INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
i
Parts C & D Enrollee Grievances, Organization/Coverage
Determinations, and Appeals Guidance
Effective July 19, 2024
Table of Contents
10
– Introduction ........................................................................................................................................... 6
10.1 – Glossary ......................................................................................................................................... 6
10.2 – Applicability to Employer-Sponsored Benefits ............................................................................. 8
10.3 – Claims Processing and Appeals for Medicare Cost Plans and Health Care Prepayment Plans
(HCPPs) .................................................................................................................................................... 9
10.4 – General Responsibilities of the Plan ............................................................................................ 10
10.4.1 – Medical Exigency Standard .................................................................................................. 10
10.4.2 – Role of the Medical Director................................................................................................. 10
10.4.3 – Delegation of Responsibilities .............................................................................................. 11
10.4.4 – Plan Communication to an Enrollee ..................................................................................... 11
10.5 – Adjudication Requirements.......................................................................................................... 12
10.5.1 –Calculation of Days for Assessing Plan Timeliness............................................................... 12
10.5.2 – When a Request is Considered Received by the Plan ........................................................... 13
10.5.3 – When Notification is Considered Delivered by the Plan ...................................................... 13
10.6 – Outreach for Additional Information to Support Coverage Decisions ........................................ 14
20 – Representatives ................................................................................................................................... 15
20.1 – Representatives Filing on Behalf of Enrollees ............................................................................. 15
20.2 – Appointment of Representative (AOR) Form or Equivalent Written Notice .............................. 16
20.2.1 – Missing or Defective Representative Form ........................................................................... 17
20.3 – Authority of a Representative ...................................................................................................... 18
30 – Grievances........................................................................................................................................... 18
30.1 – Classification between Grievances, Inquiries, Coverage Requests, and Appeals ........................ 19
30.1.1 – Inquiries Related to Non-Part D and Excluded Drugs (Part D Only) ................................... 21
30.2 – Procedures for Handling a Grievance .......................................................................................... 22
30.2.1 – Notification Requirements for Grievances ............................................................................ 23
30.3 – Quality of Care Grievances .......................................................................................................... 24
30.3.1 – Procedures for Handling a Quality of Care Grievance .................................................... 25
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
ii
30.4 – Procedure for Handling Withdrawn Grievances .......................................................................... 25
40 – Coverage Determinations, Organization Determinations (Initial Determinations) and At-Risk
Determinations ............................................................................................................................................ 26
40.1 – Part C Organization Determinations ............................................................................................ 26
40.2 – Part D Coverage Determinations ................................................................................................. 27
40.3 – Part D At-Risk Determinations .................................................................................................... 28
40.4 – Prior Authorization and Other Utilization Management Requirements ....................................... 29
40.5 – Part D Exceptions ........................................................................................................................ 31
40.5.1 – Tiering Exceptions ................................................................................................................ 31
40.5.2 – Formulary Exceptions ........................................................................................................... 31
40.5.3 – Supporting Statements for Exception Requests .................................................................... 32
40.5.4 – Adjudication Timeframes for Coverage Determinations Involving an Exception ................ 34
40.5.5 – Approval of an Exception Request ....................................................................................... 35
40.5.6 – Approval of a Tiering Exception Request ............................................................................. 36
40.6 – Who May Request an Initial Determination ................................................................................ 36
40.7 – Guidelines for Accepting Initial Determination Requests ........................................................... 37
40.8 – How to Process Requests for Expedited Initial Determinations .................................................. 38
40.9 – Who Must Review an Initial Determination ................................................................................ 42
40.10 – Processing Timeframes .............................................................................................................. 43
40.11 – Effect of Failure to Meet the Timeframe for an Initial Determination ...................................... 44
40.12 – Notification Requirements for Initial Determinations ............................................................... 45
40.12.1 – Part C Notification Requirements ....................................................................................... 45
40.12.2 – Part D Notification Requirements ....................................................................................... 49
40.12.3 – Part D Coverage Determination Notices ............................................................................. 51
40.13 – Procedures for Handling Misclassified Initial Determinations .................................................. 54
40.14 – Withdrawal of an Initial Determination Request ....................................................................... 54
40.15 – Dismissal of an Initial Determination Request .......................................................................... 55
40.15.1 – Dismissal Notice ..................................................................................................................... 55
40.15.2 – Dismissal Binding Unless Modified, Reversed or Vacated .................................................... 56
50 – Reconsiderations and Redeterminations (Level 1 Appeals) ............................................................... 57
50.1 – Who May Request a Level 1 Appeal ........................................................................................... 58
50.1.1 – Requirements for Provider Claim Appeals (Part C Only)..................................................... 59
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
iii
50.2 – Level 1 Appeal Requests.............................................................................................................. 61
50.2.1 – Guidelines for Accepting Level 1 Appeal Requests ............................................................. 61
50.2.2 – How to Process Requests for Expedited Level 1 Appeals .................................................... 63
50.3 – Good Cause Exception for Late Filing ........................................................................................ 69
50.5 – Actions the Appealing Party Can Take During a Level 1 Appeal ............................................... 70
50.5.1 – Opportunity to Submit Evidence ........................................................................................... 70
50.5.2 –Enrollee Request for Case File Content ................................................................................. 71
50.6 – Who Must Conduct a Level 1 Appeal .......................................................................................... 71
50.7 – Conducting a Level 1 Appeal ....................................................................................................... 72
50.7.1 – Processing Timeframes ......................................................................................................... 72
50.7.2 – Effect of Failure to Meet the Timeframe for Level 1 Appeals .............................................. 73
50.8 – Service or Benefit Received Prior to Notice of Decision ............................................................ 74
50.9 – Dismissal of a Level 1 Appeal Request ....................................................................................... 74
50.9.1 – Dismissal Notice ....................................................................................................................... 75
50.9.2 – Dismissal Binding Unless Modified, Reversed or Vacated ...................................................... 76
50.10 – Notification Requirements for Level 1 Appeal Decisions ......................................................... 77
50.10.1 - Part C Notification Requirements ........................................................................................ 77
50.10.2 - Part D Notification Requirements........................................................................................ 78
50.11 – Procedure for Handling Misclassified Appeals.......................................................................... 80
50.12 – Timeframes and Responsibilities for Forwarding Case Files to the Independent Review Entity
................................................................................................................................................................ 80
50.12.1 – Forwarding Case Files – Plan Responsibilities ................................................................... 80
50.12.2 – Forwarding Case Files - Timeframes .................................................................................. 81
50.12.3 – Preparing the Case File for the Independent Review Entity ............................................... 82
50.12.4 – Including Evidence of Coverage and Formulary in Case Files ........................................... 83
60 – Reconsiderations by the Independent Review Entity (Level 2 Appeal) ............................................. 83
60.1 – Who May Request a Level 2 Appeal ........................................................................................... 83
60.2 – How to Request a Level 2 Appeal (Part D Only)......................................................................... 84
60.3 – Processing Timeframes ................................................................................................................ 84
60.4 – Good Cause Extension (Part D Only) .......................................................................................... 84
60.5 – IRE Notification and Retention Requirements ............................................................................ 85
60.6 – Dismissal of a Level 2 Appeal Request ....................................................................................... 85
60.6.1 – Dismissal Notice ....................................................................................................................... 86
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
iv
60.6.2 – Dismissal Binding Unless Modified, Reversed or Vacated ...................................................... 86
60.7 – Effect of a Level 2 Appeal Determination ................................................................................... 87
60.8 – Reconsideration of Late Enrollment Penalty Determinations ...................................................... 87
60.8.1 – Summary of the LEP Reconsideration Process ..................................................................... 88
60.8.2 – Part D Plan Responsibilities under the LEP Reconsideration Process .................................. 89
60.8.3 – Requests for Information ...................................................................................................... 90
60.8.4 – Reasons for Requesting LEP Reconsideration and Presentation of Evidence ...................... 91
60.8.5 – IRE LEP Processing Timeframes.......................................................................................... 91
60.8.6 – Withdrawal of an LEP Reconsideration Request .................................................................. 92
60.8.7 - Dismissal of an LEP Reconsideration Request ...................................................................... 92
70 – Key Aspects of Administrative Law Judge (ALJ)/Attorney Adjudicator, Council, and Judicial
Review ........................................................................................................................................................ 93
70.1 – Parties to a Hearing ...................................................................................................................... 93
70.2 – Amount in Controversy ................................................................................................................ 94
70.3 – Filing Requests for Review .......................................................................................................... 95
70.4 – Review Procedures ....................................................................................................................... 98
70.4.1 – Decision-Making Timeframes .............................................................................................. 98
70.4.2 – Part D Plan Sponsor, CMS, or IRE Requesting ALJ Hearing Participation (Part D Only) .. 98
70.4.3 – Submitting Evidence at the Third Level of Review .............................................................. 99
80 – Reopening and Revising Determinations and Decisions .................................................................. 100
80.1 – Guidelines for Reopening .......................................................................................................... 100
80.2 – Reopenings Separate and Distinct from Appeals ....................................................................... 102
80.3 – Timeframes for Reopening ........................................................................................................ 102
80.3.1 – Timeframes for Initiating a Reopening ............................................................................... 102
80.3.2 – Timeframes for Processing a Reopening ............................................................................ 103
80.4 – Reopening Based on Clerical Error ........................................................................................... 104
80.5 – Good Cause for Reopening ........................................................................................................ 104
80.5.1 – New and Material Evidence ................................................................................................ 105
80.6 – Notification Requirements for Reopenings ................................................................................ 105
90 – Effectuation ....................................................................................................................................... 106
90.1 – Independent Review Entity Monitoring of Effectuation Requirements ..................................... 107
90.2 – Effectuation Requirements for Former Plans ............................................................................. 108
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
v
100 – Provider Notices in Hospital, SNF, HHA, and CORF Settings (Part C Only) ............................... 108
100.1 – Hospital Settings – Important Message from Medicare and Detailed Notice .......................... 108
100.1.1 MA Plan Responsibilities Following BFCC-QIO Notification of Appeal .......................... 109
100.2 – Skilled Nursing Facility (SNF), Home Health (HH), and Comprehensive Outpatient
Rehabilitation Services (CORF) Settings ............................................................................................. 109
100.2.1 – MA Plan Responsibilities Following BFCC-QIO Notification of Appeal........................ 110
100.3 – Part A Medicare Outpatient Observation Notice (MOON) ..................................................... 110
110 – Part C Data ...................................................................................................................................... 111
Appendices ................................................................................................................................................ 112
Appendix 1 – Medicare Managed Care (Part C) Appeals Process Overview ....................................... 112
Appendix 2 – Medicare Prescription Drug (Part D) Appeals Process Overview ................................. 113
Appendix 3 – Resources ....................................................................................................................... 114
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
6
10 – Introduction
This guidance covers the appeal provisions set forth at 42 CFR Part 422 Subpart M and 42 CFR
Part 423 Subparts M and U. It addresses grievances, coverage/organization determinations and
appeals for beneficiaries enrolled in a plan provided by a Medicare Advantage (MA)
organization, a Medicare cost plan, health care prepayment plan (HCPP), or a stand-alone Part D
plan.
The contents of this document do not have the force and effect of law and are not meant to bind
the public in any way, unless specifically incorporated into a contract. This document is intended
only to provide clarity to the public regarding existing requirements under the law.
Additional information related to Part C and Part D grievances, coverage/organization
determinations, and appeals may be found on the following Appeals and Grievances guidance
webpages:
https://www.cms.gov/Medicare/Appeals-and-Grievances/MMCAG/index.html
https://www.cms.gov/Medicare/Appeals-and-Grievances/MedPrescriptDrugApplGriev/index
10.1 – Glossary
For purposes of this guidance, the following terminology will be used as described in the
corresponding instances:
Refers to Part C Only Refers to Part D Only
Refers to Both
Parts C & D
Medicare Advantage (MA)
plan, Medicare Advantage
Organization (MAO),
Medicare cost plan or health
care prepayment plan (HCPP)
Part D plan sponsor or plan
sponsor
Plan
Request for Organization
Determination
Request for Coverage
Determination
Coverage Request
Organization Determination
Coverage Determination
Initial Determination
Reconsideration*
Redetermination
Level 1 Appeal
*This term is also used to refer to the IRE level of appeal (level 2 appeal) under both Part C and Part D.
Note: The term “coverage decision” will be used throughout this guidance in circumstances
where the term applies to both an initial determination and a level 1 appeal decision.
Unless otherwise stated in this guidance, the following definitions apply:
Appeal: As defined at 42 CFR §422.561 and §423.560, the procedures that deal with the review
of adverse initial determinations made by the plan on health care services or benefits under Part
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
7
C or D the enrollee believes he or she is entitled to receive, including a delay in providing,
arranging for, or approving the health care services or drug coverage (when a delay would
adversely affect the health of the enrollee) or on any amounts the enrollee must pay for a service
or drug as defined in 42 CFR §422.566(b) and §423.566(b). These appeal procedures include a
plan reconsideration or redetermination (also referred to as a level 1 appeal), a reconsideration by
an independent review entity (IRE), adjudication by an Administrative Law Judge (ALJ) or
attorney adjudicator, review by the Medicare Appeals Council (Council), and judicial review.
Beneficiary and Family Centered Care Quality Improvement Organization (BFCC-QIO):
Organizations comprised of practicing doctors and other health care experts under contract to the
federal government to monitor and improve the care given to Medicare enrollees. The BFCC-
QIOs review enrollee complaints about the quality of care provided by physicians, inpatient
hospitals, hospital outpatient departments, hospital emergency rooms, skilled nursing facilities
(SNFs), home health agencies (HHAs), Medicare managed care plans, Medicare Part D
prescription drug plans, and ambulatory surgical centers. The BFCC-QIOs also review
continued stay denials in acute inpatient hospital facilities as well as coverage terminations in
SNFs, HHAs, and comprehensive outpatient rehabilitation facilities (CORFs). In some cases, the
BFCC-QIO can provide informal dispute resolution between the health care provider (e.g.,
physician, hospital, etc.) and enrollee.
Clean Claim (Part C Only): As defined at 42 CFR §422.500(b), a claim that has no defect,
impropriety, lack of any required substantiating documentation (consistent with 42 CFR
§422.310(d)), or particular circumstance requiring special treatment that prevents timely
payment and that otherwise conforms to the clean claim requirements for equivalent claims
under Original Medicare.
Dismissal: A decision not to review a request for a grievance, initial determination, or appeal
because it is considered invalid or does not otherwise meet Medicare Advantage or Part D
requirements.
Effectuation: Authorization or provision of a benefit that a plan has approved, payment of a
claim or compliance with a complete or partial reversal of a plan’s original adverse
determination.
Enrollee: An eligible individual who has elected a Medicare Advantage, Prescription Drug, or
cost plan or health care prepayment plan (HCPP).
Grievance: An expression of dissatisfaction with any aspect of the operations, activities or
behavior of a plan or its delegated entity in the provision of health care items, services, or
prescription drugs, regardless of whether remedial action is requested or can be taken. A
grievance does not include, and is distinct from, a dispute of the appeal of an organization
determination or coverage determination or an LEP determination.
Independent Review Entity (IRE): An independent entity contracted by CMS to review
adverse level 1 appeal decisions made by the plan. Under Part C, an IRE can review plan
dismissals.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
8
Inquiry: Any verbal or written request for information to a plan or its delegated entity that does
not express dissatisfaction or invoke a plan’s grievance, coverage or appeals process, such as a
routine question about a benefit.
Non-Contract Provider: A provider or supplier that does not contract with a MA organization
to provide services covered by the MA plan.
Quality of Care Grievance: A grievance related to whether the quality of covered services
provided by a plan or provider meets professionally recognized standards of health care,
including whether appropriate health care services have been provided or have been provided in
appropriate settings.
Reconsideration: Under Part C, the first level in the appeals process which involves a review of
an adverse organization determination by an MA plan, the evidence and findings upon which it
was based, and any other evidence submitted by a party to the organization determination, the
MA plan or CMS. Under Part D, the second level in the appeals process which involves a
review of an adverse coverage determination by an independent review entity (IRE), the
evidence and findings upon which it was based, and any other evidence the enrollee submits or
the IRE obtains. As used in this guidance, the term may refer to the first level in the Part C
appeals process in which the MA plan reviews an adverse Part C organization determination or
the second level of appeal in both the Part C and Part D appeals process in which an independent
review entity reviews an adverse plan decision.
Redetermination: First level in the Part D appeal process in which the plan sponsor reviews an
adverse Part D coverage determination, including the findings upon which the decision was
based and any other evidence submitted or obtained.
Reopening: A remedial action taken to change a binding determination or decision even though
the determination or decision may have been correct at the time it was made based on the
evidence of record.
Representative: Under Part C, as defined in §422.561, an individual appointed by an enrollee
or other party, or authorized under state or other applicable law, to act on behalf of an enrollee or
other party involved in a grievance, organization determination, or appeal. Under Part D
§423.560 defines “representative” as an individual either appointed by an enrollee or authorized
under state or other applicable law to act on behalf of the enrollee in filing a grievance, obtaining
a coverage determination, or in dealing with any of the levels of the appeals process. For both
Part C & Part D, unless otherwise provided in the applicable law, the representative will have all
of the rights and responsibilities of an enrollee or other party, as applicable.
Withdrawal: A voluntary verbal or written request to rescind or cancel a pending grievance,
initial determination, or appeal request submitted by the same party.
10.2 – Applicability to Employer-Sponsored Benefits
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
9
Part C Only
Managed care appeal procedures apply to all benefits offered under an MA plan, including
optional supplemental benefits. However, determinations on benefits purchased by an employer,
over and above the Medicare approved benefit package provided by the MA plan, such as
payments of premiums or enrollee cost sharing provided by the employer, are not subject to the
requirements outlined in this guidance.
Part D Only
Part D appeal procedures apply to all Part D benefits offered under an Employer/Union-Only
Group Waiver Plan (EGWP). These plans are offered by Medicare Advantage Organizations,
PDP Sponsors, or Cost Plan Sponsors. Part D plan sponsors of EGWPs must follow Part D
determination, grievance, and appeal procedures in cases in which the provision of employer
other health insurance is intertwined with drugs offered under the Part D benefit such that the
two cannot be separated as a practical matter. See Chapter 12 of the Prescription Drug Benefit
Manual for additional information on prescription drug benefits for EGWPs.
10.3 – Claims Processing and Appeals for Medicare Cost Plans and Health
Care Prepayment Plans (HCPPs)
It is often appropriate for the Medicare Administrative Contractors (MACs) to process claims for
enrollees in cost plans (except as specified otherwise, these rules affecting cost plans also apply
to HCPPs) as regular Part B claims (e.g., when enrollees see an out-of-network physician
without plan authorization or for certain services such as physical therapy [see Chapter 17(B),
§300 of the Medicare Managed Care Manual]). It may also be appropriate for MACs to process
Part B emergency or urgently needed services (See 42 CFR §417.558).
Similarly, it may be appropriate for a MAC to process claims for cost plan enrollees as regular
Part A claims (e.g., when enrollees use an out-of-network facility or for certain services such as
home health or hospice services [see Chapter 17(B), §300 of the Medicare Managed Care
Manual]). Also, if a cost plan with a contract under section 1876 of the Social Security Act (the
Act) elects “billing option 1” (i.e., chooses to have CMS pay for all hospital and skilled nursing
facility (SNF) services – see 42 CFR §417.532(c)), the MAC would process any claims received
(including Part B hospital outpatient claims).
However, regardless of who pays Part A or Part B claims, if an enrollee has received services
through the cost plan’s network, or out-of-network at the direction of the cost plan/network
provider (e.g. referral), or because of an emergency inpatient admission, appeals concerning a
denial of payment of such services are subject to the rules that apply to cost plan services.
Pursuant to §417.600, those rights, procedures, and requirements pertaining to appeals contained
in 42 CFR Part 422 Subpart M are applicable to enrollees in 1876 cost plans. (In the case of an
HCPP, §417.840 requires HCPPs to apply the MA regulations at §§422.568 through 422.626 to
appeals related to Part B services. Part A services are not covered under the HCPP agreement,
and would always be processed under the 42 CFR Part 405 fee-for-service appeals rules.)
Furthermore, the enrollee cannot be held liable for a Part A or Part B service just because a MAC
denied the claim under these circumstances. This is true even though the cost plan has no

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
10
influence on the MAC decision. The 42 CFR Part 405 fee-for-service appeals rules apply to the
first level appeal in an 1876 cost plan only in a case in which the enrollee self-referred out of the
cost plan’s provider network or hospital/SNF network without the cost plans involvement
(including outpatient emergency services at an out-of-network hospital). Any disputes involving
applicable cost-sharing are subject to the rules that apply to cost plan services at 42 CFR Part
422 Subpart M.
If an enrollee files an appeal with the cost plan when the appeal should have been filed with the
MAC, the cost plan must inform the enrollee that the appeal should be filed with the MAC that
denied the payment. The cost plan should direct the enrollee to the Medicare Summary Notice
(MSN) for an explanation of the 42 CFR Part 405 fee-for-service appeals process. The cost plan
must inform the enrollee in the Evidence of Coverage (EOC) that the cost plan’s appeals process
is only for disputes relating to organization determinations made by the plan or certain
emergency admissions.
10.4 – General Responsibilities of the Plan
10.4.1 – Medical Exigency Standard
The medical exigency standard requires a plan and the independent review entity to make
decisions as “expeditiously as the enrollee’s health condition requires.” This standard is set forth
in regulations at Part 422 Subpart M and Part 423 Subpart M with respect to coverage requests
and effectuation of favorable decisions.
This standard requires that the plan or the independent review entity apply, at a minimum,
established accepted standards of medical practice in assessing an individual’s medical
condition. Evidence of the individual’s condition can be obtained from the treating provider or
from the individual’s medical record (e.g., diagnosis, symptoms, or test results).
This standard was established by regulation to ensure that plans develop a standard for
determining the urgency of coverage requests, triage incoming requests against established
criteria, and prioritize each request according to these standards. Plans must treat each case in a
manner that is appropriate for the facts and circumstances of the enrollee’s medical condition.
Plans should not routinely take the maximum time permitted for adjudicating coverage requests.
10.4.2 – Role of the Medical Director
In accordance with 42 CFR §422.562(a)(4) and 423.562(a)(5), all plans must employ a medical
director who is responsible for ensuring the clinical accuracy of all coverage decisions made by
the plan that involve medical necessity. CMS expects plans to have processes in place for
elevating issues of clinical concern to the medical director; however, it is not expected that a
plan’s medical director will review every medical necessity decision. CMS considers the medical
director to be fulfilling their responsibility through the plan’s established process for when a
medical director must be involved.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
11
The medical director has overall responsibility for the plan’s clinical decision-making, and as
such, is expected to be involved in various aspects of related plan policies and operations which
may include: medical and utilization review, benefits and claims management, formulary
administration, processing coverage decisions in accordance with adjudication timeframes and
notice requirements, provider/prescriber outreach, staff training, and oversight of delegated
entities. The medical director must be a physician, as defined in section 1861(r) of the Act, with
a current license to practice medicine in a state, territory, Commonwealth of the United States, or
the District of Columbia.
10.4.3 – Delegation of Responsibilities
With the exception of the employment of the medical director, a plan may delegate any of the
responsibilities discussed in this guidance to another entity or individual that provides or
arranges Part C or Part D benefits. In cases of delegation, the plan remains responsible and must
therefore ensure that requirements are met completely by the delegated entity and/or individual.
The plan must have a comprehensive and on-going monitoring and auditing process in place to
validate the performance of the delegated entities’ compliance with applicable CMS
requirements.
10.4.4 – Plan Communication to an Enrollee
Plans must establish and maintain procedures for standard and expedited initial determinations,
appeals and grievances. Written information about these procedures (including the quality of
care grievance process available through the Beneficiary and Family Centered Care Quality
Improvement Organization (BFCC-QIO)) must be provided or made available to enrollees at
initial enrollment, upon an enrollee’s request and annually thereafter, as well as in any
circumstance that provides enrollee rights to initial determinations, appeals, and grievances as
described throughout this guidance, including but not limited to:
• Grievance procedures available upon involuntary disenrollment initiated by the plan;
• Any changes to the plan’s grievance or appeals procedures 30 days in advance of the
effective date of the changes; and
• Appeals procedures upon notification of an adverse initial determination or a service or
coverage termination (e.g., hospital, CORF, HHA, or SNF settings).
All written communication and notifications must be written in a manner that is understandable
to the enrollee. Where applicable in standardized notices, the plan must use the approved notice
language. (See §§40.12 and 50.10 for notification requirements for initial determinations and
level 1 appeals, respectively.)
Plans must also provide written communications and notices described in this guidance in
alternate formats and languages consistent with Section 1557 of the Affordable Care Act and
Section 504 of the Rehabilitation Act of 1973. In addition, a plan’s fax and e-mail or web-based
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
12
portal systems must meet the Health Insurance Portability and Accountability Act of 1996
(HIPAA) privacy and security requirements. If the enrollee agrees, the MA plan may deliver
written notices by fax or e-mail. Please see Medicare Marketing Guidelines regarding electronic
communication with enrollees.
Enrollees requesting an alternate format should have the same level of access to information as
an individual not requesting information in an alternate format. The term “access” includes
providing information in a format that the given individual can understand.
Delays in providing materials in alternate formats can impact timeframes that enrollees may have
to take certain actions. For example, a disabled enrollee received services from a non-contract
provider and now requests instructions in an alternate format as to how to request that the plan
reimburse the enrollee for the out-of-pocket claim. If the plan requires the claim to be filed
within a certain amount of time, it must take into account the additional time that may be needed
to provide the instructions in the alternate format and for the enrollee to submit the claim. The
plan should accept the individual’s claim as timely when it is submitted.
Additionally, plans should ensure enrollees with limited English proficiency are able to
communicate with plans regarding initial determinations, appeals, and grievances. Enrollees with
limited English proficiency should have the same level of access to plan representatives and
information regarding initial determinations, appeals, and grievances as enrollees who are
proficient in English. Plans may view the CMS Office of Minority Health website for strategies
on how to ensure plan services to enrollees are culturally and linguistically equitable. For
example, plans may incorporate the Building an Organizational Response to Health Disparities
Resource Guide into plan operations. Plans may also incorporate the Office of Minority Health’s
Disparity Impact Statement into their operations to reduce health disparities among enrollees.
10.5 – Adjudication Requirements
10.5.1 –Calculation of Days for Assessing Plan Timeliness
For the purpose of assessing the timeliness of a plan’s completion of a grievance, initial
determination, or level 1 appeal, the day a plan receives the request is not counted as “day
one”. “Day one” is the day after receipt of the request. (Day/days are calendar days unless
otherwise specified and includes weekends and holidays). Timeframes measured in hours must
be met within the number of hours indicated. For example:
An MA plan receives a request for a standard prior organization determination on May 1. The
plan must notify the enrollee of its determination as expeditiously as the enrollee’s health
condition requires, but no later than 14 calendar days after the date the plan receives the request,
which would be May 15.
A Part D plan sponsor receives a request for an expedited coverage determination on May 1 at
10:30 am. Given the 24-hour timeframe, the plan must notify the enrollee of its determination no
later than 10:30 am on May 2.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
13
10.5.2 – When a Request is Considered Received by the Plan
Plans must have processes in place to accept requests (grievance, coverage, and appeal requests)
24 hours a day, 7 days a week (including holidays). Requests (and for Part D, prescriber
supporting statements for exception requests) are deemed "received" on the date and time:
• The plan initially stamps a document received by regular mail (i.e., U.S. Postal Service);
• A delivery service that has the ability to track when a shipment is delivered (e.g., U.S.
Postal Service, UPS, FedEx, or DHL) delivers the document;
• A faxed document is successfully transmitted to the plan, as indicated on the fax
transmission report;
• A verbal request is made by telephone with a customer service representative;
• A message is left on the plan’s voicemail system if the plan utilizes a voicemail system to
accept requests or supporting statements after normal business hours; or
• A request is received through the plan’s website, provided the website and/or portal meets
all applicable regulatory requirements.
Note: For standard requests, the processing timeframe begins when the plan, any unit in the
plan, or a delegated entity (including a delegated entity that is not responsible for processing)
receives a request. For expedited requests, the processing timeframe begins when the
appropriate department receives the request. Plan material should clearly state where pre-
and post-service requests should be sent, thus ensuring requests are received at the correct
location and giving the plan the greatest amount of time to process the request. Plan policy
and procedures should clearly indicate how to route requests that are received in an incorrect
location to the correct location as expeditiously as possible.
10.5.3 – When Notification is Considered Delivered by the Plan
Unless otherwise specified (e.g., Section 40.8 of this guidance), written notification is considered
delivered on the date (and time, if applicable) the notice has left the possession of the plan or
delegated entity. Generally, this occurs when the notice has been deposited into the courier drop
box or external outgoing mail receptacle (e.g., U.S. Postal Service or FedEx bin) or for electronic
delivery of required materials, the date the plan sends the materials to the enrollee (see Section
100.2.2 of the Medicare Marketing Guidelines for requirements on delivering electronic
materials to enrollees). Placement into the plan or delegated entity’s internal outgoing mail
receptacle is not considered delivered. For electronic payments (i.e., EFTs), delivery occurs on
the date (and time, if applicable) the plan distributes the funds for payment.
Verbal notification is considered delivered on the date (and time, if applicable) a plan speaks

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
14
directly to or leaves a voicemail for an enrollee or enrollee’s representative. Plans may initially
provide verbal notification to enrollees prior to issuing written notification.
In circumstances when verbal notification is permitted per regulatory requirements and the plan
successfully provides verbal notice (e.g., spoke with the person that submitted the request or was
able to leave a voicemail message), the required written notification must be sent by the plan
within 3 calendar days of the verbal notice. If the plan is not able to successfully provide verbal
notice (i.e., when a plan has an enrollee’s telephone number on file, but is unable to reach the
enrollee at the number provided because, for example, it is either incorrect, out-of-service, or no
person (or no voicemail system) answers), written notice must be sent within the applicable
timeframe. Information regarding verbal notification for expedited requests can be found at
§40.8 for initial determinations and §50.2.2 for level 1 appeals.
The regulations applicable to adjudication timeframes for standard Part C plan reconsiderations
at 42 CFR § 422.590(a) and (c) and standard Part D redeterminations at 42 CFR § 423.590(a) do
not address verbal notification. However, the plan may choose to initially provide verbal
notification of the decision, but the required written notification must be issued within the
applicable adjudication timeframe. For Part C reconsiderations, the plan must issue the
determination as expeditiously as the enrollee's health condition requires, but no later than 30
calendar days from the date it receives the request for a standard reconsideration.
For Part D redeterminations, the plan must notify the enrollee in writing of its redetermination as
expeditiously as the enrollee's health condition requires, but no later than 7 calendar days from
the date it receives the request for a standard redetermination.
10.6 – Outreach for Additional Information to Support Coverage Decisions
Plans must have processes in place for making timely coverage decisions (initial requests and
appeals), which includes soliciting clinical documentation, such as medical records, when
necessary. If a plan does not have enough information to make an approval decision on an item,
service, or drug request, it should make reasonable and diligent efforts to obtain all necessary
information.
Plans are only required to conduct outreach to request additional information from a provider if
the plan does not have all necessary information to make a coverage or appeal decision. In
instances when outreach is necessary to make a coverage or appeal decision, a minimum of one
attempt to obtain additional information is sufficient. Plans may adopt best practices for
outreach, such as making multiple attempts, using multiple methods for requesting information
(e.g., telephone, fax, e-mail, etc.), and/or and involving plan physicians in order to increase the
likelihood of obtaining necessary information. If the plan does not receive any additional
information, the plan should make the best decision it can based on the information available
within the required adjudication timeframes. Plans are not required to conduct outreach prior to
denying claims payments if they believe they have all the necessary information needed to make
a coverage decision.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
15
Part C Only
For expedited organization determination and reconsideration requests, if medical information is
needed from a non-contract provider, the MA plan must request the necessary information within
24 hours of receipt of the request.
Part D Only
For expedited redetermination requests, if medical information is needed, the Part D plan
sponsor must request the information within 24 hours of receipt of the request.
Plans should document all requests for information and maintain that documentation within the
case file. If the plan issues an adverse decision due to the inability to obtain clinical information
needed to approve coverage, the plan should clearly identify that basis and the necessary
information in the written denial notice. See §§ 422.568(d) and (e), 422.570(d), and 422.572(d)
for denials related to Part A and B services, items and Part B drugs, and 423.568(f) and (g) for
denials related to Part D benefits.
Note: See § 20.2.1 for information regarding missing documentation for representation (i.e.,
no appointment of representation form on file), and § 50.1.1 for missing Waiver of Liability
form (WOL) for non-contract providers.
20 – Representatives
20.1 – Representatives Filing on Behalf of Enrollees
Individuals who represent enrollees may either be appointed or authorized (for purposes of this
guidance, both are referred to as “representatives”) to act on behalf of the enrollee in filing a
grievance, requesting an initial determination, or in dealing with any of the levels of the appeals
process.
Who Can Act or be Appointed as a
Representative
Requirement for Representation
Any individual appointed by the enrollee (e.g.,
relative, friend, advocate, attorney)
The enrollee must submit Form CMS-1696,
Appointment of Representative (AOR) or an
equivalent written notice (hereinafter,
collectively referred to as a representative
form).
An individual authorized under state or other
applicable law.* Could include, but is not
limited to:
• Court appointed guardian
• Individual with durable power of attorney
• A health care proxy
• A person designated under a health care
consent statute
• A representative form is not required.
• Authorized individual must produce
appropriate legal papers supporting his or
her status under state or other applicable
law.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
16
Who Can Act or be Appointed as a
Representative
Requirement for Representation
• Executor of an estate
*Plans with service areas comprising more than one state should be aware of the different state representation
requirements and are responsible for determining whether a person or entity who asserts surrogate status is an
appropriate surrogate under state law.
When verbally submitting grievances, initial determinations, and reconsiderations, when
applicable, enrollees cannot verbally appoint a representative and must submit a valid
representative form. However, if a purported representative makes a verbal request and the
enrollee verbally confirms they want to file the request described by the purported representative,
the request must be documented and processed as a request from the enrollee, not a
representative. All communication (written and verbal) must be delivered to the enrollee until a
valid, written representative form is on file. In these instances, plans are not required to make
efforts to obtain a written representative form.
20.2 – Appointment of Representative (AOR) Form or Equivalent Written
Notice
If an appointment is made using the OMB-approved Form CMS-1696, Appointment of
Representative (AOR), or an equivalent written notice (see requirements below) the plan must
accept it. Plans are prohibited from requiring the use of a specific form for appointments. The
AOR contains the necessary elements to meet representation requirements, and is preferred.
(Note: Section 4 of the AOR does not apply to MA plans or Part D plan sponsors.) Plans may
not require information beyond what is included in the AOR or the requirements outlined below
for an equivalent written notice.
Plans are required to accept an AOR with electronic signatures if the form is submitted through
the plan’s secure portal or other secure electronic means (e.g., a secure messaging system),
provided all applicable regulatory and CMS website/electronic communication requirements are
met. AORs contain an enrollee’s HICN (Health Insurance Claim Number), Medicare
Beneficiary Identifier (MBI) or plan ID number and should be treated as protected information.
An equivalent written notice includes the following:
• Name, address, and telephone numbers of the enrollee and the individual being appointed;
• Enrollee’s HICN or Medicare Beneficiary Identifier, or plan ID number;
• The appointed representative’s professional status or relationship to the party;
• A written explanation of the purpose and scope of the representation;
• A statement that the enrollee is authorizing the representative to act on his or her behalf
for the claim(s) at issue, and a statement authorizing disclosure of individually identifying
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
17
information to the representative;
• A statement by the individual being appointed that he or she accepts the appointment; and
• Is signed and dated by the enrollee and the individual being appointed.
A representative form is valid for one year from the date it has signatures for both the enrollee
and the appointee, unless revoked. For example, if the enrollee signs the form on January 1,
2019 and the representative signs on January 3, 2019 (or vice versa), the form is effective for one
year starting on January 3, 2019.
If the enrollee would like the same individual to continue serving as a representative after one
year, the enrollee must reappoint that person by submitting a new representative form.
A form is valid for the life of a grievance, coverage request, or appeal if the grievance, coverage
request, or appeal was received within one year of the date a representative form is signed by
both the enrollee and appointee.
If the representative form is maintained and accessible by the plan, a photocopy of the signed
representative form is not required to be filed with future grievances, coverage requests, or
appeals made on behalf of the enrollee in order to continue representation. If the plan uses a
representative form that is on file for requests, it must include a copy when sending a case file to
higher level adjudicators, if applicable.
Note: A representative form submitted with a request that specifically limits the
appointment to MA or Part D benefits is not valid for requests that involve Part D or MA
benefits, respectively. In these instances, the enrollee must properly execute separate
representative forms.
20.2.1 – Missing or Defective Representative Form
When a request for a grievance, initial determination, or level 1 appeal is filed by a person
claiming to be a representative, but the party does not provide the valid representative
documentation to show that the individual is authorized to act on the enrollee’s behalf, the plan
should:
• For expedited requests, develop procedures to ensure that expedited requests are not
inappropriately delayed.
• Inform the enrollee and purported representative, in writing, that the grievance, coverage
request, or appeal is not valid until documentation is provided.
• Make and document its reasonable efforts to secure the necessary representative
documentation.
The plan must not issue a decision until or unless such documentation is obtained. The plan is

