LEAD 345
CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION
4.1 CHEMICAL IDENTITY
Pb is a naturally occurring element with an abundance of 0.0016% in the earth’s crust (Davidson et al.
2014). It is a member of Group 14 (IVA) of the periodic table. Natural Pb is a mixture of four stable
isotopes:
204
Pb (1.4%),
206
Pb (24.1%),
207
Pb (22.1%), and
208
Pb (52.4%). The Pb isotopes
206
Pb,
207
Pb, and
208
Pb are the stable decay product of the naturally occurring decay series of uranium, actinium, and
thorium, respectively (Haynes 2014).
Pb is found in concentrated and easily accessible Pb ore deposits that are widely distributed throughout
the world (King et al. 2014). Its properties, such as corrosion resistance, density, and low melting point,
make it a familiar metal in pipes, solder, weights, and storage batteries. The chemical identities of Pb and
several of its compounds are provided in Table 4-1.
Table 4-1. Chemical Identity of Lead and Compounds
Characteristic
Lead
Lead(II) acetate
Lead(II) azide
Lead(II) bromide
Synonym(s) and
registered trade
name(s)
C.I. 77575; C.I.
Pigment metal 4;
Glover; Lead flake;
Lead S2; Omaha;
Omaha & Grant; SI;
SO
a
Acetic acid
lead(2+) salt (2:1);
neutral lead
acetate; plumbous
acetate; normal
lead acetate; sugar
of lead; salt of
Saturn
b
Lead azide
b
Lead bromide
(PbBr
2
); plumbous
bromide
b
Chemical formula
Pb
b
Pb(CH
3
CO
2
)
2
b
Pb(N
3
)
2
b
PbBr
2
b
Chemical structure
Not applicable
Not applicable
Not applicable
Not applicable
CAS Registry
Number
7439-92-1
b
301-04-2
b
13424-46-9
b
10031-22-8
b
LEAD 346
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Lead and Compounds
Characteristic
Lead(II) chloride
Lead(II) chromate
Lead(II)
tetrafluoroborate
c
Lead(II) iodide
Synonym(s) and
registered trade
name(s)
Lead chloride
(PbCl
2
); Lead(2+)
chloride; Plumbous
chloride
b
Chromic acid
(H
2
CrO
4
lead(2+)
salt (1:1); Chrome
yellow; Cologne
yellow; King’s
yellow; Leipzig
yellow; Paris
yellow; C.I.
Pigment Yellow 34;
lead chromium
oxide (PbCrO
4
);
plumbous
chromate; C.I.
77600
b
Tetrafluoro
borate(1-)
Lead(2+)
a
Lead iodide (PbI
2
);
Plumbous iodide
b
Chemical formula
PbCl
2
b
PbCrO
4
b
Pb(BF
4
)
2
a
PbI
2
b
Chemical structure
Not applicable
Not applicable
Not applicable
Not applicable
CAS Registry
Number
7758-95-4
b
7758-97-6
b
13814-96-5
a
10101-63-0
b
LEAD 347
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Lead and Compounds
Characteristic
Lead molybdenum
chromate
Lead(II) nitrate
Lead(II) oxide
Lead(II,II,IV) oxide
Synonym(s) and
registered trade
name(s)
Chromic acid, lead
and molybdenum salt;
chromic acid lead salt
with lead molybdate;
C.I. Pigment Red 104;
Lead chromate,
Molybdenum-Lead
chromate;
Molybdenum Orange
a
Nitric acid
lead(2+) salt (2:1);
Plumbous nitrate
b
C.I. 77577; C.I.
Pigment Yellow 46;
Lead oxide; Lead
oxide yellow; Lead
protoxide; Litharge;
Litharge Yellow
L-28; Massicot;
Massicotite;
Plumbous oxide;
Yellow lead ocher
a
Lead tetraoxide;
Lead tetroxide; Lead
oxide red; C.I.
