
The
American
Journal
of
Public
Health
to
show
what
could
be
done
by
paying
cash
instead
of
buying
on
the
instalment
plan.
In
every
room
the
board
of
health
regulations
which
could
any
way
apply
to
that
house
were
posted,
and
we
felt
that
the
neighbors
learned
a
great
deal
from
this,
especially
since
we
always
had
some
one
at
home
there
in
the
afternoon
to
talk
over
with
them
the,application
and
relation
of
our
problems
and
successes
with
our
landlord
to
their
own.
The
tenants
upstairs
became
interested,
and
the
people
soon
asked
if
they
might
use
the
rooms
for
meetings,
which
of
course
gave
the
tenement
a
larger
influence
at
once.
The
whole
house
took
on
a
different
aspect,
and
one
tenant
moved
because
she
said
she
"could
not
keep
up
to
the
pace."
This
was
considered
a
real
fall
on
her
part
by
the
people
in
her
neighbor-
hood,
and
she
has
never
really
been
reinstated
socially
because
of
it.
The
landlord
has
acquired
two
other
houses,
and
remodeled
them,
and
he
frankly
admits
tiat
he
has
been
influenced
in
his
ideas
by
what
he
learned
from
having
us
as
tenants.
The
tenement
was
not
"given
up"
in
one
sense,
but
after
three
years
was
moved
on
to
another
part
of
the
city.
We
felt
that
it
had
taught
a
real
lesson
here.
THE
SPECIFICITY
OF
DISINFECTANTS
AND
ITS
BEAR-
ING
ON
THEIR
STANDARDIZATION.
A.
L.
WALTERS,
Department
of
Experimental
Medicine,
Eli
Lilly
&
Company,
Indianapolis,
Ind.
Read
before
the
Laboratory
Section,
American
Public
Health
Association,
October
20,
1917,
at
Washington,
D.
C.
T
HE
standardization
of
disin-
fectants
has
been
an
annual
subject
of
discussion
before
health
congresses
and
societies
since
the
advocation
of
the
"drop
method"
proposed
in
1903
byRideal
and
Walker.
During
these
years
many
refinements,
changes
and
additions
have
been
made
to
this
method,
the
latest
being
the
control
of
the
acidity
of
the
media
by
determining
the
H-ion
concen-
tration.
There
is
no
doubt
that
the
method
has
been
improved
but
the
question
arises
has
not
the
broad
pur-
pose
of
the
test
been
overlooked
in
searching
out
the
details.
There
was
a
time
when
with
limited
bacteriological
knowledge,
it
was
reasonable
to
suppose
that
all
dis-
infectants
would
bear
the
same
germi-
cidal
relation
to
phenol,
no
matter
on
what
organism
tested.
This
time
has
now
passed
and
when
a
label
bears
the
statement
that
a
certain
preparation
has
a
phenol
coefficient
of
2,
it
cannot
be
taken
literally,
but
must
be
inter-
preted
in
the
light
of
the
method
used
in
determining
this
coefficient.
If
this
method
is
that
of
the
hygienic
laboratory,
it
means
that
this
prepara-
tion
under
the
conditions
of
that
test
is
twice
as
germicidal
as
phenol
on
the
bacillus
typhosus,
or
a
particular
strain
thereof.
No
conclusions
should
be
drawn
as
to
its
germi-cidal
action
on
the
staphylococcus,
streptococcus
or
any
other
organism,
not
excepting
the
bacillus
coli,
or
paratyphosus.
Nat-
urally
the
closer
the
chemical
relation
between
the
disinfectant
and
phenol,
and
the
closer
the
bacteriological
re-
lation
of
the
organisms
concerned
in
1030

Specificity
of
Disinfectants
the
comparison,
the
more
nearly
cor-
rect
will
be
any
conclusion
drawn
from
such
a
test.
On
the
other
hand,
it
is
.only
rational
to
suppose
that
disin-
fecting
agents
of
widely
different
char-
acter
will
vary
greatly
in
their
germi-
cidal
relation
to
phenol
when
tested
on
organisms
of
different
genera
or
-species.