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
18
not required to undertake a review until or unless such documentation is obtained, but may
choose to begin the review while continuing efforts to obtain the representative documentation.
The timeframe for acting on a grievance, coverage request, or appeal begins when the
representative documentation is received.
If the plan does not receive representative documentation by the conclusion of the applicable
timeframe, plus any applicable extension, the following apply:
Dismissal of coverage and appeal requests: The request is dismissed because the person or
entity making the request is not permitted to request a coverage decision or appeal or is not a
proper party. The plan must mail or otherwise transmit a written dismissal notice to the enrollee
(or other proper party) and should also send the notice to the person asserting representative
status. The dismissal notice must state all of the following: (1) the reason for the dismissal; (2)
the right to request that the plan vacate the dismissal action; and (3) the right to request review of
the dismissal. The notice should also explain how the invalid request can be cured and that the
request will be processed if the enrollee or representative submits a properly executed form. See
42 CFR §§ 422.568(g) and (h), 422.582(f) and (g), 423.568(i) and (j), 423.582(e) and (f).
See §50.9 for additional information regarding reconsideration dismissal procedures.
Grievances: MA plans and Part D plans are not required to process grievances when the person
or entity making the grievance is not permitted by 42 CFR §§ 422.564 or 423.564. In
circumstances where necessary representative documentation is missing, and not received by the
end of the grievance processing period, the plan should notify the enrollee and the purported
representative that the plan is unable to process the grievance. This notice should include
instructions on how the enrollee, or a valid representative, may resubmit the grievance.
20.3 – Authority of a Representative
The representative has all of the rights and responsibilities of an enrollee in filing a grievance,
obtaining a coverage request, or in dealing with any levels of the appeals process.
If an enrollee has identified a representative, all notices or other correspondence that must be
sent to the enrollee per the regulations at 42 CFR Part 422 or 423 Subpart M must be sent to the
enrollee’s representative instead of to the enrollee. Plans may send notices or correspondence to
both the representative and enrollee, but are not required.
30 – Grievances
A grievance is an expression of dissatisfaction with any aspect of the operations, activities, or
behavior of a plan or its delegated entity in the provision of health care or prescription drug
services or benefits, regardless of whether remedial action is requested. The regulations at 42
CFR § 422.564(a) and 423.564(a) require each plan to have meaningful procedures for the timely
resolution of grievances between enrollees and the plan or any of its delegated entities. Plans
must provide all enrollees with written grievance procedures upon initial enrollment, involuntary

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
19
disenrollment, annually, and upon request. The plan must also notify enrollees about any
changes to its grievance procedures 30 days in advance of the effective date of the change.
The grievance process is only available to enrollees or their representatives. Providers should
file complaints to plans in accordance with the applicable plans’ dispute resolution processes
and, if after communicating with the plan resolution is unsatisfactory, may contact their local
CMS Regional Office. Decisions made under the grievance process are not subject to appeal.
30.1 – Classification between Grievances, Inquiries, Coverage Requests, and
Appeals
An enrollee may contact a plan to file, make, or request a grievance, inquiry, coverage request,
or appeal. Grievance procedures are separate and distinct from initial determination and appeal
procedures. Any communication from an enrollee must be reviewed on a case-by-case basis to
determine how it should be categorized. The enrollee is not required to use any specific
language to indicate what they are requesting. Plans must determine whether the matter or the
issue is a grievance, coverage request, appeal, or combination of more than one category and
inform the enrollee (verbally or in writing) if the issue is a grievance or an appeal. If an enrollee
raises two or more issues at the same time, then each issue should be processed separately and
simultaneously (to the extent possible) under the appropriate procedure. Plans should ensure that
customer service representatives are trained to distinguish between coverage requests, appeals,
and grievances.
Note: If the plan misclassifies a grievance as an appeal and the case goes to the IRE, the IRE
will dismiss the appeal and return the case to the plan for proper processing. The plan must
notify the enrollee in writing that the case was misclassified and will be handled through the
plan’s grievance process. For initial determination requests misclassified as grievances, see
§40.13. For appeals that are misclassified as grievances see §50.11.
Examples of Inquiries, Grievances, Coverage Requests, and Appeals
Inquiries may include the following:
• An enrollee calls the plan to determine if a drug or service is covered, the plan tells the
enrollee that it is not covered, the enrollee does not complain about the exclusion or non-
coverage, does not make a request for the drug or service, nor does the enrollee argue that
it should be covered under certain circumstances.
Grievances may include the following:
• An enrollee’s involuntary disenrollment initiated by the plan;
• A change in premiums or cost sharing arrangements from one contract year to the next;
• Lack of quality of the care received;

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
20
• Plan benefit design;
• Difficulty contacting the plan via phone;
• Interpersonal aspects of care;
• The appeals process;
• The plan’s decision not to expedite a coverage or appeal request;
• General dissatisfaction about a co-payment amount, but not a dispute about the amount the
enrollee paid or is billed;
• General issue about a drug not being on the formulary or listed as an excluded drug; or
• Calculation of True Out-of-Pocket (TrOOP) costs.
Coverage Requests may include when an enrollee:
• Calls requesting or indicating they want a drug, service, or item;
• Wants to continue care with a provider who is no longer contracted with the plan (out of
network coverage);
• Wants to continue receiving services already received in accordance with the original
organization determination (this is a request for a new set of services);
• States that their drug was rejected at the pharmacy but they need it; or
• States that a drug is not excluded from Part D coverage for the indication for which it is
being prescribed.
Coverage Requests for Part D Only may include when an enrollee argues that:
• A drug is a covered Part D drug under §1860D-2(e)(1) of the Act or is covered under
§1860D-2(e)(1) for a specific indication;
• A drug is not excluded under §1860D-2(e)(2) of the Act or is not excluded under §1860D-
2(e)(2) for a specific indication;
• A drug is not excluded under §1860D-43 of the Act; or
• A drug is covered by the plan as a supplemental benefit.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
21
Conversely, if an enrollee agrees that a drug is not a covered Part D drug or is excluded from
coverage, but has a question or general issue about the drug not being covered, the transaction
should be processed as an inquiry or a grievance, respectively.
Appeals may include the following:
• An enrollee calls after receiving a bill stating they believe that the plan has required them
to pay a co-pay amount that should be the plan’s responsibility or they dispute the
calculation of the co-pay amount.
• An enrollee calls his or her plan and states, “I would like to file a grievance. You denied
my request for drug X or service Y and I need it.” When an adverse initial determination
has been made, the enrollee’s dispute should be treated as an appeal of the denial.
Therefore, either an appeal should be started or, if the plan does not accept verbal standard
appeal requests, they must inform the enrollee of how to submit a written appeal request.
Examples of Both a Grievance and a Coverage Request:
• An issue concerning untimely receipt of a service or Part D drug that has already been
covered may be treated as a grievance. If the enrollee also states that he or she was unable
to obtain the covered service or Part D drug and that the delay will adversely affect his or
her health, it should be processed as a coverage request, as well as a quality of care
grievance.
• An enrollee complains that they had to wait so long for a service or Part D drug that they
went out-of-network. This should be treated as a coverage request for the out-of-network
service or Part D drug, as well as a grievance about the timeliness of the service/benefit.
• An enrollee has a benefit that covers one pair of eyeglasses every 24 months with a
maximum contribution of $70.00. The enrollee asserts that the prescription was wrong,
and requests that the plan cover another pair of glasses. The enrollee indicates that the
previously rendered services are inadequate, or substandard in quality. Therefore, this
would be classified as a grievance (quality of care grievance) and the request for a new
pair of glasses is a new coverage request.
30.1.1 – Inquiries Related to Non-Part D and Excluded Drugs (Part D Only)
When a Part D plan sponsor receives an inquiry (that is, a question that is not a request for a
coverage determination) about a drug that is never covered by Part D or is an excluded drug, it
should explain the following to the requestor:
• Certain drugs (or the requested drug) are not covered Part D drugs under 1860D-2(e)(1) of
the Act, or are excluded from coverage under 1860D-2(e)(2) or 1860D-43 of the Act and
the plan sponsor cannot or does not offer the drug as a supplemental benefit;
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
22
• Because the drug is not a covered Part D drug under 1860D-2(e)(1) of the Act, or is
excluded from coverage under 1860D-2(e)(2) or 1860D-43 and is not offered as a
supplemental benefit, the enrollee may not obtain it through the coverage determination,
exceptions, or appeals processes;
• The enrollee should work with his or her physician or other prescriber to determine
whether a drug on the plan’s formulary is medically appropriate for treating the enrollee's
condition; and
• The enrollee, physician, or other prescriber has the right to contact the plan sponsor and
request a coverage determination if he or she believes that the requested drug is:
o A covered Part D drug under section 1860D-2(e)(1) of the Act or covered under
1860D-2(e)(1) for the indication it is being prescribed for;
o Not excluded under section 1860D-2(e)(2) of the Act or not excluded under
1860D-2(e)(2) for the purpose for which it was prescribed;
o Not excluded under section 1860D-43 of the Act; or
o Covered by the plan as a supplemental benefit.
A plan sponsor should provide this information either verbally or in writing. If a plan sponsor
chooses to deliver this information in writing, it may use the model Notice of Inquiry. If a plan
sponsor provides this information verbally, this should be documented by the plan sponsor.
30.2 – Procedures for Handling a Grievance
Procedures for processing a grievance are as follows:
Filing
Method
Filing Deadline
Processing Requirements
In writing
No later than 60
days after the
incident that
precipitates the
grievance*
Plans must:
• Complete the investigation as expeditiously as the case
requires, based on the enrollee’s heath status, but no later
than 30 days of receipt of the request, or within 24 hours
for expedited grievances.
Note: Plans may take a 14-day extension if the enrollee
requests the extension or if the plan justifies a need for
additional information and documents how the delay is in the
best interest of the enrollee. Plans must promptly notify the
enrollee in writing if the extension is going to be taken and
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
23
Filing
Method
Filing Deadline
Processing Requirements
explain the reason for the delay. See 42 CFR
§§422.564(e)(2) or 423.564(e)(2).
• Accept any information or evidence concerning the
grievance.
• Take prompt, appropriate action, including a full
investigation if necessary.
• Provide a response to all grievances raised in the written
request.
• Have procedures for tracking and maintaining records
about the receipt and disposition of grievances.
• Be able to log or capture enrollees’ grievances in a
centralized location that is readily accessible. The record
should include documentation of all telephone calls,
correspondence, and case notes related to the grievance.
• Expedite the grievance if:
o It is related to a decision not to grant an enrollee’s
request to expedite an initial determination or appeal,
and (for Part D Only) the enrollee has not yet
obtained the drug; or
o Part C Only: it involves an MA plan’s decision to
extend a timeframe related to an organization
determination or appeal.
Verbal
Same as in
writing.
Same as in writing; however, if a verbal grievance can be
resolved during the same call by the customer service
representative, the plan must document details of the
resolution and proceed to log and report the call as a
grievance.
*A plan may, but is not required to, accept and process a grievance that is filed after the 60-day deadline or
submitted with missing or defective representative documentation. If the plan chooses not to accept the invalid
filing, the plan should notify the enrollee of the plan’s decision to not accept the grievance.
30.2.1 – Notification Requirements for Grievances

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
24
For grievances made in writing, the response must:
• Be in writing,
• address all issues raised in the grievance; and
• be written in a manner that is understandable to the enrollee.
For grievances received verbally, the response may be verbal or in writing, unless specifically
requested in writing or the grievance raises a quality of care issue (see §30.3).
For standard grievances, notification must be delivered no later than 30 days from receipt (plus a
14-day extension, if applicable).
For expedited grievances, notification must be delivered no later than 24 hours from receipt.
When written notification is required for expedited grievances, plans may initially provide verbal
notification of its decision and must deliver written confirmation of its decision within 3 calendar
days of the verbal notification.
Note: If the enrollee’s representative submits a request, the representative must be notified in
lieu of the enrollee. Plans may provide notice to both the representative and enrollee, but are
not required.
30.3 – Quality of Care Grievances
A quality of care grievance is a type of grievance that is related to whether the quality of covered
services provided by a plan or provider meets professionally recognized standards of health care.
Examples of a quality of care grievance include any instances where an enrollee infers or states
they believe:
• They were misdiagnosed;
• Treatment was not appropriate; and/or
• They received, or did not receive, care that adversely impacted or had the potential to
adversely impact their health.
Quality of care grievances may be received and acted upon by the plan, the Beneficiary and
Family Centered Care Quality Improvement Organization (BFCC-QIO), or both. For any
grievance submitted to the BFCC-QIO, plans must cooperate with the BFCC-QIO in resolving
the grievance, including directing providers to respond to BFCC-QIO requests for information,
within 14 days. Plans should provide any records and requested information as quickly as
possible and within 14 days.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
25
30.3.1– Procedures for Handling a Quality of Care Grievance
Quality of care grievances submitted to the plan may be made verbally or in writing and must be
submitted no later than 60 days after the incident that precipitates the grievance. A plan may, but
is not required to, accept and process a grievance that is filed after the 60-day deadline. If the
plan chooses not to accept untimely filing, they may dismiss the grievance. Plans may take a 14-
day extension and must notify the enrollee in writing if the extension is going to be taken and
explain the reason for the delay. See 42 CFR §§ 422.564(e)(2) or 423.564(e)(2).
Plans must investigate and respond to the quality of care grievance in writing within 30 days of
receipt, or as expeditiously as the enrollee’s health condition requires.
Written notice must:
• Include a description of the enrollee’s right to file a written complaint with the BFCC-QIO
and contact information for the BFCC-QIO; and
• Be written in a manner that is understandable to the enrollee.
If the enrollee’s representative submits a request, the representative must be notified in lieu of
the enrollee. Plans may send written notice to both the representative and enrollee, but are not
required.
For grievances submitted to the BFCC-QIO, plans must cooperate with the BFCC-QIO and
comply with requirements at 42 CFR Part 476 regarding timely submission of requested
information to the BFCC-QIO if an enrollee files a grievance with the BFCC-QIO and the plan.
Note: When a plan decides to not process a quality of care grievance submitted by a person
purporting to be an enrollee’s representative but who failed to timely submit the necessary
representative documentation, the plan should, but is not required to, investigate the quality of
care grievance. The plan is not required to notify the enrollee of the outcome of the grievance
since the grievance was not properly filed.
30.4 – Procedure for Handling Withdrawn Grievances
An enrollee may submit a written withdrawal request for a grievance any time before the
decision is mailed by the plan. The plan may accept verbal withdrawals for both written and
verbal grievances received from an enrollee. The plan must clearly document in the system that
the enrollee does not want to proceed with the grievance procedures. The plan should, but is not
required to, send a written confirmation of that withdrawal to the enrollee within 3 calendar days
of receiving the withdrawal request.
If the enrollee submits a quality of care grievance verbally or in writing, but later decides to
withdraw the grievance, the plan is still required to investigate the quality of care grievance;
however, the plan is not required to notify the enrollee of the outcome of the grievance since they
decided not to pursue the grievance.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
26
40 – Coverage Determinations, Organization Determinations (Initial
Determinations) and At-Risk Determinations
A coverage determination/organization determination (hereafter referred to as an initial
determination
1
) is a decision made by the plan, or its delegated entity, concerning the payment or
provision of an item, service, or drug.
Plans must adhere to all applicable requirements set forth in 42 CFR Part 422, Subpart M and
Part 423, Subpart M when making initial determinations regardless of whether:
• Coverage is requested for an item/service/drug that is subject to a plan’s prior
authorization requirement;
• Coverage is requested for an item/service/drug that is NOT subject to a plan’s prior
authorization requirement;
• A plan makes a decision related to coverage of an item/service/drug without first
receiving a party’s request for coverage;
• A plan receives a request for coverage before the provision of the item/service/drug
that is the subject of the request to the enrollee;
• A plan receives a request for coverage during or after the provision of the
item/service/drug that is the subject of the request to the enrollee; and/or
• A plan’s decision related to coverage of an item/service/drug will be issued during or
after the provision of the item/service/drug to the enrollee.
40.1 – Part C Organization Determinations
The Part C regulations define an “organization determination” by reference to five specific
categories of decisions; this guidance provides additional guidance on what MA plan
determinations are within that definition.
An organization determination is any determination (i.e., an approval or denial) made by an MA
plan, or its delegated entity with respect to the following:
• Payment for temporarily out of the area renal dialysis services, emergency services, post-
stabilization care, or urgently needed services;
1
When necessary, this manual also generally differentiates between the types of initial determinations including
prior authorizations; voluntary requests for items, services, and/or Part B drugs (as applicable); Part D coverage
requests; and requests for payment (Part C) or reimbursement (Part D).
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
27
• Payment for any other health services furnished by a provider (other than the MA plan),
that the enrollee believes are covered under Medicare, or if not covered under Medicare,
should have been furnished, arranged for, or reimbursed by the MA plan.
• Refusal to authorize, provide, or pay for services, in whole or in part, including the type or
level of services, which the enrollee believes should be furnished or arranged by the MA
plan;
• Reduction, or premature discontinuation, of a previously authorized ongoing course of
treatment; or
• Failure of the MA plan to approve, furnish, arrange for, or provide payment for health care
services in a timely manner, or to provide timely notice of an adverse determination, such
that a delay would adversely affect the health of the enrollee.
In circumstances where there is a question whether or not the plan will cover an item or service,
the enrollee, enrollee’s representative, or the provider on behalf of the enrollee, has the right to
request approval from the plan. Such approval requests to the plan (even if to an agent or
contractor of the plan, such as a network provider) are requests for an organization determination
and must comply with the applicable regulatory requirements. Whenever an enrollee contacts an
MA plan to request a service, the request itself indicates that the enrollee believes the MA plan
should provide or pay for the service. However, when a provider declines to furnish a service
requested by an enrollee, this is not an organization determination because the provider is
making a treatment decision (which may be based on the provider’s judgment about whether the
item or service should be part of the enrollee’s treatment plan or whether the provider is willing
to furnish the item or service, regardless of coverage by the plan).
If the enrollee wishes to request information about coverage of the benefit, the enrollee must
contact the MA plan to make a coverage request for the service in question, or the provider may
make the coverage request on the enrollee’s behalf. The MA plan must educate enrollees and
providers that when there is a disagreement with a provider’s decision to decline to furnish a
service or a course of treatment, in whole or in part, the enrollee has a right to request and
receive an organization determination from the MA plan about whether coverage of the benefit
would be provided; such determination about coverage would likely address if the item or
service is medically necessary. Further, enrollees have the right to seek treatment from other
providers (such as from another provider in the network).
40.2 – Part D Coverage Determinations
A coverage determination is any determination made by the Part D plan sponsor, or its delegated
entity, with respect to the following:
• A decision about whether to provide or pay for a drug that an enrollee believes may be
covered by the plan sponsor, including a decision related to a Part D drug that is:

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
28
o not on the plan’s formulary;
o determined not to be medically necessary;
o furnished by an out-of-network pharmacy; or
o otherwise excluded under §1862(a) of the Act if applied to Medicare Part D.
• A decision on the amount of cost sharing for a drug;
• Failure to provide a coverage determination in a timely manner, when a delay would
adversely affect the health of the enrollee (see 40.11 for more information);
• Whether an enrollee has, or has not, satisfied a prior authorization or other utilization
management requirement;
• A decision about a tiering exception request under 42 CFR §423.578(a); or
• A decision about a formulary exception request under 42 CFR §423.578(b).
Note: A plan sponsor is not required to treat the presentation of a prescription at the
pharmacy as a request for a coverage determination. Accordingly, the plan sponsor is not
required to provide the enrollee with a written denial notice at the pharmacy as a result of the
transaction. However, as required under 42 CFR §423.562(a)(3), plan sponsors must arrange
with their network pharmacies to distribute the standardized pharmacy notice developed by
CMS to notify enrollees of their right to request a coverage determination. See §40.12.3 for
information about required notification at the point of sale.
40.3 – Part D At-Risk Determinations
An at-risk determination is a decision made under a plan sponsor’s drug management program
under the rules at 42 CFR §423.153(f) that involves:
• Identification of an individual as an at-risk enrollee for prescription drug abuse;
• A limitation, or the continuation of a limitation, on access to coverage for frequently
abused drugs (i.e., an enrollee specific point-of-sale (POS) edit or the selection of a
prescriber and/or pharmacy for purposes of lock-in); or
• Information sharing for subsequent Part D plan enrollments.
An at-risk determination is subject to the existing Part D benefit appeals process and timeframes
as described in this section of the manual. If an enrollee disagrees with an at-risk determination
made under a plan sponsor’s drug management program, the enrollee has the right to request a
redetermination and potentially higher levels of appeal. Also, if on redetermination the plan
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
29
sponsor affirms, in whole or in part, its decision related to an at-risk determination, the Part D
plan sponsor must forward the case to the IRE contracted with CMS within 24 hours. See §
423.590(i). For additional information and requirements on the drug management programs that
a plan sponsor may utilize, see the Improving Drug Utilization Review Controls page on the Part
D website.
40.4 – Prior Authorization and Other Utilization Management Requirements
Part C Only
An enrollee, enrollee’s representative, or a provider on behalf of the enrollee, has the right to
voluntarily request plan approval (either pre-service or concurrent to receiving services) in
circumstances where there is a question whether the plan will cover a service, item, or Part B
drug. An enrollee’s right to voluntarily receive plan approval extends to any service, item, or
Part B drug which the enrollee believes is or should be covered by the plan (this includes non-
covered services, items, and Part B drugs and those for which the plan does not require prior
authorization (PA) as a condition for coverage in its annual Evidence of Coverage).
When a plan processes a coverage request that involves mandatory PA or other utilization
management (UM) requirement, such as step therapy for Part B drugs, the plan’s determination
on whether to grant approval of a service, item, or Part B or D drug for an enrollee constitutes an
initial determination and is subject to appeal. In addition, if a plan denies coverage of a service,
item, or Part B or D drug because the enrollee failed to seek PA or failed to comply with similar
limits on coverage, the denial also constitutes an initial determination and is subject to appeal.
2
Partially adverse determinations include coverage decisions in which the MA plan approves a
PA request at a reduced level (or approves an altogether different service, item, or Part B or D
drug) than the service, item, or Part B or D drug requested. Thus, the adjudication timeframe,
notice, and other requirements applicable to coverage determinations or organization
determinations under Part 422, Subpart M & Part 423, Subpart M apply to requests that involve
a PA or other UM requirements in the same manner that they apply to all coverage requests. If
an enrollee requests coverage of a service, item, or Part B or D drug, the plan is to respond to the
PA request, and should contact the physician or prescriber for information needed to satisfy the
PA, in accordance with the outreach guidance at §10.6. Plans, however, should not use peer-to-
peer discussions to solicit substantive modification to pending PA requests in order to improve
likelihood for approval (e.g. a peer-to-peer discussion suggesting the physician or prescriber
modify a pending PA request to a lower level of service in order to receive plan approval).
Coverage and medical necessity decisions are initial determinations subject to notification and
appeal requirements. MA plans may not interfere with an enrollee’s right to receive a requested
initial determination or obstruct the enrollee’s access to the appeal process by any means.
Part D Only
The decision to place a medication on a PA list or subject it to a UM requirement is not a
coverage determination and is not subject to appeal. However, an enrollee may request that the
2
For Part D, this denial would occur after an enrollee has formally requested a coverage determination with a Part D
plan sponsor because, as indicated in §40.2
above, the presentation of a prescription at the pharmacy counter is not
considered a request for a coverage determination unless a plan sponsor chooses to treat it as such.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
30
Part D plan sponsor waive the PA or UM requirement, which would be treated as an exception
request. Plan sponsors must determine whether a request that involves PA or other UM
requirement is either a coverage determination (or redetermination) request where an enrollee is
attempting to satisfy a PA requirement, or an exception request where the enrollee is asking the
plan sponsor to waive a PA requirement. If the plan sponsor does appropriate outreach and is
still unable to determine if the enrollee/prescriber is asking for an exception to the PA criteria
(meaning the person was unresponsive to the request), the case should be treated as an attempt to
satisfy the PA criteria.
Attempting to Satisfy a PA or other UM Requirement
A case where an enrollee/physician/other prescriber is attempting to satisfy a PA requirement
(i.e., the enrollee/physician/other prescriber is aware that a PA requirement for the prescription
drug exists and, for example, submits a PA form to the plan sponsor in an attempt to satisfy the
PA requirement) should be processed as a coverage determination. The plan sponsor must notify
the enrollee (and the prescribing physician or other prescriber involved, as appropriate) of its
decision no later than 24 hours after receiving the request for expedited cases, or no later than 72
hours after receiving the request for standard cases. Where an enrollee/physician/other
prescriber is attempting to satisfy a PA requirement and the plan sponsor has a PA form available
for seeking prior authorization for the requested drug, the plan sponsor should promptly provide
the physician or other prescriber with the PA form. An enrollee, physician, or other prescriber
may use the model Medicare Part D Coverage Determination Request Form to request an
override to a PA or other UM requirement.
Asking a Plan Sponsor to Waive a PA or other UM Requirement
Where an enrollee or an enrollee's prescribing physician or other prescriber is asking a plan
sponsor to waive a PA or other UM requirement (e.g., a physician or other prescriber indicates
that an enrollee would suffer adverse effects if he or she were required to satisfy the PA
requirement), he or she is asking for an exception and the prescribing physician or other
prescriber must submit a statement to support the request consistent with the requirements set
forth in 42 CFR §423.578(b)(5). A physician or other prescriber may use the model Medicare
Part D Coverage Determination Request Form to request an exception and/or submit a
supporting statement. If the request or supporting statement is made in writing, plan sponsors
are prohibited from requiring a physician or other prescriber to submit the request or supporting
statement on a specific form. If the exception request involves benefits not yet received, the plan
sponsor must notify the enrollee (and the prescribing physician or other prescriber involved, as
appropriate) of its decision no later than 24 hours after receiving the physician’s or other
prescriber's supporting statement for expedited cases, or no later than 72 hours after receiving the
physician’s or other prescriber's supporting statement for standard cases. Under § 423.568(b),
(effective January 1, 2020), plans may toll (i.e., not begin) the timeframe by up to 14 calendar
days after receipt of the request to receive the supporting statement (see §40.5.4). If the
exception request involves reimbursement for benefits already received, the plan sponsor must
notify the enrollee (and the prescribing physician or other prescriber involved, as appropriate) of
its decision (and make payment when appropriate) no later than 14 calendar days after receiving

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
31
the request.
40.5 – Part D Exceptions
Coverage determinations include a plan sponsor’s decision on an enrollee’s exception request,
which can be a request for an exception to the plan sponsor's tiered cost-sharing structure, or
formulary or utilization management requirement. An exception request may include a request
for benefits, a request for payment, or both.
40.5.1 – Tiering Exceptions
If a plan sponsor uses a tiered cost-sharing structure to manage its drug benefits, it must establish
and maintain reasonable and complete exceptions procedures that permit enrollees to obtain a
non-preferred drug in a higher cost-sharing tier at the more favorable cost-sharing terms
applicable to alternative drugs in a lower cost-sharing tier.
Plans are permitted to limit the availability of tiering exceptions to applicable cost-sharing tiers
containing the same type of alternative drug(s) for treating the enrollee’s condition. Specifically,
the following limitations may be applied by the plan:
• Brand name drugs are eligible for tiering exceptions to the lowest applicable cost-sharing
associated with brand name alternatives.
• Biological products are eligible for tiering exceptions to the lowest applicable cost-sharing
associated with biological alternatives.
• Non-preferred generic drugs are eligible for tiering exceptions to the lowest applicable
cost sharing associated with alternatives that are either brand or generic drugs.
Plans are not required to offer tiering exceptions for brand name drugs or biological products at
the cost-sharing level of alternative drugs, where the alternatives include only generic or
authorized generic drugs.
If a plan maintains one or two specialty tiers, as defined in 42 CFR § 423.104, the plan may
design its exception process so that Part D drugs on the specialty tier(s) are not eligible for
tiering exception(s) to non-specialty tiers. However, plans with two specialty tiers must permit
tiering exception requests for drugs on the higher cost-sharing specialty tier to the lower cost-
sharing specialty tier.
40.5.2 – Formulary Exceptions
Formulary exceptions include requests for non-formulary drugs, as well as requests to have a PA
or other UM requirement waived for that enrollee. Drugs that otherwise would not be covered
(for example, because they are obtained out of network or excluded under §1862(a) of the Act),
are not considered exceptions though these coverage determination types can be appealed

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
32
through the appeals process. A coverage determination can be requested for Non-Part D and
Excluded Drugs as described in §30.1.1 of this chapter.
Unlike under the tiering exceptions process, the regulations do not specify what level of cost
sharing applies when an exception is approved under the formulary exceptions process. Instead,
a plan sponsor has the flexibility to determine what level of cost sharing will apply for non-
formulary drugs approved under the exceptions process. However, a plan sponsor is limited to
choosing a single cost-sharing level that applies to one of its existing formulary tiers. Plans may
also elect to apply a second less expensive level of cost sharing for approved formulary
exceptions for generic drugs, so long as the second level of cost sharing is associated with an
existing formulary tier and is uniformly applied to all approved formulary exceptions for generic
drugs.
Note: Under 42 CFR § 423.578(c)(4)(iii), an enrollee is prohibited from requesting a tiering
exception for a non-formulary drug approved under the formulary exception process. If an
enrollee requests a tiering exception for an approved non-formulary drug, the request is
invalid and is dismissed by the plan. See the dismissal guidance at section 40.15. However,
a drug that is subject to a UM requirement is a formulary drug (i.e., a UM requirement placed
on a formulary drug does not make that drug a non-formulary drug). Therefore, an enrollee
who requests a UM exception and receives an approval, may also request a tiering exception
for the same formulary drug.
40.5.3 – Supporting Statements for Exception Requests
The physician's or other prescriber's supporting statement is any statement, verbal or written, that
indicates the drug is medically necessary. The statement does not have to be complete with all
required information for it to be considered received and for the timeframe to start.
The adjudication timeframes for processing exception requests are the same timeframes for other
coverage determinations under Part D (see §40.10). However, when a benefit request must be
resolved under the exceptions process, the adjudication timeframe may be tolled pending receipt
of the prescriber’s supporting statement (see 42 CFR §423.568(b), 423.570(d)(1), and
423.572(a)). CMS has developed a model Request for Additional Information form that plan
sponsors may use to request a supporting statement and/or additional information.
If upon receiving the supporting statement the plan sponsor still needs additional information, the
plan sponsor must obtain the additional information, make its decision, and notify the enrollee
and/or physician or other prescriber, as appropriate, within the following timeframes after
receiving the initial written supporting statement (i.e., the timeframe may not be tolled if the plan
sponsor asks for additional information after it has received a written supporting statement):
• 24 hours for expedited requests for benefits;
• 72 hours for standard requests for benefits; or
• 14 calendar days for reimbursement requests.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
33
If the physician or other prescriber provides a verbal supporting statement, and the plan sponsor
determines that the verbal statement does not sufficiently demonstrate the medical necessity of
the requested drug, the plan sponsor may require the physician or other prescriber to
subsequently provide a written supporting statement. If the plan sponsor requires a written
statement, it must immediately contact the enrollee’s prescribing physician or other prescriber
(or the enrollee and the enrollee’s prescribing physician or other prescriber) and request the
supporting statement. The request must explicitly state that the physician or other prescriber is
required to indicate factors (1) and/or (2) for tiering exceptions, or (1), (2), and/or (3) for
formulary exceptions discussed below, as applicable, in the written supporting statement. The
plan sponsor may also request additional supporting medical documentation as part of the written
supporting statement. If the plan sponsor requires additional medical documentation, it must
clearly identify the type of information that must be submitted.
Criteria for Tiering Exceptions
The prescriber’s supporting statement must indicate that the drug(s) in the applicable lower cost-
sharing tier(s) for the treatment of the enrollee's condition would:
(1) Not be as effective as the requested drug; and/or
(2) Have adverse effects.
If the physician provides a supporting statement indicating factors (1) and/or (2), but the plan
sponsor believes it needs additional information to support one of those factors, the plan sponsor
must obtain the additional information.
Criteria for Formulary Exceptions
The prescriber’s supporting statement must indicate that the requested drug is medically
necessary for one of the following reasons:
(1) All covered Part D drugs on any tier of the plan's formulary would not be as effective for
the enrollee as the requested non-formulary drug, and/or would have adverse effects;
(2) The number of doses available under a dose restriction for the requested drug:
a. Has been ineffective in the treatment of the enrollee’s disease or medical condition;
or
b. Based on both sound clinical evidence and medical and scientific evidence, the
known relevant physical or mental characteristics of the enrollee, and known
characteristics of the drug regimen, is likely to be ineffective or adversely affect the
drug’s effectiveness or patient compliance; or
(3) The prescription drug alternative(s) listed on the formulary or required to be used in

