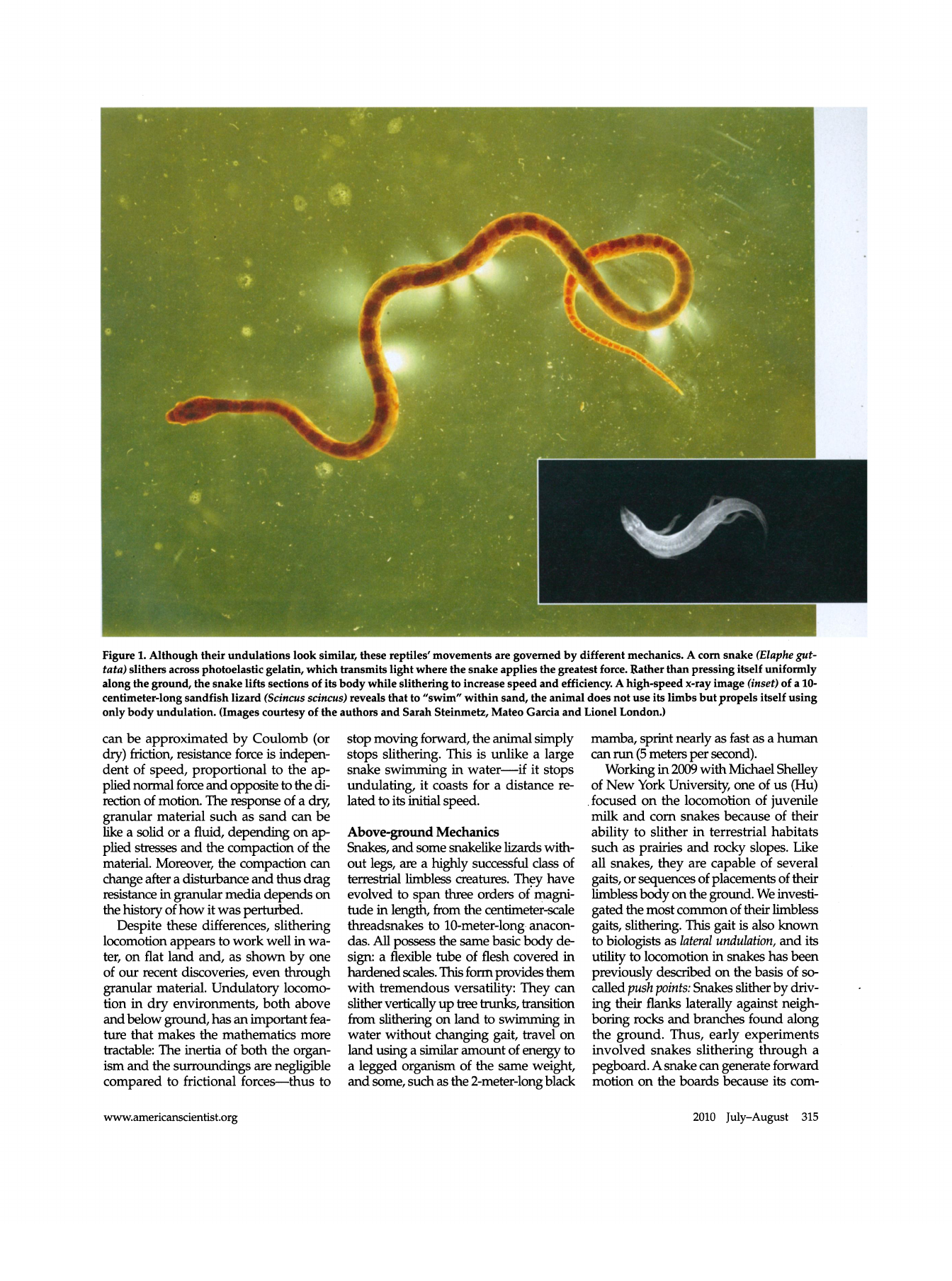
Figure 1. Although their undulations look similar, these reptiles' movements are governed by different mechanics. A corn snake (Elaphe gut
tata) slithers across photoelastic gelatin, which transmits light where the snake applies the greatest force. Rather than pressing itself uniformly
along the ground, the snake lifts sections of its body while slithering to increase speed and efficiency. A high-speed x-ray image (inset) of a 10
centimeter-long sandfish lizard (Scincus scincus) reveals that to "swim" within sand, the animal does not use its limbs but propels itself using
only body undulation. (Images courtesy of the authors and Sarah Steinmetz, Mateo Garcia and Lionel London.)
can be approximated by Coulomb (or
dry) friction, resistance force is indepen
dent of speed, proportional to the ap
plied normal force and opposite to the di
rection of motion. The response of a dry,
granular material such as sand can be
like a solid or a fluid, depending on ap
plied stresses and the compaction of the
material. Moreover, the compaction can
change after a disturbance and thus drag
resistance in granular media depends on
the history of how it was perturbed.
Despite these differences, slithering
locomotion appears to work well in wa
ter, on flat land and, as shown by one
of our recent discoveries, even through
granular material. Undulatory locomo
tion in dry environments, both above
and below ground, has an important fea
ture that makes the mathematics more
tractable: The inertia of both the organ
ism and the surroundings are negligible
compared to frictional forces?thus to
stop moving forward, the animal simply
stops slithering. This is unlike a large
snake swimming in water?if it stops
undulating, it coasts for a distance re
lated to its initial speed.
Above-ground Mechanics
Snakes, and some snakelike lizards with
out legs, are a highly successful class of
terrestrial limbless creatures. They have
evolved to span three orders of magni
tude in length, from the centimeter-scale
threadsnakes to 10-meter-long anacon
das. All possess the same basic body de
sign: a flexible tube of flesh covered in
hardened scales. This form provides them
with tremendous versatility: They can
slither vertically up tree trunks, transition
from slithering on land to swimming in
water without changing gait, travel on
land using a similar amount of energy to
a legged organism of the same weight,
and some, such as the 2-meter-long black
mamba, sprint nearly as fast as a human
can run (5 meters per second).
