
Visit the
National Academies Press online, the authoritative source for all books from the
National Academy of Sciences, the National Academy of Engineering, the Institute of
Medicine, and the National Research Council:
• Download hundreds of free books in PDF
• Read thousands of books online, free
• Sign up to be notified when new books are published
• Purchase printed books
• Purchase PDFs
• Explore with our innovative research tools
Thank you for downloading this free PDF. If you have comments, questions or just want
more information about the books published by the National Academies Press, you may
contact our customer service department toll-free at 888-624-8373,
visit us online, or
send an email to
This free book plus thousands more books are available at
http://www.nap.edu.
Copyright © National Academy of Sciences. Permission is granted for this material to be
shared for noncommercial, educational purposes, provided that this notice appears on the
reproduced materials, the Web address of the online, full authoritative version is retained,
and copies are not altered. To disseminate otherwise or to republish requires written
permission from the National Academies Press.
ISBN: 0-309-14629-1, 252 pages, 6 x 9, (2010)
This free PDF was downloaded from:
http://www.nap.edu/catalog/12793.html
Hepatitis and Liver Cancer: A National Strategy for
Prevention and Control of Hepatitis B and C
Heather M. Colvin and Abigail E. Mitchell, Editors;
Committee on the Prevention and Control of Viral
Hepatitis Infections; Institute of Medicine

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Heather M. Colvin and Abigail E. Mitchell, Editors
Committee on the Prevention and Control of Viral Hepatitis Infections
Board on Population Health and Public Health Practice
H E PAT I T I S A N D
L I V E R C A N C E R
A National Strategy for Prevention and
Control of Hepatitis B and C
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
THE NATIONAL ACADEMIES PRESS 500 Fifth Street, N.W. Washington, DC 20001
NOTICE: The project that is the subject of this report was approved by the Governing
Board of the National Research Council, whose members are drawn from the councils of
the National Academy of Sciences, the National Academy of Engineering, and the Institute
of Medicine. The members of the committee responsible for the report were chosen for their
special competences and with regard for appropriate balance.
This study was supported by Contract 200-2005-13434, TO#16, between the National Acad-
emy of Sciences and the Department of Health and Human Services (with support from the
Centers for Disease Control and Prevention, the Office of Minority Health, and the Depart-
ment of Veterans Affairs) and by the Task Force for Child Survival and Development on behalf
of the National Viral Hepatitis Roundtable. Any opinions, findings, conclusions, or recommen-
dations expressed in this publication are those of the author(s) and do not necessarily reflect
the view of the organizations or agencies that provided support for this project.
Library of Congress Cataloging-in-Publication Data
Hepatitis and liver cancer : a national strategy for prevention and control of hepatitis B
and C / Heather M. Colvin and Abigail E. Mitchell, editors ; Committee on the Prevention
and Control of Viral Hepatitis Infections, Board on Population Health and Public Health
Practice.
p. ; cm.
Includes bibliographical references and index.
ISBN 978-0-309-14628-9
1. Hepatitis B—United States. 2. Hepatitis C—United States. 3. Liver—Cancer—United
States. I. Colvin, Heather M. II. Mitchell, Abigail E. III. Institute of Medicine (U.S.).
Committee on the Prevention and Control of Viral Hepatitis Infections. IV. Institute of
Medicine (U.S.). Board on Population Health and Public Health Practice. V. National
Academies Press (U.S.)
[DNLM: 1. Hepatitis B—complications—United States. 2. Hepatitis B—prevention &
control—United States. 3. Hepatitis C—complications—United States. 4. Hepatitis C—
prevention & control—United States. 5. Liver Neoplasms—prevention & control—United
States. 6. Viral Hepatitis Vaccines—therapeutic use—United States. WC 536 H5322 2010]
RA644.H4H37 2010
616.99'436—dc22
2010003194
Additional copies of this report are available from the National Academies Press, 500 Fifth
Street, N.W., Lockbox 285, Washington, DC 20055; (800) 624-6242 or (202) 334-3313 (in
the Washington metropolitan area); Internet, http://www.nap.edu.
For more information about the Institute of Medicine, visit the IOM home page at www.
iom.edu.
Copyright 2010 by the National Academy of Sciences. All rights reserved.
Printed in the United States of America
The serpent has been a symbol of long life, healing, and knowledge among almost all cultures
and religions since the beginning of recorded history. The serpent adopted as a logotype by
the Institute of Medicine is a relief carving from ancient Greece, now held by the Staatliche
Museen in Berlin.
Suggested citation: IOM (Institute of Medicine). 2010. Hepatitis and Liver Cancer: A National
Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National
Academies Press.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
“Knowing is not enough; we must apply.
Willing is not enough; we must do.”
—Goethe
Advising the Nation. Improving Health.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
The National Academy of Sciences is a private, nonprofit, self-perpetuating society
of distinguished scholars engaged in scientific and engineering research, dedicated to
the furtherance of science and technology and to their use for the general welfare.
Upon the authority of the charter granted to it by the Congress in 1863, the Acad-
emy has a mandate that requires it to advise the federal government on scientific
and technical matters. Dr. Ralph J. Cicerone is president of the National Academy
of Sciences.
The National Academy of Engineering was established in 1964, under the charter
of the National Academy of Sciences, as a parallel organization of outstanding en-
gineers. It is autonomous in its administration and in the selection of its members,
sharing with the National Academy of Sciences the responsibility for advising the
federal government. The National Academy of Engineering also sponsors engineer-
ing programs aimed at meeting national needs, encourages education and research,
and recognizes the superior achievements of engineers. Dr. Charles M. Vest is presi-
dent of the National Academy of Engineering.
The Institute of Medicine was established in 1970 by the National Academy of
Sciences to secure the services of eminent members of appropriate professions in
the examination of policy matters pertaining to the health of the public. The Insti-
tute acts under the responsibility given to the National Academy of Sciences by its
congressional charter to be an adviser to the federal government and, upon its own
initiative, to identify issues of medical care, research, and education. Dr. Harvey V.
Fineberg is president of the Institute of Medicine.
The National Research Council was organized by the National Academy of Sci-
ences in 1916 to associate the broad community of science and technology with the
Academy’s purposes of furthering knowledge and advising the federal government.
Functioning in accordance with general policies determined by the Academy, the
Council has become the principal operating agency of both the National Academy
of Sciences and the National Academy of Engineering in providing services to the
government, the public, and the scientific and engineering communities. The Coun-
cil is administered jointly by both Academies and the Institute of Medicine. Dr.
Ralph J. Cicerone and Dr. Charles M. Vest are chair and vice chair, respectively, of
the National Research Council.
www.national -academies. org
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
v
COMMITTEE ON THE PREVENTION AND
CONTROL OF VIRAL HEPATITIS INFECTIONS
R. Palmer Beasley (Chair), Ashbel Smith Professor and Dean Emeritus,
University of Texas, School of Public Health, Houston, Texas
Harvey J. Alter, Chief, Infectious Diseases Section, Department of
Transfusion Medicine, National Institutes of Health, Bethesda, Maryland
Margaret L. Brandeau, Professor, Department of Management Science
and Engineering, Stanford University, Stanford, California
Daniel R. Church, Epidemiologist and Adult Viral Hepatitis Coordinator,
Bureau of Infectious Disease Prevention, Response, and Services,
Massachusetts Department of Health, Jamaica Plain, Massachusetts
Alison A. Evans, Assistant Professor, Department of Epidemiology
and Biostatistics, Drexel University School of Public Health,
Drexel Institute of Biotechnology and Viral Research, Doylestown,
Pennsylvania
Holly Hagan, Senior Research Scientist, College of Nursing, New York
University, New York
Sandral Hullett, CEO and Medical Director, Cooper Green Hospital,
Birmingham, Alabama
Stacene R. Maroushek, Staff Pediatrician, Department of Pediatrics,
Hennepin County Medical Center, Minneapolis, Minnesota
Randall R. Mayer, Chief, Bureau of HIV, STD, and Hepatitis, Iowa
Department of Public Health, Des Moines, Iowa
Brian J. McMahon, Medical Director, Liver Disease and Hepatitis
Program, Alaska Native Tribal Health Consortium, Anchorage, Alaska
Martín Jose Sepúlveda, Vice President, Integrated Health Services,
International Business Machines Corporation, Somers, New York
Samuel So, Lui Hac Minh Professor, Asian Liver Center, Stanford
University School of Medicine, Stanford, California
David L. Thomas, Chief, Division of Infectious Diseases, Department of
Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland
Lester N. Wright, Deputy Commissioner and Chief Medical Officer, New
York Department of Correctional Services, Albany, New York
Study Staff
Abigail E. Mitchell, Study Director
Heather M. Colvin, Program Officer
Kathleen M. McGraw, Senior Program Assistant
Norman Grossblatt, Senior Editor
Rose Marie Martinez, Director, Board on Population Health and Public
Health Practice
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
vii
Reviewers
T
his report has been reviewed in draft form by persons chosen for
their diverse perspectives and technical expertise, in accordance with
procedures approved by the National Research Council’s (NRC’s)
Report Review Committee. The purpose of this independent review is to
provide candid and critical comments that will assist the institution in mak-
ing its published report as sound as possible and to ensure that the report
meets institutional standards for objectivity, evidence, and responsiveness
to the study charge. The review comments and draft manuscript remain
confidential to protect the integrity of the deliberative process. We wish to
thank the following individuals for their review of this report:
Scott Allen, Brown University Medical School
Jeffrey Caballero, Association of Asian Pacific Community Health
Organizations
Colleen Flanigan, New York State Department of Health
James Jerry Gibson, South Carolina Department of Health and
Environmental Control
Fernando A. Guerra, San Antonio Metropolitan Health District
Theodore Hammett, Abt Associates Inc.
Jay Hoofnagle, National Institute of Diabetes and Digestive and
Kidney Diseases
Charles D. Howell, University of Maryland School of Medicine
Walter A. Orenstein, Bill & Melinda Gates Foundation
Philip E. Reichert, Florida Department of Health
Charles M. Rice III, The Rockefeller University
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
viii REVIEWERS
Tracy Swan, Treatment Action Group
Su Wang, Charles B. Wang Community Health Center
John B. Wong, Tufts Medical Center
Although the reviewers listed above have provided many constructive
comments and suggestions, they were not asked to endorse the conclusions
or recommendations, nor did they see the final draft of the report before
its release. The review of the report was overseen by Bradford H. Gray,
Senior Fellow, The Urban Institute, and Elena O. Nightingale, Scholar-in-
Residence, Institute of Medicine. Appointed by the Institute of Medicine
and the National Research Council, they were responsible for making cer-
tain that an independent examination of the report was carried out in ac-
cordance with institutional procedures and that all review comments were
carefully considered. Responsibility for the final content of the report rests
entirely with the author committee and the institution.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
ix
Acknowledgments
T
he committee acknowledges the valuable contributions made by the
many persons who shared their experience and knowledge with the
committee. The committee appreciates the time and insight of the pre-
senters during the public sessions: John Ward, Dale Hu, Cindy Weinbaum,
and David Bell, Centers for Disease Control and Prevention; Chris Taylor
and Martha Saly, National Viral Hepatitis Roundtable; Lorren Sandt, Car-
ing Ambassadors Program; Joan Block, Hepatitis B Foundation; Gary
Heseltine, Council of State and Territorial Epidemiologists; William Rogers,
Centers for Medicare and Medicaid Services; Tanya Pagán Raggio Ashley,
Health Resources Services Administration; Carol Craig, National Associa-
tion of Community Health Centers; Daniel Raymond, Harm Reduction
Coalition; and Mark Kane, formerly of the Children’s Vaccine Program,
PATH. We are also grateful for the thoughtful written and verbal testimony
provided by members of the public affected by hepatitis B or hepatitis C.
Several persons contributed their expertise for this report. The com-
mittee thanks David Hutton, of the Department of Management Science
and Engineering at Stanford University; Victor Toy, Beverly David, and
Kathleen Tarleton, of IBM; Shiela Strauss, of the New York University
College of Nursing; Ellen Chang and Stephanie Chao, of the Asian Liver
Center at Stanford University; Gillian Haney, of the Massachusetts Depart-
ment of Public Health; and all the State Adult Viral Hepatitis Prevention
Coordinators that provided information to the committee.
This report would not have been possible without the diligent assistance
of Jeffrey Efird and Daniel Riedford, of the Centers for Disease Control and
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
x ACKNOWLEDGMENTS
Prevention. We appreciate the assistance of Ronald Valdiserri, of the De-
partment of Veterans Affairs, for providing literature for the report.
The committee thanks the staff members of the Institute of Medicine,
the National Research Council, and the National Academies Press who
contributed to the development, production, and dissemination of this
report. The committee thanks the study director, Abigail Mitchell, and
program officer Heather Colvin for their work in navigating this complex
topic and Kathleen McGraw for her diligent management of the committee
logistics.
This report was made possible by the support of the Division of Viral
Hepatitis and Division of Cancer Prevention and Control of the Centers
for Disease Control and Prevention, the Department of Health and Human
Services Office of Minority Health, the Department of Veterans Affairs, and
the National Viral Hepatitis Roundtable.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
xi
Contents
ACRONYMS AND ABBREVIATIONS xvii
SUMMARY 1
The Charge to the Committee, 2
Findings and Recommendations, 2
Surveillance, 3
Knowledge and Awareness, 8
Immunization, 9
Viral Hepatitis Services, 12
Recommendation Outcomes, 17
1 INTRODUCTION 19
Prevalence and Incidence of Hepatitis B and Hepatitis C
Worldwide, 22
Prevalence and Incidence of Hepatitis B and Hepatitis C
in the United States, 25
Hepatitis B, 25
Hepatitis C, 28
Liver Cancer and Liver Disease from Chronic Hepatitis B Virus and
Hepatitis C Virus Infections, 29
The Committee’s Task, 30
The Committee’s Approach to Its Task, 32
References, 35
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
xii CONTENTS
2 SURVEILLANCE 41
Applications of Surveillance Data, 43
Outbreak Detection and Control, 44
Resource Allocation, 45
Programmatic Design and Evaluation, 45
Linking Patients to Care, 45
Disease-Specific Issues Related to Viral-Hepatitis Surveillance, 46
Identifying Acute Infections, 47
Identifying Chronic Infections, 51
Identifying Perinatal Hepatitis B, 54
Other Challenges for Hepatitis B and Hepatitis C Surveillance
Systems, 56
Infrastructure and Process-Specific Issues with Surveillance, 57
Funding Sources, 58
Program Design, 59
Reporting Systems and Requirements, 59
Capturing Data on At-Risk Populations, 61
Case Evaluation, Followup, and Partner Services, 62
Recommendations, 63
Model for Surveillance, 66
Core Surveillance, 67
Targeted Surveillance, 71
References, 72
3 KNOWLEDGE AND AWARENESS ABOUT CHRONIC
HEPATITIS B AND HEPATITIS C 79
Knowledge and Awareness Among Health-Care and Social-Service
Providers, 80
Hepatitis B, 81
Hepatitis C, 83
Recommendation, 85
Community Knowledge and Awareness, 89
Hepatitis B, 89
Hepatitis C, 93
Recommendation, 96
References, 101
4 IMMUNIZATION 109
Hepatitis B Vaccine, 109
Current Vaccination Recommendations, Requirements, and
Rates, 110
Immunization-Information Systems, 126
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
CONTENTS xiii
Barriers to Hepatitis B Vaccination, 127
Hepatitis C Vaccine, 136
Feasibility of Preventing Chronic Hepatitis C, 136
Need for a Vaccine to Prevent Chronic Hepatitis C, 137
Cost Effectiveness of a Hepatitis C Vaccine, 137
References, 138
5 VIRAL HEPATITIS SERVICES 147
Current Status, 148
Components of Viral Hepatitis Services, 154
Identification of Infected Persons, 154
Prevention, 166
Medical Management, 166
Major Gaps in Services, 170
General Population, 170
Foreign-Born People, 173
Illicit-Drug Users, 175
Pregnant Women, 181
Correctional Settings, 184
Community Health Facilities, 186
Targeting Settings That Serve At-Risk Populations, 189
References, 192
A COMMITTEE BIOGRAPHIES 209
B PUBLIC MEETING AGENDAS 215
BOXES, FIGURES, AND TABLES
Boxes
S-1 Recommendations, 4
2-1 Role of Disease Surveillance, 42
2-2 CDC Acute Hepatitis B Case Definition, 48
2-3 CDC Acute Hepatitis C Case Definition, 49
2-4 CDC Chronic Hepatitis B Case Definition, 52
2-5 CDC Hepatitis C Virus Infection Case Definition
(Past or Present), 53
2-6 CDC Perinatal Hepatitis B Virus Infection Case Definition, 55
3-1 Geographic Regions That Have Intermediate and High Hepatitis B
Virus Endemicity, 81
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
xiv CONTENTS
4-1 Summary of ACIP Hepatitis B Vaccination Recommendations, 112
5-1 Summary of Recommendations Regarding Viral Hepatitis
Services, 148
5-2 Mission Statement of Centers for Disease Control and Prevention
Division of Viral Hepatitis, 150
5-3 Components of Comprehensive Viral Hepatitis Services, 155
5-4 Summary of CDC At-Risk Populations for Hepatitis B Virus
Infection, 156
5-5 Summary of CDC At-Risk Populations for Hepatitis C Virus
Infection, 158
5-6 Hepatitis B Virus-Specific Antigens and Antibodies Used for
Testing, 160
Figures
1-1 Approximate global preventable death rate from selected infectious
diseases and other causes, 2003, 20
1-2 The committee’s approach to its task, 34
2-1 Natural progression of hepatitis B infection, 46
2-2 Natural progression of hepatitis C infection, 47
4-1 Estimated cost of adult hepatitis B vaccination per quality adjusted
life year (QALY) gained for different age groups and different rates
of acute hepatitis B virus (HBV) infection incidence, 119
4-2 Trends in private health-insurance coverage, 133
5-1 Hepatitis B services model, 157
5-2 Essential viral hepatitis services for illicit-drug users, 180
Tables
1-1 Key Characteristics of Hepatitis B and Hepatitis C, 21
1-2 Burden of Selected Serious Chronic Viral Infections in the United
States, 26
4-1 Hepatitis B Vaccine Schedules for Newborns, by Maternal HBsAg
Status—ACIP Recommendations, 114
4-2 Hepatitis B Immunization Management of Preterm Infants Who
Weigh Less Than 2,000 g, by Maternal HBsAg Status—ACIP
Recommendations, 115
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
CONTENTS xv
4-3 Estimated Chance That an Acute Hepatitis B Infection Becomes
Chronic with Age, 118
4-4 Studies of Hepatitis B Vaccination Rates in Injection-Drug
Users, 122
4-5 Public Health-Insurance Plans, 130
5-1 Summary of Adult Viral Hepatitis Prevention Coordinators
Survey, 153
5-2 Interpretation of Hepatitis B Serologic Diagnostic Test
Results, 161
5-3 Interpretation of Hepatitis C Virus Diagnostic Test Results, 164
5-4 Studies of Association Between Opiate Substitution Treatment and
Hepatitis C Virus Seroconversion, 178
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
xvii
Acronyms and Abbreviations
AASLD American Association for the Study of Liver Diseases
ACIP Advisory Committee on Immunization Practices
ACOG American College of Obstetricians and Gynecologists
AHRQ Agency for Healthcare Research and Quality
AIDS acquired immunodeficiency syndrome
ALT alanine aminotransferase
anti-HBc Hepatitis B core antibody
anti-HBs Hepatitis B surface antibody
anti-HCV Hepatitis C antibody
API Asian and Pacific Islander
AST aspartate transaminase
AVHPC adult viral hepatitis prevention coordinators
CDC Centers for Disease Control and Prevention
CHIP Children’s Health Insurance Program
CI confidence interval
CIA enhanced chemiluminescence
CMS Centers for Medicare and Medicaid Services
DIS disease intervention specialist
DTaP diptheria and tetanus toxoids and acellular pertussis
adsorbed vaccine
DUIT drug user intervention trial
DVH Division of Viral Hepatitis
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
xviii ACRONYMS AND ABBREVIATIONS
EIA enzyme immunoassay
EIP Emerging Infections Program
EPSDT early periodic screening diagnosis and treatment program
FDA Food and Drug Administration
FEHBP Federal Employee Health Benefit Program
FQHC federally qualified health center
HAV Hepatitis A virus
HBIG Hepatitis B immunoglobulin
HBsAg Hepatitis B surface antigen
HBV Hepatitis B virus
HCC hepatocellular carcinoma
HCV Hepatitis C virus
HCW health-care workers
HDHP high deductable health plan
HIAA Health Insurance Association of America
HIB haemophilus influenzae type B
HIV human immunodeficiency virus
HMO health maintenance organization
HPV human papilloma virus
HRSA Health Resources and Services Administration
IDU injection-drug user
IIS immunization information systems
IOM Institute of Medicine
IPV inactivated polio virus
MMTP methadone maintenance treatment program
NASTAD National Alliance of State and Territorial AIDS Directors
NAT nucleic acid test
NCHHSTP National Center for HIV/AIDS, Viral Hepatitis, Sexually
Transmitted Diseases, and Tuberculosis Prevention
NEDSS National Electronic Disease Surveillance System
NETSS National Electronic Telecommunications System for
Surveillance
NGO nongovernmental organization
NHANES National Health and Nutrition Examination Survey
NIDU non-injection-drug user
NVAC National Vaccine Advisory Committee
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
ACRONYMS AND ABBREVIATIONS xix
OB/GYN obstetrician/gynecologist
OMH Office of Minority Health
OR odds ratio
PEI peer education intervention
PHIN Public Health Information Network
POS point of service
PPO preferred provider organization
PY person year
QALY quality adjusted life year
RCT randomized clinical trial
RIBA recombinant immunoblot assay
RNA ribonucleic acid
RSV respiratory syncytial virus
SAMHSA Substance Abuse and Mental Health Services
Administration
SARS severe acute respiratory syndrome
SEP syringe exchange program
STD sexually transmitted disease
STRIVE Study To Reduce Intravenous Exposures
TB tuberculosis
TCM traditional Chinese medicine
USPHS US Public Health Service
USPSTF US Preventive Services Task Force
VA Department of Veterans Affairs
vCJD variant Creutzfeldt-Jakob disease
VFC Vaccines for Children
WHO World Health Organization
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
1
Summary
I
n the next 10 years, about 150,000 people in the United States will die
from liver cancer and end-stage liver disease associated with chronic
hepatitis B and hepatitis C. It is estimated that 3.5–5.3 million people—
1–2% of the US population—are living with chronic hepatitis B virus
(HBV) or hepatitis C virus (HCV) infections. Of those, 800,000 to 1.4 mil-
lion have chronic HBV infections, and 2.7–3.9 million have chronic HCV
infections. Chronic viral hepatitis infections are 3–5 times more frequent
than HIV in the United States.
Because of the asymptomatic nature of chronic hepatitis B and hepatitis
C, most people infected with HBV and HCV are not aware that they have
been infected until they have symptoms of cirrhosis or a type of liver cancer,
hepatocellular carcinoma (HCC), many years later. About 65% and 75% of
the infected population are unaware that they are infected with HBV and
HCV, respectively. Importantly, the prevention of chronic hepatitis B and
chronic hepatitis C prevents the majority of HCC cases because HBV and
HCV are the leading causes of this type of cancer.
Although the incidence of acute HBV infection is declining in the
United States, due to the availability of hepatitis B vaccines, about 43,000
new acute HBV infections still occur each year. Of those new infections,
about 1,000 infants acquire the infection during birth from their HBV-
positive mothers. HBV is also transmitted by sexual contact with an in-
fected person, sharing injection drug equipment, and needlestick injuries.
African American adults have the highest rate of acute HBV infection in
the United States and the highest rates of acute HBV infection occur in the
southern region. People from Asia and the Pacific Islands comprise the larg-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
2 HEPATITIS AND LIVER CANCER
est foreign-born population that is at risk for chronic HBV infection. The
number of people in the United States who are living with chronic HBV
infection may be increasing as a result of immigration from highly endemic
countries. On the basis of immigration patterns in the last decade, it is esti-
mated that every year 40,000–45,000 people from HBV-endemic countries
enter the United States legally.
There is no vaccine for hepatitis C. HCV is efficiently transmitted by
direct percutaneous exposure to infectious blood. Persons likely to have
chronic HCV infection include those who received a blood transfusion be-
fore 1992 and past or current injection-drug users (IDUs). Most IDUs in the
United States have serologic evidence of HCV infection (that is, they have
been exposed to HCV at some time). While HCV incidence appears to have
declined over the last decade, a large portion of IDUs, who often do not
have access to health-care services, are not identified by current surveillance
systems making interpretation of that trend complicated. African Ameri-
cans and Hispanics have a higher rate of HCV infection than whites.
THE CHARGE TO THE COMMITTEE
Despite federal, state, and local public health efforts to prevent and
control hepatitis B and hepatitis C, these diseases remain serious health
problems in the United States. Therefore, the Centers for Disease Control
and Prevention (CDC) in conjunction with the Department of Health and
Human Services Office of Minority Health, the Department of Veterans
Affairs, and the National Viral Hepatitis Roundtable sought guidance from
the Institute of Medicine (IOM) in identifying missed opportunities related
to the prevention and control of HBV and HCV infections. IOM was asked
to focus on hepatitis B and hepatitis C because they are common in the
United States and can lead to chronic disease. The charge to the committee
follows.
The IOM will form a committee to determine ways to reduce new HBV
and HCV infections and the morbidity and mortality related to chronic
viral hepatitis. The committee will assess current prevention and control
activities and identify priorities for research, policy, and action. The com-
mittee will highlight issues that warrant further investigations and oppor-
tunities for collaboration between private and public sectors.
FINDINGS AND RECOMMENDATIONS
Upon reviewing evidence on the prevention and control of hepatitis B
and hepatitis C, the committee identified the underlying factors that impede
current efforts to prevent and control these diseases. Three major factors
were found:
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 3
1. There is a lack of knowledge and awareness about chronic viral
hepatitis on the part of health-care and social-service providers.
2. There is a lack of knowledge and awareness about chronic viral
hepatitis among at-risk populations, members of the public, and
policy-makers.
3. There is insufficient understanding about the extent and seriousness
of this public-health problem, so inadequate public resources are
being allocated to prevention, control, and surveillance programs.
That situation has created several consequences:
• Inadequate disease surveillance systems underreport acute and
chronic infections, so the full extent of the problem is unknown.
• At-risk people do not know that they are at risk or how to prevent
becoming infected.
• At-risk people may not have access to preventive services.
• Chronically infected people do not know that they are infected.
• Many health-care providers do not screen people for risk factors
or do not know how to manage infected people.
• Infected people often have inadequate access to testing, social sup-
port, and medical management services.
To address those consequences, the committee offers recommendations
in four categories: surveillance, knowledge and awareness, immunization,
and services for viral hepatitis. The recommendations are discussed below,
and listed in Box S-1.
Surveillance
The viral hepatitis surveillance system in the United States is highly
fragmented and poorly developed. As a result, surveillance data do not pro-
vide accurate estimates of the current burden of disease, are insufficient for
program planning and evaluation, and do not provide the information that
would allow policy-makers to allocate sufficient resources to viral hepatitis
prevention and control programs. The federal government has provided
few resources—in the form of guidance, funding, and oversight—to local
and state health departments to perform surveillance for viral hepatitis.
Additional funding sources for surveillance, such as funding from states
and cities, vary among jurisdictions. The committee found little published
information on or systematic review of viral hepatitis surveillance in the
United States and offers the following recommendation to determine the
current status of the surveillance system:

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
4 HEPATITIS AND LIVER CANCER
BOX S-1
Recommendations
Chapter 2: Surveillance
• 2-1. The Centers for Disease Control and Prevention should conduct
a comprehensive evaluation of the national hepatitis B and
hepatitis C public-health surveillance system.
• 2-2. The Centers for Disease Control and Prevention should develop
specific cooperative viral-hepatitis agreements with all state and
territorial health departments to support core surveillance for
acute and chronic hepatitis B and hepatitis C.
• 2-3. The Centers for Disease Control and Prevention should support
and conduct targeted active surveillance, including serologic
testing, to monitor incidence and prevalence of hepatitis B virus
and hepatitis C virus infections in populations not fully captured
by core surveillance.
Chapter 3: Knowledge and Awareness about Chronic Hepatitis B
and Hepatitis C
• 3-1. The Centers for Disease Control and Prevention should work
with key stakeholders (other federal agencies, state and local
governments, professional organizations, health-care organiza-
tions, and educational institutions) to develop hepatitis B and
hepatitis C educational programs for health-care and social-
service providers.
• 3-2. The Centers for Disease Control and Prevention should work
with key stakeholders to develop, coordinate, and evaluate inno-
vative and effective outreach and education programs to target
at-risk populations and to increase awareness in the general
population about hepatitis B and hepatitis C.
Chapter 4: Immunization
• 4-1. All infants weighing at least 2,000 grams and born to hepati-
tis B surface antigen-positive women should receive single-
antigen hepatitis B vaccine and hepatitis B immune globulin in
the delivery room as soon as they are stable and washed. The
recommendations of the Advisory Committee on Immunization
Practices should remain in effect for all other infants.
• 4-2. All states should mandate that the hepatitis B vaccine se-
ries be completed or in progress as a requirement for school
attendance.
• 4-3. Additional federal and state resources should be devoted to
increasing hepatitis B vaccination of at-risk adults.
• 4-4. States should be encouraged to expand immunization-information
systems to include adolescents and adults.
• 4-5. Private and public insurance coverage for hepatitis B vaccina-
tion should be expanded.
• 4-6. The federal government should work to ensure an adequate,
accessible, and sustainable hepatitis B vaccine supply.
• 4-7. Studies to develop a vaccine to prevent chronic hepatitis C virus
infection should continue.
Chapter 5: Viral Hepatitis Services
• 5-1. Federally funded health-insurance programs—such as Medi-
care, Medicaid, and the Federal Employees Health Benefits
Program—should incorporate guidelines for risk-factor screen-
ing for hepatitis B and hepatitis C as a required core compo-
nent of preventive care so that at-risk people receive serologic
testing for hepatitis B virus and hepatitis C virus and chronically
infected patients receive appropriate medical management.
• 5-2. The Centers for Disease Control and Prevention, in conjunction
with other federal agencies and state agencies, should provide
resources for the expansion of community-based programs that
provide hepatitis B screening, testing, and vaccination services
that target foreign-born populations.
• 5-3. Federal, state, and local agencies should expand programs to
reduce the risk of hepatitis C virus infection through injection-
drug use by providing comprehensive hepatitis C virus preven-
tion programs. At a minimum, the programs should include
access to sterile needle syringes and drug-preparation equip-
ment because the shared use of these materials has been
shown to lead to transmission of hepatitis C virus.
• 5-4. Federal and state governments should expand services to
reduce the harm caused by chronic hepatitis B and hepati-
tis C. The services should include testing to detect infection,
counseling to reduce alcohol use and secondary transmission,
hepatitis B vaccination, and referral for or provision of medical
management.
• 5-5. Innovative, effective, multicomponent hepatitis C virus preven-
tion strategies for injection-drug users and non-injection-drug
users should be developed and evaluated to achieve greater
control of hepatitis C virus transmission.
• 5-6. The Centers for Disease Control and Prevention should pro-
vide additional resources and guidance to perinatal hepatitis B

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 5
BOX S-1
Recommendations
Chapter 2: Surveillance
• 2-1. The Centers for Disease Control and Prevention should conduct
a comprehensive evaluation of the national hepatitis B and
hepatitis C public-health surveillance system.
• 2-2. The Centers for Disease Control and Prevention should develop
specific cooperative viral-hepatitis agreements with all state and
territorial health departments to support core surveillance for
acute and chronic hepatitis B and hepatitis C.
• 2-3. The Centers for Disease Control and Prevention should support
and conduct targeted active surveillance, including serologic
testing, to monitor incidence and prevalence of hepatitis B virus
and hepatitis C virus infections in populations not fully captured
by core surveillance.
Chapter 3: Knowledge and Awareness about Chronic Hepatitis B
and Hepatitis C
• 3-1. The Centers for Disease Control and Prevention should work
with key stakeholders (other federal agencies, state and local
governments, professional organizations, health-care organiza-
tions, and educational institutions) to develop hepatitis B and
hepatitis C educational programs for health-care and social-
service providers.
• 3-2. The Centers for Disease Control and Prevention should work
with key stakeholders to develop, coordinate, and evaluate inno-
vative and effective outreach and education programs to target
at-risk populations and to increase awareness in the general
population about hepatitis B and hepatitis C.
Chapter 4: Immunization
• 4-1. All infants weighing at least 2,000 grams and born to hepati-
tis B surface antigen-positive women should receive single-
antigen hepatitis B vaccine and hepatitis B immune globulin in
the delivery room as soon as they are stable and washed. The
recommendations of the Advisory Committee on Immunization
Practices should remain in effect for all other infants.
• 4-2. All states should mandate that the hepatitis B vaccine se-
ries be completed or in progress as a requirement for school
attendance.
• 4-3. Additional federal and state resources should be devoted to
increasing hepatitis B vaccination of at-risk adults.
• 4-4. States should be encouraged to expand immunization-information
systems to include adolescents and adults.
• 4-5. Private and public insurance coverage for hepatitis B vaccina-
tion should be expanded.
• 4-6. The federal government should work to ensure an adequate,
accessible, and sustainable hepatitis B vaccine supply.
• 4-7. Studies to develop a vaccine to prevent chronic hepatitis C virus
infection should continue.
Chapter 5: Viral Hepatitis Services
• 5-1. Federally funded health-insurance programs—such as Medi-
care, Medicaid, and the Federal Employees Health Benefits
Program—should incorporate guidelines for risk-factor screen-
ing for hepatitis B and hepatitis C as a required core compo-
nent of preventive care so that at-risk people receive serologic
testing for hepatitis B virus and hepatitis C virus and chronically
infected patients receive appropriate medical management.
• 5-2. The Centers for Disease Control and Prevention, in conjunction
with other federal agencies and state agencies, should provide
resources for the expansion of community-based programs that
provide hepatitis B screening, testing, and vaccination services
that target foreign-born populations.
• 5-3. Federal, state, and local agencies should expand programs to
reduce the risk of hepatitis C virus infection through injection-
drug use by providing comprehensive hepatitis C virus preven-
tion programs. At a minimum, the programs should include
access to sterile needle syringes and drug-preparation equip-
ment because the shared use of these materials has been
shown to lead to transmission of hepatitis C virus.
• 5-4. Federal and state governments should expand services to
reduce the harm caused by chronic hepatitis B and hepati-
tis C. The services should include testing to detect infection,
counseling to reduce alcohol use and secondary transmission,
hepatitis B vaccination, and referral for or provision of medical
management.
• 5-5. Innovative, effective, multicomponent hepatitis C virus preven-
tion strategies for injection-drug users and non-injection-drug
users should be developed and evaluated to achieve greater
control of hepatitis C virus transmission.
• 5-6. The Centers for Disease Control and Prevention should pro-
vide additional resources and guidance to perinatal hepatitis B
continued

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
6 HEPATITIS AND LIVER CANCER
Recommendation 2-1. The Centers for Disease Control and Prevention
should conduct a comprehensive evaluation of the national hepatitis B
and hepatitis C public-health surveillance system.
The evaluation should, at a minimum,
• Include assessment of the system’s attributes, including complete-
ness, data quality and accuracy, timeliness, sensitivity, specificity,
predictive value positive, representativeness, and stability.
• Be consistent with CDC’s Updated Guidelines for Evaluating Public
Health Surveillance Systems.
• Be used to guide the development of detailed technical guidance
and standards for viral hepatitis surveillance.
• Be published in a report.
prevention program coordinators to expand and enhance the
capacity to identify chronically infected pregnant women and
provide case-management services, including referral for ap-
propriate medical management.
• 5-7. The National Institutes of Health should support a study of
he effectiveness and safety of peripartum antiviral therapy to
reduce and possibly eliminate perinatal hepatitis B virus trans-
mission from women at high risk for perinatal transmission.
• 5-8. The Centers for Disease Control and Prevention and the De-
partment of Justice should create an initiative to foster partner-
ships between health departments and corrections systems to
ensure the availability of comprehensive viral hepatitis services
for incarcerated people.
• 5-9. The Health Resources and Services Administration should
provide adequate resources to federally funded community
health facilities for provision of comprehensive viral-hepatitis
services.
• 5-10. The Health Resources and Services Administration and the
Centers for Disease Control and Prevention should provide re-
sources and guidance to integrate comprehensive viral hepatitis
services into settings that serve high-risk populations such as
STD clinics, sites for HIV services and care, homeless shelters,
and mobile health units.
BOX S-1 Continued

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 7
The committee offers the following recommendations aimed at mak-
ing viral hepatitis surveillance systems more consistent among jurisdictions
and improving their ability to collect and report data on acute and chronic
hepatitis B and hepatitis C more accurately:
Recommendation 2-2. The Centers for Disease Control and Prevention
should develop specific cooperative viral-hepatitis agreements with all
state and territorial health departments to support core surveillance for
acute and chronic hepatitis B and hepatitis C.
The agreements should include
• A funding mechanism and guidance for core surveillance
activities.
• Implementation of performance standards regarding revised and
standardized case definitions, specifically through the use of
o Revised case-reporting forms with required, standardized
components.
o Case evaluation and followup.
• Support for developing and implementing automated data-collection
systems, including
o Electronic laboratory reporting.
o Electronic medical-record extraction systems.
o Web-based, Public Health Information Network-compliant re-
porting systems.
Recommendation 2-3. The Centers for Disease Control and Preven-
tion should support and conduct targeted active surveillance, including
serologic testing, to monitor incidence and prevalence
1
of hepatitis B
virus and hepatitis C virus infections in populations not fully captured
by core surveillance.
• Active surveillance should be conducted in specific (sentinel) geo-
graphic regions and populations.
• Appropriate serology, molecular biology, and followup will allow for
distinction between acute and chronic hepatitis B and hepatitis C.
1
Incidence refers to the number of new cases within a specified period of time. Prevalence
refers to the number of existing cases in a specified population at a designated time.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
8 HEPATITIS AND LIVER CANCER
Knowledge and Awareness
The committee found that there is relatively poor awareness about
hepatitis B and hepatitis C among health-care providers, social-service
providers (such as staff of drug-treatment facilities and immigrant-services
centers), and the public, especially important, among members of specific
at-risk populations. Lack of awareness about the prevalence of chronic viral
hepatitis in the United States and the target populations and appropriate
methodology for screening, testing, and medical management of chronic
hepatitis B and hepatitis C probably contributes to continuing transmission;
missing of opportunities for prevention, including vaccination; missing of
opportunities for early diagnosis and medical care; and poor health out-
comes in infected people.
To improve knowledge and awareness among health-care pro-
viders and social-service providers, the committee offers the following
recommendation:
Recommendation 3-1. The Centers for Disease Control and Prevention
should work with key stakeholders (other federal agencies, state and
local governments, professional organizations, health-care organiza-
tions, and educational institutions) to develop hepatitis B and hepatitis
C educational programs for health-care and social-service providers.
The educational programs should include at least the following
components:
• Information about the prevalence and incidence of acute and chronic
hepatitis B and hepatitis C both in the general US population and
in at-risk populations, particularly foreign-born populations in the
case of hepatitis B, and IDUs and incarcerated populations in the
case of hepatitis C.
• Guidance on screening for risk factors associated with hepatitis B
and hepatitis C.
• Information about hepatitis B and hepatitis C prevention, hepatitis
B immunization, and medical monitoring of chronically infected
patients.
• Information about prevention of HBV and HCV transmission in
hospital and nonhospital health-care settings.
• Information about discrimination and stigma associated with hepa-
titis B and hepatitis C and guidance on reducing them.
• Information about health disparities related to hepatitis B and
hepatitis C.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 9
To increase knowledge and awareness about hepatitis B and hepatitis
C in at-risk populations and the general population, the committee offers
the following recommendation:
Recommendation 3-2. The Centers for Disease Control and Prevention
should work with key stakeholders to develop, coordinate, and evalu-
ate innovative and effective outreach and education programs to target
at-risk populations and to increase awareness in the general population
about hepatitis B and hepatitis C.
The programs should be linguistically and culturally appropriate and
should advance integration of viral hepatitis and liver-health education into
other health programs that serve at-risk populations. They should incorpo-
rate interventions that meet the following goals:
• Promote better understanding of HBV and HCV infections, trans-
mission, prevention, and treatment in the at-risk and general
populations.
• Promote increased hepatitis B vaccination rates among children
and at-risk adults.
• Educate pregnant women and women of childbearing age about
hepatitis B prevention.
• Reduce perinatal HBV infections and improve at-birth immuniza-
tion rates.
• Increase testing rates in at-risk populations.
• Reduce stigmatization of chronically infected people.
• Promote safe injections among IDUs and safe drug use among non-
injection-drug users (NIDUs).
• Provide culturally and linguistically appropriate educational infor-
mation for all persons who have tested positive for chronic HBV
or HCV infections and those who are receiving treatment.
• Encourage notification of close household and sexual contacts of
infected people to be tested for HBV and HCV and encourage
hepatitis B vaccination of close contacts.
Immunization
The longstanding availability of effective hepatitis B vaccines makes
the elimination of new HBV infections possible, particularly in children.
As noted above, about 1,000 newborns are infected by their HBV-positive
mothers at birth each year in the United States, and that number has not
declined in the last decade. To prevent transmission of HBV from moth-
ers to their newborns, the Advisory Committee on Immunization Practices
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
10 HEPATITIS AND LIVER CANCER
(ACIP) recommends that infants born to mothers who are positive for
hepatitis B surface antigen (HBsAg) receive hepatitis B immune globulin
and a first dose of the hepatitis B vaccine within 12 hours of birth. To
improve adherence to that guideline, the committee offers the following
recommendation:
Recommendation 4-1. All infants weighing at least 2,000 grams and
born to hepatitis B surface antigen-positive women should receive
single-antigen hepatitis B vaccine and hepatitis B immune globulin in
the delivery room as soon as they are stable and washed. The recom-
mendations of the Advisory Committee on Immunization Practices
should remain in effect for all other infants.
The ACIP recommends administration of the hepatitis B vaccine series
to unvaccinated children and young adults under 19 years old. School-entry
mandates have been shown to increase hepatitis B vaccination rates and to
reduce disparities in vaccination rates. Overall, hepatitis B vaccination rates
in school-age children are high (for example, about 80% of states reported
at least 95% hepatitis B vaccine coverage of children in kindergarten in
2006–2007), but there is variability in coverage among states. Additionally,
there are racial and ethnic disparities in childhood vaccination rates—Asian
and Pacific Islander (API), Hispanic, and African American children have
lower vaccination rates than non-Hispanic white children. Regarding vac-
cination of children and adults under 19 years old, the committee offers the
following recommendation:
Recommendation 4-2. All states should mandate that the hepatitis B
vaccine series be completed or in progress as a requirement for school
attendance.
Hepatitis B vaccination for adults is directed at high-risk groups—
people at risk for HBV infection from infected household contact and sex
partners, from injection-drug use, from occupational exposure to infected
blood or body fluids, and from travel to regions that have high or interme-
diate HBV endemicity. Only about half the adults who are at high risk for
HBV infection receive the hepatitis B vaccine. Low coverage of high-risk
adults is attributed to the lack of dedicated vaccine programs; limitations
of funding, insurance coverage, and cost-sharing; and noncompliance of
the involved populations. To increase the rate of hepatitis B vaccination of
at-risk adults, the committee offers the following recommendation:
Recommendation 4-3. Additional federal and state resources should be
devoted to increasing hepatitis B vaccination of at-risk adults.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 11
• Correctional institutions should offer hepatitis B vaccination to all
incarcerated persons. Accelerated schedules for vaccine administra-
tion should be considered for jail inmates.
• Organizations that serve high-risk populations should offer the
hepatitis B vaccination series.
• Efforts should be made to improve identification of at-risk
adults. Health-care providers should routinely seek risk behav-
ior histories from adult patients through direct questioning and
self-assessment.
• Efforts should be made to increase rates of completion of the vac-
cine series in adults.
• Federal and state agencies should annually determine gaps in hepa-
titis B vaccine coverage among at-risk adults and estimate the
resources needed to fill those gaps.
Immunization-information systems are used for collection and con-
solidation of vaccination data from multiple health-care providers, vaccine
management, adverse-event reporting, and tracking lifespan vaccination
histories. States have made progress on developing and implementing im-
munization-information systems, particularly with regard to collecting vac-
cination data on children. The committee believes that it is also important
to include vaccination data on adolescents and adults in immunization
information systems and offers the following recommendation:
Recommendation 4-4. States should be encouraged to expand
immunization-information systems to include adolescents and adults.
Coverage for hepatitis B vaccination is greater for children and youths
than for adults. Except for Medicaid’s Early Periodic Screening, Diag-
nosis, and Treatment entitlement, public-health insurance often contains
cost-sharing, which may create a barrier to vaccination for some people.
Private health insurance has gaps for vaccination coverage because it does
not universally cover all ACIP-recommended vaccinations for children and
adults. Furthermore, most privately insured persons are required to pay to
receive vaccinations. To reduce barriers to children and adults for hepatitis
B vaccination, the committee offers the following recommendation:
Recommendation 4-5. Private and public insurance coverage for hepa-
titis B vaccination should be expanded.
• Public Health Section 317 should be expanded with sufficient fund-
ing to become the public safety net for underinsured and uninsured
adults to receive the hepatitis B vaccination.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
12 HEPATITIS AND LIVER CANCER
• All private insurance plans should include coverage for all ACIP-
recommended vaccinations. Hepatitis B vaccination should be free
of any deductible so that first-dollar coverage exists for this preven-
tive service.
There has not been a national shortage of the hepatitis B vaccine, how-
ever, temporary supply problems occurred with this vaccine in 2008 (adult
and dialysis formulations of Recombivax HB) and 2009 (pediatric formula-
tions of Recombivax HB and Pediatric Engerix-B). A shortage was avoided
because other manufacturers were able to provide an adequate supply of the
vaccine in adult and dialysis formulations, and CDC released doses of pe-
diatric vaccine from its stockpile. To prevent future supply problems of the
hepatitis B vaccine, the committee offers the following recommendation:
Recommendation 4-6. The federal government should work to ensure
an adequate, accessible, and sustainable hepatitis B vaccine supply.
Efforts are going on to develop a vaccine for hepatitis C, which could
substantially enhance hepatitis C prevention efforts. The committee recog-
nizes the need for a safe, effective, and affordable hepatitis C vaccine and
offers the following recommendation:
Recommendation 4-7. Studies to develop a vaccine to prevent chronic
hepatitis C virus infection should continue.
Viral Hepatitis Services
Health services related to viral hepatitis prevention, risk-factor screen-
ing and serologic testing,
2
and medical management are both sparse and
fragmented among entities at the federal, state, and local levels. The com-
mittee believes that a coordinated approach is necessary to reduce the
numbers of new HBV and HCV infections, illnesses, and deaths associated
with these infections. Comprehensive viral hepatitis services should have
five core components: outreach and awareness, prevention of new infec-
tions, identification of infected people, social and peer support, and medical
management of infected people.
The committee identified major gaps in viral hepatitis services for
the general population and specific groups that are heavily affected by
HBV and HCV infections: foreign-born populations, illicit-drug users, and
2
Risk-factor screening is the process of determining whether a person is at risk for being
chronically infected or becoming infected with HBV or HCV. Serologic testing is laboratory
testing of blood specimens for biomarker confirmation of HBV or HCV infection.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 13
pregnant women. It also examined venues that provide services to at-risk
groups: correctional facilities, community health facilities, STD and HIV
clinics, shelter-based programs, and mobile health units. The committee
offers recommendations to address major deficiencies for each group and
health-care venue.
General Population
Most people who are chronically infected with HBV or HCV are un-
aware of their infection status. As treatments for chronic hepatitis B and C
improve, it becomes critical to identify chronically infected people. There-
fore, it is important that the general population have access to screening
and testing services so that people who are at risk for viral hepatitis can
be identified. The federal government is the largest purchaser of health
insurance nationally and is well positioned to be the leader in the develop-
ment and enforcement of guidelines to ensure that the people for whom it
provides health care have access to risk-factor screening, serologic testing
for HBV and HCV, and appropriate medical management.
Recommendation 5-1. Federally funded health-insurance programs—
such as Medicare, Medicaid, and the Federal Employees Health Ben-
efits Program—should incorporate guidelines for risk-factor screening
for hepatitis B and hepatitis C as a required core component of pre-
ventive care so that at-risk people receive serologic testing for hepatitis
B virus and hepatitis C virus and chronically infected patients receive
appropriate medical management.
Foreign-Born Populations
Nearly half of US foreign-born people, or 6% of the total US popula-
tion, originate in HBV-endemic countries. Thus, there is a growing urgency
for culturally appropriate programs to provide hepatitis B screening and
related services to this high-risk population. There is a pervasive lack of
knowledge about hepatitis B among Asians and Pacific Islanders, and this
is probably also the case for other foreign-born people in the United States.
The lack of awareness in foreign-born populations from HBV-endemic
countries is compounded by the gaps in knowledge and preventive practice
among health-care and social-service providers, particularly those who
serve a large number of foreign-born, high-risk patients. The committee be-
lieves that the needs of foreign-born people are best met with the approach
outlined in Recommendations 3-1 and 3-2. The community-based approach
as outlined in Recommendation 3-2 would be strengthened by additional
resources to provide screening, testing, and vaccination services.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
14 HEPATITIS AND LIVER CANCER
Recommendation 5-2. The Centers for Disease Control and Prevention,
in conjunction with other federal agencies and state agencies, should
provide resources for the expansion of community-based programs
that provide hepatitis B screening, testing, and vaccination services that
target foreign-born populations.
Illicit-Drug Users
HBV and HCV infection rates in illicit-drug users are high, particularly
in IDUs. HCV is easily transmitted among IDUs, and methods to promote
safe injection can be considered essential for HCV control. However, safe-
injection strategies alone are insufficient to control HCV transmission.
Prevention of HCV infection is a function of multiple factors—safe-injec-
tion strategies, education, testing, and drug treatment—so an integrated
approach that includes all these elements is more likely to be effective in
preventing hepatitis C.
Recommendation 5-3. Federal, state, and local agencies should expand
programs to reduce the risk of hepatitis C virus infection through
injection-drug use by providing comprehensive hepatitis C virus pre-
vention programs. At a minimum, the programs should include access
to sterile needle syringes and drug-preparation equipment because the
shared use of these materials has been shown to lead to transmission
of hepatitis C virus.
Although illicit-drug use is associated with many serious acute and
chronic medical conditions, health-care use among drug users is lower than
among persons who do not use illicit drugs. Health care for both IDUs and
NIDUs is sporadic and typically received in hospital emergency rooms,
corrections facilities, and STD clinics. Given that population’s poor access
to health care and services, it is important to have prevention and care ser-
vices in settings that IDUs and NIDUs are likely to frequent or to develop
programs that will draw them into care.
Recommendation 5-4. Federal and state governments should expand
services to reduce the harm caused by chronic hepatitis B and hepatitis
C. The services should include testing to detect infection, counseling to
reduce alcohol use and secondary transmission, hepatitis B vaccination,
and referral for or provision of medical management.
Studies have shown that the first few years after onset of injection-
drug use constitute a high-risk period in which the rate of HCV infection
can exceed 40%. Preventing the transition from non-injection-drug use
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 15
to injection-drug use will probably avert many HCV infections. The com-
mittee therefore offers the following research recommendation:
Recommendation 5-5. Innovative, effective, multicomponent hepatitis
C virus prevention strategies for injection-drug users and non-injection-
drug users should be developed and evaluated to achieve greater con-
trol of hepatitis C virus transmission. In particular,
• Hepatitis C prevention programs for persons who smoke or sniff
heroin, cocaine, and other drugs should be developed and tested.
• Programs to prevent the transition from noninjection use of illicit
drugs to injection should be developed and implemented.
Pregnant Women
States and large metropolitan areas are eligible to receive federal fund-
ing to support perinatal hepatitis B prevention programs through CDC’s
National Center for Immunization and Respiratory Diseases. Comprehen-
sive programs have been shown to be effective not only in identifying HBV-
infected pregnant women but in providing other case-management services
(for example, testing of household and sexual contacts and referral to
medical care). However, most programs are understaffed and underfunded
and cannot offer adequate case-management services.
Recommendation 5-6. The Centers for Disease Control and Prevention
should provide additional resources and guidance to perinatal hepa-
titis B prevention program coordinators to expand and enhance the
capacity to identify chronically infected pregnant women and provide
case-management services, including referral for appropriate medical
management.
Although an increasing number of effective HBV antiviral suppressive
medications have become available for the management of chronic HBV
infection, very little research has been done on the use of these medications
during the last trimester of pregnancy to eliminate the risk of perinatal
transmission. The committee believes that there is a need to fund research
to guide the effective use of antiviral medications late in pregnancy to
prevent maternofetal HBV transmission, and offers the following research
recommendation:
Recommendation 5-7. The National Institutes of Health should sup-
port a study of the effectiveness and safety of peripartum antiviral
therapy to reduce and possibly eliminate perinatal hepatitis B virus
transmission from women at high risk for perinatal transmission.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
16 HEPATITIS AND LIVER CANCER
Correctional Facilities
Incarcerated populations have higher rates of HBV and HCV infec-
tions than the general population. Screening of all incarcerated people for
risk factors can identify those who need blood tests for infection and, if
appropriate, treatment.
Recommendation 5-8. The Centers for Disease Control and Preven-
tion and the Department of Justice should create an initiative to foster
partnerships between health departments and corrections systems to
ensure the availability of comprehensive viral hepatitis services for
incarcerated people.
Community Health Centers
The Health Resources and Services Administration administers grant
programs across the country to deliver primary care to uninsured and
underinsured people in community health centers, migrant health centers,
homeless programs, and public-housing primary-care programs. In general,
funding of viral hepatitis services at community health centers is inad-
equate. Because community health centers provide primary health care for
many people who are at risk for hepatitis B and hepatitis C, it is important
for them to offer comprehensive viral hepatitis services.
Recommendation 5-9. The Health Resources and Services Adminis-
tration should provide adequate resources to federally funded com-
munity health facilities for provision of comprehensive viral-hepatitis
services.
Other Settings That Target At-Risk Populations
STD and HIV clinics, shelter-based programs, and mobile health units
are settings that serve populations that are at risk for hepatitis B and hepa-
titis C. The populations that use the settings may not have access to care
through traditional health-care venues. Integration of viral hepatitis services
into those settings creates opportunities to identify at-risk clients and to get
them other services that they need.
Recommendation 5-10. The Health Resources and Services Admin-
istration and the Centers for Disease Control and Prevention should
provide resources and guidance to integrate comprehensive viral hepa-
titis services into settings that serve high-risk populations such as STD
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SUMMARY 17
clinics, sites for HIV services and care, homeless shelters, and mobile
health units.
RECOMMENDATION OUTCOMES
The committee believes that implementation of its recommendations
would lead to reductions in new HBV and HCV infections, in medical
complications and deaths that result from these viral infections of the liver,
and in total health costs. Advances in three major categories will be needed:
in knowledge and awareness about chronic viral hepatitis among health-
care and social-service providers, the general public, and policy-makers; in
improvement and better integration of viral hepatitis services, including ex-
panded hepatitis B vaccination coverage; and in improvement of estimates
of the burden of disease for resource-allocation purposes.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
19
1
Introduction
T
he global epidemic of hepatitis B and hepatitis C is a serious
public-health problem. Using mortality data from 2003, Weiss and
McMichael (2004) ranked the public-health importance of various
infectious diseases and other conditions (see Figure 1-1). Those data under-
score that chronic hepatitis B and hepatitis C are among the leading causes
of preventable death worldwide.
Hepatitis B and hepatitis C are contagious liver diseases caused by the
hepatitis B virus (HBV) and the hepatitis C virus (HCV), respectively. HBV
is a 42-nanometer, partially double-stranded DNA virus classified in the
Hepadnaviridae family; there are eight major HBV genotypes. HCV is a
55-nanometer, enveloped, positive-strand RNA virus classified as a separate
genus, Hepacavirus, in the Flaviviridae family; there are at least six major
HCV genotypes.
Hepatitis B and hepatitis C can be either acute or chronic. The acute
form is a short-term illness that occurs within the first 6 months after a per-
son is exposed to HBV or HCV. The diseases can become chronic, although
this does not always happen and, particularly in the case of hepatitis B, the
likelihood of chronicity depends on a person’s age at the time of infection.
Chronic hepatitis B and chronic hepatitis C are serious and can result in
liver cirrhosis and a type of liver cancer, hepatocellular carcinoma (HCC).
The prevention of chronic hepatitis B and chronic hepatitis C prevents the
majority of HCC cases because HBV and HCV are the leading causes of
this type of cancer. Key characteristics of hepatitis B and hepatitis C are
summarized in Table 1-1 and discussed below and in later chapters.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
20 HEPATITIS AND LIVER CANCER
Tobacco
Other causes
Malaria
Non-HIV TB
Road accidents
vCJD
Hospital infection
Suicide
Log
10
global death rate
HIV
Caused by viruses
Dengue
HPV
Ebola
Hanta
West Nile
Flu
Polio
SARS
Measles
HBV + HCV
RSV, Rota
2
1
3
5
6
4
7
Figure 1-1 Hepatitis
R01623
vector editable
FIGURE 1-1 Approximate global preventable death rate from selected infectious
diseases and other causes, 2003.
Abbreviations: HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV,
hepatitis C virus; RSV, respiratory syncytial virus; HPV, human papilloma vi-
rus; SARS, severe acute respiratory syndrome; TB, tuberculosis; vCJD, variant
Creutzfeldt-Jakob disease.
SOURCE: Weiss and McMichael, 2004. Reprinted with permission from Macmillan
Publishers Ltd: Nature Medicine 10(12 Suppl):S70-S76, copyright 2004.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 21
TABLE 1-1 Key Characteristics of Hepatitis B and Hepatitis C
Hepatitis B Hepatitis C
Causative agent Partially double-stranded DNA
virus
Hepadnaviridae family
Enveloped, positive-strand RNA
virus
Hepacavirus genus, Flaviviridae
family
Statistics In the United States, 0.8–1.4
million people are chronically
infected with HBV
In the United States, 2.7–3.9
million people are chronically
infected with HCV
Routes of
transmission
Contact with infectious blood,
semen, and other body fluids,
primarily through:
• Birth to an infected mother
• Sexual contact with an
infected person
• Sharing of contaminated
needles, syringes, or other
injection-drug equipment
Less commonly through:
• Contact with infectious
blood through medical
procedures
Contact with blood of an infected
person, primarily through:
• Sharing of contaminated
needles, syringes, or other
injection-drug equipment
Less commonly through:
• Sexual contact with an infected
person
• Birth to an infected mother
• Contact with infectious blood
through medical procedures
Persons at risk • Persons born in geographic
regions that have HBsAg
prevalence of at least 2%
• Infants born to infected
mothers
• Household contacts of
persons who have chronic
HBV infection
• Sex partners of infected
persons
• Injection-drug users
• Sexually active persons
who are not in long-term,
mutually monogamous
relationships (for example,
more than one sex partner
during previous 6 months)
• Men who have sex with men
• Persons who have ever injected
illegal drugs, including those
who injected only once many
years ago
• Recipients of clotting-factor
concentrates made before 1987
• Recipients of blood transfusions
or solid-organ transplants
before July 1992
• Patients who have ever received
long-term hemodialysis
treatment
• Persons who have known
exposures to HCV, such as
health-care workers after
needlesticks involving HCV-
positive blood and recipients of
blood or organs from donors
who later tested HCV-positive
• All persons who have HIV
infection
continued

