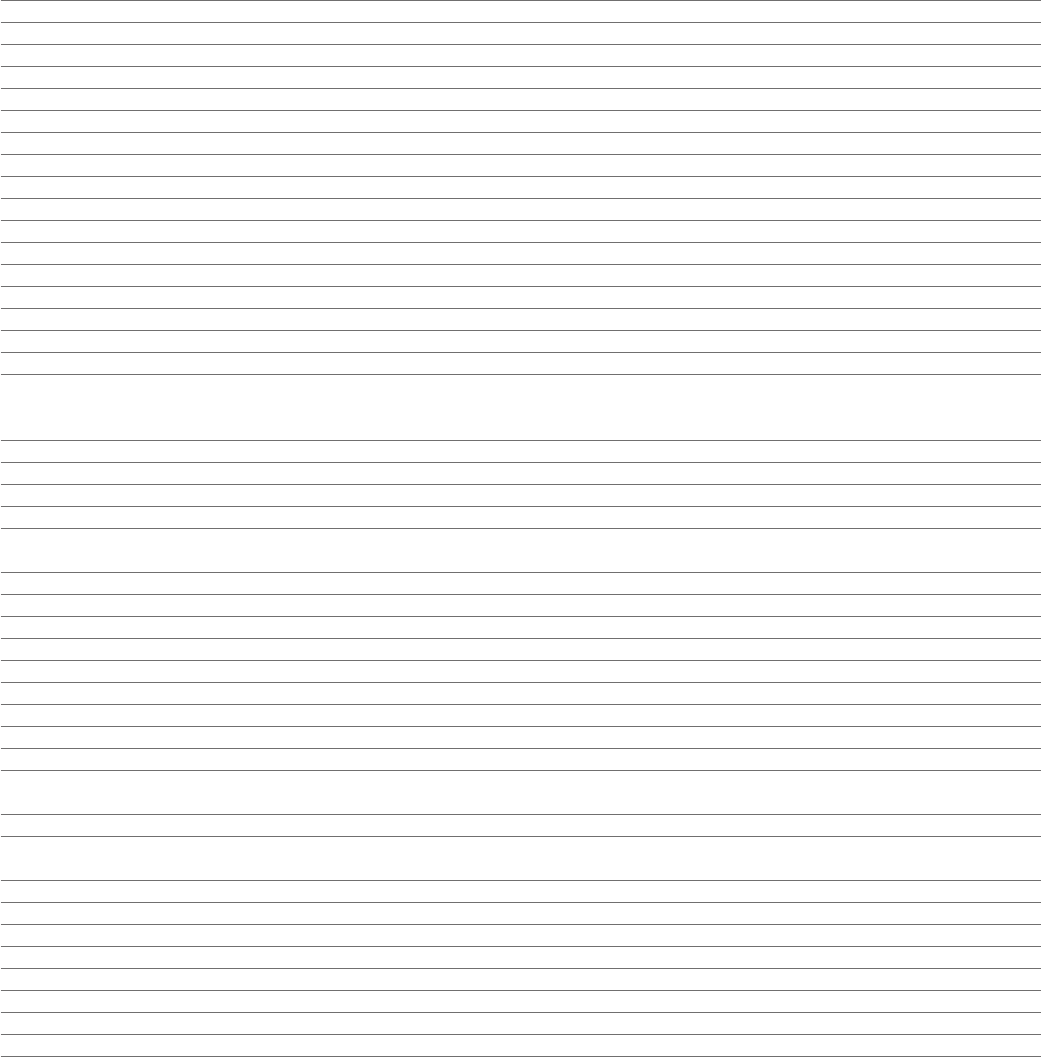
Consolidated Statement of Comprehensive Income
for the year ended 31December
2023 2022 2021
Notes $m $m $m
Product Sales 1 43,789 42,998 36,541
Alliance Revenue 1 1,428 755 388
Collaboration Revenue 1 594 598 488
Total Revenue 45,811 44,351 37,417
Cost of sales (8,268) (12,391) (12,437)
Gross profit 37,543 31,960 24,980
Distribution expense (539) (536) (446)
Research and development expense 2 (10,935) (9,762) (9,736)
Selling, general and administrative expense 2 (19,216) (18,419) (15,234)
Other operating income and expense 2 1,340 514 1,492
Operating profit 8,193 3,757 1,056
Finance income 3 344 95 43
Finance expense 3 (1,626) (1,346) (1,300)
Share of after tax losses in associates and joint ventures 11 (12) (5) (64)
Profit/(loss) before tax 6,899 2,501 (265)
Taxation 4 (938) 792 380
Profit for the period 5,961 3,293 115
Other comprehensive income:
Items that will not be reclassified to profit or loss:
Remeasurement of the defined benefit pension liability 22 (406) 1,118 626
Net gains/(losses) on equity investments measured at fair value through other comprehensive income 278 (88) (187)
Fair value movements related to own credit risk on bonds designated as fair value through profit or loss (6) 2 –
Tax on items that will not be reclassified to profit or loss 4 101 (216) 105
(33) 816 544
Items that may be reclassified subsequently to profit or loss:
Foreign exchange arising on consolidation 23 608 (1,446) (483)
Foreign exchange arising on designated liabilities in net investment hedges 23 24 (282) (321)
Fair value movements on cash flow hedges 266 (97) (167)
Fair value movements on cash flow hedges transferred to profit and loss (145) 73 208
Fair value movements on derivatives designated in net investment hedges 23 44 (8) 34
Costs of hedging (19) (7) (6)
Tax on items that may be reclassified subsequently to profit or loss 4 (12) 73 46
766 (1,694) (689)
Other comprehensive income/(expense) for the period, net of tax 733 (878) (145)
Total comprehensive income/(expense) for the period 6,694 2,415 (30)
Profit attributable to:
Owners of the Parent 5,955 3,288 112
Non-controlling interests 26 6 5 3
Total comprehensive income/(expense) attributable to:
Owners of the Parent 6,688 2,413 (33)
Non-controlling interests 26 6 2 3
Basic earnings per $0.25 Ordinary Share 5 $3.84 $2.12 $0.08
Diluted earnings per $0.25 Ordinary Share 5 $3.81 $2.11 $0.08
Weighted average number of Ordinary Shares in issue (millions) 5 1,549 1,548 1,418
Diluted weighted average number of Ordinary Shares in issue (millions) 5 1,562 1,560 1,427
Dividends declared and paid in the period 25 4,487 4,485 3,882
All activities were in respect of continuing operations.
$m means millions of US dollars.
148
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
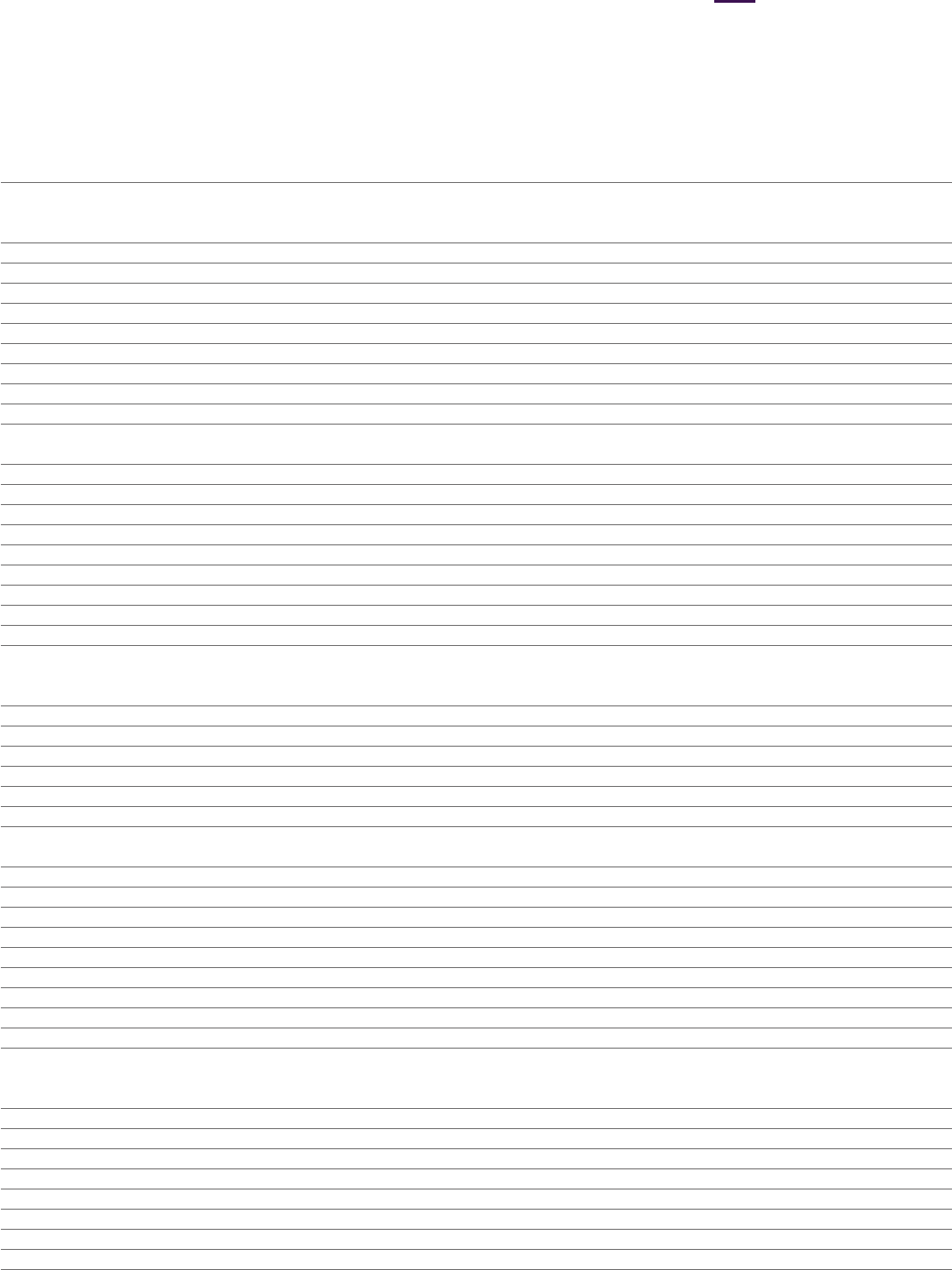
Consolidated Statement of Financial Position
at 31December
2023 2022 2021
Notes $m $m $m
Assets
Non-current assets
Property, plant and equipment 7 9,402 8,507 9,18 3
Right-of-use assets 8 1,100 942 988
Goodwill 9 20,048 19,820 19,997
Intangible assets 10 38,089 39,307 42,387
Investments in associates and joint ventures 11 147 76 69
Other investments 12 1,530 1,066 1,16 8
Derivative financial instruments 13 228 74 102
Other receivables 14 803 835 895
Deferred tax assets 4 4,718 3,263 4,330
76,065 73,890 79,119
Current assets
Inventories 15 5,424 4,699 8,983
Trade and other receivables 16 12,126 10,521 9,644
Other investments 12 122 239 69
Derivative financial instruments 13 116 87 83
Intangible assets 10 – – 105
Income tax receivable 1,426 731 663
Cash and cash equivalents 17 5,840 6,166 6,329
Assets held for sale 18 – 150 368
25,054 22,593 26,244
Total assets 101,119 96,483 105,363
Liabilities
Current liabilities
Interest-bearing loans and borrowings 19 (5,129) (5,314) (1,660)
Lease liabilities 8 (271) (228) (233)
Trade and other payables 20 (22,374) (19,040) (18,938)
Derivative financial instruments 13 (156) (93) (79)
Provisions 21 (1,028) (722) (768)
Income tax payable (1,584) (896) (916)
(30,542) (26,293) (22,594)
Non-current liabilities
Interest-bearing loans and borrowings 19 (22,365) (22,965) (28,134)
Lease liabilities 8 (857) (725) (754)
Derivative financial instruments 13 (38) (164) (45)
Deferred tax liabilities 4 (2,844) (2,944) (6,206)
Retirement benefit obligations 22 (1,520) (1,168) (2,454)
Provisions 21 (1,127) (896) (956)
Other payables 20 (2,660) (4,270) (4,933)
(31,411) (33,132) (43,482)
Total liabilities (61,953) (59,425) (66,076)
Net assets 39,166 37,058 39,287
Equity
Capital and reserves attributable to equity holders of the Company
Share capital 24 388 387 387
Share premium account 35,18 8 35,155 35,126
Capital redemption reserve 153 153 153
Merger reserve 448 448 448
Other reserves 23 1,464 1,468 1,444
Retained earnings 23 1,502 (574) 1,710
39,14 3 37,037 39,268
Non-controlling interests 26 23 21 19
Total equity 39,166 37,058 39,287
The Financial Statements from pages 148 to 215 were approved by the Board and were signed on its behalf by
Pascal Soriot Aradhana Sarin
Director Director
8 February 2024
Consolidated Statement of Financial Position 149AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
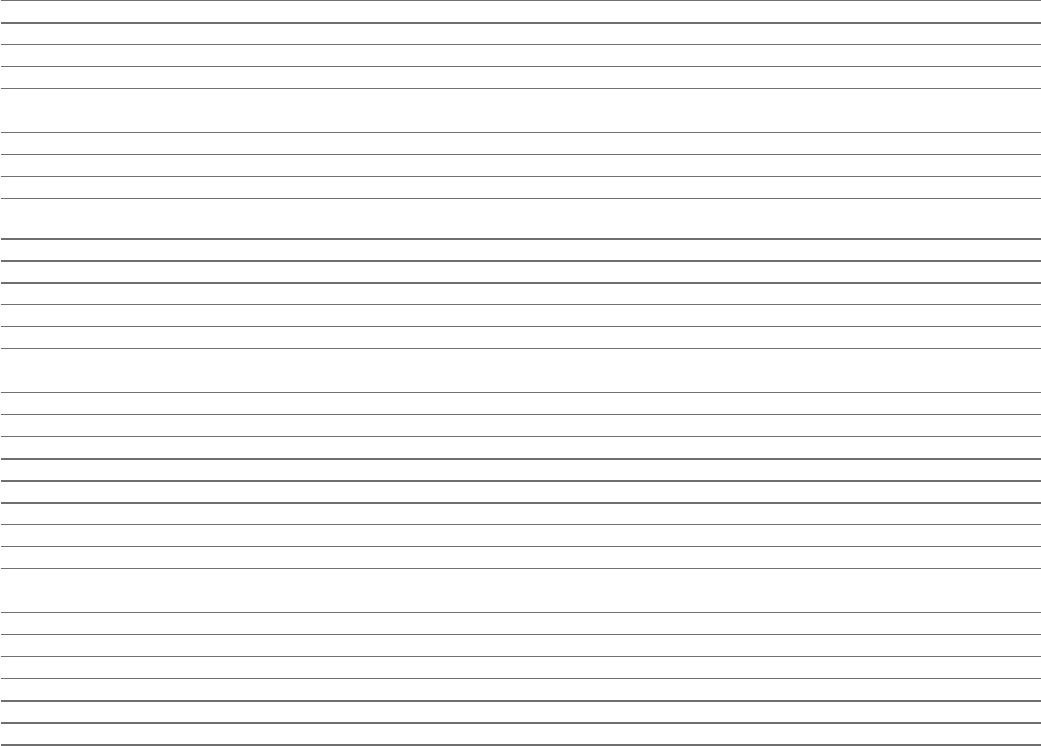
Consolidated Statement of Changes in Equity
for the year ended 31December
Share Capital Total Non-
Share premium redemption Merger Other Retained attributable controlling Total
capital account reserve reserve reserves earnings toowners interests equity
$m $m $m $m $m $m $m $m $m
At 1January 2021 328 7,971 153 448 1,423 5,299 15,622 16 15,638
Profit for the period – – – – – 112 112 3 115
Other comprehensive expense
1
– – – – – (145) (145) – (145)
Transfer to other reserves
2
– – – – 21 (21) – – –
Transactions with owners
Dividends (Note 25) – – – – – (3,882) (3,882) – (3,882)
Issue of Ordinary Shares 59 27,155 – – – – 27, 214 – 27, 214
Share-based payments charge for the period (Note 29) – – – – – 615 615 – 615
Settlement of share plan awards – – – – – (781) (781) – (781)
Issue of replacement Alexion share awards upon
acquisition (Note 27)
3
– – – – – 513 513 – 513
Net movement 59 27,155 – – 21 (3,589) 23,646 3 23,649
At 31December 2021 387 35,126 153 448 1,444 1,710 39,268 19 39,287
Profit for the period – – – – – 3,288 3,288 5 3,293
Other comprehensive expense
1
– – – – – (875) (875) (3) (878)
Transfer to other reserves
2
– – – – 24 (24) – – –
Transactions with owners
Dividends (Note 25) – – – – – (4,485) (4,485) – (4,485)
Issue of Ordinary Shares – 29 – – – – 29 – 29
Share-based payments charge for the period (Note 29) – – – – – 619 619 – 619
Settlement of share plan awards – – – – – (807) (807) – (807)
Net movement – 29 – – 24 (2,284) (2,231) 2 (2,229)
At 31December 2022 387 35,155 153 448 1,468 (574) 37,037 21 37,0 5 8
Profit for the period – – – – – 5,955 5,955 6 5,961
Other comprehensive income
1
– – – – – 733 733 – 733
Transfer to other reserves
2
– – – – (4) 4 – – –
Transactions with owners
Dividends (Note 25) – – – – – (4,487) (4,487) – (4,487)
Dividends paid to non-controlling interests (Note 25) – – – – – – – (4) (4)
Issue of Ordinary Shares 1 33 – – – – 34 – 34
Share-based payments charge for the period (Note 29) – – – – – 579 579 – 579
Settlement of share plan awards – – – – – (708) (708) – (708)
Net movement 1 33 – – (4) 2,076 2 ,10 6 2 2,108
At 31December 2023 388 35,188 153 448 1,464 1,502 39,143 23 39,166
150 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
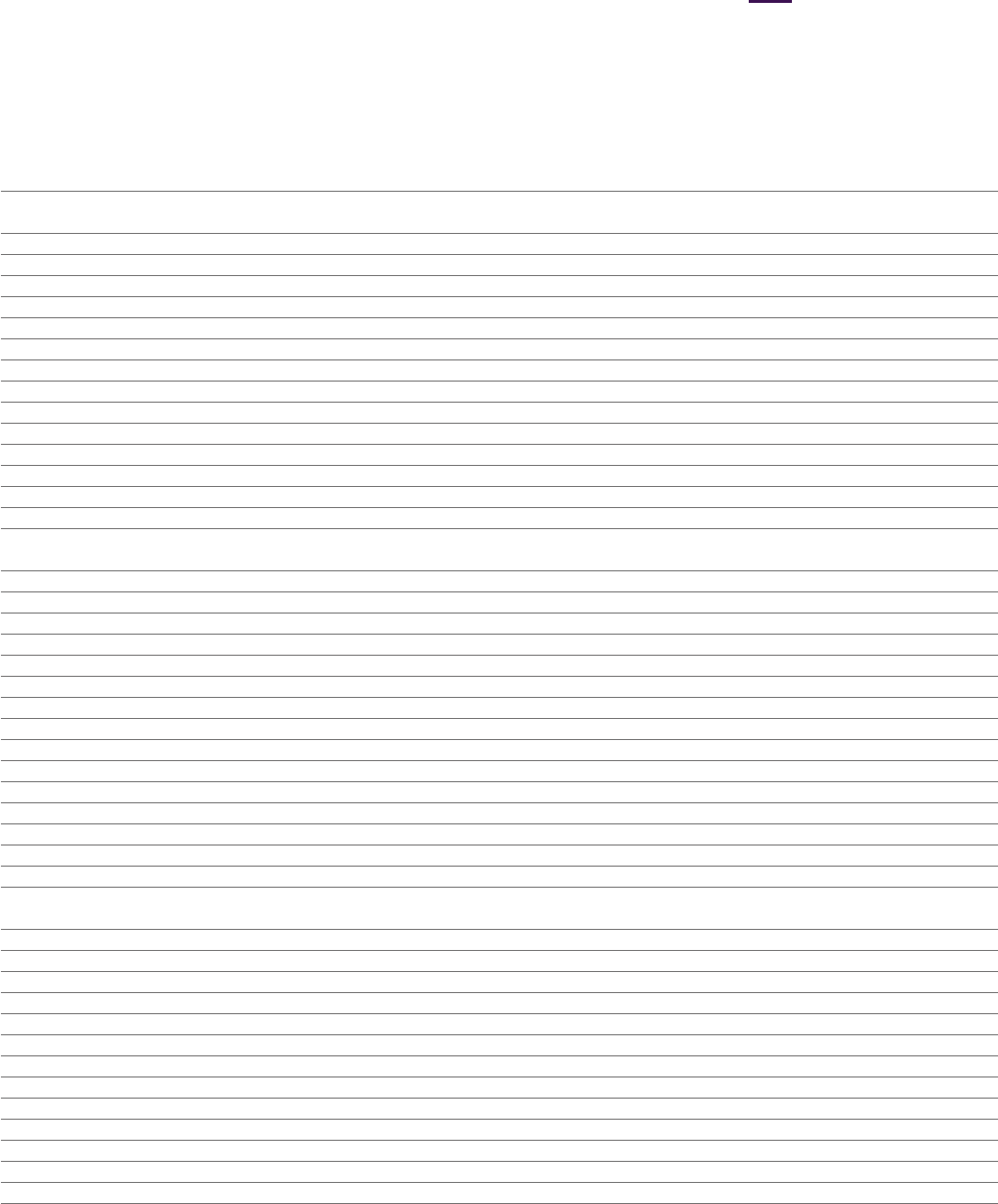
Consolidated Statement of Cash Flows
for the year ended 31December
2023 2022 2021
Notes $m $m $m
Cash flows from operating activities
Profit/(loss) before tax 6,899 2,501 (265)
Finance income and expense 3 1,282 1,251 1,257
Share of after tax losses of associates and joint ventures 11 12 5 64
Depreciation, amortisation and impairment 5,387 5,480 6,530
Increase in trade and other receivables (1,425) (1,349) (961)
(Increase)/decrease in inventories (669) 3,941 1,577
Increase in trade and other payables and provisions 2,394 1,165 1,405
Gains on disposal of intangible assets 2 (251) (104) (513)
Gains on disposal of investments in associates and joint ventures 2 – – (776)
Fair value movements on contingent consideration arising from business combinations 20 549 82 14
Non-cash and other movements 17 (386) (692) 95
Cash generated from operations 13,792 12,280 8,427
Interest paid (1,081) (849) (721)
Tax paid (2,366) (1,623) (1,743)
Net cash inflow from operating activities 10,345 9,808 5,963
Cash flows from investing activities
Acquisition of subsidiaries, net of cash acquired 27 (189) (48) (9,263)
Payments upon vesting of employee share awards attributable to business combinations 27 (84) (215) (211)
Payment of contingent consideration from business combinations 20 (826) (772) (643)
Purchase of property, plant and equipment (1,361) (1,091) (1,091)
Disposal of property, plant and equipment 132 282 13
Purchase of intangible assets (2,417) (1,480) (1,109)
Disposal of intangible assets 291 447 587
Movement in profit-participation liability 2 190 – 20
Purchase of non-current asset investments (136) (45) (184)
Disposal of non-current asset investments 32 42 9
Movement in short-term investments, fixed deposits and other investing instruments 97 (114) 96
Payments to associates and joint ventures 11 (80) (26) (92)
Disposal of investments in associates and joint ventures – – 776
Interest received 287 60 34
Net cash outflow from investing activities (4,064) (2,960) (11,05 8)
Net cash inflow/(outflow) before financing activities 6,281 6,848 (5,095)
Cash flows from financing activities
Proceeds from issue of share capital 33 29 29
Issue of loans and borrowings 3,816 – 12,929
Repayment of loans and borrowings (4,942) (1,271) (4,759)
Dividends paid (4,481) (4,364) (3,856)
Hedge contracts relating to dividend payments (19) (127) (29)
Repayment of obligations under leases (268) (244) (240)
Movement in short-term borrowings 161 74 (276)
Payments to acquire non-controlling interests – – (149)
Payment of Acerta Pharma share purchase liability (867) (920) –
Net cash (outflow)/inflow from financing activities (6,567) (6,823) 3,649
Net (decrease)/increase in Cash and cash equivalents in the period (286) 25 (1,446)
Cash and cash equivalents at the beginning of the period 5,983 6,038 7, 5 4 6
Exchange rate effects (60) (80) (62)
Cash and cash equivalents at the end of the period 17 5,637 5,983 6,038
Consolidated Statement of Cash Flows 151AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Group Accounting Policies
Basis of accounting and preparation
offinancial information
The Consolidated Financial Statements
have been prepared under the historical cost
convention, modified to include revaluation to
fair value of certain financial instruments and
pension plan assets and liabilities as described
below, in accordance with UK-adopted
international accounting standards and with
the requirements of the Companies Act 2006
as applicable to companies reporting under
those standards. The Consolidated Financial
Statements also comply fully with IFRS
Accounting Standards as issued by the
International Accounting Standards Board
(IASB) and International Accounting Standards
as adopted by the European Union.
The Consolidated Financial Statements are
presented in US dollars, which is the Company’s
functional currency.
In preparing their individual financial statements,
the accounting policies of some overseas
subsidiaries do not conform with IASB-
issued IFRSs. Therefore, where appropriate,
adjustments are made in order to present the
Consolidated Financial Statements on a
consistent basis.
New accounting requirements
Other than noted below, amendments to
accounting standards issued by the IASB and
adopted in the year ended 31 December 2023
did not have a material impact on the result or
financial position of the Group.
IAS12
On 23 May 2023, the IASB issued an
amendment to IAS 12 ‘Income Taxes’ to clarify
how the effects of the global minimum tax
framework should be accounted for and
disclosed effective 1 January 2023. This was
endorsed by the UK Endorsement Board on
19 July 2023 and has been adopted by the
Group for 2023 reporting. The Group has
applied the exemption to recognising and
disclosing information about deferred tax
assets and liabilities related to Pillar 2
income taxes.
Alliance and Collaboration Revenue
Effective 1 January 2023, the Group has
updated the presentation of Total Revenue on
the face of the Statement of Comprehensive
Income to include Alliance Revenue as a
separate element to Collaboration Revenue.
Alliance Revenue, previously reported within
Collaboration Revenue, comprises income
related to sales made by collaboration partners,
where AstraZeneca is entitled to a share of
gross profits, share of revenues or royalties,
which are recurring in nature while the
collaboration arrangement remains in place.
Alliance Revenue does not include Product
Sales where AstraZeneca is leading
commercialisation in a territory.
Collaboration Revenue arising from
collaborative arrangements where the Group
retains a significant ongoing economic interest
and receives upfront amounts and event-
triggered milestones, which arise from the
licensing of intellectual property, will continue
to be reported as Collaboration Revenue. In
collaboration arrangements either AstraZeneca
or the collaborator acts as principal in sales to
the end customer. Where AstraZeneca acts as
principal, AstraZeneca records 100% of sales
to the end customer within Product Sales. The
updated presentation reflects the increasing
importance of income arising from share of
gross profit arrangements where collaboration
partners are responsible for booking revenues
in some or all territories.
The comparative revenue reported in the
years to 31 December 2022 and 31 December
2021 has been retrospectively adjusted to
reflect the new split of Total Revenue, resulting
in Alliance Revenue being reported for the
year to 31 December 2022 of $755m and to
31 December 2021 of $388m, however the
combined total of Alliance Revenue and
Collaboration Revenue is equal to the
previously reported Collaboration Revenue
total for each prior year.
Basis for preparation of Financial
Statements on a going concern basis
The Group has considerable financial resources
available. As at 31 December 2023, the Group
has $12.7bn in financial resources (Cash and
cash equivalent balances of $5.8bn and
undrawn committed bank facilities of $6.9bn,
of which $2.0bn are available until February
2025 and the remaining $4.9bn are available
until April 2026, (in February 2024 these
facilities were extended to April 2029), with only
$5.4bn of borrowings due within one year).
The Group’s revenues are largely derived from
sales of medicines covered by patents, which
provide a relatively high level of resilience
and predictability to cash inflows, although
government price interventions in response to
budgetary constraints are expected to
continue to adversely affect revenues in some
of our significant markets. The Group,
however, anticipates new revenue streams
from both recently launched medicines and
those in development, and the Group has a
wide diversity of customers and suppliers
across different geographic areas.
Consequently, the Directors believe that, overall,
the Group is well placed to manage its business
risks successfully. Accordingly, they continue
to adopt the going concern basis in preparing
the Annual Report and Financial Statements.
Estimates and judgements
The preparation of the Financial Statements in
conformity with generally accepted accounting
principles requires management to make
estimates and judgements that affect the
reported amounts of assets and liabilities at
the date of the Financial Statements and the
reported amounts of revenues and expenses
during the reporting period. Actual results
could differ from those estimates.
The accounting policy descriptions set out the
areas where judgements and estimates need
exercising, the most significant of which
include the following Key Judgements
and
Significant Estimates :
> revenue recognition – see Revenue
Accounting Policy from page 152 and
Note 1 on page 161
> expensing of internal development expenses
– see Research and Development Policy
from page 154
> impairment reviews of Intangible assets
– see Note 10 on page 174
> useful economic life of Intangible assets –
see Research and Development Policy
from page 154
> business combinations and Goodwill –
see Business Combinations and Goodwill
Policy from page 156 and Note 27 from
page 193
> litigation liabilities – see Litigation and
Environmental Liabilities within Note 30
on page 204
> operating segments – see Note 6 on
page 167
> employee benefits – see Note 22 on
page 190
> taxation – see Note 30 from page 209 .
The Group has assessed the impact of climate
risk on its financial reporting. The impact
assessment was primarily focused on the
valuation and useful lives of intangible assets
and the identification and valuation of provisions
and contingent liabilities, as these are judged
to be the key areas that could be impacted by
climate risks. No material accounting impacts
or changes to judgements or other required
disclosures were noted.
Key Judgements are those judgements
made in applying the Group’s accounting
policies that have a material effect on the
amounts of assets and liabilities recognised
in the Financial Statements.
A Significant Estimate has a significant
risk of material adjustment to the carrying
amounts of assets and liabilities within the
next financial year.
Financial risk management policies are detailed
in Note 28 to the Financial Statements from
page 195.
AstraZeneca’s management considers the
following to be the material accounting policies
in the context of the Group’s operations.
Revenue
Revenue comprises Product Sales, Alliance
Revenue and Collaboration Revenue.
Revenue excludes inter-company revenues
and value-added taxes.
152
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Product Sales
Product Sales represent net invoice value less
estimated rebates, returns and chargebacks,
which are considered to be variable
consideration and include significant estimates.
Sales are recognised when the control of the
goods has been transferred to a third party.
This is usually when title passes to the
customer, either on shipment or on receipt of
goods by the customer, depending on local
trading terms. Revenue is not recognised in
full until it is highly probable that a significant
reversal in the amount of cumulative revenue
recognised will not occur.
Rebates are amounts payable or credited to
a customer, usually based on the quantity or
value of Product Sales to the customer for
specific products in a certain period. Product
sales rebates, which relate to Product Sales
that occur over a period of time, are normally
issued retrospectively.
At the time Product Sales are invoiced, rebates
and deductions that the Group expects to
pay are estimated based upon assumptions
developed using contractual terms, historical
experience and market-related information.
The rebates and deductions are recognised
as variable consideration and recorded as a
reduction to revenue with an accrual recorded.
These rebates typically arise from sales
contracts with government payers, third-party
managed care organisations, hospitals,
long-term care facilities, group purchasing
organisations and various state programmes.
In markets where returns are significant,
estimates of the quantity and value of goods
which may ultimately be returned are accounted
for at the point revenue is recognised. Our
returns accruals are based on actual experience
over the preceding 12 months for established
products together with market-related
information such as estimated stock levels
at wholesalers and competitor activity which
we receive via third-party information services.
For newly launched products, we use rates
based on our experience with similar products
or a predetermined percentage.
When a product faces generic competition,
particular attention is given to the possible
levels of returns and, in cases where the
circumstances are such that the level of
Product Sales are considered highly probable
to reverse, revenues are only recognised when
the right of return expires, which is generally on
ultimate prescription of the product to patients.
The methodology and assumptions used to
estimate rebates and returns are monitored
and adjusted regularly in the light of contractual
and legal obligations, historical trends, past
experience and projected market conditions.
Once the uncertainty associated with returns
is resolved, revenue is adjusted accordingly.
Under certain collaboration agreements
which include a profit sharing mechanism, our
recognition of Product Sales depends on which
party acts as principal in sales to the end
customer. In the cases where AstraZeneca
acts as principal, we record 100% of sales
to the end customer. In the cases where
AstraZeneca does not act as principal, we
record the share of gross profits received
within Alliance Revenue.
Contracts relating to the supply of certain
Vaccines & Immune Therapies medicines
relating to the COVID-19 pandemic include
conditions whereby payments are receivable
from customers in advance of the delivery of
product. Such amounts are held on the balance
sheet as contract liabilities until the related
revenue is recognised, generally upon product
delivery. Certain of these contracts contain
further provisions that restrict the use of
inventory manufactured in specified supply
chains to specified customers, resulting in an
enforceable right to payment as the activities
are performed. Under IFRS 15, such contracts
require revenue to be recognised over time
using an appropriate and reasonably
measurable method to measure progress.
Revenue is recognised on these contracts
based on the proportion of product delivered
compared to the total contracted volumes.
Certain arrangements include bill-and-hold
arrangements under which the Group invoices
a customer for a product but retains physical
possession of the product until it is transferred
to the customer at a point in time in the future.
For these types of arrangements, an
assessment is made to determine when the
performance obligation has been satisfied,
which is when control of the product is
transferred to the customer. If the customer has
obtained control of the product even though
that product remains in the Group’s physical
possession, the performance obligation to
transfer a product has been satisfied and
Product Sales are recognised. Control is
considered to have transferred when the
reason for the bill-and-hold arrangement is
substantive, the product can be identified
separately as belonging to the customer, the
product is ready for physical transfer to the
customer and AstraZeneca is unable to use
or sell the product to another customer.
Alliance Revenue
Alliance Revenue comprises income arising
from the ongoing operation of collaborative
arrangements related to sales made by
collaboration partners, where AstraZeneca
is entitled to a share of gross profits, share
of revenues or royalties, which are recurring
in nature while the collaboration agreement
remains in place. Alliance Revenue does not
include Product Sales where AstraZeneca
is leading commercialisation in a territory,
or reimbursement for AstraZeneca-incurred
expenses such as R&D or promotion
costs, which arise from the license of
intellectual property.
The Group periodically enters into transactions
where it acquires part of the rights to a product
intangible (either on-market or in-process R&D),
but for commercial reasons does not act as
principal in selling the product to the customer
and therefore does not recognise income from
the product in the form of Product Sales. This
may occur where, for example, a collaboration
partner retains the right to commercialise in a
specific territory, and has sufficient local control
over that commercialisation to book Product
Sales, while the Group instead receives a
proportion of the value generated by those
Product Sales, either in the form of a royalty,
a share of gross profits or a share of revenues.
Where the arrangement meets the definition
of a licence agreement, share of gross profits,
share of revenues and sales royalties are
recognised when achieved by applying the
royalty exemption under IFRS 15. All other
sales royalties are recognised when considered
it is highly probable there will not be a
significant reversal of cumulative income.
The determination requires estimates to be
made in relation to future Product Sales.
Collaboration Revenue
Collaboration Revenue includes income arising
from entering into collaborative arrangements
where the Group has out-licensed (sold) certain
rights associated with products and where
AstraZeneca retains a significant ongoing
economic interest in the product. Significant
interest can include ongoing supply of finished
goods, profit sharing arrangements or being
principal in the sales of medicines. These
collaborations may include development,
manufacturing and/or commercialisation
arrangements with the collaborator. Income
from out-licences may take the form of upfront
fees and milestones.
Timing of recognition of clinical and
regulatory milestones is considered to be
a key judgement. There can be significant
uncertainty over whether it is highly probable
that there would not be a significant reversal
of revenue in respect of specific milestones
if these are recognised before they are
triggered due to them being subject to the
actions of third parties. In general, where
the triggering of a milestone is subject to the
decisions of third parties (e.g. the acceptance
or approval of a filing by a regulatory
authority), the Group does not consider that
the threshold for recognition is met until that
decision is made.
Where Collaboration Revenue arises from
the licensing of the Group’s own intellectual
property, the licences we grant are typically
rights to use intellectual property which do
not change during the period of the licence
and therefore related non-conditional revenue
is recognised at the point the licence is
granted and variable consideration as soon
as recognition criteria are met.
Group Accounting Policies 153AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Group Accounting Policies
continu ed
Other performance obligations in the contract
might include the supply of product. These
arrangements typically involve the receipt of an
upfront payment, which the contract attributes
to the license of the intangible assets, and
ongoing receipts for supply, which the contract
attributes to the sale of the product we
manufacture. In cases where the transaction
has two or more components, we account for
the delivered item (for example, the transfer
of title to the intangible asset) as a separate
unit of account and record revenue on
delivery of that component. Where practicable,
consideration is allocated to performance
obligations on the basis of the standalone
selling price of each performance obligation.
However, where there is a licence of intellectual
property, it is not always possible to establish
a reliable estimate of the standalone selling
price of the licence as they are unique.
Therefore, in these rare situations, the
residual approach is used to determine the
consideration attributable to the licence.
Where fixed amounts are payable over one
year from the effective date of a contract, an
assessment is made as to whether a significant
financing component exists, and if so, the
fair value of this component is deferred and
recognised as financing income over the
period to the expected date of receipt.
Where control of a right to use licence for an
intangible asset passes at the outset of an
arrangement, revenue is recognised at the
point in time control is transferred. Where the
substance of a licence arrangement is that
of a right to access rights attributable to an
intangible asset, revenue, in the form of an
upfront fee, is recognised over time, normally
on a straight-line basis over the life of the
contract. Where the Group provides ongoing
development services, revenue in respect of
this element is recognised over the duration
of those services.
Where Collaboration Revenue is recorded
and there is a related intangible asset that is
licensed as part of the arrangement, an
appropriate amount of that intangible asset
is charged to Cost of sales based on an
allocation of cost or value to the rights that
have been licensed.
Cost of sales
Cost of sales are recognised as the
associated revenue is recognised. Cost of
sales include manufacturing costs, royalties
payable on revenues recognised, movements
in provisions for inventories, inventory
write-offs and impairment charges in relation
to manufacturing assets. Cost of sales also
includes co-collaborator sharing of profit
arising from collaborations, and foreign
exchange gains and losses arising from
business trading activities.
Research and development
Research expenditure is charged to profit and
loss in the year in which it is incurred.
Internal development expenditure is
capitalised only if it meets the recognition
criteria of IAS 38 ‘Intangible Assets’. This is
considered a key judgement. Where regulatory
and other uncertainties are such that the
criteria are not met, the expenditure is
charged to profit and loss and this is almost
invariably the case prior to approval of the
drug by the relevant regulatory authority.
Where, however, recognition criteria are
met, Intangible assets are capitalised and
amortised on a straight-line basis over their
useful economic lives from product launch.
At 31 December 2023, no amounts have met
the recognition criteria.
Payments to in-license products and
compounds from third parties for new research
and development projects (in process research
and development) generally take the form of
upfront payments, milestones and royalty
payments. Where payments made to third
parties represent consideration for future
research and development activities, an
evaluation is made as to the nature of the
payments. Such payments are expensed if they
represent compensation for sub-contracted
research and development services not
resulting in a transfer of intellectual property.
By contrast, payments are capitalised if they
represent compensation for the transfer of
identifiable intellectual property developed
at the risk of the third party. Such payments
may be made once development or regulatory
milestones are met and may also be made
on the basis of sales volumes once a product
is launched. Development and regulatory
milestone payments are capitalised as the
milestone is triggered. Sales-related payments
are accrued and capitalised with reference to
the latest Group sales forecasts for approved
indications at the present value of expected
future cash flows. Assets capitalised are
amortised, on a straight-line basis, over their
useful economic lives from product launch.
The determination of useful economic
life is considered to be a key judgement.
On product launch, the Group makes a
judgement as to the expected useful
economic life with reference to the expiry
of associated patents for the product,
expectation around the competitive
environment specific to the product and
our detailed long-term risk-adjusted sales
projections compiled annually across the
Group and approved by the Board.
The useful economic life can extend beyond
patent expiry dependent upon the nature
of the product and the complexity of the
development and manufacturing process.
Significant sales can often be achieved post
patent expiration.
Intangible assets
Intangible assets are stated at cost less
accumulated amortisation and impairments.
Intangible assets relating to products in
development are subject to impairment testing
annually. All Intangible assets are tested for
impairment when there are indications that
the carrying value may not be recoverable.
The determination of the recoverable amounts
include key estimates which are highly sensitive
to, and depend upon, key assumptions as
detailed in Note 10 to the Financial Statements
from page 172.
Impairment reviews have been carried out on
all Intangible assets that are in development
(and not being amortised), all major intangible
assets acquired during the year and all other
intangible assets that have had indicators of
impairment during the year. Recoverable
amount is determined as the higher of value-in-
use or fair value less costs to sell using a
discounted cash flow calculation, with the
products’ expected cash flows risk-adjusted
over their estimated remaining useful economic
life. Sales forecasts and specific allocated
costs (which have both been subject to
appropriate senior management review and
approval) are risk-adjusted and discounted
using appropriate rates based on our post-tax
weighted average cost of capital or for fair value
less costs to sell, a required rate of return for a
market participant. Our weighted average cost
of capital reflects factors such as our capital
structure and our costs of debt and equity.
Any impairment losses are recognised
immediately in Operating profit. Intangible
assets relating to products which fail during
development (or for which development
ceases for other reasons) are also tested for
impairment and are written down to their
recoverable amount (which is usually nil).
If, subsequent to an impairment loss being
recognised, development restarts or other
facts and circumstances change indicating
that the impairment is less or no longer exists,
the value of the asset is re-estimated and its
carrying value is increased to the recoverable
amount, but not exceeding the original value,
by recognising an impairment reversal in
Operating profit.
Government grants
Government grants are recognised in the
Consolidated Statement of Comprehensive
Income so as to match with the related
expenses that they are intended to compensate.
Where grants are received in advance of the
related expenses, they are initially recognised
in the Consolidated Statement of Financial
Position under Trade and other payables as
deferred income and released to net off
against the related expenditure when incurred.
154
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Each contract is assessed to determine
whether there are both grant elements and
supply of product which need to be separated.
In each case, the contracts set out the specified
terms for the supply of the product and the
provisions for funding for certain costs,
primarily research and development associated
with the IP. It is considered whether there are
any conditions for the funding to be refunded.
The consideration in the contract is allocated
between the grant and supply elements.
The standalone selling price for the supply
of products is determined by reference to
observed prices with other customers. The
amount allocated as a government grant is
determined by reference to the specific agreed
costs and activities identified in the contract
as not directly attributable to the supply of
product. Government grants are recorded
as an offset to the relevant expense in the
Consolidated Statement of Comprehensive
Income and are capped to match the relevant
costs incurred.
Other operating income and expense
Other operating income and expense is
generated from activities outside of the
Group’s normal course of business, which
includes Other income from divestments of or
full out-license of assets and businesses
including royalties and milestones where the
Group does not retain a significant continued
interest. Where the arrangement meets the
definition of a licence agreement, sales
milestones and sales royalties are recognised
when achieved by applying the royalty
exemption under IFRS 15. All other milestones
and sales royalties are recognised when it is
considered highly probable that there will not
be a significant reversal of cumulative income.
The determination requires estimates to be
made in relation to future Product Sales.
Joint arrangements and associates
The Group has arrangements over which it
has joint control and which qualify as joint
operations or joint ventures under IFRS 11
‘Joint Arrangements’. For joint operations, the
Group recognises its share of revenue that it
earns from the joint operations and its share of
expenses incurred. The Group also recognises
the assets associated with the joint operations
that it controls and the liabilities it incurs under
the joint arrangement. For joint ventures and
associates, the Group recognises its interest in
the joint venture or associate as an investment
and uses the equity method of accounting.
Employee benefits
The Group accounts for pensions and other
employee benefits (principally healthcare)
under IAS 19 ‘Employee Benefits’. In respect
of defined benefit plans, obligations are
determined using the projected unit credit
method and are discounted to present value
by reference to market yields on high-quality
corporate bonds, while plan assets are
measured at fair value. Given the extent of the
assumptions used to determine the value of
scheme assets and scheme liabilities, these
are considered to be significant estimates.
The operating and financing costs of such plans
are recognised separately in profit; current
service costs are spread systematically over
the lives of employees and financing costs are
recognised in full in the periods in which they
arise. Remeasurements of the net defined
benefit pension liability, including actuarial
gains and losses, are recognised immediately
in Other comprehensive income.
Where the calculation results in a surplus to
the Group, the recognised asset is limited
to the present value of any available future
refunds from the plan or reductions in
future contributions to the plan subject to
consideration of the effect any minimum
funding requirement for future service has
on the benefit available as a reduction in
future contributions.
Payments to defined contribution plans are
recognised in profit as they fall due.
Taxation
The current tax payable is based on taxable
profit for the year. Taxable profit differs from
reported profit because taxable profit
excludes items that are either never taxable or
tax deductible or items that are taxable or tax
deductible in a different period. The Group’s
current tax assets and liabilities are calculated
using tax rates that have been enacted or
substantively enacted by the reporting date.
Deferred tax is provided using the balance
sheet liability method, providing for temporary
differences between the carrying amounts of
assets and liabilities for financial reporting
purposes and the amounts used for taxation
purposes. Deferred tax liabilities are recognised
unless they arise from the initial recognition
(other than in a business combination) of assets
and liabilities in a transaction that affects
neither the taxable profit nor the accounting
profit. Deferred tax liabilities are not recognised
to the extent they arise from the initial
recognition of non-tax deductible goodwill.
Deferred tax assets are recognised to the
extent that there are future taxable temporary
differences or it is probable that future taxable
profit will be available against which the asset
can be utilised. This requires judgements to
be made in respect of the availability of future
taxable income.
No deferred tax asset or liability is recognised
in respect of temporary differences associated
with investments in subsidiaries and branches
where the Group is able to control the timing
of reversal of the temporary differences and it
is probable that the temporary differences will
not reverse in the foreseeable future.
The Group’s deferred tax assets and liabilities
are calculated using tax rates that are
expected to apply in the period when the
liability is settled or the asset realised based
on tax rates that have been enacted or
substantively enacted by the reporting date.
Deferred tax liabilities relating to assets
recognised because of a business combination
which may qualify for intellectual property
incentives are measured at the relevant
statutory tax rate. Deferred tax assets and
liabilities are offset in the Consolidated
Statement of Financial Position if, and only if,
the taxable entity has a legally enforceable
right to set off current tax assets and liabilities,
and the Deferred tax assets and liabilities
relate to taxes levied by the same taxation
authority on the same taxable entity.
Liabilities for uncertain tax positions require
management to make judgements of potential
exposures in relation to tax audit issues.
Tax benefits are not recognised unless the
tax positions will probably be accepted by
the tax authorities. This is based upon
management’s interpretation of applicable laws
and regulations and the expectation of how
the tax authority will resolve the matter. Once
considered probable of not being accepted,
management reviews each material tax benefit
and reflects the effect of the uncertainty in
determining the related taxable result.
Liabilities for uncertain tax positions are
measured using either the most likely amount
or the expected value amount depending on
which method the entity expects to better
predict the resolution of the uncertainty.
Further details of the estimates and
assumptions made in determining our recorded
liability for transfer pricing contingencies and
other tax contingencies are included in Note 30
to the Financial Statements from page 204.
Share-based payments
All plans have been classified as equity settled
after assessment. The grant date fair value
of the market-based performance elements of
employee share plan awards is calculated
using a modified Monte Carlo model, with
other elements at market price. In accordance
with IFRS 2 ‘Share-based Payment’, the
resulting cost is recognised in profit on a
straight-line basis over the vesting period
of the awards. The value of the charge is
adjusted to reflect expected and actual levels
of awards vesting, except where the failure
to vest is as a result of not meeting a market
condition. Cancellations of equity instruments
are treated as an acceleration of the vesting
period and any outstanding charge is
recognised in profit immediately.
Cash outflows relating to the vesting of share
plans for our employees are recognised within
operating activities, as they relate to employee
remuneration. The cash flows relating to
replacement awards issued to employees as
part of the Alexion acquisition (see Note 27
from page 193) are classified within investing
activities, as they are part of the aggregate
cash flows arising from obtaining control of
the subsidiary.
Group Accounting Policies 155AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Group Accounting Policies
continu ed
Property, plant and equipment
The Group’s policy is to depreciate the
difference between the cost of each item of
Property, plant and equipment and its residual
value over its estimated useful life on a
straight-line basis. Assets under construction
are not depreciated until the asset is available
for use, at which point the asset is transferred
into either Land and buildings or Plant and
equipment, and depreciated over its
estimated useful economic life.
Reviews are made annually of the estimated
remaining lives and residual values of individual
productive assets, taking account of
commercial and technological obsolescence as
well as normal wear and tear. It is impractical
to calculate average asset lives exactly.
However, the useful economic lives range from
approximately 10 to 50 years for buildings,
and three to 15 years for plant and equipment.
All items of Property, plant and equipment are
tested for impairment when there are indications
that the carrying value may not be recoverable.
Any impairment losses are recognised
immediately in Operating profit.
Leases
The Group’s lease arrangements are principally
for property, most notably a portfolio of office
premises and employee accommodation, and
for a global car fleet, utilised primarily by our
sales and marketing teams.
The lease liability and corresponding
right-of-use asset arising from a lease are
initially measured on a present value basis.
Lease liabilities include the net present value
of the following lease payments:
> fixed payments, less any lease
incentives receivable
> variable lease payments that depend on an
index or a rate, initially measured using the
index or rate as at the commencement date
> the exercise price of a purchase option if
the Group is reasonably certain to exercise
that option
> payments of penalties for terminating the
lease, if the lease term reflects the Group
exercising that option, and
> amounts expected to be payable by the
Group under residual value guarantees.
Right-of-use assets are measured at cost
comprising the following:
> the amount of the initial measurement of
lease liability
> any lease payments made at or before
the commencement date less any lease
incentives received
> any initial direct costs, and
> restoration costs.
Judgements made in calculating the lease
liability include assessing whether arrangements
contain a lease and determining the lease term.
Lease terms are negotiated on an individual
basis and contain a wide range of different
terms and conditions. Property leases will
often include an early termination or extension
option to the lease term. Fleet management
policies vary by jurisdiction and may include
renewal of a lease until a measurement
threshold, such as mileage, is reached.
Extension and termination options have been
considered when determining the lease term,
along with all facts and circumstances that may
create an economic incentive to exercise an
extension option, or not exercise a termination
option. Extension periods (or periods after
termination options) are only included in the
lease term if the lease is reasonably certain to
be extended (or not terminated).
The lease payments are discounted using
incremental borrowing rates, as in the majority
of leases held by the Group the interest rate
implicit in the lease is not readily identifiable.
Calculating the discount rate is an estimate
made in calculating the lease liability. This rate
is the rate that the Group would have to pay to
borrow the funds necessary to obtain an asset
of similar value to the right-of-use asset in a
similar economic environment with similar
terms, security and conditions. To determine
the incremental borrowing rate, the Group
uses a risk-free interest rate adjusted for
credit risk, adjusting for terms specific to the
lease including term, country and currency.
The Group is exposed to potential future
increases in variable lease payments that are
based on an index or rate, which are initially
measured as at the commencement date, with
any future changes in the index or rate excluded
from the lease liability until they take effect.
When adjustments to lease payments based
on an index or rate take effect, the lease
liability is reassessed and adjusted against the
right-of-use asset.
Lease payments are allocated between
principal and finance cost. The finance cost
is charged to the Consolidated Statement of
Comprehensive Income over the lease period
so as to produce a constant periodic rate of
interest on the remaining balance of the
liability for each period.
Payments associated with short-term leases of
Property, plant and equipment and all leases
of low-value assets are recognised on a
straight-line basis as an expense in the
Consolidated Statement of Comprehensive
Income. Short-term leases are leases with a
lease term of 12 months or less. Low-value
leases are those where the underlying asset
value, when new, is $5,000 or less and
includes IT equipment and small items of
office furniture.
Contracts may contain both lease and
non-lease components. The Group allocates
the consideration in the contract to the lease
and non-lease components based on their
relative standalone prices.
Right-of-use assets are generally depreciated
over the shorter of the asset’s useful life and
the lease term on a straight-line basis. If the
Group is reasonably certain to exercise a
purchase option, the right-of-use asset is
depreciated over the underlying asset’s useful
life. It is impractical to calculate average asset
lives exactly. However, the total lives range from
approximately 10 to 50 years for buildings,
and three to 15 years for motor vehicles and
other assets.
There are no material lease agreements under
which the Group is a lessor.
Business combinations and goodwill
In assessing whether an acquired set of
assets and activities is a business or an asset,
management will first elect whether to apply
an optional concentration test to simplify the
assessment. Where the concentration test is
applied, the acquisition will be treated as the
acquisition of an asset if substantially all of
the fair value of the gross assets acquired
(excluding cash and cash equivalents,
deferred tax assets, and related goodwill) is
concentrated in a single asset or group of
similar identifiable assets.
Where the concentration test is not applied,
or is not met, a further assessment of whether
the acquired set of assets and activities is a
business will be performed.
The determination of whether an
acquired set of assets and activities is a
business or an asset can be judgemental,
particularly if the target is not producing
outputs. Management uses a number of
factors to make this determination, which
are primarily focused on whether the
acquired set of assets and activities include
substantive processes that mean the set is
capable of being managed for the purpose
of providing a return. Key determining
factors include the stage of development
of any assets acquired, the readiness and
ability of the acquired set to produce outputs
and the presence of key experienced
employees capable of conducting activities
required to develop or manufacture the
assets. Typically, the specialised nature of
many pharmaceutical assets and processes
is such that until assets are substantively
ready for production and promotion, there
are not the required processes for a set of
assets and activities to meet the definition
of a business in IFRS 3.
156
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

