
SCCS/1557/15
Final Opinion December 2015
Scientific Committee on Consumer Safety
SCCS
OPINION ON
Methylisothiazolinone (MI) (P94)
Submission III
(Sensitisation only)
The SCCS adopted this opinion at its 10
th
plenary meeting
on 25 June 2015 and the final opinion, remaining unchanged, on 15 December 2015

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
2
About the Scientific Committees
Three independent non-food Scientific Committees provide the Commission with the
scientific advice it needs when preparing policy and proposals relating to consumer safety,
public health and the environment. The Committees also draw the Commission's attention
to the new or emerging problems which may pose an actual or potential threat.
They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee
on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and
Newly Identified Health Risks (SCENIHR) and are made up of external experts.
In addition, the Commission relies upon the work of the European Food Safety Authority
(EFSA), the European Medicines Agency (EMA), the European Centre for Disease prevention
and Control (ECDC) and the European Chemicals Agency (ECHA).
SCCS
The Committee shall provide opinions on questions concerning all types of health and safety
risks (notably chemical, biological, mechanical and other physical risks) of non-food
consumer products (for example: cosmetic products and their ingredients, toys, textiles,
clothing, personal care and household products such as detergents, etc.) and services (for
example: tattooing, artificial sun tanning, etc.).
Scientific Committee members
Ulrike Bernauer, Qasim Chaudhry, Pieter Coenraads, Gisela Degen, Maria Dusinska, Werner
Lilienblum, Elsa Nielsen, Thomas Platzek, Suresh Chandra Rastogi, Christophe Rousselle,
Jan van Benthem
Contact
European Commission
Health & Food Safety
Directorate C: Public Health
Unit C2 – Health Information and Scientific Committees
Office: HTC 03/073
L-2920 Luxembourg
©
European Union, 2015
ISSN 1831-4767 ISBN 978-92-79-56129-0
Doi:10.2875/713830 EW-AQ-16-006-EN-N
The opinions of the Scientific Committees present the views of the independent scientists
who are members of the committees. They do not necessarily reflect the views of the
European Commission. The opinions are published by the European Commission in their
original language only.
http://ec.europa.eu/health/scientific_committees/consumer_safety/index_

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
3
ACKNOWLEDGMENTS
SCCS Members
Dr. U. Bernauer
Dr. Q. Chaudhry
Prof. P.J. Coenraads
Prof. G. H. Degen (chairperson)
Dr. M. Dusinska
Dr. W. Lilienblum
Dr. E. Nielsen
Prof. T. Platzek
Dr. S. Ch. Rastogi
Dr. Ch. Rousselle
Dr. J. van Benthem
External experts
Prof. A. Bernard
Prof. J. Duus-Johansen
Dr. J. Ezendam
Prof. A. M. Giménez-Arnau (rapporteur)
Dr. E. Mikova
Dr. E. Panteri
Prof. T. Vanhaecke
This opinion has been subject to a commenting period of minimum four weeks after its
initial publication. Comments received during this time have been considered by the SCCS
and discussed in the subsequent plenary meeting.
Where appropriate, the text of the relevant sections of the opinion has been modified or
explanations have been added. Revised opinions carry the date of revision.
In the cases where the SCCS after consideration and discussion of the comments, has
decided to maintain its initial views, the opinion (or the section concerned) has remained
unchanged. Final opinions carry the date of the finalisation.
Keywords: SCCS, scientific opinion, Methylisothiazolinone (MI) (P94), sensitisation,
Regulation 1223/2009, CAS 2682-20-4, EC 220-239-6
Opinion to be cited as: SCCS (Scientific Committee on Consumer Safety), Opinion on
Methylisothiazolinone (MI) (P94) sensitisation only, 25 June 2015, SCCS/1557/15, final
opinion December 2015

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
4
TABLE OF CONTENTS
ACKNOWLEDGMENTS ........................................................................................... 3
TABLE OF CONTENTS ............................................................................................ 4
1. BACKGROUND ............................................................................................. 5
2. TERMS OF REFERENCE .................................................................................. 6
3. OPINION ..................................................................................................... 7
3.1 Chemical and Physical Specifications ....................................................... 7
3.2 Epidemiology of contact allergy to methylisothiazolinone updated ............... 7
3.3 (SCCS/1521/13) opinion on Methylisothiazolinone (P94) and rinse-off products
9
3.4 New submission ................................................................................... 9
3.5 Resubmission of comments from CE for re-consideration ......................... 10
3.6 Submission of new data from CE .......................................................... 11
3.7 Use test with rinse-off products in MI sensitised consumers. ................... 15
3.8 Compilation of cosmetovigilance data related to cosmetic products containing
Methylisothiazolinone (MI) ................................................................................ 15
3.9 DISCUSSION ..................................................................................... 17
4. CONCLUSION ............................................................................................ 19
5. REFERENCES ............................................................................................. 20
ANNEX I ........................................................................................................... 22

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
5
1. BACKGROUND
The Scientific Committee on Cosmetic Products and Non-Food Products (SCCNFP) adopted
two opinions on "Methylisothiazolinone" respectively in March 2003 (SCCNFP/0625/02) and
in April 2004 (SCCNFP/0805/04).
The SCCNFP (March 2003 - SCCNFP/0625/02) concluded that the information submitted
was insufficient at that time to allow an adequate risk assessment of Methylisothiazolinone
to be carried out. The SCCNFP required: more detailed information concerning the physico-
chemical properties of Methylisothiazolinone (e.g. LCMS analysis, pH, stability and
degradation products); information on the material used in the tests (batch numbers, purity
and impurities); an in vitro percutaneous absorption study and relevant and adequate
genotoxicity/mutagenicity studies.
In response to the opinion of the SCCNFP concerning Methylisothiazolinone, adopted during
the 23rd plenary meeting of 18 March 2003 (doc. n° SCCNFP/0625/02), additional
information on the physico-chemical properties of the substance, an in vitro percutaneous
absorption study and two studies on mutagenicity/genotoxicity were submitted to the
SCCNFP for evaluation. In April 2004 the SCCNFP (SCCNFP/0805/04) concluded that the
requested data were complete. Methylisothiazolinone was considered non
genotoxic/mutagenic.
Methylisothiazolinone (MI) was listed in Annex V/57 of Regulation (EC) No 1223/2009 to be
used as preservative at maximum concentration of 0.01% (100 ppm) in cosmetics products.
According to several Member States and a good number of published papers, the
sensitisation to MI is becoming an increasing problem all over Europe. In light of this
information, the Commission requested to the Scientific Committee (SCCS) a reassessment
of the safety of MI when it is used as preservative in cosmetics products at maximum
concentration of 100 ppm. The scientific opinion of the SCCS (SCCS/1521/13) on
Methylisothiazolinone (P94) Submission II (Sensitisation only) was delivered in March 2014
with the following conclusions:
Current clinical data indicate that 100 ppm MI in cosmetic products is not safe for the
consumer. For leave-on cosmetic products (including 'wet wipes'), no safe concentrations of
MI for induction of contact allergy or elicitation have been adequately demonstrated. For
rinse-off cosmetic products, a concentration of 15 ppm (0.0015%) MI is considered safe for
the consumer from the view of induction of contact allergy. However, no information is
available on elicitation.
Recently, the SCCS received a new mandate in order to assess safety of 100 ppm of MI
included in rinse off and hair leave on products. Data from Cosmetics Europe concerning the
safety of MI in rinse-off and hair leave on products were received in June 2014, new
cosmetovigilance data in February 2015 and data on aggregate exposures to rinse-off
products in May 2015. The concentration limit of MI to 15 ppm proposed by the SCCS for
rinse off products is based on the data available related to the mixture MCI/MI
(SCCS/1238/09). New data are submitted trying to demonstrate that 100 ppm included in
rinse-off and in leave-on hair cosmetics products is safe for the consumers. The SCCS is
requested to give an opinion about the safety of MI at 100 ppm in rinse-off and leave-on
hair cosmetic products.

