
1
AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
Modelling the COVID-19 pandemic in
context: an international participatory
approach
Ricardo Aguas ,
1,2
Lisa White,
2,3
Nathaniel Hupert,
4
Rima Shretta ,
5
Wirichada Pan- Ngum,
2
Olivier Celhay,
2
Ainura Moldokmatova,
1
Fatima Ari,
6
Ali Mirzazadeh,
7
Hamid Shari,
8
Keyrellous Adib,
9
Mohammad Nadir Sahak,
10
Caroline Franco,
11
Renato Coutinho,
12
CoMo Consortium
Practice
To cite: AguasR, WhiteL,
HupertN, etal. Modelling the
COVID-19 pandemic in context:
an international participatory
approach. BMJ Global Health
2020;5:e003126. doi:10.1136/
bmjgh-2020-003126
Handling editor Seye Abimbola
► Additional material is
published online only. To view,
please visit the journal online
(http:// dx. doi. org/ 10. 1136/
bmjgh- 2020- 003126).
Received 11 June 2020
Revised 24 September 2020
Accepted 28 September 2020
For numbered afliations see
end of article.
Correspondence to
Lisa White;
lisa. white@ ndm. ox. ac. uk
© Author(s) (or their
employer(s)) 2020. Re- use
permitted under CC BY.
Published by BMJ.
ABSTRACT
The SARS- CoV-2 pandemic has had an unprecedented
impact on multiple levels of society. Not only has the
pandemic completely overwhelmed some health systems
but it has also changed how scientic evidence is
shared and increased the pace at which such evidence
is published and consumed, by scientists, policymakers
and the wider public. More signicantly, the pandemic
has created tremendous challenges for decision- makers,
who have had to implement highly disruptive containment
measures with very little empirical scientic evidence to
support their decision- making process. Given this lack
of data, predictive mathematical models have played an
increasingly prominent role. In high- income countries,
there is a long- standing history of established research
groups advising policymakers, whereas a general lack
of translational capacity has meant that mathematical
models frequently remain inaccessible to policymakers
in low- income and middle- income countries. Here, we
describe a participatory approach to modelling that aims
to circumvent this gap. Our approach involved the creation
of an international group of infectious disease modellers
and other public health experts, which culminated in
the establishment of the COVID-19 Modelling (CoMo)
Consortium. Here, we describe how the consortium was
formed, the way it functions, the mathematical model used
and, crucially, the high degree of engagement fostered
between CoMo Consortium members and their respective
local policymakers and ministries of health.
INTRODUCTION
The novel coronavirus, SARS- CoV-2, which
causes COVID-19, has affected at least 213
countries/regions, with more than 17 million
confirmed cases and in excess of 660 000
deaths globally.
1
As a new clinical entity, the
global impact of COVID-19 is characterised
by both uncertainty and rapid discovery,
laying the grounds for mathematical model-
ling to emerge as the prominent field of
research used to provide advice for pandemic
containment strategies.
2 3
High- income Asian
countries were able to call on their system
responsiveness and experience with recent
pandemics, rapidly enforcing efficient testing
and quarantining/isolation strategies. In
contrast, European countries took a much
more measured approach (easily confused
with lack of preparedness) early on, tapping
into their modelling expertise to predict
the outcome of the pandemic and what the
best containment strategies moving forward
might be. As a result, most European coun-
tries converged and introduced suppression
Key questions
What is already known?
► The optimal approaches to tackle the COVID-19 pan-
demic depend on several contextual factors, includ-
ing population age structure, variations in available
resources (including infrastructure, nancial and
human resources) and sociocultural considerations.
► Governments across the world have been advised
by mathematical modelling projections that do
not necessarily take those contextual factors into
consideration.
What are the new ndings?
► We describe the creation of a participatory mod-
elling approach platform, the COVID-19 Modelling
Consortium, and illustrate some of its use cases.
► We demonstrate how the participatory nature of the
consortium has been critical in its success, in terms
of addresing the contextual factors that underpin
health policy interventions and gaining decision
makers' trust.
What do the new ndings imply?
► We advocate a participatory modelling approach,
where in- country experts play an essential iterative
role, being policy- facing in its dealings with policy-
makers and simultaneously delivering or facilitating
reactive modelling that can feed back, in real time,
into the decision- making processes.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from

2
AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
BMJ Global Health
strategies (including everything from the shutdown
of basic economic activity, in many instances enforced
by hastily promulgated laws or executive orders, to the
wholesale reorganisation of medical and hospital- based
care) centred around non- pharmaceutical interventions
(NPIs),
4–6
fearing that pandemic mitigation would cost
too many lives, with the notable exception of Sweden.
7
Interestingly, several low- income and middle- income
countries (LMICs) followed suit, adopting health poli-
cies informed by early modelling from developed coun-
tries, without considering how the modelling predic-
tions might be affected by contextual factors. Modelling
devoid of local context has been shown to produce far
from optimal/useful projections in the Ebola, H5N1 and
H1N1 pandemics/outbreaks.
2 8
For such high priority
and complex topics, a participatory modelling approach
seems to be particularly well suited, as it provides policy-
makers with timely and dynamic support, enabling the
modelling process to be built around the coproduction
of knowledge between modellers and policymakers.
9
This multipronged approach with collaboration among
experts of different disciplines working together to
incorporate all relevant contextual factors into pandemic
modelling has been discussed at length in Rhodes et al.
8
Here, we describe a participatory approach to model-
ling that coalesced around these contextual consider-
ations and resulted in the creation of an international
consortium of infectious disease modellers and other
public health experts, the COVID-19 Modelling (CoMo)
Consortium. Putting in- country experts at the forefront
of model development, we underscore the need to incor-
porate contextual factors (including population age
structure, resource availability—including infrastruc-
ture, financial and human resources—and sociocultural
considerations) into an iterative policy informing tool.
Our approach demands a social–ecological component
comprising a combination of the contextual factors listed
below as an integral part of the epidemiological model,
thus addressing some of the limitations observed in other
modelling exercises.
2 3 8 10 11
We appreciate a compromise
between accuracy, transparency, flexibility and timeliness
remains, but are confident a participatory approach is
the best avenue to minimise those trade- offs.
CONTEXTUAL FACTORS
Contextual factor 1: population age structure
It became clear during the early stages of the COVID-19
pandemic that a disproportionate number of older
individuals are at higher risk of severe disease and
mortality,
12 13
with 80% of deaths associated with the
disease occurring in those aged more than 65 years in
outbreaks in China and the USA.
13 14
In Italy, the propor-
tion of deaths occurring in people over the age of 70
reached 88%, presumably due to an aged population
in the Lombardy region.
15
Data from the Chinese and
Italian outbreaks suggest that the case fatality rate (CFR)
was <1% in the under- 50s, rising to almost 15% and 20%
in the over- 80s, respectively.
15 16
Children appear to suffer
from less severe symptoms
17
but are at a similar risk of
being infected as the general population; therefore, the
role played by children in the transmission of COVID-19
should be considered when developing control strate-
gies.