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
34
accordance with step therapy requirements:
a. Has been ineffective in the treatment of the enrollee’s disease or medical condition
or, based on both sound clinical evidence and medical and scientific evidence, the
known relevant physical or mental characteristics of the enrollee, and known
characteristics of the drug regimen, is likely to be ineffective or adversely affect the
drug’s effectiveness or patient compliance; or
b. Has caused or, based on sound clinical evidence and medical and scientific
evidence, is likely to cause an adverse reaction or other harm to the enrollee.
If the physician provides a supporting statement indicating factors (1), (2), and/or (3), but the
plan sponsor believes it needs additional information to support one of those factors, the plan
sponsor must obtain the additional information.
40.5.4 – Adjudication Timeframes for Coverage Determinations Involving an
Exception
The adjudication timeframes for exception requests are the same as the timeframes for other
coverage determination requests. However, if the exception request involves benefits not yet
received, the start of the timeframe may be tolled (i.e., not begin) until the plan sponsor receives
the prescriber’s supporting statement, as described in more detail below. If the exception request
is received without a supporting statement and the plan sponsor does not have sufficient
information to approve, for example, through claims history or information contained in a
previously adjudicated exception, the plan sponsor must conduct outreach to obtain it. See §10.6
for additional information regarding outreach guidelines.
Tolling the start of the adjudication timeframe is only permissible when ALL of the following
are true:
• The request is at the coverage determination level; and
• The request involves an exception; and
• The prescriber has not provided a supporting statement; and
• It involves a request for benefits (not reimbursement).
The plan must notify the enrollee (and the prescribing physician or other prescriber involved, as
appropriate) of its decision no later than 72 hours (or 24 hours in the case of an expedited
decision) after receipt of the prescriber's supporting statement or 14 calendar days after receipt of
the request, whichever occurs first (see 42 CFR §423.568(b) and 423.570(d)(1)).
If the supporting statement is not received by the end of the 14 calendar days, then the plan
sponsor must notify the enrollee (and prescriber, as appropriate) of its decision no later than 72
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
35
hours (24 for expedited cases) from the end of the 14 calendar days from receipt of the exception
request.
40.5.5 – Approval of an Exception Request
Type of
Approval
Plan Requirement
Granting an
Exception
• A plan sponsor must grant an exception when it determines that the
applicable criteria have been met.
• Once an exception is granted, the plan sponsor is prohibited from
requiring the enrollee to request approval for a refill or new
prescription to continue using the Part D prescription drug approved
under the exceptions process for the remainder of the plan year, so
long as:
o the enrollee remains enrolled in the plan;
o the physician or other prescriber continues to prescribe the drug;
and
o the drug continues to be safe for treating the enrollee’s condition.
Continuation of
Coverage Under
an Approved
Exception
• A plan sponsor may choose not to require an enrollee to resubmit an
exception request at the beginning of a new plan year.
• Whether or not a plan sponsor decides to allow coverage under an
approved exception to continue into the subsequent plan year for a
renewing enrollee the plan sponsor must send a written notice to the
enrollee at least 60 days prior to the end of the plan year, unless:
o The plan sponsor sent an approval letter to the enrollee when it
granted the exception at the coverage determination or
redetermination level which clearly identified the date that
coverage will end in the approval letter; or
o The plan sponsor sent written notice to the enrollee when it
effectuated a reversal of its adverse coverage determination or
redetermination decision by the IRE or other appeal entity, and
clearly identified the date that coverage will end in the notice.
Such notice is not the decision letter overturning the initial
adverse determination, but is a notice explaining the terms of the
approval as ordered by the IRE or other appeal adjudicator.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
36
Note: The regulations at 42 CFR §423.578(f) affirmatively state that nothing in §423.578
should be construed to mean that the prescriber's supporting statement will result in an
automatic favorable determination.
If a plan sponsor is required to send a written notice to the enrollee at least 60 days prior to the
end of the plan year or the date coverage ends, the notice must:
• Explain that the exception will not be extended,
• Provide the date that coverage will end (e.g., on December 31, 2018),
• Explain the right to request a new exception once the current exception expires, and
• Provide instructions for making a new exceptions request.
Plan sponsors are prohibited from assigning drugs approved under the exceptions process to a
designated tier, co-payment, or other cost-sharing requirement that does not otherwise exist on
the plan sponsor’s approved formulary. Additionally, drugs approved under the exceptions
process must be authorized or effectuated without the plan sponsor placing additional edits on
the request (e.g., the plan sponsor cannot place an unapproved quantity limit or duration limit on
the approved drug).
Note: For more information regarding formulary changes, such as removal of a drug from
the formulary or changing its cost-sharing status, see Chapter 6 of the Prescription Drug
Benefit Manual.
40.5.6 – Approval of a Tiering Exception Request
A plan must grant a tiering exception when it determines that factors (1) and/or (2) discussed in
§40.5.3 have been met. The regulations at 42 CFR § 423.578(f) state that nothing in the
regulations should be construed to mean that the physician’s or other prescriber’s supporting
statement will result in an automatic favorable determination. When a tiering exception is
approved, the plan sponsor must provide coverage for the drug in the higher cost-sharing tier at
the cost-sharing level that applies to the drug in the lowest applicable tier when preferred
alternatives sit on multiple lower tiers. The cost-sharing must be at the most favorable cost-
sharing tier that contains applicable alternative drugs, unless such alternative drugs are not
applicable pursuant to the limitations set forth at § 423.578(a)(6).
40.6 – Who May Request an Initial Determination
Enrollees or their representatives may make a request for all types of decisions about
coverage under both Part C and Part D.
Other parties that may request an initial determination include:
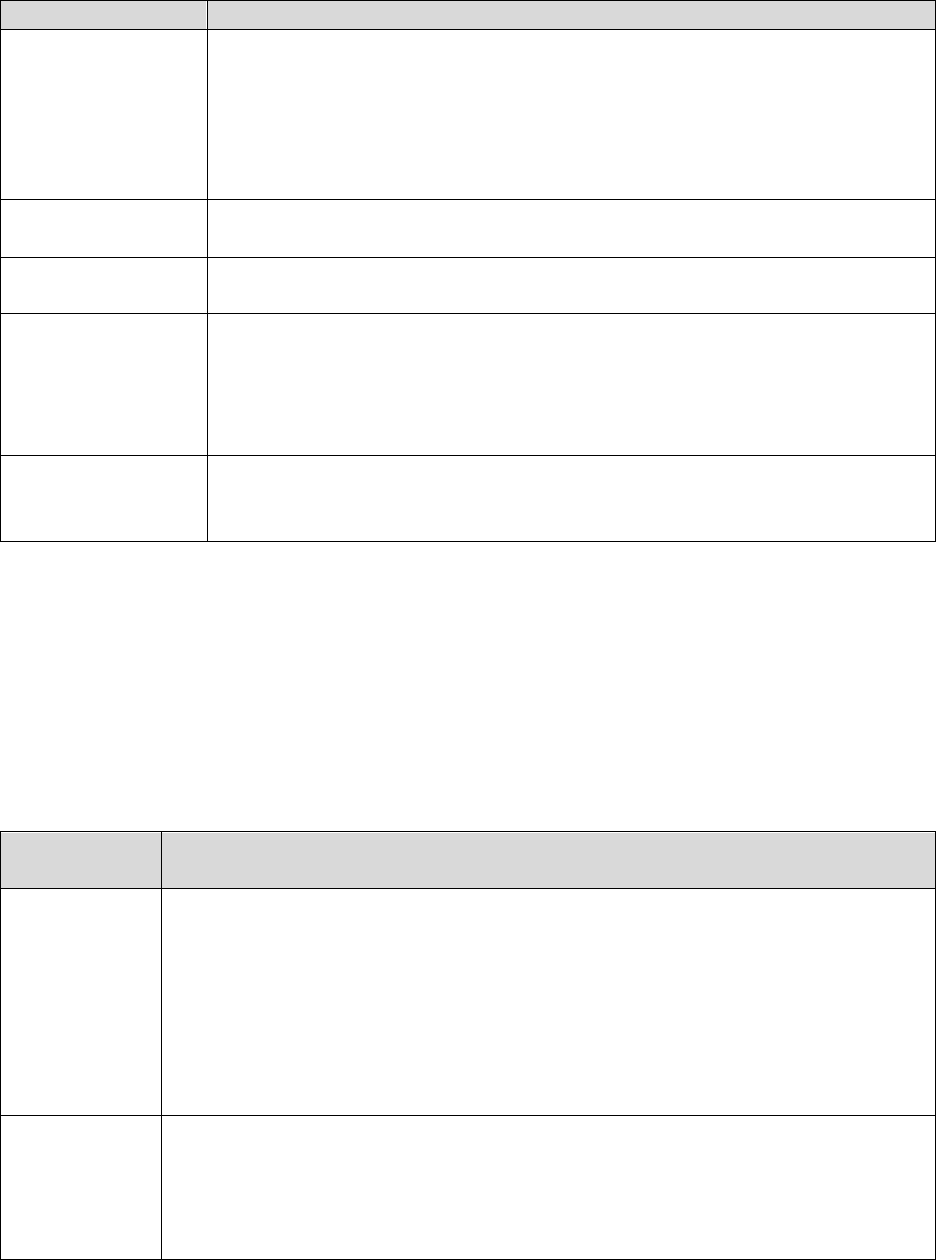
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
37
Type of Request
Who May Request
Part C, Standard
Request for Item,
Service, or Part B
Drug
• Contract or non-contract provider/physician that furnishes, or intends
to furnish, services to the enrollee.
• Staff of said provider’s/physician’s office acting on said physician’s
behalf (e.g., request is on said physician’s letterhead or otherwise
indicates staff is working under the direction of the provider).
Part C, Expedited
Request
• A physician or staff of said physician’s office acting on said
physician’s behalf (e.g., request is on said physician’s letterhead).
Part C, Payment
Request
• Contract or non-contract providers.
Part D, Standard
or Expedited
Request
• An enrollee’s prescribing physician or other prescriber.*
• Staff of said prescriber’s office acting on said prescriber’s behalf
(e.g., request is on said prescriber’s letterhead or comes from the
prescriber office fax machine).
Part D, Payment
Request
• For direct member reimbursement, only an enrollee or an enrollee’s
representative (which may be the prescribing physician or other
prescriber*) may request reimbursement under Part D.
* Pursuant to § 423.560, “other prescriber” means a health care professional other than a physician who is
authorized under State law or other applicable law to write prescriptions. It is the Part D plan sponsor’s
responsibility to identify the types of health care professionals that have prescribing authority in the states in which
the Part D plan sponsor operates.
40.7 – Guidelines for Accepting Initial Determination Requests
Plans must have processes in place for receipt and documentation of initial determination
requests, as described below.
Filing
Method
Plan Requirement
Verbal
• Establish and maintain a process for categorizing and documenting
verbal requests.
• Retain documentation of a verbal request in the case file.
• If the plan does not accept verbal requests for payment, the plan must
explain the procedures the enrollee must follow for filing a written
request.
Written
• Must accept any written request.
• The plan is prohibited from requiring requests to be on a specific form.
• Retain documentation in the case file.
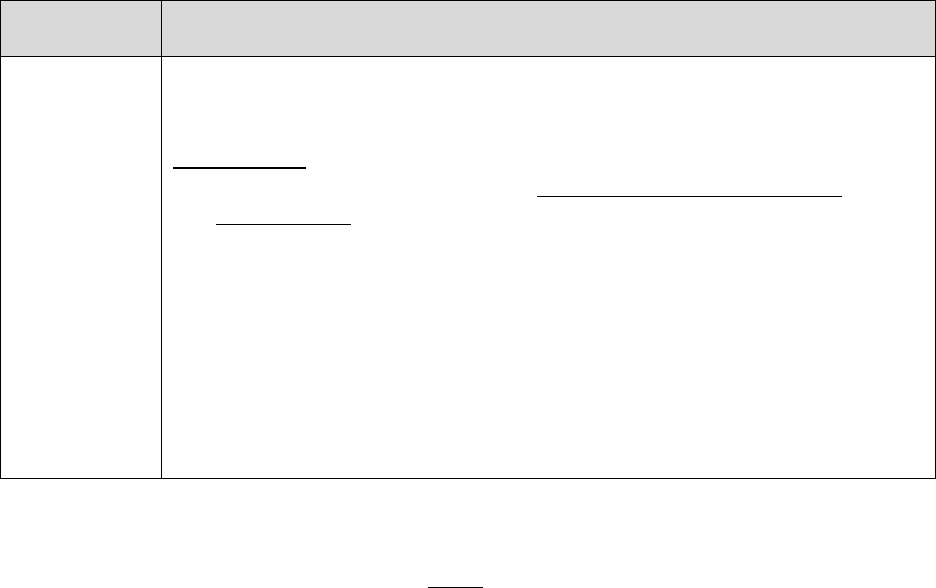
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
38
Filing
Method
Plan Requirement
• The plan or other entity may develop an approval, benefit, and/or
payment request form (for optional use).
Part D Only:
• Requests may be made on CMS’ Model Coverage Determination
Request Form.
• Part D plan sponsors may encourage enrollees to include copies of their
prescriptions with their reimbursement requests, but cannot require it.
• Plan sponsors must provide immediate access to the coverage
determination process via their internet website. The mechanism used to
accept coverage determination requests via a website is subject to the
same privacy and security safeguards as the rest of the plan sponsor’s
operations in accordance with 42 CFR §423.136.
If, upon receipt of a coverage request, a plan does not have enough information to make a
coverage decision, it must make reasonable and diligent efforts to obtain the necessary
information. For additional information see §10.6.
40.8 – How to Process Requests for Expedited Initial Determinations
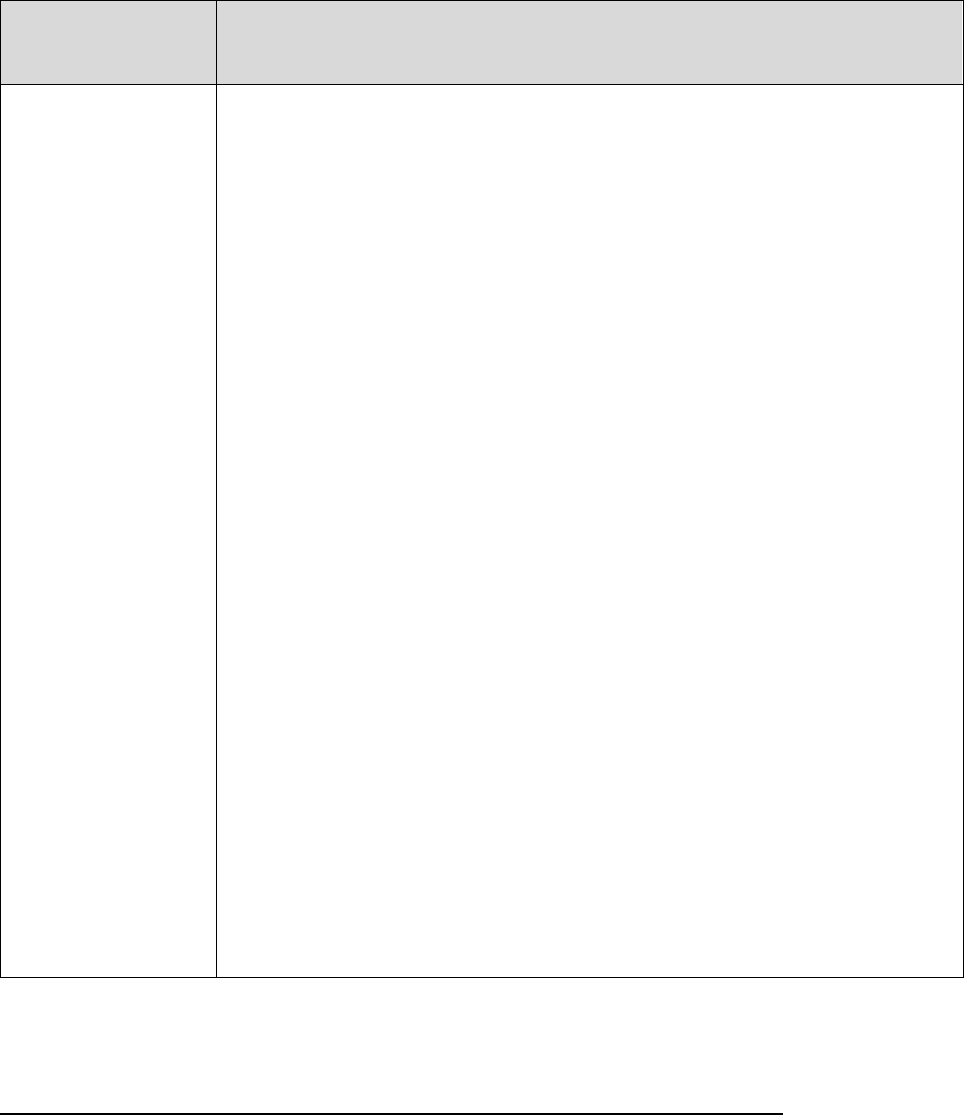
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
39
Who May Request
an Expedited
Determination
Plan Requirements
• An enrollee
• An enrollee’s
representative
• Part C: Any
physician
• Part D:
Prescribing
physician or
other prescriber
• Establish an efficient and convenient means for individuals to submit
verbal or written requests;
• Establish procedures for accepting and processing verbal and written
requests for an expedited decision;
• Develop a process for receiving the request, including designating an
office and/or department to receive both verbal or written requests and
a telephone and fax number to facilitate receipt of the requests;
• Document all verbal requests and maintain in the case file;
• Decide whether to expedite the request:
o If a request is made by an enrollee, the plan must expedite the
request if it determines that applying the standard timeframe
could seriously jeopardize the life or health of the enrollee or the
enrollee’s ability to regain maximum function.
o If a request is made or supported by a physician, prescribing
physician, or other prescriber who indicates applying the standard
timeframe could seriously jeopardize the life or health of the
enrollee or the enrollee’s ability to regain maximum function (the
physician does not have to use these exact words), the plan must
process as expedited.
o If a request involves both a payment request and a request for
approval of an item, service, or drug, the enrollee has a right to
ask for an expedited initial determination for the approval
request.
o Plans may, but are not required to, expedite payment requests.
Note: A plan must not take or threaten any punitive action against a physician who acts on
behalf or in support of a request for expedited determination.
Action Following Denial of a Request for an Expedited Initial Determination
Following denial of a request for an expedited initial determination, plans must:
• Transfer the request to the standard initial determination process;
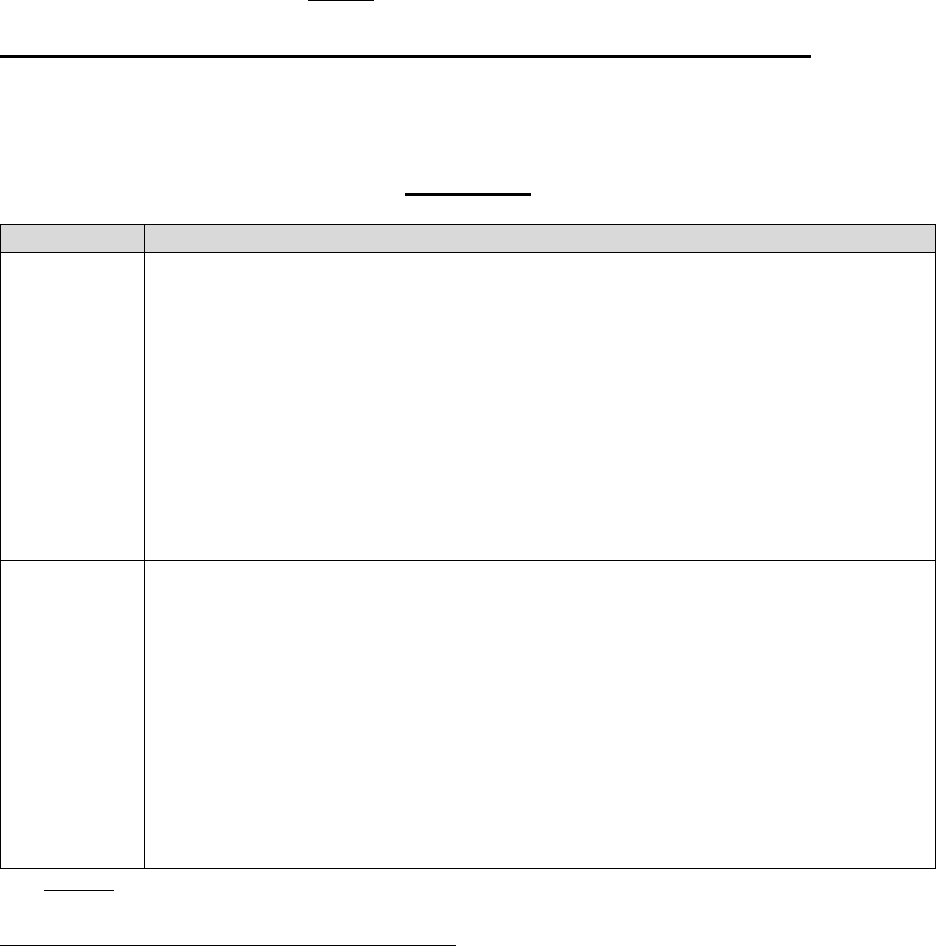
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
40
• Give the enrollee prompt verbal notice of the denial to expedite the request; and
• Deliver a written notice within 3 calendar days of the verbal notice of the denial to
expedite the request. See §40.12 for notification requirements.
Action Following Acceptance of a Request for Expedited Initial Determinations
If a plan grants a request for expedited determination, the initial determination must be made in
accordance with the following:
Part C Only
Decision
Processing Requirements for Expedited Determinations
Favorable
• Provide verbal or written notification* of favorable decision to the enrollee
(and the physician involved, as appropriate) as expeditiously as the
enrollee’s health condition requires, but no later than:
o 72 hours after receiving the request for items and services
o 24 hours after receiving the request for Part B drugs.
• If the MA plan initially provides verbal notification of its decision, it may
deliver written confirmation of its decision within 3 calendar days of the
verbal notification.
Partially
Favorable
or Adverse
• Provide written notification* to the enrollee of the decision (and the
physician involved, as appropriate) as expeditiously as the enrollee’s health
condition requires, but no later than:
o 72 hours after receiving the request for items and services.
o 24 hours after receiving the request for Part B drugs.
• If the MA plan initially provides verbal notification of its decision, it must
deliver written confirmation of its decision within 3 calendar days of the
verbal notification.
*See §40.12.1 for notification requirements.
Extension of Timeframe for Items and Services
The MA plan may only extend the 72-hour timeframe for items and services up to 14 additional
days if:
• The enrollee requests the extension; or
• The extension is justified, in the enrollee’s interest, and additional medical evidence from
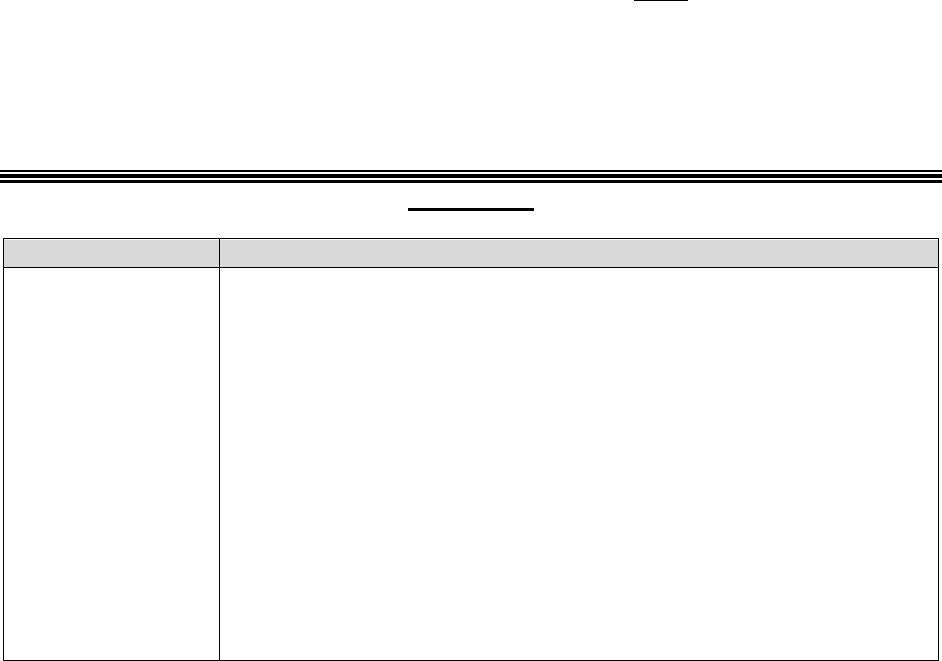
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
41
a non-contract provider is needed in order to make a decision favorable to the enrollee
(i.e., the MA plan should not extend the timeframe to get evidence to deny the coverage
request); or
• The extension is justified due to extraordinary, exigent or other non-routine circumstances
and is in the enrollee’s interest.
When the MA plan extends the timeframe, it must notify the enrollee in writing of the reasons
for the delay and inform the enrollee of the right to file an expedited grievance if they disagree
with the MA plan’s decision to grant an extension.
Note: MA plans are expected to have sufficient and appropriate contract terms to get
information and records from contract providers as necessary for expedited (and standard)
organization determinations. MA plans should not generally or regularly extend the
timeframe for an expedited organization determination to seek information or records from a
contract provider but may do so if it is justified in the enrollee’s interest and due to
extraordinary, exigent, or other non-routine circumstances.
If the MA plan needs information from a non-contract provider, the MA plan must request the
necessary information from the non-contract provider within 24 hours of the initial request for an
expedited organization determination (See 42 CFR §422.572 and §10.6 regarding Outreach for
Additional Information). Regardless of whether the MA plan needs information from non-
contract providers, the MA plan is responsible for meeting the timeframe and notice
requirements for expedited determinations.
Part B drug timeframes cannot be extended.
Part D Only
Decision
Processing Requirements for Expedited Determinations
Favorable or
Adverse
(including requests
that involve an
exception)
• The Part D plan sponsor must make the decision and deliver to the
enrollee (and the prescribing physician or other prescriber involved,
as appropriate) written notice* of its decision as expeditiously as
the enrollee’s health condition requires, but no later than 24 hours
after receiving the request or, for exceptions, receipt of the
physician's or other prescriber's supporting statement.
• If the plan sponsor initially provides verbal notification of its
decision, it must deliver written confirmation of its decision within
3 calendar days of the verbal notification.
• A plan sponsor may not extend the timeframe by dispensing a
temporary supply of the requested medication. For example, if a
plan sponsor receives a request outside of its normal business
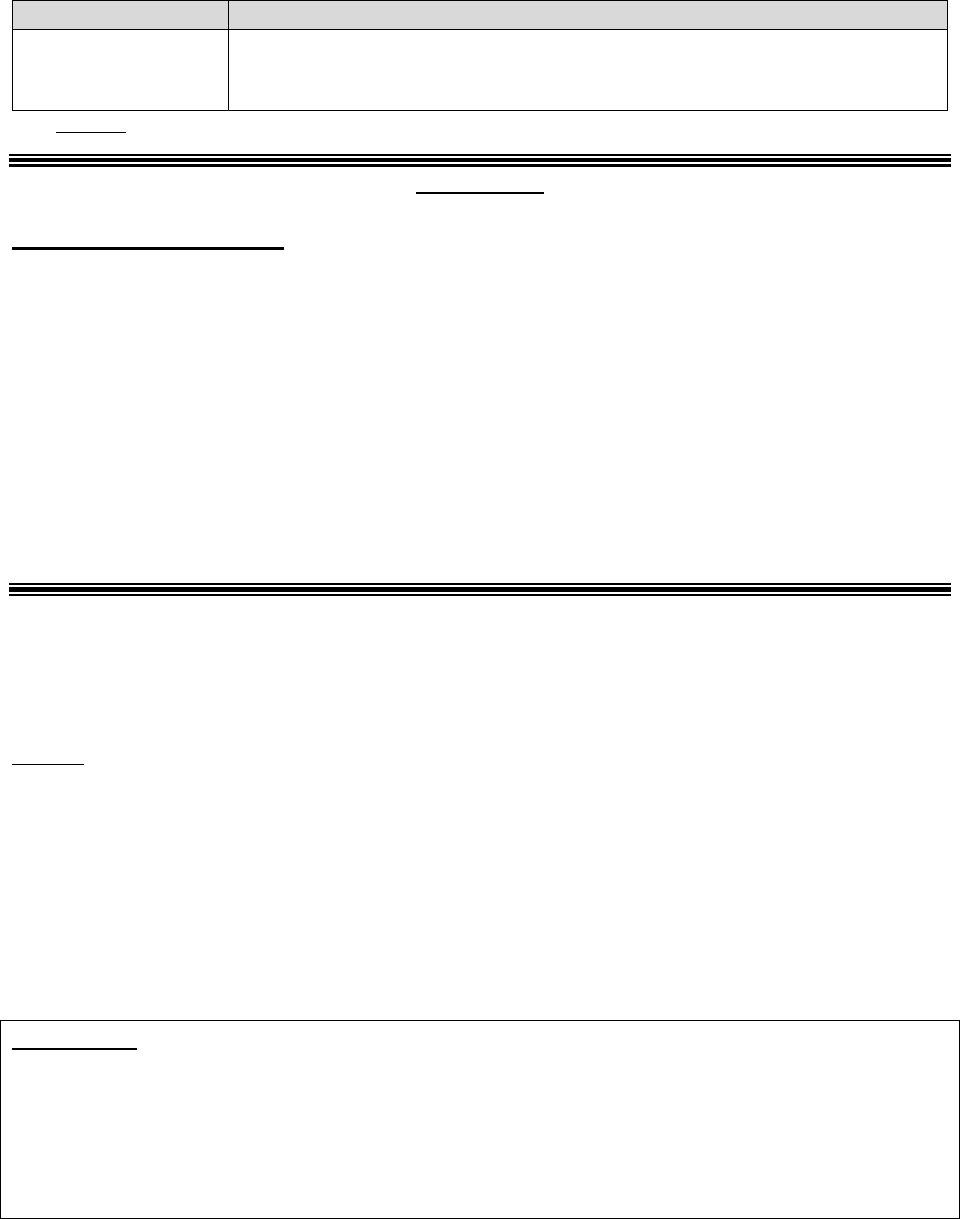
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
42
Decision
Processing Requirements for Expedited Determinations
hours, it cannot approve a 72-hour supply of the requested
medication and defer issuing a decision for 72 hours.
*See §40.12.3 for notification requirements.
Parts C & D
Change of Review Priority
After a request is initiated as a standard or expedited review, a provider may contact the plan to
change the review priority.
If the provider indicates that the enrollee’s health requires an expedited decision, the plan must
begin the applicable expedited review period at the time they receive the physician’s request to
expedite the decision.
Note: A change of priority does not allow for extra review time. If the remaining standard
review period is less than the applicable expedited review period, the original standard deadline
still applies.
40.9 – Who Must Review an Initial Determination
If a plan initially reviews a request and expects to issue a partially or fully adverse decision
based on medical necessity, the review must be completed by a physician, as defined in section
1861(r) of the Act, or other appropriate healthcare professional who has:
• Sufficient medical and other expertise;
• Knowledge of the Medicare coverage criteria; and
• A current and unrestricted license to practice within the scope of their profession in a
State, Territory, Commonwealth of the United States (that is, Puerto Rico), or the District
of Columbia.
Part C Only
With respect to the reviewing healthcare professional’s medical and other expertise, the
reviewer’s expertise must be in the field of medicine or health care that is appropriate for the
service(s) at issue.
The reviewer must apply the prudent layperson standard (as described in 42 CFR
§422.113(b)(1)) when making determinations regarding emergency services.
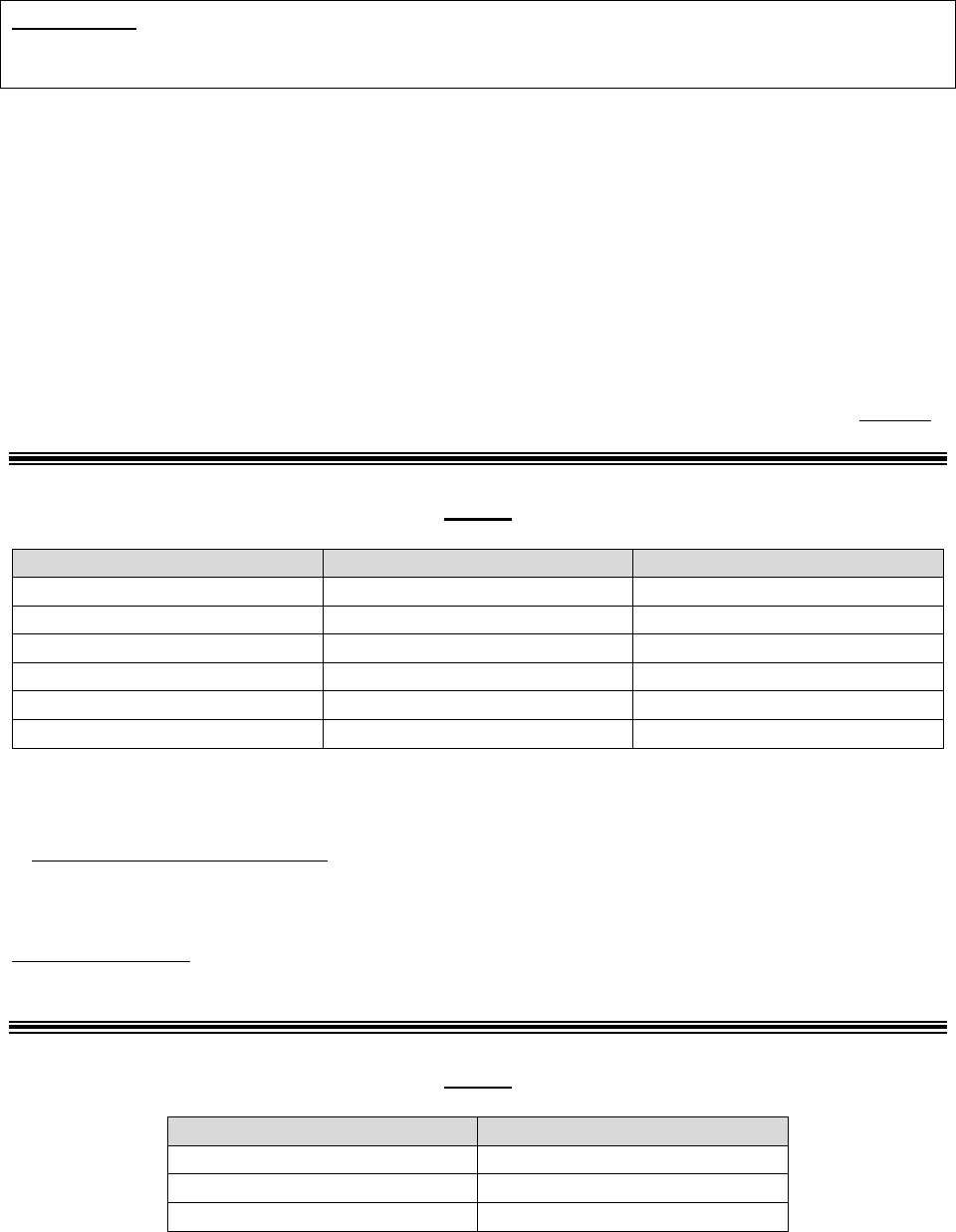
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
43
Part D Only
A pharmacist would generally be considered an appropriate health care professional for purposes
of meeting this requirement.
In general, the application of a clear statutory or contract exclusion set forth in the MA plan’s or
Part D sponsor’s Evidence of Coverage, does not constitute a decision based on the lack of
medical necessity. Conversely, an adverse decision based on a determination that the clinical
documentation supporting the coverage request is unavailable or insufficient (i.e., there is unmet
criteria) is generally considered a denial based on the lack of medical necessity.
40.10 – Processing Timeframes
Plans must have processes in place to accept coverage requests 24 hours a day, 7 days a week
(including holidays) and to notify enrollees of coverage decisions within the applicable
timeframe. For information regarding when a request is considered received, please see §10.5.2.
Part C
Type
Processing Timeframe
With Extension*
Request for Item or Service
14 calendar days
28 days
Part B Drug: Standard
72 hours
N/A
Payment: Clean Claims
30 days**
N/A
Payment: Other Claims
60 days**
N/A
Expedited
72 hours
17 days
Part B Drug: Expedited
24 hours
N/A
*14-day extension if the enrollee requests the extension or if the MA plan justifies a need for additional information
and documents how the delay is in the best interest of the enrollee. MA plan must notify enrollee in writing if
extension is going to be taken and explain the reason for the delay. Note: Part B drug and payment timeframes
cannot be extended. See 42 CFR §422.568(b)(1) and (2).
**Non-contract providers and enrollees: The MA plan must pay 95 percent of clean claims within 30 calendar days
of the request. All other claims submitted by non-contract providers or enrollees must be paid or denied within 60
calendar days from the date of the request. For additional guidance, see 42 CFR §422.520.
Contract providers: The timeframe for processing payment requests is based on the contract
terms between the MA plan and the provider. For additional guidance, see 42 CFR §422.520.
Part D
Type
Processing Timeframe
Standard
72 hours*
Expedited
24 hours*
Payment**
14 calendar days
*Or no later than 24/72 hours after receiving the physician's or other prescriber's supporting statement if the request

