Madison Public Schools
Science Program
A Framework for Integrated Teaching and Learning
Science Program
Madison Public Schools
10 Campus Drive
Madison, CT 06443
www.madison.k12.ct.us
SCIENCE CURRICULUM MADISON PUBLIC SCHOOLS
Table of Contents
Program Overview
Introduction.............................................................................................................................. i
Structure of Guide ..................................................................................................................... ii
Intent and Acknowledgements ................................................................................................... iii
Program Guide
Program Components
Philosophy ................................................................................................................................ 1
Goals ....................................................................................................................................... 1
Design ...................................................................................................................................... 1
Enduring Understandings........................................................................................................... 1
Conceptual Structure ................................................................................................................. 1
Benchmarks of Student Progress ................................................................................................ 2
Learning Environment ............................................................................................................... 2
Program Implementation ........................................................................................................... 2
Program Monitoring and Evaluation ............................................................................................ 2
Resources ................................................................................................................................. 2
Appendices ............................................................................................................................... 2
Program Framework
Vision of Science Education ....................................................................................................... 5
Vision for Scientific Literacy ....................................................................................................... 5
Mission of Science Education ..................................................................................................... 5
Goals for Science Education ....................................................................................................... 5
The Need for Science Education ................................................................................................. 5
Philosophy ................................................................................................................................ 6
Guiding Principles...................................................................................................................... 7
K – 12 Conceptual Themes and Guiding Questions ...................................................................... 7
Content Standards and Indicators for Scope and Sequence of Instruction
Elementary School
Kindergarten ....................................................................................................................... 15
Grade 1 .............................................................................................................................. 27
Grade 2 .............................................................................................................................. 47
Grade 3 .............................................................................................................................. 63
Grade 4 .............................................................................................................................. 81
Middle School
Grade 5 .............................................................................................................................. 105
Grade 6 .............................................................................................................................. 123
Grade 7 .............................................................................................................................. 141
Grade 8 .............................................................................................................................. 161
SCIENCE CURRICULUM MADISON PUBLIC SCHOOLS
High School
Grades 9 – 12
Biology Honors ................................................................................................................... 199
Integrated Science I ............................................................................................................ 227
Integrated Science II .......................................................................................................... 249
Biological Systems ............................................................................................................... 265
General Biology ................................................................................................................... 293
Human Biology ................................................................................................................... 319
Chemistry Honors ............................................................................................................... 335
Chemistry Level 2 ............................................................................................................... 357
AP Chemistry ECE 1127Q..................................................................................................... 373
AP Physics B ECE 1202 ........................................................................................................ 391
AP Physics C ECE 1401Q...................................................................................................... 433
Physics Level 2 ................................................................................................................... 443
AP Biology .......................................................................................................................... 465
Introduction to Horticulture ................................................................................................. 473
Topics in Science ................................................................................................................ 497
Biotechnology & Forensic Science ........................................................................................ 513
Anatomy & Physiology ......................................................................................................... 533
Principles of Ecology ........................................................................................................... 549
Marine Science & Technology .............................................................................................. 569
Program Implementation: Guidelines and Strategies
Instructional Delivery ................................................................................................................ 593
Instruction Requirements .......................................................................................................... 593
Instructional Time ..................................................................................................................... 594
Instructional Technology ........................................................................................................... 594
Student Support ........................................................................................................................ 595
Professional Growth .................................................................................................................. 597
Professional Supervision and Evaluation ..................................................................................... 598
Implementation ........................................................................................................................ 598
Program Monitoring and Evaluation
Program Monitoring and Evaluation ............................................................................................ 601
Resources
Safety ...................................................................................................................................... 605
Laboratory Procedures .............................................................................................................. 607
MSDS ....................................................................................................................................... 610
Web Resources ........................................................................................................................ 613
Curriculum Improvement Plan Worksheet ................................................................................... 614
Template for Unit Plan Overview ................................................................................................ 615
Works Consulted ....................................................................................................................... 616
Appendices
Scientific Process Skills for Elementary Students ......................................................................... 619
Rubric for Scoring Elementary Science Projects ........................................................................... 621
Rubric for Scoring Elementary Science Experiment Report ........................................................... 623
Laboratory Report Form for Middle School Students .................................................................... 625
Rubric for Scoring Curriculum Embedded High School Laboratory Investigations ........................... 629
Sample End of Course Tests ...................................................................................................... 630
Framework for 21
st
Century Learning ......................................................................................... 631
PROGRAM OVERVIEW
SCIENCE CURRICULUM i MADISON PUBLIC SCHOOLS
Introduction
The Madison Curriculum Renewal Process addresses the need for the continual improvement and / or
updating of the schools’ instructional programs through the periodic re-examination of curriculum. The
process is recursive and usually occurs within the same cycle as the Board of Education's Framework for
Strategic Planning. The full cycle includes fourteen steps.
The Science committee has completed the eight steps of curriculum renewal. After review of the
curriculum by the Administrative Council, the Superintendent of Schools and the Board of Education, the
steps of implementation, program monitoring and evaluation will be initiated.
Science teachers examined many resources including state and national standards and frameworks for
Science learning as well as science curricular programs nationwide. The articulation of the guide’s goals
and standards across grade levels has been examined carefully and has been achieved to the satisfaction
of the Science committee charged with the development of the guide. The committee believes that the
Madison Public School System has developed a quality Science program that is planned, ongoing, and
systematic.
David J. Klein, Anita L. Rutlin,
Superintendent Assistant Superintendent
PROGRAM OVERVIEW
SCIENCE CURRICULUM ii MADISON PUBLIC SCHOOLS
Structure of the Program Guide
The guide is organized into six (6) sections. This overview section provides direction for understanding
the contents of the guide.
The Components and Framework sections provide descriptions of the program components, including
such elements as philosophy, goals, design and understandings.
The Standards and Indicators for Scope and Sequence of Instruction section states the scope (breadth
and depth) of subject content and sequence (order of presentation) to master the subject with
understanding – to acquire knowledge and skill for handling key tasks in science. It is the overall logic for
learning: 1) a design that is back loaded from expected performances; 2) application of the content
based on clear performance goals; and 3) a sequence that enables learning and then proficient
performing. Objectives have been identified for grade levels and / or for courses. Objectives for learners
introduced at earlier grade levels may not be restated at later grade levels, even though periodic
reinforcement occurs. The curriculum facilitates learning content incrementally, progressing by tackling
increasingly complicated ideas and aspects of proficient performance.
The Program Implementation: Guidelines and Strategies section provides guidelines and strategies for
implementing the curriculum described in the preceding sections of the guide. This includes descriptions
of various components of instruction -- delivery, requirements, time, technology, student support -- as
well as professional growth and development plus coordination, supervision and evaluation.
The Program Monitoring and Evaluation section provides guidelines and procedures for assessing the
overall effectiveness of the curriculum program. There are recommendations for annual program
monitoring and questions to frame program evaluation.
The Program Resources section includes Science Safety Manuals for Elementary, Middle and High School
use as well as a guide for Scientific Process Skills for elementary students. Also included are instructional
websites and the works consulted in creating the program guide.
The Appendices follow and include references to the Connecticut Core Science Curriculum Framework
and Standards, National Science Education Standards, and the Framework for 21
st
Century Learning that
provides the foundation to insure that the science learning is current and appropriate to the needs of the
21
st
century.
PROGRAM OVERVIEW
SCIENCE CURRICULUM iii MADISON PUBLIC SCHOOLS
Intent and Acknowledgments
The Science Curriculum Committee believes that students should become confident in their skills and
realize the value of science education as they progress through a challenging curriculum that stresses
scientific reasoning and inquiry skills.
Science Faculty
Daniel Hand High School
Frank Balantic
Paul Birdsall
Erica Browne
Michael Docker
Lisa Edgerton
Bill Edwards
Paula Filippone
John Gaskell
Paul Mezick,
Dept. Chair
Katherine O’Neil
Mary O’Sullivan
Christie Peperato
David Russo
Steve Sekula
Sarah Tibbetts
Marianne Valley
Chris Walker
Island Avenue
Grade 1 Toby Andrews
Renee Pardo*
Tara Vitale
Grade 2 Maria Barnikow
Jeanette Iacobellis
Deana Perillo*
Julie Weber
Grade 3 Alicia DeNuzzo
Michelle Horn
Fran Manganello
Alisha Signore *
Laura Tanner
Grade 4 Carissa Connell*
Michael Ginsburg
Jane Kraus
Roberta Otis
Math Specialist
* Science Liaison
Walter C. Polson Middle School
Elisa Brako
Kathleen Brooks,
Prog. Coordinator
Catherine Cyr-Sinicrope
David D’Alessio
Dianna Floyd
Elizabeth Johnson
Dale Kukucka
Maud Moore
Jennifer Packevicz
Ryerson
Grade 1 Jennifer Figurelli
Clare Pinski*
Lisa Seales
Grade 2 Christine Ackerman
Erin Chester*
Alicia Dunbar*
Jennifer Maxwell
Grade 3 Scott Mongillo
Erin Smith
Lynn Voitans
Catherine Williams*
Grade 4 M. Peggy Bell*
Laleh Karimi
Eileen Martin
Kelly Ott
Tina Perry
Dr. R.H. Brown Middle School
Eric Ambler
Melinda Aresta
Kathleen Brooks,
Prog. Coordinator
Rachel Leonard
Laurine Moore
Fred Muzer
Nicole Sypher
Deb Thomas
Jeffrey
Grade 1 Denise Chabot
Debbie Lynch
Michelle Rindfleisch
Jean Stewart *
Bethany Taylor*
Grade 2 Mary Ellen Babik
Cindi Gardner
Christa Laragy
Ashley Lunn*
Stacey Ritsick*
Grade 3 Esther Magee
Diane Powers*
Tracey Rossi
Michelle Schmidt*
Pam Whalen
Grade 4 Lisa Caldwell
Lisa Caldwell
Ella Cinquino
Jennifer Hall Pflomm*
John Pluchino
Tracey Soboleski
PROGRAM OVERVIEW
SCIENCE CURRICULUM iv MADISON PUBLIC SCHOOLS
Program
Guide
PROGRAM GUIDE
PROGRAM GUIDE
SCIENCE CURRICULUM 1
Program Component Descriptions
Philosophy
An effective curriculum design needs to incorporate a philosophy, i.e., a statement of beliefs. The philosophy
reflects national trends based on research and effective practice. It also incorporates the school district’s
beliefs regarding the content area. Research studies, curriculum frameworks, and assessment are
referenced. An effective philosophy mirrors a vision statement and prepares the system to meet the needs of
its students for the 21
st
century.
Goals
Goals address what students should know and be able to do after experiencing a quality curriculum in
grades K-12. Connecticut’s Common Core of Learning states that all educated citizens must possess a core of
basic enabling skills and competencies that provide the critical intellectual foundations for broader acquisition
of knowledge. Goals that are established for Science explain those given competencies.
Design
Understanding by Design or UbD is a framework developed by Grant Wiggins and Jay McTighe and published
by the Association for Supervision and Curriculum Development. It is a tool for educational planning focused
on ―teaching for understanding.‖ The emphasis of UbD is on ―backward design,‖ the practice of looking at
outcomes in order to design curriculum units, performance assessments and classroom instruction. The
teacher starts with the classroom outcomes and then plans the curriculum, prepares assessments that help
determine student mastery, and chooses activities and materials that foster student learning.
UbD expands on ―six facets of understanding‖, which include students being able to explain, interpret, apply,
have perspective, empathize, and have self-knowledge about a topic. ―Teaching for Understanding‖ should
be evident in course design, teacher and student attitudes, and the classroom learning environment. There
should be systematic curriculum design with distinctions between the enduring understandings and essential
questions. Students should be familiar with the essential questions, performance requirements, and
evaluation criteria at the beginning of each unit or course.
Enduring Understandings
Understandings are characterized as:
Statements that summarize insights that students are expected to remember.
Inferences that students must draw, realize, or grasp, based on learning.
Insights that link facts and skills to ―big ideas‖ in meaningful ways that are related to the ―real world‖.
Conceptual Structure
The science framework is organized around eleven conceptual themes and guiding questions in the earth,
life and physical sciences. Each theme is addressed by several content standards that spiral through the
grades, each time being treated with greater depth and breadth, in accordance with developmental
appropriateness for the students.
PROGRAM GUIDE
SCIENCE CURRICULUM 2
Benchmarks of Student Progress
Benchmarks include identified assignments and assessments that serve as markers for incremental student
progress at different points in a grade level or course.
Learning Environment
The learning environment addresses the ambiance in which the students work. It is an environment which
encourages active participation through listening, watching, speaking, reading, and writing. It describes the
science classroom / lab where the student’s engagement, understanding, and development of process and
inquiry skills are nurtured.
Program Implementation
The implementation section will be dynamic. As the curriculum is available electronically, changes and
updates will be ongoing. Recent revisions to the grade level expectations in the Connecticut K-8 Science
Curriculum Standards in the Connecticut Core Science Framework have had a subsequent effect on the
curriculum.
The focus of implementation includes instructional delivery, requirements, time, and technology. Professional
development, supervision and evaluation facilitate implementation.
Program Monitoring and Evaluation
Program Evaluation addresses the effectiveness of the program from a student performance stance. The
effectiveness of the designated curriculum is determined by whether the students are progressively gaining
proficiency in science as evidenced by benchmark assignments and assessments, unit and course tests,
Connecticut Mastery Tests, and Connecticut Academic Performance Tests.
Resources
The Resources section includes Science Safety Guides for Elementary Science Safety, Middle School Science
Safety, and High School Science Safety as well as websites and works consulted in the development of this
document.
Appendices
The appendices contain reference materials such as the Connecticut Core Science Curriculum Framework,
National Science Education Standards, and the Framework for 21
st
Century Skills.
Science
Program Framework
PROGRAM FRAMEWORK
PROGRAM FRAMEWORK
SCIENCE CURRICULUM 5
Vision of Science Education: A quality science education fosters a population that:
Experiences the richness and excitement of knowing about the natural world and understanding how
it functions.
Uses appropriate scientific processes and principles in making personal decisions.
Engages intelligently in public discourse and debate about matters of scientific and technological
concern.
Applies scientific knowledge and skills to increase economic productivity.
Vision for Scientific Literacy
Global interdependence, rapid scientific and technological innovation, the need for a sustainable
environment, economy and society, and the pervasiveness of science and technology in daily life reinforce
the importance of scientific literacy. Scientifically literate individuals can more effectively interpret
information, solve problems, make informed decisions, accommodate change and create new knowledge.
Science education is a key element in developing scientific literacy and in building a strong future for
Madison's students.
Scientific literacy is an evolving combination of the science-related attitudes, skills and knowledge students
need to develop inquiry, problem-solving, and decision-making abilities, to become lifelong learners, and to
maintain a sense of wonder about the world around them. Learning experiences based on standards and
expectations will provide students with many opportunities to explore, analyze, evaluate, synthesize,
appreciate and understand the interrelationships among science, technology, society and the environment
that will affect their personal lives, careers and future.
Mission of Science Education
Scientifically literate students posses the knowledge and understanding of scientific concepts and processes
required for personal decision-making, participation in civic and cultural affairs and economic productivity.
Goals for Science Education
To promote scientific literacy, science education will…
encourage students at all grades to develop a critical sense of wonder and curiosity about
scientific and technological endeavors.
enable students to use science and technology to acquire new knowledge and solve problems, so
that they may improve the quality of their own lives and the lives of others.
prepare students to critically address science-related societal, economic, ethical and environmental
issues.
provide students with a proficiency in science that creates opportunities for them to pursue
progressively higher levels of study, prepares them for science-related occupations, and engages
them in science-related activities appropriate to their interests and abilities.
develop in students of varying aptitudes and interests a knowledge of the wide variety of careers
related to science, technology and the environment.
The Need for Science Education
―In this changed world, knowledge of math and science is paramount.‖ (U.S. Department of Education.
2006) It is essential that students are taught the skills necessary to compete and succeed in higher
education and the workforce. The study and ―work of science relies on basic human qualities such as
reasoning, insight, energy, skills and creativity – as well as on scientific habits of mind, such as intellectual
honesty, tolerance of ambiguity, skepticism, and openness to new ideas.‖ (National Science Standards)
PROGRAM FRAMEWORK
SCIENCE CURRICULUM 6
People of both genders and of all social and ethnic backgrounds with diverse talents engage in activities of
science including engineering, research and health professionals and related fields. Scientists in all fields
may work in teams or alone but they must communicate extensively with others. For progress in science,
the exchange of ideas, information, data and materials is essential. This communication goes well beyond
research facilities or universities crossing national boundaries and spanning the globe. Therefore, the
development of good communication skills is needed in addition to inquiry skills including the forming of a
scientific question, the testing of a hypothesis, designing and performing a valid experiment, collecting valid
and useful data, analyzing data, and drawing conclusions.
Philosophy
To promote scientific literacy, it is crucial to recognize how students learn, how science can best be taught,
and how learning can be assessed. Students are curious, active learners who have individual interests,
abilities and needs. They come to school with various personal and cultural experiences and prior knowledge
that generate a range of attitudes and beliefs about science and life.
Students learn most effectively when their study of science is rooted in concrete learning experiences,
related to a particular context or situation, and applied to their world. The ideas and understandings that
students develop can be progressively extended and reconstructed as students grow through their
experiences and in their ability to conceptualize. Learning involves the process of linking newly constructed
understandings with prior knowledge and adding new contexts and experiences to current understandings.
Development of scientific literacy is supported by instructional environments that engage students in the
processes of…
scientific inquiry: students address questions about natural phenomena, involving explorations as
well as focused investigations.
technological problem solving (design process): students seek answers to practical problems
requiring the application of their science knowledge in various ways.
decision making: students identify issues and pursue science knowledge that will inform the issues.
It is through these processes that students discover the significance of science in their lives and come to
appreciate the interrelationships of science, technology, society and the environment.
Each of the processes is a potential starting point for approaching science learning. These processes may
encompass a variety of learning approaches for exploring new ideas for development specific investigations
and for applying the ideas that are learned.
To achieve the vision of scientific literacy, students must increasingly become engaged in the planning,
development and evaluation of their own learning experiences. They should have the opportunity to work
cooperatively with other students, to initiate investigations, to communicate their findings, and to complete
projects that demonstrate their learning. To assist teachers in planning for instruction, assessment,
evaluation and reporting, science teachers recommend:
At the beginning of each unit of instruction, the expected student learning outcomes and
performance criteria are identified. It is important that the student learning outcomes and
performance criteria correspond with state and national standards and expectations. The
communication between students and teachers helps to clearly establish what needs to be
accomplished, thereby assisting in the learning process.
When students are aware of expected outcomes, they will be more focused on the essential
learning and more likely to assess their own progress. Furthermore, they can participate in
assessment as learning to meet expectations. Assessment must be valid, reliable and fair to
students.
PROGRAM FRAMEWORK
SCIENCE CURRICULUM 7
Guiding Principles
Guiding principles form the basis of an effective science education program. They address the complexity of
the science content and the methods by which science content is best taught. They clearly define the
attributes of a quality science curriculum at the elementary, middle, and high school levels.
Effective science programs:
are based on standards and use standards-based instructional materials.
develop students’ command of the language of science used in the standards.
reflect a balanced, comprehensive approach that includes the teaching of inquiry along with direct
instruction and reading.
use multiple instructional strategies and provide students with multiple opportunities to master the
standards.
include continual assessment of students’ knowledge and understanding.
engage all students in learning and prepare and motivate students for further instruction in
science.
use technology to teach students, assess their knowledge, develop information resources, and
enhance computer literacy.
have adequate instructional resources as well as library-media and administrative support.
use standards-based connections with other core subjects to reinforce science teaching and
learning.
K-12 Conceptual Themes and Guiding (Essential) Questions
The conceptual themes and the guiding questions together with the content standards and grade level expectations
contribute to students' abilities to have enduring understandings and to respond to the guiding (essential) questions.
Properties of Matter
How does the structure of matter affect the properties and uses of materials?
Properties of Objects (K)
Properties of Materials (2)
States of Matter (3)
Elements, Compounds and Mixtures (6)
Chemical Reactions (9)
Carbon Compounds (9)
Energy Transfer and Transformations
What is the role of energy in our world?
Electricity and Magnetism (4)
Physics of Sound (4)
Light (5)
Energy and Work (7)
Energy Conservation and Transformation (9)
Electrical Forces (9)
Forces and Motion
What makes objects move the way they do?
Position and Motion of Objects (1)
Forces and Motion (5)
Forces and Motion (8)
Matter and Energy in Ecosystems
How do matter and energy flow through ecosystems?
Food Chains - Wetlands (4)
Ecosystems (6)
PROGRAM FRAMEWORK
SCIENCE CURRICULUM 8
Structure and Function
How are organisms structured to ensure efficiency and survival?
Needs of Living Things (1)
Life Cycles of Animals (1)
Life Cycles of Plants (2)
Responses to Stimuli (5)
Human Body Systems (7)
Cell Structure and Function (10)
Heredity and Evolution
What processes are responsible for life’s unity and diversity?
Characteristics of Living Things (K)
Adaptations (3)
Reproduction and Heredity (8)
Genetics (10)
Evolution (10)
The Changing Earth
How do materials cycle through the Earth’s systems?
Properties of Soils (2)
Properties of Rocks and Minerals (4)
Cycles of Matter in Earth’s Systems (9)
Energy and Earth’s Systems
How do external and internal sources of energy affect the Earth’s systems?
Weather Patterns (K)
Land and Water Interactions (3)
Weather and Seasons (6)
The Changing Earth (7)
Earth and the Solar System
How does the position of Earth in the solar system affect conditions on our planet?
Earth, Moon and Sun (5)
The Solar System (8)
Science and Technology in Society
How do science and technology affect the quality of our lives?
Shelters (K)
Measuring Tools (1)
Food Resources - Nutrition (2)
Conservation of Materials (3)
Batteries, Bulbs and Magnets (4)
Optical Technologies (5)
Water Quality (6)
Food Technology (7)
Building Bridges (8)
Energy and Power Technologies (9)
Polymers (9)
Human Environmental Impacts (9)
Living with Microorganisms (10)
Biotechnology (10)
Human Population Growth (10)
PROGRAM FRAMEWORK
SCIENCE CURRICULUM 9
Science Practices
Understand Scientific Explanations: Students understand core concepts and principles of science
and use measurement and observation tools to assist in categorizing, representing, and interpreting the
natural and designed world.
Generate Scientific Evidence Through Active Investigations: Students master the conceptual,
mathematical, physical and computational tools that need to be applied when constructing and evaluating
claims.
Reflect on Scientific Knowledge: Scientific knowledge builds on itself over time.
Participate Productivity in Science: The growth of scientific knowledge involves critique and
communication, which are social practices that are governed by a core set of values and norms.
Benchmarks of Student Progress
Effective teaching and learning begins with the needs of students and reflects their developmental
stages. We recognize the need for a solid conceptual foundation in science and the development of
science inquiry skills in order to apply their knowledge and continue to learn.
By the end of 2
nd
grade, students will have developed a ―wonder‖ about the natural world and the
ability to observe, describe and apply basic process skills.
By the end of 5
th
grade, students will have developed ―descriptions‖ of basic natural phenomena and
the ability to perform simple experiments and record accurate data.
By the end of 8
th
grade, all students will have developed basic ―explanations‖ for natural phenomena,
and the ability to ask good questions and apply experimental procedures to collect and analyze data.
By the end of 10
th
grade, all students will have developed an ―interest‖ in global issues and the ability
to collect, analyze and use data to explore and explain related science concepts.
By the end of 12
th
grade, all students will have developed a ―deep understanding‖ of science concepts
and principles and prepared for future studies and/or careers.
(CT State Department of Education, 2005)
Integrated Science and Biology as gateway courses, which we believe will encourage students to
complete four credits of science study including advanced life, earth and physical science courses.
PROGRAM FRAMEWORK
SCIENCE CURRICULUM 10
Content Standards & Indicators
Kindergarten – Grade 4
Content Standards & Indicators
for Kindergarten
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 15 KINDERGARTEN
Course Description
ELEMENTARY SCHOOL
1. Course Title
Kindergarten General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
N/A
4. Program Contact Information
Name: Anita Rutlin
Title/Position: Assistant Superintendent
School: Central Office
Madison Town Campus
10 Campus Drive, P.O. Drawer 71
Madison, CT 06443
6. Grade Level: Kindergarten
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites: N/A
11. Brief Course Description
Kindergarten science is taught in four units throughout the school year. Students are actively engaged in
science though investigations. As part of the spiraling curriculum, aspects of life science, earth science
and physical science are taught each year. The life science unit is Heredity and Adaptations. The
physical science units are Properties of Matter and Shelters. The earth science unit is Energy in the
Atmosphere and Weather.
12. Course Goals
A guided inquiry program gives students the opportunity to explore topics and concepts though
investigations. Participating in this hands-on program helps students:
1. To foster a life long enjoyment of learning science.
2. To observe science in the world around them.
3. To meet the science standards for Connecticut Public Schools.
13. Course Outline
1. Properties of Matter 3. Energy in the Atmosphere and Weather
2. Heredity and Adaptations 4. Science and Technology in Society - Shelters
14. Instructional Methods and/or Strategies
Individual and small group work
Full class instruction and discussions
Modeling
Guided inquiry activities
15. Assessment Methods and/or Tools
Teacher Observation
Embedded Assessment Activities
Response to writing prompts and class
discussions
16. Assessment Criteria
The common assessments are based on the Madison curriculum and the Connecticut standards and
grade level expectations for science. A variety of assessment tools are employed to get the most
accurate understanding of individual achievement possible.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 16 KINDERGARTEN
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, to search out, describe, explain, and
predict natural phenomena.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing about
science.
Mathematics provides useful tools for the
description, analysis and presentation of scientific
data and ideas.
ESSENTIAL QUESTIONS
How do you make observations about
objects, organisms, and the environment?
How do you use simple measuring tools to
gather data and extend the senses?
How do you use observed patterns to make
predictions?
How do you use standard measuring tools to
collect data and nonstandard measures to
make comparisons?
How can physical properties be used to
order and sort objects and organisms?
How do you locate relevant science
information in printed resources?
How do bar graphs represent information?
KNOWLEDGE & LEARNING
The student will know…
INSTRUCTIONAL SUPPORT MATERIALS
Use the senses and simple measuring tools to
make observations and collect data.
Use standard tools to measure and describe
physical properties such as weight, length and
temperature and nonstandard measures to
estimate and compare the sizes of objects.
Count, order and sort objects by their properties.
Make predictions based on observed patterns.
Ask questions about objects, organisms and the
environment.
Read, write, listen and speak about observations of
the natural world.
Seek information in books, magazines and pictures
and present information in words and drawings.
Represent information in bar graphs.
Internet resources
Books from the Library Media Center
INSTRUCTIONAL STRATEGIES
Modeling during instruction
Inquiry activities and investigations
Guided reading
ASSESSMENT METHODS
Inquiry literacy questions
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 17 KINDERGARTEN
LEARNING STRAND
Unit: Properties of Matter --
How does the structure of matter affect the properties and uses of materials?
CT Standard K.1 – Objects have properties that can be observed and used to describe similarities and differences.
ENDURING UNDERSTANDING
Some properties can be observed with the senses,
and others can be discovered by using simple tools
or tests.
ESSENTIAL QUESTIONS
How can simple measuring tools be used to
observe common objects and sort them into
groups based on size, weight, shape or color?
How can objects be sorted according to the
materials of which they are made?
How can objects be sorted into groups based
on properties such as flexibility, attraction to
magnets, and whether they float or sink in
water?
How can objects in a group be described in
mathematical terms using quantitative
relationships such as same as, more than,
less than, equal, etc.?
UUNDERLYING CONCEPTS
Students should understand that …
INSTRUCTIONAL SUPPORT MATERIALS
Humans have five senses that they use to observe
their environment. A specific sense organ is
associated with each sense.
Objects have properties that can be observed
using the senses. Examples include size, weight,
shape, color, texture, transparency, etc. An
object’s observable properties do not include the
object’s name or its uses.
Sorting objects into groups based on one (or
more) of their properties makes it possible to
observe and describe their similarities and
differences.
Placing objects in order based on their size or
weight makes it possible to observe patterns and
describe relationships among the objects in a
group.
Objects can be described and sorted based on the
materials from which they are made (for example,
wood, paper, fabric, plastic, glass or metal).
Objects can be made of a mixture of materials.
Objects can be described and sorted based on the
results of simple tests. Simple tests include
actions such as bending, squeezing, holding it
near a magnet or putting it in water. Objects can
be described as magnetic/nonmagnetic,
flexible/not flexible, hard/soft, a floater/sinker,
etc.
The heaviness of objects can be compared using
the sense of touch. Balances and scales are
measurement tools that allow people to observe
and compare the heaviness of objects more
accurately. Objects can be sorted into groups that
have the same heaviness, or into groups that are
STC:
Comparing and Measuring
Plastic containers to hold water
Objects that can be sorted or tested for
sinking and floating
INSTRUCTIONAL STRATEGIES
Provide a variety of materials and simple
measuring tools for each group of students.
Have them measure the length of the objects
and sort them accordingly. Then have the
students sort the same objects according to
height.
Have students test to see if objects can sink
or float and sort the objects accordingly.
Have students sort the objects according to
their own criteria. Allow the students to
weigh the objects, squeeze or bend them, or
try other simple tests that allow them to sort
the objects according to a characteristic.
Have students move from group to group to
try to identify the criteria used by the original
group to sort the objects.
ASSESSMENT METHODS
Students should be able to successfully sort
objects physically or on paper in a variety of
ways according to the characteristics of the
objects using simple tools.
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
Match each of the five senses with its
associated body part and the kind of
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 18 KINDERGARTEN
―more heavy than‖ or ―less heavy than‖ a given
object.
The temperature of the air, water or bodies can
be compared using the sense of touch. A
thermometer is a measurement tool that allows
people to compare temperatures more accurately.
Objects can be sorted into groups based on
measurements of their size. Nonstandard units for
measuring size include hands, footsteps, pennies
or paper clips.
information it perceives.
Make scientific observations using the five
senses, and distinguish between an object’s
observable properties and its name or its
uses.
Classify organisms or objects by one and two
observable properties and explain the rule
used for sorting (e.g., size, color, shape,
texture or flexibility).
Use simple tools and nonstandard units to
estimate and predict properties such as
heaviness, magnetic attraction and float/sink.
Describe properties of materials such as
wood, plastic, metal, cloth or paper and sort
objects by the material from which they are
made.
Count, order and sort objects by their
observable properties.
CMT CORRELATIONS
Use the senses and simple measuring tools,
such as rulers and equal-arm balances, to
observe common objects and sort them into
groups based on size, weight, shape or color.
Sort objects made of materials such as wood,
paper and metal into groups based on
properties such as flexibility, attraction to
magnets, and whether they float or sink in
water.
Count objects in a group and use
mathematical terms to describe quantitative
relationships such as: same as, more than,
less than, equal, etc.
SCIENTIFIC LITERACY TERMINOLOGY: senses, observe, observation, property, sort, classify,
material, float, sink, flexible, heavy, magnetic, nonmagnetic, thermometer
KEY SCIENCE VOCABULARY
:

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 19 KINDERGARTEN
LEARNING STRAND
Unit: Heredity and Adaptations --
What processes are responsible for life's unity and diversity?
CT Standard K.2 - Many different kinds of living things inhabit the Earth.
ENDURING UNDERSTANDING
Living things have certain characteristics that
distinguish them from nonliving things, including
growth, movement, reproduction and response to
stimuli.
ESSENTIAL QUESTIONS
How are the appearance and behaviors of
plants, birds, fish, insects, and mammals
(including humans) similar?
How are the appearance and behaviors of
plants, birds, fish, insects, and mammals
(including humans) different?
How are the appearance and behaviors of
adults similar to their offspring?
How are the appearance and behaviors of
adults different from their offspring?
How can living and nonliving things be
distinguished from one another by describing
their characteristics?
UNDERLYING CONCEPTS
Students should understand that …
INSTRUCTIONAL SUPPORT MATERIALS
Things in our environment can be classified based
on whether they are alive, were once alive or
whether they were never alive.
Growth is an observable characteristic common to
living things.
Reproduction is an observable characteristic
common to living things. Living things can be
classified into groups based on the different ways
they reproduce. For example, some living things
lay eggs, while others produce seeds or give birth.
Offspring generally resemble their parents but are
not identical to them.
Many living things move in response to their
environment, but movement alone is not evidence
of life. For example, cars and the wind both
move, but they are not alive.
Plants and animals are living things. Plants have
characteristics (such as roots, stems, leaves and
flowers) that animals do not have. Animals have
characteristics (such as body parts and body
coverings) that plants do not have.
Animals can be classified into groups based on
generally similar characteristics such as number of
legs, type of body covering, or way of moving.
Some animal groups are reptiles, insects, birds,
fish and mammals.
Members of the same group of animals can look
and behave very differently from each other. For
example, goldfish and sharks are both fish, but
there are distinct differences in their size, color
and lifestyle. In addition, all goldfish are not
identical to each other and neither are all sharks.
Pictures of plants
Pictures of animals in different stages of life
Picture books about plants and animals
Posters of ecosystems
INSTRUCTIONAL STRATEGIES
Have students examine posters of ecosystems
and identify things as alive, once alive or
never alive.
Discuss with students what living things do
that nonliving things do not do – grow and
reproduce.
Discuss how movement alone does not
indicate something is living. Machines move
and are not alive.
Show pictures of well-know organisms and
have students sort them according to how
they reproduce (laying eggs, giving birth,
making seeds).
Have students match baby animals with what
they look like as adults. Include easy ones
such as dogs as well as more difficult ones
such as mosquitoes. Have students observe
the similarities and differences.
Have students observe the basic parts of
plants: roots, stems, leaves, flowers. These
can be seen using a classroom plant or going
outside to observe plants.
Have students compare the plant parts to
animal parts.
Show students pictures of animals and have
them sort them according to criteria of choice
such as the number of legs, type of body
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 20 KINDERGARTEN
Plants can be classified into groups based on
similarities in the appearance of their leaves,
stems, blossoms or fruits. Some plant groups are
grasses, vegetables, flowering plants and trees.
Members of the same group of plants can look
and behave very differently from each other. For
example, although oaks and palms are trees, their
size, shape, leaves and bark are very different. In
addition, all oak trees are not identical to each
other and neither are all palms.
covering, etc.
Members of the same animal group can look
very different from one another. (Examples:
fish) This can be shown using pictures or a
picture book about animals. It can also be
done by having students draw a picture of a
dog. The pictures can be shared and
compared.
Using pictures and some poster tacky
substance (such as FunTack) have the
students sort pictures of plants according to
criteria. Have them explain why they sorted
as they did.
ASSESSMENT METHODS
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
Observe and describe differences between
living and nonliving things in terms of growth,
offspring and need for energy from food.
Sort and count living and nonliving things in
the classroom, the schoolyard and in pictures.
Use nonstandard measures to estimate and
compare the height, length, or weight of
different kinds of plants and animals.
Observe and write, speak or draw about
similarities and differences between plants
and animals.
Match pictures or models of adults with their
offspring (animals and plants).
Recognize varied individuals as examples of
the same kind of living thing (e.g., different
color rabbits are all rabbits; different breeds
of dogs are all dogs).
CMT CORRELATIONS
Describe the similarities and differences in the
appearance and behaviors of plants, birds,
fish, insects and mammals (including
humans).
Describe the similarities and differences in the
appearance and behaviors of adults and their
offspring.
Describe characteristics that distinguish living
from nonliving things.
SCIENTIFIC LITERACY TERMINOLOGY: classify, reproduction, offspring, characteristics, reptile,
insect, mammal

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 21 KINDERGARTEN
LEARNING STRAND
Unit: Energy in the Earth's Systems -
How do external and internal sources of energy affect the Earth's systems?
CT Standard K.3 – Weather conditions vary daily and seasonally.
ENDURING UNDERSTANDING
Daily and seasonal weather conditions affect what we
do, what we wear and how we feel.
ESSENTIAL QUESTIONS
In what ways do weather conditions change
from day to day?
What are the seasonal weather patterns in
Connecticut?
How do changes in daily and seasonal
weather conditions affect what we do?
How do changes in daily and seasonal
weather conditions affect what we wear?
How do changes in daily and seasonal
weather conditions affect how we feel?
UNDERLYING CONCEPTS
Student should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
The sun is the source of heat and light that
warms the land, air and water. Variations in the
amount of sunlight that reach the earth cause the
weather.
Weather conditions can be observed and
described as sunny, cloudy, rainy, foggy, snowy,
stormy, windy, hot or cold. Weather observations
can be made based on how we feel, what we see
or hear, or by using weather measurement
instruments such as thermometers.
Changes in weather conditions can be recorded
during different times of day, from day to day,
and over longer periods of time (seasonal cycle).
Repeated observations can show patterns that
can be used to predict general weather
conditions. For example, temperatures are
generally cooler at night than during the day and
colder in winter than in spring, summer or fall.
Weather influences how we dress, how we feel,
and what we do outside.
Weather affects the land, animals and plants, and
bodies of water.
When the temperature is below ―freezing,‖ water
outside freezes to ice and precipitation falls as
snow or ice; when the temperature is above
freezing, ice and snow melt and precipitation falls
as rain.
Clouds and fog are made of tiny drops of water.
Clouds have different shapes, sizes and colors
that can be observed and compared. Some cloud
types are associated with precipitation and some
with fair weather.
Wind is moving air. Sometimes air moves fast and
sometimes it hardly moves at all. Wind speed can
be estimated by observing the things that it
moves, such as flags, tree branches or sailboats.
Chart
Timeline
INSTRUCTIONAL STRATEGIES
Have students record daily weather on a
chart in the classroom.
Have the students create weekly timelines of
the temperature and rainfall and paste them
on the wall adding to them each week.
(This can be done by all students or one
student can be selected to do it each day.)
Have a cardboard cut-out of a girl and a boy
wearing minimal clothing (shorts and a tee
shirt). Have cut outs of different clothing and
have a student dress the doll for the day
considering the weather conditions. (The doll
can be made as a paper doll with a magnet
on its back so it will adhere to a whiteboard
or something else magnetic. Place magnets
on the back of the clothing pieces and put the
clothes on the dolls on this way.)
Students can be asked each day how they
feel and see if a pattern arises over time as to
what the weather is and how they feel.
Activity cancellations can be discussed when
they are cancelled due to weather conditions.
A book can be read describing how animals
survive during the winter.
When the weather is snowy, students can
describe the precipitation and the melting of
the snow.
On a nice day, take the students outside for
some cloud-gazing. Have them compare the
clouds. Talk about the formation of clouds
and have the students draw pictures of what
they see. Come back into the classroom and
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 22 KINDERGARTEN
have students draw pictures of what clouds
look like at other times and talk about why
that might be.
Observe wind one day looking at how it
affects trees, flags and other things.
ASSESSMENT METHODS
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
Use the senses to observe daily weather
conditions and record data systematically
using organizers such as tables, charts,
picture graphs, or calendars.
Analyze weather data collected over time
(during the day, from day to day, and from
season to season) to identify patterns and
make comparisons and predictions.
Observe, compare and contrast cloud shapes,
sizes and colors, and relate the appearance of
clouds to fair weather or precipitation.
Write, speak or draw ways that weather
influences humans, other animals and plants.
Make judgments about appropriate clothing
and activities based on weather conditions.
CMT CORRELATIONS
Describe and record daily weather conditions.
Relate seasonal weather patterns to
appropriate choices of clothing and activities.
SCIENTIFIC LITERACY TERMINOLOGY: weather, season (winter, spring, summer, fall),
thermometer, precipitation, freezing, melt
KEY SCIENCE VOCABULARY
:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 23 KINDERGARTEN
LEARNING STRAND
Unit: Science and Technology in Society - Shelters
CT Standard K.4 - Some objects are natural, while others have been designed and made by people to improve the quality of life.
ENDURING UNDERSTANDINGS
Humans select both natural and man-made
materials to build shelters based on local climate
conditions, properties of the materials, and their
availability in the environment.
ESSENTIAL QUESTIONS
How can the types of building materials used to
build homes be described?
What properties make these materials useful?
Why do people living in different regions use
different building materials?
UNDERLYING CONCEPTS
Student should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
People need shelters to keep warm or cool,
dry and safe. Shelters are made of materials
that have properties that make them useful
for different purposes.
People in different regions of the world build
different kinds of shelters, depending on the
materials available to them, the local climate
and their customs.
Traditionally, people have built shelters using
materials that they find nearby. Today, people
build houses from materials that may come
from far away.
People who live in forested regions have
traditionally built shelters using wood and/or
leaves from nearby trees.
People who live in regions with clay soils have
traditionally built shelters using bricks or
adobe made from clay.
People who live in snowy regions have
traditionally built shelters using snow and
ice.
People who live in regions with large
animals have traditionally built shelters
using animal skins.
Although they may look quite different, most
shelters have walls, roofs and an
entrance/exit; some shelters have doors,
windows and floors. Walls, roofs and windows
are made of materials that have specific
properties. For example, walls require
materials that are rigid, windows require
materials that are transparent, and roofs
require materials that are water-resistant.
Animals build shelters using materials that are
easily available to them. The materials they
use have properties that help the animals stay
warm or cool, dry and safe.
Internet resources
Drawing materials
INSTRUCTIONAL STRATEGIES
Have students draw a picture of their home or
dream homes. Display the drawings and compare
them looking for the materials from which the
houses have been made.
Compare homes in different climates looking at the
materials from which they are made. Have
students explain why the homes were built using
those materials.
Have students identify what most shelters have in
common (walls, roofs, etc.).
Have students look at materials used, such as
windows and roofing materials, and explain why
these are required.
Look at pictures of animal homes and the materials
from which they are made.
ASSESSMENT METHODS
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Conduct simple tests to compare the properties of
different materials and their usefulness for making
roofs, windows, walls or floors (e.g., waterproof,
transparent, strong).
Seek information in books, magazines and pictures
that describes materials used to build shelters by
people in different regions of the world.
Compare and contrast the materials used by
humans and animals to build shelters.
CMT CORRELATION
Describe the types of materials used by people to
build houses and the properties that make the
materials useful.
SCIENTIFIC LITERACY TERMINOLOGY: shelter, rigid, transparent
KEY SCIENCE VOCABULARY
:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 24 KINDERGARTEN
Content Standards & Indicators
for Grade 1
MADISON PUBLIC SCHOOLS
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 27 GRADE 1
Course Description
ELEMENTARY SCHOOL
1. Course Title
Grade 1 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
N/A
4. Program Contact Information
Name: Anita Rutlin
Title/Position: Assistant Superintendent
School: Central Office
Madison Town Campus
10 Campus Drive, P.O. Drawer 71
Madison, CT 06443
6. Grade Level: 1
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
None
11. Brief Course Description
First grade science is taught in units of study throughout the school year. Students are actively engaged
in science though investigations. As part of the curriculum, aspects of life science and physical science
are taught. The life science units include the Basic Needs and Life Cycles of Plants and Animals. The
physical science topics are Measurement and Forces and Motion.
12. Course Goals
The first grade science program gives students the opportunity to explore topics and concepts though
guided investigations. Participating in this hands-on program helps students:
1. To foster a life long enjoyment of learning science.
2. To observe science in the world around them.
3. To meet the science standards for Connecticut Public Schools.
13. Course Outline
1. Forces and Motion: Shadows and Objects
2. Structure and Function: Basic Needs of Plants and Animals
3. Structure and Function: Life Cycles
4. Science and Technology in Society: Measurement (Interdisciplinary)
14. Instructional Methods and/or Strategies
Individual and small group work
Full class instruction and discussions
Modeling
Guided inquiry activities
15. Assessment Methods and/or Tools
Teacher observations with rubrics
Embedded task assessment activities
Science journal and class discussions
16. Assessment Criteria
The common assessments are based on the Madison curriculum as well as Connecticut standards and
grade level expectations for science. A variety of assessment tools are employed to get observations of
individual performance of grade level expectations.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 28 GRADE 1
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, to search out, describe, explain, and
predict natural phenomena.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing about
science.
Mathematics provides useful tools for the
description, analysis and presentation of scientific
data and ideas.
ESSENTIAL QUESTIONS
How do you make observations about
objects, organisms, and the environment?
How do you use simple measuring tools to
gather data and extend the senses?
How do you use observed patterns to make
predictions?
How do you use standard measuring tools to
collect data and nonstandard measures to
make comparisons?
How can physical properties be used to
order and sort objects and organisms?
How do you locate relevant science
information in printed resources?
How do bar graphs represent information?
UNDERLYING CONCEPTS
Student should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Use the senses and simple measuring tools to
make observations and collect data.
Use standard tools to measure and describe
physical properties such as weight, length and
temperature and nonstandard measures to
estimate and compare the sizes of objects.
Count, order and sort objects by their properties.
Make predictions based on observed patterns.
Ask questions about objects, organisms and the
environment.
Read, write, listen and speak about observations of
the natural world.
Seek information in books and pictures and
present information in words and drawings.
Represent information in bar graphs.
AIMS, Delta Modules, STC Comparing and
Measuring Module, Growing with Math Topics
Introduction to the Processes
INSTRUCTIONAL STRATEGIES
Modeling during instruction
Inquiry activities and investigations
Guided reading
ASSESSMENT METHODS
Inquiry literacy questions
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 29 GRADE 1
LEARNING STRAND
Unit: Forces and Motion -
What makes objects move the way they do?
CT Standard 1.1 - The sun appears to move across the sky in the same way every day, but its path changes gradually over the
seasons.
ENDURING UNDERSTANDINGS
An object’s position can be described by
locating it relative to another object or the
background.
An object’s motion can be described by
tracing and measuring its position over time.
ESSENTIAL QUESTIONS
Can an object be in front of, behind, next to,
inside of, above or below another object?
Can an object be paced to the left or right of
another object?
Does the size of an object change by moving
closer or farther away?
Does the sun move or does the earth move?
Why does it look like the sun moves?
What makes a shadow?
How do the length and direction of shadows
change during the day?
How can the motion of objects be changed by
pushing and pulling?
How do objects move (spinning, bouncing, rolling,
flying and sailing)?
What is motion? What is force? What sets an
object in motion? What is position?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
An object’s position can be described by
comparing it to the position of another
stationary object. One object can be
in front
of, behind, next to, inside of, above or below
another object.
The sun’s position in the daytime sky can be
described relative to stationary objects on
Earth. For example, the sun can be ―just
above the treetops,‖ ―high or low in the sky,‖
or ―on the other side of the school.‖
The description of an object’s position from
one observer’s point of view may be different
from that reported from a different observer’s
viewpoint. For example, a box of crayons
between two students is near Susan’s left
hand but near John’s right hand.
When an observer changes position,
different words may be needed to describe an
object’s position. For example, when I am
sitting on the bench the sun is ―behind‖ me;
when I move to the slide, the sun is ―in front
of‖ me.
The same object when viewed from close up
appears larger than it does when viewed from
far away (although the actual size of the
object does not change.) For example, a
beach ball held in one’s arms appears larger
than it does when viewed from across the
―How Do Objects Move?‖
―Determining an Object’s Position‖
―Which Ball is Larger?‖
Science NetLinks
Making Objects Move
AIMS lesson: ―It’s a Force, Of Course!‖
Literacy: Forces and Motion by Catherine Welch;
On the Move by Wendy Madgwick; Forces Around
Us by Sally Hewitt
Science:
Movement and Shadow
―Me and My Shadow‖
―My Shadow is Following Me‖
Literacy: The Sun is Always Shining Somewhere
by Allen Fowler; What Makes a Shadow? by Clyde
Bolla; Nothing Sticks Like a Shadow by Ann
Tompert; Me and My Shadow by Melinda Lilly;
Shadows by Carolyn Otto; The Biggest Shadow in
the Zoo by Jack Kent
INSTRUCTIONAL STRATEGIES
Teach Motion & Position Lessons
Students will observe how the size of an object
appears to change based on its position from the
child; use clear plastic clip boards to record
Teach Science NetLinks
Making Objects Move
Teach AIMS lesson
It’s a Force, Of Course!
Teach Science:
Movement and Shadow
Utilize high-touch high-tech: Force of Habit
Students will observe the position of the sun and
their shadows, during three times throughout a
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 30 GRADE 1
playground.
An object’s position can be described using
words (―near the door‖), numbers (10
centimeters away from the door) or labeled
diagrams.
Things move in many ways, such as
spinning, rolling, sliding, bouncing, flying or
sailing.
An object is in motion when its position is
changing. Because the sun’s position changes
relative to objects on Earth throughout the
day, it appears to be moving across the sky.
Motion is caused by a push or a pull. A push
or pull is called a force.
An object can be set in motion by forces that
come from direct contact, moving air,
magnets or by gravity pulling it down toward
the earth.
Pushes and pulls can start motion, stop
motion, speed it up, slow it down or change
its direction.
Changes in the sun’s position throughout the
day can be measured by observing changes in
shadows outdoors. Shadows occur when light
is blocked by an object. An object’s shadow
appears opposite the light source. Shadow
lengths depend on the position of the light
source.
day
Utilize high-touch high-tech: The Shadow Knows
Teach Scientific Literacy and Key Science
vocabulary
ASSESSMENT METHODS
Teacher observations during activities,
investigations and discussions
Responses in student’s science journal
investigation and discussions
Record of shadows
Changes in Motion: Push & Pull
Assessment
Flagpole Assessment
Completion of appropriate AIMS & Science
NetLinks projects
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
Compare and contrast the relative positions of
objects in words (in front of, behind, next to,
inside of, above or below) and numbers (by
measuring its distance from another object).
Apply direct and indirect pushes and pulls to
cause objects to move (change position) in
different ways (e.g., straight line, forward and
backward, zigzag, in a circle).
Classify objects by the way they move (e.g.,
spinning, rolling, and bouncing).
Conduct simple experiments and evaluate
different ways to change the speed and direction
of an object’s motion.
Observe record and predict the sun’s position at
different times of day (morning, noon, afternoon
or night).
Conduct simple investigations of shadows and
analyze how shadows change as the position of
the sun (or an artificial light source) changes.
CMT CORRELATIONS
Describe how the motion of objects can be
changed by pushing and pulling.
Describe the apparent movement of the sun
across the sky and the changes in the length and
direction of shadows during the day.
SCIENTIFIC LITERACY TERMINOLOGY: position, motion, shadow, force
KEY SCIENCE VOCABULARY
: behind, in front of, next to, above, below, larger, smaller, appears,
closer, further, distance, investigate, rotate, spinning, sliding, rolling, bouncing, flying, sailing, sun
Hypothesis: An educated guess
Mass: The amount of matter in an object
Movement: The act of moving
Pull To apply force to cause motion toward the source
Push To apply pressure for the purpose of moving
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 31 GRADE 1
LEARNING STRAND: Structure and Function -
How are organisms structured to ensure efficiency and survival?
Unit: Basic Needs of Plants and Animals
CT Standard 1.2 - Living things have different structures and behaviors that allow them to meet their basic needs.
ENDURING UNDERSTANDINGS
Animals need air, water and food to survive.
Plants need air, water and sunlight to survive.
Animals use structures to move around.
ESSENTIAL QUESTIONS
What do plants need to survive?
What do animals need to survive?
How do plants obtain water and sunlight?
How do animals take in air, get water and food?
How do animals move to survive?
What body parts help them to survive in their
habitat?
How do animals rely on their senses to survive?
How and what do animals eat?
What do the stem, leaf, flower or fruit do for a
plant?
How do plants make new plants?
Where do plants get their food? How do plants
stay healthy and strong?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 1.2.a.
All living things (organisms) need air, water and
food to stay alive and grow; they meet these
needs in different ways.
Most animals move from place to place to find
food and water. Some animals have two legs,
four legs, six legs or more for moving. Other
animals move using fins, wings or by slithering.
Animals get air in different ways. For example,
humans breathe with lungs, while fish breathe
with gills.
Animals get food in different ways. Some
animals eat parts of plants and others catch and
eat other animals.
Animals get water in different ways. Some
animals have special body parts, such as noses,
tongues or beaks that help them get water.
Fictional animals and plants can have structures
and behaviors that are different than real
animals and plants.
GRADE LEVEL CONCEPT 1.2.b.
Plants absorb sunlight and air through their
leaves and water through their roots.
Plants use sunlight to make food from the air
and water they absorb.
Plants have various leaf shapes and sizes that
help them absorb sunlight and air.
Plant roots grow toward a source of water.
Plant stems grow toward sunlight.
Science Anytime Dinosaur Museum
- Fossils-Inferring from Footprints
- Toothy Grin
- How Do Dinosaurs Protect Themselves?
- Circle of Life
Dinosaur Research Project
Camouflage Scavenger Hunt
Science Anytime Dinosaur Museum
- Legs, Legs, Legs
Live Birth vs. Egg Hatching
Literacy: Dinosaurs and Prehistoric Animals by
Helen Frost; Dinosaurs by Richard Fergusson; Is
It a Living Thing? by Bobbie Kalman; Who
Hops? by Katie Davis
Delta Science Module III: From Seed to Plant
-Activity 14 Caring for Plants
-Activity 7 How Big are They?
-Activity 8 How much Water?
-Activity 10 Looking at Leaves
-Activity 11 Plants and Sun
Literacy: Ten Seeds by Ruth Brown; Plants! By
Molly Smith; One Bean by Anne Rockwell
Supplementary Sheets
INSTRUCTIONAL STRATEGIES
Develop the science process of inferring by
studying fossil footprints.
Develop the science process of formulating
models:
-make a model of a plant eater’s head
-make a model of a meat eater’s head
Make a flip book of dinosaur body structures for
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 32 GRADE 1
defense.
Develop knowledge of habitat with a circle of
life including food, water, air, and shelter.
Have a scavenger hunt to teach the science
term of camouflage for survival.
To understand dinosaur movement, make
models of lizard and dinosaur legs.
Study how animals continue living through
being born or being hatched.
Plant marigold seeds to review how to take care
of plants properly.
Measure plants as they grow and compare the
rates of growth of various plants.
Water plants according to three different
watering schedules.
Compare plants’ responses to the different
watering schedules.
Draw conclusions about how much water plants
need.
Play ―Leaf Game‖ where students observe
various kinds of leaves closely and note their
different characteristics.
Examine several leaves and draw pictures.
Place plants in directional sunlight and observe
how the plants respond.
Students should discover that plants always
turn their leaves and bend their stems towards
the light.
ASSESSMENT METHODS
Teacher observations with during investigations,
experiments or activities
Science journals and class discussions
Dinosaur Research Project
How to get Food and Water (Assessment)
How Do we Move (Assessment)
From Seed to Plant
unit assessment activity
sheet 2 part A #2 & B #3 & #4
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Infer from direct observation and print or
electronic information that most animals and
plants need water, food and air to stay alive.
Identify structures and behaviors used by
mammals, birds, amphibians, reptiles, fish and
insects to move around, breathe and obtain
food and water (e.g., legs/wings/fins,
gills/lungs, claws/fingers, etc.)
Sort and classify plants by observable
characteristics (e.g., leaf shape/size, stem or
trunk covering, type of flower or fruit).
Use senses and simple measuring tools to
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 33 GRADE 1
measure the effects of water and sunlight on
plant growth.
Compare and contrast information about
animals and plants found in fiction and
nonfiction sources.
CMT CORRELATIONS
Describe the different ways that animals,
including humans, obtain water and food.
Describe the different structures plants have for
obtaining water and sunlight.
Describe the structures that animals, including
humans, use to move around.
SCIENTIFIC LITERACY TERMINOLOGY: organism, plant, animal, energy, breathe, lungs, gills,
absorb
KEY SCIENCE VOCABULARY
: structure, function, senses, danger, body parts, creeping, muscles,
skeleton, size, vision, soil, water sprinkler, seed, leaves, veins, stem, chlorophyll, flower, roots
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 34 GRADE 1
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 35 GRADE 1
LEARNING STRAND: Structure and Function
- How are organisms structured to ensure efficiency and survival?
Unit: Life Cycles
CT Standard 1.3
-
Organisms change in form and behavior as part of their life cycles.
ENDURING UNDERSTANDING
Some organisms undergo metamorphosis during
their life cycles; other organisms grow and change,
but their basic form stays essentially the same.
ESSENTIAL QUESTIONS
What are the stages in the life cycle of an
animal?
In what order do the stages of an animal life
cycle occur?
What is metamorphosis?
What animals experience metamorphosis?
What are some animals that do not experience
metamorphosis?
How do ladybugs change in form and behavior
as part of their life cycle?
How do animals that do not metamorphose
grow and carry on the life cycle?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Plants and animals have life cycles that
include a predictable sequence of stages:
they begin life, develop into adults,
reproduce and eventually die.
Animals produce offspring of their own
kind. Offspring closely resemble their
parents, but individuals vary in appearance
and behavior.
Animals are either born alive (for example,
humans, dogs and cows) or hatched from
eggs (for example, chickens, sea turtles or
crocodiles).
Animals change throughout their lives. Many
animals begin life as smaller, less capable
forms of the adult. As they develop, they
grow larger and become more independent
(for example, humans or robins).
Some animals change dramatically in
structure and function during their life
cycle in a process called metamorphosis.
Ladybugs are insects that go through
metamorphosis. Ladybug eggs hatch
into larvae that feed on aphids. Then
larvae enter a pupa stage from which adult
ladybugs emerge. As ladybugs get spots
and grow, they lay eggs and the cycle
begins again.
Frogs are amphibians that undergo
metamorphosis during their life cycle. As
they grow, frogs develop different
structures that help them meet their basic
needs in water and then on land.
o Tadpoles (polliwogs) hatch from eggs,
LHS GEMS: Ladybugs Guide
-Poster
-Activity 1: Student Observation & Ladybug
Journal
-Activity 3: Eggs & Baby Ladybugs
-Activity 4: Ladybug Pupa and Life Cycle
Life Cycles Packet
- Ladybug Life Cycle
- Butterfly Life Cycle
- Frog Life Cycle
Life Cycles with and without Metamorphosis
Literacy: The Grouchy Ladybug by Eric Carle; A
Ladybug’s Life by John Himmelman; Helpful
Ladybugs by Molly Smith; First the Egg by Laura
Seeger; Ladybug, Ladybug by Ruth Brown
Teacher Resources from the Internet
INSTRUCTIONAL STRATEGIES
Ladybugs - Students learn about ladybug body
structure, life cycle, defensive behavior, and
favorite foods. Students do observations.
Make Literacy Connections with Ladybug library
books, poems
Study other animals that metamorphose –
Butterfly and Frog
Study animals life cycles without
metamorphosis
ASSESSMENT METHODS
Teacher observations with during investigations,
experiments or activities
Life Cycle Project
Assessment – the Life Cycle of the Ladybug
Science journal responses to questions and
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 36 GRADE 1
live in water, breathe using gills, and
swim using a tail. As they
metamorphose into frogs, tadpoles
lose their gills and their tails.
o Adult frogs live on land and in water.
They breathe air using lungs and
develop webbed feet and hinged legs
for swimming in water and hopping
on land. After a female frog mates,
she lays her eggs, and the cycle
begins again.
Butterflies are insects that undergo
metamorphosis during their life cycle. As
they go through egg, larva, pupa
(chrysalis) and adult stages, butterflies
develop different structures that help them
meet their basic needs in very different
ways:
o Caterpillars hatch from eggs, live on
plants, get food by chewing leaves
and move about using legs. As they
metamorphose into butterflies
inside a chrysalis, they develop wings,
antennae and different mouth parts.
o Butterflies live on land and in the air.
They get food by sucking nectar from
flowers and move around primarily
using wings to fly. After a female
butterfly mates, she searches for the
proper host plant to lay her eggs, and
the cycle begins again.
Comparing the life cycle stages of different
organisms show how animals are alike in some
ways and unique in other ways.
class discussions
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
Explain that living things experience a life cycle
that includes birth, growth, reproduction and
death.
Distinguish between animals that are born alive
(e.g., humans, dogs, cows) and those that
hatch from eggs (e.g., chickens, sea turtles,
crocodiles).
Compare and contrast the changes in structure
and behavior that occur during the life cycles of
animals that undergo metamorphosis with those
that do not.
Analyze recorded observations to compare the
metamorphosis stages of different animals, and
make predictions based on observed patterns.
CMT CORRELATIONS
Describe the changes in organisms, such as
frogs and butterflies, as they undergo
metamorphosis.
Describe the life cycles of organisms that grow
but do not metamorphose.
SCIENTIFIC LITERACY TERMINOLOGY: life cycle, egg, metamorphosis, metamorphose, structures
(body parts), amphibian, tadpole, gills, lungs, insect, caterpillar
KEY SCIENCE VOCABULARY
: egg, spawn, polliwogs, larva, pupa, chrysalis, aphids, algae
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 37 GRADE 1
LEARNING STRAND
Science and Technology in Society
-
How do science and technology affect the quality of our lives?
Unit: Measurement
CT Standard 1.4 – The properties of materials and organisms can be described more accurately through the use of standard
measuring units.
ENDURING UNDERSTANDING
Various tools can be used to measure, describe
and compare different objects and organisms.
ESSENTIAL QUESTIONS
Which are more accurate -- non-standard units or
standard units of measure?
How can non-standard measurement units be
used to describe an object or organism?
What systems of measurement do we use in the
United States?
Can you share which specific tools are best used
to measure different quantities?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Observations can be expressed in words,
pictures or numbers. Measurements add
accuracy to observations.
Objects and organisms can be described
using nonstandard measurement units, such
as hand-lengths, pencil-lengths, handfuls, etc.
Standard measurement units are more
accurate than nonstandard units because they
have consistent values agreed on by
everyone. For example, ―My caterpillar is one
finger long‖ is much less accurate than ―My
caterpillar is 4 centimeters long.‖
Scientists and nonscientists all over the world
use the metric system of measurement.
In the United States, the customary
measurement system is used in daily life.
Equivalent values between the metric and
customary measurement systems can be
estimated (for example, 1 inch is a little more
than 2 centimeters).
Specific tools are used to measure different
quantities:
o Metric rulers are used to measure length,
height or distance in centimeters and
meters.
o Customary rulers measure length, height
or distance in inches, feet or yards.
o Balances and scales are used to compare
and measure the heaviness of objects.
o Grams and kilograms are units that
express mass; ounces and pounds are
units that express weight.
o Graduated cylinders, beakers and
measuring cups are tools used to
measure the volume of liquids.
o Volume can be expressed in milliliters
Non-standard measurement tools
Standard measurement tools
Growing with Mathematics Units 3,7, and 9
Science notebook/journals
Teacher Resources from the Internet
Meet the Measurements (Core Knowledge)
First Graders Measure Up! (Core Knowledge)
Magnificent Measurement (NCTM Illuminations)
INSTRUCTIONAL STRATEGIES
Growing with Mathematics Unit 9: Explore using
standard units of measure to communicate
measurement in a universal manner.
Growing with Mathematics Units 3,7, 9: Use
nonstandard units or physical referents to
estimate answers to measurement problems
involving length, area, weight, temperature,
volume and capacity, and then justify the
reasonableness of the answers. Use nonstandard
units, references or direct comparison of objects
(appearance, to order objects by length, area and
capacity).
Using the metric system students will measure
the length/height of their growing plants as a part
of From Seed to Plant Activity 7.
Using known size, dimension and weight of
objects as non-standard measures, students will
compare the size, dimension and weight of
dinosaurs listed in meters and kilograms.
*As a part of Morning Meeting/Calendar activities
students will be exposed to thermometers and their
use in daily living by keeping track of daily
temperatures.
ASSESSMENT METHODS
Teacher Observations during investigations,
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 38 GRADE 1
(mL), liters (L), cups or ounces.
o Thermometers are tools used to measure
temperature; thermometers can indicate
temperature in degrees Celsius or
degrees Fahrenheit, or both.
activities, and problem solving
Growing with Mathematics topic assessments
pertaining to measurement
Growing with Mathematics student workbook
pages
Science notebook/journal responses
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
Use nonstandard and standard measurements to
describe and compare the weight, length, and
size of objects and organisms. GWM 3.8, 3.9,
3.10, 7.8, 7.9, 7.10, 9.8, 9.9
Show approximate size of a centimeter, meter,
inch, foot and yard using referents such as a
finger, a hand or a book. GWM 3.8, 3.9, 3.10,
9.8, 9.9, 9.10
Select appropriate tools for measuring length,
height, weight or liquid volume.
Use metric and customary rulers to measure
length, height or distance in centimeters, meters,
inches, feet and yards. GWM 3.8, 3.9, 3.10, 9.8,
9.9, 9.10
Use balances and scales to compare and measure
the heaviness of objects and organisms in
kilograms, grams, pounds and ounces. GWM 7.8,
7.9, 7.10 (
Add scales, kilograms, grams, pounds,
ounces to math lessons)
Use graduated cylinders, beakers and measuring
cups to measure the volume of liquids in
milliliters, liters, cups and ounces. GWM 7.5, 7.6,
7.7 (
Add graduated cylinders, measuring cups,
ounces to math lessons)
Use thermometers to measure air and water
temperature in degrees Celsius and degrees
Fahrenheit. (
Daily Meeting – Class graph
)
Make graphs to identify patterns in recorded
measurements such as growth or temperature
over time. (
Temperature – Daily Meeting; growth
– From Seed To Plant: Activity 7 ―How Big Are
They?‖
CMT CORRELATION
Estimate, measure and compare the sizes and
weights of different objects and organisms using
standard and nonstandard measuring tools.
SCIENTIFIC LITERACY TERMINOLOGY: centimeter, meter, gram, kilogram, milliliter, liter,
graduated cylinder, thermometer, Celsius, Fahrenheit
KEY SCIENCE VOCABULARY
: inches, feet, yards, graphs, measuring cups, beakers, ounces, pounds,
scales, balances, weight
Volume: the space an object takes up

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 39 GRADE 1
SCIENTIFIC LITERACY TERMINOLOGY: GRADE 1
This list is intended as a guide for teachers. While not exhaustive, it includes vocabulary that should be
used, as appropriate, by teachers and students during everyday classroom discourse. It is not intended for
student memorization.
absorb
To take in, soak up
adaptation (adapt)
The process of changing to new conditions which can be physical or
behavioral
amphibian
An animal able to live both on land and in water
analyze
To study carefully
attract
To draw in by physical force
average
The typical, usual, or ordinary result of a set of data
balance
A device for weighing things
breathe
To take in and push out air
butterfly
An insect with four thin wings. Caterpillars change into butterflies.
camouflage
The disguising of people, animals, or things, to make them look like what is
around them
Celsius
The metric system for measuring temperature
centimeter
A unit of length in the metric system
characteristic
Showing a special feature or quality
classify
To put into groups or classes; sort
climate
The usual weather that occurs in a place, including the average temperature
and amounts of rain or wind
collect data
To gather information using tables and charts
compare
To look at two or more things to see how they are alike or different
conclusion
A decision made after careful thinking using evidence to support your opinion
conduct (an experiment)
To lead, guide, or direct an experiment
conserve
To use carefully, not to waste
cycle
A series of events that is regularly repeated in the same order
data
Information that is gathered during an experiment – facts and figures
decrease
To make or become less or smaller
describe
To use words to explain how something looks, feels, or acts
determine
To make a decision
diagram
A labeled drawing that shows how something works
dissolve
To mix thoroughly with a liquid
draw a conclusion
Analysis of results from an experiment
environment
Surroundings and conditions that effect natural processes
erode, erosion
To wear away or become worn
evaluate
To find out, judge, or estimate the value of
evidence
Facts or data that help find out the truth and support a conclusion
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 40 GRADE 1
experiment
A procedure that is carried out to investigate a scientific question
explain, explanation
To make clear or understandable
explore
To examine in order to discover
extinct
No longer in existence; an extinct animal or plant has died out
Fahrenheit
A measurement of temperature
fair test
A test that compares two or more things by keeping everything the same
except the thing being compared. A race is a fair test. Everyone starts at the
same place and at the same time and ends in the same place. The only thing
that is different is the speed of the runners.
findings
The results of a study or investigation
force
A force sets an object in motion by direct contact (a push or a pull) or indirect
contact (air, magnets, or gravity)
germinate
To sprout
gills
The organ of a fish that is used for taking oxygen from water
graduated cylinder
A cylindrical container used for measuring volume
gram
The metric unit for measuring mass
graph
A diagram used to show the relationship between things
gravity
A pulling force between two objects that causes smaller objects to move
towards the center of the Earth
habitat
The place where an animal or plant lives, such as a woods or a lake
hand lens
A tool used to magnify things
identify
To find out or tell exactly who a person is or what an object is
increase
To make or become greater or larger
insect
A tiny animal with six legs, three body parts (head, thorax, and abdomen), 2
antennae, and 2 eyes
investigate
To study something closely and in an organized way
kilogram
One thousand grams
layer
A single thickness or deposit of material
length
The distance from one end of something to the other
lens
A tool used to see things clearly
life cycle
All the changes a plant or animal goes through between its birth and its
death. The stages in the life of a plant. New plants come from older plants.
liter
A unit of volume i.e., capacity for liquids in the metric system
lungs
Organs used for breathing
magnifying glass
A tool used to enlarge objects
mammal
A warm blooded animal that has hair and a backbone; is warm-blooded, and
feeds their young milk
mass
The amount of matter in an object It is measure in grams.
materials
Supplies
metal
A substance that usually has a shiny surface, can be melted, and can conduct
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 41 GRADE 1
heat and electricity
metamorphosis
A complete change in appearance or form; complete transformation that
occurs when an insect or animal passes through separate stages of growth
and development
meter, meter stick
Basic unit of length in metric system, similar to a yard
milliliters
One thousandth of a liter
mineral
The ingredients that make up rocks
mixture
Something made by mixing
motion
The change in position of an object caused by a force
nutrients
Something that living things need to grow and stay healthy
object
Anything that is not alive the can be seen or touched
observe, observation
To use your senses to study something closely
opinion
A belief based on what one thinks or feels; not on actual facts
organism
Any living thing such as a plant or animal
oxygen
A colorless gas in the air that living things need to survive
pattern
Anything that repeats itself
perform an experiment
Conducting a test to prove something
photosynthesis
A process by which green plants use light energy to change carbon dioxide
and water into glucose and oxygen
position
The way in which something is placed or arranged
predict, prediction
To say what you think is going to happen; a guess based on what you know
so far
procedure
A set of specific steps that tells you how to do something
property
Something about an object that tells what it is
range
The extent to which something can vary
record (data)
Something written down to preserve facts or information
recycle
To treat materials that have been thrown away in order to use them again
reflect
To give back an image of
sand
Loose grains of worn rock
scale
Tool used to weigh things
scientific observation
To see and pay attention to an experiment in science
season
One of the four natural division of the year -- fall, winter, spring, summer
seed dispersal
The scattering of seeds
separate
To pull or take apart
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 42 GRADE 1
sequence
The order in which things occur
shadow
The dark figure cast on a surface by an object that is between the surface
and the light source
soil
The loose top layer of the Earth’s surface in which plant life can grow
sort
To put things in groups on the basis of a property, such as color, shape,
class, kind or size
speed
The condition of moving or acting rapidly
surface
The outermost layer
survive
To stay alive
temperature
Relative hotness or coldness as measured on a standard scale
texture
The fell of an object; for example, glass has a smooth texture, and sandpaper
has a rough texture
thermometer
A tool used to measure temperature
transparent
Light can pass through
weigh, weight
A measurement of how heavy something is; the pull of gravity on an object
(force that gravity exerts on a mass) Weight changes; mass doesn’t. An
astronaut in space has mass, but is weightless.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 43 GRADE 1
Science Grade 1 Books for the Library
Standards and Grade Level Expectations: 1.2.1, 1.2.2, 1.3.2
Dinosaurs and Prehistoric Animals (Pebbles Plus series) by Helen Frost Capstone Press 2005
First Encyclopedia of Dinosaurs and Prehistoric Life (Usborne Internet –Linked) by Sam Taplin Scholastic
2004
Dinosaurs by Richard Ferguson DK Publishing 2007
Is It a Living Thing? by Bobbie Kalman Crabtree 2008
Standard and Grade Level Expectation: 1.2.3
Ten Seeds by Ruth Brown Alfred Knopf 2001
Plants! (TFK Science Scoops series) by Brenda Iasevoli Harper Collins 2006
One Bean by Anne Rockwell Walker Publishing 1999
Standards and Grade Level Expectations: 1.3.1, 1.3.2, 1.3.3
A Ladybug’s Life by John Himmelman Children’s Press 1998
Helpful Ladybugs (No Backbone series) by Molly Smith Bearport 1974
First the Egg by Laura Seeger Roaring Brook Press 2007
Standard and Grade Level Expectation: 1.1.4
Forces and Motion (Fact Finders series) by Catherine Welch Capstone Press 2006
On the Move (Science Starters series) by Wendy Madgwick Raintree Steck-Vaughn 1999
Who Hops by Katie Davis Voyager Books Harcourt
Forces Around Us (It’s Science series) by Sally Hewitt Franklin Watts 1997
Standard and Grade Level Expectation: 1.1.6
Nothing Sticks Like a Shadow By Ann Tompert Houghton Mifflin 1984
Me and My Shadow (Read and Do Science series) by Melinda Lilly 2006
Shadows (Scholastic Science Readers) by Carolyn Otto Scholastic 2001.
The Biggest Shadow in the Zoo by Jack Kent Parents Magazine 1981
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 44 GRADE 1
Content Standards & Indicators
for Grade 2
MADISON PUBLIC SCHOOLS
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 47 GRADE 2
Course Description
ELEMENTARY SCHOOL
1. Course Title
Grade 2 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number: N/A
4. Program Contact Information
Name: Anita Rutlin
Title/Position: Assistant Superintendent
School: Central Office
Madison Town Campus
10 Campus Drive, P.O. Drawer 71
Madison, CT 06443
6. Grade Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites: None
11. Brief Course Description
Second grade science is taught in three units of study throughout the school year. Scientific inquiry,
literacy and numeracy are integrated throughout the units. Students are engaged in science instruction
through investigations. As part of the spiraling curriculum, aspects of life science, earth science, and
physical science are taught each year. A Nutrition Expedition is an interdisciplinary study of science and
health education. The physical science unit is Solids, Liquids and Gases. Life and earth sciences are
combined in a unit of Soil and Plants that helps student explore the relationship between soil and plants.
12. Course Goals
The second grade science program gives students the opportunity to explore topics and concepts through
investigations. Participating in this hands-on program helps student…
1. To foster a life long enjoyment of learning and the learning of science.
2. To observe science in the world around them.
3. To meet the grade level expectations of science standards for Connecticut Public Schools.
13. Course Outline
1. Properties of Matter: Solids, Liquids and Gases
2. Structure and Function & Changing Earth: Soil and Plants
3. Science and Technology in Society: Nutrition
14. Instructional Methods and/or Strategies
Individual and small group work
Interactive class instruction
Demonstrations and modeling
Guided inquiry activities and investigations
15. Assessment Methods and/or Tools
Quizzes and Unit assessments
Teacher observations
Performance tasks
Responses in student’s science journal to
investigations and class discussions
16. Assessment Criteria
Assessment of learning is based on the Madison curriculum and Connecticut standards with grade level
expectations for science. For investigations and projects, students are given direction and templates for
completing the work. Student scientists record their work in Science Journals. Observations, student responses
in science journals, and common unit assessments are employed to determine individual student
achievement.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 48 GRADE 2
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, to search out, describe, explain, and
predict natural phenomena.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing about
science.
Mathematics provides useful tools for the
description, analysis and presentation of scientific
data and ideas.
ESSENTIAL QUESTIONS
How do you make observations about
objects, organisms, and the environment?
How do you use simple measuring tools to
gather data and extend the senses?
How do you use observed patterns to make
predictions?
How do you use standard measuring tools to
collect data and nonstandard measures to
make comparisons?
How can physical properties be used to order
and sort objects and organisms?
How do you locate relevant science
information in printed resources?
How do bar graphs represent information?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Use the senses and simple measuring tools to
make observations and collect data.
Use standard tools to measure and describe
physical properties such as weight, length and
temperature and nonstandard measures to
estimate and compare the sizes of objects.
Count, order and sort objects by their properties.
Make predictions based on observed patterns.
Ask questions about objects, organisms and the
environment.
Read, write, listen and speak about observations of
the natural world.
Seek information in books, magazines and pictures
and present information in words and drawings.
Represent information in bar graphs.
FOSS Modules
Delta Science Modules
INSTRUCTIONAL STRATEGIES
Modeling during instruction
Guided inquiry activities and investigations
Guided reading
ASSESSMENT METHODS
Inquiry literacy questions
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 49 GRADE 2
LEARNING STRAND: Properties of Matter
- How does the structure of matter affect the properties and uses of materials?
Unit: Solids, Liquids and Gases
CT Standard 2.1 - Materials can be classified as solid, liquid or gas based on their observable properties.
ENDURING UNDERSTANDING
Solids tend to maintain their own shapes, while
liquids tend to assume the shapes of their
containers, and gases fully fill their containers.
ESSENTIAL QUESTIONS
What are the three states of matter?
How can we tell if matter is a solid, a liquid or a
gas?
How can you describe the differences between a
solid and a liquid, or a liquid and a gas?
How are solids different from liquids and gases?
How are solids, liquids, and gases represented in
our daily lives?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
All materials (matter) take up space. Matter can
be classified by whether it is in solid, liquid or
gas form. Each state of matter has unique
properties.
Solids are the only state of matter that keep
their own shape. A solid’s shape can only be
changed if a force is applied to it, such as
hammering, slicing or twisting. Solids can be
hard, soft, bouncy or stretchy.
Solids take up a certain amount of space
(volume); the volume does not change if the
solid is placed in different containers.
Liquids do not have their own shape; they go to
the bottom of a container and take on the shape
of the part of the container they occupy. Liquids
pour and flow from a higher point to a lower
point; some liquids flow faster than others.
Liquids have a definite volume. When a liquid is
poured into different containers, the shape of
the liquid may change, but the volume does not.
Gases do not have a definite shape; they take
on the shape of whatever container they
occupy. For example, the air in an inflated
balloon can be squeezed and reshaped.
Gases do not have a definite volume; they
spread out in all directions to fill any size
container, or they keep spreading in all
directions if there is no container. For example,
blowing even a small amount of air into a
balloon immediately fills the entire balloon; the
smell of baking bread eventually fills the entire
house and even outside.
FOSS: Solids and Liquids
Investigation 1: Solids
Part 1 - Introduce Solids
Part 2 – Sort Solid Objects
Part 3 – Construct with Solids
Investigation 2: Liquids
Part 1 – Liquids in Bottles
Part 2 – Proper
ties of Liquids
Part 3 – Liquid Level
Investigation 3: Bits and Pieces
Part 1 – Solids in Containers
Part 2 – Separating Soup Mix
Part 3 – Solids in Bottles
Part 4 – Separating Beads with Screens
Investigation 4: Solids and Liquids with Water
Part 1 – Solids and Water
Part 2 – Liquids and Water
Part 3 – Toothpaste Investigation
Dancing Raisins Experiment
http://www.fossweb.com
Literacy
related to Matter:
All About Solids, Liquids & Gases by Schlessinger
(Science Library)
Floating and Sinking by Ellen Sturm Niz
What is a Liquid? by Jennifer Boothroyd
What is a Solid? by Jennifer Boothroyd
What is a Gas? by Jennifer Boothroyd
Experiments with Solids, Liquids, and Gases
by Salvatore Tocci
Matter by Christine Webster
States of Matter by Fiona Bayrock
Change It!, Solids, Liquids, Gases and You by
By Adrienne Mason
Videos: All about Solids, Liquids & Gases; All about
Properties of Matter
High-touch high-tech program: What’s the Matter
A science notebook/journal
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 50 GRADE 2
Venn Diagram
INSTRUCTIONAL STRATEGIES
Keep Journal on Investigations & Activities
Read and discuss Matter related books
Generate a Venn diagram or ―T‖ chart to compare
the different properties of matter (liquid vs.
solids, gases vs. liquids, etc.)
ASSESSMENT METHODS
Teacher observations during investigations and
activities
Solids and Liquids assessment
Responses in science journal to investigations,
activities and class discussions
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
Compare and contrast the properties that
distinguish solids, liquids and gases.
Classify objects and materials according to their
state of matter.
Measure and compare the sizes of different solids.
Measure and compare the volume of a liquid
poured into different containers.
Design a fair test to compare the flow rates of
different liquids and granular solids.
CMT CORRELATION
Describe differences in the physical properties of
solids and liquids.
SCIENTIFIC LITERACY TERMINOLOGY: property, classify, matter, state of matter, solid, liquid, gas,
volume
KEY SCIENCE VOCABULARY
:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 51 GRADE 2
LEARNING STRANDS: Structure and Function and The Changing Earth
- How are organisms structured to ensure efficiency
and survival? How do materials cycle through the Earth's systems?
Unit: Soil and Plants
CT Standard 2.2 – Plants change their forms as part of their life cycles.
CT Standard 2.3 - Earth materials have varied physical properties which make them useful in different ways.
ENDURING UNDERSTANDINGS
Soils have different properties and
compositions that make them useful in
different ways.
Soils can be described by their color, texture
and capacity to retain water.
Soils support the growth of many kinds of
plants, including those in our food supply.
The life cycles of flowering plants include seed
germination, growth, flowering, pollination and
seed dispersal.
Flowering plants have a life cycle that involves
changes in growth and structure that ensures
production of new plants.
Other living things depend on plant
reproduction to supply the food they need.
ESSENTIAL QUESTIONS
What are the properties by which soils are
sorted?
What properties of soil are important for plant
growth?
How can you classify soils?
How can you describe the use of soil in plant
growth?
How can you describe the life cycle of a plant?
What are the conditions necessary for flowering
plants to grow?
How does the plant change during its life?
How can you describe the effects of light and
water on seed germination and plant growth?
How are plants connected with other living
things?
How do the properties of earth materials differ?
UNDERLYING CONDEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Soil is a mixture of pieces of rock (particles),
living and once living things (humus), water
and air. The components of soil can be
separated using sieves and settlement tests.
There are different types of soil that vary from
place to place. Soil properties can be observed
and compared. Soils can be classified by
properties such as color, particle size, or
amount of organic material (humus). Digging a
deep hole shows that soils are often found in
layers that have different colors and textures.
The size of the particles in soils gives the soil
its texture. Soils can be classified by how
they feel: Sandy soils feel gritty, silty soils
feel powdery, clay soils feel sticky, and soils
with small rocks feel rough and scratchy.
The broken rocks that make up soils can be
tiny (silt and clay), medium (sand), or large
(pebbles). Soils can be classified by the size
of their particles.
A soil’s texture affects how it packs together;
soils that pack together tightly hold less air
and water than soils that stay loosely packed.
There are different types of soil that vary from
place to place. Some soil types are suited for
supporting the weight of buildings and
highways; other soil types are suited for
planting food crops or forest growth.
FOSS-Pebbles, Sand, and Silt
Investigation 4: Soil Explorations Parts 1-3 and
Extensions
Literacy - Soil: Microlife that Lives in Soil by
Steve Parker; Soil by Adele Richardson
FOSS-New Plants
Investigation-1: Brassica Seeds: 1-3 & Extensions
Investigation-2: Grass and Grain Seeds: 1-3;
Extensions
Investigation-3: Stems: Parts 1-3 & Extensions
Investigation-4: Bulbs and Roots: 1-2 & Extensions
Literacy - Seed and plant: How a Seed Grows
by Helene J. Jordan, From Seed to Plant by Allan
Fowler, A Seed is Sleepy by Dianna Hutts Aston
& Sylvia Long, and The Tiny Seed by Eric Carle.
Video: Plant Life Cycle
Science Notebook/Journal
o Soil Study (AIMS)
o Which Soil Works Best?
o Look at Life in the Soil
o Make a Worm Farm
o What do Plants Need to Grow?
o The Seed Within
o Inside a Seed
o Stem Study
o Root Study
o Parts of a Flower
High-touch high-tech Programs: Give Me Dirt &
Smarty Plants
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 52 GRADE 2
GRADE LEVEL CONCEPT 2.3.b.
Many plants need soil to grow. Soil holds
water and nutrients that are taken in
(absorbed) by plant roots.
Soil is a habitat for many living things. Some
organisms live in the soil and others live on
the soil. Worms and other underground
animals create spaces for air, water and plant
roots to move through soil.
Plants we eat (―crops‖) grow in different soil
types. Plant height, root length, number of
leaves, and number of flowers can all be
affected by how much water, air and organic
material the soil holds.
To support the growth of different plants,
people can change the properties of soils by
adding nutrients (fertilizing), water (irrigating)
or air (tilling).
Flowering plants progress through a
sequenced life cycle. First, seeds sprout
(germinate), then seedlings grow into adult
plants with leaves and flowers. If the flowers
are pollinated, seeds develop that will grow
into new plants to continue the life cycle.
Roots, stems, leaves, flowers and seeds are
structures that develop during different stages
of the plant’s life cycle.
Seeds contain the beginnings of a new plant
(embryo) and the food (energy source) the
new plant needs to grow until it is mature
enough to produce its own food. Different
plant varieties produce seeds of different size,
color and shape.
Environmental conditions, such as
temperature, amount of light, amount of water
and type of soil, affect seed germination and
plant development.
A plant’s seed will grow into a new plant that
resembles but is not identical to the parent
plant or to other new plants. For example,
marigold plants produce marigold seeds that
grow into new marigold plants. Individual
marigolds, however, vary in height, number of
leaves, etc.
Seedlings are young plants that produce the
structures that will be needed by the plant to
survive in its environment. Roots and leaves
begin to grow and take in nutrients, water and
air; and the stem starts to grow towards
sunlight.
INSTRUCTIONAL STRATEGIES
ENGLISH/ LANGUAGE ARTS CONNECTIONS
-How can you make a new plant from an old
plant if you don’t have seeds?
-Farmer John planted 5 shiny blue seeds in his
garden. All of a sudden…
-A farmer is having trouble growing the corn on
his farm. The first field of corn plants started to
grow and then just died. He needs your help
before he plants the next field. Write and explain
4 things that you know about plants that you can
tell the farmer to help this corn grow corn cobs.
Draw and label a healthy corn plant.
-Flowers are beautiful and are everywhere.
There are many different colors, shapes, and
sizes of flowers located all over the world. A
citizen from Antarctica has come to your school
to visit for the week and one of his questions is
about flowers. He wants to know how a flower’s
life cycle works and why are flowers important to
people and insects.
Bobby the bee is flying from flower to flower in
search of pollen. From which part of the flower
will Bobby find the pollen, and what will he do
with it?
If you were given a class assignment to grow a
plant, what would you do to your soil to provide
your plant with the best living environment?
ASSESSMENT METHODS
Teacher observations during experiments or
activities
Soil and Plants
Science journal
Response to investigations and class discussions
in Science journal
Assessment Pre and Post study of seeds and
plants, ―Parts of a Flower"
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 53 GRADE 2
Adult plants form more leaves that help the
plant collect sunlight and air to make its
food. They produce flowers that are the
structures responsible for reproduction.
Flowers have structures that produce pollen,
attract pollinators and produce seeds that
can grow into new plants. Some flowers
have structures that develop into fruits,
berries or nuts that contain the seeds that
can grow into new plants.
Some seeds fall to the ground and germinate
close to the parent plant; other seeds are
carried (dispersed) by wind, animals, or
water to places far away. The structure of
the seed is related to the way it is dispersed.
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Use senses and simple tools (e.g., sieves and
beakers) to separate soil components such as rock
fragments, water, air, and plant remains.
Classify soils by properties such as color, particle
size (sand, silt or clay), or amount of organic
material (loam).
Explain the importance of soil to plants, animals
and people.
Evaluate the quality of different soils in terms of
observable presence of air, water, living things and
plant remains.
Conduct fair tests to investigate how different soil
types affect plant growth and write conclusions
supported by evidence.
Use senses and simple tools to observe and
describe the roots, stems, leaves, flowers and
seeds of various plants (including trees, vegetables
and grass.)
Use magnifiers to observe and diagram the parts of
a flower.
Describe the functions of roots, stems, leaves,
flowers and seeds in completing a plant’s life cycle.
Record observations and make conclusions about
the sequence of stages in a flowering plant’s life
cycle.
Compare and contrast how seeds of different plants
are adapted for dispersal by water, wind or
animals.
Conduct a fair test to explore factors that affect
seed germination and plant growth.
CMT CORRELATIONS
Describe the life cycles of flowering plants as they
grow from seeds, proceed through maturation and
produce new seeds.
Explore and describe the effects of light and water
on seed germination and plant growth.
Sort different soils by properties, such as particle
size, color and composition.
Relate the properties of different soils to their
capacity to retain water and support the growth of
certain plants.
SCIENTIFIC LITERACY TERMINOLOGY: life cycle, structures (body parts), seed, germinate,
reproduce, flower, pollen, pollinator, seed dispersal, soil, property, classify, mixture, particles, humus,
sand, silt, clay, texture, nutrients
KEY SCIENCE VOCABULARY
: alfalfa, alike, amount, different, brassica, bud, bulb, cutting, fertilizer,
grain, ingredient, carbon dioxide, sprout, structures, flower, mold, node, pollen, pollinator, potato eye,
sample, vermiculite
Humus
is plant material that has decayed or rotted.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 54 GRADE 2
MATERIALS USED FOR SOIL AND PLANTS UNIT:
Consumable
Located in Pebbles, Sand and Silt:
Potting soil
Paper plates
Self-seal freezer Ziploc bags - gallon size
9 oz. plastic cups
Plastic spoons
Located in New Plants:
Potting soil
16 oz. plastic cups
cotton balls
jumbo, clear straws
white removable labels 1 cm x 4.5 cm
bottle of liquid plant fertilizer
1 package alfalfa seeds
1 package brassica rapa seeds
1 package rye grass seeds
1 package wheat seeds
Non-consumable
Located in Pebbles, Sand, Silt:
Basins
Magnifying lenses
Screens
Metal spoons
Vials with caps
Bags of sand, gravel, pebbles
¼
liter containers
Located in New Plants:
Florescent bulbs
Lamp fixture
Planter trays
Basins
Bottle brush
Cup lids
25 ml vials with caps
BOOKS USED FOR Science Content Reading Soil and Plants Grade 2:
Flowers by Stone, Lynn M.
Fruit by Stone, Lynn M.
Seeds by Stone, Lynn M.
Stems by Stone, Lynn M.
Roots by Stone, Lynn M.
Leaves by Stone, Lynn M.
Fruits by Farndon, John
Stems by Farndon, John
Seeds by Farndon, John
Leaves by Farndon, John
Roots by Farndon, John
How do plants grow? by Stewart, Melissa
The Tiny Seed by Carle, Eric
Disgusting Plants by Miller, Connie Colwell
Microlife that Lives in Soil by Parker, Steve
Soil by Richardson, Adele
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 55 GRADE 2
LEARNING STRAND: Science and Technology in Society -
How do science and technology affect the quality of our lives?
Unit: Nutrition
CT Standard 2.4 - Human beings, like all other living things, have special nutritional needs for survival.
ENDURING UNDERSTANDINGS
The essential components of
balanced nutrition can be obtained
from plant and animal sources.
People eat different foods in order to
satisfy nutritional needs for
carbohydrates, proteins and fats.
ESSENTIAL QUESTIONS
How can you obtain the essential components of a balanced
nutrition?
Which different food groups meet our nutritional needs?
(What are the nutrition basics?)
Which sources are used to obtain the five food groups?
How can you describe which nutritional needs are necessary
for survival?
How can you describe how people in different cultures use
different food sources to meet their needs?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 2.4.a.
People need to eat a variety of foods
to get the energy and nutrients they
need to grow, move and stay
healthy. Foods are classified as
grains, fruits, vegetables, dairy,
meats and beans, and oils.
Some foods people eat come from
plants that grow wild or are planted
by farmers as crops. A fruit is the
ripened part of a flower; vegetables
are the roots, stems, leaves or
flowers of plants.
Some foods people eat come from
animals that are wild or are raised
on ranches. Meat, fish, dairy
products and eggs all come from
animals.
The types of crops that can grow in
an area depend on the climate and
soil. Some foods are grown and sold
by local farms, and some foods are
grown far away and transported to
local grocery stores.
GRADE LEVEL CONCEPT 2.4.b.
All people need the same basic
nutrients to grow, move and stay
healthy; different cultures satisfy
these needs by consuming different
foods.
The level of energy and nutrients
individuals need depends on their
age, gender and activity levels.
Most foods contain a combination of
nutrients. Labels on food packages
describe the nutrients contained in
Little D’s Nutrition Expedition Background Information,
Lessons and Materials
Little D’s Part 1: Why teach nutrition?
Little D’s Part 2: What are the nutrition basics?
Little D’s Part 3: How do you put the nutrition basics
together to eat a nutritious diet?
Little D’s Part 4: Special Nutrition Concerns for Children
Little D’s Nutrition Expedition Kit
Poster displaying the food groups (Little D, The Five-Food-
Group Dragon)
Literacy
about Nutrition
: The New Food Guide Pyramid –
Meat and Beans by Emily K. Green; The New Food Guide
Pyramid: Healthy Eating by Emily K. Green; Looking After
Myself, Health and Diet by Sally Hewitt; Eating Right,
Healthy Eating, Healthy Choices by Cathy Senker; The Edible
Pyramid Good Eating Every Day by Loreen Leedy; Good
Enough to Eat, A Kid’s Guide to Food and Nutrition by Lizzy
Rockwell
Books about Different Food in Different Cultures
: Meals
Around the World; Tony and the Pizza Champions by Tony
Gemignani; The Magic Pomegranate by Peninnah Schram;
One Hen: How One Small Loan Made a Big Difference by
Katie Milway; Let’s Eat! A comer! by Pat Mora; Hiromi’s
Hands by Lynne Barasch; The Have a Good Day Café by
Frances & Ginger Park; Grandma Lena’s Big Ol’ Turnip by
Denia Hester; Berry Magic by Teri Sloat; One Green Apple by
Eve Bunting
High touch high tech program:
Nutty Nutrients
Food package labels
Science notebook/journal
Venn Diagram
Extension Activities: Five Food Group Bingo; Brown Bagging
It; Food Group Concentration; Fishing for Foods Game
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 56 GRADE 2
the food and how much energy the
food provides (calories).
Breads, cereals, rice and pasta are
sources of carbohydrates, which
provide energy.
Meat, poultry, fish, beans, eggs and
nuts are sources of protein, which
keeps the body working properly.
Fruits and vegetables are sources of
vitamins and minerals, which keep
the body healthy.
Nuts, meats and fish are sources of
fats and oils, which provide energy.
INSTRUCTIONAL STRATEGIES
Little D’s Nutrition Expedition Activities: 1. Meet the Royal
Food Family, 2. Sir Milford and Lady Holly’s Milk Group, 3.
King Henry’s Meat Group 4. Princess Peapod’s Vegetable
Group, 5. Queen Anna Banana’s Fruit Group, 6. Prince
Waffle’s Grain Group, 7. We Need All Five, 8. The Dragon’s
Tail, 9. Smart Snacking Dragon, 10. Healthfully Ever After.
Demonstrate how to utilize the Science notebook /journal to
keep track of the student’s daily food intakes
Discuss and record how students could change their daily diet
to meet recommended nutritional needs
Create a Food Group Wall Dragon displaying the five food
groups. The students can add food to each food group
throughout the unit.
Research how other cultures use different food sources to
meet their nutritional needs. Create small groups and give
each group a country to study. The students will then
report on their country and share what they have learned.
Students will use a Venn diagram or ―T‖ chart to display
their information, in comparison to our culture. They may
also cut out various pictures of food from the internet for
display.
ASSESSMENT METHODS
Little D’s Nutrition Expedition Pre- and Post-Test
Responses to investigations and class discussions with Grade
Level Expectation Science Journal
Teacher observations of activities with Check Sheet
GRADE LEVEL EXPECTATIONS:
Assessments MUST measure the ability of students to:
Explain that food is a source of carbohydrates, protein and
fats — nutrients that animals and humans convert to energy
they use to stay alive.
Classify foods into groups based on their source and relate
common foods to the plant or animal from which they come.
Give examples of ways people can improve soil quality and
crop growth (e.g., irrigation, fertilizer, pest control).
Compare and contrast how different cultures meet needs for
basic nutrients by consuming various foods.
Evaluate the nutritional value of different foods by analyzing
package labels.
CMT CORRELATIONS
Identify the sources of common foods and classify them by
their basic food groups.
Describe how people in different cultures use different food
sources to meet their nutritional needs.
SCIENTIFIC LITERACY TERMINOLOGY: nutrient, crop,
grain, carbohydrate, protein, dairy, fats, oils,
energy
KEY SCIENCE VOCABULARY
:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 57 GRADE 2
SCIENTIFIC LITERACY TERMINOLOGY: GRADE 2
This list is intended as a guide for teachers. While not exhaustive, it includes vocabulary that should be used, as
appropriate, by teachers and students during everyday classroom discourse. It is not intended for student memorization.
absorb
To take in, soak up
adaptation (adapt)
The process of changing to new conditions
aluminum
A light weight, silver-white metal
analyze
To study carefully
atmosphere
The gas that surrounds a body in space
atom
A tiny building block of matter
attract
To draw by exciting interest or emotion
average
The typical, usual, or ordinary
balance
A device for weighing things; a tool for comparing mass
breathe
To take in and push out air
butterfly
An insect with four thin wings Caterpillars change into butterflies.
cactus
One of many kinds of plants, with thick, spiny stems without leaves
camouflage
The disguising of people, animals, or things, to make them look like what is
around them
centimeter
A unit of length in the metric system
characteristic
Showing a special feature or quality
classify
To put into groups or classes; sort
clay
A firm kind of earth made up of small particles
climate
The usual weather that occurs in a place, including the average temperature
and amounts of rain or wind
collect data
To gather information
compare
Discuss similarities and differences of items
conclusion
A decision made after careful thinking
condensation
The action of changing from a gas into a liquid
conduct (an
experiment)
To lead, guide, or direct an experiment
conserve
To use carefully, not to waste
cork
A light, spongy bark
cycle
A series of events that is regularly repeated in the same order
data
Facts or figures that are collected during an experiment
decrease
To make or become less or smaller
describe
To use words to explain how something looks, feels or acts
determine
To make a decision
diagram
A drawing that shows how something works
dissolve
To mix thoroughly To change from a solid to a liquid
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 58 GRADE 2
draw a conclusion
To make a decision after careful thinking
droplets
A small quantity of liquid
environment
Surroundings and conditions that effect natural processes
erode, erosion
To wear away or become warn
evaluate
To find out, judge, or estimate the value of
evaporate
To change into a vapor or gas
evaporation
The action of a liquid changing into a gas
evidence
Facts or signs that help one find out the truth
experiment
A procedure that is carried out to investigate a scientific question
explain, explanation
To make clear or understandable
explore
To go into or travel through an unknown place
Fahrenheit
A measurement of temperature
findings
The results of a study or investigation
flexible
Objects that can bend
float
To be at the top of liquid or air
force
Something as a pull or push that changes the speed of direction in which
something moves
freeze
To change from a liquid to a solid
gas
Matter that spreads to fill the space it’s in; matter that does not have its own
shape or volume
germinate
To begin to grow or sprout
graph
A diagram used to show the relationship between things
gravity
The natural force that causes smaller objects to move towards the center of the
Earth
hand lens
A tool used to magnify things
humid, humidity
Having a large amount of water or water vapor in the air
identify
Who a person is, or what a thing is
increase
To make or become greater or larger
insect
A tiny animal with six legs
investigate
To study something closely and in an organized way
layer
A single thickness, a coating, or sheet of material covering a surface
length
The distance of a thing measured from one end to the other
lens
A tool used to see things clearly
life cycle
The stages in the life of a plant New plants come from older plants.
liquid
Matter that flows and takes the shape of the container it is in
liter
A unit of capacity for liquids in the metric system
lungs
Organs used for breathing
magnifying glass
A tool used to enlarge objects
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 59 GRADE 2
mammal
A warm blooded animal that has hair and a backbone
materials
Tools need to do a certain job
matter
Anything that takes up space and has mass
melt
To change from a solid to a liquid
metal
A substance that is usually shiny and hard It is found underground.
meter, meter stick
A basic unit of length
mineral
The ingredients that make up rocks
mirror
A piece of glass you can see yourself in
mixture
Something made of two or more kinds of matter mixed together
motion
Any change in the position of an object
nutrients
Something that living things need to grow and stay healthy
object
Anything that is not alive that people can see or touch
observe, observation
To see and pay attention to; watch
opinion
A belief based on what one thinks or feels; not on actual facts
organism
Any living thing such as a plant or animal
oxygen
A colorless gas in the air that living things need to survive
pattern
Anything that repeats itself
pebble
A small rock
perform an experiment
Conducting a test to prove something
photosynthesis
A process by which green plants use light energy to change carbon dioxide and
water into glucose and oxygen
pitch
The degree of a slant
pluck
To remove by pulling off or out
predict, prediction
A guess based on what you know so far about you think is going to happen
procedure
A set of specific steps that tells you how to do something
property
Things we know about objects by looking at them or feeling them
range
The extent to which something can vary
record (data)
Something written down to preserve facts or information
recycle
To treat materials that have been thrown away in order to use them again
reflect
To give back an image of
result
Something that happens because of something else; consequence
reuse
To use again
sand
Loose grains of warn rock
scale
Tool used to weigh things
scientific observation
To see and pay attention to an experiment in science
seed dispersal
The scattering of seeds
separate
To pull or take apart
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 60 GRADE 2
sequence
The order in which things occur
silt
Fine particles of Earth found at the bottom of lakes and rivers
sink (float)
To go down or cause to go down under the surface
soil
The loose top layer of the Earth’s surface in which plant life can grow
solid
Matter that has its own shape and volume
solution
Mixture with two or more kinds of matter mixed evenly
sort
To arrange things on the basis of property such as color, kind, or size
speed
The condition of moving or acting rapidly
state of matter
One way matter exists -- solid, liquid, or gas
stopwatch
A device used for measuring time
surface
The outermost layer
survive
To stay alive
telescope
A tool used to make objects appear closer
temperature
Relative hotness or coldness as measured on a standard scale
texture
The look or feel of a surface
thermometer
A tool used to measure temperature
transparent
Light can pass through
weigh, weight
The measure of how heavy something is or the gravitational force on
Content Standards & Indicators
for Grade 3
MADISON PUBLIC SCHOOLS
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 63 GRADE 3
Course Description
ELEMENTARY SCHOOL
1. Course Title
Grade 3 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
N/A
4. Program Contact Information
Name: Anita Rutlin
Title/Position: Assistant Superintendent
School: Central Office
Madison Town Campus
10 Campus Drive, P.O. Drawer 71
Madison, CT 06443
6. Grade Level: 3
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites : N/A
11. Brief Course Description
Third grade science is taught in units of study that have scientific inquiry, literacy and numeracy integrated
throughout the units. Students are engaged in science instruction through activities and investigations. As part
of the spiraling curriculum, aspects of life science, earth science, and physical science are taught each year.
The life science unit is Adaptations to Habitats; the physical science unit is Properties of Matter; and the earth
science unit examines Water in relation to energy in earth’s systems.
12. Course Goals
The third grade science program gives students the opportunity to explore topics and concepts through
investigations. Participating in this hands-on program helps students:
1. To appreciate the concepts of science as part of their larger world via interdisciplinary activities.
2. To foster a life long enjoyment of learning and the learning of science.
3. To develop a growing appreciation for science as part of their evolving education.
4. To ensure students meet the science standards and grade level expectations for Connecticut Public Schools.
13. Course Outline
1. Properties of Matter / Science and Technology in Society: States of Matter and Soggy Paper
2. Heredity and Evolution: Adaptations to Habitats – Plants and Animals
3. Energy in Earth’s Systems: Water
14. Instructional Methods and/or Strategies
Individual and small group work
Full class instruction and discussions
Modeling and demonstrations
Guided inquiry activities and investigations
15. Assessment Methods and/or Tools
Quizzes and Unit assessments
Embedded task and performance assessment in class activities and investigations
16. Assessment Criteria
Assessments are based on the Madison curriculum and Connecticut standards with grade level expectations for
science. For investigations and projects, students are given a rubric or checklist of grading criteria before
doing the work. Observations, student science journals, and common unit assessments are employed to
determine individual student achievement.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 64 GRADE 3
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and
coordinated attempt, to search out,
describe, explain, and predict natural
phenomena.
Scientific literacy includes speaking,
listening, presenting, interpreting, reading
and writing about science.
Mathematics provides useful tools for the
description, analysis and presentation of
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you develop testable questions?
How do you make observations about objects,
organisms, and the environment?
How do you design and conduct simple
investigations employing simple equipment and
measuring tools to gather data and extend the
senses?
How do you use data to construct reasonable
explanations?
How do you use mathematics to analyze, interpret,
and present data?
How do you analyze, critique and communicate
investigations using words, graphs and drawings?
How do you locate relevant science information
when searching the Web?
KNOWLEDGE & LEARNING
The student will …
INSTRUCTIONAL SUPPORT MATERIALS
Identify questions that can be answered
through scientific investigation.
Make predictions.
Design a simple investigation to test a
question.
Use tools and techniques that are
appropriate for the design of the
investigation for making observations and
gathering data.
Accurately collect and record appropriate
data.
Use mathematical operations to analyze
and interpret data.
Interpret and create appropriate graphs to
present relationships between variables.
Develop logical conclusions that are based
on the analysis of experimental data.
Report findings and conclusions in various
formats using relevant vocabulary and
supporting evidence.
FOSS and Delta Science Modules
http://www.exploratorium.edu/ifi/workshops/fundamentals/index.html
INSTRUCTIONAL STRATEGIES
Modeling during instruction
Demonstrations
Guided inquiry activities and investigations
Guided internet research
Performance tasks
ASSESSMENT METHODS
CMT embedded task
―Soggy Paper‖
Selected Responses
Constructed Responses
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 65 GRADE 3
LEARNING STRAND:
Unit: Properties of Matter
- How does the structure of matter affect the properties and uses of materials?
CT Standard 3.1 – Materials have properties that can be identified and described through the use of simple tests.
CT Standard 3.4 – Earth materials provide resources for all living things, but these resources are limited and should be conserved.
ENDURING UNDERSTANDINGS
Heating and cooling cause changes in some of
the properties of materials.
Decisions made by individuals can impact the
global supply of many resources.
ESSENTIAL QUESTIONS
What are the three States of Matter?
How does heating and cooling cause changes to
the property of a material?
How do Reducing, Reusing, and Recycling
conserve earth materials?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Material has properties that are directly
observable; examples include its state of matter
or its size, shape, color or texture. Other
properties can only be observed by doing
something to the material (simple tests).
Materials can be sorted and classified based on
their testable properties.
Some materials dissolve (disappear) when
mixed in water; others accumulate on the top
or the bottom of the container. The
temperature of water can affect whether, and
at what rate, materials dissolve in it.
Some materials, such as sponges, papers and
fabrics, absorb water better than others.
Some materials float when placed in water (or
other liquids such as cooking oil or maple
syrup); others sink to the bottom of the
container.
Some materials conduct heat better than
others. Materials that are poor heat conductors
are useful for keeping things cold or hot.
The physical properties of a material can be
changed, but the material remains the same.
For example, a block of wood can be cut,
sanded or painted, but it is still wood.
Heating and cooling cause materials to change
from one state of matter to another and back
again. Adding heat can cause solids to melt into
liquids (for example, chocolate, ice cream,
butter or wax); removing heat (cooling) can
cause liquids to harden into solids (for example,
hot candle wax hardens as it cools).
Adding heat can cause water to boil and
evaporate into a gas in the air (for example,
steam rises from heated water); removing heat
(cooling) can cause water vapor to condense
into liquid water (for example, warm steam
hitting a cold mirror). Water outdoors or in an
open container evaporates without boiling (for
Delta Science Module: States of Matter
FOSS: Water
―Soggy Paper‖ CT Embedded Task
Books on Matter and Ecology
INSTRUCTIONAL STRATEGIES
DSM Activity 1 – What Is a Solid?
DSM Activity 2 – What Is a Liquid?
DSM Activity 3 – What Is a Gas?
DSM Activity 4 – Melting Ice
DSM Activity 5 – Hurry Up or Slow Down
DSM Activity 7 – Measuring Melting Points
FOSS Water Investigation 2 Part 2 Sinking and
Floating
Liquid Levels
Displacement Experiment
―Soggy Paper‖ CT Science Curriculum
Magnets
Read Ecology books, such as The Lorax by Dr.
Seuss; Reduce, Reuse and Recycle by Elizabeth
Wallace; What if We Ran Out of Fossil Fuels
?
by
Kimberly N. Miller; Water: a Vital Resource;
Waste not Want Not - Recycling
Posters promoting Earth Day
Assemblies/Programs: high-touch high-tech:
―Global Fever‖, ―Green Machine‖
Read books about Magnets: Magnets by Angela
Royston; Magnetic and Non-Magnetic by Angela
Royston
ASSESSMENT METHODS
Responses to investigations and activities in the
student’s Science Journal
Responses to assessment questions in student’s
Science Journal
―Soggy Paper‖ embedded, performance task
Properties of Matter Assessment
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 66 GRADE 3
example, puddles, ponds, fish tanks, etc.)
Water may exist as a solid, liquid or gas,
depending on its temperature. If water is
turned into ice and then the ice is allowed to
melt, the amount of water is the same as it was
before freezing.
Liquid water becomes solid water (ice) when its
temperature cools to 0 degrees Celsius (32
degrees Fahrenheit). Warming ice to a
temperature above 0 degrees Celsius (above 32
degrees Fahrenheit) causes it to melt into liquid
water.
The supply of many natural resources such as
fossil fuels, metals, fresh water and fertile soil is
limited; once they are used up or contaminated
they are difficult or impossible to replace.
Human actions can affect the survival of plants
and animals. The products of the fuels people
burn affect the quality of the air. Waste and
chemicals from factories, farms, lawns and
streets affect the quality of the water and soil.
Humans can extend the use of some natural
resources by reducing the amounts they use
(for example, driving less to reduce the amount
of gasoline used; turning off faucets not in use).
Humans can extend the use of some natural
resources by recycling, or collecting used
materials and processing them into new
materials (for example, collecting waste paper
or plastic bottles and making them into new
products).
Humans can extend the use of some natural
resources by reusing products instead of buying
new ones (for example, washing containers that
food is packaged in and using them again to
store different foods or objects).
Humans can extend the use of some natural
resources by replacing what they use (for
example, planting new trees to replace those
that are cut for lumber or paper; purifying dirty
water from storm drains and discharging clean
water back into a river).
Humans can extend the use of some natural
resources by reducing the amounts they use
(for example, driving less to reduce the amount
of gasoline used; turning off faucets when not
in use).
Some natural resources are useful to people in
their raw form (for example, fresh water, soil or
air); other natural resources must be modified
to meet human needs (for example, petroleum
must be extracted from rocks and refined into
gasoline, heating oil or plastics; wood from
Compare and contrast the properties of solids,
liquids and gases.
Demonstrate that solids, liquid and gases are all
forms of matter that take up space and have
weight.
Carry out simple tests to determine if materials
dissolve, sink or float in water and conduct heat
or attract to magnets.
Classify materials based on their observable
properties, including state of matter.
Design and conduct fair tests to investigate the
absorbency of different papers, write conclusions
based on evidence, and analyze why similar
investigations might produce different results.
Explain the role of heating and cooling in
changing matter from one state to another during
freezing, melting, evaporation and condensation.
Describe ways people use earth materials, such
as fossil fuels, trees, water, soils and rocks as
natural resources to improve their lives.
Summarize nonfiction text to explain how humans
use technology to obtain energy and make
materials from natural resources.
Explain advantages and disadvantages of
renewable and nonrenewable energy sources that
can be used for making electricity, fueling cars or
heating homes.
Design and conduct experiments to evaluate the
effectiveness of different insulating materials for
keeping a substance warm or cold (i.e.,
conducting heat).
Use mathematics to estimate, measure and graph
the quantity of a natural resource (e.g., water,
paper) used by an individual (or group) in a
certain time period.
Distinguish among reducing, reusing, recycling
and replacing as conservation techniques.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 67 GRADE 3
trees must be proceeded to make paper).
Earth materials that occur in nature include
rocks, minerals, soils, water and the gases of
the atmosphere. Earth materials are natural
resources that provide us with things we need
to live, including food, clothing, water, air,
shelter, land and energy.
CMT CORRELATIONS
Sort and classify materials based on properties
such as dissolving in water, sinking and floating,
conducting heat, and attracting to magnets.
Describe the effect of heating on the melting,
evaporation, condensation and freezing of water.
Describe how earth materials can be conserved
by reducing the quantities used, and by reusing
and recycling materials rather than discarding
them.
SCIENTIFIC LITERACY TERMINOLOGY: physical property, state of matter, solid, liquid, gas, water
vapor, dissolve, absorb, conduct, attract, melt, freeze, boil, evaporate, condense, displacement, natural
resources, recycle, reuse, replace, reduce
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 68 GRADE 3
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 69 GRADE 3
LEARNING STRAND: Heredity and Evolution
- What processes are responsible for life's unity and diversity?
Unit: Adaptations to Habitats
CT Standard 3.2 – Organisms can survive and reproduce only in environments that meet their basic needs.
ENDURING UNDERSTANDING
Plants and animals have structures and behaviors that
help them survive in different environments.
ESSENTIAL QUESTIONS
How do plant and animal physical and behavioral
adaptations allow them to survive in certain
environments?
How do plant and animal physical and behavioral
adaptations allow them to obtain food?
How do plant and animal physical and behavioral
adaptations allow them to protect themselves
from predators?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Plants have structural adaptations that allow them
to survive and thrive in certain biomes. Animals
have physical and/or behavioral adaptations that
allow them to survive in certain environments.
Adaptations are passed from parents to offspring.
Individuals that happen to be bigger, stronger or
faster can have an advantage over others of the
same species for finding food and mates.
Animals have behavioral and structural adaptations
for getting food. Structural adaptations include
things such as specialized teeth for tearing meat or
grinding grasses; specialized beaks for cracking
seeds, snatching insects, tearing meat or spearing
fish; sharp claws for grasping; keen sense of smell,
or long, sticky tongues for reaching food.
Behavioral adaptations include actions such as
following herds of prey animals, spinning webs or
stalking.
Animals have behavioral and structural adaptations
for protection from predators. Some animals have
camouflage that allows them to stay concealed by
blending in with their surroundings; some animals
look like other animals to avoid being eaten.
Structural adaptations include things such as sharp
quills, hard shells or antlers. Behavioral
adaptations include actions such as staying
absolutely still, producing a bad odor, appearing or
sounding scary, or fleeing.
Animals have behavioral and structural adaptations
for surviving harsh environmental conditions.
Animals that live in cold climates have insulating
body coverings such as blubber, down or thick
undercoats that keep them warm. Animals that live
in hot climates keep cool by releasing heat from
big ears or by panting, or by living underground.
Some animals survive seasonal changes by slowing
down body functions (hibernating in dens, tunnels
FOSS Science Stories
Structures of Life
Science DVDs and Library books
Who’s Home in the Biome
(AIMS)
Non-Fiction Read & Write
Animals & Habitats
―Plant Parts‖ & ―Making Food‖ handouts
New Plant Discovery
(AIMS)
Zoobook website
INSTRUCTIONAL STRATEGIES
Literacy - Library Books to read to students:
What Do You Know About Animal Adaptations? –
20 Questions: Science, Suzanne Slade, PowerKids Press
Cold, Colder, Coldest, Animals That Adapt to Cold
Weather by Michael Dahl, Picture Window Books
Hot, Hotter, Hottest, Animals That Adapt to Great
Heat by Michael Dahl, Picture Window Books
Animals with No Eyes: Cave Adaptation Kelly R. Barnhill
Surviving Death Valley: Desert Adaptation by Pamela Dell
Monsters of the Deep: Deep Sea Adaptation
Kelly Regan Barnhill, Capstone Press
How Do Animals Adapt? The Science of Living
Things
Bobbie Kalman, Crabtree Publishing Company
Super Survivors, Amazing Nature by Tim Knight
How Plants Survive by Kathleen V. Kudlinski
Seeds, Stems, and Stamens, The Ways Plants Fit
Into Their World Susan E. Goodman, Millbrook Press
Science Assemblies/Programs: high-touch
high-tech: Biome Sweet Home
ASSESSMENT METHODS
Teacher observation during experiments and
activities
Biome in a Box
Project
Animals & Habitats
booklet
Animal Adaptations book
Responses to investigations in student’s
Science Journal
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 70 GRADE 3
or mud) or moving to more favorable conditions
(migrating).
Plants have adaptations for getting the sunlight
they need to survive. Examples include growing or
facing toward sunlight and sending out chutes or
tendrils to get taller than neighboring plants.
Plants have adaptations for protection from
predators. Examples include spines, thorns and
toxins e.g., poison ivy.
Plants have adaptations for surviving in different
environmental conditions. Examples include
dropping leaves in winter when sunlight and water
are limited, having needle-shaped leaves that shed
snow, or surviving drought by storing water in
thick stems.
Adaptations to Habitats Assessment
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
Compare and contrast the external features
and behaviors that enable different animals
and plants (including those that are extinct) to
get food, water, and sunlight; find mates; and
be protected in specific land and water
habitats.
Explain how behaviors such as hibernation,
dormancy and migration give species
advantages for surviving unfavorable
environmental conditions.
Give examples of ways animals benefit from
camouflage.
Evaluate whether an adaptation gives a plant
or animal a survival advantage in a given
environment.
Design a model of an organism whose
adaptations give it an advantage in a specific
environment.
CMT CORRELATIONS
Describe how different plants and animals are
adapted to obtain air, water, food and
protection in specific land habitats.
Describe how different plants and animals are
adapted to obtain air, water, food and
protection in water habitats.
SCIENTIFIC LITERACY TERMINOLOGY: adaptation, advantage, camouflage, hibernation, migration
KEY SCIENCE VOCABULARY
:
Dormancy
inactive or not growing
Environment
The surroundings of a plant or animal
Habitat
where an organism naturally lives
Nutrient
a material used by a living organism to help it grow and develop
Predator
an animal that hunts and catches other animals for food
Survival
continues to live in unusual conditions
Thrive
to grow fast and stay healthy
Tundra
is cold frozen land most of the year
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 71 GRADE 3
LEARNING STRAND: Energy in Earth’s System
- How do external and internal sources of energy affect the Earth's systems?
Unit: Water
CT Standard 4.3 – Water has a major role in shaping the earth’s surface.
ENDURING UNDERSTANDINGS
Water circulates through the Earth's crust, oceans
and atmosphere.
Heating and cooling cause changes in some of the
properties of materials.
Scientists use equipment and measuring tools to
collect data about factors that affect erosion.
ESSENTIAL QUESTIONS
How does the sun’s energy impact the water
cycle?
Why does water not accumulate on the
surface of the earth after it rains?
What are the ways in which water circulates
throughout the earth?
What factors contribute to the movement of
water throughout the earth?
How does moving water affect erosion and
river formation?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Water is continuously moving between the Earth’s
surface and the atmosphere in a process called the
water cycle. Water evaporates from the surface of
the earth, rises into the air and cools, condenses,
collects in clouds, and falls again to the surface as
precipitation. The energy that causes the water
cycle comes from the sun.
Most precipitation that falls to Earth goes directly
into oceans. Some precipitation falls on land and
accumulates in lakes and ponds or is absorbed by
soil.
Rain or snowmelt in high elevations flows downhill
in streams that collect in lower elevations to form
rivers flowing downhill to an ocean.
Water moving across the earth in streams and
rivers pushes soil and breaks down pieces of rock
in a process called erosion. The moving water
carries away rock and soil from some areas and
deposits them in other areas, creating new land
forms or changing the course of a stream or river.
The amount of erosion in an area, and the type of
earth material that is moved, are affected by the
amount of moving water, by the speed of the
moving water, and by how much vegetation covers
the area.
Rivers carve out valleys as they move between
mountains or hills. The speed of the river’s flow
depends on the slope of the land. The speed of the
river’s flow affects the shape of the river’s course
(straight or meandering), the shape of the valleys
it carves (u-shaped or v-shaped) and the amount
of earth material that is pushed along or left
behind in floodplains and deltas.
Water moving in ocean waves carries sand, shells
and debris away from some coastal areas and
FOSS: Water
Water Investigation 3 Water Vapor Parts 1 & 2
Evaporation Part 4 Condensation
Water Investigation 4 Water Works
Part 1 Water in Earth Materials
Literacy:
Which Way Does It Go?
(Water
Lesson 1-2); Water Dance (1997) and Cloud
Dance (2000) by Thomas Locker Cracking
Up A Story about Erosion by Bailey and Lilly
INSTRUCTIONAL STRATEGIES
FOSS Science Stories:
The Water Cycle
&
Water Cycle Wheel
FOSS Science Stories:
Evaporation and
Condensation
Follow a River with A River’s Run activity
Erosion & Dirtmeister’s Science Lab
Science Assemblies/Programs: high-touch
high-tech:
Water Water Everywhere
CT Eli Whitney Water Center Activities
Connecticut River Museum
ASSESSMENT METHODS
Investigations and Activities in student’s
Science Journal including FOSS Investigation
Response Sheets
A River’s Run project
Dirtmeister’s Erosion Experiment & Report
Water Assessment
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Describe the role of the sun’s energy (i.e., heating
and cooling) in the continuous cycling of water
between the earth and the atmosphere through
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 72 GRADE 3
deposits them in new areas, changing the shape of
the coastline.
Erosion is constantly reshaping the earth’s land
surface. Sometimes the effects of erosion are
immediate (for example, a flash flood or a
hurricane) and sometimes the effects of erosion
take a long time (for example, the changing course
of a river or the carving of the Grand Canyon).
evaporation, condensation and precipitation.
Use models to demonstrate that topography
causes precipitation landing on earth to move
in streams and rivers from higher to lower
elevations.
Design and conduct simple investigations to
determine how moving water (flowing downhill
or in ocean waves) causes changes to the
land, the coastline or the course of a stream or
river.
Pose testable questions and employ simple
equipment and measuring tools to collect data
about factors that affect erosion (e.g., type of
earth material in an area, volume of moving
water, slope of land, vegetation coverage).
Present evidence to support a scientific claim
about the relationship between the amount
and speed of moving water and the size of
earth materials moved (e.g., silt, pebbles,
boulders).
CMT CORRELATIONS
Describe how the sun’s energy impacts the
water cycle.
Describe the role of water in erosion and river
formation.
SCIENTIFIC LITERACY TERMINOLOGY: water cycle, evaporate, condense, condensation, precipitation,
water vapor, erosion, valley, floodplain, delta
KEY SCIENCE VOCABULARY
: landform, deposition, oxbow lake, rivulets, runoff, sediment, tributaries