18
This variation in the age- dependent severity of
COVID-19 has important implications for the impact of
the disease in any given country.
Contextual factor 2: uncertainties around the characteristics
of the disease
As a newly emerged disease, considerable uncer-
tainty remains around some of the basic parameters of
COVID-19 infection. Evidence suggests that the median
incubation period is approximately 5 (95% CI: 4.5 to 5.8)
days.
19
The duration of the infectious period is extremely
uncertain, with some studies suggesting that people
become infectious before developing symptoms
20–22
and others finding that viral shedding in clinical cases
can persist for more than 20 days.
12 23
In fact, some
evidence suggests infectiousness begins before symptoms
develop and is likely to peak around the time of symptom
onset.
23–25
Several estimates for the serial interval (time
between transmission chains),
26 27
taken together with
estimates for the incubation period, strongly suggest
that asymptomatically infected people can transmit the
virus. A study from China found the median duration
from first symptoms to dyspnoea, hospital admission
and acute respiratory distress syndrome was 5, 7 and 8
days, respectively,
28
although data from New York City
suggest a more rapid progression.
29
The infection fatality
rate (IFR) measures the percentage of infected individ-
uals who later succumb to the disease; a meta- analysis
suggests an IFR for COVID-19 of around 0.20% (mean
across all ages), given all available data as of 22 March
2020.
16
However, these estimates are full of uncertain-
ties particularly to what concerns the denominator due
to challenges in reliably ascertaining how many people
are/have been infected with the virus. Two streams of
scientific research are trying to resolve this underlying
burden of infection: one relies on the use of inference
and predictive models,
30–32
making the best use of avail-
able data to disentangle the unobserved number of infec-
tions driving the force of infection; the other focuses on
diagnostic tool development to enable reliable mass sero-
logical studies to be carried out.
33–35
Reported symptomatic CFR vary widely by country, as
criteria and capacity for testing can vary considerably.
Burdens of comorbidity differ, as do demographics, and
cause of death attribution is not uniform. Both IFR and
CFR values may also be dynamic in a single setting, as
delays in deaths tend to result in the underestimation
of CFR early on in an epidemic, with surges in lethality
during healthcare system stress.
Those at higher risk of developing severe disease
include individuals with comorbidities such as hyper-
tension, cardiovascular disease and diabetes.
36
Obesity,
especially in younger patients, is emerging as a risk factor
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from

AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
3
BMJ Global Health
for more severe clinical manifestations in cohorts in
the USA.
29
There is conflicting evidence regarding the
contribution of pre- existing respiratory disease to clin-
ical severity.
37
Given the relative paucity of detailed and
accurate parameter data, it is vital that local modellers
who have intimate knowledge of their own context(s) are
engaged to ensure the optimal application and commu-
nication of any model and its outputs.
Contextual factor 3: differences in health system capacity
The COVID-19 pandemic presents myriad challenges
for healthcare systems around the world,
38
including the
need to repurpose and train healthcare staff, increase the
number of both general hospital beds and intensive care
unit beds, purchase equipment (particularly ventilators,
high- flow oxygen systems and oxygen concentrators), and
hire carers needed for specialist care and/or treatment
for comorbidities.
39
Anticipating health system demand
in comparison to capacity under various intervention
scenarios is a key aspect of national and regional stra-
tegic decision- making.
40
This underscores one of the key
roles of the disease- modelling approach in the context of
COVID-19 pandemic preparedness and response.
Contextual factor 4: socioeconomic and cultural differences
The connection between cultural values (uncertainty
avoidance, power distance, individualism vs collectivism)
and infectious diseases is well established, primarily
with literature on antibiotic prescribing and treatment
seeking behaviours.
41
Different countries have adopted
a variety of strategies and combinations of NPIs to meet
the challenges posed by COVID-19. While these differ-
ences in approach are highly dependent on local cultural
contexts and values,
42
their differential effect in the
setting of COVID-19 is debated among social scientists.
43
Early empirical work is emerging suggesting disease
spread was slower in countries with strong institutional
systems and hierarchical cultures.
44
In China, where this
novel coronavirus first emerged, a strict policy including
measures such as quarantine, self- isolation and contain-
ment immediately implemented.
17
This ultimately
involved strict physical distancing measures in social
settings, or a ‘lockdown’, as Chinese local authorities
imposed travel restrictions and severely restricted the
movement of people. Physical distancing and isolation
measures, aided by extensive testing and contact tracing,
were also used effectively in South Korea to bring their
COVID-19 epidemic under control.
45
Singapore under-
took intensive surveillance and contact tracing, followed
by isolation of suspected and confirmed cases to halt
transmission chains.
35
Some Muslim countries in the
Middle East have made a historical decision to cancel
Friday and congregational prayers and to close their
holy shrines.
46
Other countries, such as the UK, started
pursuing strategies aimed at allowing herd immunity to
gradually develop while reducing the demand on the
health service, also known as ‘flattening the curve’, but
did eventually enforce a strict lockdown policy. Notably,
in Northern Europe, the onus of practicing efficient
containment measures was transferred to the individual,
while more relaxed lockdown versions were enforced,
characterised by a minimal disruption to society. Several
recent studies have tried to estimate the impact of these
different containment/suppression strategies in different
countries,
4 47–52
but little insight has been gained into the
optimal long term strategy, assuming that a vaccine may
not be available before the end of the year.
Socioeconomic differences can critically underpin
the potential adherence of any infection containment
measure. A significant proportion of the population in
LMICs are daily- wage earners who cannot work from
home. They often rely on street vendors or local markets
for their meals, which are usually overcrowded places
where hygiene and physical distancing measures are
difficult to enforce. Large and intergenerational families,
migrants and refugees who live in densely populated areas
mean physical distancing is virtually impossible, ineffec-
tive and may cause more harm than good. Religious and
cultural festivals can seriously disrupt physical distancing
measures usually people would gather in the thousands
to celebrate Easter, Chinese New Year, Ramadan.
Contextual factor 5: a rapidly developing situation
Published models frequently remain inaccessible to poli-
cymakers in LMICs due to the lack of translational capacity
in many of these countries. This can present difficulties in
converting a prepublication or peer- reviewed model into
a practical, real- time decision- making tool. Critically, any
model requires continuous updating, usually on a daily
basis, if it is to keep pace with the situation unfolding in
a given country and the science relating to the infectious
agent it is modelling, and therefore meet the needs of
policymakers in that country. Publishing scientific papers
and online tools alone is insufficient to engage with and
inform health policymakers, because in a rapidly devel-
oping situation, the model and the modeller cannot be
separated, and policy responses must be couched within
the specific country or subnational context. This can only
be achieved via a combination of technology, training
and communication. User- friendly platforms facilitating
real- time data analysis and scenario prediction by local
epidemiologists is key for effective planning and policy
decision making to mitigate the COVID-19 pandemic.
