
Clinical Research
Reference Guide
Boston Medical Center
&
Boston University Medical Campus

Version date: 12/4/2019 2
Table of Contents
How to Use this Research Reference Guide .........................................................................................5
1. The Boston University Medical Campus .......................................................................................... 6
2. Important Places Around BMC/BU Medical Campus ...................................................................... 6
3. Useful BMC/BU Medical Campus Webpages .................................................................................. 7
4. BMC and BU ID Badges and Logins .................................................................................................. 7
A. Badges ........................................................................................................................................... 7
B. Boston University Login Information ............................................................................................ 7
C. BMC Login Information ................................................................................................................. 8
5. Occupational Health Clearance ........................................................................................................ 8
A. BMC Occupational & Environmental Medicine (OEM) ................................................................. 8
B. Research Occupational Health Program (ROHP) .......................................................................... 8
C. Obtaining Access to BMC Clinical Spaces ...................................................................................... 9
6. Facilities (Control Center & Housekeeping) .................................................................................... 9
A. BU Control Center ......................................................................................................................... 9
B. BMC Control Center ...................................................................................................................... 9
C. Environmental Services (Housekeeping) .................................................................................... 10
7. Dress Code ...................................................................................................................................... 10
8. Information Technology (IT) .......................................................................................................... 10
A. Computer Drives ......................................................................................................................... 11
9. Telecommunications (Outlook Email, Phone, Paging, Fax, Scan) ................................................. 12
A. Outlook Email .............................................................................................................................. 12
B. Email etiquette ............................................................................................................................ 13
C. Telephones .................................................................................................................................. 14
10. Office of Human Research Affairs (OHRA) ................................................................................ 14
A. Required Research Trainings ...................................................................................................... 14
B. ClinicalTrials.Gov ......................................................................................................................... 15
C. Clinical Data Warehouse (CDW) ................................................................................................. 15
11. Boston Medical Center/BU Medical Campus Institutional Review Board (IRB) ...................... 16
A. What is the Purpose of the IRB? ................................................................................................. 16
B. INSPIR-II ....................................................................................................................................... 17

Version date: 12/4/2019 3
12. Clinical and Translational Science Institute (CTSI) .................................................................... 17
A. Clinical Research Resource Office (CRRO) .................................................................................. 18
B. Research Professionals Network (RPN) ...................................................................................... 18
C. General Clinical Research Unit (GCRU) ....................................................................................... 18
13. Institutional Biosafety Committee (IBC) .................................................................................... 19
A. Safety Training for an Institutional Biosafety Committee Protocol ............................................ 19
B. Shipping Biologicals (IATA) .......................................................................................................... 19
14. Laboratories ................................................................................................................................ 20
A. BMC Central Laboratory.............................................................................................................. 20
B. GCRU Laboratory......................................................................................................................... 20
15. Investigational Pharmacy Services (IPS) .................................................................................... 21
16. Radiology .................................................................................................................................... 22
17. REDCap ........................................................................................................................................ 22
18. Velos-CT ...................................................................................................................................... 23
19. EPIC/eMerge ............................................................................................................................... 23
20. i2b2 ............................................................................................................................................. 24
21. Financial Disclosure/Conflict of Interest ................................................................................... 24
A. BMC Financial Disclosure process ............................................................................................... 24
B. BU Financial Disclosure process .................................................................................................. 24
22. Research Supplies & Equipment ................................................................................................ 25
A. BMC Process................................................................................................................................ 25
B. BU Process................................................................................................................................... 25
C. Research Equipment Calibration ................................................................................................ 26
D. Freezer and Refrigerator Calibration: ......................................................................................... 26
E. Replacing Sharps Containers ....................................................................................................... 27
23. Reimbursements .......................................................................................................................... 27
A. BMC Check Requisitions .............................................................................................................. 27
B. BU Check Disbursement .............................................................................................................. 27
C. Petty Cash ................................................................................................................................... 28
24. FedEx Shipments ........................................................................................................................ 28
A. How to set up a FedEx shipment for a study .............................................................................. 28
B. How to schedule a FedEx shipment pick up ............................................................................... 28

Version date: 12/4/2019 4
25. Recruitment ................................................................................................................................ 29
A. Recruitment Material Review ..................................................................................................... 29
B. Recruitment Resources ............................................................................................................... 29
C. Publicizing Your Study on Campus .............................................................................................. 30
26. Participant Payment ................................................................................................................... 30
A. BMC Studies ................................................................................................................................ 30
B. BU Studies ................................................................................................................................... 30
C. Parking Passes ............................................................................................................................. 30
27. Posters ........................................................................................................................................ 31
A. Ordering a Poster ........................................................................................................................ 31
B. Poster Template .......................................................................................................................... 31
28. Phlebotomy Training .................................................................................................................. 32
A. GCRU Training ............................................................................................................................. 32
B. BMC Phlebotomy Training .......................................................................................................... 32

Version date: 12/4/2019 5
How to Use this Research Reference Guide
The purpose of the Research Professionals Network (RPN) Clinical Research Reference Guide is to
provide new and existing research team members with key information about the many processes,
policies, portals, systems, technologies, etc. involved in successful conduct of research at our institutions
(Boston University School of Medicine and Boston Medical Center). While this guide is geared towards
Research Coordinators, Research Assistants, Research Project Managers, etc., it is useful for all members
of the research team, from interns to investigators.
The Clinical Research Reference Guide contains detailed information about a diverse range of research-
related processes. Review the table of contents to learn what topics this guide currently contains. This
guide is a living document; it is intended to be continuously updated, expanded, and streamlined.
Looking for something? If you hold CTRL and click the section heading in the Table of Contents, the guide
will take you to that section. Don’t see your topic listed in the table of contents? Try doing CTRL+F (Find)
to search for key words. Still don’t see your topic listed? If you discover a topic not in the guide that you
would like to see included, or the information you see is incorrect or needs updating, please fill out this
Update Request Form and the RPN manager will follow up on the request.
Sincerely,
Research Professionals Network Leadership Committee

Version date: 12/4/2019 6
RESEARCH REFERENCE GUIDE
1. The Boston University Medical Campus
Campus Maps can be found at the following link: http://internal.bmc.org/employee-center/maps
2. Important Places Around BMC/BU Medical Campus
Description
Location
ID Badge Office
710 Albany Street, Room 102
BMC Occupational Health/Working Well Clinic
Doctors Office Building, Floor 7
BMC Human Resources
85 East Concord St, 1
st
Floor
BU Human Resources
801 Massachusetts Ave, 4
th
Floor
General Clinical Research Unit (GCRU)
72 East Concord Street, 8
th
Floor
BU Research Occupational Health Program (ROHP)
72 East Concord Street, Room 825
Investigational Pharmacy Services (IPS)
Menino Basement, ME-B-378
Boston University School of Medicine
72 E Concord St
Boston University School of Public Health
715 Albany St
Institutional Review Board (IRB)
72 East Concord St, Robinson 4
th
Floor
Clinical Trials Office
660 Harrison Avenue, Gambro Building, 2
nd
Floor
Clinical Research Resources Office (CRRO)
72 East Concord St, Evans 7
th
Floor
Yawkey Cafeteria
850 Harrison Avenue, 2
nd
Floor
Chequers Cafeteria
72 East Concord St, Basement
Shapiro Cafeteria
725 Albany St, 2
nd
Floor

