
Updated: February 2024 1 | Page
New Jersey Department of Health (NJDOH),
Vaccine Preventable Disease Program
Questions and Answers on Immunization
Regulations Pertaining to Children Attending
School/ Higher Education
Frequently Asked Questions
New/updated questions are highlighted in yellow.
Please note that throughout this document, we will be referring to the
Centers for Disease Control and Prevention as the CDC and the Advisory
Committee on Immunization Practices as the ACIP.

Updated: February 2024 2 | Page
Contents
NJ Immunization Requirements ......................................................................................... 3
Childcare Pre-School Requirements .............................................................................. 4
Influenza Vaccine ................................................................................................................. 4
COVID-19 Vaccine ............................................................................................................. 11
Pneumococcal Conjugate Vaccine................................................................................ 11
Hib Vaccine ........................................................................................................................... 12
Grade Six Requirements ...................................................................................................... 13
Tdap Vaccine ........................................................................................................................ 13
Meningococcal Vaccine ..................................................................................................... 17
Other Vaccines ........................................................................................................................ 19
DTaP Vaccine ....................................................................................................................... 19
Polio Vaccine ........................................................................................................................ 20
Measles, Mumps, Rubella (MMR) Vaccine................................................................. 22
Varicella (Chickenpox) Vaccine ..................................................................................... 23
Hepatitis B Vaccine ............................................................................................................ 23
Other Vaccine Requirement Questions .......................................................................... 25
Minimum Dose Spacing Intervals ................................................................................ 25
Grace Periods and Provisional Admission ................................................................. 27
Exclusions and Exemptions ............................................................................................ 31
Serology Titers .................................................................................................................... 35
Enforcement of Immunization Regulations .................................................................. 40
Higher Education Regulations ........................................................................................... 41
NJ Immunization Information System e.g., ‘Immunization Registry’ (NJIIS) 45
Clinician Resources ................................................................................................................ 47
Updated: February 2024 3 | Page
NJ Immunization Requirements
Q: What are the minimum immunization requirements for
preschool/childcare, school, and college entry in NJ?
A: Please visit nj.gov/health/cd/imm_requirements/. The following charts
can be used to evaluate compliance:
o Childcare/Preschool
o K-12
For more information regarding Immunization of Pupils in School (N.J.A.C.
8:57-4) and Higher Education Immunization (N.J.A.C. 8:57-6), please visit
nj.gov/health/cd/imm_requirements/acode/.
Q: Are NJ Immunization Requirements in accordance with the
CDC/ACIP guidelines?
A: Yes, NJ’s immunization requirements are in accordance with the
guidelines of the American Academy of Pediatrics (AAP), the American
Academy of Family Physicians, and CDC/Advisory Committee on
Immunization Practices (ACIP). However, NJ establishes the minimum
vaccine requirements for child-care centers, preschool, and school entry and
attendance.
For example, NJ requires every child born on or after January 1, 1998, to
receive one dose of a varicella virus containing vaccine. The CDC/ACIP
schedule recommends two doses of varicella vaccine. A child would only be
required to receive one dose of a varicella virus containing vaccine for
attendance in NJ, but two doses would be recommended for optimal
protection. The NJDOH recommends following the CDC/ACIP schedule, as
periodically revised, for optimal protection.
Q: When do children need to show proof of immunization?
A: Immunization records must be presented on the first day of school.
Meningococcal and Tdap vaccines are required for all entering sixth
graders
who are 11 years of age or older. If a child is in sixth grade and under age
11, he/she must receive the vaccines within two weeks of their 11
th
birthday. Updates to immunization records must be provided to the school
as the child receives his/her immunizations.
The only exception to this rule is the 30-day grace period (see the “Grace
Periods and Provisional Admission” section for further information).

Updated: February 2024 4 | Page
Vaccine administration dates should be listed by month, day, and year.
However, if only a month and year are provided then you would need to
assess if the minimum age and dose spacing intervals can be determined.
Example:
1. An immunization record indicating a child born on January 15, 2013,
received his MMR vaccine in January 2014 would not be acceptable
because you cannot determine if the vaccine was administered prior to
the first birthday or if it was within the four-day grace period.
2. An immunization record indicating a child born on January 15, 2013,
received his MMR vaccine in February 2014 would be acceptable
because the vaccine was administered after the first birthday.
Q: Is it required for children to be in compliance with school
immunization requirements at the time of enrollment/registration or
once they are attending school?
A: Although officials may request to see an immunization record at the time
of enrollment, students are required to be in compliance with school
immunization requirements on the first day of school attendance.
The only exception to this rule is the 30-day grace period (see the “Grace
Periods and Provisional Admission” section for further information).
Childcare Pre-School Requirements
Influenza Vaccine
Q: Is the seasonal influenza vaccine a requirement for childcare and
preschool?
A: Yes, it is a requirement as set forth in N.J.A.C. 8:57-4.19 unless the
Commissioner or his or her designee temporarily suspends the requirement
due to limited vaccine availability.
N.J.A.C. 8:57-4.19 stipulates that children six months through 59 months of
age attending any licensed childcare center, or preschool facility on or after
September 1, 2008, shall annually receive at least one dose of influenza
vaccine between September 1 and December 31 of each year.
Q: How many doses of the seasonal influenza vaccine are required
for preschool/childcare attendance?

Updated: February 2024 5 | Page
A: Only one dose of seasonal flu vaccine is required for children 6 through
59 months of age attending childcare/preschool. However, the CDC/ACIP
may recommend certain children receive an additional dose for optimal
protection.
Q: Is the flu vaccine required after January 1 for children coming in
at that time or had not gotten it by December 31 of the prior year?
A: Yes, the flu vaccine is still required for children after January 1. The peak
of flu season typically occurs December through February. The flu season
can also extend until May in some cases. So, getting a flu vaccine even late
in the season is protective.
Q: Why then do the regulations specify a specific time frame?
A: It is important for children to be protected before the peak of flu season
which typically occurs December through February. In addition, health care
providers tend to have a larger supply of the flu vaccine during the earlier
part of the season.
Q: Is there a document to help childcare/preschool directors keep
track of students who need to receive the flu vaccine?
A: NJDOH recognizes the challenges in implementing the flu vaccine
requirement; therefore, the Flu Vaccine Tracking form has been developed
to assist childcare/preschool staff. This form can be used to list the names of
students who have not received the flu vaccine by December 1. Completing
this form will allow childcare/preschool directors to have ample time to send
a reminder notice to parents of students who still need to receive the
vaccine by the December 31 deadline.
For more information, please visit nj.gov/health/cd/imm_requirements/
and access the following documents from the “Resources and Tools for
School and College Health Administrators” section:
• Cover letter—flu vaccine tracking form
•
Flu vaccine tracking form
Q: Is the flu vaccine still required in childcare/preschool, if a child
already was sick with the flu?
A: Yes, the flu vaccine is still required even if the child was previously sick
with the flu. Since there are more than 100 viruses that can cause “cold and
flu” symptoms, a clinical diagnosis may not be the best indicator of what
made the child sick. Only laboratory confirmation would prove the child
actually had the flu. Even if the diagnosis is confirmed as flu, there is more

Updated: February 2024 6 | Page
than one strain of flu virus. Therefore, the child would still be required to
receive the flu vaccine as it may protect him/her from the other common
strains that are circulating. The seasonal flu vaccines protect against three
or four different types of flu viruses (depending on the type of vaccine
received).
Q: When should children be excluded for not satisfying the flu
vaccine requirement?
A: Flu vaccine is a requirement for childcare/preschool attendance for those
who are 6 through 59 months of age. At least one dose of flu vaccine is due
by December 31 of each year. Children who do not have documentation of
receiving the flu vaccine or don’t have a valid medical or religious exemption
by December 31 will need to be excluded from school until the end of flu
season, which is up until March 31 in NJ. Such students may return to school
sooner than March 31 if they…
• Submit documentation of receiving the flu vaccine or submit a
religious/medical exemption.
• They can also “age out” of the requirement. This means that once they
turn five years old (or 60 months), they are no longer subject to the
requirement.
Q: Is the flu vaccine required for children who just turned six
months of age in January since they were not age-eligible to receive
the vaccine during September 1 through December 31 of the prior
year?
A: Yes, once the child becomes age-eligible the flu vaccine is still required
until the end of flu season in NJ (through March 31).
Q: Why should a five-year-old in preschool be exempt from flu
requirement?
A: NJ’s immunization requirements reflect the ACIP recommendations
during the time the rules were written and are not updated as frequently as
ACIP revises or updates their recommendations. Therefore, you may see a
discrepancy in the vaccines or dosing schedule that are recommended
versus those that are required for school attendance in NJ. Although NJ
requires the flu vaccine for those 6 through 59 months of age, the flu
vaccine is recommended for everyone ages 6 months and older per the
ACIP, unless a person has a medical contraindication (reason for not
receiving) for the vaccine.
Q: What if a child enrolls in school in January of the following year,
will he/she be exempt from getting the mandatory flu vaccine?

Updated: February 2024 7 | Page
A: No, the flu vaccine is still required for children in January. Flu season
may not peak until February and can also extend until May in some cases.
Getting a flu vaccine even late in the season is still protective.
Children enrolled after December 31, must show proof of documentation that
they received the flu vaccine prior to entering school.
Q: Is flu vaccine required after March?
A: No, students enrolling in school after March 31 are not required to get
vaccinated; however, flu season may extend until May and therefore getting
a flu vaccine even late in the season is still protective.
Q: Is it acceptable for a child to receive flu vaccine in August when
the regulations specifically state to receive one flu dose during
September 1 through December 31 of each year?
A: Children who get vaccinated with the seasonal flu vaccine prior to
September will be considered compliant and these vaccinations will be
accepted to meet the requirement as long as the vaccine is for the
respective flu season.
Please note most seasonal flu vaccines expire on June 30.
Q: Where can a family go to get the flu vaccine if the pediatrician
does not have any more flu vaccine?
A: The influenza vaccine is recommended for all individuals > six months.
Discuss with your health care provider (HCP) what plans are in place to
ensure an adequate supply of flu vaccine for all eligible clients at the
practice.
If a national flu vaccine shortage has not been declared and your HCP
cannot guarantee an adequate supply of flu vaccine, other alternatives must
be sought by the family. Options include:
1. Asking your child’s HCP to assist with arranging for vaccination
through another health care provider
2. Seeking out another HCP who can administer flu vaccine to children;
3. Checking with your local health department to see if they will
administer flu vaccine to children less than 18 years of age;
4. Contacting your local public health clinic/Federally Qualified Health
Center (FQHC): healthapps.state.nj.us/fhs/cphc/cphcSearch.aspx
5. Checking your local newspaper for flu clinic listings and verifying that
they have flu vaccine available that is appropriate for your child’s age.
As a reminder, some local health departments and FQHCs purchase flu

Updated: February 2024 8 | Page
vaccine through the Vaccine for Children (VFC) Program. A child must
qualify to receive VFC vaccine. For more information about the VFC
Program, please visit cdc.gov/vaccines/programs/vfc/parents/qa-
flyer.html
Q: What if there is a flu vaccine shortage or a flu vaccine
distribution problem?
A: As far as distribution and shortages are concerned, the NJ regulations
state the following: In the event of a national or state vaccine supply
shortage, as determined by the CDC and Commissioner, respectively, the
Commissioner or his or her designee may temporarily suspend the
immunization requirement for the particular immunization affected by the
supply shortage, after provision of notice to the public via print and
electronic news media, NJ Local Information Network and Communications
System (NJ LINCS), electronic posting on the Department's website, etc.
Q: How is the ‘flu season’ defined?
A: Based on trend analysis of influenza seasons in NJ over the past five
years, influenza and/or influenza-like illness (ILI) have been confirmed to be
present during the months of November through to the end of March with
the peak typically occurring December through February. However, cases of
influenza can be seen at any time of the year.
Q: Is there flu vaccine available that does not contain the
preservative thimerosal?
A: Yes. Flu vaccines are currently available in both thimerosal-containing
and thimerosal-free versions. To produce enough flu vaccine for the entire
country, some of it must be put into multi-dose vials. These vials have very
tiny amounts of thimerosal to safeguard against possible contamination of
the vial once it is opened. Children can safely receive flu vaccine that
contains thimerosal. Flu vaccine that does not contain thimerosal is available
in single-dose units. The single-dose units are made without thimerosal as a
preservative because they are intended to be opened and used only once.
Additionally, the live-attenuated version of the vaccine (the nasal spray
vaccine), is produced in single-dose units and does not contain thimerosal.
For more information about vaccine safety and thimerosal, visit:
cdc.gov/vaccinesafety/Concerns/thimerosal/thimerosal_faqs.html
Q: Can individuals with egg allergies now receive the flu vaccine?
A: The ACIP recommends annual flu vaccination for everyone 6 months and
older who do not have any contraindications (medical reason for not

