Biomedicine and Biotechnology, 2014, Vol. 2, No. 1, 1-9
Available online at http://pubs.sciepub.com/bb/2/1/1
© Science and Education Publishing
DOI:10.12691/bb-2-1-1
Mechanism of DNA Binding and Cleavage
Sangeetha Gowda K.R.
1
, Blessy Baby Mathew
2
, C.N. Sudhamani
1
, H.S. Bhojya Naik
1,*
1
Department of Studies and Research in Industrial Chemistry, School of Chemical Sciences, Kuvempu University, Shankaraghatta, India
2
Department of Biotechnology, Sapthagiri College of Engineering, Bangalore, India
*Corresponding author: hsb_naik@rediffmail.com
Received November 14, 2013; Revised December 11, 2013; Accepted December 26, 2013
Abstract The necessity for cellular regulation of DNA led to the development of metallonucleases to catalyze and
repair DNA strand breaks. Due to cationic character, three-dimensional structural profiles, and propensity for
performing hydrolysis, redox, or photoreactions of metal ions and complexes, have a natural ability for interacting
with DNA. Since binding and cleavage of DNA is at the heart of cellular transcription and translation, it is an
obvious target for therapeutic intervention and the development of diagnostic structural probes. Inorganic constructs
such as cisplatin and its analogs exercise antitumor activity by inner-sphere coordination to DNA. During the last
decades, the continuous evolution of artificial metallonucleases and metal-based chemotherapeutics such as cisplatin,
photo-active octahedral metal complexes have been successfully used as DNA luminescent probes and light-driven
reactive agents during the last decades. A recent emerging trend to improve their potential as molecular tools for
studies of the genetic material is the design of bifunctional assemblies where the photo-active metal centre is
tethered through a flexible linker to a nucleic acid recognition or reactive moiety. In this view, new metal complexes
have been designed that utilize or create open coordination positions for DNA binding and hydrolysis, generate
reactive oxygen containing species or other radicals for DNA oxidation, or perform direct redox reactions with DNA.
This review briefly covers the aspects of drug molecule interaction factors, modes of DNA binding via groove
binders, intercalators and alkylators along with the cleavage patterns such as hydrolytic, oxidative and photoinduced
DNA cleavage, taking an example of Cisplatin and its mechanism.
Keywords: DNA, DNA binding, DNA cleavage, DNA drug interaction
Cite This Article: Sangeetha Gowda K.R., Blessy Baby Mathew, C.N. Sudhamani, and H.S. Bhojya Naik,
“Mechanism of DNA Binding and Cleavag.” Biomedicine and Biotechnology 2, no. 1 (2014): 1-9. doi:
10.12691/bb-2-1-1.
1. Introduction
Deoxyribonucleic acid (DNA) is of high biological
significance [1]. DNA has information stored in it in the
form of genes and these are extremely important for
various functions. DNA is the primary target molecule for
most anticancer and antiviral therapies according to cell
biology. Investigations on DNA interactions with
transition metal complexes, especially for those containing
multidentate aromatic ligands, have aroused considerable
interests owing to their potential applications as new
therapeutic agents and interesting properties that make
them as possible probes of DNA structure and
conformation [2,3]. Binding of peptides, small organic
and inorganic molecules to DNA will interfere with a
number of processes like transcription and replication
[4].
By considering this principle various disorders like cancer,
cystic fibrosis etc can be cured by using DNA as targets
for drugs. And with this emerges a whole new topic if
study called DNA drug interaction, which is of great
topical importance since 1960
[5]. Ever since then a lot of
research has happened in finding a number of metal ions
and metal complexes which have been effective in the
cancer treatment. Cleavage of DNA can be achieved by
targeting its basic constituents like base and/or sugar by an
oxidative pathway or by hydrolysis of phosphoester
linkages. Among the host of DNA-binding and cleaving
agents reported so far, transition metal complexes are of
relevance to the present work. Metal complexes have been
found to be potential to bind DNA through multitude of
interactions and to cleave the duplex by virtue of their
intrinsic chemical, electrochemical and photochemical
reactivities. Continuous demand for new anti-cancer drugs
has stimulated chemotherapeutic research based on the use
of metals since potential drugs developed in this way may
be less toxic and more prone to exhibit anti-proliferative
activity against tumors [6,7]. Transition metal complexes
have been extensively studied for their nuclease like
activity using the redox properties of the metal and
dioxygen to produce reactive oxygen species to promote
DNA cleavage by direct strand scission or base
modification [8]. Use of metal nanoparticles can be in
particular advantageous in generating singlet oxygen
[9,10]. A recent report by Geddes and coworkers
demonstrated that the presence of metal nanoparticles can
enhance singlet oxygen generation [11]. The enhanced
electromagnetic fields in proximity to metal nanoparticles
are the basis for the increased absorption and various
computational methods are available to predict the extent
of absorption and the relative increase in singlet oxygen
2 Biomedicine and Biotechnology
generation from photosensitizers [12,13]. DNA is
preferred to be used as target since its 3D structure makes
it easy to locate binding sites for the drug and to detect
changes in conformation resulting from the drug binding.
It is easy to predict the accessible chemical functional
groups for drug binding and it also has the ability to react
with a broader range of chemical species that include
water, metal ions and their complexes.
2. Mechanism
The different ways in which a drug molecule can
interact with the DNA are:
• Through control of transcription factors: Here the
drug molecule doesn’t directly interact with the DNA
instead it will interact with the protein that binds to
the DNA molecule and hence altering the functions.
• Forming DNA-RNA hybrids: By binding to RNA
molecule that in turn binds to single stranded DNA
forming DNA-RNA hybrids which will interfere with
the transcription activity.
• Direct binding of molecules: Here the small
aromatic ligand molecules directly bind to the DNA
double helix and these molecules are of many types
like groove binders, intercalators etc.
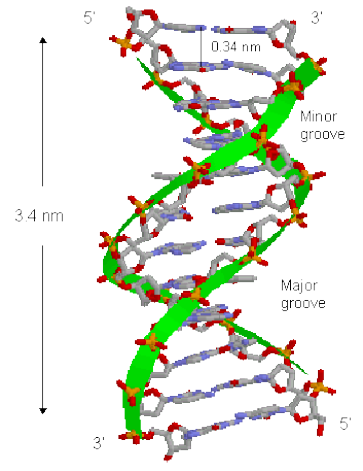
Figure 1. DNA structure and its grooves
Steps in drug development for the target DNA molecule
are:
• Based on the knowledge of the DNA of the disease
causing micro organism we can isolate specific
sequences of DNA that are of functional importance
to the organism. For example, in malaria causing
parasite P. falciparum 3 such sequences were
identified and they are TGCATGCA, GTGTCACAC
& GCACGCGCTGC.
