151
Lessons from health hazards | Tobacco industry manipulation of research
Late lessons from early warnings: science, precaution, innovation
151
Lisa A. Bero (
1
)
7 Tobacco industry manipulation of
research
(
1
) Funding and support: grants from the American Cancer Society (RPG9714301PBP) and the University of California Tobacco‑
Related Disease Research Program (6RT0025 and 9RT0193) supported this work. Authors would like to thank colleagues who have
collaborated on the studies cited in this paper: Katherine Bryan-Jones, Stanton Glantz, Miki Hong, Christina Mangurian, Theresa
Montini, and Marieka Schotland. Daniel Cook, Susan Dalton, Joshua Dunsby, Michael Givel, Anh Le, Peggy Lopippero, David Rosner
and Theo Tsoukalis for useful input to this manuscript.
(
2
) For example, two contrasting yet complementary histories of cancer in general and smoking in particular are Davies (2007) and
Keating (2009).
This chapter differs in some ways from the others in Volume 2 of Late lessons from early
warnings. The history of 'second hand', 'passive' or 'environmental tobacco smoke' (ETS), to
which non-smokers are exposed overlaps with the history of active smoking. Those affected
include the partners and children of smokers, and the bartenders and other workers who have to
work in smoky environments.
The focus in this chapter is on the strategies used by the tobacco industry to deny, downplay,
distort and dismiss the growing evidence that, like active smoking, ETS causes lung cancer
and other effects in non-smokers. It does not address the history of scientific knowledge about
tobacco and how it was used or not used to reduce lung cancer and other harmful effects
of tobacco smoke. There is much literature on this (
2
) and a table at the end of the chapter
summarises the main dates in the evolution of knowledge in this area.
The chapter concentrates on the 'argumentation' that was used to accept, or reject, the growing
scientific evidence of harm. Who generated and financed the science used to refute data on
adverse health effects? What were the motivations? What kind of science and information, tools
and assumptions were used to refute data on the adverse health of tobacco?
The release of millions of internal tobacco industry documents due to law suits in the US has
given insights into the inner workings of the tobacco industry and revealed their previously
hidden involvement in manipulating research. However, this insight is not available for most
corporate sectors. The chapter discusses the possibilities of 'full disclosure' of funding sources
and special interests in research and risk assessment in order to secure independence and
prevent bias towards particular viewpoints.
While smoking bans are now being introduced in more and more countries, other industries are
drawing inspiration from tobacco company strategies, seeking to maintain doubt about harm in
order to keep hazardous products in the marketplace.
The chapter also includes a summary of the tobacco industry's role in shaping risk assessment in
the US and Europe to serve its own interests.

Lessons from health hazards | Tobacco industry manipulation of research
152
Late lessons from early warnings: science, precaution, innovation
7.1 Introduction
This chapter describes the strategies that the
tobacco industry has used to influence the design,
conduct and publication of scientific research on
second-hand smoke; and how the tobacco industry
used this research in attempts to influence policy.
It represents an expansion of an earlier article,
'Tobacco industry manipulation of research' by Bero
(2005).
The primary motivation of the tobacco industry
has been to generate controversy about the health
risks of its products. The industry has used several
strategies including:
1. funding and publishing research that supports
its position;
2. suppressing and criticising research that does
not support its position;
3. changing the standards for scientific research;
4. disseminating interest group data or
interpretation of risks via the lay (non-academic)
press and directly to policymakers.
The strategies used by the tobacco industry have
remained remarkably constant since the early 1950s
when the industry focused on refuting data on the
harmful effects of active smoking, through to the
1990s, when the industry was more concerned with
refuting data on the harmful effects of second-hand
smoke. Tobacco industry lawyers and executives,
rather than scientists, have controlled the design,
conduct and dissemination of this research.
When data on risk appear to be controversial,
users of the data should investigate the sources of
controversy. This can be done only if interest group
involvement in all steps of the risk determination
process is transparent and fully disclosed. Since the
tobacco industry's efforts to manipulate research are
international endeavours and are shared by other
corporate interests, individuals around the world
should be aware of the strategies that the industry
has used to influence data on risk.
Communicating accurate information on risk is
essential to risk perception and risk management.
Research findings, often from basic science,
epidemiology and exposure or engineering research,
provide the basis for information on risk. These
research findings or 'facts' are, however, subject
to interpretation and the social construction of
the evidence (Krimsky, 1992). Research evidence
has a context. The roles of framing, problem
definition and choice of language influence risk
communication (Nelkin, 1985). Furthermore,
scientific uncertainties allow for a wide range of
interpretation of the same data. Since data do not
'speak for themselves' interest groups can play a
critical role in generating and communicating the
research evidence on risk.
An interest group is an organised group with
a specific viewpoint that protects its position
(Lowi, 1979). Interest groups are not exclusively
business organisations; they can comprise all kinds
of organisations that may attempt to influence
governments (Walker, 1991; Truman, 1993). Therefore,
interest groups can be expected to select and interpret
the evidence about a health risk to support their
predefined policy position (Jasanoff, 1996). For
example, public health interest groups are likely to
communicate risks in a way that emphasises harm
and, therefore, encourages regulation or mitigation of
risk (Wallack et al., 1993). Industry interest groups are
likely to communicate risks in a way that minimises
harm and reduces the chance that their product is
regulated or restricted in any way. Disputes about
whether a risk should be regulated or not are
sometimes taken to the legal system for resolution
(Jasanoff, 1995). Thus, interest groups often have two
major goals: to influence policy-making and litigation.
Beginning in the 1970s, the tobacco industry
influenced the collection, interpretation and
dissemination of data on risks of exposure to
second-hand smoke. The analysis below suggests
that this was history repeating itself; in the 1950s,
the tobacco industry had used similar strategies to
manipulate information on the risks of smoking.
Moreover, other corporate interest groups appear to
use similar tactics (Special Issue, 2005; White et al.,
2009).
Many of the strategies available to interest groups —
for example sponsoring, publishing and criticising
research — are costly. Industry might therefore be
expected to dominate examples of such activities
because corporate interest groups are more likely to
have the resources to launch expensive, coordinated
efforts. In contrast, public health groups, which tend
to act independently, are less likely to command
such resources (Montini and Bero, 2001).
Industry examples may also predominate because
some interest group activities have come to light
through the documents released during the
'discovery' process in law suits. For example, the
asbestos and tobacco industries were required to
release large amounts of internal correspondence

Lessons from health hazards | Tobacco industry manipulation of research
153
Late lessons from early warnings: science, precaution, innovation
when they were sued by groups attempting to show
that they were harmed by industry products.
7.2 Scientific community knowledge
about the hazards of second‑hand
smoke exposure
Environmental tobacco smoke, or second-hand
smoke, is a complex mixture of thousands of gases
and fine particles emitted by burning tobacco
products and from smoke exhaled by smokers, as
well as smoke that escapes while the smoker inhales
and some vapour-phase related compounds that
diffuse from tobacco products. During the 1970s
and 1980s, data on the harmful effects of exposure
to second-hand smoke began to be published in
the scientific literature. Seminal epidemiological
studies in 1981 demonstrated that second-hand
smoke exposure was associated with lung cancer
(Hirayama, 1981). United States Surgeon General
and National Academy of Sciences reports in 1986
concluded that second-hand smoke was a cause of
disease (US DHHS, 1986; NRC, 1986).
A landmark European epidemiological study on
lung cancer and second-hand smoke was initiated
by the International Agency for Research on
Cancer (IARC) in 1988 and published in 1998. The
publication reported a 16 % increase in lung cancer
risk for non-smoking spouses of smokers and a 17 %
increase for non-smokers who were exposed in the
workplace (IARC, 1998).
In 1992, the US Environmental Protection Agency
(EPA) released a risk assessment classifying ETS
as a Group A human carcinogen (US EPA, 1992).
The tobacco industry criticised the methodology of
the US EPA risk assessment for its study selection
and statistical analysis. The industry also criticised
the epidemiological design of the studies included
in the risk assessment, the ways that these studies
controlled for bias and confounding, and measured
ETS exposure (Bero and Glantz, 1993). The EPA
revised the report in response to valid criticisms
and the report was approved by the Scientific
Advisory Board. The report was improved but the
sheer volume of tobacco industry comments that
required consideration probably delayed its release.
Although the science was valid, the tobacco industry
successfully attacked the US EPA risk assessment in
court on procedural grounds (Flue-Cured Tobacco
Co-op vs. US EPA, 1998) and the tobacco industry
had similar procedural objections to the report of
Australia's National Health and Medical Research
Council, 'The health effects of passive smoking'
(NHMRC, 1997).
In 1997 the California EPA published the final
report of a risk assessment entitled 'Health Effects
of Exposure to Environmental Tobacco Smoke'
(Cal-EPA, 1997). The California risk assessment was
more comprehensive than the US EPA's assessment
because it examined the association of second-hand
smoke exposure, lung cancer and respiratory
illness, as well as cardiovascular, developmental,
reproductive and childhood respiratory effects.
The California EPA risk assessment also addressed
criticisms brought by the tobacco industry against
the US EPA risk assessment. The California risk
assessment was the result of a collaborative effort
between the Office of Environmental Health Hazard
Assessment (OEHHA) and the Air Resources Board
(ARB), two of the six constituent organisations of
the California EPA. The Scientific Review Panel that
endorsed the report concluded that second-hand
smoke is 'a toxic air contaminant' that 'has a major
impact on public health' (Cal-EPA, 1997).
In June 2005 the California Scientific Review Panel
approved an updated draft report on Identification
of Environmental Tobacco Smoke as a Toxic Air
Contaminant (Cal-EPA, 2005). The report concluded
that second-hand smoke exposure is causally
associated with developmental effects (e.g. inhibited
foetal growth, sudden infant death syndrome,
pre-term delivery), respiratory effects (e.g. asthma,
acute and chronic respiratory symptoms in children,
middle ear infections in children), carcinogenic
effects (e.g. lung cancer, nasal sinus cancer, breast
cancer in younger, premenopausal women) and
cardiovascular effects (e.g. heart disease mortality,
acute and chronic heart disease, morbidity).
The growing evidence documenting the adverse
health effects of second-hand smoke was clearly a
threat to the tobacco industry as early as the 1970s.
Restrictions on smoking could lead to reduced daily
consumption of cigarettes and a decline in sales. The
tobacco industry responded with its own science to
the independently generated data on tobacco-related
adverse health effects.
7.3 Tobacco industry strategies to
subvert scientific knowledge
Policymaking is facilitated by consensus (Kingdon,
1984; Mazmanian and Sabatier, 1989; Sabatier,
1991). Scientific research, on the other hand, is
characterised by uncertainty. The uncertainty
that is familiar to scientists poses problems when
decision-making occurs in a public forum. Thus, it
is often to the benefit of corporate interest groups to
generate controversy about evidence of a product's

