
Professional Practice Board
Conducting research with people not having
the capacity to consent to their participation
A practical guide for researchers
Prepared by Catherine Dobson on behalf of the
Mental Capacity Act Working Party
December 2008

ISBN 978 1 85433 485 5
Printed and published by the British Psychological Society.
© The British Psychological Society 2008.
The British Psychological Society
St Andrews House, 48 Princess Road East, Leicester LE1 7DR, UK
Telephone 0116 254 9568 Facsimile 0116 247 0787
E-mail [email protected] Website www.bps.org.uk
Incorporated by Royal Charter Registered Charity No 229642
If you have problems reading this document and would like it in a
different format, please contact us with your specific requirements.
Tel: 0116 252 9523; E-mail: [email protected].
About the Author
Catherine Dobson is a Consultant Clinical Psychologist working with people with learning
disabilities in Lancashire, England. She has conducted research about services for people
with learning disabilities and mental health problems and is a member of Lancashire Care
Foundation Trust’s Research Governance Committee. Catherine has contributed to
regional implementation of the Mental Capacity Act (MCA) and has both organised and
presented papers on aspects of MCA at conferences.
Acknowledgements
The author would like to thank Beverley Lowe and members of the Lancashire Care Trust’s
Research Governance Committee, in particular the Service User and Carer Subcommittee,
for sowing the seed of an idea about guidance for researchers. Secondly, thanks must be
extended to Catherine Dooley, Glynis Murphy, Nigel Atter and other members of the
Mental Capacity Act Working Party of the British Psychological Society for their
encouragement and oversight of methodology and structure of the practice guide.
A practical guide for researchers 1
Contents
Page
Foreword: Julie Jones, CEO, Social Care Institute for Excellence 3
Introduction 4
Section 1: Legal requirements for conducting research with people not having capacity to consent
1.1 The research process 6
Section 2: Ethical scrutiny
2.1 Principles of ethical scrutiny 8
2.2 Procedure for ethical review 9
2.3 Intrusive research 9
2.4. Research that does not require consent 10
2.5 Seeking consent to participation in research 10
Section 3: Assessing capacity to consent
3.1 Recruitment of participants 13
3.2 Assessing capacity to consent to participation in research 13
3.3 Applying the Five Statutory Principles of the MCA to judge whether 14
a (prospective) participant has the capacity to consent
3.4 Principle 1 16
3.5 Principle 2 16
3.6 Principle 3 17
3.7 Reaching a judgment about whether a participant lacks the capacity
to consent to research 17
3.8 Loss of capacity during a project 18
Section 4: Consulting with others
4.1 Consulting with others as a way of safeguarding the inclusion of people
who lack the capacity to consent to their participation 20
4.2 Role of Research Consultee 21
4.3 Consulting with a Research Consultee 23
4.4 Personal Consultee 24
4.5 Nominated Consultee 24
Section 5: Appraising participant involvement
5.1 Appraising whether to include or exclude a participant 25
5.2 Reviewing continuing participation in a project 25
5.3 Recommendations for good practice 27
Section 6: Case studies 28
Section 7: Concluding comments 32

Appendices
1 References 34
2 NHS Research Ethics Committees which have been ‘flagged’ to scrutinise
projects involving participants who do not have the capacity to consent 36
3 Roles and responsibilities in the research process 38
4 Additional questions and ethical implications 40
5 Contributors 41
Part 2
Checklists, sample letters and proformae 44
2 Conducting research with people without the capacity to consent

A practical guide for researchers 3
Foreword
I am very pleased to welcome and commend the research guidance as part of the
supporting materials for implementation of the Mental Capacity Act (2005).
One concern following on from the Act and its Code of Practice was that researchers might
be deterred from conducting research using people who lack capacity as participants,
because of the additional requirements. This could deny people who might wish to take
part in research projects from being included, going against the spirit of the Act and also
potentially limiting fields of enquiry and development. This guidance helps to address
these concerns by supplying practical advice and operational procedures.
This material is impressive in that it summarises the implications of the MCA for
researchers, highlights procedural changes that need to be introduced, and flags up the
ethical issues and responsibilities for researchers being mindful of the underlying
principles of the Act. It also identifies areas where further development work is required.
Of particular value are the various forms that the author has developed; these will be an
invaluable resource for researchers in helping them to construct a framework for their
approach to research and can be tailored to local circumstances.
Julie Jones
Chief Executive Officer, Social Care Institute for Excellence

4 Conducting research with people without the capacity to consent
Introduction
The practical guide is one of a series of documents commissioned by the Social Care
Institute for Excellence, addressing different aspects of the implementation of the Mental
Capacity Act (2005) and has been prepared for researchers conducting research with
human participants in the United Kingdom. The majority of researchers will be members
of professional bodies and/or be employed by academic or research organisations. Some
researchers may be independent practitioners commissioned to undertake research. The
guidance will also be of relevance to members of research ethics committees and service
user and carer organisations.
Participants of research projects may be members of the general public or be in receipt of
health or social care services.
Professional organisations contributing to the development of the guidance included:
■ The British Psychological Society;
■ The Royal College of Speech and Language Therapists; and
■ The Royal College of Psychiatrists.
Rationale for the preparation of the practical guide
The Mental Capacity Act (MCA) 2005 in England and Wales and the Incapacity Act (ICA)
2000 in Scotland have established legal frameworks within Great Britain for people lacking
the capacity to make decisions for themselves.
Sections 30–34 of MCA 2005 and Section 51 of ICA 2000 (Scotland) refer to decisions
concerning participation in research. In general, for all research, researchers should
assume that a participant or potential participant does have the capacity to decide whether
to consent or not to their participation, unless there is evidence that questions the person’s
capacity to reach this decision. The Acts and relevant Codes of Practice outline safeguards
for individual participants, carers and researchers when research projects do involve non-
consenting participants. The Code of Practice for the Mental Capacity Act was published
by the Department for Constitutional Affairs in 2007. Throughout this guide the Code of
Practice will be referred to as the MCA Code of Practice.
This practical guide offers advice and examples of good practice in connection with
conducting research with people lacking the capacity to consent to their participation.
Reference is made throughout the document to guidance produced by different
professional and research organisations within the United Kingdom.
The guidance refers primarily to changes in the research process arising from the MCA
(2005), which applies to England and Wales, with some reference to the Adults with
Incapacity Act (Scotland). At present the Mental Capacity Act does not apply to research
conducted in Northern Ireland, where the legal framework concerning decision-making
for or on behalf of people lacking capacity remains within common law and case law.
The practical guide will be disseminated to professional groups, service user and carer
groups, research organisations, university and NHS research ethics committees via
The practical guide
■ provides a number of flowcharts to assist researchers in deciding between different
courses of action when undertaking research with participants who may lack capacity
to consent;
■ offers suggestions for enhancing the decision-making capability of potential research
participants;
■ assists researchers in establishing whether a participant can or cannot consent to their
participation in research;
■ offers suggestions of who researchers can consult, how and under what circumstances,
when undertaking research with people not having the capacity to consent; and
■ provides sample letters, information sheets and declaration forms.
A practical guide for researchers 5
publications and conference presentations. The document is also available for download
from www.scie.org.uk.
This practical guide is in two parts:
Part 1 is the professional guidance and covers
■ modifications to the research process in order that a project can comply with
MCA;
■ procedures for ethical scrutiny;
■ application of the principles of the Mental Capacity Act to assess whether a
participant can consent to their participation; and
■ safeguards afforded by the Mental Capacity Act, in particular the process of
consultation with others and the appraisal of an individual’s involvement with
a project.
Part 2 provides proformas, sample correspondence and information sheets which could be adapted
by researchers as required for specific projects.
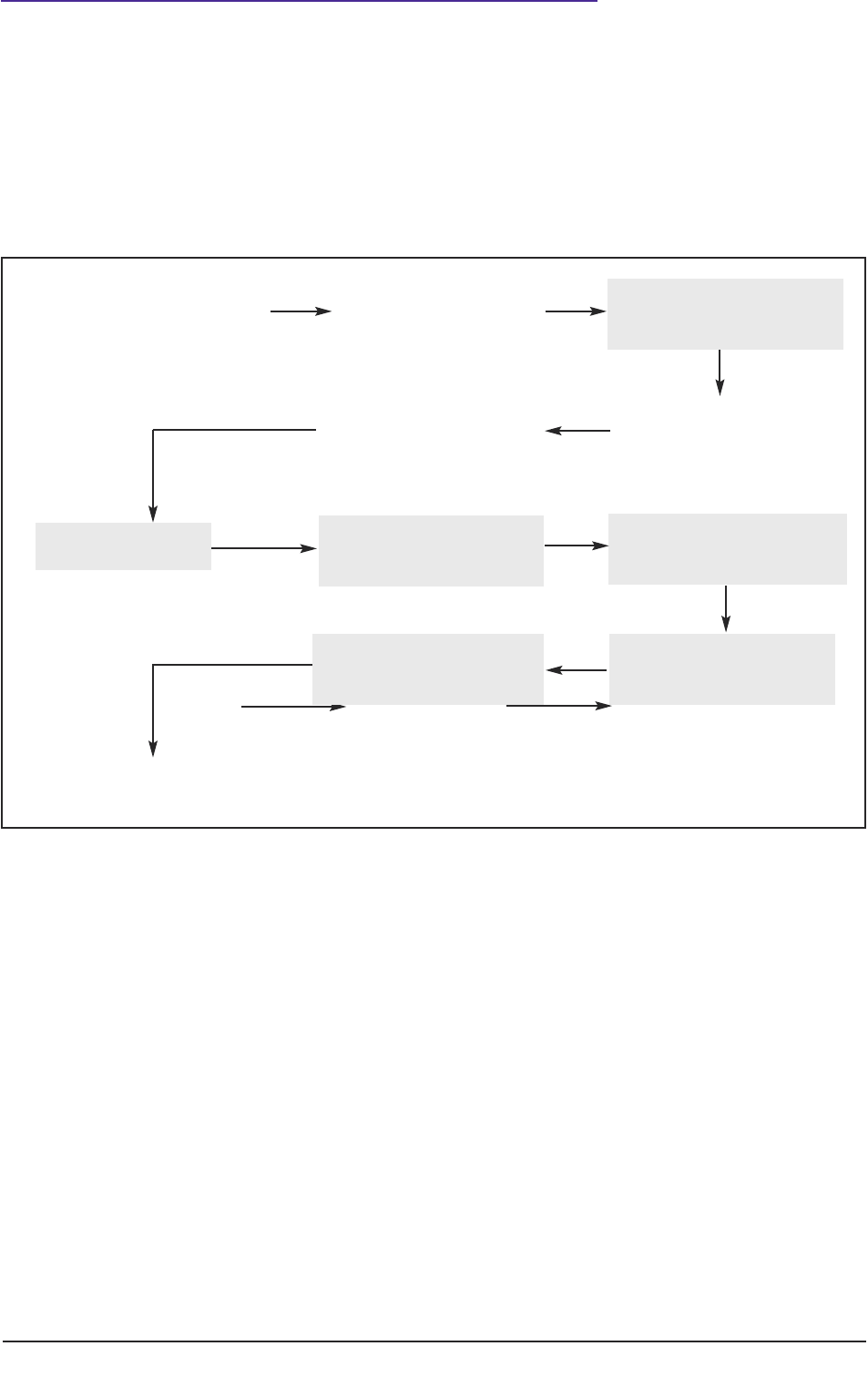
General ideas,
conduct literature review
Complete application
for ethical review
Gain favourable
ethical opinion
Recruit participants
Conduct project
Analyse data Disseminate findings
Prepare protocol
Seek consent
6 Conducting research with people without the capacity to consent
Section1: Legal requirements for conducting research with
people not having the capacity to consent
1.1 The research process
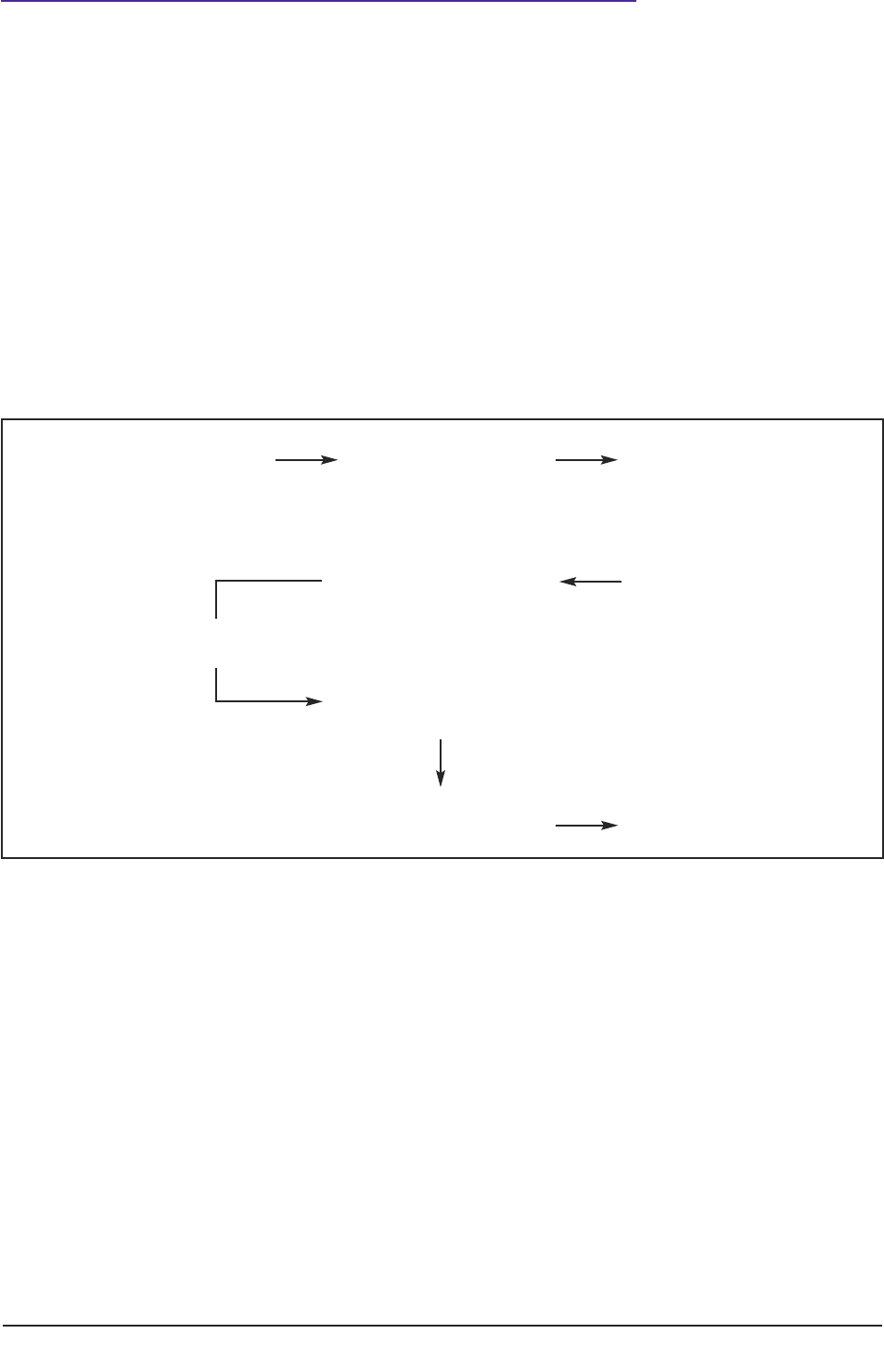
Taking stages of the ‘research process’ as a starting point (see figure 1), the practical guide
works through the detail of necessary modifications in order that projects meet the
requirements of the Mental Capacity Act.
In the United Kingdom, the Research Governance Framework for Health and Social Care
(DH, 2004b) provides a broad definition of research ‘as the attempt to derive generalisable
new knowledge by addressing clearly defined questions with systematic and rigorous methods’.
The definition is intended to cover different methodologies of research (quantitative and
qualitative) conducted in a wide range of settings with people who may be members of the
public, recipients of health or social care services or employees of such agencies.
Figure 1: Schematic representation of the research process for conducting research with human
participants.
Figure 1 depicts a schematic representation of the ‘research process’ whilst Figure 2 (page
4) indicates key modifications to that process so that the study meets legal requirements of
the Mental Capacity Act (2005), when conducting research with participants not having the
capacity to consent to their participation.
Key modifications to the research process entail:
■ changes to the process of gaining ethical approval, for example by the completion
of supplementary forms and submission to ‘flagged’ NHS Research Ethics
Committees (see 2.2);
■ explicit assessment of the capacity to consent (see 3.1–3.7);
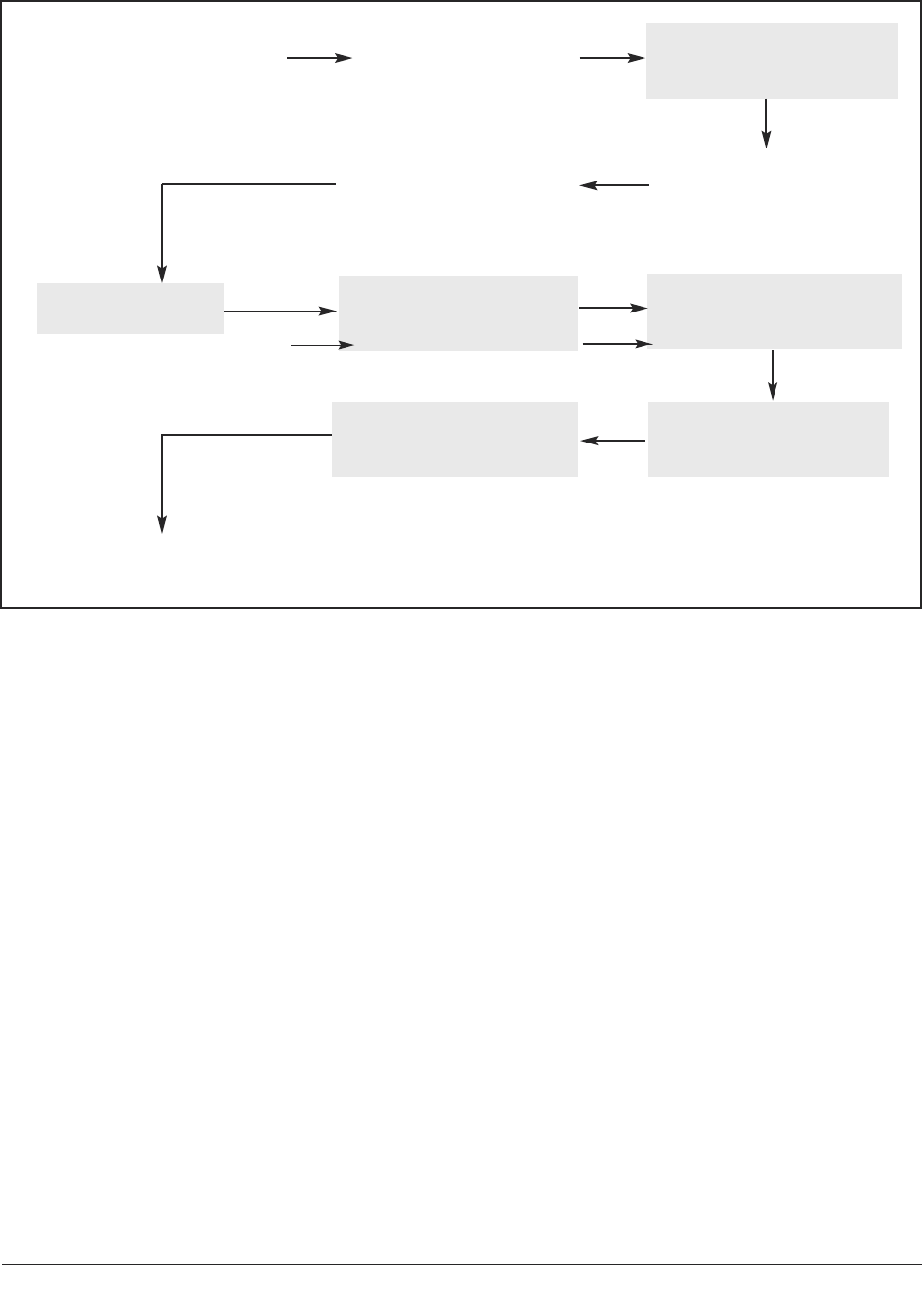
General ideas,
conduct literature review
Complete application
for ethical review
Gain favourable
ethical opinion
Recruit participants
Enhance capacity
to decide
Establish lack
of capacity
Conduct project
Analyse data
Disseminate findings
Prepare protocol
Seek consent
Consult with others
Appraise participant
involvement with project
A practical guide for researchers 7
■ procedures for ensuring consultation with others (see 4.1–4.5);
■ thorough consideration of benefit and risk to the participant; and
■ appraisal of an individual participant’s involvement with the project (see 5.1).
Figure 2: Modifications to the research process to meet requirements of the Mental Capacity Act.
Shaded areas in Figure 2 represent actions by a researcher which are different from or
additional to those required when conducting research with participants who can consent
to their participation.

