
U.S. Department of Health and Human Services
Centers for Disease Control and Prevention
Morbidity and Mortality Weekly Report
Recommendations and Reports / Vol. 67 / No. 1 January 12, 2018
Prevention of Hepatitis B Virus Infection in the
United States: Recommendations of the Advisory
Committee on Immunization Practices

Recommendations and Reports
The MMWR series of publications is published by the Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC),
U.S. Department of Health and Human Services, Atlanta, GA 30329-4027.
Suggested citation: [Author names; first three, then et al., if more than six.] [Title]. MMWR Recomm Rep 2018;67(No. RR-#):[inclusive page numbers].
Centers for Disease Control and Prevention
Brenda Fitzgerald, MD, Director
William R. Mac Kenzie, MD, Acting Associate Director for Science
Joanne Cono, MD, ScM, Director, Office of Science Quality
Chesley L. Richards, MD, MPH, Deputy Director for Public Health Scientific Services
Michael F. Iademarco, MD, MPH, Director, Center for Surveillance, Epidemiology, and Laboratory Services
MMWR Editorial and Production Staff (Serials)
Sonja A. Rasmussen, MD, MS, Editor-in-Chief
Charlotte K. Kent, PhD, MPH, Executive Editor
Christine G. Casey, MD, Editor
Teresa F. Rutledge, Managing Editor
David C. Johnson, Lead Technical Writer-Editor
Jeffrey D. Sokolow, MA, Project Editor
Martha F. Boyd, Lead Visual Information Specialist
Maureen A. Leahy, Julia C. Martinroe,
Stephen R. Spriggs, Tong Yang,
Visual Information Specialists
Quang M. Doan, MBA, Phyllis H. King,
Paul D. Maitland, Terraye M. Starr, Moua Yang,
Information Technology Specialists
MMWR Editorial Board
Timothy F. Jones, MD, Chairman
Matthew L. Boulton, MD, MPH
Virginia A. Caine, MD
Katherine Lyon Daniel, PhD
Jonathan E. Fielding, MD, MPH, MBA
David W. Fleming, MD
William E. Halperin, MD, DrPH, MPH
King K. Holmes, MD, PhD
Robin Ikeda, MD, MPH
Rima F. Khabbaz, MD
Phyllis Meadows, PhD, MSN, RN
Jewel Mullen, MD, MPH, MPA
Jeff Niederdeppe, PhD
Patricia Quinlisk, MD, MPH
Patrick L. Remington, MD, MPH
Carlos Roig, MS, MA
William L. Roper, MD, MPH
William Schaffner, MD
CONTENTS
Introduction ............................................................................................................1
New or Updated Recommendations .............................................................1
Methods ....................................................................................................................2
HBV Background ....................................................................................................3
Prophylaxis Against HBV Infection ..................................................................9
Recommendations ............................................................................................. 13
Future Directions ................................................................................................ 25
Acknowledgments ............................................................................................. 25
References ............................................................................................................. 25
Disclosure of Relationships
The 2016–2017 ACIP Hepatitis Vaccines Work Group
members wish to disclose that they have no financial or
competing interests with the manufacturers of commercial
products or suppliers of commercial services related to
hepatitis B (HepB) vaccines. Content will not include any
discussion of the unlabeled use of a product or a product under
investigational use, with the following exceptions:
• use of Pediarix vaccine for infants born to hepatitis B
surface antigen (HBsAg)–positive mothers or mothers
with an unknown HBsAg status to complete the vaccine
series after receipt of a birth dose of single-antigen HepB
vaccine and hepatitis B immune globulin (HBIG);
• alternate 3-dose vaccine administration schedules heeding
to minimum intervals of 4 weeks between the first and
second dose, 8 weeks between the second and third dose,
and 16 weeks between the first and third dose;
• modified dosing regimens (e.g., doubling of standard dose
and administration of additional doses) in certain
circumstances (e.g., for persons with immunocompromising
conditions); and
• antiviral therapy during pregnancy for the prevention of
perinatal hepatitis B virus (HBV) transmission.

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
Prevention of Hepatitis B Virus Infection in the United States:
Recommendations of the Advisory Committee
on Immunization Practices
Sarah Schillie, MD
1
Claudia Vellozzi, MD
1
Arthur Reingold, MD
2
Aaron Harris, MD
1
Penina Haber, MPH
3
John W. Ward, MD
1
Noele
P. Nelson, MD
1
1
Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC
2
University of California, Berkeley School of Public Health, Berkeley, California
3
Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC
Summary
Hepatitis B virus (HBV) is transmitted via blood or sexual contact. Persons with chronic HBV infection are at increased risk for
cirrhosis and liver cancer and require medical care. This report updates and summarizes previously published recommendations
from the Advisory Committee on Immunization Practices (ACIP) and CDC regarding the prevention of HBV infection in the
United States. ACIP recommends testing all pregnant women for hepatitis B surface antigen (HBsAg), and testing HBsAg-positive
pregnant women for hepatitis B virus deoxyribonucleic acid (HBV DNA); administration of HepB vaccine and hepatitis B immune
globulin (HBIG) for infants born to HBV-infected women within 12 hours of birth, followed by completion of the vaccine series and
postvaccination serologic testing; universal hepatitis B vaccination within 24 hours of birth, followed by completion of the vaccine
series; and vaccination of children and adolescents aged <19 years who have not been vaccinated previously. ACIP recommends
vaccination of adults at risk for HBV infection, including universal vaccination of adults in settings in which a high proportion
have risk factors for HBV infection and vaccination of adults requesting protection from HBV without acknowledgment of a
specific risk factor. These recommendations also provide CDC guidance for postexposure prophylaxis following occupational and
other exposures. This report also briefly summarizes previously published American Association for the Study of Liver Diseases
guidelines for maternal antiviral therapy to reduce perinatal HBV transmission.
Introduction
Hepatitis B virus (HBV) is transmitted through percutaneous
(i.e., puncture through the skin) or mucosal (i.e., direct contact
with mucous membranes) exposure to infectious blood or
body fluids. HBV is highly infectious, can be transmitted
in the absence of visible blood (1,2), and remains viable on
environmental surfaces for at least seven days (3). Persons
with chronic infection (e.g., those with persistent hepatitis B
surface antigen [HBsAg] in the serum for at least 6 months
following acute infection) serve as the main reservoir for HBV
transmission (4).
This report summarizes and consolidates previously
published recommendations from the Advisory Committee
on Immunization Practices (ACIP) and CDC. It also
Corresponding author: Sarah Schillie, Division of Viral Hepatitis,
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB
Prevention, CDC. Telephone: 404-718-8608; E-mail: [email protected].
contains updates to recommendations for the prevention of
HBV infection in the United States. A list of frequently used
abbreviations is provided (Box 1).
New or Updated Recommendations
The following recommendations are new or updated:
• universal hepatitis B (HepB) vaccination within
24 hours of birth for medically stable infants weighing
≥2,000 grams;
• testing HBsAg-positive pregnant women for hepatitis B
virus deoxyribonucleic acid (HBV DNA);
• postvaccination serologic testing for infants whose
mother’s HBsAg status remains unknown indefinitely (e.g.,
when a parent or person with lawful custody surrenders
an infant confidentially shortly after birth);
• single-dose revaccination for infants born to HBsAg-
positive women not responding to the initial vaccine series;
Recommendations and Reports
2 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
• vaccination for persons with chronic liver disease (including,
but not limited to, those with hepatitis C virus [HCV]
infection, cirrhosis, fatty liver disease, alcoholic liver disease,
autoimmune hepatitis, and an alanine aminotransferase
[ALT] or aspartate aminotransferase [AST] level greater than
twice the upper limit of normal); and
• removal of permissive language for delaying the birth dose
until after hospital discharge.
This report also briefly summarizes American Association for
the Study of Liver Diseases (AASLD) guidelines for maternal
antiviral therapy to reduce perinatal HBV transmission,
published previously (5). Recommendations from the
Infectious Diseases Society of America (IDSA) regarding
vaccination of the immunocompromised host are published
separately (6).
Methods
ACIP’s Hepatitis Work Group comprises professionals
from academic medicine (pediatrics, family medicine,
internal medicine, infectious disease, occupational health,
and preventive medicine specialists), federal and state public
health agencies, and medical societies.* The Work Group
reviewed epidemiology and literature, directed an economic
analysis, and deliberated upon recommendations. The
Work Group considered existing published ACIP and CDC
vaccine recommendations in summarizing recommendations
contained herein for the prevention of HBV infection.
This report updates and supplants ACIP recommendations
for HepB vaccination of children and adults published
previously (7,8). This report incorporates ACIP and CDC
recommendations published previously (9–11).
Guidelines from AASLD inform the use of antiviral
therapy among pregnant women with elevated HBV DNA
for the purpose of preventing perinatal HBV transmission.
Surveillance data were obtained from the National Notifiable
Diseases Surveillance System (NNDSS) (https://wwwn.cdc.
gov/nndss/).
Data informing clarifications to the recommendations were
summarized on the basis of findings from literature searches
that were completed on May 11, 2016. Two search terms were
used to ascertain data regarding maximum number of doses
for dialysis patients and minimum intervals for dialysis dosing:
“Hepatitis b vacc* dialysis boost*” and “Dialysis hepatitis b
vacc* schedule.” Epidemiologic and vaccine coverage data were
reviewed, as well as publicly available data on the number
of infant abandonments and safely surrendered infants. The
literature searches included clinical trials and comparative
* A list of the members appears on page 30.
BOX 1. Abbreviations used in this report
AASLD American Association for the Study of
Liver Diseases
ACIP Advisory Committee on Immunization
Practices
anti-HBc antibody to hepatitis B core antigen
anti-HBe antibody to hepatitis B e antigen
anti-HBs antibody to hepatitis B surface antigen
HBeAg hepatitis B e antigen
HBIG hepatitis B immune globulin
HBsAg hepatitis B surface antigen
HBV hepatitis B virus
HBV DNA hepatitis B virus deoxyribonucleic acid
HCP health care personnel
HCV hepatitis C virus
HepB hepatitis B
HIV human immunodeficiency virus
IDSA Infectious Diseases Society of America
IDU Injection-drug use
IgM Immunoglobulin class M
IgG Immunoglobulin class G
MSM men who have sex with men
NNDSS National Notifiable Diseases Surveillance
System
PHBPP Perinatal Hepatitis B Prevention Program
PWID persons who inject drugs
QALY quality-adjusted life-year
STI sexually transmitted infection
VAERS Vaccine Adverse Events Reporting System
VSD Vaccine Safety Datalink
studies conducted worldwide and published in English since
2000. All studies yielding pertinent information were eligible
for inclusion. Search results were supplemented by additional
relevant papers identified by subject matter experts on the
Work Group. Per the ACIP process, it was predetermined that
Grading of Recommendations Assessment, Development and
Evaluation (GRADE) was not required for these updates of
existing recommendations.
To assess vaccine safety, the Work Group searched two
postlicensure surveillance systems for adverse events from
2005 through 2015: the Vaccine Adverse Events Reporting
System (VAERS) (https://vaers.hhs.gov) and the Vaccine
Safety Datalink (VSD) (https://www.cdc.gov/vaccinesafety/
ensuringsafety/monitoring/vsd). VAERS is a national passive
surveillance system, and VSD conducts population-based
vaccine safety studies. VAERS can generate vaccine safety
hypotheses but cannot assess causality and is subject to several
limitations, including reporting biases and inconsistent data
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 3
US Department of Health and Human Services/Centers for Disease Control and Prevention
quality (12,13). VSD can be used to assess hypotheses that
arise from reviews of medical literature, reports to VAERS,
changes in immunization schedules, or the introduction of
new vaccines (14).
During February–September 2016, the Work Group held
five teleconference meetings. Work Group and ACIP members
also reviewed and commented on a draft of the statement prior
to the ACIP’s October 2016 meeting. A summary of Work
Group discussions was presented to ACIP on October 19, 2016.
At that time, ACIP members voted to approve a draft HepB
vaccine recommendations statement, including recommending
universal HepB vaccination within 24 hours of birth for
medically stable infants weighing ≥2,000 grams. In January
2017, the Work Group held a teleconference meeting to review
results of an economic analysis of single-dose revaccination
for infants born to HBsAg-positive women. Results from
that analysis were presented to ACIP on February 22, 2017.
Recommendations were not evaluated using GRADE, but
expert opinion was used to shape the recommendations. At
that time, ACIP members voted to approve language for single-
dose revaccination for infants (regardless of birth weight) born
to HBsAg-positive women. Modifications were made to the
ACIP statement during the subsequent review process at CDC
to update and clarify wording in the report.
HBV Background
Epidemiology
In 2015, a total of 3,370 cases of acute HBV infection
were reported to CDC. The actual number of acute cases is
believed to be 6.5 times the number of reported cases in any
year. It is estimated that 21,900 new cases of HBV occurred
in 2015 after under-ascertainment and under-reporting were
considered (4). The rate of reported acute HBV infections
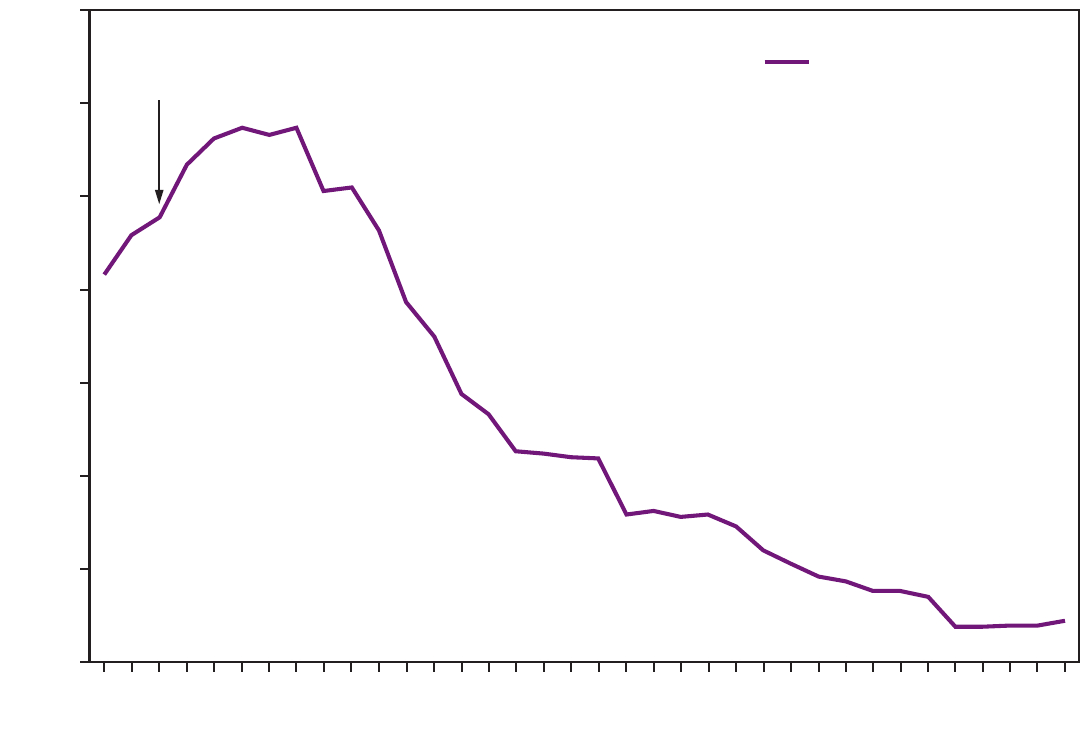
declined 88.5% since recommendations for HepB vaccination
were first issued, from 9.6 cases per 100,000 population
in 1982 to 1.1 cases per 100,000 population in 2015 (15),
although the rate of acute HBV infections remained fairly
stable during 2010–2015 (4) (Figure 1). The 2015 incidence
is greatest for persons aged 30–39 years (2.6 per 100,000
population). In 2015, persons aged ≤19 years had the lowest
incidence (0.02 cases per 100,000 population), likely a result
of routine infant vaccination. Although the incidence of acute
HBV infection is greater for males than for females, the gap
has narrowed; in 2015, the rate for males was approximately
1.6 times higher than that for females (1.3 cases and 0.8 cases
per 100,000 population, respectively) (4). During 2009–2013,
the combined incidence of acute HBV infection in three states
(Kentucky, Tennessee, and West Virginia) increased 114% and
was associated with increasing injection-drug use (16).
On the basis of national health survey data, it is estimated
that approximately 850,000 persons are living with HBV
infection (prevalence) in the United States (17,18). Studies
based on data from countries of persons migrating to the
United States and census data indicate that the total prevalence
of chronic hepatitis B might be as high as 2.2 million persons
(19), suggesting that the national health survey-based estimate
might be conservative. Foreign-born persons account for
approximately 95% of newly reported chronic infections in the
United States (20); the prevalence of chronic HBV infection
is approximately 3.5% among foreign-born persons (19), and
the majority of chronic HBV infections in the United States
are among Asians/Pacific Islanders.
Strategy to Eliminate HBV
In 1991, the United States adopted a strategy for universal
HepB vaccination of infants (21). A comprehensive strategy
to eliminate HBV transmission evolved over the ensuing three
decades and encompasses 1) routine testing of all pregnant
women for HBsAg and prophylaxis for infants born to HBsAg-
positive mothers, 2) universal vaccination of infants beginning at
birth, 3) routine vaccination of previously unvaccinated children
and adolescents, and 4) vaccination of adults at risk for HBV
infection (7–11,21–26). Preventing perinatal transmission relies
upon testing all pregnant women for HBsAg and administering
timely prophylaxis (HepB vaccine and hepatitis B immune
globulin [HBIG]) to infants born to infected mothers. Universal
HepB vaccination of all infants beginning at birth provides a
critical safeguard and prevents infection among infants born
to HBsAg-positive mothers not identified prenatally (e.g., in
situations where the mother was not tested or when testing,
interpretation, or transcription errors occurred). Vaccination
of children and adolescents not previously vaccinated and
vaccination of adults at risk for HBV infection (e.g., by sexual
or percutaneous exposure and international travelers to certain
countries) is recommended to prevent HBV transmission outside
of the perinatal setting (Box 2).
HBV prevention strategies have been implemented
successfully in the United States, but challenges remain.
Approximately 88% of commercially insured women and
84% of Medicaid-enrolled women are tested for HBsAg
during pregnancy (27). In one study of a large health system
in northern California, 93% of HBsAg-positive pregnant
women were tested for HBV DNA (28). Most (94.9%) infants
born to infected women receive recommended prophylaxis
within 12 hours of birth (29). Universal HepB vaccine birth
dose coverage, defined as 1 dose of vaccine administered
Recommendations and Reports
4 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
FIGURE 1. Incidence of hepatitis B virus infection — National Notifiable Diseases Surveillance System, United States, 1980–2015
0
50
100
150
200
250
300
350
Incidence (1,000s)
New Hepatitis B infections
1980 1982 1984 1986 1988 1990 1992 1994 1996 1998 2000 2002 2004 2010 2012 20142006 2008
Years
Vaccine recs were
rst issued in 1982
by 3 days of life, is 71.1% (30), an increase from 50.1%
during 2003–2005 prior to revised ACIP recommendations
for the birth dose before hospital discharge (31), but below
the Healthy People 2020 target of 85% (32). HepB vaccine
coverage (≥3 doses) among children aged 19–35 months and
13–17 years is 90.5% (30) and 91.4% (33), respectively.
