
Conditional Serial Release
ICWI0410.04 Page 1 of 3
Author: Jeanette M. Watson
Issue Date: 28Apr17
Source Document: ICSOP0010, Processing Serial Records
A Conditional Release allows authorized users to release a serial prior to the completion
of testing or the sample selection period and is granted when there is an emergency need
(only product available, expedite export, national program disease) and on the condition
that the serial is subject to immediate recall by the Center for Veterinary Biologics (CVB)
if tested and found unsatisfactory. Samples and APHIS Form 2008 (Form 2008) must be
received by the CVB prior to a serial being considered for Conditional Release. A written
request from the firm (letter) must accompany the Form 2008 being considered for a
conditional release. A fax of a letter can be accepted to expedite the process, but a hard
copy original signature letter must follow. The fax will be CBI’d and the original letter
becomes CVB-Inspection and Compliance’s (CVB-IC) official copy for filing.
Conditional Release
Conditional Release creates an additional parallel path to the normal Form 2008
workflow (first leg). The normal workflow (final leg) will not be affected.
Fill out ICFRM0010, Serials Ready for Conditional Action, for process/approval steps
along with performing the proper LSRTIS Actions.
1. Requesting/Authorizing the Conditional Release:
a. The Specialist completes Items 1 through 3 of the worksheet when a request has
been received.
b. The Specialist will select the type of release required depending on the sample
status (within the at-risk period or on test) in Item 4 of the worksheet.
c. Within LSRTIS, the Specialist will choose “Conditional Release” within the
LSRTIS laboratory action subcategory.
d. The Specialist should sign the form, and then obtain proper approval within
LSRTIS. A notification to the IC Section Leader should occur.
2. Approving the Conditional Release:
a. The IC Section Leader, or designate, will Approve the Conditional Release.
b. Select either “Approved” or “Denied” depending on the action needed within
LSRTIS, Product Manager Review Tab, Conditional Releases.
The first leg of the conditional release Form 2008 will then move to BCA
Ready for Approval area, if approved by Section Leader.
If the conditional release is Denied, the “first leg” Form 2008 will be complete
and no further action is necessary for the first leg. The “final leg” or now the
original Form 2008 will wait for the at-risk window or testing to be complete.
3. Processing the Conditional Release:
The Biologics Compliance Assistant (BCA) makes two copies of the Form
2008 (if it came in hard copy). This will be the “first leg” of the conditional
release. Check “Other” in Block 16 of the Form 2008 if hard copy.
If the serial is under test by our lab, type on the hard copy Form 2008, “May
be released, subject to immediate recall should this serial, now under test by
Conditional Serial Release
ICWI0410.04 Page 2 of 3
Author: Jeanette M. Watson
Issue Date: 28Apr17
Source Document: ICSOP0010, Processing Serial Records
APHIS, be found unsatisfactory. See letter from ________dated_________.”
This may also be typed a Release Criteria within LSRTIS. See below.
If the serial has not been selected for testing and still is in the testing selection
timeframe, type on the Form 2008 or Release Criteria, “May be released,
subject to immediate recall should this serial, if tested by APHIS, be found
unsatisfactory. See letter from ________dated_________.” See below.
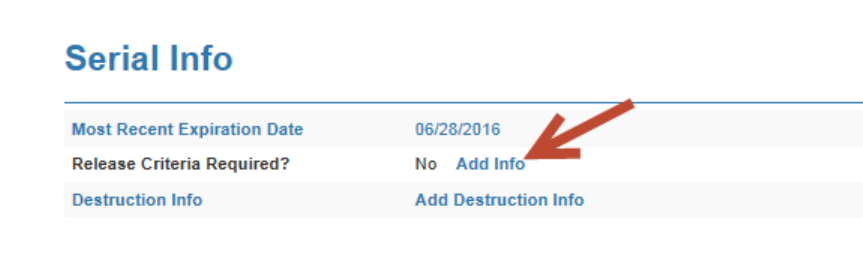
Ensure this is typed as a Serial Release Requirement in LSRTIS, so it shows
in the ENSR (Electronic Notification of Serial Release).
o Under the Serial Stat, “Serial Info” category – choose “Release Criteria
Required” – Add Info link.
o Choose the toggle of “Release Criteria Required?”
o Fill in Criteria of “Conditional Release”
o Choose Due date of either off test date or end of at risk window
o Comments – add what you type on the Form 2008 (above bullets under
3.b.)