8 Conducting research with people without the capacity to consent
Section 2: Ethical scrutiny
2.1 Principles of ethical scrutiny
Guidance and standards for the ethical conduct of research within medical and health care
settings have developed significantly over the past ten to 15 years (DH, 2005). Similar
standards of governance have been extended to ‘student research’, which apply to research
conducted as part of educational or professional training (Doyal, 2005; BPS, 2004a). Most
universities have established systems for the ethical review of research at departmental,
faculty, school or pan-university level. The equivalent systems for scrutiny within social care,
until recently, have been less well-developed (DH, 2005). From January 2009 a national
Social Care Research Ethics Committee (SCREC) will operate within the framework of the
National Research Ethics Service. (See
http://www.scie.org.uk/networks/screc/index.asp.)
A fundamental principle underlying ethical practice is ‘informed consent’. This principle
has been embedded in medical research for some time and has been extended to other
kinds of research. The Nuremberg Code and the subsequent World Medical Association
Declaration of Helsinki promoted ethical standards for the conduct of medical and
biomedical research with human participants. The two most important standards were:
■ voluntary informed consent of subjects; and
■ scientifically-valid research design that could produce fruitful results for the good
of society. (USA DH, 2004; BPS, 2004a, 2004b, 2005, 2008).
Ideally all participants of research projects should be capable of providing well-informed
and considered consent. However, the exclusion of participants who cannot decide for
themselves could deprive many people of access to the opportunity of active participation
in research and potentially of access to innovative interventions and procedures. Herring
(2006) in Medical Law and Ethics suggests
‘The Mental Capacity 2005 … establishes the right balance between the need for research to
bring benefit or information and the need for protection against exploitation and abuse. It
also seeks to ensure that any increased risk of the research, over and above that risk associated
with the condition or treatment itself, is either proportionate to the potential benefit to that
individual, or, in the case of research to provide knowledge, the risk is minimal.’
The researcher’s role, in addition to reaching a judgement about the ability of a
participant to give consent, is also to consider the balance of the benefit of participation
with an evaluation of ‘proportionate risk’.
The following paragraphs of this practice guide describe how a researcher could begin to
ensure these ethical standards are met at the project design phase of the research process.

A practical guide for researchers 9
2.2 Procedure for ethical review
All projects intending to recruit participants lacking capacity to consent must meet the
ethical approval of ‘appropriate bodies’ (MCA, Section 30(4)). As of 2008, projects can be
submitted to one of a small number of ‘flagged’ NHS research ethics committees (NHS
RECs). (See Appendix 2 for details and additional information.) The procedure of
submission to ‘flagged’ RECs applies to any project involving patients or clients of health
or social care services, and members of the public recruited via university or other research
centres. Scrutiny by a ‘flagged’ NHS REC would be in addition to ethical scrutiny internal
to the university or research organisation.
In addition to the standard online application, applicants also need to complete
supplementary forms. Supplementary forms can be downloaded from the website of the
National Research Ethics Service and are also provided in Part 2 of this practical guide.
There are three key questions on the supplementary forms requiring information
additional to that on the standard application form:
■ Can the project be as effectively undertaken with participants who have the
capacity to consent?
■ Is the research about an impairing condition that affects the person?
■ Does the research concern treatment or care of that condition?
During the course of conducting a project, researchers may become aware that
participants who initially had given valid consent have lost that capability in later stages of
the project. In order legally to conduct the research, the usual research process would
need to be halted, the protocol revised and ethical opinion re-sought before non-
consenting participants could again participate (MCA, Section 34 (2)). The Act makes
provision for such research via the Loss of Capacity During Research Project Regulations.
The relevant supplementary form is reproduced in Part 2(2) and is available on the NRES
website.
2.3 Intrusive research
The Mental Capacity Act (2005) applies in respect of research that is defined as
‘intrusive’, that is, research that would normally require the consent of a person with
capacity. It applies to clinical trials of treatments and procedures but does not apply (at
present) to trials, of medicinal products (known as CTiMPs) for which there are separate
regulations (The Medicines for Human Use (Clinical Trials) Regulations, 2004).
The MCA Code of Practice, published in 2007, provides examples of research relating
mainly to treatment and care. However, the list is followed by the statement
‘…the Act can cover more than just medical and social care research. Intrusive research which
does not meet the requirements of the Act cannot be carried out lawfully in relation to people
who lack capacity.’
The definition of ‘intrusive research’ in the Mental Capacity Act is deliberately wider than
health or medical research as it includes social care research. The following types of
research were listed in the Act’s Draft Code of Practice (DH, 2006), but omitted from the

10 Conducting research with people without the capacity to consent
published Code of Practice (the primary audience for the Code are those who are under a
duty to have regard to its provisions):
■ clinical research into new types of treatments (except clinical trials of medicines
that are covered by separate regulations);
■ health or social care services research to evaluate the effectiveness of a policy
intervention or service innovation;
■ research in other fields, (e.g. criminal justice, psychological studies, lifestyle or
socio-economic surveys);
■ research on tissue samples (i.e. blood or spare tissue removed during surgical or
diagnostic procedures) – also covered by the Human Tissue Act, 2004;
■ research on health and other personal data collected from records; and
■ observations, photography or videoing of people without capacity some of which is
done covertly so as not to distract the person
The above types of research are examples of ‘intrusive research’. Not all of them are
invasive, in the sense of physically taking something to or from a person’s body.
This meaning of ‘intrusive’ therefore leads to a broadening of the range of research which
would be unlawful if conducted with people who have the capacity to consent, but without
their consent (MCA Section 30 (2)). This also potentially applies to research which
hitherto may have been considered as not requiring the participant’s explicit consent, for
example observational studies conducted within care settings or studies using data
obtained from internet-based surveys (BPS, 2007).
Where adults lack the capacity to consent, the legal position in England and Wales is that
no other person can be authorised to give proxy consent. The sole exception at present
concerns research in connection with clinical trials of medicinal products, which require
that a legal representative give ‘informed consent’ (The Medicines for Human Use
(Clinical Trials) Regulations, 2004). For all other types of research likely to involve
participants not having the capacity to consent, researchers must demonstrate that they
have procedures in place to consult others about the involvement of prospective
participants.
2.4 Research that does not require consent
Some types of research do not require consent: this applies to all persons whether or not
they have the capacity to consent. Section 11.7 of the MCA Code of Practice lists the
following types of research:
■ Research involving data that has been anonymised and cannot be traced back to
individuals.
■ Research on human tissue that has been anonymised (Human Tissue Act 2004).
The research must have ethical approval.
■ Research using confidential patient information (Health Service (Control of
Patient Information) Regulations 2002 Section 1.2002/1438). This requires an
application to the Patient Information Advisory Group (PIAG), which can

A practical guide for researchers 11
determine ‘whether the common law duty of confidentiality can be lifted for
activities that fall within defined medical purposes, (including research) where
anonymised information will not suffice and consent is not practicable’.
In contrast to healthcare research requiring access to health and clinical records,
research using material derived from records held by social care organisations requires
the explicit consent of the individual, on the basis of the common law duty of
confidentiality. However, supplementary guidance (DH and WAG, 2008) to the MCA
Sections 30–34 states:
‘secondary legislation made under Schedule 3 (Data Protection Act, 1998) permits processing
of sensitive data where that is necessary for research, provided that the processing is in the
substantial public interest, that it does not support measures or decisions that affect
individual’s care or treatment and does not and is not likely to cause substantial damage or
distress to the subject or anyone else.’
Whilst access to NHS patient information is covered by the Act and the Code of Practice,
use of confidential information from other sources is not. The main issue is how to gain
access to partially anonymised records rather than to the full record. One respondent to
the consultation phase of this practice guide reported ‘records may be released under
special licence, the mechanism being specific to government departments/agencies; this
does not require the consent of the individual’.
In the course of preparing this practical guide, the author has encountered variation
between local authorities in the interpretation of the Data Protection Act, 1998 in relation
to the release of material for research purposes.
2.5 Seeking consent to participation in research
A critical step in the research process is the researcher seeking the consent of participants
to take part or to refuse. The process of obtaining voluntary and informed consent involves
two complementary and reciprocal decisions:
1. The participant makes a decision about whether to take part or to refuse to be
involved in a research project.
2. The researcher judges the quality of that decision. If the quality of that decision
meets certain ethical standards, the person is considered to have consented to
participate or to have refused.
Ethical standards framing this decision are:
■ freedom of choice and absence of coercion;
■ having general understanding of the research and its intentions; and
■ understanding of possible risks and benefits.
In terms of decision-making under the Mental Capacity Act, the key question for the
researcher is, does the person have the capacity to consent (or refuse) at the time the
decision needs to be made?
The National Research Ethics Service recommends that researchers allow the prospective
participant ‘sufficient time to reflect on the implication of participation in the study’

12 Conducting research with people without the capacity to consent
(NRES, 2007). This practical guide suggests that in judging the time lag between providing
information about a project and seeking consent, the researcher may need to take heed of
the nature of the project and the particular requirements of the project participants.
General ideas,
conduct literature review
Complete application
for ethical review
Gain favourable
ethical opinion
Recruit participants
Enhance capacity
to decide
Establish lack
of capacity
Conduct project
Analyse data
Disseminate findings
Prepare protocol
Seek consent
Consult with others
Appraise participant
involvement with project
A practical guide for researchers 13
Section 3: Assessing capacity to consent
3.1 Recruitment of participants
With reference to Figure 2 reproduced below, having secured a favourable ethical opinion,
the next stage in the research process is the recruitment of participants.
Figure 2: Modifications (shaded areas) to the research process to meet requirements of the Mental
Capacity Act.
In the course of prearing the project protocol the researcher will have specified criteria for
inclusion and for exclusion of participants, together with a description of procedures for
the recruitment of participants. For prospective participants who lack the capacity to
consent, the most likely recruitment methods will be via clinical or care teams, care
agencies, or service user and carer organisations. Such ‘intermediaries’ play a significant
role in the research process because of their knowledge of the sample of people from
which participants may be selected.
3.2 Assessing capacity to consent to participation in research
What is ‘mental capacity’?
Mental capacity is the ability to make a decision (MCA, Code of Practice, 2007). This
includes the ability to make a decision that affects daily life as well as the ability to make
decisions that may have legal consequences.