• Drugs are then designed to bind to these target
sequences.
• The drug should be sufficiently reactive in order to
bind to the biological target but not so reactive that it
gets deactivated by the other bio molecules in its way
[14]
.
• The frequency with which these sequences occur in
humans are determined so that the interaction doesn’t
tamper with the normal functions of humans.
DNA-drug interaction generally occurs in two steps, i.e.
binding and cleavage.
2.1. DNA Binding
The interaction of metal complexes with DNA is a
thriving area of research since discovery of cis platin 40
years ago.cis platin when binds to DNA generates
intrastrand crosslink’s that kinks the DNA structure,
inhibits transcription leading to death of cancerous cells.
Intercalation was first proposed by Leeman et.al [15] and
is defined as insertion between base pairs.
Recently, some transition metal complexes having
different ligands such as dipyridoquinoxaline or NSO-
donor Schiff base have been reported, which shows
efficient DNA binding and cleave DNA on visible light –
irradiation [16].
The larger aromatic ring system was
proved to account for the higher affinity for DNA and
consequently for higher antitumor and photocleaving
activities [17]. DNA binding and optical properties have
recently been grafted into a single molecular construct
toward the development of multifunctional
supramolecular complexes. For eg. Ru(II)/ Pt(II)
bimetallic polyazine bridging ligand (BL) structures of the
form [(tpy)RuCl(BL)PtCl2]PF. It possess optical
properties associated with the Ru(II)-polyazine scaffold
and labile chloride ligands at the Pt(II) center analogous to
cisplatin [6,18].
In this process as the name suggests the drug molecule
binds to the DNA at different positions and bind by
forming different bonds such as covalent or non covalent
bonds. Drug–DNA interactions can be classified into two
major categories, intercalation and groove binding.
2.1.1. Groove Binders
These molecules usually place themselves on the minor
groove. These bind with direct interaction with the edges
of base pairs in either of the major (G-C) or minor (A-T)
grooves of nucleic acids. Groove binding does not induce
large conformational changes in DNA and may be
considered similar to standard lock-and-key models for
ligand–macromolecular binding. Groove binders are
usually crescent-shaped molecules that bind to the minor
groove of DNA [19]
.
The way they interact can be explained as follows:
• Groove binding molecules are usually constructed of
a series of heterocyclic or aromatic hydrocarbon
rings that possess rotational freedom. This allows the
molecule to fit into the minor or major groove, with
displacement of water.
• The drug molecule first identifies targets which are
the specific DNA sequences.
• These targets are in the range of 16 to 18 base pairs.
• Molecules are bonded to the helical structure via non
covalent bonds.
• Groove binders interact with base pairs edges in
either major or minor grooves.
Biomedicine and Biotechnology 3
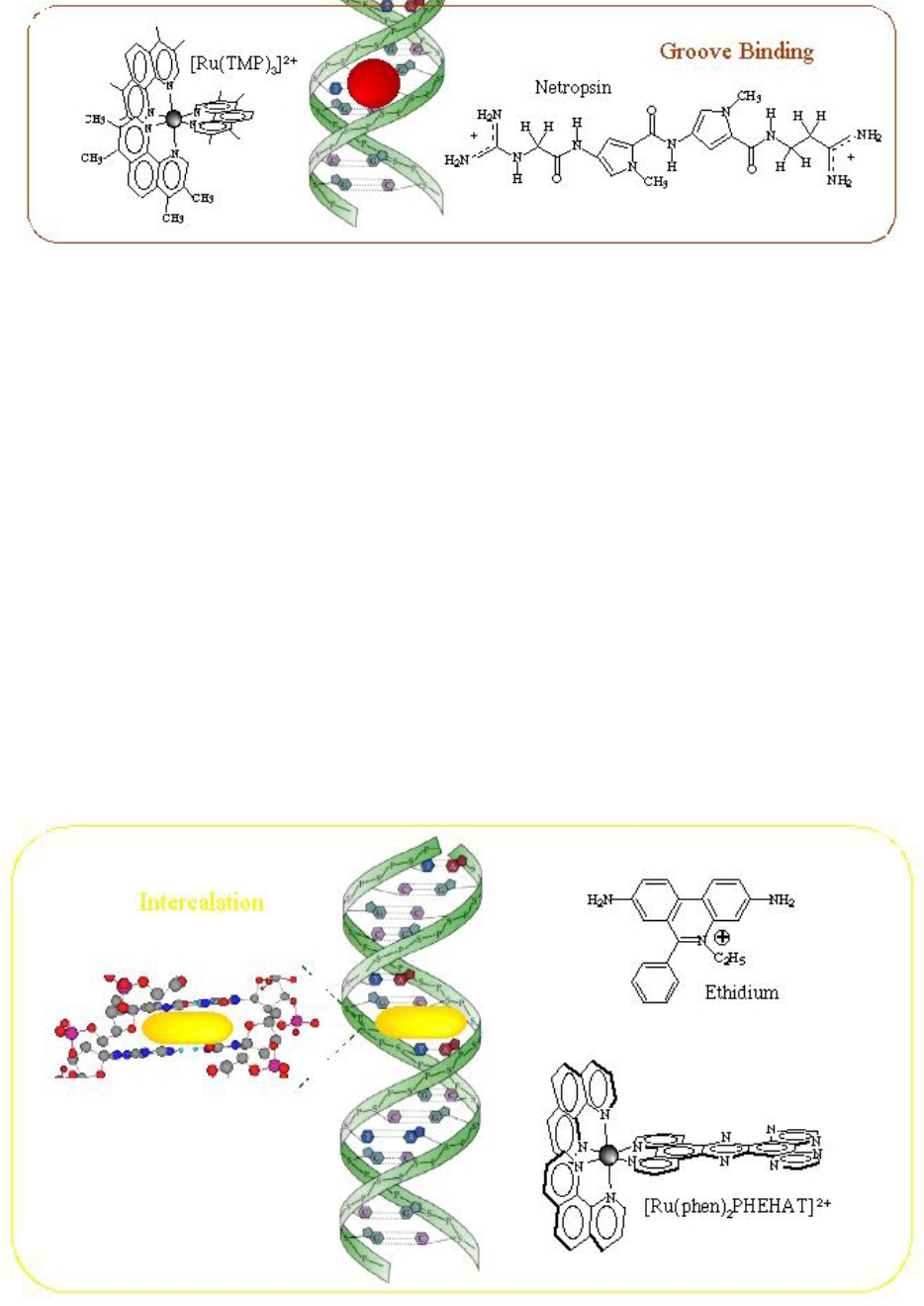
Figure 2. Adsorption of the complex in the DNA grooves [20]
Most of the drugs are groove binders as the minor and
major grooves provide a tight fit to the drug which binds
to it. As discussed earlier they are of two types, such as
minor groove binders and major groove binders [21]. A
series of heterocyclic or aromatic hydrocarbon rings get
together to form minor groove binding molecules. This
allows the displacement of water and the molecule fits
into the minor groove. The strand backbones are closer
together on one side of the helix than on the other. The
minor groove occurs where the backbones are close
together while the major groove occurs where the
backbones are far apart. It is easier for certain DNA
binding proteins to interact with the bases (the internal
parts of the DNA molecule) on the major groove side
because the backbones are not in the way. On the opposite
sides, the grooves twist around the molecule and certain
proteins bind to DNA to alter its structure or to regulate
transcription or replication [1]. Distamycin and netropsin
are natural products possessing amido groups and three
and two N-methylpyrrole rings. By means of hydrophobic
interactions and hydrogen bonding, distamycin and
netropsin interact with AT-rich regions of DNA in the
minor groove. The terminal amidine group of the small
molecule is basic in nature and attracts the drug molecule
to the negatively charged DNA phosphodiester backbone.