Version date: 12/4/2019 7
3. Useful BMC/BU Medical Campus Webpages
It is recommended that you bookmark the pages that are relevant to your position in your web browser.
BMC Internal Webpage: http://internal.bmc.org/
INSPIR II: https://inspir.bu.edu/
VelosCT: https://bmc.veloseresearch.com/velos/jsp/ereslogin.jsp
BMC IT Service Desk: https://bmc.service-now.com/
BUMC IT: http://www.bumc.bu.edu/it/
BU REDCap: https://redcap.bumc.bu.edu/
Request for REDCap Access: https://redcap.bumc.bu.edu/surveys/?s=M4ejnG
BMC/BU Medical Campus IRB Homepage: http://www.bumc.bu.edu/irb/
Clinical Research Resources Office (CRRO): http://www.bumc.bu.edu/crro/
Clinical and Translational Science Institute (CTSI): http://www.bu.edu/ctsi/
BUSM Pivot Funding Database: http://www.bumc.bu.edu/busm/research/find-funding-2/pivot-
funding-database/
BUSM Private Funding Opportunities: http://www.bumc.bu.edu/busm/research/find-funding-
2/funding-bulletin/
BMC Outlook Web Application: https://mail.bmc.org/owa/
BMC Directory: http://directory.bmc.org/WebDirectory/
BU Directory: http://www.bu.edu/directory/
BU Research Occupational Health Program (ROHP): http://www.bu.edu/rohp/
BMC Clinical Data Warehouse: http://www.bumc.bu.edu/ocr/clinical-research-clinical-warehouse-
data-accesspolicy/
4. BMC and BU ID Badges and Logins
A. Badges
You can obtain your BU or BMC ID badge at the ID Badge Office, located at 710 Albany Street, Room
102. It is on the first floor when you walk in with a window to the street. If you lose your ID badge, there
is a $40.00 fee to replace it. However, the ID Badge Office keeps a box of lost and found ID badges, so
check there before purchasing a new one. Your badge needs to be displayed in all BMC/BU Medical
Campus buildings and can be used to get a discount at the BMC cafeterias.
B. Boston University Login Information
If you are a BMC employee, you will need to obtain BU login credentials (username and password) in
order to access a number of the web portals and systems used for clinical research at BMC, namely the
IRB application submission system, INSPIR-II. You can do this two ways:
1. A BU employee can submit a request for a “Sponsored Guest Account” for you:
https://www.bu.edu/tech/support/guest/
2. Submit a Request to Access INSPIR (see INSPIR-II section for more info) to get a BU
username/password to access the INSPIR II portal: http://www.bumc.bu.edu/irb/inspir-
ii/request/
You can also contact the BUMC IT service desk for assistance or more information.
Version date: 12/4/2019 8
C. BMC Login Information
If you are a BU staff and you need a BMC login for access to BMC resources such as EPIC, your manager
should submit a ticket through BMC Service-Now (if your manager has a BMC login), or by calling BMC IT
at 617-414-4500.
5. Occupational Health Clearance
For BMC researchers and BU researchers who conduct research in BMC space there are two
occupational health clearances that you need to obtain prior to actively working on a research protocol:
BMC Occupational & Environmental Medicine (general occupational health for all BMC employees) and
BU’s Research Occupational Health Program (occupational health for all employees who are involved in
handling animals, microbiological agents, or viable samples from animals and humans).
A. BMC Occupational & Environmental Medicine (OEM)
BMC OEM has certain requirements that BMC employees (and BU staff working in BMC space) must
follow, including annual flu shots and TB tests. They also want to keep your vaccination records on file.
If you were hired as a BMC employee, then you would have obtained occupational health clearance
from OEM during your Human Resources on-boarding process. If you were hired as a BU employee, but
will be conducting research in BMC clinical space, then you will also need to be cleared by BMC’s OEM.
Bring documentation that you have been cleared by BU ROHP to BMC OEM so that OEM can clear you as
well.
B. Research Occupational Health Program (ROHP)
ROHP is specifically related to research occupational health and is not connected to the BMC
occupational health office. If you are involved in handling animals, microbiological agents, or viable
samples from animals and humans, you need to consult with ROHP to determine if you need ROHP
clearance. You can call ROHP at 617-414-7647. To obtain clearance, follow these steps:
1. Go to http://www.bu.edu/researchsupport/safety/rohp/ to access the relevant ROHP
onboarding forms.
2. Download and fill out the “Initial Health Questionnaire,” as well as any other forms you need
for your specific position (e.g. “OSHA Respiratory Medical Evaluation”).
3. Scan and email the form(s) with your vaccine records and up to date TB test results to
[email protected]. If you already provided vaccine and TB records to BMC’s Occupational &
Environmental Medicine (OEM) and were cleared by OEM, then you can provide that clearance
documentation to BU ROHP. The ROHP medical staff will review your questionnaire(s) and
contact you if additional information, testing, immunizations, or a clinic visit is required.
4. If you are working in a clinic where you have the potential to encounter a patient with a
communicable disease, you should also be fitted for a N95 respirator. Your supervisor will be
able to tell you if this is applicable to your position. The respirator fitting will be scheduled after
this form is completed and returned to ROHP http://www.bu.edu/researchsupport/forms-
policies/fit-test-sign-up-form/
5. It is recommended to forward a copy of your ROHP clearance to your supervisor to keep on file.
After you receive clearance, ROHP will send you a card with information for health care
professionals should you come down with an unexplained illness and a magnet with helpful
numbers and research safety information.
6. You will need to renew your clearance once per year.

Version date: 12/4/2019 9
C. Obtaining Access to BMC Clinical Spaces
If you need to access to specific clinic spaces, a supervisor will need to contact the ID office to request
access to the clinical space. Access to spaces can be added to BMC and BU badges. First contact the ID
office at dg-idoffice@bmc.org to find out the email address for the person who is in charge of granting
access to the clinical space (Shapiro 9, DOB 10) or wherever your staff will be working. A supervisor
should then send an email to the ID office and to the person in charge of the clinical space providing
information about the employee are requesting access for (the name, ID number, and role on the study)
and providing a brief explanation of why the access is needed (to consent patients, to collect blood
samples).
The ID office will then be able to issue you access to clinical spaces.
6. Facilities (Control Center & Housekeeping)
A. BU Control Center
BU Facilities provides routine maintenance including responding to emergencies, monitoring energy use,
renovations and improvements, cleaning buildings and office space, snow removal, and landscaping
maintenance. They also provide planning, design, and construction services for new buildings and
facilities in need of renovation. Visit the BU Facilities Management & Planning webpage to learn more
about what services they provide, contact information, or to submit a request for services:
https://www.bu.edu/facilities/
B. BMC Control Center
BMC’s Control Center is responsible for monitoring all building automation systems and dispatching staff
to respond to requests for assistance including heating/air conditioning systems, electric, fire alarm, and
other maintenance-related issues in BMC buildings. Call the control center at extension 617-638-4144 to
request a service. You can also submit an online service/maintenance request, described below.
How to submit a BMC service request
1. Go to the Service Request System on your computer desktop and click on the icon with a wrench
and screwdriver that says “Service Request.”
2. Follow the prompts to fill out the service request form.
3. When you submit your request, save your order number.
4. You can check the status of your order by clicking the Service Request icon. Click “Review Orders
History” on the top banner. Manually enter your order number and see the status of your work
order.
**If you need to get access to the system, call the Control Center at 617-638-4144. They will ask for
your Employee ID # and the Department AU#, which you can request from your supervisor or
administrator. Let them know that you are a new employee and want to be granted access to the
service request system.

Version date: 12/4/2019 10
C. Environmental Services (Housekeeping)
BMC has a housekeeping company that is contracted for the hospital. If there are repeated issues with
the cleaning company, you can contact Environmental Services, who supports BMC with managing
complaints and concerns, at: 617-414-5183.
BU Facilities has a Custodial Operations staff who clean and maintain the medical campus buildings.
Custodial requests can be submitted through the BU Facilities request described above.
7. Dress Code
There is no specific BMC or BU dress code policy specific to research staff. However, the BMC employee
dress code policy can be found in the HR Policies and Procedures subpage of the intranet here. The BU
Employee Handbook does not describe any specific dress code policy. Additional dress code guidelines
are at the discretion of each supervisor. Check with your supervisor for information on what is
appropriate to your role and work environment.
8. Information Technology (IT)
Because of the different institutional entities that are part of the BU Medical Campus (BUSM, BUSPH, BU
Dental School, BMC), there are two IT departments that we work with: BMC IT and BU Medical Campus
IT. You will know if your computer is BMC controlled if your login page has the BMC logo. A BMC
computer number is a 7-digit alphanumeric number that can be found on a white sticker on your
computer. There are two numbers on the sticker; use the one with letters and numbers.
You will know if your computer is BU Medical Campus controlled if your login page has the BU terrier
picture in the bottom left corner logo and the domain login is “AD”. A BUMC computer will have a BUMC
IT Asset # (also found on fax machines, copiers, and printers). This 6-digit number that can be found on
sticker on your computer.
If you have a question about a BMC computer, BMC network (H: and G: drives), BMC portals or
programs, you need to contact BMC IT (617-414-4500) or submit a ticket at: https://bmc.service-
now.com/. BMC IT is open 24/7.
If you have a question about BU-related technologies such as BU computers, software offered by BU,
access to any BU-managed portals like REDCap or other BU-related technology issues, call BUMC IT (617-
358-4540), or submit a ticket at: http://www.bumc.bu.edu/it/support/bumc-it/request/. BUMC IT is
open Mon-Fri, 8:30am-5pm.

