
Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=hcap20
Journal of Clinical Child & Adolescent Psychology
ISSN: 1537-4416 (Print) 1537-4424 (Online) Journal homepage: http://www.tandfonline.com/loi/hcap20
The Influence of Interactions with Dogs on Affect,
Anxiety, and Arousal in Children
Molly K. Crossman, Alan E. Kazdin, Angela Matijczak, Elizabeth R. Kitt &
Laurie R. Santos
To cite this article: Molly K. Crossman, Alan E. Kazdin, Angela Matijczak, Elizabeth
R. Kitt & Laurie R. Santos (2018): The Influence of Interactions with Dogs on Affect,
Anxiety, and Arousal in Children, Journal of Clinical Child & Adolescent Psychology, DOI:
10.1080/15374416.2018.1520119
To link to this article: https://doi.org/10.1080/15374416.2018.1520119
Published online: 30 Oct 2018.
Submit your article to this journal
View Crossmark data

The Influence of Interactions with Dogs on Affect,
Anxiety, and Arousal in Children
Molly K. Crossman, Alan E. Kazdin, Angela Matijczak, Elizabeth R. Kitt, and
Laurie R. Santos
Department of Psychology, Yale University
Interactions with animals represent a promising way to reduce the burden of childhood
mental illness on a large scale. However, the specific effects of child–animal interactions are
not yet well-established. This study provides a carefully controlled demonstration that
unstructured interactions with dogs can improve clinically relevant symptoms in children.
Seventy-eight children (55.1% female, 44.9% male) ages 10 to 13 (M = 12.01, SD = 1.13)
completed the Trier Social Stress Test for Children, followed by (a) interaction with a dog,
(b) a tactile-stimulation control condition, or (c) a waiting control condition. The Positive
and Negative Affect Schedule for Children, Short Form and the State/Trait Anxiety
Inventory for Children were completed at baseline and posttest, and salivary cortisol was
assessed at 5 time points. Adjusting for baseline scores, participants in the experimental
condition showed higher scores on the Positive Affect scale than participants in both control
conditions and lower scores on the State/Trait Anxiety Inventory for Children than partici-
pants in the waiting control condition at posttest. Negative affect was not assessed reliably,
and we detected no effect of the interactions on salivary cortisol, as measured by area under
the curve with respect to ground. Brief, unstructured interactions with dogs boosted chil-
dren’s positive emotions and reduced anxiety. Additional research is needed to further clarify
which features of the interactions produce these benefits and the extent to which interactions
with animals offer benefits that exceed the effects of other common coping strategies,
activities, and interventions.
Mental illness is the leading source of childhood disability
and a top contributor to the overall burden of disease
(Erskine et al., 2015). In the United States, approximately
one in five children are affected by mental illness each year,
with an annual financial cost of approximately $247 billion
(Centers for Disease Control, 2013; Kessler et al., 2005).
These estimates do not include consideration of subthres-
hold symptoms, which add considerably to the burden of
disease (Balzás et al., 2013). Despite the considerable pre-
valence and consequences of childhood mental illness and
subthreshold symptoms, roughly 80% of children in need
do not receive treatment (Kataoka, Zhang, & Wells, 2002).
Simple, scalable approaches to reducing the burden of
childhood mental illness and subthreshold symptoms are
urgently needed.
Animal-assisted activities (AAAs) represent a promising
type of intervention to bridge the gap between children in
need of mental health services and those receiving treat-
ment. AAAs are unstructured programs that provide parti-
cipants with opportunities to interact with animals (most
commonly domestic dogs; Canis familiaris), with the goal
of improving mental health and alleviating stress
(International Association of Human–Animal Interaction
Organizations, 2013). AAAs are promising as a method
for improving children’s mental health because they are
appealing, enjoyable, credible, low cost, and easy to imple-
ment (Crossman & Kazdin, 2015). Indeed, AAAs are
already prevalent as a strategy for improving children’s
mental health. Prominent examples include AAAs for
pediatric patients, child victim witnesses, children who
have experienced traumatic events, students learning to
read, and even those without any special status at all. The
Correspondence should be addressed to Molly K. Crossman,
Department of Psychology, Yale University, Box 208205, New Haven,
Color versions of one or more of the figures in the article can be found
online at www.tandfonline.com/hcap.
Journal of Clinical Child & Adolescent Psychology, 00(00), 1–14, 2018
Copyright © Society of Clinical Child & Adolescent Psychology
ISSN: 1537-4416 print/1537-4424 online
DOI: https://doi.org/10.1080/15374416.2018.1520119
rationale is that contact with the animals may improve
mood and affect and alleviate anxiety and stress by promot-
ing emotion-focused coping, serving as sources of social
support, providing enjoyable experiences, and/or transmit-
ting positive emotions through emotional contagion
(Crossman, 2017; Kruger, Trachtenberg, & Serpell, 2004).
Preliminary evidence in support of the effects of AAAs
on children’s affect, anxiety, and stress-related symptoms
comes from a small number of rigorously designed studies
(e.g., Beetz, Julius, Turner, & Kotrschal, 2012; Kerns,
Stuart-Parrigon, Coifman, Van Dulmen, & Koehn, 2017;
Kertes et al., 2017). These studies have focused primarily
on interactions with dogs and have documented the effects
of these interactions on self-reported, behavioral, and phy-
siological outcomes. However, the majority of research in
this area has focused on adults (Nimer & Lundahl, 2007;
Souter & Miller, 2007). In addition, most studies have
focused on whether interactions with dogs buffer against
the negative effects of stress (i.e., prevent increases in
symptoms during stress exposure; Beetz et al., 2012;
Kerns et al., 2017; Kertes et al., 2017; Krause-Parello,
Thames, Ray, & Kolassa, 2018; Vagnoli et al., 2015).
Although some AAAs focus on supporting children during
stress exposure (e.g., while giving testimony in court),
many focus on alleviating symptoms after exposure to an
acute stressor (e.g., at the sites of tragedies/natural disas-
ters), or among children facing conditions of chronic stress
(e.g., children undergoing long-term hospitalizations), and
it is not yet clear whether findings on the stress-buffering
effects of AAAs will apply in situations where the goal is to
promote recovery following a stressful event. Most impor-
tant, the bulk of this research has been exploratory in nature
(e.g., Kaminski, Pellino, & Wish, 2002; Tsai, Friedmann, &
Thomas, 2010). As a result, the effects of AAAs on chil-
dren are not yet well-established.
The preliminary state of the literature has resulted in one
particularly important gap in our understanding of the
effects of AAAs for children. Namely, it is not yet clear
whether the interactions with the animals are really the
active ingredient (Crossman, 2017; Marino, 2012). In
other words, we cannot yet say whether the improvements
observed in studies of AAAs can really be attributed to the
interactions with the animals, rather than to other aspects of
the interventions or to more general factors, such as
engagement in any distracting or appealing activity
(Marino, 2012). The issue is that the majority of studies
have relied exclusively on no-treatment control conditions,
if they have included any control condition at all (e.g.,
Braun, Stangler, Narveson, & Pettingell, 2009; Kerns
et al., 2017; Vagnoli et al., 2015). Studies that use no-
treatment control conditions demonstrate that AAAs can
alleviate clinically relevant symptoms in children and sug-
gest that the observed improvements are not simply due to
extratherapeutic factors. However, these studies do not
clarify what specific component of the intervention pro-
duced the improvements.
A handful of studies have attempted to isolate the effects
of the interactions with the animals. The most common
approach has been to compare the effects of support from
an animal to support from a human (e.g., a parent or a
friendly confederate; Beetz et al., 2012; Beetz et al., 2011;
Kertes et al., 2017
). In some cases, these studies have also
i
ncluded support from toy dogs as an additional control
(Beetz et al., 2012; Beetz et al., 2011). Findings from these
studies are mixed; some show positive effects of support
from a dog on physiological arousal but not perceived stress,
and some show positive effects on perceived stress but not
physiological arousal (e.g., Beetz et al., 2012;Kertesetal.,
2017). These findings provide initial hints that animals may
be effective sources of social support for children. However,
key questions remain. For example, do the effects of AAAs
exceed the effects of other common approaches to improving
children’s affect and anxiety? One additional study
attempted to isolate the effects of AAAs by ruling out
participation in any distracting activity as an alternative
explanation for the benefits (Barker, Knisely, Schubert,
Green, & Ameringer, 2015). However, this study showed
no differences between the effects of the two conditions,
highlighting the fact that it is not yet clear whether interac-
tions with animals convey specific benefits for children.
Observational findings from prior studies suggest one
especially important plausible alternative explanation for
the benefits of AAAs. These studies have shown that chil-
dren who engage in more physical contact with the dogs
over the course of their interactions show lower levels of
stress following those interactions, suggesting that tactile
stimulation may be driving the benefits that we typically
attribute to the animals (e.g., Beetz et al., 2012; Kertes
et al., 2017). Indeed, the benefits of tactile stimulation for
mental health are well-established (see Ardiel & Rankin,
2010, for a review). Of course, it is likely that interactions
with animals produce benefits in part because of tactile
stimulation. However, the key question in terms of isolating
the effects of AAAs is whether the benefits are attributable
entirely to the effects of tactile stimulation.
