
CLINICAL RESEARCH STUDIES
A comparison of covered vs bare expandable stents
for the treatment of aortoiliac occlusive disease
Bibombe P. Mwipatayi, MMed (Surg), FCS (SA), FRACS,
a,b
Shannon Thomas, MBBS (Hons),
a
Jackie Wong, MPH,
a
Suzanna E. L. Temple, PhD, MBA,
a,c
Vikram Vijayan, MRCS, FRCS,
a
Mark Jackson, MD, FRACS,
d
and Sally A. Burrows, BMath Grad Dip Med Stat,
e
on behalf of the
Covered Versus Balloon Expandable Stent Trial (COBEST) Co-investigators,* Perth, Western Australia
and Gold Coast, Queensland, Australia
Objective: This trial was conducted to determine if covered stents offer a patency advantage over bare-metal stents in the
treatment of aortoiliac arterial occlusive disease.
Methods: The Covered Versus Balloon Expandable Stent Trial (COBEST), a prospective, multicenter, randomized
controlled trial, was performed involving 168 iliac arteries in 125 patients with severe aortoiliac occlusive disease who
were randomly assigned to receive a covered balloon-expandable stent or bare-metal stent. Patient demographic data,
clinical signs and symptoms, TransAtlantic Inter-Society Consensus (TASC) classification, and preprocedure and
postprocedure ankle-brachial index measurements were recorded. The primary end points included freedom from binary
restenosis and stent occlusion of the treated area, as determined by ultrasound imaging or quantitative visual angiogra-
phy, or both. Postprocedural follow-up was at 1, 6, 12, and 18 months.
Results: Aortoiliac lesions treated with a covered stent were significantly more likely to remain free from binary restenosis
than those that were treated with a bare-metal stent (hazard ratio [HR], 0.35; 95% confidence interval (CI), 0.15-0.82;
P ⴝ .02). Freedom from occlusion was also higher in lesions treated with covered stents than in those treated with a
bare-metal stent (HR, 0.28; 95% CI, 0.07-1.09); however, this did not reach statistical significance (P ⴝ .07). Subgroup
analyses demonstrated a significant difference in freedom from binary restenosis for covered stents in TASC C and D
lesions compared with a bare stent (HR, 0.136; 95% CI, 0.042-0.442). This difference was not demonstrated for TASC
B lesions (HR, 0.748; 95% CI, 0.235-2.386).
Conclusions: COBEST demonstrates covered and bare-metal stents produce similar and acceptable results for TASC B
lesions. However, covered stents perform better for TASC C and D lesions than bare stents in longer-term patency and
clinical outcome. ( J Vasc Surg 2011;54:1561-70.)
Aortoiliac arterial occlusive disease is frequently en-
countered in the management of lower limb vascular insuf-
ficiency. Surgical reconstruction of the aortoiliac segment
was initially performed using thromboendarterectomy
1
;
however, advances in graft technology have made bypass
procedures more commonly performed and durable. Distal
aortic bypass has a reported surgical mortality of 4%, with
major complications seen in up to 21% of patients.
2-7
From the Department of Vascular Surgery, Royal Perth Hospital, Perth,
Western Australia
a
; School of Surgery, Faculty of Medicine, Dentistry and
Health Sciences, University of Western Australia, Perth, Western Austra-
lia
b
; Lung Institute of Western Australia and School of Medicine and
Pharmacology, University of Western Australia, Perth, Western Australia
c
;
Department of Vascular Surgery, Gold Coast Hospital, Gold Coast,
Queensland
d
; and Department of Biostatistics, School of Medicine and
Pharmacology, Royal Perth Hospital and University of Western Australia,
Perth, Western Australia.
e
*COBEST co-investigators: Dr Marek Garbowski, Dr Glen Benveniste, Dr
Michael Denton, Dr John Anderson, Dr Steve Dubenec, Dr Michael
Neale, Dr Vikram Puttaswamy, Prof John Fletcher, and Prof Geoffrey
White.
Atrium Medical Corporation provided funding for the employment of a
research assistant and had no role in study design, data collection, data
interpretation, or writing of the report. The corresponding author had full
access to all the data in the study, and the authors had final responsibility
for the decision to submit for publication.
Competition of interest: Dr Mwipatayi and Dr Jackson have received
research-funding assistance from Atrium Medical Corporation.
Presented at the VIVA meeting, Las Vegas, Nev, October 19-23, 2009; the
LINC meeting, Leipzig, Germany, January 25-28, 2010; C3 meeting,
Baltimore, Md, June 20-24, 2010; ANZSVS Vascular Meeting, Gold
Coast, State, Country, August 2-5, 2010; 10th China National Annual
Congress of Vascular Surgery, Nanjing, China, September 24-26, 2010;
Veith meeting, New York, NY, November 17-21, 2010; and CACVS
2011 Congress, Paris, France, January 27-29, 2011.
Additional material for this article may be found online at www.jvascsurg.org.
Correspondence: Prof B. Patrice Mwipatayi, University of Western Austra-
lia, School of Surgery, Royal Perth Hospital, Department of Vascular
Surgery. Level 2, MRF Bldg, Perth, Western Australia, Australia (e-mail:
The editors and reviewers of this article have no relevant financial relation-
ships to disclose per the JVS policy that requires reviewers to decline
review of any manuscript for which they may have a competition of
interest.
0741-5214/$36.00
Crown Copyright © 2011 Published by Elsevier Inc. on behalf of the
Society for Vascular Surgery. All rights reserved.
doi:10.1016/j.jvs.2011.06.097
1561
brought to you by COREView metadata, citation and similar papers at core.ac.uk
provided by Elsevier - Publisher Connector

Although a laparoscopic approach may decrease this mor-
tality and morbidity, this technique has yet to become
common place.