Lessons from health hazards | Tobacco industry manipulation of research
154
Late lessons from early warnings: science, precaution, innovation
health risks because such controversy is likely to
slow or prevent regulation of that product. Similarly,
scientific debate over the data and methods used
in risk assessment, for example, can hinder the
development of the risk assessment (Stayner, 1999).
The release of previously secret internal tobacco
industry documents as a result of the Master
Settlement Agreement in 1998 has given the
public health community insights into the tobacco
industry's motives, strategies, tactics and data (Bero,
2003). These documents show that for decades the
industry has been motivated to generate controversy
about the health risks of its products. They have
also revealed that the industry was concerned about
maintaining its credibility as it manipulated research
on tobacco (Bero, 2003).
The strategies used by the tobacco industry have
remained remarkably constant since the early
1950s. During the 1950s and 1960s, the tobacco
industry focused on refuting data on the adverse
effects of active smoking. The industry applied the
same tools it developed during that period when
it subsequently refuted data on the adverse effects
of second-hand smoke exposure during the 1970s
through the 1990s.
A 1978 report prepared by the Roper Organization
for The Tobacco Institute noted that the industry's
best strategy for countering public concern about
passive smoking was to fund and disseminate
scientific research that countered research produced
by other sources:
'The strategic and long-run antidote to
the passive smoking issue is, as we see it,
developing and widely publicizing clear-cut,
credible, medical evidence that passive
smoking is not harmful to the non-smoker's
health' (Roper Organization, 1978).
Philip Morris promoted international research
related to passive smoking in order to stimulate
controversy, as described in the notes of a meeting
of the UK [Tobacco] Industry on Environmental
Tobacco Smoke, London, 17 February 1988:
'In every major international area (USA,
Europe, Australia, Far East, South America,
Central America and Spain) we are proposing,
in key countries, to set up a team of scientists
organized by one national coordinating
scientist and American lawyers, to review
scientific literature or carry out work on ETS to
keep the controversy alive' (emphasis added)
(Boyse, 1988).
The tobacco industry organised teams of scientific
consultants all over the world with the main goal
of stimulating controversy about the adverse health
effects of second-hand smoke (Barnoya and Glantz,
2006; Chapman, 1997; Muggli et al., 2001; Grüning
et al., 2006; Assunta et al., 2004).
A variety of studies show that industry sponsorship
of research is associated with outcomes that are
favourable for the industry (Lexchin et al., 2003;
Barnes and Bero, 1998; Barnes and Bero, 1997).
One possible explanation for this bias in outcome
is that industry-sponsored research is poorly
designed or of worse 'methodological quality'
than non-industry-sponsored research. However,
there is no consistent association between industry
sponsorship and methodological quality (Lexchin
et al., 2003).
Factors other than study design can affect the
outcome of research, including:
• theframingorsocialconstructionoftheresearch
question;
• theconductofthestudy;
• thepublication(ornot)ofthestudyfindings.
The tobacco industry has manipulated these
other factors in a variety of ways. First, by using
its funding mechanisms to attempt to control the
research agenda and types of questions asked
about tobacco. Second, the industry's lawyers and
executives have been involved in the design and
conduct of industry-supported research. Third, the
tobacco industry has sponsored publications of its
own funded research, and suppressed research not
favourable to the industry.
Box 7.1 summarises the range of strategies that the
tobacco industry has used for decades to manipulate
information on the risks of tobacco. These strategies
are described in more detail in the remainder of this
section.
7.3.1 Strategy 1: fund research that supports the
interest group's position
The first element in the tobacco industry's strategy
to influence data on risk has been to sponsor
research designed to produce findings that are
favourable to the industry.
Funding research can stimulate controversy in
multiple ways. First, it can put the research agenda
Lessons from health hazards | Tobacco industry manipulation of research
155
Late lessons from early warnings: science, precaution, innovation
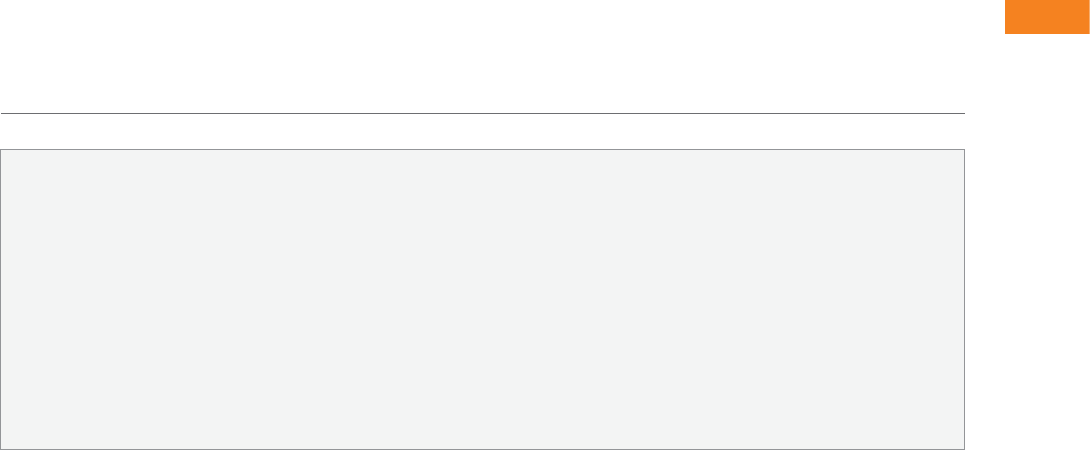
Box 7.1 Tobacco industry strategies to manipulate data on risk
1. Fund research that supports the interest group position.
2. Hide industry involvement in research.
3. Publish research that supports the interest group position.
4. Suppress research that does not support the interest group position.
5. Criticise research that does not support the interest group position.
6. Change scientific standards.
7. Disseminate interest group data or interpretation of risk in the lay press.
8. Disseminate interest group data or interpretation of risk directly to policymakers.
in the control of the interest group. Second, it can
produce data to refute research on risk conducted
by others. In addition to stimulating controversy,
funding research serves other useful purposes for the
tobacco industry. The research can be disseminated
directly to policymakers and the lay press. It can
provide good public relations for the tobacco industry
by establishing it as a philanthropic body that funds
scientific research. Similarly, funding research can
increase the industry's credibility. One of the criteria
that the Philip Morris Worldwide Scientific Affairs
Programme considered when deciding whether to
fund a research application was whether the research
would enhance the credibility of the company
(Malone and Bero, 2003).
The US tobacco industry funded research through its
trade association, The Tobacco Institute (Bero et al.,
1995; Hanauer et al., 1995), internally (e.g. internal
company research), externally (e.g. by supporting
the research of scientific consultants) and through
sponsored research organisations. Tobacco industry
lawyers and executives were involved in selecting
which research to fund. Most of the research did not
undergo any form of independent scientific peer
review but was funded on the basis of its potential
to protect the interests of the companies.
Lawyer involvement in research
In the mid-1990s, internal tobacco industry
documents were circulated by industry
whistle-blowers. By 1998, the availability of tobacco
industry documents increased exponentially as
a result of the settlement of a suit by the State of
Minnesota and Blue Cross/Blue Shield against the
major tobacco companies. The Master Settlement
Agreement between the attorneys general of 46 states
and Brown & Williamson/British American Tobacco,
Lorillard, Philip Morris, RJ Reynolds, the Council
for Tobacco Research and The Tobacco Institute
released millions of additional documents to the
public. These documents provide an unprecedented
look at how tobacco industry lawyers were involved
in the design, conduct and dissemination of tobacco
industry-sponsored research (Bero, 2003). By
involving lawyers in research, the tobacco industry
protected their research activities from public
discovery and kept their lawyers informed about
science relevant to litigation.
The internal tobacco industry documents include
descriptions of research that was funded directly by
law firms. For example, the law firms of Covington
and Burling, and Jacob and Medinger, both of
which represent a number of tobacco company
clients, funded research on tobacco (Bero et al.,
1995). Lawyers selected which projects would be
funded. The supported projects included reviews
of the scientific literature on topics ranging from
addiction to lung retention of particulate matter.
The law firms also funded research on factors
potential confounding the adverse health effects
associated with smoking. For example, projects
examined genetic factors associated with lung
disease or the influence of stress and low-protein
diets on health (Bero et al., 1995). Thus, some of the
research funded by law firms served the purpose of
deflecting attention away from tobacco as a health
hazard and protecting the tobacco companies from
litigation.
Other research was funded directly by the tobacco
companies but lawyers were involved in selecting
and disseminating these projects. For example,
tobacco companies funded individuals to serve
as consultants to prepare expert testimony for
Congressional hearings, attend scientific meetings,
review scientific literature or conduct research on
the health effects of tobacco or second-hand smoke
(Hanauer et al., 1995). At one tobacco company,
Brown & Williamson, the legal department
controlled the dissemination of internal scientific
reports (Hanauer et al., 1995). The lawyers at Brown
& Williamson developed methods for screening

Lessons from health hazards | Tobacco industry manipulation of research
156
Late lessons from early warnings: science, precaution, innovation
scientific reports from their affiliated companies
in order to ensure that scientific information
related to tobacco and health would be protected
from discovery by legal privileges. In a memo
dated 17 February 1986, J. K. Wells, the Brown &
Williamson corporate counsel, outlined one method
for protecting industry produced research data:
'The only way BAT [British American Tobacco]
can avoid having information useful to
plaintiff found at B&W is to obtain good legal
counsel and cease producing information in
Canada, Germany, Brazil and other places that
is helpful to plaintiffs' (Wells and Pepples,
1986).
Although tobacco industry public statements
claimed that the tobacco companies were funding
objective research to gather facts about the health
effects of smoking, lawyer involvement in the
research served to control the scientific debate on
issues related to smoking and health and protect
from discovery scientific documents that were
potentially damaging to the industry.
Research organisations
The tobacco industry also formed research funding
organisations, which gave the appearance that
the research they supported was independent of
influence from the industry.
The Council for Tobacco Research (CTR) was
formed by United States tobacco companies in 1954
as the Tobacco Industry Research Committee (TIRC).
The industry stated publicly that it was forming
the TIRC to fund independent scientific research
to determine whether there was a link between
smoking and lung cancer. However, internal
documents from Brown & Williamson Tobacco
Company have shown that the TIRC was actually
formed for public relations purposes, to convince
the public that the hazards of smoking had not been
proven (Glantz et al., 1996).
Research that is sponsored by federal organisations
or large foundations is typically peer reviewed
by other researchers before funding is approved.
Although the Council for Tobacco Research had
a Scientific Advisory Board consisting of well
respected researchers, not all of the research
funded by the CTR was peer reviewed by this
board. Beginning in 1966, tobacco industry lawyers
became directly responsible for many of the funding
decisions of the CTR. Between 1972 and 1991, the
CTR awarded at least USD 14 636 918 in special
project funding (Bero et al., 1995). Lawyers were not
only involved in selecting projects for funding but
also in designing the research and disseminating the
results (Bero et al., 1995).
The research funded by CTR, although initially useful
for public relations, became increasingly important
for the tobacco industry's activities in legislative and
legal settings. This evolution is described in a memo
dated 4 April 1978 from Ernest Pepples, Brown &
Williamson's vice president and general counsel,
to J. E. Edens, chairman and CEO of Brown &
Williamson Tobacco Company:
'Originally, CTR was organized as a public
relations effort. … The research of CTR
also discharged a legal responsibility. …
There is another political need for research.
Recently it has been suggested that CTR or
industry research should enable us to give
quick responses to new developments in the
propaganda of the avid anti-smoking groups.
… Finally, the industry research effort has
included special projects designed to find
scientists and medical doctors who might
serve as industry witnesses in lawsuits or in a
legislative forum' (Pepples, 1978).
The Pepples memo gives insight into why lawyers
became increasingly involved in the selection of
research projects for CTR.
The Center for Indoor Air Research (CIAR) was
formed by Philip Morris, R. J. Reynolds Tobacco
Company and Lorillard Corporation in 1988 (CIAR,
1988). The founding companies were joined by
Svenska Tobaks A.B., a Swedish domestic tobacco
company in 1994 (CIAR, 1994). The stated mission
of CIAR was 'to create a focal point organisation
of the highest caliber to sponsor and foster quality,
objective research in indoor air issues including
environmental tobacco smoke, and to effectively
communicate research findings to a broad scientific
community' (CIAR, 1989). CIAR's mission statement
was modified in 1992 to eliminate the words
referrring to environmental tobacco smoke (CIAR,
1992a; CIAR, 1992b). The elimination of research on
second-hand smoke from the mission statement was
followed by a shift in the research agenda of CIAR
to one that would prevent it investigating the health
effects of second-hand smoke.
Similar to the CTR, CIAR awarded 'peer-reviewed'
projects, which were reviewed by a Science Advisory
Board, and 'special-reviewed' projects, which
reviewed by its Board of Directors consisting of
tobacco company executives (Barnes and Bero, 1996).
From 1989 to 1993, CIAR awarded USD 11 209 388
for peer-reviewed projects and USD 4 022 723 for

Lessons from health hazards | Tobacco industry manipulation of research
157
Late lessons from early warnings: science, precaution, innovation
special-reviewed projects (Barnes and Bero, 1996).
Seventy per cent of the peer-reviewed projects funded
by CIAR examined indoor air pollutants other than
tobacco smoke. Thus, the industry appeared to be
financing peer-reviewed projects through CIAR to
enhance its credibility, to provide good publicity and
to divert attention away from second-hand smoke as
an indoor air pollutant.
In contrast to the peer-reviewed projects, almost
two-thirds of CIAR's special-reviewed projects were
related to second-hand smoke (Barnes and Bero,
1996). In addition, most special-reviewed projects
studied exposure rather than health effects. It is
therefore possible that the tobacco industry was
funding research through CIAR to develop data it
could use to support its frequent claim that persons
are not exposed to sufficient levels of passive smoke
to cause any serious adverse health effects (Tobacco
Institute, 1986).
The tobacco industry may have also been funding
special-reviewed research through CIAR to develop
scientific data that it could use in legislative and
legal settings. Six CIAR-funded investigators
have testified at government hearings. All of the
statements submitted by them supported the tobacco
industry position that second-hand smoke exposure
is not harmful to health. Data from two of CIAR's
special-reviewed projects were presented at hearings
held by the United States Occupational Safety
and Health Administration (OSHA) regarding its
proposed indoor air quality regulation. Data from
a third special-reviewed project was presented at a
Congressional hearing related to a proposed ban on
smoking on commercial aircrafts. One CIAR-funded
study was investigated extensively by the United
States Congressional Subcommittee on Health and
the Environment after it was cited in testimony before
numerous government agencies. The CIAR-funded
study had concluded that, with good building
ventilation, clean air could be maintained with
moderate amounts of smoking (Turner et al., 1992)
and was used to support testimony that indoor
smoking restrictions are not necessary. However, the
Congressional Subcommittee found that data for this
study had been altered and fabricated. An earlier
CIAR-funded study by the same organisation was
also severely compromised because The Tobacco
Institute selected the sites where passive smoking
levels were measured for the study (Barnes and Bero,
1996).
The Center for Indoor Air Research was disbanded
as part of the US Master Settlement Agreement in
1998. However, in 2000, Philip Morris re-created an
external research programme called the 'Philip Morris
External Research Program' (PMERP) with a structure
similar to that of CIAR. Like CIAR, PMERP's grant
review panel consisted of a cohort of external peer
reviewers, a science advisory board, and an internal,
anonymous review and approval committee. Three
of the six advisory board members had a previous
affiliation with CIAR. The majority of the named
reviewers also had previous affiliations with the
tobacco industry (Hirschhorn et al., 2001).
Research is an international endeavour
The tobacco industry applied the strategy of
covertly funding research on an international scale.
In Latin America and Asia, tobacco companies,
working through the law firm Covington and
Burling, developed a network of physician and
scientist consultants to prepare and present data
to refute claims about the harms of second-hand
smoke (Assunta et al., 2004; Barnoya and Glantz,
2002). In Germany, tobacco companies and the
German Association of the Cigarette Industry
developed a similar team of consultants and funded
research through various foundations and research
organisations (Grüning et al., 2006). In many cases,
the industry employed the next strategy discussed
below: hiding its support for the research.
In summary, funding research serves multiple
purposes for the tobacco industry. The research
that is directly related to tobacco has been used to
refute scientific findings suggesting that the product
is harmful and sustain controversy about adverse
effects. Tobacco industry-supported research has
been used to prepare the industry for litigation or
legislative challenges. The industry may also have
funded research not directly related to tobacco in
order to generate good publicity, enhance industry
credibility and to distract from tobacco products as a
health problem.
7.3.2 Strategy 2: hide industry involvement in
research
A defining characteristic of the tobacco industry's
response to independent evidence of the harms of
second-hand smoke has been attempts to hide its
involvement in refuting this evidence. In both of
the cases described below, tobacco companies were
secretly involved in generating data to suggest
that second-hand smoke was not harmful and
suppressing data suggesting that it was.
Philip Morris European research programme on
second-hand smoke
As early as 1968, executives at Philip Morris began
planning a new biological research facility that

