Ref: FOI/GS/ID 6480
Please reply to:
FOI Administrator
Trust Management
Maidstone Hospital
Hermitage Lane
Maidstone, Kent
ME16 9QQ
Email: mtw-tr.f[email protected]
www.mtw.nhs.uk
12 January 2021
Freedom of Information Act 2000
I am writing in response to your request for information made under the
Freedom of Information Act 2000 in relation to the policy for the prevention of
blood clots for inpatients.
You asked:
I am aware that the Trust has a policy for the prevention of blood clots for
inpatients.
Could you please advise me on how to obtain a copy of the policy?
Trust response:
Please find attached below a copy of the requested policy.
Venous thromboembolism prevention
policy and procedure
Requested/
Required by: Thrombosis Committee to comply with NICE Clinical
Guidance 92
Author: VTE Lead Nurse
Other contributors: Consultant Haematologist
Owner: Medical Director
Directorate: Corporate
Specialty: Venous Thromboembolism (VTE)
Supersedes: Venous Thromboembolism Prevention Policy and
Procedure, Version 3.0 (June 2013)
Venous Thromboembolism Prevention Policy and
Procedure, Version 3.1 (April 2014)
Venous Thromboembolism Prevention Policy and
Procedure, Version 4.0 (May 2016)
Venous Thromboembolism Prevention Policy and
Procedure, Version 4.1 (September 2016)
The policies below are now incorporated into this
policy and have been archived on Q-pulse.
Anti-embolism stockings (AES), Policy and Procedure
for the use of, Version 1.0 (February 2013)
Intermittent Pneumatic Compression Devices (IPCD),
Policy and Procedure for the use of, Version 1.0
(June 2013)
Approved by: Thrombosis Committee, 10
th
March 2016
Ratified by: Policy Ratification Committee, 12
th
May 2016
Review date: May 2019 [Extension to June 2021 approved by the
Chair of the Thrombosis Committee on 8
th
December
2020, following the arrangements for extension as set
out in the ‘Policy and procedure for the production,
approval and ratification of Trust-wide policies and
procedures ('Policy for policies') - RWF-OPPPCS-NC-
CG25]
This policy has been written for implementation during periods of
standard functioning within the Trust. Outside of those periods
(such as major incidents or national emergencies) other
‘emergency’ policies may be written to supersede or run alongside
this policy.
Disclaimer: Printed copies of this document may not be the most recent version.
The master copy is held on Q-Pulse: Organisational Wide Documentation database
This copy – REV4.2
Document history
Requirement
for
document:
To comply with national guidance on the prevention of
venous thromboembolism in hospitalised patients.
Cross
references
(external):
1. NICE. (2010). Venous thromboembolism: reducing the risk
of venous thromboembolism (deep vein thrombosis and
pulmonary embolism) in patients admitted to hospital.
NICE Clinical Guidance 92 (2010)
2. NICE. (2012). Venous thromboembolic diseases: the
management of venous thromboembolic diseases and the
role of thrombophilia testing. NICE Clinical Guidance 144
(2012)
3. Department of Health. (2007). Report of the independent
expert working group on the prevention of venous
thromboembolism in hospitalised patients. London:
Department of Health
4. Department of Health. (2008). Risk Assessment for
Venous Thromboembolism. London: Department of Health
5. National Patient Safety Agency. (2009). Patient Safety
Alert. WHO Surgical Safety Checklist.
6. The NHS Confederation (2009). Briefing paper: Reducing
Deaths from Blood Clots in Hospitals.
Associated
documents
(internal):
Acute Stroke Policy and Procedure (RWF-OPPPES-C-
SM11)
Blood clots (thrombosis), Preventing hospital acquired
[LARGE PRINT LEAFLET] [RWF-OPLF-PES92]
Blood clots (thrombosis), Preventing hospital acquired
[STANDARD PRINT LEAFLET] [RWF-OPLF-PES89]
Blood clots (thrombosis), Preventing hospital acquired;
information for maternity patients [STANDARD PRINT
LEAFLET] [RWF-OPLF-PES90]
Blood clots (thrombosis), Preventing hospital acquired;
information for maternity patients [LARGE PRINT
LEAFLET] [RWF-OPLF-PES94]
Consent to Examination or Treatment, Policy and
Procedure for [RWF-OPPPES-C-SM5]
Medical Devices Policy and Procedure [RWF-OPPPCS-NC-
EST2]
Pharmacy anti-coagulation guidance [available on the
pharmacy pages of the staff intranet]
Policy and Procedure for the Safe Management of
Anticoagulant Therapy [RWF-OPPPCSS-C-CAN3] –
Protocol for the use of Intermittent Pneumatic Compression
Devices (IPCD) for patients with Haemorrhagic Stroke (or
other contraindication to LMWH) (RWF-OPPM-ES6)
Venous Thromboembolism (VTE) in Pregnancy and
Puerperium: prophylaxis, diagnosis and management
[RWF-OPPPW&C-C-O&G2]
Venous Thromboembolism (VTE): diagnosis and
management in adults policy and procedure [RWF-
OPPPES-C-SM15]
Version control:
Issue:
Description of changes:
Date:
1.0
First iteration of the policy/procedure
July 2010
1.1
Amendment to Appendix 4 Risk Assessment
July 2010
1.2
Amendment to Appendix 4 Risk Assessment
January 2011
2.0
Revision of the policy/procedure
October 2011
3.0
Revision of the policy/procedure
June 2013
3.1
Added section 7.9 and appendix 11 to
policy/procedure; removed references to NHSLA
standards, which have ceased to exist
April 2014
4.0
Revision of the policy / procedure
Merge of this policy with Anti-embolism stockings
P&P and Intermittent Pneumatic Compression
Devices P&P
May 2016
4.1
Amended appendix table to reflect printed
stationary
September
2016
4.2
Extension to June 2021 approved by the Chair of
the Thrombosis Committee on 8
th
December 2020
December 2020
Policy statement for
Venous thromboembolism prevention
policy
The purpose of this policy is to ensure that all adult in-patients are appropriately and regularly assessed
for risk of venous thromboembolism (VTE) and if appropriate receive VTE prophylaxis according to that
risk throughout their stay at Maidstone and Tunbridge Wells NHS Trust (MTW).
Patients admitted to the Trust should have the opportunity to make informed decisions
about their care and treatment, in partnership with their health care professionals and
the Trust will offer best practice advice on reducing the risk of VTE in patients admitted
to hospital.

