
HAL Id: hal-01417725
https://hal.science/hal-01417725
Submitted on 15 Dec 2016
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-
entic research documents, whether they are pub-
lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diusion de documents
scientiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Neuroimaging and neuromodulation approaches to study
eating behavior and prevent and treat eating disorders
and obesity
David Val-Laillet, E. Aarts, B. Weber, M. Ferrari, V. Quaresima, L.E.
Stoeckel, M. Alonso-Alonso, M. Audette, Charles-Henri Malbert, E. Stice
To cite this version:
David Val-Laillet, E. Aarts, B. Weber, M. Ferrari, V. Quaresima, et al.. Neuroimaging and neuro-
modulation approaches to study eating behavior and prevent and treat eating disorders and obesity.
Neuroimage-Clinical, 2015, 8, pp.1-31. �10.1016/j.nicl.2015.03.016�. �hal-01417725�
Review
Neuroimaging and neuromodulation approaches to study eating
behavior and prevent and treat eating disorders and obesity
D. Val-Laillet
a,
⁎
, E. Aarts
b
,B.Weber
c
,M.Ferrari
d
, V. Quaresima
d
,L.E.Stoeckel
e
,M.Alonso-Alonso
f
,M.Audette
g
,
C.H. Malbert
h
,E.Stice
i
a
INRA, UR1341 ADNC, France
b
Radboud University, Donders Institute for Brain, Cognition and Behaviour, Nijmegen, The Netherlands
c
Department of Epileptology, University Hospital Bonn, Germany
d
Department of Life, Health and Environmental Sciences, University of L3Aquila, Italy
e
Massachusetts General Hospital, Harvard Medical School, USA
f
Beth Israel Deaconess Medical Center, Harvard Medical School, USA
g
Old Dominion University, USA
h
INRA, US1395 Ani-Scans, France
i
Oregon Research Institute, USA
abstractarticle info
Article history:
Received 1 December 2014
Received in revised form 18 March 2015
Accepted 19 March 2015
Available online 24 March 2015
Keywords:
Brain
Neuroimagin g
Neuromodulation
Obesity
Eating disorders
Human
Functional, molecular and genetic neuroimaging has highlighted the existence of brain anomalies and neural vul-
nerability factors related to obesity and eating disorders such as binge eating or anorexia nervosa. In particular,
decreased basal metabolism in the prefrontal cortex and striatum as well as dopaminergic alterations have
been described in obese subjects, in parallel with increased activation of reward brain areas in response to palat-
able food cues. Elevated reward region responsivity may trigger food craving and predict future weight gain. This
opens the way to prevention studies using functional and molecular neuroimaging to perform early diagnostics
and to phenotype subjects at risk by exploring different neurobehavioral dimensions of the food choices and mo-
tivation processes. In the first part of this review, advantages and limitations of neuroimaging techniques, such as
functional magnetic resonance imaging (fMRI), positron emission tomography (PET), single photon emission
computed tomography (SPECT), pharmacogenetic fMRI and functional near-infrared spectroscopy (fNIRS) will
be discussed in the context of recent work dealing with eating behavior, with a particular focus on obesity. In
the second part of the review, non-invasive strategies to modulate food-related brain processes and functions
will be presented. At th e leading edge of non-invasive brain-based technologies is real-time fMRI (rtfMRI)
neurofeedback, which is a powerful tool to better understand the complexity of human brain–behavior relation-
ships. rtfMRI, alone or when combined with other techniques and tools such as EEG and cognitive therapy, could
be used to alter neural plasticity and learned behavior to optimize and/or restore healthy cognition and eating
behavior. Other promising non-invasive neuromodulation approaches being explored are repetitive transcranial
magnetic stimulation (rTMS) and transcranial direct-current stimulation (tDCS). Converging evidence points at
the value of these non-invasive neuromodulation strategies to study basic mechanisms underlying eating behav-
ior and to treat its disorders. Both of these approaches will be compared in light of recent work in this field, while
addressing technical and practical questions. The third part of this review will be dedicated to invasive
neuromodulation strategies, such as vagus nerve stimulation (VNS) and deep brain stimulation (DBS). In combi-
nation with neuroimaging approaches, these techniques are p romising experimental tools to unravel the
NeuroImage: Clinical 8 (2015) 1–31
Abbreviations: 5-HT, serotonin; aCC,anterior cingulate cortex; ADHD, attention deficit hyperactivity disorder; AN, anorexia nervosa; ANT, anterior nucleus of the thalamus; BAT, brown
adipose tissue; BED, binge eating disorder; BMI, body mass index; B N, bulimia nervosa; BOLD,blood oxygenation level dependent; BS, bariatric surgery; CBF, cerebral blood flow; CCK,cho-
lecystokinin; Cg25, subgenual cingulate cortex; DA, dopamine; daCC, dorsal anterior cingulate cortex; DAT, dopamine transporter; DBS, deep brain stimulation; DBT, deep brain therapy;
dlPFC,dorsolateralprefrontalcortex;DTI, diffusion tensorimaging;dTMS, deep transcranial magneticstimulation;ED, eatingdisorders;EEG,electroencephalography;fMRI, functional mag-
netic resonance imaging; fNIRS, functional near-infrared spectroscopy; GP, globuspallidus; HD-tDCS, high-definition transcranial direct current stimulation; HFD, high-fat diet; HHb, deox-
ygenated-hemoglobin; LHA, lateral hypothalamus; lPFC, lateral prefrontal cortex; MER, microelectrode recording; MRS, magnetic resonance spectroscopy; Nac, nucleus accumbens; OCD,
obsessive–compulsive disorder; OFC, orbitofrontal cortex; O
2
Hb, oxygenated-hemoglobin; pCC, posterior cingulate cortex; PD, Parkinson3s disease; PET, positron emission tomography;
PFC, prefrontal cortex; PYY, peptide tyrosine tyrosine; rCBF, regional cerebral blood flow; rtfMRI, real-time functional magnetic resonance imaging; rTMS, repetitive transcranial magnetic
stimulation;SPECT,single photon emissioncomputed tomography;STN,subthalamic nucleus;tACS,transcranial alternatecurrentstimulation;tDCS,transcranial directcurrentstimulation;
TMS,transcranial magneticstimulation;TRD, treatment-resistant depression;tRNS,transcranialrandom noisestimulation; VBM, voxel-basedmorphometry; vlPFC, ventrolateralprefrontal
cortex; vmH, ventromedial hypothalamus; vmPFC, ventromedial prefrontal cortex; VN, vagus nerve; VNS, vagus nerve stimulation; VS, ventral striatum; VTA, ventral tegmental area
* Corresponding author at: INRA UR1341 ADNC F-35650, France.
E-mail address: david.val-laillet@rennes.inra.fr (D. Val-La illet).
http://dx.doi.org/10.1016/j.nicl.2015.03.016
2213-1582/© 2015 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contents lists available at ScienceDirect
NeuroImage: Clinical
journal homepage: www.elsevier.com/locate/ynicl

intricate relationships between homeostatic and hedonic brain circuits. Their potential as additional therapeutic
tools to combat pharmacorefractory morbid obesity or acute eating disorders will be discussed, in terms of tech-
nical challenges, applicability and ethics. In a general discussion, we will put the brain at the core of fundamental
research, prevention and therapy in the context of obesity and eating disorders. First, we will discuss the possi-
bility to identify new biological markers of brain functions. Second, we will highlight the potential of neuroimag-
ing and neuromodulation in individualized medicine. Third, we will introduce the ethical questions that are
concomitant to the emergence of new neuromodulation therapies.
© 2015 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contents
1. Introduction............................................................... 2
2. Utility of neuroimaging to investigate eating behavior and elucidate risk and maintenance factors for weight gain and eating disorders: towards new phenotyping
andpreventionstrategies ......................................................... 3
2.1. Predictingfutureweightgainandmaintenanceonthebasisofneuralresponsivityandfunctioning .................... 3
2.1.1. Rewardsurfeitandincentivesensitizationtheoriesofobesity ................................. 3
2.1.2. Reward deficittheoryofobesity .............................................. 4
2.1.3. Inhibitorycontrol ..................................................... 4
2.1.4. Theoreticalimplicationsandfutureresearchdirections .................................... 5
2.2. Dopaminergicimaging....................................................... 5
2.2.1. Nucleartomographicimaging ............................................... 5
2.2.2. GeneticfMRI ....................................................... 7
2.2.3. Futuredirectionsfordopaminergicimaging ......................................... 7
2.3. Thecontributionoffunctionalnear-infraredspectroscopy(fNIRS) ................................... 7
2.3.1. Briefoverviewoftheprinciples,advantagesandlimitationsoffNIRS .............................. 7
2.3.2. ApplicationoffNIRSformappinghumancorticalresponsesinthecontextoffoodstimuli/intakeandeatingdisorders........10
3. Non-invasiveneuromodulationapproaches:recentdevelopmentsandcurrentchallenges............................10
3.1. Real-timefMRIneurofeedbackandcognitivetherapy .........................................10
3.1.1. Introductiontoneurofeedbackincognitivereappraisal ....................................10
3.1.2. Cognitivereappraisal,obesity,andeatingdisorders......................................10
3.1.3. Proof-of-conceptfortheuseofrtfMRIneurofeedbackwithcognitivereappraisalfortheregulationoffoodintakebehavior .....12
3.1.4. ConsiderationforrtfMRIneurofeedbackexperimentstargetingdisordersofingestivebehavior ..................12
3.2. Transcranialmagneticstimulation(TMS)andtranscranialdirect-currentstimulation(tDCS) .......................13
3.2.1. IntroductiontoTMSandtDCS ...............................................13
3.2.2. Summaryofclinicalstudiestomodifyeatingbehaviorandeatingdisorders...........................15
3.2.3. Futureneeds:fromempirically-drivenstudiestorationalandmechanisticapproaches......................15
4. Invasiveneuromodulationstrategies:recentdevelopmentsandcurrentchallenges...............................16
4.1. Overviewoftheperipheralneuromodulationstrategiesinthecontextoffoodintakeandweightcontrol..................16
4.1.1. Changesinvagalsignalingduringobesity ..........................................16
4.1.2. Effectsofvagalstimulation.................................................16
4.1.3. Effectsofvagalblockade..................................................17
4.2. Stateoftheartofdeepbrainstimulation(DBS)anditspotentialfortacklingobesityandeatingdisorders .................17
4.2.1. OverviewonthestateoftheartinDBS ...........................................17
4.2.2. RecentDBSinnovationsandemergingDBStherapies .....................................18
4.2.3. ApplicabilityofDBSinthecontextofobesityandeatingdisorders ...............................18
5. Generaldiscussionandconclusions:thebrainatthecoreofresearch,preventionandtherapyinthecontextofobesityandeatingdisorders.....19
5.1. Towardsnewbiologicalmarkers?..................................................19
5.2. Neuroimagingandneuromodulationinthescopeofpersonalizedmedicine...............................20
5.3. Ethicsrelatedtonoveldiagnosticandtherapeutictools ........................................22
5.4. Conclusion ............................................................22
Acknowledgments...............................................................22
References ..................................................................23
1. Introduction
A recent study estimated the number of overweight adults in the
world as roughly 2.1 billion in 2013 (Ng et al., 2014). In the United
States alone, obese individuals have 42% higher health care costs than
those with healthy-weight (Finkelstein et al., 2009). Obesity is on the
rise, with severe obesity rising at a particularly alarming rate (Flegal
et al., 2010; Finkelstein et al., 2012). Because obesity is a multifactorial
condition with a complex etiology, and because success of interventions
is subject to a large interindividual variability, there is no panacea or
“one-fit-all” treatment for obesity. Bariatric surgery (BS) is the treat-
ment of choice for severe obesity due to its effectiveness compared to
behavioral and pharmacologic al interventions (Buchwald and Oien,
2013). Its utility and success rate is widely accepted. However, 20–40%
of those who undergo BS fail to lose sufficient weight (Christou et al.,
2006; Livhits et al., 2012) or regain significant weight after treatment
(Magro et al., 2008; DiGiorgi et al., 2010; Adams et al., 2012), and can
experience a number of complications during and after surgery or med-
ical and psychiatric comorbidities (Shah et al., 2006; Karlsson et al.,
2007; DiGiorgi et al., 2010; Bolen et al., 2012; Chang et al., 2014). In ad-
dition to existing methods such as BS, which annually helps thousands
of people worldwide, there is a clear need for novel approaches to obe-
sity prevention and treatment, including the development of novel di-
agnostic and phenotyping methods, as well as adjunctive therapies
2 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
that may lead to better treatment outcomes for patients who may
require invasive procedures such as BS. In comparison to the rising
obesity epidemic, eating disorders (ED) are scarcer but also certainly
underestimated and increasing at a s tartling state (Makino et a l.,
2004). In the United States, up to 24 million people across all ages and
genders suffer from ED (anorexia — AN, bulimia — BN and binge eating
disorder — BED) (Renf rew Center Foundation for Eating Disorders, 2003),
and only 1 in 10 people with ED receives treatment (Noordenbox, 2002),
even though ED have the highest mortality rate of any mental illness
(Sullivan, 1995). Epidemiology of ED was described in details (including
risk factors, incidence, prevalence, and morbidity) in recent reviews
(see Smink et al., 2012; Mitchison and Hay, 2014).
In the fight against obesity and eating disorders, improved knowl-
edge about the pathophysiological and neurobehavioral mechanisms
underlying these diseases is needed to better prevent risky behaviors,
diagnose and treat patients, and develop new therapies that are safer
and adjustable to each patient. As noted by Schmidt and Campbell
(2013), treatment of eating disorders cannot remain ‘brainless’,and
the same applies to obesity when we consider the growing amount of
literature highlighting the behavioral and brain changes/plasticity
induced by obesit y (Wang et al., 2009b; Burger and Berner, 2014),
effective bariatric surgery (Geliebter, 2013; Scholtz et al., 2014), and
neuromodulatory interventions (McClelland et al., 2013a ; Gorgulho
et al., 2014) in animal models and human subjects.
Although several excellent review papers on this subject exist (see
McClelland et al. , 2013a; Sizonenko et al., 2013; Burger and Berner,
2014; Gorgulho et al., 2014), a comprehensive work comparing a large
spectrum of exploratory and therapeutic strategies using neuroimaging
and neuromodulation technologies, in terms of advantages and limita-
tions, degree of invasiveness, and applicability to individualized medi-
cine from prevention to treatment is missing and can help provide a
road map for future research and applications. Predictive and preven-
tion studies benefiting from neuroimaging are emerging thanks to the
characterization of neural vulnerability factors that increase ri sk for
weight gain and risky eating behaviors. The first part of our review
will be dedicated to this question, as well as to the role of functional, nu-
clear, and genetic neuroimaging in fundamental research and preven-
tion programs. A particular focus will be put on obesity, because it is
the number one concern, though references to specific ED will be in-
cluded when relevant. In this first part we will also review for the first
time the contribution of a less costly and more portable cortical func-
tional neuroimaging tool (i.e. fNIRS) in the context of research on eating
behavior. The second part of our review will provide an overview of the
non-invasive neuromodulatory approaches to combat weight problems
and ED, including a pres entation of real-time fMRI neurofeedback
coupled with cognitive therapy, as well as a comparison between trans-
cranial magnetic stimulation (TMS) and transcranial direct current
st
imulation (tDCS). The third section will be dedicated to more invasive
neuromodulatory approaches to modulate homeostatic and hedonic
mechanisms through the stimulation of the vagus nerve or deep-brain
structures. Finally, we will discuss all the data presented in the perspec-
tive of obesity/ED phenotyping and individualized medicine, while ad-
dressing the ethical questions raised by new therapeutic approaches
and their promise.
2. Utility of neuroimaging to investigate eating behavior and elucidate
risk and maintenance factors for weight gain and eating disorders:
towards new phenotyping and prevention strategies
2.1. Predicting future weight gain and maintenance on the basis of neural
responsivity and functioning
An improved understanding of the risk processes that give rise to ex-
cess weight gain should guide the design of more effective preventive
programs and treatments, which is vital because extant interventions,
with the possible exception of bariatric surgery, have limited efficacy.
Theorists have focused on the reward circuitry because eating palatable
food increases activation in regions implicated in reward in both
humans and other animals, including the ventral and dorsal striatum,
midbrain, amygdala, and orbitofrontal cortex (OFC: Small et al., 2001;
Avena et al., 2006; Berridge, 2009; Stice et al., 2013) and causes dopa-
mine (DA) release in the dorsal striatum, with the amount released cor-
relating with meal pleasantness (Small et al., 2003) and caloric density
of the food (Ferreira et al., 2012) in humans. Both the orosensory prop-
erties of palatable food consumption (gustatory stimulation) and direct
intragastric infusion of high calorie food induce striatal DA release in re-
ward regions in human and animal studies (Avena et al., 2006; Tellez
et al., 2013).
2.1.1. Reward surfeit and incentive sensitization theories of obesity
The reward surfeit model holds that individuals with greater reward
region responsivity to food intake are at elevated risk for overeating
(Stice et al., 2008b). The incentive sensitization model posits that re-
peated intake of palatable foods results in an elevated responsivity of re-
ward regions to cues that are associated with palatable food intake via
conditioning, prompting elevated food intake when these cues are en-
countered (Berridge et al., 2010). According to animal studies, firin g of
striatal and ventral pallidum DA neurons initially occurs in response to
receipt of a novel palatable food, but after repeated pairings of palatable
food intake and cues that signal impending receipt of that food, DA neu-
rons begin firing in response to reward-predictive cues and no longer
fire in response to food receipt (Schultz et al., 1997; Tobler et al.,
2005). Elevated reward-related responses to food intake and cues puta-
tively override homeostatic processes of satiety, promoting excess
weight gain.
The present review focuses on prospective studies because cross-
sectional data ca nnot differentiate precursors from consequenc es of
overeating, with a focus on human studies unless otherwise indicated.
Hyper-responsivity of reward regions (striatum, amygdala, OFC) to pal-
atable food images (Demos et al., 2012), palatable food television com-
mercials (Yokum et al., 2014), geometric cues that signa l impending
palatable food image presentation (Yokum et al., 2011), palatable food
odors that predict impending palatable food receipt (Chouinard-
Decorte et al., 2010; Sun et al. , 2013), and pictorial cues that predict
impending palatable food receipt (Stice et al., 2015) predicted future
weight gain. Humans who show elevated dorsal striatum responsivity
to palatable food images show greater futu re weight gain, but only
if they are at genetic risk for higher DA signaling capacity due to
possessing an A2/A2 genotype of the TaqIA
polymorphism or a 6-
r
epeat or shorter of the 48-base pair exon 3 variable number tandem re-
peat (VNTR) polymorphis m of the DRD4 gene (Stice et al., 2010b),
which are both associated with greater DA signaling and reward region
responsivity (Jonsson et al., 1999; Bowirrat and Oscar-Berman, 2005).
The evidence from independent laboratories that elevated reward re-
gion responsivity to various food cues, including those that predict
impending palatable food receipt, predicted future weight gain provides
behavioral support for the incentive sensitization theory.
Elevated midbrain, thalamus, hypothalamus, and ventral striatum
responsivity to milk shake taste also predicted future weight gain
(Geha et al., 2013; Sun et al., 2013). Further, individuals who show ele-
vated dorsal striatum responsivity to palatable food intake show greater
future weight gain, but only if they are at genetic risk for elevated DA
signaling capacity by virtue of possessing an A2/A2 genotype of the
TaqIA polymorphism (Stice et al., 2008a; Stice et al., 2015 ). The evidence
that individuals who show elevated reward region responsivity to palat-
able food intake are more likely to enter a prolonged period of positive
energy balance and gain weight provides behavioral data in support of
the reward surfeit theory.
Although extant data provide support for both the incentive sensiti-
zation and reward surfeit theories of obesity, which are not mutually ex-
clusive, future studies should simultaneously examine individual
differences in neural response to palatable food taste, cues that signal
3D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
impending palatable food taste, and palatable food images to provide a
more comprehensive investigation of neural vulnerability factors that
predict future weight gain. Results imply that prevention programs
that reduce habitual intake of high-calorie foods should attenuate the
conditioning process that eventually leads to elevated reward region
responsivity to food cues, which may reduce future weight gain. Yet,
the fa ct that be havioral weig ht loss programs typically result in a tran -
sient reduction of high-calorie food intake, but do not produce sustained
weight loss implies that it is very difficult to reduce reward region
hyper-responsivity to food cues once it has emerged. An uncontrolled
study suggested th at humans who have been able to sustain their
weight loss over long periods of time carefully limit intake of high -
calorie foods, exercise daily, and monitor their weight (Wing a nd
Phelan, 2005). These observations imply that it would be useful to test
whether interventions that increase executive control, either by direct
modification of brain-behavior function or indirectly by modification
of the environment (which could offset the risk from elevated reward
region responsivity) result in more lasting weight loss.
2.1.2. Reward deficit theory of obesity
The reward deficit model of obesity posits that indiv iduals with
lower sensitivity of DA-based reward regions overeat to compensate
for this deficiency (Wang et al., 2002). There have only been a few pro-
spective fMRI studies that could have potentially determined whether
reduced reward region responsivity preceded weight gain, and there
have not been any prospective studies that assessed with DA function-
ing (e.g. assessed with PET) predicted future weight change. Ou t of
the six prospective studies that examined the relation of BOLD response
to palatable food images, cues that signal impending palatable food re-
ceipt, and actual palatable food receipt to future weight gain reviewed
above (Chouinard-Decorte et al., 2010; Yokum et al., 2011; Demos
et al., 2012; Geha et al., 201 3; Yokum et al., 2014; Stice et al., 2015),
none found a relation between reduced reward region responsivity to
these food stimuli and greater future weight gain. Interestingly, howev-
er, a prospective study found that young adults who showed lower re-
cruitment of striatal regions in response to milk shake receipt (Stice
et al., 2008b, 2015) and palatable food images (Stice et al., 2010b)
showed greater future weight gain if they had a genetic propensity for
reduced DA signaling capacity. The interactive effects imply that there
may be qualitatively distinct reward surfeit and rewar d deficit path-
ways to obesity, which should be investigated further.
Obese versus lean adults have shown lower striatal DA D2 receptor
availability (Volkow et al., 2008; de Weijer et al., 2011; Kessler et al.,
2014) and less striatal responsivity to high-calo rie beverage taste
(Stice et al., 2008b). I nterestingly, Guo et al. (2014) also suggested
that obese people have alterations in the DA neurocircuitry that may in-
crease their susceptibility to opportunistic overeating while at the same
time making food intake less rewarding, less goal directed and more
habitual. Whether the observed neurocircuitry alterations pre-exist or
occur as a result of obesity development is still controversial, but consid-
erable evidence suggests that overeating contributes to a down-
regulation of the DA-based reward circuitry. Lean younger subjects at
risk for future obesity due to parental obesity show hyper- rather than
hypo-responsivity of reward regions to palatable food receipt (Stice
et al., 2011). Women who gained weight over a 6-month period showed
a reduction in striatal responsivity to palatable food receipt relative to
baseline and to women who remained weight stable (St
ice et al.,
2010a). Rats randomized to overeating conditions that result in weight
gain versus control conditions show a down-regulation of post-synaptic
D2 receptors, and reduced D2 sensitivity, extracellular DA levels in the
nucleus accumbens and DA turnover, and lower sensitivit y of DA re-
ward circuitry (Kelley et al., 2003; Davis et al., 2008; Geiger et al.,
2009; Johnson and Ken ny, 2010). Minipigs randomized to a weight
gain intervention versus a stable weight condition showed red uced
prefrontal cortex, midbrain and nucleus accumbens resting activity
(Val-Laillet et al., 2011). The reduced DA signaling capacity appears to
occur because habitual intake of high-fat diets causes decreased synthe-
sis of ol eoylethanolamine, a gastrointestinal lipid messenger (Tellez
et al., 2013). Interestingly, people who report elevated intake of a partic-
ular food show reduced striatal response during intake of that food, in-
dependent of BMI (Burger and Stice, 2012; Green and Murphy, 2012;
Rudenga and Small, 2012).
Geiger et al. (2009) hypothesized that diet-induced down-regulation
of the DA circuitry may prompt overeating to increase DA signaling. Yet,
mice in which reduced striatal DA signaling from food intake was exper-
imentally induced through chronic intragastric infusion of fat worked less
for acute intragastric infusion of fat and consumed less rat chow ad lib
than control mice (Tellez et al., 2013). Further, genetically engineered
DA-deficient mice are unable to sustain appropriate levels of feeding
(Sotak et al., 2005). These dat a seem incompatible with the notion that
an induced down-regulation of DA reward circuitry leads to compensato-
ry overeating. The Tellez et al. (2013) study also provided further evi-
dence that intake of fat can result in reduced DA response to food
intake, independent of weight gain per se.
2.1.3. Inhibitory control
Vulnerabilities in reward sensitivity, habit, and inhibitory control
appear to interact to produce prolonged hyperphagia of highly palatable
foods leading to the development and maintenance of obesity
(Appelhans et al., 2011). By extension, lower activation of prefrontal-
parietal brain regions implicated in inhibitory c ontrol, may le ad to
greater s ensitivity to the rewarding effects of highly palatable foods
and greater susceptibility to the pervasive temptation of appetizing
foods in our environment, which increases overeating in the absence
of meeting homeostatic energy needs (Nederkoorn et al., 2006). In
fact, this pattern of food intake behavior appears to occur with only a
limited role for homeostatic input in modulating obesogenic food intake
behavior (Ha ll et al., 2014). Inefficient or underdeveloped inhibitory
control function may increase the risk for obesity in early childhood at
a time when rapid development is occurring in subcortical and
prefrontal–parietal brain systems that support reward and inhibitory
control functions (see Reinert et al., 2013; Miller et al., 2015 for re-
cent reviews). In addition, obesity-related alterations in adipokines,
inflammatory cytokines, and gut hormones may l ead to further dis-
ruption in neurodevelopment, especially in reward and inhibitory
control functions, which may increase the risk for poor academic perfor-
mance and even dementia risk in later life (Miller et al., 2015). For exam-
ple, obese versus lean teens sh owed less activation of prefrontal regions
(dorsolateral prefrontal cortex [dlPFC], ventral lateral prefrontal cortex
[vlPFC]) when trying to inhibit responses to high-calorie food images
and behavioral evidence of reduced inhibitory control (Batterink et al.,
2010)
and adults who had greater dlPFC activation when instructed to
“resist craving” while viewing food images had better weight loss success
following gas tric bypass surgery (Goldma n et al., 2013). Another study
found that participants who showed less recruitment of inhibitory control
regions (inferior, middle, and superior frontal gyri) during difficult versus
easy choices on a delay discounting task showed elevated future weight
gain (Kishinevsky et al., 2012; r = 0.71); however, individual differences
in delay discounting behavior did not explain weight outcomes (Stoeckel
et al., 2013b). These results converge with evidence that obese versus lean
adults showed reduced gray mater volume in the prefrontal cortex
(Pannacciulli et al., 2006), a region that modulates inhibitory control,
and with a marginal trend for reduced gray matter volume in the prefron-
tal cortex to predict weight gain over 1-year follow-up (Yokum et al.,
2011). Interestingly, obese versus lean humans also showed less recruit-
ment of inhibitory regions (ventral medial prefrontal cortex [vmPFC]) in
response to high-calorie food images (Silvers et al., 2014) and high-
calorie food TV commercials (Gearhardt et al., 2014). Further, lower
dlPFC response to high-calorie food images predicted greater ad lib food
intake over the next 3 days (Cornier et al., 2010). These find ing s are note-
worthy because all but the results from the Batterink, Kishinevsky, and
Stoeckel studies emerged in paradigms lacking a behavioral response
4 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
component. In some instances (Kishinevsky et al., 2012; Stoeckel et al .,
2013b), the neuroimaging data were a better predictor of weight out-
comes than the behavioral measure. This example highlights the future
potential for “neuromarkers” to improve outcome prediction and individ-
ualize intervention strategies to improve weight outcomes (Gabrieli et al.,
2015). Finally, it may also be possible to directly target and normalize
these brain systems using several of the neuromodulator y tools and tech-
niques described throughou t this article, such as transcrania l stimulation,
to enhance treatment outcomes (Alonso-Alonso and Pascual-Leone,
2007).
2.1.4. Theoretical implications and future research directions
Thus, most prospective and experimental studies have not provided
support for the reward deficit theory of obesity, and whereas available
data suggest that the reduced DA signaling capacity of the reward cir-
cuitry may largely result from overeating, extent data provide little sup-
port for the notion that this contributes to compensatory overeating.
Yet, there is emerging evidence that there may be qualitatively distinct
reward surfeit and reward deficit pathways to obesity that are based on
individual differences in genes that affect DA signaling and reward re-
gion responsivity to palatable food receipt, implying that it might be
useful to refine our working model regarding neural vulnerability
factors that contribute to obesity. According to what might be referred
to as the dual pathway model of obesity, we posit that individuals in
the reward surfeit pathway initially show hyper-responsivity of reward,
gustatory, and oral somatosensory regions to palatable food in take,
which increases habitual intake of energy dense foods. The reward sur-
feit pathway might be more likely for those at genetic risk for greater DA
signaling capacity. Habitual intake of palatable foods theoretically leads
to the development of hyper-responsivity of attention and reward valu-
ation regions to cues that predict food reward through conditioning
(Berr idge, 200 9), which maintains overeating because exposure to
ubiquitous food cues results in craving that prompts eating. Data sug-
gest that the hyper-responsivity of reward regions to palatable food in-
take contributes to more pr onounced cue-reward learning, whi ch
increases risk for future weight gain (Burger and Stice, 2014). We fur-
ther submit that overeating results in a down-regulation of DA-based
reward regions, producing a blunted striatal response to food intake
that emerges with obesity, but that this may not contribute to further
escalation in eating. We also theorize deficits in inhibitory control in-
crease the risk for overeating, and further that overeating leads to a sub-
sequent reduction in inhibit ory response to food stimuli, which may
also contribute to future escalation in overeating. This prediction is
based on evidence that individuals exhibit greater inhibitory control
deficits in response to frequently versus infrequently experienced re-
wards; obese versus lean individuals show a greater immediate reward
bias to food stimuli but not monetary reward (Rasmussen et al., 2010).
In contrast, individuals in the reward deficit pathway, which may be
more likely for those with a genetic propensity for lower DA-signaling
capacity, might consume more calories per eating episode because the
weaker DA-signaling may attenuate feelings of satiety, as reward re-
gions project to the hypothalamus. It is possible that the weaker DA-
signaling of reward regions attenuates the effects of gut peptides that
relay satiety. It is also possible that the lower DA signaling and reward
region responsivity operates through a completely different process,
such as by reducing physical activity because these individuals might
find exercise less rewarding, contributing to a positive energy balance.
More broadly, data imply that too much or too little reward circuitry
responsivity, which is referred to as the Goldilocks Principle, serves to
disrupt homeostatic processes that have evolved to promote sufficient,
but not excessive caloric intake. This notion would be consistent with
an allostatic load model.
With
regard to future research, additional large prospective brain
imagi ng studies should seek to identify neural vulnerability factors
that predict future weight gain. Second, environmental, social, and bio-
logical factors, including genotypes, that moderate the effects of these
vulnerability factors on future weight gain should be examined in
more detail. Third, additional prospective repeated-measures studies
should attempt to capture the plasticity of reward region responsivity
to food images/cues and food receipt, which appears to results from
overeating. Randomized controlled experiments could be used to ad-
dress these research questions, allowing much stronger inferences re-
garding these etiologic processes. It will also be important to expand
research into other relevant neuropsychological functions (e.g. motiva-
tion, working memory, multisensory processing and integration, execu-
tive function), the neural systems that mediate these functions, their
interaction with reward and homeostatic (i.e. hypothalamic, brainstem)
brain systems, and how dysfunction in these neural systems and cogni-
tive functions may impact reward and homeostatic functions in order to
have a more unified bra in–behavior model of food intake behavior
(Berthoud, 2012; Hall et al., 2014). For example, inhibitory control and
the fronto-parietal brain systems that mediate this function have been
studied; however, there are other aspects of executive function (e.g.
mental set shifting, information updating and monitoring; Miyake
et al., 2000) that are mediated by dissociable, but overlapping regions
of the fronto-parietal “executive” network and are understudied in the
context of their relationship to food intake behavior. Finally, investiga-
tors should continue to translate findings from brain imaging studies
into more effective obesity prevention and treatment interventions.
2.2. Dopaminergic imaging
As reviewed above, dopamine (DA) plays an important role in eating
behavior. Understanding the neurocognitive mechanisms by which DA
influences eating behavior is crucial for prediction, prevention and
(pharmacologic al) treatment of ob esity. To infer the in volvement of
the dopaminergic system, it is important to actually measure DA pro-
cessing. Findings of increased me tabolism or blood flow in a dopaminer-
gic target region do not necessarily imply that DA is directly involved.
For example, activation in the striatum could reflect opioid modulation
of hedonic ‘liking’ instead of dopaminergic modulation of ‘wanting’
(Berridge, 2007 ). Here, we will go into more detail about results of stud-
ies directly investigating DA.
2.2.1. Nuclear tomographic imaging
Nuclear imaging techniques such as positron emission tomography
(PET) and single photon emission computed tomography (SPECT) use
radioactive tracers and detection of gamma rays to image tissue concen-
trations of molecules of interest (e.g. DA receptors). PET and SPECT have
a very low temporal resolution (tens of seconds to minutes), usually re-
quiring one imaging session for one data point, limiting the kind of re-
search questions that ca n be targeted with the se methods.
Table 1 provides an overview of dopaminergic PET and SPECT stud-
ies that have assessed differences as a function of BMI in humans. In line
with a downregulation of dopamine signaling with obesity is the rela-
tion between lower dopamine synthesis capacity in the dorsal striatum
and an elevated BMI (Wilcox et al., 2010; Wallace et al., 2014)andlower
striatal DA D2/D 3 re ceptor binding in obese versus le an in dividuals
(Wang et al., 2001; Haltia et al., 2007; Volkow et al., 2008; de Weijer
et al., 2011; Kessler et al., 2014;
van de Giessen et al., 2014).
However,
others have found positive associations between striatal D2/D3 receptor
binding and BMI (Dunn et al., 2012; Caravaggio et al., 2015), or no asso-
ciation (Eisenstein et al., 2013). From the above-mentioned studies it is
also unclear whether differences in DA processing reflect a cause or a
consequence of an increased BMI. Some have touched upon this ques-
tion by assessing changes in DA D2/D3 receptor binding after bariatric
surgery and significant weight loss. While one study found increases
and the other found decreases in receptor binding after surgery (Dunn
et al., 2010; Steele et al., 2010), a study with a larger sample did not
find any significant changes (de Weijer et al., 2014).
Another way to investigate the involvement of DA in obesity is to as-
sess changes in extracellular DA levels induced by a psychostimulant or
5D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

Table 1
Summary of studies using SPECT or PET for dopaminergic imaging in lean, overweight or obese human subjects.
