
Morbidity and Mortality Weekly Report
Recommendations and Reports November 11, 2005 / Vol. 54 / No. RR-13
INSIDE: Continuing Education Examination
depardepar
depardepar
depar
tment of health and human sertment of health and human ser
tment of health and human sertment of health and human ser
tment of health and human ser
vicesvices
vicesvices
vices
Centers for Disease Control and PreventionCenters for Disease Control and Prevention
Centers for Disease Control and PreventionCenters for Disease Control and Prevention
Centers for Disease Control and Prevention
Good Laboratory Practices
for Waived Testing Sites
Survey Findings from Testing Sites Holding
a Certificate of Waiver Under the Clinical
Laboratory Improvement Amendments
of 1988 and Recommendations
for Promoting Quality Testing

MMWR
CONTENTS
Introduction......................................................................... 1
Background ......................................................................... 2
Surveys of Waived Testing Sites ........................................... 4
Recommended Good Laboratory Practices ........................... 8
Conclusions ....................................................................... 19
Acknowledgments ............................................................. 19
References......................................................................... 19
Terms and Abbreviations Used in this Report ..................... 22
Continuing Education Activity ......................................... CE-1
The MMWR series of publications is published by the
Coordinating Center for Health Information and Service,
Centers for Disease Control and Prevention (CDC), U.S.
Department of Health and Human Services, Atlanta, GA 30333.
Centers for Disease Control and Prevention
Julie L. Gerberding, MD, MPH
Director
Dixie E. Snider, MD, MPH
Chief Science Officer
Tanja Popovic, MD, PhD
Associate Director for Science
Coordinating Center for Health Information
and Service
Steven L. Solomon, MD
Director
National Center for Health Marketing
Jay M. Bernhardt, PhD, MPH
Director
Division of Scientific Communications
Maria S. Parker
(Acting) Director
Mary Lou Lindegren, MD
Editor, MMWR Series
Suzanne M. Hewitt, MPA
Managing Editor, MMWR Series
Teresa F. Rutledge
(Acting) Lead Technical Writer-Editor
David C. Johnson
Project Editor
Beverly J. Holland
Lead Visual Information Specialist
Lynda G. Cupell
Malbea A. LaPete
Visual Information Specialists
Quang M. Doan, MBA
Erica R. Shaver
Information Technology Specialists
SUGGESTED CITATION
Centers for Disease Control and Prevention. Good laboratory
practices for waived testing sites; survey findings from testing
sites holding a certificate of waiver under the Clinical Laboratory
Improvement Amendments of 1988 and Recommendations
for Promoting Quality Testing. MMWR 2005;54(No. RR-13):
[inclusive page numbers].
Vol. 54 / RR-13 Recommendations and Reports 1
Good Laboratory Practices for Waived Testing Sites
Survey Findings from Testing Sites Holding a Certificate of Waiver
Under the Clinical Laboratory Improvement Amendments
of 1988 and Recommendations for Promoting Quality Testing
Prepared by
Devery Howerton, PhD, Nancy Anderson, MMSc, Diane Bosse, MS, Sharon Granade, Glennis Westbrook
Division of Public Health Partnerships, National Center for Health Marketing, Coordinating Center for Health Information and Service
Summary
Under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), simple, low-risk tests can be waived and per-
formed with no routine regulatory oversight in physicians’ offices and various other locations. Since CLIA was implemented,
waived testing has steadily increased in the United States. Surveys conducted during 1999–2004 by the Centers for Medicare &
Medicaid Services and studies funded by CDC during 1999–2003 evaluated testing practices in sites holding a CLIA Certificate
of Waiver (CW). Although study findings indicate CW sites generally take measures to perform testing correctly, they raise quality
concerns about practices that could lead to errors in testing and poor patient outcomes. These issues are probably caused, in part,
by high personnel turnover rates, lack of understanding about good laboratory practices, and inadequate training. This report
summarizes study findings and provides recommendations developed by the Clinical Laboratory Improvement Advisory Commit-
tee for conducting quality waived testing. These recommendations include considerations before introducing waived testing, such
as management responsibility for testing, regulatory requirements, safety, physical and environmental requirements, benefits and
costs, staffing, and documentation. They also cover good laboratory practices for the three phases of testing: 1) before testing (test
ordering and specimen collection), 2) during testing (control testing, test performance, and result interpretation and recording),
and 3) after testing (result reporting, documentation, confirmatory testing, and biohazard waste disposal). They are intended to
be used by those who would benefit from improving their knowledge of good laboratory practices. Continued monitoring of
waived testing, with a focus on personnel education and training, is needed to improve practices and enhance patient safety as
waived testing continues to increase.
devices that are waived from most federal oversight require-
ments (and are thus designated as waived tests), including
requirements for personnel qualifications and training, qual-
ity control (QC) (unless specified as required in the test sys-
tem instructions), proficiency testing (PT), and routine quality
assessment.
Advances in technology have made tests simpler, contribut-
ing to this shift in testing. In the past, tests such as prothrom-
bin time, cholesterol, and glucose either used complex manual
methodologies or were performed using sizable instrumenta-
tion suitable for use by highly trained personnel in traditional
clinical laboratory settings. Many tests can now be performed
using compact or hand held devices by personnel with lim-
ited experience and training. These advances have enabled more
testing to be performed in emergency departments, hospital
rooms, and physicians’ offices and in nontraditional testing
sites such as community counseling centers, pharmacies, nurs-
ing homes, ambulances, and health fairs. Since the 1992
inception of the program implementing the Clinical Labora-
tory Improvement Amendments of 1988 (CLIA), the num-
bers of waived tests and the sites that perform them have
Introduction
Laboratory testing plays a critical role in health assessment,
health care, and ultimately, the public’s health. Test results
contribute to diagnosis and prognosis of disease, monitoring
of treatment and health status, and population screening for
disease. Laboratory testing affects persons in every life stage,
and almost everyone will experience having one or more labo-
ratory tests conducted during their lifetime. An estimated 7–10
billion laboratory tests are performed each year in the United
States (1,2), and laboratory test results influence approximately
70% of medical decisions (2–4). Increasingly, these decisions
are based on simple tests performed at the point-of-care using
The material in this report originated in the Coordinating Center for
Health Information and Service, Steven L. Solomon, MD, Director;
National Center for Health Marketing, Jay M. Bernhardt, PhD,
Director; and the Division of Public Health Partnerships, Robert
Martin, DrPH, Director.
Corresponding author: Devery Howerton, PhD, National Center for
Health Marketing, Coordinating Center for Health Information and
Service; 4770 Buford Hwy NE, MS G-23, Atlanta, GA, 30341. Telephone:
770-488-8126; Fax: 770-488-8275; Email: [email protected].

2 MMWR November 11, 2005
increased dramatically. This trend is expected to continue as
laboratory testing technology continues to evolve.
The purpose of this report is to highlight quality issues iden-
tified in waived testing sites on the basis of surveys conducted
on-site by the Centers for Medicare & Medicaid Services
(CMS) during 1999–2004 and studies of waived testing prac-
tices funded through CDC during 1999–2003. In addition,
this report presents recommendations developed by the Clini-
cal Laboratory Improvement Advisory Committee (CLIAC)
for improving the quality of waived testing. By following these
recommendations, errors that could potentially lead to
patient harm and the associated morbidity and mortality can
be prevented.
Background
CLIA Requirements for Waived Testing
All facilities in the United States that perform laboratory
testing on human specimens for health assessment or the
diagnosis, prevention, or treatment of disease are regulated
under CLIA (5). The CLIA program is administered by CMS
and is implemented through three federal agencies—CDC,
CMS, and the Food and Drug Administration (FDA). When
CLIA was implemented in 1992, CLIAC was chartered to
provide scientific and technical advice and guidance to the
U.S. Department of Health and Human Services (HHS) about
laboratory standards and their impact on medical and labora-
tory practice. The committee consists of 20 members selected
by the HHS secretary from authorities knowledgeable in the
fields of laboratory medicine, pathology, public health, and
clinical practice and includes consumer representatives and
an industry liaison. CLIAC also includes three ex officio mem-
bers from CDC, CMS, and FDA.
By law, CLIA regulations are based on a complexity model,
with more complicated testing subject to more stringent
requirements (6). The three categories of testing for CLIA pur-
poses are waived, moderate complexity (including the provider-
performed microscopy procedures [PPMP] subcategory), and
high complexity. Facilities performing only waived tests have
no routine oversight and no personnel requirements and are
only required to obtain a Certificate of Waiver (CW), pay bien-
nial certificate fees, and follow manufacturers’ test instructions.
Tests can be waived under CLIA if they are determined to
be “simple tests with an insignificant risk of an erroneous result”
(5). Eight tests were included in the 1992 CLIA regulations
(a ninth test was subsequently added) as meeting these crite-
ria and later, the FDA Modernization Act of 1997 clarified
that tests cleared by FDA for home use are automatically
waived. An additional route to waiver exists through a process
in which FDA evaluates studies and other information sub-
mitted by manufacturers to demonstrate that a test meets the
waiver criteria of being simple and having a low risk for error.
Approximately 1,600 test systems representing at least 76
analytes are waived under CLIA (Table 1).
Scope of Waived Testing
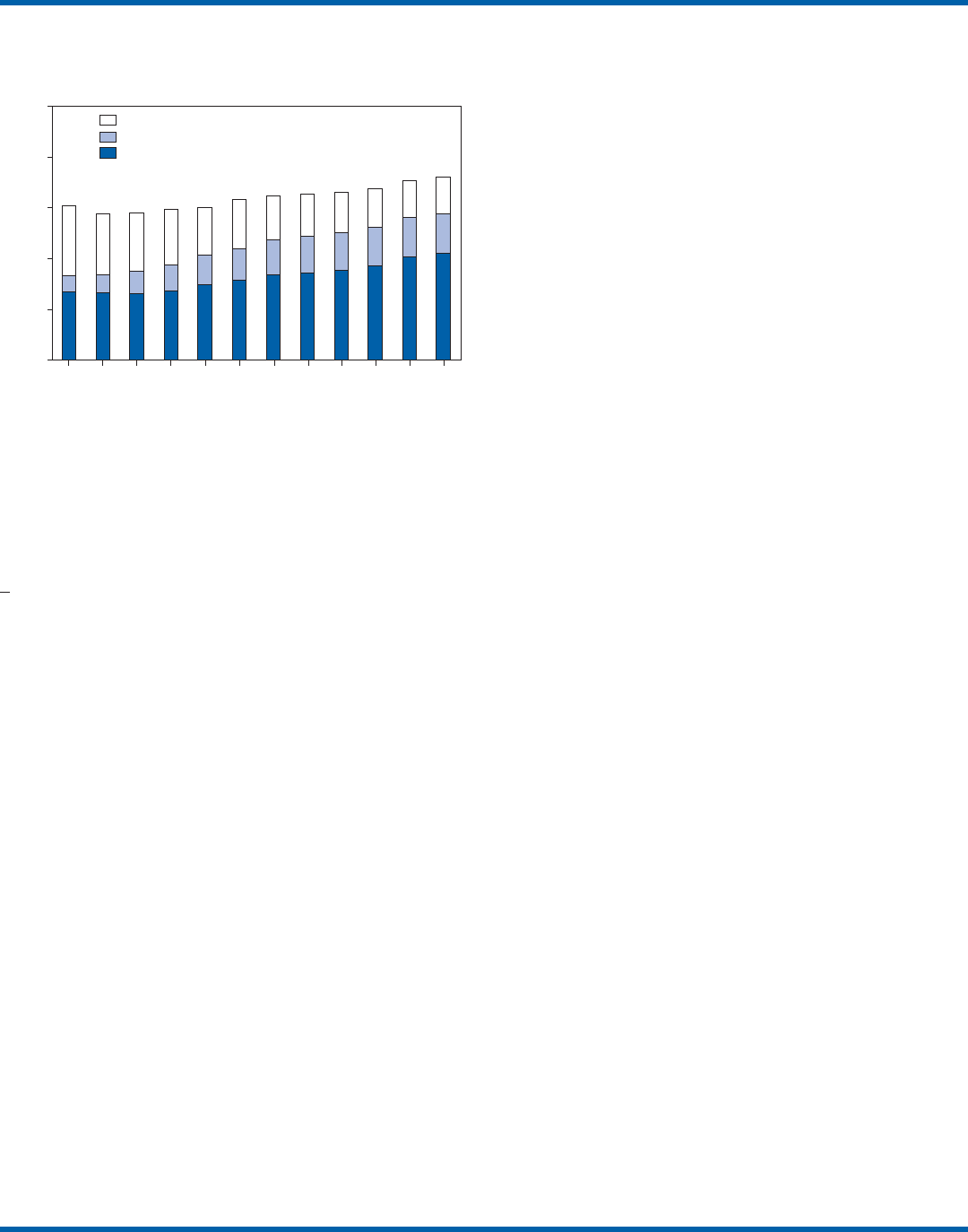
Sites performing only waived tests comprise 58% (105,138)
of the approximately 180,000 laboratory testing sites in the
United States (Table 1, Figure 1). Waived testing performed
in these sites is often wellness testing, screening tests, or other
critical testing that introduces a large population of persons
into the health-care setting. Although the testing performed
in CW sites accounts for <10% of the total U.S. testing vol-
ume, this percentage has been increasing each year since the
CLIA program began (Table 1). Most testing is not waived
and is typically performed in hospital or reference laborato-
ries (Certificate of Compliance and Certificate of Accredita-
tion), which comprise 20% of the total number of testing
sites (Figure 1). The remaining testing sites (22%) have PPMP
certificates, meaning that in addition to waived tests, direct
microscopic examinations of certain specimens can be per-
formed as part of the patient’s examination by that patient’s
TABLE 1. Increases in waived analytes and test systems, Certificate of Waiver laboratories, and Medicare Part B reimbursed waived
testing, 1993–2004
Waived testing measurement parameter 1993 1998 2000 2003 2004
No. of analytes for which waived test systems are available 9 40 53 74 76
No. of waived test systems* 203 608 832 1,495 1,638
No. of laboratories with a Certificate of Waiver
†
67,294 78,825 85,944 102,123 105,138
Percentage of laboratories with a Certificate of Waiver
†
44% 50% 52% 57% 58%
No. of Medicare Part B reimbursed waived tests NA
§
NA 14,663,751 20,781,297 23,041,693
Percentage of Medicare Part B reimbursed laboratory testing that is waived NA NA 6.5% 7.8% 8.1%
Medicare Part B payment amount for waived tests NA NA $69,765,453 $112,247,706 $128,169,398
* Numbers reflect multiple names under which individual tests are marketed and might include waived tests no longer sold.
†
Does not include Clinical Laboratory Improvement Amendments (CLIA) exempt laboratories in New York and Washington.
§
Not available.
Source: CDC and Food and Drug Administration CLIA Test categorization databases; Centers for Medicare & Medicaid Services (CMS) Medicare Part B
Utilization for CLIA-covered Laboratory Services; and CMS On-line Survey, Certification, and Reporting database.
Vol. 54 / RR-13 Recommendations and Reports 3
physician or midlevel health-care practitioner. An increasing
shift toward waived testing has resulted in a corresponding
increase in health-care expenditures for this testing. Medicare
Part B, the federal medical insurance program for persons aged
>65 years and certain disabled persons, covers diagnostic labo-
ratory testing. Payment data for 2004, provided by CMS, in-
dicated that of the $3,494,840,086 spent on reimbursed
laboratory testing for that year, $128,169,398 (3.7%) was for
waived tests. The volume of Medicare Part B reimbursed
waived laboratory testing in 2004 represented 8% of the total
reimbursed testing volume for that year, a 57% increase over
the volume in 2000 (Table 1).
Patient Safety Concerns Related
to Waived Testing
Efforts to reduce medical errors, improve health-care qual-
ity, and increase patient safety have been gaining national
attention. A report issued in 1999 by the Institute of Medi-
cine (IOM) presented a national agenda to address these
issues and recommended strategies for change that included
the implementation of safe practices at the health-care deliv-
ery level (7). As described in the IOM report, errors most
often occur when multiple contributing factors converge, and
preventing errors and improving patient safety require a sys-
tems approach. Five years after this seminal report, small but
consequential changes have occurred that have shifted the
focus to improving systems, engaging stakeholders, and moti-
vating health-care providers to adopt new safe practices (8).
Although by law waived tests should have insignificant risk
for erroneous results, these tests are not completely error-proof
and are not always used in settings that employ a systems
approach to quality and patient safety. Errors can occur any-
where in the testing process, particularly when the
manufacturer’s instructions are not followed and when test-
ing personnel are not familiar with all aspects of the test sys-
tem and how testing is integrated into the facility’s workflow.
Although data have not been systematically collected on
patient outcomes with waived testing, adverse events can
occur (9). Some waived tests have potential for serious health
impacts if performed incorrectly. For example, results from
waived tests can be used to adjust medication dosages, such as
prothrombin time testing in patients undergoing anticoagu-
lant therapy and glucose monitoring in diabetics. In addition,
erroneous results from diagnostic tests, such as those for
human immunodeficiency virus (HIV) antibody, can have
unintended consequences.
The lack of oversight and requirements for personnel quali-
fications and training for an increasingly large number of CW
sites is a concern and could contribute to errors and patient
harm. During 1999–2001, CMS conducted on-site surveys
of a representative sample of CW sites in 10 states to assess
the quality of testing in these sites. These pilot surveys identi-
fied quality issues that could result in medical errors (10).
Contributing factors included inadequate training in good
laboratory practices and high turnover rates of testing person-
nel. As a result, during 2002–2004, CMS conducted nation-
wide on-site surveys of CW facilities to collect additional data
that would provide an assessment of testing, promote good
laboratory practices and encourage improvement through
educational outreach, and make recommendations on the basis
of cumulative survey findings. The data collected from these
surveys, along with data on waived testing practices gathered
through CDC-funded studies conducted during 1999–2003
by the state health departments of Arkansas, New York, and
Washington (collectively referred to as the Laboratory Medi-
cine Sentinel Monitoring Network [LMSMN]), support the
initial CMS findings of gaps in good laboratory practices in
these sites (11–16). In addition, a 2001 report issued by the
HHS Office of Inspector General (OIG), following their in-
vestigation of CLIA certification and enrollment processes,
identified the lack of routine on-site visits to CW sites by
surveyors representing state agencies and private sector accredi-
tation organizations as presenting vulnerabilities in these sites.
The OIG report indicated that approximately half of the state
respondents reported problems related to quality issues with
the waived laboratories in their states (e.g., failure to follow
manufacturers’ instructions or failure to identify incorrect
results and performing unauthorized testing) (17). The con-
cerns noted by states were similar to those identified in the
CMS pilot studies.
FIGURE 1. Number of Clinical Laboratory Improvement
Amendments of 1988 (CLIA) certified laboratories, by
certificate type and year, 1993–2004
Source: Centers for Medicare & Medicaid Services On-line Survey,
Certification, and Reporting database.
0
50
100
150
200
250
1993 1995 1997 1999 2001 2003
1994 1996 1998 2000 2002 2004
Year
Number (in thousands)
Certificate of Accreditation/Certificate of Compliance
Provider performed Microscopy Procedures Certificate
Certificate of Waiver

