Authored by Emily Simpson
This work is licensed under a Creative Commons Attribution 4.0 International License
Chemistry 0871 Learning Centre
Naming Organic Compounds Practice
EXERCISES
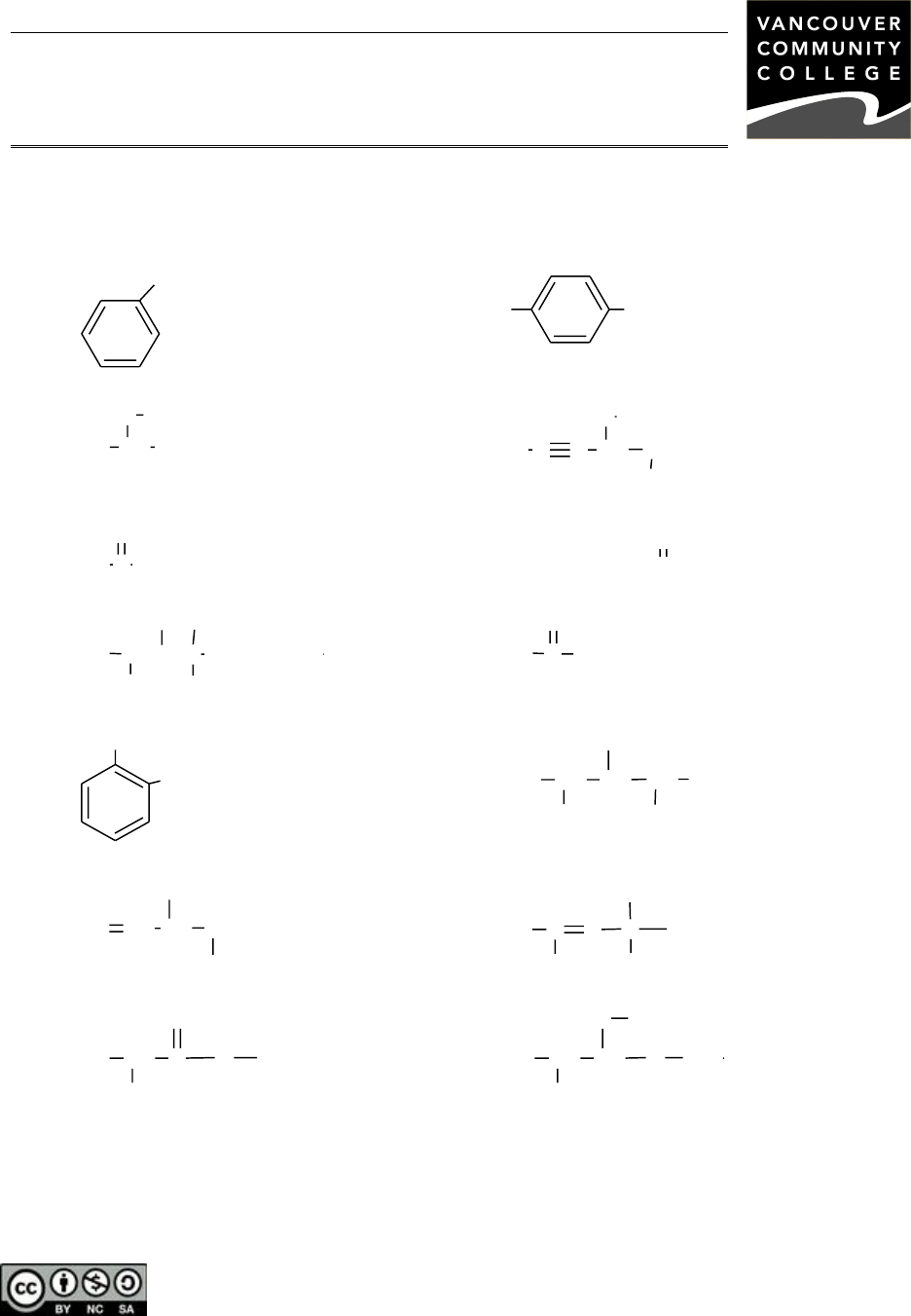
A. Identify the class of the following compounds. For any alkanes, alkenes, alkynes,
aromatic compounds, carboxylic acids or alcohols, provide the IUPAC name of the
molecule. For the four special monosubstituted benzenes, use the common name.