Subjects, status Radioligand Marker for Challenge Main findings References
SPECT studies
n = 15 obese (BMI 43 ± 5) vs.
n = 15 non-obese (BMI 22 ± 2)
[123I] iodobenzamide (IBZM) DA D2/3R 2 sessions
a
: after amphetamine
vs. baseline
Obese individuals had lower striatal DA D2/3R binding than
controls at baseline; increases in extracellular dopamine were
correlated with enhanced trait food craving in obese individuals
van de Giessen
et al. (2014)
n = 19 bariatric surgery patients
(BMI 46 ± 6 before and 41 ± 6
after)
[123I] iodobenzamide (IBZM) DA D2/3R None No significant changes in striatal DA D2/3R binding before vs.
6 weeks after bariatric surgery were found; and no correlation
with BMI before or after surgery
de Weijer et al.
(2014)
n = 123 (BMI 18–41) [(123)I]FP-CIT DAT None No association between striatal DAT binding and BMI was found van de Giessen
et al. (2013)
n = 33 (BMI 21–50) [123I] PE2I DAT None No association between striatal DAT binding and BMI was found Thomsen et al.
(2013)
n = 15 obese (BMI 47 ± 7) vs.
n = 15 non-obese (BMI 22 ± 2)
[123I] iodobenzamide DA D2/3R None Obese individuals had lower striatal DA D2/3R binding than
controls
de Weijer et al.
(2011)
n = 50 (BMI 19–31) [99mTc]-TRODAT-1 DAT None Lower DAT binding in the striatum was correlated with a higher
BMI
Chen et al. (2008)
PET studies
n = 13 obese (BMI 37–49)
n = 24 non-obese (BMI 23 ± 3)
[11C] carfentanil
[11C] raclopride
μ-Opioid R
DA D2/3R
None No difference in D2/D3R availability between obese and
non-obese women, but significantly reduced μ-opioid R
availability in obese women
Karlsson et al.
(2015)
n = 19 (BMI 21–35) [11C] raclopride DA D2/3R 2 sessions
a
: glucose (caloric) vs.
sucralose (non-caloric)
Calorie-induced increases in extracellular dopamine in ventral
striatum were correlated with a lower BMI
Wang et al. (2014)
n = 16 (BMI 20–33) 6-[18F]-Fluoro-
L-m-Tyrosine (FMT) AADC, DA synthesis None Lower dopamine synthesis in caudate nucleus was correlated
with 1) greater BMI and 2) greater preference for perceived
“healthy”, but not actual healthy, foods (independent of BMI)
Wallace et al.
(2014)
n =33(n = 16) (BMI 19–35) [18F] fallypride DA D2/3R 2 sessions (n = 16)
a
: after
amphetamine vs. baseline
Lower DA D2/3R binding in caudate and amygdala was correlated
with a higher BMI at baseline; amphetamine-induced increases in
extracellular dopamine in putamen and substantia nigra were
correlated with a higher BMI
Kessler et al. (2014)
n = 15 obese (BMI 33 − 47) vs.
n = 15 non-obese (BMI 19–28)
(N-[(11)C] methyl) benperidol
([(11) C] NMB)
DA D2R-specific,
non-displaceable
None No association between striatal DA D2 binding and BMI was
found
Eisenstein et al.
(2013)
n = 26 vs. n = 35 (BMI 19–28) [11C]-(+)-PHNO vs. [11C]
raclopride
DA D2/3R (agonist vs.
antagonist)
None Higher DA D2/3R binding in ventral striatum, as measured with
[11C]-(+)-PHNO, was correlated with a higher BMI
Caravaggio et al.
(2015)
n = 14 obese (BMI 40 ± 5) vs.
n = 8 non-obese (BMI 23 ± 2)
[18F] fallypride DA D2/3R None Lower DA D2/3R binding in caudate was correlated with a lower
BMI
Dunn et al. (2012)
n = 15 (BMI 25) 6-[18F]-Fluoro-
L-m-Tyrosine (FMT) AADC, DA synthesis None Lower DA synthesis capacity in the dorsal striatum was
correlated with a higher BMI (caudate) and increased weight loss
attempts (putamen)
Wilcox et al. (2010)
n = 5 bariatric surgery patients
(45 ± 6 before and 38 ± 7 after)
[11C] raclopride DA D2/3R None Four out of five patients showed an increase in DA D2/3R binding
in the striatum 6 weeks after bariatric surgery
Steele et al. (2010)
n = 5 bariatric surgery patients (BMI
43 ± 3 before and 38 ± 3 after)
[18F] fallypride DA D2/3R None DA D2/3R binding in striatum, (hypo) thalamus, substantia nigra
(corrected for multiple comparisons) and amygdala decreased
7 weeks after bariatric surgery
Dunn et al. (2010)
n = 10 obese (BMI 51 ± 5) vs.
n = 12 non-obese (BMI 25 ± 3)
[11C] raclopride and [18F]
fludeoxyglucose (FDG)
DA D2/3R; glucose None In obese individuals striatal D2/3R binding was lower than
controls and was positively correlated with glucose metabolism
in frontal and somatosensory cortices
Volkow et al.
(2008)
n = 12 obese (BMI 33 ± 5) vs.
n = 12 non-obese (BMI 22 ± 1)
[11C] raclopride DA D2/3R 2 sessions
a
:after i.v. glucose vs.
after i.v. placebo
Obese individuals had lower striatal DA D2/3R binding than
controls; glucose increased extracellular striatal dopamine in
men and reduced it in women
Haltia et al. (2007)
n = 10 obese (BMI 42–60) vs.
n = 10 non-obese (BMI 21–28)
[11C] raclopride DA D2/3R None Obese individuals had lower striatal DA D2/3R binding than
controls; lower striatal DA D2/3R binding was correlated with a
higher BMI in obese individuals
Wang et al. (2001)
BMI: body mass index (kg/m
2
); “x–x” reflects the range, and“x±x” reflects the average ± standard deviation; PET: positron emission tomography; DA: dopamine; D2/3R: D2/D3 receptor;
a
Increases in extracellular dopamine were observed as reductions in binding potential; i.v.: intravenous; SPECT: single photon emission tomography; DAT: dopamine transporter; AADC: aromatic l-amino acid decarboxyla se.
6 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
a food challenge (see Table 1). In such challenge studies, lower receptor
binding is interpreted as greater release of endogenous DA leading to
greater competition with the radioligand at the receptors. Challenge
studies have observed that food- or psychostimulant-induced increases
in extracellular striatal DA are associated with a lower BMI (Wang et al.,
2014), a higher BMI (Kessler et al., 2014), or have found no differences
between BMI groups (Haltia et al., 2007).
In sum, findings from nuclear imaging studies investigating differ-
ences in the striatal DA system as a function of BMI are very inconsis-
tent. In an attempt to converge on one theory of dopaminergic hypo-
activation in obesity, different authors have used different explanations
for their results. For example, DA D2/D3 receptor binding has been
interpreted to reflect DA receptor availability (e.g. Wang et al., 2001;
Haltia et al., 2007; Volkow et al., 2008; de Weijer et al., 2011; van de
Giessen et al., 2014), DA receptor affinity (Caravaggio et al., 2015), or
competition with endogenous DA (Dunn et al., 2010; Dunn et al.,
2012). Based on the data, it is often unclear whether such differences
in interpretation are valid. In addition, a very recent study by Karlsson
and colleagues showed a significant reduced μ-opioid receptor availabil-
ity in obese compared to normal-weight women, without changes in
D2-receptor availability, whic h might be an additional chan nel that
might explain the inconsisten t findings in a lo t of other studies
(Karlsson et al., 2015).
2.2.2. Genetic fMRI
By investigating the effects of common variations in DA genes the
role of predisposed vulnerability can be determined. To date, there
have only been a few studies that have combined genetics with neuro-
imaging in the domain of food reward. Most of them are functional mag-
netic resonance imaging (fMRI) studies.
Most genetic fMRI studies investigating food reward have taken into
account a common variation (i.e. polymorphism) referred to as TaqIA, of
which the A1 allele has been positively associated with BMI in several
early genetic studies (Noble et al., 1994; Jenkinson et al., 2000; Spitz
et al., 2000; Thomas et al., 2001; Southon et al., 2003). The TaqIA poly-
morphism is located in the ANKK1 ge ne, ~10 kb downstream of the
DRD2 gene (Neville et al., 2004). A1-allele carriers of the TaqIA poly-
morphism show reduced striatal D2R expression (Laruelle et al., 1998;
Pohjalainen et al., 1998; Jonsson et al., 1999). Genetic fMRI studies
have demonstrated that A1-carriers show decreased blood-oxygen-
level-dependent (BOLD) responses in DA-rich regions in the brain (dor-
sal striatum, midbrain, thalamus, orbitofrontal cortex) when consuming
a milk shake versus a tasteless solution relative to non-carriers (Stice
et al., 2008a;
Felsted et al., 2010)
. Importantly, these decreased re-
sponses for food reward consumption, as well as for imagined food in-
take, predicted future weight gain in the A1 risk allele carriers (Stice
et al., 2008a; Stice et al., 2010b). This is in line with the idea that DA
modulates the blunted response to food reward in obesity. In contrast,
when anticipating a milk shake versus a tasteless solution, A1-carriers
have demonstrated increased BOLD responses in the midbrain (Stice
et al., 2012). A multilocus composite score of dopaminergic genotypes —
including ANKK1 and four others — did not predict decreased striatal re-
sponses for the consumption of food reward, but only for the receipt of
monetary reward (Stice et al., 2012).
Thus, genetic fMRI studies suggest that individual differences in do-
paminergic genes play a role in brain responses to food reward, but their
effects are not always replicated and seem to depend on the anticipation
or the consumption of food reward.
2.2.3. Future directions for dopaminergic imaging
Together, SPECT, PET, and genetic fMRI studies suggest that brain DA
is involved in obesity. However, these neuroimaging findings are not
easily interpreted as a simple hypo- or hyper-activation of the DA sys-
tem in obesity. Moreover, there is an abundance of non-replications
and null findings, possibly due to small sample sizes. In order to use do-
paminergic imaging as a phenotyping method indicating vulnerability
for obesity or for prediction of treatment efficacy, reliability should be
increased. Genetic pathway analyses (e.g. Bralten et al., 2013) or ge-
nome wide association studies (e.g. El-Sayed Moustafa and Frogue l,
2013; Stergiakouli et al., 2014) might be more sensitive and specificin
revealing DA3s role in obesity. In the context of personalized medicine,
DA genetic fMRI studies could be combined with phar macology (see
Kirsch et al., 2006; Cohen et al., 2007; Aarts et al., 2015) to reveal the
mechanisms of anti-obesity drugs as well as individual differences in
treatment response.
Another reason for the observed inconsistencies might be that obe-
sity (i.e. BMI) is too complex and unspecific as a phenotype (see also
Ziauddeen et al., 2012), which is also evident from the fact that studies
using polygenic risk scores have only obtained small associations with
obesity phenotypes (e.g. Domingue et al., 2014). Neuroimaging studies
might more clearly reveal dopaminergic effects when using cognitive
paradigms that manipulate fo od motivation (i.e. effort provision) or
the learning of cue-reward associations, as st riatal DA is well known
for its role in these processes (Robbins and Everitt, 1992; Sch ultz et al.,
1997; Berridge and Robinson, 1998). Assessing task-related responses,
however, is a challenge during PET and SPECT due to their low temporal
resolution. Nevertheless, PET/SPECT measures could be related to off-
line task behavior (see, e.g. Wallace et al., 2014). Moreover, combina-
tions of imaging modalities such as PET and fMRI holds a strong poten-
tial for future studies (see, e.g. Sand er et al., 2013 in non-h uman
primates), making optimal use of the specificity of PET and the temporal
and spatial resolution of fMRI.
2.
3. The contribution of functional near-infrared spectroscopy (fNIRS)
Unlike the other neuroimaging techniques, such as PET and fMRI,
fNIRS does not require subjects to be in a supine position and does not
strictly restrict head movements, thus allowing to adopt a wide range
of experimental tasks suitable for properly investigating eating disor-
ders and food intake/stimuli. In addition, fNIRS us es a relatively low
cost instrumentation (with a sampling time in the order of the ms and
a spatial resolution of up to about 1 cm). On the other hand, although
EEG is a useful electrophysiological technique, its very low spatial reso-
lution makes it difficult to precisely identify the activated areas of the
brain, limiting its application to specific research questions related to
eating disorders (Jauregui-Lobera, 2012). R ecently, to deal with this
problem EEG has been combined successfully with fMRI to overcome
the spatial limitations of EEG and the temporal limitations of fMRI,
using their complementary features (Jorge et al., 2014). The parallel or
sequential use of EEG and fMRI in food related studies may provide ad-
ditional insights into neural processing cascades. However, combined
EEG–fMRI food related studies have not been reported yet. In conclu-
sion, all the above mentioned advantages of using fNIRS and EEG offer
the great promise to explore tast e-related higher cognitive brain
functions, which require tasks involving even the ingestion of food/
beverages under more natural situations.
2.3.1. Brief overview of the principles, advantages and limitations of fNIRS
The principles, advantages, and limitations of fNIRS or optical topogra-
phy or near-infrared (NIR) imaging have been summarized in recent
reviews (Hoshi, 2011; Cutini et al., 2012; Ferrari and Quaresima, 2012;
Scholkmann et al., 2014). fNIRS is a non-invasive vascular-based
neuroimaging technology that measures concentration changes of
oxygenated-hemoglobin (O
2
Hb) and deoxygenated-hemoglobin (HHb)
in cortical microcirculation blood vessels. fNIRS relies on neurovascular
coupling to infer changes in neural activity that is mirrored by changes
in blood oxygenation in the region of the activated cortical area (i.e. the
increase in O
2
Hb and the decrease in HHb). Unlike the BOLD signal of
fMRI, which is gathered from the paramagnetic properties of HHb, the
fNIRS signal is based on the changes in the intrinsic optical absorption
of both HHb and O
2
Hb (Steinbrink et al., 2006). fNIRS systems vary in
7D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

Table 2
fNIRS cognitive processing studies in patients with eating disorders, as well as healthy subj ects /patients upon food intake or food stimuli.
Food stimulus or food intake Task (s) Subjects, status Age
(years; mean ± SD)
Range (years) Device Ch Cortical
area
Main finding References
Frontal cortex reactivity in patients with eating disorders
n.u. VFT; RPST 14, HC; 10, AN;
14, BN
24.1 ± 3.0; 26.1 ± 7.1 n.a. D8 2 PFC Higher dlPFC activation in BN Sutoh et al. (2013)
n.u. VFT; control: FOT 12, HC; 16, AN 14.3 ± 1.3; 14.2 ± 1.3 n.a. D4 24 PFC VFT: AN poor PFC activation; FOT: similar
PFC activation in AN and HC
Nagamitsu et al. (2011)
n.u. VFT 27, HC; 27, ED 22.4 ± 2.0; 23.5 ± 5.2 n.a. D4 52 FT ED: bilateral OFC and right FT smaller
activation
Suda et al. (2010)
n.u. VFT 11, HC; 11, ED 26.9 ± 2.2; 21.2 ± 6.0 18–32; 14–38 D3 24 PFC Lower PFC activation in ED Uehara et al. (2007)
Effects of food taste
Sweet taste: sucrose (10%); sour taste:
citric acid (10%)
Pleasant/unpleasant
tasting task
16, HC 26.3 ± 5.5 n.a. D10 16 PFC Bilateral FP and dlPFC deactivation to
both tastes; higher right PFC activation
with citric acid
Hu et al. (2014)
Sweet snacks Taste stimulation 6, HC 21.5 ± 1.3 19–27 D5 44 PFC Bilateral primary taste area, inferior
frontal gyrus, and dlPFC activation
Ono (2012)
Different liquid taste-stimuli Encoding and retrieval of
taste memory
28, HC 32 ± 7 21–49 D12 23 PFC Bilateral FP and right DLPFC larger
activation in retrieval
Okamoto et al. (2011)
Bitter: 6-n-propylthiouracil Tasting task 48, HC n.a. 24–40 D3 24 dlPFC,
vlPFC
dlPFC and vlPFC activation Bembich et al. (2010)
Different sugar based taste-stimuli;
control: VFT, TTT
Taste stimulation 19, HC 32.1 ± 6.9 23–44 D12 17 PFC vlPFC is involved in the act of tasting. Okamoto et al. (2009)
7 green tea samples Sensory evaluation 12, HC n.a. 23–42 D12 14 lPFC Left lPFC and right inferior frontal gyrus
activation
Okamoto et al. (2006a)
Different liquid taste-stimuli; control:
TTT
Taste encoding task 18, HC n.a. 25–44 D12 17 PFC vlPFC activation Okamoto et al. (2006b)
Effects of food flavor
Sweet taste/sweet taste-lemon odor/no
taste-odor gums
Chewing test 25, HC 27.8 ± 2.8 n.a. D8 2 PFC Combination of taste/odor increases PFC
activation
Hasegawa et al. (2013)
Ethylmaltol-flavored 4% sucrose solution Sensory evaluation tasks 7, HC 31.4 ± 4.5 n.a. D4 52 PFC Ethylmaltol enhances the TC activation
when combined with a sweet taste
Saito-Iizumi et al.
(2013)
Flavored and odorless broth stimuli Sensory evaluation task 10, HC 30.5 ± 4.6 n.a. D4 52 FP, FT Bilateral TC activation upon flavored
broth taste
Matsumoto et al.
(2012)
Effects of odor food components
Irritating and hedonic odors Olfactory stimulation test 11, HC; 12, MCS n.a. n.a. D13 42 PFC PFC activation in MCS and controls Azuma et al. (2013)
Isovaleric acid (sweet smell) Olfactory stimulation test 19, HC; 36, D 42.5; 60.9 22–67; 37–81 D3 22 PFC Activation of the lower part of the PFC in
HC; no activation in D subjects
Kobayashi et al. (2012)
2-Phenyl ethanol and citral Olfactory stimulation test 14, HC 19.6 18–23 D1 2 OFC Left OFC activation; right OFC activation
upon
odor recognition
Kokan et al. (2011)
Linalool (mixed olfactory stimulant) Olfactory stimulation test 22, HC; 27, ADHD 12.4 ± 1.6; 12.7 ± 1.4 n.a. D4 48 PFC Higher TC activation in ADHD without
methylphenidate therapy
Schecklmann et al.
(2011a)
2-Phenyl ethanol; linalool (mixed
olfactory stimulant)
Olfactory stimulation test 29, HC; 29, ADHD 27.8 ± 4.1; 28.2 ± 4.5 n.a. D4 44 PFC Methylphenidate normalizes the ADHD
TC activation
Schecklmann et al.
(2011b)
8 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

Isovaleric acid (sweet smell) Olfactory stimulation test 8, HC; 5, D 28.9; 46.9 22–39; 17–69 D3 22 PFC Activation of the lower part of the PFC in
HC; no activation in D subjects
Kobayashi et al. (2009)
Isovaleric acid (sweet smell) Olfactory stimulation test 8, HC 28.9 22–39 D3 22 PFC Activation of the lower part of the PFC Kobayashi et al. (2007)
Pleasant: vanilla essence, strawberry
essence; unpleasant: scatol
Olfactory stimulation test 13, HC 23–31 D9 2 PFC PFC activation related to odor strength Harada et al. (2006)
Pleasant: vanilla substance (1%) Olfactory stimulation test 8, HC; 13, MA 66; 66 56–79; 56–72 D9 2 TC Bilateral TC activation only in HC Fladby et al. (2004)
2-Phenyl ethanol, isovaleric acid Olfactory stimulation test 12, HC 32.6 ± 14.9 n.a. D15 2 TC Bilateral TC activation (right TC higher
activation)
Ishimaru et al. (2004)
Effects of nutrition/food components
7-day essence of chicken/placebo
supplementation
Working memory and
reaction tasks
12, HC 62.3 ± 2.5 60–68 D4 24 PFC dlPFC activation only with chicken
essence upon working memory task
Konagai et al. (2013a)
12-week krill/sardine oil
supplementation
Working memory and
calculation tasks
45, HC 67.1 ± 3.4 n.a. D4 24 PFC Greater dlPFC activation with krill oil Konagai et al. (2013b)
Glucose drink (50 mg) Divided attention task 20, HC 69.4 n.a. D2 36 PFC Glucose ingestion enhances the lateral
and ventral PFC activation of the right
hemisphere to the two concurrent tasks
Gagnon et al. (2012)
12-week docosahexaenoic acid-rich fish
oil supplementation
Battery of cognitive tasks 65, HC 20.6 18–29 D14 2 PFC Dose response PFC activation Jackson et al. (2012)
Single dose green tea polyphenol
epigallocatechin gallate (135 mg)
Battery of cognitive tasks 27, HC 22 18–33 D14 12 PFC FC CBF decrease Wightman et al. (2012)
Single dose soybean peptide Battery of cognitive tasks 10, HC n.a. 20–25 D4 52 PFC FP, dlPFC activation (frequency band
amplitude increase)
Yimit et al. (2012)
Single dose caffeine (75 mg) Battery of cognitive tasks 20, HC 21.4 19–28 D14 12 PFC FC CBF decrease only in non-habitual
consumers
Kennedy and Haskell
(2011)
Single dose trans-resveratrol
(250/500 mg)
Battery of cognitive tasks 22, HC 20.2 18–25 D14 12 PFC Dose-dependent FC CBF increase Kennedy et al. (2010)
Casein hydrolysate drink ingestion;
carbohydrate drink
n.u. 11, HC 22.5 ± 2.3 21–28 D16 10 PFC Casein hydrolysate drink does not
change [tHb]; carbohydrate drink
increases [tHb]
Nakamura et al. (2010)
Single dose caffeine (180 mg) UKP calculation tests
before/after caffeine intake
14, HC n.a. 21–50 D11 2 PFC The same PFC activation before and after
caffeine intake
Higashi et al. (2004)
5-day creatine supplementation UKP calculation tests
before/after
24, HC 24.3 ± 9.1 n.a. D6 1 PFC Reduced left FC activation Watanabe et al. (2002)
Effects of food images
Visual stimulation: food photos Like/dislike test 5, HC 23.4 ± 3.4 n.a. D4 52 FP, FT FP activation Hosseini et al. (2011)
Visual: images of body types/high-calorie
food/attachment
Symptom-provocative
views
task
13, HC; 12, AN 14.3 ± 1.3; 14.4 ± 1.3 n.a. D4 24 PFC No difference in PFC activation between
HC and AN viewing body types/food; AN
higher PFC activation viewing
mother–child attachment
Nagamitsu et al. (2010)
Visual stimulation: drinks photos Preference evaluation task 9, HC 24.0 ± 4.4 n.a. D7 14 PFC Medial PFC activation Luu and Chau (2009)
Visual stimulation: food photos Preference evaluation task 8, HC 23 18–30 D13 32 PFC vmPFC activation Shimokawa et al.
(2008)
[tHb]: total hemoglobin concentration; ADHD: attention- deficit/hyperactivity diso rder; AN: anorexia nervosa; BN: bulimia nervosa; CBF: cerebral blood flow; CH: channels; D: dysosmia; dlPFC: dorsolateral prefrontal cortex; D1: BOM-L1W (Omega
Wave, Japan); D2:CW-6 (Techen, USA); D3: ETG-100 (Hitachi, Japan); D4: ETG-4000 (Hitachi, Japan); D5: ETG-7100 (Hitachi, Japan); D6: HEO-200 (Omron, Japan); D7: Imagent (ISS, USA); D8:NIRO-200 (Hamamatsu Photonics, Japan); D9:NIRO-300
(Hamamatsu Photonics, Japan); D10: OEG-16 (Spectratech, Japan); D11: OM-200 (Shimadzu, Japan); D12: OMM-2000 (Shimadzu, Japan); D13: OMM-3000 (Shimadzu, Ja pan); D14: OXYMON MkIII (Artini s, The Netherlands); D15:PSA-500 (Bio-
medical Sciences, Japan); D16: TRS-10 (Hamamatsu Photonics, Japan); ED: eating disorders; FOT: finger opposition task; FP: fr ontopolar; FT: frontotempo ral; HC: healthy controls; lPFC: lateral prefrontal cortex; MA: mild Alzheimer; MCS: multiple
chemical sensitivity; n.a.: not available; n.u.: not utilize d; OFC: orbitofrontal cortex; PFC: prefrontal cortex; RPST: rock-paper-scissors intentionallosstask;TC:temporalcortex;TTT:tonguetappingtask;UKP:Uchida–Kraepelin psychodiagnostic test;
VFT: verbal fluency task; vmPFC: ventromedial prefrontal cortex; vlPFC: ventrolateral prefrontal cortex.
9D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
complexity from dual channels to ‘whole-head’ arrays of several dozen
channels. Data processing/analysis methods permit topographical assess-
ment of real-time regional cortical hemodynamic changes. However, the
relatively low spatial resolution of fNIRS makes it difficult to precisely
identify the activated cortical regions. Moreover, the fNIRS measure-
ments, being limited to the cortical surface, cannot examine the primary
and secondary taste areas, which are located deep inside the brain
(Okamoto and Dan, 2007). Therefore, deeper brain areas, such as ventral
striatum and hypothalamus, which would be key for investigating eating
behavior, can be explored only by fMRI and/or PET.
2.3.2. Application of fNIRS for mapping human cortical responses in the
context of food stimuli/intake and eating disorders
The use of fNIRS in the context of food stimuli/intake and eating
disorders studies represents a relatively novel application, as witnessed
by the limited number of publications: 39 over the last 10 years. Table 2
summarizes these studies. The related fNIRS results mainly include: 1) a
lower frontal cortical activatio n upon different cognitive conditions/
stimuli in patients with ED, an d 2) the different activation patterns
over the frontal and temporal cortices upon different conditions/stimuli
(i.e. food taste, food flavor, odor food components, nutrition/food com-
ponents ingestion, and food images) in healthy subjects. So far, few
forms of ED have been investigated by fNIRS. Only one study has report-
ed PFC responses to visual stimuli in AN patients (Nagamitsu et al.,
2010). The other 4 ED-related studies reported in Table 2, and the ex-
tensive fMRI literature (see García-García et al., 2013 review summariz-
ing 86 studies) suggest the existence of neural differences between
normal and abnormal eating behavior in response to the sight of food.
Recently, Bartholdy et al. (2013) have reviewed the studies in which
neurofeedback was combined with neuroimaging techniques, suggest-
ing the potential use of fNIRS for evaluating ED treatments. However,
the interpretation of the fNIRS findings might be complicated by the
longer scalp-to-cortex distance in some patients with severe AN as a
consequence of their brain alteration following gray matter volume re-
duction and/or cerebrospinal fluid volume increase (Bartholdy et al.,
2013; Ehlis et al., 2014). Therefore, an assessment of the degr ee to
which cortical atrophy and scalp perfusion could affect the sensitivity
of fNIRS is essential for evaluating the usefulness of this technique first
as a research tool in patients with severe AN.
Thirty-four out of the 39 studies have been carried out only in healthy
subjects (Table 2). Twenty studies of them have demonstrated how fNIRS
can provide a useful contribution to map taste processing mainly localized
in the lateral prefrontal cortex (lPFC). Eleven studies are related to the ap-
plication of fNIRS in nutritional intervention studies in both acute and
chronic intervention paradigms (Jackson and Kennedy, 2013; Sizonenko
et al., 2013 for reviews). These studies have suggested that fNIRS is capa-
ble to detect the effect of nutrients and food components on PFC
activation.
Unfortunately, most of the studies reported in Table 2 have been
performed in small sample size, and the comparison between patients
and controls was ofte n insufficient . In addition, only a s ingle fNIRS
study, carried out using a high-cost fNIRS instrument based on time-
resolved spectroscopy, has reported absolute concentration values of
O
2
Hb and HHb.
In most of the reported studies, fNIRS probes covered only frontal
brain regions. Therefore, the involvement of other cortical areas includ-
ing parietal, fronto-temporal, and occipital regions, which might be as-
sociated with visuospatial processing, attention, and other perceptive
networks, were not investigated. In addition, most of the studies have
reported only ch anges in O
2
Hb making a comparison with fMRI findings
difficult.
These preliminary studies indicate that, when used in well-designed
studies, fNIRS neuroimaging may be a useful tool in helping to elucidate
the effects of dietary intake/supplementation. In addition, fNIRS could
be easily adopted for: 1) evaluating the efficacy of ED treatment pro-
grams and behavioral train ing pro grams, and 2) investigating the
inhibitory control of the dlPFC to visual food cues in healthy subjects
as well as in ED patients.
3. Non-invasive neuromodulation approaches: recent developments
and curren t ch allenges
3.1. Real-time fMRI neurofeedback and cognitive therapy
3.1.1. Introduction to neurofeedback in cognitive reappraisal
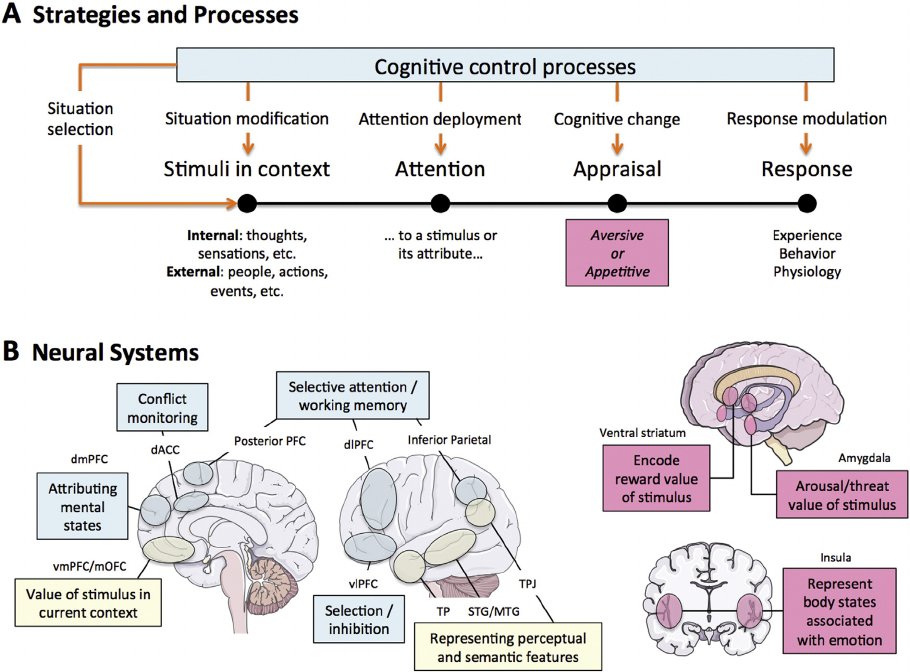
Cognitive reappraisal is an explicit emotion regulation strategy in-
volving the modification of cognitive processes in order to alter the di-
rection and/or magnitude of an emotional response (Ochsner et al.,
2012). The brain systems that generate and apply reappraisal strategies
include the prefrontal, dorsal anterior cingulate (dACC), and inferior pa-
rietal cortices (Ochsner et al., 2012). These regions function to modulate
emotional responses in the amygda la, vent ral striat um (VS), ins ula, an d
ventromedial prefrontal cortex (vmPFC) (Ochsner et al., 2012; Fig. 1).
Finally, the use of cognitive reappraisal strategies has been shown to
regulate appetitive responses to highly palatable foods via these same
neural systems (Kober et al., 2010; Hollmann et al., 2012; Siep et al.,
2012; Yokum and Stice, 2013).
Neurofeedback using functional magnetic resonance imaging (fMRI)
data is a non-invasive training method used to alter neural plasticity
and learned behavior by providing individuals with real time informa-
tion about their brain activity to support learned self-regulation of this
neural activity (Sulzer et al., 2013; Stoeckel et al., 2014; Fig. 2). Combin-
ing real time fMRI (rtfMRI) neurofeedback with cognitive reappraisal
strategies is a cutting-edge strategy for translating the latest advances
in neuroscience, clinical psychology, and technology into a therapeutic
tool that may enhance learning (Birbaumer et al., 2013), neuroplasticity
(Sagi et al., 2012), and clinical outcomes (deCharms et al., 2005). This
approach complements other existing neurotherapeutic technologies,
including deep brain and transcranial stimulation, by offering a non-
invasive alternative for brain disorders and it may add value above psy-
chotherapy alone, including cognitive behavioral therapy, by providing
information about how and where changes in cognitions are causing
changes in brain function (Adcock et al., 2005).
There appear to be abnormalities in the use of cognitive reappraisal
strategies and the brain systems that implement them that contribute to
disorders of ingestive behavior, including AN, BN, BED, obesity, and ad-
diction (Kelley et al., 2005b; Aldao and Nolen-Hoeksema, 2010; Kaye
et al., 2013). Across these disorders, there is often dysfunction in two
major brain systems that also have key roles in cognitive reappraisal:
one involving hypersensitivity to rewarding cues (e.g. VS, amygdala, an-
terior insula, vmPFC, including orbitofrontal cortex) and the other in-
volving deficient cognitive control over food or other subst ance use
(e.g. anterior cingulate, lateral prefrontal cortex — lPFC, including dorso-
lateral prefrontal cortex — dlPFC). Novel interventions designed to di-
rectly target dysfunctional emotion regulation strategies and patterns
of
neural activity may p rovide a new direction and hope for these
difficult-to-treat disorders.
3.1.2. Cognitive reappraisal, obesity, and eating disorders
Obesity is one ca ndidate di sorder tha t will be used to illustra te how
this novel, neuroscience-driven intervention approach may be imple-
mented. Different studies suggest that obese vers us lean individuals
show elevated reward region responsivity to images of high-fat/high-
sugar foods, which increases risk for weight gain (cf. Sect ion 2.1). Fortu-
nately, cognitive reappraisals, such as thinking of the long-term health
consequences of eating unhealthy food when viewing images of such
foods, increases inhibitory region (dlPFC, vlPFC, vmPFC, lateral OFC,
superior and inferior frontal gyrus) activation and decreases reward re-
gion (ventral striatum, amygdala, aCC, VTA, posterior insula) and atten-
tion region (precuneus, posterior cin gulate cortex — PCC) activation
relative to contrast conditions (Kober et al., 2010; Hollmann et al.,
2012; Siep et al., 2012; Yokum and Stice, 2013). These data suggest
10 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
that cognitive reappraisals may reduce hyper-responsivity of reward re-
gions to food cues and in crease inhibitory control re gion activation,
which is crucial because our environment is replete with food images
and cues (e.g. ads on TV) that contribute to overeati ng. Ac cordingly,
Stice et al. (2015) developed an obesity prevention program that trained
participants to use cognitive reappraisals when confronted with un-
healthy foods, reasoning that if participants learn to automatically apply
these reappraisals, they will show reduced reward and attention region
responsivity and increased inhibitory region responsivity to food images
and cues for high-fat/high-sugar food, which should reduce caloric intake.
Young adults at risk for weight gain by virtue of weight concerns (N =
148) were randomized to this new Minding Health preven tion program,
a prevention program promoting gradual reductions in caloric intake
and increases in exercise (the Healthy Weight intervention), or an obesity
education video control condition (Stice et al., 2015). A subset of Minding
Health and control participants completed an fMRI scan pre and post in-
tervention to assess neural responses to images of high-fat/sugar foods.
