
Safety Assessment of
Rosmarinus Officinalis (Rosemary)-Derived Ingredients
as Used in Cosmetics
Status: Final Report
Release Date: June 23, 2014
Panel Meeting Date: June 9-10, 2014
The 2014 Cosmetic Ingredient Review Expert Panel members are: Chairman, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V.
Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald
C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, D.P.A.
This safety assessment was prepared by Monice M. Fiume, Assistant Director/Senior Scientific Analyst.
© Cosmetic Ingredient Review
1620 L Street, NW, Suite 1200♢ Washington, DC 20036 ♢ ph 202.331.0651 ♢ fax 202.331.0088 ♢
cirinfo@cir-safety.org

ABSTRACT
The Expert Panel assessed the safety of 10 Rosmarinus officinalis (rosemary)-derived ingredients and
concluded these ingredients are safe as used in cosmetics when formulated to be non-sensitizing. The
Rosmarinus officinalis-derived ingredients are most frequently reported to function in cosmetics as skin
conditioning agents or as fragrance ingredients. The Panel reviewed the available animal and clinical
data to determine the safety of these ingredients. Because final product formulations may contain multiple
botanicals, each containing similar constituents of concern, formulators are advised to be aware of these
constituents and to avoid reaching levels that may be hazardous to consumers. Industry should use good
manufacturing practices to limit impurities that could be present in botanical ingredients.
INTRODUCTION
This report reviews the use and safety data of the following 10 Rosmarinus officinalis (rosemary)-derived ingredients as used
in cosmetics:
Rosmarinus Officinalis (Rosemary) Extract
Rosmarinus Officinalis (Rosemary) Flower Extract
Rosmarinus Officinalis (Rosemary) Flower/Leaf Stem Extract
Rosmarinus Officinalis (Rosemary) Flower/Leaf/Stem Water
Rosmarinus Officinalis (Rosemary) Leaf
Rosmarinus Officinalis (Rosemary) Leaf Extract
Rosmarinus Officinalis (Rosemary) Leaf Oil
Rosmarinus Officinalis (Rosemary) Leaf Powder
Rosmarinus Officinalis (Rosemary) Leaf Water
Rosmarinus Officinalis (Rosemary) Water
Most of the ingredients included in this review are extracts, oils, powders, or waters derived from a defined part of the
Rosmarinus officinalis (rosemary) plant.
Rosmarinus officinalis (rosemary)-derived ingredients are reported to have a number of functions, and the most common
functions in cosmetics are as a skin conditioning agent or use as a fragrance ingredient.
1
Two of the ingredients, i.e., rosmari-
nus officinalis (rosemary) flower extract and rosmarinus officinalis (rosemary) leaf extract, are reported to function as antiox-
idants. However, rosmarinus officinalis (rosemary) leaf powder is reported to function only as a flavoring agent.
CHEMISTRY
Definition
The definition of each Rosmarinus officinalis (rosemary)-derived ingredient indicates what part(s) of the plant from which
the ingredient is obtained (Table 1). In some cases, the definition also gives insight as to the method of manufacture.
General Characterization
The Rosmarinus officinalis L. plant, from the botanical family Lamiaceae, is a scented, evergreen shrub with a very pungent
odor that is native to the Mediterranean region and Portugal; the odor is sometimes defined as camphor-like.
2,3
Rosemary has
a spicy, harsh, bitter, aromatic taste. Bluish labiate flowers grow on the upper green part of the branches. Rosemary oil is
produced mostly in Spain, France, and Tunisia.
4
Rosmarinus officinalis L. is generally recognized as safe (GRAS) in foods as a spice and as a natural seasoning and flavoring.
(21CFR182.10) Rosemary has traditional or folk medicine uses, some with reported side effects.
2,5,6
The flowering dried
twig tips, the dried leaves, the fresh leaves, the fresh aerial parts, and the flowering branches are considered to be the
medicinal parts.
5
Chemical and Physical Properties
Rosmarinus officinalis (rosemary)-derived ingredients are strongly aromatic. Chemical and physical property data are
provided in Table 2.
Preparation/Extraction
Food-grade rosmarinus officinalis (rosemary) extract is prepared by extraction from the leaves of Rosmarinus officinalis.
Food-grade acetone, ethanol, hexane, or a combination of hexane and ethanol (in a two-step process) are used as extraction
solvents; the ethanol extract is sometimes deodorized or partially deodorized ethanol.
7,8
Food-grade rosmarinus officinalis
(rosemary) extract may also be extracted using supercritical carbon dioxide (CO
2
). Subsequent production steps include
filtration, purification, solvent evaporation, drying, and sieving; the extract may be deodorized, decolorized, and standardized
using diluents and carriers that are permitted in foods.
An additional method of manufacturing the cosmetic ingredients includes extraction with absolute ethanol (resulting in what
has been called “an absolute”) or a collection of the insoluble waxes (resulting in what has been called “a concrete”).
9
Both rosmarinus officinalis (rosemary) leaf extracts and rosmarinus officinalis (rosemary) leaf oil can be produced by
supercritical fluid extraction with natural CO
2
and a small amount of ethanol as a solvent.
10-13
One supplier of the leaf
extract reported that the essential oil is removed by multistep separation,
12
and a supplier of the leaf oil adds a small amount
(<4%) of sunflower oil to increase solubility when blending.
13

Food-grade rosmarinus officinalis (rosemary) leaf oil is the volatile oil obtained by steam distillation from the fresh flowering
tops or dried crushed aerial parts of Rosmarinus officinalis L.
7
The oil from Rosmarinus officinalis can also be obtained by
hydrodistillation of dried crushed aerial parts.
14
Essential oils prepared by a steam distillation process yields two distinct
fractions, a water-insoluble fraction and a water-soluble fraction.
1
The water-insoluble fraction contains the term oil in the
name and the water-soluble fraction contains the term water in the name. Therefore, rosmarinus officinalis (rosemary) leaf
water is the water-soluble fraction of the steam distillation of Rosmarinus officinalis (rosemary) leaves.
Constituents/Impurities
Rosmarinus officinalis L. is composed of an array of constituents, primarily phenolic acids, flavonoids, monoterpenes, diter-
penes, diterpenoids, and triterpenes. Structures for some of the principal components according to chemical family are
depicted in Figures 1-5.
A detailed list of chemical constituents by plant part is presented in Table 3, and a more focused listing of constituents of
Rosmarinus officinalis is provided in Table 4. Table 5 provides composition data on three rosmarinus officinalis (rosemary)
leaf extracts, based on certificates of analysis provided by suppliers of rosmarinus officinalis (rosemary) leaf extract; these
certificates report a phenolic diterpenes content of 14 or 25%.
15-18
According to the European Cosmetic Regulations, certain fragrance allergen compounds are subject to declaration on the
label if the concentration of a specified allergen exceeds 0.001% in leave-on and 0.01% in rinse-off products.
19
One supplier
declared the following concentrations of allergen compounds in a rosmarinus officinalis (rosemary) leaf extract: <0.1%
linalool and <0.2% d-limonene.
20
The principal antioxidative components of rosmarinus officinalis (rosemary) leaf extract are the phenolic diterpenes carnosol
and carnosic acid.
8
The amount of carnosol and carnosic acid present in the extract varies with the method of extraction, with
levels as low as 5-7% carnosol plus carnosic acid found in rosemary extract prepared from a partially deodorized ethanol
extract of rosemary to as high as 30% carnosol plus carnosic acid in an extract prepared with supercritical carbon dioxide.
2,7
Carnosol and carnosic acid are not the only constituents that vary with extraction method. Table 6 provides a sample of the
differences in constituent profiles in rosemary leaves based on extraction method. Some of the studies summarized in this
safety assessment provided information on the amount of constituents present in the test article; when this information was
available, it is included.
The actual amount of constituents present also varies according to the stage of development, variety of plant, season harvest-
ed, and origin of the leaves.
2,8,21,22
High-performance liquid chromatography analysis of dimethyl sulfoxide (DMSO) extracts
of rosemary leaves indicated the highest accumulation rate of the phenolic diterpenes carnosic acid, carnosol, and 12-O-
methylcarnosic acid, of rosmarinic acid, and of the flavones genkwanin and isocutellarein 7-O-glucoside was found in the
young stages of plant development.
23
The diterpenes and rosmarinic acid, but not the flavones, were found in the flower,
stem, and root extracts at lower concentrations than in the leaves during the early stages of plant growth, but the concentra-
tion of each, except for 12-O-methylcarnosic acid, tended to increase during flowering. Rosmarinic acid concentrations in
the leaves also decreased once flowering started, while the level in the flowers was slightly increased during flowering. The
flavones acted similarly to carnosic acid.
Water and light conditions also affect the amount of the constituents found in rosemary plants; for example, highly oxidized
diterpenes increase in rosemary plants exposed to drought and high light stress.
24
Although it is generally accepted that the
geographical region and stage of growth affects plant composition, some researchers reported that, within one country, the
chemical composition of rosemary essential oil (plant parts not specified) did not vary with geographical region or harvest
time.
25
Food-grade rosmarinus officinalis (rosemary) leaf extract has acceptance criteria of not more than 3 mg/kg arsenic and 2
mg/kg lead, and not more than 8.0% loss on drying.
7
Food-grade rosemary leaf oil is to have not less than 8.0% borneol and
not less than 1.5% esters, calculated as bornyl acetate.
7
Table 7 provides toxicity and other information on some constituents of Rosmarinus officinalis (rosemary)-derived ingredi-
ents. Because formulations may contain more than one botanical ingredient, caution is urged to avoid reaching levels of
toxicity for constituents of concern in the final formulation. Industry should use good manufacturing practices to limit
impurities.
USE
Cosmetic
The Rosmarinus officinalis (rosemary)-derived ingredients included in this safety assessment have a variety of functions in
cosmetics (Table 1). Most of the ingredients function as a skin conditioning agent and/or as a fragrance ingredient; rosmari-
nus officinalis (rosemary) leaf powder is reported to function only as a flavoring agent.
1
The Food and Drug Administration (FDA) collects information from manufacturers on the use of individual ingredients in
cosmetics as a function of cosmetic product category in its Voluntary Cosmetic Registration Program (VCRP). VCRP data
obtained from the FDA in 2014,
26
and data received in response to a survey of the maximum reported use concentration by
category conducted by the Personal Care Products Council (Council)
27,28
in 2013, indicate that nine of the ten ingredients in-
cluded in this safety assessment are currently used in cosmetic formulations (Table 8). Rosmarinus officinalis (rosemary) leaf
extract has the greatest number of uses, 729, followed by rosmarinus officinalis (rosemary) leaf oil, 474 uses, and rosmarinus
officinalis (rosemary) extract, 404 uses. According to the results of the concentration of use survey, most cosmetic formula-
tions contain very low concentrations of the Rosmarinus officinalis (rosemary)-derived ingredients, often much less than
0.1%. However, rosmarinus officinalis (rosemary) leaf extract is reported to be used at up to 10% in body and hand products
and 3% in eye shadow formulations and bath soaps and detergents. Rosmarinus officinalis (rosemary) flower/leaf/stem water
is the only ingredient not reported to be used.
In some cases, reports of uses were received in the VCRP, but concentration of use data were not provided. For example,
rosmarinus officinalis (rosemary) flower extract is reported to be used in 32 cosmetic formulations, but no use concentration
data were reported. In other cases, no uses were reported in the VCRP, but concentration of use data were received from
industry; rosmarinus officinalis (rosemary) flower/leaf/stem extract had no reported uses in the VCRP, but a use concentra-
tion in a deodorant was provided in the industry survey. Therefore, it should be presumed there is at least one use in a
deodorant formulation.
Products containing rosmarinus officinalis (rosemary)-derived ingredients may be applied to baby skin (e.g., 0.012% rosmari-
nus officinalis (rosemary) leaf extract in baby lotion, oils and creams), used in products that could be incidentally ingested
(e.g., 0.012% rosmarinus officinalis (rosemary) leaf in lipstick formulations), or used near the eye area (e.g., up to 3% ros-
marinus officinalis (rosemary) leaf extract in eye shadow formulations) or mucous membranes (e.g., up to 3% rosmarinus
officinalis (rosemary) leaf extract in bath soaps and detergents).
27
Additionally, Rosmarinus officinalis (rosemary)-derived
ingredients are used in cosmetic sprays and powders; for example, rosmarinus officinalis (rosemary) leaf extract is reported
to be used in other fragrance preparations at up to 0.5% and rosmarinus officinalis (rosemary) extract is used in face powders
at up to 0.05%. These products could possibly be inhaled. In practice, 95 to 99% of the droplets/particles released from
cosmetic sprays have aerodynamic equivalent diameters >10 µm.
29-32
Therefore, most droplets/particles incidentally inhaled
from cosmetic sprays would be deposited in the nasopharyngeal and bronchial regions and would not be respirable (i.e., they
would not enter the lungs) to any appreciable amount.
29,32
Rosmarinus officinalis (rosemary) extract is used in aerosol deodorants at concentrations up to 0.012%. There is some
evidence indicating that deodorant spray products can release substantially larger fractions of particulates having aerodynam-
ic equivalent diameters in the range considered to be respirable.
29
However, the information is not sufficient to determine
whether significantly greater lung exposures result from the use of deodorant sprays, compared to other cosmetic sprays.
All of the ingredients named in this safety assessment are listed in the European Union inventory of cosmetic ingredients.
33
Non-Cosmetic
Rosmarinus officinalis L. is GRAS as a spice and as a natural seasoning and flavoring when the intended use is for human
consumption (21CFR182.10) and for animal drugs, feed, and related products (21CFR582.10). It is also GRAS as an
essential oil, oleoresin (solvent-free), and natural extractive (including distillates) for human consumption (21CFR182.20)
and for animal drugs, feed, and related products (21CFR582.20). Rosemary oil can be used in the formulation of denatured
alcohol and rum (27CFR21.65).
According to The Official Journal of the European Union, extracts of rosemary contain several anti-oxidant compounds, and
although the European Food Safety Authority (EFSA) was not able to establish an acceptable daily intake due to insufficient
toxicological data, the EFSA considered the margin of safety was high enough to conclude that dietary exposure was not a
concern.
34
Extracts of rosemary are allowed in various food products at amounts of 30-1000 mg/kg, expressed as the sum of
carnosol and carnosic acid.
Rosemary leaves are used as a seasoning in cooking.
35
Rosmarinus officinalis (rosemary) leaf oil is used as a condiment and
flavoring agent in food; as an antioxidant in edible oils, meats, and other fat-containing foods; and as a dietary supplement.
Also, rosemary oil is reported to have antimicrobial activities.
4
Anti-inflammatory, antioxidant, and anti-microbial uses have been reported for rosemary.
21,36-38
Rosemary has traditional or
folk medicine uses, some with negative reported side effects.
2,5,6
Rosemary has been used as an antispasmodic in renal colic
and dysmenorrhea, and it has been used for relieving respiratory disorders. The essential oil is used internally as a carmina-
tive and as an appetite stimulant; however, large amount of the oil are reported to cause gastroenteritis and nephritis. The
essential oil is added to bath water as a circulation stimulant. As the oil or as an ointment, external application use is as an
analgesic liniment for rheumatism. Rosemary is used as a poultice for poorly healing wounds and in the treatment of eczema.
Additional folk medicine practices include use in lotions to treat baldness,
14
and use of the leaves and branches in treating
headaches.
4

TOXICOKINETICS
Penetration Enhancement
The effect of rosemary oil on the permeation of aminophylline was determined in human skin in vivo using attenuated total
reflection Fourier transform infrared (ATR-FTIR) spectroscopy.
39
Rosemary oil did enhance the permeation of aminophyl-
line; however, the increase in permeation was less than that observed with 50% ethanol.
TOXICOLOGICAL STUDIES
Single Dose (Acute) Toxicity
The acute toxicity of Rosmarinus officinalis (rosemary)-derived ingredients is not very remarkable (Table 9).
8,22,40-42
. The
dermal LD
50
of rosmarinus officinalis (rosemary) leaf oil is > 10 ml/kg.
42
The oral LD
50
of rosmarinus officinalis (rosemary)
leaves is >2 g/kg,
22
of rosmarinus officinalis (rosemary) leaf extract is >8.5 g/kg,
8
and of rosmarinus officinalis (rosemary)
leaf oil is 5.5 g/kg bw.
41
Repeated Dose Toxicity
A number of oral repeated-dose toxicity studies were performed in mice and in rats with rosmarinus officinalis (rosemary)
leaves extracted in a number of solvents (Table 10). Doses as high as 14.1 g/kg bw rosmarinus officinalis (rosemary) leaf
extract were tested (5 days by gavage), and some studies were performed for up to 3 mos (dietary) with doses of up to 400
mg/kg bw/day.
8
Increases in absolute and relative liver-to-body weights were observed in many of the studies, independent
of the extraction method; these changes were shown to be reversible, and no other signs of toxicity were observed. Oral
administration of rosmarinus officinalis (rosemary) leaf oil with carbon tetrachloride, but not without, resulted in an increase
in liver weights.
41
Ocular Irritation
Rosemary oil is reported to be a moderate ocular irritant.
21
(Details not provided.)
Anti-Inflammatory Effects
Rosmarinus Officinalis (Rosemary) Leaf Extract
Rosmarinus officinalis (rosemary) leaf extract has been shown to inhibit formaldehyde-induced plantar edema and 12-tetra-
decanoylphorbol 13-acetate (TPA)-induced and arachidonic acid-induced ear edema.
43,44
In the formaldehyde-induced plantar edema study, groups of six male Balb/C mice were given an injection of 20 µl of 3%
formaldehyde into the sub-plantar region of both hind paws.
43
After 2 h, one hind paw was treated with 10 µl of 12 mg/ml of
an ethanol extract of Rosmarinus officinalis (rosemary) leaves topically, as an injection, or both. The mice were killed after
24 h. Topical administration of the extract reduced edema by 80%, injection reduced it by 22%, and the combined applica-
tion reduced edema by 24%.
The TPA-induced ear edema study was conducted in groups of 10 male Balb/c mice.
43
The effect of pretreatment with 10-
1000 µg/cm
2
of an ethanol extract of Rosmarinus officinalis (rosemary) leaves at 30 min prior to induction of inflammation
with 25ng/cm
2
TPA was evaluated. The mice were killed after 4 h. Doses of 100, 250, 500, and 1000 µg/cm
2
of the extract
statistically significantly reduced inflammation by 38, 79, 84, and 99%, respectively.
In a TPA-induced mouse ear edema study conducted in groups of six to 10 female CD-1 mice, a single dose of 20 µl acetone,
0.5 nmol TPA, or TPA and 0.04, 0.12, or 0.36 mg of a methanol extract of Rosmarinus officinalis (rosemary) leaves in 20 µl
acetone was applied to one ear of each mouse.
44
The mice were killed after 5 h, and rosmarinus officinalis (rosemary) leaf
extract inhibited TPA-induced inflammation by 17, 75, and 92% respectively. The extract also inhibited TPA-induced
erythema.
In the arachidonic acid-induced mouse ear edema study, 0.02, 0.09, and 0.45 mg of a methanol extract of Rosmarinus offici-
nalis (rosemary) leaves in 20 µl acetone was applied to groups of 10 female CD-1 mice at 30 min prior to treatment with 0.3
mg arachidonic acid in 20 µl acetone.
44
The mice were killed after 1 h. Inflammation was inhibited by 12, 28, and 54%,
respectively.
Effect on Epidermal Hyperplasia
Two-hundred µl acetone, 1 nmol TPA, or 1 nmol TPA and 3.6 mg rosmarinus officinalis (rosemary) leaf extract in 200 µl
acetone were applied twice a day for 4 days to the dorsal skin of mice.
44
Three or four CD-1 mice were used per group.
Topical application of the extract with TPA inhibited a TPA-induced increase in the number of epidermal cell layers and
epidermal thickness.
Immunologic Effects
An aqueous extract of up to 2.5 mg/ml Rosmarinus officinalis (rosemary) leaves was found to inhibit ultraviolet (UV)-
induced up-regulation of matrix metalloproteinase-1 (MMP-1) gene transcription in dermal human fibroblasts.
45
The release
of the cytokines interleukin (IL)-1α and IL-6 was prevented by the extract.

