
TM
BIO-RAD
Giant Panda Problem Kit
for AP Biology:
A ThINQ!
™
Investigation
Catalog #17002878EDU
AP Biology Instructor’s Guide
Note: This kit contains temperature-sensitive
reagents. Open immediately upon arrival
and store components at 4°C as indicated.
Duplication of any part of this document
is permitted for classroom use only.
Please visit explorer.bio-rad.com to access
our selection of language translations
for Bio-Rad Explorer kit curricula.
For technical service, call your local Bio-Rad
offi ce or, in the U.S., call 1-800-424-6723
Dear Instructor
Thank you for inspiring the next generation of scientists, citizens, and decision
makers to be curious about the world around them. Our goal is to provide you
with tools to help your students think like real scientists. Bio-Rad’s ThINQ!
™
Investigations guide your students in asking relevant questions, making exciting
discoveries, and analyzing their results to learn from failures as well as successes.
This inquiry-based laboratory curriculum guides students through the scientifi c
process of developing and selecting a question to examine, planning and
executing experiments, documenting observations, and analyzing data. The focus
of this laboratory investigation is not solely on the answer or result, but rather
on how the result was obtained. Instead of providing students with explanations
or interpretations, the student manual poses a series of questions to focus and
stimulate thinking about all aspects of the investigation. To facilitate the teacher’s
role, explanations and interpretations are included in the instructor’s guide.
The Giant Panda Problem Kit uses two variations of an enzyme-linked
immunosorbent assay (ELISA) to determine the presence of a pregnancy-
associated disorder and to track reproductive hormones in giant pandas.
Investigation #1 in this kit engages students in technique basics and biochemical
interactions as they learn about humoral immune responses. The inquiry
investigation (#2) following Investigation #1 asks students to apply what they have
learned to a novel problem, tracking reproductive hormones using a variation of
the same assay. The Giant Panda Problem Kit is powerful in that two major
body systems (the immune system and the endocrine system)
can be explored in one laboratory activity.
The integrated nature of the biology concepts
covered by this kit is useful in building curricular
bridges to other content areas you may
teach. Connections can be made to ecology,
survival and fi tness, climate change,
and the interrelatedness between body
systems, among others. The Giant Panda
Problem Kit covers those processes in
the context of biological systems using
free energy (AP Big Idea 2) and the
interaction of biological systems
(AP Big Idea 4).
The inquiry-based curriculum of
Bio-Rad’s Giant Panda Problem Kit integrates immunity and
reproductive endocrinology into a single laboratory activity. The kit’s potential to
help students discover that body systems are connected has generated genuine
excitement among science educators. We strive to continually improve our
curriculum and products, and your input is extremely important to us. We welcome
your stories, comments, curriculum extensions, and suggestions.
Special thanks to Tim Guilfoyle, AP Biology and Microbiology teacher, for
proposing the name of this kit!
Bio-Rad Explorer
™
Team
Bio-Rad Laboratories, Inc.
6000 James Watson Drive, Hercules, CA 94547
www.explorer.bio-rad.com
DEAR INSTRUCTOR

i
explorer.bio-rad.com
Before You Start ..........................................................................................................................................................................1
Kit Storage .....................................................................................................................................................................................1
Important Notes .............................................................................................................................................................................1
Kit Summary ...................................................................................................................................................................................1
Kit Inventory Checklist ....................................................................................................................................................................2
Road Map to the Instructor’s Guide ................................................................................................................................................4
Kit Timeline .....................................................................................................................................................................................6
AP Biology Standards and Giant Panda Problem Kit Alignment ......................................................................................................9
Background for Instructors ...........................................................................................................................................................10
Instructor’s Advance Preparation ............................................................................................................................................15
Investigation #1: ELISA Antibody Test (Optional) ...........................................................................................................................17
Investigation #2: Hormone Detection ELISA ..................................................................................................................................18
Teacher Model Process ............................................................................................................................................................19
Instructor’s Guide to the Student Manual ...............................................................................................................................21
Background ..................................................................................................................................................................................21
Pre-Lab: Modeling Hormone Cycles in the Giant Panda................................................................................................................26
Investigation #1: Digital Animation Activity — ELISA Antibody Simulation ......................................................................................29
Digital Animation Activity — ELISA Antibody Simulation ..........................................................................................................30
Investigation #1: ELISA Antibody Test (Optional Structured Inquiry Activity) ...................................................................................33
Protocol ..................................................................................................................................................................................34
Post-Investigation Questions ........................................................................................................................................................36
Assignment: Investigation #1 Wrap-Up .........................................................................................................................................37
ELISA Paper Model (Optional Activity) ...........................................................................................................................................38
Investigation #2: Hormone Detection ELISA (Structured/Guided/Open Inquiry Activity) .................................................................39
Pre-Investigation Questions ..........................................................................................................................................................40
Post-Lab Assessment ..................................................................................................................................................................42
Appendices ................................................................................................................................................................................45
Appendix A: Quick Guide for Investigation #2 ..............................................................................................................................45
Appendix B: Immunological Concepts .........................................................................................................................................48
Appendix C: Glossary of Terms ....................................................................................................................................................55
Appendix D: Quantitative ELISA Laboratory Activity .....................................................................................................................58
Appendix E: References and Additional Resources ......................................................................................................................62
Appendix F: ELISA Paper Model ..................................................................................................................................................63
Contents
CONTENTS

ii

1
explorer.bio-rad.com
Kit Storage
Place the reagent bag in the refrigerator (4°C) upon arrival.
Once stock reagents are prepared, store the diluted/
reconstituted solutions at 4°C to ensure stability.
Important Notes
• Go to
bio-rad.com/PandaAPResources
to download the
Student Manual, the Teacher Model Process, the
animation question sheet (from the student manual),
and the experimental design and planning work sheet
• The printed Answer Guide is included in the kit
• Technical support is available at [email protected] or 1-800-424-6723, option 2
Kit Summary
The Giant Panda Problem Kit for AP Biology: A ThINQ!
™
Investigation allows students to explore, ask questions, make predictions
and test hypotheses, take measurements, and analyze data to explore immune responses and reproductive endocrinology.
Students investigate how antigen and antibody interactions can be detected to determine the presence of a specifi c disease agent
using an ELISA assay. Students then apply their learning to a conservation biology context wherein they model and design their
own ELISA tests to detect hormone levels in simulated giant panda urine samples. The kit contains suffi cient materials for eight
student workstations with four students each to test for the presence of antigen in samples for Investigation #2.
The kit also contains suffi cient reagents to perform optional Investigation #1 ELISA Antibody Test; however, the purchase of additional
disposable plastic transfer pipets (DPTPs) is required. Alternatively, it is possible to reuse the DPTPs in the kit, provided they are
washed and rinsed well. Reusing DPTPs introduces the possibility of cross contamination and should be undertaken with caution.
• Investigation #1: Digital Animation Activity — ELISA Antibody Simulation
ELISA Antibody test
• Investigation #2: Hormone Detection ELISA
Using a Single Assay to Study Two Body Systems
With this kit, students will investigate two distinct body systems using one central scientifi c concept and technique — an ELISA
assay. Investigation #1 familiarizes students with an antibody detection ELISA assay. Not only are students learning how an
ELISA works, they are also diagnosing giant pandas in a simulated study for a reproductive disorder. It is important that students
understand how the assay works, as they will be making their own protocol adjustments in Investigation #2 when they determine if
simulated panda urine samples contain reproductive hormones that may indicate the onset of ovulation.
As the instructor, it is your choice how much time you would like to spend discussing immune responses with your students
(Investigation #1) and reproductive endocrinology (Investigation #2). Students may require support to connect these two distinct
body systems. One way to make the connection clear for them is through the application of the ELISA assay. In both investigations
this assay is used to determine the presence or absence of an antibody (Investigation #1) or antigen (a hormone in Investigation
#2). In both investigations the interaction between antibodies (primary and secondary) and antigen is critical for diagnosis via a
colorimetric reaction.
As an extension, another means for connecting these two body systems and their function is via the stress hormone cortisol.
Several studies can support students’ understanding of stress on the body (see bibliography for further reading). Cortisol, the
primary “stress” hormone, is known to reduce the function of the reproductive system by preventing ovulation. This is particularly
problematic in giant pandas living in captivity. As giant pandas experience chronic stress due to limited access to physical
space in their enclosures, access to agreeable food and companions, exposure to unfavorable weather and caretakers and the
general public through viewing at zoos, both their immune system and reproductive system functions may suffer. Caretakers are
continually on the lookout for signs of stress in pandas in order to mitigate stressors and optimize their reproductive capacity.
Before You Start
BEFORE YOU START

2
Kit Inventory Checklist
This section lists the components provided in the ThINQ!
™
Giant Panda Problem Kit for AP Biology. It also lists the required
accessories. As soon as your kit arrives, open it and check off the listed components to familiarize yourself with the kit.
Immediately place the bag with temperature sensitive reagents in the refrigerator (4°C).
Store at 4°C Quantity per Kit (✔)
Antigen, chicken gamma globulin, lyophilized 1 vial
Primary antibody, rabbit anti-chicken polyclonal antibody
, lyophilized 1 vial
Secondary antibody, goat anti-rabbit antibody conjugated to
horseradish peroxidase (HRP), lyophilized 1 vial
HRP enzyme substrate 3,3’,5,5’-tetramethylbenzidine (TMB) 1 bottle
Store at room temperature Quantity per Kit (✔)
10x phosphate buffered saline (PBS) 1 bottle
10% T
ween 20 1 bottle
Disposable plastic transfer pipets (DPTPs) 80
Micr
oplates with 12-well strips 3 plates of 8 strips
Yellow microcentrifuge tubes, 2.0 ml 60
Colored microcentrifuge tubes, 2.0 ml 85
(17 each of green, blue, orange, violet, and brown)
Giant Panda Problem Kit for AP Biology Answer Guide, printed 1
Required Accessories (not included) Quantity (✔)
Paper towels 4 r
olls or packs
Beakers, 100–200 ml 12
Reagent bottles or tubes, 50 ml 3
Marking pens, black 12
Graduated cylinder
, 100 ml 1
Graduated cylinder, 1 L 1
Distilled water, 1 L 1
Kit Components
BEFORE YOU START
KIT INVENTORY CHECKLIST

3
explorer.bio-rad.com
Ordering Information
Catalog # Product Description
1662401EDU ELISA kit reagent r
efi ll package, includes antigen, primary antibody, secondary antibody,
10x phosphate buffered saline, 10% Tween 20, and HRP enzyme substrate
1662402EDU HRP enzyme substrate (TMB), 25 ml
1662403EDU Phosphate buffered saline, 10x, 100 ml
1610780EDU Phosphate buffered saline, 10x, 1 L
1662404EDU Tween 20, 10%, 5 ml
1610781EDU Tween 20, 10%, 1 L
1662405EDU Microplates with 12-well strips, 3 plates of 8 strips
1662406EDU Antigen, chicken gamma globulin, lyophilized
1662407EDU Primary antibody, rabbit anti-chicken polyclonal antibody, lyophilized
1662408EDU Secondary antibody, goat anti-rabbit antibody conjugated to HRP, lyophilized
1660474EDU Disposable plastic transfer pipets, sterile, 500
1660480EDU Disposable plastic transfer pipets, nonsterile, 500
1660473EDU Colored 1.5 ml microcentrifuge tubes, 6 colors, 600
2239480EDU EZ Micro
™
test tubes, 1.5 ml, natural, 500
2239430EDU EZ Micro test tubes, 2.0 ml, natural, 500
1660481EDU Microcentrifuge tube racks, set of 5 racks
Optional Accessories
Catalog # Description Quantity (✔)
1660480EDU Disposable plastic transfer pipets, nonsterile 80
1681130EDU iMark
™
Microplate Absorbance Reader 1
1660481EDU Microcentrifuge tube racks 8
1660515EDU Micropipets, 50 µl fi xed-volume 8
or
1660507EDU Micropipets, 20–200 µl adjustable volume 8
2239035EDU Standard pipet tips, 2–200 µl 150
BEFORE YOU START
KIT INVENTORY CHECKLIST

4
Road Map to the Instructor’s Guide
The Giant Panda Problem Kit for AP Biology guides you and your students through an investigation of biological systems using free
energy (AP Big Idea 2) and the interaction of biological systems (AP Big Idea 4).
The activities included in this kit are:
• Pre-lab modeling activity
• Two inquiry investigation labs
• Post-lab assessment questions
Pre-Lab Activity — this activity will set the stage for students as they consider the case of giant panda reproduction as a means
for sustaining a vulnerable species using technology. The pre-lab will help your students access and model their prior knowledge
and understandings of the immune system and specifi cally antigen and antibody interactions, why these interactions take place,
and how they can be detected. Students’ models will be revisited during the subsequent investigations. As students gather and
analyze data, they will be encouraged to revisit and revise their initial models, generated in the pre-lab.
Inquiry Investigations
These investigations use an inquiry-based approach to instill scientifi c skills and develop critical thinking in your students. They
are divided into several stages and levels of inquiry. The extent of inquiry implementation (structured, guided, and open inquiry)
is fl exible and entirely up to you. The guided and open inquiry investigation (Investigation #2) can be conducted in a structured
manner if desired. The Quick Guide to Investigation #2 (Appendix A) provides a structured inquiry protocol.
Structured Inquiry Investigation — introduces the basics of the colorimetric ELISA assay featured in this kit. Students are also
introduced to detection of antibodies as an indicator of exposure to a disease-causing agent.
• Digital Animation Activity: ELISA Antibody Simulation
• Investigation #1: ELISA Antibody Test (Optional)
Teacher Note: Two options are offered for the Antibody ELISA Test (a digital animation activity and the optional hands-on
Investigation #1). Depending on the amount of time you have to use the kit with your students you may choose one or the other.
You can also choose to use both options with your students. See the timeline options on page 6 to determine what works best
in your classroom. The kit contains suffi cient reagents to perform optional Investigation #1 ELISA Antibody Test; however, the
purchase of additional disposable plastic transfer pipets (DPTPs) is required. Alternatively, it is possible to reuse the DPTPs in the
kit, provided they are washed and rinsed well. Reusing DPTPs introduces the possibility of cross contamination and should be
undertaken with caution.
Structured, Guided, or Open Inquiry Investigation — you choose the level of inquiry (structured, guided, or open). Students
will apply knowledge gained in Investigation #1 to a novel problem involving reproductive endocrinology by designing their own
ELISA assay and testing simulated panda urine samples.
• ELISA Paper Model
• Investigation #2: Hormone Detection ELISA
Teacher Note: The kit contains suffi cient materials for eight student workstations with four students each to test for the presence
of antigen in samples for Investigation #2. There will be some extra components left over (e.g. eight microplate strips) that you may
choose to use for open inquiry experiments with your students.
Post-Lab Questions — can be used for class discussion, for post-lab synthesis, or as a means of assessing student
understanding of body systems, in particular antigen and antibody interactions in the context of immune responses and
endocrinology.
BEFORE YOU START
KIT INVENTORY CHECKLIST
5
explorer.bio-rad.com
How to Integrate the Inquiry Investigation Labs into Your Schedule
The instructor’s guide is written for you, the instructor, and includes the following:
• Background information, protocols, and sample results for the inquiry investigations
• Alignment of investigations with Learning Objectives (LO) of the Advanced
Placement (AP) Biology Curriculum
• Scheduling guide for teacher preparation and investigation activities
• Answer keys to the Pre-Lab Focus Questions, ThINQ! Exercises, and Post-Lab
Assessment Questions in the student manual
• Sidebars that call out tips and tricks, teachable moments, and AP Bio curriculum
alignment
Once the pre-lab and core Digital Animation Activity: Antibody ELISA Simulation or
Investigation #1 are performed by the entire class, you have the choice of taking a structured,
guided, or open inquiry approach for Investigation #2. If you have not conducted a guided
or open inquiry investigation previously with your students, we suggest that you review the
Teacher Model Process on page 19 for tips on how to support students as they navigate
Investigation #2.
The following is a suggested lab schedule, but it is just one of many possible scenarios. In
the suggested lab schedule, the Pre-Lab, Digital Animation Activity, Investigation #1, and
Investigation #2 are performed on separate days. You may fi nd that adding an extra day
between the investigations is helpful in preparing students as they generate their ELISA assay
designs for Investigation #2. The Post-Lab Assessment can be completed in a fi nal class period
or as homework.
These sidebars help you align each
activity to AP learning objectives.
AP Big Ideas 2 and 4 are easily
covered in this kit (see AP Biology
Curriculum Alignment on page 9 for
detailed alignments to AP LO, EK,
and SP).
AP Bio
These sidebars suggest teachable
moments to help your students
through the inquiry process.
Teachable
Moments
These sidebars correspond with the
student manual’s ThINQ! Exercises.
Please refer to the printed copy of
the Giant Panda Problem Kit for
AP Biology Answer Guide included
in the kit for the answers. These
questions can also be used for
formative assessment.
ThINQ!
Exercises
BEFORE YOU START
KIT INVENTORY CHECKLIST

6
Kit Timeline
Running the Pre-Lab and both investigations requires approximately three to fi ve 90-minute laboratory periods, possibly more if
results are analyzed during the allocated laboratory time. We also recommend 1–2 days for background review and lectures to
prepare your students for these exercises.
Background Review and Lectures (Prep time varies based on student needs)
Teacher Note: There are many ways to support students as they experience the Giant Panda Problem Kit. If you have or create
any support materials, such as slide presentations, readings, quizzes, and other resources that you would care to share with
other teachers using this kit, please upload your submissions to the Bio-Rad Explorer Community website at bio-rad.com/doc/
submissions or email us at [email protected]. In addition to providing your ideas to other teachers, using parts of the
materials shared there can save you time. Authorship of uploaded materials will be attributed to you, the teacher.
Prior Knowledge Needed by Students:
Prior to beginning the pre-lab and investigations, students should be able to demonstrate they have the following content knowledge
and understand these science practices:
• Terms and phrases that support students’ understanding of antigen and antibody interactions in general and those specifi c to
this kit (see Appendix C for a glossary of terms)
• Basic antibody structure and function
• The concept that antibodies interact with a specifi c antigen to generate an immune response
•
That constructing models is useful for thinking about real-world objects, events, and processes that are diffi cult to observe directly
• The concept that giant panda populations are threatened by anthropogenic factors and changes in climate that disrupt natural
habitat and food sources
Possible Challenges for Students:
Students may have conceptions that limit their understanding of the ELISA assay used in this kit and the science practices
emphasized in the investigations. Several supports and formative assessments are included in the curricular materials and should be
used at your discretion based on student needs. Students may require further support as they consider:
• The concept that an antigen can be any agent (such as protein or hormone) that causes an immune response
• The molecular mechanism that explains antigen and antibody interactions
• The mechanisms by which an ELISA assay can detect the presence of antigen or antibody in a sample
• Generating investigation questions
• Collaboratively designing protocols to investigate their questions
• Generating and using models to explain antigen and antibody interactions
BEFORE YOU START
KIT INVENTORY CHECKLIST

7
explorer.bio-rad.com
Timeline (3 days/2 lesson periods)
Day Activity Details
Instructor’s Advance Preparation
1 day prior Prepare Follow steps in the Instructor’s Advance Preparation (page 15) to prepare reagents
to start Lab Materials and to set up student workstations
Concepts and Connections
Day 1
Pre-Lab: Modeling Create initial models of antigen and antibody interaction mechanism
(45 min)
the ELISA Assay
Assignment: Digital Animation Activity: ELISA Antibody Simulation
Inquir
y Investigation
Day 2* Structured, Guided, or ELISA Paper Model (Optional Activity)
(90 min) Open Inquiry
Review student experimental designs and conduct investigation in groups
Investigation #2: Hormone Detection ELISA
* Day 2 can be split into two 45 minute periods.
Safety Issues
Eating, drinking, smoking, and applying cosmetics are not permitted in the work area. Wearing protective eye wear and gloves is
strongly recommended. Students should wash their hands with soap and water before and after this lab.
Teacher Note:
There is one suggested assignment in the Giant Panda Problem
Kit
. The Investigation #1 Wrap-Up is designed to
prompt students to design an investigation question and appropriate protocol to test their question. The Experimental Design and
Planning Worksheet (bio-rad.com/PandaAPResources) may be used to provide students structure as they complete this assignment.
It is not necessary to complete the assignment to do the investigations.
BEFORE YOU START
KIT INVENTORY CHECKLIST

8
Day Activity Details
Instructor’s Advance Preparation
1 day prior Prepare Follow steps in the Instructor’s Advance Preparation (page 15) to prepare reagents
to start Lab Materials and set up student workstations
Concepts and Connections
Day 1
Pre-Lab: Modeling Create initial models of antigen and antibody interaction mechanism
(45 min) the ELISA Assay
Inquir
y Investigations
Day 2* Structured Inquiry
Investigation #1: Digital Antibody ELISA Simulation and/or ELISA Antibody test (Optional)
(90 min) Investigation
Assignment: Investigation #1 Wrap-Up
Day 3* Structured, Guided, ELISA Paper Model (Optional Activity)
(90 min) or Open Inquiry
Review student experimental designs and conduct investigation in groups
Investigation #2: Hormone Detection ELISA
Synthesis
Day 4 Post-Lab Assessment Paper-based modeling activity and refl ection questions
(50 min)
* Days 2–3 can each be split into two 45 minute periods.
Extended Timeline (5 days/4 lesson periods)
BEFORE YOU START
KIT INVENTORY CHECKLIST