4 MMWR November 11, 2005
CLIAC Response
An initial CMS report of its 2002–2003 survey findings,
presented to CLIAC in 2004, supported earlier concerns about
the quality of testing practices and the need for education and
training of testing personnel in CW sites. In response, the
committee recommended publication of the 2002–2004 CMS
data in conjunction with other data pertinent to waived test-
ing performance along with recommendations for good labo-
ratory practices for waived testing sites. This information
would then be available to provide guidance to physicians,
nurses, and other health-care providers in CW facilities. As a
result, a workgroup was appointed to consider practices asso-
ciated with the waived testing process and their impact on the
quality of waived testing. This workgroup was comprised of
key stakeholders in waived testing (i.e., CLIAC members;
physicians; nurses; laboratorians; manufacturers; distributors;
and representatives from CDC, CMS, and FDA). In its evalu-
ations, the workgroup considered existing practice guidelines
from professional organizations, waived testing recommen-
dations from CMS, personal and professional experience, and
publications related to waived testing. The workgroup’s find-
ings were presented to CLIAC for its deliberations at the Feb-
ruary 2005 meeting, at which time CLIAC provided
recommendations to HHS concerning good laboratory prac-
tices for waived testing sites. CLIAC supported publication
of the recommendations, along with the data from the studies
of CW sites, and suggested the publication could serve as a
comprehensive source document that could be used to
develop additional educational tools appropriate for specific
target audiences.
Surveys of Waived Testing Sites
Methods
During 2002–2004, approximately 150 CMS and state
agency surveyors conducted on-site surveys nationwide using
a questionnaire at 4,214 sites performing testing under a CLIA
CW. Surveyors self-selected CW sites on the basis of test vol-
ume, location, and facility types. Different facilities were sur-
veyed each year so that no repetition exists among CW sites
represented in the CMS data in this report. LMSMN obtained
additional waived testing data from 1999–2003. Within
LMSMN, the Washington State Department of Health
established the Pacific Northwest Sentinel Network
(PNWSN), which included approximately 650 waived and
nonwaived laboratories in Alaska, Idaho, Oregon, and Wash-
ington. The Arkansas Sentinel Network (ASN) consisted of
94 local health units integrated into the state health agency
(mostly waived testing sites) and approximately 600 waived
and nonwaived laboratories in Arkansas and surrounding
states. PNWSN and ASN gathered data about waived testing
practices through questionnaires mailed to network members
(11). The New York Sentinel Network (NYSN) consisted of
approximately 600 limited service laboratories (facilities other
than physician office laboratories [POLs] that perform only
waived tests and PPMP). NYSN collected its data through
on-site surveys during which waived testing practices were
assessed by surveyor observation and record reviews (11).
Survey Findings
Demographics
CMS surveyed 4,214 CW sites during April 15, 2002–
November 12, 2004. This included 897 sites in 2002, 1,575
sites in 2003, and 1,742 sites in 2004. Of the CW facility
types surveyed, POLs compose the largest percentage (47%),
followed by skilled nursing facilities (14%) (Table 2). The
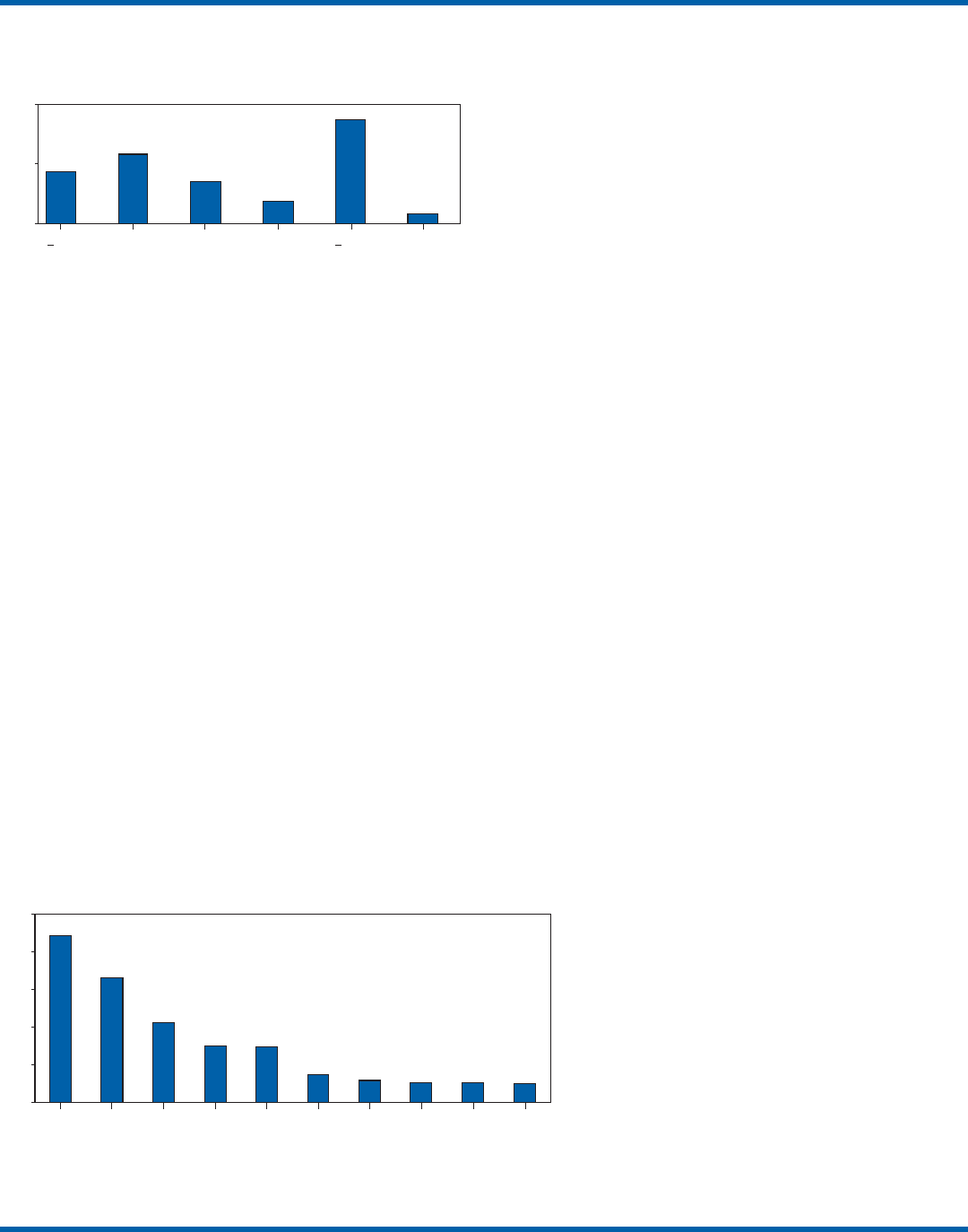
CW sites surveyed estimated performing a broad range of
annual test volumes (Figure 2). Of the facilities surveyed by
TABLE 2. Percentage of facilities with a Certificate of Waiver
(CW), by type of facility, from the Centers for Medicare &
Medicaid Services (CMS) surveyed sites, 2002–2004, and all
CW sites, February 25, 2004
CMS surveyed All CW
sites %* sites
†
%*
Facility type (n = 4,214) (n = 109,820)
Physician office 47 46
Nursing facility 14 13
Ambulatory surgery center 4 3
End-stage renal disease dialysis center 4 3
Home health agency 3 8
Community clinic 3 2
Pharmacy 2 3
School (student health) 2 1
Industrial 2 1
Hospital 1 1
Other practitioner 1 1
Ancillary test site 1 1
Ambulance 1 2
Independent 1 1
Mobile unit 1 1
Intermediate care (mentally retarded) 1 1
Rural health clinic/Federally qualified
health center 1 1
Hospice <1 1
Health fair <1 <1
Blood bank <1 <1
Health maintenance organization <1 <1
Comprehensive outpatient rehabilitation <1 <1
Public health laboratory <1 <1
Insurance <1 <1
Other 9 9
Invalid/Missing data 2 0
* Totals might not equal 100% because of rounding.
†
Data from CMS On-line Survey, Certification, and Reporting database.
Vol. 54 / RR-13 Recommendations and Reports 5
CMS during 2003–2004 (2002 data not available), 90%
reported that they performed no more than five different
waived tests, and 99% performed no more than 10 different
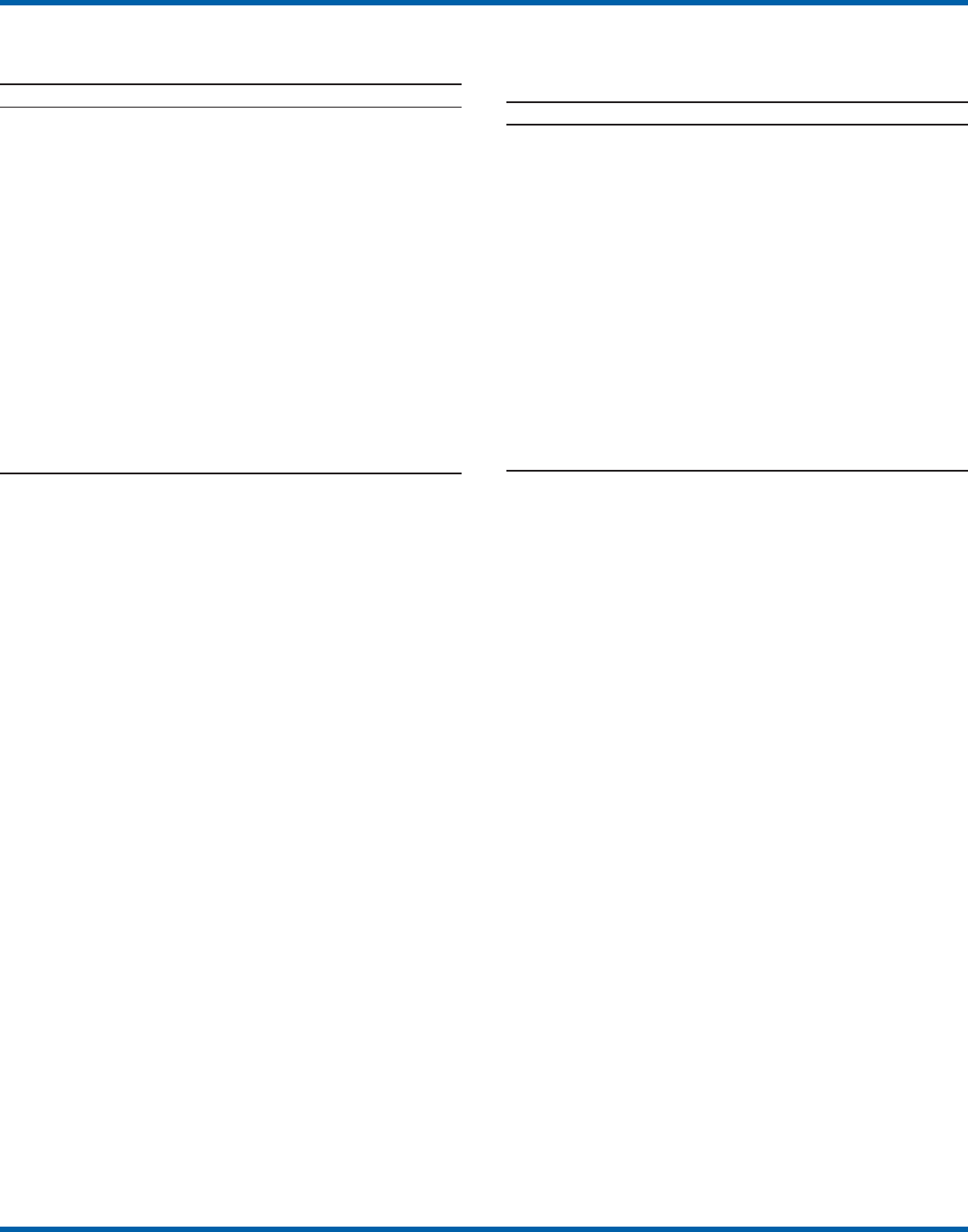
waived tests. Although the exact volume of each test performed
per site is not known, on the basis of the number of sites
testing for each analyte, the five most commonly performed
waived tests were identified as glucose, dipstick urinalysis,
fecal occult blood, urine human chorionic gonadotropin
(hCG) (visual color comparison), and group A streptococcal
antigen (direct test from throat swabs) (Figure 3). This corre-
lates with data for the top five waived tests identified through
the LMSMN, especially for POLs (11). Although not among
the most commonly performed, waived tests are available for
certain infectious diseases of public health significance and
were reportedly performed by CW sites in the CMS surveys
(influenza, 46 sites; HIV, four; and Lyme disease, one).
Personnel and Training
Under CLIA, no education or training is required for the
director or testing personnel in CW sites. The educational
background and qualifications for directors and testing per-
sonnel at CW sites were collected as part of the CMS surveys
and by LMSMN (PNWSN and NYSN). The CMS surveys
indicated that in 69% of CW sites, physicians served as direc-
tors, followed by nurses (17%) (Table 3). Similarly, 59% of
the PNWSN CW site directors were physicians, with the
remaining 41% having other backgrounds or degrees (12).
For CW testing personnel, according to the CMS data, the
top four categories were nurses (46%), medical assistants
(25%), physicians (9%), and high school graduates (7%)
(Table 3). NYSN reported that registered nurses (RNs) and
licensed practical nurses (LPNs) served as testing personnel in
84% of the limited service laboratories they surveyed (13).
Trained laboratorians (i.e., medical technologists and medical
laboratory technicians) accounted for 2% of laboratory direc-
tors and testing personnel in the CW sites surveyed by CMS
and a smaller percentage in the limited service laboratories
surveyed by the NYSN (13).
CMS surveys indicated that 43% of CW sites experienced a
change in testing personnel during the preceding 12 months.
Among the top categories of testing personnel in the PNWSN,
turnover rates were highest for medical assistants (17%), fol-
lowed by LPNs (13%), RNs (9%), and physicians (2%) (14).
Although the majority of CW sites in the CMS surveys (90%)
reported that new personnel were trained, fewer sites (85%)
evaluated staff to ensure competency. Data identifying who pro-
vided training were not submitted for all sites in the surveys.
However, according to the CW sites that provided this infor-
mation for 2003–2004 (Table 4), nurses most frequently pro-
vided waived test training (33%), followed by the manufacturer
or sales representatives (15%). Findings from a PNWSN study
indicated that the highest percentage of personnel were initially
trained by another employee (25%) or trained themselves by
using instructions provided with the waived test system (17%)
(15). Another PNWSN study indicated that most
training (77%) took place in a day or less (14).
Comments from this study reflected the thinking
that training is not always necessary or that mini-
mal time should be spent on training because per-
sons have been trained in school or on other jobs.
The time spent on training was not captured as
part of the CMS surveys.
Testing Practices
The CMS surveys indicated that the majority
of the CW sites were aware of and followed some
practices for ensuring the accuracy and reliability
of their testing. However, lapses in quality were
identified at certain sites, some of which could
result in patient harm. In some instances, CW
sites were determined to be performing testing
FIGURE 2. Estimated annual number of tests for Certificate of
Waiver (CW) sites, from the Centers for Medicare & Medicaid
Services surveyed sites, 2002–2004*
* N = 4,214.
0
20
40
101 500– 501 1,000–
1,001–1,500
No response<100
>1,501
Number of tests
% of CW sites
FIGURE 3. Percentage of Certificate of Waiver sites that perform specific
waived tests, by selected test, from the Centers for Medicare & Medicaid
Services surveyed sites, 2003–2004*
* N = 3,317. 2002 data not available.
0
10
20
30
40
50
Glucose Dipstick
urinalysis
Fecal
occult
blood
Urine
hCG
Group
A strep
antigen
Hemoglobin Cholesterol HDL
cholesterol
Triglyceride Prothrombin
time
Test
Percentage
6 MMWR November 11, 2005
that was an imminent and serious threat to the public’s health
because they were performing nonwaived testing in the
absence of CLIA-required quality measures. The CMS sur-
veys indicated that 5% of CW sites were conducting tests that
were not waived, the most frequently performed nonwaived
procedures (72%) being direct microscopic examinations (e.g.,
potassium hydroxide preparations, wet mounts, or urine sedi-
ment examinations). Surveyed CW testing sites also reported
performing various other nonwaived tests (e.g., urine and
throat cultures, Rh antigen testing, and the use of glucometers
to perform diagnostic glucose tolerance testing [an intended
use not specified in manufacturers’ instructions]). When per-
forming nonwaived tests, surveyors noted that, in some
instances, the sites were not meeting CLIA requirements for
qualified personnel, QC, PT, or test system maintenance. In
addition, these sites did not have adequate records of their
testing activities, including test system procedures, training
records, or other documentation.
Of the CW facilities CMS surveyed, 12% did not have the
most recent instructions for the waived test systems they were
using, and 21% of the sites reported they did not routinely
check the product insert or instructions for changes to the
information (Table 5). On the basis of manufacturer’s instruc-
tions, 21% of the CW sites did not perform QC testing as
specified, and 18% of the sites did not use correct terminol-
ogy or units of measure when reporting results. Among other
quality deficiencies identified were failure to adhere to proper
expiration dates for the test system, reagents, or control mate-
rials (6%) and failure to adhere to the storage conditions as
described in the product insert (3%). Six percent of CW sites
did not perform follow-up confirmatory tests as specified in
the instructions for certain waived tests (e.g., group A strep-
tococcal antigen), and 5% did not perform function checks
or calibration checks to ensure the test system was operating
correctly. Findings from the LMSMN studies were similar to
the CMS findings for these quality deficiencies (11).
Although not usually specified in the product insert (and
therefore not a CLIA requirement), proper documentation
and recordkeeping of patient and testing information are also
important elements of good laboratory practices. CMS sur-
veys indicated that 45% of CW sites did not document the
name, lot number, and expiration dates for tests performed;
TABLE 3. Percentage of Certificate of Waiver site directors and
testing personnel, from the Centers for Medicare & Medicaid
Services surveyed sites, 2002–2004
Personnel category %
Site directors (n = 3,788 responses*)
Physician (MD, DO, DPM, DDS)
†
69
Nurse (LPN, RN, NP, midwife) 17
Administrator/Nursing home administrator 3
Medical technologist/Medical laboratory technician 2
Pharmacist 2
High school, GED 1
Emergency medical technician/Paramedic 1
PhD, MS, BS degree (diverse majors) <1
Medical assistant <1
Other
§
4
Testing personnel (n = 5,511 responses*)
Nurse (LPN, RN, NP, Midwife) 46
Medical assistant 25
Physician (MD, DO, DPM, DDS) 9
High school, GED 7
Medical technologist/Medical laboratory technician 2
Emergency medical technician/Paramedic 2
Nursing assistant 1
Pharmacist 1
Physician assistant 1
Other
¶
6
* All sites did not provide this information, and some sites responded with
multiple answers for each category. For example, for the site director, a
site could have responded with Medical Technologist and Bachelor of
Science degree for the same person. For testing personnel, some sites
indicated multiple personnel types. All responses were included in the
data.
†
MD=Doctor of Medicine; DO=Doctor of Osteopathy; DPM=Doctor of
Podiatric Medicine; DDS=Doctor of Dental Surgery; LPN=licensed practical
nurse; RN=registered nurse; NP=nurse practitioner; GED=general
equivalency diploma; PhD=Doctor of Philosophy; MS=Master of Science;
and BS=Bachelor of Science.
§
Others identified as site directors were chiropractors, social workers/
counselors, physician assistants, fire chiefs, military trained personnel,
naturopaths, optometrists, physical therapists, and nutritionists.
¶
Other testing personnel were radiology technicians, patient-care
technicians, phlebotomists, hemodialysis technicians, chiropractors,
nutritionists, surgical technicians, office managers, patients/clients (self-
testing), nuclear medical technicians, social workers/counselors, medical/
nursing/pharmacy students, respiratory therapists, community-health
representatives, naturopaths, clerical staff, cardiac technicians, home
health assistants, and certified rehabilitation technicians.
TABLE 4. Number and percentage of training providers for
Certificate of Waiver testing personnel, by type of training
provider, from the Centers for Medicare & Medicaid Services
surveyed sites,* 2003–2004
Training provider No. (%)
Nurse 699 (33)
Manufacturer/Sales representative 329 (15)
Physician 220 (10)
In-service/Training coordinator 152 (7)
Other employees 144 (7)
Self-trained/Video 98 (5)
Director/Medical director 97 (5)
Medical assistant 92 (4)
Supervisor/Manager 42 (2)
Office manager 52 (2)
Laboratory director 49 (2)
Laboratory personnel 39 (2)
Hospital laboratory staff 37 (2)
Medical technologist/Medical laboratory technician 37 (2)
Laboratory consultant 19 (1)
Emergency medical technician/Paramedic 24 (1)
Pharmacist 23 (1)
Physician assistant 6 (<1)
Other 93 (4)
Physician testing only
†
54 (2)
Training not documented 51 (2)
* N = 2,139 sites. A total of 3,317 sites were surveyed. However, all sites
did not provide information on training sources, and some sites identified
more than one training provider. All responses were included in the data.
†
Sites did not specify who provided training to these physicians.

