Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 1
Higher Chemistry: Unit 1 – Chemical Changes and Structure
Part A – Periodicity and Bonding
Lesson 4 – Periodicity: Electronegativity
By the end of this lesson you should know:
1. What is meant by the term electronegativity.
2. The trends in electronegativity across a period and down a group.
3. Explain the trends in electronegativity going across a period and down a group
You will have been successful in this lesson if you:
1. Read and learn the notes given
2. Complete the tasks given.
3. Complete Exercise 1.4 and check your answers.
There is also a further reading section to help you gain more depth of
understanding for this section.
MS Teams will be monitored throughout the week by a chemistry teacher. If you
need help or clarification with either the task or the content of the lesson, just
ask.
You may wish to revise the following to help you understand this lesson:
- National 5 chemistry – atomic structure, protons, electrons and electron
arrangement
Learning Outcomes
Success Criteria
Links to Prior Knowledge
For reference, the periodic table is given in the data booklet. Download or
print a copy of the Higher Chemistry Data Booklet from MS Teams or from
the SQA website -
https://www.sqa.org.uk/sqa/files_ccc/ChemistryDataBooklet_NewH_AH-Sep2016.pdf
Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 2
Electronegativity
It is the outer electrons in any atom which are involved in making a chemical bond
with another atom. In National 5 chemistry, you would have learned that some
atoms bond by sharing a pair of electrons, this is a called a covalent bond and
usually occurs between non-metal atoms.
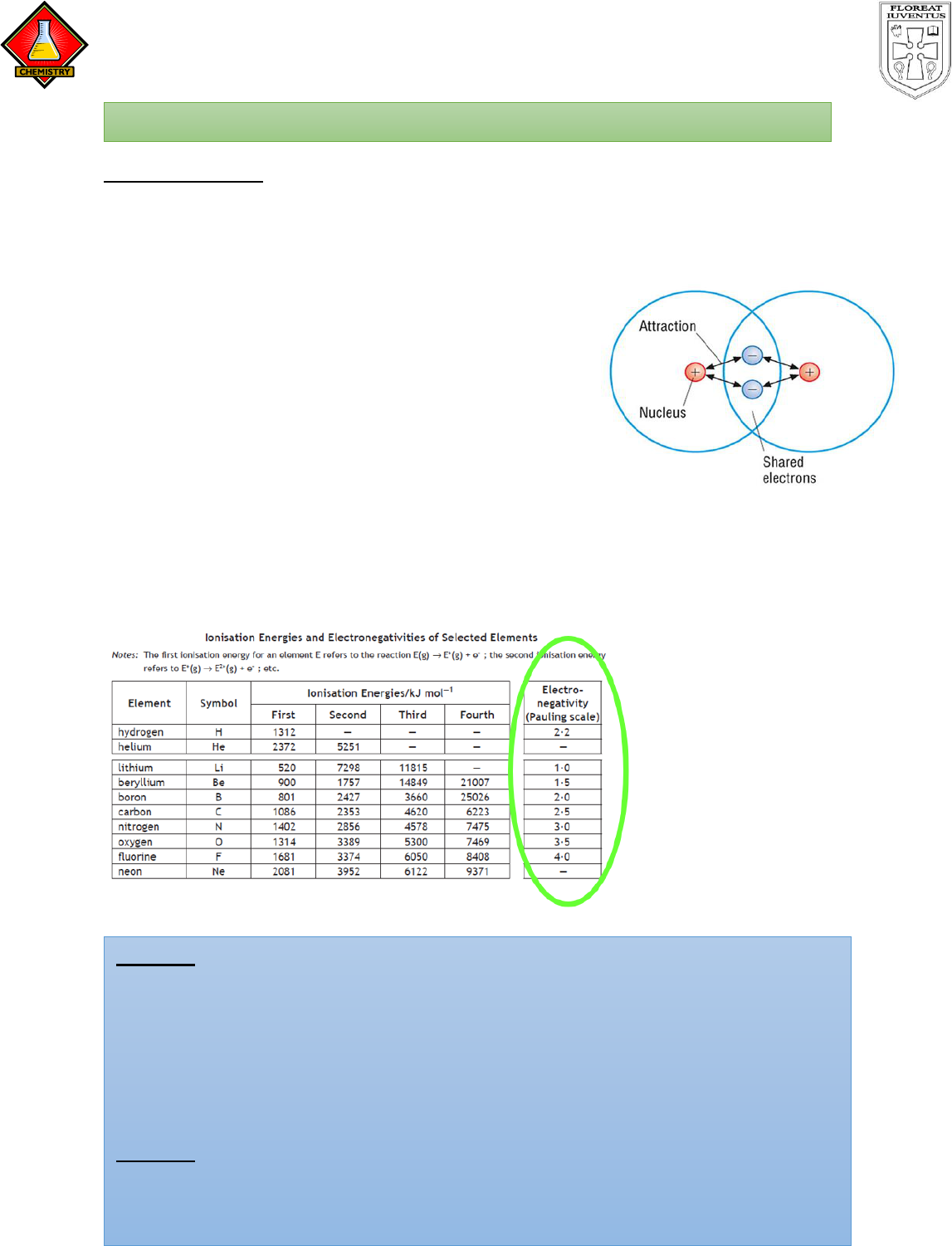
The electrons in that bond are attracted to both
atoms…. but the strength of that attraction changes
depending on the atom. Some atoms have more
“pull” on the electrons that others. This is referred
to as the “Electronegativity”.