14 Conducting research with people without the capacity to consent
What is ‘lack of capacity’?
Section 2 (1) Mental Capacity Act 2005 states:
‘For the purposes of the Act, a person lacks capacity in relation to a matter if at the material
time he is unable to make a decision for himself in relation to the matter, because of an
impairment of, or disturbance in the functioning of, the mind or brain.’
Key indicators in judging whether a person lacks capacity are:
■ the presence of an impairment or disturbance (disability, condition) that affects
the way the person is able to think;
■ whether the impairment is permanent, temporary or fluctuating;
■ the nature of the decision – the person may be able to make decisions about some
things but not others; and
■ the timing of the decision – the person may be able to make a decision on the
matter in question if the decision is delayed for another time.
In many research projects, the person who seeks consent is also the person who judges
whether or not the prospective participant has the capacity to reach this decision
themselves. This person is usually the researcher who is directly undertaking the research
activity. Researchers may need to assess the capacity of participants at different stages of the
project (if the project is to be conducted over a span of time) and possibly in connection
with different research questions.
Large-scale projects may involve teams of researchers on different sites with a Chief
Investigator taking overall responsibility for the governance of the project. The Chief
Investigator may not be the person who routinely seeks participant consent but needs to
ensure that systems are in place to safeguard the welfare of all prospective participants,
whether or not they have capacity to consent.
Responsibilities of different parties in the research process are outlined in Appendix 3.
3.3 Applying the Five Statutory Principles of the MCA to judge
whether a (prospective) participant has the capacity to
consent
The Mental Capacity Act provides a decision-making framework that researchers could
adopt to enable them to have a ‘reasonable belief’ that a person lacks capacity to consent
to participation in research. In most circumstances, researchers will be able to undertake
the assessment of capacity themselves and it is unlikely that an expert professional opinion
about capacity will be required.
In the following sections, (3.4–3.7) the five principles of the MCA 2005 are stated,
examined in some detail and applied to the research context. The reader may wish to bear
in mind the key question or decision: ‘Does the person have the capacity to consent, at the
time that the decision needs to be made?’
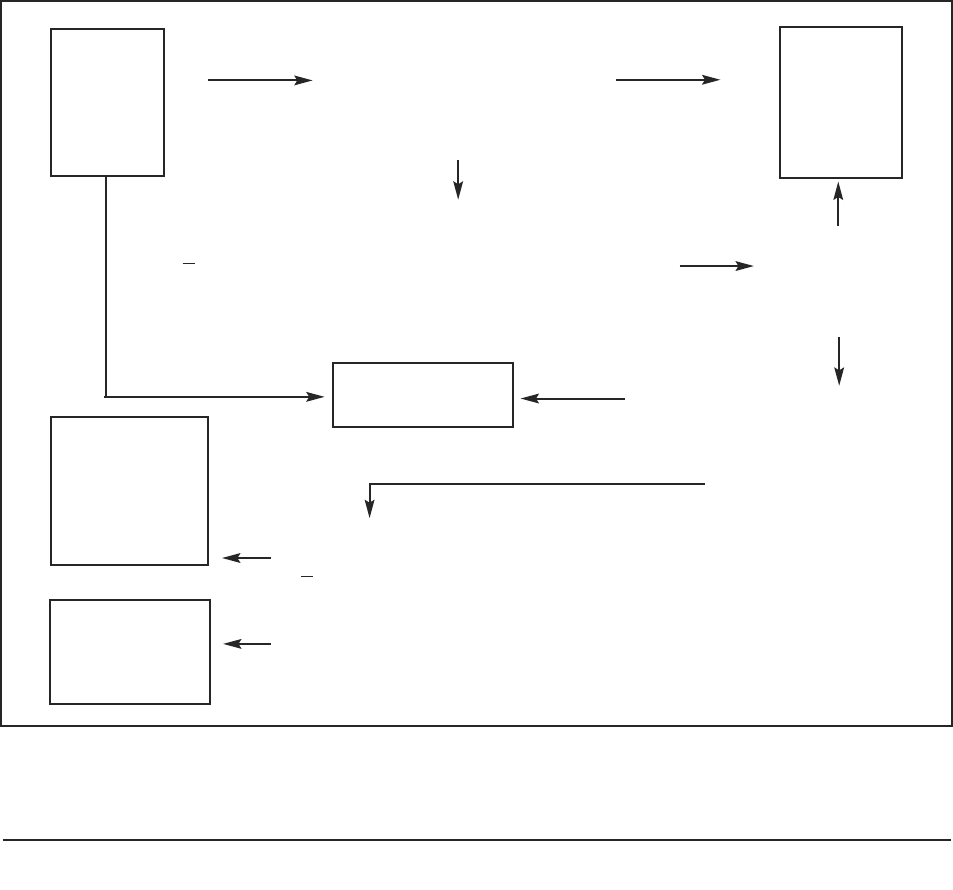
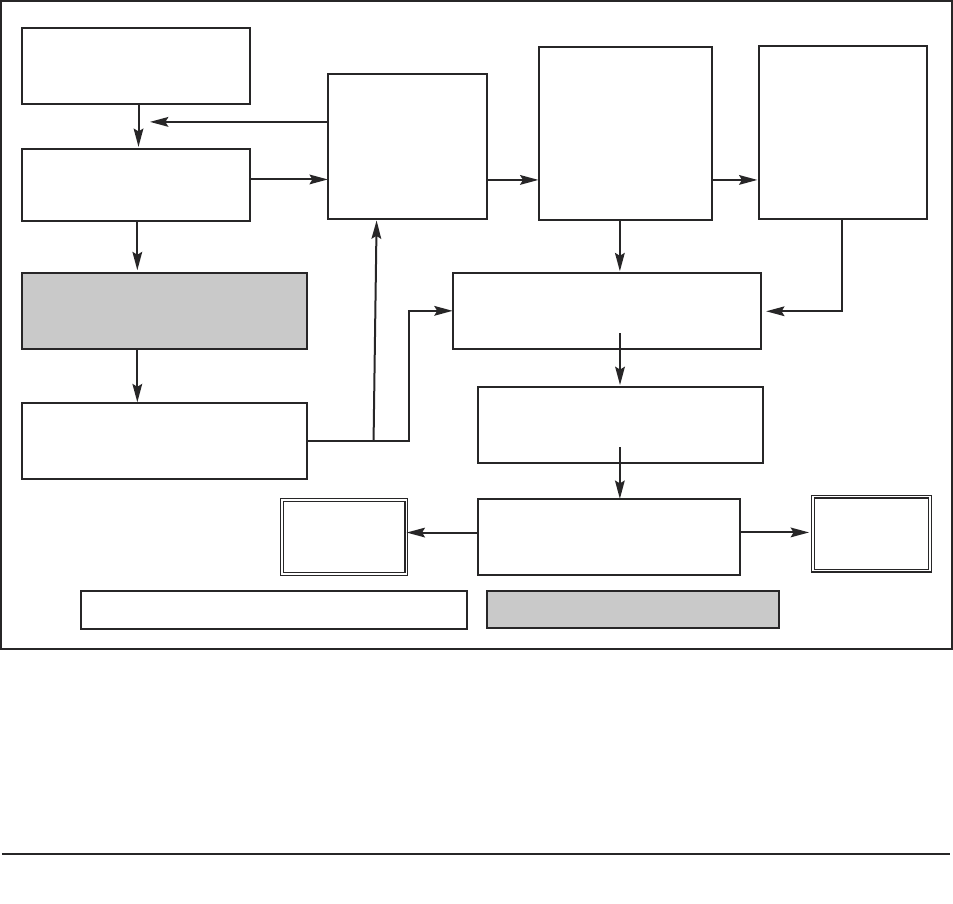
Figure 3: Decision-tree for researchers in assessing capacity to consent to participate in research.
The person
does not have
the capacity
to consent
The person
can consent
Researcher
accepts
person’s
decision
Can the
person reach
a decision?
Does the person have an
impairment affecting their
ability to reach the decision?
(see 3.7)
Can the person reach a
decision themselves to
agree or to refuse to
participate? (see 3.4)
EXCLUDE
Could the person’s decisional capacity be enhanced by
◆ Providing ‘accessible’ information about project
◆ Changing the timing and location of decision
◆ Providing education about research (see 3.5)
Does the person
◆ Understand about research?
◆ Understand consequences of taking part of refusing?
◆ retain, weigh up and use the information about the project?
◆ Communicate the decision?
(see 3.7)
yes
yes
yes
yes
no
yes
no
no
no
no
Is the
person
aged 18
or over?
A practical guide for researchers 15
The process in summary is:
1. The researcher presumes that the participant has the capacity to consent or to
refuse.
2. The prospective participant decides whether to participate or to refuse.
3. The researcher judges the quality of that decision and considers whether the
participant has agreed or refused to participate on the basis of
● freedom of choice and absence of coercion;
● having general understanding of the research and its intentions; and
● understanding of possible risks and benefits.
4. If the researcher is certain that the decision reached by the participant meets these
standards, the researcher can judge that the person has consented to or refused
participation.
5. If not certain, the researcher can use the following steps (Sections 3.4–3.7) which
are represented diagrammatically (Figure 3) as a flowchart of questions to consider
when planning and conducting this phase of the research project. By asking these
questions the researcher can reach a judgement about the nature of a participant’s
consent. A checklist based on these questions is provided in Part 2 of this guide.

The capability of a prospective participant to reach a decision themselves may be
enhanced by:
■ Amending the Information Sheet about the project, such as by the use of
‘accessible language’, using an interpreter or reader of written information,
providing only essential information about the project.
■ The National Research Ethics Service has provided extensive guidance on
improving the language and presentation of information sheets and consent
forms in order for them to be better understood by participants. (see
http://www.nres.npsa.nhs.uk/ rec-community/guidance/#informedConsent).
■ Organisations such as Connect and Medicines for Children Research Network
have produced suggestions about format and structure of information sheets.
■ Providing information in alternative formats, such as aurally for people with
visual disabilities, as well as in written formats. Consider conveying information
with diagrams and use of colour.
■ Breaking down complicated information into smaller points.
■ Altering the timing or location that consent is sought – would capacity to
consent be improved if the decision were delayed, sought at a different time of
day or in a different location?
■ Allowing the person time to reach the decision.
■ Encouraging discussion with others, such as family or friends about the project.
■ Providing education about research. Some people may not have any previous
experience of research – would training or discussion about the general idea of
research help the person to reach a decision about a particular project?
■ Responding to questions about the project.
16 Conducting research with people without the capacity to consent
3.4 Principle 1 of the MCA
A person must be assumed to have capacity unless it is established that he lacks capacity.
A researcher must assume that prospective participants for a research study have the
capacity to consent, even when they may have a condition that may question that capacity.
The researcher needs to ascertain that the person does not have capacity, and not assume
that they don’t on the basis of their condition, age, appearance or behaviour.
Proof of lack of capacity: the researcher would need to show that, on the balance of
probabilities, the individual lacks the capacity to consent to participation in the research at
the time that the consent is required to be made. See 3.7 below for detail of how this
judgement is reached.
3.5 Principle 2 of the MCA
A person is not to be treated as unable to make a decision unless all practicable steps to
help him to do so have been taken without success.
Practical support could enable a potential participant to make their own decision about
their involvement in a research project. Some examples of ways of doing this are provided
in the box below.

■ Being clear about the possible risks of participation as well as advantages and
benefits.
■ Being clear about what is actually required of the participant in conducting the
research.
■ Being clear whether the researcher is seeking consent about research that is
about to take place or is due to take place at some time in the future.
Considering systems for communicating with the prospective participant
■ Use of augmented communication or symbols or ‘talking mats’ (Cameron and
Murphy 2006)
■ Can others assist the person in communicating, whilst at the same time not
influencing the person in reaching a decision?
A person is unable to consent to their involvement if they cannot:
1. Understand information – does the person understand what the research
is about?
Does the person have a general understanding of the research project?
Can the person indicate what is expected of them (section 3.5)?
Have attempts as described above (section 2.2) to enable the participant to
make decisions for himself not been successful?
3.6 Principle 3 of the MCA
A person is not to be treated as unable to make a decision merely because he makes an
unwise decision.
Consenting to participate in research that, for example, may involve taking risks, may not
be an unwise decision even if the research is viewed by many as bizarre or unusual.
Potential examples could include studies involving sleep deprivation, extreme cold or the
effects of taking financial risks or hallucinogenic substances. Such research should be
subject as a matter of course, to ethical scrutiny within university or other research
organisations; the greater the degree of risk, the higher the level of scrutiny.
3.7 Reaching a judgment about whether a participant lacks the
capacity to consent to research
Proof of lack of capacity: the researcher would need to show that, on the balance of
probabilities, the individual lacks the capacity to consent to participation in the research at
the time that the consent is required to be made.
The researcher would have to prove that
a) the person has an impairment of the mind or brain that affects how the mind or
brain works; and
b) the impairment affects the person’s ability to consent at the time the consent is
required.
A practical guide for researchers 17

2 Retain information – can the person hold the information in their mind long
enough to use it to make a decision?
Can the person recall information about the research?
Having a poor memory per se is not sufficient grounds for saying that the
participant cannot consent.
3 Use or weigh up the information
Does the (prospective) participant consider the benefits and risks of taking part
in the research?
Can the person identify any consequences of participating or refusing to take
part?
4 Communicate their decision
Is the person unable to communicate their decision in any way, taking into
account any specific language or communication difficulties.
Fluctuating capacity
Can the decision to participate be taken at a time when capacity may have been
regained?
If the answers are NO to the first three of the above questions, then, on the balance of
probabilities, the person cannot reach a decision themselves and cannot consent to
their participation in research.
18 Conducting research with people without the capacity to consent
The researcher will need to document their judgment and consider whether to exclude
the person from the study or to include them by taking account of the safeguards provided
in the Mental Capacity Act.
A checklist for the researcher is provided in Part 2 of this guide.
3.8 Loss of capacity during a project
Some projects may be designed to include participants who are likely to lose capacity for
shorter or longer intervals during the course of a project.
In general, under the Mental Capacity Act 2005 (Loss of Capacity During Research
Project) (England) Regulations 2007 (SI2007/679) and The Mental Capacity Act 2005
(Loss of Capacity during Research Project) (Wales) Regulations 2007 (SI2007/837 (W.72),
if a person had given consent at some time in the past and that loss of capacity had not
been anticipated, the researcher would need to judge:
■ whether to make use only of data collected over the period of time that the person
had given consent;
■ whether to continue to collect data, even though the person no longer can give
consent, providing the loss of consent had not been anticipated. In this case, the

A practical guide for researchers 19
researcher would need to seek advice from a personal or nominated consultee and
appraise the participant’s involvement in the project, as described in Section 4
below. The researcher may need to re-apply for approval from a ‘flagged’ Research
Ethics Committee.
Studies designed since the MCA came into effect, would need to anticipate the possibility
of loss of capacity to consent and the researcher would need to take action as described in
earlier sections of this guide, such as the completion of supplementary forms as part of the
procedure for gaining ethical approval.

20 Conducting research with people without the capacity to consent
Section 4: Consulting with others
4.1 Consulting with others as a way of safeguarding the inclusion
of people who lack the capacity to consent to their
participation
Principle 4 of the Mental Capacity Act states that decisions made for or on behalf of the
person (are) to be made in the person’s best interests.
Principle 5 states that before the act is done, or the decision is made, regard must be had
to whether the purpose for which it is needed can be as effectively achieved in a way that is
less restrictive of the person’s rights and freedom of action
In contrast with many decisions under the remit of the Mental Capacity Act, the decision to
participate in research is not an area where consideration of an individual’s ‘best interests’
applies as described in the MCA (MCA Code of Practice, Section 5.4). The reasoning
during parliamentary debates on the MCA was that sometimes research may not be of
actual benefit to the person. If the principle of ‘best interests’ were rigorously applied,
then persons not having the capacity to consent would be restricted from opportunities for
involvement in research. However, if the research is contrary to the interests of the person
(such as if it were unduly burdensome, restrictive, etc.) then it would not gain ethical
approval and could not legally proceed.
The Mental Capacity Act provides special safeguards for the conduct of research with
participants not having the capacity to consent, which include:
■ scrutiny by an ‘appropriate body’ such as NHS Research Ethics Committee that
projects meet enhanced standards for ethical approval (as discussed in Section 2);
■ consultation with others not involved in the project about the involvement of a
person lacking capacity;
■ assurance that the interests of the participant are considered as having greater
importance than any potential benefit to others; and
■ acknowledgement of signs of objection by the participant, or contravention to an
advance decision or other form of advance statement.
The above safeguards could be considered as variations of Principle 4, the ‘best interests’
principle of the MCA and Principle 5, the ‘least restrictive’ principle.
If considering the inclusion of participants lacking the capacity to consent, the process of
consulting with others and reappraising the participant’s involvement in the light of that
consultation would have been anticipated in the course of seeking approval from a
Research Ethics Committee, prior to the recruitment of participants. However, some
projects may include participants who lose capacity during later stages of a project. In this
case researchers would still need to appraise the individual’s involvement and re-apply for
ethical approval if the project were to continue with the inclusion of non-consenting
participants (The Mental Capacity Act 2005 (Loss of Capacity during Research Project)
(England) Regulations 2007 (SI2007/679) and The Mental Capacity Act 2005 (Loss of
Capacity during Research Project) (Wales) Regulations 2007 (SI2007/837 (W.72)) .
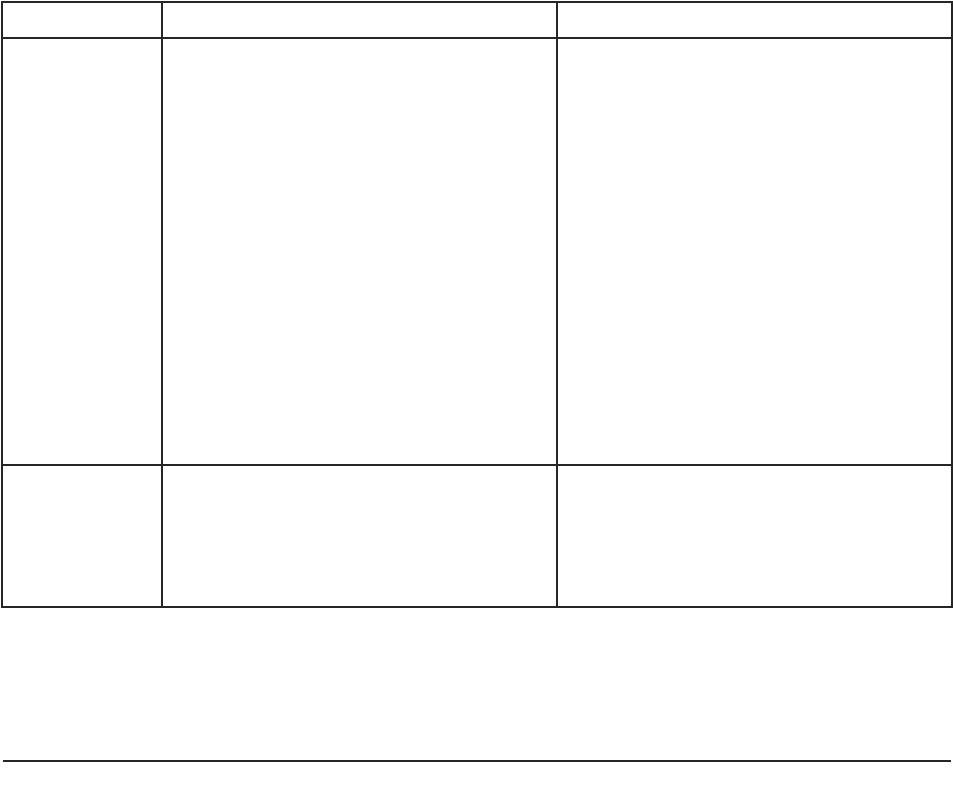
Personal Consultee Nominated Consultee
What is the
consultee’s
relationship
with the
(prospective)
participant)?
The Personal Consultee is someone the
person knows and trusts with important
decisions about their welfare and who
- is not paid to provide care to the
person;
- could be a family member, carer,
friend;
- could be an attorney or a deputy
appointed by the Court of
Protection.
The Nominated Consultee is someone
who
- may be known to the person;
- may be paid to provide care, such as
a member of staff in a care home in
which the person lives;
- may provide professional services,
such as a solicitor or a doctor;
- may not be known to the person;
- may act as a consultee for several
prospective participants.
How does the researcher decide who to
contact?
How does a
researcher
decide who to
contact?
See flow chart (Figure 4) See flow chart (Figure 4)
A practical guide for researchers 21
4.2 Role of Research Consultee
The Mental Capacity Act requires that a researcher take ‘reasonable steps’ to identify
others who could be consulted about a prospective participant’s involvement in research
(MCA para 32 (2)). The researcher is required to identify a person (someone who is
interested in that person’s welfare) who can be consulted about what the prospective
participant’s wishes and feelings about participation in the project would be if the person
had capacity. In circumstances where the prospective participant has little contact with
other than paid or professional carers, the researcher can nominate a person who can be
consulted providing they have no direct involvement with the project.
Guidance produced by the Department of Health and Welsh Assembly Government
creates roles for ‘personal’ and ‘nominated’ consultees as those consulted by the
researcher. Of particular importance the research consultee does NOT give consent on
behalf of a participant, but rather the researcher seeks advice from the consultee. The
researcher, not the consultee, makes the decision about whether to include the person as a
participant, though has to abide by information the consultee provides that suggests that
the person may object to inclusion in the project.
The following paragraphs provide further description of the roles and responsibilities of
consultees, suggest a process by which the researcher seeks advice from consultees and how
such advice can be made use of within the overall research process. The roles of personal
and nominated consultees in the research process are summarised in Table 1.
Table 1: Roles of personal and nominated consultees in the research process.
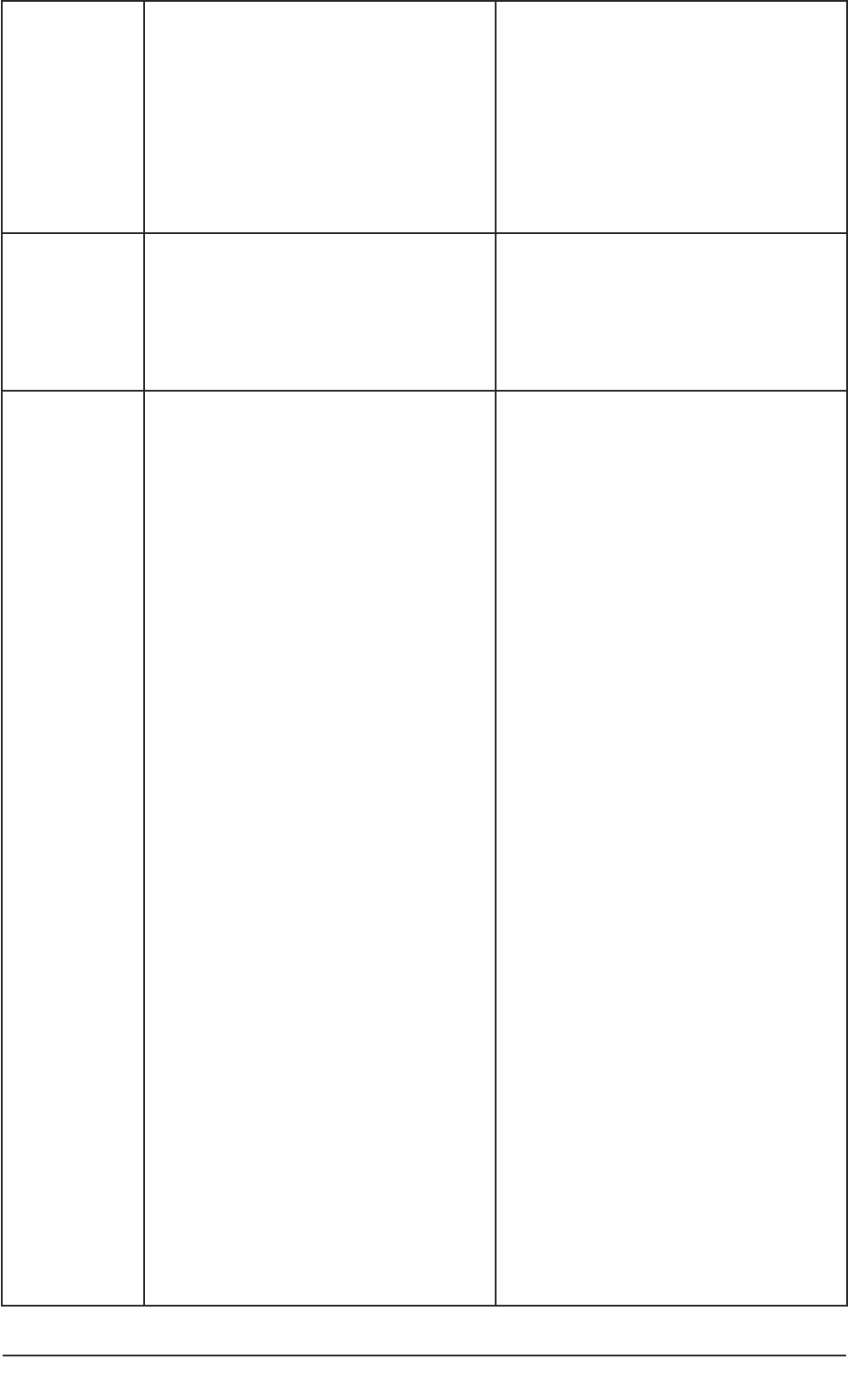
22 Conducting research with people without the capacity to consent
Who initiates
contact with a
consultee?
The clinical/care team, health or social
care agency contact the personal
consultee. Contact details are known to
the care/clinical team, health or social
care agency.
See sample letter in Part 2(4b).
The researcher contacts the Nominated
Consultee.
See sample letter in Part 2(5b) of this
guide.
What
information
does the
consultee
receive?
The researcher provides information
about the project and about the role of
a Personal Consultee.
See suggested information sheet in
Part 2 (4c).
The researcher provides information
about the project and about the role of
a Nominated Consultee.
See suggested information sheet in
Part 2 (5c).
What advice
does the
researcher seek
from the
Consultee?
Consultee’s general understanding of
the project.
Whether the participant may be
interested in taking part in the project
or whether they would object.
Whether the person may benefit in
any way by taking part.
Whether the person has previously
expressed views about involvement in
research, assuming they had such
capacity in the past.
Whether the person has made any
advance statements or has a written
advance decision for refusal of life-
sustaining treatment.
Whether participation would cause
any inconvenience or any other
difficulty for the person.
Whether the person would give any
signs, and if so, what these would be,
to indicate they were not happy
about continuing with the project.
Whether the consultee would wish to
be approached again for their views.
Whether the consultee could suggest
an alternative consultee.
Whether, from their understanding of
the person and the project, on
balance the person should or should
not take part.
Consultee’s general understanding of
the project.
Whether the consultee has any
personal or professional connections
with the project or an interest in its
outcome.
What knowledge of the person, and if
so, in what capacity.
Whether the consultee has discussed
involvement in the project with the
person.
Consultee’s views about whether the
participant may benefit from taking
part.
Consultee’s views about whether the
person may object, be upset in any
way or want to stop being involved,
and if so, how this would be shown.
Consultee’s views about whether
participation may cause any problems
or inconvenience.
Whether, from their understanding of
the person and the project, on
balance the person should or should
not take part.
A practical guide for researchers 23
yes
yes
yes
yes
yes
yes
no
no
no
no
no
no
Does the person have
capacity to consent?
Does the person have
family or friends?
Care/clinical team contacts
family or friends
Should the person be a
participant?
EXCLUDE
INCLUDE
Does the
person know
someone else?
Does the care
organisation
have a panel of
Nominated
Consultees?
Does the
research sponsor
have a panel of
Nominated
Consultees?
Key:
Questions for and action by researcher
Action by care/clinical team
Researcher discusses with
Principal/Chief Investigator
Researcher contacts
Consultee
Do family or friends
respond to invitation?
4.3 Consulting with a Research Consultee
The process of consultation starts with the clinical/care team contacting someone known
to the prospective participant and inviting them to be consulted about their relatives or
friend’s involvement in the project (see Figure 4). See Part 2 (4b) for a sample letter
together with preliminary information about the project. If the relative or friend accepts
the invitation to be a Personal Consultee, the Care/Clinical Team contacts the Personal
Consultee to make arrangements to discuss the project with the researcher. The researcher
needs to make clear that they are seeking the Personal Consultee’s views about whether or
not the prospective participant may wish to take part, not their own views about the
project. The views and opinion of the consultee will need to be documented by the
researcher for later reference. A suggested proforma is provided in Part 2 – Forms.
There is limited research about the extent to which family members are prepared to be
consulted. A study in Australia (Iacano & Murray, 2003) demonstrated that 50 per cent of
families who were approached declined to offer information about prospective participants.
The researcher may also need to approach the Personal Consultee at later stages in the
research process to confirm whether the participant would wish to continue with or to
decline taking part in the project.
Figure 4: Seeking advice from a consultee.