On the acquisition of a business, fair values
are attributed to the identifiable assets and
liabilities. Attributing fair values is a key
judgement; refer to Note 27 to the Financial
Statements from page 193 for additional
details. Contingent liabilities are also recorded
at fair value unless the fair value cannot be
measured reliably, in which case the value is
subsumed into goodwill. Where fair values of
acquired contingent liabilities cannot be
measured reliably, the assumed contingent
liability is not recognised but is disclosed in
the same manner as other contingent liabilities.
Where not all of the equity of a subsidiary
is acquired, the non-controlling interest is
recognised either at fair value or at the
non-controlling interest’s proportionate
share of the net assets of the subsidiary,
on a case-by-case basis. Put options over
non-controlling interests are recognised as a
financial liability, with a corresponding entry
in either Retained earnings or against non-
controlling interest reserves on a case-by-
case basis.
The timing and amount of future contingent
elements of consideration is an estimate.
Contingent consideration, which may include
development and launch milestones, revenue
threshold milestones and revenue-based
royalties, is fair valued at the date of acquisition
using decision-tree analysis with key inputs
including probability of success, consideration
of potential delays and revenue projections
based on the Group’s internal forecasts.
Unsettled amounts of consideration are held
at fair value within payables with changes in
fair value recognised immediately in profit.
Goodwill is the difference between the fair value
of the consideration and the fair value of net
assets acquired.
Goodwill arising on acquisitions is capitalised
and subject to an impairment review, both
annually and when there is an indication that
the carrying value may not be recoverable.
The Group’s policy up to and including
1997 was to eliminate Goodwill arising upon
acquisitions against reserves. Under IFRS 1
‘First-time Adoption of International Financial
Reporting Standards’ and IFRS 3 ‘Business
Combinations’, such Goodwill will remain
eliminated against reserves.
Subsidiaries
A subsidiary is an entity controlled, directly
or indirectly, by AstraZeneca PLC. Control is
regarded as the exposure or rights to the
variable returns of the entity when combined
with the power to affect those returns. Control
is normally evidenced by holding more than
50% of the share capital of the company,
however other agreements may be in place that
result in control where they give AstraZeneca
finance decision-making authority over the
relevant activities of the company.
The financial results of subsidiaries are
consolidated from the date control is
obtained until the date that control ceases.
Inventories
Inventories are stated at the lower of cost and
net realisable value. The first in, first out or an
average method of valuation is used. For
finished goods and work in progress, cost
includes directly attributable costs and certain
overhead expenses (including depreciation).
Selling expenses and certain other overhead
expenses (principally central administration
costs) are excluded. Net realisable value is
determined as estimated selling price less all
estimated costs of completion and costs to be
incurred in selling and distribution.
Write-downs of inventory occur in the general
course of business and are recognised in
Cost of sales for launched or approved
products and in Research and development
expense for products in development.
Assets held for sale
Non-current assets are classified as Assets
held for sale when their carrying amount is
to be recovered principally through a sale
transaction and a sale is considered highly
probable. A sale is considered highly probable
only when the appropriate level of management
has committed to the sale.
Assets held for sale are stated at the lower
of carrying amount and fair value less costs
to sell. Where there is a partial transfer of a
non-current asset to held for sale, an allocation
of value is made between the current and
non-current portions of the asset based on
the relative value of the two portions, unless
there is a methodology that better reflects the
asset to be disposed of.
Assets held for sale are neither depreciated
nor amortised.
Trade and other receivables
Financial assets included in Trade and other
receivables are recognised initially at fair value.
The Group holds the Trade receivables with the
objective to collect the contractual cash flows
and therefore measures them subsequently at
amortised cost using the effective interest
method, less any impairment, based on
expected credit losses.
Trade receivables that are subject to debt
factoring arrangements are derecognised if
they meet the conditions for derecognition
detailed in IFRS 9 ‘Financial Instruments’.
Trade and other payables
Financial liabilities included in Trade and other
payables are recognised initially at fair value.
Subsequent to initial recognition they are
measured at amortised cost using the effective
interest method. Contingent consideration
payables are held at fair value within Level 3 of
the fair value hierarchy as defined in Note 12.
Financial instruments
The Group’s financial instruments include
Lease liabilities, Trade and other receivables
and payables, liabilities for contingent
consideration and put options under business
combinations, and rights and obligations
under employee benefit plans which are dealt
with in specific accounting policies.
The Group’s other financial instruments include:
> Cash and cash equivalents
> Fixed deposits
> Other investments
> Bank and other borrowings
> Derivatives.
Cash and cash equivalents
Cash and cash equivalents comprise cash in
hand, current balances with banks and similar
institutions, and highly liquid investments
with maturities of three months or less when
acquired. They are readily convertible into
known amounts of cash and are held at
amortised cost under the hold to collect
classification, where they meet the hold to
collect ‘solely payments of principal and
interest’ test criteria under IFRS 9. Those
not meeting these criteria are held at fair
value through profit or loss. Cash and cash
equivalents in the Consolidated Statement
of Cash Flows include unsecured bank
overdrafts at the balance sheet date where
balances often fluctuate between a cash and
overdraft position.
Fixed deposits
Fixed deposits, principally comprising funds
held with banks and other financial institutions,
are initially measured at fair value, plus direct
transaction costs, and are subsequently
measured at amortised cost using the
effective interest method at each reporting
date. Changes in carrying value are
recognised in the Consolidated Statement
of Comprehensive Income.
Other investments
Investments are classified as fair value through
profit or loss (FVPL), unless the Group makes
an irrevocable election at initial recognition
for certain non-current equity investments to
present changes in Other comprehensive
income (FVOCI). If this election is made, there
is no subsequent reclassification of fair value
gains and losses to profit or loss following the
derecognition of the investment.
Bank and other borrowings
The Group uses derivatives, principally interest
rate swaps, to hedge the interest rate exposure
inherent in a portion of its fixed interest rate
debt. In such cases the Group will either
designate the debt as FVPL when certain
criteria are met or as the hedged item under
a fair value hedge.
Group Accounting Policies 157AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Group Accounting Policies
continu ed
If the debt instrument is designated as FVPL,
the debt is initially measured at fair value (with
direct transaction costs being included in profit
as an expense) and is remeasured to fair value
at each reporting date with changes in carrying
value being recognised in profit (along with
changes in the fair value of the related
derivative), with the exception of changes in
the fair value of the debt instrument relating to
own credit risk which are recorded in Other
comprehensive income in accordance with
IFRS 9. Such a designation has been made
where this significantly reduces an accounting
mismatch which would result from recognising
gains and losses on different bases.
If the debt is designated as the hedged item
under a fair value hedge, the debt is initially
measured at fair value (with direct transaction
costs being amortised over the life of the debt)
and is remeasured for fair value changes in
respect of the hedged risk at each reporting
date with changes in carrying value being
recognised in profit (along with changes in the
fair value of the related derivative).
If the debt is designated in a cash flow hedge,
the debt is measured at amortised cost
(with gains or losses taken to profit and direct
transaction costs being amortised over the
life of the debt). The related derivative is
remeasured for fair value changes at each
reporting date with the portion of the gain
or loss on the derivative that is determined to
be an effective hedge recognised in Other
comprehensive income. The amounts that have
been recognised in Other comprehensive
income are reclassified to profit in the same
period that the hedged forecast cash flows
affect profit. The reclassification adjustment is
included in Finance expense in the Consolidated
Statement of Comprehensive Income.
Other interest-bearing loans are initially
measured at fair value (with direct transaction
costs being amortised over the life of the loan)
and are subsequently measured at amortised
cost using the effective interest method at each
reporting date. Changes in carrying value are
recognised in the Consolidated Statement of
Comprehensive Income.
Derivatives
Derivatives are initially measured at fair value
(with direct transaction costs being included
in profit as an expense) and are subsequently
remeasured to fair value at each reporting
date. Changes in carrying value of derivatives
not designated in hedging relationships are
recognised in profit or loss.
The Group has agreements with some bank
counterparties whereby the parties agree to
post cash collateral, for the benefit of the other,
equivalent to the market valuation of all of the
derivative positions above a predetermined
threshold. Cash collateral received from
counterparties is included within current
Interest-bearing loans and borrowings within the
Consolidated Statement of Financial Position.
Cash collateral pledged to counterparties is
recognised as a financial asset and is included
in current Other investments within the
Consolidated Statement of Financial Position.
Cash collateral received is included in
Movement in short-term borrowings within
financing activities in the Consolidated Cash
Flow Statement. Cash collateral paid is included
in Movements in short-term investments within
investing activities in the Consolidated Cash
Flow Statement. The cash flow presentation of
cash paid and received follows the Consolidated
Statement of Financial Position presentation
of the financial asset and financial liability that
is recognised from posting the collateral.
Foreign currencies
Foreign currency transactions, being
transactions denominated in a currency other
than an individual Group entity’s functional
currency, are translated into the relevant
functional currencies of individual Group
entities at average rates for the relevant
monthly accounting periods, which
approximate to actual rates.
Monetary assets and liabilities arising from
foreign currency transactions are retranslated
at exchange rates prevailing at the reporting
date. Exchange gains and losses on loans and
on short-term foreign currency borrowings
and deposits are included within Finance
expense. Exchange differences on all other
foreign currency transactions are recognised
in Operating profit in the individual Group
entity’s accounting records.
Non-monetary items arising from foreign
currency transactions are not retranslated in the
individual Group entity’s accounting records.
In the Consolidated Financial Statements,
income and expense items for Group entities
with a functional currency other than US
dollars are translated into US dollars at
average exchange rates, which approximate
to actual rates, for the relevant accounting
periods. Assets and liabilities are translated at
the US dollar exchange rates prevailing at the
reporting date. Exchange differences arising
on consolidation are recognised in Other
comprehensive income.
If certain criteria are met, non-US dollar-
denominated loans or derivatives are
designated as net investment hedges of foreign
operations. Exchange differences arising on
retranslation of net investments, and of foreign
currency loans which are designated in an
effective net investment hedge relationship, are
recognised in Other comprehensive income in
the Consolidated Financial Statements. Foreign
exchange derivatives hedging net investments
in foreign operations are carried at fair value.
Effective fair value movements are recognised
in Other comprehensive income, with any
ineffectiveness taken to profit. Gains and
losses accumulated in the translation reserve
will be recycled to profit and loss when the
foreign operation is sold.
Provisions
Provisions are recognised when there is either
a legal or constructive present obligation as a
result of a past event, it is probable that an
outflow of economic resources will be required
to settle the obligation and a reliable estimate
can be made of the amount of the obligation.
If the effect of the time value of money is
material, provisions are discounted at the
relevant pre-tax discount rate. Where provisions
are discounted, the increase in the provision
resulting from the passage of time is recognised
as a finance cost.
Litigation and environmental liabilities
AstraZeneca is involved in legal disputes, the
settlement of which may involve cost to the
Group. A provision is made where an adverse
outcome is probable and associated costs,
including related legal costs, can be estimated
reliably. Determining the timing of recognition
of when an adverse outcome is probable is
considered a key judgement, refer to Note 30
to the Financial Statements from page 204.
Where it is considered that the Group is more
likely than not to prevail, or in the extremely
rare circumstances where the amount of the
legal liability cannot be estimated reliably,
legal costs involved in defending the claim are
charged to the Consolidated Statement of
Comprehensive Income as they are incurred.
Where it is considered that the Group has a
valid contract which provides the right to
reimbursement (from insurance or otherwise)
of legal costs and/or all or part of any loss
incurred or for which a provision has been
established, the amount expected to be
received is recognised as an asset only when
it is virtually certain.
AstraZeneca is exposed to environmental
liabilities relating to its past operations,
principally in respect of soil and groundwater
remediation costs. Provisions for these costs
are made when there is a present obligation
and where it is probable that expenditure on
remedial work will be required and a reliable
estimate can be made of the cost.
Restructuring
Restructuring costs are incurred in programmes
that are planned and controlled by the Group
which materially change either the scope of a
business undertaken by the Group, or the
manner in which that business is conducted.
A provision for restructuring costs is recognised
when a detailed formal plan is in place and
has either been announced to those affected
or has started to be implemented. The general
recognition criteria for provisions must also be
met, as described in the Provisions policy.
158
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Impairment
The carrying values of non-financial assets,
other than Inventories and Deferred tax assets,
are reviewed at least annually to determine
whether there is any indication of impairment.
For Goodwill, Intangible assets under
development and for any other assets where
such indication exists, the asset’s recoverable
amount is estimated based on the greater of
its value in use and its fair value less cost to
sell. In assessing the recoverable amount, the
estimated future cash flows, adjusted for the
risks associated with the probability of success
specific to each asset, as well as inflationary
impacts, are discounted to their present value
using a nominal discount rate that reflects
current market assessments of the time
value of money, the general risks affecting
the pharmaceutical industry and other risks
specific to each asset. For the purpose of
impairment testing, assets are grouped
together into the smallest group of assets
that generates cash inflows from continuing
use that are largely independent of the cash
flows of other assets. Impairment losses are
recognised immediately in the Consolidated
Statement of Comprehensive Income.
Applicable accounting standards
andinterpretations issued but not
yetadopted
At the date of authorisation of these financial
statements, certain new accounting standards
and amendments were in issue relating to the
following standards and interpretations but
not yet adopted by the Group:
> amendments to IAS 1 ‘Presentation of
Financial Statements’, effective for periods
beginning on or after 1 January 2024 –
endorsed by the UK Endorsement Board
(UKEB) on 21 July 2023
> amendments to IFRS 16 ‘Leases’, effective
for periods beginning on or after 1 January
2024 – endorsed by the UKEB on
11 May 2023
> amendments to IAS 7 ‘Statement of Cash
Flows’ and IFRS 7 ‘Financial Instruments:
Disclosures’, effective for periods beginning
on or after 1 January 2024 – endorsed by
the UKEB on 28 November 2023
> amendments to IAS 21 ‘The Effects of
Changes in Foreign Exchange Rates’,
effective for periods beginning on or
after 1 January 2025 – not endorsed by
the UKEB.
These new standards, amendments and
interpretations are not expected to have a
significant impact on the Group’s net results.
Group Accounting Policies 159AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
1 Revenue
Product Sales
2023 2022 2021
Emerging Rest of Emerging Rest of Emerging Rest of
US Markets Europe World Total US Markets Europe World Total US Markets Europe World Total
$m $m $m $m $m $m $m $m $m $m $m $m $m $m $m
Oncology:
Tagrisso 2,276 1,621 1,120 782 5,799 2,007 1,567 1,023 847 5,444 1,780 1,336 986 913 5,015
Imfinzi 2,317 360 758 802 4,237 1,552 287 544 401 2,784 1,245 277 485 405 2,412
Lynparza 1,254 542 734 281 2,811 1,226 488 655 269 2,638 1,087 384 618 259 2,348
Calquence 1,815 98 493 108 2,514 1,657 45 286 69 2,057 1,089 20 111 18 1,238
Enhertu – 169 60 32 261 – 51 21 7 79 – 12 4 1 17
Orpathys – 44 – – 44 – 33 – – 33 – 16 – – 16
Truqap 6 – – – 6 – – – – – – – – – –
Zoladex 14 687 133 118 952 15 657 133 122 927 13 619 147 169 948
Faslodex 31 142 28 96 297 17 159 55 103 334 30 167 113 121 431
Others 6 165 6 47 224 10 250 9 66 335 11 391 17 96 515
7,719 3,828 3,332 2,266 17,145 6,484 3,537 2,726 1,884 14,631 5,255 3,222 2,481 1,982 12,940
Cardiovascular, Renal & Metabolism:
Farxiga 1,451 2,211 1,881 420 5,963 1,071 1,665 1,297 348 4,381 732 1,195 810 263 3,000
Brilinta 744 285 271 24 1,324 744 286 282 46 1,358 735 328 346 63 1,472
Lokelma 214 50 58 90 412 170 20 30 69 289 115 3 13 44 175
roxadustat – 271 – – 271 – 197 – – 197 – 174 – – 174
Andexxa 75 – 62 45 182 77 – 41 32 150 50 – 18 – 68
Crestor 55 862 52 138 1,107 65 794 41 148 1,048 80 775 52 189 1,096
Seloken/Toprol-XL 1 621 11 7 640 – 839 14 9 862 1 928 11 11 951
Onglyza 49 131 32 15 227 76 121 38 22 257 88 179 61 32 360
Bydureon 133 3 27 – 163 242 3 35 – 280 321 3 55 6 385
Others 30 152 109 5 296 34 194 128 10 366 52 195 146 14 407
2,752 4,586 2,503 744 10,585 2,479 4,119 1,906 684 9,188 2,174 3,780 1,512 622 8,088
Respiratory & Immunology:
Symbicort 726 753 549 334
2,362 973 608 582 375 2,538 1,065 609 670 384 2,728
Fasenra 992 64 355 142 1,553 906 43 305 142 1,396 790 20 286 162 1,258
Breztri 383 161 81 52 677 239 92 33 34 398 115 55 7 26 203
Saphnelo 260 2 8 10 280 111 – 2 3 116 8 – – – 8
Tezspire – 1 48 37 86 – – 2 2 4 – – – – –
Pulmicort 28 575 68 42 713 65 462 69 49 645 72 770 73 47 962
Bevespi 34 6 17 1 58 42 5 10 1 58 39 4 11 – 54
Daliresp/Daxas 42 3 8 1 54 176 3 9 1 189 207 4 15 1 227
Others 82 206 30 6 324 143 230 42 6 421 108 287 185 14 594
2,547 1,771 1,164 625 6,107 2,655 1,443 1,054 613 5,765 2,404 1,749 1,247 634 6,034
Vaccines & Immune Therapies:
COVID-19 mAbs – 6 12 114 132 1,067 413 298 407 2,185 – 19 66 – 85
Vaxzevria – 10 2 – 12 79 729 365 625 1,798 64 2,240 1,035 578 3,917
Beyfortus 87 – 19 – 106 – – – – – – – – – –
Synagis (1) 195 175 177 546 1 173 213 191 578 23 35 203 149 410
FluMist 23 1 188 4 216 21 1 151 2 175 27 2 222 2 253
109 212 396 295 1,012 1,168 1,316 1,027 1,225 4,736 114 2,296 1,526 729 4,665
Rare Disease:
Soliris 1,734 424 670 317 3,14 5 2,180 301 805 476 3,762 1,068 170 439 197 1,874
Ultomiris 1,750 71 668 476 2,965 1,136 38 481 310 1,965 381 9 169 129 688
Strensiq 937 40 89 86 1,152 769 35 78 76 958 297 10 36 35 378
Koselugo 195 59 53 24 331 162 26 20 – 208 104 1 3 – 108
Kanuma 85 29 49 8 171 77 31 44 8 160 32 7 20 3 62
4,701 623 1,529 911 7,764 4,324 431 1,428 870 7, 0 53 1,882 197 667 364 3,110
Other:
Nexium 115 578 53 199 945 120 568 46 551 1,285 128 705 62 431 1,326
Others 18 153 52 8 231 24 220 77 19 340 43 212 109 14 378
133 731 105 207 1,176 144 788 123 570 1,625 171 917 171 445 1,704
Product Sales 17,9 61 11,751 9,029
5,048 43,789 17,25 4 11,6 34 8,264 5,846 42,998 12,000 12,161 7,604 4,776 36,541
160 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Rebates and chargebacks in the US
The major market where estimates are seen as significant is the US. When invoicing Product Sales in the US, we estimate the rebates and chargebacks
we expect to pay and we consider there to be a significant estimate associated with the rebates for Managed Care, Medicaid and Medicare Part D.
The total adjustment in respect of prior year net US Product Sales revenue in 2023 was 1.0% (2022: 1.3%; 2021: 1.5%); this represents the difference
between our prior year estimates for rebates and chargebacks against actual amounts paid for the US business. The most significant of these relate
to the Medicaid and state programmes with an adjustment in respect of prior year net US Product Sales revenue in 2023 of 0.3% (2022: 0.5%;
2021: 0.4%) and Managed Care and Medicare of 0.5% (2022: 0.8%; 2021: 0.7%).
The adjustment in respect of the prior year net US Product Sales revenue, excluding the Rare Disease therapy area in 2023, was 1.4% (2022: 1.6%;
2021: 1.8%), with Medicaid and state programmes of 0.4% (2022: 0.6%; 2021: 0.5%) and Managed Care and Medicare of 0.7% (2022: 1.1%; 2021: 0.8%).
These values demonstrate the level of sensitivity; further meaningful sensitivity is not able to be provided due to the large volume of variables that
contribute to the overall rebates, chargebacks, returns and other revenue accruals. These variables include assumptions in respect of aggregate
future sales levels, segment mix and customers’ contractual performance, and in addition for Managed Care, US Medicaid and Medicare Part D, the
channel inventory levels, and assumptions related to lag time. These assumptions are built up on a product-by-product and customer-by-customer
basis, taking into account specific contract provisions coupled with expected performance, and are then aggregated into a weighted average rebate
accrual rate for each of our products. Accrual rates are reviewed and adjusted on an as-needed basis. There may be further adjustments when
actual rebates are invoiced based on utilisation information submitted to AstraZeneca (in the case of contractual rebates) and claims/invoices are
received (in the case of regulatory rebates and chargebacks).
Alliance Revenue
2023 2022 2021
$m $m $m
Enhertu 1,022 523 197
Tezspire 259 79 –
Beyfortus 57 – –
Vaxzevria: royalties – 76 64
Other royalty income 81 68 70
Other Alliance Revenue 9 9 57
1,428 755 388
Collaboration Revenue
2023 2022 2021
$m $m $m
Lynparza: regulatory milestones 245 355 –
Lynparza: sales milestones – – 400
COVID-19 mAbs: licence fees 180 – –
Farxiga: sales milestones 29 – –
tralokinumab: sales milestones 20 110 –
Beyfortus: regulatory milestones 71 25 –
Beyfortus: sales milestones 27 – –
Nexium: sale of rights – 62 75
Other Collaboration Revenue 22 46 13
594 598 488
2 Operating profit
Operating profit includes the following significant items:
Cost of sales
In 2023, Cost of sales includes a charge of $114m (2022: charge of $3,484m) in relation to the release, in line with sales, of fair value uplift to inventory
that was recognised under IFRS 3 ‘Business Combinations’ upon the acquisition of Alexion (see Note 27).
During the year, $nil government grants were recognised within Cost of sales (2022: $nil; 2021: $290m). The grants recognised in 2021 related to
funding of manufactured Vaxzevria product for the US government, which expired prior to being accepted by the FDA.
Selling, general and administrative expense
In 2023, Selling, general and administrative expense includes a charge of $520m (2022: charge of $182m; 2021: charge of $42m) resulting from changes
in the fair value of contingent consideration arising from the acquisition of the diabetes alliance from BMS. These adjustments reflect revised
estimates for future sales performance for the products acquired and, as a result, revised estimates for future royalties payable.
In 2023, Selling, general and administrative expense also includes a charge of $1,013m (2022: charge of $789m; 2021: charge of $48m) relating to
a number of legal proceedings, including settlements in various jurisdictions in relation to several marketed products (see Note 30).
Research and development expense: Government grants
During the year $74m (2022: $113m; 2021: $531m) of government grants were recognised within Research and development expense. The grants
recognised relate to funding for Research and development and related expenses for COVID-19 mAbs of $nil (2022: $112m; 2021: $222m) and
Vaxzevria of $74m (2022: $1m; 2021: $309m).
Notes to the Group Financial Statements 161AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

2 Operating profit continued
Notes to the Group Financial Statements
continu ed
Other operating income and expense
2023 2022 2021
$m $m $m
Royalty income 107 59 62
Gains on disposal of intangible assets 251 104 513
Gains on disposal of investments in associates and joint ventures – – 776
Net gains/(losses) on disposal of other non-current assets 41 112 (4)
Update to the contractual relationships for Beyfortus (nirsevimab) 712 – –
Other income
1
393 439 453
Other expense (164) (200) (308)
Other operating income and expense 1,340 514 1,492
Gains on disposal of intangible assets in 2023 includes $241m on disposal of commercial rights to Pulmicort Flexhaler to Cheplapharm in the US.
Gains on disposal of intangible assets in 2021 includes $317m on disposal of rights to Crestor in over 30 countries in Europe, except in the UK
and Spain.
Net gains/(losses) on disposal of other non-current assets in 2022 includes a $125m gain in respect of the Waltham R&D site sale and leaseback
in MA, US (see Note 8).
Gains on disposal of investments in associates and joint ventures in 2021 relates to the disposal of the 26.7% ownership in Viela Bio, as part of the
acquisition of Viela Bio by Horizon Therapeutics plc. AstraZeneca received cash proceeds and profit of $776m upon closing, with the profit recorded
as Other operating income.
As part of the total consideration received in respect of the agreement to sell US rights to Synagis in 2019, $400m in total has been received related
to the rights to participate in the future cash flows from the US profits or losses for Beyfortus (nirsevimab), with $190m cash inflows in 2023 primarily
relating to a cash receipt from Sobi following achievement of a regulatory milestone. At 31 December 2022, the full amount of $522m was recognised
as a financial liability within non-current Other payables (the Profit Participation Liability) as the Group had not fully transferred the risks and rewards
of the underlying cash flows arising from Beyfortus to Sobi. All associated cash flows have been presented within investing activities as the Group
has received the cash in exchange for agreeing to transfer future cash flows relating to an intangible asset. In 2023, the contractual relationship
between AstraZeneca and Sobi relating to future sales of Beyfortus in the US was replaced by a royalty relationship between Sanofi and Sobi.
As a result, the Profit Participation Liability was extinguished and derecognised from the Consolidated Statement of Financial Position, with a gain
of $712m recorded in Other operating income and expense. In 2021, as a result of the Probability of Technical/Regulatory Success unwind, an
increase of $114m to the Profit Participation Liability was recorded with the cost recorded in Other operating expense.
Restructuring costs
During 2023, the Group has incurred $467m of net restructuring costs, of which $362m resulted from activities that are part of the Post Alexion
Acquisition Group Review (PAAGR), bringing the cumulative charges under this programme to $2,067m. Costs in 2023 included $109m within Cost
of sales due to the rationalisation of our manufacturing capacity and footprint across certain production sites, $207m within Selling, general and
administrative expense in relation to HR, Finance, IT & other integration costs as well as some severance costs, $212m within Research and
development expense in relation to the transformation of clinical, regulatory and other R&D data and systems, partially offset by income of $61m
in Other operating income and expense generated from the disposal of assets impacted by the restructuring.
In conjunction with the acquisition of Alexion in 2021, the enlarged Group initiated the PAAGR; a global restructuring programme aimed at integrating
systems, structure and processes, optimising the global footprint and prioritising resource allocations and investments. During 2023, the Group has
identified all remaining activities and finalised the scope of the programme. This includes the commencement of work on the planned upgrade of
the Group’s Enterprise Resource Planning IT systems (Axial Project), which is expected to be substantially complete by the end of 2030. The Group
has also continued to progress other legacy restructuring programmes.
Total restructuring costs in 2023 includes an impairment charge to Property, plant and equipment of $7m (2022: reversal of $4m; 2021: charge of
$343m), impairment of Right-of-use assets of $13m (2022: $nil; 2021: $nil) and no impairment of Intangible assets (software development costs)
(2022: reversal $17m; 2021: charge of $16m).
The tables below show the costs that have been charged in respect of restructuring programmes by cost category and type. Severance provisions
are detailed in Note 21.
2023 2022 2021
$m $m $m
Cost of sales 109 266 722
Distribution expense – 2 –
Research and development expense 212 111 223
Selling, general and administrative expense 207 405 338
Other operating income and expense (61) (67) –
Total charge 467 717 1,283
162 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

