
1
Am. J. Trop. Med. Hyg., 60(1), 1999, pp. 1–6
Copyright
q
1999 by The American Society of Tropical Medicine and Hygiene
PREDICTING MOSQUITO REPELLENT POTENCY OF N,N-DIETHYL-m-TOLUAMIDE
(DEET) ANALOGS FROM MOLECULAR ELECTRONIC PROPERTIES
DA MA, APURBA K. BHATTACHARJEE, RAJ K. GUPTA,
AND
JEAN M. KARLE
Department of Entomology, Division of Communicable Diseases and Immunology, and Department of Pharmacology, Division of
Experimental Therapeutics, Walter Reed Army Institute of Research, Washington, District of Columbia
Abstract. Specific molecular electronic properties of 30 N,N-diethyl-m-toluamide (DEET) analogs demonstrate
functional dependence with their reported duration of protection against mosquito bites, thus providing predictors of
insect repellent efficacy. No single electronic property is sufficient to predict repellent efficacy as measured by
protection time, rather a set of specific electronic properties is required. Thus, the values of the van der Waals surface
electrostatic potential by the amide nitrogen and oxygen atoms, the atomic charge at the amide nitrogen atom, and
the dipole moment must all be in optimal ranges for potent repellency. The electronic properties were calculated using
the AM1 semi-empirical quantum chemical method using commercial software. These easily calculable predictors of
repellent efficacy should be useful in predicting the relative efficacy of newly designed compounds, thus guiding the
selection of new repellents for testing.
Arthropod-borne diseases are many, but vaccines, both ef-
fective and economical, are few. Inexpensive and practical,
insect repellents become a viable and attractive alternative.
1
Since the mid 1950s, N,N-diethyl-m-toluamide (DEET, Table
1, compound 2a) has been regarded as the standard mosquito
repellent.
2,3
However, as a repellent for human use, DEET is
not equally effective against all insect and arthropod vectors
of disease.
4
It is short in duration of action and has disa-
greeable cosmetic effects.
1,4
Aside from its unpleasant odor,
DEET can penetrate the skin.
1,5
It can cause drug-drug in-
teractions and potential toxicity such as adverse reactions in
children and adults when used in concentrations exceeding
label recommendations.
1
In addition, DEET is a plasticizer
that reacts with certain plastics and synthetic rubber.
1,4, 6
Thus, a more effective mosquito repellent is needed that is
nontoxic and does not react with protective clothing.
A number of studies have found that compounds contain-
ing specific functional groups are more effective repellents
as measured by duration of protection. In 1953, after study-
ing the testing results of Morton and others
7
on 4,000 chem-
icals, Roadhouse
8
concluded that oximes are the most effec-
tive compounds and hydrocarbons make the least effective
repellents. The data indicated that an oxygen function is very
important for repellency and a phenyl group probably en-
hances repellency.
8
Examination of the test data contained
in the U. S. Department of Agriculture (USDA) handbooks
by Skinner and others
4
confirmed most of the findings of
Roadhouse. Gouck and others
9
found that adding only one
carbon atom between the cyclohexane ring and amide in
cyclohexane aliphatic amides increased the repellency of the
compound, whereas adding two or three carbon atoms re-
sulted in a decrease in repellency. In 1968, having evaluated
4,308 compounds from the same USDA data bank, Garson
and Winnike
10
found amides and imides to be more effective
repellents than phenols and alcohols.
Other studies have focused on physical properties such as
volatility, melting point, molecular weight, and polarizabil-
ity. It has been well recognized that repellents must be vol-
atile since repellents affect the olfactory chemosensilla of
the mosquito.
11–13
Duration/protection time decreases if re-
pellents are either too volatile or too nonvolatile.
10,14,15
If the
vapor concentration of the repellent decreases below the
minimum repellent concentration, a rapid loss of repellency
will result. On the other hand, if a compound is not volatile,
it will never come into contact with the olfactory end organ.
The study of the relationship between volatility and re-
pellency has been logically extended to melting and boiling
points. Christophers
16
demonstrated that compounds that
melt much above 37
8
C generally have little insect repellency.
Coumarin, with a melting point of 68–70
8
C, is an exception.
Bunker and Hirschfelder
17
noted that all of the 20 best re-
pellents had a boiling point greater than 150
8
C. Piper and
others
18
found that the preferred boiling point range was
230–260
8
C for repellents. For a series of N,N-diethylben-
zamides, Johnson and others
12
found that the optimum boil-
ing point for repellency was 120
8
C at 0.5 mm of Hg. How-
ever, a boiling point in the proper range does not guarantee
repellency.
12,19
An optimal molecular weight range of 146 to 257 for re-
pellent potency was found by Rayner and others,
13
Sugawara
and others,
20
and Alexander and others.