Working in 2009 with Michael Shelley
of New York University, one of us (Hu)
. focused on the locomotion of juvenile
milk and corn snakes because of their
ability to slither in terrestrial habitats
such as prairies and rocky slopes. Like
all snakes, they are capable of several
gaits, or sequences of placements of their
limbless body on the ground. We investi
gated the most common of their limbless
gaits, slithering. This gait is also known
to biologists as lateral undulation, and its
utility to locomotion in snakes has been
previously described on the basis of so
called push points: Snakes slither by driv
ing their flanks laterally against neigh
boring rocks and branches found along
the ground. Thus, early experiments
involved snakes slithering through a
pegboard. A snake can generate forward
motion on the boards because its com
www.americanscientist.org 2010 July-August 315
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms
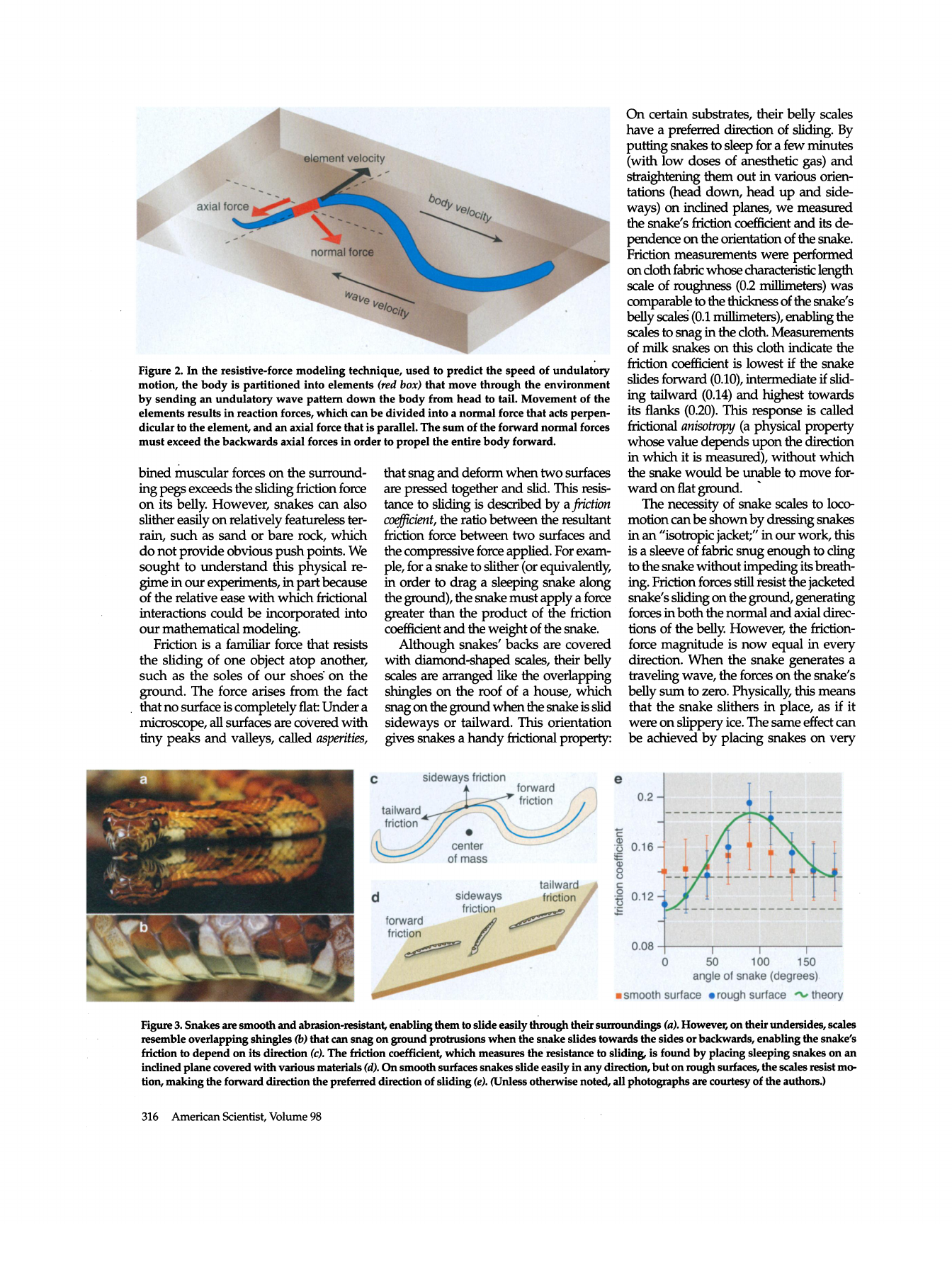
Figure 2. In the resistive-force modeling technique, used to predict the speed of undulatory
motion, the body is partitioned into elements (red box) that move through the environment
by sending an undulatory wave pattern down the body from head to tail. Movement of the
elements results in reaction forces, which can be divided into a normal force that acts perpen
dicular to the element, and an axial force that is parallel. The sum of the forward normal forces
must exceed the backwards axial forces in order to propel the entire body forward.
bined muscular forces on the surround
ing pegs exceeds the sliding friction force
on its belly. However, snakes can also
slither easily on relatively featureless ter
rain, such as sand or bare rock, which
do not provide obvious push points. We
sought to understand this physical re
gime in our experiments, in part because
of the relative ease with which frictional
interactions could be incorporated into
our mathematical modeling.
Friction is a familiar force that resists
the sliding of one object atop another,
such as the soles of our shoes on the
ground. The force arises from the fact
that no surface is completely flat: Under a
microscope, all surfaces are covered with
tiny peaks and valleys, called asperities,
that snag and deform when two surfaces
are pressed together and slid. This resis
tance to sliding is described by a friction
coefficient, the ratio between the resultant
friction force between two surfaces and
the compressive force applied. For exam
ple, for a snake to slither (or equivalently,
in order to drag a sleeping snake along
the ground), the snake must apply a force
greater than the product of the friction
coefficient and the weight of the snake.
Although snakes' backs are covered
with diamond-shaped scales, their belly
scales are arranged like the overlapping
shingles on the roof of a house, which
snag on the ground when the snake is slid
sideways or tailward. This orientation
gives snakes a handy frictional property:
On certain substrates, their belly scales
have a preferred direction of sliding. By
putting snakes to sleep for a few minutes
(with low doses of anesthetic gas) and
straightening them out in various orien
tations (head down, head up and side
ways) on inclined planes, we measured
the snake's friction coefficient and its de
pendence on the orientation of the snake.