Pigment Red 105;
C.I. 77578; Gold
satinobre; Lead
orthoplumbate; Lead
oxide (3:4); Mineral
Orange; Mineral Red;
Paris Red; Saturn
Red; Minium;
Plumboplumbic
oxide; Red Lead;
Red Lead oxide;
Trilead tetraoxide
d,e
Chemical formula
No data
Pb(NO
3
)
2
b
PbO
a
Pb
3
O
4
e
Chemical structure
Not applicable
Not applicable
Not applicable
CAS Registry
Number
12709-98-7
a
10099-74-8
b
1317-36-8
a
1314-41-6
d
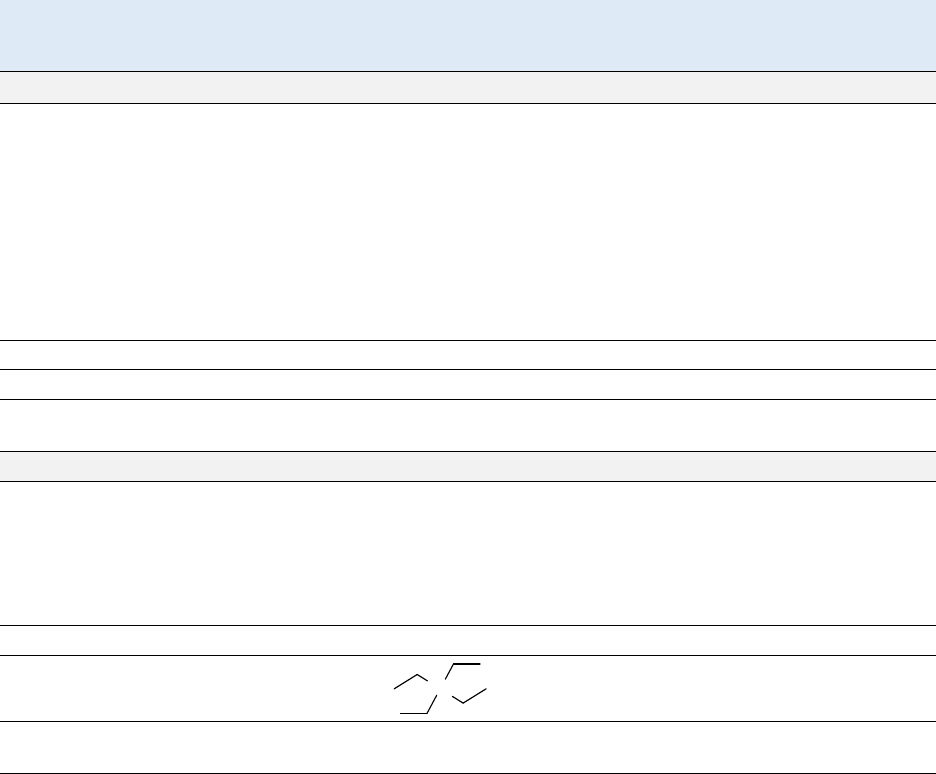
Pb
O
Pb
O
O
Pb
O
Pb
O
O
Pb
O
O
Pb
LEAD 348
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Lead and Compounds
Characteristic
Lead(II) phosphate
Lead(II) styphnate
Lead(II) sulfate
Synonym(s) and
registered trade
name(s)
C.I. 77622; Lead
orthophosphate; Lead
phosphate (3:2);
Lead(2+) phosphate;
normal lead
orthophosphate;
Phosphoric acid,
lead(2+) salt (2:3);
Plumbous phosphate;
Trilead phosphate
a
Lead trinitroresorcinate
f
Anglesite; C.I. 77630;
C.I. Pigment White 3;
Fast White;
