INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL
REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
ICH HARMONISED TRIPARTITE GUIDELINE
PHARMACEUTICAL QUALITY SYSTEM
Q10
Current Step 4 version
dated 4 June 2008
This Guideline has been developed by the appropriate ICH Expert Working Group
and has been subject to consultation by the regulatory parties, in accordance with the
ICH Process. At Step 4 of the Process the final draft is recommended for adoption to
the regulatory bodies of the European Union, Japan and USA.
Pharmaceutical Quality System
Q10
Document History
Code
History
Date
Q10
Approval by the Steering Committee under Step 2 and release
for public consultation.
9 May
2007
Current Step 4 version
Q10
Approval by the Steering Committee under Step 4 and
recommendation for adoption to the three ICH regulatory
bodies.
4 June
2008
i
PHARMACEUTICAL QUALITY SYSTEM
ICH Harmonised Tripartite Guideline
Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting
on 4 June 2008, this guideline is recommended for
adoption to the three regulatory parties to ICH
TABLE OF CONTENTS
1. PHARMACEUTICAL QUALITY SYSTEM ........................................................ 1
1.1 Introduction ............................................................................................................. 1
1.2 Scope ........................................................................................................................ 1
1.3 Relationship of ICH Q10 to Regional GMP Requirements, ISO Standards and
ICH Q7 ..................................................................................................................... 2
1.4 Relationship of ICH Q10 to Regulatory Approaches .............................................. 2
1.5 ICH Q10 Objectives ................................................................................................. 2
1.5.1 Achieve Product Realisation ........................................................................ 2
1.5.2 Establish and Maintain a State of Control ................................................. 3
1.5.3 Facilitate Continual Improvement ............................................................... 3
1.6 Enablers: Knowledge Management and Quality Risk Management ..................... 3
1.6.1 Knowledge Management .............................................................................. 3
1.6.2 Quality Risk Management ........................................................................... 3
1.7 Design and Content Considerations ....................................................................... 3
1.8 Quality Manual ....................................................................................................... 4
2. MANAGEMENT RESPONSIBILITY .................................................................. 4
2.1 Management Commitment...................................................................................... 4
2.2 Quality Policy .......................................................................................................... 5
2.3 Quality Planning ..................................................................................................... 5
2.4 Resource Management ............................................................................................ 5
2.5 Internal Communication ......................................................................................... 6
2.6 Management Review ............................................................................................... 6
2.7 Management of Outsourced Activities and Purchased Materials .......................... 6
2.8 Management of Change in Product Ownership ...................................................... 6

Pharmaceutical Quality System
ii
3. CONTINUAL IMPROVEMENT OF PROCESS PERFORMANCE AND
PRODUCT QUALITY ........................................................................................... 7
3.1 Lifecycle Stage Goals ............................................................................................... 7
3.1.1 Pharmaceutical Development....................................................................... 7
3.1.2 Technology Transfer ..................................................................................... 7
3.1.3 Commercial Manufacturing ......................................................................... 7
3.1.4 Product Discontinuation .............................................................................. 7
3.2 Pharmaceutical Quality System Elements ............................................................. 7
3.2.1 Process Performance and Product Quality Monitoring System .................. 8
3.2.2 Corrective Action and Preventive Action (CAPA) System ............................ 9
3.2.3 Change Management System ..................................................................... 10
3.2.4 Management Review of Process Performance and Product Quality ......... 11
4. CONTINUAL IMPROVEMENT OF THE PHARMACEUTICAL QUALITY
SYSTEM ................................................................................................................ 11
4.1 Management Review of the Pharmaceutical Quality System .............................. 11
4.2 Monitoring of Internal and External Factors Impacting the Pharmaceutical
Quality System ...................................................................................................... 12
4.3 Outcomes of Management Review and Monitoring .............................................. 12
5. GLOSSARY .......................................................................................................... 13
Annex 1:Potential Opportunities to Enhance Science and Risk Based Regulatory
Approaches ........................................................................................................... 16
Annex 2:Diagram of the ICH Q10 Pharmaceutical Quality System Model ...................... 17
1
PHARMACEUTICAL QUALITY SYSTEM
1. PHARMACEUTICAL QUALITY SYSTEM
1.1 Introduction
This document establishes a new ICH tripartite guideline describing a model for an
effective quality management system for the pharmaceutical industry, referred to as
the Pharmaceutical Quality System. Throughout this guideline, the term
“pharmaceutical quality system” refers to the ICH Q10 model.
ICH Q10 describes one comprehensive model for an effective pharmaceutical quality
system that is based on International Standards Organisation (ISO) quality concepts,
includes applicable Good Manufacturing Practice (GMP) regulations and complements
ICH Q8 “Pharmaceutical Development” and ICH Q9 “Quality Risk Management”. ICH
Q10 is a model for a pharmaceutical quality system that can be implemented
throughout the different stages of a product lifecycle. Much of the content of ICH Q10
applicable to manufacturing sites is currently specified by regional GMP
requirements. ICH Q10 is not intended to create any new expectations beyond current
regulatory requirements. Consequently, the content of ICH Q10 that is additional to
current regional GMP requirements is optional.
ICH Q10 demonstrates industry and regulatory authorities’ support of an effective
pharmaceutical quality system to enhance the quality and availability of medicines
around the world in the interest of public health. Implementation of ICH Q10
throughout the product lifecycle should facilitate innovation and continual
improvement and strengthen the link between pharmaceutical development and
manufacturing activities.
1.2 Scope
This guideline applies to the systems supporting the development and manufacture of
pharmaceutical drug substances (i.e., API) and drug products, including biotechnology
and biological products, throughout the product lifecycle.
The elements of ICH Q10 should be applied in a manner that is appropriate and
proportionate to each of the product lifecycle stages, recognising the differences
among, and the different goals of each stage (see Section 3).
For the purposes of this guideline, the product lifecycle includes the following
technical activities for new and existing products:
Pharmaceutical Development:
o Drug substance development;
o Formulation development (including container/closure system);
o Manufacture of investigational products;
o Delivery system development (where relevant);
o Manufacturing process development and scale-up;
o Analytical method development.