Venous thromboembolism prevention
procedure
Contents
Page
1.0 Introduction and scope 6
2.0 Definitions 7
3.0 Duties 8
4.0 Training / competency requirements 8
5.0 Care pathway 9
6.0 Patient risk assessment 10
7.0 Thromboprophylaxis 10
7.1 VTE prophylaxis in surgical patients 11
7.2 VTE prophylaxis in orthopaedic patient 11
7.3 VTE prophylaxis in medical patients 11
7.4 VTE prophylaxis in acute stroke patients 12
7.5 VTE prophylaxis in cancer patients 12
7.6 VTE prophylaxis in end of life patients 13
7.7 Patients already on anti-platelet or anticoagulant therapy 13
7.8 VTE prophylaxis in pregnancy and up to 6 weeks post-partum 13
7.9 Lower limb immobilisation and LMWH 13
8.0 Patient information and consent
14
9.0 Discharge
14
10.0 Anti-embolism stockings (AES)
15
11.0 Intermittent pneumatic compression devices (IPCD)
16
12.0 Monitoring and audit
18
Appendices
1 Process requirements
19
2 Consultation table
20
3 Equality impact assessment table
21
List of further appendices – separate documents on Q-Pulse
22
1.0 Introduction
The House of Commons Health Committee reported in 2005 that an
estimated 25,000 people a year in the UK die from preventable hospital-
acquired venous thromboembolism (VTE). This includes patients admitted
to hospital for medical care and surgery. The inconsistent use of prophylactic
measures for VTE in hospital patients has been widely reported. A UK
survey suggested that 71% of patients assessed to be at medium or high
risk of developing deep vein thrombosis (DVT) did not receive any form of
mechanical or pharmacological VTE prophylaxis.
VTE encompasses a range of clinical presentations. Venous thrombosis is
often asymptomatic; less frequently it causes pain and swelling in the leg.
Part or all of the thrombus can come free and travel to the lung as a
potentially fatal pulmonary embolism. The patient typically suffers chest
pain, breathlessness and collapse. Deep vein thrombosis carries a
considerable burden of morbidity, including long-term morbidity because of
chronic venous insufficiency. This in turn can cause venous ulceration and
development of a post-thrombotic limb (characterised by chronic pain,
swelling and skin changes). Pulmonary embolism can leave a legacy of
pulmonary hypertension.
VTE is an important cause of death in hospital patients, and treatment of
non-fatal symptomatic VTE and related long-term morbidities is associated
with considerable cost to the health service.
The purpose of this policy is to enable healthcare practitioners at MTW to
identify patients at risk of developing VTE and select the appropriate therapy
to reduce the associated mortality and morbidity risks associated with this
disease.
The diagnosis and treatment of VTE (DVT and PE) is covered in the MTW
Policy and Procedure for the Diagnosis and Management of VTE and the
MTW Policy and Procedure for the Safe Management of Anticoagulation
Therapy.
1.1 Scope
The policy applies to:
All those 18 years and older admitted to hospital as inpatients or formally
admitted to a hospital for day-case procedures, including:
surgical and medical inpatients
orthopaedic and trauma inpatients
patients admitted to Intensive Care Units
cancer inpatients
people undergoing long-term rehabilitation in hospital
patients admitted to a hospital bed for day-case medical or surgical
procedures.
Please note: Appendix 8 - this applies to patients 16 and over and is relevant
to both outpatients and inpatients with lower limb casts.
The policy does not apply to:
People younger than 18 years (but please use clinical judgement for
teenage inpatients) (exception please see Appendix 8 for lower limb
casts)
People attending hospital as outpatients (exception - please see Appendix
8 for lower limb casts)
People presenting to emergency departments without admission.
Elderly or immobile people cared for at home, or in external residential
accommodation, unless admitted to hospital.
Women in pregnancy; a separate policy exists relating to women in
pregnancy (Venous Thromboembolism (VTE) in Pregnancy and
Puerperium)
Patients admitted to hospital with a diagnosis of, or suspected diagnosis of,
deep vein thrombosis or pulmonary embolism should follow a treatment plan
and not the prophylactic plan. Please refer to the Trust Policy and Procedure
for the Diagnosis and Management of VTE and the Trust Policy and
Procedure for the Safe Management of Anticoagulation Therapy and this
policy.
2.0 Definitions
Venous Venous thrombosis is a condition in which a blood clot
Thromboembolism (thrombosis) forms in a deep vein. Blood flow along
the
(VTE) affected vein can be limited by the clot, causing
swelling and pain. Venous thrombosis most
commonly occurs in the 'deep veins' in the leg or
pelvis. This is known as a deep vein thrombosis
(DVT). An embolism is created if a part or all of the
thrombosis in the deep vein breaks off from the site
where it is created and travels along the venous
system. A leg DVT can travel to the lungs, known as
pulmonary embolism (PE). DVT and PE are the most
common manifestations of venous thrombosis. Both
conditions can be asymptomatic. DVT and PE are
known collectively as venous thromboembolism
(VTE).
Deep Vein A thrombosis occurring in a deep vein, most
commonly
Thrombosis (DVT) a deep vein of the leg or pelvis but can affect any
deep vein.
Pulmonary If the clot lodges in the lung a very serious condition,
Embolism (PE) pulmonary embolism (PE) arises.
Thromboprophylaxis Thromboprophylaxis is the treatment to prevent blood
clots forming in veins – this may be chemical or
mechanical.
Chemical Pharmaceutical agents (oral or parenteral) used to
decrease
thromboprophylaxis the clotting ability of the blood.
Mechanical Devices including anti-embolism stockings (AES) and
thromboprophylaxis intermittent pneumatic compression devices (IPCD)
can be used to increase venous blood flow and
reduce stasis within the leg veins.
Significantly reduced Used to denote patients who are bedbound, unable to
walk
mobility unaided or likely to spend a substantial proportion of
the day in bed or in a chair.
Major bleeding Refers to bleeding events that result in one or more of
the following:
death
a decrease in haemoglobin concentration of 2g/dl
or more
transfusion of two or more units of blood
bleeding into a retroperitoneal, intracranial or
intraocular site
a serious or life-threatening clinical event
a surgical or medical intervention.
Thrombosis The Thrombosis Committee was established under
the committee auspices of the Medical Director to
lead on the prevention, reduction and treatment of
VTE. It ensures best practice for the prevention and
management of VTE, based on national guidance and
other examples of best practice.
3.0 Duties (roles and responsibilities)
Chief Executive has overall responsibility and accountability for the
implementation of this policy.
Medical Director has delegated responsibility for the implementation of
this policy.
Clinical Directors will be responsible for ensuring that the clinical staff
within their area follow this policy. They will also review any audits
undertaken to ensure highlighted actions are completed and implemented
in a timely manner.