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
6
2. TERMS OF REFERENCE
1. On the basis of the data provided, does the SCCS consider Methylisothiazolinone (MI) to
be safe for consumers, when used as a preservative in rinse-off products up to
concentration limit of 100 ppm from the view of induction of contact allergy?
2. On the basis of the data provided, does the SCCS consider Methylisothiazolinone (MI) to
be safe for consumers, when used as a preservative in leave-on hair products up to
concentration limit of 100 ppm from the view of induction of contact allergy?
3. Does the SCCS have any further scientific concerns with regard to the use of
Methylisothiazolinone (MI) in cosmetic products?
SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
7
3. OPINION
3.1 Chemical and Physical Specifications
3.1.1 Chemical identity
3.1.1.1 Primary name and/or INCI name
INCI methylisothiazolinone
3.1.1.2 Chemical names
Methylisothiazolinone
IUPAC: 2-Methylisothiazol-3(2H)-one
Other: 2-Methyl-4-isothiazolin-3-one
3.1.1.3 Trade names and abbreviations
3.1.1.4 CAS / EC number
CAS no. 2682-20-4
EC 220-239-6
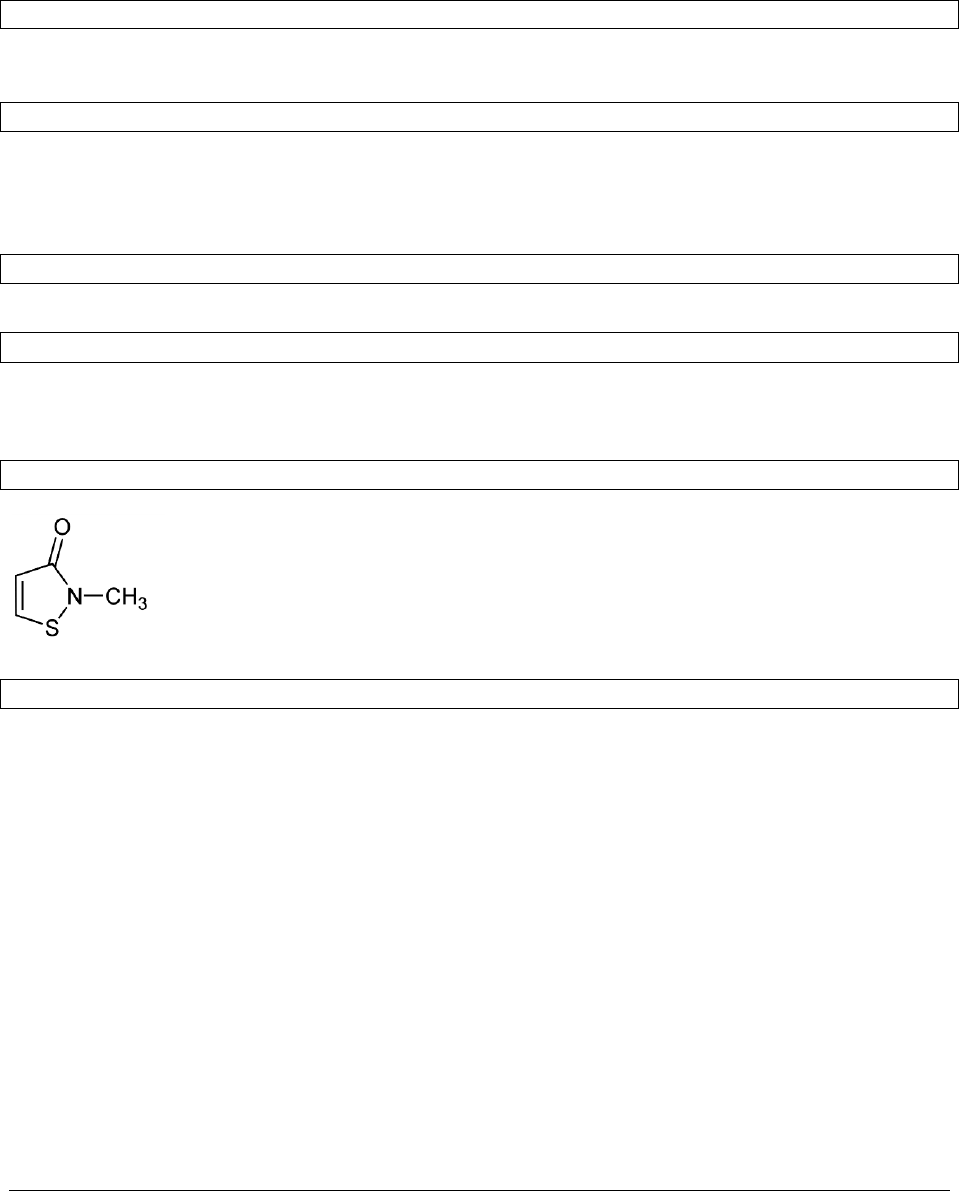
3.1.1.5 Structural formula
3.1.1.6 Empirical formula
C
4
H
5
NOS
3.2 Epidemiology of contact allergy to methylisothiazolinone updated
The SCCS Opinion on Methylisothiazolinone published 27 March 2014 includes a complete
review of the literature published about Methylisothiazolinone (MI) contact allergy up to that
date. The most important data introduced in the 2014 report are the following:
MI alone (without MCI) was introduced as a preservative in industrial products in the early
2000s, and in 2005 it was allowed as a preservative in both leave-on and rinse-off
cosmetics at a maximum concentration of 100 ppm (0.01%) (Annex V/57 of the Cosmetic
Regulation 1223/2009/ECC; Cosmetic Directive 2005/42/EC).
The first report on contact allergy from MI was published in 1987 (1). After 2000, MI was
introduced in industrial products (e.g. paints, adhesives, varnishes and cooling fluids), and

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
8
due to its weaker preservative effect was used at higher concentrations than in MCI/MI.
Allergic contact dermatitis from MI in occupational settings was reported in 2004 (2) mainly
due to exposure to paints (3, 4).
The first reports from MI contact allergy caused by cosmetics originate from 2010 (5)
mainly due to wet wipes for hygiene (baby wipes, moist tissues, moist toilet paper), hair
cosmetics (shampoos), facial cosmetics (6, 7), deodorants (8) and sunscreens (9).
Air exposure to MI induced severe cases of airborne allergic contact dermatitis and systemic
contact dermatitis, particularly from recently painted walls (10, 11), including a case in a
four-year-old child most probably sensitised to MI through baby wipes. Airborne exposures
and allergic contact dermatitis from toilet cleaners have also been reported (12).
MI has only recently been tested as a single allergen, separate from MCI/MI in the European
baseline series and in the local baseline series in several countries. In the European baseline
patch test series, MI is tested at 2000 ppm (0.2%) (17). Reactivity in patients who were
patch tested was around 1.5% until 2008 in Denmark (7) but values increased from 0.9%
in 2006 to 1.8% in 2008 in Finland (13) and very high prevalences were demonstrated in
2011/12 in Leeds (4.6%) (14), London (6%), Coimbra (4.5%) and Leuven, (5.8%), with a
very high percentage of reactions found to be actually relevant because the source of the
exposure was demonstrated (15).
In Germany, although in selected patients with suspected cosmetic or occupational
exposure, MI sensitisation rose from 1.9% in 2009 to 4.4% in 2011, particularly in female
patients (188% increase) and in patients with facial dermatitis (200% increase), suggesting
that increase in prevalence is most probably related to cosmetic exposure (16). In the US, a
similar situation seems to have occurred as MI was considered the allergen of the year 2013
(9).
Contact allergy to MI has been reported in consecutively tested dermatitis patients in
Sweden, Denmark, Germany, Finland and the UK. The contact allergy rates reported vary
between 0.5% and 6% in 2012. The rates from the UK where noticed in Leeds from 0.6% in
2009 to 4.6% in 2012 (14). In Denmark, an increase from 1.4% in 2009 to 3.1% in 2011
was recorded (11).
Other European countries have recently published their own experiences showing an
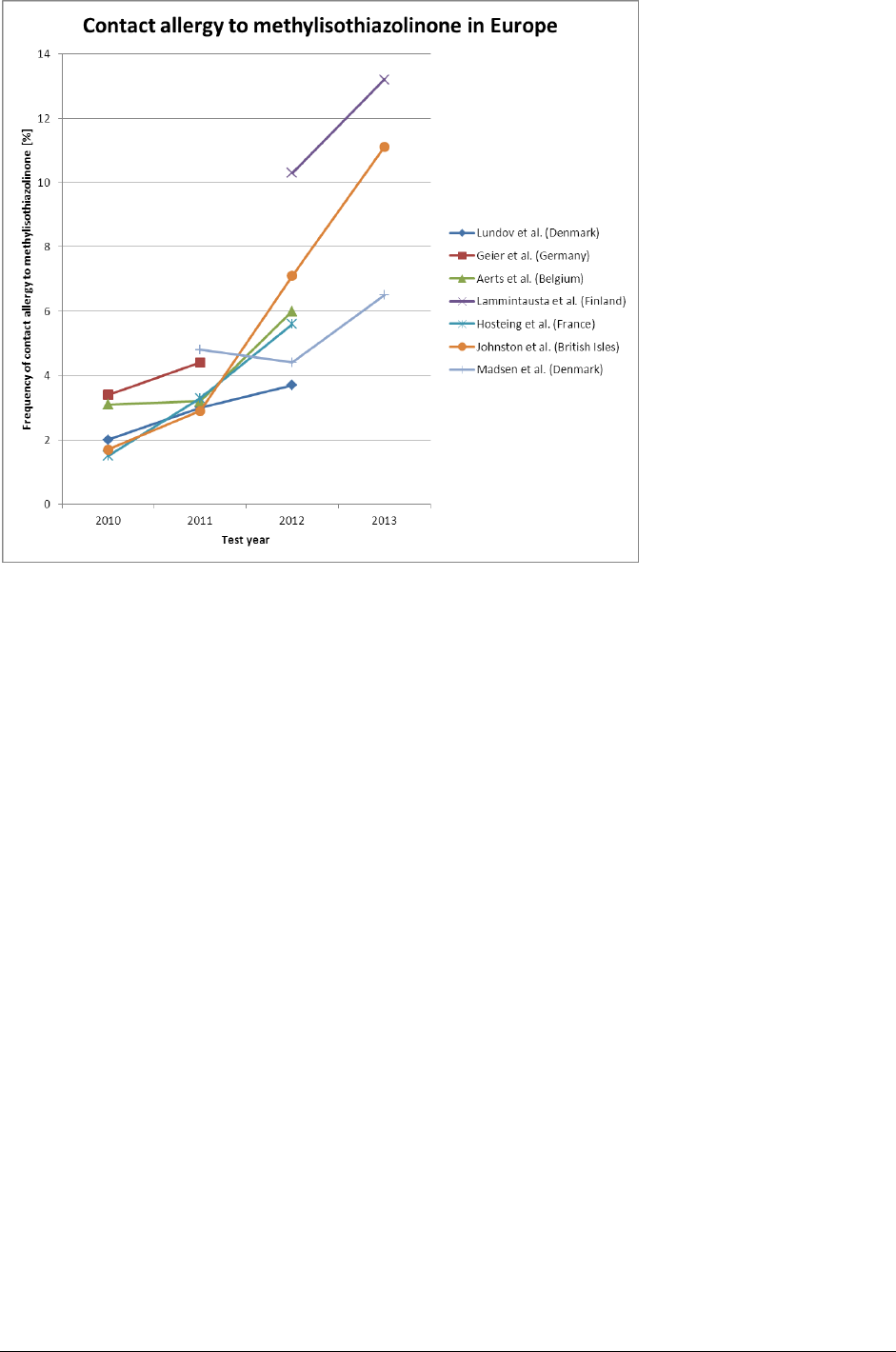
increased prevalence of contact sensitisation to MI (Figure 1). In Belgium, where in 2012,
the sensitisation rate to MCI/MI had increased to 4.5% and that for MI to 6.0%, the latter
showed a further increase to 7.2% in 2013. (18) The MCI/MI sensitisation rate increased in
the South of Gran Canaria from 3.6% in 2007 to 17.3% in 2012, and when MI was patch
tested alone at either 0.05% or 0.2%, the representative sample of this area showed a
prevalence of 8.2% (19). The French data from the REVIDAL-GERDA network, with sixteen
centres and 7874 patients tested, showed a significant increase in the proportion of MI-
positive tests in 2012 and 2011 as compared to 2010 (5.6%, 3.3%, and 1.5%, respectively;
p<0.001) when patch testing MI at 200 ppm aq. (20). In Finland a clearly increasing
incidence of MI contact allergy was found in all clinics providing data (21). It was regarded
as an epidemic of contact allergy to MI (see also Figure 1). (22)
SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
9
Figure 1. Data from recently published scientific literature concerning changes over time in MI contact allergy
among patch-tested patients in different countries, based on refs: 18, 20-23, 29, 30
3.3 (SCCS/1521/13) Opinion on Methylisothiazolinone (P94) and rinse-
off products
In the Opinion on MI from March 2014 (SCCS/1521/13), it was concluded that current
clinical data indicate that 100 ppm MI in cosmetic products is not safe for the consumer. For
leave-on cosmetic products (including ‘wet wipes’), no safe concentrations of MI for
induction of contact allergy or elicitation have been adequately demonstrated. For rinse-off
cosmetic products, a concentration of 15 ppm (0.0015) MI is considered safe for the
consumer from the view of induction of contact allergy. However, no information is available
on elicitation.
3.4 New submission
The dossier of data submitted consists of a submission letter entitled 'Industry submission
concerning safety of methylisothiazolinone (MI) in rinse-off and leave-on products', dated
12 June 2014, and eight other documents, some of which were submitted later (see below):
Resubmission of comments and data
Re-submission of the comments made by Cosmetics Europe to the draft 2013 Opinion,
consisting of:
a. Cosmetic Europe's response to the SCCS Opinion on Methylisothiazolinone,
adopted 12 December 2013 (dated 14 February 2014)
b. Hazard characterisation data for Methylisothiazolinone (MI) and
Methylisothiazolinone / Chloromethylisothiazolinone (MCI/MI)(Annex I)