Since
the
advent
of
synthetic
dyes
in
preparing
differential
culture
media
and
later
the
production
of
*diarseno-benzol,
the
specificity
of
drugs
for
certain
microorganisms
should
not
be
lost
sight
of.
Chick
and
Martin
(1)
recognize
this
speci-
ficity
of
disinfectants
when
they
say,
"Some
disinfectants
are
more
efficient
-against
a
particular
organism
than
against
any
other."
Churchman's
(2)
work
-was
quite
definite
in
showing
the
selective
affinity
of
gentian
violet
for
certain
organisms.
In
the
main
he
-could
divide
bacteria
into
two
classes,
those
which
were
inhibited
in
their
growth
by
very
weak
dilutions
of
this
dye
and
those
which
were
unaffected
by
even
much
stronger
solutions.
As
a
rule
closely
related
bacteria
reacted
similarly
to
gentian
violet
but
he
found
a
marked
exception
to
this
in
one
strain
of
B.
enteritidis.
This
strain
was
indistinguishable
by
ordi-
nary
cultural
and
staining
methods
from
four
other
strains
of
B.
enteritidis,
yet
it
was
completely
inhibited
by
weak
dilutions
(1:80,000)
of
gentian
violet,
whereas
the
other
four
were
not.
Churchman
states
that
this
instance
is
significant
because
it
establishes
the
fact
that
chemical
substances
may
be
so
specific
in
their
selective
affinity
for
microorganisms
as
to
distinguish
among
strains
of
bacteria
otherwise
indistinguishable;
and
further
that
it
indicates
to
what
extent
our
ideas
of
bactericides
must
be
modified
by
the
conception
of
chemical
affinity,
and
that
the
fact
that
a
given
bactericide
kills
a
given
organism
does
not
justify
the
conclusion
that
it
will
kill
all
closely
related
organisms,
or
even
all
strains
of
the
same
organism.
Browning
and
Gilmour
(3)
examined
a
number
of
organic
and
inorganic
compounds
for
their
bactericidal
ac-
tlon
toward
different
species
of
bac-
teria,
with
a
view
to
discovering-
(a)
substances
possessing
specific
bacte-
ricidal
properties
for
particular
organ-
isms
and-(b)
relationships
between
chemical
constitution
and
bactericidal
action.
They
found
staphylococcus
aureus
and
B.
anthracis,
organisms
more
resistant
to
phenol
than
is
B.
typhosus,
less
resistant
than
the
coli-
typhoid
group
of
organisms
to
the
action
of
certain
basic
benzol
deriva-
tives
among
which
may
be
mentioned
fuchsin,
hexamethyl
violet,
methyl-
green,
malachite-green
and
brilliant
green.
The
amount
of
hexamethyl
violet
required
to
inhibit
B.
typhosus
was
150
times
that
required
for
staphylococcus
aureus.
Furthermore
these
authors
state
that
whereas
the
action
of
malachite
green
shows
no
marked
difference
between
its
effect
on
B.
typhosus
and
B.
coli,
brilliant-
green
exerts
a
more
marked
bacteri-
cidal
action
on
B.
coli
than
on
B.
typhosus.
In
other
words,
the
bac-
tericidal
action
of
a
disinfectant
on
one
organism
is
not
necessarily
a
measure
of
its
action
on
another,
even
when
these
organisms
belong
to
the
same
genus
or
closely
related
group.
1031
1032
The
American
Jour
Another
very
interesting
feature
of
Browning
and
Gilmour's
work
is
that
they
found
the
presence
of
serum
to
increase
the
antiseptic
action
of
flavine
for
both
staphylococcus
and
B.
ty-
phosus.
This
is
certainly
contrary
to
the
action
of
serum
on
the
effect
of
any
other
class
of
disinfectants
and
should
be
taken
into
consideration
when
giving
them
a
valuation
or
phenol
coefficient.
Flavine
is
an
example
of
a
valuable
disinfectant
which
if
gauged
by
its
phenol-coefficient
would
be
practically-
worthless.
