
1
Decrease of Entropy
and Chemical Reactions
Yi
-
Fang Chang
Department of Physics, Yunnan University, Kunming, 650091, China
(e
-
mail:
)
Abstract
The chemical reactions are very complex, and include oscillation, condensation, catalyst and
self
-organization, etc. In these case changes of entropy may increase or decrease. The second law
of thermodynamics is based on an isolated system and statistical independence. If
fluctuations
magn
ified due to internal interactions exist in the system, entropy will decrease possibly. I
n
chemical reactions there are various internal interactions, so that some ordering processes with
decrease of entropy are possible on an isolated system. For example, a simplifying Fokker-
Planck
equation is solved, and the hysteresis as limit cycle is discussed.
Key words: entropy, chemical reaction, internal interaction, oscillation, catalyst.
PACS: 05.70.
-
a,
82.60.
-
s,
05.20.
-y.
1.Introduction
The measure of disorder is used by thermodynamic entropy. According to the second law of
thermodynamics, the entropy of the universe seems be constantly increasing. A mixture of two
pure substances or dissolve one substance in another is usually an increase of entropy. In a
chem
ical reaction, when we increase temperature of any substance, molecular motion increase and
so does entropy. Conversely, if the temperature of a substance is lowered, molecular motion
decrease, and entropy should decreases. In nature, the general tendency
is toward disorder.
Usual development of the second law of the thermodynamics was based on an open system,
for example, the dissipative structure theory.
Nettleton
discussed an extended thermodynamics,
which introduces the dissipative fluxes of classical nonequilibrium thermodynamics, and modified
originally rational thermodynamics with nonc
lassical
entropy flux [1]. The Gibbs equation from
maximum entropy is a statistical basis for differing forms of extended thermodynamics.
Recently, Gaveau, et al., [2] discussed the variational nonequilibrium thermodynamics of
reaction
-diffusion systems.
Cangialosi
, et al. [3], provided a new approach to describe the
component segmental dynami
cs
of miscible polymer blends combining the configurational
entropy and the self-concentration. The results show an excellent agreement between the
prediction and the experimental data. Stier [4] calculated the entropy production as function of
time in a closed system during reversible polymerization in nonideal systems, and found that the
nature of the activity coefficient has an important effect on the curvature of the entropy
production.
In particular,
Cybulski
, et al. [5], analyzed a system of two different types of Brownian
particles
confined in a cubic box with periodic boundary conditions. Particles of different types
annihilate when they come into close contact. The annihilation rate is matched by the birth rate,
thus
the total number of each kind of particles is conserved. The system evolves towards those
stationary
distributions of particles that minimize the Renyi entropy production, which decreases
monotonically during the evolution despite the fact that the topology and geometry of the interface
exhibit abrupt and violent changes.
Kinoshita
, et al. [6], developed an efficient method to evaluate

2
the translational and orientational contributions to the solute-
water
pair-correlation entropy that is
a major component of the hydration entropy in an analytical manner. They studied the effects of
the solute-water attractive interaction. A water molecule is modeled as a hard sphere of diameter,
which becomes larger, the p
ercentage
of the orientational contribution first increases, takes a
maximum value at depends on the strength of the solute-
water
attractive interaction, and
then
decreases toward a limiting value. The percentage of the orientational contribution reduces
progressively as the solute-water attractive interaction becomes stronger. They discussed the
physical origin of the maximum orientational restriction.
In fact, the second law of thermodynamics holds only for an isolated system, and it is a
probability law. This shows that transition probability from
the
molecular chaotic motion to
the
regular motion of a macroscopic body is very small. The basis of thermodynamics is the statistics,
in which a basic principle is statistical independence: The state of one subsystem does not affect
the probabilities of various states of the other subsystems, because different subsystems may be
regarded as weakly interacting [7]. It implies that various interactions among these subsystems
should not be considered. But, if various internal complex mechanism and interactions cannot be
neglected, perhaps a state with smaller entropy (for example, self-organized structure) will be able
to appear. In these cases, the statistics and the second law of thermodynamics should be different
[8
-10]. For instance, the entropy of an isolated fluid whose evolution depends on viscous pressure
and the internal friction does not increase monotonically [11].
