
This information is distributed solely for the purpose of pre-dissemination public comment under applicable
information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and
Prevention or the National Institute for Occupational Safety and Health. It does not represent and should not
be construed to represent any agency determination or policy.
NIOSH List of Hazardous Drugs
in Healthcare Settings, 2020
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Disease Control and Prevention
National Institute for Occupational Safety and Health

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
1
INSIDE FRONT COVER
PAGE LEFT INTENTIONALLY BLANK

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
2
NIOSH List of Hazardous Drugs in Healthcare
Settings, 2020
DEPARTMENT OF HEATH AND HUMAN SERVICES
Centers for Disease Control and Prevention
National Institute for Occupational Safety and Health

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
3
This document is in the public domain and may be freely copied or reprinted
Disclaimer
Mention of any company or product does not constitute endorsement by the National Institute for
Occupational Safety and Health (NIOSH). In addition, citations of websites external to NIOSH do not
constitute NIOSH endorsement of the sponsoring organizations or their programs or products.
Furthermore, NIOSH is not responsible for the content of these websites.
Ordering Information
To receive documents or other information about occupational safety and health topics, contact NIOSH at
Telephone: 1-800-CDC-INFO (1-800-232-4636)
TTY: 1-888-232-6348
CDC-INFO: http://wwwn.cdc.gov/dcs/RequestForm.aspx
or visit the NIOSH website at www.cdc.gov/niosh
For a monthly update on news at NIOSH, subscribe to NIOSH eNews by visiting
www.cdc.gov/niosh/eNews
Suggested Citation
NIOSH [2020]. NIOSH list of hazardous drugs in healthcare settings 2020. By Connor TH, MacKenzie
BA, DeBord DG, Trout DB, O’Callaghan JP, Ovesen JL, Whittaker C. Cincinnati, OH: U.S.
Department of Health and Human Services, Centers for Disease Control and Prevention, National
Institute for Occupational Safety and Health, DHHS (NIOSH) Publication Number 2020-xxx
(Supersedes 2016-161)
2020-xxx

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
4
Acknowledgements
The following NIOSH individuals screened and reviewed the information on hazardous drugs to develop
the List and prepare this document:
Marissa Alexander-Scott
Jim O’Callaghan
Doug Trout
Carissa Rocheleau
Ainsley Weston
Chris Whittaker
For significant contributions to the development and review of this document, the authors acknowledge
the following NIOSH contributors:
Ann Berry
Paul Middendorf
John Piacentino
Paul Schulte
External Reviewers
The following individuals made substantial contributions to the preparation of this List:
Shekhar Mehta, PharmD, MS
American Society of Health-System Pharmacists
Bruce Naumann, PhD, DABT
Merck
Marty Polovich, PhD, RN, AOCN
Georgia State University
Trish Weideman, PhD
Genentech, Inc.
Wendelyn Schmidt, PhD
Food and Drug Administration
Michael Hodgson, MD, MPH
Occupational Safety and Health Administration
Melissa McDiarmid, MD, MPH, DABT
University of Maryland
Michael Olson, PhD, ATS
SafeBridge Consultants, Inc
Vaiyapuri Subramaniam, PharmD, MS, FCP,
FASHP, FASCP
Department of Veterans AffairsMarian Condon,
MS, RN
University of Maryland
Chris Fausel, PharmD, MHA, BCOP
Indiana University Health
Janet Gould, PhD, DABT
Bristol-Myers Squibb Company

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
5
List of Acronyms
AHFS American Hospital Formulary Service
CFR Code of Federal Regulations
FDA Food and Drug Administration
IARC International Agency for Research on Cancer
MSHI Manufacturer’s special handling information
NIOSH National Institute for Occupational Safety and Health
NTP National Toxicology Program

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
6
Drugs Considered Hazardous
Introduction
Healthcare workers may be occupationally exposed to drugs and may experience adverse health effects
as a result. The NIOSH Alert: Preventing Occupational Exposures to Antineoplastic and Other
Hazardous Drugs in Healthcare Settings was published in September 2004,
http://www.cdc.gov/niosh/docs/2004-165/. The Alert contained a sample list of drugs identified by
NIOSH as hazardous to workers in healthcare settings. NIOSH published updated Lists in 2010, 2012,
2014, 2016, and now this in 2020.
This document supersedes previous versions of the List and presents the current list of drugs determined
by NIOSH to be hazardous.
The NIOSH List of Hazardous Drugs in Healthcare Settings (List) assists employers in providing safe and
healthy workplaces by identifying drugs approved by the FDA Center for Drug Evaluation and Research
(CDER) that have intrinsic properties that meet the NIOSH definition of a hazardous drug. The NIOSH
List creates no legal obligation for employers; it is advisory in nature and informational in content.
CAUTION: Drugs purchased and used by a facility may have entered the marketplace after the list
below was assembled. Therefore, this list may not be all-inclusive and employers should consider
creating a facility-specific hazardous drug list.
Defining Hazardous Drugs
NIOSH has formalized the methodology NIOSH uses to guide the addition of drugs to or removal of
drugs from the List, in a document entitled Procedures for Developing the NIOSH List of Hazardous
Drugs in Healthcare Settings (Procedures).
1
As stated in the Procedures, NIOSH defines a hazardous drug as a drug that is:
1
NIOSH [2020]. Procedures for Developing the NIOSH List of Hazardous Drugs in Healthcare Settings. By Whittaker C,
Ovesen JL, MacKenzie BA, Hartley T, Berry KA, Piacentino J. Cincinnati, OH: U.S. Department of Health and Human
Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH)
Publication Number 2020-xxx

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
7
1. Approved for use in humans
2
by the FDA-CDER;
3
and
2. Not otherwise regulated by the U.S. Nuclear Regulatory Commission;
4
and
3. Either:
a. Is accompanied by prescribing information in the “package insert”
5
that specifies special
handling information (Manufacturer Special Handling Information-MSHI) to protect
workers handling the drug; or
b. Is identified as a carcinogenic hazard, developmental hazard, reproductive hazard,
genotoxic hazard, or other health hazard by exhibiting one or more of the following
toxicity criteria in humans, animal models, or in vitro systems:
• carcinogenicity;
• developmental toxicity (including teratogenicity);
• reproductive toxicity;
• genotoxicity;
• organ toxicity at low doses;
6
or
• structure and toxicity profile that mimics existing drugs determined hazardous by
exhibiting any one of the previous five toxicity types;
7
unless the drug also exhibits a molecular property
8
that may limit the potential for
adverse health effects in healthcare workers from exposure to the drug.
2
Although only drugs approved by the FDA for use in humans are included in the definition of hazardous drug, some of those
drugs may be used in veterinary settings for treatment of animals and may be a hazard for veterinary care workers.
3
21 U.S.C. 301 et seq.
4
10 CFR Parts 19, 20, and 35. See https://www.nrc.gov/materials/miau/med-use.html.
5
See Drug Advertising: A Glossary of Terms at
https://www.fda.gov/drugs/resourcesforyou/consumers/prescriptiondrugadvertising/ucm072025.htm. “Prescribing information is
also called product information, product labeling, or the package insert ("the PI"). It is generally drafted by the drug company and
approved by the FDA. This information travels with a drug as it moves from the company to the pharmacist. It includes the
details and directions healthcare providers need to prescribe the drug properly. It is also the basis for how the drug company can
advertise its drug. The prescribing information includes such details about the drug as: its chemical description; how it works;
how it interacts with other drugs, supplements, foods, and beverages; what condition(s) or disease(s) it treats; who should not use
the drug; serious side effects, even if they occur rarely; commonly occurring side effects, even if they are not serious; effects on
specific groups of patients, such as children, pregnant women, or older adults and how to use it in these populations.”
6
All drugs have toxic side effects, but some exhibit toxicity at low doses. The level of toxicity reflects a continuum from
relatively nontoxic to production of toxic effects in patients at low doses (for example, a few milligrams or less). For example, a
daily therapeutic dose of 10 mg/ day or a dose of 1 mg/kg per day in laboratory animals that produces serious organ toxicity,
developmental toxicity, or reproductive toxicity has been used by the pharmaceutical industry to develop occupational exposure
limits (OELs) of less than 10 μg/m
3
after applying appropriate uncertainty factors [Sargent and Kirk 1988; Naumann and Sargent
1997; Sargent et al. 2002]. OELs in this range are typically established for potent or toxic drugs in the pharmaceutical industry.
Under all circumstances, an evaluation of all available data should be conducted to protect health care workers.
7
NIOSH [2004]. Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. By
Burroughs GE, Connor TH, McDiarmid MA, Mead KR, Power LA, Reed LD, Coyle BJ, Hammond DR, Leone MM, Polovich
M, Sharpnack DD. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease
Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
8
Properties of a drug molecule that may limit adverse effects in healthcare workers are typically chemical, physical and structural
properties that affect its absorption, distribution within the body, metabolism, or excretion e.g., chemical structure, molecular