Pine
oil,
on
the
contrary,
is
an
example
of
one
which,
based
on
its
phenol
coefficient,
should
be
of
considerable
importance,
but
which
in
practice
is
deficient
in
some
re-
spects
as
a
general
disinfectant.
This
deficiency
was
brought
to
my
atten-
tion
after
the
issuance
of
the
hygienic
laboratory's
report
(4)
on
pine
oil
as
-nal
of
Public
Health
an
efficient
liquid
disinfectant
when
I
was
asked
by
a
dentist
how
long
a
time
would
be
required
to
sterilize
instru-
ments
in
this
Pine
Oil
Disinfectant.
The
Pine
Oil
Disinfectant
was
made
by
incorporating
62.5
per
cent.
of
pine
oil
in
soap
according
to
the
di-
rections
for
making
the
"Hygienic
Laboratory
Pine
Oil
Disinfectant."
The
phenol
coefficient
of
this
solution
was
determined
and
found
to
be
3.8.
Several
different
samples
of
pine
oil
purchased
on
the
open
market
varied
in
their
phenol
coefficient.
This
vari-
ability
of
pine
oil
was
mentioned
by
Hamilton
(5)
before
this
association
last
year.
In
testing
the
ability
of
this
Pine
Oil
Disinfectant
to
sterilize
dental
or
other
instruments,
small
steel
blades
(scarifiers),
i
x
3i
centimeters
were
contaminated
with
various
organisms
and
subjected
to
the
action
of
the
dis-
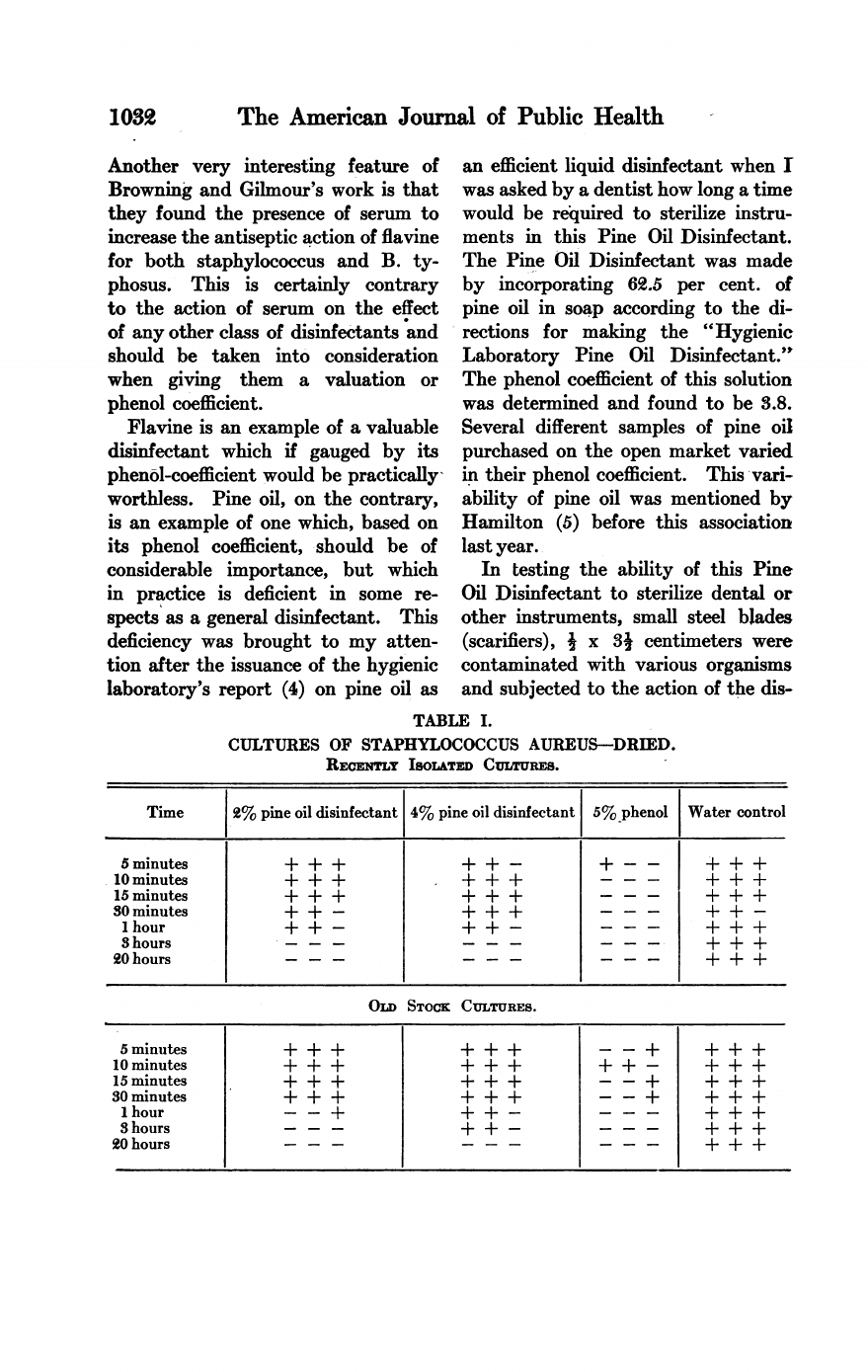
TABLE
I.