Version date: 12/4/2019 11
A. Computer Drives
There are three main network drives available which are the H drive, Y drive, and the G drive. Once you
have access to a drive, it will show up under My Computer. You or your manager will need to submit a
request to BMC IT or BUMC IT to get you access to the certain drives.
H: Drive (BMC) and Y: Drive (BU)
The H drive, also known as the Home Drive, is your personal drive where you can store all your
documents and is only available for BMC accounts. It is recommended to use the H drive to store all
your work as it is automatically backed up nightly in two separate, offsite locations. H:\ and G:\ drive
data backups are retained for 6 months. It is not recommended to keep documents on your Desktop or
Documents folder on your computer. If you run out of space on the H:Drive, you can increase the
amount of space by submitting a “increase disk quota” ticket to BMC IT. Note that the H:Drive is linked
to your BMC account, thus you can access your H:Drive at any BMC controlled computer.
The Y drive is similar to the H drive but is only available to BU faculty/staff. The Y Drive provides clients
with a centrally managed, resilient data repository for all data classifications including restricted use. By
default, clients will have access to the following storage:
Personal – Space permissioned solely for each client.
Department – A common space shared by all members of a department. Allows for sharing of
content within the department.
Project – Space for sharing data inter-departmentally based on established collaborative
projects
More information about the Y Drive, including how to connect to the drive, can be found here.
This form should be used when requesting access for a staff member to a Y drive folder(s).
G: Drive (BMC) and Z: Drive (BU)
The G and Z drives are shared department drives which contains various folders accessible to various
groups within the department. These folders typically contains important administrative documents, as
well as a folder for each study. Coordinators can use this shared folder to maintain all study documents
as it is a central location where all the study members can have access to the same information. If you
want to store sensitive information such as protected health information (PHI), BMC staff should consult
BMC IT to set up a restricted folder (or submit an ITS Service Desk request to create/gain access to a
restricted folder), and BU staff should consult BU IT to learn about alternative resources that are
available for you to store PHI.
Encrypting files
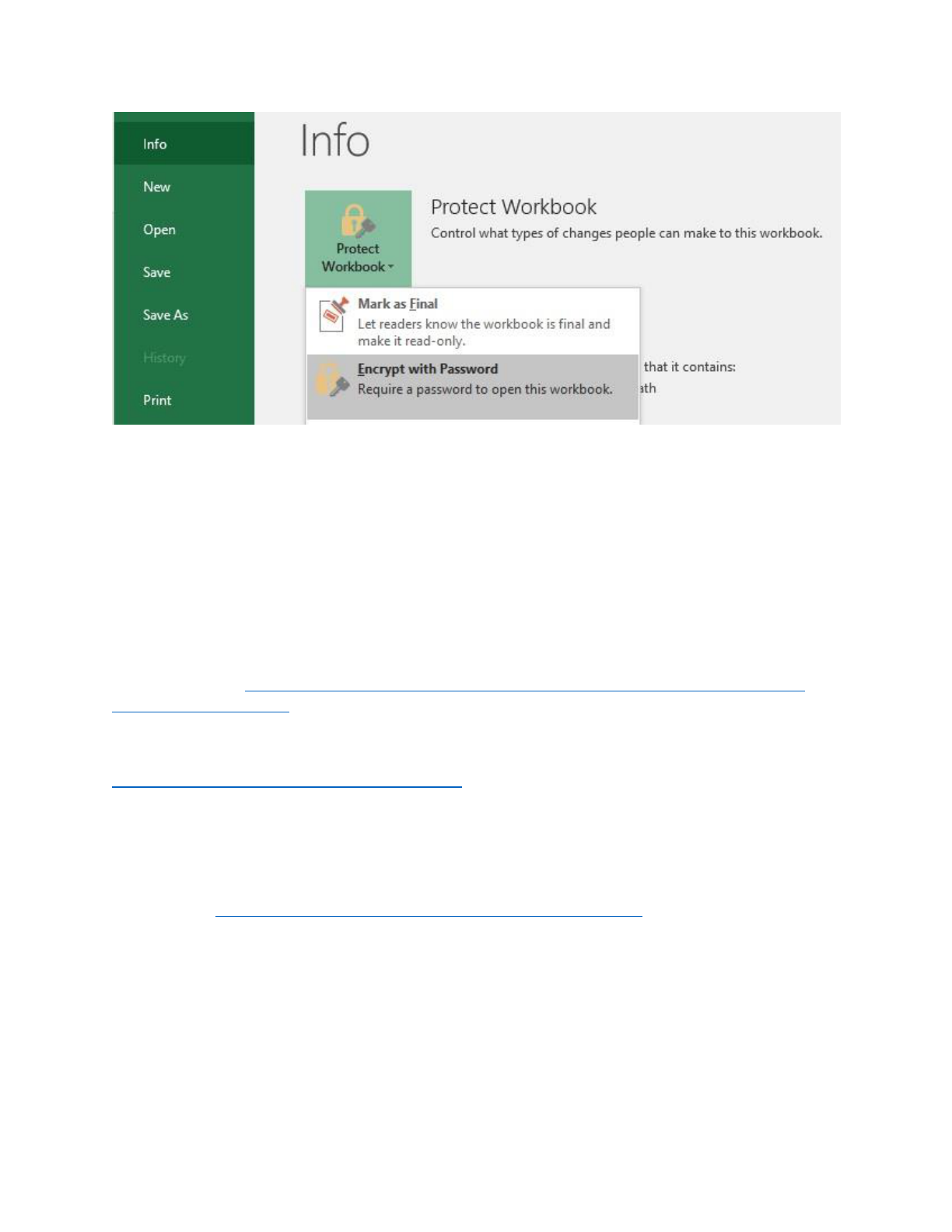
Sometimes you may want to encrypt files, such as an Excel spreadsheet. To encrypt an Excel
spreadsheet, click on “File” then “Protect Workbook” under the Info tab then “Encrypt with Password”
(please see Figure XX below for what it looks like in Excel 2016). This will prompt you to enter a case-
sensitive password twice.
Version date: 12/4/2019 12
Figure 1. Encrypting Excel file in Excel 2016.
Do not encrypt folders by setting a password to the folder, as BMC IT has difficulty accessing the file for
security purposes. If you want to have specific folders where only you and certain co-workers have
access to, please contact or create a ticket with BMC IT to create a restricted folder. Restricted folders
are not password protected, but only certain usernames can access them.
9. Telecommunications (Outlook Email, Phone, Paging, Fax, Scan)
A. Outlook Email
Microsoft Outlook is an email application available to BMC and BU staff. Please see this link for all
Outlook functions: https://support.office.com/en-us/article/basic-tasks-in-outlook-192eb102-2ee2-
4049-b7f5-aa0eb4231fbb. This website is an introduction to Outlook features such as mail, calendar,
signature, and contacts.
Additional BUMC-specific information about accessing email can be found here:
http://www.bumc.bu.edu/it/comm-collab/e-mail/
If you have both a BU and BMC email account, we recommend that you automatically forward your BU
emails to your BMC email address. However, you should not automatically forward your BMC emails to
your BU email address due to HIPAA restrictions.
Forwarding BU Email to BMC Inbox
1. Go to: https://www.bu.edu/tech/services/comm/email/forwarding/
2. Go the second part of the “Getting Started” section where it says “go directly to email
forwarding system” and click on the link.
3. You will need to use your BU username and password to access your mail settings.
4. Choose the option to “Use a Custom Email Address” and it will prove you with a window to type
in the email address you would like to have your BU emails forwarded to. Choose your BMC
email address.
5. You will get a confirmation that your email will now be forwarded to that address