Vaccine coverage (≥3 doses) is lower among adults: 27.4%
among adults who report chronic liver conditions; 31.6%
among adults who traveled outside the United States to
countries other than Europe, Japan, Australia, New Zealand,
or Canada since 1995; and 24.4% among adults with
diabetes aged 19–59 years and 12.6% of adults with diabetes
aged ≥60 years (34). Among health care personnel (HCP),
≥3-dose coverage was 64.7%, an increase from 51% in 1992
shortly after implementation of the Needlestick Safety and
Prevention Act (35), but well below the Healthy People 2020
target of 90% (32,34).
New strategies for further reducing HBV transmission in
this report include testing HBsAg-positive pregnant women
for HBV DNA to identify infants at greatest risk for infection
and guide the use of maternal antiviral therapy (36,37).
Published evidence indicates that maternal antiviral therapy
during pregnancy further reduces perinatal HBV transmission;
hence, AASLD suggests antiviral therapy when maternal HBV
DNA is >200,000 IU/mL (5,38,39).
Virus Description and Transmission
HBV is a 40–42-nm enveloped virus classified in the
Hepadnaviridae family. HBV contains a circular, partially
double-stranded DNA genome that is 3.2 kb in length. After
a susceptible person is exposed, the virus enters the liver
via the bloodstream. The liver is the primary site of HBV
replication (40–43).
HBV has been classified by two separate systems: serologic
subtype and genotype. Nine serologic subtypes initially were
described based on the heterogeneity of HBsAg: adrq+, adrq–,
ayr, ayw1, ayw2, ayw3, ayw4, adw2, and adw4 (44,45). Ten

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 5
US Department of Health and Human Services/Centers for Disease Control and Prevention
HBV genotypes, designated A–J, have been described. HBV
serotypes and genotypes vary geographically. Infection or
immunization with one genotype generally confers immunity
to all genotypes (7,44,46,47).
HBV is highly infectious, can be transmitted in the absence
of visible blood (22), and remains infectious on environmental
surfaces for at least 7 days (2,3). All HBsAg-positive persons are
infectious, but those with elevated HBV DNA or those with
hepatitis B e antigen (HBeAg), a protein from the hepatitis B
virus that circulates in the blood and is a marker of infectivity,
are most infectious. Persons with occult HBV infection (i.e.,
those who test negative for HBsAg but have detectable HBV
DNA) also might transmit infection (48).
HBV is transmitted through percutaneous, mucosal,
or nonintact skin exposure to infectious blood or body
fluids. HBV is concentrated most highly in blood, and
percutaneous exposure is an efficient mode of transmission.
Semen and vaginal secretions are infectious, and HBV also
can be detected in saliva, tears, and bile. Cerebrospinal fluid,
synovial fluid, pleural fluid, peritoneal fluid, pericardial fluid,
and amniotic fluid are also considered potentially infectious.
Urine, feces, vomitus, nasopharyngeal washings, sputum,
and sweat are not efficient vehicles of transmission unless
they contain blood because they contain low quantities of
infectious HBV. HBsAg found in breast milk is also unlikely
to lead to transmission, and hence HBV infection is not a
contraindication to breastfeeding (2,7,22).
Among adults, HBV is transmitted primarily by percutaneous
exposure to blood (e.g., by injection-drug use) and sexual
contact. HBV is transmitted efficiently by sexual contact
both among heterosexuals and among men who have sex
with men (MSM). Risk factors for sexual transmission among
heterosexuals include having unprotected sex with an infected
partner, having unprotected sex with more than one partner,
and a history of another sexually transmitted infection (STI).
Risk factors associated with sexual transmission among MSM
include having multiple sex partners, history of another
STI, and anal intercourse. Transmission can occur from
interpersonal contact (e.g., sharing a toothbrush or razor,
contact with exudates from dermatologic lesions, or contact
with HBsAg-contaminated surfaces) and in settings such as
schools, child care centers, and facilities for developmentally
disabled persons. Transmission of HBV from transfusion of
blood or blood products is rare because of donor screening
and viral inactivation procedures. Other possible sources of
infection include contaminated medical or dental instruments,
unsafe injections, needle-stick injuries, organ transplantation,
and dialysis (49).
BOX 2. Strategy to eliminate HBV transmission in the United States*
• Screening of all pregnant women for HBsAg
– HBV DNA testing for HBsAg-positive pregnant
women, with suggestion of maternal antiviral
therapy to reduce perinatal transmission when
HBV DNA is >200,000 IU/mL
– Prophylaxis (HepB vaccine and HBIG) for infants
born to HBsAg-positive
†
women
• Universal vaccination of all infants beginning at
birth
§,¶
as a safeguard for infants born to HBV-
infected mothers not identified prenatally
• Routine vaccination of previously unvaccinated
children aged <19 years
• Vaccination of adults at risk for HBV infection,
including those requesting protection from HBV
without acknowledgment of a specific risk factor
* Sources: Mast EE, Margolis HS, Fiore AE, et al. A comprehensive
immunization strategy to eliminate transmission of hepatitis B virus
infection in the United States: recommendations of the Advisory
Committee on Immunization Practices (ACIP). Part 1: immunization of
infants, children, and adolescents. MMWR Recomm Rep 2005;54(No.
RR-16):1–31; Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive
immunization strategy to eliminate transmission of hepatitis B virus
infection in the United States: recommendations of the Advisory
Committee on Immunization Practices (ACIP). Part II: immunization
of adults. MMWR Recomm Rep 2006;55(No. RR-16):1–33.
†
Refer to Table 3 for prophylaxis recommendations for infants born to
women with unknown HBsAg status.
§
Within 24 hours of birth for medically stable infants weighing ≥2,000 grams.
¶
Refer to Table 3 for birth dose recommendations for infants weighing
<2,000 grams.
Clinical Features and Natural History
Clinical manifestations of HBV infection range from
asymptomatic infection to fulminant hepatitis. The average
incubation period is 60 days (range: 40–90 days) from exposure
to onset of abnormal serum ALT levels and 90 days (range:
60–150 days) from exposure to onset of jaundice (8,42,43).
Infants, children aged <5 years, and immunosuppressed adults
with newly acquired HBV infection typically are asymptomatic,
whereas symptomatic illness is noted in 30%–50% of older
children, adolescents, and adults (7,8,44,50). When present,
signs and symptoms include nausea, vomiting, abdominal
pain, fever, dark urine, changes in stool color, hepatomegaly,
splenomegaly, and jaundice. Malaise and anorexia might
precede jaundice by 1–2 weeks. Fulminant HBV infection is
uncommon (<1%) but often results in death or liver failure
necessitating liver transplantation. Extrahepatic manifestations
of disease (e.g., skin rash, arthralgias, and arthritis) also might
occur (51). The fatality rate among persons with reported
cases of acute HBV infection is <1.5%, with the highest rates
Recommendations and Reports
6 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
in adults aged ≥55 years. Because a substantial number of
infections are asymptomatic and therefore are not reported,
the overall fatality rate among all persons with HBV infection
is likely lower (8).
Chronic infection occurs among 80%–90% of persons
infected during infancy, 30% of persons infected before
age 6 years, and <1%–12% of persons infected as an older
child or adult (7,52–54). Approximately 95% of primary
infections in immunocompetent adults are self-limited, with
elimination of the virus from blood and generally immunity
to reinfection. Chronic infection develops more frequently
in immunosuppressed persons (e.g., hemodialysis patients
and persons with human immunodeficiency virus [HIV]
infection) (54,55) and persons with diabetes (54). Chronic
HBV infection can result in cirrhosis of the liver, liver cancer,
liver failure, and death. Approximately 25% of persons who
become chronically infected during childhood and 15% of
those who become chronically infected after childhood will
die prematurely from cirrhosis or liver cancer (8,56–58).
There are four phases of chronic HBV infection: immune
tolerant, immune active, immune inactive, and reactivation.
Chronically infected persons do not necessarily pass through
these phases in a linear fashion. Persons in the immune
tolerant phase have no or minimal hepatic inflammation or
fibrosis; most chronically infected children will remain in the
immune tolerant phase until late childhood or adolescence.
The immune active phase is characterized by an active immune
response resulting in hepatic inflammation, with or without
fibrosis. Persons who remain in the immune active phase
for prolonged periods of time are at high risk for developing
cirrhosis and hepatocellular carcinoma. Persons in the immune
inactive phase have improvement of hepatic inflammation and
fibrosis. Risk for progression to hepatocellular carcinoma is
lower among persons in the immune inactive phase compared
with the active phase. Persons in the reactivation phase have
active liver inflammation with or without fibrosis (44,59–61).
HBV reactivation might occur with immunosuppressive
therapy or treatment for HCV (62).
No specific treatment exists for acute HBV infection;
supportive care is the mainstay of therapy. Guidelines for
management of chronic HBV infection in children and adults,
including disease monitoring and antiviral therapy, are available
(5). Antiviral therapy generally should be initiated in patients
with chronic HBV infection who are likely to respond to
treatment and who are at high risk for liver-related morbidity
(5). Maternal antiviral therapy to reduce perinatal transmission
is suggested for HBsAg-positive pregnant women whose HBV
DNA level is >200,000 IU/mL (5).
In areas in which HBV is highly endemic, HBV frequently
is transmitted perinatally from HBV-infected pregnant women
to their newborns. The majority of cases of perinatal HBV
transmission occur during delivery, with rare instances of in
utero transmission (63). HBV transmission might occur in germ
cell lines, as the virus has been detected in sperm, oocytes, and
embryos. Available data do not support the need for a cesarean
delivery among HBV-infected pregnant women with low HBV
DNA (63). Prior to the widespread availability of postexposure
prophylaxis, the proportion of infants born to HBsAg-positive
women acquiring HBV infection was approximately 30% for
those born to HBeAg-negative mothers and 85% for those born
to HBeAg-positive mothers. With postexposure prophylaxis,
comprised of HepB vaccine and HBIG at birth, followed by
completion of the HepB vaccine series, 0.7%–1.1% of infants
develop infection (28,29,64); infants born to mothers with high
viral loads are at greatest risk for infection despite receipt of HepB
vaccine and HBIG (29). Unvaccinated infants and children are
also at risk for horizontal transmission from infected household
and other contacts.
Interpretation of Serologic Markers
Serologic markers for HBV infection include HBsAg,
antibody to HBsAg (anti-HBs), immunoglobulin class M
(IgM) antibodies to hepatitis B core antigen (IgM anti-HBc),
and immunoglobulin class G (IgG) anti-HBc (IgG anti-HBc)
(49,65,66). At least one serologic marker is present during
the different phases of infection. HBV DNA is a measure
of viral load and reflects viral replication (49) (Table 1).
Hepatitis B e antigen (HBeAg) can be detected in persons
with acute or chronic HBV infection; the presence of HBeAg
correlates with viral replication and high infectivity; antibody
to HBeAg (anti-HBe) correlates with the loss of replicating
virus, although reversion to HBeAg positivity can occur (7).
A confirmed positive HBsAg result indicates current HBV
infection, either acute or chronic. All HBsAg-positive persons are
infectious. If HBsAg persists for >6 months, spontaneous clearance
is unlikely, and the infection is deemed chronic. HBV DNA can
be detected prior to the detection of HBsAg in an infected person.
Occult infection occurs when HBsAg is undetectable despite the
presence of HBV DNA (66–68). Transient HBsAg positivity can
occur up to 18 days following vaccination (up to 52 days among
hemodialysis patients) and is clinically insignificant (69).
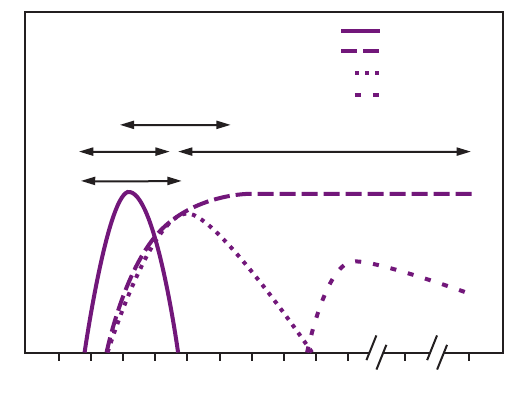
In acute HBV infection, anti-HBc (initially both IgM and
IgG) appears 1–2 weeks after the appearance of HBsAg (49)
(Figure 2). IgM anti-HBc often becomes undetectable within
6 months, and IgG anti-HBc predominates and remains
detectable for a lengthy period of time, often life-long (65,66).
The presence of IgM anti-HBc is indicative of acute infection,
while IgG anti-HBc indicates past infection (65,66). In persons
who recover from HBV infection, HBsAg is eliminated from

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 7
US Department of Health and Human Services/Centers for Disease Control and Prevention
TABLE 1. Typical interpretation of test results for hepatitis B virus infection
HBsAg Total anti-HBc IgM anti-HBc Anti-HBs HBV DNA Interpretation
- - - - - Never infected
+ - - - + or -
Early acute infection; transient (up to 18 days) after vaccination
+ + + - +
Acute infection
- + + + or - + or -
Acute resolving infection
- + - + -
Recovered from past infection and immune
+ + - - +
Chronic infection
- + - - + or -
False-positive (i.e., susceptible); past infection; “low-level” chronic infection; or passive
transfer of anti-HBc to infant born to HBsAg-positive mother
- - - + -
Immune if anti-HBs concentration is ≥10 mIU/mL after vaccine series completion;
passive transfer after hepatitis B immune globulin administration
Abbreviations: - = negative; + = positive; anti-HBc = antibody to hepatitis B core antigen; anti-HBs = antibody to hepatitis B surface antigen; HBsAg = hepatitis B
surface antigen; HBV DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
the blood and anti-HBs develops, typically within 3–4 months.
The presence of anti-HBs is generally indicative of immunity
to HBV infection (8). Anti-HBs also can be detected for 4–6
months following HBIG administration (10). Persons who
recover from natural HBV infection are typically positive for
both anti-HBs and anti-HBc, whereas persons who respond to
HepB vaccine are positive only for anti-HBs. Approximately
0.5%–2% of persons with chronic infection spontaneously
clear HBsAg yearly; anti-HBs will develop in the majority of
these persons (8).
In certain persons, anti-HBc is the only serologic marker
detected. Isolated anti-HBc-positivity can be detected
following HBV infection in persons who have recovered but
whose anti-HBs levels have waned; in populations with a
high prevalence of HBV infection, isolated anti-HBc likely
indicates previous infection with loss of anti-HBs. Some
chronically infected persons with isolated anti-HBc-positivity
have circulating HBsAg that is not detectable by a laboratory
assay. HBV DNA has been detected in <10% of persons with
isolated anti-HBc (70,71), although the presence of detectable
HBV DNA might fluctuate (72). These persons are unlikely to
transmit infection except under circumstances in which they
are the source of a large exposure, such as a blood transfusion
(8,73). Persons who are HBsAg-negative and anti-HBc-positive
can experience reactivation of infection during chemotherapy
or immunosuppressive therapy, with reappearance of HBsAg
(49). Infection with a mutant HBV strain can result in positive
laboratory tests for HBsAg, total anti-HBc, anti-HBs, and
HBV DNA, with a negative IgM anti-HBc.
Perinatal HBV infection in a child aged ≤24 months is
typically asymptomatic although fulminant hepatitis can occur;
a positive HBsAg test, positive HBeAg test, or detectable HBV
DNA may be considered laboratory evidence of perinatal HBV
in an infant born to an HBV-infected mother if timing criteria
are met (74). Infants who are born to HBsAg-positive mothers
and who do not become infected might have detectable
anti-HBc for up to 24 months after birth from passively
acquired maternal antibody (7).
Adults at Risk for HBV Infection
In 2015, CDC received 3,370 surveillance case-reports
of acute HBV infection. Of 2,207 case-reports with risk
information, 1,151 (52.2%) indicated no risk for HBV
during the 6 weeks to 6 months prior to illness onset, and the
remainder indicated at least one risk factor. Injection-drug use
and multiple sex partners were the most common reported
sources of HBV transmission (4).
Injection-drug use. Injection-drug use was reported by
30.3% of 1,657 new reported HBV cases that included
information about injection-drug use (4). Since 2009, there has
been an increase in acute HBV infection among non-Hispanic
whites aged 30–39 years residing in nonurban areas reporting
injection-drug use as a risk factor (16). Chronic HBV infection
has been identified in 3.5%-20.0% (midpoint estimate 11.8%)
of persons who inject drugs (PWID) in a variety of settings
(75) and 22.6% of PWID have evidence of past infection
(75). The proportion of HBV cases reporting injection-drug
use in three states (Kentucky, Tennessee, and West Virginia)
increased significantly, from 53% during 2006–2009 to 75%
during 2010–2013 (p<0.001, chi-square) (16).
Sexual (heterosexual and MSM) exposure. Among persons
with case-reports of HBV infection with information about
sexual exposure, 26.4% reported having two or more sexual
partners, 3.3% reported sexual contact with an HBV-infected
person, and 11.8% of males reported having had sex with
another male (4). As many as 10%–40% of adults seeking
treatment in STI clinics have evidence of current or past HBV
infection. Among adults with acute HBV infection, 39% were
screened or sought care for an STI prior to becoming infected
with HBV (76).
Household contacts. An estimated 45% of persons living
in households with others with chronic HBV infection have
serologic evidence of past HBV infection, and 16% have
Recommendations and Reports
8 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
evidence of current infection (CDC, unpublished data, 2017).
Prior to universal infant vaccination, the risk for infection was
greatest among unvaccinated children living with a person
with chronic HBV infection in a household or in an extended
family setting (67,77,78).
Developmentally disabled persons in long-term-care
facilities. Developmentally disabled persons in residential
and nonresidential facilities historically have had a chronic
HBV infection prevalence as high as 20%. The prevalence of
infection has declined substantially since the implementation
of routine HepB vaccination in these settings (79–82).
Correctional facilities. The prevalence of chronic HBV
infection has been higher among prison inmates (1.0%–3.7%)
than among the general population (83,84), reflecting an
overrepresentation of persons entering correctional facilities
with risks for HBV infection (e.g., injection-drug use and
histories of multiple sex partners).
Persons at risk for occupational exposure to HBV. Before
HepB vaccination was widely implemented, HBV infection
was recognized as a common occupational risk among HCP
(85,86). Routine HepB vaccination of HCP and the use of
standard precautions have resulted in a 98% decline in HBV
infections from 1983 through 2010 among HCP (10). The
Occupational Safety and Health Administration mandates
that employers offer HepB vaccination to all employees who
have occupational risk and that postexposure prophylaxis be
available following an exposure (10,87).
Hemodialysis patients. Since the initiation of HepB
vaccination and additional infection control precautions for
hepatitis B in dialysis centers, the incidence of HBV infection
among hemodialysis patients has declined approximately 95%
(88,89). Since 1995, the annual incidence has been stable and
HBsAg seroprevalence has remained at 1% (90). Receipt of
dialysis was reported in <1% of acute HBV surveillance cases
with information reported to CDC (4).
Persons with HCV infection. The number of reported
HCV cases in four Appalachian states (Kentucky, Tennessee,
Virginia, and West Virginia) increased 364% during 2006–
2012 among persons aged ≤30 years, with injection-drug use
as the most common reported risk factor (91). The increase
in HCV infections occurred concomitantly with an increase
in HBV infections among young adults in rural communities
in Appalachian states.
