
Clinical and Experimental Rheumatology 2022
Clinical and Experimental Rheumatology 2023; 41: 330-339.
Application of logistic regression and machine learning
methods for idiopathic inammatory myopathies
malignancy prediction
W. Zhang
1
, G. Huang
2
, K. Zheng
1
, J. Lin
1
, S. Hu
1
, S. Zheng
1
, G. Du
3
,
G. Zhang
4
, C. Bruni
5
, M. Matucci-Cerinic
5,6
, D.E. Furst
5,7,8
, Y. Wang
1
1
Department of Rheumatology and Immunology, Shantou Central Hospital, Shantou, Guangdong, China;
2
Department of Blood Purication, Shantou Central Hospital, Shantou, Guangdong, China;
3
Department of Radiology, Shantou Central Hospital, Shantou, Guangdong, China;
4
Department
of Pathology, Shantou University Medical College, Shantou, Guangdong, China;
5
Department of
Experimental and Clinical Medicine, Division of Rheumatology, University of Florence, Italy;
6
Unit of Immunology, Rheumatology, Allergy and Rare diseases (UnIRAR), IRCCS San Raffaele
Hospital, Milan, Italy;
7
Division of Rheumatology, Department of Medicine, University of California
at Los Angeles, CA, USA;
8
University of Washington, Seattle, WA, USA.
Abstract
Objective
Malignancy is related to idiopathic inammatory myopathies (IIM) and leads to a poor prognosis. Early prediction
of malignancy is thought to improve the prognosis. However, predictive models have rarely been reported in IIM.
Herein, we aimed to establish and use a machine learning (ML) algorithm to predict the possible risk factors for
malignancy in IIM patients.
Methods
We retrospectively reviewed the medical records of 168 patients diagnosed with IIM in Shantou Central hospital,
from 2013 to 2021. We randomly divided patients into two groups, the training sets (70%) for construction of the
prediction model, and the validation sets (30%) for evaluation of model performance. We constructed six types of ML
algorithms models and the AUC of ROC curves were used to describe the efcacy of the model. Finally, we set up a
web version using the best prediction model to make it more generally available.
Results
According to the multi-variable regression analysis, three predictors were found to be the risk factors to establish
the prediction model, including age, ALT<80U/L, and anti-TIF1-γ, and ILD was found to be a protective factor.
Compared with ve other ML algorithms models, the traditional algorithm logistic regression (LR) model was as
good or better than the other models to predict malignancy in IIM. The AUC of the ROC using LR was 0.900 in the
training set and 0.784 in the validation set. We selected the LR model as the nal prediction model. Accordingly,
a nomogram was constructed using the above four factors. A web version was built and can be visited on the website
or acquired by scanning the QR code.
Conclusion
The LR algorithm appears to be a good predictor of malignancy and may help clinicians screen, evaluate and
follow up high-risk patients with IIM.
Key words
machine learning, malignancy, idiopathic inammatory myopathies

331
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
Weijin Zhang, MD*
Guohai Huang, MD*
Kedi Zheng, MD
Jianqun Lin, MD
Shijian Hu, MD
Shaoyu Zheng, MD
Guangzhou Du, MD
Guohong Zhang, MD
Cosimo Bruni, MD, PhD
Marco Matucci-Cerinic, MD, PhD
Daniel E. Furst, MD
Yukai Wang, MD
*These authors contributed equally
and are to be considered co-rst authors.
Please address correspondence to:
Yukai Wang
Department of Rheumatology
and Immunology,
Shantou Central Hospital,
no. 114 Waima Road,
515041 Shantou,
Guangdong, China.
E-mail: [email protected]
Received on January 26, 2023; accepted
in revised form on February 13, 2023.
© Copyright CliniCal and
ExpErimEntal rhEumatology 2023.
Competing interests: C. Bruni reports
consultancy fees from Boehringer-
Ingelheim and Eli Lilly; grants from
Gruppo Italiano Lotta alla Sclerodermia
(GILS), Fondazione Italiana Ricerca
sull'Artrite (FIRA), European Scleroderma
Trial and Research (EUSTAR), Foundation
for Research in Rheumatology (FOREUM),
Italian Society for Rheumatology (SIR),
Scleroderma Clinical Trials Consortium
(SCTC), Scleroderma Research Foundation
(SRF) outside the submitted work.
M. Matucci-Cerinic has received honoraria
from Eli Lilly, BI and Galapagos.
D.E. Furst has received research support
from Actelion, Amgen, BMS, Prometheus,
Galapagos, GSK, NIH, Novartis, Pzer,
Sano, Roche/Genentech, Horizon and
Emerald; he has received consultancies
from Actelion, Amgen, BMS, Corbus,
Galapagos, Novartis, Pzer and Horizon,
and has been member of speaker's bureau
for CME only.
The other authors have declared
no competing interests.
Introduction
The idiopathic inammatory myopa-
thies (IIM) are a heterogeneous group
of autoimmune rheumatic diseases in-
volving skeletal muscle, the respiratory
system, skin and joints (1-3). The sub-
types of these IIM patients have differ-
ent clinical characteristics, including
proximal muscle weakness, rapid pro-
gressive interstitial lung disease and
severe skin lesions. Of note, one dis-
tinguishing feature of IIM, particularly
dermatomyositis (DM) or polymyositis
(PM), is a signicant association with
a risk of cancer (4). The relationship
between DM and cancer in a patient
with skin rash, muscle weakness and
gastric carcinoma was rst reported by
Stertz in 1916 (5). Since then, a variety
of malignancies have been reported to
be closely related with IIM, involving
multiple systems, including the naso-
pharyngeal, lung, breast and gastroin-
testinal systems (6). In a meta-analysis
of case control and cohort studies in-
cluding 4538 IIM patients in 5 studies,
the overall standardised incidence ratio
(SIR) as a risk for cancer was 4.66 and
1.75 for DM and PM correspondingly
(7). Moreover, the incidence of can-
cer is highest in the rst year after IIM
diagnosis (8), and its prognosis is ex-
tremely poor owing to the complexity
of these two diseases and the discrep-
ancy between tumour and IIM treat-
ment. Hence, it is of great importance
to predict the risk of malignance in IIM
patients as early as possible.
Recently, various studies have demon-
strated the close relationship between
cancer and risk factors with regard to
multiple demographics, clinical and
laboratory features. Patients with IIM
onset after 50 years old and male gen-
der may be at higher risk for developing
cancer (9, 10). In addition, increased
risk of malignancy is associated with
skin involvement, with skin necrosis
as the strongest association (9). Higher
levels of inammatory markers such as
C-reactive protein, erythrocyte sedi-
mentation rate, and creatine kinases
were also often observed in IIM pa-
tients with malignancy (11, 12). Nu-
merous myositis-associated antibodies
have been discovered and veried to
indicate different phenotypes of IIM,
with some indicators strongly suggest-
ing a high risk of cancer. Anti-p155/140
(anti-TIF1-γ) is associated with the
highest positive rate in patients with
cancer-associated myositis and this
is the predominant diagnostic sero-
logical indicator for malignancy (13).