Updated: February 2024 9 | Page
receiving the vaccine). People with egg allergy may get any vaccine (egg-
based or non-egg-based) that is otherwise appropriate for their age and
health status. Previously, it was recommended that people with severe
allergy to egg (those who have had any symptom other than hives with egg
exposure) be vaccinated in an inpatient or outpatient medical setting.
Beginning with the 2023-2024 season, additional safety measures are no
longer recommended for flu vaccination of people with an egg allergy
beyond those recommended for receipt of any vaccine, regardless of the
severity of previous reaction to egg. All vaccines should be given in settings
where allergic reactions can be recognized and treated quickly.
For more information about the flu, please visit cdc.gov/flu/index.htm.
Q: Will NJ accept an allergy to eggs as a valid medical exemption for
receiving the flu vaccine?
A: The NJDOH will only accept a medical exemption stating that the vaccine
is contraindicated due to a previous severe allergic reaction (e.g.,
anaphylaxis) to influenza vaccine. A previous severe allergic reaction to flu
vaccine, regardless of the component suspected of being responsible for the
reaction, is a contraindication to future receipt of the vaccine. A medical
exemption simply stating a child cannot receive flu vaccine because of an
egg allergy will no longer be accepted unless it is an anaphylactic reaction.
Q: What will be included in the current flu vaccine?
A: For more information about the current flu season, please visit
cdc.gov/flu/index.htm.
Q: What types of flu vaccines are available?
A: The ACIP recommends everyone six months of age and older receive the
flu vaccine as the single best way to prevent seasonal flu.
The "flu shot" is an inactivated vaccine (containing killed virus) that is given
with a needle. It can be given in the muscle or just under the skin. The flu
shot that is given in the muscle is approved for use in people older than 6
months, including healthy people and people with chronic medical
conditions. Most flu shots are given with a needle. One flu vaccine also can
be given with a jet injector which uses a high-pressure, narrow stream of
fluid to penetrate the skin instead of a hypodermic needle. There is also an
intradermal flu vaccine, which is injected into the skin instead of the muscle
and uses a much smaller needle than the regular flu shot.
There is also a nasal spray vaccine which is approved for use in non-
pregnant individuals, 2 through 49 years old.
Updated: February 2024 10 | Page
For detailed information about the different types of flu vaccine, please visit
cdc.gov/flu/prevent/different-flu-vaccines.htm.
Q: Why do I need to receive a flu vaccine every year?
A: A flu vaccine is needed every year because flu viruses are constantly
changing. It’s not unusual for new flu viruses to appear each year. The flu
vaccine is formulated each year to keep up with the flu viruses as they
change.
In addition, multiple studies conducted over different seasons and across
vaccine types and influenza virus subtypes have shown that the body’s
immunity to influenza viruses (acquired either through natural infection or
vaccination) declines over time. Getting vaccinated each year provides the
best protection against influenza throughout flu season.
Q: What is the difference between flu and COVID-19?
A: Influenza (flu) and COVID-19 are both contagious (easily spread)
respiratory illnesses, but they are caused by different viruses. COVID-19 is
caused by infection with a coronavirus first identified in 2019, and flu is
caused by infection with influenza viruses.
Compared to flu, COVID-19 can cause more serious illnesses in some
people. COVID-19 can also take longer before people show symptoms and
people can be contagious for longer. More information about differences
between flu and COVID-19 is available at cdc.gov/flu/symptoms/flu-vs-
covid19.htm.
Q: Will getting the flu vaccine protect me against COVID-19?
A: No. Influenza viruses and coronaviruses are different. Getting a flu
vaccine will not protect against COVID-19; however, the vaccine can reduce
flu illnesses, hospitalizations, and can help to conserve potentially scarce
health care resources during the pandemic. It’s likely that flu viruses and the
virus that causes COVID-19 will both be spreading this fall and winter,
making it more important than ever to get a flu vaccine! It is the best way to
protect yourself and others – especially those who are particularly vulnerable
to both COVID-19 and influenza such as older adults and those with chronic
health conditions.
You can get the flu and COVID-19 vaccine, including the booster dose, at the
same time. For more information about COVID-19 visit
state.nj.us/health/cd/topics/covid2019_vaccination.shtml.
Updated: February 2024 11 | Page
COVID-19 Vaccine
Q: Is COVID-19 vaccine required for school attendance in New
Jersey?
A: At this time, COVID-19 vaccination is not a requirement for school
attendance in New Jersey. However, NJDOH strongly recommends that
everyone should be up to date with age-appropriate vaccinations, per CDC’s
Advisory Committee on Immunization Practices recommendations.
Individuals and families should discuss their concerns with their health care
providers.
Q: What is the benefit of adding COVID-19 to the vaccines available
through the VFC program?
A: VFC is a federally funded and state-operated program that provides
vaccines at no cost to children who might otherwise not be vaccinated
because of inability to pay. Providing COVID-19 vaccines through the VFC
Program ensures equitable access to the vaccines as we transition COVID-19
vaccines to the commercial market. For more information about the federal
VFC Program, visit cdc.gov/vaccines/programs/vfc/parents/index.html.
Pneumococcal Conjugate Vaccine
Q: According to the regulations, your pneumococcal conjugate
vaccine (PCV) requirements of one-two doses (depending on age)
does not provide sufficient protection from the disease with the
current available formulation. Can you explain this?
A: Our regulations reflect the minimum requirements for vaccines needed to
attend school in NJ. They do not, however, comprise the full immunization
series recommended by the CDC. It is the state's intention that parents will
seek to meet their vaccination requirements for school and then begin a
dialogue with their HCP who would educate them about the importance of
completing the full vaccination series to achieve full protection from vaccine
preventable diseases and set up subsequent appointments with the intention
of giving them the age-appropriate vaccines at the next visit.
(This answer also applies to the Haemophilus influenzae type b (Hib) vaccine
as well).

Updated: February 2024 12 | Page
Q: If a child entered pre-school/childcare with four doses of PCV
vaccine administered before 12 months of age, does this child need
an additional dose?
A: Yes, even though PCV is a four-dose series, children are still required by
NJ Regulations to receive one dose after twelve months of age.
(This answer also applies to the Haemophilus influenzae type b (Hib) vaccine
as well).
Q: If a child did not attend childcare, preschool, or pre-
kindergarten, is he/she required to receive a dose of PCV before
entering kindergarten?
A: If a child is at least five years old, he/she is not required to receive PCV
prior to entry into kindergarten. NJ does not require PCV after the age of 59
months. (This answer also applies to the Haemophilus influenzae type b
(Hib) vaccine as well).
Please review the CDC Recommended Childhood Immunization Schedule to
view the guidance for child who have high-risk conditions.
Hib Vaccine
Q: According to the regulations, the Haemophilus influenzae type b
(Hib) conjugate vaccine requirements of one-two doses (depending
on age) does not provide sufficient protection from the disease with
the current available formulation. Can you explain this?
A: NJDOH regulations reflect the minimum requirements for vaccines
needed to attend school in NJ. They do not however, comprise the full
immunization series recommended by the CDC. It is the state's intention
that parents will seek to meet their vaccination requirements for school and
then begin a dialogue with their HCP who would educate them about the
importance of completing the full vaccination series to achieve full protection
from vaccine preventable diseases and set up subsequent appointments with
the intention of giving them the age-appropriate vaccines at the next visit.
(This answer also applies to the PCV as well).
Q: If a child entered pre-school/childcare with four doses of Hib
vaccine administered before 12 months of age, does this child need
an additional dose?

Updated: February 2024 13 | Page
A: Yes, even though Hib is a three-four dose series (depending on brand of
vaccine), children are still required by NJ Regulations to receive one dose
after twelve months of age.
(This answer also applies to the PCV as well).
Q: If a child did not attend childcare, preschool, or pre-
kindergarten, is he/she required to receive a dose of Hib before
entering kindergarten?
A: If a child is at least five years old, he/she is not required to receive Hib
prior to entry into kindergarten. NJ does not require Hib after the age of 59
months.
(This answer also applies to the PCV as well).
Grade Six Requirements
Tdap Vaccine
Q: Can you explain the different types of diphtheria-tetanus-and
pertussis-containing vaccines (DTaP, DT, Td, and Tdap)?
A: Vaccines used to protect against diphtheria and tetanus (i.e., DT and Td)
sometimes also include protection against whooping cough or pertussis (i.e.,
DTaP and Tdap). Babies and children younger than 7 years old receive DTaP
or DT, while older children and adults receive Tdap and Td.
Some people get confused between DTaP and Tdap and others get confused
between DT and Td. Here's a hint to help you remember. The pediatric
formulations usually have 3–5 times as much of the diphtheria component
than what is in the adult formulation. This is indicated by an upper-case "D"
for the pediatric formulation (i.e., DTaP, DT) and a lower case "d" for the
adult formulation (Tdap, Td). The amount of tetanus toxoid in each of the
products is equivalent, so it remains an upper-case "T.".
Q: Some students did not complete their primary series for
diphtheria-,tetanus-, and pertussis-containing vaccines. Is there any
guidance for getting these students caught up?
A: Yes, the CDC has a vaccine catch-up schedules accessible at
https://www.cdc.gov/vaccines/schedules/hcp/imz/catchup.html
On this same page, you will find detailed guidance specific to
DTaP/DT/Td/Tdap catch-up by age groups 4 months through 6 years, 7
through 9 years, and 10 through 18 years.