VTE Lead Nurse will be responsible for providing the education and
training to assist in the implementation of this policy, ensuring audits are
undertaken to monitor the effectiveness of the policy and ensure the policy
is revised when necessary.
Clinical staff (Medical/Nursing/Midwifery) will be responsible for
undertaking appropriate and timely risk assessments on patients (as
detailed in this policy) and entering these onto Patient Centre, as well as
ensuring that necessary prophylactic treatment is prescribed as necessary.
This applies to all patients admitted whether emergency or planned.
Theatre staff should ensure that the nursing pre-operative checklist is
performed ensuring that appropriate VTE risk assessments have been
undertaken as necessary. If no appropriate risk assessment has been
undertaken the surgical procedure should not proceed unless a life-
threatening situation.
Pharmacists: Pharmacists are responsible for checking chemical
prophylaxis is appropriately prescribed and administered. Any
discrepancies should be recorded in the patient’s healthcare record and
discussed with the prescriber and / or the patient’s medical team.
4.0 Training / competency requirements
All medical, nursing and midwifery staff are made aware of the VTE risk
assessment tool, thromboprophylaxis and patient information documents in
conjunction with this policy at local induction.
Doctors, nurses and midwives will undertake VTE training either through e-
learning or the mandatory update study day VTE session and this will be
monitored as part of their annual appraisal.
Pharmacists will undertake VTE training either through e-learning or the
mandatory update study day VTE session and this will be monitored as part of
their annual appraisal.
The above training will be monitored via the AT-Learning training database.
The training should take place every two years (see Appendix 6 for ‘Training
matrix’).
All clinical staff (Medical/Nursing/Midwifery) will be made aware of the AES
care pathway, AES patient information and the AES manufacturer’s
guidelines.
They should receive training in AES and a declaration of their competency
should be documented (see Appendix 11).
All clinical staff (medical / nursing / midwifery) will be made aware of the IPCD
care pathway and IPCD manufacturer’s guidelines. They must receive training
in IPCD and a declaration of their competency should be documented. See
Appendix 14 (IPCD competency).
6.0 Patient risk assessment
All patients admitted to Maidstone and Tunbridge Wells Trust must be
screened within two hours of admission for risk of VTE and considered
for thromboprophylaxis using the screening tool. Please refer to the VTE
Risk Assessment form: Appendix 4.
All patients must be assessed for risk of bleeding before being offered
chemical VTE prophylaxis. Patients with any of the risk factors for
bleeding will not be offered chemical prophylaxis unless the risk of VTE
outweighs the risk of bleeding. See Appendix 4.
The patient's VTE risk will be recorded on the risk assessment form and
if appropriate, thromboprophylaxis (chemical, mechanical or a
combination of both) will be commenced. See Appendix 5 for
‘Thromboprophylaxis summary’.
The risk of VTE and bleeding must be reassessed within 24 hours of
admission and whenever the clinical situation changes and treatment
adjusted accordingly, taking into account the need for extended
thromboprophylaxis in certain cases.
Treatment must take into account any contraindications to chemical or
mechanical therapies. Contraindications must be recorded in the
patient's healthcare record.
7.0 Thromboprophylaxis
Treatment should continue until the patient is fully mobile. Extended
thromboprophylaxis should be considered in high risk patients (please
refer to extended thromboprophylaxis guidelines within VTE risk
assessment: see Appendices 4 -5).
All patients who have risk factors for VTE will be prescribed low
molecular weight heparin (LMWH) from admission at 22.00 hours unless
this is contraindicated.
Patients who are at risk of VTE but who have an absolute
contraindication to LMWH must be prescribed anti-embolism stockings
unless contraindicated.
Pre-operative LMWH should not be given to elective surgical patients
unless this has been specifically requested and prescribed by the
admitting consultant.
Trauma list and non-elective surgical patients should where possible be
delayed until at least 12 hours after the last dose of LMWH has been
administered.
All patients should be adequately hydrated.
Patients should be mobilised as early as possible. Patients should be
shown how to exercise their legs if they are on bed rest.
Consider offering temporary inferior vena cava filters to patients who are
at very high risk of VTE (such as patients with a previous VTE event or
an active malignancy) and for whom mechanical and pharmacological
VTE prophylaxis are contraindicated.
Invasive procedures with a risk of bleeding (e.g. liver biopsy, endoscopy
with biopsy) should where possible be delayed until at least 12 hours
after the last dose of LMWH has been administered.
7.1 VTE prophylaxis in surgical patients
Surgical patients are at high risk if their combined surgical and anaesthetic
time is 60 minutes or longer or they have any additional risk factor as
identified on the MTW VTE risk assessment form. High risk surgical patients
should be prescribed LMWH daily as per actions on MTW VTE risk
assessment form (see Appendix 4) plus AES (unless contraindicated) and/or
intermittent pneumatic compression device.
Patients undergoing major cancer surgery in the abdomen or pelvis should
continue chemical and mechanical thromboprophylaxis for 28 days post-
surgery. Surgical patients with a body mass index of 30 or greater should
have extended chemical and mechanical thromboprophylaxis for 7 days post-
surgery. Other high risk surgical patients (including day surgery patients)
should continue thromboprophylaxis until their mobility is no longer
significantly reduced, including after discharge if necessary.
Surgical patients are at low risk if their combined surgical and anaesthetic
time is less than 60 minutes and they have no additional risk factor. Low risk
surgical patients should mobilise early and AES should be considered (unless
contraindicated).
If a spinal or epidural anaesthesia is used, additional care must be exercised
when placing or removing the catheters. Placement should be delayed until at
least 12 hours after the last LMWH dose to avoid bleeding complications at
the catheter site. Presence of a spinal or epidural catheter is not a
contraindication to LMWH use. At the discretion of the anaesthetist, LMWH
may be started immediately after the catheter is removed, or immediately after
a regional block.
For patients booked on the emergency theatre list, there needs to be a 12
hour gap between chemical thromboprophylaxis and spinal anaesthetic.
Therefore consideration must be given to the timing of the pre-operative dose,
i.e. this should be prescribed at 20.00 hours on the evening pre-surgery as a
stat dose with the 22.00 dose cancelled. Thromboprophylaxis must not simply
be omitted.
7.2 Thromboprophylaxis for orthopaedic patients
Unless contraindicated, patients admitted for elective hip or knee replacement
should have mechanical VTE prophylaxis which should continue until the
patient’s mobility is no longer significantly reduced. The patient should be
commenced on chemical thromboprophylaxis 6-10 hours post operatively.