The 2-amino group of guanine confers AT-selectivity on
the drug molecule, preventing distamycin from binding to
the minor groove of G·C base pairs by steric hindrance.
Lexitropsins
A series of dimers and trimers of distamycin and
netropsin have been synthesized and studied to increase
the DNA binding region from 3 base pairs to 10 base pairs
or more.
Dervan polyamides
Above discussed molecules do not have the ideal
crescent shape to wrap around the minor groove of DNA,
and they fail to recognize longer stretches of DNA. So a
series of oligomeric "hairpin" polyamide molecules
containing pyrrole and imidazole ring systems were
synthesized that were able to bind side-by-side in the
minor groove of DNA with high affinity and in a
sequence-specific manner [22].
2.1.2. Intercalators
Figure 3. Intercalation of a planar ligand of the complex in the DNA base pairs stack [27]
Intercalation involves the insertion of a planar molecule
between DNA base pairs, which results in a decrease in
the DNA helical twist and lengthening of the DNA [20].
They consist of planar heterocyclic groups that stack
between adjacent DNA base pairs. The complex one is
stabilized by π-π stacking interactions between the DNA
bases and drug. Intercalators show strong structural
perturbations in DNA [23].
Certain flat aromatic or
4 Biomedicine and Biotechnology
heteroaromatic molecules can fit in between the base pairs
of DNA (intercalate) and stabilize the duplex without the
disruption of base pairing pattern. Intercalation can
lengthen the duplex by around 3 Å per bound drug
molecule, causing unwinding of DNA. This prevents
DNA replication and transcription by interfering with the
action of topoisomerases. The degree of unwinding
depends on the arrangement of the intercalating molecule
and the site of intercalation. The tight ternary complex
created between the intercalated drug, the DNA and the
topoisomerase is toxic to proliferating cells, so
intercalators are often more lethal to cancer cells than to
normal cells. DNA intercalators are used in structural
studies and antisense work. Such as in the incorporation of
acridine into oligos is striking for antisense applications,
since the intercalation of acridine into the DNA-RNA
duplex significantly increases the Tm and thereby
enhances duplex stability without affecting target
specificity [24,25,26].
Acridines
They have its origin from the aniline dye industry. One
of its examples is Proflavine which contains amino groups
that interact with the negatively charged phosphates
groups on DNA due to the presence of ions, whilst the
aromatic ring arrangement intercalates.
Polypeptides
Actinomycins (polypeptide antibiotics isolated from
Streptomyces strains) by blocking chain elongation,
hinder both DNA synthesis and RNA synthesis. They
interact with G·C base pairs as they have need of the 2-
amino group of guanine for binding. The phenoxazone
ring slides into the double helix and intercalates, while the
pentapeptide side chains intermingle with the DNA minor
groove by hydrogen bonding and hydrophobic interactions.
The result of these two mechanisms of interface between
small molecule and DNA (intercalation and minor-groove
binding) is a very steady complex.
Anthracyclines
They can form antitumour antibiotics such as
doxorubicin (adriamycin) and daunorubicin (daunomycin).
Both possess an amino group on the sugar which, when
protonated, forms an ionic interaction with the negatively
charged DNA phosphate backbone. This bond helps to
hold the molecule in place, allowing the planar aromatic
ring system to slide into the double helix. Although
doxorubicin and daunorubicin differ by only one hydroxyl
group, they have different activities. Daunorubicin is
active only against leukaemia, but doxorubicin is active
against leukaemia and also a wide range of solid tumours.
2.1.3. Alkylators
Strong electrophilic compounds that react chemically
with nucleophilic groups on DNA to form covalent bonds
are known as alkylators. The resultant DNA adducts
which are produced are irreversible inhibitors of
transcription and translation. Nucleophilic substitution
reactions at the DNA bases occur by both SN1 and SN2
mechanisms. The most reactive sites are those that are
both nucleophilic and uncovered in the grooves of the
DNA duplex. The N(7) atom of guanine and the N(3)
atom of adenine complete both criteria’s. Simple
nucleophiles, for example ethyleneimines and methane
sulfonates, tend to react through a SN2 mechanism,
whereas the nitrogen mustards can form aziridinium ions
that react through an SN1 mechanism.
Ethyleneimines (aziridines)
Ethyleneimines are pre-formed aziridines and as a
result constitute a natural addition of nitrogen mustards
(mechanism of action of the mustards begins with the
formation of an electrophilic aziridinium ion by
displacement of chloride). To ensure antitumour activity,
at least two ethyleneimine groups must be available in the
molecule. To prevent protonation of the ethyleneimine,
electron-withdrawing groups are attached (protonated
ethyleneimines are too reactive). Lipophilic
ethyleneimines are intended to enter the central nervous
system.
Platinum complexes
Cisplatin and carboplatin stand for a group of anti-
cancer agents used in the treatment of testicular and
ovarian tumours. Cisplatin and carboplatin form sturdy
platinum-nitrogen bonds with guanine and adenine bases.
The cis configuration form intra-strand cross-links which
leads to unwinding of the helix, preventing transcription
and leading to cell death. The trans-isomer, trans-platin, is
not an active anti-cancer agent, perhaps because it cannot
eagerly form intra-strand cross-links. It tends to cross-link
separate strands and such lesions are repaired easily [22].