CULTURES
OF
STAPHYLOCOCCUS
AUREUS-DRIED.
RECENTLY
ISOLATED
CUTRUEES.
Time
2%
pine
oil
disinfectant
4%
pine
oil
disinfectant
5%
phenol
Water
control
5
minutes
+
+
+
+
+
-
-
+ +
+
lO
minutes
+
+
+
+
+
+
+
+
+
15
minutes
+
+ +
+
+
+
+
+
+
30
minutes
+
+
+
+
+
_
+
+
Ihour
+
+
+ +
+
+ +
8
hours
+
+
+
20
hours
+
+
+
OLD
STOCK
CULTURES.
5minutes
+ +
+
+
+
+
+
+
+
+
10
minutes
+
+
+
+
+
+
+
+
+
+
+
15
minutes
+
+
+
+
+
+
+ +
+
+
30
minutes
+
+ + +
+ +
+
+
+
+
I
hour
_ _
+
+
+
+ +
+
S
hours
+
+
+
+
+
20
hours
+
+
+
Specificity
of
DisinfectaIts
infectant,
using
phenol
as
a
control,
in
the
following
manner:
EXPERIMENT
I.
Twenty-four
hour
broth
cultures
of
recently
isolated
and
old
stock
cultures
of
staphylococcus
aureus
were
each
used
to
inoculate
84
metal
scarifiers.
Both
lots
were
placed
in
sterile
petri-
dishes
to
dry
over
night.
With
sterile
distilled
water,
2
per
cent.
and
4
per
cent.
emulsions
of
Pine
Oil
Disinfectant
and
5
per
cent.
solution
of
phenol
were
made.
Sterile
distilled
water
was
used
as
control.
Five
per
cent.
phenol
was
taken
be-
cause
it
is
the
strength
commonly
used
in
sterilizing
instruments.
The
scarifiers
were
placed
in
the
above
solutions
and
also
in
sterile
water
as
a
control,
21
in
each,
and
al-
lowed
to
remain
for
periods
of
5-10-
15-30
minutes,
1
hour,
3
hours,
and
over
night.
Those
placed
in
the
Pine
Oil
Disinfectant
were
removed,
8
at
the
end
-of
each
period
of
time,
washed
in
sterile
water
and
placed
in
fermenta-
tion
tubes
of
broth.
Those
in
5
per
cent.
phenol
were
re-
moved
and
washed
in
95
per
cent.
alcohol
before
placing
in
fermentation
tubes.
Those
in
sterile
water
were
placed
directly
in
fermentation
tubes
without
washing.
After
24
hours'
incubation,
trans-
plants
were
made
from
the
fermenta-
tion
tubes
to
agar
slants.
The
results
are
given
in
Table
I
in
which
the
plus
sign
indicates
growth
of
the
organ-
isms.
The
three
columns
following
each
interval
of
time
represent
the
three
scarifiers
used
in
each
solution.