Persons with chronic liver disease. Persons with chronic
liver disease (e.g., cirrhosis, fatty liver disease, alcoholic liver
disease, and autoimmune hepatitis) are not at increased risk
for HBV infection unless they have percutaneous or mucosal
FIGURE 2. Acute hepatitis B virus infection with recovery
Titer
Weeks after exposure
0 4 8 12 16 20 24 28 32 36 52 100
Total anti-HBc
IgM anti-HBc
Anti-HBs
HBsAg
HBV DNA
HBeAG Anti-HBe
Symptoms
Abbreviations: anti-HBc = antibody to hepatitis B core antigen; anti-HBe =
antibody to hepatitis B e antigen; anti-HBs = antibody to hepatitis B surface
antigen; HBeAg = hepatitis B e antigen; HBsAg = hepatitis B surface antigen; HBV
DNA = hepatitis B virus deoxyribonucleic acid; IgM = immunoglobulin class M.
exposure to blood or body fluids. However, concurrent chronic
HBV infection might increase the risk for progressive chronic
liver disease in these persons (92).
Travelers to countries where HBV is endemic. Short-
term travelers to countries in which HBV infection is of high
or intermediate endemicity (Box 3) typically are at risk for
infection only through exposure to blood in medical or disaster-
relief activities, receipt of medical care that involves parenteral
exposures, sexual activity, or drug use. Monthly incidence of
25‒420 per 100,000 travelers has been reported among long-
term travelers to countries where the disease is endemic (93).
Persons with HIV. Approximately 10% of HIV-positive
persons are coinfected with HBV (94–97). Chronic HBV
infection has been identified in 6%–14% of HIV-positive
persons, including in 9%–17% of MSM and in 7%–10%
of PWID (98). Coinfected persons have increased rates of
cirrhosis and liver-related mortality (99).
Persons with diabetes. Compared with adults without
diabetes, adults with diabetes have a 60% higher prevalence
of past or present HBV infection and twice the odds of
acquiring acute HBV. Repeated outbreaks of HBV infection
associated with assisted blood glucose monitoring underscore
the continued risk for this population (100–102). Data also
suggest the possibility of a higher case-fatality proportion
among persons with diabetes acutely infected with HBV
compared with those without diabetes (9).

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 9
US Department of Health and Human Services/Centers for Disease Control and Prevention
BOX 3. Prevalence of chronic hepatitis B virus infection, by country*
High (≥8% prevalence): Angola, Benin, Burkina Faso,
Burundi, Cameroon, Central African Republic, Congo,
Côte d’Ivoire, Djibouti, Equatorial Guinea, Gabon,
Gambia, Ghana, Guinea, Haiti, Kiribati, Kyrgyzstan, Laos,
Liberia, Malawi, Mali, Mauritania, Mongolia, Mozambique,
Namibia, Nauru, Niger, Nigeria, Niue, Papua New Guinea,
Senegal, Sierra Leone, Solomon Islands, Somalia, South
Sudan, Sudan, Swaziland, Togo, Tonga, Uganda, Vanuatu,
Vietnam, Yemen, and Zimbabwe.
Intermediate (5%–7.9% prevalence): Albania, Bhutan,
Cape Verde, China, Democratic Republic of the
Congo, Ethiopia, Kazakhstan, Kenya, Marshall Islands,
Moldova, Oman, Romania, Rwanda, Samoa, South
Africa, Tajikistan, Tanzania, Thailand, Tunisia, Tuvalu,
Uzbekistan, and Zambia.
Low Intermediate (2%–4.9% prevalence): Algeria,
Azerbaijan, Bangladesh, Belarus, Belize, Brunei Darussalam,
Bulgaria, Cambodia, Colombia, Cyprus, Dominican
Republic, Ecuador, Eritrea, Federated States of Micronesia,
Fiji, Georgia, Italy, Jamaica, Kosovo, Libya, Madagascar,
Myanmar, New Zealand, Pakistan, Palau, Philippines, Peru,
Russia, Saudi Arabia, Singapore, South Korea, Sri Lanka,
Suriname, Syria, Tahiti, and Turkey.
Low (≤1.9% prevalence): Afghanistan, Argentina,
Australia, Austria, Bahrain, Barbados, Belgium, Bolivia, Bosnia
and Herzegovina, Brazil, Canada, Chile, Costa Rica, Croatia,
Cuba, Czech Republic, Denmark, Egypt, France, Germany,
Greece, Guatemala, Hungary, Iceland, India, Indonesia,
Iran, Iraq, Ireland, Israel, Japan, Jordan, Kuwait, Lebanon,
Lithuania, Malaysia, Mexico, Morocco, Nepal, Netherlands,
Nicaragua, Norway, Palestine, Panama, Poland, Portugal,
Qatar, Serbia, Seychelles, Slovakia, Slovenia, Spain, Sweden,
Switzerland, Ukraine, UK, United Arab Emirates, United
States of America, and Venezuela.
No data: Andorra, Antigua and Barbuda, Armenia,
The Bahamas, Botswana, Chad, Comoros, Cook Islands,
Dominica, El Salvador, Finland, Grenada, Guinea-
Bissau, Guyana, Honduras, Latvia, Lesotho, Lithuania,
Luxembourg, Macedonia, Maldives, Malta, Mauritius,
Monaco, Montenegro, North Korea, Paraguay, Saint Kitts
and Nevis, Saint Lucia, Saint Vincent and the Grenadines,
San Marino, Sao Tome and Principe, Timor-Leste, Trinidad
and Tobago, Turkmenistan, and Uruguay.
* Source: CDC. Travelers health: infectious diseases related to travel. Atlanta, GA: US Department of Health and Human Services, CDC; 2017.
Prophylaxis Against HBV Infection
Hepatitis B Vaccines and
Hepatitis B Immune Globulins
HepB vaccination is the mainstay of HBV prevention
efforts; HBIG is generally used as an adjunct to HepB vaccine
in infants born to HBsAg-positive mothers and in certain
other postexposure prophylaxis situations. The first HepB
vaccines consisted of plasma-derived HBsAg. Recombinant
HepB vaccines containing yeast-derived HBsAg purified by
biochemical and biophysical separation techniques replaced the
plasma-derived vaccines in the United States by the late 1980s
(64,103,104). HepB vaccines recommended for use in the
United States are formulated to contain 10–40 µg of HBsAg
protein/mL and do not contain thimerosal as a preservative
(105). HBIG can augment protection until a response to
vaccination is attained. For those who do not respond to
HepB vaccination, HBIG administered alone is the primary
means of protection after an HBV exposure. HBIG provides
passively acquired anti-HBs and temporary protection (i.e.,
3–6 months). Passively acquired anti-HBs can be detected for
4–6 months after administration of HBIG (10).
HepB vaccines are available as a single-antigen formulation
and in combination with other vaccines. The two single-
antigen vaccines recommended for use in the United States,
Engerix-B (GlaxoSmithKline Biologicals, Rixensart, Belgium)
and Recombivax HB (Merck & Co., Inc., Whitehouse
Station, New Jersey), are used for the vaccination of persons
starting at birth. Of the two combination vaccines, Pediarix
(GlaxoSmithKline Biologicals, Rixensart, Belgium) is used for
the vaccination of persons aged 6 weeks–6 years and contains
recombinant HBsAg, diphtheria and tetanus toxoids and
acellular pertussis adsorbed, and inactivated poliovirus and
Twinrix (GlaxoSmithKline Biologicals, Rixensart, Belgium)
is used for the vaccination of persons aged ≥18 years and
contains recombinant HBsAg and inactivated hepatitis A virus
(Table 2). Comvax (Merck & Co., Inc., Whitehouse Station,
New Jersey), which was used previously for the vaccination of
persons aged 6 weeks–15 months and contained recombinant
HBsAg and Haemophilus b conjugate vaccine, has not been
available for purchase directly from Merck since January 1,
2015. Discontinuation of Comvax was not related to any
product safety or manufacturing issues. Aluminum salts
generally are used as adjuvants to enhance the immune response
of vaccinated persons.
Recommendations and Reports
10 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
TABLE 2. Recommended doses of hepatitis B vaccine, by group and vaccine type
Age group (yrs)
Single-antigen vaccine Combination vaccine
Recombivax Engerix Pediarix* Twinrix
†
Dose (µg) Vol (mL) Dose (µg) Vol (mL) Dose (µg) Vol (mL) Dose (µg) Vol (mL)
Birth–10 5 0.5 10 0.5 10* 0.5 N/A N/A
11–15 10
§
1 N/A N/A N/A N/A N/A N/A
11–19 5 0.5 10 0.5 N/A N/A N/A N/A
≥20 10 1 20 1 N/A N/A 20
†
1
Hemodialysis patients and other immune-compromised persons
<20 5 0.5 10 0.5 N/A N/A N/A N/A
≥20 40 1 40 2 N/A N/A N/A N/A
Abbreviation: N/A = not applicable.
* Pediarix is approved for use in persons aged 6 weeks through 6 years (prior to the 7th birthday).
†
Twinrix is approved for use in persons aged ≥18 years.
§
Adult formulation administered on a 2-dose schedule.
Two HBIG products are licensed for use in the United States:
HepaGam B (Cangene Corporation, Winnipeg, Canada)
and Nabi-HB (Biotest Pharmaceuticals Corporation, Boca
Raton, Florida). HBIG is prepared from the plasma of donors
with high concentrations of anti-HBs. Source plasma tests
negative for evidence of HIV, HBV, and HCV. Investigational
nucleic acid testing for hepatitis A virus and parvovirus B19
also is performed on pooled samples of source plasma. The
manufacturing process contains two steps to inactivate viruses
in the final product: the solvent and detergent step inactivates
enveloped viruses, and the virus filtration step removes viruses
based on their size. HBIG products licensed for use in the
United States contain no preservative and are intended for
single use only (106).
Vaccine-Induced Seroprotection
The presence of anti-HBs typically indicates immunity
against HBV infection. Immunocompetent children and adults
who have vaccine-induced anti-HBs levels of ≥10 mIU/mL
1–2 months after having received a complete HepB vaccine
series are considered seroprotected and deemed vaccine
responders (107). Vaccine-induced seroprotection is considered
a surrogate of clinical protection. Anti-HBs levels wane
over time following vaccination related in part to the age at
vaccination. Approximately 16% of persons vaccinated at age
<1 year have antibody levels of ≥10 mIU/mL 18 years following
vaccination, compared with 74% for those vaccinated at
age ≥1 year (10). However, persons initially responding to
the full 3-dose HepB vaccine series and who are later found
to have anti-HBs <10 mIU/mL remain protected. Most
persons (88%) who receive a challenge dose of HepB vaccine
30 years after HepB vaccination as children or adults develop
an antibody response of ≥10 mIU/mL indicating persistent
immunity to HBV infection (108). Data from this and other
studies suggests protection against acute symptomatic and
chronic HBV infection persists for 30 years or more among
immunocompetent persons who originally responded to HepB
vaccine (108–110).
The 3-dose HepB vaccine series produces a protective
antibody response (anti-HBs ≥10 mIU/mL) in approximately
95% of healthy infants overall (response is lower for infants
with lower birth weights) (64) and >90% of healthy adults
aged <40 years (111,112). Among healthy infants, 25% and
63% achieve anti-HBs levels ≥10 mIU/mL after the first and
second dose, respectively. Among healthy adults aged <40 years,
30%–55% and 75% achieve anti-HBs levels ≥10 mIU/mL
after the first and second dose, respectively (7,8,64). Vaccine
response is decreased among infants weighing <2000 grams
and older adults. Other factors (e.g., smoking, obesity, aging,
chronic medical conditions, drug use, diabetes, male sex, genetic
factors, and immune suppression) contribute to a decreased
response to vaccine (113–116). Although immunogenicity is
lower among immunocompromised persons, those who achieve
and maintain seroprotective antibody levels before exposure
to HBV have a high level of protection (8).
Birth dose. A birth dose of HepB vaccine serves as
postexposure prophylaxis to prevent perinatal HBV infection
among infants born to HBV-infected mothers. Although
infants requiring postexposure prophylaxis should be identified
by maternal HBsAg testing, administration of a birth dose
to all infants (even without HBIG) serves as a safeguard to
prevent perinatal transmission among infants born to HBsAg-
positive mothers not identified prenatally because of lack of
maternal HBsAg testing or failures in reporting test results.
HepB vaccine or HBIG given alone are 75% and 71% effective
in preventing perinatal HBV transmission, respectively; their
combined efficacy is 94% (29,52,117). The birth dose also
provides protection to infants at risk from household exposure
after the perinatal period (29,64).
Vaccination produces seroprotection in 98% of healthy
term infants. Vaccine response is lower among infants with
birth weights <2000 grams (64). A study among low birth

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 11
US Department of Health and Human Services/Centers for Disease Control and Prevention
weight infants demonstrated that more infants achieved
seroprotective anti-HBs levels when vaccine was initiated at
1 month of age versus within the first 3 days of life (96% vs.
68%, p<0.02) (118). Vaccine response among infants does
not vary appreciably by maternal HBsAg status or HBIG
administration (64).
Adolescents. Approximately 95% of adolescents achieve
seroprotection following HepB vaccination with a complete
series (7). The adult (10 µg) dose of Recombivax HB
administered using a 2-dose compressed schedule at 0 and
4 months or 0 and 6 months for persons aged 11–15 years
produces seroprotection proportions nearly equivalent to those
obtained with the standard regimen of 5 µg administered on
a 3-dose schedule at 0, 1, and 6 months (99.2% vs. 98.3%)
(119,120). Data on long-term antibody persistence or
protection among adolescents for 2-dose schedules are lacking.
Adults. Vaccination with a complete series results in
seroprotection in >90% of healthy adults aged <40 years.
Response decreases with age, and seroprotection is achieved
in 75% of persons aged 60 years (8).
Diabetes. A review of studies assessing HepB vaccine
response among persons with diabetes mellitus demonstrated
seroprotection in 93.9% for children with diabetes mellitus
compared with 100% for children without diabetes mellitus
(112,121).
Among adults, 88.2% of those with diabetes mellitus,
compared with 93.6% of those without diabetes mellitus,
achieved seroprotection (112). Among hemodialysis/chronic
kidney disease patients, the median proportion protected was
60.1% for those with diabetes mellitus, compared with 75.1%
for those without diabetes mellitus (112).
Immunocompromising conditions. The humoral
response to HepB vaccine is reduced in children and adults
who are immunocompromised (e.g., hematopoietic stem cell
transplant recipients, patients undergoing chemotherapy,
and HIV-infected persons) (122,123). Modified dosing
regimens, including a doubling of the standard antigen
dose or administration of additional doses, might increase
response rates. However, data on response to these alternative
vaccination schedules are limited (6).
Vaccine Safety
In prelicensure trials, adverse events following HepB
vaccination were most commonly injection site reactions
and mild systemic reactions (106). Commonly reported mild
adverse events from postmarketing data include pain (3%–
29%), erythema (3%), swelling (3%), fever (1%–6%), and
headache (3%) (124). The estimated incidence of anaphylaxis
among HepB vaccine recipients is 1.1 per million vaccine doses
(125). In 2011, the Institute of Medicine concluded that the
evidence convincingly supports a causal relationship between
HepB vaccine and anaphylaxis in yeast-sensitive persons, and
that the evidence is inadequate to accept or reject a causal
relation between HepB vaccine and several neurologic, chronic,
and autoimmune diseases (126).
During early postlicensure surveillance, several adverse
events following HepB vaccination have been described in
the scientific literature, including Guillain-Barré Syndrome
(GBS), chronic fatigue syndrome, optic neuritis, multiple
sclerosis, and diabetes mellitus; however, multiple studies
have demonstrated no association between receipt of HepB
vaccine and these conditions (126–129). In addition, no
evidence of a causal association between rheumatoid arthritis
(130), Bell’s palsy (131), autoimmune thyroid disease (132),
hemolytic anemia in children (133), anaphylaxis (134), optic
neuritis (135), Guillain-Barré Syndrome (136), sudden-onset
sensorineural hearing loss (137), or other chronic illnesses
and receipt of HepB vaccine has been demonstrated through
analysis of VSD data.
During 2005–2015, a total of 20,231 reports of adverse
events following HepB vaccination among all ages were
submitted to VAERS. The majority of primary U.S. reports
(15,787 of 20,231, 78%) were following HepB vaccine
administered with other vaccines on the same visit. Among
these, the percentage classified as serious (i.e., if one or more
of the following is reported: death, life-threatening illness,
hospitalization or prolongation of existing hospitalization,
or permanent disability)
†
was 16.7%, including 402 deaths,
of which 388 were among infants aged 6 weeks–23 months
(138). The most frequently reported adverse events for
vaccines given in combination were fever (23%), injection
site erythema (11%), and vomiting (10%) (138). Among the
4,444 single-antigen HepB reports, 6.5% were classified as
serious, including 43 deaths, of which 27 were among infants
aged ≤4 weeks. The most frequently reported adverse events
for single-antigen HepB vaccine were nausea/dizziness (8%)
and fever/headache (7%).
Vaccination Schedules
Vaccine schedules are determined on the basis of
immunogenicity data, and, for infants and children, the need
to integrate HepB vaccine into a harmonized immunization
schedule (Tables 3 and 4). Primary vaccination generally
consists of three intramuscular doses administered on a 0-,
1-, and 6-month schedule (Table 4). Recombivax HB may be
administered in a 2-dose schedule at 0 and 4–6 months for
†
Code of Federal Regulations. 21 CFR §600.80. Revised April 1, 2010. Available at
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? fr=600.80.

Recommendations and Reports
12 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
TABLE 3. Hepatitis B vaccine schedules for infants, by infant birthweight and maternal HBsAg status
Birthweight Maternal HBsAg status
Single-antigen vaccine Single-antigen + combination vaccine
†
Dose Age Dose Age
≥2,000 g Positive 1 Birth (≤12 hrs) 1 Birth (≤12 hrs)
HBIG
§
Birth (≤12 hrs) HBIG Birth (≤12 hrs)
2 1–2 mos 2 2 mos
3 6 mos
¶
3 4 mos
4 6 mos
¶
Unknown* 1 Birth (≤12 hrs) 1 Birth (≤12 hrs)
2 1–2 mos 2 2 mos
3 6 mos
¶
3 4 mos
4 6 mos
¶
Negative 1 Birth (≤24 hrs) 1 Birth (≤24 hrs)
2 1–2 mos 2 2 mos
3 6–18 mos
¶
3 4 mos
4 6 mos
¶
<2,000 g Positive 1 Birth (≤12 hrs) 1 Birth (≤12 hrs)
HBIG Birth (≤12 hrs) HBIG Birth (≤12 hrs)
2 1 mos 2 2 mos
3 2–3 mos 3 4 mos
4 6 mos
¶
4 6 mos
¶
Unknown 1 Birth (≤12 hrs) 1 Birth (≤12 hrs)
HBIG Birth (≤12 hrs) HBIG Birth (≤12 hrs)
2 1 mos 2 2 mos
3 2–3 mos 3 4 mos
4 6 mos
¶
4 6 mos
¶
Negative 1 Hospital discharge or age 1 mo 1 Hospital discharge or age 1 mo
2 2 mos 2 2 mos
3 6–18 mos
¶
3 4 mos
4 6 mos
¶
Abbreviations: HBIG = hepatitis B immune globulin; HBsAg = hepatitis B surface antigen.
* Mothers should have blood drawn and tested for HBsAg as soon as possible after admission for delivery; if the mother is found to be HBsAg positive, the infant
should receive HBIG as soon as possible but no later than age 7 days.
†
Pediarix should not be administered before age 6 weeks.
§
HBIG should be administered at a separate anatomical site from vaccine.