21
Molecules that are
bulky and have substituents attached to the main chain are
likely to be good repellents.
20, 21
Although Johnson and oth-
ers
12
could not find a correlation between repellent effec-
tiveness and Hammett substituent constants or molecular po-
larizability in a series of DEET analogs, McIver
22
suggested
that lipophilicity determined by reverse-phase high-perfor-
mance liquid chromatography is important to the degree of
repellency.
Since physical-chemical properties of repellents play a
significant role toward repellent effectiveness, we have as-
sessed the role of molecular electronic properties towards
repellent protection time using a series of DEET analogs
reported by Suryananarayana and others.
23
Using quantum
chemical methods, lowest energy conformations and molec-
ular electronic properties were calculated for 31 amides di-
vided into five different types (Table 1): Type 1, N,N-di-
methylamide; Type 2, N,N-diethylamide; Type 3, N,N-diiso-
propylamide; Type 4, N-ethylamides; and Type 5, piperidi-
neamides. The calculated structural and electronic properties
were investigated to determine any functional dependence
with protection time as measured by Suryananarayana and
others
23
to provide predictive discriminators of insect repel-
lency and provide a better understanding the structure and
repellency properties of these compounds. Although the
study specifically addresses repellent efficacy, the technique
2
MA AND OTHERS
T
ABLE
1
Structure and protection time of Type 1 N,N-dimethylamide, Type
2 N,N-diethylamide, Type 3 N,N-diisopropylamide, Type 4 N-
ethylamide, and Type 5 piperidineamide compounds
Compound
Protec-
tion
time
(hr)* Ring R R
1
5
R
2
1a
1b
1c
1d
1e
1f
o-chlorobenzamide
cyclohexamide
m-toluamide
o-ethoxylbenzamide
benzamide
p-anisamide
5
3
3
2.83
1.67
1
a
s
a
a
a
a
2-Cl
H
3-CH
3
2-OC
2
H
5
H
4-OCH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
2a
2b
2c
2d
2e
2f
m-toluamide
benzamide
cyclohexamide
o-ethoxylbenzamide
p-toluamide
p-anisamide
5
4
4
3.5
2.83
1
a
a
s
a
a
a
3-CH
3
H
H
2-OC
2
H
5
4-CH
3
4-OCH
3
C
2
H
5
C
2
H
5
C
2
H
5
C
2
H
5
C
2
H
5
C
2
H
5
3a
3b
3c
3d
3e
3f
benzamide
m-toluamide
cyclohexamide
p-anisamide
o-ethoxylbenzamide
o-chlorobenzamide
3
2.67
2
1.17
1.08
1
a
a
s
a
a
a
H
3-CH
3
H
4-OCH
3
2-OC
2
H
5
2-Cl
iC
3
H
7
iC
3
H
7
iC
3
H
7
iC
3
H
7
iC
3
H
7
iC
3
H
7
3g p-toluamide 0.5 a 4-CH
3
iC
3
H
7
4a
4b
4c
4d
4e
4f
m-toluamide
benzamide
cyclohexamide
p-toluamide
p-anisamide
o-ethoxylbenzamide
0.67
0.58
0.50
0.08
0.08
0.08
a
a
s
a
a
a
3-CH
3
H
H
4-CH
3
4-OCH
3
2-OC
2
H
5
R
1
R
2
HC
2
H
5
HC
2
H
5
HC
2
H
5
HC
2
H
5
HC
2
H
5
HC
2
H
5
5a
5b
5c
5d
5e
5f
benzamide
cyclohexamide
m-toluamide
o-chlorobenzamide
p-toluamide
p-anisamide
3
2
1.42
1
1
0.75
a
s
a
a
a
a
H
H
3-CH
3
2-Cl
4-CH
3
4-OCH
3
N,R
1
,R
2
Piperidine
Piperidine
Piperidine
Piperidine
Piperidine
Piperidine
* Values obtained from reference 23.
of linking specific molecular electronic properties to biolog-
ical activity to provide predictors of biological activity is
generally applicable to both efficacy and toxicity studies.
MATERIALS AND METHODS
Computation of geometric and electronic properties.
All computational calculations were conducted using Spar-
tan version 4.0 (Wavefunction, Inc., Irvine, CA) running on
a Silicon Graphics Indigo Extreme R4000 workstation. Ge-
ometry optimization and electronic property calculations
were performed using the Austin Model 1 (AM1) semi-em-
pirical quantum chemical method as implemented in Spar-
tan. First, a conformational search calculation together with
a population density calculation was performed for each of
the molecules. Conformation search by the double rotation
(30
8
mesh) of the dihedral angles C
2
-C
1
-C
7
-N and C
1
-C
7
-N-
R
1
(H or C) generated 144 conformations of each compound
from which the lowest energy conformer with a population
density greater than 75% was identified. Then the electronic
properties such a molecular electrostatic potentials (MEPs),
dipole moments, and individual atomic charges were calcu-
lated on the AM1-optimized geometry of the most populated
conformation. The MEP represents the energy of interaction
of a unit positive charge with the electronic charge distri-
bution generated by the nuclei and electrons of a molecule.