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
22 HEPATITIS AND LIVER CANCER
Hepatitis B Hepatitis C
Persons at risk • Health-care and public-
safety workers at risk for
occupational exposure to
blood or blood-contaminated
body fluids
• Residents and staff of
facilities for developmentally
disabled persons
• Persons who have chronic
liver disease
• Hemodialysis patients
• Travelers to countries that
have intermediate or high
prevalence of HBV infection
• Patients who have signs or
symptoms of liver disease (for
example, abnormal liver-enzyme
tests)
• Children born to HCV-positive
mothers (to avoid detecting
maternal antibody, these
children should not be tested
before the age of 18 months)
Potential for
chronic infection
Among newly infected,
unimmunized persons, chronic
infection occurs in:
• >90% of infants
• 25–50% of children aged
1–5 years
• 6–10% of older children and
adults
75–85% of newly infected persons
develop chronic infection
Clinical outcomes • 15–25% of chronically
infected persons will die from
cirrhosis, liver failure, or
hepatocellular carcinoma
• 3,000 deaths each year are
due to hepatitis B-related
liver disease in the United
States
• 60–70% of chronically infected
persons develop chronic liver
disease
• 5–20% develop cirrhosis over a
period of 20–30 years
• 1–5% will die from cirrhosis or
hepatocellular carcinoma
• 12,000 deaths each year are
due to hepatitis C-related liver
disease in the United States
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HBsAg, hepatitis B surface
antigen.
SOURCE: Adapted from CDC, 2009a.
TABLE 1-1 Continued
PREVALENCE AND INCIDENCE OF
HEPATITIS B AND HEPATITIS C WORLDWIDE
Worldwide, about 1 in 12 persons (480–520 million people) are chroni-
cally infected with HBV or HCV (Lavanchy, 2008; WHO, 2009). An
estimated 78% of cases of primary liver cancer (HCC) and 57% of cases
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 23
of liver cirrhosis are caused by chronic HBV or HCV infection (Perz et
al., 2006). Chronic liver disease due to coinfection with HBV or HCV has
become a major cause of death in persons infected with HIV (Sulkowski,
2008), and coinfection presents additional treatment challenges (Kumar
et al., 2008). It is estimated that HBV and HCV infections cause nearly a
million deaths each year (Perz et al., 2006).
Chronic viral hepatitis is a silent killer. Without testing for infection,
many chronically infected persons are not aware that they have been in-
fected until symptoms of advanced liver disease appear. Advanced liver
cancer has a 5-year survival rate of below 5% (American Cancer Society,
2009). Although much progress has been made in reducing the morbidity
and mortality through effective treatment of chronic viral hepatitis, there
is no global program to provide chronically infected persons with access to
affordable treatment.
HBV is 50–100 times more infectious than HIV (WHO, 2009). Acute
HBV infection in adults, although often asymptomatic, can cause severe ill-
ness and is associated with a 0.5–1% risk of death from liver failure (CDC,
2007). Chronic HBV infection, which occurs when the acute infection is not
cleared by the immune system, is associated with a 15–25% risk of prema-
ture death from liver cancer or end-stage liver disease (Beasley and Hwang,
1991; WHO, 2009). The World Health Organization (WHO) estimates that
up to 2 billion people worldwide have been infected with HBV; about 350
million people live with chronic HBV infection, and about 600,000 people
die from HBV-related liver disease or HCC each year (WHO, 2009).
The major transmission routes and prevalence of chronic HBV infec-
tion vary by age and geography. Primary HBV infection acquired at an
early age (through vertical transmission from an infected mother to her
newborn or horizontal transmission during early childhood) is associ-
ated with the highest risk of chronic infection and is common in people
born in or residing in the highly endemic countries of the western Pacific
region, Asia, and sub-Saharan Africa (Shepard et al., 2006). In countries
with a low prevalence of HBV carriers, primary infection usually occurs
during adolescence or young adulthood as a result of unsafe injections and
unprotected sexual activity. An estimated 21 million new HBV infections
each year are due to unsafe injections in health-care settings (Hauri et al.,
2004). Hepatitis B is also a major basis for social injustice in some endemic
countries. For example, myths and misinformation about modes of HBV
transmission have resulted in widespread discrimination against chronically
infected persons in some endemic countries, such as China, the country
with the world’s largest population of chronically infected people, who are
not allowed to work in the food industry, are often required to undergo
routine pre-employment HBV testing, and can be expelled from school or
work because of a positive test (The Economist, 2006).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
24 HEPATITIS AND LIVER CANCER
An estimated 130–170 million people live with chronic HCV infection
worldwide, and an estimated 350,000 die of HCV-related liver disease
each year (Perz et al., 2006). There are about 2.3–4.7 million new HCV
infections each year from nosocomial transmission alone (Lavanchy, 2009).
Unsafe mass immunization has led to exceedingly high HCV prevalence in
some areas, such as Egypt, where 14–20% of the population has HCV anti-
bodies (Frank et al., 2000; Lavanchy, 2008). In most populations in Africa,
North America, South America, Europe, and Southeast Asia, the prevalence
in the general population is less than 3% (Lavanchy, 2008).
HCV is efficiently transmitted via direct percutaneous exposure to in-
fectious blood. Hepatitis C became a global epidemic in the 20th century as
blood transfusions, hemodialysis, and the use of injection needles to admin-
ister licit and illicit drugs increased throughout the world (Drucker et al.,
2001; Pybus et al., 2007). For example, the extremely high prevalence of
HCV in Egypt is due to a schistosomiasis-eradication campaign that began
in the 1960s, when more than 35 million injections were administered to
about 6 million Egyptians (Deuffic-Burban et al., 2006; Frank et al., 2000;
Lehman and Wilson, 2009). The identification of the virus in 1989 led to
measures to reduce health-care–related exposure to HCV, particularly in
industrialized nations. However, more than six billion unsafe injections are
given worldwide each year (Hutin et al., 2003).
With the reduction in health-care–related exposures to HCV and the
recent introduction of the practice of illicit-drug injection in new regions
of the world, HCV infection through injection-drug use has become the
major source of exposure to HCV worldwide. Explosive increases in HCV
infection have occurred in regions of Asia and central and eastern Europe
because of poor access to sterile injection equipment and lack of drug
treatment. A recent meta-analysis reported that HCV prevalence was 84%
in injection-drug users (IDUs) surveyed in the Guangxi region bordering
the Golden Triangle in China (Xia et al., 2008). In that region, drug use
is highly stigmatized, which reduces community support for prevention ef-
forts and inhibits IDUs’ access to prevention services. Antiviral treatments
for chronic HBV and HCV infections can effectively reduce the associated
morbidity and mortality from liver disease. However, access to treatment
is often limited by high costs of care and by the asymptomatic nature of
chronic HBV and HCV infections. Therefore, many infected people are not
identified in time to benefit from antiviral treatment.
Global eradication or elimination of new HBV infections is plausible
because the infections can be prevented with the hepatitis B vaccine. No
vaccine to prevent hepatitis C has been licensed. Given the limitations of
the scope of the committee’s work, it did not assess global prevention and
control efforts for hepatitis B and hepatitis C and did not consider the in-
ternational effects of its recommendations.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 25
PREVALENCE AND INCIDENCE OF HEPATITIS B
AND HEPATITIS C IN THE UNITED STATES
HBV and HCV infections pose a major public-health problem in the
United States and are major causes of chronic liver disease. Three to five
times more people are living with chronic viral hepatitis infections than
with HIV infection. Table 1-2 presents the burden of HBV, HCV, and
HIV infections in the United States. The US Centers for Disease Control
and Prevention (CDC) estimates that 3.5–5.3 million people in the United
States—1–2% of the population—are living with chronic HBV or HCV
infection—about 800,000 to 1.4 million people with chronic hepatitis B
and an additional 2.7–3.9 million people with chronic hepatitis C (CDC,
2009d). However, an accurate estimate is difficult to obtain because there
is no national chronic-hepatitis surveillance program. Each year, about
15,000 deaths are caused by HBV- or HCV-associated liver cancer or end-
stage liver disease (CDC, 2009d). Almost half the liver transplantations in
the United States are necessitated by end-stage liver disease associated with
HBV or HCV infection (Kim et al., 2009).
The annual costs of HBV and HCV infections are difficult to determine.
The direct medical cost associated with HBV infection has been estimated
at $5.8 million based on the number of new cases in 2000 among persons
5–24 years old (Chesson et al., 2004). An estimated $1.8 billion in medical
care costs was associated with HCV infections in 1997 (Leigh et al., 2001).
Indirect costs, such as lost productivity, add to the HCV-associated cost
burden. Because of the aging of people now infected (including some people
with asymptomatic infections who will become symptomatic), HCV-related
illnesses, deaths, and costs are all expected to rise substantially during the
next two decades (Pyenson et al., 2009; Wong et al., 2000).
Hepatitis B
The national strategy for preventing new HBV infection in infants and
children—including routine screening of pregnant women for hepatitis
B surface antigen (a blood marker for chronic HBV infection), universal
infant hepatitis B immunization, and catchup vaccination of unvaccinated
children and adolescents—has resulted in a dramatic reduction in chronic
HBV infection in infants and acute HBV infection in children of all eth-
nicities (CDC, 2004; Mast et al., 2005, 2006). Despite those achievements,
the goal of eliminating perinatal HBV transmission has not been achieved,
largely because coverage of newborns with a birth dose of hepatitis B vac-
cine is incomplete (CDC, 2008c). As a result, CDC estimates that each year
about 1,000 newborns develop chronic HBV infection, which puts them at
risk for premature death from HBV-related liver disease (Ward, 2008b).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
26
TABLE 1-2 Burden of Selected Serious Chronic Viral Infections in the United States
Virus Prevalence
a,b
Percentage of
Population
Unaware
of Infection
Status
c,d,e
Deaths in 2006
Related to
Infection
a,b
Vaccine-
Preventable
Transmission
Routes
Percentage of CDC
NCHHSTP FY 2008
Budget
f
HBV 0.8–1.4 million About 65% 3,000 Yes Birth, blood, sex
2% combined
HCV 2.7–3.9 million About 75% 12,000 No Birth, blood, sex
HIV/AIDS 1.1 million About 21% 14,016 No Birth, blood, sex 69% (domestic
activities)
Abbreviations: CDC NCHHSTP, Centers for Disease Control and Prevention National Center for HIV/AIDS, Viral Hepatitis, Sexually Transmitted
Disease, and Tuberculosis Prevention; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV/AIDS, human immunodeficiency virus/acquired immu-
nodeficiency syndrome.
SOURCES:
a
CDC, 2009b;
b
CDC, 2009d;
c
Lin et al., 2007;
d
Hagan et al., 2006;
e
CDC, 2008b;
f
Ward, 2008a.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 27
Based upon surveillance data and modeling, CDC estimates that there
has been an 82% decline in incidence of acute HBV infection since 1990
with the total number of new infections in 2007 estimated at 43,000
(Daniels et al., 2009). Because many children have been vaccinated against
HBV, most reported cases of acute HBV infection are in adults. The national
strategy for preventing HBV transmission in adults—by recommending
hepatitis B vaccination selectively for high-risk adults (including men who
have sex with men, IDUs, and correctional-facility inmates)—has had only
little success in reducing the incidence of acute HBV infection in US adults
(Mast et al., 2006). Acute HBV infections are often asymptomatic or have
symptoms similar to those of other common illnesses, such as influenza, so
there is a high probability of underreporting.
In the United States, data on reported cases of acute HBV infection in
2007 indicate that the highest rate of infection is in non-Hispanic black
men: 2.3 per 100,000. The incidence is substantially lower in other popu-
lations: 0.9 per 100,000 Asians and Pacific Islanders (APIs) and 1.0 per
100,000 non-Hispanic whites and Hispanics. There also appear to be geo-
graphic variations in incidence; the highest rates of acute HBV infection are
in the South
1
(Daniels et al., 2009).
Although the incidence of acute HBV infection is declining in the United
States, the number of people who are living with chronic HBV infection
may be increasing as a result of immigration from highly endemic countries
(that is, the hepatitis B surface antigen prevalence is ≥ 2%). On the basis
of immigration patterns in the last decade, it is estimated that every year
40,000–45,000 people enter the United States legally from HBV-endemic
countries (Mast et al., 2006; U.S. Department of Homeland Security, 2009).
Some populations are at higher risk for chronic HBV infection, including
API Americans, who make up only 4.5% of the general US population (U.S.
Census Bureau, 2008) but account for more than 50% of Americans who
are living with chronic HBV infection (CDC, 2009c). The prevalence of
chronic HBV infection in API Americans is as high as 15% in some studies
and constitutes an important health disparity (CDC, 2006). Having been
born in an HBV-endemic country appears to be the major risk factor for
chronic HBV infection in the API population (Lin et al., 2007).
Recent studies suggest that routine HBV testing of all adult API Ameri-
cans is cost-effective (Hutton et al., 2007), but almost two-thirds of chroni-
cally infected API Americans are unaware of their infection status because
they have not been tested for HBV (CDC, 2006; Lin et al., 2007).
1
CDC’s southern region includes Alabama, Arkansas, Delaware, the District of Columbia,
Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma,
South Carolina, Tennessee, Texas, Virginia, and West Virginia.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
28 HEPATITIS AND LIVER CANCER
Hepatitis C
Persons likely to have chronic HCV infection include those who re-
ceived a blood transfusion before 1992 and past or current IDUs. US
veterans who use the Department of Veterans Affairs (VA) health-care sys-
tem have a higher prevalence of HCV infection (4–35%) than the general
population (about 2%) (Cheung, 2000; Dominitz et al., 2005; Groom et al.,
2008; Sloan et al., 2004), so VA has established a program to test all VA
patients for HCV infection and to manage HCV-positive patients clinically
(Kussman, 2007). As is the case with HBV infection, most patients who
have acute or chronic HCV infection are asymptomatic, and their disease
remains undiagnosed (Kamal, 2008).
In the United States, most IDUs have serologic evidence of HCV in-
fection, but the prevalence is highly variable. For example, in a study of
young IDUs in four US cities, the prevalence of HCV antibody was 35%
overall but varied from 14% in Chicago and 27% in Los Angeles to 51%
in Baltimore and New York City (Amon et al., 2008). Prevalence is strongly
associated with time engaged in risky behaviors, rising as the number of
years of drug-injecting accumulates and reaching 65–90% in longer-term
injectors (Hagan et al., 2008). HCV prevalence in IDUs in industrialized
nations has fallen in recent years. For example, in IDUs injecting for less
than 1 year, HCV prevalence fell from 46% before 1995 to 32% in a more
recent period and in IDUs injecting for 5 years or more, prevalence fell from
67% before 1995 to 53% in the period after 1995 (Hagan et al., 2008).
Most of the estimates of HCV incidence rates in IDUs in the United States
have been between 15 and 30 per 100 person years at risk, with higher
incidence found in recent-onset injectors (Garfein et al., 1998; Hagan et
al., 2001, 2008; Hahn et al., 2002; Maher et al., 2006; Smyth et al., 2000;
Thorpe et al., 2002).
The prevalence of HCV infection in the incarcerated population has
been reported to vary from 12% to 35% (Boutwell et al., 2005; Weinbaum
et al., 2003). Although some HCV transmission occurs within correctional
settings (Hunt and Saab, 2009; Macalino et al., 2004), the vast majority of
HCV-infected inmates became infected by injection-drug use in the com-
munity and not while incarcerated (Weinbaum et al., 2003).
Although reporting of acute HCV infection does not accurately reflect
the underlying incidence in the United States, the number of acute HCV
infections peaked in the late 1980s and declined throughout the 1990s
(Armstrong et al., 2006; Shepard et al., 2005). The decline observed in
the 1990s may reflect changes in IDUs’ behavior and practices, including
greater participation in needle-exchange programs (Wasley et al., 2008). It
is consistent with results of studies summarized previously that suggest that
HCV seroconversion rates in IDUs have declined since 1995 (Armstrong
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 29
et al., 2006; Shepard et al., 2005). The decline slowed and then leveled off
starting in 2003, and there was a slight increase in reported acute cases in
2006 (Wasley et al., 2008). Interpretation of those trends is complicated,
however, inasmuch as reporting is related to access to health care and di-
agnosis of acute infection; many IDUs, who often have limited access to
health care and no symptoms from infection, are not included in the trend
analysis.
LIVER CANCER AND LIVER DISEASE FROM CHRONIC
HEPATITIS B VIRUS AND HEPATITIS C VIRUS INFECTIONS
Both chronic HBV and HCV infections can lead to HCC, a type of liver
cancer, and liver disease (But et al., 2008; McMahon, 2004, 2008; Tan et
al., 2008). The two most important risk factors for HCC are chronic HBV
and HCV infections. As stated above, an estimated 78% of HCC cases and
57% of liver cirrhosis cases are caused by chronic HBV and HCV infections
(Perz et al., 2006).
In the United States, an estimated 3,000 people die each year from
HCC or chronic liver disease caused by HBV infection (CDC, 2008a).
However, risks of those outcomes vary and are higher in men and in people
who are older, ingest large amounts of alcohol, and are coinfected with HIV
(McMahon, 2004; Pungpapong et al., 2007). Outcomes of HBV infections
occur much more often in those with high blood concentrations of HBV
DNA, in persons over 40 years old, and in persons infected with HBV
genotype C (Chen et al., 2006; Dehesa-Violante and Nuñez-Nateras, 2007;
McMahon, 2004; Pungpapong et al., 2007). There are an especially high
prevalence of chronic HBV infection and a high risk of HCC in the API
American population, who make up the largest pool of chronically infected
persons in the United States and are most commonly infected with HBV
genotype C (Chang et al., 2007). HCC incidence tripled in the United States
from 1975 through 2005, and the highest incidence is in API Americans
who immigrated to the United States (Altekruse et al., 2009). American
Indian and Alaska Native peoples have been found to have the highest rate
of liver-related death of ethnic groups in the United States (Vong and Bell,
2004). The age-specific rate of death in American Indian and Alaska Native
peoples due to chronic liver disease is much higher than that in any other
population and chronic HBV infection and increasing rates of chronic HCV
infection play a large role (Vong and Bell, 2004).
In the United States, about 12,000 people die from complications of
chronic hepatitis C each year (CDC, 2008a). Deaths related to hepatitis
C have increased; the highest number of deaths are in middle-aged men,
non-Hispanic blacks, and American Indians (Wise et al., 2008). As is the
case with chronic hepatitis B, complications occur more often in men and
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
30 HEPATITIS AND LIVER CANCER
in people who are older, have metabolic syndrome secondary to obesity,
ingest large amounts of alcohol, and are coinfected with HIV (Ghany et
al., 2009; Missiha et al., 2008; Pradat et al., 2007). There are also impor-
tant ethnic and racial differences in the burden of chronic hepatitis C. The
prevalence of HCV infection is higher in blacks than in whites (Armstrong
et al., 2006; Thomas et al., 2000). Blacks also are less likely to respond
to interferon-alpha-based treatment for chronic hepatitis C; this seems to
be explained to a large extent by differences in DNA sequences near the
interferon lambda 3 gene (Ge et al., 2009; Jeffers et al., 2004; Muir et al.,
2004; Thomas et al., 2009). Likewise, there appears to be a greater burden
of chronic hepatitis C and reduced response to treatment in Hispanic whites
than in non-Hispanic whites (Armstrong et al., 2006; Bonacini et al., 2001;
Rodriguez-Torres et al., 2009). In both Hispanics and blacks, HCC risk is
increasing, in large part because of chronic hepatitis C (Altekruse et al.,
2009). However, there is less evidence than in the case of HBV infection
that different HCV genotypes or higher blood HCV concentrations increase
the risk of long-term disease outcomes. Health-care use trends from 1994
to 2001 show a 20–30% yearly increase in HCV-related hospitalizations,
length of hospital stays, total hospitalization costs, and hospital deaths
(Grant et al., 2005).
THE COMMITTEE’S TASK
CDC has developed recommendations for the prevention and control of
hepatitis B (Mast et al., 2005, 2006; Weinbaum et al., 2008) and hepatitis C
(CDC, 1998, 2001). The National Institutes of Health (NIH) has developed
consensus documents on the management of hepatitis B (NIH, 2008) and
hepatitis C (NIH, 2002). WHO has published guidelines related to hepatitis
B vaccination of children (WHO, 2001). A number of not-for-profit organi-
zations have also worked to increase awareness of the diseases, educate the
public about prevention, and advocate for those chronically infected with
HBV and HCV. Although government and nongovernment efforts have
led to a decline in the number of cases, chronic hepatitis B and hepatitis C
continue to be serious public-health problems in the United States. For that
reason, CDC in conjunction with the National Viral Hepatitis Roundtable,
a not-for-profit coalition of public, private, and voluntary organizations;
the Department of Health and Human Services Office of Minority Health;
and VA sought guidance from the Institute of Medicine (IOM) in identi-
fying missed opportunities related to the prevention and control of HBV
and HCV infections. IOM was asked to focus on hepatitis B and hepatitis
C because they are common in the United States and can lead to chronic
disease. This report does not address hepatitis A virus, hepatitis E virus, or
hepatitis D virus (also called the hepatitis delta virus) infections.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 31
The specific charge to the committee follows:
The IOM will form a committee to determine ways to reduce new HBV
and HCV infections and the morbidity and mortality related to chronic
viral hepatitis. The committee will assess current prevention and con-
trol activities and identify priorities for research, policy, and action. The
committee will highlight issues that warrant further investigations and
opportunities for collaboration between private and public sectors. In
conducting its work, the committee might want to consider:
Strategies for preventing new HBV and HCV infections:
• Improving vaccine coverage among vulnerable populations to reach
national transmission elimination goals.
• Increasing the proportion of persons aware of their chronic infection
status.
• Identifying barriers to the identification, counseling, and testing of
persons at risk for chronic hepatitis, and ways they can be reduced
and eliminated.
• Promoting prevention among adolescents and adults who engage in
risky behaviors, particularly those known to have screened positive for
HCV and HBV infection.
• Determining optimal ways to identify, develop, and implement preven-
tion programs among at-risk populations.
• Development of an effective HCV vaccine.
Strategies for reducing morbidity and mortality from chronic HBV and
HCV infections:
• Providing appropriate medical referral, evaluation, and management of
chronically infected persons.
• Assessing health-care utilization and outcomes for persons with chronic
infections, and opportunities for prevention and care to reduce health-
care-related costs.
• Reducing health disparities in morbidity and mortality from viral
hepatitis.
• Improving clinical surveillance of markers of disease progression and
stage of hepatocellular carcinoma associated with chronic viral hepatitis
and associated cirrhosis.
Assess the type and quality of data needed from state and local viral hepa-
titis surveillance systems to guide and evaluate prevention services:
• Assess the role of acute disease surveillance in monitoring new infec-
tions, detecting outbreaks, identifying vaccine failures and documenting
the elimination of HBV transmission.
• Assess the role of state and local chronic disease surveillance in describ-
ing the burden of morbidity and mortality related to chronic hepatitis
B and hepatitis C and related liver cirrhosis and cancer, the extent of
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
32 HEPATITIS AND LIVER CANCER
ongoing risk behaviors, and the impact of HIV co-infection and other
cofactors.
• Assess the role of state and local disease registries in the delivery of
prevention and care services for persons with chronic hepatitis B and
persons with hepatitis C.
• Assess the role of laboratory testing strategies for the identification of
markers for acute HCV infection.
• Assess laboratory testing strategies for identification of antiviral resis-
tance for HBV and HCV.
Finally, the committee should pay attention to addressing the special needs
of specific subpopulations at high risk, such as Asian Americans, African
Americans, and persons born in HBV-endemic countries.
THE COMMITTEE’S APPROACH TO ITS TASK
To address its charge, the committee first reviewed available evidence on
a variety of topics related to the prevention of hepatitis B and hepatitis C,
management of these diseases, and surveillance activities related to viral
hepatitis. The evidence was drawn from the published literature and from
open-session presentations by recognized experts in the field (see Appen-
dix B). Oral testimony presented by members of the public during the open
sessions was also taken into account. Additional information was obtained
from written testimony submitted to the committee (available from the
National Academies’ Public Access Records Office, public[email protected]du).
A comprehensive review and evaluation of treatments for HBV and
HCV infections (for example, which medications to use) is beyond the
scope of this report. However, treatment information can be found in guide-
lines published by the American Association for the Study of Liver Diseases
(Ghany et al., 2009; Lok and McMahon, 2009) and in NIH consensus
statements on the management of hepatitis B (NIH, 2008) and hepatitis C
(NIH, 2002).
The committee also has not been tasked with comprehensively review-
ing information about the safety of the hepatitis B vaccine. Safety issues
surrounding this vaccine were reviewed in the IOM report Immunization
Safety Review: Hepatitis B Vaccine and Demyelinating Neurological Dis-
orders (IOM, 2002). The committee that wrote that report concluded that
the evidence favored rejection of a causal relationship between hepatitis B
vaccine administered to adults and incident multiple sclerosis and multiple-
sclerosis relapse. It also found the evidence inadequate for accepting or
rejecting a causal relationship between hepatitis B vaccine and the first
episode of a central nervous system demyelinating disorder, acute dissemi-
nated encephalomyelitis, optic neuritis, transverse myelitis, Guillain-Barré
syndrome, or brachial neuritis. IOM has undertaken another review of the
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 33
safety of the hepatitis B vaccine, and the findings are expected to be avail-
able in 2011.
The committee that wrote the present report met five times in the period
December 2008–August 2009. During the meetings, the committee evalu-
ated the evidence and deliberated on issues relevant to its charge. Types of
evidence taken into consideration included international, federal, state, and
community guidelines, programs, and other activities aimed at preventing
new cases of HBV and HCV infection, identifying chronic cases of hepa-
titis B and hepatitis C, and managing those cases. It also explored federal
and state surveillance mechanisms for identifying and tracking hepatitis B
and hepatitis C cases. The committee began by identifying problems with
and gaps in the current prevention and control systems. It also examined
model programs for other infectious diseases, such as those covered under
the Ryan White CARE Act (Health Resources and Services Administration,
2009). The committee developed evidence-based recommendations to ad-
dress the problems with the current systems to reduce the numbers of new
HBV and HCV infections, to manage the care of chronically infected people
more effectively by reducing morbidity and mortality, and to improve sur-
veillance of chronic hepatitis B and hepatitis C cases.
The committee focused on making recommendations that could be
implemented with existing knowledge and available tools to advance pre-
vention and control of chronic viral hepatitis in a timely manner. Although
the committee recognizes the importance of basic research in this field,
it believes that given the scope of the problem and the lack of available
resources, its focus should be on improving prevention and control ser-
vices. As a result, the committee did not address basic-research questions
in the field extensively. Nor did it conduct cost–benefit analyses of its
recommendations.
The committee’s general approach is presented in Figure 1-2. After
defining the scope of the problem and reviewing the available evidence,
the committee identified the primary underlying factors that impede cur-
rent efforts to prevent and control hepatitis B and hepatitis C. The com-
mittee believes that a lack of awareness about viral hepatitis among both
the general public and health-care and social-service providers is leading
to continued high rates of morbidity and mortality from hepatitis B and
hepatitis C. Consistent themes were found in all the materials reviewed
by the committee; as a result, this report is organized according to four
principal categories:
• Improved disease surveillance (Chapter 2).
• Improved knowledge and awareness on the part of health-care and
social-service providers and the public (Chapter 3).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
34
FIGURE 1-2 The committee’s approach to its task.
The Problem
• 0.8–1.4 million people are chronically infected with hepatitis B virus (HBV) in United States
— 3,000 deaths each year are due to hepatitis B-related liver disease
• 2.7–3.9 million people are chronically infected with hepatitis C virus (HCV) in United States
— 12,000 deaths each year are due to hepatitis C-related liver disease
• Over 150,000 deaths due to hepatitis B and hepatitis C are projected to occur in next 10 years
Underlying issues
Lack of Public Awareness Lack of Provider Awareness
Lack of Public Resource Allocation
Consequences
• At-risk people do not know that they are at risk or how to prevent becoming infected
• At-risk people may not have access to preventive services
• Chronically infected people do not know that they are infected
• Many medical providers do not screen people or know how to manage those infected
• Infected people often have inadequate access to testing and medical management
• Inadequate disease-surveillance systems underreport both acute and chronic infections
Recommendations
Improved Disease
Surveillance
Improved Provider and
Community Education
Integration and Enhancement
of Viral Hepatitis Services
Outcomes
• Screening is widely used as a part of good primary care
• At-risk people and communities actively seek testing, preventive services, and appropriate medical management
• Better information leads to
— Improved understanding of hepatitis B and hepatitis C
— More effective and targeted prevention programs
— More research on effective vaccination and treatment options
• Infected people have better health outcomes
• Decreased transmission leads to fewer carriers of HBV and HCV and fewer cases of hepatitis B and hepatitis C
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 35
• Increased hepatitis B vaccination rates in various populations
(Chapter 4).
• Improved integration and enhancement of viral hepatitis services
for specific at-risk populations and in health-care and other settings
(Chapter 5).
REFERENCES
Altekruse, S. F., K. A. McGlynn, and M. E. Reichman. 2009. Hepatocellular carcinoma inci-2009. Hepatocellular carcinoma inci-
dence, mortality, and survival trends in the United States from 1975 to 2005. Journal of
Clinical Oncology 27(9):1485-1491.
American Cancer Society. 2009. Cancer facts & figures, 2009. Atlanta, GA.
Amon, J. J., R. S. Garfein, L. Ahdieh-Grant, G. L. Armstrong, L. J. Ouellet, M. H. Latka,
D. Vlahov, S. A. Strathdee, S. M. Hudson, P. Kerndt, D. Des Jarlais, and I. T. Williams.
2008. Prevalence of hepatitis C virus infection among injection drug users in the United
States, 1994-2004. Clinical Infectious Diseases 46(12):1852-1858.
Armstrong, G. L., A. Wasley, E. P. Simard, G. M. McQuillan, W. L. Kuhnert, and M. J. Alter.
2006. The prevalence of hepatitis C virus infection in the United States, 1999 through
2002. Annals of Internal Medicine 144(10):705-714.
Beasley, R., and L. Hwang. 1991. Overview of the epidemiology of heptocellular carcinoma.
In Viral hepatitis and liver disease. Proceedings of the 1990 international symposium on
viral hepatitis and liver disease, edited by F. B. Hollinger, S. Lemon, and H. S. Margolis.
Baltimore: Williams & Wilkins. Pp. 532-525.
Bonacini, M., M. D. Groshen, M. C. Yu, S. Govindarajan, and K. L. Lindsay. 2001. Chronic
hepatitis C in ethnic minority patients evaluated in Los Angeles county. American Journal
of Gastroenterology 96(8):2438-2441.
Boutwell, A. E., S. A. Allen, and J. D. Rich. 2005. Opportunities to address the hepatitis C
epidemic in the correctional setting. Clinical Infectious Diseases 40(s5):S367-S372.
But, D. Y., C. L. Lai, and M. F. Yuen. 2008. Natural history of hepatitis-related hepatocellular
carcinoma. World Journal of Gastroenterology 14(11):1652-1656.
CDC (Centers for Disease Control and Prevention). 1998. Recommendations for prevention
and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Mor-
bidity and Morality Weekly: Recommendations and Reports 47(RR-19):1-39.
———. 2001. Updated U.S. Public Health Service guidelines for the management of oc-
cupational exposures to HBV, HCV, and HIV and recommendations for postexpo-
sure prophylaxis. Morbidity and Morality Weekly: Recommendations and Reports
50(RR-11):1-52.
———. 2004. Incidence of acute hepatitis B—United States, 1990-2002. Morbidity and Mor-
tality Weekly Report 52(51-52):1252-1254.
———. 2006. Screening for chronic hepatitis B among Asian/Pacific Islander populations—
New York City, 2005. Morbidity and Mortality Weekly Report 55(18):505-509.
———. 2007. FAQs for health professionals: Hepatitis B. http://www.cdc.gov/hepatitis/HBV/
HBVfaq.htm#overview (accessed August 21, 2009).
———. 2008a. Disease burden from hepatitis A, B, and C in the United States. http://www.
cdc.gov/hepatitis/statistics.htm (accessed August 21, 2209)
———. 2008b. HIV prevalence estimates—United States, 2006. Morbidity and Mortality
Weekly Report 57(39):1073-1076.
———. 2008c. Newborn hepatitis B vaccination coverage among children born January 2003-
June 2005—United States. Morbidity and Mortality Weekly Report 57(30):825-828.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
36 HEPATITIS AND LIVER CANCER
———. 2009a. The ABCs of hepatitis. http://www.cdc.gov/hepatitis/Resources/Professionals/
PDFs/ABCTable.pdf (accessed November 6, 2009).
———. 2009b. HIV/AIDS statistics and surveillance. http://www.cdc.gov/hiv/topics/
surveillance/basic.htm (accessed August 24, 2009).
———. 2009c. Notice to readers: National hepatitis B initiative for Asian Americans/
Native Hawaiian and other Pacific Islanders. Morbidity and Mortality Weekly Report
58(18):503.
———. 2009d. Viral hepatitis: Statistics and surveillance. http://www.cdc.gov/hepatitis/
Statistics.htm (accessed August 24, 2009).
Chang, E. T., T. H. M. Keegan, S. L. Gomez, G. M. Le, C. A. Clarke, S. K. So, and S. L.
Glaser. 2007. The burden of liver cancer in Asians and Pacific Islanders in the greater San
Francisco Bay area, 1990 through 2004. Cancer 109(10):2100-2108.
Chen, G., W. Lin, F. Shen, U. H. Iloeje, W. T. London, and A. A. Evans. 2006. Past HBV viral
load as predictor of mortality and morbidity from HCC and chronic liver disease in a
prospective study. American Journal of Gastroenterology 101(8):1797-1803.
Chesson, H. W., J. M. Blandford, T. L. Gift, G. Tao, and K. L. Irwin. 2004. The estimated
direct medical cost of sexually transmitted diseases among American youth, 2000. Per-
spectives on Sexual and Reproductive Health 36(1):11-19.
Cheung, R. C. 2000. Epidemiology of hepatitis C virus infection in American veterans. Ameri-
can Journal of Gastroenterology 95(3):740-747.
Daniels, D., S. Grytdal, and A. Wasley. 2009. Surveillance for acute viral hepatitis—United
States, 2007. Morbidity and Mortality Weekly Report: Surveillance Summaries
58(3):1-27.
Dehesa-Violante, M., and R. Nuñez-Nateras. 2007. Epidemiology of hepatitis virus B and C.
Archives of Medical Research 38(6):606-611.
Deuffic-Burban, S., M. K. Mohamed, B. Larouze, F. Carrat, and A. J. Valleron. 2006. Expected
increase in hepatitis C-related mortality in Egypt due to pre-2000 infections. Journal of
Hepatology 44(3):455-461.
Dominitz, J. A., E. J. Boyko, T. D. Koepsell, P. J. Heagerty, C. Maynard, J. L. Sporleder,
A. Stenhouse, M. A. Kling, W. Hrushesky, C. Zeilman, S. Sontag, N. Shah, F. Ona, B.
Anand, M. Subik, T. F. Imperiale, S. Nakhle, S. B. Ho, E. J. Bini, B. Lockhart, J. Ahmad,
A. Sasaki, B. van der Linden, D. Toro, J. Martinez-Souss, V. Huilgol, S. Eisen, and
K. A. Young. 2005. Elevated prevalence of hepatitis C infection in users of United States
Veterans Medical Centers. Hepatology 41(1):88-96.
Drucker, E., P. G. Alcabes, and P. A. Marx. 2001. The injection century: Massive unsterileThe injection century: Massive unsterile
injections and the emergence of human pathogens. Lancet 358(9297):1989-1992.
The Economist. 2006. Asia: B is for bigotry; hepatitis B in China. November 18, 2006,
p. 66.
Frank, C., M. K. Mohamed, G. T. Strickland, D. Lavanchy, R. R. Arthur, L. S. Magder, T.
El Khoby, Y. Abdel-Wahab, E. S. Aly Ohn, W. Anwar, and I. Sallam. 2000. The role of
parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet
355(9207):887-891.
Garfein, R. S., M. C. Doherty, E. R. Monterroso, D. L. Thomas, K. E. Nelson, and D. Vlahov.
1998. Prevalence and incidence of hepatitis C virus infection among young adult injection
drug users. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirol-
ogy 18 Suppl 1:S11-19.
Ge, D., J. Fellay, A. J. Thompson, J. S. Simon, K. V. Shianna, T. J. Urban, E. L. Heinzen, P.
Qiu, A. H. Bertelsen, A. J. Muir, M. Sulkowski, J. G. McHutchison, and D. B. Goldstein.
2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance.
Nature 461(7262):399-401.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 37
Ghany, M. G., D. B. Strader, D. L. Thomas, and L. Seeff. 2009. Diagnosis, management, and
treatment of hepatitis C: An update. Hepatology 49(4):1335-1374.
Grant, W. C., R. R. Jhaveri, J. G. McHutchison, K. A. Schulman, and T. L. Kauf. 2005. Trends
in health care resource use for hepatitis C virus infection in the United States. Hepatol-
ogy 42(6):1406-1413.
Groom, H., E. Dieperink, D. B. Nelson, J. Garrard, J. R. Johnson, S. L. Ewing, H. Stockley,
J. Durfee, Y. Jonk, M. L. Willenbring, and S. B. Ho. 2008. Outcomes of a hepatitis C
screening program at a large urban VA medical center. Journal of Clinical Gastroenterol-
ogy 42(1):97-106.
Hagan, H., J. Campbell, H. Thiede, S. Strathdee, L. Ouellet, F. Kapadia, S. Hudson, and R. S.
Garfein. 2006. Self-reported hepatitis C virus antibody status and risk behavior in young
injectors. Public Health Reports 121(6):710-719.
Hagan, H., E. R. Pouget, D. C. Des Jarlais, and C. Lelutiu-Weinberger. 2008. Meta-regression
of hepatitis C virus infection in relation to time since onset of illicit drug injection: The
influence of time and place. American Journal of Epidemiology 168(10):1099-1109.
Hagan, H., H. Thiede, N. S. Weiss, S. G. Hopkins, J. S. Duchin, and E. R. Alexander. 2001.
Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal
of Public Health 91(1):42-46.
Hahn, J. A., K. Page-Shafer, P. J. Lum, P. Bourgois, E. Stein, J. L. Evans, M. P. Busch,
L. H. Tobler, B. Phelps, and A. R. Moss. 2002. Hepatitis C virus seroconversion among
young injection drug users: Relationships and risks. Journal of Infectious Diseases
186(11):1558-1564.
Hauri, A. M., G. L. Armstrong, and Y. J. Hutin. 2004. The global burden of disease attribut-
able to contaminated injections given in health care settings. International Journal of
STD and AIDS 15(1):7-16.
Health Resources and Services Administration. 2009. The Ryan White HIV/AIDS program.
http://hab.hrsa.gov/about/ (accessed August 21, 2009).
Hunt, D. R., and S. Saab. 2009. Viral hepatitis in incarcerated adults: A medical and public
health concern. American Journal of Gastroenterology 104(4):1024-1031.
Hutin, Y. J. F., A. M. Hauri, and G. L. Armstrong. 2003. Use of injections in health-
care settings worldwide, 2000: Literature review and regional estimates. BMJ
327(7423):1075-1080.
Hutton, D. W., D. Tan, S. K. So, and M. L. Brandeau. 2007. Cost-effectiveness of screening
and vaccinating Asian and Pacific Islander adults for hepatitis B. Annals of Internal
Medicine 147(7):460-469.
IOM (Institute of Medicine). 2002. Immunization safety review: Hepatitis B vaccine and
demyelinating neurological disorders. Edited by K. Stratton, D. Almario, and M. C.
McCormick. Washington, DC: The National Academies Press.
Jeffers, L. J., W. Cassidy, C. D. Howell, S. Hu, and K. R. Reddy. 2004. Peginterferon alfa-2a
(40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepa-
tology 39(6):1702-1708.
Kamal, S. M. 2008. Acute hepatitis C: A systematic review. American Journal of Gastroenter-
ology 103(5):1283-1297; quiz 1298.
Kim, W. R., N. A. Terrault, R. A. Pedersen, T. M. Therneau, E. Edwards, A. A. Hindman,
and C. L. Brosgart. 2009. Trends in waitlist registration for liver transplantation for viral
hepatitis in the US. Gastroenterology 137(5):1608-1686.
Kumar, R., V. Singla, and S. Kacharya. 2008. Impact and management of hepatitis B and hepa-
titis C virus co-infection in HIV patients. Tropical Gastroenterology 29(3):136-147.
Kussman, M. J. 2007. National hepatitis C program. VHA Directive 2007-022. Department
of Veterans Affairs.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
38 HEPATITIS AND LIVER CANCER
Lavanchy, D. 2008. Chronic viral hepatitis as a public health issue in the world. Best Practice
and Research. Clinical Gastroenterology 22(6):991-1008.
———. 2009. The global burden of hepatitis C. Liver International 29(s1):74-81.
Lehman, E. M., and M. L. Wilson. 2009. Epidemic hepatitis C virus infection in Egypt: Es-
timates of past incidence and future morbidity and mortality. Journal of Viral Hepatitis
16(9):650-658.
Leigh, J. P., C. L. Bowlus, B. N. Leistikow, and M. Schenker. 2001. Costs of hepatitis C. Ar-
chives of Internal Medicine 161(18):2231-2237.
Lin, S. Y., E. T. Chang, and S. K. So. 2007. Why we should routinely screen Asian Ameri-
can adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology
46:1034-1040.
Lok, A. S., and B. J. McMahon. 2009. Chronic hepatitis B: Update 2009. Hepatology
50(3):661-662.
Macalino, G. E., J. C. Hou, M. S. Kumar, L. E. Taylor, I. G. Sumantera, and J. D. Rich. 2004.
Hepatitis C infection and incarcerated populations. International Journal of Drug Policy
15(2):103-114.
Maher, L., B. Jalaludin, K. G. Chant, R. Jayasuriya, T. Sladden, J. M. Kaldor, and P. L. Sargent.
2006. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in
Australia. Addiction 101(10):1499-1508.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer,
B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate
transmission of hepatitis B virus infection in the United States: Recommendations of the
advisory committee on immunization practices (ACIP) part 1: Immunization of infants,
children, and adolescents. Morbidity and Morality Weekly: Recommendations and Re-
ports 54(RR-16):1-31.
Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald,
J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immuniza-
tion strategy to eliminate transmission of hepatitis B virus infection in the United States:
Recommendations of the advisory committee on immunization practices (ACIP) part II:
Immunization of adults. Morbidity and Morality Weekly: Recommendations and Reports
55(RR-16):1-33; quiz CE31-34.
McMahon, B. J. 2004. The natural history of chronic hepatitis B virus infection. Seminars in
Liver Disease 24(Suppl 1):17-21.
———. 2008. Natural history of chronic hepatitis B—clinical implications. Medscape Journal
of Medicine 10(4):91.
Missiha, S. B., M. Ostrowski, and E. J. Heathcote. 2008. Disease progression in chronic hepa-
titis C: Modifiable and nonmodifiable factors. Gastroenterology 134(6):1699-1714.
Muir, A. J., J. D. Bornstein, and P. G. Killenberg. 2004. Peginterferon alfa-2b and ribavirin for2004. Peginterferon alfa-2b and ribavirin for
the treatment of chronic hepatitis C in blacks and non-Hispanic whites. New England
Journal of Medicine 350(22):2265-2271.
NIH (National Institutes of Health). 2002. NIH consensus statement on management of hepa-
titis C: 2002. NIH Consensus and State-of-the-Science Statements 19(3):1-46.
———. 2008. NIH consensus development conference statement on the management of Hepa-
titis B. http://consensus.nih.gov/2008/hebB%20draft%20statement%20102208_FINAL.
pdf (accessed August 21, 2009)
Perz, J. F., G. L. Armstrong, L. A. Farrington, Y. J. Hutin, and B. P. Bell. 2006. The contribu-
tions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver
cancer worldwide. Journal of Hepatology 45(4):529-538.
Pradat, P., N. Voirin, H. L. Tillmann, M. Chevallier, and C. Trepo. 2007. Progression to cirrhosis
in hepatitis C patients: An age-dependent process. Liver International 27(3):335-339.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INTRODUCTION 39
Pungpapong, S., W. R. Kim, and J. J. Poterucha. 2007. Natural history of hepatitis B virus
infection: An update for clinicians. Mayo Clinic Proceedings 82(8):967-975.
Pybus, O. G., P. V. Markov, A. Wu, and A. J. Tatem. 2007. Investigating the endemic transmis-
sion of the hepatitis C virus. International Journal for Parasitology 37(8-9):839-849.
Pyenson, B., K. Fitch, and K. Iwasaki. 2009. Consequences of hepatitis C virus (HCV).
Costs of a baby boomer epidemic of liver disease. New York: Vertex Pharmaceuticals
Incorporated.
Rodriguez-Torres, M., L. J. Jeffers, M. Y. Sheikh, L. Rossaro, V. Ankoma-Sey, F. M. Hamzeh,
and P. Martin. 2009. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites
with hepatitis C. New England Journal of Medicine 360(3):257-267.
Shepard, C. W., L. Finelli, and M. J. Alter. 2005. Global epidemiology of hepatitis C virus
infection. The Lancet Infectious Diseases 5(9):558-567.
Shepard, C. W., E. P. Simard, L. Finelli, A. E. Fiore, and B. P. Bell. 2006. Hepatitis B virus
infection: Epidemiology and vaccination. Epidemiologic Reviews 28:112-125.
Sloan, K. L., K. A. Straits-Troster, J. A. Dominitz, and D. R. Kivlahan. 2004. Hepatitis C tested
prevalence and comorbidities among veterans in the US Northwest. Journal of Clinical
Gastroenterology 38(3):279-284.
Smyth, B. P., E. Keenan, and J. J. O’Connor. 2000. Assessment of hepatitis C infection in
injecting drug users attending an addiction treatment clinic. Irish Journal of Medical
Science 169(2):129-132.
Sulkowski, M. S. 2008. Viral hepatitis and HIV coinfection. Journal of Hepatology
48(2):353-367.
Tan, A., S. H. Yeh, C. J. Liu, C. Cheung, and P. J. Chen. 2008. Viral hepatocarcinogenesis:
From infection to cancer. Liver International 28(2):175-188.
Thomas, D. L., J. Astemborski, R. M. Rai, F. A. Anania, M. Schaeffer, N. Galai, K. Nolt,
K. E. Nelson, S. A. Strathdee, L. Johnson, O. Laeyendecker, J. Boitnott, L. E. Wilson,
and D. Vlahov. 2000. The natural history of hepatitis C virus infection: Host, viral, and
environmental factors. Journal of the American Medical Association 284(4):450-456.
Thomas, D. L., C. L. Thio, M. P. Martin, Y. Qi, D. Ge, C. O’Huigin, J. Kidd, K. Kidd, S. I.
Khakoo, G. Alexander, J. J. Goedert, G. D. Kirk, S. M. Donfield, H. R. Rosen, L. H.
Tobler, M. P. Busch, J. G. McHutchison, D. B. Goldstein, and M. Carrington. 2009.
Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature
461(7265):798-801.
Thorpe, L. E., L. J. Ouellet, R. Hershow, S. L. Bailey, I. T. Williams, J. Williamson, E. R.
Monterroso, and R. S. Garfein. 2002. Risk of hepatitis C virus infection among young
adult injection drug users who share injection equipment. American Journal of Epide-
miology 155(7):645-653.
U.S. Census Bureau. 2008. 2007 American community survey. http://factfinder.census.gov/
servlet/STTable?_bm=y&-qr_name=ACS_2008_3YR_G00_S0502&-geo_id=01000US&-
ds_name=ACS_2008_3YR_G00_&-_lang=en&-format=&-CONTEXT=st (accessed Au-
gust 20, 2009).
U.S. Department of Homeland Security. 2009. Yearbook of immigration statistics: 2008. Table
3: Persons obtaining legal permanent resident status by region and country of birth: Fis-
cal years 1999 to 2008. http://www.dhs.gov/files/statistics/publications/yearbook.shtm
(accessed August 21, 2009).
Vong, S., and B. P. Bell. 2004. Chronic liver disease mortality in the United States, 1990-1998.
Hepatology 39(2):476-483.
Ward, J. 2008a. FY 2008 domestic enacted funds. Presentation to the committee: December
4, 2008.
Ward, J. W. 2008b. Time for renewed commitment to viral hepatitis prevention. American
Journal of Public Health 98(5):779-781.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
40 HEPATITIS AND LIVER CANCER
Wasley, A., S. Grytdal, and K. Gallagher. 2008. Surveillance for acute viral hepatitis—
United States, 2006. Morbidity and Mortality Weekly Report: Surveillance Summaries
57(2):1-24.
Weinbaum, C., R. Lyerla, and H. S. Margolis. 2003. Prevention and control of infections with2003. Prevention and control of infections with
hepatitis viruses in correctional settings. Centers for Disease Control and Prevention.
Morbidity and Morality Weekly: Recommendations and Reports 52(RR-1):1-36; quiz
CE31-CE34.
Weinbaum, C. M., I. Williams, E. E. Mast, S. A. Wang, L. Finelli, A. Wasley, S. M. Neitzel,
and J. W. Ward. 2008. Recommendations for identification and public health manage-
ment of persons with chronic hepatitis B virus infection. Morbidity and Morality Weekly:
Recommendations and Reports 57(RR-8):1-20.
Weiss, R. A., and A. J. McMichael. 2004. Social and environmental risk factors in the emer-
gence of infectious diseases. Nature Medicine 10(12 Suppl):S70-S76.
WHO (World Health Organization). 2001. Introduction of hepatitis B vaccine into childhood
immunization services: Management guidelines, including information for health workers
and parents. Geneva: World Health Organization.
———. 2009. Hepatitis B fact sheet no. 204. http://www.who.int/mediacentre/factsheets/
fs204/en/ (accessed October 20, 2008).
Wise, M., S. Bialek, L. Finelli, B. P. Bell, and F. Sorvillo. 2008. Changing trends in hepatitis
C-related mortality in the United States, 1995-2004. Hepatology 47(4):1128-1135.
Wong, J., G. McQuillan, J. McHutchison, and T. Poynard. 2000. Estimating future hepatitis
C morbidity, mortality, and costs in the United States. American Journal of Public Health
90(10):1562-1569.
Xia, X., J. Luo, J. Bai, and R. Yu. 2008. Epidemiology of hepatitis C virus infection among
injection drug users in China: Systematic review and meta-analysis. Public Health
122(10):990-1003.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
41
2
Surveillance
P
ublic-health surveillance is an essential tool in the prevention and con-
trol of infectious and chronic diseases and the medical management of
people who have the diseases. Surveillance data are used to estimate
the magnitude of a health problem, to describe the natural history of a
disease, to detect epidemics, to document the distribution and spread of a
health event or disease, to evaluate control and prevention measures, and
to aid in public-health planning (Thacker, 2000). Public-health surveillance
requires standardized, systematic, continuing collection and management
of data. In addition, surveillance should encompass timely analysis and
dissemination to allow public-health action (CDC, 2001a; Thacker, 2000).
Through those steps, federal agencies and state and local health depart-
ments are able to inform stakeholders by providing reliable information
that can be used to reduce morbidity and mortality through public policy,
appropriate resource distribution, and programmatic and educational inter-
ventions. The committee has defined (see Box 2-1) the role of surveillance
for hepatitis B virus (HBV) and hepatitis C virus (HCV) that is within the
scope of its study.
This chapter describes how surveillance data are used or could be used
to determine the focus and scope of viral hepatitis prevention and control
efforts. The committee reviewed the weaknesses of the current surveillance
system for hepatitis B and hepatitis C, including the timeliness, accuracy,
and completeness of data collection, analysis, and dissemination. It found
that there were few published sources of information about viral hepatitis
surveillance. To obtain a clearer picture of the activities that were taking
place at state and local levels, the committee gathered information from

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
42 HEPATITIS AND LIVER CANCER
various sources. Its findings are based on its review of the literature and on
information gathered through surveys of and direct contact with profes-
sionals working in this field.
Much of the information gathered through surveys involved state-level
and city-level public-health department staff who were working on programs
funded by the Centers for Disease Control and Prevention (CDC). Forty-
nine states have a cooperative agreement with CDC that funds a coordina-
tor who conducts viral-hepatitis prevention activities, such as health-care
provider and consumer education, integration of viral-hepatitis prevention
services into health-care and public-health settings, and development of
state viral-hepatitis prevention plans. Although the cooperative agreements
do not include funds for viral-hepatitis surveillance, the coordinators are
good sources of information about surveillance activities being conducted
in each jurisdiction. CDC’s Division of Viral Hepatitis (DVH) performed a
brief survey of the CDC-funded hepatitis C coordinators in 2006 to gather
information about viral-hepatitis surveillance activities. At the request of
the committee, CDC again surveyed the coordinators (now called adult
viral-hepatitis prevention coordinators, AVHPCs) in April 2009. As part of
a national assessment of viral-hepatitis surveillance initiatives, the National
BOX 2-1
Role of Disease Surveillance
1. Identify acute hepatitis B virus (HBV) and hepatitis C virus (HCV)
outbreaks and individual acute cases and measure incidence
• Respond to outbreaks by
o Identifying cases
o Mobilizing appropriate resources to provide preventive services
to eliminate or minimize further transmission
• Develop accurate estimates of the burden of acute hepatitis B and
hepatitis C in United States
2. Identify chronic cases of hepatitis B and C and measure prevalence
• Develop accurate estimates of the burden of chronic disease in
United States
• Prevent secondary cases
o Hepatitis B: Education, vaccination, and screening
o Hepatitis C: Education, harm reduction, and screening
3. Link cases to appropriate services, including medical management
4. Evaluate current practices and prevention efforts
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 43
Alliance of State and Territorial AIDS Directors interviewed staff involved
in enhanced viral-hepatitis surveillance projects funded through CDC’s
Emerging Infections Programs early in 2009 (the programs are described
in more detail later in this chapter). Committee members also contacted
several AVHPCs directly in April and May 2009 to discuss their work.
The recommendations for surveillance based on the committee’s find-
ings focus on the development of a model designed to improve the quality
and accuracy of information by developing systems to collect, analyze,
and disseminate data on acute and chronic HBV and HCV infections. The
recommendations call for a two-part system: core surveillance activities,
building the capacity of state and local health departments to conduct
standard disease surveillance on newly diagnosed acute and chronic HBV
and HCV infections, and targeted surveillance to obtain data on specific
populations that are not represented fully in the collection of core surveil-
lance data. Core surveillance means those activities in which all jurisdic-
tions must engage to provide accurate, complete, and timely information to
monitor incidence, prevalence, and trends in disease diagnoses. Data from
other activities, such as targeted surveillance, supplement information from
core surveillance, and are necessary to provide accurate incidence estimates,
given the challenges of conducting hepatitis B and C surveillance, as de-
tailed in this chapter. The recommendations also include guidance regarding
the interpretation and dissemination of surveillance data.
APPLICATIONS OF SURVEILLANCE DATA
Surveillance data are used in a variety of ways by a broad base of
state health-department staff, researchers, clinicians, policy-makers, and
private industry. Federal and state health-department surveillance systems
provide population-based information that can be used to improve the
public’s health. They also offer an opportunity for public-health interven-
tion at the individual level by linking infected people to appropriate care
and support services (Klevens et al., 2009). Overall, surveillance data are
critical in estimating incidence and prevalence of HBV and HCV infections
(CDC, 2008c), and they provide a basis for studying and understanding the
mechanisms of diverse outcomes in the natural history of these infections
(Thacker, 2000).
Public health surveillance generally involves name-based reporting of
cases of specified diseases to state and local health departments. As such,
it requires the gathering of information that some people consider private.
Public health officials and state legislatures have weighed the costs and
benefits of public health surveillance and have required name-based report-
ing of specific diseases with confidentiality safeguards in place to protect
private information (Fairchild et al., 2008). Confidential name-based re-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
44 HEPATITIS AND LIVER CANCER
porting is standard practice for infectious diseases surveillance, including
HIV surveillance (CDC, 2008d). Acute HBV infections are reportable in
all states and acute HCV infections are reportable in all but one state. All
states report the cases to CDC. Chronic HBV infections are reportable in
all but six states and chronic HCV infections are reportable in all but seven
states (CSTE, 2009).
Outbreak Detection and Control
Accurate and timely surveillance data are necessary to identify out-
breaks of acute HBV and HCV infection in the health-care and community
settings. The data can assist in recognizing and addressing breaches in in-
fection control, and they can help to mitigate the size of outbreaks. There
have been several outbreaks of hepatitis B and hepatitis C in health-care
settings in recent years (CDC, 2003b, 2003d, 2005b, 2008a, 2009c; Fabrizi
et al., 2008; Thompson et al., 2009). Research on those outbreaks has
shown that they typically occurred in dialysis units, medical wards, nursing
homes, surgery wards, and outpatient clinics and resulted from breaches in
infection control (Lanini et al., 2009). In a 2009 study, researchers found
evidence of 33 outbreaks in nonhospital health-care settings in the United
States in the last 10 years. Transmission was primarily patient to patient
and was caused by lapses in infection control and aseptic techniques that
allowed contamination of shared medical devices, such as dialysis machines.
The authors stated that successful outbreak control depended on systematic
case identification and investigation, but most health departments did not
have the time, funds, personnel resources, or legal authority to investigate
health-care–associated outbreaks (Thompson et al., 2009).
Hepatitis B and hepatitis C surveillance data can be used to identify or
quantify new trends in the transmission of HBV and HCV. For example,
surveillance data can help epidemiologists to determine whether sexual
transmission of HCV reported among some cohorts of HIV-positive men
who have sex with men (Matthews et al., 2007; van de Laar et al., 2009)
is statistically significant on a population level. Surveillance data have also
been used to identify clusters of newly acquired cases of hepatitis C in ado-
lescents and young adults and to direct appropriate interventions to persons
in the clusters (CDC, 2008f). Those findings can help public-health officials
to target their resources at emerging populations being affected by HBV and
HCV, such as racial and ethnic populations or geographically linked active
injection-drug users (IDUs).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 45
Resource Allocation
Surveillance data are often used to determine how to use resources most
effectively. For example, estimates of disease burden are commonly used
to provide guidance to policy-makers on the level of funding required for
disease-related programs. If surveillance data are not available or understate
the disease burden, legislators and public-health officials will not allocate
sufficient resources to mount an appropriate public-health response.
Information on disease burden is only one factor that guides policy-
makers in allocating public-health resources. Priorities in public funding
are also driven by public awareness and advocacy. Therefore, it is im-
portant to communicate surveillance trends and disease burden clearly to
policy-makers and community advocates. For example, estimates of trends
indicate that mortality from HCV may soon exceed that from HIV (Deuffic-
Burban et al., 2007). However, despite the large number of individuals and
communities affected by hepatitis B and hepatitis C, the resources available
for addressing viral hepatitis are only a small fraction of those available
for addressing HIV. CDC’s National Center for HIV/AIDS, Viral Hepatitis,
Sexually Transmitted Diseases, and Tuberculosis Prevention had a budget
of almost $1 billion for 2008, and only 2% of it was allocated to hepatitis
B and hepatitis C (Ward, 2008). Sixty-nine percent of the budget was al-
located for HIV, 15% for sexually transmitted diseases (STDs), and 14%
for tuberculosis.
Programmatic Design and Evaluation
Public-health organizations use surveillance data to design programs
that target appropriate populations. For example, CDC requires states to
set priorities among populations for HIV prevention according to data
generated by HIV/AIDS surveillance programs and community-services
assessments (CDC, 2003a). Surveillance data can also be used to evaluate
systems for delivery of prevention and care service. A key potential role of
hepatitis surveillance programs is to evaluate the effect of HBV vaccination
programs (Wasley et al., 2007).
Linking Patients to Care
For some diseases, it is desirable to have a surveillance system closely
involved in ensuring the linkage of persons who have new diagnoses to
health-care services, often called case management (Fleming et al., 2006).
For viral-hepatitis surveillance, linking patients who have recent diagnoses
to comprehensive viral-hepatitis programs may be indicated to ensure ac-
cess to appropriate services, including clinical evaluation, regular followup
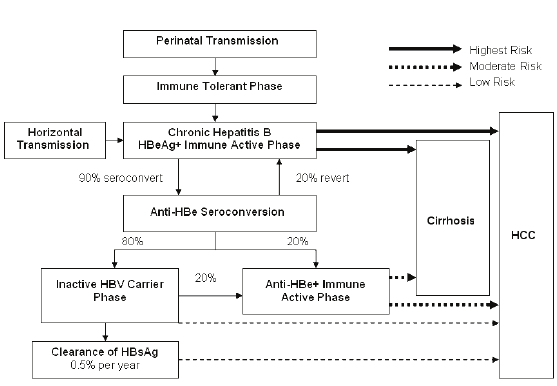
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
46 HEPATITIS AND LIVER CANCER
visits, referral to drug-treatment and harm-reduction programs, education
about liver health, and prevention of transmission to others. Chapter 5 will
discuss the components of viral-hepatitis services.
DISEASE-SPECIFIC ISSUES RELATED TO
VIRAL-HEPATITIS SURVEILLANCE
Many of the difficulties that surveillance systems face in identifying and
tracking cases of hepatitis B and hepatitis C are related to the complexity
of the infections and their associated progression (see Figures 2-1 and 2-2).
This section highlights some of those challenges. Chapter 5 will provide
more detail on issues related to screening and identification.
Figure 2-1, fixed image
a
b
c
d
d
FIGURE 2-1 Natural progression of hepatitis B infection.
Abbreviations: HBeAg, hepatitis B e antigen; anti-HBe, antibody to hepatitis B e
antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepato-
cellular carcinoma.
a
Transmission occurs in 90% of infants of HBsAg+/HBeAg+ mothers and 15%
of infants of HBsAg+/anti-HBe+ mothers.
b
30% of those infected from the age of 1–5 years and under 7% of those infected
at the age of 6 years or older.
c
About 50% of patients by 5 years and 70% of patients by 10 years will sero-
convert to anti-HBe.
d
15-25% risk of premature death from cirrhosis and HCC.
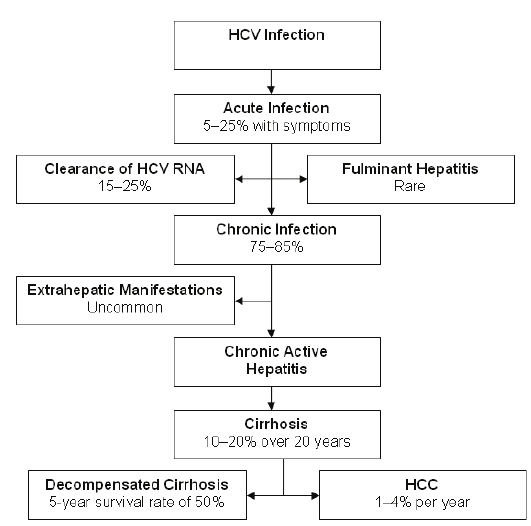
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 47
Identifying Acute Infections
Several factors contribute to the difficulty in identifying acute HBV
and HCV infections. Many newly acquired cases are asymptomatic, or
they may have symptoms similar to those of other common illnesses and
so do not prompt health-care providers to conduct serologic testing for
HBV and HCV, or the serologic tests that are conducted are inadequate
to distinguish between acute and chronic cases. About 90% of acute HBV
infections in children under 5 years of age and 70% of HBV infections in
adults are asymptomatic (McMahon et al., 1985); 75–95% of acute HCV
infections are asymptomatic (Chen and Morgan, 2006; Guerrant et al.,
2001), so few infected patients seek care for the acute illness; and there
is a very high probability of underreporting even when care is obtained
(Chen and Morgan, 2006; Cox et al., 2005; Hagan et al., 2002). Clinicians
Figure 2-2, fixed image
FIGURE 2-2 Natural progression of hepatitis C infection.
Abbreviations: HCV, hepatitis C virus; RNA, ribonucleic acid; HCC, hepatocellular
carcinoma.
SOURCE: Adapted from Chen and Morgan, 2006. Reprinted with permission from
Ivyspring International Publisher, copyright 2006.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
48 HEPATITIS AND LIVER CANCER
often are not fully aware of reporting requirements in connection with
other reportable diseases and do not initiate reports routinely (Allen and
Ferson, 2000). In addition, some persons with chronic HBV infection can
experience sudden increases in alanine aminotransferase (ALT) that may be
associated with jaundice or liver decompensation. That change may have a
variety of causes, including infection with another hepatitis virus; alcohol,
drug, or medication use; or sudden hepatitis B disease reactivation that can
be associated with the period of seroconversion from a hepatitis B e antigen
(HBeAg) state to an antibody to hepatitis B e (anti-HBe) state or reversion
from an anti-HBe state back to an HBeAg-positive state (Koff, 2004).
Therefore, in investigating acute symptomatic infections, it is important
to identify outbreaks so that preventive measures can be undertaken and,
in the case of hepatitis B, to identify and screen close contacts who might
benefit from the hepatitis B vaccine. Such information is needed if surveil-
lance staff is to determine which cases are newly diagnosed, the result of
recent exposure, or chronic (Fleming et al., 2006).
Classifying acute cases of hepatitis B and hepatitis C requires a complex
integration of clinical data, positive and negative laboratory data, and prior
or repeat testing (see Boxes 2-2 and 2-3). Many of the test results—for
BOX 2-2
CDC Acute Hepatitis B Case Definition
Clinical case definition:
An acute illness with
• discrete onset of symptoms
and
• jaundice or elevated serum aminotransferase levels
Laboratory criteria for diagnosis:
• IgM antibody to hepatitis B core antigen (anti-HBc) positive
or
• hepatitis B surface antigen (HBsAg) positive
• IgM anti-HAV negative (if done)
Case classification:
Confirmed: a case that meets the clinical case definition and is labora-
tory confirmed
Abbreviations: CDC, Centers for Disease Control and Prevention; HAV, hepatitis A virus; HBV,
hepatitis B virus.
SOURCE: CDC, 2009a.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 49
BOX 2-3
CDC Acute Hepatitis C Case Definition
Clinical case definition:
An acute illness with a discrete onset of any sign or symptom consistent
with acute viral hepatitis (e.g., anorexia, abdominal discomfort, nausea,
vomiting), and either jaundice or serum alanine aminotransferase (ALT)
levels >400 IU/L.
Laboratory criteria for diagnosis:
One or more of the following three criteria:
1) Antibodies to hepatitis C virus (anti-HCV) screening test positive
with a signal to cut-off ratio predictive of a true positive as determined
for the particular assay as defined by CDC, OR
2) Hepatitis C Virus Recombinant Immunoblot Assay (HCV RIBA)
positive, OR
3) Nucleic Acid Test (NAT) for HCV RNA positive
and, meets the following two criteria:
1) IgM antibody to hepatitis A virus (IgM anti-HAV) negative, AND
2) IgM antibody to hepatitis B core antigen (IgM anti-HBc) negative
Case classification:
Confirmed: A case that meets the clinical case definition, is laboratory
confirmed, and is not known to have chronic hepatitis C.
Abbreviations: CDC, Centers for Disease Control and Prevention; HAV, hepatitis A virus; HCV,
hepatitis C virus; RIBA, recombinant immunoblot assay; RNA, ribonucleic acid.
NOTE: URL for the signal-to-cutoff ratios: http://www.cdc.gov/ncidod/diseases/hepatitis/c/
sc_ratios.htm.
SOURCE: CDC, 2009a.
example, for ALT, aspartate transaminase, immunoglobulin M (IgM) an-
tibody to the hepatitis A virus, and IgM antibody to the hepatitis B core
antigen (HBcAg)—are difficult for health departments to obtain, particu-
larly because negative test results often are not automatically reported to
health departments (Fleming et al., 2006). Because auxiliary test results are
not systematically reported to health departments, surveillance staff must
actively follow up with health-care providers to obtain them and other
clinical indicators of acute disease. If the data cannot be obtained, either
because the proper tests were not ordered or because there is insufficient
staff to conduct followup, cases will be classified ambiguously as nonacute
infections.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
50 HEPATITIS AND LIVER CANCER
Furthermore, current CDC case definitions may miss a substantial frac-
tion of clinically apparent acute cases because they lack clinical markers
that could improve case identification and help to distinguish between acute
and chronic cases. Using data from electronic medical records, Klompas et
al. (2008) found that CDC’s case definition of acute HBV had a positive
predictive value of only 47.2% (that is, out of 1,000 people identified as
having acute hepatitis B with the CDC case definition, only 472 of them
were found to truly have acute hepatitis B). When patients with prior
positive tests for HBV infection (or International Classification of Diseases,
revision 9, codes for chronic HBV infection) were excluded, the positive
predictive value increased to 68.4%. However, the positive predictive value
was raised to above 96% by adding the requirement for peak ALT over
1,000 or total bilirubin over 1.5. Most important, when applying the most
sensitive algorithm (the algorithm that detected the greatest number of
cases of acute hepatitis B), the study found that only four of the eight cases
of acute hepatitis B were in the state’s surveillance system and only one of
the four was correctly classified as acute; this suggests that 88% of acute
hepatitis B cases may be missed if current reporting algorithms are used
(Klompas et al., 2008).
Similarly, detection of acute hepatitis C can be challenging because no
single case definition is either sensitive or specific for it. HCV seroconver-
sion may be missed, and there is no IgM-based assay that reliably distin-
guishes acute hepatitis C from chronic hepatitis C, unlike the situation with
hepatitis A virus or HBV infection. Relatively low HCV ribonucleic acid
(RNA) concentrations and more than one log fluctuation in HCV RNA
concentration are features of acute HCV infection that may be useful for
the development of more dynamic diagnostic algorithms, but the accuracy
of these algorithms has not been validated (Cox et al., 2005; McGovern et
al., 2009; Villano et al., 1999).
In summary, the identification of acute hepatitis infection is inherently
flawed because the vast majority of cases are asymptomatic and patients
do not seek medical care or testing. Such persons would be identified only
in prospective studies that include routine serial testing of liver enzyme
concentrations, such as those previously conducted to identify the incidence
of transfusion-associated hepatitis. Underreporting of diagnosed cases and
misclassification of reported cases seriously limit the accuracy of data on
cases of acute viral hepatitis collected by state and territorial surveillance
programs and transmitted to CDC. Thus, the estimates of the incidence of
acute hepatitis in the United States are based solely on symptomatic cases.
The majority of those cases may be missing from the surveillance system
because of poor access to health care, underreporting, and misclassifica-
tion. Taken together, published surveillance summaries of reported cases of
acute viral hepatitis substantially underestimate the number of cases; these
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 51
summaries may give misleading impressions of the incidence of disease to
policy-makers and program planners.
Identifying Chronic Infections
Given that both hepatitis B and hepatitis C infections are largely
asymptomatic, most people do not receive a diagnosis until the infection is
chronic. For hepatitis B, the chance of developing a chronic infection varies
with age at the time of infection.
In persons over 6 years old, the vast majority of acute HBV infections
are self-limited (Hyams, 1995). However, hepatitis B infections become
chronic in over 90% of infants who are infected at birth or in the first year
of life and in 30% of children who are infected at the age of 1–5 years
(Pungpapong et al., 2007). Although hepatitis B surface antigen (HBsAg)
is detectable within 4–10 weeks after infection, it is indicative of chronic
HBV infection only if it persists for more than 6 months (Koff, 2004). An
accurate diagnosis of chronic hepatitis B may therefore require the report-
ing of multiple serologic markers at more than one time (Koff, 2004).
For disease-surveillance purposes, it can be challenging for health de-
partments to obtain the complete laboratory results that are necessary to
classify a chronic hepatitis B case according to CDC’s case definitions (see
Box 2-4). In general, a full hepatitis B panel (including any negative results
for IgM anti-HBc) is required or two HBsAg results at least 6 months
apart. Although states govern laboratory-reporting requirements in their
jurisdictions, negative test results are generally not reportable and must be
actively obtained. CDC’s Guidelines for Viral Hepatitis Surveillance and
Case Management recommend that only positive HBsAg-test results be
reported, but this test alone is inadequate to distinguish acute from chronic
infection. Automated systems attached to electronic medical records may
help to address surveillance for chronic HBV cases in the future, but in the
meantime many diagnoses of chronic HBV infection probably will not be
correctly captured and classified as confirmed cases (CDC, 2005a).
Surveillance for chronic HCV infection also presents challenges (see
Box 2-5). In adults, about 15–25% of acute hepatitis C infections resolve
spontaneously (Villano et al., 1999). That may increase to about 45% in
children and young adults (Vogt et al., 1999). The presence of HCV RNA
is generally detected within 1 week of infection (Mosley et al., 2005), but
antibodies to HCV (anti-HCV) can be detected in only 50–70% of infected
persons at the onset of symptoms; this increases to more than 90% after 3
months (NIH, 2002). A chronic infection is characterized by the persistent
presence of HCV RNA for at least 6 months (NIH, 2002).
Typically, when a patient presents for HCV testing, the first test that
is conducted is for the presence of anti-HCV. This test is generally an en-