Vol. 54 / RR-13 Recommendations and Reports 7
35% did not maintain logs with records of their QC testing;
31% did not maintain a log or record of tests performed; and
9% did not require a requisition or test orders documented in
a patient chart before performing a test (Table 5). NYSN
observed similar findings but noted increased compliance with
state requirements for documentation/recordkeeping when
laboratories had formal affiliations with New York State-
licensed laboratories (11).
Discussion
The findings from the CMS surveys and LMSMN studies
indicated that the majority of CW testing sites performed test-
ing correctly and provided reliable service. However, in CW
sites, most directors and testing personnel did not have for-
mal laboratory training or testing experience, there was a high
turnover of personnel, and lapses in following manufacturers’
instructions and instituting practices to ensure the quality of
the testing were noted. The survey findings indicated that 485
(12%) of the 4,214 CW sites surveyed did not have the cur-
rent manufacturers’ instructions available, and 701 (21%) of
the 3,317 sites surveyed during 2003–2004 did not check to
be sure there had been no changes to the instructions. Test
system instructions can change over time and CW sites some-
times switch test systems that could have different instruc-
tions. CMS survey results also indicated that, in varying
proportions, when CW sites had the current instructions, they
did not follow critical steps in the testing process (e.g., per-
forming QC testing, reporting results correctly, adhering to
expiration dates and appropriate storage requirements, and
performing test system function checks or calibration checks).
This is a concern because the only CLIA requirement for per-
forming waived testing is to follow the manufacturer’s instruc-
tions. Neglecting to follow instructions could cause inaccurate
test results that could lead to incorrect diagnoses, inappropri-
ate or unnecessary medical treatment, and poor patient
outcomes.
CMS surveys indicated that certain CW sites (5%) were per-
forming testing more complex than waived testing without tak-
ing required measures to ensure quality. In certain CW sites,
nonwaived microscopic examinations were being performed by
personnel who lacked the education and training needed to
develop the interpretive and judgment skills necessary to accu-
rately perform these procedures. In addition, measures such as
QC, PT, adequate documentation, and monitoring are required
to ensure the accuracy and reliability of nonwaived test results.
Although direct microscopic examinations can be conducted
by a physician or midlevel health-care practitioner as part of a
patient examination, testing must be conducted under a CLIA
PPMP certificate.
The quality issues identified through these surveys might
have been caused, in part, by high turnover rates of testing
personnel in CW sites, inadequate training with respect to
waived testing, and lack of understanding of good laboratory
practices, including the importance of following all aspects of
the manufacturers’ instructions. Although the study results
indicated that most testing personnel were trained, they were
often trained for minimum periods by persons who did not
have formal education or training in clinical laboratory test-
ing and who might not have understood the importance of
measures to ensure quality testing. Certain testing personnel
also were self-trained. In addition, when testing personnel were
not evaluated to determine their competency level following
training or on an ongoing basis, no assessment was conducted
to determine whether the training was effective. The data dem-
onstrate a need for educational information among CW site
directors and testing personnel about the importance of fol-
lowing manufacturers’ instructions, adhering to expiration
dates, performing QC testing, and proper documentation and
recordkeeping. One of the recommendations in the 2001 OIG
TABLE 5. Number and percentage of quality deficiencies related
to following manufacturer’s instructions and documentation in
Certificate of Waiver sites, from the Centers for Medicare &
Medicaid Services surveyed sites,* 2002–2004
No. of (% of
Quality deficiencies sites sites)
Following manufacturer’s instructions
†
The site did not
Have current manufacturer’s instructions 485 (12)
Routinely check new product inserts for changes
§
701 (21)
Based on manufacturer’s instructions,
the site did not
Perform quality control testing 866 (21)
Report test results with terminology or units
described in package insert 744 (18)
Adhere to proper expiration dates 267 (6)
Perform required confirmatory tests 265 (6)
Perform function checks or calibration 195 (5)
Adhere to storage and handling instructions 135 (3)
Perform instrument maintenance 125 (3)
Use appropriate specimen for each test 81 (2)
Add required reagents in the prescribed order 24 (1)
Documentation
¶
The site did not
Document the name, lot number, and expiration
date for all tests performed
§
1,493 (45)
Maintain a quality-control log
§
1,151 (35)
Maintain a log of tests performed 1,318 (31)
Require test requisition (or patient chart) before
performing a test
§
304 (9)
Keep the test report in the patient’s chart
§
56 (2)
Check patient identification
§
31 (1)
* N = 4,214 sites.
†
Required for waived testing under the Clinical Laboratory Improvement
Amendments of 1988 (CLIA).
§
2003–2004 data only (n = 3,317).
¶
Not required for waived testing under CLIA.