Identifying the active ingredient in AAAs and ruling out
alternative explanations is a crucial step in ensuring that
these programs are as effective and efficient as possible
(Chambless & Hollon, 2012). This is particularly important
in light of the fact that these programs have already been
widely implemented. In addition, interventions that involve
interactions with animals introduce concerns about the wel-
fare of the animals, the possibility of exposing individuals
with allergies to and/or fears of the animals, and the risk of
contamination/disease transmission. These concerns under-
score the importance of ensuring that the animals are con-
tributing meaningfully to the process of change. As a
starting point, the field needs carefully controlled
2
CROSSMAN ET AL.
laboratory studies to isolate the effects of the types of
child–animal interactions that compose AAAs and to rule
out alternative explanations for their effects. Such investi-
gations will provide a foundation of evidence on which to
build our understanding of the role of AAAs in children’s
mental health care. Specifically, these investigations will
inform efforts to develop guidelines for practice, identify
populations of children that may be especially likely to
benefit, and determine the precise role(s) of AAAs in
clinical child psychology.
The present study evaluated the effects of brief, unstruc-
tured interactions with unfamiliar dogs on children’s anxi-
ety, affect, and physiological arousal, following exposure to
a stressful task. We compared the effects of these interac-
tions to the effects of tactile stimulation, as well as to no
intervention. A secondary goal was to evaluate whether
findings from prior studies, which have focused on whether
interactions with dogs buffer against the negative effects of
stress, would extend to interactions with dogs that occur
after stress exposure, when symptoms are already elevated.
We predicted that brief, unstructured interactions with unfa-
miliar dogs would increase children’s positive affect and
reduce negative affect, anxiety, and physiological arousal,
relative to tactile stimulation or no intervention. In addition,
we were interested in how children who did not have pets
of their own would respond to the interactions, based on
suggestions that prior experiences with animals may be
necessary to gain the full benefits of AAAs (e.g., Stewart
& Strickland, 2013). We therefore explored the role of
children’s ownership of, experience with, and feelings and
behavior toward pets in their responses to the interactions.
METHOD
Human Participants
Participants were 78 children (43 [55.1%] female, 35
[44.9%] male), ranging in age from 10 to 13
(M =12.01,SD = 1.13) and drawn from the local com-
munity surrounding a university in the northeastern
United States. The county includes a mix of urban, sub-
urban, and rural towns and has a total population of
approximately 900,000 (U.S. Census Bureau, 2016).
The county is predominantly White, Non-Hispanic, and
the median household income as of 2015 was approxi-
mately $60,000, with a poverty rate of 13.6%. Per parent
report, 44 participants ( 56.4%) were White, 6 (7.7%)
were Hispanic/Latino, 5 (6.4%) were Asian, 4 (5.1%)
were Black/African American, 1 (1.3%) was Native
American/Alaska Native, and 18 (23.1%) were multira-
cial/multiethnic. One additional participant began the
study but did not complete the full procedure and was
excluded from all analyses. For this participant, the pro-
cedure was terminated during the Trier Social Stress Test
for Children (discussed below) to prevent excessive
stress. Detailed demographic and background informa-
tion is available in Table 1.
Participants were recruited from September 2015 through
September 2016 using a range of methods including online
postings, e-mail, advertisements, fliers, and in-person at local
sites. Exclusion criteria included fears of dogs and allergies to
animal dander, saliva, or urine. Each child participant received a
$5 gift certificate, a small toy, and a certificate of appreciation;
parents also received a $15 gift certificate. Th is study was
reviewed and approved by the Institutional Review Board and
the Institutional Animal Care and Use Committee of Yale
University. Parents and child participants provided informed
consent and assent, respectively.
We elected to use an unselected community sample in light
of the need for intervention methods that can reach a broad
range of children. In addition, the goal of this investigation was
to contribute to efforts to isolate the effects of interactions with
dogs on clinically relevant symptoms in children rather than to
es
tablish the efficacy of AAAs for any full syndrome disorder.
We selected this age group (10–13) because older children
making the transition to adolescence are particularly susceptible
to barriers to treatment such as perceived stigma, discomfort
talking about mental health problems, and wanting to cope
independently (Gulliver, Griffiths, & Christensen, 2010). In
addition, AAAs are already in widespread use for children of
this age group (Friesen, 2010).
Sample Estimate
We based our target sample of 75 participants (actual
N = 78) on a sample estimate for a repeated measures
analysis of variance (ANOVA) with a small effect
(f = 0.2), three groups, a correlation of .7 between
repeated measurements, and 95% power (Faul, Erdfelder,
Lang, & Buchner, 2007). The results suggested that a
sample of 63 participants was needed. We added four
participants per condition to account for dropout, experi-
menter error, and related issues. We used this method in
light of ongoing debate about how to calculate a sample
estimate for our particular analytic approach (analysis of
covariance [ANCOVA], adjusting for baseline s cores on
the outcome measures), as well as the clear consensus that
this approach increases power relative to the ANOVA
approach (Van Breukelen, 2006; Vickers & Altman,
2001; Zhang et al., 2014). In other words, our sample
estimate reflects a conservative approach.
Measures
Positive and Negative Affect Schedule for Children,
Short Form
We used the Positive and Negative Affect Schedule for
Children, Short Form (PANAS-C-S) to assess affect at
EFFECTS OF DOGS ON ANXIETY, AFFECT, AND AROUSAL 3

baseline and after the intervention. The PANAS-C-S
includes a five-item Positive Affect scale and a five-item
Negative Affect scale (Ebesutani et al., 2012). Children
were asked to indicate the extent to which they felt each
item “right now” using a 5-point Likert scale. For example,
children were asked to rate the extent to which they felt
“happy” and “miserable” for the Positive and Negative
Affect scales, respectively. The items are summed to yield
separate Positive and Negative Affect scales.
The Positive and Negative Affect scales in the PANAS-
C-S have higher inter-item correlations than their full-
length counterparts. In the present investigation,
Cronbach’s alpha was .90 for the Positive Affect scale at
baseline. However, Cronbach’s alpha for the Negative
Affect scale at baseline was .57, indicating that negative
affect was not assessed reliably. We examined the intra-
item correlations to evaluate whether there were items with
low correlations to the rest of the scale, but we did not find
any such items and the Negative Affect scale was excluded
from further analysis. The PANAS-C-S has discriminant
validity comparable to that of the full-length measure.
Baseline scores on the Positive Affect scale were compar-
able to those reported among other nonclinical samples and
among children experiencing chronic physical illness (e.g.,
Hexdall & Huebner, 2008; Reddy, Palmer, Jackson, Farris,
& Alfano, 2017).
State/Trait Anxiety Inventory for Children
We used the State portion of the State/Trait Anxiety
Inventory for Children (STAI-C) to assess anxiety at base-
line and after the intervention period. The STAI-C consists
of 20 items, and children are asked to report how they feel
“right now, at this very moment.” For example, children are
asked to indicate whether they feel “very relaxed,”
“relaxed,” or “not relaxed” (Spielberger, Gorsuch,
Lushene, Vagg, & Jacobs, 1973). Responses on all 20
items are summed. Cronbach’s alpha at baseline was .85.
The STAI-C has demonstrated construct, concurrent, and
discriminant validity for children and adolescents (e.g.,
Seligman, Ollendick, Langley, & Baldacci, 2004;
Spielberger et al., 1973). Baseline STAI-C scores were
similar to those reported for other unselected samples but
were lower than those reported for samples of children with
clinically significant anxiety disorders (e.g., Flannery-
Schroeder & Kendall, 2000; Kendall, 1994; Muris, Rapee,
Meesters, Schouten, & Geers, 2003; Reddy et al., 2017;
Spielberger et al., 1973).
TABLE 1
Participant Characteristics
Characteristic Full Sample (N = 78) Dog (n = 26) Tactile Stimulation Control (n = 26) Waiting Control (n = 26)
Age
Range 10.00–13.96 10.13–13.92 10.27–13.94 10.00–13.96
M (SD) 12.01 (1.13) 12.16 (1.18) 12.18 (1.17) 11.69 (1.02)
Sex (%)
Female 43 (55.10) 14 (53.80) 15 (57.70) 14 (53.80)
Male 35 (44.90) 12 (46.20) 11 (42.30) 12 (46.20)
Race/Ethnicity (%)
White, Non-Hispanic 44 (56.40) 16 (61.50) 11 (42.30) 17 (65.40)
Black 4 (5.10) 1 (3.80) 2 (7.70) 1 (3.80)
Hispanic/Latino 6 (7.70) 2 (7.70) 4 (15.40) 0 (0.00)
Asian 5 (6.40) 0 (0.00) 0 (0.00) 5 (19.20)
Native American/Alaska Native 1 (1.30) 0 (0.00) 1 (3.80) 0 (0.00)
Multiracial/Multiethnic 18 (23.10) 7 (26.90) 8 (30.80) 3 (11.50)
Pet Ownership (%)
Current 62 (79.50) 22 (84.60) 18 (69.20) 22 (84.60)
Past 60 (76.90) 21 (80.80) 18 (69.20) 21 (80.80)
Dog Ownership (%)
Current 43 (55.10) 16 (61.50) 16 (61.50) 11 (42.30)
Past 34 (43.60) 12 (46.20) 10 (38.50) 12 (46.20)
CABS 28.02 (5.23) 27.94 (5.75) 27.88 (4.00) 28.21 (5.88)
EDI
Positive 31.46 (5.64) 33.16 (5.60) 31.09 (5.70) 30.04 (5.38)
Negative 10.42 (2.88) 10.40 (2.43) 9.78 (2.52) 11.04 (3.53)
PANAS-C-P M (SD)
Positive 18.36 (3.66) 18.88 (3.79) 17.88 (3.76) 18.31 (3.51)
Negative 8.23 (3.13) 7.42 (1.90) 7.40 (2.45) 9.85 (4.05)
Note: CABS = Companion Animal Bonding Scale; EDI = Experiences with Dogs Inventory; PANAS-C-P = Positive and Negative Affect Schedule for
Children-Parent Report, Short Form.
