
GUIDE FOR
ANTIMICROBIAL
USE IN
DOGS AND CATS
For more information and further resources visit
www.fvas.unimelb.edu.au/vetantibiotics
UNIVERSITY OF MELBOURNE
Version 1

Play your part in preventing
antibiotic resistant infections.
For more information visit
agriculture.vic.gov.au/amr
As part of our commitment to the implementation
of the National Antimicrobial Resistance Strategy
2015-2019, AgVic and The University of Melbourne
have created education materials about antimicrobial
resistance (AMR) and antimicrobial stewardship (AMS).
The resources aim to provide a practical guide for the
prescribing of antimicrobials that can help start the
conversation about AMR with clients.
FREE RESOURCES
• A5 antibiotic category cards for cattle, horses, sheep, chickens and pigs
• Pocket guide for antimicrobial use in horses • A3 waiting room posters
• A5 prescribing tearaway pads • A4 fact sheet on MRSP • A6 sticker sheets
• DL Double-sided prescribing leaflets • A4 S4 medicated feed order posters
• A2 Australian Prescribing Guidelines for horses • Antibiotic Guardian lapel pins
You can order our resources by emailing
animal.biosecurity@agriculture.vic.gov.au
We all have an important
role to play in the fight
against antimicrobial
resistance.

Antibiotic Pharmacokinetics & Pharmacodynamics
Dogs & Cats
Intrinsic resistance
All members of a bacterial genus or species have
properties that make them naturally resistant to
certain antimicrobials.
Acquired resistance
Previously susceptible bacteria acquire new
genes or a mutation occurs conferring resistance.
Intrinsic resistance Vs Acquired resistance
Bacteriostatic
“ECSTaTiC for bacteriostatic”
Erythromycin (macrolides)
Clindamycin
Sulphonamides
Trimethoprim
Tetracyclines
Chloramphenicol
Bactericidal
“Very Proficient For Complete Cell Murder”
Vancomycin
Penicillin
Fluoroquinolones
Cephalosporins
Carbapenems
Metronidazole
Bacteriostatic Vs Bactericidal Time-Dependent Vs Concentration Dependent
Time-Dependent
• Optimise killing by maximising time above MIC.
• More frequent administration or extended infusion increases
efcacybyextendingT>MIC.
• Goal exceed MIC by 1-5 times for 50-80% of dosage interval.
• E.g.penicillin,cephalexin,TMS,tetracyclines,clindamycin.
C
max
Concentration Dependent
• Optimise killing by maximising peak concentration.
• Higher doses at less frequent intervals (ie. once daily) increases
efcacybymaximisingC
max
:MICratio.
• Goal C
max
:MIC >8.
• E.g.aminoglycosides,uoroquinolones,metronidazole.
C
max
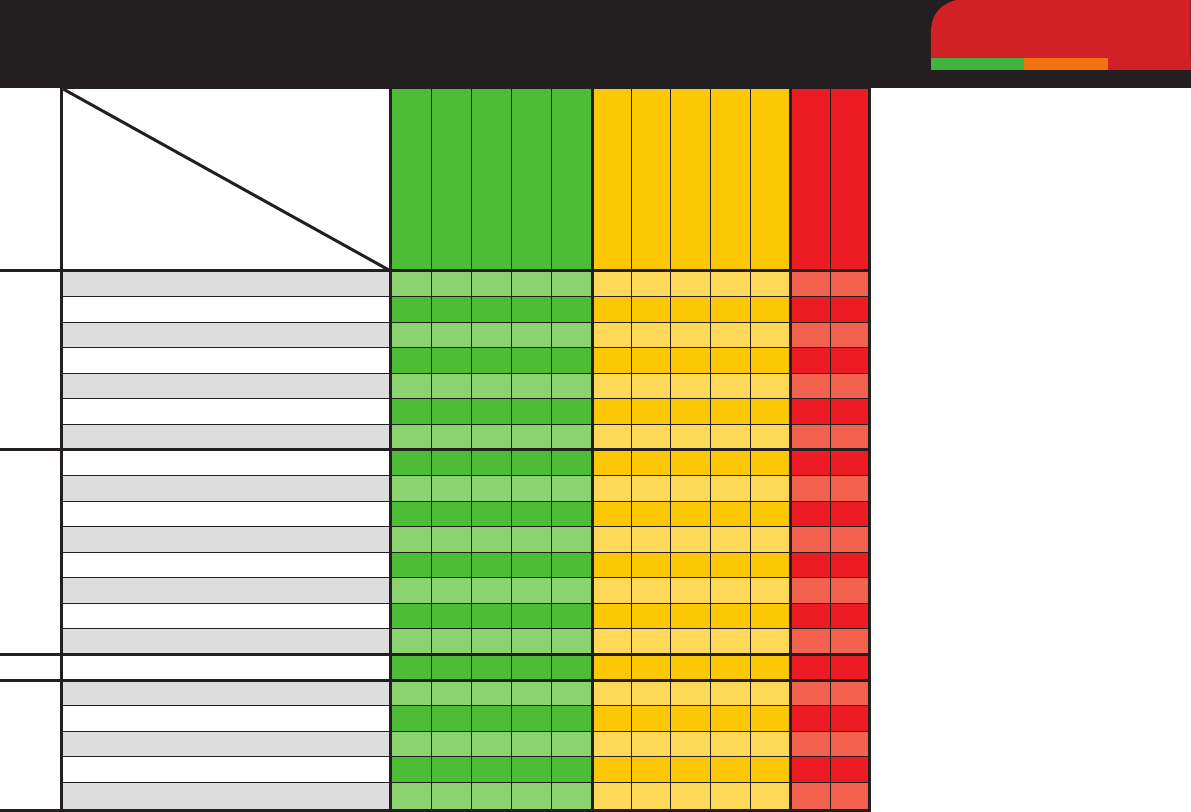
Trafc-light system is based
on the ASTAG antimicrobial
importance rating system.
4 Drug of choice.
+
Good susceptibility.
±
Variable susceptibility.
IR Intrinsicallyresistant.
CoP Coagulasepositive.
CoN Coagulasenegative.
§ Susceptibilitypoorly
pr
edictable, multidrug
resistance increasing in
fr
equency, culture and
susceptibility testing
is strongly recommended.
‡
Multidrugresistant
Staphylococcus
aureus
likely to be of human
origin. Review aseptic
technique.
*
Klebsiella aerogenes
intrinsically r
esistant.
**
Proteus vulgaris
intrinsically resistant.