According to the Boltzmann and Einstein fluctuation theories, all possible microscopic states
of a system are equal-probable in thermodynamic equilibrium, and the entropy tends to a
maximum value finally. In statistical mechanics fluctuations may occur, and always bring the
entropy to decrease [7,12
-
14]. Under certain condition fluctuations can be
magnified [12
-
14].
Chichigina
[15] proposed a new method for describing selective excitation as the addition of
information to a thermodynamic isolated system of atoms, decreasing the entropy of the system as
a result. This information approach is used to calculate the light-induced drift velocity. The
computational results are in good agreem
ent with experimental data.
2.Fokker
-
Planck Equation, Oscillation and Change of Entropy
There are various internal interactions in chemical reactions, and here noise and internal
fluctuations cannot be neglected often. The nonlinear chemical reactions belong to the
non
-equilibrium phase transformation, and fluctuations magnified may derive far-
equilibrium
states.
It is known that change of entropy may increase or decrease in chemical reactions [16,17]. If
the chemical heterogeneous reaction, multistability and hysteresis loop become to an entropy
function, which will be not a monotone increasing function, these phenomena will
possess
possibly
new property of entropy.
Gaveau, et al., introduced a standard Master equation [2,18]:
r
iiriiiir
i
tnPnWtrnPrnW
t
tnP
)]
},
({})({
)
},
({})({
[
)
},
({
, (1)
and an approximate Fokker
-
Planck equation:
1,
2
1
)(
2
1
)]
,()([
),(
ji
ij
ji
i
s
i
i
pD
xxV
txpxA
xt
txp
, (2)
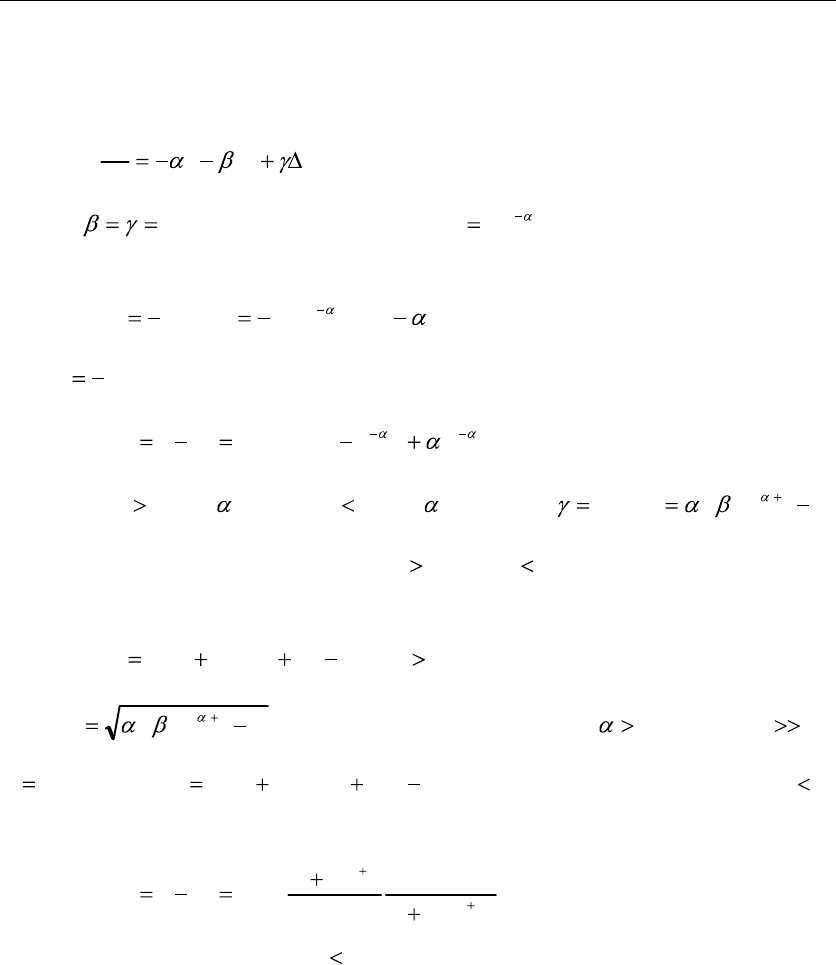
3
where
p(x,t)
is the density of the probability distribution function. This is a kinetic equation of the
distribution function [12,19].