The electrostatic potentials were sampled over the entire ac-
cessible surface of the molecule (corresponding roughly to
a van der Waals contact surface) and plotted onto a surface
of constant electron density (0.002 e/au
3
). Additionally, MEP
isoenergy contours extending beyond the van der Waals sur-
face at
2
10 kcal/mol were generated.
Evaluation of the functional dependence of individual
stereoelectronic properties was performed using S-Plus4
(MathSoft, Inc., Seattle, WA). A matrix of graphs with pro-
tection time versus specific electronic property and electron-
ic property versus electronic property (see list of properties
in Table 2) were first created using a subset of the four most
and five least potent compounds to identify potential func-
tional relationships. Only protection time versus electronic
property graphs in which the electronic property had dis-
tinctly different numerical values for the most and least po-
tent compounds or electronic property versus electronic
property graphs in which the most and least potent com-
pounds formed distinct clusters were selected for further ex-
amination. Examination of these graphs using all of the com-
pounds resulted in identification of a set of electronic prop-
erties linked to repellent potency.
Biologic testing. Biologic testing was performed and re-
ported by Suryananarayana and others.
23
Briefly, protection
time (PT) was determined by applying test compound at a
dose of 1 mg/cm
2
onto the external surface of a human fist
followed by exposure to 200 females (5–7 days old) of the
day-biter mosquito Aedes aegypti for 5 min every 30 min.
The PT is defined as the period of protection offered at given
doses until two consecutive bites are obtained at a 30-min
interval.
24
The reported protection times represent the aver-
age of multiple determinations.
RESULTS
Conformation. All compounds shared a similar backbone
conformation with the dihedral angle C
1
-C
7
-N-C
R2
equal to
180
86
8.1
8
.
Negative potentials by the amide group. The MEP is a
representation of the charge distribution at the van der Waals
surface (Figure 1, second column). Figure 1 shows the lo-
cation of the most negative potential (colored deepest red)
by the carbonyl oxygen atom, indicating that this atom is
intrinsically the most nucleophilic site in the molecule, rang-
ing from
2
77.3 to
2
72.6 kcal/mol (Table 2). A negative
potential region less negative than by the carbonyl oxygen
atom also occurs by the amide nitrogen atom in the range
of
2
38.6 to
2
16.1 kcal/mol. These negative potential
3
MOSQUITO REPELLENT POTENCY
T
ABLE
2
Molecular electronic properties*
Compound
Protection
time (hr)
Maximum negative
potential by
oxygen atom
(kcal/mol)
Maximum negative
potential by
nitrogen atom
(kcal/mol)
Maximum
positive
potential
(kcal/mol)
Site of maximum
positive potential
Dipole
moment
(Debye)
Electrostatic charges
OC
7
N
1a
1b
1c
1d
1e
1f
5
3
3
2.83
1.67
1
2
73.1
2
75.2
2
76.2
2
73.8
2
74.7
2
75.7
2
22.8
2
23.2
2
17.0
2
23.8
2
16.1
2
18.7
21.1
17.8
18.3
18.5
19.2
24.3
4-H
HofCH
3
6-H
4-H
2-H
3-H
3.82
3.27
3.45
2.21
3.55
4.37
2
0.50
2
0.53
2
0.51
2
0.55
2
0.52
2
0.52
0.50
0.58
0.54
0.70
0.56
0.59
2
0.30
2
0.36
2
0.29
2
0.33
2
0.30
2
0.33
2a
2b
2c
2d
2e
2f
5
4
4
3.5
2.83
1
2
75.0
2
74.9
2
74.5
2
71.4
2
76.4
2
75.5
2
23.2
2
24.1
2
25.1
2
27.8
2
28.6
2
30.0
16.9
18.9
16.2
17.9
17.0
23.3
5-H
2-H
HofCH
2
4-H
3-H
3-H
3.68
3.61
3.25
2.23
3.68
3.55
2
0.54
2
0.54
2
0.55
2
0.56
2
0.55
2
0.55
0.65
0.66
0.68
0.74
0.76
0.75
2
0.45
2
0.49
2
0.51
2
0.50
2
0.56
2
0.54
3a
3b
3c
3
2.67
2
2
75.9
2
76.9
2
75.9
2
20.3
2
23.3
2
25.9