By
the
hygienic
laboratory
method
of
testing
disinfectants,
Pine
Oil
Dis-
infectant
would
be
classed
as
having
4
times
the
germicidal
power
of
phenol.
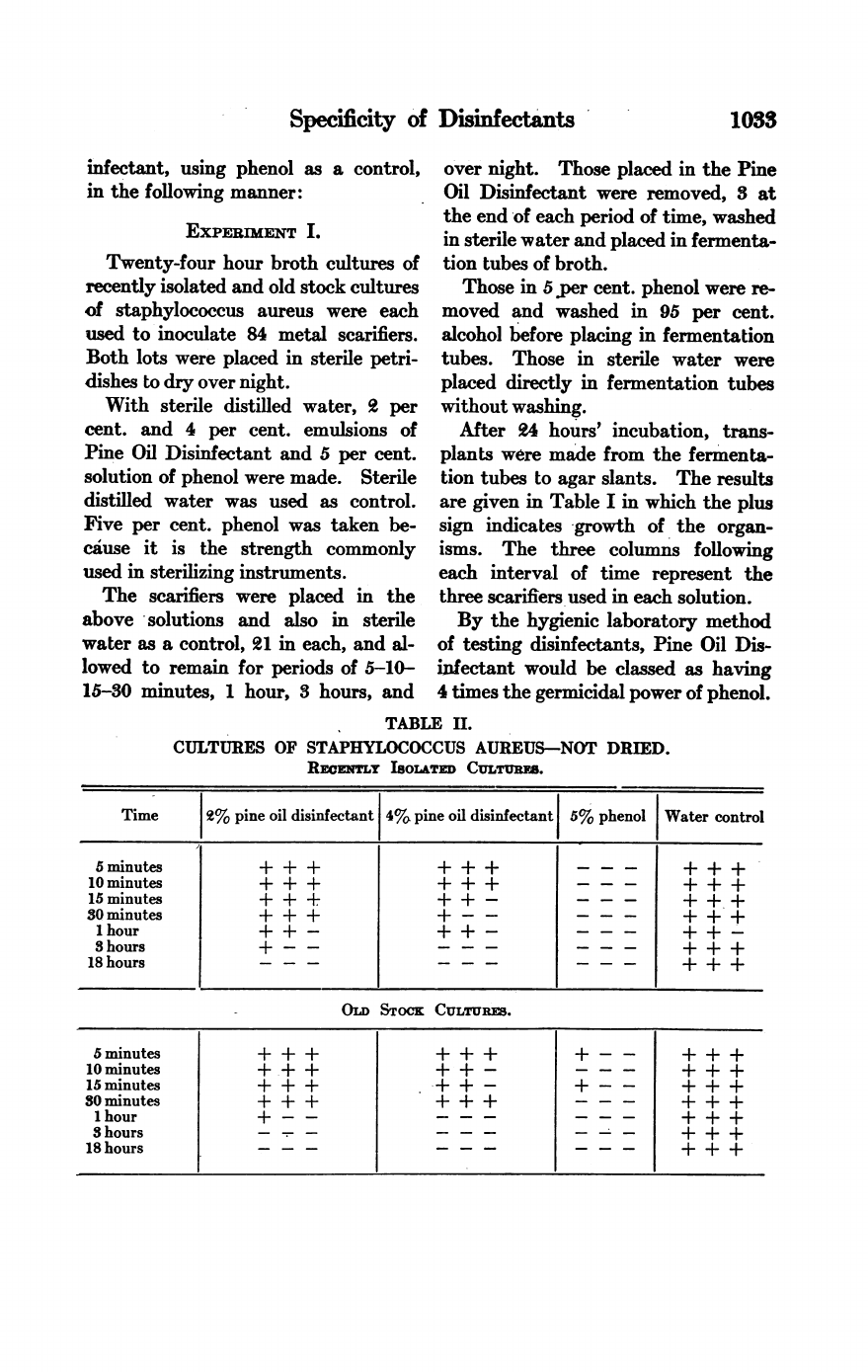
TABLE
II.
CULTURES
OF
STAPHYLOCOCCUS
AUREUS-NOT
DRIED.