Version date: 12/4/2019 13
You can also set up Outlook to receive both BU and BMC emails display concurrently. This may help as
you can see both your BMC and BU emails on the same platform and it also allows you to archive your
emails easily.
Alternative way for setting up Outlook for both BU and BMC Emails
1. Go to Outlook and click on “File”
2. In the “Info” tab and under “Account Information”, click “Add Account”.
3. Enter your BU or BMC email (order does not matter).
Note that BMC email accounts have a relatively small amount of space available (900MB) and BU email
accounts have a much larger space (50GB). If you forward all your BU emails to your BMC email, it may
fill your BMC email account and you will have to archive more often. To save space in your BMC email
inbox, you should set up Outlook Email Archives so that you can save and archive your emails without
them contributing to your inbox size. Call BMC IT and have them walk you through setting up your BMC
Outlook Email Archives.
Alternative Ways of Accessing BMC Email
In certain situations, you may need to access your email at a computer where your BMC email is not set
up in Outlook such as when you are at home or at a conference. To access your BMC email, go to
www.mybmc.org and click on “BMC email >>”. Type in “BMC\your BMC username” and your BMC
password to login. Once you are done, make sure to log out even though the system will automatically
log you out after a few minutes of inactivity.
B. Email etiquette
Remember that every email you send is a representation of both yourself and your department. Your
manager may provide you with in depth training on email professionalism and etiquette. Understand
that any email you send could be forwarded to anyone in or outside of your department/hospital,
therefore one should think carefully about what is put in email.
Some key professional emailing tips include:
Respond to emails within 24 hours, even if just to acknowledge receipt of the email
Use informative subject lines
Strive for conciseness
Proofread every email
Use email signatures
Check your assumptions, spell out acronyms
Use appropriate honorifics (Dr., Ms., Prof., etc…)
DO NOT write angry emails
Keep your inbox clean; use email folders to maintain email organization
Please make sure to cc/bcc appropriately
Email signature example (you can see how to set this up in the link provided previously):
John Doe
Research Assistant
Department of Wizardry
Boston Medical Center
720 Harrison Avenue

Version date: 12/4/2019 14
Doctors Office Building, Suite XYZ
Boston, MA 02118
T: 617-123-4567| F: 617-765-4321
C. Telephones
BMC phone numbers begin with (617) 414- or (617) 638-. BU phone numbers begin with (617) 352-,
(617) 353-, or (617) 358-. If you are calling a phone number within your institution, you can dial the last
5 digits of the phone number (8-5432).
BU telephones
To call a BU phone from a BU phone, dial the extension (8-####).
To call a BMC phone from a BU phone, dial the 10 digit phone number (617-414-1234).
To place an outside call, dial 1+ the 10 digit phone number.
Instructions for setting up voicemail and using BU telephones can be found on the BUMC IT website.
BMC telephones
To call a BMC phone from a BMC phone, dial the extension (4-#### or 8-####).
To call non-BMC numbers from a BMC phone, you must dial 9-1-(area code)-(phone number).
To transfer someone to another phone: While on phone with a person/subject/patient, hit transfer and
the extension of the staff member you need. Once that staff member picks up, notify them who is
calling. You can now hang up and the phone ID display should say ‘Transfer Complete.’ Make sure the
staff member stays on the line and the caller will be patched through.
Voicemail Set Up: Call BMC IT to reset your voicemail password. Then call the voice mail access number
at 46565 or 86565 to set up your voicemail. The system will ask you to type your temporary password.
This is your 5 digit extension number and add a zero to the end. The system will then walk you through
the steps to set up your voicemail.
10. Office of Human Research Affairs (OHRA)
The Office of Human Research Affairs (OHRA) is an entity that covers both BU Medical Campus and BMC.
Its mission is to support investigators as they design and conduct human research studies. The Director
of OHRA is Dr. John Ennever.
The OHRA includes the Human Research Protection Program (HRPP), the BMC/BU Medical Campus
Institutional Review Board (IRB), the Research Quality Assurance program, the Clinical Data Warehouse
(CDW) the Clinical Research Resources Office (CRRO), and support for Clinicaltrials.gov registration and
results reporting. It facilitates Funding Announcement Opportunities and publishes a monthly
newsletter called the Clinical Research Times. More information about all of these programs is available
on the OHRA website: http://www.bumc.bu.edu/ohra/
A. Required Research Trainings

Version date: 12/4/2019 15
BMC and Boston University Medical Campus require that all researchers involved in human research
must receive formal training. Depending on the type of research you conduct, there are several levels of
required training.
You can find information about what trainings are required here:
http://www.bumc.bu.edu/ohra/required-training/
WARNING: Currently, if you renew your training more than 30 days prior to the expiration date, CITI
will NOT update your expiration date and you will need to re-do the training at a later date.
B. ClinicalTrials.Gov
ClinicalTrials.gov is a U.S. governmental site for registering all clinical trials in the United States. The
purpose of this site is to be a central mechanism to ensure that clinical trials are registered and their
summary results are reported, primarily in order to inform potential subjects of the results, and to
increase the likelihood that negative results of trials will be publicized. Each trial only needs to be listed
once, after which it will receive a clinicaltrials.gov NCT registration number.
Each clinical trial listed in clinicaltrials.gov must have a “Responsible Party” who is responsible for
upkeep of the clinicaltrials.gov record and reporting of summary results. For investigator-initiated
clinical trials, the Principal Investigator is considered the Responsible Party, and is the one who has to
complete the registration. For multi-center clinical trials, someone other than the local PI usually fulfills
this responsibility. The Responsible Party who initially registered the clinical trial is also the one who
must maintain the information about the trial, including updating recruitment information at least every
12 months and reporting final results.
As of November 2016, all investigators with NIH funding whose research meets clinicaltrials.gov
registration requirements must ensure their study is registered on clinicaltrials.gov or may face steep
financial penalties and may not be able to publish on their research.
For access to clinicaltrials.gov or assistance preparing registrations and/or results reporting, contact
Karla Damus (damus[email protected]), Administrator, BMC/BU Medical Campus ClinicalTrials.gov and Clinical
Research Education. More information about clinicaltrials.gov at BMC/BU Medical Campus can be found
here: http://www.bumc.bu.edu/ohra/clinicaltrials-gov/
The public clinicaltrials.gov website can be found here: https://clinicaltrials.gov/
Investigators may sign-in to access their clinicaltrials.gov records here: https://register.clinicaltrials.gov/
C. Clinical Data Warehouse (CDW)
The Clinical Data Warehouse is a project established in 2005 to bring data at the hospital into a single
relational data warehouse. This means that clinical researchers who have IRB/HIPAA approval can, with
the help of a database developer:
get data with a single query (rather than searching manually)
cross-reference data from EPIC, Logician, SDK, SCM, OR and ED
get simple patient counts defined by some user specified criteria
relate local data with BMC data
search text files (physician notes, pathology reports, etc.) for keywords

Version date: 12/4/2019 16
and more, depending on the researcher’s needs
IRB approval must be obtained prior to requesting data from the CDW. The primary contact for the CDW
is Linda Rosen, MSEE, Research Manager, Clinical Data Warehouse.
11. Boston Medical Center/BU Medical Campus Institutional Review Board (IRB)
The BMC/BU Medical Campus Institutional Review Board (IRB) is the IRB for BMC and all institutions on
the BU Medical Campus (BUSM, BUSPH, BU Dental School, and BMC). The BU Charles River Campus has
a separate IRB. The IRB website contains a wealth of guidance documents such as protocol templates,
informed consent form templates, and step-by-step instructions for completing IRB applications through
their application submission portal: INSPIR II (more information below).
A. What is the Purpose of the IRB?
The IRB provides ethical review of human subjects research to protect the rights and welfare of human
subjects of research and to assure that human research is conducted according to applicable federal,
state, and local laws and regulations and the relevant policies of the Human Research Protection
Program (HRPP), Boston Medical Center, and Boston University.
The HRPP Policies and Procedures are available on the IRB website and can be found here:
http://www.bumc.bu.edu/ohra/hrpp-policies/. There is both a PDF version, along with a website
version. New versions are uploaded every time there is a policy change – be sure to update accordingly
if needed!
IRB information that is often requested by industry-sponsors, such as IRB Federalwide Assurance (FWA)
number and the IRB roster are available on the IRB website: http://www.bumc.bu.edu/irb/bumcirb/
The BMC/BU Medical Campus IRB website provides extensive guidance on application submission
procedures and the IRB staff are accessible and happy to answer questions. The IRB wants to hear from