We solve a simplify
ing Fokker
-
Planck equation:
ppp
dt
dp
3
. (3)
1.When
0, the distribution function is
t
Ce
p . It corresponds to the Maxwell-
Boltzmann distribution, and its entro
py is
)
(ln
ln
tC
KCe
p
Kp
s
t
. (4)
Let
C
KC
s
ln
0
, so
])1(
[ln
0
tt
te
eC
KC
ss
ds
. (5)
Therefore,
0
ds
for
>0, and
0
ds
for
<0. 2.When
0
,
)1(/
22 ct
ep
.
In this case the equation has two solutions: 0p and 0p , but the latter is meaningless. It
corresponds to the Bose
-
Einstein distribution [13,
20], and its entropy is
0]
ln
)1
ln(
)1
[(
ppppKs . (6)
Here
)1(/
2 ct
ep
decreases with increasing t for 0 . When 0p
,
pKs
ln
. Let ]
ln
)1
ln(
)1
[(
00000
ppppKs , where
0
p corresponds to tt
0
,
so
]
)1(
)1(
ln[
0
0
1
0
0
1
0
p
p
p
p
p
p
p
p
Kss
ds
. (7)
This is a decreasing function, 0
ds
. It is consistent with the Bose-Einstein statistics
corresponding condensation. Further, the general equations with the nonlinear term, which
corresponds often to various interactions, may have different phase transformations.
In chemical reactions more intriguing is the Belousov-
Zhabotinskii
(BZ)
class of oscillating
reactions some of which can continue for hours [21-24]. Field and Dutt, et al., discussed the limit
cycles and the discontinuous entropy production changes in the reversible Oregonator [25,26],
which may describe the oscillations in the BZ
reaction.
The chemical oscillations as one type of
the limit cycle imply at least that entropy cannot be
increase monotonically.
The Fokker-Planck equation may be the reaction-diffusion equation. It can be extended to
several equations. The two equations may describe Brusselator in chemical reactions. The three
equations may describe
Oregonator
and BZ reaction.
Dutt, et al., reported changes discontinuously
at Hopf bifurcation points leading to limit cycle oscillations out of an unstable steady state [27].
Under some cases the factual changes of systems controlled by parametric values can be
achieved through the existing dynamical process inside systems, in which hysteresis loop may be
formed spontaneously. The character of the hysteresis loop can bring states of system to periodic

4
change s
pontaneously with time.
Steady states in the Raleigh-Benard convection have exhibited hysteresis in entropy
production [28]. At different steady states
D
utt calculated entropy production [29], and the average
entropy production for the unstable steady stat
es generating limit cycle oscillations. The hysteresis
in the entropy production as a function of the rate constant k of the steps in Oregonator (k
decreasing or increasing) as time evolves showed the stable and unstable (limit cycle) phases. In
the case the Floquet exponent is positive or negative for different critical points of Hopf
bifurcations, i.e., the entropy productions ha
ve
two values for the same chemical reaction. The
entropy production is calculated in the model in a subcritical Hopf bifurcation region. Dutt, et al.,
calculated the entropy production for steady and oscillatory states in another region [27,30,31].
Dutt
discussed the hysteresis in entropy production in a model exhibiting oscillations in the
BZ
reaction, and a bistability in the entropy production between a steady state and an oscillatory
state in the neighborhood of a subcritical Hopf bifurcation. He identified this region in the past by
a perturbation method. The numerical results present a thermodynamic formulation of multistable
structures in
a nonlinear dissipative system [29].
3.Decrease of Entropy and Condensed Pro
cess
We discussed fluctuations magnified due to internal interactions, etc., so that the equal-
probability does not hold. In this case, the entropy with time would be defined as [10]
r
rr
tPtPktS )(
ln
)()( . (8)
From this a possible decrease of entropy is calculated in an internal condensed process. Internal
interactions, which bring about inapplicability of the statistical independence, cause possibly
decreases of entropy in an isolated system. The possibility is researched for attractive process,
internal energy, system entropy and nonlinear interactions, etc. An isolated system may form a
self
-
organized structure [8
-
10].