17.1
16.7
15.4
3-H
5-H
HofCH
3.63
3.65
3.25
2
0.55
2
0.55
2
0.55
0.68
0.69
0.68
2
0.55
2
0.56
2
0.57
3d
3e
3f
3g
1.17
1.08
1
0.5
2
76.7
2
72.6
2
74.4
2
77.3
2
27.8
2
24.6
2
25.7
2
22.1
22.6
17.8
20.4
16.7
3-H
5-H
5-H
3-H
3.25
2.25
3.90
3.74
2
0.54
2
0.57
2
0.53
2
0.55
0.69
0.76
0.57
0.68
2
0.60
2
0.57
2
0.51
2
0.56
4a
4b
4c
4d
4e
4f
0.67
0.58
0.50
0.08
0.08
0.08
2
74.5
2
74.4
2
75.4
2
75.5
2
74.7
2
75.7
2
25.8
2
24.3
2
26.7
2
25.2
2
24.3
2
38.6
33.4
34.0
33.5
33.6
35.2
22.1
HofNH
HofNH
HofNH
HofNH
HofNH
HofNH
3.22
3.37
3.46
3.50
4.50
3.55
2
0.54
2
0.53
2
0.56
2
0.53
2
0.54
2
0.56
0.69
0.65
0.69
0.68
0.71
0.78
2
0.63
2
0.61
2
0.65
2
0.63
2
0.64
2
0.71
5a
5b
5c
5d
5e
5f
3
2
1.42
1
1
0.75
2
73.8
2
76.3
2
75.6
2
74.4
2
74.8
2
75.7
2
33.0
2
26.8
2
33.1
2
25.5
2
35.1
2
30.2
17.6
17.1
18
20.5
17.2
22.8
4-H
1-H
6-H
4-H
3-H
3-H
3.52
3.40
3.56
3.91
3.60
3.27
2
0.53
2
0.55
2
0.53
2
0.53
2
0.54
2
0.54
0.71
0.66
0.68
0.63
0.73
0.68
2
0.55
2
0.48
2
0.52
2
0.51
2
0.55
2
0.46
* Underlined values are outside of the range of values for the compounds with 4 or 5 hr protection times.
regions indicate the likely sites for binding to the electron-
poor regions of receptor molecules.
Most positive potential. Regions of positive potentials at
the van der Waals surface indicate the electrophilic or acidic
sites. The site for the most positive potential is regarded as
intrinsically the most electrophilic or acidic site in the mol-
ecule. Although the location of the most positive potential
is by different hydrogen atoms on different molecules, the
magnitude of the most positive potential appears to be re-
lated to protection time. All compounds that protect for at
least 2.8 hr have a maximum positive potential in the range
of 16.2–21.1 kcal/mol, whereas all compounds with a most
positive potential higher than 21.1 kcal/mol protected for no
more than 1 hr (Table 2). Thus, the intrinsic electrophilicity
of these compounds appears to have a role toward repellen-
cy.
Isopotential contour at
2
10 kcal/mol. Three-dimension-
al electrostatic isopotential maps at
2
10 kcal/mol (Figure 1,
third column) are the electrostatic features beyond the van
der Waals surface of the molecules that are considered to be
key features through which a molecule recognizes its recep-
tor at longer distances and accordingly promotes interaction
between complimentary sites with the receptor.
25
Figure 1
shows that all repellents have a large extended negative po-
tential region extending out from the carbonyl group. Al-
though this potential characterizes the primary level of rec-
ognition interaction with the receptor, there is no apparent
relationship with the size or shape of these surfaces to pro-
tection time.
Dipole moment. The dipole moment indicates the intrin-
sic polarity of a molecule. Its magnitude is a good indicator
of intrinsic lipophilicity or hydrophobicity; the larger the
magnitude, the more likely the compound is hydrophilic.
The magnitude of the dipole moment for the most active
repellents (PT
.
3.5 hr, Table 2) ranges between 3.25 and
3.82 Debye, an indication that an optimal lipophilicity or
hydrophobicity for this class of compounds is necessary for
the molecule to be an active repellent. All compounds with
a dipole moment outside of this range protected for less than
3 hr. Since some of the poor repellents have a dipole moment
in the optimal range, the dipole moment by itself does not
serve as a predictor of protection time. The orientation of
the dipole moment is not linked to protection time as the
dipole moment points toward the carbonyl oxygen atom for
all compounds.