REPRODUCTIVE AND DEVELOPMENTAL TOXICITY
Non-Human
Rosmarinus Officinalis (Rosemary) Leaf Extract
Oral administration of high doses of rosmarinus officinalis (rosemary) leaf extract adversely affected fertility in male rats.
46
Groups of 10 male Sprague Dawley rats were fed a diet with 0, 250 or 500 mg/kg bw/day of an ethanol extract of Rosmarinus
officinalis (rosemary) leaves in distilled water. After 53 days of dosing, each male rat was mated with two untreated female
rats for 10 days; the female rats had been given a subcutaneous (s.c.) dose of 5.0 mg estradiol benzoate 54 h and 0.5 mg
progesterone at 54 and 6 h, respectively, prior to being placed with the males. The males were dosed during, and killed after,
the 10-day mating period, and the reproductive organs were examined. The females were killed 1 wk after the mating period,
and the reproductive tract of each female was examined to determine pregnancy and the number of implantation sites, viable
fetuses, and fetal resorptions.
Body weights of the male rats of the test groups were similar to those of the control group. However, the high dose group
exhibited statistically-significantly reduced absolute weights and organ-to-body weight ratios of testes and male accessory
sex organs, diameters of seminiferous tubules and Leydig cell nuclei, height of epithelia of the epididymes and seminal
vesicles, germinal and interstitial cell counts, levels of sex hormones, and sperm density and motility when compared to the
controls. The numbers of interstitial degenerating cells were statistically-significantly increased in the high-dose group.
Exposure of the males to the high dose resulted in a reduced number of pregnant females, implantations and viable fetuses,
and an increased the number of resorptions. Results from the low-dose groups suggested dose-response trends in these
parameters, although statistically-significant differences were observed only with the high-dose group.
Rosmarinus Officinalis (Rosemary) Flower/Leaf/Stem Extract
A group of 12 gravid female Wistar rats was dosed by gavage with 26 mg/day of a 30% aq. extract of rosmarinus officinalis
(rosemary) flower/leaf/stem extract (13 mg/ml solids) on days 1-6 of gestation (preimplantation), and a group of 14 gravid
rats was dosed with the extract on days 6-15 of gestation (organogenesis).
47
Negative control groups of 12 or 11 gravid rats
were given saline by gavage on days 1-6 or 6-15 of gestation, respectively. All dams were killed on day 21 of gestation. No
signs of maternal toxicity were observed, and maternal weight gains were similar for treated and control groups.
In the rats dosed on days 1-6 of gestation, a non-statistically significant increase in preimplantation loss was observed. No
changes in post-implantation loss were seen as compared to controls, and no other reproductive parameters were affected. In
the group treated on days 6-15 of gestation, a non-statistically significant increase in post-implantation loss rate (2.54%) was
reported; analysis of the resorptions found that they occurred during the early post-implantation period. No other changes in
reproductive parameters were observed when compared to the negative control group. Developmental effects were not ob-
served in either group.
Human
According to the PDR for Herbal Medicines, rosemary preparations should not be used as a drug during pregnancy; very
large quantities of the leaves reportedly can be misused as an abortifacient.
5
According to Herbal Drugs and Phytopharma-
ceuticals, toxic side effects may occur with components of the essential oil.
48
(Details were not provided.)
Effects on Estrogenic Activity
Non-Human
Rosmarinus Officinalis (Rosemary) Leaf Extract
Groups of seven or eight 6-wk old ovariectomized CD-1 mice were fed either a diet containing 2% of a methanol extract of
Rosmarinus officinalis (rosemary) leaves or the basal diet.
49
After 3 wks, the animals were given an i.p. injection of 0, 45, or
100 ng/mouse estradiol or estrone in 50 µl corn oil, once daily for 3 days. Eighteen hours after the last injection, the animals
were killed and the uterus was removed. In the mice fed the basal diet, estradiol and estrone increased the uterine wet weight
in a dose-dependent manner. Rosemary inhibited 35-50% of the uterine response; this was statistically significant.
Human
Rosmarinus Officinalis (Rosemary) Leaf Extract
In a study investigating the effects of a botanical supplement on sex steroid hormones and metabolic markers in premeno-
pausal women, a few changes were found, however, the changes were not very remarkable.
50
A group of 15 premenopausal
women were asked to take a supplement containing 100 mg Rosmarinus officinalis (rosemary) leaf 5:1 extract; 100 mg Cur-
curma longa (turmeric) root extract standardized to 95% curcumin; 100 mg Cyanara scolymus (artichoke) leaf 6:1 extract;
100 mg Silybum marinum (milk thistle) seed extracted; 100 mg Taraxacum officinalis (dandelion) root 4:1 extract; and 50 mg
Schidandra chinensis (berry) 20:1 extract. Four capsules were to be taken twice a day with meals. Rice powder placebo
capsules were given to a group of 15 premenopausal women using the same dosing regimen. Blood and urine samples were
collected during the early-follicular and mid-luteal phases of study menstrual cycles 1 and 5.
On average, test subjects took 6.3 capsules/day, and controls took 7.1 capsules/day. Compared to the placebo group, the fol-
lowing changes from Cycle 1 to Cycle 5 in early-follicular phase serum hormone concentrations were statistically significant
or borderline significant: decreases in serum dehydroepiandrosterone (-13.2%, p= 0.02); dehydroepiandrosterone sulfate (-
14.6%, p=0.07); androstenedione (-8.6%, p=0.05); and estrone sulfate (-12.0%, p=0.08). No other statistically significant
changes or trends were observed for other serum sex steroid hormones, serum metabolic markers, or urinary estrogen metab-
olites at either phase.
GENOTOXICITY
In vitro, rosemary extract (solvent not specified)
51
and rosmarinus officinalis (rosemary) leaf oil
52
were not mutagenic in an
Ames test, and rosmarinus officinalis (rosemary) leaf extract was not genotoxic in an Ames test, a chromosomal aberration
assay in human lymphocytes, or a gene-locus mutation assay in human lymphocytes
8
(Table 11). In in vivo studies in mice
and rats, oils that were extracted by hydrodistillation induced statistically significant increases in chromosomal aberrations
without gaps in a chromosomal aberration assay at 2000 mg/kg bw, increases in micronucleated polychromatic erythrocytes
(MNPCEs) in several micronucleus tests at 1000 and 2000 mg/kg bw, and increases in DNA damage in a comet assay at
≥300 mg/kg bw;
14,41
however, no genotoxic effects were seen in mice in a micronucleus test at 1500 mg/kg bw/day with
leaves extracted with absolute ethanol.
41
A hydro-alcoholic extract of rosemary was not genotoxic in a chromosomal aberra-
tion assay or a micronucleus test in rats.
53
A mixture containing 19% Rosmarinus officinalis (rosemary) leaves, 71.5% St.
John’s Wort, and 9.5% spirulina induced in mice statistically significant increases in MNPCEs at 760 and 1520 mg/kg
bw/day in a micronucleus test; in frequency of aneuploidy, percent polyploidy, and total percent aberrations with 760 and
1520 mg/kg bw/day in a chromosomal aberration assay; and in frequency of banana-shaped, swollen acrosome, and triangu-
lar head sperm abnormalities and percent total spermatozoa abnormalities at 1520 mg/kg bw/day in a spermatozoa abnormal-
ity assay.
54
Rosmarinus officinalis (rosemary) leaf extract was shown to have anti-mutagenic potential, in vitro, in an Ames test with
Salmonella typhimurium and in Comet assays in a human hepatoma cell line..
51
In vivo, in micronucleus assays, rosmarinus
officinalis (rosemary) leaf extract did not decrease the number of MNPCEs induced in mice by a genotoxic agent.
41
CARCINOGENICITY
Effects on Tumor Promotion
Topical application of methanol and double distilled water extracts of Rosmarinus officinalis (rosemary) leaves statistically
significantly decreased skin tumors in mice; in these studies, 7,12-dimethylbenz[a]anthracene (DMBA) or benzo[a]pyrene
(B(a)P )was used for initiation and TPA
44
or croton oil
55,56
was used for promotion (Table 12). Dietary administration of
1.0% rosmarinus officinalis (rosemary) leaf extract decreased the incidence of palpable mammary tumors in rats caused by
DMBA.
57
IRRITATION AND SENSITIZATION
Skin Irritation/Sensitization
An ointment containing 4.4% rosmarinus officinalis (rosemary) leaf oil (and other essential oils), applied at concentrations up
to 40%, was not irritating to rat skin (Table 13).
58
However, in a rabbit study, occlusive application to intact and abraded
skin produced moderate irritation.
42
In clinical testing, Rosmarinus officinalis (rosemary) leaves produced irritation (scores of +/-, +, or ++) in 44/234 patients
with contact dermatitis or eczema (Table 13).
59
A supercritical extract and the absolute of Rosmarinus officinalis (rosemary)
leaves were considered weak irritants in a small study with test populations of 20-25 subjects; the extracts were not
phototoxic.
9
Formulations containing up to 0.2% rosmarinus officinalis (rosemary) leaf extract were not irritants or
sensitizers.
60-62
Rosmarinus officinalis (rosemary) leaf oil, 10% in petrolatum, was not an irritant in a 48-h closed patch test,
or a sensitizer in a maximization study;
42
a formulation containing 1.5% rosmarinus officinalis (rosemary) leaf oil was not an
irritant or a sensitizer in an HRIPT.
63
Phototoxicity
Rosmarinus Officinalis (Rosemary) Leaf Extract
The phototoxicity of rosmarinus officinalis (rosemary) leaf extract extracted with supercritical CO
2
, as a concrete (insoluble
wax) extracted in hexane, or as a concrete extracted in hexane, was evaluated.
9
Photopatch tests were performed on two of
three test sites; one site was irradiated with 10 J/cm
2
UVA and the second site with 75% of the minimal erythema dose of
UVB. The test sites were scored after 48 and 72 h, and were compared to the non-irradiated site. None of the extracts were
phototoxic.
Case Reports
Several cases of allergic reactions to Rosmarinus officinalis (rosemary) have been reported (Table 14).
64-72
In some of the
studies, follow-up patch testing included photopatch tests; generally, reactions were stronger in the photopatch tests when
compared to standard testing.
68,69
Some of the follow-up patch testing included carnosol; testing with 0.1 and 1.0% carnosol
resulted in positive reactions.
65,69

SUMMARY
This report addresses the safety of 10 Rosmarinus officinalis (rosemary)-derived ingredients as used in cosmetics. Most of
the ingredients included in this review are extracts, essential oils, powders, or waters derived from a defined part of the
Rosmarinus officinalis (rosemary) plant. The Rosmarinus officinalis (rosemary)-derived ingredients are reported to have a
number of functions in cosmetics, and the most common functions are as a skin conditioning agent or as a fragrance ingredi-
ent. According to VCRP data obtained from the FDA, rosmarinus officinalis (rosemary) leaf extract has the most uses, 729,
followed by rosmarinus officinalis (rosemary) leaf oil, 474 uses, and rosmarinus officinalis (rosemary) extract, 404 uses.
Most of the reported use concentrations for Rosmarinus officinalis (rosemary)-derived ingredients are well below 0.1%.
However, rosmarinus officinalis (rosemary) leaf extract has higher concentrations of use reported, specifically, use at up to
10% in body and hand products and 3% in eye shadow formulations and bath soaps and detergents. Rosmarinus officinalis
(rosemary) flower/leaf/stem water is the only ingredient not reported to be used.
Rosmarinus officinalis (rosemary) extract is prepared by extraction from the leaves of Rosmarinus officinalis with acetone,
ethanol, hexane, a combination of hexane and ethanol (in a two-step process), or supercritical CO
2
; it can also be prepared
from a deodorized or partially deodorized ethanol extract of rosemary. Additional methods include extraction with absolute
ethanol (resulting in an absolute) or a collection of the insoluble waxes (resulting in a concrete).
Rosmarinus officinalis L. is composed of an array of constituents, primarily phenolic acids, flavonoids, monoterpenes, diter-
penes, diterpenoids, and triterpenes. The principal antioxidative components of rosmarinus officinalis (rosemary) leaf extract
are the phenolic diterpenes carnosol and carnosic acid. The actual amount of constituents present varies according to the
stage of development, variety of plant, season harvested, origin of the leaves, and extraction method.
Rosemary oil increased the permeation of aminophylline through human skin, but the increase was not as great as that seen
with 50% ethanol.
The acute toxicity of Rosmarinus officinalis (rosemary)-derived ingredients is not very remarkable. The dermal LD
50
of ros-
marinus officinalis (rosemary) leaf oil is > 10 ml/kg. The oral LD
50
of rosmarinus officinalis (rosemary) leaves is >2 g/kg,
of rosmarinus officinalis (rosemary) leaf extract is >8.5 g/kg, and of rosmarinus officinalis (rosemary) leaf oil is 5.5 g/kg bw.
A number of oral repeated-dose toxicity studies were performed in mice and in rats with Rosmarinus officinalis (rosemary)
leaves extracted in a various solvents. Doses as high as 14.1 g/kg bw rosmarinus officinalis (rosemary) leaf extract were
tested (5 days by gavage), and some studies were performed for up to 3 mos (dietary) with doses of up to 400 mg/kg bw/day.
Increases in absolute and relative liver-to-body weights were observed in many of the studies, independent of the extraction
method; these changes were shown to be reversible, and no other signs of toxicity were observed. Oral administration of
rosmarinus officinalis (rosemary) leaf oil with carbon tetrachloride, but not without, resulted in an increase in liver weights.
Rosmarinus officinalis (rosemary) leaf extract has been shown to have anti-inflammatory activity. Rosmarinus officinalis
(rosemary) leaf extract inhibited a TPA-induced increase in the number of epidermal cell layers and epidermal thickness in
mouse skin.
A high dose (500 mg/kg/day) of Rosmarinus officinalis (rosemary) leave extract was a reproductive toxicant in a dietary
study in male rats. In a study in gravid female Wistar rats, no statistically significant changes were observed after oral dosing
with 26 mg/day of a 30% aq. rosmarinus officinalis (rosemary) flower/leaf/stem extract during preimplantation or during
organogenesis. In a dietary study in ovariectomized CD-1 mice, 2% of a methanol extract of Rosmarinus officinalis (rose-
mary) leaves inhibited the uterine response in a statistically significant manner.
In a clinical study investigating the effects on sex steroid hormones and metabolic markers of a botanical supplement contain-
ing 100 mg Rosmarinus officinalis (rosemary) leaf 5:1 extract (and other botanical ingredients) in premenopausal women, a
few changes were found. Overall, the changes were not remarkable.
In vitro, rosemary extract (solvent not specified) and rosmarinus officinalis (rosemary) leaf oil were not mutagenic in an
Ames test, and rosmarinus officinalis (rosemary) leaf extract was not genotoxic in an Ames test, a chromosomal aberration
assay in human lymphocytes, or a gene-locus mutation assay in human lymphocytes. In in vivo studies in mice and rats, oils
that were extracted by hydrodistillation induced statistically significant increases in chromosomal aberrations without gaps in
a chromosomal aberration assay at 2000 mg/kg bw, increases in micronucleated polychromatic erythrocytes (MNPCEs) in
several micronucleus tests at 1000 and 2000 mg/kg bw, and increases in DNA damage in a comet assay at ≥300 mg/kg bw;
however, no genotoxic effects were seen in mice in a micronucleus test at 1500 mg/kg bw/day with leaves extracted with ab-
solute ethanol. A hydro-alcoholic extract of rosemary was not genotoxic in a chromosomal aberration assay or a micronucle-
us test in rats. A mixture containing 19% Rosmarinus officinalis (rosemary) leaves, 71.5% St. John’s Wort, and 9.5% spiru-
lina induced in mice statistically significant increases in MNPCEs at 760 and 1520 mg/kg bw/day in a micronucleus test; in
frequency of aneuploidy, percent polyploidy, and total percent aberrations with 760 and 1520 mg/kg bw/day in a chromo-
somal aberration assay; and in frequency of banana-shaped, swollen acrosome, and triangular head sperm abnormalities and
percent total spermatozoa abnormalities at 1520 mg/kg bw/day in a spermatozoa abnormality assay.
Rosmarinus officinalis (rosemary) leaf extract was shown to have anti-mutagenic potential in vitro. In vivo, in micronucleus
assays, rosmarinus officinalis (rosemary) leaf extract did not decrease the number of MNPCEs induced by a genotoxic agent.