8
Currently, the decision to perform an endovascular
procedure or open surgical bypass depends on various
factors, most importantly, the severity and anatomic distri-
bution of disease. The TransAtlantic Inter-Society Consen-
sus (TASC) I and II working group published a consensus
of recommendations for management of peripheral arterial
disease.
9,10
The recommendation for patients with focal or
short lesions (ie, TASC A and TASC B lesions) is endovas-
cular intervention with an evidence basis suggesting long-
term patency rates close to 80% at 2 years and 60% at 5
years.
11,12
Lower patency rates with TASC C and D lesions
led to the recommendation for an open surgical approach,
except in patients deemed to be high-risk.
The use of covered stents to treat aortoiliac occlusive
disease has been proposed as a method to reduce intimal
hyperplasia and improve patency rates.
13-15
Although the
results of numerous reports are promising, present evidence
is insufficient to support their widespread application. Cur-
rently, covered stent use is largely reserved for the treat-
ment of aneurysms, ruptures, and arteriovenous fistulas.
16-18
No published randomized controlled trials (RCTs) to date
have compared the efficacy of covered stents vs bare-metal
stents (BMSs) for aortoiliac occlusive disease. We hypoth-
esized that via intimal exclusion, covered stents will provide
a patency advantage and improved clinical outcome in
advanced iliac occlusive disease compared with BMS.
METHODS
Study design. The Covered Versus Balloon Expand-
able Stent Trial (COBEST) was a prospective, multicenter,
RCT (registration number: ISRCTN89458845) compar-
ing the use of the Advanta V12 balloon-expandable cov-
ered stent (Atrium Medical Corp, Hudson, NH) with
commercially available bare-metal stents (BMS) in patients
with aortoiliac occlusive disease. The study was conducted
between January 2006 and December 2008 with 13 phy-
sicians (11 vascular surgeons and 2 interventional radiolo-
gists) at eight major centers across Australia. The study
design allowed an assessment of the safety and efficacy of
covered stents vs BMSs in the treatment of hemodyna-
mically significant aortoiliac occlusive disease. In particular,
COBEST aimed to determine if the theoretical advantage
of covered stents resulted in clinical improvements.
The institutional review board and ethics committees at
each hospital approved the study protocol, and written
informed consent was obtained from all patients before
enrollment. The criterion for study entry and exclusion are
listed in Table I.
Study end points. The primary study end point was
the rate of binary restenosis (defined by ⱖ50% reduction in
lumen diameter) and freedom from stent occlusion at 18
months. These were determined by duplex ultrasound
(DUS) imaging of the aortoiliac segment, computed to-
mography angiography (CTA), or catheter biplane digital
subtraction angiography (DSA). Patients were assessed
clinically with an ankle-brachial index (ABI) and aortoiliac
arterial DUS at intervals of 1, 6, 12, and 18 months. If the
DUS scan was inconclusive, a CTA or DSA, or both, were
performed using a prespecified protocol to determine if the
primary end point had been achieved.
The secondary end points were determined anato-
mically, clinically, and hemodynamically. Anatomic end
points included stent patency, as assessed by the TASC B,
C, and D classification, stent integrity, and target vessel
revascularization (TVR), defined as any repeat percutane-
ous intervention or surgical bypass of any segment of the
target vessel. The target vessel was defined as the entire
aortoiliac vessel proximal and distal to the target lesion.
TVR was typically driven by clinical evidence of symptoms
or positive stress-induced significant symptoms (treadmill
exercise ABI).
The clinical end points were major amputation above
the ankle and major adverse events resulting in patient
hospitalization or prolongation of existing hospitalization,
significant physical disability, or death. The hemodynamic
end point was a change in ABI between baseline and
measurements at 1, 6, 12, and 18 months.
Randomization. Eligible patients were randomized
to receive a covered stent or a BMS by an online computer-
ized randomization program (https://io.atriummed.com/
Cobest/default.aspx). Unstratified randomization was
used to randomize patients in a 1:1 ratio with a minimiza-
tion algorithm to allow balanced allocation of participants
across intervention groups. All randomized patients were
irrevocably included in the study, regardless of eligibility or
Table I. Inclusion and exclusion criteria for the Covered
Versus Balloon Expandable Stent study
Inclusion criteria
● Men and women aged ⱖ18 years
● Informed consent obtained
● Evidence of TASC B, C, or D lesions
● Hemodynamically significant dissections and recurrent stenosis
after angioplasty
Exclusion criteria
● Life expectancy ⬍12 months (patients had to be followed up
for at least 18 months)
● Uncontrolled hypertension
● TASC A lesion
● Pregnant women or women of childbearing potential who
were not using an effective method of contraception
● Prior enrollment in this trial, or a patient who had had any
procedure performed at the aortoiliac level
● Extensive common femoral artery disease or multiple groin
procedures
● Contraindication to aspirin or clopidogrel usage
● Occluded superficial and profunda femoral arteries
● Mental condition rendering the individual unable to
understand the nature, scope and possible consequences of the
study, or a language barrier preventing the individual from
providing informed consent
● Uncooperative attitude or potential for noncompliance with
the protocol requirements, making study participation
impractical
TASC, TransAtlantic Inter-Society Consensus.
JOURNAL OF VASCULAR SURGERY
December 2011
1562 Mwipatayi et al

if they received the allocated treatment, and were moni-
tored for 18 months. A subgroup of patients had bilateral
lesions and each side of the iliac artery disease (left or right)
was randomized in these patients rather than the patient
per se.
Stents. The investigational devices consisted of the
Advanta V12 covered stent and commercially available
BMSs. The Advanta V12 is encapsulated with expanded
polytetrafluroethylene (ePTFE), premounted on an Aner-
tia noncompliant balloon (Atrium Medical Corp), and
compatible with a 6F to 7F sheath. Stents were sized
perioperatively, with covered and BMS oversized by 1 mm
beyond the calculated diameter of the iliac artery treated.