Atomic charges. These values indicate the intrinsic re-
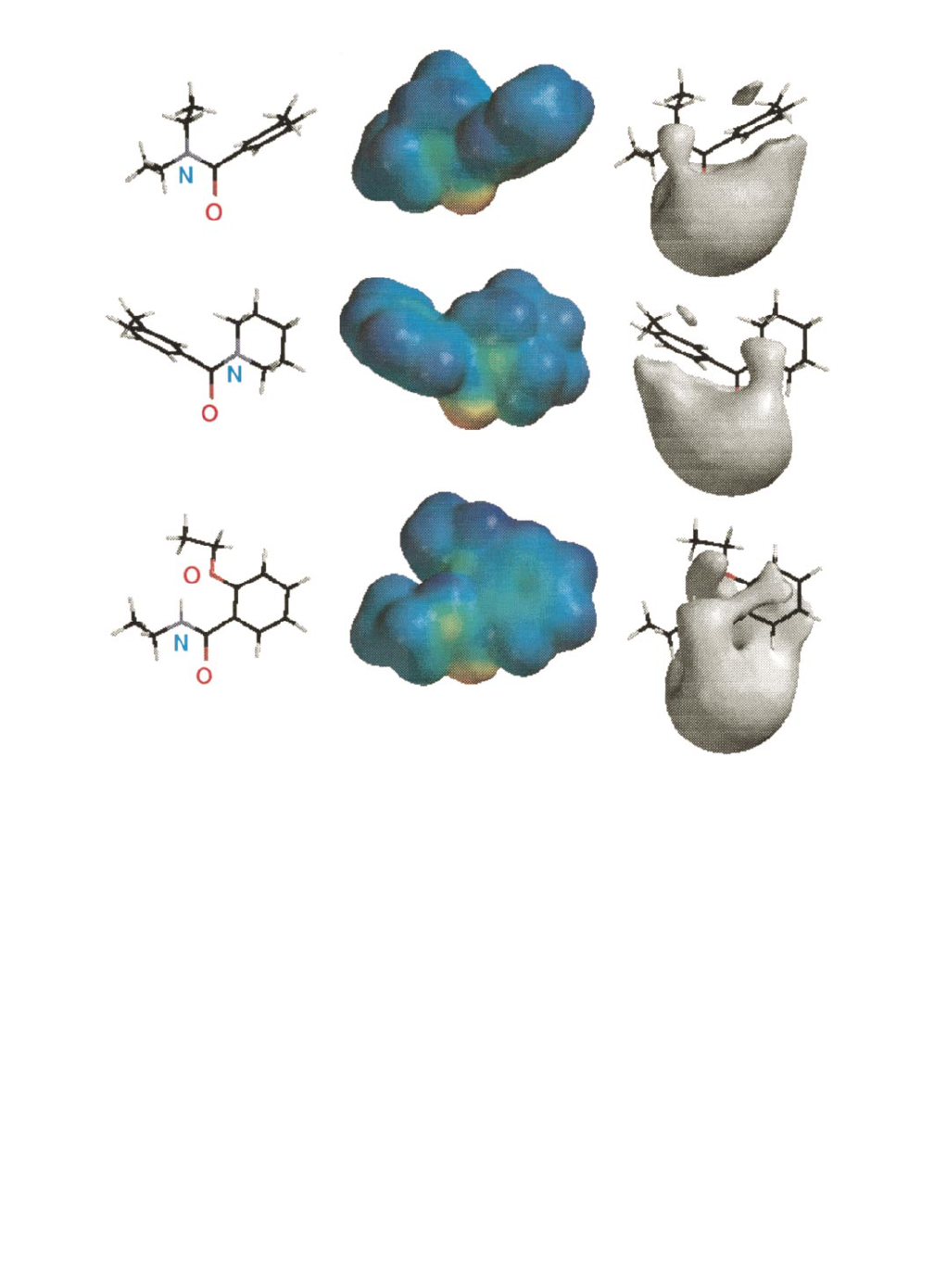
4
MA AND OTHERS
F
IGURE
1. Optimized geometry (first column), color-coded molecular electrostatic potential plotted onto surface of constant electron (0.002
e/au
3
)(second column), and
2
10 kcal/mol isopotential surface (third column) of compounds 2a (top row, protection time [PT]
5
5 hr), 5c
(middle row, PT
5
1.4 hr), and 4f (bottom row, PT
5
0.08 hr). Atoms are colored black for carbon, red for oxygen, blue for nitrogen, and
gray for hydrogen. The deepest blue surface is the most positive, and the deepest red surface is the most negative.
active character of the individual atoms constituting the mol-
ecule. The magnitude of negative charge on an atom will
characterize the nucleophilic nature of the atom whereas the
magnitude of positive charge will correspondingly charac-
terize the electrophilic nature of the atom. A low atomic
charge of the amide nitrogen atom is associated with low
protection times such that all compounds with a charge more
negative than
2
0.51 e protected no longer than 3 hr (Table
2). Protection time was even poorer, less than 1 hr, when the
amide nitrogen atom has an electrostatic charge more neg-
ative value than
2
0.60 e. Thus, the intrinsic nucleophilic
character of the nitrogen atom seems to have a significant
role toward repellency. In general, as the electrostatic charge
of the nitrogen atom becomes more negative, the electro-
static charge of the carbonyl carbon atom becomes more
positive (Table 2).