Version date: 12/4/2019 17
you and is happy to provide guidance. We recommend that you familiarize yourself with the IRB website
so that you are aware of all the resources available to you!
Resources for connecting with IRB staff:
IRB office hours (schedule can be found on website)
Setting up appointments with IRB staff outside of office hours
You can call the main number (617-358-5372) and request to speak to an IRB staff member for
any questions about a new or ongoing IRB application.
If you have INSPIR-II questions contact Khaled Khattar at ([email protected] or 617-358-5351).
B. INSPIR-II
INSPIR-II is the portal through which all BMC/BU Medical Campus IRB submissions are done. The
BMC/BU Medical Campus IRB website provides extensive step-by-step instructions for all types of IRB
submissions, including screenshots of each step in the INSPIR II submission portal. These instructions are
available at the following link: http://www.bumc.bu.edu/irb/inspir-ii/inspir-ii-instructions-for-
investigators/
You need a BU username/password to access INSPIR-II. If you are a BMC employee and do not have a BU
username, then you will need to request one in order to access INSPIR-II. To do so, you can submit an
INSPIR II access request form at the following link (from the BMC/BU Medical Campus IRB webpage,
INSPIR II tab): http://www.bumc.bu.edu/irb/inspir-ii/request/
Additional instructions for getting access to INSPIR-II can be found here:
https://www.bumc.bu.edu/irb/files/2016/10/Access-to-INSPIR.pdf
12. Clinical and Translational Science Institute (CTSI)
The BU Clinical and Translational Science Institute (CTSI) is a partnership between Boston University and
Boston Medical Center launched in 2008 with funding from the National Institutes of Health. Its mission
is to facilitate and expedite the complex and lengthy process of translational science: transforming
biomedical discoveries into clinical diagnostics and therapeutics. The CTSI is a center of expertise that
provides tools, services, and resources to clinical and translational investigators in the BMC/BU Medical
Campus community. The Director of the CTSI is Dr. David Center.
The BU CTSI’s training and education programs include:
Mentored Career Development (KL2)
Pre- and Post-doctoral Training Program (TL1)
Clinical Research Training Program (CREST)
Seminars and Workshops
Research consultation sessions (IRB, DSMPs, ICFs, HIPAA requirements, etc..) via the CRRO
Good Clinical Practice (GCP) training sessions (via the CRRO)
RPN Peer-led competency based interactive workshops
The BU CTSI’s research support programs include:
The Clinical Research Resources Office (CRRO)

Version date: 12/4/2019 18
CTSI’s General Clinical Research Unit (GCRU)
Consultation services (study design, biostatistical analysis, data management...)
Funding support, pilot grants
Recruitment and retention resources
Software tools
Collaborative research and networking (ARC Mechanism)
Biostatistics, Epidemiology & Research Design (BERD) program, free biostatistics support
Core Resources Program – guidance for researchers on BUMC Cores
Grant Writing & Editing services in collaboration with BMC Office of Development
A. Clinical Research Resource Office (CRRO)
The Clinical Research Resource Office is a BMC/BU Medical Campus entity that is part of the HRPP under
OHRA, with funding from BU CTSI that supports all types of clinical research taking place both on the at
BMC and BU Medical Campus. The first thing you should do regarding the CRRO is to get onto the
mailing list to receive information about trainings and events. Fill out the CRRO mailing list form here:
http://www.bumc.bu.edu/crro/home/email-signup/. Here are some of the services the CRRO provides
clinical research teams at BMC/BU Medical Campus:
Frequent professional development trainings and seminars
Fundamentals in the Conduct of Clinical Research and PI Training courses
Overviews of changes to IRB policies, documents, and guidelines
Audit preparations and internal audits (QA reviews)
Filmed and recorded courses and seminars from past
One on one meetings to discuss research conduct, design, working with the IRB
Written and online materials about BMC/BU Medical Campus clinical research practices
Study documentation templates
B. Research Professionals Network (RPN)
The Research Professionals Network (RPN) is an initiative on the BMC/BU Medical Campus to enhance
the quality of human/clinical research on campus through an effective network for research
professionals. The RPN is open to any research staff member involved in coordinating human subjects
research. The RPN offers workshop trainings, study management tools, a networking platform, and
career development opportunities.
You can find out more information about the RPN and join the network on the CRRO website:
http://www.bumc.bu.edu/crro/research-professional-network/. Contact Gina Daniels
([email protected]u) if you want more information or have questions about the RPN.
C. General Clinical Research Unit (GCRU)
The General Clinical Research Unit (GCRU) is a center within the BU CTSI that offers physical space and
support to conduct research. Services include performing clinical procedures and assessments, CPR and

Version date: 12/4/2019 19
phlebotomy training, provision of dry ice, and administration of investigational drugs, devices, or
interventions. See the website for a full list of services, the fee schedule, and contact information.
13. Institutional Biosafety Committee (IBC)
The Institutional Biosafety Committee (IBC) is an institutional committee created under the NIH
Guidelines to review research involving recombinant DNA and synthetic nucleic acid research. The IBC
also reviews all research involving biohazardous materials (such as blood samples) and has overall
oversight and responsibility for the Biosafety program at BU and BMC. The IBC carries out these
functions pursuant to requirements set forth by federal, state, and local agencies, as well as BU. More
information about IBC can be found on their website:
http://www.bu.edu/researchsupport/compliance/ibc/
All studies that involve biospecimens (blood, urine, etc.) must have an IBC profile and must get annual
approval from the IBC. Information about this application process can be found here:
http://www.bu.edu/researchsupport/compliance/ibc/applications/
A. Safety Training for an Institutional Biosafety Committee Protocol
If you will be working in a laboratory space, you will need to complete training to work safely. Trainings
include: Laboratory Safety training, Bloodborne Pathogens Safety training, and Shipping Biologicals
(IATA) training. Additional trainings may be required based on your IBC study profile (see above). Once
your IBC profile is created and you have been added as lab personnel to that profile, the IBC system will
generate the list of trainings you will be required to complete. Most trainings are completed via the
BioRAFT portal, except for the IATA training which is completed in person.
Training can be found at http://www.bu.edu/rims/
Click on BioRaft training (note: Not accessible via Internet Explorer)
Log in with your BU username and password.
If your supervisor has added you to an Institutional Biosafety Committee (IBC) protocol already,
you will see a list of required training courses. If you have not already been added to an IBC
protocol, you will need to ask your supervisor which courses you should complete.
If you will be conducting a clinical trial that involves the collection and/or processing of biological
samples, you need IBC to sign off on the IRB application in INSPIR. The application will automatically be
routed to IBC for signature after you submit it. If you have questions or concerns about working with
biological materials collected for clinical studies, contact any of the IBC staff at
http://www.bu.edu/researchsupport/compliance/ibc/
B. Shipping Biologicals (IATA)
If you will be shipping biological specimens (e.g. blood, urine, cells, or animal tissues) off campus, you
need to be trained in shipping biologics. After completion of training you will receive an International Air
Transport Association (IATA) certificate valid for two years from the date of issue. A list of training
sessions can be found at http://www.bu.edu/rims/
Click on BioRaft training (note: Not accessible via Internet Explorer)