The BMS used was chosen at the discretion of the
interventionist, but Australian Therapeutic Goods Ad-
ministration approval was required. The bare-metal bal-
loon-expandable stents used included Palmaz Genesis
(Cordis Corp, East Bridgewater, NJ) in 32.5%, Express
LD iliac stent (Boston Scientific, Natick, Mass) in 28%,
Assurant Cobalt iliac stent (Medtronic, Minneapolis,
Minn) in 18.6%, Peiron (Biotronik, Berlin, Germany) in
12.8%, and AVE-Bridge (Medtronic) in 2.3%. The two
self-expandable BMSs used were the Smart (Cordis
Corp) in 3.5% and Edwards Life Stent (Bard Peripheral
Vascular Inc, Tempe, Ariz) in 2.3%. No drug-eluting
stents were used.
Eligible
170 Limbs
Excluded – 2 Limbs
Missing data
Analysed – 81 Limbs
Lost to follow-up – 1 Limb (1 Patient)
1 Month - 1 Limb
6 Months - 0 Limb
12 Months - 0 Limb
18 Months - 0 Limb
V12
83 Limbs
Lost to follow-up – 8 Limbs (8 Patients)
1 Month - 4 Limb
6 Months - 1 Limb
12 Months - 1 Limb
18 Months - 2 Limb
Bare Stent
85 Limbs
Analysed – 78 Limbs
Allocation
Analysis
Follow-Up
Randomised
168 Limbs
(125 Patients)
Enrolment
V12
82 Limbs
Bare Stent
86 Limbs
Baseline
Crossover
–
1 Limb
Fig 1. Flow chart shows the trial profile, including descriptions of the patients enrolled in the study and patients who
were lost to follow-up.
JOURNAL OF VASCULAR SURGERY
Volume 54, Number 6
Mwipatayi et al 1563
Medical therapy. All patients received aspirin (100-
150 mg daily) indefinitely and clopidogrel (75 mg daily) for
a minimum of 1 month after the intervention. A 300-mg
loading dose of clopidogrel was given during or after the
intervention.
Study procedures. Access to the iliac lesion was
achieved at the investigator’s discretion via an antegrade
approach from the contralateral femoral artery with the use
of a dedicated 6F or 7F “cross-over” sheath or via a
retrograde (ipsilateral) approach. A brachial approach was
used at the discretion and experience of the investigator
using a Shuttle sheath (Cook Medical, Bloomington, Ind).
After sheath placement, a heparin bolus (3000-5000 units)
was administered intravenously.
DSA was performed to assess and confirm the DUS
imaging or CTA findings. The patency status of the ipsilat-
eral femoral, popliteal, and infrapopliteal arteries was doc-
umented. After the target lesion was successfully crossed
with a hydrophilic 0.018-inch or 0.035-inch wire, patients
were randomized and received a covered balloon-expandable
stent or a BMS.
At baseline and each follow-up, all patients were strat-
ified by symptoms according to categories outlined previ-
ously by Rutherford et al.
19
The status of the runoff vessels
was determined using an objective scoring system.
19
The
superficial and deep femoral arteries, which are the runoff
vessels for iliac artery procedures, were evaluated by the
presence of an occlusion or stenosis, or if they were patent.
Mandatory clinical evaluations combined with mea-
surement of ABIs were performed at each postprocedural
follow-up at 1, 6, 12, and 18 months. DUS imaging of the
aortoiliac segment was also performed at each visit. Binary
restenosis was defined by a doubling of peak systolic veloc-
ities (PSV) across a lesion or a PSV ⬎300 cm/s with
monophasic Doppler waveforms in the distal common
femoral artery.
20-22
Medical events, hospitalizations, access
interventions, and adverse events were documented.
Statistical analysis. Sample size was calculated using
the method proposed by Armitage et al.
23
Because there
has been no previous RCT comparison between groups
with the two types of stent, an initial limit of noninferiority
between the two groups was estimated to be 10% of the
true population restenosis rate, which we assumed to be 6%.
With power set at 80% and ␣ set at 5% (on a two-sided test),
the number of iliac arteries required was calculated to be 70
in each group, giving a total of 140 limbs. To compensate
for patients lost to follow-up, we included an additional 14
limbs. Thus, the total target sample size was calculated to
be at least 154 limbs. One patient did not receive the
allocated treatment (cross-over treatment). This was a con-
scious decision by the surgeon because the randomized
stent was not available.
Table II. Characteristics of the study patients at baseline
according to treatment groups
Characteristic
a
V12 stent Bare stent
(n ⫽ 83) (n ⫽ 85)
Patients, No. 62 63
Age, years 65.34 ⫾ 1.43 67.21 ⫾ 1.29
Male sex 67.7 57.1
Race
White 96.8 95.2
Aboriginal 1.6 1.6
Asian 1.6 3.2
Side of lesion
Left 59.0 42.4
Right 39.8 56.5
Central 1.2 1.1
Rutherford-Becker
Category 1 3.2 4.8
Category 2 27.4 30.2
Category 3 40.3 50.8
Category 4 24.2 12.7
Category 5 4.8 1.6
Risk factors
Diabetes 24.2 28.6
Hypertension 56.5 71.4
Smoking 59.7 58.7
Hyperlipidemia 41.9 46.0
Chronic renal failure 3.2 3.2
COAD 9.7 9.5
Coronary artery disease 37.1 38.1
Hypercoagulable states 6.5 3.2
Pre-op antiplatelets 88.7 93.7
Aspirin 87.1 84.1
Clopidogrel 9.7 19.0
COAD, Chronic obstructive airway disease.
a
Continuous data are expressed as mean ⫾ standard error of the mean, and
categoric data as number or percentage.