¶
The final dose in the vaccine series should not be administered before age 24 weeks (164 days).
persons aged 11–15 years using the adult formulation. Pediarix
is administered at ages 2, 4, and 6 months; it is not used for
the birth dose. Twinrix may be administered before travel or
any other potential exposure on an accelerated schedule at 0,
7, and 21–30 days, followed by a dose at 12 months. HepB
vaccination of adult hemodialysis patients consists of high-dose
(40 µg) Recombivax HB administered on a 0-, 1-, and 6-month
schedule or high-dose (40 µg) Engerix-B administered on a
0-, 1-, 2-, and 6-month schedule (106).
Alternative vaccination schedules (e.g., 0, 1, and 4 months
or 0, 2, and 4 months) have been demonstrated to elicit dose-
specific and final rates of seroprotection similar to those obtained
on a 0-, 1-, and 6-month schedule. Increasing the interval
between the first 2 doses has little effect on immunogenicity
or the final antibody concentration (139–141). The third dose
confers the maximum level of seroprotection and provides
long-term protection (142). Longer intervals between the
last 2 doses (e.g., 11 months) result in higher final antibody
levels (142) but might increase the risk for acquisition of
HBV infection among persons who have a delayed response
to vaccination. Higher geometric mean titers are associated
with longer persistence of measurable anti-HBs.
Response to Revaccination
A challenge dose of HepB vaccine may be used to determine
the presence of vaccine-induced immunologic memory
through generation of an anamnestic response. The term
“booster dose” has been used to refer to a dose of HepB vaccine
administered after a primary vaccination series to provide
rapid protective immunity against significant infection (i.e.,
infection resulting in serologic test results positive for HBV
and/or clinically significant disease). Among persons who were
vaccinated prior to age 1 year and found to have anti-HBs levels
<10 mIU/mL 6–18 years later, a single challenge dose of HepB
vaccine resulted in anti-HBs levels ≥10 mIU/mL in 60%–97%
of those tested. Similar results were found among persons
initially vaccinated at age ≥1 year (10). Immunocompetent
persons with a response ≥10 mIU/mL following a challenge
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 13
US Department of Health and Human Services/Centers for Disease Control and Prevention
dose are considered protected, regardless of subsequent declines
in anti-HBs (10,109).
One study found that of infants born to HBsAg-positive
women who were not infected at birth and who did not
respond to a primary vaccine series, all developed seroprotective
levels of anti-HBs after receipt of 3 additional doses (143). No
data exist that suggest that children who have no detectable
antibody after 6 doses of vaccine benefit from additional doses.
Maternal Antiviral Therapy for Preventing
Perinatal HBV Transmission
Antiviral therapy (i.e., lamivudine, telbivudine, and
tenofovir) has been studied as an intervention to reduce
perinatal HBV transmission among pregnant women with
high HBV DNA levels (e.g., average HBV DNA levels of
7.6 log10 IU/mL) (144). Maternal antiviral therapy started
at 28–32 weeks’ gestation, as an adjunct to HepB vaccine and
HBIG administered to the infant shortly after delivery, has
been associated with significantly reduced rates of perinatal
HBV transmission (5). The use of lamivudine and telbivudine
is limited by viral resistance and mutations. Tenofovir is not
associated with resistance and is the preferred agent (5).
Available data support the safety of tenofovir during pregnancy,
although its use might be associated with reduced bone mineral
content in infants with in utero exposure (5,39,63,144–146).
AASLD suggests antiviral therapy to reduce perinatal HBV
transmission when maternal HBV DNA is >200,000 IU/mL.
Maternal therapy is generally discontinued at birth to 3 months
postpartum (5).
Cost-Effectiveness Considerations
HBV prevention strategies targeting perinatal transmission
are considered very cost-effective (i.e., an incremental
cost-effectiveness ratio <$25,000). The current strategy of
administering HepB vaccine and HBIG within 12 hours of
birth for infants born to HBsAg-positive mothers and universal
infant vaccination prior to hospital discharge has an incremental
cost-effectiveness ratio of $6,957 per quality-adjusted life year
(QALY) saved when compared with a strategy of universal
infant HepB vaccination prior to hospital discharge alone
(147). CDC’s U.S. Perinatal Hepatitis B Prevention Program
(https://www.cdc.gov/hepatitis/partners/perihepbcoord.
htm), which provides case management services to infants
born to HBsAg-positive women, also has been demonstrated
to decrease infections, increase QALYs saved, and be a cost-
effective use of resources (148). A strategy of testing HBsAg-
positive pregnant women for HBV DNA, followed by maternal
antiviral prophylaxis for women with high HBV DNA, would
TABLE 4. Hepatitis B vaccine schedules for children, adolescents, and adults
Age group
Schedule* (interval represents time
in months from first dose)
Children (1–10 yrs) 0, 1, and 6 mos
0, 1, 2, and 12 mos
Adolescents (11–19 yrs) 0, 1, and 6 mos
0, 12, and 24 mos
0 and 4–6 mos
†
0, 1, 2, and 12 mos
0, 7 days, 21–30 days, 12 mos
§
Adults (≥20 yrs) 0, 1, and 6 mos
0, 1, 2, and 12 mos
0, 1, 2, and 6 mos
¶
0, 7 days, 21–30 days, 12 mos
§
* Refer to package inserts for further information. For all ages, when the HepB
vaccine schedule is interrupted, the vaccine series does not need to be
restarted. If the series is interrupted after the first dose, the second dose should
be administered as soon as possible, and the second and third doses should
be separated by an interval of at least 8 weeks. If only the third dose has been
delayed, it should be administered as soon as possible. The final dose of vaccine
must be administered at least 8 weeks after the second dose and should follow
the first dose by at least 16 weeks; the minimum interval between the first and
second doses is 4 weeks. Inadequate doses of hepatitis B vaccine or doses
received after a shorter-than-recommended dosing interval should be
readministered, using the correct dosage or schedule. Vaccine doses
administered ≤4 days before the minimum interval or age are considered valid.
Because of the unique accelerated schedule for Twinrix, the 4-day guideline
does not apply to the first three doses of this vaccine when administered on a
0-day, 7-day, 21–30-day, and 12-month schedule (new recommendation).
†
A 2-dose schedule of Recombivax adult formulation (10 µg) is licensed for
adolescents aged 11–15 years. When scheduled to receive the second dose,
adolescents aged >15 years should be switched to a 3-dose series, with doses 2 and
3 consisting of the pediatric formulation administered on an appropriate schedule.
§
Twinrix is approved for use in persons aged ≥18 years and is available on an
accelerated schedule with doses administered at 0, 7, 21–30 days, and 12 months.
¶
A 4-dose schedule of Engerix administered in two 1 mL doses (40 µg) on a 0-,
1-, 2-, and 6-month schedule is recommended for adult hemodialysis patients.
cost an additional $3 million but would save 2,080 QALYs and
prevent 324 chronic HBV infections, and therefore would be
considered cost-effective, with an incremental cost-effectiveness
ratio of $1,583 per QALY saved (36).
Cost-effectiveness also has been assessed for HBV prevention
strategies outside of the perinatal setting. Vaccinating adults
aged 20–59 years with diabetes mellitus costs $75,094 per
QALY saved; cost-effectiveness ratios increase with age at
vaccination (149). Among previously vaccinated current
HCP (including those in training), pre-exposure anti-HBs
testing followed by revaccination and retesting (if necessary,
based on anti-HBs levels), compared with no intervention,
was not considered cost-effective with an incremental cost per
QALY saved of $3–$4 million at year one and approximately
$800,000 over 10 years (150).
Recommendations
This section contains guidance for the prevention of HBV
infection, including ACIP recommendations for HepB
vaccination of infants, children, adolescents, and adults

Recommendations and Reports
14 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
(Box 4) and CDC and ACIP recommendations for HBV
prophylaxis following occupational and nonoccupational
exposures, respectively.
Prevention of Perinatal HBV Transmission
Identification and Management of HBV-Infected
Pregnant Women
• All pregnant women should be tested for HBsAg during
an early prenatal visit (e.g., first trimester) in each
pregnancy, even if they have been vaccinated or tested
previously. Testing those pregnant women known to be
chronically infected with HBV provides documentation
of the positive HBsAg test result obtained during
pregnancy and helps to ensure that their infants will be
identified for timely prophylaxis.
– All HBsAg-positive pregnant women should be tested
for HBV DNA to guide the use of maternal antiviral
therapy during pregnancy for the prevention of
perinatal HBV transmission (new recommendation).
– AASLD suggests maternal antiviral therapy when the
maternal HBV DNA is >200,000 IU/mL
(new recommendation).
– All HBsAg-positive pregnant women should be referred
to their jurisdiction’s Perinatal Hepatitis B Prevention
Program (PHBPP) for case management to ensure that
their infants receive timely prophylaxis and follow-up.
A copy of the original laboratory report indicating the
pregnant woman’s HBsAg-positive status should be
provided to the hospital or birthing facility where the
delivery is planned and to the HCP who will care for
the newborn infant.
– All HBsAg-positive pregnant women should receive
information concerning HBV that discusses the
potential use of antiviral therapy, the importance of
prophylaxis for their infant (HepB vaccine and HBIG
within 12 hours of birth), completion of the vaccine
series, and postvaccination serologic testing.
• Women not tested prenatally, those with clinical hepatitis,
and those whose behaviors place them at high risk for
HBV infection (e.g., recent or current injection-drug use,
having had more than one sex partner in the previous
6 months or an HBsAg-positive sex partner, having been
evaluated or treated for a STI) should be tested at the time
of admission to the hospital or birthing facility for delivery.
• All laboratories that provide HBsAg testing of pregnant
women should use a Food and Drug Administration–
licensed or approved HBsAg test and should perform
testing according to the manufacturer’s labeling, including
BOX 4. Persons recommended to receive hepatitis B vaccination
• All infants
• Unvaccinated children aged <19 years
• Persons at risk for infection by sexual exposure
– Sex partners of hepatitis B surface antigen
(HBsAg)–positive persons
– Sexually active persons who are not in a long-term,
mutually monogamous relationship (e.g., persons
with more than one sex partner during the previous
6 months)
– Persons seeking evaluation or treatment for a
sexually transmitted infection
– Men who have sex with men
• Persons at risk for infection by percutaneous or
mucosal exposure to blood
– Current or recent injection-drug users
– Household contacts of HBsAg-positive persons
– Residents and staff of facilities for developmentally
disabled persons
– Health care and public safety personnel with
reasonably anticipated risk for exposure to blood
or blood-contaminated body fluids
– Hemodialysis patients and predialysis, peritoneal
dialysis, and home dialysis patients
– Persons with diabetes aged 19–59 years; persons
with diabetes aged ≥60 years at the discretion of
the treating clinician
• Others
– International travelers to countries with high or
intermediate levels of endemic hepatitis B virus
(HBV) infection (HBsAg prevalence of ≥2%)
– Persons with hepatitis C virus infection
– Persons with chronic liver disease (including, but
not limited to, persons with cirrhosis, fatty liver
disease, alcoholic liver disease, autoimmune
hepatitis, and an alanine aminotransferase [ALT]
or aspartate aminotransferase [AST] level greater
than twice the upper limit of normal)
– Persons with HIV infection
– Incarcerated persons
– All other persons seeking protection from HBV
infection
testing of initially reactive specimens with a licensed
neutralizing confirmatory test. When pregnant women
are tested for HBsAg at the time of admission for delivery,
shortened testing protocols may be used and initially
reactive results reported to expedite administration of
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 15
US Department of Health and Human Services/Centers for Disease Control and Prevention
postexposure prophylaxis of infants. Commercial
laboratories should be encouraged to capture pregnancy
status for women tested for HBsAg to aid in identification
of HBV-infected pregnant women.
Management of Infants Born to Women Who Are
HBsAg-Positive
• All infants born to HBsAg-positive women should receive
HepB vaccine and HBIG within 12 hours of birth,
administered at different injection sites (e.g., separate
limbs). Only single-antigen HepB vaccine should be used
for the birth dose (Table 3).
• Infants born to women for whom HBsAg testing results
during pregnancy are not available but other evidence
suggestive of maternal HBV infection exists (e.g., presence
of HBV DNA, HBeAg-positive, or mother known to be
chronically infected with HBV) should be managed as if
born to an HBsAg-positive mother (new recommendation).
• The HepB vaccine series should be completed according
to the recommended schedule for infants born to HBsAg-
positive mothers. The final dose in the series should not
be administered before age 24 weeks (164 days). Although
not indicated in the manufacturers’ package labeling,
Pediarix may be used for infants aged ≥6 weeks born to
HBsAg-positive mothers to complete the vaccine series
after receipt of a birth dose of single-antigen HepB vaccine
and HBIG.
• For infants weighing <2,000 grams, the birth dose (i.e.,
the initial HepB vaccine dose) should not be counted as
part of the vaccine series because of the potentially reduced
immunogenicity of HepB vaccine in these infants; 3
additional doses of vaccine (for a total of 4 doses) should
be administered beginning when the infant reaches age
1 month. The final dose in the series should not be
administered before age 24 weeks (164 days).
• Postvaccination serologic testing for anti-HBs and HBsAg
should be performed after completion of the vaccine series
at age 9–12 months (generally at the next well-child visit
following completion of the HepB vaccine series).
Postvaccination serologic testing should be performed for
infants born to HBsAg-positive mothers and infants whose
mother’s HBsAg status remains unknown (i.e., those
infants who are safely surrendered shortly after birth) (new
recommendation). Anti-HBs testing should be performed
using a method that allows detection of the protective
concentration of anti-HBs (≥10 mIU/mL). Testing should
not be performed before age nine months to avoid
detection of passive anti-HBs from HBIG administered
at birth and to maximize the likelihood of detecting late
HBV infection. Anti-HBc testing of infants is not
recommended because passively acquired maternal anti-
HBc might be detected in infants born to HBsAg-positive
mothers up to age 24 months.
– HBsAg-negative infants with anti-HBs levels ≥10 mIU/
mL are protected and need no further medical management.
– HBsAg-negative infants with anti-HBs <10 mIU/mL
should be revaccinated with a single dose of HepB
vaccine and receive postvaccination serologic testing
1–2 months later (new recommendation). Infants
whose anti-HBs remains <10 mIU/mL following single
dose revaccination should receive two additional doses
of HepB vaccine to complete the second series followed
by postvaccination serologic testing 1–2 months after
the final dose.
– Based on clinical circumstances or family preference,
HBsAg-negative infants with anti-HBs <10 mIU/mL may
instead be revaccinated with a second, complete 3-dose
series, followed by postvaccination serologic testing
performed 1–2 months after the final dose of vaccine.
– Available data do not suggest a benefit from
administering additional HepB vaccine doses to infants
who have not attained anti-HBs ≥10 mIU/mL
following receipt of two complete HepB vaccine series.
– HBsAg-positive infants should be referred for
appropriate follow-up.
• Infants who are born to HBsAg-positive mothers and
receive postexposure prophylaxis may be breastfed
beginning immediately after birth.
• For infants transferred to a different facility after birth
(e.g., hospital with higher level of neonatal care), staff at
the transferring and receiving facilities should communicate
regarding the infant’s HepB vaccination and HBIG receipt
status to ensure prophylaxis is administered in a timely
manner (new recommendation).
Management of Infants Born to Women with
Unknown HBsAg Status
• Infants born to women for whom HBsAg testing results
during pregnancy are not available but other evidence
suggestive of maternal HBV infection exists (e.g., presence
of HBV DNA, HBeAg-positive, or mother known to be
chronically infected with HBV) should be managed as if
born to an HBsAg-positive mother (new recommendation).
The infant should receive both HepB vaccine and HBIG
within 12 hours of birth.
• Women admitted for delivery without documentation of
HBsAg test results should have blood drawn and tested as
soon as possible.
• While maternal HBsAg test results are pending, infants
with birth weights ≥2,000 grams born to women with an
Recommendations and Reports
16 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
unknown HBsAg status should receive the first dose of
HepB vaccine (without HBIG) within 12 hours of birth.
Only single-antigen HepB vaccine should be used for the
birth dose (Table 3).
– If the mother is determined to be HBsAg-positive, the
infant should receive HBIG as soon as possible but no
later than age seven days, and the vaccine series should
be completed according to the recommended schedule
for infants born to HBsAg-positive mothers. The final
dose in the series should not be administered before
age 24 weeks (164 days). If the mother is determined
to be HBsAg-negative, the vaccine series should be
completed according to the recommended schedule for
infants born to HBsAg-negative mothers. The final
dose in the series should not be administered before
age 24 weeks (164 days).
• Because of the potentially decreased immunogenicity of
vaccine in infants weighing <2,000 grams, these infants
should receive both single-antigen HepB vaccine and
HBIG, administered at different injection sites (e.g.,
separate limbs), if the mother’s HBsAg status cannot be
determined within 12 hours of birth. The birth dose of
vaccine should not be counted as part of the 3 doses
required to complete the vaccine series; 3 additional doses
of vaccine (for a total of 4 doses) should be administered
according to a recommended schedule on the basis of the
mother’s HBsAg test result. The final dose in the series
should not be administered before age 24 weeks (164 days).
– If it is not possible to determine the mother’s HBsAg
status (e.g., when a parent or person with lawful custody
safely surrenders an infant confidentially shortly after
birth), the vaccine series should be completed according
to a recommended schedule for infants born to HBsAg-
positive mothers (new recommendation). The final
dose in the series should not be administered before
age 24 weeks (164 days). These infants should receive
postvaccination serologic testing at age 9–12 months,
and revaccination if necessary (new recommendation).
• Anti-HBs testing should be performed using a method
that allows detection of the protective concentration of
anti-HBs (≥10 mIU/mL). Testing should not be performed
before age nine months to avoid detection of passive anti-
HBs from HBIG administered at birth and to maximize
the likelihood of detecting late HBV infection. Anti-HBc
testing of infants is not recommended because passively
acquired maternal anti-HBc might be detected in infants
born to HBsAg-positive mothers up to age 24 months.
– HBsAg-negative infants with anti-HBs levels ≥10 mIU/mL
are protected and need no further medical management.
– HBsAg-negative infants with anti-HBs <10 mIU/mL
should be revaccinated with a single dose of HepB
vaccine and receive postvaccination serologic testing
1–2 months later (new recommendation). Infants
whose anti-HBs remains <10 mIU/mL following single
dose revaccination should receive two additional doses
of HepB vaccine to complete the second series, followed
by postvaccination serologic testing 1–2 months after
the final dose.
– Based on clinical circumstances or family preference,
HBsAg-negative infants with anti-HBs <10 mIU/mL may
instead be revaccinated with a second, complete 3-dose
series, followed by postvaccination serologic testing
performed 1–2 months after the final dose of vaccine.
– Available data do not suggest a benefit from
administering additional HepB vaccine doses to infants
who have not attained anti-HBs ≥10 mIU/mL
following receipt of two complete HepB vaccine series.
– HBsAg-positive infants should be referred for
appropriate follow-up.
• Infants born to mothers with unknown HBsAg status may
be breastfed beginning immediately after birth.
• For infants transferred to a different facility after birth
(e.g., a hospital with a higher level of neonatal care), staff
at the transferring and receiving facilities should
communicate regarding the infant’s HepB vaccination and
HBIG receipt status to ensure prophylaxis is administered
in a timely manner (new recommendation).
Persons Recommended for
HepB Vaccination
Universal Vaccination of Infants
• All infants should receive the HepB vaccine series as part
of the recommended childhood immunization schedule,
beginning at birth as a safety net (Box 4; Table 3).