Spectrum of Activity Against Common Bacteria
A guide to empirical therapy while awaiting susceptibility results.
Gram +veGram -veUrinary isolates
Staphylococcus pseudintermedius (CoP)
§
Staphylococcus aureus (CoP)
‡
Staphylococcus felis (CoN)
β-haemolytic Streptococci (eg S. canis)
Enterococcus faecalis
§
Enterococcus faecium
§
Actinomyces spp.
Escherichia coli
Enterobacter spp.
§
Klebsiella spp.
§
Proteus spp.
Pseudomonas spp.
§
Pasteurella spp.
Bordetella bronchiseptica
Mycoplasma spp.
Anaerobes
Staphylococcus pseudintermedius
Enterococcus faecalis
Escherichia coli
Proteus mirabilis
Proteus spp. (excluding P. mirabilis)
Drug
Bug
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
±
±
±
±
±
±
±
±
± ±
±
± ±+
+ + +
+
+
+
+
+
+
+
+ +
+ +
+
+
+
+
+
+
+ +
+
+
+
+
+
+
+
+
+
+
+
+
*
+**
+*
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
IR
IR
IR
IR
IR
IR IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IR
IRIR
IR
IR
IR IR
IR
IR
IR
IR
IRIR
IR
IR
IR
IR
IR
IR IRIR
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+++
+
+ + ++
±
±
±
±
±
±
±
±
±
Enrooxacin
Cefovecin
Clindamycin
Metronidazole
Gentamicin
Cefazolin/cephalexin
Amoxycillinclavulanate
Chloramphenicol
Trimethoprim sulpha
Doxycycline
Ampicillin/amoxycillin
Penicillin
Dogs & Cats
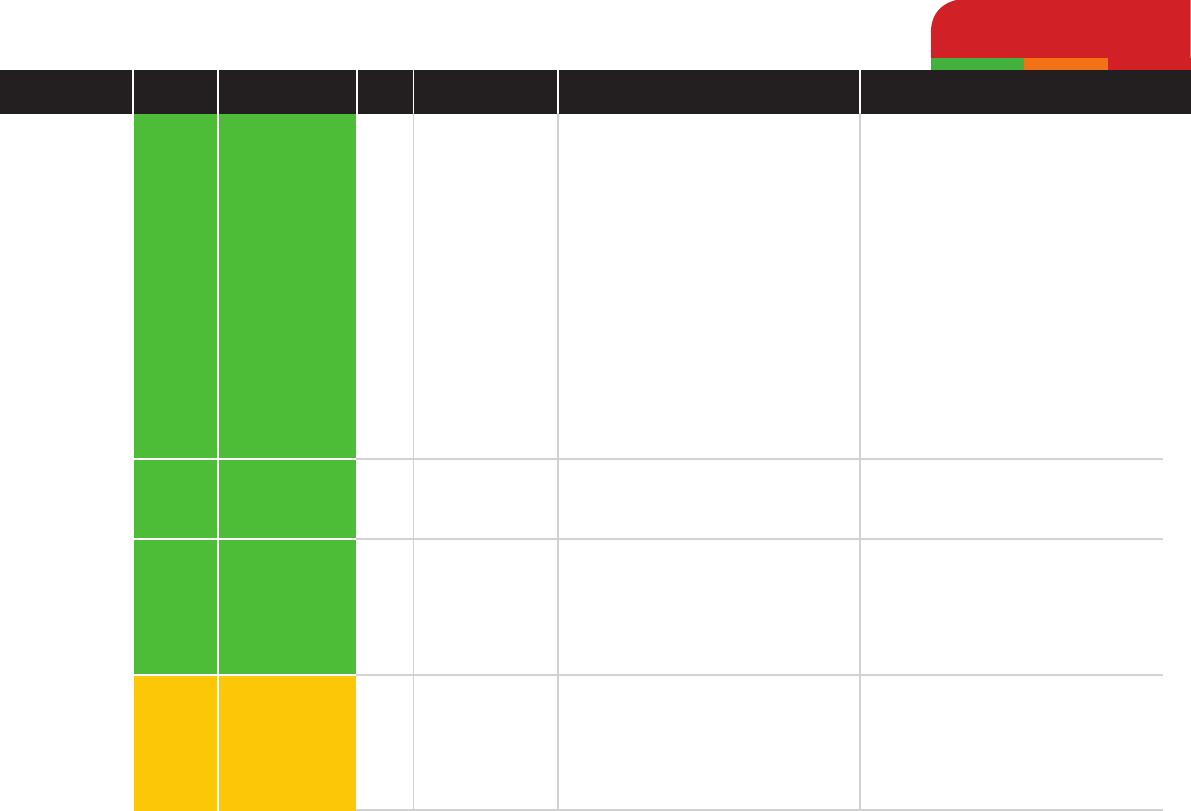
Amoxycillin
Ampicillin
Penicillin
Amoxycillin
clavulanic acid
Low
Low
Low
Medium
Antibiotic Pharmacotherapy by Class
Beta-lactams
Clinical Pearls
Anaerobic activity useful for cat-bite
infections, periodontal disease, tooth
abscesses, wound infections.
Drug of choice for streptococci,
clostridia, actinomycosis and
Pasteurella multocida.
Greater activity against Gram-
negative bacteria than penicillin,
including E. coli and Proteus mirabilis.
Very high urinary concentrations,
usefulforUTIs,evenpenicillinase-
producing S. aureus.
Not recommended for pyelonephritis
or prostatitis.
Excreted in bile, therefore good for
cholestatic infections.
SlowIV(over3mins).
Spectrumofactivityequivalent
to amoxycillin.
IndicatedforGram-positive
aerobic and anaerobic bacteria
(streptococci, clostridia) and for
infections caused by susceptible
Gram-negative bacteria eg.
P. multocida.
Clavulanic
acidextendstherange
of amoxycillin against
β-lacatamase
producing pathogens, such as
methicillin-susceptible staphylococci.
Higher dose recommended for
Gram-negative infections.
Adverse Reactions
Anaphylaxis rare, other mild
hypersensitivity reactions more
common (urticaria, fever,
angioneurotic oedema).
Anorexia, vomiting, diarrhoea.
Hypersensitivity reactions
and gastrointestinal disturbance
possible.
Hypersensitivity reactions.
Pain on injection.
Anorexia, vomiting, diarrhoea.
Hypersensitivity reactions.
Anaphylaxis after intravenous
administration during general
anaesthesia.