THE COVID-19 MODELING (COMO) CONSORTIUM
CoMo Consortium—policy rationale
During the acute phase of the pandemic, the focus of
most CoMo Consortium modelling work has been to
address immediate questions driven by policymakers
(figure 1). These have focused on exploring optimal strat-
egies to achieve specific aims, such as minimising cases,
mortality or demand on the health system. As the situa-
tion evolves, more thought is now being directed towards
economic impacts and the direct and indirect costs of
the disease and the measures taken to control its spread.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from
4
AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
BMJ Global Health
The challenges of increasing and repurposing health
system capacity, in addition to worker absenteeism due
to COVID-19 morbidity and mortality, represent some
of the direct costs associated with the pandemic.
53
The
economic and behavioural responses adopted to reduce
SARS- CoV-2 transmission form a substantial proportion
of the indirect costs associated with the pandemic. These
include suspension of manufacturing and production;
employment losses and reductions in consumer spending
in many sectors, notably travel and tourism;
53
interrup-
tions in services and the supply chain of products for the
prevention and control of other major diseases, such as
vaccine- preventable diseases, HIV, malaria and active
cancers. A fully comprehensive set of cost efficiency/
effectiveness analyses will be of great value to inform
countries seeking scientific evidence evaluating current
and future containment strategies.
CoMo Consortium—a participatory approach
Historically, modelling expertise has been concentrated
in high- income countries (HICs), where multiple model-
ling groups, often with large teams, have tended to form
consortia. While these consortia mainly inform policy
in HICs, they often act as external service providers to
LMICs. Modelling inputs to LMICs have, therefore, gener-
ally involved two groups of professionals: modellers from
HICs and policymakers from LMICs; a situation which
is far from ideal. We sought to avoid this by adopting a
participatory approach when establishing the interna-
tional CoMo Consortium. A participatory approach is
key for policymakers to fully appreciate the uncertainties
subjacent to assumed parameter values, implemented
mechanisms of action and general model structure. The
immediate consequence of that understanding is clarity
on the relevance of critical data in circumventing uncer-
tainties, and the understanding that continual validation
frameworks are key to guarantee the best possible policy
is implemented at all times. The use of a participatory
approach can even be viewed as part of the intervention
package, as argued in other studies,
3
and is highly desir-
able,
54
as evidenced by the number of policymakers from
multiple countries around the world who have requested
to actively participate in the CoMo Consortium. Being a
Consortium member also facilitates information sharing
among countries with comparable contexts that might be
addressing similar questions.
The CoMo Consortium mathematical model was devel-
oped by three groups of professionals, with each group
forming one of three nodes: a development node, an
in- country expert node and a policymaker node. Each
node comprises a variety of relevant professionals (table 1,
figure 2). Where there were existing in- country experts,
the CoMo Consortium sought to build on existing close
working relationships (or establish such relationships)
with these experts.
CoMo Consortium—development phase
The CoMo Consortium was established as a response to
the analytical demands of LMICs trying to prepare for
the COVID-19 epidemic. Several in- country experts were
approached for support by their own policymakers and
later reached out to the Oxford Modelling for Global
Health group for additional technical advice and support.
This sparked an initiative to provide technical support
and mentoring to these individuals which evolved into a
precursor for the CoMo consortium. The consortium was
officially formed when the mathematical model and its
accompanying online application were introduced to the
first in- country teams. The model is an age- dependent
susceptible- exposed- infected- recovered model, adapted
from
55
to reflect SARS- CoV-2 transmission/virological
traits and the interventions being deployed in different
countries—a detailed model description can be found
in the online supplemental materials. A key focus was to
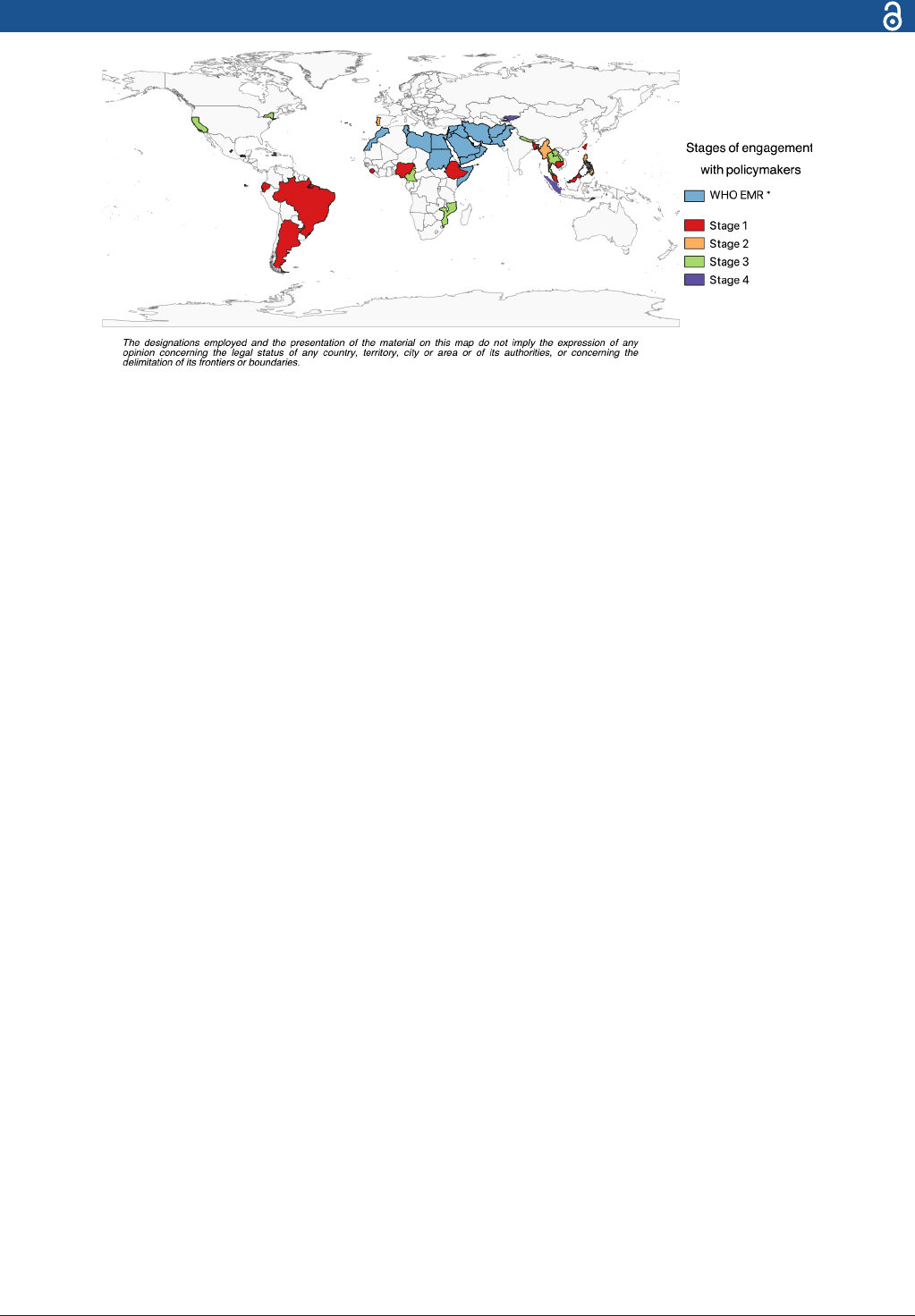
Figure 1 CoMo Consortium participants. Individual country participants, colour coded by the stages of engagement with
policymakers (table2). *Refers to the 22 countries/territories using the CoMo model through the WHO Regional Ofce for the
Eastern Mediterranean (EMRO). The designations employed and the presentation of the material on this map do not imply the
expression of any opinion concerning the legal status of any country, territory, city or area or of its authorities or concerning the
delimination of its frontiers or boundaries. CoMo, COVID-19 Modelling.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from
AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
5
BMJ Global Health
build a SARS- CoV-2 specific model that could be seam-
lessly updated as new information became available and
different sets of interventions were considered. A stand-
alone Excel- based tool, the Cornell COVID-19 Caseload
Calculator with Capacity and Ventilators model, formed
the basis for the healthcare components of the CoMo
Consortium model.