Table III. Clinical and radiologic characteristics of the
study patients at baseline according to treatment groups
Characteristic
a
V12 stent Bare stent
(n ⫽ 83) (n ⫽ 85)
Preoperative assessment
Resting ABI (side of lesion) 0.65 ⫾ 0.03 0.63 ⫾ 0.03
No. 75 78
Duplex scan
Performed 62 (74.7) 65 (76.5)
⬎50% stenosis 87.1 81.5
Occlusion 12.9 18.5
Angiogram
Performed 67 (80.7) 73 (85.9)
TASC B 50.7 72.6
TASC C 34.3 20.5
TASC D 14.9 6.8
CT angiogram
Performed 32 (38.6) 36 (42.4)
TASC B 56.3 63.9
TASC C 31.3 30.6
TASC D 12.5 5.6
Runoff
Performed 74 (89.1) 73 (85.9)
0 0 1.4
1 12.2 5.5
2 20.3 21.9
3 23.0 37.0
4 44.6 34.2
ABI, Ankle-brachial index; TASC, TransAtlantic Inter-Society Consensus.
a
Data are number (%) or mean ⫾ standard error of the mean.
JOURNAL OF VASCULAR SURGERY
December 2011
1564 Mwipatayi et al

Because this patient did not receive the allocated treat-
ment, we undertook two main analyses. An intention-to-
treat analysis was performed for all baseline and primary end
point data for all randomized patients irrespective of
whether the patient received the randomized study device
or the patient’s compliance with the study protocol. A
per-protocol analysis was performed for all subgroup anal-
yses and secondary end point data because the one cross-
over patient’s data did not create any difference between
the intention-to-treat analysis and the per-protocol analy-
sis.
Baseline characteristics of the two groups were de-
scribed using means and standard errors (SE) for continu-
ous data and proportions for qualitative variables. Freedom
from binary restenosis and freedom from stent occlusion at
1, 6, 12, and 18 months were analyzed with the use of
Kaplan-Meier survival estimates. Cox proportional hazards
models were used to determine if differences between the
estimates were significant after establishing the requisite
proportional hazards assumptions were met. Alternative
end points, occlusion, and binary restenosis at 18 months,
were analyzed using logistic regression.
Subgroup analyses of both restenosis end points were
performed by testing the interaction of subgroup and ran-
domized treatment. There were insufficient numbers of
vessels with an occlusion for subgroup analysis to be per-
formed. Logistic regression was also used to investigate the
differences in proportions of patients with limb amputation
between the two randomized groups. Results are reported
as hazard ratios (HR) or odds ratios (OR) with 95% confi-
dence intervals (CI).
When two iliac arteries from the same patient were
randomized, it was necessary to recognize that the vessels
were genetically identical and therefore the outcomes
would be correlated. To adjust for the intracluster correla-
tion, robust sandwich estimators, as proposed by Lin and
Wei (1989),
24
were used to produce cluster adjusted SEs
and P values for all logistic regression and survival analyses.
Analysis of the change in ABI indexes and TVR over time
was performed using a hierarchic longitudinal mixed-models
analysis. The interaction between randomized treatment
and time was used to determine if there were differences
in the treatment slopes over time for the linear ABI model.
The small number of TVR cases precluded investigation of
the interaction in the logistic model.
All hypotheses tests were performed using two-sided
tests, and the critical value for statistical significance was set
at a value of P ⬍ .05. Analyses were conducted using PASW
18 (SPSS, Chicago, Ill), SAS 9.2 (SAS Institute Inc, Cary,
NC), and Stata 11 (StataCorp LP, College Station, Tex)
statistical software.
RESULTS
The trial recruited 125 patients (168 individual iliac
arteries). Randomization assigned 83 vessels to receive a
covered stent and 85 to receive a BMS (Fig 1). Patients
were assessed at the prespecified intervals for 18 months.
One patient in the covered-stent group and eight in the
BMS group were lost to follow-up.
At baseline, demographic and clinical characteristics
were similar between the two groups, with no significant
differences in the frequency of atherothrombotic risk fac-
Fig 2. Kaplan-Meier curves are shown for freedom from binary restenosis for the intention-to-treat population. The
sandwich variance estimate method (Lin and Wei) was used to determine the log-rank test adjusted for clustering (P ⫽
.0243). CI, Confidence interval; HR, hazard ratio.
JOURNAL OF VASCULAR SURGERY
Volume 54, Number 6
Mwipatayi et al 1565
tors or coexisting cardiovascular conditions (Table II).
Most patients in both groups were already receiving anti-
platelet agents, and only three patients were taking oral
anticoagulation medications (Table II). In the BMS group,
Palmaz Genesis, Express LD, Assurant Cobalt, and Peiron
stents were used predominantly.
Preoperative imaging demonstrated 72.6% in the BMS
group had TASC B lesions vs 50.7% in the covered-stent
group (Table III).
Procedural complications included hemorrhage, flow-
limiting dissection, lymph leak, and seroma formation, with
no statistical significance between the groups. Intravascular
anticoagulation was administered to 97.6% of patients dur-
ing the procedure. No conversions to open aortoiliac sur-
gery during the perioperative period were reported. Proce-
dural success, defined as ⬍30% residual stenosis during the
angiographic intervention, was achieved in 100% of cases in
both groups.
Primary outcome. Aortoiliac lesions treated with a
covered stent were significantly more likely to remain free
from binary restenosis at 18 months than those that were
treated with a BMS (HR, 0.35; 95% CI, 0.15-0.82; P ⫽
.02). Binary restenosis was documented in 8 arteries in the
covered-stent group and in 20 in the BMS group (Fig 2).