Drug Dose
11-22mg/kg
q8-12h
10-20mg/kg
q6-8h
20-40,000IU/kg
q12h
12.5-25mg/kg
q8-12h
Route
IV
IM
PO
IV
IM
SC
IM
PO
IM
SC
IV
Antibiotic
Importance
Rating
Drug
Class
Dogs & Cats
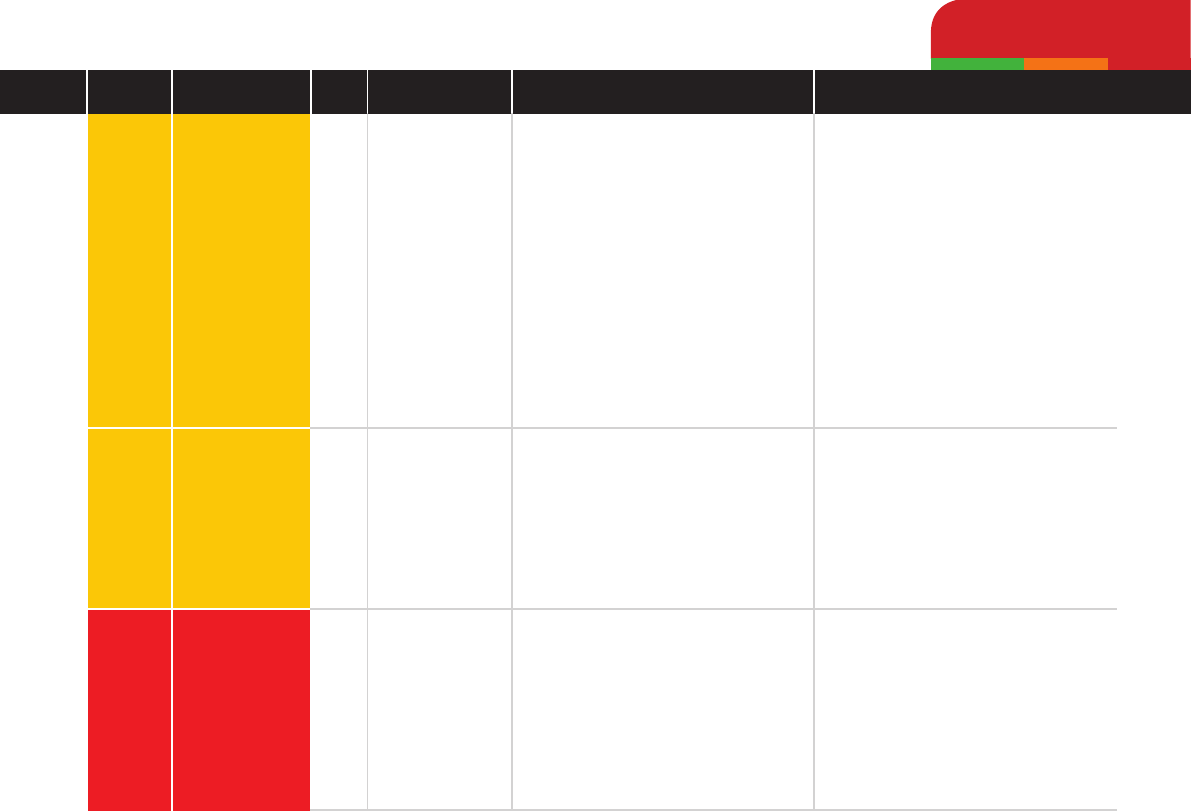
Cefazolin
Cephalexin
Cefovecin
Medium
Medium
High
Antibiotic Pharmacotherapy by Class
Beta-lactams
Clinical Pearls
1st generation cephalosporin active
against methicillin-susceptible
staphylococci, streptococci,
some Gram-negative aerobes,
unpredictable against anaerobes.
Greater Gram-negative activity
than cephalexin and cephalothin.
Good bone penetration.
For surgical prophylaxis administer
IV30-60minsbeforerstincision.
Repeat intra-operative dosing
interval q4hrs for common skin
ora(staphylococci,streptococci),
q2hrs for E. coli.
1st generation cephalosporin, similar
activity to cefazolin except less
Gram-negative activity.
GivewithfoodtoreduceGITside
effects, can also lower dose if side
effects occur. Only use for skin
disease when topical therapy
insufcienttocontrolpyoderma.
3rd
generationcephalosporin.
Similar
spectrumofactivityto
amoxycillin clavulanate.
Reserve** for infections where no
effective alternative.
LabelrestraintFORUSEONLY
in dogs and cats where indicated
by antibiotic sensitivity testing
according to principles of prudent use.
Adverse Reactions
Hypersensitivity reactions,
painonIMinjection.
Vomiting and diarrhoea common
when administered without food.
Hypersensitivity reactions possible.
Vomiting, diarrhoea,
hypersensitivity.