Minding Health participants showed significantly greater reductions in
body fat than controls and percentage of caloric intake from fat and
sugar than Healthy Weight participants, though these effects attenuated
by 6-month follow-up. Further, Minding Health participants showed
greater activation of an inhibitory control region (inferior frontal gyrus)
and reduced activation of an attention/expectation region (mid cingulate
gyrus) in response to palatable food images relative to pretest and con-
trols. Although the Minding Health intervention produced some of the
hypothesized effects, it only affected some outcomes and the effects
often showed limited persistence.
It is possible that the addition of rtfMRI neurofeedback training to
the Minding Health intervention may lead to more persistent effects
and improved treatment outcomes. Given the emphasis on the use of
cognitive reappraisal in the Minding Health intervention, fMRI-based
neurofeedback was preferred compared to other, complementary tech-
nologies su ch as electroencephalography (EEG) due to th e superior
spati al resolution of fMRI, including the ability to target su bcortical
brain structures critical to the re gulation of food intake behavior for
neurofeedback. The first study demonstrating the therapeutic potential
of rtfMRI neurofeedback was published in 2005 (deCharms et al.,
2005). There have been several studies now demonstrating rtfMRI
neurofeedback-induced changes in brain function in multiple structures
of relevance to disorders of ingestive behavior, including the amygdala
(Zotev et al., 2011; Zotev et al., 2013; Bruhl et al., 2014), insula (Caria
et al., 2007; Caria et al., 2010; Frank et al., 2012), aCC (deCharms et al.,
2005; Chapin et al., 2012; Li et al., 2013), and PFC (Rota et al., 2009;
Sitaram et al., 2011). Several groups have also reported successful appli-
cation of rtfMRI to modify cognitive and behavioral processes relevant
for the treatment of clinical disorders (for review of these studies see
deCharms, 2007
; W
eiskopf et al., 2007; deCharms, 2008; Bi rbaumer
et al. , 2009; Caria et al. , 2012; Chapin et al., 2012; Weiskopf, 2012;
Sulzer et a l., 2013), including an application in the area of obesity
(Frank et al., 2012). For a revi ew of potential applications of rtfMRI
Fig. 1. A model of the cognitive control of emotion (MCCE). (A) Diagram of the processing steps involved in generating an emotion and the ways in which cognitive control processes (blue
box) might be used to regulate them. As described in the text, the effects of different emotion regulation strategies (the red arrows descending from the cognitive control processes box)
can be understood in terms of the stages of the emotion generation sequence that they influence. The pink box seen at the appraisal stage is meant to indicate that neural systems involved
in generating emotion support this process. (B) Neural systems involved in using cognitive strategies, such as reappraisal, to regulate emotion (left, blue boxes), systems involved in gen-
erating those responses (left, pink boxes), and systems with an undefined or intermediary role in reappraisal (left, yellow boxes; adapted from Ochsner et al., 2012 with permission). Brain
schematic representations were provided by Servier Medical Art (http://www.servier.fr).
11D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
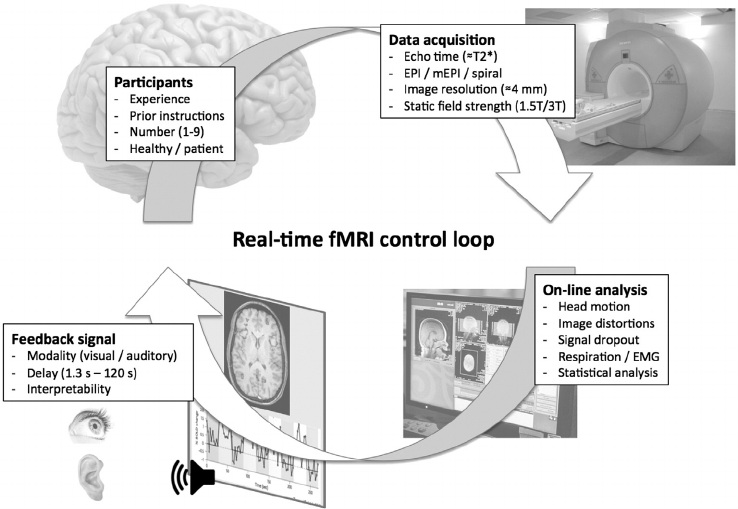
neurofeedback for disorders of ingestive behavior, see Bartholdy et al.
(2013).
3.1.3. Proof-of-concept for the use of rtfMRI neurofeedback with cognitive
reappraisal for the regulation of food intake behavior
As a proof-of-concep t, Stoec kel et al. (2013a) completed a study
combining the use of cognitive reappraisal strategies (described
above) and rtfMRI neurofeedback in 16 healthy-weight participants
(BMI b 25) without a history of disordered eating who we re acutely
fasted. In a pilot study, an independent sample of 5 participants were
able to improve control of inhibition-related (lateral inferior frontal cor-
tex), but not reward-related (ventral striatum), brain activation using
rtfMRI neurofeedback (Stoeckel et al., 2011). Therefore, lateral inferior
frontal cortex was selected as the target bra in region of interest for
neurofeedback. Participants completed two neurofeedback visits,
1 week apart. At each visit, participants initially performed a functional
localizer task, the stop signal task, which is a well-known test of
inhibitory control (Logan et al., 1984) that activates lateral inferior fron-
tal co rtex (Xue et al., 2008). Participants then attempted to self-regulate
brain activity within this region of interest using cognitive regulation
strategies while viewing highly palatable food images. While viewing
the food images, participants were asked to either mentalize their
urge to eat the food (crave or 'upregulation’) or consider the long-
term future consequences of over-consuming the food (cognitive reap-
praisal or ‘downregulation’). At the end of each neurofeedback training
trial, participants received feedback from the brain region identified by
the localizer scan using custom in-house software developed at the
Massachusetts Institute of Technology (for technical details, see Hinds
et al., 2011). Participants also recorded their subjective cravings in re-
sponse to the food images throughout the session. Compared to upreg-
ulation trials, participants had less reward circuit activity (ventral
tegmental area (VTA), VS, amygdala, hypothalamus, and vmPFC) and
decreased craving when using reappraisal strategies (ps b 0.01). In addi-
tion, the difference in activity in the VTA and hypothalamus during up-
regulation vs. reappraisal was correlated with craving (rs = 0.59 and
0.62, ps b 0.05). Neurofeedback training led to improved control of lat-
eral inferior frontal cortex; however, this was not related to mesolimbic
reward circuit activation or craving. rtfMRI neurofeedback training led
to increased control of brain activity in healthy-weight participants ;
however, neurofeedback did not enhance the effect of cognitive regula-
tion strategies on mesolimbic reward circuit activity or craving after two
sessions (Stoeckel et al., 2013a).
3.1.4. Consideration for rtfMRI neurofeedback ex periments targeting
disorders of ingestive behavior
Before testing this protocol in individuals with disorders of ingestive
behavior, including obesity, it will be important to consider which brain
region(s) are good targets for rtfMRI neurofeedback training and how
best to represent neuropsychological functions at the neural systems
level. For example, the hypothalamus has a central role in the regulation
of ingestive behavior; however, it is a relatively small structure with sev-
eral subnuclei with heterogeneous functional properties that contribute
to the regulation of hunger, satiety, and metabolism, but also less closely
related functions such as sleep. Given the resolution of rtfMRI, it is possi-
ble that a neurofeedback signal from the hypothalamus would include in-
formation from a combination of these subnuclei, which may impact the
effective ness of efforts to improve voluntary regulation of a specificfunc-
tion (e.g. hunger). It is also important to consider the likelihood that the
targeted function is amenable to training. For example, it is possible that
targeting the homeostatic control of feeding represented in the hypothal-
amus and brainstem may lead to compensatory behaviors to defend the
set point of body weight given that these are central, highly conserved
neural circuits that control normal en
ergy homeostasis. However, it may
be possible to target hedonic, cognitive control, or other “non-homeostat-
ic” mechanisms (and their supporting neural circuits) that may help indi-
viduals more effectively to adapt to their environment while minimizing
compensatory behaviors that may lead to persistent obesity. It is also un-
clear whether better outcomes would be expected from ne urofeedback
from an anatomically-restricted brain region or set of brain regions or
whether a network approach using connectivity-based feedback or
multi-voxel pattern classification (MVPA) may be preferable given the
regulation of ingestive behavior involves both homeostatic and non-
homeostatic mechanisms represented in a distributed neural circuitry in
the brain (Kelley et al., 2005a). An ROI-based approach could be used to
Fig. 2. Schematic of real-time functional magnetic resonance imaging (rtfMRI) control loop. Typically, echo planar imaging (EPI) images are extracted from the magnetic resonance (MR)
scanner online, analyzed by third-party software, and then presented back to the subject for the purposes of neural self-regulation (adapted from Weiskopf et al., 2004) mEPI: multi-echo
EPI; EMG: electromyography.
12 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

target a specific brain region (e.g., vmPFC for the regulation of subjective
reward value of highly palatable food cues). Another option is to
normalize disrupted functional connections between a set of brain
regions instantiating a well-characterized function (e.g., the entire
mesocorticolimbic reward system consisting of VTA-amygdala-VS-
vmPFC). MVPA may be preferable if there is a distributed set of multiple
brain networks that underlie a complex neuropsychological construct
such as cue-induce food craving. It may also be necessary to augment
rtfMRI neurofeedback training by incl uding a psychological or cognitive
training intervention, such as Minding Health, prior to neurofeedback. Fi-
nally, it may be necessary to augment psychological or cognitive training
with adjunctive pharmacotherapy or device-based neuromodulation
such as TMS to enhance the efficacy of neurofeedback training. For a
more detailed discussion of these and other issues of relevance to the de-
sign of rtfMRI neurofeedback studies of disorders of ingestive behavior,
see Stoeckel et al. (2014).
3.2. Transcranial magnetic stimulation (TMS) an d transcranial dir ect-
current stimulation (tDCS)
3.2.1. Introduction to TMS and tDCS
Non-invasive neuromodulation techniques allow the external ma-
nipulation of the human brain in a safe manner, without the require-
ment of a neurosurgical procedure. Over the past two decades there
has been growing interest in the use of non-invasive neuromodulation
in neuro logy and p sychiatry, mo tivated by the shortage of effective
treatments. The most commonly used techniques are transcranial mag-
netic stimulation (TMS) and transcranial direct current simulation
(tDCS). TMS is based on the application of rapidly changing magnetic
fields that are delivered with a coil encased in plastic that is placed
over the scalp of the subject (Fig. 3A). These varying magnetic fields
cause an induction of se condary currents in the adjacent c ortex that
can be strong enough to trigger neuronal action potentials (Barker,
1991; Pascual-Leone et al., 2002; Hallett, 2007; Ridding and Rothwell,
2007). TMS can be administered in single or multiple pulses, also called
repetitive TMS (rTMS). In the case of tDCS, mild DC currents (typically in
the order of 1–2 mA) are applied directly over the head through a pair of
saline-soaked electrode pads connected to a battery-like device
(Fig. 3B). Approximately 50% of the current delivered by tDCS pene-
trates the scalp and can raise or decrease the resting membrane poten-
tial of neurons in underlying areas (anodal or cathodal tDCS stimulation,
respectively), causing changes in spontaneous firing (Nitsche et al.,
2008). rTMS and tDCS can induce transient/lasting changes that are be-
lieved to be mediated by changes in synaptic strength. A comprehensive
overview of these techniques and their mechanisms of action are be-
yond the scope of this section and can be found elsewhere (Pascual-
Leone et al., 2002; Wassermann et al., 2008; Stagg and Nitsche, 2011).
Table 3 presents a summary of key differences between TMS and
tDCS. While TMS and tDCS have been and still remain the dominant
techniques in the field, other novel or modified forms of non-invasive
neuromodulation have been developed in recent years and are actively
under investigation, such as deep TMS (dTMS) (Zangen et al. , 2005),
high-definition tDCS (HD-tDCS) (Datta et al., 2009), transcranial
alternate current simulation (tACS) (Kanai et al., 2008), or transcran ial
random noise stimulation (tRNS) (Terney et al., 2008
). Additional tech-
ni
ques for neuromodulation are those that are invasive (cf. Section 4),
such as deep brain stimulation (DBS), or those that target peripheral
nerves, such as vagus nerve stimulation (VNS).
Over the past two decades there has been remarkable progress in
our understanding of the neurocognitive basis of human eating behav-
ior, obesity and eating disorders. A number of neuroimaging and neuro-
psychology studies have identified the crosstalk between reward and
cognition as a central component in the regulation of eating behavior
and body weight in humans (Alonso-Alonso and Pascual-Leone, 2007;
Wang et al., 2009a; Kober et al., 2010; Hollmann et al., 2012; Siep
et al., 2012; Vainik et al., 2013; Yokum an d Stice, 2013). As research con-
tinues in this field, the available knowledge makes it possible to begin
exploring interventions that shift from behavior to neurocognition as
the primary targ et. Overall, neuromodulatory techniques can bring
valuable insights and open novel therapeutic avenues in this new
Fig. 3. Pictures of (A) butterfly coils for transcranial magnetic stimulation (TMS) and (B) electrodes and battery for transcranial direct current stimulation (tDCS).
Table 3
Comparative between TMS and tDCS.
Characteristics Transcranial magnetic stimulation (TMS) Transcranial direct current stimulation (tDCS)
Spatial resolution Very good (approximately 1 cm
3
) Poor (conventional tDCS) to good (HD-tDCS)
Temporal resolution Excellent (ms) Poor (s)
Tolerability Very good to fair, depending on protocols Excellent to very good
Safety Good (can rarely cause seizures) Excellent
Cost High range (typically $30,000–$100,000) Low to middle range ($250 –$10,000)
Portability Fair Excellent
Regulatory status Cleared for some specific devices and applications (depression, cortical mapping, migraine) Not cleared. Only off label application
Consumer versions No Yes
13D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

Table 4
Summary of studies with TMS and tDCS in the field of human eating behavior.
Study characteristics Subjects, status Stimulation protocol Main outcome measures Main findings References
TMS studies
Acute effects (single session);
parallel design, randomized,
double-blind, sham-controlled
n = 37 subjects (mean age: 30;
86.8% of women) with
bulimic-type eating disorders
Target: DLPFC; two groups: active (left DLPFC, 5 cm
anterior to hand motor area) and control (sham rTMS);
parameters: 1000 pulses, 10 Hz rTMS, 20 min,
intensity 110% motor threshold
Food craving (VAS) while exposed
to real food and a movie of food;
frequency of bingeing in a 24-hour
follow-up period
Decrease in food craving; reduction in bingeing
in 24 h post rTMS
Van den Eynde
et al. (2013)
Acute effects (single session);
crossover design, randomized,
single-blind, sham-controlled;
improved sham condition
matched for perceived
painfulness of the stimulation
n = 10 women (mean age: 28.3)
with frequent food cravings
(≥3 times/week during the past
month); 3-hour fasting
Target: DLPFC; two conditions: active (left DLPFC) and
control (sham rTMS); Parameters: 3000 pulses, 10 Hz
rTMS, 15 min, intensity 100% motor threshold
Food craving (VAS) while exposed
to food images
No differences between conditions Barth et al. (2011)
3-week intervention; parallel
design, randomized,
double-blind, sham-controlled;
preceded by 1-week of sham
rTMS in all participants
n = 14 women (mean age: 27.4)
with bulimia nervosa
Target: DLPFC; 1 week with sham rTMS before
randomization to avoid high placebo responders; two
groups: active (left DLPFC) and control (sham rTMS);
parameters: 3 weeks, 15 sessions, 2000 pulses per
session. 20 Hz rTMS, intensity 120% motor threshold
Change in binges and purges;
mood and compulsive symptoms
No differences between groups Walpoth et al.
(2008)
Acute effects (single session);
parallel design, randomized,
double-blind, sham-controlled
n = 28 women (mean age: 25.8)
with frequent food cravings
(≥3 times/week); 3–4 h fasting
Target: DLPFC; two groups: active (left DLPFC) and
control (sham rTMS); parameters: 1000 pulses, 10 Hz
rTMS, 20 min, intensity 110% motor threshold
Food craving (VAS); consumption
of snack foods
Decrease in food craving; no effect on snack
consumption
Uher et al. (2005)
tDCS studies
Acute effects (single session);
crossover design, randomized,
double-blind, sham-controlled
n = 9 women (mean age: 23.4);
all lean with frequent food
cravings (≥3 times/day); 3-hour
fasting
Target: DLPFC; two conditions: active (anode over
F4/cathode over F3) and control (sham tDCS);
parameters: 2 mA, 20 min, 35 cm
2
sponge electrodes
EEG event-related potentials
during an Go/No-Go task; food
craving (VAS) while exposed to
real food and a movie of food;
snack intake; attentional bias for
food (eye tracking)
Reduction of the frontal N2 component and
enhancement of the P3a component of No-Go
responses; reduction in caloric intake
Lapenta et al.
(2014)
8-day intervention; crossover
design, randomized,
single-blind, sham-controlled
n = 14 men (mean age: 24.8), all
lean, with low scores in
three-factor eating
questionnaire; 6-hour fasting
Target: DLPFC; two conditions: active (anode over an
area 5 cm anterior to the right motor cortex/cathode
over the left forehead) and control (sham tDCS);
parameters: 1 mA, 20 min, 35 cm
2
sponge electrodes
Subjective appetite (ratings and
VAS); free eating from a
standardized multi-choice test
buffet
14% decrease in total calorie consumption, at
the expense of carbohydrates; decrease in
appetite: nonspecific and specific (sweets and
savory food)
Jauch-Chara et al.
(2014)
Acute effects (single session);
crossover design, randomized,
double-blind, sham-controlled
n = 17 women (mean age: 26.4;
29.4% of overweight) with
frequent food cravings (≥1/day)
Target: DLPFC; two conditions: active (anode over
F4/cathode over F3) and control (sham tDCS);
parameters: 2 mA, 20 min, 4 cm
2
sponge electrodes
Food craving ratings while
viewing movies of food; temporal
discounting task; free eating test
Decrease in craving for sweets; no effect on
temporal discounting no change in free eating;
moderating effect of temporal discounting:
participants with more reflective choice
behavior showed more susceptibility to
anticraving effects of tDCS
Kekic et al. (2014)
Acute effects (single session), in
combination with an exercise
bout of about 200 calories;
crossover design, randomized,
single-blind, sham-controlled
n = 9 subjects (mean age: 24;
55% of men; all overweight or
obese), 2- to 3-hour fasting
Target: DLPFC; two conditions: active (anode over
F3/cathode over Fp2) and control (sham tDCS);
parameters: 2 mA, 20 min, 35 cm
2
pads
Subjective appetite (VAS) Decrease in desire to eat with tDCS; greater
appetite suppression with the combination of
tDCS and exercise
Montenegro et al.
(2012)
Acute effects (single session);
crossover design, randomized,
single-blind, sham-controlled
n = 19 subjects (mean age: 32.5;
68.4% of women; about 58% of
overweight or obese) with
frequent food cravings
(≥3 times/week during the past
month); 4-hour fasting
Target: DLPFC; two conditions: active (anode over
F4/cathode over F3) and control (sham tDCS);
parameters: 2 mA, 20 min, standard sponge electrodes
Food craving and ability to resist
tasting (VAS) while viewing food
images; free consumption of
previously presented foods
Decrease in food craving, particularly for sweets
and carbohydrates; no change in food
consumption
Goldman et al.
(2011)
Acute effects (single session);
crossover design, randomized,
double-blind, sham-controlled
n = 23 subjects (mean age: 23.7;
91% of women) with frequent
food cravings (≥3 times/day);
3-hour fasting
Target: DLPFC; three conditions: active 1 (anode over
F3/cathode over F4), active 2 (anode over F4/cathode
over F3), control (sham tDCS); parameters: 2 mA,
20 min, 35 cm
2
sponge electrodes
Food craving (VAS) while exposed
to real food and a movie of food;
snack intake; attentional bias for
food (eye tracking)
Decrease in food craving only in condition
active 1; decrease in snack intake in conditions
active 1 and 2; decrease in attentional bias for
food only in condition active 1
Fregni et al.
(2008)
rTMS: repetitive transcranial magnetic stimulation; tDCS: transcranial di r ect current stimulation; DLPFC: dorsolateral prefrontal co r tex; VAS: visual analogue scale; Electrode montage for tDCS: F3 (left DLPFC), F4 (right DLPFC), Fp2 (right supra-
orbital); EEG: electroencephalography; N2, P3a: specific EEG electrophysiological measures.
14 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
scenario that places neurocognition as a central component of human
eating behavior.
3.2.2. Summary of clinical studies to mo dify eating behavior and eating
disorders
Eating behavior is a recent application in the field of non-invasive
neuromodulation, with the earliest study dating back to 2005 (Uher
et al., 2 005). TMS and tDCS are the only techniques that have been
used in this context. Table 4 provides a summary of randomized, con-
trolled, proof-of-concept studies. To date, these studies have tested
acute, single-session effects only, with two exceptions: one study with
rTMS in bulimic patients (3 weeks), and a recent study with tDCS in
healthy men (8 days). The targeted area, dorsolateral prefrontal cortex
(dlPFC), is a complex brain region related to executive functions that
supports cogn itive control of food intake. Overall, the underlying
hypothesis is that enhancin g dlPFC activ ity may alte r the reward –
cognition balance towards facilitation of cognitive control and possibly
suppression of reward-related mechanisms tha t drive food craving and
overeating. The specific dlPFC-dependent cognitive processes being
affected by rTMS or tDCS and mediating the observed behavioral effects
remain largely unknown. Possibilities include changes in reward valua-
tion mechanisms (Camus et al., 2009), attentional biases (Fregni et al.,
2008), or inhibitory control (Lapenta et al., 2014). rTMS studies have
targeted the left dlPFC only, via excitatory protocols (10 and 20 Hz).
tDCS studies have targeted both the right and left dlPFC, with slightly dif-
ferent approaches/montages. The majority of studies — allwithtDCSand
one with rTMS — have evaluated effects on food craving, subjective appe-
tite and food intake. Altogether, they have consistently found an acute
suppression in the scores of self-reported food craving and appetite mea-
sured by ratings or visual analogue scales (VAS). There is some indication
that the effect with tDCS may be more specific for craving of sweets.
Changes in food intake have been rather inconsistent with a single session
of rTMS or tDCS. In the longest study to date with tDCS (8 days), the au-
thors found a 14% decrease in calorie consumption (Jauch-Chara et al.,
2014). An important bias in some studies is the use of a sham procedure
without any current flow as control, instead of sham stimulation in areas
that are irrelevant to food intake for example. Since the stimulation is
sometimes perceptible by the patient, we cannot exclude a placebo effect
in some cases.
Studies with eating disorder pati ents so far have used only rTMS.
Several case reports (Ka molz et al., 2008; McClelland et al., 2013b)
and an open-label study (Van den Eynde et al., 2013) (not included in
the table) suggest potential for rTMS in anorexia nervosa, but findings
should be replicated in placebo-control led trials. For the case of B N, an
early case report suggested potential benefits with rTMS (Hausmann
et al., 2004), but this was not confirmed in a subsequent clinical trial
that used this technique over 3 weeks (Walpoth et al., 2008). A recent
case study reported beneficial effects using 10 Hz rTMS applied over a dif-
ferent target, the dorsomedial prefrontal cortex, in a refractory patient
with BN (20 sessions, 4 weeks) (Downar et al., 2012). This brain region
represents a promising target given its general role in cognitive control,
specifically performance monitoring and action selection (
Bush et al.,
20
00; Krug and Carter, 2012), and its link with th e clinical course of AN
and BN (McCormick et al., 2008; Goddard et al., 2013; Lee et al., 2014).
3.2.3. Future needs: from empirically-driven studies to rational and mecha-
nistic approaches
Results from these initial studies provide a good proof of concept for
the translation of non-invasive neuromodulation into the field of eating
behavior. Potential applications can be the enhancement of cognitive
control and underlying brain regions to support successful weight loss
maintenance in obesity (DelParigi et al., 2007; McCaffery et al., 2009;
Hassenstab et al., 2012), or rebalancing ventral and dorsal brain systems
in AN and B N (Kaye et al., 2010). While the overall rationale is quite
clear, the specifics of using noninvasive neuromodulation in the treat-
ment of obesity and eating disorders are currently under investigation
and the best approaches and protocols remain to be defined. Noninva-
sive neuromodulation could be used alone or in combination with
other strategies such as behavioral therapy, cognitive training, physical
fitness and nutrition, to create synergistic effects. Aside from therapeu-
tic applications, neuromodulation techniques can be used to inform
disease mechanisms, e.g. examining the causal involvement of a specific
region in a gi ven cognitive process or behavioral manifestation
(Robertson et al., 2003). Recent st udies hav e examined the potential
of TMS to quantify reward responses (Robertson et al., 2003) and results
from this line of work could eventually lead to the development of ob-
jective biomarkers that can help study eating phenotypes.
While there is a high potential for future uses of neuromodulation in
the field of eating behavior, there are still many limitations and open
questions. Blinding is a key issue, called into question by one rTMS
study in food craving and a tDCS study where subjects were able to
guess the condition they had received with 79% accuracy (Barth et al.,
2011; Goldman et al., 2011). Future studies should consider parallel de-
signs to overcome this problem, or at least rule out the possibility of in-
complete blinding when crossover designs are used. Another need to
address in future studies is the addition of more clinically meaningful
outcomes. rTMS and tDCS have caused changes in measures that are
sensitive and valid in an experimental setting, e.g. visual analogue
scales, but their clinical relevance remains uncertain.
All studies to date have targeted the DLPFC, as in other applications
of tDCS and rTMS in neuropsychiatry. There is need to explore addition-
al targets; dorsomedial prefrontal cortex/dorsal anterior cingulate cor-
tex (daCC), parietal regions and anterior insular cortex are particularly
promising. Both rTMS and tDCS are currently optimized to target brain
regions located on the surface. Reaching deeper brain structures may
be more feasible with HD-tDCS, or with dTMS for the case of mid-
depth areas such as insular cortex (Zangen et al., 2005). A recently de-
scribed method for rTMS consists of guiding stimulation on the basis
of intrinsic functional connectivity determined by resting-state fMRI
(Fox et al., 2012a; Fox et al., 2012b). Aside from targeting brain regions
alone, non-invasive neuromodulation can be administered with simul-
taneous cognitive training. This approach may lead to more functional
effects (Martin et al., 2013; Martin et al., 2014) and is articularly suited
for eating disorders and obesity, where there are impairments in specif-
ic neurocognitive domains, such as executive functions, even though the
picture is complex (Alonso-Alonso, 2013; Balodis et al., 2013
). The use
of
cognitive performance and/or ways of measuring brain activity can
also facilitate target monitoring and overall contribute to optimize the
delivery of neuromodulation. A recent tDCS study points in that direc-
tion, with a combination of EEG event-related potentials and behavioral
measures of food craving and food intake (Lapenta et al., 2014).
More work is needed to understand potential sources of variability
in the response to neuromodulation. The majority of participants in
these rTMS/tDCS studies have been young women, with variable BMI.
Gender effects remain unaddressed, with no direct comparisons so far
between women and men, but differences are likely based on the effect
of gender on brain correlates of appetite (Del Parigi et al., 2002; Wang
et al., 2009a). When studying food-related processes and mechanisms,
it is also important to consider the underlying variability in brai n activ-
ity related to metabolic state. As mentioned in Table 4, subjects have
been stimulated typically in an intermediate state, i.e. about 2–4h
after a meal. It is unknown whether different conditions can cause bet-
ter results. Another potential confounder that remains unaddressed is
the role of dieting. Patients with eating disorders and obesity usually
follow diets that can be quite restrictive and, more importantly, could
have substa ntial eff ects on brain excitability and also in the se nsitivity/
response to neuromodulation (Alonso-Alonso, 2013). An additional fac-
tor is whether a person receives TMS or tDCS in a weight-reduced state
or in a weight-stable state, which would also have consequences in the
resting brain state and neuromodulatory response (Alonso-Alonso,
2013). Lastly, at a more technical level, individual head anatomy can
alter electric or electromagnet ic tra nsmission. This issue has been
15D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
extensively addressed using computational models of tDCS ( Bikson
et al., 2013). A particular concern in this regard is whether head fat, a
relatively resistive tissue, could affect current density distribution
(Nitsche et al., 2008; Truong et al., 2013).
Regarding side effects, both TMS and tDCS are non-invasive, safe and
rather painless techniques that are very well tolerated in the vast major-
ity of cases (Nitsche et al., 2008; Rossi et al., 2009). The most common
adverse effects with rTMS is headache, which occurs approximately in
25–35% of patients during dlPFC stimulation, followed by neck pain
(12.4%) (Machii et al., 2006). With tDCS, a substantial proportion of peo-
ple (N50%) report transient sensations under the electrode that can be
defined as tingling, itching, burning or pain, and are usually mild or
moderate (Bruno ni et al., 2011). When designing a study it is important
to exclude participants with contraindications to receive either TMS or
tDCS, and collect adverse events in a systematic manner. There are stan-
dardized questionnaires available for that purpose (Rossi et al., 2009;
Brunoni et al., 2011). The most worrisome adverse effect of non-
invasive neuromodulation is the induction of seizure, which has been
reported onl y a few times with rTMS (Rossi et al., 2009).
The field of neuromodulation is expanding very quickly and it has
started to cross boundaries beyond the medical and research community
to curious individual consumers and recreational users. It is important
that we, the community of scientists working in neuromodulation,
remain committed to guarantee research integrity and maintain high eth-
ical standards in the use of these methods. The possibility of manipulating
the human brain can be as fascinating and tempting as trying a new diet
to curb appetite, but it is important to remind that the current state of sci-
ence in this field is far from being conclusive. And, as importantly, trans-
cranial devices are not playthings (Bikson et al., 2013).
4. Invasive neuromodulation strategies: recent developments and
current challenges
4.1. Overview of the peripheral neuromodulation strategies in the context of
food intake and weight control
4.1.1. Changes in vagal signaling during obesity
The homeostatic control of food intake involves a complex, bidirec-
tional communication system between the periphery and the central
nervous system that h as been extensively reviewed (Williams and
Elmquist, 2012). The vagus nerve, because it contains mainly afferents
neurons that arise from the gut, the pancreas and the liver, plays a key
role in this communication. In non-obese individuals, chemosensory
(acid-sensing ion channels) and mechanosensory vagal receptors signal
immediate availability of food (Page et al., 2012). Further, several
hormones including ghrelin, cholecystokinin (CCK) and peptide tyro-
sine tyrosine (PYY) have the capability to activate vagal afferents
(Blackshaw et al., 2007).
Aside from an excessive accumulation of fat, a substantial body of ev-
idence suggests that obesity and/or high fat diet is associated with alter-
ation of peripheral responses to nutrients. Studies in rodents subjected
to a high-fat diet (HFD), or in diet-induced obesity consistently show re-
duced suppressive effects of intestinal nutrients on food intake com-
pared to control animals (Covasa and Ritter, 2000; Little, 2010). This is
associated with a reduced sensitivity of jejunal afferents (primarily
vagal) to low-level distension and reduced excitability of identified jeju-
nal vagal afferents within the nodose ganglion to CCK and 5-HT expo-
sure (
Daly et al., 2011).
Corresponding reductions in vagal afferent
expression of receptors for CCK, 5-HT and other anorexic GI peptides
have been reported in the nodose ganglion (Donovan and Bohland,
2009). Additionally, HFD reduced the responses of gastric vagal tension
receptors to distension and augmented the inhibitory effect of ghrelin
on vagal afferents. Alternatively, while leptin potentiated vagal mucosal
afferent responses, potentiation of mucosal afferents by leptin was lost
after HFD (Kentish et al., 2012). The loss of vagal afferent signaling to-
gether with the altered processing of vagal signals within the dorsal
vagal complex suggest that resetting these sensitivities by chronic
vagal stimulation (VNS) might reduce overeating.
4.1.2. Effects of vagal stimulation
Unilateral left cervical vagal stimulation is approved for treatment-
resistant depression and intractable epilepsy in the European Union,
the United States and Canada. Epileptic patients reported frequently
changes in eating behavior with alteration in diet preferences (Abubakr
and Wambacq, 2008). These reports generated further investigations, ini-
tially through pure serendipity, which subsequently used animal models
to evaluate the effects of VNS on food intake and related weight control
(for synthetic tables on VNS studies, please see Val-Laillet et al., 2010;
McClelland et al., 2013a). The original studies in 2001 of Roslin and
Kurian (2001) in dogs and the other from Krolczyk et al. (2001) in rat s
suggested a decrease in weight gain or a weight loss during chronic
vagal stimulation. Surprisingl y, despite different surgical approache s, the
results demonstrated by these authors were identical. Indeed, Roslin
and Kurian (2001) used a bilateral cuff placement within the thorax
(hence stimulating both dorsal and ventral vagal trunks) while Krolczyk
et al. (2001) used a cervical placement on the sole left vagus to be similar
with the clinical setup for intractable epilepsy. Since these pioneering
studies, several research groups, including us, have published positive re-
sults using various electrodes locations, electrodes set-up and stimulation
parameters. The first attempt to evaluate the adequate location of the
electrodes for food intake control was performed by Laskiewicz et al.
(2003). They demonstrated that bilateral VNS is more effective than uni-
lateral stimulation. Using a large animal pre-clinical model, we used
juxta-abdominal bilateral vagal stimulation on the longest longitudinal
study performed to date. We show that chronic vagus nerve stimulation
decreased weight gain, food consumption and sweet craving in adult
obese minipigs (Val-Laillet et al., 2010). Further, unlike others studies per-
formed in smaller animal models, efficacy improves over time in a man-
ner comparable that already exemplified in intractable epilepsy patients
(Arle and Shils, 2011).
Unfortunately, the positive results observed in almost all animal pre-
clinical studies have not been confirmed in humans. Because of regula-
tory restraints, all human studies have been performed using left cervi-
cal vagal cuff only with stimulation settings similar or closely identical
to those used for depression or epilepsy. Despite using long-term stim-
ulation, weight loss was found in about half of the subjects (Burneo
et al., 2002; Pardo et al., 2007; Verdam et al., 2012). At present, no
clear explanation for these non-responsive subjects can be offered. A re-
cent study by Bodenlos et al. (2014) suggests that large BMI individuals
are less responsive to VNS than lean people. Indeed, in their study, VNS
suppressed food intake in lean patients only.
Several authors have investigated the physiological basis of VNS
with specific reference to the left cervical placement of the electrode.
Vijgen et al. (2013) have demonstrated in an elegant study combining
PET imaging of the brown adipose tissue (BAT) and a cohort of VNS ep-
ileptic patients that VNS signific
antly increases energy expenditure.