This relationship between TIF1-γ and
cancer-associated myositis is so tight
with odds ratios reaching as high as 23
(95% CI 5.23-101.2) (14). Recent stud-
ies also showed a higher prevalence of
malignancy in IIM patients with anti-
nuclear matrix proteins (NXP)-2 and
anti-3-hydroxy-3-methyglutaryl-coen-
zyme A reductase (HMGCR) antibod-
ies. To date, a quantitative predictive
model has rarely been developed to
predict the risk for malignancy in IIM
patients. A nomogram risk prediction
model by Zhong et al. (15) showed that
patients older than 50-year-old, dys-
phagia, refractory itching and elevat-
ed creatine kinase were risk factors,
while interstitial lung disease was a
protective factor for dermatomyositis-
related-malignancy, with an area under
curve (AUC) of 0.756. However, this
model did not incorporate TIF1-γ and
only applied to patients with DM.
Notably, machine learning (ML) algo-
rithms have been widely utilised in re-
cent years to develop predictive models
which appear to have better predictive
ability than the traditional regression
approaches (16). In this retrospective,
case-control study, we aimed to use
machine learning to predict and com-
pare algorithms to establish the best
risk factors algorithm for malignancy
in IIM patients.
Materials and methods
Patients
We retrospectively reviewed the medi-
cal records of patients diagnosed with
IIM in Shantou Central Hospital, Chi-
na, from 2013 to 2021. We included
168 patients after excluding 1 patient
with too much missing information.
This study was approved by the Shan-
tou Central Hospital Ethics Commit-
tee (no. 2022-037). Patients included
into this study met the classication
criteria for IIM (1), including dermato-
myositis (DM), polymyositis (PM),
immune-mediated necrotising myopa-

332
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
thy (IMNM), anti-synthetase syndrome
(ASS), and inclusion body myositis
(IBD). Exclusion criteria were: 1) ab-
sence of complete clinical data; 2) un-
conrmed diagnosis of IIM; 3) diagno-
sis of hepatitis.
Data collection
The following data were collected:
(i) baseline information including age
and gender; (ii) clinical symptoms in-
volving muscle weakness, myalgia,
arthralgia, rash (typical skin involve-
ment of Gottron’s rash, Gottron’s sign,
mechanical hand, heliotrope rash, V-
neck sign, shawl sign and holster sign),
pruritus, dry mouth and dry eye, dys-
phagia, respiratory syndrome, fever,
oedema, cutaneous ulcer, and Raynaud
phenomenon; (iii) clinical signs includ-
ing rash. For high-risk IIM patients, es-
pecially those with several risk factors
including the elderly, DM, dysphagia,
tumour markers positivity and TIF-1γ
positivity, patients were required to be
screened for malignancy through PET/
CT, as well as gastrointestinal endo-
scope if digestive symptoms occurred
or with their consent. Otherwise, espe-
cially those with protective factors in-
cluding interstitial lung disease (ILD)
and negative TIF-1γ antibody, age-
appropriate screening, including naso-
pharyngeal MR, chest CT, abdominal
CT, gastrointestinal endoscope, breast
ultrasound and thyroid ultrasound were
performed as clinically indicated; (iv)
laboratory data including white blood
cell (WBC), lymphocyte (LY), alanine
transaminase (ALT), aspartate ami-
notransferase (AST), creatinine (Cr),
blood urea nitrogen (BUN), lactic de-
hydrogenase (LDH), creatine kinase
(CK), D-dimer, C-reaction protein
(CRP), erythrocyte sedimentation rate
(ESR), ferritin, carcino-embryonic an-
tigen (CEA), alpha fetoprotein (AFP),
carbohydrate antigen 199 (CA199),
carbohydrate antigen 125 (CA125),
complement 3 (C3), complement 4
(C4), antinuclear antibody (ANA) and
myositis antibody prole.
Statistical analysis
Statistical analysis was performed us-
ing R (v. 4.05) and SPSS 22.0 (IBM,
USA) software. Continuous variables
were expressed as mean±SD and were
analysed by Student’s t-test when
they were normally distributed. Vari-
ables were analysed by nonparametric
methods and described using medians
(Q1, Q3) when their distribution was
skewed or kurtotic. Categorical vari-
ables were analysed using χ
2
. When
univariate analysis revealed variables
with a p<0.05, they were included in
the predictive models and p>0.1 as the
criterion for removing variables.
Odds ratios (ORs) and 95% condence
intervals (95% CIs) were calculated for
all potential predictors of malignancy
when using multivariate regression.
When the regression showed a variable
to have a p<0.10, it was included in the
prediction model.
The R packages of “glmnet”, “rms”,
“caret”, “rpart”, “partykit”, “e1071”,
“MASS”, “randomForest”, “xgboost”,
and “neuralnet” were used to establish
the prediction model of ML algorithms.
The R packages of “pROC” and “rmda”
were used to validate the prediction
ability of the model. The R packages of
“corrplot” and “ggcorplot” were used
to establish the heat map. The R pack-
age of “shiny” and “shinyPredict” was
used to establish the web application.
The R packages of “ingredients” and
“DALEX” were used to show the rela-
tive importance of variables of predic-
tion model.
In this study, we randomly split pa-
tients into two groups, namely the
training sets (70%) for construction of
prediction model, and the validation
sets (30%) for evaluation of model per-
formance. We constructed six types of
ML algorithms models, i.e. Logistic re-
gression (LR), Support vector machine
(SVM), random forest (RF), Classi-
cation and regression tree (CART),
Extreme gradient boosting (XGBoost),
and Neural network (NNET). Then we
used the area under curve (AUC) of
the receiver operating characteristic
(ROC) curve to evaluate and compare
the predictive ability of the models in
the training and validation sets. The
value of the AUC of the ROC curve
was used to describe the efcacy of the
model. Finally, we set up the web ver-
sion using the best prediction model.
The prediction probability of malig-
nancy in IIM can be easily calculated
and displayed on the website after in-
putting clinical features.
Results
Population characteristics
We identied 168 patients with IIM di-
agnosed between 2013 and 2021. Twen-
Table I. Demographics, subtypes and ma-
lignancy distribution in IIM patients.
n =168
Age (years, IQR) 56.0 (44.0, 64.8)
Male n (%) 50 (29.8)
Diagnosis
DM n (%) 86 (51.2)
PM n (%) 40 (23.8)
ASS n (%) 29 (17.2)
IMNM n (%) 13 (7.7)
Malignancy (n=37)
Nasopharyngeal cancer n (%) 11 (29.7)
Breast cancer n (%) 10 (27.0)
Lung cancer n (%) 7 (18.9)
Oesophagus cancer n (%) 5 (13.5)
Cervical adenocarcinoma n (%) 1 (2.7)
Ovarian cancer n (%) 1 (2.7)
Mediastinum cancer n (%) 1 (2.7)
Multiple cancers n (%) 1 (2.7)
Time relationship between tumourigenesis
and disease diagnosis
simultaneous n (%) 20 (54.1)
before n (%) 5 (13.5)
after n (%) 12 (32.4)
DM: dermatomyositis, PM: polymyositis, ASS:
anti-synthetase syndrome, IMNM: immune-me-
diated necrotic myopathy.