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 73 GRADE 3
Science – Grade 3
DVDs
Science Clips for Children: Characteristics of Plants
Schlessinger Media – A Division of Library Video Company
18 minutes, Closed Caption
Plant Life for Children: All About Plant Adaptation
Schlessiinger Science Library – A Division of Library Video Company
23 minutes, Closed Caption
Animal Life for Children: All About Animal Adaptations
Schlessiinger Science Library – A Division of Library Video Company
23 minutes, Closed Caption
Properties of Matter, Part 1
School Videos
SchoolMedia, Inc. – Physical Science Grades K-4
20 minutes
Physical Science for Children: All About Properties of Matter
Schlessiinger Science Library – A Division of Library Video Company
23 minutes, Closed Caption
Phases of Matter – Bill Nye the Science Guy
Disney Educational Productions
26 minutes, Closed Caption

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 74 GRADE 3
Science – Grade 3
Nonfiction and Fiction Books for Science from the Library
Water Science Unit
Which Way Does It Go?
(Water Lesson 1-2)
Water Dance
Thomas Locker
Cloud Dance
Thomas Locker
Cracking Up: A Story about Erosion
Jacqui Bailey and Matthew Lilly
Properties of Matter Science Unit
Magnets, My World of Science
Angela Royston, Heinemann Library a Division of Pearson
Magnetic and Nonmagnetic, My World of Science
Angela Royston, Heinemann Library a Division of Pearson
Floating and Sinking, Our Physical World
Ellen Sturm Niz, First Facts by Capstone Press
Matter, Discovering Science
Rebecca Hunter, Raintree Steck-Vaughn Publishers, Harcourt
Matter, Solids, Liquids, and Gases, Science All Around Me
Mir Tamim Ansary, Rigby Interactive Library, Rigby Education
What is a Gas?
Jennifer Boothroyd, First Step Nonfiction
What is a Liquid?
Jennifer Boothroyd, First Step Nonfiction
What is a Solid?
Jennifer Boothroyd, First Step Nonfiction
Experiments with Solids, Liquids, and Gases
Salvatore Tocci, Children’s Press, Division of Scholastic
Solids, Liquids, Gases, Simply Science
Charnan Simon, Compass Point Books
Solids, Liquids, and Gases, Science Around Us
Darlene R. Stille, The Child’s World
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 75 GRADE 3
Everyday Physical Science Experiments with Liquids, Science Surprises
Amy French Merrill, The Rosen Publishing Group’s PowerKids Press
Everyday Physical Science Experiments with Solids, Science Surprises
Amy French Merrill, The Rosen Publishing Group’s PowerKids Press
Solids, Liquids, and Gases, Starting with Science
Ontario Science Centre, Kids Can Press
The Lorax
Dr. Suess
Reduce, Reuse and Recycle
Elizabeth Wallace
What if We Ran Out of Fossil Fuels?
Kimberly N. Miller
Adaptations in Habitats Science Unit
What Do You Know About Animal Adaptations? – 20 Questions: Science
Suzanne Slade, PowerKids Press
Cold, Colder, Coldest, Animals That Adapt to Cold Weather
Michael Dahl, Picture Window Books
Hot, Hotter, Hottest, Animals That Adapt to Great Heat
Michael Dahl, Picture Window Books
Animals with No Eyes, Cave Adaptation
Kelly Regan Barnhill, Capstone Press
Surviving Death Valley, Desert Adaptation
Pamela Dell, Capstone Press
Monsters of the Deep, Deep Sea Adaptation
Kelly Regan Barnhill, Capstone Press
How Do Animals Adapt? The Science of Living Things
Bobbie Kalman, Crabtree Publishing Company
Super Survivors, Amazing Nature
Tim Knight, Heinemann Library, Division of Reed Elsevier
How Plants Survive
Kathleen V. Kudlinski, Chelsea House Publishers
Seeds, Stems, and Stamens, The Ways Plants Fit Into Their World
Susan E. Goodman, Millbrook Press, Lerner Publishing Group
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 76 GRADE 3
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 77 GRADE 3
SCIENTIFIC LITERACY TERMINOLOGY: Grade 3
This list is intended as a guide for teachers. While not exhaustive, it includes vocabulary that should be
used, as appropriate, by teachers and students during everyday classroom discourse. It is not intended for
student memorization.
absorb
To take in, soak up
adaptation (adapt)
The process of changing to new conditions
aluminum
A light weight, silver-white metal
analyze
To study carefully
atmosphere
The gas that surrounds a body in space
attract
To draw by exciting interest or emotion
average
The typical, usual, or ordinary
balance
A device for weighing things
beaker
A cylindrical container used in a laboratory for liquids
biome
A biome is an area on Earth that has similar geography, climate, plants, and
animals. There are land biomes and water biomes.
breathe
To take in and push out air
camouflage
The disguising of people, animals, or things, to make them look like what is
around them
Celsius
The metric system for measuring temperature
centimeter
A unit of length in the metric system
characteristic
Showing a special feature or quality
classify
To put into groups or classes; sort
clay
A firm kind of earth made up of small particles
climate
The usual weather that occurs in a place, including the average temperature
and amounts of rain or wind
collect data
To gather information
compare
Discuss similarities and differences of items
conclusion
A decision made after careful thinking
condense
To make more dense or compact
condensation
The action of changing from a gas into a liquid
conduct (an experiment)
To lead, guide, or direct an experiment
conserve
To use carefully, not to waste
cork
A light, spongy bark
cycle
A series of events that is regularly repeated in the same order
data
Facts or figures collected during an experiment
decrease
To make or become less or smaller
describe
To use words to explain how something looks, feels or acts
determine
To make a decision

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 78 GRADE 3
diagram
A drawing that shows how something works
displacement
To remove from the normal position or location
dissolve
To mix thoroughly To change from a solid to a liquid
draw a conclusion
Analysis of results from an experiment
droplets
A small quantity of liquid
drought
The lack of water
ecosystem
A balance of plants and animals living and working together in a self
contained environment
environment
Surroundings and conditions that effect natural processes
erode, erosion
To wear away or become worn
evaporate
To change into a vapor or gas
evaporation
The action of a liquid changing into a gas
evidence
Facts or signs that help one find out the truth
experiment
A procedure that is carried out to investigate a scientific question
explain, explanation
To make clear or understandable
explore
To go into a travel through an unknown place
extinct
No longer alive anywhere on earth
Fahrenheit
A measurement of temperature
fair test
An experiment where the variables are held consistent
findings
The results of a study or investigation
float
To be at the top of liquid or air
freeze
To change from a liquid to a solid
gas
Matter that spreads to fill the space it’s in
germinate
To begin to grow or sprout
graduated cylinder
A cylindrical container used for measuring volume
gram
The metric unit for measuring mass
graph
A diagram used to show the relationship between things
gravity
The natural force that causes smaller objects to move towards the center of
the Earth
habitat
Where an organism naturally lives
hand lens
A tool used to magnify things
hibernate, hibernation
Extended period of sleep
humid, humidity
Having a large amount of water or water vapor in the air
hypothesis
Question or purpose for conducting an experiment
identify
To find out or tell exactly who or what an object is
increase
To make or become greater or larger
insect
A tiny animal with six legs
insulate, insulator
Type of material used for keeping objects warm or cold

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 79 GRADE 3
investigate
To study something closely and in an organized way
kilogram
One thousand grams
layer
A single thickness, a coating, or sheet of material covering a surface
length
The distance of a thing measured from one end to the other
lens
A tool used to see things clearly
life cycle
The stages in the life of a plant New plants come from older plants
liquid
Matter that flows and takes the shape of the container it is in
liter
A unit of capacity for liquids in the metric system
magnifier
To make larger
magnifying glass
A tool used to enlarge objects
mass
The amount of matter in an object
materials
Supplies
melt
Physical change of states from solid to liquid
meter, meter stick
A metric unit of linear measure
migrate, migration
To move or change location
milliliters
One thousandth of a liter
mineral
The ingredients that make up rocks
mixture
Something made by mixing
natural resources
Any substance that comes from the earth
nutrients
Something that living things need to grow and stay healthy
object
Anything that is not alive that people that people can see or touch
observe, observation
To see and pay attention to; watch
offspring
The result of animals reproducing (babies)
opinion
A belief based on what one thinks or feels; not on actual facts
organism
Any living thing such a as a plant or animal
oxygen
A colorless gas in the air that living things need to survive
pattern
Anything that repeats itself
perform an experiment
Conducting a test to prove something
photosynthesis
A process by which green plants use light energy to change carbon dioxide
and water into glucose and oxygen
precipitation
Any form of water falling from a cloud
predict, prediction
A guess based on what you know so far about what you think is going to happen
procedure
Sequence of steps to perform an experiment
process
The scientific method
property
A characteristic of a material something that you can observe such as color,
smell, and taste
range
The extent to which something can vary
record (data)
Something written down to preserve facts or information
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 80 GRADE 3
recycle
To treat materials that have been thrown away in order to use them again
reflect
To give back an image of
reproduce
To produce new plants or animals
result
Something that happens because of something else; consequence
reuse
To use again
sand
Loose grains of worn rock
scale
Tool used to weigh things
scientific observation
To see and pay attention to an experiment in science
seed dispersal
The scattering of seeds
separate
To pull or take apart
sequence
The order in which things occur
sink (float)
To go down or cause to go down under the surface
soil
The loose top layer of the Earth’s surface in which plant life can grow
solid
Matter that holds its own shape
sort
To arrange according to property such as color, class, shape, size
state of matter
Solid, liquid, or gas.
surface
The outermost layer
survive
To stay alive
temperature
Relative hotness or coldness as measured on a standard scale
tension(surface)
The skin like surface on the water that pulls it together into the smallest
possible volume
testable
Able to test
texture
The look or feel of a surface
thermometer
A tool used to measure temperature
transparent
Light can pass through
volume
How much space matter takes up
water cycle
The sequence of condensation and evaporation of water on earth causing
clouds and rain and other forms of precipitation
weigh, weight
The measure of how heavy something is or of the gravitational force on an object
Content Standards & Indicators
for Grade 4
MADISON PUBLIC SCHOOLS
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 83 GRADE 4
Course Description
ELEMENTARY SCHOOL
1. Course Title
Grade 4 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
N/A
4. Program Contact Information
Name: Anita Rutlin
Title/Position: Assistant Superintendent
School: Central Office
Madison Town Campus
10 Campus Drive, P.O. Drawer 71
Madison, CT 06443
6. Grade Level: 4
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites: None
11. Brief Course Description
Fourth grade science is taught in units of study throughout the school year. Students are engaged in science class
through activities and investigations. As part of the spiraling curriculum, aspects of life science, earth science, and
physical science are taught each year. The life science component is integrated within the Wetlands unit. The earth
science unit is Earth Materials. The physical science units include Magnetism and Electricity and the Physics of Sound.
12. Course Goals
The fourth grade science program gives students the opportunity to explore topics and concepts through investigations.
Participating in this hands-on program helps students:
1. To appreciate the concepts of science as part of their larger world via interdisciplinary activities.
2. To foster a life long enjoyment of learning and the learning of science.
3. To develop a growing appreciation for science as part of their evolving education.
4. To ensure students meet the science standards and grade level expectations for Connecticut Public Schools.
13. Course Outline
1. Core Scientific Inquiry, Literacy and Numeracy
2. Energy Transfer and Transformations: Physics of Sound
3. Matter and Energy in Ecosystems: Wetlands
4. The Changing Earth: Earth Materials
5. Science and Technology in Society: Magnetism and Electricity
14. Instructional Methods and/or Strategies
Individual and small group work
Full class instruction and discussions
Modeling
Guided inquiry activities and investigations
15. Assessment Methods and/or Tools
Quizzes and Unit assessments
Embedded task and assessments in class activities and investigations
16. Assessment Criteria
The common assessments are based on the Madison curriculum and Connecticut standards and grade level expectations
for science. For authentic assessments and projects, students are given a rubric or checklist of grading criteria before
doing the work. A variety of assessment tools are employed to get the most accurate understanding of individual
student achievement possible.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 84 GRADE 4
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, to search out, describe, explain, and
predict natural phenomena.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing about
science.
Mathematics provides useful tools for the
description, analysis and presentation of scientific
data and ideas.
ESSENTIAL QUESTIONS
How do you develop testable questions?
How do you make observations about
objects, organisms, and the environment?
How do you design and conduct simple
investigations employing simple equipment
and measuring tools to gather data and
extend the senses?
How do you use data to construct
reasonable explanations?
How do you use mathematics to analyze,
interpret, and present data?
How do you analyze, critique and
communicate investigations using words,
graphs and drawings?
How do you locate relevant science
information when searching the Web?
KNOWLEDGE & LEARNING
The student will …
INSTRUCTIONAL SUPPORT MATERIALS
Identify questions that can be answered through
scientific investigation.
Make predictions.
Design a simple investigation to test a question.
Use tools and techniques that are appropriate for
the design of the investigation for making
observations and gathering data.
Accurately collect and record appropriate data.
Use mathematical operations to analyze and
interpret data.
Interpret and create appropriate graphs to present
relationships between variables.
Develop logical conclusions that are based on the
analysis of experimental data.
Report findings and conclusions in various formats
using relevant vocabulary and supporting evidence.
FOSS and Delta Science Modules
INSTRUCTIONAL STRATEGIES
Modeling during instruction
Guided-inquiry activities and investigations
Guided internet research
Performance tasks
ASSESSMENT METHODS
Connecticut Framework embedded task ―Go
with the Flow‖
Research projects/activities
Inquiry literacy questions
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 85 GRADE 4
LEARNING STRAND: Energy Transfer and Transformations
- What is role of energy in our world?
Unit: Physics of Sound
CT Standard 5.1 - Sound and light are forms of energy.
ENDURING UNDERSTANDING
Sound is a form of energy that is produced by the
vibration of objects and is transmitted by the vibration
of air and objects.
ESSENTIAL QUESTIONS
What are the properties of sound that make
them identifiable?
How are sounds made?
How are volume and pitch different?
How does the length affect the rate of
vibration and therefore the pitch?
How does tension affect the rate of vibration
and therefore the pitch?
What materials and variables affect how you
hear sound?
How does sound travel?
How is sound affected when it travels
through the three states of matter?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
There are a variety of sounds in our environment.
Sounds have characteristics --loudness, pitch and
quality, timbre, that allow them to be identified.
For sound to occur, there must be a vibrating
object, a material through which the vibrations are
transferred e.g., air or water, and a receiver e.g.,
an ear, to perceive the sound.
Objects can be caused to vibrate by actions i.e.,
striking, strumming, bowing, plucking or blowing.
Sounds can vary in loudness, volume. Volume is
affected by the strength of the force causing the
vibration. For example, striking a drum forcefully or
gently produces sounds with different volumes.
Sounds can have a high or low tone, pitch. Pitch
depends on the speed of the vibration. Objects
that vibrate quickly have a high pitch, while those
that vibrate slowly have a low pitch.
Pitch is affected by characteristics such as the
shape, length, tension or thickness of the vibrating
material e.g., the vibrating material may be a
string, a glass, a wire or a drum.
Sound travels (is transmitted) through materials
by causing them to vibrate. Sound is not
transmitted if there are no materials to vibrate.
Solids, liquids and gases (air) transmit sound
differently.
Sounds can be reflected or absorbed, depending
on the properties of the material it hits.
Sound tends to bounce off smooth, hard surfaces,
producing an echo; sound tends to be absorbed by
soft, porous surfaces, producing a muffled sound.
FOSS – ―Physics of Sound‖
STOMP Curriculum Guide
Literacy: Adventures in Sound with Max Axiom,
Super Scientist by Emily Sohn
High touch high tech:
Vibes
INSTRUCTIONAL STRATEGIES
FOSS Investigation 1 Dropping In
Part 1 Drop Challenge
Part 3 Sound and Vibrations & STOMP ―Salt Voice
Prints‖
FOSS Investigation 2 Good Vibrations
Part 1 Vibration and Pitch
Part 2 Length and Pitch & Exploratorium ―Sound
Sandwich‖
Part 3 Tension and Pitch
FOSS Investigation 3 How Sound Travels
STOMP Lesson 6 ―How Sounds Get Where They’re
Going‖
Part 1: Sounds through Air and Water
Part 2 Sounds Through Solids
FOSS Investigation 4
Part 1 Sound Challenges
STOMP Lesson 3 ―Amplifying Sounds‖
ASSESSMENT METHODS
Responses to Investigations and Assessment
Questions in student’s Science Journal:
-Response Sheet: Dropping In
-Science Journal: How are sounds made? What
happens to make a sound louder or quieter?
Describe in words and draw a picture.
-Response Sheet: Good Vibrations
-Science Journal: What happens to the pitch and
vibrations when the length of the sound source
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 86 GRADE 4
changes?
Science Journal: What happens to the pitch when
the tension on a sound source changes? What
happens to the pitch when different thicknesses of
string or rubber bands are plucked?
Sounds through Air Investigation Student Sheet
No.13
Sounds Through Water Investigation Student
Sheet No. 14
Sounds Through Solids Investigation Student
Sheet No. 16
Assessment: Rubber-Band Instrument
Science Journal: Which types of materials reflect
sound well? Which types of materials absorb
sound? How could you tell?
Sound Assessment
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
Generalize that vibrating objects produce
sound if the vibrations are transferred from
the object through another material (e.g., air,
solid, and liquid).
Demonstrate how the loudness, pitch and
quality/timbre of sound can be varied.
Design and conduct investigations to
determine factors that affect pitch.
Describe the properties of materials that
reflect or absorb sound.
Analyze properties of materials that cause
sound to be reflected or absorbed, then apply
findings to design a device that reflects or
absorbs sound.
Construct simple musical instruments (e.g.,
rubber band guitars, drums, etc.) that
produce sounds with various pitches, volume
and timbres.
CMT CORRELATIONS
Describe the factors that affect the pitch and
loudness of sound produced by vibrating
objects.
Describe how sound is transmitted, reflected
and/or absorbed by different materials.
SCIENTIFIC LITERACY TERMINOLOGY: vibration, volume, pitch, transmit, reflect, absorb
KEY SCIENCE VOCABULARY
: amplitude, amplification, frequency, transfer, intensity, quality, resonant
frequencies, nodes, waves
Volume: loudness or softness
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 87 GRADE 4
LEARNING STRAND: Matter and Energy in Ecosystems
- How do matter and energy flow through ecosystems?
Unit: Wetlands
CT Standard 4.2 - All organisms depend on the living and nonliving features of the environment for survival.
ENDURING UNDERSTANDING
When the environment changes, some organisms
survive and reproduce and others die or move to
new locations.
ESSENTIAL QUESTIONS
What are the characteristics of wetlands and why
are they important to wildlife and humans?
How do temperature, soil, and wildlife vary in
different locations moving from the ocean beach to
the salt marsh?
What physical and behavioral characteristics of
wildlife such as beavers, birds, fiddler crabs, and
snails allow them to thrive in wetland habitats?
What are the structures of plants such as cattails,
quillwort, and glasswort that help them thrive in
wetland habitats?
What are the components of a salt marsh food
chain and what are the steps in this cycle?
What are the components of a food web in a salt
marsh and how are they interconnected?
How does habitat loss and degradation affect
populations of migrating water birds?
How are the lives of the plants and animals in the
salt marsh affected by the changing tides, and how
are these organisms equipped to live in this
dynamic ecosystem?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Living and nonliving things interact in land
and water environments called ecosystems.
Every ecosystem has certain conditions
(abiotic factors) and a variety of living things
(organisms) that are adapted for survival in
those conditions. Abiotic factors include the
quality and amount of air, sunlight, water and
soil, as well as the terrain and climate.
Organisms depend on other organisms and on
the nonliving things in an ecosystem to meet
their basic needs for food, water and
protection.
Plants use energy from the sun to produce
their own food from air and water. The type
of soil, amount of water and temperature
range in an area determine the plants that
grow there.
Animals that live in an area get their energy
and nutrients either directly or indirectly from
plants that grow there; herbivores consume
only plants, carnivores consume animals,
and omnivores consume both animals and
plants. Decomposers consume plant and
animal waste and remains, returning
Project WILD Aquatic K-12 Curriculum and Activity
Guide
WOW! The Wonders of Wetlands, An Educator’s
Guide
Department of Environmental Protection guided
Field Studies
Project Learning Tree Activity Guide
Literacy: Weird Friends by Jose Aruego & Ariane
Dewey. Gulliver Books; Marvels in the Muck by
Doug Wechsler; Animal Survivors of the Wetlands
by Barbara Somervill; Marshes and Swamps by
Phillip Johansson; Butternut Hollow Pond by Brian
Heinz; Saving Oceans and Wetlands by Jen Green
Oil Spill! by Melvin Berger & Paul Mirocha
INSTRUCTIONAL STRATEGIES
Field Study (Fall and Spring) at Hammonasset State
Park in wetlands environment
Fall Field Study Activities
:
- Marsh Munchers, Aquatic WILD, p.34
- Hot Foot, Adapted from VA: Your Backyard
Classroom
- Marsh March, Adapted from VA: Your
Backyard Classroom
- Wetlands Weirdos (Fiddler Crabs/Glasswort)
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 88 GRADE 4
nutrients to the soil where they are used
again by plants.
Some of the sun’s energy is transferred from
one organism to another when a plant or
animal is consumed by another animal. A
food chain is a simple model that illustrates
the passage of energy from one organism to
another. Food webs are more realistic
models that show the varied energy-passing
relationships among plants and animals in an
ecosystem.
Environments are always changing. Some
changes occur naturally; examples include
disease outbreaks, violent storms, forest fires
sparked by lightning. Other changes are
caused by human activity; examples include
establishing conservation areas, passing laws
to control pollution, clearing forests for
agriculture or construction, applying
chemicals to lawns and crops, burning fossil
fuels, etc.
Changes in an environment are sometimes
beneficial to organisms and sometimes
harmful. For example, a newly created beaver
pond provides habitat that attracts frogs and
raccoons to an area; but trees, earthworms
and moles are no longer able to survive in the
area.
When environments change, some organisms
can accommodate the change by eating
different foods or finding different shelters
e.g., hawks nest on city buildings and
consume pigeons and rats. Those organisms
that can no longer meet their basic needs die
or move to new locations.
Spring Field Study Activities
:
- Wetlands Metaphors, Aquatic WILD
(Refresher)
- Just Ducky, Flying WILD
- Basics of Birding
- Bird Behavior Scavenger Hunt, Flying WILD
- Migration Headache, Aquatic WILD, p. 15
Classroom Instructional Activities
:
- ―Wetland Metaphors,‖ Project WILD Aquatic p.39
- ―Marsh Market‖ part II: ―What’s for Lunch?‖ WOW:
Wonders of Wetlands p.111
-―Marsh Munchers‖ Project WILD Aquatic p.34
-Crash: A Tale of Two Species‖ video thirteen WNET
NY cpb
-―Marsh Market‖ part I: ―Make a ―Living‖ Wetland
Food Web‖ WOW: Wonders of Wetlands p.109
- ―Salt Marsh Players‖, Project WET p.99
- ―Hydropoly‖ WOW: Wonders of Wetlands p.260
- ―Bill Nye: Wetlands‖ Video
ASSESSMENT METHODS
Performance assignments are designed to evaluate
student participation and comprehension, including
posters, models, webs, and chains.
Response to assessment questions in student’s
Science Notebooks
Life in a Salt Marsh
project
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Give examples of ways that living and nonliving
things are interdependent within an ecosystem.
Draw diagrams showing how the sun’s energy
enters and is transferred from producers to
consumers in a local land or aquatic food chain.
Design and conduct simple investigations to record
interactions among producers, consumers,
herbivores, carnivores, omnivores and decomposers
in an ecosystem.
Analyze food webs to describe how energy is
transferred from plants to various animals in an
ecosystem.
Distinguish between naturally occurring changes in
ecosystems and those caused by human activity.
Predict the effect an environmental change, such as
drought or forest destruction, might have on the
community of living things.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 89 GRADE 4
CMT CORRELATIONS
Describe how animals, directly or indirectly, depend
on plants to provide the food and energy they need
in order to grow and survive.
Describe how natural phenomena and some human
activities may cause changes to habitats and their
inhabitants.
SCIENTIFIC LITERACY TERMINOLOGY: ecosystem, habitat, organism, abiotic factors, nutrients,
producer, consumer, herbivore, carnivore, omnivore, decomposer, food chain, food web
KEY SCIENCE VOCABULARY: tertiary consumer, secondary consumer, primary consumer, primary
producer, hydrology, marsh, wetland
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 90 GRADE 4
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 91 GRADE 4
LEARNING STRAND: The Changing Earth
- How do materials cycle through the Earth's systems?
Unit: Earth Materials
CT Standard 3.3 - Earth materials have different physical and chemical properties.
ENDURING UNDERSTANDING
Rocks and minerals have properties that may be
identified through observation and testing; these
properties determine how earth materials are used.
ESSENTIAL QUESTIONS
How is inquiry used to investigate our
environment?
How do rocks and minerals cycle through our
environment?
What are the three types of rocks and how are
they formed?
What properties can we use to describe and
classify rocks?
What properties can we use to identify
minerals?
What are the similarities and differences
between rocks and minerals?
How can you use different tools to determine
the hardness a rock?
Based on specific properties, what are the best
uses for different rocks and minerals?
What can you conclude when one mineral can
scratch another?
What kind of tests can be conducted to
determine what minerals are inside a rock?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Earth is mainly made of rock. Rocks on the earth’s
surface are constantly being broken down into
smaller and smaller pieces, from mountains to
boulders, stones, pebbles and small particles that
make up soil.
Rocks can be sorted based on properties, such as
shape, size, color, weight or texture.
Properties of rocks can be used to identify the
conditions under which they were formed.
Igneous rocks are formed when melted rock
cools, hardens and forms crystals. Melted rock
that cools slowly inside a volcano forms large
crystals as it cools. Melted rock that cools rapidly
on the earth’s surface forms small crystals (or
none at all).
Sedimentary rocks are formed underwater when
small particles of sand, mud, silt or ancient
shells/skeletons settle to the bottom in layers that
are buried and cemented together over a long
period of time. They often have visible layers or
fossils.
Metamorphic rocks are formed when igneous or
sedimentary rocks are reheated and cooled or
pressed into new forms. They often have bands,
streaks or clumps of materials.
FOSS – ―Earth Materials‖
Sedimentary Rocks, Igneous Rocks,
Metamorphic Rocks, The Rock Cycle
Bill Nye The Science Guy ―Rocks & Minerals‖
The Pebble in My Pocket by Meredith Hooper
Rocks in His Head by Carol Otis Hurst
Everybody Needs a Rock by Byrd Baylor
INSTRUCTIONAL STRATEGIES
FOSS Investigation 1 Mock Rocks
Part 1 Investigating Mock Rocks
Part 2 Taking Rocks Apart
Part 3 Observing Crystals
FOSS Investigation 2 Scratch Test
Part 1: Observing Minerals
Part 2: Testing for Hardness
Part 3: Observing Crystals (second activity)
FOSS Investigation 3 Calcite Quest
Part 1: Detecting Calcite
Part 2: Looking for More Evidence
FOSS Science Stories: Earth Materials ―Where
Do Rocks Come From?‖ Investigation #4 p.34
Browse websites listed in Pacing Guide
Sedimentary Rocks
Igneous Rocks
Metamorphic Rocks
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 92 GRADE 4
Rock properties make them useful for different
purposes. Rocks that can be cut into regular
shapes are useful for buildings and statues; rocks
that crumble easily are useful for making mixtures
such as concrete and sheetrock.
All rocks are made of materials called minerals
that have properties that may be identified by
testing. Mineral properties include color, odor,
streak, luster, hardness and magnetism.
Minerals are used in many ways, depending on
their properties. For example, gold is a mineral
that is easily shaped to make jewelry; talc is a
mineral that breaks into tiny grains useful for
making powders.
Earth materials that occur in nature include rocks,
minerals, soils, water and gases of the
atmosphere. Earth materials are natural
resources that provide us with things we need to
live, including food, clothing, water, air, shelter,
land and energy.
Some natural materials are useful to people in
their raw form e.g., fresh water, soil or air. Other
natural resources must be modified to meet
human needs e.g., petroleum must be extracted
from rocks and refined into gasoline, heating oil
or plastics; wood from trees must be processed to
make paper.
The Rock Cycle
Literacy: Rocks in His Head by Carol Otis Hurst
FOSS Investigation 4 Take It for Granite
(optional)
Interactive Site: Learning Zone : Rock Cycle
http://www.oum.ox.ac.uk/thezone/rocks/cycle/index.htm#
map
Interactive Rock Cycle Animation (optional)
www.classzone.com/books/earth_science/terc/conte
nt/investigations/es0602/es0602page02.cfm
Make rock candy (optional guided lesson) This
activity takes several days to see the desired
results and if chosen needs to be done early in
the unit. The lesson takes one period but
observations are done for 3-10 days.
Fieldtrip of CT Geology (Joe Oslander)
Take students on a bus tour of various rock
formations:
-Great Eastern Border Fault on Route 79 –
sedimentary & metamorphic rocks
-Lake Quonnipaug (North Madison) – sedimentary
& igneous rocks
ASSESSMENT METHODS
Teacher observations
Rock information organizer for each type of
rock – igneous, sedimentary, and metamorphic
Maintain responses to investigations and
discussions in student’s science notebook
Rocks and Minerals Unit Assessment
Constructed Response- What are the three
types of rocks and how are they formed?
Constructed Response- Describe the process in
which earth materials change.
Constructed Response- Draw conclusions and
defend the best uses for several (3 or more)
rock properties.
- Mock Rocks Response Sheet (No. 11)
- Scratch Test Response Sheet (No. 15)
- Mineral Properties Response Sheet (No. 14)
- Calcite Quest Response Sheet (No.17)
- Performance Assessment Scratch Test (No.7)
- Performance Assessment Vinegar Test (No.8)
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Differentiate between rocks and minerals.
Use the senses and simple measuring tools to
gather data about various rocks and classify
them based on observable properties (e.g.,
shape, size, color, weight, visible markings).
Conduct simple tests to determine properties of
different minerals (e.g., color, odor, streak,
luster, hardness, magnetism), organize data in
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 93 GRADE 4
a table, and use the data and other resources
to identify unknown mineral specimens.
Summarize nonfiction text to compare and
contrast the conditions under which igneous,
metamorphic and sedimentary rocks are
formed.
Observe and analyze rock properties (e.g.,
crystal size or layers) to infer the conditions
under which the rock was formed.
Evaluate the usefulness of different rock types
for specific applications (e.g., construction,
countertops, statues or monuments).
CMT CORRELATIONS
Describe the physical properties of rocks and
relate them to their potential uses.
Relate the properties of rocks to the possible
environmental conditions during their
formation.
SCIENTIFIC LITERACY TERMINOLOGY: property, classify, texture, igneous, sedimentary,
metamorphic, fossil, crystal, mineral, natural resources
KEY SCIENCE VOCABULARY
: magma, extrusive, intrusive, sediment, streaks, luster, magnetism
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 94 GRADE 4
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 95 GRADE 4
LEARNING STRAND: Energy Transfer and Transformations
- What is the role of energy in our world?
Unit: Electricity and Magnetism
CT Standard 4.4 – Electrical and magnetic energy can be transferred and transformed.
ENDURING UNDERSTANDINGS
Electricity in circuits can be transformed into light,
heat, sound and magnetic effects.
Magnets can make objects move without direct
contact between the object and the magnet.
ESSENTIAL QUESTIONS
What kind of materials do magnets attract?
What happens when you bring two or more
magnets together?
How do magnets interact with other objects?
Does an iron object have to touch a magnet to
become a temporary magnet?
Does magnetic force go through all materials?
How do you get electricity from a source to a
receiver?
How does electricity flow through a circuit?
What does a switch do in a circuit?
How do batteries and wires conduct electricity
to light a bulb and make a motor run?
What types of materials are conductors of
electricity and what materials are insulators?
How do you make a magnet that turns on and
off? (combine understanding of electricity and
magnetism)
How can the strength of an electromagnet be
changed?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 4.4.b.
Magnets pull on (
attract
) objects made of iron or
that have iron in them. Materials can be identified
using magnets, and mixtures of materials can be
separated using magnets. (CT 3.1 Standard –
Some materials are attracted to magnets.
Magnetic materials contain iron.)
Some areas of a magnet have stronger magnetic
attraction than other areas.
Magnets can pull (attract) or push (repel) other
magnets.
The ends of a magnet are called
poles
. A magnet’s
poles are often referred to as
north
and
south
.
When the north pole of one magnet is placed near
the north pole of another magnet, they repel each
other; when the south pole of one magnet is
placed near the south pole of another magnet,
they repel each other; when the north pole of one
magnet is placed near the south pole of another
magnet, they attract each other.
A magnet’s push or pull can cause a magnetic
object or another magnet to move without direct
contact. The strength of a magnet’s attractive
force can be measured by recording the number or
mass of the objects it attracts or the distance
FOSS ―Magnetism and Electricity‖
www.fossweb.com
Delta Science Content Reader
Electricity and
Magnetism
School visit by Connecticut Light and Power
including "Electrical Safety World" booklets
and lesson plans.
INSTRUCTIONAL STRATEGIES
FOSS Investigation 1-The Force
Part 1:Investigating Magnets & Materials Steps 9-22
Sheet #3 Magnetic Observations; Science Journal
FOSS Investigation 2 – Making Connections
Part 1: Lighting a Bulb
Part 2: Making a Motor Run
Part 3: Finding Conductors and Insulators; Science Journal
Sheet #10
FOSS Investigation 3 – Advanced Connections
Part 1: Building Series Circuits
FOSS Investigation 4 – Current Attractions
Part 1 Building an Electromagnet
Part 2 Changing Number of Winds Sheet #18
―Winding Electromagnets‖
Delta Science Content Reader:
Electricity and
Magnetism
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 96 GRADE 4
across which it attracts objects.
When a magnet, or a magnetized object such as a
compass needle, is allowed to swing freely, its
ends will point toward the earth’s magnetic north
and south poles.
Magnets and electromagnets have many uses in
everyday life. Examples may include paper clip
containers, refrigerator door seals, shower curtain
weights, or a compass.
GRADE LEVEL CONDEPT 4.4.a.
Electric current flows (
is transferred
) from an
energy source (
battery)
through a continuous loop
(
circuit
) and back to the source. A complete circuit
(
closed circuit
) forms a closed loop that allows
electric current to flow; an incomplete circuit (
open
circuit
) has a break in the loop that prevents the
flow of electric current.
Complete circuits can be made by connecting
wires, batteries and bulbs in certain sequences.
Circuits are completed only when certain parts of a
battery, a bulb or a wire are touching (
making
contact
). Circuit diagrams show the relative
positions of batteries, bulbs and wires in complete
circuits.
Conductors
are materials that allow electric current
to flow through them in an electric circuit. An open
circuit can be completed by inserting a conductive
material. If a bulb stays lit when an object is added
to an electric circuit, the material is a conductor.
Insulators are materials that do not allow electric
current to flow through them in an electric circuit.
If a bulb does not stay lit when an object is added
to an electric circuit, the material is an insulator.
Conductors can be tested to compare how easily
they allow electricity to flow through them.
Electrical energy is changed (
transformed
) into
light and heat energy as it passes through a bulb
in a circuit. Electrical energy can be transformed
into sound energy as it passes through a bell or a
radio in a circuit.
Adding batteries or bulbs to a circuit can produce
observable changes.
Electricity flowing through an electrical circuit
produces magnetic effects in the wires. The
electromagnet can be turned on and off, and its
strength can be varied and measured.
ASSESSMENT METHODS
Responses to Assessment Questions in student’s
Science Journal
Science Journal: Sheet #7 ―The Flow of Electricity‖
Science Journal: Sheet #9
Science Journal: Sheet #10
Science Journal: Sheet #16 ―Response Sheet Circuit
Design‖
Science Journal: Sheet #18 ―Winding
Electromagnets‖
Delta Science Content Reader
Electricity and
Magnetism
Booklet Test
Curriculum Embedded Performance Task, Content
Standard 4.4, Go With the Flow (mandatory)
Magnetism and Electricity Assessment
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
Construct complete (closed) and incomplete (open)
series circuits in which electrical energy is
transformed into heat, light, sound and/or motion
energy.
Draw labeled diagrams of complete and incomplete
circuits and explain necessary components and how
components must be arranged to make a complete
circuit.
Predict whether diagrammed circuit configurations
will light a bulb.
Develop a method for testing conductivity and
analyze data to generalize that metals are good
electrical conductors and nonmetals are not.
Observe magnetic effects associated with electricity
and investigate factors that affect the strength of
an electromagnet.
Describe materials that are attracted by magnets.
Design procedures to move objects and separate
mixtures of solids by using magnets.
Investigate how magnets react with other magnets
and analyze findings to identify patterns in the
interactions between north and south poles of
magnets.
Give examples of uses of magnets (e.g., motors,
generators, household devices).
CMT CORRELATIONS
Describe how batteries and wires can transfer
energy to light a bulb.
Explain how simple electrical circuits can be
used to determine which materials conduct
electricity.
Describe the properties of magnets, and how
they can be used to identify and separate
mixtures of solid materials.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 97 GRADE 4
SCIENTIFIC LITERACY TERMINOLOGY: magnet, attract, repel, iron, pole, force, electric current,
energy source, battery, contact, complete (closed) circuit, incomplete (open) circuit, conduct, insulate,
insulator
KEY SCIENCE VOCABULARY
: electric circuit, contact, circuit, insulation, filament, Fahnstock clip
conductor: through which current electricity passes easily
electromagnet: a temporary magnet made when electric current flows through a wire coil wrapped
around an iron or steel core
generator: a device that uses motion to produce electric current
magnetic field: the area around a magnet where its force to attract metals acts
parallel circuit: a circuit that has more than one path for electric current to follow
series circuit: a circuit that has only one path for electric current to follow
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 98 GRADE 4
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 99 GRADE 4
SCIENTIFIC LITERACY TERMINOLOGY: Grade 4
This list is intended as a guide for teachers. While not exhaustive, it includes vocabulary that should be
used, as appropriate, by teachers and students during everyday classroom discourse. It is not intended
for student memorization.
absorb
To take in, soak up
adaptation (adapt)
The process of changing to new conditions which can be physical or
behavioral
attract
To draw in by physical force
average
The typical, usual, or ordinary result of a set of data
balance
A device for weighing things
battery
A source of electricity
binoculars
A device that magnifies a far away object (such as a bird)
camouflage
The disguising of people, animals, or things, to make them look like what is
around them
Celsius
The metric system for measuring temperature
centimeter
A unit of length in the metric system
characteristic
Showing a special feature or quality
circuit
A pathway through which electric current flows
classify
To put into groups or classes; sort
climate
The usual weather that occurs in a place, including the average
temperature and amounts of rain or wind
collect data
To gather information using tables and charts
compare
Discuss similarities and differences of items
conclusion
A decision made after careful thinking using evidence to support opinion
conduct (an experiment)
To lead, guide, or direct an experiment
conserve
To use carefully, not to waste
crystal
A regularly shaped piece with angles and flat surfaces into which many
substances solidify
cycle
A series of events that is regularly repeated
data
Facts, figures, and observations that are recorded from an experiment
decrease
To make or become less or smaller
describe
To use words to explain how something looks, feels or acts
determine
To make a decision
diagram
A labeled drawing that shows how something works
dissolve
To mix thoroughly with a liquid
draw a conclusion
Analysis of results from an experiment
ecosystem
A balance of plants & animals living & working together in a particular environment

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 100 GRADE 4
environment
Surroundings and conditions that effect natural processes
erode, erosion
To wear away or become worn
evaluate
To find out, judge, or estimate the value of
evidence
Facts or data that help support a conclusion
experiment
A procedure that is carried out to investigate a scientific question
explain, explanation
To make clear or understandable
explore
To examine in order to discover
extinct
No longer alive anywhere on earth
Fahrenheit
A customary measurement of temperature
fair test
An experiment where all but one the variables are held constant
findings
The results of a study or investigation
force
Force sets an object in motion by direct contact (push or pull) or indirect
contact (air, magnets or gravity)
gills
The organ of a fish that is used for breathing
graduated cylinder
A cylindrical container used for measuring volume
gram
The metric unit for measuring mass
guitar string
The part of a guitar that is plucked with the fingers or a pick
habitat
Where an organism naturally lives
hand lens
A tool used to magnify things
hibernate
Extended period of sleep
hypothesis
Question or purpose for conducting an experiment
identify
To find out or tell exactly who or what an object is
increase
To make or become greater or larger
insulate, insulator
A material that prevents the flow of electricity
investigate
To study something closely and in an organized way
kilogram
A metric measurement of mass equal to thousand grams
layer
A single thickness or deposit of material
length
The distance of a thing measured from one end to the other
lens
A tool used to see things clearly
life cycle
The stages in the life of an organism
liter
A unit of capacity for liquids in the metric system
magnet
An object that is attracted to iron, cobalt, and nickel
magnifier
To tool to make an object appear larger
magnifying glass
A tool used to enlarge objects
mass
The amount of matter in an object
materials
Supplies
metal
A substance that usually has a shiny surface, can be melted, and can
conduct heat and electricity

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 101 GRADE 4
metamorphosis
A complete change in appearance or form
meter, meter stick
A metric unit of linear measure.
migrate
To move or change location
migration
The movement of populations
milliliters
One thousandth of a liter
mineral
An ingredient of rock that cannot be broken down further
mixture
Something made by mixing
natural resources
Any substance that comes from the earth
nutrients
Something that living things need to grow and stay healthy
object
Anything that is not alive that people can see or touch
observe, observation
To see and pay attention to; watch
offspring
The result of animals reproducing (babies)
opinion
A belief based on what one thinks or feels; not on actual facts
organism
Any living thing such a as a plant or animal
oxygen
A colorless gas in the air that living things need to survive
particles
Very small piece or amount; a speck Ex: grain of sand
pattern
Anything that repeats itself
pebble
A small round stone
perform an experiment
Conducting a test to prove something
photosynthesis
A process by which green plants use light energy to change carbon dioxide
and water into glucose and oxygen
pitch (sound)
A highness or lowness of a musical sound
pluck (a string)
To pull at or let go; sharp pull or tug
predict, prediction
A guess based on what you know so far about what you think is going to happen
pressure
Force applied by one object to another object that is touching
procedure
Sequence of steps to perform an experiment
process
A series of steps that lead to a result (scientific method)
property
A characteristic of a material something that you can observe such as
color, smell, and taste
range
The extent to which something can vary
record (data)
Something written down to preserve facts or information
recycle
To treat materials that have been thrown away in order to use them again
reflect
To send back light rays, heat, or sound from a surface
repel
When two magnets come together to push apart
reproduce
To produce new plants or animals
result
Something that happens because of something else; consequence
reuse
To use again
sand
Loose grains of worn rock
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 102 GRADE 4
scale
Tool used to weigh objects
scientific observation
To look carefully, to analyze in an experiment in order to collect data
seed dispersal
The scattering of seeds
separate
To pull or take apart
sequence
The order in which things occur
soil
To go down or cause to go down under the surface
solid
Having a definite shape and mass Not liquid or gas
sort
To arrange according to property such as color, size, shape, class
stopwatch
Used for measuring short periods of time precisely
strum (a string)
Play on a string instrument by stroking the strings lightly with the fingers
surface
The outermost layer
survive
To stay alive
temperature
Relative hotness or coldness as measured on a standard scale
tension
Act of stretching or the condition of being stretched
testable
Able to test
texture
The look or feel of a surface
thermometer
A tool used to measure temperature
transparent
Light can pass through
vibrate, vibration
To move or cause to move back and forth rapidly
weigh, weight
To measure the mass or the gravitational force on an object

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 103 GRADE 4
Science Grade 4
Fiction and Nonfiction Books for Science in the Library
Earth Materials Science Unit
The Pebble in My Pocket by Meredith Hooper
Rocks in His Head by Carol Otis Hurst
Everybody Needs a Rock by Byrd Baylor
Wetlands Science Unit
A Day in the Salt Marsh by Kevin Kurtz
Weird Friends: Unlikely Allies in the Animal Kingdom by Jose Aruego
Oil Spill! By Melvin Berger and Paula Mirocha
Marshes and Swamps: A Wetland Web of Life by Philip Johansson
Butternut Hollow Pond by Brian J. Heinz
Marvels in the Muck by Doug Wechsler
Animal Survivors of the Wetlands by Barbara Somervill
Saving Oceans and Wetlands by Jen Green
Magnetism and Electricity Science Unit
Electricity: From Amps to Volts by Christopher Cooper, Heinemann Library, Chicago, IL 2004
Circuits, Shocks, and Lightning by Celeste A. Peters, Raintree Steck-Vaughn, Austin, TX 2000
Thomas A. Edison by Paul Mason, Raintree Steck-Vaughn, New York, NY 2002
Physics of Sound Science Unit
Adventures in Sound with Max Axiom, Super Scientist by Emily Sohn
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 104 GRADE 4
Content Standards & Indicators
Grades 5 - 8
Content Standards & Indicators
for Grade 5
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 109 GRADE 5
Course Description
MIDDLE SCHOOL
1. Course Title
Grade 5 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
4. Program Contact Information
Name: Kathleen Brooks
Title/Position: Middle School Science Coordinator
School: Dr. Robert H. Brown Middle School
980 Durham Road
Madison, CT 06443
Phone: 245-6475 X7082
6. Grade Level: 5
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
N/A
11. Brief Course Description
The fifth grade science course is a general science course. It is part of a spiraling curriculum in which aspects of
life science, physical science and earth/space science are addressed each school year. In grade five the life science
topics include ecosystems and human senses. The physical science topics include the study of forces and motion
and the properties of light. The earth/space science unit is a study of the Earth, moon and sun and the relationship
between these celestial bodies.
12. Course Goals
The fifth grade science program is based on the belief that students should appreciate science as a process of
inquiry. This is achieved through research, discussion, and hands-on experiences. Participating in an inquiry-based
environment helps
To foster a life long enjoyment of learning and the learning of science.
To view, through interdisciplinary connections, the concepts of science as part of the students' larger world.
To develop a growing appreciation for the outcomes of learning science as part of their evolving education.
To ensure students meet the science standards for Connecticut Public Schools.
13. Course Outline
Light
Sense Perception
- Sight
- Other senses
Earth, Moon and Sun
Forces and Motion
Ecosystems
- Organisms
- Food Chains and Food Webs
- Connecticut Ecosystems
Innovations
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 110 GRADE 5
14. Instructional Methods and/or Strategies
Individual and small group work
Full class instruction and discussions
Lecture
Modeling
Inquiry-based activities
PowerPoint presentations and notes
Research
15. Assessment Methods and/or Tools
Pencil and paper quizzes
Common unit assessments
Performance assessments
Lab reports
Research papers and/or projects
Assessments embedded in class activities
16. Assessment Criteria
The common assessments are based on the Madison curriculum as well as Connecticut standards and grade level
expectations for science. For performance assessments and projects students are given a rubric or grading criteria
before doing the work. A variety of assessment tools are employed to get the most accurate understanding of
individual student achievement possible.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 111 GRADE 5
LEARNING STRAND Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt to search out, describe, explain and
predict natural phenomena.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing
about science.
Mathematics provides useful tools for the
description, analysis and presentation of scientific
data and ideas.
ESSENTIAL QUESTIONS
How do you develop testable questions?
How do you make observations about objects,
organisms, and the environment?
How do you design and conduct simple
investigations employing simple equipment and
measuring tools to gather data and extend the
senses?
How do you use data to construct reasonable
explanations?
How do you use mathematics to analyze,
interpret, and present data?
How do you use words, graphs and drawings
to analyze, critique and communicate
investigations?
How do you locate relevant science information
when searching the Web?
KNOWLEDGE & LEARNING
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify questions that can be answered through
scientific investigation.
Make predictions.
Design a simple investigation to test a question.
Use tools and techniques that are appropriate for
the design of the investigation for making
observations and gathering data.
Accurately collect and record appropriate data.
Use mathematical operations to analyze and
interpret data.
Interpret and create appropriate graphs to
present relationships between variables.
Develop logical conclusions that are based on the
analysis of experimental data.
Report findings and conclusions in various formats
using relevant vocabulary and supporting
evidence.
Calculators
Rulers
Protractors
Computers
Graph paper
Smart Board Technology
Sentence strips
Sharpie markers
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during instruction
Inquiry activities and investigations
Guided Internet research
Integrate technology
Performance tasks
SUGGESTED ASSESSMENT METHODS
Lab Reports
Research projects/activities
CMT-like inquiry questions
Open-ended questions requiring constructed
responses
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 112 GRADE 5
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 113 GRADE 5
LEARNING STRAND Energy Transfer and Transformations
- What is the role of energy in our world?
Unit: Light
CT Standard 5.1 - Sound and light are forms of energy.
ENDURING UNDERSTANDING
Light is a form of energy that travels in a straight
line and can be reflected by a mirror, refracted by
a lens, or absorbed by objects.
ESSENTIAL QUESTIONS
Why do different materials reflect, refract or
absorb light?
How do different surfaces affect the
properties of light?
What affect do lenses have on light and the
images we see?
What affect do mirrors have on light and
the images we see?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Light travels in straight paths away from a source of
illumination in all directions until it hits an object.
Some sources of illumination produce their own light
(e.g., the sun, fire, light bulb); other sources of
illumination reflect light produced by something else
(e.g., the moon or a mirror).
Light interacts with objects in various ways; it can
be reflected off the object, absorbed by the
object, or refracted through the object.
Materials can be classified based on how much light
passes through them. Transparent materials allow
most light to pass through them. Translucent
materials allow some light to pass through them.
Opaque materials do not allow any light to pass
through them.
Objects that have flat, smooth surfaces reflect light
and produce a mirror-like image. Objects that have
curved or uneven surfaces scatter the reflected light
and produce distorted or blurry images.
Light always reflects away from a mirror at the
same angle that it hits the mirror. The angle of
incoming light equals the angle of reflected light.
Objects that block light traveling from a source
produce shadows. The shape, length, direction and
clarity of a shadow depend on the shape and
position of the object.
Light changes direction (refracts) as it passes from
one transparent material to another (e.g., as it
passes from air to water or through lenses).
DSM II Lenses and Mirrors
Prisms
Flashlights
Lenses
Magnifiers
Plastic cups
Beakers
Teacher-created materials
Miscellaneous products displaying the
properties of transparent, translucent,
opaque
Laser pointers
SUGGESTED INSTRUCTIONAL STRATEGIES
Mirror Maze lab
Build a Periscope
Pitch Black Box
Looking through convex and concave lenses
Light reflection off flat, convex and concave
mirrors
Prism Observation
Law of Reflection Activity
SUGGESTED ASSESSMENT METHODS
Performance Task:
Mirror Maze lab activity
Other Assessments:
Teacher-created quizzes
Common unit test
Research project using technology tools
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
1.
Provide evidence that light travels in
straight lines away from a source in all
directions.
2.
Investigate how light is refracted as it
passes through a lens or through one
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 114 GRADE 5
transparent material to another.
3.
Demonstrate that white light is composed
of many colors.
4.
Explain that all visible objects are reflecting
some light to the human eye.
5.
Contrast the way light is reflected by
smooth, shiny objects (e.g., mirror or pool
of water) and how it is reflected by other
objects.
6.
Measure angles to predict the path of light
reflected by a mirror.
7.
Determine whether a material is opaque,
transparent or translucent based on how
light passes through it.
8.
Design and conduct light absorption
experiments that vary the size, length,
direction and clarity of a shadow by
changing the position of the light-blocking
object or the light source.
CMT CORRELATION
Describe how light is absorbed and/or
reflected by different surfaces.
SCIENTIFIC LITERACY TERMINOLOGY: reflect, absorb, refract, transparent, translucent, opaque,
angle, transfer
KEY SCIENCE VOCABULARY
: shadow
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 115 GRADE 5
LEARNING STRAND Structure and Function -
How are organisms structured to ensure efficiency and survival? How do scienc
e
and technology affect the quality of our lives?
Unit: Sense Perception
CT Standard 5.2 - Perceiving and responding to information about the environment is critical to the survival of organisms.
CT Standard 5.4 - Humans have the capacity to build and use tools to advance the quality of their lives.
ENDURING UNDERSTANDINGS
The structure and function of the sense organs
influence how humans and animals perceive
the world.
The sense organs perceive stimuli from the
environment and send signals to the brain
through the nervous system.
Advances in technology allow individuals to
acquire new information about the world.
ESSENTIAL QUESTIONS
How do lenses correct vision?
How is the structure of the eye like a camera?
How does the structure of the eye help us see
light and color?
What affect does light have on the colors we
perceive?
What role do the senses play in an organism’s
survival?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Animals have sense organs that are structured
to gather information about their environment.
Information perceived by the senses allows
animals to find food, water, mates and
protection.
Each sense organ perceives specific kinds of
stimuli. Some human senses are more or less
developed than the senses of other animals.
Sense organs transfer information through a
network of nerves to the brain where it is
interpreted and responded to. The brain
responds by sending messages to all parts of
the body. The type of response and the amount
of time it takes for the response to occur vary
depending on the stimulus.
The human eye is structured to collect light
through the cornea and the pupil. The amount
of light that enters the eye is controlled by the
iris. The cornea and the lens refract the light
and focus it onto the retina and the optic
nerve where it is transformed into electrical
signals that are sent to different parts of the
brain.
For anything to be visible, light must be
present. For a person to see an object, the light
it reflects or produces must have a straight,
unobstructed path to the eye.
Human eyes have receptors for perceiving
shades of red, orange, yellow, green, blue,
indigo and violet.
Sunlight (or “white light”) is a combination of
colors. White light passed through prisms, water
droplets or diffraction gratings can be refracted
to show its component colors: red, orange,
yellow, green, blue, indigo and violet.
FOSS Kit: Human Brain and Senses
DSM II Color and Light
Chromatography paper
Mr. Sketch Markers
Prisms/ CD’s
Optical Illusion instructional supplies
Flashlights
Color Wheels and Color Paddles
Convex and Concave Lenses
Chromatography paper
Spin-art wheels
Batteries
Plastic test tubes and storage racks
Food coloring
U.V. Reading beads
SUGGESTED INSTRUCTIONAL STRATEGIES
Kaleidoscope Project
Chromatography Activity
Spinning Wheels
Eye Diagram Investigation
Mixing Light and Mixing Color Activities
UV Reacting Beads
Corrective Vision Investigation
Build a Model of the Eye
SUGGESTED ASSESSMENT METHODS
Performance Task:
CMT Embedded Task:
Catch It!
Other Assessments:
Teacher-created quizzes
Common unit test
Lab Reports
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 116 GRADE 5
The perceived color of an object depends on the
color of the light illuminating it and the way the
light interacts with the object. The color humans
see is the color that is reflected by the object.
For example, an object that appears green is
absorbing all colors except green, which is
reflected to the eye.
Human skin is structured to detect information
related to texture, temperature, pressure
and vibration. Each sensation has different
receptors distributed around the body; some
areas of the body have greater concentrations
of receptors for certain sensations, making
those areas more sensitive than others to
texture, temperature, or pressure.
Human noses are structured to collect and
detect chemicals floating in the air (odors). Tiny
hairs behind the nose have special receptors
that respond to airborne chemicals and produce
electrical signals that are transmitted to
different parts of the brain by the olfactory
nerve.
Human tongues are sense organs that are
structured for detecting chemicals dissolved in
saliva (flavors). Taste buds respond to 4 basic
tastes: salty, sweet, sour and bitter. Special
receptors in taste buds respond to tastes and
produce electrical signals that transmit
information through nerves to different parts of
the brain.
People design optical tools (e.g., binoculars,
telescopes, eyeglasses or periscopes) that
enable them to see things better or to see what
cannot be seen by human eyes alone. Optical
tools change the path of light by reflecting or
refracting it.
Throughout history new optical technologies
have led to new discoveries and understandings
that change people’s lives.
Periscopes allow people to see things that are
not within their line of sight (for example,
around corners, over walls, under a table, or
above the ocean’s surface from a submerged
submarine).
Telescopes make distant objects appear larger
(and therefore closer).
Magnifiers, such as hand lenses, microscopes
or make-up mirrors, make objects appear
larger.
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
1. Explain the role of sensory organs in perceiving
stimuli (e.g., light/dark, heat/cold, flavors,
pain, etc.) and sending signals to the brain.
2. Pose testable questions a
nd design experiments to
determine
factors that affect human reaction
time.
3. Conduct simple tests to explore the capabilities
of the human senses.
4. Summarize nonfiction text to explain the role of
the brain and spinal cord in responding to
information received from the sense organs.
5. Identify the major structures of the human
eye, ear, nose, skin and tongue, and explain
their functions.
6. Draw diagrams showing the straight path of
light rays from a source to a reflecting object
to the eye, allowing objects to be seen
7. Describe the properties of different materials
and the structures in the human eye that
enable humans to perceive color.
8. Generalize that optical tools, such as
binoculars, telescopes, eyeglasses or
periscopes, change the path of light by
reflecting or refracting it.
9. Construct simple periscopes and telescopes,
and analyze how the placement of their lenses
and mirrors affects the quality of the image
formed.
10. Evaluate the best optical instrument to
perform a given task.
11. Design and conduct simple investigations to
determine how the shape of a lens or mirror
(concave, convex, flat) affects the direction in
which light rays travel.
12. Explain how eyeglasses or contact lenses
improve vision by changing the path of light to
the retina.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 117 GRADE 5
The shape of a lens or mirror (concave,
convex or flat) affects the direction in which
light travels.
o Telescopes focus light using a lens that
refracts the light (refracting telescope) or a
curved mirror that reflects the light
(reflecting telescope).
o Periscopes use flat mirrors to reflect light to
change its path.
o Magnifying glasses use convex lenses to
refract light so that objects appear larger.
Some human eyes do not focus light properly
onto the retina. Eyeglasses are lenses that
improve vision by changing the path of light
(refracting it) so that it forms an image on the
retina.
Cameras have parts that function similarly to
the human eye.
HUMAN EYE
CAMERA
FUNCTION
Eyelid
Lens cap
Protect
interior parts
Pupil
Lens opening
(aperture)
Control
amount of
light entering
Cornea, lens
Lens
Focus light
rays on a
point
Retina
Film (or digital
medium)
Respond to
light resulting
in an image
13. Analyze the similarities and differences
between structures of the human eye and
those of a simple camera.
CMT CORRELATIONS
Describe how light absorptions and reflection
allow one to see the shapes and colors of
objects.
Describe the structure and function of the
human senses and the signals they perceive.
Compare and contrast the structures of the
human eye with those of a camera.
Describe the uses of different instruments, such
as eyeglasses, magnifiers, periscopes and
telescopes to enhance our vision.
SCIENTIFIC LITERACY TERMINOLOGY: sense organ, receptor, stimulus, response, nervous system,
vibration, reflect, refract, cornea, iris, pupil, lens, retina, white light, absorb, optical tool, hand lens,
magnifying glass, telescope, periscope, mirror, concave, convex, focus, camera, eye parts (See chart
above.)
KEY SCIENCE VOCABULARY
: temperature, texture, pressure
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 118 GRADE 5
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 119 GRADE 5
LEARNING STRAND Earth in the Solar System -
How does the position of Earth in the solar system affect conditions on our
planet?
Unit: Earth, Moon, Sun
CT Standard 5.3 - Most objects in the solar system are in a regular and predictable motion.
ENDURING UNDERSTANDING
The positions of the Earth and moon relative to
the sun explain the cycles of day and night,
and the monthly moon phases.
ESSENTIAL QUESTIONS
What affect does gravity have on celestial
bodies?
How does Earth’s movement through space
explain recurrent phenomenon?
What affect does the moon’s position in space
have on its appearance to us on Earth?
What properties of a celestial body affect its
gravitational pull?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
The sun, Earth and its moon are spherical
objects that move in two ways: they spin
(rotate) and they change positions relative to
each other (revolve).
The sun is a star that produces light that travels
in straight lines away from the sun in all
directions. Light from the sun illuminates objects
that reflect light, including Earth and its moon.
The side of the Earth that is facing the sun
experiences daylight; the side of the Earth
facing away from the sun experiences night. All
parts of the Earth experience a cycle that
includes both day and night, providing evidence
that the Earth is rotating on its axis.
The amount of time it takes for the Earth to
rotate once on its axis is regular and predictable
(24 hours), and is called ―a day.‖ Earth’s
rotation makes it appear as if the sun is
moving across the sky from east to west.
The moon is a rocky object that revolves around
the Earth in a circular path called an orbit. The
amount of time it takes for the moon to revolve
once around the Earth is about 29 days and is
called a ―lunar month.‖
Half of the moon is always illuminated by the
sun. Phases of the moon occur because a
different portion of the lit half of the moon is
visible from Earth each day as the moon
revolves around the earth.
The changes in the moon’s phases occur in a
regular and predictable sequence. At predictable
periods during the lunar cycle, the moon is
visible in either the daytime or the nighttime
sky.
At the beginning of a lunar month, no lit part of
the moon is visible from Earth (new moon). As
Delta Kit: Earth, Moon, and Sun
Moon Boxes
Bill Nye videos
Smart Board
Lap tops
Library Media Center resources
Earth, moon, and sun model
Light bulb fixtures
String
Duct tape
Poster board
Solar balloons
SUGGESTED INSTRUCTIONAL STRATEGIES
Create scale models of Earth, moon, and sun
Demonstrate scale distance between celestial
bodies
Model of Earth’s rotation and revolution on axis
and influence on seasonal changes
Compare and contrast the forces of magnetism
and gravity
Mass vs. Weight activity
Google Sky using laptops and Smart Board
Looking for micrometeorites
―what’s out there‖ activity
Internet Research: Duration of Daylight Hours
comparison
Moon phase investigation
SUGGESTED ASSESSMENT METHODS
Performance Task:
Moon journals
Other Assessments:
Teacher-created quizzes
Common unit test
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 120 GRADE 5
the moon progresses through the first two
quarters of its complete trip around the Earth,
larger portions of the right side of the moon are
illuminated each day. When the moon has
completed half its trip around the Earth, the full
moon is illuminated. During the third and fourth
quarters of the moon’s trip around the Earth,
the illuminated portion gradually decreases so
only the left side is illuminated and finally no lit
portion of the moon is visible from Earth again.
Like the sun, the moon appears to rise at the
eastern horizon and set at the western horizon
due to the earth’s rotation. From one day to the
next, when observed at the same time from the
same location, the moon’s position in the sky
varies in predictable ways.
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
1. Explain the motion of the Earth relative to the
sun that causes Earth to experience cycles of
day and night.
2.
Construct models demonstrating Earth’s
rotation on its axis, the moon’s revolution
around the Earth, and the Earth and moon
revolving around the sun.
3.
Distinguish between the sun as a source of
light and the moon as a reflection of that light.
4.
Observe and record the moon’s appearance
over time and analyze findings to describe the
cyclical changes in its appearance from Earth
(moon phases).
5. Relate the moon phases to changes in the
moon’s position relative to the Earth and sun
during its 29-day revolution around the Earth.
CMT CORRELATIONS
Explain the cause of day and night based on
the rotation of Earth on its axis.
Describe the monthly changes in the
appearance of the moon, based on the moon’s
orbit around the Earth.
SCIENTIFIC LITERACY TERMINOLOGY: sphere, illuminate, reflect, rotate, day/night cycle (24-hour
rotation period), horizon, orbit, revolve, month (one lunar cycle), moon phase, new moon
KEY SCIENCE VOCABULARY:
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 121 GRADE 5
LEARNING STRAND: Forces and Motion -
What makes objects move the way they do?
Unit: Forces and Motion
CT Standard 4.1 – The position and motion of objects can be changed by pushing or pulling.
ENDURING UNDERSTANDINGS
The size of the change in an object’s motion is
related to the strength of the push or pull.
The more massive an object is, the less effect
a given force will have on its motion.
ESSENTIAL QUESTIONS
Why do objects move?
How do forces affect the motion of an object?
What affect does the mass/ weight have on the
motion of an object?
How are force and mass related?
Why do we wear seatbelts?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 4.1.a.
An object is in motion when its position is
changing. Speed describes how far an object
moves in a given amount of time (e.g., miles
per hour).
A force is a push or pull that can cause an
object to move, stop, change speed or
direction.
The greater the force, the greater the change in
motion. For example, two people can push a
heavy box that could not be pushed by one
person alone.
Given an object, changing the amount of force
applied to it causes measurable effects.
When an object does not move in response to a
push or a pull, it is because another equal-sized
force, such as gravity or friction, is
counteracting the push or pull. Gravity (the
earth’s pulling force) and friction (the force
between two surfaces) are common forces that
work against motion.
GRADE LEVEL CONCEPT 4.1.b.
The amount of force needed to move an object
is related to the object’s mass.
The greater the object’s mass, the greater the
force needed to move it, stop it or change its
speed or direction.
An object with a small mass is easier to stop or
cause a change in motion than an object with a
large mass.
Given the same amount of force, changing the
mass of an object has measurable effects.
Lego cars
Onobots
Meter sticks
Latex-free rubber bands
Marbles of different materials
Weights
Ramps
Legos (general bricks)
Newton’s Cradle
Coins
Rulers
Golf pencils
Poster board
Timers
Note cards
String
Nuts/bolts/washers
200 gram weights
SUGGESTED INSTRUCTIONAL STRATEGIES
Marble Run
Paper Football
Botmobile Car launch
Botmobile Tractor Pull with weight
Design a friction efficient vehicle
Car crash- inertia activity
Pop Rockets
Collision ball activity
Parachute lab
SUGGESTED ASSESSMENT METHODS
Performance Task:
Marble Ramp
Other Assessments:
Teacher-created quizzes
Common unit test
Lab Activities
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 122 GRADE 5
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
1. Demonstrate that a force can cause an object
to start moving, stop, or change speed or
direction.
2.
Use measurement tools and standard units to
compare and contrast the motion of objects
such as toy cars, balls, model rockets or planes
in terms of change in position, speed and
direction.
3.
Design and conduct experiments to determine
how the motion of objects is related to the
mass of the object and the strength of the
force applied.
4.
Describe how friction forces caused by air
resistance or interactions between surface
materials affect the motion of objects.
5.
Predict the effect of an object’s mass on its
motion.
CMT CORRELATIONS
Describe the effects of the strengths of pushes
and pulls on the motion of objects.
Describe the effect of the mass of an object on
its motion.
SCIENTIFIC LITERACY TERMINOLOGY: motion, force, speed, gravity, mass, friction
KEY SCIENCE VOCABULARY
:
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 123 GRADE 5
LEARNING STRAND Matter and Energy in Ecosystems -
How do matter and energy flow through ecosystems?
Unit: Connecticut Ecosystems
CT Standard 6.2 – An ecosystem is composed of all the populations that are living in a certain space and the physical factors
with which they interact.
ENDURING UNDERSTANDINGS
Populations in ecosystems can be categorized as
producers, consumers, and decomposers of
organic matter.
An ecosystem is the complete interplay between
the living organisms and physical environment in a
specific area.
Abiotic and biotic factors interact within
ecosystems.
Populations in ecosystems are affected by biotic
factors, such as other populations, and abiotic
factors such as soil and water supply.
ESSENTIAL QUESTIONS
What influences do biotic and abiotic factors
have on different ecosystems?
How are populations affected by interactions
of predator-prey and consumer-producer
relationships?
How can common food webs in different
Connecticut ecosystems be described?
What effect does the sun’s energy have on
producers, consumers and decomposers
within an ecosystem?
How are populations in ecosystems affected
by limiting factors?
How are ecosystems affected by the impact
of organisms living within their specific
niche?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPTS 6.2.a.
An ecosystem is the complex interplay between
the living organisms and physical environment in a
specific area.
Ecosystems can be categorized into abiotic and
biotic components. Abiotic components include
nonliving things such as soil, minerals, climate,
water, sunlight, and wind. Biotic components
include all living things.
Interactions among biotic and abiotic factors
support the flow of energy and cycling of materials
in ecosystems. For example, air temperature,
availability of water and amount of wind influence
the growth of certain species of plants in an area,
plant species provide food for animal populations,
and plants and animals cycle oxygen and carbon
dioxide.
Soil is a mixture of materials that includes
weathered rocks and decomposed organic
material, as well as air and water. Soils vary from
place to place. The composition of soils affects how
air and water move through the soil, and this
influences the kinds of plants that can grow in it.
Water is a mixture of materials that includes
dissolved oxygen and minerals as well as
suspended sediments and debris.
Soil and water provide important habitats for plants
and animals within ecosystems.
Prentice Hall: Science Explorer Environmental
Science
Owl pellets
Forceps
Instructional posters
Hand lenses
Bill Nye video
Planet Earth video
Stick bug kit
Thermometers
Magazines
SUGGESTED INSTRUCTIONAL STRATEGIES
Adaptations investigation
Design an animal
Explore the diversity of frogs in Connecticut
Report on Explorer magazine article on
ecosystems
Dissect owl pellets
Blubber Gloves
Habitat models of Connecticut
Outdoor classroom- pond studies
Meigs Point Nature Center
Kellogg Environmental Center lessons
Create a creature
Tadpole study, when available
Stick bug lab
Animal cards activity
Heat loss lab
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 124 GRADE 5
GRADE LEVEL CONCEPT 6.2.b.
The sun is the main source of energy on Earth.
During photosynthesis, green plants use the
energy of sunlight to change the elements in
carbon dioxide (CO
2
) and water (H
2
0) into materials
(simple carbohydrates) that are a source of energy
for the plant to carry on its life processes.
Photosynthesis is affected by abiotic factors such
as amount of sunlight, availability of water and air
temperature.
Green plants are the producers in an ecosystem;
they rely directly on sunlight to produce the
materials they use for energy.
Plants are a source of energy (food) and nutrients
for animals that consume them. Energy passed to
consumers that eat plants came indirectly from
the sun as a result of photosynthesis. Some
animals consume plants, and other animals
consume animals that eat plants in predator-prey
relationships.
Consumers are adapted for eating different foods:
herbivores
are consumers that eat only plants;
carnivores
are consumers that eat only animals;
omnivores
are consumers that eat both plants
and animals.
Decomposers (mainly bacteria and fungi) consume
dead plants and animals and break down the
organic materials, thus returning nutrients to the
environment for reuse by other organisms.
Plants and animals within an ecosystem interact in
various ways as they compete for limited
resources. Relationships among organisms can be
beneficial or harmful to one or both organisms.
Food chains are models that show how materials
and energy are transferred from producers to
different levels of consumers in an ecosystem. The
basis of every food chain is the energy stored in
green plants.
Food webs are models that show the complex
variety of energy sources available to most
consumers in an ecosystem.
Connecticut has forest and park ecosystems, as
well as fresh water and marine ecosystems that
include a variety of plants and animals.
An energy pyramid is a model that shows the use of
energy in an ecosystem. A large number of
producers and primary consumers support a smaller
number of higher-level consumers due to the
consumption and loss of energy at each consumer
level.
SUGGESTED ASSESSMENT METHODS
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Analyze and interpret how biotic and
abiotic factors interact within a given
ecosystem.
2.
Design and conduct a scientific investigation
to explore the porosity and permeability of
soils and their ability to support different
plant life.
3.
Defend the statement, ―The sun is the main
source of energy on Earth.‖
4.
Express in general terms how plants and
other photosynthetic organisms use the
sun’s energy.
5.
Investigate and report on the effects of
abiotic factors on a plant’s ability to
photosynthesize.
6.
Compare and contrast how energy and
matter flow in a Connecticut ecosystem,
emphasizing the interactions among
producers, consumers and decomposers.
7.
Identify local examples of predator-prey
relationships and justify the impact of each
type of population on the other.
8.
Create and interpret graphs that illustrate
the fluctuation of populations over time.
9.
Distinguish a food chain from a food web
and identify local examples of each.
10.
Explain the impact of environmental
conditions such as climate, elevation,
topography or water quality on food chains.
11. Predict what will happen to a population
based on current trends (fires, disease, over
hunting, development) and defend the
prediction.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 125 GRADE 5
Populations of species within an ecosystem are
affected by the availability of resources such as
food, water, living space, or mates. Populations can
be reduced or increased by environmental changes
caused by nature (for example, droughts, forest
fires or disease) and by humans (climate change,
land development or overhunting).
Predator-prey relationships help to maintain a
balanced ecosystem. Increases or decreases in prey
populations result in corresponding increases or
decreases in predator populations. Fluctuations over
time in populations of interacting species can be
represented in graphs.
All organisms cause changes in the environment
where they live. Some of the changes caused by
organisms can be helpful to the ecosystem and
others can damage the ecosystem.
CMT CORRELATIONS
Describe how abiotic factors, such as
temperature, water and sunlight, affect the
ability of plants to create their own food
through photosynthesis.
Explain how populations are affected by
predator-prey relationships.
Describe common food webs in different
Connecticut ecosystems.
SCIENTIFIC LITERACY TERMINOLOGY: ecosystems, organism, population, biotic factor, abiotic factor,
food chain, photosynthesis, producer, consumer, herbivore, carnivore, omnivore, food web, predator, prey
KEY SCIENCE VOCABULARY:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 126 GRADE 5
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 127 GRADE 5
LEARNING STRAND Science and Technology in Society -
How do science and technology affect the quality of our lives?
Unit: Innovations
CT Standard 8.4 – In the design of structures there is a need to consider factors such as function, materials, safety, cost and
appearance.
ENDURING UNDERSTANDING
The form of a structure follows functions.
ESSENTIAL QUESTIONS
What factors could influence the design of a
product?
How does design influence the way something
works?
Why does innovation occur?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Understand that materials, cost, and time
affect the design of structures.
Understand how structure can improve
function.
Appreciate that what consumers want
influences the design of the product.
Know that Science and Technology affect the
quality of our lives.
FOSS kit: Ideas and Inventions
Washers
Onobots
String
Paper
Plastic bags
Art supplies
SUGGESTED INSTRUCTIONAL STRATEGIES
Design a Parachute
Create a paper airplane
Longest workable straw
Paper cup pendulum
Egg drop design
Science in the News
SUGGESTED ASSESSMENT METHODS
Performance Task:
Design a Parachute
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
1. Use technology to simulate how engineers plan,
test and revise designs given parameters including
cost, time, safety and aesthetics.
KEY SCIENCE VOCABULARY
: balanced/unbalanced forces
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 128 GRADE 5
Content Standards & Indicators
for Grade 6
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 131 GRADE 6
Course Description
MIDDLE SCHOOL
1. Course Title
Grade 6 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
4. Program Contact Information
Name: Kathleen Brooks
Title/Position: Middle School Science Coordinator
School: Dr. Robert H. Brown Middle School
980 Durham Road
Madison, CT 06443
Phone: 245-6475 X7082
6. Grade Level: 6
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites- N/A
11. Brief Course Description
The sixth grade science course is a general science course. It is part of a spiraling curriculum in
which aspects of life science, physical science and earth and space science are addressed in each
school year. In grade six, the life science units include a study of the musculoskeletal system of the
human body, cells, microbes, and plants. The physical science topic is an introduction to chemistry.
The earth/space science topics are weather and climate.
12. Course Goals
The lower middle school science program is designed to provide students with opportunities to ―do‖
science. Activities that are appropriate to the developmental levels of students, moving from
concrete to abstract, are emphasized; the mode of instruction is inquiry-based. Investigations foster
logical and independent thought, and group interaction is part of the classroom experience.
Participating in an inquiry-based environment
Provides opportunities for students to learn the skills of observation, investigation,
experimentation, and research.
Assists students in the development of science literacy to make them more aware and
understanding of the natural and physical world around them.
Ensures that students meet the science standards for Connecticut public schools.
13. Course Outline
Weather and Climate
- Atmosphere
- Climate
- Air Masses and Fronts
- Winds
Matter
- Phases

MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 132 GRADE 6
- Physical and Chemical Properties
- Physical and Chemical Change
Cells
- Plant Cells
- Animal Cells
Microbes
- Food Preservation
Human Musculoskeletal System
Plants
- Structure and Function
14. Instructional Methods and/or Strategies
Individual and small group work
Whole class instruction and discussions
Lecture
Modeling
Inquiry-based activities
PowerPoint presentations and notes
Research
15. Assessment Methods and/or Tools
Quizzes
Common unit assessments
Authentic assessments
Lab reports
Research papers and/or projects
Embedded performance assessment in class activities
16. Assessment Criteria
The common assessments are based on the Madison curriculum and Connecticut standards and
grade level expectations for science. For authentic assessments and projects students are given a
rubric or grading criteria before doing the work. A variety of assessment tools are employed to get
the most accurate understanding of individual student achievement possible.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 133 GRADE 6
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry progresses through a continuous
process of questioning, data collection, analysis
and interpretation.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing
about science.
Scientific literacy includes also the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
mathematical operations and procedures to
calculate, analyze and present scientific data and
ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that
is logically connected to the problem and the
design of the experiment?
Why is it critical to design and conduct
appropriate types of scientific investigations,
using the appropriate tools and techniques,
to make observations and gather data to
answer various questions?
How do you identify independent and
dependent variables?
Why is it important to identify variables that
need to be kept constant?
Why is it essential to assess the data that
was collected, using mathematical operations
to analyze and interpret data, and present
relationship between variables in appropriate
graphs?
Why is it essential to assess the validity of
the experimental design identifying sources
of error and the credibility of scientific claims
in different sources of information?
Why is it important to communicate findings,
using relevant scientific vocabulary and clear
logic that are based on the results generated
during the experiment?
KNOWLEDGE & LEARNING
The student will...
INSTRUCTIONAL SUPPORT MATERIALS
Identify questions that can be answered through
scientific investigation.
Formulate a testable hypothesis, in the "If…,
then… because" format that is logically connected
to the problem.
Design an experiment in which the independent
and dependent variables are accurately identified
and variables, which need to be, are kept
constant.
Use appropriate tools and techniques that are
appropriate for the design of the experiment for
making observations and gathering data.
Accurately collect and record appropriate data.
Use mathematical operations to analyze and
interpret data.
Interpret and create appropriate graphs to
present relationships between variables.
Develop logical conclusions that are based on the
analysis of experimental data.
Report findings and conclusions in various formats
(i.e., lab reports) using relevant vocabulary,
supporting evidence, and clear logic.
General lab equipment
Safety equipment
Prentice Hall Explorer Series Texts
Unit specific materials as noted
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during instruction
Inquiry activities and investigations
Guided internet research
Performance tasks
SUGGESTED ASSESSMENT METHODS
Lab Reports
Research projects/activities
Inquiry literacy questions
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 134 GRADE 6
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 135 GRADE 6
LEARNING STRAND Properties of Matter -
How does the structure of matter affect the properties and uses of materials?
Unit: Matter
CT Standard 6.1 – Materials can be classified as pure substances or mixtures, depending on their chemical and physical properties.
ENDURING UNDERSTANDINGS
Mixtures are made of combinations of elements
and/or compounds, and they can be separated by
using a variety of physical means.
Pure substances can be either elements or
compounds, and they cannot be broken down by
physical means.
ESSENTIAL QUESTIONS
How do changing levels of energy effect
physical changes of matter?
How can mixtures be separated using the
properties of the substances from which they
are made, such as particle size, density,
solubility, and boiling point?
How can general properties of matter be
used to identify specific substances?
How does the physical property of a
substance determine its appropriate use?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 6.1.a.
Everything is made of matter. Matter has two
fundamental properties: it has weight (mass) and
it takes up space (volume).
All matter has a variety of properties, some of
which are characteristic of the substance.
Characteristic properties do not depend on the
amount of the substance as mass and volume do.
Properties such as magnetic attraction,
conductivity, density, pH, boiling point and
solubility are characteristic properties that can be
used to identify substances.
Solids, liquids or gases can be combined to form
mixtures. In a mixture, each substance keeps its
individual properties. In some mixtures, each of
the components can be seen (e.g., rocks, twigs,
insects and leaves are visible components of soil);
in other mixtures, the individual substances blend
so well that they appear to be a single substance
(e.g., oxygen, nitrogen and carbon dioxide are
mixed together to form air).
Mixtures can be separated using different methods,
depending on the physical properties of the
component substances. Filtering, evaporating,
floating/settling, dissolving, and using magnets
are all methods for separating mixtures based on
the properties of their components.
Solutions are mixtures that appear to be single
substances because particles have dissolved and
spread evenly throughout the mixture. Not all
separation methods are effective for separating the
components of solutions.
General lab equipment
General safety equipment
Prentice Hall module: Matter
Density cubes
Marbles
Clay
Pebbles
Food containers
Iron filings
Packing peanuts
Cotton balls
Blow dryer or other heat source
SUGGESTED INSTRUCTIONAL STRATEGIES
Density Cube lab
Displacement lab
Flinking (Float/Sink) lab
Practice using triple beam balance
Determine the volume of regular shaped
objects using mathematical formulas
Mass versus weight planet poster
Implementation of matter concepts through
experimentation or demonstration
Mass lab using food nutritional facts
Boiling Point lab
Separating mixtures-iron filings
SUGGESTED ASSESSMENT METHODS
Performance Task:
Students will be asked to perform a lab
activity involving determining the density of
different cubes to identify what the
substance is. They will formulate a
hypothesis and perform the activity
recording both qualitative and quantitative
data. The data will be analyzed and
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 136 GRADE 6
conclusions will be drawn.
Other Assessments:
Teacher-created quizzes
Common unit test
Learning activities
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Explain that density (mass/volume) is a
characteristic property that can be used to
identify an element or substance.
2.
Differentiate between a mixture and an
element or compound and identify
examples.
3.
Conduct and report on an investigation that
uses physical means such as particle size,
density, solubility and magnetism to
separate substances in a mixture.
CMT CORRELATIONS
Explain how mixtures can be separated by
using properties of the substances from
which they are made, such as particle size,
density solubility and boiling point.
SCIENTIFIC LITERACY TERMINOLOGY: characteristic, property, mass, weight, volume, density,
solubility, boiling point, mixture, solution, particle, atom, element, molecule, compound
KEY SCIENCE VOCABULARY
: substance, evaporation, dissolve
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 137 GRADE 6
LEARNING STRAND Structure and Function --
How are organisms structured to ensure efficiency and survival?
Unit: Cells
CT Standard 7.2 – Many organisms, including humans, have specialized organ systems that interact with each other to maintain
dynamic internal balance.
ENDURING UNDERSTANDINGS
All organisms are composed of one or more cells;
each cell carries on life-sustaining functions.
Multicellular organisms need specialized structures
and systems to perform basic life functions.
ESSENTIAL QUESTIONS
How do the basic structures of a cell, such
as nucleus, cytoplasm, mitochondria, and
cell membrane, function to support life?
How are organisms structured to ensure
efficiency and survival?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 7.2.a.
Living things have characteristics that distinguish them
from nonliving things. Living things use energy,
respond to their environment, grow and develop,
produce waste and reproduce.
Organisms are made of tiny cells that perform the
basic life functions and keep the organism alive. Many
organisms (e.g., yeast, algae) are single-celled and
many organisms (e.g., plants, fungi and animals) are
made of millions of cells that work in coordination.
All cells come from other cells and they hold the
genetic information needed for cell division and
growth. When a body cell reaches a certain size, it
divides into two cells, each of which contains identical
genetic information. This cell division process is called
mitosis.
The cell is filled with a fluid called
cytoplasm
; cells
contain discrete membrane-enclosed structures
called
organelles
. Each of the organelles performs a
specific cellular function and it can be identified by its
shape.
o The nucleus contains the genetic materials
(chromosomes), and it directs the cell activities,
growth and division.
o The mitochondrion contains enzymes that
break down sugars and release chemical energy.
One cell can contain hundreds of mitochondria.
o The entire cell is surrounded by the plasma
membrane which controls the flow of materials
into and out of the cell.
GRADE LEVEL CONCEPT 7.2.b.
Systems consist of parts that interact with and
influence each other. Parts of a system work together
to make the whole entity work. Similarly, each part of
an animal body has a specific job to do, and all the
different parts work together to support life.
Although all cells have similar basic structures, in
multicellular organisms cells have specialized shapes
that enable them to perform specific roles (e.g.,
muscle, nerve, and skin cells can be identified by their
General lab equipment
Safety equipment
Prentice Hall module: Cells
Prepared slides
Microscopes
Microviewers
SUGGESTED INSTRUCTIONAL STRATEGIES
Build a model of a cell
Microviewer activities
Draw and label diagrams of a plant and
animal cells
Compare cell to a real world model
Look at plant and animal cells under
microscopes
Appropriate drawing and labeling of
microscope view field
Flow chart of interaction of cells, tissues,
organs, and organ systems
SUGGESTED ASSESSMENT METHODS
Performance Tasks:
Build a model of a cell and identify the
cell parts
Compare the structure and functions of a
cell to a real-world model
Illustrate the structural differences and
function of various cell types found in
multicellular organisms (muscle, bone)
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
1. Compare and contrast single-celled
organisms with multicellular organisms.
2. Illustrate and describe in writing the
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 138 GRADE 6
distinct shapes).
Groups of similar cells are organized in tissues that
have specific functions (e.g., providing support,
connecting parts, carrying messages, protecting
internal and external surfaces).
Different tissues work together to form an organ, and
organs work together as organ systems to perform
essential life functions.
structure and the function of the following
cell structures: cell membrane, cytoplasm,
mitochondria and nucleus in an animal
cell.
3. Explain how the structure and function of
multicellular organisms (animals) is
dependent on the interaction of cells,
tissues, organs and organ systems.
CMT CORRELATION
Describe the basic structures of an
animal cell, including the nucleus,
cytoplasm, mitochondria, and cell
membrane, and how they function to
support life.
SCIENTIFIC LITERACY TERMINOLOGY: structure, function, cell, organelle, cytoplasm, nucleus, cell
membrane, mitochondria, tissues, organism, system
KEY SCIENCE VOCABULARY
: chromosomes, enzymes
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 139 GRADE 6
LEARNING STRAND Science and Technology in Society -
How do science and technology affect the quality of our lives?
Unit: Microbes
CT Standard 7.4 - Technology allows us to improve food production and preservation, thus improving our ability to meet the
nutritional needs of growing populations.
ENDURING UNDERSTANDING
Various microbes compete with humans for the
same sources of food.
ESSENTIAL QUESTIONS
How can freezing, dehydration, pickling, and
irradiation prevent food spoilage caused by
microbes?
How do microbes impact human life?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
1. Microorganisms (microbes) are microscopic
organisms, such as bacteria, yeast and mold, that
are found almost everywhere: in air, soil and
water, inside our bodies and in our foods.
2. Bacteria are single-celled organisms that differ
from other single-celled organisms in that they do
not have organelles such as a nucleus,
mitochondrion or chloroplast.
3. Bacteria are an essential component of any food
web because they break down complex organic
matter into simple materials used by plants. Some
bacteria can produce their own food through
photosynthesis and others are consumers that
compete for foods that humans eat.
4. Some bacteria can be beneficial to humans. Certain
bacteria live symbiotically in the digestive tracts
of animals (including humans) and help break
down food. Other bacteria are used by humans to
purify waste water and to produce foods such as
cheese and yogurt.
5. Some bacteria are harmful to humans. They can
spoil food, contaminate water supplies and cause
infections and illness.
6. Food preservation methods create conditions that
kill bacteria or inhibit their growth by interfering
with the bacterium’s life processes. Food
preservation methods include removing moisture
by dehydration or salting, removing oxygen by
vacuum-packing, lowering pH by pickling,
lowering temperature by refrigerating or freezing,
and destroying the bacterial cells by irradiation or
heat (pasteurizing and cooking).
7. Throughout history, humans have developed
different methods to ensure the availability of safe
food and water to people around the world.
Prentice Hall module: Bacteria to Plants
General lab equipment
General lab safety equipment
Dehydrator
Pickling materials
Plastic baggies
Microviewers
Magazines
Yogurt
SUGGESTED INSTRUCTIONAL STRATEGIES
Microviewer activities
View pre-prepared slides and student-made
slides under microscopes
5 Station lab activity; Pro-scope,
microscopes, microviewers, research history
of microscope, power point presentations
Pickling lab
Dehydrating lab
Magazine activity
Picture activity
Yogurt activity
SUGGESTED ASSESSMENT METHODS
Performance Tasks:
Microbe Board Game
Analyze and record the various ways
bacteria function in food webs.
Compare and contrast the effectiveness
and safety of past and current methods
of food preservation.
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
1. Investigate and describe in writing different
types of microbes and the environmental
conditions necessary for their survival.
2. Describe the optimum conditions for rapid
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 140 GRADE 6
bacterial growth.
3. Illustrate and describe the structural
differences between bacterial and animal
cells.
4. Discover and discuss how humans use
bacteria to produce food and identify
examples.
5. Compare and contrast the role of bacteria in
food production and food spoilage.
6. Evaluate and report how each method of
food preservation including dehydration,
pickling, irradiation and refrigeration works
to stop or inhibit bacterial growth and give
examples of each.
CMT CORRELATION
Describe how freezing, dehydration, pickling
and irradiation prevent food spoilage caused
by microbes.
SCIENTIFIC LITERACY TERMINOLOGY: microbe, bacteria, single-celled organism, dehydration,
pickling, irradiation
KEY SCIENCE VOCABULARY
: symbiotically
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 141 GRADE 6
LEARNING STRAND Structure and Function -
How are organisms structured to ensure efficiency and survival?
Unit: Musculoskeletal System
CT Standard 7.2 – Many organisms, including humans, have specialized organ systems that interact with each other to maintain
dynamic internal balance.
ENDURING UNDERSTANDING
Multicellular organisms need specialized
structures and systems to perform basic
life functions.
ESSENTIAL QUESTIONS
How does the musculo-skeletal system support life
functions?
How does the musculo-skeletal system allow
movement?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 7.2.b.
The human skeletal system includes bones
joined together by ligaments. The skeletal
system functions to shape and support the
body, protect internal organs, enable
movement, form blood cells, and store
minerals such as calcium and phosphorous.
Joints are places where two bones come
together and body movement can occur.
The structure of a joint (e.g., ball and
socket, hinge or pivot) determines the kind
of movement possible at that point.
The human muscular system includes
skeletal, smooth and cardiac muscles. The
skeletal muscles are attached to bones by
tendons and they are responsible for the
movement of the body. The cardiac muscle
is responsible for the pumping action of the
heart and the smooth muscles are related
to the movement of the internal organs.
The muscular and skeletal systems interact
to support the body and allow movement.
General lab equipment
General lab safety equipment
X-rays
SUGGESTED INSTRUCTIONAL STRATEGIES
Interactive games for musculo-skeletal system
Webquest games for musculo-skeletal system, such
as build and label a skeleton
Activities for gender differences of the musculo-
skeletal system such as ―Great Bone Mysteries‖
Joint isolation activity
Muscle fatigue ―tests‖
Create a creature flip book
Model arm demonstration
SUGGESTED ASSESSMENT METHODS
Performance Tasks:
Unit activity including cells, microorganisms, and
musculo-skeletal system
Compare and contrast the structure and function of
skeletal muscle with cardiac and smooth muscle.
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to
meet these state expectations:
1. Investigate and explain in writing the basic
structure and function of the human skeletal
system.
2. Differentiate between the structures and range of
motion associated with ball, socket and hinge
joints and relate human joints to simple machines.
3. Demonstrate how the muscles, tendons, ligaments
and bones interact to support the human body and
allow movement.
CMT CORRELATION
Explain how the human musculoskeletal system
supports the body and allows movement.
SCIENTIFIC LITERACY TERMINOLOGY: structure, function, tissue, organ, system
KEY SCIENCE VOCABULARY
: tendons, ligaments, joints
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 142 GRADE 6

MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 143 GRADE 6
LEARNING STRAND Energy in Earth's Systems -
How do external and internal sources of energy affect the Earth's systems
Unit: Weather and Climate
CT Standard 6.3 – Variations in the amount of the sun’s energy hitting the Earth’s surface affect daily and seasonal weather
patterns.
ENDURING UNDERSTANDING
Local and regional weather are affected by the
amount of solar energy the area receives and the
proximity to a large body of water.
ESSENTIAL QUESTIONS
How do external and internal sources of
energy affect the Earth’s System?
How does heating affect the movement of
molecules?
How do temperature, pressure and water
content in the atmosphere affect local
weather?
How does the unequal heating of the Earth’s
surface cause winds?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Earth is surrounded by layers of gases
(atmosphere) that influence the environment and
support life. Weather on Earth is caused by the
daily changes in the temperature, pressure and
amount of moisture in the lower atmosphere.
Regions of the earth experience distinct long-term
climate conditions caused, in part, by different
amounts of solar energy they receive.
Heat energy causes molecules to move. The
molecules that make up all matter are in constant
motion. Solids, liquids and gases differ in the
movement and arrangements of their molecules.
Molecules in gases move randomly and
independently of one another. Molecules in liquids
move around each other randomly, but are loosely
held together by an attraction force. Molecules in
solids are closely locked in a patterned position and
can only vibrate back and forth.
When heat energy is added to a substance, its
molecules move faster (increased temperature)
and spread apart from each other (become less
densely arranged). When heat energy is removed,
molecules move slower (decreased temperature)
and come together (become more densely
arranged).
If enough heat energy is absorbed by a solid or a
liquid, the molecules may overcome the forces
holding them together and change to a new state
of matter. Solids change to liquids (melt) and
liquids change to gases (vaporization) when heat
energy is absorbed from the surroundings.
Conversely, heat energy is given off when gases
change to liquids (condensation) or liquids
change to solid (freezing).
Different surfaces on Earth absorb and release
solar energy at different rates. Land has a lower
Prentice Hall module Weather and Climate
(2009)
General lab equipment
Thermometers
Magdeburg and vacuum pump
Internet access / reference materials
Weather maps
Power Point Software
Sand and lamps
Zip-lock baggies
Hot plate
SUGGESTED INSTRUCTIONAL STRATEGIES
Weigh a flat Zip-lock bag and a Zip-lock bag
filled with air to show that air has mass.
Perform the activity on page 16 in the
Prentice Hall module Weather and Climate.
Graph the layers of the atmosphere
according to temperature and altitude.
Perform the Lab ―How Clean is the Air?‖ on
pages 26-27 in the Prentice Hall module
Weather and Climate.
Do demonstrations showing the significance
of air pressure utilizing imploding soda cans,
magdeburg sphere and Cartesian divers.
Perform demonstrations showing methods of
heat transfer.
Perform the activity ―Heating Earth’s Surface‖
on pages 40-41 in the Prentice Hall module
Weather and Climate using it to help explain
land breezes and sea breezes.
Perform ―What Is the Greenhouse Effect?‖ on
page 135 in the Prentice Hall module
Weather and Climate. Two beakers with dirt
may be used to replace the shoeboxes with
construction paper.
Draw a diagram of the water cycle.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 144 GRADE 6
heat capacity than water; therefore land
temperatures change more rapidly than water
temperatures do. The surface temperature of large
bodies of water, such as the oceans that cover a
great deal of the earth, affects the temperature of
the air above them.
Earth’s atmosphere (air) is a mixture of different
amounts of gases (mainly nitrogen, followed by
oxygen, carbon dioxide and water vapor). Air
molecules constantly press on and around objects
on Earth (air pressure). Due to the pulling force of
Earth’s gravity, air close to Earth is denser than air
higher in the atmosphere; denser air causes
greater air pressure.
Wind is caused by air moving from areas of high
pressure to low pressure. Cool, dense air is high
pressure and tends to sink; warm, less dense air is
low pressure and tends to rise. Local and global
winds move in predictable patterns based on
uneven heating of Earth’s surface.
Local winds can be influenced by atmospheric
conditions, terrain (mountain, deserts) and
closeness to large bodies of water. Near coastal
areas, the day to night temperature and pressure
differences between land and water cause local
winds to blow from ocean to land (―sea breeze‖)
during day and from land to ocean (―land
breeze‖) at night.
Global winds are caused by the circulation of cold,
dense polar air and warm, less dense equatorial
air. The rotation of the earth, combined with the
location of the continents, causes bands of wind
patterns on the earth. For example, weather tends
to move generally from west to east.
Large bodies of water absorb heat energy, causing
water to evaporate. The amount of water vapor
in the atmosphere (humidity) is dependant on the
temperature of the air. Warm air holds more water
vapor than cool air. As warm, humid air rises and
cools, its molecules become more closely spaced
and the water vapor condenses into tiny water
droplets that are less dense than air (clouds).
Weather on Earth is caused by daily variations in
the temperature, pressure and humidity of
different bodies of air (air masses). Warm, moist,
less dense air masses rise, thus decreasing air
pressure usually indicates that cloudy, wet, warmer
weather is approaching. Cool, dry, denser air
masses sink, thus increasing air pressure usually
indicates clear, dry, cooler weather is approaching.
When masses of warm, moist air interact with
masses of cool, dry air, the boundary is called a
warm front. The way in which the air masses
Measure relative humidity using two
thermometers or a sling psychrometer.
Demonstrate how hail is made.
View Weather Channel broadcasts.
Perform the density of three waters lab.
Using weather maps, examine the weather
patterns for three consecutive days and
predict the weather for the next day.
Perform the Edd-Head’s Internet activity
predicting weather.
Cloud layering activity
Heat absorption activity
Cloud maker activity
Demonstrate the Coriolis Effect
SUGGESTED ASSESSMENT METHODS
Performance Tasks:
The Density of Three Waters lab
Compare the density of cold air to warm
air and predict the impact of each on
weather patterns
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Compare the composition and structure of
the Earth’s atmospheric layers.
2. Demonstrate how changes in temperature,
pressure, moisture, and density of air affect
weather patterns (e.g., air masses and air
pressure.)
3. Describe in writing how solar energy drives
Earth’s weather systems.
4. Investigate and report on how the
introduction of heat affects the motion of
particles and the distance between them.
5. Illustrate the transfer of energy as matter
changes phase.
6. Design, conduct and report in writing an
investigation that reveals different
substances absorb and release heat at
different rates.
7. Research and give examples of heat transfer
and local weather differences in
Connecticut.
8. Investigate and explain the movement of
local winds, including ―sea breezes‖ and
―land breezes,‖ based upon the uneven
heating of the Earth’s surface and a change
in air pressure.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 145 GRADE 6
move past one another influences the type of
weather that results. Weather predictions can be
made based on the pattern of warm, wet, low
pressure air being typically followed by cool, dry,
high pressure air.
Connecticut, and the northeast in general, often
has rapidly changing weather because three
patterns of moving air interact here: cold, dry air
from the north, warm, moist air from the Atlantic
ocean coastline, and air moving across the US from
west to east.
9. Examine and explain that global winds are
caused by uneven heating of the Earth’s
surface and the rotation of the Earth.
10. Design a weather forecast based upon
collected weather data.
CMT CORRELATIONS
Describe the effect of heating on the
movement of molecules in solids, liquids and
gases.
Explain how local weather conditions are
related to the temperature, pressure and
water content of the atmosphere and the
proximity to a large body of water.
Explain how the uneven heating of the
Earth’s surface causes winds and affects the
seasons.
SCIENTIFIC LITERACY TERMINOLOGY: molecule, dense, solid, liquid, gas, melting, freezing,
condense, evaporate, air pressure, humidity, air mass, cold/warm front, precipitation, global wind, sea
breeze, land breeze.
KEY SCIENCE VOCABULARY
: vaporization
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 146 GRADE 6
MADISON PUBLIC SCHOOLS 147 GRADE 6
LEARNING STRAND Matter and Energy in Ecosystems -
How do matter and energy flow through ecosystems?
Unit: Plants
CT Standard 6.2 – An ecosystem is composed of all the populations that are living in a certain space and the physical factors
which they interact.
ENDURING UNDERSTANDINGS
Populations in ecosystems are affected by biotic
factors, such as other populations, and abiotic
factors, such as soil and water supply.
Populations in ecosystems can be categorized as
producers, consumers and decomposers of
organic matter.
ESSENTIAL QUESTIONS
How do basic cell structures specialize to support
life in plants?
How do soil properties affect plant growth?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Ecosystems can be categorized into abiotic and
biotic components. Abiotic components include
nonliving things such as soil, minerals, climate,
water, sunlight, and wind. Biotic components
include all living things.
An ecosystem is the complex interplay between
the living organisms and physical environment in
a specific area.
Interactions among biotic and abiotic factors
support the flow of energy and cycling of
materials in ecosystems. For example, air
temperature, availability of water and amount of
wind influence the growth of certain species of
plants in an area, plant species provide food for
animal populations, and plants and animals cycle
oxygen and carbon dioxide.
Soil is a mixture of materials that includes
weathered rocks and decomposed organic
material, as well as air and water. Soils vary from
place to place. The composition of soils affects
how air and water move through the soil, and
this influences the kinds of plants that can grow
in it.
Water is a mixture of materials that includes
dissolved oxygen and minerals as well as
suspended sediments and debris.
Soil and water provide important habitats for
plants (and animals) within ecosystems.
GRADE LEVEL CONCEPT 6.2.b.
The sun is the main source of energy on Earth.
During photosynthesis, green plants use the
energy of sunlight to change the elements in
carbon dioxide (CO
2
) and water (H
2
0) into
materials (simple carbohydrates) that are a source
of energy for the plant to carry on its life
processes.
Photosynthesis is affected by abiotic factors such
as amount of sunlight, availability of water and
Prentice Hall module: Bacteria to Plants
Various seeds
Plastic baggies
Microscopes
Plastic cups
Iodine
General lab safety equipment
Various soil samples
Plastic water bottles (20 oz.)
Cheese cloth
Clay
Plants
Stereoscopes
Gloves
SUGGESTED INSTRUCTIONAL STRATEGIES
Visit Outdoor Classroom, sketch plants and
research various species
Dissecting a Seed
Demonstrate geotropism with radish seeds
Seed germination with sandwich baggies
Examination of roots and root hairs
Leaf rubbings
Examination of underside of leaves with
stereoscopes
Flower dissection using rhododendron
Prediction of germination & germination rate
Examination of soil
CMT Embedded Task:
"Dig In!"
SUGGESTED ASSESSMENT METHODS
Performance Tasks:
CMT Embedded Task:
Dig In!
Soil properties and water absorption
Other Assessments:
Teacher-created quizzes
Common unit test
Classroom labs
MADISON PUBLIC SCHOOLS 148 GRADE 6
air temperature.
Green plants are the producers in an
ecosystem; they rely directly on sunlight to
produce the materials they use for energy.
Plants are a source of energy (food) and
nutrients for animals that consume them.
Energy passed to consumers that eat plants
came indirectly from the sun as a result of
photosynthesis. Some animals consume plants,
and other animals consume animals that eat
plants in predator-prey relationships.
Plants (and animals) within an ecosystem interact
in various ways as they compete for limited
resources. Relationships among organisms can be
beneficial or harmful to one or both organisms.
Food chains are models that show how materials
and energy are transferred from producers to
different levels of consumers in an ecosystem.
The basis of every food chain is the energy
stored in green plants.
Food webs are models that show the complex
variety of energy sources available to most
consumers in an ecosystem.
Connecticut has forest and park ecosystems, as
well as fresh water and marine ecosystems that
include a variety of plants and animals.
Populations of species within an ecosystem are
affected by the availability of resources such as
food, water, living space, or mates. Populations
can be reduced or increased by environmental
changes caused by nature (for example,
droughts, forest fires or disease) and by humans
(climate change, land development or
overhunting).
All organisms cause changes in the environment
where they live. Some of the changes caused by
organisms can be helpful to the ecosystem and
others can damage the ecosystem.
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Analyze and interpret how biotic and abiotic
factors interact within a given ecosystem.
2. Design and conduct a scientific investigation to
explore the porosity and permeability of soils
and their ability to support different plant life.
3. Defend the statement, ―The sun is the main
source of energy on Earth.‖
4. Express in general terms how plants and other
photosynthetic organisms use the sun’s energy.
5. Investigate and report on the effects of abiotic
factors on a plant’s ability to photosynthesize.
6. Compare and contrast how energy and matter
flow in a Connecticut ecosystem, emphasizing
the interactions among producers, consumers
and decomposers.
7. Identify local examples of predator-prey
relationships and justify the impact of each type
of population on the other.
8. Explain the impact of environmental conditions
such as climate, elevation, topography or water
quality on food chains.
9. Predict what will happen to a population based
on current trends (fires, disease, over hunting,
development) and defend the prediction.
CMT CORRELATIONS
Describe how abiotic factors, such as
temperatures, water and sunlight, affect the
ability of plants to create their own food through
photosynthesis.
SCIENTIFIC LITERACY TERMINOLOGY: ecosystem, organisms, population, biotic factor, abiotic
factor, food chain, photosynthesis, producer, consumer, herbivore, carnivore, omnivore, food web,
predator, prey
KEY SCIENCE VOCABULARY
: decomposer, predator-prey relationships
Content Standards & Indicators
for Grade 7
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 151 GRADE 7
Course Description
MIDDLE SCHOOL
1. Course Title
Grade 7 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
4. Program Contact Information
Name: Kathleen Brooks
Title/Position: Middle School Science Coordinator
School: Walter C. Polson Middle School
302 Green Hill Road
Madison, CT 06443
Phone: 245-6475 X7082
6. Grade Level: 7
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
N/A
11. Brief Course Description
The seventh grade science course is a general science course. It is part of a spiraling curriculum in which
aspects of life science, physical science and earth/space science are addressed each school year. In grade seven
the life science topics include the study of the circulatory, digestive, and excretory systems of the human body.
The physical science topics include the study of forces and motion, simple machines and bridge design. The
earth/space science topic is geology. In addition, an earth science / ecology unit on Connecticut’s water
resources is included.
12. Course Goals
The upper middle school science program combines the development of logical, scientific thought processes with
current scientific theories, terminology, and factual information. Participating in an inquiry-based learning
environment
Provides opportunities for students to practice the skills of observation, investigation, experimentation, and
research.
Assists students in the development of scientific literacy to make them more aware and understanding of the
natural and physical world around them.
Ensures students meet the science standards for Connecticut public schools.
Encourages students to become interested in science as well as to learn about careers in science.
13. Course Outline
Circulation, Respiration, Excretion
- Structure and Function
- Defense Against Disease
Geology
- Plate Tectonics
- Erosion and Glaciation
- Basic Rock Types and Rock Formation
Connecticut Water Resources
- Fresh Water

MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 152 GRADE 7
- Salt Water
- Septic and Sewer Systems
Forces and Motion
- Forces: Friction, Buoyant, Gravitational
- Newton’s Laws of Motion
- Momentum, Speed, and Acceleration
Work and machines
- Forces and Work
- Simple Machines
Structures (Bridges)
- Structural Shapes and Materials
- Bridge Designs
14. Instructional Methods and/or Strategies
Individual and small group work
Whole class instruction and discussions
Lecture
Modeling
Inquiry-based activities
PowerPoint presentations and notes
Research
15. Assessment Methods and/or Tools
Quizzes
Common unit assessments
Authentic assessments
Lab reports
Research papers and/or projects
Embedded performance assessment in class activities
16. Assessment Criteria
The common assessments are based on the Madison curriculum and Connecticut standards and grade level
expectations for science. For authentic assessments and projects students are given a rubric or grading criteria
before doing the work. A variety of assessment tools are employed to get the most accurate understanding of
individual student achievement possible.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 153 GRADE 7
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry progresses through a continuous
process of questioning, data collection, analysis
and interpretation.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing
about science.
Scientific literacy includes also the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
mathematical operations and procedures to
calculate, analyze and present scientific data and
ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
Why is it critical design and conduct
appropriate types of scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you identify independent and
dependent variables?
Why is it important to identify variables that
need to be kept constant?
Why is it essential to assess the data that was
collected, using mathematical operations to
analyze and interpret data, and present
relationship between variables in appropriate
graphs?
Why is it essential to assess the validity of the
experimental design identifying sources of
error and the credibility of scientific claims in
different sources of information?
Why is it important to communicate your
findings, using relevant scientific vocabulary
and clear logic that are based on the results
generated during the experiment?
KNOWLEDGE & LEARNING
The student will...
INSTRUCTIONAL SUPPORT MATERIALS
Identify questions that can be answered through
scientific investigation.
Formulate a testable hypothesis, in the ―If…,
then… because‖ format that is logically connected
to the problem.
Design an experiment in which the independent
and dependent variables are accurately identified
and variables, which need to be, are kept
constant.
Use appropriate tools and techniques that are
appropriate for the design of the experiment, for
making observations and gathering data.
Accurately collect and record appropriate data.
Use mathematical operations to analyze and
interpret data.
Interpret and create appropriate graphs to
present relationships between variables.
Develop logical conclusions that are based on the
analysis of experimental data.
Report findings and conclusions in various formats
(i.e., lab reports) using relevant vocabulary,
supporting evidence, and clear logic.
Prentice Hall modules
Lab materials
Safety equipment
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during instruction
Inquiry activities and investigations
Guided internet research
Performance tasks
SUGGESTED ASSESSMENT METHODS
Lab Reports
Research projects/activities
Inquiry literacy questions
Benchmarks
CMT Embedded Tasks (Modified Versiona):
-
―Feel the Beat‖
- ―Shipping and Sliding‖ Friction Lab
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 154 GRADE 7
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 155 GRADE 7
LEARNING STRAND Structure and Function -
How are organisms structured to ensure efficiency and survival?
Unit: Human Body (Circulation, Respiration, Excretion)
CT Standard 7.2 – Many organisms, including humans, have specialized organ systems that interact with each other to maintain
dynamic internal balance.
ENDURING UNDERSTANDING
Multicellular organisms need specialized
structures and systems to perform basic life
functions.
ESSENTIAL QUESTIONS
Why are the basic anatomical structures of the
human respiratory, circulatory, and excretory
system so vital?
How do the organ systems bring oxygen and
nutrients to the cells and expel wastes?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
The major parts of the human respiratory
system are the nose, trachea, bronchi and
lungs. This system is responsible for breathing
and exchange of gases between the body and
its surroundings.
The major parts of the human circulatory
system are the heart, arteries, veins and
capillaries. The right side of the heart pumps
blood to the lungs for gas exchange; the left
side of the heart pumps the oxygenated blood
around the body.
The blood is made up of plasma, red and
white blood cells, and platelets. Its main role
is to carry small food molecules and respiratory
gases (oxygen and carbon dioxide) to and from
cells. Blood cells are also responsible for
destroying invading particles, preventing
diseases and stopping bleeding after injuries.
The respiratory and circulatory systems work
together to provide all cells with oxygen and
nutrients. When the body’s need for oxygen
changes, the circulatory and respiratory
systems respond by increasing or decreasing
breathing and heart rates. These changes can
be measured by counting breaths, heartbeats
or pulses per minute.
Prentice Hall Science Explorer Human Biology
and Health
Lab equipment
Lab Safety equipment
Microscopes
Timers
Stethoscopes
Thermometers
Limewater
Bill Nye's ―Circulation‖ video
Food coloring and small beakers or cups
Apples and plastic bags
SUGGESTED INSTRUCTIONAL STRATEGIES
Label the major anatomic parts of the human
body corresponding to the circulatory,
respiratory, and excretory systems.
View microscopic specimens of the specialized
cells, tissue and organ systems.
Perform research on circulatory conditions.
Perform the state embedded task ―Feel the
Beat.‖
Demonstrate how perspiration affects body
temperature.
Show the Bill Nye's video ―Circulation.‖
Perform a ―Circulatory Walk.‖
Perform the activity ―Excretion and Exercise.‖
Design a poster or create a story, cartoon, poem,
or song to show the complete path of a red blood
cell through the human body.
Demonstrate how more pressure affects blood
flow using a squeeze bottle with water.
Perform the A-B-O Lab (
p. 103 Human Biology
and Health).
Perform the activity ―How Does Filtering a Liquid
Change the Liquid?‖ (
p. 127 Human Biology and
Health).
Perform testing for the presence of glucose and
protein
(pp 132-133 Human Biology and Health).
Perform ―The Skin as Barrier‖ as demonstration
(
pp 152-153 Human Biology and Health).
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 156 GRADE 7
SUGGESTED ASSESSMENT METHODS
Benchmark: CMT Embedded Task:
―Feel the Beat‖
Performance Tasks:
Demonstrate how the heart functions to
circulate and re-oxygenate blood in the human
body.
Compare the structures and functions of the
basic components of blood (plasma, platelets,
red and white cells).
Analyze the interaction between the circulatory
and respiratory systems as the demand for
oxygen changes.
Other Assessments:
Teacher-created quizzes
Common unit test
Lab activities
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to meet these state expectations:
1. Label the major parts of the human respiratory
system and explain in writing the function of
each part (nasal cavity, trachea, bronchi, lungs
and diaphragm).
2. Label the major parts of the human circulatory
system and explain in writing the function of
each part (heart, veins, arteries and capillaries).
3. Design and conduct controlled variable
experiments to analyze the interaction between
the circulatory and respiratory systems as the
demand for oxygen changes.
CMT CORRELATION
Describe the structures of the human digestive,
respiratory and circulatory systems and explain
how they function to bring oxygen and nutrients
to the cells and expel waste materials.
SCIENTIFIC LITERACY TERMINOLOGY: structure, function, cell, mitosis, organelle, cytoplasm, nucleus,
cell membrane, mitochondrion, tissue, organ, system
KEY SCIENCE VOCABULARY
: trachea, bronchi, arteries, veins, capillaries, plasma, platelets
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 157 GRADE 7
LEARNING STRAND Energy in the Earth's Systems -
How do external and internal sources of energy affect the Earth's
Systems?
Unit: Geology
CT Standard 7.3 – Landforms are the result of the interaction of constructive and destructive forces over time.
ENDURING UNDERSTANDINGS
Volcanic activity and the folding and faulting of
rock layers during the shifting of the Earth’s crust
affect the formation of mountains, ridges, and
valleys.
Glaciation, weathering, and erosion change the
Earth’s surface by moving earth materials from
place to place.
ESSENTIAL QUESTIONS
How do external and internal sources of
energy affect the Earth’s systems?
How do folded and faulted rock layers
provide evidence of the gradual up and down
motion of the Earth’s crust?
How do glaciation, weathering and erosion
create and shape valleys and floodplains?
How can the boundaries of tectonic plates be
inferred from the location of earthquakes and
volcanoes?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 7.3.a.
Earth's surface is constantly being shaped and
reshaped by natural processes. Some of these
processes, like earthquakes and volcanic
eruptions, produce dramatic and rapid change.
Others, like weathering and erosion, usually
work less conspicuously over longer periods of
time.
Glaciers form in areas where annual snowfall is
greater than the seasonal melt, resulting in a
gradual build-up of snow and ice from one season
to the next.
Glaciers increase and decrease in size over long
periods of time, depending on variations in Earth’s
climate.
Glaciers move slowly, spreading outward across a
region or moving down a slope.
Moving glaciers reshape the land beneath them by
scraping, carving, transporting and depositing soil
and rock.
Glacial landforms have identifiable shapes.
Connecticut’s landscape provides many examples
of glacial movement and deposition.
Weathering and erosion work together as
destructive natural forces. Both are forces that
break down rock into small particles called
sediments.
Weathering is caused by physical, chemical or
biological means. Rock properties, such as
hardness, porosity or mineral content, influence
susceptibility to weathering.
Erosion loosens and transports sediment formed by
weathering. Moving water and wind cause changes
to existing landforms and create new landforms
such as valleys, floodplains, plateaus,
Prentice Hall module Earth’s Changing
Surface (2009)
Prentice Hall Module Inside Earth(2008)
Maps (on the computer and hard copies)
GEMS kit for making topographic maps
Stream tables
Rock and mineral samples
Mineral testing equipment
General lab supplies
Lab safety equipment
Stereoscopes
Proscope
GEMS magnetic boards
Internet access
GPS
Sand, toilet paper rolls, rulers
―Shake, Rattle, and Roll‖ Set
SUGGESTED INSTRUCTIONAL STRATEGIES
Do the cut and paste activity showing the
major lithospheric plates.
Use maps and globes to find locations.
Use maps to show the large variety of maps
made for different purposes.
Show students how to interpret a
topographic map. Perform an activity how to
make a topographic map. (Page 31 Earth’s
Changing Surface)
Perform a rock shake activity to see how
shaking and/or acid affect the rate of
weathering of limestone.
Examine rocks testing for their mineral
content.
Perform the ―Sand Hill‖ activity (Page 70
Earth’s Changing Surface)
Perform a stream table activity (i.e., pages
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 158 GRADE 7
canyons, caves or dunes.
GRADE LEVEL CONCEPT 7.3.b.
Earth’s surface features, such as mountains,
volcanoes and continents, are the constantly-
changing result of dynamic processes and forces at
work inside the Earth.
The solid Earth has a core, mantle and crust
each with distinct properties.
Earth’s crust is broken into different ―tectonic
plates‖ that float on molten rock and move very
slowly. Continental drift is driven by convection
currents in the hot liquid mantle beneath the
crust.
The presence of plant and animal fossils of the
same age found around different continent shores,
along with the matching coastline shapes of
continental land masses, provides evidence that
the continents were once joined.
Tectonic plates meet and interact at divergent,
convergent or transform boundaries. The way in
which the plates interact at a boundary affects
outcomes such as folding, faulting, uplift or
earthquakes.
The folding and faulting of rock layers during the
shifting of the Earth’s crust causes the constructive
formation of mountains, ridges and valleys.
Mountain formation can be the result of
convergent tectonic plates colliding, such as the
Appalachians and the Himalayas; mountains may
also be formed as a result of divergent tectonic
plates moving apart and causing rifting as in East
Africa or Connecticut.
Most volcanoes and earthquakes are located at
tectonic plate boundaries where plates come
together or move apart from each other. A
geographic plot of the location of volcanoes and
the centers of earthquakes allows us to locate
tectonic plate boundaries.
The geological makeup of Connecticut shows
evidence of various earth processes, such as
continental collisions, rifting, and folding that have
shaped its structure.
82-83 Earth’s Changing Surface)
Demonstrate the affect of moving air on
sediment by blowing air through a straw at a
container with a flat layer of cornmeal being
careful not to blow it in the direction of
students.
Using photographs, show evidence of
processes that shape the surface of the Earth
(i.e., glaciers, volcanoes, weathering and
erosion, faulting, folding).
Show virtual tours from Eastern Connecticut
State University website.
Locate the epicenter and focus of an
earthquake on a diagram.
Plot the location of volcanoes and/or
earthquakes comparing it to the location of
plate boundaries.
Utilize the ―Shake, Rattle, and Roll‖
equipment.
Use a Dichotomous Key to classify igneous,
metamorphic and sedimentary rocks.
Use the magnetic boards from the GEMS kit
to explore the concept of magnetic pole
reversal.
Use the fault models or sponges to
demonstrate faulting.
Use the interactive CD ―Plate Tectonics‖ to
show island formation from plumes, the
formation of island arcs, and volcanic arcs,
and evidence for plate tectonics as well as to
review general concepts.
SUGGESTED ASSESSMENT METHODS
Performance Task:
Stream table activity
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
1. Illustrate and describe in writing the
composition of the three major layers of the
Earth’s interior.
2.
Explain how Earth’s internal energy is
transferred to move tectonic plates.
3.
Demonstrate the processes of folding and
faulting of the Earth’s crust.
4.
Correlate common geological
features/events (deep sea trenches,
mountains, earthquakes, volcanoes) with
the location of plate boundaries.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 159 GRADE 7
5.
Compare geological features that result
from constructive forces (e.g., mountains
and ridges) with geological features that
result from destructive forces (e.g., canyons
and flood plains).
6.
Analyze and interpret data about the
location, frequency and intensity of
earthquakes.
7.
Compare and contrast the major agents of
erosion and deposition of sediments:
running water, moving ice, wave action,
wind and mass movement due to gravity.
8.
Investigate and determine how glaciers
form and affect the Earth’s surface as they
change over time.
9.
Distinguish between weathering and
erosion.
10.
Observe and report on the geological events
that are responsible for having shaped
Connecticut’s landscape.
CMT CORRELATIONS
Describe how folded and faulted rock layers
provide evidence of gradual up and down
motion of the Earth’s crust.
Explain how glaciation, weathering and
erosion create and shape valleys and
floodplains.
Explain how the boundaries of tectonic plates
can be inferred from the location of
earthquakes and volcanoes.
SCIENTIFIC LITERACY TERMINOLOGY: Erosion, weathering, glacier, valley, floodplain, core,
mantle, folds, fault/fault line, continent, tectonic plate, plate boundary, convection, mountains, volcano,
earthquake.
KEY SCIENCE VOCABULARY
: igneous, sedimentary, metamorphic, plateaus, canyons, caves, dunes,
lithosphere, asthenosphere, Pangaea
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 160 GRADE 7
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 161 GRADE 7
LEARNING STRAND Science and Technology in Society -
How do science and technology affect the quality of our lives?
Unit: Connecticut Water Resources
CT Standard 6.4 – Water moving across and through earth materials carries with it the products of human activities.
ENDURING UNDERSTANDING
Most precipitation that falls on Connecticut
eventually reaches the Long Island Sound.
ESSENTIAL QUESTIONS
What is the affect of septic and sewage
systems on the quality of surface and
ground water?
How might human activity impact water
resources in Connecticut such as ponds,
rivers, and the Long Island Sound?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Water is essential for life and is a distinguishing
feature of Earth among the planets in our solar
system. Humans and other organisms use water in
various ways.
The surface of Earth is largely covered with water,
most of which is saltwater found in oceans. Only
freshwater is drinkable, and it is found on the land
(surface water), beneath the ground (groundwater),
and frozen in glaciers.
Water is a universal solvent that dissolves and
carries many substances through the environment
(e.g., acid rain, calcium, carbon dioxide, oxygen,
salt, metals, etc). Many substances that are
dissolved in water may be either harmful
(pollutants) or beneficial to organisms (minerals,
oxygen, nutrients). Water temperature affects its
ability to dissolve substances such as oxygen and
salt.
Some water that falls to Earth as precipitation soaks
into the ground, some evaporates almost
immediately, and some moves across earth’s
surfaces filling streams, rivers and reservoirs.
Factors affecting whether water seeps into the
ground include the amount of rainfall, the length of
time it falls, the permeability of the ground
surface and subsurface, the saturation of the soil,
and the steepness (slope) of the land.
Water moving beneath the earth’s surface is
influenced by size of and spaces between the
particles in rock and soils.
Water moving across the earth’s surface is affected
by the shape and slope of the land and the
properties of the surface materials it encounters.
The area draining into a river system or other body
of water is a watershed. Folds and faults in
Connecticut’s landform cause water to move
generally from north to south, eventually draining
into Long Island Sound.
Prentice Hall Science Explorer Earth’s
Waters (2008)
Lab materials
Internet access
Water quality testing materials
Topographic maps of watersheds
Easel paper, markers, materials
representing pollutants, masking tape
SUGGESTED INSTRUCTIONAL STRATEGIES
Explain the role of septic and sewage
systems. Compare and contrast them.
Perform some water quality tests.
Using topographic maps identify the
watershed of a stream or river in
Connecticut.
Perform the Project O suggested activity
demonstrating point source and non point
source pollution in the Connecticut River.
Design and perform an experiment to clean
water after pollutants have contaminated it
(filtering).
Trace a drop of water as it falls to Earth.
Have different groups of students trace the
drop from a variety of locations making a
flow chart or drawing of its travel.
Perform a demonstration to show the
desalination process (Page 56 in the
Prentice Hall module Earth’s Waters).
Collect data on the amount of water
students use each day (using averages for
showers, etc.) and compare their usage to
national and global usage per capita.
Brainstorm ways to conserve water and to
reduce water pollution (i.e., rain collection
to water gardens, drip irrigation).
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 162 GRADE 7
Water moving through a watershed picks up,
suspends or dissolves various substances produced
by nature and by human activities. The quality and
usability of water depends on what materials have
been picked up, carried and concentrated in the
water.
Water quality is important to support a variety of
aquatic life and for human consumption. Water
quality is evaluated by measuring indicators such as
levels of dissolved oxygen, pH, turbidity and the
presence of other dissolved substances. Substances
such as heavy metals (e.g., lead and aluminum),
sulfur, fertilizers, road salt are pollutants that may
be dissolved in surface water or ground water,
making the water unhealthy.
Water entering Long Island Sound carries with it the
products of human use. These pollutants negatively
impact the aquatic life, commercial and recreational
uses of the Sound.
Point source pollution, such as untreated
sewage, industrial or recreational waste, can be
discharged directly into the Sound if it is not
regulated and controlled.
Non-point source pollution is difficult to trace or
control because it originates across the large
watershed area that drains into Long Island Sound.
A major contaminant reaching Long Island Sound by
way of watersheds is nitrogen.
Drinking water may come from groundwater sources
accessed by drilling wells, or from surface water
reservoirs.
People’s use of water adds waste products and
harmful materials to the water which must be
removed before returning the water to the
environment. Wastewater can be purified using
various physical, biological and chemical processes.
Septic systems use settling and bacterial digestion
to break down wastes in a holding tank; then the
water is further purified as it is spread across a
leaching field and percolates through layers of soil.
Sewage treatment facilities are required in densely
populated areas. Sewage treatment facilities use
multiple filtrations, biological and chemical methods
to purify water before returning the water to the
environment.
Laws, regulations and remedial actions have helped
to protect and restore water resources.
Research laws that oversee the regulation
of water use and water quality. Debate
fabricated controversial issues regarding
water quality such as the dumping of
wastes in the ocean or a land fill being
proposed for property near the school.
SUGGESTED ASSESSMENT METHODS
Performance Task:
Design and perform an experiment to
clean water after pollutants have
contaminated it (i.e., filtering).
Other Assessments:
Teacher-created quizzes
Common unit test
Student research and debate on
environmental issue
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Discuss and chart the reasons why water is
essential for life.
2. Observe, analyze and record the unique
physical and chemical properties of water.
3. Research the differences in quantities
between fresh water (solid and liquid) and
salt water covering the Earth’s surface and
report on the impact to humans.
4. Investigate and explain in writing how
substances, both harmful and beneficial,
dissolve in and are carried by surface and
ground water.
5. Use appropriate maps to locate and
identify the major watersheds that drain
into Long Island Sound and analyze how
the topography influences the way water
moves in the Long Island Sound
watershed.
6. Research and evaluate in writing the
effects of common point and nonpoint
water pollutants in Connecticut.
7. Compare and contrast the general
structures, processes and limitations of a
septic system to a secondary wastewater
treatment plant.
8. Debate the effectiveness of a law designed
to protect water resources.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 163 GRADE 7
CMT CORRELATIONS
Explain the role of septic and sewage
systems on the quality of surface and
ground water.
Explain how human activity may impact
water resources in Connecticut, such as
ponds, rivers, and the Long Island Sound
ecosystems.
SCIENTIFIC LITERACY TERMINOLOGY: surface water, ground water, fresh water, salt water,
pollutant, watershed, point source pollution, nonpoint source pollution, well, septic system, wastewater,
KEY SCIENCE VOCABULARY
: runoff, sewer, turbidity, universal solvent, permeability, slope,
topographic, brackish
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 164 GRADE 7
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 165 GRADE 7
LEARNING STRAND Force and Motion -
What makes objects move they way they do?
Unit: Forces and Motion
CT Standard 8.1 – An object’s inertia causes it to continue moving the way it is moving unless it is acted upon by a force.
ENDURING UNDERSTANDINGS
The motion of an object can be described by its
position, direction of motion and speed.
An unbalanced force acting on an object
changes its speed and/or direction of motion.
Objects moving in circles must experience force
acting toward the center.
ESSENTIAL QUESTIONS
How is the speed of a moving object calculated?
How is motion illustrated in graphs?
How can the qualitative relationships among
force, mass and changes in motion be described?
How can the forces acting on an object moving in
a circular path be described?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
An object is said to be in motion when its
position changes in relation to a point of
reference. An object’s motion can be described
and represented graphically according to its
position, direction of motion, and speed.
Speed describes the change in an object’s
position over a period of time, and is measured
in units such as meters per second or miles per
hour.
Average speed takes into account the different
speeds at which an object moves over a period
of time. Average speed is calculated by dividing
the total distance traveled by the change in time,
regardless of any changes in motion or direction
during its travel.
Motion of objects can be represented on a
distance vs. time line graph, with distance
traveled as the vertical (―y‖) axis and time as the
horizontal (―x‖) axis. The steepness and slant of
the motion line vary depending on the speed and
direction of the moving objects. A straight
horizontal line indicates an object at rest.
In order for an object to change its motion, a
push/pull (force) must be applied over a
distance.
Forces can act between objects that are in direct
contact, or they can act over a distance. There
are forces of attraction, such as gravity or
magnetism, and forces of resistance, such as
friction and drag (air resistance). Forces are
measured in Newtons or pounds using scales.
Forces can act simultaneously on an object from
all directions with different strengths
(magnitudes). When the magnitude and
direction of all the forces acting on an object are
combined, or added together, the total force
(net force) determines the object’s motion.
Forces in opposite directions are subtracted;
forces in the same direction are added.
If the strength of all the forces acting on an
Prentice Hall module Forces, Motion and Energy
(2009)
Wooden blocks, bricks, boxes
Spring scales
Carts and metal weights
Magnets
Friction blocks
Toys (i.e., rattlebacks, rubber balls, remote-
controlled car, gyroscopes, airplanes)
Timers
Various surfaces
Newtonian demonstrator
Circular motion demonstrator
SUGGESTED INSTRUCTIONAL STRATEGIES
Explore the effects of forces by working through
stations involving buoyant force, magnetic force,
elastic force, gravitational force, static electrical
force, and friction.
Perform the state embedded task ―Shipping and
Sliding.‖
Design an experiment comparing rolling and
sliding friction.
Solve problems using the formulas for force,
momentum, and acceleration.
Demonstrate a noncontact force using a super
magnet.
Explore motion using toys such as tops,
gyroscopes, Frisbees, rattlebacks, and rubber
balls.
Perform an activity flying toy airplanes or racing a
remote-controlled car and calculate the speed of
the object.
Demonstrate Newton’s Laws of Motion.
Predict the projectile motion of different masses
as they roll down an incline and off a table to the
floor.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 166 GRADE 7
object from one direction is equivalent to the
strength of the forces from the opposite
direction, then the forces cancel each other out,
and are said to be balanced. Balanced forces
keep an object moving with the same speed and
direction, including keeping it at rest.
If the net force acting on an object is not zero,
then the forces are said to be unbalanced, and
the object’s speed or direction will change,
changing its motion (acceleration).
Acceleration is any change in motion, and occurs
when something speeds up, slows down or
changes direction. On a position time graph,
this would be indicated by a change in the
steepness of the motion line, or by a curved line.
The greater the unbalanced force on an
object, the greater its change in motion
(acceleration). The greater the mass of an
object, the greater the force needed to change
its acceleration. Given the same amount of force,
an object with a greater mass will change
acceleration less. The total net force acting on
an object can be determined by measuring its
mass and change in motion (acceleration).
Some objects continuously change direction
without changing speed, causing them to move
in a circular path. Circular motion is caused by
a constant unbalanced force that is constantly
changing direction and pulling towards the
center. If there were no force pulling the object
toward the center, it would continue to move in
a straight line in the direction it was moving
before the force was removed.
SUGGESTED ASSESSMENT METHODS
Benchmark Task:
CMT Embedded Task: Revised
―Shipping and
Sliding‖
Friction Lab
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Demonstrate how forces, including friction, act on an
object to change its position over time in relation
to a fixed point of reference.
2. Calculate the average speed of a moving object,
and distinguish between instantaneous speed and
average speed of an object.
3. Create and interpret distance-time graphs for
objects moving at constant and nonconstant
speeds.
4. Predict the motion of an object given the
magnitude and direction of forces acting upon it
(net force).
5. Investigate and demonstrate how unbalanced
forces cause acceleration (change in speed and/or
direction of an object’s motion).
6. Assess in writing the relationship between an
object’s mass and its inertia when at rest and in
motion.
7. Express mathematically how the mass of an
object and the force acting on it affect its
acceleration.
8. Design and conduct an experiment to determine
how gravity and friction (air resistance) affect a
falling object.
9. Illustrate how the circular motion of an object is
caused by a center-seeking force (centripetal
force) resulting in the object’s constant
acceleration.
CMT CORRELATIONS
Calculate the average speed of a moving object
and illustrate the motion of the object in graphs of
distance over time.
Describe the qualitative relationships among force,
mass and changes in motion.
Describe the forces acting on an object moving in
a circular path.
SCIENTIFIC LITERACY TERMINOLOGY: motion, point of reference, speed, constant speed, average
speed, position-time graph, slope, force, friction, gravity, inertia, mass, acceleration, balanced/unbalanced
forces, net force, circular motion, Newtons
KEY SCIENCE VOCABULARY
: point of reference, centripetal force, magnitudes
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 167 GRADE 7
LEARNING STRAND Energy Transfer and Transformations -
What is the role of energy in our world?
Unit: Work and Machines
CT Standard 7.1 – Energy provides the ability to do work and can exist in many forms.
ENDURING UNDERSTANDINGS
Work is the process of making objects move through
the application of force.
Energy can be stored in many forms and can be
transformed into the energy of motion.
ESSENTIAL QUESTIONS
How does the formula W=FD allow
problems to be solved for force, distance,
and work when lifting heavy objects?
How are simple machines, such as inclined
planes, pulleys, and levers used to create
mechanical advantage?
How are different types of stored (potential)
energy used to make objects move?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 7.1.a.
In order for an object to change its motion, a
push/pull (force) must be applied over a distance.
Forces can act between objects that are in direct
contact, such as pulling directly on a string or friction
acting on a sliding block. Forces can act over a
distance, such as gravity or magnetism. Forces are
measured in Newtons or pounds using scales.
Work is a scientific concept that expresses the
mathematical relationship between the amount of
force needed to move an object and how far it moves.
For work to be done, a force must be applied for a
distance in the same direction as the motion. An
object that does not move has no work done on it,
even if forces are being applied.
Work (measured in Joules) is calculated by
multiplying the force (measured in newtons) times the
distance (measured in meters). When an object is
lifted, the work done is the product of the force of
gravity (weight) times the height the object is lifted.
The amount of work done is increased if more force is
applied or if the object is moved a greater distance.
Simple machines can be used to move objects.
People do ―input‖ work on a simple machine which, in
turn, does ―output‖ work in moving an object. Simple
machines are not used to change the amount of work
to move or lift an object; rather, simple machines
change the amount of effort force and distance for the
simple machine to move the object.
Simple machines work on the principle that a small
force applied over a long distance is equivalent work
to a large force applied over a short distance.
Some simple machines are used to move or lift an
object over a greater output distance (snow shovel),
or change direction of an object’s motion, but most
are used to reduce the amount of effort (input
force) required to lift or move an object (output
Prentice Hall module Forces, Motion, and
Energy (2009)
Levers and pennies
Inclined plane
Weights
Spring scales
Pulleys, string, ring stands
Internet access
Samples of levers
SUGGESTED INSTRUCTIONAL STRATEGIES
Perform a pennies and Levers activity and
compare the forces when either the
resistance force is closer to the fulcrum or
the effort force is closer to the fulcrum.
Use simple tools (i.e., hammer, wrench,
pliers, broom) to demonstrate classes of
levers.
Perform a pulley activity to compare the
mechanical advantage of various
arrangements.
Perform an inclined plane activity to
determine what effect height has on the
mechanical advantage of an inclined plane.
Complete the Simple Machines Computer
lab activity by visiting
www.edheads.org/activities/simple-
machines/
Learn more about simple machines and to
determine what simple machines make up
four common compound machines.
SUGGESTED ASSESSMENT METHODS
Performance Task
Inclined plane activity
Other Assessments:
Teacher-created quizzes
Common unit test
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 168 GRADE 7
force).
An inclined plane is a simple machine that reduces
the effort force needed to raise an object to a given
height. The effort force and distance and output force
and distance depend on the length and height
(steepness) of the inclined plane.
A pulley is a simple machine that reduces the effort
force needed to lift a heavy object by applying the
force through a greater distance (pulling more rope
through the pulley). The effort force and distance,
output force and distance, and direction of motion all
depend on the number of pulleys and their position.
A lever is a simple machine that reduces the effort
force needed to lift a heavy object by applying the
force at a greater distance from the fulcrum of the
lever. The effort force and distance, output force and
distance, and direction of motion all depend on the
position of the fulcrum in relationship to the input and
output forces.
The mechanical advantage of a simple machine
indicates how useful the machine is for performing a
given task by comparing the output force to the input
force. The mechanical advantage is the number of
times a machine multiplies the effort force. The longer
the distance over which the effort force is applied, the
greater the mechanical advantage of the machine.
The mechanical advantage of a machine can be
calculated by dividing the resistance force by the
effort force. Most of the time the resistance force is
the weight of the object in newtons.
Simple machines always produce less work output
than work put in, because some motion energy is
converted to heat and sound energy by friction.
GRADE LEVEL CONCEPT 7.1.b.
Energy is the ability to cause objects to change
position (motion).
Potential energy is the capacity for doing work that
a body possesses because of its position or condition.
Gravitational potential energy (an object about to roll
down a hill), elastic potential energy (a stretched
rubber band) and chemical potential energy
(carbohydrates in foods).
Kinetic energy is energy a body possesses because
it is in motion.
Energy is changed (transformed) from one form to
another. For example, potential chemical energy of
foods, which is often measured in Calories, is
transformed by cells into heat, electrical and kinetic
energy used in the body.
When energy is transformed, the total amount of
energy stays constant (is conserved).
Work is done to lift an object, giving it gravitational
Word problem applications
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
1. Conduct simple experiments that show and
explain how forces work to change the
motion of an object.
2.
Calculate work done on an object as force
or distance varies.
3.
Explain in writing how the six simple
machines make work easier but do not
alter the amount of work done on an
object, and demonstrate how everyday
objects function as simple machines.
4.
Determine ways to modify a simple
machine (inclined plane, pulley and lever)
to improve its mechanical advantage.
5.
Defend the statement, ―Work output of a
machine is always less than work input
because of energy lost due to friction.‖
6.
Design and create a working compound
machine from several simple machines.
7.
Use a diagram or model of a moving object
(roller coaster, pendulum, etc.) to describe
the conversion of potential energy into
kinetic energy and vice versa.
8.
Discuss different forms of energy and
describe how they can be converted from
one form to another for use by humans
(e.g., thermal, electrical, light, chemical,
mechanical).
9.
Trace energy conversions that occur in the
human body once food enters and explain
the conversions in writing.
10.
Calculate potential and kinetic energy and
relate those quantities to total energy in a
system.
____________________________________
CMT CORRELATIONS
Explain the relationship among force,
distance and work and use the relationship
(W=FxD) to calculate work done in lifting
heavy objects.
Explain how simple machines, such as
inclined planes, pulleys and levers are used
to create mechanical advantage.
Describe how different types of stored
(potential) energy can be used to make
objects move.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 169 GRADE 7
potential energy (weight x height). The gravitational
potential energy of an object moving down a hill is
transformed into kinetic energy as it moves, reaching
maximum kinetic energy at the bottom of the hill.
Some kinetic energy is always transformed into heat
by friction; therefore, the object will never reach the
same height it started from again without added
energy.
SCIENTIFIC LITERACY TERMINOLOGY: force, friction, gravity, weight, Newton, scale, work, joule,
effort (input) force, output force, simple machine, lever, fulcrum, pulley, inclined plane, mechanical
advantage, energy, potential energy, kinetic energy, energy transformation, conservation of energy
KEY SCIENCE VOCABULARY
: output (resistance/load) force, Ideal Mechanical Advantage, Actual
Mechanical Advantage
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 170 GRADE 7
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 171 GRADE 7
LEARNING STRAND Science and Technology in Society -
How do science and technology affect the quality of our lives?
Unit: Structures (Bridges)
CT Standard 8.4 – In the design of structures there is a need to consider factors such as function, materials, safety, cost, and
appearance.
ENDURING UNDERSTANDING
Bridges can be designed in different ways to
withstand certain loads and potentially
destructive forces.
ESSENTIAL QUESTION
How are beam, truss and suspension bridges
designed so they can withstand the forces that
act on them?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
A force is a push or a pull and is described by
its strength and direction and can be caused by
a moving or a stationary object. Forces are
measured in newtons or pounds using scales.
Forces can act simultaneously on an object from
all directions with different strengths
(magnitudes). When the magnitude and
direction of all the forces acting on an object
are combined, or added together, the total force
(net force) determines the object’s motion.
Forces in opposite directions are subtracted;
forces in the same direction are added.
If the strength of all the forces acting on an
object from one direction is equivalent to the
strength of the forces from the opposite
direction, then the forces cancel each other out,
and are said to be balanced.
Bridges are elevated structures designed to
support the movement of objects over a span.
Two important forces at work in bridges are
tension
and
compression
.
Bridges must support their own weight (dead
load) and the weight of those objects that will
cross over them or act on them from time to
time, such as wind, snow and ice (live load).
Bridges are kept stable by balancing the load
forces with the supporting forces of the
structure. These forces can cause parts of the
bridge structure to push together (compression)
or pull apart (tension).
Different bridge designs distribute tension and
compression forces in different ways, depending
on the shapes of the parts of the structure. The
biggest difference among bridge designs is the
distances they can cross in a single span.
Shapes commonly used in bridge design include
arches, triangles and rectangles.
Bridges are constructed of different materials
whose properties and costs vary. Some
materials are strong against compression forces
but weak against tension forces; some materials
resist fire, corrosion or weathering. Materials
commonly used in bridge design include wood,
Index cards and masking tape
K-nex kits
Internet access
Pictures of bridges
SUGGESTED INSTRUCTIONAL STRATEGIES
Build a bridge with four index cards to support
various metal weights.
Perform a pendulum lab and compare it to the
motion of skyscrapers.
Build structures using K-nex kits.
Build towers or bridges out of straws, toothpicks
and clay to support the weight of nails.
Identify bridges in town and worldwide that fit
the classification of four major bridge types.
Use the interactive website on bridges
http://www.pbs.org/wgbh/buildingbig/bridge
Compare the Tacoma-Narrows Bridge that
collapsed to the one that replaced it.
SUGGESTED ASSESSMENT METHODS
Performance Task:
Building bridge/towers to support nails
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Identify the forces acting on a truss, beam and
suspension bridge, including compression,
tension and gravity using models, pictures or
diagrams.
2. Explain in writing the advantages and
disadvantages of truss, beam and suspension
bridge design and visually identify each bridge.
3. Conduct an experiment to discover and report
on a bridge’s ability to support a load based on
the interplay of tension and compression forces
that result in a net force of zero.
4. Use technology to simulate how engineers plan,
test and revise bridge designs given
parameters including cost, time, safety and
aesthetics.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 172 GRADE 7
rope, aluminum, concrete and steel.
A beam bridge balances the load by
concentrating it entirely onto the two piers that
support the bridge at either end. When a force
pushes down on the beam, the beam bends. Its
top edge is pushed together (compression), and
its bottom edge is pulled apart (tension). The
amount of bend depends on the length of the
beam.
A truss bridge uses rigid, interlocking beams
to form a system of triangles that distribute the
load among all parts of the structure, increasing
the structural strength of the bridge.
A suspension bridge uses cables suspended
from tall towers to hold up the deck and
distribute the load. The tension and
compression forces acting on the beam are
distributed among the cables (which experience
tension) and the towers (which experience
compression).
Engineers and scientists build models of
bridges, conduct controlled experiments to learn
how they will withstand various stresses, and
consider the benefits and trade-offs of various
design alternatives.
Bridge design is influenced by the length of the
span, the properties of the materials and the
environmental conditions, as well as by practical
considerations, such as the bridge’s appearance,
cost of materials or construction site challenges.
Bridges can fail because they have faulty parts,
are used in ways that exceed what was
intended by the design, or were poorly designed
to begin with.
_____________________________________
CMT CORRELATION
Explain how beam truss and suspension bridges
are designed to withstand the forces that act on
them.
SCIENTIFIC LITERACY TERMINOLOGY: balanced/unbalanced forces, net force, load, tension force,
compression force, beam bridge, truss bridge, suspension bridge force
KEY SCIENCE VOCABULARY
: dead load, live load, Newtons, tension, weathering, scale, motion,
materials, span, I-beam, arch bridge
Content Standards & Indicators
for Grade 8
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 175 GRADE 8
Course Description
MIDDLE SCHOOL
1. Course Title
Grade 8 General Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Science
3. Transcript Course Code/Number
4. Program Contact Information
Name: Kathleen Brooks
Title/Position: Middle School Science Coordinator
School: Walter C. Polson Middle School
302 Green Hill Road
Madison, CT 06443
Phone: 245-6475 X7082
6. Grade Level: 8
7. Seeking ―Honors‖ Distinction?
Yes No Not Applicable
8. Unit Value
Full Year
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
N/A
11. Brief Course Description
The eighth grade science course is a general science course. It is part of a spiraling curriculum in which aspects of
life science, physical science and earth/space science are addressed each school year. In grade eight the life
science topics include the study of life processes at both the cellular and multi-cellular levels and genetics. The
physical science topics include the study of chemistry, electromagnetic systems, light and sound. The earth/space
science topic is astronomy. A unit of forensic science is also taught.
12. Course Goals
The upper middle school science program combines the development of logical, scientific thought processes with
current scientific theories, terminology, and factual information. Participating in an inquiry-based learning
environment
Provides opportunities for students to practice the skills of observation, investigation, experimentation, and
research.
Assists students in the development of science literacy to make them more aware and understanding of the
natural and physical of the world around them.
Ensures students meet the science standards for Connecticut public schools.
Encourages students to become interested in science as well as to learn of careers in science.
13. Course Outline
Astronomy
- Planetary Motion
- Gravity in space
Chemistry
- Atoms, Elements, Compounds
- The Periodic Table of Elements
Life Processes
- Life Processes in the Cell
- Respiration vs. Photosynthesis

MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 176 GRADE 8
- Human Nervous System and Senses
- Human Digestive System
Genetics
- Mendel’s Principles
- Punnett Squares
- Pedigree
- Genetic Disorders
Electromagnetic Systems
- Static Electricity
- Current Electricity
- Magnetism
Light and Sound
- Waves
- Light
- Sound
Forensic Science
14. Instructional Methods and/or Strategies
Individual and small group work
Whole class instruction and discussions
Lecture
Modeling
Inquiry-based activities
PowerPoint presentations and notes
Research
15. Assessment Methods and/or Tools
Quizzes
Common unit assessments
Authentic assessments
Lab reports
Research papers and/or projects
Embedded performance assessment in class activities
16. Assessment Criteria
The common assessments are based on the Madison curriculum and Connecticut standards and grade level
expectations for science. For authentic assessments and projects students are given a rubric or grading criteria
before doing the work. A variety of assessment tools are employed to get the most accurate understanding of
individual student achievement possible.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 177 GRADE 8
LEARNING STRAND
Unit: Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry progresses through a continuous
process of questioning, data collection, analysis and
interpretation.
Scientific inquiry requires the sharing of findings and
ideas for critical review by colleagues and other
scientists.
Scientific literacy includes speaking, listening,
presenting, interpreting, reading and writing about
science.
Scientific literacy includes also the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
mathematical operations and procedures to
calculate, analyze and present scientific data and
ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the design
of the experiment?
Why is it critical to design and conduct appropriate
types of scientific investigations, using the
appropriate tools and techniques, to make
observations and gather data to answer various
questions?
How do you identify independent and dependent
variables?
Why is it important to identify variables that need
to be kept constant?
Why is it essential to assess the data that was
collected, using mathematical operations to analyze
and interpret data, and present relationship
between variables in appropriate graphs?
Why is it essential to assess the validity of the
experimental design identifying sources of error and
the credibility of scientific claims in different sources
of information?
Why is it important to communicate your findings,
using relevant scientific vocabulary and clear logic
that are based on the results generated during the
experiment?
How do you make connections to what is learned in
science class to the real world?
KNOWLEDGE & LEARNING
The student will ...
INSTRUCTIONAL SUPPORT MATERIALS
Identify questions that can be answered through
scientific investigation.
Formulate a testable hypothesis, in the ―If..., then…
because‖ format that is logically connected to the
problem.
Design an experiment in which the independent and
dependent variables are accurately identified and
variables, which need to be, are kept constant.
Use appropriate tools and techniques that are
appropriate for the design of the experiment for
making observations and gathering data.
Accurately collect and record appropriate data.
Use mathematical operations to analyze and
interpret data.
Interpret and create appropriate graphs to present
relationships between variables.
Develop logical conclusions that are based on the
analysis of experimental data.
Report findings and conclusions in various formats
(i.e., lab reports) using relevant vocabulary,
supporting evidence, and clear logic.
Prentice Hall modules
Lab equipment
Safety equipment
Internet access
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during instruction
Inquiry activities and investigations
Guided internet research
Performance tasks
SUGGESTED ASSESSMENT METHODS
Performance Task:
―Ice and Salt‖ lab
Other Assessments:
Lab Reports
Research projects/activities
CMT-like inquiry questions
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 178 GRADE 8
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 179 GRADE 8
LEARNING STRAND Earth in the Solar System -
How does the position of Earth in the solar system affect conditions on our planet?
Unit: Astronomy
CT Standard 8.3 – The solar system is composed of planets and other objects that orbit the sun.
ENDURING UNDERSTANDINGS
Gravity is the force that governs the motion of
objects in the solar system.
The motion of the Earth and moon relative to the
sun causes daily, monthly and yearly cycles on
Earth.
ESSENTIAL QUESTIONS
How does the position of Earth in the solar
system affect conditions on our planet?
How does gravity affect the orbital movement of
planets in the solar system?
How do the relative motion and relative position
of the sun, Earth, and moon affect the seasons,
phases of the moon and eclipses?
How does the motion of Earth in relation to the
sun explain the phenomena of the day and the
year?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 8.3.a.
Earth is part of a system of celestial bodies that are
grouped together around a central star, the Sun.
This system includes objects of different masses
and composition such as planets, moons, asteroids,
minor planets, and comets. These objects move in
predictable paths determined by gravity.
Gravity is a force of attraction between two
objects. The strength of gravitational force
depends on the total mass of the two objects and
the distance between them. The greater the total
mass, the greater the force of gravity. The greater
the distance between two objects, the less the
force of gravity.
The difference between an object’s mass and its
weight is explained by gravity. Mass is the
measure of the amount of matter in an object;
weight is the force of gravity between an object
and the celestial body it is on. Bodies in the solar
system have different masses; therefore the same
object has a different weight on each celestial
body.
Objects in the solar system are held in their
predictable paths by the inward-pulling
gravitational attraction of the very massive sun.
The interaction of the center-pulling force of
gravity with a moving object’s inertia (tendency to
keep moving) keeps one object in circle-like motion
(revolution) around another. This causes planets to
orbit around the center of the solar system and
moons to orbit around planets.
The Earth and other planets move through space in
two ways: rotation on an axis and revolution
around the sun. Earth revolves around the sun in
a near-circular path, explaining cyclical phenomena
Module: Prentice Hall Science Explorer
Astronomy (2007)
Models, balls, flashlights, Internet access, NASA
materials including videos
Lunar Samples on loan from NASA to a certified
presenter
Internet access
Tide charts
SUGGESTED INSTRUCTIONAL STRATEGIES
Using a model of the sun, Earth and moon, show
the positions during eclipses.
Draw a diagram of a lunar eclipse and a solar
eclipse.
Show the class a NASA video on living in space.
Using the Internet, investigate the day and night
sky using websites.
Research astronomy topics and present them to
the class.
Make a travel brochure or poster inviting people
to visit a planet.
Create a question/answer book about what
causes a day, month, year, etc.
Make/interpret a graph of a local tide table and
how it relates to the moon.
Use the overhead projector and PowerPoint
presentations to enhance lectures.
Complete book assignments.
See a presentation of the lunar samples on loan
from NASA to a certified presenter.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 180 GRADE 8
such as seasons and changes in visible star.
patterns (constellations).
The time it takes for an object to complete one
revolution around the sun depends on the speed
at which it is moving and the size of its orbit.
Objects more distant from the sun’s gravitational
pull move slower than those that are closer.
Earth’s period of revolution is about 365 days
(year); planets that are more distant from the sun
take longer to orbit (revolve) around the sun,
resulting in longer years.
GRADE LEVEL CONCEPTS 8.3.b.
Earth rotates around an axis or rotation, a line
going through the center of the earth from the
north pole to the south pole. The tilt of Earth’s
axis relative to its orbital path, combined with the
spherical shape of the earth, cause differences in
the amount and intensity of the sun’s light striking
different latitudes of the earth.
Earth experiences seasons as northern or
southern hemispheres are tilted toward the sun
over the course of its 365-day revolution period.
Earth’s tilt causes seasonal differences in the
height of the perceived path of the sun and the
number of hours of sunlight. Seasons are not
related to a change in distance between the Earth
and the Sun, since that distance changes very
little.
The moon changes its position relative to the earth
and sun as it revolves around the earth in a period
of about 29 days. The same half of the moon is
always reflecting light from the Sun; some of the
reflected light reaches Earth. Phases of the moon
are explained by changes in the angle at which the
sun’s light strikes the moon and is reflected to
Earth. The relative position of the Sun, Earth and
moon can be predicted given a diagram of a moon
phase.
Performance Tasks:
Create a Venn Diagram to compare and contrast
a lunar eclipse and a solar eclipse.
Other Assessments:
Write an explanation of the effect of gravity on
the orbital movements of planets in the solar
system.
Make a drawing to explain how the relative
motion and relative position of the sun, Earth
and moon affect the seasons, phases of the
moon and eclipses.
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Relate the strength of gravitational force
between two objects to their mass and the
distance between the centers of the two objects
and provide examples.
2.
Describe in writing how gravitational attraction
and the inertia of objects in the solar system
keep them on a predictable elliptical pathway.
3.
Distinguish between rotation of Earth on its axis
and its elliptical revolution around the sun.
4.
Investigate and report in writing how the Earth’s
revolution around the sun affects changes in
daylight and seasons.
5.
Compare the revolution times of all the planets
and relate it to their distance from the sun.
6.
Conduct and report on an investigation that
shows how the Earth’s tilt on its axis and
position around the sun relates to the intensity
of light striking the Earth’s surface.
7.
Use a model to demonstrate the phases of the
moon relative to the position of the sun, Earth
and moon.
8.
Develop a model or illustration to show the
relative positions of the Earth, sun and moon
during a lunar and solar eclipse and explain how
those positions influence the view from Earth.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 181 GRADE 8
SUGGESTED ASSESSMENT METHODS
Eclipses occur when the moon, Earth and sun
occasionally align in specific ways. A solar
eclipse occurs when the when the moon is directly
between the Earth and the sun (during new moon
phase) and the moon blocks the sun’s light,
creating a moving shadow on parts of the earth. A
lunar eclipse occurs when the Earth is directly
between the moon and the sun (full moon
phase), the Earth blocks the sun’s light, casting a
shadow over the moon.
Ocean tides on Earth are caused by the moon’s
gravitational force pulling on large bodies of
water as the Earth and moon move around each
other daily. The regular daily and monthly
movement of the water (tides) can be
predicted.
CMT CORRELATIONS
Explain the effect of gravity on the orbital
movements of planets in the solar system.
Explain how the relative motion and relative
position of the sun, Earth, and moon affect the
seasons, phases of the moon and eclipses.
SCIENTIFIC LITERACY TERMINOLOGY: force, gravity, orbit, revolution, year, period, mass, weight,
rotation, hemisphere, season, phase, new moon, solar eclipse, lunar eclipse, tides
KEY SCIENCE VOCABULARY
: axis, phase, full moon, latitude, tilt waxing crescent, waning crescent,
waxing gibbous, waning gibbous, first quarter, third/last quarter
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 182 GRADE 8
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 183 GRADE 8
LEARNING STRAND Properties of Matter – How does the structure of matter affect the properties and uses of materials?
Unit: Chemistry
CT Standard 6.1 – Materials can be classified as pure substances or mixtures, depending on their chemical and physical properties.
ENDURING UNDERSTANDINGS
Pure substances can be either elements or
compounds and they cannot be broken down
by physical means.
Mixtures are made of combinations of elements
and/or compounds, and they can be separated
by using a variety of physical means.
ESSENTIAL QUESTIONS
How does the structure of matter affect the
properties and uses of materials?
How does the arrangement of the Periodic Table
describe the properties of an element?
How are the properties of elements different
from the properties of compounds?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
All matter is made of particles called atoms
that are too small to be seen without special
magnification. For example, a gold ring can be
broken into smaller and smaller pieces until the
pieces are no longer visible.
All matter is made of different combinations of
about 100 pure substances called elements.
The smallest particle of an element is an atom.
Iron is an example of an element that is made
up of only iron atoms.
Each element has distinct characteristic
properties. The Periodic Table of Elements is
used to organize elements based on properties
such as their reactivity, state of matter,
conductivity or density. Element names are
represented by letter symbols on the Periodic
Table.
Some elements, such as iron (―Fe‖) and
aluminum (―Al‖), are classified as
metals
because they have similar properties.
Individual metallic elements have distinct
characteristic properties (for example, sodium
(―Na‖) is a light, soft metal that is
nonmagnetic, while iron is a magnetic metal
that is denser than sodium and aluminum).
Some elements, such as carbon (―C‖),
hydrogen (―H‖), oxygen (―O‖) and chlorine
(―Cl‖), are classified as
nonmetals
. Carbon is
a nonmetal that occurs in several different
forms (graphite, diamond, and coal), each of
which has distinct properties. Hydrogen and
oxygen are nonmetals that are similar in that
they are both gases; however, each gas has
distinct characteristic properties such as color
and odor.
Atoms can combine chemically to make a
molecule of a new substance with new
properties called a compound. A molecule is
the smallest part of a compound and is made
of atoms of different elements in specific
Module: Prentice Hall Science Explorer Chemical
Interactions (2008)
General lab equipment
Lab safety equipment
Ice, salt, metals, HCl, calcium, pH indicators
Internet access
Plastic atoms of elements for overhead projector
Periodic Table of Elements wall charts and
student charts
SUGGESTED INSTRUCTIONAL STRATEGIES
Present safety guidelines.
Lecture using the overhead projector, computer
images, or Power Point for enhancement.
Give a lecture on the significant scientists who
impacted the discovery of the atom.
Create a list of identifying characteristics of a
solid, a liquid, and a gas.
Model how elements combine using the cross-
over method and Lewis Dot Diagrams.
Using the board, an overhead projector and
manipulatives, show students how to
demonstrate the Law of Conservation of Mass by
balancing equations.
Use a chart of the Periodic Table to describe the
properties of common elements and the general
organization of the table.
Draw posters of the elements.
Perform labs or demonstrations for the following:
- Calcium and Water
- Neutralization Reactions, and
- Acid and Metal Reactions.
Complete an Element Spreadsheet utilizing
Microsoft Excel.
Draw a Bohr model of an atom.
Facilitate a sing-along with students singing ―The
Element Song‖ following the words and music on
an interactive website.
SUGGESTED ASSESSMENT METHODS
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 184 GRADE 8
amounts
.
Unlike mixtures, compounds
cannot be separated using the physical
properties of the component elements.
Compounds have different properties than the
individual elements of which they are made.
For example, table salt (NaCl) is a compound
with different characteristic properties than the
elements sodium and chlorine from which it is
made; water (H
2
0) is a compound with
different characteristic properties than the
elements hydrogen and oxygen from which it is
made. Different amounts of the same
elements can produce compounds with
different properties (e.g., water (H
2
0) and
hydrogen peroxide (H
2
0
2
).
In a chemical reaction, atoms can rearrange
to form different molecules of new compounds.
During photosynthesis, carbon dioxide (CO
2
) is
taken in by green plants and combined with
water (H
2
O). The carbon, hydrogen and
oxygen atoms rearrange to make two new
compounds: glucose (made of atoms of
carbon, oxygen, and hydrogen) and oxygen
gas (made of atoms of oxygen).
In a chemical reaction, the same amount of
matter (mass) is present at the start and the
end, since the atoms are not created or
destroyed but simply rearrange.
Performance Tasks:
Perform the Ice and Salt lab and write a lab report
utilizing formal lab writing techniques.
Complete lab investigations abiding by all lab
safety standards.
Develop an explanation of the patterns by which
the periodic table is organized, including the
structure of atoms and the resulting properties of
the elements.
Use a periodic table to identify elements in a
compound; to locate metals, semimetals and
nonmetals; and predict the general
characteristics of an element.
Other Assessments:
Identify the different types of reactions when
given chemical equations.
Complete a worksheet describing the properties
of common elements, such as oxygen, hydrogen,
carbon, iron and aluminum.
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students
to:
1. Describe the structure of the atom and its
component parts.
2. Explain that density (mass/volume) is a
characteristic property that can be used to
identify an element or substance.
3. Compare and contrast the properties of a metal
(aluminum, iron, etc.) with a nonmetal (oxygen,
carbon, etc.).
4. Illustrate the differences in the physical and
chemical properties of a molecule and the
individual atoms that bonded to form that
molecule.
5. Differentiate between a mixture and an element
or compounds and identify examples.
6. Conduct and report on an investigation that uses
physical means such as particle size, density,
solubility and magnetism to separate substances
in a mixture.
7. Use the patterns of the Periodic Table to locate
metals, semimetals and nonmetals and predict
the general characteristics of an element.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 185 GRADE 8
CMT CORRELATIONS
Describe the properties of common elements,
such as oxygen, hydrogen, carbon, iron and
aluminum
Describe how the properties of simple
compounds, such as water and table salt, are
different from the properties of the elements of
which they are made.
Explain how mixtures can be separated by using
the properties of the substances from which they
are made such as particle size, density, solubility
and boiling point.
SCIENTIFIC LITERACY TERMINOLOGY: characteristic, property, mass, weight, volume, density, solubility,
boiling point, mixture, solution, particle, atom, element, molecule, compound, metal, non-metal, chemical reaction
KEY SCIENCE VOCABULARY
: state of matter, conductivity, soluble
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 186 GRADE 8
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 187 GRADE 8
LEARNING STRAND Structure and Function – How are organisms structured to ensure efficiency and survival?
Unit: Life Processes
CT Standard 7.2 – Many organisms, including humans, have specialized organ systems that interact with each other to maintain
dynamic internal balance.
ENDURING UNDERSTANDINGS
All organisms are composed of one or more
cells.
Each cell carries on life-sustaining functions.
Multicellular organisms need specialized
structures and systems to perform basic life
functions.
ESSENTIAL QUESTIONS
How do the basic structures of an animal cell
function to support life?
How do the structures of the human digestive system
function to bring nutrients to the cells and expel
wastes?
How do the structures of the human nervous system
function to support life?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
All cells come from other cells and they hold
the genetic information needed for cell
division and growth. When a body cell
reaches a certain size, it divides into two
cells, each of which contains identical
genetic information. This cell division
process is called mitosis.
The cell is filled with a fluid called
cytoplasm; cells contain discrete
membrane-enclosed structures called
organelles. Each of the organelles
performs a specific cellular function and it
can be identified by its shape.
- The nucleus contains the genetic materials
(chromosomes), and it directs the cell
activities, growth, and division.
- The mitochondrion contains enzymes
that break down sugars and release chemical
energy. One cell can contain hundreds of
mitochondria.
- The entire cell is surrounded by the
plasma membrane.
Systems consist of parts that interact with
and influence each other. Parts of a system
work together to make the whole entity
work. Similarly, each part of an animal
body has a specific job to do, and all the
different parts work together to support life.
Although all cells have similar basic
structures, in multicellular organisms cells
have specialized shapes that enable them to
perform specific roles (e.g., muscle, nerve,
and skin cells can be identified by their
distinct shapes.)
The major parts of the human digestive
system are the mouth, esophagus, stomach,
small intestine and large intestine. This
system is responsible for breaking down
Prentice Hall Science Explorer Cells and Heredity
(2008)
Lab ware
Lab safety equipment
Microscopes
Stereoscopes
Digital microscope
Proscope
Video flex cam
LED projector
Dialysis tubing
Karo syrup
Ammonia
String
Prepared slides of cells
pH indicators
HCl
Neutralizing substances
―Optical Illusions‖ Power Point presentation
Materials for wet slides
SUGGESTED INSTRUCTIONAL STRATEGIES
Review basic life processes such as digestion,
respiration and excretion occur at the cellular level.
Review cells and cell structures by observing them
using a microscope.
Use dialysis tubing with Karo syrup suspended in a
beaker of water to observe osmosis.
Demonstrate diffusion of a gas by inverting a test
tube with water and a few drops of phenolthelein
covered by a single layer of dialysis tubing over a
beaker containing a small amount of ammonia.
Label the main parts of the digestive system on a
diagram.
Draw a flow chart of how an impulse passes from
one neuron to another.
Project slides of neurons and a CT scan of a human
brain for students to examine.
Compare and contrast respiration and
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 188 GRADE 8
food, absorbing nutrients and water, and
eliminating waste. The liver and pancreas
support the functions of the major digestive
organs by producing and releasing digestive
liquids into the digestive tract.
The nervous, immune and excretory systems
interact with the digestive, respiratory and
circulatory systems to maintain the body’s
dynamic internal balance (homeostasis).
photosynthesis.
Show the PowerPoint presentation ―Optical Illusions.‖
SUGGESTED ASSESSMENT METHODS
Performance Tasks:
Perform a lab to test products of their choice to
settle stomach acid (i.e. antacids, milk, Tums,
crackers, ginger ale).
Determine and report on how a similar group of cells
are organized in tissues that have specific functions.
Analyze and illustrate how tissues form organs with
specific functions that contribute to the larger
system.
Trace energy conversions that occur in the human
body once food enters and explain the conversions
in writing.
Research and defend the statement, ―Body systems
are interdependent and act together to maintain the
body’s dynamic internal balance‖ (homeostasis).
Other Assessments:
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of students to:
1. Illustrate and describe in writing the structure and
the function of the following cell structures: cell
membrane, cytoplasm, mitochondria and nucleus in
an animal cell.
2. Label the major parts of the human digestive
system and explain in writing the function of each
part in the chemical and physical breakdown of food
(mouth, esophagus, stomach, small intestine, large
intestine and rectum).
CMT CORRELATIONS
Describe the basic structures of an animal cell,
including the nucleus, cytoplasm, mitochondria and
cell membrane, and how they function to support
life.
Describe the structures of the human digestive,
respiratory and circulatory systems and explain how
they function to bring oxygen and nutrients to the
cells and expel waste materials.
Explain how the human musculoskeletal system
supports the body and allows movement.
SCIENTIFIC LITERACY TERMINOLOGY: structure, function, cell, cytoplasm, nucleus, cell membrane,
tissue, organ system
KEY SCIENCE VOCABULARY
: cell division, genetic, mitosis, cytoplasm, organelles, cellular function,
nucleus, chromosomes, mitochondrion, enzymes, mitochondria, plasma membrane, multicellular
organisms, digestive system, digestive organs
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 189 GRADE 8
LEARNING STRAND Heredity and Evolution -
What processes are responsible for life's unity and diversity?
Unit: Genetics
CT Standard 8.2 – Reproduction is a characteristic of living systems and it is essential for the continuation of every species.
ENDURING UNDERSTANDINGS
Heredity is the passage of genetic information
from one generation to another.
Some of the characteristics of an organism are
inherited and some result from interactions with
the environment.
ESSENTIAL QUESTIONS
What processes are responsible for life’s
unity and diversity?
What are the similarities and differences in
meiosis and mitosis cell division?
How do the male sperm and the female egg
explain sex determination in offspring?
How do inherited traits get passed to
offspring?
How is the genetic information organized in
genes on chromosomes?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
GRADE LEVEL CONCEPT 8.2.a.
Living organisms must reproduce to continue the
existence of their species. Through reproduction
new individuals which resemble their parents are
formed. All the organisms alive today arose from
preexisting organisms.
All the cells in a multicellular organism result
from a single fertilized egg cell, through a process
of continuous cell divisions (mitosis).
Instructions for how an organism develops are
stored in DNA molecules which are part of the
chromosomes inside the cell nucleus.
The chromosomes occur in matching pairs, and
each cell in a multicellular organism contains the
number of chromosomes that are typical of that
species. For example, cells in human beings
contain 23 pairs of chromosomes, 46 in all.
Organisms grow by increasing the number of body
cells. During
mitosis
, a body cell first duplicates
the chromosomes and then divides into two
identical daughter cells, each one with a complete
set of chromosomes.
Most multicellular organisms reproduce by
sexual
reproduction, in which new cells are produced by
the combination of two germ cells (gametes).
During
meiosis
, matching chromosomes in each
pair separate from each other so that each germ
cell contains only half of the chromosomes of the
original cell.
Mitosis and meiosis are similar processes in that
they both result in the separation of existing cells
into new ones. They differ in that the germ cells
produced during meiosis have only one copy of
each chromosome. When two germ cells unite
during fertilization, the resulting zygote has two
copies of each chromosome, one from each
parent, ensuring maternal and paternal genetics
Module: Prentice Hall Explorer Cells and
Heredity (2008)
DNA model
Videos
Microsoft Publishing software
Coins, construction paper, glue, scissors
Lego Genetics Kits
Internet access
SUGGESTED INSTRUCTIONAL STRATEGIES
Use a Lego Genetics Kit to create
chromosomes and go through cell division.
Use 3-D models of cells during cell division
to show mitosis.
Observe prepared slides of cells are various
stages of cell division.
Model how to use a Punnett Square.
Complete the worksheets practicing the use
of Punnett Squares.
Model how to read a pedigree chart.
Perform the Gene/Jean activity forming
facial features with the toss of two coins.
Analyze a pre-made pedigree chart tracing
a genetic condition through multiple
generations.
Create a pedigree chart tracing a particular
genetic condition through several
generations.
Show the video on genetics.
SUGGESTED ASSESSMENT METHODS
Performance Task:
Research and create professional looking
genetic pamphlets regarding a rare genetic
disorder.
Other Assessments:
List in order the stages of mitosis.
Identify the characteristics of each stage of
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 190 GRADE 8
at birth. Males produce millions of sperm over the
course of their adult life. Females are born with a
finite number of immature eggs in the ovaries that
are released one at a time in a monthly cycle.
Meiosis and gamete formation takes place in the
reproductive organs;
testes
in males produce the
sperm and
ovaries
in females produce the eggs.
In humans, the reproductive organs are in place
at birth, but are readied to perform their
reproductive functions by hormones released
during adolescence. Males produce millions of
sperm over the course of their adult life. Females
are born with a finite number of immature eggs in
the ovaries that are released one at a time in a
monthly cycle.
In humans, if an egg is fertilized by a sperm in the
female’s fallopian tube, the resulting
zygote
may develop into a fetus in the female uterus. If
the egg is not fertilized, it will leave the female’s
body in a monthly discharge of the uterine lining
(menstrual cycle).
A segment of DNA that holds the information for a
specific trait is called a gene. Each chromosome
in a pair carries the same genes in the same
place, but there are different versions of each
gene.
In sexual reproduction, offspring of the same
parents will have different combinations of genes
and traits, creating genetic variability within the
species. Sexual reproduction is the basis for the
evolution of living organisms.
GRADE LEVEL CONCEPT 8.2.b.
Gender in humans is a trait determined by genes
carried by a special pair of chromosomes
identified as ―X‖ and ―Y‖. Female gametes give
only an ―X‖ chromosome; male gametes can give
either an ―X‖ or a ―Y‖. The sperm that fertilizes
the egg determines the sex of the offspring: a
zygote containing two X chromosomes will
develop into a female and a zygote containing X
and Y chromosomes will develop into a male.
Most human traits are inherited from parents, but
some are the result of environmental conditions.
For example, eating and exercising habits may
affect the body mass and shape of individuals in
the same family.
mitosis.
Compare and contrast mitosis and meiosis.
Use a Punnett Square to demonstrate
Mendel’s principles of heredity by
determining the expressed allele and
identifying the genotype and phenotype
ratios.
Draw or build a model of the double helix
shape of DNA.
Teacher-created quizzes
Common unit test
GRADE LEVEL EXPECTATIONS
Assessments MUST measure the ability of
students to:
1. Relate the continued existence of any
species to its successful reproduction and
explain in writing the factors that contribute
to successful reproduction.
2.
Describe the structure, location and
function of chromosomes, genes and DNA
and how they relate to each other in the
living cell.
3.
Illustrate and chart the purpose, cell type
(somatic and germ) and resulting
chromosome count during cell division in
mitosis and meiosis.
4.
Identify the major structures in human male
and female reproductive systems and
explain where meiosis and gamete
formation take place.
5.
Investigate and report on the role of
hormone production as it initiates and
regulates the creation of male and female
germ cells from birth through adolescence
and into adulthood.
6.
Compare and contrast the events and
processes that occur when a human egg is
fertilized or not fertilized.
7.
Demonstrate the relationship of
corresponding genes on pairs of
chromosomes to traits inherited by
offspring.
8.
Describe in writing the role of the germ cells
in the formation of the human zygote and
its resulting 23 pairs of chromosomes, the
23rd of which determines gender and the
other 22 of which determine the
characteristics of that offspring.
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 191 GRADE 8
CMT CORRELATIONS
Explain the differences in cell division in
somalic and germ cells.
Describe the structure and function of the
male and female human reproductive
systems including the process of egg and
sperm production.
Describe how genetic information is
organized in genes on chromosomes and
explain sex determination in humans.
SCIENTIFIC LITERACY TERMINOLOGY: multicellular organism, heredity, trait, chromosome, gene,
DNA, species, mitosis, meiosis, gamete, adolescence, hormone, testes, sperm, ovary, egg, fallopian tube,
uterus
KEY SCIENCE VOCABULARY
: germ cell, genotype, phenotype, double helix
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 192 GRADE 8
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 193 GRADE 8
LEARNING STRAND
Unit: Electromagnetic Systems
CT Standard 9.2 – The electrical force is a universal force that exists between any two charged objects.
ENDURING UNDERSTANDINGS
Electric charges can accumulate on a surface as
in static electricity or flow through a wire as in
current electricity.
Moving electrical charges produce magnetic
forces and moving magnets can produce
electrical force.
Electrical current can be transformed into light
through the excitation of electrons.
ESSENTIAL QUESTIONS
What is the role of electrical energy in our
world?
What is the relationship among voltage, current
and resistance in a simple series circuit?
How is electricity used to produce heat and light
in incandescent bulbs and heating elements?
How are current and magnetism related?
UNDERLYING CONCEPTS
Students should understand that….
INSTRUCTIONAL SUPPORT MATERIALS
An electric charge results when an object either
gains or loses electrons.
Static electricity is the accumulation of
electrical charges on the surface of an object.
The Law of Electric Charges states that like
charges repel and unlike charges attract.
The distance between two charged objects
affects the strength of an electric force
(Coulomb’s Law).
Charges that build up on an object are static
electricity; charges that flow through objects are
current electricity.
Amperage is the amount of current passing a
point in one second.
Voltage is the push of electricity.
Resistance is the opposition to current in an
electric current.
Resistance produces heat.
An ohm is the resistance of electricity.
Charges easily flow through a conductor; charges
cannot flow through an insulator.
Ohm’s Law shows the relationship between
voltage, current, and resistance.
A transformer is a device used to raise or lower
voltage.
A generator produces electricity.
An electrical circuit with a single path is a series
circuit; an electrical circuit with multiple paths is
a parallel circuit.
Electric current produces a magnetic field.
Module: Prentice Hall Science Explorer
Electricity and Magnetism (2007)
Magnets
Wires, bulbs, batteries, , buzzers, meters (i.e.;
ammeter, volt meter, galvanometer)
Vande Graaff generator, plasma ball and other
materials
Styrofoam cup
Internet access
SUGGESTED INSTRUCTIONAL STRATEGIES
Demonstrate how to make an electromagnet
noting the relationship of the number of coils to
the strength of the magnet.
Perform activities using batteries, wires, lights,
and meters.
Determine the maximum voltage possible using
three D cell batteries.
Produce a series circuit using a battery and
three lights.
Produce a parallel circuit using a battery and
three lights.
Determine how amperage is affected by the
number of devices using on battery and up to
three lights.
Produce a circuit that allows one switch to
control two devices.
Produce a circuit that allows one switch to
control one of two devices.
Produce a combination circuit with a battery and
three lights so that when one of the light bulbs
is removed, the second bulb also goes out while
the third bulb stays lit.
Produce a combination circuit using a battery
and five light bulbs so that if the first light bulb
is removed the second will not work as well;
however lights 3, 4, and 5 still work. If light 4
is removed, the fifth light will go out; however,
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 194 GRADE 8
lights 1, 2, and 3 will work.
Make a series circuit with two lights and a
parallel circuit with two lights and determine
which circuit decreases the amperage of a
circuit.
Demonstrate static electricity using an
electrostatic box and Styrofoam cup.
Perform demonstrations using a Vande Graaff
generator.
Perform demonstrations using a plasma ball.
Learn science inquiry skills
SUGGESTED ASSESSMENT METHODS
Benchmark:
Science inquiry skills activity
Other Assessments:
Teacher-created quizzes
Common unit test
CMT CORRELATIONS
Explain the relationships among voltage,
current and resistance in a simple series
circuit.
Explain how electricity is used to produce heat
and light in incandescent bulbs and heating
elements.
Describe the relationship between current and
magnetism.
KEY SCIENCE VOCABULARY
: electrons, static electricity, amperage, voltage, resistance, ohm, Ohm’s
Law, transformer, generator, circuit, conductor, insulator, parallel circuit, series circuit
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 195 GRADE 8
LEARNING STRAND
Unit: Light and Sound
National Standard: Energy is a property of many substances and is associated with heat, light, electricity, mechanical motion and
sound.
ENDURING UNDERSTANDINGS
Waves have characteristics and properties that
do not depend on the type of wave.
Waves transfer energy, have measurable
properties and behave in predictable ways.
Electrical current can be transformed into light
through the excitation of electrons.
ESSENTIAL QUESTIONS
How is energy transmitted in the form of
waves?
How does a change in energy affect the
characteristics of a wave?
What are the characteristics of wave types?
How are wavelength, frequency and wave speed
related?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Energy can be transmitted from a source as
waves.
Waves carry energy from one place to another.
Waves can transfer energy when they interact
with matter.
Transverse and longitudinal waves exist in
mechanical media, such as springs and ropes,
and in the Earth as seismic waves.
Seismic waves, sound waves, and
electromagnetic waves can be destructive or
beneficial due to the transfer of energy.
Mechanical and electromagnetic waves have
the same properties.
Wavelength, frequency and wave speed
are related.
Factors that influence the basic properties of
waves include frequency amplitude,
wavelength, and speed.
Behaviors of waves include refraction,
reflection, transmission and absorption.
Waves have characteristic behaviors, such as
interference, diffraction, and refraction.
Waves travel through different media.
Sound is a longitudinal wave whose speed
depends on the properties of the medium in
which it propagates.
The Doppler Effect results from the
characteristic behavior of waves.
The wavelength and energy of waves in
various parts of the electromagnetic
spectrum include visible light, infrared, and
ultraviolet radiation.
The electromagnetic spectrum in increasing
frequencies includes microwaves, infrared light,
visible light, ultraviolet light, X rays and
Gamma rays.
The absorption and reflection of light waves by
various materials result in the human
perception of color.
Prentice Hall module Sound and Light
Internet access
Light bench
Pig hologram
Sound tube, slinky and other demonstrators
Flashlights, mirrors, clay, easel paper
Lenses, mirrors, lasers, aquarium, and other
materials needed for laser demonstration
Tuning forks
Wave tank
Graphing calculator, CBL2, light intensity probe
SUGGESTED INSTRUCTIONAL STRATEGIES
Use lasers to demonstrate reflection and
refraction.
Demonstrate diffraction using a wave tank.
Use a slinky to demonstrate transverse and
standing waves.
Demonstrate real vs. virtual images using a
hologram of the pig and a ruler with a paper
screen.
Perform the light bench activity.
Demonstrate different types of waves using
computer-generated models.
Use prisms and spectroscopes to observe
properties of light.
Use tuning forks to experience vibration and
sound.
Perform a demonstration for students using the
sound tube and slinky with cones on the ends.
Find the angle of incidence and angle of
reflection using a flashlight taped with duct tape
and a mirror.
Determine where to place three mirrors to get a
light beam to reflect to a particular spot on
easel paper.
Demonstrate light intensity using a graphing
calculator, CBL2 and light probe.
Calculate wavelength, frequency and wave
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 196 GRADE 8
speed using a mathematical formula.
Use a PowerPoint presentation or a computer
simulation to explain the cause of the Doppler
Effect and the changes waves undergo during
the process.
SUGGESTED ASSESSMENT METHODS
Conduct investigations demonstrating the
characteristics of a wave -- wavelength,
frequency, speed, amplitude.
Conduct investigation of longitudinal and
transverse waves to determine how they are
different.
Explain how energy is transferred through
waves -- seismic, sound, electromagnetic.
Differentiate among reflection, refraction and
absorption of various types of waves.
Performance Task:
The light bench activity
Other Assessments:
Teacher-generated quizzes
Common unit test
KEY SCIENCE VOCABULARY
: amplitude, wavelength, frequency, speed, crest, trough, longitudinal,
transverse, conduction, convection, radiation, seismic, reflection, interference, diffraction, refraction,
absorption, electromagnetic spectrum
MIDDLE SCHOOL SCIENCE CURRICULUM
MADISON PUBLIC SCHOOLS 197 GRADE 8
LEARNING STRAND
Unit: Forensic Science
CT Standard: Scientific inquiry progresses through a continuous process of questions, data collection, analysis, and interpretation.
ENDURING UNDERSTANDING
Physical evidence can be used to help convict
a person in a criminal trial or to settle civil
cases.
ESSENTIAL QUESTIONS
How are tools and techniques used to make observations
and gather data in criminal and civil cases?
How is scientific knowledge communicated in a court of
law?
UNDERLYING CONCEPTS
Students should understand that…
INSTRUCTIONAL SUPPORT MATERIALS
Scientific method is used to solve forensic
problems.
Forensic science is used in criminal
investigations.
The principles of forensic science can be
applied to a hypothetical crime.
The scientific process can be used to solve a
fictional crime.
At the mock crime scene, evidence is
searched for, isolated and recorded.
Using proper forensic procedures, evidence is
collected and packaged at a mock crime scene.
Branches of forensic science include
ballistics, serology, fingerprinting,
chromatography, DNA, hair and fibers.
There are scientific ways of finding latent
prints for fingerprint analysis.
There are basic properties and unique
characteristics of fingerprints.
There are appropriate techniques used to lift
and evaluate readable latent fingerprints.
DNA has physical properties and function.
Paper chromatography can be used to
determine which pen was used on a note.
Ink pads and materials for fingerprinting and lifting latent
prints
Website for Overview of Fingerprints
Chromatography paper
Forensic Videos including ―Eyewitness News‖ on
eyewitnesses to a crime
SUGGESTED INSTRUCTIONAL STRATEGIES
View a crime and play the role of the eyewitness.
Take a description and play the role of a forensic artist.
Lift a latent print, compare to prints of a pool of
suspects, and make a correct identification based on
observation of characteristic patterns -- loop, arc and
whorl.
Identify the correct pen used on a ransom note through
chromatography.
Compare the DNA of suspects to identify the culprit.
Do the activity "Accident or Murder" in Strengthening
Your Science Instruction Using New and Innovative
Forensic Science Strategies Resource Handbook (2007).
SUGGESTED ASSESSMENT METHODS
Performance Task:
Lifting of latent fingerprints
Other Assessments:
Identifying a criminal using fingerprints
EXPECTED PERFORMANCES
Identify questions that can be answered through
scientific investigation.
Design and conduct appropriate types of scientific
investigations to answer different questions.
Use appropriate tools and techniques to make
observations and gather data.
Use mathematical operations to analyze and interpret
data.
Provide explanations to investigated problems or
questions.
KEY SCIENCE VOCABULARY
: crime scene, evidence, fingerprint analysis, forensic science, ballistics,
serology, chromatography, DNA
Content Standards & Indicators
Grades 9 - 12
BIOLOGY HONORS
SCIENCE CURRICULUM 199 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Biology - Honors
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Biology - Honors
3. Transcript Course Code/Number
00301
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 9 – 10 Level: I
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Sophomores entering the course must have earned at least an A- in both Integrated Science I and II.
Freshman entering the course must have earned at least a B+ in Algebra and an A- in 8
th
grade science.
11. Brief Course Description
This course considers life on all levels of organization with an emphasis on how molecules are
incorporated into cellular structures. The individual is considered as it relates to itself, other living things
and the biomes of the world. Higher order thinking skills as well as advanced reading skills are necessary
for success in this course. Laboratory investigations test the student’s ability to use these thinking skills, to
make observations, and formulate ideas about biological phenomena. Reflective, detailed, extensive, well
written scientific laboratory reports are an integral part of the curriculum.
12. Course Goals
1. Use the scientific/inquiry method to solve biological problems.
2. Analyze the possibilities and limits of science and technology.
3. Use technology effectively and responsibly.
4. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
5. Demonstrate respect for one's self, and strive to contribute to the success of others.
6. Understand and use safety procedures in lab investigations.
7. Integrate biochemistry with cell structure and processes.
8. Relate the fundamentals of genetics to biotechnology.
9. Discuss the interactions between humans and ecosystems.
10. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the global
community.
BIOLOGY HONORS
SCIENCE CURRICULUM 200 GRADES 9 - 12
13. Course Outline
Chapter/Unit
Activities
# of Weeks
Scientific Method/Evolution
Yeast lab with sugar- controlled experiment
Moth lab
2
Measurement/Metric System
Equipment and measurement labs
1
Chapter 1 – Basic Chemistry
Nutrients lab
Dehydration synthesis activities
pH lab
DNA activity
4
Chapter 2 – Energy Flow
Enzyme lab
ATP activity
2
Chapter 3 – Transport
Transport lab
Cell membrane posters
Human excretion
Human Respiration
4
Chapter 4 – Photosynthesis – skim 4.8,
skip section 4.9, skim section 4.6
Videos/worksheets,
Possible lab with elodea or substitute plant
Computer websites
2
Chapter 6 – Cells
Microscope labs
Animal and plant labs
Computer websites
2 ½
Chapter 5 Cellular Respiration – skim 5.8,
skip 5.10
Possible yeast lab with different sugars
Computer websites
2 ½
Chapter 8 Cell Cycle
Computer lab activities
Possible cut out activities
Computer websites
Mutation activities or pamphlets
2
Chapter 7 Transport
Stomata lab
Transport structure in leaves
Pulse rate lab
3
Chapter 12 Meiosis – only sections 12.1
and 12.2
1
Chapter 13 Genetics/Gene Expression
Human genetics activities
Investigations
Karyotyping
Probability activities
4
Chapter 15 DNA Technology
Restriction enzyme cut out activity
Electrophoresis videos and activities
Lab
3
Chapter 18 Classification
Looking at phyla specimens
2
BIOLOGY HONORS
SCIENCE CURRICULUM 201 GRADES 9 - 12
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
HONORS COURSES ONLY
17. Indicate how this honors course is different from the standard course.
The course is 1 full year and 1.5 credits. The labs are more extensive and graded with different rubrics
from the standard biology course. There is a greater emphasis on the analysis and interpretation of data
than the standard biology course.
BIOLOGY HONORS
SCIENCE CURRICULUM 202 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
Use and apply a scientific method as it applies
to biological principles.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated
during the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicates the results of a scientific
experiment.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Flinn Lab Safety Video
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Guided Internet research
Review appropriate use of lab equipment,
experimental design and lab report writing
Yeast lab with sugar (CAPT Embedded Task)
Vitamin C lab *
Moth lab *
SUGGESTED ASSESSMENT METHODS
Benchmarks *
- Meet expectation for writing a
conclusion/discussion for a lab
- Meet expectation for explaining a concept
using data in a written report
Designer Airplanes
UV Beads activity
Introduction to lab equipment
Lab safety video and quiz
Measurement lab
BIOLOGY HONORS
SCIENCE CURRICULUM 203 GRADES 9 - 12
LEARNING STRAND
Basic Chemistry: Chemistry of Life
Content Standard: 10.1 Fundamental life processes depend on the physical structure and the chemical activities of the cell.
High School Enrichment Standards - Biology -- Cell Biology - The fundamental life processes of plants and animals depend on a
variety of chemical reactions that occur in specialized areas of the organism’s cells.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
ESSENTIAL QUESTIONS
What roles do chemicals play in life?
What is the importance of the molecular
structure of molecules and their relationship to
biology?
What is the importance of chemical reactions in
living organisms and the environment?
How do various bonding types effect reactions
inside living organisms or in the environment?
What is the importance of water for the survival
of living organisms?
What is the significance of carbon as it relates
to the complexity of organic compounds?
What are the similarities and differences
between carbohydrates, proteins, lipids and
nucleic acids, including structure, function and
examples?
What is the purpose of each biological molecule
(carbohydrate, protein, lipid, nucleic acids) for
the survival of living organisms?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the importance of the structure of
atoms and molecules in terms of their function.
Cite examples of important chemical reactions
in living organisms and the environment.
Draw examples of various bonding types and
their purpose in different macromolecules.
Explain, using specific examples, how water is
important to the survival of living organisms.
Analyze why carbon is such an important
molecule in relation to living organisms.
Design a chart comparing and contrasting the
major groups of macromolecules.
Discuss the role or each macromolecule in the
survival of living organisms.
Explain the importance of maintaining pH in
living things.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
pH paper
lab supplies for organic compounds lab
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentation
Organic Compounds lab
Dehydration synthesis activities
pH lab
DNA webquest *
SUGGESTED ASSESSMENT METHODS
Benchmark *
- Meets expectation for the effective and
responsible use of technology
Quizzes
Tests – General chemistry & Organic chemistry
Teacher observations
Organic Compounds lab questions
Ph Lab analysis questions
BIOLOGY HONORS
SCIENCE CURRICULUM 204 GRADES 9 - 12
LEARNING STRAND
Energy Flow
Content Standard: 10.1 Fundamental life processes depend on the physical structure and the chemical activities of the cell.
High School Enrichment Standards - Biology--Ecology - Stability in an ecosystem is a balance between competing effects.
Cell Biology - The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in
specialized areas of the organism’s cells.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
Energy is the capacity to do work.
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
Living organisms rarely exist alone in nature.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Chemical reactions regulate life.
ESSENTIAL QUESTIONS
How do organisms use chemical energy to do
work?
How does energy flow through an ecosystem?
How do the laws of thermodynamics allow us to
predict the flow of energy in organisms and
ecosystems?
Why are enzymes essential to life?
What is ATP and how is it used as an energy
carrier?
What is the relationship among enzymes,
energy and reaction rates?
How do environmental factors affect enzyme
function?
Why is it important for most proteins,
carbohydrates and fats to be digested?
How do different organisms obtain food and
how does this relate to evolutionary adaptation?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain the flow of energy through an
ecosystem giving specific examples.
Identify examples of biotic and abiotic factors
and explain how they are related in an
ecosystem citing specific examples.
Compare and contrast movement of energy and
movement of nutrients through a food web.
Describe the first and second laws of
thermodynamics and their relationship to the
flow of energy in an ecosystem.
Define metabolism, biosynthesis, and
decomposition and explain how ATP connects
these processes.
Compare and contrast extracellular digestion
and intracellular digestion.
Explain why cells use different enzymes to
catalyze different reactions.
Demonstrate the effect of various
environmental changes on enzymes.
Describe digestion in humans.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Lab supplies
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentation
Enzyme Lab
ATP activity
Digestive Disorder pamphlet
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Enzyme lab report
Chapter check: challenge and review questions
Teacher observations
Digestive disorder pamphlet
BIOLOGY HONORS
SCIENCE CURRICULUM 205 GRADES 9 - 12
LEARNING STRAND
Transport: Exchanging Materials
High School Enrichment Standard – Biology -- Cell Biology - The fundamental life processes of plants and animals depend on a
variety of chemical reactions that occur in specialized areas of the organism’s cells.
ENDURING UNDERSTANDINGS
Movement of materials across cell membranes
is essential for maintaining homeostasis.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
There is a connection between the laws of
thermodynamics and movement of molecules
either down or against a concentration gradient.
ESSENTIAL QUESTIONS
How does the structure of the cell membrane
aid in its functions of protection, recognition
and transport?
Why do cells need both active and passive
transport methods to move materials across the
cell membrane?
What is the importance of respiration for
individual organisms and ecosystems?
What is the significance of the surface area of
an exchange membrane such as the one in the
gills of fish?
How have land-dwelling organisms adapted in
order to obtain oxygen?
How have organisms adapted in order to
remove waste products?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Draw a diagram of the structure of a cell
membrane.
Illustrate types of cell transport.
Describe the process of diffusion and the
special type called osmosis.
Chart the differences and similarities between
active and passive transport.
Explain the importance of multiple transport
methods for a cell.
Describe the process of respiration.
Compare human respiration to respiration in
plants.
Explain how excretion is essential to
maintaining homeostasis in organisms giving
specific examples using unicellular organisms.
Describe how humans excrete wastes.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Lab supplies for transport lab
Video clip on united streaming
SUGGESTED INSTRUCTIONAL STRATEGIES
Transport lab
Cell membrane posters
Video clips – passive and active transport
Respiration video
Lecture/PowerPoint presentations and notes
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Check and challenge questions
Kidney worksheet
Teacher observations
Potato core lab report
Cell membrane posters and presentations
BIOLOGY HONORS
SCIENCE CURRICULUM 206 GRADES 9 - 12
LEARNING STRAND
Photosynthesis
Content Standard: 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
High School Enrichment Standard – Biology -- Ecology – Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Everything from cells to organisms to
ecosystems is in a state of dynamic balance
that must be controlled by positive or negative
feedback mechanisms.
ESSENTIAL QUESTIONS
How is light energy conserved in ATP and
NADPH during photosynthesis?
Why is photosynthesis important to humans?
How does the structure of the chloroplast relate
to its function in photosynthesis?
What types of photosynthetic adaptations have
evolved in response to environmental
conditions?
What interactions exist between photosynthesis
and cell respiration?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the process of photosynthesis.
Describe the relationship between the light
reactions and the carbon dioxide-fixing
reactions of photosynthesis.
Describe how the structure of the chloroplast
relates to its function in photosynthesis.
Describe how the environment influences the
rate of photosynthesis.
Explain how chemoautotrophs and
photoautotrophs utilize the materials in their
environment.
Outline the similarities and differences between
photosynthesis and respiration.
Elaborate on how photosynthesis and
respiration are dependent on each other.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
SUGGESTED INSTRUCTIONAL STRATEGIES
Videos
PowerPoint and outline notes on photosynthesis
Worksheets
Diagram and written summary of the light
dependent and Calvin cycle reactions
Elodea lab
Computer websites
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Lab reports
Open-ended questions guided by textbook
Teacher observations
BIOLOGY HONORS
SCIENCE CURRICULUM 207 GRADES 9 - 12
LEARNING STRAND
Cells: Structure and Function
Content Standard: 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Everything from cells to organisms to
ecosystems is in a state of dynamic balance
that must be controlled by positive or negative
feedback mechanisms.
ESSENTIAL QUESTIONS
How is the structure related to the function in
cell organelles?
How do organelles function together in cell
processes?
What are the similarities and differences
between various types of cells (prokaryote vs.
eukaryote, plant vs. animal, uni-cellular vs.
multi-cellular)?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Elaborate how structure is related to the
function of organelles.
Cite examples of interactions between different
cell organelles.
Draw a diagram of the structure of a cell.
Compare and contrast prokaryotes and
eukaryotes.
Describe how the cell walls of plants and
bacterial cells are different and how they are
similar.
Explain how the characteristics of biological
macromolecules are important in the structure
and function of cells.
Compare and contrast uni-cellular and multi-
cellular organisms.
Describe the division of labor in a multi-cellular
organism.
Explain the levels of structure in the biosphere.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Plant and animal cell lab materials
Microscopes
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentations
Microscope labs
Animal and plant labs
Labeling various cell diagrams
Directed reading with text
Computer websites (cellsalive)
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Lab analysis questions
Open-ended questions
Teacher observations
Diagrams
BIOLOGY HONORS
SCIENCE CURRICULUM 208 GRADES 9 - 12
LEARNING STRAND
Cellular Respiration
Content Standard: 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
Everything from cells to organisms to
ecosystems is in a state of dynamic balance
that must be controlled by positive or negative
feedback mechanisms.
ESSENTIAL QUESTIONS
How is the structure related to the function in
mitochondria?
How are the characteristics of ATP important in
the energy reactions of cells?
What are the stages of cellular respiration?
What interactions exist between photosynthesis
and cell respiration?
How does the environment affect the process of
cellular respiration?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Draw a diagram of the structure of
mitochondria, label where the different stages
occur.
Compare and contrast aerobic and anaerobic
respiration.
Explain how the structure of ATP is important in
storing and releasing energy.
Explain the chemical reactions that occur in
glycolysis, Krebs cycle and electron transport
system.
Describe the relationship between
photosynthesis and respiration.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Yeast and sugars lab materials
Assignment discovery video on energy and
matter
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentations
Yeast and sugars lab
Computer websites
Assignment discovery video on energy and
matter with worksheet
Textbook reading and notes
Diagramming the stages of Cell Respiration
SUGGESTED ASSESSMENT METHODS
Quizzes (open and closed notes)
Test
Lab report on yeast respiration rate
Open-ended questions
Check and challenge questions
Teacher observations
BIOLOGY HONORS
SCIENCE CURRICULUM 209 GRADES 9 - 12
LEARNING STRAND
Cell Cycle
Content Standard: 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Everything from cells to organisms to
ecosystems is in a state of dynamic balance
that must be controlled by positive or negative
feedback mechanisms.
ESSENTIAL QUESTIONS
What structures function in cell division?
How do organelles function together in cell
processes?
What are the similarities and differences
between cell division of prokaryotes and
eukaryotes, and of plant and animal cells?
What is the significance of mitosis as it relates to
growth, development and repair of cells?
How does mitosis ensure genetic continuity?
How is the structure of DNA important in cell
division?
Why is regulation of the cell cycle important?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Draw a schematic diagram of the structure of
DNA.
Describe the structure of DNA and explain the
process of DNA replication.
Explain the major events of the cell cycle.
Draw a diagram of the cell in the various
phases of the cell cycle.
Describe how the cell cycle is regulated.
Explain how cancer is caused by uncontrolled
cell division.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Laptops
microscope
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentations
Computer websites animation of DNA replication
DNA models (pop-beads, paper cut-outs)
Mitosis models (wiki sticks or clay)
Arizona onion root online lab
Mitosis lab using onion root/whitefish
Textbook reading and notes
Diagrams of the phases of the cell cycle
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Lab analysis questions
Lab drawings (on paper and with whiteboards)
Diagrams of the cell in various stages
Open-ended questions
Teacher observations
BIOLOGY HONORS
SCIENCE CURRICULUM 210 GRADES 9 - 12
LEARNING STRAND
Transport: Systems
High School Enrichment Standard – Biology -- Physiology – As a result of the coordinated structures and functions of organ
systems, the internal environment of the human body remains relatively stable (homeostatic) despite changes in the outside
environment.
ENDURING UNDERSTANDINGS
All living organisms are active (living) because
of their abilities to link energy reactions to the
biological reactions that take place within their
cells.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Everything from cells to organisms to
ecosystems is in a state of dynamic balance
that must be controlled by positive or negative
feedback mechanisms.
ESSENTIAL QUESTIONS
What process is responsible for the movement
of water and nutrients in plants?
How do transport systems contribute to the
survival of multi-cellular organisms?
What is the difference between an open and
closed circulatory system?
How can the major structures and functions of
the human circulatory system be described?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify the transport systems found in plants.
Explain how water is transported in plants.
Explain the difference between water
movement by capillary action and water
movement explained by the cohesion-tension
hypothesis.
Explain how nutrients are transported in plants.
Compare the circulatory system of unicellular
organisms to the circulatory system in
invertebrates.
Compare and contrast the circulatory systems in
vertebrates (2, 3, 4- chambered hearts).
Describe the path of blood through the heart
and vessels of a human.
Explain what blood pressure is and what affects
it.
Describe the differences in function between
erythrocytes and leukocytes.
Compare and contrast the circulatory system
and the lymphatic system.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
―The leaf‖ CD-Rom
Blood video clip (LMC, power mediaplus)
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentation
Make a comparison chart describing the
components of blood.
Stomata lab
CD-Rom on transport structure in leaves
Cardiac 100 poster
Diagrams of human heart
Worksheets on transport systems
Internet animation on clotting
Blood typing lab
Heart rate lab
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Lab reports
Open-ended questions
Teacher observations
Label heart diagram
Blood typing analysis
Cardiac 100 poster graded by rubric
BIOLOGY HONORS
SCIENCE CURRICULUM 211 GRADES 9 - 12
LEARNING STRAND
Meiosis and Genetics
Content Standard: 10.4 – In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both
parents.
High School Enrichment Standards – Biology -- Genetics –- Mutation and sexual reproduction lead to genetic variation in a
population. -- A multi-cellular organism develops from a single zygote, and its phenotype depends on its genotype, which is
established at fertilization.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Scientific research often leads to technological
advances that can have positive and/or
negative impacts upon society as a whole.
All species tend to maintain themselves from
generation to generation using the same
genetic code.
There are genetic mechanisms that lead to
change over time, or evolution.
ESSENTIAL QUESTIONS
How does meiosis contribute to the heredity of
organisms and populations?
What are the similarities and differences
between mitosis and meiosis?
What are the errors and exceptions to Mendel’s
Laws?
What are the common patterns of inheritance?
How will genetic technologies contribute to our
understanding and treatment of common
human genetic diseases?
How does the structure of nucleic acids, genes
and chromosomes relate to their function?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Diagram the stages of meiosis.
Discuss the function of meiosis and its
importance to heredity.
Compare and contrast the processes of mitosis
and meiosis.
Relate the process of meiosis to inheritance of
traits.
Solve various types of Punnett Squares (mono
and di-hybrid crosses).
Discuss Mendel’s Laws using specific examples
in organisms.
Evaluate the errors and exceptions to Mendel’s
Laws.
Explain the difference between co-dominance
and incomplete dominance.
Explain polygenic inheritance and multiple gene
inheritance.
Discuss the causes and symptoms of common
human genetic diseases.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
PTC taste paper
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentation and lecture notes
Punnett Squares practice problems
Human genetics activities
Karyotyping (online and paper)
Probability activities
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Activity analysis questions
Punnett Squares practice problems
Open-ended questions
Teacher observations
BIOLOGY HONORS
SCIENCE CURRICULUM 212 GRADES 9 - 12
LEARNING STRAND
Gene Expression
Content Standard: 10.4 – In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both
parents.
High School Enrichment Standards – Biology -- Genetics –- Mutation and sexual reproduction lead to genetic variation in a
population. -- Genes are a set of instructions encoded in the DNA sequence of each organism that specify the sequence of amino
acids in proteins characteristic of that organism.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Scientific research often leads to technological
advances that can have positive and/or
negative impacts upon society as a whole.
All species tend to maintain themselves from
generation to generation using the same
genetic code.
There are genetic mechanisms that lead to
change over time, or evolution.
ESSENTIAL QUESTIONS
How will genetic technologies contribute to our
understanding and treatment of common human
genetic diseases?
What is the relationship between the processes
of replication, transcription, and translation?
How has our perception of DNA evolved through
the discovery of its importance?
How does the structure of nucleic acids, genes
and chromosomes relate to their function?
What are the ultimate causes of genetic errors?
How are the processes of transcription,
translation and replication regulated?
What is the importance of DNA in future
biotechnological advances?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Draw a schematic diagram of the structure of
DNA.
Illustrate the processes of replication,
transcription, and translation.
Evaluate the relationship of the processes of
replication, transcription and translation.
Discuss the history of the discovery of DNA
including relevant experiments.
Compare and contrast the three main types of
RNA including structure and function.
Discuss the ultimate cause of genetic errors as
it relates to translation.
Discuss how the processes of transcription,
translation and replication are regulated.
Identify methods cells have to prevent
mutation.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
CD-Rom on DNA molecule of life
Pop beads
SUGGESTED INSTRUCTIONAL STRATEGIES
Powerpoint presentation and lecture notes
Pop beads modeling gene expression
Cut-out activity on RNA types
Protein synthesis play
Designosaur activity
Snorks activity
Recipe for proteins
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Open-ended questions
Designosaur graded with rubric
Teacher observations
Gene expression activities
BIOLOGY HONORS
SCIENCE CURRICULUM 213 GRADES 9 - 12
LEARNING STRAND
DNA Technology
Content Standard: 10.4 – In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both
parents.
High School Enrichment Standards – Biology - Genetics -- Genes are a set of instructions encoded in the DNA sequence of each
organism that specify the sequence of amino acids in proteins characteristic of that organism. -- The genetic composition of cells can
be altered by incorporation of exogenous DNA into the cells.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Scientific research often leads to technological
advances that can have positive and/or
negative impacts upon society as a whole.
All species tend to maintain themselves from
generation to generation using the same
genetic code.
ESSENTIAL QUESTIONS
How has our perception of DNA evolved through
the discovery of its importance?
How does the structure of nucleic acids, genes
and chromosomes relate to their function?
What are the ultimate causes of genetic errors?
What is the importance of DNA in future
biotechnological advances?
How are the processes of transcription,
translation and replication regulated?
What legal and ethical problems have arisen
from new DNA technologies?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Evaluate the relationship of the processes of
replication, transcription and translation.
Discuss the history of the discovery of DNA
including relevant experiments.
Debate the importance of DNA in future
biotechnological advances.
Describe the following processes: DNA
electrophoresis, cloning, genetic engineering
and the Human Genome Project.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
Electrophoresis video
pGlo Gene transformation lab materials
Gel electrophoresis materials
Biology: Visualizing Life Holt, 1998
SUGGESTED INSTRUCTIONAL STRATEGIES
Powerpoint presentation and lecture notes
Websites on biotechnology
Article on cloning*
Read and take notes on the genetic engineering
chapter in Biology: Visualizing Life Holt, 1998
pGlo Gene transformation lab
Restrictive enzyme cut out activity
Gel electrophoresis (DNA epicenter field trip)
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Gel electrophoresis and pGlo analysis questions
Open-ended questions
Teacher observations
Benchmark*
o Meets expectations for reading critically
and responding to scientific literature
BIOLOGY HONORS
SCIENCE CURRICULUM 214 GRADES 9 - 12
LEARNING STRAND
Classification: Biological Diversity
Content Standard: 10.5 – Evolution and biodiversity are the result of genetic changes that occur over time in constantly changing
environments.
ENDURING UNDERSTANDINGS
Science is a way of knowing. It can involve a
discovery process using inductive reasoning, or
it can be a process of hypothesis testing.
Evolution is the biological change of organisms
that occurs over time. It is driven by the
process of natural selection and accounts for
the diversity of life on Earth.
Living organisms rarely exist alone in nature.
The structural levels of organisms ensure
successful functioning in all living organisms
and living systems (structure is related to
function).
Everything from cells to organisms to
ecosystems is in a state of dynamic balance that
must be controlled by positive or negative
feedback mechanisms.
ESSENTIAL QUESTIONS
What defines a species?
How do chemical and structural relationships
indicate related ancestry?
What is the Linnaean classification system?
What are the kingdoms of life?
How do advances in technology change
classification?
What are the three ways to classify a species?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Compare the differences and similarities
between the kingdoms.
Compare and contrast eubacteria and
archaeabacteria.
Explain how related ancestry can be indicated
by chemical and structural relationships.
Demonstrate how the Linnaean system of
groups and subgroups expresses the idea of a
degree of relatedness.
Compare the Linnaean system of classification
to phenetics and cladistics.
BSCS Biology: A Molecular Approach
Glencoe McGraw Hill, 2001
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture / PowerPoint presentation
Textbook reading and notes
Examine phyla specimens
How to make a cladogram
Natural history presentations
SUGGESTED ASSESSMENT METHODS
Quizzes
Test
Presentation graded with rubric
Open-ended questions
Teacher observations
Cladogram
Phyla specimen drawings and notes
BIOLOGY HONORS
SCIENCE CURRICULUM 215 GRADES 9 - 12
Biology Honors
3. Applies effective and efficient strategies for gathering information and materials,
thinking critically, and solving problems
CAPT Lab Conclusion/Discussion Rubric
Exceeds
Expectations
The student’s discussion and analysis are related t the stated problem and fully
supported by data. Their interpretation of results is thoroughly discussed.
Meets
Expectations
The student’s discussion and analysis are generally related to the stated problem
and supported by data. Minor errors in the interpretation of results may be
present. The student’s discussion of the validity of their conclusions is limited.
Meets Some
Expectations
The student’s discussion and analysis are related to the problem and supported
by data to a limited extent. Major errors in the interpretation of results may be
present. There is little discussion of the validity of the conclusions.
Does Not Meet
Expectations
The student’s discussion and analysis are not related to the stated problem, not
supported by data, or are missing. The student does not discuss the validity of
the conclusions.
BIOLOGY HONORS
SCIENCE CURRICULUM 216 GRADES 9 - 12
Testing for Vitamin C
The recommended intake of vitamin C is 60 mg per day, which can come from different food sources. To
determine the presence and the amount of vitamin C in different foods, there is a need to perform simple
chemical tests. In this task, you will use a purple indicator to test for vitamin C.
Your Task
First, you and your lab group will test a series of vitamin C solutions with known concentrations using the
vitamin C indicator. Next, you and your lab group will design and conduct an experiment to compare the
amount of vitamin C in various fruit juices. Then you will determine the concentration of vitamin C in
each of the juices.
You have been provided with the following materials and equipment. It may not be necessary to use all
of the equipment that has been provided. You may use additional materials and equipment if they are
available.
CAUTION:
The vitamin C indicator will stain clothes and hands.
Materials
Vitamin C solution (1 mg/mL) 5 test tubes
Vitamin C indicator Test tube rack
Apple juice 8 Plastic measuring cups
Pineapple juice 5 medicine droppers
White grape juice Access to tap water
Graduated cylinder Wax crayons
Paper towels for clean-up
Safety goggles and lab apron
Part I: Testing a Vitamin C Solution
First, you will find out how many drops of a vitamin C solution (with a known concentration) it takes for
the indicator to lose its purple color. You will investigate vitamin C solutions with varying concentrations
and one solution (water) that has no vitamin C added. The higher the concentration of vitamin C in
the solution, the fewer drops it will take for, the indicator to lose its purple color.
You have been given a solution containing 1.00 milligram (mg) of vitamin C per milliliter (mL) of water.
Procedure:
1. Using the table below, create vitamin C solutions with different concentrations by mixing the 1.00
mg/mL vitamin C solution with water in plastic cups. Be sure to label the cups with the corresponding
concentration.
2. Add 10 drops of the purple indicator to a clean test tube.
3. Add drops of the 1.00 mg/mL vitamin C solution, one at a time, to the test tube containing the
indicator. Shake the test tube gently after adding each drop.
4. Keep adding drops of the vitamin C solution until the indicator loses its purple color. Record your
results in the table below.
5. Repeat steps 2-4 using the other vitamin C solutions you created in step 1.
6. Create a line graph of your results.
BIOLOGY HONORS
SCIENCE CURRICULUM 217 GRADES 9 - 12
Drops of 1.00 mg/mL
Vitamin C Solution
Drops of Water
Added
Concentration of New
Vitamin C Solution
(mg/mL)
Number of Drops of
Vitamin C Solution Added
to the Indicator
40
0
1.00
30
10
.75
20
20
.50
10
30
.25
0
40
0.00
Part II: Comparing the Amount of Vitamin C in Three Fruit Juices
Now you and your lab group will design and conduct an experiment to compare the amount of vitamin C
in various fruit juices.
1. In your own words, clearly state the problem you are going to investigate. Include a clear
identification of the independent and dependent variables that will be studied.
2. Design an experiment to solve the problem. Your experimental design should match the
statement of the problem, should control for variables, and should be clearly described so that
someone else could easily replicate your experiment. Include a control if appropriate.
3. Write your experimental design and show your design your teacher before you begin your
experiment.
4. After receiving approval from your teacher, work with your lab group to carry out your
experiment. Your teacher's approval does not necessarily mean that your teacher thinks your
experiment is well designed. It simply means that, in your teacher's judgment, your experiment is not
dangerous or likely to cause an unnecessary mess.
5. While conducting your experiment, take notes. Include the results of your experiment. Tables,
charts, and/or graphs should be used where appropriate and should be properly labeled.
6. Use your results from Part I to determine the concentration of vitamin C in the juices
tested.
BIOLOGY HONORS
SCIENCE CURRICULUM 218 GRADES 9 - 12

BIOLOGY HONORS
SCIENCE CURRICULUM 219 GRADES 9 - 12
iology Honors
1A. Read Effectively Rubric
Scientific American
article with comprehension questions
Exceeds
Expectations
The student independently applies effective reading strategies to understand,
interpret, evaluate, and analyze text to acquire content knowledge about a
current topic in science.
Meets
Expectations
The student needs minimal assistance and applies effective reading strategies to
understand, interpret, evaluate, and analyze text to acquire content knowledge
about a current topic in science.
Meets Some
Expectations
The student applies some reading strategies to understand, interpret, and
attempt to analyze text to acquire content knowledge about a current topic in
science. S/he may need some assistance to read and comprehend material at
grade level.
Does Not Meet
Expectations
The student has difficulty applying reading strategies without assistance to
understand, interpret, and evaluate text to acquire content knowledge about a
current topic in science.
BIOLOGY HONORS
SCIENCE CURRICULUM 220 GRADES 9 - 12
Reading a Scientific Article
Name:
Read the following article. While reading it, write down on this sheet the following:
What is the subject/topic of the article?
What are the key ideas in the article?
What did you learn from the article (key ideas)?
Write down all the questions that you would like to ask after reading this article (remember do not
decide which of the questions are important and which are less important)
From this list of questions, select the most interesting one that you would like to investigate
(theoretically) or find more information.
How would you find out more information/answer the question you selected?
BIOLOGY HONORS
SCIENCE CURRICULUM 221 GRADES 9 - 12
Biology Honors
2. Uses technology effectively and responsibly
DNA Webquest Rubric
Exceeds
Expectations
The student independently selects a currently updated and authored website
directly related to the lab discussion.
Meets
Expectations
The student needs minimal assistance selecting an appropriate website directly
related to the lab discussion. The website may be outdated or un-authored
Meets Some
Expectations
The student needs some assistance selecting an appropriate website directly
related to the lab discussion. The website is outdated and/or un-authored.
Does Not Meet
Expectations
The student cannot select an appropriate website directly related to the lab
discussion. The website is outdated and un-authored.
BIOLOGY HONORS
SCIENCE CURRICULUM 222 GRADES 9 - 12
Name__________________________________
Using web sites on the nucleic acids, DNA and RNA, find the following answers. Please
site your sources in proper MLA form.
1. What elements make up DNA and RNA?
2. What are the monomers of nucleic acids?
3. What are the three parts of a nucleotide?
4. What are the 4 nitrogen bases found in DNA?
5. What is the name of the sugar in DNA? RNA?
6. What scientists discovered the shape of DNA? What is the shape called?
7. What nitrogen bases are purines?
8. What nitrogen bases are pyrimidines?
9. Why do purines bond to pyrimidines?
BIOLOGY HONORS
SCIENCE CURRICULUM 223 GRADES 9 - 12
Biology Honors
1 B. Writing Effectively Rubric
Peppered-moth Investigation - Analyzing data to explain
and scientific concept
Exceeds
Expectations
The student organizes, analyzes, and synthesizes the data from an activity on
evolution by natural selection in order to accurately explain this scientific concept.
The explanation is organized and developed. Word choice and syntax are
accurate and appropriate. The student shows mastery in the conventions of
Standard English in the written assignment.
Meets
Expectations
The student needs minimal assistance in organizing, analyzing, and synthesizing
data from an activity on evolution by natural selection in order to explain this
scientific concept. The explanation is somewhat organized and developed. Word
choice and syntax are accurate and appropriate. Errors in the conventions of
Standard English are few in the written assignment.
Meets Some
Expectations
The student requires additional explanations and models in order to organize,
analyze and synthesize the data from an activity on evolution by natural selection.
Writing is somewhat limited, and supporting evidence for this concept may be
slightly inaccurate or simplistic. The student may require assistance to develop or
organize his response. Word choice and syntax are consistent with grade level.
There are some errors in the conventions of Standard English in the written
assignment.
Does Not Meet
Expectations
The student requires many additional explanations, models, and/or strategies in
order to organize, analyze, and synthesize data from an activity on evolution by
natural selection. The explanation for this concept does not focus on the
supporting evidence. Ideas and concepts are often inaccurate, confusing, or
unorganized. In accurate or limited vocabulary, syntax errors, and errors in the
conventions of writing make the assignment ineffective.

BIOLOGY HONORS
SCIENCE CURRICULUM 224 GRADES 9 - 12
Investigations for Chapter 16
Origin of New Species
Investigation 16A – Natural Selection
Charles Darwin collected many facts to support
the theory of evolution by natural selection. Yet,
he never examined some remarkable examples of
natural selection that were going on around him in
the English countryside, such as the selection
process involving the peppered moth, Biston
betularia (BIS ton bet choo LAR ee a).
The Industrial Revolution began in the middle of
the eighteenth century. Since that time, tons of
soot had been deposited around the industrial
areas of England. -Soot, the ash created from
burning coal and wood, discolored and generally
darkened the surfaces of trees, rocks, and other
features of the landscape. It also destroyed the -
lichens that once grew in these areas. Lichens are
associations of algae and fungi that frequently
encrust the bark of trees. Many lichens are light in
color. Before the Industrial Revolution, the
peppered -moths that lived among the trees also
were light colored.
In 1848, the first dark-colored peppered moth was
observed and recorded. A century later, 90 percent
or more of the peppered moths in some areas were
dark in color. More than 70 species of moths in
England changed from light to dark coloration.
Similar observations have been made in other
industrial nations, including the United States.
How did this striking change in coloration come
about? Was the change related to the way in
which one species is thought to evolve normally
from another? Or was it a unique occurrence?
In this investigation, you will answer these
questions by interpreting the results of some
experiments. It will be helpful to keep the
following information in mind:
1. Hereditary characteristics of parent organisms
are passed on to their offspring.
2. Changes can occur in the hereditary material of
the parents to produce offspring with
characteristics different from those of the
parents. These changes are known as
mutations.
3. If the different form, called a mutant, survives
and reproduces, it may pass on the new trait, or
mutation, to future generations.
Materials (per team of 3)
paper and pencil
Procedure
1. Read the following description of an
experiment with chemicals from soot: The
peppered moth has a one-year life cycle. The
egg hatches into a larva (caterpillar), which
feeds on tree leaves. The animal then goes
through a dormant stage and is finally
transformed into an adult moth. In 1926, a
British scientist fed leaves treated with certain
chemicals found in soot to the larvae of light-
colored moths. The larvae then were permitted
to go through the normal life cycle. Eventually,
the larvae changed into light-colored adult
moths. These moths were allowed to mate and
produce offspring. When the scientist counted
these offspring, 8 percent of the new moths
were found to be dark-colored. Because this
rate of mutation was much higher than normal,
the scientist claimed that the chemicals found
in soot caused changes to the hereditary
material that determines body color in the
peppered moth. When the experiment was
repeated by other scientists, however, their
results showed much less than 8 percent dark-
colored offspring. Moreover, it was found that
light-colored moth larvae that were fed on
unpolluted leaves produced about as many
dark-colored moths as those fed on polluted
leaves.
2. Answer Analysis questions 1 through 3.
3. Read the following description of an
experiment in an unpolluted forest: More
recently another experiment was performed
with Riston betularia. A large number of both
the light and dark forms of the moths were
captured. The underside of each moth was
marked with a small spot of paint for
identification. Known numbers of these
marked moths were then released in an
unpolluted forest. After a period of time, moths
were collected from this forest and the marked
BIOLOGY HONORS
SCIENCE CURRICULUM 225 GRADES 9 - 12
ones were counted. Of 488 dark moths and 496
light moths released in an unpolluted forest, 34
dark moths and 62 light moths were
recaptured.
4. Answer Analysis questions 4 through 6.
5. Read the following description of an
experiment a light-colored tree:
In still another experiment, equal numbers of
light and dark moths were placed on a light-
colored tree in an unpolluted forest. These
moths were kept tinder careful observation.
Birds were seen to seize moths from the tree
and rapidly carry them away. At the end of a
day, approximately twice as many light moths
as dark moths were left on the trees. Then the
reverse experiment was performed. Equal
numbers of light and dark moths were placed
on a dark-colored tree in a polluted forest. At
the end of the day, approximately twice as
many dark- colored moths were left. Refer to
Figure 16.6 in your textbook which shows the
moths on both light and dark trees.
6. Answer Analysis questions 7 through 12.
7. Read the following passage that describes an
observation in an unpolluted forest: In an old
forest in Scotland, far removed from industrial
cities, there is a species of moth called Cleora
repandata. Of about 500 moths observed,
approximately 50, 10 percent of the total
population, were dark colored while 90 percent
were light colored. When these moths rest on
the bark of pine trees during the daytime, the
dark form is more conspicuous. Observations
have shown that many moths move from one
tree trunk to another during the day if they are
disturbed by ants or the heat of the sun. In
flight, the dark moth is visible for a distance of
about 18 meters, but the light moth is visible
for a distance of more than 90 meters.
Observers have reported seeing light moths
captured in flight by birds.
8. Answer Analysis questions 13 through 17.
Analysis
1. What is the value of an experiment that other
scientists repeat with different results?
2. What errors could have been made by the
scientist who performed the feeding
experiment in 1926?
3. What was the control in the second
experiment? Why was it necessary?
4. Why was the spot of paint placed on the under
side of the moth rather than on top?
5. What may have happened to the moths that
were not recaptured?
6. How do the results of this experiment give
evidence of natural selection? How might this
experiment be changed to give even better
evidence of natural selection?
7. What is the chief predator of the peppered
moth?
8. Assuming that equal numbers of light and dark
moths are present on a light tree, which type of
moth would most likely be preyed on? Why?
What if the moths were on a dark tree?
9. Does the last experiment described in the
procedure help support the conclusions you
have drawn thus far? Explain.
10. Which type of moth is more apt to survive in a
polluted forest? In an unpolluted forest?
11. Is it likely that dark-colored moths existed
before the Industrial Revolution?
12. If any dark colored moths did exist before the
Industrial Revolution, what probably happened
to them?
l3. Which body coloration is protective when the
moths are resting during the daytime? Give
evidence to support your view.
14. Which body coloration is protective when the
moths are in daytime flight? Give evidence to'
support your view.
BIOLOGY HONORS
SCIENCE CURRICULUM 226 GRADES 9 - 12
15. Studies have shown that light-colored moths
produce 1 dark moth in approximately 200,000
offspring. This is the mutation rate from light
to dark, only 0.0005 percent. From, these data,
how can you explain the fact that 10 percent of
the total population of moths found in an
unpolluted forest are dark?
16. On the basis of your interpretation of the
preceding experiments, write a short paragraph
using these questions as a guide: How has the
striking change in coloration of the English
peppered moth population come about? (Use
Darwin’s theory of natural selection and apply
it to what you have learned in this
investigation.) Is the change related to the
mechanisms by which one species is thought to
evolve normally from another, or is it a special
case of evolution found only in the English
peppered moth? (Apply Darwin's ideas on the
origin of new species.) Include an explanation
of how the dark moth appeared and how the
proportion of dark moths changed from 0.0005
percent to more than 90 percent in the polluted
forests.
17. Soot and factory pollution are subsiding as
pollution-control measures are practiced in
many industrial areas. Non-pollutant fuels may
replace the coal-burning furnaces of present
factories. Write a short paragraph predicting
changes you might expect in the environment
and the effect these changes will have on the:
survival and reproduction of the two colors of
peppered moth.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 227 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Integrated Science I
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Intgr. Science I
3. Transcript Course Code/Number
00316
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 9 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
This course is offered to all freshmen without a prerequisite.
11. Brief Course Description
In Integrated Science I students will explore a variety of physical phenomena and methods used to
acquire scientific knowledge. Topics considered for study include science skills, structure of matter, states
and properties of matter, chemical interactions, energy, and carbon chemistry. The course relies heavily
on inquiry based lab investigations, writing extensive lab reports, as well as mathematical problem
solving in science.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 228 GRADES 9 - 12
13. Course Outline
in presented order
:
CHAPTER
CONCEPTS
ACTIVITY / INVESTIGTAION
1) Science Skills
Using the scientific method
Introduction to significant figures
Introduction to dimensional
analysis
Presenting scientific data/results
Identifying variables*
Thickness of Aluminum Foil lab*
4) Atomic Structure
History of the atomic model
The structure of the atom
Modern atomic theory
Flame test lab
9) Carbon Chemistry
Identifying carbon compounds
Natural and synthetic polymers
Elmer’s/Borax Lab**
Paper vs. Plastic assignment **
2) Properties of Matter
Classifying matter
Physical properties
Chemical properties
Identify chemical properties lab
13.3) Buoyancy
Archimedes’ principle
Density of objects lab*
3) States of Matter
Solids, liquids, gases
Gas laws
Phase changes
Boyles law lab
Phase change demo/lab
13.1) Fluid Pressure
Water pressure
Air pressure
Pascal’s principle
Hydraulic systems
FINAL EXAMINATION
*
Benchmark Activities
** Embedded Tasks
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
Benchmark Assessments:
- Identification of independent, dependent and controlled variables in an experiment
- Differentiation of quantitative and qualitative data; representation of data on an appropriate graph
- Develop a properly designed experiment given a problem and list of materials. Students are expected to perform
the experiment using their own procedure and follow the appropriate lab report format as outlined in the rubric. This
culminating activity assesses the student's ability to apply the scientific method.
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 229 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present the relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated
during the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Physical Science: Concepts in Action
Prentice Hall, 2004
Materials for Aluminum foil lab investigation *
Internet access
Response cards
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Response cards
Aluminum Foil Lab investigation *
Significant Figure Internet investigation
Bart Simpson scientific method activity *
SUGGESTED ASSESSMENT METHODS
Benchmark:
Meet course expectations for identifying
components of the scientific method
Meet course expectations for constructing
appropriate graphs for representing experimental
data
Other Assessments
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies

INTEGRATED SCIENCE I
SCIENCE CURRICULUM 230 GRADES 9 - 12
LEARNING STRAND
Atomic Structure
CT Standard 9.4: Atoms react with one another to form new molecules.
ENDURING UNDERSTANDINGS
Atoms have a defined structure that gives each
element its unique properties.
Detailed observations can reveal information
about objects and events that cannot be
observed directly.
The evolutionary model of the atom was
developed through analysis and observations of
controlled scientific experimentation.
Atoms react with other atoms by sharing or
exchanging subatomic particles.
Atoms can combine to form more complex
forms of matter.
ESSENTIAL QUESTIONS
How does the structure of the atom help to
explain the properties of elements?
How does the structure of the atom help to
explain the ways in which atoms combine to
form new compounds?
How do the differences in the properties of
subatomic particles help to explain the current
model of the atom?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe ancient Greek models of matter.
Use historical experiments to explain the
development of the model of the atom.
Identify the location and properties of a proton,
neutron, and electron in the model of an atom.
Distinguish the atomic number of an element
from the mass number of an isotope, and use
these numbers to describe the structure of
atoms.
Demonstrate the relationship between electrons
and energy levels in an atom.
Distinguish the ground state from excited states
of an atom based on electron configurations.
Explain how radiant energy can be absorbed
and later released by an atom.
Explain how the electron cloud model and
probability represents the behavior and
locations of electrons in atoms.
Physical Science: Concepts in Action
Prentice Hall, 2004
Hoffman apparatus
Cathode ray
Materials for Flame test lab investigation
Video
Response cards
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Response cards
Hoffman apparatus demonstration
Cathode ray demonstration
Construct Bohr models activity
Flame Test lab investigation
Assignment Discovery video on matter
SUGGESTED ASSESSMENT METHODS
Quizzes
Unit Test
Investigations evaluated with rubrics
Response cards by TurningTechnologies
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 231 GRADES 9 - 12
LEARNING STRAND
Carbon Chemistry
CT Standard 9.5: Due to its unique chemical structure, carbon forms many organic and inorganic compounds.
CT Standard 9.1: Energy cannot be created or destroyed; however, energy can be converted from one form to another.
CT Standard 9.3: Various sources of energy are used by humans and all have advantages and disadvantages.
ENDURING UNDERSTANDINGS
The structure of a carbon atom allows for
millions of different arrangements.
Carbon exists in three distinct forms.
The properties of carbon are directly related
to the number and arrangement of carbon
atoms.
Hydrocarbons are molecules that contain
carbon and hydrogen.
Fossil fuels exits in three forms and produce
two primary products during combustion.
Materials produced from the cracking of
petroleum are the starting points for the
production of many synthetic compounds.
Chemical technologies products are synthetic
fibers, pharmaceuticals, plastics and fuels.
Polymers can be classified as natural or
synthetic polymers.
ESSENTIAL QUESTIONS
How is the structure of the three forms of carbon
related to their properties?
How are the arrangement and number of carbon
atoms in a hydrocarbon related to the properties?
How are the three forms of unsaturated
hydrocarbons different? Similar?
How does the carbon atom's structure affect the
type of bonds formed in organic molecules?
Where are the geographical locations where the
different forms of fossil fuels can be found?
How can a solution such as crude oil be refined
into simpler substances?
What are some characteristics of the incomplete
combustion of a fossil fuel?
What are the two ways polymers are classified?
How can simple monomers be combined to create
linear, branched and/or cross linked polymers?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Illustrate a carbon atom using a Bohr model.
Identify the valence electrons in a carbon
molecule.
Differentiate between a general formula,
molecular formula, and structural formula for
a carbon molecule.
Explain the difference between Alkanes,
Alkenes, and Alkynes.
Differentiate between an organic and an
inorganic compound.
Describe the formation, composition, and
uses of the three types of fossil fuels.
Distinguish between complete and incomplete
combustion of fossil fuels.
Describe the effects of some products of the
combustion of fossil fuels.
Distinguish a monomer from a polymer.
Describe the structures and functions of four
types of natural polymers produced by
organisms.
Physical Science: Concepts in Action
Prentice Hall, 2004 with ancillary materials
Materials for polymer lab investigation **
(CAPT Embedded Task: Synthetic Polymers)
Materials for Super Ball lab investigation
Fractional distillation apparatus
Combustion engine demonstration (virtual)
Response cards
Videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Web based instruction with Blackboard/finalsite
Research
Response cards
Molecular model kits
Fractional distillation demonstration
Combustion engine demonstration
Polymer lab investigation ** (CAPT Task)
Super ball lab investigation
Videos on carbon and polymers
Persuasive essay research assignment (paper
products vs. plastic products) ** (CAPT Task)
SUGGESTED ASSESSMENT METHODS
Quizzes and Unit Test
Investigations evaluated with rubrics
Response cards by TurningTechnologies
Persuasive essay research assignment **(CAPT)
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 232 GRADES 9 - 12
LEARNING STRAND
Properties of Matter
CT Standard 9.4: Atoms react with one another to form new molecules.
ENDURING UNDERSTANDINGS
Matter that always has the same composition is
classified as a pure substance.
Mixtures tend to retain some of the properties
of their individual substances, and can be
classified into three types.
Physical properties are characteristics of a
material that can be observed or measured
without changing the composition of the
substances.
Chemical properties can be observed only when
the substance in a sample changes into
different substances.
The characteristics of physical changes are
different than the characteristics of chemical
changes.
Properties of matter can be used to confirm the
identity of substances.
ESSENTIAL QUESTIONS
Why are elements and compounds classified as
pure substances?
How do mixtures differ from pure substances?
What is the main difference among solutions,
suspensions, and colloids?
What are some examples of physical
properties?
How can knowing a physical property be useful?
What processes can be used to separate
mixtures?
What observations might indicate that a
chemical change has occurred?
What is the difference between a physical and
chemical change?
What properties of matter make up the density
of a substance?
How can the identity of a pure substance be
determined with density?
How can you determine if an object will sink or
float?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Classify pure substances as elements or
compounds.
Distinguish between pure substances from
mixtures.
Classify mixtures as solutions, suspensions, or
colloids.
Identify substances based on their density.
Describe how properties are used to choose a
material.
Describe how distillation can be used to
separate a solution.
Explain the evidence that indicates a physical or
chemical change has occurred.
Explain how the identity of a pure substance
can be determined with density.
Explain the effect of buoyancy on the apparent
weight of an object.
Explain the relationship between the volume of
fluid displaced by an object and the buoyant
force acting on the object according to
Archimedes’ principle.
Describe the relationship among object density,
fluid density, and whether an object sinks or
floats in a fluid.
Physical Science: Concepts in Action
Prentice Hall, 2004
Ocean circulation classroom apparatus
Distillation apparatus
Density rod classroom demonstration
Materials for physical properties lab activity *
Materials for chemical properties lab activity
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web based instruction with Blackboard/finalsite
Research
Response cards
Convection demonstration with the ocean
circulation apparatus
Distillation demonstration
Density rod demonstration
Physical Properties lab investigation *
Chemical Properties lab investigation
SUGGESTED ASSESSMENT METHODS
Benchmark
: Meet course expectations for generating
a properly written scientific lab report
Other Assessments
:
Unit Test and Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 233 GRADES 9 - 12
LEARNING STRAND
States of Matter
CT Standard 9.4: Atoms react with one another to form new molecules.
CT Standard 9.1: Energy cannot be created or destroyed; however, energy can be converted from one form to another.
ENDURING UNDERSTANDINGS
Materials can be classified as solids, liquids, or
gases based on their shape and volume.
The Kinetic Theory of matter says that all particles
of matter are in constant motion.
Thermal energy can be transferred by conduction,
convection, and radiation only.
Energy provides the ability to work & exert force.
Heat flows in one direction, hot to cold.
Although there are forces of attraction among all
forms of matter, it is the weakest in gases.
The constant motion of particles in a gas allows a
gas to fill a container of any shape and size.
Pressure is the result of a force distributed over
an area.
A phase change is the reversible change that
occurs when a substance changes from one state
of matter to another.
ESSENTIAL QUESTIONS
How can shape and volume be used to classify
materials?
How can the kinetic theory and forces of
attraction be used to explain the behavior of
gases, liquids, and solids?
What is the law of conservation of energy?
How are energy and work related?
What causes gas pressure in a closed
container?
What factors affect gas pressure?
How are temperature, volume, and pressure of
a gas related?
What happens to a substance’s temperature
and a system’s energy during a phase change?
How are evaporation and boiling different?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Classify materials as solids, liquids, or gases.
Explain the behavior of gases, liquids, and solids,
using the kinetic theory of matter.
Identify factors that affect gas pressure.
Describe the conversions of energy from one form
to another.
Explain how energy is transferred by conduction,
convection and radiation.
State and apply the law of conservation of
energy.
Explain Charles’s law, Boyle’s law, and the
combined gas law.
Apply gas laws to solve problems involving gases.
Explain how temperature can be used to
recognize a phase change.
Describe the effects of adding energy to matter in
terms of the motion of atoms and molecules, and
the resulting phase changes.
Identify phase changes as endothermic or
exothermic.
Physical Science: Concepts in Action
Prentice Hall, 2004
Super heated gas demonstration
Pascal’s principle demonstration
Vacuum and Bell Jar demonstration
Molecular motion apparatus
Calculator Based Lab (CBL) temperature
probes for phase change lab
CBL pressure probes for Boyles law lab
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web instruction with Blackboard/finalsite
Research
Response card
Molecular motion demonstration
Vacuum and Bell Jar demonstration
Pascal’s principle demonstration
Boyle’s law lab activity with CBL probes
Phase change lab activity with CBL probes
SUGGESTED ASSESSMENT METHODS
Quizzes and Unit Test
Investigations evaluated with rubrics
Response cards by TurningTechnologies
Appropriate use of technology
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 234 GRADES 9 - 12
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 235 GRADES 9 - 12
Identify the Controls and Variables
Smithers thinks the drug AZT will cure
AIDS. He takes 100 patients with AIDS
and gives the drug to 50 of them (group
A). To the other 50, he gives them a drug
that looks just like AZT but is really just a .
sugar pill (group B). Both groups were
told that they were getting a drug that
would cure AIDS After 6 months, 30
patients in group A reported having fewer
symptoms. 10 people in group B reported
having fewer symptoms.
Identify the –
1. Control Group
2. Independent Variable
3. Dependent Variable
4. What should Smithers conclusion be?
5. Why was group 8 given a sugar pill?
6. Why do you think 10 people in group B
reported feeling better?
Homer notices that his shower is covered
in a strange green slime. His friend
Barney tells him that coconut juice will
get rid of the green slime. Homer decides
to test this out by spraying half of the
shower with coconut juice. He sprays the
other half of the shower with water. After
3 days of "treatment" there is no change
in the appearance of the green slime on
either side of the shower.
7. What was the initial observation?
Identify the -
8. Control Group
9. Independent Variable
10. Dependent Variable
11. What should Homer's conclusion be?
Bart believes that mice
exposed to microwaves
will become extra
strong (maybe he's
been reading
too much
RadioactiveMan). He
decides to perform this experiment by
placing 10 mice in a microwave for 10
seconds. He compared these 10 mice to
another 10 mice that had not been
exposed. His test consisted of a heavy
block of wood that blocked the mouse
food. He found that 8 out of the 10
12. What was Bart's hypothesis?
Identify the
13. Control Group
14. Independent Variable
15. Dependent Variable
16. What should Bart's conclusion be?
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 236 GRADES 9 - 12
microwaved mice were able to push the
block away. 7 out of the 10
nonmicrowaved mice were able to do the
same.
Krusty was told that a certain itching
powder was the newest best thing on the
market, it even claims to cause 50 %
longer lasting itches. Interested in this
product, he buys the itching powder and
compares it to his usual produce. One
test subject (A) is sprinkled with the
original itching powder, and another test
subject (8) is sprinkled with the
Experimental itching power. Subject A
reported having itches for 30 minutes,
Subject 8 reported to have itches for 10
hours.
Identify the-
17. Control Group
18. Independent Variable
19. Dependent Variable
20. What should Krusty's conclusion be?
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 237 GRADES 9 - 12
PHYSICAL SCIENCE
/
INTEGRATED SCIENCE I
EFFECTIVE CRITICAL THINKING STRATEGIES
IN IDENTIFYING VARIABLES
3
Exceeds Expectations
90 - 100
The student independently interprets, analyzes, and evaluates a
variety of information and data to make original predictions. S/he
accurately identifies all of the independent, dependent, and
controlled variables in an experiment.
Meets Expectations
75 - 89
The student independently interprets, analyzes, and evaluates a
variety of information and data to make specific predictions. S/he
adequately identifies most of the independent, dependent, and
controlled variables in an experiment.
Meets Some Expectations
65 - 74
The student may need some assistance to interpret a variety of data
to make general predictions. S/he poorly identifies some of the
independent, dependent, and controlled variables in an experiment.
Does Not Meet Expectations
0 - 64
The student needs assistance to interpret information to make a
prediction. S/he did not identify the independent, dependent, or
controlled variables in an experiment.
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 238 GRADES 9 - 12
Determining the Thickness of Aluminum Foil
An Exercise in Graphing and Precision
Background:
Provide a clear description of the experiment, and a rationale for why the experiment was performed
**REFERENCE PAGE 26 & 27 IN YOUR TEXTBOOK**
Problem:
1. How are the thickness and brand of aluminum foil related?
2. Which brand of foil is the cheapest/least expensive by volume?
Hypotheses:
1. State a hypothesis relating the brand of aluminum foil and thickness.
Use the (If________, then ________) format
IV:
DV:
CONSTANTS:
CONTROL: none in this lab.
Materials:
metric ruler
balance
three (3) different brands of aluminum foil
graph paper
scissors
Procedure:
1. In your lab journal duplicate three “3” copies of the data table below for each brand of
aluminum foil tested.
BRAND NAME: ______________________________
Length
(mm)
Area
(mm
2
)
Mass
(g)
Volume
(mm
3
)
Thickness
(mm)
50.0
100.0
200.0
Average
Density of Aluminum = _________________________ g/mm
3
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 239 GRADES 9 - 12
2. Cut out three squares of aluminum foil with sides of the following lengths from each brand of
foil tested: 50.0 mm, 100.0 mm, and 200.0 mm. Label each foil square.
3. Calculate the area of each foil square using proper precision. (NOTE: You may have to use
scientific notation) Record the area in your data tables.
4. Mass each square of foil. Record the mass in your data tables.
5. The density of aluminum is 2.71 g/cm
3
. Convert the density into g/mm
3
. Record the new
density below the data table.
6. Determine the volume of the foil for each length. To determine the volume divide the mass
of each length by the density in g/mm
3
. Using proper precision record the volume in the data
tables.
7. Determine the thickness of the foil for each length. Recall that volume is (length x height x
width), and area is (length x width). By dividing the volume by the area the result equals the
height “thickness” only. Using proper precision record the thickness in your data tables.
8. In your lab journal duplicate a copy of the data table below. Complete the data table.
BRAND AND AVERAGE THICKNESS
BRAND
AVG. THICKNESS (mm)
9. (The following benchmark task for the 9
th
grade science curriculum). Determine which
graph would best represent the following data: One graph relates the length and thickness of
each brand of foil tested. The second graph compares the brand and average thickness.
Construct the graphs and include them in your lab journal. Reference Chapter 1.4
10. Complete the summary questions.