Moreover, the change in energy expenditure was related to the change
in BAT activity suggesting a role for BAT in the VNS increase in energy
expenditure. VN S has been demons trated to change brain activity
throughout the entire cerebrum (Conway et al., 2012 ) and modulate
the monoaminergic systems (Manta et al., 2013). In humans, left VNS
induced rCBF (regional cerebral brain flow) decreases in the left an d
right lateral OFC and left inferior temporal lobe. Significant increases
were found also in the right dorsal anterior cingulate, left posterior
limb of the internal capsule/medial putamen, the right superior tempo-
ral gyrus. Despite the critical importance of these areas towards control
of food intake and depression, no correlation was found between brain
activation and the outcome of depression score after 12 months of VNS
therapy. Therefore, it remains to be demonstrated that the observed
brain activity changes are causative factors to explain VNS effects. The
demonstration in rats that VNS modulates visceral pain-related affective
memory (Zhang et al., 2013) might represent an alternative pathway
16 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
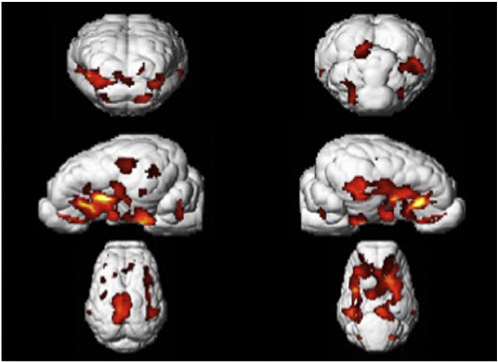
that could explain the beneficial effects observed on about half of the
patients. Our early studies on brain activation after juxta-abdominal bi-
lateral VNS performed in growing pigs (Biraben et al., 2008) using single
photon gamma scintigraphy was the first to evaluate VNS effects on the
non-pathological brain. We showed the activation of two networks. The
first one is associated with the olfactory bulb and primary olfactory
projections areas. The second one involves areas that are essential to in-
tegrate gastro-duodenal mechanosensory information (hippocampus,
pallidum) so to give a hedonic value to these. Similar results have
been reported in rats either using PET (Dedeurwaerdere et al., 2005)
or MRI (Reyt et al., 2010). Unlike behaviora l effects that take several
weeks to be identified, alterations in brain metabolism identified by
PET imaging were present 1 week only after the onset of VNS therapy.
In our porcine model of juxta-abdominal VNS, the cingulate cortex, pu-
tamen, caudate nucleus and substantia nigra/tegmental ventral area, i.e.
the main reward meso-limbic dopaminergic network, presented chang-
es in brain metabolism (Malbert, 201 3; Divoux et al., 2014)(Fig. 4). The
massive activation of the reward network at an early stage of the chron-
ic stimulation suggests that brain imaging might be used as a tool to op-
timize the vagal stimulation parameters.
As with several others therapies, the relatively poor success of VNS
in obese humans could be explained by an insufficient understanding
of the action of VNS on the brain networks controlling food intake.
Translation of animal models into clinical practice was (too) quick with-
out experimental clues towards a normalized procedure for stimulation.
For instance, as mentioned above, early human studies were performed
with unilateral cervical vagal st imulation whereas all anima l studies
suggested that bilateral juxta-abdominal location for the stimulating
cuffs was more appropriate. Furthermore, we are still in need for early
clues to refine stimulation parameters without having to wait for chang-
es in body weight. It can be speculated that brain-imaging methods to-
gether with computational model of VNS (Helmers et al., 2012)might
be of significant help towards this clinical requirement.
4.1.3. Effects of vagal blockade
Several patients after vagotomy performed as a cure for ulcer disease
report short-term loss of appetite; less commonly, prolonged loss of ap-
petite and further weight loss or failure to regain weight have been
noted (Gortz et al. , 1990). Bilateral truncal vagotomy has been used
historically as a treatment for obesity refractory to other therapies,
and has been associated with satiety and weight loss (Kral et al.,
2009). Based upon this observation and although that it has been re-
ported that the effects on body weight are lost over time (Camilleri
et al., 2008) and that truncal vagotomy was virtually ineffective to re-
duce solid food intake (Gortz et al., 1990), vagal blockade therapy was
tested in humanswith the primary objective to reduce weight of morbid
obese individuals. Vagal blockade was performed bilaterally at the ab-
dominal level using high frequency (5 kHz) current pulses. The large
scale, long lasting study called EMPOWER (Sa rr et al., 2012)demon-
strated that weight loss was not greater in treated compared to control.
Despite this therapeutic failure, Vbloc therapy in type 2 diabetic patients
(DM2) reduces the level of HbA
1c
and hypertension shortly after activa-
tion of the device (Shikora et al., 2013). This benefit and the stability of
the improvement over time suggest that the mechanisms of action may
be, at least in part, independent from weight loss. Since these parame-
ters are entirely related to fat deposition and truncal vagotomy led to
significant reductions in diet-induced visceral abdominal fat deposition
(Stearns et al., 2012), it is quite possible that the efferent neurons
blocked by the therapy might be responsible for the improvements ob-
served in DM2 patients.
4.2. State of the art of deep brain stimulation (DBS) and its potential for
tackling obesity and eating disorders
4.2.1. Overview on the state of the art in DBS
4.2.1.1. Current therapeutic applications of DBS. Deep brain stimulation
(DBS) is a technique based on implanted elect rodes for treating
neuromotor disorders such as Parkinson3sdisease(PD),aswellasepilep-
sy, while showing promise for psychological disorders like treatment-
resistant depression (TRD) and obsessive–compulsive disorders (OCD)
(Perlmutter and Mink, 2006).
The subthalamic nucleus (STN) is commonly targeted for PD, while
the anterior nucleus of the thalamus (ANT), subgenual cingulate
(Cg25), and nucleus accumbens (Nac) are respectively targeted for
epilepsy, TRD and OCD (Fig. 5). The penetration of DBS, roughly
10,000 patients per year worldwide, is minuscule compared to the prev-
alence of treatment-resis tant PD, ep ilepsy, and psychiatric disorders
(see allcountries.org; TRD: Fava, 2003; PD: Tanner et al., 2008; OCD:
Denys et al., 2010). This section is aimed at identifying these technolog-
ical developments and their potential to combat obesity and eating
disorders.
4.2.1.2. Traditional surgery planning in DBS. In the traditional deep-brain
therapy (DBT) framework, preoperative brain MRI is acquired, a stereo-
tactic frame is af fixed to the patient, who then undergoes a CT scan, and
the insertion trajectory is set based on the registered modalities and a
deep brain atlas in printed form (Sierens et al., 2008). This framework
places restrictions on the choice of approach, and surgical planning in-
volves considerable mental computation by the surgeon. Modern DBS
practice relies on intra-operative microelectrode recordings (MER) for
confirmation comes at the cost of extended operating times and greater
potential for complications (Lyons et al., 2004). Whi le MER use is com-
mon in PD, feedback on targeting success is not possible for many non-
motor disorders.
4.2.1.3. Potential complications of DBS. In traditional and image-guided
approaches, targeting does not account for brain shift, and this neglect
leads to a heightened risk of complications. While brain shift may be
negligible under some conditions (Petersen et al., 2010), other studies
suggest that shifts up to 4 mm can occur (Miyagi et al., 2007; Khan
et al., 2008). The worst case is a cerebrovascular complication, especially
when multiple trajectories are used during exploration (Hariz, 2002).
Moreover, the risk of penetration of a ventricular wall is an important
Fig. 4. Changes in glucose metabolism observed via positron emission tomography (PET)
imaging after injection of
18
FDG (fluorodeoxyglucose), between vagal stimulated vs. sham
animals. N = 8 Yucatán minipigs in both groups. VNS (vagus nerve stimulation) therapy
was applied during 8 days on ventral and dorsal vagal trunks at the level of the abdomen.
The cuff electrodes were placed surgically using a coelioscopic approach. p b 0.0001 with
FDR (false discovery rate correction) (see text for details).
17D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
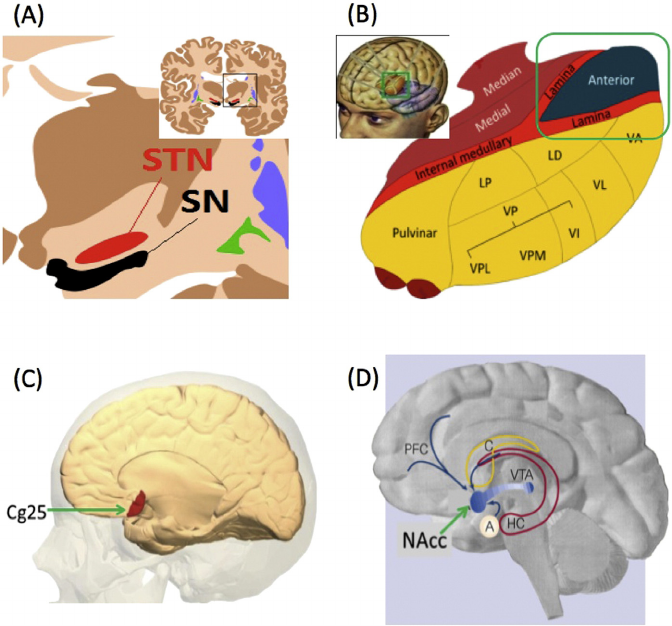
consideration (Gologorsky et al., 2011), which correlates strongly with
neurological sequelae. Despite the foregoing, DBS still has a relatively
low complication rate compared to bariatric surgery (Gorgulho et al.,
2014) and recent DBS innovations will considerably improve the safety
and accuracy of this surgery.
4.2.2. Recent DBS innovations and emerging DBS therapies
A number of innovative techniques have been proposed in image-
guided DBS, improving the functionally descriptive aspects of surgery
planning. Most groups emphasize only a small number of these tech-
niques at once, which include 1) a digital deep-brain atlas depicting
deep-brain structures in hu mans (D3Haese et al., 2005; Chak ravarty
et al., 2006) and animal models such as the pig (Saikali et al., 2010);
2) a surface model, featuring shape statistics, for registering an atlas to
patient data (Patenaude et al., 2011); 3) an electrophysiological data-
base with successful target coordinates (Guo et al., 2006); 4) a model
of venous and arterial structures, identified from the combination of
Susceptibility Weighted Imaging and Time-Of-Flight angiographic mag-
netic resonance imaging (Bériault et al., 2011); 5) multi-contrast MRI
that directly delineates the basal ganglia structures through
coregistered images weigh ted on T1, R2* (1/T2*), and susceptibility
phase/magnitude (Xiao et al., 2012); 6) validation of deep brain therapy
through animal trials, mostly confined to rodents (Bove and Perier,
2012) but also applied to (mini)pigs (Sauleau et al., 2009a; Knight
et al., 2013); 7) computer simulation of DBS (McNeal, 1976;
Miocinovic et al., 2006), using a finite element model of voltage distri-
bution of the stimulating electrode as well as an anatomical model of
the stimulated neural tissue; and 8) connectomic surgery planning for
DBS (Henderson, 2012; Lambert et al., 2012), where patient-specific
white matter tracts identified from diffusion tensor/spectrum imaging
(DTI/DSI) are exploited for effective targeting.
The above technologies relate to preoperative planning; Meanwhile,
very little effort has been devoted to intraoperative accuracy. The main
exception is intraoperative MRI (ioMRI)-guided DBS, which was pro-
posed in Starr et al. (2010), using an MRI-compatible frame. Another
recent intraoperative development is closed-loop deep-brain therapy de-
livery, based on electrical or neurochemical feedback (Rosin et al., 2011;
Chang et al., 2013).
Last, highly selective therapies have been proposed for the treat-
ment of epilepsy, which target mutated genes that modulate ion chan-
nels (Pat han et al., 201 0).
Therapies that address molecular pathways specifictoPD(LeWitt
et al., 2011), and TRD (Alexander et al., 2010) are also being developed.
In this kind of deep-brain therapy, the electrical stimulation is replaced
by the infusion of substances that modulate the neurotransmission
loc
ally.
4.2.3. Applicability of DBS in the context of obesity and eating disorders
4.2.3.1. The effects of DBS on eating behavior and body weight. In a compre-
hensive review, McClelland et al. (2013a) presented evidence from
human and animal studies on the effects of ne uromodulation on eating
behavior and body weight. Four studies observed clinical improvements
and weight gain in patients with anorexia nervosa (AN) treated with
DBS (in the Cg25, Nac, or ventral capsule/striatum – VC/VS) (Israel et al.,
2010; Lipsman et al., 2013; McLaughlin et al., 2013; Wu et al., 2013); a sin-
gle case report showed a significant weight loss in a DBS-trea ted patient
suffering from obsessive–compulsive disorders (M antione et al., 2010);
and eleven studies reported either over-eating and/or increases in
Fig. 5. DBT targets: (A) subthalamic nucleus (coronal view, yellow, labeled “STN”); (B) anterior nucleus of thalamus (3D rendering, dark blue, labeled “anterior”); (C) subgenual anterior
cingulate (medial view, region high-lighted in red); (D) nucleus accumbens (medial view, blue circle) (Wiki).
18 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
cravings, weight gain and BMI following DBS of the STN and/or globus
pallidus — GP (Macia et al., 2004; Tuite et al., 2005; Montaurier et al.,
2007; Novakova et al., 2007; Bannier et al., 2009; Sauleau et al., 2009b;
Walker et al., 2009; Strowd et al., 2010; Locke et al., 2011; Novakova
et al., 2011; Zahodne et al., 2011). In patients treated for PD, we can as-
sume that the decrease in motor activity, and thus in energy expenditure ,
might explain part of the increased weight gain, even though Amami et al.
(2014) recently suggested that compulsive eating may be specifically re -
lated to STN stimulation.
Amongst the 18 animal studies (mainly rats) assessing food intake
and weight further DBS (McClelland et al., 2013a), only two stimulated
the Nac or dorsal striatum, while the others focused on the lateral (LHA)
or ventromedial (vmH) hypothalamus. Halpern et al. (2013) showed
that DBS of Nac can reduce binge eating, while van der Plasse et al.
(2012) interestingly revealed different effects on sugar motivation and
food intake according to the sub-area of Nac stimulated (core, lateral
or med ial shell). LHA stimulation mos tly induced food intake and
weight gain (Delgado and Anand, 1953; Mogenson, 1971; Stephan
et al., 1971; Schallert, 1977; Halperin et al ., 1983), even though Sani
et al. (2007) showed a decreased weight gain in rats. vmH stimulation
decreased food intake and/or weight gain in most cases (Brown et al.,
1984; Stenger et al., 1991; Bielajew et al., 1994; Ruffin and Nicolaidis,
1999; Lehmkuhle et al., 2010), but two studies showed increased food
intake (Lacan et al., 2008; Torres et al., 2011).
Tomycz et al. (2012) published the theoretical foundations and de-
sign of the first human pilot study aimed at using DBS to combat obesity
specifically. Preliminary results from this study (Whiting et al., 2013)in-
dicate that DBS of the LHA may be applied safely to humans with intrac-
table obesity, and induce some weight loss under metabolically optimized
settings. Two clinical trials on DBS for AN are also in progress according to
Gorgulho et al. (2014), which demonstrate that DBS is a hot topic and
promising alternative strategy to combat obesity and eating disorders.
4.2.3.2. What the future has to offer. Mos
t of the DBS studies aimed at
modifying eating behavior or body weight in animal models were per-
formed one to several decades ago, and almost exclusively focused on
the hypothalamus, which plays a pivotal rol e in homeostatic regula-
tions. The explosion of functional brain imaging studies and the descrip-
tion of brain anomalies in the reward and dopaminergic circuits of
subjects suffering from obesity or eating disorders show that hedonic
regulations ar e of the utmost imp ortance for food intake control.
The most effective treatment against obesity remains bariatric sur-
gery, and especially the gastric bypass surgery. We have a lot to learn
from the effectiveness of this treatment in terms of brain mechanisms
and potential targets for DBS, and recent studies managed to describe
the surgery-induced remodeling of bra in responses to food reward,
hunger or satiety (Geliebter, 2013; Frank et al., 2014; Scholtz et al.,
2014). The Nac and PF C are part of the brain areas impa cted. Knight
et al. (2013) showed in pigs that DBS of the Nac can modulate the activ-
ity of psychiatrically important brain areas, such as the PFC, for which
anomalies were described in obese humans (Le et al., 2006; Volkow
et al ., 2008) and minipigs (Val-La illet et al., 201 1). All the DBS improve-
ments described beforehand will help targeting the best structures and
coping with brain shift, and large animal models such as the minipig are
an asset in perfecting surgical strategies.
Basal nuclei have a complex ‘somatotopy’ (Choi et al., 2012), and DA
spatial and temporal release involves distinct neural microcircuits with-
in subregions of these nu clei (Besson et al., 2010; Bassareo et al., 2011;
Saddoris et al ., 2013), which means that small errors in terms of
targeting can have dramatic consequences in terms of neural networks
and neurotransmission processes impacted. Once this challenge will be
achie ved, highly innovative deep-brain therapies could target some
functions of the dopaminergic system for example, which is altered in
patients suffering from obes ity (Wang et al., 2002; Volkow et al.,
2008) and animal mod els of addictive-like cravings or bingeing
(Avena et al., 2006; Avena et al., 2008), with the aim of normalizing
the functional processes of the DA system (as in Parkinson3s for the
motor disorders). Even though findings relating obesity and DA abnor-
malities appear sometimes inconsistent, it is probably because incorrect
interpretations or comparisons have been done. Most of the discrepan-
cies in the DA literature arose because different pathological stages (dif-
ferent degrees of obesity with different comorbidities, reward deficit vs.
surfeit phenotypes), brain processes (basal activity vs. response to food
stimuli), or cognitive processes (liking vs. wanting, occasional vs.
habitual consumption) were compared. Before proposing a DBS strate-
gy, there is a need for phenotyping patients in terms of neural circuits/
functions impacted. For example, the individual reward sensitivity phe-
notyp e may determine the treatment target in terms of goal brain
change (i.e. increased/decreased DA regions responsivity for deficit vs.
surfeit phenotypes, respectively). In other patients for whom there is
no alteration of the reward circuit but rather neural abnormalities in
metabolic centers (such as the hypothalamus), the DBS strategy might
be completely different (e.g. modulate the LHA or vMH activity in AN
or obese patients to stimulate or decrease food intake, respectively).
Real-time fMRI neurofeedback combined with cognitive therapy (cf.
Section 3.1) might also be used for closed-loop DBS therapy. Even
though it has never been tested in our knowledge, the efficacy of
targeting specific nuclei for DBS might be validated through its ability
to improve real-time brain and cognitive processes related to self-
control over highly palatable food stimuli (Ma
ntione et al., 2014). This
approach might be used to finely tune the DBS parameters and location
to maximize its impact on specific cognitive tasks or processes (e.g. self-
control over palatable foods).
Overall, these data offer a large field of research and developments
to improve DBS surgery and make it, one day, a safer, flexible and re-
versible alternative to classical bariatric surgery.
5. General discussion and conclusions: the brain at the core of
research, prevention and therapy in the context of obesity and
eating disorders
As described in this review, neuroimaging and neuromodulation ap-
proaches are emergent and promising tools to explore the neural vul-
nerability factors and obesity-related brain anomalies, and eventually
to provide innovative therapeutic strategies to combat obesity and ED.
The different sections of this review article can raise several questions
in terms of implementation of these tools in fundamental research, pre-
vention programs and therapeutic plans. How can these new technolo-
gies and exploratory approaches find a place within the current medical
workflow, from prevention to treatment? What are the requisites for
their implementation, for which added value in comparison to existing
solutions, and where could they slot into the current therapeutic plan?
To answer these questions, we propose to initiate three debates that will
inevitably need further work and reflection. First, we will discuss the
possibility to identify new biologic al markers of key brain functions.
Second, we will h ighlight the potential role of neuroimaging and
neuromodulation in individualized medicine to improve the clinical path-
ways and strategies. Third, we will introduce the ethical questions that are
unavoidably concomitant to the emergence of new neuromodulation
therapies in humans.
5.1. Towards new biological markers?
“It is far more important to know what person the disease has than
what disease the person has.” This quote from Hippocrates bears the
quintessence of preventive medicine. Indeed, reliable prediction and ef-
ficient prevention are the ultimate objective in public health. Similarly,
accurate diagnosis, prognosis and treatment are mandatory for a good
medical practice. But all of these cannot be reached without a good
knowledge of the healthy and ill (or at risk) indi vidual phenotypes,
19D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
which can be achieved through the description and validation of consis-
tent biological markers.
Psychiatric studies ext ensively des cribed the symptomology as well
as the environmental and behavioral risk factors underlying ED, while
obesity has been described through the lenses of multiple disciplines
as a multifactorial disease with a complex etiology. Despite all of this
knowledge, accurate biomarkers or clinical criteria are still lacking and
obsolete indices (such as BMI) are still used all over the world to define
and categorize patients. Yet, as reminded by Denis and H amilton
(2013), many persons classified as obese (BMI N 30) are healthy and
should not be treated and categorized as diseased. On the contrary, sub-
jects that are not considered at risk with classical clinical criteria might
show a real vulnerability with more accurate markers, as described for
the TOFI sub-phenotype (i.e. thin-on-the-outside, fat-on-the-inside),
characterizing individuals at increased metabolic risk with normal
body mass, BMI and waist circumference, but with abdominal adiposity
and ectopic fat that MRI and MRS phenotyping can help to diagnose
(Thomas et al., 2012). In the context of neuroimaging, neural vulnerabil-
ity factors could help predicting a risk for further weight gain or suscep-
tibility to contract a contentious relationship with food, as described in
Burger and Stice (2014). For obvious practical and economical reasons,
this approach could not be used for a systematic screening, but might
be proposed to subjects that are particularly at risk, because of an unfa-
vorable genetic or en vironmental ground. Since plasmatic gut-brain
obesity-associated biomarkers were fo und to be associated with
neurocognitive sk ills (Miller et al., 2015), their detection could advocate
the collection of further functional bio markers at the brain level and
contribute to a step-by-step diagnosis. Identifying neural risk factors
in people at risk, preferably in the young age, might guide further inter-
ventions (e.g. cognitive therapy) for pre-symptomatic treatment of
obesity or eating disorders. For example, reward sensitivity phenotype
may dictate the treatment target in terms of goal brain change (i.e.
increased/decreased reward regions responsivity for deficit vs. surfeit
phenotypes, respectively). Another example is the case of patients pre-
senting symptoms that are common to different diseases and for which
specific explorations are required. Some gastrointestinal diseases com-
monly mimic the presentation of eating disorders, which incites the cli-
nician to consider a broad dif ferential diagnosis when evaluating a
patient for an eating disorder (Bern and O3Brien, 2013). New neuropsy-
chiatric markers would con sequently help diagnosis and should be
added to the battery of decision criteria available.
Omics approaches, referring to innovative technology platforms such
as genetics, genomics, proteomics, and metabolomics, can provide exten-
sive data of which the computation might lead to the formulation of new
biomarkers for prediction and diagnosis (Katsareli and Dedoussis, 2014;
Cox et al., 2015; van Dijk et al., 2015). But the integration between
omics and imaging technologies should potentiate the definition of
these biomarkers, through the identification of organ-specific(notably
brain-specific) metabolis ms an d culprits associated with diseases
(Hannukainen et al., 2014). As described in the first section of this re-
view, neural vulnerability factors could appear before the onset of ED
or weight problems, highlighting the possible existence of subliminal
predictors that brain imaging only might reveal.
Radiomics is a new discipline referring to the extraction and analysis
of large amounts of advanced quantitative imaging features with high
throughput from medical images obtained with computed tomography,
PET, or structural and functional MRI (Kumar et al., 2012; Lambin et al.,
2012). Radiomics has been initially developed to decode tumor pheno-
types (Aerts et al., 2014
), including brain tumors (Co
query et al., 2014),
but could be applied to other medical fields than oncology, such as eat-
ing disorders and obesity. As reminded in Section 2.2, the combination
of imaging modalities holds potential for future studies to decipher
the neuropathological mechanisms of a disease or disorder. Radiomics
(or neuromics when applied to brain ima ging) could merge in the
same individual some information about brain activity and cognitive
processes (via fMRI, fNIRS, PET or SPECT) (see Section 2.1), availability
of neurotransmitters, transporters or receptors (via PET or SPECT) (see
Section 2.2), focal differences in brain anatomy (via voxel-based mor-
phometry — VBM) or connectivity (via diffusor tensor imaging – DTI)
(Karlsson et al., 2013; Shott et al., 2015), brain infla mmatory status
(via PET or MRI) (Cazettes et al., 2011; Amhaoul et al., 2014), etc. On
the basis of these multimodal information, neuromics could further
generate synthetic brain mapping to provide an integrative/holistic in-
sight on brain anomalies associated with loss of food intake control or
ED. Moreover, this combination of neurological information might
help clarifying some discrepancies between studies, or apparent incon-
sistent findings such as those highlighted in the literature relating BMI
and DA signaling for example. Indeed, these discrepancies might de-
pend on the interpretation of studies that have looked at different as-
pects of dopamine sign aling, or that compared processes (associated
to cognitive functions) that were not comparable.
These biomarkers could be used to phenotype patients with a diag-
nosis of obesity and/or ED, as well as establish prognosis further specific
interventions. They could also be used in prevention programs to iden-
tify subjects with neural vulnerability fac tors and provide some recom-
mendations to prevent the onset of behavioral and health problems. In
terms of therapy, radiomics/neuromics might also be used before
selecting brain target(s) for neuromodulation, because the information
gathered through this method might help predicting the consequences
of neurostimulation on the activation of neural networks or the modu-
lation of neurotransmission.
5.2. Neuroimaging and neuromodulation in the scope of personalized
medicine
Personalized (or individualized) medicine is a medical model that
proposes the customization of healthcare using all clinical, genetic and
environmental information available, with medical decisions, practices,
and/or products being tailored to the individual patient. As reminded by
Cortese (2007), individualized medicine is in a pivotal position in the
evolu tion of national and global health care in the 21st century, and
this assertion is particularly true for nutritional disorders and diseases,
given the societal and economical burden that obesity represents in
the world for example, as well as the complexity and diversity of
obese phenotypes (Blundell and Cooling, 2000; Pajunen et al., 2011).
Advances in computational power and medical imaging are paving the
way for personalized medical treatments that consider a patient3s
genetic, anatomical, and physiological chara cteristics. In addition to
these criteria, cognitive measurements related to eating behavior (see
Gibbons et al., 2014 for a review) should be used in conjunction with
brain imaging because linking imaging data with cognitive processes
(or biological measures) can potentiate the analysis and discrimination
power.
Once the patient and the disease are well portrayed, the question of
thebestsuitabletherapyarises.Ofcourse, individual history (and notably,
previously unsuccessful therapeutic attempts) is particularly important.
There is a graduation in both the severity of the disease and the degree
of invasiveness of treatments available (Fig. 6A).Obviously,basicrequire-
ments for a healthy lifestyle (i.e. balanced diet, minimal physical activity,
good sleep and social life, etc.) are sometimes difficult to achieve for many
people, and never sufficie
nt for those who went beyond a particular
threshold in the disease progression. The classical therapeutic treatment
plan then includes psychological and nutritional interventions, pharma-
cological treatments and, in pharmacorefractory patients, the logical
next step is bariatric sur gery (for morbid obesity) or hospitalization (for
severe eating disorders). All the neuroimaging and neuromodulation
strategies presented in this review can slot into the possible therapeutic
plan at different levels, therefore at different stages of a disease, from
identificati on of neural vulnerability traits to treatment of severe forms
of the disease (Fig. 6A). Moreover, as illustrated in Fig. 6B, all the
neuromodulation approaches presented do not target the same brain
20 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
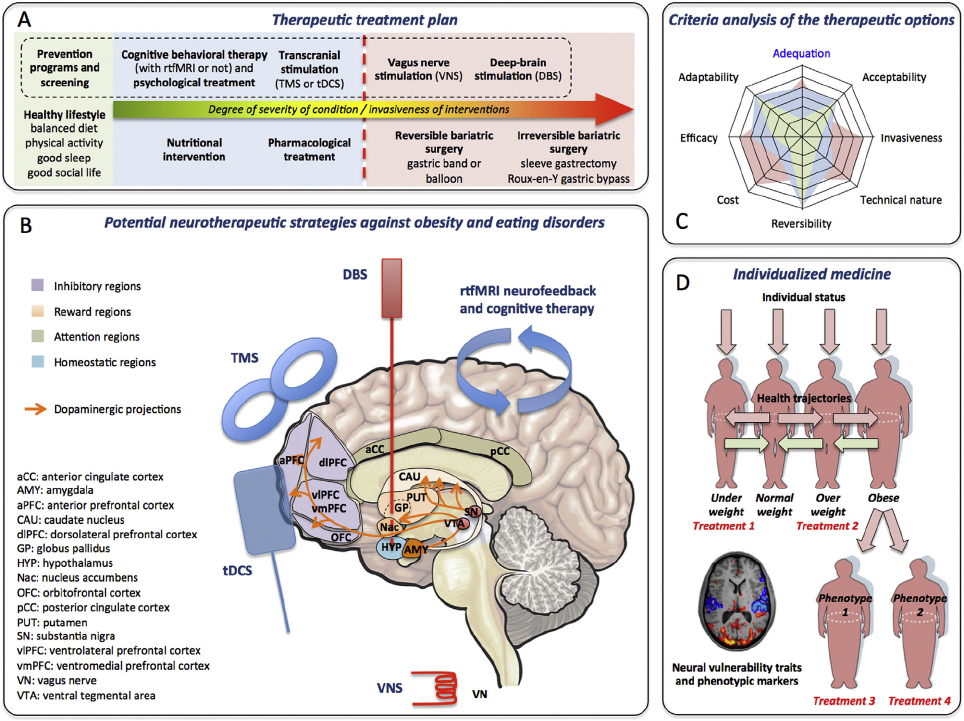
structures or networks. The PFC, which is the primary target for transcra-
nial neuromodulation strategies (e.g. TMS and tDCS), sends inhibitory
projections to the orexigenic network but also has a major role in mood,
food stimuli valuation, decision-making processes, etc. While rtfMRI
neurofeedback could target virtually any moderate-sized brain region,
existing studies mainly focused on the PFC, the ventral striatum, but
also the cingulate cortex, which is very important for attentional process-
es. Lastly, in the context of nutritional disorders, DBS itself can target very
different deep-brain structures, such as reward or homeostatic regions
(Fig. 6B). As a consequence, the choice of a neuromodulation strategy
cannot rest on a single criterion (e.g. balance between the severity of dis-
ease — e.g. high BMI with comorbidities — and the invasiveness of thera-
py), but on multiple assessment criteria, of which some of these are
directly related to the patient3s phenotype and some others to the interac-
tion between patient and therapeutic option (Fig. 6C). For some obese pa-
tients, stimulating the hypothalamus via DBS for example might be
ineffective or counterproductive if their condition takes its roots in
anomalies of the brain reward circuit. There is consequently a great
danger (t he least being wasting time and money, the worst being
worsening t he patient3s condition) in testing neuromodulation in
patients before knowing which regulation process to target — and
if the patient indeed develops iatrogenic neurobehavioral anoma-
lies relate d to this proc ess.
In the future, computational brain network models should play a
major role in integrating, reconstructing, computing, simulating an d
predicting structural and functional brain data from various imaging
modalities, from individual subjects to entire clinical populations. Such
models could integrate functionalities for the reconstruction of structur-
al connectivity from tractographic data, the simulation of neural mass
models connected by realistic parameters, the computation of individu-
alized mea surements used in human brain imaging and their web-
based 3D scientific visualization (e.g. The Virtual B rain, Jirsa et al.,
2010), leading eventually to pre-operative modeling and predictions
in the field of therapeutic neuromodulation.
Fig. 6. Schematic representation showing how potential neurotherapeutic strategies could be included in the therapeutic treatment plan for patients suffering from obesity and/or eating
disorders. (A) Simplified therapeutic treatment plan categorizing the different options according to the degree of severity of the patient3s condition (BMI, comorbidities, etc.) and/or the
degree of invasiveness of the interventions (in green: prevention programs and basic behavioral requirements for a healthy lifestyle; in blue: minimally invasive interventions; in red:
invasive interventions requiring surgery/anesthesia). In the dotted box are indicated the therapeutic options discussed in the review. (B) Potential neurotherapeutic strategies against obe-
sity and/or eating disorders, which target specific brain areas or complete neural networks regulating food intake, reward, attention, and homeostasis. (C) Examples of criteria analysis for
the assessment of therapeutic options for an individual patient. Acceptability (pre- or post-intervention) of the therapy is patient-dependent. Some criteria are therapy-dependent, such as
the invasiveness, technical nature, reversibility, and cost. The efficacy and adaptability of the therapy depend on the interaction between patient and therapy, and can be estimated upon
data from a characterized clinical population. Adequation between therapy and patient is conditioned by all the aforementioned criteria, but also by external factors such as the social en-
vironment, the geographical/temporal availability of therapy, and the healthcare system the patient depends on. On the schematic three hypothetical intervention strategies to treat obe-
sity in a lambda patient, e.g. a diet (in green), a minimally invasive therapy (in blue), and an invasive therapy (red) are represented. (D) In the context of individualized medicine, the
absolute requirement is a good phenotyping of the clinical populations and individual patients, but also a good knowledge of the population/individual health trajectories. According
to the type of disease/disorder, the individual history, and the degree of severity of the patient3s condition, different therapeutic options can be considered. But within a given clinical pop-
ulation (e.g. morbidly obese), different phenotypes can exist and condition the choice of the treatment. Neuroimaging can help identifying neural vulnerability factors and markers,
selecting the best treatment option, and shaping therapeutic strategies (e.g. rtfMRI neurofeedback or brain target identification for neuromodulation protocols).
21D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
5.3. Ethics related to novel diagnostic and therapeutic tools
As described in this paper, the battle against obesity and eating dis-
orders has given rise to many new interdisciplinary developments.
Novel less invasive treatments (in comparison to classical bariatric sur-
gery for example) are within scrutiny in research and clinics. However, a
sound critical attitude towards these novel techniques should be main-
tained especially before their clinical a pplication. As reminded in
Section 3.2, even minimally invasive neuromodulation techniques are
not playthings (Bikson et al., 2013), and can have neuropsychological
consequences that are not anodyne. Due to our current inability to un-
derstand the intricacies of brain modulations and their consequences
on cognitive processes, eating behavior and body functions, it is crucial
to remember another Hippocrates3 aphorism: “first do no harm”. Fur-
ther preclinical studies in relevant animal models (e.g. p ig mo dels,
Sauleau et al., 2009a; Clouard et al., 2012; Ochoa et al., 2015) are thus
mandatory, along with extensive brain imaging programs to reveal
the individual phenotypes and histories (Fig. 6D) that could shape pre-
vention programs and possibly justify the use of neuromodulation
therapy.
To be implemented in the therapeutic treatment plan against obesi-
ty and eating disorders, neuromodulation strategies must have higher
assessment scores than classical options, and this assessment must inte-
grate various criteria such as acceptability, invasiveness, technical na-
ture (i.e. technologies and skills required), reversibility, cost, efficacy,
adaptability and finally, adequation with the patient (Fig. 6C). The
main advantages of neuromodulation approaches in comparison to
classical bariatric surgery are: minimal invasiveness (e.g. DBS does not
systematically require general anesthesia and leads to less comorbidi-
ties than a gastric by-pass), high reversibility (neuromodulation can
be stopped immediately if problematic — even though insertion of
deep-brain electrodes can induce residual lesions throughout the de-
scent), adaptability/flexibility (brain target and/or stimulation parame-
ters can be easily and quickly modified). But these advantages are not
sufficient. The cost/advantage balance of each approach must be studied
accurately, and the efficiency (cross between efficacy and level of
investment, i.e. time, money, energy) of the alternative technique in im-
proving life expectancy must compete with that of classical techniques.