For the systems with internal interactions, we proposed that the total entropy should be
extended
to [10]
ia
dSdSdS
, (9)
where
a
dS
is an additive part of entropy, and
i
dS
is an interacting part of entropy. Eq.(9) is
similar to a well known for
mula:
SdSddS
ei
, (
10
)
in the theory of dissipative structure proposed by Prigogine. Two formulae are applicable for
internal or external interactions, respectively.
The entropy of non
-i
deal gases is:
)/1
log(
V
Nb
NSS
id
. (
11
)
This is smaller than one of ideal gases, since b is four times volume of atom, 0b
.
This
corresponds to the existence of interactions between the gas molecules, and average of forces
betw
een molecules
is
attractive [13].
The entropy of a solid is:
332
)(15/2 u
VT
S , (12)
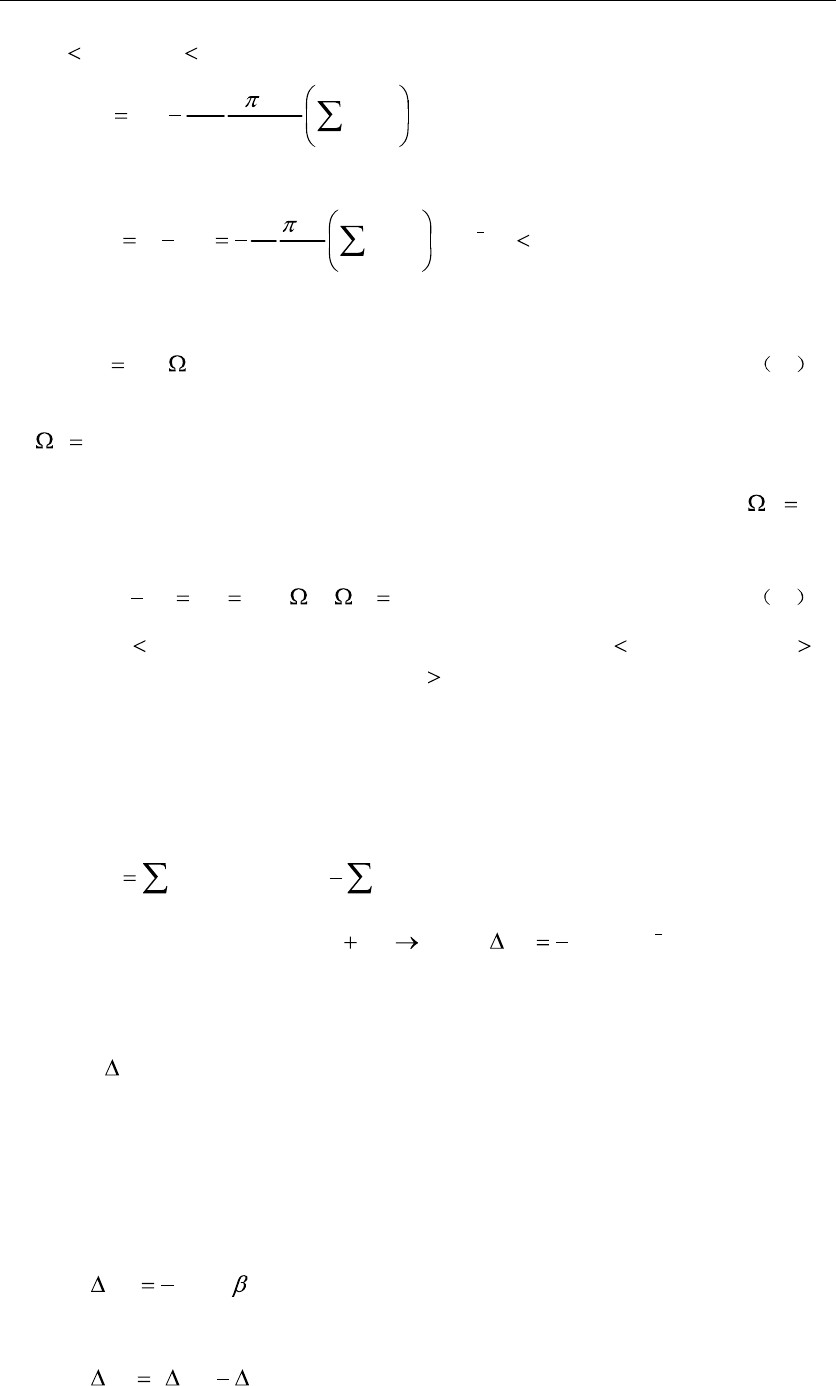
5
so
0
dS
for 0
dT
. The free energy with the
correlation part of plasma is [13]:
2/3
2
2/1
2/13
)(
3
2
a
aa
id
zN
VT
e
FF
. (13)
Correspondingly, the entropy is:
0
3
2/3
2/3
2
2/1
2/13
TzN
V
e
SS
dS
a
aa
id
. (14)
In these cases, there are
the
electric attractive forces between plas
ma.