Table II. Prevalence rate of malignancy in different subtypes of IIM patients.
Category DM PM ASS IMNM p
n 86 40 29 13
male n (%) 24 (27.9) 17 (42.5) 4 (13.8) 5 (38.5) 0.055
age (year) 56.0 (44.0, 63.0) 57.0 (41.3, 63.8) 58.0 (49.5, 66.5) 41.0 (31.5, 61.5) 0.137
malignancy n (%) 27 (31.4) 4 (10.0) 6(20.7) 0 0.002*
DM: dermatomyositis, PM: polymyositis, ASS: anti-synthetase syndrome, IMNM: immune-mediated
necrotic myopathy. *p<0.05.

333
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
ty-nine and eight tenths of them are
male. Median age at IIM diagnosis was
56.0 (44.0, 64.8) (Table I). Eighty-six
patients (51.2%) were classied as DM
(with 31.4% malignancy), 40 patients
(23.8%) as PM (with 10% malignancy)
and 29 patients (17.2%) as ASS (with
20.7% malignancy) the rest (7.7%) as
other IIM patients (Table II). Among
these 168 patients, 37 patients had a
malignancy (the Malignancy group).
The top three malignant tumours were
nasopharyngeal cancer (29.7%), breast
cancer (27%) and lung cancer (18.9%).
The remaining 131 patients were des-
ignated as the Non-Malignancy group.
When contrasting these two groups, we
found that patients with malignancy
were statistically signicantly older
and more frequently diagnosed as DM
(p<0.05 for both). Clinically, patients
with the following characteristics were
more likely to develop malignancies:
dysphagia (p=0.021), Gottron’s sign
(p=0.011), V-neck sign (p<0.001), and
shawl sign (p=0.016). In contrast, the
following characteristics were associ-
ated with a lower likelihood of malig-
nancy: arthralgia (p=0.011), respira-
tory involvement (p=0.04), and ILD
(p=0.003). There were no differences in
gender, muscle weakness, myasthenia,
myalgia, pruritus, dry mouth and dry
eye, fever, oedema, cutaneous ulcer,
Raynaud’s phenomenon, mechanical
hand, heliotrope rash, and holster sign
(Table III).
Among the laboratory data, the Ma-
lignancy group had statistically high-
er likelihood of a positive TIF1-γ
(p<0.001) and a lower ALT (p=0.011).
Categorical variables of ALT level were
adopted because it did not meet with
linear correlation, and ALT<80U/L
was selected as the threshold. The two
groups did not differ in term of WBC,
LY, AST, Cr, BUN, LDH, CK, D-dimer,
CRP, ESR, ferritin, CEA, AFP, CA199,
CA125, C3, C4 and the rest of the my-
ositis antibody prole (Table IV and V).
Risk factors for malignancy
among IIM patients
In the univariable analysis, age, DM,
arthralgia, dysphagia, respiratory in-
volvement, Gottron’s sign, V-neck
sign, shawl sign, ILD, ALT<80U/L,
CEA>2.0 ng/ml and anti-TIF1-γ were
statistically signicantly different and
were included in the multi-variable
regression analysis (Table VI). The
relationships among variables are il-
lustrated by a heat map analysis (Fig.
1). Based on the multivariable analysis,
the only independent risk factors that
Table III. Patients’ characteristics and symptoms in the malignancy group and non-malig-
nancy group. Data are expressed with interquartile range (Q1, Q3) if the distribution was
abnormal, and otherwise with mean ± SD for continuous data. For categorical variables,
data are expressed with number (%).
Malignancy Non-malignancy Statistics p
n=37 n=131
Age (years, IQR) 59.0 (51.5, 65.0) 55.0 (39.0, 63.0) 2.100 0.036
*
Male n (%) 15 (40.5) 35 (26.7) 2.637 0.104
DM n (%) 27 (73.0) 59 (45.0) 9.011 0.003
*
Myasthenia n (%) 28 (75.7) 87 (66.4) 1.146 0.284
Myalgia n (%) 15 (40.5) 64 (48.9) 0.801 0.371
Arthralgia n (%) 2 (5.4) 32 (24.4) 6.467 0.011
*
Pruritus n (%) 10 (27.0) 26 (19.8) 0.883 0.347
Dry mouth and dry eye n (%) 3 (8.1) 12 (9.2) 0.039 0.843
Dysphagia n (%) 13 (35.1) 23 (17.6) 5.295 0.021
*
Respiratory involvement n (%) 7 (24.3) 54 (41.2) 3.515 0.040
*
Fever n (%) 0 11 (8.4) 2.094 0.148
Oedema n (%) 3 (8.1) 4 (3.1) 0.797 0.372
Cutaneous ulcer n (%) 2 (5.4) 5 (3.8) 0.000 1.000
Raynaud phenomenon n (%) 0 6 (4.6) 0.679 0.410
Gottron’s rash n (%) 13 (35.1) 33 (25.2) 1.435 0.231
Gottron’s sign n (%) 18 (48.6) 35 (26.7) 6.426 0.011
*
Mechanical hand n (%) 4 (10.8) 16 (12.2) 0.000 1.000
Heliotrope rash n (%) 18 (48.6) 42 (32.1) 3.458 0.063
V-neck sign n (%) 18 (48.6) 23 (17.6) 15.117 <0.001
*
Shawl sign n (%) 11 (29.7) 17 (13.0) 5.830 0.016
*
Holster sign n (%) 3 (8.1) 6 (4.6) 0.708 0.400
ILD n (%) 11 (29.7) 75 (57.3) 8.747 0.003
*
DM: dermatomyositis; ILD: interstitial lung disease. *p<0.05.
Table IV. Comparison of laboratory data between malignancy group and non-malignancy
group.