Minimally invasive and less costly neuroimaging and neuromodulation
methods must receive a particular interest because they will permit a
more important and widespread penetration in healthcare systems
and populations. We gave the example of fNIRS and tDCS as non-
invasive, relatively cheap and portable technologies, in comparison to
other imaging and neuromodulation modalities that are costly, depen-
dent on high-tech infrastructures, and consequently not readily avail-
able. Also, it is important to remind that, in the case of bariatric
surgery, the aim is not to lose the most weight possible but to limit mor-
tality and comorbidities a ssociated with obesity. Some therapeutic
options might be less effective than class ical bariatric surgery to lose
weight quickly but could be as efficient (or even better) to improve
health on the long term, which means that the success criteria of (pre)-
clinical trials should sometimes be revised or augmented with criteria
related to the improvement of neurocognitive processes and control be-
havior, rather than mere weight loss (which is very often the case).
Once again, a lot of obese people are satisfied with their own lives/
conditions (sometimes wrongfully) and some obese are indeed
completely healthy. As a matter of fact, recent sociological phenomena,
especia lly in Nor th America, led for example to the emergence of fat
acceptance movements (Kirkland, 2008). Such a phenomenon is far
from being anecdotic or minor in terms of sociological impact on politics
and healthcare systems, because it focuses on civil rights consciousness,
freewill and disc rimination, i.e. que stions tha t affect direct ly a lot of peo-
p
le (in the USA, two thirds of the population is overweight, one third is
obese). First, some people might perceive neuroimaging-based preven-
tion and diagnosis as stigmatizing tools, which necessitates to focus sci-
entific communication on the main objectives of this approach, i.e.
improving vulnerability detection and healthcare solutions. Second,
whatever the method employed, artificially modifying brain activity is
not trivial, because the intervention can modify conscious and uncon-
scious functions, self-control, and decision-making processes, which is
very different than aiming at corre cting motor functions such as for
DBS and Parkinson3s disease. Soda taxes and other dissuasive measures
to fight obesity are usually unpopular and reproved, because it is some-
times perceived as paternalism and an affront against freewill (Parmet,
2014). But let3s think about neuromodulation: Instead of increasing the
monetary value of palatable foods, the aim of neuromodulation is to de-
crease the hedonic value people attribute to these foods, within their
brain. We must foresee that a technology that could change or correct
mental processes will inexorably hatch a serious debate on bioethics,
similarly to cloning, stem cells, genetically modified organisms, and
gene therapy. Scientists, sociologists and bioethicists must be ready to
address these questions because new exploratory tools and therapies
cannot find their place without being accepted at every level of the so-
ciety, i.e. individual patient, medical authorities, politics, and public
opinion. Even if the decision to be subjected to a particular therapy be-
longs to the patient, individual decisions are always influenced by ideas
that are conveyed at all levels of society, and medical authorities must
approve all therapies. In a recent paper, Petersen (2013) stated that
the rapid development of the life sciences and related technologies (in-
cluding neuroimaging) has underlined the limitations of bioethics3 per-
spectives and reasoning for addressing emergent normative questions.
The author pleads in favor of a normative sociology of bio-knowledge
that could benefit from the principles of justice, beneficence and
nonmaleficence, as well as on the concept of human rights (Petersen,
2013). Even if some approaches are not biologically invasive, they can
be psy chologically and philosoph ically inv asive.
5.4. Conclusion
The technologies and ideas presented in this paper rejoin the state-
ment and conclusions of Schmidt and Campbell (2013), i.e. treatment
of eating disorders and obesity cannot remain ‘brainless’. A biomarker
approach combining genetic, neuroimaging, cognitive and other biolog-
ical measures will facilitate development of early effective precision
treatments (Insel, 2009; Insel et al., 2013), and serve individuali zed
prevention and medicine. Even tho ugh recent scientificdiscoveries
and innovative technology breakthrough pave the way to new medical
applications, our knowle dge of the neuropsychological mechanisms
governing eating behavior and favoring the emergence of a disease is
still embryonic. Fundamental research in animal models and rigorous
bioethics approach are consequently mandatory for a good translational
science in this field.
Acknowledgments
This review topic was proposed by the NovaBrain International Con-
sortium that was created in 2012 with the aim to promote innovative
re
search to explore the relationships between brain functions and
eating behaviors (Coordinator: David Val-Laillet, INRA, France). The
founding members of the NovaBrain Consortium were: Institut National
de la Recherche Agronomique (INRA, France), INR A Transfert S.A.
(France), Wageningen University (The Netherlands), Institute of Agri-
culture and Food Research and Technology (IRTA, Spain), University
Hospital Bonn (Germany), Institut Européen d3 Administration des Af-
faires (INSEAD, France), University of Surrey (UK), Radboud University
Nijmegen, The Netherlands, Noldus Information Technology BV (The
Netherlands), University of Queensland (Australia), Oregon Research
Institute (USA), Pennington Biomedical Research Centre (USA), Centre
National de La Recherche Scientifique (CNRS, France), Old Dominion
University (USA), Stichting Dienst Landbouwkundig Onderzoek —
Food & Biobased Research, The Netherlands, Aix-Marseille University
22 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

(France), i3B Innovations BV (The Netherlands), Jožef Stefan Institute
(Slovenia), University of Bologna (Italy). The preparation a nd initial
meetings of the NovaBrain Consortium were co-funded by INRA and
the Britta ny Region (France) in the context of the FP7 European
Program. Dr. Alons o-Alonso is a reci pient of grants fro m the Boston Nu-
trition and Obesity Research Center (BNORC), 5P30 DK046200, and the
Nutrition Obesity Research Center at Harvard (NORCH), P30 DK040561.
Dr. Eric Stice benefited from the following grants for the research men-
tioned herein: Roadmap Supplement R1MH64560A; R01 DK080760;
and R01 DK092468. Bernd Webe r was supported by a Heisenberg
Grant of the German Research Council (DFG; We 4427/3-1). Dr. Esther
Aarts was supported by a VENI grant of The Netherlands Organization
for Scientific Research (NWO) (016.135.023) and an AXA Research
Fund fellowship (Ref: 2011). Luke Stoeckel received a financial support
from the National Institutes of Health (K23DA032612; R21DA030523),
the Norman E. Zinberg Fellowship in Addiction Psychiatry at Harvard
Medic al School, the Charles A. King Trust, the McGovern Institute
Neurotechnology Program, and private funds to the Massachusetts Gen-
eral Hospital Department of Psychiatry. Some research presented in this
paper was carried out in part at the Athinoula A. Martinos Center for
Biomedical Imaging at the McGovern Institute for Brain Research at
the Massachusetts Institute of Technology. All the authors state that
they have no conflict of int erests related to this manuscript.
References
Aarts,E.,VanHolstein,M.,Hoogman,M.,Onnink,M.,Kan,C.,Franke,B.,Buitelaar,J.,Cools,R.,
2015. Reward modulation of cognitive function in adult attention-deficit/hyperactivity
disorder: a pilot study on the role of striatal dopamine. Behav. Pharmacol. 26 (1–2),
227–240. http://dx.doi.org/10.1097/FBP.000000000000011625485641.
Abubakr, A., Wambacq, I., 2008. Long-term outcome of vagus nerve stimulation therapy in
patients with refractory epilepsy. J. Clin. Neurosci. 15 (2), 127–129. http://dx.doi.org/
10.1016/j.jocn.2007.07.08318068991.
Adams,T.D.,Davidson,L.E.,Litwin,S.E.,Kolotkin,R.L.,LaMonte,M.J.,Pendleton,R.C.,Strong,
M.B., Vinik, R., Wanner, N.A., Hopkins, P.N., Gress, R.E., Walker, J.M., Cloward, T.V.,
Nuttall, R.T., Hammoud, A., Greenwood, J.L., Crosby, R.D., McKinlay, R., Simper, S.C.,
Smith, S.C., 2012. Health benefits of gastric bypass surgery after 6 years. JAMA 308
(11), 1122–1131. http://dx.doi.org/10.1001/2012.jama.1116422990271.
Adcock, R.A., Lutomski, K., Mcleod, S.R., Soneji, D.J., Gabrieli, J.D., 2005. Real-time fMRI
during the psychotherapy session: toward a methodology to augment therapeutic
benefit, exemplary data Human Brain Mapping Conference.
Aerts, H.J., Velazquez, E.R., Leijenaar, R.T., Parmar, C., Grossmann, P., Cavalho, S., Bussink, J.,
Monshouwer, R., Haibe-Kains, B., Rietveld, D., Hoebers, F., Rietbergen, M.M., Leemans,
C.R., Dekker, A., Quackenbush, J., Gillies, R.J., Lambin, P., 2014. Decoding tumour phe-
notype by noninvasive imaging using a quantitative radiomics approach. Nat.
Commun. 5, 4006. http://dx.doi.org/10.1038/ncomms500624892406.
Aldao, A., Nolen-Hoeksema, S., 2010. Specificity of cognitive emotion regulation strate-
gies: a transdiagnostic examination. Behav. Res. Ther. 48 (10), 974–983. http://dx.
doi.org/10.1016/j.brat.2010.06.00220591413.
Alexander, B., Warner-Schmidt, J., Eriksson, T., Tamminga, C., Arango-Lievano, M., Arango-
Llievano, M., Ghose, S., Vernov, M., Stavarache, M., Stavarche, M., Musatov, S., Flajolet,
M., Svenningsson, P., Greengard, P., Kaplitt, M.G., 2010. Reversal of depressed behav-
iors in mice by p11 gene therapy in the nucleus accumbens. Sci. Transl. Med. 2 (54),
54ra76. http://dx.doi.org/10.1126/scitranslmed.300107920962330.
Allcountries.org. Epilepsy: aetiology, epidemiology and prognosis. Available:
http://www.
allcountries.org/health/epilepsy_aetiogy_epidemiology_and_prognosis.html
Alonso-Alonso, M., 2013. Translating tDCS into the field of obesity: mechanism-driven ap-
proaches. Front. Hum. Neu rosci. 7, 512. http://dx.doi.org/10.3389/fnhum.2 013.
0051223986687.
Alonso-Alonso, M., Pascual-Leone, A., 2007. The right brain hypothesis for obesity. JAMA
297 (16), 1819–1822. http://dx.doi.org/10.1001/jama.297.16.181917456824.
Amami,P.,Dekker,I.,Piacentini,S.,Ferré,F.,Romito,L.M.,Franzini,A.,Foncke,E.M.,Albanese,
A., 2014. Impulse control behaviours in patients with Parkinson3s disease after subtha-
lamic deep brain stimulation: de novo cases and 3-year follow-up. J. Neurol. Neurosurg.
Psychiatry http://dx.doi.org/10.1136/jnnp-2013-30721425012201.
Amhaoul, H., Staelens, S., Dedeurwaerdere, S., 2014. Imaging brain inflammation in epi-
lepsy. Neuroscience 279, 238–252. http://dx.doi.org/10.1016/j.neuroscience.2014.
08.04425200114.
Appelhans, B.M., Woolf, K., Pagoto, S.L., Schneider, K.L., Whited, M.C., Liebman, R., 2011.
Inhibiting food reward: delay discounting, food reward sensitivity, and palatable
food intake in overweight and obese women. Obesity Silver Spring 19 (11),
2175–2182. http://dx.doi.org/10.1038/oby.2011.5721475139.
Arle, J.E., Shils, J.L., 2011. Essential Neuromodulation. Academic Press.
Avena, N.M., Rada, P., Hoebel, B.G., 2008. Underweight rats have enhanced dopamine release
and blunted acetylcholine response in the nucleus accumbens while bingeing on su-
crose. Neuro science 156 (4), 865–871. http://dx.doi.org/ 10.1016/j.neuroscience.2008.
08.01718790017.
Avena, N.M., Rada, P., Moise, N., Hoebel, B.G., 2006. Sucrose sham feeding on a binge
schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine
satiety response. Neuroscience 139 (3), 813–820. http://dx.doi.org/10.1016/j.
neuroscience.2005.12.03716460879.
Azuma, K., Uchiyama, I., Takano, H., Tanigawa, M., Azuma, M., Bamba, I., Yoshikawa, T.,
2013. Changes in cerebral blood flow during olfactory stimulation in patients with
multiple chemical sensitivity: a multi-channel near-infrared spectroscopic study.
PLOS One 8 (11), e80567. http://dx.doi.org/10.1371/journal.pone.008056724278291.
Balodis, I.M., Molina, N.D., Kober, H., Worhunsky, P.D., White, M.A., Rajita Sinha, S., Grilo,
C.M., Potenza, M.N., 2013. Divergent neural substrates of inhibitory control in binge
eating disorder relative to other manifestations of obesity. Obesity Silver Spring 21
(2), 367–377. http://dx.doi.org/10.1002/oby.2006823404820.
Bannier, S., Montaurier, C., Derost, P.P., Ulla, M., Lemaire, J.J., Boirie, Y., Morio, B., Durif, F.,
2009. Overweight after deep brain stimulation of the subthalamic nucleus i n
Parkinson disease: long term follow-up. J. Neurol. Neurosurg. P sychiatr y 80 (5),
484–488. http://dx.doi.org/10.1136/jnnp.2008.15857619060023.
Barker, A.T., 1991. An introduction to the basic principles of magnetic nerve stimulation.
J. Clin. Neurophysiol. 8 (1), 26–37. http://dx.doi.org/10.1097/00004691-199101000-
00005 2019648 .
Barth, K.S., Rydin-Gray, S., Kose, S., Borckardt, J.J., O3Neil, P.M., Shaw, D., Madan, A., Budak,
A., George, M.S., 2011. Food cravings and the effects of left prefrontal repetitive trans-
cranial magnetic stimulation using an improved sham condition. Front. Psychiatry 2,
9. http://dx.doi.org/10.3389/fpsyt.2011.0000921556279.
Bartholdy, S., Musiat, P., Campbell, I.C., Schmidt, U., 2013. The potential of neurofeedback
in the treatment of eating disorders: a review of the literature. Eur. Eat. Disord. Rev.
21 (6), 456–463. http://dx.doi.org/10.1002/erv.225024115445
.
Ba
ssareo, V., Musio, P., Di Chiara, G., 2011. Reciprocal responsiveness of nucleus accum-
bens shell and core dopamine to food- and drug-conditioned stimuli. Psychopharma-
cology (Berl.) 214 (3), 687–697. http://d x.doi.org/10.1007/s00213-010-2072-
82111 0007.
Batterink, L., Yokum, S., Stice, E., 2010. Body mass correlates inversely with inhibitory con-
trol in response to food among adolescent girls: an fMRI study. Neuroimage 52 (4),
1696–1703. http://dx.doi.org/10.1016/j.neuroimage.2010.05.05920510377.
Bembich, S., Lanzara, C., Clarici, A., Demarini, S., Tepper, B.J., Gasparini, P., Grasso, D.L.,
2010. Individual differences in prefrontal cortex activity during perception of bitter
taste using fNIRS methodology. Chem. Senses. 35 (9), 801–812. http://dx.doi.org/
10.1093/chemse/bjq08020801896.
Bériault, S., Al Subaie, F., Mok, K., Sadikot, A.F., Pike, G.B., 2011. Automatic trajectory plan-
ning of DBS neurosurgery from multi-modal MRI datasets. Medical Image Computing
and Computer Assisted Intervention — MICCAI. Springer, Toronto, pp. 259–267.
Bern, E.M., O3Brien, R.F., 2013. Is it an eating d isorder, gastrointestinal disorder, or
both? Curr. Opin. Pediatr. 25 (4), 463–470. http://dx.doi.org/10.1097/MOP.
0b013e328362d1ad23838835.
Berridge, K.C., 2007. The debate over dopamine3s role in reward: the case for incentive sa-
lience. Psychopharmacology (Berl.) 191 (3), 391–431. http://dx.doi.org/10.1007/
s00213-006-0578-x17072591.
Berridge, K.C., 2009. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eat-
ing disorders. Physiol. Behav. 97 (5), 537–550. http://dx.doi.org/10.1016/j.physbeh.
2009.02.04419336238.
Berridge, K.C., Ho, C.Y., Richard, J.M., Difeliceantonio, A.G., 2010. The tempted brain eats:
pleasure and desire circuits in obesity and eating disorders. Brain Res. 1350, 43–64.
http://dx.doi.org/10.1016/j.brainres.2010.04.00320388498.
Berridge, K.C., Robinson, T.E., 1998. What is the role of dopamine in reward: hedonic im-
pact, reward learning, or incentive salience ? Brain Res. Br ain Res. Rev. 28 (3),
309–369. http://dx.doi.org/10.1016/S0165-0173(98)00019-89858756.
Berthoud, H.R., 2012. The neurobiology of food intake in an obesogenic environment. Proc.
Nutr.Soc.71(4),478–487. http://dx.doi.org/10.1017/S002966511200060222800810.
Besson, M., Belin, D., Mcnamara, R., Theobald, D.E., Castel, A., Beckett, V.L., Crittenden,
B.M., Newman, A.H., Everitt, B.J., Robbins, T.W., Dalley, J.W., 2010. Dissociable control
of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of
the nucleus accumbens. Neuropsychopharmacology 35 (2), 560–569. http://dx.doi.
org/10.1038/npp.2009.16219847161.
Bielajew, C., Stenger, J., Schindler, D., 1994. Factors that contribute to the reduced weight
gain following chronic ventromedial hypothalamic stimulation. Behav. Brain Res. 62
(2), 143–148. http://dx.doi.org/10.1016/0166-4328(94)90021-37945964.
Bikson, M., Bestmann, S., Edwards, D., 2013. Neuroscience: transcranial devices are not
playthings. Nature 501 (7466), 167.
http://dx.doi.org/10.1038/501167b24025832.
Bi
raben, A., Guerin, S., Bobillier, E., Val-Laillet, D., Malbert, C.H., 2008. Central activation
after chronic vagus nerve stimulation in pigs: contribution of functional imaging.
Bull. Acad. Vet. Fr. 161.
Birbaumer, N., Ramos Murguialday, A., Weber, C., Montoya, P., 2009. Neurofeedback and
brain–computer interface clinical applications. Int. Rev. Neurobiol. 86, 107–117.
http://dx.doi.org/10.1016/S0074-7742(09)86008-X19607994.
Birbaumer, N., Ruiz, S., Sitaram, R., 2013. Learned regulation of brain metabolism. Trends
Cogn. Sci. 17 (6), 295–302. http://dx.doi.org/10.1016/j.tics.2013.04.00923664452.
Blackshaw, L.A., Brookes, S.J.H., Grundy, D., Schemann, M., 2007. Sensory transmission in
the gastrointestinal tract. Neurogastroenterol. Motil. 19 (1 Suppl), 1–19. http://dx.
doi.org/10.1111/j.1365-2982.2006.00871.x17280582.
Blundell, J.E., C ooling, J., 2000. Routes to obesity: phenotypes, food choices and
activity. Br. J. Nutr. 83 (Suppl. 1), S33– SS38. http://dx.doi.org/10.1017/
S000711450000093310889790.
Bodenlos, J.S., Schneider, K.L., Oleski, J., Gordon, K., Rothschild, A.J., Pagoto, S.L., 2014.
Vagus nerve stimulation and food intake: effect of body mass index. J. Diabetes Sci.
Technol. 8 (3), 590–595. http://dx.doi.org/10.1177/193229681452518824876624.
Bolen, S.D., Chang, H.Y., Weiner, J.P., Richards, T.M., Shore, A.D., Goodwin, S.M., Johns, R.A.,
Magnuson, T.H., Clark, J.M., 2012. Clinical outcomes after bariatric surgery: a five-year
23D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
matched cohort analysis in seven US states. Obes. Surg. 22 (5), 749–763. http://dx.
doi.org/10.1007/s11695-012-0595-222271357.
Bové, J., Perier, C., 2012. Neurotoxin-based models of Parkinson3s disease. Neuroscience
211, 51–76. http://dx.doi.org/10.1016/j.neuroscience.2011.10.05722108613.
Bowirrat,A.,Oscar-Berman,M.,2005.Relationship between dopaminergic neurotransmis-
sion, alcoholism, and reward deficiency syndrome. Am. J. Med. Genet. B Neuropsychiatr.
Genet. 132B (1), 29–37. http://dx.doi.org/10.1002/ajmg.b.3008015457501.
Bralten, J., Franke, B., Waldman, I., Rommelse, N., Hartman, C., Asherson, P., Banaschewski,
T., Ebstein, R.P., Gill, M., Miranda, A., O ades, R.D., Roeyers, H., Ro thenberger, A.,
Sergeant, J.A., Oosterlaan, J., Sonuga-Barke , E., Steinhausen, H.C., Faraone, S.V. ,
Buitelaar, J.K., Arias-Vásquez, A., 2013. Candidate genetic pathways for attention-
deficit/hyperactivity disorder (ADHD) show association to hyperactive/impulsive
symptoms in children with ADHD. J. Am. Acad. Child Adolesc. Psychiatry 52 (11),
1204–1212. http://dx.doi.org/10.1016/j.jaac.2013.08.02024157394.
Brown, F.D., Fessler, R.G., Rachlin, J.R., Mullan, S., 1984. Changes in food intake with elec-
trical stimulation of the ventromedial hypothalamus in dogs. J. Neurosurg. 60 (6),
1253–1257. http://dx.doi.org/10.3171/jns.1984.60.6.12536726369.
Brühl, A.B., Scherpiet, S., Sulzer, J., Stämpfli, P., Seifritz, E., Herwig, U., 2014. Real-time
neurofeedback using functional MRI could improve down-regulation of amygdala ac-
tivity during emotional stimulation: a proof-of-concept study. Brain Topogr. 27 (1),
138–148. http://dx.doi.org/10.1007/s10548-013-0331-924241476.
Brunoni, A.R., Amadera, J., Berbel, B., Volz, M.S., Rizzerio, B.G., Fregni, F., 2011. A systematic
review on reporting and assessment of adverse effects associated with transcranial
direct current stimulation. Int. J. Neuropsychopharmacol. 14 (8), 1133–1145. http://
dx.doi.org/10.1017/S146114571000169021320389.
Buchwald, H., Oien, D.M., 2013. Metabolic/bariatric surgery worldwide. Obes. Surg. 2011,
427–436. http://dx.doi.org/10.1007/s11695-012-0864-0.
Burger, K.S., Berner, L.A., 2014. A functional neuroimaging review of obesity, appetitive
hormones and ingestive behavior. Physiol. Behav. 136, 121–127. http://dx.doi.org/
10.1016/j.physbeh.2014.04.02524769220.
Burger, K.S., Stice, E., 2012. Frequent ice cream consumption is associated with reduced
striatal response to receipt of an ice cream-based milkshake. Am. J. Clin. Nutr. 95
(4), 810–817. http://dx.doi.org/10.3945/ajcn.111.02700322338036.
Burger, K.S., Stice, E., 2014. Greater striatopallidal adaptive coding during cue-reward
learning and food reward habituation predict future weight gain. Neuroimage 99,
122–128. http://dx.doi.org/10.1016/j.neuroimage.2014.05.06624893320.
Burneo, J.G., Faught, E., Knowlton, R., Morawetz, R., Kuzniecky, R., 2002. Weight loss asso-
ciated with vagus nerve stimulation. Neurology 59 (3), 463–464. http://dx.doi.org/10.
1212/WNL.59.3.46312177391.
Bush, G., Luu, P., Posner, M.I., 2000. Cognitive and emotional influences in anterior cingu-
late cortex. Trends Cogn. Sci. 4 (6), 215–
222. h
ttp://dx.doi.org/10.1016/S1364-
6613(00)01483-210827444.
Camilleri, M., Toouli, J., Herrera, M.F., Kulseng, B., Kow, L., Pantoja, J.P., Marvik, R., Johnsen,
G., Billington, C.J., Moody, F.G., Knudson, M.B., Tweden, K.S., Vollmer, M., Wilson, R.R.,
Anvari, M., 2008. Intra-abdominal vagal blocking (VBLOC therapy): clinical results
with a new implantable medical device. Surgery 143 (6), 723–731. http://dx.doi.
org/10.1016/j.surg.2008.03.01518549888.
Camus, M., Halelamien, N., Plassmann, H., Shimojo, S., O3Doherty, J., Camerer, C.,
Rangel, A ., 2009. Repetitive transcranial magnetic stimulation over the right dor-
solateral prefrontal cortex decreases valuations during food choices. Eur.
J. Neurosci. 30 (10), 1980–1988. http://dx.doi.org/10.1111/j.1460-9568.2009.
06991.x19912330.
Caravaggio, F., Raitsin, S., Gerretsen, P., Nakajima, S., Wilson, A., Graff-Guerrero, A., 2015.
Ventral striatum binding of a dopamine D2/3 receptor agonist but not antagonist pre-
dicts normal body mass index. Biol. Psychiatry 77, 196–202. http://dx.doi.org/10.
1016/j.biopsych.2013.02.01723540907.
Caria, A., Sitaram, R., Birbaumer, N., 2012. Real-time fMRI: a tool for local brain
regulation. Neuroscientist 18 (5), 487– 501. http://dx.doi.org/10.1177/
107385841140720521652587.
Caria, A., Sitaram, R., Veit, R., Begliomini, C., Birbaumer, N., 2010. Volitional control of an-
terior insula activity modulates the response to aversive stimuli. A real-time function-
al magnetic resonance imaging study. Biol. Psychiatry 68 (5), 425–432. http://dx.doi.
org/10.1016/j.biopsych.2010.04.02020570245.
Caria,A.,Veit,R.,Sitaram,R.,Lotze,M.,Weiskopf,N.,Grodd,W.,Birbaumer,N.,
2007. Regulation of anterior insular cortex activity using real-ti me fMRI.
Neuroimage 35 (3), 1238– 1246. http://dx.doi.org/10.1016/j.neuroimage.
2007.01.01817336094.
Cazettes, F., Cohen, J.I., Yau, P.L., Talbot, H., Convit, A., 2011. Obesity-mediated inflamma-
tion may damage the brain circuit that regulates food intake. Brain Res. 1373,
101–109. http://dx.doi.org/10.1016/j.brainres.2010.12.00821146506.
Chakravarty, M.M., Bertrand, G., Hodge, C.P., Sadikot, A.F., Collins, D.L., 2006. The creation
of a brain atlas for image guided neurosurgery using serial h istological data.
Neuroimage 30 (2), 359–376. http://dx.doi.org/10.1016/j.neuroimage.2005.09.
04116406816.
Chang, S.H., Stoll, C.R., Song, J., Varela, J.E., Eagon, C.J., Colditz, G.A., 2014. The effectiveness
and risks of bariatric surgery: an updated systematic review and meta-analysis,
2003–2012. J.A.M.A. Surg. 149 (3), 275–287. http://dx.doi.org/10.1001/jamasurg.
2013.365424352617.
Chang, S.Y., Kimble, C.J., Kim, I., Paek, S.B., Kressin, K.R., Boesche, J.B., Whitlock, S.V., Eaker,
D.R., Kasasbeh, A., Horne, A.E., Blaha, C.D., Bennet, K.E., Lee, K.H., 2013. Development
of the Mayo investigational neuromodulation control system: toward a closed-loop
electrochemical feedback system for deep brain stimulation. J. Neurosurg. 119 (6),
1556–1565. http://dx.doi.org/10.3171/2013.8.JNS12214224116724.
Chapin, H., Bagarinao, E., Mackey, S., 2012. Real-time fMRI applied to pain management.
Neurosci. Lett. 520 (2), 174–181. htt p://dx.doi.org/ 10.1016/j.neulet.2012.02.
07622414861.
Chen, P.S., Yang, Y.K., Yeh, T.L., Lee, I.H., Yao, W.J., Chiu, N.T., Lu, R.B., 2008. Correlation
between body mass index and striatal dopamine transporter availability in healthy
volunteers
— a
SPECT study. Neuroimage 40 (1) , 275–279. http://dx.doi.org/10.
1016/j.neuroimage.2007.11.00718096411.
Choi, E.Y., Yeo, B.T., Buckner, R.L., 2012. The organization of the human striatum estimated
by intrinsic functional connectivity. J. Neurophysiol. 108 (8), 2242–2263. http://dx.
doi.org/10.1152/jn.00270.201222832566.
Chouinard-Decorte, F., Felsted, J., Small, D.M., 2010. Increased amygdala response and de-
creased influence of internal state on amygdala response to food in overweight com-
pared to healthy weight individuals. Appetite 54 (3), 639. http://dx.doi.org/10.1016/j.
appet.2010.04.042.
Christou, N.V., Look, D., Maclean, L.D., 2006. Weight gain after short- and long-limb gastric
bypass in patients followed for longer than 10 years. Ann. Surg. 244 (5), 734–740.
http://dx.doi.org/10.1097/01.sla.0000217592.04061.d517060766.
Clouard, C., Meunier-Salaün, M.C., Val-Laillet, D., 2012. Food preferences and aversions in
human health and nutrition: how can pigs help the biomedical research? Animal 6
(1), 118–136. http://dx.doi.org/10.1017/S175173111100131522436160.
Cohen, M.X., Krohn-Grimberghe, A., Elger, C.E., Weber, B., 2007. Dopamine gene predicts
the brain3s response to dopaminergic drug. Eur. J. Neu rosci. 26 (12), 3652–3660.
http://dx.doi.org/10.1111/j.1460-9568.2007.05947.x18088284.
Conway, C.R., Sheline, Y.I., Chibnall, J.T., Bucholz, R.D., Price, J.L., Gangwani, S., Mintun,
M.A. , 2012. Brain blood-flow change with acute vagus nerve stimulation in
trea tment-re fractory major depressive disorder. Brain Stimul. 5 (2), 163–171.
http://dx.doi.org/10.1016/j.brs.2011.03.00122037127.
Coquery, N., Francois, O., Lemasson, B., Debacker, C., Farion, R., Rémy, C., Barbier, E.L., 2014.
Microvascular MRI and unsupervised clustering yields histology-resembling images
in two rat models of glioma. J. Cereb. Blood Flow Metab. 34 (8), 1354–1362. http://
dx.doi.org/10.1038/jcbfm.2014.9024849664.
Cornier, M.A., Salzberg, A.K., Endly, D.C., Bessesen, D.H., Tregellas, J.R., 2010. Sex-based dif-
ferences in the behavioral and neuronal responses to food. Physiol. Behav. 99 (4),
538–543. http://dx.doi.org/10.1016/j.physbeh.2010.01.00820096712.
Cortese, D.A., 2007. A vision of individualized medicine in the context of global
health.Clin.Pharmacol.Ther.82(5),491–493. http://dx.doi.org/10.1038/sj.
clpt.610039017952101.
Covasa, M., Ritter, R.C., 2000. Adaptation to high-fat diet reduces inhibition of gastric emp-
tying by CCK and intestinal oleate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278
(1), R166–RR17010644635.
Cox, A.J., West, N.P., Cripps, A.W., 2015. Obesity, inflammati on, and the gut microbiota. Lan-
cet Diabetes Endocrinol. 3, 207–215. http://dx.doi.org/10.1016/S2213-8587(14) 70134-
2.
Cutini, S., Basso Moro, S., Bisconti, S., 2012. Review: Functional near infrared optical imag-
ing in cognitive neuroscience: an introductory review. J. Near Infrared Spectrosc. 20
(1), 75–92. http://dx.doi.org/10.1255/jnirs.969.
D3Haese, P.F., Cetinkaya, E., Konrad, P.E., Kao, C., Dawant, B.M., 2005. Computer-aided
placement of deep brain stimulators: from planning to intraoperative guidance. I.
E.E.E. Trans. Med. Imaging 24 (11), 1469
–14
78. http://dx.doi.org/10.1109/TMI.2005.
85675216279083.
Daly, D.M., Park, S.J., Valinsky, W.C., Beyak, M.J., 2011. Impaired intestinal afferent nerve
satiety signalling an d vagal afferent excitability in diet induced obesity i n the
mouse. J. Physiol. 589 (11), 2857–2870. http://dx.doi.org/10.1113/jphysiol.2010.
20459421486762.
Datta, A., Bansal, V., Diaz, J., Patel, J., Reato, D., Bikson, M., 2009. Gyri-precise head model of
transcranial direct current stimulation: improved spatial focality using a ring elec-
trode versus conventional rectangular pad. Brain Stimul. 2 (4), 201–207. http://dx.
doi.org/10.1016/j.brs.2009.03.00520648973.
Davis, J.F., Tracy, A.L., Schurdak, J.D., Tschöp, M.H., Lipton, J.W., Clegg, D.J., Benoit, S.C., 2008.
Exposure to elevated levels of dietary fat attenuates psychostimulant reward and
mesolimbic dopamine turnover in the rat. Behav. Neurosci. 122 (6), 1257–1263.
http://dx.doi.org/10.1037/a001311119045945.
De Weijer, B.A., Van De Giessen, E., Janssen, I., Berends, F.J., Van De Laar, A., Ackermans,
M.T., Fliers, E., La Fleur, S.E., Booij, J., Serlie, M.J., 2014. Striatal dopamine receptor
binding in morbidly obese women before and after gastric bypass surgery and its re-
lationship with insulin sensitivity. Diabetologia 57 (5), 1078–1080. http://dx.doi.org/
10.1007/s00125-014-3178-z24500343.
De Weijer, B.A., Van De Giessen, E., Van Amelsvoort, T.A., Boot, E., Braak, B., Janssen, I.M.,
Van De Laar, A., Fliers, E., Serlie, M.J., Booij, J., 2011. Lower striatal dopamine D2/3 re-
ceptor availability in obese compared with non-obese subjects. E.J.N.M.M.I. Res. 1 (1),
37. http://dx.doi.org/10.1186/2191-219X-1-3722214469.
Decharms, R.C., 2007. Reading and controlling human brain activation using real-time
functional magnetic resonance imaging. Trends Cogn. Sci. 11 (11), 473–481. http://
dx.doi.org/10.1016/j.tics.2007.08.01417988931.
Decharms, R.C., 2008. Applications of real-time fMRI. Nat. Rev. Neurosci. 9 (9), 720–729.
http://dx.doi.org/10.1038/nrn241418714327.
Decharms, R.C., Maeda, F., Glover, G.H., Ludlow, D., Pauly, J.M., Soneji, D., Gabrieli, J.D.,
Mackey, S.C., 2005. Control over brain activation and pain learned by using real-
time functional MRI. Proc. Natl. Acad. Sci. U. S. A. 102 (51), 18626–18631. http://dx.
doi.org/10.1073/pnas.050521010216352728.
Dedeurwaerdere, S., Cornelissen, B., Van Laere, K., Vonck, K., Achten, E., Slegers, G., Boon,
P., 2005. Small animal positron emission tomography during vagus nerve stimulation
in rats: A pilot st udy. Epilepsy Res. 67 (3 ), 133–141. http://dx.doi.org/10.1016/j.
eplepsyres.2005.09.00816289508.
Del Parigi, A., Chen, K., Gautier, J.F., Salbe, A.D., Pratley, R.E., Ravussin, E., Reiman, E.M.,
Tataranni, P.A., 2002. Sex differences in the human brain3s response to hunger and sa-
tiation. Am. J. Clin. Nutr. 75 (6), 1017–102212036808.