Freemans White
Lead; Lead bottoms;
Milk white; Mulhouse
White; Sulfuric acid,
lead(2+) salt (1:1)
a
Chemical formula
Pb
3
(PO
4
)
2
a
Pb(C
6
HN
3
O
8
)
2
f
PbSO
4
b
Chemical structure
Not applicable
Not applicable
Not applicable
CAS Registry
Number
7446-27-7
a
15245-44-0
f
7446-14-2
b
Characteristic
Lead(II) sulfide
Tetraethyl lead
Lead(II) carbonate
Synonym(s) and
registered trade
name(s)
C.I. 77640; Galena;
Natural lead sulfide;
Plumbous sulfide
a
Tetraethylplumbane; Lead tetraethyl;
TEL
b
Carbonic acid,
lead(2+) salt (1:1);
Cerussite; Dibasic
lead carbonate;
Lead(2+) carbonate;
White lead
a
Chemical formula
PbS
a
Pb(C
2
H
5
)
4
a
PbCO
3
a
Chemical structure
Not applicable
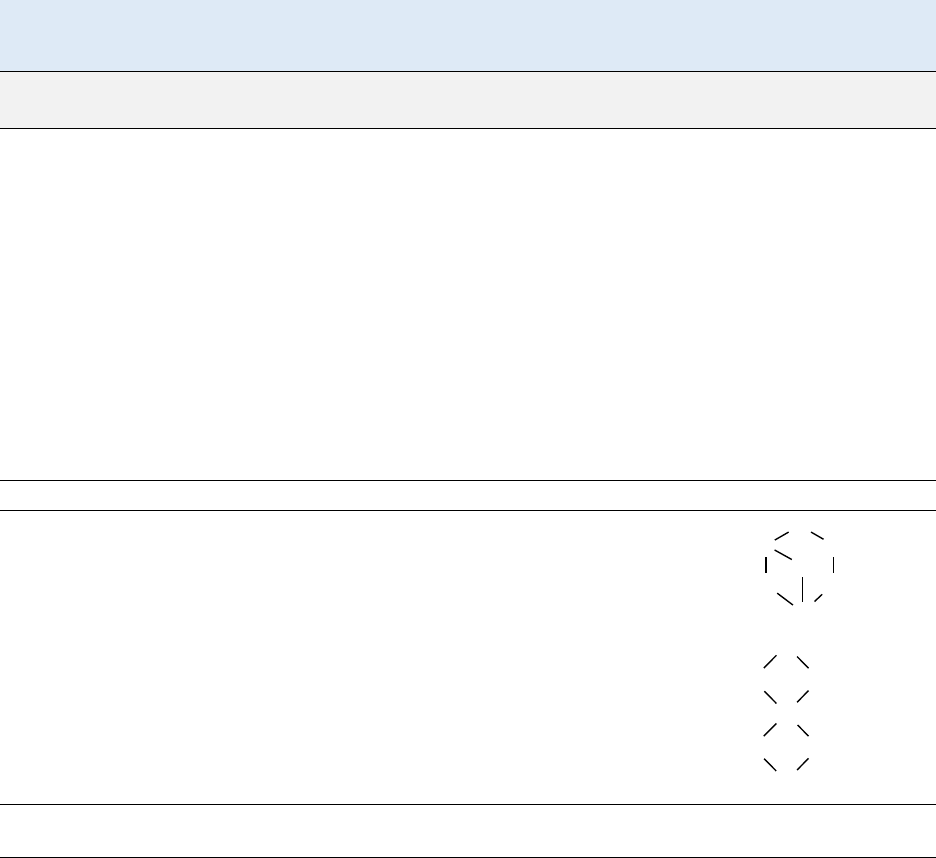
P
b
Not applicable
CAS Registry
Number
1314-87-0
a
78-00-2
b
598-63-0
a
a
Lewis 2012.
b
O’Neil et al. 2013.
c
Stable only in aqueous solution (Haynes 2014).
d
NLM 2020.
e
Haynes 2014.
f
Boileau et al. 2012.