Updated: February 2024 14 | Page
Q: What if a child received DTaP or Tdap at the wrong age
indication?
A: Please follow the guidance below:
• Tdap given to a child younger than age 7 years as either dose 1, 2, or
3, is not valid. Repeat with DTaP as soon as feasible.
• Tdap given to a child younger than age 7 years as either dose 4 or 5
can be counted as valid for DTaP dose 4 or 5.
• Tdap or DTaP given to a fully vaccinated child age 7–9 years: the child
should receive the routine adolescent Tdap dose at age 11–12 years.
• Tdap or DTaP given to a fully vaccinated child age 10 years: count this
dose as the routine adolescent Tdap dose recommended at age 11–12
years.
• DTaP given to an undervaccinated child age 7–9 years: count this dose
as a Tdap dose of the catch-up series. The child should receive the
routine adolescent booster dose of Tdap at age 11–12 years.
• DTaP given to an undervaccinated child age 10 years: count this dose
as the routine adolescent Tdap dose recommended at age 11–12
years.
• DTaP given to a person age 11 years or older: count this dose as a
routine Tdap dose.
Q: Some sixth graders are not 11 years old. I’m guessing that a
10-year-old would not have to receive Tdap until age 11 to meet the
requirement for school attendance, is that correct?
A: Yes, a 10-year-old would not be required to receive the Tdap vaccine
until 11 years of age and in grade six or higher per NJ’s immunization
regulations. The Department recommends the dose be received within two
weeks of the 11
th
birthday.
Although a Tdap given at age 10 is not required, it can count towards NJ’s
immunization requirement for sixth grade and higher. In accordance with
ACIP recent guidelines, a dose of Tdap is recommended to be given at 10
years and older. Tdap given before age 10 (7-9 years) would require
revaccination (see question below).
Q: lf a child received a dose of Tdap before the 10
th
birthday, can
this be counted towards the sixth-grade requirement?
A: Beginning the 2020-2021 school year, children who receive a Tdap
before age 10 would need to receive an additional dose to meet NJ’s
immunization requirements for sixth grade and higher and as long as five
Updated: February 2024 15 | Page
years have elapsed from the last tetanus-and diphtheria-containing dose
(see question below). According to the current ACIP recommendation,
doses of Tdap should be given on or after the 10
th
birthday to count towards
the adolescent dose routinely given between ages 11-12.
Q: ACIP no longer recommends a minimum interval between Td and
Tdap, but NJ still allows for the five-year interval. How does this
impact NJ’s school immunization requirements?
A: ACIP recommendations change more frequently than NJ rules are
updated; therefore, you may see an inconsistency in rule language versus
the recommendations. According to NJ’s immunization regulations, a student
will not be required to receive an additional dose of Tdap unless five years
have elapsed from the last tetanus-and diphtheria-containing dose. The
school will need to flag this student to comply once they are eligible to
receive the vaccine (five years have elapsed). See example below:
Example: Child is up-to-date with the DTP/DTaP/Td/Tdap primary
series, but the last Tdap dose was given at age nine. The child is now
10 and will need to receive an additional dose of Tdap to meet NJ’s
immunization requirements for sixth grade and higher. According to
ACIP, this Tdap can be given now, but NJ rules allows a five-year
minimum interval. Therefore, this child will be required to receive the
Tdap dose at age 14 (five years later).
Q: According to the recent ACIP recommendations, Tdap may be
administered in any situations where Td was previously
recommended. Does this mean a child can receive three Tdap at ages
seven and older to complete the primary series? Would this would
mean an additional dose (fourth dose) would be required if they
were all administered before the 10
th
birthday?
A: Yes, technically a child could receive three Tdap at age seven and older.
This child would be required to receive another dose of Tdap (fourth dose) if
the previous doses were before the 10
th
birthday. NJ immunization rules
state Tdap must be given no earlier than age 10 to count towards the sixth-
grade requirement for school attendance.
Please note, according to NJ’s current immunization regulations, a student
will not be required to receive an additional dose of Tdap unless five years
have elapsed from the last tetanus-and diphtheria-containing dose. The
school will need to flag this student to comply once they are eligible to
receive the vaccine (five years have elapsed). Please see the question above
for more information.

Updated: February 2024 16 | Page
Q: If a student was inadvertently overlooked for the sixth grade
Tdap requirement, would he/she still need to meet this requirement
in the higher-grade levels?
A: Yes, all children born after January 1, 1997, attending or transferring
into a NJ school at grade six or higher-grade level from another state or
country are subject to the Tdap requirement, provided at least five years
have elapsed from the last documented tetanus-and diphtheria-containing
dose.
Please note, the ACIP recommends that Tdap vaccine can be given earlier
regardless of the interval since the last Td. However, NJ will not require this
dose until five years have elapsed.
Q: NJ’s regulations for Tdap states that a dose is required for
students entering or attending grade six, or a comparable age level
special education program with an unassigned grade. What if a child
is 11 years old, but has the mental abilities of a five-year-old, would
he still need to receive the vaccine for Tdap?
A: Yes, the child would still need to follow NJ’s immunization requirements
and receive one dose of Tdap vaccine. The vaccine recommendations refer to
the age-appropriate grade for the child’s biological age, and not the child’s
mental capacity.
(This answer also applies to all NJ Immunization Requirements).
Q: If a child is medically contraindicated from receiving pertussis
vaccine, would receiving the Td vaccination suffice for the sixth
grade Tdap requirement?
A: The NJ immunization requirement is for all sixth graders to receive the
Tdap vaccine. The purpose of this requirement is to provide protection to
this age cohort whose immunity to pertussis wanes from their last DTaP
vaccination at four-six years of age. If a child cannot receive the pertussis
component, then they cannot receive Tdap and therefore would need to
provide a medical exemption from their health care provider.
In this circumstance, the Td vaccine is not a required vaccine for sixth grade
entry as long as the child is up-to-date with their diphtheria-, and tetanus-
containing vaccines.
See CDC’s catch-up schedule for guidance,
cdc.gov/vaccines/schedules/hcp/imz/catchup.html.

Updated: February 2024 17 | Page
The catch-up guidance job aids on this webpage can help you to access the
doses recommended based on age and previous vaccination history.
Meningococcal Vaccine
Q: There are different vaccines for meningococcal disease. Can you
please clarify the difference between these vaccines?
A: There are three types of meningococcal vaccines for preteens and teens:
• Meningococcal conjugate vaccines (MenACWY)
• Serogroup B meningococcal vaccines (MenB)
• Pentavalent meningococcal vaccine (MenABCWY)
NJDOH requires that children in grade 6 or higher be immunized against the
four serogroups (A, C, W, Y) that are present in the meningococcal-
containing vaccines licensed for use in the United States.
Q: Is the serogroup B meningococcal vaccine (MenB)required for
school attendance
A: No. The serogroup B meningococcal vaccine is not required for secondary
school attendance. The NJDOH requires vaccines that are recommended for
general use by the ACIP.
Serogroup B vaccines are recommended for people 10 and older who are
identified as being at an increased risk for the disease.
ACIP recommends that a MenB series may be administered to people 16
through 23 years of age with a preferred age of vaccination of 16 through 18
years. This recommendation allows for shared clinical decision-making
between the provider and the student based on the risk and benefit for the
individual patients. If you get this vaccine, you still must get the vaccine that
covers serogroups A, C, W, and Y in order to meet the requirement for sixth
grade or higher. For more information, visit
cdc.gov/vaccines/vpd/mening/public/adolescent-vaccine.html.
Q: Should schools provide information about serogroup B disease
and the serogroup B vaccine to students?
A: There are currently statutes and regulations requiring distribution of
meningococcal educational materials.
Summary of N.J.S.A. 18A:40-21.2 (P.L.2006, c.64):
The Commissioner of Health, in consultation with the Commissioner of
Education, shall develop an educational fact sheet concerning meningococcal
meningitis for distribution to parents or guardians of students grades 6
through 12. These fact sheets have been distributed in a manner prescribed
by the Commissioner of Education since 2007. This is required for public

Updated: February 2024 18 | Page
schools and voluntary for private schools. The VPDP has prepared a
meningococcal educational brochure titled, “Meningococcal Disease: Are You
Protected?”. It is available in English and Spanish at the following website,
nj.gov/health/cd/topics/meningo.shtml.
Q: Some sixth graders will not be 11 years old. I’m guessing that a
10-year-old would not have to be in compliance with the sixth-grade
meningococcal vaccine requirement until he or she reaches 11, is
that correct?
A: Yes, a 10-year-old entering sixth grade will not be required to receive
the meningococcal-containing vaccine until they turn 11 years of age.
However, in accordance with ACIP recommendations, meningococcal vaccine
(MenACWY) given at age 10 or older would be acceptable and meet NJ’s
immunization requirements for school attendance. (See the question
below for further information).
Q: A child received a meningococcal vaccine prior to 11 years of
age. Would this satisfy NJ’s Immunization requirement?
A: When meningococcal vaccine was licensed in January 2005, data were
lacking on long-term efficacy and the need for additional vaccination.
Therefore, NJDOH previously accepted doses given prior to 11 years of age
without the need for revaccination. Since that time, studies have indicated
that antibody levels decline. ACIP now recommends any meningococcal
vaccination given prior to the tenth birthday does NOT count toward
routinely recommended doses (ages 11 and older). Beginning the 2012-
2013 school year, children who received the vaccine prior to the tenth
birthday will need to be revaccinated for NJ school attendance.
However, there are exceptions to this rule. Meningococcal conjugate vaccine
is recommended for certain children ages 2 months through 10 years.
Students who travel to countries where meningococcal disease is endemic,
have certain medical conditions such as complement component deficiencies
and functional or anatomic asplenia (including sickle cell disease), or who
are present during a meningococcal disease outbreak may have previously
received meningococcal vaccine. These children may need to receive booster
doses of vaccine and should consult with their physician to determine the
appropriate vaccination schedule. According to the ACIP, eight weeks is the
minimum interval between doses of meningococcal conjugate vaccine;
however, a health care provider may determine the most appropriate
interval based on his/her clinical assessment. Such students will satisfy
the meningococcal vaccine requirement by submitting a medical
exemption written by a health care provider. Please see the following
link for detailed information about those at high risk, immunize.org/wp-
content/uploads/catg.d/p2018.pdf.

Updated: February 2024 19 | Page
Q: If a student was inadvertently overlooked for the sixth-grade
meningococcal requirement, would he/she still need to meet this
requirement in the higher-grade levels?
A: Yes, all children born after January 1, 1997, attending or transferring
into a NJ school at grade six or higher-grade level from another state or
country are subject to the meningococcal vaccine requirement.
(This answer also applies to the Tdap vaccine requirement).
Q: I heard the CDC/ACIP recommends a booster dose of MenACWY.
Will the booster dose be required for attendance at a NJ secondary
school (grades 6 through 12)?
A: A booster dose of MenACWY is not required for attendance or entry into a
NJ secondary school but following the CDC/ACIP recommendations would be
recommended for optimal protection. Additionally, a dose of MenACWY
between the ages of 16-18 will be required for students enrolling into a NJ
institution of higher education. (See Higher Ed section for more
information)
Q: A child transferred to a NJ school from out of the country. In the
child’s country, he received a vaccine for meningococcal disease, but
the vaccine did not protect from all the types present in the US
vaccine. Does the child need to be revaccinated with a
meningococcal vaccine licensed in the US to meet NJ immunization
requirements?
A: NJDOH is requiring that children be immunized against the four
serogroups (A, C, W, Y) that are present in the meningococcal-containing
vaccines licensed for use in the United States. If any vaccines administered
in foreign countries do not match the strains in US licensed vaccines, these
vaccinations will not be accepted, and the child will require revaccination to
achieve optimal protection.
Other Vaccines
DTaP Vaccine
Q: How many doses of DTaP are required for school entry in NJ?
A: A child will need four-five doses of DTaP. The following two scenarios
are acceptable:
• A total of four doses of a DTaP-containing vaccine with one of these four
doses administered on or after the child’s fourth birthday.