1)
I
8)
CH
3
Br
2)
CH
3
CH
3
CH
2
CHCH
3
CH
2
CH
2
9)
CH
3
CH
3
C
C CH
CHCH
3
CH
2
CH
3
3)
CH
3
C
CH
2
CH
3
O
10)
CH
2
CH
2
CH
O
CH
2
CH
3
4)
CH
3
CH
CH
C CH
2
CH
2
CH
2
CH
3
CH
3
CH
2
CH
3
CH
3
Cl
11)
CH
2
CH
2
CH
3
CH
2
CH
2
C
O
OH
5)
CH
2
CH
3
CH
2
CH
3
12)
CH
3
CH CH
CH
CH
3
CH
3
CH
3
OH
6)
CH
2
CH CH
CH
2
CH
3
CH
3
13)
CH
3
C C
C
CH
3
CH
3
CH
2
CH
3
CH
3
7)
CH
3
CH C
O
CH
3
CH
3
O
14)
CH
3
CH CH
O
CH
3
CH
2
CH
2
CH
3
CH
3

This work is licensed under a Creative Commons Attribution 4.0 International License
2
B. Draw the structural formulas for the following compounds:
1) 1-pentene 7) 4-methylhexanoic acid
2) 2-methyl-3-heptyne 8) 2,3-dichloro-4-ethyl-2-hexene
3) 3-ethyl-4,5-dimethylpentane 9) 2,4-dinitrotoluene
4) 2-ethyl-1-pentanol 10) 3-ethyl-2,3-dimethyl-2-pentanol
5) m-bromophenol 11) 5-chloro-4-methyl-3-heptanone
6) 3,3,6,6-tetraethyl-4-octyne 12) 3-phenyl-1-propyne
C. Draw all possible open-chain structures for the following molecular formulas and
name them:
1) C
5
H
12
2) C
5
H
10
3) C
3
H
8
O

This work is licensed under a Creative Commons Attribution 4.0 International License
3
SOLUTIONS
A. (1) aromatic compound: iodobenzene (2) alkane: 3-methylhexane (3) ketone
(4) alkane/alkyl halide: 3-chloro-4-ethyl-2,4-dimethyloctane (5) aromatic compound:
o-diethylbenzene or ortho-diethylbenzene (6) alkene: 3-methylpentene (7) ester
(8) aromatic compound: p-bromotoluene or para-bromotoluene (9) alkyne:
4-ethyl-5-methyl-2-hexyne (10) aldehyde (11) carboxylic acid: hexanoic acid
(12) alcohol: 2,4-dimethyl-3-pentanol (13) alkene: 2,4,4-trimethyl-2-hexene
(14) ether
B. (1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
(11)
(12)
C. (1) pentane 2-methylbutane 2,2-dimethylpropane
(2) 1-pentene 2-pentene 2-methyl-1-butene
3-methyl-1-butene 2-methyl-2-butene
(3) 1-propanol 2-propanol methoxyethane (methyl ethyl ether)
H
2
C=CH−CH
2
−CH
2
−CH
3
H
3
C−CH−C≡C−CH
2
−CH
2
−CH
3
CH
3
H
3
C−CH
2
−CH−CH−CH
2
−CH
3
CH
2
−CH
3
CH
3
HO−CH
2
−CH−CH
2
−CH
2
−CH
3
CH
2
−CH
3
OH
Br
H
3
C−CH
2
−C−C≡C−C−CH
2
−CH
3
CH
2
−CH
3
H
3
C−CH
2
CH
2
−CH
3
H
3
C−CH
2
O=C−CH
2
−CH
2
−CH−CH
2
−CH
3
OH
CH
3
H
3
C−C=C−CH−CH
2
−CH
3
Cℓ
CH
2
−CH
3
Cℓ
CH
3
NO
2
NO
2
H
3
C−C−C−CH
2
−CH
3
CH
2
−CH
3
H
3
C
CH
3
HO
H
3
C−CH
2
−C−CH−CH−CH
2
−CH
3
H
3
C
O
Cℓ
HC≡C−CH
2
H
3
C−CH
2
−CH
2
−CH
2
−CH
3
H
3
C−CH
2
−CH
2
−CH
3
CH
3
H
3
C−CH
2
−CH
3
CH
3
CH
3
H
2
C=CH−CH
2
−CH
2
−CH
3
H
3
C−CH=CH−CH
2
−CH
3
H
2
C=C−CH
2
−CH
3
CH
3
H
2
C=CH−CH−CH
3
CH
3
H
3
C−C=CH−CH
3
CH
3
HO−CH
2
−CH
2
−CH
3
H
3
C−CH−CH
3
OH
H
3
C−O−CH
2
−CH
3