Delgado, J.M., Anand, B.K., 1953. Increase of food intake induced by electrical stimulation
of the lateral hypothalamus. Am. J. Physiol. 172 (1), 162–16813030733.
24 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
Delparigi, A., Chen, K., Salbe, A.D., Hill, J.O., Wing, R.R., Reiman, E.M., Tataranni, P.A., 2007.
Successful dieters have increased neural activity in cortical areas involved in the con-
trol of behavior. Int. J. Obes. (Lond) 31 (3), 440–448. http://dx.doi.org/10.1038/sj.ijo.
080343116819526.
Demos, K.E., Heatherton, T.F., Kelley, W.M., 2012. Individual differences in nucleus accum-
bens activity to food and sexual images predict weight gain and sexual beh avior.
J. Neurosci. 32 (16), 5549–5552. http://dx.doi.org/1 0.1523/ JNEUROSCI.5958-11.
201222514316.
Denis, G.V., Hamilton, J.A., 2013. Healthy obese persons: how can they be identified and
do metabolic profiles stratify risk? Curr. Opin. Endoc rinol. Diabetes Obes. 20 (5),
369–376. http://dx.doi.org/10.1097/01.med.0000433058.78485.b323974763.
Denys, D., Mantione, M., Figee, M., Van Den Munckhof, P., Koerselman, F., Westenberg, H.,
Bosch, A., Schuurman, R., 2010. Deep brain stimulation of the nucleus accumbens for
treatment-refractory obsessive–compulsive disorder. Arch. Gen. Psychiatry 67 (10),
1061–1068. http://dx.doi.org/10.1001/archgenpsychiatry.2010.12220921122.
Digiorgi, M., Rosen, D.J., Choi, J.J., Milone, L., Schrope, B., Olivero-Rivera, L., Restuccia, N.,
Yuen, S., Fisk, M., Inabnet, W.B., Bessler, M., 2010. Re-emergence of diabetes after gas-
tric bypass in patients with mid- to long-term follow-up. Surg. Obes. Relat. Dis. 6 (3),
249–253. http://dx.doi.org/10.1016/j.soard.2009.09.01920510288.
Divoux, J.L., [!(%xInRef|ce:surname)!], B., [!(%xInRef|ce:surname)!], M., Malbert, C.H.,
Watabe, K., Mat ono, S., Ayabe, M., Kiyonaga, A., Anzai, K., Higaki, Y., Tanaka, H.,
2014. Early changes in brain metabolism following vagal stimulation. Obes. Facts 7
(1), 26–35. http://dx.doi.org/10.1159/000358576.
Domingue, B.W., Belsky, D.W., Harris, K.M., Smolen, A., Mcqueen, M.B., Boardman, J.D.,
2014. Polygenic risk predicts obesity in both white and black young adults. PLOS
One 9 (7), e101596. http://dx.doi.org/10.1371/journal.pone.010159624992585.
Donovan, C.M., Bohland, M., 2009. Hypoglycemic detection at the portal vein: absent in
humans or yet to be elucidated? Diabetes 58 (1), 21–23. http://dx.doi.org/10.2337/
db08-143719114726.
Downar, J., Sankar, A., Giacobbe, P., Woodside, B., Colton, P., 2012. Unanticipated rapid re-
mission of refractory bulimia nervosa, during high-dose repetitive transcranial mag-
netic stimulation of the dorsomedial prefrontal cortex: a case report. Front.
Psychiatry 3, 30. http://dx.doi.org/10.3389/fpsyt.2012.0003022529822.
Dunn, J.P., Cowan, R.L., Volkow, N.D., Feurer, I.D., Li, R., Williams, D.B., Kessler, R.M.,
Abumrad, N.N., 2010. Decreased dopamine type 2 receptor availability after bariatric
surgery: preliminary findings. Brain Res. 1350, 123–130. http://dx.doi.org/10.1016/j.
brainres.2010.03.06420362560.
Dunn, J.P., Kessler, R.M., Feurer, I.D., Volkow, N.D., Patterson, B.W., Ansari, M.S., Li, R.,
Marks-Shulman, P., Abumrad, N.N., 2012. Relationship of dopamine type 2 receptor
binding potential with fasting neuroendocrine hormones and insulin sensitivity in
human obesity. Diabetes Care 35 (5), 1105–1111. http://dx.doi.org/10.2337/dc11-
225022432117.
Ehlis, A.C., Schneider, S., Dresler, T., Fallgatter, A.J., 2014. Application of functional near-
infrared spectroscopy in psychiatry. Neuroimage 85 (1), 478–488. http://dx.doi.org/
10.1016/j.neuroimage.2013.03.06723578578.
Eisenstein, S.A., Antenor-Dorsey, J.A., Gredysa, D.M., Koller, J.M., Bihun, E.C., Ranck, S.A.,
Arbeláez, A.M., Klein, S., Perlmutter, J.S., Moerlein, S.M., Black, K.J., Hershey, T., 2013.
A comparison of D2 receptor specific binding in obese and normal-weight individuals
using PET with (N-[(11)C]methyl)benperidol. Synapse 67 (11), 748–
756. htt
p://dx.
doi.org/10.1002/syn.2168023650017.
El-Sayed Moustafa, J.S., Froguel, P., 2013. From obesity genetics to the future of personal-
ized obesity therapy. Nat. Rev. Endocrinol. 9 (7), 402–413. http://dx.doi.org/10.1038/
nrendo.2013.5723529041.
Fava, M., 2003. Diagnosis and definition of treatment-resistant depression. Biol. Psychia-
try 53 (8), 649–659. http://dx.doi.org/10.1016/S0006-3223(03)00231-212706951.
Felsted, J.A., Ren, X., Chouinard-Decorte, F., Small, D.M., 2010. Genetically determined dif-
ferences in brain response to a primary food reward. J. Neurosci. 30 (7), 2428–2432.
http://dx.doi.org/10.1523/JNEUROSCI.5483-09.201020164326.
Ferrari, M., Quaresima, V., 2012. A brief review on the history of human functional near-
infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63
(2), 921–935. http://dx.doi.org/10.1016/j.neuroimage.2012.03.04922510258.
Ferreira, J.G., Tellez, L.A., Ren, X., Yeckel, C.W., de Araujo, I.E., 2012. Regulation of fat intake
in the absence of flavour signalling. J. Physiol. 590 (4), 953–972. http://dx.doi.org/10.
1113/jphysiol.2011.21828922219333.
Finkelstein, E.A., Khavjou, O.A., Thompson, H., Trogdon, J.G., Pan, L., Sherry, B., Dietz, W.,
2012. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 42 (6),
563–570. http://dx.doi.org/10.1016/j.amepre.2011.10.02622608371.
Finkelstein, E.A., Trogdon, J.G., Cohen, J.W., Dietz, W., 2009. Annual medical spending at-
tributable to obesity: payer-and service-specific estimates. Health Aff (Millwood)
28 (5), w822–ww831. http://dx.doi.org/10.1377/hlthaff.28.5.w82219635784.
Fladby, T., Bryhn, G., Halvorsen, O., Rosé, I., Wahlund, M., Wiig, P., Wetterberg, L., 2004. Ol-
factory response in the temporal cortex of the elderly measured with near-infrared
spectroscopy: a preliminary feasibility study. J. Cereb. Blood Flow Metab. 24 (6),
677–680. http://dx.doi.org/10.1097/01.WCB.0000119966.74298.5C15181375.
Flegal, K.M., Carroll, M.D., Ogden, C.L., Curtin, L.R., 2010. Prevalence and trends in obesity
among US adults, 1999–2008. JAMA 303 (3), 235–241. http://dx.doi.org/10.1001/
jama.2009.201420071471.
Fox, M.D., Buckner, R.L., White, M.P., Greicius, M.D., Pascual-Leone, A., 2012a. Efficacy of
transcranial magnetic stimulation targets for depression is related to intrinsic func-
tional connectivity with the subgenual cingulate. Biol. Psychiatry 72 (7), 595–603.
http://dx.doi.org/10.1016/j.biopsych.2012.04.02822658708.
Fox, M.D., Halko, M.A., Eldaief, M.C., Pascual-Leone, A., 2012b. Measuring and
manipulating brain connectivity with resting state functional connectivity
magnetic res onance imag ing (fcMRI) and tra nscrania l m agnetic sti mulation
(TMS). Neuroimage 62 (4), 2232–2243. ht tp://dx.doi.org/10.1016/j.neuroimage.2012.
03.03522465297.
Frank, S., Lee, S., Preissl, H., Schultes, B., Birbaumer, N., Veit, R., 2012. The obese brain
athlete: self-regulation of the anterior insula in adiposity. PLOS One 7 (8), e42570.
http://dx.doi.org/10.1371/journal.pone.004257022905151.
Frank, S., Wilms, B., Veit, R., Ernst, B., Thurnheer, M., Kullmann, S., Fritsche, A., Birbaumer,
N., Preissl, H., Schultes, B., 2014. Altered brain activity in severely obese women may
recover after Roux-en Y gastric bypass surgery. Int. J. Obes. (Lond) 38 (3), 341–348.
http://dx.doi.org/10.1038/ijo.2013.6023711773.
F
regni, F., Orsati, F., Pedrosa, W., Fecteau, S., Tome, F.A., Nitsche, M.A., Mecca, T., Macedo,
E.C., Pascual-Leone, A., Boggio, P.S., 2008. Transcranial direct current stimulation of
the prefronta l cortex modulates the desire for spe cific foods. Appetite 51 (1),
34–41. http://dx.doi.org/10.1016/j.appet.2007.09.01618243412.
Gabrieli, J.D., Ghosh, S.S., Whitfield-Gabrieli, S., 2015. Prediction as a humanitarian and
pragmatic contribution from human cognitive neuroscience. Neuron 85 (1), 11–26.
http://dx.doi.org/10.1016/j.neuron.2014.10.04725569345.
Gagnon, C., Desjardins-Crépeau, L., Tournier, I., Desjardins, M., Lesage, F., Greenwood, C.E.,
Bherer, L., 2012. Near-infrared imaging of the effects of glucose ingestion and regula-
tion on prefrontal activation during dual-task execution in healthy fasting older
adults. Behav. Brain Res. 232 (1), 137–147. http://dx.doi.org/10.1016/j.bbr.2012.03.
03922 487250.
García-García, I., Narberhaus, A., Marqués-Iturria, I., Garolera, M., Rădoi, A., Segura, B.,
Pueyo, R., Ariza, M., Jurado, M.A., 2013. Neural responses to visual food cues: insights
from functional magnetic resonance imaging. Eur. Eat. Disord. Rev. 21 (2), 89–98.
http://dx.doi.org/10.1002/erv.221623348964.
Gearhardt, A.N., Yokum, S., Stice, E., Harris, J.L., Brownell, K.D., 2014. Relation of obesity to
neural activation in response to food commercials. Soc. Cogn. Affect. Neurosci. 9 (7),
932–938. http://dx.doi.org/10.1093/scan/nst05923576811.
Geha, P.Y., Aschenbrenner, K., Felsted, J., O3Malley, S.S., Small, D.M., 2013. Altered hypo-
thalamic response to food in smokers. Am. J. Clin. Nutr. 97 (1), 15–22. http://dx.doi.
org/10.3945/ajcn.112.04330723235196.
Geiger, B.M., Haburcak, M., Avena, N.M., Moyer, M.C., Hoebel, B.G., Pothos, E.N., 2009. Def-
icits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience
159 (4), 1193–1199. http://dx.doi.org/10.1016/j.neuroscience.2009.02.00719409204.
Geliebter, A., 2013. Neuroimaging of gastric distension and gastric bypass surgery. Appe-
tite 71 , 459–465. http://dx.doi.org/10.1016/j.appet.2013.07.00223932915.
Gibbons, C., Finlayson, G., Dalton, M., Caudwell, P., Blundell, J.E., 2014. Metabolic pheno-
typing guidelines: studying eating beh aviour in humans. J. Endocrinol. 222 (2),
G1–G12. http://dx.doi.org/10.1530/JOE-14-002025052364.
Goddard, E., Ashkan, K., Farrimond, S., Bunnage, M., Treasure, J., 2013. Right frontal lobe
glioma presenting as anorexia nervosa: further evidence implicating dorsal anterior
cingulate as an area of dysfunction. Int. J. Eat. Disord. 46 (2), 189–192. http://dx.
doi.org/10.1002/eat.2207223280700.
Goldman, R.L., Borckardt, J.J., Frohman, H.A., O3Neil, P.M., Madan, A., Campbell, L.K., Budak,
A., George, M.S., 2011. Prefrontal cortex transcranial direct current stimulation (tDCS)
temporarily reduces food cravings and increases the self-reported ability to resist
food in adults with frequent food craving. Appetite 56 (3), 741–746. http://dx.doi.
org/10.1016/j.appet.2011.02.01321352881.
Goldman, R.L., Canterberry, M., Borckardt, J.J., Madan, A., Byrne, T.K., George, M.S., O3Neil,
P.M., Hanlon, C.A., 2013. Executive control circuitry differentiates degree of success in
weight loss following g astric-bypass surgery. Obesity Silver Spring 21 (11),
2189–2196. http://dx.doi.org/10.1002/oby.2057524136926.
Gologorsky, Y., Ben-Haim , S., Moshier, E.L., Godbold
, J., Tagliati, M., Weisz, D., Alterman, R.L.,
2011. Transgressing the ventricular wall during subthalamic deep brain stimulation sur-
gery for Parkinson disease increases the risk of adverse neurological sequelae. Neurosur-
gery 69 (2), 294–299. http://dx.doi.org/10.1227/NEU.0b013e318214abda21389886.
Gorgulho, A.A., Pereira, J.L., Krahl, S., Lemaire, J.J., De Salles, A., 2014. Neuromodulation for
eating disorders: ob esity and anorexia. Neurosurg. Clin. N. A m. 25 (1), 147–157.
http://dx.doi.org/10.1016/j.nec.2013.08.00524262906.
Gortz, L., Bjorkman, A.C., Andersson, H., Kral, J.G., 1990. Truncal vagotomy reduces food
and liquid i ntake in man. Physiol. Behav. 48 (6), 779–781. http://dx.doi.org/10.
1016/0031-9384(90)90226-T2087506.
Green, E., Murphy, C., 2012. Altered processing of sweet taste in the brain of diet soda
drinkers. Physiol. Behav. 107 (4), 560–567. http://dx.doi.org/10.1016/j.physbeh.
2012.05.00622583859.
Guo, J., Simmons, W.K., Herscovitch, P., Martin, A., Hall, K.D., 2014. Striatal dopamine D2-
like receptor correlation patterns with human obesity and opportunistic eating be-
havior. Mol. Psychiatry 19 (10), 1078–1084. http://dx.doi.org/ 10.1038/mp.2014.
10225 199919.
Guo, T., Finnis, K.W., Parrent, A.G., Peters, T.M., 2006. Visualization and navigation system de-
velopment and application for stereotactic deep-brain neurosurge ries. Comput. Aided
Surg. 11 (5), 231–239. http://dx.doi.org/10.3109/1092908060099723217127648.
Hall, K.D., Hammond, R.A ., Rahmandad, H., 2014. Dynamic interplay amon g homeo-
static, hedonic, a nd cognitive feedback circuits regulating bod y weight. Am.
J. Public. Health 104 (7), 1169–1175. http://dx.doi.org/10.2105/AJPH.2014.
30193124832422.
Hallett, M., 2007. Transcranial magnetic stimulation: a primer. Neuron 55 (2), 187–199.
http://dx.doi.org/10.1016/j.neuron.2007.06.02617640522.
Halperin, R., Gatchalian, C.L., Adachi, T.J., Carter, J., Leibowitz, S.F., 1983. Relationship of ad-
renergic and electrical brain stimulation induced feeding responses. Pharmacol.
Biochem. Behav. 18 (3), 415–422. http://dx.doi.org/10.1016/0091-3057(83)90464-
16300 936.
Halpern, C.H., Tekriwal, A., Santollo, J., Keating, J.G., Wolf, J.A., Daniels, D., Bale, T.L., 2013.
Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in
mice involves D2 receptor modulation. J. Neurosci. 33 (17), 7122–7129. http://dx.
doi.org/10.1523/JNEUROSCI.3237-12.201323616522.
Haltia, L.T., Rinne, J.O., Merisaari, H., Maguire, R.P., Savontaus, E., Helin, S., Någren, K.,
Kaasinen, V., 2007. Effects of intravenous glucose on dopaminergic function in the
25D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
human b rain in vivo. Synapse 61 (9), 748–756. http://dx.doi.org/10.1002/syn.
2041817568412.
Hannukainen, J., Guzzardi, M., Virtanen, K., Sanguinetti, E., Nuutila, P., Iozzo, P., 2014. Im-
aging of organ metabolism in obesity and diabetes: treatment perspectives. Curr.
Pharm. Des.24745922
Harada, H., Tanaka, M., Kato, T., 2006. Brain olfactory activation measured by near-
infrared spectroscopy in humans. J. Laryngol. Otol. 120 (8), 638–643. http://dx.doi.
org/10.1017/S002221510600123X16884548.
Hariz, M.I., 2002 . Complications of deep brain stimulation surgery. Mov. Disord. 17
(Suppl. 3), S162–SS166. http://dx.doi.org/10.1002/mds.1015911948772.
Hasegawa, Y., Ta chibana, Y., Sakagami, J., Zhang, M., Urade, M., Ono, T., 2013. Flavor-
enhanced modulation of cerebral blood flow during Gum chewing. PLOS One 8 (6),
e66313. http://dx.doi.org/10.1371/journal.pone.006631323840440.
Hassenstab, J.J., Sweet, L.H., Del Parigi, A., Mccaffery, J.M., Haley, A.P., Demos, K.E., Cohen,
R.A., Wing, R.R., 2012. Cortical thickness of the cognitive control network in obesity
and successful weight loss maintenance: a preliminary MRI study. Psychiatry Res.
202 (1), 77–79. http://dx.doi.org/10.1016/j.pscychresns.2011.09.00822595506.
Hausmann, A., Mangweth, B., Walpoth, M., Hoertnagel, C., Kramer-Reinstadler, K., Rupp,
C.I., Hinterhuber, H., 2004. Repetitive transcranial magnetic stimulation (rTMS) in
the double-blind treatment of a depressed patient suffering from bulimia nervosa:
a case report. Int. J. Neuropsychopharmacol. 7 (3), 371–373. http://dx.doi.org/10.
1017/S146114570400442015154975.
Helmers, S.L., Begnaud, J., Cowley, A., Corwin, H.M., Edwards, J.C., Holder, D.L., Kostov, H.,
Larsson, P.G., Levisohn, P.M., De Menezes, M.S., Stefan, H., Labiner, D.M., 2012. Appli-
cation of a computational model of vagus nerve stimulation. Acta Neurol. Scand. 126,
336–343. http://dx.doi.org/10.1111/j.1600-0404.2012.01656.x22360378.
Henderson, J.M., 2012. “Connectomic surgery”: diffusion te nsor imaging (DTI)
tractography as a targeting modality for surgical modulation of neural networks.
Front. Integr. Neurosci. 6, 15. http://dx.doi.org/10.3389/fnint.2012.0001522536176.
Higashi, T., Sone, Y., Ogawa, K., Kitamura, Y.T., Saiki, K., Sagawa, S., Yanagida, T., Seiyama , A.,
2004. Changes in regional cerebral blood volume in frontal cortex during mental work
with and without caffeine intake: functional monitoring using near-infrared spectrosco-
py.J.Biomed.Opt.9(4),788–793. http://dx.doi.org/10.1117/1.175523315250767.
Hinds, O., Ghosh, S., Thompson, T.W., Yoo, J.J., Whitfield-Gabrieli, S., Triantafyllou, C.,
Gabrieli, J.D., 2011. Computing moment-to-moment BOLD activation for real-time
neurofeedback. Neuroimage 54 (1), 361–368. http://dx.doi.org/10.1016/j.
neuroimage.2010.07.06020682350.
Hollmann, M., Hellrung, L., Pleger, B., Schlögl, H., Kabisch, S., Stumvoll, M., Villringer, A.,
Horstmann, A., 2012. Neural correlates of the volitional regulation of the desire for
food. Int. J. Obes. (Lond) 36 (5), 648–655. http://dx.doi.org/10.1038/ijo.2011.
12521712804.
Hoshi, Y., 2011. Towards the next generation of near-infrared spectroscopy. Philos. Trans.
A Math. Phys. Eng. Sci. 369 (1955), 4425–4439. http://dx.doi.org/10.1098/rsta.2011.
026222006899.
Hosseini, S.M., Mano, Y., Rostami, M., Takahashi, M., Sugiura, M., Kawashima, R., 2011.
Decoding what one likes or dislikes from single-trial fNIRS measurements. Neuroreport
22 (6), 269
–273. h
ttp://dx.doi.org/10.1097/WNR.0b013e3283451f8f21372746.
Hu, C., Kato, Y., Luo, Z., 2014. Activation of human prefrontal cortex to pleasant and aver-
sive taste using functional near-infrared spectroscopy. FNS 5 (2), 236–244. http://dx.
doi.org/10.4236/fns.2014.52029.
Insel, T.R., 2009. Translating scientific opportunity into public health impact: a strategic
plan for research on mental illness. Arch. Gen. Psychiatry 66 (2), 128–133. http://
dx.doi.org/10.1001/archgenpsychiatry.2008.54019188534.
Insel, T.R., Voon, V., Nye, J.S., Brown, V.J., Altevogt, B.M., Bullmore, E.T., Goodwin, G.M.,
Howard, R.J., Kupfer, D.J., Malloch, G., Marston, H.M., Nutt, D.J., Robbins, T.W., Stahl,
S.M., Tricklebank, M.D., Williams, J.H., Sahakian, B.J., 2013. Innovative solutions to
novel drug development in mental health. Neurosci. Biobehav. Rev. 37 (10 1),
2438–2444. http://dx.doi.org/10.1016/j.neubiorev.2013.03.02223563062.
Ishimaru, T., Yata, T., Horikawa, K., Hatanaka, S., 2004. Near-infrared spectroscopy of the
adult human olfactory cortex. Acta Otolaryngol. Suppl. 95–98 (553), 95–98. http://
dx.doi.org/10.1080/0365523041001775115277045.
Israël, M., Steiger, H., Kolivakis, T., Mcgregor, L., Sadikot, A.F., 2010. Deep brain stimulation
in the subgenual cingulate cortex for an intractable eating disorder. Biol. Psychiatry
67 (9), e53–ee54. http://dx.doi.org/10.1016/j.biopsych.2009.11.01620044072.
Jackson, P.A., Kennedy, D.O., 2013. The application of near infrared spectroscopy in nutri-
tional intervention studies. Front. Hum. Neurosci. 7, 473. http://dx.doi.org/10.3389/
fnhum.2013.0047323964231.
Jackson, P.A., Reay, J.L., Scholey, A.B., Kennedy, D.O., 2012. Docosahexaenoic acid-rich fish
oil modulates the cerebral hemodynamic response to cognitive tasks in healthy
young adults. Biol. Psychol. 89 (1), 183–190. http://dx.doi.org/10.1016/j.biopsycho.
2011.10.00622020134.
Jauch-Chara, K., Kistenmacher, A., Herzog, N., Schwarz, M., Schweiger, U., Oltmanns, K.M.,
2014. Repetitive electric brain stimulation reduces food intake in humans. Am. J. Clin.
Nutr.100,1003–1009. http://dx.doi.org/10.3945/ajcn.113.07548125099550.
Jáuregui-Lobera, I., 2012. Electroencephalography in eating disorders. Neuropsychiatr.
Dis. Treat. 8, 1–11. http://dx.doi.org/10.2147/NDT.S2730222275841.
Jenkinson, C.P., Hanson, R., Cray, K., Wiedrich, C., Knowler, W.C., Bogardus, C., Baier, L.,
2000. Association of dopamine D2 receptor polymorphisms Ser311Cys and TaqIA
with obesity or type 2 diabetes mellitus in Pima Indians. Int. J. Obes. Relat. Metab.
Disord. 24 (10), 1233–1238. http://dx.doi.org/10.1038/sj.ijo.080138111093282.
Jirsa, V.K., Sporns, O., Breakspear, M., Deco, G., Mcintosh, A.R., 2010. Towards the virtual
brain: network modeling of the intact and the damaged brain. Arch. Ital. Biol. 148
(3), 189–20521175008.
Johnson, P.M., Kenny, P.J., 2010. Dopamine D2 receptors in addiction-like reward dysfunc-
tion and compulsive eating in obese rats. Nat. Neurosci. 13 (5), 635–641. http://dx.
doi.org/10.1038/nn.251920348917.
Jönsson, E.G., Nöthen, M.M., Grünhage, F., Farde, L., Nakashima, Y., Propping, P., Sedvall,
G.C., 1999. Polymorphisms in the dopamine D2 receptor gene and their relationships
to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry 4 (3),
290–296. http://dx.doi.org/10.1038/sj.mp.400053210395223
.
Jo
rge, J., Van Der Zwaag, W., Figueiredo, P., 2014. EEG–fMRI integration for the study of
human brain function. Neuroimage 102, 24–34. http://dx.doi.org/10.1016/j.
neuroimage.2013.05.11423732883.
Kamolz, S., Richter, M.M., Schmidtke, A., Fallgatter, A.J., 2008. Transcranial magnetic stim-
ulation for comorbid depression in anorexia. Nervenarzt 79 (9), 1071–1073. http://
dx.doi.org/10.1007/s00115-008-2537-818661116.
Kanai, R., Chaieb, L., Antal, A., Walsh, V., Paulus, W., 2008. Frequency-dependent electrical
stimulation of the visual cortex. Curr. Biol. 18 (23), 1839–1843. http://dx.doi.org/10.
1016/j.cub.2008.10.02719026538.
Karlsson, H.K., Tuominen, L., Tuulari, J.J., Hirvonen, J., Parkkola, R., Helin, S., Salminen, P.,
Nuutila, P., Nummenmaa, L., 2015. Obes ity is associated with decreased μ-opioid
but unaltered dopamine D2 receptor availability in the brain. J. Neurosci. 35 (9),
3959–3965. http://dx.doi.org/10.1523/JNEUROSCI.4744-14.201525740524.
Karlsson, H.K., Tuulari, J.J., Hirvonen, J ., Lepomäki, V., Parkkola, R., Hiltunen, J.,
Hannukainen, J.C., Soinio, M., Pham, T., Salminen, P., Nuutila, P., Nummenmaa, L.,
2013. Obesity is associated with white matter atrophy: a combined diffusion tensor
imaging and voxel-based morphometric study. Obesity Silver Spring 21 (12),
2530–2537. http://dx.doi.org/10.1002/oby.2038623512884.
Karlsson, J., Taft, C., Rydén, A., Sjöström, L., Sullivan, M., 2007. Ten-year trends in health-
related quality of life after surgical and conventional treatment for severe obesity:
the SOS intervention study. Int. J. Obes. (Lond) 31 (8), 1248–1261. http://dx.doi.
org/10.1038/sj.ijo.080357317356530.
Katsareli, E.A., Dedoussis, G.V., 2014. Biomarkers in the field of obesity and its related co-
morbidities. Expert Opin. Ther. Targets. 18 (4), 385–401. http://dx.doi.org/10.1517/
14728 222.2014.88232124479492.
Kaye,W.H.,Wagner,A.,Fudge,J.L.,Paulus,M.,2010.Neurocircuitryofeatingdisor-
ders.Curr.Topol.Behav.Neurosci.6,37–57. http://dx.doi.org/10.1007/7854_
2010_85.
Kaye, W.H., Wierenga, C.E., Bailer, U.F., Simmons, A.N., Wagner, A., Bischoff-Grethe, A.,
2013. Does a shared neurobiology for foods an d drugs of abuse contribute to ex-
tremes of food ingestion in anorexia and bulimia nervosa? Biol. Psychiatry 73 (9),
836–842. http://dx.doi.org/10.1016/j.biopsych.2013.01.00223380716.
Kekic, M., Mcclelland, J., Campbell, I., Nestler, S., Rubia, K., David, A.S., Schmidt, U., 2014.
The eff ects of prefrontal cortex transcranial direct current stimulation (tDCS) on
food craving and temporal discounting in women with frequent food cravings. Appe-
tite 78, 55–62. http://dx.doi.org/10.1016/j.appet.2014.03.01024656950.
Kelley, A.E., Baldo, B.A., Pratt, W.E., Will, M.J., 2005a. Corticostriatal-hypothalamic circuitry
and food motivation: integration of energy, action and reward. Physiol. Behav. 86 (5),
773–795. http://dx.doi.org/10.1016/j.physbeh.2005.08.06616289609.
Kelley, A.E., Schiltz, C.A., Landry, C.F., 2005b. Neural systems recruited by drug- and food-
related cues: studies of gene activation in corticolimbic regions. Physiol. Behav. 86
(1–2), 11–14. http://dx.doi.org/10.1016/j.physbeh.2005.06.01816139315.
Kelley, A.E., Will, M.J., Steininger, T.L., Zhang, M., Haber, S.N., 2003. Restricted daily con-
sumption of a highly palatable food (chocolate ensure(R)) alters striatal enkephalin
gene expression. Eur. J. Neurosci. 18 (9), 2592
–25
98. http://dx.doi.org/10.1046/j.
1460-9568.2003.02991.x14622160.
Kennedy, D.O., Haskell, C.F., 2011. Cerebral blood flow and behavioural effects of caffeine
in habitual and non-habitual consumers of caffeine: a near infrared spectroscopy
study. Biol. Psychol. 86 (3), 298–306. http://dx.doi.org/10.1016/j.biopsycho.2010.12.
01021 262317.
Kennedy, D.O., Wightman, E.L., Reay, J.L., Lietz, G., Okello, E.J., Wilde, A., Haskell, C.F., 2010.
Effects of resveratrol on cerebral blood flow variables and cognitive performance in
humans: a double-blind, placebo-controlled, crossover in vestigation. Am. J. Clin.
Nutr. 91 (6), 1590–1597. http://dx.doi.org/10.3945/ajcn.2009.2864120357044.
Kentish, S., Li, H., Philp, L.K., O3Donnell,T.A.,Isaacs,N.J.,Young,R.L.,Wittert,G.A.,Blackshaw,
L.A.,Page,A.J.,2012.Diet-inducedadaptationofvagalafferentfunction.J.Physiol.590
(1), 209–221. http://dx.doi.org/10.1113/jphysiol.2011.22215822063628.
Kessler, R.M., Zald, D.H., Ansari, M.S., Li, R., Cowan, R.L., 2014. Changes in dopamine re-
lease and dopamine D2/3 receptor levels with the development of mild obesity. Syn-
apse 68 (7), 317–320. http://dx.doi.org/10.1002/syn.2173824573975.
Khan, M.F., Mewes, K., Gross, R.E., Skrinjar, O., 2008. Assessment of brain shift related to
deep brain stimulation surgery. Stereotact. Funct. Neurosurg. 86 (1), 44–53. http://
dx.doi.org/10.1159/00010858817881888.
Kirkland, A., 2008. Think of the hippopotamus: rights consciousness in the fat acceptance
movement. Law Soc. Rev. 42 (2), 397–432. http://dx.doi.org/10.1111/j.1540-5893.
2008.00346.x.
Kirsch, P., Reuter, M., Mier, D., Lonsdorf, T., Stark, R., Gallhofer, B., Vaitl, D., Hennig, J., 2006.
Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism
and the dopamine agonist bromocriptine on the brain activation during the anticipa-
tion of reward. Neurosci. Lett. 405 (3), 196–201. http://dx.doi.org/10.1016/j.neulet.
2006.07.03016901644.
Kishinevsky, F.I., Cox, J.E., Murdaugh, D.L., Stoeckel, L.E., Cook 3rd, E.W., Weller, R.E., 2012.
fMRI reactivity on a delay discounting task predicts weight gain in obese women. Ap-
petite 58 (2), 582–592. http://dx.doi.org/10.1016/j.appet.2011.11.02922166676.
Knight, E.J., Min, H.K., Hwang, S.C., Marsh, M.P., Paek, S., Kim, I., Felmlee, J.P., Abulseoud,
O.A., Bennet, K.E., Frye, M.A., Lee, K.H., 2013. Nucleus accumbens deep brain stimula-
tion results in insula and prefrontal activation: a large animal FMRI study. PLOS One 8
(2), e56640. http://dx.doi.org/10.1371/journal.pone.005664023441210.
Kobayashi, E., Karaki, M., Kusaka, T., Kobayashi, R., Itoh, S., Mori, N., 2009. Functional op-
tical hemodynamic imaging of the olfacto ry cortex in normosmia subjects and
dysosmia subjects. Acta Otolaryngol. Suppl. 79–84 http://dx.doi.org/10.1080/
0001648090296432519848246.
26 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

Kobayashi, E., Karaki, M., Touge, T., Deguchi, K., Ikeda, K., Mori, N., Doi, S., 2012. Olfactory
assess ment using n ear-infrare d spectroscopy. ICME. International Conference on
Complex Medical Engineering. (Kobe, Japan).
Kobayashi, E., Kusaka, T., Karaki, M., Kobayashi, R., Itoh, S., Mori, N., 2007. Functional op-
tical hemodynamic imaging of the olfactory cortex. Laryngoscope 117 (3), 541–546.
http://dx.doi.org/10.1097/MLG.0b013e31802ffe2a17334319.
Kober, H., Mende-Siedlecki, P., Kross, E.F., Weber, J., Mischel, W., Hart, C.L., Ochsner, K.N.,
2010. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc.
Natl. Acad. Sci. U. S. A. 107 (33), 14811–14816. http://dx.doi.org/10.1073/pnas.
100777910720679212.
Kokan, N., Sakai, N., Doi, K., Fujio, H., Hasegawa, S., Tanimoto, H., Nibu, K., 2011. Near-infrared
spectroscopy of orbitofronta l cortex during odorant stimulation. Am. J. Rhinol. Allergy
25 (3), 163–165. http://dx.doi.org/10.2500/ajra.2011.25.363421679526.
Konagai, C., Watanabe, H., Abe, K., Tsuruoka, N., Koga, Y., Effects of essence of chicken on
cognitive brain function: a near-infrared spe ctroscopy study, vol. 77(1) (2013a).
Biosci Biotechnol Biochem, pp. 178–181
10.1271/bbb.120706] [Pubmed: 23291775].
Konagai, C., Yanagimoto, K., Hayamizu, K., Han, L., Tsuji, T., Koga, Y., 2013b. Effects of krill
oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain
function: a randomized controlled trial in healthy elderly volunteers. Clin. Interv.
Aging 8, 1247–1257. http://dx.doi.org/10.2147/CIA.S5034924098072.
Kral, J.G., Paez, W., Wolfe, B.M., 2009. Vagal nerve function in obesity: therapeutic impli-
cations. World J. Surg. 33 (10), 1995–2006. http://dx.doi.org/10.1007/s00268-009-
0138-819618240.
Krolczyk, G., Zurowski, D., Sobocki, J., Słowiaczek, M.P., Laskiewicz, J., Matyja, A., Zaraska,
K., Zaraska, W., Thor, P.J., 2001. Effects of continuous microchip (MC) vagal
neuromodulation on gastrointestinal function in rats. J. Physiol. Pharmacol. 52 (4 1),
705–71511787768.