Topical application of methanol and double distilled water extracts of rosmarinus officinalis (rosemary) leaves statistically
significantly decreased skin tumors in mice; in these studies, DMBA or benzo[a]pyrene was used for initiation and TPA or
croton oil was used for promotion. Dietary administration of 1.0% rosmarinus officinalis (rosemary) leaf extract decreased
the incidence of palpable mammary tumors in rats caused by DMBA.
An ointment containing 4.4% rosmarinus officinalis (rosemary) leaf oil (and other essential oils), applied at concentrations up
to 40%, was not irritating to rat skin. However, in a rabbit study, occlusive application to intact and abraded skin produced
moderate irritation.
In clinical testing, Rosmarinus officinalis (rosemary) leaves produced irritation (scores of +/-, +, or ++) in 44/234 patients
with contact dermatitis or eczema. A supercritical extract and the absolute of Rosmarinus officinalis (rosemary) leaves were
considered weak irritants in a small study with test populations of 20-25 subjects; the extracts were not phototoxic. Formula-
tions containing up to 0.2% rosmarinus officinalis (rosemary) leaf extract were not irritants or sensitizers. Rosmarinus
officinalis (rosemary) leaf oil, 10% in petrolatum, was not an irritant in a 48-h closed patch test, or a sensitizer in a maximi-
zation study in 25 subjects; a formulation containing 1.5% rosmarinus officinalis (rosemary) leaf oil was not an irritant or a
sensitizer in an HRIPT in 104 subjects.
Several cases of allergic reactions to Rosmarinus officinalis (rosemary) have been reported. In some of the studies, follow-up
patch testing included photopatch tests; generally, reactions were stronger in the photopatch tests, compared to standard
testing. Some also evaluated the effect of carnosol; testing with 0.1 and 1.0% carnosol resulted in positive reactions.
DISCUSSION
Upon initial review of the safety assessment of Rosmarinus officinalis (rosemary)-derived ingredients, the Panel issued an
Insufficient Data Announcement requesting the following:
1. Dermal sensitization data for 10% rosmarinus officinalis (rosemary) leaf extract (i.e., a human repeated-insult patch
test in a sufficient number of subjects at concentration of use);
2. Chemical characterization of the flower, if available;
3. Additional information on the deodorizing process performed during preparation of some of the ingredients,
including information on what by-products may form; and
4. Information as to why the PDR of Herbal Medicines states that rosemary preparations should not be used during
pregnancy.
The majority of these data were not received. Rosmarinus officinalis is GRAS as a spice, and although that alleviates the
concern of oral exposure with cosmetic use, dermal irritation and sensitization data are necessary to determine safety.
Additional information on the deodorizing process that is part of the preparation of some of the ingredients also was not
received. After further discussion, the Panel stated that because the deodorizing process is part of the preparation of food-
grade rosmarinus officinalis (rosemary) extract, and because data are included in this safety assessment on some ingredients
that were deodorized and no adverse effects were found, the Panel was not concerned with obtaining additional information
on this process or the by-products that might form.
The Panel did note that because botanical ingredients, derived from natural plant sources, are complex mixtures, there is con-
cern that multiple botanical ingredients may each contribute to the final concentration of a single constituent. Therefore,
when formulating products, manufacturers should avoid reaching levels in final formulation of plant constituents that may
cause sensitization or other adverse effects. Specific examples of constituents that could possibly induce sensitization are
linalool or monoterpenes, and those that could possibly cause adverse effects are caffeic acid and terpenes, such as thujone,
limonene, and methyleugenol.
The Panel expressed concern about pesticide residues and heavy metals that may be present in botanical ingredients. They
stressed that the cosmetics industry should continue to use current good manufacturing practices (cGMPs) to limit impurities.
One study evaluated the irritation potential of Rosmarinus officinalis (rosemary) leaves in patients with contact dermatitis or
eczema. The Panel stated that because the test subjects were patients with eczematous skin, the report of irritation could not
be interpreted for relevance to cosmetic use.
The Panel discussed the positive results observed in a reproductive toxicity study in male rats fed 500 mg/kg/day rosmarinus
officinalis (rosemary) leaf extract, as well as the caution in the PDR for Herbal Medicines stating that rosemary preparations
should not be used as a drug during pregnancy. The effects in the rat study were observed at exposure concentrations that
would be well above those used in cosmetic products, and the PDR refers to the use of rosemary as a drug at very high con-
centrations. Because these effects were observed only at very high concentrations, and because no statistically significant
effects were reported in a study in rats dosed orally with 26 mg/day of a 30% aq. extract of rosmarinus officinalis (rosemary)
flower/leaf/stem extract, reproductive and developmental toxicity is not a concern with cosmetic use of Rosmarinus
officinalis (rosemary)-derived ingredients, which are mostly used at very low concentrations.

Finally, the Panel discussed the issue of incidental inhalation exposure to Rosmarinus officinalis (rosemary)-derived ingredi-
ents. The Panel stated that although there were no inhalation data available, the Rosmarinus officinalis (rosemary)-derived
ingredients are used at very low concentrations in products that could incidentally be inhaled; e.g., rosmarinus officinalis
(rosemary) leaf extract is used in other fragrance preparations and rosmarinus officinalis (rosemary) extract is used in face
powders. The Panel noted that in aerosol products, 95% – 99% of droplets/particles would not be respirable to any appreci-
able amount. Furthermore, droplets/particles deposited in the nasopharyngeal or bronchial regions of the respiratory tract
present no toxicological concerns based on the chemical and biological properties of these ingredients. Coupled with the
small actual exposure in the breathing zone and the concentrations at which the ingredients are used, the available informa-
tion indicates that incidental inhalation would not be a significant route of exposure that might lead to local respiratory or
systemic effects. A detailed discussion and summary of the Panel’s approach to evaluating incidental inhalation exposures to
ingredients in cosmetic products is available at http://www.cir-safety.org/cir-findings
.
CONCLUSION
The CIR Expert Panel concluded that the following ten Rosmarinus officinalis (rosemary)-derived ingredients are safe in the
present practices of use and concentration in cosmetics described in this safety assessment when formulated to be non-
sensitizing:
Rosmarinus Officinalis (Rosemary) Extract
Rosmarinus Officinalis (Rosemary) Flower Extract
Rosmarinus Officinalis (Rosemary) Flower/Leaf/Stem Extract
Rosmarinus Officinalis (Rosemary) Flower/Leaf/Stem Water*
Rosmarinus Officinalis (Rosemary) Leaf
Rosmarinus Officinalis (Rosemary) Leaf Extract
Rosmarinus Officinalis (Rosemary) Leaf Oil
Rosmarinus Officinalis (Rosemary) Leaf Powder
Rosmarinus Officinalis (Rosemary) Leaf Water
Rosmarinus Officinalis (Rosemary) Water
*Not reported to be in current use. If this ingredient was to be used in the future, the expectation is that it would be used in product
categories and at concentrations comparable to others in this group.
FIGURES
Figure 1. Principal diterpenes
O
H
H
O
C
H
3
C
H
3
H
3
C
C
H
3
O
O
1a. Carnosol
O
H
H
O
C
H
3
C
H
3
H
3
C C
H
3
H
O
O
1b. Carnosic acid
O
H
H
O
C
H
3
C
H
3
H
3
C C
H
3
O
O
H
O
1c. Rosmanol
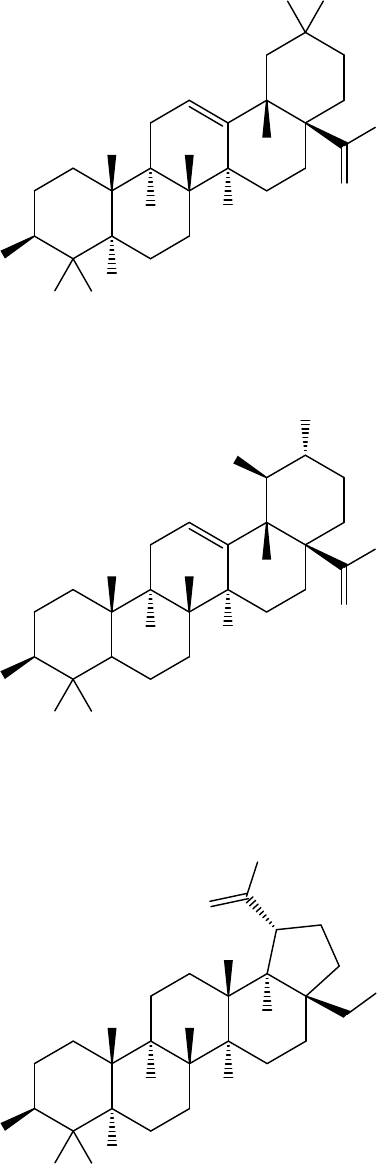
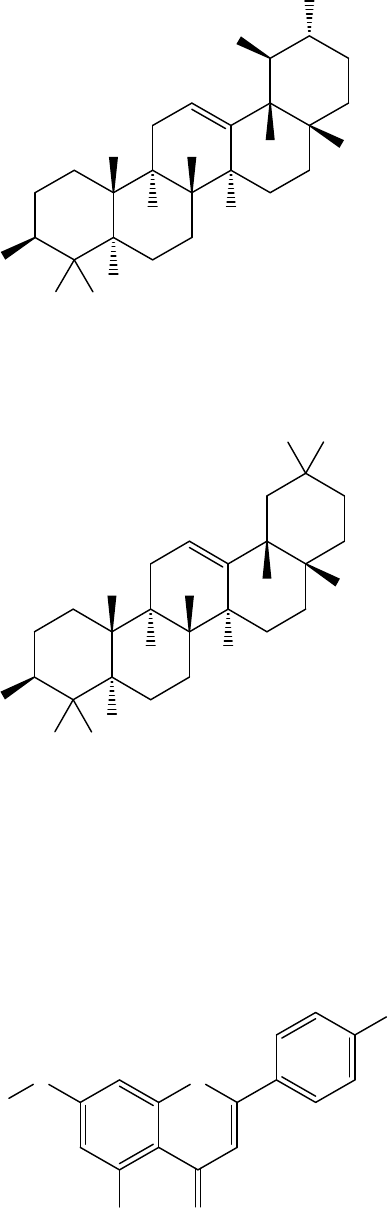
Figure 2. Principal triterpenes
H
O
C
H
3
H
3
C
H
C
H
3
C
H
3
H
3
C C
H
3
H
C
H
3
O
O
H
H
2a. Oleanolic acid
H
O
C
H
3
H
3
C
C
H
3
C
H
3
C
H
3
H
3
C
H
C
H
3
O
O
H
H
2b. Ursolic acid
O
H
C
H
3
C
H
3
H
C
H
3
H
3
C
H
O
C
H
3
H
H
H
C
H
3
H
2
C
2c. Betulin
C
H
3
H
O
C
H
3
H
3
C
H
C
H
3
C
H
3
H
C
H
3
H
C
H
3
H
3
C
2d. α-Amyrin
C
H
3
H
O
C
H
3
H
3
C
H
C
H
3
C
H
3
H
C
H
3
H
C
H
3
H
3
C
2e. β-Amyrin
Figure 3. Principal flavonoids
O
O
O
H
O
H
O
H
3
C
3a. Genkwanin
O
H
O
O
O
H
3
C
O
H
3
C
O
O
O
H
O
H
O
H
O
H
3b. Cirsimarin
O
O
O
H
O
H
H
O
O
H
3c. Luteolin
O
O
O
O
H
H
O
O
H
C
H
3
3d. Diosmetin
O
O
O
H
O
H
H
O
3e. Apigenin
Figure 4. Phenolic acids
H
O
H
O
O
O
H
4a. Caffeic acid
H
O
H
O
O
O
O
H
O
H
O
H
O
H
O
4b. Chlorogenic acid
H
O
H
O
O
O
O
H
O
H
O
H
O
H
O
4c. Neochlorogenic acid
H
O
H
O
O
O
O
H
O
H
O
H
O
4d. Labiatic acid
Figure 5. Principal Volatiles
O
C
H
3
C
H
3
H
3
C
5a. 1,8-Cineole
O
H
3
C
C
H
3
H
3
C
5b. Camphor
C
H
3
H
3
C
C
H
3
5c. α-Pinene
H
O
H
3
C
H
3
C
C
H
3
5d. Borneol

TABLES
Table 1. Definitions and reported functions
Ingredient (CAS No.)
Definition
1
Reported Function(s)
1
Rosmarinus Officinalis (Rosemary) Extract
(84604-14-8)
the extract of the whole plant Rosmarinus officinalis
skin-conditioning agent – misc
Rosmarinus Officinalis (Rosemary) Flower
Extract
the extract of the flowers of Rosmarinus officinalis
antioxidant; deodorant agents; skin-
conditioning agents – misc
Rosmarinus Officinalis (Rosemary)
Flower/Leaf/Stem Extract
the extract of the flowers, leaves and stems of
Rosmarinus officinalis
fragrance ingredients; skin-conditioning
agents - misc
Rosmarinus Officinalis (Rosemary)
Flower/Leaf/Stem Water
the aqueous solution of the steam distillates obtained
from the flowers, leaves and stems of
Rosmarinus
officinalis
fragrance ingredient
Rosmarinus Officinalis (Rosemary) Leaf
the leaf of Rosmarinus officinalis
skin-conditioning agents – misc
Rosmarinus Officinalis (Rosemary) Leaf
Extract (84604-14-8)
the extract of the leaves of Rosmarinus officinalis
antimicrobial agents; antioxidant; fragrance
ingredients; skin-conditioning agents -
miscellaneous; skin-conditioning agents –
occlusive
Rosmarinus Officinalis (Rosemary) Leaf
Oil (8000-25-7)
the essential oil obtained from the flowering tops and
leaves of Rosmarinus officinalis
fragrance ingredients; skin-conditioning
agents – misc
Rosmarinus Officinalis (Rosemary) Leaf
Powder
the powder derived from the dried, ground leaves of
Rosmarinus officinalis
flavoring agents
Rosmarinus Officinalis (Rosemary) Leaf
Water
an aqueous solution of the steam distillate obtained from
the leaves of Rosmarinus officinalis
fragrance ingredient
Rosmarinus Officinalis (Rosemary) Water
an aqueous solution of the steam distillate obtained from
Rosmarinus officinalis
fragrance ingredient
Table 2. Chemical and physical properties
Property
Description
Reference
Rosmarinus Officinalis (Rosemary) Leaf
odor
strongly aromatic
36
Rosmarinus Officinalis (Rosemary) Leaf Extract
physical state and appearance
powder or liquid
colorless, volatile oil
dark brown viscous liquid with a characteristic smell and taste (as the extract (and)
Helianthus Annuus Seed Oil)
7
8
10,11
solubility
insoluble in water
7
refractive index
1.4710 - 1.4740
16
density
0.9165 - 0.9220
16
Rosmarinus Officinalis (Rosemary) Leaf Oil
physical state and appearance
colorless or pale yellow liquid with characteristic odor and a warm, camphoraceous
taste
colorless, pale yellow, or pale green liquid with a camphorous odor
7,35
73
solubility
almost insoluble in water
soluble in most vegetable oils; insoluble in alcohol and in propylene glycol
35
7
density (d
25
25
)
0.894-0.912
0.907-0.920
35
73
index of refraction (n
D
20
)
1.464-1.476
35
Rosmarinus Officinalis (Rosemary) Leaf Powder
physical state and appearance
greyish-green to yellowish-green powder
36

Table 3. Chemical constituents by plant part (ppm)
74
Constituent*
Plant
Leaf
Flower
Shoot
Resin,
Exudate, Sap
Essential Oil
carbohydrates
640,600-
704,660
-
-
-
-
-
fiber
165,420-
206,338
-
-
-
-
-
fat
134,020-
187,418
-
-
-
-
-
water
77,900-108,300
-
-
-
-
-
ash
61,900-75,570
-
-
-
-
-
protein
40,700-62,568
-
-
-
-
-
ursolic acid
28,000-41,000
-
-
20
-
-
rosmarinic acid
25,000
3500
-
13,500
-
-
EO
3300-25,000
-
-
-
-
-
calcium
10,919-16,150
-
-
-
-
-
potassium
8842-11,284
-
-
-
-
-
oleanolic acid
10,500
-
-
20
-
-
carnosol
-
530-9803
cineole
168-9728
-
-
-
-
-
1,8-cineole
8125
-
-
-
-
-
camphor
60-5800
-
-
-
-
-
myrcene
25-5605
-
-
-
-
-
bornyl acetate
5054
-
-
-
-
-
α –pinene
235-4750
-
-
-
-
-
borneol
12-4237
-
-
-
-
-
magnesium
2142-2483
-
-
-
-
-
rosmaric acid
3000-3500
-
-
-
-
-
camphene
23-2350
-
-
-
-
-
β-caryophyllene
12-2075
-
-
70-2075
toluene
436-2071
-
-
-
-
-
limonene
1950
-
-
-
-
-
α –terpineol
24-1555
-
-
-
-
-
β-pinene
17-1425
-
-
-
-
-
phosphorus
490-1000
-
-
-
-
-
p-cymene
25-950
-
-
-
-
-
carvone
16-760
-
-
-
-
-
α-humulene
-
-
-
725
salicylates
-
70-680
-
-
-
-
ascorbic acid
612-673
-
-
-
-
-
α-amorphene
70-665
-
-
-
-
-
γ-muurolene
70-665
1
-
-
-
-
phytosterols
580-640
-
-
-
-
-
sodium
462-592
-
-
-
-
-
linalool
585
-
-
-
-
-
α –terpinene
4-555
-
-
-
-
-
terpinen-4-ol
4-521
-
-
-
-
-
α –thujene
1-475
-
-
-
-
-
δ-terpineol
7-418
-
-
-
-
-
iron
220-400
-
-
-
-
-
α –thujone
84-399
-
-
-
-
-
(E)-β-ocimene
-
-
-
380
verbenone
10-375
-
-
-
-
-
geraniol
50-370
-
-
-
-
-
3-hexanone
74-351
-
-
-
-
-
terpinolene
12-350
-
-
-
-
-
caryophyllene
16-340
-
-
-
-
-
δ-3-carene
330
-
-
-
-
-
fenchone
250
-
-
-
-
-
β-thujone
11-209
-
-
-
-
-
β-elemene
-
-
-
3-200
sabinene
190
-
-
-
-
-
mesityl alcohol
40-190
-
-
-
-
-
linalool acetate
32-152
-
-
-
-
-
α –phellandrene
133
-
-
-
-
-
α- fenchyl alcohol
28-133
-
-
-
-
-
p-menth-3-en-1-ol
28-133
-
-
-
-
-
3,5,5-trimethylhexan-1-ol
28-133
-
-
-
-
-
trans-ocimene
4-130
-
-
-
-
-
cis-pinan-3-one
-
17-110
-
-
-
-
4-terpinenyl-acetate
-
12-110
-
-
-
-
safrole
32-95
-
-
-
-
-