Updated: February 2024 20 | Page
OR
• A total of any five doses of a DTaP-containing vaccine
As a clarification to the DTaP requirements, a child needs four-five doses of
DTaP; however, it is dependent on when the child enters school. Please
review the following examples:
Children who are 18 months and older will need four doses if
attending/entering childcare/preschool. The requirement to receive the
fourth birthday booster dose (fifth dose) will not apply until the child
attends Kindergarten. Please note all other children must be age-
appropriately vaccinated for childcare/preschool entry.
Children who are first entering a preschool program at four years of age or
older will also need four doses prior to entry. If one of these four doses was
given on or after the fourth birthday, this child will NOT need an additional
dose for Kindergarten.
Persons aged seven years and older who are not fully immunized with DTaP
vaccine should use the CDC catch-up schedule to receive or have a history of
receiving at least three doses of DTaP, Td, and/or Tdap. Tdap given at ages
10 and older can count towards the sixth-grade school requirement. CDC
schedules and catch-up guidance is available at
cdc.gov/vaccines/schedules/hcp/imz/catchup.html
Polio Vaccine
Q: How many doses of polio are required for school entry in NJ?
A: Students will need three-four doses of a polio-containing vaccine
depending upon the age of school entry. The following two scenarios are
acceptable:
• A total of three doses of a polio vaccine with one of these three
doses administered on or after the child’s fourth birthday.
OR
• A total of any four doses of polio-containing vaccine
As a clarification to the Polio requirements, a child needs three-four doses of
Polio, however it is dependent on when the child enters school. Please
review the following examples:
Children who are 18 months and older will need three doses if
attending/entering childcare/preschool. The requirement to receive the

Updated: February 2024 21 | Page
fourth birthday booster dose (fourth dose) will not apply until the child
attends Kindergarten. Please note all other children must be age-
appropriately vaccinated for childcare/preschool entry.
Children who are first starting a preschool program at four years of age will
also need three doses prior to entry. If one of these three doses was given
on or after the fourth birthday, this child will NOT need an additional dose
for Kindergarten.
Children seven years of age and older attending school must have a
minimum of three doses of polio vaccine. If these children do not have
documentation of receiving at least three doses, use the
CDC catch-up schedule available at
cdc.gov/vaccines/schedules/hcp/imz/catchup.html. If you scroll
down this page, you will also see a detailed guidance
specific to Polio vaccine catch-up.
Q: I have a seven-year-old student who just received his third dose
of polio, but it was given just four weeks after the second dose. Is
this acceptable for school attendance?
A: This dose would be considered invalid because the spacing between the
second and third dose should be six months. A dose of polio would need to
be administered six months from this invalid dose to comply with NJ’s school
immunization requirements.
In 2018, the CDC revised the minimum intervals between polio doses. For
children younger than age four, there is a minimum interval of one month
between doses two and three. For children, ages four and older, the
minimum interval is six months between dose two and three. Since the
adoption of the four-day grace period in January 2008, schools need to
make sure that all vaccines meet the recommended minimum age and dose
spacing intervals. Please contact the Vaccine Preventable Disease Program
to discuss your specific scenario/question if you need further guidance.
Q: What polio vaccines satisfy the NJ immunization requirements
for school attendance?
There are two types of vaccine that protect against polio: inactivated
poliovirus vaccine (IPV) and oral poliovirus vaccine (OPV). IPV is the only
polio vaccine that has been given in the United States since 2000. It protects
against poliovirus types 1, 2, and 3. OPV is used in other countries.
Before April 1, 2016, OPV also helped to protect against the three types of
poliovirus. Last year, all countries that use OPV switched to using an OPV
that only protects against types 1 and 3.

Updated: February 2024 22 | Page
CDC/ACIP recommends age-appropriate U.S. IPV schedule which protects
against poliovirus types 1, 2, and 3 for U.S. infants and children. Therefore,
only the following conditions would satisfy the polio vaccination
requirements for school attendance in NJ:
• OPV doses given before April 1, 2016
OR
• Inactivated Polio Vaccine (IPV) doses
Q: Is polio vaccine required for students 18 and older?
Students 18 and older are not required to receive the polio vaccine for
school attendance, but it can be recommended for optimal protection.
According to the ACIP, adults who are known or suspected to be
unvaccinated or incompletely vaccinated against polio should complete a
primary vaccination series with inactivated polio vaccine (IPV). In addition,
adults who have received a primary series of trivalent oral polio vaccine
(tOPV) or IPV in any combination and who are at
increased risk of poliovirus exposure may receive another dose of IPV.
Q: If a student’s immunization record shows no history or
questionable documentation of polio vaccine, can lab evidence of
immunity (serology) be used?
Serology to assess polio immunity will no longer be an available option
because of increasingly limited availability of antibody testing against type 2
poliovirus. In this case, the student would need to be vaccinated or
revaccinated in accordance with the age-appropriate IPV schedule.
However, previous serologic testing, which was obtained when testing for
type 2 poliovirus was still available in the U.S., will still be accepted as
evidence of polio immunity if the test documents a separate positive result
for each of the three poliovirus serotypes.
Measles, Mumps, Rubella (MMR) Vaccine
Q: If a child received the MMR vaccine at 6-11 months of age
because of international travel, will the dose count for school
attendance?

Updated: February 2024 23 | Page
A: No. Although MMR can be given prior to 12 months of age for
international travel, this dose would not count towards the required doses
for school attendance unless it was given either 4 days before age 12
months. (See “Grace Periods and Provisional Admission” section for more
information about the four-day grace period).
Q: MMR and Varicella were given two weeks apart. Why is this not
acceptable for school attendance?
A: MMR and Varicella are both live vaccines. Two live vaccines can be
administered on the same day or at least 28 days apart. If vaccines are
given too close together, it can result in a less than optimal immune
response. In this instance, the second vaccine administered should not be
counted and the dose should be repeated at least 4 weeks later.
Varicella (Chickenpox) Vaccine
Q: Is the varicella vaccine required for children entering a licensed
childcare and less than 19 months of age?
A: Per the ACIP recommendations, the first dose of varicella vaccine may be
given between the ages of 12-15 months of age. However, for requirements
for school entry into a licensed childcare facility in NJ, you do not need a
varicella vaccination until 19 months of age.
Q: Is the second dose of varicella vaccine a requirement for school
entry?
A: No, the second dose of varicella vaccine is not required but is strongly
recommended by NJDOH. The ACIP recommends a second dose of varicella
vaccine to be given between four to six years of age for optimal protection.
Q: Per NJ immunization regulations, who needs the varicella
vaccine?
A: All children, born on or after January 1, 1998, and is at least 19 months
of age or older and attending a NJ school is required to receive one dose of
varicella vaccine. This applies to all transfer students, both out of state/out
of country and those transferring from another school district within the
state.
Hepatitis B Vaccine
Q: How many doses of hepatitis B are required for school entry?
Updated: February 2024 24 | Page
A: Per NJ immunization regulations, the three-dose hepatitis B series is not
required until a child enters kindergarten. By kindergarten entry, a child
must enter school with three doses of hepatitis B vaccine. Previously
unvaccinated adolescents, between the ages of 11-15 years, can receive a
two-dose hepatitis B vaccine adolescent/adult series. Please see the
following handout to ensure students are receiving the appropriate dosing,
immunize.org/catg.d/p2081.pdf .
Q: Can an adolescent receive the two-dose adolescent series outside
the licensed age?
A: No, the two-dose adolescent series is only licensed for persons 11-15
years of age. Talk with your health care provider for further guidance.
Q: What are the minimum intervals between hepatitis B vaccine
doses?
The introduction of new vaccines and combination vaccines can make it
difficult for health care providers to keep track of minimum dose spacing
intervals.
There has been confusion regarding the hepatitis B vaccine schedule for
children. NJDOH supports the recommendation of the CDC to vaccinate
children at birth.
Please note the following minimum intervals after the birth dose:
The minimum interval between the first and second dose:
Weeks after first dose - 4 weeks (28 days)
There are three minimum intervals that must be met for the third dose:
Weeks after first dose - 16 weeks (112 days)
Weeks after second dose - 8 weeks (56 days)
Weeks after birth - 24 weeks (168 days)
Please use the ACIP’s minimum age and dose spacing intervals table for
further guidance:
cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/A/age-interval-
table.pdf.
If the minimum interval or age is defined in terms of weeks, then use weeks
to calculate the minimum interval or age. If the minimum interval or age is