According to
ln
kS , 15
in an isolated system there are the n-particles, which are in different states of energy respectively,
so
!
1
n . Assume that internal attractive interaction exists in the system, and the n-
particles
will cluster to m-particles. If they are in different states of energy still, there will be !
2
m
.
Therefore, in this process
)!/!
ln(
)/
ln(
1212
nmkk
dS
SS . 16
So long as
n
m
for the condensed process, entropy decreases 0
dS
. Conversely,
n
m
for the dispersed process, entropy increases 0
dS
. In these cases it is independent that each
cl
uster includes energy more or less. In an isolated system, cluster number is lesser, the degree of
irregularity and entropy are smaller also. This is consistent with a process in which entropy
decreases from gaseous state to liquid and solid states.
In the chemical reactions the entropy changes may be calculated from the standard molar
entropy [32]
)()(
000
reactions
Sv
products
SvS
rp
,
(1
7)
for example, for the conversion
10
22
5.146,32
JK
S
NO
O
NO
. Further, Petrucci,
et al., proposed to be able to apply some qualitative reasoning: Because three moles of gaseous
reactants produce only two moles of gaseous products, they can expect the entropy to decrease;
that is that
0
S
should be negative [17
].
When gaseous ions change over to aquosity in hydration reactions, their standard molar
entropy remarkably decreases. When ions are smaller or their charges increase, entropy of solution
decreases more. This is consistent with our conclusion [8-10]. A dissolved process may be an
automatic one.
Based on the thermodynamic relationships
ln
0
RT
G , (18)
and the change of the entropy
TGHS /)(
000
, (19)
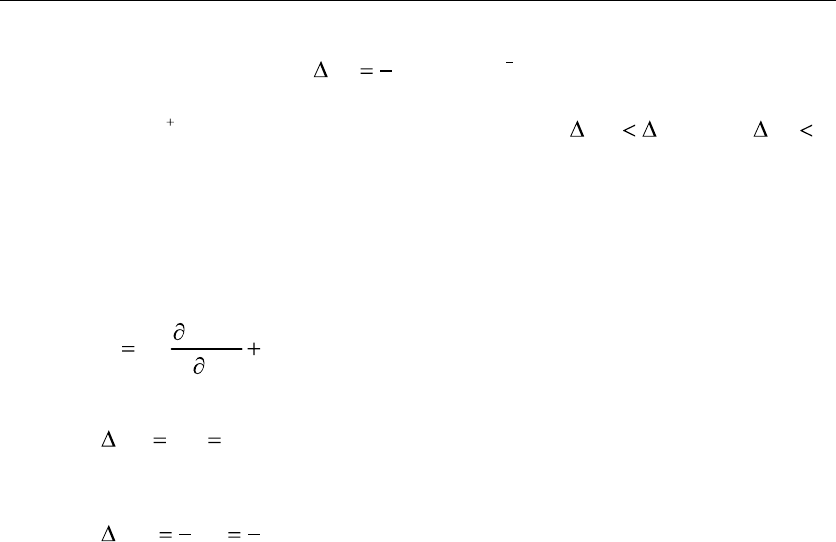
6
the thermodynamic function
10
91
.
19
kJmolST
at
C
0
25
for the complex
2
42
])([
Me
NH
Cd
[33]. For
3224
,,, OOH
COCH
, there is
00
GH
, i.e.,
0
0
S
[1
7
].