Table 3. Chemical constituents by plant part (ppm)
74
Constituent*
Plant
Leaf
Flower
Shoot
Resin,
Exudate, Sap
Essential Oil
cis-β-terpineol
20-95
-
-
-
-
-
α- fenchyl acetate
20-95
-
-
-
-
-
longifolene
20-95
-
-
-
-
-
isoborneol
7-95
-
-
-
-
-
rosmanol
-
92
-
-
-
-
(+)-limonene
16-76
-
-
-
-
-
δ-cadinene
75
-
-
-
-
-
caryophyllene oxide
75
-
-
-
-
-
(Z)-β-ocimene
-
-
-
75
-
-
trans-pinocarveol
-
32-42
-
-
-
-
3-octanone
20-40
-
-
-
-
-
boron
22-39
-
-
-
-
-
zinc
30-38
-
-
-
-
-
AR-curcumene
8-38
-
-
-
-
-
methyl heptenone
8-38
-
-
-
-
-
myrtenol
8-38
-
-
-
-
-
lavandulol
7-34
-
-
-
-
-
trans-β-terpineol
7-34
-
-
-
-
-
trans-myrtenol
-
32
-
-
-
-
benzyl alcohol
7-32
-
-
-
-
-
elemol
7-32
-
-
-
-
-
γ-eudesmol
7-32
-
-
-
-
-
rosmadial
-
30
-
-
-
-
α-amyrenone
-
-
-
30
-
-
β-amyrenone
-
-
-
30
-
-
epirosmanol
-
26
-
-
-
-
β-carotene
19-21
-
-
-
-
-
rofficerone
-
-
-
20
-
-
trans-sabinene hydrate
19
-
-
-
-
-
manganese
18-19
-
-
-
-
-
cis-α-bisabolene
4-19
-
-
-
-
-
isopinocarveol
4-19
-
-
-
-
-
isopulegol
4-19
-
-
-
-
-
3-octanol
4-19
-
-
-
-
-
dimethyl styrene
1-19
-
-
-
-
-
7-methoxy-rosmanol
-
-
-
18
isorosmanol
-
-
17
-
-
-
cis-myrtenol
-
11-17
-
-
-
-
cisimaritrin
-
-
-
16
-
-
α-amyrin
NS
-
-
13
-
-
β-amyrin
NS
-
-
13
-
-
botulin
-
-
-
12.1
-
-
α –muurolene
NS
2-12
-
-
-
-
3-o-acetyloleanolic acid
-
-
-
11
-
-
3-o-acetylursolic acid
-
-
-
11
-
-
niacin
10-11
-
-
-
-
-
peperitenone
-
4-8
-
-
-
-
eugenol methyl ether
-
5-7
-
-
-
-
copper
5-6
-
-
-
-
-
thiamin
5-6
-
-
-
-
-
carvacrol
NS
5-6
-
-
-
-
α -terpinenyl acetate
-
5-6
-
-
-
-
allo-aromadendrene
-
4-5
-
-
-
-
neo-thujol
-
1.5-5
-
-
-
-
calamenene
1-5
-
-
-
-
-
trans-carveol
1-5
-
-
-
-
-
p-cymen-8-ol
1-5
-
-
-
-
-
nopol
1-5
-
-
-
-
-
γ-candinene
NS
1-5
-
-
-
-
α-copaene
-
2-4
-
-
NS
-
epi-
α
-bisabolol
-
3
-
-
-
-
sabinyl acetate
-
1.5
-
-
-
-
β-gurjunene
-
0.5
-
-
-
-
cis-sabinene hydrate
NS
0.4
-
-
-
-
β-phellandrene
trace
-
-
-
-
-
tricyclene
trace
-
-
-
-
-
α-fenchol
-
trace
-
-
-
-
p-menth-cis-en-1-ol
-
trace
-
-
-
-

Table 3. Chemical constituents by plant part (ppm)
74
Constituent*
Plant
Leaf
Flower
Shoot
Resin,
Exudate, Sap
Essential Oil
p-menth-trans-en-1-ol
-
trace
-
-
-
-
trans-anethole
NS
-
-
-
-
-
apigen-7-glucoside
NS
-
-
-
-
-
betulin
NS
-
-
-
-
-
bornylene
NS
-
-
-
-
-
cadalene
NS
-
-
-
-
-
caffeic acid
NS
-
-
-
-
-
calacorene
NS
-
-
-
-
-
carnosic acid
NS
-
-
-
-
-
chlorogenic acid
NS
-
-
-
-
-
cirsilion
NS
-
-
-
-
-
cubenene
NS
-
-
-
-
-
diosmetin
NS
-
-
-
-
-
epi-
α
-amyrin
NS
-
-
-
-
-
eriodictiol
NS
-
-
-
-
-
ethanol
NS
-
-
-
-
-
α-fenchene
NS
-
-
-
-
-
β-fenchene
NS
-
-
-
-
-
genkwanin-4’-methyl ether
NS
-
-
-
-
-
glycolic acid
NS
-
-
-
-
-
genkwanin
NS
-
-
-
-
-
hesperidin
NS
-
-
-
-
-
hispidulin
NS
-
-
-
-
-
hispiduloside
NS
-
-
-
-
-
humulene epoxide I
NS
-
-
-
-
-
humulene epoxide II
NS
-
-
-
-
-
5-hydroxy-4',7-dimethoxyflavone
NS
-
-
-
-
-
hydroxybenzoic acid-4-
β
-D-glucoside
NS
-
-
-
-
-
4-hydroxybenzoyl glucoside
NS
-
-
-
-
-
α-hydroxyhydrocaffeic acid
NS
-
-
-
-
-
2-
β
-hydroxyoleanolic acid
NS
-
-
-
-
-
3-β-hydroxyurea-12,20(30)-dien-17-on
acid
NS
-
-
-
-
-
19-
α
-hydroxyursolic acid
NS
-
-
-
-
-
isobornyl acetate
NS
-
-
-
-
-
isobutyl acetate
NS
-
-
-
-
-
isorosmaricine
NS
-
-
-
-
-
labiatic acid
NS
-
-
-
-
-
ledene
NS
-
-
-
-
-
luteolin
NS
NS
-
-
-
-
luteolin-7-glucoside
NS
-
-
-
-
-
6-methoxy-genkwanin
NS
-
-
-
-
-
6-methoxy-luteolin
NS
-
-
-
-
-
6-methoxy-luteolin-7-glucoside
NS
-
-
-
-
-
6-methoxyluteolin-7-methyl ether
NS
-
-
-
-
-
methyl ether
NS
-
-
-
-
-
methyl eugenol
NS
-
-
-
-
-
N-methyl rosmaricine
NS
-
-
-
-
-
neo-chlorogenic acid
NS
-
-
-
-
-
nepetin
NS
-
-
-
-
-
nepetrin
NS
-
-
-
-
-
1-octen-3-ol
NS
-
-
-
-
-
picrosalvin
NS
-
-
-
-
-
rosmadiol
NS
-
-
-
-
-
rosmaricine
NS
-
-
-
-
-
rosmaridiphenol
NS
-
-
-
-
-
rosmarinol
NS
-
-
-
-
-
rosmariquinone
NS
-
-
-
-
-
salvigenin
NS
-
-
-
-
-
santene
NS
-
-
-
-
-
salicylic-acid-2-β-D-glucoside
NS
-
-
-
-
-
α –selinene
NS
-
-
-
-
-
sinensetin
NS
-
-
-
-
-
β-sitosterol
NS
-
-
-
-
-
squalene
NS
-
-
-
-
-
syringic-acid-4-β-D-glucoside
NS
-
-
-
-
-
tannin
NS
-
-
-
-
-
thymol
NS
-
-
-
-
-

Table 3. Chemical constituents by plant part (ppm)
74
Constituent*
Plant
Leaf
Flower
Shoot
Resin,
Exudate, Sap
Essential Oil
trimethylalkane
NS
-
-
-
-
-
o-o-N-trimethylrosmaricine
NS
-
-
-
-
-
vanillic-acid-4-β-D-glucoside
NS
-
-
-
-
-
verbenol
NS
-
-
-
-
-
betulinic acid
-
NS
-
-
-
-
δ-4-carene
-
NS
-
-
-
-
diosmin
-
NS
-
-
-
-
7-ethoxy-rosmanol
-
NS
-
-
-
-
luteolin-3’-o-(3”-o-acetyl)-
β
-D-
glucuronide
-
NS
-
-
-
-
luteolin-3’-o-(4”-o-acetyl)-
β
-D-
glucuronide
-
NS
-
-
-
-
luteolin-3’-o-
β
-D-glucuronide
-
NS
-
-
-
-
monomethyl alkane
-
NS
-
-
-
-
pristane
-
NS
-
-
-
-
protocatechuic-acid-4-β-D-glucoside
-
NS
-
-
-
-
pectin
-
-
-
NS
-
-
acetic acid
-
-
-
-
NS
-
butan-2-ol
-
-
-
-
NS
-
caproic acid
-
-
-
-
NS
-
deca-trans-2,trans-4-dien-1-al
-
-
-
-
NS
-
hept-trans-2-en-1-al
-
-
-
-
NS
-
heptan-1-al
-
-
-
-
NS
-
heptan-2-ol
-
-
-
-
NS
-
heptanoic acid
-
-
-
-
NS
-
hexan-1-al
-
-
-
-
NS
-
hexan-1-ol
-
-
-
-
NS
-
3-methyl-butan-1-ol
-
-
-
-
NS
-
β-ocimene
-
-
-
-
NS
-
octan-1-ol
-
-
-
-
NS
-
octane-2,3-dione
-
-
-
-
NS
-
octanoic acid
-
-
-
-
NS
-
pentan-1-al
-
-
-
-
NS
-
pentan-1-ol
-
-
-
-
NS
-
pentan-2-ol
-
-
-
-
NS
-
zingiberene
-
-
-
-
NS
-
dipentene
-
-
-
-
-
NS
*constituents reported in ppm
NS – amount not specified
“ – “ means not reported

Table 4. Constituent data by plant part
Reference
Plant part not specified
- volatile oil (0.5-2.5%): 1,8-cineole (20-50%); camphor (10-25%); α-pinene (up to 25%); other monoterpenes (including borneol
and limonene)
- rosmarinic acid
- diterpene bitter substances: carnosol; carnosolic acid (picrosalvin); isorosmanol; rosmanol; rosmadiol; rosmaridiphenol
rosmariquinone
- triterpene acids: ursolic acid; oleanolic acids; rosmanol; 7-ethoxyrosmanol; betulic acid; carnosol; traces of 19α-
hydroxyursolic, 2β-hydroxyoleanolic, and 3β-hydroxyurea-12,20(30)-dien-17-oic acids
- triterpene alcohols: α-amyrin; β-amyrin; betulin
- flavonoids: luteolin; genkwanin (7-O-methlylapigenin); diosmetin; diosmin; genkwanin-4’-methyl ether; 6-methoxygenkwanin;
6-methyoxyluteolin; 6-methoxyluteolin-7-glucoside; 6-methoxyluteolin-7-methylether; hispidulin; apigenin
- corresponding glycosides
2,4,5
Leaf
- volatile oil (1.0-2.5%): 1,8-cineole (15-55%); camphor (5-25%); α-pinene (9-26%); camphene (2.5-12%); β-pinene (2-9%);
borneol (1.5-6%); limonene (1.5-5%); bornyl acetate (1-5%); isobutyl acetate; β-caryophyllene; p-cymene; linalool; myrcene; α-
terpineol (12-24%); verbenol
- diterpenes (up to 4.6%): carnosic acid; carnosol; isorosmanol; rosmadiol; rosmaridiphenol; rosmanol; rosmariquinone;
triacetylrosmanol; dimethylrosmanol
- triterpenes: oleanolic acid (10%); ursolic acid (2-5%); α-amyrin; β-amyrin; epi-α-amyrin; 19-α-ursolic acid; 2-β-hydroxy
oleanolic acid; betulin
- phenolic acids (2-3%): rosmarinic acid (3.5%); chlorogenic acid; neo-chlorogenic acid; caffeic acid; labiatic acid
- flavonoids: genkwanin; cirsimarin; diosmetin; apigenin; luteolin; nepetin; nepitrin; diosmin; hesperidin; homoplantiginin;
phegopolin
- alkaloids: rosmaricin; isorosmaricine
- tannins
- saponins
- glycolic acid and glyceric acid
- vitamin C; vitamin P
- choline
5,22,35,36,75
Leaf Oil
- α-pinene (8-25%), β-pinene (7.6%); eucalyptol (20-50%), camphor (10-27.6%), borneol (20%), 1,8-cineole (15.8%); β-myrcene
(10%); camphene (5.2-5.8%), limonene (5.9%); p-cymene (4.8%); β-caryophyllene (3.1%); verbenone (2.6%); linalool
35,40,41,73,76
- From one sample (concentration in the oil):
- monoterpenoid esters (24.76%): bornyl acetate (20.86%); linalyl acetate (2.90%); terpinyl acetate (1.0%)
- monoterpenoid alcohols (23.78%): borneol (8.25%); linalool (5%); isoborneol (4.13%); γ-terpineol (2.94%); α-terpineol
(1.9%); terpinene 4-ol (1.43%); carveol (0.13%)
- monoterpenoid ketones (18.67%): L-camphor (14.06%); verbenone (2.56%); carvone (1.9%); α-thujone (0.15%)
- monoterpenoid ethers (10.86%): methyl eugenol (5.46%); 1,8-cineole (5.05%); linalool oxide (0.35%)
- sesquiterpenes (8.96%): β-caryophellene (4.31%); caryophellene oxide (3.19%); spathulenol (1.27%); α-copene (0.19%)
- phenols (4.06%): thymole (3.06%); carvacrol (0.91%); methyl chavicol (0.19%)
- monoterpenes (3.4%): p-cymene (1.15%);
α
-pinene (0.95%); camphene (0.81%); myrcene (0.22%); limonene (0.15%)
41
Flower
- carnosic acid, carnosol, 12-O-methylcarnosic acid, at levels that are less than that found in the leaves
- highest levels of rosmarinic acid are found in the flower
- the flavones genkawanin and isoscutellarein 7-O-glucoside were not found in a DMSO extract
23
Seed
- 560.5 µg/g
α
-tocotrienol; 300.3 µg/g β-tocotrienol; 109.4 µg/g
γ
-tocotrienol
77
Essential Oil
- mainly monoterpenes:
α
-pinene (20.1-21.7%),
β
-pinene; camphene; limonene; 1,8-cineole (23.5-26.5%); eucalyptol (4.5%);
and borneol
- camphor (7.2%); berbonone (7.6%); linalool; verbenol; terpineol; 3-octanone; isobornyl acetate
4,78,79
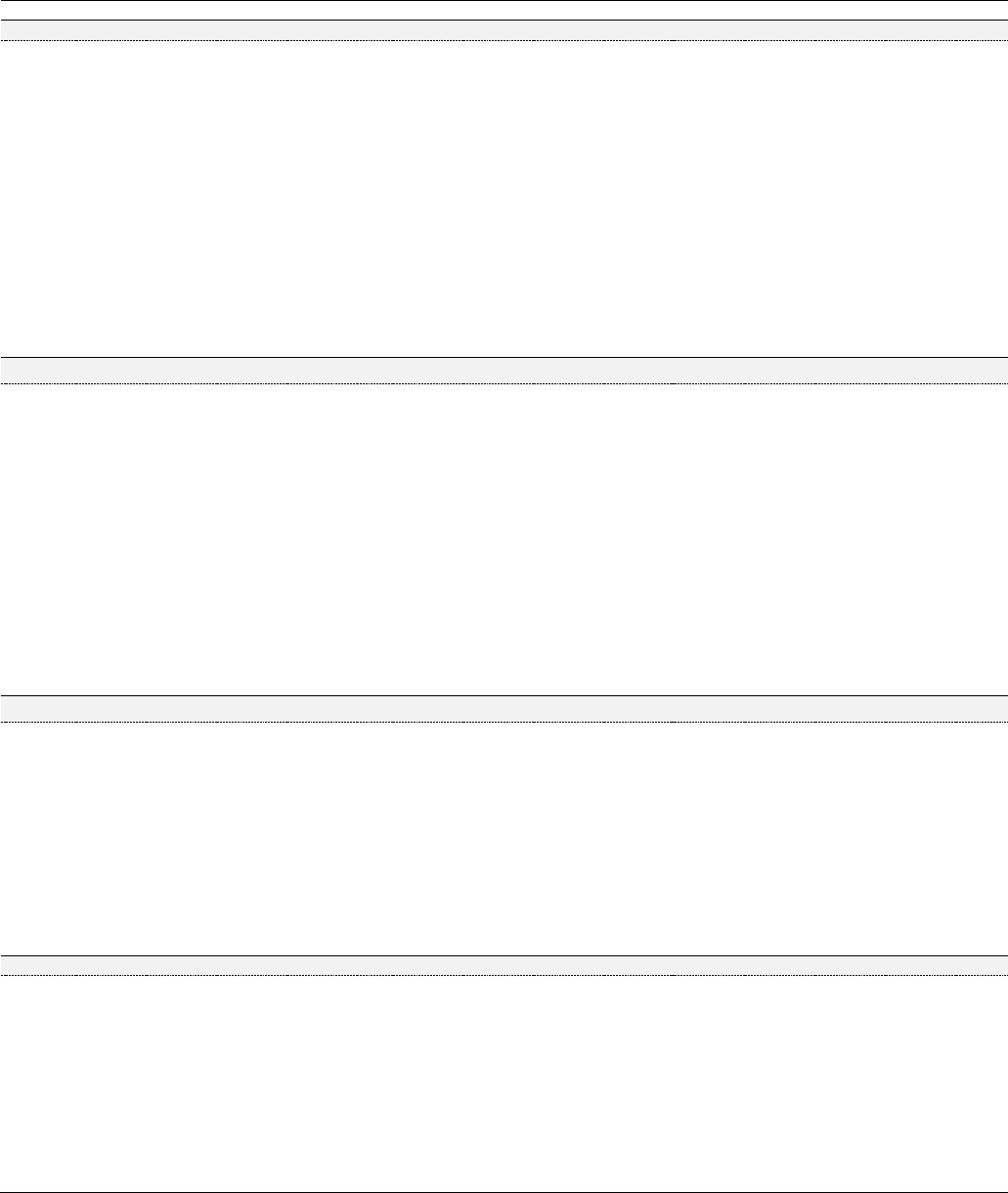
Table 5. Rosmarinus Officinalis (Rosemary) Leaf Extracts (CO
2
extract) – Certificates of Analysis
Analytical Detail
Specifications (%)
Results (%)
Rosmarinus Officinalis (Rosemary) Extract (CO
2
)
16
Essential Oil Content
78-88
78
Volatile components:
α
-pinene
8-12
11.4
camphene
n.s.
4.0
β
-pinene
n.s.
3.7
myrcene
n.s.
2.7
p-cymene
n.s.
1.2
limonene
2-4
2.4
1,8-cineole
>40
41.3
linalool
n.s.
0.83
camphor
6-13
13.0
borneol
n.s.
3.8
α
-terpineol
n.s.
3.9
verbenone
n.s.
0.45
bornyl acetate
n.s.
0.94
carophyllene
3-10
4.7
Rosmarinus Officinalis (Rosemary) Leaf Extract (CO
2
; 14% diterpene phenols) (and) Helianthus Annuus Seed Oil
17
Essential Oil Content
<2
1.9
Phenolic diterpenes:
rosmanol
n.s.
0.07
7-methyl-rosmanol
n.s.
0.09
carnosol
n.s.
1.2
carnosolic acid
n.s.
10.5
12-methyl-carnosolic acid
n.s.
2.4
sum of phenolic diterpenes
13-15
14.3
Reference antioxidant compounds (carnesol +
carnosic acid, calculated as carnosic acid)
n.s.
9.5
Ursolic Acid
n.s,
0.43
Oleanolic Acid
n.s.
0.62
residual ethanol
<2
0.71
water content
<1
0.30
Rosmarinus Officinalis (Rosemary) Leaf Extract (CO
2
; 25% diterpene phenols) (and) Helianthus Annuus Seed Oil
18
Essential Oil Content
<4
3.0
Phenolic diterpenes:
rosmanol
n.s.
0.13
7-methyl-rosmanol
n.s.
0.18
carnosol
n.s.
1.4
carnosolic acid
n.s.
18.7
12-methyl-carnosolic acid
n.s.
4.5
sum of phenolic diterpenes
24-26
24.9
Ursolic Acid
n.s.
0.29
Oleanolic Acid
n.s.
0.51
residual ethanol
<2
0.39
water content
<1
0.91
Rosmarinus Officinalis (Rosemary) Leaf Extract (CO
2
; 25% diterpene phenols) (and) Helianthus Annuus Seed Oil
12,15
Essential Oil Content
<4
1.7
Phenolic diterpenes:
rosmanol
n.s.
0.13
7-methyl-rosmanol
n.s.
0.32
carnosol
n.s
2.9
carnosic acid
> 16
20.6
12-methyl-carnosic acid
n.s.
1.0
sum of phenolic diterpenes
24-26
25.0
Ursolic Acid
n.s.
0.42
Oleanolic Acid
n.s.
0.52
residual ethanol
<2
0.33
water content
<1
0.15
n.s. – not specified
Table 6. Differences in constituent profiles in Rosmarinus officinalis (rosemary) Leaf Extract based on extraction method
*
8
Extraction Method
Constituent (ppm) dried leaves
supercritical CO
2
acetone
ethanol extract,
partially deodorized
ethanol extract,
deodorized
decolorized and deodorized
using hexane and ethanol
Triterpenes
betulin
<4760
6000
5600
8450
9460
6790
amyrin
<500
34
200
160
230
360
oleanic+ursolic acid
148,100
48,500
100,500
119,800
164,500
60,000
Flavonoids
genkwanin
2.9
0.65
1.60
2.30
3.66
2.1
Volatiles
1,8-cineole
56,100
80
1700
1320
53
30
camphor
25,200
220
2360
2080
120
20
borneol
10,000
90
960
840
40
10
Heavy Metals
lead
2.90
0.09
0.03
0.13
0.15
0.18
arsenic
1.14
<0.034
0.05
0.25
0.25
0.32
* standardized to 10% carnosic acid + carnosol content
Table 7. Toxicity information on constituents of Rosmarinus officinalis (rosemary)
Component
Toxicity information
Phenol Acids
Caffeic Acid
- in a MMC-induced SCE assay in human lymphocytes, 100 μM caffeic acid enhanced MMC-induced SCEs by 55%; 100 μM
caffeic acid alone enhanced MMC-induced SCEs by 26%
80
- caffeic acid is reported to penetrate skin and have UV photoprotective activity
81
- humans and animals metabolize caffeic acid to the same metabolites, and hydrolyze chlorogenic acid to caffeic acid; IARC con-
cluded that there is sufficient evidence for carcinogenicity in animals of caffeic acid; no data were available on the carcinogenicity
in humans, and IARC concluded that caffeic acid is possibly carcinogenic to humans
82
- the carcinogenic potency of caffeic acid, estimated based on an average human intake of 1 mg/kg bw/day, was less than 1000 can-
cer cases per 1,000,000 individuals; in rats 1 or 2% (10,000 or 20,000 ppm) caffeic acid in the diet for 51 wks to 2 yrs induced pap-
illomas of the forestomach and renal adenomas; one study, in which rats were exposed to 2% (20,000 ppm) caffeic acid in the diet
for 2 yrs, showed treatment-induced carcinomas of the forestomach, whereas two studies with shorter exposure durations showed no
such effect; caffeic acid was shown to exert strong promotion activity for forestomach carcinogenesis; chronic exposure to caffeic
acid in the diet induced hyper
plasia of the forestomach (mice, rats, and hamsters), hyperplasia of the kidney (mice and rats), and in
-
creased liver and kidney wts (rats); few toxic effects resulted from acute exposure; subchronic dietary exposures did not induce clin-
ical symptoms of toxicity, however, hyperplasia of the forestomach was observed; some genotoxic effects seen in vitro but not in
vivo
83
Chlorogenic Acid
-an antioxidant that inhibited tumor promotion by phorbol esters in mice; some controversy exists over allergic reactions in green
coffee beans, but it was accepted that chlorogenic acid was not the allergen
81
-in mice, 2% (20,000 ppm) chlorogenic acid in the diet for 96 weeks induced papillomas and carcinomas of the forestomach,
alveolar type II-cell tumors of the lung, and renal cell adenomas; few toxic effects resulted from acute exposure; subchronic dietary
exposures did not induce clinical symptoms of toxicity, however, reduced kidney and adrenal wts and hyperplasia of the fore-
stomach were observed; some genotoxic effects seen in vitro but not in vivo
83
Flavonoids
epidemiological studies implicated high dietary intake levels of flavonoids in heart disease, but a study of cancer risk failed to find a
link; some evidence of genotoxicity in bacterial assays, but a European Organization of Cosmetic Ingredients Industries and
Services (UNITIS) report stated that flavonoids do not appear to be genotoxic to mammals in vivo; flavonoids are not considered
allergens
81
Diterpenes
Carnosic Acid
- is a known antioxidant;
84
in a toxicokinetic study in male Sprague-Dawley rats, carnosic acid was absorbed into the blood stream
after oral administration and was bioavailable, traces of the acid were found in the intestinal content, liver, and muscle tissue of the
abdomen and legs, carnosic acid was present in its free form, and the main route of elimination was the feces;
84
not mutagenic in an
Ames test, with or without metabolic activation, at doses equivalent to the concentration present in up to 6000 µg/plate of a
decolorized and deodorized rosemary leaf extract
8
Carnosol
- topical application of carnosol isolated from rosemary inhibited TPA-induced ear inflammation and tumor promotion in mice;
44
not mutagenic in an Ames test, with or without metabolic activation, at doses equivalent of the concentration present in up to 6000
µg/plate of a decolorized and deodorized rosemary leaf extract
8
Monoterpenes
these chemicals may be skin sensitizers
81
d- Limonene
- d-limonene consumption has been estimated as 0.2 -2 mg/kg bw/day; in men, oral intake induced transient proteinuria
82
- developmental toxicity in the form of delayed prenatal growth has been observed in mice, rats and rabbits exposed to d-limonene
during gestation, and skeletal anomalies have also been observed in the fetuses of exposed mice and rabbits;
85
- the few genotoxicity studies available indicated that d-limonene and its 1,2-epoxide metabolite are not genotoxic
85
- IARC found there are sufficient evidence for carcinogenicity in animals, concluding that d-limonene produces renal tubular
tumors
in male rats by a non-DNA-reactive mechanism, through an α
2u
-globulin-associated response, and therefore, the mechanism by
which d-limonene increases the incidence of renal tubular tumors in male rats is not relevant to humans; no data were available on
the carcinogenicity in humans, and IARC concluded that d-limonene is not classifiable as to its carcinogenicity in humans
85
α-Pinene
negative in the Ames assay and a mouse micronucleus test
86

