International Journal of Research Publication and Reviews, Vol 4, no 12, pp 2765-2768 December 2023
International Journal of Research Publication and Reviews
Journal homepage: www.ijrpr.com ISSN 2582-7421
Pharmacovigilance and its Methods: An Recent Review
Atul Sanjay Rathod ¹, Amol Anil Talnikar ²
¹PRMSS Anuradha college of pharmacy, Chikhali, Buldhana.
²Terna Public Charitable Trust college of engineering department of pharmacy Dharashiv (Osmanabad).
A B S T R A C T:
Pharmacovigilance is a scientific branch that plays a vital role in ensuring drug safety and effectiveness. It focuses on detecting adverse reactions to drugs,
particularly those not identified during clinical trials but observed in marketed drugs. The World Health Organization defines pharmacovigilance as "the science
and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems." Various methods, including
passive surveillance, active surveillance, and stimulated reporting, are employed to identify adverse drug reactions.
Keywords: Surveillance, consolidation, Pharmacovigilance, Investigation.
Introduction:
Pharmacovigilance is a study of safety and effectiveness of a drug and different health related products. Pharmacovigilance play an important role in
detection, assignment, understanding and prevention of drug related problems[¹,²,³].Identification of adverse drug reaction (ADR) is important for the
safety of patient who take a drug. Monitoring drug usage thoroughly, including pharmacovigilance inspections, ADR reporting, periodic safety report
collection, and post-authorization safety studies, is essential. Information technology plays a crucial role in advancing the healthcare industry, significantly
enhancing clinical safety practices. Therefore, ensuring the safety, efficacy, and cost-effectiveness of drugs remains highly significant at present. [⁴].An
important and integral part of clinical research is pharmacovigilance[⁵].
Objectives:
1) Enhancing patient care and safety in connection with the utilization of medicines and all medical and paramedical interventions.
2) Enhancing public health and safety related to the utilization of medicines.
3) Identifying complications linked to the use of medicines and communicating them appropriately.
4) Assessing the benefits, efficacy, and risks of medicines while advocating for their safe, rational, and more effective (including cost-effective)
use.
5) Promoting education, comprehension, and clinical training in pharmacovigilance, coupled with its effective communication to healthcare
professionals and the general public.
Scope:
1.Enhance patient care and safety concerning the utilization of medications, as well as all medical and paramedical interventions.
2.Enhance public health and safety regarding the usage of medicines.
3.Play a role in evaluating the benefits, risks, effectiveness, and overall safety of medications, fostering their secure, logical, and optimized (including
cost-effectiveness) application.
4. Foster comprehension, education, and clinical training in pharmacovigilance, ensuring effective communication to the public[⁶].
International Journal of Research Publication and Reviews, Vol 4, no 12, pp 2765-2768 December 2023 2766
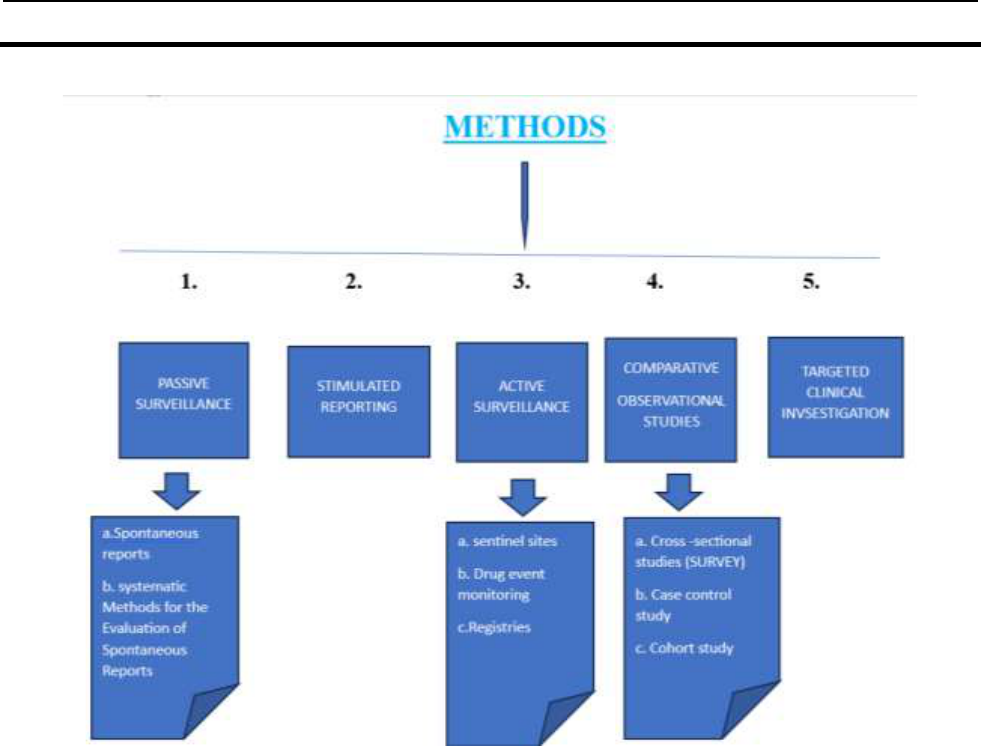
Methods of pharmacovigilance:
1. PASSIVE SURVEILLANCE:
Passive surveillance is when we don't actively do anything to control negative effects, except encourage health professionals and others to report safety
concerns—it relies on their initiative and motivation to report[⁶].
A. Spontaneous reports:
In spontaneous reports healthcare workers or patients can freely tell a company, regulator, or group about adverse reactions to drugs without it being part
of a study or planned data collection.[⁴].
In a spontaneous report, a doctor or patient shares their observations about a drug reaction to a company or organization.