56
From inception, the goal was to allow the user to define
critical aspects of the model structure, model inputs, both
in terms of parameter values and interventions consid-
ered, interface options and output reporting. This was
key to the model being well accepted and easily adopted
by so many different countries.
The consortium has grown organically since 16 March
2020 and now includes approximately 100 members,
representing more than 30 countries (figure 1). Regular
communication channels were established, and several
developmental working groups were formed. These
working groups are either country- specific, question-
specific or technique- specific, with the latter including
working groups for the mechanistic model, developing
the web- based interface, exploring spatial formulations
of the model and a hospital capacity simulation tool.
Question- specific working groups include those centred
around lockdown- release strategies, or screening and
diagnostic strategies. The in- country experts are engaged
in continuous communication with their respective poli-
cymakers, enabling the rapid adaptation of the CoMo
Consortium model and other in- country models (where
available) to address the fast- paced changes occurring
during the course of the outbreak in each particular
setting.
CoMo Consortium—dissemination phase
Following its initial development phase, the CoMo
Consortium moved into the dissemination phase, once
the model code had been sufficiently scrutinised and the
interface—figure 3—had been redesigned to facilitate
model calibration to data. A health economics working
group was formed to identify the most appropriate use of
available funds and inform future cost efficiency analyses.
In- country working groups continued to work on their
local COVID-19 situations while also contributing to and
benefitting from the question- specific and technique-
specific working groups. The full model code was made
available to all members and a special online ‘code
reading’ session was held. Members were then able to
take the code and adapt it for their own local context. For
Table 1 The three nodes of the CoMo Consortium Development phase
Development node In- country expert nodes Policymaker nodes
► Lead modeller
► Experienced modellers
► Clinician modeller (public health
specialist)
► App developer
► Coordinator (junior modeller)
► Economist
► Research clinician/preparedness modellers
► Epidemiologists/surveillance specialists
(eld/public health)
► Modellers (early stage/senior)
► Public health specialists
► Health economics modellers
► Medical statisticians
► Representatives from WHO/other NGOs
► State government
► State ministries of public health
► National ministries of public
health
► Local governing bodies and their
health departments
CoMo, COVID-19 Modelling.
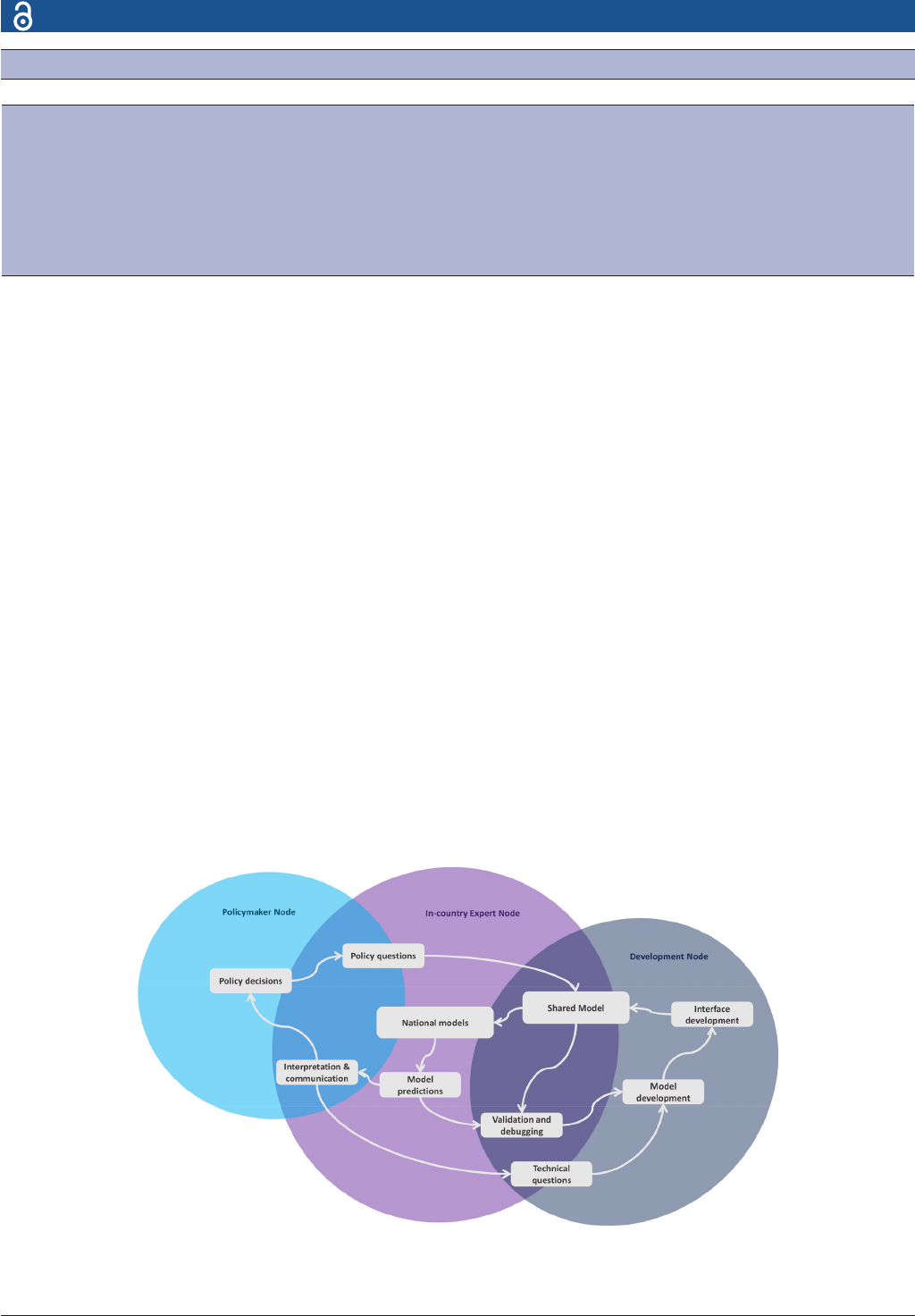
Figure 2 CoMo Consortium outlook and interaction ows. This diagram illustrates how the different partners interact in order
to digest policy questions into model simulations through the in- country expert node and the development node and ultimately
result in actionable predictions informing policy decisions. CoMo, COVID-19 Modelling.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from
6
AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
BMJ Global Health
example, the Brazil group has incorporated a modelling
fitting algorithm while simplifying the hospital simulator
submodel, while the Nigeria group has changed the age
structure to reflect the age classes used by the Nigerian
disease surveillance system.