2023 2022 2021
$m $m $m
Severance costs 57 187 217
Accelerated depreciation and impairment charges 68 135 371
Other
1
342 395 695
Total charge 467 717 1,283
Financial instruments
Included within Operating profit are the following net gains and losses on financial instruments:
2023 2022 2021
$m $m $m
Gains/(losses) on forward foreign exchange contracts 42 150 (21)
Losses on receivables and payables (260) (203) (42)
Total (218) (53) (63)
Impairment charges
Details of impairment charges for 2023, 2022 and 2021 are included in Notes 7, 8 and 10.
3 Finance income and expense
2023 2022 2021
$m $m $m
Finance income
Returns on deposits and equity securities 291 78 12
Fair value gains on debt and interest rate swaps 43 14 –
Interest income on income tax balances 10 3 31
Total 344 95 43
Finance expense
Interest on debt, leases and other financing costs (1,132) (889) (774)
Net interest on post-employment defined benefit plan net liabilities (Note 22) (38) (29) (26)
Net exchange losses (34) (16) (20)
Discount unwind on contingent consideration arising from business combinations (Note 20) (132) (168) (226)
Discount unwind on other long-term liabilities
1
(200) (216) (248)
Fair value losses on debt and interest rate swaps (3) – (4)
Interest expense on income tax balances (87) (28) (2)
Total (1,626) (1,346) (1,300)
Net finance expense (1,282) (1,251) (1,257)
There was no interest capitalised during the year.
Financial instruments
Included within finance income and expense are the following net gains and losses on financial instruments:
2023 2022 2021
$m $m $m
Interest and fair value adjustments in respect of debt designated at fair value through profit or loss, net of derivatives 13 (9) (5)
Interest and changes in carrying values of debt designated as hedged items in fair value hedges, net of derivatives – – (9)
Interest and fair value changes on fixed and short-term deposits, equity securities, other derivatives and tax balances 177 54 16
Interest on debt, commercial paper, overdrafts and lease liabilities held at amortised cost (1,004) (837) (738)
The interest rate fair value hedges were closed in 2021. Fair value gain or loss of $nil (2022: $nil; 2021: loss of $33m) on interest rate fair value hedging
instruments and $nil fair value gain or loss (2022: $nil; 2021: gain of $29m) on the related hedged items have been included within Interest and changes
in carrying values of debt designated as hedged items in fair value hedges, net of derivatives.
Fair value loss of $1m (2022: loss of $25m; 2021: loss of $19m) on derivatives related to debt instruments designated at FVPL and $7m fair value gain
(2022: gain of $26m; 2021: gain of $19m) on debt instruments designated at FVPL have been included within Interest and fair value adjustments in
respect of debt designated at fair value through profit or loss, net of derivatives.
Notes to the Group Financial Statements 163AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
continu ed
4 Taxation
Taxation charge/(credit) recognised in the Consolidated Statement of Comprehensive Income is as follows:
2023 2022 2021
$m $m $m
Current tax
Current year 2,417 1,823 1,200
Adjustment to prior years 28 (187) (5)
Total 2,445 1,636 1,195
Deferred tax
Origination and reversal of temporary differences (1,473) (2,563) (1,417)
Adjustment to prior years (34) 135 (158)
Total (1,507) (2,428) (1,575)
Taxation charge/(credit) recognised in the profit for the year 938 (792) (380)
Taxation credit/(charge) recognised in Other comprehensive income is as follows:
2023 2022 2021
$m $m $m
Current and deferred tax
Items that will not be reclassified to profit or loss:
Remeasurement of the defined benefit liability 102 (231) (117 )
Equity investments measured at fair value through Other comprehensive income (1) 15 27
Movement in deferred taxes relating to changes in tax rates – – 195
Total 101 (216) 105
Items that may be reclassified subsequently to profit or loss:
Foreign exchange arising on designated liabilities in net investment hedges (24) 73 43
Fair value movement on cash flow hedges 12 – (5)
Movement in deferred taxes relating to changes in tax rates – – 8
Total (12) 73 46
Taxation credit/(charge) recognised in Other comprehensive income 89 (143) 151
The reported tax rate in the year was 14% and included a favourable adjustment of $828m to deferred taxes arising from a UK group company
undertaking a routine intragroup purchase of certain intellectual property. This intragroup purchase resulted in additional amortisable tax basis in
the UK which can be fully utilised against forecast UK taxable profits. Deferred tax has been recognised on this additional tax basis in the year.
This is offset by updates to tax liabilities following progress of reviews by tax authorities and administrative appeal processes and derecognition
of deferred tax assets following changes to forecast taxable income of specific subsidiaries.
The income tax paid for the year was $2,366m.
Taxation has been provided at current rates on the profits earned for the years covered by the Group Financial Statements. The 2023 prior year
current tax adjustment relates mainly to tax accrual to tax return adjustments and updates to provisions for tax contingencies. The 2022 prior
year current tax adjustment relates mainly to tax accrual to tax return adjustments and updates to provisions for tax contingencies. The 2021
prior year current tax adjustment relates mainly to tax accrual to tax return adjustments.
The 2023 prior year deferred tax adjustment relates mainly to tax accrual to tax return adjustments and adjustments to the recognition of deferred
tax assets. The 2022 prior year deferred tax adjustments relate mainly to tax accrual to tax return adjustments and updates to provisions for tax
contingencies. The 2021 prior year deferred tax adjustments relate mainly to tax accrual to tax return adjustments and updates to estimates of
prior year tax liabilities following settlements with tax authorities.
To the extent that dividends remitted from overseas subsidiaries, joint ventures and associates are expected to result in additional taxes, appropriate
amounts have been provided for. Unremitted earnings or differences in the carrying value and tax basis of investments may be liable to additional
taxes if distributed as dividends or on a liquidation event. Deferred tax is provided for such differences in relation to Group entities where management
is intending to remit earnings in the foreseeable future. The aggregate amount of gross temporary differences associated with investments in
subsidiaries, partnerships and branches for which deferred tax liabilities have not been recognised totalled approximately $7,565m at 31 December
2023, $3,221m of which has a corresponding deductible temporary difference of the same gross value which is not recognised as it is not probable
of reversing in the foreseeable future but on which different tax rates apply.
164
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Factors affecting future tax charges
As a group with worldwide operations, AstraZeneca is subject to several factors that may affect future tax charges, principally the levels and mix
of profitability in different jurisdictions, transfer pricing regulations, tax rates imposed and tax regime reforms. On 11 July 2023, Finance (No.2)
Act 2023 was enacted in the UK, introducing a global minimum effective tax rate of 15%. The legislation implements a domestic top-up tax and a
multinational top-up tax, effective for accounting periods starting on or after 31 December 2023. A Pillar 2 Effective Tax Rate (ETR) is calculated
for every jurisdiction in which the Group operates and Pillar 2 Income Taxes will arise when the Pillar 2 ETR is less than 15%. Pillar 2 Income Taxes
could be payable in the UK, or the local jurisdiction if it has introduced a Qualifying Domestic Minimum top-up Tax. AstraZeneca is continuing to
monitor potential impacts as further guidance is published by the OECD and territories implement legislation to enact the rules. Management has
performed an assessment of the impact of the UK’s Pillar 2 rules based on our 2023 data and no Pillar 2 Income Taxes are expected to arise for
most jurisdictions in which the Group operates. It is anticipated that AstraZeneca may, in some jurisdictions, incur additional tax liabilities, but the
effect on the reported tax charge is reasonably estimated to be immaterial.
The Group has applied the exemption under the IAS 12 ‘Income Taxes’ amendment for recognising and disclosing information about deferred tax
assets and liabilities related to top-up income taxes.
Tax reconciliation to UK statutory rate
The table below reconciles the UK statutory tax charge to the Group’s total tax charge/(credit):
2023 2022 2021
$m $m $m
Profit/(loss) before tax 6,899 2,501 (265)
Notional taxation charge at UK corporation tax rate of 23.5% (2022: 19%; 2021: 19%) 1,621 475 (50)
Differences in effective overseas tax rates
1
(224) (59) 1
Deferred tax (credit)/charge relating to change in tax rates
2
(66) (108) 54
Unrecognised deferred tax asset
3
341 68 32
Items not deductible for tax purposes 46 90 208
Items not chargeable for tax purposes – – (163)
Intellectual Property incentive regimes
4
(367) (265) –
Other items
5
(406) (941) (299)
Adjustments in respect of prior years
6
(7) (52) (163)
Total tax charge/(credit) for the year 938 (792) (380)
AstraZeneca is domiciled in the UK but operates in other countries where the tax rates and laws are different to those in the UK. The impact on
differences in effective overseas tax rates on the Group’s overall tax charge is noted above. Profits arising from our manufacturing operation in
Puerto Rico are granted special status and are taxed at a reduced rate compared with the normal rate of tax in that territory under a tax incentive
grant continuing until 2031. The Group receives intellectual property incentives in certain jurisdictions, resulting in a reduction to the tax charge in
the income statement of $367m in 2023.
Notes to the Group Financial Statements 165AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

4 Taxation continu ed
Notes to the Group Financial Statements
continu ed
Deferred tax
The total movement in the net deferred tax balance in the year was $1,555m. The movements are as follows:
Intangibles, Pensionand Elimination of Lossesand
Property,plant post-retirement unrealised profit Untaxed taxcredits Accrued
and equipment
1
benefits on inventory reserves
2
carriedforward expenses Other Total
$m $m $m $m $m $m $m $m
Net deferred tax balance at 1January 2021 (2,627) 656 1,807 (801) 714 660 111 520
Income statement 782 (166) (59) (139) 307 697 153 1,575
Other comprehensive income 52 83 – – – – 40 175
Equity – – – – – 4 10 14
Additions through business combinations
3
(3,744) 13 166 – 507 (1,263) 147 (4,174)
Exchange 57 (33) (53) 78 (10) (13) (12) 14
Net deferred tax balance at 31December 2021 (5,480) 553 1,861 (862) 1,518 85 449 (1,876)
Income statement
4
1,414 (55) 274 38 (126) 778 105 2,428
Other comprehensive income 72 (231) – – – – 16 (143)
Equity – – – – – – 38 38
Exchange 63 (36) (111) 108 (134) 17 (35) (128)
Net deferred tax balance at 31December 2022 (3,931) 231 2,024 (716) 1,258 880 573 319
Income statement
4
1,518 (69) 426 96 (308) (23) (133) 1,507
Other comprehensive income (16) 106 – – – – (23) 67
Equity – – – – – – (21) (21)
Additions (24) – – – 50 – (1) 25
Exchange (38) 15 (64) (40) 106 32 (34) (23)
Net deferred tax balance at 31December 2023
5
(2,491) 283 2,386 (660) 1,106 889 361 1,874
Imfinzi
The net deferred tax balance, before the offset of balances within countries, consists of:
Intangibles, Pensionand Elimination of Lossesand
Property,plant post-retirement unrealised profit Untaxed taxcredits Accrued
andequipment benefits on inventory reserves carriedforward expenses Other Total
$m $m $m $m $m $m $m $m
Deferred tax assets at 31December 2021 1,476 574 1,910 – 1,571 1,117 618 7, 26 6
Deferred tax liabilities at 31December 2021 (6,956) (21) (49) (862) (53) (1,032) (169) (9,142)
Net deferred tax balance at 31December 2021 (5,480) 553 1,861 (862) 1,518 85 449 (1,876)
Deferred tax assets at 31December 2022 1,499 276 2,048 – 1, 274 1,005 609 6,711
Deferred tax liabilities at 31December 2022 (5,430) (45) (24) (716) (16) (125) (36) (6,392)
Net deferred tax balance at 31December 2022 (3,931) 231 2,024 (716) 1,258 880 573 319
Deferred tax assets at 31December 2023 1,883 313 2,386 – 1,141 1,011 488 7,222
Deferred tax liabilities at 31December 2023 (4,374) (30) – (660) (35) (122) (127) (5,348)
Net deferred tax balance at 31December 2023 (2,491) 283 2,386 (660) 1,10 6 889 361 1,874
Analysed in the Consolidated Statement of Financial Position, after offset of balances within countries, as follows:
2023 2022 2021
$m $m $m
Deferred tax assets 4,718 3,263 4,330
Deferred tax liabilities (2,844) (2,944) (6,206)
Net deferred tax balance 1,874 319 (1,876)
166 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Unrecognised deferred tax assets
Deferred tax assets (DTA) of $1,251m (2022: $807m; 2021: $719m) have not been recognised in respect of deductible temporary differences because
it is not probable that future taxable profit will be available against which the Group can utilise the benefits therefrom.
2023 2023 2022 2022 2021 2021
Temporary Unrecognised Temporary Unrecognised Temporary Unrecognised
differences DTA differences DTA differences DTA
$m $m $m $m $m $m
Temporary differences expiring:
Within 10 years 87 22 104 26 4 1
More than 10 years 153 32 153 32 53 11
Indefinite 2,788 595 686 163 300 79
3,028 649 943 221 357 91
Tax credits and State tax losses expiring:
Within 10 years 152 115 101
More than 10 years 363 384 441
Indefinite 87 87 86
602 586 628
Total 1,251 807 719
5 Earnings per $0.25 Ordinary Share
2023 2022 2021
Profit for theyear attributable to equity holders ($m) 5,955 3,288 112
Basic earnings per Ordinary Share $3.84 $2.12 $0.08
Diluted earnings per Ordinary Share $3.81 $2.11 $0.08
Weighted average number of Ordinary Shares in issue for basic earnings (millions) 1,549 1,548 1,418
Dilutive impact of share options outstanding (millions) 13 12 9
Diluted weighted average number of Ordinary Shares in issue (millions) 1,562 1,560 1,427
The earnings figures used in the calculations above are post-tax. The weighted average number of Ordinary Shares in issue is calculated by taking
the number of Ordinary Shares outstanding each day weighted by the number of days that those shares were outstanding.
6 Segment information
The Group has reviewed its assessment of reportable segments under IFRS 8 ‘Operating Segments’ and concluded that the Group continues to
have one reportable segment.
This determination is considered to be a Key Judgement and this judgement has been taken with reference to the following factors:
1 The level of integration across the different functions of the Group’s pharmaceutical business:
AstraZeneca is engaged in a single business activity of pharmaceuticals and the Group does not have multiple operating segments. AstraZeneca’s
pharmaceuticals business consists of the discovery and development of new products, which are then manufactured, marketed and sold. All
of these functional activities take place (and are managed) globally on a highly integrated basis. These individual functional areas are not
managed separately.
2 The identification of the Chief Operating Decision Maker (CODM) and the nature and extent of the financial information reviewed by the CODM:
The SET, established and chaired by the CEO, is the vehicle through which the CEO exercises the authority delegated to him from the Board for
the management, development and performance of AstraZeneca as a whole. It is considered that the SET is AstraZeneca’s Chief Operating Decision
Making body (as defined by IFRS 8). The operation of the SET is principally driven by the management of the Commercial operations, R&D,
manufacturing and supply and enabling functions. All significant operating decisions are undertaken by the SET. While members of the SET have
responsibility for implementation of decisions in their respective areas, operating decision making is at SET level as a whole. Where necessary,
these are implemented through cross-functional sub-committees that consider the Group-wide impact of a new decision. For example, product
launch decisions would be initially considered by the SET and, on approval, passed to an appropriate sub team for implementation. The ability
of the enterprise to develop, produce, deliver and commercialise a wide range of pharmaceutical products are central to the SET decision-
making process.
In assessing performance, the SET reviews financial information on an integrated basis for the Group as a whole, substantially in the form of, and
on the same basis as, the Group’s IFRS Financial Statements. The high upfront cost of discovering and developing new products, coupled with
the relatively insignificant and stable unit cost of production, means that there is not the clear link that exists in many manufacturing businesses
between the revenue generated on an individual product sale and the associated cost and hence margin generated on a product. Consequently,
the profitability of individual drugs or classes of drugs is not considered a key measure of performance for the business and is not monitored by
the SET. The focus of additional financial information reviewed is at brand sales and Gross Margin level within specific geographies. Expenditure
analysis is completed for the science units, operations and enabling functions; there is no allocation of these centrally managed Group costs to
the individual product or brands. The bonus of SET members’ continues to be derived from the Group scorecard outcome as discussed in our
Directors’ Remuneration Report.
3 How resources are allocated:
Resources are allocated on a Group-wide basis according to need. In particular, capital expenditure, in-licensing, and R&D resources are allocated
between activities on merit, based on overall therapeutic considerations and strategy under the aegis of the Group’s Early-Stage Product
Committees and Late-Stage Product Committees.
Notes to the Group Financial Statements 167AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
6 Segment information continued
Notes to the Group Financial Statements
continu ed
Geographic areas
The following table shows information for Total Revenue by geographic area and material countries. The additional tables show the Operating profit
and Profit before tax made by companies located in that area, together with Non-current assets, Total assets, Assets acquired, Net operating
assets, and Property, plant and equipment owned by the same companies. Product Sales by geographic market are included in the area/country
where the legal entity resides and from which those sales were made.
Total Revenue
2023 2022 2021
$m $m $m
UK 3,368 3,117 3,245
Rest of Europe
France 1,152 1,107 915
Germany 2,099 1,902 1,486
Italy 813 735 577
Spain 847 738 578
Sweden 1,704 1,721 2,322
Others 3,110 2,706 1,949
9,725 8,909 7,8 27
The Americas
Canada 967 1,166 772
US 18,121 17, 278 12,047
Others 1,683 1,175 1,203
20,771 19,619 14,022
Asia, Africa& Australasia
Australia 390 571 547
China 5,872 5,743 6,002
Japan 3,640 3,986 3,395
Others 2,045 2,406 2,379
11,947 12,706 12,323
Total Revenue 45,811 44,351 37,417
Total Revenue outside of the UK totalled $42,443m for the year ended 31 December 2023 (2022: $41,234m; 2021: $34,172m).
Operating profit/(loss) Profit/(loss) before tax
2023 2022 2021 2023 2022 2021
$m $m $m $m $m $m
UK 665 1,120 (950) (577) 272 (1,477)
Rest of Europe 4,885 2,945 2,999 4,999 2,709 2,682
The Americas 1,495 (954) (1,936) 1,328 (1,140) (2,401)
Asia, Africa & Australasia 1,14 8 646 943 1,149 660 931
Continuing operations 8,193 3,757 1,056 6,899 2,501 (265)
Non-current assets
1, 2
Total assets
2023 2022 2021 2023 2022 2021
$m $m $m $m $m $m
UK 8,626 8,208 7,310 19,616 16,786 16,615
Rest of Europe 32,905 34,301 38,286 40,638 40,669 48,383
The Americas 26,524 25,425 26,333 34,754 32,990 34,301
Asia, Africa & Australasia 910 929 1,078 6,111 6,038 6,064
Continuing operations 68,965 68,863 73,007 101,119 96,483 105,363
Assets acquired
3
Net operating assets
4
2023 2022 2021 2023 2022 2021
$m $m $m $m $m $m
UK 812 2,301 810 5,275 3,863 3,239
Rest of Europe 1,770 522 26,527 32,920 32,726 40,161
The Americas 1,925 421 10,810 22,746 23,290 24,786
Asia, Africa & Australasia 117 51 94 1,405 1,895 736
Continuing operations 4,624 3,295 38,241 62,346 61,774 68,922
168 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
Property, plant and equipment
2023 2022 2021
$m $m $m
UK 2,831 2,526 2,542
Ireland 1,164 1,040 969
Sweden 1,678 1,472 1,593
US 2,371 2,176 2,660
Rest of the world 1,358 1,293 1,419
Continuing operations 9,402 8,507 9,18 3
Geographic markets
The table below shows Product Sales in each geographic market in which customers are located.
2023 2022 2021
$m $m $m
UK 978 996 1,206
Rest of Europe 8,201 7, 503 6,792
The Americas 20,855 20,126 14,893
Asia, Africa & Australasia 13,755 14,373 13,650
Continuing operations 43,789 42,998 36,541
Product Sales are recognised when control of the goods has been transferred to a third party. A significant proportion of this is upon delivery of
the products to wholesalers. One wholesaler (2022: one; 2021: one) individually represented greater than 10% of Product Sales. The value of Product
Sales to this wholesaler was $6,513m (2022: $5,387m; 2021: $4,862m).
7 Property, plant and equipment
Assetsin TotalProperty,
Landand Plantand course of plantand
buildings equipment construction equipment
$m $m $m $m
Cost
At 1January 2021 5,851 7,738 2,478 16,067
Additions through business combinations (Note 27) 542 339 254 1,135
Capital expenditure 9 31 1,112 1,152
Transfer of assets into use 236 611 (847) –
Disposals and other movements (92) (469) (200) (761)
Exchange adjustments (169) (347) (69) (585)
At 31December 2021 6,377 7,9 0 3 2,728 17,0 0 8
Capital expenditure 5 19 1,042 1,066
Transfer of assets into use 226 683 (909) –
Transfer of Assets held for sale (Note 18) (434) (293) – (727)
Disposals and other movements (425) (146) 28 (543)
Exchange adjustments (309) (610) (236) (1,15 5)
At 31December 2022 5,440 7,5 5 6 2,653 15,649
Additions through business combinations (Note 27) 2 10 – 12
Capital expenditure 9 43 1,402 1,454
Transfer of assets into use 959 1,15 8 (2 ,117) –
Disposals and other movements (6) (255) (11) (272)
Exchange adjustments 65 192 118 375
At 31December 2023 6,469 8,704 2,045 17, 218
Depreciation and impairment
At 1January 2021 2,826 4,990 – 7,816
Depreciation charge for the year 231 493 – 724
Impairment (reversal)/charge (1) 121 223 343
Disposals and other movements (74) (428) (223) (725)
Exchange adjustments (105) (228) – (333)
At 31December 2021 2,877 4,948 – 7,825
Depreciation charge for the year 286 566 – 852
Impairment charge/(reversal) 20 8 (28) –
Transferred to Assets held for sale (Note 18) (300) (277) – (577)
Disposals and other movements (227) (188) 28 (387)
Exchange adjustments (167) (404) – (571)
At 31December 2022 2,489 4,653 – 7,142
Depreciation charge for the year 241 492 – 733
Impairment charge 4 4 – 8
Disposals and other movements (13) (220) – (233)
Exchange adjustments 44 122 – 166
At 31December 2023 2,765 5,051 – 7, 816
Notes to the Group Financial Statements 169AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
continu ed
Assetsin TotalProperty,
Landand Plantand course of plantand
buildings equipment construction equipment
$m $m $m $m
Net book value
At 31December 2021 3,500 2,955 2,728 9,183
At 31December 2022 2,951 2,903 2,653 8,507
At 31December 2023 3,704 3,653 2,045 9,402
Impairment charges in 2021 totalling $343m were recognised for Plant and equipment and Assets in course of construction due to the rationalisation
of our manufacturing capacity and footprint across certain production sites as a result of restructuring programmes, including the PAAGR (see
Note 2). These charges were recognised in Cost of sales. The revised carrying value of the impacted assets is $nil, under fair value less costs to sell.
2023 2022 2021
$m $m $m
The net book value of land and buildings comprised:
Freeholds 2,976 2,555 2,985
Leaseholds 728 396 515
8 Leases
Right-of-use assets
Total
Land and Motor Right-of-use
buildings vehicles Other assets
$m $m $m $m
Cost
At 1January 2021 735 272 36 1,043
Additions through business combinations (Note 27) 255 8 – 263
Additions – separately acquired 145 98 2 245
Disposals and other movements 25 (44) (4) (23)
Exchange adjustments (27) (13) (1) (41)
At 31December 2021 1,13 3 321 33 1,487
Additions through business combinations (Note 27) 4 – – 4
Additions – separately acquired 140 81 14 235
Disposals and other movements (33) (58) (13) (104)
Exchange adjustments (62) (15) (2) (79)
At 31December 2022 1,182 329 32 1,543
Additions through business combinations (Note 27) 8 – – 8
Additions – separately acquired 220 219 5 444
Disposals and other movements (71) (57) (2) (130)
Exchange adjustments 13 4 1 18
At 31December 2023 1,352 495 36 1,883
Depreciation and impairment
At 1January 2021 247 117 13 377
Depreciation charge for the year 144 85 6 235
Disposals and other movements (54) (42) – (96)
Exchange adjustments (11) (6) – (17)
At 31December 2021 326 154 19 499
Depreciation charge for the year 160 80 6 246
Impairment charge 2 – – 2
Disposals and other movements (54) (50) (10) (114)
Exchange adjustments (23) (8) (1) (32)
At 31December 2022 411 176 14 601
Depreciation charge for the year 170 98 7 275
Impairment charge 14 – – 14
Disposals and other movements (53) (61) (2) (116)
Exchange adjustments 7 2 – 9
At 31December 2023 549 215 19 783
Net book value
At 31December 2021 807 167 14 988
At 31December 2022 771 153 18 942
At 31December 2023 803 280 17 1,100
7 Property, plant and equipment continued
170 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Lease liabilities
2023 2022 2021
$m $m $m
The present value of lease liabilities is as follows:
Within one year (271) (228) (233)
Later than one year and not later than five years (657) (549) (544)
Later than five years (200) (176) (210)
Total lease liabilities (1,12 8) (953) (987)
The interest expense on lease liabilities included within Finance expense was $33m (2022: $24m; 2021: $22m).
The total cash outflow for leases in 2023 was $301m (2022: $268m; 2021: $262m).
The Group has entered into lease contracts that have not yet commenced. The nominal value of estimated future lease payments under these lease
contracts approximates $1,615m as of 31 December 2023. Of this value, $1,348m relates to a property lease in the US which is expected to commence
in 2026 with a lease term of 15 years.
In 2022 the Group entered into a sale and leaseback agreement in relation to the Waltham R&D site in MA, US. Prior to the sale, the carrying value
of the Property, plant and equipment was $124m. Cash proceeds of $265m were received, recorded within Disposal of property, plant and equipment
within the Consolidated Statement of Cash Flows, and a gain on disposal of $125m was recorded within Other operating income and expense
within the Consolidated Statement of Comprehensive Income. A lease liability and a corresponding right-of-use asset were recorded of $28m and
$13m, respectively.
9 Goodwill
2023 2022 2021
$m $m $m
Cost
At 1January 20,131 20, 311 12,16 4
Additions through business combinations (Note 27) 158 15 8,287
Exchange and other adjustments 72 (195) (140)
At 31December 20,361 20,131 20,311
Amortisation and impairment losses
At 1January 311 314 319
Exchange and other adjustments 2 (3) (5)
At 31December 313 311 314
Net book value
At 31December 20,048 19,820 19,997
Goodwill is tested for impairment at the operating segment level, this being the level at which goodwill is monitored for internal management purposes.
As detailed in Note 6, the Group does not have multiple operating segments and is engaged in a single business activity of pharmaceuticals.
Recoverable amount is determined on a fair value less costs to sell basis using the market value of the Company’s outstanding Ordinary Shares.
Our market capitalisation is compared to the book value of the Group’s net assets and this indicates a significant surplus at 31 December 2023
(and 31 December 2022 and 31 December 2021). No goodwill impairment was identified.
Notes to the Group Financial Statements 171AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
continu ed
10 Intangible assets
Product, Software
marketingand Other development
distributionrights intangibles costs Total
$m $m $m $m
Cost
At 1January 2021 42,677 2,642 1,288 46,607
Additions through business combinations (Note 27) 26,455 430 70 26,955
Additions – separately acquired 587 6 119 712
Transferred to Assets held for sale (Note 18) (1,266) (47) – (1,313)
Disposals (801) (402) (23) (1,226)
Exchange and other adjustments (1,062) (18) (22) (1,102)
At 31December 2021 66,590 2,611 1,432 70,633
Additions through business combinations (Note 27) – 46 – 46
Additions – separately acquired 2,051 12 105 2,168
Disposals (57) (105) (36) (198)
Exchange and other adjustments (1,799) (122) (106) (2,027)
At 31December 2022 66,785 2,442 1,395 70,622
Additions through business combinations (Note 27) 65 35 – 100
Additions – separately acquired 2,530 200 170 2,900
Disposals (669) – (14) (683)
Exchange and other adjustments 496 30 24 550
At 31December 2023 69,207 2,707 1,575 73,489
Amortisation and impairment losses
At 1January 2021 22,564 2,128 968 25,660
Amortisation for year 2,908 172 63 3,143
Impairment charges 2,067 – 18 2,085
Transferred to Assets held for sale (Note 18) (931) (14) – (945)
Disposals (797) (402) (21) (1,220)
Exchange and other adjustments (535) (21) (26) (582)
At 31December 2021 25,276 1,863 1,002 28,141
Amortisation for year 3,899 181 76 4,156
Impairment charges 236 82 – 318
Impairment reversals (77) – (17) (94)
Disposals (55) (105) (20) (180)
Exchange and other adjustments (887) (76) (63) (1,026)
At 31December 2022 28,392 1,945 978 31,315
Amortisation for year 3,771 75 80 3,926
Impairment charges 434 – – 434
Disposals (667) – (12) (679)
Exchange and other adjustments 336 41 27 404
At 31December 2023 32,266 2,061 1,073 35,400
Net book value
At 31December 2021 41,314 748 430 42,492
At 31December 2022 38,393 497 417 39,307
At 31December 2023 36,941 646 502 38,089
2023 2022 2021
$m $m $m
Net book value
Current intangible assets – – 105
Non-current intangible assets 38,089 39,307 42,387
At 31December 38,089 39,307 42,492
Other intangibles consist mainly of research and device technologies and the Alexion brand name. Included within Software development costs are
assets currently in development that will commence amortisation when ready for use.
Included within Additions − separately acquired are amounts of $625m (2022: $1,135m; 2021: $124m), relating to deferred payments and other non-cash
consideration for the acquisition of Product, marketing and distribution rights, which are not reflected in the current year Consolidated Statement of
Cash Flows. Disposals include amounts related to fully depreciated assets that are no longer in use by the Group.
172
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Amortisation charges are recognised in profit as follows:
Product, Software
marketingand Other development
distributionrights intangibles costs Total
$m $m $m $m
Year ended 31December 2021
Cost of sales 66 – – 66
Research and development expense – 33 – 33
Selling, general and administrative expense 2,842 138 63 3,043
Other operating income and expense – 1 – 1
Total 2,908 172 63 3,143
Year ended 31December 2022
Cost of sales 32 – – 32
Research and development expense – 30 – 30
Selling, general and administrative expense 3,867 151 76 4,094
Total 3,899 181 76 4,156
Year ended 31December 2023
Cost of sales 32 – – 32
Research and development expense – 28 – 28
Selling, general and administrative expense 3,739 47 80 3,866
Total 3,771 75 80 3,926
Net impairment charges are recognised in profit as follows:
Product, Software
marketingand Other development
distributionrights intangibles costs Total
$m $m $m $m
Year ended 31December 2021
Research and development expense 1,464 – – 1,464
Selling, general and administrative expense 603 – 18 621
Total 2,067 – 18 2,085
Year ended 31December 2022
Research and development expense 95 – – 95
Selling, general and administrative expense 64 82 (17) 129
Total 159 82 (17) 224
Year ended 31December 2023
Research and development expense 417 – – 417
Selling, general and administrative expense 17 – – 17
Total 434 – – 434
Impairment charges and reversals
We perform a rigorous impairment trigger assessment for all our intangible assets. Intangible assets under development and not available for use
are tested annually for impairment and other intangible assets are tested when there is an indication of impairment loss or reversal. Where testing
is required, the recoverable amount of the assets is estimated in order to determine the extent of the impairment loss or reversal. Where it is not
possible to estimate the recoverable amount of an individual asset, the Group estimates the recoverable amount of the Cash Generating Unit (CGU)
to which it belongs. The Group considers that as the intangible assets are linked to individual products and that product cash flows are considered
to be largely independent of other product cash flows, the CGU for intangibles is at the product level. Group-level budgets and forecasts include
forecast capital investment and operational impacts related to sustainability projects, as well as inflationary impacts, and form the basis for the
value in use models used for impairment testing.
An asset’s recoverable amount is determined as the higher of an asset’s or CGU’s fair value less costs to sell or value in use, in both cases using
discounted cash flow calculations where the asset’s expected post-tax cash flows are risk-adjusted over their estimated remaining period of
expected economic benefit. Where the value in use approach is used, the post-tax risk-adjusted cash flows are discounted using AstraZeneca’s
post-tax weighted average cost of capital (7.5% for 2023, 7% for 2022 and 2021) which is a nominal rate. There is no material difference in the
approach taken to using pre-tax cash flows and a pre-tax rate compared to post-tax cash flows and a post-tax rate, as required by IAS 36. Where
fair value less costs to sell is used to determine recoverable value, the discount rate is assessed with reference to a market participant; this is not
usually materially different to the AstraZeneca post-tax weighted average cost of capital of 7.5%. Intangible assets have been tested for impairment
under the value in use basis at risk-adjusted post-tax discount rates ranging between 7.5% to 9.5%.
Notes to the Group Financial Statements 173AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
10 Intangible assets co n tinued
Key assumptions and significant estimates used in calculating the recoverable amounts are highly sensitive and specific to the nature of the
Group’s activities including:
> outcome of R&D activities
> probability of technical and regulatory success
> market volume, share and pricing (to derive peak year sales)
> amount and timing of projected future cash flows
> sales erosion curves following patent expiry.
Whilst the intangible assets portfolio is generally exposed to significant impairment risk within the next financial year, no sensitivities have been
disclosed since no specific asset has been identified as having a significant risk of a material impairment arising from reasonably possible changes
in key assumptions.
For assets held at fair value less costs to sell, we make appropriate adjustments to reflect market participant assessments.
In 2023, the Group recorded impairment charges of $17m in respect of launched products. Impairment charges recorded against products in
development totalled $417m, including $244m related to ALXN1840 which was fully impaired following the decision to discontinue development.
In 2022, the Group recorded impairment charges of $146m in respect of launched products. Impairment charges recorded against products in
development totalled $172m due to decisions made to terminate the related activities.
In 2021, the Group recorded impairment charges of $603m in respect of launched products, including Bydureon ($469m, revised carrying amount
of $50m) under value in use model, roxadustat ($121m, revised carrying amount of $215m) under value in use model and other launched products
totalling $13m.
Impairment charges recorded against products in development in 2021, based on fair value less costs to sell, totalled $1,464m, principally Ardea
($1,172m) which was fully impaired following the decision to discontinue development of verinurad. The remaining impairments relate to full
impairments of various products in development, due to either management’s decision to discontinue development as part of a Group-wide
portfolio prioritisation review, or due to the outcome of research activities.
The Group has performed an assessment on assets which have had impairments recorded in previous periods to determine if any reversals of
impairments were required. No impairment reversals were recorded in 2023. Impairment reversals of $94m were recorded in 2022, including $77m
in respect of products in development. No impairment reversals were recorded in 2021.
When launched products are partially impaired, the carrying values of these assets in future periods are particularly sensitive to changes in forecast
assumptions, including those assumptions set out above, as the asset is impaired down to its recoverable amount.
Significant assets
Carryingvalue Remainingamortisation
$m period
C5 franchise (Soliris/Ultomiris) intangible assets arising from the acquisition of Alexion 14,356 4 to 12 years
Intangible assets arising from the acquisition of Acerta Pharma 4,335 9 years
Strensiq, Kanuma, Andexxa intangible assets arising from the acquisition of Alexion 4,147 9 to 15 years
Enhertu intangible assets acquired from Daiichi Sankyo 2,831 10 years
Intangible asset products in development arising from the acquisition of Alexion
1
2,489 Not amortised
Intangible assets arising from the acquisition of ZS Pharma Inc. 1,838 8 years
Other intangible assets acquired from Daiichi Sankyo
1
989 Not amortised
Baxdrostat intangible asset acquired from CinCor Pharma, Inc.
1
780 Not amortised
Airsupra intangible asset 524 11 years
Intangible assets arising from the restructuring of a historical joint venture with MSD 472 3 to 6 years
Farxiga/Forxiga intangible assets acquired from BMS 426 3 years
Intangible assets arising from the acquisition of Pearl Therapeutics, Inc 412 5 to 6 years
Monalizumab intangible assets acquired from Innate Pharma
1
370 Not amortised
RSV franchise assets arising from the acquisition of MedImmune 305 2 years
Rare disease portfolio assets acquired from Pfizer
1
300 Not amortised
The intangible asset baxdrostat recognised on acquisition of CinCor Pharma, Inc. in 2023 was assessed under the optional concentration test
in IFRS 3 and was determined to be an asset acquisition, as substantially all of the value of the gross assets acquired was concentrated in this
single asset.
The acquisition of Pfizer’s pre-clinical rare disease gene therapy portfolio in 2023 was assessed under IFRS 3 and the transaction was treated as
an asset acquisition.
174
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