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
8
Determining Whether a Drug Is Hazardous
NIOSH uses a sequential approach for assessing and interpreting scientific information in order to
determine whether an FDA-approved drug meets the NIOSH definition of hazardous drug. NIOSH’s
approach to evaluating the hazard potential of a drug includes: (1) reviewing FDA databases to identify
drugs that have the potential to meet the NIOSH definition of hazardous drug; (2) reviewing molecular
properties and information in the manufacturer-provided drug package insert to identify information
relevant to making a determination about placing a drug on the List, excluding a drug from the List, or
removing a drug from the List; (3) assessing, integrating, and synthesizing evidence from human, animal,
and in vitro studies of drug toxicity; (4) using molecular property, toxicity and hazard characterization
criteria established in the Procedures in making a decision to place a drug on the List or to exclude a drug
from the List; and (5) allowing for reconsideration of a NIOSH decision to place a drug on the List or to
remove a drug from the List.
The methodology used by NIOSH to evaluate chemical properties, pre-clinical information, and clinical
information about each drug is detailed in the Procedures.
Developing a Facility-Specific List of Hazardous Drugs
The NIOSH List is an aid designed to enable employers to identify which drugs handled by employees
are considered by NIOSH to be hazardous drugs. Because new drugs and new formulations are
continuously brought to market between NIOSH’s periodic updates hazardous drug evaluation should be
a continual process. Employers should establish their own procedures to identify and evaluate new drugs
as they enter their workplace and, when appropriate, reassess their presence on hazardous drug lists as
toxicological data become available to support re-categorization.
In developing a facility-specific list of hazardous drugs, workplaces may consider facility-specific
criteria, including the specific product formulations and packaging within their facility, which NIOSH
cannot utilize when developing the List. In addition to the NIOSH List, non-governmental organizations
have developed various approaches to identifying and classifying hazardous drugs [Chaffee et al. 2010;
Badry et al. 2013; Kaestli et al. 2013]. When creating a facility specific list some facilities may find they
handle investigational drugs, which have not been approved by FDA-CDER or reviewed by NIOSH.
Toxicological data may be incomplete or unavailable for investigational drugs. If the mechanism of
action suggests that there may be a concern, it is prudent to handle them as hazardous drugs until
adequate information becomes available to exclude them.
A site-specific risk assessment is outside of the scope of the List and includes consideration of dose,
potency, and exposure potential during formulation and use from events such as: routine handling,
weight or mass. See Clementi F, Fumagalli G. Molecular Pharmacology. Hoboken, NJ: Wiley & Sons;2015; Di L, Kerns EH.
Drug-Like Properties: Concepts, Structure, Design, and Methods. Oxford, UK: Elsevier;2016; Mattson P, Kihlberg J. How big is
too big for cell permeability. J Med Chem. 2017;60:1662-1664. https://doi.org/10.1021/acs.jmedchem.7b00237 .

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
9
compounding, spills, broken device, needle stick, inadvertent contact, or surface contamination. When
using a drug on the List, NIOSH encourages employers to do a site-specific risk assessment that informs
effective risk management procedures. More information about managing the risk of handling hazardous
drugs can be found in Managing Hazardous Drug Exposures: Information for Health Care Settings
(NIOSH, 2020). A facility-specific list along with Managing Hazardous Drug Exposures: Information
for Health Care Settings [NIOSH 2020] and other guidance from American Society of Health System
Pharmacist (ASHP), United States Pharmacopeia (USP), Oncology Nursing Society (ONS) and other
organizations, can help employers establish effective hazardous drug management procedures specific to
their workplace
References
Badry N, Fabbro J, de Lemos ML [2014]. Hazards in determining whether a drug is hazardous. J Oncol
Pharm Pract 20:312–315.
Chaffee BW, Armistead JA, Benjamin BE, Cotugno MC, Forrey RA, Hintzen BL, Pfeiffenberger T,
Stevenson JG [2010]. Guidelines for the safe handling of hazardous drugs: consensus recommendations.
Am J Health-Syst Pharm 67:1545–1546.
Kaestli L-Z, Fonzo-Christe C, Bonfillon C, Desmueles J, Bonnabry P [2013]. Development of a
standardized method to recommend protective measures to handle hazardous drugs in hospitals. Eur J
Hosp Pharm 20:100–105.
Naumann BD, Sargent EV [1997]. Setting occupational 7 exposure limits for pharmaceuticals. Occup
Med 12(1):67–80.
NIOSH [2004]. NIOSH alert: preventing occupational exposure to antineoplastic and other hazardous
drugs in health care settings. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for
Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH)
Publication No. 2004-165.
NIOSH [2020] Procedures for Developing the NIOSH List of Hazardous Drugs in Healthcare Settings.
Whittaker C, Ovesen JL, MacKenzie BA, Hartley T, Berry KA, Piacentino, J. Cincinnati, OH: U.S.
Department of Health and Human Services, Centers for Disease Control and Prevention, National
Institute for Occupational Safety and Health, DHHS (NIOSH) Publication Number 2020-xxx.
Sargent EV, Kirk GD [1988]. Establishing airborne exposure control limits in the pharmaceutical
industry. Am Ind Hyg Assoc J 49(6):309–313.
Sargent EV, Naumann BD, Dolan DG, Faria EC, Schulman L [2002]. The importance of human data in
the establishment of occupational exposure limits. Hum Ecol Risk Assess 8(4):805–822.

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
10
NIOSH List of Hazardous Drugs in Healthcare Settings
2020
NIOSH performed a hazard identification and characterization of each drug on the List, in accordance
with the NIOSH Procedures for Developing the NIOSH List of Hazardous Drugs in Healthcare Settings.
The 2020 List supersedes previous versions.
2020 Hazardous Drugs List Changes
The 2020 List adds 16 drugs, three of which have special handling
9
information from the manufacturers
and removes five drugs
10
from the list. Drugs reviewed for this update were new drug approvals or
received safety related new warnings from FDA in the period between January 2014 and December 2015.
In addition to these updates, the tables categorizing hazardous drugs have been reorganized and are
discussed below.
Table 1 now includes drugs that meet the NIOSH definition of a hazardous drug and contain MSHI in the
package insert; and/or are classified by the NTP as “known to be a human carcinogen,” or classified by
IARC as “carcinogenic” or “probably carcinogenic.” In the 2016 List this table identified antineoplastic
drugs, however, in this update not all of the drugs on Table 1 are antineoplastic drugs.
Table 2 contains drugs that meet one or more of the NIOSH definition of a hazardous drug but are not
drugs which have MSHI or are classified by the NTP as “known to be a human carcinogen,” or classified
by the IARC as “carcinogenic” or “probably carcinogenic,” some of which also have adverse
reproductive effects for populations at risk. This table now also includes drugs that only meet the NIOSH
criteria as a developmental (including teratogenicity) and/or reproductive hazard. In the 2016 update of
the List this table did not include drugs that only posed a developmental and/or reproductive hazard.
In the 2016 List, Table 3 provided a list of drugs that met the NIOSH criteria of a reproductive hazard
(damaging to a male or female person’s ability to conceive or carry to term an offspring) or
developmental hazard (able to cause disruption in the development of unborn children including
teratogenic outcomes). In this 2020 List, those drugs that only meet NIOSH’s criteria as a developmental
and/or reproductive hazard are identified in the supplemental information column with a blue
notification; a separate Table is no longer provided.
9
When NIOSH becomes aware of recently approved drugs that include MSHI, it adds them to the List immediately. The
notification of these additions are posted to the NIOSH website at: https://www.cdc.gov/niosh/docs/2016-161/default.html. These
drugs would have been officially on the previous version of the list from the date of the notification and are only now being
added into the publication.
10
When NIOSH removes a drug from the List, the notification of these removals are posted to the NIOSH website at:
https://www.cdc.gov/niosh/docs/2016-161/default.html

