© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 1 of 9
PAEDIATRIC INTENSIVE CARE – CLINCIAL PRACTICE GUIDELINE
DIABETIC KETOACIDOSIS PROTOCOL
(Call consultant on admission)
1. Introduction
DKA is a potentially life-threatening medical emergency due to absolute or relative insulin deficiency
coupled with counter-regulatory hormones excess.
2. Aim
To provide a guideline for management of the patient presenting with DKA.
The objective of this guideline is to:
• Recognise DKA early
• Correct metabolic disturbances (dehydration, ketoacidosis, hyperglycemia)
• Prevent complications
• Identify and treat precipitating events
3. Parameters of the guideline:
These guidelines are intended for the management of children who present with DKA
4. Definition:
A. DKA: Diabetes Ketoacidosis
Clinical & Biochemical Criteria
Clinical history
Clinical signs
Biochemical
Polyuria, polydipsia,
polyphagia, wt loss or
abdominal pain
or vomiting
Varying degree of dehydration,
Kussmaul respiration,
fruity (acetone) smell,
altered sensorium
RBS >11mmol/1,
Venous Blood Gas (pH <7.3mmHg,
HCO
3
<15 mmol/l), ketonemia or
ketonuria and glycosuria
B. CBG – Capillary Blood Glucose
6. Emergency Management:
A. Resuscitation
Airway: If comatose, insert airways & NG tube
Breathing: Give oxygen via face mask (even if O2 Sat > 95% in RA)
Circulation: Insert IV cannula + IA line & take blood samples (see below)
Cardiac monitor (ECG for hypo/hyperkalemia)
+ IDC
If in shock, give 10ml/kg normal saline bolus ½-1hr, maximum of 30mls/kg to restore
circulation. (N.B. Discuss with the Consultant if the patient has received 30mls/kg)
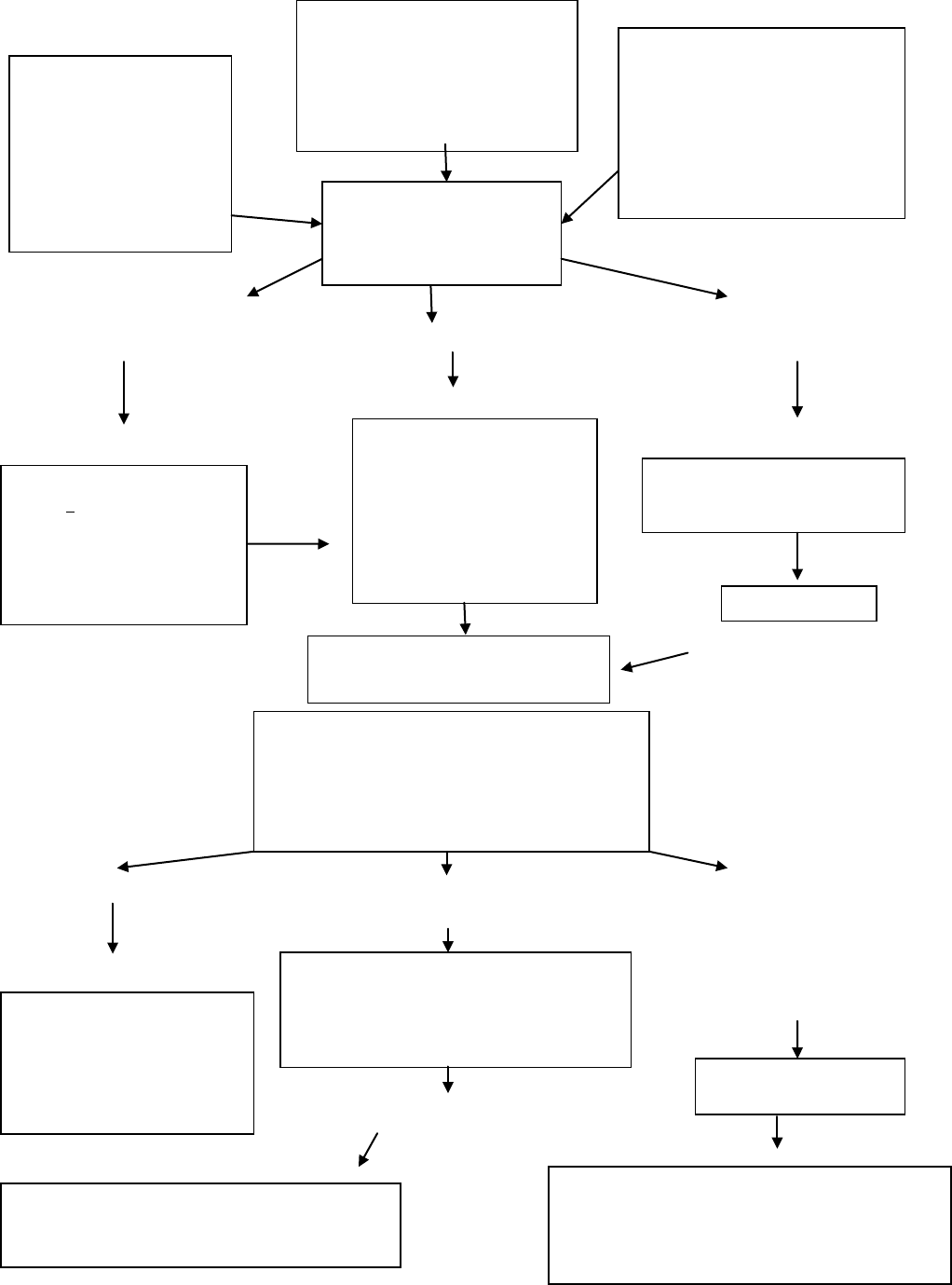
Algorithm for the management of diabetic ketoacidosis. Source: adapted from Dunger et al. Karger Publ. 1999
© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 2 of 9
Immediate assessment
Shocked (reduced peripheral pulses) Dehydration >5% Minimal