8 MMWR November 11, 2005
report was that CMS should provide educational outreach to
directors of waived and PPMP laboratories about the CLIA
requirements (17).
The findings in the 2002–2004 CMS surveys are subject to
at least three limitations, and caution should be used in
extrapolating the survey data to make generalizations about
waived testing. First, the CMS surveys were not intended to
be a scientific study of a random sample of CW sites. Waived
testing data were collected by CMS to provide an assessment
of testing practices, promote good laboratory practices, and
encourage improvement through educational outreach.
Although surveyors attempted to include a wide variety of
CW sites in the sample, the sites were self-selected by survey-
ors and selection was based, to some degree, on convenience
to the surveyors and willingness of the sites to participate in
the voluntary surveys. However, few sites refused to partici-
pate in the surveys. Overall, the sites represent a nationwide
sample and the distribution of CW facility types is similar to
the distribution of CW facility types in the United States
(Table 2). In addition, the 2002–2004 CMS survey findings
resulted in the same general conclusions as the earlier CMS
pilot studies, which were conducted on a random sample of
laboratories (10). Second, the CMS data were collected and
entered into the database by a large number of persons, intro-
ducing variability. Although training was provided before the
surveys were conducted, the intent of the survey questions
was subject to individual interpretation. Because the phrasing
of some questions differed slightly from 2002 to 2003–2004,
in certain cases, the meanings of the questions also changed.
Finally, the CMS surveys did not assess the frequency of erro-
neous test results in CW sites or whether lapses in following
manufacturers’ instructions directly affected test results or
patient outcomes. Similar limitations to these were identified
in the LMSMN studies (11).
The findings of the CMS and LMSMN studies are strik-
ingly similar. Even though the majority of CW sites meet the
CLIA requirement to follow manufacturers’ instructions for
test performance, and many sites follow additional good labo-
ratory practices, over the years these studies have demonstrated
that a persistent percentage of CW sites do not meet minimal
requirements and are not aware of recommended practices to
help ensure quality testing. Because surveying all CW sites is
not feasible, the proposed actions to improve and promote
quality testing in CW sites emphasize the importance of edu-
cation and training for CW site directors and testing person-
nel. To provide a guide that can be adapted for use, either in
part or as a whole, by persons or facilities considering the
initiation of waived testing and personnel performing waived
testing, CLIAC provided recommendations for good labora-
tory practices. By implementing these recommendations, CW
sites could improve quality, reduce testing errors, and enhance
patient safety.
Recommended Good
Laboratory Practices
Overview
These recommendations are intended to promote the use
of good laboratory practices by physicians, nurses, and other
providers of waived testing in a variety of CW sites. They
were developed on the basis of recommendations and other
resources that provided additional information for promot-
ing patient safety and the quality of CLIAC waived testing in
laboratories or nontraditional testing sites (18–22). These rec-
ommendations address decisions that need to be made and
steps to be taken as a facility begins offering waived testing or
adds a new waived test. They also address developing proce-
dures and training CW personnel and describe recommended
practices for each phase of the total testing process, or path of
workflow, including the important steps or activities before,
during, and after testing. The activities that occur in each of
these phases are critical to providing quality testing (Table 6).
Considerations Before Introducing
Waived Testing or Offering
a New Waived Test
Forethought, planning, and preparation are critical to initi-
ating high-quality waived testing in any type of setting. This
section describes factors to consider before opening a waived
testing site or offering an additional waived test. Questions to
address include the following:
• Management responsibility for testing. Who will be
responsible and accountable for testing oversight at the
CW site, and does this person have the appropriate train-
ing for making decisions on testing?
• Regulatory requirements. What federal, state, and local
regulations apply to testing, and is the site adequately pre-
pared to comply with all regulations?
TABLE 6. Activities within each phase of the total testing
process
Before testing During testing After testing
Control testing/checks
Test performance
Results interpretation
Recording results
Test ordering
Patient identification,
preparation
Specimen collection,
handling
Preparing materials,
equipment, and
testing area
Reporting results
Documenting
Confirmatory testing
Patient follow-up
Disease reporting
Biohazard
waste disposal

Vol. 54 / RR-13 Recommendations and Reports 9
• Safety. What are the safety considerations for persons con-
ducting testing and those being tested?
• Testing space and facilities. What are the physical and
environmental requirements for testing?
• Benefits and costs. How will the care offered in the site
benefit by introduction of testing or the addition of a
new test, and what will it cost?
• Staffing. How will introduction of testing affect the cur-
rent work flow, are there sufficient personnel to conduct
testing, and how will they be trained and maintain test-
ing competency?
• Documents and records. What written documentation
will be needed, and how will test records be maintained?
Management Responsibility
Each testing site should identify at least one person respon-
sible for testing oversight and decision-making, later referred
to as the CW site director. In POLs, this might be a physician
or someone in a senior management position who has the
appropriate background and knowledge to make decisions
about laboratory testing. Ideally, the person signing the CW
application (CMS Form 116) is responsible for management
of the testing operations. The management staff should dem-
onstrate a commitment to the quality of testing service by
complying with applicable regulatory requirements and pro-
moting good laboratory practices.
Regulatory Requirements
CLIA certification. Each site offering only waived testing
that is not included under any other type of CLIA certificate
must obtain a CLIA CW before testing patient specimens.
Certain sites offering waived testing can be certified as part of
a larger health-care organization that holds a CLIA Certifi-
cate of Compliance or Certificate of Accreditation. In addi-
tion, certain public health testing sites offering only waived
testing can be included under a limited public health or
mobile testing exception. A valid CLIA certificate is required
for Medicare reimbursement.
To apply for a CLIA certificate, CMS Form 116 (http://
www.cms.hhs.gov/clia/cliaapp.asp) must be completed and
sent to the state agency for the state in which the testing site is
located. This form asks for specific information, including
the type of testing site (laboratory type), hours of operation,
estimated total annual volume of waived testing, and the total
number of persons involved in performing waived testing. The
form must be signed by the facility owner or the facility direc-
tor. Specific state agencies and contacts are available at
http://www.cms.hhs.gov/clia/ssa-map.asp. The state agency
will process the application and send an invoice for the regis-
tration fee. If additional assistance is required, contact the
appropriate CMS regional office (http://www.cms.hhs.gov/
clia/ro-map.asp).
CLIA requirements that apply to testing sites operating
under a CW include the following:
• Renew the CW every 2 years.
• Perform only waived tests. Waived tests include test sys-
tems cleared by FDA for home use, and simple, low-risk
tests categorized as waived under CLIA. Sometimes a test
that can be performed using different specimens or pro-
cedures might be waived only for certain specimen types
or procedures. Because the list of waived tests is constantly
being revised as new test systems are added, the most cur-
rent information about waived tests and appropriate speci-
mens is available at http://www.accessdata.fda.gov/scripts/
cdrh/cfdocs/cfCLIA/search.cfm.
• Follow the instructions in the most current manufacturer’s
product insert, without modification, when performing
the test. Changes to the timing of the test or physical
alteration of the test components (e.g., cutting test cards
or strips to increase the number of specimens tested per
kit) are examples of modifications. If modified, tests are
no longer waived tests and become subject to the more
stringent CLIA requirements for nonwaived testing.
• Permit announced or unannounced on-site inspections
by CMS representatives.
State and local regulations. States and local jurisdictions
vary as to the extent to which they regulate laboratory testing.
Some states and localities have specific regulations for testing,
some require licensure of personnel who perform testing, and
some have phlebotomy requirements. State and local jurisdic-
tions often regulate biohazard safety, including handling and
disposal of medical waste. The person responsible for testing
oversight should ensure that all state and local requirements
are met. These requirements might be more or less stringent
than federal requirements. When state or local regulations
governing laboratory testing are more stringent than the fed-
eral CLIA requirements, they supersede what is required
under CLIA.
Safety requirements. The Occupational Safety and Health
Administration (OSHA) and individual state standards require
employers to provide a safe and healthy work environment
for employees. Each CW site must comply with OSHA stan-
dards pertinent to workplace hazards (23). Regulatory require-
ments for all OSHA standards, including specific information
for medical and dental offices (24), are available at http://
www.osha.gov and by telephone, 800-321-6742.
The OSHA Bloodborne Pathogens Standard applies to sites
where workers have potential occupational exposure to blood
and infectious materials (25). The requirements for compli-
ance with this standard include, but are not limited to:

10 MMWR November 11, 2005
• A written plan for exposure control, including
postexposure evaluation and follow-up for the employee
in the event of an “exposure incident;”
• Use of Universal Precautions, an approach to infection
control in which all human blood and certain human body
fluids are treated as if known to be infectious for HIV,
hepatitis B virus, hepatitis C virus, and other bloodborne
pathogens. Universal Precautions is one component of
Standard Precautions, a broader approach designed to
reduce the risk for transmission of microorganisms from
both recognized and unrecognized sources of infection in
hospitals;
• Use of safer, engineered needles and sharps;
• Use of personal protective equipment (PPE) such as gloves
and protective eyewear;
• Provision of hepatitis B vaccination at no cost for those with
possible occupational exposure who want to be vaccinated;
• Safety training for handling blood, exposure to bloodborne
pathogens, and other infectious materials; and
• Equipment for the safe handling and disposal of
biohazardous waste (e.g., properly labeled or color-coded
sharps containers and biohazard trash bags and bins).
Additional safety practices for performing testing are:
• Prohibit eating, drinking, or applying makeup in areas
where specimens are collected and where testing is being
performed (i.e., where hand-to-mouth transmission of
pathogens can occur);
• Prohibit storage of food in refrigerators where testing sup-
plies or specimens are stored;
• Provide hand-washing facilities or antiseptic hand-
washing solutions; and
• Post safety information for employees and patients.
Specific information on the Bloodborne Pathogens Stan-
dard and needlestick prevention is available at http://www.
osha.gov/SLTC/bloodbornepathogens/index.html.
CDC and the Clinical and Laboratory Standards Institute
(CLSI) (formerly NCCLS) have also published information
about biosafety and precautions for preventing transmission
of bloodborne pathogens in the workplace (26–30).
Privacy and confidentiality requirements. The Health
Insurance Portability and Accountability Act of 1996 (HIPAA)
established federal privacy standards to protect patients’ medi-
cal records and other health information provided to health
plans, doctors, hospitals, and other health-care providers.
Under HIPAA, CW sites are required to establish policies and
procedures to protect the confidentiality of health informa-
tion about their patients, including patient identification, test
results, and all records of testing. These medical records and
other individually identifiable health information must be
protected, whether on paper, in computers, or communicated
orally. In addition, CW sites should be aware that applicable
state laws that provide more stringent privacy protections for
patients supersede HIPAA. Additional information on HIPAA
is available at http://www.hhs.gov/ocr/hipaa.
Physical Requirements for Testing
Testing should be performed in a separate designated area
where adequate space to safely conduct testing and maintain
patient privacy is available. In addition, some tests have spe-
cific environmental requirements described in the
manufacturer’s product insert that need to be met to ensure
reliable test results. Meeting these environmental conditions
can be challenging in nontraditional settings (e.g., health fairs)
or community outreach venues (e.g., shopping malls, meet-
ing rooms, parks, parking lots, mobile vans, and buses). Fac-
tors to consider include:
• Humidity — Unusually high, low, or extreme fluctua-
tions in humidity can cause deterioration of reagents and
test components, affect the rate of chemical reactions and
specimen interaction, or make test endpoints blurred and
difficult to read.
• Temperature — Temperature ranges for storage of test
components and controls and for test performance are
defined by the manufacturer to maintain test integrity.
Extreme temperatures can degrade reagents and test com-
ponents, impact reaction times, cause premature expira-
tion of test kits, and affect the test results.
• Lighting — Inadequate lighting can negatively affect speci-
men collection, test performance, and interpretation of
test results.
• Work space — Work surfaces should be stable and level
and be able to be adequately disinfected; work space should
be adequate in size for patient confidentiality, ease of speci-
men collection, test performance, and storage of supplies
and records.
Benefit and Cost Considerations
Evaluating the benefits of a particular test. Evaluate the
test system, its intended use, performance characteristics, and
the population to be tested when assessing whether to intro-
duce waived testing or a new waived test. Information for this
evaluation can be obtained from the test manufacturer’s prod-
uct insert (Table 7) or by speaking with the manufacturer’s
technical representatives. Specific considerations include:
• Intended use – Be aware of the intended medical use for
which FDA approved the test system as explained in the
product insert. This section describes what is being mea-
sured by the test, the type of specimen for which it is
approved, and whether it is a quantitative or qualitative
measurement.

Vol. 54 / RR-13 Recommendations and Reports 11
• Performance characteristics — Assess the information on
performance provided by the test manufacturer or pub-
lished data. Review data that includes the test’s accuracy,
precision, sensitivity, specificity, and interferences.
• Patient population — Consider the population that will
be tested before offering a test. Some tests have not been
evaluated for use in specific age groups (e.g., pediatric
populations). The predictive value for certain types of test
results in a specific patient population depends on the
test’s sensitivity, specificity, and the prevalence of the con-
dition in the population. For example, when testing for a
certain condition or disease in a low-prevalence popula-
tion, the predictive value of a positive result will be low
compared with the predictive value of a negative result.
Refer to the product insert for limitations for use in par-
ticular patient populations.
• Need for supplemental testing or patient follow up —
Some waived tests provide preliminary results as part of a
multitest series (e.g., rapid HIV testing) or results that
must be considered in conjunction with other medical
information. These test results might require additional
testing before a definitive test result is obtained, and
patients might need posttest counseling about the mean-
ing of the test result. Assess the potential need for addi-
tional time, documentation, and staffing and a mechanism
to refer additional testing to another laboratory when
offering such tests.
• Test system considerations — Consider the simplicity of
operating the test system, length of time to obtain a result,
and the level of technical support provided by the manu-
facturer or distributor. Sales restrictions, such as special
training requirements, development of a quality assurance
program, or provision of information to patients, might
apply to some waived tests and require additional plan-
ning and resources.
Cost considerations. A fiscal assessment of testing is part
of a good management program. Before offering a new test,
consider the level of reimbursement and factors that contrib-
ute to total test cost. These factors include:
• Test kits or instruments, supplies not provided with the
test, control and calibration materials, inventory require-
ments for anticipated test volume (including seasonal test-
ing), and the shelf life of test components and supplies.
• Equipment maintenance, such as repairs or preventive
maintenance contracts.
• Additional safety and biohazard equipment.
• Personnel training, competency assessment, and the
potential need for additional personnel.
• Recordkeeping and information systems.
• Required supplemental/confirmatory testing.
• Regulatory compliance.
• Resource needs to manage public health reporting, if
required nationally or by the state.
Personnel Considerations
Personnel competency and turnover are important factors
affecting the quality and reliability of waived testing results.
No CLIA requirements exist for waived testing personnel quali-
fications; however, applicable state or local personnel regula-
tions must be met. Personnel issues to consider include:
• Is staffing adequate?
— Determine whether employees have sufficient time and
skills to reliably perform all activities needed for test-
ing in addition to their other duties.
— Be aware that temporary or parttime personnel might
be less proficient in performing testing.
— Evaluate staff for color-blindness because this can limit
their ability to interpret test results based on color end-
points.
• How much training will be needed?
— Take into account the staff turnover rate and the
ongoing need to provide training for new personnel.
— Factor in the time and resources for adequate training
and competency evaluation of staff before they per-
form testing.
— Consider how testing personnel will maintain compe-
tency, especially when testing volume is low.
Developing Procedures and Training
Personnel
After the decision is made to offer waived testing, it is good
practice to develop written policies and procedures so that
responsibilities and testing instructions are clearly described
for the testing personnel and facility director. The testing pro-
cedures form the basis of training for testing personnel. These
procedures should be derived from the manufacturer’s instruc-
tions and should be in a language understandable to testing
personnel.
Written Test Procedures
To comply with CLIA requirements and provide accurate
testing, CW sites must adhere to the manufacturer’s current
testing instructions. These instructions, as outlined in the prod-
uct insert, include directions for specimen collection and han-
dling, control procedures, test and reagent preparation, and
instructions for test performance, interpretation, and report-
ing (Table 7). In addition, certain manufacturers provide quick
reference instructions formatted as cards or small signs con-
taining essential steps in conducting a test. Quick reference
instructions should be clearly posted where testing is per-