Lessons from health hazards | Tobacco industry manipulation of research
158
Late lessons from early warnings: science, precaution, innovation
would focus on examining the effects, including
carcinogenic effects, of second-hand smoke
exposure in various animal species. In 1970,
Philip Morris purchased a research facility in
Germany, Institut für Industrielle und Biologische
Forschung GmbH (INBIFO) (Diethhelm et al.,
2004). Philip Morris hired Ragnar Rylander, a
Swedish university professor, as the coordinator
of INBIFO. Rylander communicated INBIFO's
research findings to Philip Morris executives
in the United States, who would then decide
whether to disseminate the research more
widely or keep it secret. Research that remained
unpublished included studies providing evidence
that 'sidestream smoke' (which enters the air
from a burning cigarette, cigar or pipe) is more
toxic than 'mainstream smoke' (which is inhaled
directly) (Diethhelm et al., 2004). Published
research included an epidemiological study
suggesting an association between lung cancer and
green tea, a finding that would be useful to the
tobacco industry in distracting from the harms of
second-hand smoke (Tewes et al., 1990).
One of the most striking features of the INBIFO
programme was that its coordinator, Ragnar
Rylander, had long-standing and secret links to
the tobacco industry. Thus, he conferred a false
sense of credibility to the programme. Professor
Rylander's association with the tobacco industry
was investigated by an official university committee,
the Fact Finding Commission of the University of
Geneva. The committee concluded that Rylander
was acting as a sponsored agent of the tobacco
industry, rather than as an independent researcher
when he testified as a scientific expert, organised
scientific congresses and directed research at
INBIFO (Fact Finding Commission, 2004). An
extensive analysis of internal tobacco industry
documents found that Rylander took no initiatives
'in the area of [second-hand smoke research] without
first consulting extensively with his contacts within
the tobacco industry'(Fact Finding Commission,
2004).
Tobacco industry creation and dissemination of
a study on second-hand smoke
The tobacco industry's development of the Japanese
Spousal Smoking Study provides another example
of industry involvement in designing, conducting
and disseminating research, and its efforts to hide
this involvement.
In 1981, Takeshi Hirayama published an influential
study showing an association of second-hand smoke
exposure and lung cancer (Hirayama, 1981). The
Hirayama study has been voted the most influential
paper ever on second-hand smoke (Chapman,
2005) and was the most frequently cited study
in regulatory hearings on smoking restrictions
(Montini et al., 2002). In these hearings, tobacco
industry representatives have argued that the
Hirayama study is flawed due to misclassification
bias (Bero and Glantz, 1993; Schotland and Bero,
2002). Furthermore, analysis of internal tobacco
industry documents by Hong and Bero (2002) has
shown how the tobacco industry hid its involvement
in creating a study, the Japanese Spousal
Smoking Study, to support its arguments about
misclassification bias.
The tobacco industry documents reveal that
although the Japanese Spousal Smoking Study
was undertaken by named Japanese investigators,
project management was conducted by Covington
and Burling (a tobacco industry law firm), the
research was supervised by a tobacco industry
scientist and a tobacco industry consultant assisted
in reviewing the study design and interpreting
the data (Hong and Bero, 2002). The documents
show that the tobacco companies that funded
the study did not want any of these individuals
named as co-authors on any of the resulting
scientific publications. Although the tobacco
companies considered using the Center for Indoor
Air Research (CIAR) as 'a cover' to fund the study,
three companies agreed to fund the study directly.
Progress reports for the study were prepared on
Covington and Burling stationery. When the study
was prepared for publication, the tobacco industry
consultant was the sole author (Lee, 1995). The
publication acknowledged 'financial support from
several companies of the tobacco industry' (Lee,
1995). This acknowledgement tells the reader little
about who was actually involved in the design,
conduct and publication of the study. The hidden
roles of the tobacco company lawyers and scientist
raise questions about who was accountable for the
research (Hong and Bero, 2002).
The analysis of tobacco industry documents (Hong
and Bero, 2002) was noticed by Dr E. Yano, one
of the Japanese investigators who was originally
involved in the Japanese Spousal Smoking study.
Dr Yano had been unaware that Dr Lee had
published the study. He had retained the original
data from the study and has reported that Dr Lee's
published analysis excluded data that did not
support misclassification bias (Yano, 2005). Dr Yano
demonstrated that using the full data from the
Japanese Spousal Smoking Study changes the
conclusion of Lee's published report. After 10 years,
the scientific community was able to obtain data that
had been suppressed by the tobacco industry.

Lessons from health hazards | Tobacco industry manipulation of research
159
Late lessons from early warnings: science, precaution, innovation
7.3.3 Strategy 3: publish research that supports the
interest group position
Research has little impact unless it can be cited. The
tobacco industry has realised that funding research
that supports its interests must be followed by the
dissemination of such research in scientific literature.
The tobacco industry uses several vehicles to publish
the findings of its sponsored research, including
funding the publication of symposia proceedings,
books, journal articles and letters to the editors of
medical journals. To suggest that the research it funds
meets scientific standards and that there is substantial
support for its position, the tobacco industry then
cites its industry-funded, non-peer-reviewed
publications in scientific and policy arenas.
Symposium proceedings
Scientific meetings or symposia often result in
the publication of books or journal articles that
summarise the research presented there. The
pharmaceutical industry, for example, publishes
reports of symposia containing poor quality and
unbalanced articles favourable to particular drugs
(Bero et al., 1992; Rochon, 1994). The tobacco industry
has sponsored numerous symposia on second-hand
smoke (Bero et al., 1994) and paid for scientific
consultants to organise and attend these meetings
(Barnoya and Glantz, 2002, 2006; Muggli et al., 2001).
Between 1965 and 1993, reports on 11 symposia on
passive smoking were published. Six were published
as special issues of medical journals, while five were
published independently as books. None of the
symposia was peer reviewed; six were sponsored
by the tobacco industry or its affiliates such as the
Center for Indoor Air Research, The Tobacco Institute
and Fabriques de Tabac Reunies. Two of the six
industry-sponsored symposia did not explicitly
acknowledge industry sponsorship. The tobacco
industry sometimes sponsored conferences through
independent organisations so that their sponsorship
would be hidden (Bero et al., 1995; Bero et al., 1994).
The symposia on passive smoking were attended by
an international group of scientists and held across
the world, including Europe, the United States,
Canada, Japan and Argentina. One symposium report
was published in Spanish. CTR special projects were
often used to support scientists to prepare talks for
conferences and to send scientists to conferences
(Glantz et al., 1996).
On the surface, articles from symposia look like
articles from peer-reviewed journals. To test the
hypothesis that symposium articles on second-hand
smoke differ in content from articles on second-hand
smoke appearing in scientific journals, Bero et al.
(1994) compared the symposia articles to a random
sample of articles on passive smoking from the
scientific literature and to two consensus reports on
the health effects of passive smoking (US DHHS,
1986; NRC, 1986). Of the symposium articles,
41 % (122/297) were reviews, compared with 10 %
(10/100) of journal articles. Symposia articles were
significantly more likely than journal articles to
agree with the tobacco industry position that
tobacco is not harmful (46 % compared to 20 %),
less likely to assess the health effects of passive
smoking (22 % compared to 49 %), less likely to
disclose their source of funding (22 % compared
to 60 %), and more likely to be written by tobacco
industry-affiliated authors (35 % compared to 6 %).
Symposium authors published a lower proportion of
peer-reviewed articles than consensus report authors
(71 % compared to 81 %) and were more likely to be
affiliated with the tobacco industry (50 % compared
to 0 %) (Bero et al., 1994).
Symposia proceedings can potentially influence
policy because they are often cited as if they are
peer-reviewed articles and balanced reviews of
the scientific literature, with no disclosure of
their industry sponsorship. For example, tobacco
industry-sponsored symposia on second-hand
smoke have been used to attempt to refute both
peer-reviewed journal articles and risk assessments
of second-hand smoke (Bero and Glantz, 1993;
Schotland and Bero, 2002; Chapman et al., 1990).
Symposia articles have also been cited in tobacco
industry public relations materials and the press
(e.g. Tobacco Institute, 1986); and as the consensus
of a gathering 'of leading experts from around the
world' who disagree with the published literature on
passive smoking (Johnston and Sullum, 1994).
In summary, tobacco industry-sponsored symposia
articles on second-hand smoke consist, in large part,
of review articles that reach different conclusions
about the health effects of passive smoking than
peer-reviewed journal articles or consensus reports.
Furthermore, symposia are more likely to publish
research that discusses issues that distract from
tobacco as a health problem. The tobacco industry
affiliations of symposia authors suggest that industry
control over publication and research funding is
likely to influence the presentation of findings.
Quality of tobacco industry-funded symposium
publications
When policymakers, judges, lawyers, journalists
and scientists are presented with tobacco
industry-sponsored symposium articles, they must
decide whether to incorporate these publications into

Lessons from health hazards | Tobacco industry manipulation of research
160
Late lessons from early warnings: science, precaution, innovation
their deliberations. Although the lack of balance and
peer review suggests that tobacco industry-sponsored
literature may lack scientific rigour, the issue of peer
review and study quality is a contentious subject.
Methodological quality is determined by the presence
or absence of study design characteristics aimed at
reducing bias, such as blinding, follow-up, controlling
for confounding and controlling for selection bias.
Barnes and Bero (1997) assessed the methodological
quality of the research presented at symposia.
As articles from pharmaceutical industry
sponsored symposia have been found to be of poor
methodological quality (Rochon, 1994; Cho and
Bero, 1996) it was hypothesised that articles from
tobacco industry-sponsored symposia would be
poorer in methodological quality than peer-reviewed
journal articles. Other characteristics of articles were
evaluated that might be associated with quality, such
as disclosure of the source of research sponsorship
and the study's conclusions, topics and design.
Original research articles on the health effects of
second-hand smoke published in peer-reviewed
journals were compared to those published in
non-peer-reviewed symposium proceedings from
1980 to 1994.
The study found that peer-reviewed articles were
better quality than symposium articles independent
of their source of funding, their conclusions on the
health effects of second-hand smoke and the type of
study design. Peer-reviewed articles received higher
scores than symposium articles for most of the criteria
evaluated by the quality assessment instrument.
Quality of tobacco industry-sponsored review
articles
Policymakers and clinicians often rely on review
articles to provide accurate and up-to-date
overviews of a topic of interest (Montini and Bero,
2001). As already noted, a large proportion of
symposium articles are reviews of the health effects
of second-hand smoke (Bero et al., 1994) and are
frequently cited in response to government requests
for information (Bero and Glatz, 1993; Montini
et al., 2002; Schotland and Bero, 2002). In view of
their importance in guiding policy, it is somewhat
disconcerting that published review articles often
reach markedly different conclusions about the
adverse health effects of second-hand smoke.
Barnes and Bero (1998) conducted a study to evaluate
the quality of review articles on the health effects
of passive smoking and to determine whether
the conclusions of review articles are primarily
associated with their quality or with other article
characteristics. The a priori hypotheses were that
review articles concluding that passive smoking is not
harmful would tend to be poor in quality, published
in non-peer-reviewed symposium proceedings
and written by investigators with tobacco industry
affiliations. The topic of the review and the year
of publication were also reviewed as potential
confounding factors.
In the sample of 106 review articles, the only factor
associated with concluding that passive smoking is
not harmful was whether the author of the review
article was affiliated with the tobacco industry
(Barnes and Bero, 1998). As shown in Table 7.1,
review articles concluding that passive smoking is
not harmful were about 90 times more likely to be
funded by the tobacco industry than those concluding
that second-hand smoke is harmful. Methodological
quality, peer-review status, outcomes studied
in the reviews, and year of publication were not
associated with the conclusions of the articles. Thus,
sponsorship of review articles by the tobacco industry
appears to influence the conclusions of these articles,
independent of methodological quality.
The tobacco industry has argued that independent
reviews of second-hand smoke are flawed because
studies with statistically significant results are more
likely to be published than studies with statistically
non-significant results (Dickersin et al., 1992; Shook,
Hardy and Bacon, 1993). The industry argues that
publication bias — the tendency to publish work
with statistically significant results — prevents the
identification of all relevant studies for reviews of the
health effects of second-hand smoke (e.g. Armitage,
1993). Bero et al. (2004) conducted a preliminary
Photo: © istockphoto/Richard Clark
Lessons from health hazards | Tobacco industry manipulation of research
161
Late lessons from early warnings: science, precaution, innovation
Table 7.1 Factors associated with review articles concluding that passive smoking is not
harmful to health: multiple logistic regression analysis
Factor Odds ratio (95 % CI) P value
Quality score 1.5 (< 0.1–67.5) 0.83
Not peer reviewed v. peer reviewed 1.3 (0.3–5.4) 0.70
Tobacco industry sponsored v. not tobacco industry sponsored 88.4 (16.4–476.5) < 0.001
Outcomes — lung cancer v. other clinical outcomes 1.6 (0.2–10.3) 0.63
Heart disease v. other clinical outcomes 1.6 (0.2–14.7) 0.67
Year of publication 1.1 (0.9–1.3) 0.45
Source: Barnes and Bero, 1998.
study of publication bias showing that statistically
non-significant studies are published: approximately
20 % of the published peer-reviewed articles on passive
smoking present statistically non-significant findings.
By interviewing investigators studying second-hand
smoke and health effects, Misakian and Bero
(1998) determined that studies with statistically
non-significant results take about two years longer to
be published than those with statistically significant
results. For studies conducted in humans, only
statistical significance was predictive of time to
publication, not study design or sample size. Thus,
the tobacco industry's argument that statistically
non-significant results are not published is invalid.
Since statistically non-significant results are published
but take longer to be published than statistically
significant results, reviews of research should attempt
to include unpublished data and be periodically
updated. Reviews conducted by the Cochrane
Collaboration, for example, attempt to identify
unpublished studies and include them in reviews
if they meet quality standards. Cochrane reviews,
which are published online, are also regularly
updated (Bero and Rennie, 1995).
7.3.4 Strategy 4: suppress research that does not
support the interest group position
Interest groups are eager to fund and publish
research that supports their position and hesitant
to publicise research that does not. In some
cases, tobacco industry lawyers and editors have
edited their externally funded scientific research
publications; in other cases they have prevented
publication of the research (Hong and Bero, 2002;
Muggli et al., 2001; Barnoya and Glantz, 2002).
Editing has included attempts to obscure evidence
on adverse health effects by using the code word
'zephyr' for 'cancer' in internal memos about health
effects research (Glantz et al., 1996; BAT, 1956).
Research conducted internally by tobacco companies
or through industry-controlled research organisations
is likely to be suppressed if it is unfavourable to
the industry. For example, the German tobacco
industry-supported research organisation, INBIFO,
did not publish its research showing that sidestream
smoke is more toxic than mainstream smoke
(Grüning et al., 2006).
Tobacco companies have also conducted internal
research on the use of chemical additives to reduce,
mask or otherwise alter the visibility, odour, irritation
or emission of second-hand smoke. Some of these
studies showed that the additives increased emissions
of toxins such as carbon monoxide or the carcinogenic
substances, N'-nitrosoniornicotine and benzo(a)
pyrene (Conolly et al., 2000). Virtually none of this
research has been published in scientific literature,
however, and data on additives is not typically
available to public health policymakers.
7.3.5 Strategy 5: criticise research that does not
support the interest group position
Another strategy that the tobacco industry has used
to stimulate controversy about research on risk has
been to criticise research that is not favourable to
its position. Science is improved by constructive
criticism. However, the tobacco industry has misused
legitimate means of scientific debate, such as letters
to the editor of scientific journals and editorials.
The tobacco industry has also used less legitimate
methods to criticise research, including attacking the
integrity of researchers or obtaining data through
lawsuits and reanalysing it using inappropriate
techniques (Barnes et al., 1995).
In order to get its views into public commentary on
risk assessments (Bero and Glantz, 1993; Schotland
and Bero, 2002) or the lay press (Chapman et al.,
1990), the tobacco industry has frequently cited
letters to the editor as if they were peer-reviewed