dehydration
Reduced conscious level/coma Not in shock Tolerating oral
fluids Acidotic (hyperventilation)
Vomiting
Acidosis not improved Blood glucose 17mmol/l Neurological deterioration
Or Warning signs:
Blood glucose falls >5mmol/l/h headache,
slowing heart rate, irritability,
decreased conscious level
Incontinence, specific
neurological
signs
Improvement
Clinically well, tolerating oral fluids
B. Initial investigation
• Blood glucose (formal sugar & CBG)
• Urea & electrolytes (electrolytes from blood gases can be used)
• Blood gases (venous give very similar pH & CO
2
to arterial)
Clinical History
Polyuria
Polydipsia
Wt loss (Weigh)
Abdominal pain Weakness
Vomiting
Confusion
Clinical Signs
Assess dehydration
Deep sighing respiration (Kussmaul)
Smell of ketones
Lethargy/drowsy +/- vomiting
Biochemical features & investigation
Ketones in urine
Elevated blood glucose
Acidaemia
Blood gases urea, electrolytes Others
investigations as indicated
Diagnosis confirmed
Diabetic Ketoacidosis
Call senior staff/Consultant.
Resuscitation
Airway + NG tube
Breathing (100% O
2
)
Circulation (0.9% saline 10-20
ml/kg over 1-2h & repeat until
circulation is restored) but do
not exceed 30ml/kg
IV Therapy
Calculates fluid requirement
Correct over 48 hrs
Saline 0.9%
ECG for T wave changes
Add KCL 40mmol per litre fluid
Therapy
Start with SC insulin. Continue
oral fluids
Continuous insulin infusion
0.1ml/kg/hr
Clinical observations
Hourly blood glucose
Hourly fluids input & output
Neurological status at least hourly
Electrolytes at least 2 hourly after start of IV therapy
Monitor ECG for T-wave changes
IVF Therapy
Change to 0.45% saline + 5% glucose
Adjust sodium infusion to promote an increase
in measured serum sodium
Re-evaluate
IV fluid calculations
Insulin delivery system & dose
need for additional resuscitation
Consider sepsis
Exclude hypoglycemia
Is it cerebral edema?