• For all medically stable infants weighing ≥2,000 grams at
birth and born to HBsAg-negative mothers, the first dose
of vaccine should be administered within 24 hours of birth
(new recommendation). Only single-antigen HepB
vaccine should be used for the birth dose.
• Infants weighing <2,000 grams and born to HBsAg-
negative mothers should have their first vaccine dose
delayed to the time of hospital discharge or age 1 month
(even if weight is still <2,000 grams). For these infants, a
copy of the original laboratory report indicating that the
mother was HBsAg negative during this pregnancy should
be placed in the infant’s medical record. Infants weighing
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 17
US Department of Health and Human Services/Centers for Disease Control and Prevention
<2,000 grams at birth have a decreased response to HepB
vaccine administered before age 1 month (118).
• For infants transferred to a different facility after birth
(e.g., a hospital with a higher level of neonatal care), staff
at the transferring and receiving facilities should
communicate regarding the infant’s HepB vaccination and
HBIG receipt status to ensure prophylaxis is administered
in a timely manner (new recommendation).
• The final dose in the vaccine series should not be
administered before age 24 weeks (164 days).
• In populations with currently or previously high rates of
childhood HBV infection (e.g., Alaska Natives; Pacific
Islanders; and immigrant families from Asia, Africa, and
countries with intermediate or high endemic rates of
infection), the first dose of HepB vaccine should be
administered at birth and the final dose at age 6–12 months.
Vaccination of Children and Adolescents
• HepB vaccination is recommended for all unvaccinated
children and adolescents aged <19 years (Box 4).
• Children and adolescents who have not previously received
HepB vaccine should be vaccinated routinely at any age
(i.e., children and adolescents are recommended for
catch-up vaccination) (Table 4).
Vaccination of Adults
• HepB vaccination is recommended for all unvaccinated
adults at risk for HBV infection and for all adults requesting
protection from HBV infection. Acknowledgement of a
specific risk factor should not be a requirement for
vaccination (Box 4).
• Adults recommended to receive HepB vaccine:
– Persons at risk for infection by sexual exposure (e.g.,
sex partners of HBsAg-positive persons, sexually active
persons who are not in a mutually monogamous
relationship [e.g., persons with more than one sex
partner during the previous 6 months], persons seeking
evaluation or treatment for a sexually transmitted
infection, and MSM).
– Persons with a history of current or recent injection-
drug use are at increased risk for HBV infection. An
increased incidence of HBV incidence among young
adults in rural U.S. communities has been associated
with an increase in injection-drug use.
– Other persons at risk for infection by percutaneous or
mucosal exposure to blood (household contacts of
HBsAg-positive persons; residents and staff of facilities
for developmentally disabled persons; health care and
public safety personnel with reasonably anticipated risk
for exposure to blood or blood-contaminated body
fluids; hemodialysis patients and predialysis, peritoneal
dialysis, and home dialysis patients; persons with
diabetes mellitus aged <60 years and persons with
diabetes mellitus aged ≥60 years at the discretion of the
treating clinician).
– Others (international travelers to countries with high
or intermediate levels [HBsAg prevalence of ≥2%]
[Box 3] of endemic HBV infection, persons with HCV
infection, persons with chronic liver disease [including,
but not limited to, those with cirrhosis, fatty liver
disease, alcoholic liver disease, autoimmune hepatitis,
and an ALT or AST level greater than twice the upper
limit of normal] [new recommendation], persons with
HIV infection, incarcerated persons, all other persons
seeking protection from HBV infection without
acknowledgement of a specific risk factor).
Vaccination of Pregnant Women
• Pregnant women who are identified as being at risk for
HBV infection during pregnancy (e.g., having more than
one sex partner during the previous 6 months, been
evaluated or treated for an STI, recent or current injection-
drug use, or having had an HBsAg-positive sex partner)
should be vaccinated.
• Pregnant women at risk for HBV infection during
pregnancy should be counseled concerning other methods
to prevent HBV infection.
Implementation Strategies
Delivery Hospital Policies and Procedures
• All delivery hospitals and birthing facilities should implement
policies and procedures to ensure identification of infants
born to HBsAg-positive mothers and infants born to mothers
with unknown HBsAg status, initiation of prophylaxis for
these infants, and routine birth dose for medically stable
infants weighing ≥2,000 grams within 24 hours of birth. Such
policies and procedures should include standing orders and
electronic medical record reminders or prompts.
Case-Management Programs to Prevent Perinatal
HBV Infection
• States and localities should establish case-management
programs, including appropriate policies, procedures, laws,
and regulations to ensure that all pregnant women are
tested for HBsAg during each pregnancy, and that those
who are HBsAg-positive are tested for HBV DNA to guide
maternal antiviral therapy. Infants born to HBsAg-positive
women and women with unknown HBsAg status also
should receive case management.
Recommendations and Reports
18 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
Settings Providing Services to Adults
• In settings in which a high proportion of persons have risk
factors for HBV infection (e.g., health care settings
targeting services to injection-drug users, correctional
facilities, institutions and nonresidential day care facilities
for developmentally disabled persons), all adults should
be assumed to be at risk for HBV infection and should be
offered HepB vaccination if they have not previously
completed vaccination.
• HCP should implement standing orders to administer
HepB vaccine as part of routine services to adults who
have not completed the vaccine series and make HepB
vaccination a standard component of evaluation and
treatment for STIs and HIV/AIDS.
• When feasible, HepB vaccination should be offered in
outreach and other settings in which services are provided
to persons at risk for HBV infection (e.g., needle-exchange
programs, HIV testing sites, HIV prevention programs,
and homeless shelters).
• In medical settings, HCP should implement standing orders
to identify adults recommended for HepB vaccination and
administer vaccination as part of routine services.
Postexposure Prophylaxis
This section provides recommendations for management
of persons who are exposed to HBV through a distinct,
identifiable exposure to blood or body fluids that contain
blood, in occupational and nonoccupational settings.
• Wounds and skin sites that have been in contact with blood
or body fluids should be washed with soap and water; mucous
membranes should be flushed with water. Using antiseptics
(e.g., 2%–4% chlorhexidine) for wound care or expressing
fluid by squeezing the wound further have not been shown
to reduce the risk for HBV transmission; however, the use of
antiseptics is not contraindicated. The application of caustic
agents (e.g., bleach) or the injection of antiseptics or
disinfectants into the wound is not recommended.
Occupational Settings
Vaccinated HCP
• For vaccinated HCP (who have written documentation
of a complete HepB vaccine series) with subsequent
documented anti-HBs ≥10 mIU/mL, testing the source
patient for HBsAg is unnecessary. No postexposure
prophylaxis for HBV is necessary, regardless of the source
patient’s HBsAg status (Table 5).
• For vaccinated HCP (who have written documentation
of a complete HepB vaccine series) without previous anti-
HBs testing, the HCP should be tested for anti-HBs and
the source patient (if known) should be tested for HBsAg
as soon as possible after the exposure. Anti-HBs testing
should be performed using a method that allows detection
of the protective concentration of anti-HBs (≥10 mIU/mL).
Testing the source patient and the HCP should occur
simultaneously; testing the source patient should not be
delayed while waiting for the HCP anti-HBs test results,
and likewise, testing the HCP should not be delayed while
waiting for the source patient’s HBsAg results (Table 5).
– If the HCP has anti-HBs <10 mIU/mL and the source
patient is HBsAg-positive or has an unknown HBsAg
status, the HCP should receive 1 dose of HBIG and
be revaccinated as soon as possible after the exposure.
HepB vaccine may be administered simultaneously
with HBIG at a separate anatomical injection site (e.g.,
separate limb). The HCP should then receive the
second 2 doses of HepB vaccine to complete the second
series (likely 6 doses total when accounting for the
original series) according to the vaccination schedule.
So the HCP’s vaccine response status can be documented
TABLE 5. Postexposure management of health care personnel after occupational percutaneous or mucosal exposure to blood or body fluids,
by health care personnel HepB vaccination and response status
HCP status
Postexposure testing Postexposure prophylaxis
Postvaccination
serologic testing
Source patient
(HBsAg) HCP testing (anti-HBs) HBIG Vaccination
Documented responder after complete series No action needed
Documented nonresponder after two
complete series
Positive/unknown * HBIG x2 separated by
1 month
N/A
Negative No action needed
Response unknown after complete series Positive/unknown <10 mIU/mL HBIG x1 Initiate revaccination Yes
Negative <10 mIU/mL None Initiate revaccination Yes
Any result ≥10 mIU/mL No action needed
Unvaccinated/incompletely vaccinated or
vaccine refusers
Positive/unknown
HBIG x1 Complete vaccination Yes
Negative
None Complete vaccination Yes
Abbreviations: anti HBs = antibody to hepatitis B surface antigen; HBIG = hepatitis B immune globulin; HBsAg = hepatitis B surface antigen; HCP = health care
personnel; N/A = not applicable.
* Not indicated.
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 19
US Department of Health and Human Services/Centers for Disease Control and Prevention
for future exposures, anti-HBs testing should be
performed 1–2 months after the final vaccine dose.
– If the HCP has anti-HBs <10 mIU/mL and the source
patient is HBsAg-negative, the HCP should receive an
additional single HepB vaccine dose, followed by repeat
anti-HBs testing 1–2 months later. HCP whose anti-
HBs remains <10 mIU/mL should undergo
revaccination with two more doses (likely 6 doses total
when accounting for the original series). So the HCP’s
vaccine response status can be documented for future
exposures, anti-HBs testing should be performed
1–2 months after the final dose of vaccine.
– If the HCP has anti-HBs ≥10 mIU/mL at the time of
the exposure, no postexposure HBV management is
necessary, regardless of the source patient’s HBsAg status.
• For vaccinated HCP with anti-HBs <10 mIU/mL after
two complete HepB vaccine series, the source patient
should be tested for HBsAg as soon as possible after the
exposure. If the source patient is HBsAg-positive or has
unknown HBsAg status, the HCP should receive 2 doses
of HBIG (1,10). The first dose should be administered as
soon as possible after the exposure, and the second dose
should be administered 1 month later. HepB vaccine is
not recommended for the exposed HCP who has
previously completed two HepB vaccine series. If the
source patient is HBsAg-negative, neither HBIG nor HepB
vaccine is necessary (Table 5).
Unvaccinated HCP
• For unvaccinated or incompletely vaccinated HCP, the
source patient should be tested for HBsAg as soon as
possible after the exposure. Testing unvaccinated or
incompletely vaccinated HCP for anti-HBs is not
necessary and is potentially misleading, because anti-HBs
≥10 mIU/mL as a correlate of vaccine-induced protection
has only been determined for persons who have completed
an approved vaccination series (107) (Table 5).
• If the source patient is HBsAg-positive or has an unknown
HBsAg status, the HCP should receive 1 dose of HBIG
and 1 dose of HepB vaccine administered as soon as
possible after the exposure. HepB vaccine may be
administered simultaneously with HBIG at a separate
anatomical injection site (e.g., separate limb). The HCP
should complete the HepB vaccine series according to the
vaccination schedule. To document the HCP’s vaccine
response status for future exposures, anti-HBs testing
should be performed approximately 1–2 months after the
final vaccine dose. Anti-HBs testing should be performed
using a method that allows detection of the protective
concentration of anti-HBs (≥10 mIU/mL). Because
anti-HBs testing of HCP who received HBIG should be
performed after anti-HBs from HBIG is no longer
detectable (6 months after administration), it might be
necessary to defer anti-HBs testing for a period longer
than 1–2 months after the last vaccine dose in these
situations (Table 5).
– HCP with anti-HBs ≥10 mIU/mL after receipt of the
primary vaccine series are considered immune.
Immunocompetent persons have long-term protection
and do not need further periodic testing to assess anti-
HBs levels.
– HCP with anti-HBs <10 mIU/mL after receipt of the
primary series should be revaccinated. For these HCP,
administration of a second complete series on an
appropriate schedule, followed by anti-HBs testing
1–2 months after the final dose, is usually more
practical than conducting serologic testing after each
additional dose of vaccine. So the HCP’s vaccine
response status can be documented for future exposures,
anti-HBs testing should be performed 1–2 months after
the final vaccine dose.
• If the source patient is HBsAg-negative, the HCP should
complete the HepB vaccine series according to the
vaccination schedule. So the HCP’s vaccine response status
can be documented for future exposures, anti-HBs testing
should be performed approximately 1–2 months after the
final vaccine dose (Table 5).
– HCP with anti-HBs ≥10 mIU/mL after receipt of the
primary vaccine series are considered immune.
Immunocompetent persons have long-term protection
and do not need further periodic testing to assess anti-
HBs levels.
– HCP with anti-HBs <10 mIU/mL after receipt of the
primary series should be revaccinated. For these HCP,
administration of a second complete series on an
appropriate schedule, followed by anti-HBs testing
1–2 months after the final dose, is usually more practical
than conducting serologic testing after each additional
dose of vaccine. So the HCP’s vaccine response status
can be documented for future exposures, anti-HBs
testing should be performed 1–2 months after the final
vaccine dose.
Clinical Management of Exposed HCP
• HCP who have anti-HBs <10 mIU/mL (or who are
unvaccinated or incompletely vaccinated) and sustain an
exposure to a source patient who is HBsAg-positive or has
an unknown HBsAg status should undergo baseline testing
for HBV infection as soon as possible after the exposure,
and follow-up testing approximately 6 months later. Testing
Recommendations and Reports
20 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
immediately after the exposure should consist of total anti-
HBc, and follow-up testing approximately 6 months later
should consist of HBsAg and total anti-HBc.
• HCP exposed to a source patient who is HBsAg-positive
or has an unknown HBsAg status do not need to take
special precautions to prevent secondary transmission
during the follow-up period; however, they should refrain
from donating blood, plasma, organs, tissue, or semen
(10). The exposed HCP does not need to modify sexual
practices or refrain from becoming pregnant (10). If an
exposed HCP is breastfeeding, she does not need to
discontinue (7,10). No modifications to an exposed HCP’s
patient-care responsibilities are necessary to prevent
transmission to patients based solely on exposure to a
source patient who is HBsAg-positive or has an unknown
HBsAg status.
Previously Vaccinated HCP
• Providers should only accept written, dated records as
evidence of HepB vaccination (151).
• An increasing number of HCP have received routine HepB
vaccination during childhood. No postvaccination
serologic testing is recommended after routine infant or
adolescent HepB vaccination. Because vaccine-induced
anti-HBs wanes over time, testing HCP for anti-HBs years
after vaccination might not distinguish vaccine
nonresponders from responders. Pre-exposure assessment
of current or past anti-HBs results upon hire or
matriculation, followed by one or more additional doses
of HepB vaccine for HCP with anti-HBs <10 mIU/mL
and retesting anti-HBs, if necessary, helps to ensure that
HCP will be protected if they have an exposure to HBV-
containing blood or body fluids (Box 5; Figure 3).
– HCP who cannot provide documentation of 3 doses
of HepB vaccine should be considered unvaccinated
and should complete the vaccine series. Postvaccination
serologic testing for anti-HBs is recommended
1–2 months after the third vaccine dose. HCP who are
inadvertently tested before receiving 3 documented
doses of HepB vaccine and have anti-HBs ≥10 mIU/mL
should not be considered immune because anti-HBs
≥10 mIU/mL is a known correlate of protection only
when testing follows a documented 3-dose series.
Health care facilities are encouraged to try to locate
vaccine records for HCP and to enter all vaccine doses
in their state immunization information system.
Nonoccupational Settings
HBsAg-Positive Source
This section provides recommendations for management
of persons who are exposed to HBV through a distinct,
identifiable exposure to blood or body fluids that contain
blood, in nonoccupational settings (Table 6). The exposed
person does not need to undergo postvaccination serologic
testing following vaccination based solely on being exposed.
• Exposed persons who have written documentation of a complete
HepB vaccine series and who did not receive postvaccination
testing should receive a single dose of HepB vaccine.
• Exposed persons who are in the process of being vaccinated
but who have not completed the vaccine series should receive
a dose of HBIG and complete the HepB vaccine series (it
is not necessary to restart the HepB vaccine series). HepB
vaccine may be administered simultaneously with HBIG
at a separate anatomical injection site (e.g., separate limb).
• Exposed unvaccinated persons should receive both HBIG
and HepB vaccine as soon as possible after exposure
(preferably within 24 hours). HepB vaccine may be
administered simultaneously with HBIG at a separate
anatomical injection site (e.g., separate limbs). Studies are
limited on the maximum interval after exposure during
which postexposure prophylaxis is effective, but the
interval is unlikely to exceed 7 days for percutaneous
exposure and 14 days for sexual exposures. The HepB
vaccine series should be completed according to the
vaccination schedule.
HBsAg-Unknown Source
• Exposed persons with written documentation of a
complete HepB vaccine series require no further treatment.
• Exposed persons who are in the process of being vaccinated
but who are not fully vaccinated should complete the
HepB vaccine series (it is not necessary to restart the
vaccination series).
• Exposed unvaccinated persons should receive the HepB
vaccine series with the first dose administered as soon as
possible after exposure, preferably within 24 hours. Studies
are limited on the maximum interval after exposure during
which postexposure prophylaxis is effective, but the interval
is unlikely to exceed 7 days for percutaneous exposure and
14 days for sexual exposures. The vaccine series should be
completed according to the vaccination schedule.

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 21
US Department of Health and Human Services/Centers for Disease Control and Prevention
Immunization Management Issues
Prevaccination Testing
• Vaccination of persons immune to HBV because of current
or previous infection or HepB vaccination does not
increase the risk for adverse events (8). However, in
populations that have high rates of previous HBV
infection, prevaccination testing might reduce costs by
avoiding vaccination of persons who are already immune.
Prevaccination testing consists of testing for HBsAg, anti-
HBs, and anti-HBc. Serologic testing should not be a
barrier to vaccination of susceptible persons, especially in
populations that are difficult to access. Testing is not a
requirement for vaccination, and in settings where testing
is not feasible, vaccination of recommended persons
should continue.
• The first dose of HepB vaccine should typically be
administered immediately after collection of the blood for
serologic testing. Prevaccination testing is recommended
for the following persons (Box 6):
– household, sexual, or needle-sharing contacts of
HBsAg-positive persons;
– HIV-positive persons;
– persons with elevated alanine aminotransferase (ALT)/
aspartate aminotransferase (AST) of unknown etiology;
– hemodialysis patients (refer to 2001 CDC
recommendations [88] for additional information);
– MSM; and
– past or current injection-drug users.
Testing for HBV Infection
• Testing for HBV infection (consisting of testing for
HBsAg, anti-HBs, and anti-HBc) is also recommended
for the following persons:
– persons born in countries of high and intermediate
HBV endemicity (HBsAg prevalence ≥2%);
– U.S.-born persons not vaccinated as infants whose
parents were born in countries with high HBV
endemicity (≥8%);
– persons needing immunosuppressive therapy, including
chemotherapy, immunosuppression related to organ
transplantation, and immunosuppression for
rheumatologic or gastroenterologic disorders; and
– donors of blood, plasma, organs, tissues, or semen.
• All pregnant women should be tested for HBsAg
during each pregnancy. Pregnant women with positive
HBsAg tests should be tested for HBV DNA.
Postvaccination Serologic Testing
• Serologic testing for immunity is not necessary after
routine vaccination of infants, children, or adults.