Table 7. Toxicity information on constituents of Rosmarinus officinalis (rosemary)
Component
Toxicity information
1,8-Cineole
positive in a sister chromatid exchange assay; negative in a chromosomal aberration assay; negative in an Ames test
87
β-Myrcene
has been reported to cause dermatitis and conjunctivitis in humans; in Wistar rats, the NOAEL for embryotoxicity was 0.5 g/kg
bw/day and the NOAEL for peri- and post-natal developmental toxicity was 0.25 g/kg bw/day; was not genotoxic in vitro in SCE
and chromosomal aberration assays in Chinese hamster cells or human lymphocytes, but it did induce a slight increase is SCE in
cultured hepatic tumor cells; was not genotoxic in vivo in rat bone marrow cells;
88
up to 1.0 g/kg bw was administered in corn oil,
by gavage, to rats and mice, and there was clear evidence of carcinogenic activity in male rats (increased incidences of renal tubule
neoplasms) and male mice (increased incidences of hepatocellular adenoma, hepatocellular carcinoma, and hepatoblastoma), and
equivocal evidence in female rats (increased incidences of renal tubule adenoma) and female mice (marginally increased incidences
of hepatocellular adenoma and carcinoma)
89
Linalool
- safe at up to 4.3% (20% in consumer fragrance); listed as a fragrance allergen by the European Commission
81
- International Fragrance Association (IFRA) stated pure linalool is not a sensitizer, but hydroperoxides and other oxidation
products have shown sensitizing properties; one of the major oxidation products of linalool was isolated and identified as 7-
hydroperoxy-3,7-dimethyl-octa-1,5-diene-3-ol; in sensitization studies in guinea pigs, linalool of high purity gave no reactions,
while linalool that had been oxidized for 10 wks sensitized the animals; it was concluded that autoxidation of linalool is essential for
its sensitizing potential
90
α,β-Thujone
α,β-thujone was not mutagenic in the Ames test; in the micronucleus test, negative in male and positive in female mice; β-thujone:
some evidence of carcinogenicity in male rats – significant incidence of cancers of the preputial gland in male rats given 25 mg/kg
by gavage, and an increase in adrenal gland tumors in male rats may have been due to β-thujone; no increase in in cancer incidence
in female rats (dosed with up to 50 mg/kg by gavage) or male or female mice (dosed with up to 25 mg/kg by gavage); all rats dosed
with 50 mg/kg and all female mice dosed with 25 mg/kg died
91
Methyleugenol
- IARC concluded that there is sufficient evidence in experimental animals for carcinogenicity; no data were available on the
carcinogenicity in humans, and IARC concluded that methyleugenol is possibly carcinogenic to humans
92
- IFRA stated available metabolic, biochemical and toxicological data in laboratory species provide clear evidence of non-linearity
in the dose-response relationship with respect to metabolic activation and mechanisms associated with carcinogenic effects; con-
sideration of these data indicates an NOEL in the rat in the dose-range of 1-10 mg/kg bw/day; based on the lower end of the NOEL
and applying a 1000x safety factor for systemic effects a daily dose of 60 μg/day is supported; taking into account a dermal penetra-
tion factor of 40% leads to an acceptable dose of 150 μg/day
93
Terpene Alcohols
α-Terpineol
- oral LD50 in mice, 2830 mg/kg; 1000 mg/kg bw/day for 2 wks caused reduced body wt gains and an increase in serum cholesterol;
not mutagenic in an Ames test or mouse lymphoma assay; did not induce pulmonary tumors in mice given i.p. injections; a dermal
irritant in animals studies, but not a dermal irritant in a 4-h clinical study; not a sensitizer in guinea pigs; in clinical patch tests, 5%
in pet. had 1/1606 positive and 11/1606 questionable reactions in one study and 2/1200 positive reactions in another
94
Ursolic acid
topical application of ursolic acid isolated from rosemary inhibited TPA-induced ear inflammation and tumor promotion in mice
44
Triterpene Alcohols
hepatoprotective and anti-carcinogenic activity has been suggested for lupeol; no toxicity data were available; triterpene alcohols
were considered to have intermediate risk
81
Table 8. Frequency and concentration of use according to duration and type of exposure
# of Uses
26
Max. Conc. of Use (%)
27
# of Uses
26
Max. Conc. of Use (%)
27
# of Uses
26
Max. Conc. of Use (%)
27
Rosmarinus Officinalis (Rosemary)
Extract
Rosmarinus Officinalis (Rosemary)
Flower Extract
Rosmarinus Officinalis (Rosemary)
Flower/Leaf/Stem Extract
Totals*
404
0.00004-0.16
32
NR
NR
0.0024
Duration of Use
Leave-On
247
0.00096 – 0.051
14
NR
NR
0.0024
Rinse Off
154
0.00004 -0.16
18
NR
NR
NR
Diluted for (Bath) Use
3
NR
NR
NR
NR
NR
Exposure Type
Eye Area
20
0.01-0.05
2
NR
NR
NR
Incidental Ingestion
7
0.011
1
NR
NR
NR
Incidental Inhalation-Spray 93
a
; 64
c
0.00096-0.001
a
1
2
a
; 1
c
NR NR NR
Incidental Inhalation-Powder
64
c
0.05
1
c
NR
NR
NR
Dermal Contact
306
0.00096-0.16
6
NR
NR
0.0024
Deodorant (underarm) NR
not spray: 0.0098
aerosol: 0.0098-0.012
NR NR NR not spray: 0.0024
Hair - Non-Coloring
116
0.00004-0.003
25
NR
NR
NR
Hair-Coloring
1
NR
NR
NR
NR
NR
Nail
1
NR
NR
NR
NR
NR
Mucous Membrane
26
0.0005-0.16
1
NR
NR
NR
Baby Products
NR
NR
NR
NR
NR
NR
Rosmarinus Officinalis (Rosemary)
Leaf
Rosmarinus Officinalis (Rosemary)
Leaf Extract
Rosmarinus Officinalis
(Rosemary) Leaf Oil
Totals*
16
0.002
729
0.00001-10
474
0.00001-1.5
Duration of Use
Leave-On
1
0.002
473
0.00001-10
308
0.0003-1.5
Rinse Off
14
NR
254
0.00001-3
141
0.00001-0.12
Diluted for (Bath) Use
1
NR
2
0.0002-0.04
25
0.5-0.97
Exposure Type
Eye Area
NR
NR
27
0.002-3
8
NR
Incidental Ingestion
NR
NR
27
0.00001-0.009
3
0.008
Incidental
Inhalation-Spray 1
a
0.002
a
8; 185
a
;
110
c
0.001-0.5
aerosol: 0.0016
pump spray: 0.0001-0.005
0.00002-0.5
a
25
90
a
; 106
c
0.011-1.5
aerosol: 0.007
Incidental Inhalation
-Powder NR NR
2
7
b
; 110
c
0.0002
2
1
b
; 106
c
0.0003
Dermal Contact
4
NR
460
0.00001-10
381
0.0003-1.5
Deodorant (underarm)
NR
NR
1
a
NR
NR
NR
Hair - Non-Coloring
12
0.002
219
0.00001-0.5
89
0.00001-1.5
Hair-Coloring
NR
NR
22
0.04
1
NR
Nail
NR
NR
1
0.005-0.053
NR
NR
Mucous Membrane
1
NR
71
0.00001-3
64
0.0002-0.97
Baby Products
NR
NR
8
0.012
4
NR
Rosmarinus Officinalis (Rosemary)
Leaf Powder
Rosmarinus Officinalis (Rosemary)
Leaf Water
Rosmarinus Officinalis
(Rosemary) Water
Totals*
2
0.05
25
0.000069-1
2
---
Duration of Use
Leave-On
1
NR
11
0.000069-1
2
NR
Rinse Off
1
0.05
14
0.00015-0.25
NR
NR
Diluted for (Bath) Use
NR
NR
NR
NR
NR
NR
Exposure Type
Eye Area
NR
NR
NR
0.000069-0.00016
NR
NR
Incidental Ingestion
NR
NR
NR
0.005
NR
NR
Incidental Inhalation-Spray
1
c
NR
4
a
; 4
c
NR
1
a
; 1
c
NR
Incidental Inhalation-Powder
1
c
NR
4
c
NR
1
c
NR
Dermal Contact
2
NR
10
0.00009-0.36
2
NR
Deodorant (underarm)
NR
NR
NR
NR
NR
NR
Hair - Non-Coloring
NR
0.05
15
0.00019-1
NR
NR
Hair-Coloring
NR
NR
NR
NR
NR
NR
Nail
NR
NR
NR
NR
NR
NR
Mucous Membrane
NR
NR
NR
0.005
NR
NR
Baby Products
NR
NR
NR
NR
NR
NR
Table 8. Frequency and concentration of use according to duration and type of exposure
# of Uses
26
Max. Conc. of Use (%)
27
# of Uses
26
Max. Conc. of Use (%)
27
# of Uses
26
Max. Conc. of Use (%)
27
Rosemary
#
Totals*
12
---
Duration of Use
Leave-On
4
---
Rinse Off
7
---
Diluted for (Bath) Use
1
---
Exposure Type
Eye Area
NR
---
Incidental Ingestion
NR
---
Incidental Inhalation-Spray
1; 2
c
---
Incidental Inhalation-Powder
1; 2
c
---
Dermal Contact
8
---
Deodorant (underarm)
NR
---
Hair - Non-Coloring
4
---
Hair-Coloring
NR
---
Nail
NR
---
Mucous Membrane
2
---
Baby Products
NR
---
* Because each ingredient may be used in cosmetics with multiple exposure types, the sum of all exposure types may not equal the sum of total uses
NR – not reported
a
Includes products that can be sprays, but it is not known whether the reported uses are sprays
b
Includes products that can be powders, but it is not known whether the reported uses are powders
c
Not specified whether a spray or a powder, but it is possible the use can be as a spray or a powder, therefore the information is captured in both categories
#
Plant part and type of preparation not known