Definition: Electronegativity is a measure of the attraction an atom has for the
electrons involved in a bond.
Electronegativity values are given page 11 of the data booklet.
Notes
TASK 1 - Using the information on page 11 of the data booklet decide which
atom in the following molecules would have a greater attraction the electrons
in the bond.
1. NH
3
2. HF
3. CO
2
TASK 2 - On your data booklet, find the element with the highest
electronegativity and highlight this. Where does this element appear in the
periodic table? Now find the element with the lowest electronegativity. Where
does this element appear on the periodic table?
Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 3
Electronegativity: Trends
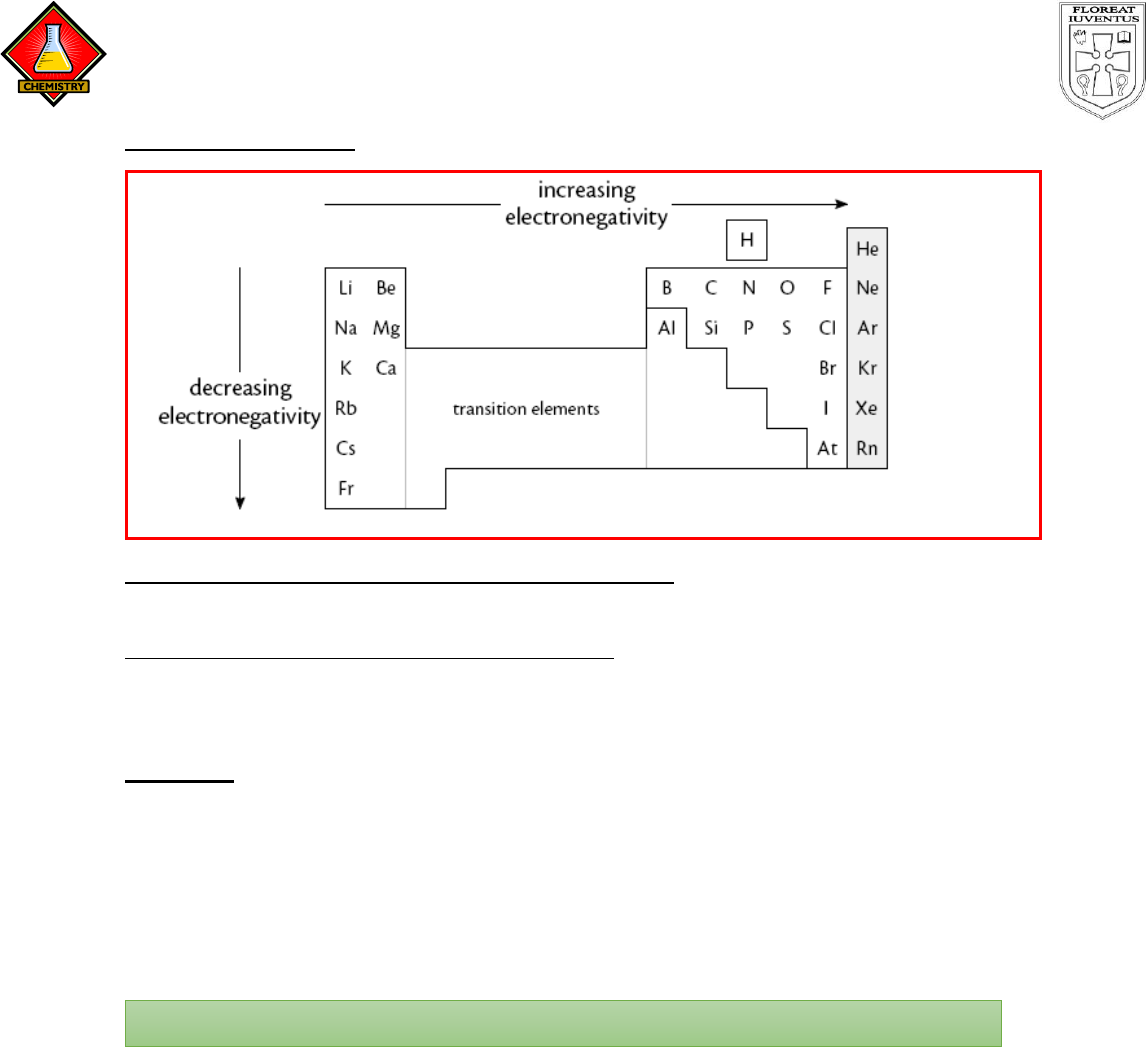
Trend in Periods:
Electronegativity increases as you go along a period due to an increase in nuclear
charge meaning the bonding electrons are held by a stronger force.
Trend in Groups:
Electronegativity decreases as you go down a group due to the atoms having more
occupied electron shells. This means:
- There is less pull on the electrons as they are increasingly further away from
nuclear attraction.
AND
- Electrons in inner shells have a screening effect shielding the bonding
electrons from nuclear attraction.
TASK 3 - Using the information on page 11 of the data booklet find the
electronegativity values for
Group 1 elements from lithium caesium
Group 2 elements beryllium strontium
Period 2 elements lithium fluorine
Period 3 elements sodium chlorine
Having gathered the data above, what general trends have you observed?
Take a moment to think about why these patterns may occur…
Sound familiar?
The explanation of the trends in electronegativity are the same as the
explanations for the trends in ionisation energy. This is because both involve
the attraction of outer electrons. The stronger the “pull” on the outer
electrons, the harder it will be for the electron to leave (ionisation energy).
Similarly the stronger the pull on the other electrons, the stronger to pull not