An ongoing series of training sessions on the use of the
model and the communication of results to policymaking
partners was initiated by the consortium leader and then
taken up by the members. Code version release notes are
continually made available to all consortium members,
highlighting any changes made and the reasons for those
changes. A concerted effort was made to bridge the
model code with the app code and test the performance
of the model across code versions to ensure consistency.
At least one consultation was held between the devel-
opment node and each country’s expert node to discuss
parameter value assumptions and the contextual nature
of some parameters, given how interventions are imple-
mented locally. Some of these meetings instigated
updates to the model structure and app interface custo-
misation options.
CoMo Consortium—use-cases
Three use- cases have emerged for the CoMo Consor-
tium model and its accompanying web- based interface.
First, in settings where there is already considerable
translational capacity, the CoMo Consortium model is
used by in- country modellers to crosscheck the models
they have written, thus helping to refine their outputs
and to maintain high accuracy and validity. Second, in
settings where there is some coding expertise but less
capacity to develop bespoke models, the CoMo Consor-
tium’s primary code can be modified by in- country
modellers to create country- specific models that are
being used to assist policymakers’ decision- making.
Third, in contexts where there is a desire among poli-
cymakers to use modelling to inform development of
their strategies based on local parameter values, but
limited capacity in terms of modelling or coding, the
primary code is used via the CoMo Consortium model’s
web- based interface.
Regardless of the use- case, CoMo operations are ruled
by the flows of interaction depicted in figure 2. Clearly, the
main catalysts are policy questions that shape the shared
model that is adapted by each in- country expert team to
generate country specific models. The in- country expert
teams liaise with the technical experts to validate and
debug their models and later proceed to communicate the
model predictions to the policymakers in a comprehen-
sible way. We cannot emphasise enough how critical the
role played by the in- country experts is. It falls on them to
engage with policymakers and explain to them the features
and capabilities of the model, guide them in addressing
appropriate modelling questions, and later package those
questions alongside all relevant contextual factors to the
technical team to ensure the model can represent the
desired context appropriately. A few examples of the appre-
ciation of the contextual nature of questions addressed by
countries follow:
► In Syria, the main concern in the early stages of the
epidemic was how the virus would spread in displaced
populations and refugee camps. This could not be
explored with the standard CoMo model, so a task
team was put together to develop a bespoke refugee
camp model.
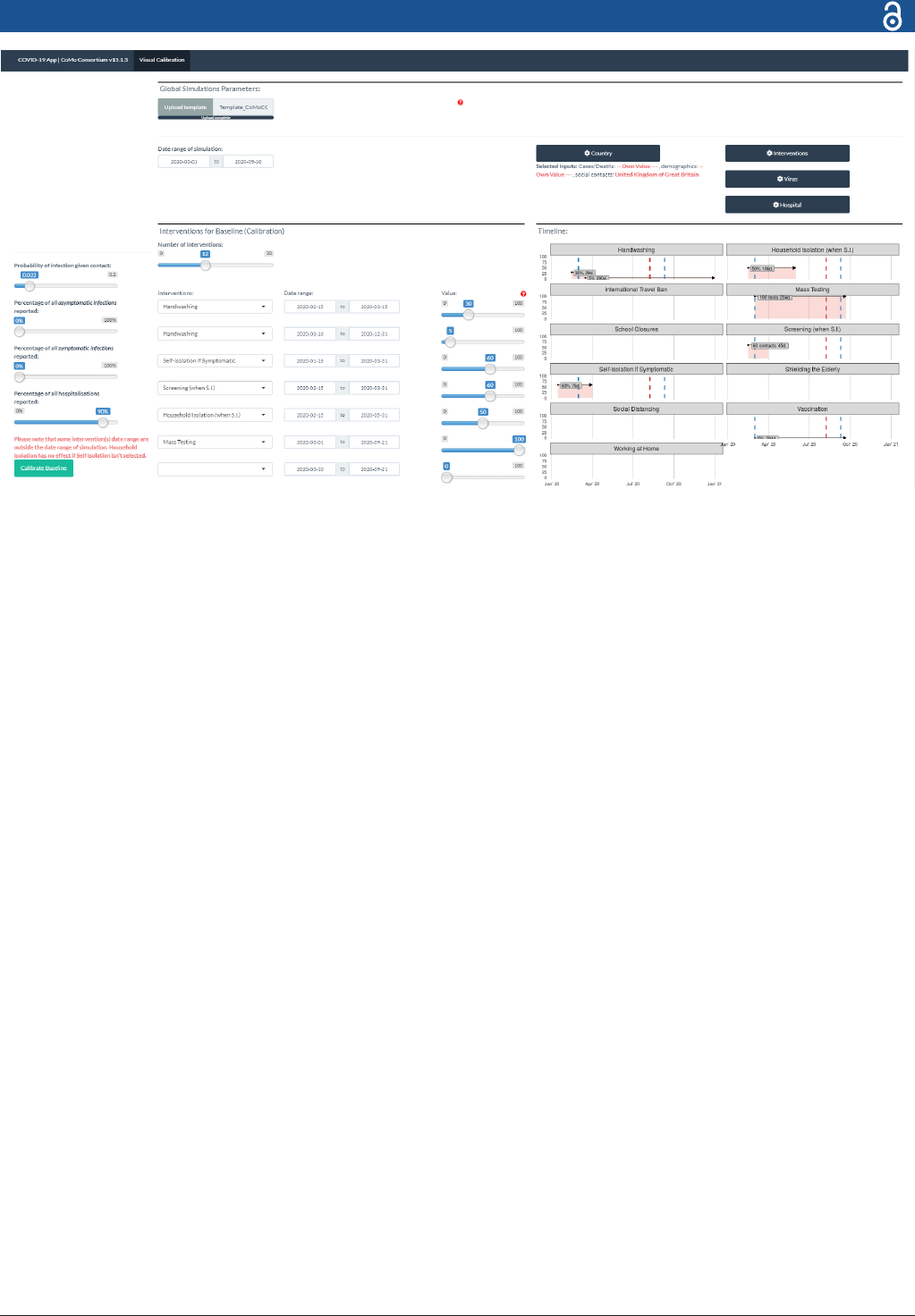
Figure 3 The CoMo model online application. Users can either upload a lled- in template or input all parameter values in the
app directly. User can specify up to 30 intervention periods, dening the start and end dates, as well as the assumed coverage
for each.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from

AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
7
BMJ Global Health
► In New York, special attention was paid to the very
fast upsurge in patients with respiratory distress in
most city hospitals. This raised serious concerns on
how countries with lower health capacity would fare,
specifically to what concerns the number of available
intensive care unit beds and ventilators. As a conse-
quence, we invested quite some time developing the
hospital submodel and have made it a central point of
discussion with in- country modellers, as it is their role
to assess the current and likely health capacity in their
country and convey the message of how those limi-
tations impact on the predicted epidemic mortality
burden to their respective policymakers.