Version date: 12/4/2019 20
Log in with your BU username and password.
o Click on Course Directory.
o Under Research Safety, select Shipping Biologicals Sign Up.
14. Laboratories
Many research studies, and most clinical trials, include a laboratory component (collection of
blood/serum/plasma samples and/or urine samples). Multi-center, industry-sponsored research studies
often utilize one “central” laboratory for all sites; therefore, each site ships lab samples to the central
lab which processes the samples and sends lab results back to the sites. Single-site, investigator-initiated
research studies often utilize their “local” laboratory, such as BMC’s central lab. If your study uses a
central lab, the study sponsor should provide you will all necessary central lab information and logistics,
including a Laboratory Reference Manual. If your study uses a local lab, please see the relevant section
below.
A. BMC Central Laboratory
BMC has one central laboratory that provides all hospital clinical laboratory services as well as supports
labs drawn for research studies at BMC and BU Medical Campus. It is part of the BMC Department of
Pathology and Laboratory Medicine. The Office Manager for Laboratory Medicine is Susan Mallard-Shea
(Susan.Mallard-Sh[email protected]; 617-638-7800). You can reach out to Susan Mallard-Shea for assistance
setting up a new study. The Operations Manager for Laboratory Medicine is Neil O’Neill
(Neil.O’Neill@bmc.org; 617-414-4737) and is available as back-up.
Lab Reports and Invoices
BMC Central Lab reports get delivered via inter-office mail. Lab reports should be reviewed and signed
by an MD or qualified staff before being recorded on the CRF and/or entered into a database. Be aware
that it may take a few days to receive lab reports via interoffice mail.
If you see the lab did not run a lab test, contact Susan immediately and they may be able to add/run the
test (specimens are held for only a couples of days after initial blood draw/urinalysis).
Laboratory invoices are sent via interoffice mail to the designated recipient on a monthly basis. When
you receive an invoice, cross-check it with your research records to ensure that you are being billed for
the correct tests. Once you have verified that all charges listed are correct and complete, give a copy of
this verified list to your study team member who handles research invoicing for your study, so they can
email the verified invoice to their contact in the Clinical Trials Office.
**If there are any billing discrepancies** contact Susan Mallard-Shea (Susan.Mall[email protected];
617-638-7800). If Susan is on vacation or doesn’t return your calls after a couple days, you can try Neil
O’Neill. Susan (or Neil) will send out a new invoice if necessary.
B. GCRU Laboratory
The GCRU laboratory is staffed with an experienced Laboratory Manager and Laboratory Assistant with
years of experience in laboratory services and phlebotomy experience combined in pediatric and adult
participants. The GCRU laboratory provides the following services:
Processing of blood, urine, CSF, sputum.
Packaging and shipping (dry ice for shipping and transport)

Version date: 12/4/2019 21
Storage of frozen samples with matching log
Informational resource before and during the implementation process
Bio-repository services (offers term storage of research samples by BUMC investigators)
To learn more about the GCRU lab services and fees, visit the GCRU section of this page:
https://www.bu.edu/ctsi/support-for-research/protocol-implementation/
15. Investigational Pharmacy Services (IPS)
All clinical trials at BMC/BU Medical Campus that involve an investigational drug or protocol-required
ancillary medications are required to utilize the Investigational Pharmacy Services (IPS) for drug storage,
handling and dispensation. The IPS is staffed by a team of pharmacists and technicians who are all
extremely well-versed in medication management for clinical research. The IPS Manager is Stephen
Zalewski, PharmD, CCRC.
Contact information and Shipping Address:
Boston Medical Center
Investigational Pharmacy Service (ME-B378)
840 Harrison Ave
Boston, MA 02118
Phone: (617) 638-6774
Fax: (617) 638-6748
Email: IPS@BMC.org
Available services include:
Protocol Review and Editing
Study Start-Up/Close-Out
Institutional Logistics and Operations
Inventory Control
Storage – temperature monitoring
Drug Information
Assist with database maintenance (Vestigo,
EPIC)
Pharmacy Binders
Dispensing and Accountability
Randomization
Blinding
Compounding
Repackaging
Collaborating with monitors and auditors for
site and remote visits.
Providing tours for site qualification visits
IRB membership
IPS Involvement with a New Project may include:
Review of protocol/draft to determine feasibility
Determine the scope of the project
Inpatient vs Outpatient vs Ambulatory Infusion Center
Clinic setting vs GCRC

Version date: 12/4/2019 22
24h/7d enrollment
Nursing/Pharmacy training needs
IPS cost analysis
Project timelines
Steps to follow for using IPS for your study
1. As you plan for a new protocol, please contact IPS as early as possible with a draft of the protocol
and the IPS Protocol Planning Worksheet.
2. IPS will review the protocol for feasibility, level of required service and provide you with an IPS
Budget Agreement. No IPS resources will be assigned until the PI returns a signed IPS Budget
Agreement.
3. IPS will then work with the PI and the research team to get the protocol “IPS Ready.” IPS Readiness
involves setting up protocol binders with all the necessary documents to operationalize the study
(i.e. protocols, informed consents, investigator’s brochures, IRB approval letters, operations
manuals, etc.).
4. IPS and the Research Team will work together to obtain protocol specific training and delegation of
responsibilities, obtain IXRS access, receive study drugs and supplies as we prepare to initiate the
protocol.
5. IPS may meet to discuss Site Logistics if the research plan is complicated or the PI/Research Team
has not worked with IPS on prior studies.
6. Once all of the necessary new protocol pre-requisites have been met, IPS will notify the PI/Research
Team that they are “IPS Ready” and screening and enrollment may commence.
16. Radiology
If your study requires a radiological procedure (X-ray, MRI, ultrasound, etc.), you will need to contact the
Department of Radiology. You can get in touch with Margaret Lavoye (mlavoye@bu.edu) and/or Dr.
Stephan Anderson (san[email protected]u, Stephan.anderson@bmc.org) to discuss your study requirements.
17. REDCap
REDCap is an online database system used by many academic centers and research programs. The BU
Medical Campus REDCap portal can be found here: https://redcap.bumc.bu.edu/
To request access to REDCap, complete and submit the online Request for REDCap Access form, which
can be found here: https://redcap.bumc.bu.edu/surveys/?s=M4ejnG. Note that you will need to know
your BU username to complete this form. Follow-up with your supervisor if you have further questions
about completing this request form.
Once you have access to REDCap, you can log in to the BU Medical Campus REDCap portal and review
training videos in the Training Resources tab. Recommended videos include: Brief Overview, Detailed
Overview, Data Entry Overview as well as any of the short videos under the ‘Types of REDCap Projects’
section.

Version date: 12/4/2019 23
Tasha Coughlin is the BU Medical Campus REDCap Administrator. She can be reached at
REDCap Drop-in sessions occur twice per month. You can find more information about the Drop-in
session schedule here: http://www.bu.edu/ctsi/tools/
18. Velos-CT
Velos-CT is the web portal through which clinical protocols, draft clinical trials agreements/ contracts
and budgets are submitted to the BMC Clinical Trials Office (CTO). It is also the portal through which we
attest to every procedure done at every study visit for every participant so that the hospital can
correctly allocate charges to either research funds or a patient’s insurance. The Velos-CT portal can be
found here: https://bmc.veloseresearch.com/velos/jsp/ereslogin.jsp
To get access to Velos-CT, you first need to complete the in-person Velos-CT training. To sign up for a
training, email BMC Research Business Intelligence (RBI) at RBI@bmc.org to ask when the next
training(s) are scheduled for and to request to be enrolled in one of these trainings.
After you attend the training, you will receive an email from BMC RBI. This email will contain further
instructions to get access to Velos-CT.
Additional Resources for Velos-CT, including training videos, can be found at
https://www.bmc.org/research-operations/systems.
19. EPIC/eMerge
In order to gain access to EPIC, the electronic medical record system utilized at Boston Medical Center,
all users must first have an account created with BMC IT. Users must complete eMerge training in order
to have their EPIC account activated. For view-only access, users can complete an online training. For
edit-level access, users must attend an in person training. Visit: http://internal.bmc.org/emerge/, click
on “Looking for training registration? Click here!”, and follow the instructions.
Once training has been completed, proceed to requesting account access. Please note, requests for
access must be submitted by a manager or supervisor.
1. Log in to the BMC ITS Service Now website at https://bmc.service-now.com
2. Under the “Self Service” Tab, select “Service Catalog”
3. If requesting as part of getting a new hire a BMC IT account: Under “BMC IT On-Boarding/Off-
Boarding Access” select “New BMC Customer IT Access”. After filling in the contact information
for the supervisor and the new user, select “Other” for “Account Access Type”.
If staff already has BMC IT account: Submit an IT request through the BMC ITS Service Desk for
“eMerge/Epic Access” (located under the eMerge section)
4. For “Application Access”, select “eMerge/Epic Account”. This is the platform in which EPIC is
housed.
Contact the BMC service desk at 617-414-4500 with account access or creation questions.

