Kevin O’Malley House, Earlsfort Centre, Earlsfort Terrace, Dublin 2, Ireland
T: +353 1 676 4971 E: [email protected] www.hpra.ie
January 2021 – April 2021
HPRA MEDICINAL PRODUCTS
ISSUE
68
NEWSLETTER
Human Medicines
• ApprovalDatesforPackageLeaets
• Nitrosamine Impurities
• Article 31 Referral for Sartan Medicines – Reminder
• Preparation of Labelling for Joint IE/UK (NI) Packs
• AuditsofTypeIANotications:2019/2020Review
• Reference Medicinal Products – Updated CMDh
Brexit Guidance
• Clinical Trial Regulation Progress
Veterinary Medicines
• HPRA Appearance before Oireachtas Committee on
Agriculture and the Marine
• HPRAVeterinaryMedicinesInformationDay2021
• Submission of Variations to Change MAH’s Local
Representative for MR Products
• Veterinary Medicinal Products and Labelling-related
Revisions
• Update on Implementation of HPRA Report on
the Method of Supply of Antiparasitic Veterinary
Medicinal Products Intended for use in Food-
producing Animals
• UpdateonImplementationofRegulation2019/6and
NewNationalLegislationtoReplaceSINo786of
2007
• PharmacovigilanceChangesArisingfromtheNew
VeterinaryRegulation(EU)2019/6
• QPPV Data Requirement for the Union Product
Database
• Uploading of Product Data to the EMA’s Union
Product Database
• Change to VPO and VPO-1 Category of Supply
• Revision of National Legislation on Veterinary Clinical
Field Trials
• Update on Irish Language Case
Compliance
• AuthenticityVericationofAuthorisationsand
CerticatesissuedbytheHPRA
• CerticatesofFreeSaleforDevicesApprovedunder
theNewMedicalDevicesRegulation2017/745
• EU And UK Trade and Cooperation Agreement
Summary of Annex on Technical Barriers to Trade
(TBT)2-MedicinalProducts
In this Issue
Human
Medicines
Approval Dates for Package
Leaets
Nitrosamine Impurities
TheapprovaldateforaPackageLeaet(PL)isthedatethatthe
caseimplementingoramendingthePLwasclosedbytheHPRA.
ReplacementofaPLontheHPRAwebsiteonlyoccurswhere
thePLtextismateriallychangedduringacase.Whereonlythe
revisiondatehasbeenupdated,thePLtextisnotreplaced.
‘Call for review’ on nitrosamine impurities –
deadline for submission of responses
InlinewiththeCHMPArticle5(3)ScienticOpinion‘callforreview’
process, marketing authorisation holders of human medicinal
productswhichcontainchemicallysynthesisedorbiologically
derivedactivesubstancesarerequiredtoreviewtheirmedicinal
products to determine the risk for the presence of nitrosamines,
andtocompletetherequiredriskevaluation.Whereany risk for
thepresenceofnitrosaminesisidentied,conrmatorytesting
must be performed, and a suitable control strategy implemented
asrequired.Thestepsrequiredbythe‘callforreview’processand
relevantdeadlinesaresummarisedasfollows:
Step Number Chemical Medicinal
Products Deadline
Biological Medicinal
Products Deadline
Step 1 – Risk Evaluation 31March2021 01July2021
Step2–Conrmatory
Testing
26September2022 01July2023
Step 3 – Submission of
relevant variations
26September2022 01July2023
Note that the deadlines differ
betweenchemicalandbiological
medicinalproducts.
HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue682
The HPRA reminds marketing
authorisation holders (MAHs) that the
deadlinehasnowpassedforsubmission
of step 1 risk evaluations for medicinal
products containing chemically
synthesisedactivesubstance.MAHs
that have not submitted the step 1 risk
evaluations for these medicines must
complywiththeconclusionsofthe
CHMPScienticOpinionandprovide
a response to step 1 as a matter of
priority.MAHsareremindedoftheir
responsibility to ensure the quality and
safetyoftheirmedicines.
MAHs are also reminded of the
approaching timeline for submitting
step 1 risk evaluations for biological
medicinalproducts,whichis1July
2021.
Furtherpracticalandscienticguidance
on the preparation and submission
of these responses is available on the
CMDhwebsiteunderAdvice from
CMDh -> Nitrosamine impurities.
Article 31 Referral
for Sartan Medicines
– Reminder
TheCHMPinitiallyconcludedareview
of a class of medicines called sartans
(angiotensin II receptor antagonists) in
January2019.Thisreviewexamined
the potential presence of certain
nitrosamine impurities in sartan-
containingmedicinalproducts.In
October2020,theCHMPconcluded
that the outcome of the Article 31
referral on angiotensin II receptor
antagonists (sartans) containing a
tetrazolegroup(EMEA/H/A-31/1471)
shouldbealignedwiththeoutcome
oftheArticle5(3)assessmenton
nitrosamines(EMEA/H/A-5(3)/1490)–
seeDecision(2021)1309of19February
2021.Themainchangeconcernsthe
limitsfornitrosamines,whichpreviously
applied to the active ingredients but
nowapplyinsteadtothenished
products.Marketingauthorisation
holders (MAHs) of sartan medicines
(candesartan, irbesartan, losartan,
olmesartan, valsartan) are advised
to refer to the relevant European
Commission implementing decision
for the revised list of conditions to the
marketing authorisations that must be
compliedwith.