12 MMWR November 11, 2005
TABLE 7. Components of the manufacturer’s product insert*
Component Information provided
Describes the test purpose, the substance being detected or measured, test methodology, appropriate specimen type and the
Food and Drug Administration-cleared conditions for use. Might address whether the test is to be used for diagnosis or screen-
ing the target population and whether it is for professional use or self-testing.
Explains what the test detects and a short history of the methodology, including the disease process or health condition being
detected or monitored. Might include the response to disease (e.g., development of IgM antibodies), the symptoms and their
severity, and the disease prevalence. Includes literature citations as applicable.
States the methodology used in the test. Details the technical aspects (chemical, physical, physiologic, or biologic reactions) of
the test, and explains how the components of the test system interact with the patient specimen to detect or measure a specific
substance.
Alerts the user of practices or conditions that might affect the test and warns of potential hazards (e.g., handling infectious
specimens or toxic reagents). Frequent precautions include directions to not mix components from different lot numbers, to not
use products past expiration dates, and the need for safe disposal of biohazardous waste. Might address conditions for
specimen acceptability.
Specifies conditions for storing reagents and test systems to protect their stability. Includes recommended temperature ranges
and, as applicable, physical requirements (e.g., protection from humidity and light). Also addresses the stability of reagents and
test systems when opened or after reconstitution and/or mixing. Describes indicators of reagent deterioration.
Lists the reagents and materials supplied in the test system kit and the concentration and major ingredients used to make the
reagents.
Lists materials needed to perform the test but not provided in the test system kit.
Details the procedures for specimen collection, handling, storage, and stability, including, as applicable, instructions for
performing a fingerstick, appropriate anticoagulant or swab type, and directions for specimen preparation. Might address
conditions for specimen acceptability.
Provides step-by-step instructions for performing the test and frequently includes visual aids (e.g., pictures or graphs). Critical
information (e.g., the order of reagent addition, timing of test steps, mixing and temperature requirements, and reading of the
test results) is included.
Describes how to read and interpret the test results and often includes visual aids. Alerts the user when the results are invalid
and gives instructions on what to do when the results cannot be interpreted. Might include precautions against reporting results
unless supplementary/confirmatory testing is performed.
Explains what aspects of the test system are monitored by QC procedures and provides instructions on how to perform QC.
Includes recommendations on how frequently QC should be performed, acceptable QC results, and what to do when QC values
are not acceptable. Might include specific information about external QC and, as applicable, internal procedural QC.
Describes conditions that might influence the test results or for which the test is not designed. Limitations could include:
• possible interferences from medical conditions, drugs, or other substances.
• warning that the test is not approved for use with alternate specimen types or in alternate populations (e.g., pediatric).
• indications of the need for additional testing that might be more specific or more sensitive.
• warning that the test does not differentiate between active infection and carrier states.
• statement that the test result should be considered in the context of clinical signs and symptoms, patient history, and other test
results.
Describes the test result the user should expect (positive/negative or within/outside of a reference interval). Explains, as
applicable, how results can vary depending on disease prevalence and the season of the year. Might include a brief description
of studies conducted to derive this information.
Details the results of studies conducted to evaluate test performance. Included are data used to determine accuracy, precision,
sensitivity, specificity, and reproducibility of the test and results of cross-reactivity studies with interfering substances.
* Product inserts vary in format, but the majority contain the information described above. Some information might appear in different sections than listed
above because of format variations between manufacturers. Certificate of Waiver site directors and testing personnel should read this information for a
complete understanding of each test.
Intended use
Summary
Test principle
Precautions
Storage/Stability
Reagents and
materials supplied
Materials required
but not provided
Specimen collection
and preparation
Test procedure
Interpretation of
results
Quality control (QC)
Limitations
Expected values
Performance
characteristics

Vol. 54 / RR-13 Recommendations and Reports 13
formed. The specific test system name should be on the quick
reference instructions to avoid confusion.
A comprehensive procedure manual is a valuable resource
for CW sites. Although product inserts can be used as test
procedures, these instructions will typically need to be supple-
mented with testing information that is unique to the CW
site’s operations and workflow (31). A procedure manual can
also include examples of forms used (e.g., charts to record
daily test kit storage temperatures, infectious disease report-
ing forms, or logs for recording control testing and test results)
and check lists for personnel training. New testing procedures
should be reviewed and signed by the CW site director before
incorporating them into the procedure manual. The manual
should be updated as tests or other aspects of the testing ser-
vice change and should be reviewed by the director whenever
changes are made. When procedures are no longer used, they
should be removed from the manual and retained with a
notation of the dates during which they were in service.
When writing procedures for each CW site, it might be
helpful to:
• Use a template with standard component headings to
facilitate writing a new procedure and promote ease of
use when performing testing;
• List all materials needed and how to prepare them before
testing;
• Include instructions for patient preparation and specimen
collection;
• Highlight key steps in the procedure (e.g., test incuba-
tion time);
• List test limitations;
• Describe actions to take when the test does not perform
as expected;
• Integrate control procedures with the steps for perform-
ing patient testing to assure control testing is performed;
• Include established reference intervals and critical values
for the test; and
• Describe how to record and report results and how to
handle critical values.
Personnel Training
Trained and competent testing personnel are essential to
good quality testing and patient care. Data from CDC and
CMS surveys demonstrate that waived testing sites are subject
to a high rate of personnel turnover. Personnel should be
trained and competent in each test they will perform before
reporting patient results (32,33). In addition, training should
include aspects of safety (including Universal Precautions) and
QC. The CW site director or other person responsible for
overseeing testing should ensure that testing personnel receive
adequate training and are competent to perform the proce-
dures for which they are responsible. Training checklists are
helpful to ensure the training process is comprehensive and
documented.
The training process. Training should be provided by a
qualified person (e.g., experienced co-worker, facility expert,
or outside consultant) with knowledge of the test performance,
good laboratory practices, and the ability to evaluate the effi-
cacy of the training. On-the-job training should include the
following steps:
1. The trainee reads the testing instructions.
2. The trainer demonstrates the steps for performing the test.
3. The trainee performs the test while the trainer observes.
4. The trainer evaluates test performance, provides feedback
and additional instruction, and follow-up evaluations to
ensure effective training.
5. Both trainer and trainee document completion of training.
Training resources. Resources for training are available from
various sources. Tools for training continue to evolve and are
not limited to traditional methods. Instructional videos, work-
shops, computer-based programs, and other methods can be
used. The manufacturer’s test system instructions and instru-
ment operating manuals should be the primary resource for
information and training in CW sites. Other sources for train-
ing on waived testing or specific tests include:
• Manufacturers and distributors who often provide tech-
nical assistance, product updates or notifications, and lim-
ited training.
• Professional organizations that can provide workshops or
other training tools.
• State health departments or other government agencies
that can provide limited training.
Competency Assessment
To ensure testing procedures are performed consistently and
accurately, periodic evaluation of competency is recommended,
with retraining, as needed, on the basis of results of the compe-
tency assessment (32). Assessment activities should be conducted
in a positive manner with an emphasis on education and pro-
moting good testing practices. Competency can be evaluated
by methods such as observation, evaluating adequacy of docu-
mentation, or the introduction of mock specimens by testing
control materials or previously tested patient specimens. Exter-
nal quality assessment or evaluation programs, such as volun-
tary PT programs, are another resource for assessment.
Additional Measures to Help Testing Staff
Ensure Reliable Results
The CW site director or person overseeing testing should pro-
mote quality testing and encourage staff to ask questions and
seek help when they have concerns. Recommendations include:

14 MMWR November 11, 2005
• Identifying a resource person or expert (e.g., a consultant
or manufacturer’s technical representative), available
either off-site or on-site, to answer questions and be of
assistance.
• Posting telephone numbers for manufacturers’ technical
assistance representatives.
• Designating an appropriately trained person, who under-
stands the responsibilities and impact of changing from
one test system to another, to discuss new products with
sales representatives. Uninformed personnel might mis-
takenly use a promotional test kit, provided by a distribu-
tor or manufacturer’s representative, for patient testing
without realizing the consequences of test substitution.
Recommended Practices Before Testing
Preparations before performing patient testing are a critical
element in producing quality results. Paying attention to test
orders, properly identifying and preparing the patient, col-
lecting a good quality specimen, and setting up the test sys-
tem and testing area all contribute to reliable test results.
Test Orders, Patient Identification,
and Preparation
Before collecting the specimen, confirm the test(s) ordered and
the patient’s identification and verify that pretest instructions or
information, as applicable, have been provided. This includes:
• Test orders — CW sites performing various waived tests
should routinely confirm that the written test order is
correct. If there is a question, check with the ordering
clinician. Standing orders for certain tests might apply,
but they should be documented.
• Patient identification — Identify the patient before col-
lecting the specimen. Because names can be similar and
lead to confusion, use birth dates, middle initials, identi-
fication numbers, or other means to ensure the specimen
is collected from the correct patient.
• Pretest instructions — Some tests require special prepa-
ration on the patient’s part (e.g., a fasting state for glu-
cose testing). Provide the patient with pretest instructions,
when appropriate, and when special preparation is needed,
verify that patients received instructions before testing.
To determine if patients followed the instructions, ask
them to explain how they prepared for the test.
• Pretest information — Discuss factors, test limitations,
or medical indications that can affect test results with the
patient, as appropriate, and provide pertinent informa-
tion such as pamphlets supplied by the test manufacturer,
when specified in the product insert.
Specimen Collection and Handling
The product insert provides details on proper collection,
handling, and storage of patient specimens. Collect waived
test specimens exactly as described in the test system instruc-
tions, using the appropriate collection device and method to
obtain a quality specimen (33–36). Improperly collected,
stored, or compromised specimens should not be tested. Speci-
mens and, in some cases, test devices need to be appropriately
labeled to prevent mix-up.
Waived test specimens. Waived tests are approved for use
only with direct, unprocessed specimens that do not require
operator manipulation (Table 8). Specimens that are processed
or manipulated by the user (e.g., serum or plasma) require
centrifugation, dilution, extraction, or other preparation steps
that require special training or instrumentation and are not
appropriate for waived tests. Sometimes, tests can be performed
using both processed and unprocessed specimen types, but
are waived only for the unprocessed specimens, in which case
the product insert should identify the appropriate specimen
for the waived test. For example, a single product insert might
include instructions for performing a waived test using
unprocessed whole blood and for performing the same test
using plasma, which would not be waived. Other examples
include group A streptococcal antigen testing, which is waived
only when performed on a throat swab and not when per-
formed on a microbiology culture, and visual color compari-
son tests for hCG (pregnancy tests) using urine that are waived,
whereas serum or plasma hCG tests are not waived.
Specimen collection. The person collecting the patient
specimen or giving the collection instructions should have a
thorough understanding of the specimen type, proper collec-
tion method (including the need to wear gloves or other PPE
as appropriate), and handling to assure a quality specimen
(33–36). Directions for specimen collection, handling, and
storage are included in the product insert and must be fol-
lowed explicitly. For example, instructions might specify one
TABLE 8. Types of direct, unprocessed specimens suitable for
waived testing
Specimen type Examples of waived tests
Whole blood (fingerstick or Glucose, cholesterol, prothrombin
anticoagulated blood collected time, infectious mononucleosis,
by venipuncture) and HIV antibody
Urine Dipstick urinalysis and pregnancy
test (hCG)
Throat swab Group A streptococcal antigen
Nasopharyngeal swab, Influenza
nasal wash, or aspiration
Stool Occult blood
Saliva Alcohol
Oral fluid HIV antibody
Gastric biopsy
H. pylori
Vol. 54 / RR-13 Recommendations and Reports 15
drop of capillary blood or include precautions to use the sec-
ond drop of blood from a fingerstick rather than the first.
When gloves are worn during specimen collection, they should
be removed and discarded in an appropriate waste receptacle
before contact with another patient. Hand hygiene should be
performed between patients.
Collection devices. Manufacturers might provide or specify
specimen collection devices. These devices, whether supplied
with the test system or specified in the product insert, are
integral to the test system and should be used to ensure the
correct specimen type and volume to provide reliable results.
Containers and collection devices might have additives that
affect the specimen or are part of the test and should not be
substituted or altered. For example, throat swab collection kits
used with group A streptococcal antigen tests might look the
same; however, they might be made from a variety of fibers or
contain different materials that could interfere with the test
or affect organism viability. Whole blood capillary tubes (e.g.,
used for cholesterol, hemoglobin A
1
C, or Helicobacter pylori
testing) can have additives or hold different specimen vol-
umes which affect test reactions and results.
Fingerstick and venipuncture collection devices are for one-
time use only. Never reuse needles, syringes, or lancets. To
avoid transmission of hepatitis B virus, hepatitis C virus, HIV,
and other bloodborne pathogens, appropriately discard sharps,
lancets, and platforms for spring-loaded lancets and disinfect
instruments contaminated by blood (9, 28).
Specimen labeling. Labeling procedures should meet the
needs of the testing site and should be adequate to prevent
specimen mix-up. To prevent errors, always label specimens
with pertinent information (e.g., unique patient name or other
unique identifier). Depending on workflow, specimen label-
ing also might include the date and time of collection and
identification of the collector. For waived tests in which the
specimen is applied directly to the test device (e.g., throat swabs
for group A streptococcal antigen), the test strip, cassette, or
other device should be labeled with the patient identification
before collecting the specimen, especially if more than one
test is being performed at the same time.
Preparing the Testing Area, Test Materials,
and Equipment
Preparing the testing area and materials (e.g., kits, reagents,
control materials, and equipment) before testing patient speci-
mens is essential to maintaining efficient workflow and good
quality testing (Table 9). Before beginning the test, read and
understand the test instructions specified in the product
insert and included in the CW site’s procedures. Verify that
the instructions are current for the test in use and that no
changes have been made. Do not use product inserts that are
out of date for the test system currently in use. When opening
a new kit, check for notifications in the external labeling or
special notices that might be included with product inserts or
packaging.
Additional considerations for good testing practices are:
• Abide by expiration dates and discard expired reagents
and test kits as soon as the expiration date elapses.
• When preparing to perform testing, allow time for any
refrigerated items, including reagents or patient specimens,
to reach room temperature before testing, if specified in
the product insert.
Recommended Practices During Testing
When the testing area is prepared and the specimen has
been collected, the process continues to the testing phase.
Important activities during this phase include QC testing, test
performance, result interpretation and recording.
Quality Control Testing
Performing QC testing procedures provides assurance that
the test performs as expected and alerts the user when prob-
lems occur. QC testing is designed to detect problems that
might arise because of operator error, reagent or test kit dete-
rioration, instrument malfunction, or improper environmen-
tal conditions. Test procedures should describe the type of
TABLE 9. Pretesting task checklist for waived tests
Testing area
Clean work surfaces and remove clutter or trash
Ensure adequate lighting
Check and record temperatures (e.g., testing environment and
refrigerators)
Replenish supplies (e.g., specimen collection, biohazard waste
containers, and forms)
Test system and reagents
Check the product insert
and exterior labeling on kits and reagents for
changes
Check and record expiration dates (Do not use expired reagents or kits)
Check and record lot numbers for test kits, test devices and controls
(Do not mix reagents from different products or lot numbers. If new
lot, set up quality control as needed and refer to product insert for any
changes in control ranges)
Visually inspect reagents or vials for damage, discoloration, or
contamination
Prepare reagents according to instructions (If opening new reagents,
write the date opened on the outside of the vial or test kit)
Inspect equipment and electrical connections for integrity
If the test system incorporates internal calibration steps that need to be
checked before testing, conduct the calibration check or set the test
system as specified by the manufacturer*
* Portable equipment, if moved, might be subject to inaccurate results. To
verify proper test system functioning, perform control testing or calibration
check procedures even if not specified by the manufacturer after moving
the equipment.