INTEGRATED SCIENCE I
SCIENCE CURRICULUM 240 GRADES 9 - 12
SUMMARY QUESTIONS
1. How many significant figures were there in your measurement of the length of each square of foil
tested?
2. What effect, if any, did the length of foil have on the thickness of the foil?
Explain ...
3. Which length of foil resulted in the most precise estimate off oil thickness?
Explain why.
4. What factor(s) may have limited the precision of your measurements?
5. What is the cost ($) per volume (mm
3
) for each brand of foil tested? To answer this question you
will need the total area (see box), average thickness (reference your calculations), and the cost of
each brand of foil tested. You must show your work for full credit
CHALLENGE QUESTION REQUIRED
1. Aluminum, like all matter, is composed of tiny particles called atoms. Each aluminum atom has a
diameter of 2.86 x 10
-10
m. Calculate how many atoms make up the average thickness of each
brand of foil tested.
WHAT YOU SHOULD CONSIDER WHEN DEVELOPING YOUR CONCLUSION AND
DISCUSSION:
1. Does your data support or refute your hypothesis?
2. Is there evidence of error in your data?
a. Explain how your know there is error (analyze data table and graphs)
b. Identify the error as instrumental, human, or a combination of the two.
3. Provide information from your procedure and/or your data that would help you answer the
problem(s). Try to include as much detail as possible. Offer a logical explanation for your
results. (the amount of explaining varies among experiments)
4. Explain how you think the experiment could be improved.
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 241 GRADES 9 - 12
PHYSICAL SCIENCE / INTEGRATED SCIENCE I
EFFECTIVE CRITICAL THINKING STRATEGIES
IN CONSTRUCTING GRAPHS
3
Exceeds Expectations
The student independently collects, interprets, analyzes, and
evaluates data to solve a problem. S/he accurately identifies data as
quantitative or qualitative. S/he constructs the appropriate graph to
represent the data. The graph is titled, properly scaled, labeled and
accurately charted. The structure of the graph does not contain any
errors.
Meets Expectations
The student independently collects, interprets, analyzes, and
evaluates data to solve a problem. S/he accurately identifies data as
quantitative or qualitative. S/he constructs the appropriate graph to
represent the data. The graph is titled, scaled, labeled and charted.
Minor errors in structure of the graph are present.
Meets Some Expectations
The student independently collects, interprets, analyzes, and
evaluates data to solve a problem. S/he accurately identifies data as
quantitative or qualitative. S/he constructs the appropriate graph to
represent the data. The graph is titled, scaled, labeled and charted.
Significant errors in structure of the graph are present.
Does Not Meet Expectations
The student independently collects data but may require assistance
to interpret, analyze, and evaluate the data to solve a problem. S/he
inaccurately identifies data as quantitative or qualitative. S/he
constructs the wrong graph type or requires assistance to
construct a graph to represent the data.

INTEGRATED SCIENCE I
SCIENCE CURRICULUM 242 GRADES 9 - 12
LAB REPORT RUBRIC
THICKNESS OF ALUMINUM FOIL
F= 0 - 1.75 D = 2.0 C = 2.25 B = 2.5 A= 2.75 - 3.0
REPORT FORMAT .......................................................................................................................
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
Lab report contains a completed introduction which
includes the background, two problems, hypotheses,
and variables for the problem
Lab report is written in a lab journal "IPS COURSE"
Lab report contains a materials and a complete
procedure section. The steps are listed in a numbered
format.
Lab report contains a results section, which includes 4
data tables and two separate graphs
Lab report contains a summary question section. All
questions including challenge question are attempted.
Lab report contains a conclusion and discussion
section. A simple conclusion and a developed response
that explains the results are included.
0= incomplete lab
The overall report is complete, legible, and properly
organized. Data tables, graphs, and other charts are
neatly constructed.
Total Points:
/ 21
Comments related to REPORT FORMAT:
INTRODUCTION AND EXPERIMENTAL DESIGN .............................................................................
Number next to each standard reflects
the order of the lab report
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
1. Background: Provides a clear description of the
experiment and a rationale for why the experiment was
performed
2. Problem: Identifies one testable problem directly
related to the investigation/hypothesis
3. Hypothesis: Formulates a properly written and
testable hypothesis directly related to problem # 1
4. Variables: All variables including IV, DV,
CONSTANTS, and CONTROL for the problem are
correctly identified, labeled, and are related to the
procedure and results
Total Points:
/ 12
Comments related to INTRODUCTION AND EXPERIMENTAL DESIGN:
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 243 GRADES 9 - 12
PROCEDURE AND RESULTS ....................................................................................................
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
1. Materials: Provides a detailed list of all the materials
used in the investigation.
2. Procedure: Presents easy to follow numbered steps,
which are logically sequenced, complete, and detailed.
3. Data Table: Tables are correctly constructed and
labeled with appropriate units. All data is present and
accurate. Proper precision is used in all calculations.
4. Graphs: Appropriate line and/or bar graph(s) are
constructed on graph paper to represent the data
Both Graphs are titled, correctly scaled and axes are
correctly labeled with appropriate units. Data is
correctly plotted on the graph.
Total Points:
/ 15
Comments related to PROCEDURE AND RESULTS:
CONCLUSION AND DISCUSSION ..........................................................................................................
Number next to each standard reflects
the order of the lab report
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
5. Questions: All responses to the summary questions
including the challenge question are correctly
answered. Proper precision is used in the challenge
question.
6. Conclusion: The hypothesis is either supported or
refuted, which is backed by student observations and
cited with data collected during the investigation.
Discusses the major findings and attempts to offer a
logical explanation for the findings. Student attempts to
provide answers to both problems in the activity.
Provides recommendations for further study based on
observed error during the investigation. Use of correct
grammar / Spelling correct
Total Points:
/ 12
Comments related to CONCLUSION AND DISCUSSION:
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 244 GRADES 9 - 12
PHYSICAL PROPERTIES LAB
CH. 2 and 13.3 Density * *BENCHMARK* *
PROBLEM: (Reference Chapters 2 & 13.3 for help)
1. How is an object's ability to float in water related to its density?
IV.
Less than water
Equal to water
Greater than water
CONTROL:
DV:
C:
TASK:
1. Write a hypothesis for the problem. Remember: YOUR VARIABLES SHOULD REFLECT
YOUR HYPOTHESIS.
2. Predict if the objects will float or sink in water. Record your predictions in data table II before you
begin collecting data.
3. Write a procedure to determine the mass, volume, and density of the substances.
4. Conduct the experiment and record your results (using proper precision) in the data tables
5. Calculate the % error for the experimental density values
6. Answer the questions
7. Complete a formal lab report in your lab journal (see rubric)
FAQ'S BY STUDENTS:
1. How will I determine the volume of an irregular object? What if the irregular object floats?
2. How will I determine the mass of water?
3. How can I be consistent with accuracy and precision?
4. How do I graph all the points on the graph?
MATERIALS:
2 Tekaform cylinders of different sizes Ruler (if needed)
2 Aluminum cylinders of different sizes Balance
2 PVC cylinders of different sizes Paper clip
2 Wooden marbles of different sizes Graduated cylinder 25mL, 50mL, and 100
I White plastic anchor
Water (room temperature 24°C)
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 245 GRADES 9 - 12
DATA TABLE I
X Y
OBJECTS
VOLUME(cm
3
)
MASS (g)
sm Wood Marble
DATA TABLE II
X Y
OBJECTS
VOLUME(g/cm
3
)
FLOAT OR SINK
Predicted Observed
sm Wood Marble
AT THIS POINT YOU SHOULD COPY THE KNOWN DENSITY
VALUES FROM THE BOARD AND CALCULATE % ERROR
Your Value - Known Value
____________________________________ X 100%
Known Value
GRAPH: Construct a graph to show the relationship between the mass and volume of substances in table
1. Include a legend .. Construct a second graph comparing the substances to their measured densities.
Include a legend.
QUESTIONS #1-7
1. What physical property of matter does the slope of the lines in graph # 1 represent?
2. Examine your graphs and data tables. What appears to be true about the density of objects that float in
water? Objects that sink in water
3. What would be true about the density of an object that was suspended in water?
4. How would the density of an object change if it were cut in half? What if the sample size of each
object was doubled? Explain your reasoning.
5. Would substances/objects that float in water also float in other liquids? What would you need to
know about the other liquids to make an accurate prediction?
6. How might a change in temperature affect the density of a substance? Explain. (Hint: Think about
how temperature affects the volume of a substance)
7. Can density be used to help identify a pure substance, a mixture or both? Explain
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 246 GRADES 9 - 12
PHYSICAL SCIENCE / INTEGRATED SCIENCE I
WRITING A LAB REPORT RUBRIC
1B
Exceeds Expectations
The problem and hypothesis are stated clearly and completely.
Accurate identification of the independent and dependent variables.
The experimental design matches the stated problem. Variables are
held constant. The procedures are clear, complete and replicable.
A control is included when appropriate. Data are well organized and
presented in an appropriate manner. Conclusions are fully supported
by data and address the hypothesis. Reliability of data and validity of
conclusions are thoroughly discussed.
Meets Expectations
The problem and hypothesis are stated adequately. Adequate
identification of the independent and dependent variables. The
experimental design generally matches the stated problem. Attempt
at holding variables constant is made. Procedures are generally
complete. Data are organized and presented in an appropriate
manner. Minor errors or omissions may be present. Conclusions are
generally supported by the data and address the hypothesis. Minor
errors in interpretation of results may be present. Discussion and
reliability of data and validity of conclusions is limited.
Meets Some Expectations
The problem and/or hypothesis are poorly stated. Limited
identification of independent and dependent variable. The
experimental design matches the stated problem to some extent.
Little attempt to hold variables constant. Procedures are incomplete.
Data are poorly organized or presented in an appropriate manner.
Major omissions or errors may be present. Conclusions are
supported by data and address the hypothesis to a limited extent.
Major errors in interpretation or results may be present. There is
little discussion of the reliability of the data or validity of conclusions.
Does Not Meet Expectations
The statement of the problem and/or hypothesis is very limited or
missing. No identification of independent or dependent variables.
The experimental design does not match the stated problem, is
very incomplete or missing. There is no attempt to hold variables
constant. Data are very poorly organized or presented in an
inappropriate manner or missing. Conclusions are not supported by
data, do not address the hypothesis or are missing. There is no
discussion of the reliability of data or validity of conclusions.
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 247 GRADES 9 - 12
LAB REPORT RUBRIC
DENSITY OF OBJECTS
F= 0 - 1.75 D = 2.0 C = 2.25 B = 2.5 A= 2.75 - 3.0
REPORT FORMAT .......................................................................................................................
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
Lab report contains a completed introduction which
includes the background, problem, hypotheses,
and all the variables.
Lab report is written in a lab journal
Lab report contains a complete list of all materials and a
complete procedure section. The steps are listed in a
numbered format.
Lab report contains a results section, which includes Data
Table 1 and 2, known values, % error calculations and
two graphs
Lab report contains a summary question section. All
questions are attempted.
Lab report contains a conclusion and discussion
section. A simple conclusion and a developed response
that explains the results and answers the problem are
included.
0= incomplete lab
The overall report is complete, legible, and properly
organized. Data tables, graphs, and other charts are
neatly constructed.
Total Points:
/ 21
Comments related to REPORT FORMAT:
INTRODUCTION AND EXPERIMENTAL DESIGN .............................................................................
Number next to each standard reflects
the order of the lab report
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
1. Background: Provides a clear description of the
experiment and a rationale for why the experiment was
performed
2. Problem: Identifies one testable problem that is
directly related to the investigation’s procedure and results
3. Hypothesis: Formulates a properly written and
testable hypothesis directly related to the problem
4. Variables: All variables including IV, DV,
CONSTANTS, and CONTROL for the problem are
correctly identified and are related to the procedure and
results
Total Points:
/ 12
Comments related to INTRODUCTION AND EXPERIMENTAL DESIGN
INTEGRATED SCIENCE I
SCIENCE CURRICULUM 248 GRADES 9 - 12
PROCEDURE AND RESULTS ....................................................................................................
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
5. Materials: Provides a detailed list of all the materials
used in the investigation.
6. Procedure: Presents easy to follow numbered steps,
which are logically sequenced, complete, accurate, and
detailed. Procedure must show that the experiment can
be replicated to produce the same results
7. Data Table: Tables are correctly constructed and
labeled with appropriate units. All data is present and
correct, all calculations including % error are correct,
and all known values are included
8. Graphs: Appropriate graph types are constructed on
graph paper to represent the data in tables 1 and 2
Both Graphs are titled, correctly scaled and axes are
correctly labeled with appropriate units. All data is
correctly plotted on the graph.
Total Points:
/ 15
Comments related to PROCEDURE AND RESULTS:
CONCLUSION AND DISCUSSION ..........................................................................................................
Number next to each standard reflects
the order of the lab report
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
9. Questions: All responses to the summary questions
are correctly answered.
10. Conclusion: The hypothesis is either supported or
refuted, which is backed by student observations and
cited with data collected during the investigation.
Discusses the major findings and attempts to offer a
logical explanation (apply concept of buoyancy) for the
findings. Student provides an accurate answer to the
problems based on collected data and knowledge of the
topic. Provides recommendations for further study based
on observed error during the investigation.
Use of correct grammar / Spelling correct
Total Points:
/ 12
Comments related to CONCLUSION AND DISCUSSION:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 249 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Integrated Science II
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Intgr. Science II
3. Transcript Course Code/Number
00317
4. Program Contact Information
Name: Paul Mezick
Title/Position: Chairperson, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 9 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other:___________________________
9. Approval
BOE Approved
Anticipated Approval ___________________(date)
10. Pre-Requisites
This course is offered to all freshmen without a prerequisite. This course may be taken prior to Integrated Science I.
11. Brief Course Description
In Integrated Science II, students will explore the origin of our planet and the processes that continue to shape the
Earth system. Topics considered for study include astronomy, Earth’s structure and motion, resources and
environment, plate tectonics, and physical oceanography. Students will engage in inquiry based lab investigations.
Students are required to complete a research assignment.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 250 GRADES 9 - 12
13. Course Outline
in presented order
:
CHAPTER
CONCEPTS
INVESTIGATION
2) Nature of Science
The Scientist's Mind
The Scientific Method of Inquiry
Scientists’ Tools
28) Stars and Galaxies
Closer Look at Light
Star Characteristics
Stellar Evolution
Galaxies
Spectroscope Lab
Parallax Lab
The Sun and the Solar System
26) The Sun
The Sun’s Size, Heat, and
Structure
4) Earth’s Structure & Motion
Earth’s Formation
Earth’s Rotation
Earth’s Revolution
Cooperative group projects
Retrograde Motion Lab
1) Earth as a System
New View of the Earth (Gaia)
The Earth’s Four Spheres
Cycles of the Earth
ES0103 investigation
ES0104 investigation
Research paper *
7) Environment Resources
Mineral Resources
Energy Resources
Environmental Issues
17.4 Human Impacts on
Atmosphere
Atmosphere activity
ES01087 investigation
Solar Energy Lab
Brownfield Sites **
Acid Rain Activity **
Earth’s Changing Surface
8) Plate Tectonics
What is Plate Tectonics?
Types of Plate Boundaries
CEEP Plate Boundary Lab
23) The Ocean Floor
Studying the Ocean Floor
The Continental Margin
The Ocean Basin
CEEP Ocean Topography Lab
11) Mountain Building
Where Mountains Form
How Mountains Form
Types of Mountains
Relative Dating Activity
*
Benchmark Activities
** Embedded Tasks
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research

INTEGRATED SCIENCE II
SCIENCE CURRICULUM 251 GRADES 9 - 12
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum as well as Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 252 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated
during the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If...,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Earth Science by Namowitz, Spaulding
McDougal Littell, 2003
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Inquiry investigations
Response cards
SUGGESTED ASSESSMENT METHODS
Class participation
Lab Reports
Unit Test
Quizzes
Response cards by TurningTechnologies
Note:
emphasis on scientific inquiry, literacy, and
numeracy occurs within Integrated Science II
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 253 GRADES 9 - 12
LEARNING STRAND
Astronomy: “Stars and Galaxies”
CT Standard 9.1: Energy cannot be created or destroyed; however, energy can be converted from one form to another.
CT Standard 9.3: Various sources of energy are used by humans and all have advantages and disadvantages.
ENDURING UNDERSTANDINGS
Stars differ from one another in mass, size,
temperature, and distance from Earth.
Astronomers analyze light from objects in space
in order to learn about the composition and
movement of the objects.
All stars are composed of similar matter, but
mass regulates stars' color, brightness and life
expectancy.
Stars are born, and they mature, grow old, and
die; their lifespan and final form depend on
their masses.
In nuclear fusion, matter is transformed directly
into energy in a process that is several million
times as energetic as chemical burning.
Billions of galaxies make up the universe,
which, according to the big bang model, formed
between 10 and 20 billion years ago.
ESSENTIAL QUESTIONS
What is the electromagnetic spectrum, and how
does it help astronomers learn about stars?
What are the characteristics of a star?
What are the phases of a star's life cycle?
What are galaxies?
From where do scientists think the universe
came?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the characteristics of the
electromagnetic spectrum.
Explain techniques for analyzing light to obtain
information about stars.
Explain the Doppler effect and how it gives
information about star motions.
Explain why the positions of constellations in
the sky change with the seasons.
List three units astronomers use to measure
distances to stars.
Describe characteristics of stars, including
mass, size, temperature, color, and luminosity.
Describe the birth of a star.
Compare and contrast the life cycle of a various
stars.
Describe the remnants of supernovae.
Tell what a galaxy is and describe the various
types of galaxies.
Explain the origin of the universe according to
the big bang model.
Earth Science by Namowitz, Spaulding
McDougal Littell, 2003
Spectrum discharge tubes
Diffraction gratings / spectroscopes
Doppler effect apparatus
Video
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Response cards
Doppler effect demonstration
Spectroscopy lab investigation
Parallax lab investigation
Video: Journey into the Solar System
SUGGESTED ASSESSMENT METHODS
Quizzes
Unit Test
Investigations evaluated by rubrics
Video response summary
Response cards by TurningTechnologies
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 254 GRADES 9 - 12
LEARNING STRAND
Astronomy: “The Sun and The Solar System”
CT Standard 9.1: Energy cannot be destroyed; however, energy can be converted from one form to another.
ENDURING UNDERSTANDINGS
The sun is vastly larger than any of the rest of
the objects in the solar system.
The sun gets its energy from the fusion of light
elements into heavier ones.
Throughout history scientists have developed
models to account for their observations of the
stars and the planets.
Earth formed from a whirling cloud of gas and
debris into a multilayered sphere, which has
since been losing heat.
Earth rotates on its axis once approximately
every 24 hours, resulting in day and night.
Earth revolves around the sun in an elliptical
orbit, causing seasonal variations.
ESSENTIAL QUESTIONS
What is the sun’s structure and source of
energy?
How have observations made by scientists in
the past contributed to our understanding of
the sun and the universe today?
How was Earth formed, and what are some
characteristics of its structure?
What is rotation and what are its effects?
What is revolution and what are its effects?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain the structure of the sun and its energy
source.
Describe the effects of sunspots, solar wind,
and magnetic storms on Earth and explain the
role of Earth’s magnetic field.
Describe the early models of the movements of
the planets and stars.
Explain Newton’s Law of Gravitation.
Explain how most scientists explain the
formation of the solar system.
Describe the Earth’s size and shape and the
arrangement of its layers.
List three sources of Earth’s heat.
Describes Earth’s magnetic field.
Give evidence for Earth’s rotation.
Relate Earth’s rotation to the day-night cycle.
Give evidence for Earth’s revolution around the
sun.
Describe Earth’s path and rate of revolution.
Explain why seasons occur.
Earth Science by Namowitz, Spaulding
McDougal Littell, 2003
Solar energy lab materials
Angular momentum apparatus
Gyroscope platform apparatus
Videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Response cards
Solar energy lab investigation (CAPT Embedded
Task)
Retrograde motion lab investigation
Chapter 4 group presentations
Video: Secrets of The Sun
Video: Origins of Earth
SUGGESTED ASSESSMENT METHODS
Quizzes
Unit Test
Investigations / presentations evaluated with
rubrics
Video response summaries
Response cards by TurningTechnologies
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 255 GRADES 9 - 12
LEARNING STRAND
Earth as a System
CT Standard 9.7: Elements on Earth move among reservoirs in the solid earth, oceans, atmosphere and organisms as part of
biogeochemical cycles.
CT Standard 9.9: Some materials can be recycled, but others accumulate in the environment and may affect the balance of the
Earth systems.
ENDURING UNDERSTANDINGS
Scientists and others are beginning to view
Earth as a system of interconnected and
interacting parts, instead of a collection of
unrelated parts.
The Earth system consists of four spheres that
all affect one another: the atmosphere, the
geosphere, the hydrosphere, and the biosphere.
Elements of Earth exist in essentially fixed
amounts and are located in various chemical
reservoirs.
The water cycle, carbon cycle, and the energy
cycle all involve interactions among the four
spheres of Earth.
The cyclical movement of matter between
reservoirs is driven by the Earth’s internal and
external sources of energy.
ESSENTIAL QUESTIONS
What is Earth system science?
What are the Earth system’s four spheres, and
how do they affect one another?
What are Earth’s natural cycles and how do
they work?
How does the internal energy of the Earth
cause matter to cycle through its major
reservoirs?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe how scientists view Earth today.
Compare and contrast open and closed
systems.
Explain the significance of Earth as essentially a
closed system.
Explain how internal and external energy of the
Earth causes matter to cycle through it.
Describe the characteristics of the water,
carbon, and energy cycles.
Analyze how humans interact with the water,
carbon, and energy cycles.
Describe human efforts to reduce the
consumption of raw materials and improve air
and water quality.
Explain the short and long-term impacts of
landfills and incineration of waste materials on
the quality of the environment.
Earth Science by Namowitz, Spaulding
McDougal Littell, 2003
LMC classroom for internet activities
Portable laptop cart
ES0103 investigation handout
ES0104 investigation handout
Video
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Response cards
ES0103 internet investigation
ES0104 internet investigation
Video: The Next Industrial Revolution
SUGGESTED ASSESSMENT METHODS
Quizzes
Unit Test
Investigations evaluated with rubrics
Video response summary
Response cards by TurningTechnologies
Appropriate use of technology
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 256 GRADES 9 - 12
LEARNING STRAND
Environment Resources
CT Standard 9.3: Various sources of energy are used by humans and all have advantages and disadvantages.
CT Standard 9.6: Chemical technologies present both risks and benefits to the health and well-being of humans, plants and
animals.
CT Standard 9.8: The use of resources by human populations may affect the quality of the environment.
ENDURING UNDERSTANDINGS
Earth has renewable and nonrenewable
resources. Humans’ demand for and use of
resources sometimes exceeds the available
supply.
Humans depend on a variety of energy
resources, both renewable and nonrenewable,
to meet their energy needs.
Human use of Earth’s resources affects the
living and nonliving parts of the environment.
Human activities affect the atmosphere by
producing air pollutants and other substances
that contribute to problems such as acid rain
and ozone depletion.
ESSENTIAL QUESTIONS
What types of resources are parts of Earth’s
environment, and how are they important to
humans?
What are nonrenewable and renewable energy
resources?
How does the use of Earth’s resources affect
Earth’s environment?
How are combustion by-products from
industries and vehicles a major source of air
pollution?
How can land development, transportation
options, and consumption of resources affect
the environment?
How do changes in the composition of the
atmosphere lead to changes in the global
climate?
How are the byproducts of modern industry
deteriorating water quality?
What alternative energy sources are currently
being explored to address the disadvantages of
using fossil fuels?
LEARNING OBJECTIVES
The student will….
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish between renewable and
nonrenewable resources.
Explain how the availability and use of minerals
determine how long mineral reserves will last
Identify renewable and nonrenewable energy
resources.
Explain how fossil fuels form.
Explain how heat is used to generate electricity.
Describe how humans use renewable and
nonrenewable energy resources to meet their
energy needs.
Describe how the use of renewable and
nonrenewable resources affects the
environment.
Explain how humans can slow the depletion of
resources.
Discuss how human activities can affect the
atmosphere.
Compare and contrast acid rain, smog, ozone
depletion, and global warming.
Earth Science by Namowitz, Spaulding
McDougal Littell, 2003
LMC instruction for renewable energy research
assignment *
LMC classroom for internet activities
ES01087 investigation handout **
Video(s)
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web-based instruction with Blackboard/finalsite
Research
Response cards
ES01807 internet investigation **
Renewable energy research assignment *
(CAPT Energy Uses in Connecticut)
Atmospheric CO
2
lab investigation
Video: Dimming the Sun
Video: Global Warming – What You Need to
Know
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 257 GRADES 9 - 12
SUGGESTED ASSESSMENT METHODS
Benchmark:
Meet course expectations for independently
generating a properly written and properly cited
research assignment
Other Assessments:
Quizzes
Unit Test
Investigations evaluated with rubrics
Video response summary
Response cards by TurningTechnologies
Appropriate use of technology
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 258 GRADES 9 - 12
LEARNING STRAND
Earth’s Changing Surface
CT Standard 9.7: Elements on Earth move among reservoirs in the solid earth, oceans, atmosphere and organisms as part of
biogeochemical cycles.
ENDURING UNDERSTANDINGS
The lithosphere is broken into rigid plates that
move in relationship to one another on the
asthenosphere.
Boundaries between plates are described
generally as divergent, convergent, or transform,
depending on how the plates move relative to
each other.
Three hypotheses describe how mantle
convection, ridge push, and slab pull may cause
plate movements.
Plate movements have caused Earth’s continents
to change their positions on the globe over time.
The continental margins are the underwater
edges of continents and include several types of
topographical features.
The ocean basin has a wide range of
topographical features. Natural forces change
these features over time.
ESSENTIAL QUESTIONS
What evidence have scientists found to support
the theory of plate tectonics?
What are important features of different types of
plate boundaries?
What are some of the hypotheses scientists have
about the cause of plate movement?
How have plate movements caused changes in
the positions and shapes of Earth’s landscapes?
What tools and methods do scientists use to
study the ocean floor?
What are continental margins?
What are the topographical features of the ocean
basin?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Discuss evidence used by Alfred Wegener to
support his idea of continental drift.
Explain how the theory of plate tectonics helps to
predict the locations of earthquakes and
volcanoes.
Discuss the differences among the three types of
plate boundaries.
Contrast the three different types of convergent
boundaries.
Discuss mantle convection as a possible cause of
plate movements.
Compare and contrast ridge push and slab pull.
Explain how the Earth’s landmasses have
changed positions over the past 200 million
years.
Discuss the roles of plate tectonics, igneous
activity, and deposition in the formation of
continental landmasses.
Describe the parts of the continental margin
Compare and contrast active and passive
continental margins.
Describe the features of the ocean basin.
Explain how ocean basin features change over
time.
Earth Science McDougal Littell, 2003
Plate tectonic model
World seismicity map
Pacific Ocean topographic maps (12)
Atlantic Ocean topographic map
Video(s)
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Textbook ancillary materials
Web based instruction-Blackboard/finalsite
Research
Response cards
CEEP Lab #1 ―How Fast is The Ocean Floor
Moving?‖
CEEP Lab #2 ―Lithospheric Plates and Ocean
basin Topography‖
Video: The Wave That Shook The World
Video: The Living Machine
SUGGESTED ASSESSMENT METHODS
Quizzes and Unit Test
Investigations evaluated with rubrics
Video response assessment
Response cards
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 259 GRADES 9 - 12
Energy and the Environment
Research Assignment
CAPT Embedded Task: Energy Uses in Connecticut
Each student is responsible for selecting an alternative renewable or nonrenewable
energy technology and completing the following assignment.
Please type (12 pt font, double space, 1 inch margins) your response to the target
questions that apply to your alternative energy technology. Your response must be a
minimum of 4 pages in addition to your citations.
The United States and the rest of the Globe currently rely heavily on the use of fossil
fuels as the primary energy source for fueling our economies. Alternative energy forms
(solar, wind, nuclear, hydro, geothermal, biomass and others) are frequently debated as
alternative ways of providing energy while decreasing our reliance on fossil fuels. Your
assignment is to select an alternative energy source and examine its potential as a fuel
source for the future. To keep the topic relevant to your daily lives, I ask that you also
compare your chosen alternative energy to Connecticut's current energy profile. You will
want to discuss some of the following issues with respect to the fuel source you choose
when completing your assignment.
TARGET QUESTIONS
1. How does the technology work? What are the principles (technical aspects) of operation?
2. How expensive "$$" is the technology? (compare to costs "$$" of fossil fuels)
3. What is/are the primary constraint(s) or limiting factor(s) associated with this technology?
4. Are there specific environmental concerns associated with the manufacture, distribution, use, or
disposal of the technology?
5. Identify the stakeholders? (Who or what does/does not benefit from the manufacture, distribution,
use or disposal of the energy technology?)
6. Is this technology currently used today? If so, how much, where, and in what form?
7. What % of the United State's total energy consumption is currently attributed to the application of
the technology?
8. Examine Connecticut's energy profile. Is your energy technology a practical alternative to
Connecticut's current source(s) of energy?
9. What are your personal feelings and observations? Are you in favor or against the use of the
technology? Be sure to summarize key points in your paper to support your position!
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 260 GRADES 9 - 12
Energies
What does this
energy consist of?
Does it involve
solar energy?
Why?
Disadvantages
Advantages
Biomass
Geothermal
Hydrogen Fuel
Hydro-Power
Nuclear
Wind
Tidal
Sun
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 261 GRADES 9 - 12
EARTH SCIENCE /INTEGRATED SCIENCE II
RESEARCH PAPER RUBRIC
1B & 2
Exceeds Expectations
90 – 100
The student understands not only the objective but also the implications
of assignments. S/he writes in a variety of modes, with a clear focus or
thesis. Supporting details are well developed and organized, showing
both analysis and synthesis of ideas. Word choice and syntax are
accurate and appropriate. The student shows mastery in the conventions
of Standard English. The student successfully completes all parts of the
writing process.
Meets Expectations
75 – 89
The student understands the objective of assignments and selects an
appropriate mode of written expression with a focus or thesis. Supporting
details show an understanding of the subject matter and an analysis of
ideas. They are somewhat developed and organized. Word choice and
syntax are accurate and appropriate. Errors in the conventions of
Standard English are few. The student completes most parts of the
writing process.
Meets Some Expectations
65 – 74
The student requires some additional explanations and models in order to
understand the objective of assignments or to complete the writing
process. With direction, s/he selects an appropriate mode. Writing has
somewhat limited focus or thesis, and supporting ideas may be
inaccurate, simplistic, and/or confused. The student may require
assistance to develop or organize his response. Word choice and syntax
are consistent with grade level. There are some errors in the conventions
of Standard English.
Does Not Meet
Expectations
0 - 64
The student misinterprets significant elements of writing assignments,
selecting an inappropriate mode or using it incorrectly. The student
requires many additional explanations, models, graphic organizers,
and/or strategies in order to complete parts of the writing process. The
writing has no clear focus or a very limited thesis. Ideas and concepts are
often unorganized or inaccurate. Inaccurate or limited vocabulary, syntax
errors, and errors in the conventions of writing make the writing
ineffective.
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 262 GRADES 9 - 12
ENERGY AND THE ENVIRONMENT RESEARCH PAPER RUBRIC
PAPER FORMAT
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
Cover page including name, title, class and period
Approved font, size, spacing and margins
4 page minimum length excluding pictures and Works
Cited page
Works cited/bibliography for cited text and pictures
(MLA format). alphabetized
*note* include all sources used for reference
All notes are turned in with the paper
Completed energy comparison chart
Total Points:
Comments related to PAPER FORMAT section:
USE OF SOURCES
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
Sources are current (dates provided in works cited)
Sources are consistently related to the topic
A variety of sources other than required sources (books,
E-books journals, periodicals, and authored websites)
were used. (min. of four)
*note * All sources including those that were not cited
within the text o{your paper
Source information (text & pictures) cited within text
(MLA format)
Total Points:
Comments related to USE OF SOURCES section:
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 263 GRADES 9 - 12
RESEARCH PAPER CONTENT
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
Principles of operation are detailed and clearly described
The stakeholders are clearly identified and discussed
Costs associated with the technology are compared to fossil fuels. Monetary
comparisons are made and cited within the text. Data supports statements
Current use/uses of the technology are discussed
Constraints associated with the technology are discussed. Data supports
statements
Environmental concerns associated with the manufacture, distribution, use,
and disposal of the technology are discussed. Data supports statements
Comparisons to Connecticut’s current energy profile are discussed.
The writer takes a position based on his/her interpretation and analysis of the
research. The writer provides persona! opinions on points discussed within
the text
All information on the renewable energy comparison chart is accurate and
complete
Total Points:
Comments related to CONTENT section:
MECHANICS / ORGANIZATION
Undeveloped
0 – 1.75
Beginning
2.0 – 2.25
Developing
2.5
Accomplished
2.75 – 3.0
Introduction engaging and directed toward the body of the paper
Text organization (paragraphs follow a logical sequence)
Writing sophistication (varied sentences, mature vocabulary)
Mixture of writer’s words with paraphrased/quoted (MLA format) sources
woven into text
Use of correct grammar / Spelling correct
Clear conclusion containing the topic sentence and a summary of the
research
Total Points:
Comments related to MECHANICS / ORGANIZATION section:
INTEGRATED SCIENCE II
SCIENCE CURRICULUM 264 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 265 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Biological Systems
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Biological Systems
3. Transcript Course Code/Number
00312
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 10 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.25 (2.5 trimester equivalent)
1.5 (three trimester equivalent
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
C or better in Integrated Science I and II
11. Brief Course Description
This course involves the study of the broad fundamental principles governing living things. The cell,
classifications of living things, and various living systems are examined. It includes the study of plants
and animals and how they function in their environment, the biosphere. Inquiry based laboratory
investigations and scientific laboratory reports are designed to support concepts discussed in class and to
teach concepts by inquiry.
12. Course Goals
1. Demonstrate proficiency and fluency in communication to meet the literacy demands of the global
community.
2. Use technology effectively and responsibly.
3. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
4. Demonstrate respect for one’s self, and strive to contribute to the success of others.
5. Demonstrate the ability to successfully complete homework and laboratory assignments
independently.
6. Conduct lab experiments safely using appropriate scientific protocols.
7. Analyze and synthesize scientific information as it relates to everyday living.
8. Demonstrate skills in using various types of biological instruments and scientific methodologies.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 266 GRADES 9 - 12
13. Course Outline
in presented order
:
UNIT / Chapter
CONCEPT
ACTIVITY
THE NATURE OF LIFE
1) The Science of Biology
Lab safety
Lab equipment
Scientific method
pH lab
Yeast and Temperature lab
2) The Chemistry of Life
Enzymes
Chemical reactions
Carbon compounds
Enzymes Lab (CAPT Embedded Task)
Acid Rain (CAPT Embedded Task)
Organic compounds in food lab
ECOLOGY
3) The Biosphere
Energy flow
Cycles of matter
Ecosystem WebQuest
Bauer Farm study (local habitat)
4) Ecosystems and Communities
Climate
Biomes
Aquatic ecosystems
Biome research project
5) Populations
Effects of growth
Human population
Yeast Population Dynamics lab
CAPT Embedded Task
CELLS
7) Cell Structure and Function
History of the cell
Eukaryotic cell
Cell boundaries
Diversity of the cell
Microscope lab
Plant vs. Animal cell lab
Osmosis diffusion lab
8) Photosynthesis
Energy and life
Photosynthesis overview
Reactions of
photosynthesis
9) Cellular Respiration
The Krebs cycle
Electron transport
10) Cell Growth and Division
Cell growth
Cell division
Regulating the cell cycle
Internet ―Root Mitosis‖ activity
GENETICS
11) Introduction to Genetics
Gregor Mendel
Probability
Punnett Square
Meiosis
Human Traits Investigation (CAPT
Embedded Task: Human Population
Dynamics)
Probability lab
Human Karyotyping lab
Pipe cleaner cheerios meiosis lab
12) DNA and RNA
DNA
Chromosome replication
RNA & protein synthesis
Mutations
Design-o-saur activity
DNA extraction from cells
EVOLUTION
15) Darwin’s Theory of Evolution
Life’s diversity
Natural selection
Kingdoms and Domains
Modern Classification
Jelly bellicus lab
Bio-engineered Food (CAPT Task)
Newspaper mice activity
Mystic Aquarium field trip
Natural history project
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 267 GRADES 9 - 12
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 268 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated
during the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicates the results of a scientific
experiment.
Biology Prentice Hall, 2008
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lecture instruction
Inquiry investigation
Supplement textbook ancillary materials
Guided Internet research
SUGGESTED ASSESSMENT METHODS
Yeast Lab
**Benchmark**
CAPT Embedded Performance Task: Laboratory
Investigation of Yeast Population Dynamics
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 269 GRADES 9 - 12
LEARNING STRAND: THE NATURE OF LIFE
Science of Biology and The Chemistry of Life
CT Standard 10.3: Similarities in the chemical and structural properties of DNA in all living organisms allow the transfer of genes
from one organism to another.
CT Standard 10.4: In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both parents.
ENDURING UNDERSTANDINGS
Genetic information is stored in genes that are
located on chromosomes inside the cell nucleus.
Most organisms contain homologous
chromosomes in the cell nucleus inherited from
parents.
Most organisms have two genes for each trait,
one on each of the homologous chromosomes
in the cell nucleus.
The principles of genetics and cellular chemistry
can be used to produce new foods and
medicines in biotechnological processes.
Organic compounds make up all living things.
ESSENTIAL QUESTIONS
How can the genetic information of organisms
be altered to make them produce new
materials?
How does meiosis contribute to the genetic
variability of an organism?
How can the Punnett Square technique be used
to predict the distribution of traits in mono- and
di–hybrid crossings?
How can the probable mode of inheritance of
traits (e.g., recessive/dominant, sex-linked) be
deduced from pedigree diagrams showing
phenotypes?
What is the difference between genetic
disorders and infectious diseases?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the four groups of organic compounds.
Identify the functions of the organic compounds
in living things.
Identify and describe the structure and function
of nucleic acids.
Identify the chemical properties of water.
Identify acids and bases.
Identify the use of enzymes in living cells.
Identify the importance of enzymes in living
cells.
Identify and use the principles of probability
and genetics.
Explain Mendelian genetics.
Use Pedigree diagrams.
Use effective and efficient strategies for
gathering information and materials, and
thinking critically.
Biology Prentice Hall 2008 and ancillary
materials
AV materials (LMC and departmental)
General lab equipment
SUGGESTED INSTRUCTIONAL STRATEGIES
Have students complete the worksheets from
chapters 2 and 11 and 12
Use appropriate websites to illustrate genetic
problems
Do enzyme lab with pectin, amylase, or
enzyme of choice (CAPT Task: Enzymes)
Have students perform PH lab
Have students do activities with organic
chemistry (food lab, cut outs etc.)
Have students use Punnett Square technique to
predict distribution of traits
Have students view department PowerPoint
presentations
Lab activities for probability, human
karyotyping, and human traits (CAPT Task)
SUGGESTED ASSESSMENT METHODS
Quizzes, activities and homework
Benchmark: CAPT Performance Task - Enzymes
CAPT Performance Task - Acid Rain
Computer based tests on chapters 2, 11 and 12
Nucleic acid and genetics websites activities
Internet activity on mitosis
Ph lab
Pedigree activity
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 270 GRADES 9 - 12
LEARNING STRAND: ECOLOGY
The Biosphere, Ecosystems & Communities, and Populations
CT Standard - 10.6: Living organisms have the capability of producing populations of unlimited size, but the environment can
support only a limited number of individuals from each species.
ENDURING UNDERSTANDINGS
Human populations grow due to advances in
agriculture, medicine, construction and the use
of energy.
The size of a population of organisms is
affected by many environmental factors.
Humans modify ecosystems as a result of rapid
population growth, use of technology and
consumption of resources.
Technological advances have effected human
population growth.
ESSENTIAL QUESTIONS
- How do populations grow?
- How does competition affect growth?
- What are the factors that affect population size?
- Why does population growth differ in countries
around the world?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the factors that affect the carrying
capacity of the environment.
Discuss how energy flows through an
ecosystem.
Explain the energy pyramid.
Explain how technological advances have
affected the size and growth rate of human
populations throughout history.
Analyze population graphs and or charts.
Explain how change in population density is
affected by emigration, immigration, birth and
death rates; relate these factors to the
exponential growth of human populations.
Identify the biotic and abiotic components in
the ecosystem.
Identify the key characteristics of land and
aquatic biomes.
Biology Prentice Hall, 2008
Ecosystem webquest
Bauer Farm or DHHS land field study
Worksheets and Audio Visual material for
Biology chapters 3, 4, and 5
Websites on population growth
CAPT Embedded Task: Human Population
Dynamics
CAPT Embedded Task: Yeast Population
Dynamics
SUGGESTED INSTRUCTIONAL STRATEGIES
Inquiry activities
Investigating growth of a population of bacteria
Yeast population growth lab
SUGGESTED ASSESSMENT METHODS
- Lab reports
- Laboratory investigation scored with CAPT rubric
- Powerpoint slide show for Human Population
Dynamics
- Section quizzes and chapter assessments
- Unit tests
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 271 GRADES 9 - 12
LEARNING STRAND: CELLS
Cell Structure and Function, Photosynthesis, Cellular Respiration, and Cell Growth and Division
CT Standard - 10.1: Fundamental life processes depend on the physical structure and the chemical activities of the cell.
CT Standard - 10.2: Microorganisms have an essential role in life processes and cycles on earth.
ENDURING UNDERSTANDINGS
The cell is the basic unit of structure and function of
all living things.
Complex living things are organized at different
levels.
Living things are composed of cells which can
interact in such a way that results in evolving
chemical, physiological, and biological processes.
Cells and living things have optimal ranges for
different conditions under which they perform life
processes.
Most of the chemical activities of the cell are
catalyzed by enzymes that function only in a narrow
range of temperatures and acidity conditions.
The cell membrane regulates many of the cell's
properties.
Microorganisms are an essential role in life processes
and cycles on Earth.
Understanding the growth and spread patterns of
bacteria and viruses enables the development of
methods to prevent and treat infectious diseases.
The cellular processes of photosynthesis and
respiration involve transformation of matter and
energy.
ESSENTIAL QUESTIONS
How do cell structures differ among living
things?
What is the general role of enzymes in
metabolic cell processes?
What is the function of the cell membrane in
supporting cell functions?
What are the processes of active and
passive transport as it relates to cells?
What is the importance of cells in their role
of homeostasis?
What are the similarities and differences
between bacteria and viruses?
How are bacterial and viral infectious
diseases transmitted?
How are bacteria and viruses both helpful as
well as harmful to human beings?
How are bacteria and yeasts used to
produce foods for human consumption?
How do environmental factors affect living
things?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Biology Prentice Hall, 2008
Prepared slides of cells
Audio Visual materials on cells and cell
functions (LMC and departmental)
Websites on cells such as Cells Alive and
www.accessexcellence.org
Microscopes
Explain the relationship between cell respiration and
photosynthesis.
Discuss the importance of cell metabolism and its
effect on homeostasis.
Identify the main steps in cellular respiration.
Identify the main steps in photosynthesis.
Explain the difference between meiosis and mitosis.
Relate the structure to the function of cells & to the
structures in which they make up in an organism.
Describe the processes of cell transport.
Differentiate between the cells of the six kingdoms.
Compare and contrast eukaryotic and prokaryotic
cells and viruses.
Explain the life cycle of viruses and bacteriophages.
Describe how some infectious diseases are
transmitted to humans.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 272 GRADES 9 - 12
Examine the role of antibiotics in the prevention and
treatment of infectious diseases.
Explain the importance of bacteria and yeasts to
human beings.
Relate the importance of meiosis and mitosis to
genetic recombination.
Relate the importance of energy conversions in cells
(ATP).
SUGGESTED INSTRUCTIONAL STRATEGIES
Diffusion lab (i.e., potato, cucumber or
elodea)
CAPT investigation on cell transport
Worksheets from Biology chapter 7-10
Simulations of meiosis and mitosis
Internet worksheet on mitosis – Cells Alive
Posters or charts comparing meiosis and
mitosis
Worksheets on ATP/ADP cycle
Microscope lab activities
CD Rom on bacteria and viruses
SUGGESTED ASSESSMENT METHODS
Teacher generated tests/quizzes
Lab reports on cell transport
Posters or worksheets evaluated with rubrics
Worksheets on videos and DVD’s
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 273 GRADES 9 - 12
LEARNING STRAND: EVOLUTION
Darwin's Theory of Evolution
CT Standard - 10.5: Evolution and biodiversity are the result of genetic changes that occur over time in constantly changing
environments
CT Standard - 10.3: Similarities in the chemical and structural properties of DNA in all living organisms allow the transfer of genes
from one organism to another.
ENDURING UNDERSTANDINGS
Species survival is affected by many factors
including the environmental changes as well as
genetic factors.
There is evidence supporting the theory of
evolution.
Mutations and recombination of genes create
variability in populations.
Changes in the environment may result in the
selection of organisms that are better able to
survive and reproduce.
The principles of genetics and cellular chemistry
can be used to produce new foods and
medicines in biotechnological processes.
ESSENTIAL QUESTIONS
What is the process of altering genetic material
to make new products?
What is an example of how gene therapy has
altered the lives of human beings for the better
and for the worse?
What are some risks and/or benefits of altering
the genetic composition and cell products of
organisms (bacteria etc.)?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain that Darwin’s observations led to his
theory of natural selection.
Examine how mutations and adaptations apply
to Darwin’s theory of evolution
Explain the scientific evidence that disproves
Lamark’s theory of evolution.
Explain how the difference between artificial
and natural selection affects human beings and
the process of evolution of many living things.
Understand how genetics plays a role in
evolution.
Understand that vestigial structures such as the
appendix provide evidence of human evolution.
Identify how bioengineering/biotechnology is
used to benefit humans
Biology Prentice Hall 2008
Worksheets for Biology chapters 15, and parts
of 13
Cloning videos
Extracting DNA lab
Simulation labs in biotechnology
CAPT Embedded Task: Bio-engineered Food
SUGGESTED INSTRUCTIONAL STRATEGIES
Responses to questions in lab reports
Response sheets for cloning videos
Evidences of Evolution reports evaluated with
rubrics
Mice lab questions and answers
Evolution videos
SUGGESTED ASSESSMENT METHODS
Response sheets to evolution videos, cloning
videos, biotechnology simulations
Lab reports for questions on mice lab and
extracting DNA lab
Questions and discussion regarding the cloning
of organisms
Stem cell discussion
Discussion of the benefits of genetic
engineering for humans, for medicine and food
Issues in Biology p. 330, ―Do genetically
modified foods need stricter controls?‖
Persuasive Pamphlet regarding Bio-engineered
Food (CAPT Embedded Task)
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 274 GRADES 9 - 12
How to Write a Lab Report
General directions
Use section headings in the order given below
Use full sentences.
Make new data tables: do not attach tables from the lab.
Title - Use the one provided or create a title that is appropriate.
Problem - Compose a sentence or two describing the purpose of the investigation. What you are trying to
solve?
Independent and Dependent Variables -List variables when asked for Control and Constants
Hypothesis - This is your prediction of what will occur based on prior knowledge or information from
your textbook or notes.
Materials - List materials provided or materials you selected for use.
Procedure
Use numbers to label the steps in your procedure. See the lab reports that I have given you as examples.
List any changes in the handout you might have been given.
Make the procedure easy to read and follow, so that the reader can repeat the steps you have given and get
the same results. Check carefully for accuracy, completeness and precision. Use units when appropriate.
Data Table
Make a neat, concise data table with vertical and horizontal columns and INCLUDE UNITS and labels.
Be sure to include averages when appropriate or the derived units (density, speed etc.).
For some reports, there will be data or analysis questions to answer in this section of the lab report.
Graph
Use a title for the graph.
Draw and label the X and Y axes of the graph.
Determine appropriate scale for X and Y axes.
The independent variable is the X axis, and the dependent variable is the Y axis.
Discussion
This is usually the most important part of the lab. This section usually has the highest point value. You
should include the following topics in paragraph form. You should use more than one sentence for each
answer.
-What if any trends did you notice?
-What were the major findings of the lab?
-Was the hypothesis supported by the data? Use data to support this response.
-How did your findings compare to the findings of others?
-What could be sources of error?
-What possible explanations can you offer for your findings?
-How does the experiment relate to what you are learning in class?
-Summarize your findings supported by data.
Conclusion
Write one or two sentences that summarize the findings of the lab.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 275 GRADES 9 - 12
Biological Systems
Factors Affecting the Rate of Yeast Respiration
Problem
How does temperature affect the rate of respiration in yeast?
Hypothesis
Make a hypothesis based on your research and knowledge for YOUR Problem ONLY!
Use "If…, then…" format.
Materials
Yeast solution Beakers
Droppers Stirrers
Test tubes
Stoppers for test tubes markers for test tubes
Glucose solution
Hot plate boiling stones
Ice Water
Thermometers rulers
Graduated cylinders
General directions and cautions
Take 50 ml total of yeast solution (stir beaker before obtaining solution)
Be sure to label test tubes.
Use 15 ml of yeast in each test tube.
Use 10 ml of glucose.
Stir or swirl test tubes.
Use boiling stones in beakers of boiling water.
The lab experiment will have a reaction occur.
Don't measure the time.
Perform the experiment with all test tubes at same time.
Do not leave the thermometer in boiling water =100 degrees all the time
Due Date:
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 276 GRADES 9 - 12
Biological Systems Benchmark
Rubric for Respiration Lab with Yeast
Problem - given
Hypothesis - 5 points for "If…, then…" format
Materials - given
Variables -15 points; list dependent, independent, control and constants (at least 3)
Procedure - 20 points; steps are labeled and numbered and can be followed; they are in full
sentences
Results/Data Tables - 20 points; tables must include units, as well as indicate change in the
height of bubbles. The report must be neat and clearly reflect the data.
Discussion - 30 points The discussion includes:
sources of error i.e., validity of experiment and results compared to other groups (5 points).
a discussion of hypothesis i.e., was it correct or incorrect; explain (5 points).
an answer to the problem (5 points).
a discussion of fermentation (5 points).
a discussion of why a warm temperature was the best (5 points).
Conclusion - 10 points; write two or three sentences in a succinct scientific manner
summarizing the results or findings of the lab.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 277 GRADES 9 - 12
Biological Systems
Yeast Lab
B Writing
Exceeds Expectations
90 – 100
The student understands not only the objective but also the
implications of the lab. S/he uses the proper format. S/he writes with
a clear focus / hypothesis. Supporting evidence is well developed and
organized, showing both analysis and synthesis of ideas. Word choice
and syntax are accurate and appropriate. The student shows mastery
in the conventions of Standard English.
Meets Expectations
80 – 89
The student understands the objective of the lab, uses the proper
format, and has focus / hypothesis. Supporting evidence shows an
understanding of the subject matter and an analysis of ideas. Is
somewhat developed and organized. Word choice and syntax are
accurate and appropriate. Errors in the conventions of Standard
English are few.
Meets Some Expectations
70 – 79
The student requires some additional explanations and models in
order to understand the objective of the lab or to complete the
process. With direction, s/he selects an appropriate format. Writing
has a somewhat limited focus or hypothesis, and supporting evidence
may be inaccurate, simplistic, and/or confused. The student may
require assistance to develop or organize his response. Word choice
and syntax are consistent with grade level. There are some errors in
the conventions of Standard English.
Does Not Meet Expectations
0 - 69
The student misinterprets significant elements of the lab, selecting an
inappropriate format. The student requires many additional
explanations, models, and/or strategies in order to complete parts of
the process. The writing has no clear focus or a very limited
hypothesis. Ideas and concepts are often unorganized or inaccurate.
Inaccurate or limited vocabulary, syntax errors, and errors in the
conventions of writing make the writing ineffective.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 278 GRADES 9 - 12
Peppered Moth Simulation
Objectives:
Describe the importance of coloration in avoiding predation
Relate environmental change to changes in organisms
Explain how natural selection causes populations to change
Materials:
Sheet of white paper
Newspaper
Forceps
Colored pencils
Clock with second hand
30 newspaper circles (made with hole punch)
30 white circles (made with hole punch)
Purpose: In this lab, you will simulate how predators locate prey in different environments. You
will analyze how color affects an organism's ability to survive in certain environments.
Industrial melanism is a term used to describe the adaptation of a population in response to
pollution. One example of rapid industrial melanism occurred in populations of peppered moths
in the area of Manchester, England from 1845 to 1890. Before the industrial revolution, the
trunks of the trees in the forest around Manchester were light grayish-green due to the
presence of lichens. Most of the peppered moths in the area were light colored with dark spots.
As the industrial revolution progressed, the tree trunks became covered with soot and turned
dark. Over a period of 45 years, the dark variety of the peppered moth became more common.
Procedure:
1. Place a sheet of white paper on the table and have one person spread 30 white circles and
30 newspaper circles over the surface while the other person isn't looking.
2. The "predator" will then use forceps to pick up as many of the circles as he can in 15
seconds.
3. This trial will be repeated with white circles on a newspaper background, newspaper circles
on a white background, and newspaper circles on a newspaper background. Record the data in
chart below.
Starting Population
Number Picked up
Trial
Background
Newspaper
White
White
Newspaper
1
white
30
30
2
white
30
30
3
newspaper
30
30
4
newspaper
30
30
Analysis
1. What did the experiment show about how prey are selected by predators?
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 279 GRADES 9 - 12
2. What moth coloration is the best adaptation for a dark (newspaper) background? How do
you know?
3. What would you expect the next generation of moths to look like after trial 1? What about the
next generation after trial 3?
4. How does the simulation model natural selection?
5. Examine the table and construct a graph, Plot the years of the study on the X-axis, and the
number of moths captured on the Y axis. You should have 2 lines on your graph -- one for light
moths, and one for dark moths.
Year
# of Light
Moths
Captured
# of Dark
Moths
Captured
2
537
112
3
484
198
4
392
210
5
246
281
6
225
337
7
193
412
8
147
503
9
84
550
10
56
599
6. Explain in your own words
what the graph shows.
7. Describe a situation where
this type of selection might
occur.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 280 GRADES 9 - 12
CHAPTER
10
Evolution and
Natural Selection
Reteaching
Natural Selection in Action
In the 1800s there were two varieties of a certain species of English moth-a light colored variety and a
dark-colored variety. Both rested on trees, which had light colored bark. The coloring of the light moths
served as camouflage on the light-colored trees, so the light moths were not as easily seen by predators as
the dark moths were. But as industry in the cities increased, the trees became covered with dark soot.
Over many generations, the moth populations adapted to the increased amount of soot.
Examine the diagrams and then answer the questions that follow.
1. H.B.D. Kettlewell's experiments demonstrated that natural selection was at work in the process shown
above. His work had three steps. Referring to page 182 of your textbook, state what each of the steps of
Kettlewell's work proved.
Step 1 ______________________________________________________________________________
___________________________________________________________________________________
Step 2 ______________________________________________________________________________
___________________________________________________________________________________
Step 3 ______________________________________________________________________________
___________________________________________________________________________________
2. Why was the percentage of dark moths so small in 1845? ____________________________________
___________________________________________________________________________________
3. How did natural selection cause the percentage of dark moths to increase? ______________________
___________________________________________________________________________________
___________________________________________________________________________________
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 281 GRADES 9 - 12
Biological Systems
Writing Rubric for
Natural Selection / Evolution Activity
1B
Exceeds Expectations
Using supporting evidence from an activity on evolution by natural
selection the student will organize, analyze and synthesize the data
from the activity. Word choice and syntax are accurate and
appropriate. The student shows mastery in the conventions of
Standard English in the written assignment.
Meets Expectations
Using some supporting evidence from an activity on evolution by
natural selection the student will organize, analyze and synthesize
the data from the activity. Is somewhat developed and organized.
Word choice and syntax are accurate and appropriate. Errors in the
conventions of Standard English are few in the written assignment.
Meets Some Expectations
The student requires additional explanations and models in order to
organize, analyze and synthesize the data from the activity on
evolution by natural selection. With direction, s/he selects an
appropriate format. Writing is somewhat limited, and supporting
evidence may be slightly inaccurate or simplistic. The student may
require assistance to develop or organize his response. Word choice
and syntax are consistent with grade level. There are some errors in
the conventions of Standard English in the written assignment.
Does Not Meet Expectations
The student requires many additional explanations, models, and/or
strategies in order to complete parts of the writing process. The
writing has no clear focus on the data. Ideas and concepts are often
inaccurate, confusing or unorganized. Inaccurate or limited
vocabulary, syntax errors, and errors in the conventions of writing
make the assignment ineffective.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 282 GRADES 9 - 12
Names: ______________________________________________ Date: ___________________
Biological Systems
Diffusion Lab
In this lab you will design your own experiment to test the permeability of polyethylene
sandwich bags to starch and iodine.
Problem statement: Will the polyethylene membrane allow iodine to cross the membrane? Will
the polyethylene membrane allow starch, a larger molecule than iodine, to cross the membrane?
Hypothesis:
Materials: (list all materials you use below)
Procedures: (list your procedures so specifically that someone else could repeat your
experiment exactly by following your procedures alone)
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 283 GRADES 9 - 12
Observations:
Initial observations:
Observations after 24 hours:
Analysis:
1. Did iodine molecules move through the membrane? __________________________________
How do you know? ____________________________________________________________
2. Did starch molecules pass through the membrane? ___________________________________
How do you know? ____________________________________________________________
3. What can you infer from this experiment about movement of large and small molecules through
a thin polyethylene membrane?
4. Does your data support your hypothesis? Explain why or why not.
Conclusions:
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 284 GRADES 9 - 12
Biological Systems
Rubric for
Gathering Information and Solving Problems
#3
Exceeds Expectations
The student will independently write a detailed, well organized,
controlled reproducible procedure to solve the problem stated in the
lab. (For example, How do different enzymes affect the rate of a
chemical reaction?)
Meets Expectations
With minimal assistance the student will write a fairly well organized
controlled reproducible procedure to solve the problem stated in the
lab.
Meets Some Expectations
With some assistance the student will write a procedure to solve the
problem stated in the lab. The procedure is not well organized, is
confusing to follow and it contains a control.
Does Not Meet Expectations
With a great deal of assistance the student writes a procedure to
solve the problem stated in the lab. The procedure is not well
organized, is confusing to follow and does not contain a control.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 285 GRADES 9 - 12
Name: _______________________________________________ Date: ___________________
Lab partners:___________________________________________
Testing for Vitamin C
The recommended intake of vitamin C is 60 mg per day, which can come from different food
sources. To determine the presence and the amount of vitamin C in different foods, there is a
need to perform simple chemical tests. In this task, you will use a purple indicator to test for
vitamin C.
Your Task
First, you and your group will test a series of vitamin C solutions with known concentrations
using the vitamin C indicator. Next, you and your lab group will design and conduct an
experiment to compare the amount of vitamin C in various fruit juices. Then you will determine
the concentration of vitamin C in each of the juices.
You have been provided with the following materials and equipment. It may not be necessary to
use all of the equipment that has been provided. You may use additional materials and equipment
if they are available.
CAUTION: The vitamin C indicator will stain clothes and hands.
Materials
Vitamin C solution (1 mg/mL) 5 test tubes
Vitamin C indicator Test tube rack
Apple juice 8 Plastic measuring cups
Pineapple juice 5 medicine droppers
White grape juice Access to tap water
Graduated cylinder Wax crayons
Paper towels for clean-up
Safety goggles and lab apron
Part I: Testing a Vitamin C Solution
First, you will find out how many drops of a vitamin C solution (with a known concentration) it
takes for the indicator to lose its purple color. You will investigate vitamin C solutions with
varying concentrations and one solution (water) that has no vitamin C added. The higher the
concentration of vitamin C in the solution, the fewer drops it will take for the indicator to
lose its purple color.
You have been given a solution containing 1.00 milligram (mg) of vitamin C per milliliter (mL)
of water.
Procedure:
1. Using the table below, create vitamin C solutions with different concentrations by mixing the
1.00 mg/mL vitamin C solution with water in plastic cups. Be sure to label the cups with the
corresponding concentration.
2. Add 10 drops of the purple indicator to a clean test tube.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 286 GRADES 9 - 12
Names: ______________________________________________ Date: ___________________
Lab partners:___________________________________________
3. Add drops of the 1.00 mg/mL vitamin C solution, one at a time, to the test tube containing the
indicator. Shake the test tube gently after adding each drop.
4. Keep adding drops of the vitamin C solution until the indicator loses its purple color.
Record your results in the table below.
5. Repeat steps 2-4 using the other vitamin C solutions you created in step 1.
6. Create a line graph of your results.
Drops of 1.00 mg/mL
Vitamin C Solution
Drops of Water Added
Concentration of New
Vitamin C Solution
(mg/mL)
Number of Drops of
Vitamin C Solution
Added to the Indicator
40
0
1.00
30
10
.75
20
20
.50
10
30
.25
0
40
0.00
Part II: Comparing the Amount of Vitamin C in Three Fruit Juices
Now you and your lab group will design and conduct an experiment to compare the amount of
vitamin C in various fruit juices.
1. In your own words, clearly state the problem you are going to investigate. Include a clear
identification of the independent and dependent variables that will be studied.
2. Design an experiment to solve the problem. Your experimental design should match the statement of
the problem:, should control for variables, and should be clearly described so that someone else could
easily replicate your experiment. Include a control if appropriate.
3. Write your experimental design and show your teacher your design before you begin your experiment.
4. After receiving approval from your teacher, work with your lab group to carry out your
experiment. Your teacher's approval does not necessarily mean that your teacher thinks your
experiment is well designed. It simply means that, in your teacher's judgment, your experiment is not
dangerous or likely to cause an unnecessary mess.
5. While conducting your experiment, take notes. Include the results of your experiment. Tables,
charts, and/or graphs should be used where appropriate and should be properly labeled.
6. Use your results from Part I to determine the concentration of vitamin C in the juices tested.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 287 GRADES 9 - 12
Biological Systems
Rubric for
Gathering Information and Solving Problems
#3
Exceeds Expectations
The student will independently write a detailed, well organized,
controlled reproducible procedure to solve the problem stated in the
lab. (For example, How do different enzymes affect the rate of a
chemical reaction?)
Meets Expectations
With minimal assistance the student will write a fairly well organized
controlled reproducible procedure to solve the problem stated in the
lab.
Meets Some Expectations
With some assistance the student will write a procedure to solve the
problem stated in the lab. The procedure is not well organized, is
confusing to follow and it contains a control.
Does Not Meet Expectations
With a great deal of assistance the student writes a procedure to
solve the problem stated in the lab. The procedure is not well
organized, is confusing to follow and does not contain a control.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 288 GRADES 9 - 12
Biological Systems
Scientific Method Investigation
Problem - How does salt water concentration affect the germination of seeds?
Hypothesis - Make one in your group. You might need to research seed germination using
the books available or Internet resources.
Materials
10 seeds
saltwater concentrations of 0.3%, 0.6%, 1% and 2%
paper towels for seeds in which to germinate
Each day, water will be available to moisten the paper towels containing the seeds.
Beakers if necessary
Any other materials you need can be obtained by asking the teacher
Be sure to include the parts of an experiment discussed in class and that you
were quizzed on, i.e., variables, constants etc.
Procedure - Write a step by step procedure using numbers to solve the problem.
Make a data table to collect the data over the next 3-4 days (Time depends on room
temperature and/or light.) Use good observation and measurement skills.
Investigate factors that affect seed germination.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 289 GRADES 9 - 12
Biological Systems
Rubric for
Gathering Information and Solving Problems
#3
Exceeds Expectations
The student will independently write a detailed, well organized,
controlled reproducible procedure to solve the problem stated in the
lab. (For example, How do different enzymes affect the rate of a
chemical reaction?)
Meets Expectations
With minimal assistance the student will write a fairly well organized
controlled reproducible procedure to solve the problem stated in the
lab.
Meets Some Expectations
With some assistance the student will write a procedure to solve the
problem stated in the lab. The procedure is not well organized, is
confusing to follow and it contains a control.
Does Not Meet Expectations
With a great deal of assistance the student writes a procedure to
solve the problem stated in the lab. The procedure is not well
organized, is confusing to follow and does not contain a control.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 290 GRADES 9 - 12
Names: ______________________________________________ Date: ___________________
Biological Systems
Group Project
Cell Structure and Function
Background:
Cells are microscopic units that make up all living things. They are alive and exhibit all of the
features of things The cell is made up of many different parts and each of these parts has a role in
the cell. These parts that make up a cell are called organelles, meaning "little organs". Without
these organelles, the cell would not be able to survive.
Assignment:
Over the next 2-3 days, you will work in small groups to research two or three organelles. I will
assign groups as well as which organelles you will be researching. Your research will include:
1. The name of the organelle
2. A description of the organelle's function in your own words
3. Why the organelle is important
4. Where the organelle is located in the cell
5. A citation page in MLA format
Once you have researched your organelle, you will draw a diagram of it that will be used in a
large scale model of the cell made by the entire class. Once your research and drawing are
complete, you will put together a presentation to teach the class about the functions of your
assigned organelles. You decide how you would like to present your information. Some
ideas/suggestions are PowerPoint presentations, posters, etc.
Expectations:
I expect that you will work as a team. One person should not be doing all the work! Your
behavior and effort each class day will be graded and will become part of your final project
grade. Finally, not only will you receive a grade from me, but also your group members will
have a chance to evaluate your work.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 291 GRADES 9 - 12
Biological Systems
Rubric for
Cell Structure and Function
2. Uses technology effectively and responsibly
Exceeds Expectations
The student independently selects a currently updated and authored
on-line print source related to the topic and creates a source citation
page that follows correct MLA format.
Meets Expectations
The student needs minimal assistance selecting a currently updated
and authored on-line appropriate website directly related to the topic
and creates a source citation page.
Meets Some Expectations
The student needs assistance selecting an appropriate website
directly related to the topic and needs assistance preparing the
source citation page. The website might be outdated and/or
un-authored.
Does Not Meet Expectations
The student cannot select an appropriate website directly related to
the topic. The website is outdated and un-authored. The source
citation page is incorrect or missing.
BIOLOGICAL SYSTEMS
SCIENCE CURRICULUM 292 GRADES 9 - 12
Cell Project Grading Rubric
Name: _________________________________ Organelle: _____________________________
Daily Points
Behavior in class (5 points each)
Day 1 __________________________________
Day 2 __________________________________
Day 3 __________________________________
Effort in class (5 points each)
Day 1 __________________________________
Day 2 __________________________________
Day 3 __________________________________
What is your organelle’s function and why it is important? (20 points)
_______________________________________
Where is your organelle found in the cell? (20 points)
_______________________________________
Diagram of your organelle (15 points)
_______________________________________
Quality of Presentation (10 points)
_______________________________________
Citation Page (5 points)
Benchmark: Using technology effectively
Exceeds Expectations
The student independently selects a currently updated and authored
on-line print source related to the topic and creates a source citation
page that follows correct MLA format.
Meets Expectations
The student needs minimal assistance selecting a currently updated
and authored on-line appropriate website directly related to the topic
and creates a source citation page.
Meets Some Expectations
The student needs assistance selecting an appropriate website
directly related to the topic and needs assistance preparing the
source citation page. The website might be outdated and/or
un-authored.
Does Not Meet Expectations
The student cannot select an appropriate website directly related to
the topic. The website is outdated and un-authored. The source
citation page is incorrect or missing.
Total Points Possible = 100
Your Score: __________/100
Comments: ____________________________________________________________________
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 293 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
General Biology
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
General Biology
3. Transcript Course Code/Number
00313
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade:10 Level: 3
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
None
11. Brief Course Description
This introductory course will explore the basic structure and function of living things. Unicellular and
multicellular forms of life will be examined as they relate to living things and the environment with
emphasis on laboratory experiences. During the first trimester, a scientific method will be used to explore
how living things continue to exist as part of the biospheres of the world. Emphasis will be placed on how
organisms have evolved and how they have come to occupy specific places in the ecosystem. In the
second trimester, major emphasis is the study of the human body and its impact on the biological world.
Heredity and evolutionary concepts will be examined in relation to man.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.
7. Analyze and synthesize scientific information in relation to everyday living.
GENERAL BIOLOGY
SCIENCE CURRICULUM 294 GRADES 9 - 12
13. Course Outline Part 1:
Chapters taught in order of the text.
Chapters Taught
Topics
Activities
# of weeks
1: Biology and You
- Scientific Method
- Measurement
- Scientific tools
- What is biology?
- ―A Controlled Experiment‖
(yeast temperature lab)
- Measurement Lab
- Designer Airplanes Lab
2
3: Chemistry of Life
- Matter and Substances
- Water and solutions
- Carbon compounds
- Enzymes
- Molecular models
- Telltale Cabbage (pH)
- Organic compound Lab
2
4: Ecosystems
- What is an ecosystem?
- Energy flow in ecosystems
- Water, Carbon, Oxygen,
and Nitrogen cycles
- Field trip to Bauer Farm
2
5. Populations and
Communities
- Populations
- Interactions in
Communities
- Shaping Communities
- Population Growth (Quick
Lab)
- The Effect of Herbivores
on a Plant Species (Quick
Lab)
- Yeast Population Growth
Lab
2
7: Cell Structure
(include Ch. 8 and 9 –
photosynthesis and
cellular respiration)
- Cell Theory
- Structure and function of
cell organelles
- Overview of cell
respiration and
photosynthesis
- Prokaryotes vs.
Eukaryotes
- Introduction to
microscopes (letter ―e‖)
- Plant and Animal Cell Lab
- Cell size and function
activity
3
10: Cell Growth and
Division
- Cell Cycle
- Stages of mitosis
- Cell cycle regulation and
cancer
- Online mitosis (onion root
tip, University of Arizona)
- Cell Cycle Lab (from
Visualizing Life book)
4
11: Meiosis and Sexual
Reproduction
(sections 1 and 2)
- Reproduction
- Chromosome number
- Stages of Meiosis
- Meiosis lab (with pipe
cleaners)
- Meiosis flip book
2
12: Mendel and
Heredity
- Mendel’s experiments
- Dominant and recessive
alleles
- Genotype and phenotype
- Homozygous and
heterozygous
- Punnett Squares
- Incomplete dominance
and codominance
- Human inheritance Lab
- Probability and dominant
and recessive alleles activity
(creating a human face)
- Punnett Squares
3
GENERAL BIOLOGY
SCIENCE CURRICULUM 295 GRADES 9 - 12
13: DNA, RNA, and
Proteins (sections 1, 2,
some of 3)
- Experiments to identify
DNA
- Structure of DNA
- DNA replication
- Gene expression and RNA
(basic overview)
- DNA models (pop beads
or paper helix)
- DNA extractions
2
16: Evolutionary
Theory
- Charles Darwin
- Natural Selection
- Evidence for evolution
- Patterns of evolution
- Homologous structures
coloring activity
- Natural selection Lab
2
20. Bacteria and
Viruses
- Bacteria
- Viruses
- Bacteria, Viruses, and
Humans
2
13. Course Outline Part 2: Learning Strands taught in order of presentation.
Learning Strand
Chapters in Book
CT Standards
Weeks
Biology and You
1
10.1
2
Chemistry of Life
3
10.1
2
Ecosystems
4
N/A
2
Population Ecology
5
10.6
2
Cells
7, parts of 8 & 9
10.2
3
Cell Growth and Cell
Division
10, 11
10.3
4
DNA and Heredity
12, 13
10.4
5
Evolutionary Theory
16
10.5
2
Bacteria and Viruses
20
10.2
2
Microorganisms
20
10.3
2
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
GENERAL BIOLOGY
SCIENCE CURRICULUM 296 GRADES 9 - 12
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
GENERAL BIOLOGY
SCIENCE CURRICULUM 297 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
that are based on the results generated during
the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Biology, Holt Rinehart Winston, 2008
Laboratory instrumentation
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research
SUGGESTED ASSESSMENT METHODS
Scientific Method Practice worksheets
CAPT embedded, open ended lab investigations
―A Controlled Experiment‖ (yeast temperature
lab)
Measurement Lab
Designer Airplanes Lab
UV Beads Lab
GENERAL BIOLOGY
SCIENCE CURRICULUM 298 GRADES 9 - 12
LEARNING STRAND
Biology and You
CT Content Standard 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
ENDURING UNDERSTANDINGS
Biology is the study of life.
There are many branches of biology including
biochemistry, botany, cell biology, ecology,
evolutionary theory, genetics, microbiology,
physiology, and zoology.
All living things share seven properties of life
(cellular organization, homeostasis, metabolism,
responsiveness, reproduction, heredity, and
growth).
Recognize how homeostasis plays a role in the
maintenance of living systems.
ESSENTIAL QUESTIONS
What is biology?
How are all of Earth’s organisms tied together
by common traits?
How do all of Earth’s organisms rely on one
another for their survival?
What are the seven properties of life?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Define biology.
Describe the various branches of biology.
Identify and define the seven properties of life.
Biology, Holt Rinehart Winston, 2008
PowerPoint presentation
Internet access
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture enhanced by PowerPoint presentations
Have students relate five of the characteristics
of life to an organism of their choice.
SUGGESTED ASSESSMENT METHODS
Poster illustrating the seven features of life
Directed Reading worksheets
Active Reading worksheets
GENERAL BIOLOGY
SCIENCE CURRICULUM 299 GRADES 9 - 12
LEARNING STRAND
Chemistry of Life
CT Content Standard 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
Most of the chemical activities of the cell are catalyzed by enzymes that function only in a narrow range of temperature and acidity.
ENDURING UNDERSTANDINGS
All living and nonliving things are made of
matter.
Matter is composed of atoms that bond
together to make molecules.
Without the unique properties of water, life as
we know it could not exist.
Living things are complex systems of interacting
and evolving chemical, physical and biological
processes.
Energy provides the ability to do work.
Energy is requires by all living things to carry
out life processes.
Proteins, carbohydrates, lipids and nucleic acids
are the fundamental organic groups found in
living things.
Enzymes decrease the amount of energy
needed for reactions to occur in living systems.
DNA is responsible for storing the information
needed for cell reproduction and survival.
ESSENTIAL QUESTIONS
How is water unique and how do substances
dissolved in water affect its properties?
How do living things use energy?
What chemical processes occur in living things?
How do they happen?
How are enzymes important in the chemical
reaction of living systems?
What are the fundamental types of organic
molecules found in living things? How are they
used?
Why is DNA a critical component to modern
biology?
What is the relationship between DNA, proteins
and traits?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish between elements and compounds.
Describe the difference between covalent and
ionic bonding.
Analyze how macromolecules are broken down
into monomers for energy and other life
processes.
Identify the function of the main nutrients
needed by living things.
Identify the structure of DNA.
Describe how enzymes increase the speed of
biochemical reactions.
Biology, Holt Rinehart Winston, 2008
Items to represent elements
Molecular models
Food samples (simple sugar, starch, protein)
Indicators (iodine, methylene blue, etc.)
http://www.accessexcellence.org/ (Access
Excellence)
SUGGESTED INSTRUCTIONAL STRATEGIES
Molecular modeling
Telltale Cabbage (pH)
Organic Compound Lab
Concept mapping
Directed Reading
Active Reading
Demonstrations
SUGGESTED ASSESSMENT METHODS
Concept map/web of molecules of life, elements
of which they are composed, descriptions of
monomers and polymers and their functions
Telltale Cabbage Lab conclusion (Benchmark)
Oral and written observations
Molecular Models – check for understanding
GENERAL BIOLOGY
SCIENCE CURRICULUM 300 GRADES 9 - 12
LEARNING STRAND
Ecosystems
CT Content Standard – Ecology: Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
The current state of any environment is
maintained by the dynamic exchange of the
processes that dictate its nature. Changes in
any of the interacting processes will impact the
current state (for better or worse).
Energy drives systems and cycles in our
universe.
The elements of living things take many forms.
Structural and behavioral characteristics help an
organism to survive in its environment.
Diverse habitats provide the ―needs of life‖ for a
variety of organisms.
The cycling of matter and the flow of energy
are required for the functioning of ecosystems.
ESSENTIAL QUESTIONS
How do matter and energy move through
biospheres?
What relationships exist between living things?
How do structures differ among living things?
How is structure related to function?
How do ecosystems change over time?
How have human activities effected the
environment?
What needs are met by an organism’s
surroundings?
What energy roles do organisms play in an
ecosystem?
How are living things linked to each other and
their environment?
How do human activities impact and alter the
environment?
How can we conserve and protect our
environment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Relate the availability of resources to
growth/decline of populations.
Demonstrate the flow of energy in an
ecosystem.
Examine the ways in which populations within
communities interact.
Examine the carbon, nitrogen, oxygen, and
water cycles.
Describe how structural and behavioral
adaptations increase organisms' chances for
survival in their environment.
Explain the importance of biodiversity.
Distinguish between the major terrestrial
biomes.
Analyze human impact on ecosystems.
Biology, Holt Rinehart Winston, 2008
Nets, buckets, etc.
Models
Predator-prey game materials
Books on Connecticut ecology and
environmental law
Planet Earth DVD
SUGGESTED INSTRUCTIONAL STRATEGIES
Field trip to Bauer Farm/nature walk
Cooperative group activities
Ecosystems demonstrations
Construct energy pyramids and food webs
Stream, pond or wetland field studies
Observations of biotic, abiotic and
relationships between organisms
Research and present an environmental issue
and its impact on the living ecosystem or on
endangered species.
SUGGESTED ASSESSMENT METHODS
Observations of field studies
Lab reports
Summary of article for class discussion
Test/quizzes
Research projects and presentations
Energy flow diagrams
GENERAL BIOLOGY
SCIENCE CURRICULUM 301 GRADES 9 - 12
LEARNING STRAND
Population Ecology: Populations and Communities
CT Content Standard 10.6 – Living organisms have the capability of producing populations of unlimited size, but the environment
can support only a limited number of individuals from each species.
Human populations grow due to advances in agriculture, medicine, construction and the use of energy.
Humans modify ecosystems as a result of rapid population growth, use of technology and consumption of resources.
ENDURING UNDERSTANDINGS
Population size is affected by many factors,
including environmental factors.
Technological advances have effected human
population growth and size.
The rapid increase in human population size has
effected the environment.
Interactions between organisms are the basis of
communities and are shaped by evolution.
ESSENTIAL QUESTIONS
What are the factors that affect the size of a
population?
How can population graphs be analyzed to
explain population growth?
How have technological advances affected
human population growth and size?
What are types of interactions between
organisms in a community?
How does competition for resources affect
species in a community?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify biotic and abiotic factors that determine
the carrying capacity of an environment.
Describe the parts of an exponential growth
curve.
Name and describe some technological
advances that have affected human population
growth and size.
Compare and contrast the various interactions
that take place within a community.
Describe the results of competition among
organisms in a community.
Biology, Holt Rinehart Winston, 2008
Articles on human population and its effects
SUGGESTED INSTRUCTIONAL STRATEGIES
Human Populations Dynamics STS
Yeast Population Dynamics embedded task
Labs – population, predator-prey
Discuss examples of feeding relationships and
symbiotic relationships that exist in a chosen
ecosystem.
The Effect of Herbivores on a Plant Species Lab
Population Growth Lab
SUGGESTED ASSESSMENT METHODS
Test/quizzes
Lab reports
Projects
Presentations
Creating & interpreting graphs – Population
growth
GENERAL BIOLOGY
SCIENCE CURRICULUM 302 GRADES 9 - 12
LEARNING STRAND
Cells: Cell Structure
CT Content Standard 10.3 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
Most of the chemical activities of the cell are catalyzed by enzymes that function only in a narrow range of temperature and acidity
conditions.
The cellular processes of photosynthesis and respiration involve transformation of matter and energy.
ENDURING UNDERSTANDINGS
Cells are the basic units of life.
Complex living things are organized in different
levels.
Living things are complex systems of interacting
and evolving chemical, physical and biological
processes.
Change in any interacting process will impact
cells/living things.
Cells/living things have optimal ranges for
different environmental conditions in which they
perform life processes.
The cell membrane regulates movement of
substances in and out of a cell based on their
size and chemical charge.
ESSENTIAL QUESTIONS
How do (cell) structures differ among living
things?
How is structure related to function?
How is the cell membrane organized?
How does the cell membrane control movement
of substances into and out of the cell?
How do environmental factors affect living
things?
How are cells organized in complex multicellular
organisms?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Relate the structure and function of cellular
organelles to the overall performance of the cell
and therefore the organism.
Analyze how materials move in and out of cells.
Differentiate between plant and animal cells.
Differentiate between prokaryotic and
eukaryotic cells.
Biology, Holt Rinehart Winston, 2008
Cell models
Prepared slides – mitosis, plant, animal, etc.
Play-Doh
Assignment Discovery: The Cell DVD
Microscopes
Internet for selected websites
SUGGESTED INSTRUCTIONAL STRATEGIES
Microscope work
Cooperative group activities
Diagrams
Research projects
Directed Reading
Active Reading
Demonstrations
Venn Diagrams
SUGGESTED ASSESSMENT METHODS
Tests and quizzes
Microscope Labs (letter ―e‖, Plant and Animal
Cells)
Research Project grades with rubric
Structure and Function of Organelles
(Benchmark)
Class participation
Plant and Animal Cell diagrams
Cell City Activity
GENERAL BIOLOGY
SCIENCE CURRICULUM 303 GRADES 9 - 12
LEARNING STRAND
Cell Growth and Cell Division: Meiosis and Sexual Reproduction
CT Content Standard 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
Most of the chemical activities of the cell are catalyzed by enzymes that function only in a narrow range of temperature and acidity.
ENDURING UNDERSTANDINGS
Cells divide to produce other cells.
DNA directs cell division.
Cells go through a cell cycle to ensure new cells
are identical to the old cells.
Cell growth, division, and the cell cycle are
highly regulated by protein and environmental
signals.
Cancer is caused by uncontrolled cell growth.
Understanding how to control cell growth may
be the key to curing cancer.
ESSENTIAL QUESTIONS
Why do cells divide?
How do cells prepare for cell division?
How do cells divide to produce more cells?
What are some of the factors that control cell
growth and division?
How do feedback signals relate to the cell
cycle?
How does cancer relate to the cell cycle?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the stages of the cell cycle.
Identify and describe the phases of mitosis.
Describe the role of DNA in cell division.
Describe how cell division is regulated.
Explain the importance of learning how to
control cell growth.
Biology, Holt Rinehart Winston, 2008
Internet for selected websites
Prepared slides
Models of the stages of mitosis
Wiki sticks
Video: Ultimate Discovery: Human Body
SUGGESTED INSTRUCTIONAL STRATEGIES
Cell Cycle Lab (from Visualizing Life book)
Mitosis in Plant Cells Lab
Online Mitosis (Onion root tips from University
of AZ)
Visuals – video clips, diagrams, drawings
Active Reading
Directed Reading
Cancer websites
SUGGESTED ASSESSMENT METHODS
Internet Activities (Cell Cycle, Mitosis drawings
and descriptions and Onion Root Tips)
Phases of Mitosis cut out activity
Phases of Mitosis drawings
Tests and quizzes
Class participation
Phases of Mitosis modeling (with Wiki Sticks)
GENERAL BIOLOGY
SCIENCE CURRICULUM 304 GRADES 9 - 12
LEARNING STRAND
DNA and Heredity
CT Content Standard 10.3 – Similarities in the chemical and structural properties of DNA in all living organisms allow the transfer of
genes from one organism to another.
CT Content Standard 10.4 – In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both
parents.
Genetic information is stored in genes that are located on chromosomes inside the cell nucleus.
Changes in the environment may result in the selection of organisms that are better able to survive and reproduce.
ENDURING UNDERSTANDINGS
There is a process of inheriting traits from
parents to offspring through genes.
Meiosis allows genetic information from two
parents to combine and form offspring that are
different from both parents.
Sexual reproduction increases variation in
offspring.
Modern genetics is based on the work of
Mendel and his explanations of heredity.
Predictions can be made using Punnett squares,
pedigree or other methods to determine
frequencies of genotypes and/or phenotypes in
offspring.
Genetic mutations can be harmful and cause
genetic disorders or they can be beneficial and
offer advantages to organisms.
DNA is the blueprint from which all living things
are made.
Traits are determined by proteins that are built
according to instructions coded in DNA.
ESSENTIAL QUESTIONS
What are the stages of meiosis?
How are mitosis and meiosis similar? How are
they different?
How are characteristics of living things passed
on through generations?
How is information organized in a DNA
molecule?
How does Mendel’s work explain the simple
patterns of heredity in pea plants?
How can Punnett squares and pedigrees be
used to determine genotypic and phenotypic
frequencies of offspring?
How does the process of meiosis and sexual
reproduction affect the variation of offspring?
How do heredity and the environment interact
to influence phenotype?
How are genetic disorders different from
infectious diseases?
What is the shape of a DNA molecule?
What is the process of gene expression?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Compare sexual and asexual reproduction.
Describe the stages of meiosis.
Describe Mendel’s Theory and how it explains
simple patterns of inheritance.
Apply the Punnett Squares to solve genetic
problems.
Explain how events that occur during meiosis
contribute to genetic variation and/or
mutations.
Deduce the probable mode of inheritance of
traits from pedigree diagrams showing
phenotypes.
Compare and contrast genetic disorders and
infectious diseases.
Describe the shape of DNA molecule.
Explain the major steps of DNA replication and
why this process is important.
Biology, Holt Rinehart Winston, 2008
DNA model or craft materials or pop beads to
create a model
Clay (for meiosis modeling)
Meiosis video (from United Streaming)
Assignment Discovery Genetics (DVD)
Internet for selected websites
SUGGESTED INSTRUCTIONAL STRATEGIES
Visuals (DVDs, video clips, animations,
drawings)
Modeling – phases of meiosis with clay
Active Reading
Directed Reading
Sequencing Skills – phases of meiosis
PowerPoints – Mendel’s experiments
Human inheritance lab
Probability and dominant and recessive alleles
activity (creating a human face)
Punnett Squares
DNA models (pop beads or paper helix)
Genetic disorder research project
Labs – Blood typing – prepared slides or
simulated lab
GENERAL BIOLOGY
SCIENCE CURRICULUM 305 GRADES 9 - 12
Concept mapping
SUGGESTED ASSESSMENT METHODS
Quiz on Meiosis video
Presentations – Genetic disorders project
Tests and quizzes
Class participation
Genetic problems (probability and Punnett
Squares)
Teacher observations of drawings, meiosis
modeling with clay, DNA pop beads
Lab analysis questions (Blood Typing)
GENERAL BIOLOGY
SCIENCE CURRICULUM 306 GRADES 9 - 12
GENERAL BIOLOGY
SCIENCE CURRICULUM 307 GRADES 9 - 12
LEARNING STRAND
Evolutionary Theory
CT Content Standard 10.5 – Evolution and biodiversity are the result of genetic changes that occur over time in constantly changing
environments.
Mutations and recombination of genes create genetic variability in populations.
Changes in the environment may result in the selection of organisms that are better able to survive and reproduce.
ENDURING UNDERSTANDINGS
Many aspects of biology are explained by
evolutionary theory.
Adaptation is any structural or behavioral
changes in a species that takes place over time
and helps the species to survive in its habitat.
Structural and behavioral characteristics help an
organism to survive in its environment.
Changes in the environment may result in the
selection of organisms that are better able to
survive and reproduce.
There is evidence that supports the theory of
evolution including fossil evidence, anatomical
evidence, and DNA evidence.
ESSENTIAL QUESTIONS
What is evolution by natural selection?
How do species become more diverse?
How do some species survive environmental
changes while others do not?
How do species become extinct?
What evidence is used to support the Theory of
Evolution, including the fossil record?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain how Darwin developed this theory of
evolution from his experiences.
Describe the steps of natural selection as a
mechanism for evolution.
Examine how mutations and adaptations apply
to Darwin’s theory of natural selection.
Explain how the many pieces of evidence
scientists have discovered supports the theory
of evolution.
Explain how new species arise as a result of
evolution.
Biology, Holt Rinehart Winston, 2008
Internet for selected websites
Materials for jelly bellicus (jelly beans, boxes, wood
shavings)
SUGGESTED INSTRUCTIONAL STRATEGIES
Cooperative group activities to illustrate natural
selection– Jelly Bellicus
Peppered Moth internet activity
Create simulated model/poster of natural selection
principles.
Active Reading
Directed Reading
Visuals - Homologous structures coloring activity,
skeletons, PowerPoint pictures and diagrams
SUGGESTED ASSESSMENT METHODS
Lab analysis questions – Jelly Bellicus, peppered
moth internet activity
Presentations
Tests and quizzes
Class participation
Homologous structures coloring activity
Teacher observations
GENERAL BIOLOGY
SCIENCE CURRICULUM 308 GRADES 9 - 12
LEARNING STRAND
Microorganisms: Bacteria and Viruses
CT Content Standard 10.2 – Microorganisms have an essential role in life processes and cycles on Earth.
Understanding the growth and spread patterns of viruses and bacteria enables the development of methods to prevent and treat
infectious diseases.
ENDURING UNDERSTANDINGS
Microorganisms play an essential role in life
processes and cycles on Earth.
Understanding the growth and spread patterns
of bacteria and viruses enables the
development of methods to prevent and treat
infectious diseases.
Bacteria and viruses have a large impact on
humans, from benefiting the environment to
causing disease.
ESSENTIAL QUESTIONS
What are the differences between bacteria and
viruses?
How are bacteria and viruses both helpful and
harmful to humans?
How can humans prevent and treat infectious
diseases?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Compare and contrast the differences in
structure of bacteria and viruses.
Compare and contrast the differences in the life
cycles of bacteria and viruses.
Describe how infectious diseases are
transmitted to humans by bacteria, viruses and
other microorganisms.
Describe the roles of bacteria and viruses in the
environment, industry, and research.
Examine the role of sanitation, vaccination, and
antibiotic medications in the prevention and
treatment of infectious diseases.
Biology, Holt Rinehart Winston, 2008
Internet for selected websites
SUGGESTED INSTRUCTIONAL STRATEGIES
Research and present infections diseases
(symptoms, vectors, treatments. Etc.)
Debate and explain the rationale behind the ―over
prescribed‖ use of antibiotics.
Guest speaker – microbiologist, epidemiologist, lab
technician, etc.
Read and summarize articles on related current
issues of disease.
Lab activity – grow bacterial cultures, look at the
effect of antibiotics on bacterial growth
Web quests
Venn diagrams (viruses vs. bacteria)
Visuals – posters, animations
Demonstrations
Active Reading
Directed Reading
SUGGESTED ASSESSMENT METHODS
Posters (comparison of structures and life cycles of
bacteria and viruses)
Tests and quizzes
Lab reports – growing bacteria
Projects and presentations – research on infectious
diseases
Article summaries
Class participation
Debate – overuse of antibiotics

GENERAL BIOLOGY
SCIENCE CURRICULUM 309 GRADES 9 - 12
Name _____________________________________ Class ___________ Date ___________________________
Quick Lab
Telltale Cabbage
Red cabbage contains a natural indicator that can be used to identify how acidic or basic a
solution is.
Procedure
1. Cut up a cabbage leaf into very tiny clippings by using scissors.
2. Put on safety goggles. Place the clippings in a beaker of warm water. Swirl the mixture. Wait
several minutes until the water changes color.
3. Add several drops of lemon juice. Note any changes in appearance.
4. Add about 114 tsp of baking soda. Note any changes in appearance. Continue adding small
amounts of baking soda, and observe-additional color changes.
Analysis
1. Describe what happened when the leaf clippings were placed in warm water.
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
2. Describe what happened when lemon juice (an acid) was added to the indicator solution.
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
3. Describe what happened when the baking soda was added to the acidic solution.
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
4. Critical Thinking Inferring Relationships from your observations, what do you think caused
the reaction. when the baking soda and acidic solution were combined? ____________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
Original content Copyright @by Holt, Rinehart and Winston. Additions and changes to the original content are the responsibility of the instructor.
Holt Biology 44 Chemistry of Life
GENERAL BIOLOGY
SCIENCE CURRICULUM 310 GRADES 9 - 12
General Biology
Telltale Cabbage Lab - using data to write a conclusion
1 B. Writing Effectively
Exceeds Expectations
The student uses supporting evidence and data from the lab to write a
well developed and organized conclusion. Word choice and syntax are
accurate and appropriate. The student shows mastery in the
conventions of Standard English.
Meets Expectations
The student uses some supporting evidence and from the lab to write
a fairly well developed and organized conclusion. Word choice and
syntax are accurate and appropriate. Errors in the conventions of
Standard English are few.
Meets Some Expectations
The student uses supporting evidence and data which may be
inaccurate, simplistic, and/or confused to write the lab conclusion. The
student may require assistance to develop or organize his/her
response. Word choice and syntax are consistent with grade level.
There are some errors in the conventions of Standard English.
Does Not Meet Expectations
The student misinterprets significant evidence and data in the lab,
selecting an inappropriate format. The student requires many
additional explanations, models, and/or strategies in order to complete
parts of the process. The writing has no clear focus. Ideas and
concepts are often unorganized or inaccurate. Inaccurate or limited
vocabulary, syntax errors, and errors in the conventions of writing
make the writing ineffective.

GENERAL BIOLOGY
SCIENCE CURRICULUM 311 GRADES 9 - 12
Name _________________________________________ Date ___________________________________
Research Project
Cell Structure and Function
Backqround:
Cells are microscopic units that make up all living things. They are alive and exhibit all of the features of
living things. The cell is made up of many different parts and each of these parts has a role in the cell.
These parts that make up a cell are called
organelles,
meaning "little organs". Without these organelles,
the cell would not be able to survive.
Your Assignment:
Over the next 2-3 days, you will research an assigned organelle and then create a PowerPoint
presentation to teach the class about your organelle. Your PowerPoint presentation should include:
A minimum of 5 slides
A unique slide design
Animation
The name of the organelle
A description of the organelle (what it looks like, what it's made of, etc.)
An explanation of the organelle's function
in your own words.
Where the organelle is located in the cell
A picture of the organelle
A list of the resources you used cited in MLA Format
Expectations:
I expect that everyone will create a PowerPoint presentation. Your behavior and effort each class day will
be graded and will become part of your final project grade. This includes the day of the presentations. I
expect that you will listen quietly to your classmates' presentations, ask questions when appropriate, and
take notes. Finally, not only will you receive a grade from me, but your classmates will also have a
chance to evaluate your presentation.
GENERAL BIOLOGY
SCIENCE CURRICULUM 312 GRADES 9 - 12
Name _________________________________________ Date ___________________________________
Cell Project Grading Rubric
Criteria
Points Possible
Points Earned
Behavior in Class
Day 1
4
Day 2
4
Day 3
4
Effort in Class
Day 1
4
Day 2
4
Day 3
4
Minimum of 5 slides
2
Unique slide design
2
Animation
2
Name of organelle is included in
presentation and spelled correctly
5
Description of organelle is accurate and
complete
15
Explanation of function is accurate and
complete
20
Location of organelle is accurate
10
Picture of organelle
5
Sources are current, authored, and cited
in MLA format
5
Class evaluation
10
Total
100
Teacher Comments: _____________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
GENERAL BIOLOGY
SCIENCE CURRICULUM 313 GRADES 9 - 12
General Biology
Cell Organelle Research Project
2. Uses technology effectively and responsibly
Exceeds Expectations
The student independently selects a currently updated and authored
websites directly related to the research project.
Meets Expectations
The student needs minimal assistance selecting an appropriate
website directly related to the research project. The website may be
outdated or un-authored
Meets Some Expectations
The student needs some assistance selecting an appropriate website
directly related to the research project. The website is outdated
and/or un-authored.
Does Not Meet Expectations
The student cannot select an appropriate website directly related to
the research project. The website is outdated and un-authored.
GENERAL BIOLOGY
SCIENCE CURRICULUM 314 GRADES 9 - 12
Name _________________________________________ Date ___________________________________
A Controlled Experiment
Yeast Lab
The control is an important part of any scientific experiment. The control is the part of the experiment
that remains unchanged throughout the experiment. It serves as a basis for comparison with any variable
that you may introduce .
PROBLEM: Test the effect of temperature on the metabolic activity of yeast cells.
HYPOTHESIS: Create a hypothesis before proceeding.
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
MATERIALS: Listed below are the materials that will be supplied to your group.
If you think you will need something that is not provided, please ask. The teacher will make available
additional materials if possible.
Test tubes (3)
Test tube rack
Beaker (3)
Thermometer
Marker
Graduated cylinder
Yeast culture (25 ml)
Eyedropper
Room temperature water (21° - 23°C)
Warm water (30° - 35°C)
Stopwatch
Metric ruler
PROCEDURE: In groups of two you will design an experiment to test the metabolic activity of yeast. You
will then carry out the experiment with your lab partner.
Note: It will take at least 15 minutes for the yeast to become active.
GENERAL BIOLOGY
SCIENCE CURRICULUM 315 GRADES 9 - 12
PROCEDURE
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Control: ______________________________________________________________________________
______________________________________________________________________________________
Independent Variable: ______________________________________________________
________________________________________________________________________
Dependent Variable: ________________________________________________________
________________________________________________________________________
GENERAL BIOLOGY
SCIENCE CURRICULUM 316 GRADES 9 - 12
ANALYSIS QUESTIONS
1. In the lab that you have just completed, describe the things that are considered the constants.
A. ____________________________________
B. ____________________________________
C. ____________________________________
D. ____________________________________
2. Describe the control in this lab. ___________________________________________________________
______________________________________________________________________________________
3. Describe the independent variable in this lab. _______________________________________________
______________________________________________________________________________________
4. Describe the dependent variable in this lab. _________________________________________________
______________________________________________________________________________________
5. What can you conclude about the effects of temperature on the activity of yeast cells?______________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
6. Why does a good experiment only have one independent variable? _____________________________
______________________________________________________________________________________
______________________________________________________________________________________
7. In this lab we examined how higher temperatures affected the activity of yeast cells. Describe how you
would set up an experiment to determine how colder temperatures effect the activity of yeast cells.
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
______________________________________________________________________________________
GENERAL BIOLOGY
SCIENCE CURRICULUM 317 GRADES 9 - 12
TABLE 1: TEAM DATA
Temperature of
Yeast Solution
Height at
Start (mm)
Solution Height
at Finish (mm)
Change in
Height (mm)
TABLE\2: CLASS DATA
Team Number
Change in Height (mm)
1
2
3
4
5
6
GENERAL BIOLOGY
SCIENCE CURRICULUM 318 GRADES 9 - 12
General Biology
Yeast Lab - Writing a Procedure
3. Applies effective and efficient strategies for gathering information and materials, thinking
critically, and solving problems
Exceeds Expectations
The student independently writes a detailed, well organized,
controlled, and reproducible procedure to solve the problem stated in
the lab. (For example, What is the effect of temperature on the
metabolic activity of yeast cells?)
Meets Expectations
With a minimal assistance the student writes a fairly well organized,
controlled, and reproducible procedure to solve the problem stated in
the lab.
Meets Some Expectations
With some assistance, the student will write a procedure to solve the
problem stated in the lab. The procedure is not well organized,
confusing to follow, but does contain a control.
Does Not Meet Expectations
With a great deal of assistance the student writes a procedure to solve
the problem stated in the lab. The procedure is not well organized, is
confusing to follow and does not contain a control.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 319 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Human Biology
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Human Biology
3. Transcript Course Code/Number
00320
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 10 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisite
Successful completion of Biological Systems
11. Brief Course Description
Students will study the structure and function of each major system in the human body. Particular
emphasis is placed on understanding how the ―well‖ body functions to better understand the body when
it becomes ill and requires the skills of the health care professional. Substantial time will be spent
studying the disease process as it relates to the different body systems. Dissection of a fetal pig is
included in this course in order to give students first hand experience with the various organs associated
with the body systems. (Alternative assignments are available.)
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Use technology effectively and responsibly.
3. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
4. Demonstrate respect for one’s self, and strive to contribute to the success of others.
5. Understand how the Human Body works in a healthy and diseased state.
6. Read articles in newspapers and popular journals with adequate understanding for topics of Human
Biology.
HUMAN BIOLOGY
SCIENCE CURRICULUM 320 GRADES 9 - 12
13. Course Outline: (2 Trimesters)
Unit/Chapter
Topics / Activities
# of Weeks
UNIT I
Organization of the Human Body –
An Introduction to Human Biology
Overview of body systems
Interconnected nature of the body systems
- Activity: Asian Tsunami Dominoes
Planes of division
- Activity: Play-doh animal sections
Metric system review
1
Biochemistry
Atoms, elements, molecules, compounds
Organization of the periodic table
Atomic structure
Acids, bases, and pH
- Lab: Find the pH of common household solutions
Test: Organization of the Human Body and Biochemistry
3 Days
Cells and Their Function
Cells, organelles, and mitosis
Cell transport, diffusion, osmosis, and tissues
- Activity: Gummy bear diffusion/osmosis
Cancer
- Research project: oral PowerPoint presentation
(Benchmark)
- Video: Cancer Warriors
2
UNIT 2
Nervous System
Video: Human Body – Pushing the Limits: Sensations
Organization of the nervous system, nerve cells, nerve
impulses, reflexes, and reaction time
- Lab: Card sorting reaction time lab
- Lab: Card sorting reaction time inquiry lab
Structure of the spinal cord and spinal cord disorders
Structure and function of the brain
- Video: Human Body – Pushing the Limits: Brain Power
2
Quiz: Organization of the Nervous System and Spinal
Cord
Activity: Nerve and brain poster
Lab: Fetal pig nervous system dissection
- Dissection journal
Nervous System – Senses
Sight
- Video: Human Body – Pushing the Limits: Sight
Hearing and smell
- Lab: Olfactory fatigue lab
- Lab: Olfactory fatigue with coffee beans inquiry lab
1
Test: The Nervous System
Endocrine System
Introduction to the endocrine system, hormones, receptors,
and target cells.
Endocrine glands and disorders
- Research project: Endocrine gland and disorder poster
(Benchmark)
- Activity: Endocrine poster scavenger hunt
1.5
UNIT 3
Circulatory System – The Heart
Anatomy of the heart
- Demonstration: Cow heart dissection
- Activity: Heart tissue microscopy
- Lab: Fetal pig circulatory system dissection
Heart physiology
- Activity: Taking pulse and blood pressure (resting and
active)
- Lab/Activity: ECG/EKG
2
HUMAN BIOLOGY
SCIENCE CURRICULUM 321 GRADES 9 - 12
Heart disease and prevention
- Video: The Mysterious Human Heart - The Silent Killer
Heart disease treatments
- Activity: Online virtual heart transplant (maybe)
- Video: The Mysterious Human Heart - The Spark of Life
Midterm Exam
Blood
Functions of the blood
Blood constituents
- Lab: Microscopy – red and white blood cells
Blood types
- Lab: ―Who Trashed My Locker?!‖ (blood typing)
Blood genetics
Counting blood cells
- Lab: Hemocytometers
2
Respiratory System
Anatomy of the respiratory system
Physiology of the respiratory system
- Lab: Lung capacity
- Activity: Build-a-Lung
Regulation of breathing
- Lab: Human respiration (LoggerPro)
Test: The Respiratory System
2
Test: The Blood
UNIT 4
Immune System
Bacteria and Viruses
- Structure
- Reproductive cycles
- Activity: Bacteria and Virus Poster
- Lab: ―Bacteria Everywhere!‖
- Movie: ―Influenza 1918‖
Quiz: Bacteria and Viruses
Immune System
- Components
- How the immune system works
- Activity: ―Operation Antibody‖
- Movie: ―Inside Look at the Flu‖
Vaccines and Antibiotics
Test: The Immune System
4
Digestive System
Anatomy and physiology of the digestive system
- Lab: Digestive system dissection
- Movie: ―Evolve: Guts‖
- Activity: Digestive system disorder brochure
1
UNIT 5
Reproductive System
Movie: ―Life’s Greatest Miracle‖
Activity: Human development timeline
Male reproductive system anatomy and physiology
Female reproductive system anatomy and physiology
2
Final Exam

HUMAN BIOLOGY
SCIENCE CURRICULUM 322 GRADES 9 - 12
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response cards
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative assessments
Midterm examination
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response cards
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments:
- Cancer research project. Assesses the student's ability to validate information obtained through
research. (IC: Speaking/Listening/Viewing and 2: Technology)
- Endocrine system poster project. Assesses the student's ability to validate information obtained
through research. (IC: Speaking/Listening/ Viewing and 2: Technology)
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
HUMAN BIOLOGY
SCIENCE CURRICULUM 323 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic
that are based on the results generated during
the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
The Human Body in Health and Disease
Lippincott Williams and Wilkins 2000
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
SUGGESTED ASSESSMENT METHODS
Lab using metric units to measure length, mass,
volume, and density.
Lab reports reflecting the usage of appropriate
language and terminology including the
concepts of a hypothesis, dependent and
independent variables, and graphs.
Use appropriate tools and techniques to make
observations and gather data.
HUMAN BIOLOGY
SCIENCE CURRICULUM 324 GRADES 9 - 12
LEARNING STRAND
Body Organization, Biochemistry and Cells
Fundamental life processes depend on the physical structure and the chemical activities of the cell.
Similarities in the chemical and structural properties of DNA in living organisms allow the transfer of genes from one organism to
another.
CT Standard 10.4 – In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both parents.
CT Standard: Enrichment High School Biology – As a result o the coordinated structures and functions of organ systems, the
internal environment of the human body remains relatively stable (homeostatic) despite changes in the outside world.
ENDURING UNDERSTANDINGS
The body is organized into atoms, molecules,
cells, tissues, organs and organ systems.
All body systems are interconnected and rely on
each other to work properly.
Constant input of energy is required to maintain
this level of organization.
Cellular function is dependent on programmed
instructions found in the DNA of the cell.
Cellular organization and thus life is dependent
on the principles of physics and chemistry as
they apply to a living changing and dynamic
system.
ESSENTIAL QUESTIONS
What are the physical and chemical principles
that apply to the homeostatic condition and
thus maintain life?
How do the various body systems work
together so that they work properly and
efficiently?
How does glucose operate in the cell to provide
the energy necessary for the continuation of life
functions?
Where is DNA found in the cell and how does it
communicate its blueprint to the cell?
What happens to a cell that causes it to die?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the flow of energy from a level of high
potential to lower potential.
Describe how the body is organized into
increasing levels of complexity.
Define homeostasis as a state of constant
harmony (ease) within the body.
Explain the process by which DNA is used to
create proteins.
Explain the vital role that enzymes play in the
development of a homeostatic environment
within the cell.
Interpret the periodic table in terms of how the
elements are organized.
Illustrate the atomic structure of various
biologically active elements use the periodic
table as a guide.
Use the periodic table to predict which elements
are more likely to interact with each other to
form compounds.
The Human Body in Health and Disease
Lippincott Williams and Wilkins 2000
PowerPoint slides
Response cards
Dominoes
Gummy Bears
Household solutions of varying pH
Play-doh
Metric rulers and scales
Online video: Cancer Warriors
Laptop carts for lessons on the Internet
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Use dominoes to construct a domino course
that models how body systems are connected
Use Gummy Bears to practice use of the metric
system and determine changes in length, mass,
volume, and density.
Research various topics surrounding cancer and
present orally research (Benchmark)
SUGGESTED ASSESSMENT METHODS
Formative quizzes
Summative test
Research project
Assessments evaluated with rubrics
Benchmark assessment
HUMAN BIOLOGY
SCIENCE CURRICULUM 325 GRADES 9 - 12
LEARNING STRAND
The Nervous System, Spinal Cord and Endocrine System
The brain, spinal cord and endocrine system are the three controlling systems of the body. Each system plays a specific role but all
system work together to provide for balance and coordination.
CT Standard 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
CT Standard: Enrichment High School Biology – As a result o the coordinated structures and functions of organ systems, the internal
environment of the human body remains relatively stable (homeostatic) despite changes in the outside world.
ENDURING UNDERSTANDINGS
There are two main control systems in the
body.
The brain and spinal cord have the fastest
impulse speed and the most localized effect.
The endocrine system employs hormones, is
slower and has a generalized effect, often
over a long period of time.
Each system demonstrates the principle that
structure often determines function in a body
system.
The neuroendocrine mechanism illustrates the
―marriage‖ between the nervous system and
the endocrine system.
ESSENTIAL QUESTIONS
How does the structure of a neuron influence its
function?
What is the mechanism that determines when a
neuron will ―fire‖ or become functional?
What happens in the brain when learning occurs?
What is the connection between sensory experience
and learning?
What determines if a hormone will be successful in
influencing a particular cell to ―turn on or off‖?
Why do some cells respond to certain hormones
and other cells do not?
What are some hormonal abnormalities and what
kinds of treatment are available?
How does the brain control the endocrine system?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Illustrate the anatomy of a typical neuron.
Describe the physiology of the ―action
potential‖ and the function of myelin.
Identify the four lobes of the brain.
List one function associated with each lobe of
the brain.
Explain the process by which we are able to
see a focused image.
Explain why certain people are unable to see
a focused image.
Explain the process of smell.
Explain the concept of olfactory fatigue.
Explain the relationship between hormones,
receptors, and target cells.
Research the names and functions of the
most important hormones.
Research various endocrine glands, their
associated diseases, and their treatments.
The Human Body in Health and Disease
Lippincott Williams and Wilkins 2000
PowerPoint slides
Playing cards
Fetal pigs and dissection kits
Laptop carts for lessons on the Internet
Poster paper and other materials
Essential oils, Q-tips, coffee beans
Videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint presentations and notes
Research
Laboratory investigations
Cooperative grouping
Audio Visual presentations
Response cards
Create posters illustrating the anatomy of a nerve
cell and the anatomy of the brain
Tests of students' reaction time.
Olfactory fatigue experiences
Research various endocrine glands and their
associated disorders. (Benchmark)
SUGGESTED ASSESSMENT METHODS
Informational posters constructed from research of
endocrine glands and associated disorders
HUMAN BIOLOGY
SCIENCE CURRICULUM 326 GRADES 9 - 12
LEARNING STRAND
The Heart, Blood and Respiration
The blood, the heart, and respiration provide the mechanism for driving the life forces in the body. Each system provides for life
giving oxygen to be provided so as to maintain cellular homeostasis.
CT Standard 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
CT Standard: Enrichment High School Biology – As a result o the coordinated structures and functions of organ systems, the internal
environment of the human body remains relatively stable (homeostatic) despite changes in the outside world.
ENDURING UNDERSTANDINGS
There is an interrelationship between the
blood, the heart and the lungs.
Each of the organ systems performs a
different but vital role in maintaining the
homeostasis of the healthy body.
The blood has many functions including
maintaining body temperature, oxygenating
tissues, and carrying antibodies from place to
place.
The heart maintains the blood pressure and is
the driving forced behind circulation.
The lungs cause the body to excrete carbon
dioxide and take in needed oxygen.
ESSENTIAL QUESTIONS
What is the relationship between the blood, heart
and lungs with regard to maintaining homeostasis?
What is the role of erythrocytes and how are they
formed in the blood?
What is the mechanism that keeps the human heart
pumping at a regular rate?
What effect does blood pressure have on the
normal functioning of the heart?
What is a heart attack and what causes it to
happen?
Why does the chest have to move to take in air?
How does the volume/pressure relationship allow a
person to breath?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the interrelationships between the
blood, heart and lungs and their associated
systems.
List the seven types of blood cells.
Describe what causes blood cells to be
formed in the bone marrow.
Illustrate the anatomy of the human heart.
Describe the electrical mechanism that causes
the heart to beat in a continuous manner.
Describe the mechanism and causative agents
in a myocardial infarction.
Explain the mechanism by which the chest
expands and contracts so as to cause inflation
of the lungs.
Explain why individuals have different blood
types.
Explain what Rh incompatibility is.
The Human Body in Health and Disease
Lippincott Williams and Wilkins 2000
Blood typing test kit
Sphygmomanometers and stethoscopes
Videos
Cow hearts
Fetal pigs
Dissection kits
Prepared slides of cardiac tissue and blood
Blood cell counting kit with hemocytometers
Laptop carts and Vernier EKG units
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint notes
Blood typing lab
Determining blood pressure using blood pressure
cuff
Audio Visual presentations
Cow heart dissection demonstration
Fetal pig dissection
SUGGESTED ASSESSMENT METHODS
Lab reports
Response cards
Formative quizzes
Summative test
HUMAN BIOLOGY
SCIENCE CURRICULUM 327 GRADES 9 - 12
LEARNING STRAND
Disease and Immunity
The disease process and immunity are in a constant struggle to win control of the body. The immune system is the final defense
again the death of the human.
CT Standard 10.2 - Microorganisms have an essential role in life processes and cycles on Earth.
CT Standard: Enrichment High School Biology – As a result o the coordinated structures and functions of organ systems, the
internal environment of the human body remains relatively stable (homeostatic) despite changes in the outside world.
CT Standard: Enrichment High School Biology – Organisms have a variety of mechanisms to combat disease.
ENDURING UNDERSTANDINGS
The body spends much energy attempting to
prevent the onset of disease.
The body has various levels of defense or
prevention mechanisms.
The body repairs itself and generates new
cells as required.
Immunity is a condition that we are either
born with or acquired to prevent the spread
of disease.
Not everyone has the ability to develop all
types of immunity.
Vaccines were created to help the immune
system battle both viral and bacterial
diseases.
ESSENTIAL QUESTIONS
What causes disease to occur?
Why do we feel tired and lethargic when we
become sick?
What are some of the ways our body responds
when it realizes we are getting sick?
What are the three levels of defense we have
against the disease process?
Why is it that some become sick and others do
not?
What is immunity?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the structural parts of a virus and
bacterium.
Describe the process by which viruses and
bacteria reproduce.
Explain the value of prevention as opposed to
therapy.
List the three levels of defense that the body
employs to prevent the disease process.
Describe the process by which the body fights
infection.
The Human Body in Health and Disease
Lippincott Williams and Wilkins 2000
PowerPoint slides
Videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeled instruction
PowerPoint notes
Audio Visual presentations
Research
Response cards
Bacteria/Virus poster project
―Bacteria Everywhere‖ lab
―Operation Antibody‖ audio/visual project
SUGGESTED ASSESSMENT METHODS
Formative quiz
Summative test
Lab report
Research project
Assessments evaluated with rubrics
HUMAN BIOLOGY
SCIENCE CURRICULUM 328 GRADES 9 - 12
LEARNING STRAND
Reproduction, Development, and Heredity
Reproduction, development, and heredity provide for cellular repair and continuation of the species.
Content Standard 10.4 - In sexually reproducing organisms, each offspring contains a mix of characteristics inherited from both
parents.
Content Standard 10.5 - Evolution and biodiversity are the result of genetic in constantly changing environments changes that occur
over time.
ENDURING UNDERSTANDINGS
Reproduction is the mechanism by which the
body repairs itself and passes on traits to the
next generation.
The goal of all living things is to continue the
species and improve the next generation.
Sexual reproduction increases variability and
thus helps to insure resistance from disease.
The male body is designed to manufacture and
deliver sperm and the female body is designed
to manufacture ova and nourish the developing
fetus.
Sperm and ova are carriers of the DNA message
and must be united for the next generation to
occur.
The female body is specifically designed and
hormonally driven to nourish the developing
fetus for at least nine months.
The concepts of pair bonding and love are
designed to provide two parents to care and
nourish the young child.
ESSENTIAL QUESTIONS
Why is sexual reproduction better than asexual
reproduction (parthenogenesis)?
Why do the male and female members of the
human race have different anatomical
reproductive structures?
Can a human be produced if no sex act occurs
between a male and female?
Why do we have ―love‖ in the human world and
not in other forms of animal life?
INSTRUCTIONAL SUPPORT MATERIALS
The Human Body in Health and Disease
Lippincott Williams and Wilkins 2000
PowerPoint slides
Video: ―Life’s Greatest Miracle‖
Poster/timeline materials
Laptop carts
Internet lessons
LEARNING OBJECTIVES
The student will…
SUGGESTED INSTRUCTIONAL STRATEGIES
Learn the anatomical structures in the male and
female human reproductive system.
Learn how the process of gametogenesis
occurs.
Appreciate the need for both sperm and ovum
to be present at the moment of conception.
Illustrate the development of a human from
conception to birth.
Debate the validity of human cloning.
Research the concept of stem cells and how
they can be used to treat disease.
Modeled instruction
PowerPoint presentations and notes
Cooperative grouping
Audio Visual presentations
Research
Human development timeline project
Human cloning debate
SUGGESTED ASSESSMENT METHODS
Assessments evaluated with rubrics
Research project
Final examination
HUMAN BIOLOGY
SCIENCE CURRICULUM 329 GRADES 9 - 12
LEARNING STRAND
Digestive System
CT Standard 10.1 – Fundamental life processes depend on the physical structure and the chemical activities of the cell.
CT Standard: Enrichment High School Biology – As a result o the coordinated structures and functions of organ systems, the internal
environment of the human body remains relatively stable (homeostatic) despite changes in the outside world.
ENDURING UNDERSTANDINGS
The function of the digestive system is to break
down nutrients and deliver them to the blood.
The primary organs of the digestive system
including the stomach, small and large
intestines, and liver.
ESSENTIAL QUESTION
What happens when various digestive system
organs fail to work properly?
INSTRUCTIONAL SUPPORT MATERIALS
Hole’s Essentials of Anatomy and Physiology
David Shier, Jackie Butler, Ricki Lewis (2008)
PowerPoint notes
Laptops
Dissection supplies
Videos dealing with the digestive system.
LEARNING OBJECTIVES
The student will…
SUGGESTED INSTRUCTIONAL STRATEGIES
learn the anatomy and physiology of the
digestive system organs.
learn the pathology of various digestive system
disorders.
Short lecture and diagram on the anatomy and
physiology of the digestive system.
Fetal pig dissection.
Research project on the subject of digestive
system disorders.
Video: ―Evlove – Guts‖
SUGGESTED ASSESSMENT METHODS
Research project on the subject of digestive
system disorders (brochure).
HUMAN BIOLOGY
SCIENCE CURRICULUM 330 GRADES 9 - 12
Cancer Research Project
Introduction:
In this unit we are learning about cells and tissues. Cancer is a disease that starts in the cells,
spreads into tissues, and eventually, if not treated promptly, can lead to death. Cancer affected
12 million people in the year 2007, and is one of the leading causes of death in the United States.
It is, therefore, very important to be exposed to the subject of cancer and learn as much as
possible about how the disease starts, how it progresses, current treatments, cutting edge
treatments, causes and risk factors, etc.
In groups of two (2) or three (3) you will conduct in-depth research on one (1) of the following
topics:
Topic #1: What is cancer?
Explain how cancer starts.
Explain how cancer spreads.
Explain what cancer does that makes it so deadly.
Topic #2: Causes and risk factors associated with cancer.
Select a minimum of four (4) causes and/or risk factors.
Explain how each of the selected causes and/or risk factors is associated with the
onset of cancer.
Describe how people can avoid the selected causes and/or risk factors and how they
can, therefore, reduce their risk of cancer.
Topic #3: Cancer treatments
Current treatments and how they work (radiation, chemotherapy, surgery, etc.).
Cutting edge treatments and how they work (if you can find any).
Future treatments (if you can find any).
You will use the results of your research to construct a PowerPoint presentation. You will then
present your research orally in front of the class using your PowerPoint presentation.
Requirements:
1. Oral Presentation:
o You will prepare a five to ten minute oral presentation on your selected topic.
o You will use PowerPoint to present your research to the class. The PowerPoint slides
should contain written information about your selected topic as well as visuals
(pictures, diagrams, charts, etc.).
o Each member of the group should be involved in an equal part of the presentation.