Technology Transfer:
o New product transfers during Development through Manufacturing;
o Transfers within or between manufacturing and testing sites for
marketed products.

Pharmaceutical Quality System
2
Commercial Manufacturing:
o Acquisition and control of materials;
o Provision of facilities, utilities, and equipment;
o Production (including packaging and labelling);
o Quality control and assurance;
o Release;
o Storage;
o Distribution (excluding wholesaler activities).
Product Discontinuation:
o Retention of documentation;
o Sample retention;
o Continued product assessment and reporting.
1.3 Relationship of ICH Q10 to Regional GMP Requirements, ISO
Standards and ICH Q7
Regional GMP requirements, the ICH Q7 Guideline, “Good Manufacturing Practice
Guide for Active Pharmaceutical Ingredients”, and ISO quality management system
guidelines form the foundation for ICH Q10. To meet the objectives described below,
ICH Q10 augments GMPs by describing specific quality system elements and
management responsibilities. ICH Q10 provides a harmonised model for a
pharmaceutical quality system throughout the lifecycle of a product and is intended to
be used together with regional GMP requirements.
The regional GMPs do not explicitly address all stages of the product lifecycle (e.g.,
Development). The quality system elements and management responsibilities
described in this guideline are intended to encourage the use of science and risk based
approaches at each lifecycle stage, thereby promoting continual improvement across
the entire product lifecycle.
1.4 Relationship of ICH Q10 to Regulatory Approaches
Regulatory approaches for a specific product or manufacturing facility should be
commensurate with the level of product and process understanding, the results of
quality risk management, and the effectiveness of the pharmaceutical quality system.
When implemented, the effectiveness of the pharmaceutical quality system can
normally be evaluated during a regulatory inspection at the manufacturing site.
Potential opportunities to enhance science and risk based regulatory approaches are
identified in Annex 1. Regulatory processes will be determined by region.
1.5 ICH Q10 Objectives
Implementation of the Q10 model should result in achievement of three main
objectives which complement or enhance regional GMP requirements.
1.5.1 Achieve Product Realisation
To establish, implement and maintain a system that allows the delivery of products
with the quality attributes appropriate to meet the needs of patients, health care
professionals, regulatory authorities (including compliance with approved regulatory
filings) and other internal and external customers.

Pharmaceutical Quality System
3
1.5.2 Establish and Maintain a State of Control
To develop and use effective monitoring and control systems for process performance
and product quality, thereby providing assurance of continued suitability and
capability of processes. Quality risk management can be useful in identifying the
monitoring and control systems.
1.5.3 Facilitate Continual Improvement
To identify and implement appropriate product quality improvements, process
improvements, variability reduction, innovations and pharmaceutical quality system
enhancements, thereby increasing the ability to fulfil quality needs consistently.
Quality risk management can be useful for identifying and prioritising areas for
continual improvement.
1.6 Enablers: Knowledge Management and Quality Risk Management
Use of knowledge management and quality risk management will enable a company to
implement ICH Q10 effectively and successfully. These enablers will facilitate
achievement of the objectives described in Section 1.5 above by providing the means
for science and risk based decisions related to product quality.
1.6.1 Knowledge Management
Product and process knowledge should be managed from development through the
commercial life of the product up to and including product discontinuation. For
example, development activities using scientific approaches provide knowledge for
product and process understanding. Knowledge management is a systematic approach
to acquiring, analysing, storing and disseminating information related to products,
manufacturing processes and components. Sources of knowledge include, but are not
limited to prior knowledge (public domain or internally documented); pharmaceutical
development studies; technology transfer activities; process validation studies over
the product lifecycle; manufacturing experience; innovation; continual improvement;
and change management activities.
1.6.2 Quality Risk Management
Quality risk management is integral to an effective pharmaceutical quality system. It
can provide a proactive approach to identifying, scientifically evaluating and
controlling potential risks to quality. It facilitates continual improvement of process
performance and product quality throughout the product lifecycle. ICH Q9 provides
principles and examples of tools for quality risk management that can be applied to
different aspects of pharmaceutical quality.
1.7 Design and Content Considerations
(a) The design, organisation and documentation of the pharmaceutical quality
system should be well structured and clear to facilitate common understanding
and consistent application.
(b) The elements of ICH Q10 should be applied in a manner that is appropriate
and proportionate to each of the product lifecycle stages, recognising the
different goals and knowledge available for each stage.
(c) The size and complexity of the company’s activities should be taken into
consideration when developing a new pharmaceutical quality system or

Pharmaceutical Quality System
4
modifying an existing one. The design of the pharmaceutical quality system
should incorporate appropriate risk management principles. While some
aspects of the pharmaceutical quality system can be company-wide and others
site-specific, the effectiveness of the pharmaceutical quality system is normally
demonstrated at the site level.
(d) The pharmaceutical quality system should include appropriate processes,
resources and responsibilities to provide assurance of the quality of outsourced
activities and purchased materials as described in Section 2.7.
(e) Management responsibilities, as described in Section 2, should be identified
within the pharmaceutical quality system.
(f) The pharmaceutical quality system should include the following elements, as
described in Section 3: process performance and product quality monitoring,
corrective and preventive action, change management and management review.
(g) Performance indicators, as described in Section 4, should be identified and
used to monitor the effectiveness of processes within the pharmaceutical
quality system.
1.8 Quality Manual
A Quality Manual or equivalent documentation approach should be established and
should contain the description of the pharmaceutical quality system. The description
should include:
(a) The quality policy (see Section 2);
(b) The scope of the pharmaceutical quality system;
(c) Identification of the pharmaceutical quality system processes, as well as their
sequences, linkages and interdependencies. Process maps and flow charts can
be useful tools to facilitate depicting pharmaceutical quality system processes
in a visual manner;
(d) Management responsibilities within the pharmaceutical quality system (see
Section 2).