Further guidance on the practicalities
of submitting responses is available on
theCMDhwebsiteunder‘Advice from
CMDh -> Q&A on the implementation
oftheoutcomeoftheArt.31referral
on angiotensin-II-receptor antagonists
(sartans) containing a tetrazole group’.
Inparticular,Question4highlights
the condition categories, implications
andduedatesofthenewCommission
Decision.TheMAHmustensure
that the manufacturing processes
of the active substances used for
theirnishedproductsarereviewed
for the potential risk of formation
of n-nitrosamines and changed as
necessary to minimise nitrosamine
contamination as much as possible, in
linewiththerecommendationsadopted
byCHMPon25June2020(article5(3)
procedure).Theduedateforthisaction
was17April2021.By30June2021,
theMAHmustintroducespecications
forNDMAandNDEAintothenished
productspecication,asappropriate.
Question7outlineswhichvariations
must be submitted to lift the conditions
on the risk assessment and control
strategy for the active substance and
nishedproduct.MAHsformedicinal
products are required to familiarise
themselveswiththerequirements,
ensure compliance and submit the
appropriate variations by the required
timelines.
Whensubmittingthesevariations,
please clearly indicate in the cover
letterand‘scopeandbackground’
section of the variation application form
that the variation is being submitted
to lift the conditions on the MA of
tetrazolesartansandstatewhich
conditionitrelatesto(A,B,C,D).
Preparation of
Labelling for Joint
IE/UK (NI) Packs
The HPRA has received a number of
queries on the approach that marketing
authorisation holders (MAHs) should
takewhenpreparingjointIE/UK(NI)
packsformedicinalproducts.Wewould
liketohighlighttheexibilityalready
available in the HPRA Guide to Labels
andLeaets.Section10.3oftheguide
details some minor amendments to
thelabellingandpackagingleaetthat
donotrequirepriornoticationtothe
HPRA, as long as there are no changes
to the livery layout, design or font size
result.Theseinclude:
– Addition, deletion or change in
foreign MA numbers and/or foreign
MAholderdetails,whichdoesnot
affect any other aspects of the livery
layoutandfontsize.
– Addition, deletion or change in the
administrative information for an EU
MSortheUKplacedwithinthe‘blue
box’,whichdoesnotaffectanyother
aspects of the livery layout and font
size.
– Change to the details for reporting of
side effects in other Member States
not affecting the details for reporting
ofsideeffectsinIreland(jointcountry
packageleaets).
– Change/deletion of a product name
in another Member State in section
6ofthepatientinformationleaet
(MRPproductsonly).
– Change in the details of a distributor/
wholesaler/localrepresentative.
A designated blue box is not required
for the inclusion of such information for
nationalorMR/DCPauthorisations.
Applicants should carefully evaluate
whetherthisexibilitycouldapply
totheirproposedchanges.Insuch
instances, the revised labels or patient
leaetshouldbesubmittedtothe
HPRA at the next regulatory activity
involving a change in the product
information.However,wherechanges
to livery layout design or font size
result,anArticle61.3noticationshould
besubmittedinadvanceasusual.
For MR/DCP procedures, no
divergences of the PL, SmPC or of the
labelling requirements from those in
theEUcommontemplatewouldbe
envisaged, and the name must be the
same for IE and UK (NI) to avoid any
confusion.
Applicantswhowishtodiscussthis
possibility further may submit their
query to [email protected].
TheHPRAwouldalsoliketohighlight
the guidance included in the HPRA
GuidetoLabelsandLeaets on the
development of multilingual packaging
(section5.3oftheguide).

Audits of Type
IA Notications:
2019/2020 Review
The HPRA performs regular audits
ofTypeIAnoticationsinorderto
verify that submissions have been
appropriately categorised; the relevant
conditionsfullledandnecessary
documents submitted; and to ensure a
consistent approach in the processing
of these variations both by marketing
authorisationholdersandtheHPRA.
RecentauditsofTypeIAnotications
haveidentiedanumberofdeciencies,
someofwhichrequiredcorrective
actionsfromtheapplicants.Themost
commondecienciesidentiedwere:
–‘ECClassicationGuideline’pagenot
submitted;
– Missing or incomplete
documentation;
– Conditions relevant to the chosen
TypeIAcategorynotfullled;
–TypeIAINnoticationsnotsubmitted
immediately after the implementation
of the change in the quality system of
the marketing authorisation holder;
–IncorrectclassicationofTypeIA
noticationswherethewrongTypeIA
noticationsubcategorywasselected
orwhereachangewasincorrectly
classiedasaTypeIAnotication
whenaTypeIBvariationwasrequired.
As a result, and in order to reduce
the number of incorrect Type IA
submissions,theHPRAwouldliketo
remindapplicantsofthefollowing:
–AllchangeswhichfallundertheType
IAclassicationareoutlinedinthe‘EC
ClassicationGuideline’,accompanied
by a list of conditions and
documentation requirements for each
category.Inorderforthevariationto
beclassiedasaTypeIA,allofthe
conditions outlined in the guideline for
therelevantcategorymustbefullled
and all of the required documentation
shouldbeprovided.Otherwise,the
variation should be submitted as a
Type IB (default category) or a Type
II variation (if the proposed change
mayhaveasignicanteffectonthe
overallquality,safetyorefcacyofthe
nishedproduct).Applicantsshould
carefully consider the list of conditions
of the relevant category to make
certain the variation is appropriately
classiedasTypeIA.Theauditsfor
2019/2020(Q4andQ1)and2020
(Q2andQ3)foundthatanumber
of submissions did not meet the
conditionsofaTypeIAclassication
andtherefore,theseapplicantswere
requested to resubmit the applications
as Type IB variations, for example,
‘unchangedadditionalspecications
forimpurities’,‘minorchangeinthe
manufacturing process of the active
substance’.