4 CROSSMAN ET AL.
Salivary Cortisol
Salivary cortisol is a reliable measure of activation of
the Hypothalamic-Pituitary-Adrenal axis, which regulates
the body’s stress response, and has been extensively vali-
dated as a measure of physiological stress (see Kirschbaum
& Hellhammer, 1989, for a review). Salivary cortisol was
assessed as a physiological indicator of stress at five time
points: Baseline; immediately after the stress induction; and
at 5, 10, and 15 min into the intervention period. Thus,
accounting for the time to collect the cortisol samples, the
last cortisol sample was collected approximately 41 min
after stressor onset and 23 min after its conclusion.
Although a longer time line of assessment (45 to 60 min
post-exposure) is commonly used to capture complete cor-
tisol recovery, the timing of the cortisol assessments was
selected based on consideration of a number of factors.
Previous research examining the use of an intervention to
promote cortisol recovery following stress exposure
showed group differences at approximately 20 min post-
exposure (Khalfa, Bella, Roy, Peretz, & Lupien, 2003).
Studies of cortisol recovery in children and adolescence
additionally show recovery at approximately 20 min post-
exposure (Hankin, Badanes, Abela, & Watamura, 2010).
Finally, in previous pilot work, our research group found
that absent any intervention, child participants showed
nearly complete cortisol recovery using this time line. In
light of these findings and because our priority was to
detect differences in the rate of cortisol recovery between
groups, we chose to prioritize more frequent assessments to
maximize our chances of capturing between-group differ-
ences in the rate of cortisol recovery. In addition, we
selected the length of the interaction (15 min, not including
breaks for cortisol assessments) based on our expectation of
what would be maximally effective for the children and in
the best interests of the dogs. Extending the cortisol assess-
ment period would thus have meant continuing the assess-
ments beyond the conclusion of the interactions, which
might have diluted the between-group effects.
Participants were instructed not to consume any food in the
hour before coming to the lab (Kirschbaum & Hellhammer ,
1989). Salivary cortisol was collected using Saliva Bio Oral
Swabs (Salimetrics, Carlsbad, CA), which participants
chewed for 1 min per sample. After collection, samples
were frozen (below −20 °C) until assay. Samples were
assayed in duplicate according to the manufacturer’s recom-
mended protocol (#1–3002, Salimetrics, Carlsbad, CA) using
a highly sensitive enzyme immunoassay designed for analyz-
ing saliva samples and a sample test volume of 25 μLof
saliva per determination. The lower limit of sensitivity of the
assay is 0.007 μg/dL, and the standard curve ranges between
0.012 μg/dL to 3.0 μg/dL. The average intra-assay coefficient
of variability (CV) was 7.92%, indicating that cortisol con-
centration was assessed reliably.
To evaluate cortisol output over the course of the study,
we computed the area under the curve with respect to
ground (AUC
G
; Pruessner, Kirschbaum, Meinlschmid, &
Hellhammer, 2003). AUC
G
provides a summary of the
overall cortisol output, allowing for straightforward analy-
sis of repeated measures data. AUC
G
takes into account
change over time with respect to baseline scores, as well as
the overall magnitude of the cortisol response (Khoury
et al., 2015; Pruessner et al., 2003).
Positive and Negative Affect Schedule for
Children–Parent Report, Short Form
We hypoth esized that children’s affect would change over
the course of the study. However, change in affect may be
influenced by more long-term moods. Accordingly, we
included the Positive and Negative Affect Schedule for
Children–Paren t Report, Short Form (PANAS-C-P) as a possi-
bleadjustmentvariable.ThePANAS-C-Pisthesameasthe
child-report version (see earlier), but in this case parents were
asked to respond based on how their children have felt “during
the past few weeks.” Cronbach’salphawas.86forthePositive
Affect scale and .81 for the Negative Affect scale. The
PANAS-C-P has demonstrated construct validity (Ebesutani,
Okamura, Higa-McMillan, & Chorpita, 201 1). Scores on the
PANAS-C-P in the present sample were similar to those
reported previously among a large, school-based sample
(Ebesutani et al., 2011).
Companion Animal Bonding Scale
We included the Companion Animal Bonding Scale
(CABS) to explore the role of children’s feelings and behavior
toward companion animals in their responses to the interactions
in our study. The CABS is a self-report measure that assesses
children’s day-to-day interac tions with and feelin gs toward their
companion animals. Children indicate the frequency of each of
eight behaviors and feelings relating to their bonding with their
pet (e.g., “How often do you feel that you have a close relation-
ship with your companion animal?”) on a 5-point Likert scale.
The CABS has established convergent validity and internal
consistency (Cohen, 2002; Poresky, Hendrix, Mosier , &
Samuelson, 1987; Triebenba cher, 1999). Cronbach’salphafor
the CABS was .70. Participants in all three condition s who had a
pet at the time of the study completed the CABS. Participants
who did not have a pet at the time of participation did not
complete the CABS.
Experiences with Dogs
We were interested in exploring the role of each child’s
general prior experiences with dogs, in addition to their level
of bonding with a particular companion animal. In addition, we
wished to quantify children’s general exposure to dogs, regard-
less of whether they themselves had a dog at the time of the
EFFECTS OF DOGS ON ANXIETY, AFFECT, AND AROUSAL 5

study. We therefore asked parents of child participants to report
on current and past pet ownership and to specify the species of
those pets. This allowed us to analyze the effects of pet owner-
ship in general, as well as dog ownership in particular. In
addition, we included an adapted version of the Experiences
with Dogs Inventory (EDI; Crossman, Kazdin, & Knudson,
2015). The EDI asked parents to rate how frequently their
childrenhadengagedineachof20experiencesinvolving
dogs, including 13 positive/neutral experiences (e.g., “My
child has touched/petted a dog”) and seven negative experi-
ences (e.g., “My child has been harmed by a dog”), which yield
separate Positive and Negative sum scores. Responses are made
using a Likert scale ranging from 1 (never)to5(very fre-
quently). Cronbach’s alpha for the Positive dimension of the
EDI was .69. W e therefore removed one item (“My child has
worked with dogs to earn money [e.g., dog walker, dog sitter],”
which had a small, negative correlation; r = −.01) with the rest
of the scale. Cronbach’s alpha for the resulting 12-item Positive
dimension was .70, and we therefore used this version.
Cronbach’s alpha for the Negative dimension was .72. The
EDI has established convergent validity (Crossman et al.,
2015).
Stress Task
We used the Trier Social Stress Test for Children (TSST-C)
to induce moderate psychosocial stress (Buske-Kirschbaum
et al., 1997). The TSST-C involves an anticipation phase, a
public speaking phase, and a mental arithmetic phase. The
TSST-C reliably elicits moderate psychosocial stress in
children ages 7 to 14 (Gunnar, Talge, & Herrera, 2009).
Selection and Protection of the Dogs
Eight dogs (four male, four female) participated in data collec-
tion. Two additional dogs participated but were not involved in
data collection for the experimental condition (i.e., only parti-
cipants in the control conditions interacted with these dogs, after
completing the study). Although it would have been preferable
to limit the number of dogs (and consequ ently the amount of
variability in the nature of the interactions), this number of dogs
was needed to facilitate participant scheduling. In addition, the
variability introduced by the eight dogs included in the present
study was less than is introduced in studies evaluating interac-
tions between children and their own dogs (e.g., Kerns et al.,
2017;Kertesetal.,2017). All dogs were certified or registered
as therapy dogs with leading therapy dog or ganizations (e.g.,
The Good Dog Foundation, Pet Partners) or had successfully
completed an equivalent behavioral evaluation. Veterinary
records were provided for all dogs to indicate that they were
up-to-date on vaccinations and free of parasites.
1
Before
enrollment, each dog completed an orientation visit at the lab,
which included time for acclimation to the facility, orientation
of the handler and dog to the study procedures, and evaluation
of the dog’s behavioral response to the procedures.
A key component of the hypothesis was that interaction
with a dog (rather than a dog and handler) would alleviate
stress and improve affect. In addition, a primary limitation
of past human–animal interaction research has been the
failure to disentangle the effects of interaction with the
dogs from interaction with the dogs’ handlers and other
program participants (Marino, 2012). Accordingly, it was
important that participants in the present study interact
independently with the dogs without extensive intervention
from human handlers. We achieved this goal by having a
member of the research team sit in the corner of the room
to supervise the interactions, intervening only as necessary
to protect the well-being of the dog and/or child. The dog’s
handler waited in a separate room.
