
Volume 21: Mini Workshops
Association for Biology Laboratory Education (ABLE) ~ http://www.zoo.utoronto.ca/able
492
The Use of Writing in Investigative Biology Laboratories
Ralph W. Preszler
Department of Biology, Foster Hall
New Mexico State University
Las Cruces, NM 88003-8001
505-646-5346
Fax 505-646-5665
Ralph Preszler was introduced to the study of biology and developed an interest in
botany and plant ecology at Southern Oregon State College (now Southern
Oregon University) in Ashland Oregon where he earned his B.S. in biology. He
then moved to Northern Arizona University, where he earned his M.S. and Ph.D.
studying interactions between plants and herbivores and where he was given the
opportunity to discover the fascinating and challenging nature of teaching.
Many schools across the country have recently changed from primarily observational
laboratory exercises to more investigative, hypothesis-testing experiments. This shift in the
nature of laboratory activities has dramatically altered the purpose of laboratory reports. What
once were fairly descriptive reports which could be successfully written outside of class have
become writing exercises that help the students synthesize and interpret their experiments. It is
no longer realistic to expect that students can successfully complete this challenging component
of the experimental process without considerable guidance. We have been developing activities
in the laboratory that help students learn the synthetic and creative reasoning skills that they will
need to complete their scientific investigation successfully as they write their reports.
After in-class activities that help students distinguish hypotheses from predicted results,
and after learning to recognize and describe the relevant patterns (or lack of patterns) in their
observed results, most students are able to form a conclusion regarding a hypothesis. This initial
conclusion is the starting point of our students’ discussion sections. This conclusion should be
consistent with and supported by the comparison between their predicted and observed results. It
is the subsequent components of the discussion — critical evaluation of experimental
Reprinted From: Preszler, R. W. 2000. The use of writing in investigative biology laboratories. Pages
492-496 in Tested studies for laboratory teaching, Volume 21 (S. J. Karcher, Editor). Proceedings of the
21st Workshop/Conference of the Association for Biology Laboratory Education (ABLE), 509 pages.
- Copyright policy: http://www.zoo.utoronto.ca/able/volumes/copyright.htm
Although the laboratory exercises in ABLE proceedings volumes have been tested and due consideration
has been given to safety, individuals performing these exercises must assume all responsibility for risk. The
Association for Biology Laboratory Education (ABLE) disclaims any liability with regards to safety in
connection with the use of the exercises in its proceedings volumes.
Volume 21: Mini Workshops
493
assumptions and discussion of implications — that our students find the most challenging. This
paper provides a detailed description of a laboratory experiment that we have developed
(Preszler and Haas 1999) that helps students learn to identify and evaluate the consequences of
assumptions that are imbedded within their experimental design. I close with a brief description
of assignments that have helped our students explore the implications of their work.
This experiment, derived from the work of Reinking et al. (1994), uses enzymes found in
two dietary supplements to evaluate the lock and key model of enzyme specificity. The first
dietary supplement, Lactaid® contains beta-galactosidase which breaks the beta-linkage in
lactose to produce glucose and galactose; in contrast, Beano® contains alpha-galactosidase
which breaks the alpha-linkage in melibiose which also produces glucose and galactose (Figure
1, Step One). Yeast is also included in each solution to produce a bioassay that generates carbon
dioxide from glucose if the first reaction has occurred (Figure 1, Step Two). The four treatments
shown in Table One provide a test of the specificity of the two galacotsidases.
Before they have conducted the experiment, I ask each group of students to identify an
assumption in the experimental design. To help them discover underlying assumptions, I discuss
the following categories of assumptions that are present in nearly all experiments:
♦ We are able to measure the dependent variable accurately. In this experiment we hope to
measure the rate of the initial reaction, but we are actually measuring the production of a
gas during the bioassay.
♦ We are able to manipulate the independent variable in the intended fashion. Are we
really controlling combinations of enzymes and sugars?
♦ There are no additional variables that may bias our results.
I then ask each group to contribute to the class list of assumptions. After we have identified
the assumptions associated with the design of the experiment, students design their control
variables. Table One illustrates the initial four treatments that test the specificity of the enzymes
followed by control variables that can be used to evaluate the validity of the following
assumptions. (After each assumption I’ve listed in parentheses the number of the corresponding
control variable):
♦ Yeast cannot metabolize lactose (5);
♦ Yeast cannot metabolize melibiose (6);
♦ The dietary supplements do not contain any ingredients that can be metabolized by yeast
(7&8);
♦ Yeast can metabolize glucose (9).
The results of this experiment generally support the hypothesis that the enzymes are specific
to their respective sugars, but there is an interesting exception to this correspondence between
the predicted and observed results. As indicated in Table One, Beano and lactose produce a
small amount of carbon dioxide. However, if students understand the purpose of their control
variables, they can use them to identify the assumption that was not met and that accounts for the
CO
2
that is produced by this treatment. Notice Control #8 indicates that Beano contains
Volume 21: Mini Workshops
Association for Biology Laboratory Education (ABLE) ~ http://www.zoo.utoronto.ca/able
494
substrates which can be reduced by yeast. These ingredients within the dietary supplement help
account for the unexpected CO
2
produced by the combination of Beano and lactose.