Malignancy Non-malignancy Statistics p
n=37 n=131
WBC 10
9
/L 7.68 ± 3.14 8.71 ± 4.27 -1.369 0.173
LY 1 0
9
/L 1.2 (0.6, 1.7) 1.3 (0.9, 2.0) -1.188 0.235
ALB g/L n=166 35.03 ± 5.75 35.2 ± 6.4 -0.102 0.919
ALT U/L 35.0 (19.0, 69.0) 68.0 (29.0, 160.0) -2.540 0.011*
AST U/L 62.0 (27.0, 128.5) 88.0 (37.0, 190.0) -1.678 0.093
Cr umol/L 50.7 (45.6, 70.4) 55.0 (42.9, 66.7) 0.239 0.811
BUN mmol/L 4.3 (3.6, 5.5) 4.5 (3.5, 6.0) -0.136 0.892
LDH U/L n=163 405.0 (318.0, 705.5) 459.0 (331.0, 777.0) -0.750 0.453
CK U/L n=165 724.0 (156.3, 2489.0) 696.0 (171.0, 4830.0) -0.572 0.567
D-dimer ug/L n=138 880.0 (430.0, 2020.0) 696.0 (432.5, 1662.5) 0.207 0.836
CRP mg/L n=159 5.5 (3.0, 12.3) 6.4 (2.3, 14.6) 0.465 0.642
ESR mm/h n=147 14.0 (9.0, 29.0) 22.0 (10.0, 45.0) -1.928 0.054
Ferritin ng/mL n=110 704.4 (382.0, 1033.3) 583.3 (279.5, 1217.0) 0.960 0.337
CEA ng/mL n=161 2.4 (1.6, 4.5) 1.8 (1.2, 3.1) 1.889 0.059
AFP IU/ml n=160 2.1 (1.5, 3.0) 1.9 (1.4, 3.2) 0.713 0.476
CA199 U/mL n=131 7.5 (5.5, 16.2) 9.3 (5.4, 18.4) -0.628 0.530
CA125 U/mL n=130 10.2 (7.2, 15.7) 11.2 (7.3, 18.6) -0.691 0.489
C3 g/L n=146 0.91 ± 0.17 0.88 ± 0.22 0.667 0.661
C4 g/L n=146 0.23 ± 0.07 0.22 ± 0.08 0.439 0.914
WBC: white blood cell; LY: lymphocyte; ALB: albumin; ALT: alanine transaminase; AST: aspartate
aminotransferase; Cr: creatinine; BUN: blood urea nitrogen; LDH: lactic dehydrogenase; CK: creatine
kinase; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; CEA: carcino-embryonic anti-
gen; AFP: alpha fetoprotein; CA199: carbohydrate antigen 199; CA125: carbohydrate antigen 125; C3:
complement 3; C4: complement 4. *p<0.05.

334
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
predicted malignancy were age (per
ten years, OR=1.612; 95% CI [0.997,
2.607]; p=0.052- included despite be-
ing slightly greater than 0.05 based on
the literature (9, 15, 17) and clinical
judgement), ALT<80 U/L (OR=11.175;
95% CI [1.367, 91.355]; p=0.024),
and anti-TIF1-γ (OR=4.963; 95% CI
[1.193, 20.642]; p=0.028). Intersti-
tial lung disease (OR=0.193; 95% CI
[0.058, 0.643]; p=0.007) was a negative
predictive factor.
The use of machine learning
algorithms
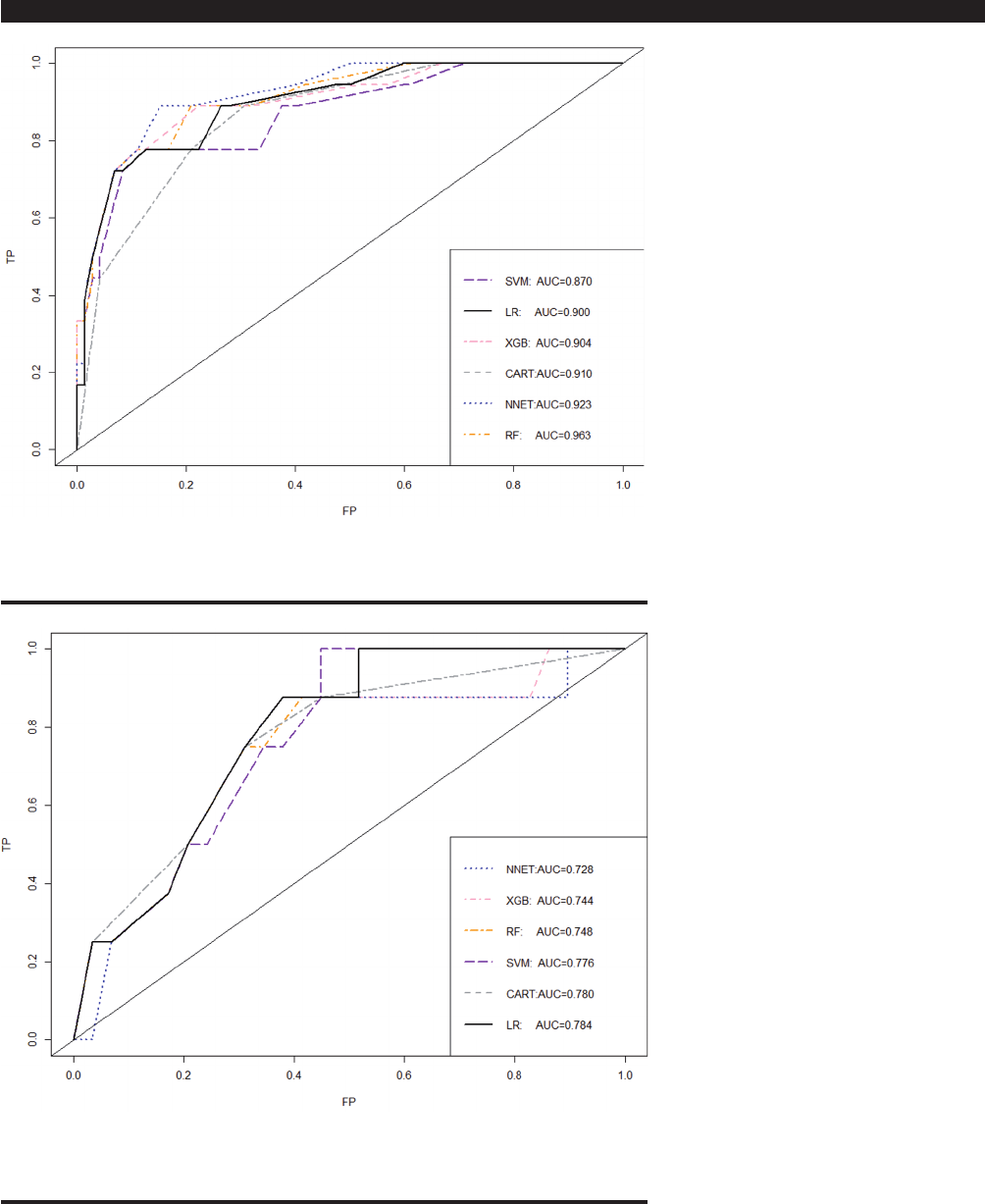
The performance of six different ML
algorithm models as predictors of ma-
lignancy in training sets and validation
sets are shown and compared in Fig-
ures 2 and 3 and Table VII and VIII,
respectively. The results showed that
the NNET and RF model possessed ex-
cellent predictive ability in the training
set, but did not do well in the validation
set. The traditional Logistic Regression
algorithm model did as well or better
than other machine learning algorithms
in predicting malignancy of IIM (AUC
of ROC was 0.900 in the training set
and 0.784 in the validation set) (Table
VII and VIII). Therefore, we selected
the LR model as the nal prediction
model.