This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
11
In the 2016 List, Table 4 provided a list of the drugs removed from the List. In this 2020 List, a new
section identifies changes to the placement of drugs on the List, including drugs that are no longer
considered hazardous and those that have been moved from one table to another.
In the 2016 List, Table 5 provided information on recommended exposure controls for hazardous drugs
based on formulations. NIOSH has removed the table from the List. Risk management is outside the
scope of the List document. NIOSH addresses risk management issues in Managing Hazardous Drug
Exposures: Information for Healthcare Settings which includes information on using engineering
controls, administrative controls and personal protective equipment for working with hazardous drugs in
healthcare settings. It is available on the NIOSH website at: www.cdc.gov/niosh/topics/hazdrug/.
In previous Lists, the supplemental information column has contained information that may be useful for
individual drugs, including pregnancy categories. This information may not have been related to
NIOSH’s decision to place the drug on the List. As of 2015, FDA no longer uses the letter pregnancy
categories for drugs and NIOSH has removed that information from the supplemental information
column. For drugs listed prior to this 2020 List, all other supplemental information has been retained,
though that information may not be related to the listing of the drug. For drugs listed in this 2020 List,
NIOSH has identified the relevant hazard criteria of the drug in the supplemental information column.
CAUTION: Drugs purchased and used by a facility may have entered the marketplace after the list below
was assembled. This list is not all-inclusive. Drugs reviewed for this update were new drug approvals or
received safety related new warnings from FDA in the period between January 2014 and December 2015.
More recent information on drugs, including updated product inserts and information on new safety
related changes to labels, can be found at:
FDA Approved Drugs:
www.accessdata.fda.gov/scripts/cder/daf/index.cfm
FDA Safety-related Labeling Changes:
www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/
DailyMed:
dailymed.nlm.nih.gov
DrugBank:
www.drugbank.ca
The National Library of Medicine Drug Portal:
druginfo.nlm.nih.gov
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
12
NIOSH List of Hazardous Drugs in Healthcare Settings
2020
The drugs in Table 1 meet the following classification criteria:
Drugs which contain manufacturers’ special handling information; and/or
Drugs which meet the NIOSH definition of a hazardous drug and are classified by the NTP as “known to
be a human carcinogen,” and/or classified by the IARC as “carcinogenic” or “probably carcinogenic”
Many of these drugs are cytotoxic and the majority are hazardous to males or females who are actively
trying to conceive, women who are pregnant or may become pregnant, and women who are breast
feeding, because the drugs may be excreted in breast milk.
Not all drugs in Table 1 are antineoplastic drugs.
Drugs reviewed for this update were new drug approvals or received safety related new warnings from
FDA in the period between January 2014 and December 2015.
Drugs underlined and in red font were added in 2020.
MSHI = manufacturer’s special handling information
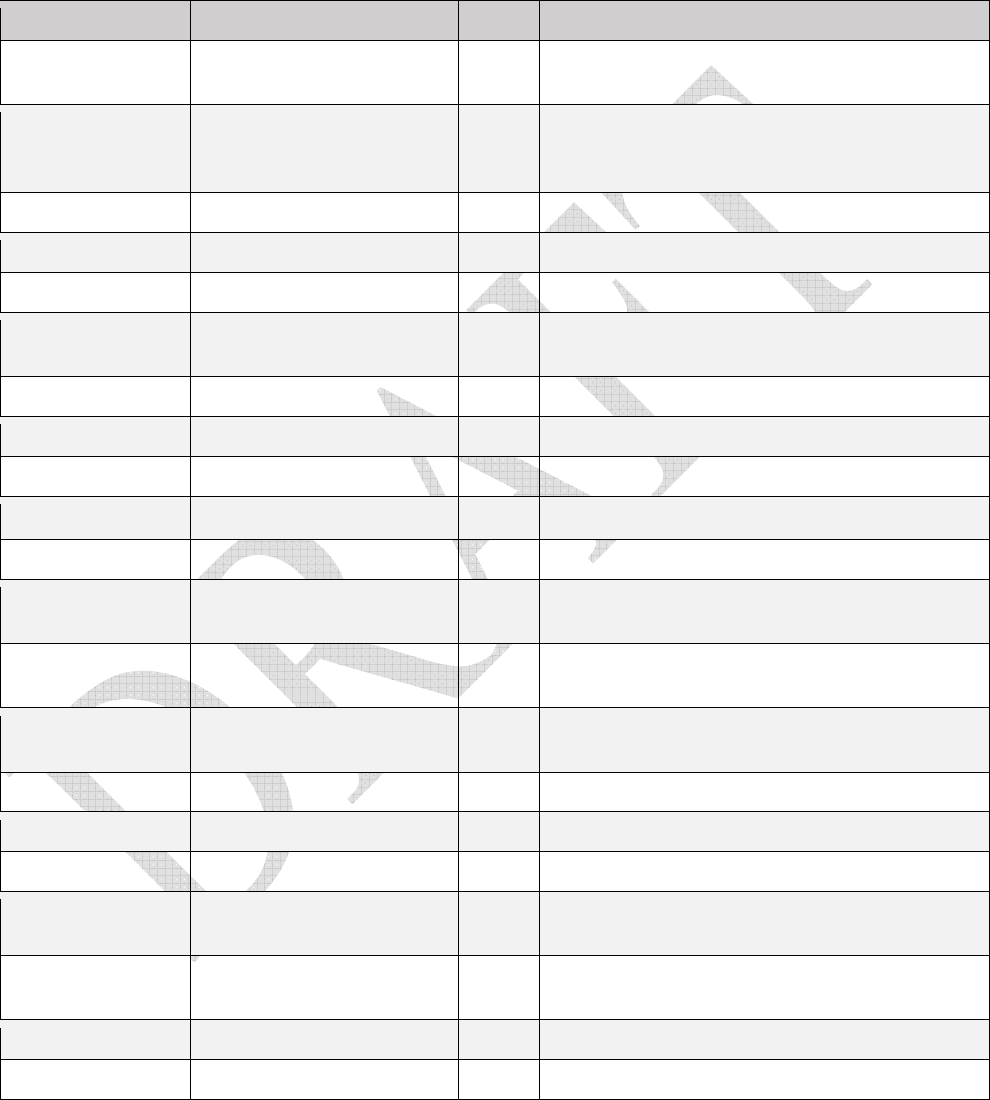
Table 1.
Table 1. Table 1.
Table 1.
D
rugs that contain MSHI in the package insert
and/
or
meet the NIOSH definition of a hazardous
drug and are classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.”
Drug AHFS classification MSHI Supplemental Information
11
trastuzumab
emtansine
10:00 antineoplastic agents yes Monoclonal antibody conjugated to mertansine
(emtasine)
altretamine 10:00 antineoplastic agents yes
amsacrine NA antineoplastic agents yes IARC Group 2B
arsenic trioxide 10:00 antineoplastic agents yes IARC Group 1 carcinogen
azacitidine 10:00 antineoplastic agents yes IARC Group 2A carcinogen
11
Drugs identified as IARC Group 2B are listed in Table 1 because they have MSHI.
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
13
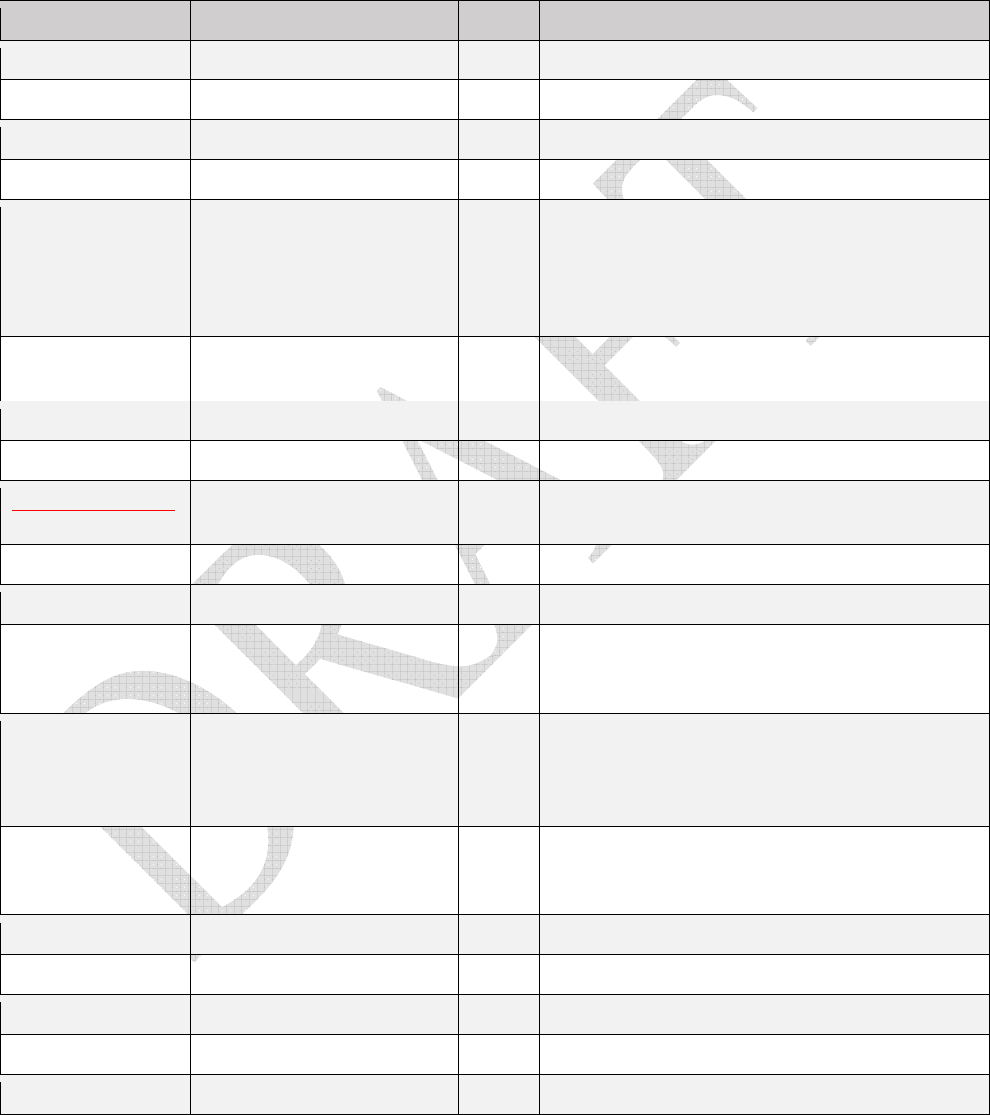
Table 1.
Table 1. Table 1.
Table 1.
D
rugs that contain MSHI in the package insert
and/
or
meet the NIOSH definition of a hazardous
drug and are classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.”
Drug AHFS classification MSHI Supplemental Information
11
azathioprine 92:44 immunosuppressant yes
IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
belinostat 10:00 antineoplastic agents yes May cause teratogenicity and/or embryo-fetal
lethality because it is a genotoxic drug and targets
actively dividing cells
bendamustine 10:00 antineoplastic agents yes Cytotoxic; Developmental toxicity
bleomycin 10:00 antineoplastic agents yes IARC Group 2B
bortezomib 10:00 antineoplastic agents yes
brentuximab
vedotin
10:00 antineoplastic agents yes Monoclonal antibody conjugated to vedotin
busulfan 10:00 antineoplastic agents yes IARC Group 1 carcinogen
cabazitaxel 10:00 antineoplastic agents yes
capecitabine 10:00 antineoplastic agents yes Metabolized to 5-fluorouracil
carboplatin 10:00 antineoplastic agents yes
carmustine 10:00 antineoplastic agents yes IARC Group 2A carcinogen
chlorambucil 10:00 antineoplastic agents yes IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
chloramphenicol 8:12:08 chloramphenicols IARC Group 2A carcinogen; NTP “known to be
human carcinogen”
cidofovir 8:18:32 nucleoside and
nucleotides
yes
cisplatin 10:00 antineoplastic agents yes IARC Group 2A carcinogen
cladribine 10:00 antineoplastic agents yes
clofarabine 10:00 antineoplastic agents yes
cyclophosphamide 10:00 antineoplastic agents yes IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
cyclosporine 92:44 immunosuppressive
agents
IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
cytarabine 10:00 antineoplastic agents yes
dacarbazine 10:00 antineoplastic agents yes IARC Group 2B
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
14
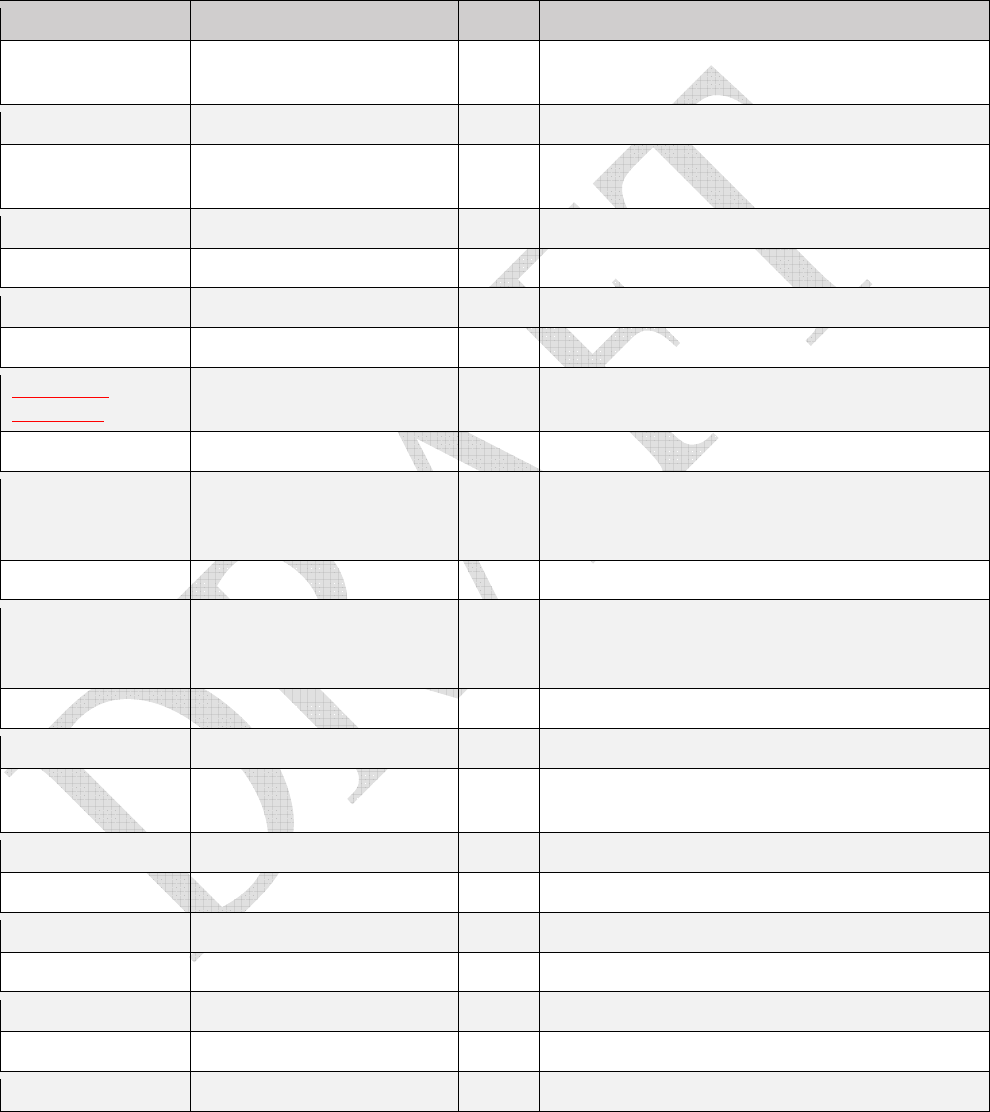
Table 1.
Table 1. Table 1.
Table 1.
D
rugs that contain MSHI in the package insert
and/
or
meet the NIOSH definition of a hazardous
drug and are classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.”
Drug AHFS classification MSHI Supplemental Information
11
dactinomycin 10:00 antineoplastic agents yes
dasatinib 10:00 antineoplastic agents yes
daunorubicin 10:00 antineoplastic agents yes IARC Group 2B; AKA daunomycin
decitabine 10:00 antineoplastic agents yes
dexrazoxane 92:56 protective agents yes Secondary malignancies observed in patients
treated long term with Razoxane (a racemic mixture
containing dexrazoxane); Genotoxic in vitro and in
vivo; in laboratory studies, Testicular atrophy
observed at or below the human dose
diethylstilbestrol NA IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
docetaxel 10:00 antineoplastic agents yes
doxorubicin 10:00 antineoplastic agents yes IARC Group 2A carcinogen
enfortumab vedotin 10:00 antineoplastic agents yes Monoclonal antibody conjugated to vedotin;
Cytotoxic; Developmental toxicity
epirubicin 10:00 antineoplastic agents yes
estramustine 10:00 antineoplastic agents yes
estrogen/
progesterone
combinations
68:12 contraceptives IARC Group 1 carcinogen;
NTP “known to be human
carcinogen”
estrogens,
conjugated
68:12 contraceptives NTP “known to be human carcinogen”; Black Box
warning for endometrial cancer and cardiovascular
risks; Long-term use in women and laboratory
studies increases frequency of several cancers
estrogens,
esterified
68:12 contraceptives NTP “known to be human carcinogen”; Black Box
warning for endometrial cancer and cardiovascular
risks
etoposide 10:00 antineoplastic agents yes IARC Group 1 carcinogen
everolimus 10:00 antineoplastic agents yes
floxuridine 10:00 antineoplastic agents yes
fludarabine 10:00 antineoplastic agents yes
fluorouracil 10:00 antineoplastic agents yes
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
15
Table 1.
Table 1. Table 1.
Table 1.
D
rugs that contain MSHI in the package insert
and/
or
meet the NIOSH definition of a hazardous
drug and are classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.”
Drug AHFS classification MSHI Supplemental Information
11
ganciclovir 8:18:32 nucleosides
nucleotides
yes
gemcitabine 10:00 antineoplastic agents yes
gemtuzumab
ozogamicin
10:00 antineoplastic agents yes Monoclonal antibody conjugated to ozogamicin;
Cytotoxic; Developmental toxicity
hydroxyurea 10:00 antineoplastic agents yes Special warning on handling bottles and capsules
idarubicin 10:00 antineoplastic agents yes
ifosfamide 10:00 antineoplastic agents yes
imatinib 10:00 antineoplastic agents yes
inotuzumab
ozogamicin
10:00 antineoplastic agents yes Monoclonal antibody conjugated to ozogamicin;
Cytotoxic; Developmental toxicity
irinotecan 10:00 antineoplastic agents yes
ixazomib 10:00 antineoplastic agents yes Male and female patients of childbearing potential
must use effective contraceptive measures during
and for 3 months following treatment
ixabepilone 10:00 antineoplastic agents yes
lenalidomide 92:20 biologic response
modulators
yes Analog of thalidomide; FDA Black box warnings for
limb abnormalities; in laboratory studies, caused
thalidomide-type limb defects in monkey offspring
lomustine 10:00 antineoplastic agents yes IARC Group 2A carcinogen
mechlorethamine 10:00 antineoplastic agents yes
melphalan 10:00 antineoplastic agents yes IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
mercaptopurine 10:00 antineoplastic agents yes
methotrexate 10:00 antineoplastic agents yes
mitomycin 10:00 antineoplastic agents yes IARC Group 2B
mitotane 10:00 antineoplastic agents yes
mitoxantrone 10:00 antineoplastic agents yes IARC Group 2B
nelarabine 10:00 antineoplastic agents yes
omacetaxin 10:00 antineoplastic agents yes
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
16
Table 1.
Table 1. Table 1.
Table 1.
D
rugs that contain MSHI in the package insert
and/
or
meet the NIOSH definition of a hazardous
drug and are classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.”
Drug AHFS classification MSHI Supplemental Information
11
oxaliplatin 10:00 antineoplastic agents yes
paclitaxel 10:00 antineoplastic agents yes
panobinostat 10:00 antineoplastic agents yes Special warnings on contraception for females while
taking and one month post- treatment
pemetrexed 10:00 antineoplastic agents yes
pentostatin 10:00 antineoplastic agents yes
polatuzumab
vedotin
10:00 antineoplastic agents yes Monoclonal antibody conjugated to vedotin;
Cytotoxic; Developmental toxicity
pomalidomide 10:00 antineoplastic agents yes Analog of thalidomide; Females of reproductive
potential must use 2 forms of contraception or
continuously abstain from heterosexual sex during
and for 4 weeks after stopping treatment
pralatrexate 10:00 antineoplastic agents yes
procarbazine 10:00 antineoplastic agents yes IARC Group 2A carcinogen
romidepsin 10:00 antineoplastic agents yes
streptozocin 10:00 antineoplastic agents yes IARC Group 2B
tamoxifen 10:00 antineoplastic agents;
68.16.12 estrogen agonist-
antagonist
IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
temozolomide 10:00 antineoplastic agents yes
temsirolimus 10:00 antineoplastic agents yes
teniposide 10:00 antineoplastic agents yes IARC Group 2A carcinogen
thalidomide 92:20 biologic response
modulators
yes
thioguanine 10:00 antineoplastic agents yes
thiotepa 10:00 antineoplastic agents yes IARC Group 1 carcinogen; NTP “known to be human
carcinogen”
topotecan 10:00 antineoplastic agents yes
trabectedin 10:00 antineoplastic agents yes Cytotoxic; Genotoxic
trastuzumab
deruxtecan
10:00 antineoplastic agents yes Monoclonal antibody conjugated to deruxtecan;
Cytotoxic
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
17
Table 1.
Table 1. Table 1.
Table 1.
D
rugs that contain MSHI in the package insert
and/
or
meet the NIOSH definition of a hazardous
drug and are classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.”
Drug AHFS classification MSHI Supplemental Information
11
trifluridine 10:00 antineoplastic agents yes Embryo-fetal lethality and embryo-fetal toxicity at
doses lower than or similar to exposures at the
recommended human dose
uracil mustard NA yes IARC Group 2B
valganciclovir 8:18:32 nucleosides and
nucleotides
yes
valrubicin 10:00 antineoplastic agents yes
vandetanib 10:00 antineoplastic agents yes
vinblastine 10:00 antineoplastic agents yes
vincristine 10:00 antineoplastic agents yes
vinorelbine 10:00 antineoplastic agents yes
vorinostat 10:00 antineoplastic agents yes Adverse embryo-fetal effects at less than the
recommended human dose
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
18
The drugs in Table 2 meet the NIOSH definition of a hazardous drug but are not drugs which have MSHI
and are not classified by the NTP as “known to be a human carcinogen,” and/or classified by the IARC as
“carcinogenic” or “probably carcinogenic.” These drugs exhibit one or more of the types of toxicity
described in the NIOSH definition of hazardous drug. Some of these drugs may present an occupational
hazard to males or females who are actively trying to conceive, women who are pregnant or may become
pregnant, and women who are breast feeding, because they may be present in breast milk.
Drugs reviewed for this update were new drug approvals or received safety related new warnings from
FDA in the period between January 2014 and December 2015.
Drugs underlined and in red font were added in 2020.
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
abacavir 8:18.08.20 nucleoside and
reverse transcriptase
inhibitors
Malignant tumors observed in male and female mice
and rats; Genotoxic in vivo micronucleus test.
abiraterone 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Women who are pregnant
or women who may be pregnant should not handle without
protection (e.g., gloves)
acitretin 88:04 vitamin A Only met the NIOSH criteria as a developmental
and/or reproductive hazard
afatinib 10:00 antineoplastic agents Special warnings on contraception for females while
taking and two weeks post- treatment
aflibercept
10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Embryotoxic and
teratogenic in rabbits at exposure levels lower than human
exposures at the recommended dose, with increased
incidences of external, visceral, and skeletal fetal
malformations
alefacept 84:92 skin and mucous
membrane agents,
miscellaneous
Increased frequency of malignancies observed in
treated patients
alitretinoin 84:92 skin and mucous
membrane agents,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
ambrisentan 24:12:92 vasodilating agents,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
19
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
anastrozole 68.16.04 antiestrogens; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
apomorphine 28:36.20.08 Nonergot-
derivative dopamine receptor
agonists
Genotoxic in several in vitro assays
axitinib 10:00 antineoplastic agents Teratogenic, embryotoxic and fetotoxic in mice at
exposures lower than human exposures
bexarotene 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
bicalutamide 10:00 antineoplastic agents
blinatumomab 10:00 antineoplastic agents Organ Toxicity at Low Dose - Neurotoxicity
bosentan 24:12:92 vasodilating agents,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
bosutinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
cabergoline 28:36:20:04 ergot-derivative
dopamine receptor agonists
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
cabozantinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Embryo lethal in rats at
exposures below the recommended human dose
carbamazepine 28:12:92 anticonvulsants,
miscellaneous
Black Box warning for aplastic anemia; Congenital
malformations in offspring of mothers who took
drug; Rapid transplacental passage
carfilzomib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Special warnings on
contraception while taking and two weeks post-
treatment
ceritinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
cetrorelix 92:40 gonadotropin-releasing
hormone antagonists
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
choriogonadotropin 68:18 gonadotropins Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
clobazam 28:12.08 benzodiazapines Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity;
Reproductive toxicity-male; Reproductive toxicity-
female
clomiphene 68:16:12 estrogen agonist-
antagonists
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
clonazepam 28:12:08 benzodiapines Only met the NIOSH criteria as a developmental
and/or reproductive hazard
cobimetinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity;
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
20
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
Reproductive toxicity-male; Reproductive toxicity-
female
colchicine 92:16 antigout agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
crizotinib 10:00 antineoplastic agents
dabrafenib 10:00 antineoplastic agents Special warnings on contraception for females while
taking and two weeks post-treatment
deferiprone 64:00 Heavy metal antagonists Genotoxic in vitro and in vivo
degarelix 68:18.04 antigonadropins;
10:00 antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
dihydroergotamine 12:16.00 sympatholytic
(andrenergic blocking) agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
dinoprostone 76:00 oxytocics Only met the NIOSH criteria as a developmental
and/or reproductive hazard
divalproex 28:12:92 anticonvulsants,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Black box warning on
embryo-fetal death or severe birth defects; Recommend
effective contraception for females during therapy and for
seven months after treatment; Present in semen; No sperm
donation during and three months post-treatment
dronedarone 24:04:04 antiarrythmics Only met the NIOSH criteria as a developmental
and/or reproductive hazard
dutasteride 92:08 5-alpha reductase
inhibitors
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
entecavir 8:18:32 nucleosides and
nucleotides
enzalutamide 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Embryo-fetal toxicity in
mice at exposures that were lower than in patients
receiving the recommended dose
ergonovine/methylergonovine 76:00 oxytocics Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
– third trimester
eribulin 10:00 antineoplastic agents
erlotinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
eslicarbazepine 28:12:92 anticonvulsants,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
estradiol 68:16:04 estrogens Black Box warning for malignant neoplasms;
Increased risk of endometrial cancer, breast cancer,
and ovarian cancer; in laboratory studies, increased
frequency of carcinomas of the breast, uterus, cervix,
vagina, testis, and liver; Present in breast milk
estropipate 68:16:04 estrogens Black Box warning for endometrial carcinoma in
postmenopausal women and use during pregnancy
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
21
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
exemestane 68.16.04 Antiestrogens; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
exenatide 68:20.06 incretin mimetics Carcinogenicity; Developmental toxicity
finasteride 92:08 5-alpha reductase Only met the NIOSH criteria as a developmental
and/or reproductive hazard
fingolimod 92:20 biologic response
modifiers
In laboratory studies, increased malformations and
embryo-fetal deaths at less than the RHD; Malignant
lymphomas observed in male and female mice
fluconazole 8:18.08 azoles Only met the NIOSH criteria as a developmental
and/or reproductive hazard
fluoxymesterone 68:08 androgens Tumors in mice and rats and possibly humans
flutamide 10:00 antineoplastic agents
Indicated only for men
fosphenytoin 28:12.12 hydantoins Metabolized to phenytoin
fulvestrant 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
ganirelix 92:40 gonadotropin- releasing
hormone antagonists
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
gonadotropin, chorionic 68:18 gonadotropins
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
goserelin 68:16.08 gonadotropins; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
histrelin 68:16.08 gonadotropins; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Can cause fetal harm
when administered to a pregnant patient with the
possibility of spontaneous abortion
icatibant 92:32 complement inhibitors Only met the NIOSH criteria as a developmental
and/or reproductive hazard
isotretinoin 84:92.00 misc. skin and
mucous membrane agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
ivabradine 24:04.90 misc. cardiac agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
leflunomide 92:36 disease-modifying
antirheumatic agents
Teratogenic in laboratory studies at 1/10 HD; Marked
postnatal survival at 1/100 HD; Severe liver injury
reported in patients; Carcinogenicity observed at
doses below HD
lenvatinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
letrozole 68.16.04 Antiestrogens; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
leuprolide 68:16.08 gonadotropins; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
liraglutide
recombinant
68:20.06 incretin mimetics Black Box warning for thyroid C-cell tumors, with
supporting evidence in laboratory studies; In
laboratory studies, teratogenic at or below the MRHD
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
22
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
lomitapide 24:06:92 antilipemic agents,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
macitentan 48:48 vasodilating agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
medroxyprogesterone acetate 68:32 progestins Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
IARC Group 2B
megestrol 10:00 antineoplastic agents
Nursing should be discontinued if megestrol is required;
Women at risk of pregnancy should avoid exposure
menotropins 68:18 gonadotropins Only met the NIOSH criteria as a developmental
and/or reproductive hazard
methimazole 68:36:08 antithyroid agents
Appears in human breast milk
methyltestosterone
68:08 androgens Only met the NIOSH criteria as a developmental
and/or reproductive hazard
mifepristone 76:00 oxytocics Only met the NIOSH criteria as a developmental
and/or reproductive hazard
miltefosine 8:30.92 misc. antiprotozoals Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity;
Reproductive toxicity – male; Reproductive toxicity –
female
mipomersen 24:06:92 antilipemic agents,
miscellaneous
Black box warning of hepatotoxicity
misoprostol 56:28.28 prostaglandins Only met the NIOSH criteria as a developmental
and/or reproductive hazard
mycophenolate mofetil 92:44 immunosuppressive
agents
Black Box warning for embryo fetal toxicity,
malignancies and serious infections; Increased risk of
first- trimester pregnancy loss and increased risk of
congenital malformations; Special warning: tablets
should not be crushed and capsules should not be
opened or crushed. Avoid inhalation or direct contact
with skin or mucous membranes of the powder
contained in capsules and oral suspension (before or
after constitution). If such contact occurs, wash
thoroughly with soap and water; rinse eyes with plain
water.
mycophenolic acid 92:44 immunosuppressive
agents
Black Box warning for embryo fetal toxicity,
malignancies and serious infections; Increased risk of
first- trimester pregnancy loss and increased risk of
congenital malformations; Black Box warning for
lymphomas and other malignancies; genotoxic in
vitro and in vivo
nafarelin 68:18 gonadotropins Only met the NIOSH criteria as a developmental
and/or reproductive hazard
nevirapine 8:18.08.16 nonnucleoside
reverse transcriptase
inhibitors
In laboratory studies, hepatocellular adenomas and
carcinomas at doses lower than human dose
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
23
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
nilotinib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
olaparib 10:00 antineoplastic agents Genotoxicity; Developmental toxicity
ospemifene 68:16:12 estrogen agonist-
antagonists
Black box warning on increased risk of endometrial
cancer in certain populations; Risk of adverse
outcomes during pregnancy and labor
oxcarbazepine 28:12:92 anticonvulsants,
miscellaneous
Tumors observed in laboratory studies at 1/10 MRHD
oxytocin 76:00 oxytocics Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
– third trimester
palifermin 84:16 cell stimulants and
proliferants
Potential for stimulation of tumor growth
pamidronate 92:24 bone resorption
inhibitors
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
paroxetine 28:16:04:20 selective serotonin
uptake inhibitors
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
pasireotide 68:29:04 somatostatin agonists Only met the NIOSH criteria as a developmental
and/or reproductive hazard
pazopanib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
peginesatide 20:16 hematopoetic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
pentetate calcium trisodium NA Only met the NIOSH criteria as a developmental
and/or reproductive hazard
phenoxybenzamine 12:16:04:04 non-selective
alpha-andrenergic blocking
agents
IARC Group 2B
phenytoin 28:12.12 hydantoins IARC Group 2B
pipobroman NA
plerixafor 20:16 hematopoietic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
ponatinib 10:00 antineoplastic agents
progesterone 68:32 progestins IARC Group 2B
progestins 68:12 contraceptives
propylthiouracil 68:36.08 antithyroid agents IARC Group2B
raloxifene 68:16:12 estrogen agonists-
antagonists
Abortion and developmental abnormalities seen at
low doses in laboratory studies; Evidence of tumors
at low doses in laboratory studies
rasagiline 28:36 antiparkinsonian agents
regorafenib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Black box warning on
severe and sometimes fatal hepatotoxicity; Total loss of
pregnancy at doses lower that recommended human dose
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
24
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
ribavirin 8:18:32 nucleosides and
Teratogenic and embryotoxic
nucleotides
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
riociguat 48:48 vasodilating agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
sirolimus 92:44 immunosuppressive
agents
AKA rapamycin; Increased risk of lymphomas and
other malignancies; Embryotoxic and fetotoxic at 0.2
HD
sonidegib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity;
Reproductive toxicity – female
sorafenib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
spironolactone 24:32.20 mineralocorticoid
receptor antagonists
Black box warning for tumorogenicity in laboratory
studies
sunitinib 10:00 antineoplastic agents
tacrolimus 92:44 immunosuppressive
agents
Increased risk of lymphomas and other malignancies;
Reproductive effects seen in laboratory studies below
the MRHD; Excreted in breast milk
temazepam 28:24:08 benzodiazepines Only met the NIOSH criteria as a developmental
and/or reproductive hazard
teriflunomide 92:20 immunomodulatory
agents
Black box warning on severe hepatotoxicity and
teratogenicity including major birth defects
testosterone 68:08 androgens Only met the NIOSH criteria as a developmental
and/or reproductive hazard
tofacitinib 92:36 disease modifying
antirheumatic drugs
Black box warning for lymphoma and other
malignancies
topiramate 28:12.92 anticonvulsants,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
toremifene 68.16.12 estrogen agonist-
antagonist; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
trametinib 10:00 antineoplastic agents Embryotoxic and abortifacient at doses less than
recommended human dose
tretinoin 84:16 cell stimulants and
proliferants
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
triptorelin 68:18.08 gonadotropins; 10:00
antineoplastic agents
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
ulipristal 68:12 contraceptives Only met the NIOSH criteria as a developmental
and/or reproductive hazard
urofollitropin 68:18.00 gonadotropins Only met the NIOSH criteria as a developmental
and/or reproductive hazard; Developmental toxicity
valproate/valproic acid 28:12:92 anticonvulsants,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
This information is distributed solely for the purpose of pre-dissemination public comment under
applicable information quality guidelines. It has not been formally disseminated by the Centers for
Disease Control and Prevention or the National Institute for Occupational Safety and Health. It does not
represent and should not be construed to represent any agency determination or policy.
25
Table 2.
Table 2. Table 2.
Table 2.
D
r
ugs that meet the NIOSH definition of a hazardous drug but are not drugs
that
have MSHI or are
classified by the NTP as “known to be a human carcinogen,” or classified by the IARC as “carcinogenic” or
“probably carcinogenic.”
(some a
(some a(some a
(some also may have adverse
lso may have adverse lso may have adverse
lso may have adverse de
dede
development and/or
velopment and/or velopment and/or
velopment and/or reproductive
reproductivereproductive
reproductive
effects)
effects)effects)
effects)
Drug AHFS classification Supplemental Information
vemurafenib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard
vigabatrin 28:12:92 anticonvulsants,
miscellaneous
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
vismodegib 10:00 antineoplastic agents Only met the NIOSH criteria as a developmental
and/or reproductive hazard;
Black box warning on
embryo-fetal death or severe birth defects; Recommend
effective contraception for females during therapy and for
seven months after treatment; present in semen; No sperm
donation during and three months post-treatment
voriconazole 8:14.08 azoles Only met the NIOSH criteria as a developmental
and/or reproductive hazard
warfarin 20:12.04.08 coumarin
derivatives
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
zidovudine 8:18:08 antiretroviral agents IARC Group 2B
ziprasidone 28:16:08:04 atypical Only met the NIOSH criteria as a developmental
and/or reproductive hazard
zoledronic acid 92:24 bone resorption
inhibitors
Only met the NIOSH criteria as a developmental
and/or reproductive hazard
zonisamide
28:12:92 anticonvulsants, Only met the NIOSH criteria as a developmental
and/or reproductive hazard