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
44
involves an exception (see §40.5.4 of this guidance for timeframes and additional information regarding exception
requests).
**Includes claims submitted by physicians, prescribers, and enrollees.
A Part D plan sponsor may not extend the applicable adjudication timeframe by dispensing a
temporary supply of the requested medication. For example, if a plan sponsor receives a request
outside of its normal business hours, it cannot approve a 72-hour supply of the requested
medication and defer issuing a decision for 72 hours; the plan sponsor must make its
determination within the applicable timeframe.
40.11 – Effect of Failure to Meet the Timeframe for an Initial Determination
Part C Only
The MA plan must explain in its annual Evidence of Coverage (EOC) that enrollees have the
right to a level 1 appeal if the MA plan fails to provide timely notice of a decision. If a plan fails
to provide the enrollee with a timely notice of its decision, this failure constitutes an adverse
decision.
Part D Only
If the Part D plan sponsor does not provide the enrollee notice of its coverage determination
within the required timeframe, this constitutes an adverse decision and the plan sponsor must
forward the complete case file to the IRE within 24 hours of the expiration of the adjudication
timeframe. The case file must contain the enrollee’s request and any verbal and/or written
evidence obtained by the plan sponsor. Refer to §50.12.3 to determine how to prepare the case
file for the IRE and what documents/items to send with the case file.
Although the plan sponsor failed to provide notice of a decision within the required timeframe,
the plan sponsor is not required to send the adverse decision notice, but instead, must notify the
enrollee that the decision was not made timely and is being forwarded to the IRE for review.
The plan sponsor should send the notification to the enrollee within 24 hours of the expiration of
the adjudication timeframe. Please see §40.12.2 for enrollee notification requirements.
Note: Because the adjudication timeframe for an exception request involving a request for
benefits does not begin until the plan sponsor receives the physician’s or other prescriber’s
supporting statement as indicated in §40.5.4, plan sponsors must not automatically forward case
files to the IRE if a supporting statement is not received.
When a plan sponsor makes a fully favorable decision on a coverage determination in less than
24 hours after the end of the adjudication timeframe, the plan sponsor should consider
effectuating and notifying the enrollee of the favorable decision (within the 24-hour period the
case must be forwarded to the IRE) in lieu of forwarding the case to the IRE.
If CMS determines that the plan sponsor has a pattern of not issuing timely decisions or not
forwarding the enrollee's request to the IRE for review within the required timeframe, the plan
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
45
sponsor may be considered to be out of compliance with the terms of its Part D contract and/or
subject to intermediate sanctions in accordance with subpart O of 42 CFR Part 423.
Transition Period Note: Plan sponsors must ensure new enrollees receive a meaningful
transition process when they have been, prior to enrollment, stabilized on a medication that is
either not on the plan formulary or is subject to utilization management requirements.
Two of the steps involve plan sponsors providing a temporary supply of the requested
medication and sending the enrollee a written notice explaining when the supply will end and the
procedures for requesting an exception. A transition process is not meaningful if an enrollee
who is in the transition period files an exception request and the plan sponsor does not make a
timely decision or does not forward the enrollee's request/case file to the IRE within the
appropriate timeframe. Therefore, when an enrollee who is in the transition period files an
exception request and the plan sponsor does not make its decision timely and/or fails to forward
a request/case file to the IRE as required, the plan sponsor must provide the enrollee with a
temporary supply of the requested prescription drug (when not medically contraindicated) until
the case is resolved by the plan sponsor or the IRE issues a reconsideration decision.
For more information about the Part D transition policy, see Chapter 6, §30.4 of the Prescription
Drug Benefit Manual.
40.12 – Notification Requirements for Initial Determinations
Plans must provide notices for initial determinations using the most efficient manner of delivery
to ensure the enrollee receives the notice in time to act. If the request was filed by the enrollee’s
representative, the representative must be notified in lieu of the enrollee. Plans may provide
notice to both the representative and enrollee, but are not required.
40.12.1 – Part C Notification Requirements
Item, Service, or Part B Drug Approvals
For favorable decisions on a request for an item, service, or Part B drug, notice may be provided
verbally or in writing to the requesting party. Verbal or written notice of a favorable decision
should explain any conditions of the approval, such as the duration of the approval. As a best
practice, MA plans are encouraged to provide written notice of favorable decisions (again,
including any applicable conditions/parameters of the approval). If a provider submits the
request on behalf of the enrollee, the MA plan must notify the enrollee as well as the provider of
its determination. If the enrollee’s representative submits a request, the representative must be
notified in lieu of the enrollee. Plans may provide notice to both the representative and enrollee,
but are not required.
If the enrollee agrees, the MA plan may send the notice by fax or e-mail. Please see Medicare
Communications and Marketing Guidelines regarding electronic communication with enrollees.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
46
Denials and Discontinuation/Reduction of Previously Authorized Ongoing Course of
Treatment
A written denial notice is required to be sent to the enrollee (and physician involved, as
appropriate) whenever an MA plan’s determination is partially or fully adverse to the enrollee.
For Part C organization determination denials, MA plans must use approved notice language
when issuing written denial notices to enrollees.
The standardized denial notice is the Notice of Denial of Medical Coverage or Payment (Form
CMS-10003-NDMCP), also known as the Integrated Denial Notice (IDN). MA plans may use a
separate written notice of denial document, such as a plan-generated claims statement to the
enrollee or provider, but must use the approved standard language. An example of a plan-
generated statement is an Explanation of Benefits (EOB), detailing what the MA plan has paid
on the enrollee’s behalf, and/or the enrollee’s liability for payment.
If an MA plan uses its existing system-generated notification (i.e., EOB) regarding payment
denials as its written notice of determination, the MA plan must ensure that the EOB contains the
OMB-approved language of the IDN verbatim in its entirety and meets the content requirements
as described in the IDN form instructions and listed below. When issuing an EOB in place of the
IDN, the MA plan must notify the enrollee via the EOB within the required timeframe. When
providing the decision, the MA plan must also take into account the enrollee’s presenting
medical condition, disabilities, and special language requirements, if any (see Section 10.4.4 for
additional information).
Note: The Advanced Beneficiary Notice of Non-Coverage (ABN), Form (CMS-R-131) does not
comply with MA organization determination requirements of 42 CFR, Part 422, Subpart M and,
therefore, shall not be used by MA plans or contracted providers. When a MA plan wishes to
inform an enrollee that a service is not covered, in whole or in part, it must issue the IDN or
include the same OMB-approved standardized language in its EOB. If a provider believes an
item, service or Part B drug may not be covered, the provider must advise the enrollee to request
prior approval from the MA plan or the provider may request prior approval on the enrollee’s
behalf. The failure to provide notice via the OMB-approved standardized language contained in
the IDN or via a clear exclusion in the plan’s EOC, consistent with the beneficiary protection
provisions in Chapter 4, means the enrollee is not liable for items, services or Part B drugs
provided by a contracted provider or upon referral from a contracted provider (see 42 CFR §
422.105; see also Chapter 4, Section 160, of the Medicare Managed Care Manual for more
information on beneficiary protections related to plan-directed care, including enrollee liability
protections).
When using the standardized IDN (see 42 CFR § 422.568(e)), the MA plan must provide:
• A specific and detailed explanation of why the medical services, items or Part B drugs
were denied, including a description of the applicable coverage rule or applicable plan
policy (e.g., Evidence of Coverage provision) upon which the action was based, and a
specific explanation about what information is needed to approve coverage must be
included, if applicable;
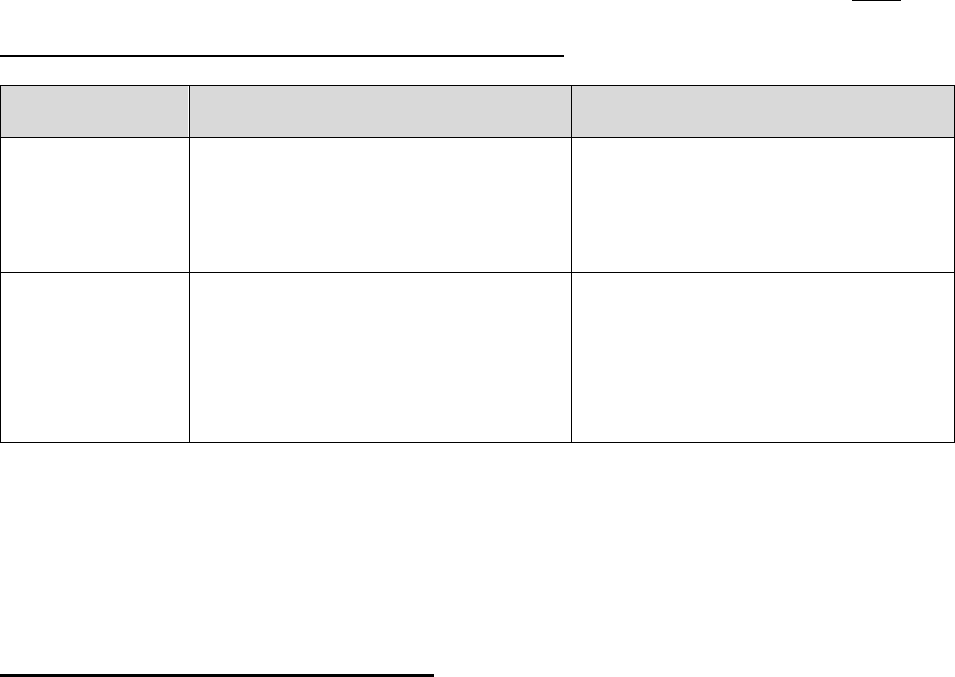
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
47
• Information regarding the enrollee’s right to appeal and the right to appoint a
representative to file an appeal on the enrollee’s behalf;
• For service denials, a description of both the standard and expedited appeal processes,
including the specific department or address for reconsideration requests and a description
of conditions for obtaining an expedited reconsideration, the timeframes for each, and the
other elements of the appeals process;
• For payment denials, a description of the standard reconsideration process and timeframes,
and the rest of the appeals process;
• The enrollee’s right to submit additional evidence in writing or in person; and
• An explanation of a provider’s refusal to furnish an item, service, or Part B drug (if
applicable).
MA plans are not required to issue an IDN if there is no enrollee liability beyond the applicable
cost sharing. An EOB would be issued and indicate any applicable cost sharing.
For provider notice requirements in hospital, SNF, HHA, and CORF settings, please see §100.
Enrollee and Non-contract Provider Payment Requests
Requestor Payment Approval Payment Denial
Enrollee or
Representative
Receives payment and EOB.
• The enrollee or representative
receives an IDN or an EOB.*
• Document must include notice of
appeal rights.
Non-contract
provider
• Provider receives payment and
remittance notice (see “Non-
Contract Provider Payment
Request section below).
• Enrollee receives EOB.
• Provider receives remittance
notice (see “Non-Contract
Provider Payment Request
section below).
• Enrollee receives EOB* with
appeal rights.
*When issuing an EOB in place of the IDN, the MA plan must notify the enrollee via the EOB within the required
timeframe. An IDN is not required if there is no enrollee liability beyond the applicable cost sharing, however, an
EOB would be issued and include any applicable cost sharing.
Note: For approved and denied payment requests from a contracted provider, the enrollee
receives an EOB. Terms of remittance for contract providers are determined by the contract
between the MA plan and the provider.
Non-Contract Provider Payment Requests
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
48
If the MA plan approves a request for payment from a non-contract provider, the provider
receives payment and a remittance advice/notice.
If the MA plan denies a request for payment from a non-contract provider, the MA plan must
notify the non-contract provider of the specific reason for the denial and provide a description of
the appeals process. MA plans must deliver either a remittance advice/notice or other similar
notification that states the non-contract provider:
• Has the right to request a reconsideration of the MA plan’s denial of payment,
• Must submit a Waiver of Liability form holding the enrollee harmless regardless of the
outcome of the appeal. (MA plans must include the form as an enclosure or attachment
and/or provide a direct link to the form);
• Has 60 calendar days from the remittance notification date to request a reconsideration;
• Should include documentation, such as a copy of the original claim or remittance
notification showing the denial, and must include any clinical records and other
documentation that supports the provider’s argument for reimbursement; and
• Return the request for reconsideration to the MA plan following the instructions provided
by the plan on where to send the request.
MA plans may not use the CMS standardized form, the IDN, to notify non-contract providers of
a claim denial. However, MA plans may use the IDN as a model template to develop a non-
contract provider denial notice with appeal rights in accordance with the above requirements.
Denial of a Request for an Expedited Organization Determination
Notice of the denial of a request for an expedited organization determination must:
• Explain that the MA plan will automatically transfer and process the request using the
required timeframe for standard requests;
• Inform the enrollee of the right to file an expedited grievance if he or she disagrees with
the MA plan’s decision not to expedite the determination (MA plans may use a Part C
model notice, Notice of Right to an Expedited Grievance);
• Provide instructions about the expedited grievance process and its timeframes; and
• Inform the enrollee of the right to resubmit a request for an expedited determination with a
physician, prescribing physician, or other prescriber’s support, including that if the
enrollee gets the support indicating that applying the standard timeframe for making
determinations could seriously jeopardize the life or health of the enrollee or the enrollee’s
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
49
ability to regain maximum function, the request will be expedited automatically.
40.12.2 – Part D Notification Requirements
Approvals
For favorable decisions, Part D plan sponsors must adhere to the following notification
requirements:
• Enrollee notification must be in writing. If the enrollee’s representative submits a
request, the representative must be notified in lieu of the enrollee. Plans may provide
notice to both the representative and enrollee, but are not required.
• Written notices must explain the conditions of the approval (the plan sponsor may
develop its own written approval notice). The conditions of approval may include (but
are not limited to):
o The duration of the approval;
o Limitations associated with an approval; and/or
o Any coverage rules applicable to subsequent refills.
Verbal notice may initially be provided to the enrollee as long as written notice is mailed within
3 calendar days of verbal notification.
If requested by an enrollee’s prescribing physician or other prescriber on behalf of the enrollee,
the plan sponsor must provide notice to the prescriber and written notice to the enrollee.
If a plan sponsor successfully notifies the physician or prescriber verbally, the plan sponsor does
not need to send a written follow-up.
Denials
For requests denied in whole or in part, plan sponsors must adhere to the following notification
requirements:
• Enrollee notification must be in writing. If the enrollee’s representative submits a request,
the representative must be notified in lieu of the enrollee. Plans may provide notice to
both the representative and enrollee, but are not required.
• If notice is delivered within required timeframe, enrollee receives Notice of Denial of
Medicare Prescription Drug Coverage, Form CMS-10146 (see §40.12.3 for specific CMS-
10146 requirements).

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
50
Verbal notice may initially be provided to the enrollee as long as written notice is mailed within
3 calendar days of verbal notification.
If the request was made by an enrollee’s prescribing physician or other prescriber on behalf of
the enrollee, the plan sponsor must provide notice to the prescriber and written notice to the
enrollee.
If a plan sponsor successfully notifies the physician or prescriber verbally, the plan sponsor does
not need to send a written follow-up to the physician or prescriber, but must still send written
notice to the enrollee.
Denial of a Request for an Expedited Review
If the plan sponsor denies a request to expedite a coverage determination, it must transfer the
request to the standard coverage determination process (as described in §40.8), provide prompt
verbal notice of the denial, and subsequently deliver (i.e. mail) written notice within 3 calendar
days after providing verbal notice.
• If an enrollee has identified a representative, the plan sponsor must provide notice to the
enrollee's representative instead of the enrollee.
• If an enrollee's prescribing physician or other prescriber files a request on behalf of an
enrollee, the plan sponsor must notify both the prescriber and the enrollee. The enrollee
must receive written notice of the decision.
The verbal notice and written follow-up notice must:
• Explain that the plan will automatically transfer and process the request using the 72-hour
time frame for standard determinations;
• Inform the enrollee of the right to file an expedited grievance if he or she disagrees with
the plan’s decision not to expedite the determination;
• Inform the enrollee of the right to resubmit a request for an expedited determination and
that, if the enrollee gets his or her prescribing physician’s or other prescriber's support
indicating that applying the standard time frame for making determinations could
seriously jeopardize the life or health of the enrollee or the enrollee’s ability to regain
maximum function, the request will be expedited automatically; and
• Provide instructions about the expedited grievance process and its time frames.
CMS has developed a model notice, Notice of Right to an Expedited Grievance, that Part D plan
sponsors can use whenever a request to expedite is denied.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
51
Untimely Decisions
If the plan sponsor fails to provide notice of a decision within the required timeframe, the plan
sponsor is not required to send the adverse decision notice, but instead, the plan sponsor should
notify the enrollee that the decision was not made timely and is being forwarded to the IRE for
review. The notice must:
• Advise the enrollee of his/her right to submit additional evidence that may be pertinent to
the enrollee’s case, if the enrollee chooses;
• Direct the enrollee to submit such evidence to the independent review entity; and
• Include information on how to contact the independent review entity.
CMS has developed a model Notice of Case Status that plan sponsors can use in lieu of the
adverse decision notice to notify enrollees whenever cases are forwarded to the IRE.
40.12.3 – Part D Coverage Determination Notices
Notification by Network Pharmacies: Medicare Prescription Drug Coverage and Your
Rights
When a pharmacist explains to an enrollee that a drug is not on a Part D plan's formulary, or is
subject to prior authorization, step therapy, or other limitation, the transaction does not constitute
a coverage determination, unless the plan sponsor treats the presentation of the prescription as a
request for a coverage determination.
Plan sponsors must arrange with network or preferred pharmacies to provide enrollees with a
written copy of the standardized pharmacy notice (Medicare Prescription Drug Coverage and
Your Rights, Form CMS-10147) when the enrollees’ prescription cannot be filled under the Part
D benefit and the issue cannot be resolved at the point of sale. Permissible exceptions to this
requirement are detailed below. CMS expects plan sponsors to have internal controls in place to
reasonably ensure that network pharmacies are complying with this requirement and must
arrange with their network pharmacies (including mail-order and specialty pharmacies) to
distribute the notice to enrollees. The pharmacy notice must be delivered to the enrollee if the
pharmacy receives a transaction response indicating the claim is not covered by Part D and the
designated NCPDP response code is returned.
The designated NCPDP response code is NOT returned in the following scenarios (this list is not
all-inclusive):
• The claim rejects only because it does not contain all necessary data elements for
adjudication;
• The drug in question is an over the counter (OTC) drug that is not covered by the

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
52
enrollee’s plan;
• The prescription is written by a sanctioned provider who has been excluded from
participation in the Medicare program;
• The drug is not listed on the participating CMS Manufacturer Labeler Code List;
• The drug is not listed on the FDA Electronic List—NDC Structured Product Labeling
Data Elements File (NSDE);
• The Part D plan sponsor rejects the claim for the drug in question only because of a “refill
too soon/early refill” edit;
• The claim is rejected at POS due to a limitation on access to coverage for frequently
abused drugs under a plan sponsor’s drug management program (DMP), such as a POS
edit that limits the quantity, dose, or specific drugs that will be covered for the patient or a
prescriber or pharmacy lock-in edit.
• The drug in question is rejected by the Part D plan benefit, but is covered by a co-
administered insured benefit managed by a single processor. In this scenario, the
pharmacy submits a single claim transaction for the drug and the drug is covered by the
co-administered insured benefit after being rejected by Part D and processed in
accordance with the benefits offered by the supplemental payer.
Note: If the drug is not covered by the Part D plan, but the enrollee pays for the cost of the
drug pursuant to plan-sponsored negotiated pricing or a discount card program (which may
provide a lower price but leaves the enrollee responsible for 100 percent of the drug cost), a
designated NCPDP response code will be returned notifying the pharmacy to deliver a copy
of the pharmacy notice to the enrollee.
For Mail Order Pharmacies:
The notice should be delivered to the enrollee via the enrollee’s preferred method of
communication (fax, electronic, or first-class mail) as expeditiously as the enrollee’s health
condition requires, but no later than 72 hours from the pharmacy’s receipt of the original
transaction response indicating the claim is not covered by Part D.
For Home Infusion Pharmacies:
Enrollees brought on service by the home infusion pharmacy, the pharmacy can also choose to
deliver the notice in person with delivery of home infusion drugs or through an infusion nurse, as
long as the next scheduled visit is within 72 hours of the receipt of the transaction code
indicating the claim cannot be covered by Part D.
For Pharmacies Serving Long-Term Care Facilities:

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
53
Given the uniqueness of the long-term care (LTC) setting, there is typically no point-of-sale
encounter between the pharmacy and the enrollee (LTC resident) and, therefore, no practical
means for the pharmacy to deliver the notice directly to the enrollee. In most instances where
there is an issue with the prescription, CMS expects that the pharmacist will contact the
prescriber or an appropriate staff person at the LTC facility to resolve the matter and ensure the
resident receives the needed medication or an appropriate substitute, obviating the need to
deliver the notice. If the matter cannot be resolved, the pharmacy must fax or otherwise deliver
the notice to the enrollee, the enrollee’s representative, prescriber, or an appropriate staff person
at the LTC facility as expeditiously as the enrollee’s health condition requires, but no later than
72 hours from the pharmacy’s receipt of the original transaction response indicating the claim is
not covered by Part D.
Note: If the enrollee is a self-pay resident and the pharmacy cannot fill the prescription
under the Part D benefit, the pharmacy must, upon receipt of the transaction response, fax or
otherwise deliver the notice to the enrollee, the enrollee’s representative, prescriber, or an
appropriate staff person at the LTC facility. After distribution of the notice, the LTC
pharmacy should continue to work with the prescriber or facility to resolve the matter and
ensure the resident receives the needed medication or an appropriate substitute.
For Indian Health Service, Tribe and Tribal Organization, and Urban Indian Organization
(I/T/U) Pharmacies:
Because IHS enrollees’ prescription drugs, when dispensed through I/T/U pharmacies, are filled
and dispensed at no cost to the enrollee regardless of whether the drug is rejected at POS by the
Part D plan, I/T/U pharmacies are exempt from the requirement to distribute the pharmacy
notice.
Note: This exemption applies only to I/T/U pharmacies that dispense prescriptions at no cost
to the enrollee. Any network commercial pharmacy providing services to IHS-eligible Part D
enrollees must distribute the notice in accordance with the requirements in this section.
Standardized Denial Notice: Notice of Denial of Medicare Prescription Drug Coverage
The Part D plan sponsor must use the approved standardized denial notice (Notice of Denial of
Medicare Prescription Drug Coverage, Form CMS-10146). The standardized denial notice has
been written in a manner that is understandable to the enrollee and provides:
• The specific reason for the denial that takes into account the enrollee’s presenting medical
condition, disabilities, and special language requirements, if any (see section 10.4.4 for
additional information);
• A description of any applicable Medicare coverage rule or any other applicable Part D
plan policy upon which the denial decision was based, including any specific formulary
criteria that must be satisfied for approval.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
54
• If the drug could be approved under the exception rules, the denial notice must explicitly
state the need for a prescriber’s supporting statement and clearly identify the type of
information that should be submitted when seeking a formulary or tiering exception;
• Information regarding the right to appoint a representative to file an appeal on the
enrollee’s behalf;
• For coverage denials, a description of both the standard and expedited redetermination
processes and timeframes, including conditions for obtaining an expedited
reconsideration, and the rest of the appeals process; and
• For payment denials, a description of the standard redetermination process and
timeframes, and the rest of the appeals process.
The denial rationale must be specific to each individual case and written in a manner calculated
for an enrollee to understand. See the Notice of Denial of Medicare Prescription Drug Coverage,
along with the instructions and examples of the denial rationale for additional guidance.
Plan sponsors must complete the applicable sections of the model Request for Redetermination
of Medicare Prescription Drug Denial form and send it to the enrollee (and physician or other
prescriber when appropriate) with each adverse coverage determination notice. Enrollees are not
required to use the model notice when submitting a redetermination request.
Note: Plan sponsors that do not deliver notice within the required timeframe should not use
the Notice of Denial of Medicare Prescription Drug Coverage form, but should provide
notice that the case has been forwarded to the IRE (plan sponsors may use the model Notice
of Case Status).
40.13 – Procedures for Handling Misclassified Initial Determinations
If the plan misclassifies a coverage request as a grievance and later discovers the error, the plan
must notify the enrollee in writing that the issue was misclassified and will be handled as a
coverage request. The timeframe for processing the request begins on the date the request is
received by the plan, not the date the plan discovers its error (please see §40.11 when a plan fails
to meet the timeframe for processing an initial determination). Plans are expected to audit their
own coverage and grievance processes for the presence of errors and institute appropriate quality
improvement projects as needed.
40.14 – Withdrawal of an Initial Determination Request
A request for an initial determination be withdrawn at any time before the decision is issued.
This request must come from the party who requested the initial determination. If a request to
withdraw is filed with the plan, the plan will dismiss the initial determination request. The
request to withdraw may be either written or verbal. See guidance related to dismissals at §
40.15.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
55
40.15 – Dismissal of an Initial Determination Request
Plans must dismiss a request for an initial determination under any of the following
circumstances:
• The individual or entity making the request is not permitted to request an initial determination
under the applicable regulation.
• The plan determines that the individual or entity making the request failed to make a valid
request for an initial determination that substantially complies with 42 CFR §§ 422.568(a) or
423.568(a). A valid request, as contemplated in §§ 422.568(a) and 423.568(a), includes
sufficient information to identify the enrollee to allow the plan to adjudicate the request
(or, at a minimum, make contact with the enrollee to clarify the request), including a full
name or member ID number or at least one means of contact (e.g., address, telephone
number, email). In addition, under Part D, an enrollee may not request a
tiering exception for an approved non-formulary prescription drug. See 42 CFR §
423.578(c)(4)(iii). In this circumstance, a plan would dismiss the request and issue a
dismissal notice in accordance with the notice requirements at § 40.15.1.
• The enrollee dies while the request is pending and the enrollee’s spouse or estate has no
remaining financial interest in the case and no other individual or entity with a financial interest
in the case wishes to pursue the initial determination. Financial interest means having financial
liability for the item(s) or service(s) underlying the coverage request.
• The individual or entity who requested the review submits a timely verbal or written request for
withdrawal of their request for an initial determination with the plan.
When the plan’s dismissal is due to a timely withdrawal request, the plan is required to dismiss the initial
determination request and issue a dismissal notice in accordance with the notice requirements at section
40.15.1 in order to preserve the rights of other proper parties to the decision who may wish to request
review of the dismissal.
The guidance in 40.15 does not alter reporting requirements. Withdrawn requests and dismissals
should continue to be reported separately in their distinct categories, per existing reporting
requirements.
NOTE: The above list of circumstances (taken from the applicable regulations) under which a
plan must dismiss a request for an initial determination is exhaustive. A plan may not deem a
request invalid or dismiss a request for an initial determination for any reason not explicitly
included in 42 CFR §§ 422.568(g) and 423.568(i), as applicable.
40.15.1 – Dismissal Notice
If a plan dismisses an initial determination request, the plan must mail or otherwise transmit a
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
56
written notice of the dismissal to the parties at their last known address by the conclusion of the
applicable adjudication timeframe.
The dismissal notice must state all of the following:
(1) The reason for the dismissal;
(2) The right to request that the plan vacate the dismissal action; and
(3) The right to request review of the dismissal.
Consistent with the timeframe for requesting a timely appeal of an initial determination, a
request for review of a dismissal must be filed within 60 calendar days from the date of the
plan’s dismissal notice.
Plans may use, and modify as necessary, the model Coverage Dismissal Notice when notifying
an enrollee of a dismissal.
40.15.2 – Dismissal Binding Unless Modified, Reversed or Vacated
A plan’s dismissal of an initial determination request is binding unless it is modified or reversed
by the plan upon appeal or the dismissal is vacated for good cause. Upon receipt of a request to
review a dismissal, the plan will conduct an appeal in accordance with §50 of this guidance,
including the applicable adjudication timeframes for redeterminations and reconsiderations.
Requests for Review of a Dismissal of an Initial Determination Request
If a party appeals a plan’s dismissal of an initial determination request and the plan determines
that its dismissal was in error, the plan reverses the dismissal and processes the request for
coverage in accordance with applicable adjudication timeframes and notice requirements. See
Section 40.10. The timeframe for the initial determination begins on the date/time of the plan’s
decision to reverse its dismissal.
If a party appeals a plan’s dismissal of an initial determination request and the plan upholds its
dismissal, there is no further right to appeal the dismissal to a higher-level adjudicator.
However, in addition to the right to appeal a dismissal, an enrollee has the right to request that
the plan vacate the dismissal action.
Requests to Vacate Dismissal of an Initial Determination Request
A plan may vacate its own dismissal if good cause is established within 6 months of the date of
the notice of the dismissal. A plan may find good cause to vacate a dismissal if, for example, the
plan determines the dismissal was issued in error because the documentation in the
administrative case file shows the reason for dismissing the request was incorrect. For examples
of where good cause may exist, please see § 50.3. If a party submits a request to vacate a
dismissal of an initial determination request and the request contains sufficient evidence or other
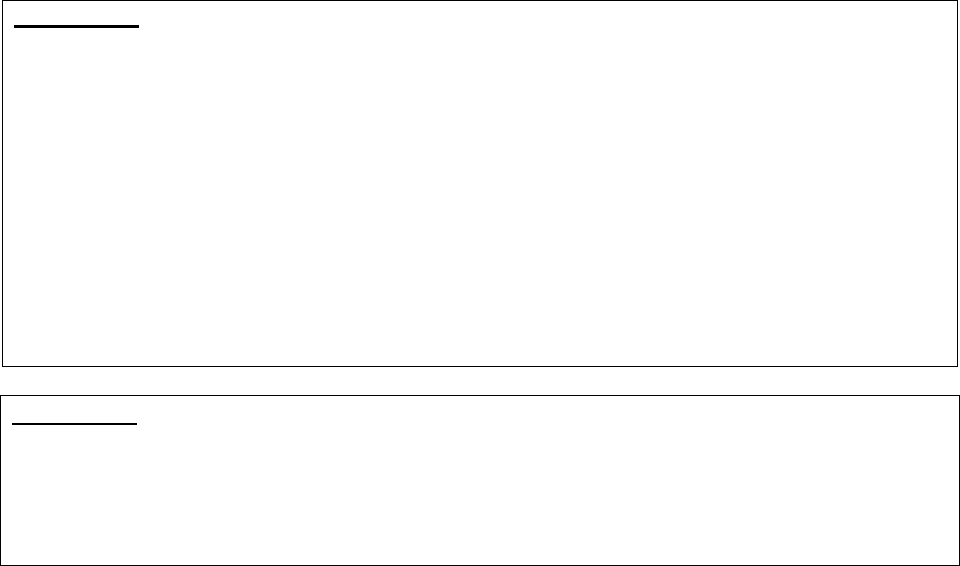
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
57
documentation that supports a finding of good cause for vacating, the plan makes a favorable
good cause determination. Once the plan makes a favorable good cause determination, it vacates
its prior dismissal action and performs an initial determination consistent with the timeframes at
§ 40.10. Where a finding for good cause is made, the plan should document the reason for that
finding in the case file.
If the plan does not find good cause to vacate the dismissal, the dismissal remains in effect. The
plan issues a letter (not a dismissal notice) explaining that good cause has not been established
and the dismissal cannot be vacated. The plan should explain in clear language why the
information submitted with the request to vacate the dismissal does not establish good cause to
vacate the dismissal action.
50 – Reconsiderations and Redeterminations (Level 1 Appeals)
A party (as described below) to an adverse initial determination has a right to a reconsideration
(Part C) or redetermination (Part D) by the plan. A reconsideration or redetermination
(hereinafter referred to as a level 1 appeal) consists of a review of an adverse initial
determination, the evidence and finding upon which it was based, and any other evidence that the
parties submit or that is obtained by the plan.
Part C Only
The parties to an organization determination for purposes of an appeal include:
• The enrollee (including his or her representative);
• An assignee of the enrollee (i.e., a physician or other provider who has furnished a service
to the enrollee and formally agrees to waive any right to payment from the enrollee for
that service);
• The legal representative of a deceased enrollee’s estate; or
• Any other provider or entity (other than the MA plan) determined to have an appealable
interest in the proceeding.
Part D Only
The parties to a coverage determination include the enrollee and the enrollee's representative, if
applicable. In some cases, as described in this section, the enrollee's prescribing physician or
other prescriber is also a party. However, an enrollee's prescribing physician or other prescriber
does not have all of the rights and responsibilities of the enrollee with respect to party status,
unless the physician or other prescriber is the enrollee's representative.
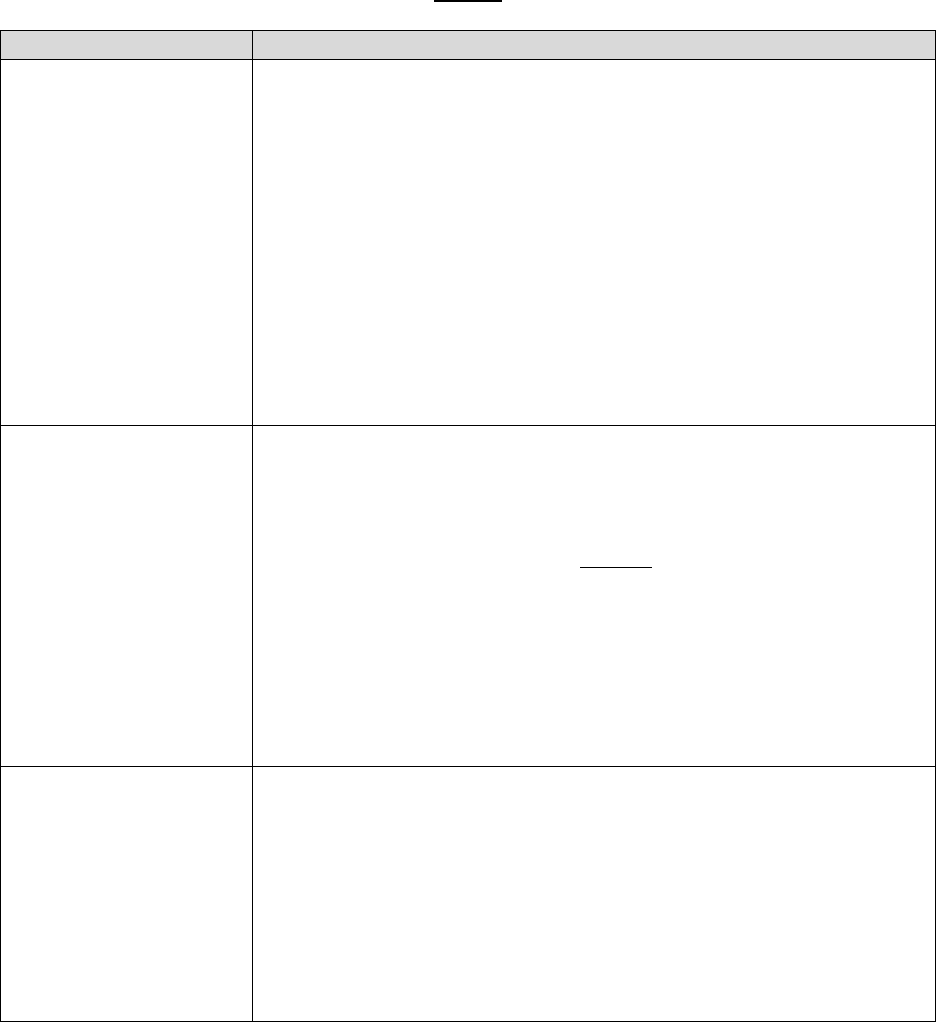
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
58
50.1 – Who May Request a Level 1 Appeal
Part C
Type of Request
Who May Request An Appeal
Standard
Reconsideration:
Requests for Item,
Service, or Part B
Drug
• An enrollee;
• An enrollee’s representative;
• The enrollee’s treating physician acting on behalf of the
enrollee* or staff of physician’s office acting on said
physician’s behalf (e.g., request is on said physician’s
letterhead or otherwise indicates staff is working under the
direction of the provider).; or
• Any other provider or entity (other than the MA plan)
determined to have an appealable interest in the proceeding.
Standard
Reconsideration:
Payment
• An enrollee;
• An enrollee’s representative;
• Non-contract provider (see §50.1.1 for non-contract provider
payment appeals);
• The legal representative of a deceased enrollee’s estate; or
• Any other provider or entity (other than the MA plan)
determined to have an appealable interest in the proceeding.
Expedited
Reconsideration
• An enrollee;
• An enrollee’s representative;
• Any physician or staff of physician’s office acting on said
physician’s behalf (e.g., request is on said physician’s
letterhead or otherwise indicates staff is working under the
direction of the provider) acting on behalf of the enrollee.
*If the enrollee’s records indicate that he or she has not previously visited the requesting physician, the MA plan
should undertake reasonable efforts to confirm that the enrollee has received appropriate notification of the appeal.
Note: Contract providers (including subcontracted entities) do not have appeal rights under
the provisions discussed in this guidance. Contract provider disputes involving plan payment
denials are governed by the appeals/dispute resolution provisions in the contract between the
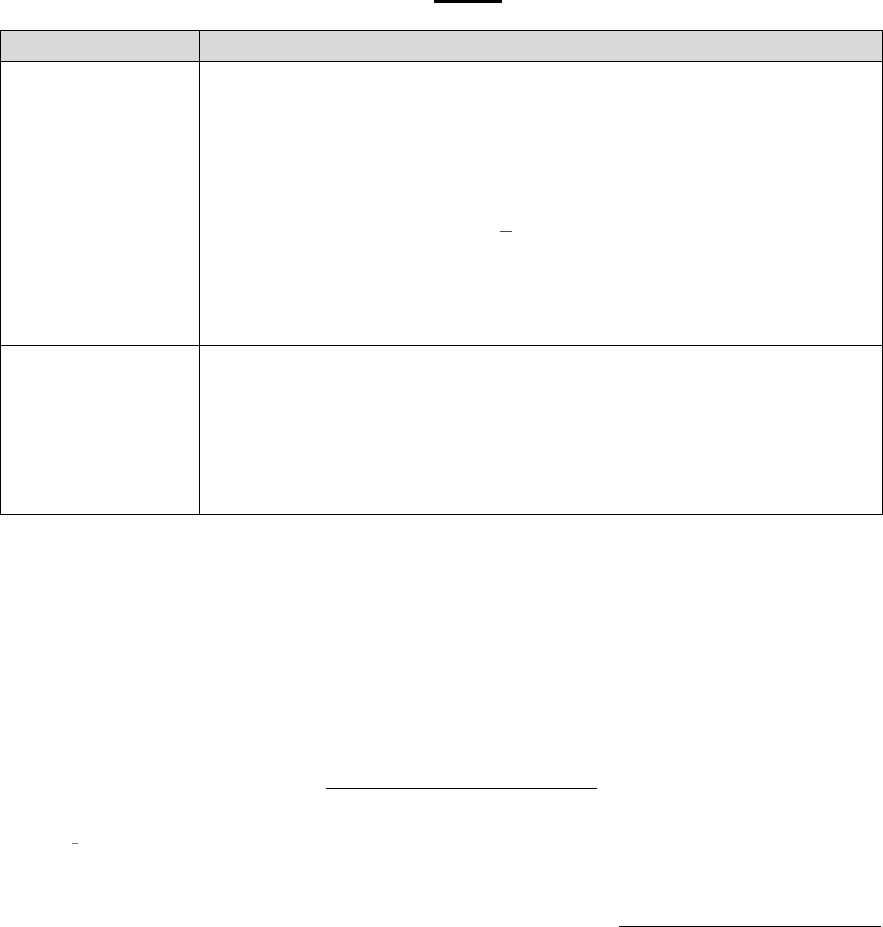
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
59
provider and the plan.
Part D
Type of Request
Who May Request An Appeal
Standard or
Expedited
Redetermination
• An enrollee;
• An enrollee’s representative;
• An enrollee’s prescribing physician or other prescriber* acting
on behalf of the enrollee**; or
• Staff of a physician’s office acting on a physician’s behalf (e.g.,
request is on the office’s letterhead)
Standard
Payment
Redetermination
• For direct member reimbursement, only an enrollee or an
enrollee’s representative (which may be the prescribing
physician or other prescriber) may appeal an adverse
reimbursement decision under Part D.
* Pursuant to § 423.560, “other prescriber” means a health care professional other than a physician who is
authorized under State law or other applicable law to write prescriptions. It is the Part D plan sponsor’s
responsibility to identify the types of health care professionals that have prescribing authority in the states in which
the Part D plan sponsor operates.
**If the enrollee’s records indicate that he or she has not previously visited the requesting physician or prescriber,
the plan sponsor should undertake reasonable efforts to confirm that the enrollee has received appropriate
notification of the appeal.
50.1.1 – Requirements for Provider Claim Appeals (Part C Only)
The appeal provisions set forth at 42 CFR Part 422 Subpart M and described in this guidance are
designed to protect enrollee rights related to grievances, organization determinations, and
appeals, and non-contracted provider rights related to organization determinations and appeals.
A non-contract provider, on his or her own behalf, may request a reconsideration (i.e., an appeal)
for a denied claim only if the non-contract provider completes a Waiver of Liability (WOL)
statement, which provides that the non-contract provider will not bill the enrollee regardless of
the outcome of the appeal.
If an appeal is submitted, the WOL must be filed with the appeal. The appeal should include
other supporting documentation (e.g., copy of remittance advice/notice and clinical records).
Non-contract providers who have executed a WOL are not required to complete the
representative form because the provider is not representing the enrollee, and thus does not need
a written representative form. Furthermore, because the enrollee no longer has an appealable
interest under 42 CFR Part 422 Subpart M, plan notices/correspondence regarding the non-