2. MANAGEMENT RESPONSIBILITY
Leadership is essential to establish and maintain a company-wide commitment to
quality and for the performance of the pharmaceutical quality system.
2.1 Management Commitment
(a) Senior management has the ultimate responsibility to ensure an effective
pharmaceutical quality system is in place to achieve the quality objectives, and
that roles, responsibilities, and authorities are defined, communicated and
implemented throughout the company.
(b) Management should:
(1) Participate in the design, implementation, monitoring and maintenance
of an effective pharmaceutical quality system;
(2) Demonstrate strong and visible support for the pharmaceutical quality

Pharmaceutical Quality System
5
system and ensure its implementation throughout their organisation;
(3) Ensure a timely and effective communication and escalation process
exists to raise quality issues to the appropriate levels of management;
(4) Define individual and collective roles, responsibilities, authorities and
inter-relationships of all organisational units related to the
pharmaceutical quality system. Ensure these interactions are
communicated and understood at all levels of the organisation. An
independent quality unit/structure with authority to fulfil certain
pharmaceutical quality system responsibilities is required by regional
regulations;
(5) Conduct management reviews of process performance and product
quality and of the pharmaceutical quality system;
(6) Advocate continual improvement;
(7) Commit appropriate resources.
2.2 Quality Policy
(a) Senior management should establish a quality policy that describes the overall
intentions and direction of the company related to quality.
(b) The quality policy should include an expectation to comply with applicable
regulatory requirements and should facilitate continual improvement of the
pharmaceutical quality system.
(c) The quality policy should be communicated to and understood by personnel at
all levels in the company.
(d) The quality policy should be reviewed periodically for continuing effectiveness.
2.3 Quality Planning
(a) Senior management should ensure the quality objectives needed to implement
the quality policy are defined and communicated.
(b) Quality objectives should be supported by all relevant levels of the company.
(c) Quality objectives should align with the company’s strategies and be consistent
with the quality policy.
(d) Management should provide the appropriate resources and training to achieve
the quality objectives.
(e) Performance indicators that measure progress against quality objectives
should be established, monitored, communicated regularly and acted upon as
appropriate as described in Section 4.1 of this document.
2.4 Resource Management
(a) Management should determine and provide adequate and appropriate
resources (human, financial, materials, facilities and equipment) to implement
and maintain the pharmaceutical quality system and continually improve its
effectiveness.
(b) Management should ensure that resources are appropriately applied to a

Pharmaceutical Quality System
6
specific product, process or site.
2.5 Internal Communication
(a) Management should ensure appropriate communication processes are
established and implemented within the organisation.
(b) Communications processes should ensure the flow of appropriate information
between all levels of the company.
(c) Communication processes should ensure the appropriate and timely escalation
of certain product quality and pharmaceutical quality system issues.
2.6 Management Review
(a) Senior management should be responsible for pharmaceutical quality system
governance through management review to ensure its continuing suitability
and effectiveness.
(b) Management should assess the conclusions of periodic reviews of process
performance and product quality and of the pharmaceutical quality system, as
described in Sections 3 and 4.
2.7 Management of Outsourced Activities and Purchased Materials
The pharmaceutical quality system, including the management responsibilities
described in this section, extends to the control and review of any outsourced activities
and quality of purchased materials. The pharmaceutical company is ultimately
responsible to ensure processes are in place to assure the control of outsourced
activities and quality of purchased materials. These processes should incorporate
quality risk management and include:
(a) Assessing prior to outsourcing operations or selecting material suppliers, the
suitability and competence of the other party to carry out the activity or
provide the material using a defined supply chain (e.g., audits, material
evaluations, qualification);
(b) Defining the responsibilities and communication processes for quality-related
activities of the involved parties. For outsourced activities, this should be
included in a written agreement between the contract giver and contract
acceptor;
(c) Monitoring and review of the performance of the contract acceptor or the
quality of the material from the provider, and the identification and
implementation of any needed improvements;
(d) Monitoring incoming ingredients and materials to ensure they are from
approved sources using the agreed supply chain.
2.8 Management of Change in Product Ownership
When product ownership changes, (e.g., through acquisitions) management should
consider the complexity of this and ensure:
(a) The ongoing responsibilities are defined for each company involved;

Pharmaceutical Quality System
7
(b) The necessary information is transferred.
3. CONTINUAL IMPROVEMENT OF PROCESS PERFORMANCE AND
PRODUCT QUALITY
This section describes the lifecycle stage goals and the four specific pharmaceutical
quality system elements that augment regional requirements to achieve the ICH Q10
objectives, as defined in Section 1.5. It does not restate all regional GMP
requirements.
3.1 Lifecycle Stage Goals
The goals of each product lifecycle stage are described below.
3.1.1 Pharmaceutical Development
The goal of pharmaceutical development activities is to design a product and its
manufacturing process to consistently deliver the intended performance and meet the
needs of patients and healthcare professionals, and regulatory authorities and
internal customers’ requirements. Approaches to pharmaceutical development are
described in ICH Q8. The results of exploratory and clinical development studies,
while outside the scope of this guidance, are inputs to pharmaceutical development.
3.1.2 Technology Transfer
The goal of technology transfer activities is to transfer product and process knowledge
between development and manufacturing, and within or between manufacturing sites
to achieve product realisation. This knowledge forms the basis for the manufacturing
process, control strategy, process validation approach and ongoing continual
improvement.
3.1.3 Commercial Manufacturing
The goals of manufacturing activities include achieving product realisation,
establishing and maintaining a state of control and facilitating continual
improvement. The pharmaceutical quality system should assure that the desired
product quality is routinely met, suitable process performance is achieved, the set of
controls are appropriate, improvement opportunities are identified and evaluated, and
the body of knowledge is continually expanded.
3.1.4 Product Discontinuation
The goal of product discontinuation activities is to manage the terminal stage of the
product lifecycle effectively. For product discontinuation, a pre-defined approach
should be used to manage activities such as retention of documentation and samples
and continued product assessment (e.g., complaint handling and stability) and
reporting in accordance with regulatory requirements.