Table 1. DNA alkylating agents [28]
Alkylating agents Examples
DNA -targeted
mustards
• Oilgopyrrole and oligoimidazole carriers
• Bis-(benzimidazole) carriers
•
Polybenzamide carriers
• 9-Anilinoacridine-4-carboxamide carriers
Guanine-specific
alkylating agents
• Mitomycins
• Carmethizole analogues
•
Pyrrolobenzodiazepines
•
Ecteinascidin analogues
Adenine-specific
alkylating agents
• Duocarmycin and analogues
• Benz[e]indolones
• Analogues of KW-2189
•
Amino analogues
• Bizelesin and other bis analogues
2.2. DNA Cleavage
Cleavage of DNA is a vital process in all living systems.
For example, topoisomerase enzymes resolve topological
problems of DNA in replication, transcription and other
cellular transactions by cleaving one or both strands of the
DNA [25]. Another example are restriction enzymes (or
restriction endonucleases), which protect the cell against
virus infection by cleavage of the foreign DNA [29], or by
degrading cellular DNA during apoptosis of the affected
cell
[30]. Finally, the activity of many anticancer drugs
rely on their ability to introduce extended damage to the
DNA in the (affected) cells (e.g. bleomycin) [31], which
can trigger apoptosis [32], leading to the cell death [33]. In
general, three different types of DNA cleavage can be
distinguished, namely i) DNA hydrolysis, ii)
photochemical cleavage, and iii) oxidative cleavage,
although the last two categories are quite closely related.
2.2.1. Hydrolytic Cleavage
It can be defined as a method of DNA cleavage by the
cleavage of phosphor diester bonds to generate fragments
in the presence of water. The fragments produced here can
be relegated. The half life of a typical phosphate diester
bond of DNA in neutral water under ambient conditions
Biomedicine and Biotechnology 5
(25°C) is estimated to be in the order of tens to hundred
billions of years. This means that a catalyst has to
accelerate this reaction 1017-fold to achieve an effective
hydrolysis of the phosphate backbone of DNA within an
acceptable timeframe (i.e. a couple of min). General
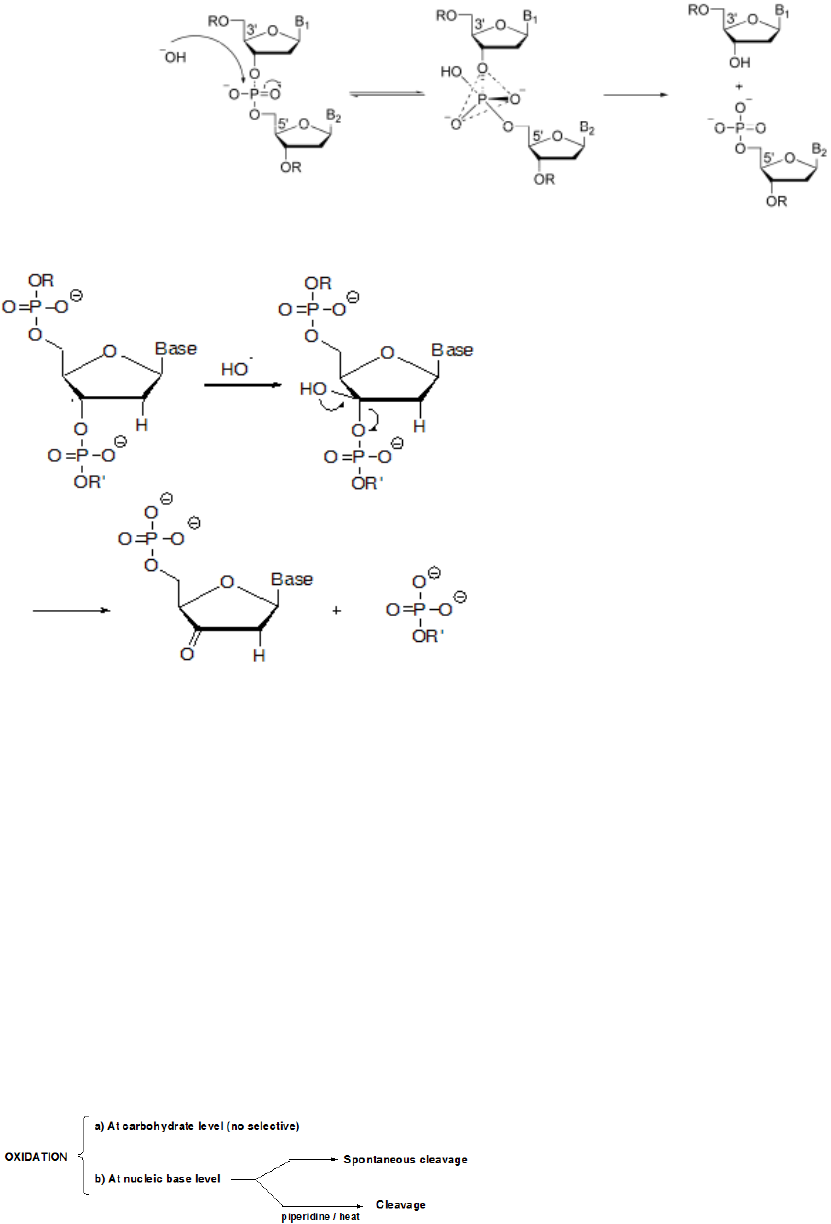
mechanism of this method is the hydrolysis reaction is
facilitated by the presence of metal ions, acting as Lewis
Acids. These Lewis acids can activate the phosphate
group towards nucleophilic attack, activate water or
hydroxide as nucleophile or increase the leaving group
ability of the departing alcohol. The general accepted
mechanism of the DNA hydrolysis reaction is a
nucleophilic attack at the DNA phosphate backbone, to
form a five coordinate intermediate, which can be
stabilized by the catalyst. Subsequent cleavage of either
the 3’-PO (as seen is most often in enzymatic systems) or
the 5’-PO results in a strand scission. After this
nucleophillic attack one group leaves as an alcohol.