Friction measurements were performed
on cloth fabric whose characteristic length
scale of roughness (0.2 millimeters) was
comparable to the thickness of the snake's
belly scales (0.1 millimeters), enabling the
scales to snag in the cloth. Measurements
of milk snakes on this cloth indicate the
friction coefficient is lowest if the snake
slides forward (0.10), intermediate if slid
ing tailward (0.14) and highest towards
its flanks (0.20). This response is called
frictional anisotrop)) (a physical property
whose value depends upon the ctoection
in which it is measured), without which
the snake would be unable to move for
ward on flat ground.
The necessity of snake scales to loco
motion can be shown by dressing snakes
in an "isotropie jacket;" in our work, this
is a sleeve of fabric snug enough to cling
to the snake without impeding its breath
ing. Friction forces still resist the jacketed
snake's sliding on the ground, generating
forces in both the normal and axial direc
tions of the belly. However, the friction
force magnitude is now equal in every
direction. When the snake generates a
traveling wave, the forces on the snake's
belly sum to zero. Physically, this means
that the snake slithers in place, as if it
were on slippery ice. The same effect can
be achieved by placing snakes on very
sideways friction
c
0.16
0.12
0.08?
0 50 100 150
angle of snake (degrees)
smooth surface ?rough surface *v theory
Figure 3. Snakes are smooth and abrasion-resistant, enabling them to slide easily through their surroundings (a). However, on their undersides, scales
resemble overlapping shingles ) that can snag on ground protrusions when the snake slides towards the sides or backwards, enabling the snake's
friction to depend on its direction (c). The friction coefficient, which measures the resistance to sliding, is found by placing sleeping snakes on an
inclined plane covered with various materials (d). On smooth surfaces snakes slide easily in any direction, but on rough surfaces, the scales resist mo
tion, making the forward direction the preferred direction of sliding (e). (Unless otherwise noted, all photographs are courtesy of the authors.)
316 American Scientist, Volume 98
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms

Figure 4. To test the necessity of snake scales to slithering locomotion, a snake is fitted with an
"isotropie jacket" that prevents its scales from interacting with the ground. When the jacketed
snake tries to slither, it creates frictional propulsive forces whose magnitude is independent of the
direction of motion. Consequently, these forces sum to zero, causing the snake to slither in place.
smooth surfaces such as plastic. On such
substrates, snakes are unable to prog
ress forward unless they lift their bodies
while slithering or transition to another
gait (such as sidewinding, or concertina
mode, in which the snake folds itself like
the pleats of an accordion).
In our mathematical model, we used
the snake's scale properties to determine
the steady speed of the snake's center
of mass. The inputs to the model in
clude the belly friction coefficients and
characteristics of the snake's undulation
kinematics (frequency, wavelength and
amplitude). We assume that along the
snake's length, its weight is uniformly
applied to the ground. Figure 6 shows
our virtual snake juxtaposed by green
arrows denoting the magnitude and di
rection of the friction forces acting on
the snake's belly. The components, of the
arrows pointing in the snake's direction
of motion are responsible for its propul
sion; the remaining components indicate
directions in which the snake's energy is
wasted. Upon adding all the frictional
forces, we were surprised to find that the
speed of our virtual snake was only half
that of the snake we observed in the lab
(8 centimeters per second, or 0.2 body
lengths per second). Some part of snake
behavior clearly was not being captured
in our simplified mathematical model.
Previous investigators have observed
snakes altering their weight distribution
by lifting the peaks and troughs of their
undulating bodies, concentrating their
weight on the rentiaining points of contact
with the ground. This body lifting is most
clear in sidewinding, in which the snake
travels laterally and leaves a trail in sand
that resembles discrete "footsteps" rather
than a continuous winding path. Our
experiments with snakes on mirrored
surfaces and photoelastic gelatin (which
transmits light when compressed) indi
cate that snakes are also capable of lifting
their bellies while they slither forward.
In our model, we showed theoretically
that such dynamic load balancing leads
to increases in speed of 35 percent and in
efficiency of 50 percent. Why such a large
advantage? Figure 6 shows the directions
of propulsive forces (friction) everywhere
along the snake. The peaks and troughs
of the curves show propulsive-force ar
rows that point normal to the direction
of snake motion, the direction in which
energy is wasted. Because friction is pro
portional to the weight applied, the snake
generates more thrust if it lifts its body in
these regions and increases its weight
elsewhere. Thus, slithering shares certain
features with human walking. When we
walk, we transfer weight from our hind
foot to the leading foot by lifting the hind
foot rather than dragging it. Similarly, a
snake lifts the parts of its body that are
doing the least useful work. This adjust
ment is a simple change to their weight
distribution that snakes can perform. By
working to understand weight distri
butions further, we may one day know
how speedier snakes such as the black
mamba can move so quickly.
Underground Mechanics
Many desert organisms, including
snakes, moles, lizards and scorpions,
disappear into sand to avoid predators
and heat as well as to catch prey. The
sandfish, which one of us (Goldman)
studies, is a 10-centimeter-long desert
lizard with fringed toes on its four limbs,
a shovel-shaped snout and a flattened
belly and flanks?these features are hy
pothesized to aid it in burying itself and
sand-swimming. Like snakes, ite scales
are smooth and abrasion resistant. Un
like snakes, the belly scales on the sand
fish do not overlap. Although there have
been many hypotheses about how such
organisms move within a medium, un
til our work there had been almost no
detailed studies of kinematics, in part
Figure 5. A time-lapse illustration shows the differing results of a snake slithering across a rough
surface (top) versus a smooth surface (bottom). On both surfaces, the snake generates waves of
constant amplitude, wavelength and frequency. However, only on the rough surface do the fric
tional forces generated create forward motion. On the smooth surface, the snake slithers in place.
www.americanscientist.org
2010 July-August 317
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms

Figure 6. A corn snake is placed on a mirrored surface (a) in order to see when there is a reflection of the belly, indicating that the snake has
lifted its body. Models use the snake's frictional properties and the kinematics of its body wave to calculate the frictional propulsive forces for
snakes with a uniform (b) and a nonuniform (c) weight distribution. The blue curves trace the snake's orientation, the orange dots show its cen
ter of mass, and green arrows indicate the direction and magnitude of the friction force applied by the ground to the snake as it moves from left
to right. Arrows pointing to the left are thrust forces; ones in other directions show energy wasted. Orange lines show sections of the body with
a normal force less than the weight of the section. Lifting increases the amount of forward force at the snake's inflection points (black dots).
because opaque sand makes subsurface
visualization challenging.