► In Afghanistan and Timor- Leste there was a lot of
concern regarding the role of migrants in causing
local outbreaks and seed local transmission. This led
to the addition of a user defined parameter setting
the number of daily imported infections.
► Interventions are not implemented in the same way
everywhere, as country contextual idiosyncrasies crit-
ically affect the potential efficiency and practicability
of any intervention.
– Shielding of the elderly should be a major com-
ponent of mortality burden reduction strategies
in HICs, but are not feasible to employ in most
LMICs, where people live in large familial house-
holds and it would not be possible for the elder-
ly to isolate (and in some countries not culturally
acceptable).
– Self- isolation was a core component of initial con-
tainment strategies in Europe where it was quite
straightforward and easily achievable for the indi-
vidual person. In LMICs that is not that case, for
the reasons highlighted above. Due to these issues
with household structures, in Thailand, the min-
istry of health decided to isolate people fitting a
clinical algorithm in governmental facilities, where
they were kept for up to 14 days, or until they test
negative for the virus. This type of testing centre
was implemented early on in South Korea and
Singapore to great effect.
The stage of engagement with local policymakers is
quite heterogeneous across CoMo Consortium members
(table 2). Members with existing channels of communi-
cation with Ministries of Health have gone through the
process of providing feedback to their relevant authori-
ties at a much faster rate. Some members of the academic
community have needed to foster links with appropriate
points of contact at Ministries of Health, specially appointed
government entities, or COVID-19 taskforces, and establish
appropriate channels of communication and gain the trust
of policymakers. In some instances, it has been necessary
for members to navigate a complex landscape, sometimes
involving finding consensus among several different model-
ling groups offering advice to their government.
At the time of writing, 11 of the 22 countries from the
WHO Regional Office for the Eastern Mediterranean
(EMRO) have been using the CoMo Consortium model.
Table 2 CoMo Consortium member countries’ stages of engagement with policymakers
Stage 1
Preliminary analyses and model calibration to explore optimal containment strategies
► Argentina
► Bangladesh
► Brazil
► Cambodia
► Ecuador
► Ethiopia
► Nigeria
► Taiwan
► Sierra Leone
► Malaysia
Stage 2
Have engaged with local Ministries of Health (MoH) or relevant policymakers and are in
the process of analysing the different strategies under consideration
► Philippines
► Portugal
► Myanmar
► Northwest Syria
Stage 3
Have on at least one occasion presented CoMo Consortium model results to the local
MoH or relevant policymakers
► Afghanistan
► Cameroon
► Haiti
► Iran
► Lao PDR
► Tabasco Province, Mexico
► Queretaro State, Mexico
► Thailand
► Mozambique
► New York State, USA
► Orange County, California, USA
► Timor- Leste
► Nepal
Stage 4
The local MoH has made policy decisions based on CoMo Consortium model predictions
► Kyrgyzstan
► North Sumatra Province, Indonesia
CoMo, COVID-19 Modelling.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from

8
AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
BMJ Global Health
Some are engaged in independent research using either
the CoMo Consortium model (table 2), their own models,
or both, while others are receiving active support from
the WHO EMRO COVID-19 Modelling Support Group.
The countries are distributed across all stages of policy
engagement documented in table 2.
CONCLUSION
Faced with the most significant pandemic in more than a
century, we chose a participatory approach to create the
international CoMo Consortium and develop a dynamic
infectious disease model that addressed a global need.
The key to the success of this participatory approach lies
with the in- country expert node. The in- country experts
include professionals with a wide range of expertise that
play an essential iterative role, being both policy- facing in
its dealings with policymakers and simultaneously deliv-
ering or facilitating reactive modelling that can feed back,
in real time and based on the latest data, into the decision-
making processes. Importantly, this continuous cooper-
ation and feedback loop has been a valuable part of the
process to facilitate collaboration and develop trust. A static
online tool alone would not be sufficient to achieve this.
The biggest strength of participatory approaches can ulti-
mately be its largest limitation, as everything is reliant of the
in- country expert being able to reach policymakers and/
or gain access to the relevant data, perform data quality
control, and liaise with the model development team to
ensure the interventions being implemented in the field
are well captured in the model.
Critically, our approach allows for tailoring of the
model and online app to meet each country’s needs
and facilitates translation of the analytical requirements
into easily digestible outputs that can inform policy. The
CoMo Consortium approach brings modelling to a broad
range of people who will benefit from its participatory
nature, through a combination of technology, training
and effective communication.
Author afliations
1
Nufeld Department of Medicine, University of Oxford Centre for Tropical Medicine
and Global Health, Oxford, UK
2
MAEMOD, Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand
3
Center for Tropical Medicine and Global Health, University of Oxford Centre for
Tropical Medicine, Oxford, UK
4
Weill Cornell Medicine, Cornell Institute for Disease and Disaster Preparedness,
New York, New York, USA
5
Nufeld Department of Medicine, University of Oxford, Oxford, UK
6
Department of Epidemiology, Florida International University, Miami, Florida, USA
7
School of Medicine, University of California San Francisco, San Francisco,
California, USA
8
WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in
Health, Kerman University of Medical Sciences, Kerman, Iran, Kerman, Iran (the
Islamic Republic of)
9
Independent Researcher (No afliation), no afliation, UK
10
Regional Ofce for the Eastern Mediterranean, World Health Organization, Kabul,
Afghanistan
11
Waves and Non- Linear Patterns Research Group, São Paulo State University
(UNESP), Institute of Theoretical Physics, Sâo Paulo, Sao Paulo, Brazil
12
Centre for Mathematics, Computation and Cognition, Federal University of ABC
Center of Mathematics Computing and Cognition, Santo Andre, São Paulo, Brazil
Twitter Rima Shretta @rimashretta and Caroline Franco @_francocarol
Collaborators CoMo Consortium: Proochista Ariana; Penny Hancock; Roberto
A Kraenkel; Sompob Saralamba; Nantasit Luangasanatip; Sheetal Prakash Silal;
Jared Norman; Rachel Hounsell; Sai Thein Than Tun; Yu Nandar Aung; Bakare
Emmanuel A; Biniam Getachew; Sandra Adele; Semeeh A Omoleke; Rashid U
Zaman; Nicholas Letchford; Daniel M Parker; Sunil Pokharel; Dipti Lata; Siyu Chen;
Shwe Sin Kyaw; Inke N D Lubis; Ivana Alona; John Robert C Medina; Chris Erwin
G Mercado; Sana Eybpoosh; Ibrahim Mamadu; Manar Marzouk; Nicole Feune de
Colombi; Lorena Suárez- Idueta; Francisco Obando; Luzia Freitas; Michael G Klein;
David Scales; Dooronbekova Aizhan; Chynar Zhumalieva; Aida Estebesova; Aibek
Mukambetov; Shamil Ibragimov; Aisuluu Kubatova; Phetsavanh Chanthavialy;
Amel H Salim; Sudhir Venkatesan; Sarin K C; Priyanka Shrestha; Sayed Ataullah
Saeedzai; Jenny Hsieh; Mick Soukavong; Yuki Yunanda; Handoyo Harsono; Mahnaz
Hossain Fariba; Viviana Mabombo; Nicole Advani; Nusrat Jabin; Reshania Naidoo;
Parinda Wattanasri; Amen- Patrick Nwosu; Sopuruchukwu Obiesie.