Figure 4. Proposed reaction mechanism for the hydrolysis of DNA
Figure 5. Cleavage at nucleobases
2.2.2. Oxidative Cleavage
This method of cleavage involves the oxidation of
deoxy ribose by abstraction of sugar hydrogen or
oxidation of nucleobases. Oxidative cleavage is usually
mediated by the presence of additives and photo induced
DNA cleaving agents i.e. an external agent like light or
H
2
O
2
is required to initiate cleavage. Like in hydrolytic
cleavage in this method the DNA fragments cannot be
religated. Oxidative cleavage can occur both at the
carbohydrate level and at the nucleic base level. Oxidative
cleavage of DNA can result in the damage of all four
nucleobases or the deoxy ribose sugar. Generally
Hydroxyl radical species of O
2
(OH) are involved in this
oxidative cleavage. The mechanism of oxidative cleavage
occurs in 3 ways: hydrogen abstraction, addition and
electron transfer.
Cleavage at deoxyribose sugar: If the oxidative
cleavage occurs
at the carbohydrate, abstraction of one
hydrogen of deoxyribose can initiate the oxidative
cleavage process. In the next figure the process following
the C-3’ abstraction of deoxyribose is shown in Figure 5.
The
oxidation at the nucleic base level occurs
preferably at
guanine because it’s lower oxidation
potential. Hydroxyl radical reacts with the heterocyclic
bases in DNA by addition. In pyramidines OH adds to the
C5 or C6 double bond leading to cleavage. In purines the
hydroxyl ion binds to the C4, C5 & C8
[34].
2.2.3. Photoinduced DNA Cleavage
Photocleavage of nucleic acids allows the use of light to
trigger nuclease activity. Nucleases that are activated by
visible or near-UV light can be used for examination of
processes such as transcription and to probe nucleic acid
structure as photofootprinting and photo-sequencing
agents.On the other hand, photosensitization of DNA by
drugs may be useful as a potential anti-tumor therapy.
DNA photocleavage can occur by a wide variety of
mechanisms such as [35] hydrogen atom abstraction from
the sugar ring by photochemically generated radicals [36],
direct electron transfer from the base (usually guanine) to
the photoexcited cleaver [37], singlet oxygen production
by transfer of energy from the excited photocleaver, and
[38] formation of base adducts.
DNA damage initiated by photosensitization can be
divided in two major types; Type I process a one electron
process and Type II process a pathway involving singlet
oxygen [39,40].
In the first type (Type I process), the cleaving agent is
excited and generates sequentially a superoxide radical
from molecular oxygen via an electron transfer step.
Superoxide itself is a rather poor oxidant [41], and it can
be further reduced (leading to H
2
O
2
and OH) or it can
function as a reductant. The DNA damage observed via
this pathway is mainly guanine oxidation, formed via
guanine radical cations [39,40]. This results in the
formation of base labile sites in the DNA.
In a Type II process, the photo excited compound
generates singlet oxygen, which only modifies guanine
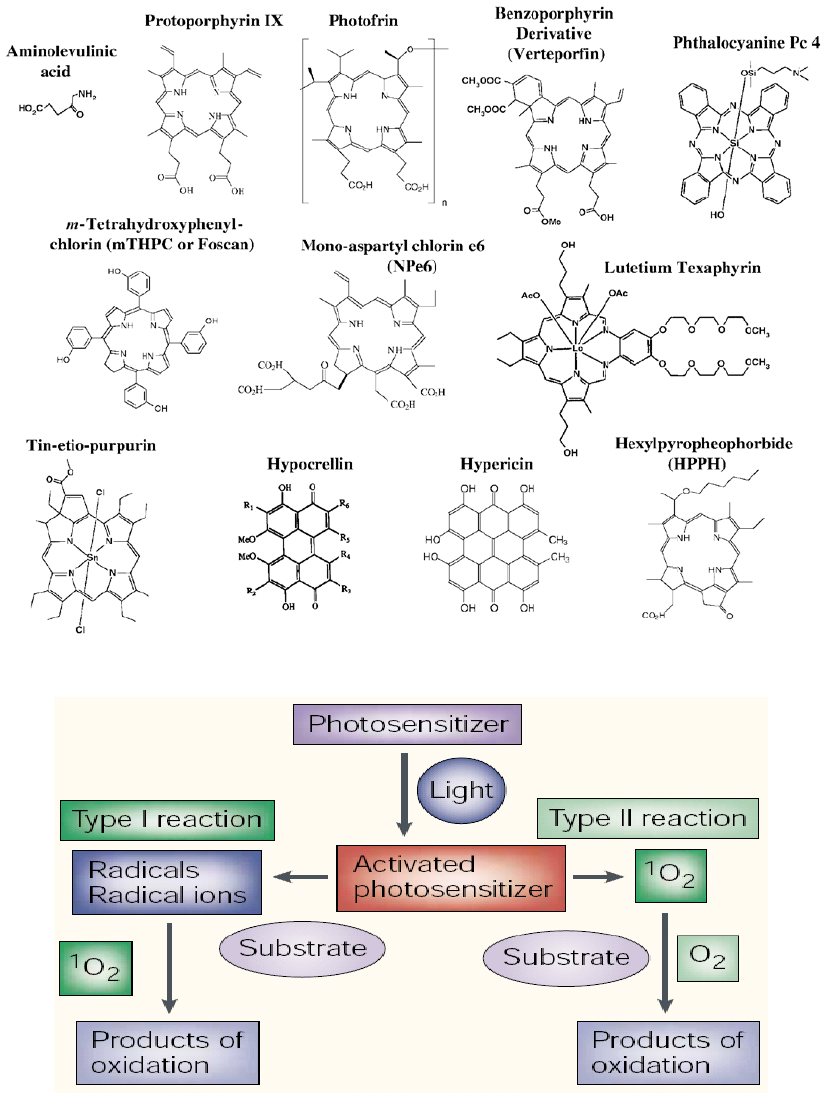
6 Biomedicine and Biotechnology
residues, in contrast to superoxide. Two pathways can be
distinguished [39,40,42,44] A Diels-Alder reaction with
singlet oxygen results in the formation 4,8-dihydro-4-
hydroxy-8-oxo-dG, and after further reduction in 8-oxo-
dG. A [2+2] cycloaddition with singlet oxygen results
after a cascade of reactions in the formation of cyanuric
acid. The modified residues are base labile positions in the
DNA and alkaline.
Figure 6. Names and structures of selected photosensitizers in clinical or pre-clinical studies
Figure 7. Schematic representation of PDT mechanism
2.3. Cisplatin a Classical Example of DNA
Binding and Cleavage Agent
The inorganic compound cis-
diamminedichloroplatinum (II) cis-[Pt(NH
3
)
2
(Cl)
2
]
commonly referred to as cisplatin, also called as Peyrone's
salt was named after Michel Peyrone who first synthesized
it in 1845. It was the first member of a class of platinum-
containing anti-cancer drugs, which now also includes
carboplatin and oxaliplatin. Cisplatin and its analogs are
heavy metal complexes containing a central atom of
platinum surrounded by two chloride atoms and two
ammonia molecules in the cis position. Cisplatin is a
white lyophilized powder soluble in water or saline at
1mg/ml and in diethylformamide at 24 mg/ml with a
melting point of 270°C. Cisplatin has biochemical
properties similar to that of bifunctional alkylating agents,
producing interstrand, intrastrand and monofunctional
adduct cross-linking in DNA.