just on an atoms own electrons, but also on that of the other electrons in a
bond.
Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 4
Metals & Non-metals
Non-metals tend to have higher electronegativities than metals, because they are
on the top-right of the table.
Metals tend to have lower electronegativities than non-metals, because they are
on the lower-left area of the table.
SUMMARY
Electronegativity increases across a period due to increasing nuclear charge.
Electronegativity decreases down a group due to the atoms having more energy
levels. This means bonding electrons are further from the nuclear charge and are
shielded by inner electrons.
You should now know:
1. Atoms of different elements have different attractions for bonding electrons.
2. Electronegativity is a measure of the attraction an atom involved in a bond has
for the electrons of the bond.
3. The trends in electronegativity across periods and down groups can be
rationalised in terms of covalent radius, nuclear charge and the screening effect
due to inner shell electrons.
Learning Outcomes

Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 5
To learn more about electronegativity. Follow the links below:
BBC Bitesize: https://www.bbc.co.uk/bitesize/guides/zxc99j6/revision/1
Read page 8 and try the TEST
Evans2 chem web: https://www.evans2chemweb.co.uk/login/index.php#
Username: snhs password: giffnock
Select any teacher revision material CfE Higher Periodicity
Royal Society of Chemistry: http://www.rsc.org/periodic-table/trends
Have a look at this periodic table, you can play around to see the periodicity
patterns in the table.
Complete Exercise 1.4 and check your answers
Further Reading
Questions
Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 6
1. The data book lists the electronegativity of many elements.
(a) What is meant by the term electronegativity?
(b) Which type of elements have the highest electronegativity?
(c) Which type of elements have the lowest electronegativity?