To address concerns raised by separating dogs from their
handlers, our procedure included a number of protections for
both the dogs and the child participants. First, a central compo-
nent of the orientation visit was to evaluate the dog’s response to
being separated from the handler. After familiarization with the
facility and the members of the research team, each dog was left
with a member of the research team for a test period of
5–10 min. During this test period, the handler was free to
observe the dog through a two-way mirror or closed-circuit
television system. If dogs showed signs of stress, the test was
ended. Dogs that showed signs of stress or otherwise demon-
strated inappropria te behavior were not accepted into the study.
2
During the study, handlers were again able to watch all
interactions through a two-way mirror and had the option to
stop any interaction to protect their dog’s safety or well-
being. However, an interaction was terminated early (after
10 min) on only one occasion, due to experimenter con-
cerns about signs of stress (e.g., barking).
3
Finally, partici-
pants were given instructions for safe interactions with
dogs and were provided brief, scripted prompts to remind
them of these instructions as needed. All members of the
research team were trained to detect signs of stress and
1
Therapy dog registration/certification was used as an indicator of
dogs’ behavioral suitability for interactions with unfamiliar children.
Because the interactions in this study were conducted without the handler
in the room, these interactions are not considered therapy dog visits/
interactions.
2
One dog that demonstrated appropriate behavior and did not show
signs of stress at the orientation visit later showed signs of stress during its
first days of participation in the study. This dog was accordingly with-
drawn from participation following consideration of changes in her beha-
vior in and around the lab by the members of the research team and the
dog’s handler.
3
In that instance, the dog was reunited with its handler, and the child
waited for the remaining 5 min of the interaction period. Because the
majority of the interaction was completed, that participant was retained for
all analyses. However, we also confirmed that the pattern of results
(including main and supplementary analyses) does not change when that
participant is removed from the analyses.
6 CROSSMAN ET AL.

aggression in dogs and received hands-on training for mon-
itoring child–dog interactions. To prevent fatigue, dogs
never participated in the study 2 days in a row and never
had more than two sessions per day, with breaks provided
between sessions.
Study Conditions
Assignment to the three study conditions was made using a
random number generator (https://www.randomizer.org/).
4
The experimental condition involved a 15-min interaction
with a dog, which was supervised by an experimenter.
Participants were permitted to interact freely with the
dogs so long as they maintained appropriate behavior
(e.g., no touching the dogs’ eyes/ears/mouths, no yelling)
and the dogs did not show signs of stress or aggression.
To establish whether the interactions conveyed benefits
beyond the effects of tactile stimulation, we included a
tactile-stimulation control condition in which children
were given a soft blanket. The goal was to evaluate whether
something about the act of interacting with the dogs, rather
than just the tactile stimulation they provide or the presence
of any novel and comforting object, would reduce chil-
dren’s stress. We also included a waiting control condition
to evaluate whether interaction with a dog is more effective
than children’s independent coping skills and to rule out the
effects of factors such as completing the measures repeat-
edly, familiarization with the setting, and the simple pas-
sage of time as alternative explanations. As in the
experimental condition, the experimenter remained in the
corner of the room during both control conditions.
Procedure
Participant sessions were scheduled to begin between 1 and
6 p.m. After providing informed consent, parents of child
participants completed a background questionnaire about
their children, which included the PANAS-C-P. After pro-
viding assent, child participants provided baseline saliva
samples and then completed baseline self-report measures
(PANAS-C-S, STAI-C). The TSST-C was then completed,
followed immediately by collection of the second cortisol
sample. Participants then engaged in their respective con-
ditions for 15 min, with the third, fourth, and fifth cortisol
samples collected at 5-min intervals. Participants then com-
pleted the posttest PANAS-C-S and STAI-C, and partici-
pants in both control conditions were given the chance to
interact with a dog. Participants were then debriefed and
provided their toys, gift certificates, and certificates of
participation.
Data Analytic Plan
Treatment of Missing Data
We computed sum scores for all self-report measures.
For cases in which individual items were missing, missing
data were prorated using the mean of the completed items.
Measures missing more than one fourth of the items were
excluded. The average number of missing items per mea-
sure per participant was 0.57.
Preliminary Analysis
We chec ked f or dif ferences on background and demo-
graphic variables by condition using chi-squares and one-way
ANOVAs. We used Pearson product–moment correlations to
evaluate the relations among baseline variables and to check for
redundancy of measures. We used a threshold of .71 (indicating
a shared variance of 50%) to evaluate redundancy of measures.
In case of correlations exceeding .71, we evaluated the correla-
tions between change scores on those measures, with the inten-
tion that we would combine measures where change scores
were also highly correlated. For participants in the experimental
condition, we used one-way ANOVAs to check for differences
in change scores for the STAI-C and the Positive Affect scale,
as well as for differences in AUC
G
, based on the dog with
which participants interacted.
Effects of the Interactions
We used a one-way ANCOVA to evaluate the effect of
condition on each self-report measure (the Positive Affect
scale and the STAI-C), adjusting for scores on that same
measure at baseline. We used this approach (rather than a
repeated-measures ANOVA) because baseline measures
were collected before the stress induction and the interven-
tion (rather than immediately before the intervention). In
cases of significant effects, planned contrast were con-
ducted to evaluate the difference between the experimental
condition and each of the two control conditions. For
ANCOVAs, adjusted means are presented unless otherwise
noted. For planned contrasts, Cohen’s d was calculated
using adjusted means and the square root of the mean
square error. We used a one-way ANOVA to evaluate
differences in AUC
G
based on study condition.
Supplementary Analysis
We conducted exploratory analyses to examine the
importance of children’s current and past ownership of
pets and of dogs in particular, experiences with dogs, and
their self-reported feelings and behaviors toward their dogs.
For this analysis, we focused only on participants in the
4
After Participant 44, random assignment was split up by gender (i.e.,
there were separate random assignment lists for male and female partici-
pants) to ensure similar proportions of male and female participants in
each condition.
EFFECTS OF DOGS ON ANXIETY, AFFECT, AND AROUSAL
7

experimental condition. We used Pearson’s partial correla-
tions to examine the relations between the continuous ani-
mal experience variables (EDI, CABS) and each self-report
outcome (PANAS-C-S, STAI-C), adjusting for baseline
scores on the same self-report measure. We additionally
used Pearson product–moment correlations to evaluate the
relations between AUC
G
and EDI and CABS scores. For
the pet ownership variables (current and past pet owner-
ship, current and past dog ownership), we used one-way
ANCOVAs to evaluate the effects on posttest scores on
each self-report measure, adjusting for baseline scores on
the same self-report measure and one-way ANOVAs to
evaluate the effects of these pet ownership variables on
AUC
G
.
RESULTS
Preliminary Analysis
Participants in the three conditions did not differ in terms of
age, race/ethnicity, sex, current or past dog ownership, EDI
scores, or CABS scores (ns).
5
In light of the low number of
participants who did not have pets at the time of participa-
tion (n = 11) or in the past (n = 13), the sample size was
insufficient to evaluate whether the conditions differed in
terms of current and/or past pet ownership of participants.
Frequencies of current and past pet ownership are presented
in Table 1. Participants in the different conditions did not
differ at baseline on the Positive Affect scale, the STAI-C,
or cortisol (ns). Parent reports of participants’ positive
affect “during the past few weeks” using the PANAS-C-P
also did not differ by condition (ns). However, parent
reports on the Negative Affect scale of the PANAS-C-P
did vary across conditions, F(2, 74) = 5.87, p = .004, η
2
p
= 0.14. Parent-reported Negative Affect scale scores were
higher for participants in the waiting control condition
(M = 9.85, SD = 4.05) than for participants in the experi-
mental condition ( M = 7.42, SD = 1.90), a mean difference
of 2.42 points, 95% CI [0.79, 4.05], p = .004, d = 0.77.
Scores for participants in the waiting control condition
were also higher than for those in the tactile-stimulation
control condition (M = 7.40, SD = 2.45), a mean difference
of 2.45 points, 95% CI [0.80, 4.09], p = .004, d = 0.73.
However, parent-reported Negative Affect scores were not
significantly correlated with change in Positive Affect scale
scores, r(73) = .06, p = .623, change in STAI-C scores, r
(73) = −.05, p = .690, or AUC
G
, r(73) = .07, p = .582. As a
result, and in consideration of the fact that self-reports on
the Negative Affect scale were excluded as an outcome
measure, parent-reported Negative Affect was not included
in subsequent analyses.
In terms of the relations among baseline measures,
Positive Affect scores were negatively correlated with
STAI-C scores, r(75) = −.78, p < .001. The magnitude of
this correlation raised concerns about the possibility of
redundancy of measures. However, change in Positive
Affect scores and change in STAI-C scores from baseline
to posttest were only moderately negatively correlated, r
(74) = −.46, p < .001. We consequently retained the
Positive Affect scale and STAI-C as separate measures.
Baseline salivary cortisol was not significantly correlated
with baseline Positive Affect scale scores, r(72) = −.01,
p = .926, or STAI-C scores, r(72) = .03, p = .800. Within
the experimental condition, there were no significant differ-
ences in change in Positive Affect scores, F(7, 17) = 1.12,
p = .396, η
2
p
= 0.32; change in STAI-C scores, F(7,
17) = 0.96, p = .488, η
2
p
= 0.28; or AUC
G
, F(7, 17) = 0.79,
p = .603, η
2
p
= 0.25, based on the dog with which participants
interacted.