4.4 Personal Consultee
The Personal Consultee is someone who the (prospective) participant knows and whom
they trust with important decisions about their welfare. The Personal Consultee could be a
relative, a friend or someone having a Lasting Power of Attorney for personal welfare
(including healthcare and consent to medical treatment) or a deputy appointed by the
Court of Protection. The Personal Consultee could NOT be someone who is a paid carer
or who has a professional relationship with the prospective participant.
The researcher seeks an opinion from the Personal Consultee about
■ whether the person should take part in the research;
■ what the person’s wishes and feelings would be about such a project;
■ whether it is likely that the person would decline to take part, had they the capacity
to decide.
If the consultee advises the researcher that the person would not wish to take part, then
the person should be withdrawn from the project
Given that Section 32(2) of the Mental Capacity Act (2005) suggests that ‘reasonable steps’
should be taken to identify a Personal Consultee and has also proposed the appointment of
Nominated Consultees, the researcher appears to have a choice about who to approach. A
flowchart (Figure 4) is offered as a means of assisting the researcher in deciding whether to
seek consultation from a Personal or a Nominated Consultee.
4.5 Nominated Consultee
A Nominated Consultee may be a paid carer or someone who has a professional
relationship with the person, such as a solicitor or a doctor, but who has no financial or
other interest in the outcome of the project.
Guidance prepared by the Department of Health and the Welsh Assembly Government
(2008) suggests that Nominated Consultees could be drawn from a list of potential
Consultees, convened by a research active care organisation, such as a NHS Trust, research
sponsors, such as the Wellcome Foundation, or universities. Research networks which have
evolved as part of the NHS Research Strategy, Best Research for Best Health, may also
provide a convenient host for panels of Research Consultees. Alternatively, Independent
Mental Capacity Advocates may be in a position to contribute to consultation about the
involvement of participants in research, depending on local arrangements.
During the course of discussions for the preparation of this guide, a number of
respondents expressed concern about inviting an opinion from consultees who were not
known to the prospective participant.
Suggestions for good practice therefore are that a consultee adopts an approach similar to
that of an advocate, in which the consultee meets the prospective participant, carers,
relatives and friends (if available) in order to gain relevant information on which to base
advice to the researcher.
24 Conducting research with people without the capacity to consent

Section 5: Appraising partipant involvement
5.1 Appraising whether to include or exclude a participant
Section 31 of MCA states that the researcher must ensure that the project meets the
following five requirements.
1. The research project is associated with the condition which impairs the participant
and/or any treatment of the condition. An ‘impairing condition’ is one which
causes or contributes to any disturbance of the mind or brain (and on which the
assessment of lack of capacity is based).
2. The research project could not be undertaken as effectively solely with participants
who have capacity to consent.
3. The research must be intended to provide knowledge of the causes, treatment or
care of people affected by the same or similar impairing condition or that it
concerns treatment or care of the condition.
The MCA Code of Practice provides further examples of ‘similar’ conditions or
impairments that may not have the same cause, such as cognitive impairment
associated with brain injury acquired in adulthood and intellectual impairment
consequential to a genetic disorder.
4. The participant is likely to benefit from undertaking the research and that the
benefit is not disproportionate to any burden in taking part.
5. If there are no benefits to the person and if the research concerns the gaining of
knowledge about the condition, then there should be negligible risk to the
participant. In addition, participation in the project should not interfere with the
participant’s freedom of action or privacy in a significant way, or be unduly invasive
or restrictive.
Figure 5 provides a diagrammatic representation of the decisions or judgments the
researcher needs to make. Requirements 1–4 above can be debated in principle in the
study protocol and in the application for ethical approval (shaded boxes). The fifth
requires information about the impact of involvement in the project for the individual
participant, information which could only be obtained in the course of consultation with
others.
A practical guide for researchers 25
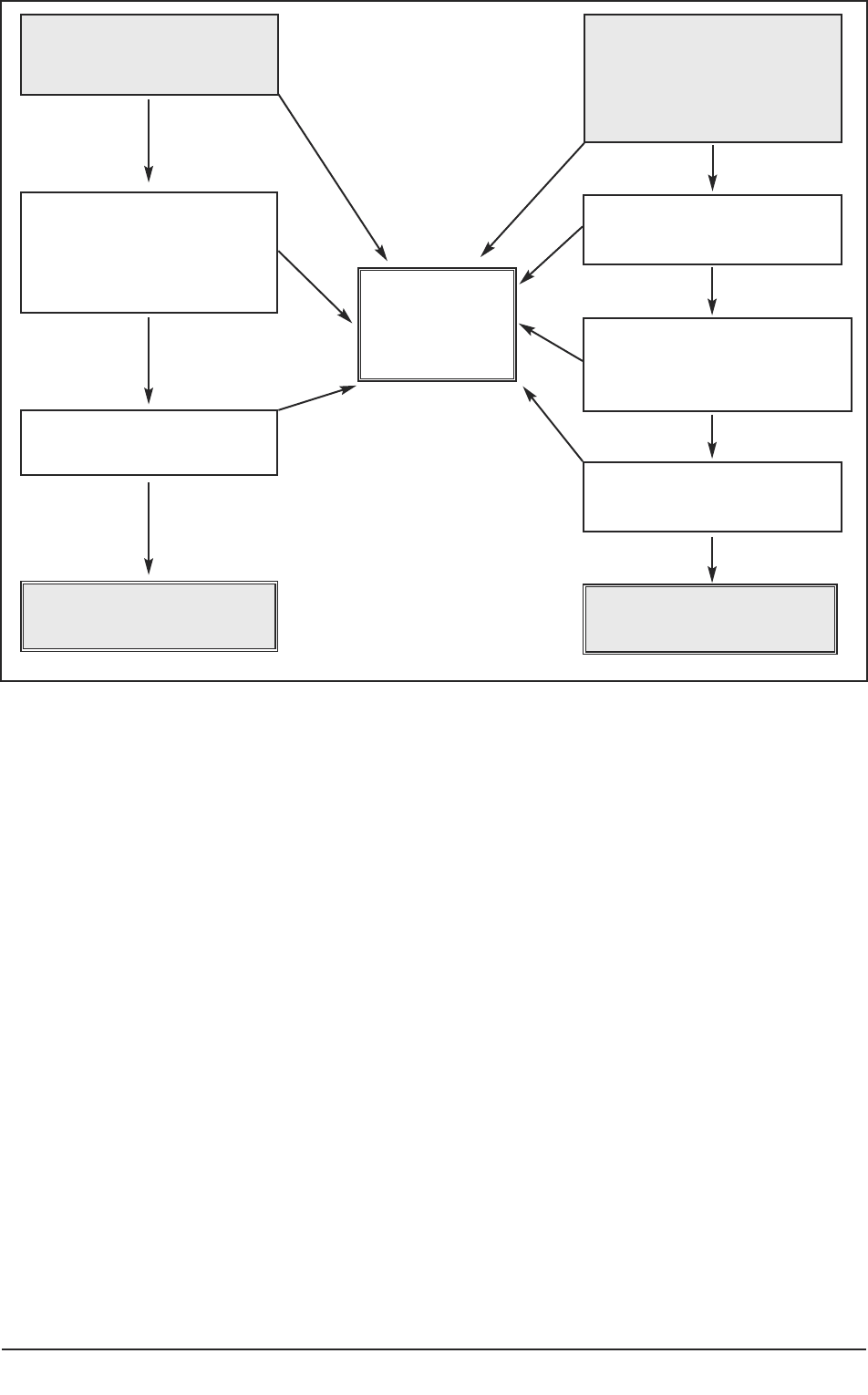
Figure 5: Appraising an individual’s involvement in a project.
Figure 5 summarises questions the researcher needs to consider. A checklist for researchers
is available in Part 2(6) of the practice guide.
5.2 Reviewing continuing participation in a project
A key feature of consent to participate is the freedom to continue or to withdraw from a
study. Participants having significant cognitive impairment (language, memory and
problem-solving abilities) may be susceptible to influence as a result of pressure from
researcher or carer or by merely being in the ‘research environment’ (Gudjonsson, 2002).
Researchers may need to become aware of potential behaviour, both verbal and non-verbal
which indicates that the person may wish to withdraw (MCA, Section 33(4) and Code of
Practice 11.31). Examples may be that in addition to a clear expression of no longer
wishing to participate, the person pushes the equipment away or takes themselves away
from the researcher. In research based on observational methods, identifying behaviour
that is non-consenting to research may be difficult to distinguish from other behaviour or
activities being observed. Such matters would also have been addressed in the application
for ethical approval.
26 Conducting research with people without the capacity to consent
Is there negligible risk
to the participant?
Can research be undertaken
as effectively with
participants having
capacity to consent?
Is the research about
knowledge of causes,
treatment or care of persons
with an impairing condition?
Is the research about
treatment or care of an
impairing condition?
INCLUDE the person
EXCLUDE
the person
INCLUDE the person
Do benefits of participation
outweigh burden?
Does doing the research affect
the participant’s freedom of
action or privacy?
Is the research unduly
invasive or restrictive?
yes
no
no
yes
yes
yes
no
no
yes
yes
no
no
no
yes

If in doubt, the researcher may need to seek additional advice from a Personal or
Nominated Consultee. If the consultee advises that the person should be withdrawn, then
this must be respected by the researcher (MCA, Section 32 (5)). The researcher may find it
helpful to refer to Figure 5 (Appraising an individual’s involvement with a project)
together with checklist 6 in Part 2 of the Guide
Finally, if any of the requirements of the Act are no longer met, the individual must be
withdrawn from the project (Code of Practice, 11.31).
5.3 Recommendations for good practice
1. The researcher appraises the project in relation to each individual participant
lacking consent, even though these matters would have been anticipated in
principle during the process of gaining ethical approval. The benefits, burdens
and risks cannot be assumed to be similar for all participants.
2. Relevant evidence and decisions are documented.
3. The appraisal or re-appraisal of a participant’s involvement with the project is
conducted by means of a discussion between the researcher (who has collected
information from a consultee) and the Principal or Chief Investigator. A major
responsibility of the Principal or Chief Investigator is the overall welfare of
participants. Documentation recording aspects of the discussion is included in
Part 2(6) of the guide.
A practical guide for researchers 27

Section 6: Case studies
Case study 1 demonstrates how a researcher could undertake an assessment of capacity
and an appraisal of the involvement of an individual in a study.
Questions below offer the researchers a step-by-step examination of Susan’s capacity to
consent and consideration of whether she should be included in the study. See Figures 3,
4 and 5 and checklists provided in Part 2. (Additional material is provided for purposes of
illustration).
Q: Can Susan consent to her participation in research?
If yes – Susan can decide whether or not to participate.
If no or unsure – the researcher needs to assess Susan’s capacity to consent.
Whether Susan indicates her willingness to participate is a separate matter from
her capacity to consent to participation.
Q: Does Susan have a condition impairing her ability to make decisions?
A: A Susan suffers from psychosis which impairs her capability in making decisions,
including about participating in the research study.
28 Conducting research with people without the capacity to consent
Susan, aged 25, married with two children, has been experiencing some mental health
problems since the age of 16. She has been experiencing low mood, anxiety and low
levels of paranoia but has been able to cope with her problems to date. She has been
treated by her GP who has prescribed anxiolytics and anti-depressants and at times she
has also been receiving input from the Community Mental Health Team (CMHT) in the
area. Susan’s marriage recently broke down and her ex-husband has been making threats
to take the children away from her because of her mental health condition. Her mother
died when she was still very young, her father is an alcoholic, and she has no brothers and
sisters. Her friends have rejected her due to her mental health problems and Susan has
no one to talk to and feels isolated.
As a result of these experiences her mental health problems have now become
unmanageable and Susan has experienced a mental breakdown. She is now on a female
ward and her ability to reason and make judgements is significantly impaired. After a
detailed assessment of her symptoms by the psychiatrist Susan is diagnosed with a first
episode of psychosis. Susan fulfils the necessary criteria to participate in your first episode
study. The study involves participation in lengthy questionnaires that could take up to
three hours to complete some of which need to be videotaped.
Would Susan participate in the research study?