4. Releasing the Conditional Release:
a. The first leg of the conditional release should be in BCA Ready for Approval.
Ensure the APHIS Disposition shows “Other - Conditional Release Granted” in
the Action Sheet. Approve the Form 2008.
b. The Form 2008 should move to Ready for Signature. The Specialist will sign the
“first leg” of the conditional release both electronically and hard-copy.
c. If hard copy:
Stamp the photocopy of the Form 2008 with the appropriate stamps in Blocks
18 and 19, and then give to the initiating Specialist for signature.
Mail photocopy of Form 2008 to firm, Contact the firm of Conditional
Release action, and document the contact on ICFRM0010 and the Phone Log
#.
Drop file the CVB photocopy with original Form 2008 and any other
paperwork in the corresponding product code file in the pending 2008 file
drawer.
5. Releasing the Original Form 2008:
a. Once testing has been verified or the at-risk window complete, the “final leg” of
release of the electronic Form 2008 will move to BCA Ready for Approval area,
which can be approved and moved on for Signature by the Specialist.
Conditional Serial Release
ICWI0410.04 Page 3 of 3
Author: Jeanette M. Watson
Issue Date: 28Apr17
Source Document: ICSOP0010, Processing Serial Records
b. Once the serial comes up for final release in BCA Ready for Approval, pull the
previous photocopy and paperwork from the pending drawer. This is the “final
leg” of the conditional release.
c. Complete the APHIS disposition (Block 16) on the original Form 2008: The
APHIS disposition of the serial on the Action Sheet will be based on either the
Firm’s disposition or CVB Laboratory testing results.
If the serial was tested as “SAT” by our lab, check “SAT” and “Other”, and
type, “This completes the conditional release granted per Specialist name on
initial date of conditional release” (from worksheet).
or
If the serial was not tested and the Action Sheet shows to release as “Not to
Be Tested,” check “NBT” and “Other,” and type, “This completes the
conditional release granted per Specialist name on initial date of conditional
release” (from worksheet).
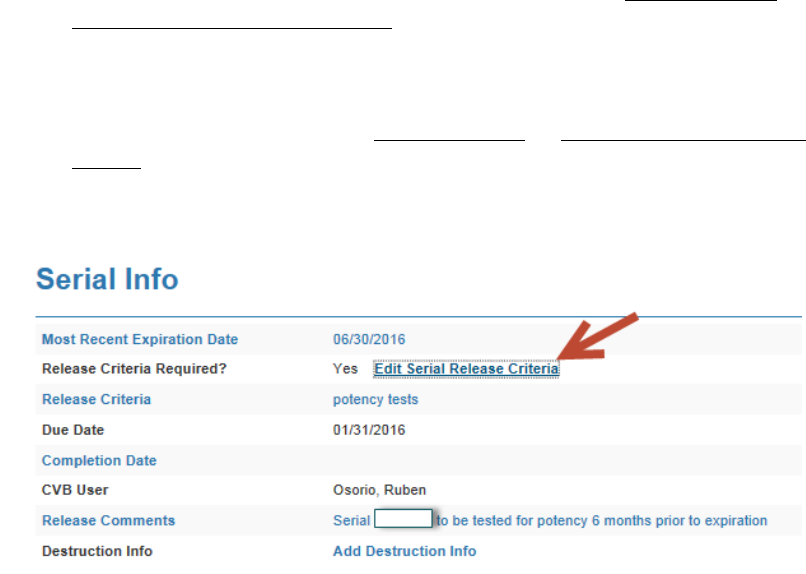
Complete the Release Requirements in LSRTIS (so it does not show up on the
ENSR report). This will show in the Serial Stat again.
o Choose the “Edit Serial Release Criteria”
o Fill in the “Completion Date” with the date of Release of final Leg.
d. Stamp the Form 2008 with the appropriate stamps in Blocks 18 and 19. The date
on the Form 2008 is the date of the Action Sheet.
e. Give the Form 2008 to the Program Coordinator for signature with any other
serials up for release that day. (Clip “first leg” copy to the original Form 2008.)
f. Staple the original Form 2008 on top of the “first leg” Form 2008 of the
conditional release. File Form 2008 in file room.
g. Mail firm’s copy of the Form 2008.
h. Complete Section 9 of the ICFRM0010, indicating the date of release and initials
of the BCA processing the form.
i. File the completed worksheet with product correspondence in the file room. A
copy is included with the Action Sheets in the pending 2008 file drawer.