Krug, M.E., Carter, C.S., 2012. Conflict control loop theory of cognitive control. In: Mangun,
G.R. (Ed.), The Neuroscience of Attention: Attentional Control and Selection. Oxford
University Press, New York, pp. 229–249.
Kumar, V., Gu, Y., Basu, S., Berglund, A., Eschrich, S.A., Schabath, M.B., Forster, K., Aerts, H.J.,
Dekker, A., Fenstermacher, D., Goldgof, D.B., Hall, L.O., Lambin, P., Balagurunathan, Y.,
Gatenby, R.A., Gillies, R.J., 2012. Radiomics: the process and the challenges. Magn.
Reson. Imaging 30 (9), 1234–1248. http://dx.doi.org/10.1016/j.mri .2012.06.
01022898692.
Laćan, G., De Salles, A.A., Gorgulho , A.A., Krahl, S.E., Frig hetto, L., Behn ke, E.J.,
Melega, W.P., 2008. Modulation of food intake following deep brain stimula-
tion of the ventromedial hypothalamus in the vervet monkey. Laboratory in-
vestigation. J. Neurosurg. 108 (2), 336–342. http://dx.doi.org/10.3171/JNS/
2008/108/2/033618240931.
Lambert, C., Zrinzo, L., Nagy, Z., Lutti, A., Hariz, M., Foltynie, T., Draganski, B., Ashburner, J.,
Frackowiak, R., 2012. Confirmation of functional zones within the human subthalam-
ic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted im-
aging. Neuroimage 60 (1), 83–94. http://dx.doi.org/10.1016/j.neuroimage.2011.11.
08222173294.
Lambin, P., Rios-Velazquez, E., Leijenaar, R., Carvalho, S., Van Stiphout, R.G., Granton, P.,
Zegers, C.M., Gillies, R., Boellard, R., Dekker, A., Aerts, H.J., 2012. Radiomics: extracting
more information from medical images using advanced feature analysis. Eur.
J. Cancer 48 (4), 441–446. http://dx.doi.org/10.1016/j.ejca.2011.11.03622257792.
Lapenta, O.M., Sierve, K.D., de Macedo, E.C., Fregni, F., Boggio, P.S., 2014. Transcranial di-
rect current stimulation modulates ERP-indexed inhibitory control and reduc es
food consumption. Appetite 83, 42–48. ht tp://dx.doi.org/10.1016/j.appet.2014.08.
00525128836.
Laruelle, M., Gelernter, J., Innis, R.B., 1998. D2 receptors binding potential is not affected
by Taq1 polymorphism at the D2 receptor gene. Mol. Psychiatry 3 (3), 261–265.
http://dx.doi.org/10.1038/sj.mp.40003439672902.
Laskiewicz, J., Królczyk, G., Zurowski, G., Sobocki, J., Matyja, A., Thor, P.J., 2003. Effects of
vagal neuromodulation and vagotomy on control of food intake and body weight in
rats. J. Physiol. Pharmacol. 54 (4), 603–61014726614.
Le, D.S., Pannacciulli, N., Chen, K., Del Parigi, A., Salbe, A.D., Reiman, E.M., Krakoff, J., 2006.
Less activation of the left dorsolateral prefrontal cortex in response to a meal: a fea-
ture of obesity. Am. J. Clin. Nutr. 84 (4), 725–73117023697.
Lee, S., Ran Kim, K., Ku, J., Lee, J.H., Namkoong, K., Jung, Y.C., 2014. Resting-state synchrony
between anterior cingulate cortex and precuneus relates to body shape concern in
an
orexia nervosa and bulimia nervosa. Psychiatry Res. 221 (1), 43–48. http://dx.doi.
org/10.1016/j.pscychresns.2013.11.00424300085.
Lehmkuhle, M.J., Mayes, S.M., Kipke, D.R., 2010. Unilateral neuromodulation of the ven-
tromedial hypothalamus of the rat through deep brain stimulation. J. Neural Eng. 7
(3), 036006. http://dx.doi.org/10.1088/1741-2560/7/3/03600620460691.
LeWitt, P.A., Rezai, A.R., Leehey, M.A., Ojemann, S.G., Flaherty, A.W., Eskanda r, E .N.,
Kostyk,S.K.,Thomas,K.,Sarkar,A.,Siddiqui,M.S.,Tatter,S.B.,Schwalb,J.M.,
Poston,K.L.,Henderson,J.M.,Kurlan,R.M.,Richard,I.H.,VanMeter,L.,Sapan,
C.V., Du ring, M.J., Kaplitt, M.G., 2011. AAV2-GAD gene therapy for advanced
Parkinson3s disease: a double-blind, sham-surgery controlled, randomised
trial. Lancet Neurol. 10 (4), 309– 319. http://dx.doi.org/10.1016/S1474-
4422(11)70039-421419704.
Li, X., Hartwell, K.J., Borckardt, J., Prisciandaro, J.J., Saladin, M.E., Morgan, P.S., Johnson, K.A.,
Lematty, T., Brady, K.T., George, M.S., 2013. Volitional reduction of anterior cingulate
cortex activity produces decreased cue craving in smoking cessation: a preliminary
real-time fMRI study. Addict Biol. 18 (4), 739–748. http://dx.doi.org/10.1111/j.
1369-1600.2012.00449.x22458676.
Lipsman, N., Woodside, D.B., Giacobbe, P., Hamani, C., Carter, J.C., Norwood, S.J., Sutandar,
K., Staab, R., Elias, G., Lyman, C.H., Smith, G.S., Lozano, A.M., 2013. Subcallosal cingu-
late deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1
pilot trial. Lancet 381 (9875), 1361–1370. http://dx.doi.org/10.1016/S0140-
6736(12)62188-623473846.
Little, T.J., Feinle-Bisset, C., 2010. Oral and gastrointestinal sensing of dietary fat and appe-
tite regulation in humans: modification by diet and obesity. Front. Neurosci. 4, 178.
http://dx.doi.org/10.3389/fnins.2010.0017821088697.
Livhits, M., Mercado, C., Yermilov, I., Parikh, J.A., Dutson, E., Mehran, A., Ko, C.Y., Gibbons,
M.M., 2012. Preoperative predictors of weight loss following bariatric surgery: sys-
tematic review. Obes. Surg. 22 (1), 70–89. http://dx.doi.org/10.1007/s11695-011-
0472-421833817.
Locke, M.C., Wu, S.S., Foote, K.D., Sassi, M., Jacobson, C.E., Rodriguez, R.L., Fernandez, H.H.,
Okun, M.S., 2011. Weight changes in subthalamic nucleus vs globus pallidus internus
deep brai n stimulation: results from the COMPARE Parkinson disease deep brain
stimulation cohort. Neurosurgery 68 (5), 1233–1237. http://d x.doi.org/10.1227/
NEU.0b013e31820b52c521273927.
Logan, G.D., Cowan, W.B., Davis, K.A., 1984. On the ability to inhibit simple and choice re-
action time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform.
10 (2), 276–291. http://dx.doi.org/10.1037/0096-1523.10.2.2766232345.
Luu, S., Chau, T., 2009. Neural representation of degree of preference in the medial pre-
frontal cortex. Neuroreport 20 (18), 1581–1585. http://dx.doi.org/10.1097/WNR.
0b013 e32832d598919957381.
Lyons, K.E., Wilkinson, S.B., Overman, J., Pahwa, R., 2004. Surgical and hardware complica-
tions of subthalamic stimulation: a series of 160 procedures. Neurology 63 (4),
612–616. http://dx.doi.org/10.1212/01.WNL.0000134650.91974.1A15326230.
Machii, K., Cohen, D., Ramos-Estebanez, C., Pascual-Leone, A., 2006. Safety of rTMS to non-
motor cortical areas in healthy participants and patients. Clin. Neurophysiol. 117 (2),
455–471. http://dx.doi.org/10.1016/j.clinph.2005.10.01416387549.
Macia, F., Perlemoine, C., Coman, I., Guehl, D., Burbaud, P., Cuny, E., Gin, H., Rigalleau, V.,
Tison, F., 2004. Parkinson3s disease patients with bilateral subthalamic deep brain
stimulation gain weight. Mov. Disord. 19 (2), 206–212. http://dx.doi.org/10.1002/
mds.1063014978678
.
Ma
gro, D.O., Geloneze, B., Delfini, R., Pareja, B.C., Callejas, F., Pareja, J.C., 2008. Long-term
weight regain after gastric bypass: a 5-year prospective study. Obes. Surg. 18 (6),
648–651. http://dx.doi.org/10.1007/s11695-007-9265-118392907.
Makino, M., Tsuboi, K., Dennerstein, L., 2004. Prevalence of eating disorders: a comparison
of western and non-western countries. MedGenMed 6 (3), 4915520673.
Malbert, C.H., 2013. Brain imaging during feeding behaviour. Fundam. Clin. Pharmacol. 27,
26.
Manta, S., El Mansari, M., Debonnel, G., Blier, P., 2013. Electrophysiological and neuro-
chemical effects of long-term vagus nerve stimulation on the rat monoaminergic sys-
tems. Int. J. Neuropsychopharmacol. 16 (2), 459–470. http://dx.doi.org/10.1017/
S146114571200038722717062.
Mantione, M., Nieman, D.H., Figee, M., Den ys, D., 2014. Cognitive-behavioural therapy aug-
ments the effects of deep brain stimulation in obsessive–compulsive disorder. Psychol.
Med. 44, 3515–3522 . http://dx.doi.org/10.1017/S003329171400095625065708.
Mantione, M., Van De Brink, W., Schuurman, P.R., Denys, D., 2010. Smoking cessation and
weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeu-
tic and research implications: case report. Neurosurgery 66 (1), E218. http://dx.doi.
org/10.1227/01.NEU.0000360570.40339.6420023526.
Martin, D.M., Liu, R., Alonzo, A., Green, M., Loo, C.K., 2014. Use of transcranial direct current
stimulation (tDCS) to enh ance cognit ive training : effect of timing of stimulation. Exp.
Brain Res. 232, 3345–3351. http://dx.doi.org/10.1007/s00221-014-4022-x24992897.
Martin, D.M., Liu, R., Alonzo, A., Green, M., Player, M.J., Sachdev, P., Loo, C.K., 2013. Can
transcranial direct current stimulation enhance outcomes from cognitive training?
A randomized controlled trial in healthy participants. Int. J. Neuropsychopharmacol.
16 (9), 1927–1936. http://dx.doi.org/10.1017/S146114571300053923719048.
Matsumoto, T., Saito, K., Nakamura, A., Saito, T., Nammoku, T., Ishikawa, M., Mori, K., 2012.
Dried-bonito aroma components enhance salivary hemodynamic responses to broth
tastes detected by near-infrared spectroscopy. J. Agric. Food Chem. 60 (3), 805–811.
http://dx.doi.org/10.1021/jf203725r22224859.
Mccaffery, J.M., Haley, A.P., Sweet, L.H., Phelan, S., Raynor, H.A., Del Parigi, A., Cohen, R.,
Wing, R.R., 2009. Differential functional magnetic resonance imaging response to
food pictures in successful weight-loss maintainers relative to normal-weight and
obese controls. Am. J. Clin. Nutr. 90 (4), 928–934. http://dx.doi.org/10.3945/ajcn.
2009.2792419675107.
Mcclelland, J., Bozhilova, N., Campbell, I., Schmidt, U., 2013a. A systematic review of the
effects of neuromodulation on eating and body weight: evidence from human and
animal studies. Eur. Eat. Disorders Rev. 21 (6), 436–455. http://dx.doi.org/10.1002/
erv.2256.
Mcclelland, J., Bozhilova, N., Nestler, S., Campbell, I.C., Jacob, S., Johnson-Sabine, E. ,
Schmidt, U., 2013b. Improvements in symptoms following neuronavigated repetitive
transcranial magnetic stimulation (rTMS) in severe and enduring anorexia nervosa:
findings from two case studies. Eur. Eat. Disord. Rev. 21 (6), 500–506. http://dx.doi.
org/10.1002/erv.226624155247.
Mccormick, L.M., Keel , P.K., Brumm, M.C., Bowers, W., Swayze, V., Andersen, A. ,
Andreasen, N., 2008. Implications of starvation-induced change in right dorsal anteri-
or cingulate volume in anorexia nervosa. Int. J. Eat. Disord. 41 (7), 602–610. http://dx.
doi.org/10.1002/eat.2054918473337.
Mclaughlin, N.C., Didie, E.R., Machado, A.G., Haber, S.N., Eskandar, E.N., Greenberg, B.D.,
2013. Improvements in anorexia symptoms after deep brain stimulation for intracta-
ble obsessive–compulsive disorder. Biol. Psychiatry 73 (9), e29–ee31.
http://dx.doi.
o
rg/10.1016/j.biopsych.2012.09.01523128051.
Mcneal, D.R., 1976. Analysis of a model for excitation of myelinated nerve. I. E.E.E. Trans.
Biomed.Eng.23(4),329–337. http://dx.doi.org/10.1109/TBME.1976.3245931278925.
Miller, A.L., Lee, H.J., Lumeng, J.C., 2015. Obesity-associated biomarkers and executive
function in children. Pediatr. Res. 77 (1–2), 143–147. http://dx.doi.org/10.1038/pr.
2014.15825310758.
Miocinovic, S., Parent, M., Butson, C.R., Hahn, P.J., Russo, G.S., Vitek, J.L., Mcintyre, C.C.,
2006. Computational an alysis of subthalamic nucleus and lenticular fasciculus
27D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
activation during therapeutic deep brain stimulation. J. Neurophysi ol. 96 (3),
1569–1580. http://dx.doi.org/10.1152/jn.00305.200616738214.
Mitchison, D., Hay, P.J., 2014. The epidemiology of eating disorders: genetic, environmen-
tal, and societal factors. Clin. Epidemiol. 6, 89–97. http://dx.doi.org/10.2147/CLEP.
S4084124728136.
Miyagi, Y., Shima, F., Sasaki, T., 2007. Brain shift: an error factor during implantation of
deep brain stimulation electrodes. J. Neurosurg. 107 (5), 989–997. http://dx.doi.org/
10.3171/JNS-07/11/098917977272.
Miyake, A., Friedman, N.P., Emerson, M.J., Witzki, A.H., Howerter, A., Wager, T.D., 2000.
The unity and diversity of executive functions and their contributions to complex
“frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 41 (1), 49–100. http://
dx.doi.org/10.1006/cogp.1999.073410945922.
Mogenson, G.J., 1971. Stability and modification of consummatory behaviors elicited by
electrical stimulation of the hypothalamus. Physiol. Behav. 6 (3), 255–260. http://
dx.doi.org/10.1016/0031-9384(71)90036-94942176.
Montaurier, C., M orio, B., Bannier, S., Derost, P., Arn aud, P., Brandolini-Bunlon, M.,
Giraudet, C., Boirie, Y., Durif, F., 2007. Mechanisms of body weight gain in patients
with Parkinson3s disease after subthalamic stimulation. Brain 130 (7), 1808–1818.
http://dx.doi.org/10.1093/brain/awm11317535833.
Montenegro, R.A., Okano, A.H., Cunha, F.A., Gurgel, J.L., Fontes, E.B., Farinatti, P.T., 2012.
Prefrontal cortex transcranial direct current stimulation associated with aerobic exer-
cise change aspects of appetite sensation in overweight adults. A ppetite 58 (1),
333–338. http://dx.doi.org/10.1016/j.appet.2011.11.00822108669.
Nagamitsu, S., Araki, Y., Ioji, T., Yamashita, F., Ozono, S., Kouno, M., Iizuka, C., Hara, M.,
Shibuya, I., Ohya, T., Yamashita, Y., Tsuda, A., Kakuma, T., Matsuishi, T., 2011. Prefron-
tal brain function in children with anorexia nervosa: a near-infrared spectroscopy
study. Brain Dev. 33 (1), 35–44. http://dx.doi.org/ 10.1016/j.braindev.200 9.12.
01020129748.
Nagamitsu, S., Yamashita, F., Araki, Y., Iizuka, C., Ozono, S., Komatsu, H., Ohya, T.,
Yamashita, Y. , Kakuma, T., Tsuda, A., Matsuishi, T., 2010. Characteristic prefrontal
blood volume patterns when imaging body type, high-calorie food, and mother–
child attachment in childhood anorexia n ervosa: a near infrared spectroscopy
study. Brain Dev. 32 (2), 162–167. http://dx.doi.org/10.1016/j.braindev.2009.01.
00219216042.
Nakamura, H., Iwamoto, M., Washida, K., Sekine, K., Takase, M., Park, B.J., Morikawa, T.,
Miyazaki, Y., 2010. Influences of casein hydrolysate ingestion on cerebral activity, au-
tonomic nerve activity, and anxiety. J. Physiol. Anthropol. 29 (3), 103–108. http://dx.
doi.org/10.2114/jpa2.29.10320558968.
Nederkoorn, C., Smulders, F.T., Havermans, R.C., Roefs, A., Jansen, A., 2006. Impulsivity in
obese women. Appetite 47 (2), 253–256. http://dx.doi.org/10.1016/j.appet.2006.05.
00816782231.
Neville, M.J., Johnstone, E.C., Walton, R.T., 2004. Identification and characterization of
ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1.
Hum. Mutat. 23 (6), 540–545. http://dx.doi.org/10.1002/humu.2003915146457.
Ng, M., Fleming, T., Robinson, M., Thomson, B., Graetz, N., Margono, C., Mullany, E.C.,
Biryu
kov, S., Abbafati, C., Abera, S.F., Abraham, J.P., Abu-Rmeileh, N.M., Achoki, T.,
Albuhairan, F.S., Alemu, Z.A., Alfonso, R., Ali, M.K., Ali, R., Guzman, N.A., Ammar, W.,
Anwari, P., Banerjee, A ., Barquera, S., Basu, S., Bennett, D.A., Bhutta, Z., Blore, J.,
Cabral, N., Nonato, I.C., Chang, J.C., Chowdhury, R., Courville, K.J., Criqui, M.H.,
Cundiff, D.K., Dabhadkar, K.C., Dandona, L., Davis, A., Dayama, A., Dharmaratne, S.D.,
Ding, E.L., Durr ani, A.M. , Esteghamati, A., Farzadfar, F., Fay, D.F., Feigin, V. L.,
Flaxman, A., Forouzanfar, M.H., Goto, A., Green, M.A., Gupta, R., Hafezi-Nejad, N.,
Hankey, G.J., Harewood, H. C., Havmoeller, R., Hay, S., Hernandez, L., Husseini, A.,
Idrisov, B.T., Ikeda, N., Islami, F., Jahangir, E., Jassal, S.K., Jee, S.H., Jeffreys, M., Jonas,
J.B., Kabagambe, E.K., Khalifa, S.E., Kengne, A.P., Khader, Y.S., Khang, Y.H., Kim, D.,
Kimokoti, R.W., Kinge, J.M., Kokubo, Y., Kosen, S., Kwan, G., Lai, T., Leinsalu, M., Li,
Y., Liang, X., Liu, S., Logroscino, G., Lotufo, P.A., Lu, Y., Ma, J., Mainoo, N.K., Mensah,
G.A., Merriman, T.R., Mokdad, A.H., Moschandreas, J., Naghavi, M., Naheed, A., Nand,
D., Narayan, K.M., Nelson, E.L., Neuhouser, M.L., Nisar, M.I., Ohkubo, T., Oti, S.O.,
Pedroza, A., et al., 2014. Global, regional, and national prevalence of overweight and
obesity in children and adults during 1980–2013: a systematic analysis for the Global
Burden of Disease Study. Lancet 384, 766–781. http://dx.doi.org/10.1016/S0140-
6736(14)60460-8.
Nitsche, M.A., Cohen, L.G., Wassermann, E.M., Priori, A., Lang, N., Antal, A., Paulus, W.,
Hummel, F., Boggio, P.S., Fregni, F., Pascual-Leone, A., 2008. Transcranial direct current
stimulation: state of the art 2008. Brain Stimul. 2008 (3), 206–223. http://dx.doi.org/
10.1016/j.brs.2008.06.00420633386.
Noble, E.P., Noble, R.E., Ritchie, T., Syndulko, K., Bohlman, M.C., Noble, L.A., Zhang, Y.,
Sparkes, R.S., Grandy, D.K., 1994. D2 dopamine receptor gene and obesity. Int. J. Eat.
Disord. 15 (3), 205–217. http://dx.doi.org/10.1002/1098-108X(199404)15:3b205::
AID-EAT2260150303N3.0.CO;2-P8199600.
Noordenbos, G., Oldenhave, A., Muschter, J., Terpstra, N., 2002. Characteristics and treat-
ment of patients with chronic eating disorders. UEDI 10 (1), 15–29. http://dx.doi.
org/10.1080/106402602753573531.
Novakova, L., Haluzik, M., Jech, R., Urgosik, D., Ruzicka, F., Ruzicka, E., 2011. Hormonal reg-
ulators of food intake and weight gain in Parkinson3s disease after subthalamic nucle-
us stimulation. Neuro Endocrinol. Lett. 32 (4), 437–44121876505.
Novakova, L., Ruzicka, E., Jech, R., Serranova, T., Dusek, P., Urgosik, D., 2007. Increase in
body weight is a non-motor side effect of deep brain stimulation of the subthalamic
nucleus in Parkinson3s disease. Neuro Endocrinol. Lett. 28 (1), 21–2517277730.
Ochoa, M., Lallès, J.P., Malbert, C.H., Val-Laillet, D., 2015. Dietary sugars: their detection by
the gut-brain axis and their peripheral and central effects in health and diseases. Eur.
J.Nutr.54(1),1–24. http://dx.doi.org/10.1007/s00394-014-0776-y25296886.
Ochsner, K.N., Silvers, J.A., Buhle, J.T., 2012. Functional imaging studies of emotion regulation:
a synthe tic review and evolving model of the cognitive control of emotion. Ann. N. Y.
Acad. Sci. 1251, E1–E24. http://dx.doi.org/10.1111/j.1749-6632.2012.06751.x23025352.
Okamoto , M., Dan, H., Clowney, L., Yamaguchi, Y., Dan, I., 2009. Activation in ventro-
lateral prefrontal cortex during the act of tasting: an fNIRS study. Neurosci. Lett.
451 (2), 129–133. http://dx.doi.org/10.1016/j.neulet.2008.12.01619103260.
Okamoto, M., Dan, H., Singh, A.K., Hayakawa, F., Jurcak, V., Suzuki, T., Kohyama, K., Dan, I.,
2006a. Prefrontal activity during flavor difference test: application of functional near-
infrared spectroscopy to sensory evaluation studies. Appetite 47 (2), 220–232. http://
dx.doi.org/10.1016/j.appet.2006.04.00316797780.
Okamoto, M., Dan, I., 2007. Functional near-infrared spectroscopy for human brain map-
ping of taste-related cognitive functions. J. Biosci. Bioeng. 103 (3), 207–21
5. http://dx.
doi.org/10.1263/jbb.103.20717434422.
Okamoto, M., Matsunami, M., Dan, H., Kohata, T., Kohyama, K., Dan, I., 2006b. Prefrontal
activity during taste encoding: an fNIRS study. Neuroimage 31 (2), 796–806. http://
dx.doi.org/10.1016/j.neuroimage.2005.12.02116473020.
Okamoto, M., Wada, Y., Yamaguchi, Y., Kyutoku, Y., Clowney, L., Singh, A.K., Dan, I., 2011.
Process-specific prefrontal contributions to episodic encoding and retrieval of tastes:
a functional NIRS study. Neuroimage 54 (2), 1578–1588. http://dx.doi.org/10.1016/j.
neuroimage.2010.08.01620832483.
Ono, Y., 2012. Prefrontal activity correlating with perception of sweetness during eating.
ICME. International Conference on Complex Medical Engineering.(Kobe,Japan)2012.
Page, A.J., Symonds, E., Peiris, M., Blackshaw, L.A., Young, R.L., 2012. Peripheral neural tar-
gets in obesity. Br. J. Pharmacol. 166 (5), 1537–1558. http://dx.doi.org/10.1111/j.
1476-5381.2012.01951.x22432806.
Pajunen, P., Kotronen, A., Korpi-Hyövälti, E ., Keinänen-Kiukaanniemi, S., Oksa, H.,
Niskanen, L., Saaristo, T., Saltevo, J.T., Su ndvall, J., Vanhala, M., Uusi tupa, M.,
Peltonen, M., 2011. Metabolically healthy and unhealthy obesity phenotypes in the
general population: the FIN-D2D survey. B.M.C. Public. Health 11, 754. http://dx.doi.
org/10.1186/1471-2458-11-75421962038.
Pannacciulli, N., Del Parigi, A., Chen, K., Le, D.S., Reiman, E.M., Tataranni, P.A., 2006. Brain
abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 31
(4), 1419–1425. http://dx.doi.org/10.1016/j.neuroimage.2006.01.04716545583.
Pardo, J .V., Sheikh, S. A., Kuskowski, M .A., Surerus-Johns on, C., Hagen, M.C., Lee, J.T.,
Rittberg, B.R., Adson, D.E., 2007. Weight loss during chronic, cervical vagus nerve
stimulation in depressed patients with obesity: an observation. Int. J. Obes. (Lond.)
31, 1756–1759. http://dx.doi.org/10.1038/sj.ijo.080366617563762.
Parmet, W.E. (2014), Beyond paternalism: rethinking the limits of public health law. Con-
necticut Law Review Northeastern University School of Law Research Paper No. 194-
2014
Pascual-Leone, A., Davey, N., Rothwell, J., Wassermann, E., Puri, B., 2002. Handbook of
Transcranial Magnetic Stimulation. Arnold, London.
Patenaude, B., Smith, S.M., Kennedy, D.N., Jenkinson, M., 2011. A Bayesian model of shape
and appearance for subcortical brain segmentation. Neuroimage 56 (3), 907–922.
http://dx.doi.org/10.1016/j.neuroimage.2011.02.04621352927.
Pathan, S.A., Jain, G.K., Akhter, S., Vohora, D., Ahmad, F.J., Khar, R.K., 2010. Insights into the
novel three ‘D’s of epilepsy treatment: drugs, delivery systems and devices. Drug
Discov. Today 15 (17–18), 717–732. http://dx.doi.org/10.1016/j.drudis.2010.06.
01420 603226.
Perlmutter, J.S., Mink, J.W., 20 06. Deep brain stimulation. Annu. Rev. Neurosci. 29,
229–257. http://dx.doi.org/10.1146/annurev.neuro.29.051605.11282416776585.
Petersen, A., 2013. From bioethics to a sociology of bio-knowledge. Soc. Sci. Med. 98,
264–270. http://dx.doi.org/10.1016/j.socscimed.2012.12.03023434118.
Petersen, E.A., Holl, E.M., Martinez-Torres, I., Foltynie, T., Limousin, P., Hariz, M.I., Zrinzo, L.,
2010. Minimizing brain shift in stereotactic functional neurosurgery. Neurosurgery
67 (3 Suppl), 213–221. http://dx.doi.org/10.1227/01.NEU.0000380991.23444.
08206 79927
.
P
ohjalainen, T., Rinne, J.O., Någren, K., Lehikoinen, P., Anttila, K., Syvälahti, E.K., Hietala, J.,
1998. The A1 allele of the human D2 dopamine receptor gene predicts low D2 recep-
tor availability in healthy volunteers. Mol. Psychiatry 3 (3), 256–260. http://dx.doi.
org/10.1038/sj.mp.40003509672901.
Rasmussen, E.B., Lawyer, S.R., Reilly, W., 2010. Percent body fat is related to delay and
probability discounting for food in humans. Behav. Processes 83 (1), 23–30. http://
dx.doi.org/10.1016/j.beproc.2009.09.00119744547.
Reinert, K.R., Po3e, E.K., Barkin, S.L., 2013. The relationship between executive function and
obesity in children and adolescents: a systematic literature review. J. Obes. 2013,
82095 6. http://dx.doi.org/10.1155/2013/82095623533726.
Renfrew Center Foundation for Eating Disorders., 2003. Eating Disorders 101 Guide: A
Summary of Issues, Statistics and Resources. Renfrew Center Foundation for Eating
Disorders.
Reyt, S., Picq, C., Sinniger, V., Clarençon, D., Bonaz, B., David, O., 2010. Dynamic causal
modelling and physiological confounds: a functional MRI study of vagus nerve stim-
ulation. NeuroImage 52, 1456–1464. http://dx.doi.org/10.1016/j.neuroimage.2010.
05.02120472074.
Ridding, M.C., Rothwell, J.C., 2007. Is there a future for therapeutic use of transcranial
magnetic stimulation? Nat. Rev. Neurosci. 8 (7), 559–567. http://dx.doi.org/10.
1038/ nrn216917565358.
Robbins, T.W., Everitt, B.J., 1992. Functions of dopamine in the dorsal and ventral striatum.
Seminars in Neuroscience 4 (2), 119–127. http://dx.doi.org/10.1016/1044-
5765( 92)90010-Y.
Robertson, E.M., Théoret, H., Pascual-Leone, A., 2003. Studies in cognition: the problems
solved and created by transcranial magnetic stimulation. J. Cogn. Neurosci. 15 (7),
948–960. http://dx.doi.org/10.1162/08989290377000734414614806.
Rosin,B.,Slovik,M.,Mitelman,R.,Rivlin-Etzion,M.,Haber,S.N.,Israel,Z.,Vaadia,E.,Bergman,
H., 2011. Closed-loop deep brain stimulation is superior in am eliorating Parkinsonism.
Neuron 72 (2), 370–384. http://dx.doi.org/10.1016/j.neuron.2011.08.02322017994.
Roslin, M., Kurian, M., 2001. The use of electrical stimulation of the vagus nerve to treat
morbid obesity. epilepsy &. Behaviour 2, S11–SS16. http://dx.doi.org/10.1006/
ebeh.2001.0213.
28 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
Rossi, S., Hallett, M., Rossini, P.M., Pascual-Leone, A., Safety of TMS Consensus Group,
2009. Safety, ethical considerations, and application guidelines for the use of trans-
cranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol.
120 (12), 2008–2039. http://dx.doi.org/10.1016/j.clinph.2009.08.01619833552.
Rota, G., Sitaram, R., Veit, R., Erb, M., Weiskopf, N., Dogil, G., Birbaumer, N., 2009. Self-
regulation of regional cortical activity using real-time fMRI: the right inferior frontal
gyrus and linguistic processing. Hum. Brain Mapp. 30 (5), 1605–1614. http://dx.doi.
org/10.1002/hbm.2062118661503.
Rudenga, K.J., Small, D.M., 2012. Amygdala response to sucrose consumption is inversely
related to artificial sweetener use. Appetite 58 (2), 504–507. http://dx.doi.org/10.
1016/j.appet.2011.12.00122178008.
Ruffin, M., Nicolaidis, S., 1999. Electrical stimulation of the ventromedial hypothalamus
enhances both fat utilization and metabolic rate that precede and parallel the inhibi-
tion of feeding behavior. Brain Res. 846 (1), 23–29. http://dx.doi.org/10.1016/S0006-
8993(99)01922-810536210.
Saddoris, M.P., Sugam, J.A., Cacciapaglia, F., Carelli, R.M., 2013. Rapid dopamine dynamics
in the accumbens core and shell: learning and action. Front. Biosci. Elite Ed. 5,
273–288. http://dx.doi.org/10.2741/E61523276989.
Sagi, Y., Tavor, I., Hofstetter, S., Tzur-Moryosef, S., Blumenfeld-Katzir, T., Assaf, Y., 2012.
Lear ning in the fast lane: new insights into neuroplasticity. Neuron 73 (6),
1195–1203. http://dx.doi.org/10.1016/j.neuron.2012.01.02522445346.
Saikali, S., Meurice, P., Sauleau, P., Eliat, P.A., Bellaud, P., Randuineau, G., Vérin, M., Malbert,
C.H., 2010. A three-dimensional digital segmented and deformable brain atlas of the
domestic pig. J. Neurosci. Methods 192 (1), 102–109. http://dx.doi.org/10.1016/j.
jneumeth.2010.07.04120692291.
Saito-Iizumi, K., Nakamura, A., Matsumoto, T., Fujiki, A., Yamamoto, N., Saito, T.,
Nammoku, T., Mori, K., 2013. Ethylmaltol odor enhances salivary hemodynamic re-
sponses to sucrose taste as detected by near-infrared spectroscopy. Chem. Percept.
6(2),92–100. http://dx.doi.org/10.1007/s12078-013-9142-3.
Sand er, C.Y., Hooker, J.M., Catana, C., Normandin, M.D., Alpert, N.M., Knudsen, G .M.,
Vanduffel, W., Rosen, B.R., Mandeville, J.B., 2013. Neurovascular coupling to D2/D3
dopamine receptor occupancy using simultaneous PET/functiona l MRI. Proc. Natl.
Acad.Sci.U.S.A.110(27),11169–11174. http://dx.doi.org/10.1073/pnas.
122051211023723346.
Sani, S., Jobe, K., Smith, A., Kordower, J.H., Bakay, R.A., 2007. Deep brain stimulation for
treatment of obesity in rats. J. Neurosurg. 107 (4), 809 –813. http://dx.doi.org/10.
3171/JNS-07/10/080917937228.
Sarr, M.G., Billington, C.J., Brancatisano, R., Brancatisano, A., Toouli, J., Kow, L., Nguyen, N.T.,
Blackstone, R., Maher, J.W., Shikora, S., Reeds, D.N., Eagon, J.C., Wolfe, B.M., O3Rourke,
R.W., Fujioka, K., Takata, M., Swain, J.M., Morton, J.M., Ikramuddin, S., Schweitzer, M.,
2012. The EMPOWER Study: randomized, prospective, double-blind, multicenter trial
of vagal blockade to induce weight loss in morbid obesity. Obes. Surg. 22 (11),
1771–1782. http://dx.doi.org/10.1007/s11695-012-0751-822956251.
Sauleau, P., Lapouble, E., Val-Laillet, D., Malbert, C.H., 2009a. The pig model in brain imag-
ing and neurosurgery. Animal 3 (8), 1138–1151. http://dx.doi.or g/10.1017/
S175173110900464922444844.
Sauleau, P., Leray, E., Rouaud, T., Drapier, S., Drapier, D., Blanchard, S., Drillet, G., Péron, J.,
Vérin, M., 2009b. Comparison of weight gain and energy intake after subthalamic ver-
sus pallidal stimulation in Parkinson
3s
disease. Mov. Disord. 24 (14), 2149–2155.
http://dx.doi.org/10.1002/mds.2276519735089.
Schallert, T., 1977. Reactivity to food odors during hypothalamic stimulation in rats not
experienced with stimulation-induced eating. Physiol. Behav. 18 (6), 1061–10 66.
http://dx.doi.org/10.1016/0031-9384(77)90012-9928528.
Schecklmann, M., Schaldecker, M., Aucktor, S., Brast, J., Kirchgässner, K., Mühlberger, A.,
Warnke, A., Gerlach, M., Fallgatter, A.J., Romanos, M., 2011a. Effects of methylpheni-
date on olfaction and frontal and temporal brain oxygenation in children with
ADHD. J. Psychiatr. Res. 45 (11), 1463–1470. http://dx.doi.org/10.1016/j.jpsychires.