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
52 HEPATITIS AND LIVER CANCER
BOX 2-4
CDC Chronic Hepatitis B Case Definition
Clinical description
Persons with chronic HBV infection may have no evidence of liver dis-
ease or may have a spectrum of disease ranging from chronic hepa-
titis to cirrhosis or liver cancer. Persons with chronic infection may be
asymptomatic.
Laboratory criteria
• IgM antibodies to anti-HBc negative
and
• a positive result on one of the following tests: hepatitis B surface an-
tigen HBsAg, HBeAg, or hepatitis B virus (HBV) DNA,
or
• HBsAg positive or HBV DNA positive or HBeAg positive two times
at least 6 months apart (Any combination of these tests performed 6
months apart is acceptable.)
Case classification
Confirmed: a case that meets either laboratory criteria for diagnosis
Probable: a case with a single HBsAg positive or HBV DNA positive or
HBeAg positive lab result when no IgM anti-HBc results are available
Comment
Multiple laboratory tests indicative of chronic HBV infection may be
performed simultaneously on the same patient specimen as part of a
“hepatitis panel.” Testing performed in this manner may lead to seemingly
discordant results, e.g., HBsAg-negative and HBV DNA-positive. For
the purposes of this case definition, any positive result among the three
laboratory tests mentioned above is acceptable, regardless of other test-
ing results. Negative HBeAg results and HBV DNA levels below positive
cutoff level do not confirm the absence of HBV infection.
Abbreviations: CDC, Centers for Disease Control and Prevention; anti-HBc, hepatitis B core
antigen; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBV, hepatitis B
virus; DNA, deoxyribonucleic acid.
SOURCE: CDC, 2009a.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 53
BOX 2-5
CDC Hepatitis C Virus Infection Case Definition
(Past or Present)
Clinical description:
Most HCV-infected persons are asymptomatic. However, many have
chronic liver disease, which can range from mild to severe including cir-
rhosis and liver cancer.
Laboratory criteria for diagnosis:
• Anti-HCV positive (repeat reactive) by EIA, verified by an additional
more specific assay (e.g., RIBA for anti-HCV or nucleic acid testing
for HCV RNA),
or
• HCV RIBA positive,
or
• Nucleic acid test for HCV RNA positive,
or
• Report of HCV genotype,
or
• Anti-HCV screening-test-positive with a signal to cut-off ratio predic-
tive of a true positive as determined for the particular assay (e.g., ≥3.8
for the enzyme immunoassays) as determined and posted by CDC.
Case classification:
Confirmed: a case that is laboratory confirmed and that does not meet
the case definition for acute hepatitis C.
Probable: a case that is anti-HCV positive (repeat reactive) by EIA and
has ALT or SGPT values above the upper limit of normal, but the anti-
HCV EIA result has not been verified by an additional more specific
assay or the signal to cutoff ratio is unknown.
Abbreviations: CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus; EIA,
enzyme immunoassay; RIBA, recombinant immunoblot assay; RNA, ribonucleic acid; ALT or
SGPT, alanine aminotranferase.
SOURCE: CDC, 2009a.
zyme immunoassay (EIA). A repeatedly reactive EIA is followed by a more
specific assay to detect viremia, such as the recombinant immunoblot assay
(RIBA) for anti-HCV, or by nucleic acid testing for HCV RNA. In some
cases, an EIA with a high signal-to-cutoff ratio predictive of a true positive
will be used in the place of a confirmatory RIBA. However, all confirmed
anti-HCV test results should be followed by a test for the presence of HCV
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
54 HEPATITIS AND LIVER CANCER
RNA (Alter et al., 2003; Ghany et al., 2009). The difficulty in identifying
chronic cases often revolves around the need for two separate tests (or
other supplemental antibody tests) and the 6-month timeframe required for
a diagnosis of chronic HCV infection. Many infected people are tested at
public or nonclinical testing sites—such as drug-treatment facilities, sites for
testing for HIV or STDs, or community-based organizations—that conduct
only the less expensive anti-HCV tests. Persons tested at those sites might
not have access to an HCV RNA test, or the laboratory conducting the
initial EIA test might not routinely test for HCV RNA when an EIA has
been positive. The process leads to incomplete diagnoses and inaccurate
reporting of the number of chronic cases.
The CDC-recommended case definition of nonacute HCV infection
also poses some problems in interpreting the collected data. Although it is
assumed that the majority of nonacute hepatitis C cases represent chronic
infections, this case definition also includes acute cases that do not meet the
confirmed acute case classification (CDC, 2005a). However, a case defined
only by the presence of anti-HCV could be a late acute infection, a chronic
infection, a resolved infection, or a false-positive assay result. It is therefore
essential that anti-HCV testing be supplemented by testing for HCV RNA
and by followup samples to classify cases correctly and to use the data for
program-planning purposes.
Identifying Perinatal Hepatitis B
Since 1992, CDC has awarded funds to 64 grantees to support peri-
natal hepatitis B prevention programs through its Immunization Services
Division’s cooperative agreements with health departments. The funds sup-
port perinatal hepatitis B coordinators, who are charged with identifying all
HBsAg-positive pregnant women, ensuring the administration of appropri-
ate immunoprophylaxis to all infants born to these women, ensuring the
completion of postvaccination serologic testing of the infants, and ensuring
the completion of the hepatitis B vaccine series. Most coordinator programs
also include ensuring vaccination of household contacts and sexual partners
of HBsAg-positive women in their mission (CDC, 2009g).
In an economic analysis of immunization strategies to prevent hepatitis
B transmission in the United States, Margolis et al. (1995) found that pre-
vention of perinatal infection and routine infant vaccination would reduce
the lifetime risk of HBV infection by at least 68%. They estimated that
prevention of perinatal HBV infections would save $41.8 million in medi-
cal and work-loss costs. Routine hepatitis B vaccination of infants would
provide additional savings of $19.7 million. Both strategies were found to
be cost-effective, with estimated costs per year of life saved of $164 for
preventing perinatal HBV infection and of $1,522 for infant vaccination.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 55
In 1992, the Connecticut Department of Public Health found that
hiring a state-level perinatal coordinator led to significantly better compli-
ance with the recommendation to administer hepatitis B immune globulin
(HBIG) than followup by local health departments. They also found that
completion of the three-dose vaccination series was higher in cases followed
by the state coordinator than in those followed by local public-health of-
ficials (CDC, 1996).
Two major problems occur in the identification and management of
possible cases (see Box 2-6). The first involves linking pregnancy status
with positive HBsAg laboratory reports on females of child-bearing age in
a timely manner. In 2005, the Advisory Committee on Immunization Prac-
tices called for improving prevention of perinatal and early childhood HBV
transmission by improving laws and regulations to improve identification
of HBsAg-positive and HBsAg-undetermined mothers (Mast et al., 2005).
BOX 2-6
CDC Perinatal Hepatitis B Virus Infection Case Definition
Clinical description
Perinatal hepatitis B in the newborn may range from asymptomatic to
fulminant hepatitis.
Laboratory criteria
HBsAg positive
Case classification
HBsAg positivity in any infant aged >1–24 months who was born in the
United States or in US territories to an HBsAg-positive mother
Comment: Infants born to HBsAg-positive mothers should receive hepa-
titis B immune globulin (HBIG) and the first dose of hepatitis B vaccine
within 12 hours of birth, followed by the second and third doses of vac-
cine at 1 and 6 months of age, respectively. Postvaccination testing for
HBsAg and anti-HBs (antibody to HBsAg) is recommended from 3 to 6
months following completion of the vaccine series. If HBIG and the initial
dose of vaccine are delayed for >1 month after birth, testing for HBsAg
may determine if the infant is already infected.
Abbreviations: CDC, Centers for Disease Control and Prevention; HBsAg, hepatitis B surface
antigen; anti-HBs, antibody to HBsAg; HBIG, hepatitis B immune globulin.
SOURCE: CDC, 2009a.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
56 HEPATITIS AND LIVER CANCER
Implementation of automated electronic systems can greatly increase the
speed with which cases can be identified (LaPorte et al., 2008), but they
are not available in most states. The second problem involves a lack of
resources to follow up on all potential and known cases and their contacts
in a timely manner. Followup of an infant can take up to 2 years. In addi-
tion, a substantial number of HBsAg-positive mothers are not identified in
time to ensure the required followup of the mothers and their infants (see
Chapter 4).
Other Challenges for Hepatitis B and Hepatitis C Surveillance Systems
Repeat testing in high-risk populations can confuse the number of sus-
pected acute versus chronic infections. Members of some populations, such
as IDUs, may repeatedly incur HCV infection that resolves spontaneously
without ever becoming chronic (Mehta et al., 2002). Those cases could mis-
takenly be classified as chronic infections based on antibody results alone.
Many of the people affected by hepatitis B and hepatitis C have limited
access to health care (for example, active IDUs, homeless people, some
Pacific Islanders, legal immigrants living in poverty, and undocumented
immigrants) and are less likely to be diagnosed appropriately, to provide
complete and accurate demographic and behavioral information, or to
access followup care. Structural and political barriers, stigma, and fear
of legal repercussions contribute to the limitations on their access. Each
HBV-infected or HCV-infected person who does not enter into appropriate
medical care represents a missed opportunity for secondary prevention and
may contribute to the collection of inaccurate and less detailed surveillance
data. Finding ways to ensure that patients receive comprehensive and cul-
turally appropriate care and referrals not only would increase the likelihood
of improving their health outcomes but is likely to affect surveillance-data
collection favorably.
Finally, because of the chronic nature of viral hepatitis, it is important
that surveillance staff communicate well between jurisdictions. Persons
with chronic disease can be misclassified as having acute cases if earlier
diagnoses made in other jurisdictions are not identified. Not infrequently,
a previous diagnosis has been reported in another state or jurisdiction. The
ability of state and local surveillance-program staff to track cases across
jurisdictions is hampered by various factors, including inadequacy of staff
resources, nonstandardized surveillance software systems, and the lack of a
national database that could be used to identify potential matches in other
jurisdictions.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 57
INFRASTRUCTURE AND PROCESS-SPECIFIC
ISSUES WITH SURVEILLANCE
Current public-health surveillance systems for hepatitis B and hepati-
tis C are poorly developed and are inconsistent among jurisdictions. As a
result, surveillance data do not provide accurate estimates of the current
burden of disease, are insufficient for program planning and evaluation,
and do not provide the information that would allow policy-makers to al-
locate sufficient resources to address the problem. The AVHPCs, funded by
CDC in state and territorial health departments, are tasked with identifying
mechanisms for educating the public, at-risk populations, and medical-
service and social-service providers about viral hepatitis; for managing and
coordinating viral-hepatitis prevention activities; and for integrating viral-
hepatitis screening programs and related services into health-care settings
and public-health programs that serve at-risk adults. In most cases, how-
ever, CDC funding covers only the AVHPC’s salary. No funding is provided
for viral-hepatitis testing, hepatitis B immunizations, or other services.
Most important, AVHPCs are not funded to conduct surveillance activities,
although many of them provide technical assistance for such programs.
In addition, CDC’s DVH has scant resources for providing funding and
guidance to local and state health departments to perform surveillance for
viral hepatitis. The resources provided for viral-hepatitis surveillance con-
trast sharply with the resources that CDC provides for HIV surveillance.
For example, CDC has specific cooperative agreements with the states and
territories to conduct core HIV/AIDS surveillance activities. The coopera-
tive agreements are accompanied by dedicated funding, specific CDC proj-
ect officers and epidemiologists, regular technical-assistance meetings and
training, and a help desk that has trained staff to answer database-related
questions. The guidance for HIV/AIDS surveillance is a three-volume set
containing more than 500 pages of detailed instructions, standards, and
guidelines. In contrast, CDC’s cooperative agreements with state and ter-
ritorial health departments for viral hepatitis do not include surveillance
activities. In addition, although CDC’s DVH has produced guidelines for
viral-hepatitis surveillance for state and territorial health departments, they
are presented in fewer than 50 pages (CDC, 2005a). Given that the guide-
lines cover three distinct and complex diseases (hepatitis A, hepatitis B, and
hepatitis C), they lack the detail necessary to create surveillance practices
that are consistent among jurisdictions. As a result of the deficiency of
resources dedicated to hepatitis surveillance, data are incomplete, variable,
and inaccurate. Inconsistency between jurisdictions seriously undermines
the validity of the data provided at the state, regional, and national levels.
The inability of health departments to track all diagnosed cases also se-
riously undermines case-management and prevention efforts. For example,
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
58 HEPATITIS AND LIVER CANCER
studies have shown that vaccinating close contacts of persons chronically
infected with HBV (that is, ring vaccination) is cost-effective (Hutton et al.,
2007). The strategy remains cost-effective even in populations in which the
prevalence of chronic HBV infection is as low as 2%. However, without
funding and staffing for surveillance and identification of new cases of HBV,
ring vaccination is not a public-health activity that is typically supported
by most health departments.
CDC has funded seven enhanced projects through the Viral Hepatitis
Surveillance Emerging Infections Programs (EIPs). The projects began in
2004 and were scheduled to end in 2009. CDC plans to extend the pro-
gram for 2 more years (personal communication, J. Efird, CDC, May 18,
2009). They are in Colorado, Connecticut, Minnesota, New York state,
New York City, Oregon, and San Francisco. Although the projects focus on
surveillance for hepatitis A, hepatitis B, and hepatitis C, they all take differ-
ent approaches, including multiple approaches in individual jurisdictions.
Project funding supports epidemiologic and data-entry staffing. Methods
used in the seven programs include verification of diagnoses with medical
providers, chart review, followup calls to infected persons that focus on
education or data collection, educational mailings (for example, letters
and booklets), followup with persons that are household or close contacts
(especially acute HBV cases), sampling for followup of cases of chronic
HBV and HCV, review of all data, and matching with HIV and STD pro-
grams. There is no uniform evaluation of the projects.
In February and March 2009, staff of the National Alliance of State
and Territorial AIDS Directors (NASTAD) interviewed the coordinators of
the seven EIPs. From those interviews, staff identified additional program-
matic issues that affect reporting. They include resource issues, such as
the varied capacity of county and city health departments (which leads to
inconsistencies in data collection and data systems, in some instances in the
same state); the staffing requirements needed to collect, process, and man-
age data; and the staff and time needed to investigate health-care–related
outbreaks adequately. Other issues are the need to educate medical provid-
ers better on which laboratory tests are needed for appropriate diagnosis
(also noted by Fleming et al., [2006]), and the difficulty that staff face in
obtaining demographic data (including data on race, ethnicity, and country
of origin) and data on risk history (Klevens et al., 2009; NASTAD, 2009).
Funding Sources
Funding for hepatitis surveillance is highly fragmented. No federal
funds are dedicated to chronic-hepatitis surveillance except for the seven
jurisdictions that receive funds from CDC’s DVH to perform enhanced
surveillance activities. State, territorial, or city health-department viral-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 59
hepatitis surveillance units, where they exist, often receive no dedicated fed-
eral funding for this activity. Others may receive funding directly from their
state or city, or they may be integrated into or receive funding from other
programs or units that receive federal funding from CDC programs whose
missions are related to epidemiology or viral hepatitis. The CDC programs
include the Immunization Services Division (related to perinatal hepatitis
B), the Epidemiology and Laboratory Capacity for Infectious Diseases pro-
gram (acute hepatitis B), EIPs, and the DVH. Funding may also be avail-
able through private organizations or foundations. Some surveillance units
receive funding from multiple sources. Each funding source may require
different activities and may provide varied guidance on the receiving unit’s
activities. The recent survey of AVHPCs conducted by NASTAD found that
fewer than one-fourth of the 43 responding jurisdictions reported receiving
funding for surveillance for either chronic HBV or chronic HCV infection
(NASTAD, 2009).
Program Design
Variability among jurisdictions is also due to a wide array of program
structures. In a 2006 survey, 33% of the 52 hepatitis C coordinators funded
by CDC reported being in their jurisdictions’ communicable-diseases or epi-
demiology programs, 25% in HIV–STD programs, 14% in HIV programs,
and the remainder in the immunization or STD programs (CDC, 2006). The
hepatitis C coordinators’ locations within public health departments may
or may not correspond with the health department program responsible
for conducting surveillance, which can lead to reduced involvement and
oversight by the coordinator of viral hepatitis surveillance activities.
In a later survey of the (renamed) AVHPCs by CDC in April 2009,
only 32 states reported having state viral-hepatitis plans. Of the 32, 29
included surveillance as a component. However, fewer than two-thirds of
the program coordinators reported being able to implement the surveillance
components. The reasons listed for not implementing plan components
were lack of staff and lack of funding (CDC, 2009h).
Reporting Systems and Requirements
Reporting of surveillance data to CDC by state and territorial health
departments is voluntary, and in general little federal funding is provided
for HBV and HCV surveillance activities (Klein et al., 2008). Chronic
hepatitis B is reportable in 42 states, but only 38 states conduct surveil-
lance and maintain systems, and only 20 report cases to CDC (George,
2004). Chronic hepatitis C is reportable in 40 states, but only 20 report
cases to CDC (George, 2004). CDC collects data from states that report
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
60 HEPATITIS AND LIVER CANCER
chronic HBV and HCV infections (and received data on 133,520 cases of
HCV infection in 2007 alone), but these data are not presented routinely
in Morbidity and Mortality Weekly Report, which is published by CDC, or
elsewhere (Klevens et al., 2009). Although all states but one perform some
degree of surveillance for acute HBV and HCV (Daniels et al., 2009b),
much of this surveillance is passive at best.
There are significant barriers to implementing more comprehensive
surveillance activities. In the previously mentioned survey conducted by
NASTAD in 2009, it was reported that of the 43 responding jurisdictions,
almost half received between 1,000 and 10,000 HBV laboratory results
annually, and over 70% reported the same range for the number of HCV
laboratory results received annually (NASTAD, 2009). Many states do not
have the staffing or systems to keep up with such a high volume of informa-
tion received and are often unable to follow up with medical providers to
address underreporting or to obtain demographic and risk-history informa-
tion, such as race, ethnicity, and drug-use details (Klein et al., 2008). The
lack of funding to hire adequate staff is the fundamental barrier to complete
and accurate surveillance.
Although CDC provides case-reporting forms for the collection of
viral-hepatitis surveillance data, the forms do not have required elements,
and they ask for data that are often difficult to obtain. Moreover, the use
of the forms is inconsistent among states and local jurisdictions. Of the 43
health departments that responded to inquiries from the present commit-
tee, 26 have developed their own HBV case-reporting form, and 21 have
developed an HCV case-reporting form. The forms were created to capture
behavioral-risk information not included on CDC’s form or to improve
data collection and entry into the separate jurisdictions’ specific software
systems. For example, the CDC case-reporting form does not collect risk-
behavior information specific to chronic HBV. Finally, 8 states do not use a
case-reporting form at all for reporting HBV, and 12 states do not use one
for HCV; these jurisdictions rely solely on the reporting of laboratory-test
results.
Paradoxically, efforts to modernize and enhance public-health surveil-
lance systems have led to greater inconsistency in data collection. In 1984,
CDC began work on the Epidemiologic Surveillance Project. The goal of
the project was to develop computer-based transmission of public-health
surveillance data between states and CDC. By 1989, all 50 states were
participating in the reporting system for certain acute infectious diseases,
and the system was renamed the National Electronic Telecommunications
System for Surveillance (NETSS) (CDC, 2009f). Data were transmitted
weekly to CDC in a standard record format. However, the system quickly
became dated with advances in information and surveillance technology,
such as electronic laboratory reporting and electronic medical records.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 61
Moreover, other surveillance systems and software were used for reporting
of HIV/AIDS, STDs, tuberculosis, and some vaccine-preventable diseases.
In 1999, CDC developed the concept of the National Electronic Disease
Surveillance System (NEDSS), which was designed to promote the develop-
ment of interoperable surveillance systems (that is, the Public Health Infor-
mation Network, or PHIN) at federal, state, and local levels (CDC, 2009e).
The NEDSS initiative describes information-system standards to which all
systems must adhere but does not require use of CDC-produced software.
CDC provides the NEDSS Base System, a software system that may be used,
but only 16 jurisdictions have opted to use it. Most jurisdictions use PHIN-
compliant systems, which are either purchased from a commercial vendor
or developed specifically for a particular jurisdiction. A few jurisdictions
continue to use the NETSS system while their PHIN-compliant systems are
being developed (personal communication, J. Efird, CDC, April 1, 2009).
The result is that CDC no longer provides a standardized database for in-
putting and reporting data on viral hepatitis. Consequently, there is a wide
array of state systems with an even wider array of capabilities. The lack of
standardization makes it difficult for states to share information efficiently.
In addition, creating and modifying their systems can lead to substantial
expenses for states and jurisdictions (CDC, 2009i).
Even states and jurisdictions that have PHIN-compliant systems in
place may not have the staff to enter the high volume of viral-hepatitis data
received. Four of the 43 states that responded to the recent questionnaire
for this committee reported not having any staff to enter data. They do
not include states that may not be able to enter all received data fully. In
the 2009 NASTAD survey of AVHPCs, it was reported that 27 of the 43
reporting jurisdictions had backlogs of HCV data, with an average of 6,200
cases that needed to have data entered (NASTAD, 2009).
Capturing Data on At-Risk Populations
As discussed previously, current surveillance systems do not adequately
capture cases of acute and chronic HBV and HCV infections. That is par-
ticularly true for members of marginalized populations. IDUs are at high
risk for both HBV and HCV infections. The incidence of HCV infection
in IDUs ranges from 2% to 40% per year, with most rates in the range of
15–30% per year
1
(Maher et al., 2006; Mathei et al., 2005; van den Berg et
al., 2007), and the incidence of HBV infection from 10% to 12% per year
(Ruan et al., 2007). A study of IDUs in Seattle looked at those who became
infected with HBV or HCV during the 12-month study period. The study
matched study participants who had become infected to those identified in
1
HCV prevalence is calculated in percentages from person years.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
62 HEPATITIS AND LIVER CANCER
the health department’s surveillance database. Of the 113 study participants
who became infected, only two cases of those identified in the study were
picked up by the state’s surveillance system (Hagan et al., 2002). None of
the 65 acute HCV infections in IDUs was reported.
Enhanced sero-surveillance studies, such as the National Health and
Nutrition Examination Survey (NHANES), which are often used to provide
supplemental data and prevalence estimates when surveillance systems are
inadequate or incomplete, have serious limitations when addressing viral
hepatitis. For example, NHANES, the study most commonly used to es-
timate the disease burden of chronic HBV and HCV infections, excludes
or underrepresents populations that are most at risk for HBV and HCV
infections. Those populations include homeless persons, institutionalized
and incarcerated persons, and persons of Asian and Pacific Island descent.
About 1.5 million people are in state or federal prisons in the United States
(Department of Justice, 2009). CDC estimated chronic HBV prevalence
in the adult prison population to be around 1–3.7%, and the prevalence
of chronic HCV infection has been reported to vary from 12–35% (CDC,
2003c). Thus, the national estimate of 2.7–3.9 million people chronically
infected with HCV (CDC, 2009b; Daniels et al., 2009a) potentially excludes
several hundred thousand cases. Similarly, Asians, Pacific Islanders, Ameri-
can Indian, and Alaska Native people are undersampled among NHANES
participants (Coleman et al., 1998). Given the higher prevalence of HBV
in those populations, NHANES underreports the number of chronic HBV
cases in the national estimates (Kim, 2007).
A supplemental HIV surveillance project funded by CDC’s Division of
HIV/AIDS Prevention is the National HIV Behavioral Surveillance System.
The system surveys persons at high risk for HIV infection in cities with high
rates of AIDS diagnoses to determine their risk behavior, testing behavior,
and use of prevention services. Even though IDUs are one of the targeted
populations studied through the system (CDC, 2009d), HCV testing has
not been included systematically as part of the study design, and this leads
to missed opportunities to define the injection and injection-related behav-
iors as they apply to HCV and HCV prevention.
Case Evaluation, Followup, and Partner Services
The reporting of a case of hepatitis B or hepatitis C by a public test site
or private clinic provides an opportunity for public-health followup. Part of
the followup generally involves ensuring that the persons with the reported
diagnoses and their partners receive proper medical evaluation, counseling,
vaccination, and referrals to support services as needed (Fleming et al.,
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 63
2006). An inability of a health department to identify a case becomes a
missed opportunity to prevent future cases and to ensure that HBV-infected
and HCV-infected people receive the care that they need.
In 2007, the Massachusetts Department of Public Health piloted the
use of STD disease intervention specialists (DISs) to follow up on cases
of HCV infection in people 15–25 years old. The DISs were tasked with
collecting risk-history data and providing partner-notification services for
drug-sharing partners of the infected people. There was some success in
reaching a small sample of the high volume of infected people, but no
funding was available to support the staff. In addition, the DISs had com-
peting priorities that kept the department from being able to implement
its methods fully so that they could be appropriately evaluated (Onofrey
et al., 2008).
Given the demands on staff, most state health-department surveillance
units indicated that they were barely able to keep up with the basics of
data collection. The 2009 NASTAD survey found that only 18 of the 43
jurisdictions conducted any type of followup; in the ones that did conduct
followup, there was wide variation in what that comprised (NASTAD,
2009). Followup can consist of making calls to providers or cases to collect
demographic, clinical, or risk-history data and contacting infected people
by mail, by telephone, or in person to provide education or referral to medi-
cal services. For the most part, even the best resourced surveillance units are
able to conduct only very limited case management (Fleming et al., 2006).
Furthermore, the utility and cost effectiveness of conducting partner
services for persons who test positive for HBV or HCV is yet to be deter-
mined. Currently, states conduct partner notification for HIV and some
STDs. Services include notifying sex or needle-sharing partners of exposure
to disease and testing, counseling, and referrals for other services. Newly
revised CDC guidelines on partner services strongly recommend partner
services for persons reported to have HIV or early syphilis; services for
gonorrhea and chlamydial infection are recommended for high-priority
cases as resources allow (CDC, 2008e). Recommendations for persons
who have viral hepatitis were not included in the HIV and STD integrated
guidelines.
Recommendations
Recommendation 2-1. The Centers for Disease Control and Prevention
should conduct a comprehensive evaluation of the national hepatitis B
and hepatitis C public-health surveillance system.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
64 HEPATITIS AND LIVER CANCER
The evaluation should, at a minimum,
• Include assessment of the system’s attributes, including complete-
ness, data quality and accuracy, timeliness, sensitivity, specificity,
predictive value positive, representativeness, and stability.
• Be consistent with CDC’s Updated Guidelines for Evaluating Public
Health Surveillance Systems.
• Be used to guide the development of detailed technical guidance
and standards for viral hepatitis surveillance.
• Be published in a report.
The committee found little published information on or systematic
review of viral-hepatitis surveillance in the United States. Specific informa-
tion was obtained from a series of surveys of the AVHPCs. In contrast,
the history and status of national HIV surveillance is well reviewed and
documented (CDC, 1999; Glynn et al., 2007; Nakashima and Fleming,
2003).
In July 2001, CDC published updated guidelines for evaluating public-
health surveillance systems (CDC, 2001b). According to the guidelines,
the evaluation should “involve an assessment of system attributes, includ-
ing simplicity, flexibility, data quality, acceptability, sensitivity, predictive
value positive, representativeness, timeliness, and stability.” The lack of
sensitivity of state hepatitis-surveillance systems is well documented for
acute cases, and many states do not perform surveillance for chronic HBV
or chronic HCV infections. Moreover, the movement of CDC away from
NETSS, a CDC-provided system, and toward a national network of PHIN-
compliant systems has left state and territorial health departments with a
wide array of software systems and capabilities (CDC, 2009i). A compre-
hensive review is needed to document the current systems and capacities
of public-health jurisdictions. The evaluation should focus on developing
guidance to improve consistency of data, guide the development of detailed
technical guidance and standards for hepatitis-surveillance programs, and
allow CDC to improve understanding and description of the limitations of
the data collected. Completion of this task should not delay the implemen-
tation of other components of the surveillance-related recommendations
in this report.
Recommendation 2-2. The Centers for Disease Control and Prevention
should develop specific cooperative viral-hepatitis agreements with all
state and territorial health departments to support core surveillance for
acute and chronic hepatitis B and hepatitis C.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 65
The agreements should include
• A funding mechanism and guidance for core surveillance activities.
• Implementation of performance standards regarding revised and
standardized case definitions, specifically through the use of
o Revised case-reporting forms with required, standardized
components.
o Case evaluation and followup.
• Support for developing and implementing automated data-collection
systems, including
o Electronic laboratory reporting.
o Electronic medical-record extraction systems.
o Web-based, PHIN-compliant reporting systems.
CDC should provide more comprehensive guidance to states on sur-
veillance for viral hepatitis. The committee suggests that CDC use the HIV
surveillance system as a model. The committee focused on that surveillance
model as an alternative to the current model because of its organization,
availability of technical assistance, and provision of detailed guidelines. The
strength of the model is in its centralized guidance, mandatory process and
outcome standards, and oversight at a national level, all of which provide
consistency in data among jurisdictions (Hall and Mokotoff, 2007).
CDC is able to oversee its national effort through separate cooperative
agreements with each state and territory for specific core HIV surveillance
activities (CDC, 2007). The agreements not only provide funding for enough
dedicated staff to provide followup directly with providers and to conduct
active surveillance but commit states and territories to specific methods and
performance expectations. States are also provided with detailed guidance
on case investigation, classification, and followup requirements (CDC/
CSTE, 2006). To ensure consistency, CDC holds required disease-specific
technical-assistance meetings for all grantees. Project officers and epide-
miologists are assigned to each grantee. As of April 2008, all states and
territories had implemented the confidential, name-based reporting method
used for all other reportable infectious diseases (CDC, 2008d). The national
HIV surveillance system will soon be able to achieve case counts with no
duplicates among jurisdictions through an interstate reciprocal notification
system wherein CDC provides quarterly reports on cases that might be
duplicated between states on the basis of matching soundex codes,
2
dates
2
A soundex code is a coded last name index based on the way a surname sounds rather
than the way it is spelled. It is a census coding system developed so you can find a surname
even if it may have been recorded under various spellings. A soundex code consists of a letter
and three numbers (CDC/CSTE, 2006).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
66 HEPATITIS AND LIVER CANCER
of birth, race, and sex. There is also guidance and sufficient staffing to be
able to investigate cases of public-health importance, including clusters of
unusual clinical, laboratory, or geographic occurrences; cases with unusual
modes of transmission; cases without detectable antibody response; and
cases with unusual strains of HIV, such as HIV-2 and non-B subtypes.
Another aspect of the HIV surveillance model is that all jurisdictions
use standardized HIV/AIDS case-report forms. Specific information must
be completed before the software system will classify an entry as a case;
this information includes laboratory or provider diagnosis confirmation,
and patient’s date of birth, race, ethnicity, and sex. The case-report form
includes the collection of behavioral-risk information, measures of immu-
nologic function (CD4+ cell count and percentage), and viral load.
Most important, the HIV model includes process and outcome stan-
dards that all jurisdictions must strive to achieve (CDC, 1999). The outcome
standards include completeness, timeliness, accuracy, risk ascertainment,
and collection of first CD4+ cell count. Because the new software system is
document-based, it will enable evaluation of the completeness of national
case ascertainment with a capture-recapture method (Hall et al., 2006). The
resulting information can be used to determine weaknesses in the reporting
system and to help interpret data appropriately.
Finally, the process and outcome standards have been incorporated into
CDC’s updated framework for evaluating public-health surveillance sys-
tems (Hall and Mokotoff, 2007). CDC assesses national HIV surveillance
data against the required outcome standards annually. The HIV/AIDS sur-
veillance evaluation framework promotes continuous improvement in the
quality of data through technical guidance, measurement of performance,
reporting of assessment results to state and local health departments, and
adjustments in guidance, training, or technical assistance according to as-
sessment results.
The cooperative agreement and the associated funding have allowed the
development of the national HIV surveillance system. Both are imperative
for the development of an accurate, timely, and complete hepatitis surveil-
lance system that will provide accurate incidence and prevalence data to
inform proper resource allocation, program development and evaluation,
and policy-making.
The following section details the committee’s recommended model for
structuring surveillance for hepatitis B and hepatitis C.
MODEL FOR SURVEILLANCE
The committee recommends that a two-tiered model be developed:
core surveillance and targeted surveillance. The initial focus of the program
should be the development and implementation of standardized systems
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 67
among all states to maximize their capacity to perform core surveillance for
acute and chronic HBV and HCV infection. Standardization will be accom-
plished through cooperative agreements, improved guidance, and adequate
and consistent funding. Systems should be integrated into existing HIV or
other disease surveillance infrastructure where feasible. Complementary
efforts need to be made in building enhanced supplemental surveillance
systems to describe trends in underrepresented at-risk populations better
and to address the gaps identified in the current surveillance system. Both
types of surveillance activities will provide better information to policy-
makers and service-delivery systems to improve care for people who are at
risk for or living with HBV or HCV infection. Changes should be phased
and prioritized, with the first step focused on the development and funding
of core surveillance systems for each state.
Core Surveillance
Core surveillance—including collection, processing, analysis, and
dissemination of data on cases of acute and chronic HBV and HCV
infection—is needed in all states. Because of the public-health importance
of quick identification of outbreaks and nosocomial transmission, acute-
disease surveillance has had the highest priority in surveillance programs
in the past. However, chronic-disease surveillance is also critical in that, if
funded appropriately, it will assist in the recognition of acute cases, aid in
moving people with recent diagnoses into appropriate care, contribute to an
increased understanding of disease burden, allow evaluation of prevention
efforts, and, given appropriate case management, save on costs associated
with treatment of patients who have cirrhosis, hepatocellular carcinoma, or
liver transplantation. Proper chronic-disease surveillance can also improve
acute-disease surveillance by enhancing the accuracy and efficiency of re-
lated data collection. Evaluation of the core surveillance system should be
ongoing to ensure that it is meeting emerging needs.
Funding Mechanism
In the proposed model, the state would be the primary unit of surveil-
lance. Funding should be earmarked for viral-hepatitis surveillance through
cooperative agreements with the states. CDC should ensure that all states
have sufficient infrastructure to identify and appropriately investigate all
suspected cases of acute and chronic HBV and HCV infection. Cooperative
agreements should require reporting of standardized viral-hepatitis sur-
veillance data within 3 years of implementation. The agreements should
include funding for states to hire staff to process laboratory results, enter
data, and follow up cases of acute and chronic HBV and HCV infections.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
68 HEPATITIS AND LIVER CANCER
Case Definitions
CDC should revise and standardize definitions and methods. Revised
case definitions should reflect active and resolved hepatitis C infection (for
example, a case should not be confirmed if only antibody test results are
available). Recommended testing for hepatitis C should include, where pos-
sible, HCV RNA tests to determine whether a person is actively infected.
The case definition for acute HBV and HCV infection should be revised
to remove the need for symptoms for classification as a confirmed case.
Classification as a suspected case of acute HCV infection should be used
to encourage active followup of likely recent infections (for example, in
adolescents and young adults) (CDC, 2008f).
Case-Reporting Form
The case-reporting form should be standardized, and core components
of it should be required of all jurisdictions to permit better capture of
information on cases of acute and chronic HCV and HBV infection. The
required elements should be such that they could reasonably be found in a
patient’s medical record. For example, the current CDC form requests the
number of sexual partners in a given period. That information is not typi-
cally found in a medical record or known by a medical provider. Additional,
more comprehensive epidemiologic studies could be funded to provide for
patient interviews and a detailed assessment of risk factors (see Recom-
mendation 2-3). Furthermore, the case-reporting form should collect more
detailed demographic data on racial and ethnic populations to identify and
address disparities among populations. For example, the case-reporting
form should include categories for different ethnicities and should disag-
gregate Asians and Pacific Islanders (for example, Chinese, Vietnamese,
Japanese, and Marshallese).
Automated Data-Collection Systems
Automated or passive methods of accessing and processing test results
should be supported and improved. Enhancing and expanding automated
methods of collecting data (for example, Web-based disease-reporting sys-
tems, electronic laboratory reporting, and electronic medical records) reduce
staff time, increase timeliness and completeness, and minimize data-entry
errors (Klevens et al., 2009; Klompas et al., 2008; Lazarus et al., 2001;
Panackal et al., 2002; Vogt et al., 2006; Wurtz and Cameron, 2005). Given
the volume of viral-hepatitis data, automated systems clearly are indicated
(Hopkins, 2005). However, it has been noted that although electronic
laboratory reporting can greatly increase the timeliness and accuracy of
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 69
reporting, it does not remove the need for health departments to conduct
additional followup to obtain information not contained in laboratory
reports, such as symptoms, race and ethnicity, and risk history (Hopkins,
2005; Klevens et al., 2009).
A pilot study of a surveillance system based on electronic medical re-
cords in Massachusetts found a 39% increase in reported cases of chlamydia
and a 53% increase in reported cases of gonorrhea over a 12-month period
compared with cases reported through the existing passive surveillance
system. The system was also able to identify 81 instances of pregnancy not
identified by passive surveillance in patients with chlamydia or gonorrhea
(CDC, 2008b). The system was shown to identify cases of acute HBV in-
fection reliably, including cases that had not yet been reported to state au-
thorities (Klompas et al., 2008). Other studies have found a similar benefit
of improving surveillance for infectious diseases via automatic notification
with electronic medical records (Allen and Ferson, 2000; Hopkins, 2005).
CDC should promote the use of surveillance systems based on electronic
medical records and open-source platforms that will enable the extraction
and transmission of data to state and local health departments.
Standardized Laboratory Reporting It is essential that laboratory data be
standardized and that health departments have automated access to them.
Automated electronic laboratory reporting improves the completeness and
timeliness of disease surveillance (Effler et al., 1999, 2002; Overhage et
al., 2008; Panackal et al., 2002; Ward et al., 2005). Currently, many
laboratory-data collection systems do not integrate or link the multiple
laboratory tests needed to satisfy a case definition (CDC, 2008b). That
could be more easily addressed with electronic laboratory reporting. CDC
should work with states and laboratories to develop and standardize elec-
tronic systems. In addition, it may be useful for CDC to document and
monitor which laboratory tests are reportable in each state, as is done for
the HIV surveillance system.
Identifying Pregnant Women There is a strong need to identify pregnant
women who have chronic HBV to ensure that appropriate followup of
the newborn is conducted with regard to receipt of HBIG and hepatitis B
vaccine. Currently, most health departments lack an automated means of
determining whether the subject of a reported positive HBsAg test was a
pregnant woman. Local health departments have to investigate all positive
hepatitis B tests in women of childbearing age, and this creates a substantial
workload. CDC should work with national laboratory vendors to identify
ways of reporting whether positive HBV tests are linked with prenatal pan-
els. Web-based surveillance systems may be useful for improving capture of
data on pregnant women who have HBV infection (LaPorte et al., 2008).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
70 HEPATITIS AND LIVER CANCER
PHIN-Compliant Systems CDC needs to contribute to more timely de-
velopment of PHIN-compliant systems in all jurisdictions. A review of the
literature evaluating the timeliness of reporting of infectious diseases found
that reporting lag and the variability among states limit the usefulness of
data. The inconsistency in reporting limits CDC’s ability to identify and
respond to multistate outbreaks in a timely manner. The review called for
a more standardized approach in evaluating and describing surveillance-
system timeliness (Jajosky and Groseclose, 2004). Although it did not look
specifically at hepatitis B or hepatitis C, its conclusions are relevant to the
present report.
Electronic Medical Records The reporting of relevant infectious-disease
test results should be a component of electronic medical-record systems.
CDC should support state and local jurisdictions in working with clinical
and community health-center partners to develop algorithms for auto-
matic viral-hepatitis disease reporting based on electronic medical records.
It has already been shown to be effective in enhancing acute-HBV report-
ing without adding to the burden on medical providers (Klompas et al.,
2008).
Case Investigation and Followup
Standards for case investigation and followup should be developed
and implemented to ensure that newly diagnosed patients receive ad-
equate information and referrals. An effective surveillance system should
identify most of the diagnosed cases of both acute and chronic HBV and
HCV infections. Identification of infected people by health departments
should be the first step in getting them into appropriate care. Because
of resource and system inadequacies, it is not. Most health departments
indicated that they were unable to do more than follow up on potentially
pregnant HBV-positive women (personal communication, Adult Viral
Hepatitis Prevention Coordinators, May 2009). If state health depart-
ments had appropriate funding to follow up recently diagnosed cases of
HBV and HCV infection directly, more people would be able to receive
appropriate education and referral into the array of medical and social-
service care that may be indicated.
Analyzing, Reporting, and Disseminating Findings
Once the capacity for state health departments to conduct HBV and
HCV surveillance is improved, CDC should report accurate results that are
based on the improved data. As discussed earlier in this chapter, there are
important concerns about underreporting, particularly of the incidence of
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 71
acute HCV infection. Until the quality of the data collected has improved,
reports should clearly indicate the limitations of the data. For example,
• Trends in acute HBV and HCV infections should be interpreted
with caution because of systematically missing cases that represent
the burden of disease in particular risk groups.
• Discussions of data on acute HBV and HCV infections should
reflect the issue of the large number of chronic infections to ensure
appropriate understanding of the scope of the problem.
• Reported incidences should be presented as ranges rather than
single numbers to reflect the uncertainty of the estimates.
Targeted Surveillance
Once core hepatitis B and hepatitis C surveillance activities are well
established, supplemental or pilot projects should be tested. CDC should
develop and support innovative supplemental surveillance programs.
Recommendation 2-3. The Centers for Disease Control and Preven-
tion should support and conduct targeted active surveillance, including
serologic testing, to monitor incidence and prevalence of hepatitis B
virus and hepatitis C virus infections in populations not fully captured
by core surveillance.
• Active surveillance should be conducted in specific (sentinel) geo-
graphic regions and populations.
• Appropriate serology, molecular biology, and followup will allow for
distinction between acute and chronic hepatitis B and hepatitis C.
Enhanced Surveillance
Supplemental surveillance projects should be funded or conducted by
CDC and should include serosurveillance among targeted populations.
Serosurveillance projects will provide data for improved estimation of
the scope of the problem in underrepresented populations such as certain
racial and ethnic groups, and at-risk populations, including institutional-
ized, homeless, immigrant, and refugee populations. Enhanced surveillance
projects should be structured to obtain information in both rural and urban
regions of the United States. Serosurveillance programs should be flexible
and allow researchers to focus on emerging behavioral risks, for example,
in adolescents and young adults and in HIV-positive men who have sex with
men (Klevens et al., 2009). Conducting serosurveillance or screening among
at-risk populations in correctional facilities may provide opportunities to
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
72 HEPATITIS AND LIVER CANCER
collect more detailed data and to refer people directly into appropriate
medical care, including treatment for acute HCV infection (McGovern et
al., 2006). Other enhanced surveillance projects should include
• Determining the level of care that patients receive after diagno-
sis, including medical and social-service referrals and treatment
(Fleming et al., 2006).
• Following subsets of cases to improve understanding of natural
history (Global Burden of Hepatitis C Working Group, 2004).
• Matching data on chronic hepatitis B and hepatitis C with cancer
registries (Global Burden of Hepatitis C Working Group, 2004).
• Matching data on chronic HBV and HCV infections with HIV/AIDS
data to determine the burden of coinfection in communities.
• Measuring the vaccination status of acute HBV infection cases and
identifying missed opportunities for vaccination.
• Ensuring that viral hepatitis is addressed and integrated with ap-
propriate projects for the National HIV Behavioral Surveillance
System.
• Measuring HBV and HCV seroconversion rates in selected
populations.
Partner Services
Partner services have been found to be effective in identifying un-
diagnosed cases of HIV (Hogben et al., 2007). Similar programs could
potentially be useful identifying cases of hepatitis B and hepatitis C (CDC,
2008e; Hogben and Niccolai, 2009; Marcus et al., 2009). State and local
health departments should be funded to pilot and evaluate partner-services
programs for suspected acute and chronic cases of HBV infection and acute
cases of HCV infection, especially in young people. Integration with exist-
ing partner service programs should be explored. Evaluation should focus
on the efficacy of referral into care services and on screening of exposed
partners—sexual partners for hepatitis B and drug-sharing partners for
hepatitis B and hepatitis C (CDC, 2007).
REFERENCES
Allen, C. J., and M. J. Ferson. 2000. Notification of infectious diseases by general practitioners:
A quantitative and qualitative study. Medical Journal of Australia 172(7):325-328.
Alter, M. J., W. L. Kuhnert, and L. Finelli. 2003. Guidelines for laboratory testing and result2003. Guidelines for laboratory testing and result
reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention.
Morbidity and Morality Weekly: Recommendations and Reports 52(RR-3):1-13, 15;
quiz CE11-CE14.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 73
CDC (Centers for Disease Control and Prevention). 1996. Prevention of perinatal hepatitis
B through enhanced case management—Connecticut, 1994-95, and the United States,
1994. Morbidity and Mortality Weekly Report 45(27):584-587.
———. 1999. Guidelines for national human immunodeficiency virus case surveillance, includ-
ing monitoring for human immunodeficiency virus infection and acquired immunodefi-
ciency syndrome. Centers for Disease Control and Prevention. Morbidity and Morality
Weekly: Recommendations and Reports 48(RR-13):1-27, 29-31.
———. 2001a. National hepatitis C prevention strategy: A comprehensive strategy for the
prevention and control of hepatitis C virus infection and its consequences. Atlanta, GA:
CDC. http://www.cdc.gov/hepatitis/HCV/Strategy/PDFs/NatHepCPrevStrategy.pdf (ac-
cessed August 21, 2009).
———. 2001b. Updated guidelines for evaluating public health surveillance systems: Recom-
mendations from the guidelines working group. Morbidity and Mortality Weekly Report
50(RR-13):1-36.
———. 2003a. 2003-2008 HIV prevention community planning guidance. http://www.cdc.
gov/hiv/topics/cba/resources/guidelines/hiv-cp/pdf/hiv-cp.pdf (accessed May 18, 2009).
———. 2003b. Hepatitis C virus transmission from an antibody-negative organ and tissue
donor—United States, 2000-2002. Morbidity and Mortality Weekly Report 52(13):273-
274, 276.
———. 2003c. Prevention and control of infections with hepatitis viruses in correctional set-
tings. Morbidity and Mortality Weekly Report 52(RR01):1-33.
———. 2003d. Transmission of hepatitis B and C viruses in outpatient settings—New York,
Oklahoma, and Nebraska, 2000-2002. Morbidity and Mortality Weekly Report 52(38):
901-906.
———. 2005a. Guidelines for viral hepatitis surveillance and case management. Centers for
Disease Control and Prevention.
———. 2005b. Transmission of hepatitis B virus among persons undergoing blood glucose mon-
itoring in long-term-care facilities—Mississippi, North Carolina, and Los Angeles county,
California, 2003-2004. Morbidity and Mortality Weekly Report 54(9):220-223.
———. 2006 (unpublished). 2006 assessment tool: Hepatitis C coordination and integration
activities.
———. 2007. Program announcement ps08-802: HIV/AIDS surveillance. http://www.cdc.
gov/od/pgo/funding/PS08-802.htm (accessed August 19, 2009).
———. 2008a. Acute hepatitis C virus infections attributed to unsafe injection practices at
an endoscopy clinic—Nevada, 2007. Morbidity and Mortality Weekly Report 57(19):
513-517.
———. 2008b. Automated detection and reporting of notifiable diseases using electronic medi-
cal records versus passive surveillance—Massachusetts, June 2006-July 2007. Morbidity
and Mortality Weekly Report 57(14):373-376.
———. 2008c. Disease burden from hepatitis A, B, and C in the United States.
———. 2008d. HIV infection reporting. http://www.cdc.gov/hiv/topics/surveillance/reporting.
htm (accessed July 28, 2009).
———. 2008e. Recommendations for partner services programs for HIV infection, syphilis,
gonorrhea, and chlamydial infection. Morbidity and Morality Weekly: Recommendations
and Reports 57(RR-9):1-83; quiz CE81-84.
———. 2008f. Use of enhanced surveillance for hepatitis C virus infection to detect a cluster
among young injection-drug users—New York, November 2004-April 2007. Morbidity
and Mortality Weekly Report 57(19):517-521.
———. 2009a. Case definitions. http://www.cdc.gov/ncphi/disss/nndss/casedef/case_definitions.
htm (accessed August 21, 2009).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
74 HEPATITIS AND LIVER CANCER
———. 2009b. FAQs for health professionals: Hepatitis C. http://www.cdc.gov/hepatitis/
HCV/HCVfaq.htm (accessed August 21, 2009).
———. 2009c. Hepatitis C virus transmission at an outpatient hemodialysis unit—New York,
2001-2008. Morbidity and Mortality Weekly Report 58(8):189-194.
———. 2009d. HIV-associated behaviors among injecting-drug users—23 cities, United States,
May 2005-February 2006. Morbidity and Mortality Weekly Report 58(13):329-332.
———. 2009e. National electronic disease surveillance system. http://www.cdc.gov/nedss/
(accessed July 28, 2009).
———. 2009f. National electronic telecommunications system for surveillance. http://www.
cdc.gov/ncphi/disss/nndss/netss.htm (accessed July 28, 2009).
———. 2009g. Perinatal hepatitis B prevention coordinators. http://www.cdc.gov/hepatitis/
Partners/PeriHepBCoord.htm (accessed August 21, 2009).
———. 2009h. (unpublished) Report on the status of state viral hepatitis plans for the Institute
of Medicine.
———. 2009i. Status of state electronic disease surveillance systems—United States, 2007.
Morbidity and Mortality Weekly Report 58(29):804-807.
CDC/CSTE. 2006. Technical guidance for HIV/AIDS surveillance programs, volumes I-III
Centers for Disease Control and Prevention.
Chen, S. L., and T. R. Morgan. 2006. The natural history of hepatitis C virus (HCV) infection.
International Journal of Medical Sciences 3(2):47-52.
Coleman, P. J., G. M. McQuillan, L. A. Moyer, S. B. Lambert, and H. S. Margolis. 1998.1998.
Incidence of hepatitis B virus infection in the United States, 1976-1994: Estimates from
the national health and nutrition examination surveys. Journal of Infectious Diseases
178(4):954-959.
Cox, A. L., D. M. Netski, T. Mosbruger, S. G. Sherman, S. Strathdee, D. Ompad, D. Vlahov,
D. Chien, V. Shyamala, S. C. Ray, and D. L. Thomas. 2005. Prospective evaluation of
community-acquired acute-phase hepatitis C virus infection. Clinical Infectious Diseases
40(7):951-958.
CSTE (Council of State and Territorial Epidemiologists). 2009. State reportable condi-
tions assessment (SRCA). http://www.cste.org/dnn/ProgramsandActivities/PublicHealth
InformaticsOLD/StateReportableConditionsQueryResults/tabid/261/Default.aspx (ac-
cessed December 15, 2009).
Daniels, D., S. Grytdal, and A. Wasley. 2009a. Surveillance for acute viral hepatitis—
United States, 2007. Morbidity and Mortality Weekly Report: Surveillance Summaries
58(3):1-27.
———. 2009b. Surveillance for acute viral hepatitis—United States, 2007. Morbidity and
Mortality Weekly Report: Surveillance Summaries 58(3):1-27.
Department of Justice. 2009. Office of Justice Programs, Bureau of Justice Statistics. http://
www.ojp.usdoj.gov/bjs/ (accessed August 30, 2009).
Deuffic-Burban S., T. Poynard, M. S. Sulkowski, and J. B. Wong. 2007. Estimating the future
health burden of chronic hepatitis C and human immunodeficiency virus infections in
the United States. Journal of Viral Hepatitis 14(2):107-115.
Effler, P., M. Ching-Lee, A. Bogard, M.-C. Ieong, T. Nekomoto, and D. Jernigan. 1999.
Statewide system of electronic notifiable disease reporting from clinical laboratories:
Comparing automated reporting with conventional methods. Journal of the American
Medical Association 282(19):1845-1850.
Effler, P. V., M. C. Ieong, T. Tom, and M. Nakata. 2002. Enhancing public health surveillance
for influenza virus by incorporating newly available rapid diagnostic tests. Emerging
Infectious Diseases 8(1):23-28.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 75
Fabrizi, F., A. Marzano, P. Messa, P. Martin, and P. Lampertico. 2008. Hepatitis B virus infec-
tion in the dialysis population: Current perspectives. International Journal of Artificial
Organs 31(5):386-394.
Fairchild, A. L., R. Bayer, and J. Colgrove. 2008. Privacy, democracy and the politics of disease
surveillance. Public Health Ethics 1(1):30-38.
Fleming, D. T., A. Zambrowski, F. Fong, A. Lombard, L. Mercedes, C. Miller, J. Poujade, A.
Roome, A. Sullivan, and L. Finelli. 2006. Surveillance programs for chronic viral hepatitis
in three health departments. Public Health Reports 121(1):23-35.
George, P. 2004 (unpublished). New directions for hepatitis surveillance. Division of Viral
Hepatitis, CDC.
Ghany, M. G., D. B. Strader, D. L. Thomas, and L. Seeff. 2009. Diagnosis, management, and
treatment of hepatitis c: An update. Hepatology 49(4):1335-1374.
Global Burden of Hepatitis C Working Group, GBHCWG. 2004. Global burden of disease
(GBD) for hepatitis C. Journal of Clinical Pharmacology 44(1):20-29.
Glynn, M. K., L. M. Lee, and M. T. McKenna. 2007. The status of national HIV case surveil-
lance, United States 2006. Public Health Reports 122(Suppl 1):63-71.
Guerrant, R. L., D. H. Walker, and P. F. Weller, eds. 2001. Essentials of tropical infectious
diseases. Philadelphia, PA: Churchill Livingstone.
Hagan, H., N. Snyder, E. Hough, T. Yu, S. McKeirnan, J. Boase, and J. Duchin. 2002. Case-
reporting of acute hepatitis B and C among injection drug users. Journal of Urban Health
79(4):579-585.
Hall, H. I., and E. D. Mokotoff. 2007. Setting standards and an evaluation framework for hu-
man immunodeficiency virus/acquired immunodeficiency syndrome surveillance. Journal
of Public Health Management and Practice 13(5):519-523.
Hall, H. I., R. Song, J. E. Gerstle, 3rd, and L. M. Lee. 2006. Assessing the completeness of
reporting of human immunodeficiency virus diagnoses in 2002-2003: Capture-recapture
methods. American Journal of Epidemiology 164(4):391-397.
Hogben, M., and L. M. Niccolai. 2009. Innovations in sexually transmitted disease partner2009. Innovations in sexually transmitted disease partner
services. Current Infectious Disease Reports 11(2):148-154.
Hogben, M., T. McNally, M. McPheeters, and A. B. Hutchinson. 2007. The effectiveness of
HIV partner counseling and referral services in increasing identification of HIV-positive
individuals a systematic review. American Journal of Preventive Medicine 33(2 Suppl):
S89-S100.
Hopkins, R. S. 2005. Design and operation of state and local infectious disease surveillance
systems. Journal of Public Health Management and Practice 11(3):184-190.
Hutton, D. W., D. Tan, S. K. So, and M. L. Brandeau. 2007. Cost-effectiveness of screening
and vaccinating Asian and Pacific Islander adults for hepatitis B. Annals of Internal
Medicine 147(7):460-469.
Hyams, K. C. 1995. Risks of chronicity following acute hepatitis B virus infection: A review.
Clinical Infectious Diseases 20(4):992-1000.
Jajosky, R. A., and S. L. Groseclose. 2004. Evaluation of reporting timeliness of public health
surveillance systems for infectious diseases. BMC Public Health 4:29.
Kim, W. R. 2007. Epidemiology of hepatitis B in the United States. Current Hepatitis Reports
6(1):3-8.
Klein, S. J., C. A. Flanigan, J. G. Cooper, D. R. Holtgrave, A. F. Carrascal, and G. S. Birkhead.
2008. Wanted: An effective public health response to hepatitis C virus in the United
States. Journal of Public Health Management and Practice 14(5):471-475.
Klevens, R. M., C. Vonderwahl, S. Speers, K. Alelis, K. Sweet, E. Rocchio, T. Poissant, T. Vogt,
and K. Gallagher. 2009 (unpublished). Hepatitis C virus infection from population-based
surveillance in six U.S. sites, 2006-2007.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
76 HEPATITIS AND LIVER CANCER
Klompas, M., G. Haney, D. Church, R. Lazarus, X. Hou, and R. Platt. 2008. Automated
identification of acute hepatitis b using electronic medical record data to facilitate public
health surveillance. PLoS ONE 3(7):e2626.
Koff, R. S. 2004. Hepatitis B and hepatitis D. In Infectious diseases, edited by S. L. Gorbach,
J. G. Bartlett, and N. R. Blacklow. Philadelphia, PA: Lippincott, Williams & Wilkins.
Pp. 765-784.
Lanini, S., V. Puro, F. Lauria, F. Fusco, C. Nisii, and G. Ippolito. 2009. Patient to patient
transmission of hepatitis B virus: A systematic review of reports on outbreaks between
1992 and 2007. BMC Medicine 7(1):15.
LaPorte, T., M. Conant, M. McGarty, S. Troppy, S. Barrus, S. Lett, M. O’Donnell, G. Haney,
and A. DeMaria. 2008 (March). Improved case finding of hepatitis B positive women
of child-bearing age through implementation of a web-based surveillance system. Paper
presented at National Immunization Conference, Atlanta, Georgia.
Lazarus, R., K. P. Kleinman, I. Dashevsky, A. DeMaria, and R. Platt. 2001. Using automated
medical records for rapid identification of illness syndromes (syndromic surveillance):
The example of lower respiratory infection. BMC Public Health 1:9.
Maher, L., B. Jalaludin, K. G. Chant, R. Jayasuriya, T. Sladden, J. M. Kaldor, and P. L. Sargent.
2006. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in
Australia. Addiction 101(10):1499-1508.
Marcus, J. L., K. T. Bernstein, and J. D. Klausner. 2009. Updated outcomes of partner no-2009. Updated outcomes of partner no-
tification for human immunodeficiency virus, San Francisco, 2004-2008. AIDS 23(8):
1024-1026.
Margolis, H. S., P. J. Coleman, R. E. Brown, E. E. Mast, S. H. Sheingold, and J. A. Arevalo.
1995. Prevention of hepatitis B virus transmission by immunization. An economic
analysis of current recommendations. Journal of the American Medical Association
274(15):1201-1208.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer,
B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate
transmission of hepatitis B virus infection in the United States: Recommendations of the
advisory committee on immunization practices (ACIP) part 1: Immunization of infants,
children, and adolescents. Morbidity and Morality Weekly: Recommendations and Re-
ports 54(RR-16):1-31.
Mathei, C., G. Robaeys, P. van Damme, F. Buntinx, and R. Verrando. 2005. Prevalence of
hepatitis C in drug users in Flanders: Determinants and geographic differences. Epide-
miology and Infection 133(1):127-136.
Matthews, G. V., M. Hellard, J. Kaldor, A. Lloyd, and G. J. Dore. 2007. Further evidence of
HCV sexual transmission among HIV-positive men who have sex with men: Response
to Danta et al. AIDS 21(15):2112-2113.
McGovern, B. H., C. E. Birch, M. J. Bowen, L. L. Reyor, E. H. Nagami, R. T. Chung, and
A. Y. Kim. 2009. Improving the diagnosis of acute hepatitis C infection using expanded
viral load criteria. Clinical Infectious Diseases 49.
McGovern, B. H., A. Wurcel, A. Y. Kim, J. Schulze zur Wiesch, I. Bica, M. T. Zaman, J. Timm,
B. D. Walker, and G. M. Lauer. 2006. Acute hepatitis C virus infection in incarcerated2006. Acute hepatitis C virus infection in incarcerated
injection drug users. Clinical Infectious Diseases 42(12):1663-1670.
McMahon, B. J., W. L. M. Alward, D. B. Hall, W. L. Heyward, T. R. Bender, D. P. Francis,
and J. E. Maynard. 1985. Acute hepatitis B virus infection: Relation of age to the clinical
expression of disease and subsequent development of the carrier state. Journal of Infec-
tious Diseases 151(4):599-603.
Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D.
Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet
359(9316):1478-1483.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
SURVEILLANCE 77
Mosley, J. W., E. A. Operskalski, L. H. Tobler, W. W. Andrews, B. Phelps, J. Dockter, C.
Giachetti, M. P. Busch, f. t. T.-t. V. Study, and R. E. D. S. Groups. 2005. Viral and host
factors in early hepatitis C virus infection. Hepatology 42(1):86-92.
Nakashima, A. K., and P. L. Fleming. 2003. HIV/AIDS surveillance in the United States, 1981-
2001. Journal of Acquired Immune Deficiency Syndromes 32(Suppl 1):S68-S85.
NASTAD (National Alliance of State and Territorial AIDS Directors). 2009 (unpublished).
Viral hepatitis surveillance survey.
NIH (National Institutes of Health). 2002. NIH consensus statement on management of hepa-
titis C: 2002. NIH Consensus and State-of-the-Science Statements 19(3):1-46.
Onofrey, S., D. Church, D. Heisey-Grove, P. Briggs, T. Bertrand, and A. J. DeMaria. March
10-13, 2008. Utilizing disease intervention specialist for follow-up on hepatitis C in indi-
viduals between the ages of 15 and 25 years: A 3-month pilot program. Paper presented
at 2008 National STD Prevention Conference, Chicago, IL.
Overhage, J. M., S. Grannis, and C. J. McDonald. 2008. A comparison of the completeness
and timeliness of automated electronic laboratory reporting and spontaneous reporting
of notifiable conditions. American Journal of Public Health 98(2):344-350.
Panackal, A. A., M. M’Ikanatha N, F. C. Tsui, J. McMahon, M. M. Wagner, B. W. Dixon, J.
Zubieta, M. Phelan, S. Mirza, J. Morgan, D. Jernigan, A. W. Pasculle, J. T. Rankin, Jr.,
R. A. Hajjeh, and L. H. Harrison. 2002. Automatic electronic laboratory-based reporting
of notifiable infectious diseases at a large health system. Emerging Infectious Diseases
8(7):685-691.
Pungpapong, S., W. R. Kim, and J. J. Poterucha. 2007. Natural history of hepatitis B virus
infection: An update for clinicians. Mayo Clinic Proceedings 82(8):967-975.
Ruan, Y., G. Qin, L. Yin, K. Chen, H. Z. Qian, C. Hao, S. Liang, J. Zhu, H. Xing, K. Hong,
and Y. Shao. 2007. Incidence of HIV, hepatitis C and hepatitis B viruses among injec-
tion drug users in southwestern China: A 3-year follow-up study. AIDS 21(Suppl 8):
S39-S46.
Thacker, S. B. 2000. Historical development. In Principles and Practice of Public Health Sur-
veillance, edited by S. Teutsch and R. E. Churchill. Oxford, United Kingdom: Oxford
University Press.
Thompson, N. D., J. F. Perz, A. C. Moorman, and S. D. Holmberg. 2009. Nonhospital health
care-associated hepatitis B and C virus transmission: United States, 1998-2008. Annals
of Internal Medicine 150(1):33-39.
van de Laar, T., O. Pybus, S. Bruisten, D. Brown, M. Nelson, S. Bhagani, M. Vogel, A.
Baumgarten, M. L. Chaix, M. Fisher, H. Gotz, G. V. Matthews, S. Neifer, P. White,
W. Rawlinson, S. Pol, J. Rockstroh, R. Coutinho, G. J. Dore, G. M. Dusheiko, and M.
Danta. 2009. Evidence of a large, international network of HCV transmission in HIV-
positive men who have sex with men. Gastroenterology 136(5):1609-1617.
van den Berg, C. H., C. Smit, M. Bakker, R. B. Geskus, B. Berkhout, S. Jurriaans, R. A.
Coutinho, K. C. Wolthers, and M. Prins. 2007. Major decline of hepatitis C virus2007. Major decline of hepatitis C virus
incidence rate over two decades in a cohort of drug users. European Journal of
Epidemiology 22(3):183-193.
Villano, S. A., D. Vlahov, K. E. Nelson, S. Cohn, and D. L. Thomas. 1999. Persistence of
viremia and the importance of long-term follow-up after acute hepatitis C infection.
Hepatology 29(3):908-914.
Vogt, M., T. Lang, G. Frosner, C. Klingler, A. F. Sendl, A. Zeller, B. Wiebecke, B. Langer, H.
Meisner, and J. Hess. 1999. Prevalence and clinical outcome of hepatitis C infectionPrevalence and clinical outcome of hepatitis C infection
in children who underwent cardiac surgery before the implementation of blood-donor
screening. New England Journal of Medicine 341(12):866-870.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
78 HEPATITIS AND LIVER CANCER
Vogt, R. L., R. Spittle, A. Cronquist, and J. L. Patnaik. 2006. Evaluation of the timeliness and
completeness of a web-based notifiable disease reporting system by a local health depart-
ment. Journal of Public Health Management and Practice 12(6):540-544.
Ward, J. 2008. FY 2008 domestic enacted funds. Presentation to the committee: December
4, 2008.
Ward, M., P. Brandsema, E. van Straten, and A. Bosman. 2005. Electronic reporting improves
timeliness and completeness of infectious disease notification, the Netherlands, 2003.
Euro Surveillance 10(1):27-30.
Wasley, A., J. T. Miller, and L. Finelli. 2007. Surveillance for acute viral hepatitis—United States,
2005. Morbidity and Mortality Weekly Report: Surveillance Summaries 56(3):1-24.
Wurtz, R., and B. J. Cameron. 2005. Electronic laboratory reporting for the infectious diseases
physician and clinical microbiologist. Clinical Infectious Diseases 40(11):1638-1643.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
79
3
Knowledge and Awareness About
Chronic Hepatitis B and Hepatitis C
A
n estimated 0.8–1.4 million people in the United States are chroni-
cally infected with hepatitis B virus (HBV) and 2.7–3.9 million peo-
ple are chronically infected with hepatitis C virus (HCV). However,
there is relatively poor awareness about these infections among health-care
providers, social-service providers, and the general public. Lack of aware-
ness about the prevalence of chronic viral hepatitis in the United States and
about the proper methods and target populations for screening and medical
management of chronic hepatitis B and hepatitis C probably contributes to
continuing transmission; missing of opportunities for prevention, including
vaccination; missing of opportunities for early diagnosis and medical care;
and poor health outcomes in infected people.
As discussed in Chapters 1 and 2, surveillance data on the numbers of
people acutely and chronically infected with HBV and HCV are imprecise
and can be difficult to interpret. The prevalence of chronic infections remains
high for several reasons, and the aging of the chronically infected population
has contributed to the tripling of liver-cancer incidence during the last three
decades (Altekruse et al., 2009; McGlynn et al., 2006). The persistently high
prevalence of chronic HBV infection can be attributed in part to immigra-
tion of chronically infected people from HBV-endemic regions—including
East Asia, Southeast Asia, and sub-Saharan Africa—to the United States.
The high prevalence of chronic HCV infection is due in part to the lack of
access to preventive measures, such as harm-reduction programs, and lack
of access to antiviral treatments in high-risk populations.
This chapter is divided into two sections. The first addresses knowledge
and awareness about hepatitis B and hepatitis C in health-care providers
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
80 HEPATITIS AND LIVER CANCER
and social-service providers; the second addresses the topic with reference
to the general population and at-risk populations. Each section begins by
describing what is known about the levels of knowledge and awareness
about hepatitis B and hepatitis C and how gaps in education about these
diseases are affecting prevention, screening and testing, and treatment op-
portunities. Those summaries are followed by the committee’s recommen-
dations for addressing the gaps and the rationale and supportive evidence
for the recommendations.
KNOWLEDGE AND AWARENESS AMONG
HEALTH-CARE AND SOCIAL-SERVICE PROVIDERS
The committee found that knowledge about chronic hepatitis B and
hepatitis C among health-care providers, particularly primary-care pro-
viders (for example, physicians, physician assistants, and nurse practitio-
ners), and social-service providers (for example, staff of drug-treatment
programs, needle-exchange programs, and immigrant services centers) is
generally poor. Although there have been no large-scale, controlled studies
of health-care providers’ knowledge about chronic hepatitis B and hepatitis
C, it is clear that knowledge has been imperfect among providers in all the
surveys whose results have been published. Subjects of deficient knowledge
include
• The prevalence of chronic hepatitis B and hepatitis C in the general
and high-risk populations in the United States.
• The clinical sequelae of chronic viral hepatitis.
• The characteristics of at-risk persons who should be tested for
chronic HBV and HCV infection and vaccinated to protect them
from hepatitis B.
• The approaches to primary and secondary prevention in addition
to hepatitis B vaccination.
• The proper methods of testing and interpretation of test results.
• The proper followup management for chronic infection.
Provider guidelines for hepatitis screening, prevention, treatment, and
followup have been in place for decades and are updated regularly (CDC,
1991, 1998, 2005, 2008b, 2008c; Ghany et al., 2009; Lok and McMahon,
2009; Mast et al., 2005, 2006). However, current studies of provider
knowledge about chronic viral hepatitis have not identified why health-care
providers fail to follow national recommended guidelines.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 81
Hepatitis B
Studies have shown that many primary care providers cannot differenti-
ate between adult populations that should be screened for chronic hepatitis
B because of their high prevalence of chronic infection (for example, people
born in geographic regions with high HBV endemicity; see Box 3-1) and
populations that should be vaccinated against HBV because of their high
risk of becoming newly infected (for example, health-care workers, men
who have sex with men, prison inmates, and household and sexual contacts
of chronically infected individuals) (Euler et al., 2003b; Ferrante et al.,
2008; Lai et al., 2007).
In a survey of primary care providers in San Francisco, all 91 respon-
dents correctly answered that Chinese immigrants have a higher prevalence
of chronic hepatitis B than non-Hispanic white or US-born Chinese people.
However, a portion of the same group incorrectly identified HIV-infected
BOX 3-1
Geographic Regions That Have Intermediate and
High Hepatitis B Virus Endemicity
Africa: all countries
Asia and Middle East: all countries
South and Western Pacific: all countries and territories but only indig-
enous persons in Australia and New Zealand
Eastern Europe: all countries except Hungary
Western Europe: Greece, Malta, Portugal, and Spain and indigenous
populations of Greenland
North America: Alaska natives and indigenous populations of northern
Canada
Central America: all countries
South America: Argentina, Bolivia, Brazil, Ecuador, Guyana, Suriname,
Venezuela, and Amazonian areas of Colombia and Peru
Caribbean: Antigua and Barbuda, Dominica, Dominican Republic, Gre-
nada, Haiti, Jamaica, Puerto Rico, St. Kitts and Nevis, St. Lucia, St. Vin-
cent and Grenadines, Trinidad and Tobago, and Turks and Caicos
SOURCE: Modified from Mast et al., 2006.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
82 HEPATITIS AND LIVER CANCER
persons (16%), men who have sex with men (18%), and injection-drug
users (IDUs; 23%) as having a higher prevalence of chronic hepatitis B
than Chinese immigrants (Lai et al., 2007). In the same study, 30% of the
respondents were not able to identify the correct test to use for diagnosing
chronic HBV infection.
A cross-sectional survey conducted among 217 members of the New
Jersey Academy of Family Physicians found that a higher proportion of
family physicians recommended screening for hepatitis B among men who
have sex with men (93%), IDUs (95%), and HIV-infected patients (96%)
than for immigrants from Southeast Asia (68%) or sub-Saharan Africa
(57%)—areas that are highly endemic for HBV with over 8% seropreva-
lence of hepatitis B surface antigen (HBsAg) (Ferrante et al., 2008). Only
50% of survey participants recommended screening household contacts of
persons who had chronic HBV infection—an established high-risk popula-
tion. Finally, 21% of the New Jersey family physicians did not know what
step to take next if a patient tested positive for HBsAg or would refer such
a patient to a specialist for followup (Ferrante et al., 2008). However, 83%
of the respondents were interested in receiving education about chronic
viral hepatitis.
Chu (2009) presented data at the 2009 International Symposium on
Viral Hepatitis and Liver Disease that showed that only 18–30% of Asian
American primary care providers who treat Asian American adult patients
reported routinely testing them for HBV infection in their practice. That
finding illustrates the incomplete knowledge even among primary care
providers who themselves constitute a group at high risk for chronic HBV
infection.
At the 2007 Society of General Internal Medicine annual meeting,
Dulay et al. (2007) reported on the results of a multiple-choice hepatitis
B knowledge survey completed by 196 attendees at a university-based
continuing-medical-education conference for primary care providers, in-
cluding nurse practitioners and physician assistants. Of the respondents,
55% were not able to identify HBsAg as the determinant for chronic HBV
infection. Knowledge about the appropriate use of the HBsAg test was sub-
stantially higher among primary care providers who were Asian (68%) than
those of other ethnicities (43%), among physicians (56%) than nonphysi-
cians (23%), and among providers who had more years of experience or
more time spent in the clinic. Some 44% of the respondents did not know
that chronic HBV infection could be controlled with medication, and 25%
incorrectly responded that chronic HBV infection is curable.
Given that the probability of developing chronic hepatitis B is highest
when infants are exposed to HBV through their mothers at birth, both the
US Preventive Services Task Force and the US Centers for Disease Control
and Prevention (CDC) recommend testing all pregnant women for HBsAg
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 83
during an early prenatal visit even if they have been previously vaccinated
or tested (CDC, 1991; U.S. Preventive Services Task Force, 2009). Cur-
rently, only 27 states have maternal HBsAg screening laws (CDC, 2008c).
State screening laws do not necessarily translate into higher testing rates,
because they often do not include an enforcement mechanism or sanctions
for noncompliance (Euler et al., 2003b). In a study of family physicians in
New Jersey, a state with a maternal screening law, Ferrante et al. (2008)
found that 22% of respondents indicated that they did not recommend
testing pregnant women for HBV infection.
At the 2009 International Symposium on Viral Hepatitis and Liver
Disease, Chao et al. (2009b) presented results of a study of perinatal
health-care providers’ knowledge about hepatitis B and the management
of HBsAg-positive pregnant patients recommended by the Advisory Com-
mittee on Immunization Practices (ACIP). Questionnaires were mailed or
administered to 100 practicing obstetrician/gynecologists (OB/GYNs) and
31 peripartum nurses in Santa Clara County, CA, an area with one of the
largest annual numbers of HBsAg-positive pregnant women in the United
States. Although most of the OB/GYNs reported that they tested pregnant
women for HBsAg and properly advised HBsAg-positive women that their
newborns should receive the hepatitis B vaccine and hepatitis B immuno-
globulin within 12 hours of birth, overall knowledge about hepatitis B was
low. Only 26% of OB/GYNs and 10% of peripartum nurses knew that the
prevalence of chronic hepatitis B is higher in Asians than in other ethnic
populations; only 33% of OB/GYNs and 17% of peripartum nurses knew
that there is a high risk of HBV infection becoming chronic in exposed
newborns; and only 22% of OB/GYNs and 37% of peripartum nurses
knew about the risk of death conferred by chronic hepatitis B. Only 62% of
the OB/GYNs referred their HBsAg-positive pregnant patients for chronic-
hepatitis management.
Hepatitis C
Health-care providers’ knowledge about hepatitis C appears to be
similarly insufficient, although there is far less published research on this
topic (Ascione et al., 2007; Ferrante et al., 2008; Shehab et al., 1999, 2001;
Strauss et al., 2006).
In the study of New Jersey family physicians described above, Ferrante
et al. (2008) found that although 95% would recommend testing of IDUs
for HCV infection, only 81% would recommend HCV testing for people
who received blood transfusions before 1992, and only 65% would recom-
mend testing of incarcerated persons—all populations that are at high risk
for HCV infection and that fall within national testing guidelines. Although
HCV testing of pregnant women is not supported by any evidence-based
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
84 HEPATITIS AND LIVER CANCER
recommendations or guidelines, 34% of family physicians surveyed by
Ferrante et al. would nevertheless recommend it. Of the respondents, 31%
did not know what to do next or would refer a patient to a specialist after
a positive test for HCV antibody, and 2% incorrectly assured patients that
those who tested positive were immune to HCV. Physicians in practice
for more than 20 years were found to have the lowest level of knowledge
about HCV risk factors, whereas those in practice for 5 years or less had
the highest knowledge level.
A survey of 593 fellows of the American College of Obstetricians and
Gynecologists (ACOG), half of whom considered themselves to be primary
care providers, assessed screening and counseling practices for HCV infec-
tion. About half (49%) reported that they tested for HCV infection in all
obstetric and gynecologic patients who self-reported ever having injected il-
licit drugs, and 35% tested all patients who reported having received blood
transfusions before 1992 (Boaz et al., 2003). Nearly half counseled HCV-
infected patients to avoid breastfeeding, and 70% counseled HCV-infected
patients to use condoms with their steady sexual partners; both kinds of
advice are inconsistent with recommendations of CDC (CDC, 1998) and
ACOG (2000, as cited in Boaz et al., 2003). Only 64% recommended that
patients who had HCV infection avoid alcohol, which has been found to
increase the risk of disease progression (Ascione et al., 2007).
An earlier mailed survey of 1,412 primary care providers in the United
States also assessed knowledge about risk factors for HCV infection and
management of hepatitis C (Shehab et al., 2001). Nearly three-fourths
(73%) of the respondents had seen fewer than five hepatitis C patients
within the preceding year, and almost half (44%) had no experience with
treatment for HCV infection. Almost all knew the most common risk fac-
tors for HCV infection—injection-drug use, blood transfusion during the
1980s, and multiple sex partners. One-fourth incorrectly indicated that
blood transfusion continues to be a risk factor, and 19% erroneously be-
lieved that casual household contact is a major risk factor. Some 50% of the
providers reported that they routinely ask their patients about risk factors
for HCV infection; 78% test for HCV infection among patients who have
increased liver enzymes with or without HCV risk factors, and 70% test
all patients who have risk factors regardless of liver enzyme levels. When
presented with a scenario on how to treat a hypothetical patient for chronic
HCV infection, 27% of the respondents did not know which therapy to use.
A previous study by the same researchers had also found substantial gaps in
primary care providers’ knowledge about hepatitis C (Shehab et al., 1999).
The gaps persisted even though 95% of the respondents in the 2001 study
reported having used at least one educational tool about hepatitis C in the
preceding 2 years; this suggests that primary care providers misreport their
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 85
exposure to educational materials about hepatitis C or that such materials
do not communicate accurate information effectively.
HCV-positive patients perceive that health-care providers are judgmen-
tal toward those with HCV infection because of its association with illicit
drug use (Janke et al., 2008). Numerous studies have shown that health-
care workers have extremely negative views of IDUs and characterize them
as manipulative, unpleasant, and uncooperative (McLaughlin et al., 2000;
Paterson et al., 2007). Such attitudes among health-care providers can have
a number of deleterious effects, including discouraging of at-risk persons
from accessing testing and other services and reducing the effectiveness of
HCV education and counseling messages (Zickmund et al., 2003).
Additional research has examined HCV knowledge among drug-
treatment providers. Research conducted with 104 members of the staffsResearch conducted with 104 members of the staffs
of two drug-free and two methadone-maintenance treatment programs
(MMTPs) in the New York metropolitan area demonstrated that knowl-
edge about hepatitis C is inadequate (Strauss et al., 2006). Five of 20 items
on an HCV knowledge assessment were not answered correctly by the ma-
jority of the participating staff, suggesting that staff may be systematically
misinformed rather than merely uninformed about some important HCV
issues that affect their clients. Total scores on the assessment averaged 70%,
71%, and 45% among the medically credentialed staff, noncredentialed
staff in the MMTPs, and noncredentialed staff in the drug-free programs,
respectively. The majority of those in the latter group had never participated
in training specifically devoted to HCV; these staff may be sharing incorrect
information with patients or, aware of their limitations in HCV knowledge,
failing to provide patients much needed HCV information. It is critical that
both medically credentialed and noncredentialed staff in the programs re-
ceive effective HCV training so that they can support their patients’ HCV
education and information needs appropriately.
Recommendation
Many providers are not aware of the high prevalence of chronic hepa-
titis B and hepatitis C in some populations. Improved understanding of
risk factors for acute and chronic HBV and HCV infections and collection
of data on them, including country of birth and ethnicity, and the use of
risk-factor screening will lead to increased identification of cases, increased
provision of preventive resources, increased vaccination to protect those at
risk for hepatitis B infection, and reduction in disparities in the burden of
chronic viral hepatitis.
On the basis of the evidence described above, the committee concludes
that insufficient provider knowledge leads to critical missed opportunities
for providers to educate patients about prevention of hepatitis B and hepa-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
86 HEPATITIS AND LIVER CANCER
titis C, to identify patients who may be at risk for these infections, and to
test for chronic HBV and HCV infection in patients and their sexual, family,
and household contacts in the case of hepatitis B and in drug-use networks
in the case of hepatitis C. To address that issue, the committee offers the
following recommendation:
Recommendation 3-1. The Centers for Disease Control and Prevention
should work with key stakeholders (other federal agencies, state and
local governments, professional organizations, health-care organiza-
tions, and educational institutions) to develop hepatitis B and hepatitis
C educational programs for health-care and social-service providers.
Educational programs and materials for health-care and social-service
providers should focus on improving provider awareness and adherence to
practice guidelines for hepatitis B and hepatitis C. The educational programs
should be targeted to primary care providers, appropriate social-service
providers (such as staff of drug-treatment facilities and immigrant-services
centers), and licensed and unlicensed alternative-medicine professionals
(such as acupuncturists and traditional Chinese medicine practitioners) that
serve at-risk populations. At-risk populations include foreign-born people
from HBV- or HCV-endemic countries, clients of sexually-transmitted-
disease (STD) clinics and HIV clinics, IDUs, others at risk because of a
history of percutaneous exposures, and close contacts of people who have
chronic hepatitis B and chronic hepatitis C.
The educational programs should include at least the following
components:
• Information about the prevalence and incidence of acute and chronic
hepatitis B and hepatitis C both in the general US population and
in at-risk populations, particularly foreign-born populations in the
case of hepatitis B, and IDUs and incarcerated populations in the
case of hepatitis C.
• Guidance on screening for risk factors associated with hepatitis B
and hepatitis C.
• Information about hepatitis B and hepatitis C prevention, hepatitis
B immunization, and medical monitoring of chronically infected
patients, specifically,
o Information about methods of testing and interpretation of
results.
o Information about medical management and long-term care:
< How to select candidates for antiviral therapy.
< Importance of liver-cancer screening.
< When to refer patients to a specialist.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 87
• Information about prevention of HBV and HCV transmission in
hospital and nonhospital health-care settings.
• Information about discrimination and stigma associated with hepa-
titis B and hepatitis C and guidance on reducing them.
• Information about health disparities related to hepatitis B and
hepatitis C.
CDC should examine interventions that target several venues and
types of providers, such as educational institutions, health-care facilities,
substance-abuse service providers, and alternative-care providers.
Educational Institutions
Schools of medicine, nursing, physician assistants, complementary and
alternative medicine, and public health should develop improved curricula
to ensure that their graduates are knowledgeable about chronic hepatitis B
and hepatitis C. The curricula should include information on disease preva-
lence, risk factors, preventive actions, appropriate diagnostics, selection
of persons for testing, and appropriate followup for chronically infected
patients and those susceptible to infection.
Continuing-medical-education courses and activities about viral hepa-
titis conducted online or at provider meetings should be regularly offered
to family-practice physicians, internists, OB/GYNs, pediatricians, nurses,
and physician assistants. Drug-treatment counselors’ education and certifi-
cation examinations should also include hepatitis B and hepatitis C. Ques-
tions about chronic hepatitis B should be included on board-certification
or recertification examinations for internists, family-practice physicians,
pediatricians, and OB/GYNs; and questions about chronic hepatitis C
should be included in board examinations for internists and family-practice
physicians. Although there has been no systematic effort to determine
whether continuing-medical-education courses and certification examina-
tions include questions about hepatitis B and hepatitis C, the shortcomings
in knowledge among health-care providers suggest that current efforts are
insufficient, and that new approaches are needed to improve knowledge.
Educational programs should include targeted outreach to and enroll-
ment of providers who work in high-risk venues (for example, STD and
HIV clinics) and in areas where there are many at-risk foreign-born clients,
such as hospitals, clinics, and community health centers that serve large
populations of Asian and Pacific Islander (API) and foreign-born patients
from other highly endemic regions.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
88 HEPATITIS AND LIVER CANCER
Hospital and Other Health-Care Facilities
Health-care workers and their patients are at risk for exposure to
infected blood and body fluids and therefore vulnerable to infection with
HBV and HCV. As discussed in Chapter 2, there have been several out-
breaks of hepatitis B and hepatitis C in health-care settings in recent years
(CDC, 2003b, 2003c, 2005, 2008a, 2009a; Fabrizi et al., 2008; Thompson
et al., 2009). Hospitals and nonhospital health-care facilities (such as di-
alysis units, endoscopy clinics, and long-term-care facilities) should develop
educational programs to reinforce the importance of adhering to recom-
mended standard precautions and procedures to prevent the transmission of
bloodborne infections in both inpatient and outpatient health-care settings
(Thompson et al., 2009). Health-care workers should be routinely vac-
cinated to protect them from hepatitis B. Although the ACIP recommends
that health-care workers receive the hepatitis B vaccine, and the Occupa-
tional Safety and Health Administration requires employers to offer the
hepatitis B vaccine to all health-care workers who may be exposed to blood
(29 CFR 1910.1030), about 25% of health-care workers remain unvacci-
nated (Simard et al., 2007). Successful interventions to prevent exposures
known to transmit bloodborne infections have included general safety train-
ing; training specific to prevention of needle-stick injuries; modification of
practice, staffing, and workload adjustments; and use of protective devices,
such as needles that automatically retract (Clarke et al., 2002; Holodnick
and Barkauskas, 2000; Hooper and Charney, 2005; Stringer et al., 2002;
Trim, 2004).
Substance-Abuse–Related Service Providers
Staff of drug-treatment programs, needle-exchange programs, and cor-
rectional facilities should be participants in viral-hepatitis educational pro-
grams. Studies have shown that IDUs who used needle-exchange programs
or who had been in drug treatment were more likely than others to report
their HCV-antibody status accurately (Hagan et al., 2006). Very high pro-
portions of IDUs have been in jail or prison (Milloy et al., 2008); therefore,
periods of incarceration may present a prime opportunity for providing
hepatitis C education to this high-risk population. In many communities
that have needle-exchange programs, the majority of IDUs have partici-
pated in them (Hagan et al., 1999; Lorvick et al., 2006). Over the period
during which a person may inject illicit drugs, the likelihood that he or she
has been in a drug-treatment program rises (Galai et al., 2003; Hagan et al.,
1999). Thus, the committee believes that providing standardized education
to staff of drug-treatment and needle-exchange programs and correctional
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 89
facilities will increase the likelihood that at-risk and HCV-infected persons
in these settings receive consistent and accurate information.
Alternative-Care Providers
Alternative-care providers would also benefit from participating in
educational programs about viral hepatitis. In California, four annual
educational symposia, in 2004–2007, were arranged by a collaboration of
academic, professional, and community-based organizations to improve
HBV-related knowledge among traditional Chinese medicine practitioners
and acupuncturists—providers who serve a largely API population, a pa-
tient population that has a high prevalence of chronic hepatitis B and the
associated risk of hepatocellular carcinoma (Chang et al., 2007). A pre-
course survey was administered; about half the participants did not know
ways to prevent HBV transmission, the age group most likely to develop
chronic infection, which blood test to use to diagnose chronic infection, or
the risk of death from liver disease or cancer in people who had chronic
hepatitis B. The postcourse survey showed a statistically significant im-
provement in HBV-related knowledge: about 80% of participants were able
to answer questions about prevention and diagnosis of and treatment for
HBV infection correctly.
COMMUNITY KNOWLEDGE AND AWARENESS
The committee has found that knowledge and awareness about hepa-
titis B and hepatitis C are lacking in members of the public and, most im-
portant, in members of specific at-risk populations. Lack of knowledge and
awareness about hepatitis B and hepatitis C in the community often leads
to misinformation, missing of opportunities for prevention and treatment,
and stigmatization of infected populations. The consequences for members
of at-risk communities are important in that missing opportunities for
prevention can lead to infection of additional people with HBV and HCV.
Once infected, they frequently are unaware of their infection and so run
the risk of unknowingly infecting others and of not receiving appropriate
medical management. Although there have been no large-scale, population-
based, controlled studies of community knowledge about hepatitis B and
hepatitis C, all published surveys have shown that knowledge about these
diseases is sparse.
Hepatitis B
As mentioned earlier, APIs are at high risk for chronic hepatitis B. A
number of studies have assessed awareness and knowledge about hepatitis B
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
90 HEPATITIS AND LIVER CANCER
in API populations, including Vietnamese, Cambodian, Korean, and Chinese
Americans, who are known to have a higher prevalence of chronic HBV
infection than the general US population (Hwang et al., 2008; Ma et al.,
2007b, 2008; Taylor, 2006; Taylor et al., 2000, 2002, 2004, 2005a, 2005b;
Thompson et al., 2002; Wu et al., 2007). For example, among Vietnamese
Americans, about 64% had never heard of the hepatitis B vaccine (Ma et
al., 2007b), about 70% were unaware that Asian Americans are at high risk
for chronic hepatitis B (Hwang et al., 2008), most were uninformed about
routes of HBV transmission (Taylor et al., 2000, 2005a, 2005b), and only
one-third had a doctor’s recommendation to undergo HBV testing (Taylor
et al., 2004). In populations of low socioeconomic status, fewer than 10%
had been tested for or vaccinated against HBV (Ma et al., 2007a, 2007b).
In a group of Cambodian Americans, fewer than 50% had ever heard of
or been tested for HBV, and fewer than 25% knew that chronic infection
is lifelong and incurable (Taylor et al., 2002).
Misinformation about HBV transmission creates obstacles for pre-
vention and treatment. In qualitative interviews, most Korean Americans
expressed the belief that sharing of contaminated food and eating utensils
was the most common route of HBV transmission, whereas few mentioned
that HBV can be sexually or parenterally transmitted, and none mentioned
vertical mother-to-child transmission (Choe et al., 2005). Among Chinese
Americans, fewer than half had been tested or vaccinated (Taylor et al.,
2006; Thompson et al., 2002), up to 53% believed HBV could be trans-
mitted by contaminated food (Wu et al., 2007), up to 61% were unaware
that chronic hepatitis B is typically asymptomatic, and 46% believed that
there is a curative treatment for chronic hepatitis B (Wu et al., 2007); about
65% of those who were chronically infected were unaware of their infection
status (Lin et al., 2007).
The committee was unable to find studies that looked at hepatitis B
awareness among other foreign-born immigrants from highly endemic re-
gions such as sub-Saharan Africa, the Middle East, and Eastern European
nations (see Box 3-1). Some educational resources have been translated into
a few languages. For example, New York City has translated its hepatitis
B educational materials into Chinese, Korean, Spanish, and French (New
York Department of Health and Mental Hygiene, 2008).
The incarcerated population has a high risk of being infected with HBV.
About 30% of patients who had acute hepatitis B reported a history of
incarceration before HBV infection (Charuvastra et al., 2001; Goldstein et
al., 2002). Knowledge about HBV transmission in this population is poor
and results in missing of opportunities for vaccination and prevention. A
voluntary, anonymous survey of 153 male and female inmates of the Rhode
Island Department of Corrections revealed that over half the 30% who
reported having risk factors for HBV infection did not consider themselves
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 91
to be at risk for hepatitis B (Vallabhaneni et al., 2004), and 44% of the
inmates were not aware that HBV can be transmitted through unprotected
sexual activity.
Several studies have found that knowledge about hepatitis B is low
among men who have sex with men, another population at high risk for
HBV infection (McCusker et al., 1990; Neighbors et al., 1999; Rhodes et
al., 2000). A 1990 study found that 68% of men who have sex with men
and are patients at a community health center reported that they were
aware of the vaccine, and 25% of those who knew about it had been vac-
cinated (McCusker et al., 1990). Most of the participants who knew about
the hepatitis B vaccine had learned about it from newspapers targeting the
gay population (64%); a minority had learned about it from health-care
providers (44%), friends (37%), and brochures from health-care facilities
or gay organizations (36%). A 1999 study had similar findings: 33% of
the participants were unaware of the hepatitis B vaccine, and 63% had not
been tested for hepatitis B; of those who were aware of the vaccine, only
22% had received the full vaccine series (Neighbors et al., 1999). A simi-
larly low level of hepatitis B knowledge was found among patrons of gay
bars in Birmingham, Alabama, where 32% reported having no information
about hepatitis, 96% reported engaging in high-risk sexual behavior, and
those who had not been vaccinated against HBV (58% of respondents) had
much poorer knowledge about hepatitis B prevention than those who had
been vaccinated (Rhodes et al., 2000).
Stigma
For many people born outside the United States, a cultural stigma is
attached to a diagnosis of chronic hepatitis B. For example, in China, there
is pervasive discrimination against people who are chronically infected
with hepatitis B, who are frequently expelled from schools, fired from jobs,
and shunned by other community members despite the recent passage of
national antidiscrimination laws (China Digital Times, 2009). In a 2007
survey covering 10 major cities in China, hepatitis B was cited as one of
the top three reasons for job discrimination (China Daily, 2007). Given
the deeply ingrained stigma of hepatitis B in some endemic countries, it is
not surprising that many immigrants remain reluctant to undergo testing
and seek medical attention for a positive test result even after moving to
the United States. Because that cultural aversion to hepatitis B testing and
management is due largely to a lack of knowledge about routes of HBV
transmission and means of prevention, any effort to deliver viral-hepatitis
services to the foreign-born population must include an educational com-
ponent to dispel myths (for example, that HBV can be transmitted through
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
92 HEPATITIS AND LIVER CANCER
food, water, and casual contact) and to establish facts, particularly ones
that encourage testing, vaccination, and followup.
Education Programs
Several educational programs targeting API communities have been
successful in disseminating hepatitis B awareness and promoting preven-
tion. Successful programs often build on community partnerships and com-
bine educational resources with increased access to testing, prevention, and
care for participants (CDC, 2006; Chao et al., 2009a; Juon et al., 2008; Lin
et al., 2007). The Hepatitis B Initiative is a community-based hepatitis B
outreach program that partnered with nine Korean American and Chinese
American churches in the Baltimore and Washington, DC, metropolitan
area to provide culturally and linguistically tailored, faith-based HBV edu-
cation, testing, and vaccination (Juon et al., 2008). The initiative has gener-
ated community support and awareness through word of mouth, articles in
local Asian ethnic media, educational sessions and luncheons for API com-
munity leaders, and a national conference for API pastors. In 2003–2006,
the program tested 1,775 participants for HBV and found that 2% were
chronically infected and 61% were not vaccinated. Among 924 unvacci-
nated participants, nearly all received the first dose of hepatitis B vaccine,
89% received the second, and 79% completed the three-dose series. The
Asian American Hepatitis B Program, a collaboration of community groups
and academic and community health centers in New York City, provides
hepatitis B screening, vaccination, and treatment. The program found that
about 15% of newly tested persons had chronic HBV infection, all of whom
were born outside the United States and half of whom had been in the
country for more than 10 years (CDC, 2006).
The Jade Ribbon Campaign is a program focused on reducing the
nationwide health disparity in hepatitis B. This program sponsors com-
munity HBV screening and education clinics and partners with over 400
community-based organizations and federal and state agencies to provide
culturally and linguistically tailored information and multimedia public-
service announcements about hepatitis B burden, risk factors, transmis-
sion, prevention, detection, treatment, and followup to the API community
and health professionals (Asian Liver Center, 2009; CDC, 2009b). The
program’s clinics have found that about 45% of participants were not
vaccinated against HBV, 9–13% of participants were chronically infected,
and up to two-thirds of those who were chronically infected were unaware
of their infection status. Of those who said that they had been vaccinated
against HBV, 20% were unprotected and 5% chronically infected (Chao et
al., 2009a; Lin et al., 2007). This model has been adapted by a number of
cities around the country (Chang et al., 2009; Fernandez, 2008; Hsu et al.,
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 93
2007; Larkin, 2007; McBride, 2008; San Francisco HepB Free, 2009; Tsai
et al., 2008; Zola et al., 2009).
As discussed in Chapter 4, the ACIP recommends that all newborns,
previously unvaccinated children and adolescents, and previously unvac-
cinated adults at high risk for infection be vaccinated against hepatitis
B (Mast et al., 2005, 2006). The latter group includes adults at risk for
infection by sexual exposure, IDUs, household contacts of chronically
infected persons, developmentally disabled persons in long-term-care fa-
cilities, persons at risk for occupational exposure to HBV, hemodialysis
patients, persons with chronic liver disease, and travelers to HBV-endemic
regions, including Asia, Africa, much of Eastern Europe, the Amazon Basin,
the Caribbean, and the Pacific Islands (see Box 4-1). There is a shortage
of hepatitis B education, vaccine promotion, and awareness programs for
nearly all those at-risk populations, and programs need to be developed to
target HIV-positive people, IDUs, and people from highly HBV-endemic
regions (Rein et al., 2009). Although a handful of studies have evaluated
cross-sectional hepatitis B knowledge levels in some of the populations, the
committee knows of no programs that have demonstrated a quantitative
improvement in knowledge about hepatitis B after the implementation of a
targeted, evidence-based educational program.
A potential model to target at-risk populations is to develop pilot sites
similar to CDC’s Racial and Ethnic Approaches to Community Health,
REACH 2010. The REACH 2010 program provided grants to communi-
ties to address services for specified illnesses in particular racial and ethnic
populations. The program targeted blacks, American Indians, Alaska Na-
tives, Asian Americans, Hispanics, and Pacific Islanders—all populations
that have a high prevalence or incidence of hepatitis B and some hepatitis C
also. Viral hepatitis was not part of the program (Collins, 2006; Giles et
al., 2004).
Hepatitis C
Although fewer studies have been conducted to assess awareness of
hepatitis C in specific populations, the literature suggests that knowledge
about this disease is poor. In a cohort of 3,768 women who had or were at
risk for HIV infection, about one-fourth of those with chronic HCV infec-
tion were not aware of their infection status (Cohen et al., 2007). Younger
and black women were less likely to be aware of their HCV infection status,
whereas women who had past alcohol treatment, a history of injection-drug
use, or increased alanine aminotransferase (a liver enzyme) were more likely
to be aware that they were positive for HCV infection. Of those aware of
their chronic HCV infection, the health-care providers of 47% had recom-
mended that they have a liver biopsy, and 56% of these had undergone a
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
94 HEPATITIS AND LIVER CANCER
biopsy; 39% of those who were aware of their HCV infection status had
been offered treatment, and 57% of these had received treatment.
Similarly, in the Collaborative Injection Drug User Study Drug User In-
tervention Trial (DUIT), which enrolled 3,004 young IDUs in five US cities,
72% of anti-HCV-positive and 46% of anti-HCV-negative IDUs were not
aware of their HCV serologic status (Hagan et al., 2006). History of drug
treatment or needle exchange was associated with increased awareness of
HCV serologic status, so these programs may be key locations for provision
of HCV screening in this population. In a questionnaire survey given to 150
patients who were seeking substance-abuse treatment at a Department of
Veterans Affairs medical center, 90% of patients who were HCV-infected
were not aware of their status, and 41% of the IDUs did not know or were
unsure of how HCV is transmitted or about the complications of hepatitis
C (Dhopesh et al., 2000)..
Stein et al. (2001) surveyed 306 former IDUs about their knowledge of
HCV transmission, infection status, and risk of liver disease. They foundThey found
that nearly all the participants knew that HCV is transmitted by sharing
contaminated needles. Among people who had not been tested or did not
know their test results, some 82% were HCV seropositive. One-third of the
people reporting that they were seronegative were actually seropositive—a
demonstration that, as in other surveys, self-reported infection status is
unreliable. Of respondents, 81% estimated their risk of developing liver
disease, specifically cirrhosis, in the next 10 years at 50% or greater.
The risk associated with the shared use of injection paraphernalia other
than syringes is poorly understood (Rhodes et al., 2004). Among IDUs who
have chronic HCV infection and are aware of their infection, the pattern is
similar: the majority understand that they can transmit their infection by
passing on their used syringes to others, but there is less certainty regarding
the shared use of cookers, cottons, and rinse water (Rhodes and Treloar,
2008; Wright et al., 2005).
There is substantial confusion among IDUs regarding the interpretation
of HCV screening tests. In an Australian study, 42% of IDUs believed that
being antibody-positive meant that they were immune to HCV infection
(O’Brien et al., 2008). Misunderstanding of the meaning of antibody-test
results was also observed in a qualitative study of IDUs in London, England
(Rhodes et al., 2004).
Stigma
A number of studies have examined the psychologic consequences of
HCV infection and concluded that hepatitis C is a highly stigmatized dis-
ease, owing in large part to its association with injection-drug use (Conrad
et al., 2006; Crofts et al., 1997; Dunne and Quayle, 2001; Grundy and
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 95
Beeching, 2004). There is also a public perception that HCV is highly con-
tagious and that it is life-threatening in most cases (Conrad et al., 2006);
this has led to discrimination on the part of people who inappropriately
perceive themselves to be at risk from casual contact with an HCV-positive
person. In a study of patients in a liver clinic in Iowa, 57% of HCV-positive
people reported having experienced stigma associated with their infection
(Zickmund et al., 2003). Many patients who have HCV infection wish to
disclose their HCV status to family, intimate partners, and others in an
effort to protect them from infection and to obtain psychosocial support.
Even in work settings, people have been eager to disclose their HCV status
so that in the event of injury co-workers would take extra care in avoid-
ing exposure to contaminated blood. However, many people report that
informing others of their HCV status has led to inappropriate reactions,
such as “[I’m] not allowed to use the cups because they don’t really know
. . . how to pass it on” (Conrad et al., 2006). In another study, a patient
reported that “they didn’t want me drinking out of the water fountain”
(Zickmund et al., 2003).
Education Programs
Although only a handful of studies have examined the influence of edu-
cation on HCV-related risk behavior in IDUs, the results are consistent in
showing that enhanced education and counseling are associated with safer
injection practices (Garfein et al., 2007; Latka et al., 2008; Tucker et al.,
2004). In the DUIT study by Garfein et al. (2007), young HIV-seronegative
and HCV-seronegative IDUs were enrolled in a randomized trial of an inter-
vention that sought to train them to be peer educators. The goals of the
peer-education intervention (PEI) were to develop mastery over knowledge
and skills necessary for prevention of HIV and HCV infection so that they
could pass the knowledge on to their peers. Behavior change was measured
in the PEI subjects and in subjects randomized to an equal-attention control
group. Reductions in injection risk behavior were observed in both study
arms, but the PEI group reported significantly greater reductions.
A parallel study, the Study to Reduce Intravenous Exposures (STRIVE),
enrolled young HCV-seropositive IDUs (most of whom were chronically
infected) and randomized them into a PEI or control condition. Significantly
greater reductions in injection practices that could transmit HCV to other
IDUs were observed in the PEI group (Latka et al., 2008). Thus, enhanced
education and skill-building can lead to safer injection practices and may
contribute to avoidance of infection in susceptible IDUs and reduction in
transmission of infection to other IDUs. That strategy parallels the Preven-
tion for Positives initiatives for HIV (CDC, 2003a).
Patients in drug-treatment programs have considerable needs for educa-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
96 HEPATITIS AND LIVER CANCER
tion about hepatitis C. In spite of the disproportionate prevalence of HCV
infection in drug users, research conducted with 280 patients who never
injected drugs (non-injection-drug users, NIDUs) and past or current IDUs
in 14 US drug-treatment programs showed that many remain uninformed
or misinformed about the disease (Strauss et al., 2007). The 280 partici-
pating patients scored, on the average, 56% on a 20-item true–false HCV
knowledge assessment, demonstrating inadequate knowledge about hepa-
titis C. IDUs scored significantly higher, on the average, than did NIDUs
(60% vs 51%), but their scores also suggest many gaps in their knowledge
about hepatitis C. Fewer than half of all the patients correctly endorsed
facts concerning HCV transmission, the duration of hepatitis C treatment,
the potential effectiveness of hepatitis C medication in active drug users,
the course of HCV infection, and the possibility of spontaneous clearance
of the infection.
To address the knowledge gaps, all the programs offered at least one
form of hepatitis C education: all offered one-on-one sessions with staff,
12 of the programs offered hepatitis C education in a group format, and
11 offered education through pamphlets and books. However, only 60% of
all the participating patients used any of their programs’ hepatitis C educa-
tion services. Those who did avail themselves of the hepatitis C education
opportunities generally assessed them favorably. Of all the patients, many
were unaware that hepatitis C education was offered in their programs
through individual sessions with staff, group meetings, and books and pam-
phlets (42%, 49%, and 46% of the patients, respectively), and 22% were
unaware that any hepatitis C education opportunities existed (Strauss et al.,
2007). Thus, efforts need to focus especially on ensuring that all drug-treat-
ment program patients are made aware of and encouraged to use hepatitis
C education services in their programs. Such awareness and encouragement,
however, will be useful only if staff of drug-treatment programs have up-
to-date knowledge about the virus and treatment options so that they can
share hepatitis C information with their patients accurately.
Recommendation
On the basis of the above findings, the committee offers the follow-
ing recommendation to increase educational and awareness opportunities
about hepatitis B and hepatitis C.
Recommendation 3-2. The Centers for Disease Control and Prevention
should work with key stakeholders to develop, coordinate, and evalu-
ate innovative and effective outreach and education programs to target
at-risk populations and to increase awareness in the general population
about hepatitis B and hepatitis C.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 97
CDC should work with other federal agencies and state and local
governments to form partnerships with health-care providers, private or-
ganizations (including employers and nonprofit organizations), schools,
and appropriate community organizations to develop awareness programs
and campaigns to educate the general public and at-risk populations about
hepatitis B and hepatitis C. The programs should include shared resources
that are linguistically and culturally appropriate and support integration of
education about viral hepatitis and liver health into other health programs
that serve at-risk populations. Successful programs like those discussed
above should serve as models for interventions and existing materials, such
as the American Congress of Obstetricians and Gynecologists patient edu-
cation materials on viral hepatitis (American College of Obstetricians and
Gynecologists, 2007, 2008, 2009), should be used as a basis for producing
linguistically and culturally relevant materials.
Innovative approaches should be developed to address populations that
have access to few educational programs including foreign-born people
from the highly HBV endemic regions, men who have sex with men, IDUs,
and household and sexual contacts of people who are chronically infected
with HBV and HCV.
Programs should be evaluated to ensure that they are effectively tar-
geting the general public and at-risk people and populations. The general
public should be targeted because HBV and HCV infections occur in people
not easily identifiable as belonging to an at-risk population or people who
fail to report potential risk factors (Daniels et al., 2009). The results of
evaluation of the programs will inform future initiatives. The programs
should incorporate interventions that meet the following goals:
• Promote better understanding of HBV and HCV infections,
transmission, prevention, and treatment in at-risk and general
populations.
• Promote increased hepatitis B vaccination rates among children
and at-risk adults.
• Educate pregnant women and women of childbearing age about
hepatitis B prevention.
• Reduce perinatal HBV infections and improvement of at-birth im-
munization rates.
• Increase testing rates in at-risk populations.
• Reduce stigmatization of chronically infected people.
• Promote safe injections among IDUs and safe drug use among
NIDUs.
• Provide culturally and linguistically appropriate educational infor-
mation for all persons who have tested positive for chronic HBV or
HCV infections and those who are receiving treatment.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
98 HEPATITIS AND LIVER CANCER
• Encourage notification of household and sexual contacts of infected
people to be tested for HBV and HCV and encourage hepatitis B
vaccination of close contacts.
General Public Awareness and Education
Lack of knowledge about HBV and HCV transmission contributes to
the stigma of infection and is a barrier to testing, prevention, and care.
Public HIV-awareness campaigns led to reduced stigma and discrimina-
tion toward patients with HIV infection (Brown et al., 2003). As in the
case of HIV/AIDS, increasing general public knowledge about hepatitis B
and hepatitis C can be expected to reduce discrimination toward infected
people, reduce transmission, and increase early diagnosis and treatment
that ultimately save lives.
Broader community education should include print and multimedia
educational materials about viral hepatitis for the public, large employers,
and health insurers. It should work to mobilize and facilitate a grassroots
movement among community stakeholders, including health-care provid-
ers, employers, mainstream and ethnic media, community-based organiza-
tions, and students. Large employers, such as multinational corporations,
are potentially important partners in hepatitis prevention and control in
that they provide health benefits to about two-thirds of Americans who
have health insurance and are commonly employers of foreign-born people
from HBV-endemic countries both in the United States and overseas.
The lack of knowledge and awareness about hepatitis B and hepatitis
C in the general population suggests that integration of viral-hepatitis and
liver-health education into existing health-education curricula in schools
will help to eliminate the stigma of those chronically infected and improve
prevention of viral hepatitis. There is evidence that adolescents are unaware
of hepatitis B and hepatitis C risks and how to prevent becoming infected
(Moore-Caldwell et al., 1997; Slonim et al., 2005). Many schools already
require health education on HIV, which has transmission routes similar to
those of hepatitis B and hepatitis C (CDC, 2008b). Several school-based
programs have been demonstrated to reduce HIV risk in students and could
serve as models for viral hepatitis education initiatives (Gaydos et al., 2008;
Kennedy et al., 2000).
Community-Based Outreach to Foreign-Born
Immigrants from HBV-endemic countries make up the largest popula-
tion of people who have chronic hepatitis B in the United States, and it is
essential that they receive culturally and linguistically tailored information
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 99
about transmission and risks of HBV infection and that it promote testing,
vaccination, and medical management.
Rein et al. (2009) estimated that there are 55 active community-based
hepatitis B outreach programs in the country that were targeting mostly
APIs, of which they contacted 31. Although those programs have done
much to inform APIs about hepatitis B, there is a need for additional
programs that target APIs, given the burden of hepatitis B within that
population.
There is also a need for education programs that target foreign-born
people from other HBV-endemic regions. The models used by programs de-
signed for APIs could be modified to address the needs of other populations.
Community-based education and screening programs—including outreach
at cultural festivals, health fairs, and places of worship—have been shown
to be effective in improving APIs’ knowledge about hepatitis B (Chao et
al., 2009a; Hsu et al., 2007; Juon et al., 2008; Lin et al., 2007) and could
potentially be effective with other ethnic populations. Each year, around
20,000 people are tested through those programs, and HBsAg is detected
in about 8% of the tested population (Rein et al., 2009). Some 30% of the
programs were supported by local government funding, 27% by state fund-
ing, and 10% by federal funding. Other sources include pharmaceutical and
insurance companies, research and service grants, community hospitals, and
other private funding sources (Rein et al., 2009).
Rein et al. (2009) also found that there were few or no hepatitis B
outreach programs in most regions of the United States (the Southeast, the
Midwest, and the Southwest outside of California and the Houston area).
Education and prevention programs should be expanded to provide services
in underserved regions of the United States given that the highest rates of
acute hepatitis B incidence are in the south (Daniels et al., 2009).
Correctional Facilities
About 2 million people are incarcerated in the US correctional system.
The major risk factors for viral hepatitis in people in correctional facilities
are injection-drug use, tattooing, and sexual activity (see Chapters 4 and 5
for additional information about incarcerated populations). Because people
in the correctional system are more likely to be infected or to become in-
fected with HBV and HCV than the US general population, it is important
to provide educational opportunities about hepatitis B and hepatitis C in
correctional facilities. Increased knowledge and awareness about the dis-
eases will lead to a greater understanding among inmates about how to
prevent them, the advantages of hepatitis B vaccination, why they should
be tested for chronic hepatitis B and hepatitis C, and what to do about a
positive test result for either infection. Niveau (2006) reviewed risk factors
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
100 HEPATITIS AND LIVER CANCER
for acquiring infectious diseases in correctional settings and found that
effective preventive measures included information dissemination and edu-
cation. Inmate peer-based health education has been effective in primary
prevention of HIV (Hammett, 2006). The addition of hepatitis education to
existing peer-based inmate educational programs is feasible and will prob-
ably incur minimal additional cost. Boutwell et al. (2005) called education
of prisoners about hepatitis C as part of a larger program of prevention,
testing, and treatment a “cornerstone of the public health response to the
hepatitis C epidemic in the United States” and recommended research into
program implementation.
Drug-Treatment Facilities and Needle-Exchange Programs
Drug-treatment and needle-exchange programs reach a substantial pro-
portion of active injectors who have HCV infection or are at risk of acquir-
ing it. Because the programs have regular, long-term contact with many
IDUs, there are multiple opportunities to disseminate information about
hepatitis B and hepatitis C, including the benefits of hepatitis B vaccination,
how to avoid reinfection with HCV, and the importance of followup care
for those chronically infected.
Although education programs developed for needle exchange, drug
treatment, and corrections facilities will reach substantial proportions of
those at risk, important segments of IDU populations will not be reached by
them. Women and young people who inject drugs are less likely than others
to attend needle-exchange and drug-treatment programs (Bluthenthal et al.,
2000; Miller et al., 2001). Novel programs are needed that will access the
hidden injectors, and outreach and peer-education programs are potentially
effective ways to achieve this goal.
Perinatal Facilities That Care for Pregnant Women
The risk of chronic infection after exposure to HBV is highest in early
life, and most people who have chronic hepatitis B were infected at birth
or during early childhood. Each year in the United States, about 24,000
HBsAg-positive women give birth and about 1,000 newborns develop
chronic HBV infection (Ward, 2008). The latter occurs largely because of
failure to adhere to ACIP recommendations and timely administration of
the birth dose of the hepatitis B vaccine and hepatitis B immunoglobulin.
Although it is recommended that household contacts be tested because
of high risk of infection, fewer than 50% are tested, and fewer than 50%
of those tested and found to be HBV-negative or of unknown status are
vaccinated (Euler et al., 2003a). Therefore, perinatal-care facilities and their
staffs (including OB/GYNs and their clinic staffs) provide an excellent op-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 101
portunity to educate pregnant women about the importance of HBsAg test-
ing, interpretation of the results, and the importance of newborn hepatitis
B vaccination. The women should be given culturally and linguistically ap-
propriate educational information about the importance of administration
of the birth dose of the hepatitis B vaccine and hepatitis B immunoglobulin
within 12 hours of birth if needed, completion of the hepatitis B vaccine
series by the age of 6 months, and postvaccination testing. There is a need
to develop a novel program to educate pregnant women in perinatal-care
facilities about hepatitis B to prevent perinatal transmission, to refer women
who are chronically infected for medical care, and to refer family and
household contacts for testing, vaccination, and care if needed.
REFERENCES
Altekruse, S. F., K. A. McGlynn, and M. E. Reichman. 2009. Hepatocellular carcinoma inci-2009. Hepatocellular carcinoma inci-
dence, mortality, and survival trends in the United States from 1975 to 2005. Journal of
Clinical Oncology 27(9):1485-1491.
American College of Obstetricians and Gynecologists. 2007. ACOG practice bulletin no. 86:
Viral hepatitis in pregnancy. Obstetrics and Gynecology 110(4):941-956.
———. 2008. Hepatitis B virus in pregnancy. http://www.acog.org/publications/patient_
education/bp093.cfm (accessed August 21, 2009).
———. 2009. Protecting yourself against hepatitis B. http://www.acog.org/publications/
patient_education/bp125.cfm (accessed October 24, 2009).
Ascione, A., T. Tartaglione, and G. G. Di Costanzo. 2007. Natural history of chronic hepatitis
C virus infection. Digestive and Liver Disease 39(Suppl 1):S4-S7.
Asian Liver Center. 2009. Information resources. http://liver.stanford.edu/Public/index.html
(accessed August 21, 2009).
Boaz, K., A. E. Fiore, S. J. Schrag, B. Gonik, and J. Schulkin. 2003. Screening and counseling
practices reported by obstetrician-gynecologists for patients with hepatitis C virus infec-
tion. Infectious Diseases in Obstetrics and Gynecology 11(1):39-44.
Bluthenthal, R. N., A. H. Kral, L. Gee, E. A. Erringer, and B. R. Edlin. 2000. The ef-
fect of syringe exchange use on high-risk injection drug users: A cohort study. AIDS
14(5):605-611.
Boutwell, A. E., S. A. Allen, and J. D. Rich. 2005. Opportunities to address the hepatitis C
epidemic in the correctional setting. Clinical Infectious Diseases 40(s5):S367-S372.
Brown, L., K. Macintyre, and L. Trujillo. 2003. Interventions to reduce HIV/AIDS stigma:
What have we learned? AIDS Education and Prevention 15(1):49-69.
CDC (Centers for Disease Control and Prevention). 1991. Hepatitis B virus: A comprehensive
strategy for eliminating transmission in the United States through universal childhood
vaccination: recommendations of the Immunization Practices Advisory Committee. Mo-
bility and Mortality Weekly Report 40(No. RR-13):1-25.
———. 1998. Recommendations for prevention and control of hepatitis C virus (HCV) in-
fection and HCV-related chronic disease. Centers for Disease Control and Prevention.
Morbidity and Morality Weekly: Recommendations and Reports 47(RR-19):1-39.
———. 2003a. Advancing HIV prevention: New strategies for a changing epidemic—United
States, 2003. Morbidity and Mortality Weekly Report 52(15):329-332.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
102 HEPATITIS AND LIVER CANCER
———. 2003b. Hepatitis C virus transmission from an antibody-negative organ and tissue
donor—United States, 2000-2002. Morbidity and Mortality Weekly Report 52(13):273-
274, 276.
———. 2003c. Transmission of hepatitis B and C viruses in outpatient settings—New York,
Oklahoma, and Nebraska, 2000-2002. Morbidity and Mortality Weekly Report 52(38):
901-906.
———. 2005. Transmission of hepatitis B virus among persons undergoing blood glucose moni-
toring in long-term-care facilities—Mississippi, North Carolina, and Los Angeles county,
California, 2003-2004. Morbidity and Mortality Weekly Report 54(9):220-223.
———. 2006. Screening for chronic hepatitis B among Asian/Pacific Islander populations—
New York City, 2005. Morbidity and Mortality Weekly Report 55(18):505-509.
———. 2008a. Acute hepatitis C virus infections attributed to unsafe injection practices at
an endoscopy clinic—Nevada, 2007. Morbidity and Mortality Weekly Report 57(19):
513-517.
———. 2008b. HIV prevention education and HIV-related policies in secondary schools—
selected sites, United States, 2006. Morbidity and Mortality Weekly Report 57(30):
822-825.
———. 2008c. Maternal hepatitis B screening and reporting requirements. http://www2a.cdc.
gov/nip/stateVaccApp/StateVaccsApp/HepatitisScreenandReport.asp (accessed November
7, 2008).
———. 2009a. Hepatitis C virus transmission at an outpatient hemodialysis unit—New York,
2001-2008. Morbidity and Mortality Weekly Report 58(8):189-194.
———. 2009b. Patient education resources. http://www.cdc.gov/hepatitis/B/PatientEduB.htm
(accessed September 9, 2009).
Chang, E. T., S. Y. Lin, E. Sue, M. Bergin, J. Su, and S. K. So. 2007. Building partnerships
with traditional Chinese medicine practitioners to increase hepatitis B awareness and
prevention. Journal of Alternative and Complementary Medicine 13(10):1125-1127.
Chang, E. T., E. Sue, J. Zola, and S. K. So. 2009. 3 for life: A model pilot program to prevent
hepatitis B virus infection and liver cancer in Asian and Pacific Islander Americans.
American Journal of Health Promotion 23(3):176-181.
Chao, S. D., E. T. Chang, P. V. Le, W. Prapong, M. Kiernan, and S. K. So. 2009a. The Jade Rib-
bon Campaign: A model program for community outreach and education to prevent liver
cancer in Asian Americans. Journal of Immigrant and Minority Health 11(4):281-290.
Chao, S. D., C. Cheung, A. Yue, and S. K. So. 2009b. Low hepatitis B knowledge among peri-
natal healthcare providers serving county with nation’s highest rate of births to mothers
chronically infected with hepatitis B. Paper presented at Poster presentation at the 13th
Internationational Symposium on Viral Hepatitis and Liver Disease, Washington, DC.
March 20-24, 2009.
Charuvastra, A., J. Stein, B. Schwartzapfel, A. Spaulding, E. Horowitz, G. Macalino, and J. D.
Rich. 2001. Hepatitis B vaccination practices in state and federal prisons. Public Health
Reports 116(3):203-209.
China Daily. 2007 (June 14). Discrimination in job market common. http://www.china.org.
cn/english/features/cw/213887.htm (accessed March 30, 2009).
China Digital Times. 2009. China news tagged with: hepatitis B. http://chinadigitaltimes.
net/china/hepatitis-b/ (accessed August 21, 2009).
Choe, J. H., N. Chan, H. H. Do, E. Woodall, E. Lim, and V. M. Taylor. 2005. Hepatitis B and
liver cancer beliefs among Korean immigrants in western Washington. Cancer 104(12
Suppl):2955-2958.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 103
Chu, D. 2009. Hepatitis B virus screening practices of Asian-American primary care physicians
who treat Asian adults living in the United States. Paper presented at oral presentation
at the 13th International Symposium on Viral Hepatitis and Liver Disease, Washington,
DC. March 20-24, 2009.
Clarke, S. P., J. L. Rockett, D. M. Sloane, and L. H. Aiken. 2002. Organizational climate,
staffing, and safety equipment as predictors of needlestick injuries and near-misses in
hospital nurses. American Journal of Infection Control 30(4):207-216.
Cohen, M. H., D. Grey, J. A. Cook, K. Anastos, E. Seaberg, M. Augenbraun, P. Burian, M.
Peters, M. Young, and A. French. 2007. Awareness of hepatitis C infection among women
with and at risk for HIV. Journal of General Internal Medicine 22(12):1689-1694.
Collins, J. 2006. Addressing racial and ethnic disparities: Lessons from the REACH 2010 com-
munities. Journal of Health Care for the Poor and Underserved 17(2 Suppl):1-5.
Conrad, S., L. E. Garrett, W. G. Cooksley, M. P. Dunne, and G. A. MacDonald. 2006. Living
with chronic hepatitis C means “you just haven’t got a normal life any more.” Chronic
Illness 2(2):121-131.
Crofts, N., R. Louie, and B. Loff. 1997. The next plague: Stigmatization and discrimination re-
lated to Hepatitis C virus infection in Australia. Health and Human Rights 2(2):86-97.
Daniels, D., S. Grytdal, and A. Wasley. 2009. Surveillance for acute viral hepatitis—United
States, 2007. Morbidity and Mortality Weekly Report: Surveillance Summaries 58(3):
1-27.
Dhopesh, V. P., K. R. Taylor, and W. M. Burke. 2000. Survey of hepatitis B and C in addiction
treatment unit. American Journal of Drug and Alcohol Abuse 26:703.
Dulay, M., J. Zola, J. Hwang, A. Baron, and C. Lai. 2007. Are primary care clinicians knowl-
edgeable about screening for chronic hepatitis B infection? Presented at the 30th annual
meeting of the Society of General Internal Medicine (SGIM), Toronto, Canada. Journal
of General Internal Medicine 22(Suppl 1):100.
Dunne, E. A., and E. Quayle. 2001. The impact of iatrogenically acquired Hepatitis C infec-
tion on the well-being and relationships of a group of Irish women. Journal of Health
Psychology 6(6):679-692.
Euler, G. L., J. Copeland, and W. W. Williams. 2003a. Impact of four urban perinatal hepatitis
B prevention programs on screening and vaccination of infants and household members.
American Journal of Epidemiology 157(8):747-753.
Euler, G. L., K. G. Wooten, A. L. Baughman, and W. W. Williams. 2003b. Hepatitis B surface
antigen prevalence among pregnant women in urban areas: Implications for testing,
reporting, and preventing perinatal transmission. Pediatrics 111(5):1192-1197.
Fabrizi, F., A. Marzano, P. Messa, P. Martin, and P. Lampertico. 2008. Hepatitis B virus infec-
tion in the dialysis population: Current perspectives. International Journal of Artificial
Organs 31(5):386-394.
Fernandez, E. 2008. Hepatitis B plan seeks to aid high-risk groups. San Francisco Chronicle,
September 19, 2008.
Ferrante, J. M., D. G. Winston, P. H. Chen, and A. N. de la Torre. 2008. Family physi-
cians’ knowledge and screening of chronic hepatitis and liver cancer. Family Medicine
40(5):345-351.
Galai, N., M. Safaeian, D. Vlahov, A. Bolotin, and D. D. Celentano. 2003. Longitudinal pat-
terns of drug injection behavior in the ALIVE study cohort, 1988-2000: Description and
determinants. American Journal of Epidemiology 158(7):695-704.
Garfein, R. S., E. T. Golub, A. E. Greenberg, H. Hagan, D. L. Hanson, S. M. Hudson, F.
Kapadia, M. H. Latka, L. J. Ouellet, D. W. Purcell, S. A. Strathdee, and H. Thiede. 2007.
A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C
virus infection in young injection drug users. AIDS 21(14):1923-1932.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
104 HEPATITIS AND LIVER CANCER
Gaydos, C. A., Y.-H. Hsieh, J. S. Galbraith, M. Barnes, G. Waterfield, and B. Stanton. 2008.
Focus-on-teens, sexual risk-reduction intervention for high-school adolescents: Impact on
knowledge, change of risk-behaviours, and prevalence of sexually transmitted diseases.
International Journal of STD and AIDS 19(10):704-710.
Ghany, M. G., D. B. Strader, D. L. Thomas, and L. Seeff. 2009. Diagnosis, management, and
treatment of Hepatitis C: An update. Hepatology 49(4):1335-1374.
Giles, W. H., P. Tucker, L. Brown, C. Crocker, N. Jack, A. Latimer, Y. Liao, T. Lockhart, S.
McNary, M. Sells, and V. B. Harris. 2004. Racial and ethnic approaches to community
health (REACH 2010): An overview. Ethnicity and Disease 14(3 Suppl 1):S5-S8.
Goldstein, S. T., M. J. Alter, I. T. Williams, L. A. Moyer, F. N. Judson, K. Mottram, M. Fleenor,
P. L. Ryder, and H. S. Margolis. 2002. Incidence and risk factors for acute hepatitis B in
the United States, 1982-1998: Implications for vaccination programs. Journal of Infec-
tious Diseases 185(6):713-719.
Grundy, G., and N. Beeching. 2004. Understanding social stigma in women with hepatitis C.
Nursing Standard 19(4):35-39.
Hagan, H., J. Campbell, H. Thiede, S. Strathdee, L. Ouellet, F. Kapadia, S. Hudson, and R. S.
Garfein. 2006. Self-reported hepatitis C virus antibody status and risk behavior in young
injectors. Public Health Reports 121(6):710-719.
Hagan, H., J. P. McGough, H. Thiede, N. S. Weiss, S. Hopkins, and E. R. Alexander. 1999.
Syringe exchange and risk of infection with hepatitis B and C viruses. American Journal
of Epidemiology 149(3):203-213.
Hammett, T. M. 2006. HIV/AIDS and other infectious diseases among correctional inmates:
Transmission, burden, and an appropriate response. American Journal of Public Health
96(6):974-978.
Holodnick, C. L., and V. Barkauskas. 2000. Reducing percutaneous injuries in the or by edu-
cational methods. AORN Journal 72(3):461-464, 468-472, 475-466.
Hooper, J., and W. Charney. 2005. Creation of a safety culture: Reducing workplace injuries
in a rural hospital setting. AAOHN Journal 53(9):394-398.
Hsu, C. E., L. C. Liu, H. S. Juon, Y. W. Chiu, J. Bawa, U. Tillman, M. Li, J. Miller, and M.
Wang. 2007. Reducing liver cancer disparities: A community-based hepatitis-B preven-
tion program for Asian-American communities. Journal of the National Medical As-
sociation 99(8):900-907.
Hwang, J. P., C. H. Huang, and J. K. Yi. 2008. Knowledge about hepatitis B and predictors
of hepatitis B vaccination among Vietnamese American college students. Journal of
American College Health 56(4):377-382.
Janke, E. A., S. McGraw, G. Garcia-Tsao, and L. Fraenkel. 2008. Psychosocial issues in hepa-
titis C: A qualitative analysis. Psychosomatics 49(6):494-501.
Juon, H. S., C. Strong, T. H. Oh, T. Castillo, G. Tsai, and L. D. Oh. 2008. Public health model
for prevention of liver cancer among Asian Americans. Journal of Community Health
33(4):199-205.
Kennedy, M. G., Y. Mizuno, R. Hoffman, C. Baume, and J. Strand. 2000. The effect of
tailoring a model HIV prevention program for local adolescent target audiences. AIDS
Education and Prevention 12(3):225-238.
Lai, C. L., and M. F. Yuen. 2007. The natural history of chronic hepatitis B. Journal of Viral
Hepatitis 14(Suppl 1):6-10.
Larkin, M. 2007. Hepatitis B awareness spread on the web. The Lancet Infectious Diseases
7(6):383.
Latka, M. H., H. Hagan, F. Kapadia, E. T. Golub, S. Bonner, J. V. Campbell, M. H. Coady,
R. S. Garfein, M. Pu, D. L. Thomas, T. K. Thiel, and S. A. Strathdee. 2008. A randomized
intervention trial to reduce the lending of used injection equipment among injection drug
users infected with hepatitis C. American Journal of Public Health 98(5):853-861.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 105
Lin, S. Y., E. T. Chang, and S. K. So. 2007. Why we should routinely screen Asian Ameri-
can adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology
46(4):1034-1040.
Lok, A. S., and B. J. McMahon. 2009. Chronic hepatitis B: Update 2009. Hepatology
50(3):661-662.
Lorvick, J., R. N. Bluthenthal, A. Scott, M. L. Gilbert, K. S. Riehman, R. L. Anderson, N. M.
Flynn, and A. H. Kral. 2006. Secondary syringe exchange among users of 23 California
syringe exchange programs. Substance Use and Misuse 41(6-7):865-882.
Ma, G. X., C. Y. Fang, S. E. Shive, J. Toubbeh, Y. Tan, and P. Siu. 2007a. Risk perceptions and
barriers to hepatitis B screening and vaccination among Vietnamese immigrants. Journal
of Immigrant and Minority Health 9(3):213-220.
Ma, G. X., S. E. Shive, C. Y. Fang, Z. Feng, L. Parameswaran, A. Pham, and C. Khanh. 2007b.
Knowledge, attitudes, and behaviors of hepatitis B screening and vaccination and liver
cancer risks among Vietnamese Americans. Journal of Health Care for the Poor and
Underserved 18(1):62-73.
Ma, G. X., S. E. Shive, J. I. Toubbeh, Y. Tan, and D. Wu. 2008. Knowledge, attitudes, and
behaviors of Chinese hepatitis B screening and vaccination. American Journal of Health
Behavior 32(2):178-187.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer,
B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate
transmission of hepatitis B virus infection in the United States: Recommendations of the
Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants,
children, and adolescents. Morbidity and Mortality Weekly Report Recommandations
and Reports 54(RR-16):1-31.
Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald,
J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immuniza-
tion strategy to eliminate transmission of hepatitis B virus infection in the United States:
Recommendations of the advisory committee on immunization practices (ACIP) part II:
Immunization of adults. Morbidity and Morality Weekly: Recommendations and Reports
55(RR-16):1-33; quiz CE31-34.
McBride, G. 2008. Hepatitis B virus-induced liver cancer in Asian Americans: A preventable
disease. Journal of the National Cancer Institute 100(8):528-529.
McCusker, J., E. M. Hill, and K. H. Mayer. 1990. Awareness and use of hepatitis B vaccine
among homosexual male clients of a Boston community health center. Public Health
Reports 105(1):59-64.
McGlynn, K. A., R. E. Tarone, and H. B. El-Serag. 2006. A comparison of trends in the in-
cidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United
States. Cancer Epidemiology, Biomarkers and Prevention 15(6):1198-1203.
McLaughlin, D. F., H. McKenna, and J. C. Leslie. 2000. The perceptions and aspirations illicit
drug users hold toward health care staff and the care they receive. Journal of Psychiatric
and Mental Health Nursing 7(5):435-441.
Miller, M., A. Eskild, I. Mella, H. Moi, and P. Magnus. 2001. Gender differences in syringe2001. Gender differences in syringe
exchange program use in Oslo, Norway. Addiction 96(11):1639-1651.
Milloy, M. J., E. Wood, W. Small, M. Tyndall, C. Lai, J. Montaner, and T. Kerr. 2008. Incar-
ceration experiences in a cohort of active injection drug users. Drug and Alcohol Review
27(6):693-699.
Moore-Caldwell, S. Y., M. J. Werner, L. Powell, and J. W. Greene. 1997. Hepatitis B vaccina-
tion in adolescents: Knowledge, perceived risk, and compliance. Journal of Adolescent
Health 20(4):294-299.
Neighbors, K., C. Oraka, L. Shih, and P. Lurie. 1999. Awareness and utilization of the hepatitis
B vaccine among young men in the Ann Arbor area who have sex with men. Journal of
American College Health 47(4):173-178.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
106 HEPATITIS AND LIVER CANCER
New York Department of Health and Mental Hygiene. 2008. Hepatitis B: The facts booklet.
http://www.nyc.gov/html/doh/html/cd/cdhepb.shtml (accessed October 28, 2009).
Niveau, G. 2006. Prevention of infectious disease transmission in correctional settings: A
review. Public Health 120(1):33-41.
O’Brien, S., C. Day, E. Black, and K. Dolan. 2008. Injecting drug users’ understanding of
hepatitis C. Addictive Behaviors 33(12):1602-1605.
Paterson, B. L., M. Backmund, G. Hirsch, and C. Yim. 2007. The depiction of stigmatization
in research about hepatitis C. International Journal on Drug Policy 18(5):364-373.
Rein, D. B., S. B. Lesesne, P. J. Leese, and C. M. Weinbaum. 2010. Community-based
hepatitis B screening programs in the United States in 2008. Journal of Viral Hepatitis
17(1):28-33.
Rhodes, S. D., R. J. Diclemente, L. J. Yee, and K. C. Hergenrather. 2000. Hepatitis B vaccina-
tion in a high risk MSM population: The need for vaccine education. Sexually Transmit-
ted Infections 76(5):408-409.
Rhodes, T., and C. Treloar. 2008. The social production of hepatitis C risk among injecting
drug users: A qualitative synthesis. Addiction 103(10):1593-1603.
Rhodes, T., M. Davis, and A. Judd. 2004. Hepatitis C and its risk management among drug
injectors in London: Renewing harm reduction in the context of uncertainty. Addiction
99(5):621-633.
San Francisco HepB Free. 2009. http://sfhepbfree.org (accessed September 9, 2009).
Shehab, T. M., S. S. Sonnad, and A. S. F. Lok. 2001. Management of hepatitis C patients
by primary care physicians in the USA: Results of a national survey. Journal of Viral
Hepatitis 8(5):377-383.
Shehab, T. M., S. S. Sonnad, M. Jeffries, N. Gunaratnum, and A. S. Lok. 1999. Current prac-
tice patterns of primary care physicians in the management of patients with hepatitis C.
Hepatology 30(3):794-800.
Simard, E. P., J. T. Miller, P. A. George, A. Wasley, M. J. Alter, B. P. Bell, and L. Finelli. 2007.
Hepatitis B vaccination coverage levels among healthcare workers in the United States,
2002-2003. Infection Control and Hospital Epidemiology 28(7):783-790.
Slonim, A. B., A. J. Roberto, C. R. Downing, I. F. Adams, N. J. Fasano, L. Davis-Satterla, and
M. A. Miller. 2005. Adolescents’ knowledge, beliefs, and behaviors regarding hepatitis B:
Insights and implications for programs targeting vaccine-preventable diseases. Journal of
Adolescent Health 36(3):178-186.
Stein, M. D., J. Maksad, and J. Clarke. 2001. Hepatitis C disease among injection drug us-
ers: Knowledge, perceived risk and willingness to receive treatment. Drug and Alcohol
Dependence 61(3):211-215.
Strauss, S. M., J. M. Astone-Twerell, C. Munoz-Plaza, D. C. Des Jarlais, M. Gwadz, H. Hagan,
A. Osborne, and A. Rosenblum. 2006. Hepatitis C knowledge among staff in U.S. drug
treatment programs. Journal of Drug Education 36(2):141-158.
———. 2007. Drug treatment programs patients’ hepatitis C virus (HCV) education needs and2007. Drug treatment programs patients’ hepatitis C virus (HCV) education needs and
their use of available HCV education services. BMC Health Services Research 7(39).
Stringer, B., C. Infante-Rivard, and J. A. Hanley. 2002. Effectiveness of the hands-free tech-
nique in reducing operating theatre injuries. Occupational and Environmental Medicine
59(10):703-707.
Taylor, V. M., J. H. Choe, Y. Yasui, L. Li, N. Burke, and J. C. Jackson. 2005a. Hepatitis B
awareness, testing, and knowledge among Vietnamese American men and women. Jour-
nal of Community Health 30(6):477-490.
Taylor, V. M., J. C. Jackson, N. Chan, A. Kuniyuki, and Y. Yasui. 2002. Hepatitis B knowledge
and practices among Cambodian American women in Seattle, Washington. Journal of
Community Health 27(3):151-163.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
KNOWLEDGE AND AWARENESS ABOUT CHRONIC HEPATITIS B AND C 107
Taylor, V. M., J. C. Jackson, M. Pineda, P. Pham, M. Fischer, and Y. Yasui. 2000. Hepatitis B
knowledge among Vietnamese immigrants: Implications for prevention of hepatocellular
carcinoma. Journal of Cancer Education 15(1):51-55.
Taylor, V. M., S. P. Tu, E. Woodall, E. Acorda, H. Chen, J. Choe, L. Li, Y. Yasui, and T. G.
Hislop. 2006. Hepatitis B knowledge and practices among Chinese immigrants to the
United States. Asian Pacific Journal of Cancer Prevention 7(2):313-317.
Taylor, V. M., Y. Yasui, N. Burke, J. H. Choe, E. Acorda, and J. C. Jackson. 2005b. Hepatitis
B knowledge and testing among Vietnamese-American women. Ethnicity and Disease
15(4):761-767.
Taylor, V. M., Y. Yasui, N. Burke, T. Nguyen, A. Chen, E. Acorda, J. H. Choe, and J. C.
Jackson. 2004. Hepatitis B testing among Vietnamese American men. Cancer Detection
and Prevention 28(3):170-177.
Thompson, M. J., V. M. Taylor, J. C. Jackson, Y. Yasui, A. Kuniyuki, S. P. Tu, and T. G. Hislop.
2002. Hepatitis B knowledge and practices among Chinese American women in Seattle,
Washington. Journal of Cancer Education 17(4):222-226.
Thompson, N. D., J. F. Perz, A. C. Moorman, and S. D. Holmberg. 2009. Nonhospital health
care-associated hepatitis B and C virus transmission: United States, 1998-2008. Annals
of Internal Medicine 150(1):33-39.
Trim, J. C. 2004. Raising awareness and reducing the risk of needlestick injuries. Professional
Nurse 19(5):259-264.
Tsai, N. C., P. S. Holck, L. L. Wong, and A. A. Ricalde. 2008. Seroepidemiology of hepa-
titis B virus infection: Analysis of mass screening in Hawaii. Hepatology International
2(4):478-485.
Tucker, T., C. L. Fry, N. Lintzeris, S. Baldwin, A. Ritter, S. Donath, and G. Whelan. 2004.
Randomized controlled trial of a brief behavioural intervention for reducing hepatitis C
virus risk practices among injecting drug users. Addiction 99(9):1157-1166.
U.S. Preventive Services Task Force. 2009. Screening for hepatitis B virus infection in preg-
nancy: U.S. Preventive Services Task Force reaffirmation recommendation statement.
Annals of Internal Medicine 150(12):869-873, W154.
Vallabhaneni, S., G. E. Macalino, S. E. Reinert, B. Schwartzapfel, F. A. Wolf, and J. D.
Rich. 2004. Prisoners’ attitudes toward hepatitis B vaccination. Preventive Medicine
38(6):828-833.
Ward, J. W. 2008. Time for renewed commitment to viral hepatitis prevention. American
Journal of Public Health 98(5):779-781.
Wright, N. M., C. N. Tompkins, and L. Jones. 2005. Exploring risk perception and behaviour
of homeless injecting drug users diagnosed with hepatitis C. Health & Social Care in the
Community 13(1):75-83.
Wu, C. A., S. Y. Lin, S. K. So, and E. T. Chang. 2007. Hepatitis B and liver cancer knowledge
and preventive practices among Asian Americans in the San Francisco bay area, Califor-
nia. Asian Pacific Journal of Cancer Prevention 8(1):127-134.
Zickmund, S., E. Y. Ho, M. Masuda, L. Ippolito, and D. R. LaBrecque. 2003. “They treated
me like a leper.” Stigmatization and the quality of life of patients with hepatitis C. Journal
of General Internal Medicine 18(10):835-844.
Zola, J., E. M. Bachus, M. Bergin, T. Fang, and S. So. March 30-April 2, 2009. San Francisco
Hep B Free: A proactive approach to promoting hepatitis B immunization in conjunc-
tion with screening and care. Poster presentation at the 43rd National Immunization
Conference Dallas, Texas.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
109
4
Immunization
H
epatitis B is a vaccine-preventable disease for which a safe and ef-
fective vaccine has been available for nearly three decades. The first
part of this chapter reviews current federal vaccination recommenda-
tions and state vaccination requirements for hepatitis B. It also summarizes
what is known about hepatitis B vaccination rates in specific populations
(for example, infants, children, and adults, including subgroups of at-risk
adults, such as incarcerated people and occupationally exposed people).
The committee identified missed opportunities for hepatitis B vaccination
and makes recommendations to increase the vaccination rate among vari-
ous populations.
A vaccine for hepatitis C does not exist. The second part of this chapter
summarizes current efforts to develop a hepatitis C vaccine and challenges
that have been encountered. The committee makes a recommendation
about hepatitis C vaccine development.
HEPATITIS B VACCINE
The first hepatitis B vaccine, a plasma-derived vaccine, was licensed by
the US Food and Drug Administration (FDA) in 1981 (IOM, 1994). By the
late 1980s, the plasma-derived vaccine was replaced with a recombinant
version, which expresses the hepatitis B surface antigen (HBsAg) and is
produced in Saccharomyces cerevisiae (common baker’s yeast). The recom-
binant vaccine was licensed by FDA in 1986 and is the type of hepatitis B
vaccine currently used in the United States. It is an anticancer vaccine: by
preventing hepatitis B, it prevents hepatocellular carcinoma.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
110 HEPATITIS AND LIVER CANCER
Hepatitis B vaccine is available both as single-antigen formulations
and as multiantigen formulations in fixed combination with other vac-
cines (Mast et al., 2005). The two single-antigen vaccines are Recom-
bivax HB
®
(Merck & Co., Inc., Whitehouse Station, NJ) and Engerix-B
®
(GlaxoSmithKline Biologicals, Rixensart, Belgium). Of the three licensed
combination vaccines, Twinrix
®
(GlaxoSmithKline Biologicals, Rixensart,
Belgium) is used for vaccination of adults, and Comvax
®
(Merck & Co.,
Inc., Whitehouse Station, NJ) and Pediarix
®
(GlaxoSmithKline Biologicals,
Rixensart, Belgium) are used for vaccination of infants and young children.
Twinrix contains recombinant HBsAg and inactivated hepatitis A virus.
Comvax contains recombinant HBsAg and Haemophilus influenzae type
b (Hib) polyribosylribitol phosphate conjugated to Neisseria meningitidis
outer-membrane protein complex. Pediarix contains recombinant HBsAg,
diphtheria and tetanus toxoids and acellular pertussis adsorbed (DTaP),
and inactivated poliovirus.
The hepatitis B vaccine is administered in a three-dose series: two
priming doses administered 1 month apart and a third dose administered 6
months after the second (Mast and Ward, 2008). Alternative schedules have
been used successfully. Administration of the three-dose series results in
protective concentrations of anti-HBs in more than 95% of healthy infants,
children, and adolescents and in more than 90% of healthy adults aged 40
years old and younger. Immunogenicity drops below 90% in adults over
the age of 40 years. The hepatitis B vaccine has a pre-exposure efficacy of
80–100% and a postexposure efficacy of 70–95%, depending on whether
hepatitis B immune globulin (HBIG) is given with the vaccine. The duration
of immunity appears to be long-lasting, and booster doses of the vaccine
are not routinely recommended (Mast and Ward, 2008).
HBIG is derived from plasma and is used prophylactically to prevent
infection with the hepatitis B virus (HBV). It provides passively acquired
antibody to hepatitis B surface antigen (anti-HBsAg) and temporary protec-
tion (3–6 months). HBIG is typically used as an adjunct to hepatitis B vac-
cine for postexposure immunoprophylaxis to prevent HBV infection (Mast
et al., 2005). HBIG administered alone is the primary means of protection
after an HBV exposure for people who do not respond to hepatitis B vac-
cination. It is also used after liver transplantation for end-stage hepatitis B
to prevent recurrence of the disease in the transplanted liver.
Current Vaccination Recommendations, Requirements, and Rates
The Advisory Committee on Immunization Practices (ACIP) provides
advice and guidance to the US Department of Health and Human Services
and the US Centers for Disease Control and Prevention (CDC) on the con-
trol of vaccine-preventable diseases. It develops written recommendations
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 111
for the routine administration of vaccines to children and adults in the
civilian population. The ACIP recommendations for who should receive
the hepatitis B vaccine are summarized in Box 4-1. The American Academy
of Pediatrics in its Report of the Committee on Infectious Diseases follows
the ACIP recommendations for the hepatitis B vaccine (American Academy
of Pediatrics, 2009).
Perinatal Vaccination
ACIP first recommended universal hepatitis B vaccination of infants in
1991 (ACIP, 1991). Despite the recommendation, each year about 1,000
newborns in the United States acquire chronic HBV infection (Ward, 2008),
a number that has not declined in the last decade. That constitutes an im-
portant gap that needs to be addressed in future prevention efforts.
ACIP currently recommends that the first dose—that is, the birth
dose—be administered before hospital discharge in infants born to HbsAg-
negative women and within 12 hours of birth in infants born to women
who are HbsAg-positive or of unknown status (Mast et al., 2005). It also
recommends that infants born to HBsAg-positive mothers should be given
HBIG within 12 hours of birth. There is no evidence of appreciable ben-
efit if HBIG is administered more than 72 hours after birth. The timely
identification of HBsAg-positive mothers to prevent perinatal transmis-
sion underscores the need for rapid hepatitis B tests (discussed further in
Chapter 5). The hepatitis B vaccine series should be completed by the age
of 18 months (see Table 4-1). Depending on which type of vaccine (single-
antigen or combination) is administered, the series can consist of three or
four vaccinations.
Current ACIP hepatitis B vaccine recommendations for preterm infants
who weigh less than 2,000 g are summarized in Table 4-2. For preterm
infants, the first dose of the vaccine is given within 12 hours of birth if the
mother is HBsAg-positive or is of unknown status. If the mother is known
to be HbsAg-negative, the first dose is administered at the age of 1 month
or at hospital discharge (Mast et al., 2005). The preterm-infant schedule is
based on the recognition that preterm infants have a decreased response to
hepatitis B vaccine administered before the age of 1 month.
Data from National Immunization Surveys demonstrate that national
newborn hepatitis B vaccination coverage did not change appreciably after
implementation of the 2005 ACIP hepatitis B vaccination recommenda-
tion (CDC, 2009b). Using National Immunization Survey data that were
collected before implementation of the 2005 ACIP hepatitis B vaccination
recommendation, CDC estimated that the national newborn hepatitis B
vaccination coverage was 46%, 47.9%, and 42.8% at the age of 1 day
in the 2004, 2005, and 2006 surveys (CDC, 2008c, 2009b). Using data