Contributors RA, LW and WP- N developed the CoMo model. OC developed the
online model application. CF and RC consulted on the model structure. NH, RS,
AMo, FA, AMi, HS, KA and MNS are early model users that helped revise the model
structure and the online application. RA and LW wrote the initial manuscript draft.
All authors revised the draft manuscript.
Funding RA is funded by the Bill and Melinda Gates Foundation (OPP1193472).
LW is funded by the Li Ka Shing Foundation. CF is funded by grant #2017/26770-8,
São Paulo Research Foundation (FAPESP). The CoMo Consortium has support from
the Oxford University COVID-19 Research Response Fund (ref: 0009280). Scientic
writing assistance and editorial support was provided by Adam Bodley, according
to Good Publication Practice guidelines.
Map disclaimer The depiction of boundaries on this map does not imply the
expression of any opinion whatsoever on the part of BMJ (or any member of its
group) concerning the legal status of any country, territory, jurisdiction or area or of
its authorities. This map is provided without any warranty of any kind, either express
or implied.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement There are no data in this work.
Supplemental material This content has been supplied by the author(s). It has
not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been
peer- reviewed. Any opinions or recommendations discussed are solely those
of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and
responsibility arising from any reliance placed on the content. Where the content
includes any translated material, BMJ does not warrant the accuracy and reliability
of the translations (including but not limited to local regulations, clinical guidelines,
terminology, drug names and drug dosages), and is not responsible for any error
and/or omissions arising from translation and adaptation or otherwise.
Open access This is an open access article distributed in accordance with the
Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits
others to copy, redistribute, remix, transform and build upon this work for any
purpose, provided the original work is properly cited, a link to the licence is given,
and indication of whether changes were made. See:https:// creativecommons. org/
licenses/ by/ 4. 0/.
ORCID iDs
RicardoAguas http:// orcid. org/ 0000- 0002- 6507- 6597
RimaShretta http:// orcid. org/ 0000- 0001- 5011- 5998
REFERENCES
1 COVID-19 Dashboard: Johns Hopkins University. Available: https://
coronavirus. jhu. edu/ map. html
2 Van Damme W, Dahake R, Delamou A, etal. The COVID-19
pandemic: diverse contexts; different epidemics- how and why? BMJ
Glob Health 2020;5:e003098.
3 Rhodes T, Lancaster K. Mathematical models as public troubles in
COVID-19 infection control: following the numbers. Health Sociology
Review 2020;29:177–94.
4 Flaxman S. Report 13: estimating the number of infections and the
impact of non- pharmaceutical interventions on COVID-19 in 11
European countries, 2020.
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from

AguasR, etal. BMJ Global Health 2020;5:e003126. doi:10.1136/bmjgh-2020-003126
9
BMJ Global Health
5 Davies NG, Kucharski AJ, Eggo RM, etal. Effects of non-
pharmaceutical interventions on COVID-19 cases, deaths, and
demand for hospital services in the UK: a modelling study. Lancet
Public Health 2020;5:e375–85.
6 Cowling BJ, Ali ST, Ng TWY, etal. Impact assessment of non-
pharmaceutical interventions against coronavirus disease 2019 and
inuenza in Hong Kong: an observational study. Lancet Public Health
2020;5:e279–88.
7 Habib H. Has Sweden's controversial covid-19 strategy been
successful? BMJ 2020;369:m2376.
8 Rhodes T, Lancaster K, Lees S, etal. Modelling the pandemic:
attuning models to their contexts. BMJ Glob Health 2020;5:e002914.
9 Freebairn L, Rychetnik L, Atkinson J- A, etal. Knowledge
mobilisation for policy development: implementing systems
approaches through participatory dynamic simulation modelling.
Health Res Policy Syst 2017;15:83.
10 Mansnerus E. Using model- based evidence in the governance of
pandemics. Sociol Health Illn 2013;35:280–91.
11 Leach M, Scoones I. The social and political lives of zoonotic
disease models: narratives, science and policy. Soc Sci Med
2013;88:10–17.
12 Zhou F, Yu T, Du R, etal. Clinical course and risk factors for mortality
of adult inpatients with COVID-19 in Wuhan, China: a retrospective
cohort study. Lancet 2020;395:1054–62.
13 CDC COVID-19 Response Team. Severe Outcomes Among Patients
with Coronavirus Disease 2019 (COVID-19) - United States, February
12- March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:343–6.
14 nychealth / coronavirus- data, 2020. Available: https:// github. com/
nychealth/ coronavirus- data/ blob/ master/ by- age. csv
15 Onder G, Rezza G, Brusaferro S. Case- Fatality rate and
characteristics of patients dying in relation to COVID-19 in Italy.
JAMA 2020;323:1775-1776.
16 Oke J, Heneghan C. Global Covid-19 case fatality rates, 2020.
Available: https://www. cebm. net/ covid- 19/ global- covid- 19- case-
fatality- rates/
17 Wu Z, McGoogan JM. Characteristics of and Important Lessons
From the Coronavirus Disease 2019 (COVID-19) Outbreak in
China: Summary of a Report of 72 314 Cases From the Chinese
Center for Disease Control and Prevention. JAMA 2020;323:1239-
1242.
18 Bi Q, Wu Y, Mei S, etal. Epidemiology and transmission of
COVID-19 in Shenzhen China: analysis of 391 cases and 1286 of
their close contacts. medRxiv 2020.
19 Lauer SA, Grantz KH, Bi Q, etal. The incubation period of
coronavirus disease 2019 (COVID-19) from publicly reported
conrmed cases: estimation and application. Ann Intern Med
2020;172:577–82.
20 Wei WE, Li Z, Chiew CJ, etal. Presymptomatic Transmission of
SARS- CoV-2 - Singapore, January 23- March 16, 2020. MMWR Morb
Mortal Wkly Rep 2020;69:411–5.
21 Arons MM, Hateld KM, Reddy SC, etal. Presymptomatic SARS-
CoV-2 infections and transmission in a skilled nursing facility. N Engl
J Med 2020;382:2081–90.
22 Tong Z- D, Tang A, Li K- F, etal. Potential presymptomatic
transmission of SARS- CoV-2, Zhejiang Province, China, 2020.
Emerg Infect Dis 2020;26:1052–4.
23 To KK- W, Tsang OT- Y, Leung W- S, etal. Temporal proles of viral
load in posterior oropharyngeal saliva samples and serum antibody
responses during infection by SARS- CoV-2: an observational cohort
study. Lancet Infect Dis 2020;20:565–74.
24 He X, Lau EHY, Wu P, etal. Temporal dynamics in viral shedding and
transmissibility of COVID-19. Nat Med 2020;26:672–5.