Updated: February 2024 25 | Page
defined in terms of months, then use months to calculate the minimum age
or interval.
Q: I recently heard that the hepatitis B requirement, specifically the
intervals between doses, has changed. What will happen with
students that may have an incorrect interval?
A: The hepatitis B regulations have not changed but with the adoption of
the four-day grace period in January 2008, schools need to make sure that
all vaccines meet the recommended minimum age and dose spacing
intervals. This applies to all vaccines—not just hepatitis B. Any child who
received hepatitis B vaccine after the four-day grace period was adopted
must have proper minimum age and dose spacing intervals to be counted as
valid doses.
Please see the “Minimum Dose Spacing Intervals” and “Grace Periods
and Provisional Admission” sections of this document for further
information.
Q: A student's immunization record indicates that the first dose of
hepatitis B vaccine was given “at Hospital” or “at Birth” rather than
specifying a date of administration. Would this be an acceptable
form of documentation?
A: Yes, you can accept “at Hospital” or “at Birth” as the date of
administration for the first dose.
Other Vaccine Requirement Questions
Minimum Dose Spacing Intervals
Q: What is meant by "minimum intervals" between vaccine doses?
A: Vaccination schedules are generally determined by clinical trials, usually
prior to licensure of the
vaccine. The spacing of doses in the clinical trial
usually becomes the recommended schedule. A "minimum interval" is the
shortest time between two doses of a vaccine series in which an adequate
response to the second dose can be expected. The concern is t
hat a dose
given too soon after the previous dose may reduce the response to that
dose.
Q: What is considered acceptable documentation for receipt of a
vaccine?
Updated: February 2024 26 | Page
A: Ideally all immunization dates should include a month, day, and year;
however, NJ will accept a documented date of just month and year if the
doses administered are determined to be in compliance with the minimum
age or dose spacing intervals.
For example, a student born on August 20, 2011, received a dose of MMR
vaccine in August 2012. Since you cannot determine when the MMR vaccine
was administered, a documented date of just month and year would not be
sufficient. This dose could have been administered on August 1, 2011, which
would be prior to the child’s first birthday.
Q: Why is it important to make sure vaccines meet the minimum age
and interval?
A: Doses administered too close together or at too young an age can lead to
a suboptimal (inadequate or poor) immune response.
Q: Where can I find the accepted minimum age and intervals? In
some places, I see the minimum age in months and in some places I
see it written in weeks.
A: You should consult the ACIP/CDC recommended minimum age and
intervals document located at
cdc.gov/vaccines/schedules/downloads/child/catchup-schedule-
pr.pdfhttp://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/A
/age-interval-table.pdf. If the minimum interval or age is defined in terms of
weeks, then use weeks to calculate the minimum interval or age. If the
minimum interval or age is defined in terms of months, then use months to
calculate the minimum age or interval.
Q: We sometimes have differences of opinion among our staff in
determining the minimum interval or age for administering vaccines.
Recommendations are sometimes written in months, weeks, or days.
Can you help clarify?
A: Customarily, if the dosing interval is 4 months or more, it is common to
use calendar months (e.g., 6 months from October 1 is April 1). If the
interval is less than 4 months, it is common to convert months into days or
weeks (e.g., 1 month = 4 weeks = 28 days).
For common questions about the scheduling of vaccine doses, visit the
Immunization Action Coalition’s webpage:
immunize.org/askexperts/scheduling-vaccines.asp.
Q: Is there a tool that can help you calculate intervals between
doses quickly?
Updated: February 2024 27 | Page
A: There may be several date calculator tools available on the internet such
as the time and date tool which is accessible at:
timeanddate.com/date/duration.html. (Please note, NJDOH does not
endorse, control, or guarantee the accuracy or completeness of information
contained on the time and date website.)
Grace Periods and Provisional Admission
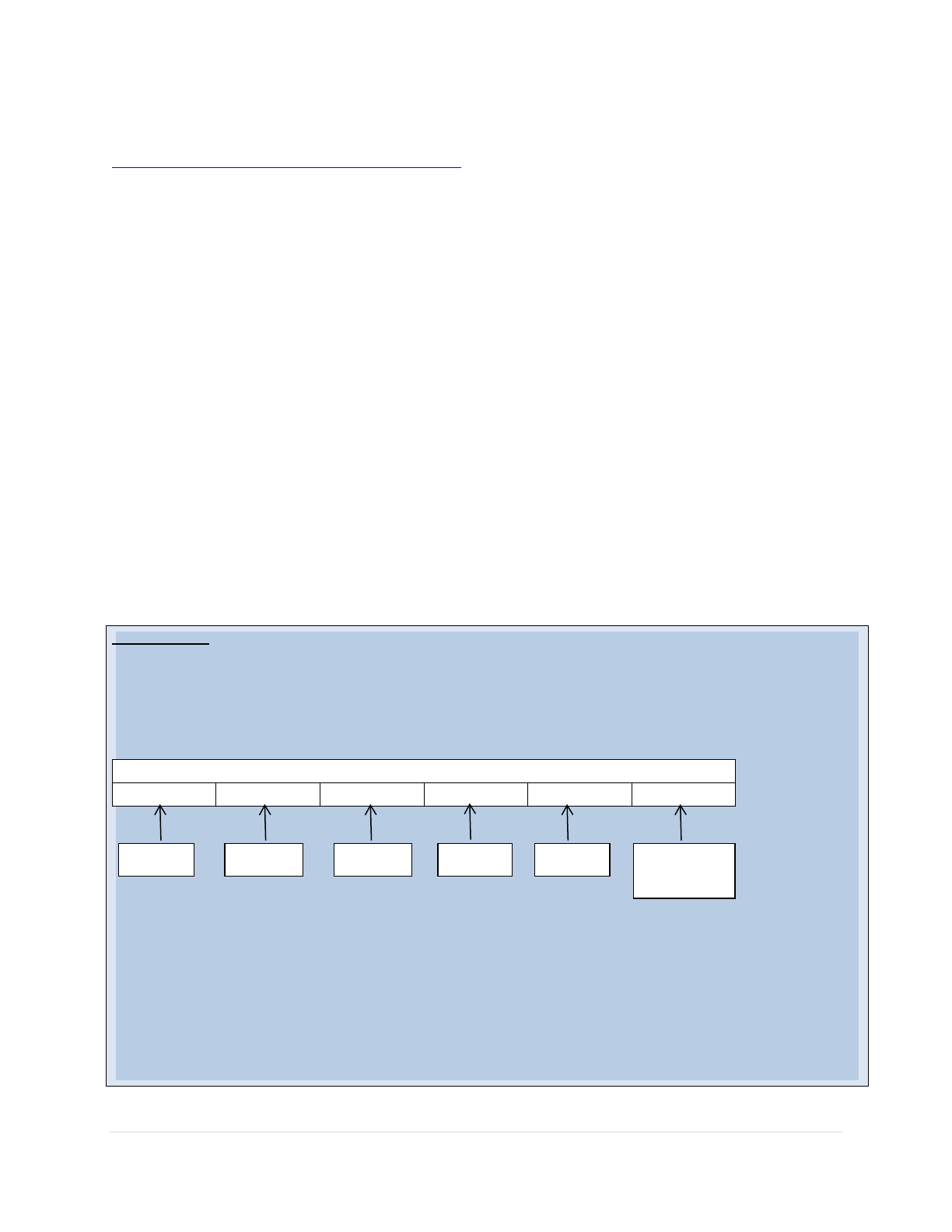
Q: Can you please explain the four-day grace period?
A: All vaccines administered less than or equal to 4 days before either the
specified minimum age or dose spacing intervals shall be counted as valid
and shall not require revaccination in order to enter or remain in a school,
preschool, or childcare facility.
Day 1 is the day before the minimum age or minimum interval for a vaccine.
Doses of any vaccine administered ≥5 days earlier than the minimum
interval or age should not be counted as valid doses and should be repeated
as age appropriate. Please see the following example:
Example:
A child born on November 6, 2013, received the MMR vaccine on November
3, 2014. The minimum age for this vaccine is 12 months which would be
November 6, 2014.
November
1
2
3
4
5
6
Since November 6 is the minimum age, doses administered on or after
November 2 would be considered valid (November 6 - 4 days = November
2). If the child received the dose on November 1, the dose would have been
considered invalid.
Please note that ACIP does not recommend applying the four-day grace
period for the dose spacing interval between two live vaccines. However, for
school attendance and auditing purposes, this will be acceptable.
Minimum
age
Day 1
Day 2
Day 3
Day 4
Day 5
Updated: February 2024 28 | Page
Q: What if a dose of vaccine is administered too soon after the
previous dose. When can we give another (valid) dose?
A: If vaccines are given too close together, it can result in a less than
optimal immune response. However, in most instances, a difference of a few
days is unlikely to have a negative effect on immune response. With the
exception of rabies vaccine, ACIP allows a grace period of 4 days (i.e.,
vaccine doses administered up to 4 days before the recommended minimum
interval or age can be counted as valid). However, if a dose was
administered 5 or more days earlier than the recommended minimum
interval between doses, it is not valid and must be repeated.* The repeat
dose should be spaced after the invalid dose by the recommended minimum
interval.
*The only exceptions to this rule are the mRNA COVID-19 (Pfizer and Moderna) vaccines:
ACIP does not recommend administration of an additional dose following an incorrect dosing
interval.
Q: We often find it confusing to determine the minimum intervals
for hepatitis B vaccine doses. Will the four-day grace period make
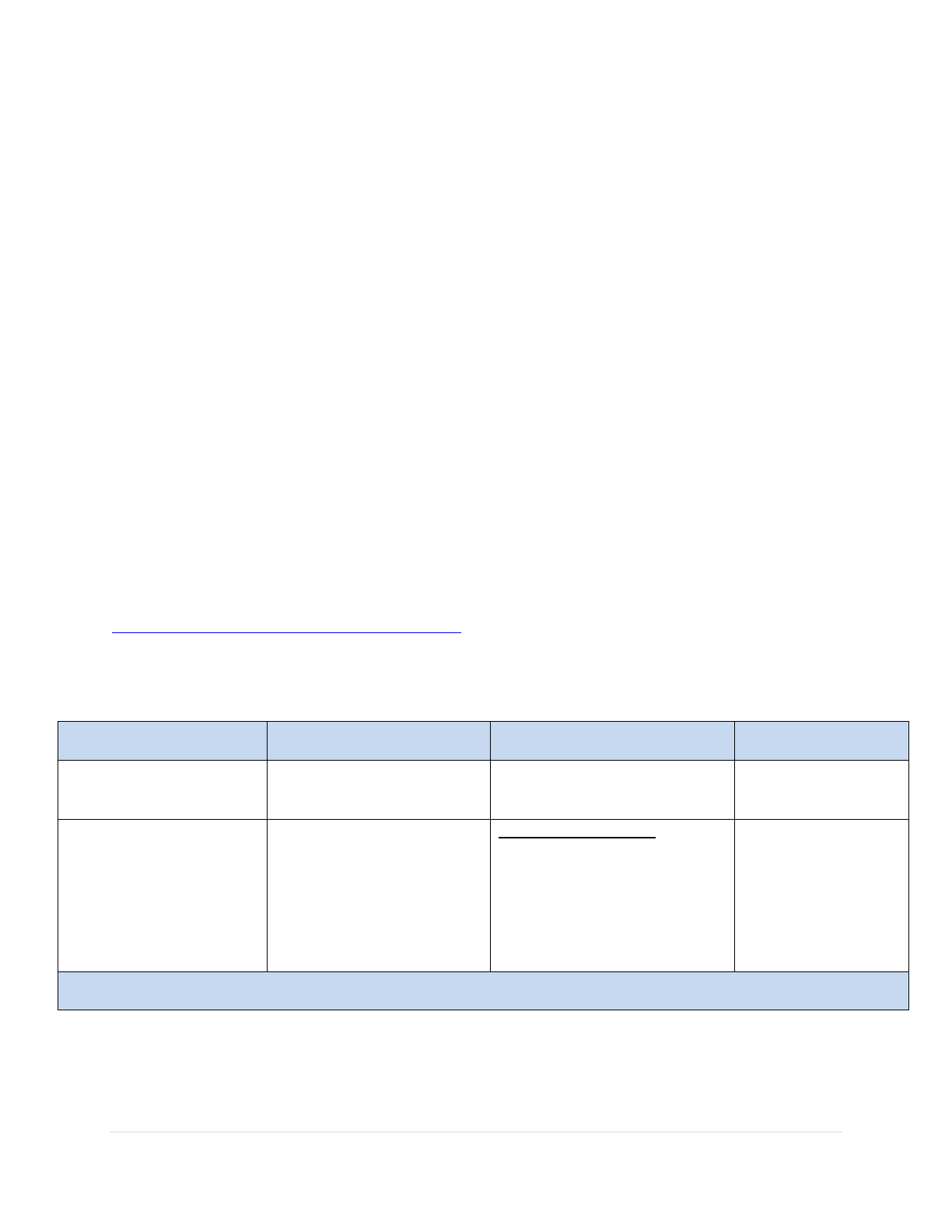
certain doses valid? Could you please provide an example?
A: In order to help with the calculation of minimum intervals, you may want
to utilize tools on the internet such as the time and date tool accessible at
timeanddate.com/date/duration.html for the example below. (Please note,
NJDOH does not endorse, control, or guarantee the accuracy or
completeness of information contained in external websites.)
Vaccine
Administration Date
Minimum Interval
Comments
Acceptable () or
Unacceptable ()
DOB: March 25, 2012
(given at birth)
The minimum age for the
hepatitis B vaccine is at
birth.
March 25, 2012, is the date
of birth and the date of
vaccine administration.
April 25, 2012
The minimum interval
between the first and
second dose is 4 weeks
(28 days).
NOTE:
April 22 would mark the
minimum interval.
March 25-April 25
4 weeks and 3 days (total of
31 days).
This satisfies the minimum
interval of 28 days (April
22).
There are three criteria for the third dose of hepatitis B vaccine. All criteria need to be met in order for
the dose to be considered valid.
Updated: February 2024 29 | Page
September 1, 2012
The minimum interval
between the second and
third dose is 8 weeks
(56 days).
NOTE:
June 20 would mark the
minimum interval.
April 25-September 1
18 weeks and 3 days (total
of 129 days)
This satisfies the minimum
interval
(June 20).
The minimum interval
between the first and
third dose is 16 weeks
(112 days).
NOTE:
July 15 would mark the
minimum interval.
March 25—September 1.
22 weeks and 6 days (total
of 160 days)
This satisfies the minimum
interval
(July 15).
The minimum age for the
third dose is 24 weeks
(168 days).
NOTE:
September 9 would mark
the minimum age.
March 25 is the date of
birth.
March 25-September 1
22 weeks and 6 days
(160 days) after birth
This dose would be
considered invalid because
the third dose was given
prior to 24 weeks (168
days) of age
*
*Since the dose was administered ≥5 days earlier than the minimum interval or age, the last dose is considered
invalid. The dose would be considered valid if it were given on or after September 9 (the minimum age). If you
applied the four-day grace period, you can accept doses administered on or after September 5 (September 9 - 4
days = September 5).
Q: Can you please explain the 30-day grace period?
A: Students entering a NJ school from out of state or out of country are
allowed up to 30 days to provide proof of immunization history before their
provisional status begins.
If after the 30 days have elapsed and no documentation of previous
vaccination is provided; the child may not attend school until one dose of all
age-appropriate required vaccines are received before being provisionally
admitted.
Q: When does the 30-day grace period begin?
A: The 30-day grace period would begin within 30 calendar days of the
registration date OR upon school entry, whichever comes later.
Updated: February 2024 30 | Page
Q: To whom does the 30-day grace period apply?
A: According to the NJ immunization regulations, the 30-day grace period
only applies to transfer students, coming from out of state/out of country.
This does not apply to in-state transfer students.
Q: What is Provisional Admission?
A: Provisional admission allows a child to enter/attend school after having
received a minimum of one dose of each of the required vaccines. Pupils
must be actively in the process of completing the series and on schedule to
receive subsequent doses as rapidly as medically feasible. A school nurse
or school administrator shall review the immunization status of a
provisionally enrolled student every 30 days to ensure continued compliance
in completing the required doses of vaccine(s).
Provisional status can only be granted one time to students entering or
transferring into schools, preschools, or childcare centers in NJ. Information
on this status will need to be sent by the original school to the new school.
Students who are 4 months through 18 years whose vaccinations have been
delayed or who are more than one month behind, need to follow the
minimum age and dose spacing intervals in accordance with the Advisory
Committee on Immunization Practices (ACIP) Recommended Catch-Up
Schedule, cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-
combined-schedule.pdf#page=3
Q: How do you define, “as rapidly as medically feasible”?
A: The phrase, “as rapidly as medically feasible” is in reference to meeting
the minimum age and dose spacing intervals in accordance with the ACIP
Recommended Catch-Up Immunization Schedule. Please see the following
example:
Example: A child was provisionally admitted to Kindergarten because he
had received one dose of hepatitis B vaccine (NJ requires three doses of
hepatitis B vaccine for school attendance). The school nurse/administrator
would need to assess when the next dose in the vaccine series is due by
consulting the ACIP Recommended Catch-Up Immunization Schedule.
According to this schedule, the minimum dose spacing interval between
hepatitis B dose one and two is four weeks. Therefore, this child will need to
receive the second dose of hepatitis B vaccine once four weeks has elapsed
from his first dose. It would not be medically feasible for the child to receive
this dose prior to four weeks. If the minimum interval has exceeded (i.e. the
child has not shown documentation of receiving the second dose after the
four weeks have elapsed), this student would be considered out of
Updated: February 2024 31 | Page
compliance and may not be allowed to attend school until he receives this
required dose.
Q: Is there a document that school nurses can use to keep track of
students who are enrolled provisionally?
A: The NJDOH recognizes the challenge in keeping track of students who
have been provisionally admitted; therefore, you may use the Provisional
Admission Student Tracking form to streamline the process. For more
information, please visit nj.gov/health/cd/imm_requirements/
and access the following documents from the “Resources and Tools for
School Administrators and section:
• Cover letter—provisional admission student tracking form
• Provisional admission student tracking form
Q: When is a student considered out-of-compliance?
A student would be considered out of compliance if he/she:
• Does not have an immunization record (only those children entering a
NJ school from out of state or out of country are allowed up to 30 days
to provide proof of immunization history before their provisional status
begins).
• Does not have serology or proof of immunity for missing vaccines
• Does not have a religious or medical exemption on file
• Does not meet the provisional admission definition since the minimum
age and dose spacing interval to receive the next dose in the
vaccination series has been exceeded.
Q: How do you determine whether or not the student should be
enrolled, excluded, admitted provisionally, or allowed a 30-day grace
period?
A: Visit nj.gov/health/cd/imm_requirements/ and access the “Immunization
Compliance Flow Charts” from the “Resources and Tools for School
Administrators and section to guide you through the process.
Exclusions and Exemptions
Q: When would a child need to be excluded from school?
A: There are two situations in which a child would be excluded from school:
1. Non-compliance with vaccine requirements: A child must be in compliance
with vaccination requirements by the time they enter school. In the instance