This information is distributed solely for the purpose of pre-dissemination public comment under applicable
information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and
Prevention or the National Institute for Occupational Safety and Health. It does not represent and should not be
construed to represent any agency determination or policy.
26
Changes to the Placement of Drugs on the List
This table identifies drugs that were either removed after the 2016 update to the List or were placed in a table
different than they were placed in the 2016 update to the List.
Changes to the placement of drugs from the 2016 List.
Drugs removed from the List
Drug
Notation
Bacillus Calmette Guerin
(BCG)
BCG was removed from the NIOSH list because it is an
infectious agent and not
classified as a drug by FDA. For handling recommendations see drug package
insert.
p
aliperidone
NIOSH
reviewed data from studies provided by the manufacturer and
determined
it is unlikely that paliperidone poses a carcinogenic, reproductive, or
developmental hazard to workers in a healthcare setting and is no longer
considered a hazardous drug by NIOSH.
pertuzumab
NIOSH reviewed data concerning the developmental effects related to pertuzumab
treatment and has determined that it is unlikely that pertuzumab poses a
reproductive threat to workers in healthcare settings and is no longer considered a
hazardous drug by NIOSH.
r
isperidone
NIOSH
reviewed data from studies provided by the manufacturer and
determined
it is unlikely that risperidone poses a carcinogenic, reproductive, or developmental
hazard to workers in a healthcare setting and is no longer considered a hazardous
drug by NIOSH.
televancin
Televancin was removed from the NIOSH list based o
n
data from reproductive
studies provided by the manufacturer concerning its lack of reproductive toxicity.
Drugs moved to a different table
Abiraterone
Moved from
Table
1 to
Table
2
Acitretin
Moved from
Table
3 to
Table
2
afatinib
Moved from
Table
1 to
Table
2
Aflibercept
Moved from
Table
1 to
Table
2