These pointed out that change of entropy is negative value, namely,
entropy decrease in these
cases.
Moreover, the standard product entropy may be negative value in metal ions in aqueous
solution [17,33].
The entropy is expressed in terms of the partition function Z as
Zk
T
Z
kT
S
ln
)
(ln
. (20)
For order and disorder the increase of entropy in fusion is
nR
kN
S
fu
, (2
1)
which is called the communal entropy [17
]. Contrarily, the entropy in condensation sho
uld is
nRkN
S
con
, (
22
)
which decreases. In the general case, fusion and condensation are in the open systems. But, a few
condensations with internal interactions may be in some isolated systems. Further, the general
phase transformation is in the open systems. But, a spontaneous magnetization may be in an
isolated system. This is an ordering process, in which entropy should decrease.
4.Catalyst and Discussion
The catalysts may substantially lower the activation energy, and increase the rates of some
chemical reactions without itself being consumed in the reactions, and may control direction of
reactions. The mass of the catalyst remains constant [34,35].
In fact, the catalyst in chemical reactions possesses some character of the auto-
control
mechanism in an isolated system. If it does not need the input energy, at least in a given time
interval, the self
-
catalyst is similar with auto
-
control like a type of Maxwell demon, which is just a
type of internal interactions. The demon may be a permeable membrane. For the isolated system,
this is possible that the catalyst and other substance are mixed to produce new order substance
with smaller entropy. Ordering is the formation of structure through the self-organization from a
disordered state.
Dutt proposed the result interesting to biophysical processes like glycolytic oscillations, and
may be considered useful for better efficiency of the biochemical engines [27], namely if their
structures is bette
r, it will implies that there is smaller entropy for the same process.
In the chemical and biological self-organizing processes some isolated systems may tend to
the order states spontaneously. Ashby pointed out [36]: Ammonia and hydrogen are two gases, bu
t
they mix to form a solid. There are about 20 types of amino acid in germ, they gather together, and
possess a new reproductive property. Generally solid is more order than gas, and corresponding
solid entropy is smaller than gaseous entropy. Germ should
be more order than amino acid yet.
Prigogine and Stengers [14] discussed a case: When a circumstance of Dictyostelium
discoideum becomes lack of nutrition, discoideum as some solitary cells will unite to form a big
cluster spontaneously. In this case these cells and nutrition-liquid may be regarded as an isolated

7
system.
Jantsch [37] pointed out: When different types of sponge and water are mixed up in a
uniform suspension, they rest after few hours, and then separate different types automatically. It is
a
more interesting order process, a small hydra is cut into single cell, and these cells will
spontaneously evolve, firstly form some cell-clusters, next form some malformations, finally they
become a normal hydra.
The decrease of entropy relates possibly to the thermodynamics of enzyme-
catalyzed
reactions. An enzyme is a complex protein molecule that can act as a biological catalyst. In
biology there are self
-
organize and self
-
assembly [38], etc.
These phenomena show that nature does not tend toward disorder always, and are some
ordering processes with decrease of entropy.
Our conclusion is: The chemical reactions are complex processes. When there are various
internal interactions and fluctuations in an isolated system: 1. It should be discussed that all
midd
le change process from begin to end is always entropy increase. 2.The entropy increase
principle in these processes may not hold always. 3. All of world does not trend to disorder always.
Further,
a negative temperature is based on the Kelvin scale and the condition dU>0 and dS<0.
Conversely, there is also
the
negative temperature for dU<0 and dS>0. We find that
the
negative
temperature derives necessarily decrease of entropy [39]
.
Moreover,
the
negative temperature is
a
query, and is contradiction with usual meaning of temperature and with some basic concepts of
physics and mathematics. It is a
notable
question in nonequilibrium thermodynamics.
In this paper,
we attend mainly to thermodynamic results and change of entropy in the chemical reactions.
Referen
ces:
1.
R. E. Nettleton
, J. Chem. Phys. 93,8247(1990); 97, 8815(1992).
2.
B.Gaveau, M.Moreau
and J.Toth, J.Chem.Phys. 111,7736,7748(1999); 115,680(2001).
3.