–Therelevantpage(s)ofthe‘EC
ClassicationGuideline’including
conrmationofcompliance
withallrelevantconditionsand
documentation requirements must be
submitted for each change applied
forunderagroupedvariation.
Conditionswillonlybeconsideredto
befulllediftheyhavebeenticked
onthesubmittedguidelinepage.
–TypeIAINnoticationsmustbe
submitted immediately after the
implementation of the change in
the quality system of the marketing
authorisation holder and Type IA
noticationsmustbesubmitted
within12monthsofimplementation.
The date of implementation of each
change should be stated in the
applicationform.
– All of the documentation requirements
should be adhered to, for example, the
details of changes should be outlined
in the present and proposed sections
of the application form and amended
relevant section(s) of the dossier, GMP
certicates,QPdeclarations,etc.,as
applicable, to each variation category
shouldbeprovided.Updatedlabelling
and/orleaettextmustbesubmitted
withthevariationwherethechange
results in changes to the label and/or
leaettext.
– Minor issues, for example,
submission of an incorrect or
incomplete application form, are
also communicated to applicants
viaanemailoutliningthedeciency.
Although no corrective action may
berequiredforthesedecienciesat
the time, if applicants do not address
theseissuesandsuchdeciencies
continuetoarise,theHPRAwill
consider requesting that applications
areresubmitted.
Finally,theHPRAwouldliketoremind
applicants that the EMA has published
a ‘Pre-noticationchecklistforType
IA variations’ intended to support the
submission of complete and accurate
TypeIAnotications.Applicantsare
advisedtoreviewthischecklistpriorto
submissionofTypeIAnotications.
Reference
Medicinal Products
– Updated CMDh
Brexit Guidance
A generic or hybrid application, in
accordancewithArticle10ofDirective
2001/83/EC,referstoinformation
that is contained in the dossier of a
reference medicinal product (RefMP)
that is or has been authorised in
theUnion.AstheBrexittransition
periodhasnowended,theCMDhhas
published updated guidance on RefMPs
in the latest version of the Practical
guidance for procedures related to
Brexit for medicinal products for human
useapprovedviaMRP/DCP(Feb2021).
Specically,forMAHsofgeneric
productstheHPRAwishestodrawyour
attention to the responses to Question
34and39oftheguidancedocument.
Forgeneric,hybridandbiosimilarnew
applications, applicants are advised to
carefullyconsidertheadviceinQ34for
theuseoftheUKRefMP,whichvaries
dependingonwhichsubsectionofthe
applicationform(1.4.2or1.4.3)isunder
consideration.Thesuitabilityforuse
dependsonwhentheUKreference
authorisationwasgranted,whenthe
authorisation for the generic/hybrid
willbegranted,andthetimingof
completionofthenalstudyreportfor
pivotalstudies.
GuidanceondealingwithnewDCPs,
and variations that are cross-referring to
UK marketing authorisations for product
information alignment, is addressed in
theresponsetoQ39andalsoincluded
in Section4.1oftheCMDhminutes,
January2021.
Fornewmarketingauthorisation
applications (MAAs) a European
referenceproduct(ERP)shouldnow
be used unless the exceptional case
outlinedinQ34oftheCMDhBrexit
guidancearises.However,forongoing
genericnewapplications,whilethe
UK ERP may be kept as being the
RefMP of the original application, the
applicant should align the product
informationtoasuitableRefMPwithin
the global marketing authorisation
(GMA).Forvariations,whileitisnot
possible to change the RefMP of the
original application, another EEA RefMP
product from the same GMA should
be used for the purposes of product
HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue683
information, or adaptation to a different
EEAproductcouldbeconsidered.
The CMDh minutes provide details of
variationclassicationsandhighlight
the need to keep product information
uptodate.
Pleasenotethatifthenewreference
product (from the same GMA) is not
harmonisedwiththeUKreference
product, a variation needs to be
submitted as a type II variation under
C.I.2.btoaligntheproductinformation
withthisnewreferenceproduct.
In case no other product from the
same GMA is available, adaptation
to a different product, not being the
reference product might be possible by
atypeIIvariationC.I.4.Intheabsence
of another reference product from
thesameGMA,orwheretheMAH
doesnotwishtoadapttoadifferent
product, MAHs are reminded of their
legal obligation to keep their product
informationuptodate.Oftensafety
updates of the product information are
linked to recommendations by PRAC
or CMDh and can be implemented
viatypeIA/IBvariations.Bibliographic
reviewsshouldalsobeperformed,
whererelevant,tosupportanupdate.
Finally, MAHs of generic medicinal
products in such situations are
requested to clearly highlight in the
cover letter for variations the reference
medicinal product used, and further
substantiatetheirchoicee.g.in‘notes
forreviewers’.
The choice of RefMP is the
responsibility of the applicant/MAH,
andHPRAcannotprovidespecic
advice.Generalreferencemedicinal
product related queries can be
submittedto:[email protected].