• Testing for anti-HBs after vaccination is recommended
for the following persons whose subsequent clinical
management depends on knowledge of their immune
status (Box 7):
– infants born to HBsAg-positive women and infants
born to women whose HBsAg status remains unknown
BOX 5. Testing anti-HBs for health care personnel (HCP) vaccinated
in the past
The issue: An increasing number of HCP have received
routine hepatitis B (HepB) vaccination during childhood.
No postvaccination serologic testing is recommended after
routine infant or adolescent HepB vaccination. Because
vaccine-induced antibody to hepatitis B surface antigen
(anti-HBs) wanes over time, testing HCP for anti-HBs
years after vaccination might not distinguish vaccine
nonresponders from responders.
Guidance for health care institutions: Health
care institutions may measure anti-HBs upon hire or
matriculation for HCP who have documentation of a
complete HepB vaccine series in the past (e.g., as part
of routine infant or adolescent vaccination). HCP with
anti-HBs <10 mIU/mL should receive one or more
additional doses of HepB vaccine and retesting (Figure 3).
Institutions that decide to not measure anti-HBs upon hire
or matriculation for HCP who have documentation of a
complete HepB vaccine series in the past should ensure
timely assessment and postexposure prophylaxis following
an exposure (Table 5).
Considerations: The risk for occupational HBV
infection for vaccinated HCP might be low enough in
certain settings so that assessment of anti-HBs status
and appropriate follow-up should be done at the time of
exposure to potentially infectious blood or body fluids. This
approach relies on HCP recognizing and reporting blood
and body fluid exposures and therefore may be applied
on the basis of documented low risk, implementation,
and cost considerations. Certain HCP occupations have
lower risk for occupational blood and body fluid exposures
(e.g., occupations involving counseling versus performing
procedures), and nontrainees have lower risks for blood
and body fluid exposures than trainees. Some settings
also will have a lower prevalence of HBV infection in the
patient population served than in other settings, which will
influence the risk for HCP exposure to HBsAg-positive
blood and body fluids.
Recommendations and Reports
22 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
FIGURE 3. Pre-exposure evaluation for health care personnel previously vaccinated with complete, ≥3-dose HepB vaccine series who have
not had postvaccination serologic testing*
Measure antibody to hepatitis B surface antigen (anti-HBs)
anti-HBs
≥10 mIU/mL
anti-HBs
<10 mIU/mL
anti-HBs
<10 mIU/mL
anti-HBs
≥10 mIU/mL
Administer 1 dose of HepB vaccine,
postvaccination serologic testing
Administer 2 more doses
of HepB vaccine,
postvaccination serologic testing
anti-HBs <10 mIU/mL anti-HBs ≥10 mIU/mL
No action for
hepatitis B prophylaxis
(regardless of source patient
hepatitis B surface antigen status)
Health care
personnel need
to receive
hepatitis B
evaluation for
all exposures
Source: Adapted from CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations
of the Advisory Committee on Immunization Practices (ACIP). Part II: immunization of adults. MMWR 2006;55(No. RR-16).
* Should be performed 1–2 months after the last dose of vaccine using a quantitative method that allows detection of the protective concentration of anti-HBs
(≥10 mIU/mL) (e.g., enzyme-linked immunosorbent assay [ELISA]).

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 23
US Department of Health and Human Services/Centers for Disease Control and Prevention
TABLE 6. Postexposure management after distinct nonoccupational percutaneous or mucosal exposure to blood or body fluids
Exposure*
Management
Unvaccinated person Previously vaccinated person
HBsAg-positive source HepB vaccine series and HBIG HepB vaccine dose
HBsAg status unknown for source Hep B vaccine series No management
Abbreviations: HepB = hepatitis B; HBsAg = hepatitis B surface antigen; HBIG = hepatitis B immune globulin.
* Exposures include percutaneous (e.g., bite or needlestick) or mucosal exposure to blood or body fluids, sex or needle-sharing contact, or victim of sexual assault/abuse.
(e.g., infants safely surrendered shortly after birth).
Postvaccination serologic testing should consist of
testing for anti-HBs and HBsAg;
– HCP and public safety workers at risk for blood or
body fluid exposure;
– hemodialysis patients (and other persons who might
require outpatient hemodialysis), HIV-infected
persons, and other immunocompromised persons (e.g.,
hematopoietic stem-cell transplant recipients or persons
receiving chemotherapy), to determine the need for
revaccination and the type of follow-up testing; and
– sex partners of HBsAg-positive persons, to determine
the need for revaccination and for other methods of
protection against HBV infection.
• Testing should be performed 1–2 months after
administration of the final dose of the vaccine series using
a method that allows determination of a protective
concentration of anti-HBs (≥10 mIU/mL).
– Persons found to have anti-HBs concentrations of
≥10 mIU/mL after the primary vaccine series are
considered to be immune.
– Immunocompetent persons have long-term protection and
do not need further periodic testing to assess anti-HBs levels.
– Immunocompromised persons might need annual testing
to assess anti-HBs concentrations (See Revaccination).
– Persons found to have anti-HBs concentrations of
<10 mIU/mL after the primary vaccine series should
be revaccinated. Administration of all doses in the
second series, on an appropriate schedule, followed by
anti-HBs testing 1–2 months after the final dose, is
usually more practical than serologic testing after one
or more doses of vaccine (except for when revaccinating
infants born to HBsAg-positive mothers).
– Persons who do not have a protective concentration of
anti-HBs after revaccination should be tested for HBsAg.
– If the HBsAg test result is positive, the person should
receive appropriate management, and any household,
sexual, or needle-sharing contacts should be identified and
vaccinated. Prevaccination testing (consisting of anti-HBc,
HBsAg, and anti-HBs) to identify infected persons is
recommended for household, sexual, or needle-sharing
contacts of HBsAg-positive persons; serologic testing
should not be a barrier to vaccination, and the first HepB
vaccine dose should be administered immediately after
collection of the blood for serologic testing.
– Persons who test negative for HBsAg should be
considered susceptible to HBV infection and should
be counseled about precautions to prevent HBV
infection and the need to obtain HBIG postexposure
prophylaxis for any known or likely exposure to an
HBsAg-positive source (10).
• Testing HCP with documentation of complete HepB
vaccination for anti-HBs upon hire or matriculation (i.e.,
pre-exposure assessment of prior response to HepB
vaccination), followed by one or more additional doses of
HepB vaccine for HCP with anti-HBs <10 mIU/mL, helps
to ensure that HCP will be protected if they have an
exposure to HBV-containing blood or body fluids.
• Anti-HBs levels of ≥10 mIU/mL are generally considered
seroprotective; however, different assays have different
assay cutoff values based on which reported levels of anti-
HBs might vary depending on the assay used. Refer to the
package insert of the test for the determination of actual/
correct levels of anti-HBs antibodies.
Revaccination
• Revaccination (i.e., booster dose, challenge dose, or
revaccination with a complete series) is not generally
recommended for persons with a normal immune status
who were vaccinated as infants, children, adolescents, or
adults. Available data do not suggest a maximum number
of booster doses. Revaccination when anti-HBs is
<10 mIU/mL is recommended for the following persons:
– Infants born to HBsAg-positive mothers. HBsAg-
negative infants with anti-HBs <10 mIU/mL should
be revaccinated with a single dose of HepB vaccine,
and retested 1–2 months later. Infants whose anti-HBs
remains <10 mIU/mL following single dose
revaccination should receive two additional doses of
HepB vaccine on a vaccine schedule to complete the
second series, followed by anti-HBs testing 1–2 months
later. Alternatively, these infants may be revaccinated
with a second 3-dose series and retested (HBsAg and
anti-HBs) 1–2 months after the final dose of vaccine.

Recommendations and Reports
24 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
BOX 6. Persons recommended to receive serologic testing prior
to vaccination*
• Household, sexual, or needle contacts of hepatitis B
surface antigen (HBsAg)–positive persons
†
• HIV-positive persons
†
• Persons with elevated alanine aminotransferase/
aspartate aminotransferase of unknown etiology
†
• Hemodialysis patients
†
• Men who have sex with men
†
• Past or current persons who inject drugs
†
• Persons born in countries of high and intermediate
hepatitis B virus (HBV) endemicity (HBsAg
prevalence ≥2%)
• U.S.-born persons not vaccinated as infants whose
parents were born in countries with high HBV
endemicity (≥8%)
• Persons needing immunosuppressive therapy,
including chemotherapy, immunosuppression related
to organ transplantation, and immunosuppression for
rheumatologic or gastroenterologic disorders
• Donors of blood, plasma, organs, tissues, or semen
* Serologic testing comprises testing for hepatitis B surface antigen (HBsAg),
antibody to HBsAg, and antibody to hepatitis B core antigen.
†
Denotes persons also recommended for hepatitis B vaccination. Serologic
testing should occur prior to vaccination. Serologic testing should not be a
barrier to vaccination of susceptible persons. The first dose of vaccine should
typically be administered immediately after collection of the blood for
serologic testing.
– HCP. Completely vaccinated HCP with anti-HBs
<10 mIU/mL should receive an additional dose of HepB
vaccine, followed by anti-HBs testing 1–2 months later.
HCP whose anti-HBs remains <10 mIU/mL should
complete the second series (usually 6 doses total), followed
by repeat anti-HBs testing 1–2 months after the final dose.
Alternatively, it might be more practical for very recently
vaccinated HCP with anti-HBs <10 mIU/mL to receive
the second complete series (usually 6 doses total), followed
by anti-HBs testing 1–2 months after the final dose.
– Hemodialysis patients. For hemodialysis patients
treated in outpatient centers, the need for booster doses
should be assessed by annual anti-HBs testing. A
booster dose should be administered when anti-HBs
levels decline to <10 mIU/mL. Anti-HBs testing
1–2 months following the booster dose to assess
response is not recommended.
– Other immunocompromised persons. For other
immunocompromised persons (e.g., HIV-infected
persons, hematopoietic stem-cell transplant recipients, and
persons receiving chemotherapy), the need for booster
doses has not been determined. Annual anti-HBs testing
and booster doses should be considered for persons with
an ongoing risk for exposure.
Interrupted Schedules and Minimum
Dosing Intervals
• For all ages, when the HepB vaccine schedule is
interrupted, the vaccine series does not need to be
restarted. If the series is interrupted after the first dose,
the second dose should be administered as soon as possible,
and the second and third doses should be separated by an
interval of at least 8 weeks. If only the third dose has been
delayed, it should be administered as soon as possible. The
final dose of vaccine must be administered at least 8 weeks
after the second dose and should follow the first dose by
at least 16 weeks; the minimum interval between the first
and second doses is 4 weeks. Inadequate doses of
HepB vaccine or doses received after a shorter-than-
recommended dosing interval should be readministered,
using the correct dosage or schedule.
• Vaccine doses administered ≤4 days before the minimum
interval or age are considered valid. Because of the unique
accelerated schedule for Twinrix, the 4-day guideline does
not apply to the first 3 doses of this vaccine when
administered on a 0-day, 7-day, 21–30-day, and 12-month
schedule (new recommendation).
• In infants, administration of the final dose is not
recommended before age 24 weeks.
Other Immunization Management Issues
• No differences in immunogenicity have been observed
when one or 2 doses of HepB vaccine produced by one
manufacturer are followed by doses from a different
manufacturer (8). Whenever feasible, the same vaccine
should be used for the subsequent doses; however, if a
different brand is administered, the dose should be
considered valid and does not need to be repeated.
• Providers should only accept dated records as evidence of
HepB vaccination.
• Although vaccinations should not be postponed if records
cannot be found, an attempt to locate missing records
should be made by contacting previous health care
providers, reviewing state or local immunization
information systems, and searching for a personally held
record. If records cannot be located within a reasonable
time, these persons should be considered susceptible and
started on the age-appropriate vaccination schedule. An
anti-HBs ≥10 mIU/mL is a serologic correlate of protection
only when following a documented, complete series.

Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 25
US Department of Health and Human Services/Centers for Disease Control and Prevention
BOX 7. Persons recommended to receive postvaccination serologic
testing* following a complete series of HepB vaccination
• Infants born to hepatitis B surface antigen (HBsAg)–
positive mothers or mothers whose HBsAg status
remains unknown (e.g., when a parent or person with
lawful custody safely surrenders an infant confidentially
shortly after birth infants safely surrendered at or
shortly after birth)
†
• Health care personnel and public safety workers
• Hemodialysis patients and others who might require
outpatient hemodialysis (e.g., predialysis, peritoneal
dialysis, and home dialysis)
• HIV-infected persons
• Other immunocompromised persons (e.g.,
hematopoietic stem-cell transplant recipients or
persons receiving chemotherapy)
• Sex partners of HBsAg-positive persons
* Postvaccination serologic testing for persons other than infants born to
HBsAg-positive (or HBsAg-unknown) mothers consists of anti-HBs.
†
Postvaccination serologic testing for infants born to HBsAg-positive (or
HBsAg-unknown) mothers consists of anti-HBs and HBsAg. Persons
with anti-HBs <10 mIU/mL after the primary vaccine series should be
revaccinated. Infants born to HBsAg-positive mothers or mothers with
an unknown HBsAg status should be revaccinated with a single dose of
HepB vaccine and receive postvaccination serologic testing 1–2 months
later. Infants whose anti-HBs remains <10 mIU/mL following single dose
revaccination should receive two additional doses of HepB vaccine,
followed by postvaccination serologic testing 1–2 months after the final
dose. Based on clinical circumstances or family preference, HBsAg-
negative infants with anti-HBs <10 mIU/mL may instead be revaccinated
with a second, complete 3-dose series, followed by postvaccination
serologic testing performed 1–2 months after the final dose of vaccine.
For others with anti-HBs <10 mIU/mL after the primary series,
administration of 3 additional HepB vaccine doses on an appropriate
schedule, followed by anti-HBs testing 1–2 months after the final dose,
is usually more practical than serologic testing after ≥1 dose of vaccine.
• In all settings, vaccination should be initiated even though
completion of the series might not be ensured.
• Adverse events occurring after administration of any vaccine
should be reported to VAERS. Reports should be submitted
to VAERS online, by facsimile, or by mail. More information
about VAERS is available by calling 1-800-822-7967 (toll-
free) or online at https://vaers.hhs.gov.
Future Directions
ACIP and CDC will review these recommendations as new
epidemiology or other information related to HepB vaccines
(including licensure of additional HepB-containing vaccines),
HepB vaccine adverse events, and the experience gained in the
implementation of these recommendations becomes available.
Revised recommendations will be developed as needed.
Acknowledgments
Mona Doshani, Alaya Koneru, Henry Roberts, PhD, Division of
Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis,
STD, and TB Prevention, CDC.
References
1. CDC. Updated U.S. Public Health Service guidelines for the
management of occupational exposures to HBV, HCV, and HIV and
recommendations for postexposure prophylaxis. MMWR Recomm Rep
2001;50(No. RR-11):1–52.
2. Preboth M. PHS guidelines for management of occupational exposure
to HBV, HCV and HIV: management of occupational blood exposures.
Am Fam Physician 2001;64:2012–4.
3. Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE.
Survival of hepatitis B virus after drying and storage for one week. Lancet
1981;317:550–1. https://doi.org/10.1016/S0140-6736(81)92877-4
4. CDC. Viral hepatitis—statistics and surveillance. Atlanta, GA: US
Department of Health and Human Services, CDC; 2017. https://www.
cdc.gov/hepatitis/statistics/
5. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM,
Murad MH; American Association for the Study of Liver Diseases.
AASLD guidelines for treatment of chronic hepatitis B. Hepatology
2016;63:261–83. https://doi.org/10.1002/hep.28156
6. Rubin LG, Levin MJ, Ljungman P, et al.; Infectious Diseases Society of
America. 2013 IDSA clinical practice guideline for vaccination of the
immunocompromised host. Clin Infect Dis 2014;58:e44–100. https://
doi.org/10.1093/cid/cit684
7. Mast EE, Margolis HS, Fiore AE, et al.; Advisory Committee on
Immunization Practices (ACIP). A comprehensive immunization strategy
to eliminate transmission of hepatitis B virus infection in the United
States: recommendations of the Advisory Committee on Immunization
Practices (ACIP). Part 1: immunization of infants, children, and
adolescents. MMWR Recomm Rep 2005;54(No. RR-16):1–31.
8. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive
immunization strategy to eliminate transmission of hepatitis B virus
infection in the United States: recommendations of the Advisory
Committee on Immunization Practices (ACIP). Part II: immunization
of adults. MMWR Recomm Rep 2006;55(No. RR-16):1–33.
9. CDC. Use of hepatitis B vaccination for adults with diabetes mellitus:
recommendations of the Advisory Committee on Immunization
Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011;60:1709–11.
10. Schillie S, Murphy TV, Sawyer M, et al.; CDC. CDC guidance for
evaluating health-care personnel for hepatitis B virus protection and
for administering postexposure management. MMWR Recomm Rep
2013;62(No. RR-10):1–19.
11. Schillie S, Murphy TV, Fenlon N, Ko S, Ward JW. Update: shortened
interval for postvaccination serologic testing of infants born to hepatitis
b-infected mothers. MMWR Morb Mortal Wkly Rep 2015;64:1118–20.
12. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring
in the Vaccine Adverse Event Reporting System (VAERS). Vaccine
2015;33:4398–405. https://doi.org/10.1016/j.vaccine.2015.07.035
13. Zhou W, Pool V, Iskander JK, et al. Surveillance for safety
after immunization: Vaccine Adverse Event Reporting System
(VAERS)—United States, 1991–2001. MMWR Surveill Summ
2003;52(No. SS-1):1–24.
14. McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety
Datalink: successes and challenges monitoring vaccine safety. Vaccine
2014;32:5390–8. https://doi.org/10.1016/j.vaccine.2014.07.073
15. CDC. Surveillance data for acute viral hepatitis—United States, 2008.
Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
https://www.cdc.gov/hepatitis/statistics/2008surveillance/index.htm
Recommendations and Reports
26 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
16. Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus
infections—Kentucky, Tennessee, and West Virginia, 2006–2013.
MMWR Morb Mortal Wkly Rep 2016;65:47–50.
17. Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic
hepatitis B virus (HBV) infection in U.S. households: National Health
and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology
2016;63:388–97. https://doi.org/10.1002/hep.28109
18. Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of
hepatitis B virus infection in the United States in the era of vaccination.
J Infect Dis 2010;202:192–201. https://doi.org/10.1086/653622
19. Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence
of chronic hepatitis B among foreign-born persons living in the United
States by country of origin. Hepatology 2012;56:422–33. https://doi.
org/10.1002/hep.24804
20. Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The
increasing burden of imported chronic hepatitis B—United States,
1974–2008. PLoS One 2011;6:e27717. https://doi.org/10.1371/
journal.pone.0027717
21. CDC. Hepatitis B virus: a comprehensive strategy for eliminating
transmission in the United States through universal childhood
vaccination. Recommendations of the Immunization Practices Advisory
Committee (ACIP). MMWR Recomm Rep 1991;40(No. RR-13):1–25.
22. CDC. Recommendation of the Immunization Practices Advisory
Committee (ACIP). Inactivated hepatitis B virus vaccine. MMWR Morb
Mortal Wkly Rep 1982;31:317–22, 327–8.
23. CDC. Changing patterns of groups at high risk for hepatitis B in the
United States. MMWR Morb Mortal Wkly Rep 1988;37:429–32, 437.
24. CDC. Update: universal precautions for prevention of transmission of
human immunodeficiency virus, hepatitis B virus, and other bloodborne
pathogens in health-care settings. MMWR Morb Mortal Wkly Rep
1988;37:377–82, 387–8.