This should continue for 35 days for hip replacements and 14 days for knee
replacements. Each patient should be reviewed on a case by case basis. Hip
fracture patients should be prescribed LMWH daily as per actions on MTW
VTE risk assessment form (see Appendix 4) plus AES (unless
contraindicated) and/or intermittent pneumatic compression device.
Thromboprophylaxis should be continued for 28 days. Please see Appendix
5 for summary of extended thromboprophylaxis for specific orthopaedic
surgery.
It is acceptable to reduce thromboprophylaxis to 2,500 units subcutaneous
daily for trauma patients with a weight of 49kg or under.

For patients booked on the orthopaedic trauma list, there needs to be a 12
hour gap between chemical thromboprophylaxis and spinal anaesthetic.
Therefore consideration must be given to the timing of the pre-operative dose,
i.e. this should be prescribed at 20.00 hours on the evening pre surgery as a
stat dose with the 22.00 dose cancelled. Thromboprophylaxis must not simply
be omitted.
7.3 VTE prophylaxis in medical patients
Patients expected to have ongoing reduced mobility plus one or more risk
factors and no contraindications identified should be prescribed LMWH daily
as per actions on the MTW VTE risk assessment form.
Patients who have a bleeding risk or an absolute contraindication to LMWH
must be prescribed AES instead unless contraindicated. If AES also
contraindicated (e.g. acute stroke patients) then an intermittent compression
device should be considered.
Please see Appendix 7 for guidance on thromboprophylaxis for patients with
a bleed or suspected bleed.
7.4 VTE prophylaxis in acute stroke patients
Patients admitted with an acute stroke should have a VTE risk assessment on
admission. LMWH should be withheld until CT imaging has excluded a
haemorrhage and the size of any infarct has been able to be assessed. Once
haemorrhage has been excluded and the patient has been reviewed by the
stroke/medical consultant to assess the size of the infarct, the VTE risk
assessment should be repeated within 24 hours. Unless there are further
contraindications, prophylactic LMWH should be prescribed within the first 72
hours of admission. Care should be taken if there are large territorial infarcts,
if there is a risk of haemorrhagic transformation or if there is a high risk of intra
or extra cranial haemorrhage (this decision will be made the Stroke
Consultant).Patients who have received thrombolysis should not be
prescribed LMWH until they have undergone a follow up CT brain imaging
after 24 hours. A second VTE risk assessment should take place following
the repeat CT head scan. It is expected that the majority of patients will be
able to commence VTE chemical prophylaxis following this assessment but
there will be some patients where this will not be appropriate due to possible
post thrombolysis complications such as haemorrhagic conversion or due to
the size or location of the established infarct. These patients should be
reviewed by the responsible stroke consultant for a decision and future
management in relation to VTE prevention including consideration of using
IPCD after the initial 12 hours if not already commenced. This decision will be
documented in the patient’s record.
All patients with a bleeding risk e.g. haemorrhagic stroke or other
contraindication to LMWH and who have reduced mobility must be assessed
for the use of an intermittent pneumatic compression device (IPCD) using the
IPCD proforma (see Appendix 15). All patients commenced on the IPCD’s
must have the care plan completed within the stroke integrated care pathway
document. Any removal of the IPCDs for anything other than physical therapy
or brief inspection and care of the legs for longer than 2 hours must be
documented on the IPCD monitoring sheet within the stroke integrated care
pathway. If the IPCD have been left off for more than 2 hours the patient’s
legs must be checked carefully for any signs of DVT before reapplying IPCD.
Please see Acute Stroke Policy and Procedure for stroke integrated care
pathway.
If the use of an IPCD is indicated the IPCD will be prescribed on the patient’s
prescription chart by the doctor or appropriate non-medical prescriber. Do
NOT offer AES for VTE prophylaxis to patients who are admitted for stroke.
7.5 VTE prophylaxis in cancer patients
Definition: active cancer should be considered to be:
1. A diagnosis of cancer (other than basal cell or squamous cell
carcinoma of the skin) within the previous six-month period
2. Any treatment for cancer within the previous six-month period
3. Recurrent or metastatic cancer
7.5.1 Patients with active cancer should receive thromboprophylaxis
throughout their hospital admission unless contraindicated.
7.5.2 Extended surgical thromboprophylaxis should be offered to those
requiring surgery for the management of abdominal and pelvic cancer (NICE
2010).
7.5.3 Thromboprophylaxis is not routinely recommended to prevent catheter-
related thrombosis.
Please refer to the Cancer Thrombosis Guideline within the Policy and
Procedure for the Safe Management of Anticoagulant Therapy for further
information.
7.6 VTE prophylaxis in end of life patients
Patients in palliative care, who have potentially acute reversible pathology,
should be considered for LMWH. However, if the patient is in terminal care or
on an end-of-life care pathway, pharmacological or mechanical VTE
prophylaxis should not routinely be offered. In both cases, decisions about
VTE prophylaxis should be reviewed daily, taking into account potential risks
and benefits and views of the patient, family and/or carers and
multidisciplinary team.
7.7 Patients already on anti-platelet or anticoagulant therapy
Consider offering additional mechanical or pharmacological VTE prophylaxis if
the patient is at risk of VTE. Take into account the risk of bleeding and of co-
morbidities such as arterial thrombosis.
If the risk of VTE outweighs the risk of bleeding, consider offering
pharmacological VTE prophylaxis according to the reason for admission.
If the risk of bleeding outweighs the risk of VTE, offer mechanical VTE
prophylaxis.
Do not offer additional pharmacological or mechanical VTE prophylaxis to
patients who are taking vitamin K antagonists (warfarin) and who are within
their therapeutic range, or direct oral anticoagulants (rivaroxaban, apixaban,
dabigatran, edoxaban), providing anticoagulant therapy is continued.

Do not offer additional pharmacological or mechanical VTE prophylaxis to
patients who are having full anticoagulant therapy (for example, Fondaparinux
sodium, LMWH or UFH).
7.8 VTE prophylaxis in pregnancy and up to six weeks post-partum
Please refer to separate policy for Obstetrics: Maidstone and Tunbridge
Wells NHS Trust. Venous Thromboembolism (VTE) in Pregnancy and
Puerperium
7.9 Lower limb immobilisation and LMWH
Immobilisation is defined as rigid immobilisation in plaster or fibre-glass. This
specifically excludes removable devices such as walking boots or cricket-pad
splints as there is no evidence for VTE prophylaxis in these patients. There is
no evidence for VTE prophylaxis in upper limb injuries.