The relative importance of
variables in prediction models
The relative importance of variables
in each prediction model is shown in
Figure 4. Although the importance of
different variables in different models
was variable, and differences among
the variables were small, anti-TIF1-γ
and low ALT were numerically the two
most importance positive predictive
variables among the 6 models and ILD
was most useful as negative predictor.
Prediction and validation of
model for malignancy in IIM patients
On the basis of the four factors selected
by multivariate analysis (Age, TIF1-γ,
ALT and ILD), for the convenience of
clinical application, we constructed a
nomogram to predict the probability
of malignancy in IIM patients (Fig. 5).
One determines the numerical value of
each factor based on the vertical line
intersection between the variable and
the point axis, and then adds all vari-
able points to calculate the total risk
score, with each risk score correspond-
ing to the probability of malignancy.
The usefulness of this nomogram will
need to be tested in several other data-
sets but it seemed useful as applied in
our patients.
The web version of model
For extending the application of the
model established in this study, a web
version was built and can be visited
on the website https://hgh-163.shin-
yapps.io/DynNomapp/ or acquired by
scanning the QR code (Fig. 6) with
a smartphone. The algorithm deter-
Table VI. Univariate and multivariable logistic regression analysis of risk factors for idio-
pathic inammatory myopathies. (Step backward, Wald test, entry condition 0.05, deletion
condition 0.10).
Factors Univariate Multivariate
p OR(95%CI) p OR (95%CI)
Age (per 10 year) 0.009 1.456 (1.099-1.931) 0.052* 1.612 (0.997-2.607)
DM 0.004 3.295 (1.476-7.355)
Arthralgia 0.022 0.177 (0.040-0.776)
Dysphagia 0.024 2.543 (1.130-5.725)
Respiratory involvement 0.065 0.458 (0.200-1.049)
Gottron’s sign 0.013 2.598 (1.225-5.512)
V-neck sign <0.001 4.449 (2.027-9.765)
Shawl sign 0.019 2.837 (1.189-6.771)
ILD 0.004 0.316 (0.144-0.693) 0.007* 0.193 (0.058-0.643)
ALT <80U/L 0.006 3.739 (1.460-9.577) 0.024* 11.175 (1.367-91.355)
CEA >2.0 ng/ml 0.031 2.316 (1.081-4.964)
TIF1γ <0.001 15.781 (4.415-56.410) 0.028* 4.963 (1.193-20.642)
DM: dermatomyositis; ILD: interstitial lung disease; ALT: alanine transaminase; CEA: carcino-embry-
onic antigen; TIF1γ: transcription intermediary factor 1γ. *p<0.10.
Table V. Comparison of myositis antibody prole between malignancy group and non-
malignancy group.
Malignancy Non-malignancy Statistics p
n=37 n=131
ANA (n=162) 21 (60.0) 69 (54.3) 0.357 0.550
MDA5 1 (3.8) 19 (17.9) 2.217 0.137
TIF1γ 10 (38.5) 4 (3.8) 22.965 <0.001
*
NXP2 0 9 (8.5) 1.221 0.269
SAE1 1 (3.8) 1 (0.9) 0.356
Mi-2 0 4 (3.8) 0.585
ARS
EJ 0 4 (3.8) 0.585
OJ 1 (3.8) 1 (0.9) 0.356
PL-7 2 (7.7) 4 (3.8) 0.112 0.738
PL-12 0 1 (0.9) 1.000
Jo-1 (n=161) 4 (11.4) 23 (18.3) 0.914 0.339
HA 0 1 (0.9) 1.000
SRP 0 11 (10.4) 1.767 0.121
HMGCR 0 1 (0.9) 1.000
Cn1a 0 2 (1.9) 1.000
PMSCL75 0 2 (1.9) 1.000
KU 1 (3.8) 1 (0.9) 0.356
RNA-PIII 0 1 (0.9) 1.000
Th/To 0 2 (1.9) 1.000
Ro-52 (n=161) 16 (45.7) 56 (44.4) 0.018 0.894
ANA: antinuclear antibody; MDA5: melanoma differentiation-associated gene 5; TIF1γ: transcription
intermediary factor 1γ; NXP2: nuclear matrix protein 2; SAE1: small ubiquitin-like modier 1; ARS:
aminoacyl tRNA synthetases; EJ: glycyl; OJ: isoleucyl; PL-7: threonyl; PL-12: alanyl; Jo-1: histidyl;
HA: tyrosyl; SRP: signal recognition particle; HMGCR: 3-hydroxy 3-methylutaryl coenzyme A re-
ductase; Cn1a: cytoplasmic 5’ nucleotidase 1A; PMSCL75: polymyositis-scleroderma 75; RNA-PIII:
RNA polymerase III. *p<0.05.

335
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
mines the predicted probability of
malignancy by inputting the clinical
characteristics.
Discussion
Malignancy may be disguised as in-
ammatory myositis, and myositis may
be a manifestation of the paraneoplas-
tic syndrome of malignancy. Thus, the
rashes of IIM may be a skin window
for recognising malignancy. About one-
third of patients with myositis develop
a malignancy within 3 years of “IIM”
diagnosis, while patients with IIM have
the highest risk of developing malig-
nancy within 1 year of diagnosis (18).
Further, malignancy remains one of the
leading causes of death in IIM patients
(19, 20). Thus, it is of great importance
to be able to predict malignancy in IIM
patients, and as early as possible, so
that treatment of the malignancy can
begin quickly, thus increasing the prob-
ability of a good outcome.
The aim of our work was to establish,
in a retrospective, case-control study,
a potentially clinically useful, multi-
variate risk prediction model for malig-
nancy in IIM patients. After examining
and comparing 6 machine learning al-
gorithms, the logistic regression model
remained at least as good as and per-
haps slightly better than the machine
learning algorithms. Based on LR, four
factors were selected as the nal com-
ponents in the model to predict malig-
Fig. 1. The relationship between different variables and malignancy.
Mali: malignant; Age: age per ten years; DM: dermatomyositis; Arth: arthralgia; Rash: rash; dysp: dysphagia; Resp: respiratory involvement; Gott: Gottron’s
sign; V nec: V-neck sign; Shaw: shawl sign; ILD: interstitial lung disease; ALT: alanine transaminase <80U/L; CEA: carcino-embryonic antigen >2.0 ng/
ml; TIF1γ: transcription intermediary factor 1γ.
The Pearson coefcient value shown on the left of the heat map. On the right of the heat map, the red ball indicates negative correlation, and the blue ball
indicates positive correlation.
The size and colour depth is positively proportional to the correlation coefcient. *p<0.05, **p<0.01.