25. CDC. Prevention of perinatal transmission of hepatitis B virus: prenatal
screening of all pregnant women for hepatitis B surface antigen. MMWR
Morb Mortal Wkly Rep 1988;37:341–6, 351.
26. CDC. Update: recommendations to prevent hepatitis B virus
transmission—United States. MMWR Morb Mortal Wkly Rep
1999;48:33–4.
27. Kolasa MS, Tsai Y, Xu J, Fenlon N, Schillie S. Hepatitis B surface antigen
testing among pregnant women, United States 2014. Pediatr Infect Dis
J 2017;36:e175–80. https://doi.org/10.1097/INF.0000000000001516
28. Kubo A, Shlager L, Marks AR, et al. Prevention of vertical transmission of
hepatitis B: an observational study. Ann Intern Med 2014;160:828–35.
https://doi.org/10.7326/M13-2529
29. Schillie S, Walker T, Veselsky S, et al. Outcomes of infants born to
women infected with hepatitis B. Pediatrics 2015;135:e1141–7. https://
doi.org/10.1542/peds.2014-3213
30. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination
coverage among children aged 19–35 months—United States, 2016.
MMWR Morb Mortal Wkly Rep 2017;66:1171–7. https://doi.
org/10.15585/mmwr.mm6643a3
31. CDC. Newborn hepatitis B vaccination coverage among children born
January 2003-June 2005—United States. MMWR Morb Mortal Wkly
Rep 2008;57:825–8.
32. US Department of Health and Human Services, Office of Disease
Prevention and Health Promotion. Healthy people 2020. Washington,
DC: US Department of Health and Human Services; 2014. https://
www.healthypeople.gov/
33. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional,
state, and selected local area vaccination coverage among adolescents
aged 13–17 yearsUnited States, 2016. MMWR Morb Mortal Wkly
Rep 2017;66:874–82.
34. Williams WW, Lu PJ, O’Halloran A, et al.; CDC. Surveillance of
vaccination coverage among adult populations—United States, 2015.
MMWR Surveill Summ 2017;66(No. SS-11):1–28.
35. Agerton TB, Mahoney FJ, Polish LB, Shapiro CN. Impact of the
bloodborne pathogens standard on vaccination of healthcare workers with
hepatitis B vaccine. Infect Control Hosp Epidemiol 1995;16:287–91.
https://doi.org/10.2307/30143095
36. Fan L, Owusu-Edusei K Jr, Schillie SF, Murphy TV. Cost-effectiveness of
testing hepatitis B-positive pregnant women for hepatitis B e antigen or
viral load. Obstet Gynecol 2014;123:929–37. https://doi.org/10.1097/
AOG.0000000000000124
37. CDC; The American College of Obstetricians and Gynecologists.
Screening pregnant women for hepatitis B virus (HBV) infection.
Atlanta, GA: US Department of Health and Human Services, CDC;
2015. https://www.cdc.gov/hepatitis/hbv/pdfs/prenatalhbsagtesting.pdf
38. Pan CQ, Duan ZP, Bhamidimarri KR, et al. An algorithm for risk
assessment and intervention of mother to child transmission of
hepatitis B virus. Clin Gastroenterol Hepatol 2012;10:452–9. https://
doi.org/10.1016/j.cgh.2011.10.041
39. Pan CQ, Han GR, Jiang HX, et al. Telbivudine prevents vertical
transmission from HBeAg-positive women with chronic hepatitis B.
Clin Gastroenterol Hepatol 2012;10:520–6. https://doi.org/10.1016/j.
cgh.2012.01.019
40. Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus
infection. Immunol Cell Biol 2007;85:16–23. https://doi.org/10.1038/
sj.icb.7100009
41. Gish RG, Given BD, Lai CL, et al. Chronic hepatitis B: Virology,
natural history, current management and a glimpse at future
opportunities. Antiviral Res 2015;121:47–58. https://doi.org/10.1016/j.
antiviral.2015.06.008
42. Hoofnagle JH, Di Bisceglie AM. Serologic diagnosis of acute and
chronic viral hepatitis. Semin Liver Dis 1991;11:73–83. https://doi.
org/10.1055/s-2008-1040426
43. Krugman S, Overby LR, Mushahwar IK, Ling CM, Frösner GG,
Deinhardt F. Viral hepatitis, type B. Studies on natural history and
prevention re-examined. N Engl J Med 1979;300:101–6. https://doi.
org/10.1056/NEJM197901183000301
44. Long SS, Prober CG, Fischer M. Principles and practice of pediatric
infectious diseases. 5th ed. Amsterdam, the Netherlands: Elsevier; 2017.
45. Magnius LO, Norder H. Subtypes, genotypes and molecular epidemiology
of the hepatitis B virus as reflected by sequence variability of the S-gene.
Intervirology 1995;38:24–34. https://doi.org/10.1159/000150411
46. Kramvis A. Genotypes and genetic variability of hepatitis B virus.
Intervirology 2014;57:141–50. https://doi.org/10.1159/000360947
47. Kramvis A. The clinical implications of hepatitis B virus genotypes and
HBeAg in pediatrics. Rev Med Virol 2016;26:285–303. https://doi.
org/10.1002/rmv.1885
48. Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol
2004;11:18–25. https://doi.org/10.1016/j.tracli.2003.11.007
49. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet
2014;384:2053–63. https://doi.org/10.1016/S0140-6736(14)60220-8
50. McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B
virus infection: relation of age to the clinical expression of disease
and subsequent development of the carrier state. J Infect Dis
1985;151:599–603. https://doi.org/10.1093/infdis/151.4.599
51. Dienstag JL. Immunopathogenesis of the extrahepatic manifestations
of hepatitis B virus infection. Springer Semin Immunopathol
1981;3:461–72. https://doi.org/10.1007/BF01951493
52. Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally
transmitted hepatitis B virus infections with hepatitis B immune
globulin and hepatitis B vaccine. Lancet 1983;322:1099–102. https://
doi.org/10.1016/S0140-6736(83)90624-4
53. Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The
influence of age on the development of the hepatitis B carrier state. Proc
Biol Sci 1993;253:197–201. https://doi.org/10.1098/rspb.1993.0102
54. Hyams KC. Risks of chronicity following acute hepatitis B virus
infection: a review. Clin Infect Dis 1995;20:992–1000. https://doi.
org/10.1093/clinids/20.4.992
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 27
US Department of Health and Human Services/Centers for Disease Control and Prevention
55. Hadler SC, Judson FN, O’Malley PM, et al. Outcome of hepatitis B
virus infection in homosexual men and its relation to prior human
immunodeficiency virus infection. J Infect Dis 1991;163:454–7. https://
doi.org/10.1093/infdis/163.3.454
56. Beasley RP, Lin CC, Hwang LY, Chien CS. Hepatocellular carcinoma and
hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet
1981;318:1129–33. https://doi.org/10.1016/S0140-6736(81)90585-7
57. Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A
mathematical model to estimate global hepatitis B disease burden and
vaccination impact. Int J Epidemiol 2005;34:1329–39. https://doi.
org/10.1093/ije/dyi206
58. McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis
B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-
positive Alaska native carriers. Arch Intern Med 1990;150:1051–4.
https://doi.org/10.1001/archinte.1990.00390170087019
59. Jonas MM, Lok AS, McMahon BJ, et al. Antiviral therapy in management
of chronic hepatitis B viral infection in children: a systematic review and
meta-analysis. Hepatology 2016;63:307–18. https://doi.org/10.1002/
hep.28278
60. Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for
chronic hepatitis B viral infection in adults: A systematic review and
meta-analysis. Hepatology 2016;63:284–306. https://doi.org/10.1002/
hep.28280
61. McMahon BJ. Chronic hepatitis B virus infection. Med Clin North Am
2014;98:39–54. https://doi.org/10.1016/j.mcna.2013.08.004
62. Wang C, Ji D, Chen J, et al. Hepatitis due to reactivation of
hepatitis B virus in endemic areas among patients with hepatitis C
treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol
2017;15:132–6. https://doi.org/10.1016/j.cgh.2016.06.023
63. Nelson NP, Jamieson DJ, Murphy TV. Prevention of perinatal
hepatitis B virus transmission. J Pediatric Infect Dis Soc 2014;3(Suppl
1):S7–12. https://doi.org/10.1093/jpids/piu064
64. Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B
vaccination among newborn infants: a review. Vaccine 2013;31:2506–16.
https://doi.org/10.1016/j.vaccine.2012.12.012
65. Pondé RA. Atypical serological profiles in hepatitis B virus infection. Eur
J Clin Microbiol Infect Dis 2013;32:461–76. https://doi.org/10.1007/
s10096-012-1781-9
66. Pondé RA, Cardoso DD, Ferro MO. The underlying mechanisms for
the ‘anti-HBc alone’ serological profile. Arch Virol 2010;155:149–58.
https://doi.org/10.1007/s00705-009-0559-6
67. Franks AL, Berg CJ, Kane MA, et al. Hepatitis B virus infection
among children born in the United States to Southeast Asian
refugees. N Engl J Med 1989;321:1301–5. https://doi.org/10.1056/
NEJM198911093211905
68. Mortensen E, Kamali A, Schirmer PL, et al. Are current screening
protocols for chronic hepatitis B virus infection adequate? Diagn
Microbiol Infect Dis 2016;85:159–67. https://doi.org/10.1016/j.
diagmicrobio.2015.12.005
69. Calisti G, Herman O, Powley M, Haque T. Persistence of hepatitis B surface
antigen in blood in a chronic haemodialysis patient following vaccination
booster. BMJ Case Rep 2014;2014(jun10 1):bcr2013202191. https://
doi.org/10.1136/bcr-2013-202191
70. Grob P, Jilg W, Bornhak H, et al. Serological pattern “anti-HBc alone”:
report on a workshop. J Med Virol 2000;62:450–5. https://doi.
org/10.1002/1096-9071(200012)62:4<450::AID-JMV9>3.0.CO;2-Y
71. Silva AE, McMahon BJ, Parkinson AJ, Sjogren MH, Hoofnagle JH, Di
Bisceglie AM. Hepatitis B virus DNA in persons with isolated antibody
to hepatitis B core antigen who subsequently received hepatitis B vaccine.
Clin Infect Dis 1998;26:895–7. https://doi.org/10.1086/513918
72. Candotti D, Lin CK, Belkhiri D, et al. Occult hepatitis B infection
in blood donors from South East Asia: molecular characterisation and
potential mechanisms of occurrence. Gut 2012;61:1744–53. https://
doi.org/10.1136/gutjnl-2011-301281
73. De Feo TM, Poli F, Mozzi F, Moretti MP, Scalamogna M; Collaborative
Kidney, Liver and Heart North Italy Transplant Program Study
Groups. Risk of transmission of hepatitis B virus from anti-HBC
positive cadaveric organ donors: a collaborative study. Transplant Proc
2005;37:1238–9. https://doi.org/10.1016/j.transproceed.2004.12.041
74. Council of State and Territorial Epidemiologists. Hepatitis B, perinatal
virus infection 2017 case definition. Atlanta, GA: Council of State
and Territorial Epidemiologists; 2017. https://wwwn.cdc.gov/nndss/
conditions/hepatitis-b-perinatal-virus-infection/case-definition/2017/
75. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of
hepatitis B and hepatitis C in people who inject drugs: results of
systematic reviews. Lancet 2011;378:571–83. https://doi.org/10.1016/
S0140-6736(11)61097-0
76. CDC. Viral hepatitis—CDC recommendations for specific
populations and settings. Atlanta, GA: US Department of Health
and Human Services, CDC; 2015. https://www.cdc.gov/hepatitis/
populations/stds.htm
77. Hurie MB, Mast EE, Davis JP. Horizontal transmission of hepatitis B virus
infection to United States-born children of Hmong refugees. Pediatrics
1992;89:269–73.
78. Chaudhary RK, Perry E, Cleary TE. Prevalence of hepatitis B infection
among residents of an institution for the mentally retarded. Am J Epidemiol
1977;105:123–6. https://doi.org/10.1093/oxfordjournals.aje.a112363
79. Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital
health care-associated hepatitis B and C virus transmission: United
States, 1998-2008. Ann Intern Med 2009;150:33–9. https://doi.
org/10.7326/0003-4819-150-1-200901060-00007
80. Williams RE, Sena AC, Moorman AC, et al. Hepatitis B vaccination
of susceptible elderly residents of long term care facilities during
a hepatitis B outbreak. Vaccine 2012;30:3147–50. https://doi.
org/10.1016/j.vaccine.2012.02.078
81. Woodruff BA, Vazquez E. Prevalence of hepatitis virus
infections in an institution for persons with developmental
disabilities. Am J Ment Retard 2002;107:278–92. https://doi.
org/10.1352/0895-8017(2002)107<0278:POHVII>2.0.CO;2
82. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV,
hepatitis B, and hepatitis C in people with severe mental illness. Am J
Public Health 2001;91:31–7.
83. Weinbaum CM, Williams I, Mast EE, et al. Recommendations for
identification and public health management of persons with chronic
hepatitis B virus infection. MMWR Recomm Rep 2008;57(No. RR-8):1–20.
84. Weinbaum C, Lyerla R, Margolis HS. Prevention and control of
infections with hepatitis viruses in correctional settings. MMWR
Recomm Rep 2003;52(No. RR-1):1–36.
85. Dienstag JL, Ryan DM. Occupational exposure to hepatitis B virus
in hospital personnel: infection or immunization? Am J Epidemiol
1982;115:26–39. https://doi.org/10.1093/oxfordjournals.aje.a113277
86. Hadler SC, Doto IL, Maynard JE, et al. Occupational risk of
hepatitis B infection in hospital workers. Infect Control 1985;6:24–31.
https://doi.org/10.1017/S0195941700062457
87. US Department of Labor. Occupational Safety and Health
Administration. OSHA Law and Regulations. https://www.osha.gov/
law-regs.html
88. CDC. Recommendations for preventing transmission of infections
among chronic hemodialysis patients. MMWR Recomm Rep
2001;50(No. RR-5):1–43.
89. Alter MJ, Favero MS, Maynard JE. Impact of infection control
strategies on the incidence of dialysis-associated hepatitis in the United
States. J Infect Dis 1986;153:1149–51. https://doi.org/10.1093/
infdis/153.6.1149
Recommendations and Reports
28 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
90. Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance
of dialysis-associated diseases in the United States, 2002. Semin Dial
2005;18:52–61. https://doi.org/10.1111/j.1525-139X.2005.18108.x
91. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus
infection related to injection drug use among persons aged ≤30 years—
Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012.
MMWR Morb Mortal Wkly Rep 2015;64:453–8.
92. Bell BP. Hepatitis A and hepatitis B vaccination of patients with chronic
liver disease. Acta Gastroenterol Belg 2000;63:359–63.
93. Johnson DF, Leder K, Torresi J. Hepatitis B and C infection in
international travelers. J Travel Med 2013;20:194–202. https://doi.
org/10.1111/jtm.12026
94. Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence
of chronic hepatitis B and incidence of acute hepatitis B infection in
human immunodeficiency virus-infected subjects. J Infect Dis
2003;188:571–7. https://doi.org/10.1086/377135
95. Chun HM, Fieberg AM, Hullsiek KH, et al.; Infectious Disease Clinical
Research Program HIV Working Group. Epidemiology of Hepatitis B
virus infection in a US cohort of HIV-infected individuals during the
past 20 years. Clin Infect Dis 2010;50:426–36. https://doi.
org/10.1086/649885
96. Homann C, Krogsgaard K, Pedersen C, Andersson P, Nielsen JO. High
incidence of hepatitis B infection and evolution of chronic hepatitis B
infection in patients with advanced HIV infection. J Acquir Immune
Defic Syndr 1991;4:416–20.
97. Thio CL. Hepatitis B in the human immunodeficiency virus-infected
patient: epidemiology, natural history, and treatment. Semin Liver Dis
2003;23:125–36. https://doi.org/10.1055/s-2003-39951
98. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J
Hepatol 2006;44(Suppl):S6–9. https://doi.org/10.1016/j.
jhep.2005.11.004
99. Thio CL, Seaberg EC, Skolasky R Jr, et al.; Multicenter AIDS Cohort
Study. HIV-1, hepatitis B virus, and risk of liver-related mortality in
the Multicenter Cohort Study (MACS). Lancet 2002;360:1921–6.
https://doi.org/10.1016/S0140-6736(02)11913-1
100. Reilly ML, Schillie SF, Smith E, et al. Increased risk of acute hepatitis B
among adults with diagnosed diabetes mellitus. J Diabetes Sci Technol
2012;6:858–66. https://doi.org/10.1177/193229681200600417
101. Schillie SF, Xing J, Murphy TV, Hu DJ. Prevalence of hepatitis B
virus infection among persons with diagnosed diabetes mellitus in the
United States, 1999–2010. J Viral Hepat 2012;19:674–6. https://doi.
org/10.1111/j.1365-2893.2012.01616.x
102. Polish LB, Shapiro CN, Bauer F, et al. Nosocomial transmission of
hepatitis B virus associated with the use of a spring-loaded finger-stick
device. N Engl J Med 1992;326:721–5. https://doi.org/10.1056/
NEJM199203123261101
103. Emini EA, Ellis RW, Miller WJ, McAleer WJ, Scolnick EM,
Gerety RJ. Production and immunological analysis of recombinant
hepatitis B vaccine. J Infect 1986;13(Suppl A):3–9. https://doi.
org/10.1016/S0163-4453(86)92563-6
104. Stephenne J. Development and production aspects of a recombinant
yeast-derived hepatitis B vaccine. Vaccine 1990;8(Suppl):S69–73,
discussion S79–80.
105. CDC. Update: expanded availability of thimerosal preservative-free
hepatitis B vaccine. MMWR Morb Mortal Wkly Rep 2000;49:642–51.
106. US Food and Drug Administration. Licensed biological products with
supporting documents. Silver Spring, MD: US Department of Health
and Human Services, Food and Drug Administration; 2017. https://
www.fda.gov/biologicsbloodvaccines/ucm133705.htm
107. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of
hepatitis B antibody is protective? J Infect Dis 1999;179:489–92.
https://doi.org/10.1086/314578
108. Bruce MG, Bruden D, Hurlburt D, et al. Antibody levels and protection
after hepatitis B vaccine: results of a 30-year follow-up study and
response to a booster dose. J Infect Dis 2016;214:16–22. https://doi.
org/10.1093/infdis/jiv748
109. Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose.
Clin Infect Dis 2011;53:68–75 https://doi.org/10.1093/cid/cir270.
110. Simons BC, Spradling PR, Bruden DJ, et al. A longitudinal hepatitis B vaccine
cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity
despite loss of antibody against HBV surface antigen. J Infect Dis
2016;214:273–80. https://doi.org/10.1093/infdis/jiw142
111. André FE. Summary of safety and efficacy data on a yeast-derived
hepatitis B vaccine. Am J Med 1989;87(3a):S14–20. https://doi.
org/10.1016/0002-9343(89)90525-1
112. Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine
among persons with diabetes: a systematic review of the literature. Diabetes
Care 2012;35:2690–7. https://doi.org/10.2337/dc12-0312
113. Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis
H. Immunogenicity of hepatitis B Vaccines. Implications for persons
at occupational risk of hepatitis B virus infection. Am J Prev Med
1998;15:1–8. https://doi.org/10.1016/S0749-3797(98)00003-8
114. Shaw FE Jr, Guess HA, Roets JM, et al. Effect of anatomic
injection site, age and smoking on the immune response to
hepatitis B vaccination. Vaccine 1989;7:425–30. https://doi.
org/10.1016/0264-410X(89)90157-6
115. Bowman S, Grau LE, Singer M, Scott G, Heimer R. Factors associated
with hepatitis B vaccine series completion in a randomized trial for
injection drug users reached through syringe exchange programs
in three US cities. BMC Public Health 2014;14:820. https://doi.
org/10.1186/1471-2458-14-820
116. Drachman R, Isacsohn M, Rudensky B, Drukker A. Vaccination
against hepatitis B in children and adolescent patients on dialysis.