Effects of the Interactions
Baseline scores and unadjusted posttest scores on each of
the outcome measures are presented in Table 2. The
ANCOVA evaluating the effect of the interactions on
Positive Affect scores revealed a significant effect of con-
dition, F(2, 72) = 4.37, p = .016, η
2
p
= 0.11. Planned
contrasts revealed that posttest Positive Affect scores for
participants in the experimental condition (M = 18.82,
SE = 0.60) were significantly higher than those of partici-
pants in the waiting control condition (M = 16.90,
SE = 0.59), a mean difference of 1.92 points, 95% CI
[0.24, 3.60], p = .025, d = 0.65.
6
Positive Affect scores
for participants in the experimental condition were also
significantly higher than those of participants in the tac-
tile-stimulation control condition (M = 16.48, SE = 0.59), a
mean difference of 2.35 points, 95% CI [0.66, 4.03],
p = .007, d = 0.79. Following exposure to a stressful task,
child participants who interacted with a dog showed higher
levels of positive affect than participants who received
tactile stimulation without any interaction or waited.
Detailed statistics for planned contrasts are presented in
Table 3.
5
Because the majority of the sample was White and cell sizes for all
ethnicities other than White were small (< 7), we collapsed across all
ethnicities other than White (Asian, Black/African American, Hispanic/
Latino, Native American/Alaska Native, and “other”) for the purposes of
this analysis. The total number of White participants was 44, and the total
number of non-White participants was 34.
6
Initially, we conducted the planned contrasts for the self-report out-
comes using a Bonferroni correction for multiple comparisons. However, a
reviewer raised the possibility that this approach might have been too
conservative, and we consequently removed the correction. When the
Bonferroni correction is applied, the comparison between participants in
the experimental condition and those in the waiting control condition is
not significant for the Positive Affect scale (Bonferroni corrected thresh-
old = .025). The results for the STAI-C are unchanged.
8 CROSSMAN ET AL.
There was also a significant effect of condition for
STAI-C scores, F(2, 72) = 4.69, p = .012, η
2
p
= 0.12.
Participants in the experimental condition (M = 26.47,
SE = 0.85) had significantly lower scores than participants
in the waiting control condition (M = 30.09, SE = 0.83), a
mean difference of 3.63 points, 95% CI [1.25, 6.01],
p = .003, d = 0.86. However, the difference between
STAI-C scores in the experimental condition and those in
the tactile-stimulation control condition (M = 28.72,
SE = 0.85) was not significant, p = .065, 95% CI [−0.14,
4.65], d = 0.53. These findings convey that interaction with
the dog did reduce anxiety relative to waiting without
intervention but raise questions about whether this anxio-
lytic effect is specific to interaction with a dog, or whether
tactile stimulation from any soothing object might convey a
similar benefit.
The ANOVA for AUC
G
failed to reveal a significant
effect of condition, F(2, 73) = 0.98, p =.381,η
2
p
= 0.03.
Participants who interacted with the dogs did not differ
significantly from participants in the other conditions in
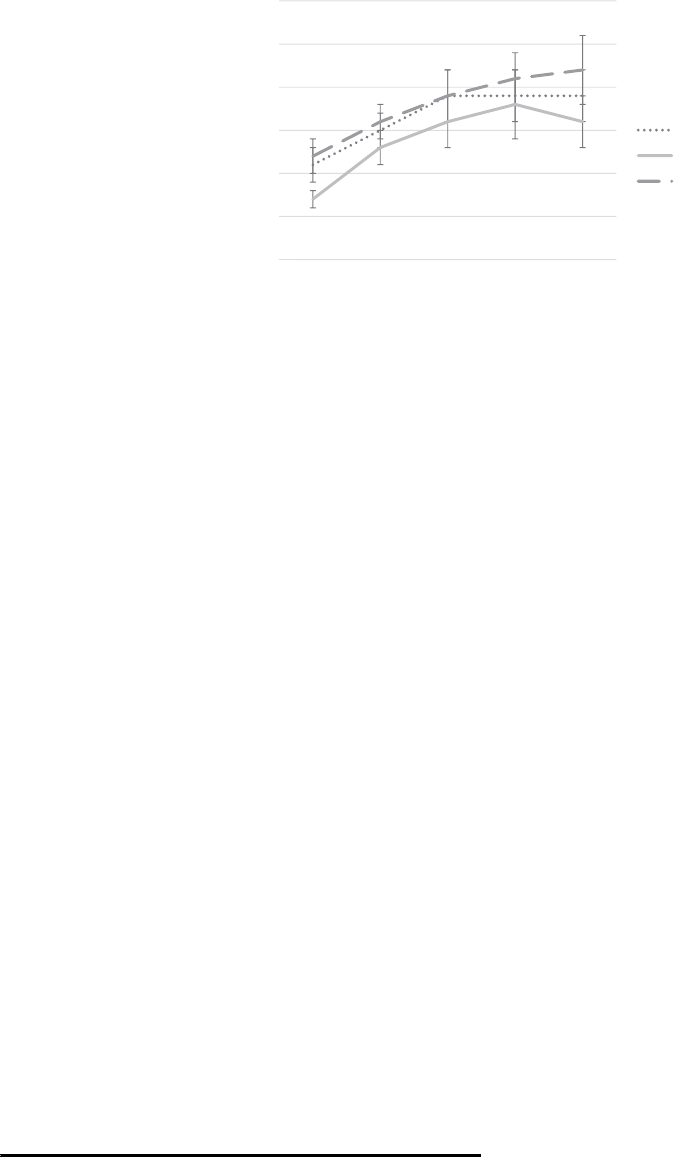
terms of overall physiological arousal. A visual depiction
of change in salivary cortisol concentration for each
condition is presented in Figure 1.
Supplementary Analysis
Among participants in the experimental condition, we
explored whether participants’ responses to the interactions
varied based on their EDI and CABS scores and based on
current and past pet ownership.
EDI
Among participants in the experimental condition, scores
on the Positive dimension of the EDI were significantly
correlated with posttest Positive Affect scale scores, adjust-
ing for baseline Positive Affect scale scores, r(21) = .42,
p = .047. However, the Positive dimension of the EDI was
not significantly correlated with either posttest STAI-C
scores (adjusting for baseline STAI-C scores), r(21) = −.23,
p = .288, or AUC
G
, r(22) = −.21, p = .329. Scores on the
negative dimension of the EDI were not associated with
posttest Positive Affect scores, r(21) = .16, p = .465, or
STAI-C scores (adjusting for baseline scores on the same
measures), r(21) = .05, p = .839. Surprisingly, however,
higher scores on the Negative dimension of the EDI were
associated with lower AUC
G
, r (22) = −.47, p =.021.
CABS
Adjusting for baseline scores, the CABS was not asso-
ciated with posttest Positive Affect scale scores, r(14) = .08,
p = .776, or STAI-C scores, r(14) = −.04, p = .897. The
CABS was also not associated with AUC
G
, r(14) = −.37,
p =.161.
Pet ownership
Participants who had pets at the time of participation
showed significantly higher Positive Affect scale scores at
posttest (M = 20.45, SE = 0.79) than participants who did not
have pets at the time of participation (M =15.89,SE = 1.88),
when adjusting for baseline Positive Affect scale scores,
F(1, 22) = 4.85, p =.038,η
2
p
= 0.18. However, STAI-C
scores, F(1, 22) = 0.25, p =.622,η
2
p
= 0.01 and AUC
G
,
F(1, 23) = 0.93, p =.346,η
2
p
= 0.04, did not vary based on
TABLE 2
Unadjusted Means and Standard Deviations for Primary Outcome
Measures at Baseline and Posttest
Measures:
M (SD)
Full
Sample
(N = 78)
Dog
(n = 26)
Tactile Stimulation
Control (n = 26)
Waiting
Control
(n = 26)
Baseline
Scores
PANAS-
C-S:
Positive
17.58
(5.22)
18.69
(4.76)
17.40 (4.86) 16.65 (5.96)
STAI-C 28.08
(4.66)
27.15
(3.84)
28.32 (4.85) 28.77 (5.22)
Cortisol
(μg/dL)
0.10 (0.09) 0.11
(0.10)
0.07 (0.04) 0.12 (0.11)
Posttest
Scores
PANAS-
C-S:
Positive
17.39
(5.51)
19.72
(5.18)
16.36 (4.65) 16.15 (6.03)
STAI-C 28.64
(5.86)
25.80
(4.94)
29.42 (5.43) 30.58 (6.24)
Cortisol
(μg/dL)
0.19 (0.18) 0.19
(0.17)
0.16 (0.17) 0.22 (0.20)
Note. PANAS-C-S = Positive and Negative Affect Schedule for
Children, Short Form; STAI-C = State/Trait Anxiety Inventory for
Children (state portion).