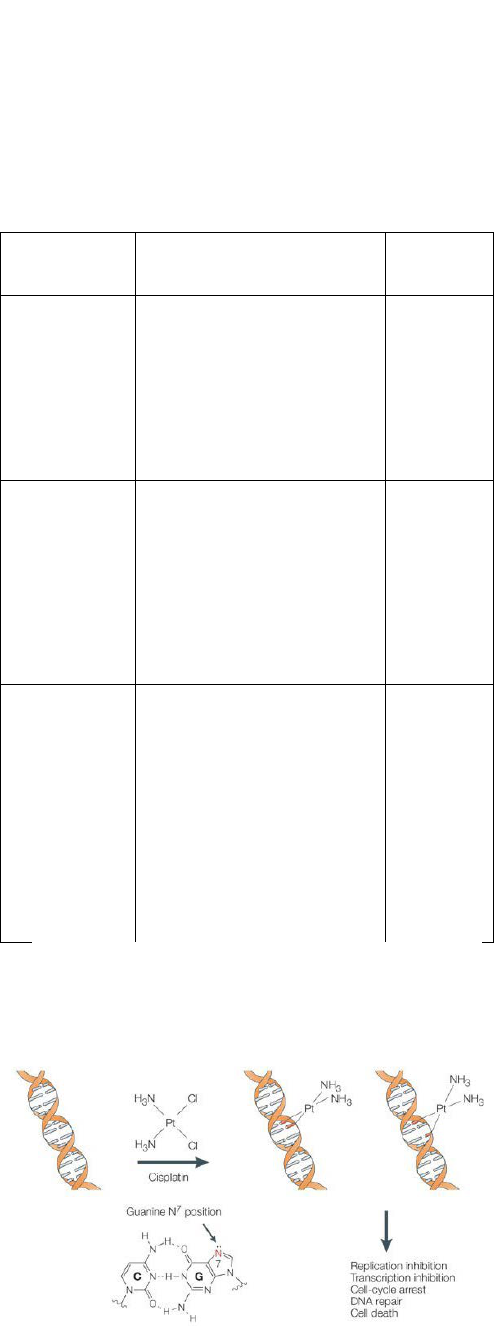
Biomedicine and Biotechnology 7
2.3.1. Mechanism of Cisplatin
One chloride ligand is slowly displaced by water
resulting in the formation of [PtCl (H
2
O)(NH
3
)
2
] [16].
Because of this the complex can bind to bases easily
especially to guanine [PtCl (guanine-DNA) (NH
3
)
2
]
+
. On
displacement of another Chlorine molecule by water the
cisplatin molecule can bind to another guanine in the same
DNA molecule forming a cross link between the two
strands. After complete binding of the Cisplatin the DNA
molecule will bend at a 30 deg. Angle. This leads to DNA
damage. The damaged DNA elicits DNA repair
mechanism which leads to apoptosis.
Table 2. DNA Cleavage agents
Types of
cleavage
Examples References
Hydrolytic
cleavage
Cu(II)TACH complex
copper-ATCUN complexes
copper(II)−l-histidine complex
[CoII(CysGly)(HisSer)]
[CoII(CysGly)(HisPhe)]
[45]
[46]
[47]
[48]
Oxidative
cleavage
Zn(F-BDPA)(NO3)2
[Cu(mbpzbpy)Br2](H2O)2.5
[Cu(mpzbpya)Cl](CH3OH)
[Cu2(mTPXA)Cl4]3 H2O
[Cu2(pTPXA)Cl4]3 H2O
[Cu3(HPTAB)Cl5]Cl3 H2O
[49]
[50]
[50]
[51]
Photo induced
cleavage
(photosensitizers)
Photofrin® (HpD)
Levulan® (ALA)
Metvix® (M-ALA)
Visudyne® (Vertiporfin)
Antrin® (Lutexaphyrin)
Foscan® (Temoporfin)
LS11 (Talaporfin)
Photosens® (Phthalocyanine)
[52]
Cisplatin is administered intravenously as short-term
infusion in normal saline for treatment of solid
malignancies. It is used to treat various types of cancers
like ovarian, bladder, cervical, lungs etc.
Figure 8. Mechanism of Cisplatin [53]
3. Conclusion
Recent advances in medicinal inorganic chemistry
demonstrate significant prospects for the utilization of
metal complexes as drugs, presenting a flourishing arena
for inorganic chemistry. Compounds containing metal
ions have and will continue to play an important role in
biomedical technology. Whether as imaging agents,
therapeutics or probes for chemical genetics, inorganic
compounds will continue to provide the research
community with tools that cannot be achieved with
organic chemistry alone. Significant progress in platinum
based anticancer agents has been achieved, based in part
on a mechanistic understanding of the DNA-binding and
pharmacological effects of cisplatin. Several new
compounds with reduced toxicity and high specificity
have been developed. Ruthenium complexes with
antitumor activity are also emerging rapidly. The future
development of medicinal inorganic chemistry requires an
understanding of the physiological processing of metal
complexes or drugs with DNA, to provide a rational basis
for the design of new metal-based drugs. In this direction
of designing new drugs understanding the mechanism of
DNA drug interaction is vital. Application of new
methodologies such as combinatorial chemistry,
extensively used in organic drug discovery, will be
beneficial for the development of inorganic compounds as
therapeutics.
Abbreviations
TACH: 1,3,5-triaminocyclohexane
ATCUN: amino terminal copper nickel
CysGly: cysteinylglycine
HisSer: histidylserine
HisSer: histidylphenylalanine
BDPA: N,N′-bis(benzyl)-N,N′-bis(2-
pyridylmethyl)-6,6′-bis(aminomethyl)-
2,2′-bipyridine)
mbpzbpy: 6,6-bis(3,5-dimethyl-N-pyrazolmethyl)-
2,2-bipyridine)
Hmpzbpya: 6-(3,5-dimethyl-N–pyrazolmethyl)-2,2-
bipyridine-6-carboxylic acid)
mTPXA: N,N,N',N'-tetra-(2-pyridylmethyl)-m-
xylylene diamine
pTPXA: N,N, N',N'-tetra-(2-pyridylmethyl)-p-
xylylenediamine
HPTAB: N,N,N',N',N'',N''-hexakis(2-
pyridylmethyl)-1,3,5-tris-
(aminomethyl)benzene
HpD: Hematoporphyrin derivative
ALA: 5-Aminolevulinic acid
M-ALA: Methylated 5-Aminolevulinic acid
BPD: Benzoporphyrin derivative
LS11: talaporfin Sodium.