The sandfish uses its limbs to move
rapidly on the surface of the sand but
when startled, it points its snout down
and quickly disappears (within half a
second) beneath the sand. Once fully
submerged, the sandfish is quite chal
lenging to locate. The physics that gov
erns the propulsive forces in the granular
world into which the sandfish descends
are quite different from the frictional
forces that are important for slithering on
solid surfaces, mainly because granular
materials such as sand can yield (flow)
and solidify in response to perturbation.
Desert sand is typically dry and com
posed of roughly spherical particles from
0.1 to 0.3 millimeters in diameter that
only interact on contact through energy
dissipating forces such as viscoelastic
ity, plastic deformation and friction. De
pending on the applied stress, granular
media can display a range of physical
behaviors with features characteristic of
gases, fluids and solids. For example,
a pile of grains on a flat board behaves
like a yield-stress fluid: It acts like a solid
if the pile is not tilted too much, but at
sufficiently high angles of inclination
it undergoes a transition to a fluid that
flows downhill. There is not yet a funda
mental comprehension of the mechanics
of these materials at the level known for
fluids such as water and air.
The behavior of granular media is
sensitive to their conditions and prepa
ration: One of us (Goldman) has recently
investigated how a parameter called the
packing fraction?the ratio of the material
volume of grains to the occupied vol
ume?controls the material's response
to sustained perturbation (such as the
movement of an object through the
sand). Although the range of naturally
occurring disordered packings occupied
by approximately spherical dry grains
is small (58 percent at the loosest and 63
percent at the most tightly packed), these
different states behave quite differently:
A loosely packed collection of grains be
haves in a more fluidlike manner, flow
ing smoothly in response to disturbance,
whereas in a tightly packed collection of
grains the drag force nearly doubles and
the grains flow in a halting, abrupt man
ner when an object is dragged through
them. This is a consequence of the ability
of loose packings to flow by particles
pushing into free volume, whereas tight
packings flow as groups of particles cre
ate new volume by expansion (called di
lation). Unlike in fluids such as water, in
which force on a moving object increases
with rising velocities, in granular me
dia for low enough velocities, forces on
objects are approximately independent
of speed, because velocity-independent
frictional interactions dominate the par
ticle interactions in this regime.
Modeling such behavior is also a chal
lenge. Although there has been much
progress in describing the gaslike state
of shaken granular media, drag laws are
not available for flows in which fluid
and solid states coexist. A successful ap
proach to modeling these materials is
to instead apply a more "brute force"
approach?let a computer follow the
motion and interaction of millions of in
dividual grains subject to collision rules
and gravity. This procedure is called mo
lecular dynamics. Once validated, such
models can give insight into particle
level flows, Auctioning as a virtual mi
croscope into the medium.
A Quick Burial
It is fascinating to contemplate how a
sandfish moves within sand. Does the
animal use its limbs to paddle or does
it undulate like an eel, or both? Once
its head breaks up the material, does
it remain a fluid or does it solidify fast
enough that subsequent portions of the
body have to refluidize it? Do chang
es in material compaction (loosely to
tightly packed sand) change the behav
ior and movement pattern of the sand
318 American Scientist, Volume 98
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms

Figure 7. It is difficult to see the pattern of a snake adjusting its weight as it slithers, because the snake's body is often in contact with the ground. One
way to visualize regions of higher applied force is to film a snake slithering on gelatin, illuminated from below, between cross-polarizing filters (left).
The orientation of the filters initially blocks the polarized light from reaching the imager, but when the snake pushes on the gelatin, the light is ro
tated and can be detected by the camera, as shown by the luminous regions (middle and right). Although the adhesiveness of the gelatin can interfere
with the snake's natural locomotion, this technique suggests that less of the snake may be in contact with the ground than was previously believed.
fish? To investigate questions such as
these, one of our (Goldman's) doctoral
students, Ryan Maladen, uses a combi
nation of x-ray imaging techniques to
visualize subsurface motion.
To control the properties of the sand
encountered by the animal, we use a
custom-made fluidized bed, a device in
which a collection of grains placed into a
container with a porous bottom is driven
upward by a flow of air. Below a critical
flow rate, the grains remain in a solid
state, but above this threshold, the grains
take on the properties of a fluid. Once the
airflow is stopped, the grains settle into
a loosely packed state; subsequent per
turbations by either pulses of air or con
trolled vibrations to the bed can create
repeatable states of different compaction.
EHiring experiments, the sandfish is
placed in a holding pen connected to
the bed, then a gate on the pen is lifted.
Once the animal realizes it has access to
the sand, it immediately runs out of the
pen toward the material, with its back
straight and using its limbs for propul
sion. High-speed video reveals that to
bury itself the animal uses a combina
tion of its limbs and its body to push
itself into the sand. The burial time is
rapid and does not depend significantly
on the packing fraction of the sand.
Once below the surface of the sand,
direct visualization with high-speed
cameras become impossible. To image
movement within the material, we rely
on high-speed x-ray video. The sand
(and the lizard) are placed between an
x-ray source and a scintillating material
(called an image intensifier), which con
verts the x-ray photons into electrons,
Figure 8. The sandfish lizard, native to the deserts of north Africa, uses its shovel-shaped
snout, smooth skin and powerful body to undulate through sand at speeds of up to two body
lengths per second. The 10-centimeter-long reptile can bury itself in less than a second. On
the surface it uses its limbs for locomotion, but once buried, it holds its limbs at its sides and
moves solely by propagating a sinusoidal wave along its body.
www.americanscientist.org 2010 July-August 319
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms

time (seconds)
Figure 9. Drag force on a small rod as a function of time demonstrates the difference in resistance
between loosely (blue) and tightly (red) packed granular media. The force in loosely packed par
ticles is constant after an initial transient increase when material flows around the rod. Tightly
packed material displays large oscillations in force as material in front of the rod periodically
yields and a block of solidified material is forced to the surface. The rod (inset) is 2 centimeters
long and is dragged at 1 centimeter per second; the particles are 0.3-millimeter glass beads.
which strike another surface that emits
visible light, which is then captured by a
conventional high-speed camera.