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
10
c. The Efficacy of Methylisothiazolinone (MI) and Methylchloroisothiazolinone/
Methylisothiazolinone (CMI/MI) and the Microbiological Safety of Cosmetic
Products (Annex II)
Submission of new data
Submissions of new data were accepted in 3 rounds, June 2014, February 2015 and May
2015. Data received were:
a. Summary of the data reviewed by the SCCNFP in its opinions on MI from 2003
to 2004.
b. Skin allergy assessment and the Quantitative Risk Assessment.
c. Methylisothiazolinone and the mixture of chlorinated CMI/MI (3:1 ratio) are
two different preservatives with different safety and efficacy profiles (dated 26.
May 2014)
d. Compilation of cosmeto-vigilance data related to cosmetic products
containingMI (submitted February 2015).
e. Assessment of impact on the risk of induction of skin sensitisation from
aggregated exposure arising from use of rinse-off cosmetic products
containing 100ppm methylisothiazolinone (MI) (submitted May 2015).
3.5 Resubmission of comments from CE for re-consideration
Cosmetics Europe re-submitted a dossier with their previous response to the Opinion of
SCCS dated 12 December 2013 consisting of the 3 documents mentioned above.
In the submission letter (dated 12
Th
June 2014, CE justifies this re-submission with the fact
that they do not feel their comments were adequately addressed in the final SCCS Opinion,
especially concerning the following points:
Clinical data in isolation is insufficient to establish safe induction levels for MI.
MCI is at least one order of magnitude more potent than MI. The animal data and data
from Human Repeated Insult Patch Tests are given in the re-submission (annex I) and it
is concluded that ‘applying identical specific concentration limits to both MCI/MI and MI
is not justified based on the available hazard characterization data.'
SCCS comment
The use of MI in cosmetic has caused an unprecedented high rate of sensitised individuals in
Europe as reflected by the patch test data from dermatology clinics mentioned above.
Clinical data have established that current uses of MI at 100 ppm are unsafe. The risk
assessment based on predictive assays using the methods available at the time (SCCS
opinion 2004) and later has failed to protect the consumer with regard to induction of
contact allergy to MI and allergic contact dermatitis.
Data from humans who have developed contact sensitisation and allergic contact dermatitis
through the use of consumer products are highly relevant for risk assessment and should
never be disregarded, especially not when risk assessment based on predictive assays has
failed. Below, the SCCS comments further on some of these data.

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
11
Concerning the difference in potency between MCI and MI, the prediction of potency is
based on experimental studies in animals and sometimes humans. These are models that
may or may not accurately reflect the true difference between substances. MI has been
used since its inclusion in cosmetics in up to 8.8 times higher concentrations (100 ppm)
than MCI (11.25 ppm in the mixture MCI/MI 3:1 at 15 ppm). According to the submission,
the difference in potency is at least one order of magnitude: the NESIL derived from HRIPTs
MCI is 18 times more potent than MI (NESIL MCI: 0.83µg/cm
2
and MI: 15µg/cm
2
).
Nevertheless, the use of MI in up to 8.8 times higher concentrations than MCI for the past
10 years in cosmetic products has led to the current situation of exceptionally high rates of
contact allergy to MI in consumers.
The SCCS also replied to these comments by Cosmetics Europe following the consultation
concerning the SCCS Opinion (SCCS/1521/13). In this Opinion it is suggested that MI at 15
ppm in rinse-off products should be safe for the consumer. There is, at present, no data to
indicate that a higher level is ‘safe’ for either induction or elicitation. Therefore, 15 ppm was
chosen for safety reasons, as clearly discussed in the Opinion.
3.6 Submission of new data from CE
3.6.1 Quantitative Risk Assessment applied to Methylisothiazolinone in rinse of
products and leave-on hair cosmetics
In the submission, Cosmetics Europe applied the Quantitative Risk Assessment (QRA)
methodology (24, 25) to predict maximum safe exposure levels, i.e. exposure levels that
are assumed not to cause induction of skin sensitisation.
3.6.1.1 QRA methodology
According to the submission, the QRA approach for allergens in consumer products follows
the same four fundamental steps as identified for general toxicology risk assessment: a)
hazard identification b) dose-response assessment or hazard quantification c) exposure
assessment and d) risk characterisation. The induction of skin sensitisation is a threshold-
based event; the metric for risk assessment for this toxicological endpoint is accepted to be
dose per unit area of skin (or g/cm
2
). The key steps of the QRA process are determination
of known safe benchmarks, application of sensitisation assessment factors, and calculation
of consumer exposure through normal products use. With these parameters, an acceptable
exposure level (AEL) can be calculated and compared with the consumer exposure level
(CEL). When the AEL exceeds the CEL, it is predicted that induction of skin sensitisation is
unlikely to occur.
SCCS comment
The SCCP adopted an Opinion concerning Dermal Sensitisation Quantitative Risk
Assessment (Citral, Farnesol and Phenylacetaldehyde) on 24 June 2008 (SCCP/1153/08).
The QRA model mentioned and applied in the new submission is identical with the QRA
model assessed in the Opinion (SCCP/1153/08), leading to the following conclusion, as
stated in that Opinion:
The dermal sensitisation QRA model is based primarily on data from experimental
sensitisation tests in humans e.g. Human Repeated Insult Patch Tests (HRIPT).
There is a lack of in-depth method description, and the experience with this test,
its validity, sensitivity and reliability is sparse outside industry. Performing this
type of experimental sensitisation tests on humans is considered unethical.