References
[1] Shaikh, S.A., Jayaram, B., “DNA Drug Interaction”, Department
of Chemistry and Supercomputing Facility for Bioinformatics and
Computational Biology, Indian Institute of Technology.
[2] Gajendragad, M. R., Agarwala, U., Anorg, Z., “1, 3, 4-
Thiadiazole-2, 5-dithiol as a Complexing Agent II. Complexes of
NiII, RhI, PdII, PtII, AuIII, and CuII”, Allg. Chem., 415, 84, 1975.

8 Biomedicine and Biotechnology
[3] Ronconi,L., Sadler,P.J., “Using coordination chemistry to design
new medicines”, Coord. Chem. Rev., 251, 1633, 2007.
[4] Kennard, O., Pure & App. Chem, Cambridge Crystallographic
Data Centre, Vol 65, pp 6. , 1993.
[5] Yunus,G., Sreevatsava,S., Gupta, V.D.,”A theoretical analysis of
drug-DNA interactions: stability of poly D (at) binding with
aminosteroid dipyrandium”, Department of Physics, Integral
University.
[6] Beaudoin, A.R., “Teratogenic activity of 2-amino-1,3,4-
thiadiazole hydrochloride in Wistar rats and the protection
afforded by nicotinamide”, Teratology, 7, 65-71, 1973.
[7] Looker, J.H., Wilson Jr. L.W., “1, 2, 3-Thiadiazoles as potential
antineoplastic agents I. Synthesis of novel 4-monosubstituted and
4,5-disubstituted derivatives”, J. Heterocyclic. Chem., 2, 348,
1965.
[8] Clerici,F., Pocar,D., Brufani,M., “Synthesis of 2-Amino-5-
sulfanyl-1,3,4-thiadiazole Derivatives and Evaluation of Their
Antidepressant and Anxiolytic Activity”, J. Med. Chem., 44, 931,
2001.
[9] Gowda, K.R.S., Naik, H.S.B., Kumar, B.V., Sudhamani, C.N.,
Sudeep, H.V., Naik, T.R.R., Krishnamurthy, G. “Synthesis,
antimicrobial, DNA-binding and photonuclease studies of
Cobalt(III) and Nickel(II) Schiff base complexes”, Spectrochimica
Acta Part A, 105: 229-237, 2013.
[10] Arjmand,F., Muddassir,M., “A mechanistic approach for the DNA
binding of chiral enantiomeric L- and D-tryptophan-derived metal
complexes of 1,2-DACH: Cleavage and antitumor activity”,
Chirality, 23, 250, 2011.
[11] Liu,J., Zou, X.H., Zhang, Q.L., Mei, W.J., Liu, J.Z., Ji, L.N.,
“Synthesis, Characterization and Antitumor Activity of a Series of
Polypyridyl Complexes”, Met. Based Drugs, 7 343, 2000.
[12] Pindur, U., Haber, M., Sattler, K., “Antitumor active drugs as
intercalators of deoxyribonucleic acid: Molecular models of
intercalation complexes“, J. Chem. Educ., 70, 263, 1993.
[13] Sudhamani, C.N., Naik, H.S.B., Girija, D., Gowda, K.R.S.,
Giridhar, M., Arvinda, T., “Novel complexes of Co(III) and Ni(II)
containing peptide ligands:Synthesis, DNA binding and
photonuclease activity“, Spectrochimica Acta Part A, 2014; 118:
271-278.
[14] Sabine, H., Rijt, V., Sadler, P.J., “Current application and future
potential for bio inorganic chemistry in the development of anti
cancer drug”, University of Warwich, Vol 14, pp: 23-24, 2009.
[15] Mizyed, S., Kiwan, R., Marji, D., “Synthesis of New Azacrown
Ether Schiff-Bases and their Complexes with C60”, Jordan
Journal of Chemistry, 8, 71-78, 2013.
[16] Dhar, S., Nethaji, M., Chakravarty, R.A., “Synthesis, crystal
structure and photo-induced DNA cleavage activity of ternary
copper(II) complexes of NSO-donor Schiff bases and NN-donor
heterocyclic ligands”, Inorganica Chimica Acta, 358 (7). pp.
2437-2444, 2005.
[17] Leung-Toung, R., Wodzinska, J., Li, W., Lowrie, J., Kukreja, R.,
Desilets, D., Karimian, K., Tam, T. F., ”1,2,4-thiadiazole: a novel
Cathepsin B inhibitor”, Bioorg. Med. Chem. Lett., 11, 5529, 2003.
[18] Matysiak, J., Skrzypek, A., Feewiadomy, A., ” Synthesis and
antifungal activity of novel 5-substituted 4-(1,3,4-thiadiazol-2-
yl)benzene-1,3-diols” , Heteroatom Chem., 21, 533, 2010.
[19] Palchaudhuri, R., Hergenrother, P.J., “DNA as a target for
anticancer compounds: methods to determine the mode of binding
and the mechanism of action”, Current Opinion in Biotechnology,
Volume 18, Issue 6, Pages 497-503, 2007.
[20] Mei, H., Barton, J., "A Chiral Probe for A-form Helices of DNA
and RNA: Tris(tetramethylphenanthroline)ruthenium(II)", J. Am.
Chem. Soc., 108, 7414, 1986.
[21] Raman, N., Selvan, A., “Investigation of DNA binding mechanism,
photoinduced cleavage activity, electrochemical properties and
biological functions of mixed ligand copper(II) complexes with
benzimidazole derivatives: synthesis and spectral characterization”,
Journal of Enzyme Inhibition and Medicinal Chemistry, 27, 380-
389.
[22] Brown, T., Brown (Jr), T., Nucleic Acids Book, ATDBIO.
[23] Wu, L., “Unveiling biomacromolecule interactions- NMR and
optical spectroscopy studies on ligand binding to DNA and
lysozyme”, Chalmers University of Technology, Gothenburg,
Sweden, 2013.