The use of prophylactic LMWH is effective at reducing incidence of VTE in
ambulatory patients with lower limb immobilisations. If commenced,
prophylactic LMWH should be given for the duration of immobilisation. The
use of LMWH is associated with low rates of heparin induced
thrombocytopenia (HIT) and major bleeding when used for
thromboprophylaxis in ambulatory patients with cast immobilisation.
See Appendix 8 for the Lower limb immobilisation and LMWH pathway.
8.0 Patient information and consent
Treatment and care should take into account patients’ needs and preferences.
People admitted to hospital should have the opportunity to make informed
decisions about their care and treatment, in partnership with their healthcare
professionals. If patients do not have the capacity to make decisions,
healthcare professionals should follow the Policy and Procedure for Consent
to Examination or Treatment written with regard to the Department of Health’s
advice on consent and the Mental Capacity Act.
Good communication between healthcare professionals and patients is
essential. It should be supported by evidence-based written information
tailored to the patient’s needs. Treatment and care, and the information
patients are given about it, should be culturally appropriate. It should also be
accessible to people with additional needs such as physical, sensory or
learning disabilities, and to people who do not speak or read English.
All patients admitted to hospital will receive a patient information document,
produced by the Trust, relating to the prevention of thromboembolism, i.e. one
of the following:
Blood clots (thrombosis), Preventing hospital acquired [STANDARD PRINT
LEAFLET] [RWF-OPLF-PES89]
Blood clots (thrombosis), Preventing hospital acquired [LARGE PRINT
LEAFLET] [RWF-OPLF-PES92]
Blood clots (thrombosis), Preventing hospital acquired; information for
maternity patients [STANDARD PRINT LEAFLET] [RWF-OPLF-PES90]
Blood clots (thrombosis), Preventing hospital acquired; information for
maternity patients [LARGE PRINT LEAFLET] [RWF-OPLF-PES94]
This information will be used to obtain verbal consent from the patient allowing
healthcare staff to assess and where necessary provide prophylactic
treatment to the patient to reduce the risk of VTE.
Ward medical and nursing staff must be completely familiar with the patient
information provided, enabling them to answer general questions that may
arise while obtaining verbal consent.
9.0 Discharge
On discharge, all patients and/or their families / carers will be informed of the
signs and symptoms of DVT and PE and the importance of seeking medical
help if this is suspected.
If discharged with VTE prophylaxis (pharmacological and/or mechanical),
patients and/or their families or carers will be given verbal and written
information on the correct use and duration of VTE prophylaxis at home and
who to contact if problems occur.
If VTE prophylaxis is either pharmacological or mechanical, the patient and/or
their family should be shown how to use it. The patient’s GP must be notified
that the patient is being discharged with VTE prophylaxis.
Patients should be advised to wear their anti-embolism stockings until they
regain their usual mobility or longer if advised. When patients are discharged
with anti-embolism stockings, it should be ensured that they are able to
remove and replace the stockings for daily hygiene needs or that they have
someone who can do this for them. Patients should also be informed to check
for skin marking, blistering or discolouration, particularly over heels and bony
prominences.
10.0 Anti-embolism stockings (AES)
Anti-embolism stockings (AES) exert graduated circumferential pressure from
distal (below knee) to proximal (above knee) regions of the leg. They work to
help prevent the formation of thrombosis (blood clot) by promoting increased
blood flow velocity in the leg veins by compression of the deep venous
system, and by reducing venous dilatation.
One of the purposes of this Policy is to ensure that anti-embolism stockings
are correctly supplied, applied and utilised, and that their use is not
contraindicated.
Correctly applied, in the absence of contra-indications, anti-embolism
stockings are a safe and non-invasive therapy for reducing the incidence of
venous thromboembolism by the promotion of venous blood flow.
Anti-embolism stockings can be used as a therapy on their own or as an
adjunct to low molecular weight heparin in patients who have been clinically
assessed as having a risk of venous thromboembolism. AES can be used in
conjunction with IPCD.
It is important to ensure safe application through assessment, measurement
and application to ensure patients receive the correct management.
Patients should be informed of decisions about their care and treatment.
10.1 Indications for AES

Indication for use of AES - All patients admitted to hospital are eligible for anti-
embolism stockings as part of their thromboprophylaxis based on an up to
date VTE risk assessment.
Surgical patients: All surgical patients with a risk of VTE (unless anti-embolism
stockings contraindicated).
Medical patients: Medical patients with a risk of VTE and for whom
anticoagulation therapy (chemical thromboprophylaxis) is contraindicated
(unless anti-embolism stockings are also contraindicated).
Obstetric patients: All obstetric patients with a risk of VTE (unless anti-
embolism stockings are contraindicated).
Patients admitted to hospital with a diagnosis of, or suspected
diagnosis of, deep vein thrombosis or pulmonary embolism should
follow the Policy and Procedure for Diagnosis and Management of VTE
and not this prophylactic plan.
10.2 Contraindications for AES
Acute stroke patients
Patients with suspected or confirmed peripheral arterial disease
Patients who are receiving high dose vasopressor inotropes
Patients with severe dermatitis
Patients with very fragile ‘tissue paper’ skin
Patients who have recently undergone skin grafting
Patients with an allergy to the stocking fabric
Patients with peripheral neuropathy
Patients with cardiac failure
Patients with massive leg oedema
Patients who have an unusual leg size or shape or deformity preventing
correct fit
Venous ulcers or wounds
10.3 Caution for use of AES
Use caution in patients with sensory impairment e.g. patients undergoing
mechanical ventilation, sedated patients or those with an epidural – check
skin integrity twice daily.
Use caution in patients with diabetes mellitus and assess for sensory
impairment.
10.4 Pre requisites for practitioners
Registered Nurse / Midwife / Doctor with appropriate training and experience.
Clinical Support Workers (CSW) with appropriate training and experience.
Student nurse / midwife under the supervision of a registered nurse / midwife.
Use in accordance with manufacturer’s guidelines.
Please see Appendix 9 for standard operating procedure (SOP) for the use of
AES. Please see Appendix 10 for care plan for a patient with AES.
11.0 Intermittent pneumatic compression devices (IPCD)

Intermittent pneumatic compression devices (IPCD) work by augmenting
venous blood flow velocity, thus reducing venous stasis and by enhancing
early fibrinolytic activity to reduce the risk of clot formation.
The garments, which can be either leg sleeves or foot cuffs, compress the
limbs to enhance venous blood movement. After the compression and then
release, the controller waits for a period of time before the next compression
is initiated.
Correctly applied, in the absence of contra-indications, intermittent pneumatic
compression devices are a safe and non-invasive therapy for reducing the
incidence of venous thromboembolism by the promotion of venous blood flow.