11 Investments in associates and joint ventures
2023 2022 2021
$m $m $m
At 1January 76 69 39
Additions 80 26 92
Share of after tax losses (12) (5) (64)
Exchange and other adjustments 3 (14) 2
At 31December 147 76 69
On 1 November 2023, AstraZeneca entered into an agreement with Cellectis, a clinical-stage biotechnology company, to accelerate the development
of next generation therapeutics in areas of high unmet medical need, including oncology, immunology and rare diseases. Under the terms of the
agreement, AstraZeneca contributed $80m in funds and holds a 22% interest in the associate entity.
On 29 January 2021, AstraZeneca entered into an agreement with IHP Holdings Limited to create and run an online platform (iHospital) offering
consultations with physicians, repeat prescriptions and e-pharmacy in China. The agreement resulted in the formation of a new entity, IHP HK 27
Holdings Limited. AstraZeneca contributed $30m in initial funds and holds a 50% interest in the associate entity.
On 1 December 2020, AstraZeneca and China International Capital Corporation (CICC) entered into an agreement to set up a Global Healthcare
Industrial Fund to drive healthcare system innovation by leveraging local capital and accelerating China-related innovation incubation. The agreement
resulted in the formation of a new entity, Wuxi AstraZeneca-CICC Venture Capital Partnership (Limited Partnership). AstraZeneca holds a 22%
interest in the associate entity and contributed $1m in initial funds in 2020, with contributions of $45m and $21m made in 2021 and 2022 respectively.
On 23 September 2021, AstraZeneca entered into an agreement with VaxEquity Limited to collaborate and develop self-amplifying RNA technology
with the aim of generating treatments for target diseases. AstraZeneca contributed $14m in initial funds and holds a 40% interest in the associate entity.
On 23 February 2018, AstraZeneca entered into an agreement with a consortium of investors to form a new, US-domiciled standalone company
called Viela Bio. In February 2021, AstraZeneca agreed to divest its 26.7% ownership in Viela Bio, as part of the acquisition of Viela by Horizon
Therapeutics plc. AstraZeneca received cash proceeds and profit of $776m upon closing with the profit recorded as Other operating income. In 2021,
prior to divestment, the Group provided transitional research and development services to Viela Bio, comprising $1m of passed-through third-party
costs incurred by the Group on behalf of Viela Bio.
On 27 November 2017, AstraZeneca entered into a joint venture agreement with Chinese Future Industry Investment Fund (FIIF), to discover, develop
and commercialise potential new medicines to help address unmet medical needs globally, and to bring innovative new medicines to patients in
China more quickly. The agreement resulted in the formation of a joint venture entity based in China, Dizal (Jiangsu) Pharmaceutical Co., Limited
(Dizal). Since its establishment, AstraZeneca has contributed $80m in cash to the joint venture entity and has a 27% interest in the joint venture.
On 1 December 2015, AstraZeneca entered into a joint venture agreement with Fujifilm Kyowa Kirin Biologics Co., Ltd. to develop a biosimilar using
the combined capabilities of the two parties. The agreement resulted in the formation of a joint venture entity based in the UK, Centus Biotherapeutics
Limited (Centus). Since its establishment, AstraZeneca has contributed $135m in cash to the joint venture entity and has a 50% interest in the joint
venture. On 26 April 2023, Centus entered a voluntary liquidation process.
All investments are accounted for using the equity method. At 31 December 2023, unrecognised losses in associates and joint ventures totalled
$140m (2022: $92m; 2021: $73m) which have not been recognised due to the investment carrying value reaching $nil value.
Aggregated summarised financial information for the associate and joint venture entities is set out below:
2023 2022 2021
$m $m $m
Non-current assets 424 290 215
Current assets 362 300 506
Total liabilities (287) (72) (99)
Net assets 499 518 622
Amount attributable to AstraZeneca 85 91 65
Goodwill 52 – –
Exchange adjustments 10 (15) 4
Carrying value of investments in associates and joint ventures 147 76 69
Joint contractual arrangements were entered into between AstraZeneca and Daiichi Sankyo Company Limited (Daiichi Sankyo); in March 2019 for
the co-development and co-commercialisation of Enhertu and in July 2020 for the co-development and co-commercialisation of Dato-DXd. Each
party shares global pre-tax net income from the collaboration on a 50:50 basis (with the exception of Japan where Daiichi Sankyo maintains exclusive
rights and AstraZeneca receives a royalty). The joint operation is not structured through a separate legal entity, and it operates from AstraZeneca
and Daiichi Sankyo’s respective principal places of business.
Notes to the Group Financial Statements 175AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
12 Other investments
2023 2022 2021
$m $m $m
Non-current investments
Equity securities at fair value through Other comprehensive income 1,530 1,056 1,168
Fixed income securities at fair value through profit or loss – 10 –
Total 1,530 1,066 1,168
Current investments
Fixed income securities at fair value through profit or loss 20 13 16
Cash collateral pledged to counterparties 102 162 –
Fixed deposits – 64 53
Total 122 239 69
Other investments held at FVOCI include equity securities which are not held for trading and which the Group has irrevocably elected at initial
recognition to recognise in this category. Other investments held at FVPL mainly comprise fixed income securities that the Group holds to sell.
The fair value of listed investments is based on year end quoted market prices. Fixed deposits and Cash collateral pledged to counterparties are
held at amortised cost with carrying value being a reasonable approximation of fair value given their short-term nature.
Cash collateral pledged to counterparties relates to collateral pledged on derivatives entered into to hedge the Group’s risk exposures. In 2022,
following significant foreign currency volatility increasing the collateral requirements, the Group revised its presentation to ‘Other investments’.
In 2021 amounts of $47m are presented within Cash and cash equivalents.
Fair value hierarchy
The table below analyses equity securities and bonds, contained within Other investments and carried at fair value, by valuation method. The different
levels have been defined as follows:
> Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities
> Level 2: inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices)
or indirectly (i.e. derived from prices)
> Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs).
2023 2023 2022 2022 2021 2021
FVPL FVOCI FVPL FVOCI FVPL FVOCI
$m $m $m $m $m $m
Level 1 20 1,217 13 880 16 1,064
Level 2 – – – – – –
Level 3 – 313 10 176 – 104
Total 20 1,530 23 1,056 16 1,168
Assets are transferred in or out of each Level on the date of the event or change in circumstances that caused the transfer.
Equity securities that are analysed at Level 3 include investments in private biotech companies. In the absence of specific market data, these unlisted
investments are held at fair value based on the cost of investment and adjusting as necessary for impairments and revaluations on new funding
rounds, which approximates to fair value. Movements in Level 3 investments are detailed below:
2023 2023 2022 2022 2021
FVPL FVOCI FVPL FVOCI FVOCI
$m $m $m $m $m
At 1January 10 176 – 104 217
Additions – 127 10 32 1
Revaluations 3 14 – 50 –
Net transfers out from Level 3 to Level 1 – – – (4) (113)
Disposals (13) (8) – (5) –
Impairments and exchange adjustments – 4 – (1) (1)
At 31December – 313 10 176 104
176 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

13 Derivative financial instruments
Non-current Current Current Non-current
assets assets liabilities liabilities Total
$m $m $m $m $m
Interest rate swaps related to instruments designated at fair value through profit or loss
1
25 – – – 25
Cross currency swaps designated in a net investment hedge 62 – – (2) 60
Cross currency swaps designated in a cash flow hedge – – – (43) (43)
Forward FX designated in a cash flow hedge
2
– 13 – – 13
Other derivatives 15 70 (79) – 6
31December 2021 102 83 (79) (45) 61
Non-current Current Current Non-current
assets assets liabilities liabilities Total
$m $m $m $m $m
Interest rate swaps related to instruments designated at fair value through profit or loss
1
– 1 – – 1
Cross currency swaps designated in a net investment hedge 55 – – (4) 51
Cross currency swaps designated in a cash flow hedge – – – (160) (160)
Forward FX designated in a cash flow hedge
2
– 1 (13) – (12)
Other derivatives 19 85 (80) – 24
31December 2022 74 87 (93) (164) (96)
Non-current Current Current Non-current
assets assets liabilities liabilities Total
$m $m $m $m $m
Cross currency swaps designated in a net investment hedge 100 – – (1) 99
Cross currency swaps designated in a cash flow hedge 116 – (30) (37) 49
Forward FX designated in a cash flow hedge
2
– 19 (4) – 15
Other derivatives 12 97 (122) – (13)
31December 2023 228 116 (156) (38) 150
All derivatives are held at fair value and fall within Level 2 of the fair value hierarchy as defined in Note 12, except for an equity warrant which falls
within Level 3 (valued at $12m (2022: $19m; 2021: $15m), held within Non-current assets). None of the derivatives have been reclassified in the year.
The fair value of interest rate swaps and cross currency swaps is estimated using appropriate zero coupon curve valuation techniques to discount
future contractual cash flows based on rates at the current year end.
The fair value of forward foreign exchange contracts and currency options are estimated by cash flow accounting models using appropriate yield
curves based on market forward foreign exchange rates at the year end. The majority of forward foreign exchange contracts for existing transactions
had maturities of less than one month from year end.
The interest rates used to discount future cash flows for fair value adjustments, where applicable, are based on market swap curves at the reporting
date, and were as follows:
2023 2022 2021
Derivatives 0.1% to 5.3% 0.1% to 4.7% (0.5)% to 3.6%
14 Non-current other receivables
2023 2022 2021
$m $m $m
Prepayments 274 243 391
Accrued income 52 44 61
Retirement benefit scheme surpluses (Note 22) 92 90 –
Other receivables 385 458 443
Non-current other receivables 803 835 895
Prepayments include $nil (2022: $nil; 2021: $92m) in relation to our research collaboration with Moderna. Other receivables include $51m (2022: $71m;
2021: $44m) owed by FibroGen, Inc. for promotional activity in China pursuant to the roxadustat collaboration.
15 Inventories
2023 2022 2021
$m $m $m
Raw materials and consumables 1,531 1,422 1,755
Inventories in process 2,325 1,864 5,216
Finished goods and goods for resale 1,568 1,413 2,012
Inventories 5,424 4,699 8,983
The Group recognised $6,038m (2022: $9,618m; 2021: $9,640m) of inventories as an expense within Cost of sales during the year.
Inventory write-downs in the year amounted to $574m (2022: $479m; 2021: $552m), principally arising from the reassessment of usage or demand
expectations prior to inventory expiration.
Notes to the Group Financial Statements 177AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
16 Current trade and other receivables
2023 2022 2021
$m $m $m
Trade receivables 8,452 7, 271 6,054
Less: Expected credit loss provision (Note 28) (45) (59) (23)
8,407 7,212 6,031
Other receivables 1,639 1,659 1,808
Prepayments 1,617 1,329 1,512
Government grants receivable 11 25 –
Accrued income 452 296 293
Trade and other receivables 12,126 10,521 9,644
Trade receivables include $1,977m (2022: $2,470m; 2021: $1,865m) measured at FVOCI classified ‘hold to collect and sell’ as they are due from
customers that the Group has the option to factor, or relate to bank acceptance drafts received in settlement of trade receivables per common
practice in China.
All other financial assets included within Current trade and other receivables are held at amortised cost with carrying value being a reasonable
approximation of fair value.
17 Cash and cash equivalents
2023 2022 2021
$m $m $m
Cash at bank and in hand 1,325 1,411 1,461
Short-term deposits 4,515 4,755 4,868
Cash and cash equivalents 5,840 6,166 6,329
Unsecured bank overdrafts (203) (183) (291)
Cash and cash equivalents in the cash flow statement 5,637 5,983 6,038
AstraZeneca invests in constant net asset value funds, low-volatility net asset value funds and short-term variable net asset value funds with same day
access for subscription and redemption. These investments fail the ‘solely payments of principal and interest’ test criteria under IFRS 9. They are
therefore measured at FVPL, although the fair value is materially the same as amortised cost.
Non-cash and other movements, within operating activities in the Consolidated Statement of Cash Flows, includes:
2023 2022 2021
$m $m $m
Share-based payments charge for the period 579 619 615
Settlement of share plan awards (650) (592) (570)
Pension contributions (188) (205) (174)
Pension charges recorded in operating profit 55 101 136
Long-term provision charges recorded in operating profit 460 87 270
(Gain)/loss on disposal of tangible assets (41) (112) 4
Update to the contractual relationships for Beyfortus (nirsevimab) (729) – –
Foreign exchange and other
1
128 (590) (186)
Total operating activities non-cash and other movements (386) (692) 95
18 Assets held for sale
Assets held for sale amount to $nil (2022: $150m; 2021: $368m).
In 2022, Assets held for sale comprised Property, plant and equipment assets relating to the West Chester site in Ohio, US. The transaction closed
on 30 January 2023.
In 2021, Assets held for sale comprised Intangible assets relating to the rights to certain respiratory assets acquired from Almirall and Actavis plc.
(including Tudorza and Duaklir). The transaction closed on 4 January 2022.
178
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
19 Interest-bearing loans and borrowings
Repayment 2023 2022 2021
dates $m $m $m
Current liabilities
Bank overdrafts On demand 203 183 291
Other short-term borrowings excluding overdrafts 97 78 3
Collateral received from derivative counterparties 215 89 93
Lease liabilities 271 228 233
Floating rate notes US dollars 2022 – – 250
2.375% Callable bond US dollars 2022 – – 999
0.3% Callable bond US dollars 2023 – 1,399 –
2023 Floating bank loan US dollars 2023 – 2,000 –
Floating rate notes US dollars 2023 – 400 –
3.5% Callable bond US dollars 2023 – 849 –
7% Guaranteed debentures US dollars 2023 – 294 –
0.75% Callable bond euros 2024 995 – –
0.7% Callable bond US dollars 2024 1,600 – –
2024 Floating rate bank loans US dollars 2024 2,000 – –
Other loans (including commercial paper) Within one year 19 22 24
Total 5,400 5,542 1,893
Non-current liabilities
Lease liabilities 857 725 754
0.3% Callable bond US dollars 2023 – – 1,397
2023 Floating bank loan US dollars 2023 – – 1,998
Floating rate notes US dollars 2023 – – 400
3.5% Callable bond US dollars 2023 – – 848
7% Guaranteed debentures US dollars 2023 – – 320
0.75% Callable bond euros 2024 – 957 1,014
0.7% Callable bond US dollars 2024 – 1,598 1,598
2024 Floating bank loans US dollars 2024 – 1,998 1,997
3.375% Callable bond US dollars 2025 1,994 1,992 1,988
0.7% Callable bond US dollars 2026 1,19 6 1,195 1,193
1.2% Callable bond US dollars 2026 1,248 1,246 1,245
3.625% Callable bond euros 2027 829 – –
3.125% Callable bond US dollars 2027 747 746 745
4.875% Callable bond US dollars 2028 1,095 – –
1.25% Callable bond euros 2028 879 845 896
1.75% Callable bond US dollars 2028 1,246 1,245 1,244
4% Callable bond US dollars 2029 995 995 994
0.375% Callable bond euros 2029 881 846 898
4.9% Callable bond US dollars 2030 645 – –
1.375% Callable bond US dollars 2030 1,294 1,293 1,292
2.25% Callable bond US dollars 2031 747 747 746
5.75% Non-callable bond pound sterling 2031 444 420 470
3.75% Callable bond euros 2032 827 – –
4.875% Callable bond US dollars 2033 497 – –
6.45% Callable bond US dollars 2037 2,725 2,724 2,724
4% Callable bond US dollars 2042 989 988 988
4.375% Callable bond US dollars 2045 981 981 980
4.375% Callable bond US dollars 2048 738 737 737
2.125% Callable bond US dollars 2050 487 487 486
3% Callable bond US dollars 2051 735 735 734
Other loans US dollars 146 190 202
Total 23,222 23,690 28,888
Total interest-bearing loans and borrowings
1, 2
28,622 29,232 30,781
Notes to the Group Financial Statements
179AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
19 Interest-bearing loans and borrowings c ontinued
Notes to the Group Financial Statements
continu ed
Total Total Total
loans and loans and loans and
borrowings borrowings borrowings
2023 2022 2021
$m $m $m
At 1January 29,232 30,781 20,380
Changes from financing cash flows
Issue of loans and borrowings 3,816 – 12,929
Repayment of loans and borrowings (4,942) (1,271) (4,759)
Movement in short-term borrowings 161 74 (276)
Repayment of obligations under leases (268) (244) (240)
Total changes in cash flows arising on financing activities from borrowings (1,233) (1,441) 7,6 5 4
Movement in overdrafts 20 (85) 31
New lease liabilities 444 253 503
Additions through business combinations – 5 2,523
Exchange 187 (287) (378)
Other movements (28) 6 68
At 31December 28,622 29,232 30,781
Also included within cash flows arising from financing activities within the Consolidated Statement of Cash Flows is a $867m cash outflow (2022:
outflow of $920m; 2021: $nil) related to the Acerta Pharma share purchase liability which has a closing liability at 31 December 2023 of $833m
(2022: $1,646m; 2021: $2,458m) within Trade and other payables (see Note 20).
Set out below is a comparison by category of carrying values and fair values of all the Group’s interest-bearing loans and borrowings:
Instruments Instruments Total
designated designatedin Amortised carrying Fair
atfairvalue
1
cashflowhedge
2
cost value value
$m $m $m $m $m
2021
Overdrafts – – 291 291 291
Lease liabilities due within one year – – 233 233 233
Lease liabilities due after more than one year – – 754 754 754
Loans and borrowings due within one year – – 1,369 1,369 1,378
Loans and borrowings due after more than one year 320 1,910 25,904 28,134 30,596
Total at 31December 2021 320 1,910 28,551 30,781 33,252
2022
Overdrafts – – 183 183 183
Lease liabilities due within one year – – 228 228 228
Lease liabilities due after more than one year – – 725 725 725
Loans and borrowings due within one year 294 – 4,837 5,131 5,105
Loans and borrowings due after more than one year – 1,802 21,163 22,965 21,657
Total at 31December 2022 294 1,802 27,13 6 29,232 27,898
2023
Overdrafts – – 203 203 203
Lease liabilities due within one year – – 271 271 271
Lease liabilities due after more than one year – – 857 857 857
Loans and borrowings due within one year – 995 3,931 4,926 4,887
Loans and borrowings due after more than one year – 2,535 19,830 22,365 21,769
Total at 31December 2023 – 3,530 25,092 28,622 27,987
The fair value of fixed-rate publicly traded debt is based on year end quoted market prices; the fair value of floating rate debt is nominal value, as
mark-to-market differences would be minimal given the frequency of resets. The carrying value of loans designated at FVPL is the fair value; this falls
within the Level 1 valuation method as defined in Note 12. For loans designated in a fair value hedge relationship, carrying value is initially measured
at fair value and remeasured for fair value changes in respect of the hedged risk at each reporting date. All other loans are held at amortised cost.
Fair values, as disclosed in the table above, are all determined using the Level 1 valuation method as defined in Note 12, with the exception of
overdrafts and lease liabilities, where fair value approximates to carrying values.
180
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

A loss of $6m was made during the year on the fair value of bonds designated as FVPL. A gain of $25m has been made on these bonds since
designation. Under IFRS 9, the Group records the component of fair value changes relating to the component of own credit risk through Other
comprehensive income. Changes in credit risk had no material effect on any other financial assets and liabilities recognised at fair value in the
Group Financial Statements. The change in fair value attributable to changes in credit risk is calculated as the change in fair value not attributable
to market risk.
The interest rates used to discount future cash flows for fair value adjustments, where applicable, are based on market swap curves at the reporting
date, and were as follows:
2023 2022 2021
Loans and borrowings n/a to n/a
1
4.3% to 4.9% 0.1% to 0.6%
20 Trade and other payables
2023 2022 2021
$m $m $m
Current liabilities
Trade payables 3,267 2,550 2,824
Value-added and payroll taxes and social security 492 468 463
Rebates, chargebacks, returns and other revenue accruals 7,817 6,078 5,298
Clinical trial accruals 1,424 1,417 1,047
Other accruals 6,112 5,551 5,649
Collaboration Revenue contract liabilities 7 12 12
Vaccine contract liabilities 142 169 1,003
Deferred government grant income – 1 67
Contingent consideration 966 757 849
Acerta Pharma share purchase liability (Note 26) 833 867 920
Other payables 1,314 1,170 806
Total 22,374 19,040 18,938
Non-current liabilities
Accruals 36 37 25
Collaboration Revenue contract liabilities 7 14 26
Contingent consideration 1,171 1,465 2,016
Acerta Pharma share purchase liability (Note 26) – 779 1,538
Other payables 1,446 1,975 1,328
Total 2,660 4,270 4,933
Included within Rebates, chargebacks, returns and other revenue accruals are contract liabilities of $102m (2022: $87m; 2021: $99m). The revenue
recognised in the year from opening contract liabilities is $88m, comprising $76m relating to other revenue accruals and $12m Collaboration Revenue
contract liabilities. The major markets with Rebates, chargebacks, returns and other revenue accruals are the US where the liability at 31 December
2023 amounted to $5,116m (2022: $3,961m; 2021: $3,172m), of which Rare Disease comprises $190m (2022: $139m; 2021: $127m), and China where
the liability at 31 December 2023 amounted to $567m (2022: $579m; 2021: $814m).
Trade payables includes $123m (2022: $67m; 2021: $44m) due to suppliers that have signed up to a supply chain financing programme, under which
the suppliers can elect on an invoice-by-invoice basis to receive a discounted early payment from the relationship bank rather than being paid in line
with the agreed payment terms. If the option is taken, the Group’s liability is assigned by the supplier to be due to the relationship bank rather than
the supplier. The value of the liability payable by the Group remains unchanged. The Group assesses the arrangement against indicators to assess
if debts which vendors have sold to the funder under the supplier financing scheme continue to meet the definition of trade payables or should be
classified as borrowings. At 31 December 2023, the payables met the criteria of Trade payables. The supply chain financing programme operates
in the US, UK, Sweden, China and Germany, and as at 31 December 2023, the programme had 461 suppliers enrolled across these countries.
Vaccine contract liabilities relate to amounts received from customers, primarily government bodies, in advance of supply of product.
Deferred government grant income relates to government grants received or receivable but for which the related expenses have not been incurred.
Included within current Other payables are liabilities to Daiichi Sankyo totalling $199m (2022: $100m; 2021: $nil) resulting from the collaboration
agreement in relation to Enhertu entered into in March 2019 and $nil (2022: $nil; 2021: $324m) in relation to Dato-DXd entered into in July 2020.
Additionally, included within non-current Other payables are liabilities totalling $774m (2022: $1,125m; 2021: $100m) as a result of the Enhertu
collaboration agreement and $464m (2022: $nil; 2021: $nil) as a result of the Airsupra collaboration agreement.
In November 2020, Calquence received marketing approval in the EU, which removed all remaining conditionality in respect of the Acerta Pharma
put and call options regarding the non-controlling interest; the option was exercised in April 2021 (see Note 26). The payments will be made in similar
annual instalments in 2022 through to 2024, with the first payment of $920m made in 2022 and the second payment of $867m made in 2023, with
a closing liability as at 31 December 2023 of $833m (2022: $1,646m; 2021: $2,458m). Interest arising from amortising the liability is included within
Finance expense (see Note 3). The associated cash flows are disclosed as financing activities within the Consolidated Statement of Cash Flows.
With the exception of Contingent consideration payables of $2,137m (2022: $2,222m; 2021: $2,865m) which are held at fair value within Level 3 of the
fair value hierarchy as defined in Note 12, all other financial liabilities are held at amortised cost with carrying value being a reasonable approximation
of fair value.
Notes to the Group Financial Statements 181AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
20 Trade and other payables contin ued
Contingent consideration
2023 2022 2021
$m $m $m
At 1January 2,222 2,865 3,323
Additions through business combinations 60 – –
Settlements (826) (772) (643)
Disposals – (121) –
Revaluations 549 82 14
Reclassification to Other payables – – (55)
Discount unwind (Note 3) 132 168 226
At 31December 2,137 2,222 2,865
Contingent consideration arising from business combinations is fair valued using decision-tree analysis, with key inputs including the probability of
success, consideration of potential delays and the expected levels of future revenues.
Revaluations of Contingent consideration are recognised in Selling, general and administrative expense and include an increase of $520m in 2023
(2022: an increase of $182m; 2021: an increase of $42m) based on revised milestone probabilities, and revenue and royalty forecasts, relating to
the acquisition of BMS’s share of the Global Diabetes Alliance. Discount unwind on the liability is included within Finance expense (see Note 3).
The discount rate used for the Contingent consideration balances range from 5% to 8%. The most significant Contingent consideration balance is
the Global Diabetes Alliance which is discounted at 8% and is reviewed against comparable benchmarks on a regular basis.
Management has identified that reasonably possible changes in certain key assumptions, including the likelihood of achieving successful trial results,
obtaining regulatory approval, the projected market share of the therapy area and expected pricing for launched products, may cause the calculated
fair value of the above contingent consideration to vary materially in future years.
The contingent consideration balance relating to BMS’s share of Global Diabetes Alliance of $1,945m (2022: $2,124m; 2021: $2,544m) would increase/
decrease by $195m with an increase/decrease in sales of 10% as compared with the current estimates.
The maximum development and sales milestones payable under outstanding Contingent consideration arrangements arising on business combinations
are as follows:
Natureof Maximumfuturemilestones
Acquisitions Year contingentconsideration $m
Spirogen 2013 Milestones 180
Amplimmune, Inc. 2013 Milestones 150
Almirall
1
2014 Milestones and royalties 345
Neogene 2023 Milestones 110
The amount of royalties payable under the arrangements is inherently uncertain and difficult to predict, given the direct link to future sales and the
range of outcomes. The maximum amount of royalties payable in each year is with reference to net sales.
21 Provisions
Employee Other
Severance Environmental benefits Legal provisions Total
$m $m $m $m $m $m
At 1January 2021 214 100 128 348 770 1,560
Additions through business combinations (Note 27) – – 41 73 27 141
Charge for year 238 23 46 109 456 872
Cash paid (172) (32) (49) (285) (84) (622)
Reversals (62) – – (5) (175) (242)
Exchange and other movements (6) (1) 29 (1) (6) 15
At 31December 2021 212 90 195 239 988 1,724
Charge for year 227 61 1 830 365 1,484
Cash paid (223) (19) (41) (814) (185) (1,282)
Reversals (43) – (27) (94) (98) (262)
Exchange and other movements (8) (1) 15 – (52) (46)
At 31December 2022 165 131 143 161 1,018 1,618
Charge for year 123 21 22 1,102 245 1,513
Cash paid (87) (41) (14) (219) (404) (765)
Reversals (28) (3) (3) (23) (143) (200)
Exchange and other movements 3 4 20 (5) (33) (11)
At 31December 2023 176 112 168 1,016 683 2,155
182 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

2023 2022 2021
$m $m $m
Due within one year 1,028 722 768
Due after more than one year 1,127 896 956
Total 2,15 5 1,618 1,724
Provisions are often subject to substantial uncertainties with regard to the timing and final amounts of any payments. Once established, these amounts
remain in Provisions even after settlement is reached and uncertainty resolved, with no transfer to Trade and other payables prior to payment. This
is to provide more transparent disclosure of subsequent movements in brought forward and carried forward balances. Settled legal claims included
within provisions are held at amortised cost with carrying value being a reasonable approximation of fair value.
Severance provisions arise predominantly in connection with global restructuring initiatives, including the PAAGR, which involve rationalisation of
the global supply chain, the sales and marketing organisation, IT and business support infrastructure, and R&D.
In conjunction with the acquisition of Alexion in 2021, the enlarged Group initiated the PAAGR; a global restructuring programme, aimed at integrating
systems, structure and processes, optimising the global footprint and prioritising resource allocations and investments. This includes the commencement
of work on the planned upgrade of the Group’s Enterprise Resource Planning IT systems (Axial Project). The Group has also continued to progress
other legacy restructuring programmes.
Employee costs in connection with the initiatives are recognised in severance provisions when a detailed formal plan has been communicated to
those employees affected. Final severance costs are often subject to the completion of the requisite consultations on the areas impacted, with the
majority of the cost expected to be paid within one year. AstraZeneca endeavours to support employees affected by restructuring initiatives to
seek alternative roles within the organisation. Where the employee is successful, any severance provisions will be released.
Details of the Environmental provisions totalling $112m (2022: $131m; 2021: $90m) and ongoing matters are provided in Note 30. These uncertainties
can also cause reversal in previously established provisions once final settlement is reached.
Legal issues are often subject to substantial uncertainties with regard to the timing and final amounts of any payments. A significant proportion of
the total legal provision, $616m (2022: $30m; 2021: $15m) due within one year and $372m (2022: $92m; 2021: $105m) due after more than one year
1
,
relates to matters settled, but not paid, in previous periods, further details are provided in Note 30.
The majority of Employee benefit provisions relate to Executive Deferred Compensation Plans, which include uncertainty over the ultimate timing
and amount of payment to be made to the executives.
Other provisions comprise amounts relating to specific contractual or constructive obligations and disputes. Included within Other provisions are
amounts associated with long-standing product liability settlements that arose prior to the merger of Astra and Zeneca, which given the nature
of the provision, the amounts are expected to be settled over many years; the final settlement values and timings are uncertain. Also included in
Other provisions is an amount of $163m (2022: $165m; 2021: $185m), in relation to third-party liability and other risks (including incurred but not yet
reported claims); the claims are considered to be uncertain as to timing and amount. Charges to Other provisions in 2023 included $87m (2022: $12m;
2021: $243m) in relation to the PAAGR restructuring programme, which has a closing provision of $49m (2022: $143m; 2021: $243m), including $8m
(2022: $95m; 2021: $158m) held in non-current provisions expected to be settled over time by 2025. In 2022, charges to Other provisions included
$301m in relation to termination fees and onerous contracts with contract manufacturing organisations, the vast majority of which was settled in 2023.
No provision has been released or applied for any purpose other than that for which it was established.
22 Post-retirement pension and other defined benefit schemes
Background
This section predominantly covers defined benefit arrangements like post-retirement pension and medical plans which make up the vast bulk of
the Group’s liabilities. However, it also incorporates other benefits which fall under IAS 19 rules and which require an actuarial valuation, including
but not limited to: lump sum plans, long service awards and defined contribution pension plans which have some defined benefit characteristics
(e.g. a minimum guaranteed level of benefit). In total, over 50 plans in 28 countries are covered.
The Group and most of its subsidiaries offer retirement plans which cover the majority of employees. The Group’s policy is to provide defined
contribution (DC) orientated pension provision to its employees unless otherwise compelled by local regulation. As a result, many of these retirement
plans are DC, where the Group contribution and resulting charge is fixed at a set level or is a set percentage of employees’ pay. However, several
plans, mainly in the UK and Sweden, are defined benefit (DB), where benefits are based on employees’ length of service and salary. The major DB
plans are largely legacy arrangements as they have been closed to new entrants since 2000, apart from the collectively bargained Swedish plan
(which is still open to employees born before 1979). During 2010, following consultation with its UK employees’ representatives, the Group introduced
a freeze on pensionable pay at 30 June 2010 levels for DB members of the UK Pension Fund. The number of active members in the Fund continues
to decline and is now 400 employees.
The major DB plans are funded through separate, fiduciary-administered assets. The cash funding of the plans, which may from time to time involve
payments from the Group, is designed, in consultation with independent qualified actuaries, to ensure that the assets are sufficient to meet future
obligations as and when they fall due. The funding level is monitored by the Group and local fiduciaries, who take into account the strength of the
Group’s covenant, local regulation, cash flows, and the solvency and maturity of the pension plan.
Notes to the Group Financial Statements
183AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

22 Post-retirement and other defined benefit schemes continued
Notes to the Group Financial Statements
continu ed
Financing Principles and Funding Framework
Eighty six per cent of the Group’s total DB obligations (or 66% of net obligations) at 31 December 2023 are in schemes within the UK and Sweden.
In these countries, the pension obligations are funded in line with the Group’s financing principles, as disclosed in prior years.
The Group has developed a long-term funding framework to implement these principles. This framework targets either full funding on a low-risk
funding measure, or buyout with an external insurer as the pension funds mature, with affordable long-term de-risking of investment strategy along
the way. Unless local regulation dictates otherwise, this framework determines the cash contributions payable.
UK
The UK Pension Fund represents approximately 65% of the Group’s DB obligations at 31 December 2023. The financing principles are modified in
light of the UK regulatory requirements (summarised below) and resulting discussions with the Trustee.
Role of Trustee and Regulation
The UK Pension Fund is governed and administered by a corporate Trustee which is legally separate from the Group. The Trustee Directors are
comprised of representatives appointed by both the employer and employees and include an independent professional Trustee Director. The Trustee
Directors are required by law to act in the interest of all relevant beneficiaries and are responsible in particular for investment strategy and the
day-to-day administration of the benefits. They are also responsible for jointly agreeing with the employer the level of contributions due to the UK
Pension Fund.
The UK pensions industry is regulated by The Pensions Regulator whose statutory objectives and regulatory powers are described on its website,
www.thepensionsregulator.gov.uk.
The Pension Scheme Act 2021 became effective in the UK from 1 October 2021. A section of this Act places additional legal requirements on
companies who sponsor UK defined benefit pension schemes, to monitor and assess corporate activity, with a focus on the potential impact of
such activity on the ongoing security of these benefits. The Group maintains a framework to ensure it meets its responsibilities under the Act.
There have been two UK High Court Rulings relating to Guaranteed Minimum Pensions (GMP) equalisation in 2018 and 2020. Following the publication
of guidance around implementation in 2021, the Trustee, with input from the Group, has now completed the equalisation of benefits for the vast
majority of pensioner members, with the project expected to complete in 2024. Further details are set out later in this Note. An estimate of the
impact of these changes has already been recognised in 2018 and 2020, and actual experience is in line with the estimates previously recognised.
In June 2023, the UK High Court (Virgin Media Limited v NTL Pension Trustees II Limited) ruled that certain historical amendments for contracted-
out defined benefit schemes were invalid if they were not accompanied by the correct actuarial confirmation. The judgment is subject to appeal.
The Trustee and Group are monitoring developments and will consider if there are any implications for the UK Pension Fund, if the ruling is upheld.
Funding requirements
UK legislation requires that an actuarial valuation is completed for all DB pension schemes every three years, which compares the schemes’
liabilities to its assets. As part of the triennial valuation process, the Trustee and the Group must agree on a set of assumptions to value the liabilities
and determine the contributions required, if any, to ensure the UK Pension Fund is fully funded over an appropriate time period and on a suitably
prudent measure. The assumptions used to value the liabilities for the triennial actuarial valuation are required to be prudent, whereas the assumptions
used to prepare an IAS 19 accounting valuation are required to be ‘best estimate’.
The last full actuarial valuation of the UK Pension Fund was carried out by a qualified actuary as at 31 March 2022 and finalised in May 2023, ahead
of the statutory deadline.
Under the funding assumptions used to set the statutory funding target, the key assumptions from the actuarial valuation as at 31 March 2022 (shown
as a single-equivalent rate) were as follows: salary increases at 0% per annum (as a result of pensionable pay levels being frozen in 2010); pension
increases at 3.64% per annum; and discount rate at 3.03% per annum. The resulting valuation of the Fund’s liabilities on that basis was £5,951m
($7,820m) compared to a market valuation of assets at 31 March 2022 of £5,604m ($7,364m).
Aspects of the triennial actuarial valuation are governed by a long-term funding agreement, effective since October 2016, which sets out a path to
full funding on a low-risk measure. Under this agreement, if a deficit exists, the Group is required to provide security. This security takes the form
of a charge in favour of the Trustee over all land and buildings on the Group’s Cambridge Biomedical Campus site. This charge was enacted in
December 2023, and provides long-term security to the Trustee in respect of the Group’s future deficit recovery contributions. The value of the
charge is currently £317m ($404m) and it is capped at £350m ($446m). The value of the charge will vary and is expected to reduce over time, before
falling away. Under the terms of the charge, the Trustee can only exercise its right over the ownership of the site in a Group insolvency event.
184
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

In relation to deficit recovery contributions, a lump sum contribution of £39m ($48m) was made in March 2023, with a further annual contribution
of £39m ($50m) due before 31 March 2024, and each year up to March 2028.
Further progress was made over 2023 in equalising GMP for members of the UK Pension Fund. The method of equalisation converts GMP to non-
GMP pension to simplify the structure and administration of benefits. As at 31 December 2023, almost all pensioner and dependent members have
had their benefits equalised and, for non-pensioner members, a process will be in place in 2024 to equalise their benefits at their point of retirement.
As part of the project, a Pension Increase Exchange (‘PiE’) option was also made available to the majority of pensioner members, at the Group’s
discretion. This option provided the member with a choice to opt for a higher pension right away, but with no, or fewer, inflation-linked increases
in the future. Take-up of this option resulted in a reduction to expected future liabilities and a $16m past service credit was taken to the income
statement in March 2023.
Under the governing documentation of the UK Pension Fund, any future surplus in the Fund would be returnable to the Group by refund assuming
gradual settlement of the liabilities over the lifetime of the Fund. In particular, the Trustee has no unilateral right to wind up the Fund without Company
consent nor does it have the power to unilaterally use surplus to augment benefits prior to wind-up. As such, there are no adjustments required in
respect of IFRIC 14 ‘IAS 19 – The Limit on a Defined Benefit Asset, Minimum Funding Requirements and their Interaction’.
On current bases, it is expected that ongoing contributions (excluding those in respect of past service deficit contributions) during the year ending
31 December 2024 for the UK scheme will be approximately $18m.
United States
In May 2023, AstraZeneca Pharmaceuticals LP agreed a buy-out of its qualified US Defined Benefit Pension Plan with an external insurer. All Plan
liabilities (approximately $840m) have now been discharged (via a mix of cash payments to participants and purchase of insured annuities), with an
impact of $1.7m on the income statement and a net Group cash contribution of approximately $25m. The Plan is wound up and the Trust is closed.
The transaction will be completed in 2024, pending approval of Group annuity contracts from State Regulators.
There are three remaining immaterial US post-retirement benefit plans and therefore from 2024, these will not be individually disclosed.
Sweden
The Swedish plans account for 20% of the Group’s defined benefit obligations. They are governed by Fiduciary Bodies with responsibility for the
investment of the assets. These plans are funded in line with the Group’s financing principles and local regulations.
The Swedish defined benefit pension plans were actuarially valued at 31 December 2022, when plan obligations were estimated to amount to $1,312m
and plan assets were $946m. The local Swedish GAAP funding position can influence contribution policy. Over 2023, for the main pension fund
the Group did not request a reimbursement of benefit payments made throughout the year as the funding level was below 100% on the Swedish
GAAP basis. The benefit payments over 2023, totalling approximately $47m, are therefore regarded as Group contributions.
On current bases, it is expected that ongoing contributions (excluding those in respect of past service deficit contributions) during the year ending
31 December 2024 for Sweden will be approximately $53m.
Other defined benefit plans
The Group provides benefit plans other than pensions which have to be reported under IAS 19. These include lump sum plans, long service awards
and defined contribution pension plans which have a guaranteed minimum benefit. However, the largest category of these ‘other’ non-pension
plans are healthcare benefits.
In the US, and to a lesser extent in certain other countries, the Group’s employment practices include the provision of healthcare and life assurance
benefits for eligible retired employees. As at 31 December 2023, some 2,673 retired employees and covered dependents currently benefit from
these provisions and some 2,133 current employees will be eligible on their retirement. The Group accrues for the present value of such retiree
obligations over the working life of the employee. In practice, these benefits will be funded with reference to the financing principles.
In the US, the Post Retirement Welfare Plan which provides retiree medical benefits has a surplus of $66m. As a result, the investment strategy
has been fully de-risked. The Group has concluded that under current legislation, the surplus would be repayable in the future to subsidise other
medical benefits offered to employees.
The cost of post-retirement benefits other than pensions for the Group in 2023 was $1m (2022: $1m; 2021: $1m). Plan assets were $161m and plan
obligations were $114m at 31 December 2023. These benefit plans have been included in the disclosure of post-retirement benefits under IAS 19.
Notes to the Group Financial Statements 185AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