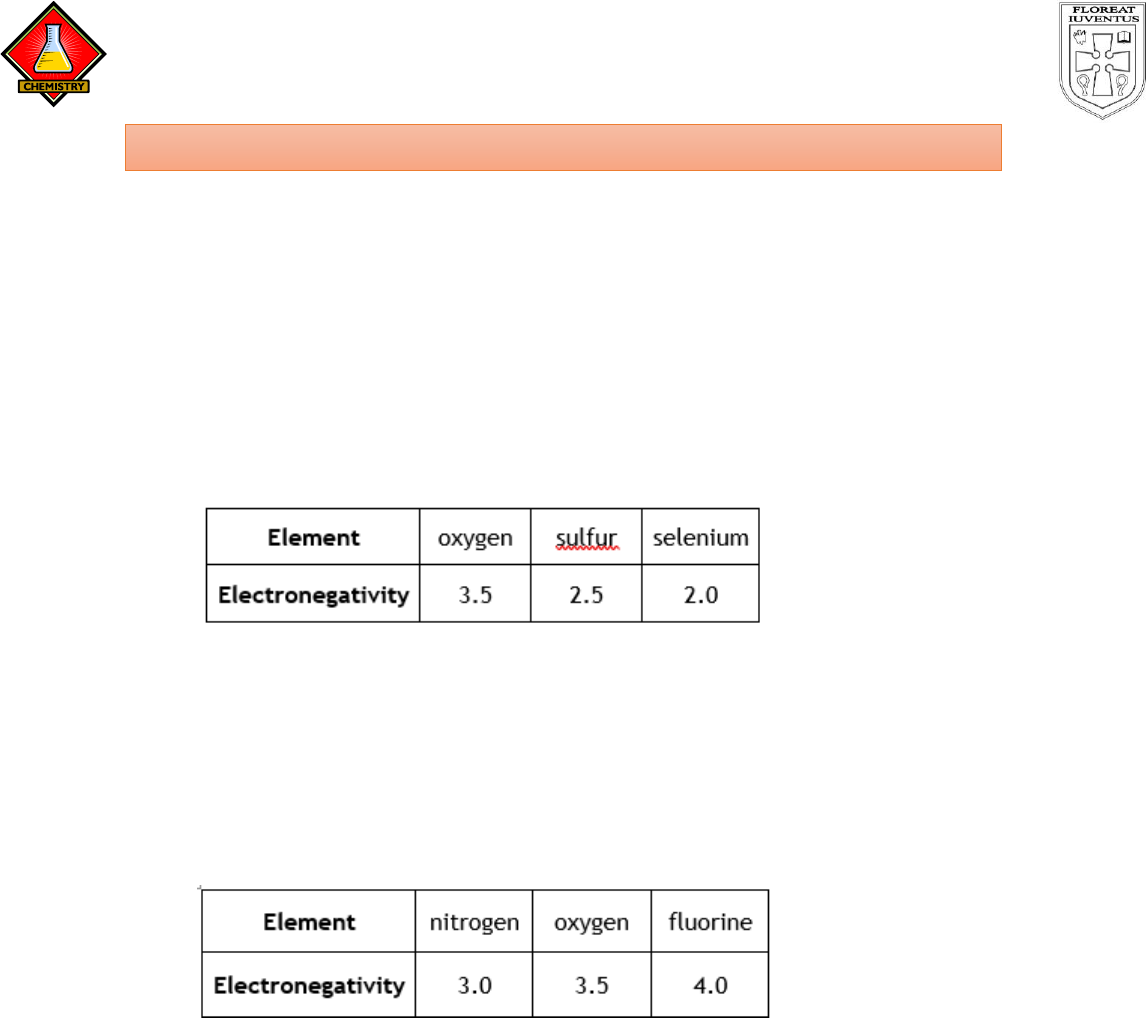
2. The table below shows the electronegativities of some group six elements:
Explain the trend in electronegativity as you go down a group in the periodic
table.
3. The table below shows the electronegativities of some second period
elements:
Explain the trend in electronegativity as we cross a period in the periodic
table from left to right.
Exercise 1.4 – Periodicity: Electronegativity
Higher Chemistry St. Ninian’s High School
CCS: Part A Lesson 4 – Periodicity: Electronegativity Page 7
1. (a) Electronegativity is the measure of attraction that an atom has for
bonded electrons.
(b) Non-metals
(c) Metals
2. As you descend group 6, electronegativities decrease because:
There is less pull on the electrons as they are increasingly further away
from nuclear attraction.
AND
Electrons in inner shells have a screening effect shielding the bonding
electrons from nuclear attraction.
3. Electronegativity increases as you go along a period due to an increase in
nuclear charge meaning the bonding electrons are held by a stronger force.
Exercise 1.4 – ANSWERS