Version date: 12/4/2019 24
20. i2b2
i2b2 is an NIH funded effort to provide a standardized data architecture and informatics capabilities to
combine clinical patient data with demographic, biologic, and genomic data for use in clinical research
projects. Their primary focus is on the use of de-identified electronic health data to better understand
and improve the health of the residents of Boston and to develop new tools and approaches to support
the effective use of i2b2 with its broader community.
The request for access form can be filled out here.
Instructions on how to navigate the i2b2 Query Tool can be found here.
21. Financial Disclosure/Conflict of Interest
Financial Disclosure/Conflict of Interest forms allow institutional compliance staff, the IRB, and study
sponsors to know if you or your immediate family members (spouse, dependent children, etc.) have
financial dealings that might cause you to be impacted by the outcome of a study, working with a
certain sponsor, or institution.
A. BMC Financial Disclosure process
If the grants you work on are awarded to BMC PI(s), you must disclose through the BMC Compliance
Department’s web-based system, COI-SMART. This can be accessed through mybmc.org using your BMC
login credentials. You must disclose financial interests once per year.
To get access to COI-SMART, contact BMC’s Compliance Department at COI-Complian[email protected] and
provide your name, email address, department and section (e.g., Pediatrics/Pediatric Infectious
Diseases; Medicine/General Internal Medicine; Medicine/Cardiology).
B. BU Financial Disclosure process
If the grants you work on are awarded to BU PI(s), you must disclose through the BU Online Financial
Interest Disclosure Module. You must disclose financial interests each time a new project is started, and
then annually for each project, as well as any time a new significant financial interest is discovered or
acquired.
Submit your financial interest disclosure for this project as follows:
1) Go to https://share.bu.edu/sites/fcoi/
2) Sign in with your BU email address as ID and Kerberos password
3) Click on "My Financial Disclosure Forms"
4) Click on “[+NewDisclosure]”
5) Go through the form and then click "Submit"
Optional: a delegate (e.g., department admin) can pre-fill grant information in disclosure forms for
PI's this way:
1) Go to https://share.bu.edu/sites/fcoi/

Version date: 12/4/2019 25
2) Click on “Manage Projects”
3) Click on “+Add Project”, in the top left corner
4) After saving, the delegate clicks on “Send Invitation to PI”, in the right-most column of the
“Manage Projects” page
5) Then when the PI signs in, they see the project with an INVITED to disclose status
You can find more information about the BU Conflict of Interest/Financial Disclosure process on the BU
Research Compliance website.
22. Research Supplies & Equipment
Most studies will require purchase of some type of research supplies, such as phlebotomy supplies, ECG
electrodes, CRF binders, phlebotomy tubes, etc. Some study sponsors may provide certain research
supplies, while other sponsors ask sites to procure these supplies individually. Be sure that you have a
clear understanding of what research supplies will be provided by the sponsor and which you will be
responsible for procuring.
A. BMC Process
Lawson Ordering System
To place an order to purchase basic laboratory supplies such as gloves, gauze, bandaids or alcohol
swabs, the Lawson Ordering System must be used. To get access to the system, go to this link (http://fis-
web.bmc.org/intranet/userForms.php) and fill out the Lawson User Request form.
For more information on how to submit an order, please visit this link
(http://internal.bmc.org/departments/supply-chain-operations/procurement), where you can review
procurement policies, Lawson tutorials, and register for an upcoming training.
Purchase Order
Purchase orders (POs) can be used to purchase new study equipment or supplies that are NOT available
through the Dept. of Materials Distribution. Your administrator will likely have physical copies of POs.
B. BU Process
There are several ordering methods available to purchase products and services. The primary ordering
method BU Sourcing & Procurement manages and administers is the shopping cart method. Alternate
ordering methods are available for specific types of purchases to help expedite the purchasing process.
Terrier Marketplace
The Terrier Marketplace integrates with SAP and allows BU rapid enablement of supplier electronic
catalogs over a supplier network. It has a large selection of contracted supplier products including lab,
office, and facility supplies.

Version date: 12/4/2019 26
C. Research Equipment Calibration
Once a year your lab and medical equipment should be calibrated. Calibration determines that the
numbers you are reading on your equipment are accurate.
BMC process:
BMC’s Department of Clinical Engineering does most calibrations but there are some items that
are not covered by this department.
Steps for Calibrating or Servicing Lab and Medical Equipment (NOT freezers and blood
pressure cuffs):
1. Contact Clinical Engineering at [email protected] and provide them with the location of the
lab and the biosafety level or the location of the clinical space. Check with your
manager about who in the Department is responsible for maintaining the logs of
equipment calibration and maintenance.
2. A member of the Clinical Engineering Department will place a sticker on equipment that
is serviced.
3. Calibrations and maintenance for most equipment must be done once a year.
BU process:
If you have information about this topic, please share!
D. Freezer and Refrigerator Calibration:
If you want a basic confirmation that the temperatures shown on the freezer and refrigerator are
accurate, follow the steps below. If you want a formal reading from a trained professional, you will need
to look into an outside source to calibrate the freezer or refrigerator.
BMC Process
1. Fill out a service request with facilities. In the “Work Requested Of” section, select “Heating and
Ventilation.” Make sure to describe in detail what calibration is requested.
2. Facilities will send someone to look at the freezer. Make sure to describe to this person what
you need them to do.
3. Once the facilities person has verified that the temperature reading in the refrigerator and
freezer are accurate, ask them to either place a sticker with the date or send you a dated
written document stating that the freezer and refrigerator are calibrated.
BU Process
1. Contact Facilities at 617-638-4211 to have them set up an alarm should the refrigerator or
freezer temperature be outside a certain range of temperatures.
2. The Control center will be notified in the case of an alarm and they will contact the people listed
on the front of the refrigerator or freezer so that samples can be moved before they are spoiled.
Refrigerators and freezers that contain important samples should be plugged in to Red outlets,
which maintain power in the event that the electricity supply is interrupted.

Version date: 12/4/2019 27
E. Replacing Sharps Containers
Sharps waste are defined as needles, syringes, Pasteur pipettes, pipette tips, razor blades and other
metal sharps, regardless of whether they are contaminated with biohazardous materials. Sharps waste
must be placed in approved sharps containers.
BMC Process
Page the Sharps Removal Program at BMC at 5038 to request the removal of any of the filled sharps
containers.
To page for removal of the sharps container from your computer, go to
https://directory.bmc.org/webxchange/ and type in “Sharp Box” in the field titled Last Name and
“Transporter” in the field titled First Name. Type in your office phone number in the field titled “Enter 7
or 10 digit callback number”. Write the appropriate message (including the location) requesting for the
removal of the sharps containers in the field titled Text Message and send your request.
BU Process
Sharps containers for the laboratory are available from Facilities Management & Planning (FMP)
custodial staff. FMP staff removes full sharps containers from laboratories, brings them to the building’s
designated biological waste storage room, and replaces the container with an empty one.
To request a pickup call the Medical Campus Control Center at 617-638-4144
For additional guidance see also:
Biological Waste Management Guidelines (https://www.bu.edu/researchsupport/forms-
policies/biological-waste-management-guidelines/)
BU Facilities Management and Planning Request Services page (https://www.bu.edu/facilities/request-
services/)
23. Reimbursements
A. BMC Check Requisitions
Check requisitions can be used to pay invoices or to reimburse for a receipt that is over $35. Check
requisitions can be downloaded on the BMC Financial Forms page (http://fis-
web.bmc.org/intranet/forms.php).
B. BU Check Disbursement
A BU check disbursement is a formal request to pay vendors and individuals for goods and services that
do not require a Boston University purchase order. This online form can be found on the BU Accounts
Payable Resources page (https://www.bu.edu/ap/resources/forms/disbursement-form/). Login is
Version date: 12/4/2019 28
required.
For information regarding BU purchase orders, including which services and goods do or do not require
a purchase order, see your department financial administrator and/or BU Sourcing & Procurement
(https://www.bu.edu/sourcing/)
C. Petty Cash
BMC Process
Petty cash is generally used when a research team member has purchased something study related for
under $35 and needs to be reimbursed. The petty cash form can be downloaded from the BMC Financial
Forms page (http://fis-web.bmc.org/intranet/forms.php).
1. Complete form using your accounting unit and activity number.
2. Refer to detail accounts for codes
3. Redeem at cashiers office
BU Process
Petty Cash funds are relatively small amounts of cash entrusted to the Petty Cash Custodian within a
department for reimbursing individuals for small, miscellaneous business expenses. You must complete
two forms to establish a Petty Cash fund. Information about this process can be found on the
Comptroller Office website
(https://www.bu.edu/cfo/comptroller/departments/cashier/resources/petty-cash-medical-campus/)
24. FedEx Shipments
Some study and clinical trial protocols will require using FedEx shipments for laboratory samples,
research and regulatory documentation, study medication shipments and other study-related tasks. You
should work with your administrator to set up a new account or gain access to a departmental FedEx
account.
A. How to set up a FedEx shipment for a study
Order shipping materials (boxes, labels, etc.) from FedEx.com using your study login
Create/print shipping labels from FedEx.com using your study login
There is a mailroom in the basement of the medical school building that often has large boxes if
they are needed.
B. How to schedule a FedEx shipment pick up
When sending out a FedEx shipment, be sure that you consider how much advance notice FedEx needs
in order to pick up the package before your office closes, or whether the pick-up location is a restricted
access area. Pick-ups may require up to 3 hours advance notice so the pick-up should be scheduled by
2pm.
1. Call 1-800-GOFEDEX.