22 Post-retirement and other defined benefit schemes continued
Notes to the Group Financial Statements
continu ed
Financial assumptions
Qualified independent actuaries have updated the actuarial valuations under IAS 19 for the major defined benefit schemes operated by the Group
to 31 December 2023. The assumptions used may not necessarily be borne out in practice, due to the inherent financial and demographic uncertainty
associated with making long-term projections. These assumptions reflect the changes which have the most material impact on the results of the
Group and were as follows:
2022
UK US Sweden RestofGroup
1
Inflation assumption 3.2% – 1.9% 2.5%
Rate of increase in salaries –
2
– 3.4% 4.0%
Rate of increase in pensions in payment 3.1% – 1.9% 2.5%
Discount rate – defined benefit obligation 4.9% 5.0% 4.1% 3.7%
Discount rate – interest cost 5.0% 4.9% 4.0% 3.8%
Discount rate – service cost 4.8% n/a 4.0% 3.7%
2023
UK US Sweden RestofGroup
1
Inflation assumption 3.1%
3
– 1.6% 2.2%
Rate of increase in salaries –
2
– 3.1% 3.7%
Rate of increase in pensions in payment 2.9% – 1.6% 2.2%
Discount rate – defined benefit obligation
4
4.6% 4.7% 3.3% 3.3%
Discount rate – interest cost
5
4.6% 4.7% 3.3% 3.3%
Discount rate – service cost
5
4.5% n/a 3.3% 3.3%
The weighted average duration of the post-retirement scheme obligations is approximately 11 years in the UK, 16 years in Sweden and 13 years
for the Rest of the Group (including Germany).
Demographic assumptions
The mortality assumptions are based on country-specific mortality tables. These are compared to actual experience and adjusted where sufficient
data are available. Additional allowance for future improvements in life expectancy is included for all major schemes where there is credible data
to support a continuing trend.
The table below illustrates life expectancy assumptions at age 65 for male and female members retiring in 2023 and male and female members
expected to retire in 2043 (2022: 2022 and 2042 respectively).
Lifeexpectancyassumptionforamalememberretiringatage65 Lifeexpectancyassumptionforafemalememberretiringatage65
Country 2023 2043 2022 2042 2023 2043 2022 2042
UK 22.1 23.1 22.2 23.2 23.7 24.8 23.8 24.9
US 22.2 24.6 22.0 23.2 23.3 26.2 23.4 25.0
Sweden 21.8 23.6 21.8 23.6 23.9 26.0 23.9 26.0
In the UK, the Group adopted the CMI 2022 Mortality Projections Model with a 1% long-term improvement rate. No other demographic assumptions
have changed since they were updated in 2022 following the actuarial valuation. The Group has continued to assume that 25% of members
(2022: 25%) will transfer out of the defined benefit section of the AstraZeneca Pension Fund at the point of retirement.
In the US and Sweden, the mortality assumptions are unchanged from 2022.
186
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Risks associated with the Group’s defined benefit pension schemes
The UK defined benefit plan accounts for 65% of the Group’s defined benefit obligations and exposes the Group to a number of risks, the most
significant of which are:
Risk Description Mitigation
Asset
pricingrisk
The Defined Benefit Obligation (DBO) is calculated using a discount rate
set with reference to AA-rated corporate bond yields; asset returns that
differ from the discount rate will create an element of volatility in the
solvency ratio. Approximately 45% of the UK Pension Fund is allocated
to growth assets. Although these growth assets are expected to
outperform AA-rated corporate bonds in the long term, they can lead to
volatility and mismatching risk in the short term. The allocation to growth
assets is monitored to ensure it remains appropriate given the UK Pension
Fund’s long-term objectives.
In order to mitigate investment risk, the Trustee invests in a suitably
diversified range of asset classes, return drivers and investment managers.
The investment strategy will evolve to further improve the expected risk/return
profile as opportunities arise. De-risking of the investment strategy took
place over 2023, as the Fund moved ahead of its long-term target, with the
benchmark allocation to Growth Assets reducing from 62.5% to 47.5%.
The Trustee has hedged approximately 92% of unintended non-sterling,
overseas currency risk within the UKPension Fund assets.
Interest
raterisk
A decrease in corporate bond yields will increase the present value
placedon the DBO for accounting purposes.
The interest rate hedge of the UK Pension Fund is predominantly implemented
via holding gilts (and gilt repurchase agreements or ‘gilt repo’) of appropriate
duration. This hedge protects to a large degree against falls in long-term
interest rates and the UK Pension Fund is approximately 98% hedged as a
percentage of assets at the end of 2023 (versus target of 100%). Nonetheless,
there remain differences in the bonds and instruments held by the UK Pension
Fund to hedge interest rate risk on the statutory and long-term funding basis
(gilts and gilt repo) and the bonds analysed to set the DBO discount rate on
an accounting basis (AA corporate bonds). As such, there remains some
mismatching risk on an accounting basis should yields on gilts diverge
compared to AA corporate bonds.
Inflation risk The majority of the DBO is indexed in line with price inflation (mainly
inflation as measured by the UK Retail Price Index (RPI) but also for
some members a component of pensions is indexed by the UK
Consumer Price Index (CPI)) and higher inflation will lead to higher
liabilities (although, in the vast majority of cases, this is capped at an
annual increase of 5%, known as Limited Price Indexation or LPI).
The UK Pension Fund holds RPI index-linked gilts and gilt repo. The inflation
hedge of the UK Pension Fund protects to some degree against higher-than-
expected inflation increases on the DBO (approximately 100% hedged as a
percentage of assets at the end of 2023). Over 2023, work was carried out
by the Trustee to improve the accuracy of the hedge to LPI linked liabilities.
Life
expectancy
The majority of the UK Pension Fund’s obligations are to provide
benefits for the life of the member, so increases in life expectancy
willresult in an increase in the liabilities.
In 2013 the Trustee entered into a longevity swap to hedge against the risk
of increasing life expectancy over the next 75 years. The swap currently
covers approximately 8,000 of the UK Pension Fund’s pensioners, equivalent
to $2.4bn of Pension fund liability. A one-year increase in life expectancy
would result in a $214m increase in pension fund obligations, which would
be partially offset by a $108m increase in the value of the longevity swap
and hence the pension fund assets.
Cash flow and
liquidity risk
The UK Pension Fund is maturing and cash flow negative. Assets
areliquidated to meet benefit outgo and potentially from time to time,
to supplement the collateral pool required to post margin for
derivativeholdings.
There is a risk of the Trustee requesting liquidity support from the Group
to meet margin calls or expenditure, if the liquidity position of the UK
Pension Fund is not effectively monitored and managed.
The Trustee invests in a diversified portfolio of highly liquid assets to
manage sequencing risk and operates a collateral management policy,
maintaining a minimum liquidity ‘buffer’ above recommended regulatory
guidelines, which can be quickly supplemented in an orderly manner.
Over 2023, in addition to the Growth and Liability Hedging portfolios,
theTrustee allocated 7% of assets to a new, cash flow driven investment
portfolio, consisting of investment grade corporate bonds. The purpose of
this portfolio is to generate income to help meet the Fund’s benefit outgo.
The portfolio is expected to grow over time as further de-risking occurs.
Other risks
There are a number of other risks of administering the UK Pension Fund which the Trustee manages with Group input. Some of the major risks
include counterparty risks from using derivatives (mitigated by using a specialist investment manager to oversee a diversified range of counterparties
of high standing and ensuring positions are collateralised daily). Furthermore, there are operational risks (such as paying out the wrong benefits)
and legislative risks (such as the UK government introducing new legislation). These are mitigated so far as possible via the governance structure
in place which oversees and administers the pension funds.
The Group’s pension plans in Sweden also manage these key risks, where relevant, in a similar way, with the local fiduciary bodies investing in a
diversified manner and employing a framework to hedge interest rate risk where practicable.
Local fiduciary boards are aware of Environmental, Social and Governance (ESG) risks as they pertain to investment policy, and where local regulation
allows, have policies in place to monitor and manage such risks and comply with local legislation and disclosure requirements. The Trustee of the
UK Pension Fund published its inaugural Task Force for Climate-related Disclosures (TCFD) report in October 2023.
Notes to the Group Financial Statements 187AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

22 Post-retirement and other defined benefit schemes continued
Notes to the Group Financial Statements
continu ed
Assets and obligations of defined benefit schemes
The assets and obligations of the defined benefit schemes operated by the Group at 31 December 2023, as calculated in accordance with IAS 19,
are shown below. The fair values of the schemes’ assets are not intended to be realised in the short term and may be subject to significant change
before they are realised. The present value of the schemes’ obligations is derived from cash flow projections over long periods and is therefore
inherently uncertain.
Scheme assets
2022
UK US Sweden RestofGroup Total
Quoted Unquoted Quoted Unquoted Quoted Unquoted Quoted Unquoted Quoted Unquoted Total
$m $m $m $m $m $m $m $m $m $m $m
Government bonds
1
1,931 – 104 – – – 60 – 2,095 – 2,095
Corporate bonds
2
– – 622 – – – 11 – 633 – 633
Derivatives
3
– (608) (2) (3) – 325 (2) – (4) (286) (290)
Investment funds: Listed Equities
4
– 265 – – – – 49 4 49 269 318
Investment funds:
Absolute Return/Multi Strategy
4
– 1,701 – – – 475 6 – 6 2,176 2,182
Investment funds: Corporate Bonds/Credit
4
– 817 – – – 144 49 10 49 971 1,020
Cash and cash equivalents 52 415 285 – – 2 – 4 337 421 758
Other – – – 2 – – 1 311 1 313 314
Total fair value of scheme assets/(liabilities)
5
1,983 2,590 1,009 (1) – 946 174 329 3,166 3,864 7,030
2023
UK US Sweden
RestofGroup Total
Quoted Unquoted Quoted Unquoted Quoted Unquoted Quoted Unquoted Quoted Unquoted Total
$m $m $m $m $m $m $m $m $m $m $m
Government bonds
1
2,383 – 61 – – – 51 – 2,495 – 2,495
Corporate bonds
2
373 – 94 – – – 6 – 473 – 473
Derivatives
3
– (532) – – – 440 – – – (92) (92)
Investment funds: Listed Equities
4
– 321 – – – – 53 3 53 324 377
Investment funds:
Absolute Return/Multi Strategy
4
– 1,131 – – – 461 5 8 5 1,600 1,605
Investment funds: Corporate Bonds/Credit
4
– 667 – – – 165 48 – 48 832 880
Cash and cash equivalents 53 363 5 – – 2 – 3 58 368 426
Other – – – – – – (1) 316 (1) 316 315
Total fair value of scheme assets
5
2,809 1,950 160 – – 1,068 162 330 3,131 3,348 6,479
Scheme obligations
2022
UK US Sweden RestofGroup Total
$m $m $m $m $m
Present value of scheme obligations in respect of:
Active membership (212) (54) (430) (424) (1,120)
Deferred membership (804) (437) (369) (299) (1,909)
Pensioners (3,785) (531) (513) (250) (5,079)
Total value of scheme obligations (4,801) (1,022) (1,312) (973) (8,10 8)
2023
UK US Sweden RestofGroup Total
$m $m $m $m $m
Present value of scheme obligations in respect of:
Active membership (233) (45) (553) (442) (1,273)
Deferred membership (853) (2) (443) (294) (1,592)
Pensioners (4,075) (107) (606) (254) (5,042)
Total value of scheme obligations (5,161) (154) (1,602) (990) (7,907)
188 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Net (deficit)/surplus in the scheme
2022
UK US Sweden RestofGroup Total
$m $m $m $m $m
Total fair value of scheme assets 4,573 1,008 946 503 7,0 3 0
Total value of scheme obligations (4,801) (1,022) (1,312) (973) (8,108)
Deficit in the scheme as recognised in the
Consolidated Statement of Financial Position (228) (14) (366) (470) (1,078)
Included in Non-current other receivables – 62 – 28
1
90
Included in Retirement benefit obligations (228) (76) (366) (498) (1,168)
(228) (14) (366) (470) (1,078)
2023
UK US Sweden RestofGroup Total
$m $m $m $m $m
Total fair value of scheme assets 4,759 160 1,068 492 6,479
Total value of scheme obligations (5,161) (154) (1,602) (990) (7,907)
(Deficit)/surplus in the scheme as recognised in the
Consolidated Statement of Financial Position (402) 6 (534) (498) (1,428)
Included in Non-current other receivables – 66 – 26
1
92
Included in Retirement benefit obligations (402) (60) (534) (524) (1,520)
(402) 6 (534) (498) (1,428)
Fair value of scheme assets
2023 2022
UK US Sweden RestofGroup Tot al UK US Sweden RestofGroup Total
$m $m $m $m $m $m $m $m $m $m
At beginning ofyear 4,573 1,008 946 503 7,0 30 7,333 1,413 1,234 584 10,564
Interest income on scheme assets 229 22 38 11 300 123 29 18 5 175
Expenses (9) (1) – (1) (11) (5) (2) – – (7)
Actuarial (losses)/gains (59) 2 37 (45) (65) (1,964) (295) (153) (55) (2,467)
Exchange and other adjustments 262 (1) 48 20 329 (728) – (152) (34) (914)
Employer contributions 65 35 46 42 188 118 7 43 37 205
Participant contributions 1 4 – 7 12 1 5 – 5 11
Benefits paid (303) (68) (47) (45) (463) (305) (149) (44) (39) (537)
Settlements – (841) – – (841) – – – – –
Scheme assets’ fair value at end ofyear 4,759 160 1,068 492 6,479 4,573 1,008 946 503 7, 0 3 0
The actual return on the plan assets was a gain of $235m (2022: loss of $2,292m).
Movement in post-retirement scheme obligations
2023 2022
UK US Sweden RestofGroup Tot al UK US Sweden RestofGroup Total
$m $m $m $m $m $m $m $m $m $m
Present value of obligations in scheme at beginning ofyear (4,801) (1,022) (1,312) (973) (8,108) (7,941) (1,404) (2,373) (1,300) (13,018)
Current service cost (6) (2) (13) (35) (56) (14) (1) (35) (38) (88)
Past service credit/(cost) 12 – (2) 2 12 (5) – (4) 3 (6)
Participant contributions (1) (4) – (7) (12) (1) (4) – (5) (10)
Benefits paid 303 68 47 45 463 305 149 44 39 537
Interest expense on post-retirement scheme obligations (239) (22) (50) (27) (338) (132) (29) (31) (12) (204)
Actuarial (losses)/gains (155) (12) (202) 28 (341) 2,243 268 806 268 3,585
Exchange and other adjustments (274) 1 (70) (34) (377) 744 (1) 281 72 1,096
Settlements – 839 – 11 850 – – – – –
Present value of obligations in scheme at end ofyear (5,161) (154) (1,602) (990) (7,907) (4,801) (1,022) (1,312) (973) (8,108)
The obligations arise from over 50 plans in 28 countries:
2023 2022
UK US Sweden RestofGroup Tot al UK US Sweden RestofGroup Total
$m $m $m $m $m $m $m $m $m $m
Funded– pension schemes
1
(5,151) – (1,599) (868) (7,618) (4,787) (851) (1,310) (842) (7,79 0 )
Funded– post-retirement healthcare – (94) – – (94) – (111) – – (111)
Unfunded– pension schemes
1
– (60) (3) (113) (176) – (60) (2) (122) (184)
Unfunded– post-retirement healthcare (10) – – (9) (19) (14) – – (9) (23)
Total (5,161) (154) (1,602) (990) (7,9 07) (4,801) (1,022) (1,312) (973) (8,10 8)
Notes to the Group Financial Statements
189AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

22 Post-retirement and other defined benefit schemes continued
Notes to the Group Financial Statements
continu ed
Consolidated Statement of Comprehensive Income disclosures
The amounts that have been charged to the Consolidated Statement of Comprehensive Income, in respect of defined benefit schemes for the year
ended 31 December 2023, are set out below.
2023 2022
UK US Sweden RestofGroup Tot al UK US Sweden RestofGroup Total
$m $m $m $m $m $m $m $m $m $m
Operating profit
Current service cost (6) (2) (13) (35) (56) (14) (1) (35) (38) (88)
Past service credit/(cost) 12 – (2) 2 12 (5) – (4) 3 (6)
Expenses (9) (1) – (1) (11) (5) (2) – – (7)
Total charge to Operating profit (3) (3) (15) (34) (55) (24) (3) (39) (35) (101)
Finance expense
Interest income on scheme assets 229 22 38 11 300 123 29 18 5 175
Interest expense on post-retirement scheme obligations (239) (22) (50) (27) (338) (132) (29) (31) (12) (204)
Net interest on post-employment defined benefit plan liabilities (10) – (12) (16) (38) (9) – (13) (7) (29)
Charge before taxation (13) (3) (27) (50) (93) (33) (3) (52) (42) (130)
Other comprehensive income
Difference between the actual return and the expected return
onthepost-retirement scheme assets (59) 2 37 (45) (65) (1,964) (295) (153) (55) (2,467)
Experience (losses)/gains arising on the post-retirement
schemeobligations (25) (2) (67) (13) (107) 55 (16) (99) (6) (66)
Changes in financial assumptions underlying the present value
ofthe post-retirement scheme obligations (142) (10) (135) 44 (243) 2,272 284 896 275 3,727
Changes in demographic assumptions 12 – – (3) 9 (84) – 9 (1) (76)
Remeasurement of the defined benefit liability (214) (10) (165) (17) (406) 279 (27) 653 213 1,118
Past service cost includes granting early retirement in UK and Sweden.
Total Group pension costs in respect of defined contribution and defined benefit schemes during the year are set out below (see Note 29).
2023 2022
$m $m
Defined contribution schemes 482 445
Defined benefit schemes − Current service cost and Expenses 67 95
Defined benefit schemes − Past service (credit)/cost (12) 6
Pension costs 537 546
Rate sensitivities
The following table shows the US dollar effect of a change in the significant actuarial assumptions used to determine the retirement benefits obligations
in our three main defined benefit pension obligation countries.
2023 2022
+0.5% −0.5% +0.5% −0.5%
Discount rate
UK ($m) 269 (308) 262 (289)
US ($m) 4 (4) 46 (49)
Sweden ($m) 109 (123) 95 (107)
Total ($m) 382 (435) 403 (445)
2023 2022
+0.5% −0.5% +0.5% −0.5%
Inflation rate
1
UK ($m) (189) 184 (173) 165
US ($m) n/a n/a n/a n/a
Sweden ($m) (116) 104 (104) 93
Total ($m) (305) 288 (277) 258
2023 2022
+0.5% −0.5% +0.5% −0.5%
Rate of increase in salaries
UK ($m) n/a n/a n/a n/a
US ($m) n/a n/a n/a n/a
Sweden ($m) (46) 42 (47) 43
Total ($m) (46) 42 (47) 43
190 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

2023 2022
+1year −1year +1year −1year
Mortality rate
UK ($m) (214)
2
212
3
(191) 193
US ($m) (2) 2 (20) 20
Sweden ($m) (51) 51 (44) 44
Total ($m) (267) 265 (255) 257
In consideration of current market conditions, additional sensitivities have been calculated for the UK and Sweden schemes for 2023. The effect
on retirement benefit obligations of a 1.0% change in assumption is as follows: $525m (UK) and $210m (Sweden) if the discount rate is increased;
$(634)m (UK) and $(254)m (Sweden) if the discount rate is decreased; $(384)m (UK) and $(240)m (Sweden) if the inflation rate is increased; and
$363m (UK) and $201m (Sweden) if the inflation rate is decreased.
The sensitivity to the financial assumptions shown above has been estimated taking into account the approximate duration of the liabilities and the
overall profile of the plan membership.
The inflation sensitivity allows for the impact of a change in inflation on salary increases and pension increases (where these assumptions are
inflation-linked).
The salary increase sensitivity reflects the impact of an increase of only salary relative to inflation.
The sensitivity to the life expectancy assumption is estimated based on a revised mortality assumption that extends/reduces the current life
expectancy by one year for a particular age.
23 Reserves
Retained earnings
The cumulative amount of goodwill written off directly to reserves resulting from acquisitions, net of disposals, amounted to $595m (2022: $591m;
2021: $615m) using year end rates of exchange.
At 31 December 2023, 1,580,137 shares, at a cost of $129m, have been deducted from Retained earnings (2022: 1,671,446 shares, at a cost of $112m;
2021: 3,922,122 shares, at a cost of $239m) to satisfy future vesting of employee share plans.
There are no significant statutory or contractual restrictions on the distribution of current profits of subsidiaries; undistributed profits of prior years
are, in the main, permanently employed in the businesses of these companies. The undistributed income of AstraZeneca companies overseas might
be liable to overseas taxes and/or UK taxation (after allowing for double taxation relief) if they were to be distributed as dividends (see Note 4).
2023 2022 2021
$m $m $m
Cumulative translation differences included within Retained earnings
At 1January (3,694) (1,934) (1,143)
Foreign exchange arising on consolidation 608 (1,446) (483)
Exchange adjustments on goodwill (recorded against other reserves) 4 (24) (21)
Foreign exchange arising on designated liabilities in net investment hedges
1
24 (282) (321)
Fair value movements on derivatives designated in net investment hedges 44 (8) 34
Net exchange movement in Retained earnings 680 (1,760) (791)
At 31December (3,014) (3,694) (1,934)
The cumulative loss with respect to costs of hedging is $22m (2022: loss of $3m; 2021: gain of $4m) and the loss during the year was $19m (2022:
loss of $7m; 2021: loss of $6m).
The balance remaining in the foreign currency translation reserve from net investment hedging relationships for which hedge accounting no longer
applied is a gain of $527m. For further detail relating to hedging balances, please see the Hedge accounting section within Note 28, from page 200.
Other reserves
The other reserves arose from the cancellation of £1,255m of share premium account by the Company in 1993 and the redenomination of share
capital of $157m in 1999. The reserves are available for writing off goodwill arising on consolidation and, subject to guarantees given to preserve
creditors at the date of the court order, are available for distribution.
Notes to the Group Financial Statements 191AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
24 Share capital
Allotted,called-upandfullypaid
2023 2022 2021
$m $m $m
Issued Ordinary Shares ($0.25 each) 388 387 387
Redeemable Preference Shares (£1 each– £50,000) – – –
At 31December 388 387 387
The Redeemable Preference Shares carry limited class voting rights and no dividend rights. This class of shares is capable of redemption at par at
the option of the Company on the giving of seven days’ written notice to the registered holder of the shares.
The Company does not have a limited amount of authorised share capital.
The movements in the number of Ordinary Shares during the year can be summarised as follows:
No.ofshares
2023 2022 2021
At 1January 1,549,800,030 1,549,400,665 1,312,668,724
Issue of share capital (business combinations) – – 236,321,411
Issue of shares (share schemes) 362,596 399,365 410,530
At 31December 1,550,162,626 1,549,800,030 1,549,400,665
Share issues
Issue of share capital (business combinations) represents share capital issued as part of the acquisition of Alexion (see Note 27).
Share repurchases
No Ordinary Shares were repurchased by the Company in 2023 (2022: nil; 2021: nil).
Shares held by subsidiaries
No shares in the Company were held by subsidiaries in any year.
25 Dividends to shareholders
2023 2022 2021 2023 2022 2021
Pershare Pershare Pershare $m $m $m
Second interim (March 2023) $1.97 $1.97 $1.90 3,047 3,046 2,490
First interim (September 2023) $0.93 $0.93 $0.90 1,440 1,440 1,392
Total $2.90 $2.90 $2.80 4,487 4,486 3,882
The Company has exercised its authority in accordance with the provisions set out in the Company’s Articles of Association, that the balance of
unclaimed dividends outstanding past 12 years be forfeited. Unclaimed dividends of $nil (2022: $1m; 2021: $nil) have been adjusted for in Retained
earnings in 2023.
The 2022 second interim dividend of $1.97 per share was paid on 27 March 2023. The 2023 first interim dividend of $0.93 per share was paid on
11 September 2023.
Reconciliation of dividends charged to equity to cash flow statement:
2023 2022 2021
$m $m $m
Dividends charged to equity 4,487 4,486 3,882
Exchange losses on payment of dividend 5 5 3
Hedge contracts relating to payment of dividends (cash flow statement) (19) (127) (29)
Dividends paid to non-controlling interests 4 – –
Net movement of unclaimed dividends in the year 4 – –
Dividends paid (cash flow statement) 4,481 4,364 3,856
192 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

26 Non-controlling interests
The Group Financial Statements at 31 December 2023 reflect equity of $23m (2022: $21m; 2021: $19m) and total comprehensive income of $6m
(2022: $2m; 2021: $3m) attributable to the non-controlling interests in AstraZeneca Pharma India Limited, P.T. AstraZeneca Indonesia, Beijing Falikang
Pharmaceutical (China) Co. Limited, and AstraZeneca Algeria Pharmaceutical Industries SPA.
In February 2016, AstraZeneca acquired a 55% controlling stake in Acerta Pharma where the non-controlling interest was subject to put and call
options. The put option gave rise to a liability (see Note 20). AstraZeneca exercised its option to acquire the remaining 45% of shares in Acerta
Pharma in April 2021.
As part of the acquisition of Alexion in July 2021, a pre-existing non-controlling interest in Caelum Biosciences was recognised (Note 27). This was
valued at $150m, the agreed-upon exercise price for the exclusive option to acquire the remaining equity. The option was exercised on 28 September
2021 and the acquisition of Caelum Biosciences closed shortly thereafter on 5 October 2021.
27 Acquisition of business operations
Acquisitions of business operations in 2023
On 16 January 2023, AstraZeneca completed the acquisition of Neogene Therapeutics Inc. (Neogene), a global clinical-stage biotechnology company
pioneering the discovery, development and manufacturing of next-generation T-cell receptor therapies (TCR-Ts). The purchase price allocation exercise
has completed, with the fair value of total consideration determined at $267m. Intangible assets of $100m and goodwill of $158m were recognised
in the acquisition balance sheet, as well as a cash outflow of $189m net of cash acquired. Future contingent milestones-based and non-contingent
consideration is payable to a maximum of $120m. Neogene’s results have been consolidated into the Group’s results from 16 January 2023.
Acquisitions of business operations in 2022
On 16 November 2022, AstraZeneca completed the acquisition of 100% of the issued shares of LogicBio Therapeutics, Inc. (LogicBio) based in
Lexington, MA, US. LogicBio is a clinical-stage genetic medicine company pioneering genome editing and gene delivery platforms to address rare
and serious diseases from infancy through adulthood. The total consideration was $72m. Cash of $68m was paid on the completion date, with $4m
of outstanding options, which will be settled in cash, recorded in current Trade and other payables. Goodwill of $15m, assets of $82m, including
$46m of intangible assets, and liabilities of $25m were recognised on acquisition. LogicBio’s results have been consolidated into the Group’s results
from 16 November 2022.
Acquisitions of business operations in 2021
On 21 July 2021, AstraZeneca completed the acquisition of 100% of the issued shares of Alexion Pharmaceuticals, Inc (Alexion), based in Boston,
MA, US. Alexion is a global biopharmaceutical company focused on serving patients and families affected by rare diseases and devastating conditions
through the discovery, development and commercialisation of life-changing medicines.
At closing, Alexion shareholders received 2.1243 AstraZeneca American Depositary Shares (ADSs) and $60 in cash for each of their Alexion shares.
Unvested Alexion employee share awards were converted to equivalent AstraZeneca share awards. The fair value of the purchase consideration
was $41,058m, comprising AstraZeneca ADSs of $27,196m, cash of $13,349m and replacement employee share awards of $513m.
The Group funded the cash element of the acquisition with $8bn of new long-term debt, issued in May and June 2021, $4bn of term loans drawn in
July 2021 under the $17.5bn committed bank facilities entered into in December 2020 to secure the acquisition financing, and existing cash balances.
The Group cancelled the remaining $13.5bn of the facilities in June, July and October 2021. Loans and borrowings of $2.3bn acquired with Alexion
were repaid in full shortly following completion of the acquisition.
The acquisition was accounted for as a business combination using the acquisition method of accounting in accordance with IFRS 3 ‘Business
Combinations’ and consequently the Alexion assets acquired, and liabilities assumed, were recorded by AstraZeneca at fair value, with the excess
of the purchase price over the fair value of the identifiable assets and liabilities being recognised as goodwill.
As part of the Alexion acquisition in 2021, we identified the assets (comprising principally launched products and IPR&D post pre-clinical stage)
and liabilities acquired. Attributing fair values to assets acquired and liabilities assumed as part of business combinations is considered to be a
key judgement. The purchase price allocation was performed with assistance from an independent valuer to advise on the valuation techniques
and key assumptions in the valuation, in particular in respect of the valuation of the intangible assets and inventory.
Notes to the Group Financial Statements 193AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
continu ed
27 Acquisition of business operations continued
The fair values assigned to the Alexion business combination in 2021 were:
Fair value
$m
Non-current assets
Property, plant and equipment 1,135
Right-of-use assets 263
Intangible assets 26,855
Other non-current assets 301
28,554
Current assets
Inventories 6,769
Trade and other receivables 2,096
Intangible assets 100
Cash and cash equivalents 4,086
13,051
Current liabilities
Interest-bearing loans and borrowings (2,336)
Trade and other payables (1,192)
Other current liabilities (40)
(3,568)
Non-current liabilities
Lease liabilities (228)
Deferred tax liabilities (4,191)
Other non-current liabilities (697)
(5,116)
Total net assets acquired 32,921
Less: non-controlling interests (150)
Goodwill 8,287
Total fair value of consideration 41,058
Less: fair value of equity consideration (27,19 6)
Less: fair value of replacement employee share awards (513)
Less: cash and cash equivalents acquired (4,086)
Net cash outflow 9,263
The estimated fair value and useful lives of intangible assets were as follows:
Fair value Useful lives
$m Years
Launched products – C5 franchise (Soliris/Ultomiris) 18,480 6 to 15
Launched products – Strensiq, Kanuma, Andexxa 5,215 11 to 17
Products in development 2,760 Not amortised
Other intangibles 500 5 to 10
26,955
The fair value attributed to intangible assets was $26,955m and primarily represents intellectual property rights over launched products of $23,695m
and products under development of $2,760m. These were fair valued using the multi-period excess earnings method, which uses a number of
estimates regarding the amount and timing of future cash flows. The key assumptions in the cash flows are the probability of technical and regulatory
success, peak year sales and revenue erosion curves. In accordance with the Group’s policy on impairment assessments as set out on page 159,
the assets were assessed for impairment in the final quarter of 2023, 2022 and 2021. Future milestones have been included in the valuation of the
intangible assets (as a deduction of cash flows).
The fair value of inventory, which includes raw materials, work in progress and finished goods related to the launched products was estimated at
$6,769m, an uplift of $5,635m on the carrying value prior to the acquisition. The fair value adjustment relates only to work in progress and finished
goods and was calculated as the estimated selling price less costs to complete and sell the inventory, associated margins on these activities and
holding costs. As at 31 December 2023, the fair value uplift has been fully unwound.
Property, plant and equipment principally comprises the manufacturing facilities in Dublin and Athlone, Ireland and was fair valued using a cost
approach. The estimated fair value of $1,135m represents an uplift of $111m over carrying value.
The estimated fair value of contingent liabilities was $76m, relating to various claims and disputes in each case where there is a possible, but not
probable, future financial exposure, and involve an assessment of the likelihood of a number of scenarios in relation to those matters. This amount
has been included within other non-current liabilities of $697m.
The estimated fair value of trade and other receivables was $2,096m, which approximated the contractual cash flows.
The net deferred tax position reflected an adjustment of $5,215m related to the deferred tax impact of the fair value uplifts on intangible assets,
inventories, property, plant and equipment and contingent liabilities as described above.
194
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Goodwill amounting to $8,287m was recognised on acquisition and is underpinned by a number of elements, which individually could not be quantified.
Most significant among these is the premium attributable to a pre-existing, well-positioned business in the innovation-intensive, high-growth rare
diseases market with a highly skilled workforce and established reputation. Other important elements include the potential unidentified products
that future research and development may yield and the core technological capabilities and knowledge base of the company. Goodwill is not
expected to be deductible for tax purposes.
Non-controlling interests reflect Alexion’s pre-existing minority equity interest in Caelum Biosciences and have been valued at $150m, the agreed-
upon exercise price for the exclusive option to acquire the remaining equity. The option was exercised on 28 September 2021 and the acquisition
of Caelum Biosciences closed shortly thereafter on 5 October 2021 (Note 26).
Alexion’s results have been consolidated into the Group’s results from 21 July 2021. For the period from acquisition to 31 December 2021, before
reflecting the fair value adjustments arising on the acquisition, Alexion’s Total Revenues were $3,071m and Profit after tax was $889m. If the
acquisition had taken effect at the beginning of the reporting period in which the acquisition occurred (1 January 2021), on a pro forma basis, after
reflecting the fair value adjustments arising on the acquisition, the Total Revenue of the combined Group for the year ended 31 December 2021
would have been $41,132m and the Loss after tax would have been $1,152m. This pro forma information does not purport to represent the results
of the combined Group that actually would have occurred had the acquisition taken place on 1 January 2021 and should not be taken to be
representative of future results.
Total acquisition-related costs of $5m (2022: $4m; 2021: $171m) have been incurred by the Group, which include advisory, legal and other
professional fees. These costs are presented in the Statement of Comprehensive Income within Selling, general and administrative expense and
Finance expense.
The terms of the acquisition include a retention bonus plan for legacy Alexion employees whereby up to $50m may be used for retention bonus awards
to employees at the level of Vice President or below. In 2023, $nil costs were recorded in the Statement of Comprehensive Income (2022: $3m;
2021: $24m). These bonuses vested and were paid six months after the acquisition, or earlier.
Upon completion of the acquisition, all unvested Alexion employee share awards were converted into AstraZeneca restricted stock awards that
continue to have, and shall be subject to, the same terms and conditions as applied in the corresponding Alexion awards immediately prior to
completion. Alexion Performance Stock Plan (PSU) awards that included performance-based vesting conditions were converted using the greater
of the original target level and Alexion’s assessment of the level of achievement immediately prior to completion (subject to a limit of 175% for the
awards granted in 2019 and a limit of 150% for the awards granted in 2020). In the year, a cost of $48m (2022: $257m; 2021: $257m) has been
recorded in the Statement of Comprehensive Income, $nil (2022: $9m; 2021: $9m) in Cost of sales, $16m (2022: $92m; 2021: $73m) in Research
and development expense and $32m (2022: $156m; 2021: $175m) in Selling, general and administrative expense. Payments made to the Employee
Benefit Trust upon vesting of share awards recognised as part of the consideration for the acquisition of Alexion are recognised within investing
activities in the Group’s Statement of Cash Flows as the cash payment relates to the settlement of the obligation that arose on the acquisition of
Alexion that was included as part of the consideration for the acquisition.
28 Financial risk management objectives and policies
The Group’s principal financial instruments, other than derivatives, comprise bank overdrafts, loans and other borrowings, lease liabilities, current
and non-current investments, cash and short-term deposits. The main purpose of these financial instruments is to manage the Group’s funding
and liquidity requirements. The Group has other financial assets and liabilities such as trade receivables and trade payables, which arise directly
from its operations.
The principal financial risks to which the Group is exposed are those of liquidity, interest rate, foreign currency and credit. Each of these is managed
in accordance with Board-approved policies. These policies, together with the Group’s approach to capital management, are set out below.
Capital management
The capital structure of the Group consists of Shareholders’ equity (Note 24), Debt (Note 19), Other current investments (Note 12) and Cash (Note 17).
For the foreseeable future, the Board will maintain a capital structure that supports the Group’s strategic objectives through:
> managing funding and liquidity risk
> optimising shareholder return
> maintaining a strong, investment-grade credit rating.
The Group utilises factoring arrangements and bank acceptance drafts discounting for selected trade receivables. These arrangements qualify for
full derecognition of the associated trade receivables under IFRS 9. Amounts due on invoices that have not been factored at year end, from customers
that are subject to these arrangements, are disclosed in Note 16.
Funding and liquidity risk are reviewed regularly by the Board and managed in accordance with the policies described below.
The Board regularly reviews its shareholders’ distribution policy, which comprises a regular cash dividend and potentially a share repurchase
component. No share repurchases have been made since 2012.
The Group’s net debt position (loans and borrowings net of Cash and cash equivalents, Other investments and Derivative financial instruments)
has decreased from a net debt position of $22,923m at the beginning of the year to a net debt position of $22,510m at 31 December 2023.
Notes to the Group Financial Statements 195AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

28 Financial risk management objectives and policies continued
Notes to the Group Financial Statements
continu ed
Liquidity risk
The Board reviews the Group’s ongoing liquidity risks annually as part of the planning process and on an ad hoc basis. The Board considers short-term
requirements against available sources of funding, taking into account forecast cash flows. The Group manages liquidity risk by maintaining access
to a number of sources of funding which are sufficient to meet anticipated funding requirements. Specifically, the Group uses US and European
commercial paper, bank loans, committed bank facilities and cash resources to manage short-term liquidity and manages long-term liquidity by
raising funds through the capital markets. At 31 December 2023, the Group was assigned short-term credit ratings of P-1 by Moody’s and A-1 by
Standard and Poor’s. The Group’s long-term credit rating was A2 Stable outlook by Moody’s and A Stable outlook by Standard and Poor’s.
In addition to Cash and cash equivalents of $5,840m, short-term fixed income investments of $20m, less overdrafts of $203m at 31 December 2023,
the Group has committed bank facilities of $6,875m available to manage liquidity. These committed bank facilities have no financial covenants.
$2,000m mature in February 2025. The maturity of the $4,875m facilities was extended in February 2024 from April 2026 to April 2029. The Group
regularly monitors the credit standing of the banks providing the facilities and currently does not anticipate any issue with drawing on the committed
facilities should this be necessary. Advances under these facilities currently bear an interest rate per annum based on SOFR (Secured Overnight
Financing Rate) plus a margin.
At 31 December 2023, the Group has $4,855m outstanding from debt issued under a Euro Medium Term Note programme and $19,959m under a
SEC-registered programme. The funds made available under these facility agreements may be used for the general corporate purposes of the Group.
The maturity profile of the anticipated future contractual cash flows including interest in relation to the Group’s financial liabilities, on an undiscounted
basis and which, therefore, differs from both the carrying value and fair value, is as follows:
Bank Total Derivative Derivative Total
overdrafts Trade non-derivative financial financial derivative
andother Bonds and Lease andother financial instruments instruments financial
loans bank loans liability payables instruments receivable payable instruments Total
$m $m $m $m $m $m $m $m $m
Within oneyear 387 1,981 256 19,007 21,631 (11,766) 11,774 8 21,639
In one to twoyears – 5,647 210 2,521 8,378 (55) 66 11 8,389
In two to threeyears – 5,242 163 1,669 7, 074 (1,060) 1,079 19 7,0 9 3
In three to fouryears – 2,591 130 862 3,583 (35) 39 4 3,587
In four to fiveyears – 2,970 96 233 3,299 (118) 111 (7) 3,292
In more than fiveyears – 19,727 221 2,212 22,160 (1,521) 1,480 (41) 22,119
387 38,158 1,076 26,504 66,125 (14,555) 14,549 (6) 66,119
Effect of interest – (8,609) – – (8,609) 299 (325) (26) (8,635)
Effect of discounting, fair values and issue costs – (142) (89) (2,633) (2,864) (36) 7 (29) (2,893)
31December2021 387 29,407 987 23,871 54,652 (14,292) 14,231 (61) 54,591
Bank Total Derivative Derivative Total
overdrafts Trade non-derivative financial financial derivative
andother Bonds and Lease andother financial instruments instruments financial
loans bank loans liability payables instruments receivable payable instruments Total
$m $m $m $m $m $m $m $m $m
Within oneyear 365 5,777 249 19,065 25,456 (12,445) 12,478 33 25,489
In one to twoyears – 5,233 208 2,086 7,527 (1,012) 1,078 66 7,593
In two to threeyears – 2,608 172 872 3,652 (34) 38 4 3,656
In three to fouryears – 2,983 128 595 3,706 (103) 103 – 3,706
In four to fiveyears – 1,267 84 814 2,165 (32) 35 3 2,168
In more than fiveyears – 18,156 184 3,177 21,517 (1,436) 1,378 (58) 21,459
365 36,024 1,025 26,609 64,023 (15,062) 15,110 48 64,071
Effect of interest (15) ( 7, 9 82) – – ( 7, 9 97 ) 227 (249) (22) (8,019)
Effect of discounting, fair values and issue costs – (113) (72) (3,299) (3,484) 63 7 70 (3,414)
31December2022 350 27,929 953 23,310 52,542 (14,772) 14,868 96 52,638
Bank Total Derivative Derivative Total
overdrafts Trade non-derivative financial financial derivative
andother Bonds and Lease andother financial instruments instruments financial
loans bank loans liability payables instruments receivable payable instruments Total
$m $m $m $m $m $m $m $m $m
Within oneyear 542 5,469 313 22,401 28,725 (11,302) 11,366 64 28,789
In one to twoyears – 2,764 261 1,482 4,507 (100) 114 14 4,521
In two to threeyears – 3,137 208 788 4,133 (164) 179 15 4,148
In three to fouryears – 2,230 138 625 2,993 (924) 883 (41) 2,952
In four to fiveyears – 3,822 88 12 3,922 (949) 971 22 3,944
In more than fiveyears – 17,995 271 35 18,301 (1,507) 1,340 (167) 18,134
542 35,417 1,279 25,343 62,581 (14,946) 14,853 (93) 62,488
Effect of interest (27) (8,270) – – (8,297) 589 (644) (55) (8,352)
Effect of discounting, fair values and issue costs – (168) (151) (309) (628) 44 (46) (2) (630)
31December2023 515 26,979 1,128 25,034 53,656 (14,313) 14,163 (150) 53,506
Where interest payments are on a floating rate basis, it is assumed that rates will remain unchanged from the last business day of each year ended
31 December.
196
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