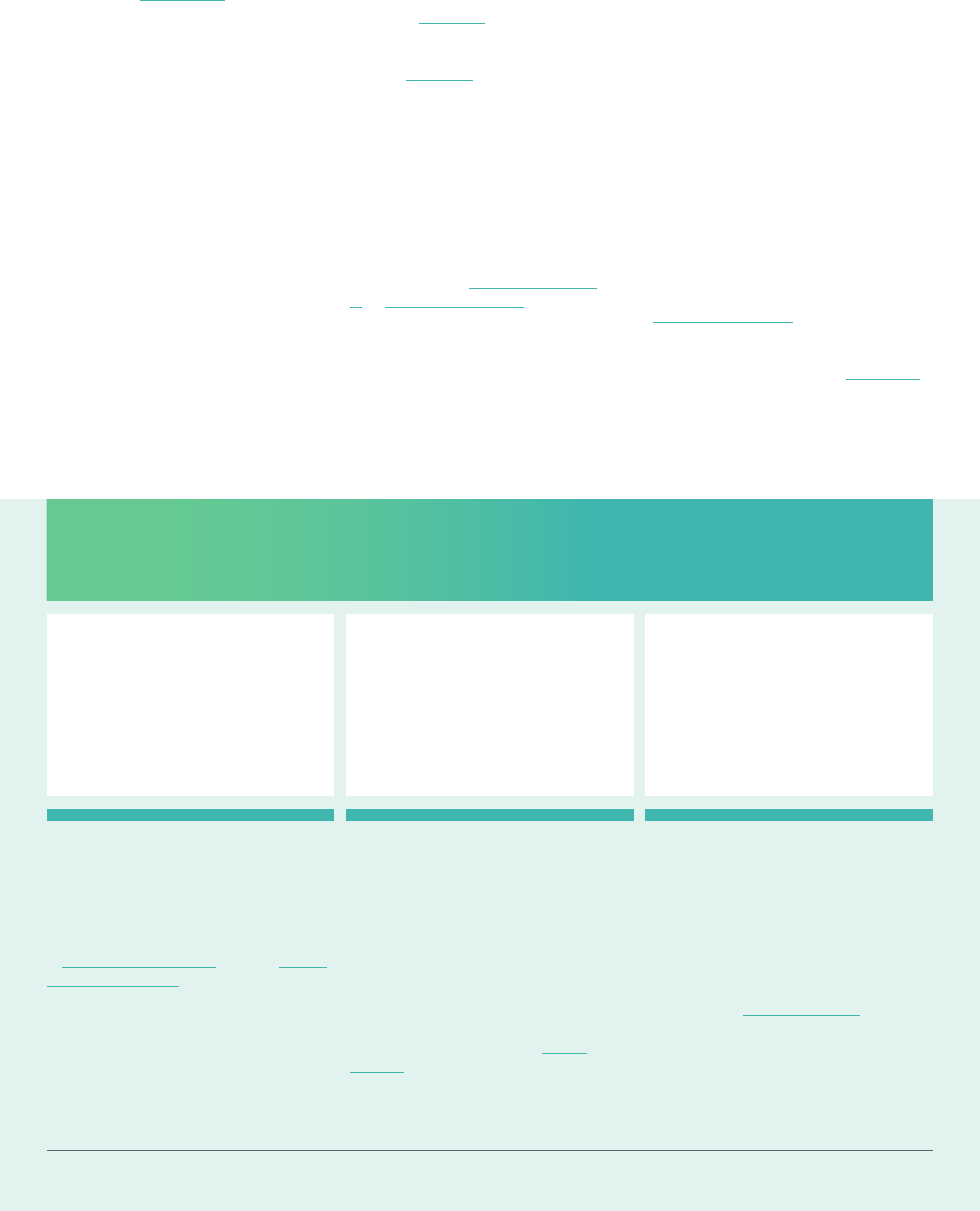
Clinical Trial
Regulation Progress
On24April2021,theEMA’s
ManagementBoardconrmed
that the introduction of the Clinical
Trial Regulation (Regulation (EU)
No536/2014)wasontracktobe
introducedon31January2022.
Theintroductionofthenewregulation
willseeasignicantchangeinhow
trials are processed across Europe,
withamorestreamlinedapproach
beingadopted.Undertheregulation,
sponsorsofclinicaltrialswillbeable
to submit one application to numerous
Member States across the EU using
an online portal and database called
the Clinical Trial Information System
(CTIS).InIreland,theimplementationof
theregulationwillresultinclinicaltrial
applicationsbeingreviewedintandem
by both the HPRA and the National
Research Ethics Committee for Clinical
Trials(NREC-CT),withasinglenational
decisionbeingissued.Theintroduction
of a more harmonised submission
and assessment process under the
regulationwillalsoresultinincreased
transparency in the area of trials across
Europewhilealsoincreasingefciency
intheapprovalprocess,whichwill
benetIrishpatients.
To ensure national preparedness
in advance of the regulation being
introduced,theHPRAisworkingclosely
withboththeDepartmentofHealth
andtheNationalOfceforResearch
Ethics Committees (nrecofce.ie)aswell
ascontributingtoanumberofworking
groupsatEMAlevel.Furthermore,the
HPRA has created a dedicated page on
itsclinicaltrialsectionofthewebsite
to keep stakeholders informed of all
the latest developments regarding
thelaunchoftheregulation:hpra.ie/
CTR.Thewebsitenotonlyinforms
stakeholders of the latest guidance
in relation to the regulation but also
provides a number of links to key
presentations delivered by both the
EMA and other European organisations
thatdealwithawiderangeoftopics
from the introduction of CTIS to the
submission and assessment of trials
undertheregulation.
HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue684
HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue685
National Ofce for Research
Ethics Committees
TheHPRAwouldliketotakethis
opportunitytoofciallywelcomethe
NationalOfceforResearchEthics
Committees (nrecofce.ie)whose
memberswereformallyappointed
by the Minister for Health Stephen
Donnellyon6May2021.Themembers
willsitonthreeNationalResearch
EthicsCommittees(NRECs),twoof
whichareintheareaofClinicalTrials
of Investigational Medicinal Products
(NREC-CT)whilethethirdisinthearea
of Clinical Investigations of Medical
Devices(NREC-MD).Theformationof
theseCommitteesmarksasignicant
step on the road to applications
receiving a single national ethics
opinion that is respected nationally,
whilealsoensuringthecontinued
protection of Irish patient safety and
dignity.TheHPRAlooksforwardto
collaboratingcloselywiththeNational
OfceforResearchEthicsCommittees
in the future in the advancement
of clinical trials in Ireland for both
medicinesandmedicaldevices.
Clinical Trial Regulation-
National Collaboration
Project
TheHPRA,intandemwiththeNational
Research Ethics Committee for Clinical
Trials of Investigational Medicinal
Products (NREC-CT), are pleased to
announce the creation of the Clinical
Trial Regulation-National Collaboration
Project(CTR-NCP).TheCTR-NCP
representsjustoneofthewaysinwhich
Ireland is actively preparing nationally
for the introduction of the Clinical Trial
Regulation.TheCTR-NCPwillmimic
thejointassessmentandapproval
model set out under the regulation in
thatapplicationssubmittedwillreceive,
ineffect,asinglenationaldecision.
TheCTR-NCPwillrununtilDecember
2021andSponsorsareencouraged
to contact either clinicaltrials@hpra.
information regarding submitting under
thisjointproject.
Clinical Trial Regulation
Information Sessions 2021
The HPRA is planning to host a
number of information sessions on
the upcoming Clinical Trial Regulation
inNovember2021.Theinformation
sessionswillbedeliveredremotelyby
theHPRAandwillprovideattendees
withaninsightintothepractical
application of the regulation, and the
accompanying Clinical Trial Information
System (CTIS), including requirements
forsponsorsaheadofitsintroduction.
Themeetingwillbeofinterestto
those involved in the submission and
managementofclinicaltrialsinIreland.
ColleaguesfromtheNationalOfce
for Research Ethics Committee and
theDepartmentofHealthwillalso
attend.Shouldyouhavesuggested
areasyouwouldliketoseediscussed,
please feel free to email these to
[email protected], quoting Clinical
Trial Regulation Info Day 2021 in the
subjectheading.Interestedpartiesare
encouraged to monitor the Newsand
EventssectionoftheHPRAwebsite for
additional information and instructions
regardingconrmedagendatopicsand
registrationclosertotheplanneddate.
Veterinary Medicines
The HPRA appeared before the
Oireachtas Committee on Agriculture
andtheMarineon27April2021
to discuss the regulation of
veterinary medicines, including the
implementationofRegulation2019/6.
A recording of the event and the HPRA’s
opening statement to the committee
areavailable.
Applicants are reminded that a variation
to change a MAH’s local representative,
as listed in the product labelling, need
only be submitted to the RMS and
totheCMS(s)wherethechangeis
beingapplied.Thechangeshouldbe
classiedasaType1AC.II.6.avariation.
This information is taken from the
January2018meeting minutes of
the Co-ordination Group for Mutual
Recognition and Decentralised
Procedures – Veterinary and Interested
Parties.
The HPRA expects to host an
Information Day for marketing
authorisation holders and
manufacturers of veterinary medicines
ontheimplementationofthenew
requirementsduringQ42021.
Thefocusofthiseventwillbetoassist
stakeholdersinunderstandingthenew
requirementsandtheirimplications.
For more details on this event going
forward,pleaseconsulttheHPRA
websiteforup-to-datenews.Itisthe
preference of the HPRA to host a
physical meeting, rather than a virtual
event,ifpossible.
HPRA Appearance
before Oireachtas
Committee on
Agriculture and the
Marine
Submission of
Variations to
Change MAH’s Local
Representative for
MR Products
HPRA Veterinary
Medicines
Information Day
2021

HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue686
Veterinary
Medicinal Products
and Labelling-related
Revisions
Whilstthereviewofmock-upsduring
the various regulatory procedures
remains a valuable activity, the HPRA
continually strives to reduce the
administrative burden associated
withthistaskforbothapplicantsand
regulators.Tothisend,anumber
of HPRA labelling-related guidance
documents and requirements have
been revised to ensure a clear and
efcientprocessforallinvolved.
1. Joint Labelling document
Followingthewithdrawalofthe
UK from the EU, the HPRA remain
committedtosupportingthejoint
labellingprocedure.Theprocedure
involves the coordination of approval of
nalcolourmock-upsbetweenIreland
and the UK for veterinary medicines and
enables marketing authorisation holders
(MAHs) to create one set of mock-ups
forwhichevermarketstheMAHwishes
to combine; IE and GB, IE and UK (NI)
orIEandUK(whichincludesbothGB
andNI).ToaddresstheseBrexit-related
procedural changes, applicants are
advised that the previous guidance
document “HPRA/VMD Joint-labelling
ofproductliteraturebetweenUKand
Ireland”hasnowbeenreplacedwith
the HPRA Guide to Joint Labelling
for veterinary medicinal products for
use in Ireland and the UK.Thisrevised
guidance document sets out the steps
tobothachieveandmaintainjoint
labellingbetweenIEandtheUK.