Lessons from health hazards | Tobacco industry manipulation of research
162
Late lessons from early warnings: science, precaution, innovation
journal articles. Letter authors affiliated to the tobacco
industry often fail to disclose their affiliation. These
findings support the suggestions by a number of
journal editors that letter writers should disclose
potential conflicts of interest and that journals should
peer review letters (Rennie, 1993).
As mentioned above, the tobacco industry has
maintained large teams of international scientific
consultants (Chapman, 1997; Muggli et al., 2001;
Barnoya and Glantz, 2002). A major goal of the
tobacco industry's scientific consultancy programme
was to refute data about the harmful effects of
tobacco. Industry consultants were paid to criticise
independent research on tobacco and second-hand
smoke via participation in scientific conferences;
publications such as conference proceedings, journal
articles and books; media appearances; testimony at
tobacco litigation trials; forming a scientific society on
indoor air; and preparing statements for government
committees. The industry consultant programmes
were international and were used to discredit
research conducted by non-industry scientists around
the world (Chapman, 1997; Muggli et al., 2001;
Barnoya and Glantz, 2002).
7.3.6 Strategy 6: changing scientific standards
As described above, the tobacco industry has devoted
enormous resources to attacking and refuting
individual scientific studies. In addition, the industry
has attempted to manipulate scientific methods and
regulatory procedures for its benefit. The tobacco
industry has influenced the debate around 'sound
science' (Ong and Glantz, 2001), standards for
risk assessment (Hirschhorn and Bialous, 2001),
international standards for tobacco and tobacco
products (Bialous and Yach, 2001) and laws related to
data access and quality (Baba et al., 2005).
The tobacco industry has played a major role in
developing ventilation standards for indoor air
quality and in establishing international standards
for tobacco and tobacco products. The International
Organization for Standardization (ISO) develops
international standards for tobacco and tobacco
products, including the measurement of tar and
nicotine yield. The tobacco industry, working through
the Cooperation Centre for Scientific Research
Relative to Tobacco (CORESTA) gathered scientific
evidence for ISO and suggested the standards
that were adopted (Bialous and Yach, 2001). These
standards incorrectly imply that there are health
benefits from low-tar and low-nicotine products
(Djordjevic et al., 1995). The tobacco industry
has also been involved in developing ventilation
standards for over 20 years. The industry influenced
the development of ventilation standards by the
American Society of Heating, Refrigeration, and Air
Conditioning Engineers (ASHRAE) by generating
data and presenting it to the committee (Bialous and
Glantz, 2002). This resulted in a standard that ignores
the health effects of second-hand smoke exposure,
concentrating instead on a 'comfort' standard.
In the early 1990s, the tobacco industry launched a
public relations campaign about 'sound science' and
'good epidemiological practices' (GEP) and used this
rhetoric to criticise government reports, particularly
on the harms of environmental tobacco smoke. All
scientists agree that research should be rigorously
conducted. But the 'sound science' and 'GEP'
campaigns were public relations efforts controlled
by industry executives and lawyers to promote
unreasonably high standards of proof about the harm
caused by the industry's products. For example,
'sound science' rhetoric argues that epidemiological
studies can never establish evidence of harm because
they cannot 'prove' causality. This approach ignores
the fact that a comprehensive assessment of risk
involves considering all the evidence related to a
toxin, not just the epidemiology (Ong and Glantz,
2001).
The tobacco industry also developed a campaign
to criticise the technique of risk assessment of low
doses of a variety of toxins (Hirschhorn and Bialous,
2001). The tobacco industry worked with the
chemical, petroleum, plastics and chlorine industries
to develop its criticisms of risk assessment. In fact,
the first version of GEP was drafted by the Chemical
Manufacturers Association. After about ten years, by
the late 1990s, the industry's 'sound science' public
relations campaign ended. The tobacco industry
then turned to advancing the 'sound science' concept
through legislation (Baba et al., 2005).
One major goal of the tobacco industry has been to
obtain data from independent studies and reanalyse
it using 'sound science' criteria to reach different
conclusions. Philip Morris, for example, used a
three-step strategy to obtain data:
1. asking the researchers for the data directly;
2. litigation;
3. encouraging the enactment of policies that release
data (Baba et al., 2005).
The industry's efforts resulted in 'sound science
legislation': laws that influenced access to data and
standards for data analysis.

Lessons from health hazards | Tobacco industry manipulation of research
163
Late lessons from early warnings: science, precaution, innovation
In 1998, the United States Congress enacted a data
access law as a rider to the Fiscal Year 1999 Omnibus
Appropriations Act (US Congress, 1999). The law,
for the first time, made all data produced under
federally funded research studies available on
request through the Freedom of Information Act
(FOIA) (Zacaroli, 1998). Two years after the adoption
of the data access provision, another amendment
was added to the 2001 Omnibus Appropriations
Act. The Data Quality Act (2000) requires the
Office of Management and Budget to develop
government-wide standards for data quality in the
form of guidelines. Individual federal agencies must
promulgate their own conforming guidelines based
on OMB's model and adopt standards that 'ensure
and maximise the quality, objectivity, utility and
integrity of information disseminated' by federal
agencies (OMB Watch, 2002). The standards to be
adopted were created by the industry sponsors, not
independent researchers.
While the public had an opportunity to comment
on implementing the laws, these amendments
were initially passed and adopted without a
legislative hearing, committee review or debate
(Renner, 2002). The scientific, academic research
and public health communities voiced concerns
during the public comment period about
potential problems with confidentiality of medical
information, discouragement of research subjects,
misinterpretation of incomplete or prematurely
released data sets, delay of research, protection of
national security information, and administrative
and financial burdens (AAAS, 1999). The research
community was also concerned that these measures
were supported by industry groups seeking to
contest environmental and other regulations
(Zacaroli, 1998).
Although the tobacco industry intended to hide
its involvement in the data access and quality acts,
internal industry documents reveal that these policies
were driven by tobacco industry efforts to coordinate
corporate interests. Tobacco industry strategies to
advance sound science legislation included (Baba
et al., 2005):
• demonstratingthatthepubliccaresaboutthe
issue by sponsoring a poll on issues of data access
and rules of epidemiological studies that can be
made public;
• leveragingalliesandgroupsthathavealready
taken a stand on the issue;
• usingscientistsandtechnicalconferencestofocus
on the issue;
• encouragingasmallgroupofmembersof
Congress to take a stand on the issue;
• encouragingtheAdministrationtotakeastand
for sound science;
• mobilisingalliedindustries(i.e.fishing,utilities,
waterworks) to lobby their local representatives;
• helpingtoorganisecoalitionsforother
epidemiological issues coming up soon
(e.g. fishing industry, mercury, methyline
chloride);
• educatingandmobilisingthebusiness
community on sound science v. junk science and
the federal legislative/regulatory process;
• usingstatestogenerateaction—conducting
briefings in states on epidemiological studies
and the need for uniform standards and
encouraging the passage of state laws;
• developingbroadbipartisansupportfor
'freedom of information' with regards to the data
behind regulations and laws;
• leveraginglobbyiststocontactkeylegislative
members;
• briefingthemedia;
• briefingbusinesscoalitionsontheneedfordata
access;
• usingtheCongressionalScienceCommitteeto
influence Congress.
Together, the data access and data quality acts
provide a mechanism for challenging the scientific
merit of data outside scientific journals and other
channels of scientific review (McGarity, 2004;
Kaiser, 1997). As scientists, legal experts and
environmentalists have pointed out, however,
the data access and data quality riders have the
potential to block agencies from using emerging
science from non-industry sources and to slow the
regulatory process (Kaiser, 2003; Hornstein, 2003;
Shapiro, 2004). The laws can be used to prevent
future policies and to repeal existing policies that
do not meet the data quality standards. The laws
could shift the scientific standards of data used for
policy purposes to favour standards promulgated
by industry. Finally, access to data and quality
standards are not applied equitably; they only apply
to data generated with government funding, not
industry funding.

Lessons from health hazards | Tobacco industry manipulation of research
164
Late lessons from early warnings: science, precaution, innovation
Panel 7.1 Shaping risk assessment in the US and the EU: the role of the tobacco industry
Katherine Smith, Anna Gilmore and Gary Fooks
The interest of Philip Morris in shaping risk assessment was precipitated by the US EPA risk assessment
of environmental tobacco smoke (ETS), which resulted in ETS being categorised as a class A human
carcinogen (Hirschhorn and Bialous, 2001). Philip Morris challenged the assessment as part of its broader
'sound science' campaign (Ong and Glantz, 2001). This involved lobbying for laws requiring that:
• epidemiological studies meet a particular set of criteria or standards before they can officially inform
policy decisions;
• epidemiological data used in publicly funded studies be made available through freedom of information
requests.
Although the tobacco industry's campaign was ultimately unsuccessful in overturning the EPA's
classification of ETS, it did manage to place 'a cloud over its validity' until 2002 (Muggli et al., 2004),
leading to delays in subsequent introduction of protective legislation. Further, Philip Morris had some
success in introducing data access laws and shaping the Data Quality Act (2000) (Baba et al., 2005).
Philip Morris believed that a similar campaign might be even more effective in Europe, where officials had
not yet taken up the scientific threat of ETS to the same extent. From the mid-1990s onwards, therefore,
it focused its campaign more heavily on Europe. Here, informed by what the Chemical Manufacturers
Association had termed 'good epidemiological practice' (GEP), Philip Morris concentrated on lobbying for a
mandatory set of criteria or standards that epidemiological studies would have to meet before they could
be officially considered by policymakers in Europe (Ong and Glantz, 2001). The Philip Morris standards for
'good epidemiological practice' included a requirement for evidence relating to relative risks of less than
2.0 to be disregarded as too weak to warrant policy intervention.
As of late 2000 Ong and Glantz (2001) concluded that, despite the efforts of Philip Morris, 'no European
Union resolution on GEP had been produced'. As far as we are aware, this remains the case.
British American Tobacco (BAT) managers studied the Philip Morris campaigns carefully and from
1995 onwards considered lobbying for a mandatory requirement for 'structured risk assessment' in
EU policymaking because they believed it could be used to prevent the introduction of public smoking
restrictions (Smith et al., 2010; BAT, 1995 and 1996). By this stage, the industry was well aware
of the negative health impacts of second-hand smoke and was simultaneously trying to influence
the evidence-base on this issue. BAT managers believed that 'a legislated demand for structured
risk assessment', governed by strict 'rules for the assessment of epidemiological and animal data'
would 'remove the possibility of introducing public smoking restrictions that are based on risk claims'
(BAT, 1995).
Our analysis of BAT's internal documents has not yet established precisely what BAT managers meant
when they used the term 'structured risk assessment'. All of the documents with titles indicating that they
include detailed information on this issue have been redacted (
3
).
A 1995 BAT document makes it clear that the company's interpretation of 'risk assessment' involved a
set of 'rules for the assessment of epidemiologic and animal data', which BAT managers believed would,
if applied, make it 'apparent that ETS has not been proven to be a cause of disease in non-smokers'
(BAT, 1995).
BAT managers wanted to use risk assessment as a way of limiting officials' discretion. For example, a
document discussing the company's efforts to influence risk assessment says: 'The challenge will be
to persuade government departments to subordinate policy or judgemental considerations in favour of
scientific rigour in risk assessment' (Gretton, undated [circa 1995]).
(
3
) See for example BAT (1991 and 1996). The Legacy Tobacco Documents Library website, which hosts these documents, states that
the term 'redacted', 'Indicates whether the document contains words or sentences that were erased (redacted) by the tobacco
company due to confidentiality issues (i.e. trade secrets, attorney/client privileges) before the document was publicly released'
(University of California, 2011).