The Group has $2bn of bank loans that mature in July 2024 which the Group can repay before maturity at face value. Other than that, it is not expected
that the cash flows in the maturity profile could occur significantly earlier or at significantly different amounts, with the exception of $2,137m of
contingent consideration held within Trade and other payables (see Note 20).
Market risk
Interest rate risk
The Group maintains a Board-approved mix of fixed and floating rate debt and uses underlying debt, interest rate swaps and forward rate agreements
to manage this mix.
The majority of surplus cash is currently invested in US dollar liquidity funds and investment-grade fixed income securities.
The interest rate profile of the Group’s interest-bearing financial instruments are set out below. In the case of current and non-current financial
liabilities, the classification includes the impact of interest rate swaps which convert the debt to floating rate.
2023 2022 2021
Fixedrate Floatingrate Total Fixedrate Floatingrate Total Fixedrate Floatingrate Total
$m $m $m $m $m $m $m $m $m
Financial liabilities
Current 2,885 2,515 5,400 2,476 3,066 5,542 1,232 661 1,893
Non-current 23,222 – 23,222 21,511 2,179 23,690 23,985 4,903 28,888
Total 26,107 2,515 28,622 23,987 5,245 29,232 25,217 5,564 30,781
Financial assets
Fixed deposits – – – 64 – 64 53 – 53
Cash collateral pledged to counterparties – 102 102 – 162 162 – – –
Cash and cash equivalents – 5,840 5,840 250 5,916 6,166 – 6,329 6,329
Total – 5,942 5,942 314 6,078 6,392 53 6,329 6,382
In addition to the financial assets above, there are $11,288m (2022: $9,546m; 2021: $8,765m) of other current and non-current asset investments and
other financial assets.
The Group is also exposed to market risk on other investments.
2023 2022 2021
$m $m $m
Equity securities at fair value through Other comprehensive income (Note 12) 1,530 1,056 1,168
Non-current fixed income securities at fair value through profit or loss (Note 12) – 10 –
Total 1,530 1,066 1,168
Foreign currency risk
The US dollar is the Group’s most significant currency. As a consequence, the Group results are presented in US dollars and exposures are managed
against US dollars accordingly.
Translational
Approximately 60% of Group external sales in 2023 were denominated in currencies other than the US dollar, while a significant proportion of
manufacturing, and research and development costs were denominated in pound sterling and Swedish krona. Surplus cash generated by business
units is substantially converted to, and held centrally in, US dollars. As a result, operating profit and total cash flow in US dollars will be affected by
movements in exchange rates.
This currency exposure is managed centrally, based on forecast cash flows. The impact of movements in exchange rates is mitigated significantly
by the correlations which exist between the major currencies to which the Group is exposed and the US dollar. Monitoring of currency exposures
and correlations is undertaken on a regular basis and hedging is subject to pre-execution approval.
As at 31 December 2023, before the impact of derivatives, 2% of interest-bearing loans and borrowings were denominated in pound sterling and
16% were denominated in euros. Where there is non-US dollar debt and an underlying net investment of that amount in the same currency, the
Group applies net investment hedging. Exchange differences on the retranslation of debt designated as net investment hedges are recognised in
Other comprehensive income to the extent that the hedge is effective. Any ineffectiveness is taken to profit. For details of non-US dollar debt in a
designated hedging relationship please see the Hedge accounting section within this Note 28 from page 200.
The Group holds cross-currency swaps to hedge against the impact of fluctuations in foreign exchange rates. Fair value movements on the revaluation
of the cross-currency swaps are recognised in Other comprehensive income to the extent that the hedge is effective, with any ineffectiveness taken
to profit.
As at 31 December 2023, the Group operates in three countries designated as hyperinflationary, being Argentina, Venezuela and Turkey. The foreign
exchange risk of these markets has been assessed and deemed to be immaterial.
Transactional
The Group aims to hedge all its forecasted major transactional currency exposures on working capital balances, which typically extend for up to three
months. Where practicable, these are hedged using forward foreign exchange contracts. In addition, external dividend payments in pound sterling
to UK shareholders and in Swedish krona to Swedish shareholders are fully hedged from announcement date to payment date. Foreign exchange
gains and losses on forward contracts transacted for transactional hedging are taken to profit or to Other comprehensive income if the contract is
in a designated cash flow hedge.
Notes to the Group Financial Statements 197AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

28 Financial risk management objectives and policies continued
Notes to the Group Financial Statements
continu ed
Sensitivity analysis
The sensitivity analysis set out below summarises the sensitivity of the market value of our financial instruments to hypothetical changes in market
rates and prices. The range of variables chosen for the sensitivity analysis reflects our view of changes which are reasonably possible over a one-year
period. Market values are the present value of future cash flows based on market rates and prices at the valuation date. For long-term debt, an
increase in interest rates results in a decline in the fair value of debt.
The sensitivity analysis assumes an instantaneous 100 basis point change in interest rates in all currencies from their levels at 31 December 2023,
with all other variables held constant. Based on the composition of our long-term debt portfolio and cash reserves as at 31 December 2023, a 1%
increase in interest rates would result in an additional $25m in interest expense on the debt and an additional $58m interest income on the cash
reserves. The exchange rate sensitivity analysis assumes an instantaneous 10% change in foreign currency exchange rates from their levels at
31 December 2023, with all other variables held constant. The +10% case assumes a 10% strengthening of the US dollar against all other currencies
and the -10% case assumes a 10% weakening of the US dollar.
Each incremental 10% movement in foreign currency exchange rates would have approximately the same effect as the initial 10% detailed in the
table below and each incremental 1% change in interest rates would have approximately the same effect as the 1% detailed in the table below.
Interestrates Exchangerates
31December2021 +1% −1% +10% −10%
Increase/(decrease) in fair value of financial instruments ($m) 1,978 (2,10 6) 82 (85)
Impact on profit: gain/(loss) ($m) – – 24 (9)
Impact on equity: gain/(loss) ($m) – – 58 (76)
Interestrates Exchangerates
31December2022 +1% −1% +10% −10%
Increase/(decrease) in fair value of financial instruments ($m) 1,317 (1,490) 81 (89)
Impact on profit: gain/(loss) ($m) – – 26 (15)
Impact on equity: gain/(loss) ($m) – – 55 (74)
Interestrates Exchangerates
31December2023 +1% −1% +10% −10%
Increase/(decrease) in fair value of financial instruments ($m) 1,361 (1,534) 196 (212)
Impact on profit: gain/(loss) ($m) – – 134 (128)
Impact on equity: gain/(loss) ($m) – – 62 (83)
Credit risk
The Group is exposed to credit risk on financial assets, such as cash investments, derivative instruments, and Trade and other receivables. The
Group was also exposed in its Net asset position to its own credit risk in respect of the 2023 debentures which are accounted for at FVPL. Under
IFRS 9, the effect of the losses and gains arising from own credit risk on the fair value of bonds designated at FVPL are recorded in Other
comprehensive income.
Financial counterparty credit risk
The majority of the AstraZeneca Group’s cash is centralised within the Group treasury entity and is subject to counterparty risk on the principal
invested. The level of the Group’s cash investments and hence credit risk will depend on the cash flow generated by the Group and the timing of
the use of that cash. The credit risk is mitigated through a policy of prioritising security and liquidity over return and, as such, cash is only invested
in high credit-quality investments. Counterparty limits are set according to the assessed risk of each counterparty and exposures are monitored
against these limits on a regular basis.
The Group’s principal financial counterparty credit risks at 31 December 2023 were as follows:
Current assets
2023 2022 2021
$m $m $m
Cash at bank and in hand 1,325 1,411 1,461
Money market liquidity funds 4,425 4,486 4,772
Other short-term cash equivalents 90 269 96
Total Cash and cash equivalents (Note 17) 5,840 6,166 6,329
Fixed income securities at fair value through profit or loss (Note 12) 20 13 16
Cash collateral pledged to counterparties (Note 12) 102 162 –
Fixed deposits (Note 12) – 64 53
Total derivative financial instruments (Note 13) 116 87 83
Current assets subject to credit risk 6,078 6,492 6,481
Non-current assets
2023 2022 2021
$m $m $m
Derivative financial instruments (Note 13) 228 74 102
Non-current assets subject to credit risk 228 74 102
198 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

The majority of the Group’s cash is invested in US dollar AAA-rated money market liquidity funds. The money market liquidity fund portfolios are
managed by six external third-party fund managers to maintain an AAA rating. The Group’s investments represent no more than 10% of each overall
fund value. There were no other significant concentrations of financial credit risk at the reporting date.
All financial derivatives are transacted with commercial banks, in line with standard market practice. The Group has agreements with some bank
counterparties whereby the parties agree to post cash collateral, for the benefit of the other, equivalent to the market valuation of the derivative
positions above a predetermined threshold. The carrying value of such cash collateral held by the Group at 31 December 2023 was $215m (2022: $89m;
2021: $93m) and the carrying value of such cash collateral posted by the Group at 31 December 2023 was $102m (2022: $162m; 2021: $47m).
The impairment provision for other financial assets at 31 December 2023 was immaterial.
Trade receivables
Trade receivable exposures are managed locally in the operating units where they arise and credit limits are set as deemed appropriate for the
customer. The Group is exposed to customers ranging from government-backed agencies and large private wholesalers to privately owned pharmacies,
and the underlying local economic and sovereign risks vary throughout the world. Where appropriate, the Group endeavours to minimise risks by
the use of trade finance instruments such as letters of credit and insurance. The Group applies the expected credit loss approach to establish an
allowance for impairment that represents its estimate of expected losses in respect of Trade receivables.
The Group applies the IFRS 9 simplified approach to measuring expected credit losses which uses a lifetime expected loss allowance to Trade
receivables. To measure expected credit losses, Trade receivables have been grouped based on shared credit characteristics and the days past due.
The expected loss rates are based on payment profiles over a period of 36 months before 31 December 2023, 31 December 2022 or 31 December
2021 respectively and the corresponding historical credit losses experienced within this period. The historical loss rates are adjusted to reflect
current and forward-looking information on macroeconomic factors affecting the ability of the customer to settle the receivables.
On that basis, the loss allowance was determined as follows:
0-90 days 90-180 days Over 180 days
31December 2021 Current past due past due past due Total
Expected loss rate 0.1% 1.2% 22.6% 11.0%
Gross carrying amount ($m) 5,617 328 18 91 6,054
Loss allowance ($m) 5 4 4 10 23
0-90 days 90-180 days Over 180 days
31December 2022 Current past due past due past due Total
Expected loss rate 0.03% 0.3% 32.0% 40.6%
Gross carrying amount ($m) 6,791 331 50 99 7, 271
Loss allowance ($m) 2 1 16 40 59
0-90 days 90-180 days Over 180 days
31December 2023 Current past due past due past due Total
Expected loss rate 0.01% 0.3% 0.8% 15.0%
Gross carrying amount ($m) 7,709 342 121 280 8,452
Loss allowance ($m) 1 1 1 42 45
Trade receivables are written off where there is no reasonable expectation of recovery.
Impairment losses on Trade receivables are presented as net impairment losses within Operating profit, any subsequent recoveries are credited
against the same line.
In the US, sales to three wholesalers accounted for approximately 80% of US sales (2022: three wholesalers accounted for approximately 73%;
2021: three wholesalers accounted for approximately 94%).
The movements of the Group expected credit losses provision are follows:
2023 2022 2021
$m $m $m
At 1January 59 23 23
Net movement recognised in income statement (14) 37 (2)
Amounts utilised, exchange and other movements – (1) 2
At 31December 45 59 23
Given the profile of our customers, including large wholesalers and government-backed agencies, no further credit risk has been identified with the
Trade receivables not past due other than those balances for which an allowance has been made. The income statement credit or charge is recorded
in Operating profit.
Notes to the Group Financial Statements 199AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
28 Financial risk management objectives and policies continued
Hedge accounting
The Group uses foreign currency borrowings, foreign currency forwards and swaps, currency options, interest rate swaps and cross-currency
interest rate swaps for the purpose of hedging its foreign currency and interest rate risks. The Group may designate certain financial instruments
as fair value hedges, cash flow hedges or net investment hedges in accordance with IFRS 9. Hedge effectiveness is determined at the inception
of the hedge relationship, and through periodic prospective effectiveness assessments to ensure that an economic relationship exists between
the hedged item and hedging instrument. Sources of hedge effectiveness will depend on the hedge relationship designation but may include:
> a significant change in the credit risk of either party to the hedging relationship
> a timing mismatch between the hedging instrument and the hedged item
> movements in foreign currency basis spread for derivatives in a fair value hedge
> a significant change in the value of the foreign currency-denominated net assets of the Group in a net investment hedge.
The hedge ratio for each designation will be established by comparing the quantity of the hedging instrument and the quantity of the hedged item
to determine their relative weighting; for all of the Group’s existing hedge relationships the hedge ratio has been determined as 1:1. Designated
hedges are expected to be effective and therefore the impact of ineffectiveness on profit is not expected to be material. The accounting treatment
for fair value hedges and debt designated as FVPL is disclosed in the Group Accounting Policies section from page 152.
The following table represents the Group’s continuing designated hedge relationships under IFRS 9.
2021
Other comprehensive income
Fair value
loss
Opening Fair value recycled Closing
Nominal balance (gain)/loss to the balance Average
amounts Carrying 1January deferred Income 31December Average Average pay
in local value 2021 to OCI statement 2021 maturity USD FX interest
currency $m $m $m $m $m year rate rate
Cash flow hedges – foreign currency and interest rate risk
1, 3, 4
Cross currency interest rate swaps – Euro bonds EUR 1,700m (43) 46 182 (201) 27 2026 1.14 USD 2.85%
FX Forwards − short-term FX risk USD 1,220m 12 (5) – (7) (12) 2022 – –
Net investment hedge – foreign exchange risk
2, 3
Transactions matured pre-2021 – (565) – – (565) – – –
Cross currency interest rate swap – JPY investment JPY 58.3bn 62 (19) (43) – (62) 2029 108.03 JPY 1.53%
Cross currency interest rate swap – CNY investment CNY 458m (2) 2 – – 2 2026 6.68 CNY 4.80%
Foreign currency borrowing – GBP investment GBP 350m 470 (233) (5) – (238) 2031 n/a GBP 5.75%
Foreign currency borrowing – EUR investment
5
EUR 450m – 85 (47) – 38 2021 n/a EUR 0.88%
Foreign currency borrowing – EUR investment
6
EUR 800m 898 – (50) – (50) 2029 n/a EUR 0.38%
Contingent consideration liabilities and Acerta Pharma share
purchase liability – AZUK and AZAB USD investments USD 2,658m (2,658) 1,411 421 – 1,832 – – –
2022
Other comprehensive income
Fair value
(gain)/loss
Opening Fair value recycled Closing
Nominal balance (gain)/loss to the balance Average
amounts Carrying 1January deferred Income 31December Average Average pay
in local value 2022 to OCI statement 2022 maturity USD FX interest
currency $m $m $m $m $m year rate rate
Cash flow hedges – foreign currency and interest rate risk
1, 3, 4
Cross currency interest rate swaps – Euro bonds EUR 1,700m (160) 27 118 (111) 34 2026 1.14 USD 2.85%
FX Forwards − short-term FX risk USD 1,126m (12) (12) (14) 38 12 2023 – –
Net investment hedge – foreign exchange risk
2, 3
Transactions matured pre-2022 – (527) – – (527) – – –
Cross currency interest rate swap – JPY investment JPY 58.3bn 55 (62) 7 – (55) 2029 108.03 JPY 1.53%
Cross currency interest rate swap – CNY investment CNY 458m (4) 2 2 – 4 2026 6.68 CNY 4.80%
Foreign currency borrowing – GBP investment GBP 350m 420 (238) (50) – (288) 2031 n/a GBP 5.75%
Foreign currency borrowing – EUR investment
6
EUR 800m 846 (50) (52) – (102) 2029 n/a EUR 0.38%
Contingent consideration liabilities and Acerta Pharma share
purchase liability – AZUK and AZAB USD investments USD 2,093m (2,093) 1,832 384 – 2,216 – – –
200 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
2023
Other comprehensive income
Fair value
(gain)/loss
Opening Fair value recycled Closing
Nominal balance (gain)/loss to the balance Average
amounts Carrying 1January deferred Income 31December Average Average pay
in local value 2023 to OCI statement 2023 maturity USD FX interest
currency $m $m $m $m $m year rate rate
Cash flow hedges – foreign currency and interest rate risk
2, 4, 5
Cross currency interest rate swaps – Euro bonds EUR 3,200m 49 34 (210) 139 (37) 2027 1.10 USD 3.80%
FX Forwards − short-term FX risk USD 2,009m 15 12 (33) 6 (15) 2024 – –
Net investment hedge – foreign exchange risk
3, 4
Transactions matured pre-2023 – (527) – – (527) – – –
Cross currency interest rate swap – JPY investment JPY 58.3bn 100 (55) (45) – (100) 2029 108.03 JPY 1.53%
Cross currency interest rate swap – CNY investment CNY 458m (1) 4 (3) – 1 2026 6.68 CNY 4.80%
Foreign currency borrowing – GBP investment GBP 350m 444 (288) 24 – (264) 2031 n/a GBP 5.75%
Foreign currency borrowing – EUR investment
7
EUR 800m 881 (102) 33 – (69) 2029 n/a EUR 0.38%
Contingent consideration liabilities and Acerta Pharma share
purchase liability – AZUK and AZAB USD investments USD 1,937m (1,937) 2,216 (81) – 2 ,135 – – –
Key controls applied to transactions in derivative financial instruments are to use only instruments where good market liquidity exists, to revalue
all financial instruments regularly using current market rates and to sell options only to offset previously purchased options or as part of a risk
management strategy. The Group is not a net seller of options, and does not use derivative financial instruments for speculative purposes. The Group
held no options during the reporting period.
29 Employee costs and share plans for employees
Employee costs
The monthly average number of people, to the nearest hundred, employed by the Group is set out in the table below. In accordance with the
Companies Act 2006, this includes part-time employees.
2023 2022 2021
Employees
UK 10,700 9,800 8,900
Rest of Europe 23,000 20,600 18,300
The Americas 22,400 20,900 18,800
Asia, Africa& Australasia 30,300 30,700 33,600
Continuing operations 86,400 82,000 79,600
Geographical distribution described in the table above is by location of legal entity employing staff. Certain staff will undertake some or all of their
activity in a different location.
The number of people employed by the Group at the end of 2023 was 89,900 (2022: 83,500; 2021: 83,100).
The costs incurred during the year in respect of these employees were:
2023 2022 2021
$m $m $m
Wages and salaries 9,341 8,656 7,6 3 3
Social security costs 1,100 991 886
Pension costs 537 546 564
Other employment costs 1,357 1,338 1,192
Total 12,335 11, 531 10,275
Severance costs of $123m are not included above (2022: $227m; 2021: $238m).
The charge for share-based payments in respect of share plans is $579m (2022: $619m; 2021: $615m). Payments made to the Employee Benefit
Trust upon vesting of share awards are recognised within operating cash flows, reflecting the substance of the arrangement in place between the
Group and the Trust. The plans are equity settled.
The Directors believe that, together with the basic salary system, the Group’s employee incentive schemes provide competitive and market-related
packages to motivate employees. They should also align the interests of employees with those of shareholders, as a whole, through long-term
share ownership in the Company. The Group’s current US, UK and Swedish schemes are described below; other arrangements apply elsewhere.
Notes to the Group Financial Statements 201AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
continu ed
29 Employee costs and share plans for employees continued
Bonus and share plans
US
In the US, there are two employee short-term performance bonus plans in operation to differentiate and reward strong individual performance.
Performance bonuses are paid in cash. The AstraZeneca Performance Share Plan and the AstraZeneca Global Restricted Share Plan operate in
respect of relevant employees in the US. AstraZeneca ADRs necessary to satisfy the awards are purchased on the market or funded via a trust.
UK
The AstraZeneca UK Performance Bonus Plan
Employees of participating AstraZeneca UK companies are invited to participate in this bonus plan, which rewards strong individual performance.
Bonuses are paid in cash.
The AstraZeneca UK All-Employee Share Plan
The Company offers UK employees the opportunity to buy Partnership Shares (Ordinary Shares). Employees may invest up to £150 a month to
purchase Partnership Shares in the Company at the current market value. In 2010, the Company introduced a Matching Share element, the first
award of which was made in 2011. Currently one Matching Share is awarded for every four Partnership Shares purchased. Partnership Shares and
Matching Shares are held in the HM Revenue & Customs (HMRC)-approved All-Employee Share Plan. At the Company’s AGM in 2002, shareholders
approved the issue of new shares for the purposes of the All-Employee Share Plan.
Sweden
In Sweden, an all-employee performance bonus plan is in operation, which rewards strong individual performance. Bonuses are paid 50% into a
fund investing in AstraZeneca equities and 50% in cash. The AstraZeneca Executive Annual Bonus Scheme, the AstraZeneca Performance Share
Plan and the AstraZeneca Global Restricted Stock Plan all operate in respect of relevant AstraZeneca employees in Sweden.
Other bonus and share plans that operate across the Group are described below.
The AstraZeneca Executive Annual Bonus Scheme
This scheme is a performance bonus scheme for Directors and senior employees who do not participate in the AstraZeneca UK Performance
Bonus Plan. Annual bonuses are paid in cash and reflect both corporate and individual performance measures. The Remuneration Committee has
discretion to reduce or withhold bonuses if business performance falls sufficiently short of expectations in any year such as to make the payment
of bonuses inappropriate.
The AstraZeneca Deferred Bonus Plan
This plan was introduced in 2006 and is used to defer a portion of the bonus earned under the AstraZeneca Executive Annual Bonus Scheme into
Ordinary Shares in the Company for a period of three years. The plan currently operates only in respect of Executive Directors and members of
the SET (with awards granted as AstraZeneca ADRs for members of SET employed within the US). Awards of shares under this plan are typically
made in March each year, the first award having been made in February 2006.
The AstraZeneca Performance Share Plan
This plan was approved by shareholders in 2020 for a period of 10 years (subsequently amended by approval of shareholders in 2021) and replaces
the 2014 AstraZeneca Performance Share Plan. Generally, awards can be granted at any time, but not during a closed period of the Company.
The first grant of Performance Share Plan awards was made in May 2014 under the 2014 AstraZeneca Performance Share Plan. Awards granted
under the plan vest after three years, or in the case of Executive Directors and members of the SET, after an additional two-year holding period,
and is subject to the achievement of performance conditions. For awards granted to all participants in 2023, vesting is subject to a combination
of measures focused on science and innovation, revenue growth, financial performance and carbon reduction. The Remuneration Committee has
responsibility for agreeing any awards under the plan and for setting the policy for the way in which the plan should be operated, including agreeing
performance targets and which employees should be eligible to participate.
The AstraZeneca Investment Plan
This plan was introduced in 2010 and approved by shareholders at the 2010 AGM. The final grant of awards under this plan took place in March
2016. Awards granted under the plan vest after eight years and are subject to performance conditions measured over a period of four years.
The AstraZeneca Global Restricted Stock Plan
The Global Restricted Stock Plan (GRSP) was introduced in 2010. This plan provides for the grant of restricted stock unit (RSU) awards to selected
below SET-level employees and is used in conjunction with the AstraZeneca Performance Share Plan to provide a mix of RSUs and performance
share units (PSUs). Awards typically vest on the third anniversary of the date of grant and are contingent on continued employment with the
Company. The Remuneration Committee has responsibility for agreeing any awards under the plan and for setting the policy for the way in which
the plan should be operated.
The AstraZeneca Restricted Share Plan
This plan was introduced in 2008 and provides for the grant of restricted share unit (RSU) awards to key employees, excluding Executive Directors.
Awards are made on an ad hoc basis with variable vesting dates. The plan has been used five times in 2023 to make awards to 305 employees.
The Remuneration Committee has responsibility for agreeing any awards under the plan and for setting the policy for the way in which the plan
should be operated.
202
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

The AstraZeneca Extended Incentive Plan
This plan was introduced in 2018 and provides for the grant of awards to key employees, excluding Executive Directors. Awards are made on an
ad hoc basis and 50% of the award will normally vest on the fifth anniversary of grant, with the balance vesting on the tenth anniversary of grant.
The award can be subject to the achievement of performance conditions. The Remuneration Committee has responsibility for agreeing any awards
under the plan and for setting the policy for the way in which the plan should be operated, including agreeing performance targets (if any) and which
employees should be invited to participate.
Details of share options outstanding during the year for the main share plans are shown below.
The AstraZeneca
Performance Share Plan
The AstraZeneca
Global Restricted Stock Plan
The AstraZeneca
Restricted Share Plan
The AstraZeneca
Extended Incentive Plan
Ordinary
Shares
ADR
Shares
Ordinary
Shares
ADR
Shares
1
Ordinary
Shares
ADR
Shares
Ordinary
Shares
ADR
Shares
‘000 ‘000 ‘000 ‘000 ‘000 ‘000 ‘000 ‘000
Outstanding at 1January 2021 3,045 4,791 1,626 9,175 161 506 300 65
Granted 1,275 2,082 902 4,509 139 481 – 175
Forfeited (220) (494) (158) (1,254) (18) (42) (18) (45)
Cancelled (9) – (1) (8) – – – –
Exercised (632) (1,201) (341) (2,881) (27) (182) – –
Outstanding at 31December 2021 3,459 5,178 2,028 9,541 255 763 282 195
Granted 1,059 2,339 1,237 6,478 75 216 – –
Forfeited (132) (570) (190) (1,627) (25) (136) (23) –
Cancelled – – – (3) – – – –
Exercised (756) (1,223) (606) (2,706) (72) (165) – –
Outstanding at 31December 2022 3,630 5,724 2,469 11,6 83 233 678 259 195
Granted 976 2,071 1,185 6,343 208 436 71 95
Forfeited (148) (437) (187) (1,417) (20) (59) (8) –
Cancelled – – – (3) – – – (34)
Exercised (813) (1,470) (570) (2,738) (86) (288) (107) (9)
Outstanding at 31 December 2023 3,645 5,888 2,897 13,868 335 767 215 247
The AstraZeneca
Performance Share Plan
The AstraZeneca
Global Restricted Stock Plan
The AstraZeneca
Restricted Share Plan
The AstraZeneca
Extended Incentive Plan
WAFV
1
WAFV WAFV WAFV WAFV WAFV WAFV WAFV
pence $ pence $ pence $ pence $
WAFV of 2021 grants 6012 41.56 6893 47.75 7415 53.96 – 56.83
WAFV of 2022 grants 8328 55.73 9167 61.21 9894 63.35 – –
WAFV of 2023 grants 9929 59.95 10822 65.38 11135 65.37 11748 74.78
Alexion employee share award plan
At acquisition in 2021 Alexion employee share awards were converted into AstraZeneca restricted stock awards that continue to have, and shall be
subject to, the same terms and conditions as applied in the corresponding Alexion awards immediately prior to completion. The fair value at the
grant date was $57.54 and of the 15,220,000 shares outstanding at 31 December 2021, 8,627,000 were exercised and 980,000 were forfeited during
2022. During 2022, Alexion employees had the option to defer awards due to vest in July 2022 until February 2023 when they would also receive
an additional vest equivalent to 15% of the shares deferred. As a result, 1,780,000 shares were deferred, resulting in an additional 267,000 shares
being issued with a grant date fair value of $65.62, that vested in 2023. During 2023, 2,060,000 shares vested, 531,000 were forfeited/cancelled
and the closing balance of these awards as of 31 December 2023 was 3,022,000.
The weighted average fair value for awards granted under the AstraZeneca Performance Share Plan is primarily based on the market price at the
point of grant adjusted for the market-based performance elements which are valued using a modified version of the Monte Carlo method. The fair
values of all other plans are set using the market price at the point of award. These awards are settled in equity including dividends accumulated
from the date of award to vesting.
Notes to the Group Financial Statements 203AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

Notes to the Group Financial Statements
continu ed
30 Commitments, contingent liabilities and contingent assets
2023 2022 2021
Commitments $m $m $m
Contracts placed for future capital expenditure on Property, plant and equipment andsoftware development costs
not provided for in these financial statements 1,368 502 388
Guarantees and contingencies arising in the ordinary course of business, for which no security has been given, are not expected to result in any
material financial loss.
Research and development collaboration payments
The Group has various ongoing collaborations, including in-licensing and similar arrangements with development partners. Such collaborations
may require the Group to make payments on achievement of stages of development, launch or revenue milestones, although the Group generally
has the right to terminate these agreements at no cost. The Group recognises research and development milestones as an intangible asset once
it is committed to payment, which is generally when the Group reaches set trigger points in the development cycle. Revenue-related milestones
are recognised as intangible assets on product launch at a value based on the Group’s long-term revenue forecasts for the related product. The
table below indicates potential development and revenue-related payments that the Group may be required to make under such collaborations.
Years5
Total Under1year Years1and2 Years3and4 andgreater
$m $m $m $m $m
Future potential research and development milestone payments 10,971 1,256 3,798 1,764 4,153
Future potential revenue milestone payments 20,195 43 491 2,400 17,261
The table includes all potential payments for achievement of milestones under ongoing research and development arrangements. Revenue-related
milestone payments represent the maximum possible amount payable on achievement of specified levels of revenue as set out in individual contract
agreements, but exclude variable payments that are based on unit sales (e.g. royalty-type payments) which are expensed as the associated sale
is recognised. The table excludes any payments already capitalised in the Financial Statements for the year ended 31 December 2023 which have
been capitalised with reference to the latest Group sales forecasts for approved indications.
The future payments we disclose represent contracted payments and, as such, are not discounted and are not risk-adjusted. As detailed in the
Risk section from page 54, the development of any pharmaceutical product candidate is a complex and risky process that may fail at any stage
in the development process due to a number of factors (including items such as failure to obtain regulatory approval, unfavourable data from key
studies, adverse reactions to the product candidate or indications of other safety concerns). The timing of the payments is based on the Group’s
current best estimate of achievement of the relevant milestone.
Environmental costs and liabilities
The Group’s expenditure on environmental protection, including both capital and revenue items, relates to costs that are necessary for implementing
internal systems and programmes, and meeting legal and regulatory requirements for processes and products. This includes investment to conserve
natural resources and otherwise minimise the impact of our activities on the environment.
They are an integral part of normal ongoing expenditure for carrying out the Group’s research, manufacturing and commercial operations and are
not separated from overall operating and development costs. There are no known changes in legal, regulatory or other requirements resulting in
material changes to the levels of expenditure for 2021, 2022 or 2023.
In addition to expenditure for meeting current and foreseen environmental protection requirements, the Group incurs costs in investigating and cleaning
up legacy land and groundwater contamination. In particular, AstraZeneca has environmental liabilities at some currently or formerly owned, leased
and third-party sites.
In the US, Zeneca Inc., and/or its indemnitees, have been named as potentially responsible parties (PRPs) or defendants at a number of sites where
Zeneca Inc. is likely to incur future environmental investigation, remediation, operation and maintenance costs under federal, state, statutory or
common law environmental liability allocation schemes (together, US Environmental Consequences). Similarly, Stauffer Management Company LLC
(SMC), which was established in 1987 to own and manage certain assets of Stauffer Chemical Company acquired that year, and/or its indemnitees,
have been named as PRPs or defendants at a number of sites where SMC is likely to incur US Environmental Consequences.
AstraZeneca has also given indemnities to third parties for a number of sites outside the US. These environmental liabilities arise from legacy operations
that are not currently part of the Group’s business and, at most of these sites, remediation, where required, is either completed or in progress.
AstraZeneca has made provisions for the estimated costs of future environmental investigation, remediation, operation and maintenance activity
beyond normal ongoing expenditure for maintaining the Group’s R&D and manufacturing capacity and product ranges, where a present obligation
exists, it is probable that such costs will be incurred and they can be estimated reliably. With respect to such estimated future costs, there were
provisions at 31 December 2023 in the aggregate of $112m (2022: $131m; 2021: $90m), mainly relating to the US. Where we are jointly liable or
otherwise have cost-sharing agreements with third parties, we reflect only our share of the obligation. Where the liability is insured in part or in
whole by insurance or other arrangements for reimbursement, an asset is recognised to the extent that this recovery is virtually certain.
It is possible that AstraZeneca could incur future environmental costs beyond the extent of our current provisions. The extent of such possible
additional costs is inherently difficult to estimate due to a number of factors, including: (1) the nature and extent of claims that may be asserted in the
future; (2) whether AstraZeneca has or will have any legal obligation with respect to asserted or unasserted claims; (3) the type of remedial action,
if any, that may be selected at sites where the remedy is presently not known; (4) the potential for recoveries from or allocation of liability to third
parties; and (5) the length of time that the environmental investigation, remediation and liability allocation process can take. As per our accounting
policy on page 158, Provisions for these costs are made when there is a present obligation and where it is probable that expenditure on remedial
work will be required and a reliable estimate can be made of the cost. Notwithstanding and subject to the foregoing, we estimate the potential
additional loss for future environmental investigation, remediation, remedial operation and maintenance activity above and beyond our provisions
to be, in aggregate, between $114m and $191m (2022: $113m and $188m; 2021: $99m and $165m) which relates mainly to the US.
204
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Legal proceedings
AstraZeneca is involved in various legal
proceedings considered typical to its
business, including actual or threatened
litigation and actual or potential government
investigations relating to employment matters,
product liability, commercial disputes, pricing,
sales and marketing practices, infringement of
IP rights, and the validity of certain patents
and competition laws. The more significant
matters are discussed below.
Most of the claims involve highly complex
issues. Often these issues are subject to
substantial uncertainties and, therefore, the
probability of a loss, if any, being sustained
and/or an estimate of the amount of any loss
is difficult to ascertain.
We do not believe that disclosure of the
amounts sought by plaintiffs, if known, would
be meaningful with respect to these legal
proceedings. This is due to a number of
factors, including (i) the stage of the
proceedings (in many cases trial dates have
not been set) and the overall length and extent
of pre-trial discovery; (ii) the entitlement of the
parties to an action to appeal a decision; (iii)
clarity as to theories of liability, damages and
governing law; (iv) uncertainties in timing of
litigation; and (v) the possible need for further
legal proceedings to establish the appropriate
amount of damages, if any.
While there can be no assurance regarding
the outcome of any of the legal proceedings
referred to in this Note 30, based on
management’s current and considered view of
each situation, we do not currently expect
them to have a material adverse effect on our
financial position including within the next
financial year. This position could of course
change over time, not least because of the
factors referred to above.
In cases that have been settled or
adjudicated, or where quantifiable fines and
penalties have been assessed and which are
not subject to appeal (or other similar forms of
relief), or where a loss is probable and we are
able to make a reasonable estimate of the
loss, we generally indicate the loss absorbed
or make a provision for our best estimate of
the expected loss.
Where it is considered that the Group is more
likely than not to prevail, legal costs involved
in defending the claim are charged to profit as
they are incurred.
Where it is considered that the Group has a
valid contract which provides the right to
reimbursement (from insurance or otherwise)
of legal costs and/or all or part of any loss
incurred or for which a provision has been
established, and we consider recovery to be
virtually certain, the best estimate of the
amount expected to be received is recognised
as an asset.
Assessments as to whether or not to
recognise provisions or assets, and of the
amounts concerned, usually involve a series of
complex judgements about future events and
can rely heavily on estimates and assumptions.
AstraZeneca believes that the provisions
recorded are adequate based on currently
available information and that the insurance
recoveries recorded will be received. However,
given the inherent uncertainties involved in
assessing the outcomes of these cases, and in
estimating the amount of the potential losses
and the associated insurance recoveries, we
could in the future incur judgments or insurance
settlements that could have a material adverse
effect on our results in any particular period.
IP claims include challenges to the Group’s
patents on various products or processes and
assertions of non-infringement of patents.
A loss in any of these cases could result in loss
of patent protection on the related product.
The consequences of any such loss could be
a significant decrease in Product Sales, which
could have a material adverse effect on our
results. The lawsuits filed by AstraZeneca for
patent infringement against companies that
have filed abbreviated new drug applications
(ANDAs) in the US, seeking to market generic
forms of products sold by the Group prior to the
expiry of the applicable patents covering these
products, typically also involve allegations of
non-infringement, invalidity and unenforceability
of these patents by the ANDA filers. In the event
that the Group is unsuccessful in these actions
or the statutory 30-month stay expires before
a ruling is obtained, the ANDA filers involved
will also have the ability, subject to FDA
approval, to introduce generic versions of the
product concerned.
AstraZeneca has full confidence in, and will
vigorously defend and enforce, its IP.
Over the course of the past several years,
including in 2023, a significant number of
commercial litigation claims in which
AstraZeneca is involved have been resolved,
particularly in the US, thereby reducing potential
contingent liability exposure arising from such
litigation. Similarly, in part due to patent litigation
and settlement developments, greater certainty
has been achieved regarding possible generic
entry dates with respect to some of our patented
products. At the same time, like other companies
in the pharmaceutical sector and other
industries, AstraZeneca continues to be subject
to government investigations around the world.
Patent litigation
Legal proceedings brought against AstraZeneca
for which a provision has been taken
Imfinzi and Imjudo
US and ROW patent proceedings
In February 2022, in Japan, Ono Pharmaceuticals
filed a lawsuit in Tokyo District Court, Civil
Division against AstraZeneca alleging that
AstraZeneca’s marketing of Imfinzi in Japan
infringed several of their patents.
In March 2022, Bristol-Myers Squibb Co. and
E.R. Squibb & Sons, LLC filed a lawsuit in the
US District Court for the District of Delaware
(District Court) against AstraZeneca alleging
that AstraZeneca’s marketing of Imfinzi
infringed several of their patents. In April 2023,
Bristol-Myers Squibb Co., E.R. Squibb & Sons,
LLC, Tasuku Honjo, Ono Pharmaceutical Co.,
Ltd., and the Dana-Farber Cancer Institute Inc.
filed a separate lawsuit in the District Court
against AstraZeneca alleging that AstraZeneca’s
marketing of Imfinzi infringed another of
their patents.
In January 2023, Bristol-Myers Squibb Co.
and E.R. Squibb & Sons, LLC filed a lawsuit
in the District Court against AstraZeneca
alleging that AstraZeneca’s marketing of
Imjudo infringed two of their patents.
In July 2023, AstraZeneca entered into a global
settlement agreement with Bristol-Myers
Squibb Co., E.R. Squibb & Sons, LLC, and
Ono Pharmaceutical Co., Ltd. that resolves
all patent disputes between the companies
relating to Imfinzi and Imjudo. In June 2023,
a provision was taken totaling $510m.
These matters are now concluded.
Legal proceedings brought against AstraZeneca
considered to be contingent liabilities
Enhertu
US patent proceedings
In October 2020, Seagen Inc. (Seagen) filed
a complaint against Daiichi Sankyo Company,
Limited (Daiichi Sankyo) in the US District Court
for the Eastern District of Texas (District Court)
alleging that Enhertu infringes a Seagen patent.
AstraZeneca co-commercialises Enhertu with
Daiichi Sankyo, Inc. in the US. After trial in April
2022, the jury found that the patent was infringed
and awarded Seagen $41.82m in past damages.
In July 2022, the District Court entered final
judgment and declined to enhance damages
on the basis of wilfulness. In October 2023,
the District Court entered an amended final
judgment that requires Daiichi Sankyo to pay
Seagen a royalty of 8% on US sales of Enhertu
from April 1, 2022, through November 4, 2024, in
addition to the past damages previously awarded
by the Court. AstraZeneca and Daiichi Sankyo
have appealed the District Court’s decision.
In December 2020 and January 2021,
AstraZeneca and Daiichi Sankyo, Inc. filed
post-grant review (PGR) petitions with the US
Patent and Trademark Office (USPTO) alleging,
inter alia, that the Seagen patent is invalid for
lack of written description and enablement.
The USPTO initially declined to institute the
PGRs, but, in April 2022, the USPTO granted
the rehearing requests, instituting both PGR
petitions. Seagen subsequently disclaimed
all patent claims at issue in one of the PGR
proceedings. In July 2022, the USPTO reversed
its institution decision and declined to institute
the other PGR petition. AstraZeneca and Daiichi
Sankyo, Inc. requested reconsideration of the
decision not to institute review of the patent.
Notes to the Group Financial Statements 205AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
30 Commitments, contingent liabilities and contingent assets con tinued
Notes to the Group Financial Statements
continu ed
In February 2023, the USPTO reinstituted the
PGR proceeding. An oral hearing took place in
August 2023. In January 2024, the USPTO
issued a decision that Seagen’s patent is
unpatentable, invalidating all claims asserted
against Enhertu. The USPTO’s decision
does not overturn the Texas District Court’s
decision unless and until the USPTO’s decision
is affirmed on appeal by the US Court of
Appeals for the Federal Circuit. No such appeal
has been filed.
Faslodex
Patent proceedings outside the US
In 2021 in Japan, AstraZeneca received notice
from the Japan Patent Office (JPO) that Sandoz
K.K. (Sandoz) and Sun Pharma Japan Ltd.
(Sun) were seeking to invalidate the Faslodex
formulation patent. AstraZeneca defended the
challenged patent, and Sun withdrew from the
JPO patent challenge. In July 2023, the JPO
issued a final decision upholding various claims
of the challenged patent and determining that
other patent claims were invalid. In August
2023, Sandoz appealed the JPO decision to
the Japan IP High Court.
Tagrisso
US patent proceedings
In September 2021, Puma Biotechnology, Inc.
and Wyeth LLC filed a patent infringement
lawsuit in the US District Court for the District
of Delaware against AstraZeneca relating to
Tagrisso. Trial has been scheduled for May 2024.
Legal proceedings brought by AstraZeneca
considered to be contingent assets
Brilinta
US patent proceedings
In 2015 and subsequently, in response to
Paragraph IV notices from ANDA filers,
AstraZeneca filed patent infringement lawsuits
in the US District Court for the District of
Delaware (District Court) relating to patents
listed in the FDA Orange Book with reference
to Brilinta. In 2022, AstraZeneca entered into
several separate settlements and the District
Court entered consent judgments to dismiss
each of the corresponding litigations.
Additional proceedings are ongoing in the
District Court. No trial date has been set.
Calquence
US patent proceedings
In February 2022, in response to Paragraph IV
notices from multiple ANDA filers, AstraZeneca
filed patent infringement lawsuits in the US
District Court for the District of Delaware. In its
complaint, AstraZeneca alleges that a generic
version of Calquence, if approved and marketed,
would infringe patents listed in the FDA Orange
Book with reference to Calquence that are
owned or licensed by AstraZeneca. Trial has
been scheduled for March 2025.
In February 2023, Sandoz Inc. filed a petition
for inter partes review with the US Patent and
Trademark Office of certain Calquence patent
claims. AstraZeneca has asserted claims for
patent infringement against Sandoz and
other defendants in the US ANDA litigation.
In August 2023, the US Patent Trial and
Appeal Board issued a decision denying
institution of inter partes review.
Daliresp
US patent proceedings
In 2015 and subsequently, in response to
Paragraph IV notices from ANDA filers,
AstraZeneca filed patent infringement lawsuits
in the US District Court for the District of New
Jersey (District Court) relating to patents listed
in the FDA Orange Book with reference to
Daliresp. In 2022, AstraZeneca entered into a
settlement agreement and the District Court
entered a consent judgment to dismiss the
corresponding litigation. Additional ANDA
challenges are pending.
Farxiga
US patent proceedings
In May 2021, AstraZeneca proceeded to trial
against ANDA filer Zydus Pharmaceuticals (USA)
Inc. (Zydus) in the US District Court for the
District of Delaware (District Court). In October
2021, the District Court issued a decision finding
the asserted claims of AstraZeneca’s patent as
valid and infringed by Zydus’s ANDA product. In
August 2022, Zydus appealed the District Court
decision. Zydus’s appeal has been dismissed.
In December 2023, AstraZeneca initiated
ANDA litigation against Sun Pharmaceutical
Industries Ltd. and Sun Pharmaceutical
Industries, Inc. in the District Court. No trial
date has been set.
Lokelma
US patent proceedings
In August 2022, in response to Paragraph IV
notices, AstraZeneca initiated ANDA litigation
against multiple generic filers in the US District
Court for the District of Delaware. Trial has been
scheduled for March 2025.
Lynparza
US patent proceedings
In December 2022, AstraZeneca received a
Paragraph IV notice from an ANDA filer relating
to patents listed in the FDA Orange Book with
reference to Lynparza. In February 2023,
in response to the Paragraph IV notice,
AstraZeneca, MSD International Business
GmbH, and the University of Sheffield initiated
ANDA litigation against Natco Pharma Limited
(Natco) in the US District Court for the District
of New Jersey. In the complaint, AstraZeneca
alleged that Natco’s generic version of Lynparza,
if approved and marketed, would infringe patents
listed in the FDA Orange Book with reference
to Lynparza. No trial date has been scheduled.
In December 2023, AstraZeneca received a
Paragraph IV notice from an ANDA filer relating
to patents listed in the FDA Orange Book
with reference to Lynparza. In February 2024,
in response to the Paragraph IV notice,
AstraZeneca, MSD International Business
GmbH, and the University of Sheffield initiated
ANDA litigation against Sandoz Inc. (Sandoz)
in the US District Court for the District of New
Jersey. In the complaint, AstraZeneca alleged
that Sandoz’s generic version of Lynparza, if
approved and marketed, would infringe patents
listed in the FDA Orange Book with reference
to Lynparza. No trial date has been scheduled.
Soliris
US patent proceedings
In January 2024, Alexion initiated patent
infringement litigation against Samsung
Bioepis Co. Ltd. in the US District Court
for the District of Delaware alleging that
Samsung’s biosimilar eculizumab product,
for which Samsung is currently seeking
FDA approval, will infringe six Soliris-related
patents. No trial date has been scheduled.
Five of the six asserted patents are also the
subject of inter partes review proceedings
before the US Patent and Trademark Office.
Tagrisso
Patent proceedings outside the US
In Russia, in August 2023, AstraZeneca filed
lawsuits in the Arbitration Court of the
Moscow Region (Court) against the Ministry
of Health of the Russian Federation and
Axelpharm LLC related to Axelpharm’s
improper use of AstraZeneca’s information
to obtain authorisation to market a generic
version of Tagrisso. In December 2023, the
Court dismissed the lawsuit against the
Ministry of Health of the Russian Federation.
In January 2024, AstraZeneca filed an appeal,
which is pending. The lawsuit against
Axelpharm remains pending before the Court.
In Russia, in November 2023, Axelpharm LLC
filed a compulsory licensing action against
AstraZeneca in the Arbitration Court of the
Moscow Region (Court) related to a patent
that covers Tagrisso. The lawsuit remains
pending before the Court.
Legal proceedings brought against AstraZeneca
which have been concluded
Movantik
US patent proceedings
AstraZeneca has resolved by settlement
agreement the previously disclosed patent
infringement lawsuit brought by Aether
Therapeutics, Inc. in the US District Court for the
District of Delaware against AstraZeneca, Nektar
Therapeutics and Daiichi Sankyo, Inc., relating
to Movantik. This matter is now concluded.
Legal proceedings brought by AstraZeneca
which have been concluded
Symbicort
US patent proceedings
In February 2023, AstraZeneca resolved
by settlement agreement the previously
disclosed ANDA litigations with Mylan
Pharmaceuticals Inc. and Kindeva Drug
Delivery L.P. (together, defendants). In those
actions, AstraZeneca alleged that the
defendants’ generic versions of Symbicort,
206
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

