Chemistry of Swimming
Swimming, one of the oldest and most universal sports, has been practiced as a sport since
Ancient Greek times. Although not practised at the ancient Olympic Games it was one of only five
sports included in the first modern Olympic Games in 1896. Nowadays a whole range of sports
take place in the swimming pool including swimming, water polo, synchronized swimming, diving
and aqua hockey. Swimming pools have one of the clearest links to chemistry we see in the
sporting environment. Nearly anybody who has ever been to a public swimming pool will say they
have encountered chlorine - although maybe not quite as they thought. Strangely, water sports
taking place in the sea would also encounter chlorine, but in the form of sodium chloride - this is
what makes sea water salty - although there would be many impurities and other salts also
present. The following questions are exploring some of the chemistry surrounding chlorine and the
periodic group it is from.
1. Chlorine, the chemical so readily associated with the swimming pool, can also be found in
common salt. What is the chemical name and chemical formula for salt?
2. Fluorine, chlorine and bromine are all group 7 elements.
What is another name given to this group of elements?
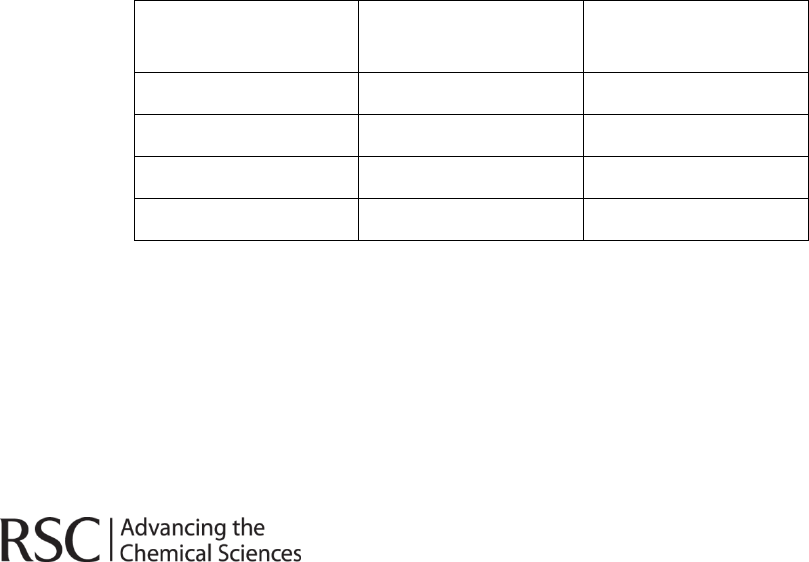
3. In the table below write the chemical symbol corresponding to
the element and also the appearance of that element when in molecular form at room
temperature. Fluorine has been included as the example.
Element
Symbol
Appearance at
room temperature
Fluorine
F
Pale yellow gas
Chlorine
Bromine
Iodine
4. What type of bonding has occurred when two chlorine atoms have
bonded together to form a molecule of chlorine?
Fact
Fluorine and chlorine
are both present in
gases harmful to the
atmosphere known
as CFCs, or
chlorofluorocarbons.
athletes’ wetsuits.
Fact
Tracy Caulkins is
the only swimmer
ever, man or
woman, to own
American records in
every stroke.
Fact
As you descend the halogen group the
elements become less reactive. Fluorine is
the most reactive halogen.

5. In question 1 you wrote down the chemical formula for salt. Identify which atom has lost an
electron and which atom has gained an electron to form the ionic bond in the compound.
6. Chlorine forms salts with other metals as well as those in group 1. A common chloride is
iron(III) chloride. Balance the following equation between iron and chlorine to produce
iron(III) chloride.
__Fe(s) + __Cl
2
(g) 2FeCl
3
(s)
7. At room temperature, only one of the first four halogens can be found in a solid state. This
halogen, when in a solution, can be used to test for starch. What is the halogen? What
colour does the starch go when tested with this halogen?
Fact
When a group 7 element, such as chlorine, reacts with a group 1 metal a salt is formed. Salts are ionic
compounds. When two elements bond to form an ionic compound like this, one element will lose an
electron and one element will gain an electron.