DISCUSSION
An examination of the electronic properties of the amides
that protect for at least 4 hr results in the following profile:
a maximum positive potential in the range of 16.2 to 21.1
kcal/mol, a maximum negative potential of
2
75.0 to
2
73.1
kcal/mol, a negative potential by the amide nitrogen atom
of
2
25.1 to
2
22.8 kcal/mol, a dipole moment of 3.25 to
3.82 Debye, and atomic charges at the carbonyl oxygen
atom, the carbonyl carbon atom, and the amide nitrogen
atom of
2
0.55 to
2
0.50 e, 0.50 to 0.68 e, and
2
0.51 to
2
0.30 e, respectively. All of the other compounds have at
least one of these values outside of these ranges. Since no
single electronic property guarantees highly efficacious re-
pellency, the results demonstrate that to be highly effica-
cious, i.e., protect for at least 4 hr, a compound must have
all of the listed electronic properties in the numerical ranges
listed above.
Two of the above listed electronic properties, the electro-
static potential by the amide nitrogen atom and the atomic
charge of the amide nitrogen atom, exclude all but com-
pounds 1b (3 hr protection time) and 1d (2.83 hr protection
time) from the most efficacious group or 25 of the 27 less
efficacious compounds, thus showing the importance of the
nucleophilicity of the amide nitrogen atom (Figure 2). Al-
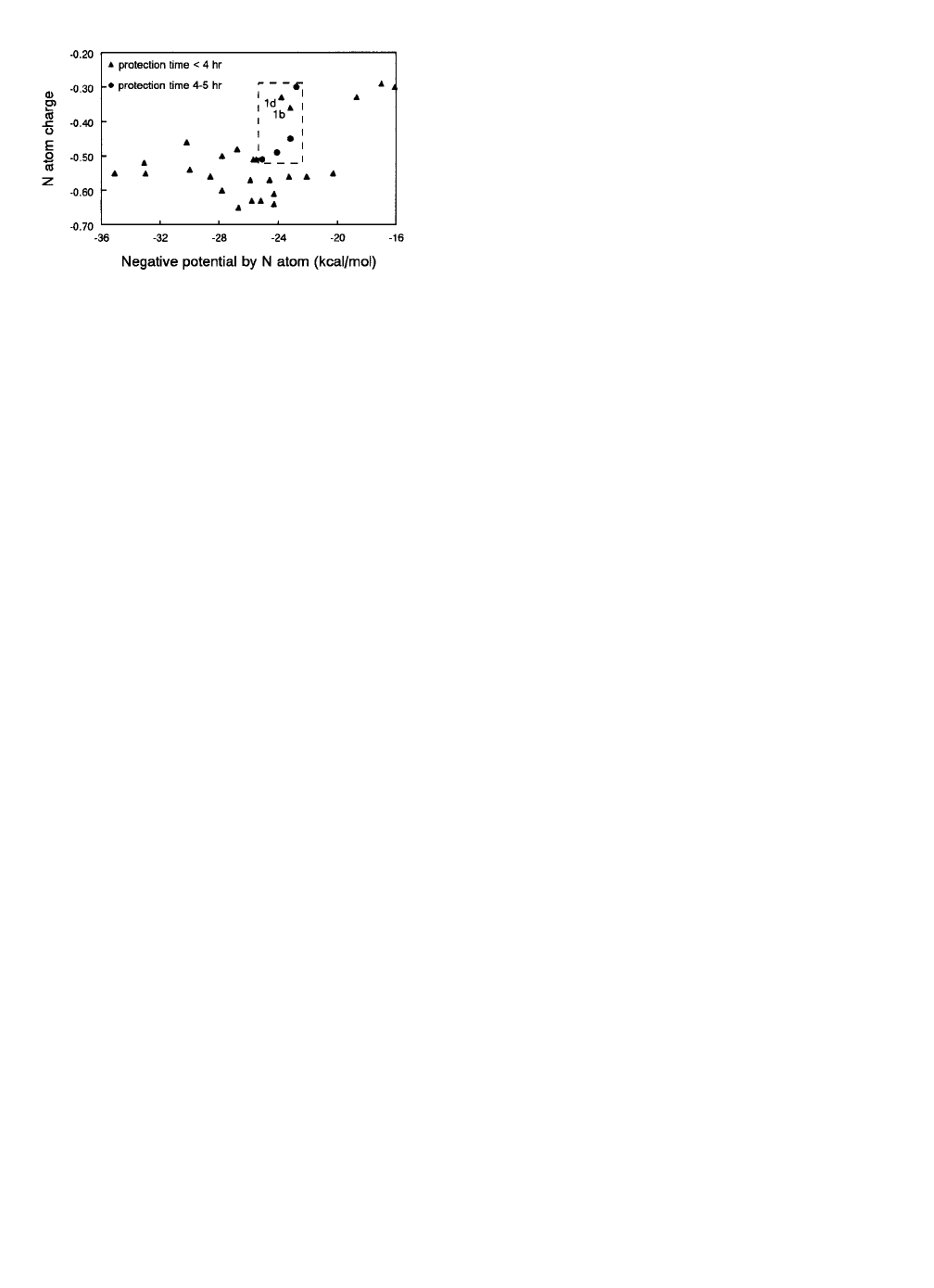
5
MOSQUITO REPELLENT POTENCY
F
IGURE
2. Plot of the atomic charge of the amide nitrogen atom
versus the maximum negative potential by the amide nitrogen atom,
showing that all compounds that protect less than 4 hr, except for
compounds 1b and 1d, have values that fall outside the range of
values of the compounds that protect for at least 4 hr (see box with
dashed lines).