Drug Dose
20-35mg/kgq8h
for therapy,
22 mg/kg
surgical
prophylaxis
22-30mg/kgq12h
8mg/kg
Route
IV
IM
PO
SC
Antibiotic
Importance
Rating
Drug
Class
Dogs & Cats
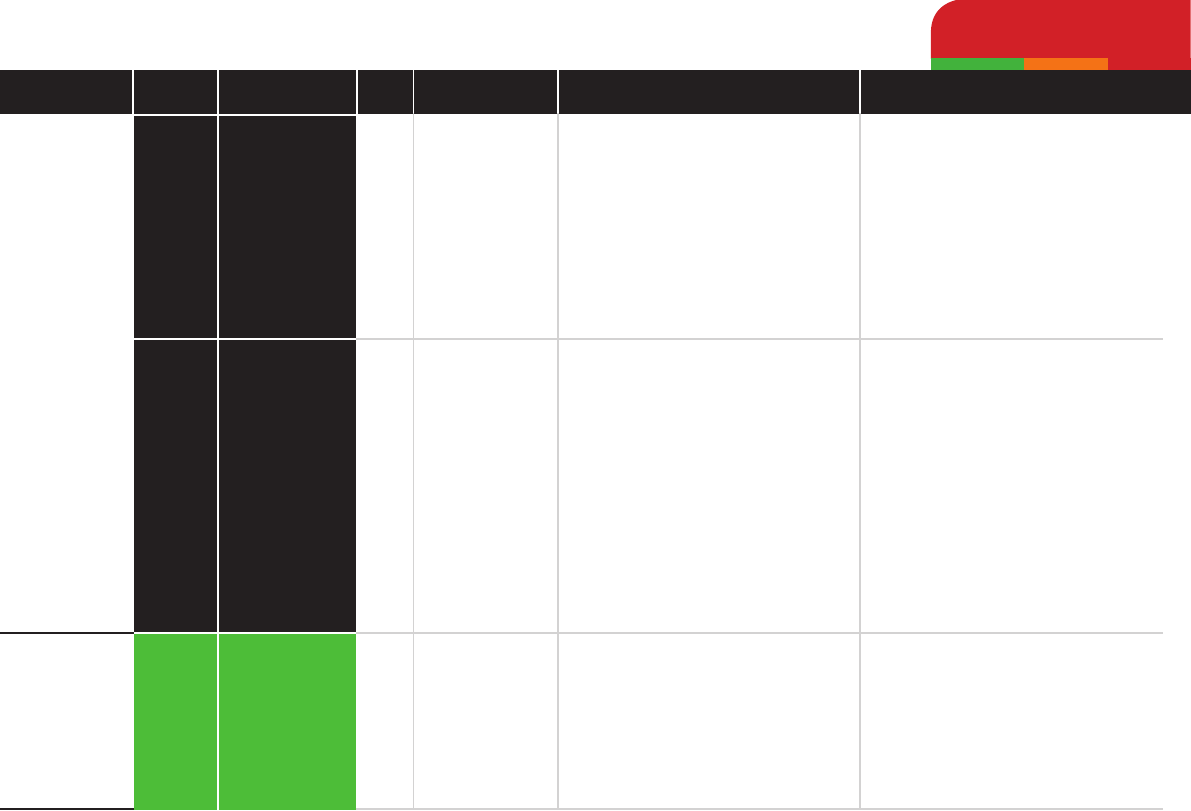
Ceftazidime
Cefotaxime
Doxycycline
High
High
Low
Antibiotic Pharmacotherapy by Class
Beta-lactamsTetracyclines
Clinical Pearls
3rdgenerationcephalosporinwith
10 times greater activity against
P. aeruginosa.
Slightlylessactiveagainstall
other organisms than other
cephalosporins.
Reserve** for P. aeruginosa
infectionswithconrmed
susceptibility.
3rdgenerationcephalosporin.
Due to expense and potential to
select for resistant infections,
these drugs should be reserved**
for life-threatening infections,
such as bacterial meningitis caused
by Gram-negative bacteria
(especially Enterobacteriaceae).
Maybeusedincombinationwithan
aminoglycosideforMDRinfections
in compromised animals
(neutropaenic).
Excellent penetration into most
tissues (including prostate).
Broad spectrum acitivity, including
many intracellular pathogens such
as Chlamydia, Coxiella, Nocardia
and some Mycoplasma species.
Adverse Reactions
Gastrointestinal disturbance.
Pain
onIMinjection,gastrointestinal
disturbances common due to
broad antibacterial action.
Superinfection
withresistant
microorganisms, including yeasts,
may be anticipated.
Administration to growing puppies
and pregnant bitches results in
yellow discolouration of teeth.
Drug Dose
25-50mg/kg
q8-12h
Toexceed
P. aeruginosa
MIC30mg/kg
q4h or constant
IVinfusionof
4.1mg/kg/h
20-40mg/kg q8h
5mg/kg q12h or
10mg/kg q24h
Route
IV
IM
PO
Antibiotic
Importance
Rating
Drug
Class
Dogs & Cats
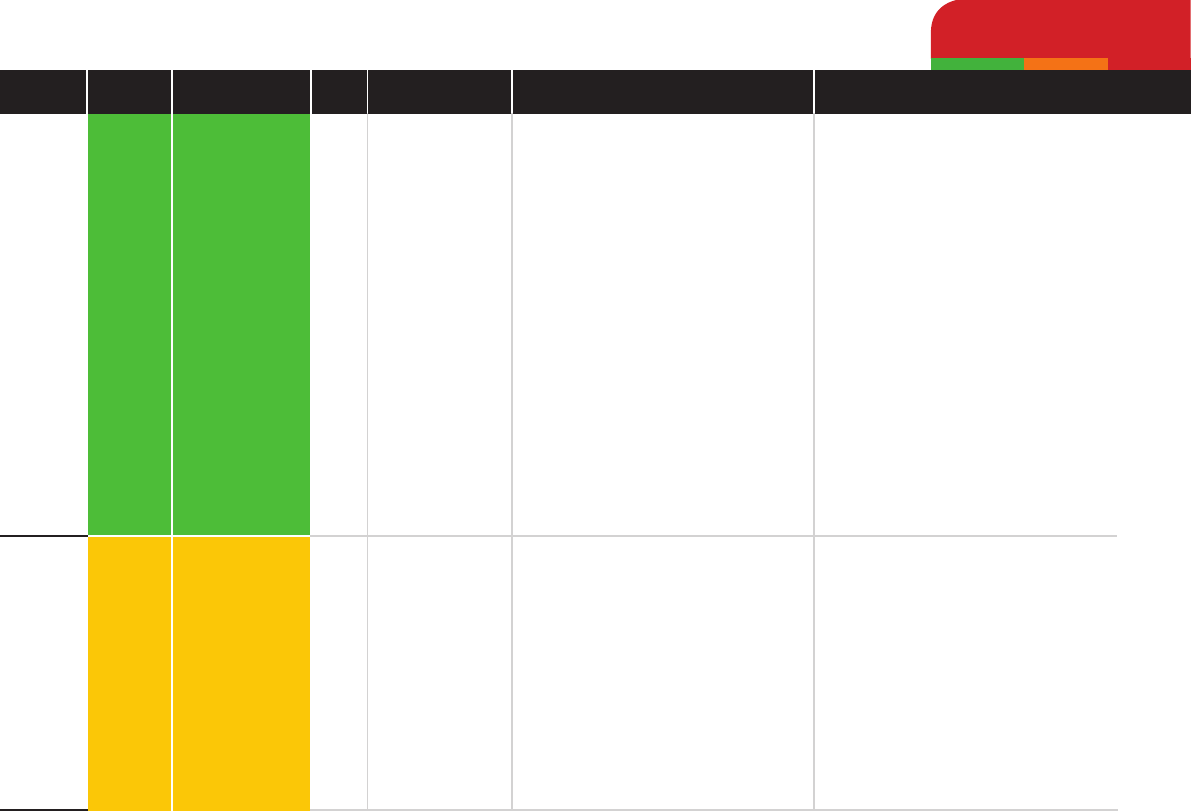
Trimethoprim
sulphonamide
Gentamicin
Low
Medium
Antibiotic Pharmacotherapy by Class
SulphonamidesAminoglycosides
Clinical Pearls
Broad spectrum activity, including
Nocardia spp., Toxoplasma spp. and
other protozoa.