REemmY
ISOLATED
CuLTURS.
Time
2%
pine
oil
disinfectant
4%
pine
oil
disinfectant
5%
phenol
Water
control
5
minutes
+
+
+
+ +
+
+
+
+
10
minutes
+
+
+
+ +
+
+
+
+
15
minutes
+
+
+
+
+
+
+
+
30
minutes
+
+
+
+
+
+
+
Ihour
+
+
+
+
+
8
hours
+
+
+
18
hours
+
+
+
OLD
STOCE
CULTURES.
5
minutes
+
+
+
+
_
+
+
+
10
minutes
+
+
+
+
+
+
+
+
15
minutes
+
+
+
+
+
+ + +
80
minutes
+
+
+
+
+
+
+
+
I
hour
+
+ +
8
hours
+
+
+
18
hours
1033

The
Amenrcan
Journal
of
Public
Health
From
the
above
experiment
in
which
staphylococci
were
used
as
test
organ-
isms,
Pine
Oil
Disinfectant,
2
per
cent.
or
even
4
per
cent.
does
not
destroy
the
organisms
in
one
hour;
whereas
they
are
usually
killed
by
5
per
cent.
phenol
within
15
minutes.
Another
striking
point
brought
out
in
Table
I
is
that
Pine
Oil
Disinfectant,
4
per
cent.,
is
no
more
effective
than
the
2
per
cent.
dilution.
In
order
to
check
these
results
a
second
series
was
carried
out
differing
from
the
former
only
in
that
the
in-
oculated
scarifiers
were
not
dried
be-
fore
placing
them
in
the
disinfectant
solutions
and
that
the
fermentation
tubes
were
incubated
48
hours
before
transplants
were
made
from
them.
These
results
confirm
those
of
Ex-
periment
I,
and
again
show
that
Pine
Oil
Disinfectant
diluted
with
water
to
make
a
4
per
cent.
mixture
or
emulsion
is
little,
if
any,
more
effective
than
the
2
per
cent.
emulsion.
Other
similar
tests
were
made
using
B.
typhosus,
streptococcus
pyogenes,
and
B.
diphtheria
and
showed
that
in
practically
all
instances
the
test
organ-
isms
were
killed
within
15
minutes
by
the
disinfectants
employed;
and
that
of
the
four
bacteria
used
in
the
ex-
periments,
the
staphylococcus
alone
stood
out
as
being
disproportionately
resistant
to
pine
oil
solutions
in
soap.
Pine
oil
cannot,
then,
be
termed
a
general
disinfectant
of
high
germicidal
value,
for
although
showing
to
ad-
vantage
when
tested
on
the
typhoid
bacillus,
it
is
much
less
efficient
than
phenol
or
cresol
when
the
infecting
organism
is
the
staphylococcus
aureus.
This
is
another
instance
of
the
fallacy
of
the
present
generally
accepted
method
of
determining
the
value
of
disinfectants,
namely,
the
so-called
hygienic
laboratory
method.
The-
physician
or
dentist
who
does
not
know
the
intricacies
or
inaccuracies
of
the
present
methods
of
testing
or'
standardizing
disinfectants
is
justified
in
thinking
that
a
phenol
coefficient
of
4
means
that
the
preparation
is:
four
times
as
active
as
phenol,
and.
that
a
1
or
2
per
cent.
solution
of
it,
will
sterilize
his
instruments
within
5
or
10
minutes.
He
is
further
justi-
fied
in
reposing
confidence
in
the'
phenol
coefficient
of
a
disinfectant
because
'the
method
of
determining
this
is
sanctioned
by
the
government
and
by
the
Council
on
Pharmacy
and
Chemistry
of
the
American
Medical
Association.
One
state
requires
that
disinfectants
must
have
the
phenol'
coefficient
on
the
label,
and
before
a
disinfectant
will
be
admitted
to
New
and
Non-Official
Remedies,
this
same
condition
must
be
complied
with.