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
12
Epidemiological and experimental data, providing information on
sensitisation/elicitation reactions in consumers by the substance evaluated (e.g
preservative or fragrance) in marketed products, are not integrated in the dermal
sensitisation QRA model. It is of concern that the model operates with multiple
product categories without considering risk from aggregated exposures and that
scientific consensus has not been achieved concerning the choice of safety
factors. Occupational exposures are not considered although they have been
identified as an important area of development of the dermal sensitisation QRA.
The data provided shows that the application of the dermal sensitisation QRA
approach would allow increased exposures to allergens, already known to cause
allergic contact dermatitis in consumers. The model has not been validated and
no strategy of validation has been suggested. There is no degree of certainty that
the levels of skin sensitisers identified by the dermal sensitisation QRA are safe
for the consumer.
Identification of safe levels of exposure to existing substances known to cause
allergic contact dermatitis in the consumer should be based on clinical data
and/or elicitation low effect levels. Currently these are the only methods that
have proven efficient in reducing/preventing existing problems of
sensitisation/allergic contact dermatitis in the consumer.
The QRA model in the new submission from June 2014 has not been updated or modified
concerning any of the points raised above. In the additional submission dated 25 May 2015
the effect of aggregate exposures to MI in rinse–off products on risk of induction of
sensitisation by QRA was addressed. See below.
3.6.1.2 Application of quantitative risk assessment to MI
In the submission by CE, the QRA methodology was applied to MI in cosmetic products
according to the principles explained above. As stated above, the SCCS has no faith in the
model in its current form (SCCP/1153/08), but has nevertheless chosen to comment on
substance specific data in this section used in the QRA, as these comments may have
relevance for further development of the model and future quantitative risk assessment of
MI.
The maximum permitted amount of MI in cosmetic products is 100 ppm. The consumer
exposure to 100 ppm (0.01%) MI is calculated by multiplying the amount of product used
per day by 0.01% and is expressed as dose/surface area (i.e. µg/cm
2
). The range of
products for which suitable exposure data have been identified, along with the amount of
product used, is listed in Table 1 (Annex I of this Opinion).
According to the submission, No Expected Skin Sensitisation Level (NESIL) is a benchmark
that is derived from animal and human data through the application of a Weight of Evidence
(WoE) approach using all relevant data.
For the determination of a WoE NESIL for MI, the data from 5 Human Repeat Insult Patch
Tests (HRIPT) and 4 local lymph node assays (LLNA) were considered. In the HRIPTs, no
positive responses were observed up to an exposure level of 15 µg/cm
2
MI in water.
Sensitisation was induced at exposures of 20 and 25 µg/cm
2
. Based on these data, CE
concludes that MI can be considered to be a strong sensitiser.
In the LLNA, MI had EC3 values between 0.4% (100µg/cm
2
) and 11% (2750µg/cm
2
)
depending on the vehicle used. The EC3 values indicate that MI is a strong sensitiser.
SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
13
Taking all of the data together, since the HRIPT threshold is the lowest no observed effect
level (NOEL) available, it shall, according to the submission, take precedence in deriving the
NESIL for use in the QRA. Therefore, the WoE NESIL for MI is 15µg/cm
2
. A summary of the
considered HRIPT studies (26) conducted on MI is given in the submission as table 2 and
reproduced below:
Vehicle, Dose Volume,
Patch Size
Induction
Concentration
(ug/cm
2
)
Challenge
Concentration
Positive
Responses
Water; 0.2ml, 4 cm
2
200ppm (10 µg/cm
2
)
200ppm
0 / 100
Water; 0.2ml, 4 cm
2
300ppm (15 µg/cm
2
)
300ppm
0 / 98
Water; 0.2ml, 4 cm
2
400ppm (20 µg/cm
2
)
400ppm
1 / 116
Water; 0.2ml, 4 cm
2
500ppm (25 µg/cm
2
)
500ppm
1 / 210
Water; 0.2mL,4 cm
2
600ppm (30 µg/cm
2
)
600ppm
0 / 214
Table 2: Summary of the HRIPT studies conducted on MI (from the submission)
SCCS comment
In the submission, the NESIL is determined to be 15 µg/cm
2
by WoE approach based on
data from HRIPT given above and as table 2 in the submission.
The sensitivity and predictivity of the HRIPT does not only depend on the choice of
concentration for induction, but also the choice of challenge concentration.
It can be seen from table 2 above (from the submission) that not only the induction
concentrations varied between experiments, but also the challenge concentration of MI. The
high dose induction group has been challenged with a high dose (max 600 ppm) and the
low dose induction group with a low dose (min 200 ppm). It is a general principle in patch
testing that the maximal concentration that can be tolerated without causing skin irritation
should be used for demonstrations of sensitisation (27).
This means that the lower levels in these experiments, which seemingly cause no induction,
are not put to a sufficient test at challenge and that induction may have occurred but may
not have been revealed. The NESIL for MI may be lower than 15 µg/cm
2
, as the
experiments have not been performed in a way so that conclusions on no-effect levels can
be made.
The SCCS also has comments regarding AEL/CEL ratio and predicted risk from rinse-off
products and stay-on hair cosmetics.
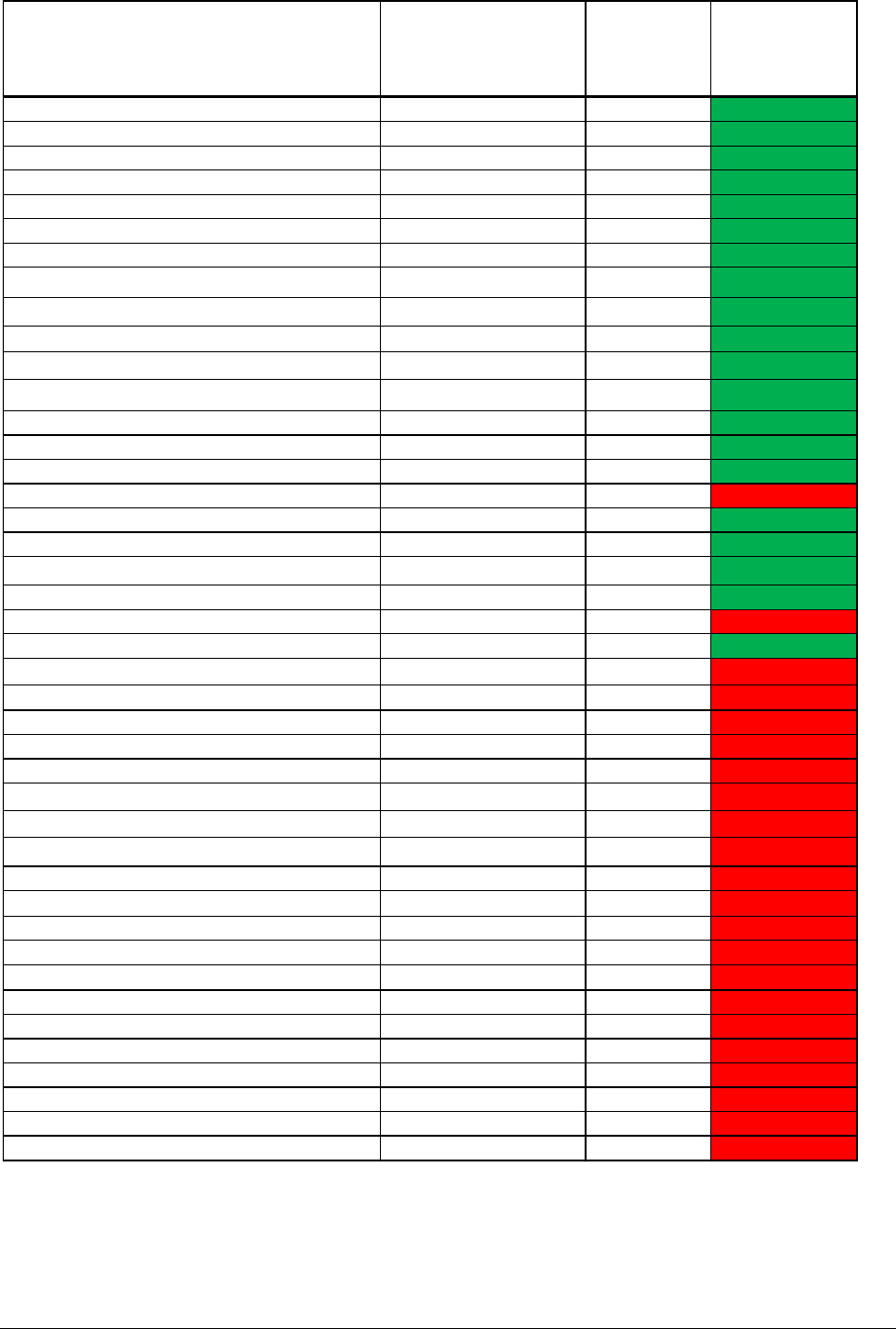
In the submission table 3 (reproduced in Annex 1), 20 products categories are green:
ranging from an AEL/CEL of 1.0 (body lotion and after-sun cream) to 140.6 (shower gel).
According to the submission product, categories with an AEL/CEL >1 are unlikely to cause
induction. This would also mean that body lotion and after-sun cream lotion with an