Nephrol Dial Transplant 1989;4:372–4. https://doi.org/10.1093/
oxfordjournals.ndt.a091892
117. Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of
hepatitis B immunisation in newborn infants of mothers positive for
hepatitis B surface antigen: systematic review and meta-analysis. BMJ
2006;332:328–36. https://doi.org/10.1136/bmj.38719.435833.7C
118. Patel DM, Butler J, Feldman S, Graves GR, Rhodes PG. Immunogenicity
of hepatitis B vaccine in healthy very low birth weight infants. J Pediatr
1997;131:641–3. https://doi.org/10.1016/S0022-3476(97)70078-7
119. Marsano LS, West DJ, Chan I, et al. A two-dose hepatitis B
vaccine regimen: proof of priming and memory responses in
young adults. Vaccine 1998;16:624–9. https://doi.org/10.1016/
S0264-410X(97)00233-8
120. Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ.
A randomized trial of alternative two- and three-dose hepatitis B
vaccination regimens in adolescents: antibody responses, safety, and
immunologic memory. Pediatrics 2001;107:626–31. https://doi.
org/10.1542/peds.107.4.626
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 29
US Department of Health and Human Services/Centers for Disease Control and Prevention
121. Fabrizi F, Dixit V, Messa P, Martin P. Hepatitis B virus vaccine in
chronic kidney disease: improved immunogenicity by adjuvants? A
meta-analysis of randomized trials. Vaccine 2012;30:2295–300. https://
doi.org/10.1016/j.vaccine.2012.01.064
122. Hovi L, Valle M, Siimes MA, Jalanko H, Saarinen UM. Impaired
response to hepatitis B vaccine in children receiving anticancer
chemotherapy. Pediatr Infect Dis J 1995;14:931–4. https://doi.
org/10.1097/00006454-199511000-00002
123. Zuin G, Principi N, Tornaghi R, et al. Impaired response to
hepatitis B vaccine in HIV infected children. Vaccine 1992;10:857–60.
https://doi.org/10.1016/0264-410X(92)90050-T
124. World Health Organization. Information sheet observed rate of vaccine
reactions: hepatitis B vaccine. Geneva, Switzerland: World Health
Organization; 2012. http://www.who.int/vaccine_safety/initiative/
tools/Hep_B_Vaccine_rates_information_sheet.pdf
125. Bohlke K, Davis RL, Marcy SM, et al.; Vaccine Safety Datalink Team.
Risk of anaphylaxis after vaccination of children and adolescents.
Pediatrics 2003;112:815–20. https://doi.org/10.1542/peds.112.4.815
126. Stratton K, Ford A, Rusch E, Clayton EW, eds. Adverse effects of vaccines:
evidence and causality. Washington, DC: National Academy Press; 2012.
127. Stratton K, Almario D, McCormick MC, editors. Immunization safety
review: hepatitis B vaccine and demyelinating neurological disorders.
Washington, DC: National Academies Press; 2002.
128. Halsey NA, Duclos P, Van Damme P, Margolis H; Viral Hepatitis
Prevention Board. Hepatitis B vaccine and central nervous system
demyelinating diseases. Pediatr Infect Dis J 1999;18:23–4. https://
doi.org/10.1097/00006454-199901000-00007
129. DeStefano F, Weintraub ES, Chen RT. Hepatitis B vaccine and risk
of multiple sclerosis. Pharmacoepidemiol Drug Saf 2007;16:705–7,
author reply 707–8. https://doi.org/10.1002/pds.1408
130. Ray P, Black S, Shinefield H, et al.; Vaccine Safety Datalink Team. Risk
of rheumatoid arthritis following vaccination with tetanus, influenza
and hepatitis B vaccines among persons 15–59 years of age. Vaccine
2011;29:6592–7. https://doi.org/10.1016/j.vaccine.2011.06.112
131. Rowhani-Rahbar A, Klein NP, Lewis N, et al. Immunization and
Bell’s palsy in children: a case-centered analysis. Am J Epidemiol
2012;175:878–85. https://doi.org/10.1093/aje/kws011
132. Yu O, Bohlke K, Hanson CA, et al. Hepatitis B vaccine and risk
of autoimmune thyroid disease: a Vaccine Safety Datalink study.
Pharmacoepidemiol Drug Saf 2007;16:736–45. https://doi.
org/10.1002/pds.1354
133. Naleway AL, Belongia EA, Donahue JG, Kieke BA, Glanz JM; Vaccine
Safety Datalink. Risk of immune hemolytic anemia in children
following immunization. Vaccine 2009;27:7394–7. https://doi.
org/10.1016/j.vaccine.2009.09.023
134. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis
after vaccination in children and adults. J Allergy Clin Immunol
2016;137:868–78. https://doi.org/10.1016/j.jaci.2015.07.048
135. Baxter R, Lewis E, Fireman B, DeStefano F, Gee J, Klein NP. Case-
centered analysis of optic neuritis after vaccines. Clin Infect Dis
2016;63:79–81. https://doi.org/10.1093/cid/ciw224
136. Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-
Barré syndrome with vaccinations. Clin Infect Dis 2013;57:197–204.
https://doi.org/10.1093/cid/cit222
137. Baxter R, Lewis N, Bohrer P, Harrington T, Aukes L, Klein NP. Sudden-
onset sensorineural hearing loss after immunization: a case-centered
analysis. Otolaryngol Head Neck Surg 2016;155:81–6. https://doi.
org/10.1177/0194599816639043
138. Haber P, Moro PL, Ng C, et al. Safety of currently licensed hepatitis
B surface antigen vaccines in the United States, Vaccine adverse event
reporting system (VAERS), 2005–2015. Vaccine 2017. http://www.
sciencedirect.com/science/article/pii/S0264410X1731722X
139. Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of
hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine
1989;7:106–10. https://doi.org/10.1016/0264-410X(89)90046-7
140. Halsey NA, Moulton LH, O’Donovan JC, et al. Hepatitis B vaccine
administered to children and adolescents at yearly intervals. Pediatrics
1999;103:1243–7. https://doi.org/10.1542/peds.103.6.1243
141. Wiström J, Ahlm C, Lundberg S, Settergren B, Tärnvik A. Booster
vaccination with recombinant hepatitis B vaccine four years after
priming with one single dose. Vaccine 1999;17:2162–5. https://doi.
org/10.1016/S0264-410X(99)00012-2
142. Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B:
comparison of three different vaccination schedules. J Infect Dis
1989;160:766–9. https://doi.org/10.1093/infdis/160.5.766
143. Tan KL, Goh KT, Oon CJ, Chan SH. Immunogenicity of recombinant
yeast-derived hepatitis B vaccine in nonresponders to perinatal
immunization. JAMA 1994;271:859–61. https://doi.org/10.1001/
jama.1994.03510350069039
144. Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic
hepatitis B viral infection during pregnancy: A systematic review and meta-
analysis. Hepatology 2016;63:319–33. https://doi.org/10.1002/hep.28302
145. Jourdain G, Ngo-Giang-Huong N, Cressey TR, et al. Prevention of
mother-to-child transmission of hepatitis B virus: a phase III, placebo-
controlled, double-blind, randomized clinical trial to assess the efficacy
and safety of a short course of tenofovir disoproxil fumarate in women
with hepatitis B virus e-antigen. BMC Infect Dis 2016;16:393. https://
doi.org/10.1186/s12879-016-1734-5
146. Han GR, Jiang HX, Yue X, et al. Efficacy and safety of telbivudine
treatment: an open-label, prospective study in pregnant women for
the prevention of perinatal transmission of hepatitis B virus infection.
J Viral Hepat 2015;22:754–62. https://doi.org/10.1111/jvh.12379
147. Fan L, Owusu-Edusei K Jr, Schillie SF, Murphy TV. Cost-effectiveness
of active-passive prophylaxis and antiviral prophylaxis during
pregnancy to prevent perinatal hepatitis B virus infection. Hepatology
2016;63:1471–80. https://doi.org/10.1002/hep.28310
148. Barbosa C, Smith EA, Hoerger TJ, et al. Cost-effectiveness analysis
of the national Perinatal Hepatitis B Prevention Program. Pediatrics
2014;133:243–53. https://doi.org/10.1542/peds.2013-0718
149. Hoerger TJ, Schillie S, Wittenborn JS, et al. Cost-effectiveness of
hepatitis B vaccination in adults with diagnosed diabetes. Diabetes Care
2013;36:63–9 10.2337/dc12-0759. https://doi.org/10.2337/dc12-0759
150. Hoerger TJ, Bradley C, Schillie SF, Reilly M, Murphy TV. Cost-
effectiveness of ensuring hepatitis B protection for previously vaccinated
healthcare personnel. Infect Control Hosp Epidemiol 2014;35:845–54.
https://doi.org/10.1086/676865
151. CDC. General recommendations on immunization—recommendations
of the Advisory Committee on Immunization Practices (ACIP).
MMWR Recomm Rep 2011;60(No. RR-2):1–64.
Recommendations and Reports
30 MMWR / January 12, 2018 / Vol. 67 / No. 1
US Department of Health and Human Services/Centers for Disease Control and Prevention
Advisory Committee on Immunization Practices
Membership as of September 16, 2016
Chair: Nancy Bennett, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York.
Executive Secretary: Amanda Cohn, MD, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia.
Members: Robert Atmar, MD, Baylor College of Medicine, Houston, Texas; Edward Belongia, MD, Marshfield Clinic Research Foundation, Marshfield,
Wisconsin; Echezona Ezeanolue, MD, University of Nevada, Las Vegas, Nevada; Paul Hunter, MD, City of Milwaukee Health Department, Milwaukee,
Wisconsin; Allison Kempe, MD, University of Colorado School of Medicine, Denver, Colorado; Grace Lee, MD, Boston Children’s Hospital, Boston,
Massachusetts; Kelly Moore, MD, Tennessee Department of Health, Nashville, Tennessee; Cynthia Pellegrini, March of Dimes, District of Columbia;
Arthur Reingold, MD, University of California, Berkeley School of Public Health, Berkeley, California; Laura Riley, MD, Harvard Medical School, Boston,
Massachusetts; José Romero, MD, University of Arkansas for Medical Sciences, Little Rock, Arkansas; David Stephens, MD, Emory University, Atlanta,
Georgia; Peter Szilagyi, MD, University of California, Los Angeles, California; Emmanuel (Chip) Walter, Jr., MD, Duke University School of Medicine,
Durham, North Carolina.
Ex Officio Members: Bruce Gellin, MD, National Vaccine Program Office, District of Columba; Richard Gorman, MD, National Institutes of Health, Bethesda,
Maryland; Amy Groom, MPH, Indian Health Service, Albuquerque, New Mexico; Mary Beth Hance, Centers for Medicare and Medicaid Services, Baltimore,
Maryland; Jane Kim, MD, Department of Veterans Affairs, Durham, North Carolina; Narayan Nair, MD, Health Resources and Services Administration,
Rockville, Maryland; Eric Sergienko, MD, Department of Defense, Atlanta, Georgia; Wellington Sun, MD, Food and Drug Administration, Bethesda, Maryland.
Liaison Representatives: American Academy of Family Physicians, Margot Savoy, MD, Wilmington, Delaware; American Academy of Pediatrics, Carrie
Byington, MD, Salt Lake City, Utah; American Academy of Pediatrics, Red Book Editor, David Kimberlin, MD, Birmingham, Alabama; American Academy of
Physician Assistants, Marie-Michèle Léger, MPH, Alexandria, Virginia; American College Health Association, Susan Even, MD, Columbia, Missouri; American
College of Nurse Midwives, Carol Hayes, MN, MPH, Atlanta, Georgia; American College of Nurse Midwives Alternate, Pamela Meharry, PhD, Chicago,
Illinois; American College of Obstetricians and Gynecologists, Kevin Ault, MD, Kansas City, Kansas; American College of Physicians, Sandra Fryhoffer, MD,
Atlanta, Georgia; American College of Physicians Alternate, Gregory Poland, MD, Rochester, Minnesota; American Geriatrics Society, Kenneth Schmader,
MD, Durham, North Carolina; America’s Health Insurance Plans, Mark Netoskie, MD, Houston, Texas; American Medical Association, Sandra Fryhoffer,
Atlanta, Georgia; American Nurses Association, Charles Rittle, DNP, Pittsburgh, Pennsylvania; American Osteopathic Association, Stanley Grogg, DO, Tulsa,
Oklahoma; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association of Immunization Managers, Christine Finley,
MPH, Burlington, Vermont; Association for Prevention and Teaching Research, Paul McKinney, MD, Louisville, Kentucky; Association of State and Territorial
Health Officials, Terry Dwelle MD, Bismarck, North Dakota; Biotechnology Industry Organization, Phyllis Arthur, District of Columbia.; Council of State
and Territorial Epidemiologists, Christine Hahn, MD, Boise, Idaho; Canadian National Advisory Committee on Immunization, Ian Gemmill, MD, Kingston,
Ontario, Canada; Infectious Diseases Society of America, Kathleen Neuzil, MD, Seattle, Washington; Infectious Disease Society of America Alternate, Carol
Baker, MD, Houston, Texas; National Association of County and City Health Officials, Matthew Zahn, MD, Santa Ana, California; National Association
of County and City Health Officials, Jeffrey Duchin, MD, Seattle, Washington; National Association of Pediatric Nurse Practitioners, Patricia Stinchfield,
MPH, St. Paul, Minnesota; National Foundation for Infectious Diseases, William Schaffner, MD, Nashville, Tennessee; National Immunization Council
and Child Health Program, Mexico; Ignacio Villasenor Ruiz, MD, Mexico; National Medical Association, Patricia Whitley-Williams, MD, New Brunswick,
New Jersey; National Vaccine Advisory Committee, Kimberly Thompson, ScD, Orlando, Florida; Pediatric Infectious Diseases Society, Sean O’Leary, MD,
Denver, Colorado; Pediatric Infectious Diseases Society Alternate, Mark Sawyer, MD, San Diego, California; Pharmaceutical Research and Manufacturers
of America, David Johnson, Swiftwater, Pennsylvania; Society for Adolescent Health and Medicine, Amy Middleman, MD, Houston, Texas; Society for
Healthcare Epidemiology of America, David J. Weber, MD, University of North Carolina, Chapel Hill, North Carolina.
The ACIP Hepatitis Work Group
Membership as of September 16, 2016
Chair: Arthur Reingold, MD, University of California, Berkeley School of Public Health, Berkeley, California
Members: Natali Aziz, MD, MD, Stanford University School of Medicine, Stanford, California; Sharon Balter, MD, New York City Department of Health
and Mental Hygiene, New York, New York; Elizabeth Barnett, MD, Boston University School of Medicine, Boston, Massachusetts; Kathleen Harriman,
PhD, California Department of Public Health, Richmond, California; Susan Even, MD, University of Missouri, Columbia, Missouri; Christine Finley,
Vermont Department of Health, Burlington, Vermont; Robert Frenck, MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Kathleen
Harriman, PhD, California Department of Public Health, Richmond, California; Susan M. Lett, MD, Massachusetts Department of Public Health, Jamaica
Plain, Massachusetts; Marian Major, PhD, Food and Drug Administration, Silver Spring, Maryland; Brian McMahon, MD, Alaska Native Tribal Health
Consortium, Anchorage, Alaska; Amy Middleman, MD, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; David A. Nace, MD,
University of Pittsburgh, Pittsburgh, Pennsylvania; Gregory Poland, MD, Mayo Clinic, Rochester, Minnesota; Pamela Rockwell, DO, University of Michigan,
Ann Arbor, Michigan; José Romero, MD, University of Arkansas for Medical Sciences, Little Rock, Arkansas; Brenna Simons-Petrusa, PhD, Alaska Native
Tribal Health Consortium, Anchorage, Alaska; Ann Thomas, MD, Oregon Health Authority, Portland, Oregon; David J. Weber, MD, University of North
Carolina, Chapel Hill, North Carolina, Matthew Zahn, MD, Children’s Hospital of Orange County, Orange, California; Jennifer Zipprich, PhD, California
Department of Public Health, Richmond, California.
Contributors (CDC): Carolyn Bridges, MD, Maria Cano, MD, Melissa Collier, MD, Mona Doshani, MD, Mark Gershman, MD, Penina Haber, MPH,
Aaron Harris, MD, Beth Hibbs, MPH, Scott Holmberg, MD, Ruth Jiles, PhD, David Kim, MD, Alaya Koneru, MPH, Noele Nelson, MD, PhD, Sarah
Schillie, MD, Philip Spradling, MD, Claudia Vellozzi, MD, John Ward, MD, Tureka Watson, MS, Donna L. Weaver, MN, Matthew Wise, PhD.
Recommendations and Reports
MMWR / January 12, 2018 / Vol. 67 / No. 1 31
US Department of Health and Human Services/Centers for Disease Control and Prevention
CDC Adoption of ACIP Recommendations
Recommendations for the routine use of vaccines in children, adolescents, and adults are developed by the Advisory Committee on Immunization Practices
(ACIP). ACIP is chartered as a federal advisory committee to provide expert external advice and guidance to the Director of CDC on use of vaccines and related
agents for the control of vaccine-preventable diseases in the civilian population of the United States. Clinical recommendations for routine use of vaccines
are harmonized to the greatest extent possible with recommendations made by others (e.g., the American Academy of Pediatrics, the American Academy of
Family Physicians, the American College of Obstetricians and Gynecologists, and the American College of Physicians).
ACIP recommendations adopted by the CDC Director become agency guidelines on the date published in MMWR. The accompanying recommendations
that summarize the ACIP findings and conclusions were drafted based on the recommendations and revised based on feedback from ACIP voting members.
The CDC Director approved these recommendations prior to publication. Opinions of individual members of ACIP might differ to some extent from the
recommendations in this report as these recommendations are the position of CDC based on the ACIP recommendations to the CDC Director. Additional
information regarding ACIP is available at https://www.cdc.gov/vaccines/acip.

ISSN: 1057-5987 (Print)
The Morbidity and Mortality Weekly Report (MMWR) Series is prepared by the Centers for Disease Control and Prevention (CDC) and is available free of
charge in electronic format. To receive an electronic copy each week, visit MMWR’s free subscription page at https://www.cdc.gov/mmwr/mmwrsubscribe.html.
Paper copy subscriptions are available through the Superintendent of Documents, U.S. Government Printing Office, Washington, DC 20402; telephone
202-512-1800.
Readers who have difficulty accessing this PDF file may access the HTML file at https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm?s_
cid=rr6701a1_w. Address all inquiries about the MMWR Series, including material to be considered for publication, to Executive Editor, MMWR Series,
Mailstop E-90, CDC, 1600 Clifton Rd., N.E., Atlanta, GA 30329-4027 or to [email protected].
All material in the MMWR Series is in the public domain and may be used and reprinted without permission; citation as to source, however, is appreciated.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations
or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of these sites. URL addresses
listed in MMWR were current as of the date of publication.