Management
Give mannitol 0.5-1g/kg , Restrict IV fluids by 1/3 , Call
senior staff
Move to ICU, Consider cranial imaging only after
patient is stable
Transition to SC insulin
Start SC insulin then stop IV insulin after 30 minutes
No improvement

© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 3 of 9
• HbA1c
• Urine ketones and glucose (N.B. Blood ketones is more superior to urine ketones)
• Islet cell antibodies, insulin antibodies, GAD antibodies, antiendomyseal lgA antibodies
and TFTs for all newly diagnosed patients.
• Other investigations only if indicated; FBC, Urinalysis, Chest X-Ray and Cultures
(blood, urine, throat, CSF) then give appropriate antibiotics
(N.B. leukocytosis is common in DKA and does not necessarily indicates sepsis, unless there is fever)
9. Management
A. Fluids
• Treat shock with bolus 10mls/kg 0.9% saline over 1/2-1hr, (max. of 30mls/kg), (if further
fluid boluses required at this stage, discuss with consultant
)
• After restoration of BP, all children with DKA and unequivocal signs of dehydration
should be given the following amount of IVF (based on maintenance
requirements of 80% of normal, and 3-5% of dehydration corrected over 48hrs)
irrespective of their apparent degree of dehydration (see table below):
Wt (kg)
5
6
7
8
9
10
12
14
16
18
20
25
30
35
40
45
(ml/hr)
24
28
32
36
40
45
50
55
65
70
75
80
90
95
105
110
(N.B. this fluid rate (ml/hr) includes deficit AND maintenance fluid needs)
• Fluid therapy should be reviewed if oliguria develops due to tubular necrosis caused by
severe hypotension before resuscitation may require fluid restriction. But persistent
hypovolemia may require extra fluids
• Initial fluids should be 0.9% saline or Lactated Ringers with 40mmol KCL in 1 L for the
1
st
48 hrs (see section on Potassium).
• Change IVF to 5% dextrose (add 100mls of D50% in 900mls 0.9 N/S), you can increase
the dextrose concentration to as high as 12.5% (discuss with consultant)
1
• Indications of adding glucose in the maintenance fluids:
Serum glucose is 14 mmol/l
Rapid drop in blood glucose level (>5-8 mmol/l per hr) even if the serum
glucose is >14 mmol/l
Ketacidosis remains despite correction of hyperglycemia
• Give 2-5ml/kg D10% bolus if CBG 3-4mmol/l
• Do not change the IVF to 0.45% saline if the corrected Na level does not rise
1
• Start oral fluids when clinically improved. (N.B these oral fluids should be subtracted from IVF if
still within 48 hrs)
B. Insulin
• No initial insulin bolus
• Start insulin after 1 hr of initial IVF
© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 4 of 9
• Add 50 units insulin “short acting” (regular) to 49.5mls of 0.9% saline to make 1units/ml
solution. Prime giving set prior to commencing infusion
• Run at 0.1unit/kg/hr, (N.B. in <5yrs and neonates start with 0.05unit/kg/hr).
• Correct ketoacidosis first before hyperglycemia.
• Do not reduce or stop insulin infusion, instead add glucose in the IVF (see Fluids
• )
• Blood glucose fall rate should be 3-5 mmol/l per hour
• Keep blood glucose at 8-12mmol/l
• The best time to change insulin infusion to SC is just before a meal, when the child is alert
and metabolically stable (glucose 8-12mmol/l, pH >7.3 & HCO3 >15). The insulin
infusion should only be stopped 30mins after the 1
st
SC insulin injection.
C. Potassium
• Start KCl at a concentration of 40-60mmol/l (40mmol if Body Wt <30kg, and 60mmol if
>30kg)
• Extreme care should be taken if the initial serum K is >5.5mmol/l or if the patient is
anuric. Check ECG monitor for peak T wavesECG monitor( Discuss with Consultant)
D. Bicarbonate
• Discuss with the Consultant.
E Monitoring & Observation
Hourly blood glucose
Hourly fluid input and output
Neurological status at least hourly
Electrolytes and blood gases 2 - 4hourly after start of IV therapy
BP, PR, RR 1-2 hourly & Temperature 4 hourly.
Urine ketones and glucose 4 hourly or every voids
Monitor ECG for T- wave changes
F. Complications of therapy
• Hypoglycemia
• Hypokalemia
• Aspiration pneumonia
• Cerebral oedema
10. Cerebral edema
• If suspected, exclude hypoglycemia and inform Consultant immediately.

© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 5 of 9
• Treatment:
1. Keep NBM, give 100% O
2
, and elevate the head of the bed by 30º
2. Reduce the rate of IVF to 2/3 of the calculated IVF
3. Give mannitol 0.5-1g/kg IV over 20 mins, may repeat if no initial response in 30
mins to 2hrs.
4. Hypertonic saline (2.7-3%) 5-10 ml/kg over 30mins may be an alternative or a
second line of therapy if no initial response to mannitol
5. Intubation and mechanical ventilation for impending repiratory failure, avoid
aggressive hyperventilation (keep PCO
2
at 30-35 mmHg)
11. Transition from Insulin infusion to Subcutaneous:
Step1. Determine the Total daily Insulin Requirement using a Sliding Scale
• Subcutaneous insulin therapy is initiated with regular (short acting) insulin given in a
dose of 0.2U/kg/dose, with the subsequent dose being adjusted every 6 h, depending
on the response as judged by the blood glucose levels and ketonuria
• Monitor CBG 4 times/day (pre-meals & after midnight)
• Use Table Appendix 2 Column 1, 2 & 5 for calculating and adjusting a sliding
scale
Step 2. Use Twice Daily Insulin Regimen
• Divide the Total daily Insulin Requirement into (2/3 before breakfast, 1/3 before
dinner)
Step 3. Determine components of each dose
• 2/3 of each dose as intermediate-acting (Isophane) insulin.
• 1/3 as short-acting (Regular)
• If using mixtard insulin, give 2/3 dose in the morning and 1/3 in the afternoon
NOTE:
• CBG can still be fluctuating even after a fixed insulin-meal regimens as calculated
above
• First exclude inter-current illness or stress etc.
• Use “10% rule” for insulin dose changes after determining the time at which
abnormal CBG occurs.
• Modify insulin doses by 10 %, e.g. if CBG is persistently out of range before dinner
then the dose modification applies only to the morning or lunch time insulin and
the amount is determined by 10% of the morning dose.
Appendix 1
• Anion gap: (12 + 2mmol/l): (Na + K) – (Cl + HCO3).
© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 6 of 9
• Osmolality: (280 – 300mmol/l): 2(Na + K) + Glucose(mmol/l) + Urea
• Corrected Na: measured Na + [2(plasma glucose (mmol/l) – 5.6)] ÷ 5.6 to calculate the
corrected Na use this link Tools\correctedNA.pdf
• Fluid calculation, use this link for calculating fluid rates;
http://www.bsped.org.uk/professional/guidelines /docs/DKACalculator.pdf
Appendix 2
TABLE1: Subcutaneous Insulin Dosing
BOLUS INSULIN
AGE
(YR)
TARGET
GLUCOSE
(MMOL/L)
TOTAL
DAILY
INSULIN
(U/KG/D)
*
BASAL
INSULIN, % OF
TOTAL DAILY
DOSE
Units Added per
5.5mmol/l above
Target
0–5
5.5-11.1
0.6–0.7
25–30
0.50
5–12
4.4-8.3
0.7–1.0
40–50
0.75
12–18
4.4-8.3
1.0–1.2
40–50
1.0–2.0
[‡]
*
Newly diagnosed children in the “honeymoon” may only need 60–70% of a full replacement dose.
Total daily dose per kg increases with puberty.
‡
For finer control, extra insulin may be added in 2.8mmol/l increments.
TABLE2: A 6 Hourly Sliding Scale for SC insulin
Age
(yrs)
Insulin
SC
CBG (mmol/l)
5-10
10-15
15-20
>20
0-5
Insulin
0.15U/kg/dose
0.15U/kg/dose +
0.5Unit
0.15U/kg/dose +
1Unit
GO BACK
TO INSULIN
INFUSION
5-12
Insulin
0.2U/kg/dose
0.2U/kg/dose +
0.75Unit
0.2U/kg/dose +
1.5Units
12-18
Insulin
0.25U/kg/dose
0.25U/kg/dose +
1Unit
0.25U/kg/dose +
2Units
(N.B. The above Sliding scale table is derived from Table 1)
Appendix 3: Diagnostic criteria for Cerebral Edema in children with DKA
1. Diagnostic criteria for cerebral edema

© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 7 of 9
• Abnormal motor or verbal response to pain
• Decorticate or decerebrate posture
• Cranial nerve palsy (especially lll, l V and Vl)
• Abnormal neurogenic respiratory pattern (e.g. grunting,
Cheyne-Stroke respiration, apneoa)
2. Major criteria
• Altered mentation/fluctuating level of
consciousness
• Sustained heart rate deceleration (decrease
of >20 beats/min) not attributing to
improved intravascular volume or sleep
state.