CAS = Chemical Abstracts Services
4.2 PHYSICAL AND CHEMICAL PROPERTIES
Pb, a blueish-white metal with bright luster, is very soft, highly malleable, ductile, a poor conductor of
electricity, and is very resistant to corrosion (Haynes 2014). A clean Pb surface will not be attacked by
dry air; however, in moist air, the surface will react and become coated with a layer of lead(II) oxide
(PbO). This coating may be hydrated and combine with carbon dioxide to form lead(II) carbonate
(PbCO
3
) (Carr et al. 2004). This protective coating of insoluble Pb compounds slows or halts corrosion
of the underlying metal. Pb is rarely found in its metallic form in nature and commonly occurs as a
LEAD 349
4. CHEMICAL AND PHYSICAL INFORMATION
mineral with sulfur or oxygen. The most important Pb mineral is galena (PbS). Other common
Pb-containing minerals include anglesite (PbSO
4
), cerussite (PbCO
3
), and minium (Pb
3
O
4
) (Carr et al.
2004; Davidson et al. 2014; Haynes 2014).
Pb can exist in the 0 oxidation state in metallic Pb and in compounds as the +2 or +4 oxidation states. In
the environment, Pb is primarily found in the +2 state in inorganic compounds. The chemistry of
inorganic Pb compounds is generally similar to that of the Group 2(II) or alkaline earth metals. There are
three common oxides of Pb: lead(II) oxide (PbO); lead(II,IV) oxide or lead tetroxide (Pb
3
O
4
); and
lead(IV) oxide or lead dioxide (PbO
2
). The +4 state is only formed under strongly oxidizing conditions.
Inorganic Pb(+4) compounds are relatively unstable and would not be expected to be found under
ordinary environmental conditions. Pb is amphoteric, meaning that it can react with acids and bases. In
acid, Pb forms Pb(+2) (plumbous) and Pb(+4) (plumbic) salts and in basic solution, it forms plumbites
(PbO
2
2-
) and plumbates (Pb(OH)
6
2-
) (Carr et al. 2004). In organolead compounds, Pb is typically in the
tetravalent (+4) oxidation state (Carr et al. 2004; Haynes 2014).
Data on the physical and chemical properties of Pb and several of its compounds are provided in
Table 4-2.
LEAD 350
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Lead and Compounds
Property
Lead
Lead(II) acetate
Lead(II) azide
Lead(II) bromide
Molecular weight
207.2
a
325.3
b
291.24
a
367.0
b
Color
Bluish-white,
silvery, gray metal
a
White crystals
b
Needles or white
powder
a
White orthorhombic
crystals
b
Physical state
Solid
Solid
Solid
Solid
Melting point
327.4°C
a
280°C
b
Decomposes at
190°C
c
371°C
b
Boiling point
1,740°C
a
Decomposes
b
No data
892°C
b
Density
11.34 g/cm
3
at
20°C
a
3.25 g/cm
3b
4.17 g/cm
3
at
20°C
c
6.69 g/cm
3b
Odor
No data
Slightly acetic odor
(trihydrate)
a
No data
No data
Odor threshold:
Water
No data
No data
No data
No data
Air
No data
No data
No data
No data
Solubility:
Water
Insoluble
d
443,000 mg/L at
20°C
b
230 mg/L at 18°C
a
9,750 mg/L at 25°C
b
Acids
Soluble in dilute
nitric acid
d
; reacts
with sulfuric acid
a
Soluble in acid
e
Freely soluble in
acetic acid
a
No data
Bases
No data
Soluble in alkali
e
No data
No data
Organic solvents
Soluble in glycerin;
slightly soluble in
alcohol
e
Slightly soluble in
alcohol; freely
soluble in glycerol
d
No data
Insoluble in alcohol
b
Partition coefficients:
Log K
ow
No data
No data
No data
No data
Log K
oc
No data
No data
No data
No data
Vapor pressure
1.77 mmHg at
1,000°C
a
No data