Updated: February 2024 32 | Page
of sixth grade entry, where a child is younger than the licensed age to be
given a vaccine, the child can wait until they are age eligible to receive the
adolescent vaccine. The child should be given two weeks to comply with
vaccination requirements by either providing documentation that they
received the vaccine, or a note from the health care provider with an
appointment date to receive the vaccine. This documentation needs to be
provided to the school nurse to include in their immunization record.
Depending on individual circumstances, a scheduled appointment outside the
two-week period may be acceptable. The Department's goal is not to exclude
anyone, but if the child does not receive the vaccine in a reasonable period,
he/she will be asked to leave school.
2. In the event of an outbreak: N.J.A.C. 8:57-4.19 Emergency powers of the
Commissioner of Health
(a) In the event that the Commissioner, Department of Health or his or her
designee determines either that an outbreak or threatened outbreak of
disease or other public health immunization emergency exists, the
Commissioner or his or her designee may issue either additional
immunization requirements to control the outbreak or threat of an outbreak
or modify immunization requirements to meet the emergency.
(b) All children failing to meet these additional requirements shall be
excluded from a school, preschool, or childcare center until the outbreak or
threatened outbreak is over.
(c) These requirements or amendments to the requirements shall remain in
effect until such time as the Commissioner, Department of Health or his or
her designee determines that an outbreak or a threatened outbreak no
longer exists or the emergency is declared over, or for three months after
the declaration of the emergency, whichever one comes first.
The Commissioner, Department of Health or his or her designee may re-
declare a state of emergency if the emergency has not ended.
N.J.A.C. 8:57-4.4 Religious exemptions
(d) Those children with religious exemptions from receiving
immunizing agents may be excluded from the school, preschool, or
childcare center during a vaccine-preventable disease outbreak or
threatened outbreak as determined by the Commissioner, Department
of Health or his or her designee.
N.J.A.C. 8:57-4.3 Medical exemptions
(d) Those children with medical exemptions to receiving specific
immunizations may be excluded from the school, preschool, or
childcare facility during a vaccine-preventable disease outbreak or
threatened outbreak as determined by the Commissioner, Health or his
or her designee.
N.J.A.C. 8:47-4.5 Provisional admission
Updated: February 2024 33 | Page
(g) Those children in provisional status may be temporarily excluded
from the school, preschool, or childcare center during a vaccine-
preventable disease outbreak or threatened outbreak as determined
by the Commissioner, Department of Health or his or her designee.
Q: What type of health care provider can write an acceptable
medical exemption?
A: Per the NJDOH Vaccine Preventable Disease Program, only a physician
licensed to practice medicine/ osteopathic medicine and a nurse practitioner
can write a medical exemption.
Q: What is considered grounds for filing a medical exemption?
A: A medical exemption must indicate a specific period of time in which the
child cannot receive specific vaccinations. Reason(s) for medical
contraindication must be enumerated by the ACIP and the American
Academy of Pediatrics (AAP). Precautions to receiving a vaccine are not
contraindications but a provider must take into consideration
immunize.org/catg.d/p3072a.pdf.
Q: Is there a medical exemption form?
A: Yes, the NJ Department of Health (NJDOH) recently created a Request
for Medical Exemption from Mandatory Immunization Form (IMM-53) and
guidance document available at nj.gov/health/forms/imm-53.pdf. Health
care providers who submit medical exemptions for mandatory vaccinations
must ensure that the information submitted is accurate and verifiable. The
use of this form is not mandated or required. It is a tool that may be used
by health care providers, schools, preschools, childcare facilities, and local
health department to help determine the validity of a medical exemption
from mandatory immunization.
Q: Do medical exemptions have to be renewed annually?
A: Medical exemptions need to be reviewed, but not necessarily updated,
annually. A medical exemption must indicate a specific period of time in
which the child cannot receive specific vaccinations. Once the time period
ends, the child will be required to obtain the immunization(s) from which
he/she has been exempted.
For example, if a child was granted a medical exemption because he/she
was on medication that was contraindicated for one or more vaccines, that
child would not be required to receive those specific vaccinations until the
specified time period has elapsed. If the child is still medically
Updated: February 2024 34 | Page
contraindicated and the time period has elapsed, a new medical exemption
would need to be submitted.
Q: What should be included in an acceptable religious exemption?
A: A religious exemption is not the same as a philosophical, moral, or
conscientious exemption. A religious exemption does not have to include the
name of the religion, nor does it need to be notarized nor does it need to be
signed by a religious leader. It can be filed by a parent or guardian of a
minor or by an adult individual. The parent/guardian’s written statement
must be signed and dated.
All schools, childcare centers, and local health officers may be advised that
the religious exemption extends to private, parochial, and public institutions.
When a parent or guardian submits their written religious exemption to
immunization, which contains some religious reference, those persons
charged with implementing administrative rules at N.J.A.C. 8:57–4.4, should
not question whether the parent’s professed religious statement or stated
belief is reasonable, acceptable, sincere and bona fide. In practice, if the
written statement contains the word “religion” or “religious” or some
reference thereto, then the statement should be accepted, and the religious
exemption of mandatory immunization(s) granted. Please note, religious-
affiliated schools cannot be challenged on their decision.
Q: Do religious exemptions have to be renewed annually?
A: Religious exemptions do not need to be updated yearly. However, a
religious exemption would become null and void if a child received either a
vaccine that was recommended or required for school attendance.
Example: In the beginning of the school year, a child was granted a
religious exemption and did not have to receive any of the required vaccines.
Later in the school year, the child provides documentation of receiving one
dose of Tdap. Since the child now has received a vaccine from which he was
previously exempted, the religious exemption is now null and void. This
means he would now be responsible for receiving all of the required vaccines
from which he was previously exempted.
If a religious exemption was granted for a specific vaccine (i.e. varicella),
the child would only be exempted from that particular vaccine and would be
responsible for meeting all other vaccine requirements to continue attending
school.
Q: If a child has a religious exemption, but then receives a COVID-19
vaccine, does this make the religious exemption null and void?

Updated: February 2024 35 | Page
A: At this time, COVID-19 vaccine is not a school requirement, However,
NJDOH strongly recommends that everyone should be up-to-date with age-
appropriate vaccinations, per CDC’s Advisory Committee on Immunization
Practices recommendations.
When you file for an exemption, you are stating that immunizations conflict
with your religious beliefs and therefore you will not be receiving them. If a
child has a general/blanket religious exemption on file, then any vaccine that
is received after the filing date, whether recommended (i.e., HPV) or
required (i.e., MMR) would nullify and void the religious exemption. The child
will be responsible for receiving all the vaccines he/she was previously
exempted from.
Q: Are there any forms parents can complete for religious
exemptions?
A: The NJ Department of Health does not have template religious exemption
forms. Please refer to the above questions to see what constitutes a valid
religious exemption.
Q: Are philosophical or moral objections now acceptable in NJ?
A: No, currently the only two exemptions allowed in NJ are religious and
medical exemptions.
Serology Titers
Q: Are serology titers acceptable as laboratory evidence of
immunity in lieu of completing a vaccination series?
A: The subchapter 8:57-4 on immunization requirements specifically
addresses the acceptance of serology titers. According to the NJ
Administrative Code 8:57-4.6(c):
“Laboratory evidence of protective immunity, as enumerated by the ACIP of
the United States Public Health Service, shall be accepted as evidence of
immunization if a parent or guardian cannot produce a documented history
of immunization.”
In addition, The Antibody Titer Law (Holly’s Law, NJSA 26:2N-8-11), passed
on January 14, 2004, requires the NJ Department of Health (NJDOH) to
accept serologic evidence of protective immunity to measles, mumps and
rubella in lieu of the second ACIP recommended measles, mumps and
rubella vaccine.
The tests used to document immunity must be approved by the U.S. Food
and Drug Administration (FDA) for this purpose and performed by a

Updated: February 2024 36 | Page
laboratory that is CLIA certified. The reference ranges and interpretation
must be included with the laboratory results and the documentation must be
placed in the record. Borderline, equivocal and negative titers necessitate
vaccination/re-vaccination.
The use of serology to evaluate exposure or immunity to infectious diseases
is complicated and is the topic of a great deal of medical literature. There
are considerations that need to be addressed when one considers serology
titer results. For example, the time interval from receiving the last
vaccination and when the serology titer sample is drawn may produce a false
sense of security that an individual is fully protected (as immune levels may
initially peak immediately after receiving a dose but decrease over time).
Likewise, for some vaccines, the ACIP and NJDOH do not recognize serology
as an alternative to vaccination since serologic correlates for protection do
not exist for some diseases (e.g. Bordetella pertussis).Providers should
review the ACIP/CDC recommendations for serology testing and ensure they
are ordering the appropriate tests (e.g., it is never appropriate to order an
IgM to assess vaccine-induced immunity).
NJDOH does not support the use of serology to “abort” a vaccine schedule as
approved by the US Food and Drug Administration and recommended by the
ACIP (e.g., check serology after 1 dose of hepatitis B vaccine). However,
NJDOH recognizes that serology is useful for individuals to:
• Document natural infection to certain diseases.
• Document immunity in an individual who received a complete
vaccination series but lacks documentation – and revaccination is not
practical (e.g., refugees).
• Document immunity in an individual who received a complete
vaccination series but vaccination practices were questionable – and
revaccination is not practical (e.g., vaccination with expired vaccine).
• Document post-vaccination response in those individuals who are at
high risk of infection with a particular disease (hepatitis BsAb in infants
born to sAg positive mothers, health care workers).
As more reliable data on serology titers becomes available from the ACIP,
we will incorporate that into our consideration of the use of serology titers
for acceptable laboratory evidence of immunity.
Q: What serology titer tests are currently available for mandatory
vaccines and how will the serology results be evaluated?
• Measles, Mumps and Rubella
In most cases, an antibody level