Defects
of
this
method
have
been
re-
peatedly
pointed
out,
but
have
been
apparently
disregarded
by
its
advo-
cates
and
entirely
overlooked
by
others
interested
in
having
it
made
a
legal
method
for
standardizing
disinfectants.
Hamilton
and
Ohno
(6)
have
shown
that
this
method
in
different
hands
gives
widely
varying
results
when
testing
the
same
disinfectant
on
the
same
strain
of
typhoid
organism.
If
comparable
results
cannot
be
obtained
when
the
same
organism
is
used,
one
would
certainly
expect
a
disinfectant
to
vary
widely
in
germicidal
power
when
tested
on
different
organisms.
In
fact,
Anderson
and
McClintic,
the
1084

Specificity
of
Disinfectants
originators
of
the
Hygienic
Laboratory
Method,
say
(7)
"
Unless
different
observers
use
the
same
species
of
organism,
there
can
be
no
possibility
of
uniformity
in
results.
The
co-
efficient
obtained
with
different
species
may
vary
as
much
as
300
per
cent."
If
this
be
the
case,
what
is
the
prac-
tical
value
of
the
phenol
coefficient
as
determined
by
the
Hygienic
Labora-
tory
Method?
Certainly
only
a
small
proportion
of
any
commercial
dis-
infectant
is
employed
to
destroy
ty-
phoid
bacilli.
In
the
case
of
pine
oil,
we
have
an
agent
considerably
more
germicidal
than
phenolwhen
acting
on
the
typhoid
bacillus,
and
very
much
less
active
then
phenol
on
the
staphylococcus.
This
proves
conclusively
that
the
com-
parative
disinfectant
value
of
pine
oil
and
phenol
cannot
be
determined
by
any
one
test.
Other
defects
in
the
Hygienic
Lab-
oratory
Method
have
been
pointed
out.
Duyser
and
Lewis
(8)
call
at-
tention
to
three
factors
which
cause
unreliable
results.
As
stated
they
are
"
(a)
The
use
of
an
excessive
num-
ber
of
bacteria
depletes
the
disinfecting
solution
before
the
culture
is
rendered
sterile.
(b)
An
unknown
volume
is
withdrawn
for
testing,
in
that
the
volume
of
the
standard
loop
is
not
constant.
(c)
It
is
impossible
to
de-
termine
from
the
broth
tube
inocu-
lated,
how
complete
the
killing
was
at
the
time
the
sample
was
withdrawn."
Duyser
and
Lewis
showed
that
the
so-called
standard
loop,
which
is
sup-
posed
to
transfer
a
constant
amount
of
culture
from
a
tube
or
flask
to
the
disinfectant,
varies
under
the
best
conditions
at
least
30
per
cent.
from
the
average,
and
when
not
very
care-
fully
used
may
vary
as
much
as
80
per
cent.
This
inaccuracy
of
the
standard
loop
was
verified
by
A.
D.
St.
John
(9)
who
was
able
to
obtain
with
the
same
loop
a
variation
of
300
per
cent.
by
slowly
withdrawing
the
loop
edgewise
and
quickly
withdrawing
it
parallel
with
the
surface.
The
lack
of
uniformity
of
different
loops
in
the
hands
of
different
bacteriologists
can
readily
be
appreciated.
So
that,
as
a
matter
of
fact,
the
stating
of
the
phenol
coefficient
on
a
label
may
be
misleading
and,
instead
of
informing
the
public
as
to
the
value
of
any
particular
agent,
may
misinform
and
give
a
false
sense
of
security.
At
the
same
time
it
would
afford
ample
opportunity
for
litigation
and
a
wide
difference
of
opinion
among
experts.
The
value
of
any
method
of
testing
disinfectants
has
been
well
stated
by
Phelps
(10),
"The
results
of
any
dis-
infection
experiments
are
fundamen-
tally
influenced
by
such
conditions
as
temperature,
character
of
the
organ-
ism
employed,
number
of
organisms
in
unit
volume,
and
character
of
the
medium.
In
the
absence
of
complete
data
covering
these
points
results
are
practically
worthless,
at
least
for
purposes
of
comparison.