This information is distributed solely for the purpose of pre-dissemination public comment under applicable
information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and
Prevention or the National Institute for Occupational Safety and Health. It does not represent and should not be
construed to represent any agency determination or policy.
27
Alitretinoin
Moved from
Table
3 to
Table
2
Anastrozole
Moved from
Table
1 to
Table
2
axitinib
Moved from
Table
1 to
Table
2
Azathioprine
Moved from
Table
2 to
Table
1
Bexarotene
Moved from
Table
1 to
Table
2
Bicalutamide
Moved from
Table
1 to
Table
2
Bosentan
Moved from
Table
3 to
Table
2
Bosutinib
Moved from
Table
1 to
Table
2
Cabergoline
Moved from
Table
3 to
Table
2
Cabozantinib
Moved from
Table
1 to
Table
2
Carfilzomib
Moved from
Table
1 to
Table
2
Ceizotinib
Moved from
Table
1 to
Table
2
Cetrorelix
Moved from
Table
3 to
Table
2
Chloramphenicol
Moved from
Table
2 to
Table
1
Choriogonadotropin
Moved from
Table
3 to
Table
2
cidofovir
Moved from
Table
2 to
Table
1
Clomiphene
Moved from
Table
3 to
Table
2
Clonazepam
Moved from
Table
3 to
Table
2
Colchicine
Moved from
Table
3 to
Table
2
Cyclosporine
Moved from
T
able
2 to
Table
1
Dabrafenib
Moved from
Table
1 to
Table
2
Degarelix
Moved from
Table
1 to
Table
2
Dexrazoxane
Moved from
Table
2 to
Table
1
Diethylstilbestrol
Moved from
Table
2 to
Table
1
Dinoprostone
Moved from
Table
3 to
Table
2