Complete occlusions of the stented lesion during the
follow-up period occurred in 3 patients in the covered-stent
group and in 10 patients in the BMS group; however, this
difference was not statistically significant (HR, 0.28; 95%
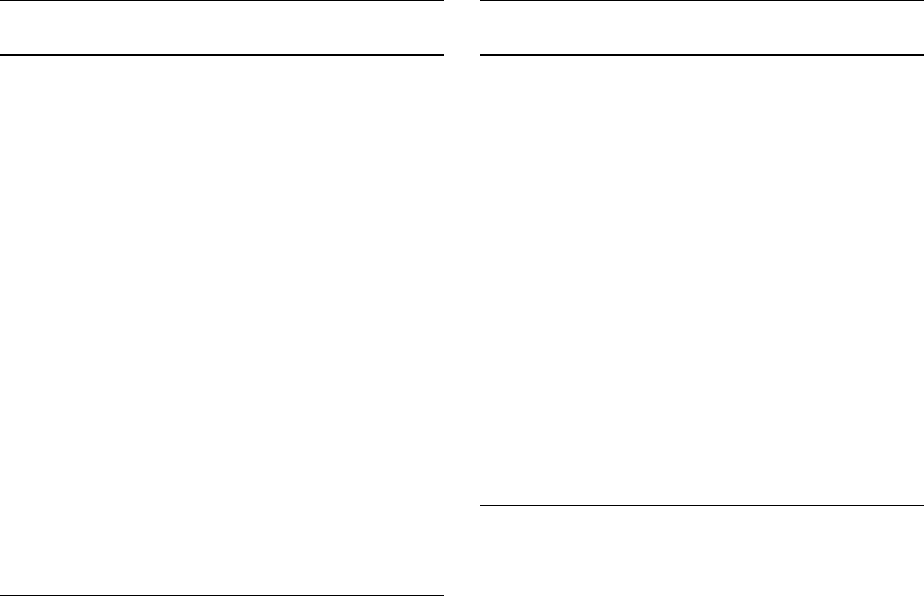
CI, 0.07-1.09; P ⫽ .07; Fig 3).
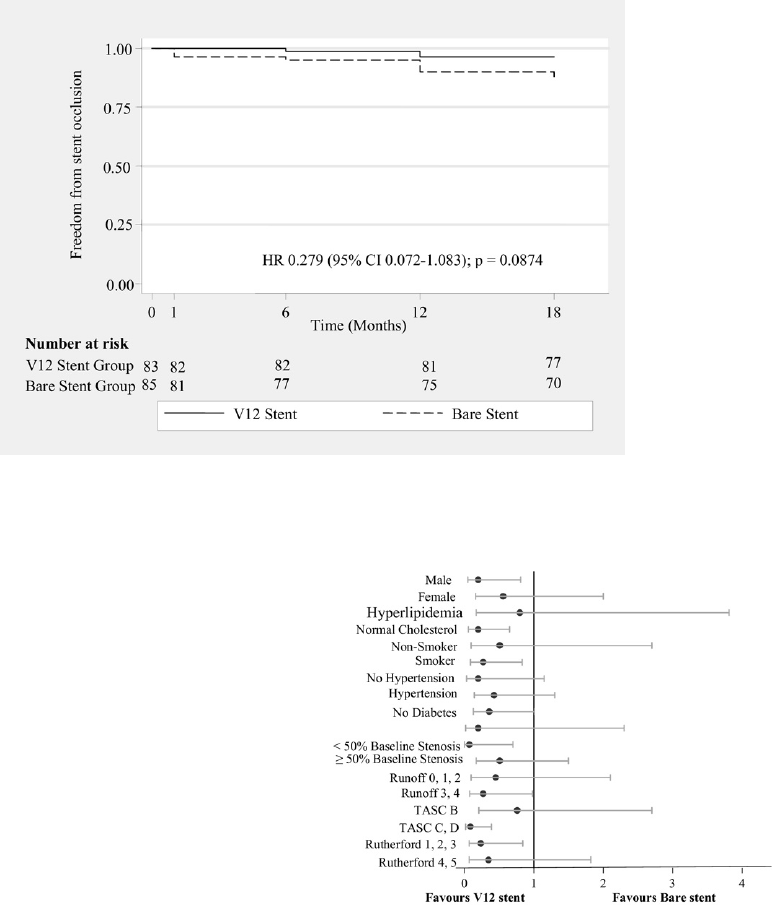
Subgroup analyses. The subgroup analysis specifically
looked at freedom from binary restenosis according to the
initially designated TASC lesions. A significant interaction
was found between the TASC classification and the stent
type for binary restenosis at 18 months (P ⫽ .03). None of
the factors investigated were significant (Fig 4). This study
was not powered to investigate interactions; hence, further
research is required to confirm these results. The Kaplan-
Meier survival estimates showed a statistically significant
benefit when covered stents were used in TASC C and D
Fig 3. Kaplan-Meier curves are shown for freedom from stent occlusion for the intention-to-treat population. The
sandwich variance estimate method was used to determine the log-rank test adjusted for clustering (P ⫽ .0874). CI,
Confidence interval; HR, hazard ratio.
Fig 4. Odds ratios of 18-month restenosis of ⬎50% in selected
patient subgroups. For most of the variables, the 95% confidence
interval crosses the line of identity, indicating lack of statistical
significance. The probability values for interaction indicate that
only TransAtlantic Inter-Society Consensus (TASC) variables in-
teracted on the level of statistical significance with the treatment
modality.
JOURNAL OF VASCULAR SURGERY
December 2011
1566 Mwipatayi et al

lesions compared with BMSs (HR, 0.136; 95% CI, 0.042-
0.442; Fig 5; Appendix Fig, online only). However, this
significant difference in freedom from binary restenosis was
not demonstrated for TASC B lesions (HR, 0.748; 95% CI,
0.235-2.386; Fig 6).