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
14
AEL/CEL of 1 should have been coloured red in the table 3 as these products are likely to
induce sensitisation.
According to the submission 100 ppm is safe for use concerning induction of sensitisation in
rinse-off products and stay-on cosmetics as the AEL/CEL is above 1.
The AEL/CEL is calculated in the following way with data used for shower gels:
AEL= NESIL (15 µg/cm
2
) /SAF (100)= 0.15 µg/cm
2
CEL= 0.0011 µg/cm
2
(table 2- Annex 1)
AEL/CEL= 0.15 µg/cm
2
/0.0011 µg/cm
2
= 136 (incorrectly given as 140.6 in the
submission (table 3-Annex 1).
A product with an AEL/CEL of 1.1 is in theory unlikely to cause induction. This means that
the QRA model predicts that not only 100 ppm (0.01%) in a shower gel is unlikely to cause
induction but also 1.2% MI (12000 ppm). For shampoos 0.18% (1800 ppm) is the predicted
maximum concentrations unlikely to induce sensitisation and for hairstyling products 0.03%
(300 ppm) given an AEL/CEL ratio of 1.1.
The data provided show that the application of the dermal sensitisation QRA approach to
rinse-off products would allow increased exposures to MI, a strong allergen already known
to cause many cases of allergic contact dermatitis in consumers. This alone makes it difficult
to have confidence in the model in its current version.
The comments above also apply to stay-on hair cosmetic products. Furthermore, it is not
clear if the QRA model for stay-on hair cosmetics in the original submission (June 2014)
also takes exposure to the hands into account. Hands are bound to be exposed to the hair
products either during application or by touching the hair unintentionally.
3.6.1.3 Assessment of aggregate exposure in the QRA
In the new submission by CE (May 2015), aggregate exposures to a number of rinse-off
products such as shower gels and shampoos are calculated. Aggregate exposure is
calculated using an interim/pragmatic approach in which the CEL is calculated for different
body parts relevant for MI exposure by rinse-off products, e.g. hands, face, scalp and the
rest of the body. Aggregate exposure is the sum of the exposure level estimated for the
individual products used on the respective body part. In all cases concerning aggregate
exposures to rinse-off cosmetic products yields an AEL:CEL ratio greater than 1. The
following AEL:CEL ratios were reported:
Hands = 2.1
Face = 1.8 (females) and 5.3 (males)
Scalp = 8.8
Rest of the body = 140.
CE considers it an interim assessment of impact of aggregate exposures and concludes that
the risk of induction from aggregate exposure to rinse-off products is very low.
SCCS comment
The SCCS assessed the QRA in 2008 and pointed to several shortcomings in the model
including the lack of considerations of aggregate exposures. The SCCS is aware that
updating of the QRA model is currently ongoing in industry. The QRA presented by CE is
using the same approach, except the aggregate exposure assessments, as defined in the
initial QRA approach. Hence, the criticism raised previously by the SCCS (SCCP1153/08) is
the same. Furthermore, the aggregate exposure assessment, which is presented as an
interim approach, needs to be evaluated and accepted by the SCCS before it can be applied
to specific substances.

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
15
3.7 Use test with rinse-off products in MI sensitised consumers.
In the submission letter (dated 12 June 2014), a paragraph is devoted to the subject of
performing a use test study – also called ROAT study - in sensitised consumers.
A negative use test study with a rinse-off product would confirm safety concerning not only
elicitation, but also induction. CE states that: 'The cosmetics industry is studying different
possibilities to further confirm the safety of MI-preserved rinse-off products in MI-patch test
positive consumers’.
SCCS comment
In the meantime a use test study in MI sensitised consumers has been performed and
published in February 2015 (28). Here 19 MI-allergic subjects and 19 controls without MI
allergy applied 2 liquid hand soaps five times per day on areas of 5*10 cm on the ventral
side of their forearms. One soap contained 100 ppm MI, the maximum allowed
concentration in cosmetics, and was used by 10 allergic subjects and all controls. Another
liquid soap with 50 ppm MI was used by 9 allergic subjects. As the negative control, all
subjects used a similar soap that did not contain MI. The repeated open applications (ROAT)
proceeded for up to 21 days or until a positive reaction occurred. The study was conducted
in a randomised and blinded fashion. Ten (10) out of 10 MI-allergic subjects developed
positive reactions to the soap with 100 ppm and 7 out of 9 reacted to the 50 ppm soap,
while none of the 19 controls had a positive reaction during 21 days of application
(p=0.0001). The authors concluded that rinse-off products preserved with 50 ppm MI or
more are not safe for consumers. A no-effect level was not determined (28).
The results of this study do not support safety of MI in rinse-off products at either 100 ppm
or at 50 ppm for elicitation or induction.
3.8 Compilation of cosmetovigilance data related to cosmetic products
containing Methylisothiazolinone (MI)
Five major manufacturers of cosmetic products from the Cosmetics Europe MI Task Force
collated all reported undesirable events associated with products containing MI and products
from the same categories without MI for a period of five years and 6 months (1 January
2009 – 30 June 2014). The categories for which data were identified were: rinse-off
products (face wash, shampoo, conditioner, and shower products), hair leave-on products
(hair styling products), skin leave-on products (face wipes, deodorants, face care, baby
wipes, after-shave products).
The causal relationship of each reported event to the product was assessed using a 5-level
scale and was assigned to one of the following categories: “very likely”, “likely”, “not clearly
attributable”, “unlikely” and “excluded”, in accordance with the causality assessment
method recommended by the European Commission. Undesirable events given a causality
assessment “likely” and “very likely” were considered as undesirable effects; they were
further assessed by a qualified assessor and those which were compatible with the
symptoms and chronology of allergic contact dermatitis and skin irritation were given the
respective designation.
Reporting rates were calculated as the number of undesirable events (separately for MI-
containing and non-MI-containing products) per millions of units sold for the time period
considered. Overall ‘industry rates’ were calculated by dividing the sum of all reported
undesirable events by the sum of all units sold by the five companies during the five years

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
16
and six months (in millions). The results are reported separately for leave-on skin products,
rinse-off skin and hair products and leave-on hair products.
Once leave-on products were assessed there was an approximately 5-fold difference in
confirmed undesirable effects (allergic contact dermatitis and skin irritation) between leave-
on skin products containing MI and leave-on skin products without MI. There was no
increase in reporting rates for rinse-off products containing MI (0.71) as compared to rinse-
off products without MI (2.0). No increase in reporting rates for leave-on hair products
containing MI (0.09) was observed as compared to leave-on hair products without MI
(0.15). It is concluded in the report that the reporting rates are generally low for both MI-
containing and non-MI-containing products.
SCCS comment
The submission does not provide detailed information about methodology or data
concerning numbers and types of the adverse events, the number of products in each
category from each company or the number of adverse events disregarded. It only provides
end results as given above. It is therefore not possible to assess the data.
A number of recent peer-reviewed scientific papers from different countries address the
same question as the cosmetovigilance study by CE concerning product types involved in
allergic contact dermatitis to MI (18, 20, 30). They all show that rinse-off products play a
role in allergic reactions in consumers diagnosed with MI contact allergy.
A restropective, nationwide and multicentre French report-based study (20) involved an
analysis of all cases reported by French doctors belonging to the REVIDAL-GERDA group
and performing patch tests from 2010 to 2012. Sixteen centers participated in the study
and 7874 patients were tested. MI-positive tests rose from 1.5% in 2010 to 5.5% in 2012.
Tests were clinically relevant in 80.2% to 90.3% of cases. Information about the products
used was available for 83.7% (247/295) of MI-positive patients. Cosmetics accounted for
73.1% of causative products. Among the cosmetics that were specified, the majority were
rinse-off, mainly soaps, particularly industrial soaps, toilet products, and hair products (20).
A study from Belgium (18) reviewed the medical charts of patients who were investigated
between 2010 and 2012 by members of the Belgian Contact and Environmental Dermatitis
Group for MCI/MI and MI allergy. All together 8680 patients were patch tested for MCI/MI
allergy and 5979 with MI alone, and 373 (4.3%) and 324 (5.4%), respectively, turned out
positive. The youngest patient was 2 years of age. Cosmetics were allergen sources for MI
and or MCI/MI in 53.7% to 61.3% of cases. Although the exact cosmetic was reported only
for a subgroup of patients, some specific leave-on products were mentioned including wet
wipes and deodorants. Also a considerable number of rinse-off products were involved (e.g.
shampoos), but the specific number was not given (18).
In Germany (30), contact allergy surveillance data collected by the Information Network of
Departments of Dermatology in the years 2009–2012 were analysed. For 602 MI-positive
patients, their own products had been patch tested (altogether, 4933 different products
causing a total of 372 positive patch test reactions). In particular, leave-on products caused
a high proportion of positive patch test reactions to the tested products. In total 5.6% out
of the MI positive patients without fragrance allergy was positive to liquid soaps/shower gels
and 3.9% to shampoo. In comparison 7.5% were positive to face cream and 17.5% to
moisturisers. Patch tests with rinse-off products may be quite non-sensitive for detecting
sensitisation to any of their ingredients including MI, as the product is diluted before testing
(30).
These studies differ from the CE cosmetovigilance study in methodology as the consumer
(patient) with an adverse reaction to a cosmetic product has been seen by a dermatologist