9
explorer.bio-rad.com
AP Biology Standards and Giant Panda Problem Kit Alignment
Pre-Lab Investigation Post-Lab
AP Curriculum ELISA Kit Alignment with AP LO Q’s 1 2 Q’s
LO 2.29 The student can create representations and models
to describe immune responses. [SP 1.1, 1.2; EK2.D.4]
LO 2.31 The student can connect concepts in and across
domains to show that timing and coordination of specifi c
events are necessary for normal development in an organism
and that these events are regulated by multiple mechanisms.
[SP 7.2; EK 2.E.1]
LO 2.32 The student is able to use a graph or diagram
to analyze situations or solve problems (quantitatively or
qualitatively) that involve timing and coordination of events
necessary for normal development in an organism.
[SP 1.4; EK 2.E.1]
LO 2.33 The student is able to justify scientifi c claims with
scientifi c evidence to show that timing and coordination of
several events are necessary for normal development in an
organism and that these events are regulated by multiple
mechanisms. [SP 6.1; EK2.E.1]
LO 2.35 The student is able to design a plan for collecting
data to support the scientifi c claim that the timing and
coordination of physiological events involve regulation.
[SP 4.2; EK 2.E.2]
LO 2.43 The student is able to connect the concept of cell
communication to the functioning of the immune system.
[SP 7.2; EK 2.D.4]
Students create representations and models of the
hormone interactions and assays to track hormones.
Students examine and describe the interactions of
different hormones that produce an ovulation event.
Students examine diagrams of the reproductive system of
the giant panda in order to determine which hormone(s) to
track when predicting an ovulation event.
Students generate explanations based on scientifi c
evidence to support their claims about tracking hormones
and disease causing agents.
Students develop an ELISA protocol for tracking a specifi c
hormone in female giant pandas.
Students generate explanations that tie reproductive
endocrinology to the interaction of antigens and
antibodies.
✓ ✓ ✓ ✓
✓ ✓ ✓ ✓
✓ ✓ ✓
✓ ✓ ✓
✓ ✓ ✓ ✓
✓ ✓
✓ ✓ ✓ ✓
Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow,
to reproduce, and to maintain dynamic homeostasis.
Pre-Lab Investigation Post-Lab
AP Curriculum ELISA Kit Alignment with AP LO Q’s 1 2 Q’s
LO 4.8 The student is able to evaluate scientifi c questions
concerning organisms that exhibit complex properties due to
the interaction of their constituent parts. [SP 3.3; EK 4.A.4]
LO 4.9 The student is able to predict the effects of a change in
a component(s) of a biological system on the functionality of an
organism(s). [SP 6.4; EKe 4.A.4]
LO 4.10 The student is able to refi ne representations and
models to illustrate biocomplexity due to interactions of the
constituent parts. [SP 1.3; EK 4.A.4]
LO 4.19 The student is able to use data analysis to refi ne
observations and measurements regarding the effect of
population interactions on patterns of species distribution and
abundance. [SP 5.2; EK 4.B.3]
LO 4.20 The student is able to explain how the distribution
of ecosystems changes over time by identifying large-scale
events that have resulted in these changes in the past.
[SP 6.3; EK 4.B.3]
LO 4.21 The student is able to predict consequences of
human actions on both local and global ecosystems.
[SP 6.4; EK 4.B.3]
LO 4.22 The student is able to construct explanations based
on evidence of how variation in molecular units provides cells
with a wider range of functions. [SP 6.2; EK 4.C.1]
LO 4.26 The student is able to use theories and models to
make scientifi c claims and/or predictions about the effects of
variation within populations on survival and fi tness.
[SP 6.4; EK 4.C.3]
LO 4.27 The student is able to make scientifi c claims and
predictions about how species diversity within an ecosystem
infl uences ecosystem stability. [SP 6.4; EK 4.C.4]
Students analyze data and generate models that explain
the relationship between parts of complex systems such
as the reproductive and immune systems.
Students generate explanations of system function under
different conditions (e.g., presence or absence of antigen).
Students generate initial models and refi ne their models as
they gather new evidence.
Students draw connections between species distribution
and abundance in relation to need for conservation.
Students name specifi c conditions that affect the
ecosystem that can be detrimental for species distribution
and abundance.
Students predict the effect of human impact on the
environment and its consequences to vulnerable and
endangered species.
Students model and explain antigen and antibody
interactions within an ELISA.
Students are asked to consider the effect of human
impacts on the environment for vulnerable and
endangered species in terms of their survival and fi tness.
Students make predictions and claims about the
abundance of species in an ecosystem based on threats
to species survival.
Big Idea 4: Biological systems interact, and these systems and their interactions
possess complex properties.
✓ ✓ ✓ ✓
✓ ✓ ✓ ✓
✓ ✓ ✓ ✓
✓ ✓ ✓
✓ ✓ ✓ ✓
✓
✓
✓ ✓
✓
✓
✓ ✓
✓ ✓
Big Idea 4: Biological systems interact, and these systems and their interactions
possess complex properties.
BEFORE YOU START
AP STANDARDS
10
Background for Instructors
With the ThINQ!
™
Giant Panda Problem Kit for AP Biology students explore antigen and antibody interactions and develop an
ELISA assay of their own design to track an ovulation hormone in giant pandas. Tracking hormones like estrogen and luteinizing
hormone is important to predict the onset of ovulation. Using an ELISA assay is a simple way for students to determine if an
interaction between an antigen and antibody is taking place. The practical applications of this assay give students a real world
experience that will make the abstract concepts involved in reproductive endocrinology relevant.
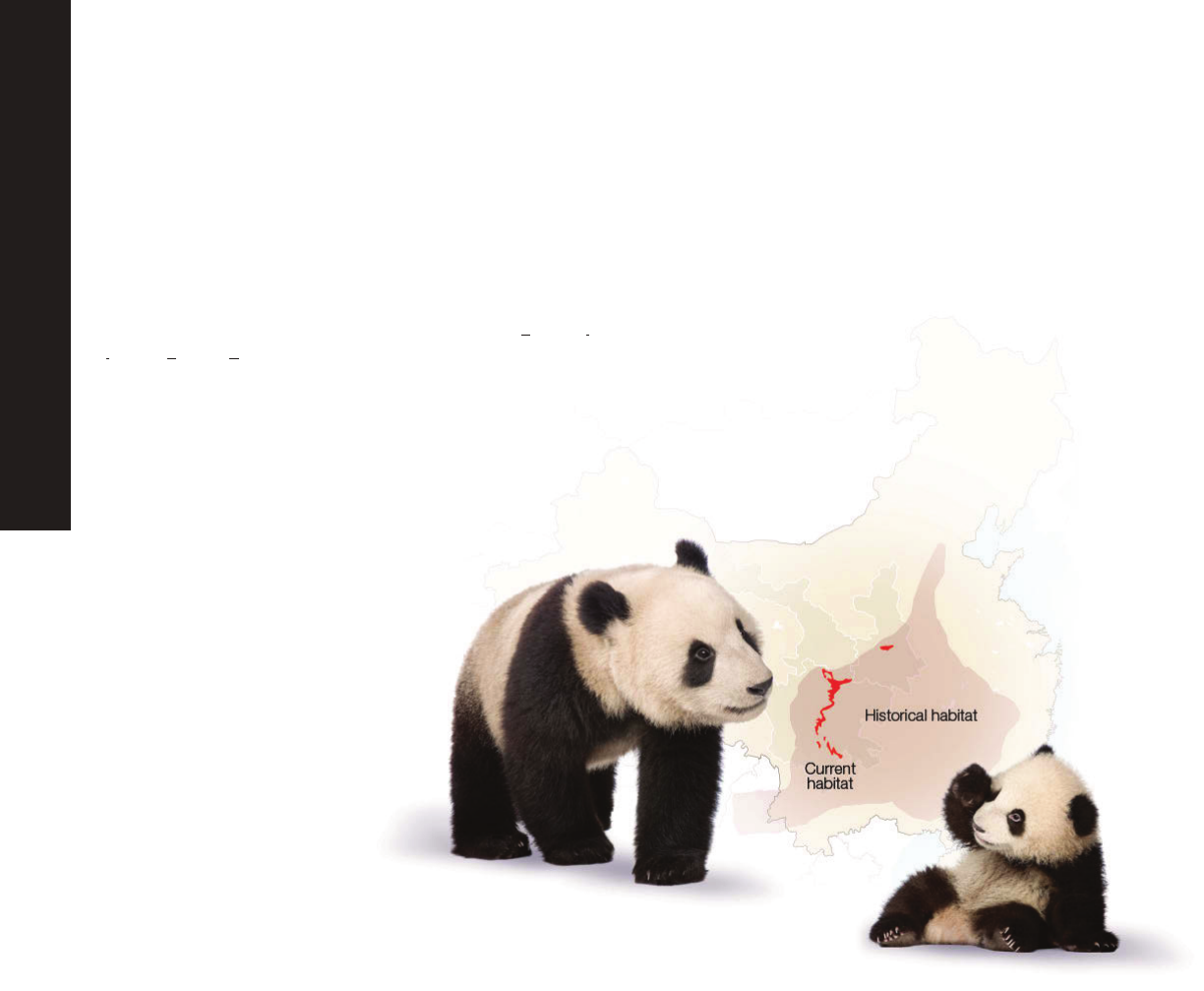
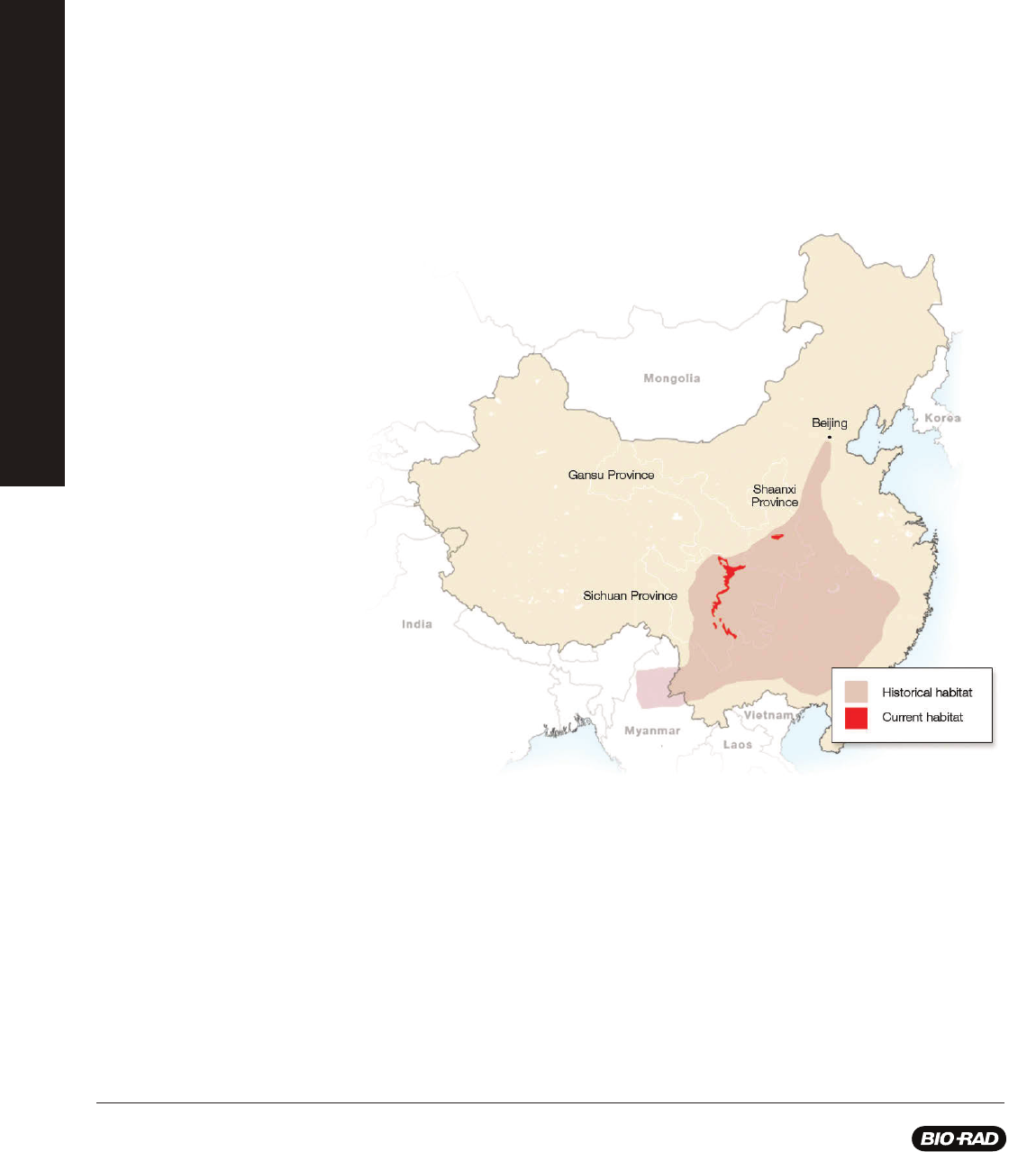
Giant Pandas: Saving a Species From Extinction
Giant pandas living in the wild are found among about forty, small, fragmented areas in three provinces of China: Shaanxi, Gansu,
and Sichuan. Destruction of the giant panda’s habitat with farming, deforestation, and urban development along with climate
change and poaching have all contributed to the decline of the giant panda. As a conservation measure in 1984, the giant panda
was listed as an endangered species under the United States Endangered Species Act. Due to an increase in research about
panda reproduction, advances in reproductive technologies, and the enforcement of laws protecting endangered species, in 2016
the giant panda’s status shifted from endangered to vulnerable marking a major advance in conservation efforts. This, however,
does not mean the giant panda is out of danger as climate change and industrialization continue to threaten bamboo forests in
China - the panda’s primary food source.
One of the unique characteristics of the giant panda that also lends to its vulnerable status is
its reproductive cycle. Unlike most other mammals, female giant pandas only ovulate once
per year — typically between February and June — with a fertility window
of about 72 hours. In the wild, this makes successful
breeding quite diffi cult as male pandas typically live
solitary lives and females are not always available
due to geographic barriers, such as cities, roads,
mountains, and rivers. In captivity, breeding giant
pandas is met with higher rates of success;
however, diffi culties still arise as females
tend to be choosy and often require
assistance using artifi cial insemination.
Once a female panda’s egg is fertilized
it will fl oat freely in the female’s fallopian
tube and uterus for many months. Until
implantation occurs, pregnancy cannot be
confi rmed. Often caretakers are unaware of
pregnancy until a few weeks before birth.
Giant pandas can typically have 1–3 offspring at
a time, but often care for only one during any given birth.
Pandas born in captivity have a greater chance of survival as
human caretakers step in and ensure each cub is nursed.
A two pronged approach is required to continue increasing
numbers of giant pandas. Those in the wild require protection by government agencies
capable of establishing and maintaining crucial habitats containing abundant bamboo forests and enforcing strict punishments
for poachers. In captivity more sensitive tests are required to better track female panda hormones indicating that ovulation is
imminent. Currently, caretakers at zoos will collect urine and fecal samples and test levels of reproductive hormones to pinpoint
the window of opportunity for mating and artifi cial insemination. It is important to continue developing and improving such tests to
increase their sensitivity and reliability and ensure successful panda pregnancies.
Mammalian Reproductive Endocrinology
Dozens of hormones and enzymes are required in order to support ovulation in female mammals, such as the giant panda. Here
we describe an essential set that will be discussed in this kit. They include gonadotropin-releasing hormone (GnRH), progesterone,
estrogen, luteinizing hormone (LH), and follicle stimulating hormone (FSH).
BEFORE YOU START
BACKGROUND FOR INSTRUCTORS
11
explorer.bio-rad.com
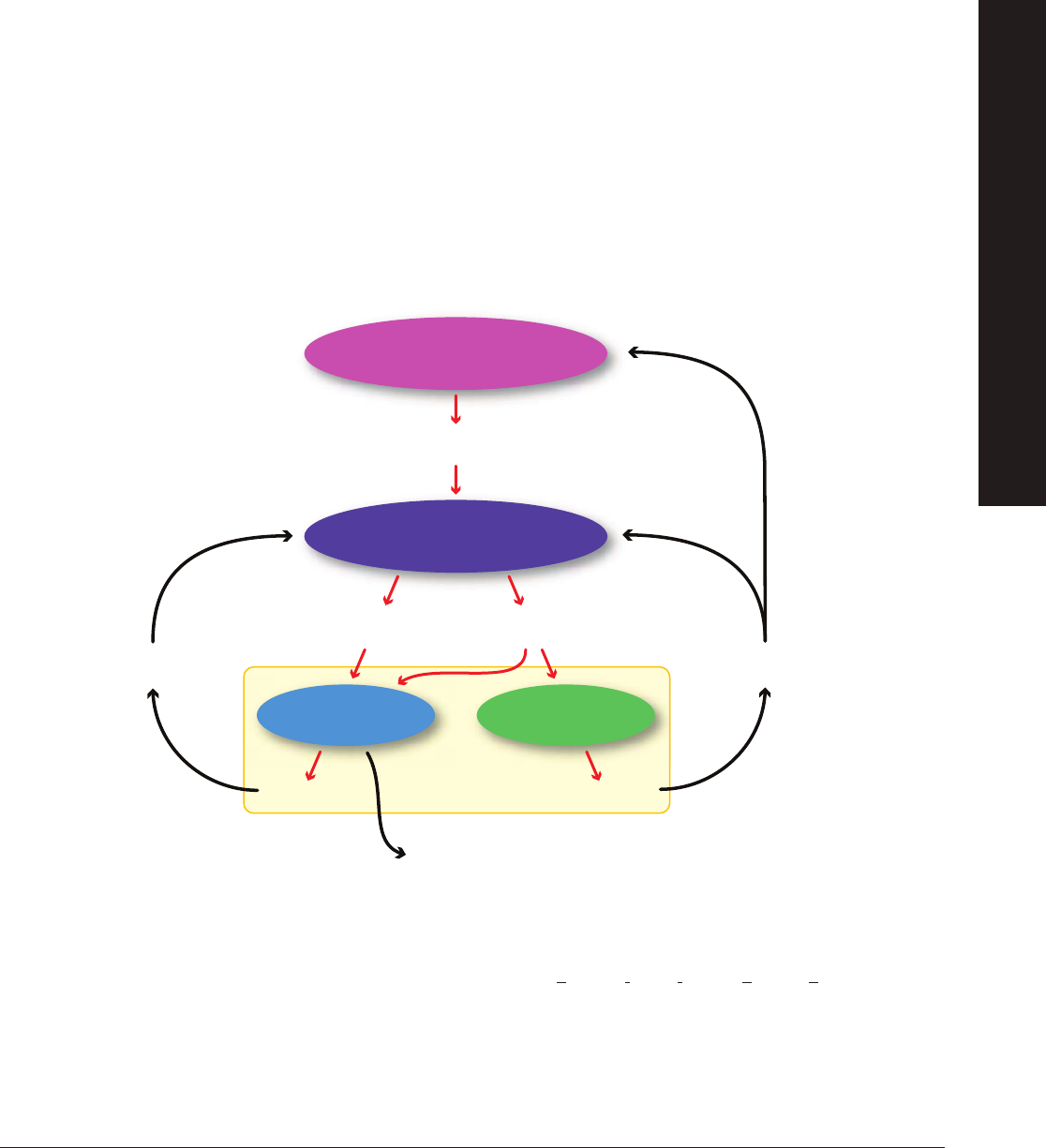
The hypothalamus, an area at the forefront of the brain that serves as the primary neurohormone producer, connects both
the nervous system and endocrine system. The hypothalamus can be stimulated both extrinsically (e.g., scent marking left by
a potential mate) and intrinsically (e.g., presence or absence of coitus). Upon receiving specifi c stimuli that trigger reproductive
behaviors, the hypothalamus releases gonadotropin-releasing hormone (GnRH). Once released in the brain, GnRH travels
through a series of blood vessels to the anterior pituitary gland in the brain. The anterior pituitary gland then produces and
releases FSH and LH.
FSH promotes the growth and development of follicles in the ovary that produce estrogen. The release of estrogen at this point
has a positive feedback effect on the hypothalamus whereby more GnRH is released and therefore more LH and FSH. Estrogen
also plays a key role in preparing the uterine lining for the potential implantation of an embryo after fertilization takes place. Once
the follicle is mature, a large amount of estrogen is produced that in turn stimulates a surge in LH production triggering release of
the egg from the follicle. At this point ovulation has occurred. The remaining follicle becomes the corpus luteum — a hormone
secreting structure in the ovary that forms from the follicle once the egg is released from the ovary into the fallopian tube.
The corpus luteum’s primary function is the production of progesterone which supports and maintains pregnancy. Over time, the
corpus luteum produces increasing amounts of progesterone. During this time, progesterone acts as a negative feedback signal
to the hypothalamus indicating it should reduce production of GnRH, which reduces the production of LH and FSH, thus inhibiting
follicular growth in the ovaries. If implantation of an embryo does not occur, the corpus luteum reduces in size and another round
of follicular development occurs. The level of progesterone will also decrease and menstruation will occur.
Being a mammal, the female giant panda experiences these hormone cycles, yet only once per year. This makes determining
the timing of ovulation in female pandas critical for reproductive success, especially in captivity and with the use of artifi cial
insemination. One way to determine the presence of reproductive hormones in pandas is to test whether the hormones are
present or absent and if present, how much is there. This can done using an enzyme-linked immunosorbent assay (ELISA).
Using an ELISA, researchers can determine the presence of a hormone in a sample and, if quantifying, can determine how much
of the hormone is present in the sample. The next section describes how the ELISA assay featured in this kit works.
Hypothalamus
Pituitary
Gonadotropin-Releasing
Hormone (GnRH)
Ovulation
Follicle Corpus Luteum
Follicle Stimulating
Hormone (FSH)
Luteinizing
Hormone (LH)
Estrogen Progesterone
Stimulation
Inhibition
Ovary
BEFORE YOU START
BACKGROUND FOR INSTRUCTORS
12
How Does an ELISA Assay Work?
There are several different types of ELISA protocols. The protocols in this kit rely on indirect
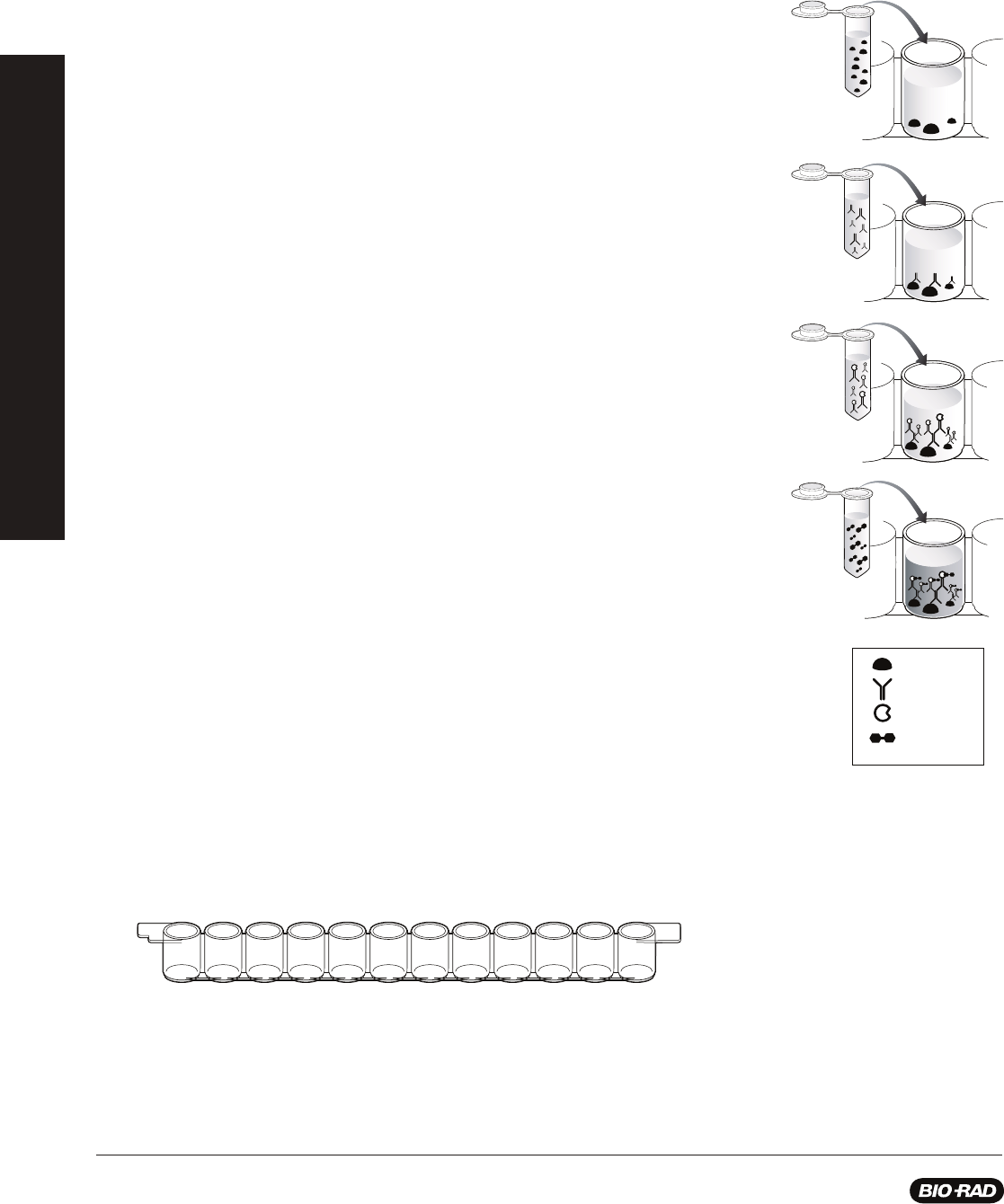
antibody capture ELISA. The steps in this assay are:
Step 1: Antigen is added to the wells of the microplate strip and incubated to allow
binding, after which unbound antigen is washed from the wells with buffer containing
detergent. The detergent also serves as a blocking agent, binding to all unused protein
binding sites in the wells and preventing nonspecifi c binding of antibody.
Step 2: Primary antibody solution is added to the wells and incubated to allow the
antibody to bind to the antigen. Then unbound primary antibody is washed from the wells.
Step 3: Enzyme-labeled secondary antibody solution is added to the wells and
incubated to allow the secondary antibody to bind to the primary antibody. Then unbound
secondary antibody is washed from the wells.
Step 4: Chromogenic (color-producing) enzyme substrate is added to the wells and
incubated to allow color to develop. Results of the assay are evaluated. Wells that remain
colorless are negative and wells that turn blue are positive.
To create a relevant and meaningful classroom context for this activity, the in-depth
information in Appendices B and C provides background vocabulary and factual and
conceptual lecture points. In addition, useful reading and web sites are included in
Appendix E. The following section briefl y describes the technical and conceptual points
that are directly related to the investigations in this curriculum.
Microplate strips: Microplates are made of polystyrene which adsorbs (binds) proteins
by hydrophobic interaction. The plates provided in this kit have 96 wells, arranged in
8 removable rows of 12-well strips. A student group shares one strip. Each well holds
approximately 250 microliters (µl).
Antigen
Antibody
Enzyme
Enzyme
Substrate
BEFORE YOU START
BACKGROUND FOR INSTRUCTORS
13
explorer.bio-rad.com
Antigen: In this kit, the antigen is chicken gamma-globulin (purifi ed from egg yolks) which serves as a generic representative of
any hypothetical antigen, protein or otherwise. In the Giant Panda Problem Kit the antigen represents a hormone, such as GnRH,
estrogen, FSH, or LH, that indicates the beginning of ovulation.
Incubation times: The rate of binding depends on the incubation temperature and the concentrations of the reagents. This
kit has been optimized so that each incubation can be performed for 5 minutes at room temperature. Exceeding this time or
temperature will cause an increase in color intensity and possibly some background color in the negative controls and samples.
Blocking: Blocking agents are added after antigen adsorption to prevent nonspecifi c binding of antibodies to the plastic, which
would produce false positive results. The blocking agent may be a protein or a detergent (or both). Common blocking agents
include Tween 20 (a nonionic detergent that is used in this kit), nonfat dry milk, gelatin, and bovine serum albumin (BSA).
Primary (1°) antibodies: The antibodies that recognize and bind to the antigen in an immunoassay are primary antibodies. In
this kit, the primary antibodies are polyclonal rabbit antibodies raised against chicken gamma globulin and are called rabbit
anti-chicken antibodies. In the ELISA Antibody Test starting on page 33, this primary antibody represents giant panda antibodies
in a sample of panda urine.
Secondary (2°) antibodies: Secondary antibodies recognize and bind to primary antibodies. They are made in animals of a
different species than that used to make the primary antibody. For this kit, goats were immunized with rabbit IgG to make the
secondary antibodies, and are called goat anti-rabbit antibodies. In this kit, the secondary antibody represents the antibody
engineered to bind to the simulated giant panda antibodies (see primary antibodies).
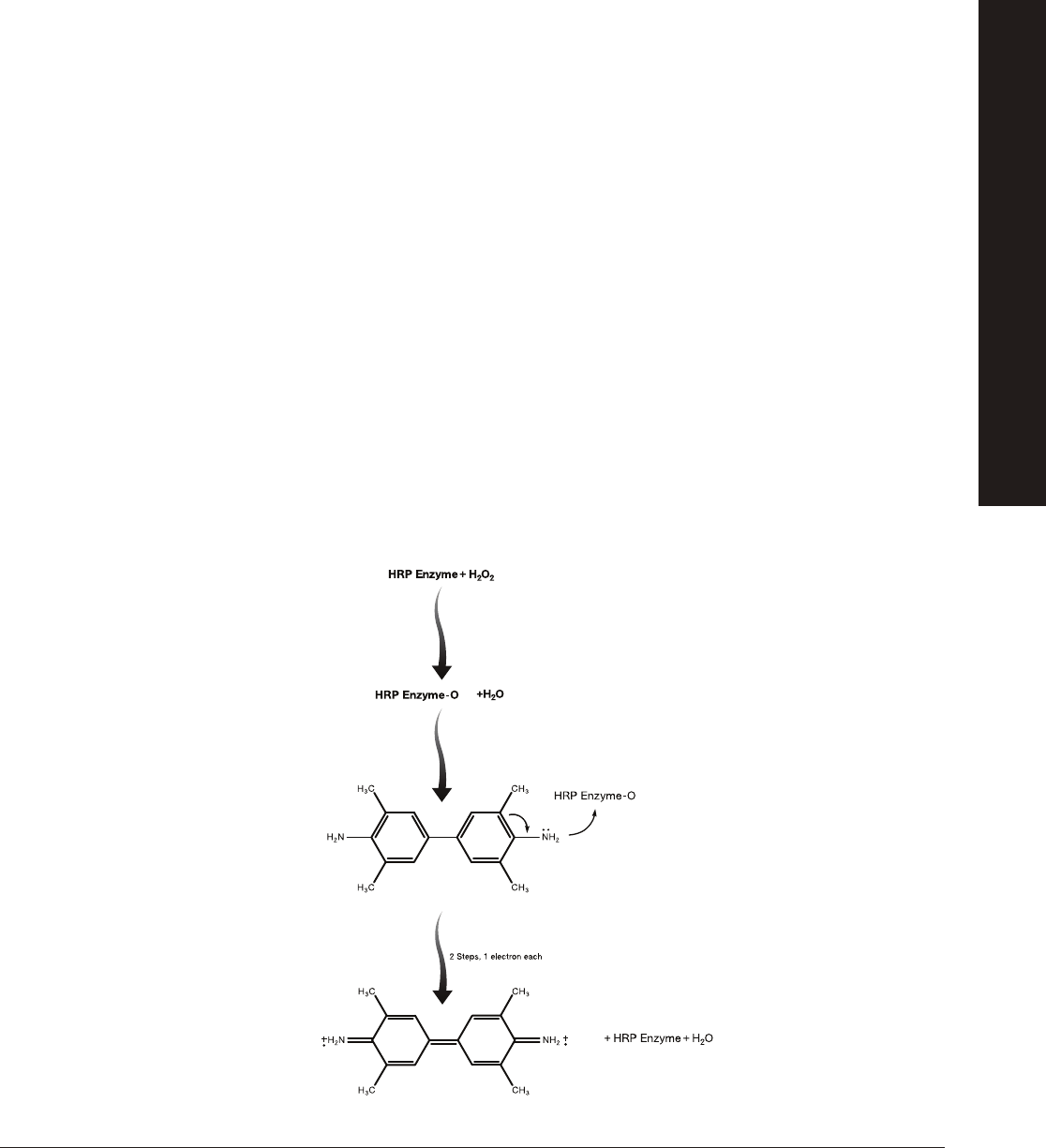
Colorimetric detection: Secondary antibodies for this type of ELISA are linked to enzymes. Detection of secondary antibodies
that are bound to primary antibodies occurs by an enzyme-substrate reaction. In this kit, the secondary antibody is linked to
the enzyme horseradish peroxidase (HRP). In the presence of hydrogen peroxide (H
2
O
2
), HRP catalyzes the oxidation of the
chromogenic substrate 3,3',5,5'-tetramethylbenzidene (TMB). This oxidation of TMB by HRP forms a blue product.
Note: TMB is light sensitive, and the assay results should be determined 5–10 minutes after the substrate is added to the wells. If
the microplate strips sit longer, nonspecifi c color may develop. Color that develops after the 5–10 minute incubation should not be
considered in the assay results. After 20–30 minutes, the blue color may begin to fade as TMB precipitates out of solution.
3,3’,5,5’
-tetramethylbenzidine
(TMB)
(Colorless)
Quinone iminium
double cation radical
of TMB
(Blue color)
BEFORE YOU START
BACKGROUND FOR INSTRUCTORS

14
Controls: Controls should always run side by side with actual samples to make sure that the procedure is working correctly.
Controls can resolve ambiguous results that occur due to human error or contaminated reagents; controls must be included in any
valid ELISA. For the negative control, the antigen or primary antibody is either omitted (as in this kit) or the antigen is replaced by a
factor that will not bind specifi cally to the antibody. The positive control always contains the target antigen or antibody. A negative
sample that gives a positive assay result is called a false positive. A positive sample that gives a negative assay result is called a
false negative.
Many diagnostic assays give a percentage of false positive or false negative results, so confi rmation of diagnosis by a second
type of assay is important. For example, immunoassays for antibodies to human immunodefi ciency virus (HIV) can give either
false positive or false negative results. False positives can result from recent vaccinations, and false negatives can result from
immunosuppression (e.g., from drugs given after transplants) or from administering the test too soon after infection with HIV.
(Antibodies against HIV do not appear until some weeks after HIV infection; the appearance of specifi c antibodies is called
seroconversion.) Because of this, positive HIV ELISA results are always confi rmed by western blot.
In an ELISA assay like that in Investigation #1 (in which antibody concentration is the experimental variable), an appropriate negative
control would be wells with the antibody sample omitted. Any color product in those wells would be the result of 1) nonspecifi c
binding of the secondary antibodies, or 2) experimental error. An appropriate positive control would be a sample known to contain
the antibody we are trying to detect.
In an ELISA test like that in Investigation #2 (in which antigen concentration is the experimental variable), an appropriate negative
control would be wells with antigen omitted. Any color product in those wells would be the result of either 1) nonspecifi c binding of
the primary or secondary antibody, or 2) experimental error. An appropriate positive control would be a sample known to contain
antigen. For many clinical ELISAs, control solutions containing antigen are provided with the commercial kits.
Analysis of Results: An ELISA can give qualitative (yes or no) or quantitative (how much?) information. Qualitative results can
be determined visually without the use of complicated instrumentation. Quantitative results can be estimated visually and scored
symbolically, e.g., (++) for strong signal, (+) for weak signal, (+/–) for an ambiguous signal, and (–) for no detectable signal. For
accurate and precise determination of concentrations, a microplate reader is required. Microplate readers quantitate the absorbance
of light by the colored substrate in each well of a microplate. They use the negative control wells to set a baseline and then read the
absorbance of each well at a specifi ed wavelength. For example, the peak absorbance for TMB is at 655 nm. Quantitative ELISA
controls include a dilution series of known concentrations that is used to create a standard curve. This standard curve allows the
concentration of antigen in a sample to be quantitated, which in turn may help a researcher, clinician, or physician determine the
infection level of a particular disease. A lesson extension to analyze a data set from a quantitative ELISA is included in Appendix D.
ELISAs are performed so routinely in both clinical and research laboratories that assays for many antigens are available in kit form.
Kits normally include all components and controls needed for a given test except for the experimental samples. For example,
Bio-Rad’s Clinical Diagnostics Group and Food Science Division produce over 100 kits that are used to detect autoimmune
diseases, blood viruses, genetic disorders, microorganisms, toxins, bovine spongiform encephalopathy (BSE or mad cow
disease), and chronic wasting disease (CWD).
Volume Measurements
This kit contains graduated disposable plastic transfer pipets (DPTPs) to use for preparing some of the reagents where volumes
between 250 microliters (µl) and 5 milliliters (ml) are required. In addition, adjustable- or fi xed-volume micropipets may be used to
more easily and accurately measure 50 µl volumes. The illustration shows the marks on the DPTP corresponding to the volumes to
be measured. Volumes over 1 ml will require multiple additions. For each step of the laboratory preparation, use a fresh DPTP or a
fresh pipet tip. Measuring liquids that contain detergents that foam (e.g., the wash buffer) requires that you read the volume at the
interface of the liquid and the bubbles.
1 ml
750 µl
500 µl
250 µl
100 µl
25 µl
BEFORE YOU START
BACKGROUND FOR INSTRUCTORS
15
explorer.bio-rad.com
Material Needed for Advance Preparation Quantity
Antigen, chicken gamma globulin, lyophilized 1 vial
Primary antibody
, rabbit anti-chicken polyclonal antibody, lyophilized 1 vial
Secondary antibody, goat anti-rabbit antibody conjugated to (HRP), lyophilized 1 vial
HRP enzyme substrate (TMB) 1 bottle
10x phosphate buffered saline (PBS) 1 bottle
10% Tween 20 1 bottle
Distilled water, sterile is recommended 1 L
Procedure (Estimated time — 60 min)
1. Prepare buffers.
W
e recommend you use a 100 ml and a 1 liter (L) graduated cylinder for preparing the buffer solutions. You will also need 1 L
of distilled water.
2. Rehydrate the freeze-dried antigen, primary antibody, and secondary antibody to create stock solutions.
Carefully remove the stopper from the three lyophilized reagents and add the indicated reagents below to make 50x stock
solutions.
NOTE: You must not use wash buffer in this step.
Buffer Volume Reagent Used for
1x PBS, 100 ml 90 ml Distilled water Rehydrating antigen, primary and secondary
antibodies to make 50x reagent stock solutions
10 ml 10x PBS Diluting 50x antigen to make positive control and
student samples
Wash Buffer, 900 ml 805.5 ml Distilled water Dilution of 50x antibody and plate washing
90 ml 10x PBS
4.5 ml 10% Tween 20
Instructor’s Advance Preparation
Vial Rehydration Volume
Antigen 0.5 ml 1x PBS
Primary antibody 0.5 ml 1x PBS
Secondary antibody
0.5 ml 1x PBS
INSTRUCTOR’S ADVANCE PREPARATION
16
3. Dilute 50x stock reagents.
Label one 50 ml bottle or tube for each of the diluted solutions below. Add the contents of the appropriate 50x concentrated
stock to the corresponding 50 ml bottle or tube.
4. Dispense reagents for student workstations.
See instructions for dispensing reagents for student workstations on the next two pages. Be careful to follow the instructions
precisely as the workstation setup for Investigations #1 and #2 differ only slightly.
Teacher Note: Throughout this kit, reagents are referred to in different ways depending on their use in each investigation.
The table below serves as a guide for understanding how reagents are named in each investigation for you, the teacher, and
for your students.
Diluted solution Volume Reagent Used for
1x antigen, label one 50 ml 15 ml 1x PBS
1x Antigen
bottle or tube 300 µl 50x antigen stock
NOTE: You must not add any buffer containing Tween 20 to the antigen, or the
experiment will not work — therefore do NOT use wash buffer to dilute the antigen.
1x primary antibody 24.5 ml Wash buffer
Primary antibody
label one 50 ml bottle or tube 0.5 ml 50x primary antibody stock
Rinse out the vial with some of the diluted reagent to ensure that all of the stock solution
is used.
1x secondary antibody 24.5 ml Wash buffer
Secondary antibody
label one 50 ml bottle or tube 0.5 ml 50x secondary antibody stock
Dilute the secondary antibody less than 24 hours before the start of the lesson.
Rinse out the vial with some of the diluted reagent to ensure that
all of the stock solution is used.
Reagent Investigation #1 Investigation #2
1x antigen antigen purifi ed antigen antigen positive control, panda
samples, or antigen of interest
1x PBS PBS negative control PBS negative control
1x primary primary antibody positive control, primary antibody primary antibody
antibody panda samples, or
antibody of interest
1x secondary secondary antibody secondary antibody secondary antibody secondary antibody
antibody
Substrate substrate substrate substrate substrate
(
teacher-facing term) (student-facing term) (teacher-facing term) (student-facing term)
INSTRUCTOR’S ADVANCE PREPARATION

17
explorer.bio-rad.com
In this investigation students will learn the technique of conducting an ELISA while determining if simulated urine samples from
giant pandas contain disease antibodies. Learning this technique will support their design of an ELISA for the presence of a panda
hormone in Investigation #2.
Procedure (Estimated time — 30 min)
1. Label colored tubes as described in the table below.
2.
Dispense volumes of reagents as indicated.
3.
If preparing student workstations in advance, store all materials at 4
o
C until needed.
4. Set up student workstations as indicated below. One workstation serves 4 students.
Stopping points: Although this procedure is designed to fi t into a single lesson period, you may stop the laboratory activity after
adding simulated panda urine samples to the wells and place all reagents in the refrigerator at 4°C overnight. Alternatively, if you wish
to stop during the ELISA you may add wash buffer to the microplate wells at any stage after the addition of antigen and prior to the
addition of enzyme substrate. Place the microplate strips and all the reagents in the refrigerator at 4°C overnight.
Investigation #1: Antibody ELISA Test (Optional)
Item (Label) Contents Quantity per Workstation
☐ Yellow tubes Set of panda urine samples (P1, P2; 200 µl each) 1
☐ Violet tube (+) Positive control (200 µl) 1
☐ Blue tube (–) Negative control (200 µl) 1
☐ Green tube (AG) Purifi ed antigen (800 µl) 1
☐ Orange tube (SA) Secondary antibody (800 µl) 1
☐ Brown tube (SUB) Enzyme substrate (800 µl) 1
☐ 12-well microplate strip 1
☐ 50 µl fi xed-volume micropipet, or 20–200 µl adjustable micropipet (optional) 1
☐ Yellow tips (optional) 10–20
☐ Disposable plastic transfer pipet (DPTPs) Not included in kit* 10
☐ 35 ml wash buffer in beaker PBS with 0.05% Tween 20 1
☐ Large stack of paper towels 1
☐ Black marking pen 1
*
The kit contains suffi cient reagents to perform optional Investigation #1 ELISA Antibody Test; however, the purchase of additional
disposable plastic transfer pipets (DPTPs) is required. Alternatively, it is possible to reuse the DPTPs in the kit, provided they are
washed and rinsed well. Reusing DPTPs introduces the possibility of cross contanimation and should be undertaken with caution.
Tubes Description Label Contents (Each Tube)
Violet tubes, 8 Positive contr
ols (+) 200 µl, 1x primary antibody
Blue tubes, 8 Negative controls (–) 200 µl, 1x PBS
Green tubes, 8 Purifi ed antigen (AG) 800 µl, 1x antigen
Orange tubes, 8 Secondary antibody (SA) 800 µl, 1x secondary antibody
Brown tubes, 8 Enzyme substrate (SUB) 800 µl, HRP enzyme substrate
NOTE: HRP enzyme substrate is light sensitive, so its important to use the dark tubes to store this reagent.
Yellow tubes, 1 tube per Positive panda urine samples — 1 per student pair (P1) 200 µl, 1x primary antibody
pair of students (16 max) Negative panda urine sample — 1 per student pair (P2) 200 µl, 1x PBS
We recommend that you design the experiment so that 50% of the urine samples will test positive and 50% of the urine
samples will test negative. However, the fi nal ratio is up to you. Make two
urine samples for each student pair in your class as
indicated above. Mix up the tubes before distributing.
INSTRUCTOR’S ADVANCE PREPARATION

18
Investigation #2: Hormone Detection ELISA
In this investigation students will design their own ELISA assay to track the presence of a reproductive hormone of their choice
(decided during the Pre-lab activity). Students will test four simulated giant panda urine samples for the presence or absence of
the hormone.
Procedure (Estimated time — 30 min)
1. Label colored tubes as described in the table below.
2.
Dispense volumes of reagents as indicated.
3.
If preparing student workstations in advance, store all materials at 4
o
C until needed.
4. Set up student workstations as indicated below. One workstation serves 4 students.
Stopping points: Although this procedure is designed to fi t into a single lesson period, you may stop the laboratory activity after
adding simulated panda urine samples to the wells and place all the reagents in the refrigerator at 4°C overnight. Alternatively, if you
wish to stop during the ELISA you may add wash buffer to the microplate wells at any stage after the addition of antigen and prior to the
addition of enzyme substrate. Place the microplate strips and all the reagents in the refrigerator at 4°C overnight.
Item (Label) Contents Quantity per Workstation
☐ Yellow tubes Set of panda urine samples (P1, P2, P3, P4; 200 µl each) 1
☐ Violet tube (+) Positive control (200 µl) 1
☐ Blue tube (–) Negative control (200 µl) 1
☐ Green tube (PA) Primary antibody (800 µl) 1
☐ Orange tube (SA) Secondary antibody (800 µl) 1
☐ Brown tube (SUB) Enzyme substrate (800 µl) 1
☐ 12-well microplate strips 1
☐ 50 µl fi xed-volume micropipet, or 20–200 µl adjustable micropipet (optional) 1
☐ Yellow tips (optional) 10–20
☐ Disposable plastic transfer pipet (DPTPs) 10
☐ 35 ml wash buffer in beaker PBS with 0.05% Tween 20 1
☐ Large stack of paper towels 1
☐ Black marking pen 1
Tubes Description Label Contents (Each Tube)
Violet tubes, 8 Positive controls (+)
200 µl, 1x antigen
Blue tubes, 8 Negative controls (–) 200 µl, 1x PBS
Green tubes, 8 Primary antibody (PA) 1 ml, 1x primary antibody
Orange tubes, 8 Secondary antibody (SA) 1 ml, 1x secondary antibody
Brown tubes, 8 Enzyme substrate (SUB) 1 ml, HRP enzyme substrate
NOTE: HRP enzyme substrate is light sensitive, so its important to use the dark tubes to
store this reagent.
Yellow tubes, 4 per Positive panda urine samples — 2 per student pair (P1, P4) 200 µl, 1x antigen
workstation (32 max) Negative panda urine sample — 2 per student pair (P2, P3) 200 µl, 1x PBS
We recommend that you design the experiment so that 50% of the urine samples will test positive
and 50% of the urine samples will test negative. However, the fi nal ratio is up to you. You may want to
m
ix up the tubes for random distribution.
INSTRUCTOR’S ADVANCE PREPARATION
19
explorer.bio-rad.com
Teacher Model Process
This table is designed to highlight specifi c steps during protocol design (for Investigation #2), where students may
require additional support. As students design their protocols, you may fi nd it useful to support their thinking and writing
by using the questions and prompts below. This table can be used in conjunction with the Experimental Planning and
Design Worksheet (bio-rad.com/PandaAPResources) as a formative or summative assessment tool and during class
time to support students in the protocol design
process.
Inquiry Lesson Step Suggested Questions and Prompts to Support Protocol Design for Investigation #2 Kit-Specifi c Applications
Identifying the
components of the
ELISA and explaining
their interactions
Making
Observations
Making observations that lead to an investigation question
In Investigation 1 and/or the Digital Animation Activity, what is the role of the antigen
in the wells?
What is added to the positive control wells to achieve positive results (blue color)?
What is missing from the negative control wells so that results are negative?
Why do positive samples turn blue?
What would happen if the secondary antibodies were not added to the wells?
Defi ning
the Purpose
of the
Investigation
Tracking a particular
hormone in panda urine
to determine fertility
Clarifying the purpose of the investigation
What was the purpose of Investigation 1?
How does the purpose of Investigation 1 differ from the purpose of this investigation?
What small changes could you make to the protocol in Investigation 1 to meet the
purpose of this investigation?
What steps from the protocol in Investigation 1 can you use to design this investigation?
Hypothesis
Formation
Understanding how
an ELISA for hormone
detection can
determine the fertility
of female pandas
Clarifying goals for the investigation
Can you explain in your own words what the investigation question is asking?
What do you already know about how an ELISA works?
Knowing this, how would you modify the protocol for Investigation 1 and/or the
Digital Animation Activity to determine which pandas are about to ovulate?
What evidence would you need in order to answer the investigation question?
TEACHER MODEL PROCESS
20
Inquiry Lesson Step Suggested Questions and Prompts to Support Protocol Design for Investigation #2 Kit-Specifi c Applications
Presence or absence
of blue color
What variables are
relevant to antibody and
antigen reactions; what
variables affect antibody
and antigen reactions
Analyzing
Evidence
Identifying what counts as supportive evidence
What is the investigation question?
Your classmates are trying to answer the investigation question; what pieces of
evidence would you expect them to use?
How do you know whether evidence should or should not be used to answer the
investigation question?
What justifi cations can you provide to support what counts as evidence in this investigation?
Working within the constraints of classroom time and supplies
What are the capabilities and limitations of the materials available to you?
What protocol could you use as a template to create a protocol for this investigation?
How could you revise the template protocol to achieve the goal of this investigation
with the allotted materials/time/etc.?
Use of reagents
such as panda urine
samples, antibodies to
panda hormone, and
secondary antibodies
Determining
Protocol Scope
Assumptions about the
interaction of reagents
to produce reliable
test results
Assumptions about
reaction mechanisms
How to set up
an ELISA that is
reliable and provides
information about each
sample and controls
Presence and absence
of hormone of interest
Determining appropriate steps and detail
What questions might one of your classmates have if they read your protocol
(that is, too few or unnecessary details?)
How does step X meet the goal of the investigation (that is, unnecessary detail)?
Understanding use of controls
What is the purpose of a control?
What controls might be useful in this protocol?
Outlining
Protocol
Steps
Understanding “givens” and what may be assumed
What are your assumptions about the hormone, about the antibodies, about the
substrate, and about how they interact with each other?
What justifi cations validate your assumptions?
The experimental planning worksheet has students articulate and draw individual protocol steps. But having the
students draw an overview of the experiment (reagents drawn and labeled in order of addition to each well) may help
them conceptualize the experiment more easily prior to writing out the individual protocol steps. This supports their
development in creating and refi ning a model.
Teachable
Moments
TEACHER MODEL PROCESS

21
explorer.bio-rad.com
Instructor’s Guide to the Student Manual
Background
Giant Pandas: Saving a Species From Extinction
Giant pandas living in the wild are found among about forty, small, fragmented areas
in three provinces of China: Shaanxi, Gansu, and Sichuan. Destruction of the giant
panda’s habitat with farming, deforestation, and urban development along with climate
change and poaching have all contributed to the decline of the giant panda. As a
conservation measure in 1984, the giant panda was listed as an endangered species
under the United States Endangered Species Act. Due to an increase in research about
panda reproduction, advances in reproductive technologies, and the enforcement of
laws protecting endangered species, in 2016 the giant panda’s status shifted from
endangered to vulnerable marking a major advance in conservation efforts. This,
however, does not mean the giant panda is out of danger as climate change continues
to threaten bamboo forests in China — the panda’s primary food source, poachers are
still active, and urban sprawl continues to threaten panda habitat.
One of the unique characteristics of the giant panda is its reproductive cycle. Unlike
most other mammals that ovulate on a monthly basis, female giant pandas only ovulate
once per year — typically between February and June — with a fertility window of about
72 hours. In the wild, this makes successful breeding quite diffi cult as male pandas
typically live solitary lives and females are not always available due to geographic
barriers, such as cities, roads, mountains, and rivers. In captivity, breeding giant pandas
is met with higher rates of success; however, diffi culties still arise as females tend to
be choosy and often require assistance using artifi cial insemination. Once a female
panda’s egg is fertilized it will fl oat freely in the her fallopian tube and uterus for many
months. Until implantation occurs, pregnancy cannot be confi rmed. Often caretakers are
unaware of pregnancy until a few weeks before birth. Giant pandas typically have
1–3 offspring at a time, but often only care for only one
during any given birth. Pandas born in captivity have a
greater chance of survival as human caretakers
step in and ensure that each
cub is nursed.
Collaborate and use outside
resources to answer the
following questions:
Conservation status of species
changes often as new threats
arise and current threats
subside. Fish and marine life
are often threatened by human
activities such as overfi shing,
pollution of ocean and fresh
water habitats, and climate
changes. When it comes to
making choices about what
seafood to purchase and
consume, what resources are
available to consumers to know
that fi sh they are buying is not
vulnerable or endangered?
Please refer to the printed copy of
the Giant Panda Problem Kit for AP
Biology Answer Guide included in
the kit for the answer
.
ThINQ!
Exercises
As your students read about the
reasons that giant pandas became
threatened you may also want
to make connections to natural
selection and its role in contributing
to changes in population among
various species.
This discussion could serves as a
segue for determining the role of
natural selection in the increase
and decrease of populations
over time.
Teachable
Moments
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
BACKGROUND

22
A two pronged approach is required to continue increasing numbers of giant pandas. Those
in the wild require protection by government agencies capable of establishing and maintaining
crucial habitats containing abundant bamboo forests and enforcing strict punishments for
poachers. For pandas in captivity, sensitive tests are required to track female panda hormones
indicating that ovulation is imminent. Currently, caretakers at zoos will collect urine and fecal
samples and test levels of reproductive hormones to pinpoint the window of opportunity for
mating and artifi cial insemination. It is important to continue developing and improving such
tests to increase their sensitivity and reliability and ensure successful panda pregnancies.
Mammalian Reproductive Endocrinology
Reproductive endocrinology is the study of hormones that support reproductive function.
Dozens of hormones and enzymes are required in order to support ovulation in female
mammals, such as the giant panda. Here we describe an essential set that will be discussed
in this lab. They include gonadotropin-releasing hormone, progesterone, estrogen, luteinizing
hormone (LH), and follicle stimulating hormone (FSH).
Collaborate and use outside
resources to answer the
following questions:
Antibodies are proteins that bind
to specifi c antigens. Why might
the specifi city of antibody and
antigen interactions be useful in
an immune response?
Please refer to the printed copy of
the Giant Panda Problem Kit for AP
Biology Answer Guide included in
the kit for the answer
.
What role do antibodies play
when a person receives a blood
transfusion from an incompatible
blood donor?
Please refer to the printed copy of
the Giant Panda Problem Kit for AP
Biology Answer Guide included in
the kit for the answer
.
ThINQ!
Exercises
Hypothalamus
Pituitary
Gonadotropin-Releasing
Hormone (GnRH)
Ovulation
Follicle Corpus Luteum
Follicle Stimulating
Hormone (FSH)
Luteinizing
Hormone (LH)
Estrogen Progesterone
Stimulation
Inhibition
Ovary
Connections to the male
reproductive system can also
be discussed with your students
as they learn how the female
reproductive system works.
Many of the hormones in the
female reproductive system are
also present in males. Another
connection may be to disorders
of the endocrine system that
could jeopardize fertility in
both males and females of a
species and how this may affect
populations over time.
Teachable
Moments
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
BACKGROUND
23
explorer.bio-rad.com
The hypothalamus, an area at the forefront of the brain that serves as the primary
neurohormone producer, connects both the nervous system and endocrine system. The
hypothalamus can be stimulated both extrinsically (e.g., scent marking left by a potential
mate) and intrinsically (e.g., presence or absence of coitus). Upon receiving specifi c
stimuli that trigger reproductive behaviors, the hypothalamus releases gonadotropin-
releasing hormone (GnRH). Once released in the brain, GnRH travels through a series
of blood vessels to the anterior pituitary gland in the brain. The anterior pituitary gland
then produces and releases FSH and LH.
FSH promotes the growth and development of follicles in the ovary that produce
estrogen. The release of estrogen at this point has a positive feedback effect on
the hypothalamus whereby more GnRH is released and therefore more LH and
FSH. Estrogen also plays a key role in preparing the uterine lining for the potential
implantation of an embryo after fertilization takes place. Once the follicle is mature, a
large amount of estrogen is produced that in turn stimulates a surge in LH production
triggering release of the egg from the follicle. At this point ovulation has occurred. The
remaining follicle becomes the corpus luteum — a hormone secreting structure in
the ovary that forms from the follicle once the egg is released from the ovary into the
fallopian tube.
The corpus luteum’s primary function is the production of progesterone which supports
and maintains pregnancy. Over time, the corpus luteum produces increasing amounts of
progesterone. During this time, progesterone acts as a negative feedback signal to the
hypothalamus to reduce production of GnRH which reduces the production of LH and
FSH thus inhibiting follicular growth in the ovaries. If implantation of an embryo does not
occur, the corpus luteum reduces in size and another round of follicular development
occurs. The level of progesterone will also decrease and menstruation will occur.
Being a mammal, the female giant panda experiences these hormone cycles, but only
once per year. This makes determining the timing of ovulation in female pandas critical
for reproductive success, especially in captivity and with the use of artifi cial insemination.
One way to determine the presence of reproductive hormones in pandas is to use
an enzyme-linked immunosorbent assay (ELISA). Using an ELISA, researchers can
determine the presence of a hormone in a sample and, if quantitation is necessary,
can determine how much of the hormone is present in the sample. The next section
describes how the ELISA featured in this lab works.
How Does the ELISA Work?
An ELISA is designed to detect proteins called antibodies produced by the body
during an infection or when foreign molecules are found in the body as part of the
body’s immune response. Immunology is the study of the immune system and how
the body protects itself against disease causing agents. Over 100 years ago, biologists
found that animals’ internal immune systems respond to invasion by “foreign entities”
or antigens. When an invader enters the body, it provokes an immune response that
includes the production of proteins called antibodies. Like magic bullets, antibodies seek
out and attach themselves to invading entities (foreign antigens), fl agging the invaders
for destruction by other cells of the immune system. The invaders may be any molecules
foreign to the body, including components of infectious agents like bacteria, viruses,
and fungi. Today, antibodies have become vital scientifi c tools, used in biotechnology
research and to diagnose and treat disease as well as track specifi c hormones in the
body. Antibodies make up to 15% of your total blood serum protein, so there is usually
an antibody ready to deal with any antigen. Antibodies are very specifi c; each antibody
recognizes only a single portion of an antigen.
Collaborate and use outside
resources to answer the
following questions:
When detecting a specifi c
hormone in a urine sample,
many other proteins (including
other hormones) are present in
the sample. How is the primary
antibody for a hormone of
interest able to detect a specifi c
hormone versus all the others in
a sample?
Please refer to the printed copy of
the Giant Panda Problem Kit for AP
Biology Answer Guide included in
the kit for the answer
.
ThINQ!
Exercises
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
BACKGROUND
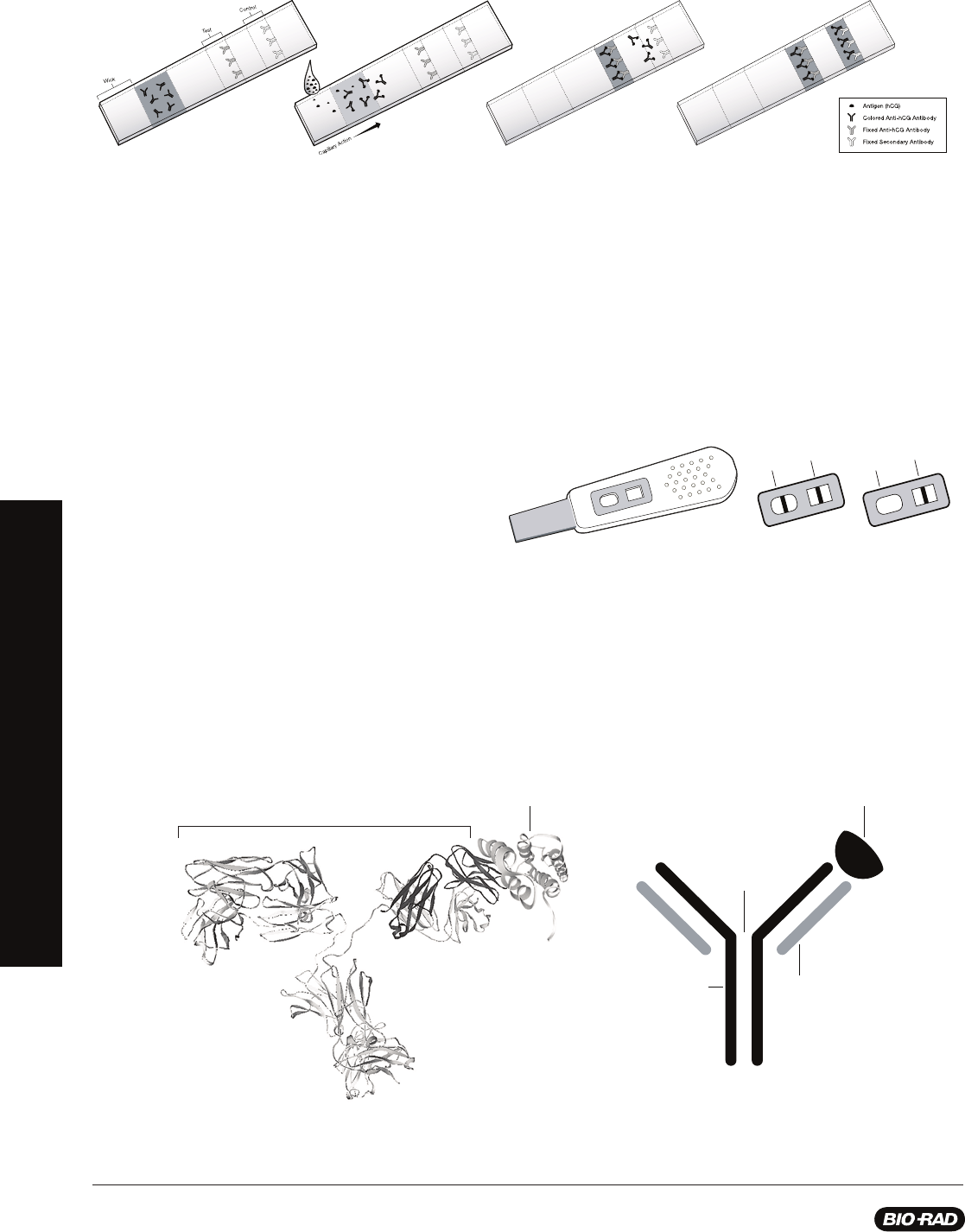
24
The ELISA relies on antibodies to detect the presence of antibodies or antigens in liquid samples. Because they are antibody-
based, ELISAs are called immunoassays. ELISAs can detect minute amounts of disease agents in samples such as bodily fl uids
(before the body has had a chance to mount a detectable immune response). Other applications for ELISA include testing for
hormones such as human chorionic gonadotropin (hCG) in pregnancy tests and LH in ovulation tests, illegal steroids in drug tests,
bacteria in food safety tests, and the presence of genetically modifi ed organisms contaminating non-GMO food.
Some tests give positive or negative results in a matter of minutes. For example, home pregnancy dipstick tests detect levels of hCG
in the urine of pregnant women within days of fertilization. The wick area of the dipstick is coated with anti-hCG antibody labeled
with a pink compound (see step 1 of the fi gure above). When the strip is dipped in urine, if hCG is present it will bind to the pink
antibody, and the pink hCG-antibody complex will migrate up the strip via capillary action (see step 2 of the fi gure above). When
the pink complex reaches the fi rst test zone, a narrow strip containing an unlabeled fi xed anti-hCG antibody, the complex will bind
and concentrate there, making a pink stripe (see step 3 of the fi gure above). The dipsticks have a built-in control zone containing an
unlabeled secondary antibody that binds unbound pink complex (present in
both positive and negative results) in the second stripe (see step 4
of the fi gure above). Thus, every valid test will give a second
pink stripe (control line), but only a positive pregnancy test
will give two pink stripes.
How Are Antibodies Made?
When exposed to foreign antigens, all mammals generate an immune response and produce antibodies, proteins that recognize
and bind tightly to the specifi c antigens. Each antibody recognizes only a single portion of an antigen. Animals such as goats,
rabbits, and mice can be injected with a foreign antigen and, after a period of time, their serum will contain antibodies that
specifi cally recognize that antigen. If the antigen was a disease-causing agent, the antibodies can be used to develop diagnostic
tests for the disease. Not all immunoassays detect foreign antigens that cause disease. Recall the examples above of ELISA tests
that confi rm ovulation or pregnancy. These immunoassays detect the presence of molecules that are naturally produced in our
bodies and do not cause disease, such as hormones. In these cases, an animal would be injected with the hormone we want to
detect in order to produce antibodies that will recognize that antigen. In an immunoassay, the antibodies used to recognize foreign
antigens like disease agents are called primary antibodies.
Antigen
-
S
-
S
-
-
S
-
S
-
-
S
-
S
-
-
S
-
S
-
Disulfide
Bonds
H Chain
L Chain
Antigen
A B
Antibody
Positive
Negative
Control
Test
Control
Test
Step 1 Step 2 Step 3 Step 4
Structure of antibodies
A, Structure of IgG bound to the HIV capsid protein p24 as determined by X-ray crystallography (Harris et al. 1998, Momany et al. 1996). These structures can be
downloaded and manipulated from the Protein Data Bank (rcsb.org/pdb/home/home.do, Berman et al. 2000) using the PDB identifi cation codes 1IGY and 1AFV.
B, A commonly used representation of an antibody bound to an antigen.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
BACKGROUND

25
explorer.bio-rad.com
Secondary antibodies recognize and bind to primary antibodies in an immunoassay.
They are prepared by injecting antibodies produced by one species of animal into
another species. This works because the antibodies produced by different species
are different enough from each other that they will be recognized as foreign and will
provoke an immune response. For example, if you want a secondary antibody that
will recognize a human primary antibody, inject human antibodies into an animal like a
rabbit. After the rabbit mounts an immune response against the human antibody, the
rabbit serum will contain antibodies that recognize and bind to human antibodies. In
this experiment, the secondary antibodies you will be working with are conjugated to
an enzyme named horseradish peroxidase (HRP); HRP in the presence of its substrate,
3,3’,5,5’-tetramethylbenzidine (TMB), produces a blue color.
Controls in Immunoassays
For any immunoassay to be interpretable, it must include both positive and negative
controls; for example, samples that will give known results. Controls are always run
side by side with experimental samples. If you do not run a positive control and the
experiment gives negative results, how can you be sure the results are truly negative?
What if the assay simply did not work? If a positive sample gives a negative assay result,
it is called a false negative. Conversely, if you do not run a negative control and the
experiment gives all positive results, how can you be sure the results are truly positive?
What if the assay was contaminated with antigen? If a negative sample gives a positive
assay result, it is called a false positive.
Controls are also needed to guard against experimental error and to ensure that the
assay is working correctly. There can be problems with reagents, which can degrade due
to age or poor storage conditions. Operators can make mistakes by choosing the wrong
reagents, making errors in dilutions or in pipetting, or failing to remove unbound reagents.
Poor record keeping is another source of false assay results. Most of these possibilities
can be verifi ed within the assay with the appropriate controls.
In this lab students are introduced
to an indirect ELISA. There are
four basic types of ELISAs: direct,
indirect, sandwich, and competition
or inhibition ELISA. Making
comparisons to the ELISA used in
this lab to other types may be helpful
when explaining how the assay
works and how to apply one type
versus another.
Students could also explain the
mechanism supporting each
in order to demonstrate their
understanding of antigen and
antibody interactions.
Teachable
Moments
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
BACKGROUND
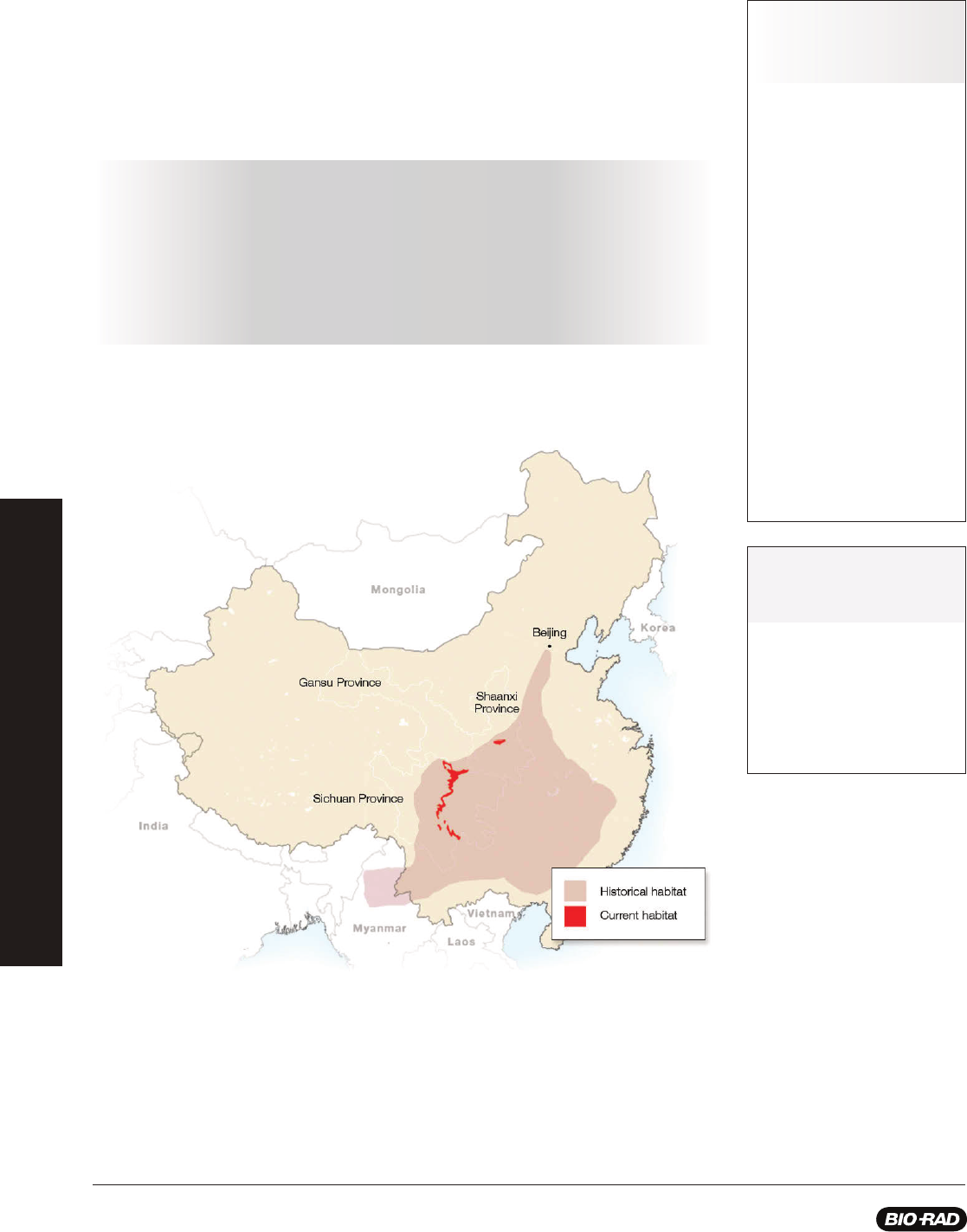
26
Pre-Lab Activity: Modeling Ovulation in Giant Pandas
Learning Goals:
• Students identify prior knowledge about the mammalian reproductive cycle
• Students consider hormones that can infl uence the ovulation cycle
• Students generate initial models about hormone interactions that support ovulation
Teacher Note: Providing students an opportunity to assess their prior knowledge of the
female mammalian reproductive cycle is useful as they build understanding over the course
of this lab. Modeling their ideas supports students in making their thinking visible and
communicating their ideas. The model they create in the pre-lab will be revisited during later
investigations and revised based on the data they collect and analyze. This process will
reinforce students’ thinking about the mechanism supporting the ELISA used in the kit. At this
point there should not be a “right answer” — this is simply a time when students can share
their initial ideas with each other.
Refer to the AP Biology Curriculum
Alignment tables on page 9 for more
details on how this activity aligns to
AP Curriculum Learning Objectives
(LO), Essential Knowledge (EK), and
Science Practices (SP).
Big Idea 2
LO 2.29 [EK 2.D.4 & SP 1.1, 1.2]
LO 2.31 [EK 2.E.1 & SP 7.2]
LO 2.32 [EK 2.E.1 & SP 1.4]
LO 2.33 [EK 2.E.1 & SP 6.1]
LO 2.43 [EK 2.D.4 & SP 7.2]
Big Idea 4
LO 4.8 [EK 4.A.4 & SP 3.3]
LO 4.9 [EK 4.A.4 & SP 6.4]
LO 4.10 [EK 4.A.4 & SP 1.3]
LO 4.19 [EK 4.B.3 & SP 5.2]
LO 4.20 [EK 4.B.3 & SP 6.3]
LO 4.21 [EK 4.B.3 & SP 6.4]
LO 4.26 [EK 4.C.3 & SP 6.4]
LO 4.27 [EK 4.C.4 & SP 6.4]
AP Bio
For decades giant pandas were considered an endangered species. Giant pandas in the
wild live in isolated, or fragmented, groups nestled high in the mountains of four provinces
in China. Giant pandas did not always live in small communities. In fact their habitat once
ranged across most of China and into the neighboring countries
of Myanmar and Northern Vietnam. Today the majority of
giant pandas are found in the Min Mountains in Sichuan
and Gansu provinces and the Qinling Mountains in
Shaanxi Province.
As students learn about giant
pandas during the Pre-Lab
Activity you may want to make
connections to topics such as
extinction, speciation, and evolution
that support the rise and fall of
populations of organisms over time.
Teachable
Moments
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
PRE-LAB ACTIVITY

27
explorer.bio-rad.com
In your group, answer the following questions:
1. Name at least three reasons that could explain why the giant panda’s habitat has been reduced to small and isolated areas of
China when it once spanned nearly the entire country and into neighboring countries.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
2.
Of these reasons, choose one that could be addressed with human intervention. Explain what humans could do to restore the
habitat of the giant panda.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
One way humans attempt to save endanger
ed and vulnerable species from extinction is through the establishment of breeding
programs where conservationists study the reproductive cycles of an animal and provide interventions to support successful mating,
pregnancy, birth, and survival of offspring. For the giant panda, conservation efforts were successful enough that in 2016 the
International Union for Conservation of Nature (IUCN) changed their status from endangered to vulnerable. This does not mean the
giant panda is “safe” from extinction. The classifi cation of vulnerable species means that the species may be very likely to return to
endangered status due to threats to its habitat and reproductive success. Learning more about the reproductive cycle of pandas is
critical to conserving the species.
In your group, answer the following question:
3. How might learning about the giant pandas’ reproductive cycle be important for preventing the pandas’ return to the
endangered species list?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer
.
One of the major obstacles to the breeding success of giant pandas is that in the wild male pandas generally live solitary lives with
very few interactions with female pandas. Adding to the geographic barriers, female pandas are only fertile 1–3 days out of a year
and only begin to bear young at 5 years of age. Zoos and other conservation centers have stepped in to increase the chances
of successful panda pregnancies and births by ensuring that male and female pandas have access to one another during critical
breeding days and by using artifi cial insemination to increase the chance of fertilization. With artifi cial insemination, animal caretakers
gather sperm from male pandas and inject the sperm into the uterus of the female panda during the time she is likely to ovulate,
releasing an egg from her ovary into her fallopian tube. To understand this process and to best determine when a female panda is
about to conceive, conservationists must fi rst understand the reproductive biology of female pandas that leads to an ovulation event.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
PRE-LAB ACTIVITY

28
4. Listed in the table below are the fi ve major reproductive hormones that support ovulation in female mammals, including the
giant panda. Describe the role of each hormone in this process and the approximate time during the ovarian cycle that it
reaches peak levels.
Endocrinologists, scientists and medical doctors who study how hormones work in the body, can track reproductive hormones
in female mammals to determine when and how much of each of the hormones listed in the table above are present in the body.
Tracking the levels of hormones and when they are released provides valuable information for developing tests that can predict
when events, like ovulation, will occur.
5. Given the information in the table above, identify at least three hormones that, if tracked, would be good indicators that
ovulation is about to occur in a female giant panda.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
In order to best support panda reproduction by natural mating and artifi cial insemination, highly sensitive and specifi c tests are
needed to determine when a female panda is most likely to conceive. In the next investigation you will learn about a test that can
be used to detect the presence of certain molecules, like hormones, but fi rst it is important to determine which hormones are most
likely to provide accurate information about the timing of ovulation.
6. In the space below, describe and/or draw how you would design a test that could track the hormones you identifi ed in
question 5. Consider what you might use as a sample for testing, when you might do your tests, how often, and how your
test would be constructed. What controls would you use?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
Hormone Role in Regulation of the Menstrual Cycle Timing of Peak Level
Gonadotropin-releasing
hormone (GnRH)
Estrogen
Luteinizing
hormone (LH)
Follicle stimulating
hormone (FSH)
Progesterone
Please refer to the printed copy of the Giant
Panda Problem Kit for AP Biology Answer
Guide included in the kit for the answers.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
PRE-LAB ACTIVITY

29
explorer.bio-rad.com
Investigation #1: Digital Animation Activity — ELISA Antibody
Simulation
Teacher Note: Direct your students to
bio-rad.com/PandaAPResources
to view the antibody
detection ELISA animation. Download and print the blank animation activity questions sheet
from the student manual from
bio-rad.com/PandaAPResources
for your students to use
along with the animation preparing for Investigation #2. The printed Answer Guide can be
found in the kit.
If you are using the extended timeline for this kit, Investigation #1 (page 33) will use a
hands-on approach to support students in running an ELISA and thinking about protocol
design for Investigation #2. You can also review how the ELISA in this lab works by reading
page 23 of the background information in this manual.
Learning Goals:
• Students learn about the interaction between antigens and antibodies as a mechanism
underlying an immune response
• Students learn that the structure of antibodies allows for interactions with specifi c antigens
• Students learn how to set up and run an ELISA for antibody detection
• Students refl ect on how to design a scientifi c protocol to answer a research question
• Students learn how to refi ne scientifi c models based on evidence
Refer to the AP Biology Curriculum
Alignment tables on page 9 for more
details on how this activity aligns to
AP Curriculum Learning Objectives
(LO), Essential Knowledge (EK), and
Science Practices (SP).
Big Idea 2
LO 2.29 [EK 2.D.4 & SP 1.1, 1.2]
LO 2.31 [EK 2.E.1 & SP 7.2]
LO 2.32 [EK 2.E.1 & SP 1.4]
LO 2.33 [EK 2.E.1 & SP 6.1]
LO 2.35 [EK 2.E.2 & SP 4.2]
LO 2.43 [EK 2.D.4 & SP 7.2]
Big Idea 4
LO 4.8 [EK 4.A.4 & SP 3.3]
LO 4.9 [EK 4.A.4 & SP 6.4]
LO 4.10 [EK 4.A.4 & SP 1.3]
LO 4.19 [EK 4.B.3 & SP 5.2]
LO 4.22 [EK 4.C.1 & SP 6.2]
AP Bio
In the Pre-Lab you learned about the reproductive hormones in female mammals, like the
giant panda. This information is needed in order to design a test that tracks reproductive
hormones in pandas that may indicate an upcoming ovulation event. As you may have
discussed with your classmates, tests can be developed to track hormones in the body. One
test that can be used for this purpose is an ELISA. Typically, ELISAs are used to determine
if an organism has been exposed to a disease causing agent. The test typically uses a
sample of an organism’s blood, saliva, urine, or feces to identify whether or not antibodies
to a particular disease causing agent are present. An ELISA for antibody detection is a
good place to start when thinking about how you will develop a test to track hormones in a
panda during Investigation #2. In this activity you will view a digital animation of an antibody
detection ELISA.
Go to
bio-rad.com/PandaAPResources
to view the antibody detection ELISA animation. Your
teacher will provide you with a question sheet to guide your thinking about the animation. You
can also download and print the question sheet yourself at
bio-rad.com/PandaAPResources
.
As part of your assignment you will design an ELISA protocol to test panda urine samples for
an ovulation hormone during Investigation #2. Be sure to pr
ovide your ELISA protocol to your
teacher for review before beginning Investigation #2.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
DIGITAL ANIMATION ACTIVITY

30
Digital Animation Activity: ELISA Antibody Simulation
Instructions: Go to
bio-rad.com/PandaAPResources
to view the antibody detection ELISA animation. Click through the animation
and read the description for each step. After viewing the animation, answer the questions below.
1. What is the purpose of the ELISA featured in the animation?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
2. Why is it important to add purifi ed antigen to the wells fi rst?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
3. The animation did not include any contr
ol(s). Why are controls important to include?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer
.
4. What would be appropriate positive and negative controls for an antibody detection ELISA?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
5. The antibody detection ELISA in the animation shows one well for the experimental setup. Is it appropriate to use just one
well when setting up an ELISA test? Why or why not?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer
.
6. The secondary antibody is attached to an enzyme (HRP) that chemically changes the enzyme substrate, turning it from a
colorless solution to a blue solution. If you ran an antibody detection ELISA with positive control wells, negative control
wells, and experimental wells, predict which wells of your experiment should turn blue, which should remain colorless,
and which wells you are not sure about and why.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
7. In the space below, draw and annotate what is happening in wells that turned blue to explain what is causing the
blue color.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer
.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
DIGITAL ANIMATION ACTIVITY
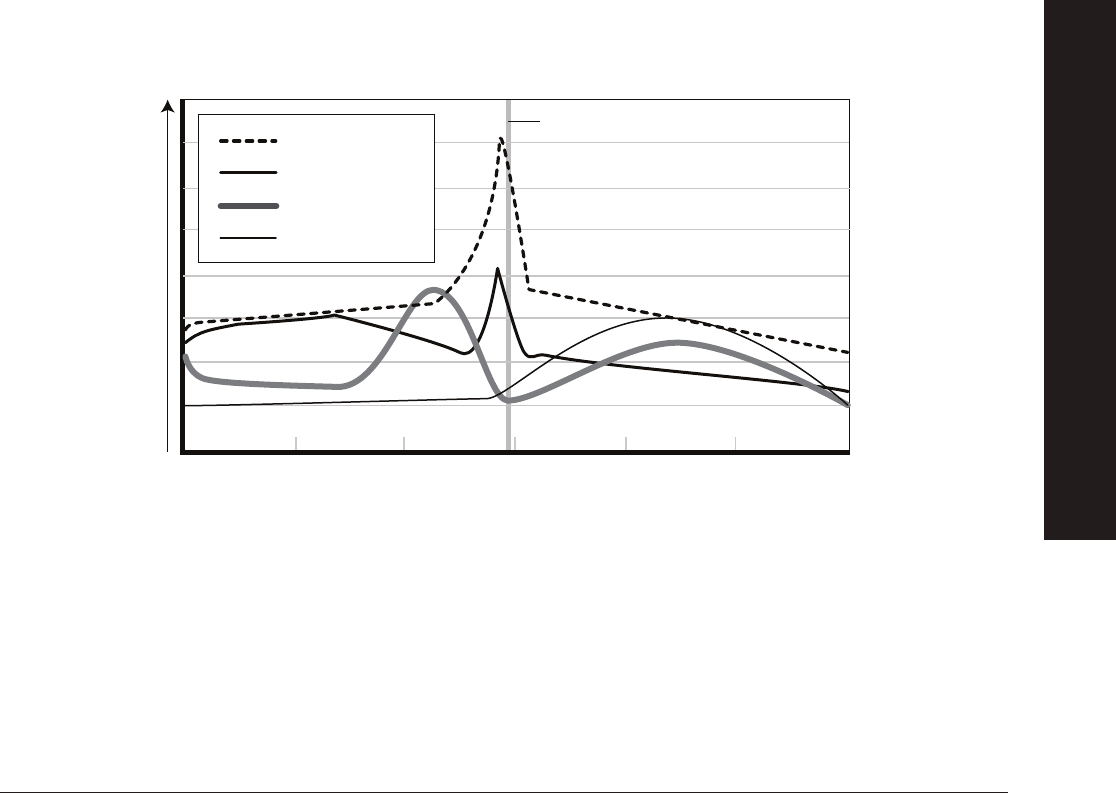
31
explorer.bio-rad.com
During this activity you observed a digital animation of an antibody detection ELISA. Thinking back to the background reading in
this manual, you learned that ELISAs can also track hormones in the body, like reproductive hormones in female giant pandas.
However, when tracking a hormone, the ELISA would be detecting an antigen (the hormone of choice) instead of the presence or
absence of antibodies to a particular disease.
8. How can antibodies be engineered to detect the presence of a molecule that does not cause disease, such as a hormone?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
9. Based on the animation and your answer to question 7 draw a well and label the components in order of addition to detect
the presence of an antibody potentially found in a panda urine sample. A real-world example of this is testing a panda for
pre-eclampsia. Pre-eclampsia is an autoimmune pregnancy disorder resulting in high blood pressure and protein in the
urine and can have fatal results for both mother and offspring. In many cases, antiphospholipid (fatty acid) antibodies are
indentifi ed using an ELISA and serve as an indicator for pre-eclampsia.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
10. During the Pre-Lab you identifi ed at least three hormones that could be tracked in order to determine the onset of ovulation
in a giant panda. Given the information in the graph below, which of the three reproductive hormones would provide the
greatest accuracy for onset of ovulation?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
Follicular Luteal
Hormone level
Ovarian Cycle Phases
FSH
LH
Estrogen
Progesterone
Ovulation
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
DIGITAL ANIMATION ACTIVITY

32
11. What is your reasoning for choosing this particular hormone?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
12. What controls would you need to include to test for the presence or absence of your hormone in samples from
giant pandas?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
13. Looking back at your Pre-Lab model (question 6) and your choice of hormone to track from this activity (question 10),
develop an ELISA protocol that can detect hormone levels in panda urine. Be sure to include controls in your protocol.
Your teacher will review your work prior to beginning Investigation #2.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer
.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
DIGITAL ANIMATION ACTIVITY
33
explorer.bio-rad.com
Investigation #1: ELISA Antibody Test (Optional Structured
Inquiry Activity)
Teacher Note: Direct your students to
bio-rad.com/PandaAPResources
for the antibody
detection ELISA animation. Download and print the blank animation question sheet from
the student manual at
bio-rad.com/PandaAPResources
for your students to use along
with the animation preparing students for Investigation #2. The printed Answer Guide can
be found in the kit.
If you are using the extended time line for this kit, optional Investigation #1 (this page)
uses a hands-on approach to support students in running an ELISA and thinking about
protocol design for Investigation #2. For a refresher on how the ELISA in this investigation
works, see page 23 of the background information in this manual.
Learning Goals:
• Students learn about the interaction between antigens and antibodies as the
mechanism underlying an immune response
• Students learn that the structure of antibodies allows for interactions with
specifi c antigens
• Students learn how to set up and run an ELISA for antibody detection
• Students refl ect on how to design a scientifi c protocol to answer a research question
• Students learn how to refi ne scientifi c models based on evidence
Refer to the AP Biology Curriculum
Alignment tables on page 9 for more
details on how this activity aligns to
AP Curriculum Learning Objectives
(LO), Essential Knowledge (EK), and
Science Practices (SP).
Big Idea 2
LO 2.29 [EK 2.D.4 & SP 1.1, 1.2]
LO 2.31 [EK 2.E.1 & SP 7.2]
LO 2.32 [EK 2.E.1 & SP 1.4]
LO 2.33 [EK 2.E.1 & SP 6.1]
LO 2.35 [EK 2.E.2 & SP 4.2]
LO 2.43 [EK 2.D.4 & SP 7.2]
Big Idea 4
LO 4.8 [EK 4.A.4 & SP 3.3]
LO 4.9 [EK 4.A.4 & SP 6.4]
LO 4.10 [EK 4.A.4 & SP 1.3]
LO 4.19 [EK 4.B.3 & SP 5.2]
LO 4.22 [EK 4.C.1 & SP 6.2]
AP Bio
In the Pre-Lab you learned about the reproductive hormones in female mammals, like the giant panda. This information is needed
in order to design a test that tracks reproductive hormones in pandas that may indicate an upcoming ovulation event. As you may
have discussed with your classmates, tests can be developed to track hormones in the body. One test that can be used for this
purpose is an ELISA. Typically, ELISAs are used to determine if an organism has been exposed to a disease causing agent. The test
uses a sample of an organism’s blood, saliva, urine, or feces to identify whether or not antibodies to a particular disease causing
agent are present. An ELISA for antibody detection is a good place to start when thinking about how you will develop a test to track
hormones in a panda during Investigation #2.
In this investigation you will run an ELISA to diagnose which of two female giant pandas has pre-eclampsia. Pre-eclampsia is an
autoimmune pregnancy disorder resulting in high blood pressure and protein in the urine and can have fatal results for both mother
and offspring. In many cases, anti-phospholipid (fatty acid) antibodies are identifi ed using an ELISA and serve as an indicator for
pre-eclampsia.
Item (Label) Contents Quantity
☐ Yellow tubes Set of panda urine samples (P1, P2; 200 µl each) 1
☐ Violet tube (+) Positive control (200 µl) 1
☐ Blue tube (–) Negative control (200 µl) 1
☐ Green tube (AG) Purifi ed antigen (800 µl) 1
☐ Orange tube (SA) Secondary antibody (800 µl) 1
☐ Brown tube (SUB) Enzyme substrate (800 µl) 1
☐ 12-well microplate strip 1
☐ 50 µl fi xed-volume micropipet, or 20–200 µl adjustable micropipet (optional) 1
☐ Yellow tips (optional) 10–20
☐ Disposable plastic transfer pipet (DPTP) 10
☐ 35 ml wash buffer in beaker PBS with 0.05% Tween 20 1
☐ Large stack of paper towels 1
☐ Black marking pen 1
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
ELISA ANTIBODY TEST
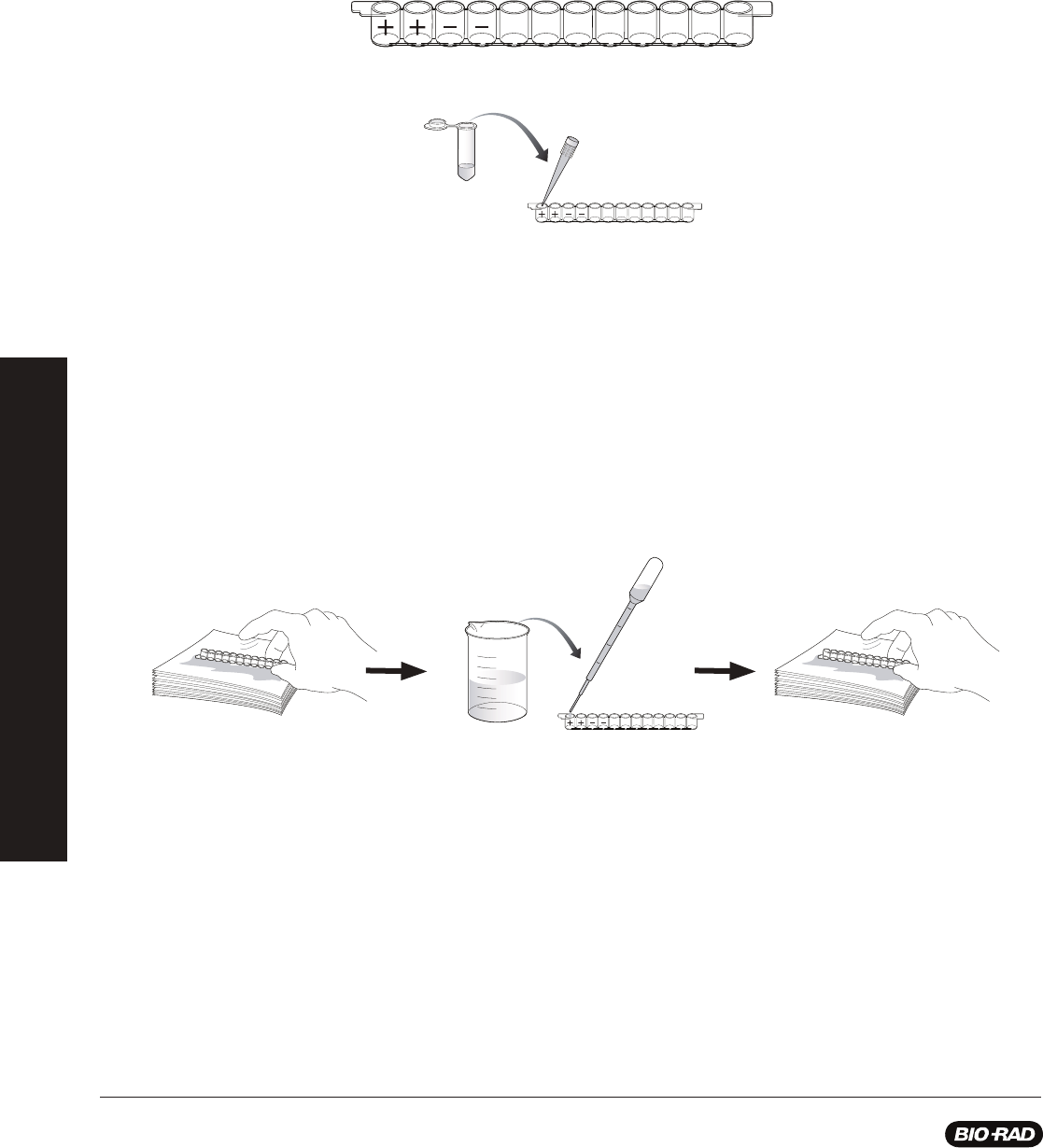
34
Protocol
1. The yellow tubes contain the urine samples that will be tested for the presence of anti-phospholipid antibodies. Label each
yellow tube to identify the sample being tested.
2. Label the outside wall of each well of your 12-well strip. On each strip label the fi rst two wells with a (+) for the positive
controls and the next two wells with a (–) for the negative controls. Label the remaining wells in duplicate to identify the
samples being tested. You will have four unused wells in your strip. For example, Panda 1 (P1) and Panda 2 (P2) like this:
3. Use a pipet to transfer 50 µl of the purifi ed antigen (AG) from the green tube into the fi rst 8 wells. The antigen in this case
is phospholipids.
4. Wait 5 min while the antigen binds to the plastic wells.
5. Wash unbound antigen out of the wells:
a. Tip the microplate strip upside down onto the paper towels so that the samples drain out, then vigorously tap the
strip a few times upside down on the paper towels to get rid of all the liquid and bubbles in the wells.
b. Discard the wet paper towels.
c. Use a pipet fi lled with wash buffer from the beaker to fi ll each well with wash buffer, taking care not to spill over into
neighboring wells. The same transfer pipet will be used for all washing steps. Take care not to touch the tip of the pipet
to the wells of the strip.
d. Tip the microplate strip upside down onto the paper towels so that the wash buffer drains out, then gently tap the strip
a few times upside down on the paper towels to get rid of all the liquid in the wells.
e. Discard the wet paper towels.
6. Repeat wash steps 5 c–e one time.
7. Use a fresh pipet to transfer 50 µl of the positive control (+) from the violet tube into the two (+) wells. The positive control
contains anti-phospholipid antibodies.
P1 P1 P2 P2
P1 P1 P2 P2
Purifi ed
antigen
P1 P1 P2 P2
Wash
A. C. D.
WASH
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
ELISA ANTIBODY TEST
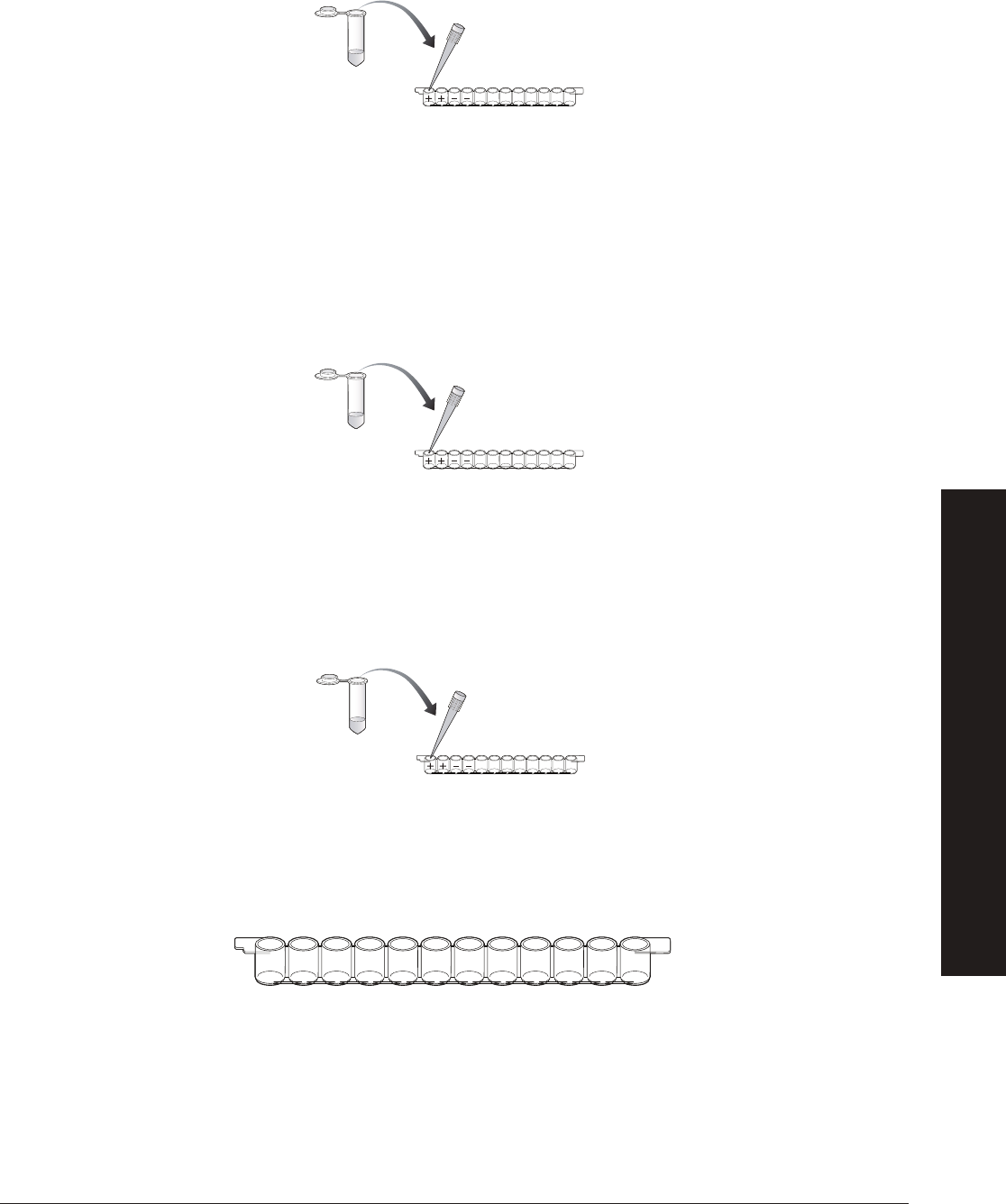
35
explorer.bio-rad.com
8. Use a fresh pipet to transfer 50 µl of the negative control (–) from the blue tube into the two (–) wells.
9. Use a fresh pipet to transfer 50 µl of each urine sample (P1 and P2) into the appropriately labeled two wells.
10. Wait 5 min to allow the serum antibodies in the controls and samples to bind to the antigen (phospholipids).
11. Wash the samples out of the wells by performing wash steps 5–6. This will wash the wells out two times.
12. Use a fresh pipet to transfer 50 µl of secondary antibody (SA) from the orange tube into the fi rst 8 wells of the microplate
strip. The secondary antibody will bind only to the anti-phospholipid antibodies.
13. Wait 5 min for the secondary antibody to bind to the primary antibody.
14. Wash the unbound secondary antibody out of the wells by performing wash step 5 one time. Then perform wash step 6
two times. This will wash the wells out a total of three times.
15. Use a fresh pipet to transfer 50 µl of enzyme substrate (SUB) from the brown tube into the fi rst 8 wells of the microplate strip.
16. Wait 5 min. Observe and record your results.
Results
Label the fi gure below with the same labels you wrote on the wells in step 1. In each of the wells, put a (+) if the well turned blue and
a (–) if there is no color change.
P1 P1 P2 P2
Control
or sample
WASH 2x
P1 P1 P2 P2
Secondary
antibody
WASH 3x
P1 P1 P2 P2
Enzyme
substrate
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
ELISA ANTIBODY TEST

36
Post-Investigation Questions
Teacher Note: Just like learning science content knowledge, students also require practice learning how to design protocols.
These post-investigation questions are designed to prompt students’ refl ections about the protocol they completed in Investigation
#1. There are several ways students can answer the question presented in Investigation #1. Students should be given time to
discuss their thinking and share their ideas with the class.
1.1 What is the purpose of this protocol?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
1.2 Why is it important to add purifi
ed antigen to the wells fi rst?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
1.3 Why is it important to include a positive and a negative control?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
1.4 The secondary antibody is attached to an enzyme (HRP) that chemically changes the enzyme substrate, turning it from
a colorless solution to a blue solution. Predict which wells of your experiment should turn blue, which should remain
colorless, and which wells you are not sure about.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
1.5 In the space below, draw and annotate what is happening in your “+” wells that turned blue to explain what is causing the
blue color.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
During this investigation, you conducted an antibody detection ELISA. Thinking back to the background reading in this
manual, you learned that ELISAs can also track hormones in the body, like reproductive hormones in female giant pandas.
However, when tracking a hormone the ELISA would be detecting an antigen (the hormone of choice) instead of the
presence or absence of antibodies to a particular disease.
1.6 What changes might you make to this protocol to track the presence of a hormone in
a urine sample instead of the presence of an antibody?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology
Answer Guide included in the kit for the answer
.
It may be useful to hold a whole
class discussion where students
generate a list of important
considerations when designing
an experimental protocol. This
list should include general factors
such as use of a control, defi ning
an investigation question, and
careful collection of data, among
others. This list could be posted
on the classroom wall to serve as
a reminder in future investigations
when students generate their own
protocols.
Teachable
Moments
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
ELISA ANTIBODY TEST
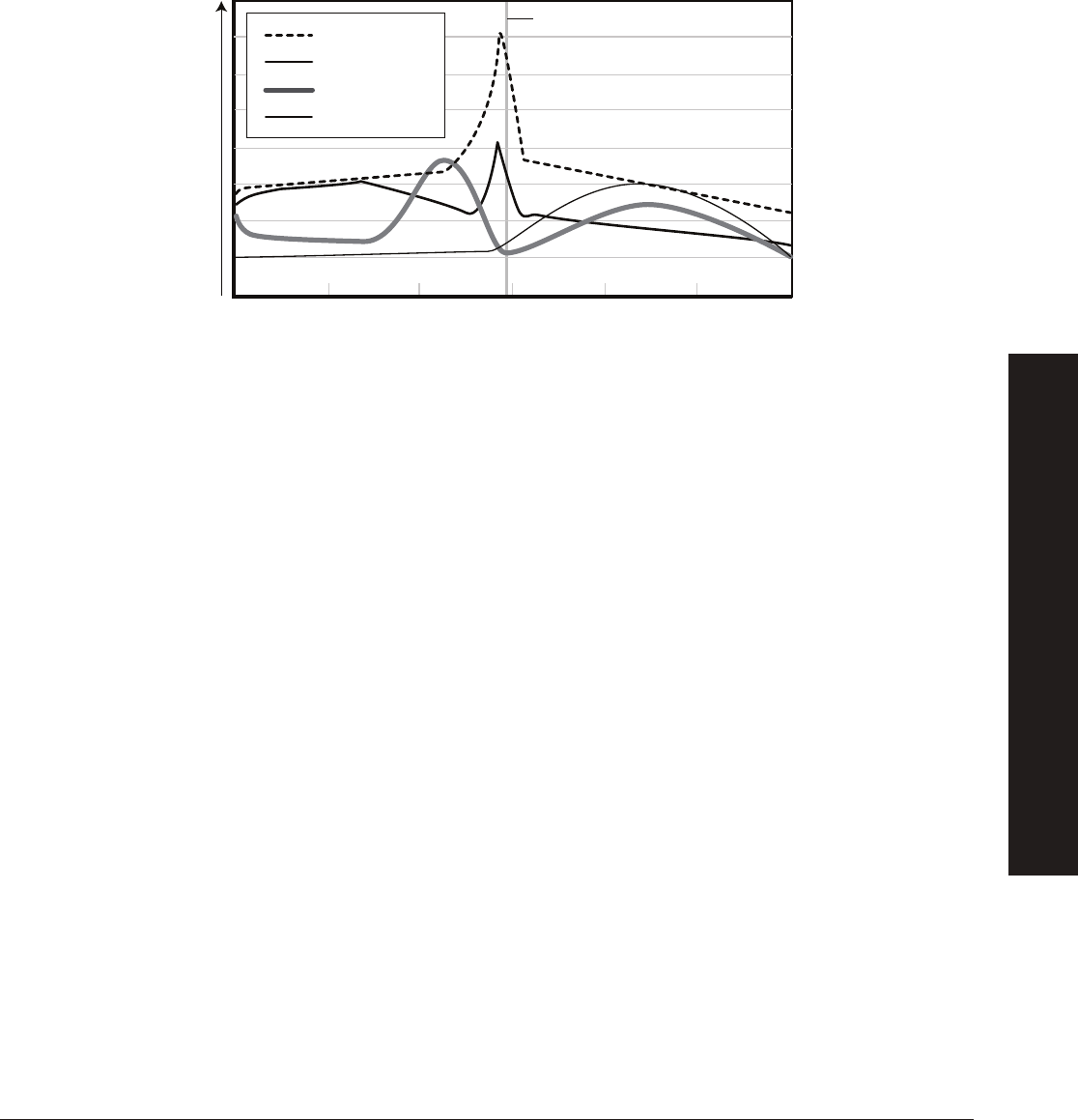
37
explorer.bio-rad.com
1.7 During the Pre-Lab you identifi ed at least three hormones that could be tracked to determine the onset of ovulation
in a giant panda. Given the information in the graph below, which of the three reproductive hormones would provide the
greatest accuracy for onset of ovulation?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
1.8 What is your reasoning for choosing this particular hormone?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
1.9 How can antibodies be engineered to detect the presence of a molecule that does not cause disease, such as a hormone?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
1.10 Using your response to question 1.6, what controls would you need to include to test for the presence or absence of your
hormone in samples from giant pandas?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
The data you generated during this investigation provide insight about how an ELISA can be used to determine if a panda has
anti-phospholipid antibodies, an indicator for pre-eclampsia. However, further investigations are required to answer the question:
How can we test whether the pandas in captivity are ready to conceive?
Assignment: Investigation #1 Wrap-Up
Looking back at your pre-lab model (question 6) and your choice of hormone to track from Investigation #1 (question 1.7), develop
an ELISA protocol that can detect hormone levels in panda urine. Be sure to include controls in your protocol. Your teacher will
review your work prior to beginning Investigation #2.
Follicular Luteal
Hormone level
Ovarian Cycle Phases
FSH
LH
Estrogen
Progesterone
Ovulation
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
ELISA ANTIBODY TEST

38
ELISA Paper Model (Optional Activity)
Teacher Note: This paper model activity will prepare your students as they think about their protocol design in Investigation #2.
The paper model can be used to test students’ understanding of an antibody detection ELISA and an antigen (hormone) detection
ELISA. See Appendix F on page 63 for the ELISA paper model. You can make copies and cut the pieces out yourself ahead of
class, or ask your students to cut the model pieces out at the start of class.
1. In your group use the paper model pieces to model an antibody detection ELISA. Be sure to explain each step of the ELISA
and why each step is necessary.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer
.
2.
In your group use the paper model pieces to model a hormone (antigen) detection ELISA. Be sure to explain each step of
the ELISA and why each step is necessary
.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
ELISA PAPER MODEL
39
explorer.bio-rad.com
Investigation #2: Hormone Detection ELISA
(Structured/Guided/Open Inquiry Activity)
Teacher Note: Conducting inquiry investigations can be both enriching and challenging for
students. Depending on your students’ familiarity with the inquiry process you may decide
to pursue structured, guided, or open inquiry for Investigation #2. If you choose to use a
structured approach, see the quick guide in Appendix A. In both guided and open styles of
inquiry, students are provided the opportunity to develop an experimental protocol to answer
a question. With guided inquiry, the teacher provides the investigation question and with
open inquiry students develop their own question to investigate. In both cases, the teacher’s
role is to provide support, facilitate discussions among groups, and guide students’ science
understanding and practice development as needed. For a refresher on how the ELISA in this
investigation works, see page 23 of the background information in this manual.
Learning Goals:
• Students learn how to design a scientifi c protocol to answer a research question
• Students can model and explain how an ELISA for hormone detection works
• Students learn how to refi ne scientifi c models based on evidence
In the Pre-Lab you learned about the reproductive hormones in female mammals, like the giant
panda. During the Investigation #1 Digital Animation Activity and/or the ELISA Antibody Test
you learned how an ELISA works to detect specifi c antibodies. This information is needed
in order to design an ELISA that tracks a specifi c reproductive hormone in pandas that may
indicate an upcoming ovulation event. In this investigation you will run an ELISA to determine
which of four female giant pandas is about to ovulate. Your results will be used to help
caretakers determine which female pandas are nearing their fertility window — an important
step in the conservation of giant pandas as a species.
Student Workstation Checklist
Refer to the AP Biology Curriculum
Alignment tables on page 9 for more
details on how this activity aligns to
AP Curriculum Learning Objectives
(LO), Essential Knowledge (EK), and
Science Practices (SP).
Big Idea 2
LO 2.29 [EK 2.D.4 & SP 1.1, 1.2]
LO 2.31 [EK 2.E.1 & SP 7.2]
LO 2.32 [EK 2.E.1 & SP 1.4]
LO 2.33 [EK 2.E.1 & SP 6.1]
LO 2.35 [EK 2.E.2 & SP 4.2]
LO 2.43 [EK 2.D.4 & SP 7.2]
Big Idea 4
LO 4.8 [EK 4.A.4 & SP 3.3]
LO 4.9 [EK 4.A.4 & SP 6.4]
LO 4.10 [EK 4.A.4 & SP 1.3]
LO 4.19 [EK 4.B.3 & SP 5.2]
LO 4.22 [EK 4.C.1 & SP 6.2]
AP Bio
Item (Label) Contents Quantity
☐ Yellow tubes Set of panda urine samples (P1, P2, P3, P4; 200 µl each) 1
☐ Violet tube (+) Positive control (200 µl) 1
☐ Blue tube (–) Negative control (200 µl) 1
☐ Green tube (PA) Primary antibody (1 ml) 1
☐ Orange tube (SA) Secondary antibody (1 ml) 1
☐ Brown tube (SUB) Enzyme substrate (1 ml) 1
☐ 12-well microplate strips 1
☐ 50 µl fi xed-volume micropipet, or 20–200 µl adjustable micropipet (optional) 1
☐ Yellow tips (optional) 10–20
☐ Disposable plastic transfer pipet (DPTP) 10
☐ 35 ml wash buffer in beaker PBS with 0.05% Tween 20 1
☐ Large stack of paper towels 1
☐ Black marking pen 1
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
HORMONE DETECTION ELISA

40
Pre-Investigation Questions
In this investigation you will design an experimental protocol to answer your questions and
test your ideas to generate an ELISA to detect a specifi c ovulation hormone of your choice in
panda urine samples. For this investigation, all materials and reagents from Investigation #1
will be provided to you.
2.1 With your group, determine your investigation question:
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer
Guide included in the kit for the answer.
2.2 Given what you learned about the antibody detection ELISA in Investigation #1,
what procedural steps will you take in order to answer your investigation
question?
Remember that antibodies can be engineered to detect molecules, such as
hormones, that do not cause disease. Look back at the protocol and data analysis
sections of Investigation #1
for ideas and draw and/or describe your steps below.
Don’t forget to include an experimental control.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer
Guide included in the kit for the answer.
Teacher Note: You may fi nd it helpful to use an Experimental Design and Planning
Worksheet to guide your students through the procedure writing process. Go to
bio-rad.com/PandaAPResources
to download the worksheet.
Collaborate and use outside
resources to answer the
following questions:
Typically, urine samples do
not contain consistent levels
of hormones. For example,
pregnancy tests for humans
become reliable only about
10 days after implantation
of the embryo in the uterine
lining when human chorionic
gonadotropin (hCG), the
“pregnancy hormone,” is
produced. Test results become
more and more reliable
with increasing time after
implantation as greater levels of
hCG are produced. How might
this information infl uence when
and how often an ovulation test
for giant pandas is conducted?
Please refer to the printed copy of
the Giant Panda Problem Kit for AP
Biology Answer Guide included in
the kit for the answer
.
ThINQ!
Exercises
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
HORMONE DETECTION ELISA

41
explorer.bio-rad.com
Sample Results
2.3 Revisit your drawing and explanation of how the antibody detection ELISA works from Investigation #1 (question 1.5)
or from the Digital Animation Activity: ELISA Antibody Test (question 7). Using these models as a reference, draw and
explain what is happening in the wells of the ELISA for this investigation.
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
2.4 What recommendations would you provide the panda caretakers in terms of the reproductive capacity of the four giant
pandas you tested?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the
answer.
P1 P1 P2 P2 P3 P3 P4 P4
P
P1
P1
P1
P1
P1
P1
P1
P1
P1
P2
P2
P2
P2
P2
P2
P3
P3
P3
P4
P4
P4
P4
P4
P4
P4
P4
P4
P4
P
P1
P1
P1
P1
P1
P1
P1
P1
P1
P3
P3
P3
P4
P4
P4
P4
P4
P4
P4
P4
P4
P4
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
HORMONE DETECTION ELISA

42
Post-Lab Assessment
Teacher Note: The following Post-Lab Assessment is designed to provide your students
an opportunity to apply their understanding of both an antibody detection ELISA (like the
Investigation #1 Digital Animation Activity Antibody ELISA Simulation) and an antigen detection
ELISA (like Investigation #2). Students answer refl ection questions that draw on learning from
the Pre-Lab activity and investigations. See Appendix F on
page 63
for the paper models. You
can make copies and cut the pieces out yourself ahead of class, or ask your students to cut
the model pieces out at the start of class.
Ruffed lemurs found in the eastern rainforests of Madagascar typically live arboreally, in the
crowns of large trees, with most of their time spent 15 to 25 meters (50–80 feet) above the
forest fl oor. Their diet comprises mainly of fruit, fl owers, and young leaves accessible year
round.
Prized for their meat and fur, ruffed lemurs have been hunted nearly to extinction. Many
residents of Madagascar fi nd a large portion of the protein in their diet from animals hunted
in the rainforest. Called bushmeat, this form of protein can be a valuable dietary resource to
people without access to other food. However, many wild animals carry diseases that can be
transmitted through their blood to humans who are processing the meat for consumption. For
example, the Ebola epidemic of 2014 likely began with the transmission of the virus from a
fruit bat to humans in Guinea.
In addition to hunting, ruffed lemurs also suffer from habitat loss due to deforestation, climate
changes, and urban development. Because of these threats, the ruffed lemur became critically
endangered in 2008 meaning the species faces a very high risk of extinction. Fortunately
ruffed lemurs reproduce easily in captivity so they make an excellent species for reintroduction
into the wild.
Recently conservation biologists noticed that a conspiracy, or group, of lemurs in a
Madagascar wildlife preserve were behaving oddly. Several lemurs were seen on the ground
acting lethargic. The biologists sedated three lemurs to run some tests and see if they could
understand this strange new behavior. All three of the lemurs had elevated body temperatures
and appeared to be dehydrated and underweight as if they had not been eating regularly
despite the availability of food in the tree tops of the preserve.
Refer to the AP Biology Curriculum
Alignment tables on page 9 for more
details on how this activity aligns to
AP Curriculum Learning Objectives
(LO), Essential Knowledge (EK), and
Science Practices (SP).
Big Idea 2
LO 2.29 [EK 2.D.4 & SP 1.1, 1.2]
LO 2.33 [EK 2.E.1 & SP 6.1]
LO 2.43 [EK 2.D.4 & SP 7.2]
Big Idea 4
LO 4.8 [EK 4.A.4 & SP 3.3]
LO 4.9 [EK 4.A.4 & SP 6.4]
LO 4.19 [EK 4.B.3 & SP 5.2]
LO 4.20 [EK 4.B.3 & SP 6.3]
LO 4.21 [EK 4.B.3 & SP 6.4]
LO 4.26 [EK 4.C.3 & SP 6.4]
LO 4.27 [EK 4.C.4 & SP 6.4]
AP Bio
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
POST-LAB ASSESSMENT

43
explorer.bio-rad.com
1. What type of ELISA would you recommend using to determine if the lemurs have a disease and why?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
The biologists performed several ELISAs to identify the presence of antibodies for different disease causing agents. They ran tests
for antibodies indicating an immune response to Encephalitozoon intestinalis, Toxoplasma gondii (T. gondii), Lesavirus 2, and
Encephalomyocarditis (EMCV) virus. Interestingly, only the test for T. gondii antibodies in the lemurs’ blood generated a positive
result. However, the biologists questioned whether the result was reliable or not since the colorimetric result for two of the lemurs
was extremely faint compared to their positive controls and for the third lemur the result appeared to be negative for T. gondii
antibodies.
2. What could be the reason for a very faint colorimetric result in two of the samples compared to the positive control, and a
possible negative result for the third sample?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer
.
The biologists wer
e curious about the results and wanted to know more about the sensitivity of the ELISA test they were using.
Using a quantitative ELISA, the biologists could determine the amount of antibodies present in each of the samples from the
lemurs. They fi rst set up a series of standards to use as a comparison. Each well contained an decreasing amount of antibody
from 1000 ng/ml (well 1) to 0 ng/ml (well 12). As each well contains a known concentration of antibodies, the two positive lemur
samples (A and B) could be compared to the standards and the unknown concentration in each sample could be determined.
Immediately the biologists could visually compare the lemur samples to the standards and could roughly estimate the
concentration of antibody in each sample. Wanting to be more precise, they used a microplate reader, an instrument designed
to determine the concentration of a chemical in a sample, to determine the exact concentration of T. gondii antibodies in each
sample.
Top: 12-well strip containing positive control (+), negative control (-), lemur sample 1 (A), and lemur sample 2 (B). Bottom: 12-well strip containing serial dilution of
standards from 1000 ng/ml antibody (well 1) to 0 ng/ml antibody (well 12).
ELISA samples Concentration of antibodies, ng/ml
Positive control 1,000
Negative control 0
Sample A 16
Sample B 125
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
POST-LAB ASSESSMENT

44
3. What do these data tell you about the condition of the two lemurs that tested positive for antibodies to T. gondii?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
4. Is the third lemur that tested negative for antibodies to T. gondii
at risk of contracting toxoplasmosis? What about other
lemurs in the conspiracy? Why or why not?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for
the answer.
INSTRUCTOR’S GUIDE TO THE STUDENT MANUAL
POST-LAB ASSESSMENT
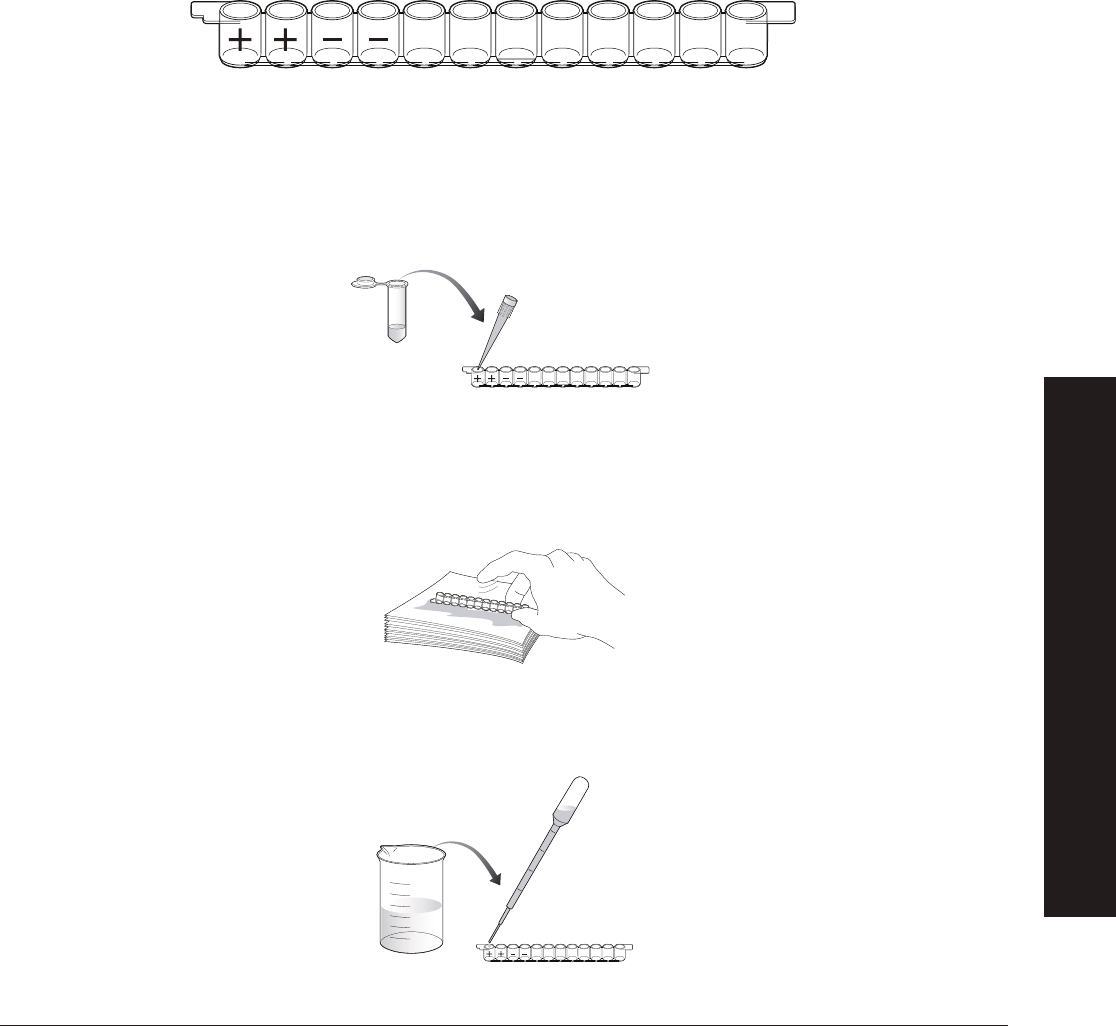
45
explorer.bio-rad.com
Appendix A
Quick Guide
Investigation #2: Hormone Detection ELISA
Investigation question: How can we use the antibody detection ELISA test as a model to generate our own test for hormone
levels in pandas?
Protocol
1. Find panda urine samples in yellow tubes provided by your teacher.
2. Label the outside wall of each well of your 12-well strip. On each strip label the fi rst two wells with a “+” for the positive controls
and the next two wells with a “–” for the negative controls. Label the remaining wells in duplicate to identify the samples being
tested. All twelve wells will be used in this investigation. For example, Panda 1 (P1), Panda 2 (P2), Panda 3 (P3), and Panda 4
(P4) like this:
3. Bind the antigen to the wells:
a. Use a pipet to transfer 50 µl of the positive control (+) from the violet tube into the two “+” wells.
b. Use a fresh pipet to transfer 50 µl of the negative control (–) from the blue tube into the two “–” wells.
c. Use a fresh pipet for each sample and transfer 50 µl of each of your team’s panda urine samples into the
appropriately initialed two wells.
4. Wait 5 min while all the proteins in the samples bind to the plastic wells.
5. Wash the unbound sample out of the wells:
a. Tip the microplate strip upside down onto the paper towels so that the samples drain out, then vigorously tap the
strip a few times upside down on the paper towels to remove all liquid and bubbles from the wells.
b. Discard the wet paper towels.
c. Use a pipet fi lled with Wash buffer from the beaker to fi ll each well with wash buffer, taking care not to spill over into
neighboring wells. The same transfer pipet will be used for all washing steps. Take care not to touch the tip of the pipet
to the wells of the strip.
P1 P1 P2 P2 P3 P3 P4 P4
P1 P1 P2 P2 P3 P3 P4 P4
Wash
P1 P1 P2 P2 P3 P3 P4 P4
Control
or sample
APPENDIX A
QUICK GUIDE
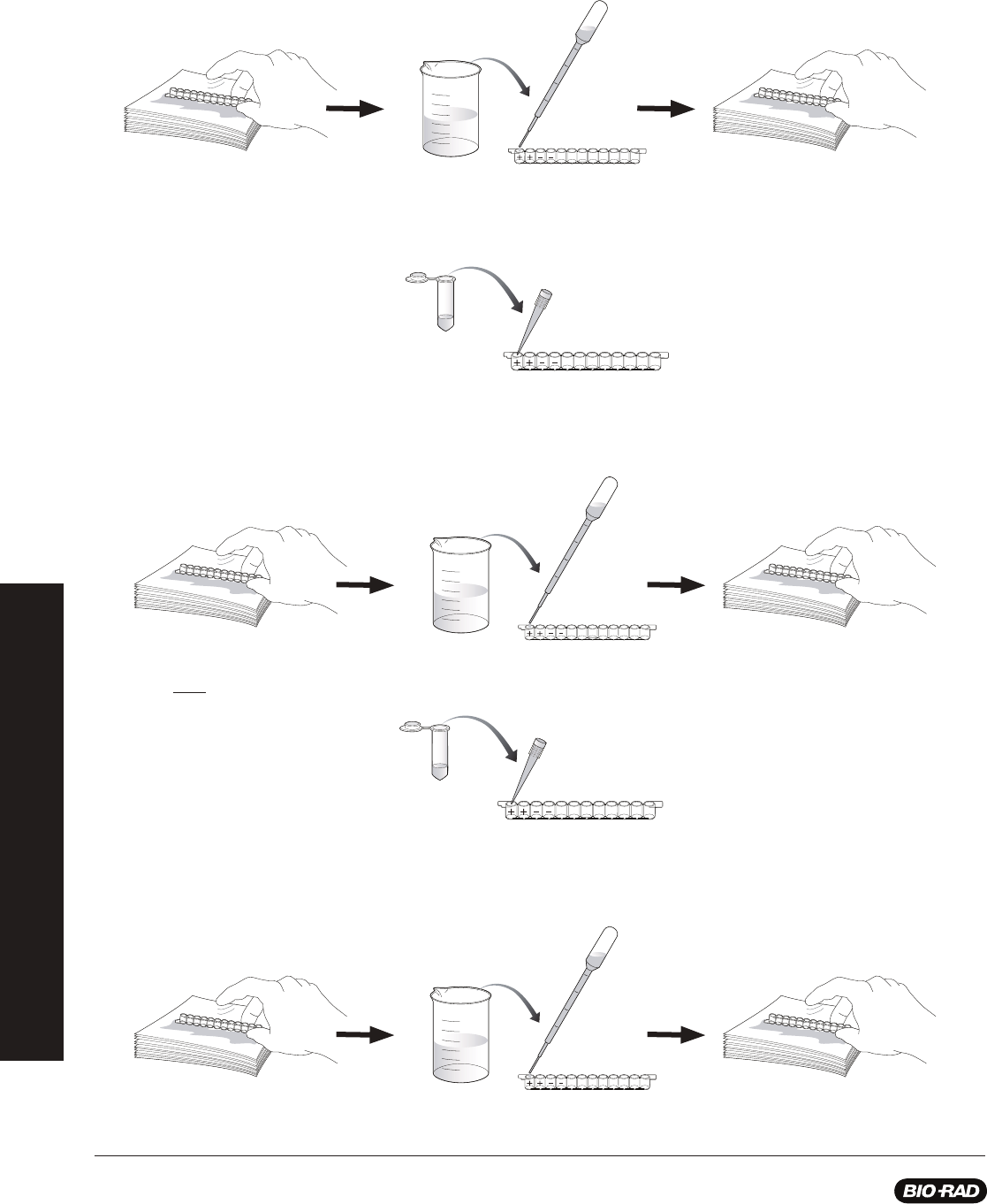
46
d. Tip the microplate strip upside down onto the paper towels so that the wash buffer drains out, then vigorously tap the
strip a few times upside down on the paper towels to remove all liquid and bubbles from the wells.
e. Discard the wet paper towels.
6. Repeat wash steps 5 c–e one time.
7. Use a fresh pipet to transfer 50 µl of primary antibody (PA) from the green tube into all 12 wells of the microplate strip.
8. Wait 5 min for the primary antibody to bind.
9. Wash the unbound primary antibody out of the wells by performing wash steps 5–6. This will wash the wells out two times.
10. Use a fresh pipet to transfer 50 µl of secondary antibody (SA) from the orange tube into all 12 wells of the microplate strip.
11. Wait 5 min for the secondary antibody to bind to the primary antibody.
12. Wash the unbound secondary antibody out of the wells by performing wash step 5 one time. Then perform wash step 6 two
times. This will wash the wells out a total of three times.
P1 P1 P2 P2 P3 P3 P4 P4
Wash
A. C. D.
P1 P1 P2 P2 P3 P3 P4 P4
Secondary
antibody
P1 P1 P2 P2 P3 P3 P4 P4
Primary
antibody
P1 P1 P2 P2 P3 P3 P4 P4
Wash
A. C. D.
P1 P1 P2 P2 P3 P3 P4 P4
Wash
A. C. D.
APPENDIX A
QUICK GUIDE
47
explorer.bio-rad.com
The secondary antibody is attached to an enzyme (HRP) that chemically changes TMB (the enzyme substrate), turning it
from a colorless solution to a blue solution. Predict which wells of your experiment should turn blue and which should remain
colorless and which wells you are not sure about and state why.
13. Use a fresh pipet to transfer 50 µl of enzyme substrate (SUB) from the brown tube into all 12 wells of the microplate strip.
14. Wait 5 min. Observe and record your results.
Results Section
15. Label the fi gure below with the same labels you wrote on the wells in step 1. In each of the wells, put a “+” if the well turned
blue and a “–” if there is no color change.
P1 P1 P2 P2 P3 P3 P4 P4
Enzyme
substrate
APPENDIX A
QUICK GUIDE
48
Immunological Concepts
Immunity
Immunology is the study of the immune system. The body protects itself from infection using physical and chemical barriers,
antibodies that circulate in the blood, and immune cells that attack foreign substances and invading microorganisms. Some
types of immune cells adapt to “remember” (recognize) specifi c invaders, in case of future attacks. A person is born with certain
immunological defenses against pathogens. This is called innate immunity and includes circulating macrophages and natural killer
cells. These defenses do not change with exposure to pathogens and do not have much specifi city for particular pathogens.
Passive immunity is the acquisition of antibodies from an external source, for example, antibodies passed from mother to infant, or
certain post-exposure vaccines such as that for rabies. Passive immunity lasts only a few weeks, and also does not change with
multiple exposures.
Acquired or adaptive immunity is a specifi c response to specifi c foreign substances. Although individuals are born with the
ability to respond to these invaders, the system must be activated by an initial contact with the invader. The initial contact, or
immunization, begins a cascade of events that allows the body to mount a specifi c response on subsequent exposure to the
invader, hence the term acquired immunity, as initial contact is necessary to acquire the immunity. Acquired immunity is split
into two categories: humoral immunity involves production of antibodies that circulate in the bloodstream and lymph and bind
specifi cally to foreign antigens, and cell-mediated immunity involves the production of T lymphocytes (T cells) that bind and
destroy infected cells.
Acquired immunity is the basis for the series of vaccinations that we undergo as we grow up. In the 1790s, long before we had
any understanding of the immune system, it was discovered that inoculation with pus from a cowpox lesion prevented infection
with smallpox, a disease related to cowpox. The US Centers for Disease Control (CDC) currently recommends childhood
vaccination against 12 diseases: measles, mumps, rubella (German measles), diphtheria, tetanus (lockjaw), pertussis (whooping
cough), polio, Haemophilus infl uenzae type b (Hib disease), hepatitis B, varicella (chicken pox), hepatitis A, and pneumococcal
disease. For travelers abroad, additional vaccinations are recommended (or required, in the case of the US military). The
recommendations are based on the traveler’s destination. For example, the CDC recommends that travelers to tropical South
America be vaccinated against hepatitis A, hepatitis B, rabies (if the traveler will be exposed to animals), typhoid, and yellow fever,
plus booster doses for tetanus, diphtheria, and measles.
Components of the Acquired Immune Response
In an immune response, an invasion by something foreign to the body (an antigen) generates antibody production by B lymphocytes
(B cells). Each B lymphocyte generates a unique antibody that recognizes a single shape on an antigen called an epitope and thus
helps the immune cells (including B cells, T cells, and macrophages) to recognize and attack foreign invaders. Everyone (except
those who are immune-compromised) has circulating antibodies and lymphocytes that collectively recognize a huge number of
antigenic substances.
Appendix B
Antigen
-
S
-
S
-
-
S
-
S
-
-
S
-
S
-
-
S
-
S
-
Disulfide
Bonds
H Chain
L Chain
Antigen
A B
Antibody
Structure of antibodies
A, Structure of IgG bound to the HIV capsid protein p24 as determined by X-ray crystallography (Harris et al. 1998, Momany et al. 1996). These structures can be
downloaded and manipulated from the Protein Data Bank (rcsb.org/pdb/home/home.do, Berman et al. 2000) using the PDB identifi cation codes 1IGY and 1AFV.
B, A commonly used representation of an antibody bound to an antigen.
APPENDIX B
IMMUNOLOGICAL CONCEPTS

49
explorer.bio-rad.com
Antigens can be microorganisms (e.g., viruses and bacteria), microbial products (e.g., toxins produced by some bacteria, or
protein components of the microbes), foreign proteins, DNA and RNA molecules, drugs, and other chemicals. Antibodies are
proteins also called immunoglobulins (Ig), that are produced by B cells and can remain attached to B cells or become freely
circulating. There are fi ve classes of immunoglobulins: IgG, IgM, IgA, IgE, and IgD. IgG is the most abundant in the internal body
fl uids, comprising about 15% of total serum protein in adults, and each IgG molecule can bind two antigen molecules. IgM is also
in serum and is responsible for the primary immune response. IgA is found in external secretions such as tears, saliva, milk, and
mucosal secretions of the respiratory, genital, and intestinal tracts and is a fi rst line of defense against invading microorganisms.
IgA is also the only antibody passed from mother to infant. IgD may be involved in regulating the immune response, and IgE is a
primary component in allergic reactions.
Epitopes are the specifi c parts of antigens that are recognized by antibodies. Each antibody recognizes a single epitope, thus
multiple antibodies may recognize and bind to different epitopes on a single antigen. For example, an HIV virus particle (virion)
has many potential epitopes on its surface that may be recognized by many different antibodies. One particular antibody may
recognize the amino terminus of p24, an HIV capsid protein, while another may recognize the carboxy terminus of p24. Immune
cells are the soldiers of the acquired immune response. Macrophages serve two primary functions: 1) removing foreign cells and
molecules from the blood, and 2) processing antigens and presenting them on their cell surfaces. Macrophages present antigenic
epitopes on their cell surfaces to be recognized by T cells. The T cells draw more immune cells to the site of infection, causing
infl ammation. Both B cells and T cells are lymphocytes (white blood cells), and each recognizes a single specifi c epitope. T cells
mature in the thymus, and B cells mature in the bone marrow. B cells produce antibodies; antibodies make up to 15% of your
total blood serum protein, so there is usually an antibody ready to deal with any antigen. The huge number and diversity of
different antibodies are possible because B cells have the ability to rearrange their DNA to make different antibody genes. Like
macrophages, B cells present antigenic epitopes on their surface to attract T cells. T cells have two main functions: they stimulate
the proliferation of B cells that have bound to an antigen, and they kill whole cells that are infected by a virus to prevent the virus
infecting other cells.
Why We Need an Immune System
Even bacteria have innate and adaptive immune responses. As part of the innate immune response, bacteria make restriction
enzymes that destroy foreign DNA from bacterial viruses (bacteriophages), and they protect their own DNA by labeling it as “self”
through methylation. As part of an adaptive immune response, bacteria incorporate short stretches of foreign DNA into their own
bacterial chromosome. These loci are called CRISPR or clustered regularly interspaced short palindromic repeats. The next time
a bacterium encounters foreign DNA with these sequences, bacterial enzymes degrade it, protecting the host cell from potential
bacteriophage infection, conjugation, or natural transformation events. Our immune system is at work every day, protecting us
from thousands of potential threats, but it is so effi cient that we usually don’t notice it. Disease can result from infection, genetic
defect, or environmental toxins. Infection is an invasion by and multiplication of pathogenic (disease-causing) microorganisms.
The infection can be 1) transmitted from person to person, like a cold or the fl u, 2) transmitted from animals to people (called
zoonosis), like rabies or psittacosis, or 3) contracted from the environment, like parasites contracted from water or soil. The CDC
and World Health Organization (WHO) state that infectious diseases are the leading cause of death worldwide. Organisms that can
cause disease are called pathogens and include bacteria, viruses, fungi, infectious proteins called prions, and parasites. Infectious
diseases spread in a variety of ways:
Pathogen Transmission Example
Exchange of body fl uids HIV, SARS, Epstein-Barr virus (EBV), sexually transmitted diseases
Food Foodborne agents like E. coli O157:H7, which causes diarrheal disease; prions, which cause
Creutzfeldt-Jakob disease (mad cow disease in cattle); or nematodes, which cause trichinosis
Water Waterborne agents like the bacteria that cause cholera or the protozoa that cause giardiasis
Inhalation Microorganisms like the viruses that cause the fl u or the bacteria that cause tuberculosis
Absorption through the skin Nematodes like hookworms
Vector transfer (vectors are Malaria, West Nile virus, dengue fever, and yellow fever (mosquito vector); Lyme disease
organisms such as ticks or and Rocky Mountain spotted fever (both tick vectors); Plague (fl ea vector); Some diseases,
mosquitoes that carry pathogens such as Ebola hemorrhagic fever, are presumed to have vectors, but the vectors have not
from one host to another) yet been identifi ed
APPENDIX B
IMMUNOLOGICAL CONCEPTS

50
Problems with the Immune System
We depend on our immune system to protect us from disease, but when the immune system fails to function correctly, it
can cause severe health problems. These problems fall in to three basic categories: hypersensitivity, immunodefi ciency, and
autoimmune diseases. Hypersensitive reactions occur when the immune system overreacts to an antigen. The immune system
functions are normal in a hypersensitive reaction, just exaggerated in scope, and this can result in illness or even death. There
are four types of hypersensitive reactions: 1) anaphylactic reactions or immediate hypersensitivity, generally called allergies, such
as food, dust mite, and pollen allergies (the antigen that causes the reaction is called an allergen); 2) cytotoxic reactions, such as
transfusion reactions and Rh incompatibility reactions; 3) immune complex reactions, such as farmer’s lung, a disease caused by
inhaling mold spores; and 4) delayed-type hypersensitivity, such as contact sensitivity (e.g., poison ivy dermatitis and contact
dermatitis after exposure to chemicals or environmental agents ranging from metallic nickel to cosmetics).
Immunodefi ciency means that an individual is unable to mount an effective immune response, resulting in increased vulnerability to
opportunistic infections. There are two types of immunodefi ciency: 1) Primary immunodefi ciency has a genetic basis. Severe
combined immunodefi ciency (SCID, “bubble boy” disease) is an example of primary immunodefi ciency. Treatments for primary
immunodefi ciency may include gene therapy. 2) Secondary immunodefi ciency has an external cause and is more common than
primary immunodefi ciency. Secondary immunodefi ciency may be caused by an infection, as in the case of HIV/AIDS, by drug
treatments, such as immunosuppressive drugs given after organ transplant, or by other health factors, such as poor nutrition,
stress, or aging.
Autoimmune disease results from the immune system making a mistake and mounting an immune response against one’s own
body. Some examples of autoimmune disease include systemic lupus erythematosus (lupus, SLE), rheumatoid arthritis, multiple
sclerosis (MS), insulin-dependent diabetes mellitus (IDDM), and celiac disease. Infectious diseases are diagnosed by observing
symptoms and performing laboratory tests. Diagnostic tests may look for the microorganism itself or some part of it (e.g., bacterial
or viral antigens), microbial products (e.g., bacterial toxins), or reactions of the body to the disease agent. The latter may include
testing for signs of an immune response to the disease agent (e.g., antibodies) or for indications of effects of the disease agent
on the body (e.g., abnormal enzyme activity or protein levels). In the last decade, tests to detect microbial RNA and DNA have
become common.
Laboratory tests cover a wide variety of methods, some of which have been in use for decades and others, like the tests for RNA
and DNA from disease agents, which are very new. Depending on the test and putative diagnosis, laboratory tests may look for
signs of disease in most body fl uids, including blood, urine, stool samples, cerebrospinal fl uid, and saliva. In the US, the Food and
Drug Administration regulates laboratory tests. The fi rst tests for detecting and identifying microorganisms from clinical samples
used antisera directed against specifi c microbes. The antibodies were labeled with a fl uorescent tag, and the microorganisms
could be detected with microscopy when the antibodies bound to them. Other early diagnostic tests include: 1) culture methods,
in which microorganisms from clinical samples are grown on different culture media and their growth and appearance observed
(frequently takes weeks to get results); 2) identifi cation of microbe-specifi c antibodies in serum by immunoassays such as ELISA;
and 3) agar diffusion assays, in which antisera and antigens are placed in holes in agar plates. Both diffuse into the agar, and
where antibodies encounter antigens for which they are specifi c, they bind. Upon antibody-antigen binding, a visible precipitation
band forms. Many of these tests are still in use.
Boosting the Immune System with Vaccination
Doctors use the immune response to give us resistance to infectious diseases before we are exposed to them. Through
vaccination, we are exposed to non-harmful forms of the pathogen that invoke an immune response. We also frequently need
booster shots to invoke the secondary response to maintain the antibody levels in our blood. Vaccines used in immunization may
be of several types:
1. Live attenuated vaccines are weakened (attenuated) microbes, that are nonpathogenic. Using current technology, deletion or
inactivation of microbial genes weakens the pathogens so they can be used in vaccines; previously, less pathogenic strains
were selected from natural populations. Examples of live vaccines include those against polio (Sabin type), measles, mumps,
and smallpox.
2. Killed or inactivated vaccines are made of microbes killed by heat or chemicals. Killed vaccines are much safer than live
vaccines, particularly for individuals with compromised immune systems, but they do not usually provoke as strong an
immune response as do live vaccines. Examples of killed vaccines include those against rabies, cholera, polio (Salk type),
and infl uenza.
APPENDIX B
IMMUNOLOGICAL CONCEPTS
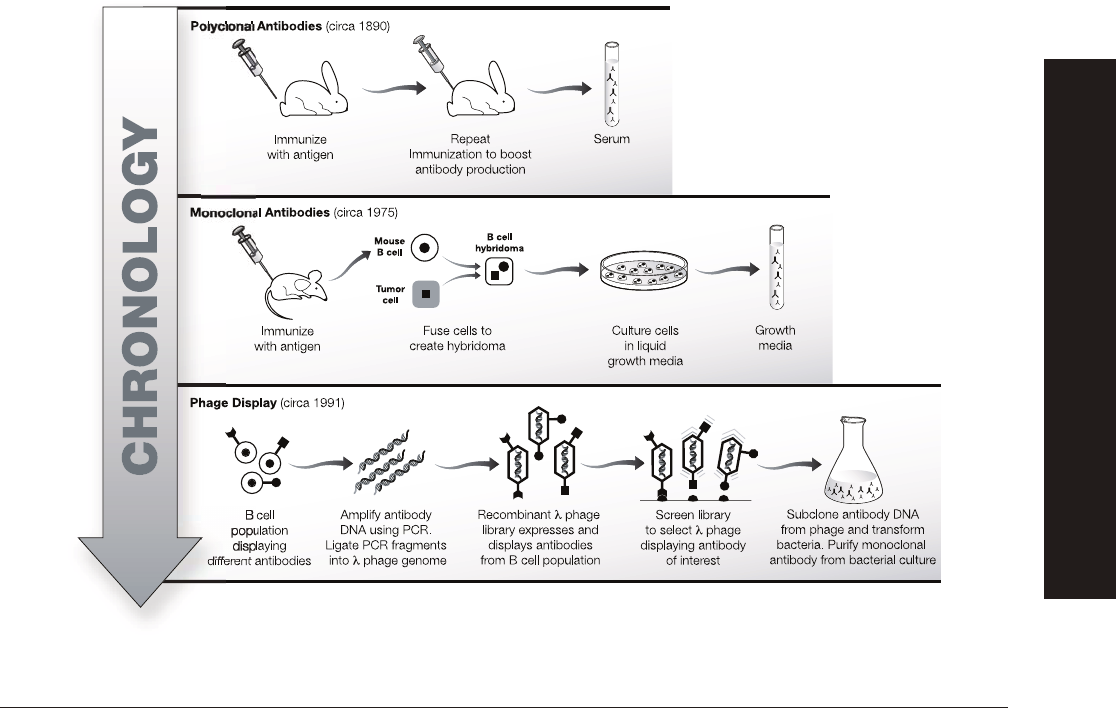
51
explorer.bio-rad.com
3. Subunit vaccines are made from pieces of microbes. They consist of one or more antigens from either the disease agent or a
microbial product, and they may be derived from the organisms or engineered using molecular biology. Examples of subunit
vaccines include those against hepatitis B, anthrax, and tetanus.
4. DNA vaccines are a recent approach to vaccine development. DNA that codes for microbial antigens is cloned into a vector,
and the naked DNA is injected into the patient. The DNA is taken up by cells, transcribed, and translated, and the resulting
antigenic protein elicits an immune response. No DNA vaccines are yet available, but some are in clinical trials.
5. Antibody vaccines are another innovation in vaccine development. The ability to construct human monoclonal antibodies
using recombinant DNA technology means that antibodies prepared against specifi c antigens may be used safely in humans.
For example, in 2012 Raxibacumab - a human monoclonal antibody against an antigen involved in anthrax infection - became
available for use in humans.
6. Post-exposure vaccines (immunotherapy) are used to treat a disease. Some immunotherapies have been used for years (e.g.,
administering immune serum globulin after exposure to hepatitis and administering equine antivenin for snakebite), but there
are not many other current vaccine-based immunotherapies. Probably the best known is post-exposure rabies vaccination,
consisting of 5 doses of rabies vaccine over 30 days. If the vaccine regimen is begun promptly after exposure, it is
100% effective in preventing disease. Smallpox vaccination also provides protection even when administered 2–3 days
postexposure. If the smallpox vaccine is administered as late as 5 days after exposure, it may prevent smallpox from being
fatal, although it will not prevent the disease.
Tapping Nature’s Toolkit: Manufacturing Antibodies
Antibodies used in research can be manufactured in the laboratory, both in vivo and in vitro. In vivo techniques have been in use
for over 100 years. There are two types of traditionally produced antibodies: polyclonal antibodies and, in the last 30 years,
monoclonal antibodies. Currently, antibody production is being revolutionized by recombinant DNA technology and, while
most antibodies are still produced by traditional methods using animals or animal cells, techniques for making antibodies using
recombinant DNA technology are becoming more common.
Timeline of antibody production technology.
APPENDIX B
IMMUNOLOGICAL CONCEPTS

52
Polyclonal Antibodies
Polyclonal antibodies are generated by immunizing an animal (usually a rabbit, goat, or sheep) and obtaining serum. For example,
purifi ed HIV gp120 protein can be injected into a goat, which will then generate antibodies directed against the many epitopes of
gp120. (Remember that the goat will produce many different antibodies to the multiple epitopes of an antigen.) Blood containing
the antibodies is drawn from the goat and the cells of the blood are removed, leaving the serum. The product is antiserum towards
gp120, and the antiserum can be used directly or the antibodies can be purifi ed from it. The antibodies are called polyclonal
because the antibodies are from many (poly) B cell clones (clonal) in the goat’s blood. Polyclonal antiserum has the advantage of
being simple and inexpensive to produce, but the disadvantage is that no two batches, even made in the same animal, will be
exactly the same.
Monoclonal Antibodies
For many antibody applications such as diagnostic tests, polyclonal antibodies are too variable. In these cases, one antibody type
from a single B cell clone is preferable. B cell clones producing single antibodies can be isolated from the spleens of immunized
mice, but these cells die after a few weeks in the laboratory, limiting production of the large amounts of antibody generally needed
for research and commercial applications. However, B cells can be made to live (and produce antibodies) indefi nitely if they are
fused with tumor-like immortal cells. The fusion generates hybrid cells (a hybridoma cell line), which can be cultured indefi nitely;
the monoclonal antibodies generated by the hybrid cells can be collected and purifi ed from the growth medium with almost no
batch-to-batch variability.
Genetically Engineering Antibodies
The ability of antibodies to act like magic bullets and home in on their targets makes them ideal candidates for medical therapies.
For example, an antibody that recognizes a tumor antigen can be attached to a chemotherapy drug or radioactive molecule
and be used to deliver the drug specifi cally to targeted tumor cells, sparing the patient many of the side effects of conventional
chemotherapy or radiation treatment. However, traditional antibodies made in animals are seen by the human immune system
as foreign and elicit an immune response that results in their destruction. Recombinant DNA technology can be used to produce
antibodies that look human to the human immune system and so can be used as therapeutic agents in people. (For example,
Herceptin is a “humanized” antibody used to treat breast cancer.) Using genetic engineering to manufacture antibodies also
obviates the sacrifi ce of laboratory animals. Two of the methods used to engineer antibodies are described below.
Hybridoma Immortalization
Recombinant DNA technology allows the antigen recognition site from a known mouse monoclonal antibody to be camoufl aged
within a human antibody by combining part of the mouse gene with the human antibody gene. Bacteria transformed with this
DNA are capable of producing humanized monoclonal antibodies indefi nitely, with the added bonus that culturing bacteria requires
much less time and expense then the culture of a mouse hybridoma cell line.
Phage Display
Novel antibodies to antigens are being generated using modern biotechnology. Libraries of billions of potentially useful antibodies
are being created by inserting shuffl ed antibody genes from billions of human B cells into the genomes of bacteriophage lambda
(bacteriophages, or phages, are viruses that infect bacteria; lambda phage is a specifi c species of phage), so that the lambda
phages display the binding sites from human antibodies on their surfaces. This phage library is screened to fi nd a phage that binds
to a specifi c antigen. The phage can then be used directly as an antibody would be used. Alternatively, the DNA from the selected
phage can be cloned into a human antibody gene and transformed into bacteria. Large amounts of the antibody can then be
produced for therapeutic use. Phage display is a robust methodology used in immunotherapy.
Labeling and Detecting Antibodies
Antibodies are used in diagnosis and research as labeling tools. As labels they have to be made visible, so antibodies are
covalently linked (or conjugated) to chemical labels that emit detectable signals. Detection systems can be low-tech or high-tech,
and the detection system determines the type of label used. For example, a fl uorescently labeled antibody allows you to localize
an antigen in a cell using a high-tech fl uorescent microscope. Antibodies are also linked to enzymes that oxidize a chromogenic
(color-producing) substrate, producing visible color only where
the enzyme-linked antibody has bound. Enzyme-linked antibodies
are commonly used in western blots, microscopy, and ELISA. Antibody targets or antigens can be detected directly by labeling the
antibody specifi c for
the antigen and looking for signal.
APPENDIX B
IMMUNOLOGICAL CONCEPTS
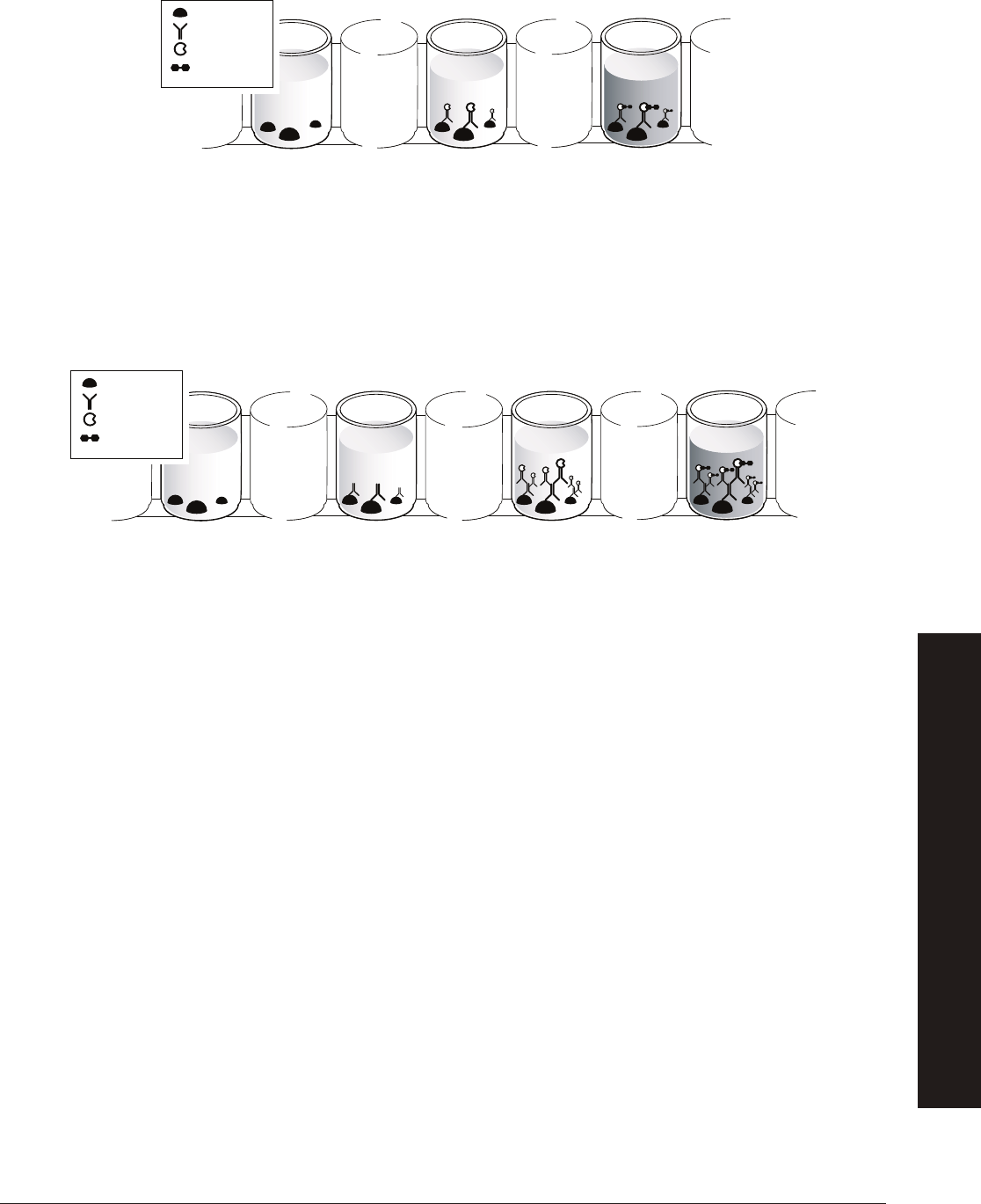
53
explorer.bio-rad.com
However, labeling every type of antibody scientists might wish to use is time-consuming and costly. Thus, a more common method
to visualize antigens is called indirect detection. This technique relies on the use of polyclonal secondary antibodies. Secondary
antibodies recognize primary antibodies. The primary antibody binds specifi cally to the antigen, and the secondary antibody binds
specifi cally to the primary antibody. The indirect method means that only one type of enzyme-linked secondary antibody is needed
to visualize all antibodies produced in one type of animal (e.g., in rabbits), reducing time and cost. Indirect detection adds a bonus,
since the primary antibody is effectively an antigen to the secondary antibody. The primary antibody has many different epitopes and
so is bound by multiple secondary antibodies. Thus, more labels accumulate around the antigen, amplifying the signal.
Secondary antibodies are produced by injecting the antibodies of one animal into a different species of animal. For example, if the
primary antibody is a mouse monoclonal antibody, secondary antibodies are generated by immunizing a goat with any mouse
antibody. Goat polyclonal anti-mouse IgG is purifi ed from the goat serum and linked to an enzyme for detection. Secondary
antibodies are commercially available, either unlabeled or with a wide variety of fl uorescent or enzymatic labels for many applications.
Putting Antibodies to Use
Antibodies have been used for decades as research tools, but in recent years the expansion of technology to produce antibodies
has yielded a myriad of new applications that take advantage of the specifi city of antibody binding. The basis of all immunoassays
is the specifi c binding of an antibody to its antigen, and there are many ways that binding can be utilized. Here are some of those
uses: Immunostaining localizes antigens in organelles, cells, tissues, or whole organisms, and can also be used to distinguish
one cell type from another. For example, pathologists can identify cancer cells using immunostaining. Cancer cells frequently
look identical to normal cells under the microscope, but when they are immunostained, variations in the amount and kinds of cell
surface proteins (antigens) are revealed. Studying this information helps diagnose cancer, and it can help in our understanding of
how cancer cells cause harm.
Immunostaining tissues or organisms can tell us in what cell types a protein is normally found, which can help us understand
the protein’s function. For instance, immunostaining of plant seedlings at different stages of maturation allows us to follow how a
protein’s abundance and localization change as the plant grows. Antibodies for immunostaining are labeled with either fl uorescent
molecules or enzymes that produce colored signals upon addition of a substrate.
A special application of immunostaining is fl uorescence-activated cell sorting (FACS),
in which a population of cells is stained
with a fl uorescently labeled antibody and then physically separated into labeled and unlabeled cells. The cell sorter uses lasers to
detect the fl uorescent labels and an electrostatic charge to sort the cells in solution. Cell sorters can separate as many as 100,000
cells per second!
Antigen
Antibody
HRP
Enzyme
Substrate (TMB)
Antigen
Antibody
HRP
Enzyme
Substrate (TMB)
Direct detection of antibodies.
Indirect detection of antibodies.
APPENDIX B
IMMUNOLOGICAL CONCEPTS

54
Immunoblotting or western blotting tells us about a protein’s size and relative abundance in a given sample. In western blotting, an
antibody picks out a specifi c protein from a complex sample (usually lysed cells or tissue) that has been separated by size using
SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins separated in SDS-PAGE gels are transferred (electroblotted) from
the gel to the surface of a nylon or nitrocellulose membrane using an electrical current. The membrane is probed with a primary
antibody that is specifi c for the protein of interest, and then an enzyme-linked secondary antibody is used to visualize the protein.
The enzyme oxidizes a colorimetric substrate, producing a colored band on the membrane. Alternatively, the oxidized substrate
may emit light (chemiluminescent substrate) that is detected as a band on photographic fi lm. The size of the protein is determined
by comparing the position of the band to the position of known protein standards that are run alongside it on the SDS-PAGE
gel. The abundance of the protein is determined by comparing band intensity to known amounts of protein standards run on the
same gel.
A modifi cation of immunoblotting is called dot blotting, in which a sample is spotted onto a membrane directly rather than
being blotted from a gel. Dot blotting is used for rapid screening of a large number of samples. This technique provides a rapid
determination of whether a particular protein or antigen is present, as many samples may be spotted on a membrane and
processed simultaneously, but dot blotting provides no information about the size of the protein.
APPENDIX B
IMMUNOLOGICAL CONCEPTS

55
explorer.bio-rad.com
Appendix C
Glossary
3,3',5,5'-tetramethylbenzidine (TMB): A soluble colorimetric substrate, oxidized to a blue color by horseradish peroxidase and
frequently used in ELISA assays.
Acquired immunity: A specifi c response to specifi c foreign substances that adapts with multiple exposures. Also called adaptive
immunity.
Anterior Pituitary Gland: A major organ of the endocrine system found in that brain that regulates several physiological
processes including stress, growth, reproduction and lactation.
Antibody: Immunoglobulin protein formed in response to a challenge of the immune system by a foreign agent. Antibodies bind to
specifi c antigens.
Antigen: Any agent that provokes an immune response and is bound specifi cally by either antibodies or T cells.
Antiserum: Blood serum containing antibodies raised against a specifi c antigen.
Assay: A test for qualitatively assessing or quantitatively measuring the presence or amount or functional activity of a target entity.
Autoimmune disease: Disease that results from the immune system making a mistake and mounting an immune response
against one’s own body. Examples are systemic lupus erythematosus (lupus, SLE), rheumatoid arthritis, and multiple sclerosis
(MS).
Bacteriophage: A virus that infects bacteria; also called a phage. Can be used to introduce foreign DNA into a bacterial genome.
Chromogenic: Color-producing. Substrates that produce a colored product when acted upon by an enzyme are termed
chromogenic substrates; for example, 3,3’,5,5’-tetramethylbenzidine (TMB) produces a blue product when oxidized by
horseradish peroxidase.
Clone: In the context of molecular biological techniques, “to clone” means to obtain a fragment of DNA from a genome and
ligate it into another piece of DNA, such that the ligated DNA now has an identical copy of that gene fragment. In the context of
cell biology, “a clone” is a cell or group of cells that are all derived through cell division from the same parent cell and thus have
identical genetic information.
Conjugate: A substance formed by the covalent bonding of two types of molecules, such as horseradish peroxidase linked to
(“conjugated to”) an antibody.
Corpus Luteum: A hormone-secreting structure that develops in an ovary after an ovum (egg cell) has been discharged but
degenerates after a few days unless pregnancy has begun.
Clustered regularly interspaced short palindromic repeats (CRISPR): Segments of prokaryotic DNA containing short
repetitions of base sequences. Each repetition is followed by short segments of “spacer DNA” from previous exposures to a
bacteriophage virus or plasmid. The CRISPR/Cas system is a prokaryotic immune system that confers resistance to foreign
genetic elements such as those present within plasmids and phages, and provides a form of acquired immunity. CRISPR
associated proteins (Cas) use the CRISPR spacers to recognize and cut these exogenous genetic elements in a manner
analogous to RNA interference in eukaryotic organisms.
Enzyme: A protein with catalytic activity. The molecule that an enzyme acts on is called its substrate. Enzymes are classifi ed (and
frequently named) on the basis of the reactions that they catalyze. For example, a peroxidase oxidizes its substrate.
Epitope: A specifi c site on an antigen that is recognized by an antibody. Also called antigenic determinant.
Estrogen: Any of a group of steroid hormones that promote the development and maintenance of female characteristics of the
body. The main sources of estrogen in the body are the ovaries and the placenta.
Follicle-Stimulating Hormone (FSH): A glycoprotein polypeptide hormone that is synthesized and secreted by the gonadotropic
cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the
body.
APPENDIX C: GLOSSARY
APPENDIX C
GLOSSARY

56
Gonadotropic Cells: Endocrine cells in the anterior pituitary gland that produce the gonadotropins, such as the follicle-stimulating
hormone (FSH) and luteinizing hormone (LH).
Gonadotropin-Releasing Hormone (GnRH): A hormone released by the hypothalamus in the brain. GnRH acts on receptors
in the anterior pituitary gland. GnRH signals the pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing
hormone (LH).
Horseradish peroxidase (HRP): An enzyme frequently used to label secondary antibodies. HRP oxidizes substrates (e.g., TMB)
for colorimetric detection.
Immune cell: Any cell of the immune system, including lymphocytes (B and T cells) and macrophages.
Hybridoma: A hybrid cell used as the basis for the production of antibodies in large amounts for diagnostic or therapeutic use.
Hypothalamus: A region of the forebrain below the thalamus that coordinates both the autonomic nervous system and the
activity of the pituitary glands, controlling body temperature, thirst, hunger, and other homeostatic systems, and involved in sleep
and emotional activity.
Immunodefi ciency: Weakening or defects of the immune response such that an individual is unable to mount an effective
immune response. May have a genetic basis, result from a disease or other health factor, or be caused by immunosuppressive
drugs.
Immunogen: Any agent that provokes an immune response. Immunogens that provoke a response from the immune system are
called antigens.
Immunoglobulin (Ig): General term for all types of antibodies.
Immunology: The study of the immune system, the body system that protects the body from foreign substances, cells, and
tissues by producing an immune response.
Ligate: To connect pieces of DNA together, for example, inserting a fragment of an antibody gene into a phage genome.
Luteinizing Hormone (LH): A hormone secreted by the anterior pituitary gland that stimulates ovulation in females and the
synthesis of androgen in males.
Lymphocyte: Type of white blood cell. Component of the immune system, includes T cells (thymus-derived) and B cells (bone
marrow-derived).
Macrophage: A type of white blood cell that binds and engulfs foreign materials and antigens in a process called phagocytosis.
Macrophages serve two primary functions: 1) removing foreign cells and molecules from the blood; and 2) processing antigens
and presenting them on their cell surfaces.
Menstruation: The process in a female mammal of discharging blood and other materials from the lining of the uterus at regular
intervals, except during pregnancy.
Microplate: Molded plastic plate consisting of multiple small wells, usually in a 96-well format.
Monoclonal Antibody: An antibody produced by a single clone of cells or cell line and consisting of identical antibody molecules.
Ovulation: Discharge of an ovum (egg cell) or ovules (egg cells) from the ovary.
Pathogens: An organism that can cause disease. Pathogens include bacteria, viruses, fungi, infectious proteins called prions, and
parasites.
Phage: Short for bacteriophage, a virus that parasitizes a bacterium by infecting it and reproducing inside it.
Polyclonal Antibody: Antibodies that are secreted by different B cell lineages within the body (whereas monoclonal antibodies
come from a single cell lineage). They are a collection of immunoglobulin molecules that react against a specifi c antigen, each
identifying a different epitope.
Primary antibody: In an immunoassay, the antibody that binds a specifi c antigen, conferring specifi city to the assay.
Progesterone: A steroid hormone released by the corpus luteum that stimulates the uterus to prepare for pregnancy.
APPENDIX C
GLOSSARY

57
explorer.bio-rad.com
Secondary antibody: In an immunoassay, the antibody that recognizes the primary antibody, which is from a different species.
Secondary antibodies are frequently labeled for easy detection.
Serum (plural sera): The clear fl uid obtained when the solid components (e.g., red and white blood cells) are removed from
whole blood.
Substrate: The target molecule for an enzyme. TMB: see 3,3’,5,5’-tetramethylbenzidine.
Vector: An organism that carries pathogens from one host to another. Vectors are frequently arthropods; e.g., ticks or
mosquitoes.
Zoonosis (plural zoonoses): An infection transmitted to humans from an animal host; e.g., SARS and rabies.
APPENDIX C
GLOSSARY

58
Quantitative ELISA Laboratory Activity
Teacher Note: You will not have enough reagents to complete the investigations in this kit and perform a quantitative ELISA with
your students. Instead we offer a sample data set from a quantitative ELISA for your students to analyze. If you would like your
students to perform a hands-on quantitative ELISA, go to
bio-rad.com/PandaAPResources
to purchase additional reagents as
needed. You can also go to
bio-rad.com/PandaAPResources
to download the ELISA Immuno Explorer Kit Instruction Manual.
Appendix D of the Instruction Manual describes the teacher preparation and student protocol to perform a quantitative ELISA.
Using a microplate reader such as the iMark
™
Microplate Absorbance Reader with a 655 nm fi lter (catalog #1681130EDU),
students can read the absorbance values of their wells, generate a standard curve, and calculate the concentration of their
samples. If no microplate reader is available, the students can visually match the intensity of their samples with the samples in their
dilution series and estimate the concentration of antigen in their samples.
While ELISA gives a defi nitive qualitative (yes/no) answer, a major strength lies in that it can also give quantitative (how much?)
information. ELISA data can be interpreted in comparison to a standard curve (a serial dilution of a known, purifi ed antigen) in
order to precisely calculate the concentrations of antigen in various samples. In other words, to determine how much antigen or
antibody of interest is in a sample, the results must be compared to a series of standards that contain a known quantity of antigen
or antibody of interest. This lesson extension provides a quantitative ELISA data set for your students to analyze.
Using Serial Dilution to Generate the Standard
In order to determine how much antigen or antibody of interest a sample contains, standards containing a known amount of
antigen or antibody of interest must be generated for comparison. Serial dilution, the stepwise dilution of a substance, is often
used to generated a series of standards for comparison. In this case, a known concentration of antigen or antibody of interest
(for example, 1000 ng/ml antigen or antibody of interest) is diluted across a 12-well strip and an ELISA is performed. The
colorimetric results demonstrate a more intense blue color in the 1000 ng/ml well (well 1) and no observable color change in
the 0 ng/ml well (well 12).
Quantitative results can be estimated visually and scored symbolically, for example, (++) for strong signal, (+) for weak signal, (+/–)
for an ambiguous signal, and (–) for no detectable signal. For accurate and precise determination of concentrations, a microplate
reader is required. Microplate readers quantitate the absorbance of light by the colored substrate in each well of a microplate. They
use the negative control wells to set a baseline and then read the absorbance of each well at a specifi ed wavelength. For example,
the peak absorbance for TMB is at 655 nm.
Microplate readers measure the amount of light at a specifi c wavelength (in this case, 655 nm) that is absorbed by the liquid in
the wells of the microplate. The absorption of light by the liquid is directly related to the intensity of the colored product in the
wells, which in turn is determined by the amount of enzyme activity in the wells. The amount of enzyme activity is governed by the
amount of antigen that originally bound to the wells.
Quantitative ELISA controls include a dilution series of known concentrations that is used to create a standard curve. In this case,
a standard curve is created by plotting the known concentrations of each well on the y-axis and the corresponding absorbance
values from the microplate reader on the x-axis (See example on next page). (Note: The unit of measurement for absorbance is
absorbance units, or AU.) Since the resulting curve will be logarithmic, you will need to linearize it by plotting the data on semilog
graph paper. The concentrations of the test samples are determined by drawing vertical lines from their absorbance values on the
x-axis to the standard curve and then reading horizontally from the points where the vertical lines intersect with the standard curve
to the concentration values on the y-axis.
Appendix D
1 2 3 4 5 6 7 8 9 10 11 12
1000 500 250 125 63 31 16 8 4 2 1 0
Concentrations (ng/ml)
APPENDIX D
QUANTITATIVE ELISA LAB ACTIVITY
59
explorer.bio-rad.com
0 0.2 0.4 0.6 0.8 1.0 1.2
Absorbance at 655 nm, AU
1,000
100
10
1
0.1
Concentration, ng/ml
Test Sample A
Test Sample B
An example of a standard curve from a dilution series from 1,000 ng/ml to 1 ng/ml, read at 655 nm. Test sample A had an absorbance of 0.456 AU, and test
sample B had an absorbance of 0.208 AU. Thus, their concentrations were 125 ng/ml and 35 ng/ml, respectively.
APPENDIX D
QUANTITATIVE ELISA LAB ACTIVITY
60
Quantitative Results for Students’ Analysis
Purpose:
Researchers working with giant panda conservationists were interested in determining which of two female pandas were nearing
their ovulation point.
Research Question: How much ovulation indicating hormone is present in each of four giant panda urine samples?
Methods:
Urine samples were collected from each panda and the samples were frozen and shipped to the lab for analysis. Once at the lab,
the samples were thawed and an antigen detection ELISA protocol was followed in order to determine the amount of ovulation
indicating hormone in each panda urine sample. The researchers knew that a minimum of 120 ng of ovulation indicating hormone
per ml of urine would be needed to indicate an ovulation event in the next 1–2 days.
The r
esear
chers added purifi ed ovulation indicating hormone to three positive control wells in a microplate and PBS to three
negative control wells. The researchers then added urine samples from each of the pandas to three wells each. After washing all
of the wells, the researchers added purifi ed primary antibody that would bind with the ovulation indicating hormone in the positive
control wells and in the sample wells if any were present. After washing, the researchers added enzyme linked secondary antibody
that would bind any remaining primary antibody. After a fi nal wash, the researchers added substrate to all of the wells that would
produce a color change indicating the presence of ovulation indicating hormone. The researchers used a microplate reader set at
655 nm to determine the absorbance (Abs) of each of the wells.
Results
Standard
Experiment
Analysis of Results
On the next page, generate a standard curve using the Standard results from the fi rst table above. Use the example on the
previous page as a guide. Then using the results from the Experiment (second table above) determine the concentration of
ovulation indicating hormone in ng/ml for the positive control, negative control, and the two panda urine samples by drawing
vertical lines from their absorbance values on the x-axis to the standard curve and then reading horizontally from the points where
the vertical lines intersect with the standard curve to the concentration values on the y-axis.
Once you have graphed the data, answer the following questions:
How much ovulation indicating hormone is present in each of the two giant panda urine samples?
Pandas are likely to ovulate when the concentration of ovulation indicating hormone in their urine reaches 120 ng/ml. Which of the
two pandas is about to ovulate?
Please refer to the printed copy of the Giant Panda Problem Kit for AP Biology Answer Guide included in the kit for the answers.
Ovulation indicating
hormone, ng/ml 1,000 500 250 125 63 31 16 8 4 2 1 0
Well 1 2 3 4 5 6 7 8 9 10 11 12
Abs 0.760 0.752 0.725 0.569 0.416 0.290 0.180 0.168 0.125 0.106 0.086 0.072
Positive Control Negative Control Panda 1 Panda 2
Well 1 2 3 4 5 6 7 8 9 10 11 12
Abs 0.727 0.768 0.779 0.066 0.083 0.081 0.560 0.561 0.563 0.174 0.199 0.182
APPENDIX D
QUANTITATIVE ELISA LAB ACTIVITY

61
explorer.bio-rad.com
APPENDIX D
QUANTITATIVE ELISA LAB ACTIVITY
0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
Absorbance
1,000
100
10
1
0.1
Concentration, ng/ml
Semilog graph paper

62
Appendix E
References
BBC News (2016). Giant Panda No Longer an Endangered Species. www.bbc.co.uk/newsround/37273632, accessed
October 12, 2016.
Benjamini E et al. (1996). Immunology: A Short Course, Wiley-Liss, New York.
Berman HM et al. (2000). The Protein Data Bank, Nucleic Acids Res 28, 235–242.
Breitling F and Dübel S (1999). Recombinant Antibodies, John Wiley & Sons, New York.
China Highlights (2016). Giant Pandas’ Habitat - 20 Isolated Patches in Western China.
www.chinahighlights.com/giant-panda/habitat.htm, accessed October 12, 2016.
Harris LJ et al. (1998). Crystallographic structure of an intact IgG monoclonal antibody, J Mol Biol 275, 861–872.
Harlow E and Lane D (1999). Using Antibodies: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor.
Kaufmann SHE et al. (2002). Immunology of Infectious Diseases, ASM Press, Washington, DC.
Kersey DC (1999). Reproductive and adrenal endocrinology of the giant panda (Ailuropoda melanoleuca). Unpublished
dissertation. ebot.gmu.edu/bitstream/handle/1920/3428/Kersey_David.pdf?sequence=1&isAllowed=y, accessed
October 12, 2016.
Kinoshita K et al. (2012). Spectral pattern of urinary water as a biomarker for estrus in the giant panda. Sci Rep 856, 1–9.
Lashley FR and Durham JD (eds) (2002). Emerging Infectious Diseases, Springer Publishing Company, New York.
Law B (1996). Immunoassay: A Practical Guide, Taylor & Francis, Bristol.
Momany C et al. (1996). Crystal structure of dimeric HIV-1 capsid protein, Nat Struct Biol 3, 763–770.
SciJourner (2012). New light on giant panda reproduction problems.
www.scijourner.org/2012/04/24/new-light-on-giant-panda-reproduction-problems/, accessed October 12, 2016.
Truant AL (2002). Manual of Commercial Methods in Clinical Microbiology, ASM Press, Washington, DC.
Willis EL et al. (2011). The acute phase protein ceruloplasmin as a non-invasive marker of pseudopregnancy, pregnancy, and
pregnancy loss in the giant panda. PLOS One. journals.plos.org/plosone/article?id=10.1371/journal.pone.0021159, accessed
October 12, 2016.
Zhang H et al. (2009). Delayed implantation in giant pandas: The fi rst comprehensive empirical evidence. Reproduction, 138,
979–986.
Useful Websites
www.who.int/en/
World Health Organization (WHO)
www.cdc.gov/
Centers for Disease Control and Prevention (CDC)
www.niaid.nih.gov/
National Institute of Allergy and Infectious Diseases (NIAID)
www.usamriid.army.mil/
Legal Notices
AP and Advanced Placement are trademarks of The College Board.
Tween is a trademark of ICI Americas Inc.
APPENDIX E
REFERENCES, USEFUL WEBSITES, LEGAL NOTICES
63
explorer.bio-rad.com
Appendix F
ELISA Paper Model
The optional paper model activity for Investigation #2 asks students to model their understanding of the ELISA. You can make
copies and cut the pieces out yourself ahead of class, or ask your students to cut the model pieces out at the start of class.
For a picture of the completed paper model activity please refer to the printed copy of the Giant Panda Problem Kit for AP Biology
Answer Guide included in the kit.
Primary Antibodies in Serum
Wash
Wash WashWash Wash WashWash
Wash WashWash WashWash
APPENDIX F
ELISA PAPER MODEL
64
HRP Enzyme Linked Secondary Antibody
HRP Enzyme Substrate (TMP)
Antigens
APPENDIX F
ELISA PAPER MODEL

65
explorer.bio-rad.com

66

67
explorer.bio-rad.com

TM
BIO-RAD
10000074713 Ver A (12005498)
0817 Sig 1216
Web site bio-rad.com USA 1 800 424 6723 Australia 61 2 9914 2800 Austria 43 1 877 89 01 177 Belgium 32 (0)3 710 53 00 Brazil 55 11 3065 7550
Canada 1 905 364 3435 China 86 21 6169 8500 Czech Republic 420 241 430 532 Denmark 45 44 52 10 00 Finland 358 09 804 22 00
France 33 01 47 95 69 65 Germany 4
9 89 31 884 0 Hong Kong 852 2789 3300 Hungary 36 1 459 6100 India 91 124 4029300
Israel 972 03 963 6050 Italy 39 02 216091 Japan 81 3 6361 7000 Korea 82 2 3473 4460 Mexico 52 555 488 7670 The Netherlands 31 (0)318 540 666
New Zealand 64 9 415 2280 Norway 47 23 38 41 30 Poland 48 22 331 99 99 Portugal 351 21 472 7700 Russia 7 495 721 14 04
Singapore 65 6415 3188 South Africa 27 (0) 861 246 723 Spain 34 91 590 5200 Sweden 46 08 555 12700 Switzerland 41 026 674 55 05
Taiwan 886 2 2578 7189 Thailand 66 2 651 8311 United Arab Emirates 971 4 8187300 United Kingdom 44 020 8328 2000
Bio-Rad
Laboratories, Inc.
Life Science
Group
(01)03610521157823