No data
0.0075 mmHg at 374°C
b
Henry’s law
constant
No data
No data
No data
No data
Autoignition
temperature
No data
No data
No data
No data
Flashpoint
No data
No data
No data
No data
Flammability limits
No data
No data
No data
No data
Conversion factors
Not relevant
f
Not relevant
f
Not relevant
f
Not relevant
f
Explosive limits
No data
No data
Explodes at
350°C
a
No data
Valence state
0
+2
+2
+2
LEAD 351
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Lead and Compounds
Property
Lead(II) chloride
Lead(II) chromate
Lead(II)
tetrafluoroborate
Lead iodide
Molecular weight
278.1
g
323.19
a
380.8
b
461.05
g
Color
White, orthorhombic
needles
g
Yellow or orange-
yellow powder
a
No data
Yellow
hexagonal
crystals
g
Physical state
Solid
Solid
Stable only in
aqueous solution
b
Solid
Melting point
501°C
g
844°C
a
No data
402°C
g
Boiling point
950°C
g
No data
No data
954°C
g
Density
5.85 g/cm
3g
6.12 g/cm
3b
No data
6.16 g/cm
3g
Odor
No data
No data
No data
No data
Odor threshold
No data
No data
No data
No data
Solubility:
Water
9,900 mg/L at 20°C
g
0.2 mg/L
a
Soluble
b
630 mg/L at
20°C
g
Acids
Slightly soluble in
dilute hydrochloric
acid
g
Soluble in dilute
nitric acid; insoluble
in acetic acid
a
No data
No data
Bases
Slightly soluble in
dilute ammonia
g
No data
No data
No data
Organic solvents
Insoluble in alcohol
g
No data
No data
Insoluble in
alcohol
g
Partition coefficients:
Log K
ow
No data
No data
No data
No data
Log K
oc
No data
No data
No data
No data
Vapor pressure
7.5 mmHg at 637°C
b
No data
No data
0.75 mmHg at
470°C
b
Henry’s law
constant
No data
No data
No data
No data
Autoignition
temperature
No data
No data
No data
No data
Flashpoint
No data
No data
No data
No data
Flammability limits
No data
No data
No data
No data
Conversion factors
Not relevant
f
Not relevant
f
Not relevant
f
Not relevant
f
Explosive limits
No data
No data
No data
No data
Valence state
+2
+2
+2
+2

LEAD 352
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Lead and Compounds
Property
Lead molybdenum
chromate
Lead(II) nitrate
Lead(II) oxide
Lead(II,II,IV) oxide
Molecular weight
No data
331.23
g
223.21
g
685.57
e
Color
No data
Cubic or monoclinic
colorless crystals
g
Reddish-yellow;
yellow (above
489°C)
g
Bright red heavy
powder
a
; red
tetrahedral crystals
b
Physical state
No data
Solid
Solid
Solid
Melting point
No data
Begins to
decompose above
205°C
g
897°C (begins to
sublime before
melting)
g
830°C
b
; 500°C
e
Boiling point
No data
No data
Decomposes at
1,472°C
g
Decomposes between
500-530°C
d
Density
No data
4.53 g/cm
3g
9.53 g/cm
3
(Litharge)
g
;
9.6
g/cm
3
(Massicot)
g
8.92 g/cm
3b
;
9.1 g/cm
3e
Odor
No data
No data
No data
No data
Odor threshold:
No data
No data
No data
No data
Solubility:
Water
No data
56:5 g/100 mL at
20°C
g
50.4 mg/L at 25°C
(Litharge)
g
;
106.5 mg/L at
25°C (Massicot)
g
Insoluble in water
d
Acid
No data
Insoluble in
concentrated nitric
acid
a
Soluble
g
Dissolves in acetic
acid or hot
hydrochloric acid
b,g
Base
No data
Soluble in alkali and
ammonia
g
Soluble
g
No data
Organic solvents
No data
87.7 mg/L (43%
aqueous ethanol) at
22°C
g
Insoluble in
alcohol
a
Insoluble in alcohol
g
Partition coefficients:
Log K
ow
No data
No data
No data
No data
Log K
oc
No data
No data
No data
No data
Vapor pressure
No data
No data
0.0075 mmHg at
724°C
b
No data
Henry’s law
constant
No data
No data