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
17
and also patch tested. This means that a diagnosis can be made and a causal relationship to
product types established. The studies do not offer a comparison with other preservatives.
In addition to these studies RIVM in the Netherlands took a multi-stakeholder approach
entailing spontaneous reports from consumers, general practitioners and dermatologists
regarding undesirable effects to cosmetic products. The four most frequently reported
cosmetic products involved in undesirable reactions were moisturisers, make-up, hair care
products and soaps. Dermatologists reported more cases than consumers of undesirable
effects of hair care products (predominately shampoos, constituting 82% of the products)
and soaps (bath and shower products). The most commonly reported allergens in the
patients were isothiazolinones (23%), whereof almost half were found have a causal link
between the undesirable effect and the cosmetic product, however no direct link was made
between the specific allergens and product types in the report (31).
3.9 DISCUSSION
In the previous Opinion on MI from March 2014 (SCCS/1521/13), it was concluded that no
safe concentrations of MI for induction of contact allergy or elicitation have been adequately
demonstrated for leave-on cosmetic products. For rinse-off cosmetic products, a
concentration of 15 ppm (0.0015 %) MI was considered safe for the consumer from the
view of induction of contact allergy, while no data were available concerning elicitation.
The present SCCS Opinion addresses safety concerns regarding the use of MI at 100 ppm in
rinse-off and leave-on hair cosmetic products. The arguments to defend this concentration
used by Cosmetic Europe are based on the results of a QRA including new data on
aggregate exposures and the information obtained from the cosmetovigilance system.
According to the QRA methodology, rinse-off and leave-on hair cosmetic products are
considered safe with a low risk of inducing contact sensitisation. The data obtained from the
cosmetovigilance system established by Industry do not show an excess of adverse events
due to MI in rinse–off and leave-on hair cosmetic products compared to products without
MI.
Nevertheless, the most recently published peer-reviewed literature shows an increase in
contact allergy to methylisothiazolinone in Europe. New data from Belgium, Gran Canaria,
France, Germany, Finland and the United Kingdom demonstrate an extraordinary increase
and high rate of contact allergy to MI. In some countries, the increase has more than tripled
in just a few years and has reached epidemic proportions. New cases are also seen in very
young children of 1-2 years of age, which is unusual for contact allergy.
The QRA for induction of contact sensitisation has previously been evaluated by the
Scientific Committee (SCCP1153/08), which amongst others concluded that: ‘The model has
not been validated and no strategy of validation was suggested. There is no confidence that
the levels of skin sensitizers identified by the dermal sensitisation QRA are safe for the
consumer.’ The QRA model used in this new submission about MI in rinse-off and leave-on
hair cosmetic products is similar to the QRA previously evaluated by the SCCP.
The QRA data provided specifically on MI in the current submission predict that 100 ppm
(0.01%) MI is unlikely to induce sensitisation in rinse-off products and stay-on hair
cosmetics. However, this QRA approach does predict that even higher concentrations of MI -
up to 12.000 ppm (1.2%) - in such products would be unlikely to induce sensitisation. The
fact that the QRA model permits such high levels of a strong sensitiser in rinse-off products
seriously questions its predictions and makes it difficult to have confidence in the presented
QRA model, as also highlighted in the Opinion SCCP1153/08. Aggregate exposure is not
considered in the first submission of QRA data from June 2014, but in an additional

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
18
submission from Cosmetics Europe from May 2015, QRA interim data considering aggregate
exposures to rinse-off products were provided.
The SCCS is aware that the QRA model is currently being updated. All the criticism raised in
Opinion SCCP1153/08 needs to be addressed and the new model needs to be assessed and
scientifically accepted before it can be applied to specific substances. The aggregate
exposure model also needs to be evaluated and accepted by the SCCS before it can be
applied to specific substances.
Predictive models are important to avoid adverse health effects in humans from cosmetic
ingredients. However in situations where the adverse health effects have already occurred
in humans, it is appropriate to consider the epidemiological data as these represent the
relevant end-point at which preventive actions are to be directed. Such data exist from
dermatology clinics and as cosmetovigilance data, either as spontaneous reports or active
surveillance.
The cosmetovigilance system established by industry is based on spontaneous reports
primarily from consumers and rarely supported by dermatological assessment or allergy
testing, which makes the causality assessment difficult and subject to variation among
companies. Such cosmetovigilance data may be useful in indicating a problem with certain
ingredients or specific products, but is in general of limited value in establishing safety or
disproving a problem. The data submitted by Cosmetics Europe lacked details in reporting,
such as numbers of adverse reaction to different product types, and could thus not be
assessed.
Cosmetovigilance data have recently been published from several countries (2014-2015). A
multi-stakeholder approach was taken by The Netherlands entailing spontaneous reports
from consumers, general practitioners and dermatologists. Dermatologists reported more
cases than consumers of undesirable effects of hair care products (predominately
shampoos) and soaps (bath and shower products). The most commonly reported allergens
in the patients were isothiazolinones, but this study did not allow to causally link the specific
allergens to certain product types.
In Belgium and France, all data on MI contact allergic patients from multiple dermatological
centers were reviewed. In the Belgium study it was concluded that although the exact
cosmetic was reported only for a subgroup of patients, a considerable number of rinse-off
cosmetics (e.g. shampoos) were involved. No distinction was made between MI and/or
MCI/MI. In the French study concerning MI allergy, the majority of causative products were
rinse-off, mainly soaps, particularly industrial soaps, toilet products, and hair products. In a
German multi-centre study patients had been tested with their own cosmetic products.
Stay-on cosmetic products were clearly more often positive in MI allergic patients than
rinse-off products. Nevertheless, rinse-off products also gave reactions. Testing of rinse-off
products requires dilution and may make the test less sensitive in picking up allergies.
These data represent consumers who have been exposed sufficiently to develop the disease
allergic contact dermatitis. This may be caused by one product or multiple products
simultaneously or in sequence.
There is no doubt from the clinical data as presented in the previous and present Opinion
that stay-on cosmetic products, especially wet wipes, are important causes of MI allergy.
This is also acknowledged by CE in their submission and they have advised their members
to discontinue the use of MI in such products. Rinse-off cosmetic products also play a
significant role in allergic contact dermatitis to MI according to recent epidemiological
studies. This is supported by a new use test study performed in patients sensitised to MI,
where a soap preserved with 100 ppm or 50 ppm MI used five times a day elicited allergic
reactions in all or almost all sensitised patients and not in controls. This study may not
directly show that rinse-off products are implicated in induction of contact allergy to MI, but
may indicate a role. It is generally accepted that concentrations/doses of allergens, which

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
19
do not elicit reactions, would also be safe for induction in the majority of individuals e.g.
The Nickel Directive (Nickel Directive (76/769/EEC - now 94/27/EEC)) and the recent
REACH regulation of chromium VI in leather (regulation EU 301/2014, which adds a
Chromium VI restriction to Annex XVII of regulation 1907/2006 (REACH)) are based on this
principle. Thus as almost all the participants in the use test study developed allergic contact
dermatitis to a soap with 100 ppm or 50 ppm, the result of this study do not support safety
of MI at current use concentrations in terms of induction.
In the scientific Opinion (SCCS/1521/13) on methylisothiazolinone (MI), the conclusion
concerning safe use of MI in rinse-off products at 15 ppm was based on bench-marking to
the experience with the use of the mixture MCI/MI at 15 ppm for the past 30 years. In the
new submission, industry submits that MCI and MI are very different in their sensitising
potency and therefore imposing identical concentration limits is not warranted.
In the current Opinion it is highlighted that MI has been used in up to 8.8 times higher
concentrations than MCI for the past 10 years in cosmetic products, which has led to the
current situation of high rates of contact allergy to MI in consumers. There are, at present,
no convincing data to indicate that a higher level is ‘safe’ for either induction or elicitation.
Therefore, 15 ppm was chosen for safety reasons given in the previous Opinion
(SCCS/1521/13).
4. CONCLUSION
1. On the basis of the data provided, does the SCCS consider Methylisothiazolinone
(MI) to be safe for consumers, when used as a preservative in rinse-off products
up to concentration limit of 100 ppm from the view of induction of contact allergy?
The information provided does not support the safe use of MI as a preservative in rinse-off
cosmetic products up to a concentration limit of 100 ppm from the view of induction of
contact allergy.
For rinse-off cosmetic products, a concentration of 15 ppm (0.0015%) MI is considered safe
for the consumer from the point of view of induction of contact allergy.
2. On the basis of the data provided, does the SCCS consider Methylisothiazolinone
(MI) to be safe for consumers, when used as a preservative in leave-on hair
products up to concentration limit of 100 ppm from the view of induction of
contact allergy?
The information provided does not support the safe use of MI as a preservative in leave-on
hair cosmetic products up to a concentration limit of 100 ppm from the point of view of
induction of contact allergy.
3. Does the SCCS have any further scientific concerns with regard to the use of
Methylisothiazolinone (MI) in cosmetic product
The concerns and opinions raised in SCCS Opinion SCCS/1521/13 (12 December 2013 with
revision 27 March 2014) remain. The results of the recent Scandinavian study do not
support safety of MI in rinse-off products at either 100 ppm or at 50 ppm for elicitation or
induction.