16 MMWR November 11, 2005
controls to be used, how to perform QC testing (including
QC testing frequency), and actions to be taken when QC
results are unacceptable.
Types of controls. Two types of controls typically found in
waived tests are:
• Internal, procedural, or built-in controls — evaluate
whether certain aspects of the test system are working
properly. They are designed to verify that the test system
is working as expected, that sufficient specimen was added
and, for unitized test devices, whether it migrated through
the test strip properly. Certain test systems might have
electronic internal controls to monitor electronic func-
tions.
• External controls — mimic patient specimens and moni-
tor the testing process, from specimen application to
result interpretation, to assure proper test performance.
They might be provided as liquid or other materials simi-
lar to patient specimens and might be included with the
test system or purchased separately.
Frequency of control testing. For certain test systems, the
product insert describes the minimum conditions or recom-
mended frequencies for testing internal and/or external con-
trols. Each site should determine the appropriate control
testing frequency for each test system and the frequency should
not be less than specified in the product insert. When deter-
mining the frequency for running external controls, consider
the robustness of the test, stability of the environment, and
skills and knowledge of the testing personnel. At a minimum,
external controls should be tested with each new shipment of
utilized test devices, when testing a new lot number, and by
each new operator before conducting testing. Controls should
be tested either before or concurrent with patient specimens
by the same personnel who routinely perform patient testing.
Corrective action when control testing fails. If controls
do not perform as expected, patient testing should not be per-
formed or results reported until the problem is identified and
corrected. The product insert should provide information on
procedures for handling unexpected control results, identify-
ing sources of error (including interfering substances), and
manufacturer contact information for technical assistance. This
information might be incorporated into the facility’s proce-
dures or posted for quick reference. The test site should have
telephone numbers or other contact information readily avail-
able (e.g., numbers for manufacturers’ technical assistance,
the facility’s director, consultant, or public health departments).
Documentation. Documenting and monitoring control
testing results provides an indication that the test was prop-
erly performed by the operator and the test system (reagents,
instruments, or any components) performed as expected.
Records of control results should be periodically reviewed to
detect shifts or changes in performance over time.
Performing the Test
The following points are important to remember when per-
forming the test:
• Follow the steps in the test procedure in the exact order
described in the product insert.
• Test controls at the frequency determined by the CW site.
• Pay attention to timing for waived tests, particularly unit-
ized test devices that must be read during specific time
intervals. Incorrect timing of these types of tests can give
erroneous test results. Insufficient timing can result in false
negative or invalid results because the specimen might
not react completely with test system reagents. Time
intervals longer than those specified in the product insert
can result in false positive, false negative, or invalid
results because of exaggerated color development, fading
of reaction products, or migration beyond a visible range.
Therefore, it is important to have a system established to
read results during the correct timeframe, especially if con-
ducting more than one test at a time. Suggestions for help-
ing to ensure correct timing of tests include using timers
that beep until turned off, using timers that can easily be
worn or attached to clothing, using multiple timers when
performing more than one test at a time, and maintain-
ing extra timers and batteries.
Test Results Interpretation
When the test is complete, interpret the results according
to instructions in the product insert (including the quick ref-
erence guide). Test results are of the following two types:
• Quantitative — Tests that provide numerical results gen-
erated by the test device or instrument. Numerical results
are values corresponding to the concentration of the spe-
cific substance being measured. The value includes spe-
cific measurement units (e.g., such as a glucose result of
100 mg/dL). No interpretation is necessary to read the
result.
• Qualitative — Tests that detect whether a particular sub-
stance, condition, or microbiological organism is present
or absent. Results are interpreted as positive/reactive, nega-
tive/nonreactive, or invalid. Invalid results might indi-
cate a problem with the specimen or the test system.
Diagrams, color photographs, and color-comparison
charts are often part of the product insert and quick ref-
erences and serve as guides for interpretation.
Resolving Problems
If a discrepancy is identified between the patient’s test
results and the clinical information or if the results are invalid
Vol. 54 / RR-13 Recommendations and Reports 17
or otherwise compromised, testing should be repeated.
Results should not be reported until the problem is resolved.
Follow the steps in the product insert to resolve problems with
test results. Unitized test system instructions usually suggest
repeating the test with a new device and referring to QC or
trouble-shooting procedures. If repeat testing does not resolve
the problem, contact the manufacturer or product technical
representative. Quantitative results can be obtained that are
beyond the measuring range of the instrument or test device.
Each site should have documentation of quantitative test
measuring ranges and a procedure for handling test results
that are beyond the reportable ranges, either low or high.
Recording Results
Record test results according to the site’s policy. Results can
be recorded directly in a patient’s chart, in log books, or on a
separate report form. Records or logs of test results should
have enough detail so the test site can retrieve information.
Quantitative results should be recorded using the units of
measurement of the test system. Qualitative test results should
be recorded using interpretive words or abbreviations such as
positive, negative, reactive or R, or nonreactive or NR instead
of symbols like plus and minus (+, -) to help avoid clerical
errors because a negative (-) sign can easily be changed to a
positive (+) sign. If a test result is not acceptable or requires
repeat testing (e.g., out of range or invalid), record the initial
result, noting it was unacceptable, take steps necessary to
resolve the problem, then record the correct result. Good labo-
ratory practices include recording what happens, whether
acceptable or not, and what is done to correct problems
encountered during testing.
Recommended Practices After Testing
After-testing activities include issuing test reports, supple-
mental or confirmatory testing, public health disease report-
ing (if required), testing area cleanup, biohazard waste disposal,
and documentation of testing activities.
Test Reports
After the completion of the test, results are documented
and reported. Patient reports should be legible and reported
in a timely manner to the appropriate person. Reports should
meet the needs of the testing site and should be appropriately
standardized so reports generated on-site are easily distinguish-
able from referral laboratory reports. Verbal reports of test
results should be documented and followed by a written report.
Waived testing sites, such as point-of-care sites or physicians’
offices, might accurately and legibly record results directly in
the patient’s record as a matter of practice. If results are not
recorded directly in a patient’s chart, they should be recorded
in a written report format that includes all information needed
to correctly identify and interpret the results as determined by
the testing site (Table 10).
Critical values are test results necessary for patient evalua-
tion or treatment that require immediate notification to the
clinician. Each site should define the critical values, if appro-
priate, for the tests in use and ensure that testing personnel
are aware of these values and the procedure for alerting the
clinician. Procedures should be in place to ensure documen-
tation of critical values and timely notification of the proper
medical personnel.
Supplemental or Confirmatory Testing
The product insert should explain when supplemental test-
ing is needed to confirm a waived test result or when the test
is to be used as part of a multitest algorithm. A confirmatory
test could be a different waived test (performed at the testing
site or another CW site) or a nonwaived test performed by a
CLIA-certified referral laboratory (37) (Table 11). When
nonwaived confirmatory testing is needed, the patient can be
sent to another facility for specimen collection and testing, or
the specimen can be collected at the CW site and sent to a
referral laboratory.
The CW site should have written policies to ensure confir-
matory and supplemental testing is performed when needed.
For each waived test that requires additional testing, the CW
site should document the processes and procedures necessary
to manage referral or confirmatory testing. When a CW site
collects specimens for referral, procedures should include the
following:
• Instructions for ordering additional tests, contact infor-
mation for the referral laboratory used, and examples of
completed test request forms.
• Specimen collection and labeling procedures with
examples of forms used to track referred specimens.
• Safe specimen transport or shipping information as nec-
essary, including special packaging and shipping require-
TABLE 10. Examples of test report information
Testing facility
information Patient information Test information
Name, anonymous
identifier
Record/billing number
Birth date, sex, and age
Name
Address, site, or clinic
number
Telephone number
Facility director
Test ordered
Test result
Units of measure
Interpretation
Reference intervals
Comments or
qualifying statement
Date completed
and/ or reported
Person reporting

18 MMWR November 11, 2005
ments for confirmatory or supplemental tests for infec-
tious diseases (e.g., HIV).
Maintaining records of referred testing is important for
patient care and follow-up. Logs and other records should
have sufficient information to track and retrieve the test
results and reports, such as:
• Information linking the referred specimen to patient
identification,
• The name and contact information for the referral
laboratory,
• The test name and date referred,
• Complete test results and the date received, and
• The date the final report is issued.
Public Health Reporting
Federal and state public health agencies require testing
facilities to report confirmed positive results for certain infec-
tious diseases (e.g., HIV, influenza, and Lyme disease) (38,39).
Testing facilities should confer with local public health agen-
cies for the most current information on required reporting
procedures since diseases identified for reporting can change
over time, and state requirements might vary.
Biohazard Waste Disposal
Dispose of the biohazardous waste generated in specimen
collection and testing according to site procedures that need
to be in compliance with local ordinances, state, and federal
OSHA regulations as previously discussed.
Documents and Records
Documentation is essential to assure quality waived testing.
Proper documentation is necessary for monitoring and assess-
ing test performance, identifying and resolving problems that
could affect patient testing, retrieving and verifying informa-
tion, and maintaining adequate patient and personnel records.
Log books or electronic systems can be used for maintaining
and tracking information. In some cases, records might be
part of the patient’s medical chart. Testing records should be
maintained in chronological order to facilitate retrieval of
information if needed. In addition, control records should be
kept in the order in which they were completed so they can
easily be compared with test records if there are questions about
testing performed within a specific time period. The person
responsible for testing oversight and decision-making should
review records periodically. State regulations or other govern-
mental agencies might require CW sites to retain documents
and records for a specific length of time.
Aspects of testing for which records or documentation are
recommended include:
• Test orders
• Test procedures or work instructions (e.g., written proce-
dures specific to the CW site and current product inserts)
• Records of testing materials used, test system and equip-
ment function checks, and maintenance
— Daily records of temperatures for refrigerators, freez-
ers, and the testing area, as needed for the tests per-
formed
— Lot numbers, dates used, and expiration dates of test
systems and reagents
— Date and time (if applicable) of equipment function
checks and any maintenance performed
— As applicable, notifications from manufacturers about
product recalls or other problems, especially if the recalls
or warnings refer to specific lot numbers or test systems
• Test results, including any confirmatory or supplemental
testing
• QC testing results and corrective action taken if control
results are unacceptable
— Date and time (if applicable) of control testing
— Lot number and expiration date of external controls
• Records of any test system failures, troubleshooting, and
corrective action taken when problems are identified,
including related communication with testing personnel
• Personnel training and competency assessment
• Records of PT or other external quality assessment
Quality Assessment
Good laboratory practices can be expanded to include
assessment activities to evaluate and improve the quality of
CW site testing. Assessment activities can be either internal
or external, depending on the needs, resources, and practices
of the site.
Internal Assessment
Objective internal assessment offers flexible, low-cost
options for evaluating quality such as self-conducted inspec-
tions, supervisory review of documented problems that occur
TABLE 11. Examples of supplemental/confirmatory testing
for waived infectious disease tests
Waived test method result Supplemental/Confirmatory test
Western blot or immunofluores-
cence assay
Viral culture
Western blot
Throat culture
Preliminary positive for HIV-1
antibody
Presumptive negative for
influenza A or B
Presumptive positive for
Borrelia
burgdorferi
(Lyme disease)
antibodies
Negative for group A streptococ-
cal antigen (screen from
children and adolescents)

Vol. 54 / RR-13 Recommendations and Reports 19
in the different phases of the testing process, review of QC
documentation, and testing and reporting procedures. Test
performance can be assessed, if specimens are suitable, by
exchanging specimens with another testing facility using the
same test method(s) and comparing the results. Results from
these assessment activities should be documented and evalu-
ated, noting any irregularities and the actions taken to resolve
problems or improve processes or procedures.
External Assessment
Because CW sites are not routinely inspected by CMS, vol-
untary inspections by peers or consultants can offer additional
educational opportunities and feedback on current practices
along with ideas for quality improvement. Voluntary external
inspections evaluate the testing site practices and documenta-
tion systems, and a more narrowly focused assessment of test
performance can be accomplished by participating in perfor-
mance evaluation programs or subscribing to PT programs.
These programs provide challenge samples to test as if they were
patient specimens and the results are evaluated with respect to
how close they are to the intended target values. Participation
in these types of programs can be used to evaluate overall test-
ing performance and as a training or educational tool for test-
ing personnel.
Conclusion
This report summarizes the findings of multiple surveys of
sites performing waived testing throughout the United States.
Although the surveys were conducted through several mecha-
nisms, the findings lead to similar conclusions about lapses in
quality in CW sites, and they highlight the need for addi-
tional education and training related to waived testing for CW
site directors and testing personnel. The recommendations
provided in this report are intended to serve as a guide to
improve the quality of testing in CW sites and enhance
patient safety. They can be disseminated by a variety of indi-
viduals and organizations and adapted for use in different set-
tings where waived testing is conducted. Continued
surveillance and monitoring of waived testing performance is
needed to determine the effectiveness of these recommenda-
tions on protecting and improving the public’s health.
Acknowledgments
The preparers acknowledge the contributions and assistance
provided by John Hancock, James Handsfield, MPH, and Rhonda
Whalen, MS, of the Division of Public Health Partnerships, National
Center for Health Marketing, Coordinating Center for Health
Information and Service, CDC; Daralyn Hassan, MS, and the CMS
Waived Laboratory Project Team, Division of Laboratory Services,
Survey and Certification Group, Center for Medicare and State
Operations, CMS.
References
1. Steindel SJ, Rauch WJ, Simon MK, Handsfield J. National inventory
of clinical laboratory testing services (NICLTS): development and test
distribution for 1996. Arch Pathol Lab Med 2000;124:1201–8.
2. Anonymous. How reliable is laboratory testing? Lab Tests Online.
Available at http://www.labtestsonline.org/understanding/features/
reliability.html.
3. Forsman RW. Why is the laboratory an afterthought for managed care
organizations? Clin Chem 1996;42:813–6.
4. Becich MJ. Information management: moving from test results to clini-
cal information. Clin Leadersh Manag Rev 2000;14:296–300.
5. Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C.
263a PL100-578 (1988).
6. Laboratory Requirements, 42 C.F.R. Chapter IV, Part 493 (2003).
7. Kohn LT, Corrigan JM, Donaldson MS, eds. To err is human: build-
ing a safer health system. Committee on Quality of Health Care in
America, Institute of Medicine. Washington, DC: National Academy
Press; 2000.
8. Leape LL, Berwick DM. Five years after To Err is Human. JAMA 2005;
293:2384–90.
9. CDC. Transmission of hepatitis B virus among persons undergoing
blood glucose monitoring in long-term-care facilities—Mississippi,
North Carolina, and Los Angeles County, California, 2003–2004.
MMWR 2005;54:220–3.
10. Centers for Medicare and Medicaid Services. CMS certificate of waiver
and provider performed microscopy procedures pilot project final
report. Baltimore, MD: Centers for Medicare and Medicaid Services;
2001. Available at http://www.cms.hhs.gov/clia/ppmpfr2001.pdf.
11. Steindel SJ, Granade S, Lee J, et al. Practice patterns of testing waived
under the clinical laboratory improvement amendments. Arch Pathol
Lab Med 2002;126:1471–5.
12. LaBeau KM. The Pacific northwest laboratory medicine sentinel moni-
toring network: inventory of CLIA-waived tests performed in Wash-
ington State. Seattle, WA: Washington State Department of Health.
Available at http://www.phppo.cdc.gov/mlp/pdf/LMSMN/PNW/
REPORTWI.pdf.
13. Clarke LM, Jenny R, Shulman S, Reilly A, Olsen C. New York State’s
experience with assessment of waived testing and PPMP practices: are
we ready for waived HIV antibody tests? Albany, NY: New York State
Department of Health Wadsworth Center. Available at http://www.
phppo.cdc.gov/mlp/pdf/LMSMN/NY/NYreport1.pdf.
14. LaBeau KM, Granade S. The Pacific northwest laboratory medicine
sentinel monitoring network: final report of the findings of question-
naire 5—waived and PPMP sites—testing personnel turnover. Seattle
WA: Washington State Department of Health. Available at http://www.
phppo.cdc.gov/mlp/pdf/LMSMN/PNW/report0301.pdf.
15. LaBeau KM, Simon M, Granade S, Steindel SJ. The Pacific northwest
laboratory medicine sentinel monitoring network: final report of the
findings of questionnaire 1—waived and PPMP sites—training on
waived test systems. Seattle, WA: Washington State Department of
Health. Available at http://www.phppo.cdc.gov/mlp/pdf/LMSMN/
PNW/reportw1.pdf.
16. LaBeau KM, Simon M, Steindel SJ. Quality control of test systems
waived by the clinical laboratory improvement amendments of 1988:
perceptions and practices. Arch Pathol Lab Med 2000;124:1122–7.