This information is distributed solely for the purpose of pre-dissemination public comment under applicable
information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and
Prevention or the National Institute for Occupational Safety and Health. It does not represent and should not be
construed to represent any agency determination or policy.
28
Divalproex
Moved
from
Table
2 to
Table
3
Dronedarone
Moved from
Table
3 to
Table
2
Dutasteride
Moved from
Table
3 to
Table
2
Emzalutamide
Moved from
Table
1 to
Table
2
Ergovine/Methylergovine
Moved from
Table
3 to
Table
2
Eribulin
Moved from
Table
1 to
Table
2
Erloti
nib
Moved from
Table
1 to
Table
2
Eslicarbazepine
Moved from
Table
3 to
Table
2
Estrogen
-
progesterone
combinations
Moved from
Table
2 to
Table
1
Estrogens conjugated
Moved from
Table
2 to
Table
1
Estrogens; esterified
Moved from
Table
2 to
Table
1
Exe
mestane
Moved from
Table
1 to
Table
2
Finasteride
Moved from
Table
3 to
Table
2
Fluconazole
Moved from
Table
3 to
Table
2
Flutamide
Moved from
Table
1 to
Table
2
Fulvestrant
Moved from
Table
1 to
Table
2
Ganciclovir
Moved from
Table
2 to
Table
1
Gani
relix
Moved from
Table
3 to
Table
2
Goserelin
Moved from
Table
1 to
Table
2
Histrelin
Moved from
Table
1 to
Table
2
Icatibant
Moved from
Table
3 to
Table
2
Lenalidomide
Moved from
Table
2 to
Table
1
Letrozole
Moved from
Table
1 to
Table
2
Leuprolide
Moved from
Table
1 to
Table
2
Lomitapide
Moved from
Table
3 to
Table
2