D. Cangialosi
,
G. A. Schwartz
,
A. Alegr¨ª
and
J. Colmenero
, J. Chem. Phys.
123,
144908(2005).
4.
U.Stier, J. Chem. Phys.
124,
024901
(2006).
5.
O.
Cybulski
,
D.Matysiak
and
V.Babin
, J. Chem. Phys.
122,
174105
(2005).
6.
M. Kinoshita
,
N. Matubayasi
,
Y. Harano
and
M. Nakahara
, J. Chem. Phys.
124,
024512 (2006).
7.
L.D.Landau and E.M.Lifshitz, Statistical Physics (Pergamon Press. 1980).
8.
Yi
-Fang Chang, In Entropy, Information and Intersecting Science (C.Z.Yu, et al., Ed. Yunnan
Univ. Press. 1994). p53
-
60.
9.
Yi
-
Fang Chang, Apeiron, 4,97(1997).
10.
Yi
-
Fang Chang, Entropy
,
7,190(2005).
11.
J.Fort and J.E.Llebot, Phys. Lett. A205, 281(1995).
12.
H.Haken, Synergetics. An in
troduction (Springer
-
Verlag. 1977).
13.
L.E.Reichl, A Modern Course in Statistical Physics (Univ.of Texas Press. 1980).
14.
I.Prigogine and I.Stengers, Order Out of Chaos (New York: Bantam Books Inc. 1984).
15.
O.A.Chichigina, J. Exper.
Theor. Phys. 89,30(1999).
16.
D.A.Jo
hnson, Some Thermodynamic Aspects of Inorganic Chemistry. 2nd Ed.(Cambridge
Univ. Press. 1982).
17.
R.H.Petrucci, W.S.Harwood and F.G.Herring, General Chemistry: Principles and Modern
Applications, 8
th
Ed (Pearson Education, Inc. 2003).
18.
I.Prigogine, Nonequilib
rium Statistical Mechanics (New York: Wiley. 1962).

8
19.
H.
Risken, The
Fokker
-
Planck Equation (Springer
-
Verlag, 1984).
20.
J.F.Lee, F.W.Sears and D.L.Turcotte, Statistical Thermodynamics. (Addison-
Wesley
Publishing Company, Inc. 1963). p288
-
293.
21.
R.J.Field, E.Koros
and R.M.Noyes, J.Am.Chem.Soc. 94,8649(1972).
22.
P.De Kepper and J.Boissonad, J.Chem.Phys. 75,189(1972).
23.
A.T.Winfree, Spiral wave
s of chemical activity. Science
,
175,634(1972).
24.
C.Vidal. J.C.Roux and A.Rossi, J.Am.Chem.Soc. 102,1241(1980).
25.
R.J.Field, J. Chem. P
hys. 63,2289(1975).
26.
R.J.Field and H.D.Forsterling, J. Chem. Phys. 90,5400(1986).
27.
A.K.Dutt
,
D.K.Bhattacharyay
and B.C.Eu, J. Chem. Phys. 93,7929(1990).
28.
M.G.Velarde, X.L.Chu
and J.Ross, Phys. Fluids 6,550(1994).
29.
A.K.Dutt, J. Chem. Phys. 110, 1061(1999).
30.
A.K.Dutt
, J. Chem. Phys. 86,3959(1987).
31.
B.R.Irvin and J.Ross, J. Chem. Phys. 89,1064(1988).
32.
N.N.Greenwood and A.Earnshaw, Chemistry of The Elements (Pergamon Press.1984).
33.
J.P.Hunt, Metal
Ions in Aqueous Solution. (New York: W.A.Banjamin. 1963). p38
-
41.
34.
R.A.Burns, Fundamentals of Chemistry, 4
th
ed. (Pearson Education, Inc., 2003).
35.
A.T.Bell, et al., Science (Casalysis),299,1688
-
1706(2003).
36.
W.R.Ashby, An Introduction to Cybernetics (Chapman & Hall Ltd. 1956).
37.
E.Jantsch, The Self
-
Organizing (Universe. Pergamon. 1979).
38.
G.M.Whitesides, J.P.Mathias and S.T.Seto, Science, 5036,1182(1991).
39.
YI
-
Fang C
h
ang, arXiv:0704.1997.