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
112 HEPATITIS AND LIVER CANCER
BOX 4-1
Summary of ACIP Hepatitis B Vaccination Recommendations
Vaccination of infants
At birth
• Infants born to mothers who are HbsAg-positive should receive hepa-
titis B vaccine and HBIG within 12 hours of birth.
• Infants born to mothers whose HBsAg status is unknown should re-
ceive hepatitis B vaccine within 12 hours of birth. The mother should
have blood drawn as soon as possible to determine her HBsAg status;
if she is HbsAg-positive, the infant should receive HBIG as soon as
possible (no later than the age of 1 week).
• Full-term infants who are medically stable, weigh over 2,000 g, and
are born to HBsAg-negative mothers should receive single-antigen
hepatitis B vaccine before hospital discharge.
• Preterm infants weighing less than 2,000 g and born to HbsAg-
negative mothers should receive the first dose of vaccine 1 month
after birth or at hospital discharge.
After the birth dose
• All infants should complete the hepatitis B vaccine series with either
single-antigen vaccine or combination vaccine according to a recom-
mended vaccination schedule.
• Infants born to HBsAg-positive mothers should be tested for HBsAg
and antibody to HBsAg after completion of the hepatitis B vaccine
series at the age of 9–18 months.
Vaccination of children and adolescents
• All unvaccinated children and adolescents less than 19 years old
should receive the hepatitis B vaccine series.
Vaccination of adults
Persons at risk for infection by sexual exposure
• Sex partners of HbsAg-positive persons.
• Sexually active persons who are not in a long-term, mutually mo-
nogamous relationship (for example, persons with more than one sex
partner during the previous 6 months).
• Persons seeking evaluation or treatment for a sexually transmitted
disease.
• Men who have sex with men.
Persons at risk for infection by percutaneous or mucosal exposure to
blood
• Current or recent injection-drug users.
• Household contacts of HBsAg-positive persons.
• Residents and staff of facilities for developmentally disabled
persons.
• Health-care and public-safety workers who have a reasonably antici-
pated risk of exposure to blood or blood-contaminated body fluids.
• Persons with end-stage renal disease, including predialysis, hemodi-
alysis, peritoneal-dialysis, and home-dialysis patients.
• Incarcerated persons.
Others
• International travelers to regions that have high or intermediate levels
(HBsAg prevalence of at least 2%) of endemic HBV infection.
• Persons who have chronic liver disease.
• Persons who have HIV infection.
• All other persons who are seeking protection from HBV infection.
Abbreviations: ACIP, Advisory Committee on Immunization Practices; HBsAg, hepatitis B
surface antigen; HBIG, hepatitis B immune globulin.
SOURCE: Adapted from Mast et al., 2005, 2006.
from the 2007 National Immunization Survey, which were collected after
implementation of the 2005 ACIP recommendation, CDC estimates that
the national newborn hepatitis B vaccine coverage was 46% at the age of
1 day (CDC, 2009b).
As noted above, despite the ACIP recommendation to vaccinate all
newborns, about 1,000 newborns each year become chronically infected
with HBV. Even with the ACIP recommendation, birth doses of the hepati-

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 113
BOX 4-1
Summary of ACIP Hepatitis B Vaccination Recommendations
Vaccination of infants
At birth
• Infants born to mothers who are HbsAg-positive should receive hepa-
titis B vaccine and HBIG within 12 hours of birth.
• Infants born to mothers whose HBsAg status is unknown should re-
ceive hepatitis B vaccine within 12 hours of birth. The mother should
have blood drawn as soon as possible to determine her HBsAg status;
if she is HbsAg-positive, the infant should receive HBIG as soon as
possible (no later than the age of 1 week).
• Full-term infants who are medically stable, weigh over 2,000 g, and
are born to HBsAg-negative mothers should receive single-antigen
hepatitis B vaccine before hospital discharge.
• Preterm infants weighing less than 2,000 g and born to HbsAg-
negative mothers should receive the first dose of vaccine 1 month
after birth or at hospital discharge.
After the birth dose
• All infants should complete the hepatitis B vaccine series with either
single-antigen vaccine or combination vaccine according to a recom-
mended vaccination schedule.
• Infants born to HBsAg-positive mothers should be tested for HBsAg
and antibody to HBsAg after completion of the hepatitis B vaccine
series at the age of 9–18 months.
Vaccination of children and adolescents
• All unvaccinated children and adolescents less than 19 years old
should receive the hepatitis B vaccine series.
Vaccination of adults
Persons at risk for infection by sexual exposure
• Sex partners of HbsAg-positive persons.
• Sexually active persons who are not in a long-term, mutually mo-
nogamous relationship (for example, persons with more than one sex
partner during the previous 6 months).
• Persons seeking evaluation or treatment for a sexually transmitted
disease.
• Men who have sex with men.
Persons at risk for infection by percutaneous or mucosal exposure to
blood
• Current or recent injection-drug users.
• Household contacts of HBsAg-positive persons.
• Residents and staff of facilities for developmentally disabled
persons.
• Health-care and public-safety workers who have a reasonably antici-
pated risk of exposure to blood or blood-contaminated body fluids.
• Persons with end-stage renal disease, including predialysis, hemodi-
alysis, peritoneal-dialysis, and home-dialysis patients.
• Incarcerated persons.
Others
• International travelers to regions that have high or intermediate levels
(HBsAg prevalence of at least 2%) of endemic HBV infection.
• Persons who have chronic liver disease.
• Persons who have HIV infection.
• All other persons who are seeking protection from HBV infection.
Abbreviations: ACIP, Advisory Committee on Immunization Practices; HBsAg, hepatitis B
surface antigen; HBIG, hepatitis B immune globulin.
SOURCE: Adapted from Mast et al., 2005, 2006.
tis B vaccine are being missed or delayed, which the committee believes is
due to the lack of a delivery-room policy for hepatitis B vaccination. Miss-
ing or delaying the birth dose for infants born to HBsAg-positive women
substantially increases the risk that they will develop chronic hepatitis B.
To reduce the incidence of perinatal HBV infections, the committee offers
the following recommendation:

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
114 HEPATITIS AND LIVER CANCER
TABLE 4-1 Hepatitis B Vaccine Schedules for Newborns, by Maternal
HBsAg Status—ACIP Recommendations
Maternal
HbsAg Status
Single-Antigen (Stand-alone)
Vaccine
Single Antigen (Stand-alone) +
Combination Vaccine
Dose Age Dose Age
Positive
1
a
Birth (up to 12 hours) 1
a
Birth (up to 12 hours)
HBIG
b
Birth (up to 12 hours) HBIG Birth (up to 12 hours)
2 1–2 months 2 2 months
3
c
6 months 3 4 months
4
c
6 months (Pediarix)
or 12–15 months
(Comvax)
Unknown
d
1
a
Birth (up to 12 hours) 1
a
Birth (up to 12 hours)
2 1–2 months 2 2 months
3
c
6 months 3 4 months
4
c
6 months (Pediarix)
or 12–15 months
(Comvax)
Negative 1
a,e
Birth (before
discharge)
1
a,e
Birth (before
discharge)
2 1–2 months 2 2 months
3
c
6–18 months 3 4 months
4
c
6 months (Pediarix)
or 12–15 months
(Comvax)
a
Recombivax HB or Engerix-B should be used for the birth dose. Comvax and Pediarix
cannot be administered at birth or before the age of 6 weeks.
b
HBIG (0.5 mL) administered intramuscularly in a separate site from vaccine.
c
Final dose in vaccine series should not be administered before the age of 24 weeks.
d
Mothers should have blood drawn and tested for HBsAg as soon as possible after admis-
sion for delivery; if mother is found to be HbsAg-positive, infant should receive HBIG as soon
as possible but no later than the age of 7 days.
e
On a case-by-case basis and only in rare circumstances, first dose may be delayed until after
hospital discharge for an infant who weighs ≤2,000 g and whose mother is HbsAg-negative,
but only if physician’s order to withhold birth dose and copy of mother’s original HBsAg-
negative laboratory report are documented in infant’s medical record.
Abbreviations: HBsAg, hepatitis B surface antigen; ACIP, Advisory Committee on Immuniza-ACIP, Advisory Committee on Immuniza-
tion Practices; HBIG, hepatitis B immune globulin. HBIG, hepatitis B immune globulin.
SOURCE: Mast et al., 2005.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 115
Recommendation 4-1. All infants weighing at least 2,000 grams and
born to hepatitis B surface antigen-positive women should receive
single-antigen hepatitis B vaccine and hepatitis B immune globulin in
the delivery room as soon as they are stable and washed. The recom-
mendations of the Advisory Committee on Immunization Practices
should remain in effect for all other infants.
Administration of prophylaxis in the delivery room is not novel. In
the United States, vitamin K prophylaxis for vitamin K–deficiency bleeding
and tetracycline or erythromycin for prophylaxis of neonatal gonococcal
infections are routinely given to infants in the delivery room (American
Academy of Pediatrics, 1961, 1980; Workowski and Berman, 2006). The
World Health Organization recommends that the birth dose of the hepatitis
B vaccine be administered as soon after birth as possible (WHO, 2006).
A pilot project in The Lao People’s Democratic Republic demonstrated
almost 100% coverage when the hepatitis B vaccine was administered in
TABLE 4-2 Hepatitis B Immunization Management of Preterm Infants
Who Weigh Less Than 2,000 g, by Maternal HBsAg Status—ACIP
Recommendations
Maternal
HBsAg Status Recommendation
Positive HBIG + hepatitis B vaccine (within 12 hours of birth)
Continue vaccine series beginning at age of 1–2 months according
to recommended schedule for infants born to HBsAg-positive
mothers (see Table 4-1)
Do not count birth dose as part of vaccine series
Test for HBsAg and antibody to HBsAg after completion of vaccine
series at age of 9–18 months (that is, next well-child visit)
Unknown HBIG + hepatitis B vaccine (within 12 hours of birth)
Test mother for HBsAg
Continue vaccine series beginning at age of 1–2 months according
to recommended schedule based on mother’s HBsAg result (see
Table 4-1)
Do not count birth dose as part of vaccine series
Negative Delay first dose of hepatitis B vaccine until age of 1 month or
hospital discharge
Complete vaccine series (see Table 4-1)
Abbreviations: ACIP, Advisory Committee on Immunization Practices; HBIG, hepatitis B im-
mune globulin; HBsAg, hepatitis B surface antigen.
SOURCE: Mast et al., 2005.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
116 HEPATITIS AND LIVER CANCER
the delivery room (WHO, 2006). When mothers were asked to take their
newborns to a vaccination room for their hepatitis B vaccine birth dose,
vaccine coverage was low.
Childhood Vaccination
ACIP recommends that unvaccinated children and adults under 19
years old be given the hepatitis B vaccine series (Mast et al., 2005). Stud-
ies have found racial and ethnic disparities in childhood vaccination rates:
Asian and Pacific Islander (API), Hispanic, and black children had lower
vaccination rates than non-Hispanic white children (CDC, 2000; Darling
et al., 2005; Jenkins et al., 2000; Morita et al., 2008; Szilagyi et al., 2002).
However, when poverty was controlled for, the estimates did not remain sig-
nificantly lower for any racial or ethnic population than for non-Hispanic
white children (CDC, 2009c).
Studies have also found geographic variability in vaccination cover-
age (Darling et al., 2005; Morita et al., 2008; Szilagyi et al., 2002). The
disparities are seen state by state and within regions. For instance, in 2008,
Maryland had the highest percentage of children who were up to date
1
on
their vaccinations with a rate of 82.3%, compared with Montana with a
rate of 59.2% (CDC, 2009c). Szilagyi et al. (2002) looked at the use of
reminder and recall interventions by primary-care providers to increase
immunization rates for children under 2 years old. Before the intervention,
the baseline geographic disparity was an 18% difference between inner-
city children (55%) and suburban children (73%). Within 3 years of the
establishment of the intervention, the vaccination rates had increased in all
areas, including 84% in the inner city and 88% in the suburbs.
All but three states—Alabama, Montana, and South Dakota—have
a childhood hepatitis B vaccination mandate for daycare or school en-
try (Immunization Action Coalition, 2009). A retrospective cohort study
of Chicago public-school children found that the hepatitis B vaccination
school-entry mandate led to an increase in the vaccination rate among all
children and substantially decreased the disparity in the vaccination rate
between white children and black and Hispanic children (Morita et al.,
2008). Before the school-entry mandate, the study found immunizations
rates in non-Hispanic white, black, and Hispanic children of 89%, 76%,
and 74%, respectively. After the mandate was enacted, the rates changed to
1
The immunization series used in these data includes the following vaccinations—4 or more
doses of DTaP, 3 or more doses of poliovirus vaccine, 1 or more dose of any measles-contain-
ing vaccine, 3 or more doses of Hib vaccine, 3 or more doses of hepatitis B vaccine, as well
as 1 or more dose of varicella vaccine (CDC, 2009c).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 117
88%, 81%, and 87%, respectively. Although a disparity in the vaccinations
rates persisted, the gap was narrowed (Szilagyi et al., 2002).
Other studies also have found that school-entry mandates are effec-
tive in increasing hepatitis B vaccination rates (CDC, 2001b; Koff, 2000;
Olshen et al., 2007; Zimet et al., 2008) although such mandates may
not be as effective in children in daycare (Stanwyck et al., 2004). CDC
(2007) found that about 75% of states reported at least 95% hepatitis B
vaccination coverage of children in kindergarten in 2006–2007. Another
study reported that hepatitis B vaccine series coverage for children 19–35
months old in 2000–2002 ranged from 49% to 82%, depending on the
state (Luman et al., 2005).
Special attention needs to be given to vaccination coverage of foreign-
born children from countries that have a high prevalence of hepatitis B;
because of their high risk of prior infection, laboratory testing is indicated
to determine HBV-infection status.
Recommendation 4-2. All states should mandate that the hepatitis B
vaccine series be completed or in progress as a requirement for school
attendance.
Parents of foreign-born children from HBV-endemic countries should
be given information about testing for HBV and should have their children
tested before vaccination.
Adult Vaccination
Hepatitis B vaccination for adults is recommended to high-risk
populations—people at risk for HBV infection from infected household
contacts and sex partners, from occupational exposure to infected blood
or body fluids, and from travel to regions with high or intermediate levels
of endemic HBV infection (Mast et al., 2006). The estimated chance that
an acute HBV infection will become chronic decreases with increasing age
(see Table 4-3). The probability that an acute HBV infection in a 1-year-old
will become chronic is 88.5%, but only 9.0% in a 19-year-old (Edmunds et
al., 1993). Universal hepatitis B vaccination for adults is not recommended
(that is, people born before 1991 do not need to receive the hepatitis B vac-
cine unless they are at risk for HBV infection). It is not cost-effective; that
is, the health benefits achieved do not justify the cost compared with other
potential health-care interventions (Gold et al., 1996). Interventions in the
United States that cost less than $100,000 per quality adjusted life year
(QALY) gained are generally considered to be cost-effective (Owens, 1998;
WHO, 2009). Universal hepatitis B vaccination is not cost-effective even in
adult Asians and Pacific Islanders, who have a higher prevalence of HBV