3.2 Pharmaceutical Quality System Elements
The elements described below might be, required in part under regional GMP
regulations. However, the Q10 model’s intent is to enhance these elements in order to
promote the lifecycle approach to product quality. These four elements are:
Process performance and product quality monitoring system;
Corrective action and preventive action (CAPA) system;
Change management system;

Pharmaceutical Quality System
8
Management review of process performance and product quality.
These elements should be applied in a manner that is appropriate and proportionate
to each of the product lifecycle stages, recognising the differences among, and the
different goals of, each stage. Throughout the product lifecycle, companies are
encouraged to evaluate opportunities for innovative approaches to improve product
quality.
Each element is followed by a table of example applications of the element to the
stages of the pharmaceutical lifecycle.
3.2.1 Process Performance and Product Quality Monitoring System
Pharmaceutical companies should plan and execute a system for the monitoring of
process performance and product quality to ensure a state of control is maintained. An
effective monitoring system provides assurance of the continued capability of
processes and controls to produce a product of desired quality and to identify areas for
continual improvement. The process performance and product quality monitoring
system should:
(a) Use quality risk management to establish the control strategy. This can
include parameters and attributes related to drug substance and drug product
materials and components, facility and equipment operating conditions, in-
process controls, finished product specifications, and the associated methods
and frequency of monitoring and control. The control strategy should facilitate
timely feedback / feedforward and appropriate corrective action and preventive
action;
(b) Provide the tools for measurement and analysis of parameters and attributes
identified in the control strategy (e.g., data management and statistical tools);
(c) Analyse parameters and attributes identified in the control strategy to verify
continued operation within a state of control;
(d) Identify sources of variation affecting process performance and product quality
for potential continual improvement activities to reduce or control variation;
(e) Include feedback on product quality from both internal and external sources,
e.g., complaints, product rejections, non-conformances, recalls, deviations,
audits and regulatory inspections and findings;
(f) Provide knowledge to enhance process understanding, enrich the design space
(where established), and enable innovative approaches to process validation.
Pharmaceutical Quality System
9
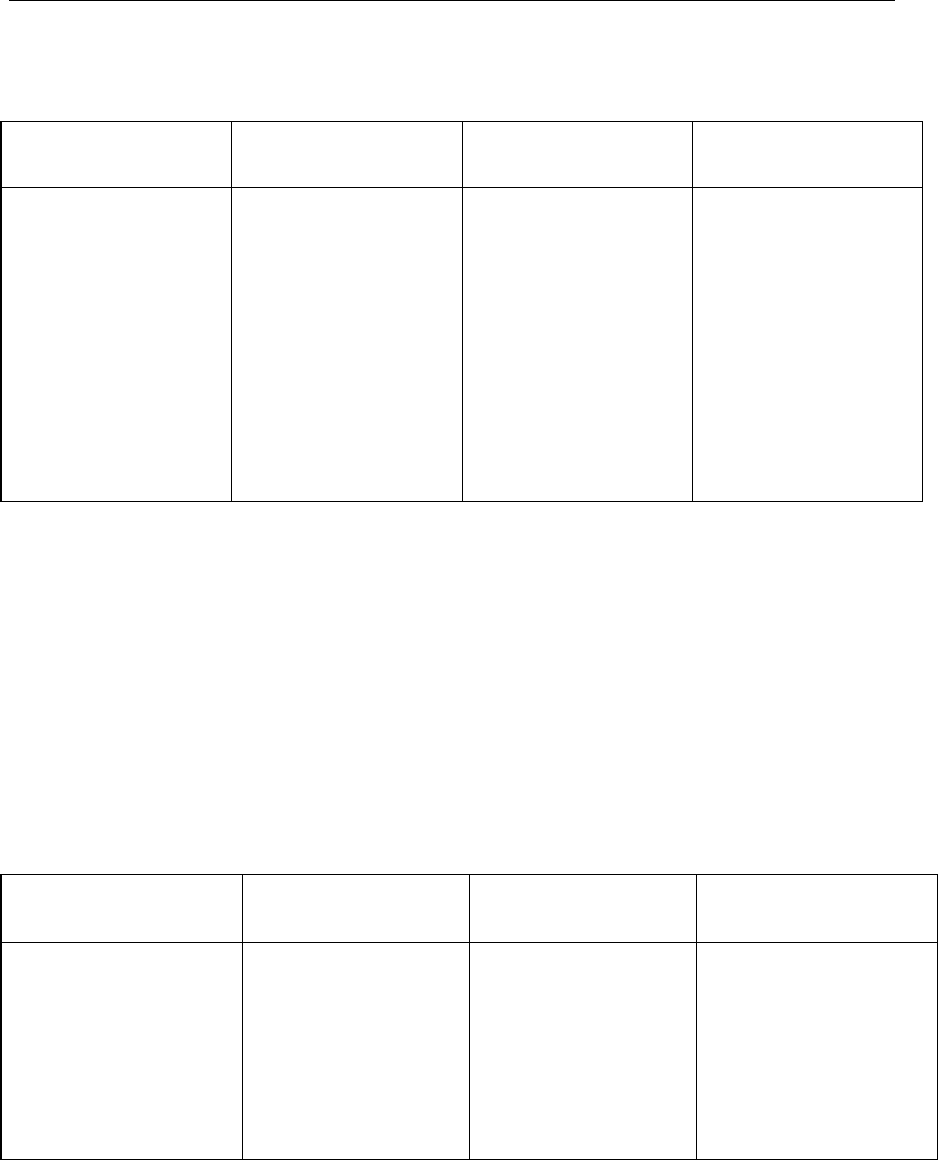
Table I: Application of Process Performance and Product Quality
Monitoring System throughout the Product Lifecycle
Pharmaceutical
Development
Technology
Transfer
Commercial
Manufacturing
Product
Discontinuation
Process and product
knowledge generated
and process and
product monitoring
conducted throughout
development can be
used to establish a
control strategy for
manufacturing.