No data
No data
Autoignition
temperature
No data
No data
No data
No data
Flashpoint
No data
No data
No data
No data
Flammability limits
No data
No data
No data
No data
Conversion factors
Not relevant
f
Not relevant
f
Not relevant
f
Not relevant
f
Explosive limits
No data
No data
No data
No data
Valence state
+2
+2
+2
+2, +2, +4
LEAD 353
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Lead and Compounds
Property
Lead(II) phosphate
Lead(II) styphnate
Lead(II) sulfate
Molecular weight
811.54
a
450.29
h
303.25
g
Color
White powder
a
Monoclinic orange-yellow
crystal (monohydrate)
b
White, heavy, crystalline
powder
a
Physical state
Solid
Solid
Solid
Melting point
1,014°C
a
No data
1,170°C
g
Boiling point
No data
No data
No data
Density
6.9 g/cm
3a
3.1 g/cm
3
(monohydrate);
2.9 g/cm
3
(anhydrous)
b
6.2 g/cm
3g
Odor
No data
No data
No data
Odor threshold:
No data
No data
No data
Solubility:
Water
Insoluble
b
Insoluble
b
42.5 mg/L at 25°C
g
Acid
Soluble in nitric acid
a
No data
Soluble in concentrated acids
g
Base
Soluble in fixed alkali
hydroxides
a
No data
Soluble in alkalies
g
Organic solvents
Insoluble in alcohol
a
No data
Insoluble in alcohol
a
Partition coefficients:
Log K
ow
No data
No data
No data
Log K
oc
No data
No data
No data
Vapor pressure
No data
No data
No data
Henry’s law
constant
No data
No data
No data
Autoignition
temperature
No data
No data
No data
Flashpoint
No data
No data
No data
Flammability limits
No data
No data
No data
Conversion factors
Not relevant
f
Not relevant
f
Not relevant
f
Explosive limits
No data
Detonates at 260°C
b
No data
Valence state
+2
+2
+2
LEAD 354
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Lead and Compounds
Property
Lead(II) sulfide
Tetraethyl lead
Lead(II) carbonate
Molecular weight
239.25
g
323.45
a
267.22
g
Color
Metallic black cubic
crystals
g
Colorless
a
Colorless rhombic crystals
g
Physical state
Solid
Liquid
a
Solid
Melting point
1,114°C
d
No data
315°C (decomposes)
g
Boiling point
Sublimes at 1,281°C
d
200 °C; 227.7°C (with
decomposition)
a
No data
Density
7.57–7.59 g/cm
3g
1.653 g/cm
3a
6.6 g/cm
3g
Odor
No data
No data
No data
Odor threshold:
No data
No data
No data
Solubility:
Water
124.4 mg/L 20°C
g
0.29 mg/L
i
1.1 mg/L at 20°C
g
Acid
Soluble in nitric acid
g
No data
Soluble
g
Base
Insoluble in alkalies
d
No data
Soluble in alkalies; insoluble in
ammonia
g
Organic solvents
Insoluble in alcohol
a
Soluble in benzene,
petroleum ether, gasoline;
slightly soluble in alcohol
a
Insoluble in alcohol
g
Partition coefficients:
Log K
ow
No data
4.15
j
No data
Log K
oc
No data
No data
No data
Vapor pressure
0.0075 mmHg at
705°C
b
0.26 mmHg at 25°C
j
No data
Henry’s law
constant
No data
No data
No data
Autoignition
temperature
No data
No data
No data
Flashpoint
No data
200°F (93°C) (closed cup)
k
No data
Flammability limits
No data
Lower flammable limit:
1.8% by volume
k
No data
Conversion factors
Not relevant
f
No data
Not relevant
f
Explosive limits
No data
No data
No data
Valence state
+2
+4
+2
a
O’Neil et al. 2013.
b
Haynes 2014.
c
Akhavan 2004.
d
Larrañaga et al. 2016.
e
Jacob 2012.
f
Since these compounds exist in the atmosphere in the particulate state, their concentrations are expressed as
μg/m
3
only.
g
Carr et al. 2004.
h
Molecular weight calculated from atomic weights.
i
Feldhake and Stevens 1963.
j
Wang et al. 1996.
k
NFPA 2002.