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
118 HEPATITIS AND LIVER CANCER
infection than the general US population (Hutton et al., 2007). However,
ring vaccination—vaccination of the close contacts of people found to be
chronically infected with HBV—is cost-effective (Hutton et al., 2007).
Figure 4-1 shows estimated cost effectiveness of hepatitis B vaccination
for different age groups and different incidences of acute hepatitis B. The
leftmost line of the graph represents a recent estimate of acute HBV inci-
dence in the general US population (Hutton et al., 2007). This estimate is
expressed as the annual percentage of people in the population who acquire
acute HBV infection. At that incidence, hepatitis B vaccination of adults
in the general US population costs more than $100,000 per QALY gained,
and is not considered to be cost-effective.
In 2004, just over half (54.6%) the adults at high risk for HBV infection
had received the hepatitis B vaccine, including about 75% of health-care
workers and 64% of public-safety workers for whom vaccination is recom-
mended (CDC, 2006; Simard et al., 2007). Of adults with acute hepatitis
B, 61% reported having missed an opportunity for vaccination (Williams
et al., 2005). Low coverage of high-risk adults is attributed to the lack of
dedicated vaccine programs, limited vaccine supply, inadequate funding,
and noncompliance by the involved populations (Mast et al., 2006).
TABLE 4-3 Estimated Chance That an Acute Hepatitis B
Infection Becomes Chronic with Age
Age (years)
Estimated Chance That Acute HBV Infection
Becomes Chronic (%)
1 88.5
2 52.5
3 41.3
4 34.6
5 29.8
6 26.1
7 23.3
8 20.9
9 19.0
10 17.3
11 15.9
12 14.7
13 13.6
14 13.0
15 11.7
16 11.0
17 10.3
18 9.6
19 9.0
NOTE: Calculated using a formula from Edmunds et al., 1993.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 119
0.0050%
0.0150%
0.0250%
0.0350%
0.0450%
0.0550%
0.0650%
0.0750%
20
30
40
50
Annual Incidence of Acute HBV Infection
Age of Person
Vaccinated
$150,000-$200,000
$100,000-$150,000
$50,000-$100,000
$0-$50,000
>$200,000
Cost per QALY Gained
Figure 4-1, editable
FIGURE 4-1 Estimated cost of adult hepatitis B vaccination per quality adjusted life
year (QALY) gained for different age groups and different rates of acute hepatitis
B virus (HBV) infection incidence. Incidence is expressed as the annual percentage
of the population becoming acutely infected with HBV (for example, incidence of
0.005% means that 5 persons per 100,000 are acutely infected with HBV each year,
and incidence of 0.075% means that 75 persons per 100,000 are acutely infected
with HBV each year). Shadings show different levels of cost per QALY gained. In-
terventions are more cost-effective as one moves down (lower age) and to the right
(higher incidence). Interventions that cost less than approximately $100,000 per
QALY gained are generally considered cost-effective in the United States (Owens,
1998; WHO, 2009). The leftmost line, incidence of 0.0050%, is based on a recent
estimate of acute hepatitis B incidence in the general US population (Hutton et al.,
2007). Analysis performed by D. Hutton using the model developed in Hutton et
al., 2007.
Adults at Risk from Sexual Exposure In a national sample of 500 sexually-
transmitted-disease (STD) clinics, the percentage that offered the hepatitis
B vaccine increased from 25% to 45% (p = 0.02) from 1997 to 2001, and
the percentage of the clinics that considered all patients eligible for the vac-
cine rose from 9% to 26% (p = 0.023) during the same period (Gilbert et
al., 2005). However, declining hepatitis B vaccination rates were reported
in a study of six STD clinics in the United States (Harris et al., 2007). The
researchers collected data on patient visits and hepatitis B vaccinations for
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
120 HEPATITIS AND LIVER CANCER
the period 1997–2005 and found that vaccination rates declined during the
later years. Possible reasons for the decline include fiscal constraints and
increasing rates of prior vaccination.
Several studies have reported that when STD clinics offered the hepa-
titis B vaccine, many patients at high risk for HBV infection opted to be
vaccinated. A study of 194 STD clinic patients found that 62% had not
previously been vaccinated for hepatitis B, and 50% of the 62% elected to
receive the vaccination (Samoff et al., 2004). A national program to vacci-
nate adults at STD clinics for hepatitis B is likely to be cost saving to society
(Miriti et al., 2008). In an anonymous HIV-testing program in Madison,
WI, 86% of patients were considered to be at high risk for HBV infection;
51% of the 86% initiated hepatitis B vaccination, and 80% who initiated
vaccination completed the vaccine series (Savage et al., 2000).
Foreign-born people from HBV-endemic countries who reside in the
United States are at risk for HBV infection from sexual exposures. Con-
tinuing sexual transmission of HBV in these communities is likely. Thus,
foreign-born adults may be at high risk for acquiring hepatitis B, and
women may transmit the virus to their newborns. Foreign-born adults
would benefit from laboratory testing to determine their infection status
and subsequent hepatitis B vaccination of susceptible people.
Adults at Risk from Injection-Drug Use CDC estimates that injection-
drug users (IDUs) account for 15% of acute HBV infections in the United
States (Daniels et al., 2009). The incidence of HBV infection in susceptible
IDUs ranges from 10 to 30 per 100 person-years (Des Jarlais et al., 2003;
Hagan et al., 1999). Hepatitis B vaccine coverage rates in IDUs are low and
estimated to be 3–20% (Altice et al., 2005; Kuo et al., 2004; Lum et al.,
2008) (see Table 4-4). The highest reported vaccination rate in US IDUs
was 22%, in new injectors studied in 2000–2002 (Lum et al., 2003). In a
study conducted in San Francisco, only 13% of IDUs over 30 years old had
ever been offered hepatitis B vaccination compared with 25% of younger
injectors (Seal et al., 2000).
On-site hepatitis B vaccination achieves higher success rates in IDUs
than referral to other locations (summarized in Table 4-4). In a multisite
study of IDUs in five US cities, IDUs participating in a randomized clinical
trial to reduce HIV and HCV transmission were offered hepatitis B vaccina-
tion under a variety of conditions. Vaccine uptake was highest when it was
provided on site and during the initial study visit (Campbell et al., 2007).
A New Haven mobile health van at a needle-exchange program found that
66% of those initially offered the hepatitis B vaccine completed all three
doses (Altice et al., 2005). Des Jarlais et al. (2001) reported a 31% comple-
tion rate in Alaskan IDUs given an off-site referral compared with 83% in
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 121
IDUs offered on-site vaccination and a $5–10 incentive at a New York City
needle-exchange program.
The committee believes that the hepatitis B vaccination rate in the
high-risk IDU population is unacceptably low. Studies of vaccine protocols
show that completion rates are substantially higher when vaccination is
offered at such a location as a needle-exchange program. Using a modeling
approach, Hu et al. (2008) found that hepatitis B vaccination of IDUs who
participate in needle-exchange programs in the United States is likely to be
cost-saving to society.
Incarcerated Populations Hennessey et al. (2009) reported that only 12%(2009) reported that only 12%
of inmates at three jails in Chicago, Detroit, and San Francisco had sero-
logic evidence of hepatitis B vaccination compared with a 25% self-reported
vaccination rate in the US population. The study also found an unexpect-
edly high rate of chronic hepatitis B infections (3.6%) and the lowest rate
of hepatitis B vaccination (10%) among Hispanic inmates.
Twenty states require that inmates receive at least some immunizations,
including the hepatitis B vaccine (CDC, 2008a). Four of those states require
vaccination of all inmates, and 16 require only that juvenile inmates be
vaccinated. Several studies reported that if offered the hepatitis B vaccine,
most inmates (60–93%) would agree to be vaccinated (Rotily et al., 1997;
Vallabhaneni et al., 2004). Alternative vaccination schedules may be effec-
tive for inmates. In a study of inmates in Denmark, 63% completed the
hepatitis B vaccination series on an accelerated 3-week schedule compared
with 20% of those on a 6-month schedule (Christensen et al., 2004).
According to CDC, 28.8% of patients who had acute hepatitis B re-
ported a history of incarceration before HBV infection (Goldstein et al.,
2002). Thus, immunization of incarcerated people could potentially prevent
nearly one-third of all acute hepatitis B cases in the United States. Although
most prison systems in the United States do not provide universal hepatitis
B vaccination for inmates, Charuvastra et al. (2001) noted that 25 of 26
states that responded to a survey reported that they would routinely vac-
cinate their inmates against HBV infection if funding for vaccination were
available.
Although the length of stay is shorter in jails than in prisons, offering
hepatitis B vaccination to jail inmates is feasible and provides a benefit to
the community after the inmates are released. Using an accelerated schedule
increases the completion rate (Christensen et al., 2004). Substantial protec-
tion is provided after even one or two of the three doses of the series. It is
important to have a health-record system that tracks immunizations so that
the vaccine series can be continued if later incarcerations occur. Ideally, im-
munizations administered in jails will be captured in an adult immunization
registry (see discussion on immunization-information systems below) so

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
122 HEPATITIS AND LIVER CANCER
TABLE 4-4 Studies of Hepatitis B Vaccination Rates in Injection-Drug
Users
Reference Location Sample; Design
Percentage Previously
Vaccinated
Percentage Ever Offered
Vaccination
Percentage Completed
Vaccination Series
Cross-sectional studies of vaccination rates
Seal et al., 2000 San Francisco, CA 135 under 30 years old, 96 at
least 30 years old
Cross-sectional
25% under 30 years old,
13% at least 30 years old
Lum et al., 2008 San Francisco, CA 831 young IDUs
Cross-sectional
22%; 18% among HCV
positive
Kuo et al., 2004 Baltimore, MD 324 IDUs, NIDUs
Cross-sectional
10% IDUs; 14% NIDUs
Additional vaccination studies
Campbell et al., 2007 5 US cities 3,181
Cohort; vaccination protocol
varied by city
Vaccination highest where
available on site (83% had at
least one dose); incentives did
not affect vaccination rates
Altice et al., 2005 New Haven, CT; mobile
health van at SEP
134 HBV-negative IDUs
Observational
3% 94% had one dose; 77% had
two doses; 66% had three
doses
Des Jarlais et al., 2001 Anchorage, AK;
New York City
AK cohort referred to clinic
(350); New York City cohort
offered on-site vaccination
at SEP (36) + small cash
incentives
Cohort
30/36 (83%) offered on site
had all three doses vs 31% of
those referred to clinic
Hutchinson et al., 2004 Glasgow, Scotland, prison
vaccination, community
assessment
In 1999, offered HBV
vaccination to all inmates;
surveyed new injectors (within
5 years)
1993, 16%
1994, 19%
1999, 15%
2002, 52%
(56% received hepatitis B
vaccine while in prison)
Abbreviations: HBV, hepatitis B virus; IDU, injection-drug user; NIDU, non-injection-drug
user; SEP, syringe-exchange program.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 123
TABLE 4-4 Studies of Hepatitis B Vaccination Rates in Injection-Drug
Users
Reference Location Sample; Design
Percentage Previously
Vaccinated
Percentage Ever Offered
Vaccination
Percentage Completed
Vaccination Series
Cross-sectional studies of vaccination rates
Seal et al., 2000 San Francisco, CA 135 under 30 years old, 96 at
least 30 years old
Cross-sectional
25% under 30 years old,
13% at least 30 years old
Lum et al., 2008 San Francisco, CA 831 young IDUs
Cross-sectional
22%; 18% among HCV
positive
Kuo et al., 2004 Baltimore, MD 324 IDUs, NIDUs
Cross-sectional
10% IDUs; 14% NIDUs
Additional vaccination studies
Campbell et al., 2007 5 US cities 3,181
Cohort; vaccination protocol
varied by city
Vaccination highest where
available on site (83% had at
least one dose); incentives did
not affect vaccination rates
Altice et al., 2005 New Haven, CT; mobile
health van at SEP
134 HBV-negative IDUs
Observational
3% 94% had one dose; 77% had
two doses; 66% had three
doses
Des Jarlais et al., 2001 Anchorage, AK;
New York City
AK cohort referred to clinic
(350); New York City cohort
offered on-site vaccination
at SEP (36) + small cash
incentives
Cohort
30/36 (83%) offered on site
had all three doses vs 31% of
those referred to clinic
Hutchinson et al., 2004 Glasgow, Scotland, prison
vaccination, community
assessment
In 1999, offered HBV
vaccination to all inmates;
surveyed new injectors (within
5 years)
1993, 16%
1994, 19%
1999, 15%
2002, 52%
(56% received hepatitis B
vaccine while in prison)
Abbreviations: HBV, hepatitis B virus; IDU, injection-drug user; NIDU, non-injection-drug
user; SEP, syringe-exchange program.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
124 HEPATITIS AND LIVER CANCER
that the vaccine series can be completed at other sites, such as drug-treat-
ment centers and STD clinics.
Hepatitis B vaccination of inmates costs the correctional system $415
per HBV infection averted, but it provides additional postincarceration
savings to society as a whole (Pisu et al., 2002).
Other At-Risk Adults
HIV-infected people. At a clinic that serves primarily HIV-infected pa-
tients in Jacksonville, FL, 45% of 1,576 HIV-infected patients were consid-
ered to be at risk for HBV infection (Bailey et al., 2008), and 30% of those
at risk were not offered hepatitis B vaccine by their health-care providers.
Routine hepatitis B vaccination at HIV clinics is highly cost-effective, with
a cost of $4,400 per QALY gained (Kim et al., 2006). Similarly, hepatitis B
vaccination at STD testing, counseling, and treatment sites has been dem-
onstrated to be highly cost-effective (Miriti et al., 2008).
Institutionalized populations. Vellinga et al. (1999) reviewed the litera-
ture and reported that among institutionalized developmentally disabled
people in the United States, the prevalence of anti-HBs antibody ranged
from 36% to 63% in residents who had Down syndrome and from 48%
to 69% in people who had other intellectual disabilities. HBsAg prevalence
was very high—27–51% in people who had Down syndrome and 6–9% in
people who had other intellectual disabilities—and this suggests that many
residents of institutions are immunized by natural infection rather than by
vaccination. The committee did not find data on rates of hepatitis B vac-
cination of institutionalized developmentally disabled people. Because they
are at risk for hepatitis B, they would benefit from vaccination.
Occupational exposure to hepatitis B virus. Only 75% of health-care
workers (HCWs) in the United States—a population at high risk for HBV
infection—have received the three-dose vaccine series in 2002-2003 (Simard
et al., 2007). The vaccination rate was highest in physicians and nurses
(81%) and lowest among black HCWs (67.6%).
Identifying At-Risk Adults
As discussed above, recommendations regarding childhood hepatitis B
vaccination are aimed at achieving universal coverage, and recommenda-
tions regarding adult vaccination focus on the identification of risk popula-
tions for targeted immunization efforts. The identification of at-risk adults
has proved problematic (CDC, 2006), and current CDC recommendations
have emphasized both site-based and individual-based risk assessment to
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 125
improve hepatitis B vaccine coverage (Mast et al., 2006). A key to the suc-
cess of such an approach is the routine availability of hepatitis B vaccine in
settings where a high proportion of persons who have risk factors are seen
(such as STD clinics), in primary-care and specialty-care medical settings,
and in occupational-health programs.
Identification of at-risk people is particularly challenging in medical
settings in that risks must be assessed in individual patients. In many
health-care settings, physicians and other providers might not be comfort-
able in asking direct questions to elicit risk history with respect to sexual or
percutaneous exposures (Ashton et al., 2002; Bull et al., 1999; Maheux et
al., 1995). Time constraints during medical appointments and inadequate
provider education in the assessment of risk histories also might lead to
insufficient assessment of risk history. In addition, there may be discrepan-
cies between a patient’s self-assessment of risk and a health-care provider’s
documented assessment (Fishbein et al., 2006). Therefore, the ACIP recom-
mends that hepatitis B vaccination be offered to any adult who requests it,
regardless of a provider’s assessment of risk (Mast et al., 2006).
Recommendation
In 2007, there were more than 40,000 new acute HBV infections in
adults (Daniels et al., 2009). To reduce the incidence of HBV infection in
adults, the committee offers the following recommendation:
Recommendation 4-3. Additional federal and state resources should be
devoted to increasing hepatitis B vaccination of at-risk adults.
• Correctional institutions should offer hepatitis B vaccination to all
incarcerated persons. Accelerated schedules for vaccine administra-
tion should be considered for jail inmates.
• Organizations that serve high-risk people should offer the hepatitis
B vaccination series.
• Efforts should be made to improve identification of at-risk adults.
Health-care providers should routinely seek risk histories from
adult patients through direct questioning and self-assessment.
• Efforts should be made to increase rates of completion of the vac-
cine series in adults.
• Federal and state agencies should determine gaps in hepatitis B
vaccine coverage among at-risk adults annually and estimate the
resources needed to fill the gaps.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
126 HEPATITIS AND LIVER CANCER
Immunization-Information Systems
Immunization registries are databases that allow the collection and
consolidation of vaccination data from multiple health-care providers.
They also make it possible to generate reminder and recall notifications
and assess vaccination coverage in defined geographic areas. Immunization-
information systems (IISs) are registries that have additional capabilities,
such as vaccine management, adverse-event reporting, lifespan vaccination
histories, and linkages with electronic data (CDC, 2005). According to a
report of the National Vaccine Advisory Committee (NVAC), IISs have been
demonstrated to improve immunization coverage, support vaccine safety,
increase timeliness of immunization, and help in the study of immuniza-
tion effectiveness in children (Hinman et al., 2007). IISs can also prevent
unnecessary immunizations by giving providers a single source for patients’
immunization histories (Yawn et al., 1998), reduce “no-show” rates, reduce
vaccine waste, save staff costs by avoiding manual review of multiple re-
cords, aid in the establishment of Healthcare Effectiveness Data and Infor-
mation Set (HEDIS) performance measures, and avoid costs associated with
the National Immunization Survey (NIS) (Bartlett et al., 2007).
The development of IISs began in 1993 when CDC started to award
planning grants to develop registries in every state (CDC, 2001a). President
Clinton established the national Childhood Immunization Initiative by
directing the secretary of health and human services to work with states
to build “an integrated immunization registry system.” That initiative led
to the Initiative on Immunization Registries, which was spearheaded by
the NVAC with support from CDC’s National Immunization Program and
the Department of Health and Human Services National Vaccine Program
Office (Bartlett et al., 2007). Since 1994, CDC’s National Center for Im-
munization and Respiratory Diseases (formerly the National Immunization
Program) has provided funding to 64 grantees (all 50 states, 6 cities, and
the US territories) through Section 317 of the Public Health Service Act for
the development of IISs. From 1994 through 2001, $181.3 million was al-
located by CDC, and an additional $20 million was provided by the Robert
Wood Johnson Foundation (CDC, 2001a). Only one state had reported no
efforts to develop and implement an IIS as of December 31, 2005 (Hinman
et al., 2007). However, three other states did not report to CDC in 2005
the percentage of children younger than 6 years old who participated in an
IIS, and this might indicate inadequacy of IISs in those states.
CDC has indicated a commitment to support the continued develop-
ment and expansion of state and community IISs (CDC, 2008b) and has a
goal of including more than 95% of children under 6 years old in grantee
IISs by 2010. To address wide variation in the performance of IISs na-
tionally, CDC required detailed business plans from grantees in 2006 to
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 127
describe operational and financial objectives of the systems. In addition, a
technical work group was established to develop approaches to measuring
performance of the systems against 12 functional standards (Hinman et al.,
2007). There are also plans to develop an IIS certification process.
As the state and community IISs develop, they are increasingly used for
broader purposes, such as emergency preparedness and response (Boom et
al., 2007), monitoring the impact of vaccine shortages (Allred et al., 2006),
and monitoring the use of new vaccines. There have also been calls to
integrate IISs with other child-information systems—such as vital registra-
tion, newborn dried-blood spot screening, and early hearing detection and
intervention (Saarlas et al., 2004)—and to expand the systems to include
adolescents and adults. The NVAC reported that as of 2005, 87% of CDC
grantees included adolescents in their IISs, and 75% included information
on persons 50 years old and older (Hinman et al., 2007).
In 2009, the NVAC issued new recommendations for federal adult im-
munization programs (HHS, 2009).One recommendation was that CDC
and the Health Resources and Services Administration (HRSA) devote
resources to the inclusion of adult immunization records in all grantee
IISs, and another was that all grantees be required to implement adult im-
munization activities and adopt ACIP recommendations for routine adult
immunization.
Recommendation 4-4. States should be encouraged to expand
immunization-information systems to include adolescents and adults.
• Systems should allow the sharing of information between states so
that immunization status can be tracked when people move from
state to state.
• Vaccine registries should include adult populations, such as incar-
cerated persons, IDUs, and people who have STDs.
• Data sharing on vaccination status should be established between
correctional facilities and public-health departments.
Barriers to Hepatitis B Vaccination
Mistrust of Vaccination
Like other childhood vaccinations, hepatitis B vaccination is some-
times refused because patients or parents of children have concerns about
the safety of a vaccine (Allred et al., 2005; Gust et al., 2008; Smith et al.,
2006a). The committee is unaware of credible evidence of serious harms
caused by the hepatitis B vaccine in its many forms. In a 2002 scientific
review by the Institute of Medicine, the hepatitis B vaccine was not found
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
128 HEPATITIS AND LIVER CANCER
to be associated with adverse health outcomes (IOM, 2002). The commit-
tee believes that it is one of the safest vaccines available. The efforts of
groups opposed to vaccination present a serious obstacle to comprehensive
vaccination coverage, which is essential for the prevention and control of
hepatitis B in the United States.
Payment for Vaccines
Insurance Coverage
Health-insurance coverage for the nonelderly population (less than
65 years old) is provided by employers (63%) and public programs (11%
by the Medicaid/Children’s Health Insurance Program and 2% by other
public programs) or is acquired by individuals in the private market (5%)
(Holahan and Cook, 2008). Some 17% of Americans under 65 years old
were reported as chronically uninsured in 2007, but as many as one-third
of Americans were uninsured for at least some of the time in 2007–2008
(Families USA, 2009b). Robust coverage for vaccinations, including hepa-
titis B vaccination, is provided by public insurance plans (Table 4-5). Pri-
vate insurance plans have variable coverage for vaccinations and various
degrees of cost-sharing. Insurance coverage for vaccinations also varies
substantially with age: children under 5 years old and people 65 years old
and over have high rates of private or public coverage (89% and nearly
100%, respectively). People 18–64 years old have much lower rates (50%)
because of lack of insurance, inadequate insurance, and the absence of a
public safety net for recommended adult vaccinations (IOM, 2003).
Insurance coverage has been demonstrated to have an important impact
on access to preventive and other health services and on health outcomes
(IOM, 2009). Studies in children involving various vaccine series, including
hepatitis B vaccine, and in adults transitioning to Medicare have shown
notable increases in vaccination rates in those with insurance coverage
(IOM, 2009). In an NIS sample of children 19–24 months old, recom-
mended vaccination completion rates were found in 76% of the children
covered by private insurance, 70% of the children covered by Medicaid or
the Children’s Health Insurance Program (CHIP), and 53% of uninsured
children (Smith et al., 2006b).
Public Vaccine Programs and Insurance
Vaccines for Children program. Children with no private insurance
may be covered up to the age of 18 years by the Vaccines for Children
(VFC) program administered by CDC (CDC, 2003). The VFC program
was created by the Omnibus Budget Reconciliation Act of 1993 as a new
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 129
entitlement program to be a required part of each state’s Medicaid plan.
The program began in October 1994. The Office of Management and
Budget approves funding for the VFC program. Funding is through the
Centers for Medicare and Medicaid Services to CDC, and awards are made
to eligible grantees. The VFC program provides a vaccination entitlement
for Medicaid-eligible, uninsured, American Indian and Alaska Native, and
underinsured children who receive vaccines at federally qualified health
centers (FQHCs). The VFC program negotiates vaccine prices at the federal
level and allocates credits to states to distribute vaccines free of charge. The
program does not cover any of the practice-based costs associated with the
administration of the vaccines. Payment for vaccine administration is gen-
erally sought from specific insurance programs, such as Medicaid, or from
parents of VFC-eligible, non-Medicaid children (CDC, 2003).
Section 317 Immunization Grant program. The Section 317 Immuni-
zation Grant Program is a federal discretionary grants program for states
and other US jurisdictions (CDC, 2009d). It was established by the 1962
Vaccination Assistance Act and was the primary source of federal funds
for vaccine purchase until it was supplanted by the VFC program in 1993.
Unlike the VFC program, Section 317 provides federal funds for both vac-
cine purchase and vaccine-related infrastructure, such as population needs
assessments, surveillance, compliance monitoring, training, and school-
based delivery systems. The program targets immunization coverage for
underinsured children and youths not eligible for the VFC program and to
a small degree uninsured and underinsured adults. In 2007, CDC created
the Adult Hepatitis B Vaccine Initiative by using savings from Section 317
funds to provide free vaccine for high-risk adults in various community
settings. Funding for vaccine costs totaled $36 million in the first 2 years;
this resulted in the delivery of over 581,000 doses, 343,000 of which re-
portedly were administered. The initiative involves 56 grantees that enroll
2,437 sites, of which 38% are local health departments, 20% STD clinics,
13% primary-care loci, 11% jails or prisons, and 18% other sites, includ-
ing substance-abuse and HIV centers (personal communication, J. Ward,
CDC, July 30, 2009). However, Section 317 funding for adult vaccination
initiatives does not support the infrastructure and medical-supply costs to
deliver vaccines to people at highest risk.
Children’s Health Insurance Program. The federal CHIP was estab-
lished in 1997 under Title IX of the Social Security Act and was expanded
in the Children’s Health Insurance Program Reauthorization Act of 2009
(CMS, 2009). It is a federal block-grant program that requires state match-
ing funds to expand health-care coverage to children under 18 years old
and pregnant women who do not meet income eligibility requirements for

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
130 HEPATITIS AND LIVER CANCER
TABLE 4-5 Public Health-Insurance Plans
Coverage Medical Management Vaccination Target Audience Cost Structure
US Department of Veterans
Affairs
7.9 million beneficiaries
Hepatitis B, hepatitis C Dedicated hepatitis program 100% Veterans
a
No cost-sharing except for
non-service-connected veterans
FEHBP
8 million beneficiaries
Hepatitis B, hepatitis C Coverage similar to private
plans
Coverage similar to private
plans
Federal employees Coverage similar to private
plans
MEDICARE Parts A, B,
C, D
49 million beneficiaries
Hepatitis B, hepatitis C Yes High-risk group coverage People at least 65 years
old, disabled, people with
end-stage renal disease
Coinsurance or copayment
applies only after yearly
deductible has been met
MEDICAID, EPSDT
43 million beneficiaries
Hepatitis B, hepatitis C Yes Yes Low-income people,
families with children,
SSI recipients, pregnant
women
b
States have option to impose
nominal copayment for
beneficiaries on basis of
income
VFC Hepatitis B No Yes Children up to 18 years
old who are uninsured,
Medicaid-eligible,
underinsured, American
Indians, Alaska Natives
Covers cost of vaccines but
not administration; providers
can charge administration
fee for non–Medicaid-eligible
children
SECTION 317
Immunization Program
Hepatitis B No Yes Children, adolescents not
served by VFC program;
small percentage (about
5%) used for adults
Can be used for infrastructure;
annual appropriations vary
CHIP
7.4 million child, 334,000
adult
beneficiaries
Hepatitis B Yes Yes Insurance benefits for
children up to 18 years old
(for families making no
more than $44,100/year)
whose income is too high
for Medicaid, too low for
private insurance
Block grant program: federal
match more than Medicaid
match
a
Household income limitations apply.
b
Covers children under 6 years old and pregnant women whose family income is no more
than 133% of federal poverty level and children 6–18 years old below 100% of federal poverty
level.
Abbreviations: EPSDT, Early Periodic Screening, Diagnosis, and Treatment program; FEHBP,
Federal Employees Health Benefits Program; VFC, Vaccines for Children; CHIP, Children’s
Health Insurance Program.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 131
TABLE 4-5 Public Health-Insurance Plans
Coverage Medical Management Vaccination Target Audience Cost Structure
US Department of Veterans
Affairs
7.9 million beneficiaries
Hepatitis B, hepatitis C Dedicated hepatitis program 100% Veterans
a
No cost-sharing except for
non-service-connected veterans
FEHBP
8 million beneficiaries
Hepatitis B, hepatitis C Coverage similar to private
plans
Coverage similar to private
plans
Federal employees Coverage similar to private
plans
MEDICARE Parts A, B,
C, D
49 million beneficiaries
Hepatitis B, hepatitis C Yes High-risk group coverage People at least 65 years
old, disabled, people with
end-stage renal disease
Coinsurance or copayment
applies only after yearly
deductible has been met
MEDICAID, EPSDT
43 million beneficiaries
Hepatitis B, hepatitis C Yes Yes Low-income people,
families with children,
SSI recipients, pregnant
women
b
States have option to impose
nominal copayment for
beneficiaries on basis of
income
VFC Hepatitis B No Yes Children up to 18 years
old who are uninsured,
Medicaid-eligible,
underinsured, American
Indians, Alaska Natives
Covers cost of vaccines but
not administration; providers
can charge administration
fee for non–Medicaid-eligible
children
SECTION 317
Immunization Program
Hepatitis B No Yes Children, adolescents not
served by VFC program;
small percentage (about
5%) used for adults
Can be used for infrastructure;
annual appropriations vary
CHIP
7.4 million child, 334,000
adult
beneficiaries
Hepatitis B Yes Yes Insurance benefits for
children up to 18 years old
(for families making no
more than $44,100/year)
whose income is too high
for Medicaid, too low for
private insurance
Block grant program: federal
match more than Medicaid
match
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
132 HEPATITIS AND LIVER CANCER
Medicaid but cannot afford private health insurance. CHIP is available to
citizens and some legal immigrants, and states can charge a premium for
coverage and impose cost-sharing based on income. States have the option
of using the grant money to establish independent insurance programs or to
expand eligibility criteria for Medicaid; in the latter case, the coverage must
conform to Medicaid requirements. Currently, 39 states have programs that
are not expansions of Medicaid. Non-Medicaid CHIP programs must pro-
vide coverage for ACIP-recommended immunizations, including hepatitis B,
and must meet a federally established minimal overall coverage.
Public programs for adults. Nonelderly adults have more limited ac-
cess to publicly funded vaccination programs and public insurance benefits
than children. Adults enrolled in Medicaid make up 25% of enrollees and
are provided coverage for vaccinations, but the coverage varies between
states. Most states provide coverage based on ACIP standards, including
hepatitis B immunization. However, cost-sharing is common, and payment
of providers varies from fixed-fee schedules, which allow separate billing
for vaccine administration (Rosenbaum et al., 2003). Elderly adults covered
under Medicare and enrolled in Medicare Part B are covered for hepatitis B
vaccination if they fall into ACIP-designated high-risk or intermediate-risk
populations. The Medicare Part B deductible must be met, and the relevant
copayment or coinsurance is applicable to the hepatitis B coverage (CMS,
2008).
Federal law generally restricts coverage for adults under CHIP to preg-
nant women but does permit coverage of adults without dependent children
under special waivers from the federal government. Eleven states are pro-
viding coverage to low-income adults under such waivers. The 2009 CHIP
reauthorization act will phase out funding for such waivers by 2011 and
thereby eliminate this public source of adult-vaccination coverage (Families
USA, 2009a). Public Health Service Section 317 grants amounted to $527
million in 2008 and allow vaccination coverage for uninsured and under-
insured adults. Nearly all the money, however, was used for vaccinating
children and youths. In 2005, it was reported that less than 5% of Section
317 funding was used for adult-vaccination efforts (Mootrey, 2007).
Private Insurance Plans
Employers provide over 66% of all health insurance for 177 million
Americans under the age of 65 years (U.S. Census Bureau, 2007). Trends in
private health-insurance coverage have reflected a shift from comprehensive
coverage with low out-of-pocket costs (health maintenance organizations,
HMOs) to broader access, network-driven, and higher-cost–sharing health
plans (preferred provider organization, PPOs) (Figure 4-2). The latter offer
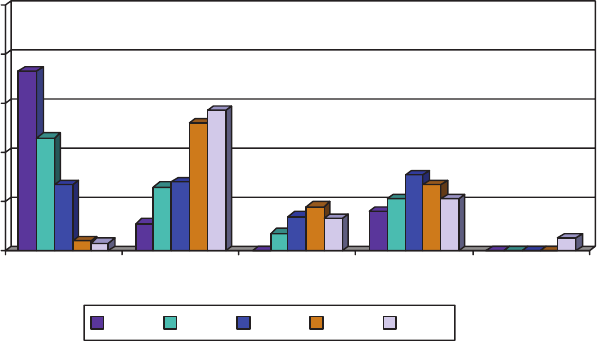
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 133
free choice of providers and hospitals but require out-of-pocket spending
(deductibles) by the consumer before coverage under the plan, cost-sharing
when the plan does provide coverage (flat dollar copayments or coinsur-
ance payments), and different levels of coverage for the same service when
acquired in-network versus out-of-network. PPO insurance arrangements
now cover more than 58% of all persons who have employment-based
health insurance (Figure 4-2).
Coverage for hepatitis B and other ACIP-recommended vaccinations is
routine in HMOs but variable in PPOs and other private insurance plans.
There is little or no cost-sharing for vaccinations and other preventive ser-
vices in HMOs, whereas it is greater in PPOs and other health plans because
of applicable deductibles and coinsurance or copayments. Cost-sharing is
greatest in health plans that have very high deductibles (high-deductible
health plans, HDHPs). In 2008, 8% of all privately insured Americans
were covered by HDHPs with annual deductibles of $1,000 or more (Kaiser
Family Foundation and HRET, 2008). HDHPs can pose formidable barri-
ers to preventive care and vaccination unless these services are specifically
exempted from the deductible or enrollees are provided a separate source
of funds to pay for them (for example, a reimbursement arrangement or a
FIGURE 4-2 Trends in private health-insurance coverage.
Abbreviations: PPO, preferred provider organization; POS, point of service; HMO,
health maintenance organization; HDHP, high-deductible health plan.
SOURCES: HIAA, 1988; Kaiser Family Foundation and HRET, 2008; KPMG,
1996.
0%
20%
40%
60%
80%
100%
Indemnity PPO POS HMO HDHP
1988 1993 1996 2002 2007
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
134 HEPATITIS AND LIVER CANCER
funded health savings account). To ensure vaccination coverage for children
under private insurance arrangements, most states have mandates for child-
hood immunizations. The regulations may also prohibit cost-sharing in the
form of deductibles or coinsurance for those services (American Academy
of Pediatrics, 2008). State mandates for recommended adult vaccinations
are less common. Employers that use national health plans are exempted
from the state mandates because their health-benefit plans operate under
the federal Employee Retirement Security Act which pre-empts state laws
that govern these plans.
Gaps and Barriers
Coverage for hepatitis B vaccination and other ACIP-recommended
vaccinations is greater for children and youths than for adults. Federal and
state funding for hepatitis B vaccination and other vaccinations provides a
safety net for the poorest children and youths, but no such program, such as
an adult version of the VFC program, exists for uninsured or underinsured
adults. Public Health Section 317 provides a potential vehicle for filling that
void, but funding has been increased only modestly since 2003 (Rodewald,
2008), and only recently has adult hepatitis B vaccination become a target
for some of the Section 317 funds. CDC has reported to Congress that it
would take about $1.6 billion—or 3 times the amount of current Section
317 funding—to fill gaps in coverage and support to states to provide a
rigorous national vaccination program for children, adolescents, and adults,
including $335 million for payments to providers for all vaccine adminis-
tration (CDC, 2009d). In that report, CDC included only $49 million for
hepatitis B vaccine purchase for 675,000 high-risk adults in a total high-risk
population of 4.5 million people who visit STD-HIV and drug-treatment
centers—a 15% uptake. If uptake at those venues reached 74% for the
first dose, as was observed at a San Diego STD clinic that combined free
vaccination with counseling (CDC, 2002), the cost for hepatitis B vaccine
purchase alone would approach $80 million.
Except for Medicaid’s Early Periodic Screening, Diagnosis, and Treat-
ment entitlement, public-health insurance often contains cost-sharing,
which may create a barrier to vaccination for some people. Adults covered
by Medicaid and Medicare and those being phased out of CHIP coverage
must share the costs of hepatitis B vaccination. Families of non-Medicaid
VFC-covered children may be responsible for the administration portion
of the vaccination cost.
Private health insurance has gaps for vaccination coverage because it
does not universally cover all ACIP-recommended vaccinations for children
and adults. Furthermore, most privately insured persons are required to pay
to receive vaccinations. More than two-thirds of privately insured persons
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 135
are enrolled in non-HMO health plans that require deductibles to be met
before plan coverage and require out-of-pocket expenditures for services.
Recommendation 4-5. Private and public insurance coverage for hepa-
titis B vaccination should be expanded.
• Public Health Section 317 should be expanded with sufficient fund-
ing to become the public safety net for underinsured and uninsured
adults to receive the hepatitis B vaccination.
• All private insurance plans should include coverage for all ACIP-
recommended vaccinations. Hepatitis B vaccination should be free
of any deductible so that first-dollar coverage exists for this preven-
tive service.
Vaccine Accessibility
The hepatitis B vaccine is available at some physician offices, des-
ignated health clinics, and some community-based outreach programs.
However, many health-care providers’ offices do not offer vaccination, and
many US cities do not have health clinics or community-based programs
that provide the hepatitis B vaccine (Rein et al., 2010). Making the vaccine
available through nontraditional settings, such as pharmacies, would prob-
ably increase hepatitis B immunization rates in the United States. Previous
studies have shown that use of nontraditional settings, such as pharmacies
and supermarkets, to deliver vaccines to US adults results in increased ac-
cessibility and convenience, reduced cost, and increased public awareness
of the need for adult immunization (Postema and Breiman, 2000). That
strategy is also likely to be cost-saving (Prosser et al., 2008). The involve-
ment of community pharmacies in vaccine distribution and administration
has been growing in recent years (Westrick et al., 2009), and enlisting
their participation in public delivery of the hepatitis B vaccine is a natural
progression.
Vaccine-Supply Concerns
Several studies of vaccine supply in the United States have expressed
concerns regarding vaccine shortages (Jacobson et al., 2006; Klein and
Myers, 2006; Santoli et al., 2004). Reasons for vaccine shortages include
cessation of production by manufacturers due to lack of profitability, li-
ability issues, problems with vaccine production, and unanticipated vaccine
demands (Klein and Myers, 2006; Santoli et al., 2004). From 2000 to 2004,
there were shortages of six pediatric vaccines: combined tetanus–diphtheria
toxoids (November 2000–June 2002), diphtheria–tetanus–acellular pertus-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
136 HEPATITIS AND LIVER CANCER
sis (March 2001–July 2002), pneumococcal conjugate (September 2001–
March 2003 and February 2004–September 2004), measles–mumps–rubella
(October 2001–July 2002), and varicella (October 2001–August 2002)
(Jacobson et al., 2006).
Although there has not been a national shortage of the hepatitis B
vaccine, temporary supply problems occurred with this vaccine in 2008
(adult and dialysis formulations of Recombivax HB) and 2009 (pediatric
formulations of Recombivax HB and Pediatric Engerix-B) (CDC, 2009a).
A shortage was avoided because other manufacturers were able to provide
an adequate supply of the vaccine in adult and dialysis formulations, and
CDC released doses of pediatric vaccine from its stockpile.
Recommendation 4-6. The federal government should work to ensure
an adequate, accessible, and sustainable hepatitis B vaccine supply.
HEPATITIS C VACCINE
Efforts are going on to develop a vaccine for hepatitis C, and several
candidates are in phase I and phase II clinical trials (Inchauspe and Michel,
2007). Although some vaccines are being developed to treat people with
chronic HCV infection (that is, therapeutic vaccines), this section focuses on
vaccines to prevent chronic HCV infection. An incomplete understanding
of how chronic HCV infection is spontaneously controlled in some people
and antigenic variability of the virus remain barriers to development of a
vaccine to prevent chronic hepatitis C.
Feasibility of Preventing Chronic Hepatitis C
The outcomes of HCV infections in humans and chimpanzees suggest
that it may be possible to develop a vaccine to prevent HCV infection.
Spontaneous clearance of the virus in 15–45% of persons after acute HCV
infection demonstrates that immunity can prevent chronic infection and its
long-term consequences, such as cirrhosis and hepatocellular carcinoma
(HCC) (Alter et al., 1992; Barrera et al., 1995; Villano et al., 1999; Vogt
et al., 1999). It also seems that immunity can be conditioned by prior ex-
posure: humans and chimpanzees that recover from HCV infection appear
to control a second infection better (the peak of viremia is lower than in
the initial infection, and the chance of recovery is greater compared with
that in persons not previously infected) (Lanford et al., 2004; Major et al.,
2002; Mehta et al., 2002). In addition, IDUs who recovered from earlier
HCV infections and have continuing HCV exposure have substantially less
viremia than those who have similar exposure but had no earlier infection
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 137
(Mehta et al., 2002). Some hepatitis C vaccine candidates have shown
similar potential (Forns et al., 2000; Weiner et al., 2001).
Although those clinical observations suggest that it is possible to de-
velop a vaccine to prevent chronic HCV infection, there are important chal-
lenges. Immunity produced by natural infection does not prevent reinfection
(that is, it is not sterilizing); such immunity reduces the frequency of chronic
infection but does not prevent it (Farci et al., 1992). Moreover, the im-
munologic correlates of those critical clinical outcomes are not sufficiently
understood for rational design or evaluation of vaccine products. Marked
genetic variability in some HCV epitopes creates an especially formidable
challenge if immunity to them is necessary for protection.
Need for a Vaccine to Prevent Chronic Hepatitis C
Although HCV infections occur in the general population of the United
States and other economically developed countries, the incidence is prob-
ably too low to justify universal HCV vaccination. A hepatitis C vaccine
is most likely to benefit populations that are at highest risk, include IDUs,
health-care workers who perform high-risk procedures, and some men who
report high-risk sexual practices with other men. A vaccine that prevents
chronic HCV infection not only might reduce the likelihood of long-term
disease, such as cirrhosis or HCC, but might reduce the likelihood of
secondary transmission by reducing the infection reservoir. It may not be
possible to produce a vaccine that prevents HCV infection, but a product
that prevents acute HCV infections from becoming chronic would probably
achieve many of the same benefits. In cases where acute HCV infection does
not resolve within a few months, early treatment can prevent most cases
from evolving into chronic HCV infection. However, because most acute
HCV infections are not recognized, a vaccine is further likely to be of great-
est benefit to populations in whom acute infection is rarely recognized and
treated (for example, IDUs).
Cost Effectiveness of a Hepatitis C Vaccine
Estimates of the cost effectiveness of hepatitis C vaccination depend
on a number of factors, including the cost of the vaccine, the target popu-
lation’s incidence, and projections of its effectiveness and duration. Several
studies have evaluated the potential cost effectiveness of an HCV vaccine
that prevents acute (and chronic) infection. Krahn et al. (2005) calculated
that if a hepatitis C vaccine with 80% efficacy was available, had a dura-
tion of effectiveness equivalent to that of the hepatitis B vaccine, and was
cost-equivalent to that of the current hepatitis A vaccine ($51 per dose plus
administration fees), it would be cost saving to vaccinate IDUs. The authors
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
138 HEPATITIS AND LIVER CANCER
also reported that vaccination of average-risk school-age children with such
a vaccine would be cost-effective. The cost would be about $18,000 per
QALY gained. Massad et al. (2009), on the basis of HCV incidence data for
Sao Paolo, Brazil, calculated that a 100% effective hepatitis C vaccine that
provides lifelong immunity and costs $300 per dose would cost $748,991
per death averted. If only high-risk people (for example, IDUs) were vacci-
nated, the cost would be $131,305 per death averted. If the hepatitis C vac-
cine had only 80% efficacy and lifelong duration, it would cost $242,667
per death averted if given only to high-risk people.
The committee recognizes the need for a safe, effective, and affordable
hepatitis C vaccine. Such a vaccine could substantially enhance hepatitis C
prevention efforts.
Recommendation 4-7. Studies to develop a vaccine to prevent chronic
hepatitis C virus infection should continue.
REFERENCES
ACIP (Advisory Committee on Immunization Practices). 1991. Hepatitis B virus: A com-
prehensive strategy for eliminating transmission in the United States through univer-
sal childhood vaccination. Recommendations of the immunization practices advisory
committee (ACIP). Morbidity and Morality Weekly: Recommendations and Reports
40(RR-13):1-25.
Allred, N. J., K. M. Shaw, T. A. Santibanez, D. L. Rickert, and J. M. Santoli. 2005. Parental
vaccine safety concerns: Results from the national immunization survey, 2001-2002.
American Journal of Preventive Medicine 28(2):221-224.
Allred, N. J., J. M. Stevenson, M. Kolasa, D. L. Bartlett, R. Schieber, K. S. Enger, and A. Shefer.
2006. Using registry data to evaluate the 2004 pneumococcal conjugate vaccine shortage.
American Journal of Preventive Medicine 30(4):347-350.
Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y.
Hu, J. K. Miller, M. A. Gerber, R. E. Sampliner, et al. 1992. The natural history of com-The natural history of com-
munity-acquired hepatitis C in the United States. The sentinel counties chronic non-A,
non-B hepatitis study team. New England Journal of Medicine 327(27):1899-1905.
Altice, F. L., R. D. Bruce, M. R. Walton, and M. I. Buitrago. 2005. Adherence to hepatitis B
virus vaccination at syringe exchange sites. Journal of Urban Health 82(1):151-161.
American Academy of Pediatrics. 1961. Vitamin K compounds and the water-soluble ana-
logues: Use in therapy and prophylaxis in pediatrics. Pediatrics 28:501-507.
———. 1980. Prophylaxis and treatment of neonatal gonococcal infections. Pediatrics 65(5):
1047-1048.
———. 2008. State legislation report. http://www.aap.org/advocacy/statelegrpt.pdf (accessed
August 21, 2009).
———. 2009. Section 3. Summaries of infectious diseases: hepatitis B. Edited by L. K.
Pickering, C. J. Baker, D. W. Kimberlin, and S. S. Long, Red book: 2009 report of
the committee on infectious diseases. Elk Grove Village, IL: American Academy of
Pediatrics.
Ashton, M. R., R. L. Cook, H. C. Wiesenfeld, M. A. Krohn, T. Zamborsky, S. H. Scholle,
and G. E. Switzer. 2002. Primary care physician attitudes regarding sexually transmitted
diseases. Sexually Transmitted Diseases 29(4):246-251.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 139
Bailey, C. L., V. Smith, and M. Sands. 2008. Hepatitis B vaccine: A seven-year study of adher-
ence to the immunization guidelines and efficacy in HIV-1-positive adults. International
Journal of Infectious Diseases 12(6):e77-e83.
Barrera, J. M., M. Bruguera, M. G. Ercilla, C. Gil, R. Celis, M. P. Gil, M. del Valle Onorato,
J. Rodes, and A. Ordinas. 1995. Persistent hepatitis C viremia after acute self-limiting
posttransfusion hepatitis C. Hepatology 21(3):639-644.
Bartlett, D. L., M. L. Washington, A. Bryant, N. Thurston, and C. A. Perfili. 2007. Cost savings
associated with using immunization information systems for vaccines for children admin-
istrative tasks. Journal of Public Health Management and Practice 13(6):559-566.
Boom, J. A., A. C. Dragsbaek, and C. S. Nelson. 2007. The success of an immunization infor-
mation system in the wake of Hurricane Katrina. Pediatrics 119(6):1213-1217.
Bull, S. S., C. Rietmeijer, J. D. Fortenberry, B. Stoner, K. Malotte, N. Vandevanter, S. E.
Middlestadt, and E. W. Hook, 3rd. 1999. Practice patterns for the elicitation of sexual
history, education, and counseling among providers of STD services: Results from the
gonorrhea community action project (GCAP). Sexually Transmitted Diseases 26(10):
584-589.
Campbell, J. V., R. S. Garfein, H. Thiede, H. Hagan, L. J. Ouellet, E. T. Golub, S. M. Hudson,
D. C. Ompad, and C. Weinbaum. 2007. Convenience is the key to hepatitis A and B vac-
cination uptake among young adult injection drug users. Drug and Alcohol Dependence
91(Suppl 1):S64-S72.
CDC (Centers for Disease Control and Prevention). 2000. Vaccination coverage among
adolescents 1 year before the institution of a seventh grade school entry vaccination
requirement—San Diego, California, 1998. Morbidity and Mortality Weekly Report
49(5):101-102, 111.
———. 2001a. Development of community- and state-based immunization registries. CDC
response to a report from the National Vaccine Advisory Committee. Morbidity and
Morality Weekly: Recommendations and Reports 50(RR-17):1-17.
———. 2001b. Effectiveness of a middle school vaccination law—California, 1999-2001.
Morbidity and Mortality Weekly Report 50(31):660-663.
———. 2002. Hepatitis B vaccination among high-risk adolescents and adults—San Diego,
California, 1998-2001. Morbidity and Mortality Weekly Report 51(28):618-621.
———. 2003. Vaccines for children: Program management. http://www.cdc.gov/vaccines/
programs/vfc/projects/program-mgmt.htm#pm (accessed August 27, 2009).
———. 2005. Immunization information system progress—United States, 2004. Morbidity
and Mortality Weekly Report 54(45):1156-1157.
———. 2006. Hepatitis b vaccination coverage among adults—United States, 2004. Morbidity
and Mortality Weekly Report 55(18):509-511.
———. 2007. Vaccination coverage among children in kindergarten—United States, 2006–07
school year. Morbidity and Mortality Weekly Report 56(32):819-821.
———. 2008a. Immunization administration requirements for correctional inmates and resi-
dents. http://www2a.cdc.gov/nip/stateVaccApp/StateVaccsApp/AdministrationbyPatient
Type.asp?PatientTypetmp=Correctional%20Inmates%20and%20Residents (accessed
July 11, 2008).
———. 2008b. Immunization information systems progress—United States, 2006. Morbidity
and Mortality Weekly Report 57(11):289-291.
———. 2008c. Newborn hepatitis B vaccination coverage among children born January 2003-
June 2005—United States. Morbidity and Mortality Weekly Report 57(30):825-828.
———. 2009a. Current vaccine shortages and delays. http://www.cdc.gov/vaccines/vac-gen/
shortages/default.htm (accessed June 11, 2009).
———. 2009b. Hepatitis B vaccine birth dose rates, national immunization survey. http://
www.cdc.gov/Hepatitis/Partners/PeriHepBCoord.htm (accessed December 14, 2009).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
140 HEPATITIS AND LIVER CANCER
———. 2009c. National, state, and local area vaccination coverage among children aged
19-35 months—United States, 2008. Morbidity and Mortality Weekly Report 58(33):
921-926.
———. 2009d. Report to congress on section 317 immunization program. Senate appropria-
tions committee. http://www.317coalition.org/legislativeupdate/senate317reportfinal.pdf
(accessed August 21, 2009).
Charuvastra, A., J. Stein, B. Schwartzapfel, A. Spaulding, E. Horowitz, G. Macalino, and J. D.
Rich. 2001. Hepatitis B vaccination practices in state and federal prisons. Public Health
Reports 116(3):203-209.
Christensen, P. B., N. Fisker, H. B. Krarup, E. Liebert, N. Jaroslavtsev, K. Christensen, and
J. Georgsen. 2004. Hepatitis B vaccination in prison with a 3-week schedule is more ef-
ficient than the standard 6-month schedule. Vaccine 22(29-30):3897-3901.
CMS (Centers for Medicare and Medicaid Services). 2008. Adult immunizations. http://www.
cms.hhs.gov/MLNProducts/downloads/Adult_Immunization.pdf (accessed August 21,
2009).
———. 2009. Overview of the Children’s Health Insurance Program (CHIP). http://www.cms.
hhs.gov/LowCostHealthInsFamChild/ (accessed August 27, 2009).
Daniels, D., S. Grytdal, and A. Wasley. 2009. Surveillance for acute viral hepatitis—United
States, 2007. Morbidity and Mortality Weekly Report: Surveillance Summaries 58(3):
1-27.
Darling, N. J., L. E. Barker, A. M. Shefer, and S. Y. Chu. 2005. Immunization coverage among
Hispanic ancestry, 2003 national immunization survey. American Journal of Preventive
Medicine 29(5):421-427.
Des Jarlais, D. C., T. Diaz, T. Perlis, D. Vlahov, C. Maslow, M. Latka, R. Rockwell, V.
Edwards, S. R. Friedman, E. Monterroso, I. Williams, and R. S. Garfein. 2003. Variabil-
ity in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C
virus infection among young injecting drug users in New York city. American Journal of
Epidemiology 157(5):467-471.
Des Jarlais, D. C., D. G. Fisher, J. C. Newman, B. N. Trubatch, M. Yancovitz, D. Paone, and
D. Perlman. 2001. Providing hepatitis B vaccination to injection drug users: Referral to
health clinics vs on-site vaccination at a syringe exchange program. American Journal of
Public Health 91(11):1791-1792.
Edmunds, W. J., G. F. Medley, D. J. Nokes, A. J. Hall, and H. C. Whittle. 1993. The influ-
ence of age on the development of the hepatitis B carrier state. Proceedings. Biological
Sciences 253(1337):197-201.
Families USA. 2009a. CHIPRA 101: Overview of the CHIP reauthorization legislation.
Washington, DC: Families USA.
———. 2009b. Hidden health tax: American pay a premium. Washington, DC: Families
USA.
Farci, P., H. J. Alter, S. Govindarajan, D. C. Wong, R. Engle, R. R. Lesniewski, I. K. Mushahwar,
S. M. Desai, R. H. Miller, N. Ogata, et al. 1992. Lack of protective immunity against
reinfection with hepatitis C virus. Science 258(5079):135-140.
Fishbein, D. B., B. C. Willis, W. M. Cassidy, D. Marioneaux, C. Bachino, T. Waddington, and
P. Wortley. 2006. Determining indications for adult vaccination: Patient self-assessment,
medical record, or both? Vaccine 24(6):803-818.
Forns, X., P. J. Payette, X. Ma, W. Satterfield, G. Eder, I. K. Mushahwar, S. Govindarajan,
H. L. Davis, S. U. Emerson, R. H. Purcell, and J. Bukh. 2000. Vaccination of chim-
panzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope e2 protein
modified the infection after challenge with homologous monoclonal HCV. Hepatology
32(3):618-625.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 141
Gilbert, L. K., J. Bulger, K. Scanlon, K. Ford, D. Bergmire-Sweat, and C. Weinbaum. 2005.2005.
Integrating hepatitis B prevention into sexually transmitted disease services: U.S. sexually
transmitted disease program and clinic trends—1997 and 2001. Sexually Transmitted
Diseases 32(6):346-350.
Gold, M. R., J. E. Siegel, L. B. Russell, and M. C. Weinstein, eds. 1996. Cost-effectiveness in
health and medicine. New York: Oxford University Press.
Goldstein, S. T., M. J. Alter, I. T. Williams, L. A. Moyer, F. N. Judson, K. Mottram, M. Fleenor,
P. L. Ryder, and H. S. Margolis. 2002. Incidence and risk factors for acute hepatitis B in
the United States, 1982-1998: Implications for vaccination programs. Journal of Infec-
tious Diseases 185(6):713-719.
Gust, D. A., N. Darling, A. Kennedy, and B. Schwartz. 2008. Parents with doubts about vac-
cines: Which vaccines and reasons why. Pediatrics 122(4):718-725.
Hagan, H., J. P. McGough, H. Thiede, N. S. Weiss, S. Hopkins, and E. R. Alexander. 1999.
Syringe exchange and risk of infection with hepatitis B and C viruses. American Journal
of Epidemiology 149(3):203-213.
Harris, J. L., T. S. Jones, and J. Buffington. 2007. Hepatitis B vaccination in six STD clinics
in the United States committed to integrating viral hepatitis prevention services. Public
Health Reports 122(Suppl 2):42-47.
Hennessey, K. A., A. A. Kim, V. Griffin, N. T. Collins, C. M. Weinbaum, and K. Sabin. 2009.2009.
Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three
jails: A case for viral hepatitis prevention in jails in the United States. Journal of Urban
Health 86(1):93-105.
HHS (Department of Health and Human Services). 2009. National Vaccine Advisory
Committee recommendations for federal adult immunization programs regarding im-
munization delivery, assessment, research, and safety monitoring. http://www.hhs.gov/
nvpo/nvac/NVACAdultImmunizationsWorkingGroupJune2009.html (accessed Septem-
ber 10, 2009).
HIAA (Health Insurance Association of America). 1988. Survey from the Health Insurance
Association of America.
Hinman, A. R., G. A. Urquhart, and R. A. Strikas. 2007. Immunization information systems:
National Vaccine Advisory Committee progress report, 2007. Journal of Public Health
Management and Practice 13(6):553-558.
Holahan, J., and A. Cook. 2008. The decline in the uninsured in 2007: Why did it happen and
can it last? Commission on Medicaid and the Uninsured, Kaiser Family Foundation.
Hu, Y., L. E. Grau, G. Scott, K. H. Seal, P. A. Marshall, M. Singer, and R. Heimer. 2008.
Economic evaluation of delivering hepatitis B vaccine to injection drug users. American
Journal of Preventive Medicine 35(1):25-32.
Hutchinson, S. J., S. Wadd, A. Taylor, S. M. Bird, A. Mitchell, D. S. Morrison, S. Ahmed,
and D. J. Goldberg. 2004. Sudden rise in uptake of hepatitis B vaccination among in-
jecting drug users associated with a universal vaccine programme in prisons. Vaccine
23(2):210-214.
Hutton, D. W., D. Tan, S. K. So, and M. L. Brandeau. 2007. Cost-effectiveness of screening
and vaccinating Asian and Pacific Islander adults for hepatitis B. Annals of Internal
Medicine 147(7):460-469.
Immunization Action Coalition. 2009. State information. Hepatitis B prevention mandates:
Prenatal, daycare, and k-12. http://www.immunize.org/laws/hepb.asp (accessed June 9,
2009).
Inchauspe, G., and M. L. Michel. 2007. Vaccines and immunotherapies against hepatitis B and
hepatitis C viruses. Journal of Viral Hepatitis 14(Suppl 1):97-103.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
142 HEPATITIS AND LIVER CANCER
IOM (Institute of Medicine). 1994. Adverse events associated with childhood vaccines: Evi-
dence bearing on causality. Edited by K. R. Stratton, C. J. Howe, and R. B. J. Johnston.
Washington, DC: National Academy Press.
———. 2002. Immunization safety review: Hepatitis B vaccine and demyelinating neurologi-
cal disorders. Edited by K. Stratton, D. Almario, and M. C. McCormick. Washington,
DC: The National Academies Press.
———. 2003. Financing vaccines in the 21st century: Assuring access and availability. Public
and private insurance coverage. Washington, DC: The National Academies Press.
———. 2009. America’s uninsured crisis. Consequences for health and healthcare. Washing-
ton, DC: The National Academies Press.
Jacobson, S. H., E. C. Sewell, and R. A. Proano. 2006. An analysis of the pediatric vaccine
supply shortage problem. Health Care Management Science 9(4):371-389.
Jenkins, C. N., S. J. McPhee, C. Wong, T. Nguyen, and G. L. Euler. 2000. Hepatitis B im-
munization coverage among Vietnamese-American children 3 to 18 years old. Pediatrics
106(6):E78.
Kaiser Family Foundation and HRET. 2008. Employer health benefits 2008 annual survey.
The Kaiser Family Foundation and Health Research & Educational Trust.
Kim, S.-Y., K. Billah, T. A. Lieu, and M. C. Weinstein. 2006. Cost effectiveness of hepatitis B2006. Cost effectiveness of hepatitis B
vaccination at HIV counseling and testing sites. American Journal of Preventive Medicine
30(6):498-498.
Klein, J. O., and M. G. Myers. 2006. Vaccine shortages: Why they occur and what needs to
be done to strengthen vaccine supply. Pediatrics 117(6):2269-2275.
Koff, R. S. 2000. Hepatitis B school-based vaccination programmes in the USA: A model for
hepatitis A and B. Vaccine 18(Suppl 1):S77-S79.
KPMG. 1996. Survey of employer-sponsored health benefits, 1993.
Krahn, M. D., A. John-Baptiste, Q. Yi, A. Doria, R. S. Remis, P. Ritvo, and S. Friedman. 2005.
Potential cost-effectiveness of a preventive hepatitis C vaccine in high risk and average
risk populations in Canada. Vaccine 23(13):1549-1558.
Kuo, I., D. W. Mudrick, S. A. Strathdee, D. L. Thomas, and S. G. Sherman. 2004. Poor valid-
ity of self-reported hepatitis B virus infection and vaccination status among young drug
users. Clinical Infectious Diseases 38(4):587-590.
Lanford, R. E., B. Guerra, D. Chavez, C. Bigger, K. M. Brasky, X. H. Wang, S. C. Ray, and
D. L. Thomas. 2004. Cross-genotype immunity to hepatitis C virus. Journal of Virology
78(3):1575-1581.
Lum, P. J., J. A. Hahn, K. P. Shafer, J. L. Evans, P. J. Davidson, E. Stein, and A. R. Moss. 2008.
Hepatitis B virus infection and immunization status in a new generation of injection drug
users in San Francisco. Journal of Viral Hepatitis 15(3):229-236.
Lum, P. J., K. C. Ochoa, J. A. Hahn, K. Page Shafer, J. L. Evans, and A. R. Moss. 2003. Hepa-
titis B virus immunization among young injection drug users in San Francisco, Calif: The
UFO study. American Journal of Public Health 93(6):919-923.
Luman, E. T., L. E. Barker, M. M. McCauley, and C. Drews-Botsch. 2005. Timeliness of
childhood immunizations: A state-specific analysis. American Journal of Public Health
95(8):1367-1374.
Maheux, B., N. Haley, M. Rivard, and A. Gervais. 1995. STD risk assessment and risk-reduction
counseling by recently trained family physicians. Academic Medicine 70(8):726-728.
Major, M. E., K. Mihalik, M. Puig, B. Rehermann, M. Nascimbeni, C. M. Rice, and
S. M. Feinstone. 2002. Previously infected and recovered chimpanzees exhibit rapid
responses that control hepatitis C virus replication upon rechallenge. Journal of Virology
76(13):6586-6595.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 143
Massad, E., F. A. Coutinho, E. Chaib, and M. N. Burattini. 2009. Cost-effectiveness analysis
of a hypothetical hepatitis C vaccine compared to antiviral therapy. Epidemiology and
Infection 137(2):241-249.
Mast, E. E., and J. W. Ward. 2008. Chapter 13. Hepatitis B vaccines. In Vaccines. 5th ed,
edited by S. A. Plotkin, W. A. Orenstein, and P. A. Offit: Elsevier Health Sciences.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer,
B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate
transmission of hepatitis B virus infection in the United States: Recommendations of the
advisory committee on immunization practices (ACIP) part 1: Immunization of infants,
children, and adolescents. Morbidity and Morality Weekly: Recommendations and Re-
ports 54(RR-16):1-31.
Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald,
J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immuniza-A comprehensive immuniza-
tion strategy to eliminate transmission of hepatitis B virus infection in the United States:
Recommendations of the advisory committee on immunization practices (ACIP) part II:
Immunization of adults. Morbidity and Morality Weekly: Recommendations and Reports
55(RR-16):1-33.
Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D.
Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet
359(9316):1478-1483.
Miriti, M. K., K. Billah, C. Weinbaum, J. Subiadur, R. Zimmerman, P. Murray, R. Gunn, and
J. Buffington. 2008. Economic benefits of hepatitis B vaccination at sexually transmitted
disease clinics in the U.S. Public Health Reports 123(4):504-513.
Mootrey, G. T. 2007. Presentation. Adult immunization at CDC. www.hhs.gov/nvpo/nvac/
documents/2007oct/Mootrey.ppt (accessed August 21, 2009).
Morita, J. Y., E. Ramirez, and W. E. Trick. 2008. Effect of a school-entry vaccination require-
ment on racial and ethnic disparities in hepatitis B immunization coverage levels among
public school students. Pediatrics 121(3):e547-e552.
Olshen, E., B. E. Mahon, S. Wang, and E. R. Woods. 2007. The impact of state policies on
vaccine coverage by age 13 in an insured population. Journal of Adolescent Health
40(5):405-411.
Owens, D. K. 1998. Interpretation of cost-effectiveness analyses. Journal of General Internal
Medicine 13(10):716-717.
Pisu, M., M. I. Meltzer, and R. Lyerla. 2002. Cost-effectiveness of hepatitis B vaccination of
prison inmates. Vaccine 21(3-4):312-321.
Postema, A. S., and R. F. Breiman. 2000. Adult immunization programs in nontraditional set-
tings: Quality standards and guidance for program evaluation. Morbidity and Morality
Weekly: Recommendations and Reports 49(RR-1):1-13.
Prosser, L. A., M. A. O’Brien, N. A. Molinari, K. H. Hohman, K. L. Nichol, M. L. Messonnier,
and T. A. Lieu. 2008. Non-traditional settings for influenza vaccination of adults: Costs
and cost effectiveness. Pharmacoeconomics 26(2):163-178.
Rein, D. B., S. B. Lesesne, P. J. Leese, and C. M. Weinbaum. 2010. Community-based2010. Community-based
hepatitis B screening programs in the United States in 2008. Journal of Viral Hepatitis
17(1):28-33.
Rodewald, L. 2008. Vaccination financing: Program funding and pressures, 317 opera-
tions funding: FY08-FY09. Presentation at AIM program managers meeting. http://
cdc.confex.com/recording/cdc/nic2009/ppt/free/4db77adf5df9fff0d3caf5cafe28f496/
paper18425_5.ppt#930,5,Section (accessed November 19, 2008).
Rosenbaum, S., L. Stewart, M. Cox, and A. Lee. 2003. Medicaid coverage of immunizations
for noninstitutionalized adults. Washington, DC: Center for Health Services Policy Re-
search, George Washington University.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
144 HEPATITIS AND LIVER CANCER
Rotily, M., C. Vernay-Vaisse, M. Bourliere, A. Galinier-Pujol, S. Rousseau, and Y. Obadia.
1997. HBV and HIV screening, and hepatitis B immunization programme in the prison
of Marseille, France. International Journal of STD and AIDS 8(12):753-759.
Saarlas, K. N., A. R. Hinman, D. A. Ross, W. C. Watson, Jr., E. L. Wild, T. M. Hastings, and
P. A. Richmond. 2004. All kids count 1991-2004: Developing information systems to
improve child health and the delivery of immunizations and preventive services. Journal
of Public Health Management and Practice (Suppl):S3-S15.
Samoff, E., A. Dunn, N. VanDevanter, S. Blank, and I. B. Weisfuse. 2004. Predictors of accep-
tance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sexually
Transmitted Diseases 31(7):415-420.
Santoli, J. M., J. O. Klein, G. Peter, and W. A. Orenstein. 2004. Disruptions in the supply2004. Disruptions in the supply
of routinely recommended childhood vaccines in the United States. Pediatric Infectious
Disease Journal 23(6):553-554.
Savage, R. B., M. J. Hussey, and M. B. Hurie. 2000. A successful approach to immunizing men
who have sex with men against hepatitis B. Public Health Nursing 17(3):202-206.
Seal, K. H., K. C. Ochoa, J. A. Hahn, J. P. Tulsky, B. R. Edlin, and A. R. Moss. 2000. Risk of
hepatitis B infection among young injection drug users in San Francisco: Opportunities
for intervention. Western Journal of Medicine 172(1):16-20.
Simard, E. P., J. T. Miller, P. A. George, A. Wasley, M. J. Alter, B. P. Bell, and L. Finelli. 2007.
Hepatitis B vaccination coverage levels among healthcare workers in the United States,
2002-2003. Infection Control and Hospital Epidemiology 28(7):783-790.
Smith, P. J., A. M. Kennedy, K. Wooten, D. A. Gust, and L. K. Pickering. 2006a. Association
between health care providers’ influence on parents who have concerns about vaccine
safety and vaccination coverage. Pediatrics 118(5):e1287-1292.
Smith, P. J., J. Stevenson, and S. Y. Chu. 2006b. Associations between childhood vacci-
nation coverage, insurance type, and breaks in health insurance coverage. Pediatrics
117(6):1972-1978.
Stanwyck, C. A., M. S. Kolasa, and K. M. Shaw. 2004. Immunization requirements for childcare
programs: Are they enough? American Journal of Preventive Medicine 27(2):161-163.
Szilagyi, P. G., S. Schaffer, L. Shone, R. Barth, S. G. Humiston, M. Sandler, and L. E.
Rodewald. 2002. Reducing geographic, racial, and ethnic disparities in childhood im-
munization rates by using reminder/recall interventions in urban primary care practices.
Pediatrics 110(5):e58.
U.S. Census Bureau. 2007. Historic health insurance tables. http://www.census.gov/hhes/www/
hlthins/historic/hihistt4.xls (accessed August 27, 2009).
Vallabhaneni, S., G. E. Macalino, S. E. Reinert, B. Schwartzapfel, F. A. Wolf, and J. D.
Rich. 2004. Prisoners’ attitudes toward hepatitis B vaccination. Preventive Medicine
38(6):828-833.
Vellinga, A., P. Van Damme, and A. Meheus. 1999. Hepatitis B and C in institutions for1999. Hepatitis B and C in institutions for
individuals with intellectual disability. Journal of Intellectual Disability Research 43(Pt
6):445-453.
Villano, S. A., D. Vlahov, K. E. Nelson, S. Cohn, and D. L. Thomas. 1999. Persistence of
viremia and the importance of long-term follow-up after acute hepatitis C infection.
Hepatology 29(3):908-914.
Vogt, M., T. Lang, G. Frosner, C. Klingler, A. F. Sendl, A. Zeller, B. Wiebecke, B. Langer, H.
Meisner, and J. Hess. 1999. Prevalence and clinical outcome of hepatitis C infectionPrevalence and clinical outcome of hepatitis C infection
in children who underwent cardiac surgery before the implementation of blood-donor
screening. New England Journal of Medicine 341(12):866-870.
Ward, J. W. 2008. Time for renewed commitment to viral hepatitis prevention. American
Journal of Public Health 98(5):779-781.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
IMMUNIZATION 145
Weiner, A. J., X. Paliard, M. J. Selby, A. Medina-Selby, D. Coit, S. Nguyen, J. Kansopon,
C. L. Arian, P. Ng, J. Tucker, C. T. Lee, N. K. Polakos, J. Han, S. Wong, H. H. Lu, S.
Rosenberg, K. M. Brasky, D. Chien, G. Kuo, and M. Houghton. 2001. Intrahepatic
genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. Journal
of Virology 75(15):7142-7148.
Westrick, S. C., S. Watcharadamrongkun, J. K. Mount, and M. L. Breland. 2009. Commu-
nity pharmacy involvement in vaccine distribution and administration. Vaccine 27(21):
2858-2863.
WHO (World Health Organization). 2006. Preventing mother-to-child transmission of hepa-
titis B: Operational field guidelines of delivery of the birth dose of hepatitis B vaccine
Manila: World Health Organization Western Pacific Region.
———. 2009. Table: Threshold values for intervention cost-effectiveness by region. http://
www.who.int/choice/costs/CER_levels/en/index.html (accessed November 4, 2009).
Williams, I. T., K. Boaz, and K. P. Openo. 2005. Missed opportunities for hepatitis B vac-
cination in correctional settings, sexually transmitted disease (STD) clinics, and drug
treatment programs [abstract 1031]. Paper presented at the 43rd Annual Meeting of the
Infectious Diseases Society of America, San Francisco, CA.
Workowski, K., and S. Berman. 2006. Sexually transmitted diseases: Treatment guidelines,
2006. Morbidity and Mortality Weekly Report 55(RR-11):1-94.
Yawn, B. P., L. Edmonson, L. Huber, G. A. Poland, R. M. Jacobson, and S. J. Jacobsen. 1998.
The impact of a simulated immunization registry on perceived childhood immunization
status. American Journal of Managed Care 4(2):185-192.
Zimet, G. D., J. Maehr, N. A. Constantine, and A. English. 2008. School-entry vaccination
requirements: A position statement of the society for adolescent medicine. Journal of
Adolescent Health 42(3):310-311.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
147
5
Viral Hepatitis Services
H
epatitis B virus (HBV) and hepatitis C virus (HCV) infections cause
substantial morbidity and mortality despite being preventable and
treatable. Deficiencies in the implementation of established guide-
lines for the prevention, diagnosis, and medical management of chronic
HBV and HCV infections perpetuate personal and economic burdens. This
chapter reviews the current status of services to prevent and manage chronic
hepatitis B and chronic hepatitis C. It then discusses the general components
of viral hepatitis services. The chapter ends with an assessment of gaps in
existing services, including a description of some models for services and
committee recommendations to improve viral hepatitis prevention and
management and to fill research needs. Services for the general US popu-
lation are considered first and then services for special populations and
service venues that have unique opportunities for interventions. Hepatitis
B immunization is covered in Chapter 4 and so is not discussed in detail
here.
The recommendations offered by the committee here are presented in
the context of the current health-care system in the United States. The com-
mittee believes strongly that if the system changes as a result of health-care
reform efforts, viral hepatitis services should have high priority in compo-
nents of the reformed system that deal with prevention, chronic disease, and
primary-care delivery. The committee’s recommendations regarding viral
hepatitis services are summarized in Box 5-1.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
148 HEPATITIS AND LIVER CANCER
CURRENT STATUS
Health services related to viral hepatitis prevention, screening, and
medical management are both limited and fragmented among entities at
the federal, state, and local levels. Numerous federal agencies administer
or fund some viral hepatitis–related services, including the Centers for
Disease Control and Prevention (CDC), the Health Resources and Services
BOX 5-1
Summary of Recommendations Regarding
Viral Hepatitis Services
General Population
• 5-1. Federally funded health-insurance programs—such as Medicare,
Medicaid, and the Federal Employees Health Benefits Program—
should incorporate guidelines for risk-factor screening for hepatitis B
and hepatitis C as a required core component of preventive care so
that at-risk people receive serologic testing for hepatitis B virus and
hepatitis C virus and chronically infected patients receive appropriate
medical management.
Foreign-Born Populations
• 5-2. The Centers for Disease Control and Prevention, in conjunction
with other federal agencies and state agencies, should provide re-
sources for the expansion of community-based programs that provide
hepatitis B screening, testing, and vaccination services that target
foreign-born populations.
Illicit Drug Users
• 5-3. Federal, state, and local agencies should expand programs to
reduce the risk of hepatitis C virus infection through injection-drug use
by providing comprehensive hepatitis C virus prevention programs. At
a minimum, the programs should include access to sterile needle
syringes and drug-preparation equipment because the shared use of
these materials has been shown to lead to transmission of hepatitis
C virus.
• 5-4. Federal and state governments should expand services to reduce
the harm caused by chronic hepatitis B and hepatitis C. The services
should include testing to detect infection, counseling to reduce alcohol
use and secondary transmission, hepatitis B vaccination, and referral
for or provision of medical management.
• 5-5. Innovative, effective, multicomponent hepatitis C virus prevention
strategies for injection drug users and non-injection-drug users should
be developed and evaluated to achieve greater control of hepatitis C
virus transmission.
Pregnant Women
• 5-6. The Centers for Disease Control and Prevention should provide
additional resources and guidance to perinatal hepatitis B prevention
program coordinators to expand and enhance the capacity to identify
chronically infected pregnant women and provide case-management
services, including referral for appropriate medical management.
• 5-7. The National Institutes of Health should support a study of the
effectiveness and safety of peripartum antiviral therapy to reduce and
possibly eliminate perinatal hepatitis B virus transmission from women
at high risk for perinatal transmission.
Incarcerated Populations
• 5-8. The Centers for Disease Control and Prevention and the Depart-
ment of Justice should create an initiative to foster partnerships be-
tween health departments and corrections systems to ensure the
availability of comprehensive viral hepatitis services for incarcerated
people.
Community Health Facilities
• 5-9. The Health Resources and Services Administration should pro-
vide adequate resources to federally funded community health facili-
ties for provision of comprehensive viral-hepatitis services.
High Impact Settings
• 5-10. The Health Resources and Services Administration and the
Centers for Disease Control and Prevention should provide resources
and guidance to integrate comprehensive viral hepatitis services into
settings that serve high-risk populations such as STD clinics, sites for
HIV services and care, homeless shelters, and mobile health units.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 149
BOX 5-1
Summary of Recommendations Regarding
Viral Hepatitis Services
General Population
• 5-1. Federally funded health-insurance programs—such as Medicare,
Medicaid, and the Federal Employees Health Benefits Program—
should incorporate guidelines for risk-factor screening for hepatitis B
and hepatitis C as a required core component of preventive care so
that at-risk people receive serologic testing for hepatitis B virus and
hepatitis C virus and chronically infected patients receive appropriate
medical management.
Foreign-Born Populations
• 5-2. The Centers for Disease Control and Prevention, in conjunction
with other federal agencies and state agencies, should provide re-
sources for the expansion of community-based programs that provide
hepatitis B screening, testing, and vaccination services that target
foreign-born populations.
Illicit Drug Users
• 5-3. Federal, state, and local agencies should expand programs to
reduce the risk of hepatitis C virus infection through injection-drug use
by providing comprehensive hepatitis C virus prevention programs. At
a minimum, the programs should include access to sterile needle
syringes and drug-preparation equipment because the shared use of
these materials has been shown to lead to transmission of hepatitis
C virus.
• 5-4. Federal and state governments should expand services to reduce
the harm caused by chronic hepatitis B and hepatitis C. The services
should include testing to detect infection, counseling to reduce alcohol
use and secondary transmission, hepatitis B vaccination, and referral
for or provision of medical management.
• 5-5. Innovative, effective, multicomponent hepatitis C virus prevention
strategies for injection drug users and non-injection-drug users should
be developed and evaluated to achieve greater control of hepatitis C
virus transmission.
Pregnant Women
• 5-6. The Centers for Disease Control and Prevention should provide
additional resources and guidance to perinatal hepatitis B prevention
program coordinators to expand and enhance the capacity to identify
chronically infected pregnant women and provide case-management
services, including referral for appropriate medical management.
• 5-7. The National Institutes of Health should support a study of the
effectiveness and safety of peripartum antiviral therapy to reduce and
possibly eliminate perinatal hepatitis B virus transmission from women
at high risk for perinatal transmission.
Incarcerated Populations
• 5-8. The Centers for Disease Control and Prevention and the Depart-
ment of Justice should create an initiative to foster partnerships be-
tween health departments and corrections systems to ensure the
availability of comprehensive viral hepatitis services for incarcerated
people.
Community Health Facilities
• 5-9. The Health Resources and Services Administration should pro-
vide adequate resources to federally funded community health facili-
ties for provision of comprehensive viral-hepatitis services.
High Impact Settings
• 5-10. The Health Resources and Services Administration and the
Centers for Disease Control and Prevention should provide resources
and guidance to integrate comprehensive viral hepatitis services into
settings that serve high-risk populations such as STD clinics, sites for
HIV services and care, homeless shelters, and mobile health units.
Administration (HRSA), the Office of Minority Health, the Agency for
Healthcare Quality and Research, the Centers for Medicare and Medicaid
Services (CMS), the Substance Abuse and Mental Health Services Adminis-
tration (SAMHSA), and the National Institutes of Health. Because there is
no coordinated federal strategy for HBV and HCV prevention and control,
those efforts are uneven in their application and funding. States, communi-