Updated: February 2024 37 | Page
(IgG) considered protective is a good indicator of immunity and must
be accepted in lieu of a second MMR vaccine as per Holly’s Law.
Serology does not need to be repeated once an antibody level in the
protective range is documented or the individual receives 2 MMR
vaccines.
• Varicella
In most cases, an antibody level
(IgG) in the protective range is a good indicator of immunity and may
be accepted in lieu of vaccination. Serology does not need to be
repeated once an antibody level in the protective range is documented
or the individual receives 2 varicella vaccines.
• Inactivated Polio Vaccine
Serology to assess polio immunity will no longer be an available option
because of increasingly limited availability of antibody testing against
type 2 poliovirus. However, previous serologic testing, which was
obtained when testing for type 2 poliovirus was still available in the
U.S., will still be accepted as evidence of polio immunity if the test
documents a separate positive result for each of the three poliovirus
serotypes.
• Diphtheria, Tetanus and Pertussis
Serologic testing for protective antibody to tetanus and diphtheria can
be obtained commercially. No established serologic correlates exist for
protection against pertussis.
• Haemophilus influenzae type b, pneumococcal, meningococcal and
influenza
There is no serology alternative to vaccination.
• Hepatitis B
Hepatitis B serology and the interpretation are complicated and
beyond the scope of this document. Pre-vaccination testing is not
routinely recommended for infants or children. Pre-vaccination testing
is recommended only for
o all persons born in Africa, Asia, the Pacific Islands, and other
regions with HBSAg prevalence of > 8%;
o household, sex, and needle-sharing contacts of HBSAg-positive
persons; and
o persons with HIV infection.
Pre-vaccination testing can be considered for groups with high risk of
HBV infection (i.e., men who have sex with men, intravenous drug
users and incarcerated persons).
Post-vaccination serology is not routinely recommended for infants,
children, adolescents, and most adults. Post-vaccination serology is
only recommended for those whose medical management is based on
knowledge of antibody status. Individuals for whom post-vaccination
serology is recommended include, chronic hemodialysis patients, other
immunocompromised patients, persons with HIV infection, sex

Updated: February 2024 38 | Page
partners of HBsAg-positive persons, infants born to HBsAg-positive
women and certain health care workers. Vaccine is 80-100% effective
in preventing infection or clinical hepatitis in those who receive the
complete course of vaccine (3 doses or 2 doses of the adolescent
formulation). Antibody levels might wane with time. However,
individuals who demonstrate an anti-HBs antibody titer of 10mIU/ml or
higher at least 1-2 months after completing the series are considered
protected for life even if detectable antibody levels wane.
Serum antibody titer cannot be used in lieu of completing the FDA-
approved/ACIP-recommended vaccine series.
Q: What are considered acceptable values for serology titer results?
A: The titer results depend on the specific test used and the reference
ranges applicable to that particular test. Equivocal and/ or borderline results
are not acceptable and require vaccination/revaccination. Negative results
require vaccination/revaccination. NJDOH recommends that they discuss
ACIP revaccination guidelines and follow-up serology with their health care
providers, as appropriate. Providers should review the ACIP/CDC
recommendations for serology testing and ensure they are ordering the
appropriate tests (e.g., it is never appropriate to order an IgM to assess
vaccine-induced immunity).
Q: What is the Antibody Titer (Holly’s Law)?
A: The Antibody Titer Law (Holly’s Law, N.J.S.A. 26:2N-8-11), passed on
January 14, 2004, requires the NJDOH to accept serologic evidence of
protective immunity to measles, mumps and rubella in lieu of the second
ACIP recommended measles, mumps and rubella vaccine. For more
information, please visit:
nj.gov/health/cd/documents/antibody_titer_law.pdf.
Q: Can you accept a positive serology titer for measles, mumps, and
rubella if the MMR vaccine administration dates are unavailable?
Wouldn’t the antibody titer law apply here?
A: The antibody titer law states that a titer can be done instead of the
second dose of a measles-containing vaccine for children who have the first
dose documented. However, in the instance where administration dates of
vaccination are not available, laboratory evidence of immunity will be
accepted. This will satisfy the requirement for school attendance, and no
additional doses of MMR vaccine will be needed.
Individuals are considered to have life-long immunity once they have
received the recommended number of MMR vaccine doses OR have other

Updated: February 2024 39 | Page
evidence of immunity. Refer to the question below regarding “What is
acceptable evidence of immunity to measles?”
Q: What is considered acceptable evidence of immunity to measles?
Acceptable presumptive evidence of immunity against measles includes at
least one of the following:
• written documentation of adequate vaccination:
o one or more doses of a measles-containing vaccine administered on
or after the first birthday for preschool-age children and adults not
at high risk
o two doses of measles-containing vaccine for school-age children,
adolescents, and adults at high risk, including college students,
health care personnel, and international travelers
• laboratory evidence of immunity (IgG)
• laboratory confirmation of measles disease (verbal history of measles
does not count).
• birth before 1957*
*Although birth before 1957 is considered acceptable evidence of measles immunity, health
care facilities should consider vaccinating unvaccinated personnel born before 1957 who do
not have other evidence of immunity with 2 doses of MMR vaccine (minimum interval 28
days).
During an outbreak of measles, health care facilities should recommend 2 doses of MMR
vaccine at the appropriate interval for unvaccinated health care personnel regardless of the
birth year if they lack laboratory evidence of measles immunity.
Q: What is acceptable proof of varicella disease?
A: A physician/licensed medical professional’s written statement of varicella
diagnosis; a written statement from parent/guardian reporting varicella
disease; a lab confirmation of protective varicella immunity are examples of
proof of varicella disease.
Q: Is a diagnosis of shingles disease acceptable proof of varicella
disease?
A: Since shingles disease occurs only in someone who has had varicella
disease previously, diagnosis of shingles by a licensed medical practitioner
would be evidence of proof of past varicella disease.
Q: If a family is requesting a serology titer to circumvent the
required immunizations and the family has health insurance which
covers immunizations, but the insurance does not cover serology
titers, whose responsibility is it to pay for the serology titers?
A: It is not a recommendation or acceptable practice by the ACIP to use
serology titers in lieu of completing a vaccination series or to avoid receiving

Updated: February 2024 40 | Page
subsequent vaccinations within a series. Additionally, in this circumstance it
would be the family’s responsibility to pay for the serology titer tests since
they are choosing not to vaccinate their child as medically appropriate.
Q
: What happens if a person receives a complete vaccine series and
for some reason has a titer done that shows the person is not
immune?
A: NJDOH and the ACIP do not recommend routine serology titer tests to
document immunity. Once a person has received the complete series of a
recommended vaccination, he/she is assumed to have produced the needed
immunity level to protect them from the disease. The ACIP has identified
specific scenarios when the use of serology titer testing is recommended. A
serology test done without a specific public health or medical reason can be
difficult to interpret and can sometimes lead to a person receiving extra
vaccines. However, a negative or equivocal serology titer might mean that
the individual is susceptible to the disease even if he/she completed the full
series of vaccines. Therefore, the NJDOH recommends that these individuals
with negative or equivocal serology titers discuss ACIP revaccination
guidelines and follow-up serology with their health care providers. Please
also refer to the question, “Q: Are serology titers acceptable as
laboratory evidence of immunity in lieu of completing a vaccination
series?”
Enforcement of Immunization Regulations
Q: What are the responsibilities of schools for ensuring
immunization compliance?
A: The NJ Immunization of Pupils in School (N.J.A.C. 8:57-4) regulations
apply to all children attending any public or private school, childcare center,
nursery school, preschool or kindergarten in NJ. According to N.J.A.C. 8:57-
2, a principal, administrator or person in charge of a school shall not
knowingly admit or retain any child whose parents have not submitted
acceptable evidence of immunizations unless, however, they have a valid
exemption. Failure to do so would be a violation of the state sanitary code
N.J.S.A. 26:1A-10 and the school may be subject to a fine. The statute
stipulates that each violation of any provision of the State Sanitary Code
shall constitute a separate offense and shall be punishable by a penalty of
not less than $50 nor more than $1,000.
Q: How do we know if a foreign immunization record is valid?
A: If you receive a foreign immunization record, you can accept it with
proper written documentation. It should ideally have a seal or stamp OR at
least signed and dated by a health care provider. You should check to see if

Updated: February 2024 41 | Page
the vaccines administered match NJ’s vaccination requirements for school
attendance. If the student has to be revaccinated, it should be done in
accordance with the ACIP Recommended Schedule, which may be simpler,
or they can have blood work done to test for immunity (when possible) to
prevent over vaccination.
If you receive records that need to be translated, the CDC has resources to
decipher foreign vaccines. Please visit the website Pink Book Appendix B:
Vaccines (cdc.gov). For further guidance please refer to the American
Academy of Pediatrics’ Red Book or the ACIP.
Q: Who is responsible for translating a child’s immunization record?
According to the immunization of pupils in school rules (N.J.A.C. 8:57-4.6),
all immunization records submitted by a parent or guardian in a language
other than English are required to be accompanied by a translation sufficient
to determine compliance with the immunization requirements.
To clarify, any person can translate a foreign immunization record as long as
compliance with New Jersey’s immunization requirements can be
determined. The NJDOH does not require the translation to come from a
health care provider, however, all translations must include the printed
name and signature of the translator.
Higher Education Regulations
Q: What are the immunization requirements for students entering
institutions of higher education?
A: Per the Higher Education Rules, N.J.A.C. 8:57-6.1, the requirements
within this subchapter apply to the following:
(a) All new or continuing full- and part-time undergraduate and graduate
students enrolled in a program of study leading to an academic degree at
any public or independent institution of higher education in NJ.
(b) Two-year institutions shall apply these rules only to those students
entering the college for the first time and registering for 12 or more credit
hours of course study per semester/term.
(c) Four-year institutions shall apply the rules to all full- or part-time
students enrolled in a program leading to an academic degree.