Monitoring during
scale-up activities can
provide a preliminary
indication of process
performance and the
successful integration
into manufacturing.
Knowledge obtained
during transfer and
scale up activities can
be useful in further
developing the control
strategy.
A well-defined system
for process
performance and
product quality
monitoring should be
applied to assure
performance within a
state of control and to
identify improvement
areas.
Once manufacturing
ceases, monitoring
such as stability
testing should
continue to
completion of the
studies. Appropriate
action on marketed
product should
continue to be
executed according to
regional regulations.
3.2.2 Corrective Action and Preventive Action (CAPA) System
The pharmaceutical company should have a system for implementing corrective
actions and preventive actions resulting from the investigation of complaints, product
rejections, non-conformances, recalls, deviations, audits, regulatory inspections and
findings, and trends from process performance and product quality monitoring. A
structured approach to the investigation process should be used with the objective of
determining the root cause. The level of effort, formality, and documentation of the
investigation should be commensurate with the level of risk, in line with ICH Q9.
CAPA methodology should result in product and process improvements and enhanced
product and process understanding.
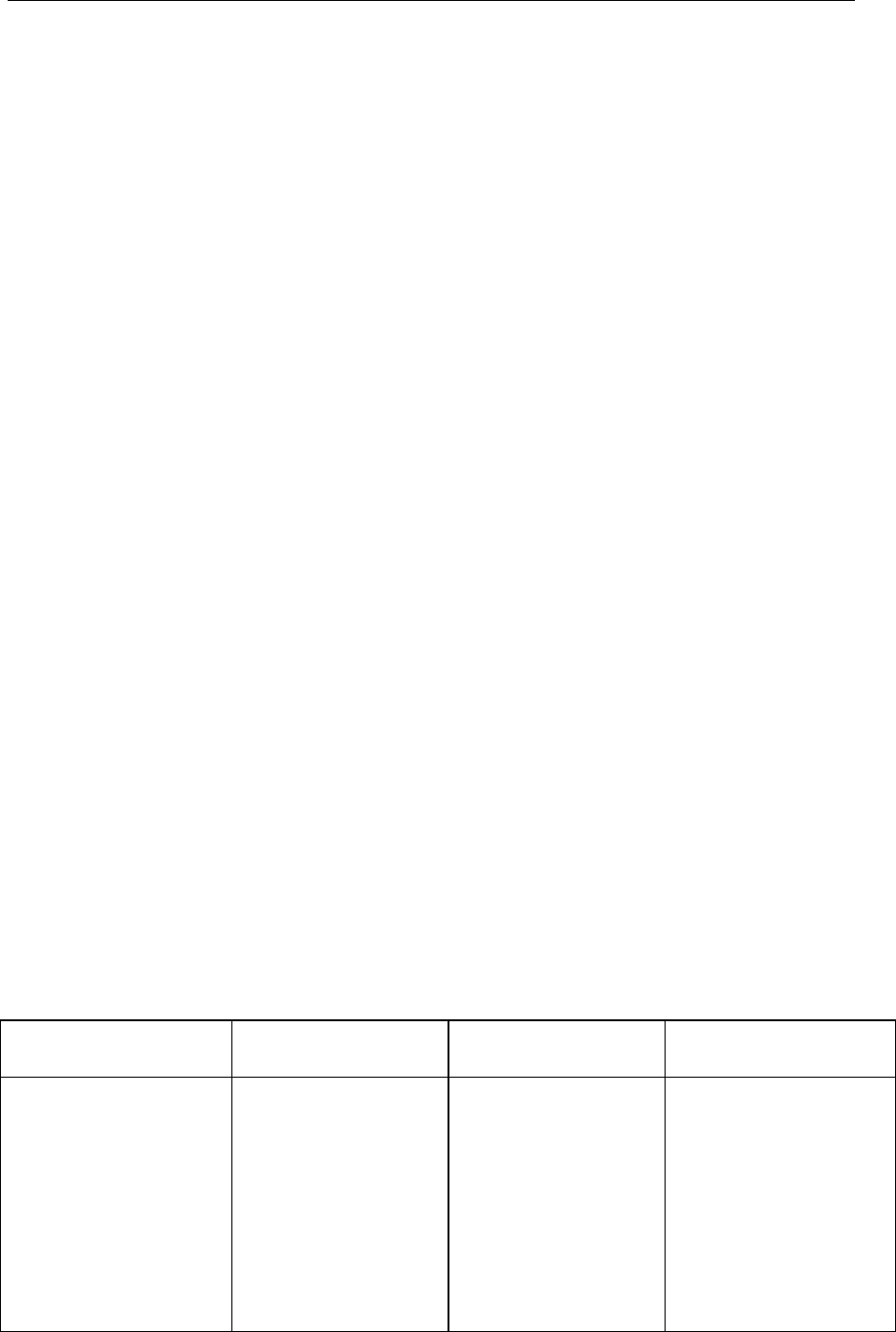
Table II: Application of Corrective Action and Preventive Action System
throughout the Product Lifecycle
Pharmaceutical
Development
Technology
Transfer
Commercial
Manufacturing
Product
Discontinuation
Product or process
variability is explored.
CAPA methodology is
useful where corrective
actions and preventive
actions are
incorporated into the
iterative design and
development process.
CAPA can be used as
an effective system
for feedback,
feedforward and
continual
improvement.
CAPA should be used
and the effectiveness
of the actions should
be evaluated.
CAPA should continue
after the product is
discontinued. The
impact on product
remaining on the
market should be
considered as well as
other products which
might be impacted.
Pharmaceutical Quality System
10
3.2.3 Change Management System
Innovation, continual improvement, the outputs of process performance and product
quality monitoring and CAPA drive change. In order to evaluate, approve and
implement these changes properly, a company should have an effective change
management system. There is generally a difference in formality of change
management processes prior to the initial regulatory submission and after
submission, where changes to the regulatory filing might be required under regional
requirements.