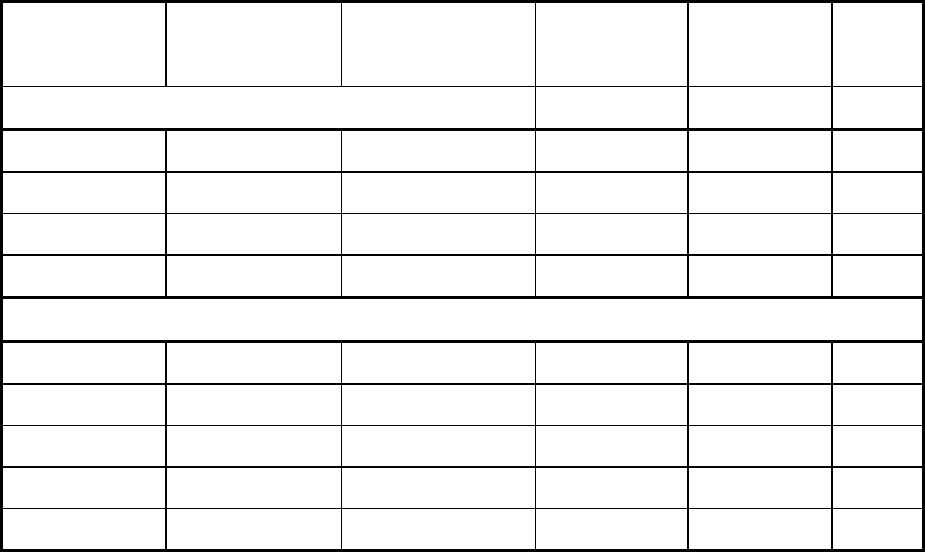
Table One. Enzyme specificity treatments and controls. Cells initially left blank to be filled
in by students have been filled in with italicized answers. Sample results, when materials are
incubated at 37° C for 25 minutes, are included in the last column.
TREATMENT SUGAR (2.5%) OR
CONTROL
(4 ml)
ENZYME (25 pills /
100 ml of water)
OR CONTROL
(2 ml)
YEAST (7%)
(10 ml)
PREDICTION
CO
2
production
or No CO
2
.
Results
(ml.)
Treatments that Test the Hypothesis
One Lactose Lactaid Yeast Yes 13
Two Melibiose Lactaid Yeast No 0
Three Lactose Beano Yeast Yes 1.5
Four Melibiose Beano Yeast No 9
Controls: Treatments that Test Assumptions
Five Lactose Water Yeast No 0
Six Melibiose Water Yeast No 0
Seven Water Lactaid Yeast No 0
Eight Water Beano Yeast No 1.3
Nine Glucose Water Yeast Yes 18
The value of this experiment is not only that it provides students with an interesting test of
enzyme specificity, but that it also helps them begin to look more critically at experimental
conclusions. As they begin to work on the implications of this experiment, I introduce another
layer of assumptions that are imbedded in scientific argument. I ask my students how
realistically does the fermentation tube represent the human body? This leads to discussions of
the need for series of experiments from in vitro work to clinical studies.
After students have evaluated the hypothesis and considered the effects of assumptions in the
design of their experiment, I ask them to discuss the implications or applications of their
conclusions. Encouraging our students to take this most challenging step in the scientific process
is essential if we hope to train students who are able to apply scientific information to societal
issues. I have used non-traditional laboratory reports and structured in-class discussions to help
students develop the ability to discuss the implications and potential applications of their work.
An example of this first approach is asking them to write a report comparing their experimental
system to an analogous system. After they have conducted a yeast complementation experiment
(originally developed by the GENE project) with prototrophic and auxotrophic yeast, I ask them
to read material describing phenylketonuria and alkaptonuria. Rather than writing a traditional
Volume 21: Mini Workshops
495
laboratory report, they write an essay discussing and explaining the analogy between the yeast
and human conditions. Many of the students are surprised by the striking similarities of these
two superficially very different systems. Exploring this analogy has improved their
understanding of the use of model systems in biology and has improved their discussions of the
implications of their experiments in subsequent reports.
As the structure of activities in teaching laboratories moves toward investigative exercises, it
is important that the use of writing assignments to facilitate the development biological literacy
also evolves toward assignments that encourage the production of scientific argument that
elucidates connections between topics within biology and is tied to larger societal issues.
References
Reinking, L.N., J.L. Reinking, and K.G. Miller. 1994. Fermentation, respiration and enzyme
specificity: a simple device and key experiments with yeast. American Biology Teacher, 56:
164-168.
Preszler, R.W., and L.L. Haas. 1999. Student investigations of cellular and organismal biology.
EMC / Paradigm Press, St. Paul, MN. ISBN 1-58175-068-4

Volume 21: Mini Workshops
Association for Biology Laboratory Education (ABLE) ~ http://www.zoo.utoronto.ca/able
496
Figure One. A bioassay of galactosidase activity.