B. Systematic Methods for the Evaluation of Spontaneous Reports:
Recently, individuals have been experimenting with novel approaches to uncover safety concerns from reports. However, many of these methods are still
in the process of development and evaluation to determine their effectiveness in identifying safety signals.[⁴].
2. STIMULATED REPORTS:
Stimulated reports can occur in situations like notifications by authorities, literature reports, or press publications. However, data from stimulated reporting
isn’t suitable for generating accurate incidence rates; it can be used to estimate reporting rates.For specific situations or limited time periods, various
methods encourage health professionals to report on new products. This includes online reporting of adverse events and systematic stimulation of reporting
based on pre-designed methods.[⁷,⁸,⁹].
3. ACTIVE SURVEILLANCE:
a. Sentinel sites:
Reviewing medical records or interviewing patients and/or physicians in selected sentinel sites allows for active surveillance, ensuring comprehensive
and accurate data on reported adverse events. These sites, chosen strategically, can offer insights like data from specific patient subgroups not accessible
through a passive spontaneous reporting system. Additionally, targeted information on drug use, including abuse, can be gathered from these sentinel
sites.
International Journal of Research Publication and Reviews, Vol 4, no 12, pp 2765-2768 December 2023 2767
b. Drug event monitoring :
In active pharmacovigilance surveillance, drug event monitoring involves identifying patients through electronic prescription data or automated health
insurance claims.
c. Registries:
Registries, whether focused on a disease or a specific exposure like drugs, compile lists of patients sharing common characteristics. These registries, such
as those for blood dyscrasias, severe cutaneous reactions, or congenital malformations, gather a comprehensive set of information through standardized
questionnaires in a prospective manner.[⁴].
4. COMPARATIVE OBSERVATIONAL STUDIES:
a. Cross-sectional Study:
A cross-sectional study, mainly used for surveys or ecological analyses, involves collecting data on inhabitants of patients during a specified interval of
time, irrespective of exposure or disease status..
b. Case-Control Study:
In a case-control study, individuals with the disease or event of interest (cases) are matched with controls who do not have the condition. Both cases and
controls are selected from the source population that gave rise to the cases. The selection of controls ensures that their exposure prevalence mirrors that
of the source population.
C. Cohort Study:
A population at risk for the disease (or event) is observed over time to record the
Occurrence of the disease (or event) in a cohort study. Exposure status
Information is available during the follow-up period for each patient. A patient Might be exposed to a medicine at one time during follow-up, but not
exposed at Another time. Meanwhile the population exposure during follow-up is Acknowledged, incidence rates can be calculated Concerning medicine
exposure, Appraisal cohorts of interest are selected on the basis of medicine use and Monitored over time in many cohort studies.[⁶].
5. TARGETED CLINICAL INVSESTIGATION :
When risks appear in preapproval trials, more studies may be needed to understand adverse reactions. Researchers might conduct pharmacodynamic and
pharmacokinetic studies to assess dosing instructions' impact on patient risk.[¹⁰,¹¹,¹²].
Conclusion:
Pharmacovigilance is a crucial aspect of drug safety and effectiveness. It reduces the risks associated with drugs and offers benefits to patients.
Pharmacovigilance detects adverse reactions of marketed drugs that may not have been identified during clinical trials, helping to overcome them. Various
methods, such as active surveillance and passive approaches, are employed to identify adverse drug reactions
Acknowledgement:
This is to acknowledge and express my thanks to all those who directly or Indirectly helped me to complete my work successfully..!!
References:
1. Management science for health USA, chapter 35 Pharmacovigilance by Christopher olsan.
2. Kumar, D.A., Reddenna, L. and Basha, S.A., 2015. Pharmacovigilance programme of India.
3. World health organization collaborating centre for international drug monitoring (2007). the importance of pharmacovigilance available at
http:/www.who_umc.org cited 18 December 2007.
4. A text book of pharmacovigilance by Dr. D. K. Tripathi , Dr. Shiv Shankar Shukla ,Dr. Ravindra kumar Pandey by Nirali Publication shivaji nagar
road, First Edition 2019.
5. WHO. Pharmacovigilance: Ensuring the Safe Use of Medicines. Geneva: WHO; 2004.
6. A text book of pharmacovigilance by Dr. Agnimitra Dinda , Monika Saxena ,thakur publication pvt ltd. Lucknow ,First Edition 2021
7. S. K. Gupta. Textbook of Pharmacovigilance. 2
nd
Edition. Jaypee Brothers Medical Publishers. 2019.
8. Elizabeth B. Andrews, Nicholas Moore. Mann’s Pharmacovigilance. 3
rd
Edition. Wiley Blackwell. 2014.
9. Borton Cobert. Cobert’s Manual of Drug Safety and Pharmacovigilance. 2
nd
Edition. Jones and Bartlett Learning. 2012.

International Journal of Research Publication and Reviews, Vol 4, no 12, pp 2765-2768 December 2023 2768
10. Fine, A. (2013). Introduction to Post -marketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER (PowerPoint slides), Retreived from:
http://www.fda.gov/downloads/AboutFDA/WorkingatFDA/FellowshipInternshipGraduateFacultyPrograms/PharmacyStudentExperientialProgramCDE
R/UCM340626.pdf
11. FDA Science Board (2011). FDA Science Board Subcommittee: Review of the FDA/CDER Pharmacovigilance Program, Retreived from:
http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/ScienceBoardtotheFoodandDrugAdministration /UCM276888.pdf
12. Cobert, Barton L, and Barton L Cobert. Cobert's Manual Of Drug Safety And Pharmacovigilance. Sudbury, Mass.: Jones & Bartlett Learning, 2012.