if approved and marketed, would infringe
various AstraZeneca patents. This matter is
now concluded.
Tagrisso
Patent proceedings outside the US
In Russia, in October 2021, AstraZeneca
filed a lawsuit in the Arbitration Court of the
Moscow Region (Court) against Axelpharm,
LLC to prevent it from obtaining authorisation
to market a generic version of Tagrisso prior
to the expiration of AstraZeneca’s patents
covering Tagrisso. The lawsuit also names the
Ministry of Health of the Russian Federation
as a third party. In March 2022, the Court
dismissed the lawsuit. In June 2022, the
dismissal was affirmed on appeal. In January
2023, the dismissal was affirmed on further
appeal. This matter is now concluded.
Product liability litigation
Legal proceedings brought against AstraZeneca
for which a provision has been taken
Nexium and Losec/Prilosec
US proceedings
AstraZeneca has been defending lawsuits
brought in federal and state courts involving
claims that plaintiffs have been diagnosed with
various injuries following treatment with proton
pump inhibitors (PPIs), including Nexium and
Prilosec. Most of the lawsuits alleged kidney
injury. In August 2017, the pending federal court
cases were consolidated in a multidistrict
litigation (MDL) proceeding in the US District
Court for the District of New Jersey for
pre-trial purposes. In addition to the MDL
cases, there were cases alleging kidney injury
filed in Delaware and New Jersey state courts.
In addition, AstraZeneca has been defending
lawsuits involving allegations of gastric cancer
following treatment with PPIs, including one
such claim in the US District Court for the
Middle District of Louisiana (Louisiana
District Court).
In October 2023, AstraZeneca resolved all
pending claims in the MDL, as well as all
pending claims in Delaware and New Jersey
state courts, for $425m, for which a provision
has been taken. The only remaining case is
the one pending in the Louisiana District
Court. The Court in that case has postponed
trial, which was previously scheduled to begin
in April 2024. No new trial date has been set.
Legal proceedings brought against AstraZeneca
considered to be contingent liabilities
Farxiga and Xigduo XR
US proceedings
AstraZeneca has been named as a defendant
in lawsuits involving plaintiffs claiming physical
injury, including Fournier’s Gangrene and
necrotising fasciitis, from treatment with
Farxiga and/or Xigduo XR. In September 2023,
the parties resolved by settlement agreement
one case, filed in state court in Minnesota,
previously scheduled for trial in October 2023.
All remaining claims are filed in Delaware state
court and remain pending.
Nexium and Losec/Prilosec
Canada proceedings
In Canada, in July and August 2017,
AstraZeneca was served with three putative
class action lawsuits. Two of the lawsuits
have been dismissed, one in 2019 and one in
2021. The third lawsuit seeks authorisation to
represent individual residents in Canada who
allegedly suffered kidney injuries from the use
of proton pump inhibitors, including Nexium
and Losec.
Onglyza and Kombiglyze
US proceedings
In the US, AstraZeneca is defending various
lawsuits alleging heart failure, cardiac injuries,
and/or death from treatment with Onglyza or
Kombiglyze. In August 2022, the US District
Court for the Eastern District of Kentucky,
presiding over the consolidated federal cases,
granted AstraZeneca’s motion for summary
judgment, which plaintiffs have appealed to
the US Court of Appeals for the Sixth Circuit.
In the California state court proceeding, the
trial court granted summary judgment for
AstraZeneca, which the California appellate
court affirmed. The California Supreme Court
has declined further review, so the California
state court proceeding has concluded.
Commercial litigation
Legal proceedings brought against AstraZeneca
considered to be contingent liabilities
340B Antitrust Litigation
US proceedings
In September 2021, AstraZeneca was served
with a class-action antitrust complaint filed in
the US District Court for the Western District
of New York (District Court) by Mosaic Health
alleging a conspiracy to restrict access to 340B
discounts in the diabetes market through
contract pharmacies. In September 2022, the
District Court granted AstraZeneca’s motion
to dismiss the Complaint. In February 2024,
the District Court denied Plaintiffs’ request to
file a new amended complaint and entered an
order closing the matter.
Anti-Terrorism Act Civil Lawsuit
US proceedings
In the US, in October 2017, AstraZeneca and
certain other pharmaceutical and/or medical
device companies were named as defendants
in a complaint filed in the US District Court for
the District of Columbia (District Court) by US
nationals (or their estates, survivors, or heirs)
who were killed or wounded in Iraq between
2005 and 2013. The plaintiffs allege that the
defendants violated the US Anti-Terrorism
Act and various state laws by selling
pharmaceuticals and medical supplies to the
Iraqi Ministry of Health. In July 2020, the District
Court granted AstraZeneca’s and the other
defendants’ motion to dismiss the lawsuit, which
the DC Circuit Court of Appeals (the Appellate
Court) reversed in January 2022. In February
2023, the Appellate Court denied a request for
en banc review. In June 2023, AstraZeneca
and the other defendants filed a petition for
review by the United States Supreme Court.
Caelum Trade Secrets Litigation
US proceedings
AstraZeneca has been defending a matter
filed by the University of Tennessee Research
Foundation in the US District Court for the
Eastern District of Tennessee (District Court)
related to CAEL-101. In October 2023,
AstraZeneca filed a motion for summary
judgment on all claims and awaits a decision by
the District Court. Trial is currently scheduled
for September 2024.
Definiens
Germany proceedings
In Germany, in July 2020, AstraZeneca received
a notice of arbitration filed with the German
Institution of Arbitration from the sellers of
Definiens AG (the Sellers) regarding the 2014
Share Purchase Agreement (SPA) between
AstraZeneca and the Sellers. The Sellers claim
that they are owed approximately $140m in
earn-outs under the SPA. The arbitration hearing
took place in March 2023 and final post-hearing
written briefs were submitted in June 2023. In
December 2023, the arbitration panel made a
final award of $46.43m in favour of the Sellers.
AstraZeneca is considering its options.
Employment Litigation
US proceedings
In December 2022, AstraZeneca was served
with a lawsuit filed by seven former employees
in the US District Court for the District of
Delaware (District Court) asserting age, religion,
and disability discrimination claims related to
AstraZeneca’s vaccination requirement. In March
2023, AstraZeneca filed a motion to dismiss the
religious and disability discrimination claims and
a motion to strike the class and collective claims.
That motion is fully briefed and the parties are
awaiting a decision by the District Court.
Pay Equity Litigation
US proceedings
AstraZeneca was defending a putative class
and collective action matter in the US District
Court for the Northern District of Illinois (District
Court) brought by three named plaintiffs, who
are former AstraZeneca employees. The case
involved claims under the federal and Illinois
Equal Pay Acts, with the plaintiffs alleging they
were paid less than male employees who
performed substantially similar and/or equal
work. In January 2023, the District Court granted
AstraZeneca’s motion to dismiss plaintiffs’
complaint. In March 2023, plaintiffs filed a
Second Amended Complaint. AstraZeneca
moved to dismiss the Second Amended
Complaint in April 2023. The motion to dismiss
was denied in October 2023, and the parties
are proceeding with discovery.
Seroquel XR (Antitrust Litigation)
US proceedings
In 2019, AstraZeneca was named in several
related complaints brought in the US District
Court for the Southern District of New York
(District Court), including several putative
class action lawsuits that were purportedly
brought on behalf of classes of direct
Notes to the Group Financial Statements 207AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Group Financial Statements
continu ed
30 Commitments, contingent liabilities and contingent assets con tinued
purchasers or end payors of Seroquel XR,
that allege AstraZeneca and generic drug
manufacturers violated US antitrust laws when
settling patent litigation related to Seroquel XR.
In July 2022, in response to AstraZeneca’s
motion to dismiss, the District Court dismissed
all claims relating to the settlement with one
of the generic manufacturers but denied the
motion with respect to all claims relating to
the second generic manufacturer and allowed
those claims to proceed. Trial is currently
scheduled for May 2025.
Syntimmune
US proceedings
In connection with Alexion’s prior acquisition of
Syntimmune, Inc., (Syntimmune) in December
2020, Alexion was served with a lawsuit
filed by the stockholders’ representative for
Syntimmune in Delaware state court that
alleged, among other things, breaches of
contractual obligations relating to the 2018
merger agreement. The stockholders’
representative alleges that Alexion failed to
meet its obligations under the merger
agreement to use commercially reasonable
efforts to achieve the milestones. Alexion also
filed a claim for breach of the representations
in the 2018 merger agreement. A trial was held
in July 2023 and a decision is expected in 2024.
Viela Bio, Inc. Shareholder Litigation
US proceedings
In February 2023, AstraZeneca was served with
a lawsuit filed in Delaware state court against
AstraZeneca and certain officers (collectively,
defendants), on behalf of a putative class
of Viela Bio, Inc. (Viela) shareholders. The
complaint alleges that defendants breached
their fiduciary duty to Viela shareholders in the
course of Viela’s 2021 merger with Horizon
Therapeutics, plc. In May 2023, AstraZeneca
filed a motion to dismiss, which is now fully
briefed and pending before the Court.
Legal proceedings brought by AstraZeneca
considered to be contingent assets
PARP Inhibitor Royalty Dispute
UK proceedings
In October 2012, Tesaro, Inc. (now wholly owned
by GlaxoSmithKline plc, (GSK)) entered into
two worldwide, royalty-bearing patent license
agreements with AstraZeneca related to GSK’s
product niraparib. In May 2021, AstraZeneca
filed a lawsuit against GSK in the Commercial
Court of England and Wales alleging that GSK
has failed to pay all of the royalties due on
niraparib sales under the license agreements.
The case was transferred to the Chancery
Division and a trial took place in March 2023.
In April 2023, the court issued a decision in
AstraZeneca’s favour. GSK has been granted
permission to appeal, and the appellate
hearing was held in January 2024.
Legal proceedings brought against AstraZeneca
which have been concluded
Alexion Shareholder Litigation
US proceedings
In December 2016, putative securities class
action lawsuits were filed in the US District
Court for the District of Connecticut (District
Court) against Alexion and certain officers and
directors (collectively, defendants), on behalf of
purchasers of Alexion publicly traded securities
during the period 30 January 2014 through
26 May 2017. The amended complaint alleged
that defendants engaged in securities fraud,
including by making misrepresentations and
omissions in their public disclosures concerning
Alexion’s Soliris sales practices, management
changes, and related investigations. In August
2021, the District Court issued a decision
denying in part defendants’ motion to dismiss
the matter. The Court granted plaintiffs’ motion
for class certification in April 2023. In August
2023, the parties reached a settlement in
principle of this matter. In September 2023,
the court granted preliminary approval of the
class settlement. A provision was taken in
September 2023. The court granted final
approval of the class settlement in December
2023, and the matter is now concluded.
AZD1222 Securities Litigation
US proceedings
In January 2021, putative securities class action
lawsuits were filed in the US District Court for
the Southern District of New York (District Court)
against AstraZeneca PLC and certain officers,
on behalf of purchasers of AstraZeneca publicly
traded securities during a period later amended
to cover 15 June 2020 through 29 January
2021. The Amended Complaint alleges that
defendants made materially false and misleading
statements in connection with the development
of AZD1222, AstraZeneca’s vaccine for the
prevention of COVID-19. In September 2022,
the District Court granted AstraZeneca’s
motion to dismiss the Amended Complaint
with prejudice. In May 2023, the US Court of
Appeals for the Second Circuit affirmed the
dismissal. The matter is now concluded.
Portola Shareholder Litigation
US proceedings
In connection with Alexion’s July 2020
acquisition of Portola Pharmaceuticals, Inc.
(Portola), Alexion assumed litigation to which
Portola is a party. In January 2020, putative
securities class action lawsuits were filed in
the US District Court for the Northern District
of California against Portola and certain officers
and directors (collectively, defendants), on
behalf of purchasers of Portola publicly traded
securities during the period 8 January 2019
through 26 February 2020. The operative
complaints alleged that defendants made
materially false and/or misleading statements
or omissions with regard to Andexxa. In June
2022, the parties reached a settlement in
principle of this matter. In March 2023, the
court granted final approval of the settlement.
The matter is now concluded.
Government investigations/proceedings
Legal proceedings brought against AstraZeneca
considered to be contingent liabilities
340B Qui Tam
US proceedings
In July 2023, AstraZeneca was served with an
unsealed civil lawsuit brought by a qui tam
relator on behalf of the United States, several
states, and the District of Columbia in the
US District Court for the Central District
of California. The complaint alleges that
AstraZeneca violated the US False Claims Act
(FCA) and state-law analogues. In September
2023, AstraZeneca filed a motion to dismiss
the relator’s claims. In response, the relator
filed a First Amended Complaint. In December
2023, AstraZeneca filed a motion to dismiss
the First Amended Complaint.
340B Administrative Proceedings
US proceedings
In September 2023, the Arkansas Insurance
Department sent AstraZeneca an administrative
complaint concerning compliance with
Arkansas’s 340B Statute, which requires
manufacturers to recognize an unlimited number
of contract pharmacies.
Previously disclosed Administrative Dispute
Resolution proceedings against AstraZeneca
remain pending before the US Health Resources
and Services Administration.
Brazilian Tax Assessment Matter
Brazil proceedings
In connection with an ongoing matter, in August
2019, the Brazilian Federal Revenue Service
provided a Notice of Tax and Description of
the Facts (the Tax Assessment) to two Alexion
subsidiaries (the Brazil Subsidiaries), as well as
to two additional entities – a logistics provider
utilised by Alexion and a distributor. The Tax
Assessment focuses on the importation of
Soliris vials pursuant to Alexion’s free drug
supply to patients programme in Brazil.
Alexion prevailed in the first level of
administrative appeals in the Brazilian federal
administrative proceeding system based on a
deficiency in the Brazil Tax Assessment. The
decision was subject to an automatic (ex officio)
appeal to the second level of the administrative
courts. In March 2023, the second level of the
administrative courts issued a decision to
remand the matter to the first level of
administrative courts for a determination on
the merits.
Texas Qui Tam
US proceedings
In December 2022, AstraZeneca was served
with an unsealed civil lawsuit brought by qui tam
relators on behalf of the State of Texas in Texas
state court, which alleges that AstraZeneca
engaged in unlawful marketing practices. In
March 2023, AstraZeneca filed a motion to
dismiss and a motion to transfer venue. In
response, relators filed an Amended Petition.
In May 2023, AstraZeneca filed a motion to
208
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

dismiss the Amended Petition and renewed its
motion to transfer venue. In September 2023,
the Texas state court denied AstraZeneca’s
motion to transfer venue and motion to dismiss.
Trial is currently scheduled for October 2024.
Turkish Ministry of Health Matter
Turkey proceedings
In Turkey, in July 2020, the Turkish Ministry
of Health (Ministry of Health) initiated an
investigation regarding payments to healthcare
providers by Alexion Turkey and former
employees and consultants. The investigation
arose from Alexion’s disclosure of a $21.5m
civil settlement with the US Securities &
Exchange Commission (SEC) in July 2020 fully
resolving the SEC’s investigation into possible
violations of the US Foreign Corrupt Practices
Act. In September 2021, the Ministry of Health
completed its draft investigation report, and
referred the matter to the Ankara Public
Prosecutor’s Office with a recommendation
for further proceedings against certain
former employees.
US Congressional Inquiry
US proceedings
In January 2024, AstraZeneca received a letter
from the US Senate Committee on Health,
Education, Labor and Pensions (HELP
Committee) seeking information related to
AstraZeneca’s inhaled Respiratory products.
AstraZeneca intends to cooperate with
the inquiry.
Vermont US Attorney Investigation
US proceedings
In April 2020, AstraZeneca received a Civil
Investigative Demand from the US Attorney’s
Office in Vermont and the Department of
Justice, Civil Division, seeking documents
and information relating to AstraZeneca’s
relationships with electronic health-record
vendors. AstraZeneca continues to cooperate
with this enquiry.
Legal proceedings brought by AstraZeneca
considered to be contingent assets
Inflation Reduction Act Litigation
US proceedings
In August 2023, AstraZeneca filed a lawsuit in
federal court in Delaware challenging aspects
of the drug price negotiation provisions of the
Inflation Reduction Act and the implementing
guidance and regulations promulgated by the
US Department of Health and Human Services.
Louisiana 340B Litigation
US proceedings
In August 2023, AstraZeneca filed a lawsuit
against the State of Louisiana alleging that
the Louisiana’s 340B statute, which requires
manufacturers to recognize an unlimited
number of contract pharmacies, is preempted
on several grounds and violates the Contracts
Clause of the U.S. Constitution. AstraZeneca
and the State of Louisiana have moved for
summary judgment on AstraZeneca’s claims.
Legal proceedings brought against AstraZeneca
which have been concluded
COVID-19 Vaccine Supply and
ManufacturingInquiries
Brazil proceedings
In February 2022, a Brazilian Public
Prosecutor filed a lawsuit against several
defendants including the Brazilian Federal
Government, AstraZeneca, and other
COVID-19 vaccine manufacturers. In April 2022,
a Brazilian Court issued an order dismissing
the lawsuit. In October 2023, the pending
appeal was dismissed. No further appeal was
made. This matter is now concluded.
Legal proceedings brought by AstraZeneca
which have been concluded
US 340B Litigation
US proceedings
In January 2021, AstraZeneca filed a lawsuit in
the US District Court for the District of Delaware
(District Court) alleging that an Advisory Opinion
issued by the Department of Health and Human
Services violates the Administrative Procedure
Act. In June 2021, the District Court found in
favour of AstraZeneca, invalidating the Advisory
Opinion. However, in May 2021, prior to the
District Court’s ruling, the US Government
issued new and separate letters to AstraZeneca
(and other companies) asserting that
AstraZeneca’s contract pharmacy policy violates
the 340B statute. AstraZeneca amended the
complaint to include allegations challenging the
letter sent in May 2021, and in February 2022,
the District Court ruled in favour of AstraZeneca
invalidating those letters sent by the US
Government. In January 2023, the Court of
Appeals affirmed the District Court’s decision
in AstraZeneca’s favour. Final judgment was
entered in favour of AstraZeneca in May 2023
and this matter is now concluded.
Other
Additional government inquiries
As is true for most, if not all, major prescription
pharmaceutical companies, AstraZeneca is
currently involved in multiple inquiries into drug
marketing and pricing practices. In addition to
the investigations described above, various law
enforcement offices have, from time to time,
requested information from the Group. There
have been no material developments in
those matters.
Tax
AstraZeneca considers whether it is probable
that a taxation authority will accept an uncertain
tax treatment. If it is concluded that it is not
probable that the taxation authority will accept
an uncertain tax treatment, where tax exposures
can be quantified, a tax liability is recognised
based on either the most likely amount method
or the expected value method depending on
which method management expects to better
predict the resolution of the uncertainty. Tax
liabilities for uncertain tax treatments can be
built up over a long period of time but the
resolution of such tax exposures usually occurs
at a point in time, and given the inherent
uncertainties in assessing the outcomes of these
exposures (which sometimes can be binary in
nature), we could, in future periods, experience
adjustments to the liabilities recognised in
respect of uncertain tax treatments that have
a material positive or negative effect on our
results in any particular period. Details of the
movements in relation to material uncertain
tax treatments are discussed below.
AstraZeneca faces a number of audits
and reviews in jurisdictions around the world
and, in some cases, is in dispute with the tax
authorities. The issues under discussion are
often complex and can require many years to
resolve. Tax liabilities recognised for uncertain
tax treatments require management to make
key judgements with respect to the outcome
of current and potential future tax audits, and
actual results could vary from these estimates.
Management does not believe a significant
risk of material change to uncertain tax
positions exists in the next 12 months.
The total net tax liability recognised in the Group
Financial Statements in respect of uncertain
tax positions is $1,336m (2022: $830m; 2021:
$768m). The net tax liability consists of $1,241m
(2022: $632m; 2021: $702m) included within
income tax payable, $441m (2022: $291m;
2021: $(33)m) included within deferred tax
asset, partially offset by $9m (2022: $(20)m;
2021: $(17)m) included within deferred tax
liabilities, and $337m (2022: $113m; 2021:
additional $82m) included within income
tax receivable.
Transfer pricing
The net tax liability included in the Group
Financial Statements to cover the worldwide
exposure to uncertain tax treatments is $401m
(2022: $260m; 2021: $77m). The increase in the
net tax liability for uncertain tax positions relating
to transfer pricing of $141m compared with 2022
is mainly as a result of an increase of tax
liabilities arising from updates to estimates of
prior period tax liabilities following progression
of tax authority reviews.
These matters can be complex and
judgemental. The liability includes uncertain
tax treatments which are estimated using the
expected value method and depend on
AstraZeneca’s assessment of the likelihood of
the approach taken by the tax authorities and
could change in the future to reflect progress
in tax authority reviews, the extent that any
tax authority challenge is concluded, or
matters lapse including following expiry of the
relevant statutes of limitation resulting in a
reduction in the tax charge in future periods.
For transfer pricing matters, including items
under tax audit, AstraZeneca estimates the
potential for additional tax liabilities above the
amount provided where the possibility of the
additional liabilities falling due is more than
remote, to be up to $386m (2022: $245m;
2021: $48m) including associated interest.
Notes to the Group Financial Statements 209AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report