The 44 patients who had bilateral iliac artery stenting
were randomized to receive 43 covered stents (SE, 0.473;
95% CI, 16.399-18.252) and 45 BMSs (SE, 0.970; 95%
CI, 12.304-16.106). The same stent was allocated to both
limbs in 20 of the 44 patients. The overall freedom from
Fig 5. Kaplan-Meier curves are shown for freedom from binary restenosis for the type of stent used according to the
TransAtlantic Inter-Society Consensus (TASC) C/D group. The sandwich variance estimate method was used to
determine the log-rank test adjusted for clustering (P ⫽ .0056). CI, Confidence interval; HR, hazard ratio.
Fig 6. Kaplan-Meier curves are shown for freedom from binary restenosis for the type of stent used according to the
TransAtlantic Inter-Society Consensus (TASC) B group. There is no statistical difference between the two stents in
patients with TASC B lesion. CI, Confidence interval; HR, hazard ratio.
JOURNAL OF VASCULAR SURGERY
Volume 54, Number 6
Mwipatayi et al 1567

binary restenosis demonstrated that there was significantly
less restenosis in covered stents compared with the BMSs
(HR, 0.14; 95% CI, 0.03-0.61; P ⫽ .009). The interaction
of TASC group and randomized stent was not significant
(P ⫽ .23).
Secondary outcomes. ABI measurements during the
follow-up period were not significantly different between
the two groups at 1 and 6 months. However, the difference
became statistically significant at 12 months (P ⫽ .014) and
had marginal significance at 18 months (P ⫽. 06), suggest-
ing long-term improvement in ABIs in the covered-stent
group (Table IV).
TVR during the study period demonstrated that there
was less reintervention in the covered-stent group com-
pared with the BMS group (OR, 21; 95% CI, 0.07-0.64;
P ⫽ .006). Most of the reinterventions were performed at
12 and 18 months (Fig 7). Three patients from the BMS
group underwent aortobifemoral bypass grafting.
Four amputations were performed during the study
period. Two patients with covered stents had below knee
amputation at 18 months, which was likely related to
uncontrollable diabetes mellitus and progression of infra-
genicular arterial disease. Two patients with BMSs had
above knee amputations at 18 months due to a combina-
tion of factors. The difference between the two groups was
not statistically significant (OR, 1.02; 95% CI, 0.089-
11.73; P ⫽ .984).
A left brachial artery approach was used in 10 patients,
with 1 patient requiring the use of the Outback LTD
re-entry catheter (Cordis Corp), with a successful outcome.
None of these patients had any major complications (no
cerebral ischemic event or vessel perforation) related to the
approach used. Multiple associated procedures were per-
formed during iliac artery stenting: femoropopliteal bypass
graft in five patients, common femoral artery endarterec-
tomy in four, and superficial femoral artery stenting in four.
External iliac artery stenting was performed in 14 patients,
with successful outcome at 12 and 18 months.
DISCUSSION
The COBEST trial is the first RCT to compare covered
stents and BMSs in the management of aortoiliac occlusive
disease. Previous studies have compared different types
of BMSs deployed in iliac arteries and failed to demons-
trate any significant differences in technical success and
follow-up outcomes.
25-27
The only published randomized
study comparing primary angioplasty, followed by selective
or primary stent placement in patients with iliac artery
obstructive disease, is the Dutch Iliac Stent Trial (DIST).
The short-term and long-term DIST results indicated that
selective stent placement should be considered as the treat-
ment of choice.
11
Angioplasty is a less invasive alternative treatment to
open bypass surgery and proven efficacy for the treatment
of patients with focal iliac artery stenosis. The procedural
technical success rate has improved significantly (up to
95%), especially when adjunctive stent placement is used.
Patency rates of 80% to 90% after 5 years have been re-
ported for short iliac stenoses, which is comparable with
patency results for open surgery.
28,29
For complex, multi-
focal, or totally occluded atherosclerotic segments of iliac
arteries (C and D lesions), TASC recommends surgery as
the procedure of choice.
10
Since their first applications by Dotter, endovascular
metallic stent placement has been widely used for the
treatment of patients with aortoiliac occlusive disease.
30,31
Vorwerk et al
32,33
reported 4-year patency rates of 78% and
82% for primary and secondary stenting procedures, respec-
tively, in 100 patients. Cikrit et al
34
reported a 5-year
patency rate of 63% in 38 limbs that were treated by Palmaz
stent placement, whereas Palmaz et al
25
initially reported a
92% patency rate at 9 months. Primary 4-year patency rates
as high as 86% were recently reported.
25,31
Covered stents are able to exclude plaque and endothe-
lium, thereby potentially mitigating late luminal loss by
halting migration and proliferation of vascular smooth
muscle cells and inflammatory cells through open stent
struts.
35
This may result in a reduction of restenosis caused
by luminal encroachment from extracellular matrix deposi-
tion intimal hyperplasia.
15,36
BMSs do not provide a
boundary that excludes the underlying plaque from the
lumen, and stent oversizing relative to the reference lumen
causes an increase in neointimal growth. Covered stents
may also offer the benefit of being less thrombogenic than
BMSs. In the United States, no covered stents are currently
approved by the U.S. Food and Drug Administration for
application in the iliac arteries for occlusive disease. How-
ever, homemade covered stents, iliac limbs from modular
aortic stent grafts, and commercially manufactured covered
stents approved for other nonvascular indications have
been placed in the iliac arteries. Because of the bulk of the
additional graft material, covered stents require larger de-
livery systems than BMSs. This may expose the patient to a
higher risk of groin complications.