This information is distributed solely for the purpose of pre-dissemination public comment under applicable
information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and
Prevention or the National Institute for Occupational Safety and Health. It does not represent and should not be
construed to represent any agency determination or policy.
29
Macitentan
Moved from
Table
3 to
Table
2
Magestrol
Moved from
Table
1 to
Table
2
Medroxyprogesterone
Moved from
Table
2 to
Table
2
Menotropins
Moved from
Table
3 to
Table
2
Methyl
testosterone
Moved from
Table
3 to
Table
2
Mifepristone
Moved from
Table
3 to
Table
2
Misoprostal
Moved from
Table
3 to
Table
2
Nafarelin
Moved from
Table
3 to
Table
2
Nilotinib
Moved from
Table
1 to
Table
2
Oxytocin
Moved from
Table
3 to
Table
2
Pam
idronate
Moved from
Table
3 to
Table
2
Paroxetine
Moved from
Table
3 to
Table
2
Pasireotide
Moved from
Table
3 to
Table
2
Pazopanib
Moved from
Table
1 to
Table
2
Peginesatide
Moved from
Table
3 to
Table
2
Pentetate
calcium
trisodium
Moved from
Table
3
to
Table
2
Plerixafor
Moved from
Table
3 to
Table
2
Ponatinib
Moved from
Table
1 to
Table
2
Regorafenib
Moved from
Table
1 to
Table
2
Ribavirin
Moved from
Table
3 to
Table
2
Riociguat
Moved from
Table
3 to
Table
2
Sorafenib
Moved from
Table
1 to
Ta
ble
2
Sunitinib
Moved from
Table
1 to
Table
2
Temazepam
Moved from
Table
3 to
Table
2
Teriflunomide
Moved from
Table
3 to
Table
2