Updated: February 2024 42 | Page
(d) Two-year institutions and Thomas Edison State College shall not be
required to apply the meningococcal rule at N.J.A.C. 8:57-6.6 and 6.7.
Below are the specific vaccination requirements for attendance:
Hepatitis B: Students entering a two- or four-year institution and enrolled
with a course study of 12 or more credit hours per semester or term shall
have received three doses of a hepatitis B containing vaccine, or
alternatively any two doses of a hepatitis B vaccine licensed and approved
for a two-dose regimen. Please see the following handout to ensure students
are receiving the appropriate dosing, immunize.org/catg.d/p2081.pdf .
Meningococcal: Institutions of higher education must ensure that newly
enrolled students receive meningococcal vaccines that are routinely
recommended by the ACIP. There are 3 types of meningococcal vaccines
available in the United States that are recommended for certain persons:
• Meningococcal conjugate vaccines (MenACWY)
• Serogroup B meningococcal vaccines (MenB)
• Pentavalent meningococcal vaccine (MenABCWY)
Please review the guidance packet available at
nj.gov/health/cd/documents/topics/meningo/meningo_requirements_higher
ed.pdf for detailed information and/or speak with your health care provider
to determine which vaccines you may need.
Measles, Mumps, Rubella: Two doses of measles vaccine and 1 dose of
mumps and rubella vaccine are required. Two MMR vaccines are also
acceptable.
Q: Are students 31 years of age and older subject to the
immunization requirements set forth in N.J.A.C. 8:57-6.4 (b)1 since
the Higher Education statute N.J.S.A. 18A:61D-1 states that the
immunization requirements specifically apply to students 30 years of
age and under?
A: The NJ Higher Education Statute, N.J.S.A. 18A:61D-1 states:
Every public and independent institution of higher education in this State
shall, as a condition of admission or continued enrollment, require every
graduate and undergraduate student who is 30 years of age or less and is
enrolled full-time or part-time in a program or course of study leading to an
academic degree, to submit to the institution a valid immunization record

Updated: February 2024 43 | Page
which documents the administration of all required immunizations against
vaccine-preventable disease, or evidence of immunity from these diseases,
in accordance with regulations promulgated by the Department of Health.
The institution shall keep the records on file in such form and manner as
prescribed by the department.
The NJDOH administrative code, N.J.A.C. 8:57-6.4, states that students born
before 1957 are exempt from the measles, mumps, and rubella (MMR)
vaccination requirement.
Since the Education Statute at N.J.S.A. 18A:61D-1 specifically states that
only students 30 years of age or less must show proof of vaccination, NJDOH
cannot require a college student over 30 years of age that meets all the
other requirements set forth at N.J.S.A. 18A:61D-1 to present proof of
vaccine or immunity for any of the required college vaccines. However,
NJDOH still highly recommends that students are age appropriately
immunized.
Q: Has the meningococcal vaccine requirements changed recently?
A: Yes. On January 13, 2020, P.L. 2019, c332 was signed into law. This new
law amends P.L.2003, c.284 (N.J.S.A.18A:62-15.1) and became effective on
June 15, 2020. In accordance with this law:
• A new student enrolling in a public or private institution of higher
education shall have received immunization for meningococcal disease
as recommended by the Advisory Committee on Immunization
Practices (ACIP) as a condition of attendance. Students must present
evidence of the vaccination(s) required.
• Each public and private institution of higher education in this State
shall offer the required meningococcal vaccines through the
institution’s student health services program or through a contracted
agreement with a community health provider.
Please note: The new meningococcal vaccine requirement applies to ALL
newly enrolled students regardless of matriculation or residential status. For
more information, please view the guidance packet available at
nj.gov/health/cd/documents/topics/meningo/meningo_requirements_higher
ed.pdf.
Q: I received a dose of MenACWY vaccine at 11 years old to attend
sixth grade in NJ. Why do I still need another dose of MenACWY for
college?

Updated: February 2024 44 | Page
A: CDC recommends a dose of MenACWY vaccine at ages 11-12 years with
a booster dose at 16 years. This is because protection from the first dose
begins to wane, so a booster dose is recommended to provide greater
protection from meningococcal disease. College students, especially
freshman living in residence halls, are at a slightly increased risk for
contracting meningococcal disease.
According to the new law, all newly enrolled students 16 through 18 will be
required to receive a dose of MenACWY on or after age 16 even if you
received a dose at a younger age. Additionally, students who are considered
high risk, including those living in residence halls, will be required to have
received a dose of MenACWY on or after the 16
th
birthday AND within 5 years
of receipt of last dose. For a list of high risk conditions, please reference the
MenACWY flow chart in the guidance packet accessible at
nj.gov/health/cd/documents/topics/meningo/meningo_requirements_higher
ed.pdf.
Please note: The meningococcal booster dose at age 16 years is not
required to attend secondary school in NJ, but you may choose to receive
the vaccine at that time in preparation for college attendance.
Q: Should schools provide information about meningococcal vaccines
to students?
A: There are currently statutes and regulations requiring distribution of
meningococcal educational materials.
Yes, it is still required for institutions to provide education on meningococcal
disease. Since 2001, institutions of higher education have been required by
law (N.J.A.C. 8:57-6.10) to provide information on meningococcal disease,
at a minimum, including its nature and severity, causes, disease prevention
and treatments, and the availability of a meningococcal vaccine to prevent
disease. The student information flyer, is available in English and Spanish at
the following links, respectively:
nj.gov/health/cd/documents/topics/meningo/are_you_protected.pdf and
nj.gov/health/cd/documents/topics/meningo/are_you_protected_spanish.pdf
This flyer may be shared to comply with this law. Alternatively, an institution
may develop their own resource to comply.
According to the recent legislation (P.L. 2019, c332), institutions of higher
education must ensure that newly enrolled students aged 16 through 23
years of age who are not routinely recommended to receive MenB vaccine
receive education on the risks and benefits of MenB vaccine and that the
vaccine be made available for students who choose to be vaccinated. ACIP
recommends that a MenB series may be administered to people 16 through
23 years of age with a preferred age of vaccination of 16 through 18 years.
Updated: February 2024 45 | Page
This recommendation allows for shared clinical decision-making between the
provider and the student based on the risk and benefit for the
individual patients.
A guidance packet which includes student information on MenB vaccine is
available at
nj.gov/health/cd/documents/topics/meningo/meningo_requirements_higher
ed.pdf
Q: Is COVID-19 vaccine required for attendance at institutions of
higher education?
A: According to the New Jersey Higher Education Rules, N.J.A.C. 8:57-6.1,
COVID-19 vaccine is not required for attendance. Institutions of Higher
Education may have set their own COVID-19 vaccine requirement. Please
check with your institution’s COVID-19 policy for more information.
NJ Immunization Information System e.g.,
‘Immunization Registry’ (NJIIS)
Q: What is NJIIS?
A: The NJ Immunization Information System (NJIIS) is a secure,
computerized, statewide immunization registry that can help parents and
health care providers keep track of immunizations given from birth through
adulthood. NJIIS is managed by the NJDOH, Vaccine Preventable Disease
Program and has been operating since 1997. For more information about
NJIIS, go to njiis.nj.gov/njiis/
Q: Who can enroll as an authorized user of NJIIS?
A: Only authorized users who have signed a confidentiality agreement can
access information on the registry. According to NJ Administrative Code
(N.J.A.C. 8:57-3.6), the following persons and entities are eligible to become
authorized users: health care providers, primary health care providers,
childcare centers, schools, colleges, universities, health benefits plans, billing
and practice management vendors, State public health or State social
services programs, local health agencies, the Department and designated
agents thereof.
Q: What is the NJIIS mandate for physicians?
A: Any health care provider that immunizes children less than seven
years of age is required by State regulation to enroll as an
authorized user of NJIIS and report vaccinations to NJIIS within 30

Updated: February 2024 46 | Page
days of administration. Mandatory participation is stipulated in the NJ
Administrative Code, N.J.A.C. 8:57-3.16.
Q: Is there a requirement for a physician’s office to input
immunizations into NJIIS within a certain time frame?
A: It is the responsibility of the health care provider—not the entity in which
he/she operates—to assure that the data are entered or sent to NJIIS. The
health care provider shall report to the NJIIS vaccines administered to
children less than seven years of age within 30 days of administration.
Practices that participate in the Vaccines for Children (VFC) program are
required to enter all VFC doses administered, regardless of the patient’s age,
into NJIIS to demonstrate accountability for all doses of VFC vaccine. The
health care provider must report all doses administered to children less than
seven years of age to NJIIS, regardless of the funding source of the vaccine
(VFC or private).
Some facilities transmit data to NJIIS through an interface. Health care
providers should check with their administrators to make sure that ongoing
data submission is being sent to NJIIS via the interface. If the data is not
submitted via an interface within this time, it is the responsibility of the
health care provider to report this information into NJIIS.
Q: Is NJIIS only for providers who vaccinate children under the age
of seven?
A: No, NJIIS is a lifespan registry and can be used for entering all vaccine
doses administered regardless of patient’s age. Clinicians who administer
vaccines to adolescents and adults are strongly recommended to become
NJIIS users to ensure that the database is as robust as possible.
Q: Does the State immunization registry, NJ Immunization
Information System (NJIIS), produce an official record that can be
used for immunization record auditing?
A: Yes, the NJIIS produces an official immunization record of a child’s
immunization history for childcare, pre-school, school, camp and college
enrollment and can be used for immunization record auditing. Other
examples of acceptable documents of immunization are the Department of
Health, Standard School/Childcare Immunization record (also known as the
IMM-8 or yellow card) and the Department of Education, State Health
History and Appraisal Form (A-45). Anyone wishing to obtain a Standard
School/ Childcare Center Immunization Record (IMM-8) can contact the NJ
Department of Health, Vaccine Preventable Disease Program at (609) 826-
4860. To obtain the A45 Health and Appraisal Record, please visit,
state.nj.us/education/students/safety/health/records/hha.shtml.
Updated: February 2024 47 | Page
Q: I see there are training for school nurses to use NJIIS. Can
childcare providers also get trained on using the registry?
Yes, childcare facilities can also take the “NJIIS School Nurse Training”.
Please visit njiis.nj.gov/core/web/index.html#/training for additional
information. These school nurse trainings are online webinars scheduled
once or twice a month. To register for an NJIIS school nurse training, please
complete the user enrollment and training request. These forms are
available at the following link:
njiis.nj.gov/docs/School%20Nurse%20New%20User%20Packet.pdf
Q: Are school nurses able to put vaccines in the registry?
A: Most school nurses have read-only access, which does not allow you to
enter vaccine doses. If school nurses would like to add historical doses, they
should contact the regional trainer for their county available at the following
link, njiis.nj.gov/core/web/index.html#/training
Q: Will vaccine doses listed as history appear on the official NJIIS
record?
A: Yes, vaccine doses entered as historical will be added to the official
immunization record.
Q: How do I merge two records in NJIIS?
A: In order to merge the records, you will need to complete the NJIIS
Duplicate Patient form (IMM-40) available at
njiis.nj.gov/core/web/index.html#/njiisDocs.
Fax the completed form to Central Maternal and Child Health Consortia
(MCHC) – NJIIS QA Unit at 732-246-3102.
Q: Can vaccine doses that were entered incorrectly be deleted from
NJIIS?
A: Only the practice who administered and entered the vaccine in NJIIS can
delete data entry errors such as entering the incorrect vaccine
administration date. In contrast, vaccine doses that were administered
outside of the ACIP recommended vaccination schedule will remain in the
system.
Clinician Resources
Q: Where can I obtain the Vaccine Declination (“Refusal to
Vaccinate”) form?
Updated: February 2024 48 | Page
A: Clinicians may refer to the American Academy of Pediatrics website
http://www2.aap.org/immunization/pediatricians/pdf/refusaltovaccinate.pdf
Q: Does NJ Department of Health (NJDOH) require a signed consent
form prior to administering a vaccination? What is required of a
health care provider before giving a vaccination?
A: No, NJDOH does not require a signed consent form prior to administering
vaccination. However, health care institutions and facilities may have their
own policies and procedures which may require a signature as a form of
consent prior to the administration of vaccine.
By Federal law, all vaccine providers must give patients, or their parents or
legal representatives, the appropriate Vaccine Information Statement (VIS)
whenever a vaccination is given. For further information about the National
Childhood Vaccine Injury Act (NCVIA) please see the following link:
immunize.org/catg.d/p2027.pdf
Q: Where can I obtain the latest Vaccine Information Statements
(VIS)?
A: All current VISs are available at the Immunization Action Coalition
website, immunize.org/vis/. VISs from these sites can be downloaded as pdf
files and printed.
Q: Where can I get a list of combination vaccinations?
A: Go to the CDC’s “Epidemiology and Prevention of Vaccine Preventable
Diseases, 13
th
edition Appendix B:
cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/B/us-vaccines.pdf
Q: I receive several patients/ students from other countries. Where
can I find a resource on vaccination schedules, by country?
A: Consider using the following tools to help with the translation of foreign
vaccine records:
• Pinkbook: Epidemiology of Vaccine Preventable Diseases | CDC
• Quick Chart of Vaccine-Preventable Terms in Multiple Languages
(immunize.org)
• International Immunization Schedules (World Health Organization)

Updated: February 2024 49 | Page
Q: Is it a violation of HIPAA to include the date that a child will be
given a vaccine dose needed for school, to be submitted by the
parent to the school for their records?
A: No, it is not a violation of HIPAA to include the appointment date that a
child plans to receive a vaccine to show documentation for the child’s
immunization record.