Even
with
such
data
given,
it
is
still
impossible
owing
to
the
variable
conditions
ob-
taining
in
practice,
to
establish
any
relationship,
or
order
of
excellence,
among
the
various
disinfectants.
At
best
we
can
only
hope
to
establish
such
relationship
under
specified
ex-
perimental
conditions."
He
further
says
"It
seems
at
present
to
be
quite
1085

The
American
Journal
of
Public
Health
necessary
to
determine
the
relative
germicidal
values
of
two
disinfectants
upon
the
actual
kind
of
germ
upon
which
they
are
to
be
employed
and
to
qualify
the
final
comparative
results
accordingly."
In
no
case
has
the
practical
value
of
this
dictum
been
so
clearly
exemplified
as
in
the
present
investigation.
Based
on
the
report
of
Stevenson
from
the
Hygienic
Labora-
tory
entitled
"An
Efficient
Liquid
Disinfectant,"
one
would
be
led
to
think
that
"Pine
Oil
Disinfectant"
is
4
to
6
times
as
effective
a
germicide
as
phenol,
without
any
exceptions.
That
this
is
not
the
case
has
been
clearly
shown
by
the
preceding
experiments
and
contrary
to
the
statement
in
the
above
report,
Pine
Oil
Disinfectant
cannot
be
satisfactorily
used
to
replace
the
ordinary
coal
tar
compounds
com-
monly
employed
as
disinfectants.
There
is
another
phase
of
the
ques-
tion
brought
up
by
these
discrepan-
cies,
namely,
legalizing
the
Hygienic
Laboratory
Method
as
a
standard
method
under
which
prosecutions
may
take
place.
That
this
is
not
desirable
is
readily
understood
when
the
in-
accuracy
of
the
method
is
considered.
Almost
every
factor
concerned
is
variable
and
is
difficult
or
impossible
to
control.
The
temperature,
the
media,
the
number
of
bacteria
used,
the
change
in
resistance
of
the
organ-
isms,
and
the
technique
are
some
of
these
factors
already
mentioned.
These
have
been
well
discussed
in
the
paper
of
J.
T.
Ainslee
Walker
(11).
Aside
from
these
considerations,
the
true
value
of
a
disinfectant
can
only
be
stated
in
terms
of
the
organism
or
organisms
on
which
it
is
to
be
used
in
actual
practice.
To
state
that
Pine
Oil
Disinfectant
is
"an
efficient
liquid
disinfectant"
which
"may
be
used
wherever
the
ordinary
coal
tar
com-
pounds
are
used"
is
certainly
drawing
erroneous
conclusions
from
a
phenol
coefficient
determination.
The
paradoxical
action
of
pine
oil
in
being
markedly
more
germicidal
than
phenol
on
the
typhoid
bacillus
and
decidedly
less
germicidal
on
the
staphylococcus
is
an
interesting
phe-
nomenon
deserving
further
study
and
is
apparently
illustrative
of
specificity
in
disinfectants.
REFERENCES.
1.
CHICK
and
MARTIN,
Jr.
Hygiene,
1908,
8,
654.
2.
CHURCHMAN,
Jr.
Exper.
Med.,
1912,
16,
r221
and
822.
S.
BROWNING
and
GILMOUR,
Jr.
Path.
and
Bact.
1913,18,
144.
4.
STEVENSON,
Public
Health
Reports,
1915,
80,
8004.
5.
HAMILTON,
Am.
Jr.
Public
Health,
1917,
7,282.
6.
OHNO
and
HAMILTON,
Am.
Jr.
Public
Health,
1914,
4,
486.
7.
ANDERSON
and
McCuwTIc,
Jr.
Infectious
Diseases,
1911,
8,
1.
8.
DUYSRR
and
LEwIs,
Jr.
Industrial
and
Engineering
Chem.,
1914,
6,
198.
9.
ST.
JOHN,
Jr.
Industrial
a-nd
Engineering
Chem.,
1914,
6,940.
10.
PHELPS,
Jr.
Infectious
Diseases,
1911,
8,
27.
11.
WALKER,
N.
Y.
Medical
Jr.,
1916,
10s,
500.
1036