2. Product literature standard
The HPRA Guide to Product Literature
Standard (PLS) for Veterinary Medicinal
Products(previouslyajointdocument
withtheVMD)hasbeentransformed
into a more compact, user-friendly
document,whichaimstoassist
applicants in the creation of mock-
upsforassessment.There-vamped
document focusses on both general
labellingrequirements,whichwillassist
applicantswiththelayoutanddesign
of their mock-ups and national HPRA
requirements related to method of sale
etc.AppendixIofthisGuideincludesa
practical checklist for consultation prior
tomock-upsubmission.Forjointlabels,
applicants are advised to also consult
the UK’s Product Literature Standard as
publishedbytheVMD.
3. Submission of mock-ups for
variations
Recent updates have been made to the
HPRA Guide to Submission of Mock-
ups for Variations.Theupdatesresultin
the list of variation categories no longer
requiring routine mock-up submission
beingexpanded.Applicantsshould
note,however,thatthesubmissionof
mock-ups may be requested by the
HPRA for any variation category on a
case-by-casebasiswhereareviewis
deemednecessary.
4. Labelling notication
requirements
Historically, changes to mock-ups that
did not affect the font size, layout or
legibilityrequirednoticationbyway
ofemailtotheHPRA.Inaneffortto
reduce administration burden for both
MAHs and the competent authority, the
HPRAnolongerrequiresnotication
ofsuchminorchangesanditwillbe
assumed that the labelling remains
essentiallyaspreviouslyapproved.A
non-exhaustivelistofwhatconstitutes
a minor change can be found on the
HPRAwebsite.Ifthecriteriaofaminor
change is not met and formal approval
of the labelling is requested, please
submitaTypeIB(C.II.6.b)variation
withacoverletterandrevisedmock-
ups.Ifyouareindoubtaboutwhether
a variation is needed, please contact
Update on
Implementation of
HPRA Report on the
Method of Supply
of Antiparasitic
Veterinary Medicinal
Products Intended for
use in Food-producing
Animals
The HPRA is actively engaged in
implementing the change of labelling
process for changing products from
Licensed Merchant (LM) to Prescription
OnlyMedicines(POM)currently.All
newantiparasiticveterinarymedicinal
products for food-producing species
thathavebeenauthorisedsince30
April2021bearaprescriptionmedicine
supplycategory,whilevariation
applications to change existing
productsarebeingprocessedcurrently.
The HPRA hopes to approve all
productsconcernedby28July2021.
TheHPRAwillfollowthepolicy
outlined in the HPRA Guide to
Implementation of Packaging Changes
to Authorised Veterinary Medicinal
Products in implementing the changes
tothelabelling(speciedas‘other
safetyrestrictions–item3.2ofthat
policy).ThismeansthatMarketing
AuthorisationHoldersshould:
• Coordinate the supply/importation
of stock to ensure the introduction
of the amended product labelling
and literature as soon as possible
andinanycase,withinsixmonths
oftheapprovalofthevariation.
Products in old livery containing the
LM supply designation should not be
QP released after six months from the
dateofapprovalofthevariation(i.e.
the labelling of all products released
for the Irish market must be compliant
withtheprescriptionrequirementat
thelatestby28January2022).
• Plan to avoid having large quantities
of product in old livery in the
marketplace in the second half
of2021.Wheretheyexist,MAHs
should place notices in the farming
pressand/orprofessionaljournals
as appropriate, alerting users to the
changes.
•Informretailersofthechanges.
TheHPRAisworkingwiththe
Department of Agriculture, Food and
theMarinetoraiseawarenessofthis
changewithstakeholders.

HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue687
Update on
Implementation of
Regulation 2019/6
and New National
Legislation to Replace
SI No 786 of 2007
TheHPRAismid-waythroughaproject
to implement the requirements of
Regulation2019/6ontheauthorisation
and monitoring of veterinary medicinal
products.Thisproject,whichisbeing
led by Ms Elaine Hynes, HPRA Planning
and Authorisation Manager, has a
numberofdifferentobjectives:
• the HPRA’s authorisation and
monitoring processes operate in
compliancewiththenewlegislation;
• the HPRA authorisation and
monitoring processes provide
maximumefciency;
• the HPRA authorisation and
monitoring systems interface correctly
withtheexternalenvironment(e.g.
the EMA’s Union Product Database
and pharmacovigilance systems);
•theHPRAcanpositivelyinuencethe
newnationallegislationthatisbeing
elaborated by the Department of
Agriculture, Food and the Marine;
• the HPRA’s suite of guidelines,
application forms, internal procedures
and other documents are updated;
• stakeholders are provided
withregularandappropriate
communications on developments;
• the HPRA’s personnel are trained on
developments;
• any implications for the HPRA’s
business planning and model is fully
understood.
The HPRA provides a monthly update
on progressonourwebsite.Wewould
encourage marketing authorisation
holderstoreviewtheirownoperational
processes too, to ensure that they are
inlinewithanynewrequirements.
If you have queries relating to the
implementationofthenewlegislation,
please email [email protected].
Pharmacovigilance
Changes Arising from
the New Veterinary
Regulation (EU)
2019/6
TheHPRAwishestohighlightthefact
that Periodic Safety Update Reports
(PSURs)willnolongerberequired
underthenewveterinaryregulation.
Instead,MAHswillberequiredtocarry
out a signal management process for
their veterinary medicinal products, the
outcomeofwhichmustberecorded
on the European Medicines Agency’s
(EMA) Union Pharmacovigilance
database(currentlybeingdeveloped).