336
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
nancy in IIM. The presence of ALT <80
U/L, increasing age and anti-TIF1-γ
predicted increased probability of ma-
lignancy, while the presence of ILD
was a protective factor.
Although age was a borderline statis-
tically signicant factor in LR, it con-
tributed to the other algorithms and age
has been associated with malignancy
in the literature. A meta-analysis in-
corporating 380 IIM patients and 1575
controls in 20 studies showed that older
age inuences susceptibility to cancer
(9). Another study reported that the
median age of DM patients with can-
cer was older than those without cancer
(17). In another study, age >50 was se-
lected as one of the factors to construct
a nomogram for predicting malignancy
in dermatomyositis patients (15). In our
study, too, the malignancy group was
older than the non-malignancy group,
with the median age 59.00 vs. 55.00,
p=0.036. And, of course, increasing
age is associated with malignancies in
general. Thus, systemic cancer screen-
ing is strongly recommended for IIM
patients older than 50 years old.
Most previous studies focused on DM
associated malignancy, while we ex-
amined the subtypes of IIM- DM, PM,
IMNM, ASS and IBM. We found that
the prevalence of malignancy in pa-
tients with DM was associated with
the highest risk of cancer-31.4% in
our cohort, which is within the 13% to
42% range found in the literature (7,
21, 22). The V-neck sign, shawl sign
and Gottron’s sign, which are typical
signs of DM, also predicted malig-
nancy (p<0.05). Non-statistical differ-
ence in Gottron’s rash, holster sign and
mechanical hand may be due to small
sample size in this study and weaker
correlation with malignancy. Thus, a
larger and prospective study should be
carried out for verication of different
rashes in malignancy prediction.
We found a relatively high prevalence
of cancer in ASS, with 6 malignan-
cies in 29 patients. This is the second
most common prevalence after DM.
The literature reports this relationship
rarely, perhaps because ASS is a rela-
tively rare disease. The literature re-
ports a prevalence of up to 16.6% and
our prevalence was 20.7%, in the same
general range as the literature (23).
Malignancy was found in 10.0% of PM
patients, ranking the third highest risk,
which was also consistent with the re-
ported risk range of 3% to 18% (21, 24).
Among the 13 patients with IMNM in our
cohort, no cancer was found and IMNM
was not a risk factor for malignancy. Of
course, the very few patients with IMNM
make any predictive algorithm suspect.
The lack of malignancy in these patients
is supported by the literature, as extra-
muscular involvement is rare in IMNM.
The two serological markers of IMNM,
Fig. 2. ROC curve analysis of different machine algorithms prediction model for malignancy of
idiopathic inammatory myopathies in the training set.
SVM: support vector machine; LR: logistic regression; XGBoost: Extreme Gradient Boosting; CART:
classication and regression tree; NNET: neural network; RF: random forest.
Fig. 3. ROC curve analysis of different machine algorithms prediction model for malignancy of
idiopathic inammatory myopathies in the validation set.
NNET: neural network; XGBoost: Extreme Gradient Boosting; RF: random forest; SVM: support
vector machine; CART: classication and regression tree; LR: logistic regression.

337
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
anti-SRP antibody and anti-HMGCR an-
tibody, were not associated with malig-
nancy in a meta-analysis (25) although
one author did nd such a relationship
(26). More research clearly needs to be
done in this area.
It was interesting to examine the use-
fulness of antibodies when predicting
malignancies in IIM. The presence of
TIF1-γ (155-kDa) increased the risk
of a malignancy vefold, and it was an
important variable as a predictor in all
models. This nding is supported by
the literature. A meta-analysis regard-
ing the usefulness of TIF1-γ in DM
showed that 80% of myositis patients
with malignancy tested positive for this
antibody, and 90% of patients without
malignancy tested negative, indicating
high sensitivity and specicity (27). Al-
though the sensitivity and specicity in
our study were not as high as the above
(31.25% and 71.42% respectively), it
might be a simple screening tool which
would alert the clinician that a given
patient needs to be closely followed.
Another well-recognised cancer-as-
sociated antibody is NXP-2, which is
linked with muscle weakness and ele-
vated CK (28). Ichimura et al. (29) col-
lected 445 cases of DM and 62 cases of
PM, of whom 7 (1.6%) and 1 (1.6%)
tested positive in NXP-2, respectively,
although another study found no differ-
ence in NXP2 positivity among those
with or without cancer (30). Of the 8
patients described by Ichimura et al., 3
cases (37.5%) developed visceral ma-
lignancies within 3 years of IIM diag-
nosis, indicating that NXP-2 might be
a marker of increased cancer risk in
IIM. This hypothesis was supported by
Fiorentino et al. (31). Thus far, no can-
cer has been observed in our 9 NXP2
positive patients, with follow-up of up
to 8 years. Larger studies are needed to
determine the usefulness of NXP2.
In our study, ILD was identied as a
protective factor for malignancy in pa-
tients with IIM, which was consistent
with the medical literature (32, 33).
According to a retrospective study by
Zhong et al., there is a negative corre-
lation between ILD and tumours in DM
patients, with an OR value as low as
Table VII. Predictive performance of different machine algorithms model for malignancy
of idiopathic inammatory myopathies in the training set.
Models Accuracy Sensitivity Specicity AUROC
LR 0.867 0.875 0.866 0.900
CART 0.856 0.727 0.873 0.910
SVM 0.856 0.727 0.873 0.870
RF 0.867 0.800 0.875 0.963
NNET 0.879 0.722 0.918 0.923
XGB 0.878 0.818 0.886 0.904
LR: logistic regression; CART: classication and regression tree; SVM: support vector machine; RF:
random forest; NNET: neural network; XGB: Extreme Gradient Boosting.
Fig. 4. Relative importance of each variable in different machine algorithms prediction model.
LR: logistic regression (A); SVM: support vector machine (B); CART: classication and regression tree (C); RF: random forest (D); XGBoost: Extreme
Gradient Boosting (E); NN: neural network (F).
Table VIII. Predictive performance of different machine algorithms model for malignancy
of idiopathic inammatory myopathies in the validation set.
Models Accuracy Sensitivity Specicity AUROC
LR 0.784 0.500 0.818 0.784
CART 0.811 0.667 0.824 0.780
SVM 0.811 0.667 0.824 0.776
RF 0.811 0.667 0.824 0.748
NNET 0.730 0.375 0.828 0.728
XGB 0.784 0.500 0.818 0.744
LR: logistic regression; CART: classication and regression tree; SVM: support vector machine; RF:
random forest; NNET: neural network; XGB: Extreme Gradient Boosting.

338
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
0.367 (95% CI [0.147, 0.913]) in mul-
tivariable analysis (15). Furthermore, a
meta-analysis conrmed the protective
role of ILD in cancer of IIM (9). On
the other hand, MDA5 positive patients
(some with lung disease) have a poor
prognosis in DM, and the medications
used to treat DM predispose to cancers
and ILD per se can increase the risk of
lung cancer (34, 35). These conicting
factors are puzzling and certainly will
require further research.