HUMAN BIOLOGY
SCIENCE CURRICULUM 331 GRADES 9 - 12
2. Written Work:
o You will prepare a written outline of the information that will be presented. This will
be distributed to your classmates before the start of your presentation.
o You will generate a quiz (no less than 10 questions) that will be completed by your
classmates at the conclusion of your presentation. DON'T MAKE THE QUIZ TOO
DIFFICULT! Remember, they are making a quiz for you to take .... you don't want
them to take revenge.
o A list of the references used in the standard MLA reference format.
Materials:
These resources will be available to you for your research:
1. Your text book
2. The Internet
3. Books from the school library (maybe)
4. High school and college-level textbooks
5. Magazines, scientific journals, and/or periodicals.
Some guidelines for proceeding:
• We will go to the library as a class or use the laptop carts to search for information that you
might need. I recommend that you start by reading about cancer in the textbook before
searching for other sources. This may be a rather brief description, but it is a good starting
point to acquaint you with cancer.
• Once you have done some research, brainstorm with your partner(s) about how you might
present your topic to the class. You might need to divide the labor - have each group
member read more about one particular aspect of your selected topic.
How it will be graded:
• Outline - 25 points
• Quiz - 25 points
• Oral Presentation - 50 points
The total points earned for these three items will count as two test grades. Each person in the
group will be graded independently on the oral presentation portion of the project.
Taking the quizzes:
Everyone will take the other groups' quizzes. These will be open notes quizzes and will be taken
immediately following a given group's oral presentation. All of your quiz scores will be
averaged to produce a single test grade. If you do not have time to finish the quiz in class it
can be finished for homework, but must be passed in the next day, or the grade will be a zero.
HUMAN BIOLOGY
SCIENCE CURRICULUM 332 GRADES 9 - 12
Cancer: Oral Presentation Rubric
CATEGORY
4
3
2
1
Content
Shows a full
understanding of the
topic.
Shows a good
understanding of the
topic.
Shows a good
understanding of
parts of the topic.
Does not seem to
understand the topic
very well.
Visual
Presentation
Visual presentation
is very well
organized and
completely supports
the content.
Visual presentation
is organized and
mostly supports the
content.
Visual presentation
is slightly
disorganized, but
mostly supports the
content.
Visual presentation
is disorganized and
does not support the
content.
Preparedness
Student is
completely prepared
and has obviously
rehearsed.
Student seems pretty
prepared but might
have needed a
couple more
rehearsals.
The student is
somewhat prepared,
but it is clear that
rehearsal was
lacking.
Student does not
seem at all prepared
to present.
Volume
Volume is loud
enough to be heard
by all audience
members throughout
the presentation.
Volume is loud
enough to be heard
by all audience
members at least
90% of the time.
Volume is loud
enough to be heard
by all audience
members at least
80% of the time.
Volume often too
soft to be heard by
all audience
members.
Vocabulary
Uses vocabulary
appropriate for the
audience. Extends
audience vocabulary
by defining words
that might be new to
most of the audience.
Uses vocabulary
appropriate for the
audience. Includes
1-2 words that might
be new to most of
the audience, but
does not define
them.
Uses vocabulary
appropriate for the
audience. Does not
include any
vocabulary that
might be new to the
audience.
Uses several (5 or
more) words or
phrases that are not
understood by the
audience.
Speaks Clearly
Speaks clearly and
distinctly all the
time, and
mispronounces no
words.
Speaks clearly and
distinctly most of
the time, but
mispronounces no
words.
Speaks clearly and
distinctly most of the
time. Mispronounces
some words.
Often mumbles or
can not be
understood.
Mispronounces a
number of words.
Time-Limit
Presentation is more
than 5 and less than
10 minutes long.
Presentation is 5
minutes long.
Presentation is 3-4
minutes long.
Presentation is less
than 3 minutes OR
more than 10
minutes.
HUMAN BIOLOGY
SCIENCE CURRICULUM 333 GRADES 9 - 12
Cancer Research Project Benchmark Rubric
#3 Applies effective and efficient strategies for gathering information and materials,
thinking critically, and solving problems.
Exceeds Expectations
The student independently collects, interprets, analyzes, and evaluates
a variety of information and data and makes original insights and
accurate conclusions.
Meets Expectations
The student independently collects, interprets, analyzes, and evaluates
a variety of information and data to make pre-established insights and
accurate conclusions.
Meets Some Expectations
The student may need some assistance collecting, interpreting,
analyzing, and evaluating a variety of information and data to make
pre-established insights and accurate conclusions.
Does Not Meet Expectations
The student needs assistance to gather information and make very
general conclusions based on that information. Errors may be present
in the student’s conclusions.
HUMAN BIOLOGY
SCIENCE CURRICULUM 334 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 335 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Chemistry - Honors
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Chemistry - Honors
3. Transcript Course Code/Number
00341
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 10 – 12 Level: 1
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of Biological Systems with an average of B+ or better or Biology-Honors with a
grade average of C+ or better, as well as completion of Level I or II Algebra I with a B+ or better and
concurrent enrollment in Level I or II Algebra II.
11. Brief Course Description
This course emphasizes chemical principles, which are deduced from experiments performed by the
student. The principles include: energy, rate and equilibrium, characteristics, chemical periodicity, and
chemical bonding in gases, liquids, and solids. The student is required to keep a detailed journal of
his/her laboratory experiments.
12. Course Goals
1. Demonstrate proficiency and fluency in communication to meet the literacy demands of the global
community.
2. Use technology effectively and responsibly.
3. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
4. Demonstrate respect for one's self, and strive to contribute to the success of others.
5. Demonstrate the ability to work on homework and laboratory assignments independently.
6. Demonstrate the ability to meet homework and lab report deadlines.
7. Demonstrate good homework habits by working on new material covered on a nightly basis.
8. Demonstrate the ability to apply chemistry concepts and synthesize connections among concepts.
CHEMISTRY HONORS
SCIENCE CURRICULUM 336 GRADES 9 - 12
13. Course Outline
CHAPTER
*LABORATORY
1) Introduction to Chemistry
Check-in, Safety Training and Safety Contract
2) Matter and Change
Physical and Chemical Properties
3) Scientific Measurement
Density
Reporting Experimental Uncertainty
4) Problem Solving in Chemistry
Changes in Physical State
5) Atomic Structure and the Periodic Table
(Atomic Theory)
Determining the Mass of Extremely
Small Objects
6) Chemical Names and Formulas
Identifying Unknown Halides
7) Chemical Quantities
Moles of Copper and Iron
Determination of the Diameter of the Copper Atom
Empirical Formula Determination
8) Chemical Reactions
(States of Matter & Energy of Chemical Changes)
Observing a Chemical Reaction
Balanced Chemical Equations
Types of Reactions
Single Replacement Reactions
Precipitation Reactions
9) Stoichiometry
Quantitative Study of a Chemical Reaction
10) States of Matter
Cooling Rates of Evaporating Liquids
11) Thermochemistry – Heat and Chemical Change
Heat of Fusion of Ice
Heat of a Chemical Reaction
Specific Heat of a Metal
Energy of Foods
12) The Behavior of Gases
(Gaseous State of Matter)
The Molar Relationship Involving Mass and Volume
Graham’s Law
Charles’ Law
The Weights of Equal Volumes of Gases
13) Electrons in Atoms
Flame Tests
14) Chemical Periodicity
The Periodic Law
15) Ionic Bonding and Ionic Compounds
16) Covalent Bonding
17) Water and Aqueous Systems
Solvent Properties of Water
18) Solutions
(The Chemistry of Solutions)
Properties of Solutions
Factors Affecting Solution Formation
Supersaturation
19) Reaction Rates and Equilibrium
Chemical Equilibrium: Qualitative Aspects
Disturbing Equilibrium: LeChatelier’s Principle
Factors Affecting Reaction Rates
20) Acids and Bases
Characteristic Reactions of Acids
Salt Hydrolysis

CHEMISTRY HONORS
SCIENCE CURRICULUM 337 GRADES 9 - 12
21) Neutralization
Neutralization Reactions
A Solubility Product Constant Buffers
Unknown Acid Titration
22) Oxidation-Reduction Reactions
Redox Titration of Iron
Oxidation-Reduction Reactions
23) Electrochemistry
Measuring the Potential of Electrochemical Cells
* Some experiments may be substituted for, or eliminated, based on availability of needed chemicals/equipment.
14. Instructional Methods and/or Strategies
1. Modeled instruction
2. Powerpoint presentations and notes
3. Laboratory investigations
4. Teacher demonstrations
5. Cooperative grouping
6. Audio visual presentations
7. Response cards by Turning Technologies
8. Web-based instruction with Blackboard/finalsite
9. Research
15. Assessment Methods and/or Tools
Chapter tests
Unit examinations
Final examination
Laboratory reports
Chapter problem sets
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response cards by Turning Technologies
Formative quizzes and test preparation quizzes
Short term projects, such as portfolio mini units, and group or individual presentations
Benchmark assessments include the following laboratory experiments:
The American Chemical Society’s Final Examination will be administered to all students taking honors chemistry.
Observing a Chemical Reaction
Physical and Chemical Properties
Density
Empirical Formula Determination
Types of Reactions
Single Replacement Reactions
Precipitation Reactions
Quantitative Study of a Chemical Reaction
Heat of Fusion of Ice
Specific Heat of a Metal
The Molar Relationship Involving Mass and Volume
Graham’s Law
Flame Tests
The Periodic Law
Solvent Properties of Water
Factors Affecting Solution Formation
Supersaturation
Chemical Equilibrium: Qualitative Aspects
Acid Base Titration
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut Standards and grade level expectations for
science. Authentic assessments are graded using a scoring rubric or grading criteria. Benchmark assignments
are graded using a common scoring rubric or grading criteria.
The technology benchmark used involves the students learning the proper use and functions of various laboratory
equipment including, but not limited to: electronic balances, analytical measuring devices such as burets, pipets,
and electronic thermometers, a wide assortment of computer-based probes and the needed software, which are
used in conjunction with a classroom set of laptop computers.
CHEMISTRY HONORS
SCIENCE CURRICULUM 338 GRADES 9 - 12
The writing benchmark is incorporated through the requirement of students to prepare and submit laboratory
reports. Some instructors accomplish this by requiring students to maintain, and submit for grading, a laboratory
notebook. Others make use of prepare laboratory report forms that accomplish this task. Which ever method is used,
the students must prepare their reports including not only all of the observations and interpretations involved in the
experiment, but also must do so using proper grammar, punctuation, spelling, and sentence structure.
The benchmarks for use of effective strategies for gathering information, critical thinking, and problem solving are
inherent with the subject of science. Starting at the beginning of the course with the Scientific Method, the students
are required to use
ALL
of these skills, on a
DAILY
BASIS
, throughout the course; it is the
ESSENCE OF SCIENCE
.
How Laboratory Reports will be Graded
Unless otherwise stated, this is how your lab report will be graded. Remember your report must reflect your
individual effort to receive credit! Please refer to the information about integrity in the lab report as needed. Each
completed lab report must have the following sections, unless otherwise stated. The sections will be evaluated using
the criteria included below.
Title
Date
Materials and Safety
Procedure
Does the procedure directly address the problem? Is the
procedure written in such a way that it could be easily
reproduced by another person?
Data and Observations
Were the measurements recorded correctly? Are the data
presented clearly and neatly? Are the data meaningful
and reliable?
Calculations
Are the calculations organized clearly? Are the
calculations done correctly, including the use of units and
significant figures?
Graphs (only required for some experiments)
Does the graph include a title? Are the axes labeled and
are units included? Are the data points plotted correctly?
Is the scale appropriate? Is the proper type of graph
used? (line vs. bar) Does the graph include the line of
best fit? Is the graph plotted correctly? (independent
variable vs. dependent variable)
Conclusion
Does the conclusion address the purpose? Does the
conclusion relate to the experimental results? Is there a
discussion of all potential sources of errors?
HONORS COURSES ONLY
17. Indicate how this honors course is different from the standard course.
Honors chemistry covers more material, performs more experiments, utilizes much more math-intensive word
problems, and delves deeper into the theoretical concepts behind the various laws governing chemistry.
Much more responsibility is placed on the student in analyzing experimental results and deducing logical conclusions
based on experimental results.
In addition, the quantity and quality of quantitative problems is also much greater than covered in the standard
course. It is assumed that all honor students have excellent, well-established study skills and good problem-solving
skills.
CHEMISTRY HONORS
SCIENCE CURRICULUM 339 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings and
ideas for critical review by colleagues and other
scientists.
Scientific literacy includes the ability to read, write,
discuss, and present coherent ideas about science.
Scientific literacy includes the ability to search for and
assess the relevance and credibility of scientific
information found in various print and electronic
media.
Scientific numeracy includes the ability to use
universal mathematical operations and procedures to
calculate, analyze and present scientific data and
ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is logically
connected to the problem and the design of the
experiment?
How do you design and conduct appropriate types of
controlled scientific investigations, using the
appropriate tools and techniques, to make observations
and gather data to answer various questions?
How do you assess the data, using mathematical
operations to analyze and interpret data, and present
relationships between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of scientific
claims in different sources of information?
How do you communicate your findings, using relevant
scientific vocabulary and clear logic, which are based
on the results generated during the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…, then…"
format, which is logically connected to the problem.
Design a controlled experiment where the
independent and dependent variables are accurately
identified.
Utilize instrument methodology that is appropriate for
the design of the experiment.
Record data in the appropriate units of measure, and
be able to convert between different units of
measure.
Use mathematical operations to analyze and interpret
data, and present relationships between variables in
appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on the
analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific experiment.
Use dimensional analysis to solve a variety of multi-
step problems.
Understand significant figures.
Chemistry: Principles and Reactions Brooks/Cole Cengage
Learning, 2009
General lab equipment (Balances, glassware, Bunsen
burners, safety goggles, aprons and gloves)
Calculators
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Performance tasks
Textbook ancillary materials
Guided Internet research
Response cards by Turning Technologies
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Teacher observations
Student class participation
Response cards by TurningTechnologies
Tests
Lab reports (design labs, collect data, analyze, draw
conclusions)
Observing a Chemical Reaction
(Benchmark
Assessment)
Physical and Chemical Properties
(Benchmark
Assessment)
Density
(Benchmark Assessment)
Determination of the diameter of a copper atom
CHEMISTRY HONORS
SCIENCE CURRICULUM 340 GRADES 9 - 12
LEARNING STRAND
Atomic Theory
CT Standard: Atomic and Molecular Structure -- The periodic table displays the elements in increasing atomic number and shows
how periodicity of the physical and chemical properties of the elements relates to atomic structure.
ENDURING UNDERSTANDINGS
The understanding of atomic structure was
developed by many scientists and scientific
thinkers over a period of more than 2000 years.
Atomic structure includes a positively charged
nucleus, containing two types of subatomic
particles (neutrons and protons), and an outer
region of negatively charged electrons.
The configuration of atoms and molecules
determines the properties of materials.
Elements exist in nature as isotopes, which
differ in number of neutrons, and atomic mass.
In nuclear fission, matter is transferred directly
into energy in a process that is several million
times more energetic than combustion.
ESSENTIAL QUESTIONS
How can early experiments that led to the
characterization of the nucleus and the discovery
of electrons be explained?
What is the description of the general structure of
the atom?
Why does atomic mass of isotopes vary for an
element?
How would you determine the chart mass of an
element, given isotope data?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe and explain the contribution that
Thomson and Rutherford made to the
development of the atomic theory.
Explain how Bohr’s model differed from its
predecessors.
Use Dalton’s Atomic Theory to define an atom.
Compare the nucleus to the atom, and include
size, mass and charge in the answer.
Explain the quantum model of the atom, in
terms of the relevance of the work of Dalton,
Thomsen, Bohr, Ruhtherford, Millikan.
Describe the mass, charge, and location of the
proton, neutron, and electron.
Understand the relationship and meaning of
atomic number and mass number.
Define isotope.
Describe the general structure of the atom, and
explain how the properties of the first 20
elements are related to their structure.
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
Videos, such as ―History of Atomic Theory‖
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations, with interactive
simulations, and imbedded practice problems
Interactive games, such as ―Its All in the Cards‖’
Lab investigations
Response cards by Turning Technologies
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data, analyze,
conclude)
Beanium
CHEMISTRY HONORS
SCIENCE CURRICULUM 341 GRADES 9 - 12
LEARNING STRAND
The Language of Chemistry
CT Standards: Atomic and Molecular Structure -- The periodic table displays the elements in increasing atomic number and shows
how periodicity of the physical and chemical properties of the elements relates to atomic structure.
Conservation of matter and Stoichiometry -- The conservation of atoms in chemical reactions leads to the principle of conservation of
matter and the ability to calculate the mass of products and reactants.
Chemical Bonds -- Biological, chemical and physical properties of matter result from the ability of atoms to form bonds from electrostatic
forces between electrons and protons and between atoms and molecules.
ENDURING UNDERSTANDINGS
Chemical symbols, formulas, and equations are
understood internationally, and are written
based upon universally accepted guidelines.
The formula of a binary compound can be
determined based on the positions of elements
on the Periodic Table.
The unit of measurement on which chemists rely
to predict quantities in chemical reactions is the
mole.
Chemical equations are presented as balanced
equations to satisfy the law of conservation of
matter.
Chemists make calculations involving quantities
of reactants and products based on balanced
chemical equations and yields.
Emission of combustion by products, such as
SO
2
, CO
2
, and NOx, by industries and vehicles is
a major source of air pollution.
During the burning of fossil fuels, stored
chemical energy is converted to useable energy.
ESSENTIAL QUESTIONS
What are the descriptions for how atoms combine
to form new substances by transferring electrons
(ionic compounds) or by sharing electrons
(molecular compounds)?
How does the Periodic Table provide for the
charge of a metal or a nonmetal, in an ionic
compound?
How can conversions be made among particles,
mass, and moles of any substance?
What is the difference between empirical and
molecular formula? What is the significance of
empirical formula in chemical analysis?
How can predictions be made about which
reactions will occur and what their products will
be?
How can you relate between different quantities in
a balanced chemical reaction?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Chemical Names and Formulas
Differentiate between ionic compounds and
molecular compounds, and between molecules
and formula units.
Explain that chemical bonds between atoms in
molecules such as H2, CH4, NH3, H2CCH2, N2,
CL2, and many large biological molecules are
covalent.
Write the chemical formula of binary or ternary
ionic compounds, molecular compounds, or
acids, given the name, and vice versa.
Describe how atoms combine to form new
substances by transferring electrons (ionic
bonding) or sharing electrons (covalent
bonding).
The Mole
Define Avogadro’s number as one mole equals
6.02 x 10 23 particles (atoms or molecules).
Define gram atomic mass, gram molecular mass,
gram formula mass, and molar mass from the
chemical formula and a table of atomic masses.
Relate mass, moles, volume, and number of
particles for a given amount of a substance, at
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
General lab equipment
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations with imbedded practice
problems
Modeling of concepts, followed by in class practice
worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Executing laboratory experiments
Empirical Formula Determination (Benchmark
Assessment)
Types of Reactions (Benchmark Assessment)
Precipitation Reactions (Benchmark Assessment)
Quantitative Study of a Reaction (Benchmark
Assessment)
Single Replacement Reactions (Benchmark
Assessment)
Balanced Chemical Reactions
CHEMISTRY HONORS
SCIENCE CURRICULUM 342 GRADES 9 - 12
standard temperature and pressure.
Relate the mole concept to the density of a gas.
Use percent composition to determine empirical
and molecular formulas.
Show that different compounds composed of the
same two elements obey the Law of Multiple
Proportions, using experimental data.
Show that different samples of the same
compound obey the Law of Definite Proportions,
using experimental data.
Chemical Equations
Identify reactants and products in a chemical
equation.
Understand that chemical reactions can be
described by writing balanced equations.
Use appropriate symbols to write an equation to
accurately describe a chemical reaction.
Classify a reaction as combination, single or
double replacement, decomposition, or
combustion.
Predict the products of each of these reaction
types, using appropriate references, such as the
activity series, or solubility rules.
Describe combustion reactions of hydrocarbons
and their resulting by-products.
Stoichiometry
Interpret a chemical equation for mole
relationships of mass, particles, or volume.
Define and apply the concepts of theoretical,
actual, and percent yield.
Explain how the release of sulfur dioxide into the
atmosphere can form acid rain, and how acid
rain affects water sources, organisms, and
human made structures.
Describe the availability, current uses, and
environmental issues related to the use of fossil
and nuclear fuels to produce electricity.
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework Quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data, analyze,
draw conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 343 GRADES 9 - 12
LEARNING STRAND
The States of Matter, and the Energy of Chemical Changes
CT Standards: Chemical Bonds -- Biological, chemical and physical properties of matter result from the ability of atoms to form
bonds from electrostatic forces between electrons and protons and between atoms and molecules.
Conservation of Matter and Stoichiometry -- The conservation of atoms in chemical reactions leads to the principle of conservation
of matter and the ability to calculate the mass of products and reactants.
ENDURING UNDERSTANDINGS
Differences in the general characteristics of the
phases of matter are related to particle energy
and the presence, or absence, of attractive or
repulsive forces between particles.
Differences in the general properties of different
substances are related to intermolecular forces
of attraction for liquid and gas phases, and the
structure in which particles are held together in
the solid phase.
Energy cannot be created or destroyed;
however, energy can be converted from one
form to another.
All chemical reactions either produce energy
(are exothermic) or absorb energy (are
endothermic), as a result of the breaking and
making of chemical bonds.
The spontaneity of a chemical reaction is a
function of the relationship between the
changes in temperature, entropy, and enthalpy.
Various forms of energy are used by humans,
and all have advantages or disadvantages.
ESSENTIAL QUESTIONS
How can you summarize the differences in the
particle arrangement and average particle
energy among particles in the solid, liquid, or
gaseous form of a substance?
How do intermolecular forces of attraction
affect the vapor pressure, boiling point, and
surface characteristics of a liquid?
What is the explanation for the components of
Gibb’s free energy equation, and the relevance
of the relevance is to spontaneity, in terms of
endergonic and exergonic reactions?
How is the enthalpy of a reaction calculated
including definitions of exothermic and
endothermic reactions?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
States of Matter
Explain the significance of absolute zero, giving
its value in degrees Celsius and Kelvin, and
relate the average kinetic energy of the
particles of a substance to temperature.
Name and characterize the three states of
matter.
Describe the motion of particles of a gas,
according to the Kinetic Theory.
Explain that the atoms and molecules in liquids
move in a random pattern, relative to one
another because the intermolecular forces are
too weak to hold the atoms or molecules in
solid form.
Describe the effects of adding energy to matter
in terms of the motion of atoms and molecules,
and the resulting phase changes.
Describe the nature of a liquid in terms of the
attractive forces between particles.
Explain why a liquid has a vapor pressure, and
why a change in temperature causes a change
in vapor pressure.
Explain the significance of the unit cell to the
shape of a crystal.
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
Vacuum pump apparatus for demonstrations
Laptop computers with Loggerpro software for
Energy of Foods / Energy of Fuels labs
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded
practice problems.
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Execute selections from suggested labs:
-Cooling Rates of Evaporating Liquids
-Heat of Fusion of Ice (Benchmark Assessment)
-Heat of a Chemical Reaction
-Specific Heat of a Metal (Benchmark Assessment)
-Energy of Foods or Energy of Fuels
Demonstrations of:
-Phase change under vacuum
-Comparative cooling rates
SUGGESTED ASSESSMENT METHODS
CHEMISTRY HONORS
SCIENCE CURRICULUM 344 GRADES 9 - 12
Differentiate between different crystal systems
and different unit cells within a crystal system.
Explain how different intermolecular forces of
attraction, such as hydrogen bonds, Van der
Waals forces, or dispersion forces affect the
vapor pressure, boiling and melting points,
volatility, and surface tension of the substance.
Thermochemistry
Find the mass, heat change, temperature
change, or specific heat, when any three of
these values are given.
Use Hess’s Law to calculate the enthalpy
change in a chemical reaction.
Use Gibb’s Free Energy equation to determine
spontaneity of a chemical reaction.
Relate changes in entropy to changes of state,
changes in temperature, and a change in the
number of product particles, compared to the
number of reactant particles.
Use standard entropies to calculate the change
in entropy of a reaction.
Characterize the spontaneous and
nonspontaneous reactions.
Distinguish between exergonic and endergonic
reactions.
Define enthalpy and determine the energy
changes that occur in a reaction.
Describe network solids and describe their
properties.
In class worksheets (collaborative)
Homework (individual)
Homework Quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data, analyze,
draw conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 345 GRADES 9 - 12
LEARNING STRAND
The Gaseous State of Matter
CT Standards: Chemical Bonds -- Biological, chemical and physical properties of matter result from the ability of atoms to form
bonds from electrostatic forces between electrons and protons and between atoms and molecules.
Conservation of Matter and Stoichiometry -- The conservation of atoms in chemical reactions leads to the principle of conservation
of matter and the ability to calculate the mass of products and reactants.
ENDURING UNDERSTANDINGS
The general behavior of a substance in the
gaseous state can be described by the Kinetic
Molecular Theory, which is based on
observation.
The average kinetic energy of particles of a
substance in the gaseous state is related to
the temperature of the substance in the
Kelvin (absolute) scale.
Particles of different substances will effuse at
different rates, depending on the size (mass)
of the particles.
The specific response to a change in
pressure, temperature, and /or volume of a
substance in the gaseous state can be
predicted by the combined gas law or one of
its corollaries, the Independent (Boyle’s,
Charles’, and Gay-Lussac’) Gas Laws.
A gas in a mixture will behave the same and
exert the same pressure as it would alone.
The Ideal Gas Law is an empirical relationship
that relates pressure, volume, temperature,
and amount of a substance in the gaseous
state of matter.
The concept of an ideal gas is hypothetical,
and an ideal gas does not actually exist; but
under most temperature and pressure
conditions, most gases behave ideally.
ESSENTIAL QUESTIONS
How does the Kinetic Molecular Theory predict
the relationship between particles of a gas, and in
turn, the behavior of a gas?
How is Graham’s Law related to the average
kinetic energy of particles of a gas?
What are the factors that determine which gas
laws are used to solve word problems?
What label (s) is (are) appropriate for each
parameter for each gas law?
Under what conditions will a real gas deviate from
an ideal gas (or, the Ideal Gas Law)?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Use Kinetic Theory to explain gas pressure.
State the value of standard temperature and
pressure.
State and explain the significance of
Avogadro’s hypothesis.
Predict the response of a gas to changes in
pressure, volume, or temperature, using the
appropriate gas law.
Understand and apply Dalton’s law of partial
pressures.
Understand Avogadro’s hypothesis as it
applies to gases.
Use the combined gas law to predict changes
in temperature, pressure, or volume.
Understand and use the Ideal Gas Law to
solve word problems.
Understand that the Ideal Gas Law is an
empirical relationship that relates pressure,
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
Glass tubes for Graham’s Law Experiment
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded practice
problems
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Execute selections from suggested labs:
-The Molar Relationship Involving Mass and
Volume (Benchmark Assessment)
-Graham’s Law (Benchmark Assessment)
-Charles’ Law
-The Weights of Equal Volumes of Gases
CHEMISTRY HONORS
SCIENCE CURRICULUM 346 GRADES 9 - 12
volume, temperature, and amount of a
substance in the gaseous state of matter.
Explain that the concept of an ideal gas is
hypothetical, and an ideal gas does not
actually exist; but under most temperature
and pressure conditions, most gases behave
ideally.
Describe how human efforts to reduce
consumption of raw materials can improve air
quality.
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework Quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data, analyze,
draw conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 347 GRADES 9 - 12
LEARNING STRAND
Electrons, The Periodic Law and Chemical Bonds
CT Standards: Atomic and Molecular Structure -- The periodic table displays the elements in increasing atomic number and
shows how periodicity of the physical and chemical properties of the elements relates to atomic structure.
Chemical Bonds -- Biological, chemical, and physical properties of matter result from the ability of atoms to form bonds from
electrostatic forces between electrons, protons, and between atoms and molecules.
ENDURING UNDERSTANDINGS
Every element has a unique electron
configuration, which is based on the structure
of the principal energy levels, energy sublevels,
and orbitals.
The electron configuration of any element is
indicated by its placement on the Periodic Table
of the Elements.
The Periodic Table is arranged in order of
increasing atomic number and grouped by
similar properties, but there are several
additional trends that progress through each
period (row) or each family of elements
(column) on the Table.
The electromagnetic spectrum is the full range
of wavelengths of radiation from the sun, and it
includes visible light, heat energy, sound waves,
and many other types of energy (microwave,
radar, X-ray, and gamma radiation).
The tendencies for metals to form cations and
for nonmetals to form anions are related to
position on the Periodic Table and electron
configuration.
The drive for atoms to form bonds is based on
the stability of the noble gases and the octet
rule.
The manner in which elements combine to form
compounds and the resulting molecular
geometries have a profound effect on the
properties exhibited by the substance.
ESSENTIAL QUESTIONS
What is electron configuration in terms of an
element’s location on the periodic table?
What is the likelihood of electron configuration
forming bonds?
What type of bonds will be formed from electron
configuration?
What are the trends of physical and chemical
properties along periods or in groups on the
Periodic Table?
What are the different groups of the periodic
table?
What is the significance of orientation and layout
of the Periodic Table?
What is the character of the ionic bonding
process?
What is the character of the molecular bonding
process?
How can the shape, bond angles and polarity be
predicted by using VSEPR theory?
How can the Molecular Bonding theory be
summarized?
What is the importance of sigma, pi, and
bonding and antibonding orbitals?
What is a description of metallic bonding?
How does metallic bonding structure affect the
properties of a metal?
What is a description of network covalent
bonding?
How does network covalent bonding structure
affect the properties of an ionic compound?
What is a description of ionic bonding, with
respect to crystal structure?
How does crystal structure affect the properties
of an ionic compound?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Electron Configuration
Describe the general shape of s, p, d, and f
orbitals.
Distinguish among principal energy level, energy
sublevel, and atomic orbital.
Use the Aufbau Principal, the Pauli Exclusion
Principal, and Hund’s Rule to write the electron
configuration of the elements.
Explain the importance of quantized energies of
electrons.
Identify the electron configuration of any element
based on its position on the Periodic Table.
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
Discharge tubes and power supplies
Spectrophotometers
Three dimensional molecular model kits
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations with imbedded
practice problems.
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions

CHEMISTRY HONORS
SCIENCE CURRICULUM 348 GRADES 9 - 12
Calculate the frequency and wavelength of light.
Use the quantum theory to explain the
photoelectric effect.
The Periodic Law (Periodicity)
Identify trends in ionization energy, electro
negativity, and the relative sizes of atom and ions.
State the Periodic Law.
Identify the different parts of the Periodic Table:
metals, nonmetals, metalloids, representative
elements, noble gases, transition metals, and rare
earth metals.
Identify the number of electrons available for
bonding from the position on the Periodic Table.
Explain why the electron configuration for
chromium and copper are different than those
assigned from the Aufbau filling diagram.
Explain how electronegativity and ionization
energy are related to bond type and formation.
Ionic Bonding
Use Lewis dot structures to provide models of
atoms, ions and molecules.
State the importance of the Noble Gas electron
configuration in the formation of ions.
State and apply the octet rule.
Describe the formation of an anion form an atom
of a nonmetallic element.
Describe the formation of a cation from an atom
of a metallic element.
Molecular Bonding
Predict the shape of simple molecules and their
polarity from Lewis dot structures.
Explain how the structure of the carbon atom
affects the type of bonds it forms in organic and
inorganic molecules.
Define single, double, and triple bonds.
Define and recognize polarity.
Define and give the characteristics of an ionic
bond, covalent bond, polar covalent bond, and
coordinate covalent bond.
Describe the VSEPR theory and the relevance to
molecular shape, specifically bond angles.
Describe the Molecular Orbital theory with respect
to pi and sigma orbitals, and bonding and
antibonding orbitals.
Describe orbital hybridization.
Explain and give an example of resonance.
Use the theory of metallic bonding to explain the
physical properties of metals.
Define bond dissociation energy.
Explain the electrical conductivity of melted and
aqueous solutions of ionic compounds.
Understand how electronegativity relates to
bonding.
Response cards by Turning Technologies
Use of three dimensional molecular models for
demonstration and also in student activities and
labs
Use of discharge tubes to illustrate atomic
emission spectra
Lab investigations should include:
-The Periodic Law (Benchmark Assessment)
-Flame Tests (Benchmark Assessment)
-Electron configuration
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw
conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 349 GRADES 9 - 12
LEARNING STRAND
The Chemistry of Solutions
Big Idea: Everything is made of matter.
ENDURING UNDERSTANDINGS
The structure of the water molecule and the presence of
hydrogen bonds in water are responsible for unique
chemical and physical properties, which dictate many
aspects of our world.
The use of the natural resource water by human
populations can affect the quality of our environment.
The significance of polarity, electrolytes and physical
conditions, such as temperature, particle size and
agitation, affect solution formation and the nature of the
solvation process.
The relative amounts of solute and solvent in a solution
are described by Molarity, Molality, mass and volume
percent.
Colligative properties depend on the amount of solute
particles present, and include boiling point elevation,
freezing point lowering, and vapor pressure lowering.
ESSENTIAL QUESTIONS
How does the accumulation of metal and
nonmetal ions used to increase agricultural
productivity become a significant source of water
pollution?
What is hydrogen bonding?
How does hydrogen bonding affect the
properties of liquids in general, and water
specifically?
What are the physical conditions that affect
solution formation, and what is the effect of each
physical condition?
What are definitions for the five terms for
concentration?
How is each term calculated?
What is the reason for the change in boiling
point, freezing point, or vapor pressure of a
solvent, to which has been added a nonvolatile
solute?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe how human efforts to reduce consumption of
raw materials can improve water quality.
Explain how the accumulation of mercury, phosphates,
and nitrates affects the quality of water and the
organisms that live in rivers, lakes, and the ocean.
Describe the hydrogen bonding that occurs in water, on
the bases of the structure of the polar water molecule,
and electronegativity.
Use the concept of hydrogen bonding to explain the high
surface tension, high boiling point, high specific heat,
and high heat of vaporization of water.
Define the terms; solution, aqueous solution, solute and
solvent.
List and explain the factors that affect the rate of
dissolving.
Understand how the concentration of a solution may be
quantitatively described.
Subjectively describe the strengths of a solution.
Use the rule of ―like dissolves like‖ to predict the
solubility of one substance in another.
Characterize colloids and suspensions and explain how
they differ from solutions.
Describe the procedure for preparing a dilute solution of
known concentration from a more concentrated solution.
Distinguish among weak electrolytes, strong electrolytes
and nonelectrolytes.
Define and describe colligative properties.
Use the concept of colligative properties to predict
boiling points or freezing points of given solutions.
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations with imbedded
practice problems
Modeling of concepts, followed by In class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Lab investigations should include:
-Solvent Properties of Water (Benchmark
Assessment)
-Properties of Solutions
-Factors Affecting Solution Formation
(Benchmark Assessment)
-Supersaturation (Benchmark Assessment)
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework Quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw
conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 350 GRADES 9 - 12
LEARNING STRAND
Reaction Rates and Equilibrium
CT Standard: Reaction Rates -- Chemical reaction rates depend on factors that influence the frequency of collision of reactant
molecules.
ENDURING UNDERSTANDINGS
Rates of chemical reactions are determined by
collisions between reacting particles.
Reaction rates depend on such factors as
concentration, temperature and pressure.
All reactions are reversible, and their
equilibrium position can be described by the
equilibrium constant.
LeChatelier’s principle predicts the response of a
system at equilibrium to a change in
concentration, pressure, volume, or
temperature.
The rate of a chemical reaction is dependant on
the kinetic order of the reaction, and the rate
constant, which in turn is dependant on the rate
determining step.
ESSENTIAL QUESTIONS
How does collision theory explain the process of a
chemical reaction?
What is the underlying concept that summarizes
LeChatelier’s Principle?
What is meant by the order of a reaction?
What is the rate constant?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Use the collision theory to explain how the rate
of a chemical reaction is influenced by the
temperature, concentration, particle size of
reactants, and catalysts.
Define chemical equilibrium in terms of equal
rate of forward and reverse reactions in a
reversible chemical reaction.
Relate the concept of the activated complex and
activation energy to the rate of a reaction.
Write the rate law for a chemical reaction, given
the order of each reactant.
Using LeChatelier’s principle, predict the
direction in which equilibrium will shift in
response to a change in concentration,
pressure, volume, or temperature.
Discuss the reaction mechanism for a chemical
reaction, given the potential energy diagram for
the reaction.
Explain that catalysts increase reaction rate by
lowering the activation energy of a chemical
reaction.
Describe that the rate of a reaction is the
increase in concentration of products over time,
and the decrease in concentration of reactants
over time.
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations with imbedded practice
problems
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Lab investigations should include:
-Qualitative Aspects of Chemical Equilibrium
(Benchmark Assessment)
-Disturbing Equilibrium, LeChatelier’s Principle
-Factors Affecting Reaction Rates
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework Quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw
conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 351 GRADES 9 - 12
LEARNING STRAND
Acids and Bases
Big Idea: Acids and bases can be defined in terms of hydrogen ions and hydroxide ions or in terms of electron pairs.
ENDURING UNDERSTANDINGS
General properties of acids and bases include taste,
ability to react with each other in a neutralization
reaction, and ability to change the color of various
indicators.
Acids and bases are electrolytes.
Acids dissociate to produce hydronium ions, and some
bases dissociate to produce hydroxide ions.
Water also dissociates into hydronium and hydroxide
ions.
The pH scale is related to the concentration of
hydronium ions logarithmically goes from 0 to 14, and
is an indication of acidity of a solution.
The pOH scale is inversely related to pH.
Acids and bases can be characterized as Lewis,
Arrhenius, or Bronsted-Lowry systems, based on
structural and behavioral characteristics of the
compounds.
The strength of acids and bases is determined by
ionization and can be measured quantitatively by Ka or
Kb.
Buffers are solutions that resist changes in pH when
limited amounts of acid or base are added.
ESSENTIAL QUESTIONS
What are the general properties of acids and bases
commonly found in households?
How can self ionization of water be explained?
How are pH and [H] related?
How are pOH and pH related?
How are pOH and [OH] related?
What is the difference between a strong acid (or
base) and a concentrated acid (or base)?
What is the chemical composition of acids?
What is the chemical composition of bases?
How does pH change in neutralization reactions?
LEARNING OBJECTIVES The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Define the general properties of acids and bases.
Define the self ionization of water.
Use the concentration of H+ OR OH- to identify a
solution as acidic or basic.
Describe acids and bases according to the three major
acid-base theories: Arrhenius, Bronsted-Lowry, and
Lewis.
Identify the conjugate acid - base pairs of the
Bronsted-Lowry Theory.
Classify substances as acids, bases, or neutral based on
the three acid base theories.
Calculate the gram equivalent mass of any acid or
base.
Perform stoichiometric calculations involving acid –
base reactions.
Calculate Ka or Kb from concentration or pH
measurements.
Identify monoprotic, diprotic, and triprotic acids.
Explain the concept of titration, and include the
relevance of the different weak / strong acid and base
combinations.
Relate among pH, pOH, [H], and [OH], given log tables
and a calculator.
Explain how a buffer system works using equations.
Use the concept of hydrolysis to explain why solutions
of some salts are acidic or basic.
Use LeChatelier’s principle to explain the common ion
effect.
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations with imbedded practice
problems.
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Lab investigations should include:
-Characteristic Reactions of Acids
-Salt Hydrolysis
-Neutralization Reactions
-A Solubility Product Constant
-Buffers
-Acid Titration (Benchmark Assessment)
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw conclusions)
CHEMISTRY HONORS
SCIENCE CURRICULUM 352 GRADES 9 - 12
LEARNING STRAND
Electrochemistry
Big Idea: Chemical energy can be converted to electric energy and electric energy to chemical energy.
ENDURING UNDERSTANDINGS
An oxidation process is one in which oxygen is gained by
a species, the oxidation number of the species is
increased, and electrons are lost by the species.
A reduction process is one in which oxygen is given up
or lost by a species, the oxidation number of the species
is decreased, and electrons are gained by the species.
If oxidation occurs, then reduction must also occur, and
this is called redox (reduction in combination with
oxidation).
There are two methods for balancing redox equation.
One method is based on keeping track of electrons lost
or gained in two half reactions; the other method is
based on determining oxidation number of all involved
species and adding coefficients as needed until balance
is achieved.
Redox reactions that are spontaneous can be used to
generate electrical energy in electrochemical cells.
In electrolytic cells, electrical energy is used to bring
about desired redox reactions.
Any cell involving a redox reaction will include an anode,
a cathode, and a bridge, or salt bridge.
ESSENTIAL QUESTIONS
What is the definition for oxidation and reduction?
Why do both the processes of oxidation and
reduction always occur together?
How is a redox reaction balanced?
What are the basic components of a voltaic cell, an
electrolytic cell, and an electrochemical cell?
How do voltaic cells work?
How do electrolytic cells work?
How do electrochemical cells work?
What are the characteristics of voltaic, electrolytic
and electrochemical cells?
What are the key differences among voltaic,
electrolytic and electrochemical cells?
INSTRUCTIONAL SUPPORT MATERIALS
Chemistry: Principles and Reactions Brooks/Cole
Cengage Learning, 2009
SUGGESTED INSTRUCTIONAL STRATEGIES
Power point presentations with imbedded practice
problems
Modeling of concepts, followed by in-class practice
worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Lab investigations should include:
-Redox Titration of iron
-Oxidation-Reduction Reactions
-Measuring Potential of Electrochemical Cells
LEARNING OBJECTIVES
The student will…
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw conclusions)
Describe the nature of electrochemical processes.
Define oxidation, reduction, oxidizing agents and reducing
agents.
Determine the oxidation number of a given atom in a
chemical equation and determine the number of electrons
lost or gained.
Balance Redox reactions.
Sketch a voltaic cell, labeling the cathode, the anode and
the direction of flow of electrons.
Identify the half cell in which oxidation takes place and the
half cell in which reduction takes place.
Identify the substance being oxidized and the substance
being reduced in a dry cell.
Define cell potential and describe how it is determined.
Define the standard electrode potential of an electrode.
Use standard electrode potentials to calculate the standard
electromotive force of a cell.
Distinguish between electrolytic and voltaic cells.
CHEMISTRY HONORS
SCIENCE CURRICULUM 353 GRADES 9 - 12
Honors Chemistry - Sample Benchmark
Students will solve word problems involving following concepts:
o dimensional analysis
o heat changes in chemical and physical processes
o gas laws problems involving temperature, volume, and the pressure of a
o contained gas
o equilibrium constant expressions, entropy and free-energy changes that
o accompany physical and chemical processes, and rate laws for simple
o chemical reactions
o oxidation-reduction reactions
(Use effective strategies for gathering information, critical thinking, problem
solving)
Example: Use the experimental data below to answer the following questions.
3A + B C
Trial
[A] in mol/L
[B] in mol/L
Initial rate (mol/Ls)
1
0.20
0.20
0.292
2
0.40
0.20
0.584
3
0.20
0.40
0.146
a) Derive the rate equation for this reaction. Include a numerical value for k, p and q. (8 points)
Grading rubric: Incorrect value for p and q -3 pts each
Correct values for p and q but k is wrong -3 pts
Incorrect units for k -1 pt
b) Calculate the rate the reaction when 3 moles of substance A and 5 moles of substance B are placed in a 10 liter
flask. (6 points)
Grading rubric: Incorrect value for concentration of A or B -3 pts each
A and B are correct but rate is wrong -3 pts
wrong units -1 pt
CHEMISTRY HONORS
SCIENCE CURRICULUM 354 GRADES 9 - 12
Benchmark
Trends In Electronic Configuration
1B, 3
Students will interpret trends in electronic configuration as they relate to physical and chemical
properties by answering questions such as free-response question* below:
―Suppose that a stable element with atomic number 119, symbol Q, has been discovered.
a) Write the ground-state electron configuration for Q, showing only the valence-shell electrons.
b) Would Q be a metal or a nonmetal? Explain in terms of electron configuration.
c) On the basis of periodic trends, would Q have the largest atomic radius in its group or would
it have the smallest? Explain in terms of electronic structure.
d) What would be the likely charge of the Q ion in stable ionic compounds?
e) Write a balanced equation that would represent the reaction of Q with water.
f) Assume that Q reacts to form a carbonate compound.
i) Write the formula for the compound formed between Q and the carbonate ion, CO
3
-1
ii) Predict whether or not the compound would be soluble in water. Explain your
reasoning.‖
(Use effective strategies for gathering information, critical thinking, problem solving)
(Write effectively)
*from the AP Chemistry 2006 Free-Response Questions‖
Exceeds
Expectations
The student independently collects, interprets, analyzes, and evaluates a variety of
information and data to make original predictions or solve problems. S/he solves problems
accurately and efficiently.
The student understands not only the objective but also the implications of assignments.
S/he writes with a clear focus or thesis. Supporting details are well developed and
organized, showing both analysis and synthesis of ideas. Word choice and syntax are
accurate and appropriate. The student shows mastery in the conventions of Standard
English.
Meets
Expectations
The student independently collects, interprets, analyzes, and evaluates a variety of
information and data to make specific predictions or solve problems. S/he solves problems
with few errors. The student understands the objective of assignments and selects an
appropriate mode of written expression with a focus or thesis. Supporting details show an
understanding of the subject matter and an analysis of ideas. They are somewhat
developed and organized. Word choice and syntax are accurate and appropriate. Errors in
the conventions of Standard English are few. The student completes most parts of the
writing process, including evaluation.
Meets Some
Expectations
The student may need some assistance to collect and interpret a variety of data to make
general predictions or solve problems. S/he solves problems with some errors.
The student requires some additional explanations and models in order to understand the
objective of assignments or to complete the writing process. With direction, s/he selects an
appropriate mode. Writing has a somewhat limited focus or thesis, and supporting ideas
may be inaccurate, simplistic, and/or confused. The student may require assistance to
develop or organize his response. Word choice and syntax are consistent with grade level.
There are some errors in the conventions of Standard English.
Does Not Meet
Expectations
The student needs assistance to gather information to make a prediction or solve problems.
S/he solves problems with inefficiently and with significant errors. The student
misinterprets significant elements of writing assignments, selecting an inappropriate mode
or using it incorrectly. The student requires many additional explanations, models, graphic
organizers, and/or strategies in order to complete parts of the writing process. The writing
has no clear focus or a very limited thesis. Ideas and concepts are often unorganized or
inaccurate. Inaccurate or limited vocabulary, syntax errors, and errors in the conventions of
writing make the writing ineffective.
CHEMISTRY HONORS
SCIENCE CURRICULUM 355 GRADES 9 - 12
Benchmark
3 Use effective strategies for gathering information, critical thinking, problem solving)
Students will solve word problems which involve the following concepts:
dimensional analysis
stoichiometry
heat changes in chemical and physical processes
gas laws problems involving temperature, volume, and the pressure of a contained gas
equilibrium constant expressions, entropy and free-energy changes that accompany physical
and chemical processes, and rate laws for simple chemical reactions
Exceeds Expectations
The student independently collects, interprets, analyzes, and evaluates a variety of
information and data to make original predictions or solve problems with a clear
approach (i.e. set up). S/he solves problems accurately and efficiently. All values
are correctly and fully labeled. Scientific notation is correct, and the approach, or
set up, is clearly defined.
Meets Expectations
The student independently collects, interprets, analyzes, and evaluates a variety of
information and data to make specific predictions or solve problems. S/he solves
problems with few errors, and with a clear approach, or set up. Most values are
correctly or fully labeled. Scientific notation is well managed.
Meets Some
Expectations
The student may need some assistance to collect and interpret a variety of data to
make general predictions or solve problems. S/he solves problems with some
errors. Approach, or solution set up is incomplete. Labels are missing or incorrect.
S/he has difficulty managing scientific notation, and solution is not fully set up.
Does Not Meet
Expectations
The student needs assistance to gather information to make a prediction or solve
problems. S/he solves problems with inefficiently and with significant errors.
Solutions are non-sequential, labels are not used, or not used correctly.
Management of scientific notation is incorrect. Solution set up is incomplete, or
missing.
CHEMISTRY HONORS
SCIENCE CURRICULUM 356 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 357 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Chemistry
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Chemistry
3. Transcript Course Code/Number
00342
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 10 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: _1.25
(two trimester + 30 days)
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of Biological Systems or Biology - Honors, as well as Algebra I (Level 1 or level 2)
with a grade average of C+ or better. Students must have completed or be concurrently enrolled in
Geometry.
11. Brief Course Description
Level II Chemistry deals with the properties of matter, changes in its composition, and practical
applications of these changes. Topics are presented in a less rigorous manner than in Chemistry-Honors.
Students are required to read scientific materials, perform laboratory experiments and write laboratory
reports.
12. Course Goals
1. Demonstrate proficiency and fluency in communication to meet the literacy demands of the global community.
2. Use technology effectively and responsibly.
3. Apply effective and efficient strategies for gathering information and materials, thinking critically and solving
problems.
4. Demonstrate respect for one's self, and strive to contribute to the success of others.
5. Demonstrate the ability to work on homework and laboratory assignments independently.
6. Demonstrate the ability to meet homework and lab report deadlines.
7. Demonstrate good homework habits by working on new material covered on a nightly basis.
8. Demonstrates the ability to apply chemistry concepts and synthesize connections among concepts.
CHEMISTRY
SCIENCE CURRICULUM 358 GRADES 9 - 12
13. Course Outline
CHAPTER
**LABORATORY
1) Introduction to Chemistry
Check-in, Safety Training and Safety Contract
2) Matter and Change (Matter, Change & Atomic Theory)
Physical & Chemical Properties
3) Scientific Measurement
Density
Reporting Experimental Uncertainty
4) Problem Solving in Chemistry
Changes in Physical State
5) Atomic Structure and the Periodic Table
Determining the Mass of Extremely
Small Objects
6) Chemical Names and Formulas (Language of Chemistry)
Identifying Unknowns
7) Chemical Quantities
Moles of Copper and Iron
Empirical Formula Determination
8) Chemical Reactions
Observing a Chemical Reaction
Types of Reactions
Precipitation Reactions
9) Stoichiometry
Quantitative Study of a Chemical
Reaction
10) States of Matter
Cooling Rates of Evaporating Liquids
*11) Thermochemistry – Heat and Chemical Change
Heat of a Chemical Reaction
12) The Behavior of Gases
(The Gaseous State of Matter)
The Molar Relationship Involving Mass and Volume
Graham’s Law
13) Electrons in Atoms
(Electrons, The Periodic Law & Chemical Bonds)
Flame Tests
14) Chemical Periodicity
The Periodic Law
15) Ionic Bonding and Ionic Compounds
16) Covalent Bonding
17) Water and Aqueous Systems
Solvent Properties of Water
18) Solutions
(The Chemistry of Solutions)
Properties of Solutions
Factors Affecting Solution Formation
*19) Reaction Rates and Equilibrium
Chemical Equilibrium: Qualitative Aspects
*20) Acids and Bases
Characteristic Reactions of Acids
* Chapters will be studied based on demonstrated student ability.
** Some experiments may be substituted for, or eliminated, based on availability of chemicals/equipment.
14. Instructional Methods and/or Strategies
1. Modeled instruction
2. PowerPoint presentations and notes
3. Laboratory investigations
4. Teacher demonstrations
5. Cooperative grouping
6. Audio visual presentations
7. Response cards by Turning Technologies
8. Web-based instruction with Blackboard/finalsite
9. Research
CHEMISTRY
SCIENCE CURRICULUM 359 GRADES 9 - 12
15. Assessment Methods and/or Tools
Chapter tests
Unit examinations
Final examinations
Laboratory reports
Chapter problem sets
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response cards by Turning Technologies
Formative quizzes and test preparation quizzes
Short term projects, such as portfolio mini units, and group or individual presentations
The American Chemical Society’s Final Examination will be administered to all students taking Level II Chemistry.
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut Standards and grade level expectations for
science. Authentic assessments are graded using a scoring rubric or grading criteria. Benchmark assignments are
graded using a common scoring rubric or grading criteria.
1. Trimester grades are based on chapter test average, homework average, laboratory report
average, unit test averages and quiz averages
2. Trimester examination averages are weighted in at the conclusion of each trimester.
CHEMISTRY
SCIENCE CURRICULUM 360 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific Inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas about
science.
Scientific literacy includes the ability to search for
and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and procedures to
calculate, analyze and present scientific data and
ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated during
the experiment?
LEARNING OBJECTIVES
The student will…
Formulate a testable hypothesis, in the "If…, then…"
format, which is logically connected to the problem.
Design a controlled experiment where the
independent and dependent variables are accurately
identified.
Utilize instrument methodology that is appropriate
for the design of the experiment.
Record data in the appropriate units of measure,
and be able to convert between different units of
measure.
Use mathematical operations to analyze and
interpret data, and present relationships between
variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on the
analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific experiment.
Use dimensional analysis to solve a variety of
multistep problems.
Understand Significant figures.
Convert measurements within the metric system.
Distinguish between qualitative and quantitative
measurements.
Define accuracy and precision.
INSTRUCTIONAL SUPPORT MATERIALS
Chemistry Prentice Hall, 2002
General Lab equipment (Balances, glassware,
Bunsen burners, safety goggles, aprons and
gloves)
Calculators
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research
Response cards by Turning Technologies
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Tests
Lab reports (design labs, collect data, analyze,
draw conclusions)
Observing a Chemical Reaction
Response cards by Turning Technologies
Density (Benchmark Assessment)
CHEMISTRY
SCIENCE CURRICULUM 361 GRADES 9 - 12
LEARNING STRAND
Matter, Change and Early Atomic Theory
CT Standards: Conservation of Matter - The conservation of atoms in chemical reactions leads to the principle of conservation of
matter and the ability to calculate the mass of products and reactants.
Chemical Bonds - Biological, chemical and physical properties of matter result from the ability of atoms to form bonds from
electrostatic forces between electrons and protons and between atoms and molecules.
Atomic and Molecular Structure - The periodic table displays the elements in increasing atomic number and shows how periodicity
of the physical and chemical properties of the elements relates to atomic structure.
ENDURING UNDERSTANDINGS
Matter is defined as anything that has mass and
takes up space.
The three phases of matter include solid, liquid,
and gas.
All matter may be categorized as a pure
substance or a mixture.
A physical change does not change the identity
of a substance, but a chemical change does
change the identity of a substance.
Compounds are composed of elements bonded
together, and their structure can only be
changed through chemical means.
Mixtures may be separated based on the
physical property differences of the components
of the mixture.
The understanding of atomic structure was
developed by many scientists and scientific
thinkers over a period of more than 2000 years.
Atomic structure includes a positively charged
nucleus containing two types of subatomic
particles (neutrons and protons) and an outer
region of negatively charged electrons.
The configuration of atoms and molecules
determines the properties of materials.
Elements exist in nature as isotopes, which
differ in number of neutrons, and atomic mass.
In nuclear fission, matter is transferred directly
into energy in a process that is several million
times more energetic than combustion.
ESSENTIAL QUESTIONS
How do you distinguish between pure
substances and mixtures?
What are the indications that a chemical
reaction (or chemical change) has taken place?
How do you distinguish between a chemical
property and a physical property?
How do you distinguish between an element
and a compound?
What are explanations for early experiments
that led to the characterization of the nucleus,
and the discovery of electrons?
What is the description of the general structure
of the atom?
Why does atomic mass of isotopes vary for an
element ?
How would you determine the chart mass of an
element, given isotope data?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Matter and Change
Name and characterize the three states of
matter.
Distinguish between matter and a substance.
Classify given samples as a substance or a
mixture.
Classify given samples as heterogeneous or
homogeneous.
Describe the difference between an element
and a compound.
Identify physical changes of matter.
Write the symbols of common elements, and
write the names of common elements, given
the symbol.
Identify changes of matter as chemical or
Chemistry Prentice Hall, 2002
General Lab equipment (Balances, glassware,
Bunsen burners, safety goggles, aprons and
gloves)
Calculators
Videos, such as ―History of Atomic Theory‖
Supplies for Beanium (dried beans)
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Response cards by Turning Technologies
Guided Internet research
CHEMISTRY
SCIENCE CURRICULUM 362 GRADES 9 - 12
physical.
Early Atomic Theory
Describe and explain the contribution that
Thomson and Rutherford made to the
development of the atomic theory.
Use Dalton’s Atomic Theory to define an atom.
Compare the nucleus to the atom, and include
size, mass and charge in the answer.
Describe the mass, charge, and location of the
proton, neutron, and electron.
Understand the relationship and meaning of
atomic number and mass number.
Define isotope.
Describe the general structure of the atom, and
explain how the properties of the first 20
elements are related to their structure.
PowerPoint presentations, with interactive
simulations, and imbedded practice problems
Interactive games, such as ―Its All in the Cards‖
Puzzle-like activities to reinforce patterns and
trends of Periodic Table
Lab investigations
Creative writing assignments, such as
―Autobiography of an Element‖
Closure tasks, such as ―Your Ticket out the
Door‖
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Tests, including a Make up Your Own Test,
assigned as a group assessment
Lab reports (design labs, collect data, analyze,
draw conclusions)
Physical and Chemical Properties(Benchmark
Assessment)
Beanium
Response cards by Turning Technologies
CHEMISTRY
SCIENCE CURRICULUM 363 GRADES 9 - 12
LEARNING STRAND
The Language of Chemistry
CT Standards: Atomic and Molecular Structure - The periodic table displays the elements in increasing atomic number and shows
how periodicity of the physical and chemical properties of the elements relates to atomic structure.
Conservation of Matter and Stoichiometry - The conservation of atoms in chemical reactions leads to the principle of conservation of
matter and the ability to calculate the mass of products and reactants.
Chemical Bonds - Biological, chemical and physical properties of matter result from the ability of atoms to form bonds from
electrostatic forces between electrons and protons and between atoms and molecules.
ENDURING UNDERSTANDINGS
Chemical symbols, formulas, and equations are
understood internationally, and are written
based upon universally accepted guidelines.
The formula of a binary compound can be
determined based on the positions of elements
on the Periodic Table.
The unit of measurement which chemists rely
on to predict quantities in chemical reactions is
the mole.
Chemical equations are presented as balanced
equations to satisfy the law of conservation of
matter.
Chemists make calculations involving quantities
of reactants and products based on balanced
chemical equations and yields.
Emission of combustion by products, such as
SO
2
, CO
2
, and NOx, by industries and vehicles
is a major source of air pollution.
During the burning of fossil fuels, stored
chemical energy is converted to useable energy.
ESSENTIAL QUESTIONS
What are the descriptions for how atoms
combine to form new substances by
transferring electrons (ionic compounds) or by
sharing electrons (molecular compounds)?
How does the Periodic Table provide for the
charge of a metal or a nonmetal, in an ionic
compound?
How can conversions be made among particles,
mass, and moles of any substance ?
What is the difference between empirical and
molecular formula?
What is the significance of empirical formula in
chemical analysis?
How can predictions be made about which
reactions will occur and what the products will
be?
How can you relate between different quantities
in a balanced chemical reaction?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Chemical Names and Formulas
Differentiate between ionic compounds and
molecular compounds, and between molecules
and formula units.
Use the Periodic Table to determine the charge
on an ion.
Explain that chemical bonds between atoms in
molecules such as H2,CH4, NH3, H2CCH2, N2,
CL2, and many large biological molecules are
covalent.
Write the chemical formula of binary or ternary
ionic compounds, molecular compounds, or
acids, given the name, and vice versa.
Describe how atoms combine to form new
substances by transferring electrons (ionic
bonding) or sharing electrons (covalent
bonding).
The Mole
Define Avogadro’s number as one mole equals
6.02 x 10 23 particles (atoms or molecules).
Properly define gram atomic mass, gram
molecular mass, gram formula mass, and molar
mass, and determine from the chemical formula
and a table of atomic masses.
Chemistry Prentice Hall, 2002
General lab equipment
Video ―Global Warming – What you Need to
Know‖
How to determine your carbon footprint
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded
practice problems
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Calculate Your Carbon Footprint, with global
warming video.
Execute laboratory experiments:
-Empirical Formula Determination (Benchmark
Assessment)
-Reactions of Halides
-Types of Reactions (Benchmark Assessment)
-Single Replacement Reactions (Benchmark
Assessment)
CHEMISTRY
SCIENCE CURRICULUM 364 GRADES 9 - 12
Relate mass, moles, volume, and number of
particles for a given amount of a substance at
standard temperature and pressure.
Relate the mole concept to the density of a gas.
Use percent composition to determine empirical
and molecular formulas.
Chemical Equations
Identify reactants and products in a chemical
equation.
Write a balanced equation when given the
names or formulas of all reactants and products
in a chemical equation.
Understand that chemical reactions can be
described by writing balanced equations.
Use appropriate symbols to write an equation to
accurately describe a chemical reaction.
Classify a reaction as combination, single or
double replacement, decomposition, or
combustion.
Predict the products of each of these reaction
types, using appropriate references, such as the
activity series, or solubility rules.
Describe combustion reactions of hydrocarbons
and their resulting by products.
Understand that all chemical reactions either
produce energy (are exothermic) or absorb
energy (are endothermic) as a result of the
breaking and making of chemical bonds.
Stoichiometry
Interpret a chemical equation for mole
relationships of mass, particles, or volume.
Define and apply the concepts of theoretical,
actual, and percent yield.
Explain how the release of sulfur dioxide into
the atmosphere can form acid rain, and how
acid rain affects water sources, organisms, and
man-made structures.
Describe the availability, current uses and
environmental issues related to the use of fossil
and nuclear fuels to produce electricity.
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data, analyze,
draw conclusions)
CHEMISTRY
SCIENCE CURRICULUM 365 GRADES 9 - 12
LEARNING STRAND
The States of Matter
CT Standards: Conservation of Matter and Stoichiometry - The conservation of atoms in chemical reactions leads to the principle of
conservation of matter and the ability to calculate the mass of products and reactants.
Chemical Bonds - Biological, chemical and physical properties of matter result from the ability of atoms to form bonds from
electrostatic forces between electrons and protons and between atoms and molecules.
ENDURING UNDERSTANDINGS
Differences in the general characteristics of the
phases of matter are related to particle energy
and the presence, or absence, of attractive or
repulsive forces between particles.
Differences in the general properties of different
substances are related to intermolecular forces
of attraction for liquid and gas phases, and the
structure in which particles are held together in
the solid phase.
Energy cannot be created or destroyed;
however, energy can be converted from one
form to another.
ESSENTIAL QUESTIONS
How can you summarize the differences in the
particle arrangement and average particle energy
among particles in the solid, liquid, or gaseous
form of a substance?
How do intermolecular forces of attraction affect
the vapor pressure, boiling point and surface
characteristics of a liquid?
How does structure on molecular level affect the
properties of a solid?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
States of Matter
Explain the significance of absolute zero, giving
its value in degrees Celsius and Kelvin, and
relate the average kinetic energy of the
particles of a substance to temperature.
Name and characterize the three states of
matter.
Describe the motion of particles of a gas
according to the Kinetic Theory.
Explain that the atoms and molecules in liquids
move in a random pattern, relative to one
another because the intermolecular forces are
too weak to hold the atoms or molecules in
solid form.
Describe the effects of adding energy to matter
in terms of the motion of atoms and molecules,
and the resulting phase changes.
Describe the nature of a liquid in terms of the
attractive forces between particles.
Explain why a liquid has a vapor pressure, and
why a change in temperature causes a change
in vapor pressure.
Explain the significance of the unit cell to the
shape of a crystal.
Differentiate between different crystal systems
and different unit cells within a crystal system.
Explain how different intermolecular forces of
attraction affect the vapor pressure, boiling and
melting points, volatility, and surface tension of
the substance.
Chemistry Prentice Hall, 2002
Vacuum pump apparatus for demonstrations
Laptop computers with Loggerpro software for
Energy of Foods / Energy of Fuels labs
Dry ice to model sublimation and for
demonstrations
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded practice
problems
Modeling of concepts, followed by In class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Execute selections from suggested labs:
-Cooling Rates of Evaporating Liquids
-Specific Heat of a Metal (Benchmark Assessment)
-Energy of Foods , or Energy of Fuels
Demonstrations of :
-Phase change under vacuum
-Comparative cooling rates
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework Quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data, analyze,
draw conclusions)
CHEMISTRY
SCIENCE CURRICULUM 366 GRADES 9 - 12
LEARNING STRAND
The Gaseous State of Matter
CT Standards: Conservation of Matter and Stoichiometry - The conservation of atoms in chemical reactions leads to the principle of
conservation of matter and the ability to calculate the mass of products and reactants.
Chemical Bonds - Biological, chemical and physical properties of matter result from the ability of atoms to form bonds from
electrostatic forces between electrons and protons and between atoms and molecules.
ENDURING UNDERSTANDINGS
The general behavior of a substance in the gaseous
state can be described by the Kinetic Molecular Theory,
which is based on observation.
The average kinetic energy of particles of a substance
in the gaseous state is related to the temperature of the
substance in the Kelvin (absolute) scale.
Particles of different substances will effuse at different
rates, depending on the size (mass) of the particles.
The specific response to a change in pressure,
temperature, and /or volume of a substance in the
gaseous state can be predicted by the Combined Gas
Law or one of its corollaries, the Independent (Boyle’s,
Charles’, and Gay-Lussac’) Gas Laws.
A gas in a mixture will behave the same and exert the
same pressure as it would alone.
The Ideal Gas Law is an empirical relationship that
relates pressure, volume, temperature, and amount of a
substance in the gaseous state of matter.
The concept of an ideal gas is hypothetical and an ideal
gas does not actually exist, but under most temperature
and pressure conditions, most gases behave ideally.
ESSENTIAL QUESTIONS
How does the Kinetic Molecular Theory
predict the relationship between particles of
a gas, and in turn, the behavior of a gas?
How is Graham's Law related to the average
kinetic energy of particles of a gas?
What are the factors that determine which
gas law to use, to solve word problems ?
What label (s) is (are) appropriate for each
parameter for each gas law?
Under what conditions will a real gas deviate
from an ideal gas (or, the Ideal Gas Law)?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Use Kinetic Theory to explain gas pressure.
State the value of standard temperature and pressure.
State and explain the significance of Avogadro’s
hypothesis.
Predict the response of a gas to changes in pressure,
volume or temperature, using the appropriate gas law).
Understand and apply Dalton’s law of partial pressures.
Understand Avogadro’s hypothesis as it applies to
gases.
Use the combined gas law to predict changes in
temperature, pressure or volume.
Understand and use the Ideal Gas Law to solve word
problems.
Use the Ideal Gas Law to calculate among pressure,
volume, temperature, and amount of a substance in the
gaseous state of matter.
Differentiate between an ideal gas and a real gas.
Describe the motion of particles of a gas according to
Kinetic Theory.
Describe how human efforts to reduce consumption of
raw materials can improve air quality.
Chemistry Prentice Hall, 2002
Glass tubes for Graham’s Law Experiment
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded
practice problems
Modeling of concepts, followed by in-class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Execute selections from suggested labs
Graham’s Law (Benchmark Assessment)
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Lab reports (design labs, collect data,
analyze, draw conclusions)
CHEMISTRY
SCIENCE CURRICULUM 367 GRADES 9 - 12
LEARNING STRAND
Electrons, The Periodic Law and Chemical Bonds
CT Standards: Chemical Bonds - Biological, chemical and physical properties of matter result from the ability of atoms to form bonds
from electrostatic forces between electrons and protons and between atoms and molecules.
Atomic and Molecular Structure - The periodic table displays the elements in increasing atomic number and shows how periodicity of
the physical and chemical properties of the elements relates to atomic structure.
ENDURING UNDERSTANDINGS
Every element has a unique electron
configuration, which is based on the structure of
the principal energy levels, energy sublevels, and
orbitals.
The electron configuration of any element is
indicated by its placement on the Periodic Table
of the Elements.
The Periodic Table is arranged in order of
increasing atomic umber and grouped by similar
properties, but there are several additional
trends that progress through each period (row)
or each family of elements (column), on the
Table.
The electromagnetic spectrum is the full range of
wavelengths of radiation from the sun, and it
includes visible light, heat energy, sound waves,
and many other types of energy (microwave,
radar, X-ray, and gamma radiation).
The tendencies for metals to form cations and
for nonmetals to form anions are related to
position on the Periodic Table and electron
configuration.
The drive for atoms to form bonds is based on
the stability of the noble gases and the octet
rule.
The manner in which elements combine to form
compounds and the resulting molecular
geometries has a profound effect on the
properties exhibited by the substance.
ESSENTIAL QUESTIONS
What is electron configuration in terms of an
element’s location on the periodic table?
What is the likelihood of electron configuration
forming bonds?
What type of bonds will be formed from electron
configuration?
What are the trends of physical and chemical
properties along periods or in groups on the
Periodic Table?
What are the different groups of the periodic
table?
What is the significance of the orientation and
layout of the Periodic Table?
What is the character of the ionic bonding
process?
What is the character of the molecular bonding
process?
How can the shape, bond angles and polarity be
predicted by using VSEPR theory?
What is a description of metallic bonding?
How does metallic bonding structure affect the
properties of a metal?
What is a description of network covalent
bonding?
How does the network covalent structure affect
the properties of an ionic compound?
What is a description ionic bonding with respect
to crystal structure?
How does crystal structure affect the properties of
an ionic compound?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Electron Configuration
Describe the general shape of s, and p orbitals.
Distinguish among principal energy level, energy
sublevel, and atomic orbital.
Use the Aufbau Principal, the Pauli Exclusion
Principal, and Hund’s Rule to write the electron
configuration of the elements.
Identify the electron configuration of any
element based on its position on the Periodic
Table.
Calculate the frequency and wavelength of light.
The Periodic Law (Periodicity)
Identify trends in (first) ionization energy,
electronegativity and the relative sizes of atoms.
Identify the different parts of the Periodic Table:
metals, nonmetals, metalloids, representative
Chemistry Prentice Hall, 2002
Discharge tubes, and power supplies.
Spectrophotometers
Three dimensional molecular model kits
Molecular models for Lewis Structures
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded
practice problems
Modeling of concepts, followed by In class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Use of three dimensional molecular models for
demonstration and also in student activities and
labs
CHEMISTRY
SCIENCE CURRICULUM 368 GRADES 9 - 12
elements, noble gases, transition metals and rare
earth metals.
Understand how physical and chemical
properties are based on an element’s placement
in a group or period of the Periodic Table.
Identify the number of electrons available for
bonding and the number of valence electrons
from the position on the Periodic Table.
Explain why the electron configuration for
chromium and copper are different than those
assigned from the Aufbau filling diagram.
Explain how electronegativity and ionization
energy are related to bond type and formation.
Ionic Bonding
Use Lewis dot structures to provide models of
atoms, ions and molecules.
State the importance of the Noble Gas electron
configuration in the formation of ions.
State and apply the octet rule.
Describe the formation of an anion form an atom
of a nonmetallic element.
Describe the formation of a cation from an atom
of a metallic element.
Molecular Bonding
Predict the shape of simple molecules and their
polarity from Lewis dot structures.
Explain how the structure of the carbon atom
affects the type of bonds it forms in organic and
inorganic molecules.
Define single, double and triple bonds.
Define resonance.
Define and recognize polarity.
Define and give the characteristics of an ionic
bond, covalent bond, polar covalent bond, and
coordinate covalent bond.
Describe the VSEPR theory and the relevance to
molecular shape, specifically bond angles.
Explain and give an example of resonance.
Use the theory of metallic bonding to explain the
physical properties of metals.
Explain the electrical conductivity of melted and
aqueous solutions of ionic compounds.
Understand how electronegativity relates to
bonding.
Use of discharge tubes to illustrate atomic
emission spectra
Labs should include:
-The Periodic Law (Benchmark Assessment)
-Electron configuration
-Properties of Solids
-Flame Tests (Benchmark Assessment)
-Lewis Structures of Molecules
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw
conclusions)
CHEMISTRY
SCIENCE CURRICULUM 369 GRADES 9 - 12
LEARNING STRAND
The Chemistry of Solutions
Big Idea: Everything is made of matter.
ENDURING UNDERSTANDINGS
The structure of the water molecule and the
presence of hydrogen bonds in water are
responsible for unique chemical and physical
properties, which dictate many aspects of our
world.
The use of the natural resource water by
human populations can affect the quality of our
environment.
The significance of polarity, electrolytes and
physical conditions (such as temperature,
particle size, and agitations) affect solution
formation and the nature of the solvation
process.
The relative amounts of solute and solvent in a
solution are described by Molarity, Molality,
mass and volume percent.
Colligative properties depend on the amount of
solute particles present, and include boiling
point elevation, freezing point lowering and
vapor pressure lowering.
ESSENTIAL QUESTIONS
How does the accumulation of metal and
nonmetal ions used to increase agricultural
productivity become a significant source of water
pollution?
What is hydrogen bonding?
How does hydrogen bonding affect the properties
of liquids in general, and water, specifically.
What are the physical conditions that affect
solution formation, and what is the effect of each
physical condition ?
What are definitions for the five terms for
concentration?
How is each term calculated?
How does the addition of a nonvolatile solute
change the boiling point, freezing point, or vapor
pressure of a solvent?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe how human efforts to reduce
consumption of raw materials can improve
water quality.
Explain how the accumulation of mercury,
phosphates, and nitrates affects the quality of
water and the organisms that live in rivers,
lakes and the ocean.
Calculate the percent water in a hydrate and
name a hydrate given the formula (or vice
versa).
Describe the hydrogen bonding that occurs in
water on the bases of the structure of the polar
water molecule and electronegativity.
Use the concept of hydrogen bonding to explain
the high surface tension, high boiling point and
low vapor pressure of water.
Chemistry Prentice Hall, 2002
Lab supplies for ―Marbling‖ activity (shaving
cream, cardstock, and food coloring)
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded practice
problems
Modeling of concepts, followed by In class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Demonstrations
Marbling (interdisciplinary art/science/history)
Labs should include:
-Supersaturation (Benchmark Assessment)
-Properties of Solutions
CHEMISTRY
SCIENCE CURRICULUM 370 GRADES 9 - 12
Define the terms solution, aqueous solution,
solute and solvent.
Understand how the concentration of a solution
may be quantitatively described.
Use the rule of ―like dissolves like‖ to predict
the solubility of one substance in another.
Describe the procedure for preparing a dilute
solution of known concentration from a more
concentrated solution.
Define and describe colligative properties.
Use the concept of colligative properties to
predict boiling points or freezing points of given
solutions.
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw
conclusions)
CHEMISTRY
SCIENCE CURRICULUM 371 GRADES 9 - 12
LEARNING STRAND
Reaction Rates and Equilibrium
CT Standard: Reaction Rates - Chemical reaction rates depend on factors that influence the frequency of collision of reactant
molecules.
ENDURING UNDERSTANDINGS
-Rates of chemical reactions are determined by
collisions between reacting particles.
-All reactions are reversible, and their equilibrium
position can be described by the equilibrium
constant.
-LeChatelier’s principle predicts the response of a
system at equilibrium to a change in
concentration, pressure, volume, or temperature.
ESSENTIAL QUESTIONS
What is the underlying concept that summarizes
LeChatelier’s Principle ?
How is the equilibrium constant, K, determined
from a chemical equation?
What is the significance of different values (very
high or very low) of K?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
-Define chemical equilibrium in terms of a
reversible reaction.
-Using LeChatelier’s principle, predict the
direction in which equilibrium will shift in
response to a change in concentration, pressure,
volume, or temperature.
-Given a chemical equation, write the expression
for the equilibrium constant.
-Given concentration information and a chemical
reaction (or equation), calculate the value for the
equilibrium constant.
Chemistry Prentice Hall, 2002
SUGGESTED INSTRUCTIONAL STRATEGIES
-Power point presentations with imbedded
practice problems
-Modeling of concepts, followed by In class
practice worksheets
-Frequent question and answer sessions
-Labs should include:
Disturbing Equilibrium
LeChatelier’s Principle
SUGGESTED ASSESSMENT METHODS
-In class worksheets (collaborative)
-Homework (individual)
-Homework quizzes (individual)
-Student class participation
-Response cards by Turning Technologies
-Tests
-Lab reports (collect data, analyze, draw
conclusions)
CHEMISTRY
SCIENCE CURRICULUM 372 GRADES 9 - 12
LEARNING STRAND
Acids and Bases
Big Idea: Acids and bases can be defined in terms of hydrogen ions and hydroxide ions or in terms of electron pairs.
ENDURING UNDERSTANDINGS
General properties of acids and bases include
taste, ability to react with each other in a
neutralization reaction, and ability to change
the color of various indicators.
Acids and bases are electrolytes.
Acids dissociate to produce hydronium ions,
and some bases dissociate to produce
hydroxide ions. Water also dissociates into
hydronium and hydroxide ions.
The pH scale is related to the concentration
of hydronium ions, logarithmically, goes from
0 to 14, and is an indication of acidity of a
solution.
The pOH scale is inversely related to pH.
Acids and bases can be characterized as
Lewis, Arrhenius, or Bronsted-Lowry systems
based on structural and behavioral
characteristics of the compounds.
The strength of acids and bases is
determined by ionization, and can be
measured quantitatively by Ka or Kb.
ESSENTIAL QUESTIONS
What are the general properties of acids and
bases commonly found in households?
How can self ionization of water be
explained?
How are pH and [H] related ?
How are pOH and pH related ?
How are pOH and [OH] related ?
What is the difference between a strong acid
(or base) and a concentrated acid (or base)?
What is the chemical composition of acids?
What is the chemical composition of bases?
How does pH change in neutralization
reactions?
INSTRUCTIONAL SUPPORT MATERIALS
Chemistry Prentice Hall 2002
LEARNING OBJECTIVES
The student will…
Define the general properties of acids and
bases.
Define the self ionization of water.
Use the concentration of H+ OR OH- to
identify a solution as acidic or basic.
Describe acids and bases according to the
three major acid-base theories: Arrhenius,
Bronsted-Lowry, and Lewis.
Identify the conjugate acid - base pairs of
the Bronsted-Lowry Theory.
Classify substances as acids, bases or neutral
based on the three acid base theories.
Calculate the gram equivalent mass of any
acid or base.
Calculate Ka or Kb from concentration or pH
measurements.
Identify monoprotic, diprotic and triprotic
acids.
Explain the concept of titration, and include
the relevance of the different weak / strong
acid and base combinations.
Relate among pH, pOH, [H], and [OH], given
log tables and a calculator.
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations with imbedded
practice problems
Modeling of concepts, followed by In class
practice worksheets
Frequent question and answer sessions
Response cards by Turning Technologies
Labs should include:
-Characteristic Reactions of Acids
-Salt Hydrolysis
-Neutralization Reactions
-A Solubility Product Constant
-Buffers
-Acid Titration
SUGGESTED ASSESSMENT METHODS
In class worksheets (collaborative)
Homework (individual)
Homework quizzes (individual)
Student class participation
Response cards by Turning Technologies
Tests
Drawings of molecules
Lab reports (collect data, analyze, draw
conclusions)
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 373 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Advanced Placement Chemistry
University of Connecticut/Early College Experience
Chemistry 1127Q/1128Q
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
AP Chemistry
3. Transcript Course Code/Number
00351
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grades: 11-12 Level: 1
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval _________________(date)
10. Pre-Requisites
Level I Chemistry and Level I Chemistry Teacher Recommendation
11. Brief Course Description
This course is designed to provide a foundation for more advanced courses in chemistry. Topics in the first half of
the course include: atomic theory, laws and theories concerning the physical and chemical behavior of gases,
liquids, solids and solutions; properties of some of the more familiar elements and their compounds; quantitative
measurements illustrating the laws of chemical combinations. Topics in the second half of the course include:
equilibrium in solutions and quantitative reactions of the common cations and anions. All students are encouraged
to take the AP Chemistry Examination.
Students start laboratory experiments and UConn examinations at 7 am.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and solving
problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the global
community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.
AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 374 GRADES 9 - 12
13. Course Outline:
CHEMISTRY 1127Q (UCONN 1
st
semester)
Chemistry: Principles and Reactions by Brooks & Cole, Cengage Learning, 2009
CHAPTER LABORATORY
1 Matter and Measurements Check-in & Safety Training and Safety Contract
2 Atoms, Molecules, and Ions Density
3 Mass Relations in Chemistry: Stoichiometry Stoichiometry, Chromatography, Fractoinal Crystallization
EXAM I (CH 1-3, Experiments)
4 Reactions in Aqueous Solutions Determination of Chemical Formula
Analysis of Unknown Chloride
5 Gases MM of a Solid Acid
EXAM II (CH. 4 –5 , Experiments)
6 Electronic Structure and the Periodic Table Fe Determination
MM of a Volatile Liquid
7 Covalent Bonding Analysis of an Al-Zn Alloy
8 Thermochemistry The Alkaline Earths & Halogens
EXAM III (CH. 6 – 8, Experiments)
9 Liquids and Solids Calorimetry
FINAL EXAM (CHAPTERS 1 – 9,Experiments)
CHEMISTRY 1128Q (UCONN 2
nd
semester)
10 Solutions Molar Mass determination by Depression of the Freezing Point
11 Rate of Reaction Rates of Chemical Reactions I: The Iodination of Acetone
12 Gaseous Chemical Equilibrium Some Nonmetals and Their Compounds
EXAM I (CH. 10-12, Experiments)
13 Acids and Bases pH – Determination of the Equilibrium
Constant for a Chemical Reaction
14 Equilibria in Acid-Base Solutions Buffers and Their Properties
15 Complex Ions Determination of the Solubility Product of Lead (II) Iodide
Exam II (CH. 13-15)
16 Precipitation Equilibria
17 Spontaneity of Reaction
18 Electrochemistry
EXAM III (CH. 16-18, Experiments)
19 Nuclear Reactions Qualitative Analysis of Group I, II, and III
Ions and General Unknowns
FINAL EXAM (CHAPTERS 10-19, Experiments)
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
Chapter problem sets
AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 375 GRADES 9 - 12
16. Assessment Criteria
The technology benchmark used involves the students learning the proper use and functions of
various laboratory equipment including, but not limited to:
a. electronic balances
b. analytical measuring devices such as balances, burets, pipets, and electronic thermometers.
c. NOTE: The University does NOT allow the use of computers in the experiments performed in this
course.
The writing benchmark is incorporated through the requirement of students to prepare and submit
laboratory reports. The students must maintain a laboratory log book while performing experiments. At
the conclusion of each lab session, students must submit initial copies of their data. They then must
prepare their laboratory reports including not only all of the observations, calculations, and
interpretations involved in the experiment, but also must do so using proper grammar, punctuation,
spelling, and sentence structure. The benchmark for use of effective strategies for gathering information,
critical thinking, and problem solving are inherent with the subject of science. Starting at the beginning of
the course with the Scientific Method, the students are required to use
ALL
of these skills, on a
DAILY
BASIS
, throughout the course; it is the
ESSENCE OF SCIENCE.
The University of Connecticut provides all benchmark assessment criteria and determines the final point
distribution (final grade). At the conclusion of each semester, the University sends a letter grade-points
earned grade distribution report. Each student’s point total is matched against this report and his/her
final letter grade for the course is determined. Below is a sample of such a report. This report was for the
second semester course (Chem 1128).
Total Points Range for Final Letter Grades
Here are the ranges for the final letter grade for Chem 1128. Numbers on the left reflect the total number
of points accumulated during the semester. Maximum points that can be accumulated = 1000
920 - 1000 = A
919 - 900 = A-
899 - 870 = B+
869 - 820 = B
819 - 800 = B-
799 - 770 = C+
769 - 720 = C
719 - 700 = C-
699 - 670 = D+
669 - 620 = D
619 - 600 = D-
599 and below F
Overall average 760/1000
Final exam average 173/250
HONORS COURSES ONLY
17. Indicate how this honors course is different from the standard course.
This is a true college course. As required, the course syllabus supplied by the University of Connecticut’s
Department of Chemistry is followed. All exams, laboratory experiments, sectional exams, final exams,
correcting rubrics, and final grading guidelines are followed so that our students are graded exactly as
students taking the course at the University of Connecticut are graded. The instructor for the course is a
University certified adjunct professor and is required to attend re-certification sessions at the University.
AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 376 GRADES 9 - 12
AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 377 GRADES 9 - 12
AP CHEMISTRY
*This course is the University of Connecticut’s CHEM 127Q (1
st
sem) and CHEM 128Q (2
nd
sem).
A. Classroom Emphasis:
1. The course is problem-solving based. Students are introduced to the various theories, laws, and
concepts through assigned readings with reinforcement provided in lectures. Strong emphasis is
placed on problem-solving and problem-solving techniques. In addition to assigned problems,
copious amounts of problems are worked out during lectures with the assistance of the
instructor.
2. Students are allowed the use of a scientific calculator but ―plotting calculators‖ are not allowed.
3. Students are encouraged to work in groups on the solution of problems during class but are
under an honor code when working on homework problems.
B. Laboratory Emphasis:
1. The laboratory emphasizes laboratory safety, laboratory techniques, observations skills, and
proper record keeping.
2. To allow sufficient time, class begins at 7 a.m. on laboratory days.
3. A pre-lab quiz is given the day before an experiment is to be performed. The quiz is based on the
―Pre-Lab Assignment‖ which accompanies each experiment in the laboratory text.
C. Homework Problems:
1. All homework problem sets are assigned by the Chemistry Department at the University of
Connecticut.
2. All homework problem sets are collected, corrected, graded, and returned to students. The
answer key is posted once the assignments are returned to the students.
3. Student are to work on homework problem sets on their own. They work under an honor code.
D. Testing:
1. One-hour chapter tests are given at the conclusion of each chapter.
2. The University of Connecticut supplies three (3) Unit Exams during each semester. Each Unit Exam
covers two (2) or three (3) chapters of work and is scheduled for a two-hour test time. The
correcting rubric is supplied by the University of Connecticut and their rubric is fully adhered to.
3. The University of Connecticut supplies a three-hour Final Examination at the conclusion of each
semester along with a correcting rubric. In addition, the university has the right to request that the
final exams be sent to them for correction.
E. Topics Covered, by Chapter
1 Matter and Measurements 1.0 weeks
a. Types of Matter
i. Elements
ii. Compounds
iii. Mixtures
b. Measurements
i. Length
ii. Volume
iii. Mass
iv. Temperature
v. Experimental Error; Significant Figures
a. Counting Significant Figures
b. Multiplication and Division
c. Addition and Subtraction
d. Exact number

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 378 GRADES 9 - 12
vi. Conversion Factors
a. One-Step Conversion
b. Multiple Conversion Factors
c. Properties of Substances
i. Density
ii. Solubility
iii. Color; Absorption Spectrum
Homework Problems: 1,3,7,9,11,13,17,19,23,25,33,39,49,53
Laboratory: Check-in & Safety Training and Safety Contract
2 Atoms, Molecules, and Ions 1.5 weeks
a. Atoms and the Atomic Theory
i. Atomic Theory
b. Components of the Atom
i. Protons
ii. Neutrons
iii. Electrons
iv. Atomic Number
v. Mass Number
vi. Nuclear Stability
c. Introduction to the Periodic Table
i. Atomic Number
ii. Mass Number
iii. Atomic Size
iv. Ionization Energy
v. Electronegativity
vi. Density
vii. Ionic Size
viii. Metallic Character
d. Molecules and Ions
i. Types of Formulas
ii. Formation of Monoatomic Ions
iii. Charges of Monoatomic Ions with Noble-Gas Structures
iv. Polyatomic Ions
v. Formulas of Compounds
e. Formulas of Ionic Compounds
f. Names of Compounds
i. Ionic
ii. Binary Molecular Compounds
iii. Acids
Homework Problems: 3,5,11,13,15,17,25,29,31,35,41,49
Laboratory: The Densites of Liquids and Solids (hands-on) (2.5 – 3 hr)
Emphasis: This experiment is performed to not only allow
students to determine, with excellent precision, the
density of a liquid and the density of a solid using the
displacement of water, but also introduces them to
the proper use of analytical balances.

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 379 GRADES 9 - 12
3 Mass Relations in Chemistry: Stoichiometry 2.0 weeks
a. Atomic Masses
i. Meaning of Atomic Masses
ii. Atomic Masses from Isotopic Composition
iii. Masses of Individual Atoms
b. The Mole
i. Meaning
ii. Molar Mass
iii. Mole-Mass Conversions
c. Mass Relations in Chemical Formulas
i. Mass Percent from Formulas
ii. Simplest Formula from Percent Composition
iii. Simplest Formula from Analytical Data
iv. Molecular Formula from Simplest Formula
d. Mass Relations in Reactions
i. Balancing
ii. Mass Relations in Reactions
iii. Limiting Reactant, Theoretical Yield
iv. Actual Yield, Percent Yield
Homework Problems: 5,7,15,23,31,41,45,47,51,55,59,67.85
Laboratory: Resolution of Matter into Pure Substances:Chromatography (hands-
on)(2.5 - 3 hr)
Emphasis: Students learn the techniques involved in paper
chromatography by separating a mixture of ions,
determining there R
f
values, and then identifying the
components of an unknown sample.
Resolution of Matter into Pure Substances:Fractional Crystallization
(hands-on)(2.5 - 3 hr)
Emphasis: Students are given a sample containing silicon carbide,
potassium nitrate, and copper (II) sulfate pentahydrate, with
the hydrate present essentially as an impurity. They learn
the proper techniques of separating and recovering the
silicon carbide and potassium nitrate and determine the
percent composition of their mixture.
EXAM I (CH 1-3, Experiments)
4 Reactions in Aqueous Solutions 2.0 weeks
a. Solute Concentrations
i. Molarity
b. Precipitation Reactions
i. Precipitation Diagram
ii. Stoichiometry
c. Acid-Base Reactions
i. Acid
ii. Base
iii. Acid-Base Reactions
iv. Acid-Base Titrations
d. Oxidation-Reduction Reactions
i. Oxidation Number
ii. Balancing Redox Reactions

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 380 GRADES 9 - 12
Homework Problems: 1,3,5,11,15,19,27,33,39,41,49,51,55,57,61,65,69,73
Laboratory: Determination of a Chemical Formula (hands-on)(2.5 – 3 hr)
Emphasis: In this experiment, the compound whose formula is
determined is CuCl
2
.
2 H
2
O. This compound is
stoichiometrically clean, in that it does not tend to lose or
absorb water, and can be obtained pure. It loses its water of
hydration completely when heated to about 110
o
C. The
copper it contains is reduced to the metal by aluminum.
Chlorine content is obtained by difference. Students
determine its percent composition and its empirical formula.
Analysis of an Unknown Chloride (hands-on)(2.5 – 3 hr)
Emphasis: Students are introduced to the proper techniques of
quantitative analysis by titration. Students watch a
demonstration of proper titration techniques and practice
the technique the day before the experiment is performed.
5 Gases 2.0 weeks
a. Measurements on Gases
i. Pressure
ii. Barometer
iii. Manometer
a. open-end
b. closed-end
b. The Ideal Gas Law
i. Variables
ii. Initial and Final State Problems
iii. Calculation of P, V, n, or T
c. Gas Law Calculations
i. Calculations of Density or Molar Mass
ii. Volumes of Gases Involved in Reactions
d. Stoichiometry of Gaseous Reactions
e. Gas Mixtures: Partial Pressures and Mole Fractions
i. Dalton’s Law
f. Kinetic Theory of Gases
i. Expression for Velocity
ii. Graham’s Law
g. Real Gases
Homework Problems: 3,7,11,19,23,29,35,39,43,49,65
Laboratory: The Standardization of a Basic Solution and the Determination of the
Molar Mass of a Solid Acid (hands-on)(2.5 – 3 hr)
Emphasis: Student use a prepared, standardized solution of
hydrochloric acid to determine the concentration of a
sodium hydroxide solution they prepare. They then use the
standardized sodium hydroxide to determine the molecular
mass of an unknown monoprotic acid.
EXAM II (CH. 4 –5 , Experiments)

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 381 GRADES 9 - 12
6 Electronic Structure and the Periodic Table 2.0 weeks
a. Light, Photon Energies, and Atomic Spectra
b. The Hydrogen Atom
i. Bohr Model
ii. Quantum Mechanical Model
c. Quantum Numbers
i. Principal Energy Levels
ii. Sublevels
iii. Azimuthal Number
iv. Magnetic Spin
d. Atomic Orbitals; Shapes and Sizes
e. Electron Configurations in Atoms
f. Orbital Diagrams of Atoms
g. Electron Arrangements in Monatomic Ions
h. Periodic Trends in the Properties of Atoms
Homework Problems: 1,5,9,17,21,23,27,31,37,39,45,49,57
Laboratory: Determination of Iron by Reaction with Permanganate – A Redox Titration
(hands-on)(2.5 – 3 hr)
Emphasis: Students use a standardized solution of potassium permanganate
to determine the percent iron in an unknown sample.
Molar Mass of a Volatile Liquid (hands-on)(2 hr)
Emphasis: Students determine the molar mass of a liquid using a vapor
density flask of known volume.
7 Covalent Bonding 2.5 weeks
a. Lewis Structures; The Octet Rule
i. Rules for Writing Lewis Structures (Single Bonds)
ii. Electron-Deficient Structures
iii. Expanded Octets
iv. Resonance Forms
b. Molecular Geometry
i. Two or Six Atoms Around Central Atom
ii. Unshared Electrons
iii. Multiple Bonding
c. Polarity of Molecules
i. Bond Polarity
ii. Molecular Polarity
d. Atomic Orbitals; Hybridization
i. Formation of Hybrid Orbitals
ii. Hybridization with 5 or 6 Electron Pairs
iii. Unshared Pairs
iv. Sigma and Pi Bonds
Homework Problems: 1,5,9,17,23,29,31,37,45,51,63
Laboratory: Analysis of an Al-Zn Alloy (hands-on)(3 hr)
Emphasis: Students determine the percent composition of an Al-Zn alloy by
reaction with hydrochloric acid. They collect the hydrogen
produced from a weighed sample of their alloy, measuring the
volume, temperature and total pressure of the gas, and then use
the Ideal Gas Law, taking into account the water vapor pressure,
to calculate the number of moles of hydrogen produced by the
sample.

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 382 GRADES 9 - 12
8 Thermochemistry 3.0 weeks
a. Principles of Heat Flow
i. Basic Concepts
ii. Endothermic
iii. Exothermic
b. Measurements of Heat Flow; Calorimetry
i. Coffee-Cup Calorimeter
ii. Bomb Calorimeter
c. Enthalpy
d. Thermochemical Equations
i. Rules of Thermochemistry
e. Enthalpies of Formation
f. Bond Enthalpy
g. The First Law of Thermodynamics
Homework Problems: 5,9,13,15,21,27,29,35,41,45,71,73
Laboratory: The Alkaline Earths and the Halogens – Two Families in the Periodic Table
(hands-on)(2 hr)
Emphasis: Students determine the relative oxidizing strengths of the
halogens. They then determine the relative solubilities of the
alkaline earth cations. Lastly, they develop a systematic
procedure for determining the presence of any Group 2 cation
and any Group 17 anion in a solution. They use this information
to determine the anion and cation present in an unknown.
EXAM III (CH. 6 – 8, Experiments)
9 Liquids and Solids 2.0 weeks
a. Liquid-Vapor Equilibrium
i. Vapor Pressure
ii. Temperature Dependence of Vapor Pressure
iii. Boiling Point
iv. Critical Temperature
b. Phase Diagrams
c. Molecular Substances; Intermolecular Forces
i. General Properties
ii. Dispersion Forces
iii. Dipole Forces
iv. Hydrogen Bonds
d. Network Covalent, Ionic, and Metallic Solids
e. Crystal Structures
i. Unit Cells in Metals
a. Simple Cubic
b. Face-Centered Cubic
c. Body-Centered Cubric
Homework Problems: 1,3,7,15,19,21,23,25,31,35,49,59
Laboratory: Heat Effects and Calorimetry (hands-on)(2.5 – 3 hr)
Emphasis: Using a calorimeter, students determine the specific heat of an
unknown metal, the heat of solution of an unknown solid, and the
heat of neutralization of known solutions of hydrochloric acid and
sodium hydroxide.
FINAL EXAMINATION – 1
st
Semester (CHAPTERS 1 – 9,Experiments)

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 383 GRADES 9 - 12
10 Solutions 2.0 weeks
a. Concentration Units
i. Molarity
ii. Mole Fraction
iii. Mass Percent Solute
iv. Molality
v. Conversion Between Units
b. Principles of Solubility
i. Nature of Solute and Solvent
ii. Effect of Temperature
iii. Effect of Pressure
c. Colligative Properties of Nonelectrolytes
i. Vapor Pressure Lowering
ii. Boiling Point Elevation
iii. Freezing Point Depression
iv. Osmotic Pressure
d. Colligative Properties of Electrolytes
Homework Problems: 3,7a,7b, 9c, 9d,11,15,17a, 17b, ,23,29,33,37,43, 59, 71
Laboratory: Molar Mass determination by Depression of the Freezing Point
(hands-on)(2.5 hr)
Emphasis: Students first determine the freezing point of pure
water, using a slurry of ice and water. They then add
a known mass of an unknown to the slurry, and find
the freezing point of the solution, thus determining
the freezing point depression. This allows them to
calculate the molality of the solution. Then then
separate the solution from the ice, weight the solution,
which contains all of the solute, and use this
information to calculate the molar mass of the solute.
11 Rate of Reaction 2.0 weeks
a. Meaning of Reaction Rate
b. Reaction Rate and Concentration
c. Reactant Concentration and Time
i. Zero-Order
ii. First-Order
iii. Second-Order
d. Models for Reaction Rate
i. Collision Model
ii. Activated Complex
e. Reaction Rate and Temperature
i. Arrhenius Equation
f. Catalysis
g. Reaction Mechanisms
Homework Problems:3, 9a, 9b, 11,13b, 13d, 17,21,25,37,43,45,51,75
Laboratory: Rates of Chemical Reactions I: The Iodination of Acetone
(hands-on)(3 hr)
Emphasis: The students study the kinetics of the reaction
between iodine and acetone. After gathering the data,
they calculate the reaction orders with respect to
acetone, hydrogen ion, and iodine;determine the rate
constant for the reaction; and determine the energy of activation.

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 384 GRADES 9 - 12
12 Gaseous Chemical Equilibrium 2.0 weeks
a. The N
2
O
4
-NO
2
Equilibrium System
b. The Equilibrium Constant Expression
i. Only Gases Involved
ii. Solids or Liquids as Well as Gases
iii. Aqueous Solutions
iv. Relation Between Equilibrium Constants
c. Determination of K
d. Application of the Equilibrium Constant
i. Determination of Direction of Reaction
ii. Extent of Reaction
e. Effect of Changes in Conditions on an Equilibrium System
i. Adding or Removing a Gaseous Species
ii. Increase in Pressure
Changes in Temperature
Homework Problems – 3, 7, 11,17,21,23,25,33,41,43,47,51
Laboratory: Some Nonmetals and Their Compounds (hands-on)(2 hr)
Emphasis: Students prepare several gases and learn to (1) test
for odor, (2) test for support of combustion, and (3)
test for acid-base properties. The gases prepared are
nitrogen, iodine, bromine, carbon dioxide, sulfur
dioxide, nitrogen dioxide, nitric oxide, ammonia, and
hydrogen sulfide. Once they have completed the
preparation and tests, they are given an unknown
compound and determine it’s composition.
EXAM I (Chapters 10 – 12, experiments)
13 Acids and Bases 2.0 weeks
a. Bronsted-Lowry Acid-Base Model
b. The Ion Product of Water
c. pH and pOH
d. Weak Acids and Their Equilibrium Constants
i. Molecules
ii. Cations
e. Weak Bases and Their Equilibrium Constants
i. Molecules
ii. Anions Derived from Weak Acids
f. Acid-Base Properties of Salt Solutions
i. Cations
ii. Anions
iii. Overall Results
Homework Problems: 1,9,13,19,23,25,31,33,39,45,49,55,59,76
Laboratory: pH – Determination of the Equilibrium Constant for a Chemical
Reaction (hands-on)(3 hr)
Emphasis: Students learn the proper use of a
spectrophotometer while determining the equilibrium
constant for the the iron (III) / thiocyanate system. This
experiment represents the most intense calculations
involved with any of the experiments and requires that
they have solid understanding of the chemical system.

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 385 GRADES 9 - 12
14 Equilibria in Acid-Base Solutions 2.5 weeks
a. Buffers
i. Capacity of Buffers
b. Acid-Base Indicators
c. Acid-Base Titrations
i. Strong Acid-Strong Base
ii. Weak Acid-Strong Base
iii. Strong Acid-Weak Base
iv. Choice of Indicator
Homework Problems: 1,,5,9a,9c,15,17,21,27,31,39,41,43,45,56
Laboratory: pH Measurements – Buffers and Their Properties (hands-on)(2 hr)
Emphasis: Students determine the pH of acid/base solutions
using indicators; determine the pH of aqueous
solutions of acidic, basic, and neutral salts; study the
buffer capacity of acetic acid-acetate ion, ammonium
ion-ammonia, and hydrogen carbonate-carbonate
buffer system; and prepare a buffer from a solution
of a weak acid.
15 Complex Ions 1.5 weeks
a. Composition of Complex Ions
i. A Complex Ion
ii. Nature of Ligands
b. Geometry of Complex Ions
i. Coordination Number = 2
ii. Coordination Number = 4
iii. Coordination Number = 6
iv. Geometric Isomerism
c. Electronic Structure of Complex Ions
i. Electronic Structure of Transition Metal Cations
ii. Crystal Field Model
a. High Energy
b. Low Energy
d. Formation Constants of Complex Ions
i. Color
Homework Problems: 3,5,7,9,13,17,21,29,37,43
Laboratory: Determination of the Solubility Product of Lead (II) Iodide
(hands-on)(2.5 – 3 hr)
Emphasis: Using a spectrophotometer, students determine the
solubility product of lead (II) iodide.
EXAM II (Chapters 13 – 15, experiments)

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 386 GRADES 9 - 12
16 Precipitation Equilibria 1.5 weeks
a. Precipitate Formation; Solubility Product Constant (K
sp
)
i. Expression for K
sp
ii. Uses of K
sp
b. Dissolving Precipitates
i. Strong Acids
ii. Aqueous Ammonia
iii. Hydroxides
Homework Problems: 1,5,,9,13,17,21,27,31,37,41,55
Laboratory: Qualitative Analysis of Group I Cations (hands-on)(4 hr)
Emphasis: Students make use of techniques learned during the
course to study the Group I cations. After completion
of all tests on known solutions, students prepare a
flow chart and analyze an unknown solution
containing one or more of the Group I cations.
17 Spontaneity of Reaction 2.0 weeks
a. Spontaneous Processe
i. Examples
ii. Factors Affecting
b. Entropy, S
c. Free Energy, G
d. Standard Free Energy Change, G
o
i. Calculation of G
o
from H
o
and H
o
ii. Calculation of G
o
at 25
o
C from G
f
o
iii. Calculation of G from G
o
e. Effect of Temperature, Pressure, and Concentration on Reaction Spontaneity
f. The Free Energy Change and the Equilibrium Constant
g. Additivity of Free Energy Changes; Coupled Reactions
Homework Problems: 1,7,11,17,19,23,29,33,37,43,51,57,63,65,69,77,79
Laboratory: Qualitative Analysis of Group II Cations (hands-on)(4 hr)
Emphasis: Students make use of techniques learned during the
course to study the Group II cations. After
completion of all tests on known solutions, students
prepare a flow chart and analyze an unknown
solution containing one or more of the Group II cations.
18 Electrochemistry 1.5 weeks
a. Voltaic Cells
i. Salt Bridge Cells
ii. Cell Notation
b. Standard Voltages
c. Relation Between E
o
, G
o
, and K
i. Determination of Whether Redox Reaction Will Occur
d. Effect of Concentration on Voltage
i. Nernst Equation
e. Electrolytic Cells
i. Cell Diagram
ii. Amount of Product Formed
f. Commercial Cells
i. Electrolysis of Aqueous Sodium Chloride
ii. Lead Storage Battery

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 387 GRADES 9 - 12
Homework Problems: 1,9,13,15,21,23,29,33,37,43,47,53,55,57
Laboratory: Qualitative Analysis of Group III Cations (hands-on)(4 hr)
Emphasis: Students make use of techniques learned during the
course to study the Group III cations. After
completion of all tests on known solutions, students
prepare a flow chart and analyze an unknown
solution containing one or more of the Group I
cations.
EXAM III (Chapters 16 – 18, experiments)
19 Nuclear Reactions 1.0 weeks
a. Radioactivity
i. Natural
ii. Induced
b. Rate of Radioactive Decay
i. Activity
ii. Age of Organic Material
c. Mass-Energy Relations
i. Relation Between E and m
Calculations
Mass Defect
d. Nuclear Fission
e. Nuclear Fusion
Homework Problems: 3,9,11,15,21,27,31,35,37
Laboratory: Analysis of a General Unknown (hands-on)(4 hr)
Emphasis: Students make use of techniques learned during the
Group I, Group II, and Group III analyses to
identify the contents of a general unknown. This
unknown may contain one or more of any of the
cations in the three groups studied.
FINAL EXAMINATION – 2
nd
Semester (Chapters 10 –19,
experiments)
F. Grading
In an attempt to be as fair as possible to any high school student enrolled in a college course, students
are given two separate grades for this course.
HIGH SCHOOL
grade:
As with any other full-year course, students receive a trimester grade which appears on their
report card and which is treated as any other high school report card grade. The following percentages
are used to determine each trimester grade.
1
st
Trimester
UConn Exam I CH. 1 – 3 15%
UConn Exam II CH. 4 – 5 15%
Laboratory 15%
Chapter Tests 21%
Homework, and Lab Quizzes 15%
My 1
st
Trimester Final Exam 19%

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 388 GRADES 9 - 12
2
nd
Trimester
UConn Exam III CH. 6 – 9 15%
UConn Exam IV CH. 10 – 12 15%
Labs 15%
Chapter Tests 12%
Homework, and Lab Quizzes 8%
UConn 1
st
Semester Exam 20%
My 2
nd
Trimester Final Exam 15%
3
rd
Trimester
UConn Exam IV CH. 10 – 12 10%
UConn Exam V CH. 13 – 15 10%
UConn Exam VI CH. 16 – 18 10%
Labs 15%
Chapter Tests 12%
Homework, and Lab Quizzes 8%
UConn 2nd Semester Exam 20%
My 3rd Trimester Exam 15%
Determination of student’s final
high school grade
for course = 1/3 Trimester I + 1/3 Trimester II + 1/3
Trimester III final grades.
University of Connecticut Grade
- this is the grade that is sent to the University of Connecticut and
which
they use
to determine whether or not to award the student college credit. This grade is based
upon the point distribution system used by the university. Following is a sample of the point distribution
used by the university. Each semester, the university sends a copy of their final point distribution, which I
use to determine the students final semester grade.
Total Points Range for Final Letter Grades (Spring 2006)
Here are the ranges for the final letter grade for Chem 128. Numbers on the left reflect the total number
of points accumulated during the semester. Maximum points that can be accumulated = 1000
920 - 1000 = A
919 - 900 = A-
899 - 870 = B+
869 - 820 = B
819 - 800 = B-
799 - 770 = C+
769 - 720 = C
719 - 700 = C-
699 - 670 = D+
669 - 620 = D
619 - 600 = D-
599 and below F
Overall average 760/1000
Final exam average 173/250

AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 389 GRADES 9 - 12
In order to be awarded four (4) credits for the 1
st
semester, a student must receive a grade of ―C‖ or
better.
In order to be awarded four (4) credits for the 2
nd
semester, a student must have received a grade of ―C‖
or better the 1
st
semester and must receive a grade of ―C‖ or better for the 2
nd
semester.
1
st
Semester Grade
UConn Exam I CH. 1 – 3 15% (150 points)
UConn Exam II CH. 4 – 5 15% (150 points)
UConn Exam III CH. 6 – 9 15% (150 points)
Laboratory 15% (200 points)
Lab Quizzes 15% (100 points)
1
st
Semester UConn Final Exam 25% (250 points)
2
nd
Semester Grade
UConn Exam IV CH. 10 – 12 15% (150 points)
UConn Exam V CH. 13 – 15 15% (150 points)
UConn Exam VI CH. 16 – 18 15% (150 points)
Laboratory 15% (200 points)
Lab Quizzes 15% (100 points)
UConn 2nd Semester Final Exam 25% (250 points)
G. Textbooks and Remedial Materials
1. Textbook: Chemistry: Principles and Reactions, Masterson and Hurley, Brooks/Cole Cengage
Learning, 2009 6
th
Edition ISBM: 978-0-495-12671-3
2. Laboratory Textbook: Chemical Principles in the Laboratory, Slowinski, Wolsey, Materson,
Brooks/Cole, 8
th
edition, 2005, ISBN: 0-534-42453-8
3. CD-ROM: Masterson & Hurley’s General Chemistry Interactive CD-ROM, version 3.0, Vining, Kotz,
Harmon, Cow Town Productions, inc., 2003, ISBN: 0-534-40885-0
4. Advanced Placement Review Textbook: AP Chemistry, Kaplan, Simon & Schuster, updated
version used each year
AP CHEMISTRY (ECE)
SCIENCE CURRICULUM 390 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 391 GRADES 9 - 12
Course Description
Grades 9 - 12
1. Course Title
Advanced Placement Physics (B)
University of Connecticut, Early College Experience
PHYS 1201 Q / PHYS 1202 Q
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
AP Physics (B)
3. Transcript Course Code/Number
00361
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 1
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
B or better in Honors Algebra II
11. Brief Course Description
This course emphasizes the basic principles of physics using algebra and trigonometry. The topics include
Newtonian mechanics (including rotation and oscillation) fluid mechanics, thermal physics, electricity and
magnetism, waves and optics, and some topics of modern physics. The level and content of the course
are typical of a first year general physics college course. This course prepares students for the Advanced
Placement Physics B examination; all students are encouraged to take the AP examination.
12. Course Goals
1. Demonstrate proficiency and fluency in communication to meet the literacy demands of the global
community.
2. Use technology effectively and responsibly.
3. Apply effective strategies for gathering information/materials, thinking critically and solving problems.
4. Demonstrate respect for one's self, and strive to contribute to the success of others.
5. Successfully complete homework and lab assignments independently.
6. Collaborate as a productive team-member of a group in accomplishing challenging inquiry based
experiments.
7. Conduct lab experiments safely using appropriate scientific protocols.
8. Analyze and synthesize the scientific information as it relates to everyday experience and
surroundings.

AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 392 GRADES 9 - 12
Physics: Principles with Applications Fifth Edition by Douglas C. Giancoli Prentice Hall, 2002
13. Course Units of Study:
Chapter
# of Weeks
1. Motion in One Dimension
Chapter 2
2 weeks
2. Vectors and Projectiles
Chapter 3
2 weeks
3. Newton’s Laws
Chapter 4
3 weeks
4 Circular Motion and Gravitation
Chapter 5
1.5 weeks
5. Work and Energy
Chapter 6
2 weeks
6. Impulse and Momentum
Chapter 7
1.5 weeks
7. Rotational Motion
Chapter 8
2 weeks
8. Statics (Bodies in Equilibrium)
Chapter 9
1 week
9. Fluids
Chapter 10
1 week
10. Vibrations and Waves
Chapter 11
1 week
11. Sound
Chapter 12
1 week
12. Temperature, Kinetic Theory, Heat
Chapters 13 & 14
2 weeks
13. Thermodynamics
Chapter 15
1 week
14. Electric Charge, Fields, Potential
Chapters 16 & 17
2 weeks
15. DC circuits
Chapters 18 & 19
1 week
16. Magnetism, EMF, Motors, Generators
Chapters 20 & 21
2 weeks
17. Electromagnetic Waves
Chapter 22
1 week
18. Optics and Light Theory
Chapters 23 - 25
2 weeks
19. Special Relativity
Chapter 26
1.5 weeks
20. Quantum Physics
Chapters 27 & 28
1.5 weeks
21. Nuclear Physics
Chapters 30 & 31
2 weeks
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 393 GRADES 9 - 12
14. Instructional Methods and/or Strategies
Cooperative group work
Differentiated instruction
Lecture
Independent learning
Project based learning
PowerPoint presentations and notes
Laboratory investigations
Web based instruction
Teacher centered and student centered discussions, demonstrations and analyses
15. Assessment Methods and/or Tools
Lab reports evaluated with rubrics
Writing
Quizzes
Exams
16. Assessment Criteria
Students will score at the ―Meets Expectations‖ level for the laboratory assignments listed according to
departmental/school-wide rubrics with exemplars of student work where appropriate; students will
perform at or above a minimum grade of C. Exam grading will be in accordance with a teacher
generated key.
Benchmark Assignments:
Computer-based Quadratic Projectile Motion Lab addresses academic expectations 1a, 1b, 1c, 2,
and 3
Optics series Labs addresses academic expectations 1a, 1b, 1c, 2, and 3
Electric circuit series of labs addresses academic expectations 1a, 1b, 1c, 2, and 3
Physics 1201 Q Exam (Exit exam provided by the University of Connecticut) addresses academic
expectation 3
Physics 1202 Q Exam (Exit exam provided by the University of Connecticut) addresses academic
expectation 3
Trimester grades are based on homework completion, performance on assessments, performance on
laboratory reports, and classroom participation/effort.
Final grades are based on a percentage of the trimester grade and up to 20% on the final exam grade.
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a common scoring rubric or grading criteria.
HONORS COURSES ONLY
17. Indicate how this honors course is different from the standard course.
This course will give the accepted students the opportunity to earn eight (8) college credits from the
University of Connecticut.
The course follows the detailed ―Curriculum Audit‖ approved by Advanced Placement (AP) and
University of Connecticut Early College Experience (ECE). This course will prepare the student for the AP
Physics B examination.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 394 GRADES 9 - 12
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 395 GRADES 9 - 12
AP
®
PHYSICS B SYLLABUS
The AP Physics B course starts the last week of August and ends in the third week of
June. The class meets for five 60-minute periods per week. The sequence and timing of the
syllabus assumes coverage of the material during the complete school year of 180 days.
The AP test is in early May and we have approximately 25 days of school after the AP
test. Approximately 85 percent of the historical AP test material is thoroughly covered in regular
classes and lab activities prior to the AP exam. The remaining material is covered in special
study sessions or as independent study assignments as sought by individual students.
Course content involves interactive lessons and frequent physical demonstrations,
participation activities, and web-based demonstration modules. The course involves 20
traditional inquiry based labs with detailed reports. In the interest of time, efficiency and
resource utilization several additional labs (“Mini labs”) are conducted by the entire class. These
experiments are teacher-directed with student volunteers performing the experiment. Data
analysis is student-directed either in lab-groups or individually as determined by the teacher.
A test is given upon completion of each unit. Several units have one or more quizzes
designed to assess bench-mark or building-block topics.
Evaluation and Assessment: Tests 30%
Quizzes 10%
Labs 20%
Homework/Mini-Labs 20%
Final Exam 20%
Textbook: Physics: Principles with Applications -Fifth Edition
Douglas C. Giancoli ISBN 0-13-061143-3
Prentice Hall, 2002
Supplemental Resources:
Problem Workbook-Holt Physics (worksheet masters)
Raymond A. Serway and Jerry S. Faughn
ISBN 0-03-036833-2
Holt, Rinehart and Winston, 2006
Section Quizzes-Holt Physics (quiz masters)
Raymond A. Serway, and Jerry S. Faughn
ISBN 0-03-036836-7
Holt, Rinehart and Winston, 2006
Supplemental Resources (cont’d):
AP Advantage Physics B
James Mooney
ISBN 1-4138-0491-8
The Peoples Publishing Group, 2005
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 396 GRADES 9 - 12
Student Study Guide for Advanced Placement Physics B
Hugh Henderson ISBN 0-471-26850-X
John Wiley & Sons, 2004
www.explorelearning demonstration and analysis “gizmos”
Unit 1: Motion in One Dimension (2 weeks)
Primary resource: Giancoli Chapter 2 p 19-47
Topics
Constant velocity motion
Frame of reference
Relative motion
Basic vectors and directional meaning
Define and apply definitions of displacement, average velocity, instantaneous velocity,
and average acceleration
Analysis involving kinematics equations, including basic problems involving free fall by
using the value of the acceleration due to gravity
Analyze motion graphs qualitatively and quantitatively, including calculations of the
slope of the tangent of an x-versus-t graph, the slope of the v-versus-t graph, the area
under the v-versus-t graph and the area under the a-versus-t graph
Lab #1 Motion in One Dimension
Analyze the motion of an object and predict the resulting x vs. t, and v vs. t graphs.
Predict when the acceleration is constant, and its sign.
Evaluate x vs. t, and v vs. t graphs and produce the correct motion?
Equipment: Pasco Motion sensor; computer.
Lab #2 Acceleration of Gravity
Compute theoretical acceleration due to gravity based upon the angle of the ramp. Use
Pasco Frictionless cart, motion sensor and computer software. Evaluate observed
acceleration using various graphs (Slope, Least Square fit). Identify and discuss sources
of error. Qualitatively assess the fractional effect of friction related to data taken at
various angles.
Equipment: Pasco Motion sensor, cart, computer, adjustable ramp, meter stick.
Unit 2: Vectors and Projectiles (2 weeks)
Primary resource: Giancoli Chapter 3 p 48-76
Topics:
Vectors and scalars
Vector addition (graphical, parallelogram and component methods)
Horizontal and vertical components of projectile motion
Analysis of projectiles fired/launched horizontally and at an angle

AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 397 GRADES 9 - 12
Lab #3: Projectile Motion
Predict where a projectile will land (ideal conditions-no error analysis) and evaluate the
estimate based upon experimental results.
Include a qualitative assessment of errors, and repeat the experiment attempting to obtain
4 out of 5 launches within a specified circular landing area.
Repeat for various angles, launch velocities and launch heights.
Equipment: projectile launcher, electronic photo-gate timer with laptop, large paper, tape
measure, and meter sticks.
Unit 3: Newton’s Laws (3 weeks)
Primary resource: Giancoli Chapter 4 p 77-111
Topics:
Contact forces, field forces and causal agents
Mass; weight; acceleration due to gravity
Static friction; kinetic friction; rolling friction; fluid friction
Effect of speed on friction
Newton’s first law of motion and objects in static equilibrium
Free-body diagrams (understanding, interpretation and application)
Newton’s second law of motion (understanding and application)
Analysis of objects in motion with constant acceleration; resultant force evaluation
(horizontal surfaces, inclined planes, and pulley systems (Atwood’s machines))
Newton’s third law of motion (understanding and application)
Analysis and application of static and kinetic friction.
Lab #4: Static Force Balance 1
Given two specified forces applied to a point, determine the third force that would be
required to establish equilibrium. Use the Pasco Force balance table to verify results, and
determine/analyze any error
Include a qualitative assessment of errors, and repeat the experiment for several specified
forces and angles.
Equipment: Pasco Force table, various masses, Newton spring scale.
Lab #5: Static Force Balance 2
Given a string suspended from two points on the ceiling, determine what angles and
tensions will result in each leg of the string when the mass is suspended from teacher
specified locations on the string. Use Newton spring scales and or Pasco Force sensors to
evaluate theoretical results.
Include a qualitative assessment of errors.
Equipment: String, Masses, Newton spring scales, Pasco force sensors, meter sticks,
protractors.
Lab #6: Dynamic Force and Uniform Accelerated Motion
Using bathroom scales, student pushers, drivers and timers, Push the instructors’ car with
a constant force, measure the rate of acceleration and determine the mass of the car in
kilograms. Several differ4ent groups with varying applied force. All groups conduct all
analysis.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 398 GRADES 9 - 12
Include a qualitative assessment of errors.
Equipment: Automobile, bathroom scales, stopwatch, cones, long tape measure.
Unit 4: Circular Motion and Gravitation (1.5 weeks)
Primary resource: Giancoli Chapter 5 p 112-144
Topics:
Uniform circular motion
Centripetal acceleration (definition and derivation)
Newton’s Law of universal gravitation
Satellites; weightlessness; Kepler’s law
Analysis of circumstances involving banking angles, the conical pendulum and motion in
a vertical circle
Lab #7: Suspended Mass Centripetal Force
Determine the period of rotation required to support various masses suspended through a
90-degree Force direction change tube. Use various radii and masses for trials. Confirm
results and identify any sources of error.
Equipment: Suspended Mass-Centripetal Force apparatus, stop-watch, and ruler.
Unit 5: Work and Energy (2 weeks)
Primary resource: Giancoli Chapter 6 p 145-179
Topics:
Work (scientific definition of work as compared to the colloquial definition)
Work done by a constant force; potential energy; kinetic energy; power.
Work analysis using a force-versus-displacement graph.
Conservative and non-conservative forces.
Conservation of mechanical energy.
Analysis using the work–energy principle involving situations of conservative and non-
conservative forces.
Lab #8: Frictional Forces
Determine the coefficients of static and kinetic friction on an aluminum surface for a
block of wood having a smooth wooden face and a fur coated face. Identify any sources
of error.
Equipment: Inclined plane, inclinometer, meter stick.
Unit 6: Impulse and Momentum (1.5 weeks)
Primary resource: Giancoli Chapter 7 p 180-208
Topics:
Impulse and momentum
Newton’s second law of motion in terms of momentum
Change in momentum analysis using a force versus time graph
Conservation of momentum (and Newton’s third law)
Elastic and inelastic collisions
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 399 GRADES 9 - 12
Conservation laws and applicability/analysis (collision types)
Conservation of momentum (collisions in one and two dimensions)
Linear vs. angular momentum
Center of mass; evaluation and analysis.
Lab #9: Conservation of Linear Momentum and Energy
Predict the outcome of several lab-cart collisions based upon specified initial mass and
speed configurations.
Equipment: Lab carts, photo-gates, masses, track, computer.
Unit 7: Rotational Motion (2 weeks)
Primary resource: Giancoli Chapter 8 p 209-240
Topics:
Work with angles measured in radians to compute angular velocity and angular
acceleration.
Moment of inertia; angular momentum; rotational kinetic energy
Conservation of angular momentum for a rotating body.
Lab #10: Conservation of Angular Momentum
Predict the period of rotation for a student on a frictionless rotating stool with arms/mass
extended who then draws the masses to his/her chest.
Experience the gyroscopic effect of the hand-held bicycle wheel demo and fully explain
the effect of manipulating the wheel perpendicular to its axis of rotation.
Equipment: Frictionless lab stool, masses, meter stick, stop-watch, hand-held bicycle
wheel.
Unit 8: Statics (Bodies in Equilibrium) (1 weeks)
Primary resource: Giancoli Chapter 9 p 241-252
Topics:
Equilibrium; tensile and compressive forces of various configurations
Static conditions based involving upon torque balance analysis
Cantilever-beam and wire configurations
Apply static concepts to basic human biomechanical activities and manipulations.
Mini Lab -group work with individual student analysis submissions. Cantilever beam and wire
analysis.
Unit 9: Fluids (1 week)
Primary resource: Giancoli Chapter 10 p 275-308
Topics:
Density; specific gravity; pressure.
Solve problems of Buoyancy and force.
Fluid Dynamics; Bernoulli’s principle; viscosity.
Venturis; capillarity; surface tension; laminar flow; turbulent flow.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 400 GRADES 9 - 12
Mini Lab -group work with individual student analysis submissions. Atmospheric can crush.
Students estimate the pressure involved and total force applied to the can.
Unit 10: Vibrations and Waves (1 week)
Primary resource: Giancoli Chapter 11 p 309--346
Topics:
Simple harmonic motion; sinusoidal mathematic representations.
Resonance; damping; interference; reflection.
Application of the concepts of wave theory to mechanical and electromagnetic waves
(velocity, wavelength, frequency, transverse, longitudinal).
Simple pendulums and springs.
Lab #11: Resonance and Waves
Predict and analyze the standing waves generated by the Pasco oscillator using stretch
chord.
Analyze and mathematically model the resonance associated with various metallic leafs
driven by the Pasco oscillator.
Analyze and model the nodal patterns associated metallic plate oscillations when driven
by the Pasco oscillator.
Unit 11: Sound (1 weeks)
Primary resource: Giancoli Chapter 12 p 347-380
Topics:
Sound wave theory, media effects, and temperature effects.
Sound intensity and the unit of decibels.
Fundamental and harmonic frequencies; basic application to music theory.
Open tube and closed tube harmonic effects.
Doppler theory/analysis/application.
Interference phenomenon.
Mini Lab -group work with individual student analysis submissions. Open tube and closed tube
exploration. Use Pasco sound frequency detector.
Unit 12: Temperature, Kinetic Theory, Heat (2 weeks)
Primary resource: Giancoli Chapter 13 p 381-416
Chapter 14 p 417-442
Topics:
Understand and use temperature scales, the gas laws and mole theory.
Thermal expansion and thermal stresses.
Properties of water (heat addition/loss, density effects, phase changes, triple-point, effects
of pressure, vapor pressure, and dew point.)
Heat, specific heat, latent heat, internal energy
Systems involving heat transfer.
Methods of heat transfer and real life connections/application/observation.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 401 GRADES 9 - 12
Unit 13: Thermodynamics (1 week)
Primary resource: Giancoli Chapter 15 p 443-475
Topics:
Laws of thermodynamics and application to system. Temperature scales, the gas laws and
mole theory.
Adiabatic, isothermal, isobaric, and isovolumetric processes.
Heat Engine cycles and efficiency. Application to several 4-stroke automobile engines,
and steam turbines.
Carnot cycle; refrigeration cycle; heat pump theory.
Entropy theory and systems involving energy transfer.
Unit 14: Electric Charge, Fields, Potential (2 weeks)
Primary resource: Giancoli Chapter 16 p 476-501
Chapter 17 p 502-526
Topics:
Charge, Static Electricity, Induced Charge,
Coulomb’s Law and associated vector analysis.
Electric Fields, Field Lines, Forces, Electric Potential, Equipotential Lines
Voltage, Energy, Capacitance, Dielectrics, Stored Charge, Potential Energy.
Lab # 12 Charge and Potential Energy
Experiment with various materials to develop a stored charge or induce a charge.
Use the Van de Graff generator to personally experience a charge in various ways, and
use the electric field developed to perform work with several “kit” experiments.
Calculate the amount of charge stored on the Van de Graff Sphere based upon observable
information.
Unit 15: DC Circuits (1 week)
Primary resource: Giancoli Chapter 18 p 527-554
Chapter 19 p 555-587
Topics:
Battery theory, Current,
Conductivity, Resistivity, Resistance, Ohm’s Law.
Parallel and Series circuits (Resistive).
Capacitors in Series and parallel
Direct Current (DC) Alternating Current (AC)
Household Circuits
Electric Power.
Circuits containing Resistors and Capacitors.
Ammeters and Voltmeters
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 402 GRADES 9 - 12
Lab # 13 Circuit Construction and Analysis
Use Pasco Battery powered Electrical Exploration kit and a digital multi-meter to design
several circuits involving parallel and series circuits of light bulbs, resistors, batteries,
and switches.
Measure currents, voltages, and resistances; compared observed values to theoreticals and
evaluate any inconsistencies.
Unit 16: Magnetism, EMF, Motors, Generators (2 weeks)
Primary resource: Giancoli Chapter 20 p 588-621
Chapter 21 p 622-659
Topics:
Magnets, Magnetic Fields, associated Vectors and Forces.
Force on a charge, force on a current.
Currents and magnetic fields; magnetic field energy.
Faraday’s law; Lenz’s law.
Mass Spectrometer and mathematical relationships.
Electromagnets, Solenoids, Inductors.
Motors and Generators.
Transformers.
AC Circuits and Impedance.
Resonance in AC circuits; oscillators.
Mini Lab -group work with individual student analysis submissions. Analyze the magnetic
field/electron beam radius/accelerator potential relationship using data taken from several
demonstration runs of the Thompson Cathode Ray tube device.
Lab # 14 DC Motor Experiment
Use the Lab-Volt variable power supply and the supplied magnets, coils, brush
assemblies, and wiring to deign and operate several DC motors. Note the speed to voltage
relationship. Measure voltage and current using the analog meters while the motor is
running and while it comes up to speed. Discuss the various relationships and
phenomena.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 403 GRADES 9 - 12
Unit 17: Electromagnetic Waves (1 week)
Primary resource: Giancoli Chapter 22 p 660-682
Topics:
Changing electric field/magnetic field relationship. Maxwell’s Equation and theory.
Electromagnetic Spectrum.
Speed wavelength frequency relationship.
Measuring the speed of light.
Visible light’s part of the spectrum and the rainbow.
Unit 18: Optics and Light Theory (2 weeks)
Primary resource: Giancoli Chapter 23 p 683-722
Chapter 24 p 723-756
Chapter 25 p 757-791
Topics:
Wave-fronts and ray-modeling.
Reflection, plane mirrors, curved mirrors.
Refraction, Snell’s law, total internal reflection.
Thin lenses, lens equation.
Particle theory, Huygens principle
Interference, Diffraction.
Thin film interference.
Spectroscopy and the spectrometer.
Polarization
Optical Instruments (the camera, the human eye, the telescope, the microscope, the
periscope.)
Aberrations of lenses and mirrors. Optical coatings. Resolution.
Lab # 15 Color addition, Color Subtraction, Prism
Determine the colors that result from the addition of two or three primary colors on a
white background.
Investigate the results of illuminating different colored paper with different colored light.
Use a prism to separate white light into its component colors and note the different angles
at which the different colors are refracted.
Investigate the refraction of different color light beams through the prism.
Lab # 16 Snell’s Law and Total Internal Reflection.
Use Snell’s Law to determine the Index of Refraction of an acrylic prism.
Determine the critical angle at which total internal reflection occurs for the acrylic prism.
Lab # 17 Reflection (Plane Mirrors and Curved Mirrors)
Study ray reflection and determine the focal length and radius of curvature for various
mirrors.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 404 GRADES 9 - 12
Lab # 18 Lenses (Concave and Convex)
Explore the difference between convex and concave lenses and determine the focal
lengths of various lenses using the optics bench.
Study ray reflection and determine the focal length and radius of curvature for various
mirrors.
Lab # 19 Telescope
Use a combination of lenses to construct a telescope and determine its magnification
Analyze the ray-paths of the telescope.
Unit 19: Special Relativity (1.5 weeks)
Primary resource: Giancoli Chapter 26 p 792-822
Topics:
Postulates of Relativity
Time dilation, length contraction
Four-dimensional space-time.
Momentum and mass. Mass and energy.
Relativistic addition of velocities
Unit 20: Quantum Physics (1.5 weeks)
Primary resource: Giancoli Chapter 27 p 823-858
Chapter 28 p 859-886
Topics:
Particle properties
Planck’s quantum hypothesis
Photons as particles, Photoelectric effect, Compton effect.
Wave particle duality
Wave nature of matter
Atomic models; Atomic Spectra; deBroglie’s hypothesis
Quantum mechanics; Uncertainty principle
Unit 21: Nuclear Physics (2 weeks)
Primary resource: Giancoli Chapter 30 p 916-942
Chapter 31 p 943-972
Topics:
Structure and properties of the nucleus
Binding Energy; nuclear forces; mass defect
Radioactivity; Periodical Table review; Conservation of nucleon number
Alpha decay; beta decay; gamma decay
Half life; decay rate; decay series
Radioactive dating
Detecting radiation; detectors
Fission; Fusion; transmutations
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 405 GRADES 9 - 12
Radiation damage to matter/tissue; dosimetry; exposure and controls
Radiation therapy
Use Pasco Battery powered Electrical Exploration kit and a digital multi-meter to
Measure currents, voltages, and resistances; compared observed values to theoreticals.
Lab # 20 Radiation Detection
Use radiation detectors with various known sources to detect and measure radiation
compared to background.
Estimate the effect of distance on the radiation detected. Compare to observed radiation
levels for several distances.
Experiment with various types of shielding and not the effect on radiation detected.
Evaluate the radiation properties of several common items. (smoke detector, glow in the
dark items)
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 406 GRADES 9 - 12
Lab Summary
Lab #
Title/Topic
Activity/Objective Summary
1
Motion in One
Dimension
Analyze the motion of an object and predict the resulting x vs. t, and v vs. t graphs. Predict when the
acceleration is constant, and its sign.
Evaluate x vs. t, and v vs. t graphs and produce the correct motion?
Equipment: Pasco Motion sensor; computer.
2
Acceleration of
Gravity
Compute theoretical acceleration due to gravity based upon the angle of the ramp. Use Pasco Frictionless cart,
motion sensor and computer software. Evaluate observed acceleration using various graphs (Slope, Least
Square fit). Identify and discuss sources of error. Qualitatively assess the fractional effect of friction related to
data taken at various angles.
Equipment: Pasco Motion sensor, cart, computer, adjustable ramp, meter stick.
3
Projectile Motion
Predict where a projectile will land (ideal conditions-no error analysis) and evaluate the estimate based upon
experimental results.
Include a qualitative assessment of errors, and repeat the experiment attempting to obtain 4 out of 5 launches
within a specified circular landing area.
Repeat for various angles, launch velocities and launch heights.
Equipment: projectile launcher, electronic photo-gate timer with laptop, large paper, tape measure, and meter
sticks.
4
Static Force
Balance 1
Given two specified forces applied to a point, determine the third force that would be required to establish
equilibrium. Use the Pasco Force balance table to verify results, and determine/analyze any error
Include a qualitative assessment of errors, and repeat the experiment for several specified forces and angles.
Equipment: Pasco Force table, various masses, Newton spring scale.
5
Static Force
Balance 2
Given a string suspended from two points on the ceiling, determine what angles and tensions will result in
each leg of the string when the mass is suspended from teacher specified locations on the string. Use Newton
spring scales and or Pasco Force sensors to evaluate theoretical results.
Include a qualitative assessment of errors.
Equipment: String, Masses, Newton spring scales, Pasco force sensors, meter sticks, protractors.
6
Dynamic Force
and Uniform
Accelerated
Motion
Using bathroom scales, student pushers, drivers and timers, Push the instructor’s car with a constant force,
measure the rate of acceleration and determine the mass of the car in kilograms. Several differ4ent groups with
varying applied force. All groups conduct all analysis.
Include a qualitative assessment of errors.
Equipment: Automobile, bathroom scales, stopwatch, cones, long tape measure.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 407 GRADES 9 - 12
7
Suspended Mass
Centripetal Force
Determine the period of rotation required to support various masses suspended through a 90-degree Force
direction change tube. Use various radii and masses for trials. Confirm results and identify any sources of
error.
Equipment: Suspended Mass-Centripetal Force apparatus, stop-watch, and ruler.
8
Frictional Forces
Determine the coefficients of static and kinetic friction on an aluminum surface for a block of wood having a
smooth wooden face and a fur coated face. Identify any sources of error.
Equipment: Inclined plane, inclinometer, meter stick.
9
Conservation of
Linear Momentum
and Energy
Predict the outcome of several lab-cart collisions based upon specified initial mass and speed configurations.
Equipment: Lab carts, photo-gates, masses, track, computer.
10
Conservation of
Angular
Momentum
Predict the period of rotation for a student on a frictionless rotating stool with arms/mass extended who then
draws the masses to his/her chest.
Experience the gyroscopic effect of the hand-held bicycle wheel demo and fully explain the effect of
manipulating the wheel perpendicular to its axis of rotation.
Equipment: Frictionless lab stool, masses, meter stick, stop-watch, hand-held bicycle wheel.
11
Resonance and
Waves
Predict and analyze the standing waves generated by the Pasco oscillator using stretch chord.
Analyze and mathematically model the resonance associated with various metallic leafs driven by the Pasco
oscillator.
Analyze and model the nodal patterns associated metallic plate oscillations when driven by the Pasco
oscillator.
12
Charge and
Potential Energy
Experiment with various materials to develop a stored charge or induce a charge.
Use the Van de Graff generator to personally experience a charge in various ways, and use the electric field
developed to perform work with several “kit” experiments.
Calculate the amount of charge stored on the Van de Graff Sphere based upon observable information.
13
Circuit
Construction and
Analysis
Use Pasco Battery powered Electrical Exploration kit and a digital multi-meter to design several circuits
involving parallel and series circuits of light bulbs, resistors, batteries, and switches.
Measure currents, voltages, and resistances; compared observed values to theoreticals and evaluate any
inconsistencies.
14
DC Motor
Experiment
Use the Lab-Volt variable power supply and the supplied magnets, coils, brush assemblies, and wiring to deign
and operate several DC motors. Note the speed to voltage relationship. Measure voltage and current using the
analog meters while the motor is running and while it comes up to speed. Discuss the various relationships and
phenomena.
15
Color addition,
Determine the colors that result from the addition of two or three primary colors on a white background.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 408 GRADES 9 - 12
Color Subtraction,
Prism
Investigate the results of illuminating different colored paper with different colored light.
Use a prism to separate white light into its component colors and note the different angles at which the
different colors are refracted.
Investigate the refraction of different color light beams through the prism.
Equipment: Pasco Light ray box and accessories
16
Snell’s Law and
Total Internal
Reflection.
Use Snell’s Law to determine the Index of Refraction of an acrylic prism.
Determine the critical angle at which total internal reflection occurs for the acrylic prism.
Equipment: Pasco Light ray box and accessories
17
Reflection (Plane
Mirrors and
Curved Mirrors)
Study ray reflection and determine the focal length and radius of curvature for various mirrors.
Equipment: Pasco Light ray box and accessories
18
Lenses (Concave
and Convex)
Explore the difference between convex and concave lenses and determine the focal lengths of various lenses
using the optics bench.
Study ray reflection and determine the focal length and radius of curvature for various mirrors.
Equipment: Pasco Light ray box, track and accessories
19
Telescope
Use a combination of lenses to construct a telescope and determine its magnification
Analyze the ray-paths of the telescope.
Equipment: Pasco Light ray box, track and accessories
20
Radiation
Detection
Use radiation detectors with various known sources to detect and measure radiation compared to background.
Estimate the effect of distance on the radiation detected. Compare to observed radiation levels for several
distances.
Experiment with various types of shielding and not the effect on radiation detected.
Evaluate the radiation properties of several common items. (smoke detector, glow in the dark items)
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 409 GRADES 9 - 12
Lab: Acceleration down an inclined plane (30 points)
Objective: Investigate acceleration down an inclined plane. Compare "Theoretical Acceleration"
(g·sinθ) to "Observed Acceleration" as measured by the Pasco CBL software.
Materials: Ramp, cart, motion sensor, meter stick, laptop with Pasco software.
Procedure:
1. Set up ramp, motion sensor, laptop. Use a cloth bundle to serve as a bumper and protect the
cart from damage.
2. Compute the theoretical acceleration for each angle prescribed in the data table.
3. Set the ramp height for each trial using trigonometric ratios to establish the required angle for
each step.
4. Coordinate lap-top operation and cart release to capture the data required. Repeat for each
angle prescribed.
Data Table:
Trial #
Angle
(degrees)
Theoretical Acceleration
Observed Acceleration
Percent Error
%100x
Theo
ObsTheo
1
0°
0.00
N/A
2
2°
3
5°
4
10°
5
15°
6
20°
7
25°
8
Spare
Analysis: (Use separate paper)
1. Show one sample calculation and diagram for how you set the angle of the ramp using
trigonometric ratios.
2. Calculate the theoretical acceleration for each trial. Show at least one sample calculation.
3. Calculate the Percent Error for each trial. Show at least one sample calculation.
4. Generate a quality line-graph (on graph paper) that clearly and accurately conveys your
results as summarized in the data table. You are not limited to only two variables. The best
graphs will include the angle vs. all 3 data columns.
Questions: (Use separate paper)
1. Does this activity confirm the relationship a = g·sinθ, where g =9.8m/s
2
?. Why?
2. Does the percent error increase or decrease as the angle increases? Why?
3. What are some possible sources of error for this lab? Which are the most significant and how
would you eliminate them.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 410 GRADES 9 - 12
PHYSICS (Level 2)
EFFECTIVE USE OF TECHNOLOGY
KINEMATICS AND DYNAMICS LABORATORY EXPERIMENTS
1 B
Exceeds Expectations
The student effectively presents raw data using a logical selection and
combination of graphs, tables, charts, scales, and colors. Where
appropriate, application software is utilized to process and present data.
Labels, scales and units clearly communicate meaning, and facilitate
understanding in the reader. Insightful and appropriate assumptions are
well expressed. Thoughts are well formulated and clearly flow throughout
the write-up referencing data, supporting calculations and the use of
technology. Real world observations are included that connect effectively
to the theory. Punctuation, spelling, and word usage are nearly flawless.
Conclusions are well versed, fluent and coherent.
Meets Expectations
Raw data is adequately presented using a logical selection and
combination of graphs, tables, charts, scales, and colors. Some lack of
clarity or missing information may occur. Labels, scales and units clearly
communicate meaning, and facilitate understanding in the reader.
Assumptions are adequately expressed and display an understanding of
the topic. Thoughts are well formulated and clearly flow throughout the
write-up. Real world observations are included that connect effectively to
the theory. Punctuation, spelling, and word usage are adequate.
Conclusions are meaningful and well-expressed.
Meets Some Expectations
Raw data is presented using a logical selection and combination of
graphs, tables, charts, scales, and colors. A significant lack of clarity or
missing information may occur, but should be addressed in accompanying
verbiage. Labels, scales and units clearly communicate meaning but the
reader may have some difficulty understanding. Assumptions are
adequately expressed but may not be fully developed or reflect complete
mastery of the theory. Thoughts are adequately expressed, but may not
always seem to flow logically through the document. Real world
observations are discussed adequately and sufficiently connect to the
theory. Punctuation, spelling, and word usage are adequate. Conclusions
are meaningful and adequately expressed.
Does Not Meet
Expectations
Raw data is poorly presented or missing. Labels, scales, and units are
missing or not useful. Assumptions are poorly expressed or completely
inappropriate. Thoughts are not adequately expressed in a fashion that
conveys information to the reader, but may not always seems to flow
logically through the document. Ideas and concepts are unorganized or
inaccurate. Punctuation, spelling, and word usage are inadequate. Errors
in the conventions of writing make the report ineffective.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 411 GRADES 9 - 12
PHYSICS
EFFECTIVE USE OF TECHNOLOGY
KINEMATICS AND DYNAMICS LABORATORY EXPERIMENTS
2
Exceeds Expectations
The student independently collects, interprets, analyzes, and evaluates
data using the Pasco lab equipment. (The student who exceeds
expectations may be seen teaching others or leading the group). S/he
independently explores ways to use the equipment to accomplish the
experiment. The student is able to transfer data to other application
software programs for further analysis or display. The student adjusts
sample rates, inputs, and detectors appropriately. S/he is able to
manipulate the various graphs and use ―best-fit‖ algorithms to mine the
most meaningful data and conclusions from the experiment.
Meets Expectations
The student independently collects, interprets, analyzes, and evaluates
data using the Pasco lab equipment, but requires occasional assistance.
S/he requires minimal coaching in using the equipment to accomplish the
experiment. The student uses the data and evaluation tools to draw
logical conclusions. The student adjust sample rates, inputs, and
detectors appropriately, but may require minor assistance or coaching.
S/he is able to manipulate the various graphs and use ―best-fit‖
algorithms to support the experiment.
Meets Some Expectations
The student effectively collects, interprets, analyzes, and evaluates data
using the Pasco lab equipment, but requires significant or repeat
assistance. S/he requires coaching and some assistance in using the
equipment to accomplish the experiment. The student makes some minor
errors in interpreting the data or using the technology, but manages to
draw logical conclusions and evidence learning. S/he is able to
manipulate the various graphs and use ―best-fit‖ algorithms to support
the experiment although s/he may require assistance from peers or
teacher.
Does Not Meet
Expectations
The student was unable to successfully use the Pasco equipment and
laptop despite significant assistance and coaching. The student collected
incomplete data and/or missed significant portions. The student was
unable to assemble the equipment and manage the technology to
accomplish the most basic aspects of the experiment.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 412 GRADES 9 - 12
PHYSICS
EFFECTIVE CRITICAL THINKING STRATEGIES
KINEMATICS AND DYNAMICS LABORATORY EXPERIMENTS
3
Exceeds Expectations
The student effectively collects, interprets, analyzes, and evaluates data
to solve a problem. S/he applies reasonable and appropriate assumptions
while displaying due diligence in the setup of the experiment. S/he
understands and clearly makes highly effective use of the capabilities of
the various detectors and interactive software to solve problems and
evaluate the meaning of data. Calculations are technically accurate,
appropriately accomplished and demonstrate clear mastery of the theory
as applied to the physical application. Conclusions are correct and
indicate insightful understanding and a readiness for higher challenges.
Meets Expectations
The student effectively collects, interprets, analyzes, and evaluates data
to solve a problem. S/he applies reasonable and appropriate assumptions
while displaying due diligence in the setup of the experiment. S/he
understands and clearly makes highly effective use of the capabilities of
the various detectors and interactive software to solve problems with
minimum of error and assistance. S/he correctly evaluates the meaning
of data using technology. Calculations are technically accurate and
appropriately accomplished, but may contain minor errors. Conclusions
are correct and appropriate.
Meets Some Expectations
The student effectively collects, interprets, analyzes, and evaluates data
to solve a problem, although some important data may be missing,
requiring the student to make logical assumptions. S/he displays due
diligence in the setup of the experiment and in correctly executing clearly
delineated steps. S/he makes reasonably effective use of the capabilities
of the various detectors and interactive software to solve problems. Some
error or difficulty with the equipment and its implementation is
acceptable. S/he correctly evaluates the meaning of data using
technology, although may have difficulty connecting the data observe to
the theory using mathematical expression and calculations. Calculations
and conclusions are technically accurate and reflect at least a qualitative
understanding.
Does Not Meet
Expectations
The student collected incomplete data and or missed significant portions.
Interpretation and evaluation of the data indicates a lack of
understanding. The student failed to demonstrate basic principals of the
scientific method in setting up equipment and conducting a meaningful
and effective controlled experiment. The equipment was not used to
gather even the most basic level of quality data. Calculations and
conclusions display a clear lack of conceptual understanding.

AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 413 GRADES 9 - 12
Experiment 1: Color Addition
EQUIPMENT NEEDED
- Ray box (color rays) - Convex lens
- Colored paper (red, yellow, green, blue)
Purpose
To determine the colors that result from the addition of two or three primary colors and to show the
effect of illuminating colored objects with different colors of light.
Procedure
Place the ray box on a white sheet of paper on the table. Adjust the box so the primary colors are
showing. If the white screen from the Optics Bench (OS-8518) is available, it can be laid flat on the
table to make a good viewing platform for this experiment. It may be helpful to raise the front end
of the box by approximately I cm (The concave lens works fine for this). This causes the colored
rays to shine out a further distance.
Place the convex lens near the ray box so it focuses the rays and causes them to cross each other
at the focal point. What is the color of the light where the three rays come together? Record the
result in Table 1.1. It may be helpful to crease the paper so it forms a wall upon which the focal
point is projected. See Figure 1.1.
Figure 1.1: Ray Box for color addition
Now block the green ray with an opaque object. What color results from adding red and blue?
Record the result in Table 1.1.
Repeat Step 3, blocking one color each in succession and completing Table 1.1.
Table 1.1 Results of Color Addition
COLORS ADDED
RESULTING COLOR
red + blue + green
red + blue
red + green
green + blue
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 414 GRADES 9 - 12
Shine the three primary colors on each of the colored sheets of paper. What color does each sheet of
paper appear to be for each color of illuminating light? Record the results in Table 1.2.
Table 1.2 Results of Reflection Off Colored Paper
COLOR OF PAPER IN
WHITE LIGHT
COLOR OF
LIGHT RAY
COLOR OF PAPER
IN COLORED LIGHT
Red
Green
Blue
Red
Green
Blue
Red
Green
Blue
Red
Green
Blue
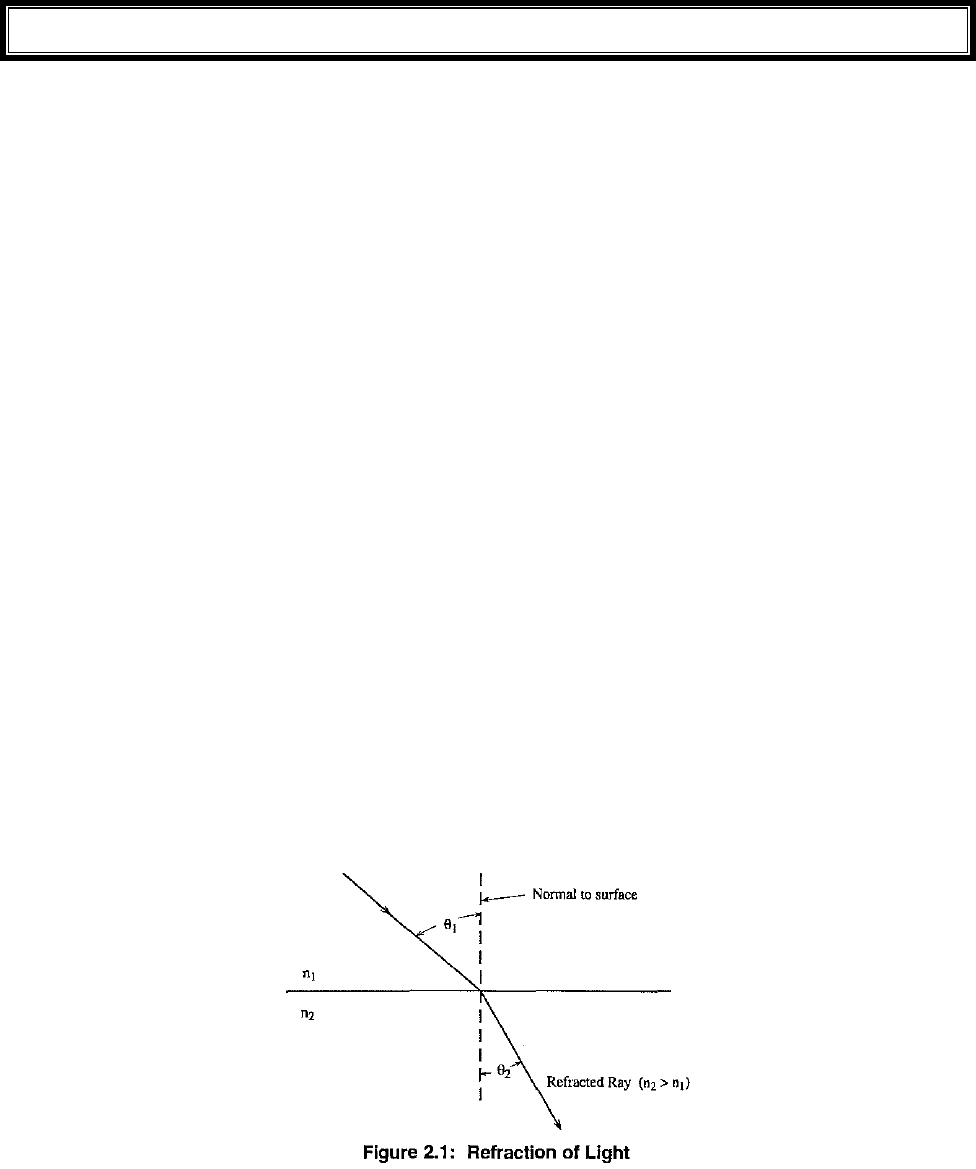
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 415 GRADES 9 - 12
Experiment 2: Prism
EQUIPMENT NEEDED
- Ray box (white ray)
- Rhombus
Purpose
To show how a prism separates white light into its component colors and to show that different
colors are refracted at different angles through a prism.
Theory
According to Snell's Law,
n
1
sin θ
1
= n
2
sin θ
2
the angle of refraction depends on the angle of incidence and the index of refraction of the
material. See Figure 2.1. Because the index of refraction for light varies with the frequency of
the light, white light which enters the material at a given angle of incidence will separate out into
its component colors as each frequency is bent a different amount.
The rhombus is made of Acrylic which has an index of refraction of 1.497 for light of
wavelength 486 nm in a vacuum, 1.491 for wavelength 589 nm, and 1.489 for wavelength 651
nm (red). Notice that in general for visible light, the index of refraction for Acrylic increases
with increasing frequency.
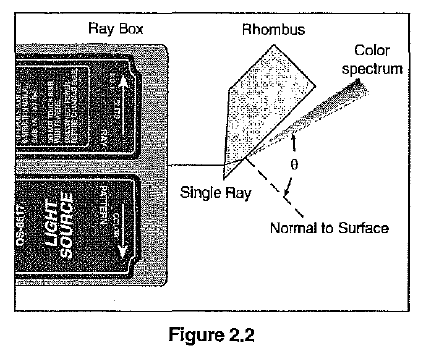
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 416 GRADES 9 - 12
Procedure for Separating White light
Place the ray box, label side up, on a white sheet of paper on the table. Adjust the box so one
white ray is showing. If the white screen from the OS-S5IS Optics Bench is available, it can
be laid flat on the table to make a good viewing platform for this experiment.
Position the rhombus as shown in Figure
2.2. The triangular end of the rhombus is
used as a prism in this experiment. Keep
the ray near the point of the rhombus for
maximum transmission of the light.
Rotate the rhombus until the angle (9) of
the emerging ray is as large as possible
and the ray separates into colors.
(a) What colors are seen and in what order
are they?
(b) Which color is refracted at the largest
angle?
(c) According to Snell's Law and the information given about the frequency dependence of
the index of refraction for Acrylic, which color is predicted to refract at the largest angle?
Turn the ray box over and shine the three primary color rays into the rhombus at the same
angle used for the white ray. Do the colored rays emerge from the rhombus parallel to each
other? Why or why not?
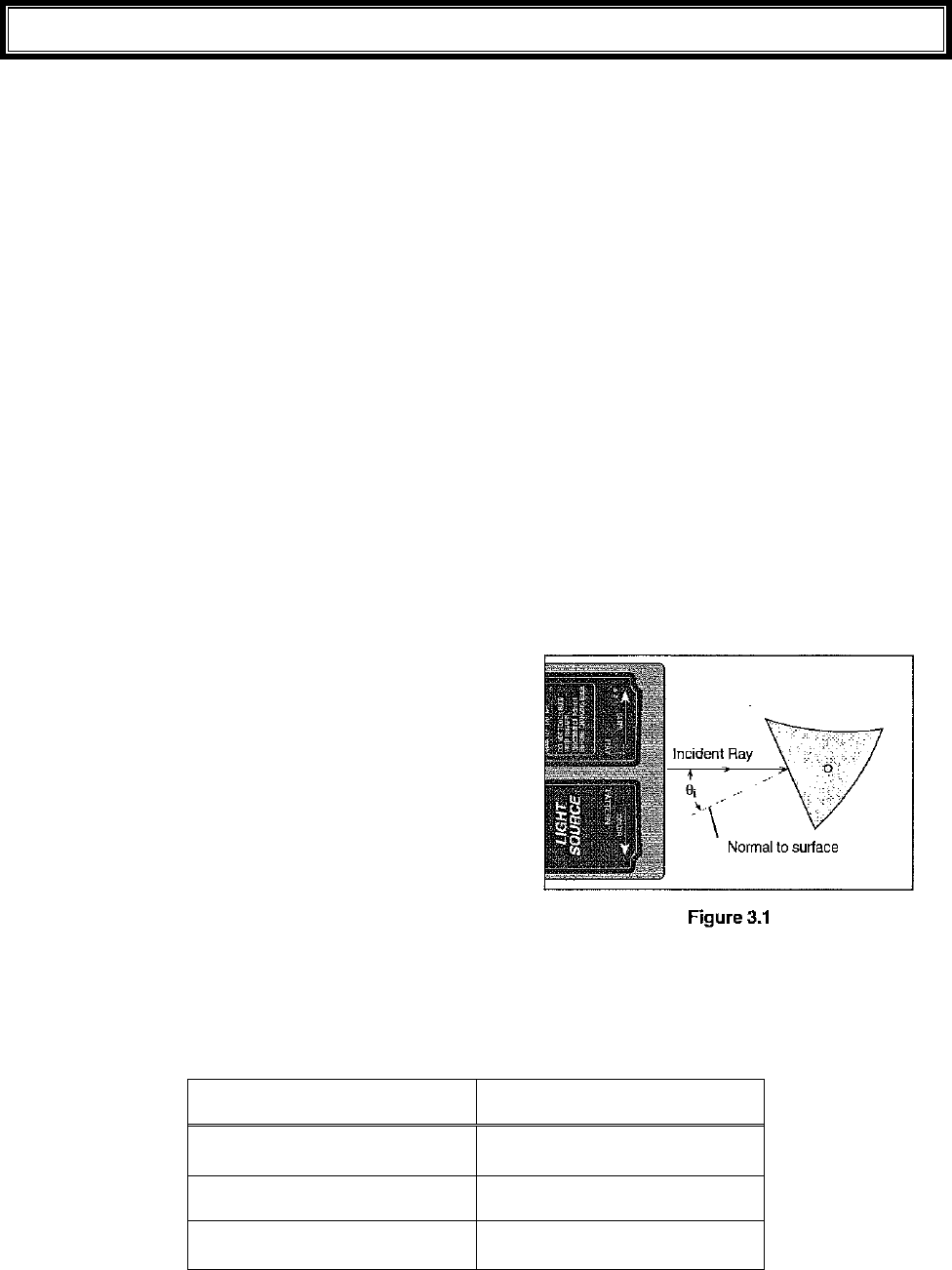
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 417 GRADES 9 - 12
Experiment 3: Reflection – Plane and Curved Mirrors
EQUIPMENT NEEDED
- Ray box (single and multiple white rays) - Plane and curved mirrors
- Protractor (SE-8732) - Drawing compass (SE-8733)
- Metric rule - White paper
Purpose
To study how rays are reflected and to determine the focal length and radius of curvature of different
types of mirrors.
Part I: Plane Mirror
Procedure
Place the ray box, label side up, on a white sheet of paper on the table. Adjust the box so one white
ray is showing.
Place the mirror on the table and position the plane surface of the mirror at an angle to the ray so that
the both the incident and reflected rays are clearly seen.
Mark the position of the surface of the plane mirror and trace the incident and reflected rays. Indicate
the incoming and the outgoing rays with arrows in the appropriate directions.
On the paper, draw the normal to the surface. See
Figure 3.1.
Measure the angle of incidence (8.,) and the angle of
reflection. Both these angles should be measured
from the normal. Record the angles in Table 3.1.
Change the angle of incidence and measure the
incident and reflected angles again. Repeat this
procedure for a total of three different incident
angles.
Adjust the ray box so it produces the three primary
color rays. Shine the colored rays at an angle to the
plane mirror. Mark the position of the surface of the plane mirror and trace the incident and reflected
rays. Indicate the colors of the incoming and the outgoing rays and mark them with arrows in the
appropriate directions.
Table 3.1 Plane Mirror Results
Angle of Incidence
Angle of Reflection
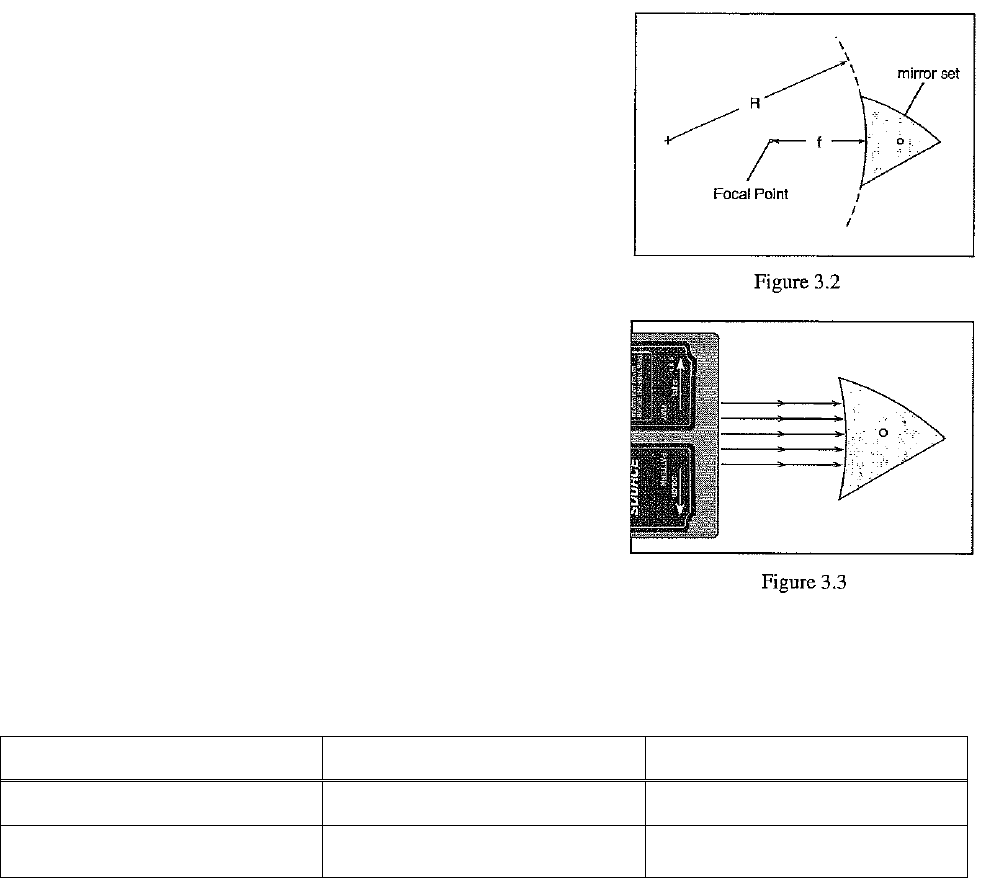
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 418 GRADES 9 - 12
Questions
What is the relationship between the angle of incidence and the angle of reflection?
Are the three colored rays reversed left-to-right by the plane mirror?
Part II: Cylindrical Mirrors
Theory
A concave cylindrical mirror will focus parallel rays
of light at the focal point. The focal length is the
distance from the focal point to the center of the
mirror surface. The radius of curvature of the mirror
is twice the focal length. See Figure 3.2.
Procedure
Using five white rays from the ray box, shine the rays straight
into the concave mirror so the light is reflected back toward
the ray box. See Figure 3.3. Draw the surface of the mirror and
trace the incident and reflected rays. Indicate the incoming and
the outgoing rays with arrows in the appropriate directions.
The place where the five reflected rays cross each other is the
focal point of the mirror. Measure the focal length from the
center of the concave mirror surface to the focal point. Record
the result in Table 3.2.
Use the compass to draw a circle that matches the curvature of
the mirror. Measure the radius of curvature using a rule and
record it in Table 3.2.
Repeat Steps I through 3 for the convex mirror. Note that in
Step 2, the reflected rays are diverging for a convex mirror and
they will not cross. Use a rule to extend the reflected rays back
behind the mirror's surface. The focal point is where these
extended rays cross.
Table 3.2 Cylindrical Mirror Results
Concave Mirror
Convex Mirror
Focal Length
Radius of Curvature using
compass
Questions
What is the relationship between the focal length of a cylindrical mirror and its radius of curvature?
Do your results confirm your answer?
What is the radius of curvature of a plane mirror?
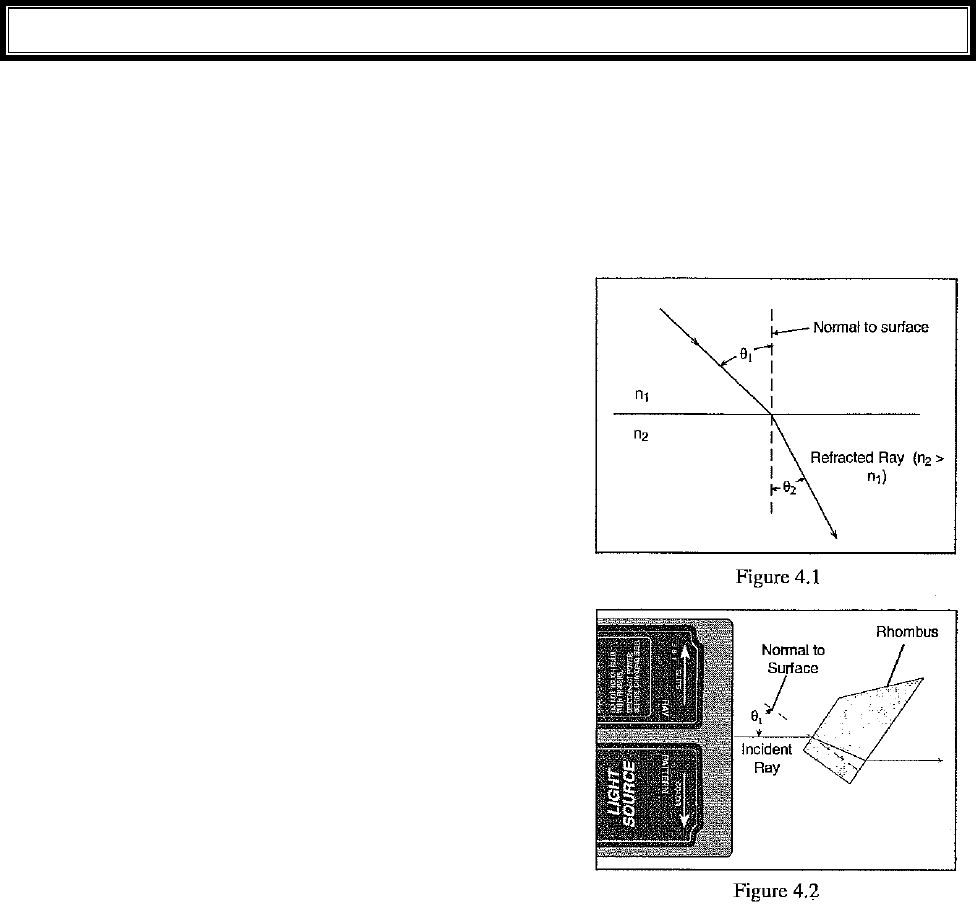
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 419 GRADES 9 - 12
Experiment 4: Snell’s Law
EQUIPMENT NEEDED
- Ray box (single white ray and colored rays)
- Rhombus
- Protractor (SE-8732)
- White paper
Purpose
To use Snell's Law to determine the index of refraction of the
acrylic rhombus.
Theory
Snell's Law states
n
1
sin θ
1
= n
2
sin θ
2
where θ
1
is the angle of incidence, θ
2
is the angle of
refraction, and n
1 and
n
2
are the respective indices of
refraction of the materials. See Figure 4.1.
Procedure
Place the ray box, label side up, on a white sheet of
paper on the table. Slide the ray mask until only one
white ray is showing.
Place the rhombus on the table and position it so the
ray passes through the parallel sides as shown in
Figure 4.2.
Mark the position of the parallel surfaces of the
rhombus and trace the incident and transmitted rays.
Indicate the incoming and the outgoing rays with
arrows in the appropriate directions. Mark carefully
where the ray enters and leaves the rhombus.
Remove the rhombus and on the paper draw a line
connecting the points where the ray entered and left the rhombus.
Choose either the point where the ray enters the rhombus or
the point where the ray leaves the rhombus. At this point, draw the normal to the surface.
Measure the angle of incidence (θ
1
) and the angle of refraction with a protractor. Both these angles
should be measured from the normal. Record the angles in Table 4.1.
Change the angle of incidence and measure the incident and refracted angles again. Repeat this
procedure for a total of three different incident angles.
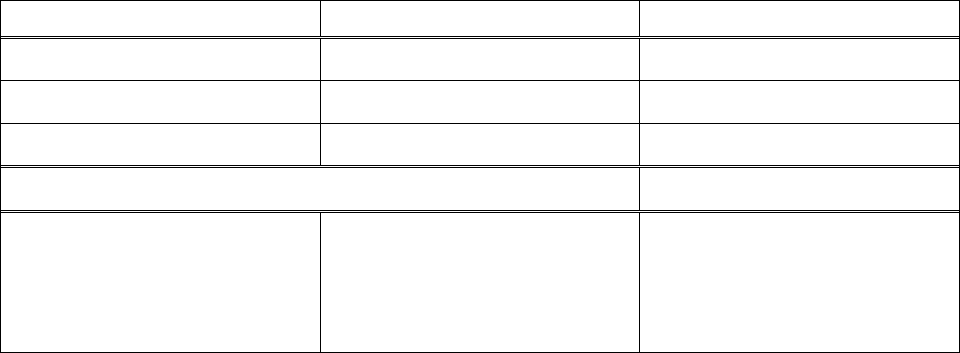
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 420 GRADES 9 - 12
Table 4.1 Data and Results
Angle of Incidence
Angle of Refraction
n rhombus
Average index of refraction
Analysis
Using Snell's Law and your data, calculate the index of refraction for the Acrylic
rhombus, assuming the index of refraction of air is one. Record the result for each of the
three data sets in Table 4.1.
Average the three values of the index of refraction and compare to the accepted value
(n = 1.5) using a percent difference.
Question
What is the angle of the ray that leaves the rhombus relative to the ray that enters the
rhombus?
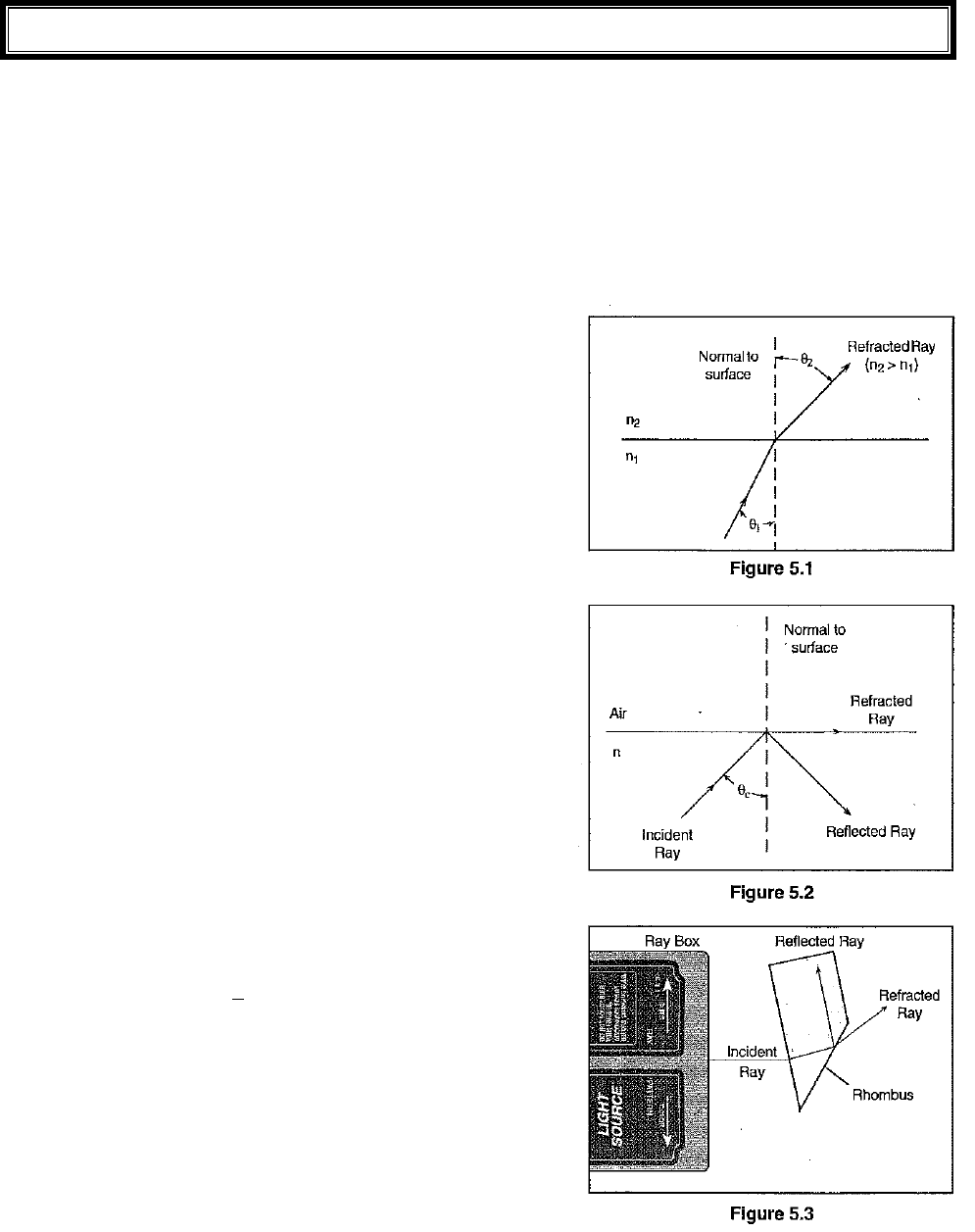
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 421 GRADES 9 - 12
Experiment 5: Total Internal Reflection
EQUIPMENT NEEDED
- Ray box (single ray)
- Rbombus
- Protractor (SE-8732)
- White paper
Purpose
To determine the critical angle at which total internal reflection occurs and to confirm it using
Snell's Law.
Theory
n
1
sin θ
1
= n
2
sin θ
2
where θ
1
is the angle of incidence, θ
2
is the angle of
refraction, and n
1 and
n
2
are the respective indices of refraction
of the materials. See Figure 5.1.
If a ray of light traveling from a medium of
greater index of refraction to a medium of
lesser index of refraction is incident with an
angle greater than the critical angle (θ
c
), there
is no refracted ray and total internal reflection
occurs. If the angle of incidence is exactly the
critical angle, the angle of the refracted ray is
90 degrees. See Figure 5.2. In this case,
using Snell's Law,
nsin θ
c
= (1)sin (90°)
assuming the medium of lesser index of refraction
is air with n
2
, = 1 and the medium of
greater index of refraction is the Acrylic rhombus
with n
1
= n = 1.5. Solving for the critical
angle gives
sin θ
c
=
n
1
Procedure
CD Place the ray box, label side up, on a white
sheet of paper on the table. Slide the ray mask
until only one white ray is showing.
Position the rhombus as shown in Figure 5.3.
Do not shine the ray through the rhombus too near the
triangular tip.
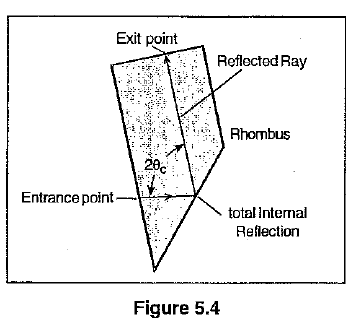
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 422 GRADES 9 - 12
Rotate the rhombus until the emerging ray just barely disappears. Just as it disappears, the ray
separates into colors. The rhombus is correctly positioned if the red has just disappeared.
Mark the surfaces of the rhombus. Mark exactly the point on the surface where the ray is internally
reflected. Also mark the entrance point of the incident ray and mark the exit point of the reflected ray.
Remove the rhombus and draw the rays that are incident upon and that reflect off the inside surface
of the rhombus. See Figure 5.4. Measure the total angle between these rays using a protractor.
If necessary, you may extend these rays to
make the protractor easier to use. Note that this
total angle is twice the critical angle because the
angle of incidence equals the angle of reflection.
Record the critical angle here:_________________
Calculate the critical angle using Snell's Law and
the given index of refraction for Acrylic. Record the
theoretical value here:_______________________
Calculate the percent difference between the measured
and theoretical values:
% difference-______________________________
Questions
How does the brightness of the internally reflected ray change when the incident angle changes from
less than θ
c
, to greater than θ
c
, ?
Is the critical angle greater for red light or violet light? What does this tell you about the index of
refraction
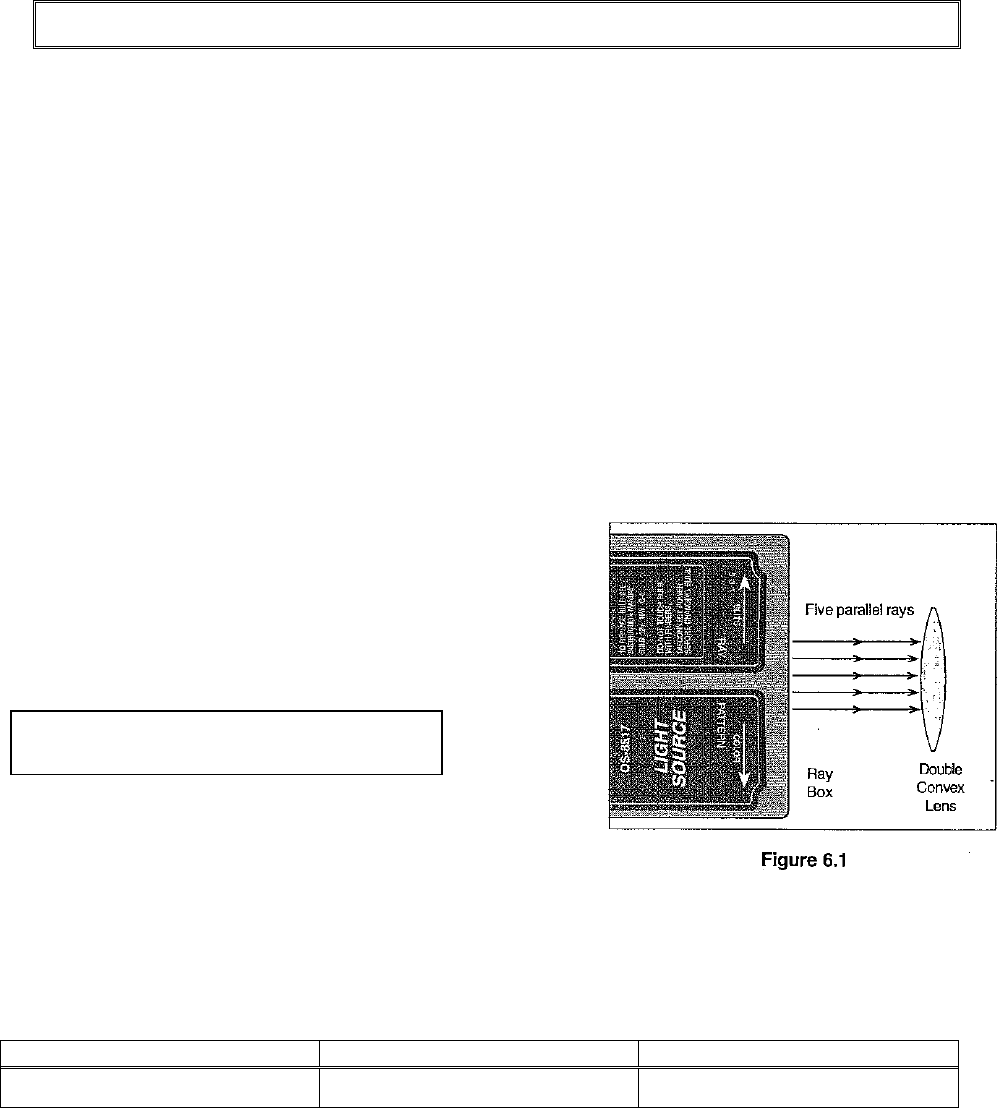
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 423 GRADES 9 - 12
Experiment 6: Refraction – Convex and Concave Lenses
EQUIPMENT NEEDED
- Ray box (multiple white rays)
- Convex lens
- Concave lens
- Metric rule
- Second convex lens (optional)
Purpose
To explore the difference between convex and concave lenses and to determine their focal
lengths.
Theory
Parallel rays of light passing through a thin convex lens cross at the focal point of the lens. The focal
length is measured from the center of the lens to the focal point.
Procedure
Place the ray box on a white piece of paper.
Using five white rays from the ray box, shine the rays straight
into the convex lens. See Figure 6.1.
Trace around the surface of the lens and
trace the incident and transmitted rays. Indicate
the incoming and the outgoing rays
with arrows in the appropriate directions.
The place where the five refracted rays cross each other is the focal point of the lens. Measure the focal
length from the center of the convex lens to the focal point. Record the result in Table 6.1.
Table 6.1 Results
Convex Lens
Concave Lens
Focal Length
Repeat the procedure for the concave lens. Note that in Step 2, the rays leaving the lens are diverging
and they will not cross. Use a rule to extend the outgoing rays straight back through the lens. The focal
point is where these extended rays cross.
Nest the convex and concave lenses together and place them in the path of the parallel rays. Trace the
rays. What does this tell you about the relationship between the focal lengths of these two lenses?
NOTE: Concave and Convex lenses have
only one flat edge. Place flat edge on surface.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 424 GRADES 9 - 12
Slide the convex and concave lenses apart to observe the effect of a combination of two lenses.
Then reverse the order of the lenses. Trace at least one pattern of this type.
Place the convex lens in the path of the five rays. Block out the center3 rays (the mirror on edge works
well) and mark the focal point for the outer two rays. Next, block out the outer two rays (or slide the
mask to the position that gives 3 rays) and mark the focal point for the inner 3 rays. Are the two focal
points the same?
If you have a second convex lens, place both convex lenses in the path of the five rays. The distance
between the lenses should be less than the focal length of the lenses. Compare the quality of the focus
of this two lens system to the focus of a single lens. Do all five rays cross in the same place?
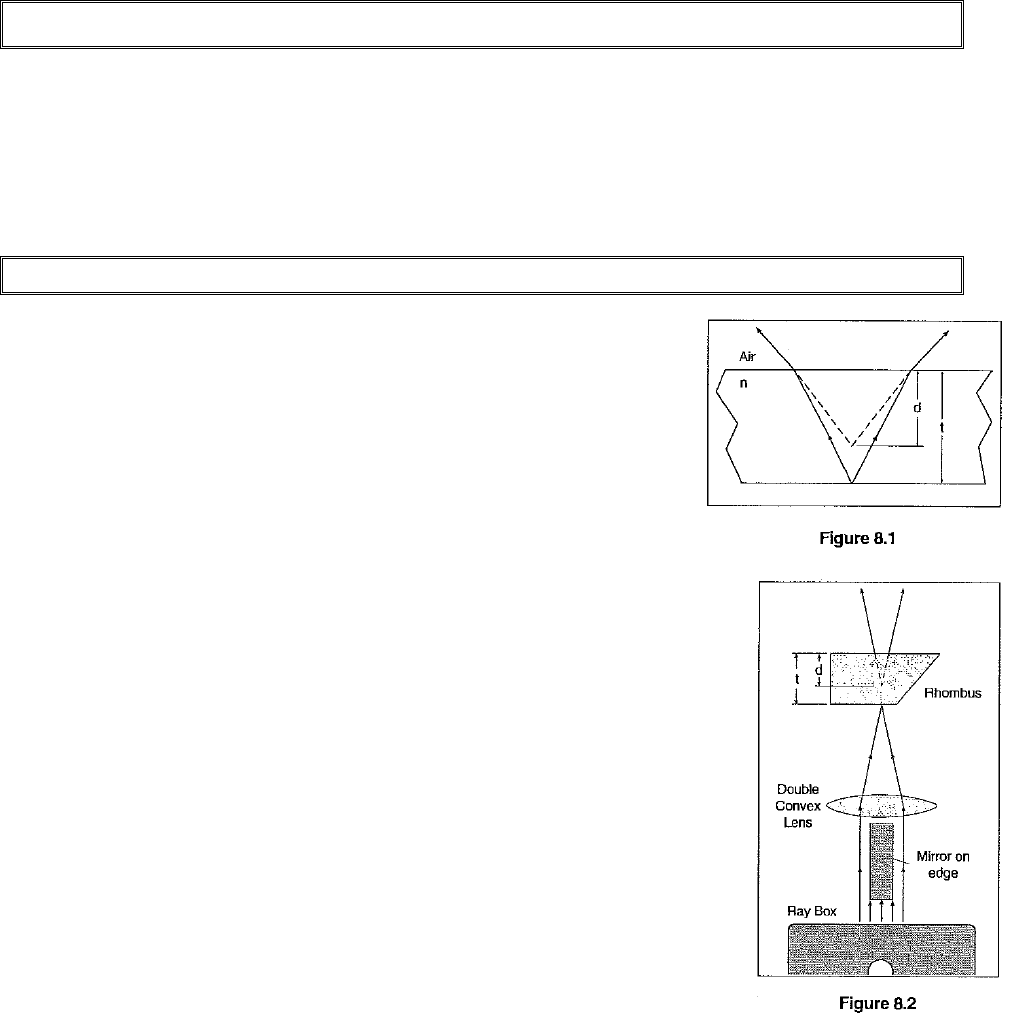
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 425 GRADES 9 - 12
Experiment 8: Apparent Depth
EQUIPMENT NEEDED
-Ray box
-Rhombus
-Mirror (Used to block center rays)
- Convex lens
- Metric rule
PART 1
Purpose
To determine the index of refraction using apparent depth.
Theory
Light rays originating from the bottom surface of a block of material
refract at the top surface as the rays emerge from the material into
the air. See Figure 8.1. When viewed from above, the apparent
depth, d, of the bottom surface of the block is less than the actual
thickness, t, of the block. The apparent depth is given by
d = tin, where n is the index of refraction of the material
Procedure
Place the ray box on a white piece of paper. Using five white rays from
the ray box, shine the rays straight into the convex lens. See Figure 8.2.
Place the mirror on its edge between the ray box and the lens so that it
blocks the middle three rays, leaving only the outside two rays.
Mark the place where the two rays cross each other.
Place the rhombus as shown in Figure 8.2. The bottom surface of the
rhombus must be exactly at the point where the two rays cross. The
crossed rays simulate the rays that emerge from the bottom of the
rhombus block discussed in the theory.
Trace the bottom and top surfaces of the rhombus and trace the rays
diverging from the top surface.
Remove the rhombus, turn off the light source, and trace the diverging
rays back into the rhombus. The place where these rays cross (inside the
rhombus) is the apparent position of the bottom of the rhombus when
viewed from the top.
Measure the apparent depth, d, and the thickness, t.
d = ____________________
t + ____________________
Calculate the index of refraction of the material using n = td. n = _____________
Calculate the percent difference between the measured value of the accepted value (n = 1.5).
% difference = _____________
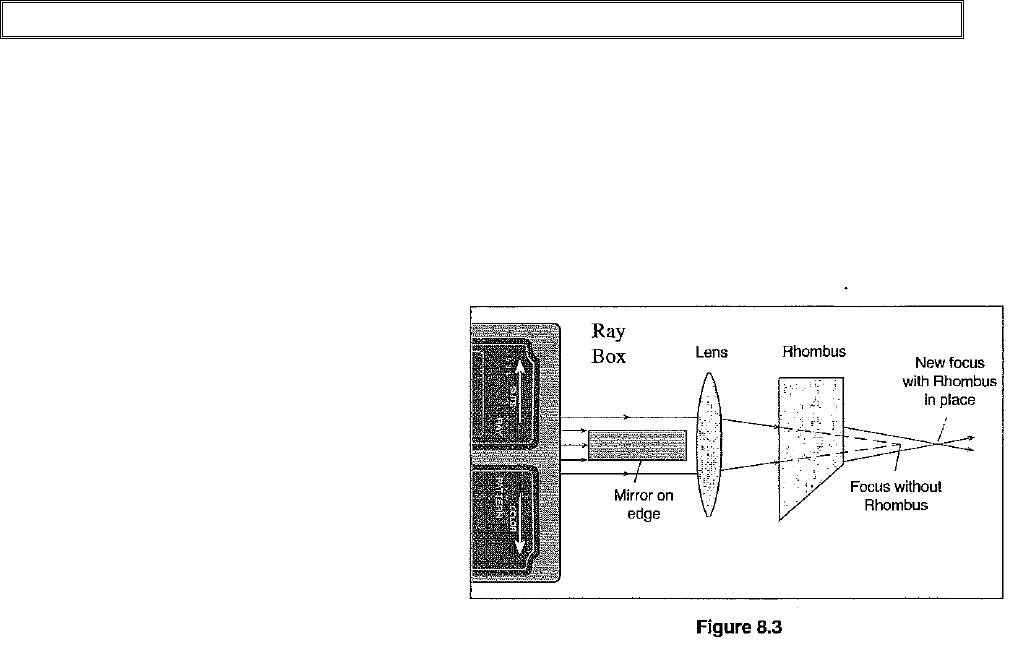
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 426 GRADES 9 - 12
PART II
Theory
Parallel rays passing through a convex lens cross at the focal point of the lens. If a block with parallel
sides is placed between the lens and the focal point, the point where the rays cross moves further from the
lens. Since the thickness, t, of the block has an apparent depth, d, that is less than the thickness (d = t/n),
the point where the rays cross must move by an amount equal the difference between the actual thickness
of the block and the apparent thickness of the block. Thus the distance, x, that the focal point moves is
given by x = t – t/n, where n is the index of refraction of the block.
Procedure
Turn the light source on. Using a new sheet
of paper, mark the place where the two rays
cross.
Set the rhombus between the lens and the
place where the rays cross. See Figure 8.3.
Mark the new place where the rays cross.
Move the rhombus to a new position, closer
to the lens. Does the position of the focal
point change?
Turn off the light source and measure the
distance, x, between the marks. x =
Using the thickness of the rhombus from Part I and the distance x, calculate the index of refraction
using n = ________
Calculate the percent difference between the measured value of the accepted value (n = 1.5).
% difference = ___________________
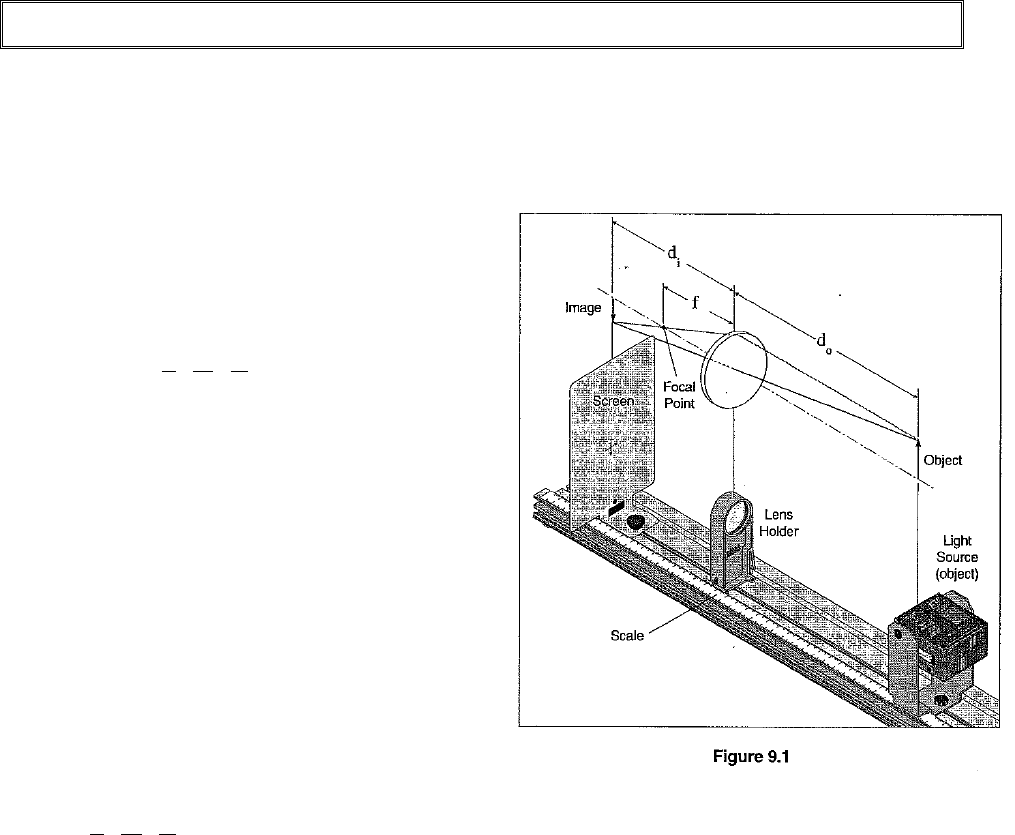
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 427 GRADES 9 - 12
Experiment 9: Focal Length of a Thin Lens
EQUIPMENT NEEDED
-Bench (OS-85 I 8) -Light source (object) (OS-8517)
-Convex lens -Screen
Purpose
To determine the focal length of a thin lens.
Theory
For a thin lens:
io
ddf
111
where f is focal length,
o
d
is the distance between
the object and the lens, and
i
d
is the distance
between the image and the lens. See Figure 9.1.
Procedure
I. FOCAL LENGTH USING AN OBJECT AT
INFINITY
Using one of the positive lenses focus a distant
light source on a paper.
Measure the distance from the lens to the paper.
This is the image distance.
Take the limit as the object distance goes to infinity in the Thin Lens Formula:
io
ddf
111
Solve for the focal length. f = _____
II. FOCAL LENGTH BY PLOTTING 1/
o
d
vs. 1/
i
d
a. On the optical bench, position the lens between a light source (the object) and a screen. Be sure
the object and the screen are at least one meter apart.
b. Move the lens to a position where an image of the object is formed on the screen. Measure the
image distance and the object distance. Record all measurements in table 9. I .
c. Measure the object size and the image size for this position of the lens.
d. Move the lens to a second position where the image is in focus (Do not move the screen or Light
Source). Measure the image distance and the object distance.
e. Measure the image size for this position also.
f. Move the screen toward the object until you can no longer find two positions of the lens where
the image will focus. Then move the screen a few centimeters further away from the object.
Repeat Parts b and d for this position of the screen and for 4 other intermediate positions of the
screen. This will give you 6 sets of data points (a total of 12 data points).
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 428 GRADES 9 - 12
g. Plot vs. using the 12 data points. This will give a straight line and the x- and y- intercepts are
each equal to 1/f.
h. Find the percent difference between the two values of the focal length found from the intercepts.
Then average these two values and find the percent difference between this average and the focal
length found in Part I.
i. For the first two sets of data points ONLY, use image and object distances to find the
magnification at each position of the lens.
objectsize
imagesize
M
Find the percent differences.
Table 9.1
Object distance
Image distance
Image size
o
d
1
i
d
1
1
2
3
4
5
6
7
8
9
10
11
12
x - intercept __________ y - intercept __________
f average__________ percent difference__________
QUESTIONS
Is the image fanned by the lens erect or inverted?
Is the image real or virtual? How do you know?
Explain why, for a given screen-object distance, there are two positions where the image is in focus.
Why is the magnification negative?
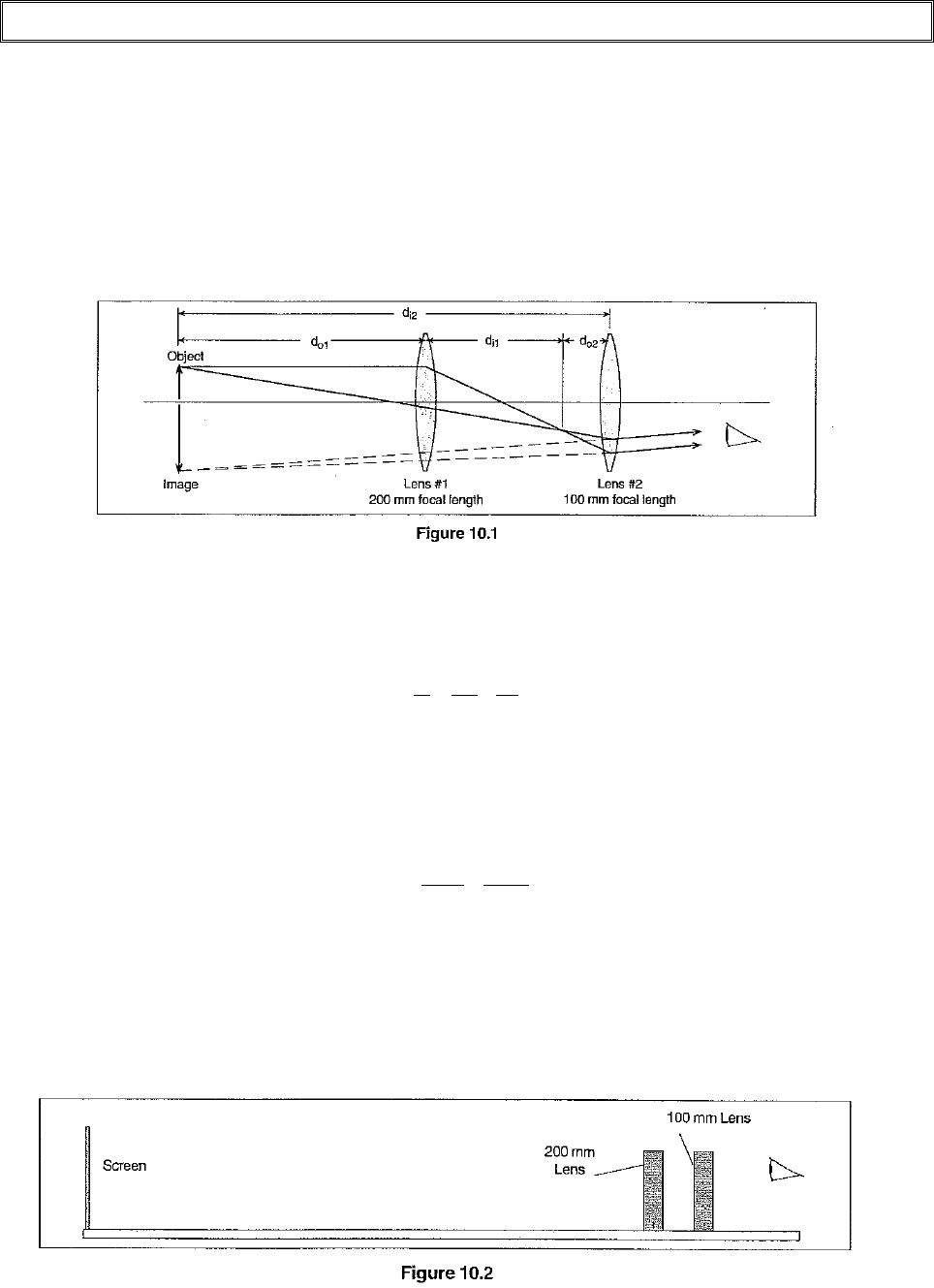
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 429 GRADES 9 - 12
Experiment 10: Telescope
EQUIPMENT NEEDED
- Bench (OS-851S) - 2 convex lenses (focal lengths 10 cm and 20 cm)
- Screen with paper pattern - Metric rule
(See back of manual for pattern)
Purpose
To construct a telescope and determine the magnification.
Theory
An astronomical telescope is constructed with two convex lenses. The ray diagram for this experiment
(shown in Figure 10.1) indicates that the image is in the same plane as the object. Having
the image in the same plane as the object allows the distance to the virtual image to be determined.
For this experiment, it is assumed that the lenses are thin compared to the other distances
involved. In this case the Thin Lens Formula may be used. This equation states:
io
ddf
111
where f is focal length,
o
d
, is the distance between the object and the lens, and
i
d
, is the distance
between the image and the lens.
The magnification of a two-lens system is equal to the multiplication of the magnifications of the
individual lenses:
2
2
1
1
21
o
i
o
i
d
d
d
d
Tape or use paper clips to fasten the paper pattern to the screen. The crosshatching on the screen acts
as the object.
The 200 mm lens is the objective lens (the one which is nearer to the object). The 100 mm lens is the
eyepiece lens (the one which is nearer to the eye). Place the lenses near one end of the optical bench
and place the screen on the other end. See Figure 10.2.
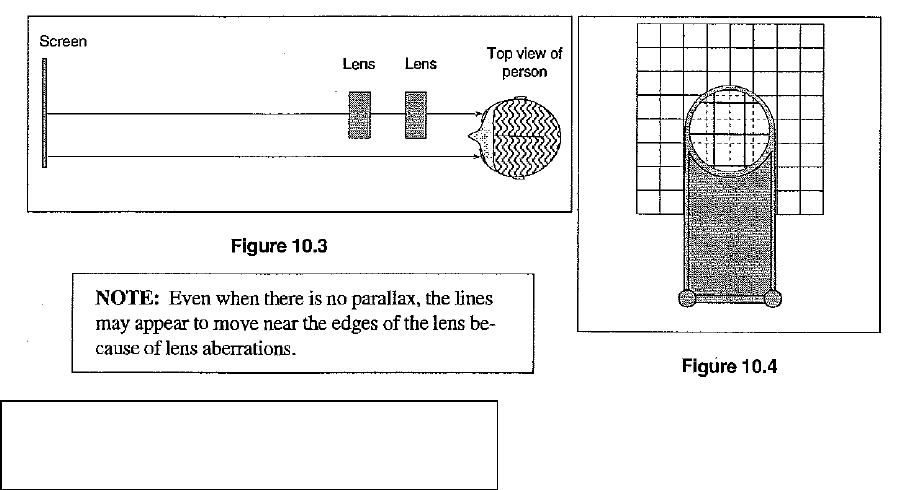
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 430 GRADES 9 - 12
Procedure
Focus the image of the object (the crosshatching on the screen) by moving the objective lens (the one
which is closer to the object). To view the image, you must put your eye close to the eyepiece lens.
Eliminate the parallax by moving the eyepiece lens until the image is in the same plane as the object
(screen). To observe the parallax, open both eyes and look through the lenses at the image with one eye
while looking around the edge of the lenses directly at the object with the other eye. See Figure 10.3. The
lines of the image (solid lines shown in Figure 10.4 inset) will be superimposed on the lines of the object
(shown as dotted lines in Figure 10.4 inset). Move your head back -and-forth or up-and- down. As you
move your head, the lines of the image will move relative to the lines of the object due to the parallax. To
eliminate the parallax, move the eyepiece lens until the image lines do not move relative to the object
lines when you move your head. When there is no parallax, the lines in the center of the lens appear to be
stuck to the object lines.
NOTE: Even when there is no parallax, the lines
may appear to move near the edges of the lens because
of lens aberrations.
With the parallax now eliminated, the virtual image is now in the plane of the object. Record the
positions of the lenses and the object in Table 10.1.
Measure the magnification of this telescope by counting the number of squares in the object that lies
along one side of one square of the image. To do this, you must view the image through the telescope
with one eye while looking directly at the object with the other eye. Record the observed magnification
in Table 10.1.
Remove the screen and look through the lenses at a distant object such as a meter stick at the opposite
side of the room. Eliminate the parallax and determine the magnification. When viewing an object at
infinity through a telescope, the magnification is the ratio of the focal lengths of the lenses. Check to
see if this is true for your telescope.
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 431 GRADES 9 - 12
Analysis
To calculate the magnification complete the following steps and record the answers in Table 10.1:
Determine
1o
d
, the distance from the object (paper pattern on screen) to the objective lens.
Determine
2i
d
, the distance from the eyepiece lens and the image. Since the image is in the plane of
the object, this is also the distance between the eyepiece lens and the object (screen).
Calculate
1i
d
using
1o
d
and the focal length of the objective lens in the Thin Lens Formula.
Calculate
2o
d
using
2i
d
and the focal length of the eyepiece lens in the Thin Lens Formula.
Calculate the magnification using:
2
2
1
1
21
o
i
o
i
d
d
d
d
Take a percent deviation between this value and the observed value.
Table 10.1 Results
Position of Objective Lens
(200mm)
Position of Eyepiece Lens
(100mm)
Position of Screen
Observed Magnification
1o
d
2i
d
1i
d
2o
d
Calculated Magnification
Percent Difference
Questions
Is the image inverted or erect?
Is the image seen through the telescope real or virtual?
AP PHYSICS (B) (ECE)
SCIENCE CURRICULUM 432 GRADES 9 - 12
PHYSICS
EFFECTIVE WRITING
OPTICS LABORATORY EXPERIMENTS
1. B
Exceeds Expectations
The student effectively presents raw data using a logical selection and
combination of graphs, tables, charts, scales, and colors. Where
appropriate, application software is utilized to process and present
data. Labels, scales and units clearly communicate meaning, and
facilitate understanding in the reader. Insightful and appropriate
assumptions are well expressed. Thoughts are well formulated and
clearly flow throughout the write-up referencing data, supporting
calculations and the use of technology. Real world observations are
included that connect effectively to the theory. Punctuation, spelling,
and word usage are nearly flawless. Conclusions are well versed, fluent
and coherent.
Meets Expectations
Raw data is adequately presented using a logical selection and
combination of graphs, tables, charts, scales, and colors. Some lack of
clarity
Meets Some Expectations
Does Not Meet
Expectations
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 433 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Advanced Placement Physics (C)
University of Connecticut, Early College
Experience (ECE)
General Physics with Calculus: PHYS 1401 Q
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
AP Physics (C)
3. Transcript Course Code/Number
00363
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 1
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of, or concurrent enrollment in AP Calculus (BC) or AP Calculus (AB)
12. Brief Course Description
AP Physics C is offered to students who have successfully completed or are concurrently enrolled in AP Calculus
(BC) or AP Calculus (AB) and who are planning to study physical science or engineering in college. This course
will prepare the student for the Mechanics portion of the AP Physics C exam. Electricity and magnetism will
also be studied. The use of calculus in problem solving and derivations will increase as the course progresses
and will be used freely in formulating principles and in solving problems when electricity and magnetism are
studied. Students who are accepted into the UCONN ECE program get University of Connecticut college credits
if they earn a UCONN grade of C or better.
Students are encouraged to take the AP Physics C Examination.
12. Course Goals
1. Demonstrate proficiency and fluency in communication to meet the literacy demands of the global
community.
2. Use technology effectively and responsibly.
3. Apply effective strategies for gathering information/materials, thinking critically and solving problems.
4. Demonstrate respect for one's self, and strive to contribute to the success of others.
5. Successfully complete homework and lab assignments independently.
6. Collaborate as a productive team-member of a group in accomplishing challenging inquiry based
experiments.
7. Conduct lab experiments safely and using appropriate scientific protocols.
8. Analyze and synthesize scientific information as it relates to everyday experience and surroundings.

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 434 GRADES 9 - 12
13. Sears and Zemansky's University Physics by Young and Freedman 12
th
Edition Pearson/Addison Wesley, 2008
Course Units of Study:
MECHANICS:
Chapter
# of Weeks
1. Units, Physical Quantities and Vectors
Chapter 1
1 week
2. Motion Along a Straight Line & Introduction to Calculus
Chapter 2
2 weeks
3 Motion in Two and Three Dimensions
Chapter 3
1.5 weeks
4. Newton's Laws of Motion and Applications
Chapters 4 & 5
3 weeks
5. Work and Kinetic Energy
Chapter 6
1.5 weeks
6. Potential Energy and Energy Conservation
Chapter 7
1.5 weeks
7. Momentum, Impulse and Collisions
Chapter 8
2 weeks
8. Rotation of Rigid Bodies
Chapter 9
1.5 weeks
9. Dynamics of Rotational Motion
Chapter 10
2 weeks
10. Equilibrium
Chapter 11
1 week
11. Periodic Motion
Chapter 13
2 weeks
12. Gravitation
Chapter 12
1 week
ELECTROMAGNETISM:
Chapter
# of Weeks
13. Electric Charge and Electric Field
Chapter 21
1.5 weeks
14. Gauss’s Law
Chapter 22
1.5 weeks
15. Electric Potential
Chapter 23
1.5 weeks
16. Capacitance and Dielectrics
Chapter 24
.5 week
17. Current, Resistance and Electromotive Force
Chapter 25
1.5 weeks
18. DC Circuits
Chapter 26
2 weeks
19. Magnetic Field & Magnetic Sources
Chapter 27
1.5 weeks
20. Sources of Magnetic Field
Chapter 28
1 week
21. Electromagnetic Induction
Chapter 29
1.5 weeks
22. Inductance
Chapter 30
.5 week
23. Alternating Current
Chapter 31
2 weeks
24. Electromagnetic Waves
Chapter 32
1 week
14. Instructional Methods and/or Strategies
Cooperative group work
Differentiated instruction
Lecture
Independent learning
Project based learning
Teacher centered and student centered discussions, demonstrations and analyses
15. Assessment Methods and/or Tools
Lab rubrics
Writing
Quizzes
Exams

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 435 GRADES 9 - 12
16. Assessment Criteria
Students will score at the ―Meets Expectations‖ level for the laboratory assignments listed according to
departmental/school-wide rubrics. Exam grading will be in accordance with a teacher generated key.
Benchmark Assignments:
1. Computer-based incline plane lab
2. Optics series of labs
3. Electric circuit series of labs addresses academic expectations 1a, 1b, 1c, 2, and 3
4. Final exam (Exit exam provided by University of Connecticut) addresses academic expectation 3
5. AP Physics C Exam addresses academic expectation 3
Trimester grades are based on homework completion, performance on assessments, performance on
laboratory reports, and classroom participation/effort
Final grades are based on a percentage of the trimester grade and up to 20% on the final exam grade.
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a common scoring rubric or grading criteria.
HONORS COURSES ONLY
17. Indicate how this honors course is different from the standard course.
This course will give the accepted students the opportunity to earn four (4) college credits from the
University of Connecticut.
The course follows the detailed ―Curriculum Audit‖ approved by Advanced Placement (AP) and the
University of Connecticut Early College Experience (ECE).
This course will prepare the student for the Mechanics portion of the AP Physics C examination.
AP PHYSICS (C) ECE
SCIENCE CURRICULUM 436 GRADES 9 - 12

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 437 GRADES 9 - 12
Syllabus
AP Physics C - Mechanics
Course Overview
This course is intended to prepare the student for the mechanics portion of the AP C
Physics exam. Electricity and magnetism will also be covered but will not be completed prior to
the administration of the AP C Physics exam. The student also has the opportunity to apply for
college credit through the UCONN Early College Experience program. First year calculus is a
co-requisite for this course.
The course is problem-solving based. Students are introduced to the various theories,
laws, and concepts of mechanics through assigned readings with reinforcement provided in
lectures. Strong emphasis is placed on problem-solving and problem solving techniques.
Homework, primarily problems will be assigned approximately 4 times per week. Classes will
include interactive lessons and frequent physical demonstrations, as well as web-based
demonstration modules. Tests will be administered upon completion of each unit.
There is also a lab component to the course. The labs are intended to reinforce and
extend the concepts learned in class. At least one session per week will be spent on laboratory
activities.
The class meets five days per week for one hour each class with every sixth class being
seventy minutes long. Class size for the 2007/2008 school year is expected to be less than fifteen
students.
Grading: Tests and Quizzes – 70%
Homework – 10%
Labs and Lab Notebook – 20 %
Text: Sears and Zemansky’s University Physics by Young & Freedman,
11
th
edition (Pearson/Addison Wesley)

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 438 GRADES 9 - 12
Course Outline
Semester 1:
1. Units, Physical Quantities, and Vectors (1 week) (Chapter 1)
2. Motion Along a Straight Line and Intro to Calculus (2 weeks) (Chapter 2)
3. Motion in Two and Three Dimensions (1½ weeks) (Chapter 3)
a. Projectile motion
b. Motion in a circle
c. Relative motion
4. Newton’s Law’s of Motion and Applications (3 weeks) (Chapters 4 and 5)
a. First law
b. Second law
c. Third law
d. Free-body diagrams
e. Friction
f. Dynamics of circular motion
5. Work and Kinetic Energy (1½ weeks) (Chapter 6)
a. Work
b. Kinetic Energy
c. Work-Energy theorem
d. Power
6. Potential Energy and Energy Conservation (1½ weeks) (Chapter 7)
a. Gravitational potential energy
b. Elastic potential energy
c. Conservative and non-conservative forces
d. Conservation of energy
e. Energy diagrams
7. Momentum, Impulse, and Collisions (2 weeks) (Chapter 8)
a. Momentum and impulse
b. Conservation of momentum
c. Elastic and inelastic collisions
d. Center of mass
8. Rotation of Rigid Bodies (1½ weeks) Chapter 9)
a. Angular velocity and acceleration
b. Relating linear and angular kinematics
c. Moment of Inertia
d. Rotational kinetic energy

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 439 GRADES 9 - 12
9. Dynamics of Rotational Motion (2 weeks) (Chapter 10)
a. Torque
b. Rigid-body rotation about a moving axis
c. Work and power in rotational motion
d. Angular momentum
e. Conservation of angular momentum
10. Equilibrium (1 week) (Chapter 11)
a. Conditions for equilibrium
b. Center of gravity
11. Periodic Motion (2 weeks) (Chapter 13)
a. Simple Harmonic motion
b. Hooke’s law
c. Pendulums
d. Damped Oscillations
12. Gravitation (1 week) (Chapter 12)
a. Newton’s law of gravitation
b. Gravitational potential energy
c. Kepler’s laws
Semester 2 - Electromagnetism:
13. Electric charge and electric field (1½ weeks) (Chapter 21)
14. Gauss’s Law (1½ weeks) (Chapter 22)
15. Electric potential (1½ weeks) (Chapter 23)
16. Capacitance and dielectrics (½ week) (Chapter 24)
17. Current, resistance, and electromotive force (1½ weeks) (Chapter 25)
18. DC Circuits (2 weeks) (Chapter 26)
19. Magnetic field and magnetic sources (1½ weeks) (Chapter 27)
20. Sources of magnetic field (1 weeks) Chapter 28)
21. Electromagnetic induction (1½ weeks) (Chapter 29)
22. Inductance (½ week) (Chapter 30)
23. Alternating Current (2 weeks) (Chapter 31)
24. Electromagnetic Waves (1 week) (Chapter 32)

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 440 GRADES 9 - 12
Lab Overview
There is also a lab component to the course. The labs are intended to reinforce and
extend the concepts learned in class. At least one session per week will be spent on laboratory
activities. Students will keep a lab notebook which will be periodically collected and assessed.
The lab grade will be 20% of the course grade.
Many of the labs are computer based using PASCO sensors and the PASCO Data Studio
software.
Lab Outline
Semester 1:
1. Composition of Vectors: A force table will be used to explore resolving vectors into
components.
2. One dimensional Motion Graphs: A sonic motion sensor will be used to generate plots
of position, velocity, and acceleration.
3. Projectile Motion
a. Projectile motion over level ground: A projectile launcher will be used to
explore the relationship between initial speed and angle on a projectile’s
flight over level ground.
b. Projectile motion: A projectile launcher, photogates and a switch pad will be
used to explore projectile motion where dy
i
≠ dy
f
.
4. Second Law
a. Free fall: Students will use various methods to explore the acceleration on an
object due to the force of gravity.
b. Acceleration on a ramp: A sonic motion sensor will be used to measure the
acceleration of a cart on an incline with a hanging mass at the top of the
incline opposing the component of force down the ramp.
5. Friction
a. Friction over level ground: A force sensor and a motion sensor will be used
to investigate friction over level ground.
b. Friction on a ramp: A force sensor and a motion sensor will be used
to investigate friction on a ramp.
6. Centripetal Force: Students will use the Pasco Rotational System to explore
centripetal force.

AP PHYSICS (C) ECE
SCIENCE CURRICULUM 441 GRADES 9 - 12
7. Bow: Students will generate a force versus pulling distance graph and use this to
determine the efficiency of a bow.
8. Simple Machine Series: Students will investigate a series of simple machines.
9. Linear Momentum in Explosions: Students will use carts on a dynamics track to
simulate an explosion.
10. Impulse: Students will use a force sensor and a sonic motion detector to explore the
relationship between impulse and change in momentum.
11. Moment of Inertia: Students will use the Pasco Rotational System to determine the
moment of inertia for several objects, including a non-symmetrical object.
12. Rotational Kinetic Energy: Students will investigate rotational kinetic energy using a
ramp, a steel ball, and the sensors of their choosing.
13. Springs: Students will use a motion sensor and a force sensor to analyze the
harmonic motion of a mass-spring system.
Semester 2:
14. Electrostatics: Students will use charged pith balls to investigate electrostatics.
15. Equipotential Lines: Students will use conductive paper to map equipotentials and
field gradients.
16. Simple Circuits
a. Ohm’s law: Students will use circuit boards and multimeters to explore
Ohm’s law.
a. Kirchhoff’s ’s Rules: Students will use circuit boards and multimeters to
explore Kirchhoff’s rules.
17. Magnetic field of a wire: Students will measure the magnetic field around a current
carrying wire when varying either the current in the wire or the distance from the wire.
18. Induction and emf: Students will use a magnetic probe and a voltmeter with the
PASCO system to investigate electromagnetic induction.
19. Alternating current: Students will use the PASCO system to display voltage versus
time to investigate inductance and capacitance in AC circuits.
AP PHYSICS (C) ECE
SCIENCE CURRICULUM 442 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 443 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Physics, Level 2
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Physics
3. Transcript Course Code/Number
00362
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
C or better in Level 2 Algebra II; B or better in Level 3 Algebra II
11. Brief Course Description
This course provides the student with an introduction to the fundamental concepts of matter and energy.
The ideas presented are developed in a progression from simpler to more complex. The principles studied
in this course are force, momentum, energy and work, and electricity and magnetism. In addition, special
topics studied may include wave motion, light and sound, and thermodynamics. Students are expected to
learn the fundamental concepts of physics, to place physics in historical and societal context, and to
further develop their understanding and application of both mathematics and the scientific process. There
is a strong laboratory component to this course.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.

PHYSICS, Level 2
SCIENCE CURRICULUM 444 GRADES 9 - 12
13. Course Outline
A. First Trimester
1. One dimensional motion 2 weeks (Newtonian Mechanics)
2. Two dimensional motion 2 weeks
3. Newton’s laws of motion, (force and friction) 2 weeks
4 Circular motion and gravitation 2 weeks
5. Work and energy 2 weeks
6. Simple machines (time permitting) 1 week
B. Second Trimester
1. Linear momentum and impulse 2 weeks
2. Thermodynamics 2 weeks (Heat & Thermodynamics)
3. Vibrations and waves 1 week (Waves and Optics)
4. Sound 1 week
5. Light 2 weeks
6. Electric charge, field, potential 1 week (Electricity and Magnetism)
7. Electric circuits 1 week
8. Magnetism and induced currents 1 week
9. Modern physics (time permitting) 1 week
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/FinalSite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
17. History of Course Development
The course stems from the earliest thinking and wonders of mankind. Many concepts and theories
developed out of necessity. Survival and the improvement of quality of life drove early inquiry, discovery
and application. Succeeding years led to improved safety and advantage over neighboring tribes. In the
modern world and in education, many of the same tenets apply as the theories of physics stretch the
imagination and challenge past understanding.
PHYSICS, Level 2
SCIENCE CURRICULUM 445 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and
coordinated attempt, through a continuous
process of questioning, data collection,
analysis and interpretation, to describe,
explain, and predict natural phenomena.
Scientific inquiry requires the sharing of
findings and ideas for critical review by
colleagues and other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to
search for and assess the relevance and
credibility of scientific information found in
various print and electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic
that are based on the results generated
during the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the ―If…,
then…‖ format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of an experiment.
Physics: Principles and Problems
Glencoe McGraw Hill, 2009
SUGGESTED INSTRUCTIONAL STRATEGIES
Cooperative group work
Differentiated instruction
Lecture
PowerPoint Presentations & Notes
Laboratory Investigations
Web based Instruction
Independent learning
Teacher centered and student centered
discussions, demonstrations and analyses
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research
SUGGESTED ASSESSMENT METHODS
Inclined Plane lab using computer software

PHYSICS, Level 2
SCIENCE CURRICULUM 446 GRADES 9 - 12
LEARNING STRAND: Motion and Forces
2.0 Newtonian Mechanics
CT Physics Standard: Newton’s laws predict the motion of most objects.
ENDURING UNDERSTANDINGS
Displacement, velocity, acceleration and time are
all interrelated.
Acceleration is the link between force and mass.
Mechanical energy is connected through force and
displacement, while momentum is connected
through force and time.
thus an object continues to move at a constant
speed or stays at rest.
The law F = ma is used to solve motion problems
that involve constant forces.
When one object exerts a force on a second object,
the second object always exerts a force of equal
magnitude and in the opposite direction.
Applying a force perpendicular to the direction of
motion causes a change of direction.
Circular motion requires the application of a
constant force toward the center of the circle.
Newton's laws are not exact, but provide very good
approximations unless an object is small enough
that quantum effects become important.
ESSENTIAL QUESTIONS
How do Newton’s Laws relate motion and
force?
How do the laws of conservation apply to
large, small and universal systems?
Why do Newton’s Laws of motion and
gravitational theories explain circular motion?
INSTRUCTIONAL SUPPORT MATERIALS
Physics: Principles and Problems
Glencoe McGraw Hill, 2009
Lab Equipment
Computer software, sensors, etc.
LEARNING OBJECTIVES
The student will…
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture, question, answer, and discussion
Laboratory models of motion
Laboratory investigations and inquiry
activities (displacement, velocity,
acceleration and time)
Problem solving
Demonstrations
Cooperative group work
Differentiated instruction
PowerPoint presentations and notes
Web based instruction
Independent learning
Teacher centered and student centered
discussions and analyses
Relate forces to linear motion and energy.
Compare circular motion (rotational) properties to
their linear counterparts.
Relate the conservation laws to each other.
Relate Newtonian Mechanics to modern
technologies such as, auto-safety and crash
analysis.
Describe simple harmonic motion.
SUGGESTED ASSESSMENT METHODS
Laboratory observation and follow-up
documentation
Laboratory reports (essay, data, calculation,
graph, synthesis and conclusion)
Projects (building mechanical devices)
Homework (readings, questions, problems)
Tests and quizzes
Student class participation
PHYSICS, Level 2
SCIENCE CURRICULUM 447 GRADES 9 - 12
LEARNING STRAND
3.0 Heat and Thermodynamics
CT Physics Standard: Energy cannot be created or destroyed although, in many processes, energy is transferred to the environment
as heat.
ENDURING UNDERSTANDINGS
Thermodynamics is the basis for heat transfer
throughout the universe.
Temperature and heat prescribe the activities of
solids, liquids and gases in their applied states.
Kinetic theory and thermodynamics show the
relationship of energy transfer between one
form of energy and another.
Heat flow and work are two forms of energy
transfer between systems.
The work done by a heat engine that is working
in a cycle is the difference between the heat
flow into the engine at high temperature and
the heat flow out at a lower temperature.
The internal energy of an object includes the
energy of random motion of the object's atoms
and molecules. The greater the temperature of
the object, the greater the energy of motion of
the atoms and molecules that make up the
object.
Most processes tend to decrease the order of a
system over time, so that energy levels
eventually are distributed more uniformly.
ESSENTIAL QUESTIONS
How does friction dissipate energy in real life
scenarios?
How do the laws of conservation apply to
energy and work?
Why does thermal expansion play such an
important role in engineering design?
a thermal medium for doing work?
What properties of water have contributed to
climate and life on the planet earth?
INSTRUCTIONAL SUPPORT MATERIALS
Physics: Principles and Problems
Glencoe McGraw Hill, 2009
Lab Equipment
Computer software, sensors, etc.
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture, question, answer, and discussion
Laboratory models of motion
Laboratory investigations and inquiry activities
(displacement, velocity, acceleration and time)
Problem solving
Cooperative group work
Differentiated instruction
PowerPoint presentations and notes
Web based instruction
Independent learning
Project based learning
Teacher centered and student centered
discussions, demonstrations and analyses
SUGGESTED ASSESSMENT METHODS
Laboratory observation with follow-up
documentation
Laboratory reports (essay, data, calculation,
graph, synthesis and conclusion)
Projects (building mechanical devices)
Homework (readings, questions, problems)
Tests and quizzes
Student class participation
LEARNING OBJECTIVES
The student will…
Apply the equations of heat transfer to lab
investigations.
Evaluate how the laws of thermodynamics
define the world around us and the kinetic
theory.
Examine how steam generators and turbines
produce electricity.
Discuss how the equations of heat transfer
affect the design of efficient devices and home
construction.
Demonstrate how thermal energy affects the
characteristics of matter.
PHYSICS, Level 2
SCIENCE CURRICULUM 448 GRADES 9 - 12
LEARNING STRAND: Waves
4.0 Waves and Optics
CT Physics Standard: Waves have characteristic properties that do not depend on the type of wave.
ENDURING UNDERSTANDINGS
Waves carry energy from one place to
another.
Transverse and longitudinal waves exist in
mechanical media, such as springs and ropes,
and in the Earth as seismic waves.
Wavelength, frequency and wave speed are
related.
Sound is a longitudinal wave whose speed
depends on the transmission medium in
which it propagates.
Radio waves, light and X-rays are different
wave length bands in the spectrum of
electromagnetic waves.
E-M wave speed depends on the media.
Wave characteristics include interference,
diffraction, refraction and polarization.
Beats and the Doppler Effect result from the
characteristic behavior of waves.
Snell’s Law provides an explanation for the
operation of optical technology.
Transverse waves provide an explanation for
the behavior of light.
Wave reflection is mathematically predictable.
Geometric optics and ray tracing illustrate
reflection and lens behavior.
ESSENTIAL QUESTIONS
What are the characteristics, behaviors and
mathematical models of waves?
How does Snell’s Law predict how light will bend as
it travels from one medium to another?
How do Young’s Double Slit experiment, Single Slit
Diffraction and the colors of soap bubbles all
support the wave nature of light?
How do the focal lengths and the radius of
curvature of mirrors and lenses locate the position
of created images based on location of an object in
front of an optical device?
INSTRUCTIONAL SUPPORT MATERIALS
Physics: Principles and Problems
Glencoe McGraw Hill
Lab Equipment
Computer software, sensors, etc.
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture, question, answer, and discussion
Laboratory models of motion
Cooperative group work
Differentiated instruction
PowerPoint presentations and notes
Web based instruction
Independent learning
Teacher centered and student centered discussions
and analyses
Laboratory investigations and inquiry activities
(displacement, velocity, acceleration and time)
Problem solving
Demonstrations
LEARNING OBJECTIVES
The student will…
Describe the characteristics and behavior of
waves.
Understand Snell’s Law and how it is the
basic of all optics.
Illustrate how the laws of reflection and
refraction apply to all optical devices.
Predict image formation from lenses and
mirrors.
Determine how Snell’s Law is the basis for all
fiber optic communications.
Analyze the laws of reflection and refraction
and predict image formation from lenses and
mirrors.
SUGGESTED ASSESSMENT METHODS
Laboratory observation and follow-up
documentation
Laboratory reports (essay, data, calculation, graph,
synthesis and conclusion)
Projects (building mechanical devices)
Homework (readings, questions, problems)
Tests and quizzes
Student class participation
PHYSICS, Level 2
SCIENCE CURRICULUM 449 GRADES 9 - 12
LEARNING STRAND: Electric and Magnetic Phenomena
5.0 Electricity and Magnetism
CT Physics Standard: Electric and magnetic phenomena are related and have many practical applications.
ENDURING UNDERSTANDINGS
Charged particles are sources of electric fields and
are subject to the forces of the electric fields from
other charges.
Protons and electrons are the essential particles of
nature that contain electric charges.
Chemical reactions are essentially electrical
reactions and the basis for DC current.
Plasma, the fourth state of matter, contains ions or
free electrons or both and conducts electricity.
Voltage is the energy per charge. Current is the
rate of flow of a charge.
Conductors, capacitors, resistors are the most basic
components of an electrical circuit.
The voltage or current in simple DC electric circuits
can be predicted using Ohm's law.
Any resistive element in a DC circuit dissipates
energy, which heats the resistor.
The power in any resistive circuit element can be
calculated using the formula Power=I
2
R.
Resistance regulates the current.
Ohm’ Law and Joule’s law describe the
relationships between voltage, current, resistance,
energy and power.
Magnetic fields are created by permanent magnets
or by electrical current.
Electromagnetism is the interaction between an
electric field and a magnetic field.
Magnetic materials and electric currents (moving
electric charges) are sources of magnetic fields and
are subject to forces arising from the magnetic
fields of other sources.
Changing magnetic fields produce electric fields,
thereby inducing currents in nearby conductors.
Electrical current and magnetic fields interact to
power electric motors or generate electric power.
ESSENTIAL QUESTIONS
How do charged particles exert forces on each
other?
How can multiple charges create positions of
zero electric field?
What is voltage?
What is current?
What is electrical resistance?
How are current, voltage and resistance
related?
What does a capacitor do?
What determines how much power is generated
in a load resistor and an electrical circuit?
How is direct current produced?
How is alternating current produced?
How is current induced in a magnetic field?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Demonstrate an understanding of fields/forces.
Create DC circuits and AC circuits.
Analyze DC circuits and AC circuits.
Explain the principles behind the fundamental
electrical components (e.g., resistors, capacitors,
inductors).
Demonstrate how electrical components combine
to create everyday electrical devices.
Investigate fields and forces created by charged
particles.
Demonstrate the vector nature of Coulomb’s law
and electric fields.
Physics: Principles and Problems
Glencoe McGraw Hill 2009
Lab Equipment
Computer software, sensors, etc.
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture, question, answer, and discussion
Laboratory models of motion
Laboratory investigations and inquiry activities
(displacement, velocity, acceleration and time)
Cooperative group work
Differentiated instruction
PHYSICS, Level 2
SCIENCE CURRICULUM 450 GRADES 9 - 12
Create and analyze DC circuits and AC circuits.
Apply the principles of Ohm’s law to electrical
components.
Use Faraday’s and Lenz’s laws to explain
electromagnetism and solve problems involving
electromagnetic induction.
PowerPoint presentations and notes
Web based instruction
Independent learning
Project based learning
Teacher centered and student centered
discussions and analyses
Problem solving
Demonstrations
SUGGESTED ASSESSMENT METHODS
Laboratory observation and follow-up
documentation
Laboratory reports (essay, data, calculation,
graph, synthesis and conclusion)
Projects (building mechanical devices)
Homework (readings, questions, problems)
Tests and quizzes
Student class participation