Intermittent pneumatic compression devices are useful as a therapy on their
own in VTE prevention for both medical and surgical patients where there is
an identified risk of VTE together with a risk of bleeding. They can be also
used as an adjunct to low molecular weight heparin in patients who have been
clinically assessed as having a risk of venous thromboembolism. IPCD can
be used in conjunction with AES.
It is important to ensure safe application through assessment, measurement
and application to ensure patients receive the correct management.
Patients should be informed of decisions about their care and treatment and
their consent obtained prior to use wherever possible. This should be
documented in the healthcare record
11.1 Indications for use of IPCD
Indication for use of IPCD - All patients admitted to hospital are indicated for
(and eligible for) intermittent pneumatic compression devices as part of their
thromboprophylaxis based on an up to date VTE risk assessment.
11.1.1 Surgical patients
IPCD are used widely within the operating department for surgical patients
with a risk of VTE who will be on the operating table for 30 minutes or more
(unless IPCD contraindicated), either alone or in conjunction with chemical
thromboprophylaxis and/or another form of mechanical thromboprophylaxis,
for example anti-embolism stockings.
IPCD are also used in the immediate post-operative period (unless IPCD
contraindicated), particularly in orthopaedic hip and knee joint replacements,
gynaecology surgery and obstetric caesarean section surgery, either alone or
in conjunction with chemical thromboprophylaxis and/or another form of
mechanical thromboprophylaxis, for example anti-embolism stockings.
11.1.2 General medical patients
IPCD are indicated for medical patients with a risk of VTE, where both
anticoagulation and anti-embolism stockings are contraindicated (unless IPCD
are also contraindicated).
11.1.3 Acute stroke medical patients
Please refer to earlier section on acute stroke patients (7.4).
Patients admitted to hospital with a diagnosis of, or suspected
diagnosis of, deep vein thrombosis or pulmonary embolism should
follow the Policy and Procedure for the Diagnosis and Management of

VTE. IPCD should not be applied until resolution of the clot has been
confirmed with diagnostic testing. The time frame for this is individual
to each patient.
11.2 Contraindications for use of IPCD
Patients with an acute or suspected DVT or PE (see above)
Patients with suspected or confirmed peripheral arterial disease
Patients with severe dermatitis
Patients with a leg wound
Patients with very fragile ‘tissue paper’ skin
Patients who have recently undergone skin grafting
Patients with an allergy to the fabric of the sleeve
Patients with peripheral neuropathy
Patients with massive oedema of the legs or pulmonary oedema from
congestive heart failure
Patients who have an unusual leg size or shape or deformity preventing
correct fit
This is not an exhaustive list.
11.3 Cautions for the use of IPCD
Use caution in patients with sensory impairment e.g. patients undergoing
mechanical ventilation, sedated patients or those with an epidural – check
skin integrity twice daily.
Use caution in patients with diabetes mellitus and assess for sensory
impairment.
Use caution in patients who are receiving high dose vasopressor inotropes.
11.4 Pre-requisites for practitioners
Registered nurse / midwife / doctor with appropriate training and
experienced
Clinical Support Workers (CSW) with appropriate training and experience.
Student nurse / midwife under the supervision of a registered nurse /
midwife.
Use in accordance with manufacturer’s guidelines
See Appendix 14 (IPCD competency).
Please see Appendix 12 for standard operating procedure (SOP) for the use
of IPCD. Please see Appendix 13 for care plan for a patient with IPCD.
12.0 Monitoring and audit
Completion of VTE risk assessments will be entered onto the Patient
Administration System and data is extracted and available daily.
Retrospective periods can also be viewed. Data is submitted via Unify as
required by the Department of Health.
Audits of VTE risk assessment and thromboprophylaxis will be
undertaken and data submitted to local commissioners if requested.
Frequency of audits will be determined by the Thrombosis Committee.
Auditors will vary, audits may be delegated to directorates to complete or
completed centrally by the VTE Lead Nurse.
Other formal audits of VTE risk assessment and thromboprophylaxis
may be undertaken with support from the audit department.
Random spot check audits will be carried out ad hoc to ensure that VTE
risk assessment, prescription of chemical thromboprophylaxis and data
capture processes are being followed. The results of which will be
shared appropriately.
Information from formal audits and the data capture will be reported at
ward level and to the Directorates, Thrombosis committee and trust
executives.
The VTE Prevention policy and procedure document will be reviewed
every three years.
All hospital acquired VTE incidents should be reported either verbally to the
VTE Lead Nurse or by using the e-reporting system. Investigation and root
cause analysis (RCA) will be undertaken on all hospital acquired VTE
incidents. Actions arising from VTE RCA and / or VTE serious incident
investigations will be added to the VTE trust wide action plan. 12.1
APPENDIX ONE
Process requirements
1.0 Implementation and awareness
Once ratified the document lead or author will submit this
policy/procedural document to the Clinical Governance Assistant who
will activate it on the Trust approved document management database
on the intranet, under ‘Trust policies, procedures and leaflets’.
A monthly publications table is produced by the Clinical Governance
Assistant which is published on the Trust intranet under “Policies”;
notification of the posting is included on the intranet “News Feed” and
in the Chief Executive’s newsletter.
On reading of the news feed notification all managers should ensure
that their staff members are aware of the new publications.
The policy will be disseminated to all clinical staff and ward areas.
The policy/procedure will be launched via cascade through directorate
nurse managers.
2.0 Review
This policy/procedure will be reviewed once every three years, as a minimum;
however, if changes in legislation or Trust practice require, amendments will
be made following Trust consultation and approval procedures.
3.0 Archiving
The Trust approved document management database on the intranet retains
all superseded files in an archive directory (obsolete register) in order to
maintain document history.