The restenosis observed in the covered-stent group was
predominantly located at the ends of the stent or outside
the stent. We hypothesis that this may be secondary to
compliance mismatch due to the use of the rigid end of the
covered stent and balloon dilatation causing the increased
stent–artery interface intimal hyperplasia. In addition, the
stent may not cover the lesion fully, resulting in progression
of the atherosclerotic process by activation from balloon
inflation but with the end of the balloon inflated firstly
Table IV. Resting ankle-brachial index (ABI) on side of
the lesion
Resting
ABI
V12 stent Bare stent
PNo. Mean ⫾ SEM No. Mean ⫾ SEM
Baseline 75 .65 ⫾ .03 78 .63 ⫾ .03 .639
1 month 71 .91 ⫾ .03 72 .91 ⫾ .03 .927
6 months 73 .89 ⫾ .02 74 .88 ⫾ .03 .653
12 months 75 .94 ⫾ .02 79 .85 ⫾ .03 .014
18 months 70 .94 ⫾ .02 73 .86 ⫾ .03 .07
SEM, Standard error of the mean.
JOURNAL OF VASCULAR SURGERY
December 2011
1568 Mwipatayi et al

outside of the stent causing a process known as the “dog
bone” effect.
We have identified three major limitations in this study:
First, the different stent types used in the BMS group may
have affected the results and led to bias. It was impractical in
the context of a multicenter study to limit the BMS type.
We suggested current practices and protocols within trial
centers should continue unchanged, with the type of BMS
selected left to the individual operator.
Second, the BMS group had fewer patients with a
TASC D lesion (7%) compared with the covered-stent
group (16.1%), which, despite the randomization, may
have influenced the results.
Third, DUS was the principal imaging tool used for
follow-up, which may have introduced bias because it is
highly operator-dependent. We decided to use DUS imag-
ing because we were reluctant to unnecessarily use invasive
investigations if not clinically required.
CONCLUSIONS
There is increasing evidence from single-center clinical
investigations that patients with complex aortoiliac lesions,
including chronic iliac artery occlusions and occlusion of
the aortoiliac bifurcation, can be treated safely and effec-
tively with a covered stent. The COBEST results demon-
strate that for patients with severe aortoiliac arterial occlu-
sive disease, there is an increased freedom from restenosis
and occlusion with covered stents compared with BMSs at
12 and 18 months. However, long-term durability data
(5-year follow-up) is desirable.
We thank Dr Michael Phillips for his statistical advice.
AUTHOR CONTRIBUTIONS
Conception and design: BM
Analysis and interpretation: BM, JW, ST
Data collection: MJ, JW, ST
Writing the article: BM ST, JW, ST, VV
Critical revision of the article: VV, ST,
Final approval of the article: BM, VV
Statistical analysis: BM, ST SB
Obtained funding: BM, MJ
Overall responsibility: BM
REFERENCES
1. Wylie EJ. Thromboendarterectomy for arteriosclerotic thrombosis of
major arteries. Surgery 1952;32:275-92.
2. de Vries SO, Hunink MG. Results of aortic bifurcation grafts for
aortoiliac occlusive disease: a meta-analysis. J Vasc Surg 1997;26:
558-69.
3. Littooy FN, Steffan G, Steinam S, Saletta C, Greisler HP. An 11-year
experience with aortofemoral bypass grafting. Cardiovasc Surg 1993;1:
232-8.
4. Schneider JR, Besso SR, Walsh DB, Zwolak RM, Cronenwett JL.
Femorofemoral versus aortobifemoral bypass: outcome and hemody-
namic results. J Vasc Surg 1994;19:43-55.
5. Huber TS, Harward TR, Flynn TC, Albright JL, Seeger JM. Operative
mortality rates after elective infrarenal aortic reconstructions. J Vasc
Surg 1995;22:287-93.
6. Erdoes LS, Bernhard VM, Berman SS. Aortofemoral graft occlusion:
strategy and timing of reoperation. Cardiovasc Surg 1995;3:277-83.
7. Passman MA, Taylor LM, Moneta GL, Edwards JM, Yeager RA,
McConnell DB, et al. Comparison of axillofemoral and aortofemoral
bypass for aortoiliac occlusive disease. J Vasc Surg 1996;23:263-9.
8. Rouers A, Meurisse N, Lavigne JP, Francart D, Quaniers J, Desiron Q,
et al. Potential benefits of laparoscopic aorto-bifemoral bypass surgery.
Acta Chir Belg 2005;105:610-5.
Fig 7. Kaplan-Meier curves for freedom from target vessel revascularization during the study period showed fewer
reinterventions occurred in the covered-stent group (P ⫽ .006 at 12 months). CI, Confidence interval; OR, odds ratio;
TVR, target vessel revascularization.
JOURNAL OF VASCULAR SURGERY
Volume 54, Number 6
Mwipatayi et al 1569
9. Dormandy JA, Rutherford RB. Management of peripheral arterial dis-
ease (PAD). TASC Working Group. TransAtlantic Inter-Society Con-
sensus (TASC). J Vasc Surg 2000;31:S1-296.
10. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes
FG, et al. Inter-Society Consensus for the Management of peripheral
Arterial Disease (TASC II). J Vasc Surg 2007;45(Suppl 1):S5-67.
11. Tetteroo E, van der Graaf Y, Bosch JL, van Engelen AD, Hunink MG,
Eikelboom BC, et al. Randomized comparison of primary stent place-
ment vs primary angioplasty followed by selective stent placement in
patients with iliac artery obstructive disease. Lancet 1998;351:1153-9.
12. Bosch JL, Hunink MG. Meta-analysis of the results of percutaneous
transluminal angioplasty and stent placement for aortoiliac occlusive
disease. Radiology 1997;204:87-96.
13. Marin ML, Veith FJ, Cynamon J, Parsons RE, Lyon RT, Suggs WD, et
al. Effect of polytetrafluoroethylene covering of Palmaz stents on the
development of intimal hyperplasia in human iliac arteries. J Vasc Interv
Radiol 1996;7:651-6.