[24] Sinha, R., Islam, M.M., Kakali, B., Gopinatha, S.K., Banerjee, A.,
Maiti, M., “The binding of DNA intercalating and non-
intercalating compounds to A-form and protonated form of
poly(rC)-poly(rG): Spectroscopic and viscometric study”, Bioorg.
& Medic. Chem., 14: 800-814, 2006.
[25] Fukui, K., Tanaka, K., “The Acridine Ring Selectively
Intercalated into a DNA Helix at Various Types of Abasic Sites:
Double Strand Formation and Photophysical Properties”, Nucleic
Acids Res., 24: 3962-3967, 1996.
[26] Salson-Behmoaras, T., Tocque, B., Rey, I., Casslgnol, M., Thuong,
N-T., Helene, C. “Short modified antisense oligonucleotides
directed against Ha-ras point mutation induce selective cleavage
of the mRNA and inhibit T24 cells proliferation”, EMBO J., 10:
1111-1118, 1991.
[27] Moucheron, C. Kirsch-De Mesmaeker, “New DNA-binding
ruthenium(II) complexes as photo-reagents for mononucleotides
and DNA C.”, J. Physical Organic Chemistry, 11, 577-583, 1998.
[28] Denny, W.A.,"DNA minor groove alkylating agents." Current
medicinal chemistry, 8, 533-544, 2001.
[29] Wang, J.C., “Cellular roles of DNA topoisomerases: a molecular
perspective”, Nat. Rev. Mol. Cell. Biol., 3, 430-440, 2002.
[30] Bickle, T.A., Krüger, D.H.,“Biology of DNA restriction”,
Microbiol. Rev., 57, 434-450, 1993.
[31] Samejima, K., Earnshaw, W.C., “Trashing the genome: the role of
nucleases during apoptosis”, Nat. Rev. Mol. Cell. Biol., 6, 677-688,
2005.
[32] Chen, J., Stubbe, J., “Bleomycins: towards better therapeutics”,
Nat. Rev. Cancer, 5, 102 112, 2005.
[33] Hengartner, M.O., “The biochemistry of apoptosis”, Nature, 407,
770-776, 2000.
[34] Kochetkov, N.K., Budovskii, E.I, Reactions Involving the
Cleavage or Rearrangement of Heterocyclic Rings of Nucleic
Acid Bases and their Derivatives. Organic Chemistry of Nucleic
Acids, Springer, 1972, pp 381-423.
[35] Kochevar, I. E.; Dunn, A., “Photosensitized reactions of DNA:
cleavage and addition”, Bioorg. Photochem., 1, 273, 1990.
[36] Paillous, N; Vicendo, P. J., “Mechanisms of photosensitized DNA
cleavage”, Photochem. Photobiol. B, 20, 203, 1993.
[37] Fernandez, M.J., Grant, K. B., Herraiz, F.,Yang, X., Lorente, A,
“DNA photocleavage by dicationic bisintercalants”, Tetrahedron
Lett., 2001, 42, 5701-04.
[38] Armitag B., “Photocleavage of Nucleic Acids”, Chem. Rev., 98,
1171, 1998.
[39] B. Meunier, G. Pratviel, J. Bernadou, “Active Species Involved in
Oxidative DNA Cleavage”, Bull. Soc. Chim. Fr., 131, 933-943,
1994.
[40] Sawyer, D.T., Valentine, J.S., “How super is superoxide? ” , Acc.
Chem. Res., 14, 393-400, 1981.
[41] Piette, J., “Biological consequences associated with DNA
oxidation mediated by singlet oxygen”, J. Photochem. Photobiol.
B, 11, 241-260, 1991.
[42] Epe, B., “Genotoxicity of singlet oxygen”, Chem. Biol. Interact.,
80(3), 239-260, 1991.
[43] Sigman, D.S., Mazumder, A., Perrin, D.M., “Chemical nucleases”,
Chem. Rev., 93, 2295-2316, 1993.
[44] Armitage, B., “ Photocleavage of nucleic acids”, Chemical
reviews, 98(3), 1171-1200, 1998.
[45] Kobayashi, T., Tobita, S., Kobayashi, M., Imajyo, T., Chikira, M.,
Yashiro, M., & Fujii, Y., “Effects of N-alkyl and ammonium
groups on the hydrolytic cleavage of DNA with a Cu(II)TACH
(1,3,5-triaminocyclohexane) complex. Speciation, kinetic, and
DNA-binding studies for reaction mechanism”, J Inorg Biochem.,
101, 348-361, 2007.
[46] Jin, Y., Cowan, J. A., “DNA cleavage by copper-ATCUN
complexes. Factors influencing cleavage mechanism and
linearization of dsDNA”, J Am Chem Soc., 8408-8415, June 2005.
[47] Ren, R., Yang, P., Zheng, W., & Hua, Z., “A Simple
Copper(II)−L-Histidine System for Efficient Hydrolytic Cleavage
of DNA”, Inorganic chemistry, 39, 5454-5463, 2000.
[48] Reddy, P. R., Manjula, P., “Ternary complexes of cobalt
cysteinylglycine with histidylserine and histidylphenylalanine–
stabilities and DNA cleavage properties” J. Chem. Sci., 119, 603-
612 November 2007.
[49] Park, H. J., Kwon, J. H., Wang, W., Lee, H. G., Kim, C., Kim, Y.,
& Cho, T. S.,“Oxidative DNA Cleavage by Zn(X-BDPA)(NO3)2
Complexes (X=F, H, and Me): Effect of Different Ligand
Substituents”, Bull. Korean Chem. Soc., 33, 1819, 2012.
[50] Maheswari, P. U., Lappalainen, K., Sfregola, M., Barends, S.,
Gamez, P., Turpeinen, U., Reedijk, J., “Structure and DNA
cleavage properties of two copper(II) complexes of the pyridine-
Biomedicine and Biotechnology 9
pyrazole-containing ligands mbpzbpy and Hmpzbpya”, Dalton
Trans., 3676-3683, 2007.
[51] Zhao, Y., Zhu, J., He, W., Yang, Z., Zhu, Y., Li, Y., Guo, Z,
“Oxidative DNA cleavage promoted by multinuclear copper
complexes: activity dependence on the complex structure”,
Chemistry., 25, 6621 August 2006.
[52] Allison, R. R., Downie, G. H., Cuenca, R., Hu, X. H., Childs, C. J.,
& Sibata, C. H., “Photosensitizers in clinical PDT”,
Photodiagnosis and Photodynamic Therapy 1, 27-42, 2004.
[53] Wang, D., Lippar, S.J., “Cellular processing of platinum
anticancer drugs”, Nature Reviews Drug Discovery 4, 307-320,
April 2005.