Version date: 12/4/2019 29
2. Request to schedule a pickup for express (Research lab samples are typically sent overnight to
the study central laboratory. Confirm with your study team if you are unsure).
3. The FedEx rep will ask for your name, address, phone number, latest time they can pick up, etc.
(Tip: Phone number is usually most efficient way for them to identify you due to the large
volume of clinics in area).
4. They’ll need to know how many packages, the weight of each package, and the airway bill
account number (source of payment). The account number is usually preprinted on the Fedex
label, or handwritten in the account number (confirm account number with study team if
unsure).
5. The representative will confirm that the pick-up has been scheduled and will give you a pick-up
confirmation number. If there are any issues with the pick-up (late, shipment error or delay,
etc.) you can call FedEx for details on the issue using confirmation number.
In some studies, there is a need to ship study medication to a participant. Check with the study team,
IPS, and/or sponsor contact to see if this is an option and if so, what the correct procedure would be. In
many cases, the procedure to ship study medication is:
1. Find an empty box or envelope for medication shipment.
2. Create a shipping label to attach to the box or envelope. To do this, go to fedex.com and log in.
Enter the login information created for this study.
3. Print label and any additional copies needed (participant binder, administrator files, etc.).
25. Recruitment
A. Recruitment Material Review
If you are interested in promoting a study to recruit participants at BMC or BUSM, you must work with
the BMC Communication and Marketing Department or the BUSM Communications Office respectively
on the development of promotional materials. You can find more information on this requirement here:
http://www.bumc.bu.edu/irb/submission-requirements/general-submission-requirements/guidance-
for-recruitment-and-screening/research-study-recruitment/
B. Recruitment Resources
Study Finder
Study Finder is a recruitment tool that has listings for studies taking place at BMC that potential
participants can search for. You must make sure that your IRB application specified that you would be
using Study Finder as a recruitment resource.
1. Log into INSPIR-II and go to “My Studies.”
2. Open the study you wish to create a Study Finder listing for.
3. Along the left hand side of the page it will list different documents associated with your study.
Go to the link that says “Study Finder.”
4. On that page, click the icon that says “Add a New Document.”
5. The form will provide you with instructions of what information to include about the study.
6. The form needs to be approved by the IRB. Notify the PI for sign off.
Version date: 12/4/2019 30
Brochures
The BMC/BU Medical Campus IRB has approved several brochures for you to provide to participants in
any approved language without being separately submitted to the IRB. You can find the full list of
approved brochures here: http://www.bumc.bu.edu/irb/submission-requirements/general-submission-
requirements/guidance-for-recruitment-and-screening/
C. Publicizing Your Study on Campus
There are a variety of communication resources available for you to publicize your studies and/or
events. You can advertise your study in BMC and BU Medical Campus weekly all staff newsletters and
flat panel screens around campus. You can find more information about e-communications and other
avenues for advertising your study and/or events around campus at this link:
https://www.bumc.bu.edu/comm/publicizing-events-at-boston-university-medical-campus/
26. Participant Payment
A. BMC Studies
ClinCard
ClinCard is the BMC method for paying participants for their time in studies. At this time, only BMC
studies are supported by the ClinCard system. ClinCards act as a reloadable debit/credit card that can be
spent in most locations. Once a research participant receives a ClinCard, they use the same ClinCard
throughout the entire study and may continue to use the same card in future studies. To use ClinCard
like a credit card, the participant simply needs to select ‘credit’ when using it, as one would do with a
regular credit card. A pin number may be set up (see ClinCard instructions) if the participant would like
to use the ClinCard as a debit card requiring a pin. More information about ClinCard, including User
Manuals and training videos can be found at the following link: https://www.bmc.org/research-
operations/systems
More detailed information about using ClinCard, including FAQs and the User Manual, can be found in
the ClinCard portal in the Support tab (must have ClinCard username/password to access the portal).
B. BU Studies
BU studies can pay participants using check, petty cash, or gift cards. You can find information about all
of these methods on the BU Research Support website: https://www.bu.edu/researchsupport/forms-
policies/human-subject-payments/.
C. Parking Passes
To reimburse or cover parking costs for participants, departments or studies can get parking validation

Version date: 12/4/2019 31
stickers from the Parking and Transportation Services office (PTS) at 710 Albany St. Office hours are 7
a.m-5 p.m., Monday thru Friday. PTS may be contacted at (617) 638-4915.
Research participants and visitors may use the parking garage at 710 Albany Street or the Doctor’s Office
Building (DOB) garage connected to the DOB. At each garage visitors will need to pay for parking (or
process their parking validation) on the 1st floor, prior to returning to their car. If they do not go to 1st
floor and instead have their parking ticket processed at the parking window, they will not be able to exit
the garage.
Parking can be validated by placing a green, red or brown sticker on the visitor’s ticket. This validation
will deduct a certain amount of money from the parking price. Updated rates can be found here:
https://www.bumc.bu.edu/parking/rate-structure/
BMC Requests for Parking Stickers
Forms to purchase parking validation stickers may be downloaded from the BUMC Parking &
Transportation Services website: http://www.bumc.bu.edu/parking/forms/. To pick up the validation
stickers, go to the PTS office at 710 Albany St (aka Parking/IDs Office). BE SURE TO WEAR YOUR BMC/BU
ID BADGE. Hand in the validation purchase form and they will give you the validation sticker booklets.
Make sure to ask for a copy of the form after they have signed to keep for your records.
BU Requests for Parking Stickers
BU departments or studies may use an Internal Service Request (ISR) for BUMC Parking Courtesy
coupons. Once an ISR is complete, print it and bring it to the PTS office to receive the validation stickers.
BE SURE TO WEAR YOUR BMC/BU ID BADGE. Hand in a copy of the approved ISR and they will give you
the validation sticker booklets. Make sure to ask for a copy of the form after they have signed to keep
for your records.
27. Posters
A. Ordering a Poster
You can order posters to be printed at the FedEx on Albany Street. The usual turnaround time is about 3
business days. They will also provide you with a sample of your printed poster to ensure that the
resolution is high enough before they print the entire thing. That FedEx is able to charge printing directly
to BMC/BU grants, but you can also pay upfront and get reimbursed (if possible) later on. You can reach
this FedEx by emailing usa5013@fedex.com, or calling 617-603-6006.
B. Poster Template
Posters can be created using PowerPoint.
For guidance about writing abstracts, creating academic posters and tips for presenting posters at
conferences, see the following links for resources:
http://www.bumc.bu.edu/jmedday/files/2011/05/Poster-Toolbox2011.pdf
https://www.bu.edu/brand/downloads-faqs/downloads/templates/

Version date: 12/4/2019 32
28. Phlebotomy Training
A. GCRU Training
The GCRU offers phlebotomy training, among a number of other resources (see GCRU section of this
guide). The GCRU phlebotomy training is a 12-week certification course that includes national
certification. You can find more information on the CTSI website: https://www.bu.edu/ctsi/support-for-
research/protocol-implementation/
B. BMC Phlebotomy Training
The BMC Phlebotomy training is offered to clinical staff and research staff performing phlebotomy on
BMC campus. You can find more information and instructions for how to sign up on the BMC internal
website: https://internal.bmc.org/departments/pathology-and-laboratory-medicine/laboratory-
medicine/research