Lessons from health hazards | Tobacco industry manipulation of research
165
Late lessons from early warnings: science, precaution, innovation
Panel 7.1 Shaping risk assessment in the US and the EU: the role of the tobacco industry
(cont.)
In practice, this constituted a way of undermining the precautionary principle as a basis for policy
decisions.
The key innovation of BAT's European campaign was the decision to focus on promoting risk assessment
within a framework of 'cost-benefit analysis', a term that BAT used interchangeably with business
impact assessment (see Smith et al., 2010). This had the additional effect of embedding economic
considerations into the risk assessment process, which would also require interventions to protect the
public against particular risks to be justified on the basis of economic costs (BAT, 1996; European Policy
Centre, 1997).
BAT initially sought advice on how to shape risk assessment in the EU from the US advisers to Philip
Morris, Covington and Burling (Covington and Burling, 1996). They advised BAT that although there was
little interest in risk assessment within the European Commission at the time it might be possible to
include 'structured risk assessment' in detailed guidance for business impact assessments, which had been
flagged as a priority for the European Commission in 1996.
BAT was aware that a campaign for regulatory reform with known links to the tobacco industry was
unlikely to succeed (Honour, 1996). It had been advised to work through a 'front group' and to recruit
other companies with similar interests, such as other large firms in regulated sectors (MacKenzie-Reid,
1995). Following this advice, BAT approached the European Policy Centre (a prominent Brussels‑based
think tank with strong links to the Commission) to lobby for regulatory reforms on its behalf (Smith et al.,
2010). BAT and the European Policy Centre then jointly set about recruiting other business interests to this
campaign (Smith et al., 2010). These companies, which included large corporations from the oil, chemical
and pharmaceuticals sectors, established an invitation‑only sub‑group within the European Policy Centre,
known as the Risk Forum (Smith et al., 2010).
These efforts contributed to certain amendments to the Treaty on European Union (EU, 1997), placing a
legal duty on the Commission to 'consult widely' and to minimise the potential 'burden' of policy changes
on 'economic operators' and others (EU, 1997). BAT interpreted this to mean that business impact
assessment and risk assessment were now mandatory within EU policymaking. The company perceived
this as 'an important victory' (BAT, undated).
The guidelines for EU officials on how to undertake impact assessment have been revised several times
since and now incorporate guidance on undertaking risk assessment (European Commission, 2009).
In 2006–2007, under pressure to open up to civil society organisations and other members of the
European Policy Centre (which was under new leadership), the coalition of companies involved in the
think tank's Risk Forum left and established a separate organisation called the European Risk Forum. This
group describes itself as 'an expert led, not-for-profit think tank' (European Risk Forum, 2008a), despite
solely representing corporate interests, virtually all of which are connected to the chemical and tobacco
industries. This was confirmed via personal correspondence from the Forum's chair, Dirk Hudig, in February
2010. The European Risk Forum is now actively encouraging the European Commission to adopt a more
structured approach to risk assessment and risk management (European Risk Forum, 2008b), although it
remains unclear precisely what this involves.
Recent analyses suggest that these corporate efforts have been somewhat successful in redefining
policymakers' understandings of and responses to risks, including those that limit use of the precautionary
principle in the EU (Löfstedt, 2004) and the United Kingdom (Dodds, 2006). However, as risk assessment
continues to be actively debated in Brussels, it is not yet possible to assess the success of the BAT or Philip
Morris campaigns.
It is of course legitimate for corporate interests to contribute to discussions on assessing scientific evidence
and weighing up risks. However, it is important to ensure that this influence is transparent, is not excessive
in comparison to other stakeholders, and does not compromise public welfare.

Lessons from health hazards | Tobacco industry manipulation of research
166
Late lessons from early warnings: science, precaution, innovation
7.3.7 Strategy 7: disseminate interest group data
or interpretation of risk in the lay press
While the tobacco industry appears to have
recognised the importance of publishing work that
supports its position in the scientific literature,
the industry also seems aware of the need to get
research data directly into the hands of the public
and policymakers. How, then, were the public
and other stakeholders involved in generating,
presenting, understanding, communicating and
using science to refute data on the adverse health
effects of tobacco?
The important role of the media in communicating
risk has been extensively studied (Nelkin, 1985;
Raymond, 1985). The tobacco industry has been
active in stimulating controversy in lay print media
about the health effects of second-hand smoke. In
a cross-sectional sample of 180 North American
newspaper and 95 magazine articles reporting on
second-hand smoke research between 1981 and
1995, Kennedy and Bero (1999) found that 66 % of
newspaper articles and 55 % of magazine articles
left readers with the impression of continuing
controversy about second-hand smoke research.
However, the proportion of those articles concluding
that the research was controversial remained
relatively constant.
Although tobacco industry-sponsored research
studies were not widely cited in lay press articles,
tobacco industry affiliated individuals were often
cited (Kennedy and Bero, 1999; Malone et al.,
2001). Among the 180 newspaper articles examined
by Kennedy and Bero (1999), 52 % cited tobacco
industry officials, whereas 56 % cited government
officials and 46 % cited independent scientists. This
citation of tobacco industry officials as experts on
scientific studies on second-hand smoke could have
contributed to the emphasis on controversy.
7.3.8 Strategy 8: present interest group data
or interpretation of risk directly to
policymakers
The last strategy in the tobacco industry's effort
to stimulate controversy about data on risk has
been to get its funded research directly into the
hands of individuals who are likely to influence
policy. A series of in-depth case studies have
been undertaken, examining the role of research
evidence in the development of two risk assessments
of second-hand smoke, two state indoor air
regulations and two United States federal tobacco
regulations (Schotland and Bero, 2002; Roth et al.,
2003, Bero et al., 2001; Bryan-Jones and Bero,
2003). Each study addressed the role of the tobacco
industry in developing risk assessments and
regulations by analysing archival data, including
written commentary and hearing transcripts, and
interviewing key policymakers.
In the United States, the processes for developing
these risk assessments and regulations involves
the appropriate government agency reviewing the
relevant scientific literature, preparing a draft report,
collecting written and oral public commentary, and
revising the report based on that public commentary
(Jasanoff, 1987 and 1996, Silbergeld, 1993). Public
participation in the process is important for
shaping the findings of the final risk assessment
or regulation, and for public acceptability of the
findings (Jasanoff, 1987). Furthermore, public
commentary could help prevent the 'capture' of the
risk assessment process by interest groups (Wilson,
1989).
Risk assessments of second-hand smoke
As noted earlier in this chapter, the US Environmental
Protection Agency (EPA) published a risk assessment
of environmental tobacco smoke (ETS) in 1992,
which concluded that passive smoking is associated
with lung cancer in adults and respiratory disease
in children. The risk assessment's development
was considerably delayed by the tobacco industry's
criticisms of the report (US EPA, 1992). Sixty-four
per cent (69/107) of submissions received by the
EPA during the public commentary period claimed
that the conclusions of the draft were invalid and,
of these, 71 % (49/69) were submitted by tobacco
industry-affiliated individuals (Bero and Glantz, 1993).
The tobacco industry-affiliated reviewers supported
their criticisms of the risk assessment by selectively
citing non-peer-reviewed literature, especially articles
from symposium proceedings (Bero and Glantz, 1993).
Thus, tobacco industry-sponsored research that was
not published in the peer-reviewed scientific literature
was submitted directly to the EPA for review.
Schotland and Bero (2002) examined the
development of the California risk assessment,
revealing that participation in the public
contribution process was not balanced among
all interested parties, and was dominated by the
tobacco industry. Critics and supporters of the risk
assessment used different criteria to evaluate the
science, suggesting that they were constructing
the evidence to support their predefined positions.
Similar to the US EPA risk assessment, the tobacco
industry was able to use its funded research to
support its arguments against the California risk
assessment.

Lessons from health hazards | Tobacco industry manipulation of research
167
Late lessons from early warnings: science, precaution, innovation
Indoor air regulation
During the 1990s, the Washington and Maryland
Occupational Safety and Health Administrations
each promulgated regulations restricting smoking
in private workplaces. The US Occupational
Safety and Health Administration also proposed
a workplace smoking restriction but this failed.
Internal tobacco industry documents show that one
strategy the industry used to defeat the proposed
federal regulation was to 'produce data to counter
the findings about the adverse health effects of
second-hand smoke' (Bryan-Jones and Bero, 2003).
Despite the tobacco industry's use of this strategy
and others to defeat the Maryland and Washington
regulations, the state regulations were passed
(Mangurian and Bero, 2000).
The two states' regulatory development processes
required a public commentary period. Opposition
to the regulation came primarily from the
tobacco industry, small businesses, and business
organisations and appeared to be coordinated (Bero
et al., 2001). Much of the business group opposition
was supported by the tobacco industry, although
this support was not disclosed in the public
commentary (Mangurian and Bero, 2000). Although
arguments not related to science were more
common than scientific arguments as a whole,
arguments about science were used more often
by opponents than supporters of the regulations
(Bero et al., 2001). As in the other examples cited
in this chapter, opponents of regulation, primarily
the tobacco industry, cited industry-sponsored
symposium proceedings or peer-reviewed journal
articles of low methodological quality to support
their criticisms of the science on which the
regulations were based.
Apparent disagreement among experts during
public testimony reinforces uncertainty about
the data underpinning risk estimates or
regulations. The studies of the Washington and
Maryland regulations suggest, however, that
the industry-supported experts used different
criteria to evaluate the science, different bodies
of evidence to support their claims and relied
on arguments about specific studies rather than
emphasising the body of evidence as a whole.
In general, the involvement of tobacco industry
lawyers and executives in the design, conduct
and dissemination of research has an impact on
how controversy can influence public opinion and
policy decisions.
Box 7.2 describes how the tobacco industry worked
to undermine tobacco control activities at the
World Health Organization.
7.4 Lessons learned
The tobacco industry has had a long-standing
strategy of funding research and disseminating
it through their sponsored, non-peer-reviewed
publications. These strategies have remained
relatively consistent as the industry has evolved
from refuting research on active smoking to
refuting research on second-hand smoke. Despite
the questionable conduct of much of this research,
the tobacco industry has widely disseminated it to
lay journalists and policymakers. In addition, the
tobacco industry has a record of suppressing and
criticising research that is unfavourable to its position.
Tobacco industry lawyers and executives, rather than
scientists, have been in control of the design, conduct
and dissemination of this research, thereby protecting
the research from public discovery. Since the
tobacco industry's efforts to manipulate research are
international endeavours, there is a need for global
awareness of the strategies that the industry has used
to influence data on risk.
When data on risk appear to be controversial,
users of the data should investigate the sources of
the controversy. Does the controversy exist only
because the findings of interest group-funded
research are contrary to data collected by others? Is
the controversy supported primarily by evidence
published in interest group-supported publications?
Is the controversy supported primarily by research
publications of low scientific quality? Is the
controversy perpetuated in the lay press through
citation of interest group-affiliated individuals? Are
the data that suggests a controversy presented to
policymakers only by the interest group?
Policymakers should apply these questions to all
situations in which a company has an interest in
creating controversy about the risks of its products.
The tobacco industry differs substantially from other
industries in the deadly nature of its products when
used as directed, and the historical lack of regulation
of tobacco products. However, the tobacco industry's
methods for influencing the design, conduct and
publication of research are similar to those of other
corporate interests. For example, studies examining
the association of pharmaceutical industry funding
and research outcomes suggest that such funding
produces studies with outcomes that are favourable
to the sponsor (Lexchin et al., 2003; Cho and Bero,
1996; Bekelman et al., 2003). The reasons for this
observed association of funding and outcome are
not clear (Bero and Rennie, 1996). For example, the
funding source does not appear to influence the
methodological quality of the published research
(Lexchin et al., 2003). Therefore, biased outcomes may
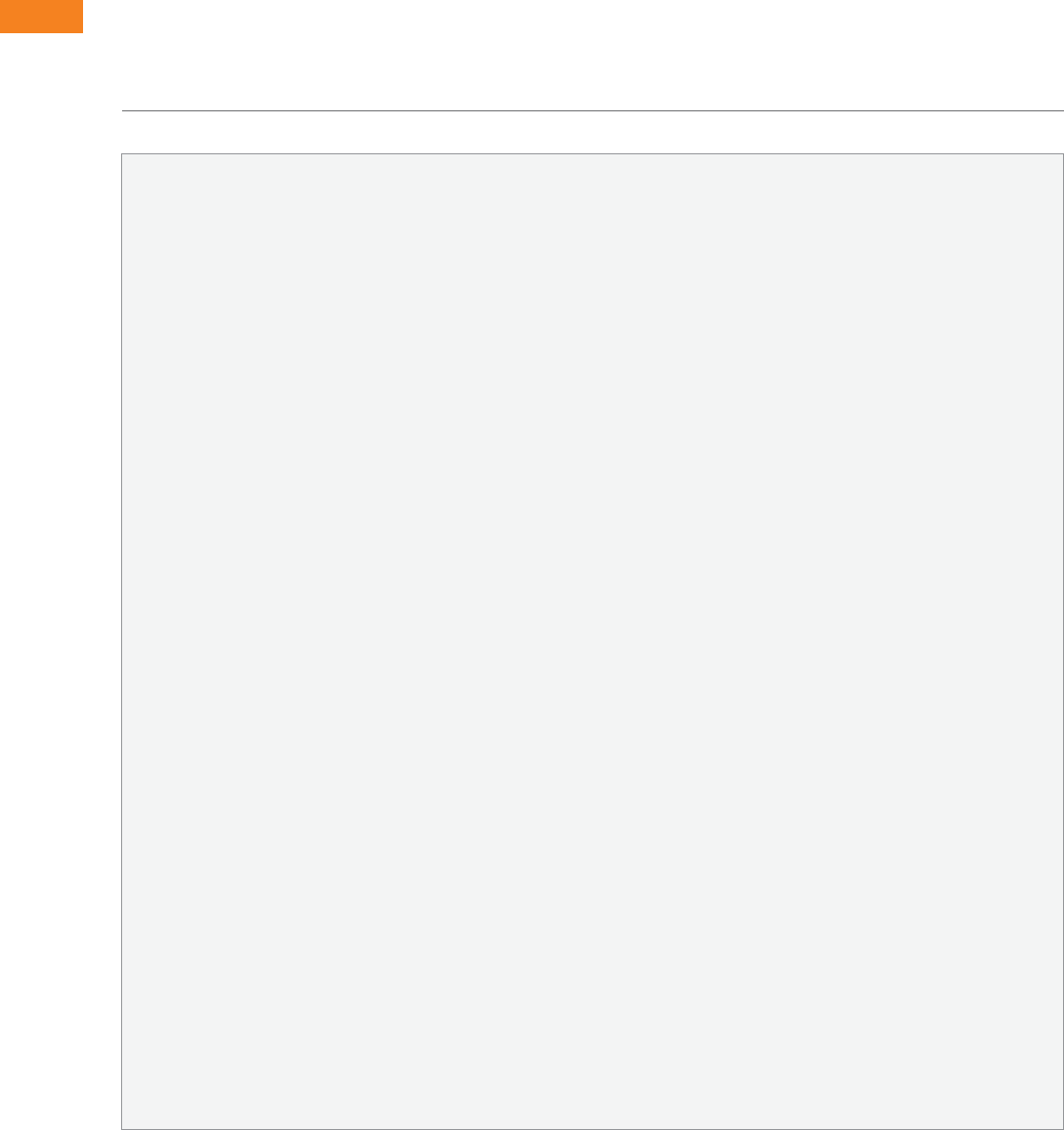
Lessons from health hazards | Tobacco industry manipulation of research
168
Late lessons from early warnings: science, precaution, innovation
Box 7.2 Tobacco industry strategies to undermine tobacco control activities at the World
Health Organization
'Tobacco industry documents reveal that, for many years, tobacco companies have deliberately subverted the
efforts of the World Health Organization (WHO) to control tobacco use. The attempted subversion has been
elaborate, well financed, sophisticated and usually invisible.
The release of millions of pages of confidential tobacco company documents as a result of lawsuits against
the tobacco industry in the United States has exposed the activities of tobacco companies in resisting tobacco
control efforts. That tobacco companies resist proposals for tobacco control comes as no surprise. What is
now clear is the scale and intensity of their often deceptive strategies and tactics.
The tobacco companies' own documents show that they viewed the WHO, an international public health
agency, as one of their foremost enemies. The documents show further that the tobacco companies instigated
global strategies to discredit and impede the WHO's ability to carry out its mission. The tobacco companies'
campaign against the WHO was rarely directed at the merits of the public health issues raised by tobacco use.
Instead, the documents show that tobacco companies sought to divert attention from the public health issues,
to reduce budgets for the scientific and policy activities carried out by the WHO, to pit other UN agencies
against the WHO, to convince developing countries that the WHO's tobacco control programme was a 'first
world' agenda carried out at the expense of the developing world, to distort the results of important scientific
studies on tobacco and to discredit the WHO as an institution.
Although these strategies and tactics were frequently devised at the highest levels of tobacco companies,
the role of tobacco industry officials in carrying out the strategies was often concealed. In their campaign
against the WHO, the documents show that tobacco companies hid behind a variety of ostensibly independent
quasi‑academic, public policy and business organisations, whose tobacco industry funding was not
disclosed. The documents also show that tobacco company strategies to undermine the WHO relied heavily
on international and scientific experts with hidden financial ties to the industry. Perhaps most disturbing,
the documents show that tobacco companies quietly influenced other UN agencies and representatives of
developing countries to resist the WHO's tobacco control initiatives.
That top executives of tobacco companies sat together to design and set in motion elaborate strategies to
subvert a public health organisation is unacceptable and must be condemned. The Committee of Experts
believes that the tobacco companies' activities slowed and undermined effective tobacco control programmes
around the world.
Given the magnitude of the devastation wrought by tobacco use, the Committee of Experts is convinced
that, on the basis of the volume of attempted and successful acts of subversion identified in its limited
search, it is reasonable to believe that the tobacco companies' subversion of the WHO's tobacco control
activities has resulted in significant harm. Although the number of lives damaged or lost as a result of the
tobacco companies' subversion of WHO may never be quantified, the importance of condemning the tobacco
companies' conduct, and taking appropriate corrective action, is overriding'.
be the results of how the research questions are asked,
how the research is actually conducted and whether
the results are published (or not published). Food
industry funding for research has also been shown to
produce outcomes favourable to the sponsor (Nestle,
2002; Levine et al., 2003).
A report by the Union of Concerned Scientists
(2007) has documented how ExxonMobil has used
the tactics of the tobacco industry to stimulate
controversy about climate science. ExxonMobil
has raised doubts about the evidence, hidden
involvement behind front groups, sponsored
scientific spokespersons to criticise the science and
attempted to shift the focus away from existing
evidence to the need for 'sound science'.
The public health community must learn more about
the internal behaviour of corporations other than the
tobacco industry in order to make conclusions about
similarities in corporate behaviour. The release of
millions of internal tobacco industry documents has
given the public health community insights into the
inner workings of the tobacco industry and revealed
Source: Summary of a report of the WHO Committee of Experts on Tobacco Industry Documents (CETID, 2000).