Well absorbed from gastrointestinal
tract, excellent penetration into
many tissues including meninges,
prostate and urinary tract.
ISCAIDrecommendedrstline
empirical treatment option for
sporadic bacterial cysitis (simple
uncomplicatedUTI)for3-5days(low
risk of adverse effects with short
course).
Fortherapy>7daysbaseline
Schirmer’steartestingrecommended
with periodic re-evaluation.
Excellent activity against
Gram-negative bacteria and
some staphylococci.
No anaerobic activity.
Synergistic
incombinationwith
β-lactam.
Inactivatedbypurulentdebris.
Ensureadequateuidand
electrolyte balance during treatment.
Clinicalmonitoringfortoxicosismay
include monitoring trough levels,
daily monitoring of urine for epithelial
casts and daily serum creatinine.
Adverse Reactions
Chronicuse(>2weeks)canlead
to crystalluria, haematuria, urinary
obstruction, haematopoietic
disorders (anaemia, leukopaenia,
thrombocytopaenia) and
dermatological reactions.
Do not use in Doberman Pinschers.
~0.25% of dogs may suffer
idiosyncratic drug reactions 10-21
days after exposure, including
fever, arthropathy, blood dyscrasia,
epistaxis, hepatopathy, skin
eruptions,uveitis,KCS.
Indogs<12kg,1weekTMSdecreases
tear production by 15%, overdose
canleadtoKCS.
Cancausehypothyroidismand/or
loweredT4indogs.
Catssalivateiftabletprotective
coating broken.
Ototoxicity possible.
Nephrotoxic especially if
hypovolaemia, hypokalaemia,
hyponatraemia, elevated trough
concentrations, pre-existing renal
disease, concurrent nephrotoxic
drug administration, prolonged
therapy
(>7-10days),age
(neonates, geriatrics).
PainonIMinjection.
Drug Dose
15-30mg/kgq12h
Dogs: 9-14mg/kg
q24h
Cats:
5-8mg/kg
q24h
Route
PO
IV
IV
IM
Antibiotic
Importance
Rating
Drug
Class
Dogs & Cats
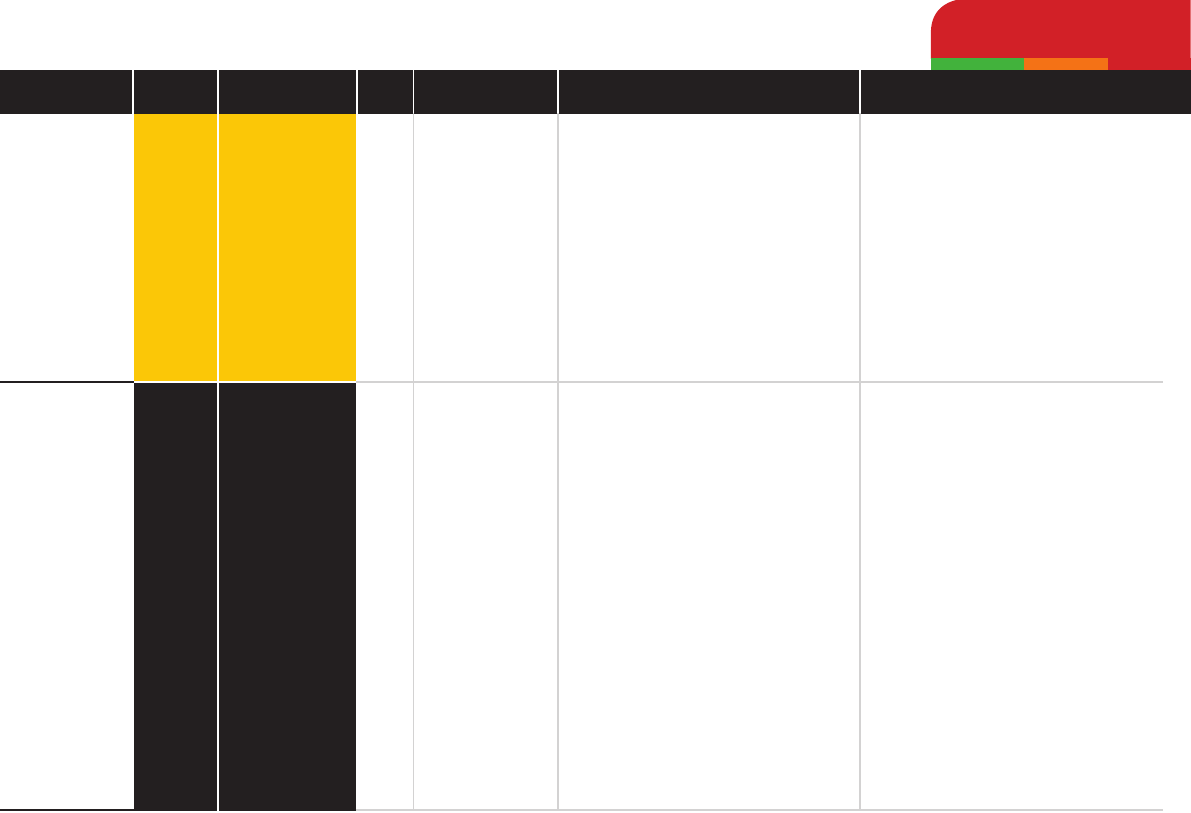
Metronidazole
Nitrofurantoin
Medium
High
Antibiotic Pharmacotherapy by Class
NitroimidazolesNitrofurans
Clinical Pearls
Not indicated in acute
gastrointestinal disease unless
evidence of sepsis.
Excellent anaerobic activity.
Criticaldrugformanaginghuman
Clostridium difcile infections.
Drug interactions: phenobarbital
may enhance metabolism;
cimetidine may decrease
metabolism and increase dose
related adverse effects.
Lower urinary tract infections only.
Reserve** for exceptional cases.
Do not use for pyelonephritis or other
conditions where tissue (vs. urine)
levels are needed.
Avoid in cases with renal impairment.
No activity against Pseudomonas,
Proteus, Serratia, Acinetobacter spp.
Probenecid inhibits renal excretion.
Antagonistictouoroquinolones.