Subsurface Swimming
When we first observed the subsurface
movement of the sandfish, we were
amazed to see that it was moving for
ward at nearly two body lengths a sec
ond using a large-amplitude undulation
of its body, and without using its limbs?
traveling faster than milk snakes move
on flat ground. The sandfish "swam" to
a depth of about 4 centimeters and then
stopped undulating, presumably feeling
safe. The sandfish moved forward by
propagating a traveling sinusoidal wave
backward from head to tail at a particu
lar frequency?the greater the frequency,
the faster it went. In tightly packed me
dia, the animal used frequencies up to
four undulations per second.
There were several interesting features
of the sandfish locomotion: We expected
that, as we increased the packing fraction
to generate more resistive media, the ani
mal should slow down. Measurements
we made on small rods in granular media
showed that drag force in tightly packed
sand is nearly double that in loosely
packed sand. Surprisingly, we found that
volume fraction had no effect on speed
for a given wave frequency. Further, the
animal's movements were the same in
both loosely and tightly packed media; it
was impossible to determine simply by
looking at the x-ray images if the animal
was swimming in more or less resistive
materials. Stranger yet, we found that on
average, the sandfish swam faster in the
tightly packed material than in the loosely
packed material. It did this by increasing
undulation frequency to a higher range
(its maximum frequency was nearly dou
bled in tight versus loose packings).
In experiments and models, we use
a number called the wave efficiency to
characterize the locomotion of the sand
fish?it is commonly used to character
ize the locomotion of other swimmers
(such as millimeter-long nematode
worms) in deformable media. Wave ef
ficiency is defined as the average swim
rrting speed divided by the wave speed
(which is a product of the frequency and
the wavelength). If there is no move
ment of the material and no slip of the
animal, the wave efficiency is one and
the animal effectively moves in a tube. If
the animal were in a vacuum with noth
ing to push against, the wave efficiency
would be zero. In granular media, we
found that the sandfish swam with a
wave efficiency of 0.5, nearly twice that
of nematodes in fluids (about 0.2) and
greater than snakes on frictional surfac
es (about 0.3). Remarkably, the wave ef
ficiency of the sandfish did not depend
on the compaction of the sand.
Modeling the Sandfish
To explain some of these phenom
ena, a doctoral student in one of our
(Goldman's) groups, Yang Ding, first
Figure 10. The physics that governs interactions with granular media can be quite complex. A container of grains (6-millimeter plastic spheres)
remains solid until an aluminum ball, 5 centimeters in diameter (left), impacts the surface, at which point the grains become a fluid (middle).
Once the ball comes to rest, the grains can resolidify. A simulation technique called molecular dynamics visualizes the forces and velocities
on the grains (right). At this instant in time, dark blue particles are moving slowly and behaving as a near-solid, whereas redder particles sur
rounding the impactor are moving more rapidly. (Two images at left courtesy of Andrei Savu.)
320 American Scientist, Volume 98
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms
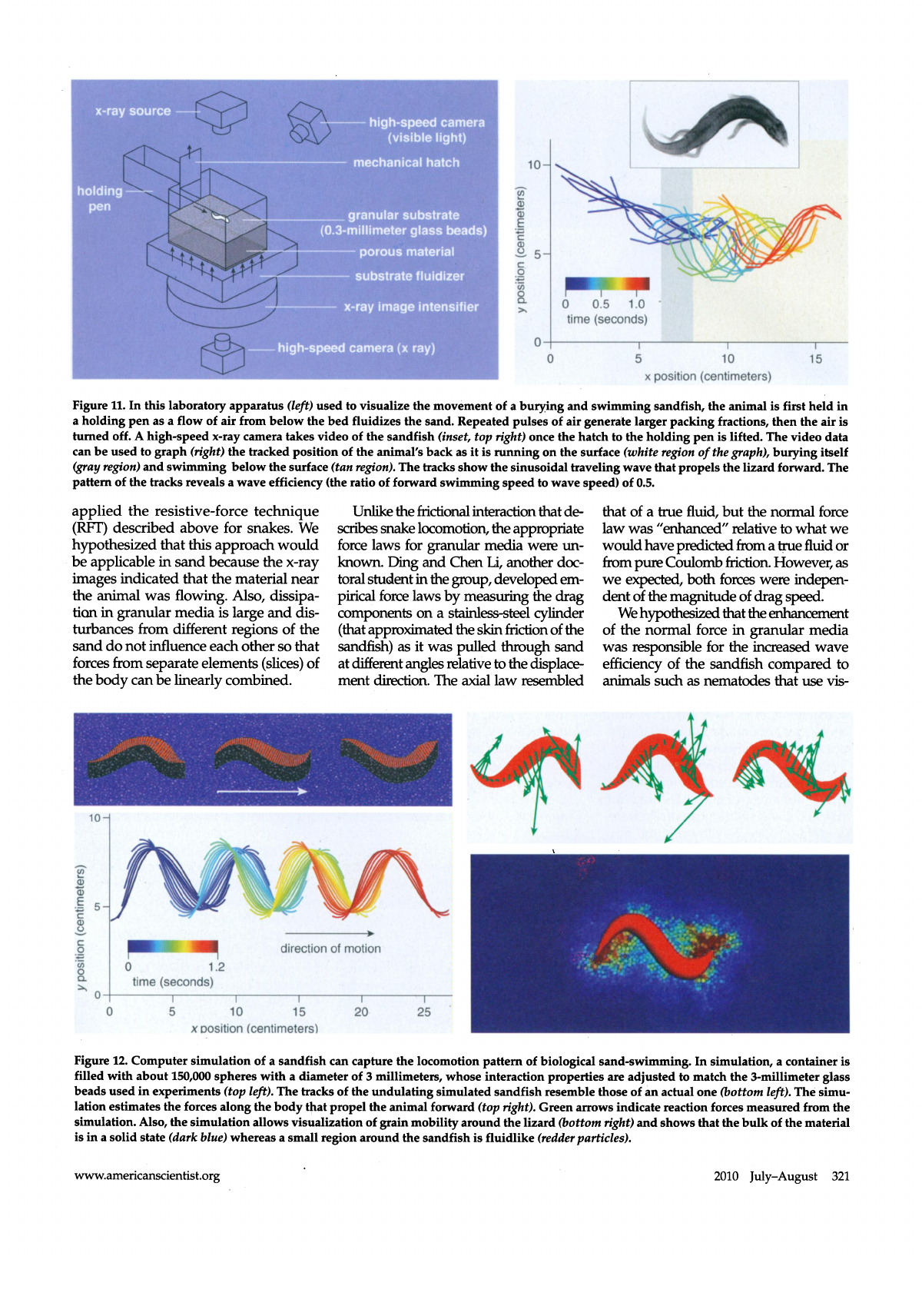
5 10
position (centimeters)
Figure 11. In this laboratory apparatus (left) used to visualize the movement of a burying and swimming sandfish, the animal is first held in
a holding pen as a flow of air from below the bed fluidizes the sand. Repeated pulses of air generate larger packing fractions, then the air is
turned off. A high-speed x-ray camera takes video of the sandfish (inset, top right) once the hatch to the holding pen is lifted. The video data
can be used to graph (right) the tracked position of the animal's back as it is running on the surface (white region of the graph), burying itself
(gray region) and swimming below the surface (tan region). The tracks show the sinusoidal traveling wave that propels the lizard forward. The
pattern of the tracks reveals a wave efficiency (the ratio of forward swimming speed to wave speed) of 0.5.