Lessons from health hazards | Tobacco industry manipulation of research
169
Late lessons from early warnings: science, precaution, innovation
their previously hidden involvement in manipulating
research (Bero, 2003). However, this insight is not
available for most corporate sectors.
In some of the few other analyses of internal
industry documents, Markowitz and Rosner
describe how the chemical, asbestos and lead
industries manipulated research about the harms of
their products (Markowitz and Rosner, 1991, 2000,
2002). Their analysis reveals that these industries
used the same tactics as tobacco companies to
create controversy about the health effects of
tetraethyl lead, asbestos, polyvinyl chloride and
other chemicals. A recent issue of the International
Journal of Occupational and Environmental Health
relies heavily on internal company documents that
the authors obtained by serving as expert witnesses
in litigation (Special Issue, 2005). It describes how
a variety of chemical companies and their trade
organisations have used the strategies outlined in
this article:
1. funding research that supports the interest
group's position;
2. hiding industry involvement in research;
3. publishing research that supports the interest
group's position;
4. suppressing research that does not support the
interest group's position;
5. criticising research that does not support the
interest group's position;
6. changing scientific standards;
7. disseminating interest group data or
interpretation of risk in the lay press;
8. disseminating interest group data or
interpretation of risk directly to policymakers.
The role of the sponsor in designing, conducting
and disseminating research can be evaluated only
if interest group involvement in all steps of the risk
determination process is fully described. Thus,
funding sources for all published research should
be fully disclosed. Our analyses show, however,
that disclosure of funding sources often provides
incomplete information about the involvement of
the sponsors in the research process. The tobacco
industry has a long history of hiding the involvement
of its lawyers and executives in the designing,
conducting and disseminating research. If internal
tobacco industry documents had not been made
available to the public, much of what is known about
the industry's manipulation of research would have
remained undiscovered.
Disclosures should not be limited to describing
the roles of the research funders in all stages of the
research process. Personal financial ties between
investigators and corporate interests (such as
consulting fees, stock ownership, honoraria etc.)
should also be fully disclosed. Personal financial
ties are increasing (Boyd and Bero, 2000) and are
associated with favourable research outcomes
for the corporate interest, even if the corporate
interest is not funding the research (Lexchin et al.,
2003). Experts who criticise research describing
the harms of a company's product should also
fully disclose their financial ties with the company.
These complete and accurate disclosures should be
found in scientific publications (including research
articles, letters to the editor and editorials),
citations in the lay press, and testimony in policy or
legal settings.
Full disclosure of a sponsor's role in designing,
conducting and publishing a study could also
improve the peer-review process. Peer reviewers are
typically limited by the information available in the
article they are reviewing. The peer review process
itself should be conducted by individuals with
adequate expertise and be independent of industry
sponsors.
The findings presented in this chapter also have
implications for how experts should be selected
to participate in the risk assessment process. As
suggested by others, professional competence,
diversity of political views, disciplines, opinions
and attitudes are important (von Winterfeldt, 1992).
However, consideration should also be given to
affiliation or interest group bias and how this will
affect risk assessment. Encouraging transparency
regarding the roles of interest groups in developing
and disseminating data on risk will not prevent
their involvement in the process. However, such
transparency will make it easier to determine which
strategies, if any, an interest group has been using to
influence the data.
Detailed and accurate financial disclosures of
research funding and financial ties are necessary, but
not sufficient, for safeguarding the integrity of the
research record. One possible benefit of disclosure
is that it might discourage scientists from entering
into financial relationships that could detract from
the perceived integrity of their research. Another
possible benefit is that transparency might improve
public trust in research (Cho, 1998). Krimsky

Lessons from health hazards | Tobacco industry manipulation of research
170
Late lessons from early warnings: science, precaution, innovation
(2003), however, has described disclosure as a
'rationalisation for creating more serious conflicts'.
He points out that disclosure is a 'public relations'
response to dealing with corporate influence on
research and not a way of potentially decreasing the
effect of the corporate sponsor on research integrity.
Although greater transparency about industry
involvement in research could facilitate evaluation
of biases in the design, conduct and reporting that
might be introduced by such sponsorship, it will
not eliminate the biases. Furthermore, if researchers
and institutions are concerned that the public
views industry-sponsored research as less credible,
regardless of any effect on bias, eliminating financial
ties may be the best way to deal with the issue.
A number of scholars have argued that there should
be a total ban on clinical investigators' financial ties
to companies that fund their research (Krimsky,
2003; Dana, 2003). These proposed bans eliminate
the need for oversight committees to 'manage' the
conflict of interest and protect against even the
appearance of conflict.
Schafer (2003) supports the 'sequestration thesis',
which would eliminate direct corporate sponsorship
of research and financial ties of investigators.
Sequestration could be achieved by forming
an independent research institute, funded by
companies, to support research. Shamoo and
Resnik (2003) have noted, however, that eliminating
financial ties and corporate funding may not be
realistic today. Some investigators advocate 'self
regulation': voluntary compliance with professional
society guidelines, or adaptation of the federal
conflict of interest policy to clinical trials funded by
private sponsors (Boyd et al., 2003).
Support for banning corporate funding of research
is most developed among academic institutions that
have policies prohibiting researchers from accepting
tobacco industry funding for research. For example,
some academic institutions, particularly schools of
medicine and public health, have developed bans on
tobacco industry funding (Herman, 2002). Examples
include Harvard University and the University
of Sydney. Some funding agencies (e.g. Legacy
Foundation) have developed policies that require
such bans as a condition for receiving funding
(Shield, 2001).
Bans on tobacco industry support for research
are warranted in view of the industry's history of
deception about its role in designing, conducting
and disseminating industry-supported research.
They are further justified by the tobacco industry's
motives for funding research, which include
distracting attention from tobacco's health risks,
gaining credibility and using the research for public
relations (Cohen, 2003).
Photo: © istockphoto/Gremlin

Lessons from health hazards | Tobacco industry manipulation of research
171
Late lessons from early warnings: science, precaution, innovation
Table 7.2 Key dates relating to knowledge of harm from active and second-hand smoke
1604 King James I of England wrote 'A Counterblaste Against Tobacco' expressing his distaste for tobacco, particularly
tobacco smoking. This was one of the earliest anti-tobacco publications
1903–1908 In the United Kingdom, the Boer War Recruits Health Report led, in 1908, to restrictions on the sale of tobacco to
children under 16 and empowered police to conscate cigarettes from children smoking in public places
1931 Argentinian oncologist Angel Roffo (1931) produced skin tumours in rabbits with tobacco tar, building on similar work
on tars and skin cancer that began with Percival Pott's UK studies of scrotal cancer and chimney sweeps (1775)
1936 US physician Alton Ochsner (1973) sees nine cases of lung cancer in six months after not seeing one in 20 years.
Noting that all the patients had begun smoking during World War I, he suggested that smoking was the cause
1938 US statistician Raymond Pearl (1938) uses insurance records to show increased death rates of smokers
1939 Franz Müller (1939) uses 86 cases of lung cancer compared to controls to show that heavy smokers had 16 times the
lung cancer deaths than non-smokers: a 'one in a million chance' nding leading to the conclusion that tobacco was
the 'single most important cause of the rise in lung cancer'
1930–1941 Schairer and Schöniger (2001) studied 195 lung cancer cases using two control groups (other cancers and no
diseases), showing that only three lung cancer cases had not smoked and that a statistical association between
tobacco and lung cancer was 'likely'
1942–1944 Seven dissertations were published on tobacco and health effects at the German National Scientic Institute for
Research on Tobacco, Jena (Zimmermann et al., 2001)
1946 Percy Stocks (1947), UK chief medical statistician to the General Register Ofce, noted a 'startling' six-fold increase in
male lung cancer between 1930 and 1944
1947 The UK Medical Research Council (MRC) met to discuss action and was attended by Bradford Hill, Alice Stewart, Ernest
Kennaway and others. Several possible causes of lung cancer were discussed: tar from roads, urban air pollution,
trafc fumes and smoking. These were all probably 'factors which prepare the soil rather than sow the seed' (Tudor
Edwards, 1946; Keeting, 2009)
1948 In an MRC study by Doll and Bradford Hill, preliminary results on 156 interviews with patients showed 'denite
association' between lung cancer and smoking, although the lack of a link between inhaling and cancer was 'surprising'
(Pollock, 1999). This was to provide the eminent statistician, Sir Ronald Fisher, with his denial of the association
between smoking and lung cancer for many years
1950 Five papers were published showing the dangers of smoking. Wynder and Graham (1950)
(concerning military veterans in the US), Doll and Bradford Hill (1950) (concerning hospital patients in the United
Kingdom) concluded that smoking was 'an important factor' in the 'induction/production' of lung cancer. Of 647 cases
in the Doll and Bradford Hill study only 0.3 % were non-smokers: a 'one in a million' chance nding. Heavy smokers
had 16 times the lung cancer deaths than non-smokers. But this result was 'largely doubted and generally ignored' by
the medical establishment (Keating, 2009)
1953 A UK Government Advisory Committee concluded that the 'association was causal' and 'young people should be
warned' (Ministry of Health, 1953a, 1953b and 1954)
1954 Preliminary results were released from the study of Doll and Hill (1954) of 40 000 doctors which was to last 50 years.
Data on 39 lung cancer cases out of 769 deaths conrmed their earlier ndings and now revealed a dose/response
effect and an association with heart disease. In the US, Hammond and Horn's (1954) study of 5 000 deaths showed
similar results
1954 Publication of scientic studies documenting tobacco's role in cancer and other fatal illnesses together with subsequent
media coverage and declining sales was referred to internally by the tobacco industry as the '1954 emergency'. The
industry responded with a public relations campaign led by Hill and Knowlton to 'manufacture doubt' about the link
between smoking and lung cancer, without actually denying it
1964 A US Surgeon General report, 'Smoking and Health', based on 29 studies, concluded that 'there is a causal relationship
between excessive smoking and lung cancer' (US DHEW, 1964)
1970–1980s The rst studies were published showing that second-hand smoking is associated with lung cancer. A US Surgeon
General report in 1986 concluded that the link is causal (US DHHS, 1986)
1993–1998 Tobacco industry subverts the WHO International Agency for Research on Cancer (IARC) study and evaluation of
second-hand smoking as a human carcinogen
Source: EEA, based on Keating, 2009 and Ong and Glantz, 2000.