Table 9. Single-dose toxicity studies
Test Article
Extraction
Solvent/Method
Species
No./Group
Vehicle
Conc/Dose Range
LD
50
/Results
Reference
DERMAL
Rosmarinus Officinalis
(Rosemary) Leaf Oil
-----
rabbits
not stated
not stated
not stated
>10 ml/kg
42
Rosmarinus Officinalis
(Rosemary) Leaf Oil
-----
rabbits
not stated
not stated
not stated
>10 g/kg
40
ORAL
Rosmarinus Officinalis
(Rosemary) Leaves – 2
samples; one harvested in
autumn (112.7, 477.8,
700.1 µg/mg extract car-
nosol, carnosic acid, total
diterpenes, respectively)
and one in spring (45.9,
245.9, 343.1 µg/mg ex
tract carnosol, carnosic
acid, total diterpenes,
respectively)
supercritical CO
2
Wistar rats
6 M/6F
corn oil
2 g/kg bw
8,22,40-
42
(gavage)
>2 g/kg
22
Rosmarinus Officinalis
(Rosemary) Leaf Extract
(see Table 5 for
composition)
ethanol extract,
partially
deodorized
mice
not stated
none stated
8.5 g/kg bw (males)
10 g/kg bw (females)
>8.5 g/kg bw (males)
>10 g/kg bw (females)
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
(see Table 5 for
composition)
ethanol extract,
deodorized
mice
not stated
none stated
24 g/kg bw (males)
28.5 g/kg bw
(females)
>24 g/kg bw (males)
>28.5 g/kg bw
(females)
8
Rosmarinus Officinalis
(Rosemary) Leaf Oil (see
Table 4 for composition)
hydrodistillation
Swiss
albino rats
20/group
-----
2-9 g/kg bw (gavage)
LD
50
= 5.50 g/kg bw
LD
10
= 1.10 g/kg bw
LD
100
= 9 g/kg bw
41
Rosmarinus Officinalis
(Rosemary) Leaf Oil (see
Table 5 for composition)
-----
rats
not stated
none stated
not stated
5 ml/kg bw
42
Table 10. Repeated-Dose Toxicity Studies
Test Article
Extraction
Solvent/Method
Animals/Group
Study Duration
Vehicle
Dose/Concentration
Parameters Examined
Results*
Reference
ORAL
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
ethanol extract,
partially deodorized
mice; no./group
not stated
5 days (gavage)
none stated
4300 mg/kg bw (males)
5000 mg/kg bw (females)
-
parameters included signs of
toxicity, body wts, feed con-
sumption, organ wts, gross
lesions
- no mortality
- body wt increased slightly in males, but no
changes were seen in females; “marked increase” in
fatty liver was observed in males after repeated
administration
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table for composition)
ethanol extract,
deodorized
mice; no./group
not stated
5 days (gavage)
none stated
11,800 mg/kg bw (males)
14,100 mg/kg bw (females)
-parameters as above
- no changes in body wts; liver wts of females were
slightly increased; fatty livers were observed in test
animals at necropsy.
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
acetone
rats; no./group not
stated
14 day (diet)
-----
up to 3800 mg/kg diet
- parameters included signs
of toxicity, body wts, feed
consumption, clinical chem
- no treatment-related signs of toxicity, mortality, or
changes in body wts or feed consumption
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
supercritical CO
2
rats; no./group not
stated
14 days (diet)
-----
up to 2400 mg/kg diet
- parameters as above
- no treatment-related signs of toxicity, mortality, or
changes in body wts or feed consumption
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
acetone
20 rats/group
13 wks (diet)
-----
300, 600, 2400, or 3800
mg/kg diet
- parameters included signs
of toxicity, body wts, feed
consumption, gross and
microscopic lesions, clinical
chem, hematology, organ wts
- variations in clinical chemistry parameters at
times were stat sig, but the researchers stated that
because the changes were inconsistent, they were
not considered dose-related
- stat. sig, decrease in alkaline phosphate in the
3800 mg/kg group
- NOAEL was 3800 mg/kg diet
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
supercritical CO
2
20 rats/group
13 wks (diet)
-----
300, 600, or 2400 mg/kg diet
- parameters as above
- variations in clinical chemistry parameters at
times were stat sig; the researchers stated that
because the changes were inconsistent, they were
not considered dose-related
- a marginal reduction in body weights and feed
consumption in the animals of the 2400 mg/kg diet
groups were attributed to a lack of palatability of
the feed
- changes were more notable in females
- NOAEL was 2400 mg/kg diet (equiv. to 180 and
200 mg/kg bw/day for males and females,
respectively)
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
supercritical CO
2
female rats; no./
group not stated
91 days (diet);
28-day recovery
period
-----
0 or 2400 mg/kg diet (equiv.
to 0 or 195 mg/kg bw/day)
-
parameters included signs of
toxicity, body wts, feed con-
sumption, gross and micro-
scopic lesions, organ wts
- slight increase in liver wts after 91-days of dosing,
but not in those killed after the 28-day recovery
period
- an increase in microsomal protein concentration
observed after 91 days of dosing was also reversible
- no notable effects on the activity of the liver
enzymes CYP1A, CYP2B, or CYP3A
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
ethanol extract,
partially deodorized
Sprague-Dawley
rats; no./group not
stated
90 days (diet)
-----
0, 500, 1500,or 5000 mg/ kg
diet (equiv. to 0, 40, 120, or
400 mg/kg bw/day)
-
parameters included signs of
toxicity, body wts, feed con-
sumption, microscopic le-
sions, organ wts, hematology
- body wts in the high dose group were very slightly
reduced, most likely as a result of decreased feed
consumption
- a dose-response relationship was observed for
relative liver-to-body wt, in which a a slight but stat
sig increase was observed
- no microscopic changes in the liver were reported
8

Table 10. Repeated-Dose Toxicity Studies
Test Article
Extraction
Solvent/Method
Animals/Group
Study Duration
Vehicle
Dose/Concentration
Parameters Examined
Results*
Reference
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
ethanol extract,
deodorized
Sprague-Dawley
rats; no./group not
stated
90 days (diet)
-----
0, 500, 1500,or 5000 mg/ kg
diet (equiv. to 0, 40, 120, or
400 mg/kg bw/day)
-parameters as above
- a dose-response relationship was observed for
relative liver-to-body wt; extracts; a slight but stat
sig increase was observed
- no microscopic changes in the liver were reported
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract (see
Table 5 for composition)
hexane and ethanol
(2-step extraction)
Sprague-Dawley
rats; no./group not
stated
3 mos (diet); 28-
day interim
group; 1-mo
recovery period
-----
0, 1000, 2500, or 5000 mg/
kg diet (equiv. to 0, 65, 164,
or 320 mg/kg bw/day)
- parameters as above
- no signs of toxicity, no mortality and no gross
lesions at necropsy
- reversible dose-dependent increases in absolute
liver wts and relative liver-to-body wts; stat sig in
the high dose group only
- treatment-related increase in bile duct hyperplasia
at the interim necropsy; the incidence was decreased
at the end of dosing and not seen after recovery
- in females, a decrease in pancreas wt was
observed at the interim necropsy
- no stat sig changes in hematology parameters, and
no microscopic changes
- the NOAEL was at least 320 mg/kg bw/day
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
(after the volatile oil [1.1%]
was removed)
absolute ethanol
Swiss albino mice;
6M/group
3 wks (gavage)
olive oil
1500 mg/kg extract
controls – olive oil
no stat sig changes in relative liver, spleen, heart, or
lung wt to body wt compared to controls; there were
no stat sig changes in clinical chemistry parameters
41
single dose CCl
4
(gavage), then 3
wks extract
(gavage)
olive oil
3.3% CCl
4
(100 mg/kg bw)
1500 mg/kg extract
- with CCl
4
only, stat sig increases in relative liver
to body wt (18%) and spleen to body wt (45.6%)
compared to olive oil controls; CCl
4
affected all
measured clinical chemistry parameters
- with the extract, the increase in relative spleen to
body wt was stat sig, but not as great as with CCl
4
alone (34.9%); there was no stat sig increase in
relative liver to body wt; many of the changes in
clinical chemistry values were reduced or were non-
stat sig
Rosmarinus Officinalis
(Rosemary) Leaf Oil (see
Table 4 for composition)
hydrodistillation
Swiss albino mice;
6M/group
3 wks (gavage)
-----
1100 mg/kg bw
controls – olive oil
no stat sig changes in relative liver, spleen, heart, or
lung wt to body wt compared to controls; there were
no stat sig changes in clinical chemistry parameters
41
single dose CCl
4
(gavage), then 3
wks oil (gavage)
olive oil
(for CCl
4
)
3.3% CCl
4
(100 mg/kg bw)
1100 mg/kg extract
- (effects of CCl
4
only are described above)
- with the oil, the increases in relative liver to body
wt (9.8%) and spleen to body wt (38.8%) were stat
sig, but not as great as with CCl
4
alone;
many of the
changes in clinical chemistry values were reduced
but were still stat sig
*if not described in the results, details on histopathology or organ weights were not provided
Abbreviations: CCl
4
: - carbon tetrachloride; conc – concentration; equiv. – equivalent; NOAEL – no-observable adverse effect level; stat sig – statistically significant
Table 11. Genotoxicity studies
Test Article
Extraction
Solvent/Method
Conc./Vehicle
Procedure
Test System
Results
Reference
IN VITRO
Rosemary Extract (not de-
fined; water-soluble; con-
tained 17% rosmarinic
acid)
-----
50, 100, or 200 µg/
plate
Ames test, with and without
metabolic activation
S. typhimurium TA98
not mutagenic
51
as above
-----
50 µg/ml (highest non-
cytotoxic dose)
comet assay
human hepatoma cell line
(HepG2)
not genotoxic
51
Rosemary Extract (not de-
fined; oil-soluble; con-
tained 50.27% carnosic
acid and 5.65% carnosol)
-----
50, 100, or 200 µg/
plate
Ames test, with and without
metabolic activation
S. typhimurium TA98
not mutagenic
51
as above
-----
5 µg/ml (highest non-
cytotoxic dose)
comet assay
human hepatoma cell line
(HepG2)
not genotoxic
51
Rosmarinus Officinalis
(Rosemary) Leaf Extract
supercritical CO
2
up to 5000 µg/plate
bacterial assay, with and without
metabolic activation
S. typhimurium TA98, TA100,
TA1535, TA1537, TA102
not mutagenic
- in TA102 only, toxicity at the highest dose
with metabolic activation
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
ethanol extract,,
partially deodorized
up to 20,000 µg/plate
bacterial assay, with and without
metabolic activation
S. typhimurium TA98, TA100,
TA1535, TA1537, TA102
not mutagenic
- some bactericidal effects in all strains; ef-
fects were reduced with metabolic activation
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
ethanol extract,
deodorized
up to 20,000 µg/plate
bacterial assay, with and without
metabolic activation
S. typhimurium TA98, TA100,
TA1535, TA1537, TA102
not mutagenic
- some bactericidal effects in all strains; ef-
fects were reduced with metabolic activation
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
hexane and ethanol
(2-step extraction)
up to 6000 µg/plate
Ames test, with and without meta-
bolic activation
S. typhimurium TA97, TA98,
TA100, TA102
-mutagenic in TA102 in one set of trials; not
reproducible with less cytotoxic conc
-not mutagenic in the other strains
- without metabolic activation: bactericidal
for all strains at 3000-6000 µg/plate; bacteri-
cidal to TA102 at almost all dose levels
-with metabolic activation, bactericidal only
at the highest dose level, if at all
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
ethanol extract,
partially deodorized
up to 100 mg/ml
chromosomal aberration assay, with
and without metabolic activation
human lymphocytes
not genotoxic
8
Rosmarinus Officinalis
(Rosemary) Leaf Extract
hexane and ethanol
(2-step extraction)
not clearly specified
but at least up to 50
µg/ml without and 35
µg/ml with metabolic
activation
gene-locus mutation assay, with
and without metabolic activation
thymidine kinase (tk) and
hgprt loci of a human lympho-
blastoid cell line (TK6)
-not genotoxic without metabolic activation
at up to 50 µg/ml
- 35 µg/ml increased mutations in the tk, but
not the hgprt, locus with activation; the in-
crease was stat sig when compared to solvent
control, but not when compared to untreated
cells; determined to be not mutagenic under
the conditions used because of a lack of a
dose-dependent increase in mutation fre-
quency and a lack of a stat sig increase of
mutation frequency compared to controls
8
Rosmarinus Officinalis
(Rosemary) Leaf Oil
-----
not stated
Ames test
not stated
negative
52
Table 11. Genotoxicity studies
Test Article
Extraction
Solvent/Method
Conc./Vehicle
Procedure
Test System
Results
Reference
IN VIVO
Rosmarinus Officinalis
hydro-alcoholic
6.43, 100, and 200
mg/kg bw
chromosomal aberration assay
Wistar rats; 6/group
not genotoxic
53
Rosmarinus Officinalis
hydro-alcoholic
6.43, 100, and 200
mg/kg bw
micronucleus assay
Wistar rats; 6/group
not genotoxic
53
Rosmarinus Officinalis
(Rosemary) Leaf Extract
(after the volatile oil
[1.1%] was removed)
absolute ethanol
1500 mg/kg bw/day in
olive oil
micronucleus test; dosed by gavage
for 7 days; negative controls were
given olive oil; positive controls
were given a single i.p. dose of 100
mg/kg bw CPA; bone marrow cells
collected 24 h after dosing
Swiss albino mice
not genotoxic; no stat sig change in the num-
ber of MNPCE or NCE or in PCE/NCE
41
Rosmarinus Officinalis
(Rosemary) Leaf Oil (see
Table 4 for composition)
hydrodistillation
1100 mg/kg bw/day
same protocol
Swiss albino mice
no stat sig change in no. of MNPCE
no. of NCE was stat sig decreased (p<0.05)
PCE/NCE was stat sig increased (p<0.01)
41
Rosmarinus Officinalis
(Rosemary) Leaf Oil
hydrodistillation
300, 1000, or 2000
mg/kg bw (by gavage)
chromosome aberration assay;
single 0.5 ml dose; negative
controls were given distilled water;
positive controls were dosed with
50 mg CPA/kg; bone marrow cells
collected 24 h after dosing
Wistar rats; 3M/3F per group
- chromosomal aberrations without gaps
were stat sig increased at 2000 mg/kg bw
- mitotic index was stat sig increased with
300 mg/kg, but not with other doses or the
positive control
14
Rosmarinus Officinalis
(Rosemary) Leaf Oil
hydrodistillation
300, 1000, or 2000
mg/kg bw (by gavage)
micronucleus test; single 0.5 ml
dose; negative controls were given
distilled water; positive controls
were dosed with 50 mg CPA/kg;
bone marrow cells collected 24 h
after dosing
Swiss mice; 3M/3F per group
- stat sig increase in MNPCEs with 1000 and
2000 mg/kg bw
- PCE/NCE was not stat sig different from
controls
14
Rosmarinus Officinalis
(Rosemary) Leaf Oil
hydrodistillation
300, 1000, or 2000
mg/kg bw (by gavage)
micronucleus test; protocol as
above; bone marrow cells collected
24 h after dosing
Wistar rats; 3M/3F per group
stat sig increase in MNPCEs with 2000
mg/kg bw
14
Rosmarinus Officinalis
(Rosemary) Leaf Oil
hydrodistillation
300, 1000, or 2000
mg/kg bw (by gavage)
comet assay; single 0.5 ml dose;
negative controls were given dis-
tilled water; positive controls were
dosed with 50 mg CPA/kg; liver
and peripheral blood cells collected
24 h after dosing
Swiss mice; 3M/3F per group
all 3 doses induced stat sig increases in DNA
damage in peripheral blood cells and liver
cells; most of the damaged cells showed
minor damage, very few had a large amount
of damage
14
mixture containing 19%
Rosmarinus officinalis
(rosemary) leaves, 71..5%
St. John’s Wort; 9.5%
spirulina
-----
0, 380, 760, or 1520
mg/ kg bw/day in
water (gavage)
micronucleus test; mice were dosed
for 7 days; femoral bone marrow
cells were used
male Swiss albino mice;
30/group
- stat. sig. increase in MNPCEs with 760 and
1520 mg/kg bw/day
- PCE/NCE was not stat sig different from
controls
54
mixture defined above
-----
0, 380, 760, or 1520
mg/ kg bw/day in
water (gavage)
chromosomal aberration assay;
mice were dosed for 7 days and
killed 19 days after last dose
male Swiss albino mice;
30/group
- stat sig increased in frequency of aneu-
ploidy with 760 and 1520 mg/kg bw/day
- % polyploids and total % aberrations were
stat sig increased at these doses
54
Table 11. Genotoxicity studies
Test Article
Extraction
Solvent/Method
Conc./Vehicle
Procedure
Test System
Results
Reference
mixture defined above
-----
0, 380, 760, or 1520
mg/ kg bw/day in
water (gavage)
assay for spermatozoa abnormality;
mice were dosed for 7 days and
killed 5 wks after last dose
male Swiss albino mice;
30/group
- stat sig increase in frequency of banana-
shaped, swollen achrosome, and triangular
head sperm abnormalities with 1520 mg/kg
bw/day
- % total spermatozoa abnormalities stat sig
increased with 1520 mg/kg bw/day
54
ANTI-MUTAGENIC EFFECTS
IN VITRO
Rosemary Extract (not
defined; contained 8.8-
10.6% carnosic acid and
1.2-1.4% carnosol) +
tBOOH
-----
≤0.8 mg/ml in medi-
um-
chain triglycerides;
only the carnosic acid
and carnosol were
soluble
Ames test; 0.5 ml rosemary extract
was incubated with 0.5 ml tBOOH
S. typhimurium TA102
stat sig reduced tBOOH-induced
mutagenicity
95
Rosemary Extract (not de-
fined; water-soluble; con-
tained 17% rosmarinic
acid) + IQ
-----
50, 100, or 200 µg/
plate extract
10 ng/plate IQ
Ames test, with metabolic
activation
S. typhimurium TA98
a stat sig reduction in IQ-induced genotoxi-
city was observed only at the highest dose
51
as above + NQNO
-----
0, 50, 100, or 200 µg/
plate extract
500 ng/plate NQNO
Ames test, without metabolic
activation
S. typhimurium TA98
no stat sig effect on NQNO-induced
genotoxicity
51
as above + tBOOH
-----
0, 0.05, 0.5, 5, or 50
µg/ml extract; 0.05
mM tBOOH
Comet assay; pretreatment with
extract for 21 h, followed by 20
min exposure to tBOOH
human hepatoma cell line
(HepG2)
stat sig reduction in tBOOH-induced DNA
damage at all doses; the reduction was not
dose-dependent – 0.05 µg/ml caused a
greater reduction than 0.5 µg/ml
51
as above + tBOOH
-----
0, 0.05, 0.5, 5, or 50
µg/ml extract; 0.05
mM tBOOH
Comet assay; co-treatment with
extract and tBOOH for 20 min
human hepatoma cell line
(HepG2)
no stat sig effect on tBOOH-induced DNA
damage
51
as above + tBOOH
-----
0, 0.05, 0.5, 5, or 50
µg/ml extract; 0.05
mM tBOOH
Comet assay; pretreatment with
extract for 21 h, followed by co-
treatment with extract and tBOOH
for 20 min
human hepatoma cell line
(HepG2)
stat sig reduction in tBOOH-induced DNA
damage at all except the lowest dose
51
as above + BaP
-----
0, 0.05, 0.5, 5, or 50
µg/ml extract; 40 µM
BaP
by co-treatment with extract and
BaP for 21 h
human hepatoma cell line
(HepG2)
stat sig reduction in BaP-induced DNA
damage only at the highest dose
51
as above + PhIP
-----
0, 0.05, 0.5, 5, or 50
µg/ml extract; 80 µM
PhIP
Comet assay; by co-treatment with
extract and PhIP for 21 h
human hepatoma cell line
(HepG2)
stat sig reduction in PhIP-induced DNA
damage only at the highest dose
51
Rosemary Extract (not de-
fined; oil-soluble; con-
tained 50.27% carnosic
acid and 5.65% carnosol)
+ IQ
-----
50, 100, or 200 µg/
plate extract
10 ng/plate IQ
Ames test, with metabolic
activation
S. typhimurium TA98
suppressed IQ-induced mutations in a stat
sig, dose-dependent, manner
51
as above + NQNO
-----
50, 100, or 200 µg/
plate extract
500 ng/plate NQNO
Ames test, without metabolic
activation
S. typhimurium TA98
suppressed NQNO-induced mutations in a
stat sig, dose-dependent, manner
51
as above + tBOOH
-----
0, 0.05, 0.5, or 5 µg/
ml extract; 0.05 mM
tBOOH
comet assay; pretreatment with
extract for 21 h, followed by 20
min exposure to tBOOH
human hepatoma cell line
(HepG2)
stat sig reduction in tBOOH-induced DNA
damage at all doses
51