TABLE 3
Planned Contrasts for Self-Report Outcomes
95% CI
Outcome Contrast
M
Difference LL UL p
Cohen’s
d
PANAS-C-S:
Positive
Dog vs. tactile
stimulation
2.35 0.66 4.03 .007 0.79
Dog vs. waiting 1.92 0.24 3.60 .025 0.65
STAI-C Dog vs. tactile
stimulation
2.25 −0.14 4.65 .065 0.53
Dog vs. waiting 3.63 1.25 6.01 .003 0.86
Note: Mean differences refl ect differences in adjusted posttest means
(posttest scores, adjusted for baseline on the same measure). Cohen’s d
was calculated using adjusted means and the square root of the mean
square error. CI = confidence interval; LL = lower limit; UL = upper limit;
PANAS-C-S = Positive and Negative Affect Schedule for Children, Short
Form; STAI-C = State/Trait Anxiety Inventory for Children (state portion).
EFFECTS OF DOGS ON ANXIETY, AFFECT, AND AROUSAL
9
current pet ownership. Neither Positive Affect scale scores,
F(1, 22) = 3.02, p =.096,η
2
p
= 0.12; nor STAI-C scores,
F(1, 22) = 0.14, p =.710,η
2
p
=0.01;norAUC
G
,
F(1, 23) = 3.26, p =.084,η
2
p
= 0.12, varied based on past
pet ownership. Positive Affect scale scores, F(1, 22) = 0.04,
p = .838, η
2
p
= 0.002; STAI-C scores, F(1, 22) = 0.04,
p =.844,η
2
p
= 0.002; and AUC
G
, F(1, 23) = 0.36, p = .553,
η
2
p
= 0.02, also did not vary based on current dog ownership.
Finally, Positive Affect scale scores, F(1, 22) = 0.08,
p = .787, η
2
p
= 0.003; STAI-C scores, F(1, 22) = 0.99,
p =.331,η
2
p
= 0.04; and AUC
G
, F(1, 23) = 0.06, p = .805,
η
2
p
= 0.003, did not vary based on past dog ownership.
7
DISCUSSION
We found that child participants who engaged in a brief,
unstructured interaction with an unfamiliar dog after expo-
sure to a moderate stressor showed higher positive affect,
relative to participants who received a soothing object or
waited for the same amount of time. Participants who inter-
acted with a dog also showed lower anxiety relative to those
who waited for the same amount of time but not relative to
those who received a soothing object. These effects on
positive mood and anxiety were medium in magnitude.
However, we did not detect any effect of the interactions
with the dogs on physiological arousal, as measured by
salivary cortisol. Overall, our findings support the effects
of brief, unstructured interactions with dogs on subjective
but not physiological indicators of affect and anxiety.
Interaction with a dog produced increases in positive
affect that exceeded the effects of tactile stimulation from a
soothing object or waiting for the same amount of time. This
finding suggests that the effects of interaction with a dog on
positive affect are not simply attributable to the effects of
tactile stimulation. Put another way, interaction with a dog
produced benefits for positive affect that exceeded the effects
of another commonly used coping strategy. However, in the
case of anxiety, the effects of the experimental condition
exceeded those of waiting but not of tactile stimulation.
This suggests that although interaction with a dog reduces
anxiety relative to children’s independent coping abilities,
children may experience similar benefits from a comforting
object that provides tactile stimulation. To summarize, we
found that interaction with a dog conveys some benefits
beyond the effects of tactile stimulation from a soothing
object. However, our findings also highlight the importance
of continuing to question what it is about the interactions
that drives the benefits (Marino, 2012).
We did not detect any effect of the interactions with the
dogs on salivary cortisol. There are two main possible inter-
pretations of this finding. First, it is possible that the inter-
actions did affect cortisol but that we failed to detect this
effect. We observed a high degree of variability in cortisol
output within each condition, which may have obscured any
effects of the interactions on cortisol. In addition, in this
study the final cortisol sample was collected approximately
41 min after the onset of the stressor (and 23 min after the
conclusion of the stressor). At that final time point, mean
cortisol levels had not returned to baseline, and it is possible
that we would have detected differences in the rate at which
cortisol returned to baseline with a longer period of interac-
tion and assessment. However, we view this possibility as
unlikely, as visual inspection of the cortisol data suggests
0
0.05
0.1
0.15
0.2
0.25
0.3
T1 T2 T3 T4 T5
Salivary Cortisol ( µg/dL )
Time Point
Dog
Tactile Stimulation
Waiting
FIGURE 1 Salivary cortisol concentration, broken down by study condition. Note: Error bars represent standard error.
7
As with the main analysis, we initially conducted the supplementary
analyses using a Bonferroni correction for multiple comparisons. However,
at the suggestion of a reviewer, we have reported the results of the supple-
mentary analyses without the correction for multiple comparisons in order
to reveal all possibly meaningful findings to inform future hypothesis
testing. When the Bonferroni correction is applied, none of the supplemen-
tary analyses are significant (Bonferroni corrected threshold = .007).
10 CROSSMAN ET AL.
that the tactile-stimulation control condition (rather than the
experimental condition) was on track to recover most
quickly of the three conditions.
The second possible interpretation is that the interactions
in our investigation did not reduce overall cortisol output.
Findings from prior studies indicate that interaction with a
dog can buffer against increases in cortisol when the inter-
action occurs during stress exposure (e.g., Beetz et al.,
2012; Vagnoli et al., 2015). However, those stress-buffering
effects may not extend to postexposure cortisol reduction.
In other words, interactions with animals may affect corti-
sol reactivity but not cortisol recovery, as these are distinct
processes (Linden, Earle, Gerin, & Christenfeld, 1997;
Ramsay & Lewis, 2003). In addition, the fact that interac-
tions with dogs appeared to affect subjective but not phy-
siological indicators of stress in the context of our
investigation is not necessarily surprising, given that
many factors (e.g., cognitive appraisal, time course) may
contribute to the generally low covariance between salivary
cortisol and subjective reports of stress (Hellhammer, Wüst,
& Kudielka, 2009). It is plausible that the interactions with
the dogs caused participants to reappraise their experiences
of the stress task without altering their already elevated
levels of cortisol or their natural patterns of cortisol recov-
ery. Prior studies have also used different control conditions
(e.g., interaction with a person) than we used in this study;
it may be that the effects of interactions with dogs on
cortisol documented in prior studies are due primarily to
the tactile stimulation the dogs provide rather than to the
interactions with the dogs per se. Finally, consistent with
our findings, a number of other studies have also failed to
detect effects of interactions with dogs on cortisol (e.g.,
Handlin et al., 2011; Kaminski et al., 2002).
Among participants who interacted with the dogs in our
study, tho se who had more extensive histo ries of previous
interactions with pets, and with dogs in particular, seemed to
benefitmost.Specifically, compared to children who did not
have pets, children who had pets at the time of participation
showed higher levels of posttest positive affect. Similarly,
children who were reported to have had more positive
experiences with dogs in the past had higher levels of
posttest positive affect. It may be that children who have
pets and/or have histories of positive experiences with dogs
are more prepared to benefit from the interactions, or else
that these factors are simply indicators that a child has a
particular affinity for pets. In addition, and somewhat sur -
prising, children who were reported to have had more nega-
tive experiences with dogs in the past showed lower overall
arousal in response to the interactions with the dogs. It is
possible that children who have had negative experiences
with dogs in the past show less excitement in response to
the interactions, or else that these children show less antici-
patory arousal precisely because they have already been
exposed to some adverse events related to dogs and have
developed a sense of mastery through those experiences.
However, none of the other measures of children’s feelings
toward and experiences with dogs were associated with
improvement on any of the three outcome measures. Thus,
it is possible that these findings represent false positives,
highlighting the need for further research to directly investi-
gate th e ch aracteristics o f children who are most likely to
benefit from AAAs.
The present findings extend the literature on the effects of
interactions with dogs on children’s affect and anxiety in
three key ways. First, participants in our study interacted
with the dogs independently and without distractions, sug-
gesting that something about the interactions with the dogs
themselves produced the benefits.Thisisnotablebecausea
failure to disentangle the effects of interactions with animals
from the effects of interactions with other people has been a
key limitation of previous studies and an important threat to
the construct validity of AAAs. Second, these findings
demonstrate that, at least for positive affect, the benefits of
AAAs are not attributable entirely to the effects of tactile
stimulation. This is important in light of prior observational
findings that show associations between the amount of touch
that occurs during an AAA and the degree of improvement
shown by the child. In addition, this finding makes a small
but important contribution to efforts to establish the extent to
w
hich the effects of AAAs can really be attributed to the
interactions with the animals specifically. Finally, our find-
ings demonstrate that AAAs can alleviate the negative
effects of stress on affect and anxiety after stress exposure,
in addition to preventing increases during exposure.
We demonstrated these effects in an unselected commu-
nity sample that was relatively diverse in terms of race,
gender, and prior experience with and ownership of pets
and that showed levels of baseline anxiety and affect that
were similar to those other community samples. As detailed
earlier , we elected to use a community sample for two
reasons. First, the primary goal of this investigation was to
provide a carefully controlled demonstration of the effects of
interactions with dogs for children. The goal was not to
establish the effects of these in teractions for any particular
disorder or population but rather to contribute to efforts to
begin to understand the basic processes involved. Second,
our interest in AAAs as a strategy for alleviating children’s
suffering comes in part from the possibility that AAAs may
be used to reach a broad range of children. Thus, demon-
strating the effects of AAAs among a community sample of
children is a strength, because it provides initial evidence for
the effects of AAAs when they are applied broadly.