Lessons from health hazards | Tobacco industry manipulation of research
172
Late lessons from early warnings: science, precaution, innovation
References
AAAS, 1999, American Association for the Advancement
of Science and Federal Focus, Inc.
Armitage, A.K., 1993, 'Environmental tobacco smoke
and coronary heart disease', Journal of Smoking‑
Related Diseases, (4/1) 27–36.
Assunta, M., Fields, N., Knight, J. and Chapman,
S., 2004, 'Care and feeding: "Our consultants
are prepared to do the kind of things they were
recruited to do" The Asian ETS Consultants
Program', Tobacco Control, (13/2) ii 4–12.
Baba, A., Cook, D., McGarity, T. and Bero, L., 2005,
'Legislating "Sound Science": Role of the tobacco
industry', American Journal of Public Health,
S1:S20–S7.
Barnes, D.E. and Bero, L.A., 1997, 'Scientific quality
of original research articles on environmental
tobacco smoke', Tobacco Control, (6/1) 19–26.
Barnes, D. and Bero. L.A., 1998, 'Why review articles
on the health effects of passive smoking reach
different conclusions', JAMA, (279) 1 566–1 570.
Barnes, D. and Bero, L.A., 1996, 'Industry-funded
research and conflict of interest: An analysis of
research sponsored by the tobacco industry through
the Center for Indoor Air Research', J Health Politics,
Policy and Law, (21) 515–542.
Barnes, D., Hanauer, P., Slade, J., Bero, L. and Glantz,
S., 1995, 'Environmental tobacco smoke: The Brown
and Williamson documents', JAMA, (274) 248–253.
Barnoya, J. and Glantz, S., 2006, 'The tobacco
industry's worldwide ETS consultants project:
European and Asian components', Eur J Public
Health, (February 2006) (16/1) 69–77.
Barnoya, J. and Glantz, S., 2002, 'Tobacco industry
success in preventing regulation of second-hand
smoke in Latin America: the "Latin Project"', Tob
Control, (11/4) 305–314.
BAT, 1956, The Zephyr Aspects of PCL, British
American Tobacco, p. 100175775.
BAT, 1991, 'Confidential communication from
industry counsel to employee of entity with whom
BAT Co shares a common legal interest with copies
sent to employee, affiliate employee and joint
defense employee containing counsel's advice
regarding assessment of EPA Scientific Advisory
Board meeting' (http://legacy.library.ucsf.edu/tid/
wvd12b00) accessed 9 December 2011.
BAT, 1995, ETS Pilot Study, Bates number(s):
700875991–700876043 (http://legacy.library.ucsf.edu/
tid/kwj63a99) accessed 15 July 2011.
BAT, 1996, 'Confidential communication from
industry counsel to affiliate counsel containing
industry counsel's advice and analysis regarding
risk assessment' (http://legacy.library.ucsf.edu/tid/
hfk42b00) accessed 9 December 2011.
BAT, 1996, EU Issues, Bates number(s): 322122073–
322122107 (http://bat.library.ucsf.edu//tid/pwt63a99)
accessed 15 July 2011.
BAT, undated, Shaping the Regulatory Environment:
Advertising and Public Smoking, Bates number(s):
322121140–322121143 (http://bat.library.ucsf.edu//
tid/xnz82a99) accessed 23 May 2008.
Bekelman, J., Li, Y. and Gross C., 2003, 'Scope and
impact of financial conflicts of interest in biomedical
research: A systematic review', JAMA, (289) 454–465.
Bero, L.A., 2003, 'Implications of the tobacco
industry documents for public health and policy',
Annu Rev Public Health, (24) 267–288.
Bero, L.A., Barnes, D., Hanauer, P., Slade, J. and
Glantz, S., 1995, 'Lawyer control of the tobacco
industry's external research program: The Brown
and Williamson documents', JAMA, (274) 241–247.
Bero, L.A., Galbraith, A. and Rennie, D., 1994,
'Sponsored symposia on environmental tobacco
smoke', JAMA, (271/8) 612–617.
Bero, L.A., Galbraith, A. and Rennie, D., 1992, 'The
publication of sponsored symposiums in medical
journals', New Eng J Med, (327) 1 135–1 140.
Bero, L.A. and Glantz, S.A., 1993, 'Tobacco industry
response to a risk assessment of environmental
tobacco smoke', Tobacco Control, (2) 103–113.
Bero, L.A., Glantz, S.A. and Rennie, D., 1994,
Publication bias and public health policy on
environmental tobacco smoke', JAMA, (272) 133–136.
Bero, L.A., Montini, T., Bryan-Jones, K. and
Mangurian C., 2001, 'Science in regulatory policy
making: case studies in the development of
workplace smoking restrictions', Tob Control, (10/4)
329–336.

Lessons from health hazards | Tobacco industry manipulation of research
173
Late lessons from early warnings: science, precaution, innovation
Bero, L.A. and Rennie, D., 1996, 'Influences on the
quality of published drug studies', Int J Technol
Assess Health Care, (12/2) 209–237.
Bero, L.A., Rennie, D., 1995, 'The Cochrane
Collaboration: Preparing, maintaining, and
disseminating systematic reviews of the effects of
health care', JAMA, (274) 1 935–1 938.
Bero, L.A., 2005, 'Tobacco industry manipulation of
research', Public Health Reports, (120) 200–208.
Bialous, S. and Glantz, S., 2002, 'ASHRAE Standard
62: Tobacco industry's influence over national
ventilation standards', Tobacco Control, (11) 315–328.
Bialous, S. and Yach, D., 2001, 'Whose standard is it,
anyway? How the tobacco industry determines the
International Organization for Standardization (ISO)
standards for tobacco products', Tobacco Control, (10)
96–104.
Boyd, E. and Bero, L., 2000, 'Assessing faculty
financial relationships with industry', JAMA, (284)
2 209–2 238.
Boyd, E.A., Cho, M.K. and Bero, L.A., 2003,
'Financial conflict-of-interest policies in clinical
research: issues for clinical investigators', Acad Med,
(78/8) 769–774.
Boyse, S., 1988, Note on a Special Meeting of the UK
Industry on Environmental Tobacco Smoke, British
American Tobacco, London, February 17, 1988,
p. 1011980–4.
Bryan-Jones, K. and Bero, L.A., 2003, 'Tobacco
industry efforts to defeat the occupational safety and
health administration indoor air quality rule', Am J
Public Health, (93/4) 585–592.
Cal-EPA, 2005, California Environmental Protection
Agency, Proposed Identification of Environmental
Tobacco Smoke as a Toxic Air Contaminant, California
Environmental Protection Agency, Sacramento.
Cal-EPA, 1997, California Environmental Protection
Agency, Health Effects of Exposure to Environmental
Tobacco Smoke, California Environmental Protection
Agency, Sacramento.
CETID, 2000, Tobacco company strategies to undermine
tobacco control activities at the World Health
Organization, Report of the Committee of Experts on
Tobacco Industry Documents.
Chapman, S., 2005, 'The most important and
influential paper ever on second-hand smoke', 2005.
Chapman, S., 1997, 'Tobacco industry memo reveals
passive smoking strategy', Bmj, (14/7094) 1 569.
Chapman, S., Borland, R., Hill, D., Owen, N. and
Woodward, S., 1990, 'Why the tobacco industry
fears the passive smoking issue', Int J Health Services,
(20/3) 417–427.
Cho, M., 1998, 'Fundamental conflict of interest',
The BioMedNet Magazine, 24.
Cho, M.K., Bero, L.A., 1996, 'The quality of drug
studies published in symposium proceedings',
Annals of Internal Medicine, (124/5) 485–489.
CIAR, 1994, Board Of Directors Meeting Sheraton
Stockholm 940222 & 940223 Agenda, Philip Morris,
p. 2028387250, Center For Indoor Air Research.
CIAR, 1992a, Research agenda and request for
applications, Center for Indoor Air Research.
CIAR, 1992b, Request For Proposals, Philip Morris,
p. 2023523350/3369, Center for Indoor Air Research.
CIAR, 1989, Request For Applications 1989–1990
(890000–900000) Research Agenda, RJ Reynolds,
p. 508146797/6836, Center for Indoor Air Research.
CIAR, 1988, Lorillard, p. 87824354/4374, Center For
Indoor Air Research.
Cohen, J., 2003, 'Accepting tobacco funding for
research: An overview of the issues', TRDRP
Newsletter Burning Issues, (6/1) 7.
Connolly, G., Wayne, G., Lymperis, D. and Doherty,
M., 2000, 'How cigarette additives are used to mask
environmental tobacco smoke', Tobacco Control, (9)
283–291.
Covington and Burling, 1996, EC Risk Assessment
Proposal, Bates number(s): 800103063–800103065
(http://bat.library.ucsf.edu//tid/hjt14a99) accessed
15 July 2011.
Dana, J., 2003, 'Harm avoidance and financial
conflict of interest', Journal of Medical Ethic, (14)
(September 12), 1–18.
Davies, D., 2007, The Secret History of the War on
Cance, Basic Books.

Lessons from health hazards | Tobacco industry manipulation of research
174
Late lessons from early warnings: science, precaution, innovation
Dickersin, K., Min, Y-I. and Meinert, C.L., 1992,
'Factors influencing publication of research
results: follow-up of applications submitted to two
institutional review boards', J Am Med Assoc, (8267)
374–378.
Diethhelm, P., Rielle, J-C. and McKee, M., 2004, 'The
whole truth and nothing but the truth? The research
that Philip Morris did not want you to see', The
Lancet, online version, 11 November 2004.
Djordjevic, M.V., Fan, J., Ferguson, S. and
Hoffmann, D., 1995, 'Self-regulation of smoking
intensity. Smoke yields of the low-nicotine, low-'tar'
cigarettes', Carcinogenesis, (16/9) 2 015–2 021.
Dodds, A., 2006, 'The Core Executive's Approach
to Regulation: From "Better Regulation" to
"Risk-Tolerant Deregulation"', Social Policy &
Administration, 40(5) 526–542.
Doll, R. and Bradford Hill, A., 1950, 'Smoking and
carcinoma of the lung, preliminary report', British
Medical Journal, (2) 739–48.
Doll, R. and Bradford Hill, A., 1954, 'The mortality
of doctors in relation to their smoking habits —
A preliminary report', British Medical Journal, (228/i)
1 451–1 455.
European Commission, 2009, Impact Assessment
Guidelines SEC(2009) 92, Europa, Brussels.
EU, 1997, Treaty of Amsterdam Amending the
Treaty on European Union, the Treaties establishing
the European Communities and Certain Related
Acts. Brussels: Office for Official Publications of the
European Communities and Europa.
European Policy Centre, 1997, Briefing Paper on Risk
Assessment, Bates number(s): 321573155 (http://
legacy.library.ucsf.edu/tid/weg63a99) accessed
15 July 2011.
European Risk Forum, 2008a, 'European Risk
Forum — ERF' (http://www.euportal2.be/index.php)
accessed 15 July 2011.
European Risk Forum, 2008b, Policy Brief 04: Judicial
Review and Risk Management at EU‑Level.
Fact Finding Commission, 2004, Report of the
inquiry into the case concerning Prof. Ragnar Rylander,
University of Geneva, Geneva, September 6, 2004.
Flue-Cured Tobacco Co-op v. US EPA, 1998; 4 F.
Supp. 2d 435 (M.D.N.C. 1998).
Glantz, S., Slade, J., Bero, L. and Hanauer, P., 1996,
The Cigarette Papers, UC Press, Berkeley, CA.
Gretton, K., undated (circa 1995), Interdepartmental
Liaison Group on Risk Assessment: Use of Assessment
within Government Departments, Bates number(s):
800148140–800148145.
Grüning, T., Gilmore, A. and McKee, M., 2006,
'Tobacco industry influence on science and scientists
in Germany', Am J Public Health, Am J Public Health,
(96/1) 20–32.
Hammond, E.C. and Horn, D., 1954, 'The
relationship between human smoking habits
and death rates', Journal of the American Medical
Association, (155) 1 316–1 328.
Hanauer, P., Slade, J., Barnes, D., Bero, L. and
Glantz, S., 1995, 'Lawyer control of internal scientific
research to protect against products liability
lawsuits: The Brown and Williamson documents',
JAMA, (274) 234–240.
Herman, R., 2002, Harvard School of Public Health
Faculty Votes Not to Accept Funds from the Tobacco
Companies and Subsidiaries, Harvard School of Public
Health, Boston, MA., January 25, 2002.
Hirayama, T., 1981, 'Non-smoking wives of heavy
smokers have a higher risk of lung cancer. A study
from Japan', Br Med J, (282) 183–185.
Hirschhorn, N. and Bialous, S., 2001, 'Second-hand
smoke and risk assessment: what was in it for the
tobacco industry?', Tobacco Control, (10) 375–382.
Hirschhorn, N., Bialous, S.A. and Shatenstein, S.,
2001, 'Philip Morris' new scientific initiative: An
analysis', Tobacco Control, (10) 247–252.
Hong, M.K. and Bero, L.A., 2002, 'How the tobacco
industry responded to an influential study of
the health effects of second-hand smoke', BMJ,
(325/7377) 1 413–1 416.
Hornstein, D.T., 2003, 'Accounting for Science:
The Independence of Public Research in the
New, Subterranean Administrative Law', Law and
Contemporary Problems, (66/227) 227–246.
Honour, H., 1996, Risk Assessment, Bates number(s):
800103062 (http://legacy.library.ucsf.edu/tid/fjt14a99)
accessed 15 July 2011.