PHYSICS, Level 2
SCIENCE CURRICULUM 451 GRADES 9 - 12
Experiment 1: Color Addition
EQUIPMENT NEEDED
- Ray box (color rays) - Convex lens
- Colored paper (red, yellow, green, blue)
Purpose
To determine the colors that result from the addition of two or three primary colors and to show the
effect of illuminating colored objects with different colors of light.
Procedure
Place the ray box on a white sheet of paper on the table. Adjust the box so the primary colors are
showing. If the white screen from the Optics Bench (OS-8518) is available, it can be laid flat on the
table to make a good viewing platform for this experiment. It may be helpful to raise the front end
of the box by approximately I cm (The concave lens works fine for this). This causes the colored
rays to shine out a further distance.
Place the convex lens near the ray box so it focuses the rays and causes them to cross each other
at the focal point. What is the color of the light where the three rays come together? Record the
result in Table 1.1. It may be helpful to crease the paper so it forms a wall upon which the focal
point is projected. See Figure 1.1.
Figure 1.1: Ray Box for color addition
Now block the green ray with an opaque object. What color results from adding red and blue?
Record the result in Table 1.1.
Repeat Step 3, blocking one color each in succession and completing Table 1.1.
Table 1.1 Results of Color Addition
COLORS ADDED
RESULTING COLOR
red + blue + green
red + blue
red + green
green + blue
PHYSICS, Level 2
SCIENCE CURRICULUM 452 GRADES 9 - 12
Shine the three primary colors on each of the colored sheets of paper. What color does each sheet of
paper appear to be for each color of illuminating light? Record the results in Table 1.2.
Table 1.2 Results of Reflection Off Colored Paper
COLOR OF PAPER IN
WHITE LIGHT
COLOR OF
LIGHT RAY
COLOR OF PAPER
IN COLORED LIGHT
Red
Green
Blue
Red
Green
Blue
Red
Green
Blue
Red
Green
Blue
PHYSICS, Level 2
SCIENCE CURRICULUM 453 GRADES 9 - 12
Experiment 2: Prism
EQUIPMENT NEEDED
- Ray box (white ray)
- Rhombus
Purpose
To show how a prism separates white light into its component colors and to show that different
colors are refracted at different angles through a prism.
Theory
According to Snell's Law,
n
1
sin θ
1
= n
2
sin θ
2
the angle of refraction depends on the angle of incidence and the index of refraction of the
material. See Figure 2.1. Because the index of refraction for light varies with the frequency of
the light, white light which enters the material at a given angle of incidence will separate out into
its component colors as each frequency is bent a different amount.
The rhombus is made of Acrylic which has an index of refraction of 1.497 for light of
wavelength 486 nm in a vacuum, 1.491 for wavelength 589 nm, and 1.489 for wavelength 651
nm (red). Notice that in general for visible light, the index of refraction for Acrylic increases
with increasing frequency.
PHYSICS, Level 2
SCIENCE CURRICULUM 454 GRADES 9 - 12
Procedure for Separating White light
Place the ray box, label side up, on a white sheet of paper on the table. Adjust the box so one
white ray is showing. If the white screen from the OS-S5IS Optics Bench is available, it can
be laid flat on the table to make a good viewing platform for this experiment.
Position the rhombus as shown in Figure
2.2. The triangular end of the rhombus is
used as a prism in this experiment. Keep
the ray near the point of the rhombus for
maximum transmission of the light.
Rotate the rhombus until the angle (9) of
the emerging ray is as large as possible
and the ray separates into colors.
(a) What colors are seen and in what order
are they?
(b) Which color is refracted at the largest
angle?
(c) According to Snell's Law and the information given about the frequency dependence of
the index of refraction for Acrylic, which color is predicted to refract at the largest angle?
Turn the ray box over and shine the three primary color rays into the rhombus at the same
angle used for the white ray. Do the colored rays emerge from the rhombus parallel to each
other? Why or why not?
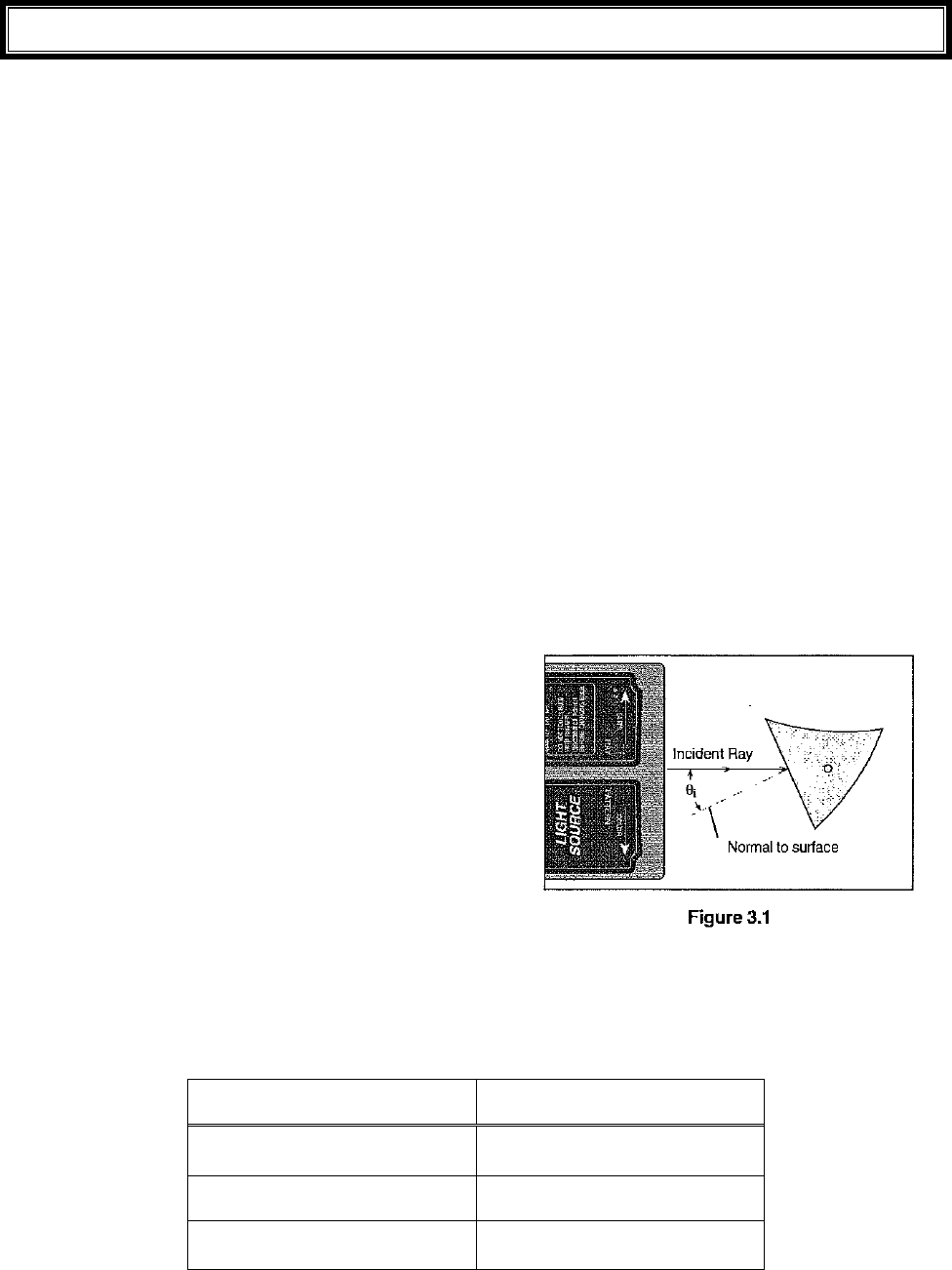
PHYSICS, Level 2
SCIENCE CURRICULUM 455 GRADES 9 - 12
Experiment 3: Reflection – Plane and Curved Mirrors
EQUIPMENT NEEDED
- Ray box (single and multiple white rays) - Plane and curved mirrors
- Protractor (SE-8732) - Drawing compass (SE-8733)
- Metric rule - White paper
Purpose
To study how rays are reflected and to determine the focal length and radius of curvature of different
types of mirrors.
Part I: Plane Mirror
Procedure
Place the ray box, label side up, on a white sheet of paper on the table. Adjust the box so one white
ray is showing.
Place the mirror on the table and position the plane surface of the mirror at an angle to the ray so that
the both the incident and reflected rays are clearly seen.
Mark the position of the surface of the plane mirror and trace the incident and reflected rays. Indicate
the incoming and the outgoing rays with arrows in the appropriate directions.
On the paper, draw the normal to the surface. See
Figure 3.1.
Measure the angle of incidence (8.,) and the angle of
reflection. Both these angles should be measured
from the normal. Record the angles in Table 3.1.
Change the angle of incidence and measure the
incident and reflected angles again. Repeat this
procedure for a total of three different incident
angles.
Adjust the ray box so it produces the three primary
color rays. Shine the colored rays at an angle to the
plane mirror. Mark the position of the surface of the plane mirror and trace the incident and reflected
rays. Indicate the colors of the incoming and the outgoing rays and mark them with arrows in the
appropriate directions.
Table 3.1 Plane Mirror Results
Angle of Incidence
Angle of Reflection
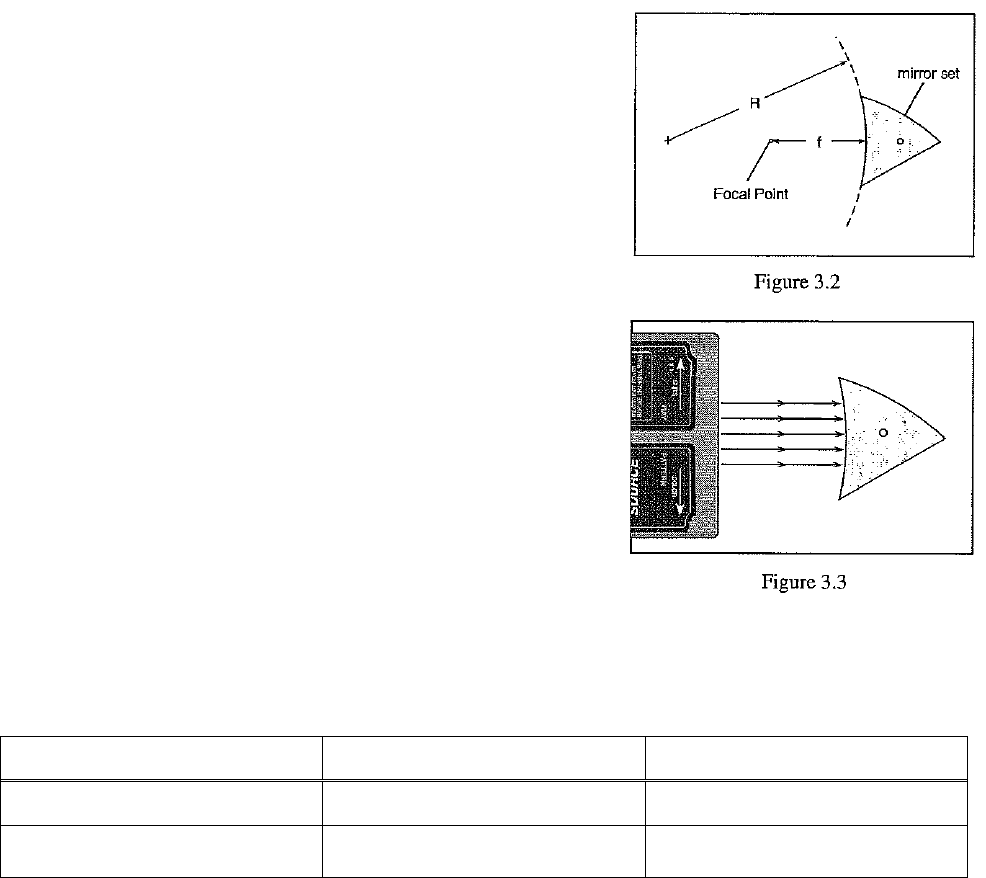
PHYSICS, Level 2
SCIENCE CURRICULUM 456 GRADES 9 - 12
Questions
What is the relationship between the angle of incidence and the angle of reflection?
Are the three colored rays reversed left-to-right by the plane mirror?
Part II: Cylindrical Mirrors
Theory
A concave cylindrical mirror will focus parallel rays
of light at the focal point. The focal length is the
distance from the focal point to the center of the
mirror surface. The radius of curvature of the mirror
is twice the focal length. See Figure 3.2.
Procedure
Using five white rays from the ray box, shine the rays straight
into the concave mirror so the light is reflected back toward
the ray box. See Figure 3.3. Draw the surface of the mirror and
trace the incident and reflected rays. Indicate the incoming and
the outgoing rays with arrows in the appropriate directions.
The place where the five reflected rays cross each other is the
focal point of the mirror. Measure the focal length from the
center of the concave mirror surface to the focal point. Record
the result in Table 3.2.
Use the compass to draw a circle that matches the curvature of
the mirror. Measure the radius of curvature using a rule and
record it in Table 3.2.
Repeat Steps I through 3 for the convex mirror. Note that in
Step 2, the reflected rays are diverging for a convex mirror and
they will not cross. Use a rule to extend the reflected rays back
behind the mirror's surface. The focal point is where these
extended rays cross.
Table 3.2 Cylindrical Mirror Results
Concave Mirror
Convex Mirror
Focal Length
Radius of Curvature using
compass
Questions
What is the relationship between the focal length of a cylindrical mirror and its radius of curvature?
Do your results confirm your answer?
What is the radius of curvature of a plane mirror?
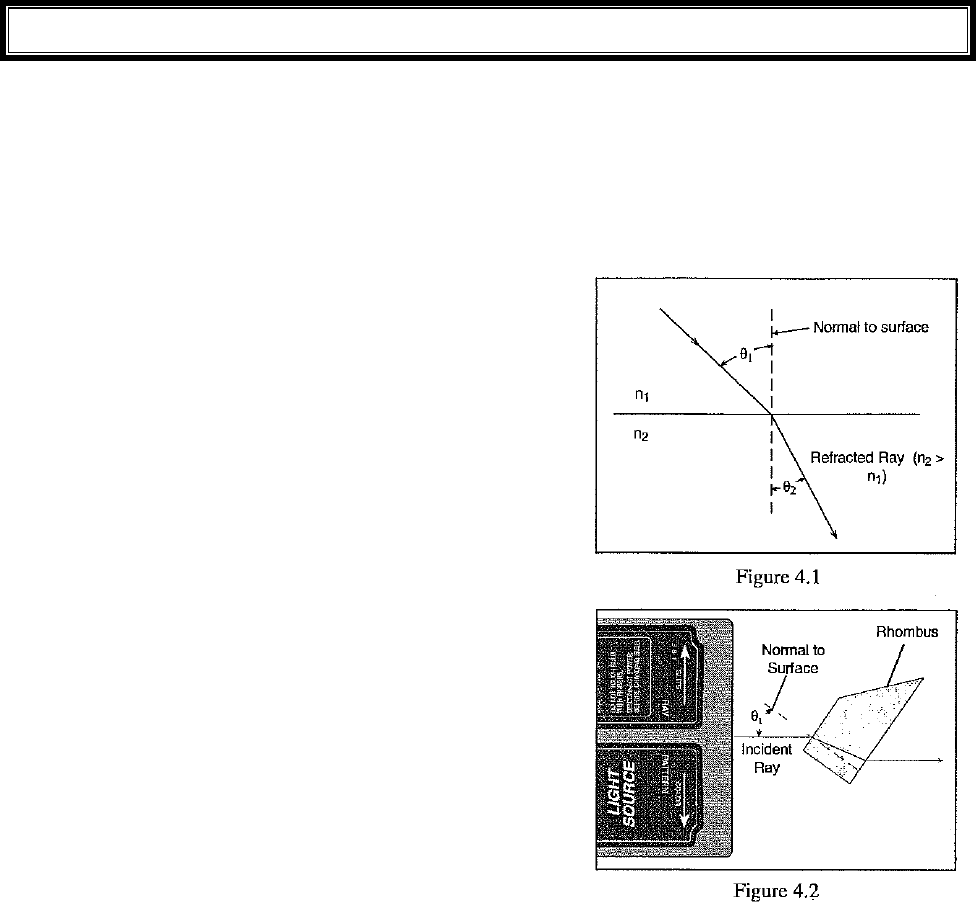
PHYSICS, Level 2
SCIENCE CURRICULUM 457 GRADES 9 - 12
Experiment 4: Snell’s Law
EQUIPMENT NEEDED
- Ray box (single white ray and colored rays)
- Rhombus
- Protractor (SE-8732)
- White paper
Purpose
To use Snell's Law to determine the index of refraction of the
acrylic rhombus.
Theory
Snell's Law states
n
1
sin θ
1
= n
2
sin θ
2
where θ
1
is the angle of incidence, θ
2
is the angle of
refraction, and n
1 and
n
2
are the respective indices of
refraction of the materials. See Figure 4.1.
Procedure
Place the ray box, label side up, on a white sheet of
paper on the table. Slide the ray mask until only one
white ray is showing.
Place the rhombus on the table and position it so the
ray passes through the parallel sides as shown in
Figure 4.2.
Mark the position of the parallel surfaces of the
rhombus and trace the incident and transmitted rays.
Indicate the incoming and the outgoing rays with
arrows in the appropriate directions. Mark carefully
where the ray enters and leaves the rhombus.
Remove the rhombus and on the paper draw a line
connecting the points where the ray entered and left the rhombus.
Choose either the point where the ray enters the rhombus or
the point where the ray leaves the rhombus. At this point, draw the normal to the surface.
Measure the angle of incidence (θ
1
) and the angle of refraction with a protractor. Both these angles
should be measured from the normal. Record the angles in Table 4.1.
Change the angle of incidence and measure the incident and refracted angles again. Repeat this
procedure for a total of three different incident angles.
PHYSICS, Level 2
SCIENCE CURRICULUM 458 GRADES 9 - 12
Table 4.1 Data and Results
Angle of Incidence
Angle of Refraction
n rhombus
Average index of refraction
Analysis
Using Snell's Law and your data, calculate the index of refraction for the Acrylic
rhombus, assuming the index of refraction of air is one. Record the result for each of the
three data sets in Table 4.1.
Average the three values of the index of refraction and compare to the accepted value
(n = 1.5) using a percent difference.
Question
What is the angle of the ray that leaves the rhombus relative to the ray that enters the
rhombus?
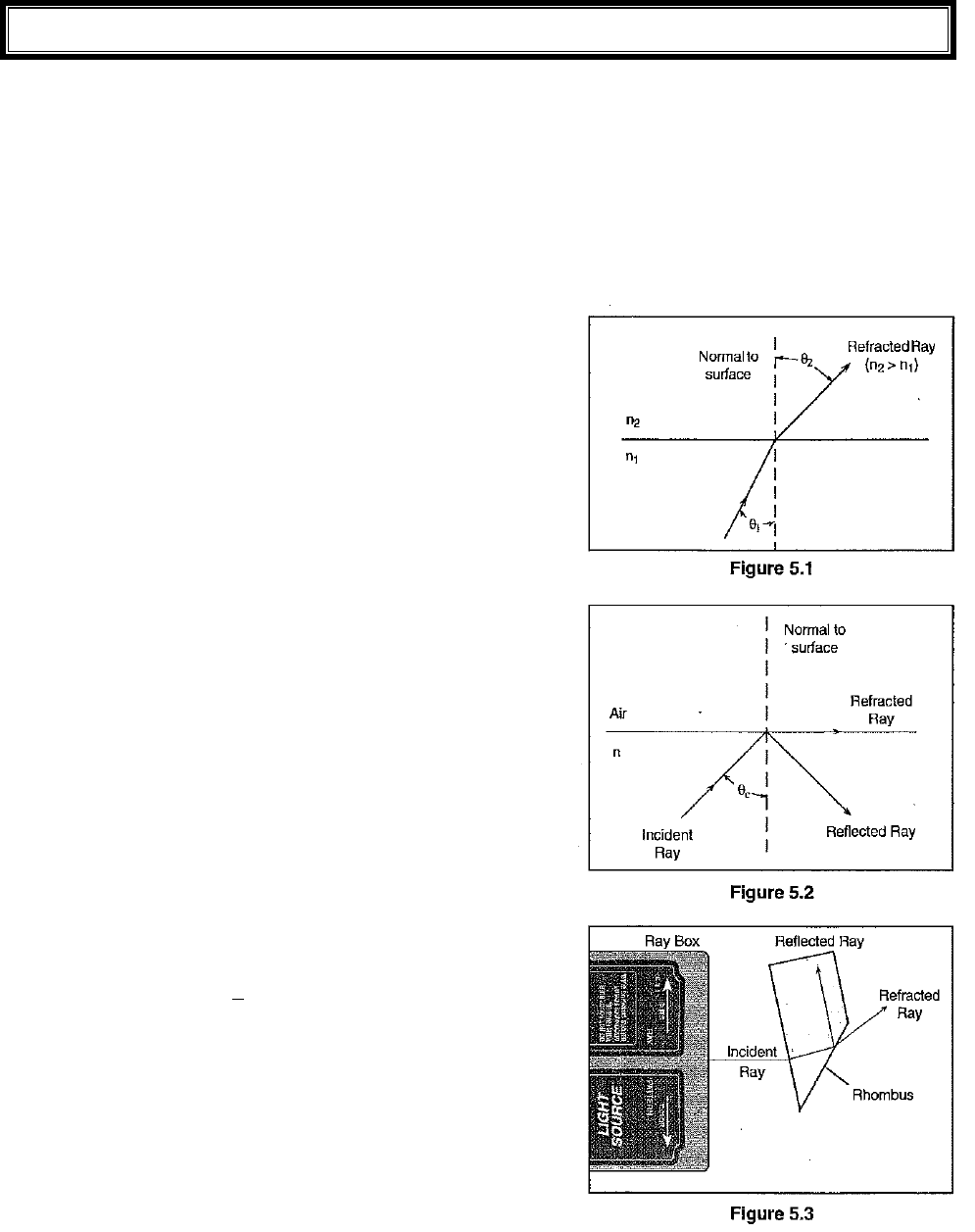
PHYSICS, Level 2
SCIENCE CURRICULUM 459 GRADES 9 - 12
Experiment 5: Total Internal Reflection
EQUIPMENT NEEDED
- Ray box (single ray)
- Rbombus
- Protractor (SE-8732)
- White paper
Purpose
To detennine the critical angle at which total internal reflection occurs and to confirm it using
Snell's Law.
Theory
n
1
sin θ
1
= n
2
sin θ
2
where θ
1
is the angle of incidence, θ
2
is the angle of
refraction, and n
1 and
n
2
are the respective indices of refraction
of the materials. See Figure 5.1.
If a ray of light traveling from a medium of
greater index of refraction to a medium of
lesser index of refraction is incident with an
angle greater than the critical angle (θ
c
), there
is no refracted ray and total internal reflection
occurs. If the angle of incidence is exactly the
critical angle, the angle of the refracted ray is
90 degrees. See Figure 5.2. In this case,
using Snell's Law,
nsin θ
c
= (1)sin (90°)
assuming the medium of lesser index of refraction
is air with n
2
, = 1 and the medium of
greater index of refraction is the Acrylic rhombus
with n
1
= n = 1.5. Solving for the critical
angle gives
sin θ
c
=
n
1
Procedure
CD Place the ray box, label side up, on a white
sheet of paper on the table. Slide the ray mask
until only one white ray is showing.
Position the rhombus as shown in Figure 5.3.
Do not shine the ray through the rhombus too near the
triangular tip.
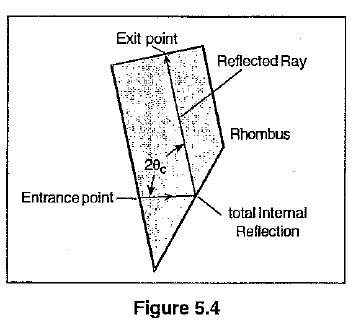
PHYSICS, Level 2
SCIENCE CURRICULUM 460 GRADES 9 - 12
Rotate the rhombus until the emerging ray just barely disappears. Just as it disappears, the ray
separates into colors. The rhombus is correctly positioned if the red has just disappeared.
Mark the surfaces of the rhombus. Mark exactly the point on the surface where the ray is internally
reflected. Also mark the entrance point of the incident ray and mark the exit point of the reflected ray.
Remove the rhombus and draw the rays that are incident upon and that reflect off the inside surface
of the rhombus. See Figure 5.4. Measure the total angle between these rays using a protractor.
If necessary, you may extend these rays to
make the protractor easier to use. Note that this
total angle is twice the critical angle because the
angle of incidence equals the angle of reflection.
Record the critical angle here:_________________
Calculate the critical angle using Snell's Law and
the given index of refraction for Acrylic. Record the
theoretical value here:_______________________
Calculate the percent difference between the measured
and theoretical values:
% difference-______________________________
Questions
How does the brightness of the internally reflected ray change when the incident angle changes from
less than θ
c
, to greater than θ
c
, ?
Is the critical angle greater for red light or violet light? What does this tell you about the index of
refraction
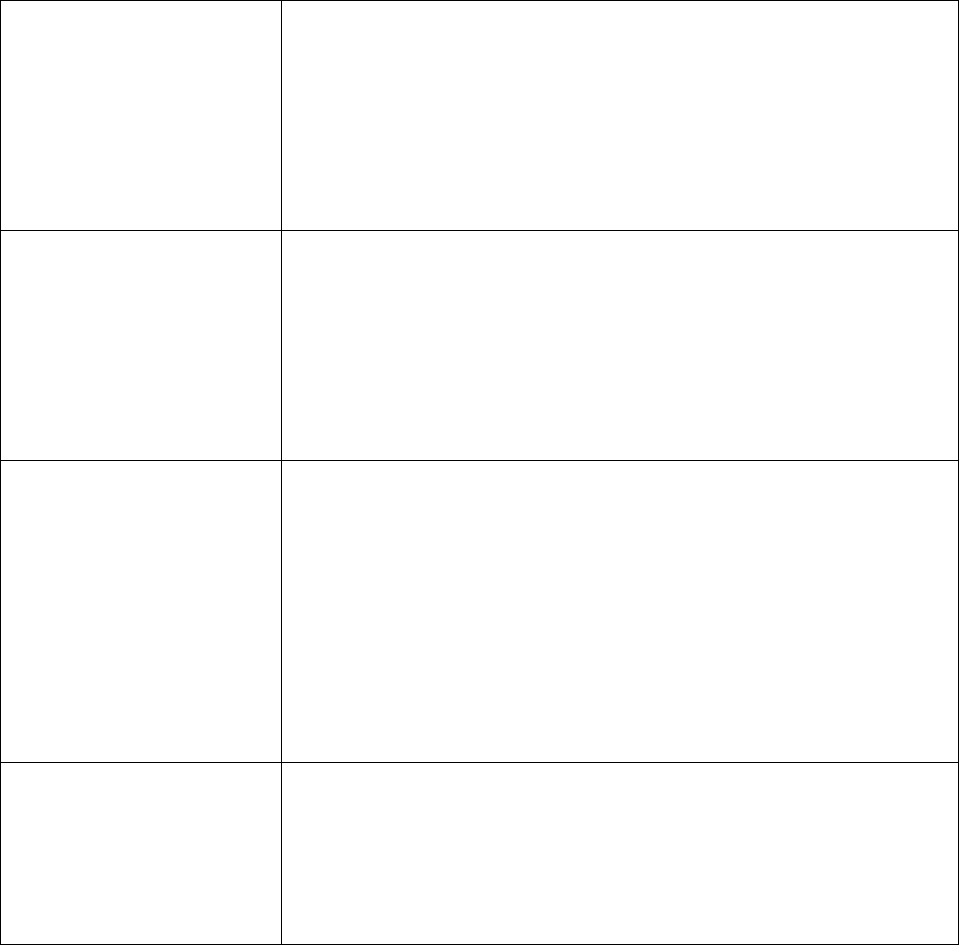
PHYSICS, Level 2
SCIENCE CURRICULUM 461 GRADES 9 - 12
PHYSICS (Level 2)
EFFECTIVE CRITICAL THINKING STRATEGIES
KINEMATICS AND DYNAMICS LABORATORY EXPERIMENTS
3
Exceeds Expectations
The student effectively collects, interprets, analyzes, and evaluates data
to solve a problem. S/he applies reasonable and appropriate assumptions
while displaying due diligence in the setup of the experiment. S/he
understands and clearly makes highly effective use of the capabilities of
the various detectors and interactive software to solve problems and
evaluate the meaning of data. Calculations are technically accurate,
appropriately accomplished and demonstrate clear mastery of the theory
as applied to the physical application. Conclusions are correct and
indicate insightful understanding and a readiness for higher challenges.
Meets Expectations
The student effectively collects, interprets, analyzes, and evaluates data
to solve a problem. S/he applies reasonable and appropriate assumptions
while displaying due diligence in the setup of the experiment. S/he
understands and clearly makes highly effective use of the capabilities of
the various detectors and interactive software to solve problems with
minimum of error and assistance. S/he correctly evaluates the meaning
of data using technology. Calculations are technically accurate and
appropriately accomplished, but may contain minor errors. Conclusions
are correct and appropriate.
Meets Some Expectations
The student effectively collects, interprets, analyzes, and evaluates data
to solve a problem, although some important data may be missing,
requiring the student to make logical assumptions. S/he displays due
diligence in the setup of the experiment and in correctly executing clearly
delineated steps. S/he makes reasonably effective use of the capabilities
of the various detectors and interactive software to solve problems. Some
error or difficulty with the equipment and its implementation is
acceptable. S/he correctly evaluates the meaning of data using
technology, although may have difficulty connecting the data observe to
the theory using mathematical expression and calculations. Calculations
and conclusions are technically accurate and reflect at least a qualitative
understanding.
Does Not Meet
Expectations
The student collected incomplete data and or missed significant portions.
Interpretation and evaluation of the data indicates a lack of
understanding. The student failed to demonstrate basic principals of the
scientific method in setting up equipment and conducting a meaningful
and effective controlled experiment. The equipment was not used to
gather even the most basic level of quality data. Calculations and
conclusions display a clear lack of conceptual understanding.
PHYSICS, Level 2
SCIENCE CURRICULUM 462 GRADES 9 - 12
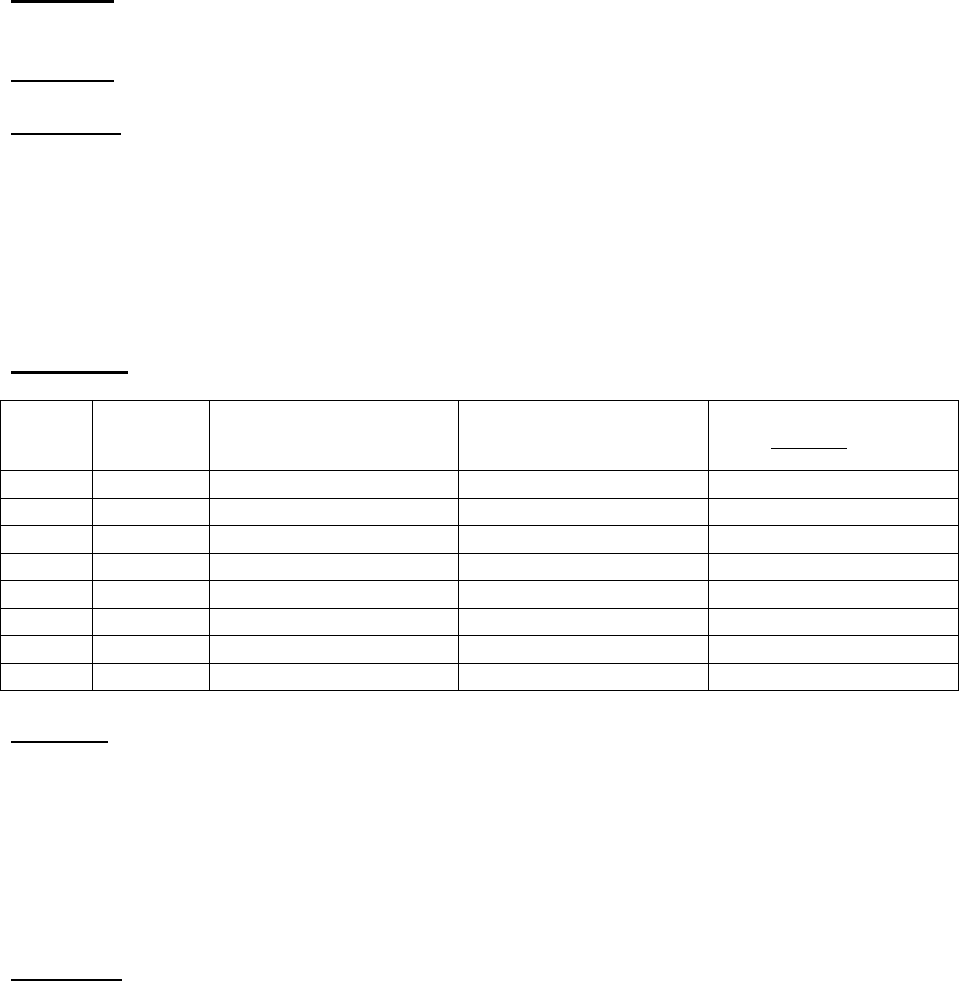
PHYSICS, Level 2
SCIENCE CURRICULUM 463 GRADES 9 - 12
Lab: Acceleration down an inclined plane (30 points)
Objective: Investigate acceleration down an inclined plane. Compare "Theoretical Acceleration"
(g·sinθ) to "Observed Acceleration" as measured by the Pasco CBL software.
Materials: Ramp, cart, motion sensor, meter stick, laptop with Pasco software.
Procedure:
5. Set up ramp, motion sensor, laptop. Use a cloth bundle to serve as a bwnper and protect the
cart from damage.
6. Compute the theoretical acceleration for each angle prescribed in the data table.
7. Set the ramp height for each trial using trigonometric ratios to establish the required angle for
each step.
8. Coordinate lap-top operation and cart release to capture the data required. Repeat for each
angle prescribed.
Data Table:
Trial #
Angle
(degrees)
Theoretical Acceleration
Observed Acceleration
Percent Error
%100x
Theo
ObsTheo
1
0°
0.00
N/A
2
2°
3
5°
4
10°
5
15°
6
20°
7
25°
8
Spare
Analysis: (Use separate paper)
5. Show one sample calculation and diagram for how you set the angle of the ramp using
trigonometric ratios.
6. Calculate the theoretical acceleration for each trial. Show at least one sample calculation.
7. Calculate the Percent Error for each trial. Show at least one sample calculation.
8. Generate a quality line-graph (on graph paper) that clearly and accurately conveys your
results as summarized in the data table. You are not limited to only two variables. The best
graphs will include the angle vs. all 3 data columns.
Questions: (Use separate paper)
4. Does this activity confirm the relationship a = g·sinθ, where g =9.8m/s
2
?. Why?
5. Does the percent error increase or decrease as the angle increases? Why?
6. What are some possible sources of error for this lab? Which are the most significant and how
would you eliminate them.
PHYSICS, Level 2
SCIENCE CURRICULUM 464 GRADES 9 - 12
PHYSICS (Level 2)
EFFECTIVE USE OF TECHNOLOGY
KINEMATICS AND DYNAMICS LABORATORY EXPERIMENTS
2
Exceeds Expectations
The student independently collects, interprets, analyzes, and evaluates
data using the Pasco lab equipment. (The student who exceeds
expectations may be seen teaching others or leading the group). S/he
independently explores ways to use the equipment to accomplish the
experiment. The student is able to transfer data to other application
software programs for further analysis or display. The student adjusts
sample rates, inputs, and detectors appropriately. S/he is able to
manipulate the various graphs and use ―best-fit‖ algorithms to mine the
most meaningful data and conclusions from the experiment.
Meets Expectations
The student independently collects, interprets, analyzes, and evaluates
data using the Pasco lab equipment, but requires occasional assistance.
S/he requires minimal coaching in using the equipment to accomplish the
experiment. The student uses the data and evaluation tools to draw
logical conclusions. The student adjust sample rates, inputs, and
detectors appropriately, but may require minor assistance or coaching.
S/he is able to manipulate the various graphs and use ―best-fit‖
algorithms to support the experiment.
Meets Some Expectations
The student effectively collects, interprets, analyzes, and evaluates data
using the Pasco lab equipment, but requires significant or repeat
assistance. S/he requires coaching and some assistance in using the
equipment to accomplish the experiment. The student makes some minor
errors in interpreting the data or using the technology, but manages to
draw logical conclusions and evidence learning. S/he is able to
manipulate the various graphs and use ―best-fit‖ algorithms to support
the experiment although s/he may require assistance from peers or
teacher.
Does Not Meet
Expectations
The student was unable to successfully use the Pasco equipment and
laptop despite significant assistance and coaching. The student collected
incomplete data and/or missed significant portions. The student was
unable to assemble the equipment and manage the technology to
accomplish the most basic aspects of the experiment.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 465 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Advanced Placement Biology
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
AP Biology
3. Transcript Course Code/Number
00370
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grades: 11 – 12 Level: 1
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ______________________(date)
10. Pre-Requisites
A in Biological Systems and Chemistry level II, B in Biology - Honors or Honors Chemistry
11. Brief Course Description
This course is designed to be the equivalent of a two–semester college introductory biology course usually
taken by biology majors during their first year. The three major areas included in the course are molecules
and cells, heredity and evolution, and organisms and populations. Major themes included are science as a
process, evolution, energy transfer, continuity and change, structure and function, regulation,
interdependence in nature and science, technology, and society. The laboratory experience is an important
component of the course. Appropriate labs will be assigned to provide students with the opportunity to
learn a variety of skills, facts, principles, and concepts of introductory level biology covered in lectures,
reading, and discussions.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically
and solving problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.

AP BIOLOGY
SCIENCE CURRICULUM 466 GRADES 9 - 12
Biology 7
th
Edition by Neil A. Campbell and Jane B Reece Benjamin-Cummings, 2007
13. Course Outline:
1. Ecology
2. Molecules and Cells
3. Genetics
4. Mechanism of Evolution
5. The Evolutionary History of Biological Diversity
6. Plant Form and Function
7. Animal Form and Function
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio visual presentations
Response Cards by TurningTechnologies
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments/projects evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Students will score at the "Meets Expectations" level for the assignments below according to
departmental/school-wide rubrics with samples of student work where appropriate.
Benchmark Assignments:
(1) Test on cellular respiration: (1A, 1B, 1C, 3); the student will meet expectations when he or she is
able to earn a grade of B on the examination.
(2) Gel electrophoresis lab: (2); the student will meet expectations when he or she is able to set-up,
run the lab, and complete the analysis for the lab.
Assessments are based on the Madison Curriculum and Connecticut standards and grade level expectations
for science. Authentic assessments are graded using a scoring rubric or grading criteria. Benchmark
assignments are graded using a
common
scoring rubric or grading criteria.
17. Indicate how this honors course is different from the standard course.
The material is either new (membrane receptors) or in much greater depth than our honors biology course.
AP BIOLOGY
SCIENCE CURRICULUM 467 GRADES 9 - 12
Audit of Advanced Placement Biology
Personal Philosophy
Biology is a fascinating and dynamic discipline. Prior to teaching, for approximately 10 years, I worked in
several Research and Development laboratories. During that time, my love of the science flourished.
Now I have the good fortune to bring that experience to the classroom where I get to share my
enthusiasm as I assist a new generation of aspiring scientists on their journey. I strive to impress the
importance of integrating all aspects of knowledge when approaching a novel situation as the key to
success. I aim to create an environment where my students may confidently mature from simply
memorizing content to actually utilizing that foundational knowledge to become pioneering problem-
solvers.
Eight Themes
There are eight major themes interwoven throughout the Advanced Placement Biology Course. These
themes are an integral part of the course and provide cohesive structure as we navigate through a wide
variety of course content
(1). Science as process
(2). Evolution
(3). Energy transfer
(4). Continuity and change
(5). Relationship of structure to function
(6). Regulation
(7). Interdependence in nature
(8). Science, technology and society
Course Overview
We meet our students five days each week for 30 weeks. Regular class periods are 60 minutes in length.
Labs take up about 25 percent of instructional time.
In the AP Biology course, our textbook is Campbell, Neil A., Jane B. Reece,
Biology
, 7
th
Edition. Benjamin
Cummings, (2007).
BSCS Biology: A Molecular Approach
, 8
th
edition
is used as a reference.
Our labs are mostly derived from the
AB Biology Lab Manual for Students
.
The students’ grades are determined from the following components:
1. Tests: Half-hour tests are given at the conclusion of each chapter and an hour exam is given
at the end of each unit.
2. Quizzes: 15 minutes quizzes are generally given after we have completed several sections of a
chapter.
3. Homework questions are given on a weekly basis. These are derived from various sources
including the text, questions I have devised, or biological material that may have been in the
news. Homework questions are collected, graded, and returned to the students. Students are to
work on homework questions on their own under the honor code.
4. Laboratory reports: In most cases students work collectively gathering data for labs. However,
they are expected to do their own analyses and conclusions.
Topics covered by chapter.
Chapter 1: Exploring life 2 days
Unit 8: Ecology Summer reading and 4 weeks
Chapter 50: An Introduction to Ecology
Lab 12: AP Dissolved oxygen and aquatic primary productivity
Chapter 51: Behavioral Ecology
Lab 11: AP Animal Behavior
Chapter 52: Population Ecology

AP BIOLOGY
SCIENCE CURRICULUM 468 GRADES 9 - 12
Chapter 53: Community Ecology
Chapter 54: Ecosystems
Unit 1: Molecules and Cells 11 weeks
Chapter 2: The chemical context of life
Homework assignments on types of bonds
Chapter 3: Water and the Fitness of the Environment
Lab on pH. Teacher generated and deals with pH of various biological substances and their
relationship to humans
Lab (virtual) How does lab precipitation affect trees?
Chapter 4: Carbon and the Molecular Diversity of Life
Lab on model building. Dry lab building types of functional groups
Chapter 5: The Structure and Function of Macromolecules
Lab on molecular identification. (Teacher generated and uses chemicals to identify types of
macromolecules
Chapter 6 A Tour of the Cell
Lab on types of cells. Teacher generated and uses live cells illustrate the differences between
prokaryotes and several types of eukaryotes.
Lab on relationship of surface area to volume from
BSCS
Biology: A Molecular Approach.
This uses agar cubes of different sizes.
Chapter 7: Membrane Structure and Function
Lab 1: AP Diffusion and osmosis
Chapter 8: An Introduction to Metabolism
Lab 2: AP Enzyme Catalysis
Chapter 9: Cellular Respiration
Lab 5: AP Cell Respiration
Chapter 10: Photosynthesis
Lab 4: AP Plant Pigments and Photosynthesis
Chapter 11: Cell Communication
Lab (Virtual) How do cell communicate with each other? from the Campbell Reece textbook
Chapter 12: The Cell Cycle
Lab on onion root cells and whitefish embryo cells. This lab involves the use of prepared slides.
It is followed by a lab quiz.
Lab (simulation) of mitotic cell division using pipe cleaner
Unit 3: Genetics 8 weeks
Chapter 13: Meiosis And Sexual Life Cycles
Lab 7: AP Genetics of organisms
Chapter 14: Mendel and the Gene Idea
Lab: continuation of lab 7
Lab on pea seeds from BSCS Biology A Molecular Approach
Chapter 15. The Chromosomal Basis of Inheritance
Lab: continuation of lab 7
Chapter 16: The Molecular Basis of Inheritance
Lab: DNA extraction from onion cells (obtained from a professional development
day workshop)
Chapter 17: From Gene to Protein
Lab: (virtual from text): How is a metabolic pathway analyzed?
Chapter 18: The Genetics of Viruses and Bacteria
Lab: techniques in microbiology. Teacher generated. This lab involves plating of organisms from
the room, identification of bacteria and fungi, and techniques of isolating bacteria from a
particular colony.
Chapter 19: Eukaryotic Genomes
Chapter 20: DNA Technology and Genomics
Lab 6: AP Molecular Biology
AP BIOLOGY
SCIENCE CURRICULUM 469 GRADES 9 - 12
Chapter 21: Genetic Basis of Development
Unit 4: Mechanisms of Evolution 3 weeks
Chapter 22: Descent with Modification
Chapter 23: The Evolution of Populations
Lab 8: AP Population Genetics and Evolution
Chapter 24: The Origin of Species
Chapter 25: Phylogeny and Systematics
Unit 5: The evolutionary history of biological diversity 3 weeks
Chapter 26: The Tree of life
Chapter 27: Prokaryotes
Activity: How has small size affected prokaryotic diversity? This is from Campbell Reece student
workbook.
Chapter 28: Protists
Survey of types of protists. Teacher generated. This lab will involve microscopic examination of
various types of protists
Chapter 29: Plant Diversity I
Chapter 30: Plant Diversity II
Chapter 31: Fungi
Survey of types of fungi. Teacher generated. This lab will involve microscopic and macroscopic
examination of various types of fungi.
Chapter 32: An Introduction to Animal Diversity
Chapter 33: Invertebrates
Survey of types. Teacher generated. This involves doing microscopic and macroscopic
examination of various types of invertebrates.
Chapter 34: Vertebrates
Unit 6: Plant Form and Function 2 weeks
Chapter 35: Plant Structure, Growth, and Development
Chapter 36: Transport in Vascular Plants
Lab 9: AP lab on transpiration
Unit 7: Animal Form and Function 5 weeks
Chapter 40: Basic Principles of Animal Form and Function
Chapter 41: Animal Nutrition
Chapter 42: Circulation and Gas Exchange
Lab 11: AP lab on the physiology of the circulatory system
Chapter 43: The Immune System
Chapter 44: Osmoregulation and Excretion
Urine lab from Carolina Biological
Chapter 45: Hormones and the Endocrine System
Chapter 48: Nervous System
Lab on nervous system and response times. Teacher generated.
Chapter 49: Sensory and Motor Mechanisms
Lab on human sense organs: Teacher generated. This lab involves how various receptors
accommodate to stimuli of hot and cold. It also involves how touch receptors are not distributed
equally over the body.
AP BIOLOGY
SCIENCE CURRICULUM 470 GRADES 9 - 12
AP BIOLOGY SCORING RUBRIC
3. Applies effective and efficient strategies for gathering information and
materials, thinking critically, and solving problems.
Exceeds Expectations
The student independently collects, interprets, analyzes, and evaluates
a variety of information and data to make original predictions or solve
problems.
S/he
solves problems accurately and efficiently.
Meets Expectations
The student independently collects, interprets, analyzes, and evaluates
a variety of information and data to make specific predictions or solve
problems.
S/he
solves problems with few errors.
Meets Some Expectations
The student may need some assistance to collect and interpret a
variety of data to make general predictions or solve problems.
S/he
solves problems with some errors.
Does Not Meet Expectations
The student needs assistance to gather information to make a
prediction or solve problems.
S/he
solves problems with inefficiently
and with significant errors.
AP BIOLOGY
SCIENCE CURRICULUM 471 GRADES 9 - 12
BENEDICT’S REAGENT TEST
PROBLEM: How do various carbohydrate (glucose, fructose, galactose, maltose,
sucrose, lactose, starch and gum Arabic) react with Benedict's reagent and Lugol' s
iodine solutions? If a color change results, what is the mechanism for the change
in color?
(2 pt) ________
MATERIALS:
Plastic cups
2% solutions of carbohydrates (glucose,
fructose, galactose, sucrose, maltose, lactose,
starch, gum arabic)
water
Lugol's iodine solution
Benedict's reagent
Goggles/apron
spot plate
marker
10 ml graduated cylinder
eye droppers
test tube clamps
water bath
test tubes
test tube rack
(3 ¾ pt) ______
PROCEDURE:
1. Put on an apron and goggles.
Benedict's reagent test
2. Label each of 8 plastic with the name of a different carbohydrate: glucose,
fructose, galactose, sucrose, maltose, lactose, starch gum arabic.
(1 pt) ________
3. Fill each plastic cup about 2/3 full with its corresponding carbohydrate from the
stock bottle.
(1 pt) ________
4. Label a 9th plastic cup "water" and fill it 2/3 full with tap water.
(1 pt) ________
5. Obtain 10 test tubes and label each of 9 with a different carbohydrate and the 10th
test tube with water.
(1 pt) ________
6. Place the labeled test tubes in a test tube rack.
(1 pt) ________
7. Using the graduated cylinder, add 10 ml of Benedict's reagent to each of the 10
test tubes.
(1 pt) ________
8. Using an eyedropper, add 30 drops of a particular carbohydrate and water to the
correspondingly labeled test tube
(1 pt) ________
9. Using the clamps, place all 9 test tubes in a boiling water bath for 5 minutes.
(1 pt) ________
10. Using the clamps, remove the 9 test tubes from the water bath.
(1 pt) ________
11. Record observations.
(1 pt) ________
Lugol's iodine test
12. Label 10 wells on the spot plate with the names of each of the 9 carbohydrate and
water.
(1 pt) ________
13. Using an eyedropper, place 3 drops of each of the 9 carbohydrates and water into
their respectively labeled wells.
(1 pt) ________
14. Using an eyedropper add 3 drops of Lugol's iodine solution to each of the 10
wells
(1 pt) ________
15. Record observations.
(1 pt) ________
AP BIOLOGY
SCIENCE CURRICULUM 472 GRADES 9 - 12
OBSERVATIONS:
Benedict's reagent test
1. water: no change in blue color
(1 pt) ________
2. glucose: from blue to brick red color
(1 pt) ________
3. fructose: from blue to brick red color
(1 pt) ________
4. glucose: from blue to brick red color
(1 pt) ________
5. sucrose: no change in blue color
(1 pt) ________
6. maltose: blue to red orange color
(1 pt) ________
7. lactose: blue to red orange color
(1 pt) ________
8. starch: no change in blue color
(1 pt) ________
9. gum arabic: no change in blue color
(1 pt) ________
Lugol's iodine test
10. With water, glucose, fructose, glucose, sucrose, maltose, lactose, and gum Arabic
all of the mixture assumed the red brown color of the iodine. (no change)
(1 pt) ________
11. With starch, the red brown color of the iodine turned blue black.
(1 pt) ________
ANALYSIS:
Benedict's reagent test
1. This test allows scientists to detect the presence of all reducing sugars
(1 pt) ________
2. or sugars with a free reactive carbonyl group (aldehydes or alpha hydroxyl
ketones)
(1 pt) ________
3. Glucose, fructose, and galactose have carbonyl groups and therefore show a
positive Benedict's test.
(1 pt) ________
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 473 GRADES 9 - 12
COURSE DESCRIPTION
HIGH SCHOOL
1. Course Title
Introduction To Horticulture
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Intro To Horticul
3. Transcript Course Code/Number
00364
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 3
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of 9
th
and 10
th
grade science.
11. Brief Course Description
This course is designed to introduce students to gardening and horticulture techniques. Time will be
spent in the greenhouse, where students will conduct plant experiments and care for individual plant
projects. In addition, students will spend time outdoors in the class garden, working on landscaping
projects and caring for the nature trail on school property. The students will learn to use reference
sources in planning and implementing their projects.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocol.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 474 GRADES 9 - 12
13. Course Outline
UNIT
CONCEPT
ACTIVITY
How to Grow Seedlings in a
Greenhouse
1. Seed and Plant Selection
Annual vs. Perennial plants
Space considerations
Cost considerations
Seed Selection Lab
2. Soil Mixtures and Containers
Sterile Soil Preparation
Seed Starter Containers
Sterile Soil Lab
Seed Starter Kit Activity
3. Seed Planting
Soaking Seeds for Germination
Soil Preparation and Use
Seed Depth
Labeling
Seed Planting Project
4. Greenhouse Management
Control of Temperature, Light, Humidity
and Air Flow
Daily Greenhouse Operations
Greenhouse Study and Use
Set-Up/Clean-Up Duties
5. Watering Seedlings
Proper Watering Techniques
Dangers of Over watering and Under
watering.
Daily Plant Monitoring and Care
6. Transplanting Seedlings
When to Transplant
How to Safely Transplant
Proper Planting Depths
Transplanting Demonstration and
Activity
7. Feeding Seedlings
Types of Fertilizers
Preparation and Application of Fertilizers
Frequency of Fertilization
Demonstration and Practice of
Fertilizer Preparation/Application
Record Keeping Lab/Practice
8. Insect and Disease Control
Types of Pesticides
Preparation and Safe Application of
Pesticides
Demonstration and Practice of
Pesticide Preparation/Application
9. Stem Cuttings
Advantages of Stem Cuttings
Selection of Plants/Cuttings
Rooting Hormones
Cuttings Planting and Care
Stem Cuttings Demonstration and
Activity
Soils and Plant Growth
10. Soil Testing
Soil Collection Techniques
Soil Texture
Major Soil Nutrients
Soil Collection Activity
Soil Texture Lab
Soil Nutrient Testing Lab
11. Fertilizing
Nutrient Effects on Plant Growth
Liquid and Solid Fertilizers
Proper Application of Fertilizers
Fertilizer Information Activity
Demonstration and Practice of
Fertilizer Preparation/Application
12. Composting
Compost Mixtures
Maintenance of Compost Pile/Bin
Uses for Compost
Weekly Oversight of Compost Bin
Application of Compost to Outdoor
Gardens
13. Mulching
Types of Mulch
Uses for Mulch
Application of Mulch
Garden Mulching Activity
14. Site Selection
Sun and Shade Gardens
Class Project: Site Selection
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 475 GRADES 9 - 12
15. Soil Preparation
Wildflower Garden Techniques
Vegetable Garden Techniques
Flower Garden Techniques
Class Project: Wildflower Plantings
Class Project: Vegetable and/or
Flower Garden
Landscape Plants and Sites
16. Landscape Design
Landscape Plant Identification
Soil, Sunlight and Water Needs of
Landscape Plants
Research Project and Power Point
Presentation on a particular
landscape plant on school property
17. Planting a Landscape
Project.
Layout of a Flower/Vegetable Garden.
Soil Preparation
Plant Handling and Transplanting
Techniques
Mulching
Class Project: Flower Bed and/or
Herb Spiral
18. Long-term Care of a
Landscape Project
Watering and Feeding Schedules
Pest Control
Harvest of Flowers, Herbs or Vegetables
Class Project: Flower Bed and/or
Herb Spiral Maintenance
Horticulture and Art:
An Interdisciplinary Project
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark Assignments:
Independent greenhouse project, growing and caring for an assortment of vegetables and
flowers of the student's choosing.
o 3-Use effective strategies for gathering information, critical thinking and problem solving.
Research project on a specific landscape plant found on school property; a PowerPoint
presentation will be made to the class.
o 1C.-Speak, listen and view effectively.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 476 GRADES 9 - 12
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 477 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated during
the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Greenhouse access.
Tools and materials pertinent to greenhouse
work
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Supplementary materials
Guided Internet research with selected websites
SUGGESTED ASSESSMENT METHODS
Independent greenhouse project
*Benchmark*
Greenhouse Set-Up/Clean-Up Duties
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 478 GRADES 9 - 12
LEARNING STRAND
How to Grow Seedlings in a Greenhouse
Content Standard 10.6 – Living organisms have the capacity of producing populations of unlimited size, but the environment can
support only a limited number of individuals of each species.
Content Standard: Enrichment High School Biology – (Ecology) Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
Plant growth is dependent on proper input of
moisture, nutrients and sunlight.
Control of plant growth factors can be
accomplished in a well-managed greenhouse.
Decisions of seed selection, container types
and soil types can enhance or deter success in
the greenhouse.
Location of work space, tools and plant
benches plays an important role in greenhouse
management.
ESSENTIAL QUESTIONS
How does pre-soaking seeds ensure higher
germination rates?
What are the four variables for plant growth
that can be controlled in the greenhouse?
How does the proper choice of container, soil
and seed lead to successful seed starting?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Determine which plants to grow in the school
greenhouse.
Prepare seeds for planting.
Select and use proper containers and soils
when planting seeds.
Maintain the proper regimen of watering,
transplanting and feeding seedlings.
Assist in maintaining a safe, clean and efficient
working environment in the greenhouse.
Use fertilizers and pesticides in a safe and
responsible manner to ensure that plant
growth is maximized.
Functioning greenhouse
Work benches
Tools and containers
Greenhouse soil mix
Vegetable, flower and herb seed packets
Fertilizers and environmentally friendly
pesticides
Greenhouse management videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lecture demonstrations
Research flower and/or vegetable types using
seed catalogs and internet sources
Daily student maintenance of greenhouse plants
Audio visual presentations on greenhouse
management
Proper use of minimum/maximum thermometer
and hygrometer, soil pH meter and soil
moisture/nutrient meter
Prepare work bench, water, feed and transplant
seedlings on a daily basis
Clean the floors, benches, sink and counters on
a daily basis
SUGGESTED ASSESSMENT METHODS
Unit test
Video response summaries
Daily assessment of ―Greenhouse Set-Up/Clean-
Up‖ crew
Ongoing assessment of growth and health of
each student’s plants
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 479 GRADES 9 - 12
LEARNING STRAND
Soils and Plant Growth
Content Standard: Enrichment High School Earth Science – (Biogeochemical Cycles) Each element on Earth moves among reservoirs
which exist in the solid earth, in oceans, in the atmosphere, and within and among organisms as part of biogeochemical cycles.
ENDURING UNDERSTANDINGS
Plant growth is dependent on soil for root
support, moisture and nutrients.
Decisions about landscape plant selection, as
well as flower and vegetable types for home
gardens, hinge on soil types and soil
preparation.
Home gardeners and commercial landscapers
must test and modify soils in order to optimize
plant growth.
Use of organic methods of soil amendment,
such as compost and mulch, can ensure
success as well as protect the surrounding
environment.
ESSENTIAL QUESTIONS
What are the essential nutrients and what are the
effects of each on plant growth?
How is soil texture measured and assessed?
What are the proper ingredients of compost and
how do you maintain a compost pile?
What are the benefits of using mulch in the
garden?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish among the various soil types found
in our region.
Determine which flowers, herbs and vegetables
are best suited to our local soil types.
Maintain the proper mix of ingredients,
moisture levels and aeration in the class
compost bins.
Measure local soils for a variety of chemical
and physical factors with the intent of
maintaining healthy soil structure.
Prepare soil in all flower, herb and vegetable
beds on school property before moving
greenhouse plants into the garden.
Compost bins
Organic mulch
Soil nutrient/Soil texture test kits/Soil meters
Soil and Plant Growth videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Research soil types using library and internet
resources
Audio visual presentations on soils and plant
growth
Learn the proper method of taking soil samples
for testing
Learn the proper use of soil meters and test kits
for soil nutrients and soil texture
Research the soil and nutrient needs of their
greenhouse-grown plants before transplanting
these to the garden beds
Learn the proper methods for applying
commercial fertilizers to maximize plant growth,
yet minimize deleterious effects on the
environment
SUGGESTED ASSESSMENT METHODS
Unit test
Video response summaries.
Soil Nutrient lab investigation
Soil Texture lab investigation
Ongoing assessment of gardening skills as applied
to outdoor projects
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 480 GRADES 9 - 12
LEARNING STRAND
Landscape Plants and Sites
Content Standard 10.6 –
Living organisms have the capacity of producing populations of unlimited size, but the environment can
support only a limited number of individuals of each species.
Content Standard: Enrichment High School Biology – (Ecology) Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
Landscape planting is both science and art,
and combines practical skills and aesthetic
sensibility.
Proper landscape design should take into
account, soils, climate, plant availability and
cost.
Maintenance of a commercially landscaped
area, such as a school, should require no
more manpower and cost than will be
available on a long-term basis.
The benefits of well thought out landscape
design include a pleasant environment for
work or recreation, as well as research
opportunities for students.
ESSENTIAL QUESTIONS
Why are certain plants so commonly used in
commercial landscaping?
What considerations for soil, moisture, sunlight
and climate must be made when creating a design?
Is the selection and placement of plants on school
property in keeping with the physical environment?
Are the plants in the school landscape likely to be
easy to maintain for the foreseeable future?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Learn to identify each of the landscape trees,
shrubs and ground cover on school property.
Determine the physical needs of selected
landscape plants.
Explain how cost and ease of care influence
the selection of landscape plants.
Cooperatively choose and prepare a site for
either a garden bed or herb spiral on school
property as a class project.
Successfully transplant greenhouse grown
flowers, herbs and/or vegetables to new
garden bed locations.
Maintain all garden beds, new and old, for the
duration of the trimester.
Prepare and give a presentation to the class
on a specific landscape plant.
Lap top computers with internet connection
Horticultural reference materials in school library
Light, soil pH and soil moisture meters
Landscaping tools
Landscaping videos
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lecture demonstrations
Audio visual presentations on landscaping
Work cooperatively in small teams on outdoor class
projects
Conduct Internet and library research on a specific
landscape plant; make PowerPoint presentation to
class
Demonstrate understanding of proper tool
selection and use on outdoor projects
Assess the soil, light and moisture conditions of
selected landscape plants
SUGGESTED ASSESSMENT METHODS
Research project on specific landscape plant
*Benchmark*
Video response summaries
Ongoing assessment of cooperative work skills as
applied to outdoor projects
Site Assessment lab
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 481 GRADES 9 - 12
LEARNING STRAND
Horticulture and Art Interdisciplinary Project
Content Standard: Scientific Inquiry – Scientific inquiry progresses through a continuous process of questioning, data collection,
analysis and interpretation. (D INQ.6 – Use appropriate tools and techniques to make observation and gather data.)
ENDURING UNDERSTANDINGS
The history of horticulture and garden design is
intertwined with the visual arts.
Wild plants are the progenitors of our modern
domestic plants.
Plant domestication began thousands of years
ago using trial-and-error methods, and
continues today using modern plant breeding
techniques.
The creation of botanically inspired art is one
application of a career choice inspired by
plants.
Botanists use certain standard techniques in
the collection and preservation of plants.
ESSENTIAL QUESTIONS
What techniques are used to collect and preserve
plants for long-term study?
What is the connection between protection of
endangered species and the collection and
preservation of museum specimens?
How does an appreciation of the botanical arts
enhance our appreciation and enjoyment of plants
in general?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Learn to distinguish among the four plant
types: tree, shrub, herb and vine.
Collect samples of each of the four plant types
on school and/or Bauer Farm property.
Preserve and mount collected plants using
botanical techniques.
Identify the plants in their collection using field
guides to trees, shrubs and wildflowers.
Learn basic drawing, painting, bookmaking
and/or other techniques as applicable to the
specific interdisciplinary project.
Internet activities on selected websites
Planning time with art department colleagues
Horticultural reference materials in school library
Plant presses
Herbarium sheets and paste
Botanical labels
Art supplies
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lecture demonstrations
Plant Collection lab
Plant Preservation lab
Introductory art skills lab
Art studio project
SUGGESTED ASSESSMENT METHODS
Botanical collection
Art project (e.g., watercolor sketches, folding
book, pen and ink illustrations, sculpture)
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 482 GRADES 9 - 12
Horticulture Scoring Rubric
Applies effective and efficient strategies for gathering information and materials, thinking
critically, and solving problems.
Exceeds
Expectations
The student independently interprets, analyzes, and evaluates a variety of information
and data to solve problems in the daily care of greenhouse plants. S/he solves problems
accurately and efficiently, and germinates, grows and maintains healthy plants.
Meets
Expectations
The student independently interprets, analyzes, and evaluates a variety of information
and data to solve problems in the daily care of greenhouse plants. S/he solves problems
with few errors, and germinates, grows and maintains healthy plants.
Meets Some
Expectations
The student may need some assistance interpreting a variety of data to solve problems
in the daily care of greenhouse plants. S/he solves problems with some errors, and
germinates, grows and maintains healthy plants.
Does Not Meet
Expectations
The student needs assistance in gathering information to solve problems in the daily care
of greenhouse plants. S/he solves problems inefficiently and with significant errors, and
germinates, grows and maintains healthy plants with difficulty.
Demonstrates proficiency and fluency in communication to meet the literacy demands of the
global community.
Speaking / Listening / Viewing
Exceeds
Expectations
The student applies effective and efficient listening and viewing strategies to understand,
interpret, evaluate, and analyze material to acquire content knowledge regarding a
specific landscape tree/shrub found on school property. S/he reflects and responds
creatively to a variety of material, and delivers a fluent and coherent power point
presentation on the tree/shrub chosen.
Meets
Expectations
The student applies effective listening and viewing strategies to understand, interpret,
evaluate, and analyze material to acquire content knowledge regarding a specific
landscape tree/shrub found on school property. S/he reflects and responds to a variety
of material and delivers a coherent power point presentation on the tree/shrub chosen.
Meets Some
Expectations
The student applies some listening and viewing strategies to understand, interpret,
evaluate, and attempt to analyze material to acquire content knowledge regarding a
specific landscape tree/shrub found on school property. S/he may need assistance to
respond to material. S/he may be reluctant to deliver a power point presentation on the
tree/shrub chosen.
Does Not Meet
Expectations
The student has difficulty applying listening and viewing strategies without assistance to
understand, interpret, and evaluate material to acquire content knowledge regarding a
specific landscape tree/shrub found on school property. The student's ability to respond
to material or to deliver a power point presentation on the tree/shrub chosen is very
limited.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 483 GRADES 9 - 12
GREENHOUSE DAILY REPORT NAME_____________________
DATE_____________________
This report will be collected and graded every Friday. Be sure to provide a written description of your greenhouse work
each day. In addition, fill out the appropriate areas on the reverse side of this sheet. Your grade depends on the
thoroughness of this report.
MONDAY
TUESDAY
WEDNESDAY
THURSDAY
FRIDAY
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 484 GRADES 9 - 12
PROCEDURE MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY____
Maximum and
Minimum Air
Temperature A
Maximum and
Minimum
Relative
Humidity A
Soil
Temperature B
Soil
PH B
Soil
Moisture B .
Watering C .
Fertilizer
Application
(list fertilizer) C .
Pesticide
Application
(list pesticide) C .
A = Daily B = Once a week C = Use as needed
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 485 GRADES 9 - 12
GREENHOUSE PLANTS
NAME ____________________________________
PLANT
NUMBER
COMMENTS
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 486 GRADES 9 - 12
GREENHOUSE SET-UP and CLEAN UP
SET-UP
SOIL IN TRAYS
SOIL MOISTENED
RECORD TEMPS.
RE-SET TEMPS.
CLEAN UP
SWEEP FLOOR
CLEAN COUNTER
CLEAN SINK
SPRAY BOTTLES
RETURNED
NEWSPAPER ON
BENCH
WATER FOR
ABSENTEES
NAME __________________________________________
NAME___________________________________________
NAME___________________________________________
DATE __________________________________________
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 487 GRADES 9 - 12
Horticulture Scoring Rubric
Applies effective and efficient strategies for gathering information and materials, thinking
critically, and solving problems.
Exceeds Expectations
The student independently interprets, analyzes, and evaluates a
variety of information and data to solve problems in the daily care of
greenhouse plants. S/he solves problems accurately and efficiently,
and germinates, grows and maintains healthy plants.
Meets Expectations
The student independently interprets, analyzes, and evaluates a
variety of information and data to solve problems in the daily care of
greenhouse plants. S/he solves problems with few errors, and
germinates, grows and maintains healthy plants.
Meets Some Expectations
The student may need some assistance interpreting a variety of data
to solve problems in the daily care of greenhouse plants. S/he solves
problems with some errors, and germinates, grows and maintains
healthy plants.
Does Not Meet
Expectations
The student needs assistance in gathering information to solve
problems in the daily care of greenhouse plants. S/he solves
problems inefficiently and with significant errors, and germinates,
grows and maintains healthy plants with difficulty.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 488 GRADES 9 - 12
DHHS TREE AND SHRUB PRESENTATION
Checklist
POWER POINT
Your Power Point should include the following information:
1. Common name ___________
2. Scientific name ___________
3. Family name ___________
4. Habit and Form
• Shape ___________
• Size (Height X Width) ___________
• Evergreen or Deciduous ___________
5. Description
• Flowers ___________
• Fruit ___________
• Foliage ___________
• Bark ___________
6. Habitat
• Native to what region of the U.S. or what country? __________
• Prefers what habitat? __________
7. Environmental Conditions! Planting Requirements
• Sunlight ___________
• Soil pH ___________
• Soil moisture __________
• Zone(s): ___________
8. Landscape Use(s) __________
9. Propagation __________
10. Insect, Disease and Other Problems __________
11. Wildlife Benefits __________
12. Other Uses __________
• Medicinal value
• Erosion prevention
• Etc.
PICTURE: __________
• Include at least one picture of the plant.
BIBLIOGRAPHY: __________
• Include a bibliography that includes websites, books
and magazine articles.
• There must be a minimum of 3 references.
MINIMUM LENGTH: __________
• Power Point should include at least 7 slides.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 489 GRADES 9 - 12
DHHS TREE AND SHRUB PRESENTATION
PURPOSE: To create a Power Point presentation about one of the trees or shrubs which we
have in our collection of landscape plants at DHHS.
PROCEDURE:
A. You are to choose from among the plants listed below.
B. Then you will research the plant using the internet and library books.
C. Once you have created your Power Point, you will give a short lecture to the class.
D. Your research will be kept on file as a reference source for future classes.
LIST OF PLANTS:
Arrowwood Pyracantha
Bayberry Redbud
Beach Rose (Rugosa rose) Red Cedar
Clethra Red maple
Heather Rhodedendron
Highbush Cranberry St. Johnswort
Inkberry Holly Silky Dogwood
Ground Juniper Spirea
Leucothoe Vinca
Star Magnolia White pine
Paper birch Oriental Cherry
White Cedar (Arborvitae) Mountain Laurel
Potentilla Ninebark
Winterberry Holly
POWER POINT
Your Power Point should include the following information:
1. Common name
2. Scientific name
3. Family name
4. Habit and Form
Shape
Size (Height X Width)
Evergreen or Deciduous
5. Description
Flowers
Fruit
Foliage
Bark
6. Habitat
Native to what region of the U.S. or what country?
Prefers what habitat? (beach, edge of stream, open field, park, etc.)
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 490 GRADES 9 - 12
7. Environmental Conditions/ Planting Requirements
Sunlight
Soil pH
Soil moisture
Zone(s): (Connecticut is zone 6.)
8. Landscape Use(s)
9. Propagation
10. Insect, Disease and Other Problems
11. Wildlife Benefits
12. Other Uses
Medicinal value
Erosion prevention
Etc.
PICTURE:
Include at least one picture of the plant.
BIBLIOGRAPHY:
Include a bibliography that includes websites, books and magazine articles.
There must be a minimum of 3 references.
MINIMUM LENGTH:
Power Point should include at least 3 slides.
SUGGESTED WEBSITES
www.gwf.org
www.gardening.cornell.edu
www.hort.uconn.edu/plants
www.dnr.state.us.forestry/Education/ohiotrees
www.ces.ncsu.edu/depts/hort/consumer/factsheets/native/common_namea-e.html
www.landscape.cornell.edu
www.google.com

INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 491 GRADES 9 - 12
POWER POINT PRESENTATION Name:____________________
HORTICULTURE
CATEGORY
1. Speaker can be heard by the audience. ____5____
2. Presentation is well organized. ___ 10 ___
3. Presentation is creative/interesting. ___ 10 ___
4. Presentation is appropriate length. ____5____
(5 minutes)
5. Speaker responds well to questions. ____5____
TOTAL POINTS __ 35 __
POWER POINT PRESENTATION Name:____________________
HORTICULTURE
CATEGORY
1. Speaker can be heard by the audience. ____5____
2. Presentation is well organized. ___ 10 ___
3. Presentation is creative/interesting. ___ 10 ___
4. Presentation is appropriate length. ____5____
(5 minutes)
5. Speaker responds well to questions. ____5____
TOTAL POINTS __ 35 __
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 492 GRADES 9 - 12
HORTICULTURE POWER POINT Name: __________________________
Common name _______________
Scientific name _______________
Family name _______________
Habit:
Shape _______________
Size _______________
Decid/Ever. _______________
Description:
Flower _______________
Fruit _______________
Foliage _______________
Bark _______________
Habitat:
Native _______________
Preference _______________
Planting Requirements:
Sunlight _______________
Soil moisture _______________
Zone _______________
Landscape uses _______________
Propagation _______________
Diseases _______________
Wildlife _______________
Other Uses _______________
Picture(s) _______________
Bibliography _______________
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 493 GRADES 9 - 12
Horticulture Scoring Rubric
Demonstrates proficiency and fluency in communication to meet the literacy demands of the
global community.
Speaking / Listening / Viewing
Exceeds Expectations
The student applies effective and efficient listening and viewing strategies
to understand, interpret, evaluate, and analyze material to acquire
content knowledge regarding a specific landscape tree/shrub found on
school property. S/he reflects and responds creatively to a variety of
material, and delivers a fluent and coherent power point presentation on
the tree/shrub chosen.
Meets Expectations
The student applies effective listening and viewing strategies to
understand, interpret, evaluate, and analyze material to acquire content
knowledge regarding a specific landscape tree/shrub found on school
property. S/he reflects and responds to a variety of material and delivers
a coherent power point presentation on the tree/shrub chosen.
Meets Some Expectations
The student applies some listening and viewing strategies to understand,
interpret, evaluate, and attempt to analyze material to acquire content
knowledge regarding a specific landscape tree/shrub found on school
property. S/he may need assistance to respond to material. S/he may be
reluctant to deliver a power point presentation on the tree/shrub chosen.
Does Not Meet Expectations
The student has difficulty applying listening and viewing strategies
without assistance to understand, interpret, and evaluate material to
acquire content knowledge regarding a specific landscape tree/shrub
found on school property. The student's ability to respond to material or
to deliver a power point presentation on the tree/shrub chosen is very
limited.

INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 494 GRADES 9 - 12
WEEKLY ARTICLE
PURPOSE:
Every week you will have the opportunity to read an article that pertains to some
aspect of this course. The goal is to make you more aware of career opportunities,
interesting discoveries and issues of importance. I hope that your interest will
continue long after you have completed this course.
METHODS:
Each Friday, at the start of class, you will hand in a 1 to 2 page review of an article
from a magazine, newspaper or internet website.
The accumulated reviews will be worth 10% of your trimester grade.
There will be 10 reviews due each trimester.
If you are absent on Friday, your review is due the day after you return to class.
Include the following information:
1. Name of magazine, newspaper or website.
2. Date of the issue.
3. Author's name.
4. Title of article.
5. Copy of any internet article.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 495 GRADES 9 - 12
Horticulture Scoring Rubric
2. Uses technology effectively and responsibly
Exceeds Expectations
The student can independently select and use appropriate internet
sources to solve problems efficiently and creatively, including summary
of and reaction to environment-related articles.
Meets Expectations
The student can select and use appropriate internet sources to solve
problems effectively without making significant errors, including
summary of and reaction to environment-related articles.
Meets Some Expectations
The student can select and use appropriate internet sources to solve
problems but makes some errors and requires some assistance,
including summary of and reaction to environment-related articles.
Does Not Meet Expectations
The student cannot select and use appropriate internet sources to solve
problems without making many significant errors and requiring
supervision; student fails to summarize and react to environment-related
articles.
INTRODUCTION TO HORTICULTURE
SCIENCE CURRICULUM 496 GRADES 9 - 12
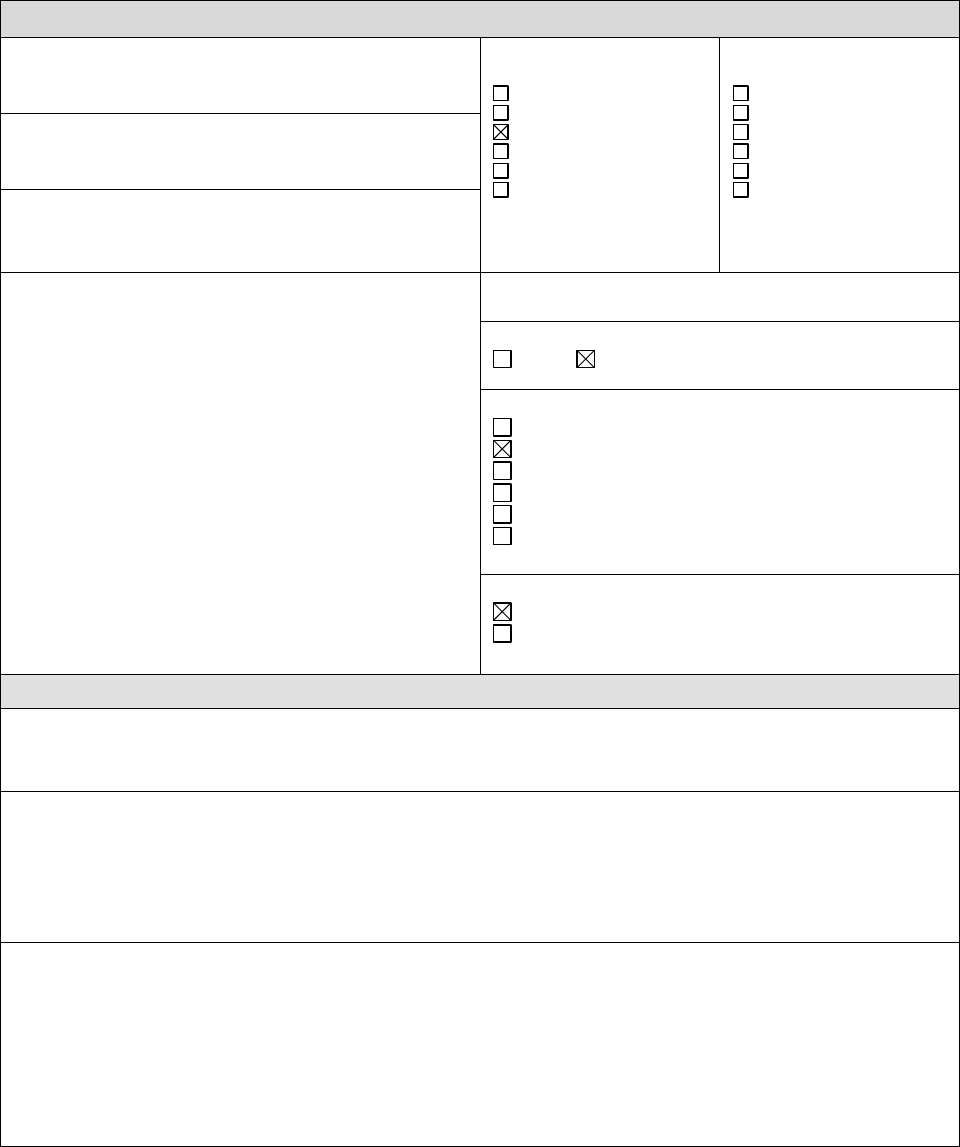
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 497 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Topics In Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Topics In Science
3. Transcript Course Code/Number
00366
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 3
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval _________________(date)
10. Pre-Requisites
Successful completion of General Biology, Biological Systems or Biology - Honors
11. Brief Course Description
This course investigates how developments in physics, chemistry and biology relate to major issues in our
society. Emphasis is placed on helping the student to develop skills in analyzing and reaching decisions
on social issues in science using specific principles in science and relating these principles to life in our
society. Discussion, reading and writing are major components of this course
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Use technology effectively and responsibly.
3. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
4. Demonstrate the ability complete assignments independently.
5. Demonstrate respect for one’s self, and strive to contribute to the success of others.

TOPICS IN SCIENCE
SCIENCE CURRICULUM 498 GRADES 9 - 12
13. Course Outline
Topics covered:
Cloning; Human Genome Project
Stem Cell Research; Human Genome Project
Surrogacy
Organ Transplants
Environmental Issues: Epidemics
Diseases
The Teenage Brain
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
Response to science articles (demonstrates proficiency and fluency in communication to meet the literacy
demands of the global community and uses technology effectively and responsibly)
Video questions and worksheets (reads, writes, speaks, listens and views effectively)
TOPICS IN SCIENCE
SCIENCE CURRICULUM 499 GRADES 9 - 12
LEARNING STRAND Cloning
Connecticut Standards
Genetics: Mutation and sexual reproduction lead to genetic variation in a population.
The genetic composition of cells can be altered by incorporation of exogenous DNA into cells.
Scientific Inquiry and Literacy: Read, interpret and examine the credibility and validity of scientific claims in different sources of
information.
ENDURING UNDERSTANDING
The cloning has ethical, legal and social
issues in today’s society.
ESSENTIAL QUESTIONS
How can cloning technologies be used?
What are ethical issues of cloning?
What are the legal issues of cloning?
What are the social issues of cloning?
Can organs be cloned for use in transplants?
What are the risks of cloning?
Should cloning be regulated?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain the process of cloning and the
different types of cloning.
Explain how cloning technologies can be used.
Discuss the ethical, legal and social issues of
cloning.
Discuss the risks of cloning.
Support positions on cloning using the
information that has been researched for
class.
Video on cloning
Video on Dolly
"Power of Genes" video
Human Genome Project Information website
Websites on genetic technology and cloning
Articles on cloning
SUGGESTED INSTRUCTIONAL STRATEGIES
Students will assess the credibility of information in
articles and write journal entries on the issues of
cloning.
Students will view the videos on cloning and
discuss the concepts associated with cloning.
Cooperative learning groups will be assigned
different articles for jigsaw activities using
PowerPoint and interactive white board
presentations.
SUGGESTED ASSESSMENT METHODS
Quizzes on cloning
Journal entries evaluated with rubrics
Presentations to the class evaluated with rubrics
TOPICS IN SCIENCE
SCIENCE CURRICULUM 500 GRADES 9 - 12
LEARNING STRAND: Stem Cell Research
Connecticut Standards
Genetics: Genes are a set of instructions encoded in the DNA sequence of each organism that specify the sequence of amino acids in
proteins characteristic of that organism.
The genetic composition of cells can be altered by incorporation of exogenous DNA into cells.
Scientific Inquiry and Literacy: Read, interpret and examine the credibility and validity of scientific claims in different sources of
information.
Communicate about science in different formats, using relevant science vocabulary, support evidence and clear logic.
ENDURING UNDERSTANDING
There are state and federal policies and
legislation govern stem cell research.
ESSENTIAL QUESTIONS
Why is stem cell research supported by some
individuals and not others?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify the importance of stem cells in the
body.
Explain the difference between stem cells and
other cells in the body.
Prepare a position paper on stem cell research.
Present the position paper on stem cell
research to the class.
Internet, periodical and news articles
Websites on stem cell research
Stem Cell videos
Science journals
Human genome Project Information website
SUGGESTED INSTRUCTIONAL STRATEGIES
Students will prepare a bibliography of reliable
useful websites on stem cell research.
Students will write journal entries of their
understanding regarding the controversy of stem
cell research.
Students will write a persuasive essay explaining
to others their point of view on stem cell research.
Students will debate the issues regarding stem
cell research.
SUGGESTED ASSESSMENT METHODS
Journal entries evaluated with rubrics
Quizzes
Position paper evaluated with a rubric
Debate evaluated with a rubric
TOPICS IN SCIENCE
SCIENCE CURRICULUM 501 GRADES 9 - 12
LEARNING STRAND: Surrogacy
Connecticut Standards
Genetics: Mutation and sexual reproduction lead to genetic variation in a population.
A multicellular organism develops from a single zygote, and its phenotype depends on its genotype, which is established at
fertilization.
Scientific Inquiry and Literacy: Read, interpret and examine the credibility and validity of scientific claims in different sources of
information.
Communicate about science in different formats, using relevant science vocabulary, support evidence and clear logic.
ENDURING UNDERSTANDINGS
Surrogacy has become a popular solution to
infertility problems.
Humans can reproduce in nontraditional
ways.
In gestational surrogacy,
in vitro
fertilization
is used to transfer the genetic mother's
fertilized embryo into the surrogate mother's
uterus.
Infertility has many causes that involve the
woman's, the man's or both partners'
reproductive systems.
ESSENTIAL QUESTIONS
What are reasons surrogacy has become more
popular?
What are reasons a woman would be willing to be
a surrogate mother?
What are some reasons couples would want/or not
want to arrange to have a surrogate mother for
their child?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish between amniocentesis, chorionic
villi sampling, and karyotyping.
Understand reproductive biology and
in vitro
fertilization.
Understand causes of fertility problems.
Periodical and news articles
Selected websites
Science journal
SUGGESTED INSTRUCTIONAL STRATEGIES
The student will read articles on Reproductive
Biology and report key concepts in class
discussions.
The student will write journal entries in response to
information presented in class about surrogate
motherhood.
Speaker from a fertility clinic to present options for
producing a baby.
The student will read articles on typical surrogate
mother profiles and surrogate motherhood and
report key concepts in class discussions.
SUGGESTED ASSESSMENT METHODS
Reports evaluated with rubrics
Journal entries evaluated with rubrics
TOPICS IN SCIENCE
SCIENCE CURRICULUM 502 GRADES 9 - 12
LEARNING STRAND: Organ Transplants
Connecticut Standards
Physiology: As a result of the coordinated structures and functions of organ systems, the internal environment of the human body
remains relatively stable (homeostatic) despite changes in the outside environment.
Scientific Inquiry and Literacy: Read, interpret and examine the credibility and validity of scientific claims in different sources of
information.
Communicate about science in different formats, using relevant science vocabulary, support evidence and clear logic.
ENDURING UNDERSTANDING
Organ transplants can save lives and are in
great demand.
ESSENTIAL QUESTIONS
Why is it so difficult to get an organ transplant when
needed?
What are some of the legal ramifications involved in
organ transplants?
What is considered ―brain-dead‖ by legal definition
and various authorities?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Recognize the advances in biotechnology
facilitating organ transplants.
Recognize the medical problems that are
associated with donated organs.
Define brain-dead.
National Geographic Channel support network
Internet articles on organ transplants
Video clips on organ transplants
SUGGESTED INSTRUCTIONAL STRATEGIES
Students will prepare a bibliography of reliable useful
websites on organ transplants.
Students will write journal entries of their
understanding regarding controversial issues of organ
transplants.
Students will debate controversial issues regarding
transplanting organs.
SUGGESTED ASSESSMENT METHODS
Quizzes
Research project about the effects of drugs needed
for successful organ transplants
Discussion of living wills
TOPICS IN SCIENCE
SCIENCE CURRICULUM 503 GRADES 9 - 12
LEARNING STRAND Environmental Issues: Epidemics
Connecticut Standards
Physiology: Organisms have a variety of mechanisms to combat disease.
Scientific Inquiry and Literacy: Read, interpret and examine the credibility and validity of scientific claims in different sources of
information.
Communicate about science in different formats, using relevant science vocabulary, support evidence and clear logic.
ENDURING UNDERSTANDING
Epidemics can be prevented with disease
control.
The diseases such as H1N1 influenza impact
our environment and the human population.
ESSENTIAL QUESTIONS
What is the impact of an epidemic?
What is the impact of an outbreak of H1N1
influenza?
Why is H1N1 influenza contagious?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Recognize the significance and impact of H1N1
influenza as well as transmission among
humans.
Explain to friends and family what steps can
be taken to reduce the chance of contracting
an infectious disease.
Internet and periodical articles on epidemics
Internet and news articles on H1N1 influenza
CDC website information and instructional
materials
SUGGESTED INSTRUCTIONAL STRATEGIES
Read and respond to articles from the World
Health Organization
Read and report on news programs and Centers
for Disease Control information regarding the
environmental issues of infectious diseases
Create a Public Service Announcement to prevent
the spread of disease
SUGGESTED ASSESSMENT METHODS
Research project on systems and methods of
disease control
Public Service Announcement
Quizzes
TOPICS IN SCIENCE
SCIENCE CURRICULUM 504 GRADES 9 - 12
LEARNING STRAND Diseases
Connecticut Standards
Physiology: Organisms have a variety of mechanisms to combat disease.
Scientific Inquiry and Literacy: Read, interpret and examine the credibility and validity of scientific claims in different sources of
information.
Articulate conclusions and explanations based on research data, and assess results based on the design of an investigation.
Communicate about science in different formats, using relevant science vocabulary, support evidence and clear logic.
ENDURING UNDERSTANDINGS
The human body has different barriers to
protect itself from diseases.
The human body defends itself differently
when infected by a virus as compared to
bacteria.
In our environment only a small percentage
of microorganisms actually cause diseases.
ESSENTIAL QUESTIONS
What is the impact of disease prevention in a
community?
How is the body protected from diseases?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe what it means to be healthy.
Learn how the body is designed so that the
systems to work together for protection.
Learn the basic physiology of the immune
system.
Define key elements of the human immune
system.
Describe ways to prevent the spread of germs
that cause common infectious diseases.
Understand the difference between passive
and active immunity.
Centers for Disease Control and Prevention
website
The Ultimate Guide: Human Body
Video
SUGGESTED INSTRUCTIONAL STRATEGIES
Online formative quizzes
Internet, periodical and news articles
Powerpoint and audio visual presentations
Science journal entries on disease topics, facts, and
treatments
SUGGESTED ASSESSMENT METHODS
Quizzes on the human immune system
Research project on a disease and its prevention
Reflection paper on personal health plan that
protects against diseases
TOPICS IN SCIENCE
SCIENCE CURRICULUM 505 GRADES 9 - 12
TOPICS IN SCIENCE
Scoring Rubric
1. Demonstrates proficiency and fluency in communication to meet the literacy demands of
the global community.
A. Reading
Exceeds
Expectations
The student applies effective reading strategies to understand, interpret, evaluate,
and analyze scientific articles to acquire content knowledge. S/he reads fluently for
assignments.
Meets
Expectations
The student applies effective reading strategies to understand, interpret, evaluate,
and analyze scientific articles to acquire content knowledge.
Meets Some
Expectations
The student applies some reading strategies to understand, interpret, evaluate,
and attempt to analyze scientific articles to acquire content knowledge. S/he may
need assistance to read material at grade level.
Does Not Meet
Expectations
The student has difficulty applying reading strategies without assistance to
understand, interpret, and evaluate scientific articles to acquire content
knowledge.
B. Writing
Exceeds
Expectations
The student writes and responds to scientific articles and/or videos with a clear
focus and can support with details that are well developed and organized, showing
both analysis and synthesis of ideas. Student responds fully to the assignments
with word choice and syntax are accurate and appropriate. The student shows
mastery in the conventions of Standard English. The student successfully
completes all parts of the writing process, including peer and self-evaluation.
Meets
Expectations
The student writes and responds to scientific articles and videos using some
supporting details to show an understanding of the subject matter and an analysis
of ideas. They are somewhat developed and organized. Word choice and syntax
are accurate and appropriate. Errors in the conventions of Standard English are
few. The student completes most parts of the writing process, including
evaluation.
Meets Some
Expectations
The student requires some additional explanations and models to write and
respond to scientific articles and videos. With direction, s/he selects an appropriate
mode. Writing has a somewhat limited supporting details.. The student may
require assistance to develop or organize his response. Word choice and syntax
are consistent with grade level. There are some errors in the conventions of
Standard English.
Does Not Meet
Expectations
The student requires many additional explanations, models, graphic organizers,
and/or strategies in order to write and respond to scientific articles and videos in
order to complete the writing process. The writing has no clear focus or no
supporting details and inaccuracies. Inaccurate or limited vocabulary, syntax
errors, and errors in the conventions of writing make the writing ineffective.
TOPICS IN SCIENCE
SCIENCE CURRICULUM 506 GRADES 9 - 12
C. Speaking / Listening / Viewing
Exceeds
Expectations
The student applies effective and efficient listening and viewing strategies to
understand, interpret, evaluate, and analyze science videos to acquire content
knowledge. S/he reflects and responds creatively to a variety of material, and
delivers fluent and coherent oral / visual presentations.
Meets
Expectations
The student applies effective listening and viewing strategies to understand,
interpret, evaluate, and analyze material to acquire content knowledge. S/he
reflects and responds to a variety of material and delivers coherent oral / visual
presentations.
Meets Some
Expectations
The student applies some listening and viewing strategies to understand, interpret,
evaluate, and attempt to analyze material to acquire content knowledge. S/he may
need assistance to respond to material. S/he may be reluctant to deliver oral /
visual presentations.
Does Not Meet
Expectations
The student has difficulty applying listening and viewing strategies without
assistance to understand, interpret, and evaluate material to acquire content
knowledge. The student's ability to respond to material or to deliver oral / visual
presentations is very limited.
2. Uses technology effectively and responsibly.
Exceeds
Expectations
The student can independently select and use appropriate technology to research
information.
Meets
Expectations
The student can select and use appropriate technology to research scientific
information effectively without making significant errors.
Meets Some
Expectations
The student can select and use appropriate technology to research information but
makes some errors and requires some assistance.
Does Not Meet
Expectations
The student cannot select and use appropriate technology to research information
without making many significant errors and requiring supervision.
3. Applies effective and efficient strategies for gathering information and materials, thinking
critically, and solving problems.
Exceeds
Expectations
The student independently collects, interprets and analyzes data related to a
complex issue using the characteristics of a critical thinker.
Meets
Expectations
The student collects, interprets, analyzes data related to a complex issue using
the characteristics of a critical thinker.
Meets Some
Expectations
The student may need some assistance to collect, interpret and analyze data using
the characteristics of a critical thinker.
Does Not Meet
Expectations
The student needs a great deal of assistance to gather information and analyze a
complex issue using the characteristics of a critical thinker

TOPICS IN SCIENCE
SCIENCE CURRICULUM 507 GRADES 9 - 12
NAME: ____________________________________ DATE: ______________________________________
The Eyes of Nye: Cloning
Thinking questions:
1. What are the pros and cons about patenting the process of nuclear transfer?
2. Dolly died at a young age. Why?
3. What is the difference between therapeutic and reproductive cloning?
4. What did scientists do to the rats? Why?

TOPICS IN SCIENCE
SCIENCE CURRICULUM 508 GRADES 9 - 12
TOPICS IN SCIENCE
1 C Speaking / Listening / Viewing
HUMAN GENOME OR CLONING VIDEO
Exceeds
Expectations
The student applies effective and efficient listening and viewing
strategies to effectively understand, interpret, evaluate, and analyze
science videos to acquire content knowledge. S/he reflects and
responds effectively and coherently to the corresponding video
sheet provided by the instructor.
Meets
Expectations
The student applies effective listening and viewing strategies to
sufficiently understand, interpret, evaluate, and analyze material to
acquire content knowledge after viewing the video on the human
genome or cloning. S/he reflects and responds coherently to the
corresponding video sheet provided by the instructor.
Meets Some
Expectations
The student applies some listening and viewing strategies to
adequately understand, interpret, evaluate, and analyze material to
acquire knowledge after viewing the video on the human genome or
cloning. S/he may need assistance to respond to the corresponding
video sheet provided by the instructor.
Does Not Meet
Expectations
The student has difficulty applying listening and viewing strategies
without assistance to understand, interpret, and evaluate material to
acquire content knowledge after viewing the video on the human
genome or cloning. S/he requires assistance to be able to respond
to the corresponding video sheet provided by the instructor.
TOPICS IN SCIENCE
SCIENCE CURRICULUM 509 GRADES 9 - 12
Bi-monthly article
Benchmark Activity
Purpose- Every other week you will have the opportunity to read an article that
pertains to some aspect of this course. The goal is to make you more aware of career
opportunities, interesting discoveries and issues of importance that might
political/scientific in nature. I hope your interest in such topics will continue long after
you have completed this course.
Methods -
Every other Friday, at the start of class, you will hand in a 1 to 2 page review of an
article from a magazine, newspaper or internet website.
The accumulated reviews with count towards 15% of your trimester grade
There will be 6 reviews due this trimester
If you are absent on Friday, your review is due the day you return to class.
If it is typed it can be no larger than font 14, double spaced. If I cannot read it, I will
ask you to type it.
Include the following information in your summary:
1. name of magazine, newspaper or website - use proper format
2. date of the article, issue etc
3. authors name
4. title of article
5. copy of the internet article
TOPICS IN SCIENCE
SCIENCE CURRICULUM 510 GRADES 9 - 12
TOPICS IN SCIENCE
WRITING SKILLS USING
BI-MONTHLY ARTICLES
1 B
Exceeds
Expectations
The student understands not only the objective but also the
implications of assignments. S/he writes in a variety of modes, with a
clear focus or thesis. Supporting details are well developed and
organized, showing both analysis and synthesis of ideas. Word
choice and syntax are accurate and appropriate. The student shows
mastery in the conventions of Standard English. The student
successfully completes all parts of the writing process.
Meets
Expectations
The student understands the objective of assignments and selects an
appropriate mode of written expression with a focus or thesis.
Supporting details show an understanding of the subject matter and
an analysis of ideas. They are somewhat developed and
organized. Word choice and syntax are accurate and appropriate.
Errors in the
conventions of Standard English are few. The student completes most
parts of the writing process.
Meets Some
Expectations
The student requires some additional explanations and models in
order to understand the objective of assignments or to complete the
writing process. With direction, s/he selects an appropriate mode.
Writing has a somewhat limited focus or thesis, and supporting ideas
may be inaccurate, simplistic, and/or confused. The student many
require assistance to develop or organize his response. Word choice
and syntax are consistent with grade level. There are some errors in
the conventions of Standard English.
Does Not
Meet Expectations
The student misinterprets significant elements of writing
assignments, selecting an inappropriate mode or using it incorrectly.
The student requires many additional explanations, models,
graphic organizers, and/or strategies in order to complete parts of the
writing process. The writing has no clear focus or a very limited
thesis. Ideas and concepts are often unorganized or inaccurate.
Inaccurate or limited vocabulary, syntax errors, and errors in the
conventions of writing make the writing ineffective.
TOPICS IN SCIENCE
SCIENCE CURRICULUM 511 GRADES 9 - 12
Assignments and Topics for Critical Thinkers
Using the 6 characteristics of a critical thinker, choose one of the following issues. You may also
see me if you have a complex issue you would like to investigate. This assignment will be worth
two test grades. The due date is Wednesday December 18
th
.
1. Decide what you think about the issue -10 pts
2. List the evidence for your point of view 20 pts - you will have the opportunity to use
resources in the classroom and the library
3. Seek other views and additional evidence. You need to interview 4 other people. Please
include 2 adults and ask them why they have that view or opinion. We will come up with
some general questions for this section in class together. 20 pts.
4. Decide which view is most reasonable and write 3 paragraphs, an introduction, a main body
and a conclusion persuading your reader to accept your view. 50 pts
a. Introduction - minimum of 3 sentences
b. Main body - minimum of 10 sentences
c. Conclusion - minimum of 5 sentences
Topics
a. Do you favor· subjecting animals to painful experiments in order to find cures for disease?
b. Do you favor using stem cells for finding cures for disease?
c. Do you believe one can be a sincere Christian and believe in the theory of Evolution?
d. Do you favor a mandatory prison term for the first time DWl convictions?
e. Why is prescription drug consumption the highest in the United States?
f. Should insurance cover car accidents in the case of DWl?
g. Who should pay for care for an orphan who terminally ill?
h. Should the life an Alzheimer's patient be prolonged?
i. Is recycling practical?
j. Is Health Care reform needed?
TOPICS IN SCIENCE
SCIENCE CURRICULUM 512 GRADES 9 - 12
TOPICS IN SCIENCE
APPLYING CRITICAL THINKING SKILLS
IN SCIENTIFIC CASE STUDIES
3
Exceeds
Expectations
The student independently collects, interprets and analyzes data related
to a complex issue using all of the characteristics of a critical thinker.
Meets
Expectations
The student independently collects, interprets and analyzes data related
to a complex issue using some of the characteristics of a critical thinker.
Meets Some
Expectations
The student may need assistance collecting, interpreting and analyzing
data related to a complex issue using some of the characteristics of a
critical thinker.
Does Not Meet
Expectations
The student requires assistance collecting, interpreting and analyzing
data related to a complex issue using some or few of the characteristics
of a critical thinker.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 513 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Introduction to Forensic Science
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Introduction to Forensic Science
3. Transcript Course Code/Number
00367
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of Biology - Honors or Biological Systems, or a B or better in General Biology
11. Brief Course Description
This course is designed for students interested in understanding what forensic science is and how it
incorporates other fields of science, such as biology, chemistry and physics. It is an introductory course for
students who have interest in pursuing a career in forensic science. A broad range of topics including
observation techniques, DNA, hair, fiber and blood analysis and fingerprinting will be explored using laboratory
techniques and activities. These topics will be applied and discussed in regards to forensic science and how
these techniques are used to solve crimes.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocol.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 514 GRADES 9 - 12
13. Course Outline
Unit
Activities
# of Weeks
Introduction to Forensic Science
Anthropometry Activity
Specialists in Forensics web quest
Backpack Mystery
1
The Crime Scene
Logic Puzzles
Deadly Picnic – Lab in Deductive
Reasoning
Clue game
Crime Scene sketch
2
Types of Evidence
Physical Evidence Flipcharts
Searching for Trace Evidence
2
Hair and Fiber Evidence
Animal hair lab
Human hair lab
Fiber analysis lab
1.5
Fingerprint Evidence
Inked fingerprint lab
Latent fingerprint lab
1
Drug Evidence
Narcotics lab
Napoleon’s Death Activity
1
Blood Evidence
Blood Typing internet activity
Blood Typing lab
Blood Spatter lab
1
DNA Evidence
Paper Helix
DNA extraction lab
Case of the Crown Jewels – DNA
fingerprinting
1
Criminal Case Project
Group research project on real life
criminal case
1.5
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison curriculum and Connecticut State standards and grade level
expectations for Science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a common scoring rubric or grading criteria.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 515 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and procedures
to calculate, analyze and present scientific data
and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the design
of the experiment?
How do you design and conduct appropriate types
of controlled scientific investigations, using the
appropriate tools and techniques, to make
observations and gather data to answer various
questions?
How do you assess the data, using mathematical
operations to analyze and interpret data, and
present relationships between variables in
appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of scientific
claims in different sources of information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic that
are based on the results generated during the
experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to the
problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is appropriate
for the design of the experiment.
Record data in the appropriate units of measure,
and be able to convert between different units of
measure.
Use mathematical operations to analyze and
interpret data, and present relationships between
variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on the
analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Laboratory materials
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research with selected websites
SUGGESTED ASSESSMENT METHODS
Laboratory analysis questions
Laboratory conclusions
Teacher observations
Research based projects
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 516 GRADES 9 - 12
LEARNING STRAND
Introduction to Forensic Science
Content Standard
Scientific Inquiry: Scientific inquiry requires the sharing of findings and ideas for critical review by colleagues and other scientists.
ENDURING UNDERSTANDINGS
Science is the method of observation and
investigation used to understand our world.
Scientists today apply theories and techniques
developed by past scientists to solve crimes.
Scientists work together and share findings in
order to effectively draw conclusions and solve
real world problems.
Forensic science is the application of science to
criminal and civil law.
Forensic science involves the collaboration of
many scientific specialists, both past and present.
ESSENTIAL QUESTIONS
What is Forensic Science?
How have the developments and research of past
scientists contributed to the development of the
field of forensic science as we know it today?
How are crimes solved?
Who is involved in solving crimes and what roles
do they play?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain how research and discoveries of past
scientists have contributed to the field of Forensic
Science.
Describe the major federal and local crime
laboratories and their functions.
Describe the roles of some of the specialists in
forensic science (i.e., entomologists, serologists,
odontologists, etc.).
List steps in pursuing justice.
Explain federal rules of evidence.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Anthropometry activity (requires Bertillon card,
meter stick, and string)
Computers with internet access
SUGGESTED INSTRUCTIONAL STRATEGIES
PowerPoint presentations
Lecture
Hands-on lab activity
Internet research with selected websites
Group work
SUGGESTED ASSESSMENT METHODS
Lab questions and discussions
Teacher observations
Student participation
Tests and quizzes
Expected Performance:
Articulate conclusions and explanations based on
research data and assess results based on the design
of the investigation.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 517 GRADES 9 - 12
LEARNING STRAND
The Crime Scene
Content Standard:
Scientific Inquiry: Scientific inquiry is a thoughtful and coordinated attempt to search out, describe, explain, and predict natural
phenomena. Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and interpretation.
ENDURING UNDERSTANDINGS
Scientific problems, including crimes, must be
solved by deductive reasoning: analyzing and
synthesizing all observations and data in order to
come to a conclusion.
Crime scenes are extremely fragile; once
disrupted or tampered with, they can never be
regained.
Observation skills are critical when investigating
crime scenes; the notes and photos taken and
the sketches drawn are used to help forensic
investigators reconstruct the crime and determine
the course of events.
ESSENTIAL QUESTIONS
What is deductive reasoning and how it is used to
solve crimes?
Why is it important for investigators to follow a
protocol when processing a crime scene?
Why is it important for crime scene investigators to
thoroughly document the crime scene? How do
they do this?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Apply deductive reasoning skills to fictitious
―Who-dun-it?‖ cases.
List and explain the appropriate steps in
processing a crime scene.
Describe the proper methods used to
document a crime scene.
Apply the documentation methods of note-
taking and sketching to a simulated crime
scene.
Explain how the chain of custody of
evidence is preserved.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
―The Deadly Picnic‖ – lab on deductive reasoning
Logic Puzzles
Clue board game
Crime Scene Information packet with questions
―The Case of the Cyanide Cocktail‖ – simulated
crime scene to set up in the classroom
Tape measures, rulers, colored pencils, graph
paper (to sketch Cyanide Cocktail crime scene)
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture and group discussion
Homework (directed reading and questions)
Group Work
Games
Performance Tasks
Crime Scene Investigation – documenting the
crime scene using notes and sketches
SUGGESTED ASSESSMENT METHODS
Teacher observations (of work on logic puzzles,
success in playing Clue, etc.)
―Cyanide Cocktail‖ Activity – crime scene sketch,
notes, and analysis questions
Tests and quizzes
Expected Performances
Identify questions that can be answered through
scientific investigation.
Design and conduct appropriate types of scientific
investigations to answer different questions.
Use appropriate tools and techniques to make
observations and gather data.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 518 GRADES 9 - 12
LEARNING STRAND
Types of Evidence
Content Standard
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation.
ENDURING UNDERSTANDINGS
There is always evidence left at a crime scene.
Matter, including forensic evidence, can be
described, organized, classified, and analyzed to
identify individual suspects.
A variety of different types of evidence are
collected from crime scenes; some types have
greater bearing on the case than others.
It is the combination of evidence collected that
ultimately makes or breaks a criminal case.
When collecting and preserving evidence, proper
scientific and legal precautions must be taken to
insure the integrity of evidence is maintained.
ESSENTIAL QUESTIONS
What is Edmond Locard’s Exchange Principle and
how is it applied to a crime scene?
What is the value of different types of evidence
found at a crime scene?
Why is it important for proper procedures to be
followed when collecting and preserving
evidence?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
List and describe the different types of evidence
(physical, direct, circumstantial, etc.).
Compare and contrast the value of each type of
evidence.
Distinguish between individual and class evidence
and list examples of each.
Describe the different types of physical evidence
that can be collected at a crime scene.
Practice using different search patterns to look
for trace evidence and determine the pros and
cons of each method.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Individual vs. Class evidence worksheet
Trace Evidence information packet with questions
Internet research with selected websites
Construction paper, markers, colored pencils,
scissors, glue (for physical evidence flipcharts)
Large open area, such as a football field, to plant
trace evidence
SUGGESTED INSTRUCTIONAL STRATEGIES
Forensic Science for High School Kendall Hunt, 2005
Lecture with class discussion
Directed Reading
Performance Tasks
Research project (Create a flipchart describing 10
types of physical evidence their methods of
collection)
Trace evidence collection using search patterns
SUGGESTED ASSESSMENT METHODS
Homework (reading and questions)
Teacher observations (searching for evidence)
Student class participation
Flipchart project graded with rubric
Tests and quizzes
Expected Performance
Use appropriate tools and techniques to make
observations and gather data.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 519 GRADES 9 - 12
LEARNING STRAND
Hair and Fiber Evidence
Content Standards
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation.
Content Standard: 9.5 – Due to its unique chemical structure, carbon forms many organic and inorganic compounds. (building block
for hair and fibers)
Enrichment Content Standards for High School Chemistry: The bonding characteristics allow the formation for many different
organic molecules of varied sizes, shapes, and chemical properties, and provide the biochemical basis of life. (allow for differences
in hair and fibers structure)
ENDURING UNDERSTANDINGS
Matter, including forensic evidence such as hair
and fibers, can be described, organized,
classified, and analyzed and can be used to
identify suspects.
Evidence can be analyzed for its chemical
components to uncover characteristics that are
not always directly observable and thus can give
insight to a crime.
ESSENTIAL QUESTIONS
What is the value of hair and fibers as trace
evidence?
What information can be gained by studying hair
and fiber evidence?
How are hair and fibers analyzed in a crime lab?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify, label, and distinguish between the main
parts of a hair.
Analyze hair samples to distinguish between
human hairs and hairs of other animal species.
Determine the identity of an unknown hair.
Describe the tools and techniques used to
analyze fibers in a crime lab.
Distinguish between natural and synthetic fibers
and give examples of each.
Analyze known fiber samples.
Determine the identity of an unknown fiber.
Explain the value of hairs and fibers as trace
evidence.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Prepared hair sample slides
Prepared fiber sample slides
Microscopes
Slides and coverslips
Hair information packet with questions
Fiber evidence case studies
SUGGESTED INSTRUCTIONAL STRATEGIES
Microscope Labs *– comparing human hair to
animal hair, comparing various human hair
samples (different individuals, different parts of
the body, etc.), comparing natural and synthetic
fiber samples
Lecture with class discussion
Directed Reading
SUGGESTED ASSESSMENT METHODS
Benchmark *
Meets expectations for gathering information and
materials, thinking critically, and solving problems.
Hair Lab and Fiber Lab drawings and analysis
questions
Homework (reading and questions)
Tests and quizzes
Expected Performance
Use appropriate tools and techniques to make
observations and gather data.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 520 GRADES 9 - 12
LEARNING STRAND
Fingerprints
Content Standard
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation
.
ENDURING UNDERSTANDINGS
Biological evidence, such as fingerprints, contains
discrete pieces of information that makes every
organism unique.
Science ideas evolve as new information is
uncovered.
Matter, including forensic evidence such as
fingerprints, can be described, organized,
classified, analyzed and used to determine the
identity of a suspect.
ESSENTIAL QUESTIONS
Why are fingerprints such a valuable piece of
evidence?
How is fingerprint evidence collected and analyzed
in order to determine the identity of a suspect?
How have computers made personal identification
easier?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Define the three basic properties that allow
individual identification by fingerprints.
Obtain an inked, readable fingerprint for each
finger.
Explain the value of fingerprints as evidence.
Distinguish between the three main classes of
fingerprints.
Examine fingerprints and classify them
appropriately.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Fingerprint ink
Charcoal powder
Fiberglass brushes
Clear adhesive tape
Index cards
Illustrations of the 3 types of fingerprints
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture with class discussion
Group work
Lab Activities – inked fingerprints and latent
fingerprints
SUGGESTED ASSESSMENT METHODS
Teacher observations
Lab analysis questions
Quizzes
Expected Performance
Assess the reliability of the data that was generated in
the investigation.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 521 GRADES 9 - 12
LEARNING STRAND
Drug Evidence
Content Standard
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation.
ENDURING UNDERSTANDINGS
Matter, including forensic evidence such as drugs,
can be described, organized, classified, and
analyzed.
Evidence can be analyzed for its chemical
components to uncover characteristics that are
not always directly observable and thus can give
insight to a crime.
ESSENTIAL QUESTIONS
How is drug evidence analyzed by the crime lab?
How are drugs classified?
What are the proper procedures for collecting and
preserving drug evidence?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish between the 6 major classes of drugs
and give examples of each.
Explain the basis for the Controlled Substances
Act’s 5 schedules of drugs.
Describe the various screening and confirmation
tests performed on drug evidence.
Explain how drugs should be properly collected
and preserved.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Narcotics Lab (from Wards)
Text
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture with class discussion
Lab Activity – testing for the presence of cocaine
SUGGESTED ASSESSMENT METHODS
Narcotics Lab analysis questions
Quizzes
Expected Performance
Assess the reliability of the data that was generated in
the investigation.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 522 GRADES 9 - 12
LEARNING STRAND
Blood Evidence
Content Standards
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation. Scientific inquiry is a thoughtful and coordinated attempt to search out, describe, explain, and predict natural
phenomena.
Enrichment Content Standards for High School Biology: Physiology – The complementary activity of major body systems provides
cells with oxygen and nutrients and removes toxic waste products such as carbon dioxide. (structure and function of blood)
Enrichment Content Standards for High School Physics: Motion and Forces – Newton’s laws predict the motion of most objects .
(blood spatter)
ENDURING UNDERSTANDINGS
Science ideas evolve as new information is
uncovered.
Matter, including forensic evidence such as blood,
can be described, organized, classified, and
analyzed and can be used to determine the
identity of a suspect.
Results from controlled experiments and research
can be used to make predictions when applied to
new circumstances.
ESSENTIAL QUESTIONS
What is the role of blood in the human body?
What is the value of blood as evidence?
How is blood type determined?
What information can be obtained by analyzing
blood spatter patterns?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish between the components of blood
and their functions.
Identify the characteristics of different blood
types.
Determine the blood type of an unknown sample.
Compare blood spatter patterns from various
circumstances.
Explore bloodstain patterns as a function of
velocity, direction and height of fall.
Describe how blood evidence is collected,
identified, and analyzed.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Simulated Blood Typing Kit (Wards)
Simulated Blood Spatter Kit (Wards)
Internet research with selected websites
Website – nobelprize.com (blood typing game)
Blood information packets and questions
SUGGESTED INSTRUCTIONAL STRATEGIES
Lecture and class discussion
Lab Activities – blood typing and blood spatter labs
Internet activity – blood typing game
Directed Reading
SUGGESTED ASSESSMENT METHODS
Lab Analysis Questions
Teacher observations - Internet activity
Homework (reading and questions)
Quizzes
Test
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 523 GRADES 9 - 12
LEARNING STRAND
DNA Evidence
Content Standards
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation. Scientific inquiry is a thoughtful and coordinated attempt to search out, describe, explain, and predict natural
phenomena.
Enrichment Standards for High School Biology: Genetics – Genes are a set of instructions encoded in the DNA sequence of each
organism that specify the sequence of amino acids in proteins characteristic of that organism. ( DNA technology)
ENDURING UNDERSTANDINGS
The DNA sequence of every organism is unique.
DNA technology allows scientists to manipulate
and analyze DNA in order to make connections
between crime scenes and suspects.
ESSENTIAL QUESTIONS
What is the value of DNA as evidence?
How is DNA extracted and characterized?
How can DNA be analyzed to make connections
between crime scenes and suspects?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the structure of DNA.
Describe what makes each person’s DNA unique.
List and describe the steps of making a DNA
fingerprint.
Analyze a DNA fingerprint to determine the
identity of a criminal.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Paper Helix Activity – templates, construction
paper, scissors, tape
―The Case of the Crown Jewels‖ Activity
DNA Extraction Lab materials (salt water, dish
detergent, ethanol, test tubes)
SUGGESTED INSTRUCTIONAL STRATEGIES
Demonstrations
Lecture with class discussion
Lab activity – DNA extraction
Create a paper model of a DNA fingerprint
SUGGESTED ASSESSMENT METHODS
Lab Analysis Questions
Teacher observations
Poster of DNA fingerprint
Test
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 524 GRADES 9 - 12
LEARNING STRAND
Criminal Case Research Project
Content Standards
Scientific Inquiry: Scientific inquiry progresses through a continuous process of questioning, data collection, analysis, and
interpretation. Scientific inquiry is a thoughtful and coordinated attempt to search out, describe, explain, and predict natural
phenomena. Scientific inquiry requires the sharing of findings and ideas for critical review by colleagues and other scientists.
ENDURING UNDERSTANDINGS
Criminal cases are solved by the coordinated
effort of many specialists, scientists, and
departments.
Scientists work together and share findings in
order to effectively draw conclusions and solve
real world problems.
Scientific problems, including crimes, must be
solved by deductive reasoning: analyzing and
synthesizing all observations and data in order to
come to a conclusion.
There will always be evidence left behind at a
crime scene.
ESSENTIAL QUESTIONS
How are real world crimes solved?
What steps are taken in order to effectively
investigate, process, and bring a criminal case to
trial?
What are some of the limitations of modern
forensic science?
What are some of the challenges faced by forensic
scientists and law enforcement officials?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Research a real life criminal case.
Explain the process of investigating a criminal
case, including the crime committed, the
collection and analysis of evidence, and the
outcome of the case.
Explain the importance of evidence in the
outcome of a criminal case.
Describe the limitations of modern forensic
science when investigating a crime.
Describe challenges faced by forensic scientists.
Forensic Science for High School Kendall Hunt, 2006
Criminalistics: An Introduction to Forensic Science,
Pearson Prentice Hall, 2004
Internet research with selected websites
PowerPoint program
SUGGESTED INSTRUCTIONAL STRATEGIES
Research project *
Student generated PowerPoint presentations
SUGGESTED ASSESSMENT METHODS
Benchmark*
- Student meets expectations for using
technology effectively and responsibly
Research project graded with rubric
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 525 GRADES 9 - 12
Name: _______________________________________ Date: ___________________________________
"Real Life" Criminal Case Project
The Assignment:
In groups of
2
or
3
you are to research any real life criminal case of your choice.
You will create a PowerPoint presentation to teach the class about your criminal case. The
PowerPoint presentation must include the following:
A minimum of 10 slides.
A unique slide design
Animation
Title
Pictures illustrating the case
Background information
What should we know about the suspects/victims/time period, etc. to help us understand
this case?
The crime committed
What happened?
The crime scene
Where?
What did it look like?
What evidence was collected?
The investigation
How long did it last?
What scientific tests were performed?
Were any mistakes made?
The suspects
Who?
Why were they suspects?
The trial (if there was one) and the outcome/verdict of the case
Any interesting facts about the case
Sources you used cited in MLA format
This assignment will count as two test grades. You will be graded on the work you do each day
in class and on the quality of the final presentation. This includes both the PowerPoint slides
and the oral presentation. It is expected that each member of the group will participate in the
presentation. The class will also evaluate your presentation.
Please see the attached rubric for the breakdown of grading.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 526 GRADES 9 - 12
Name: _______________________________________ Date: ___________________________________
"Real Life" Criminal Case Project
Grading Rubric
Criteria
Points
Possible
Points
Earned
Teacher Comments
PowerPoint:
Copy of PowerPoint is turned in
5
Minimum of 10 slides
5
Animation
3
Unique Slide Design
3
No misspellings or grammatical errors
5
Sources are cited in MLA format
5
Criminal Case
Title
2
Background Information
5
Explanation of the crime committed
5
Description of the crime scene
10
Explanation of the investigation
10
Description of the suspects
5
Description of the trial and outcome/verdict
5
Pictures enhance the text
5
Interesting facts
5
Oral Presentation
Speaks clearly and loudly
3
Well organized and understandable
3
All members share equally in the
presentation
2
Makes eye contact
2
Able to answer questions correctly
2
Class Evaluation
10
Total
100
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 527 GRADES 9 - 12
Forensics and Biotechnology
Criminal Case Research Project
2. Uses technology effectively and responsibly
Exceeds Expectations
The student can independently select appropriate internet sources to
research an actual crime. S/he selects websites that are currently
updated and authored.
Meets Expectations
The student needs minimal assistance in selecting appropriate
internet sources to research an actual crime. S/he selects websites
that may be outdated or un-authored.
Meets Some Expectations
The student requires some assistance in selecting appropriate
internet sources to research an actual crime. S/he selects websites
that are outdated and/or un-authored.
Does Not Meet Expectations
The student cannot select appropriate internet sources to research an
actual crime. S/he selects websites that are outdated and un-
authored.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 528 GRADES 9 - 12
Name: _______________________________________ Date: ___________________________________
What's Hair?
OBJECTIVE
You will identify different types of hair and determine the species of selection of
unknown hairs.
BACKGROUND INFORMATION
As a trace evidence forensic scientist, you are often asked to identify hairs found at crime
scenes. Sometimes you need to know whether or not a hair is human and if not, what type of
animal it is from. You also may need to know if a hair can differentiate two different animals of
the same species. In this lab, you will create a set of reference hairs to help answer these
questions.
MATERIALS
Cat hair Deer hair Mouse hair
Horse hair Wooly Mammoth hair Dog hair
Rat hair Human hair (3 types) Hair (5 type sample)
Unknown hairs #1, 2, 3
Compound light microscope
PROCEDURE
1. Move from microscope station to station examining each hair sample under low, medium, and
high power. Draw what you see under high power in the circles provided. Be sure to notice and
label the:
• medulla
• cuticle
• cortex
2. For two of the hair of your choice determine the medullary ratio of them by measuring the
diameter of the medulla and the diameter of the hair (this can be a rough estimate).
• Express these two numbers as a fraction - called the medullary index.
• Record this number in the data table provided.
3. Repeat step #1 for the unknown hair stations (#1, 2, and 3). Compare the unknown hair
sample to your controls to determine what types of hair they are.
• Record your guesses in the data table provided.
4. Move to the station with the slide containing the 5 types of hair. Try to determine which types
of hair they are also.
• Record your guesses in the space provided.
5. Answer the analysis questions.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 529 GRADES 9 - 12
Medullary Index
Hair Type
Medullary Index
Identity of Unknown Hair Samples
Unknown Sample
Identity
Five Types of Hair Guesses
_______________________________________
_______________________________________
_______________________________________
_______________________________________
_______________________________________
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 530 GRADES 9 - 12
Analysis Questions
1. What type of hair was each of the unknowns? What specific characteristics helped
you decide which type it was?
2. How can you tell human hair from animal hair?
3. How can investigators use hair evidence to help solve a crime? How is Locard's
Exchange Principle applied to hair evidence?
4. What are the main parts to the morphology of the hair and explain each part?
5. Explain the different types of medulla that can be used to identify hair.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 531 GRADES 9 - 12
Forensics and Biotechnology
Laboratory Analysis - Evidence Collection
3. Applies effective and efficient strategies for gathering information and materials, thinking
critically, and solving problems
Exceeds Expectations
27-30
The student independently collects, analyzes,
interprets, and evaluates evidence and data
collected from a simulated crime scene. S/he uses
that information to make original predictions and
solve problems accurately and efficiently. S/he
presents her/his findings in a coherent manner.
Meets Expectations
24/30 – 26/30
The student needs minimal assistance in collecting,
analyzing, interpreting, and evaluating evidence
and data collected from a simulated crime scene.
S/he uses that information to make specific
predictions and solve problems with few errors.
S/he presents in a coherent manner.
Meets Some Expectations
21/30 – 23/30
The student needs some assistance in collecting
evidence and interpreting data from a simulated
crime scene. S/he may need some assistance in
using that information to make general predictions
and solve problems. S/he solves problems with
some errors and presents her/his findings in a
coherent manner.
Does Not Meet Expectations
<20/30
The student needs significant assistance in
gathering evidence and interpreting information
and data from a simulated crime scene. S/he needs
assistance in making predictions and solving
problems. S/he solves problems inefficiently with
significant errors and fails to present her/his
findings in a coherent manner.
INTRODUCTION TO FORENSIC SCIENCE
SCIENCE CURRICULUM 532 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 533 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Anatomy and Physiology
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Anatomy & Phys.
3. Transcript Course Code/Number
00372
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ___________________(date)
10. Pre-Requisites
Successful completion of Biological Systems, Biology - Honors, Chemistry, or Human Biology
11. Brief Course Description
This course presents the student with an explanation of the structure of the human body and its
processes. The skeletal, muscular, nervous, circulatory, respiratory and digestive systems are studied.
Where possible, the subject matter is elucidated by laboratory investigation. This course is especially
beneficial for a student considering a medical career.
12. Course Goals
1. To help students understand the structure and function of a healthy human body.
2. To acquaint students with current research regarding application of medical science to the process of
treating and curing disease.
3. To apply effective and efficient strategies for gathering information and materials, thinking critically
and solving problems.
4. To conduct lab experiments and dissections safely.
5. To use technology effectively and efficiently.
6. To demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
7. To demonstrate the ability to complete assignments independently.
8. To demonstrate respect for one's self, and strive to contribute to the success of others.
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 534 GRADES 9 - 12
13. Course Outline
Chapter / Unit
One: The Human Body
An Orientation
Three: Cells and Tissues
Topics
Relationship between anatomy and physiology, Levels of Structural
Organization (cellorganism), Life Functions, Homeostasis,
Anatomical Vocabulary
Review of cell structure and membrane transport
Anatomy, cell physiology and four main tissue types
Seven: The Nervous System
Nervous system organization
Nervous tissue anatomy and physiology (focus on nerve impulse
conduction and reflex arc)
Types of sensory receptors in skin
Brain Dysfunctions
Structure and function of CNS and PNS (and protection)
Autonomic Nervous System
Eight: Special Senses
Major structure and function of eye in vision
Major structure and function of ear in hearing and balance
Major structure, function and location of smell and taste senses
Ten: Blood
Eleven: The Circulatory System
Composition of blood
Blood typing and transfusion reactions
Hemopoiesis
Anatomy and physiology of the heart and vessels
Pathways of blood through the heart
Cardiovascular disease and treatments
Five: The Skeletal System
Structure and function of bones and skeletal system
Classification of bones (long, short, flat, irregular)
The major anatomical areas of a long bone
Bone formation and repair
Types of fractures
Bones of axial and appendicular skeleton
Six: The Muscular System
Similarities, differences, location of three types of muscle tissue
The structure of skeletal muscle
The events of muscle cell contraction.
The different types of body movement.
Criteria used in naming muscles.
Student text:
Essentials of Human Anatomy and Physiology
by Elaine Marieb Benjamin/Cummings Publishing Co 1994
In Science office:
Anatomy and Physiology Coloring Workbook: A Complete Study Guide (Text ancillary material)
In Room 219:
Essentials of Human Anatomy and Physiology
Lab Manual, Media Manager, Test Bank (Teacher materials) 2009
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 535 GRADES 9 - 12
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/FinalSite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a common scoring rubric or grading criteria.
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 536 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
Content Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and
coordinated attempt, through a continuous
process of questioning, data collection,
analysis and interpretation, to describe,
explain, and predict natural phenomena.
Scientific inquiry requires the sharing of
findings and ideas for critical review by
colleagues and other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to
search for and assess the relevance and
credibility of scientific information found in
various print and electronic media.
Scientific numeracy includes the ability to
use universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the design
of the experiment?
How do you design and conduct appropriate types
of controlled scientific investigations, using the
appropriate tools and techniques, to make
observations and gather data to answer various
questions?
How do you assess the data, using mathematical
operations to analyze and interpret data, and
present relationships between variables in
appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of scientific
claims in different sources of information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic that
are based on the results generated during the
experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected
to the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in
recording experimental data.
Develop logical conclusions that are based
on the analysis of experimental data.
Formulate reports, using relevant
vocabulary, supporting evidence, and logic
that accurately communicate the results of a
scientific experiment.
Essentials of Human Anatomy and Physiology
Pearson/Benjamin Cummings Publishing Co., 2009
Reflex lab
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research of selected websites
Extension of reflex/reaction time lab (Students
design an experiment to test another variable that
affects reaction time.)
SUGGESTED ASSESSMENT METHODS
Benchmark:
Project for application and integration of learning:
Have students present a current treatment option
for one of the disorders presented in class
(nervous system disorder, circulatory system,
skeletal or muscular disorder).
Statistics about the procedure (e.g., survival rates,
% of population afflicted, etc.)
Credible sources used and cited correctly
Background information on the testing done to
approve the procedure (emphasize control and
experimental groups; use appropriate vocabulary)
Conclusions about success of treatment using all
relevant, credible information
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 537 GRADES 9 - 12
Format could be PowerPoint presentation, poster,
and/or lecture
Other Assessments:
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
Lab report/questions on Reaction Time lab
extension
Lab on variables affecting heart rate

ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 538 GRADES 9 - 12
LEARNING STRAND
Introduction to Anatomy and Physiology
Enrichment Content Standards for High School Science: Physiology -- As a result of the coordinated structures and functions of
organ systems, the internal environment of the human body remains relatively stable (homeostatic) despite changes in the outside
environment.
ENDURING UNDERSTANDINGS
Medical terminology is essential when describing
anatomic structure and physiology.
These terms are universally known.
classified for understanding.
ESSENTIAL QUESTIONS
What is the relationship between anatomy and
physiology?
What are the levels of organization in an
organism?
Why is homeostasis important?
How is anatomical vocabulary used?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Recognize the organization levels- from
cells to organism.
Relate homeostatic feedback mechanisms
to proper body function
Apply terminology to describe body planes
anatomical positions, etc.
Identify the body membranes and cavities.
Explain the major structures of and functions for
each of the major body systems:
· Integumentary System
· Skeletal System
· Muscular System
· Nervous System
· Endocrine System
· Circulatory System
· Lymphatic System
· Digestive System
· Respiratory System
· Urinary System
· Reproductive System
Essentials of Human Anatomy and Physiology
Pearson/Benjamin Cummings Publishing Co., 2009
Anatomy & Physiology Coloring Workbook (for
copying diagrams)
Poster paper and markers
Internet / website activities
SUGGESTED INSTRUCTIONAL STRATEGIES
Group work
Lecture
Poster summaries of 11 body systems
Question, answer, and discussion
Diagram labeling
Internet modules
http://www.wisc-
online.com/objects/index_tj.asp?objID=AP15505
The Organization of the Human Body: Body Cavities
http://www.wisc-
online.com/objects/index_tj.asp?objID=AP15305
Anatomical Terminology: Relative Position
SUGGESTED ASSESSMENT METHODS
Homework (readings, questions, and
diagramming)
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
Class work (posters, internet module)
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 539 GRADES 9 - 12
LEARNING STRAND
Cell Structure and Function
Characteristics of Tissues
Content Standard
Enrichment Content Standards for High School Science: Cell Biology -- The fundamental life processes of plants and animals depend
on a variety of chemical reactions that occur in specialized areas of the organism’s cells.
ENDURING UNDERSTANDINGS
Living things can be described, organized and
classified for understanding.
Imbalances in the cell relate to imbalances in
living things.
Cell is the basic structural and functional unit of
all living things.
Different types of cells work together to form
tissues that carry out specific functions.
ESSENTIAL QUESTIONS
What is the lowest level of organization in an
organism?
What happens when homeostasis is not
maintained?
What are the characteristics and functions of
epithelial, connective, muscle and nervous
tissues?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify the structure and function of the
cell.
Describe various membrane transport
mechanisms (endocytosis, exocytosis, etc.).
Describe the major structure and function of the
four major tissue types.
Demonstrate where each major tissue type
occurs within the human body.
Explain how glands are classified.
Illustrate and label the major tissue types
using the microscope.
Essentials of Human Anatomy and Physiology
Pearson/Benjamin Cummings Publishing Co., 2009
Student class participation
Microscopic slides of representative tissue types
Osmosis lab materials
SUGGESTED INSTRUCTIONAL STRATEGIES
Diffusion/Osmosis lab
Question, answer, and discussion
Lecture
Illustrations
Laboratory use of microscope to identify tissue
types
Direct instruction
Web sites (histology)
Label diagrams
Outline text (create study guide)
SUGGESTED ASSESSMENT METHODS
Laboratory observation and documentation
Homework (readings, questions, & problems)
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
Laboratory drawing assessments
Microscopic identification assessment
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 540 GRADES 9 - 12
LEARNING STRAND
Nervous System
Content Standard
Enrichment Content Standards for High School Science: Physiology
As a result of the coordinated structures and functions of organ systems, the internal environment of the human body remains
relatively stable (homeostatic) despite changes in the outside environment.
The nervous system mediates communication between different parts of the body and the body’s interactions with the
environment.
Feedback loops in the nervous system regulate conditions in the body.
The neurons transmit electrochemical impulses.
Sensory neurons, interneurons and motor neurons all have a role in sensation, thought an
d response.
ENDURING UNDERSTANDINGS
Human activities impact and alter cellular
environments.
The nervous system senses, processes and
responds to the environment.
Cells are a complex assemblage of interacting
and changing chemical, physical and biological
processes.
ESSENTIAL QUESTIONS
How are cellular environments impacted and
altered by human activities?
How do chemical, physical and biological
interactions take place at the cellular level?
What is the role of the nervous system?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Compare and contrast neurons to different
glial cells in terms of structure and function.
Describe the general structure of a neuron
relating to the events that lead to the
conduction of the nerve impulse.
Discuss the role of neurotransmitters in neuron
function.
Differentiate between the Central and
Peripheral Nervous System.
Identify the parts and function of the
spinal cord and brain. (reflex arc)
Describe the structure and function of the
ear and eye.
Describe the location, structure and function of
the olfactory and taste receptors.
Essentials of Human Anatomy and Physiology
Benjamin/Cummings Publishing Co., 1991, 1994
Meter stick for reflex lab
Cutaneous sensations kit
Visual Perceptions kit
DVDs:
The Human Body: Sensation
(Mezick)
The Secret life of the Brain: The Teenage Brain
(Ferron)
Optical illusions- online and document
Ear model
Eye model
SUGGESTED INSTRUCTIONAL STRATEGIES
Reflex lab
Diagrams of neurons
Nervous system PowerPoint presentations
Outline notes for PowerPoint presentations
Question, answer, and discussion
Lecture
Concept map on nervous system organization
SUGGESTED ASSESSMENT METHODS
Lab activity for reflex lab
Homework questions
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 541 GRADES 9 - 12
LEARNING STRAND
Blood and The Circulatory System
Content Standard
Enrichment Content Standards for High School Science: Physiology
As a result of the coordinated structures and functions of organ systems, the internal environment of the human body remains
relatively stable (homeostatic) despite changes in the outside environment.
ENDURING UNDERSTANDINGS
Blood supplies all cells in the body with
nutrients and removes waste.
The heart is the pump that cycles the blood
through the body (systemic) and to the lungs
(pulmonary circulation).
Imbalances in the blood relate to imbalances in
the human body.
ESSENTIAL QUESTIONS
Why is blood essential to the body?
How is blood circulated throughout the body?
What happens when homeostasis is not
maintained?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the composition and volume of whole
blood.
Describe the composition of plasma and discuss
its importance in the body.
List blood cell types compromising the formed
elements and describe the major functions of
each type.
Define anemia, polycythemia, leukopenia, and
leukocytosis, and list possible causes for each
condition.
Describe the ABO and Rh blood groups.
Explain the basis for a transfusion reaction.
Describe the location of the heart in the body
and identify its major anatomical areas on an
appropriate model or diagram.
Trace the pathway of blood through the heart.
Explain the operation of the heart valves.
Define heart sounds and murmur.
Define and explain what causes atherosclerosis
and list treatments.
Essentials of Human Anatomy and Physiology
Pearson/Benjamin Cummings Publishing Co., 2009
ABO blood test kit
Candied Blood materials (red hots, sugar etc)
Poster paper for ―cardiac 100‖
Stopwatches, heart rate monitors, blood
pressure cuffs
SUGGESTED INSTRUCTIONAL STRATEGIES
ABO blood testing
Candied blood activity
Group work labs: Cardiac 100 and Blood
Pressure/Pulse lab
SUGGESTED ASSESSMENT METHODS
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 542 GRADES 9 - 12
LEARNING STRAND
The Skeletal System
Content Standard
Enrichment Content Standards for High School Science: Physiology
As a result of the coordinated structures and functions of organ systems, the internal environment of the human body remains
relatively stable (homeostatic) despite changes in the outside environment.
ENDURING UNDERSTANDINGS
The skeletal system is instrumental in the
support, movement, and protection of the body.
Bones are dynamic organs that interact and
support other systems of the body.
ESSENTIAL QUESTIONS
What is the function of the skeletal system?
How can bones be described and what is their
relationship to other systems in the body?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Describe the general structure and function of
bone tissue.
Describe briefly the process of bone formation
in the fetus and summarize the events of bone
remodeling throughout life.
Name the four main kinds of bone. Identify the
major anatomical areas of a long bone.
Name and describe the various types of
fractures.
Differentiate between the axial and
appendicular skeleton.
Identify and describe the features of the frontal,
parietal, temporal, and occipital bones.
Identify the bones that comprise the skull,
vertebral column, thoracic cage, pectoral girdle,
upper limbs, pelvic girdle, and lower limbs.
Apply abnormal bone anatomy and
physiology to explain disease.
Essentials of Human Anatomy and Physiology
Pearson/Benjamin Cummings Publishing Co., 2009
Bone models
Skeleton
Bone diagrams
Fresh bone/joint (chicken wing)
SUGGESTED INSTRUCTIONAL STRATEGIES
Construct/illustrate compact bone
Label parts of bone models
Label diagrams of human skeleton
Dissect fresh bone and joints
Lecture
Question, answer, and discussion
At the Clinic Questions
SUGGESTED ASSESSMENT METHODS
Laboratory assessment and documentation of
chicken wing dissection
Assessment of dissection techniques
Laboratory drawing assessment
Homework (readings, questions)
Labeling of diagrams
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 543 GRADES 9 - 12
LEARNING STRAND
The Muscular System
Content Standard
Enrichment Content Standards for High School Science: Physiology
As a result of the coordinated structures and functions of organ systems, the internal environment of the human body remains
relatively stable (homeostatic) despite changes in the outside environment.
Actin, myosin, Ca2 and ATP have a role in the cellular and molecular basis of muscle contraction
.
ENDURING UNDERSTANDINGS
The muscular system plays a major role in
movement, support and homeostasis of the
human organism.
The state of an organism is maintained by the
dynamic interaction of the systems that
comprise it.
ESSENTIAL QUESTIONS
What is the function of the human muscular
system?
How does the muscular system help to maintain
homeostasis?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify the tissues and layers of skeletal
muscle.
Describe the microscopic structure of the three
types of muscle tissue.
Explain the major events of skeletal muscle
fiber contraction (Sliding Filament Theory).
Understand that major muscles are named for
their location, action, or shape.
Essentials of Human Anatomy and Physiology
Pearson Benjamin Cummings Publishing Co., 2009
Skeletal model
Chicken wing for dissection
SUGGESTED INSTRUCTIONAL STRATEGIES
Label muscle diagrams
Chicken wing dissection
Lecture
Question, answer, and discussion
Web sites (getbodysmart.com)
Video (National Geographic- LMC)
Class discussion/debate
Research
SUGGESTED ASSESSMENT METHODS
Laboratory reports with diagram assessment
Laboratory observations and documentation
Homework (readings, questions)
Unit Test
Quizzes
Investigations evaluated with rubrics
Response cards by TurningTechnologies

ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 544 GRADES 9 - 12
Name: _________________________________ Date: ___________________________
Anatomy and Physiology
Inquiry Lab: What factors affect reflexes and reaction times?
Your mission is to design an experiment, which will examine what things can affect reflexes and
reaction times using the skills you learned such as reflex testing, using the reflex hammer, the
pupil reflex, and testing someone's reaction time.
Did you ever wonder if your reaction time is faster or slower at different times of the day? What
other types of changes can affect reaction time? What could make a difference? Start by stating a
problem or making a statement to be investigated? For example “I believe that a full moon will
affect reaction time." (You cannot use that one.) Then decide what steps need to be performed to
test or investigate the problem. For example, you could test a person's reaction time during a full
moon, the day before a full moon, etc. When you are composing your procedure, you need to
figure out how to make sure you are only testing one variable at a time.
You will work with a partner(s) in constructing the experiment and gathering data, but every
person needs to write their own report.
The following guidelines are for your safety and success.
1. All lab safety rules must be followed.
2. The teacher must check your problem and experimental procedure and approve it before you
begin.
3. Every person has the right to refuse to be a test subject! Make sure you ask permission before
you test somebody for any type of test reflex or reaction time. For example, if you are going to
check a patellar reflex, you must ask the test subject for permission first, and also ask if they
have any problems with the area to be tested. (Who knows maybe they had a nasty crash on their
skateboard yesterday, you don't want to make it worse!!!)

ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 545 GRADES 9 - 12
Name: ______________________________________ Date: _________________
Lab Partners: _______________________________________________________
Inquiry Lab: What factors affect blood pressure and heart rate?
Lab Activity
Did you ever run so hard you could feel your pulse beating in your ears? Have you
ever gotten up too quickly from the couch and felt dizzy or light-headed? Have you ever felt
your heart rate quicken before a big test or competition?
Your assignment is to design an experiment to test the effect of one of the factors we
discussed in class on blood pressure and heart rate. In order to measure heart rate, you
will choose a pulse point on your body and count the number of heartbeats you feel in one
minute. In order to measure blood pressure, you will use sphygmomanometer,
stethoscope, and technique we practiced in class. Before you begin, formulate a
hypothesis to predict what effect your chosen factor will have on blood pressure and heart
rate.
Guidelines to follow:
Follow all safety rules.
Let me check your procedure and hypothesis before you begin your experiment.
Please inform me of any health concerns.
You will be working in groups of four. Two students should first test the effect of your
chosen factor on blood pressure while the other two students test the effect on heart
rate. Then switch tests. Each group member should have the opportunity to be the
subject and the tester for both blood pressure and heart rate.
Be sure to record all data (you should have data for 4 people, including yourself).
And remember, an accurate and reliable experiment is one that has been repeated at
least three times.
Materials
Stop watch
Sphygmomanometer
Stethoscope
Pencil
You will be writing a formal lab report for this lab. Your lab report should follow the
format given at the beginning of the year. Include the following analysis questions in
your lab report:
1. Would there be a difference in blood pressure and heart rate if you stood on top of a
table or sat down on the floor?
2. Compare the blood pressure and heart rate results. Are there any similarities or
differences in the changes?
3. Why is high blood pressure a health concern?
4. Research and explain why smoking causes a rise in blood pressure.

ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 546 GRADES 9 - 12
Inquiry lab: What affects reflexes and reaction times?
What factors affect blood pressure and heart rate?
Format for lab Write-up
Your lab report will have six sections.
I. Title: Title should be descriptive (describe the main idea of the experiment that was done).
This should be short (less than ten words). Include your name first then follow with name of your
lab partner.
II. Problem: State the reason the experiment was done. What was the experiment designed to
investigate?
III. Materials: List all the materials which were used in the experiment including the safety
equipment.
IV. Procedure: Summarize the experimental methods which were used to perform the experiment
or test.
V. Results: Display results of your experiment. Include tables that display the data you collected.
Also, create a graph with reaction time on the Y axis and the factors you wanted to examine on
the x-axis.
VI. Conclusions: State what you can conclude from the results. Deal directly with the data here.
Discuss how the data you collected explains, reinforces or does not reinforce the statement of the
problem which you made in part II.
***Benchmark Alert: Remember that one of the benchmark assignments for the course. The
school-wide goal "Applies effective and efficient strategies for gathering information and
materials, thinking critically, and solving problems." Will be met if you and your lab partner are
able to clearly document a procedure to test the problem you set out to investigate. ***
Exceeds Expectations
15/15-12/15 pts
The student will independently write a detailed, well organized,
controlled, reproducible procedure to solve the problem stated in the
lab. (For example, How do different activities affect the heart rate of
an individual? or What variables affect an individual’s reaction time?)
Meets Expectations
11/15-9/15 pts
With minimal assistance the student will write a fairly well organized
controlled reproducible procedure to solve the problem stated in the
lab.
Meets Some
Expectations
8/15-6/15 pts
With some assistance the student will write a procedure to solve the
problem stated in the lab. The procedure is not well organized, is
confusing to follow but it contains a control.
Does Not Meet
Expectations
5/15-0/15 pts
With a great deal of assistance the student writes a procedure to
solve the problem stated in the lab. The procedure is not well
organized, is confusing to follow and does not contain a control.
ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 547 GRADES 9 - 12
Reflexes and Reaction Time / Heart Rate Blood Pressure Inquiry Lab
Grading Rubric (65 points total)
Possible Points
Your response for maximum points
10 points
Lab report completed on time and neat.
Late labs lose ten points per day.
Lab due:_________________________
5 points
Appropriate title and headings (parts I-VI)
(all underlined)
Include name and date.
10 points
Clearly stated problem. What was the lab
designed to test or measure? Does it
consider both reflexes and reaction time?
15 points
Clearly written or outlined procedure and
list of materials. Are Both reflexes and
reaction time tested? Or Are both heart rate and
blood pressure?
* *Benchmark alert* *
10 points
Clear presentation of the results, including
tables and a graph to display reaction time
vs. your independent variable.
10 points
Clearly stated conclusions about your data
and how it related to the problem.
5 points
Sharing experimental design and data with
the class.
If the graph of reaction time vs. the independent variable is produced using excel you will
receive 3 bonus points.
Comments:

ANATOMY & PHYSIOLOGY
SCIENCE CURRICULUM 548 GRADES 9 - 12
Name: ______________________________________ Date: _________________
Lab Partners: _______________________________________________________
Inquiry Lab: What factors affect blood pressure and heart rate?
Lab Activity
Did you ever run so hard you could feel your pulse beating in your ears? Have you
ever gotten up too quickly from the couch and felt dizzy or light-headed? Have you ever felt
your heart rate quicken before a big test or competition?
Your assignment is to design an experiment to test the effect of one of the factors we
discussed in class on blood pressure and heart rate. In order to measure heart rate, you
will choose a pulse point on your body and count the number of heartbeats you feel in one
minute. In order to measure blood pressure, you will use sphygmomanometer,
stethoscope, and technique we practiced in class. Before you begin, formulate a
hypothesis to predict what effect your chosen factor will have on blood pressure and heart
rate.
Guidelines to follow:
Follow all safety rules.
Let me check your procedure and hypothesis before you begin your experiment.
Please inform me of any health concerns.
You will be working in groups of four. Two students should first test the effect of your
chosen factor on blood pressure while the other two students test the effect on heart
rate. Then switch tests. Each group member should have the opportunity to be the
subject and the tester for both blood pressure and heart rate.
Be sure to record all data (you should have data for 4 people, including yourself).
And remember, an accurate and reliable experiment is one that has been repeated at
least three times.
Materials
Stop watch
Sphygmomanometer
Stethoscope
Pencil
You will be writing a formal lab report for this lab. Your lab report should follow the
format given at the beginning of the year. Include the following analysis questions in
your lab report:
5. Would there be a difference in blood pressure and heart rate if you stood on top of a
table or sat down on the floor?
6. Compare the blood pressure and heart rate results. Are there any similarities or
differences in the changes?
7. Why is high blood pressure a health concern?
8. Research and explain why smoking causes a rise in blood pressure.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 549 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Principles of Ecology
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Princ of Ecology
3. Transcript Course Code/Number
00375
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of Biological Systems or Biology - Honors
11. Brief Course Description
Ecology is defined as the relationship of organisms to their environment. Emphasis in this course is
placed on the study of ecosystems, which are communities of living organisms and their non-living
environments functioning together. The laboratory exercises will take place in the environmental study
areas located on the school grounds, at Bauer Farm and at Hammonasset Beach State Park. Two
research projects are required of each student in this course.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocols.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the
global community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others.

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 550 GRADES 9 - 12
13. Course Outline
Chapter Topics
How the Biosphere Supports and maintains Life
1 A. Lithosphere, Hydrosphere, Atmosphere and Biosphere.
B. Gaia hypothesis as it relates to A.
3 A. Changes in the environment.
B. Needs of organisms.
C. Species, populations and communities.
Components and Functions of Ecosystems
4 A. Roles of living organisms in the ecosystem.
B. Ecosystem structure
C. Energy flow in the ecosystem
D. Chemical cycles
Interactions and Adaptations Between Organisms and the Environment
5 A. Habitats and niches
B. Evolution and adaptation
C. Populations and their limiting factors
6 A. Relationships among organisms
B. Ecological succession
C. Land biomes
Forest and Aquatic Ecosystems
9 A. Coniferous forests
B. Deciduous forests
C. Tropical rain forests
10 A. Aquatic biomes
B. Standing water Ecosystems
C. Flowing water Ecosystems
20/21 A. Uses for water
B. Water treatment
C. Types of water pollution
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 551 GRADES 9 - 12
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
Benchmark Assignments/Assessments:
Projects and Presentations on research topics of the student's choosing
Use effective strategies for gathering information, critical thinking and problem solving
Weekly reports on readings in environmental science topics from newspaper, magazine and Internet
sources
Use technology effectively and responsibly.
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 552 GRADES 9 - 12

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 553 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations,
using the appropriate tools and techniques, to
make observations and gather data to answer
various questions?
How do you assess the data, using
mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated during
the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Environmental Science: Ecology & Human Impact
by Bernstein, Winkler, Zierdt-Warshaw
Addison Wesley, 1996
Internet lessons from selected websites
Bauer Farm field trips
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
PowerPoint presentations and notes
Guided Internet research
SUGGESTED ASSESSMENT METHODS
Independent research project and presentation
*Benchmark*
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 554 GRADES 9 - 12
LEARNING STRAND
How the Biosphere Supports and Maintains Life
Content Standard: Earth Science-(Biogeochemical Cycles) Each element on Earth moves among reservoirs which exist in the solid
earth, in oceans, in the atmosphere, and within and among organisms as part of biogeochemical cycles.
Content Standard: Earth Science-(Structure and Composition of the Atmosphere) Life has changed Earth’s atmosphere, and
changes in the atmosphere affect conditions for life.
ENDURING UNDERSTANDINGS
The biosphere is subdivided into lithosphere,
atmosphere and hydrosphere.
The biosphere accounts for only a narrow
layer of the entire planet which is capable of
supporting life.
Parts of the biosphere interact with one
another and with living organisms.
Living organisms, through their activities,
can have a profound effect on the
biosphere.
The parts of the biosphere undergo change
on both short term and long term schedules.
ESSENTIAL QUESTIONS
What three conditions, unique to planet Earth, are
required to support living organisms?
How are the three rock types formed?
Where would you find examples of each rock type in
the local area?
Why is fresh water such a precious commodity?
Which layer of the atmosphere is capable of sustaining
life and what conditions prevail there?
What conditions exist in the hydrosphere that limit the
depth to which most living organisms can penetrate?
How do volcanic activities modify climate?
How does El Nino impact both human activities and
ecosystems in the Pacific basin and elsewhere?
How has glacial activity modified our local landscape?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Account for the physical conditions
necessary to support life.
Describe the nature of the lithosphere.
Describe changes that occur in the
lithosphere over time.
Describe the nature of the atmosphere.
Describe changes that occur in the
atmosphere over time.
Describe the nature of the hydrosphere.
Describe changes that occur in the
hydrosphere over time.
Describe the Gaia hypothesis and explain
how it accounts for the interaction between
living things and the biosphere.
Find examples of the three rock types in the
local area.
Environmental Science: Ecology and Human Impact
Addison Wesley, 1996 2
nd
Edition
Textbook ancillary materials, chapters 1 and 3
PowerPoint presentations, "The Biosphere" and
"Changes in the Biosphere"
Audio Visual materials: The Biosphere (LMC and
Science department)
Field Guides to the Birds
Laboratory space and materials
Outdoor field study area
Lap top computers with Internet connection
SUGGESTED INSTRUCTIONAL STRATEGIES
―Changes in the Atmosphere‖ lab
―Geographic range‖ activity
Power point presentations: ―The Biosphere‖ and
―Changes in the Biosphere‖
―New Frontiers‖ DVD
Modeling during lecture demonstrations
Teacher-lead discussion of the Gaia hypothesis and its
controversial aspects
Worksheets/Study Guides for chapters 1 and 3
―El Nino‖ unit: selected readings and videos
Weekly report on environmental science topic
*Benchmark*
SUGGESTED ASSESSMENT METHODS
Unit tests, chapters 1 and 3
Lab reports
Homework assessment and review
Worksheets or response papers to videos/DVDs
Critique of ―Weekly report‖
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 555 GRADES 9 - 12
LEARNING STRAND
Components and Functions of Ecosystems
Content Standard: Enrichment High School Biology (Ecology) Stability in an ecosystem is a balance between competing effects.
Content Standard: Enrichment High School Earth Science (Biogeochemical Cycles) Each element on Earth moves among reservoirs
which exist in the solid earth, in oceans, in the atmosphere, and within and among organisms as part of biogeochemical cycles.
ENDURING UNDERSTANDINGS
Ecosystems are composed of producers,
consumers, decomposers and the environment
which supports them.
All living things exist at one or more trophic
levels within a food web.
Energy flows through an ecosystem; much of it
is dissipated though the activity of organisms
or lost as heat.
Nutrients, such as water, move through the
carbon, nitrogen and phosphorus cycles
among/between organisms and the
environment on short or long term schedules.
ESSENTIAL QUESTIONS
Why do ecologists confine their studies to a
limited area such as a quadrant?
Why are there so few tertiary consumers?
What essential role can only be performed by
bacteria and fungi?
Why did the Osprey become a threatened
species, and how have human efforts lead to its
recovery?
What essential activity of the nitrogen cycle is
performed by the ―nitrogen-fixing‖ bacteria
Rhizopus
?
How can the rising human population be fed
efficiently without an increase in arable land?
Why do human activities in the tundra, as
compared to the rain forest, have a more drastic
and immediate impact on the ecosystem?
How has the New England hardwood forest
been impacted by human activity and what new
concerns for its continued health and
productivity do we face today?
Why does a young forest have a preponderance
of understory trees, while a mature forest has
more canopy trees?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Identify and give examples of the levels of
organization including population, community,
ecosystem and biome.
Describe how the connections in a food web
determine both the complexity and stability of
the ecosystem.
Explain the ―10% Rule‖ using an energy
pyramid.
Explain biological magnification of a toxin in an
ecosystem and relate it to the energy pyramid.
Describe the movement of nutrients through
the Nitrogen, Carbon and Phosphorus cycles.
Describe the role of producer, consumer and
decomposer in the capture and transfer of
nutrients and energy through an ecosystem.
Compare and contrast variations in
temperature and precipitation among the
major terrestrial biomes.
Environmental Science, Ecology and Human
Impact Addison-Wesley, 1996 2
nd
edition
Textbook ancillary materials, chapter 4
Audio Visual materials on components and
functions of ecosystems (LMC and Science
department)
Laboratory space and materials
Outdoor study area
Lap top computers with Internet connection
Poster board, construction paper and assorted
classroom materials
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lecture demonstrations
Students will research a specific biome and
present a poster board or power point to the
class.
―Biological Magnification‖ lab
―Forest Succession‖ lab
Bauer Farm field trip: ―Transect Study‖
Bauer Farm field trip: ―Study Area Comparison,
Field and Forest‖
Worksheets/Study Guide for chapter 4
Carbon cycle and Nitrogen cycle videos
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 556 GRADES 9 - 12
―Predators‖ DVD
Weekly report on environmental science topic
*Benchmark*
SUGGESTED ASSESSMENT METHODS
Unit test, chapter 4
Lab reports
Homework assessment and review
Worksheets or response papers to videos/DVDs
Review of and response to Bauer farm field trips
Group ―Biome‖ research and presentation
Assessment evaluated with rubric
Critique of ―Weekly report‖
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 557 GRADES 9 - 12
LEARNING STRAND
Interactions and Adaptations Between Organisms and the Environment
Content Standard 10.5: Evolution and biodiversity are the result of genetic changes that occur over time in constantly changing
environments.
Content Standard 10.6: Living organisms have the capacity of producing populations of unlimited size, but the environment can
support only a limited number of individuals of each species.
Content Standard: Enrichment High School Biology (Ecology) Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
Each species has a unique niche within an
ecosystem.
Species can evolve to adapt to changing niches.
Introduction of new species can lead to
ecosystem disruption and possible extinction of
native species.
Growth of populations is controlled by limiting
factors.
Ecosystems change over time as a result of
disruption and renewal in a process called
succession.
Chaos theory proposes the notion that relatively
small changes can have large consequences
due to the interconnectedness of organisms and
their environment.
ESSENTIAL QUESTIONS
Why do organisms rarely occupy their
fundamental niche in natural ecosystems?
How do several species of sandpiper manage to
co-exist on the same mudflat?
What evidence have you seen that Russian olive,
Multiflora rose and Oriental bittersweet are in
fact ―invasive‖ species?
Why does prey population change tend to
precede a change in the population of its
predator?
What effect does the loss of a keystone predator
have on the balance of species in an ecosystem?
What changes will occur at Bauer farm if it is not
mowed twice a year?
Why would a devastating hurricane have similar
effects on a thousand acre forest and on the
trees in your backyard?
Why do human populations worldwide seem to
be growing exponentially, and what
consequences do you see within your lifetime?
LEARNING OBJECTIVES
The student will …
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish between the fundamental niche and
the realized niche of an organism.
Explain how closely related species can avoid
direct competition with one another.
Explain the concept of convergent evolution as
a response to a similar niche of two unrelated
species.
Account for the unprecedented success of an
―invasive‖ plant or animal species in our local
environment.
Describe the difference between density-
dependent and density-independent limiting
factors.
Distinguish between primary and secondary
succession in terrestrial communities.
Explain the process of aquatic succession.
Relate an account of a small change in an
ecosystem that has had grand consequences in
the long term.
Describe the stage of succession for a local
pond, such as Horse Pond or the Bauer farm
ponds.
Environmental Science: Ecology and Human Impact
Addison Wesley, 1996
Textbook, ancillary materials, chapters 5 and 6
Audio Visual materials on interactions and
adaptations between organisms and the
environment (LMC and Science department)
Laboratory space and materials
Outdoor study area
Lap top computers with Internet connection
SUGGESTED INSTRUCTIONAL STRATEGIES
―Invasive Species‖ lab
Bauer Farm field trip: ―Old Field Succession‖
Bauer Farm field trip: ―Invasive Species Survey‖
―Predator/Prey‖ lab
―Estimating Population Size‖ lab
Worksheets/Study Guide for chapter 5 and 6
―Estuaries‖ video
―Succession‖ video
―Invaders‖ DVD
Weekly report on environmental science topic
*Benchmark*
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 558 GRADES 9 - 12
SUGGESTED ASSESSMENT METHODS
Unit tests, chapters 5 and 6
Lab reports
Homework assessment and review
Worksheets or response papers to videos/DVDs
Review of and response to Bauer farm field trips
Critique of ―Weekly report‖
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 559 GRADES 9 - 12
LEARNING STRAND
Forest and Aquatic Ecosystems
Content Standard: Enrichment High School Biology (Ecology) Stability in an ecosystem is a balance between competing effects.
Content Standard: Enrichment High School Earth Science (Energy in the Earth System) Climate is the long-term average of a region’s
weather and depends on many factors.
ENDURING UNDERSTANDINGS
Every ecosystem has a unique combination of
organisms.
Variations between ecosystems are largely
influenced by differences in temperature,
precipitation and light availability.
Dominant ecosystems in our locale include
deciduous forest, freshwater, estuarine and
salt water.
Worldwide, forests are the most productive
terrestrial biomes.
Though similar in many ways, standing and
flowing freshwater systems contain unique
communities of organisms due to physical
differences.
Soils can vary by texture and nutrient content
among terrestrial ecosystems.
ESSENTIAL QUESTIONS
Why are conifer forest food webs relatively simple
and unstable as compared to other forest types?
How does soil texture influence soil moisture?
Why is dissolved oxygen content higher in a stream
than in a pond?
Why is a pond food web more complex than a
stream food web?
How do seasonal climate changes influence the
physical and biological parameters of a pond?
What factors influence the species composition of
the deciduous forest in our area?
Which soil nutrients are most important for plant
growth? Why?
Why are temperature and dissolved oxygen such
important physical parameters in aquatic systems?
How do nitrate and phosphate levels influence plant
growth?
How does turbidity affect productivity in ponds?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Distinguish among conifer, deciduous and
tropical rain forest with regard to climatic
limiting factors.
Compare the complexity of the food webs and
biodiversity for the three forest biomes.
Perform tests for soil pH, nutrient content and
texture and explain the significance of the
results.
Participate in a Grapsid crab census and
describe limiting factors that influence crab
populations.
Collect biological, chemical and physical data
for pond and stream, and describe the
important differences between the two
systems.
Describe threats to forests worldwide,
including logging, pollution, soil erosion and
nutrient depletion.
Collect biological and physical data in a forest,
and describe the significance of the data with
regard to forestry techniques.
Construct an aquatic organism food web
based on collections made by the class in
local streams/ponds.
Environmental Science: Ecology and Human Impact
Addison Wesley, 1996
Textbook ancillary materials, chapters 9 & 10
Audio Visual materials on forest and aquatic
ecosystems (LMC and Science department)
Laboratory space and materials
Outdoor study area
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lecture demonstrations
Bauer Farm field trip: ―Stream Study‖
Bauer Farm field trip: ―Pond Study‖
―Soil Nutrients/Texture‖ lab
Hammonasset field trips: ―Rocky Shoreline, Seining
the Shallows, Willard’s Island Discovery Hike‖
Worksheets/Study Guide for chapter 9 and 10
―Deciduous Forests‖ DVD
―Troubled Waters‖ DVD
―Dangerous Catch‖ DVD
―Dirty Secrets‖ DVD
―Fresh Water‖ video
Weekly report on environmental science topic
*Benchmark*
SUGGESTED ASSESSMENT METHODS
Unit tests, chapters 9 and 10
Lab reports
Homework assessment and review

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 560 GRADES 9 - 12
Worksheets or response papers to videos/DVDs
Review of and response to Bauer Farm field trips
Review of and response to Hammonasset field trips
Critique of ―Weekly report‖
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 561 GRADES 9 - 12
Ecology Scoring Rubric
Applies effective and efficient strategies for gathering information and materials, thinking
critically, and solving problems.
Exceeds
Expectations
The student independently chooses a research topic, and collects, interprets,
analyzes, and evaluates a variety of information and data to make original predictions
or solve problems. S/he solves problems accurately and efficiently and presents
her/his findings in a coherent manner.
Meets
Expectations
The student independently chooses a research topic, and collects, interprets,
analyzes, and evaluates a variety of information and data to make specific predictions
or solve problems. S/he solves problems with few errors and presents her/his findings
in a coherent manner.
Meets Some
Expectations
The student may need some assistance in choosing a research topic, and in collecting
and interpreting a variety of data to make general predictions or solve problems.
S/he solves problems with some errors and presents her/his findings in a coherent
manner.
Does Not Meet
Expectations
The student needs assistance in choosing a research topic and in gathering
information to make a prediction or solve problems. S/he solves problems inefficiently
and with significant errors and fails to present her/his findings in a coherent manner.
Uses technology effectively and responsibly.
Exceeds
Expectations
The student can independently select and use appropriate internet sources to solve
problems efficiently and creatively, including summary of and reaction to
environment-related articles.
Meets
Expectations
The student can select and use appropriate internet sources to solve problems
effectively without making significant errors, including summary of and reaction to
environment-related articles.
Meets Some
Expectations
The student can select and use appropriate internet sources to solve problems but
makes some errors and requires some assistance, including summary of and reaction
to environment-related articles.
Does Not Meet
Expectations
The student cannot select and use appropriate internet sources to solve problems
without making many significant errors and requiring supervision; student fails to
summarize and react to environment-related articles.

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 562 GRADES 9 - 12
WEEKLY ARTICLE
PURPOSE:
Every week you will have the opportunity to read an article that pertains to some aspect of this course.
The goal is to make you more aware of career opportunities, interesting discoveries and issues of
importance. I hope that your interest will continue long after you have completed this course.
METHODS:
Each Friday, at the start of class, you will hand in a 1 to 2 page review of an article from a
magazine, newspaper or internet website.
The accumulated reviews will be worth 10% of your trimester grade.
There will be 10 reviews due each trimester.
If you are absent on Friday, your review is due the day after you return to class.
Include the following information:
1. Name of magazine, newspaper or website.
2. Date of the issue.
3. Author’s name.
4. Title of article.
5. Copy of any internet article.

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 563 GRADES 9 - 12
RESEARCH PROJECT IN ECOLOGY
PURPOSE:
This project is intended to give you practical experience in research, experimental design, and completion
of a hands-on scientific inquiry.
PROCEDURE:
You will work alone or in a group of two. Your project should examine a specific problem in a natural
ecosystem. It should not simply be a research paper nor should it be an experiment that is carried out in
a laboratory situation.
MATERIALS:
Often, the best projects are those that require a minimum of equipment. You will be allowed to borrow
equipment from this classroom as needed. Some of the things available include microscopes, nets, plant
press, tree sampling tools, water sampling kits, soil sampling kits, weather instruments, identification
guides, and other books that can provide useful background information.
TIMETABLE:
Phase 1: Due date: __________________.
A. We will use at least one class period doing library research to help you determine what
topic you will study.
B. You will write an outline or short report of at least 2 pages.
C. This document will include a statement of the problem, a discussion of your procedure,
and a list of all the materials you will use to carry out the project.
D. You must discuss your project with me before this phase is due.
Phase 2: Due date: ___________________.
A. This will be an update of your project and should include a
presentation of the work you have done. This can be written, video, photographs or slides,
specimens of plants, animals, soils, or any other information that shows me your fieldwork.
B. You should include a bibliography of references that you have used.
C. You are encouraged to discuss with me any problems that you are having well before
this phase is due.
Phase 3: Due date: ___________________.
A. The completed project will be presented to the entire class at this time.
B. You may use notes during your presentation.

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 564 GRADES 9 - 12
C. The project can be presented as a Power Point presentation, paper, video, poster,
collection of organisms, photo or 35 mm slide essay, or any other appropriate
form.
D. Your grade will be based on 60% content/ 40% presentation.
E. The 3 phases combined will total 35% (5%+5%+25%) of your trimester grade.
SUGGESTED TOPICS:
Owl pellet study
Study of the Beaver impact on Coan Pond, Rockland Preserve, Route 79.
Migration Study: Hawk, Monarch, Green Darner
Bird population census (Waterfowl, Gulls, Hawks, Swans, etc.)
Stream invertebrate study (Fast water vs. Slow water)
Invasive plant eradication project (Bittersweet, Japanese barberry, Purple loostrife, Phragmites)
Invasive plant mapping project (BAUER FARM)
Tree cavity survey
Vernal pool mapping/Amphibian survey (DHHS PROPERTY)
Live trapping study
Rocky shoreline mapping
Tree sampling project (DHHS PROPERTY)
Bluebird trail maintenance (BAUER FARM or LAND TRUST PROPERTY)
Bird feeder comparative study
Pond community comparative study (BAUER FARM)
Flying or crawling insect population study (Butterflies, beetles, bugs)
Wildflower identification (Collect and photograph)
These are simply a few ideas that have worked in the past for other students. By no means should
you limit yourself to these choices. In fact, it would be better if you devised your own plan,
something that hasn’t been done before. Please discuss your ideas with me in order to help you
formulate your plan.
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 565 GRADES 9 - 12
ECOLOGY PROJECT
GRADING CRITERIA
The final project in Ecology will be graded based on the following criteria:
SCIENTIFIC ACCURACY- Use of common names or scientific names. Use of metric or English units of
measurement. Consistency in your observations 10 POINTS
METHODS USED- A full and accurate description of your field techniques, the equipment that you used,
and any guides to identification that you used. Make it a ―Show and Tell‖ presentation. 10 POINTS
DATA COLLECTED AND PRESENTED- Your data or information should be presented in a clear and
coherent manner. Your data should be easily understood. 20 POINTS
CONCLUSION- Your conclusion should be based on the data that you have presented and should reflect
some understanding of ecosystems and how they function. 20 POINTS
ORAL PRESENTATION- See attached sheet for details. 40 POINTS
TOTAL 100 POINTS

PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 566 GRADES 9 - 12
ECOLOGY PROJECT SUMMARY Name________________________
Name ________________________
Name ________________________
Please summarize who completed which aspects of your project. Thank you.
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 567 GRADES 9 - 12
ECOLOGY FINAL PROJECT NAME_________________________
Category
Possible Points
Your Points
Scientific Accuracy
10
Methods Used
10
Data Collected/Presented
20
Conclusions
20
Oral Presentation
40
TOTAL
100
ORAL PRESENTATION
Category
Possible
Points
Your Points
1. Speaker can be heard by everyone.
5
2. Presentation is well organized.
5
3. Presentation is appropriate length. (10 minutes)
5
4. Presentation is creative and interesting, with visual
component.
15
5. Speaker responds well to questions.
10
TOTAL
40
PRINCIPLES OF ECOLOGY
SCIENCE CURRICULUM 568 GRADES 9 - 12
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 569 GRADES 9 - 12
Course Description
HIGH SCHOOL
1. Course Title
Marine Science and Technology
5. Subject Area
English
Mathematics
Science
Social Studies
World Language
Career & Tech Ed
Visual Art
Music
Physical Education
Health Education
Special Education
Library Media
2. Transcript Title/Abbreviation
Marine Science & Tech
3. Transcript Course Code/Number
00379
4. Program Contact Information
Name: Paul Mezick
Title/Position: Department Chair, Science
School: Daniel Hand High School
286 Green Hill Road
Madison, CT 06443
6. Grade: 11 – 12 Level: 2
7. Seeking ―Honors‖ Distinction?
Yes No
8. Unit Value
.25 (30 days)
0.5 (trimester equivalent)
.75 (trimester+30days)
1.0 (two trimester equivalent)
1.5 (three trimester equivalent)
Other: ___________________________
9. Approval
BOE Approved
Anticipated Approval ________(date)
10. Pre-Requisites
Successful completion of Biological Systems or Biology-Honors or a B or better in General Biology
11. Brief Course Description
Marine science and technology explores the marine environment, examines the chemical, biological and
geological properties of the sea as well as all stages of aquaculture based careers from boatbuilding and
trapping to farming and maintenance of organisms. Boat construction, fishing rod building, physical,
chemical, and ecosystem studies related to oceanography will be part of this ―hands on― course. Select
field trips will support the curriculum and provide school to career practical experience. Many forms of
coastal ecology will also be explored including, water chemistry, rocky shore, sandy shore, estuaries and
pelagic zones. In addition students who excel in this field will have an opportunity to intern in marine
related careers. Interdisciplinary projects will also be incorporated to widen the student’s understanding
of maritime based careers and life.
12. Course Goals
1. Apply effective and efficient strategies for gathering information and materials, thinking critically and
solving problems.
2. Conduct lab experiments safely using appropriate scientific protocol.
3. Use technology effectively and responsibly.
4. Demonstrate proficiency and fluency in reading and writing to meet the literacy demands of the global
community.
5. Demonstrate the ability complete assignments independently.
6. Demonstrate respect for one’s self, and strive to contribute to the success of others
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 570 GRADES 9 - 12
13. Course Outline
Group Unit
Science
Tech Ed
Field Trips
Marine Biology
And Sampling
Equipment
Marine Biology
Life in the Ocean
Evolution in the marine
environment
Field Study Sampling
Techniques
Lab Report Writing
Marine Sampling Equipment
Nets
Traps
Lures
Hammonasset
Sandy Beach Study
Marine Sampling
Equipment Overview
Ocean Life
Ocean Life Zones
Pelagic zones
Benthic Zones
Near shore
Intertidal
Fishing Rod Science
Modulus of elasticity
Materials
Evolution of the fishing
rod
Hammonasset
Marsh Study
Ecosystems
Long Island Sound
Ecosystems
Sandy Beach
Rocky Shore
Salt Marsh
Fishing Rod Building
Parts ID
Tools
Fishing Rod Construction
Hammonasset
Rocky Shore / Gaspid
Crab Study
Marine Related
Technology
Environmental Awareness
projects
Marine nomenclature
Hands-on Projects
Watercraft repair and
maintenance
Water Quality
Chemistry
Water Molecule
Salt Water
Salinity
Density
Temperature
Boating Technology
Marine Propulsion
Outboard Mechanics
Construction Materials
Fuel as a resource
Ethanol vs. Gasoline
Fence Creek
Water Testing
Marine History
Ancient Ideas
Modern Theories
Expeditions
Technology
Local History
Recreational/
Commercial Fishing
Boating
Earl Brockway
Coastal Indian Fishing
Technology
West Wharf
Clam Seeding
Boat Building
Geology of
Long Island
Sound and
Connecticut
Geology
Hutton to Plate
Tectonics
Oceans and Basins
Geology of Atlantic
Geology of LIS
Coastlines and reefs
Safe Boating
Navigation
CT Boating Safety
Hammonasset
Moraine Trail
Seal Watch
Classification
Classification
Bacteria and Protista
Cnidarians, worms
and mollusks
Mammal groups
Fish
-Cartilaginous
-Boney
Marine Sampling Equipment
Projects
Lobster Pots
Crab Traps
Boat Building
Fish Ladders
Seaview Beach
Steamer Clam Field
Study
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 571 GRADES 9 - 12
14. Instructional Methods and/or Strategies
Modeled instruction
PowerPoint presentations and notes
Laboratory investigations
Teacher demonstrations
Cooperative grouping
Audio Visual presentations
Response Cards by TurningTechnologies
Web-based instruction with Blackboard/finalsite
Research
15. Assessment Methods and/or Tools
Formative quizzes
Summative unit assessments
Final examination
Lab reports
Assessments evaluated with rubrics
Benchmark assessments
Video response summaries
Response Cards by TurningTechnologies
Research projects
16. Assessment Criteria
Assessments are based on the Madison Curriculum and Connecticut standards and grade level
expectations for science. Authentic assessments are graded using a scoring rubric or grading criteria.
Benchmark assignments are graded using a
common
scoring rubric or grading criteria.
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 572 GRADES 9 - 12
LEARNING STRAND
Core Scientific Inquiry, Literacy, and Numeracy
CT Standard: Scientific knowledge is created and communicated.
Note: This learning strand should be taught through the integration of the other learning strands. This learning strand is not meant
to be taught in isolation as a separate unit.
ENDURING UNDERSTANDINGS
Scientific inquiry is a thoughtful and coordinated
attempt, through a continuous process of
questioning, data collection, analysis and
interpretation, to describe, explain, and predict
natural phenomena.
Scientific inquiry requires the sharing of findings
and ideas for critical review by colleagues and
other scientists.
Scientific literacy includes the ability to read,
write, discuss, and present coherent ideas
about science.
Scientific literacy includes the ability to search
for and assess the relevance and credibility of
scientific information found in various print and
electronic media.
Scientific numeracy includes the ability to use
universal mathematical operations and
procedures to calculate, analyze and present
scientific data and ideas.
ESSENTIAL QUESTIONS
How do you form a testable hypothesis that is
logically connected to the problem and the
design of the experiment?
How do you design and conduct appropriate
types of controlled scientific investigations, using
the appropriate tools and techniques, to make
observations and gather data to answer various
questions?
How do you assess the data, using mathematical
operations to analyze and interpret data, and
present relationships between variables in
appropriate forms?
Why is it essential to assess the validity of the
experiment’s design and the credibility of
scientific claims in different sources of
information?
How do you communicate your findings, using
relevant scientific vocabulary and clear logic,
which are based on the results generated during
the experiment?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Formulate a testable hypothesis, in the "If…,
then…" format, which is logically connected to
the problem.
Design a controlled experiment where the
independent and dependent variables are
accurately identified.
Utilize instrument methodology that is
appropriate for the design of the experiment.
Record data in the appropriate units of
measure, and be able to convert between
different units of measure.
Use mathematical operations to analyze and
interpret data, and present relationships
between variables in appropriate formats.
Apply both precision and accuracy in recording
experimental data.
Develop logical conclusions that are based on
the analysis of experimental data.
Formulate reports, using relevant vocabulary,
supporting evidence, and logic that accurately
communicate the results of a scientific
experiment.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Laboratory instrumentation
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided internet research
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and Presentations
Projects and reports evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio

MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 573 GRADES 9 - 12
LEARNING STRAND
Marine Biology and Sampling Equipment
CT Standards: Biology - Ecology: Stability in an ecosystem is a balance between competing effects.
Technology Education Standards (2007):
D. 21 Apply organizational and time management skills to classroom and laboratory activities.
D. 22 Present information in a clear, concise and appropriate manner.
D. 23 Use research techniques to support design development.
ENDURING UNDERSTANDINGS
The environment is a complex assemblage of
interacting and evolving chemical, physical and
biological processes.
The current state of the environment is
maintained by the dynamic exchange of the
processes that dictate its nature.
Changes in any of the interacting processes will
impact the current state of the environment.
ESSENTIAL QUESTIONS
How is appropriate sampling equipment chosen
for use in conducting a field study?
Why are certain species only found in specific
areas of Hammonasset State Park?
How is data collected and analyzed in a field
study?
How is the environment changed by physical,
chemical, and biological factors?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Learn to identify each of the main species found
in Hammonasset State Park.
Use collection devices during field studies to
gather data for lab analysis.
Explain how is the environment is changed by
physical, chemical, and biological factors.
Understand proper field study techniques and
their roll in studying the environment.
Write lab reports based on field studies to show
relationships in the natural environment.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Field Study Equipment
Seine nets & Cast nets
Collection Buckets
Specimen Trays
Clip Boards & Data Sheets
Measuring devices
SUGGESTED INSTRUCTIONAL STRATEGIES
Field Study Trips
Marine Sampling Equipment Overview
Class Lecture
Modeling during lecture
Net overview and practice
Student research of marine environments
through use of field guides and internet sources
Students prepare for field work and clean up
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and Presentation
Reports and projects evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 574 GRADES 9 - 12
LEARNING STRAND
Ocean Life
10.1 Fundamental life processes depend on the physical structure and chemical activities of the cell.
10.5 evolution and biodiversity are the result of genetic changes that occur over time in constantly changing environments.
Biology - Ecology: Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
The environment is a complex assemblage of
interacting and evolving chemical, physical and
biological processes.
Estuaries are critical habitats that provide a
nursery environment for many marine
organisms.
Estuaries are highly productive, dynamic and
diverse communities that serve as a buffer zone
between land and the ocean.
Diversity of marine organisms in Long Island
Sound is a tool for measuring the health of Long
Island Sound.
Diversity of species in benthic communities
depends on their ability to adapt to the
environmental conditions of their environment.
Pelagic animals mostly live in the upper surface
waters of the open ocean; these animals have
special adaptations to help them stay near the
surface where most of the food supply is found.
ESSENTIAL QUESTIONS
How does the coastal ocean vary in terms of
salinity, temperature, and currents?
How are estuaries created and what types exist?
Why are coastal wetlands important?
How are marine organisms able to stay above
the ocean floor?
How are pelagic organisms adapted to living in
the open ocean?
What are the different groups of pelagic
organisms?
What types of organisms live in benthic
environments?
What are the environmental conditions of the
ocean floor?
What are the properties of different benthic
communities?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Discover how the coastal ocean varies
chemically and physically.
Describe how Long Island Sound was created.
Explain how a fish bladder works.
Explain the importance of an estuary for both
living and nonliving elements.
Describe the types of organisms found in the
pelagic environment.
Describe the types of organisms found in the
benthic environment.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Computer lab
Video selections
CT Sea Grant books
Long Island Sound study books
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and Presentations
Reports and projects evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 575 GRADES 9 - 12
LEARNING STRAND
Long Island Sound Ecosystems
CT Standards:
10.5 Evolution and biodiversity are the result of genetic changes that occur over time in constantly changing environments.
10.6 living organisms have the capability of producing populations of unlimited size, but the environment can support only a limited
number of individuals from each species.
Biology - Ecology: Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
Plants and animals that live in the Rocky inter-
tidal zone where land, air and sea converge,
survive in diverse and dynamic conditions that
are impacted by tides and waves.
Salt Marshes are highly productive, dynamic and
diverse communities that serve as a buffer zone
between land and the ocean.
Plants and animals that live in the sandy beach
zone where land, air and sea converge, survive
in diverse and dynamic conditions that are
impacted by tides and waves.
ESSENTIAL QUESTIONS
What kinds of organisms occur along rocky
shores?
What types of organisms live along the sandy
beach?
What types of organisms live in a salt marsh?
What are the environmental conditions found in
each Long Island Sound ecosystem?
Why are salt marshes so important to Long
Island Sound?
How are organisms adapted to live in each Long
Island Sound ecosystem?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Perform a field study of each Long Island Sound
ecosystem found at Hammonasset State Park.
Write a detailed lab report based on the findings
at each ecosystem.
Identify organisms found in each ecosystem.
Describe the importance of the salt marsh.
Identify the environmental conditions necessary
for each ecosystem found in Long Island Sound.
Explain how organisms are adapted to live in
each ecosystem.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Field study equipment
Water testing equipment
Data collection devices
Measuring devices
Identification books
Collection buckets
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects & Presentations evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 576 GRADES 9 - 12
LEARNING STRAND
Marine Related Technologies and Hands-on Projects
Technology Education Standards (2007):
D.16 Identify and explore career opportunities.
D.17 Explain the need to be a lifelong learner.
D.18 Exhibit and take responsibility for behaviors in both school and work situations.
D.19 Define and demonstrate a personal work ethic.
E.30 Fabricate a prototype to support the chosen design.
ENDURING UNDERSTANDINGS
The experience of using marine related
sampling equipment can be applied to a range
of career choices.
The participation in hands-on projects provides
an opportunity for development of a personal
work ethic.
Marine biologists use several types of marine
related technologies to access and collect
organisms and data in an aquatic environment.
Being able to understand maritime related
nomenclature will assist students in
understanding and operating in marine related
career fields.
Environmental awareness projects provide
opportunity for skill building and career field
applications.
ESSENTIAL QUESTIONS
What types of sampling equipment are used to
collect and study organisms?
What is the connection between safe boating
practices and scientific collection of data and
organisms?
How can the development of a personal work
ethic and becoming a lifelong learner help when
entering into a career?
How can hands-on projects provide an
opportunity for students to exhibit and take
responsibility for behaviors in both school and
work situations?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Learn basic skills and practices related to
building a fishing rod.
Learn basic skills and practices related to
building a boat.
Gather various marine organisms using
commercially produced and student-built
collection equipment.
Identify parts of a boat and major safety related
items dealing with water craft.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Access to work shop area
Work Benches
Woodworking tools
Fishing rod building materials and supplies
Project Storage Areas
Connecticut Safe Boating Curriculum
SUGGESTED INSTRUCTIONAL STRATEGIES
Fishing Rod building project
Boat building project
Crab/Lobster pot building activity
Environmental awareness projects
Boating technology
Watercraft repair and maintenance
SUGGESTED ASSESSMENT METHODS
Connecticut Safe Boating curriculum
assessments
Benchmark Fishing Rod building activity
Boat building activity
Teacher created quizzes, activities & homework
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 577 GRADES 9 - 12
LEARNING STRAND
Water Quality of Long Island Sound
CT Science Standards: Biology - Ecology: Stability in an ecosystem is a balance between competing effects.
Earth Science - Biogeochemical Cycles: Each element on Earth moves among reservoirs which exist in the solid earth, in oceans, in
the atmosphere, and within and among organisms as part of biogeochemical cycles.
9.4 Atoms react with one another to form new molecules.
9.5 Due to its unique form carbon forms many organic and inorganic compounds.
9.8 The use of resources by human populations may affect the quality of the environment.
ENDURING UNDERSTANDINGS
The water molecule has a bend in its geometry,
with the two hydrogen atoms on the same side
of the oxygen atom, which gives the water its
polarity and ability to form hydrogen bonds.
Water can dissolve more substances than any
other liquid, hence its name ―the universal
solvent‖.
Salinity is the total amount of dissolved solid
material in water.
Salinity varies greatly in the world’s oceans and
also in Long Island Sound.
The presence of dissolved gases in salt water is
extremely important to the organisms living
there.
Many factors affect the quality of water in Long
Island Sound.
ESSENTIAL QUESTIONS
Why does water have such unusual chemical
properties?
How salty is the ocean and Long Island Sound?
How are factors like Ph, salinity, nitrogen,
dissolved oxygen, carbon dioxide, and fecal
coliforms related to the overall health of Long
Island Sound?
What is a salt wedge and how do salt wedges
affect an estuary?
What is the quality of the water found here
along the Madison shoreline?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Study the unique chemical and physical
properties of water.
Measure the physical and chemical properties of
the water found in Long Island Sound.
Write reports based on field study findings.
Use data to help local government make
decisions for town activities such as shell
fishing.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
LaMotte water testing kits
Dissolved oxygen meter
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided Internet research
SUGGESTED INSTRUCTIONAL STRATEGIES
Field study at local shoreline sites.
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and Presentation
Reports and projects evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 578 GRADES 9 - 12
LEARNING STRAND
Maritime History in Madison and Long Island Sound
History Standards: 2.9-10.2 Locate events, peoples and places in time and place studied relative to their own location.
Scientific Inquiry & Scientific Literacy
ENDURING UNDERSTANDINGS
Madison, Connecticut is a town of 18,000
residents located on the Shore of Long Island
Sound a 20 minute drive east of New Haven.
Located on I- 95, it is easily accessible. Its area
of 37 square miles is comprised of sandy
beaches, lowland areas and hills in the
northern part of town. Incorporated as
separate town in 1826 it was originally part of
the Town of Guilford.
West Wharf was a working wharf where
fisherman dried their nets and kept their boats.
West Wharf was also a site for boat building.
Madison Beach Hotel began as a boarding
house for shipyard workers and evolved into a
hotel for summer visitors.
The four principal oceans are the Pacific,
Indian, Atlantic, and Artic Oceans.
Long Island Sound is an estuary that was
created during the last ice age by glaciers.
Indians used the shoreline to gather clams and
also used the shells for their currency.
ESSENTIAL QUESTIONS
What are the four principal oceans on planet
Earth?
What is Long Island Sound?
When was Long Island Sound discovered?
How did the Indians use our local waters?
Where is the deepest part of the ocean?
How was early exploration of the oceans
achieved?
What were the famous scientific explorations and
what did they discover?
What are the tools used to explore the world's
oceans?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
View and visit historical sites around the Town
of Madison.
Know the four principal oceans.
Know how the early explorers navigated with
out help from GPS.
Know the important findings of the first
scientific expeditions.
Know the tools used today for ocean
exploration.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Books on local maritime history
PowerPoint presentations on local history
Boat building supplies
SUGGESTED INSTRUCTIONAL STRATEGIES
Field trips to Hammonasset and other local sites
Guest speakers on native American history
Work at West Wharf with the shell fish
commission
Classroom instruction
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and presentations
Reports and projects evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 579 GRADES 9 - 12
LEARNING STRAND
Geology of Long Island Sound and Connecticut
CT Standards: Earth Science- Dynamic Earth Processes: Plate tectonics operation over geologic time has changed the patterns of
land, sea and mountains on Earth's surface.
ENDURING UNDERSTANDINGS
Geologic history in Connecticut can be summed
up in two words—"crunch and crack.‖
Evidence for this continental collision comes
from the analysis of the terranes that make up
Connecticut.
There are four major terranes in Connecticut.
Connecticut has been covered by ice at least
two times, and maybe more.
Glaciation was an important factor in shaping
and creating Connecticut’s coastline.
Glaciation is responsible for the formation of
Long Island Sound.
ESSENTIAL QUESTIONS
What is the evidence found in Connecticut which
supports Plate Tectonics?
Why is Connecticut explained as being
―crunched and cracked‖?
What are terranes?
What are the four types of terranes found in
Connecticut and where can we find them today?
How do glaciers form?
How long ago was the last ice age, and what
evidence do we see that it did in fact happen?
How did the glaciers make Long Island Sound
and shape the Connecticut shoreline?
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explore the evidence for plate tectonics here in
Connecticut.
Understand that Connecticut is made up of
different terranes.
Use a geological map to locate the terranes
and determine which terrenes are found in
Madison.
Take a field trip to look at the evidence for
glaciation in Connecticut and hike along a
recessional moraine.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Geology of Connecticut CD
Sea Grant pamphlets
DEP Website
Bedrock map of Connecticut
SUGGESTED INSTRUCTIONAL STRATEGIES
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided internet research
Field trips to Hammonasset and other local sites
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and Presentations
Reports and projects evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 580 GRADES 9 - 12
LEARNING STRAND
Classification of Marine Organisms
CT Standards: 10.5 Evolution and biodiversity are the result of genetic changes that occur over time in constantly changing
environments.
Biology- Ecology: Stability in an ecosystem is a balance between competing effects.
ENDURING UNDERSTANDINGS
To study the diversity of life, biologists use a
classification system to name organisms and
group them in a logical manner.
The six-kingdom system of classification
includes the kingdoms Eubacteria,
Archaebacteria, Protista, Fungi, Plantae, and
Animalia.
Organisms are now grouped into categories that
represent lines of evolutionary descent.
An animal is a multicellular, eukaryotic
heterotroph whose cells lack cell walls.
Animals are specialized to carry out the
following essential functions: feeding,
respiration, circulation, excretion, response,
movement, and reproduction.
Sponges are classified as animals because they
are multicellular, heterotrophic, have no cell
walls, and contain a few specialized cells.
Cnidarians are soft-bodied, carnivorous animals
that have stinging tentacles arranged in circles
around their mouth; they are the simplest
animals to have body symmetry and specialized
tissues.
Cnidarians include jellyfishes, hydras and their
relatives, sea anemones, and corals.
Flatworms are soft, flattened worms that have
tissues and internal organ systems; they are the
simplest animals to have three embryonic germ
layers, bilateral symmetry, and cephalization.
Roundworms are unsegmented worms that
have pseudocoeloms and digestive systems with
two openings, a mouth and an anus.
Annelids are worms with segmented bodies who
have a true coelom that is completely lined with
mesoderm.
Mollusks are soft-bodied animals that usually
have an internal or external shell.
Arthropods are classified based on the number
and structure of their body segments and
appendages, particularly their mouthparts.
Crustaceans typically have two pairs of
branched antennae, two or three body sections,
and chewing mouthparts called mandibles.
Echinoderms are characterized by spiny skin,
five-part radial symmetry, an internal skeleton,
a water vascular system, and suction-cup-like
structures called tube feet.
A chordate is an animal that has, for at least
some stage of its life, a dorsal, hollow nerve
ESSENTIAL QUESTIONS
How are living things classified?
How are marine organisms classified?
How many marine species exist?
What is binominal nomenclature?
What are the six kingdoms of life?
What characteristics do all animals share in
common with one another?
Why are sponges classified as animals?
What is a Cnidarian?
What are the different groups of Cnidarians?
What are the defining characteristics of
flatworms?
What are the defining characteristics of
roundworms?
What are the defining characteristics of annelids?
What are the defining characteristics of mollusks?
What are the defining characteristics of
arthropods?
What are the defining characteristics of
echinoderms?
What are the defining characteristics of
chordates?
What are the defining characteristics of fishes?
What are the defining characteristics of
mammals?
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 581 GRADES 9 - 12
cord; a notochord; pharyngeal pouches; and a
tail that extends beyond the anus.
Fishes are aquatic vertebrates that are
characterized by paired fins, scales, and gills.
In addition to having hair and the ability to
nourish their young with milk, all mammals
breathe air and are endotherms that generate
their body heat internally.
Similar ecological opportunities on the different
continents have produced some striking
examples of convergent evolution in mammals.
LEARNING OBJECTIVES
The student will…
INSTRUCTIONAL SUPPORT MATERIALS
Explain how living things are organized for
study.
Describe binomial nomenclature.
Know all of the main groups of marine
organisms and the characteristics of each.
Name the six kingdoms of life.
List the characteristics that all animals have in
common.
Explain what a sponge is and where sponges
live.
Explain what a Cnidarian is and which types
live in Long Island Sound.
Describe the defining features of flatworms,
roundworms, and annelids.
Know the characteristics of mollusks.
Know the ecology of the steamer clam and
where it can be found in Madison.
Study the life cycle of the oyster and observe
its growth at 1 year and 2 years.
Be able to explain the characteristics of
chordates including fish and mammals.
Introductory Oceanography Thurman & Trujillo
Pearson Education, 2004
Biology text
Preserved specimens
Internet sources
Sampling equipment
Dichotomous keys
Field manuals
SUGGESTED INSTRUCTIONAL STRATEGIES
Field work
Internet activities
Lab work
Lecture
Modeling during lectured instruction
Inquiry investigation
Textbook ancillary materials
Guided internet research
SUGGESTED ASSESSMENT METHODS
Constructive feedback
Performance assessment
Projects and Presentations
Reports and projects evaluated with rubrics
Self-assessment
Tests
Writing assignments
Student Portfolio

MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 582 GRADES 9 - 12
Soft Shell Clam Survey
Problem
The Madison Shellfish Commission wants you to conduct a field survey of soft shell
clams on Seaview Beach in Madison to determine if healthy populations of large sized soft
shell clams are present in sufficient numbers to be harvested.
Background Information
Location
Survey of soft shell clams Mya arenaria at Seaview Beach Land Spit - East of Waterbury
Ave. shoreline access in Madison, CT.
Surface Area
The soft -shell clam bed can be found in a spit of land that extends out from the shore at
its center approximately 140 feet from the shore. The base of the rocky spit is
approximately 100 feet at the shore tapering to 50 feet farthest off shore at full ebb tide.
Soft-shell Clams
Mya arenaria, popularly called "steamers", "softshells", "longnecks" or "Ipswich clams",
are clams that live buried in tidal mudflats most famously on the coast of New England,
but their range extends much farther north to Canada and to the Southern states.
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 583 GRADES 9 - 12
Physiology
Mya arenaria has a calcium carbonate shell, which is very thin and easily broken, hence
the name "soft-shells" (as opposed to their beach-dwelling neighbors, the thick-shelled
quahogs).
It can be found living approximately 6-10 inches under the surface of the mud and
extends a Siphon, which is used to draw in marine water that is filtered for food and
expelled, up to the surface. The holes through which the water is drawn can often be seen
at low tide and water may be visibly ejected from them when pressure is applied to the
surrounding mud. These holes are helpful in locating the clams for digging.
Clams in cooking
Soft-shell clams are edible and can be enjoyed in a variety of dishes. Before cooking, it is
generally recommended that clams be stored in saltwater for a few days to facilitate the
expulsion of sand from their digestive tracts. Some recommend that cornmeal be added to
the water to give the clams something to filter from it.
Soft-shell clams can be eaten steamed, fried, or in clam chowder. "Steamers" (steamed
soft-shell clams) are an integral part of the New England clam bake, where they are
served steamed whole in the shell, then pulled from the shell at the table and dipped, first
in the clam broth in which they were cooked, to rinse away sand, and then in butter.
Procedure:
1. First establish three transects perpendicular to the shore.
2. The eastern edge transect will be approximately 80 feet long, the center transect will be
approximately 140 ft. long and the western transect will be approximately 100 feet long.
3. Determine the surface area sampled.
4. Dig sample holes, 20" square and 12" deep, approximately 6 feet apart along each transect
beginning farthest from shore. Because of the rocky condition of the bottom, short handled
clam rakes or short handled 3 prong garden cultivators seem to work best.
5. Count all clams from each hole along each transect and record total clams indicating those
over and under 2".
6. Record the numbers of clams caught in each hole along each transect.
7. Determine the population of clams greater than 2 inches and less than 2 inches within the sample
area.
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 584 GRADES 9 - 12
Technique 1: Sampling
A technique called sampling is sometimes used to estimate population size. In this procedure, the
organisms in a few small areas are counted and projected to the entire area. For instance, if a
biologist counts 10 squirrels living in a 200 square foot area, she could predict that there are
100 squirrels living in a 2000 square foot area.
8. Project the amount of clams greater than 2 inches and less than 2 inches living in the Spit as a
whole. Use the above example to help you determine this. Remember to find the total surface
area of the spit before you do this.
9. Use these numbers to predict the overall health of the spit and make a decision as to whether
the spit should be opened for recreational shell fishing.
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 585 GRADES 9 - 12
MARINE SCIENCE
EFFECTIVE CRITICAL THINKING STRATEGIES
IN WRITING LAB REPORTS
B
Exceeds Expectations
The student understands not only the objective but also the
implications of assignments. S/he writes in a variety of modes, with a
clear hypothesis. Supporting data are well discussed and organized,
showing both analysis and synthesis of ideas into a conclusion.
Meets Expectations
The student understands the objective of assignments and selects an
appropriate mode of written expression with a hypothesis.
Supporting data show an understanding of the subject matter and an
analysis or discussion of ideas. The conclusions are somewhat
developed and organized.
Meets Some Expectations
The student requires some additional explanations and models in
order to understand the objective of assignments or to complete the
writing process. With direction, s/he selects an appropriate mode.
Writing has a somewhat limited focus or hypothesis, and data
discussed may be inaccurate, simplistic, and/or confused. The
student may require assistance to develop or organize his/her
conclusion.
Does Not Meet Expectations
The student misinterprets significant elements of writing
assignments, selecting an inappropriate mode or using it
incorrectly. The student requires many additional
explanations, models, graphic organizers, and/or strategies in order
to complete parts of the writing process. The writing has no clear
focus or a very limited hypothesis. data are often unorganized or
inaccurate. Inaccurate or limited discussion and conclusion.
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 586 GRADES 9 - 12
MARINE SCIENCE
EFFECTIVE CRITICAL THINKING STRATEGIES
INUSING TECHNOLOGY TO MANUFACTURE A
FISHING ROD OR BUILD A BOAT
2. Uses technology effectively and responsibly.
Exceeds Expectations
The student can independently select and use appropriate
technology to solve problems efficiently and creatively.
Meets Expectations
The student can select and use appropriate technology to solve
problems effectively without making significant errors.
Meets Some Expectations
The student can select and use appropriate technology to solve
problems but makes some errors and requires some assistance.
Does Not Meet Expectations
The student cannot select and use appropriate technology to solve
problems without making many significant errors and requiring
supervision.
NAME: DATE: PERIOD:
SCIENCE CURRICULUM 587 GRADES 9 - 12
Marine Science and Technology
Project: Fishing Rod
Grade
Category
Grips
Reel Seat
Spine
Guide
Placement
Wraps
Finish
Behavior/
Cleanup
Participation
0
Not assembled.
Not installed.
Not completed.
No Guides.
None
completed.
Not applied.
Rude disrespectful.
Frequently absent.
1 POOR
Grips are sloppy,
cracked and/or
incomplete. Glue
not applied or
messy.
Measurements
incorrect.
Seat not installed
and/or glues not
applied or messy.
Bushings not
constructed
correctly.
Rod not
finished
enough to
evaluate spine
placement.
Rod not
completed
enough to
evaluate guide
placement or
guides are
missing.
Rod not
assembled
enough to
evaluate quality
of the wraps.
The finish is
applied bumpy with
untidy edges and
with bubbles.
There are frayed
ends and excess
epoxy on guides or
blank
Student behavior is
poor and he/she
leaves area messy
at the end of the
period. No
cleanup tasks
completed.
Participation is
very little in
project or
activities. Cuts or
leaves class early
or is continuously
tardy.
2 WEAK
Grips are sloppy
but complete.
Glue applied
messy or not
cleaned. Gaps
present and/or
measurements
may be incorrect.
Seat not aligned
with spine and
glue is messy.
Bushings
constructed
poorly.
The rod was
built with the
spine in the
wrong
location.
Guides are
missing with the
existing guides
placed in the
wrong location.
Wraps are not
placed evenly,
finished off or
stacked
correctly. Most
are missing or
incomplete.
The finish is
applied unevenly
most edges are
sloppy. There are
some bubbles or
excess epoxy on
guides or blank.
Student behavior is
weak and he/she
does very little
during cleanup.
Participation is
weak, sometimes
tardy to class. No
cuts.
3 AVERAGE
Grips are complete
with a few epoxy
marks visible.
Measurements
correct.
Seat is somewhat
aligned with
spine but glue is
messy.
The rod was
built with the
spine close to
the correct
location.
All guides are
present but some
may be in the
wrong location or
not aligned to
spine.
Most wraps are
placed evenly,
and finished off.
Some gaps
present in
finished product.
The finish is
applied evenly
some edges are
sloppy. There are
no bubbles or
excess epoxy on
guides or blank.
Student behavior is
average and he/she
does help clean at
the end of the
period.
Average
participation in
activity and
project. No cuts or
tardies.
4
PROFICIENT
All components of
grip assembly
measured and put
together correctly.
Epoxy is applied
appropriately and
cleaned with
denatured alcohol.
Seat is aligned
with spine,
bushings were
well constructed
and glue has
been cleaned up
with denatured
alcohol.
The rod was
built correctly
with the reel
seat and guides
aligned with
the spine.
All guides are
placed correctly
according to
measurements,
aligned and
secured
correctly.
All wraps are
placed evenly,
measured for
symmetry and
stacked
correctly. No
gaps present in
finished product
The finish is
applied evenly with
neat edges and
without bubbles.
There are no frayed
ends or excess
epoxy on guides or
blank.
Student behavior is
excellent and
he/she goes above
and beyond to help
cleanup at the end
of the period.
Always engaged
during projects.
Does all
assignment and
more. Present
and on time for all
classes.
TOTAL
TOTAL:____________________________
NAME: DATE: PERIOD:
SCIENCE CURRICULUM 588 GRADES 9 - 12
Boat Building
Project: Boat __________________________________________________________
Grade
Category
Layout
Cutting
Glue
Fastener
Placement
Rails
Finish
Behavior/Cleanup
Participation
0
No layout
completed.
Not completed.
None used.
None used.
Not completed.
Not ready to
begin finishing
process.
Rude disrespectful.
Not present.
1 POOR
Components of
boat not laid out
correctly or
symmetrically
No components
of boat cut out
correctly or
symmetrically.
All glue joints
are sloppy and
much excess
glue is present
or gaps are
present in glue
joints.
All fasteners
randomly
placed. Most are
not
countersunk.
Rails not finished
or installed.
Some work has
been started to
prepare finish.
Student behavior is
poor and he/she
leaves area messy
at the end of the
period. No cleanup
tasks completed.
Participation is
very little in
project or
activities. Cuts or
leaves class early
or is continuously
tardy.
2 WEAK
Components of
boat laid out but
not symmetrically.
Weak grasp of
measuring.
Few components
of boat cut out
correctly or
symmetrically.
Many mistakes
made.
Most glue
joints are
messy with
excess glue
present.
Some fasteners
randomly
placed. Most
are not
countersunk.
Rails are almost
completed with
mistakes or
poorly placed
fasteners.
Work has been
started to prepare
finish.
Student behavior is
weak, does not stay
on task. He/she does
very little during
cleanup.
Participation is
weak, sometimes
tardy to class. No
cuts. Does not
participate in class
work.
3 AVERAGE
Most
components of
boat laid out
correctly and
symmetrically.
Good grasp of
measuring.
Some
components of
boat cut out
correctly and
symmetrically.
Few mistakes
made.
Most glue
joints are clean
and clear of any
excess glue.
Most fasteners
placed with
measurement.
Some are
countersunk.
Rails are
completed with a
few poorly placed
fasteners, planed
and sanded.
Entire project
was sanded and
ready for finish.
Student behavior is
average, stays on
task and he/she
does help clean at
the end of the
period most of the
time.
Average
participation in
activity and
project. No cuts or
tardies.
4 PROFICIENT
All components
of boat laid out
correctly and
symmetrically.
Strong grasp of
measuring.
All components
of boat cut out
correctly and
symmetrically.
No mistake
made.
All glue joints
are clean and
clear of any
excess glue.
All fasteners
placed with
precise
measurement
and care. All are
countersunk.
Rails are
completed and
planed, sanded
with no mistakes
or poorly placed
fasteners.
The finish is
applied correctly
and complete.
Student behavior is
excellent, makes
good use of class
time and he/she
goes above and
beyond to help
cleanup at the end
of the period.
Always engaged
during projects.
Does all
assignment and
more. Present and
on time for all
classes.
TOTAL
TOTAL:_______________________________________________
MARINE SCIENCE AND TECHNOLOGY
SCIENCE CURRICULUM 589 GRADES 9 - 12
Marine Science and Technology
Portfolio Evaluation Spring
Name:______________________________ Period: _____ Due Date: _______________
Portfolio Evaluation Sheet
Student Teacher
Ships Logs ______ ______
-Do you have all ships logs from the entire year?
-Are they neat and complete?
Field Trip Pictures ______ ______
-Do you haw at least 12 pictures of activities we have done in MST?
-Do they have appropriate captions regarding picture?
-Captions must reflect what is happening in the photo and must be typed.
#1 Boat Building Portfolio Reflection ______ ______
- Reflect on your boat building project.
- Did you use the 5 paragraph format? Is it typed and complete?
#2 Madison Shell Fishing Portfolio Reflection ______ ______
- Reflect on your contribution to Madison's Shell Fish Commission.
- Did you use the 5 paragraph format? Is it typed and complete?
#3 Long Island Sound Ecosystem Portfolio Reflection ______ ______
-Reflect on all of the different ecosystems studied and found in Long Island Sound.
- Did you use the 5 paragraph format? Is it typed and complete?
#4 Portfolio Reflection ______ ______
-Reflect on your activities?
-Did you use the 5 paragraph format? Is it typed and complete?
Class Notes Section ______ ______
-Coastal Ecosystems, LIS Geology, CT Geology, Marine History
-Classification - Marine Animals- Porifera - Cnidarians - Simple Worms
-Study Guides, Handouts, Work sheets
Participation ______ ______
-Did you actively participate in all class activities?
-Any class cuts?
-All Papers and Lab reports turned in and complete?
-Are all of you Ships Logs included in the portfolio?
Safety / Clean up / Behavior ______ ______
-Shop Projects, Fish tank maintenance, Field Trips
Portfolio Presentation _____ ______
-Is your portfolio neat and presentable?
-Are all components included and handed in on time? ______________
Total ___________
0 = Work is not done
1 – 3 = Work is complete but substantially below standard.
4 - 6 = Work is complete but all or part is below standard.
7 - 8 = Work meets the expectations of standard performance.
9 – 10 = Work achieves and exceeds the expectations of high-standard performance.
Program Implementation:
Guidelines & Strategies
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 593
Instructional Delivery
Instruction in science is inquiry-based. Inquiry-based instruction engages students in designing and
performing investigations to answer real world problems. During inquiry, the teacher acts as a facilitator
to student activities. Students develop the testable question, collect data, analyze the results, and
communicate the findings. As a result, connections are made between science concepts learned in the
classroom and their application to the world in which students live. In addition, student interests are
taken into account allowing some student ownership in what activities are performed in class.
Students, however, must learn basic skills needed to do inquiry. These include: the ability to identify
questions and science concepts that guide investigations; the development of good experimental design;
the use of appropriate tools and procedures for gathering data and analyzing and interpreting results; the
proper use of mathematics; the development of explanations and descriptions based on evidence; and
the communication of scientific procedures and explanations. In addition, students must learn to analyze
the validity of data and address validity in their experimental design. These skills must be systematically
taught through activities that are developmentally appropriate for students starting with simple activities,
building to guided investigations, and resulting in inquiry experiences.
This systemic instruction of inquiry skills involves a carefully planned program, delivery that implements
the objectives to be learned, and selection and sequence of the essential skills and strategies that are
necessary to achieving those objectives. It also involves the application of the skills to investigations that
enrich and enhance the learning of science content leading to a greater understanding of the content and
the ability to make connections between concepts and the real world. In implementing systematic
instruction, teachers:
Allocate sufficient time to for students to develop essential skills.
Organize information to minimize confusion that learners may experience.
Introduce new information in manageable and sequential segments.
Build on prior knowledge of the learner.
Review previously taught skills and content.
Integrate old knowledge strategically with new knowledge.
Progress from skills in more easily managed contexts to more complex contexts.
Include modifications, as necessary, for students who have special needs.
Inquiry-based instruction is demonstrated in science laboratory work. Laboratory experiences provide
opportunities for students to interact directly with natural phenomena or with data collected using tools,
materials, data collection techniques, models (including computer simulations and virtual labs) and the
theories of science. These hands-on experiences are best suited to occur in a laboratory environment
and/or field settings. At least 40% of instructional time is focused on or related to laboratory experiences.
Science classes include laboratory experiences and reflect the processes of scientific inquiry. Students are
provided multiple opportunities to connect the concepts with the techniques learned through laboratory
work to construct new knowledge/understanding. Laboratory skills and processes should be part of the
curriculum documents and instructed, modeled, practiced and assessed for understanding.
Instruction Requirements
In effective science teaching:
Teachers have and continually expand their content knowledge of science as well as their knowledge
of teaching and learning.
Teachers are able to select research-based instructional strategies that are appropriate to the
instructional goals and to students' needs.
Teachers effectively organize instruction around goals that are tied to the standards and direct
students' scientific learning.
Teachers use the results of assessment of both student learning and their own teaching to guide
instruction.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 594
Teachers build relationships with students that are based in their knowledge of how their students
think and learn.
Teachers are active as members of science-learning, collaborative communities and use this to
enhance their teaching.
Instructional Time
Priority must be given to the teaching of science, and instructional time must be protected from
interruptions.
Adequate time is allocated to science. Secondary students receive from 53 to 70 minutes of science
instruction, not including homework each school day. Elementary students receive 60 minutes of
science instruction on alternating school days. Additional instructional time is allocated for students
who are, for whatever reason, performing substantially below grade level in science. All students
need to take science courses throughout high school.
Learning time is extended through homework that increases in complexity and duration as students
mature. Homework should be valued and reviewed. The purpose of homework is to reinforce
concepts, practice skills previously taught or to have students apply their previously learned
knowledge and skills to new problems. It should be assigned in amounts that are grade-level
appropriate and, at least in the early grades, it should focus on independent practice and the
application of skills already taught. For more advanced students, homework may be used as a means
for exploring new concepts.
During the great majority of allocated time, students are active participants in the instruction. Active
can be described as the time during which students are engaged in thinking about science or doing
science.
Instructional time for science is maximized and protected from such interruptions as calls to the
office, public address announcements, and extracurricular activities.
Instructional Technology
Technology enhances the science program at Daniel Hand High School. Each of the classrooms used for
science instruction has an LCD projector, sound system and a computer from which CDs can be played.
Teachers can easily display PowerPoint presentations and access Internet sites.
All science courses include the use of appropriate tools for collecting and recording data. Students
perform activities and investigations that require the use of basic laboratory ware as well as microscopes,
probes, meters, and calculators. Specific courses may require the use of specialized equipment such as
gas pressure gauges, accelerometers, or water quality testing materials. Additionally, the installation of
SMARTBOARDS in some classes has proved not only innovative to teachers’ presentations, but also
productive for student use and reference to notes given earlier in class.
Technology is also used as an instructional tool at the middle school level. Each of the classrooms used
for science at Walter C. Polson Middle School have a TV monitor and a computer from which CDs can be
played. Three video flex-cams, several digital microscopes, a Proscope, and a few CBL2s with graphing
calculators have been purchased for classroom use. In addition, there are two computer labs that
teachers can reserve as well as two portable laptop labs. LCD projectors and SMARTBOARDS have been
purchased and are in use as well. At the Dr. Robert H. Brown Middle School, teachers have computer
work stations in the classrooms and laptops carts for classroom use. They may also reserve time in the
two computer labs in the building. LCD projectors and SMARTBOARDS have been purchased and are in
use as well. In addition, there is a projection microscope, several digital microscopes and a Proscope for
use as instructional tools.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 595
At the secondary school level, teachers have created their own web pages on finalsite, and they access
websites, research tools such as SIRS, and ICON so that students have real world experiences. The State
of Connecticut is providing access to Discovery Education Science for the middle schools. Science
teachers at both middle schools have been trained to access the video clips, virtual labs, lessons, and
other resources this site has to offer.
At the elementary schools, teachers have computer work stations in the classrooms. Teachers may also
reserve time in the computer lab in the building, in which SMARTBOARDS have been installed and are in
use as well. Students are learning to use the Internet with guided web browsing, spreadsheets, word
processing, multimedia presentations, CDs, and DVDs for scientific inquiry.
Student Support
Some students need less time and some students need more time to be able to demonstrate mastery or
proficiency for any lesson, unit, course, year or program. One way that time becomes a variable used to
better meet individual student needs is through the provision of more advanced or remedial /
compensatory instruction for those students for whom traditional time allocations are not appropriate.
Students can be supported by:
Teachers who use a variety of strategies and instructional materials and who differentiate
instruction to meet individual student needs;
Support staff who assist students with special needs; and
Before and after school assistance and reviews for all students.
To provide student support within the classroom teachers can make use of the principles of backward
design and differentiated instruction by:
1. Identify desired learning results for the subject and topics they teach.
Determine what students should know, understand, and be able to do as a result of the study.
Specify big ideas worthy of understanding.
Delineate enduring understandings on which the teacher and students will focus.
State provocative, essential questions that will guide students' exploration of the big ideas.
Articulate specific knowledge and skill that students will need for effective performance on the goals.
2. Determine acceptable evidence of student learning.
Decide what evidence will indicate that students understand the big ideas.
Consider what performances will indicate that the learners understand and can apply what they have
learned, and by what criteria those performances will be evaluated.
Determine what will constitute evidence of student proficiency with the essential knowledge,
understanding, and skill.
3. Plan learning experiences and instruction based on the first two principles.
Decide what essential knowledge, understanding, and skills need to be taught and coached.
Determine how knowledge and skills should best be taught in light of the content goals.
Plan ahead to ensure that learning is engaging and effective in the context of specified goals and
needed evidence.
4. Regard learner differences as inevitable, important, and valuable in teaching and
learning.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 596
Persist in developing greater understanding of each student’s readiness to succeed with designated
content goals to enhance individual academic growth, interests that might connect with content goals
to enhance motivation, and preferred modes of learning.
Work with students, family, and school personnel to understand and address learners' backgrounds
and experiences, including gender, culture, language, race, and personal strengths, and to address
those factors in teaching and learning plans.
5. Address learners' affective needs as a means of supporting student success.
Respond actively to students' need for affirmation, contribution, power, purpose, and challenge.
Understand and respond to the reality that these needs are met in different ways for different
students.
Understand and respond to the reality that a student's motivation to learn is tethered to a sense of
affirmation, safety, and success.
6. Periodically review and articulate clear learning goals that specify what students should
know, understand, and be able to do as a result of each segment of learning.
Ensure that each student has full access to essential knowledge, understanding, and skills in each
segment of study.
Ensure that tasks and assessments focus on essential knowledge, understanding, and skills designated
in a segment of study.
Ensure that all students reason and work at high levels.
Ensure that all students have equally engaging and equally interesting tasks.
7. Use systematic pre-assessment and ongoing assessment aligned with designated goals
to make instructional decisions and adaptations.
Provide opportunities for students to build requisite competencies when assessment results indicate a
student lacks precursor knowledge, understanding, or skill necessary for success with designated
content goals.
Provide opportunities for additional instruction, coaching, or practice when assessment results indicate
such needs for a student or group of students.
Provide opportunities to advance or extend knowledge when assessment results indicate that a student
or group of students has achieved mastery of designated content goals.
8. Employ flexibility in instructional planning and classroom routines to support success for
each learner.
Use space, time, materials, student groupings, and modes of exploring and expressing learning flexibly
to maximize the opportunity for success for a full range of learners when students work with tasks and
assessments.
Use multiple modes of presentation, illustrations linked to a wide range of cultures and experiences,
and various support systems to maximize the opportunity for a full range of learner success when
students work with tasks and assessments.
Encourage each student to work at a level of complexity or degree of difficulty that is challenging for
that student, and provide scaffolding necessary for each student to succeed at the new level of
challenge.
9. Gather evidence of student learning in a variety of formats.
Provide varied options for demonstrating what students know, understand, and can do.
Ensure that students know what "success" looks like in their work including both nonnegotiable class
requirements and student- or teacher-specified goals for individuals.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 597
Together, backward design and differentiation describe a comprehensive way of thinking about
curriculum, assessment, and instruction, stemming from a shared understanding of what constitutes
effective teaching and learning.
Professional Growth
Program Coordination
The effectiveness of the science program depends critically on the assignment of responsibility for
program coordination to the program coordinators at the middle school and high school levels and the
designated science liaisons at the elementary level. The program requires that (1) a vision be nurtured
and advocated; (2) teachers be kept abreast of changes and professional development opportunities; and
(3) curricular, instructional and assessment improvement be treated as ongoing processes.
Common means for increasing the articulation and coordination of the program are professional
development workshops, science assessment program, department meetings of two or more consecutive
grades and meetings of teachers teaching the same course or grade level. These meetings can facilitate
teachers discussing student work, assessment results, concerns and problems so that the necessary
adjustments curriculum, instruction and assessment can be made.
Professional Development
In order to implement fully any curricular/technology changes, it is essential to provide professional
development opportunities for science teachers. Training workshops and time to meet with colleagues
enable teachers to learn and adapt research based methods of teaching and learning and instructional
technology. Continued study maintains and improves the teachers’ level of proficiency, while participation
in state, regional and national science organizations provides an opportunity for exposure to the most
recent developments and studies.
The goals of professional development should be to provide classroom teachers with the knowledge and
skills they will need to implement the science content standards.
Professional development needs to provide teachers with a clear understanding of standards-based
science expectations. Students need to know the goals and uses of the science they are taught, and
teachers need to understand the basic goals of the standards and the importance of achieving those
goals.
Teachers need to understand science inquiry and how to offer opportunities for their students to develop
inquiry skills through inquiry-based instruction that is developmentally appropriate.
Teachers need to understand how the grade-level content they are teaching is related to the content
taught in previous grades and how their teaching will prepare students for the science to be introduced in
later grades.
Well designed instructional materials will greatly facilitate this goal. At the same time, in-service training
or other activities will also be needed to show teachers how their teaching is an integral part of all grade-
level standards and how they can develop strategies for linking their teaching to material for earlier and
later grades.
Phase-in strategies for new curricula must be considered carefully. To maintain momentum, teachers
should be provided the necessary support to implement new programs consistently and according to a
given timeline.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 598
Every student of science deserves to be taught by a teacher who has both the science content knowledge
and teaching skills needed to implement the standards at each student's achievement level. The teacher
must present science in ways that actively engage students in the learning of science and help them to
apply it to the world around them.
Teachers need support in developing a repertoire of effective teaching strategies that allow them to
implement a curriculum that balances problem solving, conceptual understanding and inquiry skills.
Teachers need a background in science that is considerably deeper and broader than the science they are
expected to teach. Teachers at earlier grade levels need this background to understand how their
teaching relates to the science content in later grades. Teachers at each grade level need to understand
what students will encounter in subsequent grades, because teachers will then know which foundational
skills taught at their grade level deserve the greatest attention and emphasis. To achieve this
understanding, teachers need to acquire broad science content knowledge that enables them to
comprehend the interrelationships of science concepts and inquiry skills across strands.
Professional Supervision and Evaluation
Professional evaluation that supports the science program needs to reflect the requirements of an
inquiry-based program. Administrators and the program coordinators are trained and knowledgeable
about effective science curriculum, instruction and assessment based on standards. In addition,
professional evaluation includes the preparation of an individual professional development plan,
observations, conferences, reflections and reports.
Implementation
The implementation section of this curriculum guide will be dynamic. As the teachers use the guide, they
will add learning activities and performances which are aligned or illustrative of expected outcomes /
common assessments previously agreed upon during the curriculum development process. The learning
activities and performances that will result from implementation will ensure that the curriculum is
enhanced or elaborated upon. The submitted activities and performances will become part of the school
district’s curriculum guide as suggested strategies and/or references for teachers and learners.
Program Monitoring
and Evaluation
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 601
Program Monitoring
The purpose of program monitoring is to determine what is working, what is not working, what
needs changing, and what's worth celebrating. Monitoring is a process used to determine if the
planned curriculum, instruction and assessment of the renewed / revised program is user friendly
and productive.
Monitoring in this sense is designed to examine the curriculum (design), the instruction (delivery)
and the impact on student learning (results). Monitoring of a program is concerned with the total
picture; i.e., the relationships between the teaching, the curriculum and the assessment of
student performance.
Monitoring activities begin with a review of student progress conducted at the teacher level.
Specifically, student participation in learning activities and skill development are reviewed by the
teachers in order to draw conclusions on the effectiveness of the curriculum and the
programmatic changes. In addition to student responsiveness, teachers can examine formal
assessment measures such as unit tests, common performance assessments, final examinations,
University of Connecticut ECE examinations, Generation 4 CMT Science results and Generation 3
CAPT Science results.
To measure the effectiveness of the program at schools, teachers and principals share their
observations of the program's impact on student learning. Not only will school staff examine
demonstrations of students' competencies, but also observations of safe practices and productive
use of equipment, materials and time to explore the program’s effectiveness on student learning
as it pertains to their school or a specific grade level.
To determine the overall effectiveness of a program from a K-12 perspective, information is
compiled from horizontal comparisons (grade spans across schools) and vertical (K-12) curricular
mapping. Appropriate data from many sources is considered.
Program Evaluation
Program evaluation is necessary to identify program merit and to systematically improve the
procedures of planning, implementing and evaluating instruction. Program evaluation addresses
three basic questions:
1. Is the program producing the desired results?
2. Is the program being implemented as intended?
3. Is the program plan appropriate?
Is the program producing the desire results?
This question is concerned with student outcomes. Are the students learning the objectives
targeted for each grade level? Are the students being graduated from the program having
achieved the program goals? The question informs the 12-K (backward) design and the K-12
articulation.
Is the program being implemented as intended?
This question focuses on whether the teachers are implementing the program with fidelity as
defined in the curriculum and their subsequent teaching and learning maps. Common questions
would be: Are the appropriate objectives being taught? Is enough time being spent on the
objectives? Are appropriate instructional techniques and activities being used? Are the students
being provided with sufficient practice and feedback? Program implementation evaluation can be
performed by the grade level or at the school level. The purpose is to identify problems that are
shared by several teachers with the intent of providing some form of support or assistance such
as a staff development workshop.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 602
Is the program plan appropriate?
This question is designed to look at the big picture and addresses questions such as: Should the
number of objectives in the program be increased or decreased? Should the sequence of
placement of any of the objectives be changed? Should the program objectives be revised? The
evaluation of the program plan is typically done by the entire staff collectively. Periodic evaluation
of the program plan is the process that gradually shapes and refines the program over time so
that it achieves its stated goals.
Program evaluation facilitates the dynamic evolution of a functional science education curriculum.
Program evaluation is designed to be a positive and proactive process that will ensure that
students achieve the targeted objectives and goals of the program and that instruction is
effective and efficient.
Resources

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 605
SAFETY
Teaching science through inquiry requires students to be engaged in hands-on work. Students must be
involved in their learning by developing questions and designing ways to test a hypothesis. Younger
students need the time to explore in a multi-sensory manner.
With less use of lecture and more use of investigation comes an added concern for safety. Safety rules
need to be written and discussed with all students prior to any activity insuring that all students are aware
of the rules and that they understand them. A Safety Acknowledgement Document should be sent home
for students and/or parents to sign.
It is required by law ConnOSHA safety standards that schools develop a chemical hygiene plan and
appoint a chemical hygiene officer for formal academic laboratories. For non-formal laboratory work in
elementary science, ConnOSHA requires a written Hazard Communication Program. Teachers must be
informed of the plan for their school and adhere to it. The chemical hygiene plan must be reviewed yearly
and the teachers should receive a safety update each school year.
Connecticut State Department of Education offers these science safety manuals:
High School - http://www.sde.ct.gov/sde/lib/sde/pdf/curriculum/science/safety/science_safety.pdf
Middle School - http://www.sde.ct.gov/sde/lib/sde/pdf/curriculum/science/safety/middleschool_sciencesafety.pdf
Elementary School - http://www.sde.ct.gov/sde/lib/sde/pdf/curriculum/science/safety/scisaf_cal.pdf
Professional best professional practices are also an expectation. Teachers should review safety position
statements at NSTA.org to learn more about best practices.
There are a number of safety resources that should be available to teachers and all should become
familiar with them. These include:
Print Resources
Kwan, Terry and J. Texley. 2003.
Inquiring Safely: A Guide for Middle School Teachers.
Arlington, VA: NSTA Press.
Kwan, Terry and J. Texley. 2002.
Exploring Safely: A Guide for Elementary Teachers.
Arlington,
VA: NSTA Press.
Roy, Kenneth Russell. 2007.
The NSTA Ready Reference Guide to Safer Science
. Arlington, VA:
NSTA Press.
Ryan, Kelly. 2001.
Science Classroom Safety and Law
. Batavia, IL: Flinn Scientific, Inc.
Texley, Juliana, T. Kwan and J. Summers. 2004.
Investigating Safely: A Guide for High School
Teachers.
Arlington, VA: NSTA Press.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 606

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 607
MADISON PUBLIC SCHOOLS
SCIENCE DEPARTMENT
LABORATORY PROCEDURES
1. The science laboratory can provide you with exciting opportunities while learning and doing
science. At all times the laboratory is a place for serious work. Fooling around or disruptive
behavior will result in removal from the laboratory.
2. Always prepare for an experiment by reading the directions in the manual before you come
to the laboratory. Follow the directions carefully and intelligently, noting all precautions.
NOTE THE MSDS AND NFPA PRECAUTIONS FOR EACH CHEMICAL. Do not add to, omit, or
change any of the directions unless your teacher instructs you to do so.
3. Know the location of the Chemical Safety Policy and the MSDS. The following MSDS
information MUST be shared with students by the teacher and posted for direct access by
students:
specific handling precautions
hazard identification
first aid measures
health hazards
personal protection
stability/reactivity
disposal techniques
other pertinent information (from MSDS) for each chemical.
4. Do only the experiments assigned and/or approved by your teacher. Unauthorized
experimentation is prohibited.
5. Read the label of all containers to be sure of the contents and information provided by the
MSDS and NFPA code. DO NOT USE ANY CHEMICALS STORED IN UNLABELED BOTTLES!
6. All solids and paper to be discarded must be placed in the chemical waste jar or other
location directed by the teacher. Discard chemical waste as per MSDS and NFPA
instructions. Follow directions for recycling products of your experiments per directions
from your teacher.
7. Never discard matches, filter paper, or any other slightly soluble solids in the sink.
8. Know the location of the eye wash, hood, blanket station, and chemical spill cart, as well as
the laboratory evacuation exit procedure. Sketch a diagram the lab area noting the
locations of all safety equipment, exits, fire alarms, etc. and keep it handy. Note the
location of the Chemical Safety Plan and MSDS envelope for experiments.
9. When working with corrosive materials, goggles, gloves, and lab aprons must be worn
throughout the lab period until ALL your classmates have completed the lab and the
chemicals are safely stored.
10. DO NOT TOUCH CHEMICALS WITH YOUR HANDS!
11. If acid or another corrosive chemical is spilled, wash with water for at least 15 minutes.
Notify your teacher immediately.
12. NEVER taste a chemical solution.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 608
13. No food (including candy or gum) or drink is allowed in the laboratory.
14. When observing the odor of a substance, do not hold your face directly over the container.
Fan a little of the vapor toward you by sweeping your hand over the top of the container.
15. Allow ample time for hot glass to cool. Remember that hot glass looks like cool glass.
16. REPORT ANY ACCIDENT, EVEN A MINOR INJURY, TO YOUR TEACHER!
17. Long hair must be tied back securely!
18. NEVER RETURN UNUSED CHEMICALS TO THE STOCK BOTTLES. Do not put any
object into a reagent bottle except the dropper with which it may be equipped.
19. Keep your apparatus and work area organized. Avoid spillage. If you do spill something,
clean it up immediately using proper technique. Put your own equipment into your drawer
and/or return any special apparatus to its proper place at the end of the period.
20. During clean-up time, attend to your assigned area duties. All duties must be completed
before leaving the laboratory. Wash hands thoroughly with soap at the conclusion of each
lab.
21. Respect your equipment and fellow laboratory workers.
22. Handle all spring-loaded and projectile devices with extreme caution to prevent accidental
release or discharge.*
23. Back packs and book bags must be stored under your table or on your chair out of the
aisles to accommodate proper egress from the lab/classroom.
24. STUDENTS ARE NOT TO WORK IN A LABORATORY UNLESS AN TEACHER IS
PRESENT. All student experiments are to be done under the direct supervision of an
teacher, including students completing science projects, science fair projects, etc.
25. OPEN TOE SHOES/SANDALS AND LOOSE FITTING CLOTHING OR JEWELRY ARE
NOT PERMITTED DURING SPECIFICALLY DESIGNATED LABORATORY
ACTIVITIES. Your teacher will notify you in advance of the activity.
*26. Science department regulation states that safety goggles (flexible plastic with ventilating
ports for chemical splash and glass breakage standard) must be worn by all students,
teachers and visitors in the laboratory during work periods INCLUDING CLEAN-UP TIME in
accordance with:
Science Department Policy and CT State Statute:
“Any person who is working, teaching, observing, supervising, assisting, or engaging in any
work, activity, or study in a public or private elementary or secondary school laboratory or
workshop where the process used tends to damage the eyes or where protective devices can
reduce the risk of injury to the eyes concomitant with such activity shall wear an eye protective
device of industrial quality in the manner in which such device was intended to be worn.”
27. In order to maintain a safe working environment, teachers are required by the school’s
safety compliance officer to remove from the classroom, any student out of compliance.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 609
SAFETY ACKNOWLEDGEMENT FORM
(Return this page to your science teacher after completing the required information
below.)
I have read and understood the Madison Public Schools laboratory procedures and have
been present when they were discussed in class or discussed directly with my science
teacher. I acknowledge the fact that the Science laboratory can have hazards which
potentially could make it an unsafe place to work. It is critical that I follow the attached
laboratory procedures to help make it a safer place to work and learn.
TO THE STUDENT:
Print Student Name: _____________________________________________________________
Student Signature: _____________________________ Date: ____________________________
Course: ______________________________ Teacher: ________________________________
Yes, I wear contact lenses.
TO THE PARENT/GUARDIAN:
I have read and discussed these safety rules with my child. I am confident that he/she
understands these safety rules.
Parent Signature: _____________________________ Date _____________________________
Description of my child’s allergies/sensitivities, if any:
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 610
Material Safety Data Sheets (MSDS)
Overview
A Material Safety Data Sheet (MSDS) is required under the OSHA Hazard Communication
Standard. The MSDS is a detailed informational document prepared by the manufacturer or
importer of a hazardous chemical. It describes the physical and chemical properties of the
product. MSDS’s contain useful information such as flash point, toxicity, procedures for spills and
leaks, and storage guidelines.
Information included in a Material Safety Data Sheet aids in the selection of safe products, helps
staff members understand the potential health and physical hazards of a chemical and describes
how to respond effectively to exposure situations.
A MSDS may be useful but it can not substitute for prudent practices and comprehensive risk
management. They are required as a part of any compliance obligation to be available and
displayed prominently in the school science rooms/labs.
In the United States, Material Safety Data Sheets must be written in English and contain:
the name of the chemical (same as on the label)
the chemical and common names of the substance
a listing of the ingredients
a statement of the ingredients that are known carcinogens or that present other known
hazards
In general, if a school purchases hazardous chemicals, a MSDS from the manufacture is also
provided. It is the plan for the Madison Public Schools to have a specific area and container to
house the MSDS information as labs / lessons are being taught in addition to having a notebook
containing all MSDS information.
OSHA recommends that MSDSs follow the 16-section format established by the American
National Standards Institute (ANSI) standard for preparation of MSDSs.
By following this recommended format, the information of greatest concern to students and
workers is featured at the beginning of the data sheet, including information on chemical
composition and first aid measures. More technical information that addresses topics such as the
physical and chemical properties of the material and toxicological data appears later in the
document. While some of this information (such as ecological information) is not required by the
HCS, the 16-section MSDS is becoming the international norm. The 16 sections are:
1. Identification
2. Hazard(s) identification
3. Composition/information on ingredients
4. First-aid measures
5. Fire-fighting measures
6. Accidental release measures
7. Handling and storage
8. Exposure controls/personal protection
9. Physical and chemical properties
10. Stability and reactivity
11. Toxicological information
12. Ecological information
13. Disposal considerations
14. Transport information
15. Regulatory information
16. Other information

SAMPLE MATERIAL SAFETY DATA SHEET (MSDS)
© 2003 Flinn Scientific, Inc. All Rights Reserved. Revision Date: February 24, 2003 Page 1 of 2
FLINN SCIENTIFIC INC.
"Your Safer Source for Science Supplies" MSDS #: 47.00
Material Safety Data Sheet (MSDS)
Section 1 — Chemical Product and Company Identification
Ammonia Gas
Flinn Scientific, Inc. P.O. Box 219 Batavia, IL 60510 (800) 452-1261
CHEMTREC Emergency Phone Number: (800) 424-9300
Section 2 — Composition, Information on Ingredients
Ammonia, Gas
Synonym: anhydrous ammonia
CAS#: 7664-41-7
Section 3 — Hazards Identification
Colorless gas with a pungent odor.
Moderately toxic by inhalation and ingestion. Severe irritant of eyes, respiratory tract and skin. Corrosive to
eyes.
Can cause breathing problems, chest pain, coughing and even suffocation. Can cause severe skin burns.
Moderate fire risk.
Health-2
Flammability-1
Reactivity-0
Exposure-3
Storage-0
0 is low hazard, 3 is high hazard
Section 4 — First Aid Measures
Call a physician, seek medical attention for further treatment, observation and support after first aid.
Inhalation: Remove to fresh air at once. If breathing has stopped give artificial respiration immediately.
Eye: Immediately flush with fresh water for 15 minutes.
External: Wash continuously with fresh water for 15 minutes.
Internal: Give large quantities of water. Do not induce vomiting. Call a physician or poison control at once.
Section 5 — Fire Fighting Measures
Moderate fire risk. Combustible gas.
UEL: 25% LEL: 15% Autoignition Temperature: 1203 F
Fire Fighting Instructions: Use triclass, dry chemical fire extinguisher. Firefighters should wear PPE and
SCBA with full facepiece operated in positive pressure mode.
NFPA CODE
H-3
F-1
R-0
Section 6 — Accidental Release Measures
Close cylinder at once. Remove all ignition sources and ventilate room. If cylinder is leaking, evacuate the laboratory and call fire department
with necessary breathing equipment.
Section 7 — Handling and Storage
Store with the bottled gases in a secure area.
Use and dispense in a hood.
Section 8 — Exposure Controls, Personal Protection
Avoid contact with eyes, skin and clothing. Wear chemical splash goggles, chemical-resistant gloves and chemical-resistant apron.
Use ventilation to keep airborne concentrations below exposure limits. Always wear a NIOSH-approved respirator with proper cartridges or a
positive pressure, air-supplied respirator when handling this material in emergency situations (spill or fire). Exposure guidelines: TWA 25 ppm,
STEL 35 ppm (NIOSH, ACGIH)
Section 9 — Physical and Chemical Properties
Colorless gas with a pungent odor.
Solubility: Soluble in water, moderately soluble in alcohol.
Formula: NH3
Formula Weight: 17.04
Vapor Pressure:8.75 atm (21 C)
Specific Gravity: Lighter than air.
Relative density (air = 1) : 0.597 at 25 C.
Melting Point: -77.7 C
Boiling Point: -33.35 C
Vapor Density: 0.6
Section 10 — Stability and Reactivity
Avoid contact with strong oxidizers. Will react violently with all acids. Forms explosives compounds in contact with silver or mercury.
Contact with halogens may cause spattering.
Section 11 — Toxicological Information
Acute effects: Toxic, corrosive
Chronic effects: N.A.
Target organs: Respiratory system and eyes
ORL-RAT LD50: 350 mg/kg
IHL-RAT LC50: 2000 ppm/4H
SKN-RBT LD50: N.A.
N.A. = Not available, not all health aspects of this substance have been fully investigated.
SAMPLE MATERIAL SAFETY DATA SHEET (MSDS)
© 2003 Flinn Scientific, Inc. All Rights Reserved. Revision Date: February 24, 2003 Page 1 of 2
Section 12 — Ecological Information
Data not yet available.
Section 13 — Disposal Considerations
Please consult with state and local regulations.
One option is to completely empty cylinder in an operating fume hood or outdoors. Avoid flames and sparks. Dispose of empty cylinder in trash,
if non-refillable. Another option is to bubble the ammonia gas through water, then dispose of the resulting ammonium hydroxide solution using
Flinn Disposal Method #10.
Section 14 — Transport Information
Shipping Name: Ammonia, Anhydrous, Liquefied
Hazard Class: 2.2 Non-flammable gas
UN Number: UN1005
N/A = Not applicable
Section 15 — Regulatory Information
TSCA-listed, EINECS-listed (231-635-3). RCRA D002.
Section 16 — Other Information
Consult your copy of the Flinn Scientific Catalog/Reference Manual for additional information about laboratory chemicals. This Material Safety Data
Sheet (MSDS) is for guidance and is based upon information and tests believed to be reliable. Flinn Scientific Inc. makes no guarantee of the accuracy
or completeness of the data and shall not be liable for any damages relating thereto. The data is offered solely for your consideration, investigation, and
verification. Flinn Scientific Inc. assumes no legal responsibility for use or reliance upon this data.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 613
Web Resources
Connecticut High School Science Safety: Prudent Practices and Regulations
Connecticut State Department of Education -
http://www.sde.ct.gov/sde/cwp/view.asp?a=2618&q=320890&sdenav_gid=1757
Science Safety – Key Issues in Science Laboratory Safety -
http://www.sde.ct.gov/sde/cwp/view.asp?a=2618&q=320890&sdenav_gid=1757
Connecticut High School Science Safety Prudent Practices and Regulations:
http://www.sde.ct.gov/sde/lib/sde/pdf/curriculum/science/safety/science_safety.pdf
Connecticut Middle School Science Safety Prudent Practices and Regulations:
http://www.sde.ct.gov/sde/lib/sde/pdf/curriculum/science/safety/middleschool_sciencesafety.pdf
Science Safety: Making the Connection
Council of State Science Supervisors -
http://www.sde.ct.gov/sde/cwp/view.asp?a=2618&q=320890&sdenav_gid=1757
National Science Education Leadership Association
http://www.nsela.org/publications/publications2.html
National Center for Education Statistics
NAEP Questions Tool
http://nces.ed.gov/nationsreportcard/itmrls
Safety Web Links Recommended by the Connecticut State Department of Education:
MSDS Online www.msdsonline.com
NSTA Lab Science www.nsta.org/handbook/labsci.asp
Flinn Scientific Safety Pages www.flinnsci.com
Environmental Protection Agency www.epa.gov
Centers for Disease Control www.cdc.gov
Occupational Health and Safety Administration www.osha.gov
National Fire Protection Association www.nfpa.org
NSTA best practices professional statements: http://www.nsta.org/about/positions.aspx#list
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 614
Curriculum Improvement Plan Worksheet
Identified strengths/weaknesses in student assessment and curriculum content
Recommendations
Necessary Actions
Persons responsible and completion dates
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 615
TEMPLATE FOR UNIT PLAN
Conceptual Theme
Unit Title:
Content Area Standard(s):
Enduring Understandings:
Essential Questions:
Underlying Concepts / Learning Objectives:
Evidence of Learning
Grade Level or Course Expectations:
Benchmark / Assessment / Rubric
CMT / CAPT Correlation
Learning Plan
Lessons / Activities: Scope and Sequence of Instruction:
Scientific Literacy Terminology
Instructional Resources and Materials
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 616
Works Consulted
Kwan, Terry, and Juliana Texley. Exploring Safely: A Guide for Elementary Teachers. Arlington, VA: NSTA
Press, 2002.
Kwan, Terry, and Juliana Texley. Exploring Safely: A Guide for Middle School Teachers. Arlington, VA:
NSTA Press, 2003.
Lowery, Lawrence F. Editor. Pathways to the Science Standards. Elementary School Edition. Arlington,
VA: NSTA Press, 2000.
Mariconda, Barbara and Dea Paoletta Auray. Write About Science. Trumbull, CT: Empowering Writers,
2009
Motz, LaMoine L., James T. Biehle, and Sandra S. West. Guide to Planning School Science Facilities.
Arlington, VA: NSTA Press, 2007.
National Research Council. National Science Education Standards. Washington, DC: National Academy
Press, 1996.
Rakow, Steven J. Editor. Pathways to the Science Standards. Middle School Edition. Arlington, VA: NSTA
Press, 2000.
Roy, Kenneth Russell. Ready Reference Guide to Safer Science. Arlington, VA: NSTA Press, 2007.
Texley, Juliana, Terry Kwan, and John Summers. Investigating Safely: A Guide for High School Teachers.
Arlington, VA: NSTA Press, 2004.
Texley, Juliana and Ann Wild, Editors. Pathways to the Science Standards. Second High School Edition.
Arlington, VA: NSTA Press, 2004.
__________. Science Framework for California Public Schools. Sacramento, CA: California Department of
Education, 2004.
Appendices
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 619
Scientific Process Skills for Elementary Students
The Big Picture
Scientific processes are tools that are needed to be scientifically literate
Process skills are the vehicles through which you teach content – they are the focus of science as
inquiry, or the thinking that goes along with content
Process skills are consistent across all science content areas although they are introduced and applied
as is developmentally appropriate
Although there are great resources on teaching Scientific Process Skills as an isolated unit, it is
imperative that they are included in every other unit of study as well.
Background Information
The scientific process skills are the thinking skills applied in all subject areas
The scientific process skills include but are not limited to:
Observing – the use of the five senses to gather data about objects and events. Example:
Describing a pencil as yellow.
Communicating – the use of the spoken and written words, graphs, drawings and diagrams to
share information and ideas with others. Example: Describing the change in height of a plant over
time in writing or through a graph.
Comparing – the use of observations to ascertain similarities and differences in objects and events.
Example: Placing drops of water onto different types of soil and comparing the absorption properties
of the soils.
Classifying – grouping objects or events according to similar properties Example: Placing all rocks
having certain grain size or hardness into one group.
Measuring – the use of standard or nonstandard units to determine length, mass, volume, time, etc.
Example: Using a tool to measure the length of a table using non-standard, standard or metric units.
Predicting – the use of data to forecast future events based on observations and inferences
Example: Predicting the height of a plant in two weeks time based on a graph of its growth during
the previous four weeks.
Inferring – the use of logical thought process to show a relationship between observations or
provide an explanation of an observation Example: Saying that the person who used a pencil made a
lot of mistakes because the eraser was well worn.
Defining Operationally – a definition framed in terms of your experiences. Example: Stating that
“bean growth” will be measured in centimeters per week defines how much the bean grows using the
students’ experience.
Formulating Models – developing a conceptual or physical representation of an object or event.
Examples: The students create an anatomically correct paper model of an insect.
Investigating
Formulating Hypotheses
– making an educated guess about the relationship of manipulated and
responding variable that can be tested experimentally. Example: The greater the amount of
organic matter added to the soil, the greater the bean growth.
Controlling Variables
– identifying and controlling variable in order to determine their effect on
the outcome of an experiment. Example: Realizing through past experiences that amount of light
and water need to be controlled when testing to see how the addition of organic matter affects
the growth of beans.
Experimenting
– hypothesizing, designing an experiment to test the hypothesis, controlling
variables, interpreting the data collected, and drawing conclusion. Example: The entire process of
planning and conducting the experiment on the affect of organic matter on the growth of bean
plants.

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 620
Interpreting Data
– analyzing and synthesizing data in order to draw a conclusion. Example:
Recording data from the experiment on bean growth in a data table and forming a conclusion, which
relates trends in the data to variables.
Relating
– the use of logical thought process to determine the relationships involving interactions,
dependencies, and cause-and-effect between and among objects and events. Example: Students
create a food web and investigate the interrelationship of all organisms within the food web by
removing certain organisms to discover the effects of their omission on the entire community.
Applying
– the use of a logical thought process to put scientific knowledge to use. Example:
Choosing the appropriate colored shirt on a hot summer day.
Developing Process Skills
Hands-on activities guided through well-chosen questioning strategies by teachers will enhance the
application of process skills by students.
Teachers act as facilitators not content experts, encouraging students to inquire, research and investigate
answers to their inquiries.
Guiding Questions:
Observing – Ask:
What is the most unique thing you noticed when you looked at your object or event?
How can you find out what is making a sound?
How can smells help you?
What can you find out by tasting?
What can touching tell you about an object?
Communicating – Ask:
What are all the ways you communicated about _______?
Comparing – Ask:
How is this alike or different from ________?
Classifying – Ask:
How could you divide your objects into two groups using another property?
Measuring – Ask:
How accurate was your estimate?
How could you measure your object using a different unit?
Predicting – Ask:
How could you modify your prediction?
Inferring – Ask:
What are some other explanations for your observations?
Defining Operationally – Ask:
How could you revise your definition?
Formulating Models – Ask:
How are the actual thing and the model alike?
How are the actual thing and the model different?
Investigating – Ask:
How accurate was your guess?
Could you do the investigation another way?
What other questions would you like to answer?
Was this a fair test?
What could you do to make this a fair test?
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 621
Project or Group Name________________________________________
Madison Public Schools
Elementary Science Scoring Rubric for Projects
Research Method
5 – Distinguished
(Advanced)
3 – Satisfactory
(Acceptable and Developing)
1 – Unsatisfactory
(Beginning)
0-No Response
1.Research Question / Prediction:
(What do I want to find out?)
Question - Is the problem
identified and correctly stated?
Prediction: Is the prediction
testable and related to the
problem?
Yes, the question is realistic and
appropriate limits have been set.
Yes, the prediction or hypothesis is
logical and leads directly to the
investigation. The researcher explains
reasons for their prediction from the
literature review or prior observation
or experimentation.
Yes, the question is defined but may
lead to wrong conclusions.
Yes, the prediction relates to the
question but may contain some
flawed ideas.
Yes, but has multiple predictions or
hypotheses.
No, suggests a question that is not
testable, but the reader has some idea
of what is being attempted.
No, the prediction does not relate to
the question.
No, the question is missing.
No, the prediction or hypothesis is
missing.
2. Experimental Procedure:
(How will I find out and what are
the steps I need to do?)
Is the procedure
logical and repeatable? For
example, are the steps provided?
Is the investigation designed so
that it tests the prediction or
hypothesis?
Are enough tests conducted or
samples used to provide enough
data to support or not support
the prediction or hypothesis?
Yes, the plan has a very detailed list
of equipment and materials and
specific steps that are easily followed
by some one else. Steps of the
procedure are provided.
Yes, the investigation tests the
prediction or hypothesis completely.
Yes, describes multiple tests or uses
enough tests or samples to support or
not support the hypothesis.
Yes, lists some materials and steps,
but it needs more detail and may be
difficult for someone else to repeat in
exactly the same way.
Yes, the procedure of investigation
tests the original prediction or
hypothesis but there may be some
flaws apparent.
Yes, but only describes one or two
tests or there is some doubt about
having enough tests or samples to
support or not support the hypothesis.
No, the procedure is very confusing
or has serious flaws, but there is
enough information so that the reader
has an idea of what was done.
No, there appears to be only an
awareness of question, prediction or
hypothesis.
No, there is no description of how or
if data is collected.
No, the procedure is missing. There is
no attempt to design a test of the
hypothesis.
3. Results / Data:
(What information did I collect
from my experiment?)
Are the data appropriate for the
stated prediction or hypothesis?
Are the graphs and tables accurate
and labeled correctly?
Are the results summarized
correctly in a brief narrative?
Was a mean or average used when
appropriate?
Yes, the data is appropriate for the
stated prediction or hypothesis.
Yes, the data is accurately recorded
and organized with tables, graphs, or
drawings Including labels and units.
Yes, the results are summarized
correctly in sentence form.
Yes, a mean or average was used if
appropriate.
Yes, the data is appropriate to test the
prediction or hypothesis.
Yes, the data is displayed accurately
and labeled but may have some minor
point missing.
Yes, the results were summarized but
it is incomplete.
No, a mean or average was not used
when needed.
No, the data does not help make any
judgments about the prediction or
hypothesis. There was only an
incomplete attempt to include data or
it was photocopied from a textbook.
No, results are incomplete, unlabeled
and unusable.
No, a summary was not included.
No, a mean or average was not used
when needed.
No, the data is missing.
No, there was no summary of results.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 622
Research Method
5 – Distinguished
(Advanced)
3 – Satisfactory
(Acceptable and Developing)
1 – Unsatisfactory
(Beginning)
0-No Response
4. Conclusion and Future
Research
(What did I find out? What
changes would I make to my
experiment?)
Does the student write about their
prediction or hypothesis?
Does the student indicate that
their data supported or did not
support the prediction or
hypothesis?
Are the conclusions and inferences
logical and correct?
Did the student suggest changes or
improvements to the experiment
for the future?
Yes, the prediction or hypothesis is
addressed.
Yes, the results of the data are clearly
and accurately related to the
prediction or hypothesis.
Yes, the conclusions are logical and
correct.
Yes, identifies problems with the
investigation and explains how the
project could be extended or
improved the next time it is
performed.
Yes, the prediction or hypothesis is
addressed.
Yes, the results or data are related to
the prediction or hypothesis, but there
may be some minor errors present.
Yes, the conclusions and inferences
are logical and correct with some
minor errors present.
Yes, refers to some problems with
the design of the investigation but
does not suggest improvements or
suggests future investigations, but
does not identify problems with the
investigation.
No, does not mention the prediction
or hypothesis.
No, does not relate the data to the
prediction or hypothesis.
No, there are major errors or the
conclusions are not logical or correct.
No, does not suggest problems with
the project and ways to improve it.
Does not suggest future
investigations.
No, the conclusion is missing.
Total Scores:
1 .Question / Prediction
2. Lit Review
3. Expert Procedure
4. Results / Data
5. Conc /Future Res
Scores for District Assessment:
___________
___________
___________
___________
___________
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 623
Elementary Science
Individual Student Response Scoring Rubric for Accountability
Following completion of the project, each student was to have written a response to three general topics about their
investigation. You are to read the response to these items and give one overall rating based on the student's response
to each of the three questions. This score should be an average of the score you would give on the three
parts.
A complete statement of the problem or question investigated. The student should include their prediction or
hypothesis.
A short summary explaining what they found out from their data.
An explanation about whether the data agreed or disagreed with their original prediction or hypothesis.
Use the following rubric to assess the student individual responses.
5
Distinguished
(Advanced)
3
Satisfactory
(Acceptable)
1
Unsatisfactory
(Beginning)
0
No Response
Description of the
question and
prediction
States clearly and
precisely the problem
investigated and the
prediction or
(hypothesis) tested.
Provides a great
amount of detail.
The problem and the
prediction are stated
but some points may
not be clear
Some minor
misunderstandings
may be present.
Gives a very brief or
inadequate
description.
Does not communicate
either the problem or
the prediction. (e. g.
plants)
No response or writes
about an unrelated
issue.
Explanation of the
results (conclusion)
States a complete and
logical explanation of
the results.
May quote the results.
States a reasonable
explanation of the
results.
Some minor
misunderstanding may
be present.
Response does not
explain the data or
results.
Major errors are present
or the response is so
short it does not explain
the results.
No response or writes
about an unrelated
issue.
Explanation of how
the results support or
do not support the
prediction
States a complete and
logical explanation of
the connection between
the data and the
prediction.
May quote the results.
States a reasonable
connection between the
data and the prediction.
Some minor
misunderstandings may
be present.
Response does not
explain the connection
between the data and
the prediction.
Explanation is very
confused.
No response or writes
about an unrelated
issue.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 624
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 625
LABORATORY REPORT
for Middle School
Name ________________________________
Teacher ______________________________
Date _________________________________
Title
I. Purpose:
The purpose is a statement of what you are trying to learn by performing this lab. It may be in the form of a question.
II. Hypothesis:
The hypothesis is your belief as to what the outcome of the lab will be and your reasoning behind it. This is an educated
guess and does not need to be correct.
It should be written as an “If … then” statement with an explanation.
“If…(the independent variable is changed, then ... (a changed measurement in the dependent variable) because ... (This
is based on what you know so far about how the materials interact.)
Independent Variable: _____________
Dependent Variable: ______________
III. Materials:
The materials needed are noted. They should be listed neatly in columns.
IV. Procedure:
1.) The procedure is a numbered list of directions for performing the lab.
2.) It should be a step-by-step set of instructions explaining everything that is to be done.
Control: _______________________
Constant: ______________________
V. Data / Observations:
In this section record anything that you observe or measure. The observations should be both quantitative and
qualitative. At times, measurements may be recorded in a table or a graph.
Be sure NOT to give any explanations or analysis in this section of the report.
VI. Conclusion:
The conclusion must be written in paragraph form. It should be three to five paragraphs in length and address the
following:
Restate the purpose of the lab and the hypothesis of the lab.
State the results of the lab noting if the hypothesis was correct or incorrect using essential supporting data.
Write a brief description of how the lab was performed. Note specific data such as the median or ranges of data to
support the statements made.
Discuss the overall validity of the experiment. What errors could have occurred while performing this lab? If
given the chance to perform this lab again, what could be done differently to improve accuracy?
Discuss what you learned by performing this lab. How can what was learned be applied to the real world?
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 626
Middle School
Lab Report Scoring Rubric
2
1
0
Proper Heading
The heading is complete
including name, teacher
name, core, and date.
The heading is
incomplete.
The heading is missing.
Title
The title is present.
The title is missing.
Purpose or Scientific
Question
The purpose of the lab is
stated.
The purpose of the lab is
not stated.
Hypothesis
The hypothesis is
complete with
“If…then…and
because…”
The hypothesis is
incomplete with only the
“If…then…”
The hypothesis is
missing.
Independent Variable
The independent variable
is clearly and accurately
identified.
The independent variable
is unclear or inaccurate.
The independent variable
is not identified.
Dependent Variable
The dependent variable is
clearly and accurately
identified.
The dependent variable is
unclear or inaccurate.
The dependent variable is
not identified.
Materials
The materials list is
complete and listed in
columns.
The materials list is
incomplete and/or is not
listed in columns.
The materials list is not
included.
Procedure
The procedure is written
in clear steps and easy to
follow.
The procedure is unclear
or not written in steps.
The procedure is
incomplete or missing.
Control
The control is accurately
identified.
The control is not
identified.
Constants
Two or more constants
are accurately identified.
One constant is
accurately identified.
No constant is accurately
identified.
Observation
A table showing
quantitative data is
complete and accurate
Data is incomplete or
inaccurate or not
displayed in a table.
Data is not included.
A graph of significant
data is complete, accurate
and neatly done.
A graph is incomplete or
inaccurate.
A graph is missing.
Qualitative observations
are accurate and well-
described.
Qualitative observations
are incompletely
described.
Qualitative observations
are missing.
Format
The lab report is
written/typed neatly and
in the correct format.
The lab report is not
written neatly or not in
the correct format.
The lab report is not
written neatly and not in
the correct format.
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 627
2
1
0
Conclusion
The purpose is restated.
The purpose is not
restated.
The hypothesis is noted
as being correct or
incorrect with an
explanation.
The hypothesis is noted
as being correct or
incorrect with no
explanation.
The accuracy of the
hypothesis is not
mentioned.
The lab procedure is
summarized accurately.
The lab procedure is
summarized inaccurately
or unclearly.
The lab procedure is not
included.
The results are stated
with supporting data.
The results are stated
with no supporting data.
The results are not stated.
Possible errors are
discussed.
Possible errors are not
included.
At least two things are
noted that could be done
to improve accuracy.
One thing is noted that
could be done to improve
accuracy.
Nothing is noted that
could be done to improve
accuracy.
What you learned from
the lab is noted including
how it can apply to the
real world.
What you learned from
the lab or how the lab can
apply to the real world is
noted.
What you learned from
the lab and how the lab
can apply to the real
world is not noted.
Total Score: ____/_39__ Grade: ________
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 628
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 629
Rubric for Scoring Curriculum Embedded High School
Laboratory Investigations
Statement of the Problem and Hypothesis
3 The problem and hypothesis are stated clearly and completely. Clear identification of independent and
dependent variables.
2 The problem and hypothesis are stated adequately. Adequate identification of independent and dependent
variables.
1 The problem and/or hypothesis are poorly stated. Poor identification of independent and dependent
variable.
0 The statement of the problem and/or hypothesis is very limited or missing altogether. No identification of
independent and dependent variables.
Experimental Design
3 The experimental design matches the stated problem. Variables are held constant. The procedures are
clear, complete and replicable. A control is included when appropriate.
2 The experimental design generally matches the stated problem. Attempt at holding variables constant is
made. Procedures are generally complete. Minor modifications or clarifications may be needed.
1 The experimental design matches the stated problem to some extent. Little attempt to hold variables
constant. Procedures are incomplete. Major modifications or clarifications may be needed.
0 The experimental design does not match the stated problem, is very incomplete or missing. There is no
attempt to hold variables constant.
Data Presentation
3 Data are well organized and presented in an appropriate manner.
2 Data are organized and presented in an appropriate manner. Minor errors or omissions may be present.
1 Data are poorly organized or presented in an inappropriate manner. Major omissions or errors may be
present.
0 Data are very poorly organized or presented in an inappropriate manner or missing altogether.
Conclusion
3 Conclusions are fully supported by data and address the hypothesis. Reliability of data and validity of
conclusions are thoroughly discussed.
2 Conclusions are generally supported by data and address the hypothesis. Minor errors in interpretation of
results may be present. Discussion of reliability of data and validity of conclusions is limited.
1 Conclusions are supported by data and address the hypothesis to a limited extent. Major errors in
interpretation of results may be present. There is little discussion of the reliability of the data or validity
of conclusions.
0 Conclusions are not supported by data, do not address the hypothesis or are missing. There is no
discussion of the reliability of data or validity of conclusions.
Excellent performance 10-12 points
Proficient performance 7-9 points
Marginal performance 4-6 points
Unsatisfactory performance 0-3 points

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 630
Sample End of Course Tests
1. Georgia
www.doe.k12.ga.us/ci_testing.aspx?PageReq=CI
2. North Carolina
pmhs.ucps.k12.nc.us/Academics/EOC_Review.php -Biology
Virginia Released 2004 EOC Test Interactive
Virginia Released 2005 EOC Test Interactive
Virginia Released 2006 EOC Test Interactive
Tennessee Practice Test in Science - Biology Focus Questions
NC Test Item Sample Questions
Multiple Choice Test Questions and Review Materials - from Regents
EOC Released Test from Virginia - Interactive
Texas Released 2000 EOC State Test Interactive
Texas Released 2001 EOC State Test Interactive
Texas Released 2002 EOC State Test Interactive
Lew-Ports Biology Place - Contains multiple choice interactive questions
Sample Quizzes for All Topics
Mitosis & Meiosis Review Game
Biology Review Games
Mrs. Truman's EOC Review Website
3. Virginia
www.iq.poquoson.org/2005vasol/eocbio/eocbio05.htm
4. Missouri
dese.mo.gov › Curriculum & Assessment
End-of-Course Released Items
Biology
Card
Released Form
Released Form
PE Card
Answer Key
Released Rubric
5. Louisana
www.doe.state.la.us/lde/ saa/2617.html
Biology
Biology EOC Assessment Guide PDF
6. Tennessee
www.tennessee.gov/education/assessment/doc/AYPEOCBIO1_PT.pdf
7. Arkansas
arkansased.org/testing/assessment/endofcourse.html

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 631

MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 632
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 633
21
st
Century Skills Overview
Life and Career Skills
Today’s life and work environments require far more than thinking skills and content knowledge. The
ability to navigate the complex life and work environments in the globally competitive information age
requires students to pay rigorous attention to developing adequate life and career skills.
FLEXIBILITY AND ADAPTABILITY
Adapt to Change
Adapt to varied roles, jobs responsibilities, schedules and context
Work effectively in a climate of ambiguity and changing priorities
Be Flexible
Incorporate feedback effectively
Deal positively with praise, setbacks and criticism
Understand, negotiate and balance diverse views and beliefs to reach workable solutions,
particularly in multi-cultural environments
INITIATIVE AND SELF-DIRECTION
Manage Goals and Time
Set goals with tangible and intangible success criteria
Balance tactical (short-term) and strategic (long-term) goals
Utilize time and manage workload efficiently
Work Independently
Monitor, define, prioritize and complete tasks without direct oversight
Be Self-directed Learners
Go beyond basic mastery of skills and/or curriculum to explore and expand one’s own learning and
opportunities to gain expertise
Demonstrate initiative to advance skill levels towards a professional level
Demonstrate commitment to learning as a lifelong process
Reflect critically on past experiences in order to inform future progress
SOCIAL AND CROSS-CULTURAL SKILLS
Interact Effectively with Others
Know when it is appropriate to listen and when to speak
Conduct themselves in a respectable, professional manner
Work Effectively in Diverse Teams
Respect cultural differences and work effectively with people from a range of social and cultural
backgrounds
Respond open-mindedly to different ideas and values
Leverage social and cultural differences to create new ideas and increase both innovation and
quality of work
MADISON PUBLIC SCHOOLS
SCIENCE CURRICULUM 634
PRODUCTIVITY AND ACCOUNTABILITY
Manage Projects
Set and meet goals, even in the face of obstacles and competing pressure
Prioritize, plan and manage work to achieve the intended result
Produce Results
Demonstrate additional attributes associated with producing high quality products including the
abilities to:
- Work positively and ethically
- Manage time and projects effectively
- Multi-task
- Participate actively, as well as be reliable and punctual
- Present oneself professionally and with proper etiquette
- Collaborate and cooperate effectively with teams
- Respect and appreciate team diversity
- Be accountable for results
LEADERSHIP AND RESPONSIBILITY
Guide and Lead Others
Use interpersonal and problem-solving skills to influence and guide others toward a goal
Leverage strengths of others to accomplish a common goal
Inspire others to reach their very best via example and selflessness
Demonstrate integrity and ethical behavior in using influence and power
Be Responsible to Others
Act responsibly with the interests of the larger community in mind