The change management system ensures continual improvement is undertaken in a
timely and effective manner. It should provide a high degree of assurance there are no
unintended consequences of the change.
The change management system should include the following, as appropriate for the
stage of the lifecycle:
(a) Quality risk management should be utilised to evaluate proposed changes. The
level of effort and formality of the evaluation should be commensurate with the
level of risk;
(b) Proposed changes should be evaluated relative to the marketing authorisation,
including design space, where established, and/or current product and process
understanding. There should be an assessment to determine whether a change
to the regulatory filing is required under regional requirements. As stated in
ICH Q8, working within the design space is not considered a change (from a
regulatory filing perspective). However, from a pharmaceutical quality system
standpoint, all changes should be evaluated by a company’s change
management system;
(c) Proposed changes should be evaluated by expert teams contributing the
appropriate expertise and knowledge from relevant areas (e.g., Pharmaceutical
Development, Manufacturing, Quality, Regulatory Affairs and Medical), to
ensure the change is technically justified. Prospective evaluation criteria for a
proposed change should be set;
(d) After implementation, an evaluation of the change should be undertaken to
confirm the change objectives were achieved and that there was no deleterious
impact on product quality.
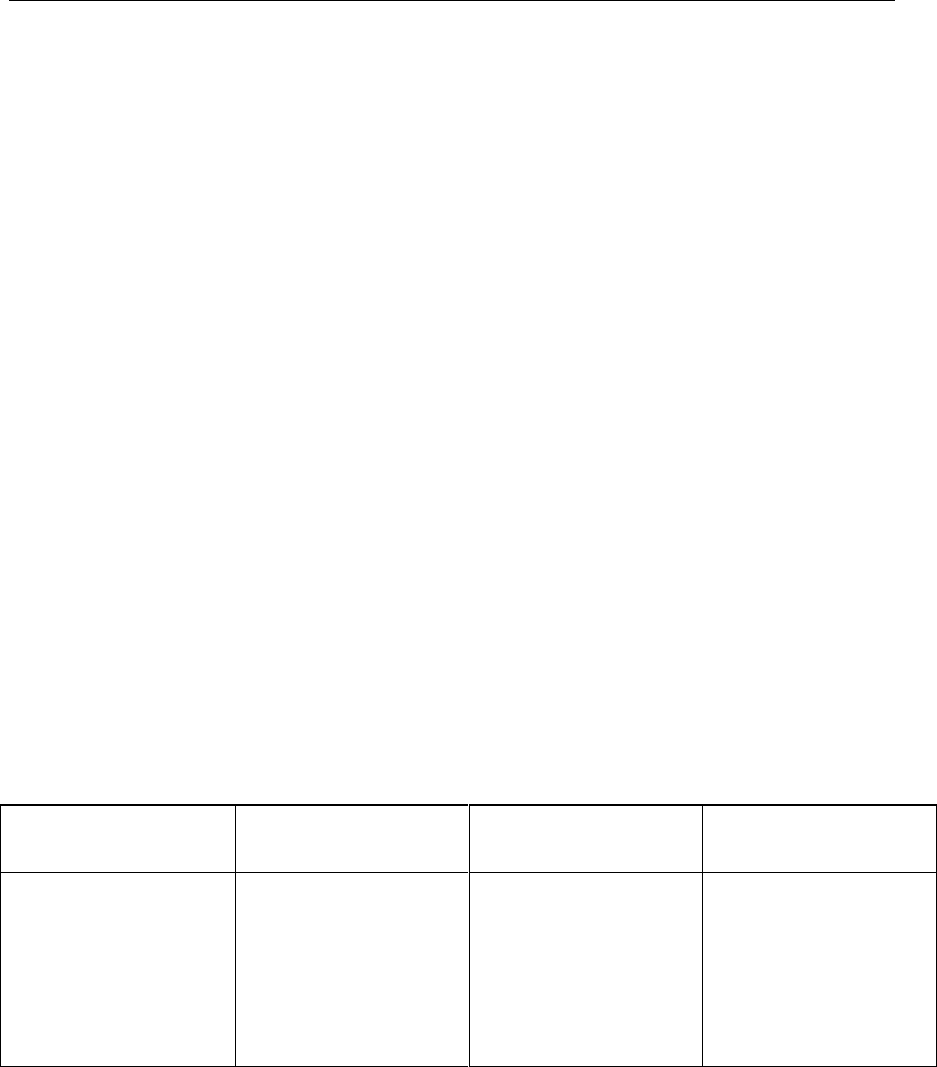
Table III: Application of Change Management System throughout the
Product Lifecycle
Pharmaceutical
Development
Technology
Transfer
Commercial
Manufacturing
Product
Discontinuation
Change is an inherent
part of the development
process and should be
documented; the
formality of the change
management process
should be consistent
with the stage of
pharmaceutical
development.
The change
management system
should provide
management and
documentation of
adjustments made to
the process during
technology transfer
activities.
A formal change
management system
should be in place for
commercial
manufacturing.
Oversight by the
quality unit should
provide assurance of
appropriate science
and risk based
assessments.
Any changes after
product
discontinuation should
go through an
appropriate change
management system.
Pharmaceutical Quality System
11
3.2.4 Management Review of Process Performance and Product Quality
Management review should provide assurance that process performance and product
quality are managed over the lifecycle. Depending on the size and complexity of the
company, management review can be a series of reviews at various levels of
management and should include a timely and effective communication and escalation
process to raise appropriate quality issues to senior levels of management for review.
(a) The management review system should include:
(1) The results of regulatory inspections and findings, audits and other
assessments, and commitments made to regulatory authorities;
(2) Periodic quality reviews, that can include:
(i) Measures of customer satisfaction such as product quality
complaints and recalls;
(ii) Conclusions of process performance and product quality
monitoring;
(iii)The effectiveness of process and product changes including those
arising from corrective action and preventive actions.
(3) Any follow-up actions from previous management reviews.
(b) The management review system should identify appropriate actions, such as:
(1) Improvements to manufacturing processes and products;
(2) Provision, training and/or realignment of resources;
(3) Capture and dissemination of knowledge.