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
20
5. REFERENCES
1. Bruze M, Dahlquist I, Fregert S, Gruvberger B, Persson K. Contact allergy to the active
ingredients of Kathon CG. Contact Dermatitis. 1987 ;16(4):183-8.
2. Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from
methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a
biocide. Dermatitis. 2004;15(4):201-5.
3. Thyssen JP, Sederberg-Olsen N, Thomsen JF, Menné T. Contact dermatitis from
methylisothiazolinone in a paint factory. Contact Dermatitis. 2006; 54(6):322-4.
4. Mose AP, Lundov MD, Zachariae C, Menné T, Veien NK, Laurberg G, Kaaber K, Avnstorp
C, Andersen KE, Paulsen E, Gotthard Mortz C, Sommerlund M, Danielsen A, Thormann J,
Kristensen O, Kristensen B, Andersen BL, Vissing S, Nielsen NH, Johansen JD. Occupational
contact dermatitis in painters: an analysis of patch test data from the Danish Contact
Dermatitis Group. Contact Dermatitis. 2012; 67(5):293-7.
5. García-Gavín J1, Vansina S, Kerre S, Naert A, Goossens A. García-Gavín J1, Vansina S,
Kerre S, Naert A, Goossens A. Methylisothiazolinone, an emerging allergen in cosmetics?
Contact Dermatitis. 2010;63(2):96-101.
6. Lundov MD1, Thyssen JP, Zachariae C, Johansen JD Prevalence and cause of
methylisothiazolinone contact allergy. Contact Dermatitis. 2010;63(3):164-7.
7. Lundov MD, Krongaard T, Menné TL, Johansen JD Methylisothiazolinone contact allergy: a
review.Br J Dermatol. 2011;165(6):1178-82.
8. Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant.
Contact Dermatitis. 2011;64(5):298-9.
9. Castanedo-Tardana MP1, Zug KA Methylisothiazolinone. Dermatitis. 2013; 24(1):2-6.
10. Aerts O, Cattaert N, Lambert J, Goossens A Airborne and systemic dermatitis, mimicking
atopic dermatitis, caused by methylisothiazolinone in a young child. Contact Dermatitis.
2013; 68(4):250-1.
11. Lundov MD, Zachariae C, Menné T, Johansen JD. Airborne exposure to preservative
methylisothiazolinone causes severe allergic reactions. BMJ. 2012; 4:345
12. Lundov MD, Menné T. Airborne exposure to methylchloroisothiazolinone and
methylisothiazolinone from a toilet cleaner. Contact Dermatitis. 2013; 68(4):252-3.
13. Ackermann L, Aalto-Korte K, Alanko K, Hasan T, Jolanki R, Lammintausta K,Lauerma A,
Laukkanen A, Liippo J, Riekki R, Vuorela AM, Rantanen T. Contact sensitisation to
methylisothiazolinone in Finland--a multicentrestudy. Contact Dermatitis 2011; 64; 49-53
14. Urwin R & Wilkinson M. Methylchloroisothiazolinone and methylisothiazolinone contact
allergy: a new “epidemic”. Contact Dermatitis 2013; 68: 253–5
15. Gonçalo M, Goossens A. Whilst Rome Burns: The Epidemic of Contact Allergy to
Methylisothiazolinone. Contact Dermatitis 2013; 68: 257-258
16. Geier J, Lessmann H, Schnuch A, Uter W. Recent increase in allergic reactions to
methylchloroisothiazolinone/methylisothiazolinone: is methylisothiazolinone the culprit?
Contact Dermatitis 2012; 67: 334-41

SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
21
17. Bruze M, Engfeldt M, Gonçalo M and Goossens A. Recommendation to include
methylisothiazolinone in the European baseline patch test series. (On behalf of the
European Society of Contact Dermatitis (ESCD) and the European Environmental and
Contact Dermatitis Research Group (EECDRG)). Contact Dermatitis 2013; 69: 263-271
18. Aerts O, Baeck M, Constandt L, Dezfoulian B, Jacobs MC, Kerre S, Lapeere H, Pierret L,
Wouters K, Goossens A. The dramatic increase in the rate of methylisothiazolinone contact
allergy in Belgium: a multicenter study. Contact Dermatitis 2014; 71: 41-48
19. Liuti F, Hernandez Hernandez Z, Borrego Hernando L. Increased sensitisation to Kathon
CG (methylchloroisothiazolinone plus methylisothiazolinone) in the South of Gran Canaria,
Spain. Actas Dermosifiliogr 2014; 105:882-883
20. Hosteing S, Meyer N, Waton J, Barbaud A, Bourrain JL, Raison-Peyron N et al. Outbreak
of contact sensitisation to methylisothiazolinone:an analysis of French data from the
REVIDAL-GERDA network. Contact Dermatitis 2014; 70: 262-269
21. Lammintausta K, Aalto-Korte K, Ackerman L, Alanko K, Berry P, Hasan T et al. An
epidemic of contact allergy to methylisotiazolinone in Finland. Contact Dermatitis 2014; 70:
184-185
22. Lundov MD, Opstrup MS, Johansen JD. Methylisothiazolinone contact allergy- a growing
epidemic. Contact Dermatitis. 2013;69:271-5.
23. Madsen JT, Andersen KE. Further evidence of the methylisothiazolinone epidemic.
Contact Dermatitis. 2014; 70: 246-247
24. Basketter DA, Clapp CJ, Safford BJ, Jowsey IR, McNamee P, Ryan CA, Gerberick GF.
Preservatives and skin sensitisation quantitative risk assessment. Dermatitis. 2008 Jan-
Feb;19(1):20-7.
25. QRA Technical Guidance Dossier 2006 and updated information booklets can be found
on the RIFM website. http://www.rifm.org/publications
26. Politano VT, Api AM. The Research Institute for Fragances Materials´human repeated
insult patch test protocol. Regulatory Toxicology and Pharmacology 2008; 52:35-38
27. Bruze M, Svedman C, Andersen KE, Bruynzeel D, Goossens A, Duus Johansen J, Matura
M, Orton D, Vigan M on behalf of the ESCD. Contact Dermatitis 2012; 66:131-136
28. Yazar K, Lundov MD, Faurschou A, Matura M, Boman A, Johansen JD, Liden C.
Methylisothiazolinone in rinse-off products causes allergic contact dermatitis: A Repeated
Open Application study. Br J Dermatol 2015: Accepted
29. Johnston GA, contributing members of the British Society for Cutaneous A. The rise in
prevalence of contact allergy to methylisothiazolinone in the British Isles. Contact Dermatitis
2014:70(4):238-40
30. Uter W, Geier J, Bauer A, Schnuch A. Risk factors associated with methylisothiazolinone
contact sensitisation. Contact Dermatitis 2013 69, 231–238
31. Salverda JG, Braqt PJ, de Wit-Bos L et al. Results of cosmetovigilance survey in The
Netherlands. Contact Dermatitis 2013: 68:139-14842
SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
22
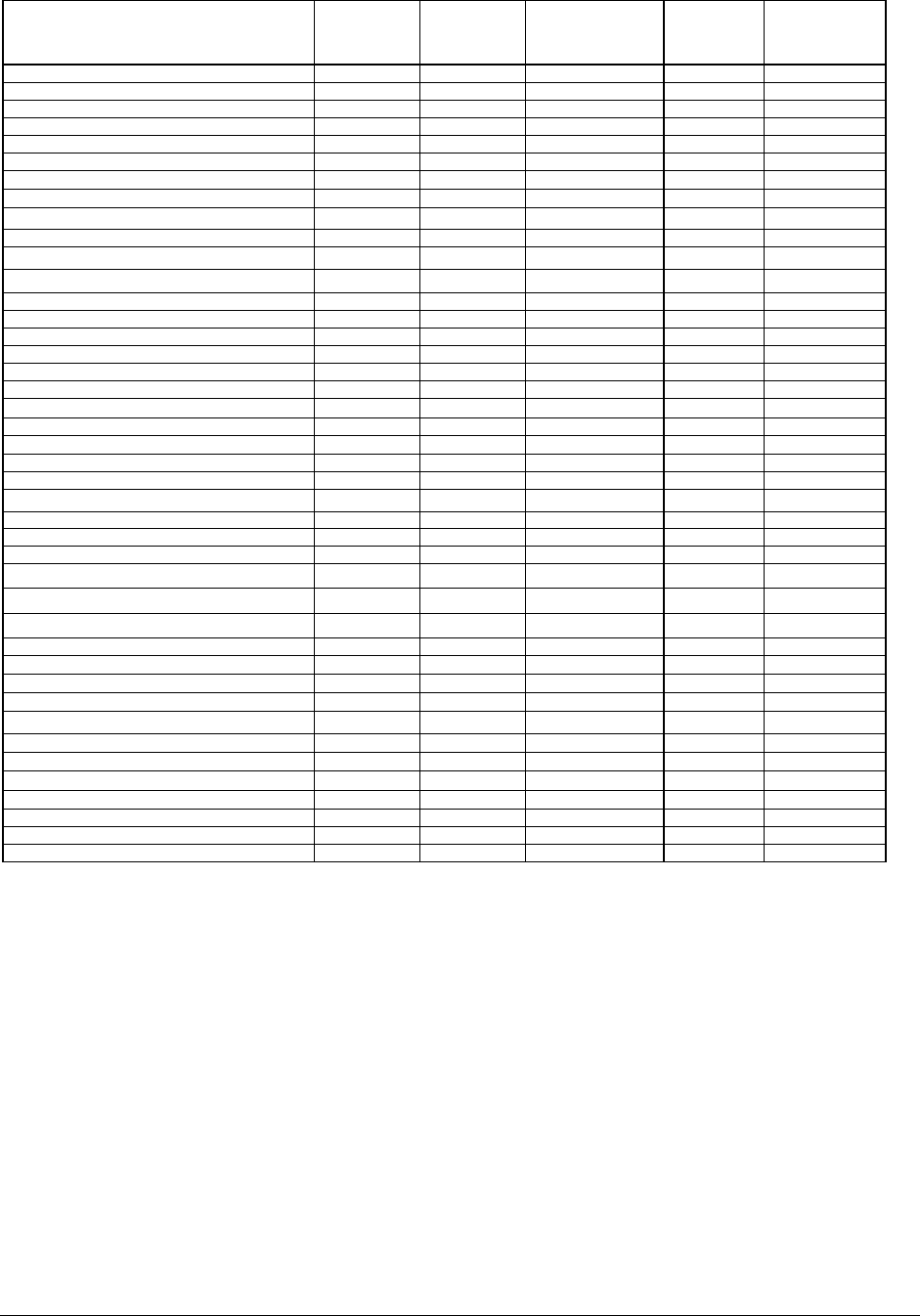
ANNEX I
Table 1: Summary of source consumer exposure data to product and product containing
100 ppm MI for use in quantitative risk assessment.
The following hierarchy of exposure data was used:
a) SCCS notes of Guidance
b) QRA Technical Guidance dossier for fragrance ingredients (IFRA)
c) Where no exposure data was available, surrogate data was derived from
the technical expertise of the Cosmetics Europe companies:
c1) surrogate exposure: face cream with 10% retention factor applied.
c2) surrogate exposure: styling aids
c3) surrogate exposure: foundation
c4) surrogate exposure: face cream
c5) surrogate exposure: non-spray deodorant
c6) surrogate exposure: deposition from film of liquid
Product type
Product
exposure
(g per day)
Product
surface area
(cm2)
Product exposure
data
(µg/cm2)
Exposure
data source
Consumer
exposure level
(μg/cm
2
)
Shower gel 18.67 17500 0.011 a 0.0011
Facial wash (liquid) 1.6 565 0.028 a 0.0028
Hand wash soap - bar 4.8 840 0.057 b 0.0057
Shaving products (male) 2 305 0.066 b 0.0066
Shampoo 10.46 1440 0.073 a 0.0073
Hair conditioner rinse off 14 1440 0.097 b 0.0097
Hand wash soap - liquid 20 840 0.238 a 0.0238
Facial cleaning lotion 1.54 565 0.273 c1 0.0273
Facial toning lotion 1.54 565 0.273 c1 0.0273
Face mask (PVA) 1.54 565 0.273 c1 0.0273
Face mask (non-PVA) 1.54 565 0.273 c1 0.0273
Hair conditioner leave on 4 1440 0.278 c2 0.0278
Hair styling products 4 1010
0.396
a
0.0396
Body lotion 7.82 15670 0.499 a 0.0499
After sun cream lotion
7.82 15670
0.50
a
0.0499
Eye shadow 0.02 24 0.83 a 0.0833
Make-up remover 5 565 0.88 a 0.0885
Liquid foundation 0.51 565 0.90 a 0.0903
concealer 0.51 565 0.90 c3 0.0903
Mouthwash 21.62 216.8 1.00 a 0.0997
Sunscreen lotion/cream/trigger 18 17500
1.03
a
0.1029
Toothpaste 2.7 216.8 1.25 a 0.1245
Eyeliner 0.005 3.2 1.56 a 0.1563
After shaving cream 1.6 775 2.065 c1 0.2065
Men's facial care 1.6 775 2.065 b 0.2065
baby nappy area Cleansing lotion
0.55 220
2.50
b
0.2500
Hand cream 2.16 860 2.512 a 0.2512
Face cream (women) 1.54 565 2.726 a 0.2726
Face mask (overnight treatment) 1.54 565 2.726 c4 0.2726
Deodorant aerosol spray (excluding propellant)
0.69
200 3.45 a 0.3450
nappy area protection cream
1.32 220
6.00
b
0.6000
Semi-permanent hair dyes (and lotions)
35 580 6.034 a 0.6034
Deodorant body spray (ethanolic)
1.43
200 7.15 a 0.7150
Deodorant non-spray 1.51 200 7.55 a 0.7550
Deodorant cosmetic pump spray
1.51 200
7.55 c5 0.7550
Lipstick, lip salve 0.057 4.8 11.88 a 1.1875
Diaper rash cream 2.64 220 12.00 b 1.2000
Facial wipes NA NA 13.00 c6 1.3000
Moist toilet tissue,
NA NA
13.00
c6
1.3000
Mascara 0.025 1.6 15.63 a 1.5625
Baby wipes
NA NA
21.00
c6
2.1000
Nail varnish product
0.25 11
22.73
b
2.2727
SCCS/1557/15
Final Opinion on Methylisothiazolinone (MI) (P94), submission III, sensitisation only
23
Table 3: The QRA outcome for 100 ppm MI in a range of cosmetic products
Product type
Consumer exposure
level to MI (μg/cm
2
)
Product
specific SAF
AEL / CEL ratio
Shower gel 0.0011 100 140.6
Facial wash (liquid) 0.0028 100 53.0
Hand wash soap - bar 0.0057 100 26.3
Shaving products (male) 0.0066 300 7.6
Shampoo 0.0073 100 20.7
Hair conditioner rinse off 0.0097 100 15.4
Hand wash soap - liquid 0.0238 100 6.3
Facial cleaning lotion 0.0273 100 5.5
Facial toning lotion 0.0273 100 5.5
Face mask (PVA) 0.0273 300 1.8
Face mask (non-PVA) 0.0273 100 5.5
Hair conditioner leave on 0.0278 100 5.4
Hair styling products 0.0396 100 3.8
Body lotion 0.0499 300 1.0
After sun cream lotion 0.0499 300 1.0
Eye shadow 0.0833 300 0.6
Make-up remover 0.0885 100 1.7
Liquid foundation 0.0903 100 1.7
concealer 0.0903 100 1.7
Mouthwash 0.0997 100 1.5
Sunscreen lotion/cream/trigger 0.1029 300 0.5
Toothpaste 0.1245 100 1.2
Eyeliner 0.1563 300 0.3
After shaving cream 0.2065 300 0.2
Men's facial care 0.2065 300 0.2
baby nappy area Cleansing lotion 0.2500 300 0.20
Hand cream 0.2512 100 0.6
Face cream (women) 0.2726 100 0.6
Face mask (overnight treatment) 0.2726 100 0.6
Deodorant aerosol spray (excl. propellant) 0.3450 300 0.1
nappy area protection cream 0.6000 300 0.08
Semi-permanent hair dyes (and lotions)
0.6034 100 0.2
Deodorant body spray (ethanolic) 0.7150 300 0.1
Deodorant non-spray 0.7550 300 0.1
Deodorant cosmetic pump spray
0.7550 300 0.1
Lipstick, lip salve 1.1875 300 0.04
Diaper rash cream 1.2000 300 0.04
Facial wipes 1.3000 100 0.1
Moist toilet tissue, 1.3000 300 0.04
Mascara 1.5625 300 0.03
Baby wipes 2.1000 300 0.02
Nail varnish product 2.2727 100 0.1