Q: Does Susan have the capacity to consent to participation in the ‘first episode’
study?
A: The researcher determines whether Susan
■ can retain information about the study;
■ understand the intentions of the project;
■ weigh up information about the project and the consequences of
participating or not;
■ convey her response.
Susan can describe some aspects of what the project is about. She understands she
has to answer some questions. Susan cannot describe how taking part or not taking
part would affect her.
The researcher kept notes of the discussion with Susan.
In discussion with the Principal Researcher, the Principal Researcher considered
that Susan did not have the capacity to consent to her involvement with the
research project at the time consent was sought, as she was not able to weigh up
information about the project or the consequences of her involvement. Susan was
unlikely to regain capacity in relation to making decisions about her involvement
with the current project.
A: Susan suffers from psychosis which may impair her capability in making decisions.
The researcher determines whether she can retain and understand the intentions
of the project, weigh up that information and the consequences of participating or
otherwise and convey her response.
If still no – then Susan does not have the capacity to consent
Whether Susan states her willingness to participate is a separate matter from her
capacity to consent.
Q Who can the researcher consult with?
A Susan is described as being isolated from family and friends. Her children are
under the age of 18, so no family member may be appropriate to act as a Personal
Consultees.
Susan is known to members of the Community Mental Health Team; hence the
researcher could contact the team to identify someone who would be willing to act
as a Nominated Consultee, providing the team did not have an interest in the
outcome of the project. If team members were connected in any way with the
project, the researcher would need to contact a different Nominated Consultee
who was independent of the project.
A practical guide for researchers 29

Q Is the research about treatment or care or about knowledge of a condition, care or
treatment?
A The study appears to be about knowledge of psychosis or its treatment or care.
Q Does Susan have an advance statement?
A The Nominated Consultee has advised that Susan has not prepared an advance
statement.
Q Would participation in the study
a) Be of negligible risk to Susan?
A The researcher uses information gained from the Nominated Consultee about
whether the interviews would potentially be of harm to Susan, perhaps, for
example, because of the nature of the interview questions.
b) Affect Susan’s freedom of action or privacy?
A The interviews could be conducted in a private room; however, the Nominated
Consultee advised that Susan participates in therapy sessions which would clash
with the intended timing of the interviews with the researcher.
c) Be unduly invasive or restrictive?
A The interviews require in-depth discussions about Susan’s family life. The
Nominated Consultee has advised that Susan has difficulty in talking about her
family life.
A conclusion could be reached that Susan may experience distress in participating in the
research interview – hence she should not be included as a participant. Of particular
importance is the key distinction between ‘research of the treatment’ and research which is
knowledge about the condition, treatment or care of persons’; if the latter, the research
may not necessarily be of benefit to the person, but would still need to meet the criterion
of ‘interests of the person outweighing those of science and society’.
Consideration of whether research is unduly invasive or restrictive may also be different for
different people. The MCA Code of Practice suggests that ‘unduly invasive’ research is that
which does not go beyond ‘the experience of daily life. Routine medical examination or
psychological assessment is considered as not being ‘unduly invasive’ (MRC, Code of
Practice, 11.19).
30 Conducting research with people without the capacity to consent

Case study 2
Case study 2 is an appraisal of the overall ethical stance of a project rather than that of the
involvement of individual participants.
In this case study, published in the Medical Research Council Ethics Guide (2007), the
Blandfordshire REC decided that the health hazard (harm) to participants was equivalent
to ‘risk’ encountered in normal daily life and approved the study. The case study also
demonstrates an approach to consultation with others which appraises the participants as a
group rather than as individuals.
A practical guide for researchers 31
The Blandfordshire REC was asked to review a proposal to study whether electronic
tagging was beneficial to the care of older people with varying degrees of dementia who
lived in residential homes. The hypothesis was that the tagging would allow the residents
more freedom while minimising their risk of getting lost. There was some discussion
about whether the tagging was an invasion of privacy when the individuals concerned
were unable to provide informed consent. However, the results of an independent
consultation, commissioned by the researchers, of relatives and carers suggested that the
benefits to the residents were perceived to outweigh this concern. The tagging device was
very small and not noticeable when worn. When the project was reviewed by the REC, it
was questioned whether the radio frequencies used constituted a health hazard in this
age group. A decision on whether the study might go ahead was deferred until the
researchers provided an updated analysis of the literature on this issue, in light of new
scientific evidence. This analysis suggested that the radio frequency risk was similar to
that of mobile telephones.

Section 7: Concluding comments
The Mental Capacity Act (2005) has opened up the possibilities that people not able to
consent should no longer be excluded from participation in research, enabling access to
experiences and potential treatments and care for which they may not hitherto have been
considered. The MCA has sought to balance such access with safeguards applying at
different stages of the research process.
Preparation of the practice guide has brought into focus a number of ethical,
philosophical and political matters, such as the nature of ‘informed consent’, the range of
decisions a researcher may need to take at different stages of the research process and
stark differences between conducting research in ‘health’ rather than in ‘social care’
settings.
The practice guide was originally envisaged as a compendium of resources, references and
‘good ideas’ for conducting research with people not having the capacity to consent to
their participation, prepared from the perspective of different parties in the research
process. After much deliberation, the author considered that taking a systemic view of the
research process would provide a meaningful structure for materials that a researcher may
find useful.
The extent to which researchers find the materials of assistance will depend on whether
researchers, sponsors and funders consider the additional safeguards feasible to
incorporate within research projects, given tight timescales and restricted budgets. An
audit of the use of the guidance materials, together with a review of experience in Scotland
and other jurisdictions, may in the future help to identify the kinds of research questions
which could not be addressed by other means.
32 Conducting research with people without the capacity to consent

Appendices
Appendix 1 References
Appendix 2 NHS Research Ethics Committees which have been
‘flagged’ to scrutinise projects involving
participants who do not have the capacity to
consent
Appendix 3 Roles and responsibilities in the research process
Appendix 4 Additional questions and ethical implications
Appendix 5 Contributors
A practical guide for researchers 33

Appendix 1
References
Article 17 of the Protocol to the Convention on Human Rights in Biomedicine or
Biomedical Research (2005).
British Psychological Society (2004a). Guidance for the minimum standards of ethical approval
for psychological research. Leicester: Author
British Psychological Society (2004b). Code of conduct, ethical principles and guidelines.
Leicester: Author.
British Psychological Society (2005). Good practice guidelines for the conduct of psychological
research in the NHS. Leicester: Author.
British Psychological Society (2008) Generic Professional Practice Guidelines (2nd edn.)
Leicester: Author.
Cameron, L. & Murphy, J. (2006). Obtaining consent to participate in research: The issues
involved in including people with a range of learning and communication disabilities.
British Journal of Learning Disabilities, 35, 113–120.
British Psychological Society (2007). Report of the working party on conducting research on the
internet: Guidelines for ethical practice in psychological research online. Leicester: Author.
Department for Constitutional Affairs (2006). The Mental Capacity Act (2005): Draft Code of
Practice. London. The Stationery Office
Department for Constitutional Affairs (2007). The Mental Capacity Act (2005): Code of
Practice. London. The Stationery Office.
Department of Health (2004a). Information about patients: An introduction to the Patient
Advisory Group for health professionals and researchers. Retrieved from
http://www.advisorybodies.doh.gov.uk/piag/InformationAboutPatients.pdf.
Department of Health (2004b). The research governance framework for health and social care –
Implementation plan for social care. London: Department of Health.
Department of Health (2005). Research governance framework for health and social care.
London: Department of Health. Retrieved 25 November 2008 from
http://www.dh.gov.uk/en/ Publicationsandstatistics/ Publications/
PublicationsPolicyAndGuidance/DH_4122427.
Department of Health (2006). Consultation on regulations made under the Mental
Capacity Act (2005). Retrieved on 27 November 2008 from http://www.dh.gov.uk/
en/ Consultations/Closedconsultations/DH_4138699.
Department of Health and Welsh Assembly Government (2008). The Mental Capacity Act
and consent for research: What research does the Act cover? . Retrieved on 27 November
2008 from http://new.wales.gov.uk/dhss/publications/health/mentalhealth/
mentalcapacityact/2117019/mcaconsente.pdf?lang=en.
34 Conducting research with people without the capacity to consent

Department of Health and Welsh Assembly Government (2008). Guidance on nominating a
consultee for research involving adults who lack capacity to consent. London: Department of
Health.
Doyle, L. (2005). The ethical governance and regulation of student projects: A proposal. Retrieved
on 25 November 2008 from http://www.dh.gov.uk/en/Researchanddevelopment/
A-Z/Researchgovernance/ index.htm/DH_4120898.
Gudjonsson, G. (2002). The psychology of interrogations and confessions: A handbook. London:
Wiley.
Herring, J. (2006). Medical law and ethics. OUP
Iacano, T. & Murray, V. (2003). Issues of informed consent in conducting medical research
involving persons with intellectual disability. Journal of Applied Research in Intellectual
Disabilities,16, 41–51.
Medical Research Council (2007). MRC ethics guide 2007: Medical research involving adults
who cannot consent. London: Author. Retrieved from http://www.mrc.ac.uk/Utilities/
Documentrecord?index.htm?d=MRC004446.
The Medicines for Human Use (Clinical Trials) Regulations 2004. London: The Stationery
Office.
The Mental Capacity Act 2005 (Loss of Capacity during Research Project) (England)
Regulations 2007.
Mental Capacity Act 2005 (Appropriate Body) (England) Regulations 2006. London: The
Stationery Office.
Mental Capacity Act 2005 (Appropriate Body) (Wales) Regulations 2006. London: The
Stationery Office.
Mental Capacity Act (2005) Chapter 9. London: Office of Public Sector Information.
National Research Ethics Service (2007). Information sheets and consent forms for
researchers and reviewers, v.3.2 May 2007. Retrieved on 25 November 2008 from
http://www.nres.npsa.nhs.uk/rec-community/Guidance/#PIS.
National Research Ethics Service (2007) Research involving adults unable to consent for
themselves v 2 September 2007. Retrieved on 27.11.08 from
http://www.nres.npsa.nhs.uk/rec-community/guidance/#consent.
Statutory Instrument 2002 No. 1438 The Health Service (Control of Patient Information)
Regulations 2002.
US Department of Health and Human Service. (2004). Research involving human subjects:
Research Regulations and ethical guidelines. Quoting from Trials of War Criminals
before the Nuremberg Military Tribunals under Control Council Law 10(2), pp. 181-182.
Washington, DC: US Government Printing Office, 1949.
A practical guide for researchers 35

Appendix 2
NHS Research Ethics Committees which have been ‘flagged’ to
scrutinise projects involving participants who do not have the
capacity to consent
The list of ‘flagged’ RECs which follows is taken from the National Research Ethics Service
document Research involving adults unable to consent for themselves (version 2, September
2007).
Flagged RECs for research involving adults unable to consent for themselves
England
Barking & Havering REC
Berkshire REC
Bolton REC
Bradford REC
Bromley REC
Cambridgeshire REC 3
Camden & Islington Community REC
Coventry REC
Dorset REC
Ealing and West London Mental Health Trust REC
Essex 2 REC
Frenchay REC
Harrow REC
Leeds West REC
Liverpool Adult REC
National Hospital for Neurology and Neurosurgery and Institute of Neurology REC
Newcastle and North Tyneside REC 2
Norfolk REC
North Staffordshire REC
Northumberland REC
Nottingham REC 1
Sandwell and West Birmingham REC
South East REC
South London and Maudsley and Institute of Psychiatry REC
36 Conducting research with people without the capacity to consent