Lessons from health hazards | Tobacco industry manipulation of research
175
Late lessons from early warnings: science, precaution, innovation
IARC, 1998, International Agency for Research on
Cancer, Passive Smoking and Lung Cancer in Europe,
IARC, Lyon.
Jasanoff, S.K., 1996, 'Is science socially constructed:
And can it still inform public policy?', Science and
Engineering Ethics, (2/3) 263–276.
Jasanoff, S., 1995, Science at the Bar: Law, Science,
and Technology in America. The Technical Discourse of
Government, Harvard University Press, Cambridge,
pp. 69–92.
Jasanoff, S., 1987, 'EPA's Regulation of Daminozide:
Unscrambling the Messages of Risk', Science,
Technology and Human Values, (12/3 & 4) 116–124.
Johnston J, (R. J. Reynolds Tobacco Company) and
Sullum, J., (Philip Morris Tobacco Company), 1994,
Collection of advertisements, American Tobacco,
p. 947086708/6724.
Kaiser, J., 2003, 'Economics. Wielding the
data-quality cudgel', Science, (299/5614) 1 837.
Kaiser, J., 1997, 'Researchers and Lawmakers Clash
Over Access to Data', Science, (277/5325) 467.
Keating, C., 2009, Smoking Kills, The Revolutionary Life
of Richard Doll, Signal Books Limited, Oxford.
Kennedy, G. and Bero, L., 1999, 'Print media
coverage of research on passive smoking', Tobacco
Control, (8) 254–260.
Kingdon, J.W., 1984, Agendas, Alternatives, and public
policies, Little, Brown and Co., Boston.
Krimsky, S., 2003, Science in the Private Interest,
Rowman & Littlefield Publishers, Inc., Lanham, MD.
Krimsky, S., 1992, 'The role of theory in risk studies',
in: Krimsky, S., Golding, D. (eds), Social Theories of
Risk, Westport, Connecticut London, pp. 3–22.
Lee, P.N.,1995, '"Marriage to a smoker" may not
be a valid marker of exposure in studies relating
environmental tobacco smoke to risk of lung cancer
in Japanese non-smoking women', Int Arch Occup
Environ Health, (67/5) 287–294.
Levine, J., Gussow, J., Hastings, D. and Eccher, A.,
2003, 'Authors' financial relationships with the food
and beverage industry and their published positions
on the fat substitute Olestra', Am J Public Health, (93)
664–669.
Lexchin, J., Bero, L.A., Djulbegovic, B. and Clark,
O., 2003, 'Pharmaceutical industry sponsorship and
research outcome and quality: Systematic review',
BMJ, (326/7400) 1 167–1 170.
Löfstedt, R.E., 2004, 'The Swing of the Regulatory
Pendulum in Europe: From Precautionary Principle
to (Regulatory) Impact Analysis', The Journal of Risk
and Uncertainty, (28/3) 237–260.
Lowi, T., 1979, The End of Liberalism, Norton, New
York.
MacKenzie-Reid, D., 1995, 'Memo from Duncan
MacKenzie-Reid to Keith Gretton enclosing paper
on risk assessment', Bates number(s): 800147230–
800147236 (http://bat.library.ucsf.edu//tid/ctt72a99)
accessed 15 July 2011.
Malone, R.E. and Bero, L.A., 2003, 'Chasing the
dollar: Why scientists should decline tobacco
industry funding', J Epidemiol Community Health,
(57/8) 546–548.
Malone, R.E., Boyd, E. and Bero, L.A., 2001, 'Science
in the news: Journalists' constructions of passive
smoking as a social problem', Social Studies of Science,
(30) 713–735.
Mangurian, C., Bero, L.A., 2000, 'Lessons learned
from the tobacco industry's efforts to prevent the
passage of a workplace smoking regulation', Am J
Public Health, (90) 1 926–1 930.
Markowitz, G., Rosner, D., 2002, Deceit and Denial:
The Deadly Politics of Industrial Pollution, University
of California Press, Berkeley, CA.
Markowitz, G and Rosner, D., 2000, "'Cater to the
children": the role of the lead industry in a public
health tragedy, 1990–1955', Am J Public Health, (90/1)
36–46.
Markowitz, G. and Rosner, D., 1991, Deadly Dust:
Silicosis and the Politics of Occupational Disease in
Twentieth Century America, Princeton University
Press, Princeton, NJ.
Mazmanian, D. and Sabatier, P., 1989,
Implementation and public policy, University Press of
America; Lanham, MD.
McGarity, T.O., 2004, 'Our Science is Sound Science
and Their Science is Junk Science: Science-Based
Strategies for Avoiding Accountability and
Responsibility for Risk-Producing Products and

Lessons from health hazards | Tobacco industry manipulation of research
176
Late lessons from early warnings: science, precaution, innovation
Activities', The University of Kansas Law Review,
(52/4) 897–937.
Michaels, D., 2008, Doubt is their product: how
industry's assault on science threatens your health,
Oxford University Press, New York.
Ministry of Health, 1953a, Smoking and lung cancer,
report of the statistical panel appointed by the CMO,
UK National Archives, London.
Ministry of Health, 1953b, Minutes of Standing
Advisory Committee, 22 December 1953, UK National
Archives, London.
Ministry of Health, 1954, Draft memorandum to
cabinet Home Affairs Committee, 26 Jan. 1954,
UK National Archives/Ministry of Health papers,
MH55/1011.
Misakian, S. and Bero, L., 1998, 'Publication bias
and research on passive smoking', JAMA, (280/13)
250–253.
Montini, T. and Bero, L.A., 2001, 'Policy makers'
perspectives on tobacco control advocates' roles
in regulation development', Tob Control, (10/3)
218–224.
Montini, T., Mangurian, C. and Bero, L.A., 2002,
Assessing the evidence submitted in the development of
a workplace smoking regulation: The case of Maryland,
Public Health Reports, 2002 May–June, (117/3)
291–298.
Muggli, M.E., Forster, J.L., Hurt, R.D. and Repace,
J.L., 2001, 'The smoke you don't see: uncovering
tobacco industry scientific strategies aimed against
environmental tobacco smoke policies', Am J Public
Health, (91/9) 1 419–1 423.
Muggli, M.E., Hurt, R.D. and Repace, J., 2004,
'The Tobacco Industry's Political Efforts to Derail
the EPA Report on ETS', American Journal of
Preventative Medicine, (26/2) 167–177.
Müller, F.H., 'Tabakmissbrauch und
Lungencarcinom', Zeitschrift für Krebsforschung,
(49) 78.
Nelkin, D. (ed.), 1985, The Language of Risk: Conflicting
Perspectives on Occupational Health, Beverly Hills: Sage
Publications.
Nestle, M., 2002, Food Politics, University of California
Press, Berkeley.
NHMRC, 1997, The health effects of passive smoking,
National Health and Medical Research Council,
Canberra.
NRC, 1986, National Research Council,
Environmental tobacco smoke: Measuring exposures
and assessing health effects, National Academy Press,
Washington, D.C.
Ochsner, A., 1973, 'Corner of history: My first
recognition of the relationship of smoking and lung
cancer', Prev Med, (2) 611–614, .
OMB Watch, 2002, Background on Data Quality
Guidelines, (updated 05/28; cited 2004 05/03)
(http://www.ombwatch.org/node/645) accessed
15 July 2011.
Ong, E.K. and Glantz, S.A., 2001, 'Constructing
"Sound Science" and "Good Epidemiology": tobacco,
lawyers, and public relations firms', Tobacco Control,
(91/11) 1–9.
Oreskes, N. and Conway, E.M., 2010, Merchants
of doubt: how a handful of scientists obscured the
truth on issues from tobacco smoke to global warming,
Bloomsbury Press.
Pearl, R., 'Tobacco smoking and longevity', Science,
1938, (87) 216–227.
Pepples, E., 1978, CTR BUDGET, Brown &
Williamson, p. 680212421/2423.
Pollock, D., 1999, Denial and delay: the political
history of smoking and health, 1951–1964 — scientists,
government and industry as seen in the papers at the
public record office, Action on Smoking and Health,
London
Raymond, C.A., 1985, 'Risk in the press: Conflicting
journalistic ideologies', in: Nelkin, D. (ed.), The
Language of Risk, Sage Publications, Beverly Hills,
p. 87–132.
Rennie, D., 1993, 'Smoke and Letters' (Editorial),
JAMA, (270) 1 742–1 743.
Renner, R., 2002, 'Little law could block major
government decisions', Environ Sci Technol, (36/23)
443A.
Rochon, P., 1994, 'Evaluating the quality of articles
published in journal supplements compared with
the quality of those published in the parent journals',
JAMA, (272) 108–113.

Lessons from health hazards | Tobacco industry manipulation of research
177
Late lessons from early warnings: science, precaution, innovation
Roffo, A.H., 1931, 'Durch Tabak beim Kaninchen
entwickeltes Carcinom', Zeitschrift für
Krebsforschung, (33) 312–322.
Roper Organization, 1978, 'A Study Of Public
Attitudes Toward Cigarette Smoking And The Tobacco
Industry In 1978, Tobacco Institute, volume 1, May
1978, p. TIMN0210766/0819.
Roth, A.L., Dunsby, J., Bero, L.A., 2003, 'Framing
processes in public commentary on US federal
tobacco control regulation', Soc Stud Sci, (33/1) 7–44.
Sabatier, P.A., 1991, 'Toward better theories of the
policy process', Political Science and Process, 147–156.
Schafer, A., 2003, 'Biomedical conflicts of interest:
a defence of the sequestration thesis', Journal of
Medical Ethics, September 2003; Online Electronic
Version: 1–40.
Schairer, E. and Schöniger, E., 2001, 'Lung cancer
and tobacco consumption', Int. J. Epidemiol, (30/1)
24–27 (an English translation of an article first
published in German in Z Krebsforsch in 1943), (34)
261–269.
Schotland, M.S. and Bero, L.A., 2002, 'Evaluating
public commentary and scientific evidence
submitted in the development of a risk assessment',
Risk Anal, (22/1) 131–140.
Shamoo, A.E. and Resnik, D.B., 2003, Responsible
Conduct of Research, Oxford University Press, New
York.
Shapiro, S.A., 2004, 'The Information Quality
Act and Environmental Protection: The Perils of
Reform by Appropriations Rider', William and Mary
Environmental Law and Policy Review, winter; (28/2)
339–374.
Shield, M., 2001, 'Policies and positions of other
organizations on the funding controversy', TRDRP
Newsletter Burning Issues, (4/1) 2 p. 4.
Shook Hardy and Bacon, 1993, Report On
Recent Ets And Iaq Developments, Philip Morris,
p. 2046361379/1406.
Smith, K.E., Fooks, G., Collin, J., Weishaar, H.,
Mandal, S. and Gilmore, A., 2010, 'Working the
System' — British American Tobacco's Influence on
the European Union Treaty and Its Implications for
Policy: An Analysis of Internal Tobacco Industry
Documents', PLoS Medicine, (7/1) e1000202,
doi:10.1371/journal.pmed.1000202.
Silbergeld, E., 1993, 'Risk assessment:
the perspective and experience of U.S.
environmentalists', Environmental Health
Perspectives, (101/2) 100–104.
Special Issue, 2005, 'Corporate Corruption of
Science', Int J of Occupational Env Health, (11/4)
311–455.
Stayner, L., 1999, 'Protecting public health in
the face of uncertain risks: the example of diesel
exhaust', American Journal of Public Health, (89/7)
991–3.
Stocks, P., 1947, 'Regional and local differences in
cancer death rates', General Register Office: studies
on medical and population subjects, No. 1., HMSO.
Tewes, F., Koo, L., Meisgen, T. and Rylander, R.,
1990, 'Lung cancer risk and mutagenicity of tea',
Environ Research, (52) 23–33.
Tobacco Institute, 1986, Tobacco smoke and the
non‑smoker: Scientific integrity at the crossroads, The
Tobacco Institute.
Truman, D., 1993, The Governmental Process, 2nd
edition, Institute of Governmental Studies Press,
Berkeley.
Tudor Edwards, A., 1946, 'Carcinoma of the
bronchus', Thorax, (1) 1–25, doi:10.1136/thx.1.1.1
Turner, S., Cyr, S. and Gross, A., 1992, 'The
measurement of environmental tobacco smoke in
585 office environments', Environment International,
(18) 19–28.
Union of Concerned Scientists, 2007, Smoke, Mirrors
and Hot Air: How ExxonMobil uses Big Tobacco's
Tactics to Manufacture Uncertainty on Climate Science,
Cambridge, MA.
University of California, 2011, 'Legacy Tobacco
Documents Library — Field Descriptions'
(http://legacy.library.ucsf.edu/help/fields_desc.jsp)
accessed 9 December 2011.
US Congress, 1999, Omnibus Consolidated and
Emergency Supplemental Appropriations Act of 1999,
Pub. L. No. 105–277.
US DHEW, 1964, Report of the Advisory Committee to
the Surgeon General of the Public Health Service, US
Department of Health, Education and Welfare.

Lessons from health hazards | Tobacco industry manipulation of research
178
Late lessons from early warnings: science, precaution, innovation
US DHHS, 1986, United States Department of
Health and Human Services, The health consequences
of involuntary smoking: A report of the Surgeon
General, United States Department of Health and
Human Services.
US EPA, 1992, United States Environmental
Protection Agency, Respiratory health effects of passive
smoking: Lung cancer and other disorders, Office of
Health and Environmental Assessment, Office of
Research and Development, Washington D.C., Report
No.: U.S. EPA Document No. EPA/600/6–90/006F.
Walker, J.L.J., 1991, Mobilizing Interest Groups in
America, University of Michigan Press, Ann Arbor.
Wallack, L., Dorfman, L., Jernigan, D. and Themba,
M., 1993, Media Advocacy and Public Health: Power for
Prevention, Sage Publications, Newbury Park, CA.
Wells, J. and Pepples, E., 1986, Re: BAT Science. UCSF
B&W, p. 1837.01.
White, J., Bandura, A. and Bero, L.A., 2009, 'Moral
disengagement in the corporate world', Accountability
in Research, (16) 41–74.
White, J. and Bero, L.A., 2010, 'Corporate
manipulation of research: Strategies are similar across
five industries', Stanford Law & Policy Review, (21/1)
105–134.
Wilson, J.Q., 1989, Bureaucracy: What Government
Agencies Do and Why They Do It, 0000Johns Hopkins
University Press, Baltimore, MD.
von Winterfeldt, D., 1992, 'Expert knowledge and
public values in risk management: the role of
decision analysis', in: Krimsky, S. and Golding, D.
(eds), Social Theories of Risk, Westport Connecticut
London, p. 321–342.
Wynder, E.L. and Graham, E.A., 1950, 'Tobacco
smoking as a possible etiologic factor in bronchogenic
carcinoma', Journal of the American Medical Association,
(143) 329–336.
Yano, E., 2005, 'Japanese spousal smoking study
revisited: How a tobacco industry funded paper
reached erroneous conclusions', Tob Control,
(14) 227–233.
Zacaroli, A., 1998, 'Budget Provision in Spending Law
Opens Access to Data Underlying Federally Funded
Studies', in: BNA Daily Environment Report, editor.
Philip Morris, p. 2065231101/1103.
Zimmermann, S., Egger, M. and Hossfeld, Uwe., 2001,
'Commentary: pioneering research into smoking and
health in Nazi Germany— The "Wissenschaftliches
Institut zur Erforschung der Tabakgefahren' in Jena"',
Int. J. Epidemiol., (30/1) 35–37.