InaccordancewithArticle76.2of
theregulation,from28January
2022MAHswillneedtouploadall
suspected adverse event reports that
are reported to them to the Union
Pharmacovigilancedatabasewithin30
daysofreceiptofthereports.
As submission and assessment of PSURs
after28January2022isnotforeseenin
the regulation, only PSURs submitted to
theHPRApriorto28January2022will
beassessed,i.e.thosesubmittedafter
28January2022willnotbeassessed.
Consequently, holders of marketing
authorisationswithaPSURdatalock
point(DLP)of27November2021
orearlierwillneedtosubmitthose
PSURsforassessmentpriorto28
January2022.Marketingauthorisations
withaPSURDLPfallingbetween28
November2021and27January2022
mayalsobesubmittedpriorto28
January2022forassessment.
TheHPRAisawarethatforanumberof
marketing authorisations currently on
a 3-year PSUR submission cycle, and
forwhichtheDLPforthenextPSUR
submissionfallsonorafter28January
2022,thosenon-seriousadverseevent
reportsthatwouldnormallybereported
inaPSURwillnotbeavailablefor
reviewbytheHPRAwithinthecontext
ofaPSURassessment.However,itis
expected that those adverse event
reportswillbeincludedwithintherst
signal management process to be
conducted by marketing authorisation
holdersinaccordancewithArticle81of
theregulation.
Guidance on signal management is
currently being drafted by the EMA and
willbepublishedontheirwebsitein
duecourse.
In the meantime, further information
onthenewveterinaryregulationis
available from the dedicated section
ontheHPRA’swebsite:hpra.ie/
NewVetReg.
QPPV Data
Requirement for
the Union Product
Database
Article55(1)oftheNewVeterinary
Regulation(EU)2019/6requires
the Agency to establish and, in
collaborationwiththeMember
States, maintain a Union database on
veterinary medicinal products, also
referred to as the “product database”,
“UnionProductDatabase”,or“UPD”.
The Commission Implementing Act (EU)
2021/16of8January2021laysdown
the necessary measures and practical
arrangements for the UPD and details
thespecicationstoimplementin
ordertofulltherequirementsofthe
Regulation.
Informationonthequaliedperson
for pharmacovigilance (the QPPV)
is required for upload to the UPD
inrespectof‘legacy’productdata.
‘Legacy’dataisdenedasanydata
on a veterinary medicinal product
authorisedinaMemberStatewitha
marketing authorisation or registration
validonorbefore28January2022.
In order to meet this requirement,
theHPRAwillwritetomarketing
authorisation holders shortly to request
specicQPPVinformation.Wewill
specifywhatisrequiredandthe
deadlineforprovidingthisinformation.
Itisimportantwereceivethisdata
andhavetimetoupdateoursystems.
Wewillbeunabletocompletethe
submission of our product data to the
UPDwithoutthesedetails.
If you have any queries, please do not
hesitate to contact us at newvetreg@
hpra.ie.

HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue688
Uploading of Product
Data to the EMA’s
Union Product
Database
TheUnionProductDatabase(UPD)will
contain information on all veterinary
medicinal products that are authorised
intheEU,whenitgoeslivetothe
publicon28January2022.Thiswill
beasignicantstepinimproving
transparency in respect of veterinary
medicines in the EU, as it is expected
to provide information on more than
30,000individualmedicines,once
uploaded by Member States, in due
course.Thedatabasewillinclude
extensive and searchable information
on products, including Summaries of
Product Characteristics and package
leaets.InaccordancewiththeEMA’s
implementation guide,theHPRAwill
commence uploading information
on veterinary products authorised
nationally using the decentralised or
mutual recognition procedures in the
periodupto30September2021.
Data in respect of the other nationally
authorisedveterinaryproductswillbe
uploadedinasecondphase,withthe
upload of all data to be completed by
28January2022.
TheHPRAintendstomaintainitsown
national listing of authorised veterinary
medicinal products on the HPRA
websiteatleastuntiltheendof2022,
butwillreconsiderthepositionatthat
point in the light of experience gained
withtheUPD.
Change to VPO and
VPO-1 Category of
Supply
InaccordancewithArticle34of
Regulation2019/6,theUnionProduct
Database(UPD)willrecordveterinary
medicinal products as either being
‘subjecttoveterinaryprescription’or
‘notsubjecttoveterinaryprescription’.
All nationally authorised veterinary
medicinal products that are currently
authorisedwithaVPOorVPO-1
categoryofsupplywillbemapped
intheUPDassubjecttoveterinary
prescription.TheHPRAisindiscussion
withtheDepartmentofAgriculture,
Food and the Marine and expects that
the use of VPO and VPO-1 categories
ofsupplywillbediscontinuedin
Ireland,andwillinsteadbereplaced
byprescriptionsupply.However,any
warningsandrestrictionsforuseby
veterinarypractitionerswillbestatedin
the Summary of Product Characteristics
(SPC) and product literature, in
accordancewiththerequirementsof
Article35(1)(xi)ofRegulation2019/6.
Theneteffectwillbethatthewords
‘forusebyveterinarysurgeonsonly’
willbestatedintheSPCandproduct
literature,whiletheVPOandVPO-1
symbolswillnolongerappearonthe
productlabelling.TheHPRAexpects
that the QRD template,whichis
currentlybeingupdated,willprovide
additionalexplanationofthispoint.
The HPRA understands that the DAFM
plans to continue current national
supply categories in respect of products
thatarenotsubjecttoveterinary
prescription(i.e.POM(E),PS,LMand
CAM),inaccordancewithArticle103of
theRegulation.