though the values of the amide nitrogen atom for compounds
1b and 1d fall within the numerical ranges for high efficacy,
their dipole moment (compound 1d) or their electrostatic po-
tential by the amide oxygen atom (compound 1b) fall outside
these optimal ranges. Thus, this group of electronic proper-
ties serves as discriminators of efficacy of repellency.
Interestingly, when the values of the calculated electronic
properties are outside of the numerical ranges of the most
potent compounds, they are usually too low for the maxi-
mum negative potential that is located by the amide oxygen
atom, too low for the atomic charge of the amide oxygen
atom, too high for the atomic charge of the carbonyl carbon
atom, and too low for the atomic charge of the amide nitro-
gen atom.
The test data of Suryananarayana and others
23
show that
DEET analogs with a secondary amine exhibit only short
protection times. This was reflected in high maximum pos-
itive electrostatic potentials on the surface of the molecule
and a highly negative atomic charge at the nitrogen atom.
The biologic data used in this study were limited to the
yellow fever mosquito Ae. aegypti; therefore, the results may
be valid for only this arthropod. The rank order of effec-
tiveness of DEET versus other repellent compounds has
been shown to be similar for Ae. aegypti and the malaria
parasite Anopheles stephensi.
26–29
The electronic properties of the amide group (N-C
5
O at-
oms) seem to be the key in determining the duration of pro-
tection against mosquito bites. The substituents attached to
the carbon and nitrogen atoms of the amide group together
influence the electronic properties of the amide group. Thus,
a balance of polarity between the two parts of the molecule
is an important contributing factor for potent repellent activ-
ity. Our investigation shows that the numerical values of the
electronic properties of the amide group fall into discrete
ranges for a lengthy duration of protection. These optimal
values are now being used as a guide for the synthesis and
testing of new potential insect repellents.
Acknowledgments: We thank the National Research Council, Wash-
ington, DC for assistance in the support of Dr. Da Ma and Dr. Apur-
ba K. Bhattacharjee. We also thank Dr. Ralf Brueckner and Dr. Mark
Marino for assistance in the use of S-Plus.
Authors’ addresses: Da Ma and Raj K. Gupta, Department of En-
tomology, Division of Communicable Diseases and Immunology,
Walter Reed Army Institute of Research, Washington, DC 20307-
5100. Apurba K. Bhattacharjee and Jean M. Karle, Department of
Pharmacology, Division of Experimental Therapeutics, Walter Reed
Army Institute of Research, Washington, DC 20307-5100.
Reprint requests: Jean M. Karle, Department of Pharmacology, Di-
vision of Experimental Therapeutics, Walter Reed Army Institute of
Research, Washington, DC 20307-5100.
REFERENCES
1. Gupta RK, Rutledge LC, 1994. Role of repellents in vector
control and disease prevention. Am J Trop Med Hyg 50: 82–
86.
2. McCabe ET, Barthel WF, Gertler SI, Hall SA, 1954. Insect re-
pellents. III. N,N-diethylamides. J Org Chem 19: 493–498.
3. Gilbert IH, Gouck HK, Smith CN, 1955. New mosquito repel-
lents. J Econ Entomol 48: 741–743.
4. Skinner WA, Johnson HL, 1980. The design of insect repel-
lents. Arien EJ, ed. Drug Design. Volume X. New York: Ac-
ademic Press, 277–302.