This information is distributed solely for the purpose of pre-dissemination public comment under applicable
information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and
Prevention or the National Institute for Occupational Safety and Health. It does not represent and should not be
construed to represent any agency determination or policy.
30
Testosterone
Moved from
Table
3 to
Table
2
Thalidomide
Moved from
Table
2 to
Table
1
Topiramate
Moved from
Table
3 to
Ta
ble
2
Toremifene
Moved from
Table
1 to
Table
2
Trametinib
Moved from
Table
1 to
Table
2
Tretinoin
Moved from
Table
3 to
Table
2
Triptorelin
Moved from
Table
1 to
Table
2
Ulipristal
Moved from
Table
3 to
Table
2
Uracil mustard
Moved from
Table
2 to
Ta
ble
1
Valganciclovir
Moved from
Table
2 to
Table
1
Valproate
Moved from
Table
3 to
Table
2
Valproic Acid
Moved from
Table
3 to
Table
2
vemurafenib
Moved from
Table
1 to
Table
2
Vigabatrin
Moved from
Table
3 to
Table
2
Voriconazole
Moved from
Table
3
to
Table
2
Warfarin
Moved from
Table
3 to
Table
2
Zif
-
afibercept
Moved from
Table
1 to
Table
2; now listed as afibercept
Ziprasidone
Moved from
Table
3 to
Table
2
Zoledronic Acid
Moved from
Table
3 to
Table
2
Zonisamide
Moved from
Table
3 to
Table
2
As noted earlier, in previous iterations of this List, Table 5 provided information on recommended exposure
controls for hazardous drugs based on formulations. Information about managing risk of exposure can now be
found in the draft NIOSH risk management document (Insert link when available).