applied the resistive-force technique
(RFT) described above for snakes. We
hypothesized that this approach would
be applicable in sand because the x-ray
images indicated that the material near
the animal was flowing. Also, dissipa
tion in granular media is large and dis
turbances from different regions of the
sand do not influence each other so that
forces from separate elements (slices) of
the body can be linearly combined.
Unlike the fractional interaction that de
scribes snake locomotion, the appropriate
force laws for granular media were un
known. Ding and Chen Li, another doc
toral student in the group, developed em
pirical force laws by measuring the drag
components on a stainless-steel cylinder
(that approximated the skin friction of the
sandfish) as it was pulled through sand
at different angles relative to the displace
ment direction. The axial law resembled
that of a true fluid, but the normal force
law was "enhanced" relative to what we
would have predicted from a true fluid or
from pure Coulomb friction. However, as
we expected, both forces were indepen
dent of the magnitude of drag speed.
We hypothesized that the enhancement
of the normal force in granular media
was responsible for the increased wave
efficiency of the sandfish compared to
animals such as nematodes that use vis
?
0 1.2
time (seconds)
-f- ?n?
10 15
position (centimeters)
Figure 12. Computer simulation of a sandfish can capture the locomotion pattern of biological sand-swimming. In simulation, a container is
filled with about 150,000 spheres with a diameter of 3 millimeters, whose interaction properties are adjusted to match the 3-millimeter glass
beads used in experiments (top left). The tracks of the undulating simulated sandfish resemble those of an actual one (bottom left). The simu
lation estimates the forces along the body that propel the animal forward (top right). Green arrows indicate reaction forces measured from the
simulation. Also, the simulation allows visualization of grain mobility around the lizard (bottom right) and shows that the bulk of the material
is in a solid state (dark blue) whereas a small region around the sandfish is fluidlike (redder particles).
www.americanscientist.org
2010 July-August 321
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms
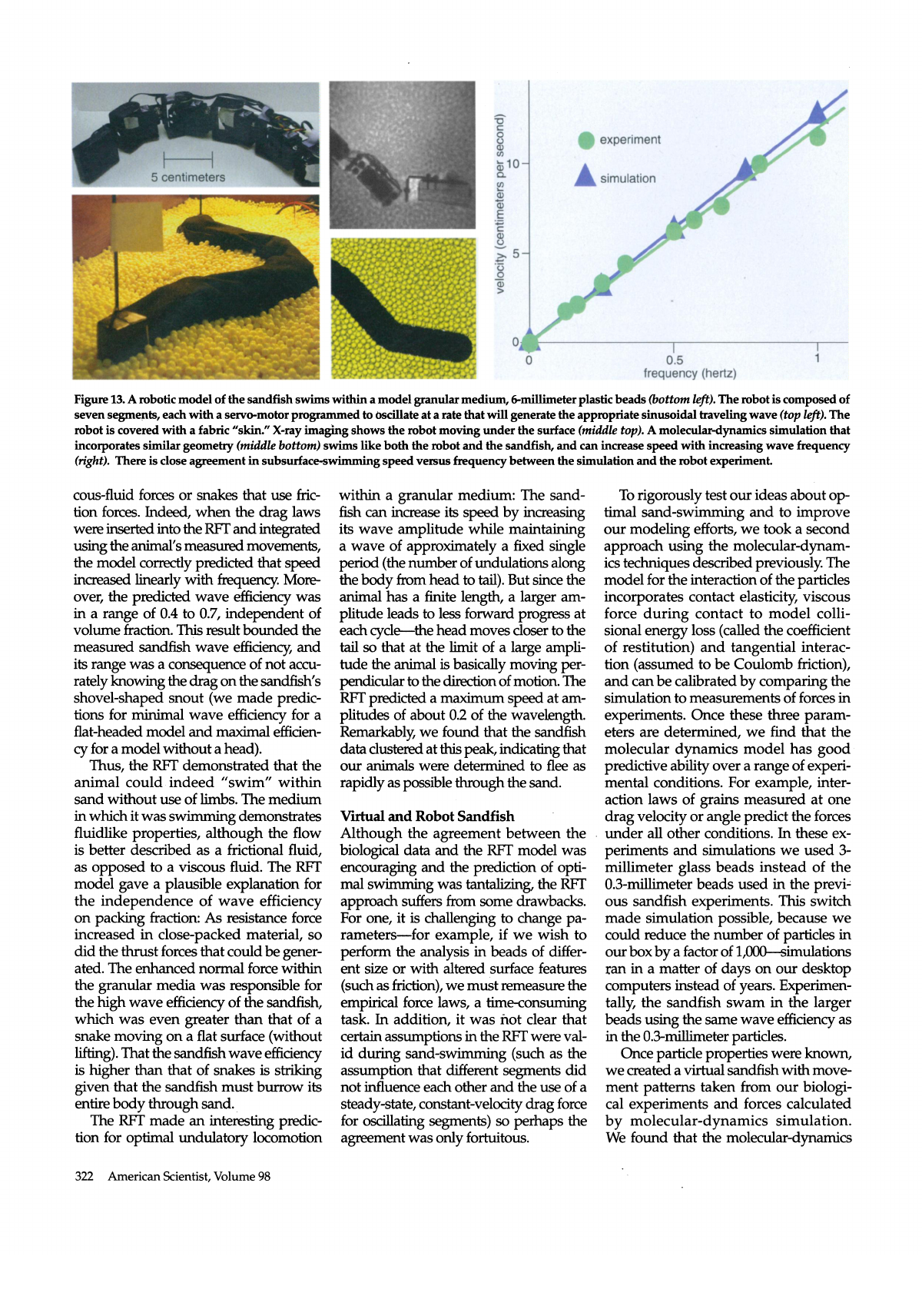
Figure 13. A robotic model of the sandfish swims within a model granular medium, 6-millimeter plastic beads (bottom left). The robot is composed of
seven segments, each with a servo-motor programmed to oscillate at a rate that will generate the appropriate sinusoidal traveling wave {top left). The
robot is covered with a fabric "skin." X-ray imaging shows the robot moving under the surface (middle top). A molecular-dynamics simulation that
incorporates similar geometry (middle bottom) swims like both the robot and the sandfish, and can increase speed with increasing wave frequency
(right). There is close agreement in subsurface-swimming speed versus frequency between the simulation and the robot experiment
cous-fluid forces or snakes that use fric
tion forces. Indeed, when the drag laws
were inserted into the RFT and integrated
using the animal's measured movements,
the model correctly predicted that speed
increased linearly with frequency. More
over, the predicted wave efficiency was
in a range of 0.4 to 0.7, independent of
volume fraction. This result bounded the
measured sandfish wave efficiency, and
its range was a consequence of not accu
rately knowing the drag on the sandfish's
shovel-shaped snout (we made predic
tions for minimal wave efficiency for a
flat-headed model and maximal efficien
cy for a model without a head).
Thus, the RFT demonstrated that the
animal could indeed "swim" within
sand without use of limbs. The medium
in which it was swimming demonstrates
fluidlike properties, although the flow
is better described as a frictional fluid,
as opposed to a viscous fluid. The RFT
model gave a plausible explanation for
the independence of wave efficiency
on packing fraction: As resistance force
increased in close-packed material, so
did the thrust forces that could be gener
ated. The enhanced normal force within
the granular media was responsible for
the high wave efficiency of the sandfish,
which was even greater than that of a
snake moving on a flat surface (without
lifting). That the sandfish wave efficiency
is higher than that of snakes is striking