South Manchester REC
South Sheffield REC
Southampton & South West Hampshire REC 1
York REC
Wales
North East Wales Health Authority Research Ethics Committee
South East Wales Research Ethics Committee Panels B, C and D
Wales Research Ethics Committee
Scotland
Scotland A Research Ethics Committee
Northern Ireland
HSC Research Ethics Committees 1-3
Please note that during 2008, NRES has initiated a Central Allocation System (CAS),
whereby researchers intending to submit a project to a ‘flagged’ REC, can do this via CAS.
For further information and relevant ‘flagged’ RECs, please see http://www.nres.npsa.nhs.uk/
news-and-publications/news/important-changes-to-booking-applications-via-the-central-
allocation-system. Retrieved on 27 November 2008.
A practical guide for researchers 37
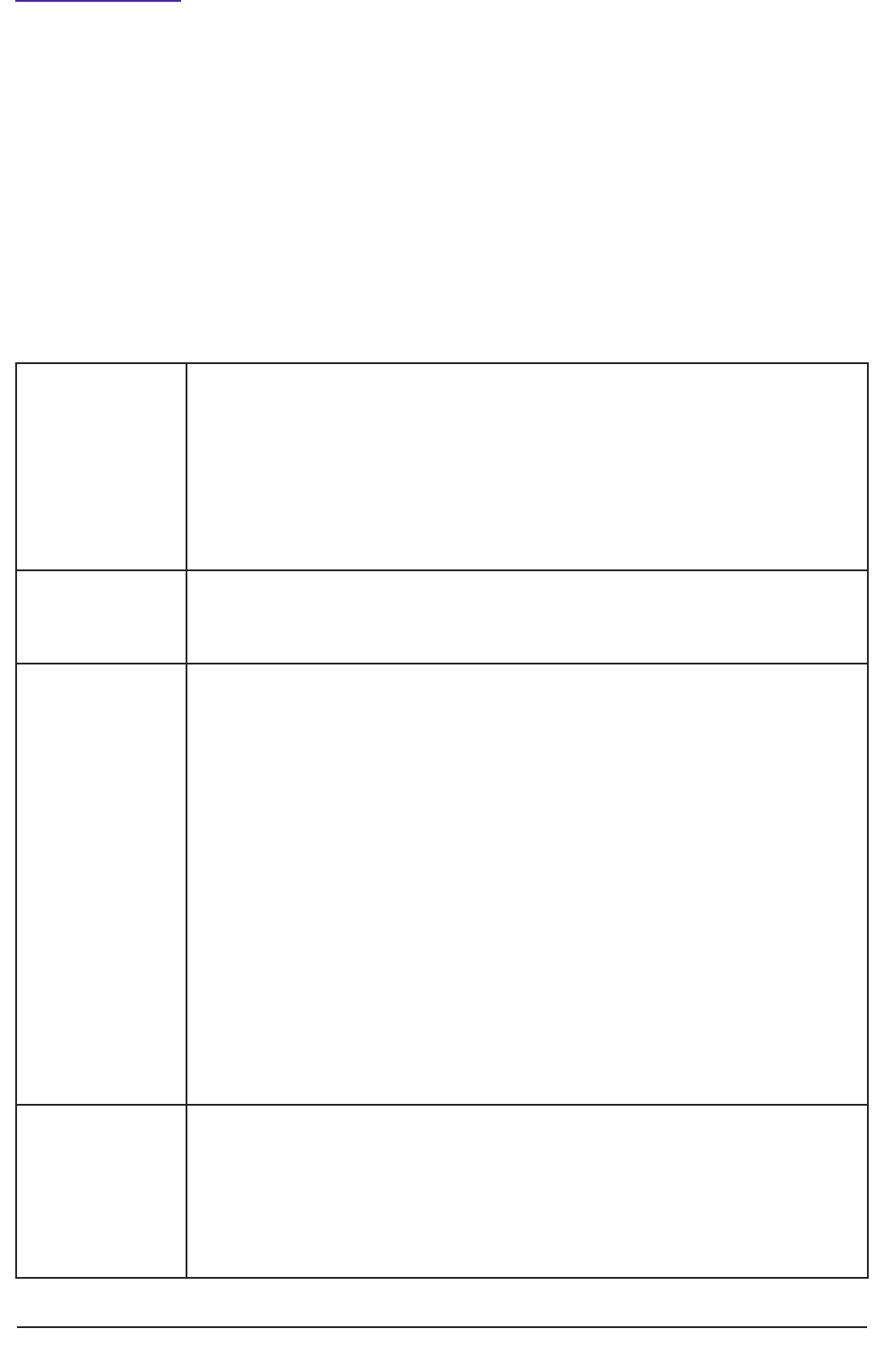
Chief Investigator,
Investigators,
other researchers
• Developing proposals that are scientifically sound and ethical.
• Seeking NHS research ethics committee approval.
• Conducting research to the agreed protocol (or proposal), in accordance
with legal requirements and guidance.
• Ensuring participants’ welfare while in the study.
• Feeding back results of research to participants.
• Assessing capacity to consent.
Research Ethics
Committee
• Providing an independent expert opinion on whether the proposed
research is ethical and respects the dignity, rights, safety and well-being of
participants.
Sponsor • Taking overall responsibility for confirming that everything is ready for the
research to begin, including:
■ putting and keeping in place arrangements for initiation and
management and funding of the study (and, for clinical trials involving
medicines, applying for authorisation and making appropriate
arrangements for investigational medicinal products for the trial);
■ satisfying itself the research protocol, research team and research
environment have passed appropriate scientific quality assurance;
■ satisfying itself the study has ethical approval before it begins;
■ satisfying itself that arrangements will be kept in place for monitoring
and reporting on the research, including prompt reporting of suspected
serious adverse incidents.
■ Ensuring the research complies with the law.
■ Convening a panel of Consultees who can be approached by a
researcher to advise about the involvement of participants in a project.
Main funder • Assessing the scientific quality of the research as proposed.
• Establishing the value for money of the research as proposed.
• Assessing the quality of the research environment in which the research
will be undertaken, and the experience and expertise of the chief
investigator, principal investigator(s) and other key researchers involved.
• Requiring that a sponsor takes on responsibility before the research begins.
Appendix 3
Roles and responsibilities in the research process
The definitions of roles and responsibilities in the research process were outlined in the
Research Governance Framework for Health and Social Care (DH, 2005) and have not been
updated in response to the Mental Capacity Act.
This practice guide proposes a number of additional responsibilities researchers may have
in connection with seeking consent and appraising participant involvement. These new
responsibilities are indicated in italics in Table 2.
Table 2: Additional responsibilities researchers may have when seeking consent and
appraising participant involvement
38 Conducting research with people without the capacity to consent
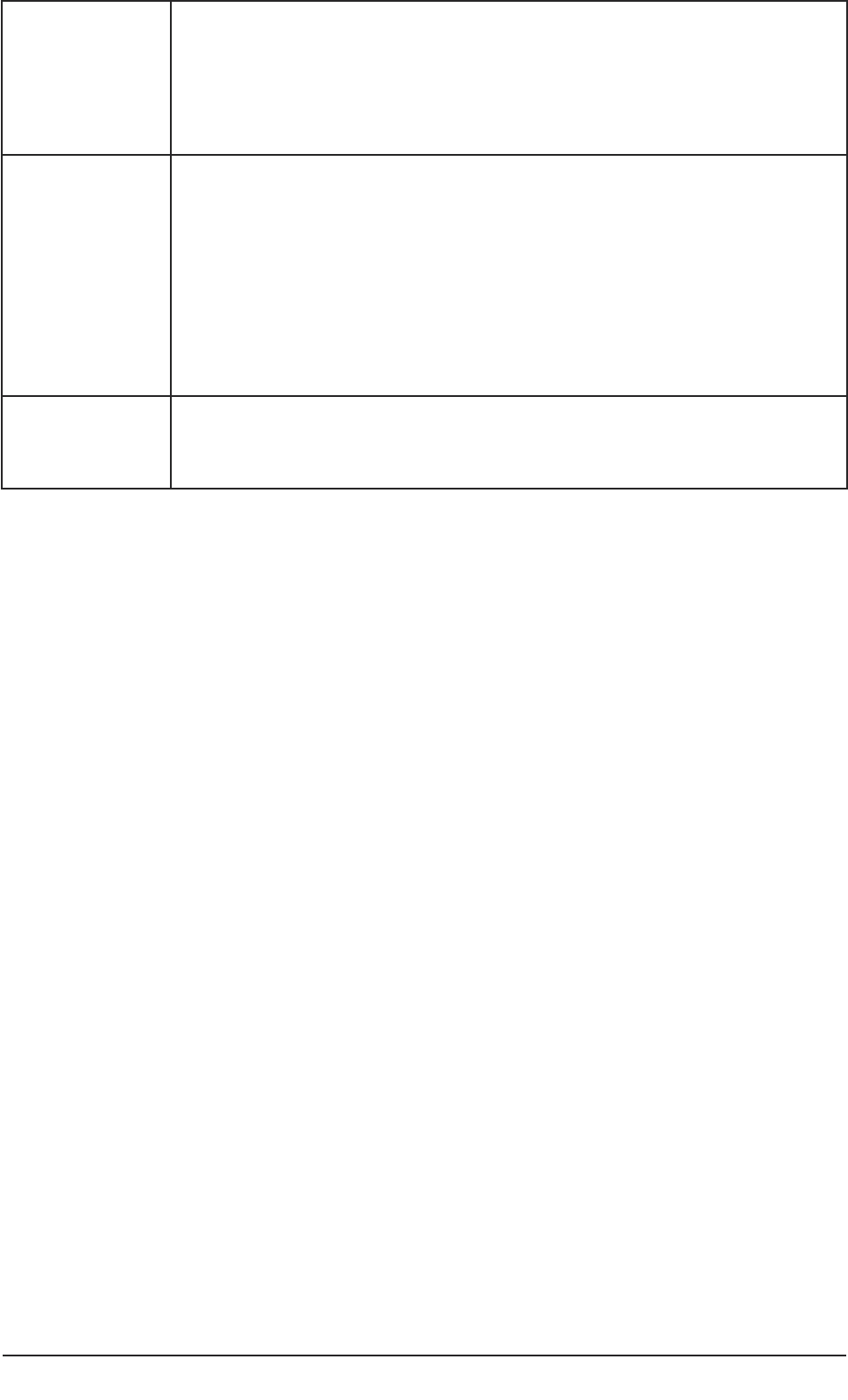
A practical guide for researchers 39
Employing
organisation
• Promoting a quality research culture.
• Ensuring researchers understand and discharge their responsibilities.
• Ensuring the research is properly designed, and that it is well managed,
monitored and reported, as agreed with the sponsor.
• Taking action if misconduct or fraud is suspected.
Care organisation/
responsible care
professional
• Ensuring that research using their patients, service users, carers or staff
meets the standard set out in the research governance framework (drawing
on the ethical review and sponsor).
• Ensuring there is ethical approval for all research for which they have a
duty of care.
• Retaining responsibility for research participants’ care.
• Appointment of Nominated Consultees or a panel of Nominated
Consultees.
Clinical Research
Network
• Providing training for researchers.
• Amongst other responsibilities, appointment of Nominated Consultees.

Appendix 4
Additional questions and ethical implications
I have data about clients obtained as part of my usual practice. Do I have to seek consent
from each person to use the data retrospectively? What if individuals are unlikely to
understand about research? Do I have to submit a proposal to REC to have results
published?
What if the Attorney (LPA for personal welfare) agrees to treatment but doesn’t know
much about research?
Research with carers or families – does the person have to consent if the basis of the
research is about them or a condition the person has?
A carer has raised concerns about actions/activities that participant is expected to
undertake in a project
Does service user or carer-led research have to comply with the same ethical standards as
professionally organised research?
What are the roles and responsibilities in collaborative research?I have concerns that the
MCA (2005) has the potential to stifle important research. For example, case-file studies of
100 per cent samples. This type of research would count as ‘intrusive’ research and require
that the permission of the participants be sought before their records are looked at. This
would prove to be an impossible task and thus this type of research method could
disappear leaving the field open only to anonymised surveys.
There needs to be at least two separate attempts to get consent, possibly separated by
consultation. For example, a person may have capacity or be more amenable on a different
day.
Concern is noted regarding the limited number of ‘appropriate bodies for ethical review’.
This inevitably will lead to increased delays given the number of project proposals that may
need to undergo review. More importantly, concern was raised that the ‘flagged’ RECS may
be ill equipped to deal with research that, whilst coming under the scope of the MCA, is
not ‘medical’ in nature.
Whilst, where strong links exist between a university research ethics committee and a local
NHS REC, the referral of projects for ethics scrutiny under the MCA, may proceed
smoothly and without undue delay for university researchers.
In the absence of such links, however, this requirement is of particular concern to
psychologists who are conducting research in accordance with the Society’s Code of Ethics
and Conduct; and Research Council Research Ethics Frameworks (such as the ESRCs) who
do not interact with the NHS, nor do they need to. Most psychological research is subject
to ethics scrutiny by university RECs – most of whom have standard operating procedures
equivalent to those in the NHS. It may therefore be appropriate for these committees to
continue to review low risk research that falls within the provisions of the MCA subject to a
clearer definition of what constitutes ‘intrusive’ research.
40 Conducting research with people without the capacity to consent

A practical guide for researchers 41
However, as ‘recognised status’ can only be granted by the Secretary of State; and there is
little evidence of a willingness on the part of the NHS to extend this status beyond those
already ‘flagged’, researchers in psychology should be very concerned about this
development. A considerable joint effort would be needed by a number of relevant
organisations (including the Research Councils, the Association of Research Ethics
Committees, and learned societies) to lobby the NHS to change its view otherwise.
The definition of ‘intrusive’ research is so broad that the MCA could potentially impact on
all psychological research. This is a matter of considerable concern for psychology
researchers in light of the comments outlined in the previous section. Clarification of what
is meant by this definition is therefore needed.
Section 11.7 of the Code of Practice states that research involving ‘data that has been
anonymised and cannot be traced back to individuals’ does not require consent (regardless
of the capacity of the participants). However, the exact nature of the data to which this
relates is ambiguous.
It is unclear whether new research that results in the collection of anonymised data would
be excluded from the requirement to obtain consent; or does this just relate to pre-existing
anonymised data sets. If the former, then this would allow for a significant amount of
psychological research to be conducted under the MCA without the need for approval
from a designated REC. If the latter, then again, the concerns outlined above remain.
Clarification should be sought on what exactly is excluded under section 11.7 of the Code
of Practice.
Have their been any evaluation of research undertaken in Scotland following the
implimentation of the Incapacity Scotland Act (2000)?
Appendix 5
Contributors
42 Conducting research with people without the capacity to consent
Nigel Atter The British Psychological Society
Dr Elizabeth Baikie Lothian University Hospitals Trust
Prof Hazel Biggs Lancaster University
Dr Catherine Dooley St Georges Hospital, London
Prof Eric Emerson Lancaster University
Beverley Gibson Lancashire Care Foundation Trust
Dr John Kincey Clinical Psychologist
Prof Glynis Murphy Kent University
Tim Rawcliffe North West Mental Health Research Network
Dr Suzanne Regan Independent social care researcher
Debra Rutter Social Care Institute for Excellence
Dr Bridget Simpson Stroke Research Network
Jane Simpson Lancaster University
Dr Louise Shelley Bangor University
Sue Williams Kent County Council
Moira Winters North West Mental Health Research Network
Dr Ann Wakefield South Manchester REC
Prof Bob Woods Bangor University
Members of Lancashire Care Trust’s Research Governance Service User and Carer Committee

Part 2: Checklists, sample letters and proformae
(Available in MS Word format)
1. National Research Ethics Service – supplementary form for approval under
Sections 30-33 of the Mental Capacity Act.
2. National Research Ethics Service – supplementary forms for approval under
Section 34 of the Mental Capacity Act.
3. Checklist – Capacity to consent to participation in research.
4. Consulting with a Personal Consultee.
a. Checklist for researcher.
b. Sample letter to partner, family member or friend from clinical/care team.
c. Sample Information Sheet.
d. Invitation to act as a Personal Consultee.
e. Personal Consultee declaration form.
5. Consulting with a Nominated Consultee.
a. Checklist for researcher.
b. Sample letter to Nominated Consultee from researcher.
c. Sample Information Sheet.
d. Invitation to act as a Nominated Consultee.
e. Nominated Consultee declaration form.
6. Checklist – appraisal of participant’s involvement in the project.
A practical guide for researchers 43
44 Conducting research with people without the capacity to consent
1 National Research Ethics Service: supplementary form for approval
under Section 30 of the Mental Capacity act 2005
Section 30 of the Mental Capacity Act 2005: Applications to NHS Research Ethics Committees
Supplementary information
REC reference number:
Name of main REC:
Full title of study:
Name of Chief Investigator:
This supplementary form should be completed if applying to a NHS Research Ethics Committee in England or
Wales for approval under section 30 of the Mental Capacity Act 2005.
Please submit the form together with the on-line REC application form (for new research) or the Notice of
Substantial Amendment form (for research already underway with a favourable opinion from a NHS REC), as
applicable. For further guidance, see http://www.nres.npsa.nhs.uk/applicants/help/guidance.htm and the
Mental Capacity Act Code of Practice.
1. What impairing condition(s) will the participants have?
2. Please justify the inclusion of participants unable to consent for themselves. It should be clear
why the research could not be carried out as effectively if confined to adults capable of giving consent.
3. How will the capacity of potential participants to consent to the research be assessed? Who in
the research team will make the assessment and what knowledge of the participant or relevant
training/experience will they have to enable them to undertake it? Please see Chapter 4 of the
MCA Code of Practice for detailed guidance on this issue.
4. Does the research have the potential to benefit participants who are unable to consent for
themselves?
Yes No
If Yes, please indicate the nature of this benefit:
5. Will the research contribute to knowledge of the causes or the treatment or care of persons with
the same impairing condition (or a similar condition)?
Yes No

If Yes, please explain how the research will achieve this:
6. Will the research involve any foreseeable risk or burden for these participants, or interfere in any
way with their freedom of action or privacy?
Yes No
If Yes, please give an assessment below. Highlight any risk, burden, restriction or invasion of privacy
specific to these participants and say what will be done to minimise it:
7. What arrangements will be made to identify and consult persons (“consultees”) able to advise
on the inclusion of each individual participant and on their presumed wishes and feelings?
Please enclose a copy of the written information to be provided to consultees. This should describe their
role under section 32 of the Mental Capacity Act and provide information about the research similar to that
which might be given to participants able to consent for themselves.
8. Is it possible that a participant might need to be treated urgently as part of the research before it
is possible to identify and consult a consultee?
Yes No
If Yes, say whether arrangements will be made instead to seek agreement from a registered medical
practitioner and outline these arrangements. Or, if this is also not feasible, outline how decisions will be
made on the inclusion of participants:
9. What arrangements will be made to consult consultees during the course of the research where
necessary? What burden could this place on consultees?
10. What steps will you take, if appropriate, to provide potential participants who are unable to
consent for themselves with information about the research, and to consider their wishes and
feelings?
A practical guide for researchers 45
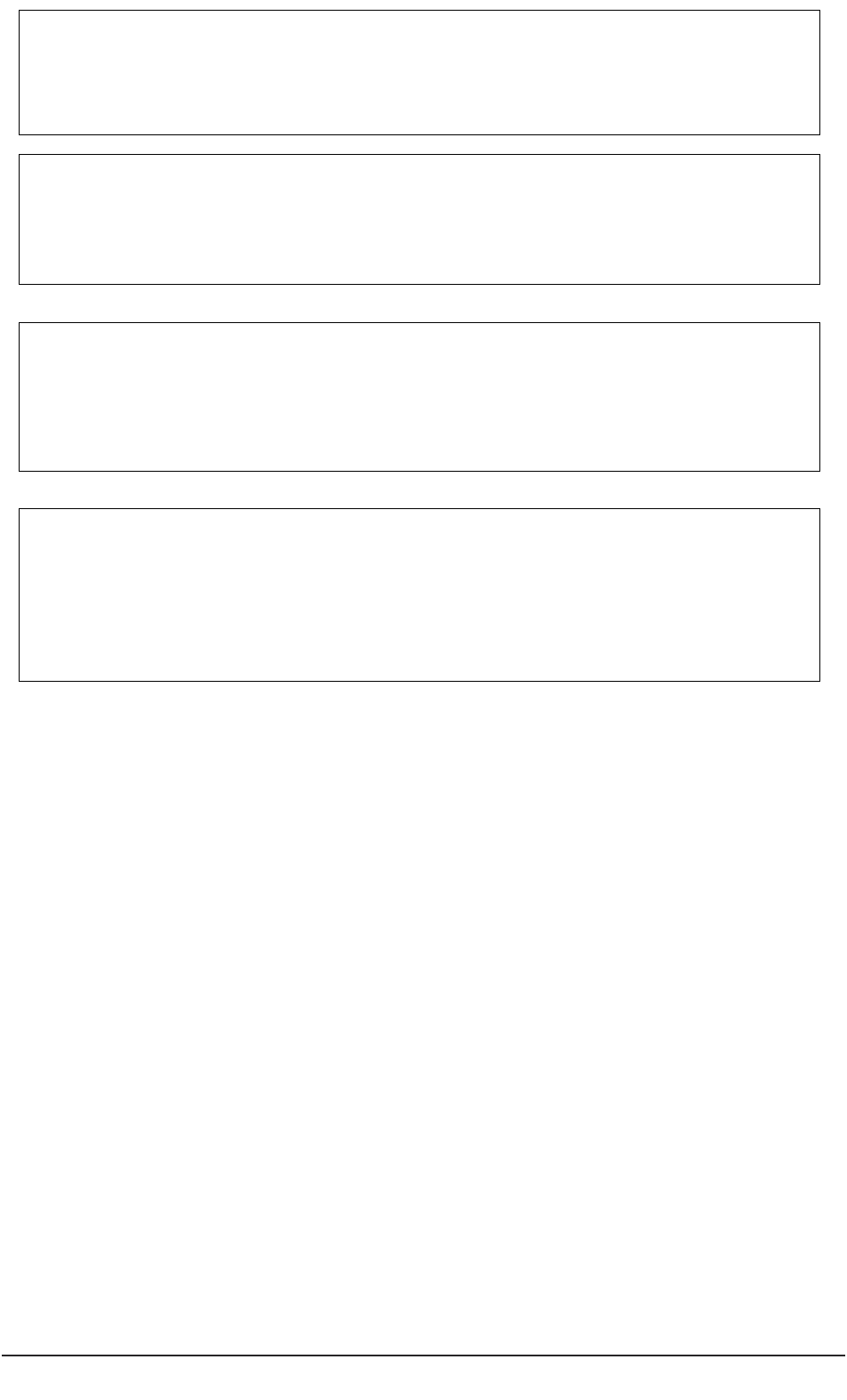
46 Conducting research with people without the capacity to consent
11. Is it possible that the capacity of participants could fluctuate during the research? How would
this be handled?
12. What will be the criteria for withdrawal of participants?
13. Describe what steps will be taken to ensure that nothing is done to which participants appear to
object (unless it is to protect them from harm or minimise pain or discomfort)?
14. Describe what steps will be taken to ensure that nothing is done which is contrary to any
advance decision or statement by the participant?