5. Moody RP, Riedel D, Ritter L, Franklin CA, 1987. The effect
of DEET (N,N-diethyl-m-toluamide) on dermal persistence
and absorption of the insecticide fenitrothion in rats and mon-
keys. J Toxicol Environ Health 22: 471–479.
6. Watanabe K, Shono Y, Kakimizu A, Okada A, Matsuo N, Satoh
A, Nishimura H, 1993. New mosquito repellent from Euca-
lyptus camaldulensis. J Agri Food Chem 41: 2164–2166.
7. Morton FA, Travis BV, Linduska JP, 1947. Results of screening
tests with materials evaluated as insecticides, miticides and
repellents at Orlando Laboratory, April, 1942 to April, 1947.
US Dept Agr Bur Entomol Plant Quaran: E-733.
8. Roadhouse LAO, 1953. Laboratory studies on insect repellency.
Can J Zool 31: 535–546.
9. Gouck HK, Hall SA, Smith CN, Gilbert IH, 1957. Repellency
of homologous series of cyclohexane aliphatic acids and am-
ides. J Econ Entomol 50: 175–177.
10. Garson LR, Winnike ME, 1968. Relationships between insect
repellency and chemical and physical parameters-a review. J
Med Entomol 5: 339–352.
11. Davis EE, Rebert CS, 1976. Lactic acid-sensitive receptor on
the antennae of mosquito. J Econ Entomol 105: 1058–1061.
12. Johnson HL, Skinner WA, Maibach HI, Pearson TR, 1967. Re-
pellent activity and physical properties of ring-substituted N,
N-diethylbenzamide. J Econ Entomol 60: 173–176.
13. Rayner HB, Wright RH, 1966. Far infrared spectra of mosquito
repellents. Can J Entomol 98: 76–80.
14. Johnson HL, Skinner WA, Skidmore D, Maibach HI, 1968.
Topical mosquito repellents. II. Repellent potency and dura-
tion in ring-substituted N,N-dialkyl- and aminoalkylbenza-
mides. J Med Chem 11: 1265–1268.
15. Dethier VG, 1956. Repellents. Annu Rev Entomol 1: 181–202.
16. Christophers SR, 1947. Mosquito repellents being a report of
the work of the mosquito repellent inquiry. J Hyg 45: 176–
231.
17. Bunker CWO, Hirschfelder AD, 1925. Mosquito repellents. Am
J Trop Med 5: 359–383.
18. Piper DE, Hall RH, Wright GF, 1951. Chemistry of insect re-
pellency. Chem Can 3: 97–98.
19. Dethier VG, 1947. Chemical Insect Attractants and Repellents.
Philadelphia: Blakiston Co.
20. Sugawara R, Tominaga Y, Suzuki T, 1977. Effects of ring un-
saturation on the activity of propyl cyclohexaneacetate as an
attractant for the German cockroach. Insect Biochem 7: 483–
485.
21. Alexander BH, Beroza M, 1963. Aliphatic amides of cyclic
amines and tolyl maleimides as mosquito repellents. J Econ
Entomol 56: 58–60.
22. McIver SB, 1981. A model for the mechanism of action of the
repellent DEET on Aedes Aegypti (Diptera: Culicidae). J Med
Chem 11: 357–361.
23. Suryanarayana MVS, Pandey KS, Parkash S, Raghuveeran CD,

6
MA AND OTHERS
Dangi RS, Swamy RV, Rao KM, 1991. Structure-activity re-
lationship studies with mosquito repellent amides. J Pharm
Sci 80: 1055–1057.
24. Sharma RK, Jain SK, Kumar S, Rao KM, 1984. Evaluation of
some insect repellent formulations. Part I. Water soluble oint-
ment bases. J Med Indian J Hosp Pharm 21: 26–29.
25. Murray JS, Ziles BA, Jayasuriya K, Politzer P, 1986. Compar-
ative analysis of the electrostatic potentials of dibenzofuran
and some dibenzo-p-dioxins. J Am Chem Soc 108: 915–918.
26. Khan AA, Maibach HI, Skidmore DL, 1973. A study of insect
repellents. 2. Effect of temperature on protection time. J Econ
Entom 66: 437–438.
27. Buescher MD, Rutledge LC, Wirtz RA, 1982. Tests of com-
mercial repellents on human skin against Aedes aegypti. Mosq
News 42: 428–433.
28. Robert LL, Hallam JA, Seeley DC, Roberts LW, Wirtz RA,
1991. Comparative sensitivity of four Anopheles (Diptera:
Culicidae) to five repellents. J Med Entom 28: 417–420.
29. Boeckh J, Breer H, Geier M, Hoever FP, Kru¨ger BW, Nentwig
G, Sass H, 1996. Acylated 1,3-aminopropanols as repellents
against bloodsucking arthropods. Pestic Sci 48: 359–373.