given that the sandfish must burrow its
entire body through sand.
The RFT made an interesting predic
tion for optimal undulatory locomotion
within a granular medium: The sand
fish can increase its speed by increasing
its wave amplitude while mamtaining
a wave of approximately a fixed single
period (the number of undulations along
the body from head to tail). But since the
animal has a finite length, a larger am
plitude leads to less forward progress at
each cycle?the head moves closer to the
tail so that at the limit of a large ampli
tude the animal is basically moving per
pendicular to the direction of motion. The
RFT predicted a maximum speed at am
plitudes of about 0.2 of the wavelength.
Remarkably, we found that the sandfish
data clustered at this peak, mcUcating that
our animals were determined to flee as
rapidly as possible through the sand.
Virtual and Robot Sandfish
Although the agreement between the
biological data and the RFT model was
encouraging and the prediction of opti
mal swimming was tantalizing, the RFT
approach suffers from some drawbacks.
For one, it is challenging to change pa
rameters?for example, if we wish to
perform the analysis in beads of differ
ent size or with altered surface features
(such as friction), we must remeasure the
empirical force laws, a time-consuming
task. In addition, it was not clear that
certain assumptions in the RFT were val
id during sand-swimming (such as the
assumption that different segments did
not influence each other and the use of a
steady-state, constant-velocity drag force
for oscillating segments) so perhaps the
agreement was only fortuitous.
To rigorously test our ideas about op
timal sand-swimming and to improve
our modeling efforts, we took a second
approach using the molecular-dynam
ics techniques described previously. The
model for the interaction of the particles
incorporates contact elasticity, viscous
force during contact to model colli
sional energy loss (called the coefficient
of restitution) and tangential interac
tion (assumed to be Coulomb friction),
and can be calibrated by comparing the
simulation to measurements of forces in
experiments. Once these three param
eters are determined, we find that the
molecular dynamics model has good
predictive ability over a range of experi
mental conditions. For example, inter
action laws of grains measured at one
drag velocity or angle predict the forces
under all other conditions. In these ex
periments and simulations we used 3
millimeter glass beads instead of the
0.3-millimeter beads used in the previ
ous sandfish experiments. This switch
made simulation possible, because we
could reduce the number of particles in
our box by a factor of 1,000?simulations
ran in a matter of days on our desktop
computers instead of years. Experimen
tally, the sandfish swam in the larger
beads using the same wave efficiency as
in the 0.3-millimeter particles.
Once particle properties were known,
we created a virtual sandfish with move
ment patterns taken from our biologi
cal experiments and forces calculated
by molecular-dynamics simulation.
We found that the molecular-dynamics
322 American Scientist, Volume 98
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms

modeling was in good agreement with
the wave efficiency predicted by the RFT
calculations (as we? as measured in the
biological data). Unlike the empirical
RFT models, molecular dynamics allows
access to particle-level information?we
could visualize the highly damped flow
of particles around the sandfish and esti
mate accurately the force on different ele
ments. Additionally confirming our RFT
model, th? simulated sandfish moved
fastest when it used the kinematics pre
dicted by the RFT and taken from obser
vations of the animal. Further work with
the molecular^dynamics model will al
low us to carefully investigate the phys
ics that creates the scaling of thrust and
drag, which leads to the independence
of wave efficiency on packing fraction.
We wanted to give our models one
more test, so we decided to move back
into the physical world: We would use
the molecular-dynamics simulation to
design a physical model of the sand
fish, a robot, that would swim within
granular material and test the prin
ciples we had learned. For our tests,
we chose segments made of servo
motors popular with hobbyists, so
we were therefore constrained in size
by commercially available actuators.