Adverse Reactions
Careinliverdisease,canpredispose
toCNStoxicity-reducedoseto
7.5mg/kg.
Gastrointestinal disturbance,
hepatotoxicity,CNSsigns,
haematuria, neutropenia.
Potentiallyteratogenicinrst
third of pregnancy.
Canimpactfaecalmicrobiome
long-term.
Gastrointestinal disturbances,
hepatopathy, male infertility in
dogs.
Drug Dose
Dogs:
10-15mg/kg
PO q12h
(10mg/kg
SLOWIV)
Cats:
10-15mg/kg q24h
4.4-5mg/kg q8h
Route
PO
IV
PO
Antibiotic
Importance
Rating
Drug
Class
Dogs & Cats
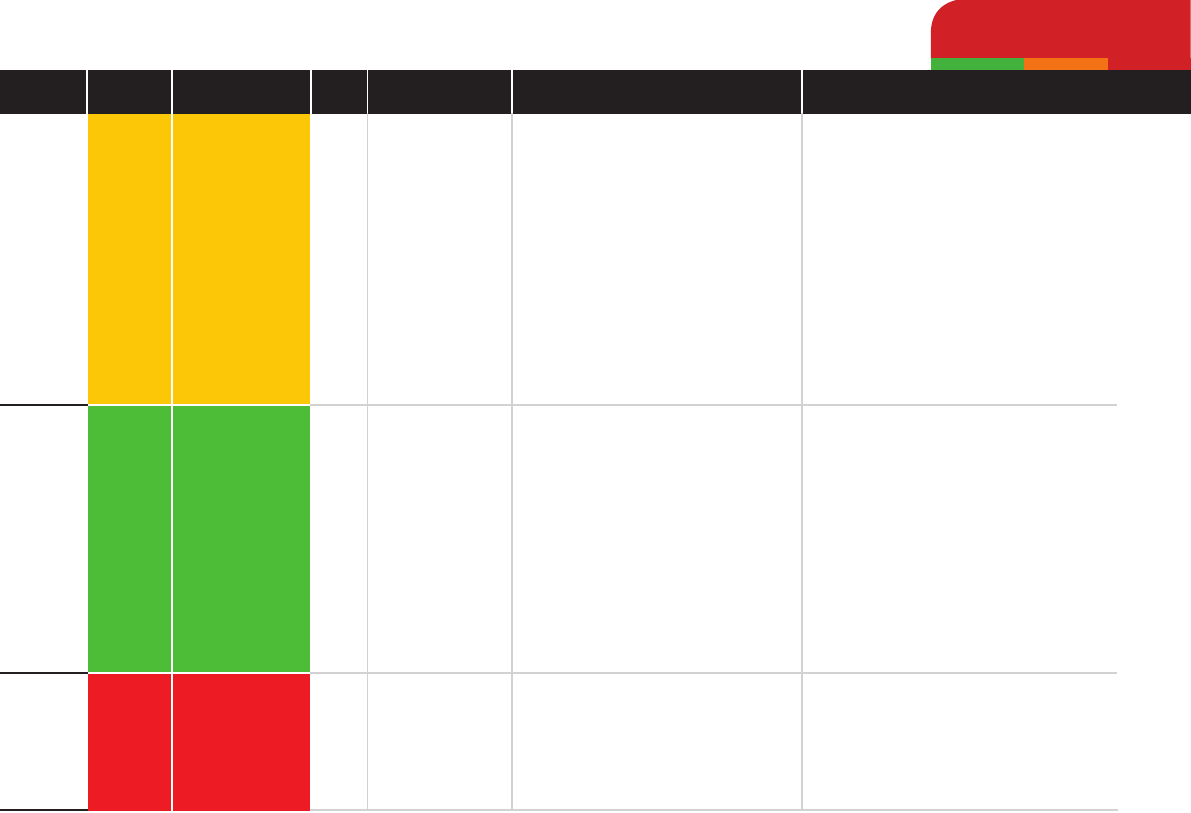
Clindamycin
Chloramphenicol
Polymyxin B
Medium
Low
High
Antibiotic Pharmacotherapy by Class
LincosamidesPhenicolsPolypeptides
Clinical Pearls
Active against staphylococci, streptococci,
Actinomyces, Nocardia, and Mycoplasma
spp. plus anaerobes (Bacteroides spp.,
Fusobacterium spp., Clostridium
perfringens).
Only u
se for skin disease when topical
therapy
insufcienttocontrolpyoderma.
Cross-resistance
tolincosamides
in bacteria resistant to macrolides.
High concentration in prostate.
Use for toxoplasmosis controversial
as may help clinical signs but not clear
inf
ection
fromCNSoreye.
Erythromycin and chloramphenicol
are antagonistic.
Mostly
usedtopically.
May
beusedsystemicallyformultidrug
resistant organisms.
Broad spectrum.
Avoid systemic use in cases with hepatic
failure, renal failure, pre-existing
haematologic abnormalities, pregnancy,
lact
ation and in young animals.
Eliminated by glucuronidation mechanisms,
cats excrete higher proportion unchanged
in urine than dogs.
Pot
ent inhibitor of P450 enzymes -
reduced hepatic clearance of
phenobarbital, pentobarbital.
Used t
opically for treatment of bacterial
keratitis, otitis externa and skin infections.
Active topically against Pseudomonas
spp. and other Gram negatives (except
Proteus, Morganella and Serratia spp.).
Inhibited
bythepresenceofpurulent
exudate.
Adverse Reactions
Oesophagitis and oesophageal
stricture have been reported in
cats associated with use of
generic capsules - follow capsules
with water or food.
Diarrhoea, neuromuscular
blockade.
Oral suspension may be
unpalatable for cats.
Pain
onIMinjection.
Anorexia, hypersalivation,
vomiting with systemic use.
Dose r
elated reversible bone
marrow suppression may develop
with pr
olonged treatment - usually
resolves within days.
Cats
moresusceptible-within2
weeks of treatment.
Wear gloves and mask when
handling medication as
idiosyncratic aplastic anaemia
can develop in people handling
this drug.
Nephrotoxic if administered
systemically.
Potentially ototoxic.
Ophthalmic formulations
associated with anaphylaxis
in cats.