Our study bears three important limitations. First, we eval-
uated only immediate changes in affect, anxiety, and arousal,
and we did not address whether any of the observed effects are
sustained in the long term. We focused on short-term changes
in light of the emerging state of this literature, as well as the
importance of even short-term symptom relief. As our
EFFECTS OF DOGS ON ANXIETY, AFFECT, AND AROUSAL 11
understanding of the strength and nature of these effects is
refined, it will be important to establish how long they are
sustained and what impact they have on mental health and
well-being in the long term. Second, due to the logistical
factors described earlier, eight dogs participated in the present
study, and casual observations by experimenters suggested
that there were differences in the nature of the interactions
with the different dogs (e.g., some engaged in more active
play, whereas others were more likely to sit with the partici-
pant and be petted throughout the interaction). As a result, it is
possible that there were undetected differences in the strength
of the effects of the intervention, depending on the dog with
which participants interacted. Third, we evaluated the influ-
ence of interactions with dogs among participants who volun-
teered to take part in a study involving dogs, introducing the
possibility of a selection effect. As a result, the benefits of
interactions with dogs observed in this study, and especially
the findings relating to participants feelings toward and
experiences with pets, may not necessarily hold for partici-
pants who have highly negative views toward dogs or no
experience at all with dogs. However, children who partici-
pate in AAAs in applied settings are also likely to have
positive attitudes toward and experiences with dogs.
The present findings suggest two important avenues
for future r esearch. First, it will be important to continue
to establish how (i.e., through what processes) interac-
tions with dogs improve affect and anxiety symptoms.
Commonly proposed mechanisms of action include tac-
tile stimulation, social support, and emotional contagion.
Studies are needed that manipulate these various pro-
cesses or use established paradigms from the study of
interpersonal relationships to investigate them. This
research will help to clarify the specificbenefits of inter-
actions with animals and inform stra tegies for maximiz-
ing those benefits.
The second area for future research stems from observa-
tions by experimenters in our studies that the different dogs
appeared to behave differently. Specifically, it will be
important for future research to evaluate whether certain
characteristics of the dogs are more effective for improving
affect and anxiety. For example, are more active dogs who
initiate more contact and interaction more engaging and
enjoyable to interact with, or do more relaxed dogs promote
more relaxation in their human interaction partners? Other
characteristics of potential importance include the size and
age of the dogs, the amount of eye contact they naturally
initiate, their responsiveness to their names or other over-
tures, and the softness of their coats. Of course, it is not
necessarily the case that certain characteristics are better for
improving affect and anxiety, whereas others are worse;
instead, it may be that certain characteristics are more
effective for certain individuals, under different circum-
stances, or with different goals in mind. We can think of
this area of research as the canine version of long-standing
efforts in psychotherapy research to tailor treatments to
individual clients and situations.
SUMMARY AND CONCLUSION
We evaluated the influence of brief, unstructured interactions
with dogs on children’s affect, anxiety, and arousal, following
exposure to a stressful task. These interactions increased
children’s positive affect, relative to waiting without any
intervention or receiving tactile stimulation from a soothing
object, and reduced children’s state anxiety relative tactile
stimulation. We did not detect any effect on physiological
arousal. These findings convey that brief, unstructured inter-
actions with dogs have a moderate impact on children’s
subjective experiences of anxiety and affect, following expo-
sure to a stressor. However, additional research is needed to
establish whether the benefits of interactions with animals
exceed the effects of other common strategies for improving
mental health and alleviating stress. The present findings
support the notion that AAAs may be an efficient strategy
for improving children’s mental health but highlight the fact
that there is not yet sufficient evidence to support their
already widespread prevalence in practice.
ACKNOWLEDGEMENT
The primary
findi
ngs reported in this article are based on data
also reported in the doctoral dissertation of the first author . We
additionally thank the Good Dog Foundation for their assistance
with this study , as well as the dogs and handlers who generously
volunteered their time to participate , and without whom this
study would not have been possible. Dr. Rajita Sinha addition-
ally provided essential assistance with the analysis of the corti-
sol data.
FUNDING
This study, in whole or in part, was funded by the Morris
Animal Foundation exclusively from a partnership with the
Human-Animal Bond Research Institute (HABRI; D15HA-
025). Additional support for this study was provided by the
Laura J. Niles Foundation and the Humane Society of the
United States.
REFERENCES
Ardiel, E. L., & Rankin, C. H. (2010). The importance of touch in
development. Paediatrics & Child Health, 15, 153–156. doi:10.1093/
pch/15.3.153
Balzás, J., Miklósi, M., Keresztény, A., Hoven, C., Carli, V., Wasserman,
C., … Wasserman, D. (2013). Adolescent subthreshold-depression and
12 CROSSMAN ET AL.
anxiety: Psychopathology, functional impairment and increased suicide
risk. Journal of Child Psychology and Psychiatry, 54, 670–677.
doi:10.1111/jcpp.12016
Barker, S. B., Knisely, J. S., Schubert, C. M., Green, J. D., & Ameringer,
S. (2015). The effect of an animal-assisted intervention on anxiety and
pain in hospitalized children. Anthrozoös, 28, 101–112. doi:10.2752/
089279315X14129350722091
Beetz, A., Julius, H., Turner , D., & Kotrschal, K. (2012). Effects of social
support by a dog on stress modulation in male children with insecure
attachment. Frontiers in Psychology , 3,1–9. doi:10.3389/fpsyg.2012.00001
Beetz, A., Kotrschal, K., Turner, D. C., Hediger, K., Uvnäs-Moberg, K., &
Julius, H. (2011). The effect of a real dog, toy dog and friendly person on
insecurely attached children during a stressful task: An exploratory study.
Anthrozoös, 24,349–368. doi:10.2752/175303711X13159027359746
Braun, C., Stangler, T., Narveson, J., & Pettingell, S. (2009). Animal-
assisted therapy as a pain relief intervention for children.
Complementary Therapies in Clinical Practice, 15, 105–109.
doi:10.1016/j.ctcp.2009.02.008
Buske-Kirschbaum, A., Jobst, S., Wustmans, A., Kirschbaum, C., Rauh,
W., & Hellhammer, D. (1997). Attenuated free cortisol response to
psychosocial stress in children with atopic dermatitis. Psychosomatic
Medicine, 59, 419–426.
Centers for Disease Control. (2013). Mental health surveillance among
children—United States, 2005-2011. Morbidity and Mortality Weekly
Report: Supplement, 62,1–35.
Chambless, D. L., & Hollon, S. D. (2012). Treatment validity for inter-
vention studies. In H. Cooper (Ed.), APA Handbook of Research
Methods in Psychology: Vol 2. Quantitative, Neuropsychological, and
Biological (pp. 1–24). Washington, D.C.: American Psychological
Association.
Cohen, S. P. (2002). Can pets function as family members? We ster n Journal
of Nursing Research, 24,621–63
8. doi:10.1177/019394502320555386
Crossman, M. K. (2017). Effects of interactions with animals on human
psychological distress. Journal of Clinical Psychology, 73, 761–784.
doi:10.1002/jclp.22410
Crossman, M. K., & Kazdin, A. E. (2015). Animal visitation programs in
colleges and universities: An efficient model for reducing student stress. In
Handbook on animal-assisted therapy: Foundations and guidelines for
animal-assisted interventions (4th ed., pp. 333–337). Waltham, MA:
Elsevier.
Crossman, M. K., Kazdin, A. E., & Knudson, K. (2015). Brief unstruc-
tured interaction with a dog reduces distress. Anthrozoös, 28, 649–659.
doi:10.1080/08927936.2015.1070008
Ebesutani, C., Okamura, K., Higa-McMillan, C., & Chorpita, B. F. (2011).
A psychometric analysis of the positive and negative affect schedule for
children–parent version in a school sample. Psychological Assessment,
23, 406–416. doi:10.1037/a0022057
Ebesutani, C., Regan, J., Smith, A., Reise, S., Higa-McMillan, C., &
Chorpita, B. F. (2012). The 10-item positive and negative affect sche-
dule for children, child and parent shortened versions: Application of
item response theory for more efficient assessment. Journal of
Psychopathology and Behavioral Assessment, 34, 191–203.
doi:10.1007/s10862-011-9273-2
Erskine, H. E., Moffitt, T. E., Copeland, W. E., Costello, E. J., Ferrari, A.