Similar to the 151 patient study of So
et al. (21), our results pointed out that
the following were risk factors for ma-
lignancy in IIM: (i) older age; (ii) ALT
<80 U/L; (iii) the presence of TIF1-γ;
and (iv) the absence of ILD. However,
we did not nd that dysphagia predicted
cancer in multivariable analysis. Coin-
cident with our result, their laboratory
data also showed that lower serum AST
was related with malignancy in IIM pa-
tients and this phenomenon was rarely
reported. Possibly, IIM patients without
malignancy may have more probability
to behave with another system involve-
ment, for instance and dominantly in
muscle injury wherein the ALT or AST
are usually elevated. This variable in-
deed surprised us due to its high weight
among the ML models (21).
Several studies (4, 6, 36) have shown
that inammatory indicators, CRP and
ESR, can be used as predictive markers
for malignancy. However, it was not the
case in our research, perhaps because of
the other factors were more important
in the multi-variable models, making
these not statistically signicant. Also,
inammation is not merely associated
with the tumour, but also may be related
to patient’s multiple other concurrent,
co-morbidities (e.g. infection, necrosis,
drugs, other inammatory conditions)
which may confound their usefulness.
Our data have some notable strengths.
It includes a relatively robust number
of patients which have been sub-set into
IIM subtypes and carefully followed
and analysed. Also, updated serologi-
cal testing has been done (e.g. TIF1-γ).
Further a relatively sophisticated anal-
ysis was undertaken using machine
learning technology, thus increasing the
robustness of the results, increasing the
probability of credible results and al-
lowing internal consistency. Further we
tried to make the results easily available
for clinical use thru the development of
the web-based nomogram.
However, our data have some limita-
tions. First, our data comes from a
single-centre, which may enrol sicker
patients and increase the prevalence of
malignancy. Furthermore, because the
machine learning algorithm itself is
closely related with sample size, better
performance of LR was probably due
to the relatively small sample. Thus, it
needs to be further veried by multi-
center studies. Second, this is a retro-
spective study and data were missing,
especially the anti-myositis antibody
proles. Third, the length of follow-up
would ideally be longer. Fourth, tests
for cryoglobulinaemia and elevated
aldolase were not available in our unit
and confounding with other diseases
may have occurred, although it was un-
likely based on clinical results.
This study summarised the possible
risk factors for malignancy in a retro-
spective and case-control study of IIM
patients, and established a multivariate
risk prediction model of LR, which has
good usefulness for clinical application
and may help clinicians screen, evalu-
ate and follow up those high-risk pa-
tients with IIM. However, it still needs
more cases to optimise this model.
References
1. MARIAMPILLAI K, GRANGER B, AMELIN D
et al.: Development of a new classication
system for idiopathic inammatory myopa-
thies based on clinical manifestations and
myositis-specic autoantibodies. Jama Neu-
rol 2018; 75: 1528-37. https://
doi.org/10.1001/jamaneurol.2018.2598
2. CARDELLI C, ZANFRAMUNDO G, COMETI L
et al.: Idiopathic inammatory myopathies:
One year in review 2021. Clin Exp Rheuma-
tol 2022; 40: 199-209. https://
doi.org/10.55563/clinexprheumatol/vskjxi
3. LI Y, LI Y, WANG Y et al.: Nomogram to
predict dermatomyositis prognosis: A pop-
ulation-based study of 457 cases. Clin Exp
Rheumatol 2022; 40: 247-53. https://
doi.org/10.55563/clinexprheumatol/0ddt88
4. MOGHADAM-KIA S, ODDIS CV, ASCHERMAN
DP, AGGARWAL R: Risk factors and cancer
screening in myositis. Rheum Dis Clin North
Am 2020; 46: 565-76.
Fig. 5. The nomogram prediction model of the malignancy probability in IIM patients. Determine the
value of each factor based on the vertical line intersection between the variable and the point axis, and
then add all variable points to calculate the total risk score, with each risk score corresponding to the
probability of malignancy.
Fig. 6. QR code to be scanned with a smart-
phone for nomogram prediction.

339
Clinical and Experimental Rheumatology 2023
Prediction model for malignancy in IIM / W. Zhang et al.
https://doi.org/10.1016/j.rdc.2020.05.006
5. PRZYBYLSKI G, JARZEMSKA A, CZERNI-
AK J, SIEMIATKOWSKA K, GADZINSKA A,
CIESLINSKI K: A case report of a patient with
dermatomyositis as a prodromal sign of lung
cancer. Pol Arch Med Wewn 2008; 118: 143-7.
6. TINIAKOU E, MAMMEN AL: Idiopathic in-
ammatory myopathies and malignancy: a
comprehensive review. Clin Rev Allergy Im-
munol 2017; 52: 20-33.
https://doi.org/10.1007/s12016-015-8511-x
7. QIANG JK, KIM WB, BAIBERGENOVA A,
ALHUSAYEN R: Risk of malignancy in der-
matomyositis and polymyositis. J Cutan Med
Surg 2017; 21: 131-6.
https://doi.org/10.1177/1203475416665601
8. CHEN D, YUAN S, WU X et al.: Incidence
and predictive factors for malignancies with
dermatomyositis: A cohort from southern
China. Clin Exp Rheumatol 2014; 32: 615-
21.
9. WANG J, GUO G, CHEN G, WU B, LU L, BAO L:
Meta-analysis of the association of dermato-
myositis and polymyositis with cancer. Br J
Dermatol 2013; 169: 838-47.
https://doi.org/10.1111/bjd.12564
10. ANDRAS C, PONYI A, CONSTANTIN T et al.:
Dermatomyositis and polymyositis associat-
ed with malignancy: A 21-year retrospective
study. J Rheumatol 2008; 35: 438-44.
11. SPARSA A, LIOZON E, HERRMANN F et al.:
Routine vs extensive malignancy search for
adult dermatomyositis and polymyositis: a
study of 40 patients. Arch Dermatol 2002;
138: 885-90.
https://doi.org/10.1001/archderm.138.7.885
12. GALLAIS V, CRICKX B, BELAICH S: [Prog-
nostic factors and predictive signs of malig-
nancy in adult dermatomyositis]. Ann Der-
matol Venereol 1996;123:722-6.
13. KAJI K, FUJIMOTO M, HASEGAWA M et al.:
Identication of a novel autoantibody reac-
tive with 155 and 140 kDa nuclear proteins
in patients with dermatomyositis: An asso-
ciation with malignancy. Rheumatology (Ox-
ford) 2007; 46: 25-8.
https://doi.org/10.1093/rheumatology/kel161
14. TRALLERO-ARAGUAS E, LABRADOR-HOR-
RILLO M, SELVA-O’CALLAGHAN A et al.:
Cancer-associated myositis and anti-p155
autoantibody in a series of 85 patients with
idiopathic inammatory myopathy. Medicine
(Baltimore) 2010; 89: 47-52. https://
doi.org/10.1097/MD.0b013e3181ca14ff
15. ZHONG J, HE Y, MA J, LU S, WU Y, ZHANG J:
Development and validation of a nomogram
risk prediction model for malignancy in der-
matomyositis patients: A retrospective study.