Table 11. Genotoxicity studies
Test Article
Extraction
Solvent/Method
Conc./Vehicle
Procedure
Test System
Results
Reference
as above + tBOOH
-----
0, 0.05, 0.5, or 5 µg/
ml extract; 0.05 mM
tBOOH
comet assay; co-treatment with
extract and tBOOH for 20 min
human hepatoma cell line
(HepG2)
no stat sig effect on tBOOH-induced DNA
damage
51
as above + tBOOH
-----
0, 0.05, 0.5, or 5 µg/
ml extract; 0.05 mM
tBOOH
comet assay; pretreatment with
extract for 21 h, followed by co-
treatment with extract and tBOOH
for 20 min
human hepatoma cell line
(HepG2)
stat sig reduction in tBOOH-induced DNA
damage at all doses; the reduction was not
dose-dependent`
51
as above + BaP
-----
0, 0.05, 0.5, or 5 µg/
ml extract; 40 µM BaP
by co-treatment with extract and
BaP for 21 h
human hepatoma cell line
(HepG2)
stat sig reduction in BaP-induced DNA
damage at the two highest doses
51
as above + PhIP
-----
0, 0.05, 0.5, or 5 µg/
ml extract; 80 µM
PhIP
by co-treatment with extract and
PhIP for 21 h
human hepatoma cell line
(HepG2)
stat sig reduction in PhIP-induced DNA
damage at the two highest doses
51
IN VIVO
Rosmarinus Officinalis
(Rosemary) Leaf Extract
(after the volatile oil
[1.1%] was removed) +
CPA
absolute ethanol
1500 mg/kg bw/day in
olive oil
micronucleus test; dosed by gavage
with the extract for 7 days, then
given a single i.p. dose of 100
mg/kg bw CPA; bone marrow cells
collected 24 h after dosing; olive
oil was used as a negative control
Swiss albino mice
stat sig increase in the number of MNPCE
and NCE compared to olive oil only; no stat
sig change in PCE/NCE
41
Rosmarinus Officinalis
(Rosemary) Leaf Oil (con-
tained 20.86% bornyl ace-
tate; 16.24% L-camphor,
and 8.25% borneol) + CPA
hydrodistillation
1100 mg/kg bw/day
micronucleus test; dosed by gavage
with the oil for 7 days, then given a
single i.p. dose of 100 mg/kg bw
CPA; bone marrow cells collected
24 h after dosing; olive oil was
used as a negative control
Swiss albino mice
stat sig increase in the number of MNPCE
and NCE, and a stat sig decrease in
PCE/NCE, compared to olive oil only
41
Abbreviations: BaP – benzo(a)pyrene; conc – concentration; CPA - cyclophosphamide: IQ – 2-amino-3-methyl-3H-imidao[4,5-F]quinoline; MMS – methyl methanesulfonate; MNPCE – micronucleated
polychromatic erythrocytes; NCE – normochromatic erythrocytes; NQNO – 4-nitroquinoline-N-oxide; PCE/NCE – ratio of polychromatic erythrocytes to normochromatic erythrocytes; PhIP – 2-amino-1-
methyl-6-phenylimidazo[4,5-b]pyridine; stat sig – statistically significant; tBOOH - t-butyl hydroperoxide

Table 12. Effects on Tumor Promotion
Test Article
Extraction
Solvent/Method
Dose/Exposure
Route
Species
No./Group
Tumor Type
Carcinogenicity Model
Results
Reference
Rosmarinus Officinalis
(Rosemary) Leaf Extract
(contained 16.5-19.2% uro-
solic acid; 3.8-4.6% carno-
sol; 0.1-0.5% carnosic acid;
trace-0.1% miltirone)
methanol
1.2 or 3.6 mg;
dermal
CD-1 mice;
30F/grp
skin
- initiation: topical treatment with 200 nmol
DMBA in 200 µl acetone
- promotion: after 1 wk, topical treatment with
200 µl acetone (controls), 5 nmol TPA in 200 µl
acetone (carc grp), or 5 nmol TPA and extract in
200 µl acetone (RE grp), 2x/wk, for 20 wks
1.2 mg: decreased tumor/mouse by 48, 27,
and 28% after 7, 11, and 15 wks TPA
promotion
3.6 mg: decreased tumor/mouse by 84, 37,
and 48% after 7, 11, and 15 wks TPA
promotion
44
as above
methanol
1.2 or 3.6 mg; 5
min prior to
B(a)P; dermal
CD-1 mice;
30F/grp
skin
- initiation: topical treatment with 200 µl acetone
(controls) or with extract in 200 µl acetone (RE
grp) 5 min prior to each 20 nmol application of
B(a)P or 2 nmol DMBA, 1x/wk, for 10 wks
- promotion: after 1 wk, promotion with 15 nmol
TPA in 200 µl acetone, 2x/wk, for 20 wks
1.2 mg: decreased tumor/mouse by 15, 42,
and 54% after 9, 13, or 21 wks TPA
promotion
3.6 mg: decreased tumor/mouse by 62, 63,
and 64% after 9, 13, or 21 wks TPA
promotion
44
as above
methanol
3.6 mg; dermal
CD-1 mice;
30F/grp
skin
- initiation: topical treatment with 200 µl acetone
(controls) or 3.6 mg extract in 200 µl acetone
(RE grp) at 120, 60, and 5 min before topical
application of 200 nmol B(a)P in 200 µl acetone
- promotion: after 1 wk, 15 nmol in 200 µl ace-
tone, 2x/wk, for 20 wks
decreased tumor/mouse by 83, 81, and
58% after 9, 13, or 21 wks TPA
promotion
44
Rosmarinus Officinalis
(Rosemary) Leaf Extract
DDW
500 mg/kg bw;
gavage
Swiss albino
mice; 12M/grp
skin
DMBA-initiated and croton oil-promoted skin
tumorigenesis
Grp 1: controls – topical treatment with 100 µl
acetone; DDW by gavage for 15 wks
Grp 2: 500 mg/kg bw/day RE in 100 µl DDW for
15 wks
Grp 3: single topical dose 100 µg DMBA in 100
µl acetone; 2 wks later, 1% croton oil in acetone,
3 x/wk; also, 100 µl by gavage for 15 wks
Grp 4: single topical dose 100 µg DMBA in 100
µl acetone; 500 mg/kg bw RE by gavage 7 days
before, during, and 7 days after DMBA; 2 wks
after DMBA, 1% croton oil in acetone, 3x/wk
Grp 5: single topical dose 100 µg DMBA in 100
µl acetone; after 2 wks, 500 mg/kg bw RE ex-
tract by gavage for 15 days and 1% croton oil in
acetone 3x/wk
Grp 6: single topical dose 100 µg DMBA in 100
µl acetone; 500 mg/kg bw RE by gavage 7 days
before DMBA until study end; 2 wks after
DMBA, 1% croton oil in acetone, 3x/wk
-a stat sig decrease in tumor number, di-
ameter, and weight and a stat sig increase
in the avg. latency period
was observed in
grps given RE compared to Grp 3 (the
carcinogen-control grp)
- blood serum and liver lipid peroxidation
level was stat sig de
creased in all RE grps
compared to grp 3
- Grp 6 had the greatest changes for all
the above parameters
- no tumors were found in animals given
RE only
- RE had no effect on body weight gains
55
Rosmarinus Officinalis
(Rosemary) Leaf Extract
DDW
1000 mg/kg bw
in DDW; gavage
Swiss albino
mice; 12M/grp
skin
DMBA-initiated and croton oil-promoted skin
tumorigenesis
-same protocol as above (Grps 1-6), except 1000
mg/kg bw RE was used
- stat sig decrease in tumor burden and
tumor yield, and a stat sig
increase in avg.
latency period, in grps given RE com-
pared to Grp 3 (the carcinogen-control
grp); tumor incidence was decreased
- blood serum lipid peroxidation level was
stat sig decreased in all RE grps, and the
liver glutathione levels stat sig increased,
compared to grp 3
- RE did not cause any adverse effects; no
tumors were seen in the RE-only grp.
56
Table 12. Effects on Tumor Promotion
Test Article
Extraction
Solvent/Method
Dose/Exposure
Route
Species
No./Group
Tumor Type
Carcinogenicity Model
Results
Reference
Rosmarinus Officinalis
(Rosemary) Extract
not specified
1.0%, in diet
Sprague-
Dawley rats;
20F/grp
mammary
- rats were fed untreated or RE-supplemented
diet throughout the study (16 wks post-DMBA)
- after 27 days of the test diet, each rat was dosed
with 30.9 mg/kg bw DMBA in corn oil by
gavage
- the incidence of palpable mammary
tumors was less in the RE-fed rats than
the controls; at study termination, the
tumor incidence was 47% less; this differ-
ence was stat sig
- the difference in tumors per tumor-bear-
ing rat was not stat sig btwn the two grps
- at study termination, 94% and 90% of
tumor-bearing rats of the control and RE
groups, respectively, possessed mammary
adenocarcinomas
- RE had no effect on body wt
57
Abbreviations: B(a)P – benzo[a]pyrene; DDW – double-distilled water; DMBA – 7,12-dimethylbenz[a]anthracene; grp – group; GR – glutathione reductase; GSH – reduced glutathione; GST –
glutathione-s-transferase; RE – Rosmarinus officinalis (rosemary) leaf extract; stat sig – statistically significant; TPA – 12-O-tetradecanoylphorbol-13-acetate
Table 13. Dermal Irritations and Sensitization
Test Article
Concentration/Dose
Test Population
Procedure
Results
Reference
NON-HUMAN
4.4% rosmarinus officinalis
(rosemary) leaf oil (and
other essential oils)
tested at concentrations
up to 40%
Lewis rats
ointment was applied to the shaved skin of Lewis rats
twice daily, for 14 days
not irritating
no gross or microscopic lesions were
reported in the skin
58
rosmarinus officinalis
(rosemary) leaf oil
undiluted
rabbits
applications were made to intact and abraded rabbit
skin under occlusion; no other details were provided
moderately irritating
42
HUMAN
Rosmarinus officinalis
(rosemary) leaves
undiluted in sufficient
petrolatum for binding
234 patients with
contact dermati-
tis or eczema
patch test
21 had +/- reactions; 18 had a + reaction; 5
had a ++ reaction; no subjects had a +++
reaction
59
Rosmarinus officinalis
(rosemary) leaves extracted
with supercritical CO
2
undiluted in petrolatum
20 subjects
epicutaneous test using Finn chambers
weak irritant
1 positive reaction
9
Rosmarinus officinalis
(rosemary) leaves as an
absolute (soluble in hexane)
undiluted in petrolatum
25 subjects
epicutaneous test using Finn chambers
weak irritant
2 positive reactions
9
2% and 10%
23 subjects pre-
viously sensi-
tized to peru
balsam and/or
perfumes or fra-
grance materials
epicutaneous test using Finn chambers
weak effect
Table 13. Dermal Irritations and Sensitization
Test Article
Concentration/Dose
Test Population
Procedure
Results
Reference
Rosmarinus officinalis
(rosemary) leaves as a
concrete (insoluble waxes)
extracted in hexane
undiluted in petrolatum
20 subjects
epicutaneous test using Finn chambers
no reactions
9
2% and 10%
23 subjects pre-
viously sensi-
tized to peru
balsam and/or
perfumes or fra-
grance materials
epicutaneous test using Finn chambers
weak effect
cream containing 0.2%
rosmarinus officinalis
(rosemary) leaf extract
undiluted
20 subjects
a 24 h single insult occlusive patch test
not an irritant
no reactions were observed, and the
primary irritation index was 0.00
60
hair spray containing
0.0013% rosmarinus
officinalis (rosemary) leaf
extract
neat
102 subjects
modified Draize HRIPT
induction: occlusive patches were applied for 24 h, and
the sites were scored prior to the application of the
next patch; patches were applied 3x/wk for 3 wks; the
material was allowed to volatilize for 30 min prior to
application
challenge: after a 2-wk non-treatment period, chal-
lenge patches were applied to a previously untreated
site; the test sites were scored 24 and 72 h after
application
not an irritant or sensitizer
transient, barely perceptible to mild
responses were observed in some subjects,
but was not considered related to skin
irritation or an allergic reaction
62
sunscreen cream containing
0.2% rosmarinus officinalis
(rosemary) leaf extract
neat
27 subjects
maximization test
induction: an occlusive patch containing 0.1 ml of
0.25% aq. SLS was applied to the upper outer arm,
volar forearm, or back of each subject for 24 h; the
SLS patch was removed and an occlusive patch with
0.1 ml test material then applied for 48 or 72 h; the
patch was then removed and the test site examined; a
total of five SLS/test material patches were applied
during induction
challenge: after a 10-day non-treatment period, an
occlusive patch with 0.1 ml of a 5% aq. SLS solution
was applied to a previously untreated site for 1 h; this
patch was removed and an occlusive patch containing
0.1 ml undiluted test material was then applied for 48
h; the challenge site was graded 1 and 24 h after patch
removal
not a contact-sensitizer
no reactions were observed
61
Rosmarinus officinalis
(rosemary) leaf oil
10% in petrolatum
not specified
48-h closed patch test; details not provided
not an irritant
42
Rosmarinus officinalis
(rosemary) leaf oil
10% in petrolatum
25 subjects
maximization test; details not provided
not a sensitizer
42
Table 13. Dermal Irritations and Sensitization
Test Article
Concentration/Dose
Test Population
Procedure
Results
Reference
leave-on massage oil con-
taining 1.5% rosmarinus
officinalis (rosemary) leaf
oil
neat
104 subjects
HRIPT
induction: an occlusive patch containing 50 µl of un-
diluted test material was applied for 48 h; the patches
were then removed and a new patch applied; 9 induc-
tion patches were applied.
challenge: performed 12-14 days after induction at the
original test site and a previously untested site for 48
h; sites were scored at 48 and 96 h
patches of 0.5% SLS were used as a positive control,
and deionized water as a negative control
did not induce allergic contact dermatitis
no reactions were observed at induction or
challenge
63
Abbreviations: human repeated insult patch test (HRIPT); sodium lauryl sulfate (SLS)

Table 14. Case reports with Rosmarinus officinalis (rosemary)
Mode of Contact
Indication
Patch Testing
Reference
cosmetics and cleansing gel con-
taining 0.1% Rosmarinus offici-
nalis (rosemary) leaf extract
itchy erythema of the face; red papules
around the eyes and on the nose and
cheeks
patch test with cosmetics and 1% aq. cleansing gel gave
positive result (+) to gel only on D3
-
patch tested gel ingredients, only positive reaction (+)
was to 0.1% aq. Rosmarinus officinalis (rosemary) leaf
extract on D3
64
occupational exposure to a Ros-
marinus officinalis (rosemary)
leaf extract
severe hand, forearm, and face
dermatitis
patch tested with 5 and 10% extract in petrolatum; + re-
action to 5 and 10% on D2 and D5; 1 control was
negative
- patch tested with carnosol in ethanol; ?+ reaction to
0.1% at 3 and D7, + reaction to 1% on D3 and D7;
controls were negative to 0.1 (n=110) and 1% (n=116)
carnosol
65
occupational use of essential
aromatherapy oils (5 cases)
hand eczema in all; other involvement
seen
- patch testing with the European baseline series, fra-
grance series, and 2% of each essential oil in petro-
latum; ++ reaction to rosemary oil in 2 subjects, + in
one, among other positive reactions
66
history of eating foods spiced
with rosemary
severe cheilitis
patch tested with 41 antigens, 21 flavoring agents and
dyes, and medications; ++ on D2 and + on D5 to rose-
mary (also + to nickel on D2 and D5; + to wood tars on
D2)
67
picked rosemary leaves
developed hand, forearm, and face
dermatitis within hours
prick-by-prick testing was negative at 15 min and
positive (++) at D2
- patch testing gave positive reactions with rosemary
(++) and thyme (+) on D2 and D4
- a photopatch test (10 J/cm) with rosemary and thyme
showed stronger reactions (+++ and ++, respectively,
on D4)
- 5 controls were negative
68
walked near, and touched,
odorous plants
cutaneous lesions on the hand and face;
developed edema and eczematous
lesions on her hands, eyelids, and face
patch and photopatch test with 1% rosemary extract
was positive (+++)
- patch and photopatch test with rosemary leaves was
positive; more intense with photopatch (++/+++)
- hydrophilic and lipophilic rosemary extracts 10%,
patch and photopatch tests were positive
- patch test with 0.1% carnosol in alcohol was positive
- patch test with sage and oregano were negative
-5 controls were negative with all
69
rosemary leaf plasters applied to
knee
after 3 days, acute dermatitis in the
application area
positive (++ on D2; +++ on D4) reactions in a patch
test with rosemary leaves, but not thyme, origanum, or
mint
- 10 controls did not react to rosemary leaves
70
applied a poultice containing
rosemary and thyme
after 24 h, acute, cutaneous, eczematous
lesion on right thigh, with vesicles and
blisters
positive patch test results with the poultice (++ on D2
and D4); rosemary (++ on D2 and D4); thyme (- on D2,
++ on D4); and colophony (+ on D2 and D4); negative
results with arnica, chamomile, and horsetails
- 12 controls were negative with rosemary and thyme
71
rosemary alcohol applied to chest
swelling of face, chest, and dorsal
aspect of arms, followed by peeling
positive reactions were found in patch test with fresh
Rosmarinus officinalis (rosemary) leaves (+++ on D2,
D3, D4), dry rosemary leaves (+ reaction on D2, D3,
D3), dry leaves wetted with water (+ reaction on D2,
D3, D3), the flower (++ reaction on D2, D3, D3), and
rosemary alcohol ((+ reaction on D2, D3, D3)
- negative reactions to 50% aq. rosemary alcohol
- positive reactions were also found with sage and
lavender
72