• Age-inappropriate incontinence
3. Minor criteria
• Vomiting
• Headache
• Lethargy or not easily arousable
• Diastolic BP >90mmHg
• Age <5 years
________________________________________________________________________________________________
To diagnose Cerebral Edema, the following criteria has to be met: (N.B. These criteria has 92%
sensitity & 4% false positive rate)
1 diagnostic criterion OR
2 major criteria OR
1 major and 2 minor criteria.
References:
© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 8 of 9
1. Wolfdorf J, Graig ME, Daneman D, et al. Diabetic Ketoacidosis in Children and Adolescents
with diabetes. (ISPED Clinical Practice Consensus Guidelines 2009 Compendium). Pediatric
Diabetes 2009: 10 (Suppl. 12): 118-133
2. Edge JA. BSPED Recommended DKA Guidelines 2009, November 2009
3. Jeha GS, Haymond MW. Clinical Features and diagnosis of Diabetic Ketoacidosis in Children.
2010 UpToDate.
4. Jeha GS, Haymond MW. Treatment and Complications of ketoacidosis in Children. 2010
UpToDate.
5. Jeha GS, Haymond MW. Cerebral Edema in Children with Diabetic Ketoacidosis. 2010
UpToDate.
6. Ismail HM, Phak NG, Thomas T. Paediatric Protocols for Malaysian Hospitals. 2
nd
Edition,
2008. Kementerian Kesihatan Malaysia.
7. Sperling MA. Type 1 Diabetes Etiology and Treatment. Humana Press Inc. 2003. New Jersey.
8. Behrman RE, Kliegman RM, Jenson HB, Stanton BF. Nelson Textbook of Pediatrics. 18
th
Edition, 2007. Saunders, Philadelphia.
9. Fogel N, Zimmerman D. Management of Diabetic Ketoacidosis in the Emergency Department.
2009: 10(4): 246-251 (not yet published)
10. Clinical Practice Guidelines – Diabetic Ketoacidosis, 2006-2009. The Royal Children’s
Hospital, Melbourne Australia.
11. Dunger DB et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric
Endocrine Society Consensus Statement on Diabetic Ketoacidosis in Children and
Adolescents. Paediatrics 2004: 113; e133-e140.
12. Jones KM, Molyneux E, Philips B, Wieteslka S. Advanced Paediatric Life Support, The
Practical Approach. 4
th
Edition, 2005. Blackwell Publishing Ltd, Massachusetts, USA.
13. Shann F. Paediatric Intensive Care Guideline, 3
rd
Edition, 2008. Royal Children’s Hospital,
Melbourne, Australia
14. Lifshitz F. Pediatric Endocrinology. Vol. 1, Obesity, Diabetes Mellitus, Insulin Resistance, and
Hypoglycemia. 5
th
Edition, 2007. Informa Healthcare, New York, USA.
15. http://www.bsped.org.uk/professional/guidelines/
Scope and Application
This CPG is intended for use by all health care
workers in their daily care of paediatric patients
© MOH_ Paediatrics Network_Diabetic Ketoacidosis_ Guideline_PICU_ 2010 Page 9 of 9
Effective Date
2010
Supercedes Policy Number Not applicable
Review Responsibilities
The Chairperson of the Paediatric CSN will
initiate the review of this guidelines every 3 years
from the date of issue or as required.
Further Information
Paediatric CSN Chairperson
RESPONSIBILITY:
CPG Owner: National Paediatric CSN
CPG Writer: Ministry of Health Date: 2010
Endorsed:
National Medicines & Therapeutic Committee, MOH
Date: 23 November 2010
Endorsed:
National Health Executive Committee, MOH
Date: 25 November 2010