14. Palmaz J. Intravascular stents: tissue-stent interactions and design con-
siderations. Am J Roentgenol 1993;160:613-8.
15. Diethrich EB. Polymeric covers and coats for metallic stents: micropo-
rous PTFE and the inhibition of intimal hyperplasia. J Endovasc Surg
1995;2:266-71.
16. Henry M, Amor M, Henry I, Klonaris C, Tzvetanov K, Buniet JM, et al.
Percutaneous endovascular treatment of peripheral aneurysms. J Car-
diovasc Surg (Torino) 2000;41:871-83.
17. Ohki T, Veith FJ. Five-year experience with endovascular grafts for the
treatment of aneurysmal, occlusive and traumatic arterial lesions. Car-
diovasc Surg 1998;6:552-65.
18. Wiesinger B, Beregi JP, Oliva VL, Dietrich T, Tepe G, Bosiers M, et al.
PTFE-covered self-expanding nitinol stents for the treatment of severe
iliac and femoral artery stenoses and occlusions: final results from a
prospective study. J Endovasc Ther 2005;12:240-6.
19. Ahn SS, Rutherford RB, Becker GJ, Comerota AJ, Johnston KW,
McClean GK, et al. Reporting standards for lower extremity arterial
endovascular procedures. Society for Vascular Surgery/International
Society for Cardiovascular Surgery. J Vasc Surg 1993;17:1103-7.
20. Zeller T, Tiefenbacher C, Steinkamp HJ, Langhoff R, Wittenberg G,
Schlüter M, et al. Nitinol stent implantation in TASC A and B superficial
femoral artery lesions: the Femoral Artery Conformexx Trial (FACT). J
Endovasc Ther 2008;15:390-8.
21. Krankenberg H, Schlüter M, Steinkamp HJ, Bürgelin K, Scheinert D,
Schulte KL, et al. Nitinol stent implantation versus percutaneous
transluminal angioplasty in superficial femoral artery lesions up to 10 cm
in length: the femoral artery stenting trial (FAST). Circulation 2007;
116:285-92.
22. Diehm N, Pattynama PM, Jaff MR, Cremonesi A, Becker GJ, Hopkins
LN, et al. Clinical endpoints in peripheral endovascular revasculariza-
tion trials: a case for standardized definitions. Eur J Vasc Endovasc Surg
2008;36:409-19.
23. Armitage P, Berry G, Matthews J. Statistical methods in medical re-
search. Oxford: Blackwell Science; 2002. p. 137-46.
24. Lin DY, Wei LJ. The robust inference for the Cox proportional hazards
model. J Am Stat Ass 1989;84:1074-8.
25. Palmaz JC, Laborde JC, Rivera FJ, Encarnacion CE, Lutz JD, Moss JG.
Stenting of the iliac arteries with the Palmaz stent: experience from a
multicenter trial. Cardiovasc Intervent Radiol 1992;15:291-7.
26. Strecker EP, Hagen B, Liermann D, Schneider B, Wolf HR,
Wambsganss J. Iliac and femoropopliteal vascular occlusive disease
treated with flexible tantalum stents. Cardiovasc Intervent Radiol 1993;
16:158-64.
27. Long AL, Sapoval MR, Beyssen BM, Auguste MC, Le Bras Y, Raynaud
AC, et al. Strecker stent implantation in iliac arteries: patency and
predictive factors for long-term success. Radiology 1995;194:739-44.
28. Motarjeme A, Keifer JW, Zuska AJ. Percutaneous transluminal angio-
plasty of the iliac arteries: 66 experiences. AJR Am J Roentgenol
1980;135:937-44.
29. Scheinert D, Schröder M, Ludwig J, Bräunlich S, Möckel M, Flachs-
kampf FA, et al. Stent-supported recanalization of chronic iliac artery
occlusions. Am J Med 2001;110:708-15.
30. Szilagyi DE, Elliott JP Jr, Smith RF, Reddy DJ, McPharlin M. A
thirty-year survey of the reconstructive surgical treatment of aortoiliac
occlusive disease. J Vasc Surg 1986;3:421-36.
31. Sacks D, Marinelli DL, Martin LG, Spies JB, Society of Interventional
Radiology Technology Assessment Committee. Reporting standards
for clinical evaluation of new peripheral arterial revascularization de-
vices. J Vasc Interv Radiol 2003;14:S395-404.
32. Vorwerk D, Guenther RW, Schürmann K, Wendt G, Peters I. Primary
stent placement for chronic iliac artery occlusions: follow-up results in
103 patients. Radiology 1995;194:745-9.
33. Vorwerk D, Günther RW, Schürmann K, Wendt G. Aortic and iliac
stenoses: follow-up results of stent placement after insufficient balloon
angioplasty in 118 cases. Radiology 1996;198:45-8.
34. Cikrit DF, Gustafson PA, Dalsing MC, Harris VJ, Lalka SG, Sawchuk
AP, et al. Long-term follow-up of the Palmaz stent for iliac occlusive
disease. Surgery 1995;118:608-13.
35. Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz
RS, et al. Pathology of acute and chronic coronary stenting in humans.
Circulation 1999;99:44-52.
36. Palmaz JC. Intravascular stents: tissue-stent interactions and design
considerations. Am J Roentgenol 1993;160:613-8.
Submitted Feb 19, 2011; accepted Jun 28, 2011.
Additional material for this article may be found online
at www.jvascsurg.org.
JOURNAL OF VASCULAR SURGERY
December 2011
1570 Mwipatayi et al

Appendix Fig (online only). Kaplan-Meier survival estimates are shown for patients with TransAtlantic Inter-Society
Consensus (TASC) B, C, and D lesions undergoing placement of the bare-metal stent and the covered (V12) stent.
JOURNAL OF VASCULAR SURGERY
Volume 54, Number 6
Mwipatayi et al 1570.e1