J., Patton, G., … Scott, J. G. (2015). A heavy burden on young minds:
The global burden of mental and substance use disorders in children and
youth. Psychological Medicine, 45, 1551–1563. doi:10.1017/
S0033291714002888
Faul, F., Erdfelder, E., Lang, A., & Buchner, A. (2007). G*Power 3: A
flexible statistical power analysis program for the social, behavioral, and
biomedical sciences. Behavior Research Methods, 39, 175–191.
doi:10.3758/BF03193146
Fl
annery-Schroeder, E. C., & Kendall, P. C. (2000). Group and individual
cognitive-behavioral treatments for youth with anxiety disorders: A
randomized clinical trial. Cognitive Therapy and Research, 24,251–
278. doi:10.1023/A:1005500219286
Friesen, L. (2010). Exploring animal-assisted programs with children in
school and therapeutic contexts. Early Childhood Education Journal,
37, 261–267. doi:10.1007/s10643-009-0349-5
Gulliver, A., Griffiths, K. M., & Christensen, H. (2010). Perceived barriers
and facilitators to mental health help-seeking in young people: A systema-
tic review. BMC Psychiatry, 10,113–1 22. doi:10.1 186/1471-24 4X-10-113
Gunnar, M. R., Talge, N. M., & Herrera, A. (2009). Stressor paradigms in
developmental studies: What does and does not work to produce mean
increases in salivary cortisol. Psychoneuroendocrinology, 34, 953–967.
doi:10.1016/j.psyneuen.2009.02.010
Handlin, L., Hydbring-Sandberg, E., Nilsson, A., Ejdebäck, M., Jansson,
A., & Uvnäs-Moberg, K. (2011). Short-term interaction between dogs
and their owners: Effects on oxytocin, cortisol, insulin and heart rate—
An exploratory study. Anthrozoös, 24, 301–315. doi: 10.2752/
175303711X13045914865385
Hankin, B. L., Badanes, L. S., Abela, J. R. Z., & Watamura, S. E. (2010).
Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children
and adolescents: Cortisol reactivity to psychosocial stress from preschool
through middle adolescence. Biological Psychiatry, 68,484–490.
doi:10.1016/j.biopsych.2010.04.004
Hellhammer,D.H.,Wüst,S.,&Kudielka,B.M.(2009). Salivary cortisol as
a biomarker in stress research. Psychoneuroen docrinology, 34, 163–171.
doi:10.1016/j.psyneuen.2008.10.026
Hexdall, C. M., & Huebner, E. S. (2008). Subjective well-being in pedia-
tric oncology patients. Applied Research in Quality of Life, 2, 189–208.
doi:10.1007/s1
1482-008-9037-7
International Association of Human– Animal Interaction Organizations.
(2013). IAHAIO white paper: The IAHAIO definitions for animal
assisted intervention and animal assisted activity and guidelines for
wellness of animals involved. Chicago, IL: Author.
Kaminski, M., Pellino, T., & Wish, J. (2002). Play and pets: The physical
and emotional impact of child-life and pet therapy on hospitalized
children. Children’s Health Care, 31, 321–335. doi:10.1207/
S15326888CHC3104_5
Kataoka, S. H., Zhang, L., & Wells, K. B. (2002). Unmet need for mental
health care among U.S. children: Variation by ethnicity and insurance
status. American Journal of Psychiatry, 159, 1548–1555. doi:10.1176/
appi.ajp.159.9.1548
Kendall, P. C. (1994). Treating anxiety disorders in children: Results of a
randomized clinical trial. Journal of Consulting and Clinical
Psychology, 62, 100 –110.
Kerns, K. A., Stuart-Parrigon, K. L., Coifman, K. G., Van Dulmen, M. H.
M., & Koehn, A. (2017). Pet dogs: Does their presence influence
preadolescents’ emotional response to a social stressor? Social
Development. doi:10.1111/sode.12246
Kertes, D. A., Liu, J., Hall, N. J., Hadad, N. A., Wynne, C. D. L., & Bhatt,
S. S. (2017). Effect of pet dogs on children’s perceived stress and
cortisol stress response. Social Development, 26, 382–401.
doi:10.1111/sode.12203
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., &
Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions
of DSM-IV disorders in the National Comorbidity Replication. Archives
of General Psychiatry, 62,593–602. doi:10.1001/archpsyc.62.6.593
Khalfa, S., Bella, S. D., Roy, M., Peretz, I., & Lupien, S. J. (2003). Effects
of relaxing music on salivary cortisol level after psychological stress.
Annals of the New York Academy of Sciences, 999,
374–376.
Khoury, J. E., Gonzalez, A., Levitan, R. D., Pruessner, J. C., Chopra, K.,
Santo Basile, V., … Atkinson, L. (2015). Summary cortisol reactivity
indicators: Interrelations and meaning. Neurobiology of Stress, 2,34–43.
doi:10.1016/j.ynstr.2015.04.002
Kirschbaum, C., & Hellhammer, D. H. (1989). Salivary cortisol in psycho-
biological research: An overview. Neuropsychobiology, 22,150–169.
doi:10.1159/000118611
EFFECTS OF DOGS ON ANXIETY, AFFECT, AND AROUSAL
13
Krause-Parello, C. A., Thames, M., Ray, C. M., & Kolassa, J. (2018).
Examining the effects of a service-trained facility dog on stress in
children undergoing forensic interview for allegations of child sexual
abuse. Assessment and Interviewing, 27, 305–320.
Kruger , K. A., Trachtenber g, S. W., & Serpell, J. A. (2004). Can animals help
humans heal? Animal-assisted interventions in adolescent mental health.
Philadelphia, PA: University of Pennsylvania School of Veterinary Medicine.
Linden, W., Earle, T. L., Gerin, W., & Christenfeld, N. (1997).
Physiological stress reactivty and recovery: Conceptual siblings sepa-
rated at birth? Journal of Psychosomatic Research, 42,117–135.
Marino, L. (2012). Construct validity of animal-assisted therapy and
activities: How important is the animal in AAT? Anthrozoös, 25,
s139–s151. doi:10.2752/175303712X13353430377219
Muris, P., Rapee, R., Meesters, C., Schouten, E., & Geers, M. (2003).
Threat perception abnormalities in children: The role of anxiety dis-
orders symptoms, chronic anxiety, and state anxiety. Anxiety Disorders,
17, 271–287. doi:10.1016/S0887-6185(02)00199-8
Nimer, J., & Lundahl, B. (2007). Animal-assisted therapy: A meta-analy-
sis. Anthrozoös, 20, 225–238. doi:10.2752/089279307X224773
Poresky, R. H., Hendrix, C., Mosier, J. E., & Samuelson, M. L. (1987).
The CABS: Internal reliability and construct validity. Psychological
Reports, 60, 743–746. doi:10.2466/pr0.1987.60.3.743
Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H.
(2003). Two formulas for computation of the area under the curve
represent measures of total hormone concentration versus time-depen-
dent change. Psychoneuroendocrinology, 28, 916–931.
Ramsay, D., & Lewis, M. (2003). Reactivity and regulation in cortisol and
behavioral responses to stress. Child Development, 74, 456–464.
Reddy, R., Palmer, C. A., Jackson, C., Farris, S. G., & Alfano, C. A.
(2017).
Impact of sleep restriction versus idealized sleep on emotional
experience, reactivity and regulation in healthy adolescents. Journal of
Sleep Research, 26, 516–525. doi:10.1111/jsr.12484
Seligman, L. D., Ollendick, T. H., Langley, A. K., & Baldacci, H. B.
(2004). The utility of measures of child and adolescent anxiety: A meta-
analytic review of the revised children’s manifest anxiety scale, the
state-trait anxiety inventory for children, and the child behavior
checklist. Journal of Clinical Child & Adolescent Psychology, 33,
557–565. doi:10.1207/s15374424jccp3303_13
Souter, M. A., & Miller, M. D. (2007). Do animal-assisted activities
effectively treat depression? A meta-analysis. Anthrozoös, 20, 167–
180. doi:10.2752/175303707X207954
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G.
A. (1973). State-trait anxiety inventory for children: Manual, instru-
ment, and scoring guide. Menlo Park, CA: Mind Garden, Inc.
Stewart, A., & Strickland, O. (2013). The companion animal in a work
situation: The roles of task difficulty and prior companion-animal guar-
dianship in state anxiety. Society & Animals, 21, 249–265. doi:10.1163/
15685306-12341287
Triebenbacher, S. L. (1999). Re-evaluation of the CABS. Anthrozoös, 12,
169–173. doi:10.2752/089279399787000200
Tsai, C., Friedmann, E., & Thomas, S. A. (2010). The effects of animal-
assisted therapy on stress responses in hospitalized children.
Anthrozoös, 23, 245–258. doi:10.2752/175303710X12750451258977
U.S. Census Bureau (2016). New Haven County, Connecticut; New Haven
city, Connecticut. Re trieved from https://www.census.gov/quickfacts/
fact/table/newhavencountyconnecticut,newhavencityconnecticut/
PST045216
Vagnoli, L., Caprilli, S., Vernucci, C., Zagni, S., Mugnai, F., & Messeri, A.
(2015). Can presence of a dog reduce pain and distress in children
during venipuncture? Pain Management Nursing, 16,8
9–95.
doi:10.1016/j.pmn.2014.04.004
Van Breukelen, G. J. P. (2006). ANCOVA versus change from baseline
had more power in randomized studies and more bias in nonrandomized
studies. Journal of Clinical Epidemiology, 59, 920 –925. doi:10.1016/j.
jclinepi.2006.02.007
Vickers, A. J., & Altman, D. G. (2001). Analysing controlled trials with
baseline and follow up measurements. BMJ, 323, 1123–1124.
Zhang, S., Paul, J., Nantha-Aree, M., Buckley, N., Shahzad, U., Cheng, J.,
… Thabane, L. (2014). Empirical comparison of four baseline covariate
adjustment methods in analysis of continuous outcomes in randomized
controlled trials. Clinical Epidemiology, 6, 227–235. doi:10.2147/CLEP.
S56554
14 CROSSMAN ET AL.