2011.05.01121689828.
Schecklmann, M., Schenk , E., Maisch, A., Kreiker, S., Jacob, C., Warnke, A., Gerlach, M.,
Fallgatter, A.J., Romanos, M., 2011b. Altered frontal and temporal brain function dur-
ing olfactory stimulati on in adult attention-deficit/hyperactivity disorder.
Neuropsychobiology 63 (2), 66–76. http://dx.doi.org/10.1159/00032344821178380.
Schmidt, U., Campbell, I.C., 2013. Treatment of eating disorders cannot remain ‘brainless’:
the case for brain-directed treatments. Eur. Eat. Disord. Rev. 21 (6), 425–427. http://
dx.doi.org/10.1002/erv.225724123463.
Scholkmann, F., Kleiser, S., Metz, A.J., Zimmermann, R., Mata Pavia, J., Wolf, U., Wolf, M.,
2014. A review on continuous wave functional near-infrared spectroscopy and imag-
ing instrumentation and methodology. Neuroimage 85 (1), 6–27. http://dx.doi.org/
10.1016/j.neuroimage.2013.05.00423684868.
Scholtz, S., Miras, A.D., Chhina, N., Prechtl, C.G., Sleeth, M.L., Dau d, N.M., I smail, N.A.,
Durighel, G., Ahmed, A.R., Olbers, T., Vincent, R.P., Alaghband-Zadeh, J., Ghatei, M.A.,
Waldman, A.D., Frost, G.S., Bell, J.D., Le Roux, C.W., Goldstone, A.P., 2014. Obese pa-
tients after gastric bypass surgery have lower brain-hedonic responses to food than
after gastric banding. Gut 63 (6), 891–902. http://dx.doi.org/10.1136/gutjnl-2013-
30500823964100.
Schultz, W., Dayan, P., Montague, P.R., 1997. A neural substrate of prediction and reward.
Science 275 (5306), 1593–1599. htt p://dx.doi.org/10.1126/science.275.5306.
15939054347.
Shah, M., Simha, V., Garg, A., 2006. Review: long-term impact of bariatric surgery on body
weight, comorbidities, and nutritional status. J. Clin. Endocrinol. Metab. 91 (11),
4223–4231. http://dx.doi.org/10.1210/jc.2006-055716954156.
Shikora, S., Toouli, J., Herrera, M.F., Kulseng, B., Zulewski, H., Brancatisano, R., Kow, L.,
Pantoja, J.P., Johnsen, G., Brancatisano, A., Tweden, K.S., Knudson, M.B., Billington,
C.J., 2013. Vagal blocking improves glycemic control and elevated blood pressure in
obese subjects with type 2 diabetes mellitus. J. Obes. 2013, 245683. http://dx.doi.
org/10.1016/j.soard.2008.09.01223984050.
Shimokawa, T., Misawa, T., Suzuki, K., 2008. Neural representation of preference
relationships. Neuroreport 19 (16), 1557–1561. http://dx.doi.org/10.1097/WNR.
0b013e32831126c618815582.
Shott, M.E., Cornier, M.A., Mittal, V.A., Pryor, T.L., Orr, J.M., Brown, M.S., Frank, G.K., 2015.
Orbitofrontal cortex volume and brain reward response in obesity. Int. J. Obes. (Lond)
39, 214–221. http://dx.doi.org/10.1038/ijo.2014.12125027223.
Siep, N., Roefs, A., Roebroeck, A., Havermans, R., Bonte, M., Jansen, A., 2012. Fighting food
temptations: the modulating effects of short-term cognitive reappraisal, suppression
and up-regulation on mesocorticolimbic activity related to appetitive motivation.
Neuroimage 60 (1), 213–220. http://dx.doi.org/10.1016/j.neuroimage.2011.12.
06722 230946.
Sierens, D.K., Kutz, S., Pilitsis, J.G., Bakay, R.a.E., 2008. St
ereotactic surgery with microelec-
trode recordings. In: Bakay, R.a.E. (Ed.), Movement Disorder Surgery. The Essentials.
Thieme Medical Publishers, New York, pp. 83–114.
Silvers,J.A.,Insel,C.,Powers,A.,Franz,P.,Weber,J.,Mischel,W.,Casey,B.J.,
Ochsner, K.N., 2014. Curbing craving: behavioral and brain evidence that chil-
drenregulatecravingwheninstructedtodosobuthavehigherbaselinecrav-
ingthanadults.Psychol.Sci.25(10),1932–1942. http://dx.doi.org/10.1177 /
095679761454600125193941.
Sitaram, R., Lee, S., Ruiz, S., Rana, M., Veit, R., Birbaumer, N., 2011. Real-time support vec-
tor classification and feedback of multiple emotional brain states. Neuroimage 56 (2),
753–765. http://dx.doi.org/10.1016/j.neuroimage.2010.08.00720692351.
Sizonenko, S.V., Babiloni, C., De Bruin, E.A., Isaacs, E.B., Jönsson, L.S., Kennedy, D.O.,
Latulippe, M.E., Mohajeri, M.H., Moreines, J., Pietrini, P., Walhovd, K.B., Winwood,
R.J., Sijben, J.W., 2013. Brain imaging and human nutrition: which measures to use
in intervention studies? Br. J. Nutr. 110 (Suppl. 1), S1–S30. http://dx.doi.org/10.
1017/ S000711451300138423902645.
Small, D.M., Jones-Gotman, M., Dagher, A., 2003. Feeding-induced dopamine release in
dorsal striatum correlates with meal pleasantness ratings in healthy human
volu nteers. Neuroimage 19 (4), 1709–1715. http://dx. doi.org/10.1016/S1053-
8119( 03)00253-212948725.
Small, D.M., Zatorre, R.J., Dagher, A., Evans, A.C., Jones-Gotman, M., 2001. Changes in brain
activity related to ea ting chocolate: from pl easure to aversion. Brain 124 (9),
1720–1733. http://dx.doi.org/10.1093/brain/124.9.172011522575.
Smink, F.R., Van Hoeken, D., Hoek, H.W., 2012. Epidemiology of eating disorders: inci-
dence, prevalence and mortality rates. Curr. Psychiatry Rep. 14 (4), 406–414.
http://dx.doi.org/10.1007/s11920-012-0282-y22644309.
Sotak, B.N., Hnasko, T.S., Robinson, S., Kremer, E.J., Palmiter, R.D., 2005. Dysregulation of
dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 1061 (2),
88–96. http://dx.doi.org/10.1016/j.brainres.2005.08.05316226228.
Southon, A., Walder, K., Sanigorski, A.M., Zi mmet, P., Nicholson, G.C., Kotowicz, M.A.,
Collier, G., 2003. The Taq IA and Ser311 Cys polymorphisms in the dopamine D2 re-
ceptor gene and obesity. Diabetes Nutr. Metab. 16 (1), 72–7612848308.
Spitz, M.R., Detry, M.A., Pillow, P., Hu, Y., Amos, C.I., Hong, W.K., Wu, X., 2000. Variant al-
leles of the D2 dopamine receptor gene and obesit y. Nutr. Res. 20 (3), 371–380.
http://dx.doi.org/10.1016/S0271-5317(00)00130-5.
Stagg, C.J., Nitsche, M.A., 2011. Physiological basis of transcranial direct current st imulation.
Neuroscientist 17 (1), 37–53. http://dx.doi.org/10.1177/107385841038661421343407.
Starr, P.A., Martin, A.J., Ostrem, J.L., Talke, P., Levesque, N., Larson, P.S., 2010. Subthalamic
nucleus deep brain stimulator placement using high-field interventional magnetic
resonance imaging and a skull-mounted aiming device: technique and application
accuracy. J. Neurosurg. 112 (3), 479–490. http://dx.doi.org/10.3171/2009. 6.
JNS08116119681683.
Stearns, A.T., Balakrishnan, A., Radmanesh, A., Ashley, S.W., Rhoads, D.B., Tavakkolizadeh,
A., 2012. Relative contributions of afferent vagal fibers to resistance to diet-induced
obesity. Dig. Dis. Sci. 57 (5), 1281–1290. http://dx.doi.org/10.1007/s10620-011-
1968-422138962.
Steele, K.E., Prokopowicz, G.P., Schweitzer, M.A., Magunsuon, T.H., Lidor, A.O., Kuwabawa,
H., Kumar, A., Brasic, J., Wong, D.F., 2010. Alterations of central dopamine receptors
before and after gastric bypass surgery. Obes. Surg. 20 (3), 369–374. http:
//dx.doi.
org/10.1007/s11695-009-0015-419902317.
Steinbrink, J., Villringer, A., Kempf, F., Haux, D., Boden, S., Obrig, H., 2006. Illuminating the
BOLD signal: combined fMRI–fNIRS studies. Magn. Reson. Imaging 24 (4), 495–505.
http://dx.doi.org/10.1016/j.mri.2005.12.03416677956.
Stenger, J., Fournier, T., Bielajew, C., 1991. The effects of chronic ventromedial hypotha-
lamic stimulation on weight gain in rats. Physiol. Behav. 50 (6), 1209–1213. http://
dx.doi.org/10.1016/0031-9384(91)90584-B1798777.
Stephan, F.K., Valenstein, E.S., Zucker, I., 1971. Copulation and eating during electrical
stimulation of the rat hypothalamus. Physiol. Behav. 7 (4), 587–593. http://dx.doi.
org/10.1016/0031-9384(71)90113-25131216.
Stergiakouli, E., Gaillard, R., Tavaré, J.M., Balthasar, N., Loos, R.J., Taal, H.R., Evans, D.M.,
Rivadeneira, F., St Pourcain, B., Uitterlinden, A.G., Kemp, J.P., Hofman, A., Ring, S.M.,
Cole, T.J., Jaddoe, V.W., Davey Smith, G., Timpson, N.J., 2014. Genome-wide associa-
tion study of height-adjusted BMI in childhood iden tifies functional variant in
ADCY3. Obesity Silver Spring 22, 2252–2259. http://dx.doi.org/10.1002/oby.
2084025044758.
Stice, E., Burger, K.S., Yokum, S., 2013. Relative ability of fat and sugar tastes to activate re-
ward, gustatory, and somatosensory regions. Am. J. Clin. Nutr. 98 (6), 1377–1384.
http://dx.doi.org/10.3945/ajcn.113.06944324132980.
Stice, E., Spoor, S., Bohon, C., Small, D.M., 2008a. Relation between obesity and blunted
stri atal response to food is moderated by TaqIA A1 allele. Science 322 (5900),
449–452. http://dx.doi.org/10.1126/science.116155018927395.
Stice, E., Spoor, S., Bohon, C., Veldhuizen, M.G., Small, D.M., 2008b. Relation of reward
from food intake and anticipated food intake to obesity: a functional magnetic reso-
nance imaging study. J. Abnorm. Psychol. 117 (4), 924–93 5. http://dx.doi.org/10.
1037/a001360019025237.
29D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31

Stice, E., Yokum, S., Blum, K., Bohon, C., 2010a. Weight gain is associated with reduced
striatal response to palatable food. J. Neurosci. 30 (39), 13105–13109. http://dx.doi.
org/10.1523/JNEUROSCI.2105-10.201020881128.
Stice, E., Yokum, S., Bohon, C., Marti, N., Smolen, A., 2010b. Reward circuitry responsivity
to f ood predicts future increases in body mass: moderating effects of DRD2 and
DRD4. Neuroimage 50 (4), 1618–1625. http://dx.doi.org/10.1016/j.neuroimage.
2010.01.08120116437.
Stice, E., Yokum, S., Burger, K., Epstein, L., Smolen, A., 2012. Multilocus genetic composite
reflecting dopamine signaling capacity predicts reward circuitry responsivity.
J. Neurosci. 32 (29), 10093–10100. http://dx.doi.org/10.1523/JNEUROSCI.1506-12.
201222815523.
Stice, E., Yokum, S., Burger, K.S., Epstein, L.H., Small, D.M., 2011. Youth at risk for obesity
show greater activation of striatal and somatosensory regions to food. J. Neurosci.
31 (12), 4360–4366. http://dx.doi.org/10.1523/JNEUROSCI.6604-10.201121430137.
Stice, E., Yokum, S., Burger, K.S., Rohde, P., Shaw, H., Gau, J.M., 2015. A pilot randomized
trial of a cognitive reappraisal obesity prevention program. Ph ysiol. Behav. 138,
124–132. http://dx.doi.org/10.1016/j.physbeh.2014.10.022.
Stoeckel, L.E., Garrison, K.A., Ghosh, S., Wighton, P., Hanlon, C.A., Gilman, J.M., Greer, S.,
Turk-Browne, N.B., deBettencourt, M.T., Scheinost, D., Craddock, C., Thompson, T.,
Calderon, V., Bauer, C.C., George, M., Breiter, H.C., Whitfield-Gabrieli, S., Gabrieli, J.D.,
LaConte, S.M., Hirshberg, L., 2014. Optimizing real time fMRI neurofeedback for ther-
apeutic discovery and development. NeuroImage Clin. 5, 245–255. http://dx.doi.org/
10.1016/j.nicl.2014.07.00225161891.
Stoeckel, L.E., Ghosh, S. , Hinds, O., Tighe,A.,Coakley,A.,Gabrieli,J.D.E.,Whitfield-
Gabrieli, S. , Evins, A., 2011. real time fMRI neurofeedback targeting r eward-
and inhibitory control-related brain re gions in cigarette smokers American
College of Neuropsychoph armacology, 50th A nnual M eeting.
Stoeckel, L.E., Ghosh, S., Keshavan, A., Stern, J.P., Calderon, V., Curran, M.T., Whitfield-
Gabrieli, S., Gabrieli, J.D.E., Evins, A.E., 2013a. (2013a). “The effect of real time fMRI
neurofeedback on food and cigarette cue reactivity” American College of
Neuropsychopharmacology, 52nd Annual Meeting.
Stoeckel, L.E., Murdaugh, D.L., Cox, J.E., Cook 3rd, E.W., Weller, R.E., 2013b. Greater impul-
sivity is associated with decreased brain activation in obese women during a delay
discounting task. Brain Imaging Behav. 7 (2), 116– 128. http://dx.doi.org/10.1007/
s11682-012-9201-422948956.
Strowd, R.E., Cartwright, M.S., Passmore, L.V., Ellis, T.L., Tatter, S.B., Siddiqui, M.S., 2010.
Weight change following deep brain stimulation for movement disorders. J. Neurol.
257 (8), 1293–1297. http://dx.doi.org/10.1007/s00415-010-5509-420221769.
Suda, M., Uehara, T., Fukuda, M., Sato, T., Kameyama, M., Mikuni, M., 2010. Dieting ten-
dency and eating be havior problems in eating disorder co rrelate with right
frontotemporal and left orbitofrontal cortex: a near-infrared spectroscopy study.
J. Psychiatr. Res. 44 (8), 547–555. http://dx.doi.org/10.1016/j.jpsychires.2009.11.
00519962158.
Sullivan, P.F., 1995. Mortality in anorexia nervosa. Am. J. Psychiatry 152 (7), 1073–1074.
http://dx.doi.org/10.1176/ajp.152.7.10737793446.
Sulzer, J., Haller, S., Scharnowski, F., Weiskopf, N., Birbaumer, N., Blefari, M.L., Bruehl, A.B.,
Cohen, L.G., Decharms, R.C., Gassert, R., Goebel, R., Herwig, U., Laconte, S., Linden, D.,
Luft, A., Seifritz, E., Sitaram, R., 2013. Real-time fMRI neurofeedback: progress and
challenges. Neuroimage 76, 386–399
. http://dx.doi.org/10.1016/j.neuroimage.2013.
03.03323541800.
Sun, X., Veldhuizen, M.G., Wray, A., De Araujo, I., Small, D., 2013. Amygdala response to
food cues in the absence of hunger predicts weight change. Appetite 60 (1),
168–174. http://dx.doi.org/10.1016/j.appet.2012.09.032.
Sutoh, C., Nakazato, M., Matsuzawa, D., Tsuru, K., Niitsu, T., Iyo, M., Shimizu, E., 2013.
Changes in self-regulation-related prefrontal activities in eating disorders: a near in-
frared spectroscopy study. PLOS One 8 (3), e59324. http://dx.doi.org/10.1371/
journal.pone.005932423527162.
Tanner, C.M., Brandabur, M., Dorsey, E.R., 2008. Parkinson Disease: A Global View. avail-
able:
http://www.parkinson.org/NationalParkinsonFoundation/files/84/84233ed6-
196b-4f80-85dd-77a5720c0f5a.pdf.
Tellez, L.A., Medina, S., Han, W., Ferreira, J.G., Licona-Limón, P., Ren, X., Lam, T.T., Schwartz,
G.J., De Araujo, I.E., 2013. A gut lipid messenger links excess dietary fat to dopamine
deficiency. Science 341 (6147), 800–802. http://dx. doi.org/10.1126/science.
123927523950538.
Terney, D., Chaieb, L., Moliadze, V., Antal, A., Paulus, W., 2008. Increasing human brain ex-
citability by transcranial high-frequency random noise stimulation. J. Neurosci. 28
(52), 14147–14155. http://dx.doi.org/10.1523/JNEUROSCI.4248-08.200819109497.
Thomas, E.L., Parkinson, J.R., Frost, G.S., Goldstone, A.P., Doré, C.J., Mccarthy, J.P.,
Collins, A.L., Fitzpatrick, J.A., Durighel, G., Taylor-Robinson, S.D., Bell , J.D.,
2012. The missing ri sk: MRI a nd MRS p henotyping of abdominal adiposity
and ectopic fat. Obesity Silver Spring 20 (1), 76– 87. http://dx.doi.org/10.
1038/oby.2011.14221660078.
Thomas, G.N., Critchley, J.A., Tomlinson, B., Cockram, C.S., Chan, J.C., 2001. Relationships
between the taqI polymorphism of the dopamine D2 receptor and blood pressure
in hyperglycaemic and normoglycaemic Chinese subjects. Clin. Endocrinol. (Oxf) 55
(5), 605–611. http://dx.doi.org/10.1046/j.1365-2265.2001.01404.x11894971.
Thomsen,G.,Ziebell,M.,Jensen,P.S.,DaCuhna-Bang,S.,Knudsen,G.M.,Pinborg,L.H.,2013.
No correlat ion between body mass index and striatal dopamine transporter availability
in healthy volunteers using SPECT and [123I]PE2I. Obesity 21, 1803–1806. http://dx.doi.
org/10.1002/oby.20225.
Tobler, P.N., Fiorillo, C.D., Schultz, W., 2005. Adaptive coding of reward value by dopamine
neurons. Science 307 (5715), 1642–1645. http://dx.doi.org/10.1126/science.
110537015761155.
Tomycz, N.D., Whiting, D.M., Oh, M.Y., 2012. Deep brain stimulation for obesity — from
theoretical foundations to designing the first human pilot study. Neurosurg. Rev. 35
(1), 37–42. http://dx.doi.org/10.1007/s10143-011-0359-921996938.
Torres, N., Chabardès, S., Benabid, A.L., 2011. Rationale for hypothalamus-deep brain stim-
ulation in food intake disorders and obesity. Adv. Tech. Stand. Neurosurg. 36, 17–30.
http://dx.doi.org/10.1007/978-3-7091-0179-7_221197606.
Truong, D.Q., Magerowski, G., Blackburn, G.L., Bikson, M., Alonso-Alonso, M., 2013. Com-
putational modeling of transcranial direct current stimulation (tDCS) in obesity: im-
pact of head fat and dose guidelines. Neuroimage Clin. 2, 759–766. http://dx.doi.org/
10.1016/j.nicl.2013.05.01124159560.
Tuite, P.J., Maxwell, R.E., Ikramuddin, S., Kotz, C.M., Kotzd, C.M., Billington, C.J., Billingtond,
C.J., Laseski, M.A., Thielen, S.D., 2005. Weight and body mass index in Parkinson3sdis-
ease patients after deep brain stimulation surgery. Parkinsonism Relat. Disord. 11 (4),
247–252. http://dx.doi.org/10.1016/j.parkreldis.2005.01.00615878586.
Uehara, T., Fukuda, M., Suda, M., Ito, M., Suto, T., Kameyama, M., Yamagishi, Y., Mikuni, M.,
2007. Cerebral blood volume changes in patients with eating disorders during word
fluency: a preliminary study using multi-channel near infrared spectroscopy. Eat.
Weight Disord. 12 (4), 183–190. http://dx.doi.org/10.1007/BF0332759618227640.
Uher, R., Yoganathan, D., Mogg, A., Eranti, S.V., Treasure, J., Campbell, I.C., Mcloughlin,
D.M., Schmidt, U., 2005. Effect of left prefrontal repetitive transcranial magnetic stim-
ulation on food craving. Biol. Psychiatry 58 (10), 840–842. http://dx.doi.org/10.1016/
j.biopsych.2005.05.04316084855
.
Vainik,
U., Dagher, A., Dubé, L., Fellows, L.K., 2013. Neurobehavioral correlates of body
mass index and eating behaviours in adults: a systematic review. Neurosci. Biobehav.
Rev. 37 (3), 279–299. http://dx.doi.org/10.1016/j.neubiorev.2012.11.00823261403.
Val-Laillet, D., Biraben, A., Randuineau, G., Malbert, C.H., 2010. Chronic vagus nerve stim-
ulation decreased weight gain, food consumption and sweet craving in adult obese
mini pigs. Appetite 55 (2), 245– 252. http://dx.doi.org/10.1016/j.appet.2010.06.
00820 600417.
Val-Laillet, D., Layec, S., Guérin, S., Meurice, P., Malbert, C.H., 2011. Changes in brain activ-
ity after a diet-induced obesity. Obesity Silver Spring 19 (4), 749–756. http://dx.doi.
org/10.1038/oby.2010.29221212769.
Van De Giessen, E., Celik, F., Schweitzer, D.H., Van Den Bri nk, W., Booij, J., 2014.
Dopamine D2/3 receptor availability and amphetamine-induced dopamine re-
leaseinobesity.J.Psychopharmacol.28(9),866– 873. http://dx.doi.o rg/10.
1177/026988111453166424785761.
Van De Giessen, E., Hesse, S., Caan, M.W., Zientek, F., Dickson, J.C., Tossici-Bolt, L., Sera, T.,
Asenbaum, S., Guignard, R., Akdemir, U.O., Knudsen, G.M., No bili, F., Pagan i, M.,
Vander Borght, T., Van Laere, K., Varrone, A., Tatsch, K., Booij, J., Sabri, O., 2013. No as-
sociation between str iatal dopamine transporter binding and body mass index: a
multi-center European study in healthy volunteers. Neuroimage 64, 61–67. http://
dx.doi.org/10.1016/j.neuroimage.2012.09.01122982354.
Van Den Eynde, F., Guillaume, S., Broadbent, H., Campbell, I.C., Schmidt, U., 2013. Repeti-
tive transcranial magnetic stimulation in anorexia nervosa: a pilot study. Eur. Psychi-
atry 28 (2), 98–101. http://dx.doi.org/10.1016/j.eurpsy.2011.06.00221880470.
Van Der Plasse, G., Schrama, R., Van Seters, S.P., Vanderschuren, L.J., Westenberg, H.G.,
2012. Deep brain stimulation reveals a dissociation of consummatory and motivated
behaviour in the medial and lateral nucleus accumbens shell of the rat. PLOS One 7
(3), e33455. http://dx.doi.org/10.1371/journal.pone.003345522428054.
Van Dijk, S.J., Molloy, P.L., Varinli, H., Morrison, J.L., Muhlhausler, B.S., Me mbers of
EpiSCOPE, 2014. Epigenetics and human obesity. Int. J. Obes. (Lond) 39, 85–97.
http://dx.doi.org/10.1038/ijo.2014.3424566855.
Verdam, F.J., Schouten, R., Greve, J.W., Koek, G.H., Bouvy, N.D., 2012. An update on less in-
vasive and endoscopic techniques mimicking the effect of bariatric surgery. J. Obes.
2012, 597871. http://dx.doi.org/10.1155/2012/59787122957215.
Vijgen, G.H.E.J., Bouvy, N.D., Leenen, L., Rijkers, K., Cornips, E., Majoie, M., Brans, B., Van
Marken Lichtenbelt, W.D., 2013. Vagus nerve stimulation increases energy expendi-
ture: relation to Brown adipose tissue activity. PLOS One 8 (10), e77221. http://dx.
doi.org/10.1371/journal.pone.0077221.t00324194874.
Volkow, N.D., Wang, G.J., Telang, F., Fowler, J.S., Thanos, P.K., Logan, J., Alexoff, D., Ding,
Y.S., Wong, C., Ma, Y., Pradhan, K., 2008. Low dopamine striatal D2 receptors are asso-
ciated with prefrontal metabolism in obese subjects: possible contributing factors.
Neuroimage 42 (4), 1537–1543. http://dx.doi.org/10.1016/j.neuroimage.2008.06.
00218 598772.
Walker, H.C., Lyerly, M. , Cutter, G., Hagood, J. , Stover, N. P., Gu thrie, S.L., Guthrie, B.L.,
Watts, R.L., 2009. Weight changes associated with unilateral STN DBS and advanced
PD. Parkinsonis m Relat. Disord. 15 (9), 709–711. http://dx.doi.org/10.1016/j.
parkreldis.2009.01.00919272829.
Wallace, D.L., Aarts, E., Dang, L.C., Greer, S.M., Jagust, W.J., D3Esposito, M., 2014. Dorsal
striatal dopamine, food preference and health perception in humans. PLOS One 9
(5), e96319. http://dx.doi.org/10.1371/journal.pone.009631924806534.
Walpoth, M., Hoertnagl, C., Mangweth-Matzek, B., Kemmler, G., Hinterhölzl, J., Conca, A.,
Hausmann, A., 2008. Repetitive transcranial magnetic stimulation in bulimia nervosa:
preliminary results of a single-centre, randomised, double-blind, sham-controlled
trial in female outpatients. Psychother. Psychosom. 77 (1), 57–60. http://dx.doi.org/
10.1159/00011006118087209.
Wang, G.J., Tomasi, D., Convit, A., Logan, J., Wong, C.T., Shumay, E., Fowler, J.S., Volkow,
N.D., 2014. BMI modulates calorie-dependent dopamine changes in accumben s
from
glucose intake. PLOS One 9 (7), e101585. http://dx.doi.org/10.1371/journal.
pone.010158525000285.
Wang, G.J., Volkow, N.D., Fowler, J.S., 2002. The role of dopamine in motivation for food in
humans: implications for obesity. Expert Opin. Ther. Targets. 6 (5), 601–609. http://
dx.doi.org/10.1517/14728222.6.5.60112387683.
Wang, G.J., Volkow, N.D., Logan, J., Pappas, N.R., Wong, C.T., Zhu, W., Netusil, N., Fowler,
J.S., 2001. Brain dopamine and obesity. Lancet 357 (9253), 354–357. http://dx.doi.
org/10.1016/S0140-6736(00)03643-611210998.
Wang, G.J., Volkow, N.D., Telang, F., Jayne, M., Ma, Y., Pradhan, K., Zhu, W., Wong, C.T.,
Thanos, P.K., Geliebter, A., Biegon, A., Fowler, J.S., 2009a. Evidence of gender differ-
ences in the ability to inhibit brain activation elicited by food stimulation. Proc.
30 D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
Natl. Acad. Sci . U. S. A. 106 (4), 1249–1254. http://dx.doi.org/1 0.1073/pnas.
080742310619164587.
Wang, G.J., Volkow, N.D., Thanos, P.K., Fowler, J.S., 2009b. Imaging of brain dopamine
pathways: implications for understanding obesity. J. Addict Med. 3 (1), 8–18.
http://dx.doi.org/10.1097/ADM.0b013e31819a86f721603099.
Wassermann, E., Epstein, C., Ziemann, U., 2008. Oxford Handbook of Transcranial Stimu-
lation. [!(sb:name)!], Press.
Watanabe, A., Kato, N., Kato, T., 2002. Effects of creatine on mental fatigue and cerebral
hemoglobin oxygenation. Neurosci. Res. 42 (4), 279–285. http://dx.doi.org/10.1016/
S0168-0102(02)00007-X11985880.
Weiskopf, N., 2012. Real-time fMRI and its application to neurofeedback. Neuroimage 62
(2), 682–692. http://dx.doi.org/10.1016/j.neuroimage.2011.10.00922019880.
Weiskopf, N., Scharnowski, F., Veit, R., Goebel, R., Birbaumer, N., Mathiak, K., 2004. Self-
regulation of local brain activity using real-time functional magnetic resonance imag-
ing (fMRI). J. Physiol. Paris 98 (4–6), 357–373. http://dx.doi.org/10.1016/j.jphysparis.
2005.09.01916289548.
Weiskopf, N., Sitaram, R., Josephs, O., Veit, R., Scharnowski, F., Goebel, R., Birbaumer, N.,
Deichmann, R., Mathiak, K., 2007. Real-time functional magnetic resonance imaging:
methods and applications. Magn. Reson. Imaging 25 (6), 989–1003. http://dx.doi.org/
10.1016/j.mri.2007.02.00717451904.
Whiting, D.M., Tomycz, N.D., Bailes, J., De Jonge, L., Lecoultre, V., Wilent, B., Alcindor, D.,
Prostko, E.R., Cheng, B.C., Ang le, C., Cantella, D., Whiting, B.B., Mizes, J.S., Finnis,
K.W., Ravussin, E., Oh, M.Y., 2013. Lateral hypothalamic area deep brain stimulation
for refractory obesity: a pilot study with preliminary data on safety, body weight,
and energy metabolism. J. Neurosurg. 119 (1), 56–63. http://dx.doi.or g/10.3171/
2013.2.JNS1290323560573.
Wightman, E.L. , Haskell, C.F., Forster, J.S., Veasey, R.C., Kennedy, D.O., 2012. Epi-
gallocatechin gallate, cerebral blood flow parameters, cognitive performance
andmoodinhealthyhumans:adouble-blind, placebo-controlled, crossover
investigation. Hum. Psychopharmacol. 27 (2), 177–186. http://dx.doi.org/10.
1002/hup.126322389082.
Wilcox, C.E., Braskie, M.N., Kluth, J.T., Jagust, W.J., 2010. Overeating behavior and striatal
dopamine with 6-[F]-fl uoro-
L-m-tyrosine PET. J. Obes. 2010. http://dx.doi.org/10.
1155/2010/909348.
Williams, K.W., Elmquist, J.K., 2012. From neuroanatomy to behavior: central integration
of peripheral signals regulating feeding behavior. Nat. Neurosci. 15 (10), 1350–1355.
http://dx.doi.org/10.1038/nn.321723007190.
Wing, R.R., Phelan, S., 2005. Long-term weight loss maintenance. Am. J. Clin. Nutr. 82 (1
Suppl), 222S–225S16002825.
Wu, H., Van Dyck-Lippens, P.J., Santegoeds, R., Van Kuyck, K., Gabriëls, L., Lin, G., Pan, G., Li,
Y., Li, D., Zhan, S., Sun, B., Nuttin, B., 2013. Deep-brain stimulation for anorexia
nervosa. World Neurosurg. 80 (3–4), S29.e1–S29.e10. http://dx.doi.org/10.1016/j.
wneu.2012.06.03922743198.
Xiao, Y., Beriault, S., Pike, G.B., Collins, D.L., 2012. Multicontrast multiecho FLASH MRI for
targeting the subthalamic nucleus. Magn. Reson. Imaging 30 (5), 627–640. http://dx.
doi.org/10.1016/j.mri.2012.02.00622503090.
Xue, G., Aron, A.R., Poldrack, R.A., 2008. Common neural substrates for inhibition of spo-
ken and manual responses. Cereb. Cortex 18 (8), 1923–1932. http://dx.doi.org/10.
1093/cercor/bhm22018245044.
Yimit, D., Hoxur, P., Amat, N., Uchikawa, K., Yamaguchi, N., 2012. Effects of soybean pep-
tide on immune function, brain function, and neurochemistry in healthy volunteers.
Nutrition 28 (2), 15 4–159. http://dx.doi.org/10.1016/j.nut.2011.05.00821872436.
Yokum, S., Gearhardt, A.N., Harris, J.L., Brownell, K.D., Stice, E., 2014. Individual differences
in striatum activity to food commercials predict weight gain in adolescents. Obesity
(Silver Spring) 22, 2544–2551. http://dx.doi.org/10.1002/oby.2088225155745.
Yokum, S., Ng, J., Stice, E., 2011. Attentional bias to food images associated with elevated
weight and future weig ht gain: an fMRI study. Obesity Silver Sp ring 19 (9),
1775–1783. http://dx.doi.org/10.1038/oby.2011.16821681221.
Yokum, S., Stice, E., 2013. Cognitive regulation of food craving: effects of three cognitive
reappraisal strategies on neural response to palatable foods. Int. J. Obes. (Lond) 37
(12), 1565–1570. http://dx.doi.org/10.1038/ijo.2013.3923567923.
Zahodne, L.B., Susatia, F., Bowers, D., Ong, T.L., Jacobson, C.E.T., Okun, M.S., Rodriguez, R.L.,
Malaty, I.A., Foote, K.D., Fernandez, H.H., 2011. Binge eating in Parkinson3sdisease:prev-
alence, correlates and the contribution of deep brain stimulation. J. Neuropsychiatry
Clin. Neurosci. 23 (1), 56–62. http://dx.doi.org/10.1176/appi.neuropsych.23.1.
5621304139.
Zangen, A., Roth, Y., Voller, B., Hallett, M., 2005. Transcranial magnetic stimulation of deep
brain regions: evidence for efficacy of the H-coil. Clin. Neurophysiol. 116 (4),
775–779. http://dx.doi.org/10.1016/j.clinph.2004.11.00815792886.
Zhang, X., Cao, B., Yan, N., Liu, J., Wang, J., Tung, V.O.V., Li, Y., 2013. Vagus nerve stimula-
tion modulates visceral pain-related affective memory. Behav. Brain Res. 236 (1),
8–15. http://dx.doi.org/10.1016/j.bbr.2012.08.02722940455.
Ziauddeen, H., Farooqi, I.S., Fletcher, P.C., 2012. Obesity and the brain: how convincing is
the addiction model? Nat. Rev. Neurosci. 13 (4), 279–286. http://dx.doi.org/10.1038/
nrn321222414944.
Zotev, V., Krueger, F., Phillips, R., Alvarez, R.P., Simmons, W.K., Bellgowan, P., Drevets,
W.C., Bodurka, J., 2011. Self-regulation of amygdala activation using real-time FMRI
n
eurofeedback. PLOS On e 6 (9), e24522. http://dx.doi.org/10.1371/journal.pone.
002452221931738.
Zotev, V., Phillips, R., Young, K.D., Drevets, W.C., Bodurka, J., 2013. Prefrontal control of the
amygdala during real-time fMRI neurofeedback training of emotion regulation. PLOS
One 8 (11), e79184. http://dx.doi.org/10.1371/journal.pone.007918424223175.
31D. Val-Laillet et al. / NeuroImage: Clinical 8 (2015) 1–31