Table IV: Application of Management Review of Process Performance and
Product Quality throughout the Product Lifecycle
Pharmaceutical
Development
Technology
Transfer
Commercial
Manufacturing
Product
Discontinuation
Aspects of
management review
can be performed to
ensure adequacy of
the product and
process design.
Aspects of
management review
should be performed
to ensure the
developed product and
process can be
manufactured at
commercial scale.
Management review
should be a structured
system, as described
above, and should
support continual
improvement.
Management review
should include such
items as product
stability and product
quality complaints.
4. CONTINUAL IMPROVEMENT OF THE PHARMACEUTICAL QUALITY
SYSTEM
This section describes activities that should be conducted to manage and continually
improve the pharmaceutical quality system.
4.1 Management Review of the Pharmaceutical Quality System
Management should have a formal process for reviewing the pharmaceutical quality
system on a periodic basis. The review should include:
(a) Measurement of achievement of pharmaceutical quality system objectives;
(b) Assessment of performance indicators that can be used to monitor the

Pharmaceutical Quality System
12
effectiveness of processes within the pharmaceutical quality system, such as:
(1) Complaint, deviation, CAPA and change management processes;
(2) Feedback on outsourced activities;
(3) Self-assessment processes including risk assessments, trending, and
audits;
(4) External assessments such as regulatory inspections and findings and
customer audits.
4.2 Monitoring of Internal and External Factors Impacting the
Pharmaceutical Quality System
Factors monitored by management can include:
(a) Emerging regulations, guidance and quality issues that can impact the
Pharmaceutical Quality System;
(b) Innovations that might enhance the pharmaceutical quality system;
(c) Changes in business environment and objectives;
(d) Changes in product ownership.
4.3 Outcomes of Management Review and Monitoring
The outcome of management review of the pharmaceutical quality system and
monitoring of internal and external factors can include:
(e) Improvements to the pharmaceutical quality system and related processes;
(f) Allocation or reallocation of resources and/or personnel training;
(g) Revisions to quality policy and quality objectives;
(h) Documentation and timely and effective communication of the results of the
management review and actions, including escalation of appropriate issues to
senior management.

Pharmaceutical Quality System
13
5. GLOSSARY
ICH and ISO definitions are used in ICH Q10 where they exist. For the purpose of
ICH Q10, where the words “requirement”, “requirements” or “necessary” appear in an
ISO definition, they do not necessarily reflect a regulatory requirement. The source of
the definition is identified in parentheses after the definition. Where no appropriate
ICH or ISO definition was available, an ICH Q10 definition was developed.
Capability of a Process:
Ability of a process to realise a product that will fulfil the requirements of that
product. The concept of process capability can also be defined in statistical terms. (ISO
9000:2005)
Change Management:
A systematic approach to proposing, evaluating, approving, implementing and
reviewing changes. (ICH Q10)
Continual Improvement:
Recurring activity to increase the ability to fulfil requirements. (ISO 9000:2005)
Control Strategy:
A planned set of controls, derived from current product and process understanding,
that assures process performance and product quality. The controls can include
parameters and attributes related to drug substance and drug product materials and
components, facility and equipment operating conditions, in-process controls, finished
product specifications, and the associated methods and frequency of monitoring and
control. (ICH Q10)
Corrective Action:
Action to eliminate the cause of a detected non-conformity or other undesirable
situation. NOTE: Corrective action is taken to prevent recurrence whereas preventive
action is taken to prevent occurrence. (ISO 9000:2005)
Design Space:
The multidimensional combination and interaction of input variables (e.g., material
attributes) and process parameters that have been demonstrated to provide assurance
of quality. (ICH Q8)
Enabler:
A tool or process which provides the means to achieve an objective. (ICH Q10)
Feedback / Feedforward:
Feedback: The modification or control of a process or system by its results or effects.
Feedforward: The modification or control of a process using its anticipated results or
effects. (Oxford Dictionary of English. Oxford University Press; 2003)
Feedback/ feedforward can be applied technically in process control strategies and
conceptually in quality management. (ICH Q10)
Innovation:
The introduction of new technologies or methodologies. (ICH Q10)

Pharmaceutical Quality System
14
Knowledge Management:
Systematic approach to acquiring, analysing, storing, and disseminating information
related to products, manufacturing processes and components. (ICH Q10)
Outsourced Activities:
Activities conducted by a contract acceptor under a written agreement with a contract
giver. (ICH Q10)
Performance Indicators:
Measurable values used to quantify quality objectives to reflect the performance of an
organisation, process or system, also known as “performance metrics” in some regions.
(ICH Q10)
Pharmaceutical Quality System (PQS):
Management system to direct and control a pharmaceutical company with regard to
quality. (ICH Q10 based upon ISO 9000:2005)
Preventive Action:
Action to eliminate the cause of a potential non-conformity or other undesirable
potential situation. NOTE: Preventive action is taken to prevent occurrence whereas
corrective action is taken to prevent recurrence. (ISO 9000:2005)
Product Realisation:
Achievement of a product with the quality attributes appropriate to meet the needs of
patients, health care professionals, and regulatory authorities (including compliance
with marketing authorisation) and internal customers requirements. (ICH Q10)
Quality:
The degree to which a set of inherent properties of a product, system or process fulfils
requirements. (ICH Q9)
Quality Manual:
Document specifying the quality management system of an organisation. (ISO
9000:2005)
Quality Objectives:
A means to translate the quality policy and strategies into measurable activities. (ICH
Q10)
Quality Planning:
Part of quality management focused on setting quality objectives and specifying
necessary operational processes and related resources to fulfil the quality objectives.