20 MMWR November 11, 2005
17. US Department of Health and Human Services, Office of Inspector
General. Enrollment and certification processes in the clinical labora-
tory improvement amendments program. Washington, DC: US
Department of Health and Human Services; 2001. Available http://
oig.hhs.gov/oei/reports/oei-05-00-00251.pdf.
18. COLA. Inside the physician office laboratory: keeping waived tests
simple. Clin Leadersh Manag Rev 2004;18:65–9.
19. Centers for Medicare and Medicaid Services. Good laboratory prac-
tices. Baltimore, MD: Centers for Medicare and Medicaid Services.
Available at http://www.cms.hhs.gov/clia/wgoodlab.pdf.
20. NCCLS. Point-of-care in vitro diagnostic (IVD) testing; approved
guideline; AST2-A, Wayne, PA: NCCLS 1999.
21. CDC. Quality assurance guidelines for testing using the OraQuick
rapid HIV-1 antibody test. Atlanta, GA: US Department of Health
and Human Services CDC; 2003. Available at http://www.phppo.cdc.
gov/DLS/pdf/HIV/QA_Guidlines_OraQuick.pdf.
22. Joint Commission for International Patient Safety. Laboratory. Oak
Brook, IL: Joint Commission for International Patient Safety.; 2005.
Available at http://www.jcipatientsafety.org/show.asp?durki=9722&
site=164&return=9344.
23. Gile TJ. Safety never takes a holiday. Clin Leadersh Manag Rev
2004;18:342–8.
24. Occupational Safety and Health Administration. Medical and dental
offices: a guide to compliance with OSHA standards. Washington, DC:
Occupational Safety and Health Administration; 2003. Available at
http://www.osha.gov/Publications/osha3187.pdf.
25. Bloodborne pathogens, 29 C.F.R., Sect. 1910.1030 (2001).
26. CDC. Exposure to blood: what healthcare personnel need to know.
Atlanta, GA: US Department of Health and Human Services
CDC;1999. Available at http://www.cdc.gov/ncidod/hip/BLOOD/
Exp_to_Blood.pdf.
27. CDC. Preventing needlestick injuries in health care settings. Atlanta, GA:
CDC; 1999. Available at http://www.cdc.gov/niosh/pdfs/2000-108.pdf.
28. NCCLS. Protection of laboratory workers from occupationally acquired
infections; approved guideline, 3rd ed. Wayne, PA: NCCLS; 2005 (pub-
lication no. M29-A3).
29. CDC. Perspectives in disease prevention and health promotion
update: universal precautions for prevention of transmission of
human immunodeficiency virus, hepatitis B virus and other bloodborne
pathogens in health-care settings. MMWR 1988;37:377–82, 387–8.
30. CDC. Selecting, evaluating, and using sharps disposal containers.
Atlanta, GA: CDC; 1998. Available at http://www.cdc.gov/niosh/
sharps1.html.
31. NCCLS. Clinical laboratory technical procedure manuals; approved
guideline, 4th ed GP02-A4. Wayne, PA: NCCLS; 2002.
32. NCCLS. Training and competence assessment; approved guideline-
second edition. Wayne, PA: NCCLS; (publication no. GP21-A2) 2004.
33. NCCLS. Blood glucose testing in settings without laboratory support;
approved guideline. Wayne, PA: NCCLS; (publication no. AST4-A) 1999.
34. NCCLS. Procedures and devices for the collection of diagnostic capil-
lary blood specimens; approved standard, 5th ed. Wayne, PA: NCCLS;
(publication no. H04-A5) 2004.
35. NCCLS. Quality microcollection. Wayne, PA: NCCLS; (publication
no. H04-A3-V) 1994.
36. NCCLS. Procedures for the collection of diagnostic blood specimens
by venipuncture; approved standard, 5th ed. Wayne, PA: NCCLS; (pub-
lication no. H03-A5) 2003.
37. NCCLS. Selecting and evaluating a referral laboratory; approved guide-
line. Wayne, PA: NCCLS; (publication no. GP9-A) 1998.
38. CDC. MMWR Summary of notifiable diseases. Atlanta, GA: CDC.
Available at http://www.cdc.gov/mmwr/summary.html.
39. CDC. Flu activity. Atlanta, GA: CDC. Available at http://www.cdc.gov/
flu/weekly/fluactivity.htm.

Vol. 54 / RR-13 Recommendations and Reports 21
Clinical Laboratory Improvement Advisory Committee Workgroup
Chair: Jared N. Schwartz, MD, Department of Pathology and Laboratory Medicine, Presbyterian Healthcare, Charlotte, North Carolina.
Co-Chair: Kathryn M. Foucar, MD, Department of Pathology, University of New Mexico, Albuquerque, New Mexico.
Members: Jennifer M. Alfisi, JD, Health Industry Distributors Association, Alexandria, Virginia; Kimberle C. Chapin, MD, Department of Pathology,
Rhode Island Hospital, Providence, Rhode Island; Mary Beth Clark, Emory Healthcare, Atlanta, Georgia; Martha H. Crenshaw, MD, Stone Mountain,
Georgia; Jacinto Del Mazo, MD, Del Mazo Medical Services, Atlanta, Georgia; Paula W. Garrott, EdM, Clinical Laboratory Science Department, University
of Illinois at Springfield, Illinois; Barbara M. Goldsmith, PhD, Caritas St. Elizabeth’s Medical Center, Boston, Massachusetts; Luann Ochs, MS, Roche
Diagnostics Corporation, Indianapolis, Indiana; Barbara E. Robinson-Dunn, PhD, William Beaumont Hospital, Royal Oak, Michigan; Lou F. Turner,
DrPH, North Carolina State Laboratory of Public Health, Raleigh; Robin Weiner, Biosite Inc., San Diego, California; Thomas L.Williams, MD, Methodist
Pathology Center, Nebraska Methodist Hospital, Omaha.
Clinical Laboratory Improvement Advisory Committee
Chair: SUNDWALL, David N. Sundwall, MD, Utah State Health Department, Salt Lake City, Utah.
Executive Secretary: Robert Martin, DrPH, National Center for Health Marketing, CDC, Atlanta, Georgia.
Members: Kimberle C. Chapin, MD, Department of Pathology, Rhode Island Hospital, Providence, Rhode Island; Joeline D. Davidson, MBA, West
Georgia Health System, LaGrange, Georgia; Kathryn M. Foucar, MD, Department of Pathology, University of New Mexico, Albuquerque; Paula W.
Garrott, EdM, Clinical Laboratory Science Dept, University of Illinois at Springfield; Patrick A. Keenan, MD, Department Family Medicine and Community
Health, University of Minnesota, Minneapolis; Michael Laposata, MD, Massachusetts General Hospital, Boston; Margaret Mary McGovern, MD, Molecular
Genetics Laboratory, Mount Sinai School of Medicine, Mount Sinai Medical Center, New York, New York; Dina R. Mody, MD, The Methodist Hospital,
Houston, Texas; Valerie L. Ng, MD, Alameda County Medical Center/Highland Hospital Clinical Laboratory, Oakland, California; Peter J. Gomatos,
MD, Fort Lauderdale, Florida; Cyril Michael Hetsko, MD, Madison, Wisconsin; Anthony N. Hui, MD, Northwest Arkansas Pathology Associates, Fayetteville,
Arkansas; Kevin P. Kandalaft, Provider Contracting & Provider Services Lovelace Health Systems, Inc., Albuquerque, New Mexico; Barbara E. Robinson-
Dunn, PhD, William Beaumont Hospital, Royal Oak, Michigan; Jared N. Schwartz, MD, Department of Pathology and Laboratory Medicine, Presbyterian
Healthcare, Charlotte, North Carolina; Albert H. Stahmer, Golden, Colorado; Lou F. Turner, DrPH, North Carolina State Laboratory of Public Health,
Raleigh; Thomas L. Williams, MD, Methodist Pathology Center, Nebraska Methodist Hospital, Omaha; Jean Amos Wilson, PhD, Focus Diagnostics, Inc.,
Cypress, California.
Ex Officio Representatives: Steven I. Gutman, MD, Office of In Vitro Diagnostic Device Evaluation & Safety, Food and Drug Administration, Washington,
DC; Thomas L. Hearn, MD, National Center for Health Marketing, CDC, Atlanta, Georgia; Judith Yost, MA, Division Laboratories Services, Center for
Medicaid and State Operations, Centers for Medicare & Medicaid Services.
Liaison Representative: Luann Ochs, MS, Roche Diagnostics Corporation, Indianapolis, Indiana.

22 MMWR November 11, 2005
accuracy
analyte
antibody
antigen
ASN
biohazard
biohazardous waste
biosafety
bloodborne pathogens
calibration (check)
centrifugation
CLIA
CLIAC
CLSI
CMS
competency assessment
Terms and Abbreviations Used in this Report
true or target value; freedom from error; the accuracy of results can be measured by comparing
them to results accepted as correct (e.g., standard methods), or by comparing them with those
from another laboratory that uses a comparable method
a substance or constituent for which a laboratory conducts testing
a protein formed in the body in response to a foreign substance (e.g., bacteria, viruses or
chemical toxins) and that interacts with the foreign substance to weaken or neutralize it
any substance that, when introduced into the body, causes the development of an immune
response, such as antibody production
Arkansas Sentinel Network
a biological agent that has the capacity to produce deleterious effects on humans, such as
microorganisms and toxins
waste containing pathogens with sufficient virulence and quantity so that exposure to the
waste by a susceptible host could result in an infectious disease
the application of combinations of laboratory practice and procedure, laboratory facilities, and
safety equipment when working with potentially infectious microorganisms to prevent infection
microorganisms that, when present in human blood, can cause disease in humans. These patho-
gens include, but are not limited to, hepatitis B virus, hepatitis C virus, and human immuno-
deficiency virus (HIV)
the process of testing and adjusting an instrument or test system to provide a known relation-
ship between the value of the substance being measured by the test and the test system’s mea-
surement response. A calibration check is a mechanism to be sure the test system has remained
stable and the results remain accurate
a process of separating blood or other body fluid cells from liquid components using a device
(centrifuge) containing compartments that spin rapidly around a central axis
Clinical Laboratory Improvement Amendments of 1988
Clinical Laboratory Improvement Advisory Committee
Clinical and Laboratory Standards Institute (formerly NCCLS)
Centers for Medicare & Medicaid Services
evaluation of a person’s ability to perform a test and to use a testing device; this includes all
aspects of testing, from specimen collection to result reporting

Vol. 54 / RR-13 Recommendations and Reports 23
confirmatory test
control
critical values
CW
CW testing site
diagnostic test
direct microscopic
examination
external controls
FDA
FDAMA
HHS
HIPAA
HIV
internal controls
kit
LMSMN
nonreactive or NR
nonwaived tests
NYSN
OIG
OSHA
an additional more specific test performed to rule out or confirm a preliminary test result to
provide a final result
a device or solution used to monitor a test system to ensure proper test performance and correct results
test results that require immediate notification to the clinician for patient evaluation or treatment
Certificate of Waiver
the location where waived testing takes place; a facility holding a CW
a test that identifies a disease or condition
the direct examination of a patient specimen using a microscope
control materials that mimic patient specimens and monitor the testing process from specimen
application to result interpretation
Food and Drug Administration
FDA Modernization Act
United States Department of Health and Human Services
Health Insurance Portability and Accountability Act of 1996
human immunodeficiency virus
procedural or built-in controls; controls that are built into a testing device and designed to
verify that the test system is working as expected
a packaged set containing test devices, instructions, reagents, and supplies needed to perform a
test and generate results
Laboratory Medicine Sentinel Monitoring Network
a result that indicates the absence of the constituent that the test is designed to detect
complex tests that do not meet the CLIA criteria for waiver and require training and specific
quality measures to ensure the accuracy and reliability of test results
New York Sentinel Network
Department of Health and Human Services Office of Inspector General
Occupational Safety and Health Administration

24 MMWR November 11, 2005
plasma
PNWSN
POL
PPE
PPMP
precision
procedure manual
product insert
PT
qualitative test
quality assessment
QC
quantitative test
quick reference
instructions
reactive or R
reagent
the liquid portion of anticoagulated blood that does not contain cells. If a chemical agent or
anticoagulant is added to a blood specimen to prevent clotting, the specimen can be separated
by centrifugation into cells and plasma
Pacific Northwest Sentinel Network
physician office laboratory
personal protective equipment; specialized clothing or equipment worn by an employee for
protection against a hazard
provider-performed microscopy procedures; a subcategory of moderate complexity testing under
CLIA
reproducibility; the measure of the closeness of the results obtained when analyzing the same
sample more than once; the measure of agreement between replicate measurements of the same
material
a handbook that contains test methods and other information needed to perform testing
written product information usually supplied by the manufacturer with each test kit or test
system containing instructions and critical details for performing the test; also referred to as
package insert
proficiency testing; an external quality assessment program in which samples are periodically
sent to testing sites for analysis
a test that detects whether a particular analyte, constituent, or condition is present or absent
a group of activities to monitor and evaluate the CW site’s entire testing process to help ensure
that test results are reliable, improve the testing process, and promote good quality testing
practices
quality control; the procedures used to detect and correct errors that occur because of test
system failure, adverse environmental conditions and variance in operator performance, as well
as the monitoring of the accuracy and precision of the test performance over time
a test that measures the concentration or amount of an analyte in a specimen, whose results are
expressed numerically
cards or small signs containing diagrams or flow charts with essential steps for conducting a
test that are often included with waived test systems
a result that indicates the presence of the constituent that the test is designed to detect
a substance that produces a chemical or biological reaction with a patient specimen that allows
detection or measurement of the analyte for which the test is designed