APPENDIX TWO
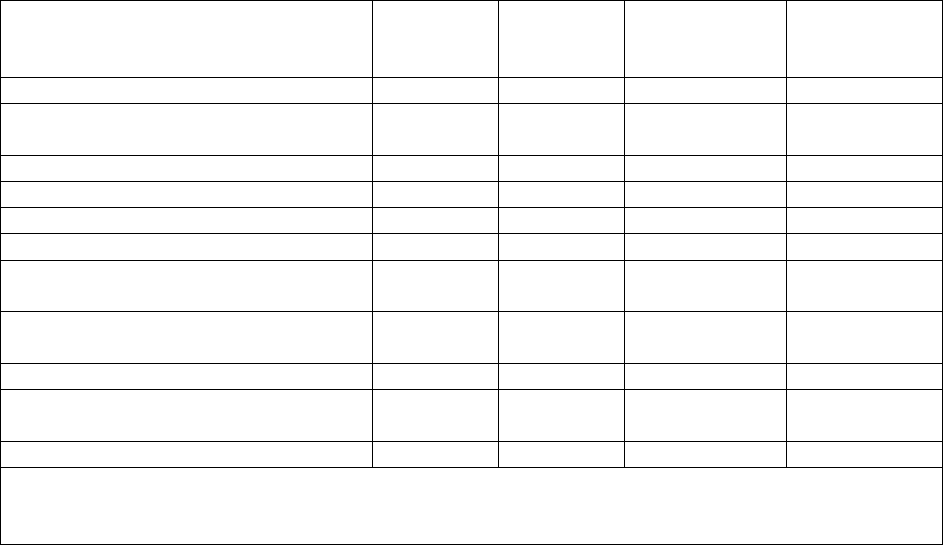
CONSULTATION ON: Revision of the Venous Thromboembolism Prevention Policy
and Procedure combining with the Anti-embolism Stocking policy and procedure and
the Intermittent Pneumatic Compression Device policy and procedure
Consultation process – Use this form to ensure your consultation has been
adequate for the purpose.
Please return comments to: VTE Lead Nurse
By date: 9
th
March 2016
The Thrombosis Committee has representation from all directorates except
paediatrics. The revision and merger of these three policies was managed
under the Thrombosis Committee. Separate sections were discussed with the
relevant departments e.g. surgical section discussed at a surgical clinical
governance meeting, stroke section discussed with all stroke consultants. The
AES and IPCD sections were discussed with the relevant company
representatives.
Job title:
Date sent
Date reply
received
Modification
suggested?
Y/N
Modification
made?
Y/N
Chief Nurse
Deputy Director of Nursing (Deputy
Chief Nurse)
ADNS
Matrons
Ward / Theatre Managers
Head of Quality and Governance
Senior Nurses Practice
Development
Thrombosis Committee
01/03/16
Discussed
at meeting
N
Medical Director
Standards Committee (no longer
exists)
Patient Safety and Risk Manager
The role of those staff being consulted upon as above is to ensure that they have shared the
policy for comments with all staff within their sphere of responsibility who would be able to
contribute to the development of the policy.
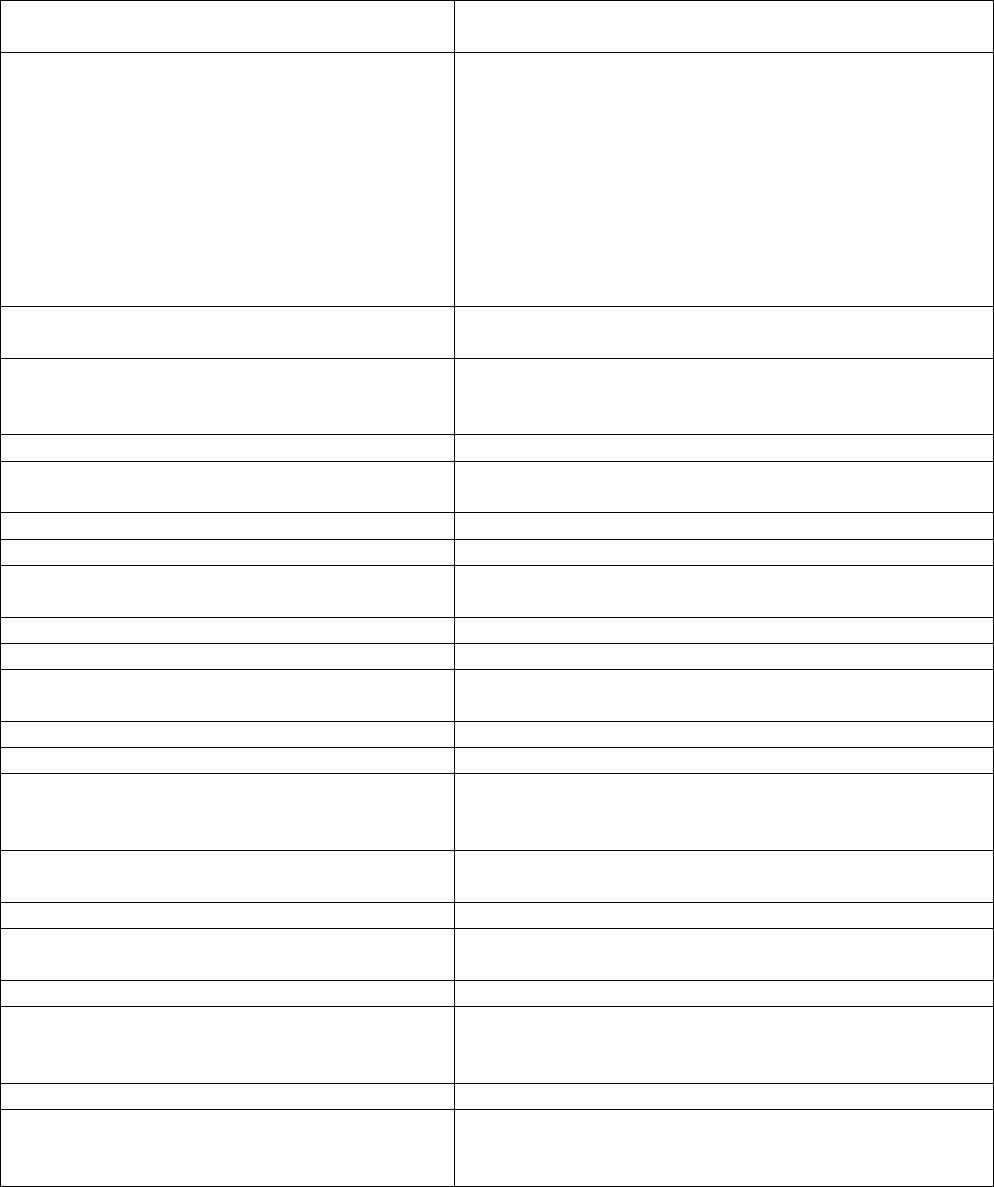
APPENDIX THREE
Equality Impact Assessment
In line with race, disability and gender equalities legislation, public bodies like MTW are
required to assess and consult on how their policies and practices affect different
groups, and to monitor any possible negative impact on equality. The completion of the
following Equality Impact Assessment grid is therefore mandatory and should be
undertaken as part of the policy development and approval process.
Title of Policy or Practice
Venous Thromboembolism Prevention Policy and
Procedure
What are the aims of the policy or
practice?