A practical guide for researchers 47
2 National Research Ethics Service. Supplementary form for approval
under Section 34 of the Mental Capacity Act: loss of capacity during a
project
Section 34 of the Mental Capacity Act 2005: Applications to NHS Research Ethics
Committees
Supplementary information
REC reference number:
Name of main REC:
Full title of study:
Name of Chief Investigator:
This supplementary form should be completed if applying to a NHS Research Ethics Committee in
England or Wales for approval under the Regulations made under section 34 of the Mental
Capacity Act 2005.
Please submit the form together with a Notice of Substantial Amendment form and a copy of the
amended protocol, incorporating procedures for complying with the above Regulations.
For further guidance, see http://www.nres.npsa.nhs.uk/applicants/help/guidance.htm and the
Mental Capacity Act Code of Practice.
1. What steps would you take to determine that a participant no longer had capacity to
consent to the research?
2. Describe the data or material, obtained prior to the loss of capacity by participants,
which you plan to retain and use in this research?
3. What further use do you plan to make of the data or material in this research?
Justify the need for further research on the data or material following loss of capacity.
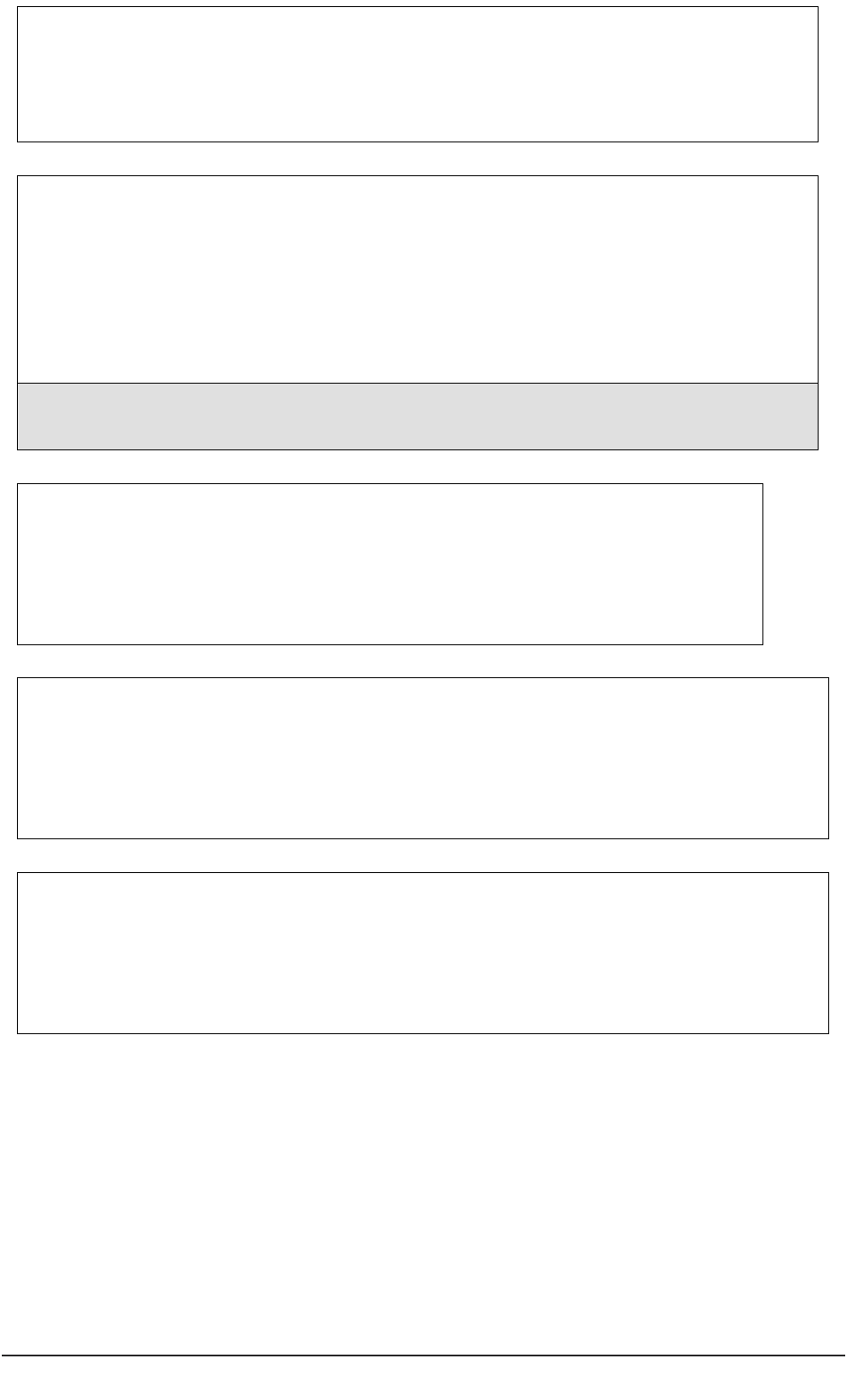
48 Conducting research with people without the capacity to consent
4. What arrangements are in place to ensure the confidentiality of any personal data
relating to participants who have lost capacity?
5. What arrangements will be made to identify and consult persons (“consultees”) able
to advise in each case on whether research should be carried out using the participant’s
data or material and on their presumed wishes and feelings?
Please enclose a copy of the written information to be provided to consultees. This should describe their role
under the Regulations made under section 34 of the Mental Capacity Act and provide information about the
research similar to that which might be given to participants able to consent for themselves.
6. What arrangements will be made to continue to consult consultees during the
course of the research?
7. What will be the criteria for discontinuing all further research using the data or
material?
8. Describe what steps will be taken to ensure that nothing is done which is contrary to
any advance decision or statement by the participant?
A practical guide for researchers 49
3 CHECKLIST for RESEACHERS 1
ASSESSING CAPACITY TO CONSENT TO PARTICIPATION IN RESEARCH
Checklist for researchers to decide whether a prospective
participant has the capacity to consent to their participation
Section A - Enabling capacity:
Have you made every effort to enable a prospective participant to make the
decision themselves to participate or refuse?
Have you used language or methods of communication that the person is
most likely to understand?
Have you given sufficient time for the person to think about the project?
Has the person conferred with others who could help explain the project?
If NO to any item in Section A, return to guidance on ‘enabling
decision-making’.
If YES to all items in Section A continue…
Section B - Diagnostic assessment
Is there evidence to demonstrate impairment of mind or brain?
Is there evidence to demonstrate that this is temporary, fluctuating or
permanent?
Is there evidence to demonstrate that the impairment affects the person’s
ability to decide about their participation in research?
If NO to any item in Section B discuss with Principal Researcher.
If YES to all items in Section B, continue…
Section C - Functional assessment
Does the person understand that they can consent to or refuse to participate
in research?
Does the person understand what the research is about?
Does the person understand and weigh-up the benefits and risks of
agreeing or refusing to take part?
Has the person communicated their decision to you in any way?
If YES to any item in Section C, return to guidance on ‘enabling
decision-making’.
If NO to the first three items in Section C – the person DOES NOT
have the capacity to consent to or to refuse to take part in the
research project.
Checklist completed by:
Date:
Participant code
50 Conducting research with people without the capacity to consent
4 Consulting with a PERSONAL CONSULTEE
4a Checklist for Researcher (Personal Consultee)
Project title:
Sample letter for partner, friend or relative – sent by care
home, care/clinical team
DONE?
Information sheet summary, contact information,
confidentiality statement sent to partner, friend or carer
Partner, friend or relative response form returned
Personal Consultee declaration completed
Participant code
A practical guide for researchers 51
4b Sample letter from clinical/care team to partner, family member or friend.
Sample letter
Service/Clinical Team/Care home headed paper
Address
Telephone
Dear Name
The XXXX service/team/home is collaborating with YYYY from (name)
Trust/Authority/University/organisation in a research project.
The project is called…………………
An important aspect of the research project is that all participants have the choice
about whether to volunteer or to refuse to take part. However some of the
residents/patients may not have the capacity to consent because of a condition/illness
they have that affects how they make some decisions.
You have been approached as you are a partner, relative or friend of a
resident/patient of this service. The researchers would like to discuss with you your
views about whether ……………….may wish to participate in the research.
I attach some information about the project, the names of the researchers and ways
that you can help.
Please have a look at the form and return to (name) at XXX using the stamped-
addressed envelope. If you have any queries, please contact (name) on 11111111 to
discuss.
Thank you for your interest in the project and taking time to read the information.
(Signed)
Manager/consultant

52 Conducting research with people without the capacity to consent
4c Sample Information Sheet
Attached information for Personal Consultees – provided by researchers
What is the project about?
o Title
o Main aims
o Recruitment of participants
o What participants are required to do
o Potential hazards
o Contact names and addresses
o Complaints
o Use headed paper from university/research organisation
We are intending to recruit participants to his project who may not have the capacity to
consent to their participation. This means that they may not be able to judge for
themselves whether they should like to take part or refuse. The project includes such
participants because we are studying about the (xxx) condition/care and treatment of
people having the (xxx) condition. We also consider that it is important for people with
the (xxx) condition to have the chance of taking part in the research project.
The project has been approved by a (named) Research Ethics Committee. We shall
make sure that the project is safe for each participant and does not cause them undue
distress. To help with this, the researchers need information from people who have
known the participant for some time.
Why have I been approached?
As a partner, friend or relative of a (prospective) participant in the study, you will have an
interest in the person’s well-being and welfare. You may have been given a Lasting
Power of Attorney to make personal welfare decisions on their behalf when they can’t.
You may be a deputy appointed by the Court of Protection.
Researcher/s in the project would like to discuss with you whether you think that your
friend or relative would like to take part. As you have known them for some time, you
may be aware of any views they may have about taking part in such a project or whether
they have made an ‘Advance Decision’. If your partner, friend or relative has made an
‘Advance Decision’ this is important as it shows that they have ready made decisions for
themselves. The researchers would like to respect the person’s wishes.

A practical guide for researchers 53
Secondly, if you think that your partner, friend or relative may be interested in taking part
in the project, you may be able to tell us about any possible difficulties they may have.
You also may be able to tell us how they may communicate that they wanted to stop
being involved.
When thinking about the wishes and interests of your partner, relative or friend, it is
important that you should set aside any of your own views about the project.
A ‘personal consultee’ is a partner, friend or relative of a prospective participant, who
provides the researchers with advice. If you would like further information about being a
‘personal consultee’, please contact xxxxx who has experience in this area.
What do I have to do now?
If you think that your partner, friend or relative would be interested in taking part, please
complete the attached form and send this back to XXXX using the stamped-addressed
envelope.
If you think that your friend, partner or relative would be interested but you are not sure
about whether you would like to talk about this with the researcher, then please suggest
who else could be approached.
If you think that your friend, partner or relative would not be interested in taking part,
then it is important that you still complete the form below.
Will information that I give be kept confidential?
Information about yourself (name, address and telephone number) is in records held by
XXX team/care team. XXX care team will contact you, should the researchers wish to
speak with you.
Information that you disclose about your partner, friend or relative concerning their
participation in the research will be held by the researcher. The researcher will not know
your name, address or telephone number. When you meet the researcher, they will talk
with you about confidentiality.