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
150 HEPATITIS AND LIVER CANCER
ties, and nongovernment organizations (NGOs) also provide viral hepatitis
services, often with funding from federal agencies.
Most viral hepatitis–related activities in CDC are administered by the
Division of Viral Hepatitis (DVH), which is part of the National Center for
HIV/AIDS, Viral Hepatitis, Sexually Transmitted Disease, and Tuberculosis
Prevention (NCHHSTP). The activities of the DVH, shown in Box 5-2,
include surveillance and epidemiologic studies and clinical and laboratory
research related to viral hepatitis. It supports viral hepatitis programs at
the national, state, and community levels; disseminates hepatitis-related
information to the public; and develops guidelines for prevention and con-
trol. In FY 2008, the DVH received $17.6 million, 2% of the NCHHSTP
BOX 5-2
Mission Statement of Centers for Disease Control and
Prevention Division of Viral Hepatitis
The Division of Viral Hepatitis (DVH) is the Public Health Service
component that provides the scientific and programmatic foundation for
the prevention, control, and elimination of hepatitis virus infections in the
United States, and assists the international public health community in
these activities.
To achieve its mission, DVH:
1. conducts surveillance and special studies to determine the epi-
demiology and disease burden associated with acute and chronic
infections and liver disease associated with hepatitis viruses;
2. conducts epidemiologic and laboratory studies, including outbreak
investigations, to determine risk factors for transmission of infec-
tions with hepatitis viruses, define the natural history and patho-
genesis of these infections, and determine their health impact;
3. conducts epidemiologic, clinical, laboratory, behavioral, and health
communications research to develop and evaluate methods and
strategies for the prevention of infections with hepatitis viruses and
their acute and chronic disease consequences;
4. develops, implements, communicates and evaluates recommen-
dations and standards for the prevention and control of infections
and liver disease associated with hepatitis viruses;
5. provides technical and programmatic leadership and assistance to
state and local health departments, non-governmental organiza-
tions and the international community to develop, implement and
evaluate programs to prevent infections with hepatitis viruses and
their consequences, including immunization to prevent hepatitis A
and eliminate transmission of hepatitis B virus infection, the pre-
vention and control of hepatitis C virus infection through counsel-
ing and testing and the prevention of transmission of bloodborne
virus infections, including hepatitis viruses, through improved
medical practices to reduce the frequency of unsafe injections
and the improvement of the safety of blood transfusions;
6. provides the leadership and coordination required to integrate viral
hepatitis prevention and control activities into other prevention
programs conducted by CDC, other Federal agencies and health
care providers;
7. conducts laboratory, clinical and epidemiologic studies to develop
and evaluate methods for the diagnosis of infections with hepatitis
viruses;
8. identifies and characterizes agents and host factors associated
with hepatitis and acute and chronic liver disease;
9. provides epidemic aid, epidemiologic and laboratory consulta-
tion, reference diagnostic services and technical assistance to
state and local health departments, other Federal agencies,
other components of CDC, and national and international health
organizations;
10. disseminates information through health communications materials,
tools and programs, scientific publications and presentations;
11. provides training opportunities for Epidemic Intelligence Service
Officers and others in CDC sponsored programs, including post-
graduate students, post-doctoral fellows, and other public health
and laboratory scientists; and
12. serves as a WHO Collaborating Center for Reference and Re-
search on Viral Hepatitis.
SOURCE: CDC, 2009a.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 151
budget (Ward, 2008a). In contrast, domestic HIV activities received 69%,
sexually transmitted diseases (STDs) received 15%, and tuberculosis re-
ceived 14% of the NCHHSTP FY 2008 budget. In FY 2009, the amount of
NCHHSTP funding received by the DVH was not much greater, at $18.3
million (NASTAD, 2009) (personal communication, J. Efird, CDC, July 9,
2009). That low level of funding for the DVH has been relatively flat for
the last 5 years.
HRSA, part of the US Department of Health and Human Services
(HHS), is charged with increasing access to health care for people who are
medically underserved. Several HRSA programs provide some direct ser-
vices for viral hepatitis, including the Bureau of Primary Health Care, the
BOX 5-2
Mission Statement of Centers for Disease Control and
Prevention Division of Viral Hepatitis
The Division of Viral Hepatitis (DVH) is the Public Health Service
component that provides the scientific and programmatic foundation for
the prevention, control, and elimination of hepatitis virus infections in the
United States, and assists the international public health community in
these activities.
To achieve its mission, DVH:
1. conducts surveillance and special studies to determine the epi-
demiology and disease burden associated with acute and chronic
infections and liver disease associated with hepatitis viruses;
2. conducts epidemiologic and laboratory studies, including outbreak
investigations, to determine risk factors for transmission of infec-
tions with hepatitis viruses, define the natural history and patho-
genesis of these infections, and determine their health impact;
3. conducts epidemiologic, clinical, laboratory, behavioral, and health
communications research to develop and evaluate methods and
strategies for the prevention of infections with hepatitis viruses and
their acute and chronic disease consequences;
4. develops, implements, communicates and evaluates recommen-
dations and standards for the prevention and control of infections
and liver disease associated with hepatitis viruses;
5. provides technical and programmatic leadership and assistance to
state and local health departments, non-governmental organiza-
tions and the international community to develop, implement and
evaluate programs to prevent infections with hepatitis viruses and
their consequences, including immunization to prevent hepatitis A
and eliminate transmission of hepatitis B virus infection, the pre-
vention and control of hepatitis C virus infection through counsel-
ing and testing and the prevention of transmission of bloodborne
virus infections, including hepatitis viruses, through improved
medical practices to reduce the frequency of unsafe injections
and the improvement of the safety of blood transfusions;
6. provides the leadership and coordination required to integrate viral
hepatitis prevention and control activities into other prevention
programs conducted by CDC, other Federal agencies and health
care providers;
7. conducts laboratory, clinical and epidemiologic studies to develop
and evaluate methods for the diagnosis of infections with hepatitis
viruses;
8. identifies and characterizes agents and host factors associated
with hepatitis and acute and chronic liver disease;
9. provides epidemic aid, epidemiologic and laboratory consulta-
tion, reference diagnostic services and technical assistance to
state and local health departments, other Federal agencies,
other components of CDC, and national and international health
organizations;
10. disseminates information through health communications materials,
tools and programs, scientific publications and presentations;
11. provides training opportunities for Epidemic Intelligence Service
Officers and others in CDC sponsored programs, including post-
graduate students, post-doctoral fellows, and other public health
and laboratory scientists; and
12. serves as a WHO Collaborating Center for Reference and Re-
search on Viral Hepatitis.
SOURCE: CDC, 2009a.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
152 HEPATITIS AND LIVER CANCER
Healthcare Systems Bureau, the HIV/AIDS Bureau, the Maternal and Child
Health Bureau, the Office of Minority Health and Health Disparities, the
Office of Planning and Evaluation, the Office of Rural Health Policy, and
the Center for Quality (Raggio Ashley, 2009). In addition, viral hepatitis
education and training activities are administered by the Bureau of Health
Professions. HRSA funding supports federally qualified health centers that
serve migrant, rural, tribal, and homeless populations. It also provides
funding for Ryan White Care Act services and maternal and child health
programs, such as Title V and Healthy Start, which provides some hepatitis
B vaccination, testing, and counseling for HBV and HCV infections. Many
people in HRSA-funded programs are foreign-born, including people from
countries that have a high prevalence of hepatitis B or have behavior risk
factors for HBV and HCV infection.
CMS, also a part of DHHS, provides health insurance through Medi-
care and Medicaid programs. Medicare covers people 65 years old or older,
people under 65 years old who have specified disabilities, and people who
have end-stage renal disease. Hepatitis B vaccination and its administration
costs are covered by Part B of Medicare for people at high or intermediate
risk for HBV infection (Rogers, 2009). People at low risk for HBV infec-
tion can receive the vaccine under Part D with a copayment that depends
on their income level. Medicare will cover laboratory testing for HBV and
HCV and treatment for chronic hepatitis B or hepatitis C. Medicaid is a
state-administered program available to low-income individuals and fami-
lies who fit into an eligibility group that is recognized by federal and state
law. Eligibility for Medicaid and coverage for viral hepatitis services vary
from state to state.
State and local (county and city) health departments obtain funds for
viral hepatitis prevention and control activities from a variety of sources,
including CDC, HRSA, SAMHSA, states, counties, cities, and private foun-
dations. CDC funding supports adult viral hepatitis prevention coordina-
tor (AVHPC) positions in 49 states and five cities (Ward, 2008a). The
total funding level is about $5 million per year, and the average award is
$90,000. CDC also funds perinatal hepatitis B coordinators in 64 states,
cities, and territories at a total program cost of $7.5 million per year (CDC,
2009d). Funding for the AVHPC and perinatal hepatitis B coordinator posi-
tions covers only the coordinators’ salaries but not programmatic activi-
ties. CDC provides viral hepatitis program support—about $900,000 per
year—in the form of grants for viral hepatitis training and education at the
state and local levels.
A number of states have developed viral hepatitis prevention plans. At
the committee’s request, the Institute of Medicine asked CDC to survey the
55 AVHPCs about the status of their jurisdiction’s plans (CDC, 2009g). All
coordinators responded to the questionnaire. Of the 55, 32 (58.2%) indi-

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 153
cated that their states had a viral hepatitis prevention plan in place, half of
which were completed in the last 5 years. Just over half of the plans include
all the components in Table 5-1. All plans address hepatitis C prevention,
and two-thirds (65.6%) address hepatitis B prevention. About 78% of the
plans include hepatitis B vaccinations whether or not other hepatitis B pre-
vention services are included. Some coordinators indicated that the CDC
Section 317 vaccination initiative resulted in substantial progress toward
implementing hepatitis B vaccination services in their jurisdictions. The
medical management component is included in the smallest percentage of
plans (62.5%) and just one-quarter of those plans have acted on this com-
ponent. Overall, the coordinator survey revealed that over 40% of juris-
dictions do not have plans; of the states that do have plans, only half have
all the components, and only 20.7% of these reported that they had made
progress in all the components. The primary barrier to plan implementation
was financial constraints on overall funding and staffing (96.9%).
A number of NGOs have been established to address the prevention
and control of HBV and HCV infections. Most of them focus on advocacy
efforts, such as raising public awareness about viral hepatitis and encour-
aging people, especially in high-risk populations, to be vaccinated for
hepatitis B, to undergo risk-factor screening for hepatitis B and hepatitis C,
and to determine whether laboratory testing and medical management are
needed. Many organizations target specific populations. For example, the
Jade Ribbon Campaign targets Asians and Pacific Islanders to reduce the
TABLE 5-1 Summary of Adult Viral Hepatitis Prevention Coordinators
Survey
Jurisdiction Plan’s Program
Components
Percentage of Jurisdictions
with Plans That Included
Component
Percentage of Jurisdictions
with Plan Components That
Have Been Acted On
Public education 96.6% 83.9%
Surveillance 90.6% 64.5%
Training for health-service,
human-service providers
87.5% 90.3%
Advocacy, community
planning
84.4% Not reported
Counseling, testing,
referral
81.3% 83.9%
Vaccination 78.1% 90.3%
Medical management 62.5% 25.8%
NOTES: All 55 adult viral hepatitis prevention coordinators completed the survey; 23 of the
55 jurisdictions do not have a viral hepatitis plan.
SOURCE: CDC, 2009f.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
154 HEPATITIS AND LIVER CANCER
prevalence of chronic HBV infection and HBV-related liver cancer (Asian
Liver Center, 2009). The Harm Reduction Coalition is an example of an
organization that develops and disseminates hepatitis C information among
illicit-drug users (Harm Reduction Coalition, 2009). Information regarding
the activities and programs supported by NGOs are presented primarily in
Chapter 3.
Health services provided by federal agencies, state and local govern-
ments, and NGOs do not form part of a coordinated national campaign.
Existing efforts at interagency information exchange, intermittent meetings
to share plans and results, and joint administration of funds for some grants
are not sufficient for the scale of the health burden presented by hepatitis
B and hepatitis C. The lack of an accountable entity to lead a coordinated
national effort has led to missed opportunities for prevention and identifica-
tion of and treatment for chronic HBV and HCV infections.
COMPONENTS OF VIRAL HEPATITIS SERVICES
The committee has identified five core functions for comprehensive
viral hepatitis services—(1) community outreach, (2) prevention, (3) iden-
tification of infected persons, (4) social and peer support, and (5) medical
management (Box 5-3). Community outreach and immunization for pri-
mary prevention are discussed in depth in Chapters 3 and 4, respectively.
Identification of infected persons, harm reduction, and medical manage-
ment are reviewed below.
Identification of Infected Persons
There are two goals for identifying people chronically infected with
HBV and HCV: to prevent transmission to close contacts (for example,
through sharing of needles and other paraphernalia and through household
and sexual contacts) and to reduce the risk of chronic liver disease through
medical treatment and support. The identification of HBV-infected and
HCV-infected people requires engagement of at-risk people and activism by
the health-care–provider community. As discussed in Chapter 3, culturally
relevant, accessible, and trusted sources of communication are required to
increase awareness and promote use of appropriate services. Health-care
and social-service providers, particularly primary-care providers, should be
knowledgeable about chronic HBV and HCV infection and identify patients
who are at risk because of their behavior or previous potential exposure to
HBV or HCV. Programs and venues that serve at-risk populations—such
as foreign-born people from HBV-endemic countries, the uninsured and un-
derinsured, illicit-drug users, and homeless people—should also be knowl-

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 155
edgeable about viral hepatitis and should have mechanisms for identifying
infected people and referring them to followup medical management.
The committee has defined a two-step process for identifying infected
people:
1. Risk-factor screening. Risk-factor screening is the process of deter-
mining whether a person is at risk for being chronically infected or
becoming infected with HBV or HCV. Risk factors include being
born in a country where the disease is prevalent, and behavior such
as illicit-drug use and having multiple sexual partners.
BOX 5-3
Components of Comprehensive Viral Hepatitis Services
Community Outreach
• Community-awareness programs
• Provider-awareness programs
Prevention
• Vaccination
• Harm reduction
• Needle-exchange programs
o Drug and alcohol treatment services
o Vaccination of hepatitis B virus-susceptible contacts
Identification of Infected Persons
• Risk-factor screening
• Laboratory testing
Social and Peer Support
• Positive prevention services
• Education and referral to other related services and care
Medical Management
• Assessment for and provision of long-term monitoring for viral hepatitis
and selection of appropriate persons for treatment (in accordance with
American Association for the Study of Liver Diseases guidelines)
• Psychiatric and other mental-health care
• Adherence support

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
156 HEPATITIS AND LIVER CANCER
2. Serologic testing. Serologic testing is laboratory testing of blood
specimens for biomarker confirmation of HBV or HCV infection.
Risk-Factor Screening
Hepatitis B Risk-Factor Screening CDC has identified risk factors for be-
coming infected or chronically infected with HBV (see Box 5-4). As discussed
in Chapter 3, improved provider awareness about risk factors is critical for
ensuring that people at risk for chronic HBV infection are identified and
that those at risk for becoming infected with HBV are vaccinated. Providers
should review patients’ backgrounds (for example, country of birth) and
discuss relevant behaviors to determine what services they need.
Figure 5-1 illustrates the pathway of services and care for people de-
pending on their risk factors identified. People who have HIV infection
or other sexually transmitted infections, men who have sex with men,
injection-drug users (IDUs), and institutionalized and incarcerated persons
BOX 5-4
Summary of CDC At-Risk Populations for
Hepatitis B Virus Infection
• Persons born in geographic regions that have HBsAg prevalence of
at least 2%
• Infants born to infected mothers
• Household contacts of persons who have chronic HBV infection
• Sex partners of infected persons
• Injection-drug users
• Sexually active persons who are not in long-term, mutually monoga-
mous relationships (for example, more than one sex partner during
previous 6 months)
• Men who have sex with men
• Health-care and public-safety workers at risk for occupational expo-
sure to blood or blood-contaminated body fluids
• Residents and staff of facilities for developmentally disabled persons
• Persons who have chronic liver disease
• Hemodialysis patients
• Travelers to countries that have intermediate or high prevalence of
HBV infection
SOURCE: Mast et al., 2005, 2006.
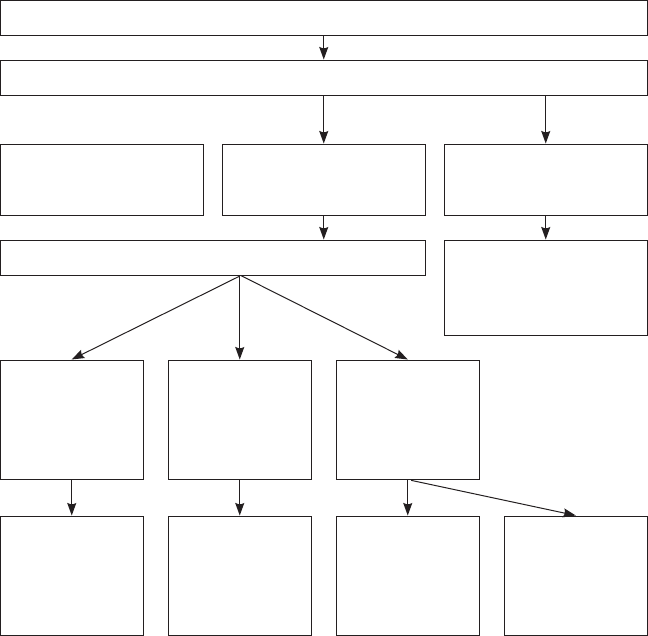
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 157
FIGURE 5-1 Hepatitis B services model.
Abbreviations: HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; anti-HBs,
antibody to hepatitis B surface antigen.
are at increased risk for HBV infection. CDC recommends that all those
populations be tested and given a first dose of vaccine at the time of testing.
However, the committee believes that an acceptable alternative is hepatitis
B vaccination without testing for all the populations except HIV-infected
persons. This approach may facilitate increased vaccination rates. All per-
sons found to have risk factors for HBV infection should receive counseling
about prevention.
Hepatitis C Risk-Factor Screening CDC has identified risk factors for peo-
ple at risk for being infected with HCV or becoming infected with HCV
(see Box 5-5). Risk-factor screening has been tested by using question-
Outreach and Awareness Activities
Risk-Factor Screening
All Pregnant Women
Foreign-Born
(see Box 3-1)
Behavioral Risk
Factors for
Hepatitis B
Test for HBV (HBsAg and Anti-HBs)
Vaccination and
Preventive Services
(select services
based on risk factor)
HBsAg-
Negative
and
Anti-HBs
Negative
Anti-HBs-
Positive
and
HBsAg
Negative
HBsAg-
Positive
and
Anti-HBs
Negative
Vaccination
for
Hepatitis B
Immune to
HBV
Continuing
Medical
Management
Testing and
Vaccinating
Household and
Sexual Contacts
for HBV

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
158 HEPATITIS AND LIVER CANCER
naires to assess a person’s potential exposure to HCV infection. It has been
found to correlate with infection status and is an effective mechanism for
identifying candidates for testing (Armstrong et al., 2006; McGinn et al.,
2008; Zuniga et al., 2006). Armstrong et al. (2006) reported that 85% of
HCV-infected people could be identified on the basis of three risk factors:
injection-drug use, blood transfusion before 1992, and abnormal serum
alanine aminotransferase levels. Additional studies have also found that
questioning patients about exposures to known risk factors for hepatitis C
is predictive of HCV infection in US veterans (Zuniga et al., 2006). People
who are current or past users of illicit drugs may not fit the stereotype of
an IDU, so all patients should be questioned about any past episode of il-
licit-drug injection. It has also been suggested that people who have tattoos
and body piercings should be tested for HCV (Carey, 2003). Researchers
who were evaluating hepatitis C incidence along the Texas–Mexico bor-
der found tattooing to be an independent risk factor for infection in their
majority-Hispanic population (Hand and Vasquez, 2005). However, it is
BOX 5-5
Summary of CDC At-Risk Populations for
Hepatitis C Virus Infection
• Persons who have ever injected illegal drugs, including those who
injected only once many years ago
• Recipients of clotting-factor concentrates made before 1987
• Recipients of blood transfusions or solid-organ transplants before July
1992
• Patients who have ever received long-term hemodialysis treatment
• Persons who have known exposures to HCV, such as
— Health-care workers after needlesticks involving HCV-positive blood
— Recipients of blood or organs from donors who later tested
HCV-positive
• All persons who have HIV infection
• Patients who have signs or symptoms of liver disease (for example,
abnormal liver-enzyme tests)
• Children born to HCV-positive mothers (to avoid detecting maternal
antibody, these children should not be tested before the age of 18
months)
Abbreviation: HCV, hepatitis C virus.
SOURCE: CDC, 2001.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 159
unclear whether tattooing and body piercing are risk factors for infection
or surrogates for other etiologic factors. As mentioned in Chapter 1, there
is a high prevalence of HCV infection in Egypt, so Egyptian immigrants to
the United States should be considered for serologic testing.
The issue of risk-factor screening and testing for HCV is controversial.
In 1998, the US Public Health Service (USPHS) recommended a process of
screening persons for HCV risk factors followed by laboratory testing for
those potentially exposed to HCV (CDC, 1998). The 1998 USPHS recom-
mendation to screen for risk factors among adults in the general population
and to test those at risk was endorsed by a number of organizations, in-
cluding the American Association for the Study of Liver Diseases (AASLD),
the Infectious Diseases Society of America, and the American College of
Physicians (Alter et al., 2004; Ghany et al., 2009). CDC recommends that
all patients be evaluated for risk factors for HCV infection (Alter et al.,
2004). In 2004, a separate group, the US Preventive Services Task Force
(USPSTF), concluded that there was no direct evidence of the benefit of
serologic testing for HCV infection in the general adult population and that
there were inadequate data for determining accurately the benefits and risks
associated with serological testing for HCV infection in otherwise healthy
asymptomatic at-risk adults (Chou et al., 2004). As outcomes of treatment
for chronic HCV infection improve, the controversy regarding screening
and testing may diminish. An example of how improvements in treatment
can change the value of identifying infected people can be seen in the ad-
vances in treatment for HIV. As effective antiretroviral therapies emerged,
recommendations for screening and testing were expanded (Myers et al.,
2009; Paltiel et al., 2005; Sanders et al., 2005).
Serologic Testing for Hepatitis B Virus and Hepatitis C Virus
Serologic tests to detect a history of exposure or to ascertain infection
or immune status with respect to HBV and HCV use virus-specific antigens
and antibodies, recombinant immunoblot assays (RIBAs), and viral nucleic
acid (DNA and RNA) tests.
Rapid HBV and HCV detection tests are not available in the United
States although they are available in other countries (Randrianirina et al.,
2008). Rapid tests for HBsAg are available in developing countries and
have high sensitivity and specificity (Randrianirina et al., 2008). Rapid te-Rapid te-
sting in HIV interventions has been demonstrated to add substantial value
in engaging hard-to-reach populations (Begley et al., 2008; Bowles et al.,
2008; Clark et al., 2008; Kassler et al., 1997; Keenan and Keenan, 2001;
Liang et al., 2005; Molitor et al., 1999; Reynolds et al., 2008; Schulden et
al., 2008; Shrestha et al., 2008; Smith et al., 2006; Spielberg et al., 2001,
2003, 2005; Sullivan et al., 2004). The availability of rapid tests in theThe availability of rapid tests in the

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
160 HEPATITIS AND LIVER CANCER
United States could enhance HBV and HCV detection and help to close
gaps in care, particularly in hard-to-reach populations.
Hepatitis B Virus Laboratory Testing Serologic markers can be used to
identify the different phases of HBV infection (Box 5-6). The preferred
laboratory test for detecting current HBV infection is for hepatitis B surface
antigen (HBsAg), and the principal test for detecting recovery from HBV
infection is for anti-HB surface antibody (anti-HBs). An alternative is to
test initially for anti-HB core antibody (anti-HBc)—which is present during
acute, chronic, and resolved HBV infection—and, if the result is positive,
to conduct followup testing for HBsAg and anti-HBs. HBV markers can be
misinterpreted by clinicians, and this can lead to clinical errors in patient
evaluations, counseling, or treatment. For example, anti-HBc and anti-HBs
can be misinterpreted as indicators of active infection (Weinbaum, 2008).
BOX 5-6
Hepatitis B Virus-Specific Antigens and Antibodies
Used for Testing
Hepatitis B surface antigen (HBsAg): A protein on the surface of hepa-
titis B virus; it can be detected at high levels in serum during acute or
chronic HBV infection. The presence of HBsAg indicates that a person is
infected and infectious. The body normally produces antibodies to HBsAg
as part of the normal immune response to infection. HBsAg is the antigen
used to make hepatitis B vaccine.
Hepatitis B surface antibody (anti-HBs): The presence of anti-HBs
is generally interpreted as indicating recovery and immunity from HBV
infection. Anti-HBs also develops in a person who has been successfully
vaccinated against hepatitis B.
Total hepatitis B core antibody (anti-HBc): This appears at the onset
of symptoms in acute hepatitis B and generally persists for life. The pres-
ence of anti-HBc indicates previous or current infection with HBV in an
undefined time frame.
IgM antibody to hepatitis B core antigen (IgM anti-HBc): Positivity
indicates recent infection with HBV (less than 6 months). Its presence
indicates acute infection.
SOURCE: CDC, 2009c.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 161
Clinicians also may not know which tests to order to test for chronic vs
acute viral HBV infection. Table 5-2 provides guidance on the interpreta-
tion of hepatitis B serologic test results.
Cost-effectiveness data on the use of laboratory testing in particular at-
risk populations are available. As mentioned above, people born in foreign
countries that have high rates of HBV (2% or more) are at the highest risk
for chronic HBV infection and constitute the largest pool of undiagnosed
persons (see Box 3-1). Laboratory testing of adult Asian and Pacific Island-
ers for HBV infection (10% prevalence of chronic HBV infection), moni-
toring and treating people who are found to be chronically infected, and
TABLE 5-2 Interpretation of Hepatitis B Serologic Diagnostic Test
Results
Antigen or Antibody Test Result Interpretation
HBsAg
Anti-HBc
Anti-HBs
All negative Susceptible
HBsAg
Anti-HBc
Anti-HBs
Negative
Positive
Positive
Immune because of natural
infection
HBsAg
Anti-HBc
Anti-HBs
Negative
Negative
Positive
Immune because of hepatitis B
vaccination
HBsAg
Anti-HBc
IgM anti-HBc
Anti-HBs
Positive
Positive
Positive
Negative
Acutely infected
HBsAg
Anti-HBc
IgM anti-HBc
Anti-HBs
Positive
Positive
Negative
Negative
Chronically infected
HBsAg
Anti-HBc
Anti-HBs
Negative
Positive
Negative
Interpretation unclear; could
be due to
• Resolved infection
• False-positive anti-HBc test
• Low-level chronic infection
• Resolving acute infection
Abbreviations: HBsAg, hepatitis B surface antigen; anti-HBc, total hepatitis B core antibody;
anti-HBs, hepatitis B surface antibody; IgM anti-HBc, IgM antibody to hepatitis B core
antigen.
SOURCE: CDC, 2009c.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
162 HEPATITIS AND LIVER CANCER
ring vaccination of their close contacts have been shown to be cost-effective
(Hutton et al., 2007). That suggests that foreign-born persons from coun-
tries that have chronic HBV rates of 2% or greater should be screened for
HBV infection. If they are negative for HBV seromarkers, they should be
offered vaccination.
Other populations to be tested for HBV include IDUs, pregnant women,
infants born to HBsAg-positive mothers, household contacts and sex part-
ners of HBV-infected persons, men who have sex with men, and people
infected with HIV. People scheduled for immunosuppressive therapy should
also be tested for HBV infection because studies have clearly shown that
persons who are HBsAg-positive have a risk of 20–50% of developing flares
of hepatitis when undergoing cancer chemotherapy (Lok et al., 1991; Yeo
and Johnson, 2006; Yeo et al., 2000). Reactivations have also been reported
to occur with other types of immunosuppressives, notably anti–tumor-
necrosis factor therapy for rheumatoid arthritis and inflammatory bowel
disease (Esteve et al., 2004; Ostuni et al., 2003). Most flares of hepatitis in
HBsAg-positive persons in this setting are asymptomatic, but icteric flares,
hepatic decompensation, and death have been reported (Lok et al., 1991;
Yeo et al., 2000). It has also been demonstrated that lamivudine prophylaxis
can cause a substantial reduction in the incidence and severity of hepatitis
flares in HBsAg-positive persons who are undergoing cancer chemotherapy
(Hsu et al., 2008; Lau et al., 2002, 2003; Li et al., 2006; Rossi et al., 2001).
Therefore, all persons scheduled to undergo cancer chemotherapy or im-
munosuppressive treatments should be screened for hepatitis B risk factors,
and followup testing for HBsAg should be performed if warranted. Persons
who are HBsAg-positive should be treated as recommended by established
practice guidelines (Lok and McMahon, 2009).
Hepatitis C Virus Laboratory Testing Persons at risk for hepatitis C should
be tested for antibodies to HCV with a licensed enzyme immunoassay
(EIA), which has a high sensitivity (Alter et al., 2003). As with all screen-
ing tests, the predictive value of a positive test varies with the population
prevalence, which for HCV is lowest in volunteer blood donors and highest
in IDUs. False-negative EIA tests may occur in immunosuppressed popula-
tions, such as patients on hemodialysis or infected with HIV (Rahnavardi
et al., 2008; Thio et al., 2000). In those settings, EIA-negative persons
suspected of having HCV infection should also be tested for HCV RNA
(Ghany et al., 2009). Table 5-3 provides guidance on the interpretation of
hepatitis C test results.
HCV EIA-positive results should be confirmed with a second test,
ideally before the information is presented to the patient to minimize the
unnecessary harm of a false-positive result. There are two separate goals
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 163
of confirming a positive EIA: to make sure that the antibodies detected in
the EIA were truly to HCV and not cross-reactive and to assess persons
with antibodies to HCV to ascertain whether HCV infection is current or
cleared spontaneously. The likelihood that a positive HCV EIA represents
antibodies to HCV (as opposed to cross-reactive antibodies) depends on the
strength of the EIA reaction. Values that exceed a particular threshold (for
example, 3.8 for tests commonly used in the United States) are likely to be
true HCV infections and unlikely to be false-positive (Alter et al., 2003;
Pawlotsky et al., 1998). Thus, some laboratories report the ratio of the test
result to the cutoff value, and ratios above that threshold can be assumed
to represent HCV antibodies. Another way to establish whether a positive
EIA reflects HCV antibodies is to run a supplemental antibody test, such
as a RIBA recombinant immunoblot assay. By separating the antigens that
are grouped in the screening EIA, the supplemental antibody test provides
better specificity (Damen et al., 1995). However, supplemental antibody
testing does not achieve the second goal of determining whether a person
has HCV infection. Thus, most authorities recommend use of HCV-RNA
testing as the next step after detection of HCV antibodies with EIA in all
settings in which HCV testing is done in at-risk persons (Alter et al., 2003;
Ghany et al., 2009). A positive result in both EIA and RNA tests means
that a person needs further counseling and medical evaluation for chronic
or acute HCV infection.
HCV-RNA testing is more expensive than antibody testing with EIA
and may require more sophisticated laboratory capability and a longer
reporting interval (Alter et al., 2003). Thus, although all reasonable efforts
should be made to confirm positive EIA HCV results before presenting
them, HCV-RNA testing may not be feasible in some settings. Lack of
available HCV-RNA testing should not be an impediment to EIA testing,
but counseling must reflect the uncertainty and the urgency of followup in
another venue for further assessment.
Laboratory testing of at-risk populations to identify HCV-infected
people has been found to be cost-effective when combined with proper
medical-management and harm-reduction strategies (Tramarin et al., 2008).
In particular, studies have found that HCV laboratory testing among cur-
rent and former IDUs is cost-effective. A 2006 study of former IDUs in a
prison in the United Kingdom found that laboratory testing and later treat-
ment of inmates cost £16,514 (about $25,000) per quality adjusted life year
(Castelnuovo et al., 2006). A 2008 study in Italy found that laboratory
testing (followed by appropriate medical management) of IDUs resulted in a
substantial difference in the incidence of premature death. In contrast, HCV
laboratory testing for people who are in the hospital for surgery but have no
other risk factors is unlikely to be cost-effective (Tramarin et al., 2008).

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
164
TABLE 5-3 Interpretation of Hepatitis C Virus Diagnostic Test Results
If HCV Test Result Is: Interpretation Action
Anti-HCV Screening Test
(EIA or CIA)
Anti-HCV Supplemental
Test: RIBA
Anti-HCV Supplemental
Test: HCV RNA HCV Status
Additional Testing or
Evaluation Required
Positive Not done Not done Not known HCV RNA; RIBA if RNA
negative
Positive Not done Negative Probably not currently
HCV infected; further
testing required
a
Obtain RIBA; if RIBA
positive, repeat RNA
Positive (high s/co ratio) Not done Not done Antibody probably true
positive; need to distinguish
past from current infection
HCV RNA
Positive Negative Not done False positive anti-HCV None
Positive Positive Not done Past or current HCV
infection
HCV RNA; if RNA
positive, evaluate for liver
disease
Positive Positive Negative Probable past HCV
infection with recovery
a
Repeat HCV RNA to rule
out active infection
a
Positive Positive or not done Positive Current acute or chronic
HCV infection
Evaluate for chronic
infection and liver disease
Positive Indeterminate Not done Not known; possible
false-positive anti-HCV or
recovery from past HCV
infection
Test for HCV RNA or
repeat anti-HCV testing
Positive Indeterminate Positive Current acute or chronic
HCV infection
Evaluate for chronic
infection and liver disease
Positive Indeterminate Negative Probably not currently
infected;
a
possible false-
positive anti-HCV or
recovery from past HCV
infection
Repeat HCV RNA or
repeat anti-HCV testing
a
A single negative HCV-RNA result cannot determine infection status, inasmuch as a person might have intermittent viremia.
Abbreviations: anti-HCV, antibody to HCV; EIA, enzyme immunoassay; CIA, enhanced chemiluminescence immunoassay; RIBA, recombinant im-
munoblot assay; RNA, ribonucleic acid; s/co ratio, signal-to-cutoff ratio.
SOURCE: Adapted from Ghany et al., 2009. Diagnosis, management, and treatment of Hepatitis C: An update. Hepatology 49(4):1335-1374.
Copyright 2009. Reprinted with permission of John Wiley & Sons, Inc.; CDC, 2009e.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
165
TABLE 5-3 Interpretation of Hepatitis C Virus Diagnostic Test Results
If HCV Test Result Is: Interpretation Action
Anti-HCV Screening Test
(EIA or CIA)
Anti-HCV Supplemental
Test: RIBA
Anti-HCV Supplemental
Test: HCV RNA HCV Status
Additional Testing or
Evaluation Required
Positive Not done Not done Not known HCV RNA; RIBA if RNA
negative
Positive Not done Negative Probably not currently
HCV infected; further
testing required
a
Obtain RIBA; if RIBA
positive, repeat RNA
Positive (high s/co ratio) Not done Not done Antibody probably true
positive; need to distinguish
past from current infection
HCV RNA
Positive Negative Not done False positive anti-HCV None
Positive Positive Not done Past or current HCV
infection
HCV RNA; if RNA
positive, evaluate for liver
disease
Positive Positive Negative Probable past HCV
infection with recovery
a
Repeat HCV RNA to rule
out active infection
a
Positive Positive or not done Positive Current acute or chronic
HCV infection
Evaluate for chronic
infection and liver disease
Positive Indeterminate Not done Not known; possible
false-positive anti-HCV or
recovery from past HCV
infection
Test for HCV RNA or
repeat anti-HCV testing
Positive Indeterminate Positive Current acute or chronic
HCV infection
Evaluate for chronic
infection and liver disease
Positive Indeterminate Negative Probably not currently
infected;
a
possible false-
positive anti-HCV or
recovery from past HCV
infection
Repeat HCV RNA or
repeat anti-HCV testing
a
A single negative HCV-RNA result cannot determine infection status, inasmuch as a person might have intermittent viremia.
Abbreviations: anti-HCV, antibody to HCV; EIA, enzyme immunoassay; CIA, enhanced chemiluminescence immunoassay; RIBA, recombinant im-
munoblot assay; RNA, ribonucleic acid; s/co ratio, signal-to-cutoff ratio.
SOURCE: Adapted from Ghany et al., 2009. Diagnosis, management, and treatment of Hepatitis C: An update. Hepatology 49(4):1335-1374.
Copyright 2009. Reprinted with permission of John Wiley & Sons, Inc.; CDC, 2009e.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
166 HEPATITIS AND LIVER CANCER
Prevention
Vaccination
A vaccine for hepatitis B has been available since the 1980s. Research
to develop a vaccine for hepatitis C continues although it is unlikely that a
vaccine will be developed and licensed in the near future. Given the com-
plexity of the issues surrounding vaccination of children and adults, this
report devotes a separate chapter (Chapter 4) to immunization.
Harm Reduction
Harm reduction refers to programs and policies that seek to reduce
the medical, social, and economic harms associated with illicit-drug use
(IHRA, 2009). Support for abstinence is an element of harm reduction but
is not a requirement for participation in harm-reduction programs. Harm
reduction focuses on providing information about safer practices (for ex-
ample, how to inject without exposing oneself to contaminated blood),
providing materials for engaging in safer practices (such as needle syringes
and condoms), and offering hepatitis B vaccination. Because harm reduc-
tion does not condemn illicit-drug use and instead seeks practical solutions
to mitigate its harmful consequences, these programs can be controversial
(Des Jarlais et al., 2009).
Medical Management
Evidence-based practice guidelines for both chronic hepatitis B and
chronic hepatitis C have been published by the American Association for
the Study of Liver Diseases (AASLD) and other organizations (Ghany et al.,
2009; Lok and McMahon, 2009). The guidelines are updated regularly to
reflect advances in care and should be referred to as the basis of appropri-
ate medical management. For the purposes of this report, the committee
specifies that the goals of medical management of chronically infected
people are to decrease the risk of developing cirrhosis, to prevent hepatic
decompensation, to decrease the risk of hepatocellular carcinoma in people
chronically infected with HBV or HCV, and to effect secondary prevention
of virus transmission.
The AASLD guidelines include recommendations for selection of
patients who have chronic hepatitis B or hepatitis C for referral to spe-
cialists and for treatment with medications (Ghany et al., 2009; Lok and
McMahon, 2009). Persons who are identified as HBsAg-positive should
have a history taken and a physical examination performed by a primary-
care provider with an emphasis on symptoms and signs of liver disease
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 167
(Lok and McMahon, 2009). Initial laboratory evaluation should include
a full liver panel, complete blood count (CBC), and hepatitis B e antigen
(HBeAg), anti-HB e antibody (anti-HBe), and HBV DNA tests. Further
management will depend on the results. Persons who are HBeAg-positive
and have increased alanine aminotransferase (ALT) should be referred for
evaluation for possible liver biopsy and treatment. Likewise, persons who
are HBeAg-negative and anti-HBe-positive, have increased HBV DNA (over
2,000 IU/mL), and have increased ALT should be referred to a specialist.
People who have normal ALT, are HBeAg-negative and anti-HBe-positive,
and have HBV DNA below 2,000 IU/mL can be followed with ALT and
aspartate aminotransferase (AST) tests every 6 months by a primary care
provider. If ALT increases above the normal limit, HBV DNA should be
tested again; if it is above 2,000 IU/mL, the patient should be referred to
a specialist. If a patient is HBeAg-positive and has normal ALT (that is,
in the immune tolerant phase), tests for ALT, AST, HBeAg, and anti-HBe
should be repeated every 6 months. If ALT rises above the normal range,
the patient should be referred to a specialist. In patients who have increased
ALT and HBV DNA, liver biopsy is often appropriate to determine the
best candidates for treatment because it is recommended that patients who
have more than mild inflammation and fibrosis on biopsy receive treat-
ment, and laboratory tests are often unable to distinguish degrees of liver
involvement (Lok and McMahon, 2004; NIH, 2008). In addition, any pa-
tient who has stigmata of liver disease—ascites, enlarged spleen, jaundice,
or encephalopathy—or a platelet count below 100,000 (which is a sign
of possible splenomegaly) should be referred immediately to a specialist.
The AASLD provides hepatitis B–specific treatment guidelines, including
how to select appropriate candidates for treatment, guidance on which
antiviral medications to use, and how to address antiviral resistance (Lok
and McMahon, 2009). All chronically HBV-infected patients, regardless of
their ALT and HBV DNA status, must be followed on a regular basis, every
3–12 months depending on the activity of their disease.
Persons who are identified as anti-HCV-positive and who have HCV
RNA present in their serum may initially be evaluated by a primary care
provider (Ghany et al., 2009). The primary care provider should take a his-
tory and perform a physical examination with emphasis on symptoms and
signs of liver disease. The initial laboratory evaluation should include a full
liver panel, CBC, and HCV genotype tests. Patients found to have signs or
symptoms of liver disease or a low platelet count (below 100,000) should
be referred to a specialist who has experience in managing persons with
advanced hepatitis C. Patients who are infected with HCV genotype 2 or 3
and who are interested in receiving treatment can be referred immediately
for treatment consideration. A primary care provider should discuss with
a patient who is infected with HCV genotype 1 the possibility of receiving
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
168 HEPATITIS AND LIVER CANCER
treatment and should emphasize that the treatment is successful in only
about 50% of cases and that the side effects can be severe. For genotype
1 patients, it may be preferable first to do a liver biopsy to determine the
degree of liver involvement and scarring before making a decision about
whether treatment should be considered sooner rather than later. The
AASLD provides hepatitis C–specific treatment guidelines, including how
to select appropriate candidates for treatment and guidance on which an-
tiviral medications to use (Ghany et al., 2009). Patients who do not want
immediate referral or treatment should be followed every 6 months with a
full liver panel and yearly CBC tests. Finally, primary care providers should
counsel patients to abstain from, or at least limit, alcohol consumption be-
cause heavy alcohol use is the greatest contributor to the rate of progressive
liver fibrosis. Patients who have a history of heavy alcohol intake should
receive counseling.
Studies have found racial and ethnic disparities in the evaluation of and
treatment for HCV infection (Butt et al., 2007; Rousseau et al., 2008). One
study of veterans reported similar rates of referral and liver biopsy for HCV-
infected persons of various racial populations but found that blacks were
less likely than whites to have complete laboratory evaluations, including
viral genotyping, and to receive antiviral treatment (Rousseau et al., 2008).
Because patient characteristics that are associated with not responding to
treatment generally are associated with not receiving treatment, it is difficult
to ascertain from available research findings the degree to which lower up-
take into treatment represents discrimination against minority populations
or appropriate implementation of treatment guidelines. For example, in
another study of veterans, less treatment was received by minority-groups
members and by persons who were older, who had a history of drug and
alcohol use, or who had comorbid illnesses (Butt et al., 2007).
Chronic HCV infection has been found to be an important cause of
liver-related death in Alaska Natives (Wise et al., 2008). The federal gov-
ernment is responsible by treaty laws to provide medical care at no cost to
American Indians and Alaska Natives, but the amount spent per person is
far less than that spent for Medicare and Medicaid recipients or for incar-
cerated persons, and is not enough to pay for treatment for HCV infection
in many tribal health-care systems. There is evidence that not all patients
who initiate therapy complete it. Over 80% of participants in clinical trials
completed the HCV antiviral therapy. However, researchers found that in
a large national cohort of veterans less than one-fourth of the patients who
began treatment for chronic hepatitis C completed a 48-week course. The
major predictors of treatment noncompletion were pretreatment anemia
and depression (Butt et al., 2009). Treatment completion rates appear to
vary among ethnic and racial populations. For example, a study found that
Hispanic patients were more likely to be candidates for treatment but were
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 169
less likely to initiate it; they were also more likely to discontinue treatment
early, and discontinuation of treatment was associated with alcohol use
(Cheung et al., 2005).
The risk of developing hepatocellular carcinoma (HCC) is a serious
concern for patients who are infected with HBV or HCV, and providers
should initiate regular monitoring for HCC (Bruix and Sherman, 2005). Pa-
tients who have chronic HBV infection and are at the highest risk for HCC
include those who have first-degree relatives who developed HCC, persons
who have cirrhosis, men 40 years old and older, and women 50 years old
and older. Of patients who have chronic HCV infection, only those who
have cirrhosis or advanced liver fibrosis (that is, bridging fibrosis) should
be monitored for HCC. Monitoring of patients at high risk for HCC should
be performed every 6 months.
Studies have found ethnic disparities in HCC treatment rates and mor-
tality (Davila and El-Serag, 2006; Siegel et al., 2008; Sonnenday et al.,
2007). Blacks and Hispanics had significantly higher HCC-related mortality
than other racial and ethnic populations. Even after adjustment for stage of
HCC and other demographic characteristics, blacks were 40% less likely
than whites to receive local or surgical therapy. Another study found that
blacks and Hispanics were 24–27% less likely than whites to receive surgi-
cal therapy (Sonnenday et al., 2007). A study that looked at liver transplants
necessitated by HCC found that in 1998–2002, black and Asian patients
were significantly less likely than white patients to receive a liver transplant
(Siegel et al., 2008). Once researchers controlled for receipt of treatment, the
difference in mortality in black patients was no longer significant (Davila and
El-Serag, 2006). Those data on racial and ethnic disparities in the outcomes
of and treatments for chronic hepatitis underscore the need for additional
research to understand the biologic and societal basis of the disparities. They
also indicate the urgency of new policies that ensure that optimal medical
care is given to all without regard to race or ethnicity.
The economic costs of chronic hepatitis B and hepatitis C are high. In
2004, the average annual medical-care costs of chronic HBV infection and
its complications per infected person in the United States were as follows:
chronic HBV infection, $761; compensated cirrhosis, $227; decompensated
cirrhosis, $11,459; liver transplantation, $86,552; transplantation care
more than 12 months after transplantation, $12,560; and hepatocellular
carcinoma, $7,533 (Lee et al., 2004). Medication costs were the largest
proportion of the chronic HBV infection and compensated cirrhosis states
and hospitalization costs made up the largest proportion of the other health
states. In the same year, Chesson et al. (2004) estimated the annual net cost
per case of chronic liver disease at $32,837 in the United States.
Although treatment costs are high, some studies have found that treat-
ment can be cost-effective. In particular, several studies compared the costs
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
170 HEPATITIS AND LIVER CANCER
of various treatments for chronic HBV infection (for example, interferon,
pegylated interferon, lamivudine, and adefovir) and found them to be cost-
effective (Kanwal et al., 2005; Rajendra and Wong, 2007). Treatments for
HCV infection with interferon or pegylated interferon plus ribavirin have
also been shown to be cost-effective (Campos et al., 2007; Lidgren et al.,
2007; Rajendra and Wong, 2007; Salomon et al., 2003).
There is evidence that people’s ability to pay affects whether they seek
and receive appropriate medical care for chronic hepatitis B and hepatitis
C. For example, among people who tested positive for HCV antibody at
public STD clinics in San Diego and an HIV test-site screening program,
the presence or absence of health insurance was strongly associated with
whether later medical care was received for HCV (Mark et al., 2007).
MAJOR GAPS IN SERVICES
The lack of comprehensive case management (that is, initial clinical
evaluation and laboratory testing, regularly scheduled clinical and labora-
tory monitoring, appropriate referral and treatment, and monitoring for
HCC) for people who have chronic hepatitis B or hepatitis C and who do
not have access to private health insurance and care is an important gap
in control of chronic viral hepatitis. The committee believes that people
who are living with chronic HBV or HCV infection should receive the
health-care services outlined in Box 5-3. The Ryan White Care program
for people who are living with HIV/AIDS is a federal approach that could
be replicated to fill the void in health-care services for patients who have
HBV or HCV infection. The committee recognizes that uncertainties in
funding and health-care reform may make implementation of such a pro-
gram challenging.
General Population
Various factors can lead to difficulties in accessing screening, preven-
tion, testing, and care related to viral hepatitis. Obstacles to obtaining such
services may be limitations in private or public insurance coverage and cost-
sharing, lack of access to public health insurance, lack of public funding
to support implementation of state viral hepatitis plans, lack of hepatitis
awareness and health literacy, inadequacy of sites or practice settings where
health-care services are received, transportation needs, social stigmas, fear
of legal prosecution related to drug use and immigration, and such cultural
factors as religious beliefs, beliefs about biologic products, health percep-
tions, and language. Among those, however, the most important barriers
to receipt of existing services are inadequacy of health-insurance coverage
and lack of money to pay for services.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 171
Having insurance, either privately or publicly funded, has a positive
association with laboratory testing for HBV infection, and those who
have private insurance have the highest testing rates. For example, Choe
et al. (2006) reported a strong relation between having ever been tested
for HBV and insurance coverage in Vietnamese American men in Seattle,
Washington. In their study, 70% of privately insured people and 51% of
people insured by the Washington State Basic Health Insurance Plan were
ever tested for HBV. As discussed in Chapter 4, health insurance must
provide strong coverage for immunization, counseling services, medical
treatment, and prescription drugs, or the insurance’s cost-sharing features
will prevent use of services. High deductibles (amounts to be paid out of
pocket before coverage begins) or benefit limits are common in insurance
policies that are provided by medium and small employers or in-network
plans (which provide different coverage in network from out of network).
That is not the case with more comprehensive insurance coverage typically
seen in integrated delivery systems and health maintenance organizations
(HMOs). As of 2007, 21% of workers who had employer-sponsored health
insurance were covered under HMOs (Claxton et al., 2007). In publicly
funded venues—where services for the poor or special risk populations,
such as STD clinics, or for IDUs are provided—inadequacies in funding
for hepatitis-related services may limit testing or other services (Boutwell
et al., 2005; Brown et al., 2007; Heseltine and McFarlane, 2007; McIntyre
et al., 2008).
The current fragmentation of viral hepatitis services involving vaccina-
tion, risk-factor screening, laboratory testing, and medical management is a
major obstacle to the effective delivery of needed services and makes com-
pliance more difficult. The lack of coordination between services can inhibit
use by requiring people to travel to multiple sites to obtain care, impairs
the development of trusting relationships among multiple providers, and
taxes a health system’s ability to transfer information where and when it is
needed for good clinical care. Studies have examined program integrationStudies have examined program integration
for HIV, STD, and viral hepatitis services and found that integration brings
more at-risk people into the system (Birkhead et al., 2007; Gilbert et al.,
2005; Gunn et al., 2007; Hennessy et al., 2007; Kresina et al., 2008; Stopka
et al., 2007; Zimmerman et al., 2007).
One important consequence of the fragmentation of viral-hepatitis ser-
vices is inconsistency in referral of people who have chronic viral hepatitis
for appropriate medical care. That gap reflects deficiencies primary-care
providers’ knowledge, and it can be substantial when there are barriers,
such as physical barriers (that is, screening and testing services in a different
location from medical-management services), economic barriers, and cultu-
ral barriers. As discussed below, the Department of Veterans Affairs (VA) is
notable for having bridged the gap integrating health services.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
172 HEPATITIS AND LIVER CANCER
The VA Medical Center in Minnesota developed a hospital-based model
that could serve as a template for health-care providers in integrated de-
livery systems, accountable care organizations, and HMOs (Groom et
al., 2008). The medical center established a method to screen patients for
HCV risk factors, to initiate appropriate viral testing, to counsel patients,
and to refer them to a dedicated hepatitis clinic for medical evaluations,
liver biopsies, and appropriate antiviral therapy. In addition, it traced the
outcome of therapy and continued to follow those who did not respond.
VA performed risk-assessment screening of 36,422 patients for HCV infec-
tion (Groom et al., 2008). The screening identified 12,485 patients (34%)
who had risk indications for anti-HCV testing. Anti-HCV antibodies were
identified in 681 (5.4% of those at risk) and HCV RNA was detected in 520
(4.2% of those at risk and 76% of those who were anti-HCV positive). Of
those who tested positive for HCV RNA, 430 (83%) were referred to the
hepatitis clinic, of whom 382 (73%) attended. A relatively large percentage
of patients (45%) were evaluated in the clinic and underwent liver biopsy.
On the basis of the extent of fibrosis on biopsy, 124 patients received anti-
viral therapy—32% of the patients referred to the clinic and 24% of those
who had viremia. A sustained virologic response occurred in 37% of the
treated patients. Thus, 46 patients could be considered cured, and 17 had
stage 3 or 4 fibrosis, which could potentially result in end-stage liver disease
and possibly HCC.
Closed systems, such as HMOs, and integrated delivery systems have
the potential to replicate the VA model. Those sources of health care
share the VA advantages of control of the various providers and care
settings, comprehensive coverage that reduces financial barriers to com-
pliance, administrative and information systems to track and share infor-
mation, team-based processes of care, and the ability to enforce standards
of performance.
The federal government is the largest purchaser of health insurance
nationally, with about 8 million people covered through the Federal Em-
ployees Health Benefits Program and those covered through Medicare,
Medicaid, and the Children’s Health Insurance Program. Given its tre-
mendous purchasing power, the federal government is well positioned to
be the leader in the development and enforcement of guidelines to ensure
that the people for whom it provides health care have access to risk-factor
screening, serologic testing for HBV and HCV, and appropriate medical
management.
Recommendation 5-1. Federally funded health-insurance programs—
such as Medicare, Medicaid, and the Federal Employees Health Ben-
efits Program—should incorporate guidelines for risk-factor screening
for hepatitis B and hepatitis C as a required core component of pre-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 173
ventive care so that at-risk people receive serologic testing for hepatitis
B virus and hepatitis C virus and chronically infected patients receive
appropriate medical management.
The committee has included recommendations regarding coverage of
vaccination for infants, children, and adults in Chapter 4.
Foreign-Born People
There are over 37 million foreign-born residents in the United States;
they represent about 12% of the nation’s population (U.S. Census Bureau,
2008). Of the foreign-born population, 27% were born in Asia, 4% in
Africa, and roughly 7% in other regions that have intermediate or high
HBV endemicity (see Box 3-1). Nearly half the US foreign-born population
(6% of the total population) originated in HBV-endemic countries (U.S.
Census Bureau, 2008), and 40,000–45,000 legal immigrants from these
countries enter the United States each year (U.S. Department of Homeland
Security, 2009; Weinbaum, 2008). It is increasingly urgent that culturally
appropriate programs provide hepatitis B screening and related services to
this high-risk population.
Efforts to deliver hepatitis B–related services to the foreign-born popu-
lation have been sparse. At the federal level, there are limited and frag-
mented resources to track and fund such services. On the local and regional
levels, some culturally tailored community-based or faith-based screening
programs target foreign-born people, such as those involving Asian and
Pacific Islander populations in San Francisco, Maryland, and New York
City (CDC, 2006; Chao et al., 2009a; Hsu et al., 2007). However, few of
the independent programs have been replicated in other communities of
at-risk foreign-born populations, so many regions in the United States that
have at-risk foreign-born populations lack community-based hepatitis B
screening (Rein et al., 2009). Few HBV screening programs are designed
for other high-risk foreign-born populations, including Africans, Middle
Easterners, eastern Europeans, and others from HBV-endemic regions. It
is unknown whether the model programs developed for Asians and Pacific
Islanders could be adapted for some of those populations or whether new
culturally tailored programs would need to be created.
The key to eliminating HBV transmission is identification of people
who are living with chronic HBV infection. As described in Chapter 3, there
is a pervasive ignorance about hepatitis B among Asians and Pacific Island-
ers, and it can be assumed that other foreign-born populations in the United
States are similarly uninformed about HBV risks, prevention, testing, and
management. That contributes to the observation that up to two-thirds of
those who are chronically infected with HBV are unaware of their infection
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
174 HEPATITIS AND LIVER CANCER
status (Lin et al., 2007). The lack of awareness in foreign-born populations
from HBV-endemic countries is compounded by gaps in knowledge and
preventive practice among health-care providers, particularly if they are
serving a large number of foreign-born, high-risk patients (see Chapter 3).
Cultural and institutional impediments are particularly important for
the foreign-born. For example, culture-specific stigmas may be attached to
a diagnosis of chronic hepatitis B. In China, there is discrimination against
people who are chronically infected with HBV, and such people reportedly
have been expelled from schools, fired from jobs, and shunned by other
community members despite the recent passage of national antidiscrimina-
tion laws (China Digital Times, 2009). Such social stigma and discrimina-
tion may contribute to the reluctance of immigrants from HBV-endemic
countries to undergo HBsAg testing or to seek medical attention for a
positive test result after settling in the United States.
Institutional barriers include administrative procedures and the absence
of culturally responsive support services. For example, a recent survey
of hospitals in the San Francisco Bay area—a region where 29% of the
population is foreign-born—found that fewer than half routinely collect
information on patients’ birthplaces (Gomez et al., 2003). The collection of
information on the birthplace of patients’ parents is even rarer—but relevant
for risk assessment. English-language proficiency and cultural preferences
of foreign-born patients may pose additional challenges to institutions that
are not prepared to work with these patient factors. Non-English-speaking
patients report that physicians are intolerant and impatient toward them
and fail to use interpreter services, even when available, to facilitate com-
munication (Barr and Wanat, 2005; Giordano and Cooper, 2009; Giordano
et al., 2009). As a result of patient–physician language discordance and
impaired communication, such patients have poorer comprehension of
medical conditions, testing, and treatment; have low compliance; and are
more likely to miss followup appointments (Giordano and Cooper, 2009;
Giordano et al., 2009; Jacobs et al., 2006; Manson, 1988; Zickmund et
al., 2004).
There is a need for evidence-based strategies and programs to dissemi-
nate information about hepatitis B transmission, infection, and treatment
to culturally and demographically diverse populations. A community-based
participatory research approach, in which communities are actively engaged
in equal partnership with scientists, is needed to ensure that the programs
are acceptable, accessible, and sustainable in the communities where they
are based. Such programs should also be flexible and scalable so that other
communities can tailor them to their own needs. The committee believes
that these tasks are best accomplished with the approach outlined in Rec-
ommendations 3-1 and 3-2 in Chapter 3. The community-based approach
as outlined in Recommendation 3-2 would be strengthened by additional
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 175
resources to provide screening, testing, and vaccination services. Therefore,
the committee offers the following recommendation:
Recommendation 5-2. The Centers for Disease Control and Prevention,
in conjunction with other federal agencies and state agencies, should
provide resources for the expansion of community-based programs
that provide hepatitis B screening, testing, and vaccination services that
target foreign-born populations.
Illicit-Drug Users
Preventing bloodborne infectious diseases, particularly hepatitis C, in
illicit-drug users is an important public-health challenge. Hepatitis C inci-
dence in IDUs has been reported to be 2–40 per 100 person-years (PY) of
observation, with most rates in the range of 15–30 per 100 PY (Maher et
al., 2006; Mathei et al., 2005; van den Berg et al., 2007b). HCV prevalence
in IDUs is typically 35–70%, depending on geographic location and dura-
tion of exposure to injection-drug use (Hagan et al., 2008). The early years
after onset of drug injection are high-risk periods when HCV seroconver-
sion rates are particularly high (Maher et al., 2006).
Non-injection-drug users (NIDUs) who sniff or snort heroin, cocaine,
and other drugs also have a high risk of HCV infection. A meta-analysis
of 26 studies showed that HCV prevalence in NIDUs was 2–35%, with
a median of 14% (Scheinmann et al., 2007). Whether drug practices,
sexual exposures, or both are the sources of HCV transmission is unclear
(Scheinmann et al., 2007). Low rates of HCV seroconversion have been
reported in NIDUs—0.4–2.7 per 100 PY—rates that are similar to those
observed in sex partners of HCV-RNA–positive persons (1.2 per 100 PY)
(Fuller et al., 2004; Neaigus et al., 2007; Rooney and Gilson, 1998). Stud-
ies have shown that HCV RNA can be detected on the surface of crack
pipes, so it is biologically plausible that drug-use practices are a route of
transmission in these people (Fischer et al., 2008). Research is needed to
explicate the etiology of HCV infection in NIDUs so that effective preven-
tion strategies can be designed.
To understand the development and opportunities for control of this
hyperendemic state of HCV infection in IDUs, it is important to consider
multiple features of the disease agent, the human host, and the environ-
ment that determine the occurrence of infection (Lillienfeld and Lillienfeld,
1980). HCV is efficiently transmitted via bloodborne exposure, and sev-
eral studies have shown that transmission can occur via the shared use of
syringes, drug cookers, and filtration cotton (Hagan et al., 2001; Hahn et
al., 2002; Thorpe et al., 2002). It takes only a very small amount of infec-
tious blood on injection equipment to result in infection. Awareness of risk
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
176 HEPATITIS AND LIVER CANCER
of HCV infection associated with the shared use of cookers and cotton is
not widespread, and about 40% of HCV transmission may be attributable
to the sharing of these items (Hagan et al., 2010). Injection often takes
place in settings that are chaotic, rushed, or otherwise not conducive to
safe practices, thereby increasing the risk of disease transmission (Rhodes
and Treloar, 2008). The persistence of moderate levels of unsafe injection
behaviors seems to be sufficient to maintain relatively high rates of new
infections (Thiede et al., 2007). The high prevalence of infectious carriers
also means that there is a high probability that one or more IDUs present
in the injection setting may be capable of transmitting HCV.
HBV infection rates in both IDUs and NIDUs are high. Seroincidence in
IDUs has been reported to be 10–12% per year (Hagan et al., 1999; Ruan
et al., 2007). A study that looked at evidence of past or present HBV infec-
tions found rates of 37% in IDUs and 19% in NIDUs (Kuo et al., 2004).
HBV transmission in these populations generally occurs as a result of drug-
related and sexual exposures to infected people. A study of more than 800
young IDUs (up to 30 years old) found low hepatitis B vaccination coverage
(22%) and a high prevalence of HBV infection (21%) (Lum et al., 2008).
Although drug use is associated with many serious acute and chronic
medical conditions, health-care utilization among drug users is low com-
pared with persons who do not use illicit drugs (Chitwood et al., 1999;
Contoreggi et al., 1998; O’Toole et al., 2007, 2008). Health care for both
IDUs and NIDUs is sporadic and generally received in hospital emer-
gency rooms, correction facilities, and STD clinics (Chitwood et al., 1999;
Huckans et al., 2005). Given this population’s limited access to health care
and services, it is important to have prevention and care services in settings
that IDUs and NIDUs are likely to frequent or to develop programs that
will draw them into care.
Program Venues
Because of its similarity to HIV in transmission routes, public-health
practitioners expected that strategies that were working for HIV would
work similarly in the case of HCV. The two major public-health interven-
tions that have been shown to reduce HIV risk in IDUs are drug-treatment
programs and syringe-exchange programs (SEPs) (Des Jarlais et al., 1996;
Metzger et al., 1998).
Drug-treatment programs offer few services related to hepatitis B and
hepatitis C and are constrained by lack of funding (Stanley, 1999). A na-
tionwide study of drug-treatment clinics found that although most clinics
educated patients about the importance of testing for HCV, only 7% tested
all clients for HCV and 22% tested none (Astone et al., 2003; Strauss et
al., 2002, 2004).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 177
Starting in the 1980s with the introduction of HIV into IDU popula-
tions, there were mass awareness and safe-injection campaigns that resulted
in substantial reductions in syringe-sharing (Des Jarlais and Semaan, 2008).
In the United States, SEPs are now available in 31 states, the District of
Columbia, and Puerto Rico (Des Jarlais et al., 2009). Several characteris-
tics support the use of SEPs as sites of care for and prevention of hepatitis
B and hepatitis C. SEPs, in addition to providing safe injection materials
and counseling services, are key access points for screening and referral
to followup medical care (Des Jarlais et al., 2009). SEPs appear to attract
and retain high-risk injectors, in particular those at highest risk for HIV
or HCV seroconversion (Hagan et al., 2001; Schechter et al., 1999). SEPs
can be referral pathways to other programs. Demand for drug treatment is
high among exchange users and SEPs are important venues for drug-treat-
ment referral (Kidorf et al., 2009; Strauss et al., 2003a). About 92% of US
SEPs offer referrals to substance-abuse treatment programs (Des Jarlais et
al., 2009), and 33% offer on-site medical care (Des Jarlais et al., 2009),
although the services offered vary and there is great geographic variability
in their distribution. Syringe coverage rates—the number of syringes avail-
able via SEPs per 100 injections—are also highly variable, ranging between
0.03 and 20 per 100 injections, and there are vast regions of the United
States where SEPs are not available (Tempalski et al., 2008). Those factors
limit the impact of the programs for HCV control (Tempalski et al., 2007,
2008). In addition, SEPs have not been as successful in reducing the shared
use of other injection equipment, such as cookers and cottons, as they have
been in reducing syringe-sharing (Hagan and Thiede, 2000).
Prevention Strategies
Several strategies to reduce HCV transmission in IDUs have been evalu-
ated. A number of studies have examined opiate-substitution treatment and
HCV seroconversion (summarized in Table 5-4). Results of those studies
suggest that retention in drug treatment is likely to be protective against
HCV seroconversion (Dolan et al., 2005; Rezza et al., 1996; Smyth et al.,
2000; Thiede et al., 2000). Retention in drug treatment may also be asso-
ciated with personal characteristics that are related to lower risk of HCV
infection, but in any case it appears that risk is reduced in persons who do
remain in treatment. It is plausible for drug treatment to reduce the risk of
HCV infection inasmuch as it reduces the frequency of injection, and some
IDUs stop injecting altogether. One limitation of drug-treatment programs
is that only a relatively small proportion of IDUs (about one-sixth) are in
treatment at any given time. In addition, the studies were limited to opiate-
substitution programs; cocaine injectors and other non-opiate injectors may
not experience similar benefits.

Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
178 HEPATITIS AND LIVER CANCER
TABLE 5-4 Studies of Association Between Opiate Substitution Treatment
and Hepatitis C Virus Seroconversion
Reference Location Design Results
Rezza et al., 1996 Italy Case-control MMTP protective against
HCV seroconversion (OR,
0.34; 95% CI, 0.1–1.1)
Crofts et al.,
1997
Melbourne,
Australia
Cohort HCV incidence in people
Continuously in MMTP:
36.9/100 PY (95% CI
19.1-70.9)
Interrupted MMTP:
14.2/100 PY (95% CI
6.3-31.6)
No MMTP: 21.4 (95% CI
8.0-57.0)
Thiede et al.,
2000
Seattle, WA Cohort
in MMTP at
enrollment
Reduced incidence in those
who continued treatment
vs those who left treatment
(adjusted OR, 0.4; 95%
CI, 0–4.2)
Patrick et al.,
2001
Vancouver,
Canada
Cohort Cumulative HCV incidence
25% in those in MMTP vs
42% in others
(p = 0.20)
Smyth et al.,
2003
Dublin, Ireland Cohort Lower incidence in those in
MMTP over 3 months vs
others (52 vs. 75 per 100
PY; p = 0.06)
Dolan et al.,
2005
Sydney,
Australia
Cohort of
incarcerated IDUs
Lowest incidence in those
in continuous MMTP
(8/100 PY) vs. those in for
less than 5 months (23/100
PY) (p = 0.01)
Maher et al.,
2006
Sydney,
Australia
Cohort Being in treatment during
the follow-up period
had no effect on HCV
seroconversion (OR = 0.83,
95% CI 0.51-1.35)
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; IDU, injection-drug user;
MMTP, methadone maintenance therapy program; NS, not significant; OR, odds ratio; PY,
person-years.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 179
Research results suggest that multicomponent risk reduction may be
needed to control HCV in active injectors. Some studies have shown that
the incidence of HCV infection continues to be high in IDUs who partici-
pate in SEPs (Hagan et al., 1999; Holtzman et al., 2009; Mansson et al.,
2000; Patrick et al., 2001). However, other studies have found associations
between SEPs and reductions in HCV infection rates. A case-control study
showed that use of a SEP in Tacoma, Washington, was associated with an
88% lower risk of HCV infection and an 82% lower risk of HBV infection
(Hagan et al., 1995). A study in Amsterdam showed that IDUs who had
“full participation in harm reduction”—they obtained all syringes from a
syringe exchange or received at least 60 mg of methadone per day—had
substantially and significantly lower rates of HCV seroconversion: fewer
than 5 per 100 PY versus more than 25 per 100 PY in others who did not
participate fully (van den Berg et al., 2007a).
A recent study of long-term IDUs who remained HIV- and HCV-
seronegative showed that they relied on a number of strategies to avoid
infection, including maintaining a regular supply of syringes and drug-prep-
aration equipment, managing their addiction by entering drug treatment as
needed to reduce their dosage, and maintaining social support to provide
stability (Mateu-Gelabert et al., 2007). Drug users who are successful in
avoiding infection have developed strategies to maintain control over their
chaotic lives. It is clear that HCV prevention is more challenging than HIV
prevention for IDUs and will require greater efforts and resources (Hagan
et al., 2008).
Research related to the practice of disinfecting syringes with bleach
indicates that it has no effect on HCV seroconversion (Hagan and Thiede,
2003; Kapadia et al., 2002). Development of new disinfecting agents that
are effective in drug-injection settings may contribute to prevention of HCV
infection in IDUs.
Other potentially useful prevention strategies focus on HCV education,
testing, and counseling. A large multicity randomized controlled trial of
young HIV-negative and HCV-negative IDUs showed that participation in
a six-session peer-education training program led to significant reductions
in unsafe injections (Garfein et al., 2007). A randomized controlled trial of
a similar intervention for HCV-positive young injectors also showed reduc-
tions in behavior that may transmit HCV (Latka et al., 2008).
Recommendations
Given the large set of factors that favor HCV transmission in IDUs, it
is not surprising that interventions that address individual aspects of risk
have not been shown to reduce incidence in individual injectors. In light
of the biology of HCV transmission—exposure to very small doses of in-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
180 HEPATITIS AND LIVER CANCER
fectious blood on injection equipment can result in infection—methods to
promote safe injection can be considered essential for HCV control. Safe-
injection strategies require access to sterile syringes and other equipment
and education to promote adoption and maintenance of safe behavior.
HCV testing and counseling to increase awareness of infection status will
also support safe practices. Access to sterile syringes and other equipment
can be increased through a combination of SEPs, pharmacy sales, and other
methods, such as the use of syringe-vending machines (Islam et al., 2008;
McDonald, 2009; Moatti et al., 2001). Drug treatment will reduce injection
frequency and assist a modest proportion of injectors to achieve abstinence.
Research has shown that none of those approaches by itself is sufficient
to eliminate HCV transmission. Because HCV prevention is a function
of multiple factors—safe-injection strategies, education, testing, and drug
treatment—an integrated program that includes all these essential elements
is more likely to be effective in preventing hepatitis C (see Figure 5-2).
Recommendation 5-3. Federal, state, and local agencies should expand
programs to reduce the risk of hepatitis C virus infection through
injection-drug use by providing comprehensive hepatitis C virus pre-
vention programs. At a minimum, the programs should include access
to sterile needle syringes and drug-preparation equipment because the
shared use of these materials has been shown to lead to transmission
of hepatitis C virus.
Recommendation 5-4. Federal and state governments should expand
services to reduce the harm caused by chronic hepatitis B and hepati-
Outreach and
other strategies to
attract drug-users
to services
Services
• HBV vaccination
• HCV testing with pretest
and posttest counseling
• Safe-injection education
• Provision of sterile
syringes, drug
preparation equipment,
and condoms
• Drug treatment
Post test
counseling
for HCV
• Seronegatives:
discussion of methods to
remain uninfected
• Seropositives: linkage to
care and discussion of
treatment and medical
management
Figure 5-2
redrawn
R01623
Hepatitis
FIGURE 5-2 Essential viral hepatitis services for illicit-drug users.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 181
tis C. The services should include testing to detect infection, counseling
to reduce alcohol use and secondary transmission, hepatitis B vaccina-
tion, and referral for or provision of medical management.
On the basis of current knowledge of the etiology and prevention of
HCV in IDUs, prevention strategies should include access to sterile injec-
tion equipment, safe-injection education, HCV testing and counseling, and
access to drug-treatment programs. Programs should include education
about safe drug use (avoiding the shared use of implements to administer
drugs by smoking or inhalation) and reduction in sex-related risks, and
all participants in the programs should be offered the hepatitis B vaccine.
The programs should be studied to elucidate the etiology of HCV infection
in IDUs and to guide the design of prevention programs. As mentioned
above, studies have shown that the first few years after onset of injection-
drug use constitute a high-risk period in which the rate of HCV infection
can exceed 40%. Preventing the transition from non-injection-drug use to
injection-drug use will probably avert many HCV infections. The commit-
tee therefore offers the following research recommendation.
Recommendation 5-5. Innovative, effective, multicomponent hepatitis
C virus prevention strategies for injection-drug users and non-injection-
drug users should be developed and evaluated to achieve greater con-
trol of hepatitis C virus transmission. In particular,
• Hepatitis C prevention programs for persons who smoke or
sniff heroin, cocaine, and other drugs should be developed and
tested.
• Programs to prevent the transition from noninjection use of il-
licit drugs to injection should be developed and implemented.
Pregnant Women
The Advisory Committee on Immunization Practices has recommended
routine screening of all pregnant women for HBsAg since 1988 (see Chap-
ter 4). The value and benefits of routine screening of pregnant women for
HBsAg were reaffirmed by the USPSTF in 2009 (U.S. Preventive Services
Task Force, 2009). Today, 27 states have maternal HBsAg-screening laws,
and 24 have specific maternal-HBsAg regulations that require health pro-
viders to report all cases of positive HBsAg blood tests to the local health
department (CDC, 2007).
More than 95% of pregnant women in the United States are tested
prenatally for HBsAg (Mast et al., 2005). States and large metropolitan
areas are eligible to receive federal funding to support perinatal hepatitis
B prevention programs through CDC’s National Center for Immunization
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
182 HEPATITIS AND LIVER CANCER
and Respiratory Disease (Jacques-Carroll et al., 2007). The programs are
administered by state and local public-health departments and vary in reach
and intensity. As mentioned in Chapter 2, many programs simply provide
surveillance, and others provide comprehensive case management that even
includes client home visits by local coordinators. That variability accounts
for the wide variation (17–59%) in rates of vaccination of household con-
tacts of HBsAg-positive pregnant women (Euler et al., 2003a).
Adequately funded perinatal hepatitis B programs are effective. Among
women enrolled in such programs for case management, the rate of admin-
istration of the birth dose of hepatitis B vaccine and HBIG was as high as
94%, with a three-dose completion rate of 71% (Jacques-Carroll, 2008).
Perinatal hepatitis B programs identify twice as many household and sexual
contacts per infant as was reported to the national database, with high rates
of programmatic compliance in households of foreign-born people (Euler
et al., 2003a). Most US programs are understaffed and underfunded, how-
ever, making adequate case management difficult. This gap has a two-fold
effect in that chronically infected women do not receive the appropriate
medical management and referral and perinatal transmission continues to
occur. CDC estimates that only 50% of HBsAg-positive pregnant women
are identified for case management (CDC, 2005). It has been estimated that
failure of perinatal HBV-prevention efforts result in about 1,000 cases of
chronic HBV infection in newborns each year (Ward, 2008b).
Hepatitis B Medical Management of Pregnant Women
An estimated 20,000 infants a year are born to women who test posi-
tive for HBV (Euler et al., 2003b). Those women require followup services
to ensure that they are knowledgeable about risks posed by their chronic
infection and that they receive appropriate referral for long-term medical
management. Their close contacts at home should be tested for HBV infec-
tion, those who are uninfected should be vaccinated, and medical referral
should be provided to those found to have chronic HBV infection. Cases
among household contacts are not uncommon when this risk group is pur-
sued aggressively for testing. Data reported by Euler et al. (2003a) showed
that 7–35% of household contacts of HBsAg-positive pregnant women
were HBsAg-positive.
Deficiencies in health-care providers’ knowledge or appropriate fol-
lowup of HBsAg-positive pregnant women are noteworthy and require
special emphasis in HBV prevention and control strategies. Obstetricians’
knowledge and preventive practices are suboptimal. In a 1997 study of
San Francisco obstetricians, over 90% of respondents acknowledged the
public-health importance of HBV infection and believed that HBV educa-
tion was feasible, but only 53% of the responding obstetricians offered
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 183
HBV information to their patients (Zola et al., 1997). In a more recent
California study of obstetrical practices in 2008, only 62% referred patients
who had new diagnoses of HBV to internists or specialists for followup of
their chronic HBV infection (Chao et al., 2009b).
Hepatitis B services for foreign-born pregnant women are in need of
improved resources that are more culturally and linguistically appropriate.
Some 73% of HBsAg-positive pregnant women in the United States were
born in East Asia or Southeast Asia (Din, 2009). Among them, Asian and
Pacific Islanders, who account for only 5.7% of all births in the United
States (CDC, 2009b), account for over two-thirds of births to mothers
who have chronic HBV infection. CDC-funded perinatal HBV prevention
coordinators are responsible for educating HBsAg-positive mothers and for
referring them for appropriate medical management. The coordinators are
restricted in their ability to fulfill that responsibility in culturally relevant
ways, because of inadequate training and resources (Chao et al., 2009a). To
strengthen the capacity and capabilities of the perinatal-HBV coordinators,
the committee offers the following recommendation:
Recommendation 5-6. The Centers for Disease Control and Prevention
should provide additional resources and guidance to perinatal hepa-
titis B prevention program coordinators to expand and enhance the
capacity to identify chronically infected pregnant women and provide
case-management services, including referral for appropriate medical
management.
Preventing Perinatal Transmission
Practice guidelines and additional recommendations focused on vac-
cination to prevent perinatal transmission are detailed in Chapter 4. There
is a need to fund research to guide the effective use of antiviral medications
late in pregnancy to prevent maternofetal HBV transmission, particularly
by high-risk pregnant women who are positive for HBeAg or who have
high HBV loads. Results of a few small studies have suggested that the use
of lamivudine in the last trimester of pregnancy reduces the rate of perina-
tal transmission from mothers with high HBV DNA (Li et al., 2003; van
Nunen et al., 2000; van Zonneveld et al., 2003). Xu et al. (2009) reported
the results of a small randomized, double-blind, placebo-controlled trial
involving 155 participants divided into treatment groups; the purpose of
the trial was to see whether lamivudine given during late pregnancy could
reduce perinatal transmission of HBV. Results suggested that lamivudine in
late pregnancy was safe and could reduce HBV transmission from mothers
who had high viral loads to their infants who also received HBIG pas-
sive immunization. However, the study was small, and large randomized,
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
184 HEPATITIS AND LIVER CANCER
double-blind, placebo-controlled trials are needed to evaluate the efficacy
and safety of oral hepatitis B antiviral therapy in eliminating perinatal HBV
transmission from women at high risk for perinatal transmission. Although
an increasing number of effective HBV antiviral suppressive medications
have become available for the management of chronic HBV infection, very
little research has been done on the use of these medications during the
last trimester of pregnancy to eliminate the risk of perinatal transmission,
particularly in the high-risk population of women who test positive for
HBeAg or have a high HBV load.
Recommendation 5-7. The National Institutes of Health should sup-
port a study of the effectiveness and safety of peripartum antiviral
therapy to reduce and possibly eliminate perinatal hepatitis B virus
transmission from women at high risk for perinatal transmission.
Correctional Settings
Incarcerated populations have higher rates of both HBV infection and
HCV infection than the general population. Correctional facilities present
a unique opportunity to bring viral hepatitis services to at-risk populations.
The period of incarceration is opportune for education about hepatitis B
and hepatitis C (see Chapter 3). Inmate peer-education programs have been
particularly effective for HIV/AIDS education and have also been used for
hepatitis education (Simmons, 2004), but relatively few prison systems
provide such programs (Collica, 2007).
Correctional facilities include both jails and prisons. Jails are operated
by county and local jurisdictions and house people who have been arrested
and are awaiting trial, people who have been convicted of misdemeanor
crimes, and people who have been convicted of felony crimes with short-
term sentences (usually less than one year). The length of stay in jail can be
a short as a few hours or longer than a year. Prisons are operated by states
and the federal government. They house people who have been convicted
of felony crimes with sentences generally of one year or longer. A few states
have combined systems that include both jail and prison inmates.
The prevalence of chronic HBV infection in correctional settings (pris-
ons and jails) is estimated to be 1–4%, and that of chronic HCV infec-
tion has been reported to vary from 12% to 35% (Boutwell et al., 2005;
Weinbaum et al., 2003). The high prevalence in this population is not pri-
marily a result of incarceration but rather indicative of people who engage
in risky behavior and were in risky settings before incarceration.
Hennessey et al. (2009) looked at HBV-infection and HCV-infection
prevalence in jail inmates. They found evidence of past HBV infections in
31% of Asian inmates, 21% of black inmates, 14% of white inmates, and
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 185
11% of Hispanic inmates. Asian inmates had the highest percentage of
chronic HBV infection (4.7%), and Hispanic inmates had the second high-
est percentage (3.6%). White inmates had the highest prevalence of HCV
infection (24%). People who had previously been incarcerated had higher
HCV prevalence in all age groups.
Correctional systems are constitutionally required to provide necessary
health care to inmates that is consistent with the community standard of
care. Screening of all incarcerated people for risk factors can identify those
for whom blood tests for infection are indicated, and the high prevalence
of HCV infection in prisons justifies such screening so that appropriate
treatment can be provided to inmates whose blood tests are positive. Al-
though screening, testing, and treatment could impose an economic burden
(Spaulding et al., 2006), a number of correctional systems have successfully
implemented medical management programs for viral hepatitis (Allen et al.,
2003; Chew et al., 2009; Farley et al., 2005; Maru et al., 2008; Sabbatani
et al., 2006; Sterling et al., 2004).
Hepatitis B vaccination in prisons is highly cost-effective; it was esti-
mated in 2002 to cost $415 per HBV infection averted (Pisu et al., 2002).
When made available, vaccinations in prisons have high uptake rates. Texas
and Michigan inmate vaccination uptake rates have been reportedly been
60–80% (Vallabhaneni et al., 2004). Vallabhaneni et al. (2004) found that
93% of 153 male inmates who were asked said that they would agree to
hepatitis B vaccination while incarcerated. Such prevention interventions save
society money because they reduce postincarceration morbidity and mortality
(Pisu et al., 2002). However, prison budgets have often not been sufficient to
provide hepatitis B vaccinations, or other HBV and HCV services.
To capitalize on inmate readiness to participate in hepatitis prevention
and control activities, correctional systems and public-health departments
need to collaborate to provide targeted testing, appropriate standard-of-
care medical management during incarceration, and followup medical ser-
vices after release into the community. However, there are several barriers
to such collaboration. Health departments and correctional facilities do
not always exchange health information, and it can be difficult to track
prisoners once they are released. State registries for hepatitis B and hepatitis
C cases are needed so that incarcerated persons with these diseases can be
quickly identified and properly managed once returned into local commu-
nities. The primary barrier to such collaboration is funding (McIntyre et
al., 2008). Most correctional systems do not initiate treatment for chronic
HCV infection unless an incarcerated person has sufficient time remaining
on his or her sentence to complete treatment, which generally takes 6–12
months (Spaulding et al., 2006).
Obstacles to collaboration between correctional systems and govern-
ment health institutions can be overcome. For example, in New York State,
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
186 HEPATITIS AND LIVER CANCER
an effort known as the Hepatitis C Continuity Program, which has been
in place since 2006, involves collaboration among the state Department of
Correctional Services, the state Department of Health, the state Division
of Parole, the New York City Health and Hospitals Corporation, medical
centers throughout the state, and manufacturers of medications for hepa-
titis C (Klein et al., 2007). Such collaborative programs should serve as a
model nationally.
Recommendation 5-8. The Centers for Disease Control and Preven-
tion and the Department of Justice should create an initiative to foster
partnerships between health departments and corrections systems to
ensure the availability of comprehensive viral hepatitis services for
incarcerated people.
The initiative should include at least the following:
• All incarcerated people should be offered screening and testing for
hepatitis B and hepatitis C.
• All susceptible incarcerated people should be offered hepatitis B
vaccine.
• Educational programs, including peer education, should include
emphasis on hepatitis B and hepatitis C.
• Systems should be developed to ensure the continuity of medical
management for hepatitis B and hepatitis C once infected persons
are released from incarceration.
Community Health Facilities
There is a great deal of variation in the types of viral hepatitis ser-
vices available within the United States. Several states—including Florida,
California, Massachusetts, and Texas—have attempted to introduce some
hepatitis services into publicly funded settings because of a lack of adequate
federal funding for hepatitis B and hepatitis C services. Florida has been
offering laboratory testing and vaccination through county health depart-
ments since 1999 by using a Hepatitis Prevention Program established
and funded by the state legislature (Baldy et al., 2007). The program has
expanded services and coordination between the county health depart-
ments, the Bureau of HIV/AIDS, the Bureau of Epidemiology, the Bureau
of Immunization, and the Bureau of Tuberculosis and Refugee Health.
Texas funded a program from 2000 to 2005 to support statewide HCV
counseling and testing among high-risk adults (Heseltine and McFarlane,
2007). The program also included a Web-based data-tracking system for
monitoring hepatitis C testing and counseling across the state. Of the al-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 187
most 40,000 tests performed, 23.2% were HCV-positive. Because funding
was inadequate, the number of tests administered dropped from almost
12,000 in 2003 to about 1,200 in 2004. As is apparent in the examples
above, a state-by-state approach of providing publicly funded viral-hepatitis
screening, testing, and care leads to wide variability in the type and quality
of services available in different regions and leaves many regions in need
without the necessary services.
As mentioned earlier in this chapter, the Health Resources and Services
Administration (HRSA) administers grant programs across the country
to deliver care to uninsured or underinsured people in community health
centers, migrant health centers, homeless programs, and public-housing pri-
mary-care programs. The role of federally funded community health facili-
ties is to provide critical and timely access to comprehensive primary-care
services to medically underserved communities. Data from HRSA’s Health
Center Program’s Uniform Data System showed that those facilities served
about 16.1 million patients in 2007 at over 7,000 service sites. Of the
patients seeking care, 91% were below the poverty level, 39% were unin-
sured, 930,589 were homeless, and 826,977 were migrant or seasonal farm
workers. The facilities also serve a high percentage of foreign-born people
(for example, refugee and immigrants). Such facilities often provide the only
health-care services available to disadvantaged populations, particularly in
rural areas. Although 20% of the US population lives in rural areas, just
11% of the nation’s physicians work there. People who live in rural areas
tend to have lower incomes and lower rates of health insurance, and they
are in poorer health than their urban counterparts (Ricketts, 2000). Com-
munity health centers are the sole source of primary care in many rural
areas (Regan et al., 2003; Ricketts, 2000; Rust et al., 2009; Wright, 2009).
For people who reside in urban areas, the barriers to health care are related
principally to health insurance, transportation, and information about af-
fordable care (Ahmed et al., 2001).
About one-third of patients who seek care at community health facili-
ties are uninsured, and uninsured patients who seek care at these facilities
are likely to use them as a medical home for primary care (Carlson et al.,
2001). For the most part, the types of services sought at these facilities are
similar to those sought by the general population and consist principally of
primary care (Henning et al., 2008). Patients at community health facilities
are also more likely to discuss health-promotion strategies than patients in
other primary-care settings (Carlson et al., 2001). The availability of these
facilities has also been shown to decrease the hospitalization rates in the
areas that they service (Probst et al., 2009).
The committee did not find published information on viral-hepatitis
services in community health facilities, but several studies have looked at
the quality of care for other chronic conditions and for preventive services,
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
188 HEPATITIS AND LIVER CANCER
such as immunizations. Those studies have found that despite serving dis-
advantaged populations, community health centers are able to offer high-
quality preventive and chronic health-care services at costs comparable
with those of facilities used by the general population (Appel et al., 2006;
Carlson et al., 2001; Christman et al., 2004; Eisert et al., 2008; Falik et al.,
2001; Hicks et al., 2006). Community health facilities have also been found
to mitigate racial and ethnic disparities in health-care delivery and services
(Appel et al., 2006; Christman et al., 2004; Eisert et al., 2008).
HRSA has oversight over its grantees and has the authority to imple-
ment health-care interventions on a national scale. HRSA facilities are well
positioned to develop and implement a national strategy to expand viral-
hepatitis services to medically underserved and often at-risk populations.
HRSA has no centralized viral-hepatitis prevention or control program
and is unable to determine the burden of hepatitis B and hepatitis C infec-
tions in the patients served in its programs (Raggio Ashley, 2009). Many
community health facilities already offer some viral-hepatitis services that
include prevention (such as immunizations), screening, testing and medical
management. However, there is little published information about these
programs.
Although there are no HRSA programs at a national level that focus
on viral hepatitis, there are programs for other health concerns that could
be used as models. For example, the Health Disparities Collaborative is a
national effort to eliminate health disparities and improve health-care de-
livery in HRSA service-delivery organizations, including community health
facilities. The initiative includes intervention to improve health-care deliv-
ery processes and chronic health conditions, such as asthma and diabetes
(Chin et al., 2004; HRSA, 2009b). It has improved the quality of care in
community health facilities for specific conditions (Landon et al., 2007).
Viral hepatitis is not one of the diseases included in the program, but this
type of program could be expanded to include viral-hepatitis services.
HRSA’s Uniform Data Systems (UDS) tracks a variety of information at
the national, state, and individual-grantee levels, such as community health
centers, migrant health centers, health-care programs for the homeless, and
public-housing primary-care programs. The information collected includes
patient demographics, services, staffing, clinical indicators, use rates, and
associated costs (HRSA, 2009a). Such data systems could potentially be
modified to include collection of data on viral-hepatitis services.
On the basis of those findings, the committee offers the following rec-
ommendation to expand the provision of viral hepatitis services:
Recommendation 5-9. The Health Resources and Services Administra-
tion should provide adequate resources to federally funded community
health facilities for provision of comprehensive viral-hepatitis services.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 189
Targeting Settings That Serve At-Risk Populations
Integrating viral hepatitis services in a broad array of settings creates
more opportunities to identify at-risk clients and to get them other services
that they need (Hoffman et al., 2004). STD/HIV clinics, shelter-based pro-
grams, and mobile health units are settings that serve populations that are
at risk for hepatitis B and hepatitis C.
STD–HIV Clinics
Clinical venues that provide screening, identification, and care for
people at risk for or infected with STDs and HIV present critical oppor-
tunities to provide similar viral hepatitis services. CDC has estimated that
almost 30% of people who have received a diagnosis of acute hepatitis B
have previously been treated for an STD (Goldstein et al., 2002). Among
HIV-infected people, rates of chronic hepatitis B are about 6–14%, and
rates of chronic hepatitis C about 33% (Alter, 2006; Sherman et al., 2002;
Sulkowski, 2008; Thio et al., 2002). In 2001, the National Alliance of State
and Territorial AIDS Directors recommended that state health programs
integrate HIV, STD, and viral hepatitis prevention services and that pro-
grams offer hepatitis A and hepatitis B vaccination; counseling and testing
for HIV, STDs, hepatitis B, and hepatitis C; and partner services and refer-
rals to additional prevention and health-care services (NASTAD, 2001).
CDC’s 2006 STD Treatment Guidelines recommend that all unvaccinated
persons attending STD clinics receive the hepatitis B vaccine (Workowski
and Berman, 2006). The concept of integrating hepatitis B vaccination into
STD clinics has been accepted and needs to be expanded to all STD clinic
venues.
Some progress has been made in the integration of viral hepatitis ser-
vices into health-care settings, such as STD or HIV clinics, that serve high-
risk populations. A study by Gilbert et al. (2005) showed that many STD
clinics have effectively introduced a policy and a plan for hepatitis B pre-
vention; 55% of STD clinics had come to consider hepatitis B vaccination
a program responsibility, and 78% had established a vaccination program.
From 1997 to 2001, there was a marked increase in the proportion of clin-
ics that offered hepatitis B vaccine (from 61% to 82%), provided hepatitis
B educational materials (from 49% to 84%), and accessed federal vaccina-
tion programs (from 48% to 84%). In areas where a state STD program
had distributed a hepatitis B prevention plan, 88% of STD clinics offered
hepatitis B vaccination compared with 50% in areas where a prevention
plan had not been developed. The main obstacles cited were the lack of re-
sources for services and low patient compliance. The need for and effect of
hepatitis B vaccination was underscored in a study of an urban STD clinic
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
190 HEPATITIS AND LIVER CANCER
in San Diego that began to offer risk-factor screening, laboratory testing,
and immunizations services in 1998 (Gunn et al., 2007); the program in-
cluded risk-factor screening of 21,631 people and found that about 69%
of patients offered the hepatitis B vaccine accepted it.
A study of risk factors for hepatitis C and laboratory testing of people
who sought care at an STD clinic found that 4.9% of the 3,367 attendees
who were tested for HCV infection were positive (Gunn et al., 2003). Al-
most 85% of those who tested positive learned of their infection for the
first time through this screening process.
Subiadur et al. (2007) found that viral hepatitis prevention services can
be incorporated into a busy STD clinic if staff and resources are available.
Similarly, an evaluation of Texas’s HCV program found that staff did not
find it difficult to integrate hepatitis C services if sufficient resources were
available, such as access to laboratory testing and adequate staffing levels
(Heseltine and McFarlane, 2007).
Integrating viral hepatitis services into existing programs increases the
opportunity for people to identify other unmet health needs or conditions.
A study that assessed the integration of viral hepatitis services (vaccination
and screening) into a New York City STD clinic found that the services
attracted at-risk people to the clinic and that they benefited from the other
services offered (Hennessy et al., 2007). Of 8,778 people in the STD clinic
who received hepatitis services, 279 (3%) were self-reported IDUs and 161
(58% of these) reported that the availability of hepatitis services was the
primary reason for their clinic visit. Among the 161, 12 new STDs and two
HIV infections were diagnosed. IDUs made up only a small proportion of
those who attended STD clinics in this demonstration project, but it seems
clear that some IDUs will seek hepatitis services if they are offered without
charge.
As with STD clinics, there are data that indicate that viral hepatitis pre-
vention and care can be integrated into HIV clinics. The USPHS guidelines
for management of opportunistic infections in HIV-infected persons include
guidance for detection and management of chronic viral hepatitis (CDC,
2002). The guidelines call for testing of all HIV-infected persons for chronic
hepatitis B and hepatitis C and for provision of hepatitis A and hepatitis B
vaccination to those who are susceptible. In addition, there are guidelines
for medical treatment of those who are chronically infected. There are
data that suggest that a much lower proportion of patients actually receive
treatment for chronic viral hepatitis. A study of 845 HIV–HCV coinfected
patients who attended the Johns Hopkins HIV Clinic in Baltimore found
277 were referred for hepatitis C care. Of those patients referred to care,
only 185 of these came for more than one appointment, 125 completed
a pretreatment assessment, and 29 started HCV treatment (Mehta et al.,
2006).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 191
Shelter-Based Programs
People who are temporarily or consistently homeless are at increased
risk for infectious diseases, including hepatitis B and hepatitis C, because of
poor living conditions, poor access to health care, high prevalence of drug
use, sexual contact with multiple partners, and sharing of personal-hygiene
equipment, such as razors (Badiaga et al., 2008; Boyce et al., 2009). The
prevalence of HIV, HBV, and HCV among drug-involved street sex workers
in Miami, Florida, was 22.4%, 53.4%, and 29.7%, respectively; and 42%
of participants were homeless (Inciardi et al., 2006). Homeless adolescents
and runaways are at particular risk because they are less likely than their
peers to be vaccinated for HBV and to have access to health care and are
more likely to engage in risky behaviors, such as drug use and sex work
(Sneller et al., 2008).
The current literature suggests that public-health programs for the
homeless should address issues related to unsafe sex, drug abuse, homeless-
ness, and other lifestyle factors that contribute to adverse health outcomes.
Reaching that population is difficult, and appropriate street-based and
shelter-based interventions are potentially effective in doing so. Collabora-
tion among providers of services to the homeless will be needed to provide
counseling, education, testing, and such interventions as condom distribu-
tion, syringe-access programs, and vaccination against HBV (Badiaga et al.,
2008; Boyce et al., 2009; Inciardi et al., 2006; Rosenheck et al., 2003; Roy
et al., 2007; Sneller et al., 2008). All homeless persons should be offered
the hepatitis B vaccine. A study of vaccination of homeless adults found
that reducing HBV-related disease through vaccinations in this population
is cost-effective and is associated with substantial improvements in quality
of life (Greengold et al., 2009).
Mobile Health Units
Community-based mobile services, such as the use of mobile health
vans, can mitigate some access issues. Programs that use mobile health-care
vans have been successful in providing HIV prevention and testing services
to at-risk people who might not seek health-care services in other settings.
Street outreach programs have been successful in reaching marginalized
populations in HIV/AIDS prevention programs (Valentine and Wright-De
Aguero, 1996). Kahn et al. (2003) found that mobile vans are a feasible
approach to community-based STD screening and treatment, are accepted
by the community, and are capable of identifying people who have STDs.
A study of polysubstance abuse and HIV/STD risk behaviors in men who
have sex with men used a mobile van as the service access point and found
that polysubstance users had high rates of uninsurance (21%) and that 96%
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
192 HEPATITIS AND LIVER CANCER
were first-time users of mobile health-van services (Mimiaga et al., 2008).
Mobile vans have some drawbacks. Shrestha et al. (2008) reviewed the
cost effectiveness of clinic-based versus mobile outreach efforts to identify
HIV cases. They found that the cost of providing a new HIV diagnosis was
considerably higher in the outreach settings than in the clinic (Clark et al.,
2008). However, a clinic setting is effective only if clients are drawn to the
facility. Hence, innovative approaches of this type should be considered for
hard-to-reach populations.
Recommendation
Integration of viral hepatitis services into venues such as STD-HIV clin-
ics, shelters, and mobile health units, is likely to have long-term benefits
because most of the people who use these types of clinics engage in high-risk
behaviors or are in high-risk settings. Therefore, the committee offers the
following recommendation:
Recommendation 5-10. The Health Resources and Services Admin-
istration and the Centers for Disease Control and Prevention should
provide resources and guidance to integrate comprehensive viral hepa-
titis services into settings that serve high-risk populations such as STD
clinics, sites for HIV services and care, homeless shelters, and mobile
health units.
REFERENCES
Ahmed, S. M., J. P. Lemkau, N. Nealeigh, and B. Mann. 2001. Barriers to healthcare access
in a non-elderly urban poor American population. Health & Social Care in the Com-
munity 9(6):445-453.
Allen, S. A., A. C. Spaulding, A. M. Osei, L. E. Taylor, A. M. Cabral, and J. D. Rich. 2003.
Treatment of chronic hepatitis C in a state correctional facility. Annals of Internal Medi-
cine 138(3):187-190.
Alter, M. J. 2006. Epidemiology of viral hepatitis and HIV co-infection. Journal of Hepatol-
ogy 44(Suppl 1):S6-S9.
Alter, M. J., W. L. Kuhnert, and L. Finelli. 2003. Guidelines for laboratory testing and result
reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention.
Morbidity and Morality Weekly: Recommendations and Reports 52(RR-3):1-13, 15;
quiz CE11-CE14.
Alter, M. J., L. B. Seeff, B. R. Bacon, D. L. Thomas, M. O. Rigsby, and A. M. Di Bisceglie.
2004. Testing for hepatitis C virus infection should be routine for persons at increased
risk for infection. Annals of Internal Medicine 141(9):715-717.
Appel, A., R. Everhart, P. S. Mehler, and T. D. MacKenzie. 2006. Lack of ethnic disparities
in adult immunization rates among underserved older patients in an urban public health
system. Medical Care 44(11):1054-1058.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 193
Armstrong, G. L., A. Wasley, E. P. Simard, G. M. McQuillan, W. L. Kuhnert, and M. J. Alter.
2006. The prevalence of hepatitis C virus infection in the United States, 1999 through
2002. Annals of Internal Medicine 144(10):705-714.
Asian Liver Center. 2009. Jade Ribbon Campaign. http://liver.stanford.edu/Outreach/JRC.html
(accessed August 29, 2009).
Astone, J., S. M. Strauss, Z. P. Vassilev, and D. C. Des Jarlais. 2003. Provision of hepatitis C
education in a nationwide sample of drug treatment programs. Journal of Drug Educa-
tion 33(1):107-117.
Badiaga, S., D. Raoult, and P. Brouqui. 2008. Preventing and controlling emerging and re-
emerging transmissible diseases in the homeless. Emerging Infectious Diseases 14(9):
1353-1359.
Baldy, L. M., C. Urbas, J. L. Harris, T. S. Jones, and P. E. Reichert. 2007. Establishing a viral
hepatitis prevention and control program: Florida’s experience. Public Health Reports
122(Suppl 2):24-30.
Barr, D. A., and S. F. Wanat. 2005. Listening to patients: Cultural and linguistic barriers to
health care access. Family Medicine 37(3):199-204.
Begley, E. B., A. M. Oster, B. Song, L. Lesondak, K. Voorhees, M. Esquivel, R. L. Merrick, J.
Carrel, D. Sebesta, J. Vergeront, D. Shrestha, and J. D. Heffelfinger. 2008. Incorporating
rapid HIV testing into partner counseling and referral services. Public Health Reports
123(Suppl 3):126-135.
Birkhead, G. S., S. J. Klein, A. R. Candelas, D. A. O’Connell, J. R. Rothman, I. S. Feldman,
D. S. Tsui, R. A. Cotroneo, and C. A. Flanigan. 2007. Integrating multiple programme
and policy approaches to hepatitis C prevention and care for injection drug users: A
comprehensive approach. International Journal of Drug Policy 18(5):417-425.
Boutwell, A. E., S. A. Allen, and J. D. Rich. 2005. Opportunities to address the hepatitis C
epidemic in the correctional setting. Clinical Infectious Diseases 40(s5):S367-S372.
Bowles, K. E., H. A. Clark, E. Tai, P. S. Sullivan, B. Song, J. Tsang, C. A. Dietz, J. Mir, A.
Mares-DelGrasso, C. Calhoun, D. Aguirre, C. Emerson, and J. D. Heffelfinger. 2008.
Implementing rapid HIV testing in outreach and community settings: Results from an
advancing HIV prevention demonstration project conducted in seven U.S. Cities. Public
Health Reports 123(Suppl 3):78-85.
Boyce, D. E., A. D. Tice, F. V. Ona, K. T. Akinaka, and H. Lusk. 2009. Viral hepatitis in a
homeless shelter in Hawai’i. Hawaii Medical Journal 68(5):113-115.
Brown, L. S., S. Kritz, R. J. Goldsmith, E. J. Bini, J. Robinson, D. Alderson, and J. Rotrosen.
2007. Health services for HIV/AIDS, HCV, and sexually transmitted infections in sub-
stance abuse treatment programs. Public Health Reports 122(4):441-451.
Bruix, J., and M. Sherman. 2005. Management of hepatocellular carcinoma. Hepatology
42(5):1208-1236.
Butt, A. A., A. C. Justice, M. Skanderson, M. O. Rigsby, C. B. Good, and C. K. Kwoh. 2007.
Rate and predictors of treatment prescription for hepatitis C. Gut 56(3):385-389.
Butt, A. A., K. A. McGinnis, M. Skanderson, and A. C. Justice. 2009. Hepatitis C treatment
completion rates in routine clinical care. Liver International 1478-3223.
Campos, N. G., J. A. Salomon, J. C. Servoss, D. P. Nunes, J. H. Samet, K. A. Freedberg, and
S. J. Goldie. 2007. Cost-effectiveness of treatment for hepatitis C in an urban cohort co-
infected with HIV. American Journal of Medicine 120(3):272-279.
Carey, W. 2003. Tests and screening strategies for the diagnosis of hepatitis C. Cleveland Clinic
Journal of Medicine 70(Suppl 4):S7-S13.
Carlson, B. L., J. Eden, D. O’Connor, and J. Regan. 2001. Primary care of patients with-
out insurance by community health centers. Journal of Ambulatory Care Management
24(2):47-59.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
194 HEPATITIS AND LIVER CANCER
Castelnuovo, E., J. Thompson-Coon, M. Pitt, M. Cramp, U. Siebert, A. Price, and K. Stein.
2006. The cost-effectiveness of testing for hepatitis C in former injecting drug users.
Health Technology Assessment 10(32): III-IV, IX-XII, 1-93.
CDC (Centers for Disease Control and Prevention). 1998. Recommendations for prevention
and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Cen-
ters for Disease Control and Prevention. Morbidity and Morality Weekly: Recommenda-
tions and Reports 47(RR-19):1-39.
———. 2001. National hepatitis C prevention strategy: A comprehensive strategy for the
prevention and control of hepatitis C virus infection and its consequences. Atlanta, GA:
CDC. http://www.cdc.gov/hepatitis/HCV/Strategy/PDFs/NatHepCPrevStrategy.pdf (ac-
cessed August 21, 2009)
———. 2002. 2001 USPHS/IDSA guidelines for the prevention of opportunistic infections in
persons infected with human immunodeficiency virus. Infectious Diseases in Obstetrics
and Gynecology 10(1):3-64.
———. 2005. A comprehensive immunization strategy to eliminate transmission of hepatitis
B virus in the United States. Part 1: Immunization of infants, children, and adolescents.
Morbidity and Mortality Weekly Report 54(RR16):1-23.
———. 2006. Screening for chronic hepatitis B among Asian/Pacific Islander populations—
New York City, 2005. Morbidity and Mortality Weekly Report 55(18):505-509.
———. 2007. National immunization survey: Hepatitis B vaccine birth-dose rates. http://
www.cdc.gov/Hepatitis/Partners/PeriHepBCoord.htm (accessed June 28, 2009).
———. 2009a. About the Division of Viral Hepatitis. http://www.cdc.gov/hepatitis/AboutUs.
htm (accessed August 21, 2009).
———. 2009b. Births: Final data for 2006. National Vital Statistics Reports 57(7). http://http://
www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_07.pdf (accessed August 21, 2009).
———. 2009c. Interpretation of hepatitis B serologic test results. http://www.cdc.gov/hepatitis/
HBV/PDFs/SerologicChartv8.pdf (accessed August 21, 2009).
———. 2009d. Perinatal hepatitis B coordinator list. http://www.cdc.gov/vaccines/vpd-vac/
hepb/perinatal-contacts.htm (accessed August 18, 2009).
———. 2009e. Reference for the interpretation of hepatitis C virus (HCV) test results. http://
www.cdc.gov/hepatitis/HCV/PDFs/hcv_graph.pdf (accessed August 21, 2009).
———. 2009f. Report on the status of state viral hepatitis plans for the Institute of Medicine
executive summary of responses (n=55). National Hepatitis TA Center, New York State
Department of Health.
———. 2009g. Status of state electronic disease surveillance systems—United States, 2007.
Morbidity and Mortality Weekly Report 58(29):804-807.
Chao, S. D., E. T. Chang, and S. K. So. 2009a. Eliminating the threat of chronic hepatitis B
in the Asian and Pacific Islander community: A call to action. Asian Pacific Journal of
Cancer Prevention 10(3):497-512.
Chao, S. D., C. Cheung, A. Yue, and S. K. So. 2009b. Low hepatitis B knowledge among pe-
rinatal healthcare providers serving county with nation’s highest rate of births to mothers
chronically infected with hepatitis B. Paper presented at Poster presentation at the 13th
Internationational Symposium on Viral Hepatitis and Liver Disease, Washington, DC.
March 20-24, 2009.
Chesson, H. W., J. M. Blandford, T. L. Gift, G. Tao, and K. L. Irwin. 2004. The estimated
direct medical cost of sexually transmitted diseases among American youth, 2000. Per-
spectives on Sexual and Reproductive Health 36(1):11-19.
Cheung, R. C., S. Currie, H. Shen, S. B. Ho, E. J. Bini, B. S. Anand, N. Brau, and T. L. Wright.
2005. Chronic hepatitis C in latinos: Natural history, treatment eligibility, acceptance,
and outcomes. American Journal of Gastroenterology 100(10):2186-2193.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 195
Chew, K. W., S. A. Allen, L. E. Taylor, J. D. Rich, and E. Feller. 2009. Treatment outcomes
with pegylated interferon and ribavirin for male prisoners with chronic hepatitis C.
Journal of Clinical Gastroenterology 43(7):686-691.
Chin, M. H., S. Cook, M. L. Drum, L. Jin, M. Guillen, C. A. Humikowski, J. Koppert,
J. F. Harrison, S. Lippold, and C. T. Schaefer. 2004. Improving diabetes care in mid-
west community health centers with the health disparities collaborative. Diabetes Care
27(1):2-8.
China Digital Times. 2009. China news tagged with: hepatitis B. http://chinadigitaltimes.
net/china/hepatitis-b/ (accessed August 21, 2009).
Chitwood, D. D., D. C. McBride, M. T. French, and M. Comerford. 1999. Health care need
and utilization: A preliminary comparison of injection drug users, other illicit drug users,
and nonusers. Substance Use and Misuse 34(4-5):727-746.
Choe, J. H., V. M. Taylor, Y. Yasui, N. Burke, T. Nguyen, E. Acorda, and J. C. Jackson. 2006.
Health care access and sociodemographic factors associated with hepatitis B testing in
Vietnamese American men. Journal of Immigrant and Minority Health 8(3):193-201.
Chou, R., E. C. Clark, and M. Helfand. 2004. Screening for hepatitis C virus infection: A
review of the evidence for the U.S. Preventive Services Task Force. Annals of Internal
Medicine 140(6):465-479.
Christman, L. K., R. Abdulla, P. B. Jacobsen, A. B. Cantor, D. Y. Mayhew, K. S. Thompson,
J. P. Krischer, and R. G. Roetzheim. 2004. Colorectal cancer screening among a sample
of community health center attendees. Journal of Health Care for the Poor and Under-
served 15(2):281-293.
Clark, H. A., K. E. Bowles, B. Song, and J. D. Heffelfinger. 2008. Implementation of rapid HIV
testing programs in community and outreach settings: Perspectives from staff at eight
community-based organizations in seven U.S. Cities. Public Health Reports 123(Suppl
3):86-93.
Claxton, G., B. DiJulio, B. Finder, E. Becker, S. Hawkins, J. Pickreign, H. Whitmore, and J.
Gabel. 2007. Kaiser and the Health Research and Education Trust survey of employer-
sponsored health benefits, 1999-2007. Chicago, IL.
Collica, K. 2007. The prevalence of HIV peer programming in American prisons: An oppor-
tunity wasted. Journal of Correctional Health Care 13:278.
Contoreggi, C., V. E. Rexroad, and W. R. Lange. 1998. Current management of infec-
tious complications in the injecting drug user. Journal of Substance Abuse Treatment
15(2):95-106.
Crofts, N., R. Louie, and B. Loff. 1997. The next plague: Stigmatization and discrimination re-
lated to hepatitis C virus infection in Australia. Health and Human Rights 2(2):87-97.
Damen, M., H. Zaaijer, H. Cuypers, H. Vrielink, C. Poel, H. Reesink, and P. Lelie. 1995.
Reliability of the third-generation recombinant immunoblot assay for hepatitis C virus.
Transfusion 35(9):745-749.
Davila, J. A., and H. B. El-Serag. 2006. Racial differences in survival of hepatocellular car-
cinoma in the United States: A population-based study. Clinical Gastroentorology and
Hepatology 4(1):104-110; quiz 104-105.
Des Jarlais, D. C., and S. Semaan. 2008. HIV prevention for injecting drug users: The first 25
years and counting. Psychosomatic Medicine 70(5):606-611.
Des Jarlais, D. C., M. Marmor, D. Paone, S. Titus, Q. Shi, T. Perlis, B. Jose, and S. R.
Friedman. 1996. HIV incidence among injecting drug users in New York city syringe-
exchange programmes. Lancet 348(9033):987-991.
Des Jarlais, D. C., C. McKnight, C. Goldblatt, and D. Purchase. 2009. Doing harm reduction
better: Syringe exchange in the United States. Addiction 104(9):1441-1446.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
196 HEPATITIS AND LIVER CANCER
Din, E. 2009. Estimating the number of births to hepatitis B surface antigen-positive women
in select US states, 2004. Paper presented at 13th International Symposium of Viral
Hepatitis and Liver Disease, Washington, DC. March 20-24, 2009.
Dolan, K. A., J. Shearer, B. White, J. Zhou, J. Kaldor, and A. D. Wodak. 2005. Four-year
follow-up of imprisoned male heroin users and methadone treatment: Mortality, re-
incarceration and hepatitis C infection. Addiction 100(6):820-828.
Eisert, S. L., P. S. Mehler, and P. A. Gabow. 2008. Can America’s urban safety net systems be
a solution to unequal treatment? Journal of Urban Health 85(5):766-778.
Esteve, M., C. Saro, F. Gonzalez-Huix, F. Suarez, M. Forne, and J. M. Viver. 2004. Chronic
hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: Need
for primary prophylaxis. Gut 53(9):1363-1365.
Euler, G. L., J. Copeland, and W. W. Williams. 2003a. Impact of four urban perinatal hepatitis
B prevention programs on screening and vaccination of infants and household members.
American Journal of Epidemiology 157(8):747-753.
Euler, G. L., J. R. Copeland, M. C. Rangel, and W. W. Williams. 2003b. Antibody response to
postexposure prophylaxis in infants born to hepatitis B surface antigen-positive women.
Pediatric Infectious Disease Journal 22(2):123-129.
Falik, M., J. Needleman, B. L. Wells, and J. Korb. 2001. Ambulatory care sensitive hospital-
izations and emergency visits: Experiences of Medicaid patients using federally qualified
health centers. Medical Care 39(6):551-561.
Farley, J., S. Vasdev, B. Fischer, E. Haydon, J. Rehm, and T. A. Farley. 2005. Feasibility and
outcome of HCV treatment in a Canadian federal prison population. American Journal
of Public Health 95(10):1737-1739.
Fischer, B., J. Powis, M. Firestone Cruz, K. Rudzinski, and J. Rehm. 2008. Hepatitis C virus
transmission among oral crack users: Viral detection on crack paraphernalia. European
Journal of Gastroenterology and Hepatology 20(1):29-32.
Fuller, C. M., D. C. Ompad, S. Galea, Y. Wu, B. Koblin, and D. Vlahov. 2004. Hepatitis C
incidence—a comparison between injection and noninjection drug users in New York
city. Journal of Urban Health 81(1):20-24.
Garfein, R. S., E. T. Golub, A. E. Greenberg, H. Hagan, D. L. Hanson, S. M. Hudson, F.
Kapadia, M. H. Latka, L. J. Ouellet, D. W. Purcell, S. A. Strathdee, and H. Thiede. 2007.
A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C
virus infection in young injection drug users. AIDS 21(14):1923-1932.
Ghany, M. G., D. B. Strader, D. L. Thomas, and L. Seeff. 2009. Diagnosis, management, and
treatment of hepatitis C: An update. Hepatology 49(4):1335-1374.
Gilbert, L. K., J. Bulger, K. Scanlon, K. Ford, D. Bergmire-Sweat, and C. Weinbaum. 2005.
Integrating hepatitis B prevention into sexually transmitted disease services: U.S. sexually
transmitted disease program and clinic trends—1997 and 2001. Sexually Transmitted
Diseases 32(6):346-350.
Giordano, C., and C. Cooper. 2009. The influence of race and language on chronic hepatitis
C virus infection management. European Journal of Gastroenterology and Hepatology
21(2):131-136.
Giordano, C., E. F. Druyts, G. Garber, and C. Cooper. 2009. Evaluation of immigration
status, race and language barriers on chronic hepatitis C virus infection management
and treatment outcomes. European Journal of Gastroenterology and Hepatology 21(9):
963-968.
Goldstein, S. T., M. J. Alter, I. T. Williams, L. A. Moyer, F. N. Judson, K. Mottram, M. Fleenor,
P. L. Ryder, and H. S. Margolis. 2002. Incidence and risk factors for acute hepatitis B in
the United States, 1982-1998: Implications for vaccination programs. Journal of Infec-
tious Diseases 185(6):713-719.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 197
Gomez, S. L., G. M. Le, D. W. West, W. A. Satariano, and L. O’Connor. 2003. Hospital policy
and practice regarding the collection of data on race, ethnicity, and birthplace. American
Journal of Public Health 93(10):1685-1688.
Greengold, B., A. Nyamathi, G. Kominski, D. Wiley, M. A. Lewis, F. Hodge, M. Singer, and
B. Spiegel. 2009. Cost-effectiveness analysis of behavioral interventions to improve vac-
cination compliance in homeless adults. Vaccine 27(5):718-725.
Groom, H., E. Dieperink, D. B. Nelson, J. Garrard, J. R. Johnson, S. L. Ewing, H. Stockley,
J. Durfee, Y. Jonk, M. L. Willenbring, and S. B. Ho. 2008. Outcomes of a hepatitis C
screening program at a large urban VA medical center. Journal of Clinical Gastroenterol-
ogy 42(1):97-106.
Gunn, R. A., M. A. Lee, P. J. Murray, R. A. Gilchick, and H. S. Margolis. 2007. Hepatitis
B vaccination of men who have sex with men attending an urban STD clinic: Im-
pact of an ongoing vaccination program, 1998-2003. Sexually Transmitted Diseases
34(9):663-668.
Gunn, R. A., P. J. Murray, C. H. Brennan, D. B. Callahan, M. J. Alter, and H. S. Margolis.
2003. Evaluation of screening criteria to identify persons with hepatitis C virus infec-
tion among sexually transmitted disease clinic clients: Results from the San Diego viral
hepatitis integration project. Sexually Transmitted Diseases 30(4):340-344.
Hagan, H., and H. Thiede. 2000. Changes in injection risk behavior associated with participa-
tion in the Seattle needle-exchange program. Journal of Urban Health 77(3):369-382.
———. 2003. Does bleach disinfection of syringes help prevent hepatitis C virus transmission?
Epidemiology 14(5):628-629; author reply 629.
Hagan, H., D. C. Jarlais, S. R. Friedman, D. Purchase, and M. J. Alter. 1995. Reduced risk of
hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange
program. American Journal of Public Health 85(11):1531-1537.
Hagan, H., J. P. McGough, H. Thiede, N. S. Weiss, S. Hopkins, and E. R. Alexander. 1999.
Syringe exchange and risk of infection with hepatitis B and C viruses. American Journal
of Epidemiology 149(3):203-213.
Hagan, H., E. R. Pouget, D. C. Des Jarlais, and C. Lelutiu-Weinberger. 2008. Meta-regression
of hepatitis C virus infection in relation to time since onset of illicit drug injection: The
influence of time and place. American Journal of Epidemiology 168(10):1099-1109.
Hagan, H., E. R. Pouget, I. T. Williams, R. L. Garfein, S. A. Strathdee, S. M. Hudson, M.
Latka, and L. Ouellet. 2010. Attribution of HCV seroconversion risk in young injection
drug users in five U.S. cities. Journal of Infectious Diseases 201(3):328-385.
Hagan, H., H. Thiede, N. S. Weiss, S. G. Hopkins, J. S. Duchin, and E. R. Alexander. 2001.
Sharing of drug preparation equipment as a risk factor for hepatitis C. American Journal
of Public Health 91(1):42-46.
Hahn, J. A., K. Page-Shafer, P. J. Lum, P. Bourgois, E. Stein, J. L. Evans, M. P. Busch, L. H.
Tobler, B. Phelps, and A. R. Moss. 2002. Hepatitis C virus seroconversion among
young injection drug users: Relationships and risks. Journal of Infectious Diseases
186(11):1558-1564.
Hand, W. L., and Y. Vasquez. 2005. Risk factors for hepatitis C on the Texas–Mexico border.
American Journal of Gastroenterology 100:2180–2185.
Harm Reduction Coalition. 2009. Hepatitis C. http://www.harmreduction.org/article.
php?list=type&type=50 (accessed August 21, 2009).
Hennessey, K. A., A. A. Kim, V. Griffin, N. T. Collins, C. M. Weinbaum, and K. Sabin. 2009.
Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three
jails: A case for viral hepatitis prevention in jails in the United States. Journal of Urban
Health 86(1):93-105.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
198 HEPATITIS AND LIVER CANCER
Hennessy, R. R., I. B. Weisfuse, and K. Schlanger. 2007. Does integrating viral hepatitis ser-
vices into a public STD clinic attract injection drug users for care? Public Health Reports
122(Suppl 2):31-35.
Henning, G. F., M. Graybill, and J. George. 2008. Reason for visit: Is migrant health care that
different? Journal of Rural Health 24(2):219-220.
Heseltine, G., and J. McFarlane. 2007. Texas statewide hepatitis C counseling and testing,
2000-2005. Public Health Reports 122(Suppl 2):6-11.
Hicks, L. S., A. J. O’Malley, T. A. Lieu, T. Keegan, N. L. Cook, B. J. McNeil, B. E. Landon,
and E. Guadagnoli. 2006. The quality of chronic disease care in U.S. community health
centers. Health Affairs 25(6):1712-1723.
Hoffman, H. L., C. A. Castro-Donlan, V. M. Johnson, and D. R. Church. 2004. The
Massachusetts HIV, hepatitis, addiction services integration (HHASI) experience: Re-
sponding to the comprehensive needs of individuals with co-occurring risks and condi-
tions. Public Health Reports 119(1):25-31.
Holtzman, D., V. Barry, L. J. Ouellet, D. C. Jarlais, D. Vlahov, E. T. Golub, S. M. Hudson,
and R. S. Garfein. 2009. The influence of needle exchange programs on injection risk
behaviors and infection with hepatitis C virus among young injection drug users in select
cities in the United States, 1994-2004. Preventive Medicine 49(1):68-73.
HRSA (Health Resources and Services Administration). 2009a. The health center program:
Uniform data system (UDS). http://bphc.hrsa.gov/uds/ (accessed November, 13, 2009).
———. 2009b. Quality healthcare collaboration. http://www.healthdisparities.net/hdc/html/
collaborativesOverview.aspx (accessed November 12, 2009).
Hsu, C., C. A. Hsiung, I. J. Su, W. S. Hwang, M. C. Wang, S. F. Lin, T. H. Lin, H. H. Hsiao,
J. H. Young, M. C. Chang, Y. M. Liao, C. C. Li, H. B. Wu, H. F. Tien, T. Y. Chao, T. W.
Liu, A. L. Cheng, and P. J. Chen. 2008. A revisit of prophylactic lamivudine for chemo-
therapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: A randomized
trial. Hepatology 47(3):844-853.
Hsu, C. E., L. C. Liu, H. S. Juon, Y. W. Chiu, J. Bawa, U. Tillman, M. Li, J. Miller, and M.
Wang. 2007. Reducing liver cancer disparities: A community-based hepatitis-B preven-
tion program for Asian-American communities. Journal of the National Medical As-
sociation 99(8):900-907.
Huckans, M. S., A. D. Blackwell, T. A. Harms, D. W. Indest, and P. Hauser. 2005. Integrated
hepatitis C virus treatment: Addressing comorbid substance use disorders and HIV infec-
tion. AIDS 19(Suppl 3):S106-S115.
Hutton, D. W., D. Tan, S. K. So, and M. L. Brandeau. 2007. Cost-effectiveness of screening
and vaccinating Asian and Pacific Islander adults for hepatitis B. Annals of Internal
Medicine 147(7):460-469.
IHRA (International Harm Reduction Association). 2009. What is harm reduction? http://
www.ihra.net/Whatisharmreduction (accessed September 14, 2009).
Inciardi, J. A., H. L. Surratt, and S. P. Kurtz. 2006. HIV, HBV, and HCV infections among drug-
involved, inner-city, street sex workers in Miami, Florida. AIDS Behav 10(2):139-147.
Islam, M., A. Wodak, and K. M. Conigrave. 2008. The effectiveness and safety of syringe
vending machines as a component of needle syringe programmes in community settings.
International Journal on Drug Policy 19(6):436-441.
Jacobs, E., A. H. Chen, L. S. Karliner, N. Agger-Gupta, and S. Mutha. 2006. The need for
more research on language barriers in health care: A proposed research agenda. Milbank
Quarterly 84(1):111-133.
Jacques-Carroll, L. 2008. Essentials of perinatal hepatitis B prevention: A training series
for coordinators and case managers—assessment and evaluation. http://www2.cdc.gov/
vaccines/ed/hepbtraining (accessed June 28, 2009).
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 199
Jacques-Carroll, L., E. E. Mast, and S. Wang. 2007. Managing a perinatal hepatitis B preven-
tion program: A guide to life as a program coordinator. http://www.cdc.gov/Hepatitis/
Partners/PeriHepBCoord.htm (accessed August 18, 2009).
Kahn, R. H., K. E. Moseley, J. N. Thilges, G. Johnson, and T. A. Farley. 2003. Community-
based screening and treatment for STDs: Results from a mobile clinic initiative. Sexually
Transmitted Diseases 30(8):654-658.
Kanwal, F., I. M. Gralnek, P. Martin, G. S. Dulai, M. Farid, and B. M. Spiegel. 2005. Treat-
ment alternatives for chronic hepatitis B virus infection: A cost-effectiveness analysis.
Annals of Internal Medicine 142(10):821-831.
Kapadia, F., D. Vlahov, D. C. Des Jarlais, S. A. Strathdee, L. Ouellet, P. Kerndt, E. E.
Morse, I. Williams, and R. S. Garfein. 2002. Does bleach disinfection of syringes protect
against hepatitis C infection among young adult injection drug users? Epidemiology
13(6):738-741.
Kassler, W. J., B. A. Dillon, C. Haley, W. K. Jones, and A. Goldman. 1997. On-site, rapid HIV
testing with same-day results and counseling. AIDS 11(8):1045-1051.
Keenan, P. A., and J. M. Keenan. 2001. Rapid HIV testing in urban outreach: A strategy for
improving posttest counseling rates. AIDS Education and Prevention 13(6):541-550.
Kidorf, M., V. L. King, K. Neufeld, J. Peirce, K. Kolodner, and R. K. Brooner. 2009. Improv-
ing substance abuse treatment enrollment in community syringe exchangers. Addiction
104(5):786-795.
Klein, S. J., L. N. Wright, G. S. Birkhead, B. A. Mojica, L. C. Klopf, L. A. Klein, E. L.
Tanner, I. S. Feldman, and E. J. Fraley. 2007. Promoting HCV treatment completion for
prison inmates: New York state’s hepatitis C continuity program. Public Health Reports
122(Suppl 2):83-88.
Kresina, T. F., R. D. Bruce, R. Lubran, and H. W. Clark. 2008. Integration of viral hepatitis
services into opioid treatment programs. Journal of Opioid Management 4(6):369-381.
Kuo, I., S. G. Sherman, D. L. Thomas, and S. A. Strathdee. 2004. Hepatitis B virus infection
and vaccination among young injection and non-injection drug users: Missed opportuni-
ties to prevent infection. Drug and Alcohol Dependence 73(1):69-78.
Landon, B. E., L. S. Hicks, A. J. O’Malley, T. A. Lieu, T. Keegan, B. J. McNeil, and E.
Guadagnoli. 2007. Improving the management of chronic disease at community health
centers. New England Journal of Medicine 356(9):921-934.
Latka, M. H., H. Hagan, F. Kapadia, E. T. Golub, S. Bonner, J. V. Campbell, M. H. Coady,
R. S. Garfein, M. Pu, D. L. Thomas, T. K. Thiel, and S. A. Strathdee. 2008. A randomized
intervention trial to reduce the lending of used injection equipment among injection drug
users infected with hepatitis C. American Journal of Public Health 98(5):853-861.
Lau, G. K., M. L. He, D. Y. Fong, A. Bartholomeusz, W. Y. Au, A. K. Lie, S. Locarnini, and
R. Liang. 2002. Preemptive use of lamivudine reduces hepatitis B exacerbation after al-
logeneic hematopoietic cell transplantation. Hepatology 36(3):702-709.
Lau, G. K., H. H. Yiu, D. Y. Fong, H. C. Cheng, W. Y. Au, L. S. Lai, M. Cheung, H. Y.
Zhang, A. Lie, R. Ngan, and R. Liang. 2003. Early is superior to deferred preemptive
lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology
125(6):1742-1749.
Lee, T. A., D. L. Veenstra, U. H. Iloeje, and S. D. Sullivan. 2004. Cost of chronic hepatitis
B infection in the United States. Journal of Clinical Gastroenterology 38(10 Suppl 3):
S144-S147.
Li, X. M., Y. B. Yang, H. Y. Hou, Z. J. Shi, H. M. Shen, B. Q. Teng, A. M. Li, M. F. Shi, and
L. Zou. 2003. Interruption of HBV intrauterine transmission: A clinical study. World
Journal of Gastroenterology 9(7):1501-1503.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
200 HEPATITIS AND LIVER CANCER
Li, Y.-H., Y.-F. He, W.-Q. Jiang, F.-H. Wang, X.-B. Lin, L. Zhang, Z.-J. Xia, X.-F. Sun, H.-Q.
Huang, T.-Y. Lin, Y.-J. He, and Z.-Z. Guan. 2006. Lamivudine prophylaxis reduces the
incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy
for lymphoma. Cancer 106(6):1320-1325.
Liang, T. S., E. Erbelding, C. A. Jacob, H. Wicker, C. Christmyer, S. Brunson, D. Richardson,
and J. M. Ellen. 2005. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS
Patient Care and STDs 19(4):253-257.
Lidgren, M., A. Hollander, O. Weiland, and B. Jonsson. 2007. Productivity improvements
in hepatitis C treatment: Impact on efficacy, cost, cost-effectiveness and quality of life.
Scandinavian Journal of Gastroenterology 42(7):867-877.
Lillienfeld, A. M., and D. E. Lillienfeld. 1980. Foundations of epidemiology. New York:
Oxford University Press.
Lin, S. Y., E. T. Chang, and S. K. So. 2007. Why we should routinely screen Asian Ameri-
can adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology
46(4):1034-1040.
Lok, A. S., and B. J. McMahon. 2004. Chronic hepatitis B: Update of recommendations.
Hepatology 39(3):857-861.
———. 2009. Chronic hepatitis B: Update 2009. Hepatology 50(3):661-662.
Lok, A. S., R. H. Liang, E. K. Chiu, K. L. Wong, T. K. Chan, and D. Todd. 1991. Reactiva-
tion of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a
prospective study. Gastroenterology 100(1):182-188.
Lum, P. J., J. A. Hahn, K. P. Shafer, J. L. Evans, P. J. Davidson, E. Stein, and A. R. Moss. 2008.
Hepatitis B virus infection and immunization status in a new generation of injection drug
users in San Francisco. Journal of Viral Hepatitis 15(3):229-236.
Maher, L., B. Jalaludin, K. G. Chant, R. Jayasuriya, T. Sladden, J. M. Kaldor, and P. L. Sargent.
2006. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in
Australia. Addiction 101(10):1499-1508.
Manson, A. 1988. Language concordance as a determinant of patient compliance and emer-
gency room use in patients with asthma. Medical Care 26(12):1119-1128.
Mansson, A. S., T. Moestrup, E. Nordenfelt, and A. Widell. 2000. Continued transmission of
hepatitis B and C viruses, but no transmission of human immunodeficiency virus among
intravenous drug users participating in a syringe/needle exchange program. Scandinavian
Journal of Infectious Diseases 32(3):253-258.
Mark, K. E., P. J. Murray, D. B. Callahan, and R. A. Gunn. 2007. Medical care and alcohol
use after testing hepatitis C antibody positive at STD clinic and HIV test site screening
programs. Public Health Reports 122(1):37-43.
Maru, D. S., R. D. Bruce, S. Basu, and F. L. Altice. 2008. Clinical outcomes of hepatitis C
treatment in a prison setting: Feasibility and effectiveness for challenging treatment po-
pulations. Clinical Infectious Diseases 47(7):952-961.
Mast, E. E., H. S. Margolis, A. E. Fiore, E. W. Brink, S. T. Goldstein, S. A. Wang, L. A. Moyer,
B. P. Bell, and M. J. Alter. 2005. A comprehensive immunization strategy to eliminate
transmission of hepatitis B virus infection in the United States: Recommendations of the
advisory committee on immunization practices (ACIP) part 1: Immunization of infants,
children, and adolescents. Morbidity and Morality Weekly: Recommendations and Re-
ports 54(RR-16):1-31.
Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald,
J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immunization
strategy to eliminate transmission of hepatitis B virus infection in the United States:
Recommendations of the advisory committee on immunization practices (ACIP) part II:
Immunization of adults. Morbidity and Morality Weekly: Recommendations and Reports
55(RR-16):1-33.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 201
Mateu-Gelabert, P., C. Treloar, V. A. Calatayud, M. Sandoval, J. C. V. Zurián, L. Maher, T.
Rhodes, and S. R. Friedman. 2007. How can hepatitis C be prevented in the long term?
International Journal of Drug Policy 18(5):338-340.
Mathei, C., G. Robaeys, P. van Damme, F. Buntinx, and R. Verrando. 2005. Prevalence of
hepatitis C in drug users in Flanders: Determinants and geographic differences. Epide-
miology and Infection 133(1):127-136.
McDonald, D. 2009. The evaluation of a trial of syringe vending machines in Canberra,
Australia. International Journal on Drug Policy 20(4):336-339.
McGinn, T., N. O’Connor-Moore, D. Alfandre, D. Gardenier, and J. Wisnivesky. 2008. Va-
lidation of a hepatitis C screening tool in primary care. Archives of Internal Medicine
168(18):2009-2013.
McIntyre, A. F., A. Studzinski, H. A. Beidinger, and C. Rabins. 2008. STD, HIV/AIDS, and
hepatitis services in Illinois county jails. Sexually Transmitted Diseases 36(2 Suppl):36(2 Suppl):
S37-S40.
Mehta, S. H., G. M. Lucas, L. B. Mirel, M. Torbenson, Y. Higgins, R. D. Moore, D. L.
Thomas, and M. S. Sulkowski. 2006. Limited effectiveness of antiviral treatment for
hepatitis C in an urban HIV clinic. AIDS 20(18):2361-2369.
Metzger, D. S., H. Navaline, and G. E. Woody. 1998. Drug abuse treatment as AIDS preven-
tion. Public Health Reports 113(Suppl 1):97-106.
Mimiaga, M. J., S. L. Reisner, R. Vanderwarker, M. J. Gaucher, C. A. O’Connor, M. S.
Medeiros, and S. A. Safren. 2008. Polysubstance use and HIV/STD risk behavior among
Massachusetts men who have sex with men accessing department of public health mobile
van services: Implications for intervention development. AIDS Patient Care and STDs
22(9):745-751.
Moatti, J. P., D. Vlahov, I. Feroni, V. Perrin, and Y. Obadia. 2001. Multiple access to sterile
syringes for injection drug users: Vending machines, needle exchange programs and legal
pharmacy sales in Marseille, France. European Addiction Research 7(1):40-45.
Molitor, F., R. A. Bell, S. R. Truax, J. D. Ruiz, and R. K. Sun. 1999. Predictors of failure to
return for HIV test result and counseling by test site type. AIDS Education and Preven-
tion 11(1):1-13.
Myers, J. J., C. Modica, M. S. Dufour, C. Bernstein, and K. McNamara. 2009. Routine rapid
HIV screening in six community health centers serving populations at risk. Journal of
General Internal Medicine 24(12):1269-1274.24(12):1269-1274.
NASTAD (National Alliance of State and Territorial AIDS Directors). 2001. Integrating viral
hepatitis into HIV/AIDS/STD programs. HIV Prevention Bulletin September.
———. 2009. Fact sheet describing need for FY2010 appropriations for hepatitis prevention.
National Alliance for State and Territorial AIDS Directors.
Neaigus, A., V. A. Gyarmathy, M. Zhao, M. Miller, S. R. Friedman, and D. C. Des Jarlais.
2007. Sexual and other noninjection risks for HBV and HCV seroconversions among
noninjecting heroin users. Journal of Infectious Diseases 195(7):1052-1061.
NIH (National Institutes of Health). 2008.2008. NIH consensus development conference state-
ment on the management of Hepatitis B. http://consensus.nih.gov/2008/hebB%20
draft%20statement%20102208_FINAL.pdf (accessed August 21, 2009)..
Ostuni, P., C. Botsios, L. Punzi, P. Sfriso, and S. Todesco. 2003. Hepatitis B reactivation in a
chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with inflix-
imab and low dose methotrexate. Annals of the Rheumatic Diseases 62(7):686-687.
O’Toole, T. P., R. A. Pollini, D. E. Ford, and G. Bigelow. 2008. The health encounter as a
treatable moment for homeless substance-using adults: The role of homelessness, health
seeking behavior, readiness for behavior change and motivation for treatment. Addictive
Behaviors 33(9):1239-1243.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
202 HEPATITIS AND LIVER CANCER
O’Toole, T. P., R. Pollini, P. Gray, T. Jones, G. Bigelow, and D. E. Ford. 2007. Factors identi-
fying high-frequency and low-frequency health service utilization among substance-using
adults. Journal of Substance Abuse Treatment 33(1):51-59.
Paltiel, A. D., M. C. Weinstein, A. D. Kimmel, G. R. Seage, 3rd, E. Losina, H. Zhang, K. A.
Freedberg, and R. P. Walensky. 2005. Expanded screening for HIV in the United States—
an analysis of cost-effectiveness. New England Journal of Medicine 352(6):586-595.
Patrick, D. M., M. W. Tyndall, P. G. Cornelisse, K. Li, C. H. Sherlock, M. L. Rekart, S. A.
Strathdee, S. L. Currie, M. T. Schechter, and M. V. O’Shaughnessy. 2001. Incidence of
hepatitis C virus infection among injection drug users during an outbreak of HIV infec-
tion. Canadian Medical Association Journal 165(7):889-895.
Pawlotsky, J.-M., I. Lonjon, C. Hezode, B. Raynard, F. Darthuy, J. Remire, C.-J. Soussy, and
D. Dhumeaux. 1998. What strategy should be used for diagnosis of hepatitis C virus
infection in clinical laboratories? Hepatology 27(6):1700-1702.
Pisu, M., M. I. Meltzer, and R. Lyerla. 2002. Cost-effectiveness of hepatitis B vaccination of
prison inmates. Vaccine 21(3-4):312-321.
Probst, J., J. Laditka, and S. Laditka. 2009. Association between community health center and
rural health clinic presence and county-level hospitalization rates for ambulatory care
sensitive conditions: An analysis across eight US states. BMC Health Services Research
9(1):134.
Raggio Ashley, T. P. 2009. Community health centers, the federal perspective: Hepatitis B and
C activities at HRSA. Presentation to the committee, March 3, 2009.
Rahnavardi, M., S. M. Hosseini Moghaddam, and S. M. Alavian. 2008. Hepatitis C in hemo-
dialysis patients: Current global magnitude, natural history, diagnostic difficulties, and
preventive measures. American Journal of Nephrology 28(4):628-640.
Rajendra, A., and J. B. Wong. 2007. Economics of chronic hepatitis B and hepatitis C. Journal
of Hepatology 47(4):608-617.
Randrianirina, F., J. F. Carod, E. Ratsima, J. B. Chretien, V. Richard, and A. Talarmin. 2008.
Evaluation of the performance of four rapid tests for detection of hepatitis B surface
antigen in Antananarivo, Madagascar. Journal of Virological Methods 151(2):294-297.
Regan, J., A. H. Schempf, J. Yoon, and R. M. Politzer. 2003. The role of federally funded
health centers in serving the rural population. Journal of Rural Health 19(2):117-124;
discussion 115-116.
Rein, D. B., S. B. Lesesne, P. J. Leese, and C. M. Weinbaum. 2009. Community-based
hepatitis B screening programs in the United States in 2008. Journal of Viral Hepatitis
17(1):28-33.
Reynolds, G. L., D. G. Fisher, L. E. Napper, K. A. Marsh, C. Willey, and R. Brooks. 2008. Re-
sults from a multiple morbidities testing program offering rapid HIV testing bundled with
hepatitis and sexually transmitted infection testing. Public Health Reports 123(Suppl
3):63-69.
Rezza, G., L. Sagliocca, M. Zaccarelli, M. Nespoli, M. Siconolfi, and C. Baldassarre. 1996.
Incidence rate and risk factors for HCV seroconversion among injecting drug users
in an area with low HIV seroprevalence. Scandinavian Journal of Infectious Diseases
28(1):27-29.
Rhodes, T., and C. Treloar. 2008. The social production of hepatitis C risk among injecting
drug users: A qualitative synthesis. Addiction 103(10):1593-1603.
Ricketts, T. C. 2000. The changing nature of rural health care. Annual Review of Public
Health 21(1):639-657.
Rogers, W. 2009. Viral hepatitis prevention polices and programs, Centers for Medicare and
Medicaid services. Presentation to the committee, March 3, 2009.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 203
Rooney, G., and R. J. Gilson. 1998. Sexual transmission of hepatitis C virus infection. Sexually
Transmitted Infections 74(6):399-404.
Rosenheck, R. A., S. G. Resnick, and J. P. Morrissey. 2003. Closing service system gaps for
homeless clients with a dual diagnosis: Integrated teams and interagency cooperation.
Journal of Mental Health Policy and Economics 6(2):77-87.
Rossi, G., A. Pelizzari, M. Motta, and M. Puoti. 2001. Primary prophylaxis with lamivudine
of hepatitis B virus reactivation in chronic HBsAg carriers with lymphoid malignancies
treated with chemotherapy. British Journal of Haematology 115(1):58-62.
Rousseau, C. M., G. N. Ioannou, J. A. Todd-Stenberg, K. L. Sloan, M. F. Larson, C. W.
Forsberg, and J. A. Dominitz. 2008. Racial differences in the evaluation and treatment
of hepatitis C among veterans: A retrospective cohort study. American Journal of Public
Health 98(5):846-852.
Roy, E., E. Nonn, N. Haley, and J. Cox. 2007. Hepatitis C meanings and preventive strategies
among street-involved young injection drug users in Montreal. International Journal on
Drug Policy 18(5):397-405.
Ruan, Y., G. Qin, L. Yin, K. Chen, H. Z. Qian, C. Hao, S. Liang, J. Zhu, H. Xing, K. Hong,
and Y. Shao. 2007. Incidence of HIV, hepatitis C and hepatitis B viruses among injec-
tion drug users in southwestern China: A 3-year follow-up study. AIDS 21(Suppl 8):
S39-S46.
Rust, G., P. Baltrus, J. Ye, E. Daniels, A. Quarshie, P. Boumbulian, and H. Strothers. 2009.
Presence of a community health center and uninsured emergency department visit rates
in rural counties. Journal of Rural Health 25(1):8-16.
Sabbatani, S., R. Giuliani, and R. Manfredi. 2006. Combined pegylated interferon and riba-
virin for the management of chronic hepatitis C in a prison setting. Brazilian Journal of
Infectious Diseases 10(4):274-278.
Salomon, J. A., M. C. Weinstein, J. K. Hammitt, and S. J. Goldie. 2003. Cost-effectiveness of
treatment for chronic hepatitis C infection in an evolving patient population. Journal of
the American Medical Association 290(2):228-237.
Sanders, G. D., A. M. Bayoumi, V. Sundaram, S. P. Bilir, C. P. Neukermans, C. E. Rydzak, L. R.
Douglass, L. C. Lazzeroni, M. Holodniy, and D. K. Owens. 2005. Cost-effectiveness of
screening for HIV in the era of highly active antiretroviral therapy. New England Journal
of Medicine 352(6):570-585.
Schechter, M. T., S. A. Strathdee, P. G. Cornelisse, S. Currie, D. M. Patrick, M. L. Rekart,
and M. V. O’Shaughnessy. 1999. Do needle exchange programmes increase the spread
of HIV among injection drug users?: An investigation of the Vancouver outbreak. AIDS
13(6):F45-F51.
Scheinmann, R., H. Hagan, C. Lelutiu-Weinberger, R. Stern, D. C. Des Jarlais, P. L. Flom, and
S. Strauss. 2007. Non-injection drug use and hepatitis C virus: A systematic review. Drug
and Alcohol Dependence 89(1):1-12.
Schulden, J. D., B. Song, A. Barros, A. Mares-DelGrasso, C. W. Martin, R. Ramirez, L. C.
Smith, D. P. Wheeler, A. M. Oster, P. S. Sullivan, and J. D. Heffelfinger. 2008. Rapid HIV
testing in transgender communities by community-based organizations in three cities.
Public Health Reports 123(Suppl 3):101-114.
Sherman, K. E., S. D. Rouster, R. T. Chung, and N. Rajicic. 2002. Hepatitis C virus prevalence
among patients infected with human immunodeficiency virus: A cross sectional analysis
of the US adult AIDS clinical trials group. Clinical Infectious Diseases 34(6):831-837.
Shrestha, R. K., H. A. Clark, S. L. Sansom, B. Song, H. Buckendahl, C. B. Calhoun, A. B.
Hutchinson, and J. D. Heffelfinger. 2008. Cost-effectiveness of finding new HIV diagno-
ses using rapid HIV testing in community-based organizations. Public Health Reports
123(Suppl 3):94-100.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
204 HEPATITIS AND LIVER CANCER
Siegel, A. B., R. B. McBride, H. B. El-Serag, D. L. Hershman, R. S. Brown, Jr., J. F. Renz, J.
Emond, and A. I. Neugut. 2008. Racial disparities in utilization of liver transplantation
for hepatocellular carcinoma in the United States, 1998-2002. American Journal of Ga-
stroenterology 103(1):120-127.
Simmons, T. M. 2004. Inmate peer education programs: 101. Infectious Diseases in Correc-
tions Report. December 2004.
Smith, L. V., E. T. Rudy, M. Javanbakht, A. Uniyal, L. S. Sy, T. Horton, and P. R. Kerndt.
2006. Client satisfaction with rapid HIV testing: Comparison between an urban sexually
transmitted disease clinic and a community-based testing center. AIDS Patient Care and
STDs 20(10):693-700.
Smyth, B. P., E. Keenan, and J. J. O’Connor. 2000. Assessment of hepatitis C infection in
injecting drug users attending an addiction treatment clinic. Irish Journal of Medical
Science 169(2):129-132.
Smyth, B. P., J. J. O’Connor, J. Barry, and E. Keenan. 2003. Retrospective cohort study exam-
ining incidence of HIV and hepatitis C infection among injecting drug users in Dublin.
Journal of Epidemiology and Community Health 57(4):310-311.
Sneller, V. P., D. B. Fishbein, C. M. Weinbaum, A. Lombard, P. Murray, J. A. McLaurin, and
L. Friedman. 2008. Vaccinating adolescents in high-risk settings: Lessons learned from
experiences with hepatitis B vaccine. Pediatrics 121(Suppl 1):S55-S62.
Sonnenday, C. J., J. B. Dimick, R. D. Schulick, and M. A. Choti. 2007. Racial and geographic
disparities in the utilization of surgical therapy for hepatocellular carcinoma. Journal of
Gastrointestinal Surgery 11(12):1636-1646; discussion 1646.
Spaulding, A. C., C. M. Weinbaum, D. T.-Y. Lau, R. Sterling, L. B. Seeff, H. S. Margolis, and
J. H. Hoofnagle. 2006. A framework for management of hepatitis C in prisons. Annals
of Internal Medicine 144(10):762-769.
Spielberg, F., B. M. Branson, G. M. Goldbaum, D. Lockhart, A. Kurth, C. L. Celum, A.
Rossini, C. W. Critchlow, and R. W. Wood. 2003. Overcoming barriers to HIV testing:
Preferences for new strategies among clients of a needle exchange, a sexually transmitted
disease clinic, and sex venues for men who have sex with men. Journal of Acquired Im-
mune Deficiency Syndromes 32(3):318-327.
Spielberg, F., B. M. Branson, G. M. Goldbaum, D. Lockhart, A. Kurth, A. Rossini, and R. W.
Wood. 2005. Choosing HIV counseling and testing strategies for outreach settings: A
randomized trial. Journal of Acquired Immune Deficiency Syndromes 38(3):348-355.
Spielberg, F., A. Kurth, P. M. Gorbach, and G. Goldbaum. 2001. Moving from apprehension
to action: HIV counseling and testing preferences in three at-risk populations. AIDS
Education and Prevention 13(6):524-540.
Stanley, A. H. 1999. Primary care and addiction treatment: Lessons learned from building
bridges across traditions. Journal of Addictive Diseases 18(2):65-82.
Sterling, R. K., C. M. Hofmann, V. A. Luketic, A. J. Sanyal, M. J. Contos, A. S. Mills, and
M. L. Shiffman. 2004. Treatment of chronic hepatitis C virus in the Virginia Department
of Corrections: Can compliance overcome racial differences to response? American Jour-
nal of Gastroenterology 99(5):866-872.
Stopka, T. J., C. Marshall, R. N. Bluthenthal, D. S. Webb, and S. R. Truax. 2007. HCV and
HIV counseling and testing integration in California: An innovative approach to increase
HIV counseling and testing rates. Public Health Reports 122(Suppl 2):68-73.
Strauss, S. M., J. M. Astone, D. D. Jarlais, and H. Hagan. 2004. A comparison of HCV anti-
body testing in drug-free and methadone maintenance treatment programs in the United
States. Drug and Alcohol Dependence 73(3):227-236.
Strauss, S. M., J. M. Astone, Z. P. Vassilev, D. C. Des Jarlais, and H. Hagan. 2003. Gaps in
the drug-free and methadone treatment program response to Hepatitis C. Journal of
Substance Abuse Treatment 24(4):291-297.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 205
Strauss, S. M., G. P. Falkin, Z. Vassilev, D. C. Des Jarlais, and J. Astone. 2002. A nationwide
survey of hepatitis C services provided by drug treatment programs. Journal of Substance
Abuse Treatment 22(2):55-62.
Subiadur, J., J. L. Harris, and C. A. Rietmeijer. 2007. Integrating viral hepatitis prevention
services into an urban STD clinic: Denver, Colorado. Public Health Reports 122(Suppl
2):12-17.
Sulkowski, M., S. 2008. Viral hepatitis and HIV coinfection. Journal of Hepatology 48(2):
353-367.
Sullivan, P. S., A. Lansky, and A. Drake. 2004. Failure to return for HIV test results among
persons at high risk for HIV infection: Results from a multistate interview project. Jour-
nal of Acquired Immune Deficiency Syndromes 35(5):511-518.
Tempalski, B., H. L. Cooper, S. R. Friedman, D. C. Des Jarlais, J. Brady, and K. Gostnell.
2008. Correlates of syringe coverage for heroin injection in 35 large metropolitan areas
in the US in which heroin is the dominant injected drug. International Journal on Drug
Policy 19(Suppl 1):S47-S58.
Tempalski, B., P. L. Flom, S. R. Friedman, D. C. Des Jarlais, J. J. Friedman, C. McKnight,
and R. Friedman. 2007. Social and political factors predicting the presence of syringe
exchange programs in 96 US metropolitan areas. American Journal of Public Health
97(3):437-447.
Thiede, H., H. Hagan, and C. S. Murrill. 2000. Methadone treatment and HIV and hepatitis
B and C risk reduction among injectors in the Seattle area. Journal of Urban Health
77(3):331-345.
Thiede, H., H. Hagan, J. V. Campbell, S. A. Strathdee, S. L. Bailey, S. M. Hudson, F. Kapadia,
and R. S. Garfein. 2007. Prevalence and correlates of indirect sharing practices among
young adult injection drug users in five U.S. Cities. Drug and Alcohol Dependence
91(Suppl 1):S39-S47.
Thio, C. L., K. R. Nolt, J. Astemborski, D. Vlahov, K. E. Nelson, and D. L. Thomas. 2000.
Screening for hepatitis C virus in human immunodeficiency virus-infected individuals.
Journal of Clinical Microbiology 38(2):575-577.
Thio, C. L., E. C. Seaberg, R. Skolasky, Jr., J. Phair, B. Visscher, A. Munoz, and D. L. Thomas.
2002. HIV-1, hepatitis B virus, and risk of liver-related mortality in the multicenter co-
hort study (MACS). Lancet 360(9349):1921-1926.
Thorpe, L. E., L. J. Ouellet, R. Hershow, S. L. Bailey, I. T. Williams, J. Williamson, E. R.
Monterroso, and R. S. Garfein. 2002. Risk of hepatitis C virus infection among young
adult injection drug users who share injection equipment. American Journal of Epide-
miology 155(7):645-653.
Tramarin, A., N. Gennaro, F. A. Compostella, C. Gallo, L. J. Wendelaar Bonga, and M. J.
Postma. 2008. HCV screening to enable early treatment of hepatitis C: A mathematical
model to analyse costs and outcomes in two populations. Current Pharmaceutical Design
14(17):1655-1660.
U.S. Census Bureau. 2008. 2007 American community survey. http://factfinder.census.gov/
servlet/STTable?_bm=y&-qr_name=ACS_2008_3YR_G00_S0502&-geo_id=01000US&-
ds_name=ACS_2008_3YR_G00_&-_lang=en&-format=&-CONTEXT=st (accessed Au-
gust 20, 2009).
U.S. Department of Homeland Security. 2009. Yearbook of immigration statistics: 2008. Table
3: Persons obtaining legal permanent resident status by region and country of birth: Fis-
cal years 1999 to 2008. http://www.dhs.gov/files/statistics/publications/yearbook.shtm
(accessed August 21, 2009).
U.S. Preventive Services Task Force. 2009. Screening for hepatitis B virus infection in preg-
nancy: U.S. Preventive Services Task Force reaffirmation recommendation statement.
Annals of Internal Medicine 150(12):869-873, W154.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
206 HEPATITIS AND LIVER CANCER
Valentine, J., and L. Wright-De Aguero. 1996. Defining the components of street outreach
for HIV prevention: The contact and the encounter. Public Health Reports 111(Suppl
1):69-74.
Vallabhaneni, S., G. E. Macalino, S. E. Reinert, B. Schwartzapfel, F. A. Wolf, and J. D.
Rich. 2004. Prisoners’ attitudes toward hepatitis B vaccination. Preventive Medicine
38(6):828-833.
van den Berg, C. H., C. Smit, M. Bakker, R. B. Geskus, B. Berkhout, S. Jurriaans, R. A.
Coutinho, K. C. Wolthers, and M. Prins. 2007b. Major decline of hepatitis C virus inci-
dence rate over two decades in a cohort of drug users. European Journal of Epidemiology
22(3):183-193.
van den Berg, C., C. Smit, G. Van Brussel, R. Coutinho, and M. Prins. 2007a. Full participa-
tion in harm reduction programmes is associated with decreased risk for human immu-
nodeficiency virus and hepatitis C virus: Evidence from the Amsterdam cohort studies
among drug users. Addiction 102(9):1454-1462.
van Nunen, A. B., R. A. de Man, R. A. Heijtink, H. G. Niesters, and S. W. Schalm. 2000.
Lamivudine in the last 4 weeks of pregnancy to prevent perinatal transmission in highly
viremic chronic hepatitis B patients. Journal of Hepatology 32(6):1040-1041.
van Zonneveld, M., A. B. van Nunen, H. G. Niesters, R. A. de Man, S. W. Schalm, and H. L.
Janssen. 2003. Lamivudine treatment during pregnancy to prevent perinatal transmission
of hepatitis B virus infection. Journal of Viral Hepatitis 10(4):294-297.
Ward, J. 2008a. FY 2008 domestic enacted funds. Presentation to the committee: December
4, 2008.
Ward, J. W. 2008b. Time for renewed commitment to viral hepatitis prevention. American
Journal of Public Health 98(5):779-781.
Weinbaum, C. M. 2008. Recommendations for identification and public health management
of persons with chronic hepatitis b virus infection. Paper presented at NIH Consensus
Development Conference: Management of Hepatitis B, Natcher Conference Center, Na-
tional Institutes of Health (Bethesda, MD).
Weinbaum, C., R. Lyerla, and H. S. Margolis. 2003. Prevention and control of infections with
hepatitis viruses in correctional settings. Centers for Disease Control and Prevention.
Morbidity and Mortality Weekly Report Recommendations and Reports 52(RR-1):1-36;
quiz CE31-CE34.
Wise, M., S. Bialek, L. Finelli, B. P. Bell, and F. Sorvillo. 2008. Changing trends in hepatitis
C-related mortality in the United States, 1995-2004. Hepatology 47(4):1128-1135.
Workowski, K., and S. Berman. 2006. Sexually transmitted diseases: Treatment guidelines,
2006. Morbidity and Mortality Weekly Report 55(RR-11):1-94.
Wright, D. B. 2009. Care in the country: A historical case study of long-term sustainability in
4 rural health centers. American Journal of Public Health 99(9):1612-1618.
Xu, W. M., Y. T. Cui, L. Wang, H. Yang, Z. Q. Liang, X. M. Li, S. L. Zhang, F. Y. Qiao, F.
Campbell, C. N. Chang, S. Gardner, and M. Atkins. 2009. Lamivudine in late pregnancy
to prevent perinatal transmission of hepatitis B virus infection: A multicentre, random-
ized, double-blind, placebo-controlled study. Journal of Viral Hepatitis 16(2):94-103.
Yeo, W., and P. J. Johnson. 2006. Diagnosis, prevention and management of hepatitis B virus
reactivation during anticancer therapy. Hepatology 43(2):209-220.
Yeo, W., P. K. Chan, S. Zhong, W. M. Ho, J. L. Steinberg, J. S. Tam, P. Hui, N. W. Leung,
B. Zee, and P. J. Johnson. 2000. Frequency of hepatitis B virus reactivation in cancer
patients undergoing cytotoxic chemotherapy: A prospective study of 626 patients with
identification of risk factors. Journal of Medical Virology 62(3):299-307.
Zickmund, S., S. L. Hillis, M. J. Barnett, L. Ippolito, and D. R. LaBrecque. 2004. Hepatitis
C virus-infected patients report communication problems with physicians. Hepatology
39(4):999-1007.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
VIRAL HEALTH SERVICES 207
Zimmerman, R., C. Finley, C. Rabins, and K. McMahon. 2007. Integrating viral hepatitis
prevention into STD clinics in Illinois (excluding Chicago), 1999-2005. Public Health
Reports 122(Suppl 2):18-23.
Zola, J., N. Smith, S. Goldman, and B. A. Woodruff. 1997. Attitudes and educational practices
of obstetric providers regarding infant hepatitis B vaccination. Obstetrics and Gynecol-
ogy 89(1):61-64.
Zuniga, I. A., J. J. Chen, D. S. Lane, J. Allmer, and V. E. Jimenez-Lucho. 2006. Analysis
of a hepatitis C screening programme for US veterans. Epidemiology and Infection
134(02):249-257.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
209
A
Committee Biographies
R. Palmer Beasley, MD (Chair), is the Ashbel Smith Professor and dean
emeritus of the University of Texas School of Public Health at Houston.
Previously, Dr. Beasley was a member of the faculty of the Department
of Epidemiology at the University of Washington and the Department of
Internal Medicine at the University of California, San Francisco. The focus
of his research has been the hepatitis B virus. His contributions to the field
include discovery of mother-to-infant transmission of the hepatitis B virus,
establishing that the hepatitis B virus is the major cause of liver cancer, and
a series of clinical trials that established the effectiveness and strategies for
the use of hepatitis B vaccine for the prevention of perinatal transmission.
Dr. Beasley has won many awards for his work, including the Charles F.
Mott General Motors International Prize for Research on Cancer, the Prince
Mahidol Award for Medicine (Thailand), and the Health Medal of the First
Order (Taiwan). He has served on numerous national and international
government advisory panels on viral hepatitis and is chair of the Associa-
tion of Schools of Public Health. He also served on the National Acad-
emies Committee on the Middle East Regional Infectious Disease Research
Program and Committee on the Assessment of Future Scientific Needs for
Variola Virus and on the Public Health and Biotechnology Review Panel.
Dr. Beasley received his MD from Harvard School of Medicine, and his MS
in preventative medicine from the University of Washington.
Harvey J. Alter, MD, is chief of clinical studies and associate director for re-
search in the Department of Transfusion Medicine at the National Institutes
of Health. Dr. Alter’s research interest is in viral hepatitis and the safety
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
210 HEPATITIS AND LIVER CANCER
of the blood supply. He was a major contributor in the fight to reduce the
incidence of transfusion-induced viral hepatitis, and he collaborated in the
discovery of hepatitis C and described its natural history. He is a member
of IOM and NAS. For his contributions, Dr. Alter has been awarded the US
Pubic Health Service Distinguished Service Medal, the Landsteiner Prize,
the Presidential Award of the International Society of Blood Transfusion,
the James Blundell Award of the British Blood Transfusion Society, and
the Distinguished Scientist Awards of both the Hepatitis B Foundation and
the American Liver Foundation, and he was elected to fellowship in the
American Association of Physicians. He was the corecipient of the 2000
Clinical Lasker Award and was made a master of the American College
of Physicians. In 2007, he was named Distinguished NIH Investigator. Dr.
Alter received his MD from the University of Rochester.
Margaret L. Brandeau, PhD, is a professor in the Department of Manage-
ment Science and Engineering of Stanford University. She also holds a cour-
tesy appointment in the Department of Medicine of the same institution.
Dr. Brandeau is an operations researcher and policy analyst with extensive
background in the development of applied mathematical and economic
models. She has conducted research on HIV, focusing on mathematical and
economic models to assess the value of different HIV and drug-abuse inter-
ventions, and on hepatitis B screening and vaccination policies. She received
her PhD in engineering and economic systems from Stanford University.
Daniel R. Church, MPH, is the adult viral hepatitis prevention coordinator
and an epidemiologist in the Division of Epidemiology and Immunization
of the Massachusetts Department of Health. He coordinates the statewide
viral hepatitis program, including disease surveillance; medical-management
services; counseling and testing programs; adult vaccination programs; edu-
cational campaigns for providers, patients, and communities; and evalu-
ation of projects. Mr. Church received his MPH in epidemiology and
biostatistics from the Boston University School of Public Health.
Alison A. Evans, ScD, is an assistant professor in the Department of Epide-
miology and Biostatistics of the Drexel University School of Public Health.
She is also the director of public-health research in the Hepatitis B Foun-
dation, Doylestown, PA, and is an adjunct associate member of the Fox
Chase Cancer Center in Philadelphia, PA. Her research interests include the
epidemiology and natural history of the hepatitis B virus and other chronic
viral infections. She received her ScD in epidemiology from the Harvard
School of Public Health.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
APPENDIX A 211
Holly Hagan, PhD, MPH, is a senior research scientist in the New York
University College of Nursing, deputy director of the Center for Drug Use
and HIV Research, and director of the center’s Interdisciplinary Research
Methods Core. Previously, she was deputy director of the Institute for AIDS
Research in the National Development and Research Institutes. She was a
senior epidemiologist in the Department of Public Health in Seattle, WA.
Her broad research interest is in the etiology and prevention of hepatitis
C and other bloodborne viral infections in drug users and other high-risk
populations; her work has also examined drug users’ access to screening
and health care. Dr. Hagan has served on several national government ad-
visory groups, including the steering committee for the National Institutes
of Health hepatitis C vaccine trial. She received her MPH in epidemiology
from the University of Massachusetts at Amherst School of Public Health
and her PhD in epidemiology from the University of Washington School of
Public Health and Community Medicine.
Sandral Hullett, MD, MPH, is the chief executive officer and medical direc-
tor of the Jefferson Health System, which consists of Cooper Green–Mercy
Hospital and Jefferson Outpatient Care. Jefferson Health System’s primary
focus is service to the underserved populations of Jefferson County, AL.
Previously, Dr. Hullett was the executive director of Family HealthCare of
Alabama, which is headquartered in Eutaw, Alabama, and provided ser-
vices to patients of west central Alabama. She has an interest in rural health
care, including health-care planning and delivery to the underserved, un-
derinsured, and poor; and she has extensive experience in research, clinical
trials, community outreach, and teaching of direct care delivery. Dr. Hullett
is a member of IOM and has served on several IOM committees, including
committees that produced America’s Health Care Safety Net: Intact but En-
dangered; Quality Through Collaboration: The Future of Rural Health; and
Measuring What Matters: Allocation, Planning, and Quality Assessment
for the Ryan White CARE Act; the Planning Committee for a Workshop
on Military Medical Ethics: Issues Regarding Dual Loyalties; and the Com-
mittee on Human Rights of NAS, NAE, and IOM. She has received many
awards and honors, including the Rural Practitioner of the Year Award in
1988 from the National Rural Health Association, the Clinical Recogni-
tion Award for Education and Training from the National Association of
Community Health Centers in 1993, the Public Health Hero Award for
Year 2000 from the University of Alabama at Birmingham School of Public
Health, the National Medical Fellowship in 2001, Lifetime Achievement
of Women in Health Care from Rutgers University in 2002, and the Local
Legends Award from the American Medical Women’s Association in Febru-
ary 2004. She received her MD from the Medical College of Pennsylvania
and her MPH from the University of Alabama at Birmingham.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
212 HEPATITIS AND LIVER CANCER
Stacene R. Maroushek, MD, PhD, MPH, is a staff pediatrician at Henne-
pin County Medical Center in Minneapolis, MN. She is also an assistant
professor in the Division of Pediatric Infectious Diseases of the University
of Minnesota. Dr. Maroushek works with immigrant pediatric patients and
has published extensively on medical evaluation and screening of immigrant
children for infectious diseases. She received her MD, her PhD in microbiol-
ogy, and her MPH from the University of Minnesota.
Randall R. Mayer, MS, MPH, is an epidemiologist and chief of the Bureau
of HIV, STD, and Hepatitis in the Iowa Department of Public Health. He
provides oversight for HIV, sexually trasmitted disease (STD), and hepatitis
prevention, care, and surveillance activities, including disease reporting,
counseling and testing, risk-reduction programs, partner services, com-
munity planning, adult immunizations for hepatitis A and hepatitis B, HIV
case management and support services, and HIV and STD drug-treatment
assistance programs. While working with the Iowa Department of Public
Health, Mr. Mayer has served as the HIV/AIDS/Hepatitis Program man-
ager and as the HIV/AIDS surveillance coordinator. He received his MPH
in epidemiology from the University of Minnesota and his MS in plant cell
physiology from Purdue University.
Brian J. McMahon, MD, is medical director of the liver disease and hepa-
titis program at the Alaska Native Tribal Health Consortium and a clini-
cal hepatologist at the Alaska Native Medical Center. He was previously
employed by the Centers for Disease Control and Prevention in Alaska.
Dr. McMahon has worked to reduce the rate of hepatitis B in the native
Alaskan population, which went from one of the highest in the world to
one of the lowest. He provides clinical care for patients who have viral
hepatitis and liver disease and conducts research in population-epidemiol-
ogy hepatitis and liver disease. He has served as a consultant on viral hepa-
titis issues to the World Health Organization and other international and
national organizations. Dr. McMahon received the Assistant Secretary for
Health Award for Exceptional Achievement in 1985; the Alvan R. Feinstein
Memorial Award from the American College of Physicians in 2003 for the
Program to Control Hepatitis B in Alaska Natives; and the 2009 Scientist
of the Year from the Hepatitis B Foundation for notable contributions in
clinical epidemiology regarding research on and control of hepatitis A,
hepatitis B, and hepatitis C in Alaska natives. He was elected a master in
the American College of Physicians. He received his MD from the Univer-
sity of Washington.
Martín J. Sepúlveda, MD, FACP, is IBM Fellow and vice president of
integrated health services for the International Business Machines Cor-
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
APPENDIX A 213
poration. His research interests and health-care reform initiatives include
patient-centered primary care and medical homes, care management and
coordination, total health management, workplace health promotion, risk-
reduction program measurement, value-based health-care purchasing, and
global occupational and health services delivery. He is a fellow of the IBM
Corporation, the American College of Physicians, the American College
of Occupational and Environmental Medicine, and the American College
of Preventive Medicine. Dr. Sepúlveda was recently chosen as an IBM Fel-
low, IBM’s highest technical achievement; was awarded honorary mem-
bership in the American Academy of Family Physicians; and received the
John D. Thompson Distinguished Fellow Award from Yale University and
the Distinguished Alumnus Award for Professional Achievement from the
University of Iowa. His team has received numerous national and interna-
tional awards in health care, health promotion, and occupational health
and safety. He serves on the IOM Board on Population Health and Public
Health Practice, the Board of Directors of the Employee Benefits Research
Institute, the Board of Advisors to the School of Public Health of the Uni-
versity of Iowa, and the Board of the National Business Group on Health;
and he chairs the Global Health Benefits Institute. He received his MD and
MPH from Harvard University.
Samuel So, MB, BS, is a professor of surgery and the Lui Hac Minh Profes-
sor at Stanford University. He is also the director of the Asian Liver Center
and director of the Multidisciplinary Liver Cancer Program at the same
institution. He has published numerous studies on solid-organ transplanta-
tion and gastric and liver cancers. Dr. So is well known for his work on
hepatitis B and liver-cancer education and prevention programs. Through
his research, Dr. So has identified the need for a public-health approach to
liver-cancer prevention in recent Asian immigrants and first- and second-
generation Asians living in the United States. Those populations have not
been the typical focus of US screening and prevention programs. Dr. So is
listed in The Best Doctors in America, published by Woodward/White, Inc.
For his work in education and prevention, he received the 2005 National
Leadership Award from the New York University Center for the Study of
Asian American Health, the 2008 American Liver Foundation Salute to
Excellence Award, and the 2009 Asian Pacific Islander Heritage Award
from the California Asian Pacific Islander Joint Legislative Caucus. He is a
member of IOM’s Board on Population Health and Public Health Practice.
Dr. So received his MB and his BS in medicine and surgery from the Uni-
versity of Hong Kong and did postdoctoral and clinical fellowships at the
University of Minnesota.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
214 HEPATITIS AND LIVER CANCER
David L. Thomas, MD, MPH, is chief of the Division of Infectious Dis-
eases, Department of Medicine, of the Johns Hopkins School of Medicine.
He is also a professor in the Department of Epidemiology in the Johns
Hopkins Bloomberg School of Public Health. His broad research interest
is viral hepatitis, and current research projects include the progression of
hepatitis C in injection-drug users, hepatitis C pathogenesis and the host
genome, and antiviral therapy for HIV–hepatitis C virus coinfection. Dr.
Thomas received his MD from the West Virginia School of Medicine and
his MPH from the Johns Hopkins School of Hygiene and Public Health.
Lester N. Wright, MD, MPH, is the deputy commissioner and chief medical
officer for the New York Department of Correctional Services. He oversees
health care for some 62,000 residents in 70 facilities, who currently include
about 4,500 HIV-positive patients and 8,000 who have hepatitis C virus
infection. Before his employment in the New York Department of Correc-
tional Services, Dr. Wright worked in several state and county health de-
partments, including the Virginia Department of Health and the Delaware
Public Health Division. He spent 7 years working in Africa on delivery
of primary health care and health-system development. He has served on
two National Academies committees: the Committee on Regulating Occu-
pational Exposure to Tuberculosis and the Committee on the Elimination
of Tuberculosis in the United States. Dr. Wright received his MD from the
Loma Linda University School of Medicine and his MPH from the Harvard
School of Public Health.
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
215
B
Public Meeting Agendas
FIRST MEETING-DECEMBER 4, 2008
National Academy of Sciences Building, Washington, DC
Welcome and opening statement
Palmer Beasley, Committee Chair
Charge to the committee
John Ward, Centers for Disease Control and Prevention (CDC)
Chris Taylor and Martha Saly, National Viral Hepatitis Roundtable
Presentations to the committee
Dale Hu, CDC
Broad Overview of Hepatitis B
Cindy Weinbaum, CDC
Broad Overview of Hepatitis C
Lorren Sandt, Caring Ambassadors Program
Hepatitis C: Moving Beyond the Silence
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
216 HEPATITIS AND LIVER CANCER
Joan Block, Hepatitis B Foundation
Hepatitis B: Time for Zero Tolerance
Public comment period
SECOND MEETING-MARCH 3, 2009
The National Academies Beckman Center, Irvine, California
Welcome and opening statement
Palmer Beasley, Committee Chair
Presentations to the committee
Gary Heseltine
Lead Consultant, Viral Hepatitis Team, Council of State and Territorial
Epidemiologists
Surveillance Strengths and Weaknesses
William Rogers
Director of CMS, Physician’s Regulatory Issues Team
Viral Hepatitis Prevention Policies and Programs, Centers for Medicare
and Medicaid (CMS)
Tanya Pagán Raggio Ashley
Director, Office of Minority Health and Health Disparities, and Chief
Medical Officer, HRSA
Community Health Centers: Health Resources and Services
Administration (HRSA) Policies and Programs
Daniel Raymond
Policy Director, Harm Reduction Coalition
Hepatitis C Prevention: Harm Reduction
David Bell
Associate Director for Science and Global Activities, Division of Viral
Hepatitis, Centers for Disease Control and Prevention
Global Viral Hepatitis Burden: Implications for the US and CDC
Response
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
APPENDIX B 217
Mark Kane
Former Director of the Children’s Vaccine Program, PATH
Global Control Programs and HBV Immunization
Question and answer period
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
Index
asymptomatic infections, 47
HBV, 11, 27, 32, 47, 93, 110, 111, 113,
117-125, 127, 128, 129, 132, 134
HCV, 51
vaccination, 11, 32, 93, 110, 111, 113,
117-125, 127, 128, 129, 132, 134
Advisory Committee on Immunization
Practices (ACIP), 4, 9-10, 11, 55, 83,
88, 93, 100, 110-111, 112-115, 116,
125, 127, 132, 133, 134, 181
African Americans/Blacks, 1, 2, 10, 27, 29,
30, 32, 93, 116, 124, 168, 169, 184
Agency for Healthcare Quality and
Research, 149
Alabama, 27 n.1, 91, 116
Alanine aminotransferase (ALT), 48, 49, 50,
53, 93, 158, 167
Alaska, 120-121, 122-123. See also
American Indians and Alaska Natives
Alcohol consumption, 5, 14, 29, 30, 48, 84,
93, 148, 155, 168, 169, 181
Alternative-care providers, educational
programs for, 86, 87, 89
American Academy of Pediatrics, 111
American Association for the Study of Liver
Diseases (AASLD), 32, 155, 159,
166, 167, 168
American College of Obstetricians and
Gynecologists, 84, 97
A
Acute disseminated encephalomyelitis, 32
Acute infections
characteristics, 19
clinical outcomes, 117, 118, 136, 137
HBV, 1, 19, 23, 27, 34, 48, 50, 59,
70-71, 99, 117, 118, 119, 120, 121,
125, 161, 189
HCV, 19, 28, 29, 34, 47, 49, 51, 71,
136, 137, 163, 165
incarcerated people, 121
injection-drug use and, 120, 137, 189
outbreak detection and control, 48, 67,
70
prevalence and incidence, 1, 50, 70-71,
99, 118, 119, 120, 121, 125
screening and testing for, 47-51, 160,
161, 163, 165
surveillance, 29, 44, 47-51, 59, 64, 67,
71
Adolescents and young adults, 7, 11, 23,
25, 31, 44, 68, 71, 93, 98, 100, 110,
112, 127, 131, 134, 191
Adult Hepatitis B Vaccine Initiative, 129
Adult viral-hepatitis prevention coordinators
(AVHPC), 42-43, 57, 59, 61, 64, 70,
152-153
Adults. See also At-risk populations; specific
populations
219
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
220 INDEX
American College of Physicians, 159
American Indians and Alaska Natives, 29,
62, 81, 93, 129, 131, 168
Anti–tumor-necrosis factor therapy, 162
Asian American Hepatitis B Program, 92
Asians and Pacific Islanders (APIs). See also
Foreign-born
access to care, 56, 169
educational programs for, 87, 92, 93,
153, 183
health-care providers, 82
incidence and prevalence of HBV
infection, 1-2, 23, 27, 29, 81-82, 83,
93, 117-118, 153-154, 161, 183,
184-185
knowledge and awareness of HBV, 13,
82, 89-90, 173
liver cancer, 29, 153-154, 169
medical management of hepatitis, 183
risk of HBV, 90
screening/testing, 161, 173
surveillance, 32, 62, 68
treatment disparities, 169
vaccination, 10, 90, 92, 116, 117-118,
161-162
Aspartate transaminase, 49, 167
Asymptomatic infected individuals,
awareness of infection, 1, 3, 24, 26,
27, 50, 51, 90
At-risk populations. See also Foreign-
born populations; Illicit-drug users;
Incarcerated populations; Men who
have sex with men; Pregnant women
access to services, 3, 56, 79
defined, 27, 86, 156
education programs, 4, 14, 85-86, 92-
93, 95-96, 97, 98-100
health service provider knowledge of,
80, 81-84, 89
immunization, 4, 9, 10-11, 27, 81, 93,
113, 120-125
knowledge and awareness of hepatitis, 3,
4, 8, 9, 34, 89-91, 93-96
prevalence and incidence of hepatitis,
62, 81
recommendations, 16-17
screening and testing, 3, 4, 5, 6, 8, 9,
11, 13-14, 16, 27, 71-72, 85, 86, 97,
124-125, 148, 153, 155, 156-159,
161, 173
services, 3, 5, 6, 13, 16-17, 56, 79, 149,
189-192
surveillance, 2, 4, 6, 7, 61-62, 67, 68,
71-72
Awareness. See Knowledge and awareness
of chronic hepatitis
B
Baltimore, 28, 92, 122-123, 190
Blacks. See African Americans/Blacks
Blood transfusions, 2, 21, 24, 28, 50, 83,
84, 151, 158
Brachial neuritis, 32
Brazil, 138
Breastfeeding, 84
Bureau of Primary Health Care, 151
C
California, 58, 81, 83, 89, 99, 120, 121,
122, 173, 174, 182, 183, 186
Cambodian Americans, 90, 92
Cancer chemotherapy, 162
Case definitions for hepatitis, 7, 48-49, 50,
51, 52-53, 54, 55, 65, 68, 69
Case management, 43, 45-46, 57-58, 62-63,
65, 68, 70, 72
Centers for Disease Control and Prevention
(CDC), 2
Adult Hepatitis B Vaccine Initiative, 129
case definitions for hepatitis B and C,
48-49, 50, 52-53, 54, 55, 68
Division of Viral Hepatitis, 150-151
educational programs, 4, 8-9, 86, 87,
97, 96
Emerging Infections Program, 43, 58, 59
Epidemiologic Surveillance Project, 60
estimates of hepatitis burden, 25, 26, 27,
62, 120, 182, 189
National Immunization Program, 126
NEDSS, 61
NETSS, 60-61, 64
partner services guidelines, 63
PHIN-compliant systems, 64, 70
prevention and control recommendations
of, 30
resource allocation for services, 5-6, 14, 15,
16-17, 26, 42, 54, 126-127, 148-150,
151, 152, 153, 175, 183, 186, 192
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INDEX 221
risk factors for hepatitis, 156, 157-158,
159
screening and counseling
recommendations of, 82-83, 84, 156-
157, 159, 183
state cooperative agreements with, 4, 7,
42, 54, 57, 64-66, 67
surveillance initiatives, 4, 6, 7-8, 42-43,
44, 45, 50, 57, 58, 59-61, 63, 64,
65-66, 67, 68, 69, 70-71
vaccination recommendations and
programs, 12, 110-111, 124-125,
126, 128-129, 134, 136, 153, 157
VFC program, 128-129, 130, 131, 134
Centers for Medicare and Medicaid
Services, 129, 149. See also
Medicaid; Medicare
Central nervous system demyelinating
disorders, 32
Chicago, 28, 116, 121
Childhood Immunization Initiative, 126
Children
asymptomatic infections, 47
HBV, 23, 25, 30, 47, 116-117, 128-132
HCV, 51
information systems on, 127-128
progression of infection in, 46, 117, 118
vaccination, 4, 9, 10, 25, 30, 93, 97, 110,
111, 112, 116-117, 128-132, 134
Children’s Health Insurance Program
(CHIP), 128, 129-132, 172
Chinese Americans, 68, 81, 82, 86, 89, 90,
92, 174
Chronic infections. See also Hepatitis B;
Hepatitis C; Knowledge and
awareness of chronic hepatitis
age at exposure and potential for, 19, 22,
46, 51, 82-83, 113, 117, 118, 156
asymptomatic nature of, 3, 23, 24, 25,
27, 28, 47, 50, 51, 52, 53, 55, 90,
159, 162
clinical outcomes, 23; see also Liver
cancer and liver cirrhosis
prevalence and incidence, 1, 34, 121
surveillance, 25, 44, 51-54, 59, 64, 67,
71
Clinical outcomes. See also Liver cancer and
liver cirrhosis
age at exposure and, 19, 22, 46, 51, 82-
83, 113, 117, 118, 156
knowledge of, 80, 83, 89
Coinfection
HBV and HCV, 23, 29, 30, 32
HIV and hepatitis, 23, 29, 72, 81-82,
190
Collaborative Injection Drug User Study
Drug User Intervention Trial, 94, 95
Colorado, 58
Committee task
approach, 32-35
charge to committee, 30-32
Community
health centers, 16, 149, 186-189
outreach, 9, 90, 91-92, 97, 98-99, 101
screening and testing programs, 5, 13
Confidentiality safeguards, 43-44, 65
Connecticut, 55, 58, 122
Contacts. See also Partner services
education of, 97, 98
vaccination, 54, 57-58, 62, 93, 117,
119-120
Correctional facilities. See also Incarcerated
populations
educational programs on viral hepatitis,
88-89, 99-100
recommendations, 16
viral hepatitis services, 6, 13, 14, 16,
149, 184-186
Counseling, 5, 14, 31, 62, 63, 84, 85, 87,
95, 124, 134, 148, 151, 152, 157,
160, 163, 168, 171, 172, 177, 179,
180, 181, 186, 189, 191
D
Deaths, preventable, by disease, 20
Denmark, 121
Department of Health and Human Services,
110
National Vaccine Program Office, 126
Office of Minority Health and Health
Disparities, 2, 30, 149, 152
Department of Justice, 6, 16, 149, 186
Department of Veterans Affairs (VA), 2, 28,
30, 94, 130, 171, 172
Detroit, 121
Discrimination. See Stigmatization and
discrimination
Drug treatment programs and facilities. See
also Illicit-drug users
educational programs on viral hepatitis,
8, 88-89, 95-96, 100, 176
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
222 INDEX
funding, 176
integrated approach, 14, 149, 179
prevention of seroconversion, 177, 178
screening and testing, 176
staff knowledge of hepatitis, 85, 88, 96
vaccination opportunities, 121, 124, 129
Drug users. See Illicit-drug users
E
Economic issues. See also Funding;
Insurance coverage
screening and testing, 27, 161-162, 163
vaccination, 54, 57-58, 117-119, 124,
137-138
Educational programs. See also Knowledge
and awareness of chronic hepatitis
advocacy efforts, 153-154
for alternative-medicine professionals,
86, 87, 89
for at-risk populations, 4, 14, 85-86, 92-
93, 95-96, 97, 98-100, 153-154
CDC initiatives, 4, 8-9, 86, 87, 96, 97
contacts, 97, 98
content, 86-87
continuing medical education, 87
educational programs, 4, 8-9, 86, 87,
96, 97
evaluation of, 97
funding, 99, 152
for general population, 4, 96, 97, 98,
99, 153
goals, 9, 97
for health-care and social service
workers, 4, 8-9, 58, 82, 84-88
integration into other programs, 9, 92,
95-96, 98
linguistically and culturally appropriate,
9, 87, 90, 92, 93, 97, 98-99, 101,
153, 183
outreach component, 96, 97, 98-99, 100
peer education, 95, 100
in perinatal facilities, 99-101
recommendations, 4, 8-9, 85-89, 96-101
safety precautions and procedures, 88
on screening and testing, 9, 58, 98
vaccination, 8, 9, 97, 101
Electronic medical records, 7, 50, 51, 60,
65, 68, 69, 70
Egyptian immigrants, 24, 159
Emerging Infections Program, 43, 58, 59
Employee Retirement Security Act, 134
End-stage renal disease patients, 113, 131,
152
Epidemiologic Surveillance Project, 60
Epidemiology and Laboratory Capacity for
Infectious Diseases program, 59
Exposure routes
knowledge and awareness, 95
sexual, 1, 23, 44, 72, 84, 119-120
unsafe vaccine injections, 24
F
Federal Employees Health Benefits Program,
5, 13, 130, 148, 172
Florida Hepatitis Prevention Program,
186-187
Food and Drug Administration, 109
Foreign-born populations. See also Asians
and Pacific Islanders; Hispanics
access to care, 56
culturally appropriate programs, 13, 56,
173-174, 183-184
educational outreach to, 9, 90, 91-92,
97, 98-99, 101, 174-175
exposure routes, 120
geographic regions of endemicity, 81-82
HBV, 1-2, 8, 13, 14, 23, 27, 81-82, 89-
90, 91-92
health disparity, 27
incidence and prevalence of HBV, 8, 27,
79, 86, 93
knowledge and awareness of risks to, 13,
79, 81-82, 86, 87, 89-90, 173-174
liver cancer and cirrhosis, 29
recommendations, 14, 175
screening and testing, 5, 13, 14, 90, 91,
148, 153-154, 155, 156, 161-162,
173
vaccination, 5, 10, 13, 14, 90, 91, 92-
93, 116, 117-118, 120, 132, 148,
157, 161-162
viral hepatitis services for, 5, 13-14, 148,
173-175
Funding
education, 99, 152
surveillance, 3, 7, 42, 57, 58-59, 63, 65,
66, 67, 71, 129
vaccination, 57, 118, 120, 129, 134
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INDEX 223
G
General population
education program, 4, 96, 97, 98, 99
knowledge and awareness of chronic
hepatitis, 3, 4, 9, 17, 33, 34, 79, 98
recommendations, 13
screening and testing, 13
viral hepatitis services, 13, 170-173
Guillain-Barré syndrome, 32
H
Health Disparities Collaborative, 188
Health Resources and Services
Administration (HRSA), 6, 16, 127,
148-149, 151-152, 187, 188-189, 192
Health-care providers and workers
APIs, 82
educational programs, 4, 8-9, 58, 82,
84-88
guidelines for, 80
immunization, 124
knowledge and awareness of hepatitis, 3,
4, 8, 17, 33, 34, 79, 80-89, 154-155,
171, 182-183
outreach to, 97
safety precautions and procedures, 88
vaccination, 88, 93, 113, 117, 118, 124,
125
Healthcare Effectiveness Data and
Information Set (HEDIS), 126
Healthcare Systems Bureau, 152
Hemodialysis, 21, 22, 24, 44, 93, 113, 156,
158, 162
Hepatitis A, 30, 48, 49, 50, 57, 58, 137,
150-151, 189, 190
Hepatitis B. See also Vaccination for
Hepatitis B; specific populations and
services
acute infection, 1, 19, 23, 27, 34, 48,
50, 59, 70-71, 99, 117, 118, 119,
120, 121, 125, 161, 189
adults, 27, 47, 117-125, 132
at-risk populations, 1-2, 21-22, 27, 81-
82, 120-125
case definition, 48, 50, 51, 52
causative agent, 19, 21
children, 23, 25, 30, 47, 116-117,
128-132
chronic infection, 19, 22, 23, 34, 48, 51,
52, 59-60, 64
community knowledge and awareness,
89-93
contact screening, 48, 82, 86
deaths, 20, 23, 26, 34, 83
economic issues, 25, 26, 128-135
education programs, 83, 90, 92-93
exposure routes, 1, 21, 23, 26, 44, 90
geographic differences, 27, 81
HBsAg determinant of infection, 10, 21,
22, 46, 48, 51, 52, 54, 55, 56, 69,
82-83, 99, 100-101, 109, 110, 111,
112, 113, 114, 115, 124, 156, 157,
159, 160, 161, 162, 166, 174, 181,
182, 183
health-care provider knowledge, 81-83
health-care use trends, 30
health-care workers, 91, 124
HIV-infected people, 29, 124
immunization, see Vaccines and
vaccination
incidence and prevalence, 1-2, 21, 23,
26-27, 29, 83, 118, 119
infants, 1, 25
institutionalized developmentally
disabled people, 124
insurance coverage, 5, 128-134
knowledge and awareness of, 81-83, 89-
93, 127-128
liver cancer and liver disease from,
29-30
medical management, 82, 90, 166-167
men who have sex with men, 91
mistrust of vaccination, 127-128
progression of infection and clinical
outcomes, 22, 23, 25, 29, 46; see
also Liver cancer and liver cirrhosis
public vaccine programs and insurance,
128-132
racial/ethnic differences, 27, 29
reactivation, 162
registries of immunization, 126-127
risk factors, 27
screening and testing, 5, 8, 13, 14, 23,
27, 47, 48-49, 51, 81, 82-83, 86, 90,
91, 124-125, 152, 156-157, 160-162
stigma/discrimination, 23, 91-92
surveillance, 44, 46, 47, 48, 50, 51, 52,
59-60, 61, 64, 71
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
224 INDEX
Hepatitis B immune globulin (HBIG), 4, 9-
10, 55, 69, 110, 111, 112-113, 114,
115, 182, 183-184
Hepatitis B Initiative, 92
Hepatitis C. See also specific populations
and services
acute infection, 19, 28, 29, 34, 47, 49,
51, 71, 136, 137, 163, 165
adults, 51
at-risk populations, 21-22, 28, 93-101,
158
case definition, 49, 50, 53, 68
causative agent, 19, 21, 137
children, 51
chronic infection, 17, 22, 34, 51-52, 59,
64, 136-138
economic issues, 25, 26, 137-138
education programs, 84-85, 95-96
exposure routes, 2, 5, 21, 24, 26, 28, 44,
84
health-care provider knowledge, 83-85
in HIV-infected people, 30
knowledge and awareness, 83-85, 93-96
medical management, 167-169
mortality, 20, 23, 26, 34, 45
prevalence and incidence, 21, 22-23, 24,
26, 28-29, 137, 138
prevention, 5, 24, 79, 136-138, 196-187
progression of infection and clinical
outcomes, 22, 24, 29-30, 47, 84; see
also Liver cancer and liver cirrhosis
racial/ethnic differences, 29-30, 168-169
risk factors, 29-30, 84
screening and testing, 5, 8, 28, 51-52,
53-54, 62, 68, 84, 85, 86, 93-94,
152, 157-159, 162-165
spontaneous resolution, 51, 136
stigma/discrimination, 24, 85, 94-95
surveillance, 28, 44, 45, 47, 49, 51-52,
53-54, 59-60, 61, 62, 63, 64, 71
vaccine development, 2, 5, 24, 136-138
Hepatitis C Continuity Program, 185-186
Hepatitis D, 30
Hepatitis E, 30
Hepatocellular carcinoma. See Liver cancer
and liver cirrhosis
High-risk populations. See At-risk
populations
Hispanics, 2, 10, 27, 30, 93, 116, 121, 159,
168-169, 184-185
HIV/AIDS, 124
burden of disease, 25, 26
coinfection, 23, 29, 72, 81-82, 190
funding for activities, 45, 150-151
HBV vaccination, 93, 113, 120, 124,
129
mortality, 20, 45
partner services, 63, 72
Prevention for Positives initiatives, 95
public awareness campaign, 98
screening and testing, 120, 156, 162
surveillance, 59, 61, 62, 63, 64, 66, 67,
72
HIV/AIDS Bureau, 152
Homeless people, 56, 62, 71, 152, 154-155,
187, 188, 191
I
Illicit-drug users, injection drug users. See
also Drug treatment programs and
facilities
access to health services, 2, 24, 29, 56,
85, 176
acute infections, 120, 137, 189
contact notification, 63, 72, 86
education programs, 95-96, 97, 154,
179
gaps in services for, 175-181
HBV, 1, 14, 23, 61-62, 82, 83, 120-121,
122-123, 176
HCV, 2, 5, 8, 14-15, 24, 28-29, 61, 62,
83, 84, 86, 93-94, 95-96, 136-137,
148, 158, 175-176
health-care use, 14, 176
high-risk period, 14-15
knowledge and awareness of risks to,
82, 83, 86, 94, 95-96
needle-exchange/safe injection programs,
5, 9, 14, 28, 80, 88-89, 94, 97, 100,
120-121, 148, 155, 166, 177, 180
prevalence and incidence of infection,
14, 27, 61-62, 82, 96, 120, 176
recommendations, 15, 179-181
referral for medical management, 148
screening, testing, and counseling, 14,
62, 83, 85, 86, 94, 148, 156-157,
158, 162, 163, 179
stigmatization and discrimination, 24,
85
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INDEX 225
surveillance, 2, 56, 61-62, 63
transmission of hepatitis, 1, 14, 24
vaccination, 14, 93, 120-121, 122-123,
124, 129, 157
viral hepatitis services, 5, 14-15, 63,
148-149, 175-181
Illicit-drug users, non-injection drug users,
9, 14, 96, 97, 122-123, 175-176
Immigrant services, 8. See also Foreign-born
populations
Immunization. See also Vaccines and
vaccination
HBIG adjuvant, 4, 9-10, 55, 69, 110,
111, 112-113, 114, 115, 182, 183-184
Immunoglobulin M (IgM) antibody, 48, 49,
50, 51, 52, 160, 161
Immunosuppresive therapy, 162
Incarcerated populations
acute infections, 121
education programs, 5, 8, 9, 99-100
HBV, 16, 27, 62, 83, 90-91, 121-124,
184
HCV, 8, 16, 28, 62, 83, 86, 100, 184
knowledge and awareness of risks to,
83, 86, 90-91
prevalence and incidence of infection,
121, 184
racial/ethnic differences, 184-185
recommendations, 186
screening and testing, 16, 156-157, 185
size of, 62, 99
surveillance, 62
vaccination, 121-124, 157, 185
viral health services, 6, 16, 149, 184-186
Incidence of hepatitis. See Prevalence and
incidence of hepatitis
Infants. See also Perinatal infections
antiviral therapy, 183-184
followup, 56
HBV case definition, 55
HBV infection, 4, 9-10, 25, 54-55, 75,
93, 100, 110, 111-116, 173, 182
immunization, 4, 9-10, 25, 54-55, 75,
93, 100, 110, 111-116, 173, 182
incidence and prevalence of hepatitis,
100, 111, 112, 182
potential for and progression to chronic
infection, 22, 46, 51, 82, 113, 156
preterm, 111, 112, 114, 115
screening/testing, 54, 162
surveillance, 182
Infectious Diseases Society of America, 159
Inflammatory bowel disease, 162
Influenza, 20, 27, 110
Information systems, 5, 11, 72, 126-127
Initiative on Immunization Registries, 126
Institute of Medicine, 127-128
Institutionalized developmentally disabled
people, 62, 93, 113, 124, 156-157
Insurance coverage
gaps and barriers, 11, 134-135, 170
private plans, 11, 12, 132-134
public plans, 11-12, 128-132, 172-173;
see also specific programs
recommendations, 11-12, 172-173
screening and testing, 13, 148
vaccination, 5, 11-12, 128-132, 135
International Symposium on Viral Hepatitis
and Liver Disease, 82, 83
Injection-drug users. See Illicit-drug users
Iowa, 95
Italy, 163
J
Jade Ribbon Campaign, 92, 153-154
K
Knowledge and awareness of chronic
hepatitis. See also Educational
programs
age and, 93
asymptomatic infected individuals, 1, 3,
24, 26, 27, 50, 51, 90
at-risk populations, 3, 4, 8, 9, 13, 34,
82, 89-91, 93-96, 173
of clinical outcomes, 80, 83, 89
community, 89-101
contact notification and screening, 9, 84
correctional facilities, 88-89, 99-100
and discrimination and stigma, 8, 9, 85,
91-92, 94-95
drug-treatment facilities and needle-
exchange programs, 5, 8, 9, 100
exposure routes, 95
general public, 3, 4, 9, 17, 33, 34, 79,
98
HBV, 81-83, 89-93, 127-128
HCV, 83-85, 93-96
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
226 INDEX
health-care and social-service providers,
3, 4, 8, 17, 33, 34, 79, 88-89, 154-
155, 171, 182-183
mistrust of vaccination, 8, 127-128
perinatal facilities, 82-83, 100-101
policy-makers, 3, 17, 98
of prevalence and incidence, 8, 79, 80,
81, 83, 89, 153-154
of prevention approaches, 80, 89
race/ethnicity and, 93
recommendations, 4, 8-9, 85-89, 96-101
or risk factors and high-risk population
characteristics, 80, 81-84, 89
of screening, testing, and management
methods, 5, 8, 9, 79, 80, 82-83, 84,
90
surveillance and, 45
Korean Americans, 90
L
Lamivudine prophylaxis, 162, 170, 183
Lao People’s Democratic Republic, 115-116
Liver cancer and liver cirrhosis
age and, 79
deaths, 1, 23, 24, 25, 29
incidence and prevalence, 22-23, 79, 154
prevention, 1, 19, 109
progression of infection to, 46-47
racial/ethnic differences, 29-30, 153-154,
169
risk factors, 29-30, 169
surveillance, 67, 72
survival rate, 23
Liver transplants, 25, 67, 110, 169
M
Maryland, 27 n.1, 116, 173
Massachusetts, 63, 69, 186
Measles, 20, 116 n.1, 136
Medicaid, 13, 128-129, 132, 148, 152, 168,
172
Early Periodic Screening, Diagnosis, and
Treatment, 11, 130-131, 134
Medical management of hepatitis, 3, 5,
166-170
access to, 56, 79, 130, 183
antiviral therapy, 6, 15, 24, 79, 86, 149,
184
coinfections, 23
components of, 155
costs and cost-effectiveness, 163,
169-170
disparities, 169
education on, 86
goals, 166
guidelines, 30, 32, 80, 155, 166-168
insurance coverage, 130, 170
interferon-alpha-based treatment, 30,
170
provider knowledge, 82, 86
racial/ethnic disparities, 168-169
referral for, 5, 6, 14, 15, 31, 56, 62-63,
70, 72, 83, 120-121, 148, 149, 153,
166, 170, 171, 177, 181, 182, 183,
189
Medicare, 5, 13, 128, 130, 132, 134, 148,
152, 168, 172
Men who have sex with men, 21, 27, 44,
71, 81, 82, 91, 97, 113, 156, 162,
191
Metabolic syndrome, 30
Minnesota, 58, 172
Mobile health units, 6, 13, 16-17, 120-121,
122, 149, 189, 191-192
Montana, 116
Multiple sclerosis, 32
N
National Alliance of State and Territorial
AIDS Directors (NASTAD), 42-43,
58, 59, 60, 61, 63, 189
National Center for HIV/AIDS, Viral
Hepatitis, Sexually Transmitted
Diseases, and Tuberculosis
Prevention, 26, 45, 150-151
National Center for Immunization and
Respiratory Diseases, 15, 126,
181-182
National Electronic Disease Surveillance
System (NEDSS), 61
National Electronic Telecommunications
System for Surveillance (NETSS),
60-61, 64
National Health and Nutrition Examination
Survey, 62
National HIV Behavioral Surveillance
System, 62, 72
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INDEX 227
National Immunization Program, 126
National Immunization Surveys, 111-112,
126, 128
National Institutes of Health, 6, 15, 30,
149, 184
National Vaccine Advisory Committee
(NVAC), 126, 127
National Viral Hepatitis Roundtable, 2, 30
Needle-stick injuries, 1, 21, 88, 158
New Haven, 120, 122-123
New Jersey Academy of Family Physicians,
82, 83
New York City, 28, 58, 85, 90, 92, 120-
121, 122, 173, 186, 190
New York state, 58, 185-186
Nosocomial infections, 24, 67, 87, 88
O
Occupational Safety and Health
Administration, 88
Office of Management and Budget, 129
Omnibus Budget Reconciliation Act of
1993, 128-129
Optic neuritis, 32
Oregon, 58
Outbreak
detection and control, 48, 67, 70
prevention, 88
P
Partner services
CDC guidelines, 63
contact notification, 9, 15, 63
cost-effectiveness, 63
funding, 72
screening and testing, 48, 82, 86, 98,
100, 154, 162
surveillance, 48, 62-63, 68, 72
vaccination, 54, 57-58, 62, 93, 113,
117, 119-120
Perinatal hepatitis B coordinators, 15, 54, 152
Perinatal infections. See also Pregnant
women
educational programs on, 99-101
HBV, 46, 111-116, 152
immunization, 9, 54-55, 111-116
knowledge and awareness, 82-83,
100-101
prevention, 5-6, 15, 54, 183-184
progression of, 46
screening and testing, 54-56
surveillance, 54-56
Peripartum antiviral therapy, 6, 15, 149, 184
Policy-makers
knowledge and awareness of chronic
hepatitis, 3, 17, 98
Polio, 20, 110, 116 n.1
Pregnant women. See also Perinatal
infections
antiviral therapy, 6, 15, 149, 184
case management, 70, 149, 182
education, 97, 100-101
educational programs on viral hepatitis,
9, 99-100
foreign-born women, 23, 182-183
HBV, 6, 15, 23, 25, 82-84, 90, 149,
182-183
HCV, 83-84
household contacts and sexual partners,
54, 182
knowledge and awareness of risks to,
82-84
lamivudine prophylaxis, 162, 170, 183
medical management, 6, 15, 149,
182-183
recommendations, 15-16, 184
screening and testing, 15, 25, 54-56,
69, 82-83, 84, 111, 120, 149, 162,
181-182
surveillance, 54, 69, 70, 182
vaccination, 4, 10, 54, 111-116, 129,
131, 132, 182
viral hepatitis services, 15, 149, 181-184
Prevalence and incidence of hepatitis
accuracy of estimates, 50-51, 56, 57, 66,
70-71
acute infections, 1, 50, 70-71, 99, 118,
119, 120, 121, 125
APIs, 1-2, 23, 27, 29, 81-82, 83,
93, 117-118, 153-154, 161, 183,
184-185
at-risk populations, 62, 81
CDC estimates, 25, 26, 27, 62, 120,
182, 189
chronic infections, 1, 34, 121
definitions, 7 n.1
immigration and, 2
knowledge and awareness of, 8, 79, 80,
81, 83, 89, 153-154
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
228 INDEX
monitoring and reporting, 71; see also
Surveillance
U.S., 1, 2, 25-29, 86
worldwide, 22-24
Prevention and control of hepatitis. See also
Counseling; Educational programs;
Medical management of hepatitis;
Screening and testing; Vaccines and
vaccination; Viral hepatitis services
barriers to, 2-3
CDC recommendation, 30
charge to committee, 31
education on, 80, 87
funding, 44, 54
harm reduction, 155, 166
knowledge and awareness of methods,
84, 86
needle-exchange/safe injection programs,
5, 9, 14, 28, 80, 88-89, 94, 97, 100,
120-121, 148, 155, 166, 180
perinatal transmission, 25, 183-184
research recommendations, 15
state plans, 152-153
strategies, 25, 31, 84, 177-179
Prevention for Positives initiatives, 95
Public Health Information Network
(PHIN), 7, 61, 64, 65, 70
R
Race/ethnicity
and knowledge and awareness of
hepatitis, 93
vaccination disparities, 10, 116-117, 121
Racial and Ethnic Approaches to
Community Health (REACH) 2010,
93
Recommendations
at-risk populations, 14, 15, 16-17, 175,
179-181, 184, 186
committee approach, 32
community health centers, 188-189
education programs, 4, 8-9, 85-89,
96-101
insurance coverage, 11-12, 172-173
integrated services, 192
outcomes of implementing, 17, 34
screening and testing, 4, 6, 13, 16, 148
vaccination, 4-5, 9-12, 93, 114, 117,
125, 127, 135, 136, 138
Referral for additional services, 5, 6, 14, 15,
31, 56, 62-63, 70, 72, 83, 120-121,
148, 149, 153, 166, 170, 171, 177,
181, 182, 183, 189
Reporting systems and requirements, 59-61,
68
Resource allocation, 45
barriers to, 3
CDC, 5-6, 14, 15, 16-17, 26, 42, 54,
126-127, 148-150, 151, 152, 153,
175, 183, 186, 192
Respiratory syncytial virus, 20
Rheumatoid arthritis, 162
Rhode Island, 90
Risk factors for hepatitis
APIs, 90
CDC, 156, 157-158, 159
knowledge and awareness of, 80, 81-84,
89
screening for, 3, 5, 8, 11, 13, 16, 85, 86,
124-125, 148, 153, 155, 156-159,
162
Ryan White CARE Act and program, 33,
152, 170
S
Safety precautions and procedures, 88
San Diego, 134, 170, 189-190
San Francisco, 58, 81, 120, 121, 122-123,
173, 174, 182
Schistosomiasis-eradication campaign, 24
Scotland, 122-123
Screening and testing
access to, 3
acute infections, 47-51, 160, 161, 163,
165
antigens and antibodies used for, 160,
161
at-risk populations, 3, 4, 5, 6, 9, 11,
13-14, 16, 27, 71-72, 91-92, 97,
120, 124-125, 148, 152, 153-154,
156-157, 158-159, 161-162, 173
barriers to, 124-125
CDC recommendations, 82-83, 84, 156-
157, 159, 183
community-based programs, 5, 13
confirmatory tests, 162-163
contacts/partners, 48, 82, 86, 98, 100,
154, 162
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INDEX 229
cost-effectiveness, 27, 161-162, 163
cultural aversion to, 91-92, 98
education on, 9, 58, 98
electronic laboratory reporting, 7, 60,
65, 68-69, 70
enzyme immunoassay, 51, 53, 54, 162-
163, 164-165
followup/repeat, 48-49, 80
general population, 13
goals, 154-155
guidelines, 80, 86
HBV, 5, 8, 13, 14, 23, 27, 48-49, 51,
81, 82-83, 86, 90, 91, 124-125, 152,
156-157, 160-162
HCV, 5, 8, 28, 51-52, 53-54, 62, 68,
84, 85, 86, 93-94, 152, 157-159,
162-165
importance, 23
insurance coverage, 13, 148, 171
interpretation of results, 94, 160-161,
162, 164-165
knowledge and awareness of methods, 5,
8, 9, 79, 80, 82-83, 84, 90
laws, 83
nucleic acid testing, 49, 53-54, 68, 159,
163, 164-165
pregnant women, 15, 25, 54-56, 69, 82-
83, 84, 111, 120, 149, 162
recombinant immunoblot assay, 49, 53,
163, 164-165
recommendations, 4, 6, 13, 16, 148
referral for medical management, 5, 6,
14, 15, 31, 56, 62-63, 70, 72, 83,
120-121, 148, 149, 153, 166, 170,
171, 177, 181, 182, 183, 189
reporting test results, 4, 6, 7, 41-56, 58,
59-61, 65, 66, 67, 68-69
resource allocation, 3, 17, 45
resources available for, 49, 54, 56, 57-58
risk-factor, 3, 5, 8, 11, 13, 16, 85, 86,
91, 124-125, 148, 153, 155, 156-
159, 162
serologic, 4, 5, 6, 7, 13, 47, 51, 53-54,
120, 148, 156, 159-165
VA program, 28, 158
Section 317 Immunization Grant program,
11, 126, 129, 130, 132, 134, 135,
153
Services. See Viral hepatitis services
Sexual exposure to hepatitis, 1, 23, 44, 72,
84, 113, 119-120
Sexually transmitted diseases (STDs)
clinic services for hepatitis, 6, 13, 14,
16-17, 54, 86, 87, 119-120, 124,
125, 129, 134, 149, 151, 170, 171,
176, 189-190, 191, 192
disease intervention specialists, 63
funding for services, 45, 151
integrating services for STD and
hepatitis, 189-190
partner notification, 63
surveillance, 59, 61, 63
Shelter-based programs, 6, 13, 16-17, 149,
189, 191, 192
Social and peer support, 3, 95, 100, 155
Social-service providers. See also Substance-
abuse services and providers
educational programs, 88-89
knowledge and awareness of hepatitis,
80-89
Society of General Internal Medicine, 82
South Dakota, 116
Southeast Asian immigrants, 24, 79, 82, 183
Standardization of data, 69
State and territorial health departments
case followup, 55
cooperative agreements with CDC, 4, 7,
42, 54, 57, 64-66, 67
funding, 152
surveillance role, 4, 6, 7
STD/HIV clinics, 87, 189-190
Stigmatization and discrimination, 8, 9, 23,
24, 56, 85, 87, 89, 91-92, 94-95, 97,
98, 170, 174
Study to Reduce Intravenous Exposures
(STRIVE), 95
Sub-Saharan African immigrants, 23, 79,
82, 90
Substance Abuse and Mental Health
Services Administration (SAMHSA),
149, 152
Substance-abuse services and providers.
See Drug treatment programs and
facilities
Surveillance
acute infections, 29, 44, 47-51, 59, 64,
67, 71
analyzing, reporting, and disseminating
findings, 67, 70-71
applications of data from, 41, 42, 43-46
at-risk populations, 2, 4, 6, 7, 32, 61-62,
67, 68, 71-72
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
230 INDEX
automated data collection, 7, 51, 56, 60,
65, 68-70
AVHPC surveys, 42-43, 54, 55, 57, 58,
59, 61, 64, 70
case definitions, 7, 48, 49, 50, 51, 52,
53, 54, 55, 65, 68, 69
case management uses, 43, 45-46, 57-
58, 62-63, 65, 68, 70, 72
CDC initiatives, 4, 6, 7-8, 42-43, 44, 45,
50, 57, 58, 59-61, 63, 64, 65-66, 67,
68, 69, 70-71, 150
challenges, 29, 47-54, 56, 60
chronic infections, 25, 44, 51-54, 59, 64,
67, 71
committee charge and approach, 31-32,
41-42
confidentiality safeguards, 43-44, 65
core activities, 4, 6, 7, 43, 66, 67, 68
current system, 3, 25, 34, 41-42
design of programs, 6, 59
disease-specific issues, 46-56
electronic medical records, 7, 50, 51, 60,
65, 68, 69, 70
enhanced projects, 58, 62, 71-72
evaluation of systems, 63-64, 66, 69, 70
funding, 3, 7, 42, 57, 58-59, 63, 65, 66,
67, 71, 129
identifying infections, 4, 6, 41-56, 69
infrastructure and process-specific issues,
57-66, 67, 70
jurisdictional issues, 56, 57, 60, 65-66
and knowledge and awareness of
heptatitis, 45
liver cancer and cirrhosis, 67, 72
model programs, 43, 65, 66-72
outbreak detection and control uses, 44,
48, 67, 70
partners of infected people, 48, 62-63,
68, 72
perinatal infections, 54-56
PHIN-compliant systems, 7, 61, 64, 65,
70
pregnant women, 54, 69, 70, 182
programmatic design and evaluation
uses, 3, 41, 45, 57, 67
quality of data, 50, 57, 64, 66, 67, 71,
79, 94
recommendations, 4, 6-7, 43, 63-66
reporting systems and requirements, 7,
48, 51, 58, 59-61, 65, 66, 67, 68-69
resource allocation uses, 3, 17, 45
serologic testing, 4, 6, 7, 47, 51, 53-54,
68, 71, 159-165
standardization issues, 6, 7, 41, 56, 61,
64, 65, 66-67, 68, 69, 70
state-CDC cooperative agreements, 4, 7,
42, 54, 57, 64-66, 67
targeted, 43, 66, 71-72
underreporting/misclassification of
infections, 3, 27, 34, 47, 50, 60, 62,
70-71
vaccinations, 59, 72, 111
T
Tattooing and piercing, 99, 158-159
Transverse myelitis, 32
Travelers, 22, 93, 113, 117, 156
Tuberculosis, 20, 26, 45, 61, 150, 151, 186
U
United Kingdom, 162
US Preventive Services Task Force, 82-83,
159, 181
US Public Health Service, 132, 150, 159, 190
V
Vaccines and vaccination, HBV
accessibility, 120-121, 124, 128, 129,
134, 135
ACIP recommendations, 4, 9-10, 11, 55,
83, 88, 93, 100, 110-115, 116, 125,
127, 132, 133, 134, 181
adults, 11, 32, 93, 110, 111, 113, 116,
117-125, 126, 127, 128, 129, 132,
134
at-risk populations, 4, 9, 10-11, 27, 81,
93, 113, 117-125
barriers to, 8, 10, 11-12, 118, 120, 124-
125, 127-136
CDC recommendations and programs,
12, 110-111, 124-125, 126, 128-129,
134, 136, 153, 157
children and adolescents, 4, 9, 10, 25,
30, 93, 97, 110, 111, 112, 116-117,
126, 127, 128-132, 134
cost-effectiveness, 54, 57-58, 117-119,
124, 137-138, 162
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
INDEX 231
coverage data, 111-112, 114-115, 116,
117, 118, 120, 121, 126, 132
education programs, 8, 9, 97, 101
efficacy, 110
evaluation of programs, 45
foreign-born people, 5, 10, 13, 14, 90,
91, 92-93, 116, 117-118, 120, 132,
148, 152, 161-162
formulations, 109-110, 136
funding for, 57, 118, 120, 129, 134, 152
geographic variability, 116
HBIG adjunct, 110, 114
health-care and social-service workers,
88, 93, 113, 117, 118, 124, 125
HIV-infected people, 93, 113, 120, 124,
129
identifying at-risk adults for, 124-125
illicit-drug users, 14, 93, 120-121, 122-
123, 124, 129
immunization-information systems, 5,
11, 72, 126-127
incarcerated people, 11, 113, 121-124,
125
incentives, 121
infants, 4, 9-10, 25, 54-55, 75, 93, 97,
110, 111-116, 120, 173, 182
institutionalized developmentally
disabled people, 93, 124
insurance coverage, 5, 11-12, 128-132,
135
liver cancer prevention, 109
liver transplants and, 110
mandatory, 116-117, 134, 153
mistrust of, 8, 127-128
partners and household members (ring
vaccination), 54, 57-58, 62, 93, 117,
119-120, 162
payment for, 57, 128-135, 152, 153
perinatal, 4, 10, 54, 111-116, 129, 131,
132
public programs and insurance, 128-132
racial and ethnic disparities, 10, 116-
117, 121
recommendations (committee), 4-5,
9-12, 93, 114, 117, 125, 127, 135,
136, 138
safety issues, 32-33, 127-128
schedules and completion of series, 11,
25, 55, 91, 101, 110, 111, 114,
116 n.1, 120, 121, 125, 127, 157
supply of vaccines, 5, 12, 118, 127,
135-136
surveillance, 59, 72, 111
travelers, 22, 93, 113, 117, 156
WHO guidelines, 30, 114
Vaccines and vaccination, HCV
development, 2, 24, 136-138, 166
feasibility, 136-137
need for, 137
recommendations, 5, 12, 138
therapeutic, 136
Vaccines for Children (VFC) program, 128-
129, 130, 131, 134
Veterans, 28, 94, 130-131, 158, 168, 171
Vietnamese Americans, 68, 90, 171
Viral hepatitis services. See also Counseling;
Educational programs; Medical
management of hepatitis; Screening
and testing; Vaccines and vaccination
access to, 2, 3, 34, 56, 79, 151, 169, 170
adult viral-hepatitis prevention
coordinators, 42-43, 57, 59, 61, 64,
70, 152-153
adults, 3, 5, 6, 13, 16-17, 56, 79, 149,
189-192
case management, 45-46, 57-58, 62-63,
70, 72, 149, 170
CDC allocations for, 5-6, 14, 15, 16-17,
26, 42, 54, 126-127, 148-150, 151,
152, 153, 175, 183, 186, 192
community-based approaches, 5, 6, 13,
14, 16, 148, 149, 174-175, 186-188
core components, 5, 12, 13, 148, 153,
154-157
current status, 148-154
design and evaluation of programs, 3,
41, 45, 57, 67
foreign-born people, 5, 12, 13-14, 16,
92, 148, 173-175
funding (public), 148-149, 150-152,
171, 172-173
gaps in, 12-17, 170-192
general population, 12, 13, 148,
170-173
HBV, 5-6, 14, 15, 148, 149, 153,
182-183
HCV, 5, 14, 148-149, 153
identifying infected persons, see
Screening and testing
illicit-drug users, 2, 5, 12, 14-15, 148-
149, 175-181
Copyright © National Academy of Sciences. All rights reserved.
Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C
http://www.nap.edu/catalog/12793.html
232 INDEX
incarcerated populations, 6, 13, 16, 149,
184-186
integrated approach, 14, 16-17, 149,
171-172, 179, 180-181, 189-192
knowledge and awareness of, 91
mobile health units, 6, 13, 16-17, 120-
121, 122, 149, 189, 191-192
model programs, 33, 152, 157, 170,
171-172
nongovernmental organizations,
153-154
pregnant women, 5-6, 13, 15, 54, 70,
149, 181-184
prevention, 3, 5, 12, 15, 166, 177-179,
183-184; see also Vaccines and
vaccination
program venues for high-risk groups, 13,
176-177
recommendations, 5-6, 12-17, 148-149,
172-173, 179-181, 192
shelter-based programs, 6, 13, 16-17,
149, 189, 191, 192
social support, 3
at STD/HIV clinics, 6, 13, 14, 16-17, 54,
86, 87, 119-120, 124, 125, 129, 134,
149, 151, 170, 171, 176, 189-190,
191, 192
Viral Hepatitis Surveillance Emerging
Infections Program, 58
Vitamin K prophylaxis, 115
W
Washington State Basic Health Insurance
Plan, 171
Whites, 2, 10, 27, 30, 33, 81, 116, 168,
169, 184-185
World Health Organization, 23, 30, 115
Collaborating Center for Reference and
Research on Viral Hepatitis, 151