Drug Dose
11mg/kg q12h
For
IV:
dilute 1:10 in
0.9% saline,
administer over
60mins
11mg/kg
q12-24h
Toxoplasmosis:
25mg/kg q12h
Dogs:
40-50mg/kg
q6-8h
Cats:
12.5-20mg/kg q12h
Route
IV
IM
PO
IV
IM
Antibiotic
Importance
Rating
Drug
Class
Topical Topical
Dogs & Cats
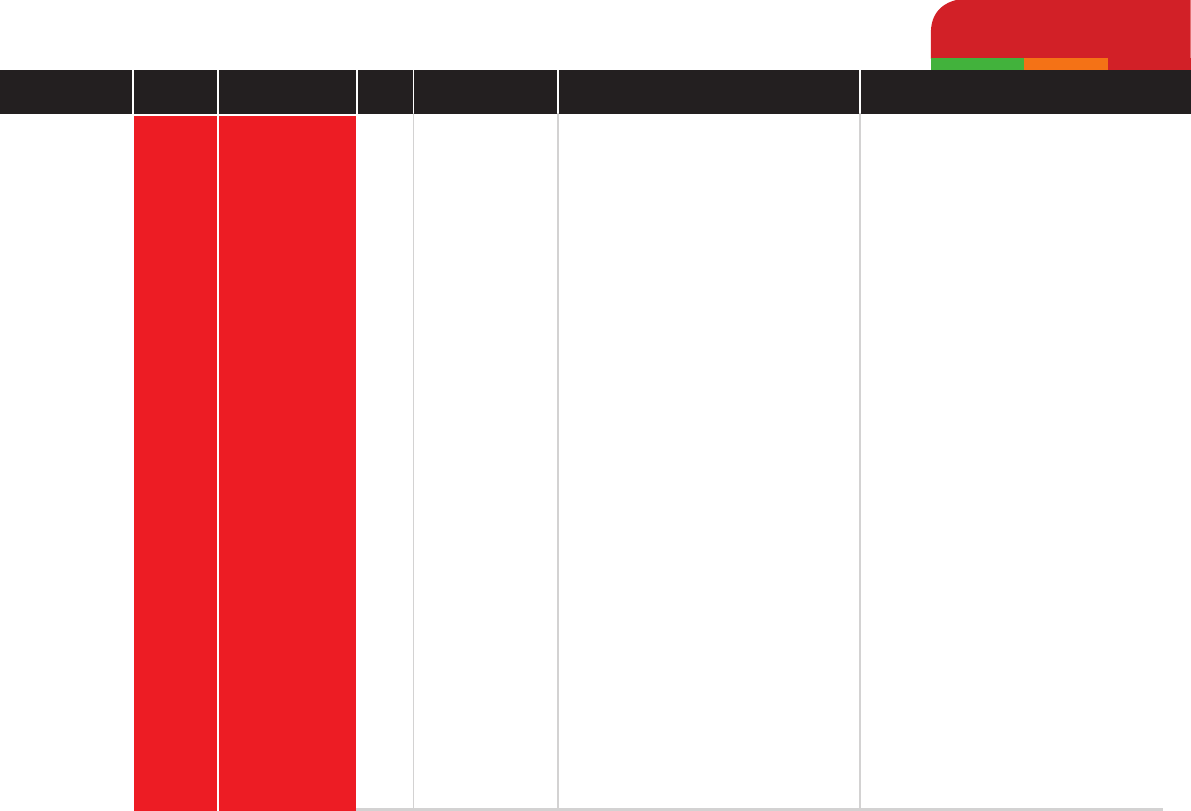
EnrooxacinHigh
Antibiotic Pharmacotherapy by Class
Fluoroquinolones
Clinical Pearls
2ndgenerationuoroquinolone
active against Pasteurella spp.,
Gram-negative enteric bacilli,
staphylococci(higherMIC).
Variable activity against Pseudomonas
aeruginosa(highestMIC).
Poor activity against streptococci,
enterococci and anaerobes.
Notindicatedinsupercialpyoderma.
Reserve** for infections where
culture and susceptibility indicate
no effective alternative.
Use is a known risk factor for
selection of methicillin-resistant
staphylococci.
Iforganismresistanttoone
uoroquinolone,typicallyresistant
to all (cross-resistance).
Good distribution to bone, prostate
andskin.Concentratedinurine,bile
and within phagocytic cells.
Enrooxacinispartially(~20%)
de-ethylatedtociprooxacin.
Oral absorption inhibited by antacids,
sucralfate, supplements containing
aluminium, calcium, iron and zinc.
Chelation/precipitation
inIVuids
with calcium or magnesium.
Reduced hepatic clearance of
theophylline.
Antagonism with chloramphenicol,
rifampicin.
Adverse Reactions
Blindness, due to retinal
detachment, and neurological
signs in cats.
Not always associated with dose
or route of administration, however
greater risk with advancing age.
Anorexia, vomiting, diarrhoea.
CNSeffectswithhighdosesor
rapidIV.
Cautioninanimalspronetoseizures.
Caninetoxicshocksyndromeand
necrotizing fasciitis caused by
uoroquinoloneusein
Streptococcus canis infections.
Arthropathy in dogs during growth,
smalldogs<8monthsold,orlarge
breeds less than 12-18 months.
Avoid use in cats - especially those
with renal disease.
Drug Dose
Dogs:
5-20mg/kg q24h
Cats:
5mg/kg q24h,
SLOWIV
Route
PO
IV
Antibiotic
Importance
Rating
Drug
Class
Dogs & Cats
2ndgenerationuoroquinolone.
Reserve** for infections where
culture and susceptibility indicate
no effective alternative.
Similaractivity,tissuedistribution,
druginteractionstoenrooxacin.
Concentratedinurine,maybeused
forconrmedpyelonephritisincats
based on susceptibility testing.
3rdgenerationuoroquinolone.
Reserve** for infections where
culture and susceptibility indicate
no effective alternative.
Greater activity against
Gram-positive cocci and anaerobes
thanotheruoroquinolones.
Similar
druginteractionsto
enrooxacin.
Avoid. Oral absorption in dogs highly
variable (~50%), lower than humans.
Only reaches therapeutic targets for
bacteriawithMIC≤0.06
µg/ml
(vs≤1
µg/ml in humans).
Generally not effective for
staphylococci or P. aeruginosa in
dogs and cats.
Marbooxacin
Pradooxacin
Ciprooxacin
High
High
High
Antibiotic Pharmacotherapy by Class
Fluoroquinolones
Clinical PearlsAdverse Reactions
Anorexia, vomiting, diarrhoea.
CNSeffectswithhighdosesor
rapidIV.
Cautioninanimalspronetoseizures.
Arthropathy in immature animals.