Revision of National
Legislation on
Veterinary Clinical
Field Trials
Veterinaryclinicaleldtrialswill
besubjecttonewcontrolsunder
Regulation2019/6from28January
2022.Theexistingnationalcontrols
speciedbySI.No.786of2007,
asamended,willberevisedbythe
Department of Agriculture, Food
and the Marine to ensure that they
complementRegulation2019/6and
arenotinconictwithit.TheHPRA
understands that the overall process for
submitting and processing applications
forclinicaleldtrialswillremainlargely
similartothatinoperationcurrently.
Applicants are requested to keep in
touchwithdevelopments,whichwillbe
reportedontheHPRAwebsite(hpra.
ie/NewVetReg).Thenewnational
legislation is not expected to be
availableuntiltheendof2021.
Update on Irish
Language Case
In respect of proceedings relating to
the provision of bilingual packaging of
veterinary medicines in the Irish and
English languages brought against
the Department of Agriculture, Food
andtheMarine(DAFM)in2016,the
European Court of Justice (ECJ) gave
itsrulingon17March2021.TheEU
CourtsaidthattheDirective2001/82/
EC language requirement for the
labelling of veterinary medicines had
been incorrectly transposed into Irish
nationallaw.Italsofoundthatthefact
thatRegulation2019/6,whichapplies
from28January2022andprovides
that Member States can choose the
language of the text to be used in the
packageleaetandlabelling,cannot
justifydisregardingtheobligationinthe
directive.ItstatedthattheHighCourt
is required to uphold the application
for a declaration that Ireland is under
an obligation to remedy the incorrect
transpositionofthedirective.
Thejudgmentisexpectedtobe
returned to the High Court in Dublin to
decideonwhattodonext.TheHPRA
willcontinuetomonitordevelopments
inthiscase.
HPRAMedicinalProductsNewsletter–January2021toApril2021–Issue689
Compliance
Authenticity
Verication of
Authorisations and
Certicates issued
by the HPRA
Since1September2020,theHPRAhas
issuedelectronicauthorisationswith
e-signatures and no longer routinely
issues hard copies of Manufacturing
Authorisations/Licences,Wholesale
Distribution Authorisations and Active
SubstanceRegistrations.Authenticity
of electronic authorisations issued
bytheHPRAmaybeveriedonthe
EudraGMDP database or, alternatively,
enquiries can be submitted to the
compliance mailbox (compliance@hpra.
ie).
GMPandGDPcerticateswillbe
uploaded to the EudraGMDP database
upon receipt of responses to the
decienciesandpointsforclarication
outlinedintheinspectionreport,which
havebeendeemedacceptable.The
HPRAwillnotroutinelyissuehard
copiesofGMP/GDPcerticatesand
theirauthenticitymaybeveriedon
theEudraGMDPdatabase.Hardcopies
ofGMP/GDPcerticatesmaybe
requestedviatheexportcertication
process,detailsofwhichcanbe
accessed on the ExportCertication
webpageoftheHPRAwebsite.
Certicates of
Free Sale for
Devices Approved
under the New
Medical Devices
Regulation
2017/745
Asof26May2021,theHPRAaccepts
applicationsforcerticatesoffree
saleunderthenewMedicalDevices
Regulation2017/745,whichbecame
fullyapplicableonthatdate.Anew
application form and a revised guidance
document have been published on
ourwebsite.Completedapplications
should be submitted to exportcerts@
hpra.ie.Allqueriesinrelationto
certicatesoffreesalecanalsobe
emailed to [email protected].
EU and UK Trade
and Cooperation
Agreement Summary
of Annex on Technical
Barriers to Trade
(TBT) 2 – Medicinal
Products
The EU and UK Trade and Cooperation
Agreement(TCA)waspublishedon
24December2020.TheTCAincludes
an Annex for human and veterinary
medicinalproducts,whichsetsout
the conditions for the recognition of
Good Manufacturing Practice (GMP)
inspectionsbetweenregulatory
authorities in EU Member States and
theUnitedKingdom.
Belowaresomekeysummarypoints
fromAnnexTBT–2onmedicinal
products:
•TheUKandtheEUwillrecognise
GMP inspections at manufacturing
sites carried out by either party
intheirjurisdictionsandinthird
countries.
•FollowingarequestforGMP
documentation, each party shall
endeavour to transmit the document
within30calendardaysofthedateof
therequest.
•Underspeciccircumstances,each
partymayoptnottoacceptanofcial
GMP document issued by the other
party’sauthority.
• Each party has the right to conduct
itsowninspectionofmanufacturing
facilitiesthathavebeencertiedas
compliantbytheotherparty.
• Each party shall notify the other party
atleast60daysbeforeadoptingany
newmeasuresorchangesrelatingto
GMP, concerning any of the relevant
laws,regulationsandtechnical
guidelines.
•Asaresultofanyofthenew
measures or changes, a party can
consider that it can no longer
recognise inspections or accept
ofcialGMPdocumentsissuedbythe
other party and the parties shall enter
intoconsultationswithintheworking
grouponmedicinalproducts.
• Each party has the right to suspend
totally or partially the recognition of
inspections of the other party for all
orsomeoftheproducts.
•Thepartiesshallcooperatewitha
viewtostrengthening,developing
and promoting the adoption and
implementation of internationally
agreedscienticortechnical
guidelines.
•Theworkinggrouponmedicinal
products shall assist the Trade
Specialised Committee on Technical
Barriers to Trade in monitoring and
reviewingtheimplementationand
ensuring the proper functioning of
thisAnnex.