To ensure that all inpatients are appropriately and
regularly assessed for risk of venous
thromboembolism (VTE) and treated according to
that risk throughout their stay at MTW. Patients
admitted to the Trust should have the opportunity to
make informed decisions about their care and
treatment, in partnership with their health care
professionals and the Trust will offer best practice
advice on reducing the risk of VTE in patients
admitted to hospital.
Identify the data and research used to
assist the analysis and assessment
NICE Guidance, VTE Exemplar Centres
Analyse and assess the likely impact on
equality or potential discrimination with
each of the following groups.
Is there an adverse impact or potential
discrimination (yes/no).
If yes give details.
Males or Females
N
People of different ages
Policy does not apply to those under the age of 18 -
based on NICE guidance
People of different ethnic groups
N
People of different religious beliefs
N
People who do not speak English as a first
language
N interpreting services available; leaflets to be made
available in different languages
People who have a physical disability
N
People who have a mental disability
N
Women who are pregnant or on maternity
leave
N
Single parent families
N
People with different sexual orientations
N
People with different work patterns (part
time, full time, job share, short term
contractors, employed, unemployed)
N
People in deprived areas and people from
different socio-economic groups
N
Asylum seekers and refugees
N
Prisoners and people confined to closed
institutions, community offenders
N
Carers
N
If you identified potential discrimination
is it minimal and justifiable and therefore
does not require a stage 2 assessment?
N
When will you monitor & review the EIA?
Alongside this policy/procedure when it is reviewed.
Where do you plan to publish the results
of your Equality Impact Assessment?
As Appendix 3 of this policy/procedure on the Trust
document management database (on Intranet, under
‘Trust policies, procedures and leaflets’).
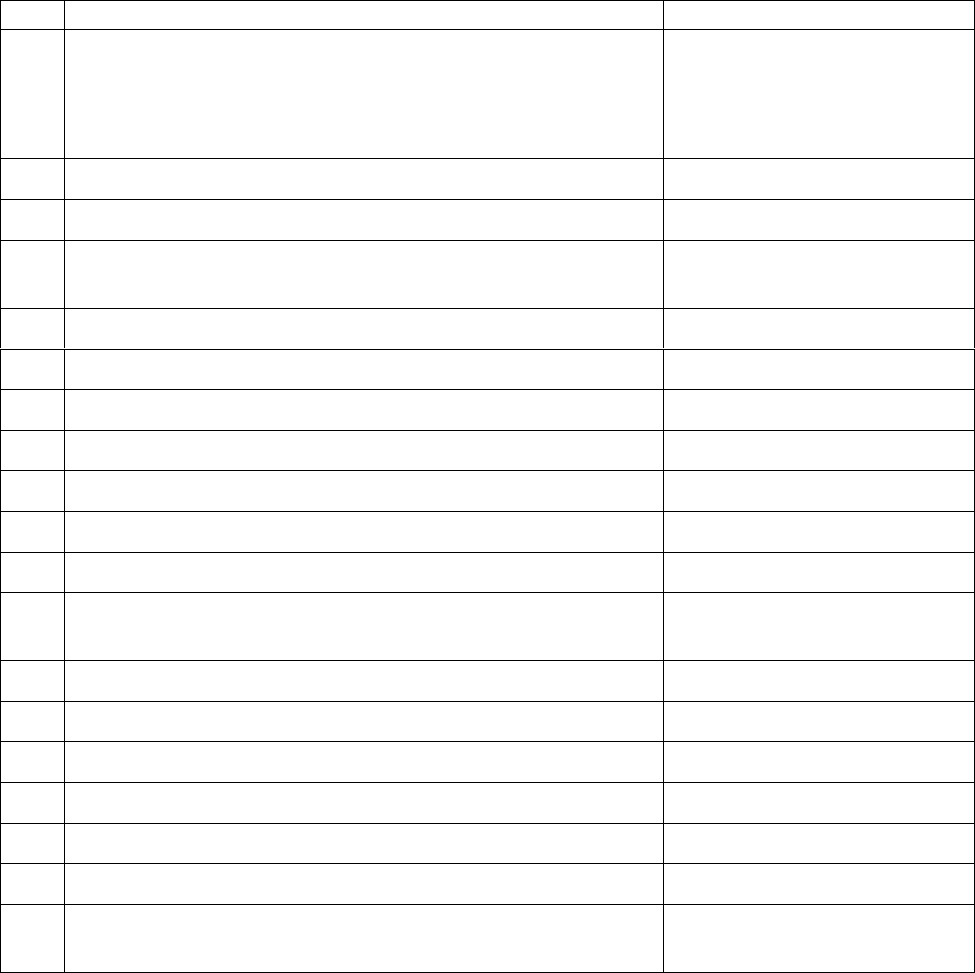
FURTHER APPENDICES
The following appendices are published as related links to the main policy
/procedure on the Trust approved document management database on the
intranet (Trust policies, procedures and leaflets):
No.
Title
Unique ID
4
VTE risk assessment form
This document is a proof of printed stationary which
should only be accesses in hard copy from clinical
areas.
RWF-OWP-APP220
5
Thromboprophylaxis summary
RWF-NUR-NUR-GUI-1
6
Training matrix
RWF-OWP-APP226
7
Guideline for thromboprophylaxis and patients with a
bleed or suspected bleed
RWF-OPG-ES7
8
Lower limb immobilisation and LMWH pathway
RWF-OPPM-ES8
9
Standard operating procedure for the use of AES
RWF-NUR-NUR-GUI-2
10
Care plan for a patient with AES
RWF-OPF-ES-C-SM2
11
AES competency
RWF-OPF-ES-C-SM1
12
Standard operating procedure for the use of IPCD
RWF-NUR-NUR-GUI-3
13
Care plan for a patient with IPCD
RWF-OPF-ES-C-SM4
14
IPCD competency
RWF-OPF-ES-C-SM3
15
Protocol for the use of IPCD for patients with
haemorrhagic stroke
RWF-OPPM-ES6
16
IPCD sizing chart for leg sleeves
RWF-OPG-ES5
17
IPCD with leg sleeves ‘Quick Guide’
RWF-OPPM-ES4
18
IPCD with leg sleeves ‘User Manual’
RWF-OPPM-ES3
19
IPCD sizing chart for foot cuffs
RWF-OPG-ES4
20
IPCD with foot cuffs ‘User Manual’
RWF-OPPM-ES5
21
IPCD trouble shooting guide
RWF-OPG-ES6
22
Patient leaflet: Kendall / SCD Express - Sequential
Compression System (Covidien)
RWF-OPLF-PES115