We also decided to scale the size of the
granular particles to 6 millimeters, so
mat we would not have to simulate bil
lions of grains and small particles would
not get into the motors. Ryan Maladen,
in collaboration with mechanical engi
neer Paul Umbanhowar at Northwest
ern University, built a device that could
undulate with the same wave pattern
as that of the sandfish. The agreement
between robot experiment and robot
simulation was within five percent. We
found, just as predicted by the models,
that the robot swam fastest in the real
world (and in simulation) using the op
timal sandfish kinematics. The optimal
wave efficiency of the robot sandfish
was 0.3 in both experiment and simula
tion. We attributed this result to the finite
number of segments of the robot?in
the simulation, when we increased the
number of segments so that the body
was nearly smooth, the optimal wave
efficiency approached 0.5.
Two Worlds Without Legs
We have studied two very different envi
ronments in which undulatory locomo
tion is effective. In above-ground snakes,
anisotropie belly friction generates
thrust to overe?me drag, whereas un
derground, an animal's sides exploit the
frictional fluidlike properties of granular
media to generate thrust. Although the
drag laws differ between these regimes,
we found that resistive-force modeling
techniques, which originated in hydro
dynamics, can be successfully applied
to organisms on and within dry land.
Our experiments and modeling consid
ered mostly planar motion of the body,
but our findings of the advantages of
dynamic body-lifting in snakes suggests
that motion in the third dimension may
be used to increase performance. In the
sandfish, we must begin to explore 3D
effects as well: Our rays reveal that the
animal does not simply swim in a fixed
horizontal plane, but actually dives into
the material at a shallow angle.
Modeling the interaction between the
organism and its environment enables us
as physical scientists to work with biolo
gists to uncover new behaviors and the
relevant neuromechanics associated with
effectively using long, slender bodies to
move. The modeling approach does
not constrain us to sandfish or snake
morphology: Using our models of the
animals, we can vary body shape and
waveform to understand benefits and
tradeoffs of different locomotor modes in
diverse environments. Of particular inter
est is the importance of gait among limb
less animals. For example, does speed
or efficiency motivate limbless animals
to shift from slithering to sidewinding?
To that end, we plan to develop models
of the internal mechanics of me animals,
which will be useful in determining their
inherent metabolic and muscular limits.
To gain this broad understanding,
we must develop force laws for both
above and below ground that can ac
commodate a wider range of substrates.
For which materials is our approxima
tion of Coulomb friction a good'one?
What happens when an animal buries
into wetted material? Can we use simi
lar empirical forces laws, or if not, how
are they modified? With improved mod
els of environments and organisms, and
their interactions, our approach can helf>
find designs for future limbless robotic
devices that can move through complex
terrain faster and more efficiently than
their natural counterparts.
Bibliography
Alexander, R. M. 2003. Principles of Animal Locomo
tion. Princeton, NJ: Princeton University Press.
Arnold, E. N. 1995. Identifying the effects of
history on adaptation: Origins of different
sand-diving techniques in lizards. Journal of
Zoology 235:351-388.
Bagnold, RA. 1954. The Physics o]-Blown Sand and
Desert Dunes. London: Methuen and Co. Ltd.
Bellairs, A. 1970. Life of Reptiles. New York, NY:
Universe Publishing.
Choset, H. M. 2005. Principles of Robot Motion:
Theory, Algorithms and Implementation. Cam
bridge, MA: MIT Press.
Cohen, N., and J. H. Boyle. 2010. Swimming at
low Reynolds number: A beginner's guide
to undulatory locomotion. Contemporary
Physics 51:103-123.
Cundall, D. 1987. Functional morphology, in
Snakes: Ecology and Evolutionary Biology, eds.
R. A. Siegel, J. T. Collins and S. S. Novak.
Caldwell, NJ: The Blackburn Press.
Dickinson, M H., C. T. Farley, R. J. Full, M. A.
R. Koehl, R. Kram and S. Lehman. 2000.
How animals move: An integrative view.
Science 288:100.
Full, R. J., and D. E. Koditschek. 1999. Templates
and anchors: Neuromechanical hypotheses
of legged locomotion on land. Journal of Ex
perimental Biology 202:3325-3332.
Gray, J. 1946. The mechanism of locomotion
in snakes. Journal of Experimental Biology
23:101-120.
Gray, J., and G. J. Hancock. 1955. The propul
sion of sea-urchin spermatozoa. Journal of
Experimental Biology 32:802-814.
Guo, Z. V., and L. Mahadevan. 2008. Limbless
undulatory locomotion on land. Proceed
ings of the National Academy of Sciences of the
U.S.A. 105:3179-3184.
Hazel, J., M. Stone, M. S. Grace and V. V. Tsuk
ruk. 1990. Nanoscale design of snake skin
for reptation locomotions via friction an
isotropy. Journal ofBiomechanics 32:477-484.
Hu, D., J. Nirody, X Scott and M. Shelley. 2009.
The mechanics of slithering locomotion.
Proceedings of the National Academy of Sci
ences of the U.S.A. 106:10081.
Jaeger, H. M., S. R. Nagel and R. P. Behring
er. 1996. The physics of granular material.
Physics Today 49:32-38.
Jayne, B. C. 1986. Kinematics of terrestrial
snake locomotion. Copeta 22:915-927.
Maladen, R. D., Y. Ding, C. Li and D. I. Gold
man. 2009. Undulatory swimming in sand:
Subsurface locomotion of the sandfish liz
ard. Science 325:314.
Nishikawa, K., et al. 2007. Neuromechanics:
An integrative approach for understanding
motor control. Integrative and Comparative
Biology 47:16-54.
Rapaport, D. C. 2004. The Art of Molecular Dy
namics Simulation, 2nd ed. Cambridge, U.K.:
Cambridge University Press.
Trueman, E. R. 1975. The Locomotion of Soft
bodied Animals. London: Edward Arnold.
Walton, M., B. G Jayne and A. E Bennett. 1990.
The energetic cost of limbless locomotion.
Science 249:524-527.
For relevant Web links, consult this
issue of American Scientist Online:
http://wv^.arnericanscientist.orq/
igsye$/ia85/pa$ta$px
www.americanscientist.org
2010 July-August
This content downloaded from
132.174.250.220 on Thu, 14 Jan 2021 18:00:08 UTC
All use subject to https://about.jstor.org/terms