Higher doses in dogs associated
with myelosuppression.
Do not use in dogs less than 1 year
of age, or in pregnant or lactating
animals.
Gastrointestinal disturbances.
Caution
inanimalspronetoseizures.
Drug Dose
2.75-5.5mg/kg
q24h
Dogs:
3-5mg/kg
q24h
Cats:
5-10mg/kg q24h
25mg/kg
Route
PO
PO
PO
Antibiotic
Importance
Rating
Drug
Class
*
Black shading represents high importance rated antibiotics not registered for use in animals
that should be avoided or ONLY used in exceptional circumstances.
**
Exceptionalcircumstancesdenedasuseinananimalbasedoncultureandsusceptibility,
where there is no effective alternative therapy and a reasonable chance of survival.
NB. Manyrecommendationsinthisguiderepresentoff-label use of antimicrobials.
Compliance
withlegalrequirementsinyourjurisdictionisyourresponsibility.
Recommendations only apply to dogs and cats and cannot be safely extrapolated to other
small animal species.
Dogs & Cats
Version 1
MRSP dermatology fact sheet
Methicillin-resistant Staphylococcus pseudintermedius (MRSP)
• Staphylococcus pseudintermedius
(SP) are normal skin/mucosal flora
found on dogs and cats.
• Methicillin resistance = resistance
to all β
-lactam antimicrobials
(including β
-lactamase inhibitor
combinations).
• Emerging opportunistic pathogen
in Australia – 12% clinical SP
infections MRSP, 8% healthy urban
dogs MRSP carriers.
• MRSP vs Methicillin-susceptible SP
• No more pathogenic
• No difference in clinical disease.
• Many MRSP carry other resistance
genes, sometimes extensive drug
resistance.
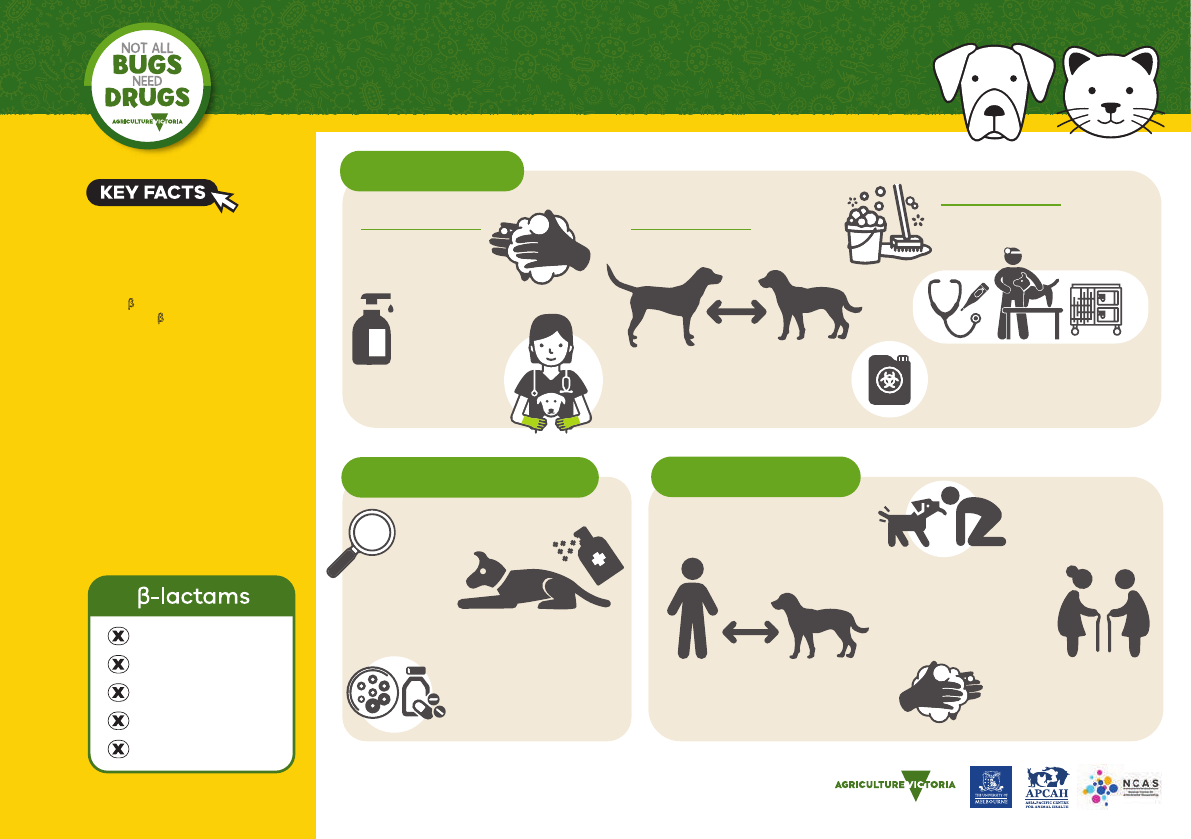
BIOSECURITY
*Except a few anti-MRSA cephalosporins
Use liquid soap
or alcohol-based
hand sanitiser.
Wear gloves when
handling patients.
Isolate MRSP patients.
Consider in-contact pets carriers.
Pets may carry MRSP after
clinical resolution.
PERSONAL
Routinely wash
hands before &
after each patient.
PATIENT
HOSPITAL
Clean gross contamination/biofilm
with detergent first then disinfect.
Routine cleaning and disinfection
all that is required.
MRSP readily inactivated by
commonly used disinfectants.
Critical to identify & address
underlying cause.
If needed, systemic therapy
based on C&S.
No dominant susceptibility
pattern, often MDR.
Try topical!
MRSP is not resistant to
antiseptics (eg. chlorhexidine, bleach).
Avoid contact with
skin, nose, mouth,
perineum and faeces
of infected dogs.
Wash or alcohol-sanitise
hands after handling
infected dog.
Warn owners of zoonotic
potential, particularly those
with breaches in their skin
barrier or poor skin integrity
(ie. wounds, elderly people).
Minimise contact between infected
dogs, other animals and people.
Exposed bedding and surfaces will
also be contaminated.
Dogs are the natural SP host.
Infection in people is rare,
but possible.
Penicillin
Amoxycillin
Cephalosporins*
Carbapenems
Amoxy/clav
TREATMENT OPTIONS ZOONOTIC RISK
For more information and further resources visit
agriculture.vic.gov.au/amr
www.fvas.unimelb.edu.au/vetantibiotics