25 Li P, Fu J- B, Li K- F, etal. Transmission of COVID-19 in the terminal
stages of the incubation period: a familial cluster. Int J Infect Dis
2020;96:452–3.
26 Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel
coronavirus (COVID-19) infections. Int J Infect Dis 2020;93:284–6.
27 Du Z, Xu X, Wu Y, etal. Serial interval of COVID-19 among publicly
reported conrmed cases. Emerg Infect Dis 2020;26:1341–3.
28 Wang D, Hu B, Hu C, etal. Clinical characteristics of 138
hospitalized patients with 2019 novel coronavirus- infected
pneumonia in Wuhan, China. JAMA 2020;323:1061.
29 Goyal P, Choi JJ, Pinheiro LC, etal. Clinical characteristics of
Covid-19 in New York City. N Engl J Med 2020;382:2372–4.
30 Verity R, Okell LC, Dorigatti I, etal. Estimates of the severity of
coronavirus disease 2019: a model- based analysis. Lancet Infect Dis
2020;20:669–77.
31 Russell TW, Hellewell J, Jarvis CI, etal. Estimating the infection and
case fatality ratio for coronavirus disease (COVID-19) using age-
adjusted data from the outbreak on the diamond Princess cruise
SHIP, February 2020. Euro Surveill 2020;25.
32 Mizumoto K, Chowell G. Estimating risk for death from coronavirus
disease, China, January- February 2020. Emerg Infect Dis
2020;26:1251–6.
33 Winter AK, Hegde ST. The important role of serology for COVID-19
control. Lancet Infect Dis 2020;20:758–9.
34 Xiang F, Wang X, He X, etal. Antibody detection and dynamic
characteristics in patients with COVID-19. Clin Infect Dis 2020.
35 Yong SEF, Anderson DE, Wei WE, etal. Connecting clusters of
COVID-19: an epidemiological and serological investigation. Lancet
Infect Dis 2020;20:809–15.
36 Yang J, Zheng Y, Gou X, etal. Prevalence of comorbidities and its
effects in patients infected with SARS- CoV-2: a systematic review
and meta- analysis. Int J Infect Dis 2020;94:91–5.
37 Halpin DMG, Faner R, Sibila O, etal. Do chronic respiratory diseases
or their treatment affect the risk of SARS- CoV-2 infection? Lancet
Respir Med 2020;8:436–8.
38 Emanuel EJ, Persad G, Upshur R, etal. Fair allocation of scarce
medical resources in the time of Covid-19. N Engl J Med Overseas
Ed 2020;382:2049–55.
39 Wang B, Li R, Lu Z, etal. Does comorbidity increase the risk of
patients with COVID-19: evidence from meta- analysis. Aging
2020;12:6049–57.
40 MacIntyre CR, Heslop DJ. Public health, health systems and
palliation planning for COVID-19 on an exponential timeline. Med J
Aust 2020.
41 Touboul- Lundgren P, Jensen S, Drai J, etal. Identication of cultural
determinants of antibiotic use cited in primary care in Europe: a
mixed research synthesis study of integrated design "Culture is all
around us". BMC Public Health 2015;15:908.
42 Hale T, Angrist N, Kira B. “Variation in Government Responses to
COVID-19” Version 5.0. Blavatnik School of Government Working
Paper, 2020.
43 Guan Y, Deng H, Zhou X. Understanding the impact of the COVID-19
pandemic on career development: insights from cultural psychology.
J Vocat Behav 2020;119:103438.
44 Messner W. The institutional and cultural context of cross- national
variation in COVID-19 outbreaks. medRxiv 2020.
45 Shim E, Tariq A, Choi W, etal. Transmission potential and severity of
COVID-19 in South Korea. Int J Infect Dis 2020;93:339–44.
46 Karamouzian M, Madani N. COVID-19 response in the middle East
and North Africa: challenges and paths forward. Lancet Glob Health
2020;8:e886–7.
47 Jarvis CI, Van Zandvoort K, Gimma A, etal. Quantifying the impact
of physical distance measures on the transmission of COVID-19 in
the UK. BMC Med 2020;18:124.
48 Prem K, Liu Y, Russell TW, etal. The effect of control strategies
to reduce social mixing on outcomes of the COVID-19 epidemic
in Wuhan, China: a modelling study. Lancet Public Health
2020;5:e261–70.
49 Tian H, Liu Y, Li Y, etal. An investigation of transmission control
measures during the rst 50 days of the COVID-19 epidemic in
China. Science 2020;368:638–42.
50 Kucharski AJ, Klepac P, Conlan AJK, etal. Effectiveness of isolation,
testing, contact tracing, and physical distancing on reducing
transmission of SARS- CoV-2 in different settings: a mathematical
modelling study. Lancet Infect Dis 2020;20:1151–60.
51 Salje H, Tran Kiem C, Lefrancq N, etal. Estimating the burden of
SARS- CoV-2 in France. Science 2020;369:208–11.
52 Di Domenico L, Pullano G, Sabbatini CE, etal. Expected impact of
lockdown in Île- de- France and possible exit strategies. medRxiv 2020.
53 Shretta R. The economic impact of COVID-19, 2020. Available:
https://www. tropicalmedicine. ox. ac. uk/ news/ the- economic- impact-
of- covid- 19
54 Freebairn L, Atkinson J- A, Kelly PM, etal. Decision makers'
experience of participatory dynamic simulation modelling: methods
for public health policy. BMC Med Inform Decis Mak 2018;18:131.
55 Pan- Ngum W, Kinyanjui T, Kiti M, etal. Predicting the relative
impacts of maternal and neonatal respiratory syncytial virus (RSV)
vaccine target product proles: a consensus modelling approach.
Vaccine 2017;35:403–9.
56 Cornell COVID caseload calculator with capacity and ventilators
(C5V Online*), 2020. Available: https:// covid19. sjsu. edu/ C5V/ default/
user/ login?_ next=/ C5V/ default/ index
on August 3, 2024 by guest. Protected by copyright.http://gh.bmj.com/BMJ Glob Health: first published as 10.1136/bmjgh-2020-003126 on 23 December 2020. Downloaded from
1
BMJ Global Health 2021;6:e003126corr1. doi:10.1136/bmjgh-2020-003126corr1
Correction
Correction for modelling the COVID-19 pandemic in context:
an international participatory approach
Aguas R, White L, Hupert N, et al. Modelling the COVID-19 pandemic in context: an
international participatory approach. BMJ Glob Health 2020;5:e003126. doi: 10.1136/
bmjgh-2020-003126.
This article has a correction. The group in South Africa has been using their own
models and not the COVID-19 Modelling Consortium model. Their inclusion in the
manuscript was an error.
Open access This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported
(CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose,
provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See:
https:// creativecommons. org/ licenses/ by/ 4. 0/.
© Author(s) (or their employer(s)) 2021. Re- use permitted under CC BY. Published by BMJ.
BMJ Global Health 2021;6:e003126corr1. doi:10.1136/bmjgh-2020-003126corr1