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
60
contract provider’s appeal would be delivered to the non-contract provider and not the enrollee.
If the WOL isn't filed with the appeal, the plan should make and document reasonable efforts to
obtain the WOL. The plan is not required to undertake a review of the appeal until or unless the
form is obtained, but it may choose to begin the review while continuing efforts to obtain a
WOL. The adjudication timeframe begins when the WOL is received by the plan. If the plan
does not receive the WOL by the end of the adjudication timeframe the plan issues a dismissal
notice per the dismissal procedures set forth in this guidance. See §50.9.
A non-contracted provider who has furnished a service to an enrollee, and submitted the WOL,
can be a party to an organization determination, in accordance with 42 C.F.R. § 422.574(b).
Thus, pursuant to 42 C.F.R. § 422.578, a non-contracted provider may request that an
organization determination be reconsidered by the plan. Even reconsideration requests submitted
by non-contracted providers that relate to the type or level of service furnished to the enrollee
must be reviewed in accordance with the administrative appeal processes outlined in 42 C.F.R.
Part 422, Subpart M.
In the following examples a non-contracted provider who is the enrollee’s assignee, and who has
submitted the WOL, must be afforded full administrative appeals rights in accordance with 42
C.F.R. Part 422 Subpart M:
• Diagnosis code/DRG payment denials. A non-contracted provider submits a claim to a
plan. The plan initially approves the claim, which is considered a favorable organization
determination (see 42 C.F.R. 422.566(b)). The plan later reopens and revises the favorable
organization determination and denies the DRG code on the basis that a different DRG
code should have been submitted and recoups funds.
• Downcoding. A plan approves coverage for inpatient services from a non-contracted
provider, which is considered a favorable organization determination (see 42 C.F.R.
422.566(b)). The plan later reopens and revises the favorable organization determination
(e.g., retrospective review) and determines the enrollee should have received outpatient
services.
• Bundling issues and disputed rate of payment. Pre- and post-pay bundling and global
payment determinations. For example, denial of procedure codes -- as mutually exclusive
to another paid procedure code, or due to inclusion in a previously paid global surgical
package.
• Level of care or rate of payment denials. Payment of a reduced fee schedule amount for a
course of treatment. For example, a provider bills a procedure code for a visit but the plan
reimburses based on a lower level of care.
Further, even if the plan partially pays for coverage (i.e., denies coverage as requested but
approves or pays for part of the service), a non-contracted provider who according to 42 C.F.R.
§422.574(b) is a party to the organization determination may request reconsideration under the
Medicare administrative appeals process; a non-contracted provider does not need to receive zero
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
61
payment to request a reconsideration or to otherwise access the Subpart M appeals process.
Note: Providers can use electronic signatures on WOL documentation when it is submitted
through the plan’s secure portal, provided the portal meets all applicable regulatory and CMS
website requirements.
An authorized party (e.g., billing agency) may submit a payment appeal request on behalf of a
non-contracted provider without a valid AOR when the billing agency can provide evidence the
provider furnished the billing agency authority to prepare a claim and receive payment on behalf
of the provider or that the billing agency otherwise has authority to pursue appeals.
Nevertheless, the billing agency may not sign the WOL on behalf of the provider.
50.2 – Level 1 Appeal Requests
A party may request a level 1 appeal by filing a written request with the plan. Plans must accept
verbal requests for expedited appeals and may accept verbal requests for standard appeals.
50.2.1 – Guidelines for Accepting Level 1 Appeal Requests
Method for
Filing Request
Standard Expedited
Written Must Accept Must Accept
Verbal
May Accept
Note: In the event that a plan
does not accept a verbal request,
the plan must explain to the party
how to file a written request.
Must Accept
Note: If a verbal request to
expedite a level 1 appeal is denied
by the plan, the plan cannot require
the party to re-file the request in
writing. Instead, the plan must
automatically transfer the request
to the standard process.
If a plan does accept verbal requests (standard or expedited), the plan’s policy should include
repeating the summarized request back to the caller. Failure to take steps to ensure that verbal
requests are properly and accurately handled may result in CMS determining that the MA plan
has inadequate policies and procedures under §§422.562, 422.582, 423.562, and 423.582. For
Part C plans, an acknowledgement letter should be sent to enrollee (in lieu of or in addition to
repeating the request verbally) to confirm the facts and basis of the appeal and that the
reconsideration request is properly and accurately noted and addressed by the MA plan. Notice
should advise the enrollee to immediately contact the plan if the acknowledgement letter does
not correctly capture the enrollee’s request.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
62
Part C Only
MA plans may, but are not required, to accept web/internet requests.
Part D Only
Part D plan sponsors must accept web/internet-based requests and protect individual health
information received via the web/internet. See requirements at §423.128(b)(7)(ii), and §423.136.
For both standard and expedited level 1 appeal requests, the following guidelines apply:
• Must be filed within 60 calendar days from the date of the notice of the initial
determination. For requests received after the 60-day filing timeframe, please see §50.3
regarding good cause exceptions for late filing.
• Request should include: Name of the enrollee, information identifying which denial is
being appealed, and contact information for the appellant. Unless the request is from a
representative, for which proof of appointment is required (see §20.2), plans cannot
require additional information in a request (e.g., appellant signature).
• The appellant is not required to use any specific language to indicate they are requesting
an appeal (e.g., “I am requesting an appeal”). The appellant may say, for example, “I do
not agree with your decision” or “Please review this decision”. Requests for appeals
should not be classified as requests for a reopening (see §80 for guidance regarding
reopenings).
• For verbal requests, plans should repeat the summarized verbal request back to the caller
and/or send an acknowledgement letter to enrollee to confirm the facts and basis of the
appeal to ensure the request is properly and accurately noted and addressed by the plan.
Notice should advise the enrollee to immediately contact the plan if the acknowledgement
letter does not correctly capture the enrollee’s request.
• For standard requests, the processing timeframe begins when the plan, any unit in the plan,
or a delegated entity (including those not responsible for processing the request) receives a
request. If an appeal request is received in the incorrect department, the plan must have
polices for prompt transfer of the call or document(s) to the appropriate department that
handles appeals.
• For expedited requests, the processing timeframe begins when the appropriate department
receives the request.
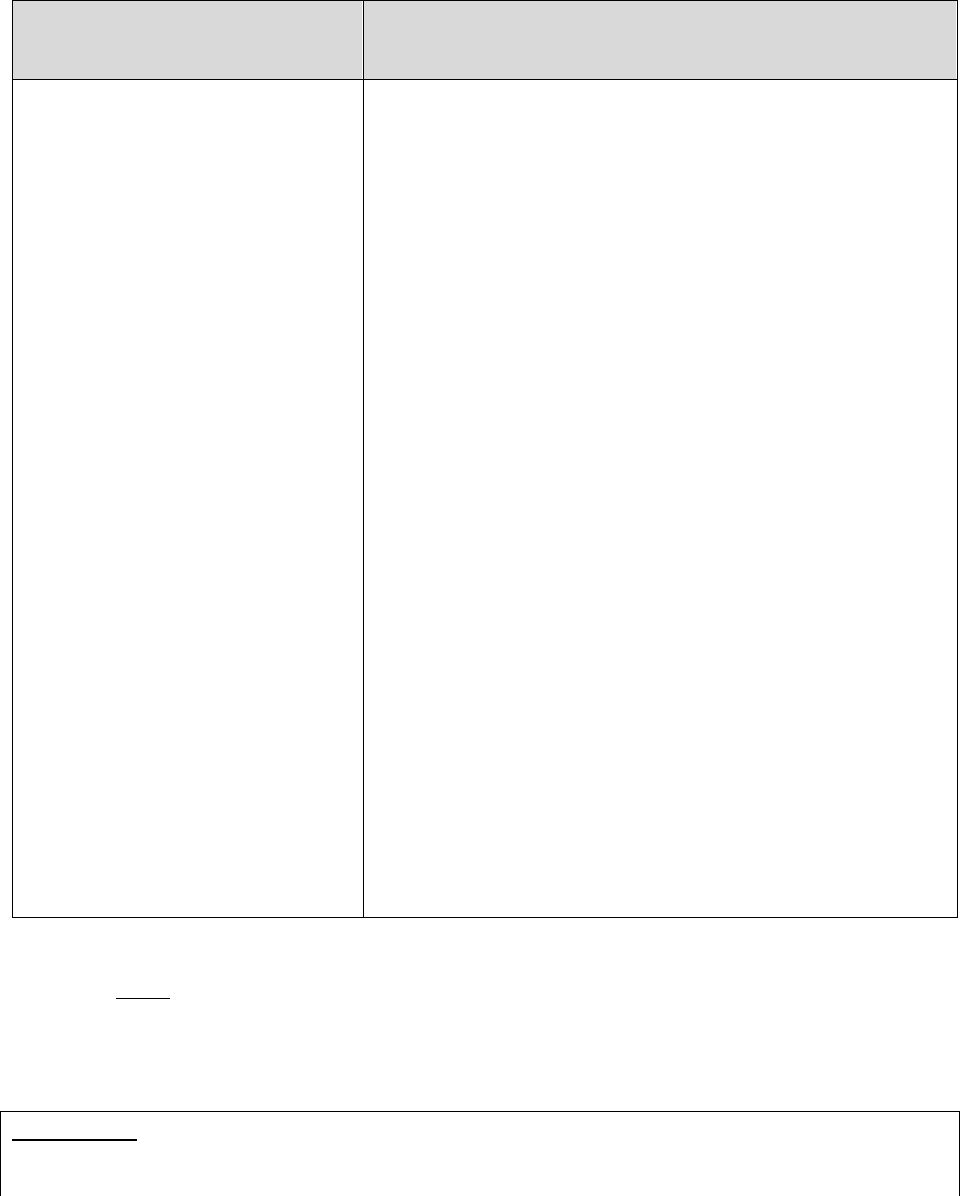
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
63
50.2.2 – How to Process Requests for Expedited Level 1 Appeals
Who May Request an
Expedited Level 1 Appeal
Plan Requirements
• An enrollee
• An enrollee’s representative
• Part C - A physician
(regardless of whether
affiliated with the plan)
• Part D – Prescribing
physician or other prescriber
acting on behalf of the
enrollee
• Establish an efficient and convenient means for
individuals to submit verbal or written requests;
• Establish procedures for accepting and processing
verbal and written requests for an expedited decision;
• Develop a process for receiving the request, including
designating an office and/or department to receive
both verbal or written requests and a telephone and fax
number to facilitate receipt of the requests;
• Document all verbal requests in writing and maintain
in the case file;
• Decide whether to expedite the request:
o If a physician (Part C)/prescribing physician or
other prescriber (Part D) makes a request or
supports an enrollee’s request for an expedited
appeal and indicates that applying the standard
timeframe could seriously jeopardize the life or
health of the enrollee or the enrollee’s ability to
regain maximum function (the physician does not
have to use these exact words), the plan must
process as expedited.
o Plans may, but are not required to, expedite appeals
for payment requests for drugs or services already
furnished.
Requests for an expedited level 1 appeal must be received within 60 days unless there is good
cause (see §50.3).
Note: A plan must not take or threaten any punitive action against a physician who acts on
behalf or in support of a request for an expedited level 1 appeal.
Part C Only
If an enrollee misses the deadline to file for immediate BFCC-QIO review of an inpatient
hospital discharge or SNF, HHA, or CORF termination decision, then the enrollee may request
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
64
an expedited reconsideration with the MA plan. MA plans should have a process in place to
distinguish between misdirected requests that should go to the BFCC-QIO (see §§422.622(b) and
422.626(a)(1)) and valid requests to the MA plan (i.e., requests made because the timeframe for
filing with the BFCC-QIO has expired).
MA plans are encouraged to automatically expedite all valid requests for reconsideration of
inpatient hospital discharges and SNF, HHA, or CORF termination decisions. If the MA plan
expedites the request, it must be processed under rules at §422.590(d) and guidance described in
this section.
If a request for review of an inpatient hospital discharge or SNF, HHA, or CORF termination
decision received by the MA plan is within the BFCC-QIO filing timeframe, MA plans should
inform the enrollee they must contact the BFCC-QIO to request the reconsideration. MA plans
should ensure that network hospitals, SNFs, HHAs, and CORFs are aware of and fulfill their
own responsibilities in connection with reviews of a hospital discharge or SNF, HHA, or CORF
termination.
MA plans are also encouraged to contact the BFCC-QIO to inform them the enrollee wants to
file an immediate BFCC-QIO review of a hospital discharge or SNF, HHA, or CORF
termination and forward a detailed notice and case file to the BFCC-QIO.
See §100 of for additional information related to hospital discharge and SNF, HHA, or CORF
termination decisions.
Part D Only
Plans may choose to expedite a redetermination request from an enrollee without requiring the
enrollee’s prescribing physician or other prescriber to submit a new statement indicating that
applying the standard timeframe could seriously jeopardize the life or health of the enrollee or
the enrollee’s ability to regain maximum function. However, if a plan sponsor chooses to do so,
it should, at a minimum, ensure the enrollee has not obtained the drug in dispute.
Action Following Acceptance of a Request for Expedited Level 1 Appeal
The plan must adhere to the following:
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
65
Part C
Reconsideration
Decision
Processing Requirements for Expedited Reconsiderations
Favorable
• Ensure the person or persons conducting the reconsideration were not
involved in the organization determination.*
• As expeditiously as the enrollee’s health condition requires, but no
later than:
o 72 hours after the request, the MA plan must:
Make the decision; and
Give notice to the enrollee (and the physician
involved, as appropriate); and
Authorize or provide the service.
Note: The 72-hour timeframe for requests for items and services
may be extended up to 14 additional days. Part B drug timeframes
cannot be extended.
• Notification must be provided within the 72-hour timeframe
• The MA plan may notify the enrollee verbally or in writing**. If the
MA plan initially provides verbal notification of its decision, it must
deliver written confirmation of its decision within 3 calendar days of
the verbal notification.
• Verbal or written notification of the decision must explain conditions
of the approval including (but not limited to):
o The duration of the approval; and
o Limitations associated with the approval.
• If the enrollee agrees, the MA plan may send the notice by fax or e-
mail. Please see Medicare Communications and Marketing
Guidelines regarding electronic communication with enrollees.
Partially
Favorable or
Adverse
• Ensure the person or persons conducting the reconsideration were not
involved in the organization determination.*
• As expeditiously as the enrollee’s health condition requires, but no

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
66
Reconsideration
Decision
Processing Requirements for Expedited Reconsiderations
later than 72 hours after the request, the MA plan must:
o Make the decision; and
o Forward the case file to the IRE within 24 hours of its
affirmation (see §50.12 for guidance on forwarding case files to
the IRE).
Note: The 72-hour timeframe for requests for items and services
may be extended up to 14 additional days. Part B drug timeframes
cannot be extended.
• MA plans are not required to notify beneficiaries upon forwarding
cases to the Part C IRE. Enrollees will receive notification from the
IRE. MA plans opting to inform parties when a case has been
forwarded to the IRE may use the model Notice of Appeal Status.
*When the issue is the denial of coverage based on a lack of medical necessity, the reconsideration must be made by
a physician with expertise in the field of medicine that is appropriate for the services at issue. The physician need
not be of the same specialty or subspecialty as the treating physician.
** Notice requirements can be found in §50.10.1.
Extension of Timeframe for Items and Services
The MA plan may only extend the 72-hour timeframe up to 14 additional days if:
• The enrollee requests the extension; or
• The extension is justified and in the enrollee’s interest due to the need for additional
medical evidence from a non-contract provider that may change an MA plan’s decision to
uphold a denial; or
• The extension is justified due to extraordinary, exigent or other non-routine circumstances
and is in the enrollee’s interest.
If the MA plan extends the timeframe, the MA plan must notify the enrollee in writing the
reasons for the extension and inform the enrollee of the right to file an expedited grievance if the
enrollee disagrees with the decision to extend the timeframe.
If the MA plan needs information from a non-contract provider, the MA plan must request the
necessary information from the non-contract provider within 24 hours of the initial request for an
expedited reconsideration. Regardless of whether the MA plan needs information from non-
contract providers, the MA plan is responsible for meeting the timeframe and notice
requirements for expedited reconsiderations. (See §10.6 for additional guidance regarding
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
67
outreach for additional information.)
MA plans are expected to have sufficient and appropriate contract terms to get information and
records from contract providers as necessary for expedited (and standard) reconsiderations. MA
plans should not generally or regularly extend the timeframe for an expedited reconsideration to
seek information or records from a contract provider but may do so if it is justified in the
enrollee’s interest and due to extraordinary, exigent, or other non-routine circumstances.
Part B drug timeframes cannot be extended.
Part D
Level 1 Appeal
Decision
Processing Requirements for Expedited Redeterminations
All Decisions
(Favorable or
Adverse, including
requests that involve
an exception)
• Ensure the person or persons conducting the redetermination
were not involved in the coverage determination.*
• Part D plan sponsors must make the decision and deliver written
notice** to the enrollee (and the prescribing physician or other
prescriber involved, as appropriate) of its decision as
expeditiously as the enrollee’s health condition requires, but no
later than 72 hours after receiving the redetermination request
(plan sponsors may not extend the timeframe by dispensing a
temporary supply of the medication).
• If the plan sponsor initially provides verbal notification of its
decision, it must deliver written confirmation of its decision
within 3 calendar days of the verbal notification.
• If the plan sponsor fails to provide the enrollee or prescribing
physician or other prescriber, as appropriate, with the decision
for the expedited redetermination within the timeframes
described above, they must forward the enrollee’s request to the
IRE within 24 hours of the expiration of the adjudication
timeframe (see §50.12 for information regarding forwarding
cases to the IRE).
*When the issue is a denial of coverage based on a lack of medical necessity, the redetermination must be made by a
physician with expertise in the field of medicine that is appropriate for the services at issue. The physician need not
be of the same specialty or subspecialty as the treating physician.
**Notice requirements can be found in §50.10.2.
If the plan needs medical information, the plan must request the necessary information within 24
hours of the initial request for an expedited level 1 appeal. Regardless of whether the plan needs
information, the plan is responsible for meeting the timeframe and notice requirements for
expedited level 1 appeals (for Part D exceptions requests, see §40.5.3 for tolling requirements).
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
68
Action Following Denial of a Request for an Expedited Level 1 Appeal
Following denial of a request for an expedited level 1 appeal, plans must:
• Transfer the request to the standard level 1 appeal process (the timeframe begins the day
the MA organization receives the request for expedited reconsideration);
• Give the enrollee prompt verbal notice of the denial including the enrollee's rights; and
• Deliver a written notice of the enrollee's rights within 3 calendar days of the verbal notice
of the denial to expedite the request.
Notice of the denial of a request for an expedited level 1 appeal must:
• Explain that the plan will automatically transfer and process the request using the required
timeframe for standard requests;
• Inform the enrollee of the right to file an expedited grievance if he or she disagrees with
the plan’s decision not to expedite the level 1 appeal (Plans may use the Notice of Right
to an Expedited Grievance for Part C and Notice of Right to an Expedited Grievance for
Part D to notify enrollees about their expedited grievance rights.);
• Inform the enrollee of the right to resubmit a request for an expedited level 1 appeal with a
physician, prescribing physician, or other prescriber’s support, including that if the
enrollee gets the physician/prescriber support indicating that applying the standard
timeframe for making determinations could seriously jeopardize the life or health of the
enrollee or the enrollee’s ability to regain maximum function, the request will be
expedited automatically; and
• Provide instructions about the expedited grievance process and its timeframes.
Parts C & D
Change of Review Priority
After a request is initiated as a standard or expedited review, a provider may contact the plan to
change the review priority (standard or expedited).
If the provider indicates that the enrollee’s health requires an expedited decision, the plan must
begin the applicable expedited review period at the time they receive the physician’s request to
expedite the decision.
Note: A change of priority does not allow for extra review time. If the remaining standard
review period is less than the applicable expedited review period, the original standard deadline

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
69
still applies.
50.3 – Good Cause Exception for Late Filing
Plans may accept a request for a standard or expedited level 1 appeal after the 60-day timeframe
if a filing party shows good cause. A request to file a level 1 appeal after the timeframe must be
in writing and state why the request for a level 1 appeal was not filed on time.
If an untimely appeal request does not include an explanation as to why the request wasn’t filed
timely, the plan may make an attempt to obtain information supporting good cause for the late
filing. The plan should consider the circumstance that kept the party from making the request on
time and whether any organizational actions might have misled the party. Plan policies
governing good cause justification when the request for a level 1 appeal is outside the 60-day
timeframe should not be discriminatory and should provide for equal and fair treatment of
enrollees.
Examples of circumstances where good cause may exist include (but are not limited to) the
following situations:
• The party did not receive the notice for the adverse initial determination, or they received
it late;
• The party was seriously ill, which prevented a timely appeal;
• There was a death or serious illness in the party’s immediate family;
• An accident (e.g., a natural or man-made disaster) caused important records to be
destroyed;
• Documentation was difficult to locate within the time limits;
• The party had incorrect or incomplete information concerning the level 1 appeal process;
• The party lacked capacity to understand the timeframe for filing a level 1 appeal; or
• The party sent the request to an incorrect address, in good faith, within the time limit and
the request did not reach the plan until after the time period had expired.
• The delay is a result of the additional time required to produce enrollee documents in an
accessible format (for example, large print or Braille). The delay is the result of an
individual having sought and received help from an auxiliary resource (such as a State
Health Insurance Assistance Program (SHIP) or senior center), on account of his or her
disability, in order to be able to file the appeal.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
70
If the plan obtains information establishing good cause, the adjudication timeframe (i.e., the
timeframe in which the plan must make its decision on the level 1 appeal) begins on the date the
plan receives that information.
If the plan denies a party’s request for a good cause extension for late filing, the request for
reconsideration is dismissed. See § 50.9 for information on dismissal procedures.
50.4 – Withdrawal of a Level 1 Appeal Request
A level 1 appeal may be withdrawn at any time before the decision is issued by filing a request
with the plan. The request to withdraw must be filed by the party who requested the level 1
appeal. If a request to withdraw is filed with the plan, the plan will dismiss the level 1 appeal
request. The request to withdraw may be written or verbal. For verbal withdrawal requests, the
plan should clearly document in their system the date and the reason why the party chose not to
proceed with the appeal. A notice of dismissal must be issued to all parties to the appeal and
include:
(1) The reason for the dismissal.
(2) The right to request that the MA organization vacate the dismissal action.
(3) The right to request review of the dismissal by the independent entity.
Part C Only: If the withdrawal request from the party that requested a reconsideration is
received after the plan has forwarded the case file to the IRE, the plan must forward the
withdrawal request to the IRE for processing.
See the content related to dismissal of a level 1 appeal at section 50.9.
50.5 – Actions the Appealing Party Can Take During a Level 1 Appeal
50.5.1 – Opportunity to Submit Evidence
The plan must provide the parties to the appeal a reasonable opportunity to present evidence
related to the appeal, in person or in writing (e.g. by telephone, fax, or hand-delivered to a plan’s
physical location). A party is not required to submit additional evidence, but each party may
exercise this right if they choose. The plan must take all of the evidence into account when
making the decision.
In the case of an expedited level 1 appeal, the opportunity to present evidence is limited by the
short timeframe for making a decision, therefore, the plan must inform the parties of the
conditions for submitting the evidence.
Part C Only
An MA plan must inform the party of their right to request a 14-day extension if the party feels

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
71
they will need additional time to submit evidence.
50.5.2 –Enrollee Request for Case File Content
Enrollees may request a copy of the contents of the case file at any point during the appeals
process. Upon an enrollee’s request, the plan must:
• Provide the enrollee with a copy of the contents of the case file, including, but not limited
to, a copy of supporting medical records and other pertinent information used to support
the decision. Where an enrollee has an alternate format preference, the case file contents
must be provided in that format.
• Make every reasonable effort to accommodate an enrollee’s request for case file material
(e.g., allowing the enrollee or authorized representative to obtain the material at a plan
location or mailing the material to any address specified by the enrollee or authorized
representative).
• Abide by all applicable federal and state laws regarding confidentiality and disclosure for
mental health records, medical records, or other health information. (See 45 CFR 164
Subpart E regarding the privacy of individually identifiable health information.)
The plan may charge the enrollee a reasonable amount for copying and mailing the case file.
When the case file is requested, the plan must inform the enrollee of the per-page copying cost
and provide an estimate of the total copying and mailing cost. The plan may not charge the
enrollee an additional cost for courier delivery of the material to a plan location that would be
over and above the cost of mailing the material to the enrollee.
50.6 – Who Must Conduct a Level 1 Appeal
The plan must designate someone other than the person involved in making the initial
determination to perform the level 1 appeal. If the initial denial was based on a lack of medical
necessity, then the level 1 appeal must be performed by a physician with expertise in the field of
medicine that is appropriate for the item, service, or drug in question.
If the physician is not of the same specialty or subspecialty as the treating physician, the
physician must have the appropriate level of training and expertise to evaluate the necessity of
the requested drug, item, or service. This does not require the physician to always have the same
specialty training as the treating physician. For example, where there are few practitioners in a
highly specialized field of medicine, a plan may not be able to hire a physician of the same
specialty or sub-specialty to review adverse initial determinations.
Part C Only
The physician performing the reconsideration must apply the prudent layperson standard (as
described in 42 CFR §422.113(b)(1)(i)) in cases involving emergency and urgently needed
services.
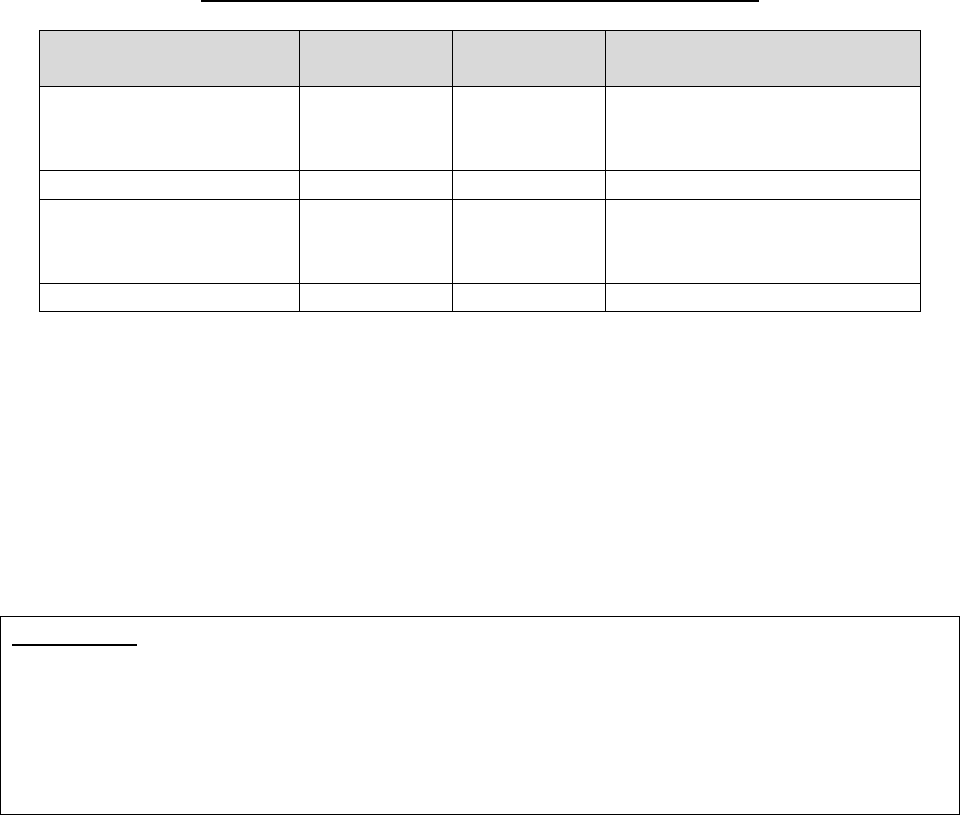
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
72
50.7 – Conducting a Level 1 Appeal
50.7.1 – Processing Timeframes
Parts C & D Level 1 Appeal Adjudication Timeframes
Type Part C
Part C with
Extension
Part D
Standard Request for
Item, Service, or Part D
Drug
30 days 44 days 7 days*
Standard Part B Drug
7 days
N/A**
N/A
Expedited
Reconsideration or
Redetermination
72 hours 17 days** 72 hours*
Payment
60 days
N/A
14 days
*Note: Part D redetermination exception requests cannot be tolled for receipt of the
prescribing physician’s supporting statement.
** Part B drug timeframes cannot be extended.
Plans must authorize or provide the service or benefit as expeditiously as the enrollee’s health
condition requires, but no later than the timeframes listed above (based on when the request was
received).
If the plan cannot obtain all relevant documentation, it must issue the decision no later than the
applicable timeframes outlined above.
Part D Only
Part D redetermination exception requests cannot be tolled for receipt of the prescribing
physician’s supporting statement.
The Part D plan sponsor must authorize payment for the benefit within 14 calendar days from the
date it receives the request and make payment (i.e., mail the payment) no later than 30 calendar
days after the date the plan sponsor receives the request.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
73
Extension of Timeframe
Part C Only
For standard and expedited reconsiderations for items and services, the MA plan may extend the
timeframe by up to 14 calendar days only if:
• The extension is requested by the enrollee;
• The extension is justified and in the enrollee’s interest due to the need for additional
medical evidence from a non-contract provider that may change an MA plan’s decision
to deny an item or service; or
• Is in the enrollee’s best interest due to extraordinary, exigent, or other non-routine
circumstances, such as a natural disaster.
When the MA plan extends the timeframe, it must notify the enrollee in writing of the reasons
for the delay, and inform the enrollee of the right to file an expedited grievance if he or she
disagrees with the MA plan’s decision to grant an extension. When extensions are used, the MA
plan must issue and effectuate its determination as expeditiously as the enrollee’s health
condition requires, but no later than upon the expiration date of the extension.
• Part B drug timeframes cannot be extended.
Part D Only
Extensions of the adjudication timeframes are not permitted in Part D. A Part D plan sponsor
may not extend the adjudication timeframe by dispensing a temporary supply of the requested
medication. For example, if a plan sponsor receives a request outside of its normal business
hours, it cannot approve a 72-hour supply of the requested medication in dispute and defer
issuing a decision for 72-hours; the plan sponsor must make its determination within the
appropriate adjudication timeframe.
50.7.2 – Effect of Failure to Meet the Timeframe for Level 1 Appeals
If a plan fails to provide the enrollee with a level 1 appeal decision within the timeframes
specified for both standard and expedited appeals, this failure constitutes an adverse decision. In
this case, the plan must forward the complete case file to the IRE (see §50.12.1 regarding
forwarding adverse level 1 appeals to the IRE). The plan’s failure to provide notice of the level 1
appeal decision within the required timeframe constitutes an adverse decision, but the plan is not
required to send the adverse decision notice to the enrollee.
Part C Only
MA plans are not required to notify beneficiaries upon forwarding cases to the Part C IRE.
Enrollees will receive notification from the IRE. MA plans opting to inform parties when a case
has been forwarded to the IRE may use the model Notice of Appeal Status.
Part D Only
Plan sponsors should notify the enrollee that the appeal decision was not made timely and is
being forwarded to the IRE for review. CMS has developed a model Notice of Case Status that
plan sponsors can use in lieu of the adverse decision notice to notify enrollees whenever cases
are forwarded to the IRE. The plan sponsor must send the notification to the enrollee within 24

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
74
hours of the expiration of the adjudication timeframe.
Note: When a plan makes a fully favorable determination on a level 1 appeal less than 24 hours
after the end of the adjudication timeframe, the plan should consider effectuating and notifying
the enrollee of the favorable appeal decision (within the 24-hour period the appeal must be
forwarded to the IRE) in lieu of forwarding the appeal to the IRE.
If CMS determines that the plan has a pattern of not processing level 1 appeals within the
required timeframes, the plan may be considered to be out of compliance with the terms of its
Medicare contract and/or subject to intermediate sanctions in accordance with 42 CFR Part 422
or Part 423, subpart O.
Part D Only
The "Transition Period Note" in §40.11 also applies to this section.
50.8 – Service or Benefit Received Prior to Notice of Decision
During the processing of an appeal of a denied coverage request, if an MA plan or a Part D
sponsor learns (by any means, including by receipt of a claim or reimbursement request) that the
enrollee received the item/service/drug that is the subject of the appeal, the MA plan or Part D
sponsor must finish adjudicating the appeal request and issue a substantive decision consistent
with the applicable requirements of 42 CFR Part 422, Subpart M or Part 423, Subpart M.
If applicable, the MA plan or Part D sponsor must separately process and issue a decision on
any related claim or reimbursement request.
Medicare Parts C and D regulations do not permit an MA plan or Part D sponsor to dismiss an
otherwise valid, timely appeal request of an adverse coverage decision solely because an
enrollee is concurrently receiving or has already received the item/service/drug that is the
subject of the appeal. MA plans or Part D sponsors may only dismiss appeal requests for the
reasons listed at 42 CFR §§ 422.582(f) and 423.582(e) (see section 50.9 of this manual for more
details).
50.9 – Dismissal of a Level 1 Appeal Request
Plans must dismiss a level 1 appeal request under any of the following circumstances:
• The individual or entity making the request is not a proper party to the appeal under the
applicable regulation. This includes the following situations: If an individual requests a
reconsideration on behalf of an enrollee, but a properly executed appointment of
representative form has not been filed (and there is no other documentation to show that
the individual is legally authorized to act on the enrollee’s behalf), the MA plan is
obligated to make attempts to secure the missing documentation (see §20.2.1).
• If a non-contracted provider requests a reconsideration of a denied claim (i.e., post-
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
75
service appeal) but fails to provide a waiver of liability statement indicating that it will not
bill the enrollee regardless of the outcome of the appeal, the MA plan should make
attempts to secure the missing documentation prior to dismissing the request. Please
note: a pre-service reconsideration request by a physician who is providing treatment to
an enrollee, upon providing notice to the enrollee, is considered a valid request.
• When the plan determines the party failed to make a valid request for an appeal that substantially
complies with the applicable regulation for making a valid request for a level 1 appeal. For
example, when the party fails to file the level 1 appeal within the proper filing timeframe in
accordance with the applicable regulation. A valid request as contemplated in §§ 422.582(a)
and 423.582(a) includes sufficient information to identify the enrollee to allow the plan to
adjudicate the request (or, at a minimum, make contact with the enrollee to clarify the
request), including a full name or member ID number or at least one means of contact
(e.g., address, telephone number, email).
• When the enrollee dies while the appeal is pending and the enrollee’s spouse or estate has no
remaining financial interest in the case and no other individual or entity with a financial interest
in the case wishes to pursue the level 1 appeal. Financial interest means having financial
liability for the item(s) or service(s) underlying the coverage request.
• When the individual or entity that requested the reconsideration submits a timely written request
to withdraw their request for a level 1 appeal.
When the dismissal is the result of a timely withdrawal request, the plan is required to mail or otherwise
transmit a dismissal notice in accordance with the notice requirements in section 50.9.1 in order to
preserve the rights of other proper parties to the decision who may wish to request review of the
dismissal. The dismissal notice will explain that the withdrawal request is the reason for dismissal. For
reporting purposes, this scenario is categorized as a withdrawal in reporting to CMS.
The guidance in section 50.9 does not alter reporting requirements. Withdrawn requests and
dismissals should continue to be reported separately in their distinct categories, per existing
reporting requirements.
NOTE: The above list of circumstances (taken from the applicable regulations) under which a
plan must dismiss a request for a level 1 appeal is exhaustive. A plan may not deem a request
invalid or dismiss a request for a level 1 appeal for any reason not explicitly included in 42 CFR
§§ 422.582(f) and 423.582(e), as applicable.
50.9.1 – Dismissal Notice
If a plan dismisses a level 1 appeal request, the plan must mail or otherwise transmit a written
notice of the dismissal to the parties at their last known address by the conclusion of the
applicable adjudication timeframe.
The dismissal notice must state all of the following:

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
76
(1) The reason for the dismissal;
(2) The right to request that the plan vacate the dismissal action; and
(3) The right to request IRE review of the dismissal.
Consistent with the timeframe for requesting a timely appeal, a request for review of a dismissal
must be filed with the IRE within 60 calendar days from the date of the plan’s dismissal notice.
Plans may use, and modify as necessary, the model Notice of Dismissal of Appeal Request when
notifying an enrollee of a dismissal.
Part C only
The rule requiring that a Part C case be automatically sent to the IRE if the plan upholds a denial
on the merits of the request does not apply in the case of a dismissal of a request for a level 1
appeal (reconsideration) because the MA organization is not making a substantive decision on
the merits of the request. If the plan dismisses a level 1 appeal request, the enrollee or other
party has the right to request IRE review of the plan’s dismissal; Part C plans must forward the
case file for a dismissal to the IRE when a proper party to the appeal requests IRE review of the
dismissal under § 422.590(i). The enrollee also has the right to request that the plan vacate the
dismissal.
50.9.2 – Dismissal Binding Unless Modified, Reversed or Vacated
The plan’s decision regarding its dismissal of a level 1 appeal request is binding unless the
enrollee or other party requests review by the Independent Review Entity or the dismissal action
is vacated by the plan. A plan may vacate its own dismissal within 6 months of the date of the
dismissal if good cause is established.
Upon receipt of a request to review a plan’s dismissal of a level 1 appeal request, the IRE will
contact the appropriate plan to obtain the case file. Plans must assemble and forward the case file
to the IRE (place in the mail or otherwise transmit the case file). The case file should be
forwarded within 24 hours of receiving the IRE’s case file request. The plan should refer to the
IRE website or the IRE Reconsideration Process Manual for information on case file content,
organization, and the most appropriate method of transmitting the case file.
If the IRE determines that the plan’s dismissal of the level 1 appeal request was in error, the IRE
vacates the dismissal and remands the case to the plan for reconsideration or redetermination
(level 1 appeal). The level 1 appeal must be conducted by the plan consistent with applicable
adjudication timeframes in § 50.7.1. The adjudication timeframe begins when the plan receives
the IRE’s remand order vacating the plan’s dismissal. The IRE’s decision regarding a plan’s
dismissal of a level 1 appeal request is binding and not subject to further review.
Requests to Vacate Dismissal of a Level 1 Appeal Request
A plan may vacate its own dismissal if good cause is established within 6 months of the date of
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
77
the notice of the dismissal. A plan may find good cause to vacate a dismissal if, for example, the
plan determines the dismissal was issued in error because there was good cause for late filing of
an appeal request. If a party submits a request to vacate a dismissal of a level 1 appeal request
and the request contains sufficient evidence or other documentation that supports a finding of
good cause for vacating, the plan makes a favorable good cause determination. Once the plan
makes a favorable good cause determination, it vacates its prior dismissal action, and performs a
redetermination or reconsideration consistent with section 50.7, including applicable
adjudication timeframes. For example, if a Part C plan dismisses a standardreconsideration
request for an item or service and the plan later finds good cause to vacate the dismissal action,
the plan must notify the enrollee (or other party) of the level 1 appeal decision within 30 calendar
days (assuming no extension is taken) of vacating the dismissal. Where a finding for good cause
is made, the plan should document the reason for that finding in the case file.
If the plan does not find good cause to vacate the dismissal (and the dismissal has not been
appealed or overturned on appeal), the dismissal remains in effect. The plan issues a letter (not a
dismissal notice) explaining that good cause has not been established and the dismissal cannot be
vacated. The plan should explain in clear language why the information submitted with the
request to vacate the dismissal does not establish good cause to vacate the dismissal action.
50.10 – Notification Requirements for Level 1 Appeal Decisions
50.10.1 - Part C Notification Requirements
Favorable Decisions
For favorable decisions, the MA plan must:
• Notify the requesting party and the enrollee in writing of its favorable determination.
• If the enrollee’s representative filed the appeal, the representative must be notified in lieu
of the enrollee. Plans may provide notice to both the representative and enrollee, but are
not required.
• Ensure written notification for appeals for service requests explain the conditions of the
approval which include (but are not limited to):
o The duration of the approval; and
o Limitations associated with the approval.
Partially Favorable, Adverse, or Untimely Decisions
For partially favorable, adverse, or untimely decisions, the MA plan must send a copy of the
complete case file with a written explanation of the MA plan’s decision to the IRE within the
applicable timeframe (see §50.12 for timeframes and case file requirements). MA plans are not

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
78
required to notify beneficiaries upon forwarding cases to the Part C IRE. Enrollees will receive
notification from the IRE. MA plans opting to inform parties when a case has been forwarded to
the IRE may use the model Notice of Appeal Status.
Note: See §50.7.2 if the MA plan makes a favorable determination for a reconsideration
request in less than 24 hours after the adjudication timeframe.
For notice requirements following denial of a request for an expedited reconsideration, see
§50.2.2.
50.10.2 - Part D Notification Requirements
Favorable Decisions
For favorable decisions, the Part D plan sponsor must:
• Notify the enrollee in writing in a readable and understandable form, in accordance with
the regulatory requirements at §423.590(h). If the enrollee’s representative filed the
appeal, the representative must be notified in lieu of the enrollee. Plans may send written
notice to both the representative and enrollee, but are not required.
• If an enrollee’s prescribing physician or other prescriber files a request on behalf of an
enrollee, the plan sponsor must provide notice to the prescriber and written notice to the
enrollee.
• If a plan sponsor provides verbal notification to a physician or other prescriber, the plan
sponsor does not need to send a written follow-up.
• Written notices must explain the conditions of the approval. The conditions of approval
may include (but are not limited to):
o The duration of the approval;
o Limitations associated with the approval; and/or
o Any coverage rules applicable to subsequent refills.
Adverse Decisions
If the request is denied, in whole or in part, the Part D plan sponsor must:
• Notify the enrollee in writing. The plan sponsor may use the model Notice of
Redetermination language, or it may develop its own notice. If the enrollee’s
representative filed the appeal, the representative must be notified in lieu of the enrollee.
Plans may send written notice to both the representative and enrollee, but are not required.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
79
• If an enrollee’s prescribing physician or other prescriber files a request on behalf of an
enrollee, the plan sponsor must provide notice to the prescriber and written notice to the
enrollee.
• If a plan sponsor provides verbal notification to a physician or other prescriber, the plan
sponsor does not need to send a written follow-up.
• In accordance with the regulatory requirements at §423.590(g), the notice must use
approved notice language in a readable and understandable form, and must:
o State the specific reason for denial that takes into account the enrollee’s presenting
medical condition, disabilities, and special language requirements, if any (see
Section 10.4.4 for additional details);
o Contain the enrollee’s HICN or MBI, plan sponsor name, plan identification
number, contract identification number, and formulary identification number;
o Provide a description of any applicable Medicare coverage rule or any other
applicable plan policy upon which the denial was based, including any specific
formulary criteria that must be satisfied for approval. If the drug could be approved
under the exception rules, the notice must explicitly state the need for a supporting
statement and clearly identify the type of information that should be submitted
when seeking a formulary or tiering exception; and
o Inform the enrollee of his or her right to a reconsideration (level 2 appeal).
Note: For adverse drug coverage redeterminations, describe both the standard and
expedited reconsideration processes, including the enrollee’s right to, and conditions for,
obtaining an expedited reconsideration and the rest of the appeals process.
For adverse payment redeterminations, describe the standard reconsideration process and
the rest of the appeals process.
For notice requirements following denial of a request for an expedited redetermination, see
§50.2.2.
Untimely Decisions
If the plan sponsor fails to provide notice of a decision within the required timeframe, the plan
sponsor is not required to send the adverse decision notice, but instead, the plan sponsor should
notify the enrollee that the decision was not made timely and is being forwarded to the IRE for
review. The notice must:
• Advise the enrollee of his/her right to submit additional evidence that may be pertinent to

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
80
the enrollee’s case, if the enrollee chooses;
• Direct the enrollee to submit such evidence to the independent review entity; and
• Include information on how to contact the independent review entity.
CMS has developed a model Notice of Case Status that plan sponsors can use in lieu of the
adverse decision notice to notify enrollees whenever cases are forwarded to the IRE.
Note: See §50.7.2 if the plan sponsor makes a favorable decision for a redetermination
request in less than 24 hours after the adjudication timeframe.
50.11 – Procedure for Handling Misclassified Appeals
If the plan misclassifies an appeal as a grievance and later discovers the error, the plan must
immediately forward the request to the appropriate division for processing and notify the
enrollee in writing that the request was misclassified and will be handled through the appeals
process. The timeframe for processing the appeal begins on the date the appeal is received by
the plan, as opposed to the date the plan discovers its error. Plans are expected to audit their own
appeals and grievance for the presence of errors and institute appropriate quality improvement
projects as needed.
50.12 – Timeframes and Responsibilities for Forwarding Case Files to the
Independent Review Entity
50.12.1 – Forwarding Case Files – Plan Responsibilities
If an MA plan appeal decision affirms the adverse initial determination (in whole or in part) or a
Part D plan sponsor does not provide notice of its standard or expedited redetermination within
the required timeframe or an enrollee has filed a reconsideration request and the IRE has
requested the enrollee's file from the plan, the plan must:
• Make reasonable and diligent efforts to gather and forward all pertinent documentation,
including medical records.
• Submit a written explanation with the complete case file (the case file must satisfy the
requirements in §50.12.3) to the IRE contracted by CMS within the forwarding
timeframes, as set forth in §50.12.2.
• Submit the case file by mail or overnight delivery service, fax, or the IRE web portal for
Part C or for Part D. Plans may access the web portal and the user’s guide on the IRE’s
website.
The plan should contact the IRE for additional information on electronic submission of case
files.
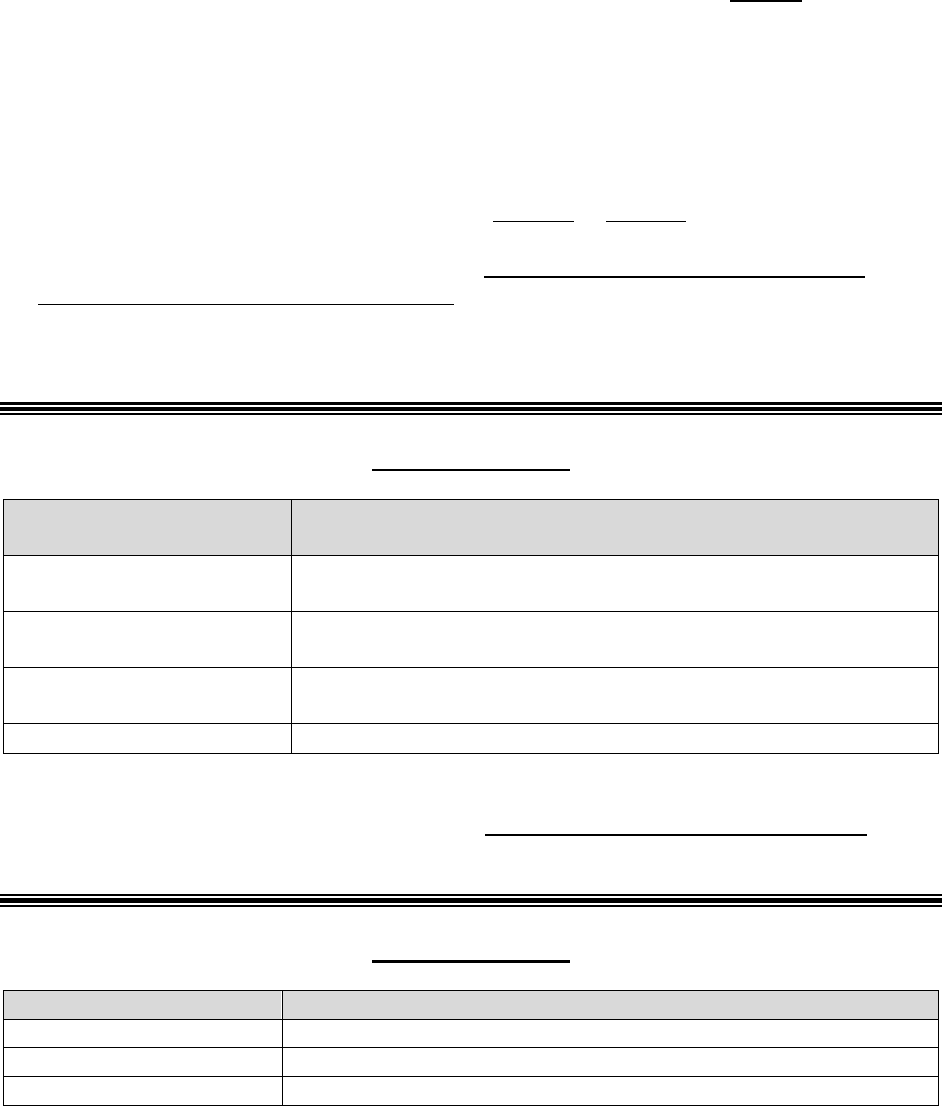
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
81
Note: When a plan makes a fully favorable determination in less than 24 hours after the end
of the applicable adjudication timeframe, the plan should consider effectuating and promptly
notifying the enrollee of the favorable decision (within the 24-hour period that the case must
be forwarded to the IRE) in lieu of forwarding the case to the IRE. See §50.7.2 for additional
information regarding untimely decisions made in less than 24 hours after the adjudication
timeframe.
For cases forwarded to the IRE, the plan must make reasonable and diligent efforts to gather and
forward all pertinent information to the IRE. If CMS determines that the plan has a pattern of
not making appropriate efforts to forward information to the IRE, the plan will be considered to
be out of compliance with the terms of its Medicare contract and/or subject to intermediate
sanctions in accordance with subpart O of 42 CFR Part 422 or Part 423.
For additional instructions, plans may refer to the Part C Reconsideration Process Manual and
the Part D Reconsideration Procedures Manual.
50.12.2 – Forwarding Case Files - Timeframes
Part C Timeframes
Case Type for Adverse
and Untimely Decisions
Forward no later than
Expedited
24 hours of the decision (or no later than expiration of
applicable extension)
Standard Items and
Services
30 calendar days of receipt of request (or no later than expiration
of extension)
Standard Part B Drug
7 calendar days of receipt of the request (no extension
permitted)
Standard Payment
60 calendar days of receipt of request
Note: For purposes of calculating timely receipt of the appeal case file by the independent
review entity, the MA plan should refer to the Part C Reconsideration Process Manual,
Section 5.2.
Part D Timeframes
Case Type for Decisions
Forward no later than
Expedited
24 hours of receipt of IRE’s request for case files
Standard
48 hours of receipt of IRE’s request for case files
Untimely
24 hours of the expiration of the adjudication timeframe
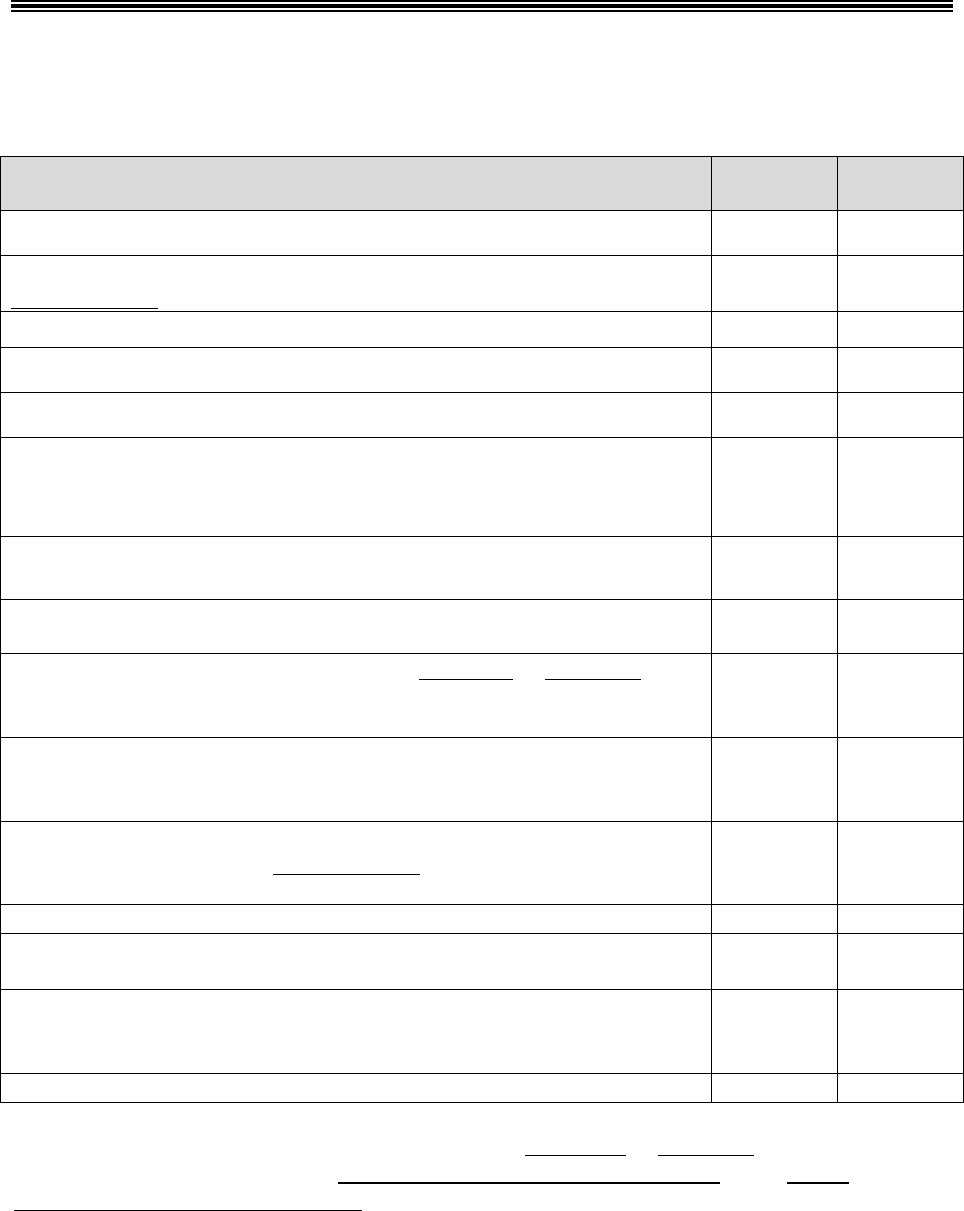
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
82
50.12.3 – Preparing the Case File for the Independent Review Entity
The following should be included in the case file forwarded to the IRE:
What to Include in A Case File When Forwarding to the IRE Part C Part D
Appeal Case File Transmittal Form/Cover Sheet ü ü
Reconsideration Background Data Form (not required if submitting via
IRE web portal)
ü N/A
Case Narrative
ü
ü
Copy of the Initial Determination Request and Notice ü ü
Copy of the Level 1 Appeal Request and Notice ü ü
Copy of information used to make the plan internal Level 1 decision,
including supporting documentation such as medical records, or
evidence submitted by the enrollee, provider, and/or prescriber.
ü ü
Expedited information regarding the Coverage Determination and
Redetermination
N/A ü
Representation documentation for representative appeals ü ü
If file is not submitted via IRE web portal for Part C or for Part D, a
complete copy of the relevant Evidence of Coverage on a universal
digital storage device (e.g. USB flash drive)
ü ü
The name and credentials of the prescribing physician or other
prescriber and contact numbers for street address, telephone, fax, and
e-mail.
N/A ü
Copy of the relevant plan formulary (on a universal digital storage
device if not submitted via IRE web portal), including descriptions of
any utilization management requirements
N/A ü
Exceptions process/criteria for determining medical necessity
N/A
ü
Any internal plan medical reviews that were obtained during
redetermination review
N/A ü
Description of medical documentation missing from the case file based
on the failure of the prescribing physician or other prescriber to submit
additional medical documentation requested by the plan.
N/A ü
Dismissal Case File Data Form
N/A
Plans may submit case files using the IRE web portal for Part C or for Part D. Plans should refer
to the most current version of the Part C Reconsideration Process Manual or the Part D
Reconsideration Procedures Manual for information concerning all required forms. Plans are
expected to fully complete all appropriate sections of the required forms in support of CMS’

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
83
appeals data collection activities.
50.12.4 – Including Evidence of Coverage and Formulary in Case Files
CMS strongly recommends that the plan include complete copies of the relevant Evidence of
Coverage (EOC) and their CMS approved formulary (Part D plans, if applicable) with any case
files sent to the IRE for review. ALJs at the Office of Medicare Hearings & Appeals (OMHA)
and the Medicare Appeals Council (Council) have indicated that these documents are needed in
their entirety in order to properly adjudicate appeals. It is in a plan's best interest to ensure that
each case file sent to the IRE includes complete versions of the EOC and/or formulary relevant
to an enrollee's specific case. Failure to include this information could result in an unfavorable
appeals decision and/or, in the case of Part D, CMS declining to refer an ALJ or attorney
adjudicator decision to the Council for review. Plans may submit case files with the EOC and
and/or formulary using the IRE web portal for Part C or for Part D. Plans may not mail or fax
paper copies of the complete EOC and/or formulary to the IRE.
If plans do not submit via the web portal, they should send information on a universal digital
storage device (e.g. USB flash drive). Include the device with the case file in the following
manner:
• The universal digital storage device must be properly labeled with the plan name and
contract number, formulary ID (Part D), enrollee name/HICN/MBI, and appeal number;
• The universal digital storage device must be securely affixed to the paper case file;
• All documents on the universal digital storage device must be in PDF or Word format and
should not be encrypted; and
• The universal digital storage device should only include the EOC and/or formulary
applicable to the specific case being adjudicated (a plan must not place copies of all of its
EOCs and formularies on the universal digital storage device).
60 – Reconsiderations by the Independent Review Entity (Level 2 Appeal)
60.1 – Who May Request a Level 2 Appeal
Part C Only
All partially favorable or adverse reconsideration decisions are forwarded to the IRE. A party
does not have to make a request for a level 2 appeal.
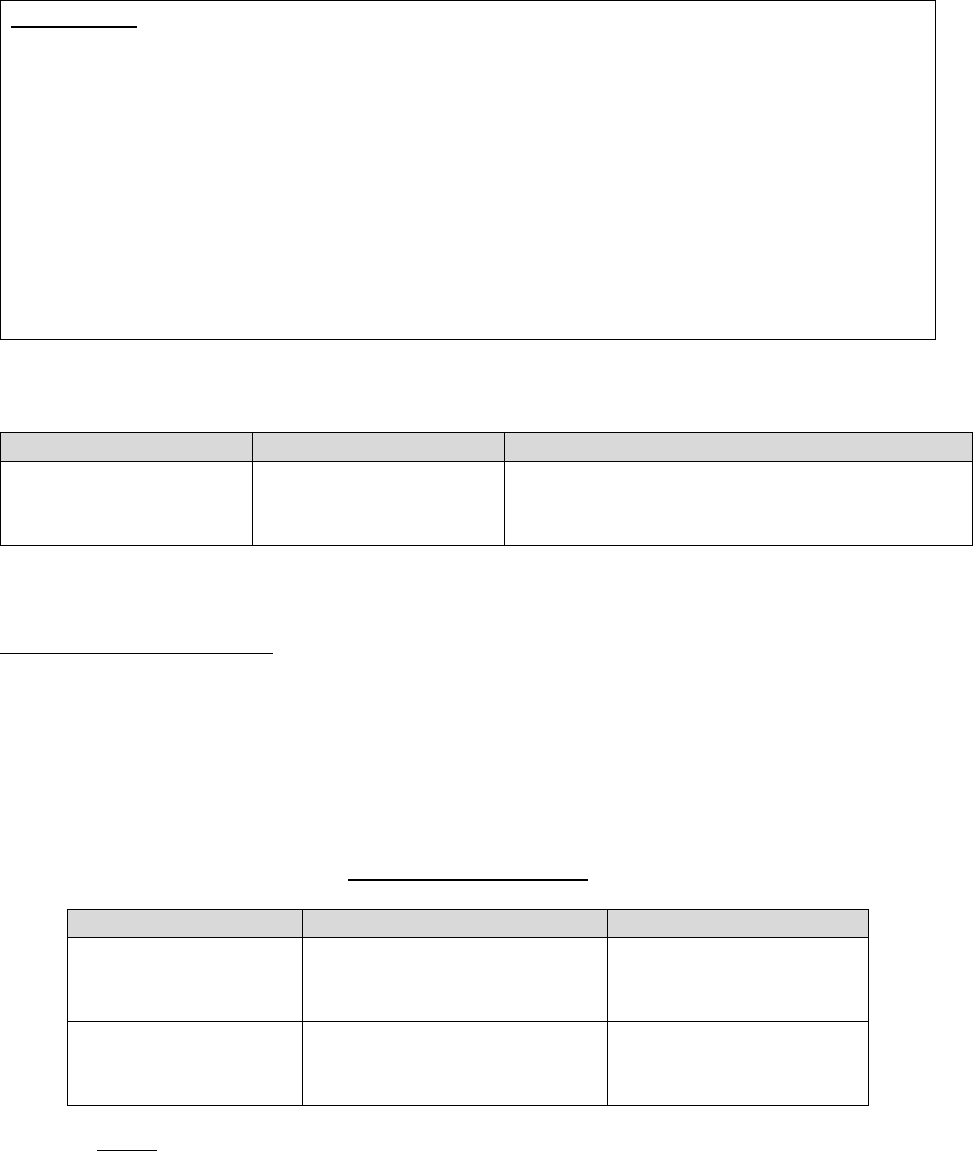
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
84
Part D Only
• An enrollee,
• An enrollee’s representative, or
• An enrollee’s prescribing physician or other prescriber (acting on behalf of an enrollee),
upon providing notice to the enrollee in accordance with §423.600(a).
• All partially favorable or adverse at-risk redetermination decisions are automatically
forwarded by the plan to the IRE within 24 hours. A party does not have to make a
request for a level 2 appeal related to an at-risk determination.
60.2 – How to Request a Level 2 Appeal (Part D Only)
Method of Filing
Type of Request
Timeframe for Filing
Written Standard or Expedited
Within 60 calendar days from the date of the
notice of the redetermination, unless the IRE
extends the timeframe for good cause.
Requests may be made by an enrollee, enrollee’s representative, or prescribing physician or other
prescriber (acting on behalf of an enrollee), upon providing notice to the enrollee on the model
Request for Reconsideration, or on any other written document. If the model notice is used, plan
sponsors should complete the applicable sections with each adverse redetermination notice.
60.3 – Processing Timeframes
The IRE must conduct the reconsideration as expeditiously as the enrollee’s health condition
requires, but not exceed the required timeframes outlined below.
Processing Timeframes
Type of Request
Part C
Part D*
Standard
Items and Services: 30 days
Payment: 60 days
Part B Drugs: 7 days
Benefit: 7 days
Payment: 14 days
Expedited
(Payment requests
cannot be expedited)
72 hours 72 hours
*For exception requests, the Part D timeframe may be tolled until the supporting statement is received.
See §40.5.4 for additional information regarding tolling.
60.4 – Good Cause Extension (Part D Only)
If a party misses the 60-day timeframe for requesting an IRE reconsideration, he or she may

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
85
request a good-cause extension. The extension request must be filed with the IRE, in writing,
and include the reason why he or she did not request a reconsideration timely. If the party shows
good cause, the IRE may extend the timeframe for filing a request for reconsideration. The IRE
should consider the circumstance that kept the party from making the request on time and
whether any actions by the Part D plan sponsor may have misled the party. Examples of
circumstances where good cause may exist include (but are not limited to) the situations
described in §50.3. The decision by the IRE on whether to grant an extension for good cause is
final and not subject to appeal.
60.5 – IRE Notification and Retention Requirements
When the IRE completes its reconsideration, it is responsible for notifying the parties of its
decision and providing a copy of the decision to the plan. Under Part C, the IRE is also
responsible for sending a copy of the decision to the CMS Regional Office that oversees the MA
plan.
The reconsideration notice must:
• Be written in a manner that is understandable to the enrollee that takes into account the
enrollee's presenting medical condition(s), disabilities, or special language requirements, if
any, and:
• Include specific reasons for the decision.
If the decision is adverse (i.e., does not completely reverse the plan’s adverse determination) the
notice must also:
• Inform the parties of the right to an ALJ hearing if the amount in controversy meets the
appropriate threshold requirement (see §70.2 for threshold requirements; plans may not
appeal to the ALJ or attorney adjudicator); and
• Describe procedures that the parties must follow to obtain an ALJ hearing, including the
filing location.
The IRE is responsible for storing reconsideration case files in accordance with CMS’ Records
Management Program. For additional retention requirements, see the Part C Reconsideration
Process Manual or the Part D Reconsideration Procedures Manual.
60.6 – Dismissal of a Level 2 Appeal Request
The IRE must dismiss a level 2 appeal request under any of the following circumstances:
• The individual or entity making the request is not a proper party to the appeal under the
applicable regulation.
• When the IRE determines the party failed to make a valid request for an appeal that substantially

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
86
complies with the applicable regulation for making a valid request.
• Part D only: When the party fails to file the appeal within the proper filing timeframe in
accordance with the applicable regulation and there is no good cause for the late filing.
• When the enrollee dies while the appeal is pending and the enrollee’s spouse or estate has no
remaining financial interest in the case and no other individual or entity with a financial interest
in the case wishes to pursue the reconsideration or redetermination. Financial interest means
having financial liability for the item(s) or service(s) underlying the coverage request.
• When the party that requested the appeal submits a timely request for withdrawal of the request
for reconsideration.
NOTE: The above list of circumstances (taken from the applicable regulations) under which the
IRE must dismiss a level 2 appeal is exhaustive. The IRE may not deem a request invalid or
dismiss a level 2 appeal for any reason not explicitly included in 42 CFR §§ 422.592(d) and
423.600(g), as applicable.
60.6.1 – Dismissal Notice
If the IRE dismisses a level 2 appeal, the IRE must mail or otherwise transmit a written notice of
the dismissal to the parties at their last known address by the conclusion of the applicable
adjudication timeframe.
The dismissal notice must state all of the following:
(1) The reason for the dismissal;
(2) The right to request that the IRE vacate the dismissal action; and
(3) The right to request ALJ review of the IRE’s dismissal.
Consistent with the timeframe for requesting a timely appeal, a request for review of a dismissal
by the IRE must be filed with the ALJ within 60 calendar days from the date of the IRE’s
dismissal notice.
When a dismissal is prompted by a timely withdrawal request, the IRE is required to mail or
otherwise transmit a dismissal notice in accordance with the notice requirements in order to
preserve the rights of other proper parties to the decision who may wish to request ALJ review of
the dismissal.
60.6.2 – Dismissal Binding Unless Modified, Reversed or Vacated
The IRE’s decision regarding its dismissal of a level 2 appeal is binding unless the enrollee or
other party requests review by the ALJ or the decision is vacated by the IRE. In addition to the
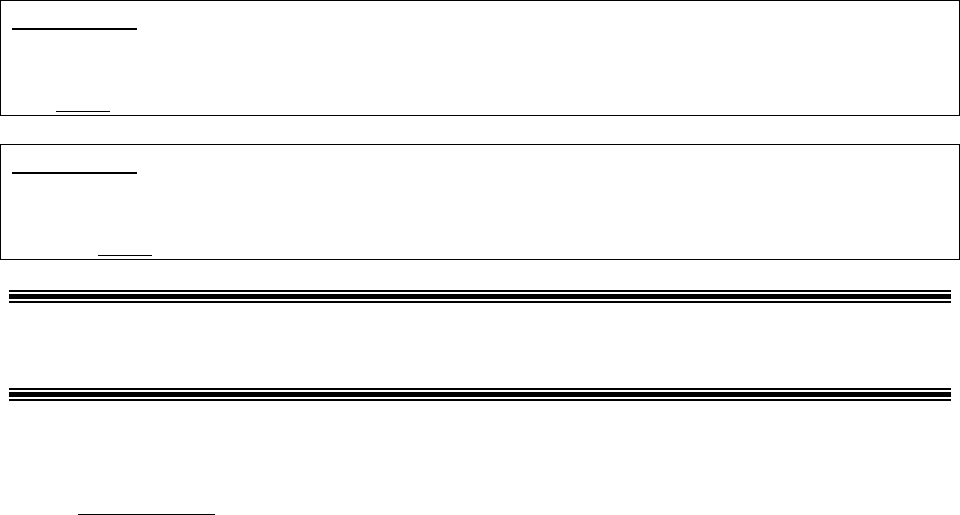
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
87
right to request ALJ review of an IRE dismissal, an enrollee (or other party) has the right to
request that the IRE vacate the dismissal action. The IRE may vacate its own dismissal within 6
months of the date of the dismissal if good cause is established.
60.7 – Effect of a Level 2 Appeal Determination
An IRE reconsideration determination is final and binding on the enrollee and the plan, unless a
party files a request for a hearing before an ALJ or attorney adjudicator (level 3 appeal).
Part C Only
Pursuant to 42 CFR §422.600, any party to the Part C reconsideration determination, except the
MA plan, has a right to request a level 3 review if the amount in controversy thresholds are met
(see §70.2 for threshold requirements).
Part D Only
Pursuant to 42 CFR §423.2000, an enrollee who is dissatisfied with the IRE reconsideration
determination has a right to request a level 3 review if the amount in controversy thresholds are
met (see §70.2 for threshold requirements).
Note: The Remainder of §60 Applies to Part D Only
60.8 – Reconsideration of Late Enrollment Penalty Determinations
Under §1860D-13(b) of the Act and 42 CFR §§423.46 and 423.56(g), the Secretary or his or her
designee imposes a late enrollment penalty (LEP) if there is a continuous period of 63 days or
more at any time after the end of the individual’s Part D initial enrollment period during which
the individual was eligible to enroll in a Part D plan, but was not enrolled in a Part D plan and
was not covered under any creditable prescription drug coverage.
“Creditable prescription drug coverage” is coverage that meets Medicare’s minimum standards
since it is expected to pay, on average, at least as much as Medicare’s standard prescription drug
coverage. This may include but is not limited to:
• Employer-based prescription drug coverage, including the Federal Employees Health
Benefits Program (FEHBP);
• State Pharmaceutical Assistance Programs (SPAPs);
• Military-related coverage (for example, VA, TRICARE coverage); and
• Certain Medicare supplemental (Medigap) policies.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
88
See 42 CFR §423.56(b) for a complete list of types of prescription drug coverage that may be
determined to be creditable.
As outlined at 42 CFR §423.56(c) and (d), with the exception of Prescription Drug Plan
Sponsors, Medicare Advantage Organizations, Section 1876 Cost-Based Contractors, and PACE
organizations offering prescription drug plans, entities that offer prescription drug coverage must
make an annual determination of creditable coverage status and provide a disclosure notice to
Medicare eligible individuals (see the appropriate plan enrollment guidance for information
related to Part D enrollment eligibility). See Chapter 4 of the Prescription Drug Benefit Manual
for additional guidance regarding creditable coverage period determinations and the calculation
and assessment of the LEP.)
Note: Prescription drug discount cards, free clinics, or drug discount websites do not
constitute creditable prescription drug coverage. Also, the “certificate of creditable coverage”
an enrollee may receive when his or her health coverage ends does not mean the prescription
drug coverage met Medicare’s minimum standards – unless the notice specifically mentioned
the enrollee had “creditable” prescription drug coverage that expected to pay as much as
Medicare’s standard prescription drug plan pays. Furthermore, workers compensation set-
aside arrangements do not constitute creditable prescription drug coverage, since this
prescription coverage is only for medication related to a work injury. For additional
guidance concerning creditable coverage-related requirements, see the material posted on the
Creditable Coverage page on the CMS website.
An enrollee, or his or her representative may request a review, or reconsideration, of a decision
to impose an LEP. An enrollee may only obtain review under the circumstances listed on the
LEP Reconsideration Request Form. Unless otherwise stated in §20.1 of this guidance, the
enrollee’s representative has all of the rights and responsibilities of an enrollee under Part D LEP
reconsideration procedures.
The LEP reconsideration is conducted by the IRE under contract with Medicare.
60.8.1 – Summary of the LEP Reconsideration Process
The LEP Reconsideration Process is described below:
• When a Part D plan sponsor sends a letter notifying an enrollee of the imposition of or
increase in the LEP (“LEP letter”), and the increase is due to reporting additional
uncovered months, except in a case where the number of uncovered months increases as a
result of an IRE decision, the plan sponsor shall include the Part D LEP Reconsideration
Notice: “Your Right to Ask Medicare to Review Your Medicare Part D Late Enrollment
Penalty” and the LEP Reconsideration Request Form.
• The information provided by the plan advises the enrollee that he or she has 60 calendar
days from the date on the LEP letter to request reconsideration of the LEP, or the request
may not be considered. If the 60-day timeframe for filing has expired, the enrollee may
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
89
request a good-cause extension, subject to the requirements described in §60.4 of this
guidance. The enrollee must explain his or her reason for filing late on a separate sheet
and send this explanation along with the LEP Reconsideration Request Form.
• The enrollee sends his or her signed, completed LEP Reconsideration Request Form to the
IRE, in accordance with the filing instructions provided on the form. Enrollees also may
write a letter requesting an LEP appeal, provided the letter contains the elements on the
LEP Reconsideration Request Form.
• The IRE shall request a copy of the case file from the plan sponsor and make a decision
based on the case file, the information supplied by the enrollee, and any other information
the IRE deems relevant.
• The IRE will inform the enrollee and the plan sponsor of the final decision.
• The plan sponsor, if applicable, shall report a revised creditable coverage determination to
CMS and notify the enrollee in writing of the new LEP amount and any refund due.
(Refer to Chapter 4 of the Prescription Drug Benefit Manual for more information.)
• The final LEP reconsideration decision is not subject to appeal (that is, is not subject to
further review by an Administrative Law Judge (ALJ) or attorney adjudicator, Council, or
in a district court of the U.S.), although CMS can review and revise a decision upon
request.
60.8.2 – Part D Plan Responsibilities under the LEP Reconsideration Process
The Part D plan sponsor shall become familiar with LEP procedures so it is able to assist
enrollees throughout the LEP reconsideration process. For example, the plan sponsor shall:
• Attempt to obtain a completed Declaration of Prior Prescription Drug Coverage form,
(Exhibit 1D in Chapter 4 of the Prescription Drug Benefit Manual) from the enrollee at the
beginning of the creditable coverage determination process, where the enrollee appears to
have a qualifying break in creditable prescription drug coverage. Obtaining the
Declaration may avoid the assessment of a LEP and the need for reconsideration.
• Send the enrollee the Part D LEP Reconsideration Notice, “Your Right to Ask Medicare to
Review Your Part D Late Enrollment Penalty”, and the LEP Reconsideration Request
Form at the same time the plan sponsor sends an enrollee his or her LEP letter.
• Assist the enrollee in completing the LEP Reconsideration Request Form upon request.
For example, the plan sponsor shall help an enrollee determine which checkbox to mark as
his or her reason for seeking reconsideration.
• The plan sponsor shall inform the enrollee that his or her request must include the
following:

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
90
o A completed, signed LEP Reconsideration Request Form or a signed, written
request for reconsideration containing the elements on the LEP reconsideration
request form; and
o If the enrollee has named a representative, proof that the individual has authority to
represent the enrollee.
Note: In addition to the items above, the plan sponsor shall inform the enrollee
that his or her request should include any additional information that may help the
enrollee’s case, including evidence that the IRE should consider (e.g., notice from
an employer sponsored health plan indicating that prior drug coverage was
creditable). See §60.8.4.
• The plan sponsor shall instruct enrollees to send this material to the IRE at the address or
fax number shown on page 2 of the LEP Reconsideration Request Form, to include their
HICN or MBI on any separate materials, and to only send photocopies of their original
documents.
• Retain a copy of the LEP letter sent to an enrollee. If the plan sponsor retains a copy of
the LEP letter in the enrollee’s file, that information will be readily and easily available if
the enrollee requests review of the LEP and the IRE requests this information.
• Send the IRE a copy of the enrollee’s case file, which includes copies of any information
the plan sponsor used in making its creditable coverage determination for the enrollee,
including, but not limited to:
o the enrollee’s Part D initial enrollment period (IEP) or subsequent IEP end date
(and how it was derived); and
o the enrollee’s creditable coverage attestation materials (“Declaration of Prior
Prescription Drug Coverage” form, Exhibit 1D in Chapter 4 of the Prescription
Drug Benefit Manual), and any documentation from CMS of the enrollee’s
enrollment in a plan or in a plan whose sponsor received the retiree drug subsidy.
If the IRE partially or fully reverses a plan sponsor’s creditable coverage determination, the plan
sponsor shall comply with the requirements described under Chapter 4 of the Prescription Drug
Benefit Manual concerning adjustment or removal of an LEP.
60.8.3 – Requests for Information
Upon request, the plan sponsor shall forward to the IRE any information necessary to make a
reconsideration decision, including all creditable coverage and LEP-related information received
in accordance with Chapter 4 of the Prescription Drug Benefit Manual, such as information from
a current or previous enrollee.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
91
Information Availability
Timeframe*
Requirement
The requested information
is available
Within 14 calendar days
Deliver (by mail or fax) a
hard copy of the requested
information.
The requested information
is not available
Within 14 calendar days
Deliver (by mail or fax) a
brief letter to the IRE
acknowledging that the
requested information is
unavailable and explain the
reason.
*after receiving the request for information
60.8.4 – Reasons for Requesting LEP Reconsideration and Presentation of
Evidence
An enrollee may request review of their LEP decision if he or she:
• Had prior creditable prescription drug coverage that the enrollee believes may have not
been considered.
• Had prior prescription drug coverage but didn’t get a notice that clearly explained whether
the drug coverage was creditable. In this case, the enrollee should submit any evidence,
such as a copy of a plan sponsor’s letter or other material, for example, a Summary of
Benefits that the enrollee found unclear or misleading.
• Believes the LEP is wrong because he or she was not eligible to enroll in a Medicare drug
plan during the period stated by the Medicare drug plan.
• Believes the LEP is wrong because he or she was unable to enroll in a Medicare drug plan
due to a serious medical emergency during the period the individual was eligible to enroll
in a drug plan.
• Has/had extra help from Medicare to pay for prescription drug coverage; that is, the low-
income subsidy for Medicare prescription drug coverage.
Refer to Chapter 4 of the Prescription Drug Benefit Manual for additional guidance on the
opportunity for certain individuals to enroll in Medicare Part D without an LEP.
60.8.5 – IRE LEP Processing Timeframes
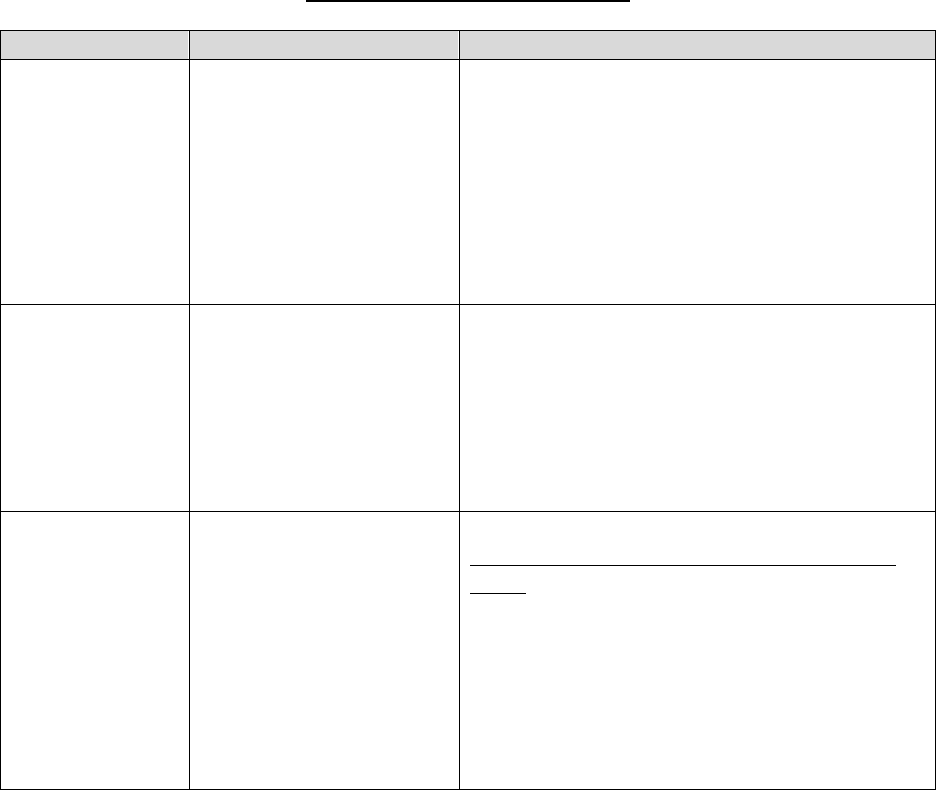
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
92
IRE Timeframes for Processing
Scenario
Timeframe
IRE Action/Notification
No extension
was requested
and good cause
for extension
was not found by
the IRE.
90 calendar days
• Notify the enrollee of the final decision
(including a decision to dismiss the
request).
• If an enrollee has identified a
representative, the IRE will send any notice
or other correspondence to the enrollee’s
representative instead of to the enrollee.
Enrollee requests
an extension or
the IRE finds
good cause to
extend.
90 calendar days with a
14-day extension
Good cause would include, for example, when
the IRE finds a need for additional information
and considers the delay to be in the interest of
the enrollee, such as receipt of additional
information that may reduce the number of
uncovered months upon which the LEP was
based.
An individual
other than the
enrollee files for
reconsideration.
The timeframe will not
begin until the IRE
receives documentation
verifying that the
individual is the
enrollee’s representative
or is authorized under
state law to act on behalf
of an enrollee.
The IRE will attempt to cure any defect in an
Appointment of Representative form (CMS-
1696) or other equivalent written notice by
requesting information from the individual
who filed the request. If the IRE cannot verify
an individual’s status as the representative
within a reasonable time period, not to exceed
30 calendar days after the date of the request,
the IRE will determine that the reconsideration
request be dismissed.
Note: In all cases, the IRE strives to notify an enrollee of its final decision as quickly as
possible. However, the IRE may take longer than the 90-day timeframe to process an LEP
reconsideration decision in certain cases depending, among other issues, on the amount of
research the IRE has to perform to verify whether an enrollee’s prior prescription drug
coverage was creditable
60.8.6 – Withdrawal of an LEP Reconsideration Request
An enrollee may withdraw his or her LEP reconsideration request in writing at any time before
the IRE mails the final decision. For purposes of a withdrawal, “enrollee” also includes a former
enrollee or his or her representative.
60.8.7 - Dismissal of an LEP Reconsideration Request
The IRE should dismiss the reconsideration under any of, but not limited to, the following

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
93
circumstances:
• When the enrollee fails to file the LEP reconsideration within the proper filing timeframe
and does not have good cause for missing the filing deadline;
• When the enrollee dies while the reconsideration is pending and the enrollee’s surviving
spouse or estate has no remaining financial interest in the reconsideration. The word
“spouse”, as used in 42 CFR §423.2052(a)(5), includes same-sex spouses as well as
opposite-sex spouses. Because civil unions and domestic partnerships are not marriages,
civil union and domestic partners are not regarded as spouses by CMS;
• When the individual requesting the reconsideration is not the enrollee, and the authority of
the individual seeking a reconsideration cannot be verified within a reasonable time
period, not to exceed 30 calendar days after the date of the reconsideration request;
• When an enrollee requests a reconsideration of an issue that is ineligible for LEP
reconsideration or is otherwise ineligible for review. For example, the IRE will not make
actuarial determinations concerning whether an enrollee’s prescription drug coverage was
creditable; that is, an enrollee may not use the LEP reconsideration process to seek review
of the decision that his or her coverage under an employer-sponsored prescription drug
plan was not creditable coverage; or
• When the enrollee submits a timely written request to withdraw their request for an LEP
reconsideration
Vacating a Dismissal
The dismissal is binding, unless the dismissal is vacated. If a Part D enrollee requests the
dismissal be vacated and he or she shows good cause that the reconsideration request should not
be dismissed, the dismissal of the reconsideration request may be vacated. The enrollee must
request that the dismissal be vacated within 60 days after the date of the dismissal notice. The
IRE will notify the enrollee and the Part D plan sponsor in writing if the dismissal is vacated.
70 – Key Aspects of Administrative Law Judge (ALJ)/Attorney Adjudicator,
Council, and Judicial Review
70.1 – Parties to a Hearing
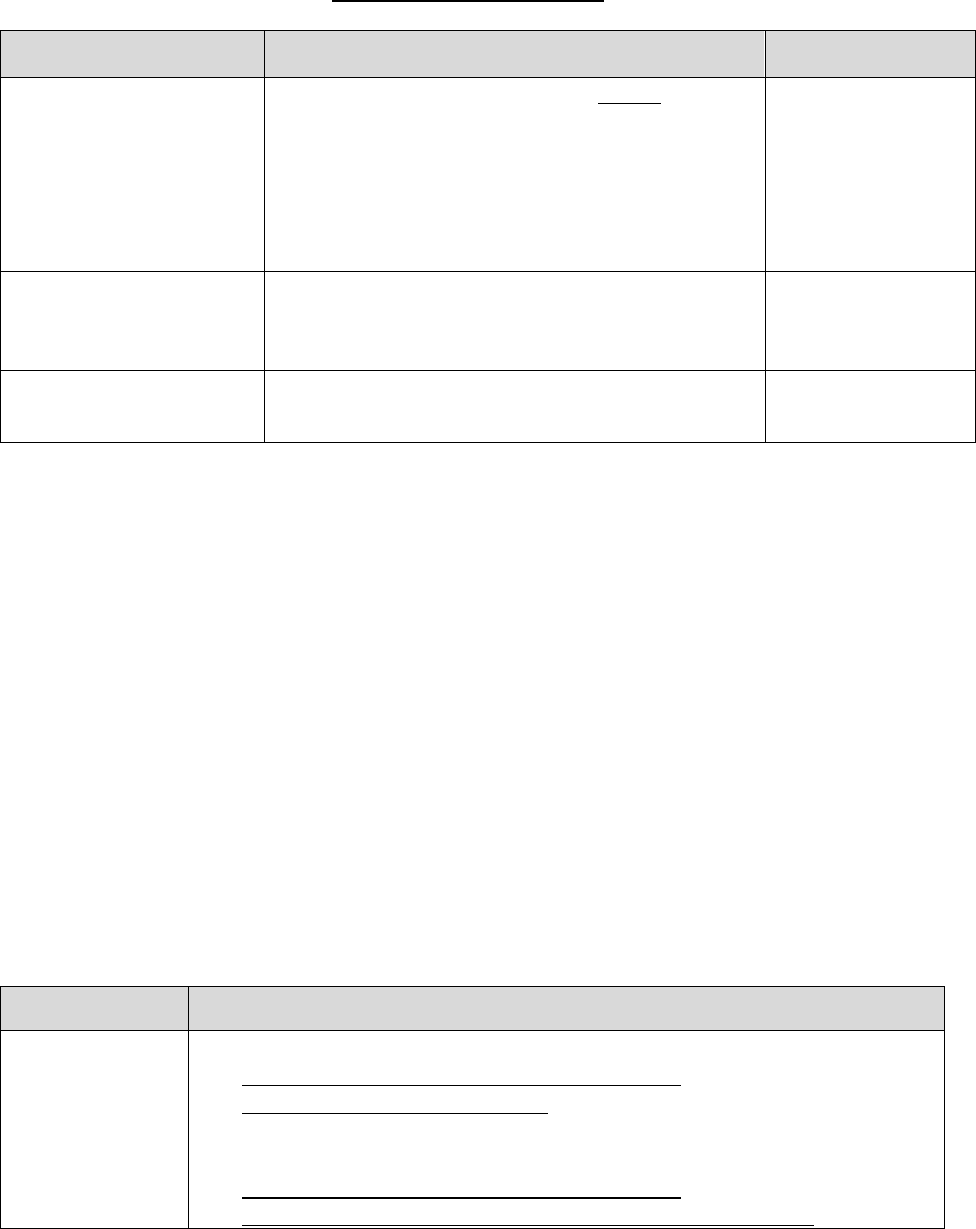
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
94
Who May Request Review
Level of Review Part C Part D*
Third Level:
ALJ/attorney adjudicator
Any party to the reconsideration, except the MA
plan, has a right to a hearing. The parties to a
hearing are the parties to the reconsideration, the
MA plan, and other person/entity whose rights
with respect to the reconsideration may be
affected by the hearing, as determined by the
ALJ or attorney adjudicator.
Enrollee
Fourth Level:
Council
Any party to the ALJ or attorney adjudicator’s
decision or dismissal, including the MA plan.
Enrollee
Fifth Level:
Federal Court
Any party, including MA plan (must notify all
other parties).
Enrollee
*An enrollee’s prescribing physician or other prescriber may request an initial determination, redetermination or
level 2 appeal, but cannot request a higher level of appeal without being the enrollee’s representative. See, for
example, 423.2002 for information on the right to an ALJ hearing.
70.2 – Amount in Controversy
In general, the amount in controversy (AIC) is computed as the actual amount charged (or
amount enrollee would have been charged) minus applicable deductible, coinsurance or
copayments. For Part D, if the basis for the appeal is the refusal by the plan to provide drug
benefits, AIC is determined by using the projected value of those benefits, reduced by any cost
sharing amounts including deductible, coinsurance or copayments that may be collected from the
enrollee. Projected value includes any costs the enrollee could incur based on the number of
refills prescribed for the disputed drugs during the plan year. For Part C, if the basis for the
appeal is the MA plan’s refusal to provide services, the AIC is computed using the projected
value of those services. Per § 422.600(b), in applying the provisions at § 405.1006(d) to the
calculation of the AIC in Part C cases, the reference to coinsurance should be read to include
coinsurance and copayment amounts. For Parts C and D, appeals may be aggregated to meet the
AIC. See §423.1970(c); [§405.1006(e) and (f)].
Level of Review
Amount in Controversy Requirement
Third Level:
ALJ/attorney
adjudicator
• Part C ALJ hearing/attorney adjudicator review:
https://www.cms.gov/Medicare/Appeals-and-
Grievances/MMCAG/ALJ.html
• Part D ALJ hearing/attorney adjudicator review:
https://www.cms.gov/Medicare/Appeals-and-
Grievances/MedPrescriptDrugApplGriev/ALJHearing.html
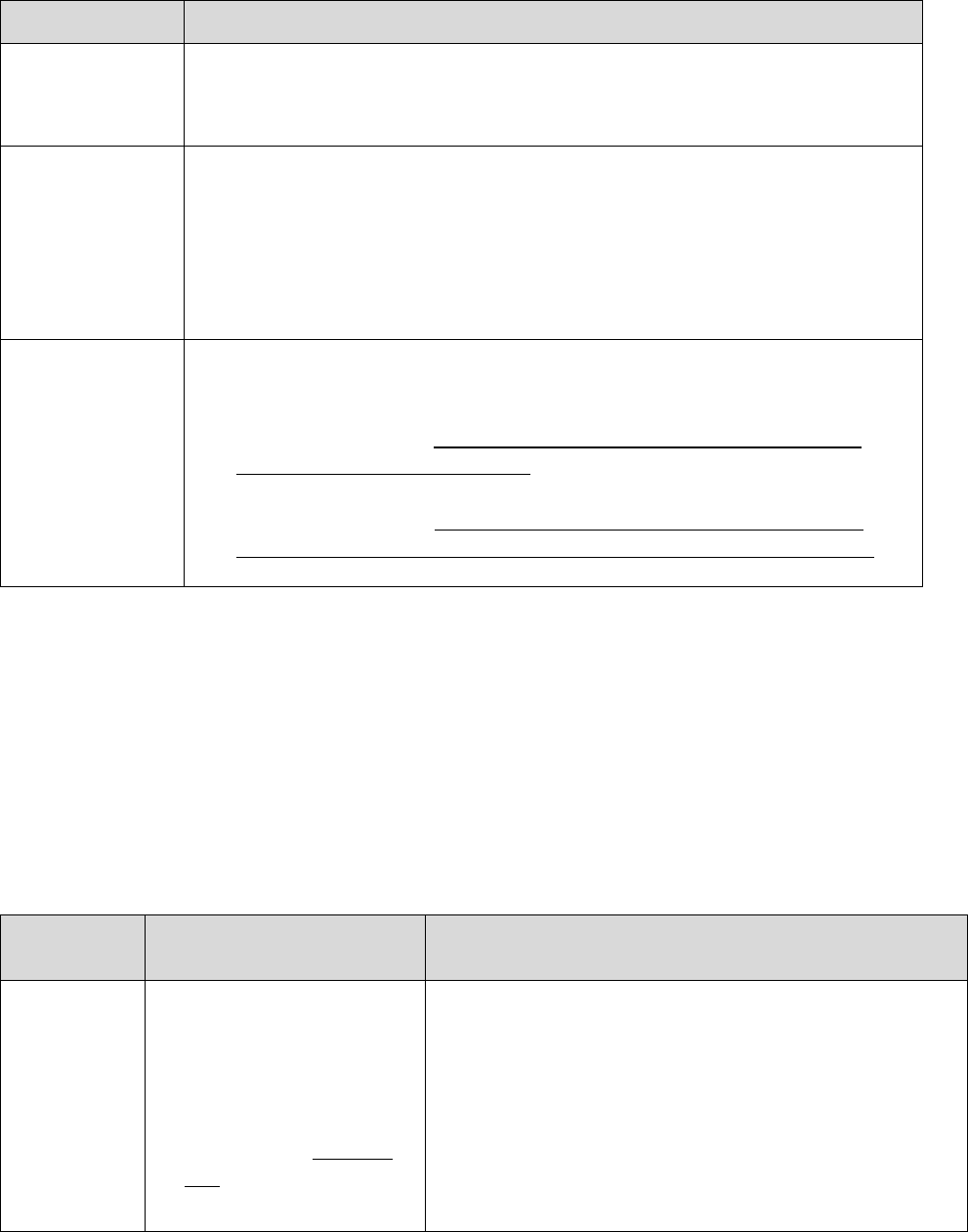
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
95
Level of Review
Amount in Controversy Requirement
If the request for ALJ hearing shows that the required AIC is not satisfied,
the appeal is dismissed at the OMHA level.
Fourth Level:
Council
• No amount in controversy requirement.
• If a case is reviewed by the Council, whether it is a review of a
dismissal or a decision at the OMHA level, there is no additional AIC
to be met with the expectation that the claim has already met the AIC
for the third level of appeal. No additional AIC is required for the
fourth level of review.
Fifth Level:
Federal Court
If a case is appealed to the fifth level, the claim must then meet the AIC
established by the Secretary for Federal Court review.
• Part C federal court: https://www.cms.gov/Medicare/Appeals-and-
Grievances/MMCAG/Fed.html
• Part D federal court: https://www.cms.gov/Medicare/Appeals-and-
Grievances/MedPrescriptDrugApplGriev/FederalCourtReview.html
70.3 – Filing Requests for Review
At the third, fourth, and fifth levels of review, the request must be filed within 60 calendar days
of receipt of a decision or dismissal from the IRE, ALJ/attorney adjudicator, or the Council,
respectively. (Unless there is evidence to the contrary, the date of receipt of a decision or
dismissal is presumed to be 5 calendar days after the date of decision or dismissal). Extension of
filing deadline may be granted for good cause. The request for good cause extension must be in
writing (except for requests for Part D expedited hearings, for which the extension may be made
verbally or in writing) and state the reason the request was late.
Level of
Review
How to file a request
If the applicable request form is not used, written
requests should include the following:
Third
Level:
ALJ/attorney
adjudicator
• With the entity/office
specified in the IRE
decision or dismissal; or
• In writing* – OMHA-
100 may be used
• Appellant’s name, address, telephone number and
Medicare number;
• The representative’s name, address, and telephone
number, if applicable.
• Case number or appeal number, if any, assigned by
the IRE;
• Reasons the appellant disagrees with the IRE
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
96
Level of
Review
How to file a request
If the applicable request form is not used, written
requests should include the following:
decision or dismissal;
• Statement of additional evidence to be submitted
and the date it will be submitted; and
• Additional request content for Part C only:
Dates of service of claim(s) being appealed, if
applicable.
• Additional request content for Part D only:
o Name of prescription drug in dispute and plan
name.
o A statement that the enrollee is requesting an
expedited hearing, if applicable. If appellant is
requesting an expedited hearing, the request
should explain why the standard timeframe may
seriously jeopardize the enrollee’s life, health,
or ability to regain maximum function.
Fourth
Level:
Council
With the Council (Address
noted in ALJ/attorney
adjudicator’s decision);
In writing* – DAB-101
may be used
Part C Only: If the MA
plan requests review, the
MA plan must
concurrently notify the
enrollee by sending a copy
of the request and
accompanying documents
submitted to the Council.
Part C Only
(if form DAB-101 is not used)
• Name
• Medicare number
• Service(s)/item(s)/Part B drug(s) for which review
is requested
• Date(s) of service
• Date of ALJ or attorney adjudicator decision or
dismissal
• Name and signature of party or party’s
representative
• Request must identify parts of the ALJ or attorney
adjudicator decision with which the party disagrees
and explain reason for disagreement.
Part D Only
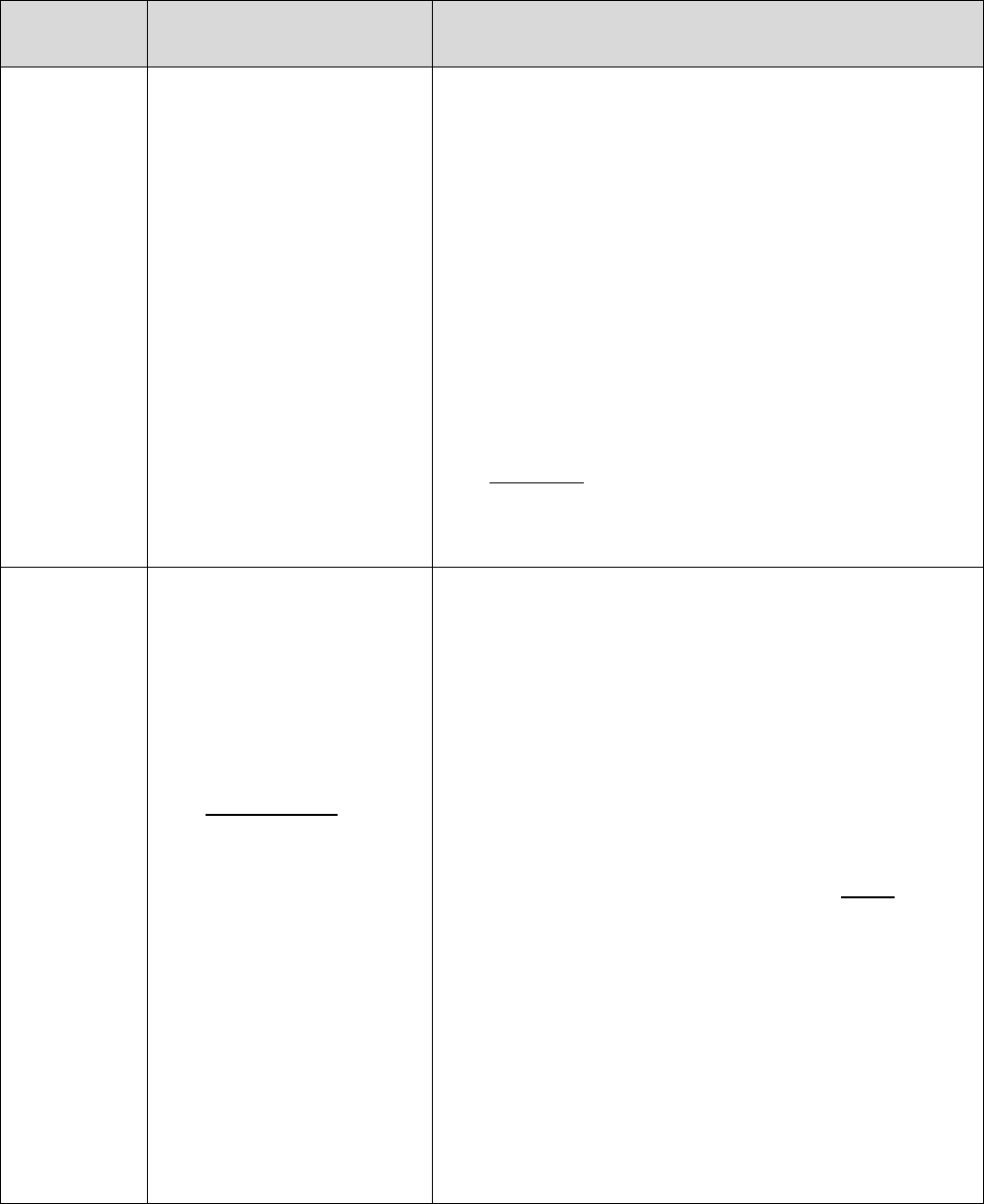
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
97
Level of
Review
How to file a request
If the applicable request form is not used, written
requests should include the following:
Same as above requirements for written requests filed
at the third level of review. Must also include:
• Case number or appeal number, if any, assigned
by OMHA;
• Reasons the appellant disagrees with the ALJ or
attorney adjudicator decision. dismissal or other
determination being appealed;
• Signature of enrollee or representative, if any; and
• A copy of the ALJ’s or attorney adjudicator’s
decision should be included with the request. The
DAB-101 form requests that the decision or
dismissal order being appealed be attached to the
request form.
Fifth Level:
Federal
Court
• Civil action must be
filed in a district court
of the U.S. where the
enrollee resides or, if
none, in the U.S. district
court for the District of
Columbia.
Part C Only: Action
may be filed in the
district court where
the party (e.g. MA
plan) has its principal
place of business.
• See §§422.612,
405.1130 – 1140 (Part
C) and §§423.2130 –
423.2140 (Part D) for
procedures related to
requesting judicial
review.
•
Not governed by Medicare regulations but an amount
in controversy threshold applies (see §70.2 for
threshold requirements).
*Under Part D, expedited requests may be submitted verbally or in writing.
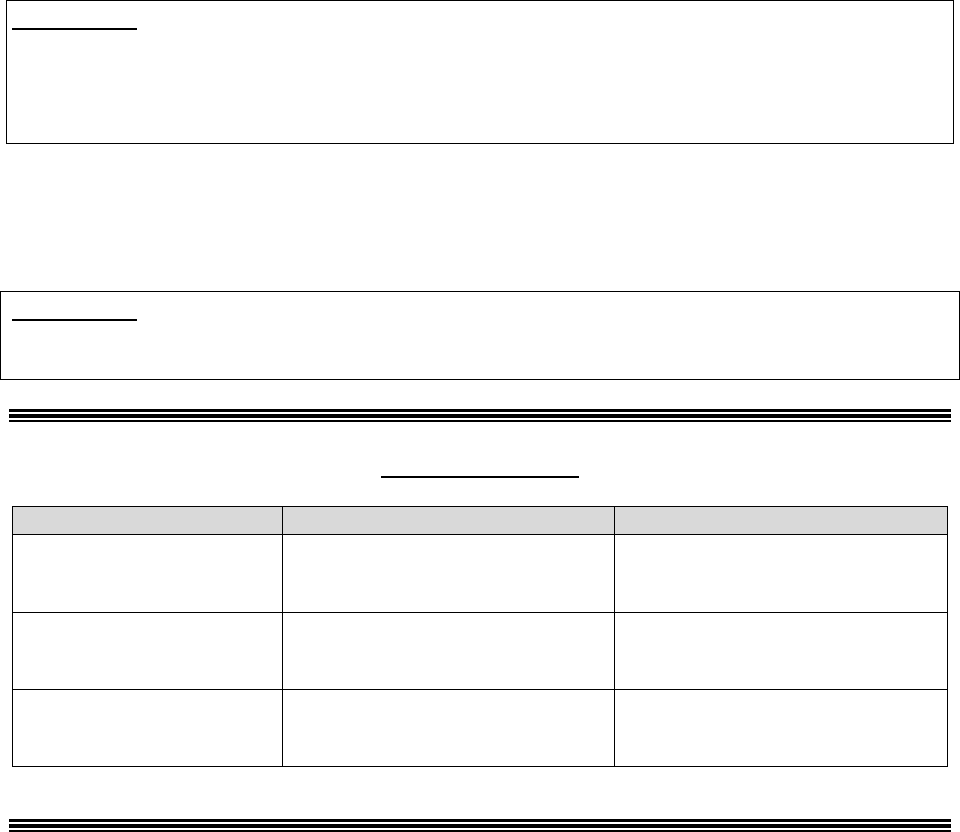
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
98
Part D Only
The Council may initiate a review on its own motion or at the request of CMS or the IRE within
60 calendar days after the date of ALJ/attorney adjudicator’s written decision or dismissal. The
Council will mail notice of the results of its own motion review to the enrollee and to CMS or
the IRE as appropriate.
70.4 – Review Procedures
70.4.1 – Decision-Making Timeframes
Part C Only
There are no statutory or regulatory decision-making timeframes for Part C appeals at the third
level of review and beyond. See 42 CFR §422.562(d).
Part D Timeframes
Level of Review
Expedited
Standard
Third Level:
ALJ/attorney adjudicator
Generally within 10 calendar
days*
Generally within 90 calendar
days
Fourth Level:
Council
Generally within 10 calendar
days*
Generally within 90 calendar
days
Fifth Level:
Federal Court
N/A N/A
* The medical exigency standard applies.
All timeframes begin on the date the request is received, unless the timeframe has been extended
as provided in the regulations. For further information on hearings and decisions by an ALJ or
attorney adjudicator, see 42 CFR §§422.600-602 and 42 CFR §§423.2000-2063.
Note: OMHA prioritizes enrollee-requested appeals (e.g., a dedicated help line, and an
enrollee mailing address that enables fast identification and processing).
70.4.2 – Part D Plan Sponsor, CMS, or IRE Requesting ALJ Hearing
Participation (Part D Only)
Participating in a hearing allows the plan sponsor, CMS, or the IRE to file position papers and/or
provide testimony to clarify factual or policy issues, but does not include calling or cross-
examining witnesses. If a plan sponsor, CMS, or the IRE requests participation in an ALJ
hearing, how and when the request to participate is made is as follows:
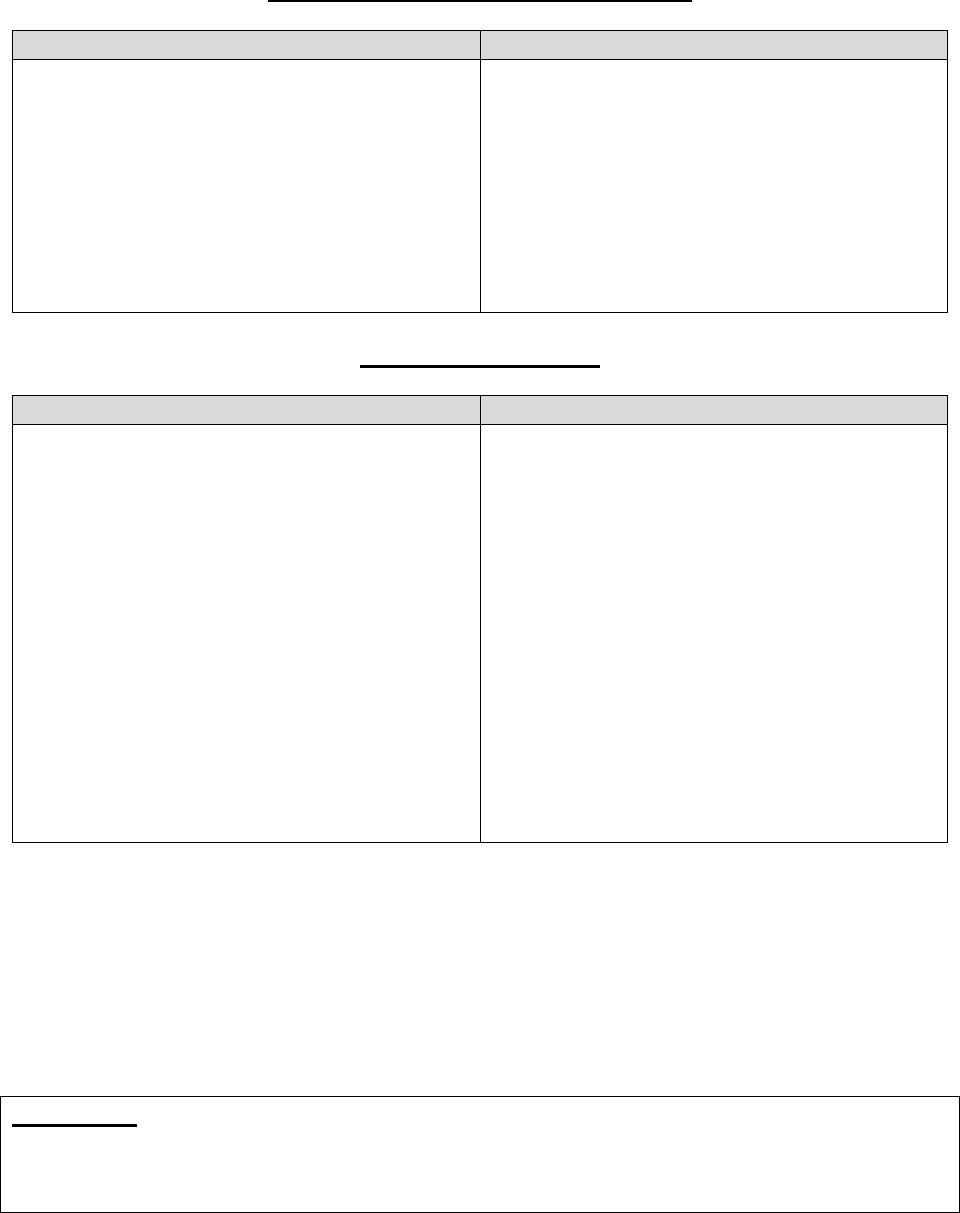
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
99
Request to Participate Filing Timeframes
Standard
Expedited
• If no hearing is scheduled, the request
must be sent within 30 calendar days
after notification that a standard request
for hearing was filed.
• If a hearing has been scheduled, the
request must be sent within 5 calendar
days after receiving the notice of hearing.
• If no hearing is scheduled, the request
must be sent within 2 calendar days after
notification that a request for expedited
hearing was filed.
• If a hearing has been scheduled, the
request must be sent within 1 calendar
day after receiving the notice of hearing.
Where to Send Request
Standard
Expedited
• If the plan sponsor, CMS, or the IRE
requests participation after receiving the
notice of hearing, written notice of a
request to participate must be sent to the
ALJ and the enrollee.
• If the plan sponsor, CMS, or the IRE
requests participation before the notice of
a hearing is received or if no notice is
required, written notice of a request to
participate must be sent to the assigned
ALJ or attorney adjudicator, or a
designee of the Chief ALJ (if the request
is not yet assigned) and the enrollee.
If the plan sponsor, CMS, or the IRE requests
participation, the request may be made
verbally for an expedited hearing and OMHA
will notify the enrollee of the request to
participate.
Note: The assigned ALJ or attorney adjudicator has discretion not to allow CMS, the IRE,
and/or plan sponsor to participate.
70.4.3 – Submitting Evidence at the Third Level of Review
An enrollee or other party must submit written or other evidence that he or she wishes to have
considered at the hearing as follows:
Part C Only
Parties must submit all written or other evidence with the hearing request, or by the date
specified in the hearing request, or, if a hearing is scheduled, within 10 calendar days of
receiving the notice of hearing. This requirement does not apply to an unrepresented enrollee.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
100
Part D Only
Submit all written or other evidence with the hearing request, or by the date specified in the
hearing request, or, if a hearing is scheduled, within:
• 10 calendar days of receiving the notice of hearing for standard hearings (these
submission requirements do not apply to unrepresented enrollees in standard Part D
appeals (see §423.2018(b)(3))).
• 2 calendar days of receiving the notice for expedited hearings.
If an enrollee wishes evidence on a change in medical condition (after the coverage
determination was made) to be considered, the ALJ/attorney adjudicator will remand the case to
the Part D IRE.
80 – Reopening and Revising Determinations and Decisions
A reopening is a remedial action taken to change a binding determination or decision even
though the binding determination or decision (i.e., initial determination or level 1 appeal) may
have been correct at the time it was made based on the evidence of record.
Given the remedial nature of a reopening, CMS expects the reopening process to be used
sparingly by plans. If a plan routinely or excessively uses the reopening process, this may
indicate that the plan does not have sufficient procedures in place for properly processing initial
determinations and level 1 appeals. For example, frequent use of the reopening process may
indicate that the plan is not thoroughly reviewing requests or is not conducting reasonable
outreach for missing information (see §10.6 for outreach requirements). Plans and other
adjudicators shall not use the reopening process in a manner that interferes with enrollee access
to the appeals process. Plans may be subject to compliance action if found to be routinely using
the reopening process in a manner that interferes with enrollee access to the appeals process.
When adjudicators reopen initial determinations or appeals, they must comply with the
reopening regulations listed below:
• 42 CFR §422.616; and
• 42 CFR §§423.1978 – 423.1986.
Pursuant to 42 CFR §422.616(a), the reopening regulations in Part 405 (i.e., §§405.980 –
405.986) are applicable to Part C, unless otherwise specified.
80.1 – Guidelines for Reopening
A decision to reopen is at the discretion of the adjudicator who has authority to conduct a
reopening. Adjudicators with authority to conduct a reopening include:

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
101
• A plan to revise the initial determination or level 1 appeal decision;
• An IRE to revise its reconsideration determination;
• An ALJ or attorney adjudicator to revise his or her decision; or
• The Council to revise the ALJ or attorney adjudicator decision or its review decision.
The filing of a request for a reopening with the IRE, ALJ, or the Council does not relieve the
plan of its obligation to make payment for, authorize, or provide benefits or services as specified
in 42 CFR §§422.618, 423.1978(b), and 423.1980(a)(3), consistent with 42 CFR §422.616(c)
and information provided in this guidance.
Part C Only
42 CFR §422.618(c)(2) does not apply to an ALJ decision following Federal District Court
remand. Pursuant to 42 CFR §405.1140(a), a decision by the Office of Medicare Hearings &
Appeals is the final decision of the Secretary, unless the Council assumes jurisdiction. Part 405
provisions (i.e., §§405.980 – 405.986) apply to Medicare Advantage Organizations, unless the
subpart provides otherwise per 42 CFR §422.562(d).
A reopening request:
• May be initiated by a plan, the IRE, ALJ or attorney adjudicator, the Council, or requested
by an enrollee or any other party to the determination or decision;
• May be made verbally or in writing;
• Should include the specific reason for requesting the reopening (a statement of
dissatisfaction is not grounds for a reopening); and
• Must be made within the timeframes permitted for reopening (as set forth in §80.3).
If the request for reopening is denied, the enrollee or other party must be notified that the
determination or decision will not be reopened. If the request was received in writing, the
adjudicator must notify the requestor in writing of the decision not to reopen. The decision on
whether to reopen is binding and not subject to appeal.
When a determination or decision is reopened and revised (including revision of the rationale for
a decision that is not revised), the plan, IRE, ALJ or attorney adjudicator, or the Council that
reopened the decision must deliver written notification to the parties to that determination or
decision, as described in §80.6.
After reopening, a revised determination or decision is binding unless it is appealed or otherwise
subsequently reopened. Only the portion of the determination or decision revised by the
reopening may be appealed. The timeframe to request an appeal of the revised determination or
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
102
decision begins on the date of the revised determination or decision.
80.2 – Reopenings Separate and Distinct from Appeals
The reopening process is separate and distinct from the appeals process. When a party has filed a
valid request for a level 1 appeal, level 2 appeal, ALJ or attorney adjudicator decision, or
Council review, no adjudicator has jurisdiction to reopen a case that is under appeal until all
appeal rights for that case are exhausted or a subsequent request by the appellant to withdraw the
appeal has been granted. For example, if a party requests a level 2 appeal and a reopening of an
initial determination simultaneously, the level 2 appeal would be the only action processed. As a
result, a party cannot have an appeal and a reopening occurring simultaneously with respect to
the same case.
Part C Only
An MA plan cannot reopen and modify its reconsideration decision after the case file for the
adverse decision has been forwarded to the IRE, as CMS considers this an issue still under
appeal.
Part D Only
A Part D plan sponsor cannot reopen and modify its decision if additional information is
received after an appellant files a request for an IRE reconsideration or the plan sponsor is
required to forward the case to the IRE, unless a subsequent request by the appellant to withdraw
the IRE reconsideration request has been granted.
Comparison of Reopenings and Appeals
Characteristic
Reopening
Appeal
Request by Adjudicator
ü
N/A
Subject to Appeal Rights* N/A* ü
Binding on all Parties
ü
ü
Clerical Errors ü N/A
*Although the decision whether or not to reopen a determination or decision is not appealable, an adverse revised
determination or decision is subject to appeal. An adverse revised determination or decision as a result of a
reopening must state the rationale and basis for the reopening and revision and any right to appeal the adverse
determination or decision.
80.3 – Timeframes for Reopening
80.3.1 – Timeframes for Initiating a Reopening
The timeframes for initiating a reopening are as follows:
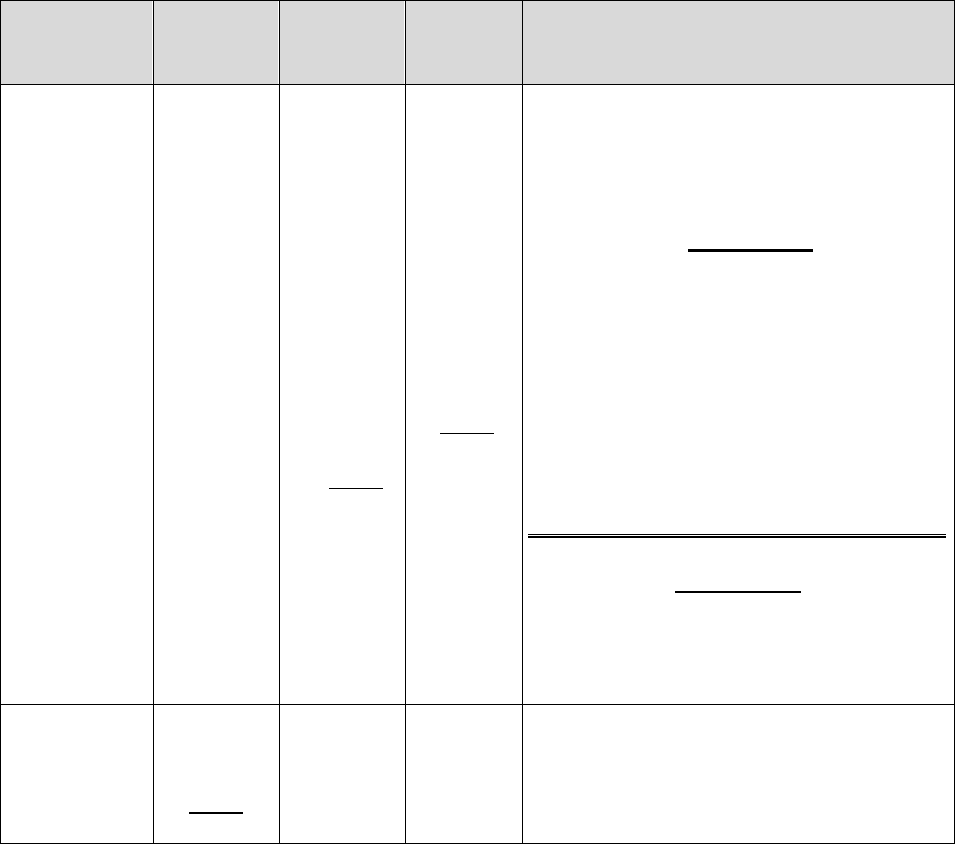
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
103
Reopening
Entity
180
Calendar
Days*
Within
1 Year*
Within
4 years*
Any Time
Plan N/A
For any
reason
(if the
case is not
under
appeal,
see §80.2)
For good
cause as
defined
in §80.5
• If reliable evidence exists (i.e., relevant,
credible, and material) that the initial
determination or level 1 appeal was
procured by fraud or similar fault.
Part C Only
• If the determination is unfavorable, in
whole or in part, but only for the
purpose of correcting a clerical error on
which that determination was based.
• To effectuate a decision issued under
the coverage (National Coverage
Determination (NCD)) appeals
process**
Part D Only
Part D plan sponsors may, but are not
required to, classify and process clerical
errors as reopenings.
IRE, ALJ or
attorney
adjudicator,
Council
For good
cause as
defined in
§80.5
N/A N/A
If the reconsideration or decision was
procured by fraud or similar fault.
*From the date of the decision.
**When reopening is initiated by the plan.
Plans must afford enrollees appropriate access to the appeals process by not repeatedly reopening
initial determinations and level 1 appeals after denial notices have been sent.
If the enrollee, provider or prescriber has submitted evidence after the initial determination or
level 1 appeal request has been denied, the plan must ascertain whether the enrollee, provider or
prescriber is seeking an appeal or a reopening (generally, these should be treated as appeals).
80.3.2 – Timeframes for Processing a Reopening
A party to an initial determination has a reasonable expectation to the administrative finality of a
determination issued by the plan. For reopenings requested by a party that the plan agrees to
reopen, the reopening action should be completed within 60 days from the date of receipt of the
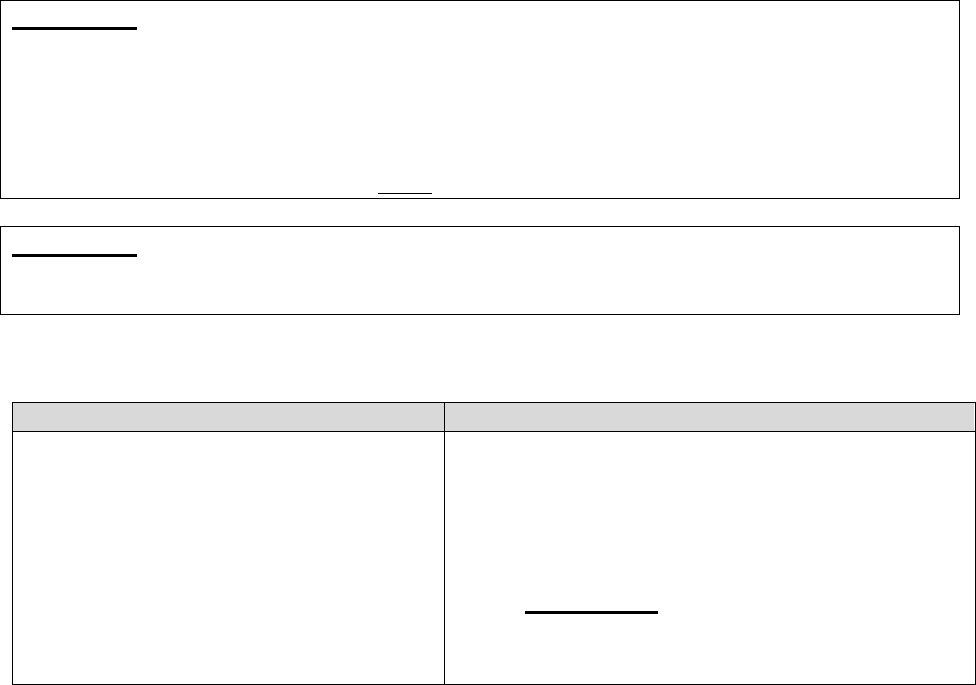
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
104
party’s reopening request.
For reopenings requested by a party at the IRE level, within 30 calendar days of receipt of the
request for reopening, the IRE will notify the party in writing as to whether or not it shall reopen
the case. If the IRE agrees to reopen the request, it will issue a revised determination within 120
days of receipt of the request.
80.4 – Reopening Based on Clerical Error
For purposes of this section, clerical error includes human and mechanical errors on the part of
the MA plan such as:
• Mathematical or computational mistakes;
• Inaccurate data entry or coding;
• Computer errors; or
• Denials of claims as duplicates.
Part C Only
An MA plan must remedy a clerical error (which include minor errors and omissions) using the
reopening process instead of the appeal process. If the MA plan receives a request for reopening
and does not agree that the issue is a clerical error, the MA plan must dismiss the reopening
request. Although a party cannot appeal an MA plan’s decision not to reopen, the MA plan must
notify the party of their rights to appeal the initial adverse decision, provided timeframes to
appeal the denial has not expired (see §80.6 for notice requirements).
Part D Only
Part D plan sponsors may, but are not required to, classify and process clerical errors as
reopenings.
80.5 – Good Cause for Reopening
Constitutes Good Cause for Reopening
Does Not Constitute Good Cause for Reopening
• There is new and material evidence
that was not available or known at the
time of the determination or decision
and may result in a different
conclusion.
• The evidence that was considered in
making the determination or decision
clearly shows on its face that an
• A change of legal interpretation or policy by
CMS in a regulation, CMS ruling, or CMS
general instruction, whether made in response to
judicial precedent or otherwise.
o Part C Only: This provision does not
preclude MA plans from conducting
reopenings to effectuate coverage decisions
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
105
Constitutes Good Cause for Reopening
Does Not Constitute Good Cause for Reopening
obvious error was made at the time of
the determination or decision. In other
words, the decision was clearly
incorrect based on all the evidence
presented in the appeal file.
issued under the authority granted by section
1869(f) of the Act. See §405.986(b).
o Part D Only: Adjudicators may reopen to
apply the current law or CMS or Part D plan
sponsor policy rather than the law or CMS or
plan sponsor policy at the time the coverage
determination is made in situations where the
enrollee has not yet received the drug and the
current law or CMS or plan sponsor policy
may affect whether the drug should be
received.
• A request to reopen a claim based upon a third-
party payer's error in making a primary payment
determination when Medicare processed the
claim in accordance with the information in its
system of records or on the claim form.
80.5.1 – New and Material Evidence
In order for evidence to be considered new and material for a plan or IRE reopening, it must
meet the following requirements:
• Was not readily available or known to the person or entity requesting/initiating the
reopening at the time of the initial determination or level 1 appeal decision;
• Does not include evidence that was or reasonably could have been, available to the
decision-maker at the time the decision was made; and
• May result in a conclusion different from that reached in the initial determination or
redetermination.
In determining whether the evidence is new and material, adjudicators must consider whether
evidence is new and material from the perspective of the person or entity requesting or initiating
the reopening.
80.6 – Notification Requirements for Reopenings
When any determination or decision is reopened and revised, (including revision of the rationale
for a decision), the plan, IRE, ALJ, or the Appeals Council must deliver written notification to
the parties to that determination or decision, including to the plan, at their last known address.
Plans should provide notification within timeframes outlined in §80.3.2.
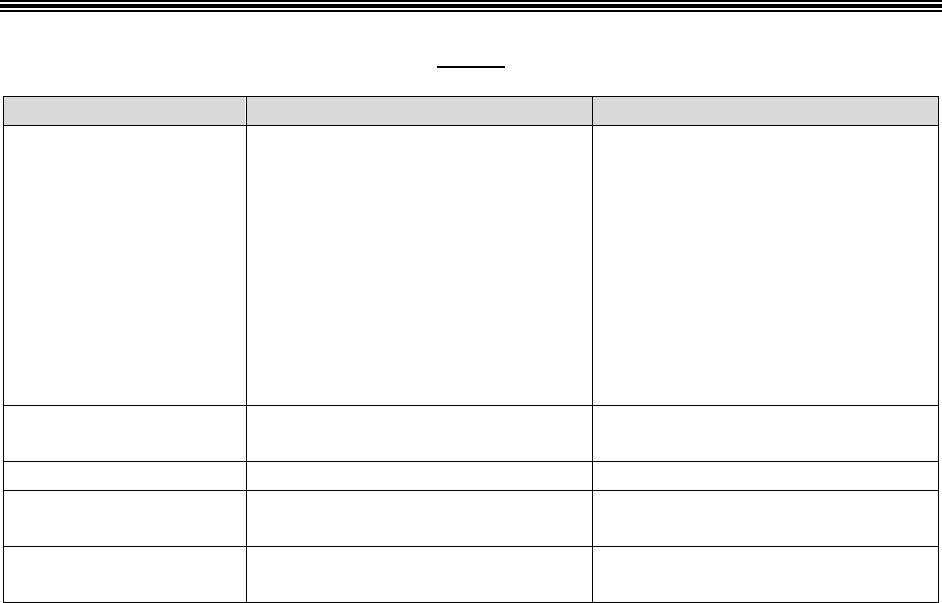
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
106
Written notification must:
• State the rationale and basis for the reopening and revision;
• State the specific reason for the revision or change in rationale, written in a manner that is
understandable to the enrollee; and
• Provide information on any appeal rights.
If a reopening results in issuance of payment to a provider, a revised remittance advice notice
must be issued.
90 – Effectuation
When a plan’s decision is reversed in whole or in part by any other appeal entity, the plan must
authorize or provide the service or benefits as expeditiously as the enrollee’s health condition
requires, however, no later than the timeframes listed below (based on when notice was
received).
Part C
Type of Request
IRE Reconsiderations
Other Entity Reconsiderations
Standard Items and
Services
• Provide as expeditiously as the
enrollee’s health condition
requires, but no later than 14
calendar days.
• If it is not appropriate to
provide within 14 calendar
days, authorize within 72
hours.
Authorize or provide no later
than 60 calendar days
Standard Part B Drug
Authorize or provide within 72
hours
Authorize or provide no later
than 60 calendar days
Standard Payment
No later than 30 calendar days
No later than 60 calendar days*
Expedited Items or
Services
Authorize or provide no later
than 72 hours
Authorize or provide no later
than 60 calendar days
Expedited Part B
Drug
Authorize or provide no later
than 24 hours
Authorize or provide no later
than 24 hours
* Special rule for when the MA plan seeks review from the Council. If the MA plan requests review of the ALJ’s
decision by the Council during the appeal (i.e. not on remand), the MA plan may await the outcome of the review
before it pays for, authorizes, or provides the service under dispute. An MA plan that files an appeal with the
Council must concurrently send a copy of its appeal request and any accompanying documents to the enrollee and
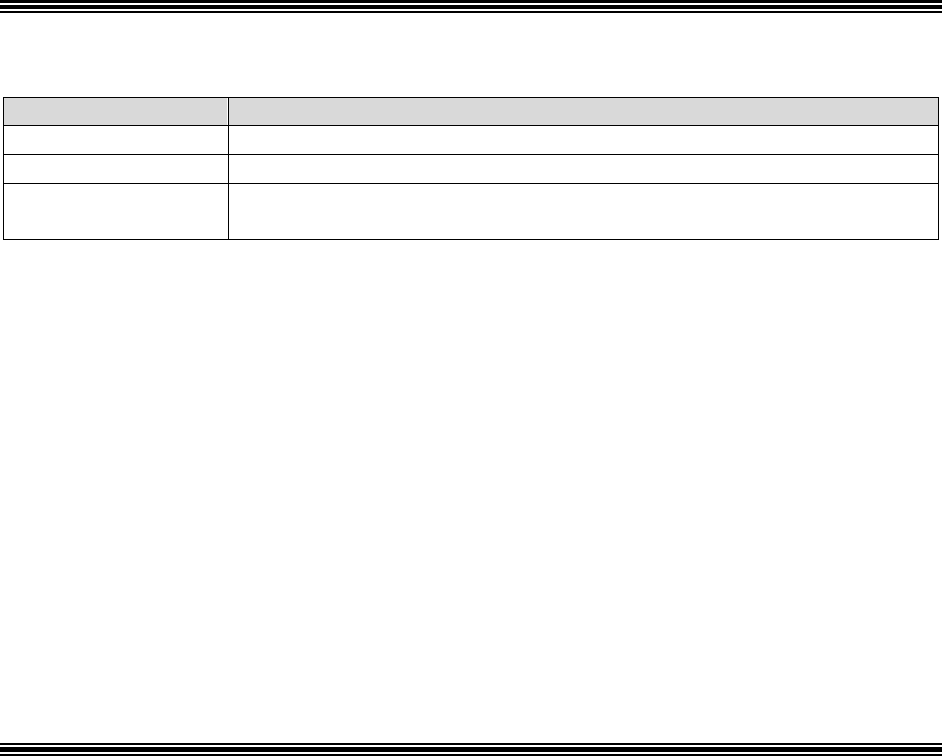
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
107
must notify the independent outside entity that it has requested an appeal. This delay is not available when the
request for review by the Council follows a remand to the ALJ by the Council or a federal court.
Part D
Type of Request
Other Entity Reconsiderations
Standard Benefit
No later than 72 hours
Expedited Benefit
No later than 24 hours
Payment*
Authorize payment with 72 hours, make payment (i.e. mail the
payment) no later than 30 calendar days.
*In addition to reimbursing the enrollee, when the plan sponsor or another appeal entity issues a favorable decision
on a reimbursement exception request, the plan sponsor must also authorize the benefit going forward. Likewise, if
a reimbursement request is approved for an enrollee who satisfied a UM requirement, the plan sponsor should
reimburse the enrollee for the amount owed, and effectuate the drug going forward in accordance with their CMS
formulary.
In general, when the plan sponsor or other appeal entity issues a favorable coverage
determination or appeal decision, the decision is retroactive to the date of the earliest request or
prescription purchase approved in a coverage determination or appeal decision. Appeal entity
means the IRE, an ALJ, the Medicare Appeals Council or a federal court with jurisdiction over
the matter.
Approved exceptions are valid for the remainder of the plan year; therefore, all prescriptions
purchased between the dates of the earliest prescription approved as an exception request and the
end of the plan year are reimbursable.
Note: A Part D plan sponsor may choose not to require enrollees to submit subsequent
requests once a coverage determine involving a UM is approved.
90.1 – Independent Review Entity Monitoring of Effectuation Requirements
CMS requires the IRE to monitor a plan’s compliance with effectuating decisions that fully or
partially reverse an original plan determination (denial). The process is as follows:
• The IRE issues a copy of the reconsidered determination to the plan. Included with this
copy is a Notice of Requirement to Comply;
• Pursuant to the compliance notice and §§422.618, 422.619, 423.636(b), and 423.638(b),
the plan is required to submit to the IRE a statement attesting the plan has effectuated the
decision in compliance with the IRE’s decision. This documentation is to confirm when
and how compliance occurred (e.g., service authorization, payment made, etc.).
Notification to the IRE that the plan “intends to pay for” or “intends to provide” the
service is not sufficient. The plan must provide the IRE with affirmative notice that the

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
108
IRE’s decision has been effectuated by payment or provision of the service. The plan’s
notice of compliance should be forwarded to the IRE concurrent with the plan’s
effectuation;
• If the IRE does not obtain the compliance notice within 2 weeks, it will mail the plan a
reminder notice; and if the IRE does not receive the plan’s compliance report within 30
days of the reminder notice, the IRE reports the plan’s failure to comply with CMS. The
plan is not copied on the notice to CMS.
90.2 – Effectuation Requirements for Former Plans
A plan is legally responsible under its contract and the regulations to authorize, provide, or pay
for all Medicare covered services or prescription drugs that are denied and upon appeal are found
to be services the plan should have authorized, provided, or paid for its enrollees. CMS policy is
that an enrollee is entitled to receive a service and/or payment of a service or prescription drug
from a plan from which the enrollee either voluntarily or involuntarily disenrolled prior to a final
decision on appeal. The plan must follow the requirements found at 42 CFR §§422.100,
423.104 422.504, and 423.505 as they relate to individual disenrollment or contract
termination/service area reduction.
100 – Provider Notices in Hospital, SNF, HHA, and CORF Settings (Part C
Only)
Note: HCPPs are not regulated by the rules in this section. Instead, HCPP enrollees follow
the Original Medicare immediate review process (42 CFR 405 Subpart J and Chapter 30 of
the Medicare Claims Processing Manual).
100.1 – Hospital Settings – Important Message from Medicare and Detailed
Notice
An enrollee has the right to request an immediate review by the Beneficiary and Family Centered
Care Quality Improvement Organization (BFCC-QIO) of a decision that inpatient hospital care is
no longer necessary. For all MA enrollees, hospitals must deliver valid, written notice of an
enrollee’s rights as a hospital inpatient, including discharge appeal rights, using the standardized
form, CMS Form R-193, An Important Message from Medicare (IM).
To request a BFCC-QIO review (immediate review), the enrollee must follow the steps listed on
the IM. If the enrollee does not make a timely request to the BFCC-QIO, the enrollee may
contact his or her MA plan to request an expedited reconsideration (see §50.2.2 for processing
requests for expedited reconsiderations), as indicated on the IM.
Delivery and form instructions for the standardized notice (Important Message from Medicare)
can be found in §200 of Chapter 30 of the Medicare Claims Processing Manual. For additional
guidance, including a copy of the IM, see the Hospital Discharge Appeal Notices webpage.

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
109
100.1.1 MA Plan Responsibilities Following BFCC-QIO Notification of
Appeal
When a BFCC-QIO notifies the MA plan that an enrollee has requested an immediate review, the
plan must:
• Properly execute and deliver (directly or by delegation) a Detailed Notice of Discharge
(DND), Form CMS-10066, to the enrollee as soon as possible, but no later than noon of
the day after the BFCC-QIO’s notification. (Instructions for the DND can be found in
§200.4.2 of Chapter 30 of the Medicare Claims Processing Manual.)
• Ensure delivery of the DND, regardless of whether it has delegated that responsibility to
its providers.
• At an enrollee’s request, the MA plan must deliver to the enrollee a copy of any
documentation that it sends to the BFCC-QIO, including written records of any
information provided by telephone. This documentation must be delivered to the enrollee
no later than close of business of the first day after the material is requested.
• Provide, directly or by delegation, all information that the BFCC-QIO needs to make its
determination, including copies of the IM and the DND (if applicable). This information
must be delivered as soon as possible, but no later than noon of the day after the BFCC-
QIO notifies the MA plan of the enrollee’s request.
Note: The delegation of notice delivery or other functions is determined by the contract
between the MA plan and its providers. The BFCC-QIO determines whether the MA plan
and the hospital should make the information available by telephone or in writing.
Pursuant to §422.622(f), the MA plan is financially responsible for continued coverage of
services during the BFCC-QIO review, regardless of whether it has delegated responsibility for
authorizing coverage or discharge determinations to its providers.
For information regarding responsibilities of the BFCC-QIO, please see §422.622(d).
100.2 – Skilled Nursing Facility (SNF), Home Health (HH), and
Comprehensive Outpatient Rehabilitation Services (CORF) Settings
An enrollee has the right to request an immediate review by the Beneficiary and Family Centered
Care Quality Improvement Organization (BFCC-QIO) when a SNF, HHA, or CORF decides to
terminate previously approved coverage (which includes an MA plan or contract provider
directing an enrollee to seek care from a non-contract provider/facility). All enrollees receiving
covered services in these settings must receive a Notice of Medicare Non-Coverage (NOMNC),
delivered by the facility or provider, before their services end. Enrollees must request an
immediate review, by telephone or in writing by noon of the first day after the day of delivery of
the NOMNC (that is, by noon of the day before the effective date on the NOMNC); if, due to an
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
110
emergency, the IRE is closed and unable to accept the enrollee's request for a fast-track appeal,
the enrollee must file a request by noon of the next day that the IRE is open for business. If the
enrollee misses the timeframe, the enrollee may request an expedited appeal from the plan.
Instructions for the NOMNC can be found in §260 of Chapter 30 of the Medicare Claims
Processing Manual. For additional guidance, including a copy of the NOMNC, see the MA
Expedited Determination Notices webpage.
100.2.1 – MA Plan Responsibilities Following BFCC-QIO Notification of
Appeal
When the BFCC-QIO notifies the MA plan that an enrollee has requested an appeal, the MA
plan must:
• Properly execute and deliver (directly or by delegation) a Detailed Explanation of Non-
coverage (DENC), to the enrollee as soon as possible, but no later than close of business
of the day of the BFCC-QIO’s notification. (Instructions for the DENC can be found in
§260.4.5 of Chapter 30 of the Medicare Claims Processing Manual. For additional
guidance, including a copy of the DENC, see the MA Expedited Determination Notices
webpage.)
• Ensure delivery of the DENC, regardless of whether it has delegated that responsibility to
its providers.
• At an enrollee’s request, the MA plan must deliver to the enrollee a copy of any
documentation that it sends to the BFCC-QIO, including written records of any
information provided by telephone. This documentation must be delivered to the enrollee
no later than close of business of the first day after the documentation is requested.
• Provide, directly or by delegation, all information that the BFCC-QIO needs to make its
determination, including copies of the notices sent to the enrollee. This information must
be delivered, as soon as possible, but no later than close of business of the day the BFCC-
QIO notifies the MA plan of the enrollee’s request.
Note: The delegation of notice delivery or other functions is determined by the contract
between the MA plan and its providers. The BFCC-QIO determines whether the MA plan
and the hospital should make the information available by telephone or in writing.
Pursuant to §422.626(b), the MA plan is financially responsible for continued coverage of
services during the BFCC-QIO review, regardless of whether it has delegated responsibility for
authorizing coverage or discharge determinations to its providers.
100.3 – Part A Medicare Outpatient Observation Notice (MOON)
Hospitals and Critical Access Hospitals (CAHs) are required to provide written and verbal
explanation to Original Medicare and Medicare Advantage enrollees who receive observation

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
111
services as outpatients for more than 24 hours.
The process for delivery of this standardized notice (Form CMS-10611), the Medicare
Outpatient Observation Notice (MOON), can be found in §400 of Chapter 30 of the Medicare
Claims Processing Manual. For additional guidance, including a copy of the MOON, see the
"Downloads" section on the Beneficiary Notices Initiative webpage.
110 – Part C Data
Note: The data disclosure requirements described in this section do not apply to HCPPs.
Upon request, MA plans are required to disclose grievance and appeals data to MA enrollees in
accordance with the regulatory requirements at 42 CFR §422.111(c)(3).
For purposes of this section, appeals data means all appeals filed with the MA plan that are
accepted for review or withdrawn upon the enrollee’s request, excluding dismissed appeals.
The MA plan should not send out a subset or partial list of the data, even if only a subset of the
data is requested. For example, if an enrollee requests data on the number of appeals received by
the MA plan, then the MA plan must send the enrollee a complete report of both its appeal and
grievance data for the reporting period.
The OMB-approved form and instructions used to report this information to the enrollee is the
Appeal and Grievance Data Form, Form CMS-R-0282.
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
112
Appendices
Appendix 1 – Medicare Managed Care (Part C) Appeals Process Overview
First
Appeal
Level
Organization
Determination
60 days to file
60 days to file
60 days to file
Automatic
forwarding to
IRE if plan
reconsideration
upholds denial
60 days
to file
Organization Determination/Appeals Process
STANDARD PROCESS
Item or Service: 14-day time limit
Payment: 60-day time limit
Part B Drug: 72-hour time limit
EXPEDITED PROCESS
Item or Service: 72-hour time limit
Part B Drug: 24-hour time limit
Health Plan Reconsideration
Item or Service: 30-day time limit
Payment: 60-day time limit
Part B Drug: 7-day time limit
Health Plan Reconsideration
72-hour time limit
IRE Reconsideration
72-hour time limit
Office of Medicare Hearings and Appeals
ALJ Hearing
AIC ≥ $180
No statutory time limit for processing
Medicare Appeals Council
No statutory time limit for processing
Federal District Court
AIC ≥ $1,850
Second
Appeal
Level
Third
Appeal
Level
Fourth
Appeal
Level
Judicial
Review
Medicare Managed Care (Part C - Medicare Advantage)
IRE Reconsideration
Item or Service: 30-day time limit
Payment: 60-day time limit
Part B Drug: 7-day time limit
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
113
Appendix 2 – Medicare Prescription Drug (Part D) Appeals Process Overview
60 days to file
60 days to file
60 days to file
Coverage Determination/Appeals Process
STANDARD PROCESS
72-hour time limit
Payment: 14-day time limit
EXPEDITED PROCESS
24-hour time limit
PDP/MA-PD
Standard Redetermination
7-day time limit
Payment: 14-day time limit
PDP/MA-PD
Expedited Redetermination
72-hour time limit
Part D IRE
Standard Reconsideration
7-day time limit
Payment: 14-day time limit
Part D IRE
Expedited Reconsideration
72-hour time limit
Office of Medicare Hearings and Appeals
ALJ Hearing
Standard Decision
AIC ≥ $180
90-day time limit
Medicare Appeals Council
Standard Decision
90-day time limit
Office of Medicare Hearings and Appeals
ALJ Hearing
Expedited Decision
AIC ≥ $180
10-day time limit
Medicare Appeals Council
Expedited Decision
10-day time limit
Coverage
Determination
Second
Appeal
Level
First
Appeal
Level
Third
Appeal
Level
Fourth
Appeal
Level
Federal District Court
AIC ≥ $1,850
Judicial
Review
60 days to file
60 days to file
Medicare Prescription Drug (Part D)

INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW: This information has not been publicly disclosed and may be
privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not
authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
114
Appendix 3 – Resources
Part C Regulations at 42 CFR Part 422 Subpart M:
https://www.ecfr.gov/cgi-bin/text-
idx?SID=1f450ef3db8f90e7c0f2c0fba827e8f3&node=sp42.3.422.m&rgn=div6
Part D Regulations at 42 CFR Part 423 Subparts M and U:
https://www.ecfr.gov/cgi-bin/text-
idx?SID=1de979b56f209e3d7eb12fabeae2b1aa&mc=true&node=pt42.3.423&rgn=div5#
sp42.3.423.m
Medicare Managed Care Appeals and Grievances Notices and Forms:
https://www.cms.gov/Medicare/Appeals-and-Grievances/MMCAG/Notices-and-
Forms.html
Medicare Prescription Drug Appeals and Grievances Notices and Forms:
https://www.cms.gov/Medicare/Appeals-and-
Grievances/MedPrescriptDrugApplGriev/PlanNoticesAndDocuments.html
https://www.cms.gov/Medicare/Appeals-and-
Grievances/MedPrescriptDrugApplGriev/Forms.html
For questions related to Parts C & D grievances, organization/coverage determinations,
and appeals, please submit to: https://appeals.lmi.org.