Peerj 2021; 9: e12626.
https://doi.org/10.7717/peerj.12626
16. WU Y, RAO K, LIU J et al.: Machine learn-
ing algorithms for the prediction of central
lymph node metastasis in patients with papil-
lary thyroid cancer. Front Endocrinol (Laus-
anne) 2020; 11: 577537.
https://doi.org/10.3389/fendo.2020.577537
17. ANTIOCHOS BB, BROWN LA, LI Z, TOSTE-
SON TD, WORTMANN RL, RIGBY WF: Malig-
nancy is associated with dermatomyositis but
not polymyositis in Northern New England,
USA. J Rheumatol 2009; 36: 2704-10.
https://doi.org/10.3899/jrheum.090549
18. YANG Z, LIN F, QIN B, LIANG Y, ZHONG R:
Polymyositis/dermatomyositis and malig-
nancy risk: A metaanalysis study. J Rheuma-
tol 2015; 42: 282-91.
https://doi.org/10.3899/jrheum.140566
19. DOBLOUG GC, GAREN T, BRUNBORG C,
GRAN JT, MOLBERG O: Survival and can-
cer risk in an unselected and complete Nor-
wegian idiopathic inammatory myopathy
cohort. Semin Arthritis Rheum 2015; 45:3
01-8. https://
doi.org/10.1016/j.semarthrit.2015.06.005
20. NUNO-NUNO L, JOVEN BE, CARREIRA PE et
al.: Mortality and prognostic factors in idio-
pathic inammatory myositis: A retrospec-
tive analysis of a large multicenter cohort of
Spain. Rheumatol Int 2017; 37: 1853-61.
https://doi.org/10.1007/s00296-017-3799-x
21. SO MW, KOO BS, KIM YG, LEE CK, YOO B:
Idiopathic inammatory myopathy associ-
ated with malignancy: A retrospective cohort
of 151 Korean patients with dermatomyosi-
tis and polymyositis. J Rheumatol 2011; 38:
2432-5.
https://doi.org/10.3899/jrheum.110320
22. SIGURGEIRSSON B, LINDELOF B, EDHAG O,
ALLANDER E: Risk of cancer in patients with
dermatomyositis or polymyositis. A popula-
tion-based study. N Engl J Med 1992; 326:
363-7. https://
doi.org/10.1056/NEJM199202063260602
23. DUGAR M, COX S, LIMAYE V, BLUMBERGS
P, ROBERTS-THOMSON PJ: Clinical hetero-
geneity and prognostic features of South Aus-
tralian patients with anti-synthetase autoanti-
bodies. Intern Med J 2011; 41: 674-9. https://
doi.org/10.1111/j.1445-5994.2010.02164.x
24. STOCKTON D, DOHERTY VR, BREWSTER
DH: Risk of cancer in patients with dermato-
myositis or polymyositis, and follow-up
implications: A Scottish population-based
cohort study. Br J Cancer 2001; 85: 41-5.
https://doi.org/10.1054/bjoc.2001.1699
25. OLDROYD A, ALLARD AB, CALLEN JP et al.:
A systematic review and meta-analysis to
inform cancer screening guidelines in idio-
pathic inammatory myopathies. Rheuma-
tology (Oxford) 2021; 60: 2615-28. https://
doi.org/10.1093/rheumatology/keab166
26. ALLENBACH Y, KERAEN J, BOUVIER AM et
al.: High risk of cancer in autoimmune ne-
crotizing myopathies: Usefulness of myositis
specic antibody. Brain 2016; 139: 2131-5.
https://doi.org/10.1093/brain/aww054
27. TRALLERO-ARAGUAS E, RODRIGO-PENDAS
JA, SELVA-O’CALLAGHAN A et al.: Useful-
ness of anti-p155 autoantibody for diagnos-
ing cancer-associated dermatomyositis: a
systematic review and meta-analysis. Arthri-
tis Rheum 2012; 64: 523-32.
https://doi.org/10.1002/art.33379
28. CASCIOLA-ROSEN L, MAMMEN AL: Myo-
sitis autoantibodies. Curr Opin Rheumatol
2012; 24: 602-8. https://
doi.org/10.1097/BOR.0b013e328358bd85
29. ICHIMURA Y, MATSUSHITA T, HAMAGUCHI
Y et al.: Anti-NXP2 autoantibodies in adult
patients with idiopathic inammatory myo-
pathies: Possible association with malignan-
cy. Ann Rheum Dis 2012; 71: 710-3. https://
doi.org/10.1136/annrheumdis-2011-200697
30. FREDI M, CAVAZZANA I, CERIBELLI A et al.:
An Italian multicenter study on Anti-NXP2
antibodies: Clinical and serological asso-
ciations. Clin Rev Allergy Immunol 2022; 63:
240-50.
https://doi.org/10.1007/s12016-021-08920-y
31. FIORENTINO DF, CHUNG LS, CHRISTOPHER-
STINE L et al.: Most patients with cancer-
associated dermatomyositis have antibodies
to nuclear matrix protein NXP-2 or transcrip-
tion intermediary factor 1gamma. Arthritis
Rheum 2013; 65: 2954-62.
https://doi.org/10.1002/art.38093
32. IKEDA S, ARITA M, MISAKI K et al.: Inci-
dence and impact of interstitial lung disease
and malignancy in patients with polymyosi-
tis, dermatomyositis, and clinically amyo-
pathic dermatomyositis: A retrospective co-
hort study. Springerplus 2015; 4: 240.
https://doi.org/10.1186/s40064-015-1013-8
33. LU X, YANG H, SHU X et al.: Factors pre-
dicting malignancy in patients with poly-
myositis and dermatomyostis: A systematic
review and meta-analysis. Plos One 2014; 9:
e94128.
https://doi.org/10.1371/journal.pone.0094128
34. BALLESTER B, MILARA J, CORTIJO J: Idio-
pathic pulmonary brosis and lung cancer:
Mechanisms and molecular targets. Int J Mol
Sci 2019; 20: 593.
https://doi.org/10.3390/ijms20030593
35. LI J, YANG M, LI P, SU Z, GAO P, ZHANG J:
Idiopathic pulmonary brosis will increase
the risk of lung cancer. Chin Med J (Engl)
2014; 127: 3142-9.
36. LEE SW, JUNG SY, PARK MC, PARK YB, LEE
SK: Malignancies in Korean patients with
inammatory myopathy. Yonsei Med J 2006;
47: 519-23.
https://doi.org/10.3349/ymj.2006.47.4.519