REFERENCES
1. Gottschalck TE and Breslawec H. International Cosmetic Ingredient Dictionary and Handbook. Washington, DC: Personal Care
Products Council, 2012.
2. Bissett NG (ed). Rosmarini folium. In: Herbal Drugs and Phytopharmaceuticals. Stuttgart: Medpharm; 1994:428-430.
3. Cronin H and Draelos ZD. Top 10 botanical ingredients in 2010 anti-aging creams. J Cosmet Dermatol. 2010;9(3):218-225.
4. Leung AY and Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. 2nd ed. New York,
NY: John Wiley & Sons, Inc., 1996.
5. PDR for Herbal Medicines. 4th ed. Montvale, NJ: Thomson Healthcare Inc, 2007.
6. Al-Sereiti MR, Abu-Amer KM, and Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic
potentialsq. Indian J Exp Biol. 1999;37:124-130.
7. Council of Experts, United States Pharmacopeial Convention. Food Chemicals Codex. 8th ed. Rockville, MD: United States
Pharmacopeia (USP), 2012.
8. European Food Safety Authority (EFSA). Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and
Materials in Contact with Food on a request from the Commission on the use of rosemary extracts as a food additive.
The EFSA Journal. 2008;721:1-29.
9. Bouhlal K, Meynadier J, Peyron J-L, and Meynadier J. The cutaneous effects of the common concretes and absolutes used in the
perfume industry. J Essent Oil Res. 1989;1(4):169-195.
10. Natural Sourcing. Rosemary antioxidant extract - 14% diterpene phenols. [pamphlet]. Oxford, CT: Natural Sourcing LLC; 2013.
11. Natural Sourcing. Rosemary antioxidant extract- 25% diterpene phenols. [pamphlet]. Oxford, CT: Natural Sourcing LLC; 2013.
12. Flavex Naturextrakte GmbH. Rosemary antioxidant extract 25% diterpene phenols, type no. 027.020 [pamphlet]. 2010.
13. Natural Sourcing. Organic rosemary oil extract. [pamphlet]. Oxford, CT: Natural Sourcing LLC; 2013.
14. Maistro EL, Mota SF, Lima EB, Bernardes BM, and Goulart FC. Genotoxicity and mutagenicity of Rosmarinus officinalis
(Labiatae) essential oil in mammalian cells in vivo. Genetics and Molecular Research. 2010;9(4):2113-2122.
15. Flavex Naturextrakte GmbH. Certificate of Analysis: Rosemary antioxidant extract, type 027.020 25% diterpene phenols
[pamphlet]. 2013.
16. Natural Sourcing. C0
2
Rosemary Extract Select Certificate of Analysis. [pamphlet]. Oxford, CT: Natural Sourcing LLC; 2011.
17. Natural Sourcing. Organic Rosemary Antioxidant C0
2
Extract 14% Diterpene Phenols Certificate of Analysis. [pamphlet].
Oxford, CT: Natural Sourcing LLC; 2012.
18. Natural Sourcing. Organic Rosemary Antioxidant C0
2
Extract 25% Diterpene Phenols Certificate of Analysis. [pamphlet].
Oxford, CT: Natural Sourcing LLC; 2013.
19. European Commission. Memorandum on the SCCNFP Opinion concerning Fragrance Allergy in Consumers adopted by the
SCCNFP during the 16th Plenary Meeting of 13 March
2001.
http://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/sccnfp_opinions_97_04/sccp_out137
_en.htm. Date Accessed 1-28-2014.
20. Flavex Naturextrakte GmbH. Allergen compounds according to Cosmetic Guideline 76/768/EEC Rosmary antioxidant extact
25% dieterpene phenols, type 027.020 [pamphlet]. 2013.
21. Prevedello M, Veggetti E, and Rapelli S. Essential oils and the antioxidant compounds from Rosmarinus officinalis L. Their
rational use in cosmetics. Journal of Applied Cosmetology. 1998;16(1):17-25.
22. Anadón A, Martínez-Larrañaga MR, Martìnez MA., Ares I, García-Risco MR, Señoráns FJ, and Reglero G. Acute oral safety
study of rosemary extracts in rats. J Food Prot. 2008;71(4):790-795.
23. del Baño MJ, Lotrente J, Castillo J, Benavente-García O, del Río JA, Ortuño A, Qurin K-W, and Gerard D. Phenolic Diterpenes,
Flavones, and Rosmarinic Acid Distribution during the Development of Leaves, Flowers, Stems, and Roots of
Rosmarinus officinalis. Antioxidant Activity. J Agric Food Chem. 2003;51(15):4247-4253.
24. Munné-Bosch S and Alegre L. Subcellular compartmentation of the dieterpene carnosic acid and its derivatives in the leaves of
rosemary. Plant Physiology. 2001;125:1094-1102.
25. Diab Y, Auezova L, Chebib H, Chalchat J-C, and Figueredo G. Chemical composition of Lebanese rosemary (Rosmarinus
officinalis L.) essential oil as a function of the geographical region and the harvest time. J.Essent .Oil Res.
2002;14(6):449-452.
26. Food and Drug Administration (FDA). Frequency of use of cosmetic ingredients. FDA Database. 2014.
27. Personal Care Products Council. 12-10-2013. Updated concentration of Use by FDA Product Category:Rosemary-Derived
Ingredients memo. Unpublished data submitted by Personal Care Products Council. 4 pages.
28. Personal Care Products Council. 7-29-2013. Concentration of use by FDA Product Category: Rosmarinic Acid. Unpublished data
submitted by Personal Care Products Council. 1 pages.
29. Bremmer HJ, Prud'homme de Lodder LCH, and Engelen JGM. Cosmetics Fact Sheet: To assess the risks for the consumer;
Updated version for ConsExpo 4. 2006. Report No. RIVM 320104001/2006. pp. 1-77.
30. Johnsen MA. The influence of particle size. Spray Technology and Marketing. 2004;14(11):24-27.
31. Rothe H. Special Aspects of Cosmetic Spray Evalulation. 9-26-2011. Unpublished data presented at the 26 September CIR
Expert Panel meeting. Washington, D.C.
32. Rothe H, Fautz R, Gerber E, Neumann L, Rettinger K, Schuh W, and Gronewold C. Special aspects of cosmetic spray safety
evaluations: Principles on inhalation risk assessment. Toxicol Lett. 2011;205(2):97-104.
33. European Commission. Cosmetics Directive
(v.1). http://ec.europa.eu/consumers/cosmetics/cosing/index.cfm?fuseaction=search.results
. Date Accessed 2-11-2013.
34. European Commission. Official Journal of the European Union. Cosmetic Directive 2010/69/EU of 22 October 2010 amending
the Annexes of the European Parliament and Council Directive 95/2/EC on food additives other than colours and
sweeteners. 2010.
35. Merck, Sharpe, & Dohme Corp. Rosemary; monograph number:
8264.
http://themerckindex.cambridgesoft.com/themerckindex/Forms/Search/ContentArea/ChemBioVizSearch.aspx?F
ormGroupId=200000&AppName=THEMERCKINDEX&AllowFullSearch=true&KeepRecordCountSynchronized=fal
se&SearchCriteriaId=23&SearchCriteriaValue=rosemary&CurrentIndex=0. The Merck Index. Date Accessed 2-12-
2013.
36. World Health Organization (WHO). WHO monographs on selected medicinal plants. Geneva, Switzerland: WHO Press, 2009.
37. Petersen M and Simmonds MSJ. Rosmarinic acid. Phytochemistry. 2003;62(2):121-125.
38. Eggensperger H, Wilker M, and Bauer P. Rosmarinic acid. A natural multiactive substance for cosmetics and dermatology. Part
2. Combinations of rosmarinic acid with other natural ingredients. SOFW Journal. 1998;124(10):634-636,639.
39. Wang L-H, Wang C-C, and Kuo S-C. Vehicle and enhancer effects on human skin penetration of aminophylline from cream
formulations: evaluation in vivo. J Cosmet Sci. 2007;58(3):245-254.
40. Aronson DB, Bosch S, Gray DA, Howard PH, and Guiney PD. A comparative human health risk assessment of p-
dichlorobenzene-based toilet rimblock products versus fragrance/surfactant-based alternatives. Journal of Toxicology
and Environmental Health, Part B: Critical Reviews. 2007;10(7):467-526.
41. Fahim FA, Esmat AY, Fadel HM, and Hassan KFS. Allied studies on the effect of Rosmarinus officinalis L. on experimental
hepatotoxicity and mutagenesis. Int J Food Sci Nutr. 1999;50(6):413-427.
42. Opdyke DL. Fragrance raw materials monographs: Rosemary oil. Food and Cosmetics Toxicology. 1974;12(7-8):977-978.
43. Mengoni ES, Vichera G, Rigano LA, Rodriguez-Puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno S, and Vojnov A.
Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive
compounds from Rosmarinus officinalis L. Fitoterapia. 2011;82(3):414-421.
44. Huang M-T, Ho C-T, Wang ZY, Ferraro T, Lou Y-R, Stauber K, Ma W, Georgiadis C, LAskin JD, and Conney AH. Inhibition of
skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Research. 1994;54:701-708.
45. Martin R, Pierrard C, Lejeune F, Hilaire P, Breton L, and Bernerd F. Photoprotective effect of a water-soluble extract of
Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and
reconstructed skin. Eur J Dermatol. 2008;18(2):128-135.
46. Nusier MK, Bataineh HN, and Daradkah HM. Adverse effects of rosemary (Rosmarinus officinalis L.) on reproductive function
in adult male rats. Exp Biol Med. 2007;232:809-813.
47. Lemonica IP, Damasceno Dc, and di-Stasi LC. Study of the embryotoxic effects of an extract of rosemary (Rosmarinus
officinalis L.). Braz J Med Biol Res. 1996;29(2):223-227.
48. Herbal Drugs and Phytopharmaceuticals. Stuttgart: Medpharm, 1994.
49. Zhu BT, Loder DP, Cai MX, Ho C-T, Huang M-T, and Conney AH. Dietary administration of an extract from rosemary leaves
enhances the liver microsomal metabolism of endogenous estrogens and decreases their uterotropic action in CD-1
mice. Carcinogenesis. 1998;19(10):1821-1827.
50. Greenlee H, Atkinson C, Stanczyk FZ, and Lampe JW. A pilot and feasibility study on the effects of naturopathic botanical and
dietary interventions on sex steriod hormon metabolism in premenopausal women. Cancer Epidemiol Biomarkers.
2007;16(8):1601-1609.
51. Žegura B, Dobnik D, Niderl MHZ, and Filipic M. Antioxidant and antigenotoxic effects of rosemary (Rosmarinus officinalis L.)
extracts in Salmonella typhimurium TA98 and HepG2 cells. Environmental toxicology and pharmacology.
2011;32(2):296-305.
52. Bersani C, Cantoni C, and Soncini G. Ames test valuation of mutagenic activity in essences and spices. Arch Vet Ital.
1981;32:10-11.
53. Gaiani TF, Carvalho JCT, Silva JMSF, and Maistro EL. Absence of clastogenic effects of the extract from medicinal plant
Rosmarinus officinalis L. on Wistar rat bone marrow cells. Cytologia. 2006;71:101-106.
54. Aleisa AM. Cytological and biochemical effects of St. John's Wort supplement (a complex mixture of St. John's Wort, Rosemary
and Spirulina) on somatic and germ cells of Swiss Albino mice. Int J Environ Res Public Health. 2008;5(5):408-417.
55. Sancheti G and Goyal PK. Effect of Rosmarinus officinalis in modulating 7,12-dimethylbenz(a)anthracene induced skin
tumorigenesis in mice. Phytother Res. 2006;20(11):981-986.
56. Sancheti G and Goyal PK. Modulatory influence of Rosmarinus officinalis on DMBA-induced mouse skin tumorigenesis. Asian
Pacific Journal of Cancer Prevention. 2006;7:331-335.
57. Singletary KW and Nelshoppen JM. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis
and of in vivo formation of mammary DMBA-DNA adducts by rosemary extract. Cancer Lett. 1991;60(2):169-175.
58. Komeh-Nkrumah SA, Nanjundaiah SM, Rajaiah R, Yu H, and Moudgil KD. Topical dermal application of essential oils
attenuates the severity of adjuvant arthritis in Lewis rats. Phytother Res. 2012;26(1):54-59.
59. Guin JD. Use of consumer product ingredients for patch testing. Dermatitis. 2005;16(2):71-77.
60. Anonymous. 1998. Human patch test of a product containing 0.2% Rosmarinus Officinalis (Rosemary) Leaf Extract.
Unpublished data submitted by Personal Care Products Council.
61. KGL Inc (Ivy Laboratories). 1998. An evaluation of the contact-sensitization potential of a topical coded product in human skin
by means of the maximization assay (product contains 0.2% Rosmarinus Officinalis (Rosemary) Leaf Extract).
Unpublished data submitted by Personal Care Products Council.
62. Reliance Clinical Testing Services, Inc. 2009. Summary of an HRIPT of a hair spray containing 0.0013% Rosmarinus Officinalis
(Rosemary) Leaf Extract. Unpublished data submitted by Personal Care Products Council. 1 pages.

63. Clinical Research Services. 2007. Human repeat insult patch test of a massage oil containing 1.5% Rosmarinus Officinalis
(Rosemary) Leaf Oil. Unpublished data submitted by Personal Care Products Council. 32 pages.
64. Inui S and Katayama I. Allergic contact dermatitis induced by rosemary leaf extract in a cleansing gel. Journal of Dermatology.
2005;3253:667179-669180.
65. Hjorther AB, Christophersen C, Hausen BM, and Menné T. Occupational allergic contact dermatitis from carnosol, a naturally-
occurring compound present in rosemary. Contact Dermatitis. 1997;37(3):99-100.
66. Trattner A, David M, and Lazarov A. Occupational contact dermatitis due to essential oils. Contact Dermatitis. 2008;58(5):282-
284.
67. Guin JD. Rosemary cheilitis: one to remember. Contact Dermatitis. 2001;45(1):63.
68. Armisén M, Rodríguez V, and Vidal C. Photoaggravated allergic contact dermatitis due to Rosmarinus officinalis cross-reactive
with Thymus vulgaris. Contact Dermatitis. 2003;48(1):52-53.
69. Serra E, Vila A, Peramiquel L, Dalmau J, Granel C, and Alomar A. Allergic contact dermatitis due to rosemary. Contact
Dermatitis. 2005;53(3):179-180.
70. Fernandez L, Duque S, Sanchez I, Quiñones D, Rodriquez F, and Garcia-Abujeta JL. Allergic contact dermatitis from rosemary
(Rosmarinus officinalis L.). Contact Dermatitis. 1997;37(5):248-249.
71. Martínez-González MC, Buján JJG, Gómez WM, and Capdevila EF. Concomitant allergic contact dermatitis due to Rosmarinus
officinalis (rosemary) and Thymus vulgaris (thyme). Contact Dermatitis. 2007;56(1):49-50.
72. González-Mahave I, Lobesa T, del Pozo MD, Blasco A, and Venturini M. Rosemary contact dermatitis and cross-reactivity with
other labiate plants. Contact Dermatitis. 2006;54(4):210-212.
73. Natural Sourcing. Rosemary Essential Oil Certificate of Analysis. [pamphlet]. Oxford, CT: Natural Sourcing LLC; 2012.
74. Duke JA. Dr. Duke's Phytochemical and Ethnobotanical Databases. Chemicals in Rosmarinus officinalis L. (Lamiaceae) --
rosemary. http://www.ars-grin.gov/duke/
. Date Accessed 2-19-2013.
75. Committee of Experts on Cosmetic Products. Plants in Cosmetics. Plants and plant preparations used as ingredients for cosmetic
products. Strasbourg: Council of Europe Publishing, 2002.
76. de Melo GAN, Grespan R, Fonseca JP, Farinha TO, Silva EL, Romero AL, Bersani-Amado CA., and Cuman RKN. Rosmarinus
officinalis L. essential oil inhibits in vivo and in vitro leukocyte migration. Journal of medicinal food. 2011;14(9):944-
946.
77. Harinantenaina L. Tocotrienols in Plants: Sources and importance. Chapter: 4. Watson RR and Preedy VR. In: Tocotrienols.
Vitamin E Beyond Tocopherols. Boca Ratopn, FL: CRC Press; 2009:43-60.
78. Jiang Y, Wu N, Fu Y-J, Wang W, Luo M, Zhao C-J, Zu Y-G, and Liu X-L. Chemical composition and antimicrobial activity of
the essential oil of Rosemary. Environmental toxicology and pharmacology. 2011;32(1):63-68.
79. Jalali-Heravi M, Moazeni RS, and Sereshti H. Analysis of Iranian rosemary essential oil: application of gas chromatography-
mass spectrometry combined with chemometrics. Journal of chromatography.A. 2011;1218(18):2569-2576.
80. Stagos D, Spanou C, Margariti M, Stathopoulos C, Mamuris Z, Kazantzoglou G, Magiatis P, and Kouretas D. Cytogenetic effects
of grape extracts (Vitis vinifera) and polyphenols on mitomycin C-induced sister chromatid exchanges (SCEs) in
human blood lymphocytes. J Agric Food Chem. 2007;55(13):5246-5252.
81. Andersen FA, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Leibler DC, Marks JG, Shank RC, Slaga TJ, and Snyder PW.
Final report of the Cosmetic Ingredient Review Expert Panel. Amended safety assessment of Calendula officinalis-
derived cosmetic ingredients. Int J Toxicol. 2010;29(4):221S-243S.
82. World Health Organization (WHO). International Agency for Research (IARC). Volume 56. Some Naturally Occurring
Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Su mmary of data reported
and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol56/volume56.pdf
. Date Accessed 2-26-2013.

83. Integrated Laboratory Systems. Chlorogenic Acid [327-97-9] and Caffeic Acid [331-39-5]. Review of toxicological
literature. http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/ChlorogenicAcid.pdf
. Date Accessed 2-
26-2013.
84. Doolaege EH, Raes K, de Vos F, Verhe R, and de Smet S. Absorption, distribution and elimination of carnosic acid, a natural
antioxidant from Rosmarinus officinalis, in rats. Plant Food Hum Nutr. 2011;66(2):196-202.
85. World Health Organization (WHO). International Agency for Research (IARC). Volume 73. Some Chemicals that Cause
Tumours of the Kidney or Urinary Bladder in Rodents and Some Other Substances. Summary of data reported and
evaluation. http://monographs.iarc.fr/ENG/Monographs/vol73/volume73.pdf
. Date Accessed 7-8-2013.
86. National Toxicology Program (NTP). Testing status of agents at NTP: α-pinene. http://ntp.niehs.nih.gov/?objectid=BD4A21C3-
123F-7908-7B76D9A5ADDD10A3. Date Accessed 3-13-2013.
87. National Toxicology Program (NTP). Testing status of agents at NTP: 1,8-
cineole. http://ntp.niehs.nih.gov/?objectid=BC9623D7-123F-7908-7BE9A208CC6CB46A
. Date Accessed 3-13-2013.
88. Integrated Laboratory Systems. β-Myrcene [123-35-3]. Review of toxicological
literature. http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/beta-myrcene_508BE.pdf. Date Accessed
3-13-2013.
89. National Toxicology Program (NTP). NTP technical report on the toxicology and carcinogenesis studies of β-myrcene (CAS No.
123-35-3) in F344/N rats and B6C3F1 mice. (Gavage studies).
2010. http://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr557.pdf
. Report No. NTP TR 557. NIH Publication No. 11-5898.
90. International Fragrance Association (IFRA). IFRA Standards, 38th Amendment: linalool. http://www.ifraorg.org/en-
us/standards-library/s/linalool#.U1r8dzXD-Uk. Date Accessed 4-25-2014.
91. National Toxicology Program (NTP). NTP Technical Report on the toxicology and carcinogenesis studies of α,β-thujone (CAS
No. 76231-76-0) in F34.N rats and B6C3F1 mice. (Gavage studies).
2011. http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/TR570.pdf
. Report No. NTP TR 570.
92. World Health Organization (WHO). IARC Monographs on the evaluation of carcinogenic risks to humans.
Methyleugenol. http://monographs.iarc.fr/ENG/Monographs/vol101/mono101-013.pdf
. Date Accessed 6-3-2013.
93. International Fragrance Association (IFRA). IFRA Standard, 36th Amendment: methyl eugenol. http://www.ifraorg.org/en-
us/search/s/methyleugenol#.U1r-RTXD-Uk. Date Accessed 4-25-2014.
94. RIFM Expert Panel, Belsito D, Bikcers D, Bruze M, Calow P, Greim H, Hanifin JM, Rogers AE, Saurat JH, Sipes IG, and
Tagami H. A toxicologic and dermatologic assessment of cyclic and non-cyclic terpene alcohols when used as
fragrance ingredients. Food Chem Toxicol. 2008;46:S1-S71.
95. Minnunni M, Wolleb U, Mueller O, Pfiefer A, and Aeschbacher HU. Natural antioxidants as inhibitors of oxygen species
induced mutagenicity. Mutat Res. 1992;269:193-200.