(ISO 9000:2005)
Quality Policy:
Overall intentions and direction of an organisation related to quality as formally
expressed by senior management. (ISO 9000:2005)
Quality Risk Management:
A systematic process for the assessment, control, communication and review of risks
to the quality of the drug (medicinal) product across the product lifecycle. (ICH Q9)

Pharmaceutical Quality System
15
Senior Management:
Person(s) who direct and control a company or site at the highest levels with the
authority and responsibility to mobilise resources within the company or site. (ICH
Q10 based in part on ISO 9000:2005)
State of Control:
A condition in which the set of controls consistently provides assurance of continued
process performance and product quality. (ICH Q10)
Pharmaceutical Quality System
16
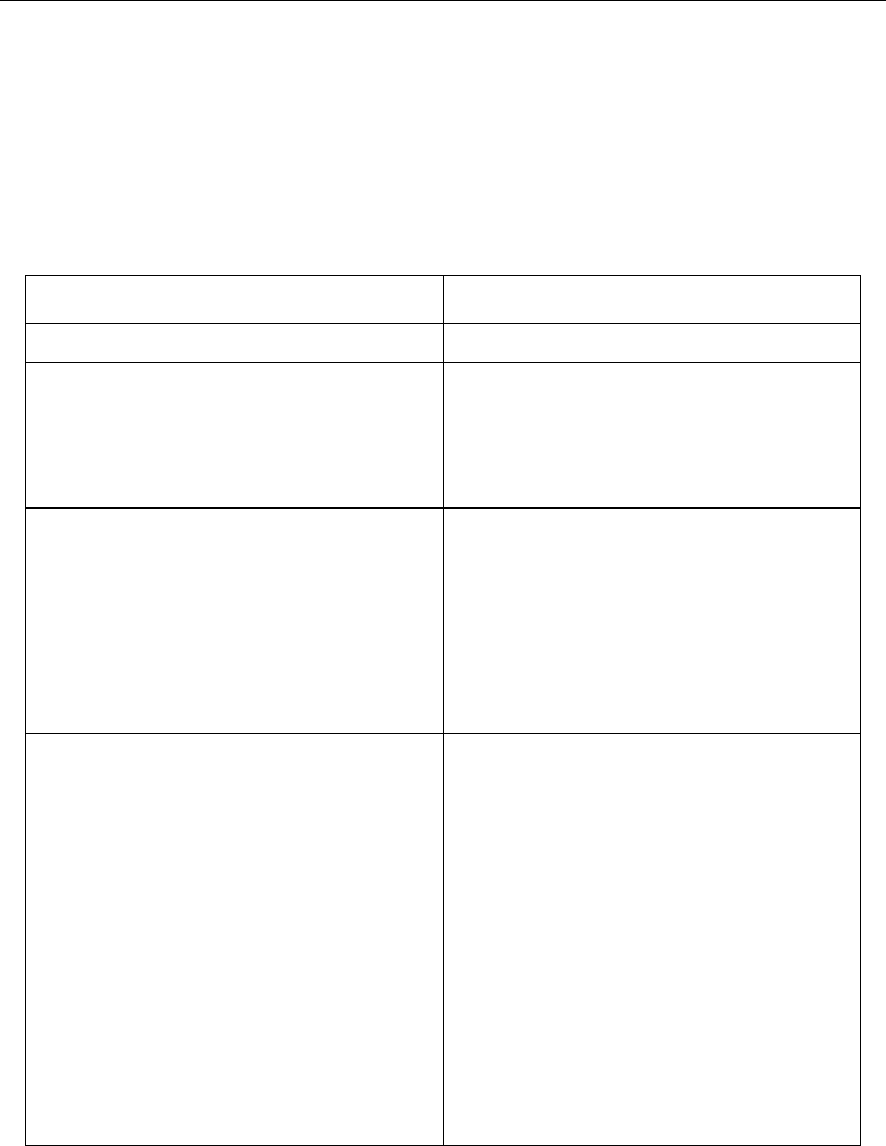
Annex 1
Potential Opportunities to Enhance Science and Risk Based Regulatory
Approaches *
*Note: This annex reflects potential opportunities to enhance regulatory approaches.
The actual regulatory process will be determined by region.
Scenario
Potential Opportunity
1. Comply with GMPs
Compliance – status quo
2. Demonstrate effective
pharmaceutical quality system,
including effective use of quality
risk management principles (e.g.,
ICH Q9 and ICH Q10).
Opportunity to:
increase use of risk based
approaches for regulatory
inspections.
3. Demonstrate product and process
understanding, including effective
use of quality risk management
principles (e.g., ICH Q8 and ICH
Q9).
Opportunity to:
facilitate science based
pharmaceutical quality
assessment;
enable innovative approaches to
process validation;
establish real-time release
mechanisms.
4. Demonstrate effective
pharmaceutical quality system
and product and process
understanding, including the use
of quality risk management
principles (e.g., ICH Q8, ICH Q9
and ICH Q10).
Opportunity to:
increase use of risk based
approaches for regulatory
inspections;
facilitate science based
pharmaceutical quality
assessment;
optimise science and risk based
post-approval change processes to
maximise benefits from innovation
and continual improvement;
enable innovative approaches to
process validation;
establish real-time release
mechanisms.

Pharmaceutical Quality System
17
Annex 2
Diagram of the ICH Q10 Pharmaceutical Quality System Model
This diagram illustrates the major features of the ICH Q10 Pharmaceutical Quality
System (PQS) model. The PQS covers the entire lifecycle of a product including
pharmaceutical development, technology transfer, commercial manufacturing, and
product discontinuation as illustrated by the upper portion of the diagram. The PQS
augments regional GMPs as illustrated in the diagram. The diagram also illustrates
that regional GMPs apply to the manufacture of investigational products.
The next horizontal bar illustrates the importance of management responsibilities
explained in Section 2 to all stages of the product lifecycle. The following horizontal
bar lists the PQS elements which serve as the major pillars under the PQS model.
These elements should be applied appropriately and proportionally to each lifecycle
stage recognising opportunities to identify areas for continual improvement.
The bottom set of horizontal bars illustrates the enablers: knowledge management
and quality risk management, which are applicable throughout the lifecycle stages.
These enablers support the PQS goals of achieving product realisation, establishing
and maintaining a state of control, and facilitating continual improvement.