Vol. 54 / RR-13 Recommendations and Reports 25
the range of test values expected for a designated population of persons (e.g., 95% of persons
presumed to be healthy [or normal])
a laboratory that receives specimens from CW sites to perform additional testing, often for follow-
up confirmatory testing; the majority of referral laboratories perform nonwaived testing
the span of test result values for which the instrument or test device can accurately measure
the lowest concentration of an analyte that can reliably be detected or measured by a test system
the cell-free liquid remaining after whole blood has clotted or coagulated
the ability of a test to detect a particular substance or constituent without interference or false
reactions by other substances
an approach used in healt-care settings to reduce the risk for transmission of microorganisms
from both recognized and unrecognized sources of infection in a wide variety of human sources.
The nature of medical procedures and testing in these settings requires expansion of Universal
Precautions to include feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus,
even when no visible blood is evident.
the state health agency or other appropriate state or local agency that has an agreement under
Section 1864 of the Social Security Act and is used by CMS to perform surveys and inspections
the instructions and all of the instrumentation, reagents, and supplies needed to perform a test
and generate results
series of activities or path of workflow for performing testing that can be divided into three
major phases; before testing, during testing, and after testing
a self-contained test device to which a specimen is added directly and in which all steps of the
testing process occur. A unitized device is used for a single test and must be discarded after
testing.
an approach to controlling infection. According to the concept of Universal Precautions, all
human blood and certain human body fluids are treated as if known to be infectious for HIV,
hepatitis B virus, hepatitis C virus, and other bloodborne pathogens.
a test system, assay, or examination that has been cleared by the FDA for home use, or HHS
has determined meets the CLIA criteria of being a simple test with an insignificant risk for an
erroneous result
blood containing all its cellular components that has not undergone centrifugation or had the
plasma removed
reference interval
referral laboratory
reportable range
sensitivity
serum
specificity
Standard Precautions
state agency
test system
total testing process
unitized test device
Universal Precautions
waived test
whole blood
Recommendations and Reports November 11, 2005 / Vol. 54 / No. RR-13
Morbidity and Mortality Weekly Report
depardepar
depardepar
depar
tment of health and human sertment of health and human ser
tment of health and human sertment of health and human ser
tment of health and human ser
vicesvices
vicesvices
vices
Centers for Disease Control and PreventionCenters for Disease Control and Prevention
Centers for Disease Control and PreventionCenters for Disease Control and Prevention
Centers for Disease Control and Prevention
Continuing Education Activity Sponsored by CDC
Good Laboratory Practices for Waived Testing Sites
Survey Findings from Testing Sites Holding a Certificate of Waiver under the Clinical Laboratory
Improvement Amendments of 1988 and Recommendations for Promoting Quality Testing
You must complete and return the response form electronically or by mail by
November 11, 2007, to receive continuing education credit. If you answer
all of the questions, you will receive an award letter for 3.0 hours Continuing
Medical Education (CME) credit; 0.3 Continuing Education Units (CEUs); or
EXPIRATION — November 11, 2007
3.4 contact hours Continuing Nursing Education (CNE) credit. If you return
the form electronically, you will receive educational credit immediately. If you
mail the form, you will receive educational credit in approximately 30 days. No
fees are charged for participating in this continuing education activity.
By Internet
1. Read this MMWR (Vol. 54, RR-13), which contains the correct answers to
the questions beginning on the next page.
2. Go to the MMWR Continuing Education Internet site at http://www.cdc.
gov/mmwr/cme/conted.html.
3. Select which exam you want to take and select whether you want to register
for CME, CEU, or CNE credit.
4. Fill out and submit the registration form.
5. Select exam questions. To receive continuing education credit, you must
answer all of the questions. Questions with more than one correct answer
will instruct you to “Indicate all that apply.”
6. Submit your answers no later than November 11, 2007.
7. Immediately print your Certificate of Completion for your records.
By Mail or Fax
1. Read this MMWR (Vol. 54, RR-13), which contains the correct answers to
the questions beginning on the next page.
2. Complete all registration information on the response form, including your
name, mailing address, phone number, and e-mail address, if available.
3. Indicate whether you are registering for CME, CEU, or CNE credit.
4. Select your answers to the questions, and mark the corresponding letters on
the response form. To receive continuing education credit, you must
answer all of the questions. Questions with more than one correct answer
will instruct you to “Indicate all that apply.”
5. Sign and date the response form or a photocopy of the form and send no
later than November 11, 2007, to
Fax: 770-488-8555
Mail: MMWR CE Credit
Division of Scientific Communications
Coordinating Center for Health Information and Service, MS K-95
Centers for Disease Control and Prevention
1600 Clifton Rd, N.E.
Atlanta, GA 30333
6. Your Certificate of Completion will be mailed to you within 30 days.
INSTRUCTIONS
ACCREDITATION
Continuing Medical Education (CME). CDC is accredited by the Accreditation Council for Continuing Medical Education to provide continuing
medical education for physicians. CDC designates this educational activity for a maximum of 3.0 hours in category 1 credit toward the AMA Physician’s
Recognition Award. Each physician should claim only those hours of credit that he/she actually spent in the educational activity.
Continuing Education Unit (CEU). CDC has been approved as an authorized provider of continuing education and training programs by the
International Association for Continuing Education and Training. CDC will award 0.3 continuing education units to participants who successfully complete
this activity.
Continuing Nursing Education (CNE). This activity for 3.4 contact hours is provided by CDC, which is accredited as a provider of continuing
education in nursing by the American Nurses Credentialing Center’s Commission on Accreditation.

CE-2 MMWR November 11, 2005
Goal and Objectives
This MMWR highlights quality issues identified in study findings of testing sites holding a Clinical Laboratory Improvement Amendments of 1988 (CLIA)
Certificate of Waiver (CW). The goal of this report is to provide recommendations for good laboratory practices to improve the quality of waived testing and enhance
patient care and safety. Upon completion of this educational activity, the reader should be able to describe 1) the quality issues reported in the study findings; 2) the
requirements for obtaining a CW; 3) management’s considerations before introducing waived testing; 4) the good laboratory practice recommendations for the three
phases of testing; and 5) resources for personnel training and quality assessment.
To receive continuing education credit, please answer all of the following questions.
1. According to CLIA regulations, facilities performing only waived tests
have…
A. routine inspections, but no requirements for director or test personnel
qualifications.
B. no routine inspections and no requirements for director or test
personnel qualifications.
C. no routine inspection, but requirements for director qualifications.
2. CLIA regulations require facilities performing only waived tests to…
A. obtain a Certificate of Waiver.
B. use Universal Precautions.
C. follow current manufacturers’ test instructions.
D. A and B.
E. A and C.
3. Factors contributing to gaps in quality found by CMS in CW sites
include…
A. inadequate training in good laboratory practices.
B. high turnover rates of testing personnel.
C. failure to follow current manufacturers’ instructions.
D. all of the above.
4. Which of the following specimen types are NOT acceptable for waived
tests?…(Indicate all that apply.)
A. urine.
B. oral fluid.
C. whole blood.
D. plasma.
E. throat swab.
5. Before performing a waived test, one should…
A. confirm the test order is correct.
B. collect test specimens exactly as described in the test system instructions.
C. prepare the testing area and materials.
D. confirm patient identity to ensure specimen collection from the correct
patient.
E. B, C and D.
F. all of the above.
6. With unitized test use devices, external controls should be tested…
A. with each new shipment of test devices.
B. by each new operator before conducting the test.
C. after testing patient specimens.
D. when testing a new lot number.
E. A, B, and D.
F. A, C, and D.
7. Waived test results can be affected by…
A. timing of the test.
B. order in which testing steps are performed.
C. storage temperature of test kits.
D. A and B.
E. all of the above.
8. Examples of waived test results that need follow-up/confirmatory
testing include…
A. presumptive positive for Lyme disease antibodies.
B. presumptive positive for influenza A or B.
C. negative group A streptococcal antigen from children and adolescents.
D. presumptive positive for HIV antibodies.
E. A, B, and C.
F. A, C, and D.
9. Essential information for which documentation should be maintained
includes…(Indicate all that apply.)
A. test kit lot numbers.
B. test kit/reagent expiration dates.
C. quality control results.
D. personnel training.
E. all of the above.
10. The person responsible for evaluating and implementing waived
testing or the CW site director should…
A. be able to perform the test better than anyone else.
B. have the appropriate background and training to understand and
evaluate a test.
C. approve written test procedures.
D. A, B and C.
E. B and C.
11. Important information contained in the product insert includes...
(Indicate all that apply.)
A. steps to perform the test and controls.
B. an explanation of the principle of the test.
C. a description of the type of specimen to be used with the test.
D. instructions for writing the CW site test procedure.
12. Which of the following is NOT true regarding considerations for
testing personnel?
A. Overall staff competency is a key element to accurate, reliable testing.
B. Personnel should have adequate training before performing patient
testing.
C. Once trained, testing personnel do not need refresher training in good
testing practices because they are using waived tests.
D. Personnel should have resources available for consultation, either on-
site or off-site.
13. Which best describes your professional activities:
A. Physician.
B. Nurse.
C. Health educator.
D. Office staff.
E. Other.
14. I plan to use these recommendations as the basis for…(Indicate all
that apply.)
A. Health education materials.
B. Insurance reimbursement policies.
C. Local Practice guidelines.
D. Public policy.
E. Other.
Vol. 54 / No. RR-13 Recommendations and Reports CE-3
Detach or photocopy.
MMWR Response Form for Continuing Education Credit
November 11, 2005/Vol. 54/No. RR-13
Good Laboratory Practices for Waived Testing Sites
Survey Findings from Testing Sites Holding a Certificate of Waiver
under the Clinical Laboratory Improvement Amendments of 1988
and Recommendations for Promoting Quality Testing
To receive continuing education credit, you must
1. provide your contact information (please print or type);
2. indicate your choice of CME, CME for nonphysicians, CEU, or CNE credit;
3. answer
all of the test questions;
4. sign and date this form or a photocopy;
5. submit your answer form by November 11, 2007.
Failure to complete these items can result in a delay or rejection of your application
for continuing education credit.
Last Name (print or type) First Name
Street Address or P.O. Box
Apartment or Suite
City State ZIP Code
Phone Number Fax Number
E-Mail Address
Signature Date I Completed Exam
Check One
(Continued on pg CE-4)
CEU Credit
CNE Credit
CME Credit
CME for
nonphysicians
Credit
15. Overall, the length of the journal article was…
A. Much too long.
B. A little too long.
C. Just right.
D. A little too short.
E. Much too short.
16. After reading this report, I am confident I can describe the quality
issues reported in the study findings.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
17. After reading this report, I am confident I can describe the
requirements for obtaining a CW.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
18. After reading this report, I am confident I can describe management’s
considerations before introducing waived testing.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
19. After reading this report, I am confident I can describe the good
laboratory practice recommendations for the three phases of testing.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
20. After reading this report, I am confident I can describe the resources
for personnel training and quality assessment.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
21. The learning outcomes (objectives) are relevant to the goals of this
report.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
22. The instructional strategies used in the report (text, figures, and
tables) help me learn the material.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
Fill in the appropriate blocks to indicate your answers. Remember, you must answer all
of the questions to receive continuing education credit!
1. [ ] A [ ] B [ ] C
2. [ ] A [ ] B [ ] C [ ] D [ ] E
3. [ ] A [ ] B [ ] C [ ] D
4. [ ] A [ ] B [ ] C [ ] D [ ] E
5. [ ] A [ ] B [ ] C [ ] D [ ] E [ ] F
6. [ ] A [ ] B [ ] C [ ] D [ ] E [ ] F
7. [ ] A [ ] B [ ] C [ ] D [ ] E
8. [ ] A [ ] B [ ] C [ ] D [ ] E [ ] F
9. [ ] A [ ] B [ ] C [ ] D [ ] E
10. [ ] A [ ] B [ ] C [ ] D [ ] E
11. [ ] A [ ] B [ ] C [ ] D
12. [ ] A [ ] B [ ] C [ ] D [ ] E
13. [ ] A [ ] B [ ] C [ ] D [ ] E
14. [ ] A [ ] B [ ] C [ ] D [ ] E
15. [ ] A [ ] B [ ] C [ ] D [ ] E
16. [ ] A [ ] B [ ] C [ ] D [ ] E
17. [ ] A [ ] B [ ] C [ ] D [ ] E
18. [ ] A [ ] B [ ] C [ ] D [ ] E
19. [ ] A [ ] B [ ] C [ ] D [ ] E
20. [ ] A [ ] B [ ] C [ ] D [ ] E
21. [ ] A [ ] B [ ] C [ ] D [ ] E
22. [ ] A [ ] B [ ] C [ ] D [ ] E
23. [ ] A [ ] B [ ] C [ ] D [ ] E
24. [ ] A [ ] B [ ] C [ ] D [ ] E
25. [ ] A [ ] B [ ] C [ ] D [ ] E
26. [ ] A [ ] B [ ] C [ ] D [ ] E
27. [ ] A [ ] B [ ] C [ ] D [ ] E
28. [ ] A [ ] B [ ] C [ ] D [ ] E
29. [ ] A [ ] B
30. [ ] A [ ] B [ ] C [ ] D [ ] E [ ] F

CE-4 MMWR November 11, 2005
Correct answers for questions 1–12.
1. B; 2. E; 3. D; 4. D; 5. F; 6. E; 7. E; 8. F; 9. E; 10. E; 11. A, B, C; 12. C.
23. The content was appropriate given the stated objective of the course.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
24. The content expert(s) demonstrated expertise in the subject matter.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
25. Overall, the quality of the journal article was excellent.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
26. These recommendations will improve the quality of my practice.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
27. The availability of continuing education credit influenced my
decision to read this report.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
28. The MMWR format was conducive to learning this content.
A. Strongly agree.
B. Agree.
C. Neither agree nor disagree.
D. Disagree.
E. Strongly disagree.
29. Do you feel this course was commercially biased?
A. Yes.
B. No.
30. How did you learn about the continuing education activity?
A. Internet.
B. Advertisement (e.g., fact sheet, MMWR cover, newsletter, or journal).
C. Coworker/supervisor.
D. Conference presentation.
E. MMWR subscription.
F. Other.

MMWR
The Morbidity and Mortality Weekly Report (MMWR) Series is prepared by the Centers for Disease Control and Prevention (CDC) and is available free of charge
in electronic format and on a paid subscription basis for paper copy. To receive an electronic copy each week, send an e-mail message to listserv@listserv.cdc.gov. The
body content should read SUBscribe mmwr-toc. Electronic copy also is available from CDC’s World-Wide Web server at http://www.cdc.gov/mmwr or from CDC’s
file transfer protocol server at ftp://ftp.cdc.gov/pub/publications/mmwr. To subscribe for paper copy, contact Superintendent of Documents, U.S. Government
Printing Office, Washington, DC 20402; telephone 202-512-1800.
Data in the weekly MMWR are provisional, based on weekly reports to CDC by state health departments. The reporting week concludes at close of business on
Friday; compiled data on a national basis are officially released to the public on the following Friday. Address inquiries about the MMWR Series, including material
to be considered for publication, to Editor, MMWR Series, Mailstop K-95, CDC, 1600 Clifton Rd., N.E., Atlanta, GA 30333; telephone 888-232-3228.
All material in the MMWR Series is in the public domain and may be used and reprinted without permission; citation as to source, however, is appreciated.
All MMWR references are available on the Internet at http://www.cdc.gov/mmwr. Use the search function to find specific articles.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or
their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of these sites. URL addresses listed in
MMWR were current as of the date of publication.
✩U.S. Government Printing Office: 2006-523-142/00122 Region IV ISSN: 1057-5987