30 Commitments, contingent liabilities and contingent assets con tinued
Management believes that it is unlikely that these
additional liabilities will arise. It is possible that
some of these contingencies may change in the
future to reflect progress in tax authority reviews,
to the extent that any tax authority challenge is
concluded or matters lapse including following
expiry of the relevant statutes of limitation
resulting in a reduction in the tax charge in
future periods. Management continues to
believe that AstraZeneca’s positions on all its
transfer pricing positions, audits and disputes
are robust, and that AstraZeneca has recognised
appropriate tax balances, including
consideration of whether corresponding relief
will be available under Mutual Agreement
procedures or unilaterally.
Other uncertain tax treatments
Included in the net tax liability is $935m (2022:
$570m; 2021: $691m) relating to a number of
other uncertain tax treatments. The increase
of $365m in the net tax liability relating to the
other uncertain tax treatments mainly relates
to an update to tax liabilities following progress
of reviews by tax authorities and administrative
appeal processes. The liability includes tax
liabilities in respect of uncertain tax treatments
which are estimated using the most likely
amount method and the expected value method
and depend on AstraZeneca’s assessment of
the likelihood of the approach taken by the tax
authorities. This could change in the future to
reflect progress in tax authority reviews, the
extent that any tax authority challenge is
concluded, or matters lapse including following
expiry of the relevant statutes of limitation
resulting in a reduction in the tax charge in
future periods.
For these other tax liabilities in respect of
uncertain tax treatments, AstraZeneca estimates
the potential for additional liabilities above the
amount provided where the possibility of the
additional liabilities falling due is more than
remote, to be up to $293m (2022: $209m;
2021: $273m) including associated interest.
It is possible that some of these liabilities may
reduce in the future if any tax authority challenge
is concluded or matters lapse following expiry
of the relevant statutes of limitation, resulting
in a reduction in the tax charge in future periods.
AstraZeneca does not believe there are any
significant other uncertain tax treatments
where the possibility of the additional liabilities
falling due is more than remote (2022: $280m;
2021: $325m) including associated interest.
Timing of cash flows and interest
The Group is currently under audit in several
countries and the timing of any resolution of
these audits is uncertain.
It is anticipated that tax payments may be
required in relation to a number of significant
disputes which may be resolved over the next
one to two years. AstraZeneca considers the tax
liabilities set out above to appropriately reflect
the expected value of any final settlement.
Some of the items discussed above are not
currently within the scope of tax authority audits
and may take longer to resolve.
Included within other payables is a net amount
of interest arising on tax contingencies of
$184m (2022: $106m; 2021: $85m).
31 Statutory and other information
2023 2022 2021
$m $m $m
Fees payable to PricewaterhouseCoopers LLP and its associates:
Group audit fee 10.2 9.9 10.5
Fees payable to PricewaterhouseCoopers LLP and its associates for other services:
The audit of subsidiaries pursuant to legislation 15.0 15.1 15.2
Attestation under s404 of Sarbanes-Oxley Act 2002 3.3 3.1 2.0
Audit-related assurance services 1.1 0.7 4.5
Other assurance services 0.2 0.2 3.4
Fees payable to PricewaterhouseCoopers Associates in respect of the Group’s pension schemes:
The audit of subsidiaries’ pension schemes 0.3 0.3 0.3
30.1 29.3 35.9
$0.7m of fees payable in 2023 are in respect of the Group audit and audit of subsidiaries related to prior years (2022: $0.6m in respect of the Group
audit and audit of subsidiaries related to prior years).
$0.3m of 2021 Group audit fees and $0.7m of 2021 Audit-related assurance services and Other assurance services relate to pre-acquisition fees
incurred by Alexion.
Included in the 2021 Audit-related assurance services and Other assurance services are $6.1m of services provided in relation to the acquisition
of Alexion and related debt issuance.
Related party transactions
The Group had no material related party transactions which might reasonably be expected to influence decisions made by the users of these
Financial Statements.
Key management personnel compensation
Key management personnel are defined for the purpose of disclosure under IAS 24 ‘Related Party Disclosures’ as the members of the Board and
the members of the SET.
2023 2022 2021
$’000 $’000 $’000
Short-term employee benefits 38,636 38,632 32,985
Post-employment benefits 1,354 1,388 1,378
Share-based payments 58,242 56,297 45,234
98,232 96,317 79,597
Total remuneration is included within employee costs (see Note 29).
32 Subsequent events
There were no material subsequent events.
210
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
At 31December 2023 Group Interest At 31December 2023 Group Interest At 31December 2023 Group Interest
Group Subsidiaries and Holdings
In accordance with section 409 of the Companies Act 2006 a full list of subsidiaries, partnerships, associates, joint ventures and joint arrangements,
the place of incorporation, registered office address, and the effective percentage of equity owned as at 31 December 2023 are disclosed below.
Unless otherwise stated, the share capital disclosed comprises ordinary shares which are indirectly held by AstraZeneca PLC.
Unless otherwise stated, the accounting year ends of subsidiaries are 31 December. The Group Financial Statements consolidate the Financial
Statements of the Company and its subsidiaries at 31 December 2023.
Wholly owned subsidiaries
Algeria
AAPM SARL 100%
Number 20, Micro-Economic Zone,
HydraBusiness Center, Dar El Medina,
Algiers, Algeria
Argentina
AstraZeneca S.A. 100%
Olga Cossettini 363, 3° floor, Buenos Aires,
Argentina
Alexion Pharma Argentina SRL 100%
Avenida Leandro N. Alem 592 Piso 6,
Buenos Aires, Argentina
Australia
AstraZeneca Holdings Pty Limited 100%
AstraZeneca Pty Limited 100%
Alexion Pharmaceuticals Australasia Pty Ltd 100%
66 Talavera Road, Macquarie Park,
NSW2113, Australia
LogicBio Australia Pty Limited 100%
Level 40, 2-26 Park Street, Sydney,
NSW2000, Australia
Austria
AstraZeneca Österreich GmbH 100%
A-1120 Wien, Rechte Wienzeile 223
Tür 16.1, Austria
Alexion Pharma Austria GmbH 100%
Donau-City-Straße 7, 30. Stock,
DCTower,Vienna 1220,Austria
Portola Österreich GmbH (in liquidation) 100%
Mooslackengasse 17, 1190 Wien, Austria
Belgium
AstraZeneca S.A. / N.V. 100%
Alfons Gossetlaan 40 bus 201
at 1702 Groot-Bijgaarden, Belgium
Alexion Pharma Belgium Sprl 100%
Alexion Services Europe Sprl 100%
de Meeûssquare 37, Bruxelles 1000, Belgium
Bermuda
Alexion Bermuda Holding ULC 100%
Alexion Bermuda Limited 100%
Alexion Bermuda Partners LP 100%
Canon’s Court, 22 Victoria St.,
Hamilton,Bermuda
Brazil
AstraZeneca do Brasil Limitada 100%
Rod. Raposo Tavares, KM 26, 9, Cotia,Brazil
Alexion Farmacêutica América Latina
Serviços de Administração de Vendas Ltda.
100%
Alexion Serviços e Farmacêutica
doBrasilLtda.
100%
Av. Dr Chucri Zaidan, 1240, 15° andar,
CEP04711-130, Ed. Morumbi Corporate
– Golden Tower Vila São Francisco,
SãoPaulo, Brazil
Bulgaria
AstraZeneca Bulgaria EOOD 100%
1057 Sofia, Izgrev Region,
36 Dragan Tsankov Blvrd, Bulgaria
Canada
AstraZeneca Canada Inc.
1
100%
Suite 5000, 1004 Middlegate Road,
Mississanga, ON, L4Y 1M4, Canada
Alexion Pharma Canada Corporation 100%
1300-1969 ST Upper Water, Halifax,
NS,B3J 3R7, Canada
Cayman Islands
AZ Reinsurance Limited 100%
18 Forum Lane, 2nd Floor, Camana Bay,
Grand Cayman, P.O. Box 69, CaymanIslands
Grey Wolf Merger Sub 100%
PO Box 309, Ugland House, Grand Cayman,
KY1-1104, Cayman Islands
Chile
AstraZeneca S.A. 100%
AstraZeneca Farmaceutica Chile Limitada 100%
Av. Isidora Goyenechea 3477, 2nd Floor,
LasCondes, Santiago, Chile
China
AstraZeneca Pharmaceutical Co., Limited 100%
No. 2, Huangshan Road, Wuxi,
JiangsuProvince, China
AstraZeneca (Wuxi) Trading Co. Ltd 100%
Building E, Huirong Plaza, Jinghui Road East,
Xinwu District, Wuxi, Jiangsu Province, China
AstraZeneca Investment (China) Co., Ltd 100%
199 Liangjing Road, China (Shanghai) Pilot
Free Trade Zone, Shanghai, China
AstraZeneca Pharmaceutical (China) Co. Ltd 100%
No. 9, Medical Avenue, Jiangsu Province,
Taizhou, China
AstraZeneca Pharmaceutical
(Beijing)Co.,Ltd
100%
1F, Building No. 4, No. 8 Courtyard,
No.1Kegu Street, Beijing Economic-
Technological Development Area,
Beijing100176, China
AstraZeneca (Guangzhou) Pharmaceutical
Co., Ltd
100%
Room 406-178, No. 1, Yichuang Street,
(China-Singapore Guangzhou Knowledge City)
Huangpu District, Guangzhou City, China
AstraZeneca Investment Consulting
(Wuxi)Co., Ltd
100%
Room 808, 8F, Building 99-2 Linghu Avenue,
Xinwu District, Wuxi, Jiangsu, China
AstraZeneca Pharmaceutical (Hangzhou)
Co., Ltd
100%
12F & 14F, Building 1, Shuli Plaza,
758 Fei Jia Tang Road, Gongshu District,
Hangzhou, Zhejiang Province, China
AstraZeneca Global R&D (China) Co., Ltd 100%
16F, 88 Xizang North Road, Jing’an District,
Shanghai, China
AstraZeneca Pharmaceutical (Chengdu)
Co., Ltd
100%
10th Floor, Building 11 (Building E11), No.366,
Hemin Street, Chengdu High-tech Zone,
China (Sichuan) Pilot Free Trade Zone, China
AstraZeneca Pharmaceutical (Shanghai)
Co., Ltd
100%
B1F, 8F & 9F, 88 Xizang North Road,
Jing’anDistrict, Shanghai, China
Alexion Pharmaceuticals (Shanghai)
Company Limited
100%
Room 702, No. 1539 West Nanjing Road,
Jing’an District, Shanghai, China
AstraZeneca Pharmaceutical
Manufacturing (Qingdao) Co., Ltd.
100%
AstraZeneca Pharmaceutical (Qingdao)
Co., Ltd.
100%
Room 806, Building 2, No. 82 Juxianqiao
Road, High-tech Zone, Qingdao City,
Shandong Province, China
Colombia
AstraZeneca Colombia S.A.S. 100%
Av Carrera 9 No. 101-67 Office 601, Bogotá,
110231, Colombia
Alexion Pharma Colombia S.A.S. 100%
Carrera 9 No. 115 - 06 /30 Edificio Tierra
Firme Oficina 2904 Bogotá D.C., Colombia
Costa Rica
AstraZeneca CAMCAR Costa Rica, S.A. 100%
San José, Escazú, Roble Corporate Center,
5to piso, Costa Rica
Croatia
AstraZeneca d.o.o. 100%
Radnicka cesta 80, 10000 Zagreb, Croatia
Group Subsidiaries and Holdings 211AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
At 31December 2023 Group Interest At 31December 2023 Group Interest At 31December 2023 Group Interest
Group Subsidiaries and Holdings
continu ed
Czech Republic
AstraZeneca Czech Republic, s.r.o. 100%
U Trezorky 921/2, 158 00 Prague 5,
CzechRepublic
Alexion Pharma Czech s.r.o. 100%
Novodvorská 994/138, Braník,
14200Prague, Czech Republic
Denmark
AstraZeneca A/S 100%
Johanne Møllers Passage 1, Dk-1799
Copenhagen V, Denmark
Egypt
AstraZeneca Egypt for Pharmaceutical
Industries SAE
100%
6th of October City, 6th Industrial Zone,
Plot2, Giza, Egypt
AstraZeneca Egypt LLC 100%
47 St. 270 New Maadi, Cairo, Egypt
Drimex LLC 100%
Plot 133, Banks’ District, 5th Settlement,
New Cairo, Cairo, Egypt
Estonia
AstraZeneca Eesti OÜ 100%
Harju maakond, Tallinn, Lasnamäe linnaosa,
Valukoja tn 8/1, 11415, Estonia
Finland
AstraZeneca Oy. 100%
Keilaranta 18, 02150 Espoo, Finland
France
AstraZeneca SAS 100%
Tour Carpe Diem-31, Place des Corolles,
92400 Courbevoie, France
AstraZeneca Reims Production SAS 100%
Chemin de Vrilly Parc, Industriel de la
Pompelle, Reims, 51100, France
AstraZeneca Dunkerque Production SCS 100%
224 Avenue de la Dordogne,
59640Dunkerque, France
Alexion Europe SAS 100 %
Alexion Pharma France SAS 100 %
103-105 Rue Anatole France 92300
Levallois-Perret, France
Germany
AstraZeneca Holding GmbH 100%
AstraZeneca GmbH 100%
Friesenweg 26, 22763, Hamburg, Germany
Sofotec GmbH 100%
Benzstrasse 1-3, 61352, Bad Homburg v.d.
Hohe, Germany
AstraZeneca Computational
PathologyGmbH
2
100%
Bernhard-Wicki-Straße 5, 80636,
Munich,Germany
Alexion Pharma Germany GmbH 100%
Landsberger Straße 300, 80687,
Munich,Germany
Greece
AstraZeneca S.A. 100%
Agisilaou 6-8 Marousi, Athens, Greece
Hong Kong
AstraZeneca Hong Kong Limited 100%
Unit 1 – 3, 11/F., China Taiping Finance Centre,
18 King Wah Road, North Point, Hong Kong
Hungary
AstraZeneca Kft 100%
1st floor, 4 building B, Alíz str., Budapest,
1117, Hungary
India
AstraZeneca India Private Limited
3
100%
Block A, Neville Tower, 11th Floor,
Ramanujan IT SEZ, Taramani, Chennai,
Tamil Nadu, PIN 600113, India
Alexion Business Services Private Limited 100%
9th Floor, Platina, G BlockPlot No. C-59,
Bandra-Kurla ComplexBandra (East),
Mumbai 400051,India
Iran
AstraZeneca Pars Company 100%
Suite 1, 1st Floor No. 39, Alvand Ave.,
Argantin Sq., Tehran 1516673114, Iran
Ireland
AstraZeneca Pharmaceuticals (Ireland)
Designated Activity Company
100%
4th Floor, South Bank House, Barrow Street,
Dublin, 4, Republic of Ireland
Alexion Pharma Holding Limited 100%
Alexion Pharma International
OperationsLimited
100%
Alexion Pharma Development Limited 100%
AstraZeneca Ireland Limited 100%
College Business & Technology Park,
Blanchardstown Road North,Dublin 15,
Republic ofIreland
Israel
AstraZeneca (Israel) Ltd 100%
Atirei Yeda 1, Building O-Tech 2, POB 8044,
Kfar Saba, 4464301, Israel
Alexion Pharma Israel Ltd 100%
4 Weizmann Str., Tel-Aviv-Jaffa, Israel
Italy
Simesa SpA 100%
AstraZeneca SpA 100%
Alexion Pharma Italy Srl 100%
Viale Decumano 39, 20157 Milan, Italy
Japan
AstraZeneca K.K. 100%
Grand Front Osaka Tower B, 3-1,
Ofuka-cho, Kita-ku, Osaka, 530-0011, Japan
Alexion Pharma GK 100%
Ebisu First Square, 18-14, Ebisu 1-chome,
Shibuya-ku, Tokyo, Japan
Kazakhstan
AstraZeneca Kazakhstan LLP 100%
Office 101, 77 Kunayev Street,
Almaty050000, Kazakhstan
Kenya
AstraZeneca Pharmaceuticals Limited 100%
L.R. No.1/1327, Avenue 5, 1st Floor,
RoseAvenue, Nairobi, Kenya
Latvia
AstraZeneca Latvija SIA 100%
Skanstes iela 50, Riga, LV-1013, Latvia
Lithuania
AstraZeneca Lietuva UAB 100%
Spaudos g., Vilnius, LT-05132, Lithuania
Luxembourg
AstraZeneca Luxembourg S.A. 100%
Rue Nicolas Bové 2A – L-1253, Luxembourg
Malaysia
AstraZeneca Asia-Pacific Business
Services Sdn Bhd
100%
12th Floor, Menara Symphony,
No. 5 Jalan Prof, Khoo Kay Kim,
Seksyen 13, 46200 Petaling Jaya,
Selangor Darul Ehsan, Malaysia
AstraZeneca Sdn Bhd 100%
Nucleus Tower, Level 11 & 12,
No. 10 Jalan PJU 7/6, Mutiara Damansara,
47800 Petaling Jaya,
Selangor Darul Ehsan, Malaysia
Mexico
AstraZeneca Health Care Division,
S.A.deC.V.
100%
AstraZeneca, S.A. de C.V. 100%
Av. Periferico Sur 4305 interior 5, Colonia
Jardines en la Montaña, Mexico City,
Tlalpan Distrito Federal, CP 14210, Mexico
Alexion Pharma Mexico S. de R.L. de C.V. 100%
Paseo de los Tamarindos 90,
Torre 1piso 6 - ACol., Bosques de la Lomas,
CP 05120 D.F,Mexico
Morocco
AstraZeneca Maroc SARLAU 100%
92 Boulevard Anfa ETG 2,
Casablanca20000, Morocco
The Netherlands
AstraZeneca B.V. 100%
AstraZeneca Continent B.V. 100%
AstraZeneca Gamma B.V. 100%
AstraZeneca Holdings B.V. 100%
AstraZeneca Jota B.V. 100%
AstraZeneca Rho B.V. 100%
AstraZeneca Sigma B.V. 100%
AstraZeneca Treasury B.V. 100%
AstraZeneca Zeta B.V. 100%
Prinses Beatrixlaan 582, 2595BM,
TheHague, The Netherlands
212 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
At 31December 2023 Group Interest At 31December 2023 Group Interest At 31December 2023 Group Interest
AstraZeneca Nijmegen B.V. 100%
Lagelandseweg 78, 6545 CG Nijmegen,
TheNetherlands
Acerta Pharma B.V. 100%
Aspire Therapeutics B.V. 100%
Kloosterstraat 9, 5349 AB, Oss,
TheNetherlands
Portola Netherlands B.V. 100%
Prins Bernhardplein 200JB Amsterdam 1097,
TheNetherlands
Alexion Holding B.V. 100%
Alexion Pharma Foreign Holdings B.V. 100%
Alexion Pharma Netherlands B.V. 100%
Prinses Beatrixlaan 582, 5895 BM,
TheHague, The Netherlands
Neogene Therapeutics B.V. 100%
Science Park 106, 1098 XG Amsterdam,
TheNetherlands
New Zealand
AstraZeneca Limited 100%
Pharmacy Retailing (NZ) Limited
t/aHealthcare Logistics,
58 Richard Pearse Drive, Mangere,
Auckland, 1142, New Zealand
Nigeria
AstraZeneca Nigeria Limited 100%
11A, Alfred Olaiya Street, Awuse Estate,
OffSalvation Street, Opebi, Ikeja,
Lagos,Nigeria
Norway
AstraZeneca AS 100%
Karvesvingen 7, 0579 Oslo, Norway
Pakistan
AstraZeneca Pharmaceuticals Pakistan
(Private) Limited
4
100%
Office No 1, 2nd Floor, Sasi Arcade, Block 7,
Main Clifton Road, Karachi, Pakistan
Panama
AstraZeneca CAMCAR, S.A. 100%
Bodega #1, Parque Logistico MIT,
CarreteraHacia Coco Solo, Colon, Panama
Peru
AstraZeneca Peru S.A. 100%
Calle Las Orquídeas N° 675, Int. 802,
Edificio Pacific Tower, San Isidro, Lima, Peru
Philippines
AstraZeneca Pharmaceuticals (Phils.) Inc. 100%
16th Floor, Inoza Tower, 40th Street,
Bonifacio Global City, Taguig 1634, Philippines
Poland
AstraZeneca Pharma Poland Sp.z.o.o. 100%
Alexion Pharma Poland Sp.z.o.o. 100%
Postepu 14, 02-676, Warszawa, Poland
Portugal
Astra Alpha Produtos Farmacêuticos Lda 100%
AstraZeneca Produtos Farmacêuticos Lda 100%
Novastra Promoção e Comércio
Farmacêutico Lda
100%
Novastuart Produtos Farmacêuticos Lda 100%
Stuart-Produtos Farmacêuticos Lda 100%
Zeneca Epsilon – Produtos
FarmacêuticosLda
100%
Zenecapharma Produtos Farmacêuticos,
Unipessoal Lda
100%
Rua Humberto Madeira, No 7,
Queluz de Baixo, 2730-097,
Barcarena,Portugal
Puerto Rico
IPR Pharmaceuticals, Inc. 100%
Road 188, San Isidro Industrial Park,
Canóvanas, 00729, Puerto Rico
Romania
AstraZeneca Pharma S.R.L. 100%
Bucharest, 1A Tipografilor Street,
MUSEOffices, 2nd and 3rd Floor,
District 1, 013714, Romania
Russia
AstraZeneca Industries, LLC 100%
8 1st Vostochniy lane, Dobrino village,
Borovskiy district, Kaluga region 249006,
Russian Federation
AstraZeneca Pharmaceuticals, LLC 100%
Building 1, 21 First Krasnogvardeyskiy lane,
floor 30, rooms 13 and 14, Moscow, 123112,
Russian Federation
Alexion Pharma OOO LLC 100%
Building 1, 21 First Krasnogvardeyskiy lane,
floor 29, Moscow, 123112, Russian Federation
Saudi Arabia
AstraZeneca Continent –
RegionalHeadquarter
100%
Al-Nakhlah Tower, Floor 13th Ath Thumamah
Road, Al Sahafa District., P.O. Box 42150,
Riyadh, Kingdom of Saudi Arabia
AstraZeneca Trading Company 100%
125 Prince Sultan, 2086 Ar Rawdah District,
23435, Jeddah, Kingdom of Saudi Arabia
Singapore
AstraZeneca Singapore Pte Limited 100%
10 Kallang Avenue #12-10, Aperia Tower 2,
339510, Singapore
South Africa
AstraZeneca Pharmaceuticals (Pty) Limited 100%
17 Georgian Crescent West, Northdowns
Office Park, Bryanston, 2191, South Africa
South Korea
AstraZeneca Korea Co. Ltd 100%
21st Floor, Asem Tower, 517,
Yeongdong-daero, Gangnam-gu,
Seoul,06164, Republic of Korea
Alexion Pharma Korea LLC 100%
41 FL.,152 Teheran-ro (Yeoksam-dong
Gangnam Finance Center),
Gangnam-gu,Seoul, Republic of Korea
Spain
AstraZeneca Farmaceutica Holding
Spain,S.A.
100%
AstraZeneca Farmaceutica Spain S.A. 100%
Laboratorio Beta, S.A. 100%
Laboratorio Lailan, S.A. 100%
Laboratorio Tau, S.A. 100%
Fundación AstraZeneca 100%
Calle del Puerto de Somport, 21-23, 28050,
Madrid, Spain
Alexion Pharma Spain S.L. 100%
Av Diagonal Num.601 P.1,
Barcelona 08028, Spain
Sweden
Astra Export & Trading Aktiebolag 100%
Astra Lakemedel Aktiebolag 100%
AstraZeneca AB 100%
AstraZeneca Biotech AB 100%
AstraZeneca BioVentureHub AB 100%
AstraZeneca Holding Aktiebolag
5
100%
AstraZeneca International Holdings
Aktiebolag
6
100%
AstraZeneca Nordic AB 100%
AstraZeneca Pharmaceuticals Aktiebolag 100%
AstraZeneca Södertälje 2 AB 100%
Stuart Pharma Aktiebolag 100%
Tika Lakemedel Aktiebolag 100%
SE-151 85 Södertälje, Sweden
Aktiebolaget Hassle 100%
Symbicom Aktiebolag
6
100%
431 83 MoIndal, Sweden
Astra Tech International Aktiebolag 100%
Box 14, 431 21 MoIndal, Sweden
Alexion Pharma Nordics Holding AB 100%
Alexion Pharma Nordics AB 100%
Kungsgatan 3,Stockholm 111 43,Sweden
Switzerland
AstraZeneca AG 100%
Evinova AG 100%
Neuhofstrasse 34, 6340 Baar, Switzerland
Spirogen Sarl
6
100%
Rue du Grand-Chêne 5, CH-1003
Lausanne,Switzerland
Alexion Pharma GmbH 100%
Giesshübelstrasse 30,
Zürich8045,Switzerland
Group Subsidiaries and Holdings 213AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Group Subsidiaries and Holdings
continu ed
At 31December 2023 Group Interest At 31December 2023 Group Interest At 31December 2023 Group Interest
Taiwan
AstraZeneca Taiwan Limited 100%
21st Floor, Taipei Metro Building 207,
TunHwa South Road, SEC 2 Taipei, Taiwan
Alexion Pharma Taiwan Ltd 100%
Room 1153, 11F, No. 1, SongZhi Rd,
Taipei11047, Taiwan
Thailand
AstraZeneca (Thailand) Limited 100%
Asia Centre 19th floor, 173/20,
SouthSathorn Rd, Khwaeng
Thungmahamek, Khet Sathorn,
Bangkok,10120, Thailand
Tunisia
AstraZeneca Tunisie SaRL 100%
Lot n°1.5.5 les jardins du lac,
bloc B les berges du lac Tunis, Tunisia
Turkey
AstraZeneca Ilac Sanayi ve Ticaret
LimitedSirketi
100%
YKB Plaza, B Blok, Kat:3-4, Levent/Besiktas,
Istanbul, Turkey
Zeneca Ilac Sanayi ve Ticaret
AnonimSirketi
100%
Büyükdere Cad., Y.K.B. Plaza, B Blok, Kat:4,
Levent/Bes¸iktas¸, Istanbul, Turkey
Alexion Ilac Ticaret Limited Sirketi 100%
Içerenköy Mahellisi Umut SK. and
Ofis Sit. No: 1012/73 Atas¸ehir,
Istanbul10-12/73,Turkey
Ukraine
AstraZeneca Ukraina LLC 100%
54 Simi Prakhovykh street, Kyiv,
01033,Ukraine
United Arab Emirates
AstraZeneca FZ-LLC 100%
P.O. Box 505070, Block D,
Dubai Healthcare City, Oud Mehta Road,
Dubai, United Arab Emirates
Alexion Pharma Middle East FZ-LLC 100%
Dubai Science Park, 501, Floor 5, EIB
Building No. 2, Dubai, United Arab Emirates
United Kingdom
Ardea Biosciences Limited 100%
Arrow Therapeutics Limited 100%
Astra Pharmaceuticals Limited 100%
AstraPharm
6
100%
AstraZeneca China UK Limited 100%
AstraZeneca Death In Service
TrusteeLimited
100%
AstraZeneca Employee Share Trust Limited 100%
AstraZeneca Finance Limited 100%
AstraZeneca Intermediate Holdings Limited
5
100%
AstraZeneca Investments Limited 100%
AstraZeneca Japan Limited 100%
AstraZeneca Nominees Limited 100%
AstraZeneca Quest Limited 100%
AstraZeneca Share Trust Limited 100%
AstraZeneca Sweden Investments Limited 100%
AstraZeneca Treasury Limited
6
100%
AstraZeneca UK Limited 100%
AstraZeneca US Investments Limited
5
100%
AZENCO2 Limited 100%
AZENCO4 Limited 100%
Cambridge Antibody Technology
GroupLimited
100%
KuDOS Horsham Limited 100%
KuDOS Pharmaceuticals Limited 100%
Zenco (No. 8) Limited 100%
Zeneca Finance (Netherlands) Company 100%
MedImmune Limited 100%
1 Francis Crick Avenue,
CambridgeBiomedical Campus,
Cambridge, CB2 0AA, United Kingdom
MedImmune U.K. Limited 100%
Plot 6, Renaissance Way, BoulevardIndustry
Park, Liverpool, L24 9JW, United Kingdom
Syntimmune Limited 100%
21 Holborn Viaduct, London, EC1A 2DY,
United Kingdom
Alexion Pharma UK Limited 100%
Portola Pharma UK Limited (in liquidation) 100%
3 Furzeground Way, Stockley Park, Uxbridge,
Middlesex, UB11 1EZ, UnitedKingdom
United States
Ardea Biosciences, Inc. 100%
Amylin Ohio LLC
7
100%
Amylin Pharmaceuticals, LLC
7
100%
AstraZeneca Collaboration Ventures, LLC
7
100%
AstraZeneca Finance LLC
7
100%
AstraZeneca Finance and Holdings Inc. 100%
AstraZeneca Pharmaceuticals LP
8
100%
Atkemix Nine Inc. 100%
Atkemix Ten Inc. 100%
BMS Holdco, Inc. 100%
Cincor Pharma Inc. 100%
Corpus Christi Holdings Inc. 100%
Isochrone Merger Sub Inc. 100%
Neogene Therapeutics, Inc. 100%
Omthera Pharmaceuticals, Inc. 100%
Optein, Inc. 100%
Stauffer Management Company LLC
7
100%
Zeneca Holdings Inc. 100%
Zeneca Inc. 100%
Zeneca Wilmington Inc.
5
100%
1800 Concord Pike, Wilmington, DE 19803,
United States
ZS Pharma Inc. 100%
1100 Park Place, Suite 300, San Mateo,
CA94403, United States
AlphaCore Pharma, LLC
7
100%
333 Parkland Plaza, Suite 5, Ann Arbor,
MI48103, United States
AZ-Mont Insurance Company 100%
100 Bank Street, Suite 630, Burlington, VT
05401, United States
MedImmune, LLC
7
100%
MedImmune Ventures, Inc. 100%
One MedImmune Way, Gaithersburg,
MD20878, United States
Pearl Therapeutics, Inc. 100%
200 Cardinal Way, Redwood City, CA 94063,
United States
Caelum Biosciences Inc. 100%
1200 Florence Columbus Road,
Bordentown, NJ 08505, United States
Alexion Services Latin America Inc. 100%
600 Brickell Ave, Miami, FL 33131,
UnitedStates
Portola USA, Inc. 100%
Portola Pharmaceuticals LLC 100%
270 East Grand Avenue,South
SanFrancisco, CA 94080, United States
Achillion Pharmaceuticals Inc. 100%
Alexion Delaware Holding LLC 100%
Alexion Pharma LLC 100%
Alexion Pharmaceuticals, Inc. 100%
Alexion US1 LLC 100%
Alexion US Holdings LLC 100%
LogicBio Therapeutics, Inc. 100%
Savoy Therapeutics Corp 100%
Syntimmune, Inc. 100%
TeneoTwo, Inc. 100%
121 Seaport Boulevard,Boston, MA 02210,
United States
Acerta Pharma LLC
7
100%
121 Oyster Point Boulevard,
SouthSanFrancisco, CA 94080,
UnitedStates
LogicBio Securities Corporation 100%
65 Hayden Avenue, Lexington, MA 92421,
United States
Alexion Holding LLC 100%
100 College Street, New Haven, CT 06510,
United States
Uruguay
AstraZeneca S.A. 100%
Yaguarón 1407 of 1205, 11.100,
Montevideo,Uruguay
Venezuela
AstraZeneca Venezuela S.A. 100%
Gotland Pharma S.A. 100%
Av. La Castellana, Torre La Castellana,
Piso5, Oficina 5-G, 5-H, 5-I, Urbanización
La Castellana, Municipio Chacao, Estado
Bolivariano de Miranda, Venezuela
Vietnam
AstraZeneca Vietnam Company Limited 100%
18th Floor, A&B Tower, 76 Le Lai, Ben Thanh
Ward, District 1, Ho Chi Minh City, Vietnam
214 AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

At 31December 2023 Group Interest At 31December 2023 Group Interest At 31December 2023 Group Interest
Subsidiaries where the effective interest
is less than 100%
Algeria
AstraZeneca Algeria Pharmaceutical
Industries SPA
49%
N° 20, Micro Zone d’Activité Hydra,
Centredes Affaires Dar El Madina, Bloc A,
6th Floor, Hydra, Algiers, Algeria
China
Beijing Falikang Pharmaceutical (China)
Co. Ltd
49%
No. 69 Fushi Road, Haidian District, Beijing,
100143, China
India
AstraZeneca Pharma India Limited
3
75%
Block N1, 12th Floor, Manyata Embassy
Business Park, Rachenahalli, Outer Ring
Road, Bangalore-560 045, India
Indonesia
P.T. AstraZeneca Indonesia 95%
Perkantoran Hijau Arkadia Tower F, 3rd Floor,
JI. T.B. Simatupang Kav. 88, South Jakarta,
12520, Indonesia
Joint Ventures
China
WuXi MedImmune Biopharmaceutical
Co.,Limited (in liquidation)
50%
Room 1902, 19/F, Lee Garden One,
33Hysan Avenue, Causeway Bay, Hong Kong
IHP HK Holdings Limited 50%
Unit 5805, 58/F., Two International Finance
Centre 8 Finance Street, Central, China
United Kingdom
Centus Biotherapeutics Limited
(inliquidation)
50%
c/o Cork Gully LLP, 40 Villiers Street,
London, WC2N 6NJ, United Kingdom
Ireland
Centus Biotherapeutics Europe Limited
(inliquidation)
50%
6th Floor, South Bank House, Barrow Street,
Dublin 4, Republic of Ireland
United States
Montrose Chemical Corporation
ofCalifornia
50%
Suite 380, 600 Ericksen Ave N/E,
BainbridgeIsland, United States
Significant Holdings
China
Dizal (Jiangsu) Pharmaceutical Co., Ltd. 26.69%
199 Liangjing Rd, Zhangjiang Hi-Tech Park,
Pudong District, Shanghai, 201203, China
Wuxi AstraZeneca-CICC Venture Capital
Partnership(Limited Partnership)
22.13%
Room 808, 8F, Building 99-2 Linghu Avenue,
Xinwu District, Wuxi, Jiangsu, China
United Kingdom
VaxEquity 40%
Lab 4 Cambridge Science Park, Unit 204
Milton Road, Cambridge, CB4 0GZ,
UnitedKingdom
United States
C.C. Global Chemicals Company 37.50%
PO Box 7, MS2901, Texas, TX76101-0007,
United States
Associated Holdings
France
Medetia SAS 10%
Institute Imagine 24, Boulevard du
Montparnasse 75015, Paris, France
Cellectis S.A. 22.35%
8, rue de la Croix Jarry, 75013 Paris, France
Israel
AION Labs Innovation Lab Ltd. 19.23%
4 Oppenheimer Street, Building B, Rehovot,
7670104, Israel
CombinAble.AI Ltd. 11.25%
5 Oppenheimer Street, Building B, Rehovot,
7670104, Israel
TenAces Biosciences Ltd. 12.50%
6 Oppenheimer Street, Building B, Rehovot,
7670104, Israel
Sweden
Swedish Orphan Biovitrum AB (publ) 9.89%
Tomtebodavägen 23A, Stockholm, Sweden
OnDosis AB 19.90%
GoCo House, 5 tr, Gemenskapens gata 9,
431 53 Mölndal, Sweden
CCRM Nordic AB 19.90%
CCRM Nordic AB, c/o GU Ventures AB,
ErikDahlbergsgatan 11 A,
41126Göteborg,Sweden
United Kingdom
Niox Group plc 16.89%
Hayakawa Building, Edmund Halley Road,
Oxford Science Park, Oxford, OX4 4GB,
United Kingdom
United States
AbMed Corporation 18%
68 Cummings Park Drive, Woburn,
MA01801, United States
Baergic Bio, Inc. 19.95%
1111 Kane Concourse, Suite 301 Bay Harbor
Islands, FL 33154, United States
Regio Biosciences 19.54%
668 Stoney Hill Road, #2, Yardley, PA 19067,
United States
Employee Benefit Trust
The AstraZeneca Employee Benefit Trust
Group Subsidiaries and Holdings
215AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Company Balance Sheet
at 31December
AstraZeneca PLC
2023 2022
Notes $m $m
Fixed assets
Fixed asset investments 1 64,189 63,555
64,18 9 63,555
Current assets
Debtors – other 4 4
Debtors – amounts owed by Group undertakings 10,928 2,608
10,932 2,612
Creditors: Amounts falling due within one year
Other payables 2 (216) (194)
Amounts owed to Group undertakings 3 – (283)
Interest-bearing loans and borrowings 3 (2,995) (2,648)
(3,211) (3,125)
Net current assets/(liabilities) 7,721 (513)
Total assets less current liabilities 71,910 63,042
Creditors: Amounts falling due after more than one year
Interest-bearing loans and borrowings 3 (16,741) (17,939)
Other payables 2 (21) (23)
(16,762) (17,9 62)
Net assets 55,148 45,080
Capital and reserves
Called-up share capital 4 388 387
Share premium account 35,188 35,155
Capital redemption reserve 153 153
Other reserves 1,779 1,927
Profit and loss account 17,640 7, 458
Shareholders’ funds 55,148 45,080
$m means millions of US dollars.
The Company’s profit for the year was $14,669m (2022: $380m).
The Company Financial Statements from pages 216 to 222 were approved by the Board and were signed on its behalf by
Pascal Soriot Aradhana Sarin
Director Director
8 February 2024
Company’s registered number 02723534
216
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Company Statement of Changes in Equity
for the year ended 31December
Share Capital
Share premium redemption Other Profitand Total
capital account reserve reserves
1
lossaccount
2
equity
$m $m $m $m $m $m
At 1January 2022 387 35,126 153 2,182 11,563 49,411
Total comprehensive income for the period
Profit for the period – – – – 380 380
Total comprehensive income for the period – – – – 380 380
Transactions with owners, recorded directly in equity
Dividends – – – – (4,485) (4,485)
Capital contributions for share-based payments – – – (255) – (255)
Issue of Ordinary Shares – 29 – – – 29
Total contributions by and distributions to owners – 29 – (255) (4,485) (4,711)
At 31December 2022 387 35,155 153 1,927 7,4 58 45,080
Total comprehensive income for the period
Profit for the period – – – – 14,669 14,669
Total comprehensive income for the period – – – – 14,669 14,669
Transactions with owners, recorded directly in equity
Dividends – – – – (4,487) (4,487)
Capital contributions for share-based payments – – – (148) – (148)
Issue of Ordinary Shares 1 33 – – – 34
Total contributions by and distributions to owners 1 33 – (148) (4,487) (4,601)
At 31December 2023 388 35,188 153 1,779 17,640 55,148
Company Statement of Changes in Equity
217AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Company Accounting Policies
Basis of presentation of
financialinformation
The Company is a private limited company,
limited by shares, incorporated and domiciled
in England & Wales. The registered address is
1 Francis Crick Avenue, Cambridge Biomedical
Campus, Cambridge, CB2 0AA.
These financial statements were prepared
in accordance with FRS 101 ‘Reduced
Disclosure Framework’.
In preparing these financial statements,
the Company applied the recognition,
measurement and disclosure requirements of
International Financial Reporting Standards as
adopted by the UK (UK-adopted international
accounting standards), but made amendments
where necessary in order to comply with the
Companies Act 2006 and to take advantage
of FRS 101 disclosure exemptions.
In these financial statements, the Company
has applied the exemptions available under
FRS 101 in respect of the following disclosures:
> Statement of Cash Flows and related notes
> disclosures in respect of transactions
with wholly owned subsidiaries
> disclosures in respect of
capital management
> the effects of new but not yet
effective IFRSs
> disclosures in respect of the compensation
of Key Management Personnel.
As the Group Financial Statements (presented
on pages 148 to 210) include the equivalent
disclosures, the Company has also taken the
exemptions under FRS 101 available in respect
of the following disclosures:
> IFRS 2 ‘Share-based Payment’ in respect
of Group settled share-based payments
> certain disclosures required by IFRS 13
‘Fair Value Measurement’ and the
disclosures required by IFRS 7 ‘Financial
Instruments: Disclosures’.
No individual profit and loss account is
prepared as provided by section 408 of the
Companies Act 2006.
Basis of accounting
The Company Financial Statements are
prepared under the historical cost convention
and on a going concern basis, in accordance
with the Companies Act 2006.
The following paragraphs describe the
main accounting policies, which have been
applied consistently.
Estimates and judgements
The preparation of the Company Financial
Statements in conformity with generally
accepted accounting principles requires
management to make estimates and
judgements that affect the reported amounts of
assets and liabilities at the date of the Financial
Statements and the reported amounts of
revenues and expenses during the reporting
period. Actual results could differ from those
estimates. There are no key judgements or
significant estimates.
Foreign currencies
Foreign currency transactions, being
transactions denominated in a currency other
than the Company’s functional currency, are
translated into US dollars at average rates
for the relevant monthly accounting periods,
which approximate to actual rates.
Monetary assets and liabilities arising from
foreign currency transactions are retranslated
at exchange rates prevailing at the reporting
date. Exchange gains and losses on loans and
on short-term foreign currency borrowings
and deposits are included within Finance
expense. Exchange differences on all other
foreign currency transactions are recognised
in Operating profit.
Non-monetary items arising from foreign
currency transactions are not retranslated in
the Company’s accounting records.
Taxation
The current tax payable is based on taxable
profit for the year. Taxable profit differs from
reported profit because taxable profit excludes
items that are either never taxable or tax
deductible or items that are taxable or tax
deductible in a different period. The Company’s
current tax assets and liabilities are calculated
using tax rates that have been enacted or
substantively enacted by the reporting date.
Deferred tax is provided using the balance
sheet liability method, providing for temporary
differences between the carrying amounts of
assets and liabilities for financial reporting
purposes and the amounts used for taxation
purposes. Deferred tax liabilities are recognised
unless they arise from the initial recognition
(other than in a business combination) of
assets and liabilities in a transaction that
affects neither the taxable profit nor the
accounting profit. Deferred tax liabilities are
not recognised to the extent they arise from
the initial recognition of non-tax deductible
goodwill. Deferred tax assets are recognised
to the extent that there are future taxable
temporary differences or it is probable that
future taxable profit will be available against
which the asset can be utilised. This requires
judgements to be made in respect of the
availability of future taxable income.
No deferred tax asset or liability is recognised
in respect of temporary differences associated
with investments in subsidiaries and branches
where the Company is able to control the timing
of reversal of the temporary differences and it
is probable that the temporary differences will
not reverse in the foreseeable future.
The Company’s deferred tax assets and
liabilities are calculated using tax rates that
are expected to apply in the period when the
liability is settled or the asset realised based
on tax rates that have been enacted or
substantively enacted by the reporting date.
Liabilities for uncertain tax positions require
management to make judgements of potential
exposures in relation to tax audit issues. Tax
benefits are not recognised unless the tax
positions will probably be accepted by the tax
authorities. This is based upon management’s
interpretation of applicable laws and regulations
and the expectation of how the tax authority will
resolve the matter. Once considered probable
of not being accepted, management reviews
each material tax benefit and reflects the
effect of the uncertainty in determining the
related taxable result.
Liabilities for uncertain tax positions are
measured using either the most likely amount
or the expected value amount depending on
which method the Company expects to better
predict the resolution of the uncertainty.
The Company has applied the exemption
under the IAS 12 ‘Income Taxes’ amendment
for recognising and disclosing information
about deferred tax assets and liabilities related
to top-up income taxes.
Investments
Fixed asset investments, including investments
in subsidiaries, are stated at cost and reviewed
for impairment if there are indications that the
carrying value may not be recoverable.
Debtors
Amounts owed by Group undertakings are
recognised initially at fair value. Subsequent
to initial recognition they are measured at
amortised cost using the effective interest
method, less any impairment losses.
The recoverability of these balances has been
assessed in accordance with IFRS 9 and no
impairment has been identified. The amounts
owed by Group undertakings are considered to
have low credit risk, due to timely payment of
interest and settlement of principal amount on
agreed due dates, limiting the loss allowance
to 12-month expected credit losses.
218
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Amounts owed by Group undertakings are
written off where there is no reasonable
expectation of recovery. Impairment losses
are presented as net impairment losses within
Operating profit, any subsequent recoveries
are credited against the same line.
Other payables
Liabilities included in Other payables are
recognised initially at fair value. Subsequent
to initial recognition they are remeasured at
either amortised cost using the effective
interest method or at fair value using an
expected credit loss model.
Financial instruments
Interest-bearing loans are initially measured at
fair value (with direct transaction costs being
amortised over the life of the loan) and are
subsequently measured at amortised cost
using the effective interest method at each
reporting date. Changes in carrying value are
recognised in profit.
Share-based payments
The issuance by the Company to employees of
its subsidiaries of a grant of awards over the
Company’s shares, represents additional
capital contributions by the Company to its
subsidiaries. An additional investment in
subsidiaries results in a corresponding increase
in shareholders’ equity. The additional capital
contribution is based on the fair value of the
grant issued, allocated over the underlying
grant’s vesting period, less the market cost of
shares charged to subsidiaries in settlement
of such share awards.
Litigation
Through the normal course of business, the
AstraZeneca Group is involved in legal disputes,
the settlement of which may involve cost to
the Company. A provision is made where an
adverse outcome is probable and associated
costs, including related legal costs, can be
estimated reliably. In other cases, appropriate
disclosures are included.
Company Accounting Policies 219AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Company Financial Statements
1 Fixed asset investments
Investments in subsidiaries
Shares Loans Total
$m $m $m
At 1January 2022 49,581 16,043 65,624
Transfer to Debtors – amounts owed by Group undertakings – (1,531) (1,531)
Capital reimbursement (380) – (380)
Exchange – (161) (161)
Amortisation – 12 12
Disposals and other movements (9) – (9)
At 31December 2022 49,192 14,363 63,555
Additions during the year – 1,588 1,588
Transfer to Debtors – amounts owed by Group undertakings – (991) (991)
Capital reimbursement (131) – (131)
Exchange – 158 158
Amortisation – 12 12
Other movements (2) – (2)
At 31December 2023 49,059 15,130 64,189
Loans to subsidiaries consists of bonds which are issued externally and are issued back to Group undertakings with comparable terms on interest
rates and are repayable on maturity, details of which are disclosed in Note 3. The recoverability of these inter-company loans has been assessed in
accordance with IFRS 9 with no impairment identified. The inter-company balances are considered to have low credit risk due to timely payment
of interest and settlement of principal amount on agreed due dates, limiting the loss allowance to 12-month expected credit losses. In 2023, there
have been no credit losses (2022: $nil).
The other movements comprise $2m representing revaluation of carrying value of a guarantee provided to Group companies as explained in
Notes 2 and 3.
2 Other payables
2023 2022
$m $m
Amounts falling due within one year
Other creditors 214 184
Deferred income 2 3
Amounts owed to Group undertakings – 7
216 194
Amounts falling due after more than one year
Other creditors 21 23
21 23
Other creditors due after more than one year include an amount representing the carrying value of the guarantee provided by the Company to its
subsidiary for the bonds issued externally as explained in Note 3. As at 31 December 2023, the carrying value of the guarantee was $21m (2022: $23m).
220
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements
3 Loans and borrowings
Repayment 2023 2022
dates $m $m
Amounts due within one year
Amounts owed to Group undertakings (unsecured)
7.2% Loan US dollars 2023 – 283
Interest-bearing loans and borrowings (unsecured)
0.3% Callable bond US dollars 2023 – 1,399
Floating rate notes US dollars 2023 – 400
3.5% Callable bond US dollars 2023 – 849
0.75% Callable bond euros 2024 995 –
2024 Floating rate bank loans US dollars 2024 2,000 –
Total amounts due within one year 2,995 2,931
Amounts due after more than one year
Interest-bearing loans and borrowings (unsecured)
0.75% Callable bond euros 2024 – 957
2024 Floating rate bank loans US dollars 2024 – 1,998
3.375% Callable bond US dollars 2025 1,994 1,992
0.7% Callable bond US dollars 2026 1,196 1,195
3.625% Callable bond euros 2027 829 –
3.125% Callable bond US dollars 2027 747 746
1.25% Callable bond euros 2028 879 845
4% Callable bond US dollars 2029 995 995
0.375% Callable bond euros 2029 881 846
1.375% Callable bond US dollars 2030 1,294 1,293
5.75% Non-callable bond pound sterling 2031 444 420
3.75% Callable bond euros 2032 827 –
6.45% Callable bond US dollars 2037 2,725 2,724
4% Callable bond US dollars 2042 989 988
4.375% Callable bond US dollars 2045 981 981
4.375% Callable bond US dollars 2048 738 737
2.125% Callable bond US dollars 2050 487 487
3% Callable bond US dollars 2051 735 735
Total amounts due after more than one year 16,741 17,939
Total loans and borrowings 19,736 20,870
2023 2022
$m $m
Loans and borrowings are repayable:
After five years from balance sheet date 11,096 11,0 51
From two to five years 3,651 3,933
From one to two years 1,994 2,955
Within one year 2,995 2,931
Total unsecured 19,736 20,870
All borrowings are issued with fixed interest rates, with the exception of the $2bn USD 2024 floating rate loans, which transitioned from LIBOR to
a rate based on compounded daily USD Secured Overnight Funding Rate (SOFR) during the year.
In addition, the Company acts as guarantor for bonds issued by its wholly owned subsidiaries, AstraZeneca Finance LLC and AstraZeneca Finance
and Holdings Inc.. AstraZeneca Finance LLC is the issuer of $1,600m 0.700% Notes due 2024, $1,250m 1.200% Notes due 2026, $1,250m 1.750%
Notes due 2028, $1,100m 4.875% Notes due 2028, $650m 4.900% Notes due 2030, $750m 2.250% Notes due 2031, and $500m 4.875% Notes due
2033 (the ‘AstraZeneca Finance Notes’) and AstraZeneca Finance and Holdings Inc., had a $2bn bank loan which was repaid during 2023. Each series
of AstraZeneca Finance Notes has been fully and unconditionally guaranteed by the Company. Each of the guarantees issued by AstraZeneca PLC
is full and unconditional and joint and several.
The guarantee by AstraZeneca PLC of the AstraZeneca Finance Notes is the senior unsecured obligation of AstraZeneca PLC and ranks equally with
all of AstraZeneca PLC’s existing and future senior unsecured and unsubordinated indebtedness. Each guarantee by AstraZeneca PLC is effectively
subordinated to any secured indebtedness of AstraZeneca PLC to the extent of the value of the assets securing such indebtedness. The AstraZeneca
Finance Notes are structurally subordinated to indebtedness and other liabilities of the subsidiaries of AstraZeneca PLC, none of which guarantee
the AstraZeneca Finance Notes.
Notes to the Company Financial Statements 221AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
Notes to the Company Financial Statements
continu ed
4 Called-up share capital
Details of share capital movements in the year are included in Note 24 to the Group Financial Statements.
5 Contingent liabilities
The Company has guaranteed the external borrowing of a subsidiary in the amount of $nil (2022: $286m).
Vermont US Attorney Investigation
In April 2020, AstraZeneca received a Civil Investigative Demand from the US Attorney’s Office in Vermont and the Department of Justice, Civil
Division, seeking documents and information relating to AstraZeneca’s relationships with electronic health-record vendors. AstraZeneca is
cooperating with this enquiry.
AZD1222 Securities Litigation
In January 2021, putative securities class action lawsuits were filed in the US District Court for the Southern District of New York (District Court)
against AstraZeneca PLC and certain officers, on behalf of purchasers of AstraZeneca publicly traded securities during a period later amended to
cover 15 June 2020 through 29 January 2021. The Amended Complaint alleges that defendants made materially false and misleading statements
in connection with the development of AZD1222, AstraZeneca’s vaccine for the prevention of COVID-19. In September 2022, the District Court
granted AstraZeneca’s motion to dismiss the Amended Complaint with prejudice. In May 2023, the US Court of Appeals for the Second Circuit
affirmed the dismissal. The matter is now concluded.
6 Statutory and other information
The Directors of the Company were paid by another Group company in 2023 and 2022.
7 Subsequent events
There were no material subsequent events.
222
AstraZeneca Annual Report & Form 20-F Information 2023 Financial Statements

Group Financial Record
2019 2020 2021 2022 2023
For the year ended 31December $m $m $m $m $m
Revenue and profits
Product Sales 23,565 25,890 36,541 42,998 43,789
Alliance Revenue 62 190 388 755 1,428
Collaboration Revenue 757 537 488 598 594
Cost of sales (4,921) (5,299) (12,437) (12,391) (8,268)
Distribution expense (339) (399) (446) (536) (539)
Research and development expense (6,059) (5,991) (9,736) (9,762) (10,935)
Selling, general and administrative expense (11,682) (11, 294) (15,234) (18,419) (19,216)
Other operating income and expense 1,541 1,528 1,492 514 1,340
Operating profit 2,924 5,162 1,056 3,757 8,193
Finance income 172 87 43 95 344
Finance expense (1,432) (1,306) (1,300) (1,346) (1,626)
Share of after tax losses in associates and joint ventures (116) (27) (64) (5) (12)
Profit/(loss) before tax 1,548 3,916 (265) 2,501 6,899
Taxation (321) (772) 380 792 (938)
Profit for the period 1,227 3,144 115 3,293 5,961
Other comprehensive income/(expense) for the period, net of tax (611) 1,608 (145) (878) 733
Total comprehensive income/(expense) for the period 616 4,752 (30) 2,415 6,694
Profit attributable to:
Owners of the Parent 1,335 3,196 112 3,288 5,955
Non-controlling interests (108) (52) 3 5 6
Earnings per share
Basic earnings per $0.25 Ordinary Share $1.03 $2.44 $0.08 $2.12 $3.84
Diluted earnings per $0.25 Ordinary Share $1.03 $2.44 $0.08 $2.11 $3.81
Dividends $2.80 $2.80 $2.80 $2.90 $2.90
Group Financial Record 223AstraZeneca Annual Report & Form 20-F Information 2023
Corporate Governance Additional InformationFinancial StatementsStrategic Report