54 Conducting research with people without the capacity to consent
What will happen to the forms when I have completed them?
The forms will be looked at by the researcher. The Care Team will contact you by (date)
to let you know whether or not the researcher would like to speak with you and arrange a
time for a discussion.
If you do not return the form, we shall assume that you do not wish to be contacted
about the project.
How can I find out more about the project?
You can contact (person) on (telephone number) to discuss the project further. The
project is lead by (person) who can be contacted at (place/number).
A practical guide for researchers 55
Invitation to act as a Personal Consultee
Project title
I think that my partner, friend or relative may
NOT like to take part in the project
Signed……………………………….
I think that my partner friend or relative may
be interested in taking part and I would like to
discuss this with the researcher.
I agree to being contacted further about the
project
Signed ………………………………….
I think that my partner, friend or relative may
like to take part in the project – but I do not
wish to be consulted.
I do not agree to being contacted further
about the project
Signed………………………………………..
Thank you for completing the form. Please send in the stamped addressed envelope to
XXX care team/care home/clinical team
Participant code
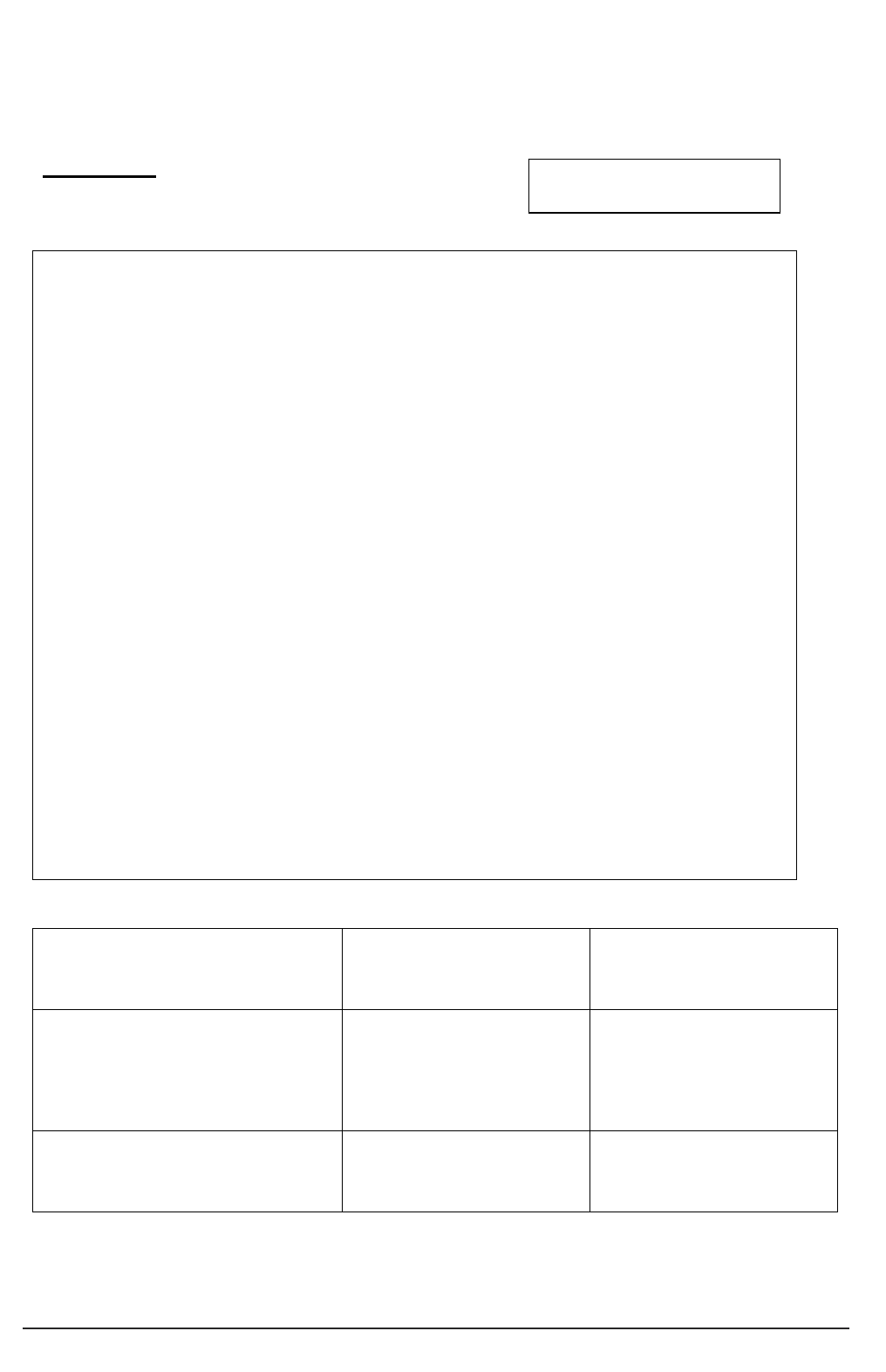
56 Conducting research with people without the capacity to consent
4e Sample Personal Consultee Declaration
Research centre/sponsor headed paper
Personal Consultee declaration
(Version ……… Date…………..)
Project title
1. I confirm that I have read and understood the Information
for Consultees (version … dated….) for the study
Please initial your
confirmation/understanding
below
2. I confirm that I have had time and opportunity to ask
questions about the study or my role as a Personal
Consultee
3. I understand the purpose of the project and what the
participant’s (my partner, friend or relative’s) involvement
would be. In my opinion, they would not object to taking
part in the study
4. I understand that participation in the project is voluntary
and that my partner, friend or relative would be withdrawn
if they do not wish to continue participating and without
giving a reason.
5. I understand that if my partner, friend or relative were
withdrawn form the project, this would not affect in any
way the care or treatment they receive, or affect their
legal rights.
6. I understand………(other features relevant to the project,
such as that my partner, friend, relative’s GP will be
informed about their involvement in the study)
Name of consultee
date signature
Name of person who has discussed
the study and provided me with
information
date signature
Principal Researcher
date signature
When completed – one copy to be retained in care/health records,
one copy for Consultee, one copy for researcher.
Participant code
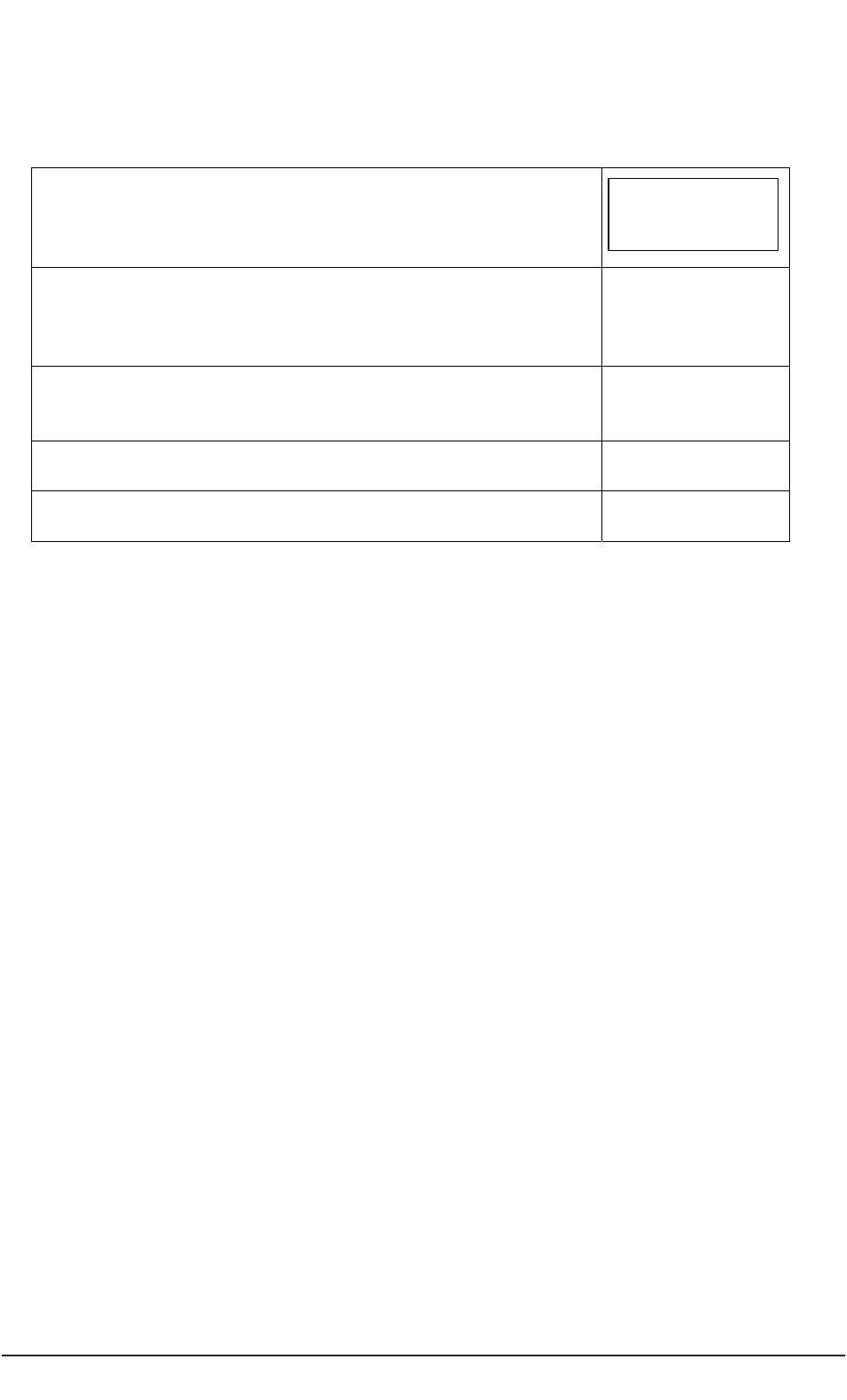
A practical guide for researchers 57
5 Documentation when consulting a NOMINATED CONSULTEE
5a Checklist for Researcher (Nominated Consultee)
Project title:
Sample letter for Nominated Consultee – sent by care
organisation/Trust/Research organisation
Information sheet summary, contact information,
confidentiality statement
Nominated Consultee agreement form returned
Nominated Consultee declaration completed
Participant code
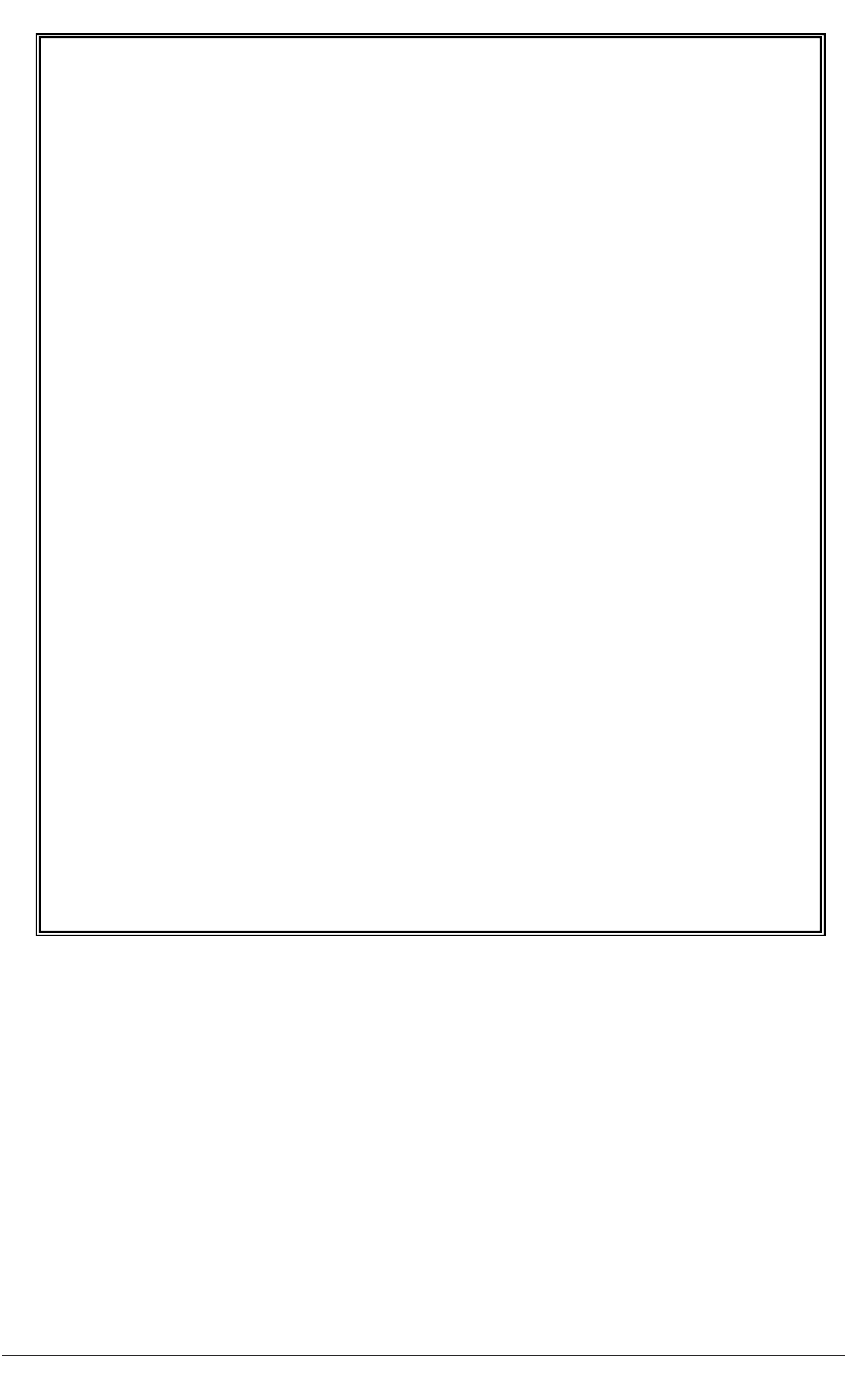
58 Conducting research with people without the capacity to consent
5b Sample letter from researcher to prospective Nominated Consultee
Sample letter
Care organisation/Trust/Research organisation
Address
Telephone
Dear Name
The XXXX service/team/home is collaborating with YYYY from (name)
Trust/Authority/University/organisation in a research project.
The project is called…………………
An important aspect of the research project is that all participants have the choice about
whether to volunteer or to refuse to take part. However some of the residents/patients may not
have the capacity to consent because of a condition/illness they have that affects how they make
some decisions.
You have been approached as you are have been named by the Care
Organisation/Trust/research organisation as someone who can be consulted on such matters.
The researchers would like to discuss with you your views about whether...………………. may
wish to participate in the research project.
I attach some information about the project, who the researchers are and in what ways you can
help.
Please write to me or telephone me on (telephone number) to discuss further. When you have
decided, please return the form in the stamped-addressed envelope.
Thank you
Signed

A practical guide for researchers 59
5c Sample Information Sheet for Nominated Consultee
Research centre/sponsor headed paper
Information for Nominated Consultees
What is the project about?
o Title
o Main aims
o Recruitment of participants
o What participants are required to do
o Potential hazards
o Contact names and addresses
o Complaints
We are intending to recruit participants to his project who may not have the capacity to
consent to their participation. This means that they may not be able to judge for
themselves whether they should take part or refuse. The project includes such
participants because we are studying about the (xxx) condition/care and treatment of
people having the (xxx) condition. We also consider that it is important for people with
the (xxx) condition to have the chance of taking part in the research project.
The project has been approved by a (named) Research Ethics Committee. We shall
make sure that the project is safe for each participant and does not cause them undue
distress. To help with this, the researchers need information from people who have
known the participant for some time or those who have agreed to be consulted on such
matters.
Why have I been approached?
You may be someone who already knows the prospective participant, working with them
as a paid carer or in a professional capacity, such as a doctor or a solicitor. Alternatively,
you may already have been approached by a care organisation, Trust or research
organisation and agreed to act as a Consultee.
If you do know the prospective participant, you may be able to advise us about any
possible difficulties they may have in taking part. You also may be able to tell us how
they may communicate that they wanted to cease being involved with the project.
When thinking about the wishes and interests of the prospective participant, it is
important that you should set aside any of your own views about the project.

60 Conducting research with people without the capacity to consent
If you would like to seek further information about being a ‘nominated consultee’, please
contact xxxxx who has experience in this area.
What do I have to do now?
If you think that the prospective participant would be interested in taking part, please
complete the attached form and send this back to XXXX using the stamped-addressed
envelope.
If you think that the prospective participant would be interested but you are not sure
about whether you would like to talk about this with the researcher, then please suggest
who else could be approached.
If you think that the prospective participant would not be interested in taking part, then it
is important that you still complete the form below.
Will information that I give be kept confidential?
Information about yourself (name, address and telephone number) will be held by the
Care organisation/Trust/Research organisation.
Information that you disclose about the prospective participant will be held by the
researcher. The researcher will not know your name, address or telephone number.
When you meet the researcher, they will talk with you about confidentiality.
What will happen to the form when I have completed it?
The forms will be looked at by the researcher. The Care organisation/Trust/Research
organisation will contact you by (date) to let you know whether or not the researcher
would like to speak with you and arrange a time for a discussion.
If you do not return the form, we shall assume that you do not wish to be contacted
about the project.
How can I find out more about the project?
You can contact (person) on (telephone number) to discuss the project further. The
Principal Researcher is (person) who can be contacted at (place/number).
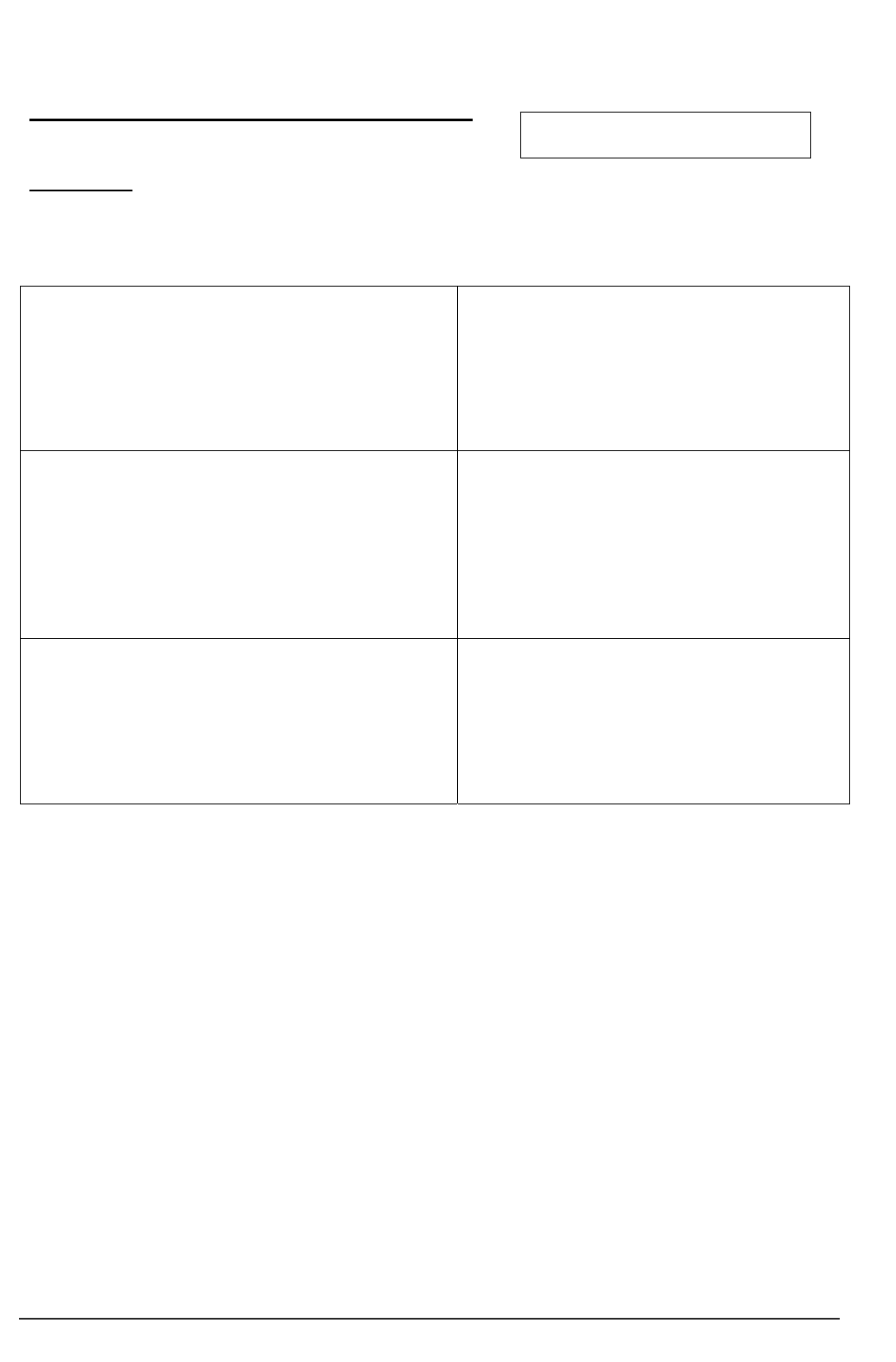
A practical guide for researchers 61
5d Invitation to act as a Nominated Consultee
Research centre/sponsor headed paper
Agreement to act as a Nominated Consultee
Project title
I think that the prospective participant may NOT
like to take part in the project
I agree with this statement
Signed……………………………….
I think that the prospective participant may be
interested in taking part and I would like to discuss
this with the researcher.
I agree to being contacted further about the
project
signed ………………………………….
I think that the prospective participant may like to
take part in the project – but I do not wish to be
consulted.
I do not agree to being contacted further
about the project
Signed………………………………………..
Thank you for completing the form. Please send in the stamped addressed envelope to
XXX Care organisation/Trust/research organisation
Participant code
62 Conducting research with people without the capacity to consent
Research centre/sponsor headed paper
5e Nominated Consultee declaration
(Version ……… Date…………..)
Project title
7.1..I confirm that I have read and understood the Information
for Nominated Consultees (version … dated….)
Please initial your
confirmation/understanding
below
8.2. I confirm that I have had time and opportunity to ask
questions about the study or my role as a Nominated
Consultee
9.3. I understand the purpose of the project and what the
participant’s involvement would be. In my opinion, they
would not object to taking part in the study
10.4.I understand that participation in the project is voluntary
and that the participant would be withdrawn if they do not
wish to continue participating and without giving a reason.
11.5.I understand that if the participant were withdrawn from
the project, this would not affect in any way the care or
treatment they receive, or affect their legal rights.
12.6.I understand………(other features relevant to the
project, such as the participant’s GP being informed about
their involvement in the study)
Name of consultee
date signature
Name of person who has discussed
the study and provided me with
information
date signature
Principal Researcher
date signature
When completed – one copy to be retained in care/health records,
one copy for Consultee, one copy for researcher.
Participant code

A practical guide for researchers 63
6 Appraisal of a participant’s involvement with a project
Checklist for researchers to appraise the inclusion of a SPECIFIC PARTICIPANT
who lacks capacity ( for projects other than Clinical Trials of Medicinal Products)
Indicate yes or
no
Has a functional assessment of capacity (for consent to research) been done?
Is it unlikely that the person would regain capacity to consent?
If YES to above continue…
Consulting with others
Does the person have an Advanced Statement about refusal of treatment?
Has the researcher consulted with a Lasting Power of Attorney for Welfare Decisions
(LPA) or a Deputy appointed by the Court of Protection?
Has the researcher consulted with family or friends?
Has the researcher consulted with a Nominated Consultee?
If YES discuss
with Principal
Researcher
If YES to above, use information gained from consultation with others to
complete the following sections
Is the research about the treatment or care of a person with an impairing condition?
Would undertaking the research be of benefit to the participant?
Is the participant likely to incur any burden by participating?
Does the benefit outweigh the burden of participation?
If NO go to
next section
If YES, continue… if NO, EXCLUDE the participant
Is the research about KNOWLEDGE of causes, treatment or care of an impairing
condition?
Are the risks of taking part negligible?
If YES continue… If NO, Exclude the participant
Is participation likely to be invasive or restrictive?
Is participation likely to interfere with the participant’s freedom or privacy?
If NO, include the participant. If YES, exclude the participant
AGREEMENT
Have the researcher and Principal/Chief Researcher agreed to INCLUDE the
participant?
Checklist completed by: Date completed:
Principal/Chief Researcher Date agreed:
