
Chapter 8
Alternatives to
Animal Use in Testing
Queen:
I will try the forces
Of these compounds on such creatures as
We count not worth the hanging, but none human . . .
Cornelius:
Your Highness
Shall from this practice but make hard your heart.
Shakespeare,
Cymbeline
Act I, Scene VI
The experimental means to be used for safety evaluations is left open to suggestion. As
unorthodox as this might sound, leaving such means open for consideration is the best
solution.
Safety evaluations should not be based on standard, specified series of tests. They
are best approached by first raising all pertinent safety questions and then searching for
the experimental means to provide the best answers. Under such circumstances, even the
standard LD test might on occasion be the best experimental means to resolve outstanding
satfety questions.
Constantine Zervos
Food and Drug Administration
Safety Evacuation and Regulation of Chemicals 2,
D. Homburger (cd.) (Base]: Karger, 1985)
—.—
CONTENTS
Page‘
Continued, But Modified, Use of Animals in Testing . . . . . . . . . . . . . . . . . . . . . ..175
Avoiding Duplicative Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 176
Reducing Pain and Distress . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
177
Use of Living Systems in Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 177
In Vitro Systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
177
Nonanimal Organisms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .179
Use of Nonliving Systems in Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
180
Chemical Systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
180
Mathematical and Computer Models . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 180
Epidemiologic Data on Humans . . . . . . . . . . . . . . . . . . . . . . . . . . ..:......
.181
The LD
50
Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ...181
Using Fewer Animals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
.182
The Limit Test and Other Refinements . . . . . . . . . . . . . . . . . . . . . . . . . . . . ..182
In Vitro and Nonanimal Methods . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .182
Skin and Eye Irritation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ]83
In Vitro Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .183
Chick Embryo . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 183
Repeated-Dose Toxicity Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
184
Hepatotoxicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ......185
Neurotoxicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 185
Mutagenicity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
185
Microorganism Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....186
In Vitro Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 186
Tests Using Insects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 186
Carcinogenicity ...,.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ..187
The Ames Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .187
Use of the Ames Test in a Battery of tests . . . . . . . . . . . . . . . . . . . . . . . . . .188
Current Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ...188
Summary and Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
190
Chapter preferences.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
191
Table
Table No.
Page
8-1. The Response of Known Human Carcinogens to Rodent Carcinogenicity
and Bacterial Mutagenicity Assays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ..188
Figure
Figure No.
Page
8-1. Chronological Sequence of Chick Embryo Chorioallantoic
Membrane Assay.... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ..184
Chapter 8
Alternatives to Animal Use in Testing
Alternatives to using animals in testing serve the
same purposes that using whole animals does—
protecting and improving human health and com-
fort. The technologies on which alternatives are
based result primarily from biomedical and bio-
chemical research. Several of them are reviewed
in this chapter, though they are discussed in
greater detail in chapter 6. Some alternatives that
might eventually replace the tests covered in chap-
ter 7 are also described here.
Notable progress in the move to alternatives has
been achieved in certain areas (78). For example,
biochemical tests to diagnose pregnancy have re-
placed those using rabbits, and the Limulus ame-
bocyte lysate test, which relies on the coagulation
of a small amount of blood from a horseshoe crab,
has replaced rabbits in testing for the presence
of bacterial endotoxins that would cause fever
(25,117). Many companies have modified the widely
used LD
5O
test to use fewer animals (22) and have
otherwise refined the methods used to test for tox-
icity (100). Mammalian cell culture assays are used
extensively in industrial laboratories for safety test-
ing of medical devices (52,53) and pharmaceutical
CONTINUED, BUT MODIFIED,
It has been suggested that many more animals
are used for testing than are needed (90) and that
changes in experimental design or improved meth-
ods of data analysis could substantially reduce the
number of animals used. Each experiment has
unique requirements (see ch. 7), and the ways in
which the number of animals might be reduced
will vary accordingly.
Many of the methods discussed in chapter 6 for
the modified use of animals in research are also
applicable to testing, such as gathering more data
from each animal or improving the analysis of re-
sults by using random block design or covariance
analysis. In random block design, animals with a
particular characteristic, such as litter mates or
animals of a certain size, are randomly assigned
to different groups to balance whatever effect
substances (1,84) and as immune response assays
(97,98).
The development of alternatives to animals in
testing has accelerated in recent years with the
establishment of programs having development
and implementation of alternatives as their goal
(see ch. 12). However, the barriers to adoption of
these tests are more than the technical barrier of
developing and validating anew technology. Test-
ing is an integral part of many regulatory schemes
and product liability law, and validation ultimately
rests on acceptance by the scientific, regulatory,
and legal communities.
Public concern over animal
to be increasing in tandem
for product and drug safety
use in testing appears
with public concern
. Ironically, the pub-
lic’s-increasing concern for safety could lead to
more testing. Yet it also provides an incentive to
develop new techniques, particularly those that
promise to be cheaper and faster than current
whole-animal methods. A further irony is that de-
veloping alternatives, as well as validating them,
sometimes requires animal use.
USE OF ANIMALS IN TESTING
these variables might have. If the groups being dis-
tributed are sufficiently large, the results can also
be analyzed to determine the effect of the mask-
ing variable (47). Covariance can be used to ana-
lyze results when some of the experimental varia-
bles are uncontrolled but known, thus estimating
their effect on the results.
As in research, the number of animals needed
as controls can be reduced by using the same group
as a control for several simultaneous experiments.
A laboratory’s ability to do this will be limited by
its size and the amount of lead time available to
allow testing to be coordinated. Another difficulty
is that environmental conditions must be exactly
the same and the tests must start and finish at ex-
actly the same times. The reduction in animal use
that simultaneous experiments brings about is
175

176 ● Alternatives to Animal Use in Research, Testing, and Education
modest because the control group should be larger
if it is being used in several simultaneous experi-
ments (34),
The use of historical data for control groups is
constrained by the difficulty of exactly duplicat-
ing the conditions of a study. However, the size
of the groups and other controlled variables can
be better planned if historical data are used to dis-
cover the background incidence of specific tumors
or other diseases before testing begins. This use
of historic controls has been recognized by the
National Cancer Institute, the world Health Orga-
nization, the Canadian Government, and the now-
defunct Interagency Regulatory Liaison Group
(104). The Federation of American Societies for
Experimental Biology has developed a data book
containing such information based on the Labora-
tory Animal Data Bank (see ch. 10) (2).
Avoiding Duplicative Testing
Animal use in testing can and has been reduced
by industry and others through improved commu-
nication and cooperation in the planning and exe-
cution of testing, thereby avoiding unintentional
duplication. Trade groups such as the Chemical
Manufacturers Association, the Pharmaceutical
Manufacturers Association, and the Soap and De-
tergent Association play important roles in this co-
ordination.
The sharing of data after testing has occurred
is often done for pesticides (see chs. 10 and 11).
And in 1978, the Food and Drug Administration
implemented a policy of permitting approval of
new drug applications solely on the basis of pub-
lished scientific papers (113). The possibility of an
unintentional repetition of an experiment is also
avoided through the work of organizations such
as the Chemical Industry Institute of Toxicology
(CIIT) (Research Triangle Park, NC). Using contri-
butions from member companies, CIIT conducts
toxicological tests and distributes the results
widely.
Governments contribute greatly to information
sharing, which allows duplicative testing to be
avoided, by providing both access to test results
and information about their own planned and on-
going tests. The International Agency for Research
on Cancer makes it easy for duplicative carcinoge-
nicity testing to be avoided by informing testing
facilities and governments about planned and on-
going testing, Federal and international databases
and publications also contain information about
planned tests and those under way (see ch. 10).
Reducing Pain and Distress
As with research, testing can be modified to re-
duce animal pain or distress in two ways: by pro-
viding relief with drugs or by changing the proce-
dures so that less pain or distress is produced (see
ch. 6). A third alternative might be to use a less
sensitive species, but there is no method by which
relative distress among species can be discerned.
Relief from pain and distress is accomplished through
analgesics, anesthetics, tranquilizers, or sedatives
and modification of the test itself.
Few pain-relieving drugs have been developed
and marketed for animals. Little information is
available on recommended doses (122) or on the
likely effect on test results. Thus, before pain re-
lief could be incorporated into a test, it would be
necessary to determine the needed dose and the
effect on the toxic response, thus using additional
animals as well as subjecting them to pain.
Several small changes that do not interfere with
the experimental design can be made by an inves-
tigator. Small needles can be substituted for large.
Animals can be comforted by petting. Social ani-
mals can be caged in groups, although there are
often reasons that multiple housing cannot be used.
Smaller doses can be used and tests can be ended
at the earliest feasible time. Sometimes, smaller
doses will actually result in increased sensitivity
of the test (38). Making such changes sometimes
depends on the attitude and expertise of individ-
ual researchers rather than the contents of test-
ing guidelines, which may not be sufficiently
detailed.

Ch. 8—Alternative to Animal Use in Testing . 177
USE OF LIVING SYSTEMS IN TESTING
As detailed in chapter 6, two kinds of living sys-
tems can reduce whole-animal use—in vitro sys-
tems based on animal or human components (cell,
tissue, and organ cultures) and systems based on
organisms not considered animals for purposes
of this report (micro-organisms and invertebrates).
(Some people consider both of these in vitro
system s.)
In Vitro Systems
Cells, tissues, and organs can be kept alive out-
side a living organism and used for testing. Al-
though animals are still required as a source for
these in vitro systems, the animal would experi-
ence distress for a much shorter time, and per-
haps less distress overall, than occurs with whole-
animal testing because it would be killed before
any experimental manipulations were carried out.
Occasionally, different cells, tissues, or organs from
the same animals can be used for different inves-
tigations. In addition, many fewer animals would
be required for a given test, in part because varia-
bility in the toxic response is smaller than it is with
whole-animal tests and in part because one ani-
mal can be used for multiple data points, further
reducing variability. The fact that human tissues
sometimes can be used confers an additional ad-
vantage because the need for extrapolation from
animal data is obviated.
These isolated components also have disadvan-
tages. They are usually unable to produce the com-
plete physiologic responses of a whole organism.
The components often become undifferentiated
and lose their ability to perform their special func-
tions when isolated from the organism, particu-
larly when the sample is broken up into its con-
stituent cells, and even more so when the cells
replicate. Another disadvantage is that the effect
of the route of exposure, a variable that can have
profound effects on test results, is often impossi-
ble to determine.
There are many measures of damage to differen-
tiated or undifferentiated cells—the rate of repro-
duction, the rate of synthesis of certain substances,
Microscopic View of Cell Culture From Rabbit Corneal Epitheliums
Photo credit: Kwan Y. Chan, University of Washington

178 ● Alternatives to Animal Use in Research, Testing, and Education
changes in membrane permeability, and damage
to some part of the cell structure. Those functions
having to do with viability and growth are most
frequently measured because they require an in-
tegration of many physiologic events within the
cell, are sensitive, and lend themselves to automa-
tion (73).
Quantifiable tests are preferred over subjective
ones, and a wide variety of quantitative approaches
are available to measure irritation, including the
release of prostaglandins (35); the production of
enzymes (46), proteins (57), antigens, antibodies,
or hormones (73); and the migration of certain
white blood cells (macrophages) to the area of ir-
ritation (12,101). Irritation can also be measured
by the extent to which cells exfoliate from the sur-
face of the tissue. The extent of damage can be
determined by counting cells and by examining
the nuclei (1O2). Another indicator of irritation,
the integrity of cell membranes, can be monitored
through the uptake of nutrients through the cell
wall. Where the nutrient uptake is active (that is,
when the cell is required to expend energy for
transport), uptake can also be used to indicate
changes in metabolism (86,102).
Liver cells have been the subject of considerable
research, in part because they play such an im-
portant role in an organism’s removal of toxic sub-
stances and in part because they retain most of
their special functions when cultured. The re-
sponse of liver ceils to toxic substances may be
measured in many ways: the use of sugar as an
indication of metabolic activity; the production of
proteins or other substances that have been cor-
related with toxicity; uptake of amino acids as an
indication of protein synthesis; changes in appear-
ance that parallel those observed in livers of whole
animals (106); and morphological changes and re-
ductions in viability (75). Other promising tech-
niques in this rapidly expanding field include cul-
turing:
●
●
●
●
beating heart cells to detect the effect of cer-
tain vapors on irregularities in heartbeat (68);
rabbit kidney tubules to detect substances that
can cause acute renal failure, and rat vaginal
tissue to test vaginal irritancy of contracep-
tives (27);
various kinds of cells to test for biocompati-
bility of implants (15,52,53); and
nerve cells to test for the synthesis of neuro-
Dispensing Apparatus for Deiivery of Cuiture Medium to Ceiis Within a Piastic Cuiture Piate
Photo credit: The Johns Hopkins University

Ch. 8—Alternative to Animal Use in Testing ● 179
transmitter chemicals, the formation of syn-
apses, and the conduction of impulses (7).
Although tissue and organ cultures may approx-
imate more closely the physiology of the human
or whole-animal model, they are more difficult to
manipulate than cell cultures (see ch. 6). Sophisti-
cated equipment must be used to monitor and con-
trol the environment and to perfuse the sample
with nutrients. Where the sample is more than
a few cell layers thick, uniform delivery of the test
substance, nutrients, and oxygen is difficult, as is
the removal of waste products. Cell differentia-
tion can usually be maintained in tissue and or-
gan cultures, albeit with some difficulty (50).
Human placentas have proved quite useful in
testing the ability of a drug to cross the placenta
from mother to fetus. There are certain logistical
problems with this method, however. The placenta
must be transferred to the perfusion apparatus
within 5 minutes after it is eliminated from the
uterus, and it is only useful for about 3 hours af-
terward (77).
Nonanimal Organisms
There are a variety of nonanimal organisms that
can replace some animals in testing, ranging from
plants to single-celled organisms to invertebrates.
All of these can respond to certain noxious stimuli,
and some may experience pain. However, many
commentators believe that they do not experience
pain or suffering in the same way that animals do,
particularly in those cases where there is no brain
or neural tissue (90). The use of such organisms,
which has never been controlled under any Fed-
eral or State law, is regarded as a replacement for
animals in this report.
Micro-organisms
In recent years, increased emphasis has been
placed on the use of bacteria and fungi to meas-
ure certain genotoxic effects. A major advantage
of these organisms is that they can be cultivated
much more easily and quickly than most animal
or human cells. Their genetic makeup is simple
compared with that of animals and humans and
the fact that a great deal is known about it facili-
tates their use, particularly in toxicological re-
search leading to new methods (74). A change in
genetic material is relatively easy to detect and
characterize. Fungal systems have been shown to
be especially useful in mutagenicity testing and
seem to be more sensitive than bacteria (126), per-
haps at the expense of falsely indicating a hazard.
Other species that have proved useful include slime
molds, algae, and protozoa (74).
Protozoa, although rather primitive overall, fre-
quently have specialized functions that mimic those
of humans. For example, the cilia of protozoa re-
spond to smoke or phenols as do the cilia in the
human bronchial tube (5). Various protozoans have
been used in toxicity testing of cigarette smoke.
protozoans are currently being evaluated for use
in screening tests for carcinogenesis, mutagene-
sis, and reproductive toxicity (93).
Invertebrates
Invertebrates have made major contributions in
biomedical research because certain aspects of
their physiology are sufficiently similar to that of
mammals (74). Although models for toxicity test-
ing require greater similarity to animals or more
thorough characterization of differences than
models for research, invertebrates offer exciting
possibilities.
Of the invertebrates, insects offer the greatest
selection of models, there being over 2 million spe-
cies from which to choose (74). Among them, the
fruit fly, Drosophila rnelanogaster, is the best un-
derstood. procedures have been developed for de-
tecting mutagenicity (18), as well as teratogenic-
ity (11) and reproductive toxicity (93).
The sea urchin has long been a favored test
organism for basic reproductive research (74). Con-
sequently, the mechanisms and procedures of
testing this invertebrate can easily be developed
and performed. The sea urchin model for fertili-
zation and development can be used in screening
for reproductive toxicity, teratogenicity, and muta-
genicity. Nematodes, annelids, and mollusks are
also used for alternative mutagenesis testing re-
gimes and, additionally, mollusks are used in the
area of reproductive toxicology. Sponges, mollusks,
crustaceans, and echinoderms are being used in
metabolism studies, as understanding metabolize
formation in nonmammalian species can lend in-
sight to interspecies variation (93).

180 ● Alternatives to Animal Use in Research, Testing, and Education
USE OF NONLIVING SYSTEMS IN TESTING
Animal use can sometimes be avoided altogether
with nonliving biochemical or physiochemical sys-
tems, although most such systems currently re-
quire animal derived components. Computer simu-
lation can also be used when there are sufficient
data available for substances related to the one
of interest and when the mechanisms of toxicity
are at least partially understood.
Chemical Systems
Whole animals have been replaced with analyti-
cal chemistry for tests involving detection of a sub-
stance or measurement of potency or concentra-
tion, such as for vaccines, anticancer drugs, and
vitamins (10). However, toxicity testing in nonliv-
ing systems is quite limited at this time.
Recently developed methods of detection or
measurement are based on the selective binding
that occurs between a particular substance and
the antibodies to it. In an assay for botulism toxin
(which traditionally required up to 200 mice), an-
tibodies obtained from rabbits are modified so that
the binding of the toxin can be detected easily. The
rabbits are initially injected with a small, harm-
less dose of the botulism toxin. Small amounts of
blood are then removed from the rabbits at regu-
lar intervals. In 4 weeks, a rabbit can produce
enough antibody, with little discomfort, to perform
tests that would otherwise require thousands of
mice (32).
Chemical systems that test for toxicity are based
on determining whether a substance undergoes
a specific reaction. For example, it is well known
that carbon monoxide binds to hemoglobin in the
blood, thus greatly reducing the blood’s ability to
carry oxygen. The extent to which a substance
would displace oxygen in hemoglobin can be a
measure of its ability to produce asphyxiation. Sub-
stances can also be tested in isolation for their ef-
fects on enzymes crucial to certain bodily functions.
An important limit of chemical systems is that
they do not indicate the extent to which an organ-
ism can recover from or prevent these reactions.
For example, a substance that binds strongly to
hemoglobin may not be a problem because it is
not absorbed. A substance will not have a signifi-
cant effect on an enzyme of interest if it is excreted
before it has an effect.
Physiochemical systems have some ability to
determine whether a substance will be absorbed
and what will happen to it. The tendency of a sub-
stance to accumulate in a biological system can
be roughly estimated by the relative proportions
that dissolve in equal volumes of water and the
organic solvent octanol (34,55). Artificial skin made
with filter paper and fats is being tried as a means
of mimicking absorption of cosmetics and drugs
(45). Reactivity and other toxicity-related proper-
ties can be deduced from chemical structure alone
(109).
Mathematical and Computer Models
Advances in computer technology during the
past 20 years have contributed to the development
of sophisticated mathematical models of quantita-
tive structure activity relationships (QSAR). These
models are used to predict biological responses
on the basis of physical and chemical properties,
structure, and available toxicological data. The limi-
tations of such models are due in part to a lack
of understanding of the mechanisms by which
toxic effects occur.
In applying QSAR, the biological effects of chem-
icals are expressed in quantitative terms. These
effects can be correlated with physiochemical
properties, composition, and/or structure. Fre-
quently used properties include an affinity for fats
versus water (octanol/water partition coefficient),
the presence of certain reactive groups, the size
and shape of molecules, and the way reactive frag-
ments are linked together.
The simplest extrapolation is for a series of
closely related chemicals. The several character-
istics they have in common need not be incorpo-
rated into the model as variables. This type of
analysis has been performed for several hundred
families of chemicals and has established that rela-
tionships within a series are fairly predictable (64).
Another approach, more broadly applicable, is
to examine the contributions of various portions
of a molecule. In more elaborate computer pro-

Ch. 8—Alternative to Animal Use in Testing . 181
grams, it is possible to identify likely reactions and
cascading physiological events in various species,
techniques first developed for pharmacology (54).
A similar approach is the use of multitiered clas-
sification schemes that use large databases to draw
semiempirical conclusions (36).
Epidemiologic Data on Humans
Perhaps the most useful alternative to animal
testing is epidemiologic studies on humans. Such
studies were used to detect carcinogenicity in hu-
mans as early as the 18th century (49,85,87). The
most well known study detected scrotal cancer
in chimney sweeps (85). A more recent example
in which epidemiologic evidence was used to de-
tect a human carcinogen was the finding that vi-
nyl chloride causes a rare liver cancer in humans
(26). A major disadvantage of epidemiologic studies
is that considerable human exposure can take place
before a toxic effect is detectable, particularly in
the case of diseases that take many years to de-
velop. Another disadvantage is that they can be
quite expensive to conduct. Privacy must also be
considered (112), preventing many data that would
be useful from being collected or analyzed.
Epidemiologic studies may be divided into three
general types: experimental, descriptive, and ob-
servational. Experimental epidemiology is the hu-
man equivalent of animal testing—providing or
withholding a substance to determine its toxic or
beneficial effects. Such studies are greatly limited
by ethical and legal considerations, as well as the
difficulties involved in securing the cooperation
of a large number of people.
Descriptive epidemiology analyzes data on the
distribution and extent of health problems or other
conditions in various populations, trying to find
correlations among characteristics such as diet,
air quality, and occupation. Such comparisons are
frequently done between countries or smaller geo-
graphic regions, as is the case for cancer statistics
collected and analyzed by the National Cancer In-
stitute (9).
observational epidemiology uses data derived
from individuals or small groups. Data would be
evaluated statistically to determine the strength
of the association between the variable of interest
and the disease. In cohort studies, a well-charac-
terized and homogeneous group is studied over
time. In case-control studies, a control group is
selected retrospectively based on variables thought
to be relevant to the effect, Both methods rely on
an accurate prediction of the variables that are
important and are subject to various selection
biases (62)112).
THE LD
50
TEST
The LD
5O
testis one of the most widely used tox-
icity tests, and the development of alternatives to
it is regarded by many as a high priority. As de-
scribed in chapter 7, this acute toxicity test meas-
ures the amount of a substance needed to kill half
the population of the test species. The LD
5O
is
used as a rough indicator of the acute toxicity of
a chemical,
The LD
5O
is useful for testing biological thera-
peutics, although there remain few such sub-
stances for which the LD
5O
is the only available
means of standardization (13)90). Other applica-
tions, perhaps not so well justified (90), are deter-
mining doses for other toxicological tests and set -
ting regulatory priorities.
There has been political pressure to abolish the
LD
5O
and it has been criticized by many toxicolo-
gists on scientific grounds. It has poor reproduci-
bility and the results are difficult to extrapolate
to humans because there are so many mechanisms
by which death could occur (70,90,125).
Despite the many criticisms of the LD
5O
, most
toxicologists agree that acute toxicity information
has valid uses, and that measurements of lethality
also are important. Nevertheless, the precision with
which the LD
5O
is measured is often unjustified
for several reasons. First, most applications of the
information do not require precision. Second, even
if the information were precise for a given spe-
cies, the LD
5O
varies so much from species to spe-

182 Ž Alternatives to Animal Use in Research, Testing, and Education
cies that extrapolation to humans is only rough.
Third, the LD
50
of a given substance varies signifi-
cantly from laboratory to laboratory, and even in
the same laboratory.
Various regulatory classification schemes make
distinctions between levels of toxicity (“highly toxic”
versus “toxic, ” versus “moderately toxic, ” versus
“nontoxic”). The LD
50
for two neighboring levels
typically differs by a factor of 4 to 10. Yet, the
reproducibility of test results does not justify even
these distinctions. A recent study, though not nec-
essarily typical, indicates the magnitude of the
problem. A series of LD
5O
tests were performed
in 60 European laboratories for five substances
on one species. The LD
5O
for one substance ranged
from 46 mg/kg body weight to 522 mg/kg, possi-
bly ranging over three toxicity levels in some clas-
sification schemes. Although the variations were
not this large for the four other chemicals tested,
the smallest variation was 350 to 1,280 mg/kg. Each
test was done with 50 or more animals so that the
results would be precise (61).
Using Fewer Animals
The standard LD
5O
requires at least three groups
of 10 animals or more each. An alternative proce-
dure for dete
rmining the Approximate Lethal Dose
(ALD) was developed as early as the
1940s (29),
in which individual animals are administered doses
that increase by 50 percent over the previous dose.
Depending on the initial dose level, the total num-
ber of animals needed is usually 4 to 10. Because
the test substance might not be cleared between
doses or because there maybe cumulative effects,
the ALD can be lower than the LD
50
, perhaps by
70 percent, though more typically by less than 20
percent (29).
Many other acute toxicity tests that require fewer
animals than the LD
50
have been developed (14,
17,33,61,69,71,94,105,107). Most require that the
doses increase sequentially, thereby allowing the
experiment to stop when a certain limit is reached.
Thus, fewer animals die in the conduct of a test,
but its duration could increase from 2 weeks to
a month or more. Although many investigators
are moving to less precise LD
5O
tests, no generally
accepted alternative seems to have emerged.
The Limit Test and
Other Refinements
If a substance is not lethal at high doses, its pre-
cise LD
5O
is not very important. In the limit test
(80), a small number of animals is given a single
oral dose, e.g., 5 g/kg body weight. If no animals
die and no major ill effects occur, no further test-
ing is needed. However, this limit is so high that
this approach may have little practical value in re-
ducing animal use (24).
Rather than determining the dose that is lethal,
studies can also be done to detect toxic effects at
doses that are not lethal. As with the LD
5O
, increas-
ing doses can be administered to a small number
of animals, perhaps stopping when some limit is
reached. This approach can be further refined so
that animals that are in distress could be sacrificed
without affecting the outcome of the test (14).
In Vitro and Nonanimal Methods
Cell toxicity—changes in cell function or death
of cells-can sometimes be used to detect acute
toxicity. However, cell toxicity cannot be expected
to function as a replacement for the LD
5O
because
lethality can occur by so many mechanisms that
are supercellular. Cell toxicity is particularly use-
ful in comparing members of chemical families,
such as alcohols and alkaloids (79).
At present, mathematical modeling has limita-
tions, although it may have some utility in range-
finding and in screening substances for testing
(109). Modeling of acute toxicity fails to meet one
of the criteria suggested by a working party on
quantitative structure activity relationships, namely
that the mechanism by which the response occurs
should involve a common rate determining step
(88). Nonetheless, in a large study involving thou-
sands of substances, a computer program was de-
veloped that predicted LD
5O
values within a fac-
tor of 2.5 for 50 percent of the substances and
within a factor of 6 for 80 percent. Considering

Ch. 8—Alternative to Animal Use in Testing Ž 183
the reproducibility of the test itself, this might be
satisfactory for some purposes, and it certainly
warrants further investigation. Furthermore, many
of the larger deviations in this study, upon fur-
ther examination, were found to involve report-
ing errors. This program relied on a multi-tiered
classification scheme based on chemical structure
(36).
SKIN AND EYE IRRITATION
The widely used Draize eye irritation test and,
to a somewhat lesser extent, the skin irritation test
have been criticized because of the amount of pain
inflicted and because they are unsatisfactory mod-
els for human irritation (91,95). First, the rabbit
eye has structural differences, such as a thinner
cornea and differing tearing apparatus (103), and
animal skin is much less sensitive and discriminat-
ing than human skin (56,63). Second, both of these
tests are sensitive to too many variables, making
reproducibility poor (83,118).
As with most tests, the number of animals used
can sometimes be reduced. Several refinements
have also been proposed. For example, screening
tests based on pH or skin irritancy might also serve
as alternatives to eye irritancy tests in limited cir-
cumstances, although preliminary studies indicate
that this approach is frequently misleading (119).
Other refinements involve local anesthetics (51,65,
110), applying smaller (43) or more dilute (120)
doses, and testing whole eyes in vitro (20). The lat-
ter method has particular appeal when cow eyes
are used because they are so readily available from
slaughterhouses. In the case of smaller doses, a
recent comparison with over 500 accidental human
exposures showed that doses smaller than those
now in use yielded results more predictive of the
human response while causing less severe irrita-
tion (38).
Skin and eye irritation are similar in many re-
spects. Thus, even though little work has been done
to develop alternatives to skin irritation tests, the
many approaches just summarized for eye irrita-
tion may eventually be applied to skin testing as
well (91).
In Vitro Tests
Several in vitro alternatives have been examined,
and it appears to some commentators that no sin-
gle alternative will be adequate, but that a battery
of in vitro tests might be a useful replacement (67).
Several types of cell cultures have been used in
developing an in vitro test for eye irritation. The
cells used are rabbit and human corneal cells (72),
mouse and hamster fibroblasts, human hepatoma
cells, and mouse macrophages (96).
A variety of effects have been used as surrogates
for eye irritation, such as the rate of uptake of uri-
dine as an indication of cell functioning and re-
covery, visible changes in cell structure, decreases
in the concentration of cell protein (96), and re-
lease of plasminogen activator from the injured
cells (21). Some techniques appear promising, par-
ticularly in their ability to rank substances based
on irritancy, Rapid progress is being made in the
development of techniques, but none can be con-
sidered validated at this time (91).
To date, little work has been done on in vitro
replacements for skin irritancy testing. However,
the growth of skin in tissue culture is of interest
for treating burn victims, and it is expected that
culture techniques currently being developed for
that purpose can be used in testing methods. In
addition, it has also been suggested that suitable
specimens can be obtained from cadavers and
surgery and from judicious use of human volun-
teers (63).
Chick Embryo
One test system receiving considerable attention
is the fertilized chicken egg. A part of the eggshell
is removed and the test substance applied to the
chorioallantoic membrane surrounding the devel-
oping embryo (see fig. 8-l). This test has the po-
tential for assessing both eye and skin irritancy.
The chorioallantoic membrane of the chick em-
bryo is a complete tissue, including arteries, capil-
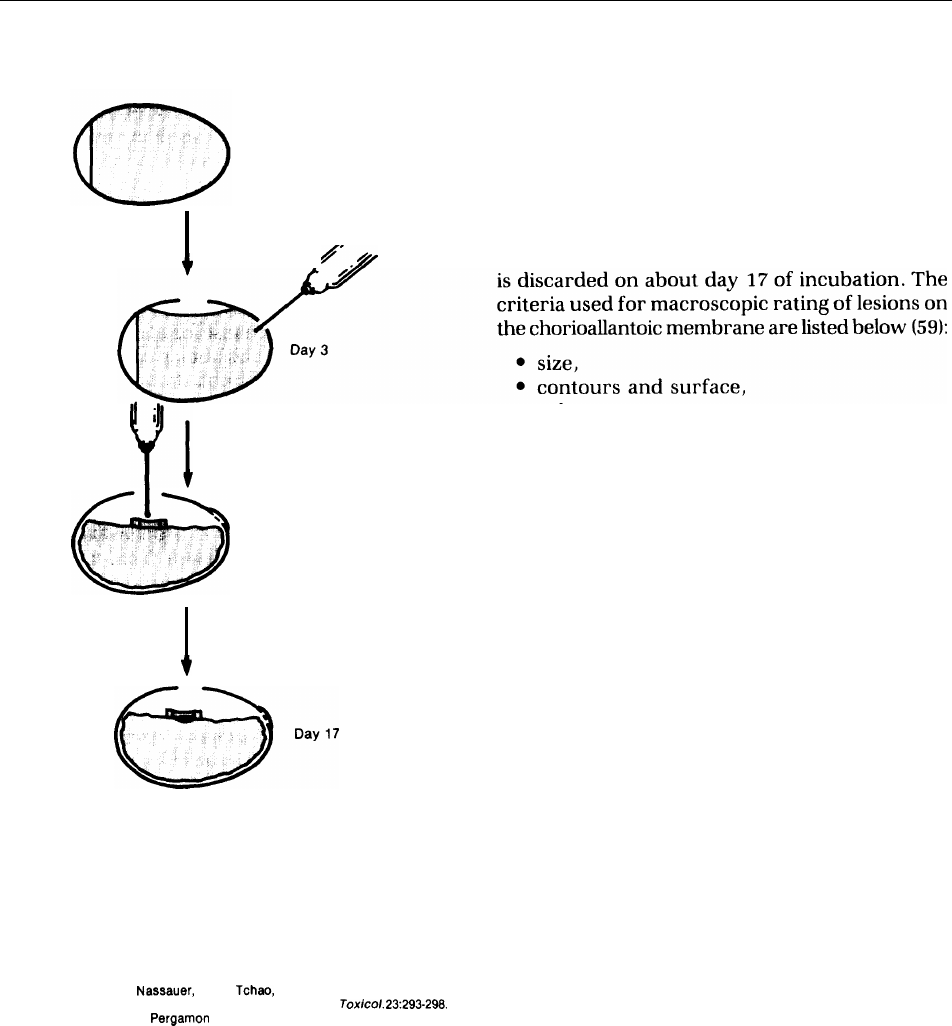
184 ● Alternatives to Animal Use in Research, Testing, and Education
Figure 8-1.—Chronological Sequence of Chick
laries, and veins, and is technically easy to study.
Embryo Chorioallantoic Membrane Assay
An embryonic membrane tested after 14 days of
incubation responds to injury with a complete in-
flammatory reaction, a process similar to that in-
Day O
duced in the conjunctival tissue of the rabbit eye.
The embryonic membrane can show a variety of
signs of irritation and has capabilities for recov-
ery (59,60).
/
Assessment of toxicity is made and the embryo
●
●
●
Day 14
size,
contours and surface,
color,
retraction of surrounding chorioallantoic
membrane,
spokewheel pattern of vessels,
● overall grade of severity, and
● necrosis (confirmed microscopically).
Although this is, strictly speaking, an in vivo test,
the chorioallantoic membrane does not have nerve
cells, and thus it is unlikely that the organism ex-
periences any discomfort. In addition, fertile eggs
are inexpensive and do not require elaborate ani-
mal room facilities.
Day O. Fertile eggs are incubated at 37” C. Day 3. The shell is
penetrated in two places: A window is cut at the top, and 1.5 to 2
milliliters of albumin is removed with a needle and discarded. The
chorioallantoic membrane forms on the floor of the air space, on
top of the embryo. The window is taped. Day 14. A test sample is
placed on the embryonic membrane and contained within a plastic
ring. Day 17. The chorioallantoic membrane is evaluated for its
response to the test substance, and the embryo is discarded.
SOURCE: J. Leighton, J. Nassauer, and R. Tchao, “The Chick Embryo in Toxicol-
ogy: An Alternative to the Rabbit Eye,”
Food Cherry.
Tox/co/.
23:293-298.
Copyright 19S5, Pergamon Press, Ltd.
REPEATED-DOSE TOXICITY TESTS
Repeated-dose toxicity testing involves the re- peated-dose testing, the long-term effects of
peated application of a substance to a biological
repeated, sublethal exposure to a substance are
assay system and subsequent measurement of
of interest, rather than acute, lethal effects. Cell
many different effects of the substance. In re-
cultures may be useful adjuncts for suspected tar-

Ch. 8—Alternative to Animal Use in Testing ● 185
Chick Embryo Chorioallantoic Membrane Assay
Photo credit: Joseph Leighton, Medical College of Pennsylvania
Typical react ion seen 3 days after certain concentrations
of household products have been placed on the 14-day-
old chorioallantoic membrane. The thin white plastic ring
has an internal diameter of 10 millimeters. The area of
injury within the ring is well defined with a distinct edge.
All of the cells in the injured area are degenerating or
dead. The severity of this positive lesion is quantified by
measuring its diameter.
get organs or tissues, but they are not a replace-
ment for whole-animal testing. The most promising
alternatives in the near future involve modifica-
tions of animal use (for example, by combining
tests), and the use of screening tests and computer
simulation for improved experimental design. The
screening tests with the greatest promise are for
hepatotoxicity and neurotoxicity.
Hepatotoxicity
Several in vitro alternatives for hepatotoxicity
have been developed, including perfused liver
(108), liver cell suspensions (39), and liver cell cul-
tures (39)44). Liver perfusions can only be main-
tained for a few hours, and with some difficulty.
Cell cultures can retain the special functions of
liver cells with specially prepared culture media
(76,81). However, the cells are viable for only a
limited period of time and do not replicate in a
reproducible manner. Although these techniques
have been used to study mechanisms of liver tox-
icity, only limited attention has been given to their
use in screening or as alternatives (91).
Neurotoxicity
The development of alternatives for neurotox-
icity is more difficult than for hepatotoxicity. The
nervous system is the most complex organ in the
body, both in terms of structure and its function.
Because many neurotoxins affect only one kind
of cell, a battery of in vitro tests would probably
be required to replace whole-animal testing–if
anything could. Substances can also affect vari-
ous areas differently, partly because of distribu-
tion factors, For example, very few substances are
able to enter the brain because of the ‘(blood-brain
barrier.” Thus, pharmacokinetic studies will con-
tinue to be very important.
Some in vitro tests (41) and tests using inverte-
brates (8) seem useful, at least for screening. As
yet, however, the primary use of in vitro tech-
niques has been the elucidation of mechanisms
of known toxic effects (31). Many toxic effects to
neural tissue have been correlated with concen-
trations of specific chemicals in or around the cells,
thus offering the means for developing in vitro
tests (31).
MUTAGENICITY
Mutation, the change in the DNA sequence of
is passed from the mutated cell to its descendants.
genes, is a mechanism by which toxic effects may
Mutation can lead to cell death or the gain or loss
be initiated. If the DNA replicates, the mutation
of certain functions. When it occurs in germ cells,
38-750 0 - 86 - 7

186 ● Alternatives to Animal Use in Research, Testing, and Education
the gene pool is affected, even if the mutation is
not expressed in the progeny. The mutations that
occur in somatic cells that are of greatest concern
are those that lead to cancer (18).
Recent advances in the techniques of cell biology
have led to an increase in the types and sophisti-
cation of mutagenicity tests available. Mutations
can be detected by analyzing DNA or its fragments
or by observing changes in the size, shape, or num-
ber of the chromosomes (which contain DNA), as
well as by observing changes in a whole organism
(34). Mutation can also be detected by measuring
the amount of DNA repair.
Micro-organism Tests
The most commonly used test for mutagenicity
is the Ames test for “reverse mutation” in Salno-
nella typhimurium
(3). Mutagenicity is detected
by exposing an already mutated strain to poten-
tial mutagens. If the mutation is reversed, the bac-
teria regain their ability to produce the amino acid
histidine and will proliferate in a histidine-deficient
culture medium.
The Ames test, as well as most other mutagenic-
ity tests involving micro-organisms, does not avoid
animal use entirely, To determine whether the meta-
bolic products of a substance might be mutagenic
even if the substance itself is not, liver prepara-
tions from rats or other rodents are used to pro-
duce at least some of the likely metabolic products.
Microorganism systems may fail to detector may
overpredict mutagenic changes that could occur
in whole animals or humans. For example, the sys-
tem provided for metabolism may not be capable
of reproducing conditions in vivo, or in the case
of screening for carcinogenicity, mutation may not
be the initiating event. on the other hand, such
systems may indicate mutagenicity when the DNA
repair system of mammals would reverse the mu-
tation.
.
Other bacterial tests have been developed using
S. typhimurium, Escherichia coli, and Bacillus sub-
tilis. These systems do not seem to offer any par-
ticular advantage over the Ames test, although
thorough evaluation is hampered by lack of a com-
parable database of results (28). Tests have also
been developed for molds (3
0)
)
fungi (16), and
yeasts (18,82).
In Vitro Tests
In vitro mutagenicity tests maybe done with cul-
tured mammalian cells that are exposed to toxic
substances, although many mammalian in vitro
tests also have an in vivo variant. Such tests typi-
cally measure acquired resistance or lost resistance
to the effects of the toxic substance. Most com-
monly used are a mouse lymphoma ceil line or ham-
ster ovary cells, but almost any well-characterized
cell can be used. ovary cells are often used be-
cause, as germ cells, they have half the number
of chromosomes to be evaluated (18).
A test known as the specific locus test can be
done with Chinese hamster ovary cells. They are
exposed to a test substance and their response to
the normally lethal 8-azaguanine or 6-thioguanine
in cell culture determined. The cell’s ability to sur-
vive, requiring the ability to metabolize the 8-
azaguanine or 6-thioguanine, is an indication of
the occurrence of mutation as a result of exposure
to the test substance. This test can also be done
with mouse lymphoma cells exposed to 5-bromo-
deoxyuridine or trifluorothymidine (23).
The sister chromatid exchange test relies on the
fact that certain substances will cause DNA break-
age and reunion. This damage can be observed
by staining the original chromosomes so that any
segments exchanged during replication can be ob-
served. Commonly used cells include human lym-
phocyte cells and rodent and human fibroblasts
(37). Both the specific locus test and the sister chro-
matid exchange can also be performed as in vivo
procedures (see ch. 7).
Although the cells are usually derived from ani-
mals, there is a considerable net savings in animal
lives when in vitro mutagenicity tests are per-
formed. For example, the rat mast cell assay can
be used to screen severe irritants, and one rat can
supply enough tissue to replace the use of 48 ani-
mals in in-vivo procedures (103).
Tests Using Insects
The most widely used insect for genetic studies
is the fruit fly, Drosophila melanogaster (114, 115).
The fruit fly has well-characterized genetics and
is similar to mammals in many key reactions, A
variety of end points can be detected. The most
common, and probably most sensitive, test is the

Ch. 8—Alternative to Animal Use in Testing • 187
sex-linked recessive lethal assay (18). Treated males
ured include the loss, gain, or breakage of chro-
are mated with untreated females, and the progeny mosomes detected by examining germ cells. With
are mated
to each other. The number and charac- the availability of mutant strains, the measurement
teristics of the male progeny are evaluated to de-
of reverse mutations can be a valuable tool. Eye
termine if lethal mutations (that is, mutations that
color is a popular method of following genetic ef-
prevent viability) have occurred.
fects in the fruit fly (18).
Other
tests involving fruit flies also exist or are
likely to be developed. End points that can be meas -
CARCINOGENICITY
Many assays meant to replace carcinogenicity
testing
are designed to detect the initiation of can-
cer rather than the formation of tumors. First, de-
tecting initiation is faster and easier than detect-
ing cancer. Second, although not all initiation leads
to cancer, certain kinds are considered reliable
surrogates for the disease.
A major problem with evaluating the predictive-
ness of alternatives to whole animals for carcinoge-
nicity testing is that very few human carcinogens
have been positively identified. Most substances
treated as human carcinogens, although docu-
mented to be known animal carcinogens, must be
viewed as probable or suspected human carcino-
gens. The development of alternatives is somewhat
hampered by a lack of epidemiologic data on hu-
mans.
Various molecular and physiochemical prop-
erties of substances have been correlated to car-
cinogenicity. Some structure-activity models de-
veloped for families of chemicals have predicted
the carcinogenic properties for 75 to 97 percent
of them. The chemicals modeled include polycyclic
aromatic hydrocarbons (123), nitrosamines (89,99,
121), and aromatic amines (124).
The Ames Test
Because mutation is often the first step in car-
cinogenesis, the Ames
test has been suggested as
a
possible screen or replacement for carcinoge-
nicity testing. It has been evaluated for this pur-
pose, both alone and as one in a battery of tests.
Alone, it is less predictive than whole-animal tests.
In a battery, it has been shown to be about as pre-
dictive as animal testing for certain families of
chemicals and substantially less predictive for
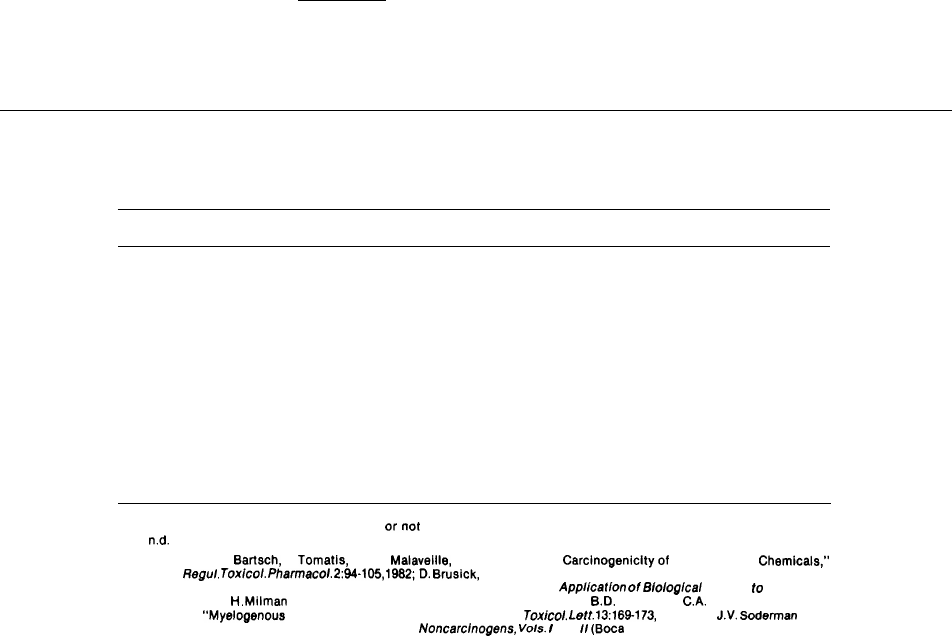
others for the substances tested. Table 8-1 shows
the predictiveness of mouse and rat bioassays and
the Ames test for some known human carcinogens.
The Ames test has been performed thousands
of times in over 2,000 laboratories throughout the
world and has provided results on over 1,000
chemical substances since it was developed less
than two decades ago. Portions of this large body
of analytical data have been reviewed in over a
dozen evaluation studies with the intent of deter-
mining the test ability to predict carcinogenicity
(6,19,66). These evaluations show that the percent-
age of human carcinogens that are also mutagens
(mutagenic carcinogens) ranges from 50 to 93 per-
cent and is most likely about 80 percent (48). About
20 percent of the human carcinogens were not
mutagens (nonmutagenic carcinogens) in the Ames
test, and it is believed that cancer associated with
these carcinogens is initiated by a mechanism other
than mutation.
A critical analysis of several studies (19) identi-
fied several sources of variation. These include
methods of chemical selection, sample coding, use
of a high proportion of chemicals known to work
well or poorly with Ames testing, and differences
in metabolic activation during the test procedure.
The conclusion was that a reasonably careful ap-
plication of the Ames technique to a nonbiased
group of chemicals would be expected to yield a
predictive accuracy of approximately 80 percent
for mouse and rat carcinogens.
The Ames test tends to be positive for a large
proportion (about 40 percent) of substances that
have not been identified as carcinogens in rodent
bioassays. It should be noted, however, that these
188 ● Alternatives to Animal Use in Research, Testing, and Education
Table 8.1.—The Response of Known Human Carcinogens to
Rodent Carcinogenicity and Bacterial Mutagenicity Assays
Rat
Mouse
Ames
Chemical
bioassay bioassay
test
4-Aminobiphenyl . . . . . . . . . . . . . . . . . . . . . . . . .
Arsenic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Asbestos . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Benzene. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Benzidine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Bis(chloromethyl)ether . . . . . . . . . . . . . . . . . . . .
Chromium; some chromium compounds . . . . .
Cyclophosphamide . . . . . . . . . . . . . . . . . . . . . . .
Diethylstilbestrol . . . . . . . . . . . . . . . . . . . . . . . . .
Melphalan . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Mustard gas . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2-Naphthylamine . . . . . . . . . . . . . . . . . . . . . . . . .
Soot, tars . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Vinyl chloride.. . . . . . . . . . . . . . . . . . . . . . . . . . .
+
—
+
—
+
+
+
+
+
+
n.d.
—
—
+
+
—
+
+
+
+
—
+
+
+
+
+
+
+
+
—
—
+
+
+
+
—
+
+
+
+
+
KEY:+ = Positive results (carcinogenic to rodents or mutagenic to bacteria)
– = Negative results (not carcinogenic
ornot mutagenic)
n.d.
= No data.
SOURCES: From H.
Bartsch, L. Tomatis, and C. Malaveille, ’’Mutagenicity and Carcinogenicityof Environmental Chemicals:’
Regu/ Tox/co/. Pharmacoi. 2:94-105, 1982;D. Brusick, devaluation of Chronic Rodent Bioaasays andAmes Assay
Tests as Accurate Modeis for Predicting Human Carcinogens,”
Application
of
Bioiogicai
Markets to Carcinogen
Testing,
H.
Milman and S. Sell (ads.) (New York: Plenum Press, 1963);
B.D.
Goldstein,
C.A.
Snyder, S. Laskin et
al.,
“Myelogenous Leukemia in Rodents Inhaling Benzene,”
TcJx/coi.
f.ett.
13:169-173,
1962; and
J.V.
Soderman (cd.),
Handbook of identified Carcinogens and
Noncarcinogens, Vols.
/
and
Ii
(Boca Raton, FL: CRC Press, 1982),
substances have not been shown to be noncarcino-
genic, and many authorities maintain that the in-
formation is insufficient to make any statement
about the proportion of noncarcinogens that are
also nonmutagens in the Ames test (4,116).
Use of the Ames Test in
a Battery of Tests
The predictive value of the Ames test, or other
mutagenicity tests, can be improved by combin-
ing it with additional short-term assays to form
a test battery. Although no US. regulatory agency
has yet recommended a specific combination, most
authorities recommend that an appropriate bat-
tery should include information from a minimum
of three types of tests:
●
●
●
gene mutation (Ames test, mouse Iymphoma
test);
chromosomal mutation (in vivo Chinese ham-
ster ovary cell cytogenetics); and
DNA damage (sister chromatid exchange,
—
unscheduled DNA repair).
At least one test should include a mammalian in
vitro cell, tissue, or organ culture assay (4).
In a recent study, 18 Ames tests averaged 66 per-
cent “accuracy” (number of chemicals correctly
identified/number of chemicals tested). Compara-
tive results from six batteries of short-term tests
that included the Ames test increased the accuracy
to 82 to 90 percent (58,111).
CURRENT TRENDS
As long as toxicological data continue to be re- thermore, there are several impediments to devel-
quired by regulators and by the courts to protect opment and implementation:
human health, animal testing will continue for the
foreseeable future. Even major progress in the de-
. A large number of scientists have been trained
velopment and implementation of alternatives will
to solve health problems and to invent new
not necessarily eliminate whole-animal tests. Fur-
products using animal models.

Ch. 8—Alternative to Animal Use in Testing ● 189
●
●
●
Regulatory schemes, product liability law, and
patent law also incorporate notions of animal
models.
A large body of animal testing information al-
ready exists that is useful in interpreting new
testing data.
There are substantial costs and delays associ-
ated with the development and adoption of
alternatives. One study indicated that it takes
about 20 years for an in vitro test to be devel-
oped, validated, adopted, and implemented
(92).
At the same time, there are several factors
facilitating the development and implementation
of alternatives:
●
●
●
●
Rapid progress is being made in techniques
for culturing mammalian cells and organs, in
instruments for detecting and quantifying
cellular and molecular changes, and in the
understanding of the cellular and molecular
processes underlying toxicity. Improved un-
derstanding is leading to the ability to predict
long-term effects and carcinogenicity from
short-term biochemical and morphological
changes.
As such advances are made, the research lab-
oratories that have developed the expertise
are often willing to apply it to the develop-
ment of new testing methods, and can do so
efficiently (42).
Organizations such as The Johns Hopkins Cen-
ter for Alternatives to Animal Testing and the
Rockefeller University laboratory have been
set up to facilitate and coordinate research
on alternatives (see ch. 12).
Many organizations have been established to
pressure those who conduct animal testing
or use data based on it to adopt alternatives
or conduct research that will lead to alter-
natives.
Strategies to speed the development and adop-
tion of alternatives will depend on the needs and
resources of the organization involved. The fol-
lowing recommendations encompass a variety of
perspectives. They were promulgated by the Tox-
icity Committee of the Fund for the Replacement
of Animals in Medical Experiments, which met
from 1979 through 1982 (40). Some involve re-
assessment of testing needs and priorities; others
involve technical strategies thought to be likely to
lead to better methods, both in testing and in evalu-
ating results:
●
●
●
●
●
●
●
●
●
●
●
●
●
Provide a mechanism for reviewing the need
for a given test.
Investigate the consequences of not requir-
ing or possessing testing data other than what
already exists. Particular attention should be
given to widely used tests such as the LD
5O
and skin and eye irritation tests with a view
toward eliminating unnecessary requirements.
Encourage flexible use of testing guidelines
and frequent reappraisal of them in light of
new knowledge.
Strive for broader-based international har-
monization and mutual recognition of data
from other countries so that duplicative test-
ing can be avoided.
Encourage detailed publication of all testing
results, particularly for costly or painful tests
or those requiring many animals.
Investigate the possibility of time limits on the
confidentiality of test results.
Make greater use of studies on absorption,
distribution, biotransformation, and excretion
in humans, as well as in test animals, to select
the most relevant exposure conditions, to aid
in extrapolation of results, and to improve the
reliability of test results.
Perform preliminary studies before undertak-
ing long-term studies so that results can be
as useful as possible.
Make greater use of the structural and con-
formational computer models used in devel-
oping drugs for the prediction of toxicity.
Standardize screening tests based on in vitro
and nonanimal tests, both to promote efficient
use of testing resources and to evaluate the
predictiveness of these tests.
Try to predict toxic reactions before testing,
both as a means for improving prediction tech-
niques and to avoid testing highly irritating
substances, particularly in the eye, if possible.
Conduct research on the mechanisms by which
toxic effects occur to facilitate the develop-
ment of new testing methods.
Develop more accurate, reproducible instru-
mentation for measuring toxic effects, avoid-

190 ● Alternatives to Animal Use in Research, Testing, and Education
●
●
●
●
●
●
●
●
●
ing subjective measurements and reducing
measurement errors.
Make greater use of depositories in standard-
izing cell lines or strains of micro-organisms
used for testing.
Study the relationship between physiochemi-
cal properties and pharmacokinetic proper-
ties, as well as between physiochemical and
toxicologic properties.
Develop techniques for detecting nonmuta-
genic carcinogens.
Develop systematic methods for objectively
evaluating new techniques.
Conduct postmarketing surveillance for ad-
verse effects, noting any discrepancies with
test results from animals.
Substitute very specific tests for the LD
5O
and
other general toxicity tests, particularly for
substances having specialized uses, such as
drugs.
Use skin irritation testing as a rough screen-
ing tool for eye irritation.
Attempt to describe specific effects in eye ir-
ritation studies, rather than reporting only
the magnitude of the response.
Investigate specific effects such as neurotox-
icity to the extent possible when conducting
general toxicity tests.
●
●
●
●
●
●
●
●
Search for cell lines that retain their special
functions upon replication and develop tech-
niques for culturing them.
Evaluate the statistical precision needed in
various circumstances with a view toward
using the smallest number of animals likely
to be adequate.
Use statistics to maximize the utility of results.
Techniques such as blocking, covariance anal-
ysis, and factorial design should be used rou-
tinely.
Improve standards of care and diet to reduce
background effects.
Take care that those conducting tests are qual-
ified to do so, including having been trained
in humane handling of animals.
Combine tests wherever possible and keep
them as short as possible, compatible with the
nature of the test,
Place greater emphasis on “no observed effect
levels” than on lethal doses when they have
greater predictive value.
Use more than one species only to answer spe-
cific questions, and not for general safety as-
sessments.
SUMMARY AND CONCLUSIONS
There has been a small but significant shift away
from whole-animal testing to in vitro and non-
animal techniques in recent years, partly as a re-
sult of advances in biological techniques and partly
in response to political and economic pressures.
Many new methods are being developed for com-
monly used tests. Most of these are not yet vali-
dated, but they already have potential uses for
screening substances for the animal testing they
may eventually replace.
There are several kinds of alternatives. The first
entails the continued, but modified, use of ani-
mals-changes in experimental design or data anal-
ysis so that fewer animals are needed or changes
in protocols to reduce pain or distress. Living tis-
sues, organs, and cells derived from humans or
animals can sometimes be used instead of whole
animals. These systems require a larger investment
of time and money to develop than do modifica-
tions of whole-animal techniques, but their advan-
tages may also be greater. They are usually faster
and often cheaper than the corresponding whole-
animal test, and they have scientific advantages
as well. However, they almost always are less
predictive than whole-animal tests and often fail
to provide reliable dose-response data, informa-
tion that is critical in estimating potential toxicity
to humans.
Data, both anecdotal and epidemiologic, on toxic
effects in inadvertently exposed humans are some-
times useful. However, these data are often con-
founded by lifestyle and exposure to other toxic
Ch. 8—Alternative to Animal Use in Testing Ž 191
factors. Another drawback is that human exposure
can be great if there are long delays between ex-
posure and observable effects.
The LD
5O
, probably the most common and most
criticized toxicity test, is well suited to the limited
use for which it was first developed. The biggest
obstacle to limiting or eliminating use of the LD
5O
is institutional: Many regulatory schemes rely on
it for classifying substances. The most promising
alternatives in the short term are testing sequences
that require fewer animals. Cell culture techniques
and computer modeling show some promise, but
they have limited value at this time.
Another common and widely criticized test is
the Draize eye irritation test. Several promising
in vitro alternatives have been developed with cell
cultures. Another technique uses the outer (chorio-
allantoic) membrane of a 14-day-old chicken em-
bryo. This technique, although it uses a whole ani-
mal embryo, is thought to involve no pain because
the membrane has no nerves. These alternatives
may also apply to skin irritation.
Alternatives to carcinogenicity testing and re-
peated dose toxicity testing are of special interest,
in part because the potential savings in testing costs
and time are quite large, and in part because these
tests require large numbers of animals. The most
promising replacements are batteries of tests in-
volving cell cultures and living, nonanimal organ-
isms. Mutagenicity testing uses many in vitro or
nonanimal protocols. Mutagenicity is of particu-
lar interest because mutation can be the first event
in other kinds of toxicity, including carcinogenic-
ity, and because it can permanently affect the hu-
man gene pool. The most well known nonanimal
mutagenicity assay is the Ames test. When it is com-
bined with other tests, the Ames shows promise
as an alternative to carcinogenicity testing, but it
is not yet validated for this use.
In general, the development of alternatives is
being facilitated by the rapid development of bio-
logical techniques, which are being applied to the
search for
-
alternatives in many different labora-
tories. Major contributions to the coordination of
these developments in the United States are being
made by Rockefeller University and The Johns
Hopkins Center for Alternatives to Animal Testing.
The implementation of alternatives is hindered
by various forms of institutional inertia, such as
regulatory schemes (see ch. 7), product liability
law (see ch. 7), and general resistance to change.
Important impediments are the large body of ex-
isting information
—derived from animals—that is
relied on for the interpretation of new data and
the lack of sufficient information to support the
use of alternatives.
CHAPTER 8 REFERENCES
1.
Adolphe
M.,
Pointet,
Y.,
Onot,
W., et
al.,
“Use of
Fibroblast
Cell Culture for the Study of Wound
Healing Drugs,
’’Znt.
~.
Cosmetic
Sci.
6:55-58,
1984.
2.
Altman,
P.L.
(cd.),
%thokgy
of Laboratory Mice
and
Rats
(New York: Pergamon Press, 1985).
3. Ames, B. N., McCann, J., and Yamasaki, E., “Meth-
ods for Detecting Carcinogens and Mutagens With
the
Sahnonella/Mammalian
Microsome
Mutagenic-
ity Test, ”
Mutat.
Res.
31:347-364,
1975.
4.
Auletta,
A., Genetic Toxicologist, U.S. Environ-
mental Protection Agency, Washington, DC, per-
sonal communication, 1984.
5.
Banerjee,
S. K., and
Ada],
T,, “Ascorbic Acid
in
the
Pars Intercerebralis Cells of Grasshopper: Its Con-
centrations During Induced Accumulation of De-
pletion of Neurosecretory Substances)
’’Anat.
Anx.
134:378-381,
1983.
6.
Bartsch,
L.,
Malaveille,
C.,
Camus,
A.M., et al., “Vali-
dation and Comparative Studies on 180 Chemicals
with S. typhimurium Strains and v79 Chinese
Hamster Cells in the Presence of Various Metabo-
lizing Systems,”
Mutat.
l?es.
76:1-50,
1980.
7.
Berky,
J., and Sherrod, C.
(eds.),
In
Vitro Toxicity
Testing
(Philadelphia, PA: Franklin Institute Press,
1977).
8. Best, J.B.,
Morita,
M.,
Ragin, J., et al., “Acute Toxic
Responses of the Freshwater Planarian
Dugesia
dorotocephala
to
Methylmercury,’
’l?ull.
Environ.
Contain.
Toxicol.
27:49-54,
1981.
9,
Blot, W. J.,
“Developing
Clues
to Environmental
Cancer: A
Stepwise
Approach With the Use of Can-
cer
Mortality Data,
’’Envir.
Health
Perspect.
32:53-
58 (1979).
10.
Borsetti,
A., Staff Scientist, U.S. Department of
192 “ Alternatives to Animal Use in Research, Testing, and Education
11
Health and Human Services, Food and Drug Ad-
ministration, Office of Science Coordination, Be-
thesda, MD, personal communication, Jan. 17,
1985.
Bournais-Vardiabasis, N.,
Teplitz,
R. L.,
Chernoff,
G. F., et al., “Detection of
Te~atogens
in the
Dro-
sophila
Embryonic Cell Culture Test: Assay of 100
Chemicals,”
Teratology
28:109-122, 1983.
12. Boyden, S.,
‘(The Chemotactic Effect of Mixtures
of Antibody and Antigen on
Polymorphonuclear
Leukocytes,” J.
Exp.
Med.
115:453-466,
1962.
13.
British Pharmacopoeia Commission, Submission
to the Advisory Committee to the Cruelty to Ani-
mals Act, 1876, London, 1977.
14. British Toxicology Society Working Party on Tox-
icity, “A New Approach to the Classification of
Substances and Preparation on the Basis of Their
Acute Toxicity,” Hum.
Toxicol.
3:85-92,
1984.
15.
Brown, V. K., “Acute Toxicity,
’’itnimals
and Aher-
natives
in
Toxicitey
Testing,
M. Balls, R.J.
Riddell,
and
A.N.
Worden
(eds,
) (New York: Academic
Press, 1983).
16. Brown, M. M., Wassom, J. S., Mailing, H. V., et al.,
“Literature Survey of Bacterial, Fungal, and
Dro-
sophila
Assay Systems Used in the Evaluation of
Selected Chemical Compounds for Mutagenic Ac-
tivity,” J.
Nat],
Cancer Inst.
62:841-871,
1979.
17, Bruce, R.D., “An
Up-anddown
Procedure for
Acute Toxicity Testing, ’’llmd.
Appl.
Toxicol.
5:151-
157, 1985.
18.
Brusick,
D.J., Principles of Genetic Toxicology
(New York: Plenum Press,
1980).
19.
Brusick,
D.J., ‘
(
Mutagenicity and Carcinogenicity
Correlations Between Bacteria and Rodents, ’’Ann.
N.Y.
Acad.
Sci.
164:176,
1983.
29.
Burton, A.B.G., York, M., and Lawrence, R. S., “The
In Vitro Assessment of Severe Eye Irritants, ’’Food
Cosmet.
Toxicol.
19:471-480,
1981.
21.
Chan,
K. Y., “An In Vitro Alternative To the Draize
Test, ’’Alternatives to the
Draize
Eye Test,
A. Gold-
berg (cd.) (New York: Mary Ann Liebert, Inc.,
1985).
22,
Chemical Week,
“Animals in Testing; How the CPI
Is Handling a Hot Issue,”
135(23):36,
1984.
23.
Clive,
D., Johnson, K.O., Spector, J.F.S., et al.,
“Vah-
dation and Characterization of the
L5178Y/TK
+/-
Mouse Lymphoma Mutagen Assay
System,
’’Mutat.
I?es.
59:61-108,
1979.
24.
Cloyd,
G. Gilbert, Director, Product Development,
25
Bone Metabolism Products, Norwich Eaton Phar-
maceuticals, Inc., Norwich, NY, personal commu-
nication, 1985.
Cooper, J. F.,
Levin,
J., and Wagner, H. N., “Quan-
titative Comparison of In Vitro and In
Vivo
Meth-
ods for the Detection of
Endotoxic,
”
J. Lab.
Clin.
Med.
78:138-148,
1971.
26.
27.
28.
29.
30.
31.
32.
33,
34.
Creech,
J. L., and Johnson, M. N.,
“Angiosarcoma
of Liver in the Manufacture of Polyvinyl Chloride,”
J.
Occup.
Med.
16:150,
1975.
Dagani,
R., “In-Vitro Methods May Offer
Aherna
-
tives to Animal Testing, ” Chem.
Eng.
News 62
(46):25-28,
1984.
Dean, B. J., and Hodges, P., “Short-Term Tests for
Genotoxicity,’’
Animals and
Ahernatives
in
Toxic-
ily
Testing, M.
Balls,
R. J. Ridden, and
A.N.
Wor-
den
(eds.)
(New York: Academic Press, 1983).
Deichmann, W
.B.,
and
Leblanc,
T.J., “Determina-
tion of the Approximate Lethal Dose With Six Ani-
mals)” J.
Ind.
Hyg.
Toxicol.
25:415-417,
1943.
De Serres, F.J., and Mailing, H. V., “Measurement
of Recessive Lethal Damage Over the Entire
Ge-
nome and Two Specific Loci of the ad-3 Region
of
Neurospora
crassa
With a Two Component Het -
erokaryon,
”
Chemical Mutagens, Principles and
Methods for Their Detection,
Vol.
H,
A.
Hollaender
(cd.) (New York: Plenum Press,
1971).
Dewar, A.J.,
“Neurotoxicity,”
Animals and Aher-
natives in Toxicity Testing,
M.
Balk,
R J.
Riddell,
and
A.N.
Worden
(eds.)
(New York: Academic
Press, 1983).
Dezfulian,
M., and Barlett, J. G., “Selective Isola-
tion and Rapid Identification of
Clostridium
botu
-
linum;
Type A and Type B by Toxin Detection,”
J.
Clin.
Microbiol.
2:231-233,
1985.
Dixon, W. J., and Mood, A. M., “A Method of Ob-
taining and Analyzing Sensitivity Data,’
)
J.
Am. Stat.
AsSOC.
43:109-126,
1948.
Doull,
J.,
Klassen,
C. D., and
Amdur,
M.O.
(eds.)
Casserett and
DouWs
Toxicology: The Basic Science
of Poisons (New
York: Macmillan Publishing Co.,
2d cd., 1980).
35. Eakins, K. E., “Prostaglandins and the
Eye,’
’Prosta
-
36
37
glandins:Physiological,
Pharmacolo#”caland
Path-
dog”cal
Aspects,
S.M.M.
Karim
(cd.) (Baltimore,
MD: University Park Press, 1976).
Enslein, K., Lander, T. R., Tomb, M.E., et al., Bench-
rnark
Papers in Toxicology: Vol. 1, A Predictive
Model for Estimating Rat
Oral
LD~O
Values
(Prince-
ton, NJ: Scientific Publishers, Inc., 1983).
Evans, H.J., and
O’Riordan,
M. L., “Human Periph-
eral Blood Lymphocytes for the Analysis of Chro-
mosome Aberration in Mutagen Tests)”
Handbook
of Mutagenicity Test Procedures,
B.J.
Kilbey,
M.
Legator, W. Nichols, et al.
(eds.)
(Amsterdam:
El-
sevier/North
Holland, 1977).
38. Freeberg, F. E., Griffith, J. F., Bruce, R. D., et al.,
“Correlation of Animal Test Method With Human
Experience for Household Products,”J.
Toxicol.
-
Cut. Ocular
Toxicol.
1:53-64,
1984.
39.
Fry, J. R., and Bridges, J. W., “The Metabolism of
Xenobiotics in Cell Suspensions and Cell Cultures,”
Progress in DrugMetabolism, Vol. 2,
J.W.
Bridges
Ch.
8—Alternative to Animal Use in Testing ● 193
and
L.F.
Chasseand
(eds.)
(New York: John Wiley
& Sons, 1977).
40.
Fund for the Replacement of Animals in Medical
Experiments,
“Report of the FRAME Toxicity Com-
mittee, ”
Animals and Alternatives in Toxicity Test-
ing,
M. Balls,
R.J.
Ridden, and A. N’.
Worden
(eds.)
(New York: Academic Press, 1983).
41. Goldberg, A. M.,
“Mechanisms
of
Neurotoxicity as
Studied
in
Tissue Culture Systems,”
Toxicology
17:201-208,
1980.
42. Goldberg,
A. M., Director, The Johns Hopkins Cen-
ter for Alternatives to Animal Testing, Baltimore,
MD, persona] communication, 1985.
43. Griffith, J. F., Nixon, G. A., Bruce, R. D., et al., “Dose-
Response Studies With Chemical Irritants in the
Albino Rabbit Eye as a Basis for Selecting optimum
Testing Conditions for Predicting Hazard to the
Human Eye,”
Toxicol.
Appl.
Pharmaco).
55:501-13,
1980.
44.
Grisham,
J. W., “Use of Hepatic Cell Cultures to De-
tect and Evaluate the Mechanisms of Action of
Toxic Chemicals,
’’lnt.
Rev.
Exp.
Pathol.
20:123-210,
1979.
45.
Guy, R. H., and Fleming, R., “Transport Across a
Phospholipid
Barrier, ”
J.
Colloid
Interface
Sci.
83:130-137,
1981.
46.
Hassid,
A., and Levine, L., “Induction of Fatty Acid
Cyclooxygenase
Activity in Canine Kidney
Cells
(MDCK)
by
Benzo(a)
Pyrene,” J.
Biol.
Chem.
252:
6591-6593,
1!377.
47.
Healey,
G. F., “Statistical Contributions to Experi-
mental
Design, ’’Animals and Alternatives in
Tox-
ici(.y
Testing,
M. Balls,
R.J.
Ridden, and
A.N.
Wor-
den
(eds.)
(New York: Academic Press, 1983).
48.
Hertzfeld,
H. R., and Myers, T. D., “Alternatives to
Animal Use in Testing and Experimentation: Eco-
nomic and Policy Considerations, ” contract report
prepared for the Office of Technology Assessment,
U.S. Congress, January 1985.
49. Hill, J.,
Cautions Against the Immoderate Use of
Snuff
(London: Baldwin and Jackson, 1761).
50.
Huot,
R., Fodart, J., Nardone, R., et al., “Differen-
tial Modulation of Human
Chorionic
Gonadotropin
Secretion by Epidermal Growth Factor in Normal
and Malignant Placental Cultures, ”
J.
Clin.
EzIdo-
crinol.
Metab,
53:1059-1063,
1981.
51. Johnson,
A. W., ‘(Use of Small Dosage and
Corneal
Anesthetic for Eye Testing In
Vivo)’’Proceedings
of the
CTFA
Ocular Safety Testing Workshop: In
Vivo
and
In
Vitro Approaches
(Washington, DC:
Cosmetic, Toiletry, and Fragrance Association,
1980).
52. Johnson, H.J., Northup, S.J., Seagraves, P. A., et al.,
“Biocompatibility
Test Procedures for Polymer
Evaluation In Vitro: I. Comparative Test System
Sensitivity,” J.
Biomed.
Mater. Res.
17:571-586,
1983.
53.
Johnson, H.J., Northup, S.J., Seagraves, P. A., et al.,
“Biocompatibility
Test procedures for Materials
Evaluation In Vitro: II. Quantitative Methods of
Toxicity Assessment, ”
J.
Biomed.
Mater. Res.
19:489-508,
1985.
54.
Kaufmann, J. J.,
Koski,
W. S., Hariharan, P. C., et
al., “Theoretical and Quantum Prediction of Toxic
Effects,” Drug Metab. Rev.
15:527-556,
1984.
55.
King, L. A., and Moffatt, A. C., “Hypnotics and Seda-
tives: An Index of Fatal Toxicity, ”
Lancet
11:387-
78, 1981.
56.
Kligman,
A.M., “Assessment of
Mild
Irritants,
’’Prin
-
ciples
of Cosmetics for the Dermatologist,
P. Frost
and
S.N.
Horwitz
(eds.
) (St Louis, MO:
C.V.
Mosby,
1982).
57. Knowles, B. B., Howe, C. C., and Aden, D. P., “Hu-
man
Hepatocellular
Carcinoma Cell Lines Secrete
the Major Plasma Proteins and Hepatitis B Surface
Antigen)” Science
209:97-99,
1980.
58.
Lave, L., Omenn, G., Hefferman, K., et al., ‘
(
Model
for
selecting
Short Term Test of
Carcinogenicity,
”
J. Am.
CO1l.
Toxicol.
2:125-130,
1983.
59.
Leighton, J., Nassauer, J., and Tchao, R., “The Chick
Embryo in Toxicology: An Alternative to the Rab-
bit Eye,” Food
Chem.
Toxicol.
23:293-298,
1985.
60.
Leighton, J., Nassauer, J., Tchao, R., et al., “Devel-
opment of a Procedure Using the Chick Egg as an
Alternative to the Draize Rabbit Test, ”
Product
Safety Evaluation,
A.M. Goldberg (cd.) (New York:
Mary Ann Liebert, Inc., 1983).
61. Lorke, D., “How Can We Save Animals in Toxicity
Testing,”
Progress Without Pain
(Lord Dowding
Fund, National Anti-Vivisectionist Society, Ltd.,
London) 22: 1984.
62. MacMahon, B., and Pugh, T. F.,
Epidemiology: Prin-
ciples and Methods
(Boston, MA: Little, Brown &
co., 1970).
63. Marks, R., “Testing for Cutaneous Toxicity, ”
Ani-
mals
and Alternatives in Toxicity Testing,
M. Balls,
R.J.
Ridden, and
A.N.
Worden
(eds,)
(New York:
Academic Press, 1983).
64. Martin, Y, C.,
QuantitativeDru gDesign
:A
Critical
Introduction (New York: Marcel
Dekker,
Inc.,
1978).
65. Maurice, D., “Pain and Acute Toxicity Testing in
the Eye, ”
Alternatives to the Draize
EwVe
Test,
A.
Goldberg (cd.) (New York: Mary Ann Liebert, Inc.,
1985).
66. McCann, J.,
Choi,
E., Yamasaki, E., et al., “Detec-
tion of Carcinogens as Mutagens in the
Salmonella/
Microsome
Tests: Assay of 300 Chemicals)”
Proc.
Natl.
Acad.
Sci.
USA
72:5135-5139,
1975.
67.
McCulley,
J. P.,
“Chairman’s Summary,”
Alterna
-

194 ● Alternatives to Animal Use in Research, Testing, and Education
tives
to the
DraizeEye
Test:
A]ternativeMethodism
Toxicology, Vol. 3,
A. Goldberg (cd.) (New York:
Mary Ann Liebert, Inc., 1985).
68.
Miletich,
D.J., Khan, A., Albrecht, R. F., et al., “Use
of Heart Cell Cultures as a Tool for the Evaluation
of
Halothan
Arrhythmia,”
Toxicol.
Appf.
Z%arrnacol.
70:181-187,
1983.
69.
Molingengo,
L., “The Curve Doses v. Survival Time
in the Evaluation of Acute Toxicity, ” J.
Pharm.
Pharmacol.
31:343-344,
1979.
70.
Morrison,
J.K,
Quinton, R. M., and
Reinert,
H., “The
Purpose and Value of
LDSO
Determinations, ’’Mod-
ern Trends in Toxicology, Vol. I,
E. Boyland and
R.
Goulding
(eds.)
(London: Butterworths, 1968).
71.
Muller,
H., and
Kley,
H. P., “Retrospective Study
of the Reliability of an Approximate
LD~O
Deter-
mined With a Small Number of Animals,”
Arch.
Toxicol.
51:189-196,
1982.
72. Nardone, R. M., and Bradlaw, J., “Toxicity Testing
With In Vitro Systems:
1.
Ocular Tissue Culture,”
J.
Toxicol.<ut.
Ocular
Toxicol.
2:81-98,
1983.
73.
Nardone, R.M., and
Ouellette,
L.A., %copeof
‘Aher-
natives’: Overview of the State of the Art, ” con-
tract report prepared for the Office of Technol-
ogy Assessment, U.S. Congress, July 1984.
74. National Research Council,
Models
for Biomedi-
75.
76.
77.
78.
79.
80,
81.
82,
cal
Research: A New Perspective
(Washington, DC:
National Academy Press, 1985).
Neal, R. A., President, Chemical Industry Institute
of Toxicology, Research Triangle Park, NC, per-
sonal communication, June 1985.
Nelson, K. F., and Acosta, D., “Long-Term Mainte-
nance and Induction of Cytochrome P-450 in Pri-
mary Cultures of Rat
Hepatocytes,’’Biochem,
Phar-
macol.
31:2211-2214,
1982.
New Scientist,
“Human Placenta Can Test Drug
Safely,”
1417:20,
Aug. 16, 1984.
Northup, S.J., “Perspectives on Alternative Meth-
ods of Toxicological
Testing,”J.
Parenter. Sci.
Tech-
nol.
37:225-226,
1983.
Northup, S.J., “Mammalian
Cell
Culture Models,”
Handbook of
Biomateria]s
Evaluation, A. Von
Recum (cd.) (in press).
Organization for Economic Cooperation and De-
velopment,
Guidelines for Testing of Chemicals,
and addenda (Paris:
1981).
Paine, A.J.,
Hockin,
L.J,
and Allen, C. M., “Long
Term Maintenance and Induction of
Cytochrome
P-450 in Rat Liver
Cell
Culture,
’’Biochem.
Pharmaco].
31:1175-1178,
1982.
Parry, J.M., Parry E.
M,,
and Parrett, J .C., ‘(Tumor
Promoters
Indu~e
Mitotic Aneuploidy in Yeast,”
Nature
294:263-265,
1981.
83.
Philips, L., Steinberg, M., Maibach, H. I., et al., “A
Comparison of Rabbit and Human Skin Response
to Certain Irritants,”
Toxicol.
Appl.
Pharmacol.
21:369-382,
1972.
84.
Pomerat, C. M., and Leake, C.D., “Short Term Cul-
tures for Drug Assays: General Considerations,”
Ann.
N.Y.
Acad. Sci.
58:1110-1128,
1954.
85.
Pott,
P.,
Chirurg”cal
Observations Relative to the
Cataract, the
PoI’ypus
of the Nose, the Cancer of
the Scrotum, the Different
R2”nds
of Ruptures, and
the Mortification of the Toes and Feet
(London:
Hawes, Clarke, and Collins, 1775).
86. Prasad, R., Shopsis, C., and Hochstadt, J., “Nutri-
ent Transport in a Bovine Lens
Epithelial
Cell Line,”
J,
Cell
Physiol.
107:231-236,
1981.
87.
Ramazzini, B.,
Diseases of Workers, 1700
(trans-
lation of the Latin text of 1713 by
Wilmer
Cage
Wright) (Chicago: University of Chicago Press,
1940).
88.
Rekker,
R. F.,
“LD~O
Values: Are They About to Be-
come Predictable?”
TIPS
383-384,
October 1980.
89. Rose, S. L., and Jurs, P.C.,
“Computer Assisted
Studies of Structure-Activity Relationships of
N-
nitroso
Compounds Using Pattern Recognition, ”
J. Med. Chem.
25:769-776,
1982.
90.
Rowan, A. N.,
Of Mice, Models, and Men: A
Criti-
cal
Evaluation of
Animal
Research
(Albany, NY:
State University of New York Press, 1984).
91. Rowan, A. N., and Goldberg, A. M., “Perspectives
92.
93.
94.
on Alternative to Current Animal Testing Tech-
niques in
Preclinica]
Toxicology,”
Ann. Rev.
Phar-
macol.
Toxico].
25:225-247,
1985.
Sabourin,
T.D., and Goss, L.B.,
Study of Aherna -
tive Species
for
Biologl”cal
Testing,
final report to
the Energy Analysis and Environment Division of
the Electric Power Research Institute,
Battelle
Lab-
oratories, Columbus, OH, 1984.
Sabourin,
T.D.,
Carlton,
B.D.,
Faulk,
R.T., et al.
(Bat-
telle
Laboratories), “Animal Testing for Safety and
Effectiveness,” contract report prepared for the
Office of Technology Assessment, U.S. Congress,
1985.
Schultz, E., and Fuchs, H., “A New Approach to
Minimizing the Number of Animals Used in Acute
Toxicity Testing and Optimizing the Information
of Test Results, ’’Arch.
Toxicol.
51:197-220, 1982.
95.
Sharpe, R., ‘
(
Four Reasons Why a Rabbit Should
Not Be Turned Into a Guinea
Pig,’ ’Progress With-
out Pain
(Lord Dowding Fund, National Anti-Vivi-
section Society, Ltd., London) 22: 1984.
96.
Shopsis, C., Borenfreund, E.,
Walberg,
J., et al.,
“In Vitro
Cytotoxicity
Assays as Potential Alterna-
tives to the Draize Ocular
Irritancy
Test,
’’Aherna
-
tive Methods in
Toxicolo~:Alterna
tive
Approaches,
A.M. Goldberg (cd.) (New York: Mary Ann
Leibert,
Inc., 1984).
97. Simpson, J. M.,
Sowell,
Z. L.,
Sarley,
J.T., et al.,
“Ef-
Ch. 8—Alternative to Animal Use in Testing “ 195
98.
99
<
100.
101.
102,
fects
of Implanted Medical Device Materials on Rat
Peritoneal
Macrophages,”
paper presented at 4th
Annual Meeting of American College of Toxicolo-
gists, Washington, DC, Nov. 30-Dec. 2, 1983.
Simpson, J. M.,
Sowell,
Z. L.,
Sarley,
J.T., et
al.,
“Cell
Culture Methods for Detecting
Immunotoxicity
of
Synthetic Polymers,” paper presented at meeting
of Society for
Biomaterials,
Washington, DC, Apr.
27-May 1, 1984.
Singer, G. M., Taylor, H
.W.,
and
Lijinsky,
W,,
“Lipo-
solubility
as an Aspect of Nitrosamine
Carcino-
genicity~uantitative
Correlations and Qualitative
Observations,”
Chem.
Biol.
Znteract.
19:133-142,
1977.
Spira,
H., “Coordinator’s Report ‘83, ” Coalition to
Abolish the
LD,O,
New York, June 1983.
Stark, D. M., and Shopsis, C., “Developing
Aherna
-
tive Assay Systems for Toxicity Testing,”
Ann.
N.
Y.
Acad.
Sci.
406:92-103,
1983.
Stark,
D. M., Shopsis, C., Borenfreund, E., et al.,
“Alternative Approaches to the Draize Assay:
Che-
motaxis,
Cytology, Differentiation, and Membrane
Transport Studies, ”
Alternative Methods in Toxi-
cology: Product
Safetev
Et~a)uation,
A.M. Goldberg
(cd.) (New York: Mary Ann Liebert, Inc., 1983).
103. Swanston, D. W., “Eye Irritancy
Testing, ’’Animals
and Alternatives in Toxicity Testing,
M. Balls, R.
J. Ridden, and
A.N.
Worden
(eds.
) (New York: Aca-
demic Press, 1983).
104. Task Force of Past Presidents, “Animal Data in Hay
and Evaluation, ”
Fund.
Appl.
Toxicol.
2:101-107,
1982.
105.
Tattersall, M. L.,
“Statistics and the
LD.O
Test, ”
Arch.
Toxicol.
[suppl.]
5:267-270,
1982.
106. Thomas, D. J., “Liver Cells Used in Toxicity Tests, ”
107
108
109.
The Johns Hopkins Center for Alternatives to Ani-
mal Testing,
2(2):3,
1984.
Thompson, W.
R,,
‘{Use of Moving Averages and
Interpolation to Estimate Median-Effective Dose:
1. Fundamental Formulas, Estimation of Error, and
Relation to other Methods,
’’l?acteriol.
Rev. 11:115-
145, 1947.
Thurman, R.G., and Reinke, L. A., “The Isolated Per-
fused Liver: A Model to Define Biochemical Mech-
anisms of Chemical Toxicity, ” Reviews
in
Biochem
-
ical
Toxicology,
t~ol.
1,
E.
Hodgson,
J.R.
Bend, and
R.M.
Philpot
(eds.)
(New York:
Elsevier,
1979).
Tute,
M .S., “Mathematical
Modeling, ’’Animals
and
Alternatives in
Toxicitev
Testing,
M:
Balls,
R.J.
Rid-
den, and
A.N.
Worden
(eds.)
(Ne\v
York: Academic
Press, 1983).
110.
Ulsamer,
A. G., Wright, P. L., and
Osterberg,
R. E.,
“A Comparison of the Effects of Model Irritants
on Anaesthetized and Nonanaesthetized Rabbits
Eyes, ”
Toxicol.
App].
Pharmacol.
41:191-192,
1977.
111.
U.S. Congress, Congressional Research Service,
112.
113.
114.
115,
116,
117,
118,
119
<
Cost Benefit Analysis in Federal Regulations: A Re-
view and Analysis of Developments, 1978-1984,
Pub. No. 84-74E (Washington, DC: May 14, 1984).
U.S. Congress, Office of Technology Assessment,
Assessment of Technologies for Determining Can-
cer Risks From the Environment,
OTA-H-138
(Washington, DC: U.S. Government Printing Of-
fice, June 1981).
vodra,
W. W., “Paper NDAs and Real Problems, ”
Food Drug
Cosmet.
Law J.
39:356-384,
1984.
Vogel, E., and Rama, C.,
“Mutagenesis Assays With
Drosophila, “Long-Term and Short -Term Screen -
ing Assays for Carcinogens: A Critical Appraisal
(Lyon,
France: International Agency for Research
on Cancer, 1980).
Vogel, E., and
Sobels,
F., “The Function of
Drosoph-
ila
in Genetic Toxicology Testing, ”
ChemicalMuta
-
gens: Principles and Methods for Their Detection,
Vol.
IV,
A.
Hollander
(cd.) (New York: Plenum
Press, 1976).
Water, M., U.S. Environmental Protection Agency,
Washington, DC, personal communication, 1984.
Weary, M., and Pearson, F.,
“Pyrogen
Testing
\Vith
Limulus
Amebocyte
Lysate,
” Med. Device
Diag.
lnd.
2(11):34-39,
1980.
Weil,
C. S., and Scala, R. A., “Study of
Intra-
and
Inter-laboratory Variability in the Results of Rab-
bit Eye and Skin Irritation Test,”
Toxicol.
Appl.
Pharmacol.
19:276-360,
1971.
Williams, S. F., “prediction of Ocular Irritancy Po-
tential From
Dermal
Irritation Test Results, ”
Food
Chem.
Toxicol.
22:157-161,
1984.
120.
Williams, S. J.,
Grapel,
G. J., and Kennedy, G. L.,
“Evaluation of Ocular
Irritancy Potential:
Intra-
laboratory Variability and Effect of
Dosage–Vol
-
ume,
”
Toxicol.
Lett.
1:235-241,
1982.
121.
Wishnok,
J. S., Archer, M. C., Edelman, A. S., et
122
123.
a],,
“N-Nitrosamine
Carcinogenicity
-Quantitative
Hansch-Taft Structure Activity Relationship ,“
Chem.
Biol.
Interact.
20:43-54,
1978.
Wright, E. M.,
Marcell,
K. L., and Woodson, J. F.,
“Animal Pain: Evaluation and Control, ”
Lab Anim.
14(4):20-35,
1985.
Yuan,
M. and
Jurs,
P.C., “Computer-Assisted
Struc
-
ture-Activity
Studies of Chemical Carcinogens: A
Polycyclic
Aromatic Hydrocarbon Data Set, ”
Tox
-
icol.
Appl.
Pharmacol.
52:294-312,
1980.
124.
Yuta,
K., and Jurs, P. C.,
“Computer-Assisted
Structure-Activity Studies of Chemical Carcino-
gens: Aromatic
Amines,”
J. Med.
Chem.
24:241,
1981.
125.
Zbinden,
G., Progress in
Toxico/ogv
(Berlin:
Springer-Verlag, 1973).
126. Zimmerman, F. K., “Mutagenicity Screening With
Fungal
Systems, ’’Ann. N. Y.
Acad.
Sci.
407:186-196,
1983.
